Abstract
Hydroxyproline-rich glycoproteins (HRGPs) are a superfamily of plant cell wall proteins that function in diverse aspects of plant growth and development. This superfamily consists of three members: hyperglycosylated arabinogalactan proteins (AGPs), moderately glycosylated extensins (EXTs), and lightly glycosylated proline-rich proteins (PRPs). Hybrid and chimeric versions of HRGP molecules also exist. In order to “mine” genomic databases for HRGPs and to facilitate and guide research in the field, the BIO OHIO software program was developed that identifies and classifies AGPs, EXTs, PRPs, hybrid HRGPs, and chimeric HRGPs from proteins predicted from DNA sequence data. This bioinformatics program is based on searching for biased amino acid compositions and for particular protein motifs associated with known HRGPs. HRGPs identified by the program are subsequently analyzed to elucidate the following: (1) repeating amino acid sequences, (2) signal peptide and glycosylphosphatidylinositol lipid anchor addition sequences, (3) similar HRGPs via Basic Local Alignment Search Tool, (4) expression patterns of their genes, (5) other HRGPs, glycosyl transferase, prolyl 4-hydroxylase, and peroxidase genes coexpressed with their genes, and (6) gene structure and whether genetic mutants exist in their genes. The program was used to identify and classify 166 HRGPs from Arabidopsis (Arabidopsis thaliana) as follows: 85 AGPs (including classical AGPs, lysine-rich AGPs, arabinogalactan peptides, fasciclin-like AGPs, plastocyanin AGPs, and other chimeric AGPs), 59 EXTs (including SP5 EXTs, SP5/SP4 EXTs, SP4 EXTs, SP4/SP3 EXTs, a SP3 EXT, “short” EXTs, leucine-rich repeat-EXTs, proline-rich extensin-like receptor kinases, and other chimeric EXTs), 18 PRPs (including PRPs and chimeric PRPs), and AGP/EXT hybrid HRGPs.
The genomics era has produced vast amounts of biological data that await examination. In order to “mine” such data effectively, a bioinformatics approach can be utilized to identify genes of interest, subject them to various in silico analyses, and extract relevant biological information on them from various public databases. Examination of such data produces novel insights with respect to the genes in question and can be used to facilitate and guide further research in the field. Such is the case here, where bioinformatics tools were developed to identify, classify, and analyze members of the Hyp-rich glycoprotein (HRGP) superfamily encoded by the Arabidopsis (Arabidopsis thaliana) genome.
HRGPs are a superfamily of plant cell wall proteins that are subdivided into three families, arabinogalactan proteins (AGPs), extensins (EXTs), and Pro-rich proteins (PRPs), and extensively reviewed (Showalter, 1993; Kieliszewski and Lamport, 1994; Nothnagel, 1997; Cassab, 1998; José-Estanyol and Puigdomènech, 2000; Seifert and Roberts, 2007). However, it has become increasingly clear that the HRGP superfamily is perhaps better represented as a spectrum of molecules ranging from the highly glycosylated AGPs to the moderately glycosylated EXTs and finally to the lightly glycosylated PRPs. Moreover, hybrid HRGPs, composed of HRGP modules from different families, and chimeric HRGPs, composed of one or more HRGP modules within a non-HRGP protein, also can be considered part of the HRGP superfamily. Given that many HRGPs are composed of repetitive protein sequences, particularly the EXTs and PRPs, and many have low sequence similarity to one another, particularly the AGPs, BLAST searches typically identify only a few closely related family members and do not represent a particularly effective means to identify members of the HRGP superfamily in a comprehensive manner.
Building upon the work of Schultz et al. (2002) that focused on the AGP family, a new bioinformatics software program, BIO OHIO, developed at Ohio University, makes it possible to search all 28,952 proteins encoded by the Arabidopsis genome and identify putative HRGP genes. Two distinct types of searches are possible with this program. First, the program can search for biased amino acid compositions in the genome-encoded protein sequences. For example, classical AGPs can be identified by their biased amino acid compositions of greater then 50% Pro (P), Ala (A), Ser (S), and Thr (T), as indicated by greater than 50% PAST. Similarly, arabinogalactan peptides (AG peptides) are identified by biased amino acid compositions of greater then 35% PAST, but the protein (i.e. peptide) must also be between 50 and 90 amino acids in length. Likewise, PRPs can be identified by a biased amino acid composition of greater then 45% PVKCYT. Second, the program can search for specific amino acid motifs that are commonly found in known HRGPs. For example, SP4 pentapeptide and SP3 tetrapeptide motifs are associated with EXTs, a fasciclin H1 motif is found in fasciclin-like AGPs (FLAs), and PPVX(K/T) (where X is any amino acid) and KKPCPP motifs are found in several known PRPs (Fowler et al., 1999). In addition to searching for HRGPs, the program can analyze proteins identified by a search. For example, the program checks for potential signal peptide sequences and glycosylphosphatidylinositol (GPI) plasma member anchor addition sequences, both of which are associated with HRGPs (Showalter, 1993, 2001; Youl et al., 1998; Sherrier et al., 1999; Svetek et al., 1999). Moreover, the program can identify repeated amino acid sequences within the sequence and has the ability to search for bias amino acid compositions within a sliding window of user-defined size, making it possible to identify HRGP domains within a protein sequence.
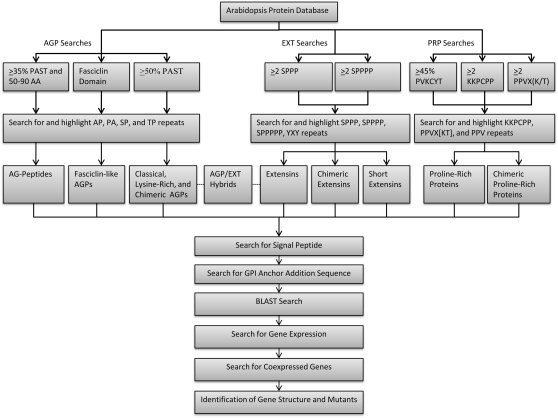
Here, we report on the use of this bioinformatics program in identifying, classifying, and analyzing members of the HRGP superfamily (i.e. AGPs, EXTs, PRPs, hybrid HRGPs, and chimeric HRGPs) in the genetic model plant Arabidopsis. An overview of this bioinformatics approach is presented in Figure 1. In addition, public databases and programs were accessed and utilized to extract relevant biological information on these HRGPs in terms of their expression patterns, most similar sequences via BLAST analysis, available genetic mutants, and coexpressed HRGP, glycosyl transferase (GT), prolyl 4-hydroxylase (P4H), and peroxidase genes in Arabidopsis. This information provides new insight to the HRGP superfamily and can be used by researchers to facilitate and guide further research in the field. Moreover, the bioinformatics tools developed here can be readily applied to protein sequences from other species to analyze their HRGPs or, for that matter, any given protein family by altering the input parameters.
Figure 1.
Bioinformatics workflow diagram summarizing the identification, classification, and analysis of HRGPs (AGPs, EXTs, and PRPs) in Arabidopsis. Classical AGPs were defined as containing greater than 50% PAST coupled with the presence of AP, PA, SP, and TP repeats distributed throughout the protein, Lys-rich AGPs were a subgroup of classical AGPs that included a Lys-rich domain, and chimeric AGPs were defined as containing greater than 50% PAST coupled with the localized distribution of AP, PA, SP, and TP repeats. AG peptides were defined to be 50 to 90 amino acids in length and containing greater than 35% PAST coupled with the presence of AP, PA, SP, and TP repeats distributed throughout the peptide. FLAs were defined as having a fasciclin domain coupled with the localized distribution of AP, PA, SP, and TP repeats. Extensins were defined as containing two or more SP3 or SP4 repeats coupled with the distribution of such repeats throughout the protein; chimeric extensins were similarly identified but were distinguished from the extensins by the localized distribution of such repeats in the protein; and short extensins were defined to be less than 200 amino acids in length coupled with the extensin definition. PRPs were identified as containing greater than 45% PVKCYT or two or more KKPCPP or PVX(K/T) repeats coupled with the distribution of such repeats and/or PPV throughout the protein. Chimeric PRPs were similarly identified but were distinguished from PRPs by the localized distribution of such repeats in the protein. Hybrid HRGPs (i.e. AGP/EXT hybrids) were defined as containing two or more repeat units used to identify AGPs, extensins, or PRPs. The presence of a signal peptide was used to provide added support for the identification of an HRGP but was not used in an absolute fashion. Similarly, the presence of a GPI anchor addition sequence was used to provide added support for the identification of classical AGPs and AG peptides, which are known to contain such sequences. BLAST searches were also used to provide some support to our classification if the query sequence showed similarity to other members of an HRGP subfamily. Note that some AGPs, particularly chimeric AGPs, and PRPs were identified from an Arabidopsis database annotation search and that two chimeric extensins were identified from the primary literature as noted in the text.
RESULTS
Finding and Classifying AGPs
The BIO OHIO program was used to identify potential classical AGPs, including the Lys-rich classical AGPs, AG peptides, and chimeric AGPs (i.e. FLAs and other chimeric AGPs) from the Arabidopsis proteome (Table I). The program initially identified 64 possible classical AGPs by searching for biased amino acid compositions of at least 50% PAST. Similarly, 86 potential AG peptides were identified by searching for proteins between 50 and 90 amino acids in length with biased amino acid compositions of at least 35% PAST. Finally, 25 potential FLAs were identified by searching for the following fasciclin H1 motif: [MALIT]T[VILS][FLCM][CAVT][PVLIS][GSTKRNDPEIV]+[DNS][DSENAGE]+[ASQM]. The 175 proteins identified by the program were further examined individually to determine if they appeared to be AGPs. The presence of a signal peptide was one such factor, as was the presence and location of AP, PA, SP, and TP repeats, since these dipeptide sequences are often present in known AGPs (Nothnagel, 1997). Finally, the presence of a GPI anchor addition sequence provided additional support, although not all AGPs have this sequence. By these criteria, 64 of the original 175 were classified as AGPs; moreover, they fall into several distinct classes: 20 classical AGPs, three Lys-rich (classical) AGPs, 16 AG peptides, 21 chimeric FLAs, three chimeric plastocyanin AGPs (PAGs), and one other chimeric AGP (Tables I and II). Additionally, one other AGP was documented in the literature, AGP30, a nonclassical or chimeric AGP, but was not identified by the program given that its PAST value of 34% was below the 50% threshold value used by the program (Baldwin et al., 2001; van Hengel and Roberts, 2003). Consequently, this AGP was added to the list of AGPs appearing in Table II but was not counted in Table I. In addition, four PRPs (PRP18, PRP5, PRP6, PRP16), 20 EXTs (EXT40, EXT17, EXT38, EXT19, EXT22, EXT18, EXT15, EXT7, EXT9, EXT10, EXT2, EXT11, EXT13, EXT16, EXT6, EXT12, EXT14, EXT8, EXT20, EXT21), and three hybrid AGP/EXTs (HAEs; HAE1, HAE3, HAE4) were identified by the program using the 50% PAST rule; further information on these HRGP sequences is presented below.
Table I. AGPs identified from the Arabidopsis genome based on biased amino acid compositions, size, and the presence of fasciclin domains.
The number in parentheses indicates the number of proteins that had a predicted signal peptide sequence.
| Search Criteria | Total | Classical AGP | Lys-Rich AGP | AG Peptide | FLA | Chimeric AGP | PRP | EXT | Hybrid | Others |
| ≥50% PAST | 64 (47) | 19 (16) | 3 (3) | 1 (1) | 0 | 4 (4) | 4 (3) | 20 (17) | 3 (2) | 10 (1) |
| ≥35% PAST and 50 to 90 amino acids | 86 (36) | 1 (1) | 0 | 16 (16) | 0 | 0 | 0 | 0 | 0 | 69 (20) |
| Fasciclin domain | 25 (21) | 0 | 0 | 0 | 21 (20) | 0 | 0 | 0 | 0 | 4 (2) |
Table II. Identification, characterization, and classification of the AGP genes in Arabidopsis.
| Locus Identifiera | Nameb | Class | AP/PA/SP/TP Repeats | PAST | Amino Acids | SPc | GPI | Organ-Specific Expression | Introns | P/5/E/I/3 Mutantsd | Top 5 BLAST Hit HRGPse |
| At1g24520 | AGP50C | Classical | 4/1/3/1 | 43% | 125 | Yes | Yes | Pollen | 0 | 1/0/1/0/0 | AGP11C, AGP6C, PAG17, AGP10C, AGP4C |
| At1g31250 | AGP51C | Classical | 1/2/10/8 | 54% | 165 | Yes | No | Siliques | 1 | 0/1/3/0/1 | AGP9C, AGP58C, AGP33I, PRP18, EXT51 |
| At1g35230 | AGP5C | Classical | 8/5/7/2 | 63% | 133 | Yes | Yes | Siliques, sepals | 0 | 0/0/1/0/0 | AGP10C, AGP7C, AGP4C, AGP2C, AGP1C |
| At1g63530 | AGP52C | Classical | 3/12/7/6 | 50% | 499 | No | No | 1 | 1/1/3/3/0 | AGP53C, AGP55C | |
| At1g63540 | AGP53C | Classical | 9/15/21/7 | 51% | 635 | No | No | Pollen | 1 | 0/0/2/0/3 | AGP52C, AGP55C |
| At2g14890 | AGP9C | Classical | 9/11/13/7 | 68% | 191 | Yes | Yes | 1 | 4/2/1/4/1 | AGP18K, AGP17K, AGP15P, PAG13, PAG8 | |
| At2g22470 | AGP2C | Classical | 8/5/6/4 | 71% | 131 | Yes | Yes | Roots | 0 | 2/6/0/0/6 | AGP3C, AGP7C, AGP4C, AGP10C, AGP5C |
| At2g28440 | AGP54C | Classical | 5/5/28/0 | 63% | 268 | Yes | No | Pollen | 0 | 2/0/3/0/0 | AGP57C, AGP9C, AGP1C, HAE1, AGP11C |
| At2g45000 | AGP55C | Classical | 15/14/14/16 | 56% | 739 | No | No | Roots, pollen | 8 | 0/6/7/4/4 | AGP53C, AGP52C, LRX5, PAG10, PAG17 |
| At2g47930 | AGP26C | Classical | 2/2/7/3 | 50% | 136 | Yes | Yes | 0 | 4/1/0/0/0 | HAE1, AGP2C, HAE4, PERK13 | |
| At3g01700 | AGP11C | Classical | 7/3/6/2 | 57% | 136 | Yes | Yes | Pollen | 0 | 0/2/4/0/2 | AGP6C, AGP21P |
| At3g06360 | AGP27C | Classical | 3/3/5/0 | 53% | 125 | Yes | Yes | 0 | 6/2/4/0/2 | AGP25C, AGP9C, AGP26C, AGP57C, AGP54C | |
| At3g22070 | AGP56C | Classical | 4/3/7/3 | 61% | 178 | Yes | No | 0 | 2/1/0/0/0 | PERK8, LRX3, LRX5, EXT51, PEX3 | |
| At3g45230 | AGP57C | Classical | 1/3/16/0 | 53% | 175 | Yes | No | 0 | 2/11/3/0/6 | AGP54C | |
| At4g09030 | AGP10C | Classical | 6/4/5/8 | 57% | 127 | Yes | Yes | 0 | 1/2/1/0/3 | AGP5C, AGP4C, AGP6C, AGP9C, AGP2C | |
| At4g16980 | AGP58C | Classical | 3/1/8/4 | 42% | 164 | Yes | Yes | 0 | 2/1/0/0/0 | AGP50C | |
| At4g40090 | AGP3C | Classical | 3/3/2/3 | 48% | 87 | Yes | No | Roots | 0 | 4/0/1/0/1 | AGP2C, PRP18 |
| At5g10430 | AGP4C | Classical | 8/11/4/9 | 54% | 135 | Yes | Yes | Roots | 0 | 3/2/2/0/0 | AGP7C, AGP5C, PRP14, EXT32 |
| At5g14380 | AGP6C | Classical | 9/3/8/1 | 48% | 150 | Yes | Yes | Pollen | 0 | 2/0/0/0/0 | AGP11C, AGP1C, AGP2C, FLA3, AGP9C |
| At5g18690 | AGP25C | Classical | 1/0/9/0 | 61% | 116 | Yes | Yes | Stems | 0 | 7/7/1/0/3 | AGP27C, AGP26C |
| At5g64310 | AGP1C | Classical | 7/8/12/1 | 72% | 131 | Yes | Yes | Roots | 0 | 0/0/0/0/0 | AGP7C, AGP2C, AGP18K, AGP4C, AGP15P |
| At5g65390 | AGP7C | Classical | 9/6/6/5 | 64% | 130 | Yes | Yes | Roots | 0 | 2/0/1/0/3 | AGP4C, AGP2C, AGP3C |
| At1g68725 | AGP19K | Lys-rich | 19/19/16/5 | 50% | 247 | Yes | No | 1 | 0/0/1/0/0 | AGP20P, AGP16P, AGP41P, AGP15P, AGP22P | |
| At2g23130 | AGP17K | Lys-rich | 13/12/10/5 | 59% | 185 | Yes | Yes | 1 | 1/0/12/0/0 | AGP18K, AGP9C, AGP15P | |
| At4g37450 | AGP18K | Lys-rich | 13/11/16/3 | 66% | 209 | Yes | Yes | 1 | 6/2/3/0/1 | AGP17K, AGP9C | |
| At1g51915 | AGP42P | AG peptide | 2/1/1/0 | 35% | 67 | Yes | No | Stamen | 1 | 0/0/1/0/0 | None |
| At1g55330 | AGP21P | AG peptide | 2/2/1/0 | 46% | 58 | Yes | Yes | 0 | 0/1/0/0/0 | AGP12P, AGP13P, AGP14P | |
| At2g41905 | AGP43P | AG peptide | 2/3/0/0 | 44% | 61 | Yes | Yes | nrf | 0 | 2/3/1/0/2 | AGP23P, PERK13 |
| At2g46330 | AGP16P | AG peptide | 3/2/0/0 | 41% | 73 | Yes | Nog | 1 | 2/1/0/0/0 | AGP20P, AGP41P, AGP22P, AGP15P, AGP21P | |
| At3g01730 | AGP44P | AG peptide | 1/0/2/1 | 45% | 87 | Yes | Yes | Roots | 0 | 1/0/3/0/3 | AGP16P, EXT38 |
| At3g13520 | AGP12P | AG peptide | 2/2/1/0 | 43% | 60 | Yes | Yes | 0 | 0/0/0/0/1 | AGP21P, AGP14P, AGP13P, AGP15P, AGP40P | |
| At3g20865 | AGP40P | AG peptide | 1/1/2/0 | 48% | 61 | Yes | Yes | Pollen | 0 | 4/1/2/0/2 | AGP2C, AGP15P |
| At3g57690 | AGP23P | AG peptide | 2/3/0/0 | 45% | 60 | Yes | Yes | Pollen | 0 | 6/1/0/0/4 | AGP43P |
| At3g61640 | AGP20P | AG peptide | 2/1/2/0 | 43% | 74 | Yes | No | 1 | 4/3/1/0/0 | AGP16P, AGP41P, AGP22P, AGP15P, PAG6 | |
| At4g26320 | AGP13P | AG peptide | 2/2/1/0 | 47% | 59 | Yes | Yes | Roots | 0 | 2/0/1/0/0 | AGP14P, AGP12P, AGP21P |
| At5g11740 | AGP15P | AG peptide | 2/1/1/0 | 50% | 61 | Yes | Yes | 0 | 2/4/0/0/1 | AGP12P, AGP13P, AGP21P, AGP41P, AGP20P | |
| At5g12880 | AGP45P | AG peptide | 1/0/3/0 | 43% | 73 | Yes | No | Roots | 0 | 6/2/2/0/3 | EXT17, EXT13, EXT20, EXT22, EXT15 |
| At5g24105 | AGP41P | AG peptide | 3/2/0/0 | 38% | 63 | Yes | Yes | nr | 1 | 3/2/0/1/0 | AGP16P, AGP20P, AGP22P |
| At5g40730 | AGP24P | AG peptide | 3/3/0/0 | 40% | 69 | Yes | Yes | Pollen | 0 | 3/0/0/0/1 | PRP8 |
| At5g53250 | AGP22P | AG peptide | 2/2/1/0 | 38% | 63 | Yes | Yes | Pollen, roots | 1 | 1/0/0/0/1 | AGP20P, AGP41P, AGP16P |
| At5g56540 | AGP14P | AG peptide | 2/1/1/0 | 41% | 60 | Yes | Yes | Roots | 0 | 3/4/2/0/1 | AGP13P, AGP12P, AGP21P, EXT31, PAG9 |
| At1g03870 | FLA9 | Chimeric | 6/4/4/0 | 31% | 247 | Yes | Yes | Roots | 0 | 4/2/3/0/2 | FLA13, FLA6, FLA11, FLA12, FLA7 |
| At1g15190 | FLA19 | Chimeric | 3/2/7/0 | 33% | 248 | Yes | No | 0 | 2/0/1/0/2 | FLA21, FLA20, LRX3, HAE1, EXT18 | |
| At2g04780 | FLA7 | Chimeric | 9/7/3/1 | 39% | 254 | Yes | Yes | 1 | 4/0/1/0/2 | FLA12, FLA9, FLA6, FLA13, FLA11 | |
| At2g20520 | FLA6 | Chimeric | 5/3/2/1 | 34% | 247 | Yes | No | Roots | 0 | 0/2/4/0/1 | FLA9, FLA13, FLA11, FLA12, FLA7 |
| At2g24450 | FLA3 | Chimeric | 11/7/4/2 | 38% | 280 | Yes | Yes | Pollen | 0 | 0/2/0/0/1 | FLA5, FLA14, FLA10, FLA8, FLA2 |
| At2g35860 | FLA16 | Chimeric | 9/6/3/1 | 28% | 445 | Yes | No | 1 | 1/1/1/2/3 | FLA15, FLA17, FLA18, FLA12, FLA13 | |
| At2g45470 | FLA8 | Chimeric | 13/6/8/3 | 43% | 420 | Yes | Yes | 0 | 4/2/5/0/1 | FLA10, FLA1, FLA2, FLA14, FLA3 | |
| At3g11700 | FLA18 | Chimeric | 8/3/1/0 | 25% | 462 | Yes | No | 2 | 8/3/7/5/0 | FLA17, FLA15, FLA16, FLA6, FLA12 | |
| At3g12660 | FLA14 | Chimeric | 2/2/4/0 | 35% | 255 | Yes | Yes | Stamen | 0 | 2/2/0/0/0 | FLA10, FLA8, FLA3, FLA1, FLA2 |
| At3g46550 | FLA4 | Chimeric | 1/4/4/1 | 37% | 420 | Yes | No | 0 | 3/3/4/0/0 | FLA10, FLA12, FLA6, FLA9, FLA11 | |
| At3g52370 | FLA15 | Chimeric | 10/4/2/1 | 28% | 436 | Yes | No | Roots | 1 | 5/6/6/1/0 | FLA16, FLA18, FLA17, FLA12, FLA6 |
| At3g60900 | FLA10 | Chimeric | 13/7/7/4 | 41% | 422 | Yes | Yes | Siliques, carpel | 0 | 10/8/5/0/3 | FLA8, FLA1, FLA2, FLA14, FLA3 |
| At4g12730 | FLA2 | Chimeric | 4/2/3/0 | 31% | 403 | Yes | No | 0 | 1/0/1/0/1 | FLA1, FLA8, FLA10, FLA14, FLA3 | |
| At4g31370 | FLA5 | Chimeric | 6/6/3/3 | 37% | 278 | Yes | Yes | 0 | 1/0/3/0/0 | FLA3, FLA14, FLA10, FLA2, FLA8 | |
| At5g03170 | FLA11 | Chimeric | 6/3/0/0 | 36% | 246 | Yes | Yes | Stems | 0 | 2/0/6/0/0 | FLA12, FLA9, FLA13, FLA6, FLA7 |
| At5g06390 | FLA17 | Chimeric | 9/5/2/0 | 26% | 458 | Yes | No | 2 | 12/2/6/1/0 | FLA18, FLA15, FLA16, FLA12, FLA13 | |
| At5g06920 | FLA21 | Chimeric | 0/0/6/2 | 32% | 353 | Yes | No | 0 | 0/2/4/0/0 | FLA19, FLA20 | |
| At5g40940 | FLA20 | Chimeric | 2/0/4/1 | 29% | 424 | No | No | 0 | 0/0/3/0/1 | FLA21, FLA19, FLA12 | |
| At5g44130 | FLA13 | Chimeric | 5/2/4/1 | 30% | 247 | Yes | Yes | 0 | 2/1/0/0/0 | FLA9, FLA6, FLA11, FLA12, FLA7 | |
| At5g55730 | FLA1 | Chimeric | 9/6/3/1 | 33% | 424 | Yes | Yes | 1 | 5/0/4/1/0 | FLA2, FLA8, FLA10, FLA14, FLA3 | |
| At5g60490 | FLA12 | Chimeric | 6/6/2/1 | 35% | 249 | Yes | Yes | Stems | 0 | 9/0/1/0/0 | FLA11, FLA13, FLA9, FLA6, FLA7 |
| At2g23990 | PAG1 | Chimeric | 7/7/3/3 | 39% | 207 | Yes | Yes | 1 | 0/1/0/0/1 | PAG12, PAG2, PAG15, PAG13, PAG7 | |
| At2g25060 | PAG2 | Chimeric | 3/3/3/0 | 31% | 182 | Yes | Yes | 1 | 3/3/2/1/0 | PAG13, PAG15, PAG12, PAG1, PAG7 | |
| At2g26720 | PAG3 | Chimeric | 1/2/3/1 | 30% | 206 | Yes | Yes | 0 | 2/0/0/0/0 | PAG4, PAG16, PAG5, PAG8, At3g53330 | |
| At2g31050 | PAG4 | Chimeric | 3/2/4/0 | 32% | 200 | Yes | Yes | Pollen | 0 | 1/0/0/0/1 | PAG3, PAG16, PAG5, PAG8, At3g53330 |
| At2g32300 | PAG5 | Chimeric | 3/4/6/2 | 46% | 261 | Yes | Yes | Roots | 2 | 0/0/1/0/0 | PAG3, PAG4, PAG16, PAG8, PAG2 |
| At2g44790 | PAG6 | Chimeric | 0/1/3/9 | 42% | 202 | Yes | Yes | Roots | 1 | 1/0/3/1/4 | PAG9, PAG8, PAG5, PAG3, PAG4 |
| At3g20570 | PAG7 | Chimeric | 4/3/4/3 | 38% | 203 | Yes | Yes | 1 | 5/1/1/0/1 | PAG2, PAG15, PAG13, PAG12, PAG17 | |
| At3g60270 | PAG8 | Chimeric | 3/1/8/1 | 38% | 187 | Yes | Yes | Roots | 1 | 8/0/2/0/0 | PAG9, PAG6, PAG4, PAG3, PAG16 |
| At3g60280 | PAG9 | Chimeric | 2/2/9/7 | 50% | 222 | Yes | Yes | Roots | 1 | 1/0/0/0/5 | PAG8, PAG6, PAG3, PAG5, PAG16 |
| At4g27520 | PAG10 | Chimeric | 10/4/20/4 | 52% | 349 | Yes | Yes | 1 | 7/4/0/0/0 | PAG17, PAG14, PAG11, PAG2, PAG7 | |
| At4g28365 | PAG11 | Chimeric | 2/2/6/1 | 31% | 199 | Yes | Yes | 1 | 4/2/1/0/0 | PAG14, PAG10, PAG17, PAG12, PAG7 | |
| At4g30590 | PAG12 | Chimeric | 4/3/3/1 | 31% | 190 | Yes | Yes | 1 | 4/3/1/0/0 | PAG1, PAG15, PAG13, PAG2, PAG7 | |
| At4g31840 | PAG13 | Chimeric | 1/1/3/1 | 31% | 177 | Yes | Yes | 1 | 0/1/7/0/5 | PAG2, PAG15, PAG12, PAG1, PAG7 | |
| At4g32490 | PAG14 | Chimeric | 5/4/6/3 | 33% | 221 | Yes | Yes | Siliques | 1 | 1/5/1/0/3 | PAG11, PAG10, PAG17, PAG2, PAG15 |
| At5g25090 | PAG15 | Chimeric | 3/4/4/0 | 32% | 186 | Yes | Yes | Shoot apex | 1 | 5/2/3/1/3 | PAG2, PAG12, PAG13, PAG7, PAG1 |
| At5g26330 | PAG16 | Chimeric | 0/2/2/1 | 40% | 187 | Yes | No | 1 | 0/0/1/1/3 | PAG3, PAG4, PAG5, PAG8, At3g53330 | |
| At5g53870 | PAG17 | Chimeric | 10/15/32/9 | 54% | 370 | Yes | Yes | 1 | 6/4/1/0/8 | PAG10, PAG11, PAG14, PAG7, PAG1 | |
| At1g03820 | AGP28I | Chimeric | 2/2/1/1 | 24% | 222 | Yes | No | 0 | 8/0/1/0/4 | PAG7 | |
| At1g28290 | AGP31I | Chimeric | 10/6/5/2 | 43% | 359 | Yes | No | Roots | 1 | 1/0/7/1/2 | AGP30I, PRP1, PRP11, PRP7, PAG17 |
| At1g36150 | AGP29I | Chimeric | 1/4/20/4 | 54% | 256 | Yes | Yes | Stamen | 2 | 2/0/1/0/0 | PEX1, PEX3, PERK8, HAE1, AGP19K |
| At2g33790 | AGP30I | Chimeric | 4/4/1/0 | 34% | 239 | Yes | No | Roots | 1 | 7/0/1/0/0 | AGP31I, PRP7, PRP11, PRP3, PRP1 |
| At5g21160 | AGP32I | Chimeric | 8/8/9/2 | 30% | 826 | No | No | 14 | 1/3/7/9/3 | LRX5, LRX3, PEX1, PEX3, LRX2 | |
| At5g56330 | AGP33I | Chimeric | 18/18/2/10 | 39% | 350 | Yes | No | Stamen | 6 | 1/2/2/3/1 | EXT51, LRX3, PRP16, PEX4, PRP17 |
Italics indicate a protein found using the Arabidopsis database annotation search.
Boldface indicates a protein that was not previously identified by Schultz et al. (2002). The letter designations in the names represent the following: C, classical AGP; P, AG peptide; K, Lys-rich classical AGP; I, chimeric AGP.
Signal peptide.
Indicates the number of mutants available in each location: P, promoter; 5, 5′ UTR; E, exon; I, intron; 3, 3′ UTR.
Underline indicates the result of a BLAST search with filtering turned off.
nr, Not reported. This indicates that data for a particular protein are not found in Genevestigator, Arabidopsis Membrane Protein Library, or MPSS.
Experimentally found to be GPI anchored (Schultz et al., 2004).
Some AGPs, particularly chimeric AGPs, can be below the 50% PAST threshold but were identified by searching the Arabidopsis protein database annotations and then subjecting such proteins to further analysis (i.e. searching for signal peptides, AP, PA, SP, and TP repeats, or GPI anchor addition sequences). With this approach, 21 additional AGPs were found, including two classical AGPs (AGP50C and AGP57C), 14 PAGs, and five other chimeric AGPs, including AGP30. The locus identifiers of these sequences are indicated in italics in Table II.
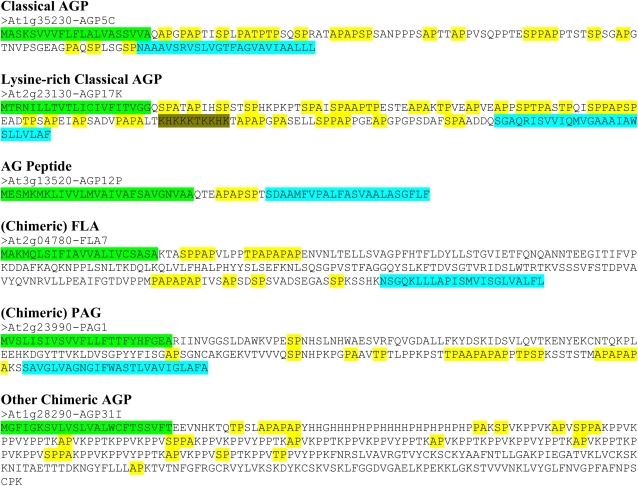
With the addition of these AGPs from the protein database annotations, the total number of potential AGPs became 85 and included 22 classical AGPs, three Lys-rich classical AGPs, 16 AG peptides, 21 chimeric FLAs, 17 chimeric PAGs, and six other chimeric AGPs (Table II). Representative amino acid sequences of these potential AGPs, including the predicted locations of their signal peptides and GPI anchor addition sequences, are displayed in Figure 2 and Supplemental Figure S1. The classical AGPs ranged in size from 87 to 739 amino acids. The majority (19 of 22) were predicted to have a signal peptide, and many (14 of 22) were also predicted to have a GPI anchor. The Lys-rich, classical AGPs ranged in size from 185 to 247 amino acids. All three were predicted to have a signal peptide, but only two were predicted to have a GPI anchor. The AG peptides ranged in size from 58 to 87 amino acids. All 16 AG peptides were predicted to have a signal peptide, but only 12 were predicted to have a GPI anchor. The FLAs ranged in size from 247 to 462 amino acids. The majority (20 of 21) were predicted to have a signal peptide, but only 11 were predicted to have a GPI anchor. The FLAs are a type of chimeric AGP; each FLA contains either one or two AGP domains. Such AGP domains were readily visualized with the BIO OHIO program by utilizing the sliding windows feature to search for biased amino acid sequences within a user-defined amino acid window size (e.g. 80% PAST in a 10-amino acid window) that slides along the protein sequence. Usually, such domains were also apparent by examining the location of the AP, PA, SP, and TP repeat units, which was easily done by the BIO OHIO program. The PAGs ranged in size from 177 to 370 amino acids. The 17 PAGs were all predicted to have a signal peptide, and 16 were predicted to have a GPI anchor. The other chimeric AGPs ranged in size from 222 to 826 amino acids. All but one (five of six) of these chimeric AGPs were predicted to have a signal peptide, and only one was predicted to have a GPI anchor as well as a signal peptide.
Figure 2.
Protein sequences encoded by representative AGP gene classes in Arabidopsis. Colored sequences at the N and C termini indicate predicted signal peptide (green) and GPI anchor (light blue) addition sequences if present. AP, PA, SP, and TP repeats (yellow) and Lys-rich regions (olive) are also indicated.
BLAST analysis was also conducted using The Arabidopsis Information Resource (TAIR) WU-Blast 2.0 to identify other potential AGP sequences and to provide insight to AGP sequences with the greatest similarity (Table II; Supplemental Table S1). BLAST searches were initially conducted with the filtering option on, but they were repeated with filtering off for those searches that found no other HRGPs. Such analysis showed that not all AGPs can be found with this method, but it did reveal sequences showing high degrees of similarity. BLAST was most successful for locating other FLAs and PAGs. In other words, a BLAST search using any one FLA sequence found most, but typically not all, other known FLA sequences.
AGP Gene Expression and Coexpressed HRGPs, GTs, P4Hs, and Peroxidases
In order to elucidate patterns of gene expression for these predicted AGPs, three public databases were searched: Genevestigator (https://www.genevestigator.ethz.ch/), the Arabidopsis Membrane Protein Library (http://www.cbs.umn.edu/arabidopsis/), and the Arabidopsis Massively Parallel Signature Sequencing (MPSS) Plus Database (http://mpss.udel.edu/at/). While about half of the AGPs had a broad range of expression throughout the plant, the other half showed organ-specific expression. Notably, several AGPs were specifically or preferentially expressed in the pollen, while others were expressed in roots, stems, leaves, and siliques (Table II; Supplemental Figs. S2–S5). Moreover, in examining the expression levels of all the AGP genes, the ones specifically or preferentially expressed in the pollen were the most highly expressed, as indicated by their high relative signal intensities. Furthermore, there was no observed correlation between organ-specific expression and a particular AGP class or between environmental stress-induced expression and a particular AGP class.
In order to elucidate HRGP gene networks and identify genes involved with AGP biosynthesis, the AGP genes were next examined with respect to coexpressed genes using The Arabidopsis Co-Response Database (http://csbdb.mpimp-golm.mpg.de/csbdb/dbcor/ath.html; Table III; Supplemental Table S2). Unfortunately, 39 of the 85 AGPs had no coexpression data available, so the following information was based on the 46 AGPs for which data were available. In analyzing the data, a focus was placed not only on other HRGPs but on GTs, P4Hs, and peroxidases, since GTs and P4Hs, and possibly peroxidases (Kjellbom et al., 1997), are responsible for posttranslational modification of AGPs. In terms of AGPs being expressed with other HRGPs, a total of 73 HRGPs were coexpressed with one or more AGPs. Among all HRGPs, FLA7 was coexpressed with the most AGPs, a total of 22 different AGPs. Interestingly, several different EXT and PRP genes were also coexpressed with numerous AGP genes. For the GTs, 27 of the 42 members of the GT2 family, 17 of the 42 members of the GT8 family, 11 of the 33 members of the GT47 family, and two of the three members of the GT29 family were coexpressed with various AGPs, to name just a few. Most notably, two members of the GT47 family (At5g22940 and At4g38040) were found to be coexpressed with 17 and 15 AGP genes, respectively. Also notable was the one member of the GT29 family (At1g08660) that was coexpressed with 14 different AGP genes and the three members of the GT8 family (At1g24170, At5g47780, At1g13250) that were coexpressed with 13, 11, and 10 different AGPs, respectively. In conducting this GT analysis, it was observed that not all of the CAZY members are annotated as GTs in the coexpression database. Consequently, coexpressed genes had to be cross-referenced against the gene identifiers listed in the CAZY database. For the P4Hs, five of 13 members of the P4H gene family were coexpressed with various AGPs. Among these, one P4H gene (At3g06300 or P4H2) was coexpressed with 10 different AGPs. Many peroxidase genes showed evidence of coexpression. The greatest amount of coexpression was exhibited by At4g26010, which was coexpressed with 13 different AGPs.
Table III. HRGPs, GTs, P4Hs, and peroxidases coexpressed with AGPs.
| HRGP Locus Identifier | Name | No. of Coexpressed AGPs | GT Locus Identifier | Name | (Family) | No. of Coexpressed AGPs | GT Locus Identifier Continued | Name | (Family) | No. of Coexpressed AGPs | P4H Locus Identifier | Name | No. of Coexpressed AGPs | Peroxidases Locus Identifier | Name | No. of Coexpressed AGPs |
| At2g04780 | FLA7 | 22 | At5g22940 | - | (GT47) | 17 | At1g05570 | Gsl06 | (GT48) | 3 | At3g06300 | P4H2 | 10 | At4g26010 | ATP13a | 13 |
| At1g03870 | FLA9 | 19 | At4g38040 | - | (GT47) | 15 | At1g06780 | - | (GT8) | 3 | At5g18900 | P4H4 | 4 | At1g05240 | - | 12 |
| At4g12730 | FLA2 | 19 | At4g39350 | CesA02 | (GT2) | 15 | At1g07240 | - | (GT1) | 3 | At2g17720 | P4H5 | 2 | At1g30870 | - | 12 |
| At4g16140 | EXT37 | 17 | At1g08660 | - | (GT29) | 14 | At1g16570 | - | (GT33) | 3 | At2g43080 | P4H1 | 1 | At3g49960 | - | 12 |
| At2g45470 | FLA8 | 16 | At1g24170 | - | (GT8) | 13 | At1g30530 | - | (GT1) | 3 | At5g66060 | P4H10 | 1 | At5g17820 | PER57 | 12 |
| At5g60490 | FLA12 | 16 | At4g02500 | - | (GT34) | 12 | At1g67880 | - | (GT17) | 3 | At5g67400 | PER73 | 12 | |||
| At4g16980 | AGP58C | 13 | At1g02730 | CslD5 | (GT2) | 11 | At1g73160 | - | (GT4) | 3 | At3g28200 | - | 10 | |||
| At5g10430 | AGP4C | 13 | At5g05170 | CesA03 | (GT2) | 11 | At2g35650 | CslA07 | (GT2) | 3 | At1g05260 | PER3 | 9 | |||
| At1g28290 | AGP31I | 12 | At5g47780 | - | (GT8) | 11 | At3g27540 | - | (GT17) | 3 | At2g43480 | - | 9 | |||
| At3g20570 | PAG7 | 12 | At5g50420 | - | (GT68) | 11 | At3g50740 | - | (GT1) | 3 | At5g24070 | - | 9 | |||
| At4g26320 | AGP13P | 12 | At1g13250 | - | (GT8) | 10 | At4g04970 | Gsl01 | (GT48) | 3 | At5g40150 | - | 9 | |||
| At5g56540 | AGP14P | 12 | At1g23480 | CslA03 | (GT2) | 10 | At4g31590 | CslC05 | (GT2) | 3 | At1g77490 | tAPX | 6 | |||
| At3g19430 | EXT51 | 11 | At1g70090 | - | (GT8) | 10 | At5g14850 | - | (GT22) | 3 | At4g09010 | - | 6 | |||
| At3g45230 | AGP57C | 11 | At2g03220 | FUT1 | (GT37) | 10 | At5g15050 | - | (GT14) | 3 | At4g21960 | PER42 | 6 | |||
| At4g37450 | AGP18K | 11 | At3g18170 | - | (GT61) | 10 | At5g38460 | - | (GT57) | 3 | At2g18980 | - | 5 | |||
| At5g55730 | FLA1 | 11 | At3g24040 | - | (GT14) | 10 | At5g41460 | - | (GT31) | 3 | At2g25080 | GPX1 | 5 | |||
| At1g62500 | PRP14 | 10 | At5g03760 | CslA09 | (GT2) | 10 | At1g14080 | FUT6 | (GT37) | 2 | At3g01190 | PER27 | 5 | |||
| At2g47930 | AGP26C | 10 | At5g19690 | - | (GT66) | 10 | At1g18580 | - | (GT8) | 2 | At4g11290 | - | 5 | |||
| At3g06750 | EXT34 | 10 | At1g34130 | - | (GT66) | 9 | At1g21480 | - | (GT47) | 2 | At4g33420 | - | 5 | |||
| At3g13520 | AGP12P | 10 | At1g74380 | - | (GT34) | 9 | At1g27120 | - | (GT31) | 2 | At4g35970 | - | 5 | |||
| At3g62680 | PRP3 | 10 | At2g15370 | FUT5 | (GT37) | 9 | At1g60470 | - | (GT8) | 2 | At2g22420 | PER17 | 4 | |||
| At4g31840 | PAG13 | 10 | At2g31750 | - | (GT1) | 9 | At1g68020 | - | (GT20) | 2 | At2g41480 | - | 4 | |||
| At5g53250 | AGP22P | 10 | At2g32620 | CslB04 | (GT2) | 9 | At1g68470 | - | (GT47) | 2 | At5g39580 | - | 4 | |||
| At5g65390 | AGP7C | 10 | At3g28180 | CslC04 | (GT2) | 9 | At1g71220 | - | (GT24) | 2 | At5g42180 | PER64 | 4 | |||
| At1g03820 | AGP28I | 9 | At5g22740 | CslA02 | (GT2) | 9 | At1g73370 | - | (GT4) | 2 | At2g18140 | - | 3 | |||
| At1g55330 | AGP21P | 9 | At1g19360 | - | (GT77) | 8 | At1g78800 | - | (GT4) | 2 | At2g43350 | - | 3 | |||
| At1g70990 | EXT33 | 9 | At2g22900 | - | (GT34) | 8 | At2g20370 | - | (GT47) | 2 | At4g30170 | - | 3 | |||
| At3g11700 | FLA18 | 9 | At3g25140 | - | (GT8) | 8 | At2g28080 | - | (GT1) | 2 | At2g37130 | PER21 | 2 | |||
| At4g27520 | PAG10 | 9 | At3g62720 | - | (GT34) | 8 | At2g29750 | - | (GT1) | 2 | At3g49120 | - | 2 | |||
| At1g52290 | PERK15 | 8 | At4g15290 | CslB05 | (GT2) | 8 | At2g35100 | - | (GT47) | 2 | At4g37530 | - | 2 | |||
| At4g13340 | LRX3 | 8 | At5g64740 | CesA06 | (GT2) | 8 | At2g41640 | - | (GT61) | 2 | At5g22410 | - | 2 | |||
| At2g10940 | PRP15 | 7 | At1g16900 | - | (GT22) | 7 | At3g21750 | - | (GT1) | 2 | At1g71695 | PER12 | 1 | |||
| At2g33790 | AGP30I | 7 | At1g27440 | - | (GT47) | 7 | At3g46970 | - | (GT35) | 2 | At2g35380 | PER20 | 1 | |||
| At3g54590 | EXT2 | 7 | At1g34270 | - | (GT47) | 7 | At3g50760 | - | (GT8) | 2 | At3g21770 | PER30 | 1 | |||
| At3g60900 | FLA10 | 7 | At1g71070 | - | (GT14) | 7 | At4g09500 | - | (GT1) | 2 | At3g63080 | - | 1 | |||
| At4g09030 | AGP10C | 7 | At2g37585 | - | (GT14) | 7 | At4g18230 | - | (GT1) | 2 | At4g08390 | sAPX | 1 | |||
| At5g06630 | EXT13 | 7 | At3g03050 | CslD3 | (GT2) | 7 | At4g18240 | - | (GT5) | 2 | At4g35000 | APX3 | 1 | |||
| At5g44130 | FLA13 | 7 | At4g00300 | - | (GT31) | 7 | At4g24000 | CslG2 | (GT2) | 2 | At5g06730 | - | 1 | |||
| At4g18670 | LRX5 | 6 | At4g02130 | - | (GT8) | 7 | At4g26940 | - | (GT31) | 2 | At5g66390 | PER72 | 1 | |||
| At5g06640 | EXT14 | 6 | At5g09870 | CesA05 | (GT2) | 7 | At5g01220 | - | (GT4) | 2 | ||||||
| At5g11740 | AGP15P | 6 | At5g11110 | - | (GT4) | 7 | At5g07720 | - | (GT34) | 2 | ||||||
| At5g25090 | PAG15 | 6 | At5g16190 | CslA11 | (GT2) | 7 | At5g16510 | - | (GT75) | 2 | ||||||
| At5g64310 | AGP1C | 6 | At5g17420 | CesA07 | (GT2) | 7 | At5g20410 | - | (GT28) | 2 | ||||||
| At1g23720 | EXT6 | 5 | At5g39990 | - | (GT14) | 7 | At5g66690 | - | (GT1) | 2 | ||||||
| At2g22470 | AGP2C | 5 | At5g61840 | - | (GT47) | 7 | At1g06000 | - | (GT1) | 1 | ||||||
| At2g25060 | PAG2 | 5 | At1g08280 | - | (GT29) | 6 | At1g06410 | - | (GT20) | 1 | ||||||
| At2g35860 | FLA16 | 5 | At1g19710 | - | (GT4) | 6 | At1g11720 | - | (GT5) | 1 | ||||||
| At3g24480 | LRX4 | 5 | At1g74800 | - | (GT31) | 6 | At1g12990 | - | (GT17) | 1 | ||||||
| At3g28550 | EXT9 | 5 | At3g02350 | - | (GT8) | 6 | At1g20575 | - | (GT2) | 1 | ||||||
| At3g52370 | FLA15 | 5 | At3g56000 | CslA14 | (GT2) | 6 | At1g23870 | - | (GT20) | 1 | ||||||
| At5g18690 | AGP25C | 5 | At4g01220 | - | (GT77) | 6 | At1g24070 | CslA10 | (GT2) | 1 | ||||||
| At5g21160 | AGP32I | 5 | At4g17770 | - | (GT20) | 6 | At1g24100 | - | (GT1) | 1 | ||||||
| At5g40730 | AGP24P | 5 | At4g32410 | CesA01 | (GT2) | 6 | At1g28710 | - | (GT77) | 1 | ||||||
| At2g24980 | EXT7 | 4 | At5g05860 | - | (GT1) | 6 | At1g43620 | - | (GT1) | 1 | ||||||
| At4g32710 | PERK14 | 4 | At5g15650 | - | (GT75) | 6 | At1g50580 | - | (GT1) | 1 | ||||||
| At5g03170 | FLA11 | 4 | At5g44030 | CesA04 | (GT2) | 6 | At1g60140 | - | (GT20) | 1 | ||||||
| At5g49280 | EXT41 | 4 | At5g55500 | - | (GT61) | 6 | At1g64910 | - | (GT1) | 1 | ||||||
| At1g23040 | EXT31 | 3 | At1g53290 | - | (GT31) | 5 | At1g64920 | - | (GT1) | 1 | ||||||
| At3g22120 | PRP16 | 3 | At2g24630 | CslC08 | (GT2) | 5 | At1g75420 | - | (GT4) | 1 | ||||||
| At3g24550 | PERK1 | 3 | At2g35610 | - | (GT77) | 5 | At1g77810 | - | (GT31) | 1 | ||||||
| At5g26330 | PAG16 | 3 | At2g44660 | - | (GT57) | 5 | At2g15480 | - | (GT1) | 1 | ||||||
| At1g09460 | PRP13 | 2 | At3g05320 | - | (GT65) | 5 | At2g19880 | - | (GT21) | 1 | ||||||
| At3g61640 | AGP20P | 2 | At3g62660 | - | (GT8) | 5 | At2g20810 | - | (GT8) | 1 | ||||||
| At5g09520 | PRP9 | 2 | At4g11350 | - | (GT31) | 5 | At2g25300 | - | (GT31) | 1 | ||||||
| At5g14920 | PRP18 | 2 | At4g23490 | - | (GT31) | 5 | At2g32430 | - | (GT31) | 1 | ||||||
| At1g26150 | PERK10 | 1 | At4g36890 | - | (GT43) | 5 | At2g37090 | - | (GT43) | 1 | ||||||
| At2g21140 | PRP2 | 1 | At5g02410 | - | (GT59) | 5 | At3g04240 | - | (GT41) | 1 | ||||||
| At2g43150 | EXT8 | 1 | At5g24300 | - | (GT5) | 5 | At3g07330 | CslC06 | (GT2) | 1 | ||||||
| At2g44790 | PAG6 | 1 | At5g62220 | - | (GT47) | 5 | At3g11670 | - | (GT4) | 1 | ||||||
| At3g57690 | AGP23P | 1 | At5g62620 | - | (GT31) | 5 | At3g15940 | - | (GT4) | 1 | ||||||
| At4g08410 | EXT10 | 1 | At1g10400 | - | (GT1) | 4 | At3g16520 | - | (GT1) | 1 | ||||||
| At4g30590 | PAG12 | 1 | At1g52420 | - | (GT4) | 4 | At3g21790 | - | (GT1) | 1 | ||||||
| At5g15780 | PRP11 | 1 | At2g38650 | - | (GT8) | 4 | At3g29630 | - | (GT1) | 1 | ||||||
| At3g11420 | - | (GT31) | 4 | At3g46720 | - | (GT1) | 1 | |||||||||
| At3g14570 | Gsl04 | (GT48) | 4 | At3g58790 | - | (GT8) | 1 | |||||||||
| At3g15350 | - | (GT14) | 4 | At4g01070 | - | (GT1) | 1 | |||||||||
| At3g29320 | - | (GT35) | 4 | At4g01750 | - | (GT77) | 1 | |||||||||
| At3g59100 | Gsl11 | (GT48) | 4 | At4g07960 | CslC12 | (GT2) | 1 | |||||||||
| At3g61130 | - | (GT8) | 4 | At4g15490 | - | (GT1) | 1 | |||||||||
| At4g31780 | - | (GT28) | 4 | At4g18780 | CesA08 | (GT2) | 1 | |||||||||
| At4g32120 | - | (GT31) | 4 | At4g19460 | - | (GT4) | 1 | |||||||||
| At5g05890 | - | (GT1) | 4 | At4g21060 | - | (GT31) | 1 | |||||||||
| At5g37180 | - | (GT4) | 4 | At4g22580 | - | (GT47) | 1 | |||||||||
| At5g53340 | - | (GT31) | 4 | At4g38240 | - | (GT13) | 1 | |||||||||
| At5g54690 | - | (GT8) | 4 | At5g05900 | - | (GT1) | 1 | |||||||||
| At5g16910 | CslD2 | (GT2) | 1 | |||||||||||||
| At5g44820 | - | (GT77) | 1 |
AGP Gene Organization and Mutants
Information was extracted from the TAIR and SALK Web sites with regard to the gene structure and available genetic mutants for each of the predicted AGP genes. The AGP genes contained few, if any, introns. Of the 85 AGPs, 46 had no introns and 32 had only one intron (Table II; Supplemental Table S3). One chimeric AGP (At5g21160 or AGP32I), however, was predicted to have 14 introns.
Examination of the various mutant lines available for research showed that nearly 99% (84 of 85) of the AGP genes had one or more mutants available. Of these mutants, 33% were in the promoter region, 19% were in the 5′ untranslated region (UTR), 25% were in an exon, 6% were in an intron, and 17% were in the 3′ UTR (Table II; Supplemental Table S4).
Finding and Classifying EXTs
The BIO OHIO program was used to identify potential EXTs by searching for SP3 and SP4 sequences repeated two or more times (Table IV). The program initially identified 114 and 63 potential EXTs by searching for these tetrapeptide and pentapeptide repeats, respectively.
Table IV. EXTs identified from the Arabidopsis genome based on SP3 and SP4 amino acid repeat units.
The number in parentheses indicates the number of proteins that had a predicted signal peptide sequence.
| Search Criteria | Total | EXT | AGP | PRP | Hybrid | Others |
| Two or more SP3 | 114 (52) | 57 (39) | 2 (2) | 0 | 4 (3) | 51 (10) |
| Two or more SP4 | 63 (41) | 50 (36) | 0 | 0 | 3 (2) | 10 (3) |
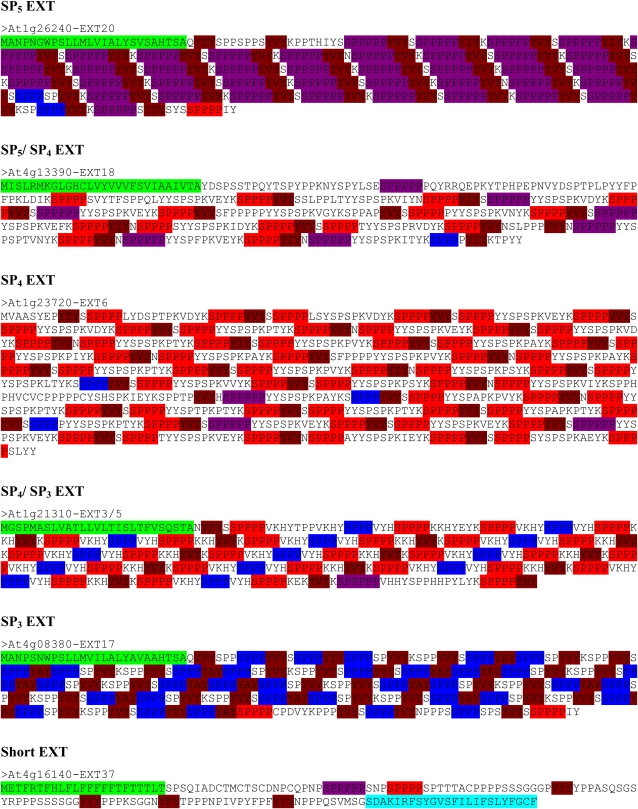
The 114 and 63 proteins identified by the program were further examined individually to determine if they appeared to be EXTs, with the realization that the 63 proteins are a subset of the 114. The presence of a signal peptide was one such factor, as was the presence and location of SP3, SP4, and SP5 repeats, since these peptide sequences are often present in known EXTs. GPI anchor addition sequences are not known to be associated with EXTs; nonetheless, testing for the presence of such a sequence was performed out of curiosity. By these criteria, 57 of the 114 and 50 of the 63 proteins were classified as EXTs. While the SP4 criteria resulted in a high percentage of EXT sequences, they did not locate all potential EXTs, given that the SP3 criteria were used to find more EXTs, but with a higher rate of false positives. Subsequent analysis involved examining the 57 EXT sequences and attempting to classify them. Based upon the repeat sequences found in these EXTs, they were placed into nine classes: three SP5 EXTs, two SP5/SP4 EXTs, 12 SP4 EXTs, two SP4/SP3 EXTs, one SP3 EXT, 12 short EXTs, 11 (chimeric) Leu-rich repeat EXTs (LRXs) that include pollen extensin-like (PEX) proteins, 11 (chimeric) Pro-rich extensin-like receptor kinases (PERKs), and three other chimeric EXTs (Tables IV and V; Fig. 3). YXY repeats were observed in most of the EXT sequences. Such sequences are involved in cross-linking EXTs (Brady et al., 1996, 1998; Schnabelrauch et al., 1996; Held et al., 2004; Cannon et al., 2008). Forty of the 59 EXTs identified contain this YXY sequence. Although YVY is the most common repeat, YIY, YYY, and YAY repeats also occur less frequently. Interestingly, several EXTs have a YPY sequence immediately following the signal peptide.
Table V. Identification, characterization, and classification of the EXT genes in Arabidopsis.
| Locus Identifiera | Nameb | Class | SP3/SP4/SP5/YXY Repeats | Amino Acids | SPc | GPI | Organ-Specific Expression | Introns | P/5/E/I/3 Mutantsd | Top Five BLAST Hit HRGPse |
| At1g26240 | EXT20 | SP5 | 2/1/40/44 | 478 | Yes | No | Roots | 0 | 1/3/2/0/0 | EXT17, EXT21, EXT22, LRX5, EXT1/4 |
| At1g26250 | EXT21 | SP5 | 7/0/28/40 | 443 | Yes | No | Roots | 0 | 1/0/5/0/3 | EXT1/4, EXT17, EXT20, EXT22, LRX5 |
| At4g08370 | EXT22 | SP5 | 3/1/13/18 | 350 | Yes | No | 2 | 1/0/0/0/0 | EXT20, EXT21, EXT17, LRX1, EXT7 | |
| At4g13390 | EXT18 | SP5/SP4 | 0/14/8/13 | 429 | Yes | No | Roots | 0 | 4/0/2/0/0 | EXT11, EXT12, EXT13, EXT14, EXT15 |
| At5g19810 | EXT19 | SP5/SP4 | 0/4/13/1 | 249 | Yes | No | Roots | 0 | 7/0/1/0/1 | EXT39, EXT35, EXT40, AGP9C |
| At1g23720 | EXT6 | SP4 | 2/61/3/34 | 895 | No | No | Roots | 0 | 1/3/0/0/0 | EXT1/4 |
| At2g24980 | EXT7 | SP4 | 3/37/0/21 | 559 | Yes | No | Roots | 0 | 0/0/1/0/1 | EXT13, EXT14, EXT11, EXT12, EXT16 |
| At2g43150 | EXT8 | SP4 | 0/22/0/9 | 212 | Yes | No | Roots | 0 | 1/0/1/0/1 | EXT10, EXT2, EXT7, EXT6, EXT9 |
| At3g28550 | EXT9 | SP4 | 3/70/0/35 | 1,018 | Yes | No | Roots | 0 | 0/0/1/0/0 | EXT10, EXT2, EXT6, EXT15, EXT14 |
| At3g54580 | EXT10 | SP4 | 2/68/0/33 | 951 | Yes | No | Roots | 0 | 0/0/1/0/2 | HAE3, EXT2, EXT9, EXT1/4, PRP3 |
| At3g54590 | EXT2 | SP4 | 2/51/0/24 | 743 | Yes | No | Roots | 0 | 2/0/0/0/0 | EXT10, EXT9, EXT1/4 |
| At4g08400 | EXT11 | SP4 | 2/31/0/26 | 513 | Yes | No | Pollen, roots | 0 | 2/1/1/0/0 | EXT12, EXT14, EXT13, EXT16, EXT7 |
| At4g08410 | EXT12 | SP4 | 2/41/0/26 | 707 | No | No | Roots | 0 | 3/0/0/0/0 | EXT11, EXT14, EXT13, EXT16, EXT7 |
| At5g06630 | EXT13 | SP4 | 1/29/0/17 | 440 | Yes | No | Roots | 0 | 3/2/1/0/0 | EXT7, EXT14, EXT11, EXT12, EXT16 |
| At5g06640 | EXT14 | SP4 | 2/42/0/25 | 689 | No | No | Roots | 0 | 1/0/2/0/0 | EXT12, EXT11, EXT13, EXT7, EXT16 |
| At5g35190 | EXT15 | SP4 | 2/12/2/8 | 328 | Yes | No | Roots | 0 | 2/0/1/0/1 | EXT11, EXT12, EXT7, EXT13, EXT16 |
| At5g49080 | EXT16 | SP4 | 0/41/0/23 | 609 | Yes | No | Roots | 0 | 1/0/0/0/0 | EXT11, EXT12, EXT7, EXT14, EXT13 |
| At1g21310 | EXT3/5 | SP4/SP3 | 13/27/1/14 | 431 | Yes | No | Radicle, roots | 1 | 0/1/0/2/0 | EXT1/4, HAE3 |
| At1g76930 | EXT1/4 | SP4/SP3 | 8/9/0/1 | 293 | Yes | No | Roots | 3 | 0/1/1/1/0 | EXT3/5, PAG10, PEX1, HAE3 |
| At4g08380 | EXT17 | SP3 | 34/2/0/49 | 437 | Yes | No | Roots | 0 | 5/1/0/0/0 | EXT20, EXT22, EXT21 |
| At1g02405 | EXT30 | Short | 0/3/0/0 | 134 | Yes | Yes | Siliques | 0 | 1/1/1/0/2 | EXT33, EXT31 |
| At1g23040 | EXT31 | Short | 0/2/0/0 | 144 | Yes | Yes | 0 | 4/5/1/0/1 | EXT33, EXT30 | |
| At1g54215 | EXT32 | Short | 0/1/1/0 | 169 | Yes | No | 0 | 1/1/0/0/0 | LRX6, LRX3, LRX2, PRP17, PEX4 | |
| At1g70990 | EXT33 | Short | 0/2/0/1 | 176 | No | Yes | Roots | 0 | 7/4/6/0/3 | EXT31, EXT30 |
| At3g06750 | EXT34 | Short | 0/1/1/1 | 147 | Yes | Yes | 0 | 5/0/2/0/8 | EXT41, EXT37 | |
| At3g20850 | EXT35 | Short | 1/0/1/2 | 134 | Yes | No | Roots | 0 | 0/0/8/0/1 | EXT40, EXT39 |
| At3g49270 | EXT36 | Short | 0/2/0/0 | 148 | Yes | No | Siliques | 2 | 1/0/2/0/55 | LRX1, LRX2, EXT32, EXT19, EXT39 |
| At4g16140 | EXT37 | Short | 0/1/1/4 | 164 | Yes | Yes | 0 | 1/0/2/0/1 | EXT41, EXT34 | |
| At5g11990 | EXT38 | Short | 4/0/1/1 | 181 | Yes | Yes | 0 | 1/2/2/0/1 | PEX4, EXT21, LRX1, LRX3, LRX5 | |
| At5g19800 | EXT39 | Short | 0/0/3/1 | 96 | Yes | No | Roots | 0 | 1/1/0/0/0 | EXT19, EXT35, EXT40 |
| At5g26080 | EXT40 | Short | 2/1/3/0 | 141 | Yes | No | Roots | 0 | 2/1/0/0/0 | EXT35, EXT39, EXT19, PERK13, PAG10 |
| At5g49280 | EXT41 | Short | 0/2/0/2 | 162 | Yes | Yes | nrf | 0 | 2/0/1/0/1 | EXT34, EXT37 |
| At1g12040 | LRX1 | Chimeric | 1/17/7/9 | 744 | Yes | No | Roots | 0 | 1/1/2/0/5 | LRX2, LRX3, LRX5, LRX4, LRX7 |
| At1g49490 | PEX2 | Chimeric | 1/13/1/0 | 847 | Yes | No | Pollen | 0 | 1/0/8/0/1 | PEX1, PEX3, PEX4, LRX5, LRX3 |
| At1g62440 | LRX2 | Chimeric | 4/12/6/3 | 826 | No | No | Roots | 2 | 3/1/7/5/1 | LRX1, LRX5, LRX4, LRX3, LRX6 |
| At2g15880 | PEX3 | Chimeric | 2/16/9/1 | 727 | No | No | Pollen | 1 | 2/1/8/0/2 | PEX4, PEX1, PEX2, LRX5, LRX3 |
| At3g19020 | PEX1 | Chimeric | 1/19/5/0 | 956 | Yes | No | Pollen | 0 | 0/3/7/0/0 | PEX2, PEX3, PEX4, LRX5, LRX4 |
| At3g22800 | LRX6 | Chimeric | 1/0/2/6 | 470 | Yes | No | Root | 0 | 3/2/5/0/1 | LRX3, LRX4, LRX5, LRX2, LRX1 |
| At3g24480 | LRX4 | Chimeric | 2/1/3/1 | 494 | Yes | No | 0 | 1/2/0/0/1 | LRX3, LRX5, LRX2, LRX6, LRX1 | |
| At4g13340 | LRX3 | Chimeric | 4/13/15/3 | 760 | Yes | No | 0 | 1/3/2/0/1 | LRX4, LRX5, LRX2, LRX6, LRX1 | |
| At4g18670 | LRX5 | Chimeric | 3/1/5/3 | 839 | Yes | No | 1 | 2/0/7/0/7 | LRX4, LRX3, LRX2, LRX6, LRX1 | |
| At4g33970 | PEX4 | Chimeric | 4/10/4/1 | 699 | Yes | No | Pollen | 0 | 2/3/7/0/1 | PEX3, PEX1, PEX2, LRX4, LRX5 |
| At5g25550 | LRX7 | Chimeric | 1/0/1/1 | 433 | Yes | No | Stamen | 0 | 3/0/1/0/2 | LRX4, LRX3, LRX5, LRX2, LRX1 |
| At1g10620 | PERK11 | Chimeric | 2/0/0/0 | 718 | No | No | Pollen | 7 | 9/0/11/2/1 | PERK12, PERK13, PERK8, PERK6, PERK10 |
| At1g23540 | PERK12 | Chimeric | 1/2/0/0 | 720 | No | No | Pollen | 7 | 9/2/3/0/0 | PERK13, PERK11, PERK8, PERK1, PERK10 |
| At1g26150 | PERK10 | Chimeric | 4/2/1/1 | 760 | No | No | 7 | 4/1/2/1/0 | PERK8, PERK13, PERK12, PERK1, PERK11 | |
| At1g49270 | PERK7 | Chimeric | 1/4/1/0 | 699 | No | No | Pollen | 6 | 2/1/3/3/0 | PERK6, PERK5, PERK1, PERK4, PERK13 |
| At1g52290 | PERK15 | Chimeric | 0/0/0/0 | 509 | No | No | 7 | 0/5/5/2/0 | PERK1, PERK5, PERK4, PERK6, PERK7 | |
| At1g70460 | PERK13 | Chimeric | 3/2/2/0 | 710 | No | No | Roots | 7 | 6/4/2/1/2 | PERK12, PERK11, PERK8, PERK1, PERK10 |
| At2g18470 | PERK4 | Chimeric | 1/0/1/1 | 633 | No | No | Pollen | 7 | 3/2/6/0/2 | PERK1, PERK6, PERK5, PERK7, PERK3 |
| At3g18810 | PERK6 | Chimeric | 1/1/2/0 | 700 | No | No | Pollen | 6 | 9/7/2/2/1 | PERK7, PERK5, PERK4, PERK1, PERK12 |
| At3g24540 | PERK3 | Chimeric | 0/1/1/0 | 509 | No | No | 8 | 0/0/5/0/0 | PERK1, PERK4, PERK5, PERK6, PERK7 | |
| At3g24550 | PERK1 | Chimeric | 3/0/0/0 | 652 | No | No | 7 | 5/3/2/0/0 | PERK4, PERK3, PERK5, PERK6, PERK7 | |
| At4g32710 | PERK14 | Chimeric | 0/0/0/0 | 388 | No | No | 7 | 0/2/4/0/2 | PERK1, PERK5, PERK15, PERK7, PERK6 | |
| At4g34440 | PERK5 | Chimeric | 2/0/0/0 | 670 | No | No | Pollen | 8 | 2/1/5/0/0 | PERK6, PERK7, PERK1, PERK4, PERK13 |
| At5g38560 | PERK8 | Chimeric | 5/2/2/3 | 681 | No | No | 7 | 4/0/5/1/0 | PERK10, PERK13, PERK12, PERK11, PERK1 | |
| At3g11030 | EXT50 | Chimeric | 0/5/0/0 | 451 | Yes | No | 4 | 23/0/2/1/0 | LRX6, LRX3, PEX2, PEX4, LRX2 | |
| At3g19430 | EXT51 | Chimeric | 0/7/0/0 | 559 | No | No | Root | 2 | 0/0/3/0/0 | LRX3, PEX3, PRP16, PEX1, LRX5 |
| At3g53330 | EXT52 | Chimeric | 0/3/0/2 | 310 | Yes | No | nr | 1 | 4/4/5/0/1 | PAG17, PAG9, PAG10 |
| At1g62760 | HAE1 | AGP/EXT hybrid | 2/0/2/0 | 312 | Yes | No | Pollen | 0 | 1/3/1/0/0 | LRX5, AGP54, PAG10, EXT51, AGP9 |
| At3g50580 | HAE2 | AGP/EXT hybrid | 1/2/1/0 | 265 | Yes | No | Stamen | 1 | 0/13/0/0/0 | PRP8 |
| At4g11430 | HAE3 | AGP/EXT hybrid | 2/0/2/0 | 219 | No | No | 1 | 0/0/0/1/0 | EXT37, LRX5, EXT19, LRX3, EXT1/4 | |
| At4g22470 | HAE4 | AGP/EXT hybrid | 2/1/0/0 | 375 | Yes | No | Leaves | 0 | 0/2/1/0/0 | PRP14, PRP16, PRP17, PRP15 |
Italics indicates a protein that did not meet our search criteria but was identified previously in the primary literature.
Boldface indicates a protein that was not previously identified in the primary literature or by Johnson et al. (2003b).
Signal peptide.
Indicates the number of mutants available in each location: P, promoter; 5, 5′ UTR; E, exon; I, intron; 3, 3′ UTR.
Underline indicates the result of a BLAST search with filtering turned off.
Not reported. This indicates that data for a particular protein are not found in Genevestigator, Arabidopsis Membrane Protein Library, or MPSS.
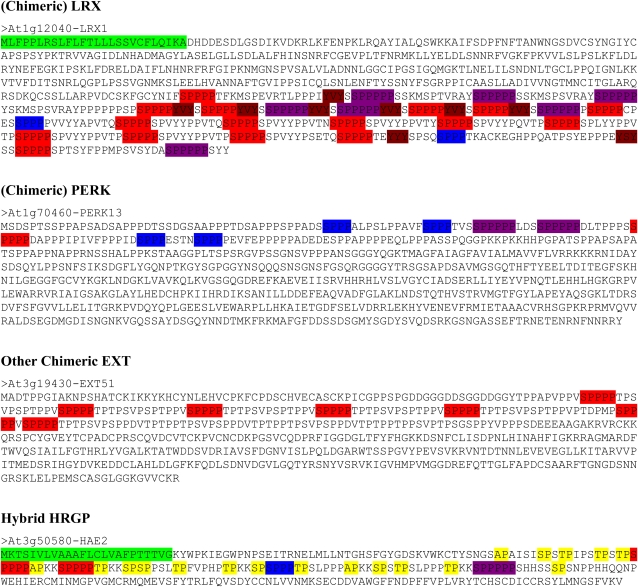
Figure 3.
(Figure continues on following page.)Protein sequences encoded by representative EXT and hybrid HRGP gene classes in Arabidopsis. Colored sequences at the N and C termini indicate predicted signal peptide (green) and GPI anchor (light blue) addition sequences if present. SP3 (blue), SP4 (red), SP5 (purple), and YXY (dark red) repeats are also indicated. AP, PA, SP, and TP (yellow) repeats are indicted on hybrid HRGP only.
The Arabidopsis protein database annotations were searched, but no additional EXTs were found beyond those already identified by the program. Additionally, four other PERKs were documented in the literature but were not identified by the program, because three (At5g24400 or PERK2, At1g68690 or PERK9, At4g32710 or PERK14) were not included in the Arabidopsis protein database and one (At1g52290 or PERK15) found in the database contained only one SPP. The PERK14 sequence was subsequently found on the TAIR Web site but lacked SP3/SP4 repeats. Nonetheless, PERK14 and PERK15, being members of the PERK family and having publicly available sequences, were added in italics to the list of EXTs appearing in Table V and subjected to subsequent analyses. PERK2 and PERK9 were described as pseudogenes on the TAIR Web site and had no sequences available. Thus, they were not added to the table or analyzed further. In addition, two AGPs (AGP9C, AGP19K) and four HAEs (HAE1, HAE2, HAE3, HAE4) were identified by the program using the SP3 rule. Analysis of these AGP sequences was already presented in the AGP section above; however, the four hybrid HRGPs were considered here along with the EXT family members.
The three other chimeric EXTs were annotated in the Arabidopsis protein database as late embryogenesis abundant protein (EXT50), expressed protein (EXT51), and plastocyanin-like protein (EXT52). EXT50, EXT51, and EXT52 contained five, seven, and three SP4 repeats, respectively. EXT51 also contained numerous TP and SP repeats, reminiscent of AGPs.
A hybrid HRGP was defined as a protein that contains sequence characteristics of different HRGPs, such as EXT and AGP sequence modules, within the same protein. The four hybrid proteins identified in the EXT search had sequence characteristics of both EXTs and AGPs. Three of these hybrids, HAE1, HAE3, and HAE4, were identified because they passed an EXT test as well as the classical AGP test, having at least 50% PAST and multiple PA and TP repeats. The other hybrid, HAE2, contained two SP4 repeats and one additional SP3 module but did not pass the 50% PAST threshold, having only 43% PAST. Nonetheless, it contained multiple AP, PA, SP, and TP repeats, which are indicative of AGPs.
BLAST analysis was also conducted with each of the EXTs, chimeric EXTs, and HAEs to identify other related sequences and to provide insight to EXT sequences with the greatest similarity (Table V; Supplemental Table S1). Such analysis showed that not all EXTs were found with this method but did reveal sequences showing high degrees of similarity and clearly showed many more potential EXT sequences compared with the results from the similar strategy for analysis of the AGPs. Such BLAST analysis of LRXs and PERKs proved especially effective, as a BLAST query using any one LRX or PERK resulted in the identification of all other members in their respective class. Analysis of the other chimeric EXTs revealed that only EXT52 resulted in BLAST hits; these hits were PAG17, PAG9, and PAG10. This result was expected, since EXT52 contains a plastocyanin domain along with the EXT motifs. BLAST analysis of the At4g11430 hybrid HRGP (HAE3) as the query sequence showed similarity to both AGP and EXT genes, providing support for its identification as a hybrid HRGP. BLAST results for the other HAEs were less informative, with HAE1 showing similarity to no other HRGPs and HAE2 and HAE4 showing similarity to only one PRP and multiple chimeric PRPs, respectively.
As seen in Table V and in Supplemental Figure S6, the 20 SP5, SP5/SP4, SP4, SP4/SP3, and SP3 EXTs ranged in size from 212 to 1,018 amino acids. The majority (17 of 20) were predicted to have a signal peptide, and none was predicted to have a GPI anchor. The 12 short EXTs ranged in size from 96 to 181 amino acids. All but one was predicted to have a signal peptide, and surprisingly, seven were predicted to have a GPI anchor. The 11 LRXs ranged in size from 433 to 956 amino acids and consisted of an N-terminal Leu-rich repeat domain and a C-terminal EXT domain. All but two were predicted to have a signal peptide, and none was predicted to have a GPI anchor. The 13 PERKs ranged in size from 509 to 760 amino acids and consisted of an N-terminal EXT domain and a C-terminal kinase domain. None was predicted to have a signal peptide or a GPI anchor. The three chimeric EXTs contained three to seven diagnostic EXT repeats; two had signal peptides, and none contained GPI anchor addition sequences. The four HAEs contained 219 to 375 amino acids; three had a signal peptide and none had GPI anchor addition sequences. The EXT domains/motifs in the LRXs, PERKs, and other chimeric EXTs as well as the EXT/AGP hybrids were readily visualized with the BIO OHIO program by observing the locations of the SP3, SP4, and SP5 repeat units.
EXT Gene Expression and Coexpressed HRGPs, GTs, P4Hs, and Peroxidases
In order to elucidate patterns of gene expression for these predicted EXTs, including the various chimeric EXTs and four HRGP hybrids, the same three public databases were searched as with the AGPs. While several EXTs had a broad range of expression throughout the plant, most of the EXT genes showed organ-specific expression. Notably, several EXTs were specifically or preferentially expressed in the root (27), while several others were specifically or preferentially expressed in the pollen/stamen (14) or siliques (one; Table V; Supplemental Figs. S7–S10). Moreover, in examining the expression levels of all the EXT genes, many of those specifically or preferentially expressed in the pollen were the most highly expressed ones, as indicated by their high relative signal intensities.
Next, the EXT and hybrid HRGP genes were examined with respect to coexpressed genes (Table VI; Supplemental Table S5). For EXTs, there was no information for 29 out of the 59 genes in The Arabidopsis Co-Response Database, and the four hybrid HRGP genes were also not listed in this database. In analyzing the data, a focus was placed not only on other HRGPs but on GTs, P4Hs, and peroxidases, since GTs, P4Hs, and EXT peroxidases are responsible for posttranslational modification of EXTs; this approach represents one potential avenue to identify genes involved in the posttranslational modification of EXTs. In terms of EXTs being expressed with other HRGPs, a total of 67 HRGPs were coexpressed with one or more EXTs. The most highly coexpressed HRGP was FLA2, which was coexpressed with a total of 15 EXTs, while FLA9 was next on the list, being coexpressed with 14 EXTs. As reported above, FLA2 and FLA9 were also coexpressed with many AGP genes. A number of EXT genes, including EXT9, EXT13, EXT14, EXT6, EXT10, EXT2, and LRX4, were also coexpressed with 10 or more EXT genes.
Table VI. HRGPs, GTs, P4Hs, and peroxidases coexpressed with EXTs.
| HRGP Locus Identifier | Name | No. of Coexpressed EXTs | GT Locus Identifier | Name | Family | No. of Coexpressed EXTs | GT Locus Identifier Continued | Name | Family | No. of Coexpressed EXTs | P4H Locus Identifier | Name | No. of Coexpressed EXTs | Peroxidase Locus Identifier | Name | No. of Coexpressed EXTs |
| At4g12730 | FLA2 | 15 | At2g32620 | CslB04 | GT2 | 9 | At4g36890 | GT43 | 2 | At3g06300 | P4H2 | 6 | At1g05240 | 8 | ||
| At1g03870 | FLA9 | 14 | At1g24170 | GT8 | 7 | At4g38040 | GT47 | 2 | At2g17720 | P4H5 | 2 | At3g49960 | 8 | |||
| At3g28550 | EXT9 | 11 | At1g74380 | GT34 | 7 | At5g03760 | CslA09 | GT2 | 2 | At2g43080 | P4H1 | 1 | At4g26010 | ATP13a | 8 | |
| At5g06630 | EXT13 | 11 | At4g15290 | CslB05 | GT2 | 7 | At5g05860 | GT1 | 2 | At5g18900 | P4H4 | 1 | At5g17820 | PER57 | 8 | |
| At5g06640 | EXT14 | 11 | At5g22940 | GT47 | 7 | At5g07720 | GT34 | 2 | At5g67400 | PER73 | 8 | |||||
| At1g23720 | EXT6 | 10 | At3g18170 | GT61 | 6 | At5g14850 | GT22 | 2 | At1g30870 | 7 | ||||||
| At3g24480 | LRX4 | 10 | At3g24040 | GT14 | 6 | At5g15050 | GT14 | 2 | At3g28200 | 7 | ||||||
| At3g54580 | EXT10 | 10 | At4g39350 | CesA02 | GT2 | 6 | At5g16910 | CslD2 | GT2 | 2 | At5g22410 | 6 | ||||
| At3g54590 | EXT2 | 10 | At1g08660 | GT29 | 5 | At5g20830 | GT4 | 2 | At4g33420 | 4 | ||||||
| At4g08410 | EXT12 | 9 | At1g13250 | GT8 | 5 | At5g24300 | GT5 | 2 | At5g39580 | 4 | ||||||
| At4g26320 | AGP13 | 9 | At3g61130 | GT8 | 5 | At5g41460 | GT31 | 2 | At1g77490 | tAPX | 3 | |||||
| At2g24980 | EXT7 | 8 | At4g00300 | GT31 | 5 | At5g44820 | GT77 | 2 | At2g25080 | GPX1 | 3 | |||||
| At3g19430 | EXT51 | 8 | At4g01750 | GT77 | 5 | At5g61840 | GT47 | 2 | At4g09010 | 3 | ||||||
| At5g10430 | AGP4C | 8 | At5g05170 | CesA03 | GT2 | 5 | At1g03520 | GT14 | 1 | At4g37530 | 3 | |||||
| At3g62680 | PRP3 | 7 | At1g02730 | CslD5 | GT2 | 4 | At1g05570 | Gsl06 | GT48 | 1 | At5g19890 | 3 | ||||
| At1g26250 | EXT21 | 6 | At1g27120 | GT31 | 4 | At1g06780 | GT8 | 1 | At5g40150 | 3 | ||||||
| At2g43150 | EXT8 | 6 | At2g03220 | FUT1 | GT37 | 4 | At1g16900 | GT22 | 1 | At1g05260 | PER3 | 2 | ||||
| At2g45470 | FLA8 | 6 | At2g31790 | GT1 | 4 | At1g19360 | GT77 | 1 | At2g31570 | 2 | ||||||
| At3g11700 | FLA18 | 5 | At3g03050 | CslD3 | GT2 | 4 | At1g19710 | GT4 | 1 | At3g03670 | 2 | |||||
| At3g13520 | AGP12P | 5 | At3g05320 | GT65 | 4 | At1g23870 | GT20 | 1 | At3g63080 | 2 | ||||||
| At4g16980 | AGP58C | 5 | At3g28180 | CslC04 | GT2 | 4 | At1g27440 | GT47 | 1 | At2g18140 | 1 | |||||
| At5g44130 | FLA13 | 5 | At4g38240 | GT13 | 4 | At1g32900 | GT5 | 1 | At2g22420 | PER17 | 1 | |||||
| At5g53250 | AGP22P | 5 | At5g05890 | GT1 | 4 | At1g34130 | GT66 | 1 | At2g37130 | PER21 | 1 | |||||
| At1g52290 | PERK15 | 4 | At5g09870 | CesA05 | GT2 | 4 | At1g34270 | GT47 | 1 | At2g41480 | 1 | |||||
| At1g55330 | AGP21P | 4 | At5g47780 | GT8 | 4 | At1g50580 | GT1 | 1 | At2g43480 | 1 | ||||||
| At2g10940 | PRP15 | 4 | At5g64740 | CesA06 | GT2 | 4 | At1g51210 | GT1 | 1 | At3g21770 | PER30 | 1 | ||||
| At3g06750 | EXT34 | 4 | At1g18580 | GT8 | 3 | At1g68020 | GT20 | 1 | At4g08770 | 1 | ||||||
| At4g13340 | LRX3 | 4 | At1g23480 | CslA03 | GT2 | 3 | At1g70090 | GT8 | 1 | At4g11290 | 1 | |||||
| At4g27520 | PAG10 | 4 | At1g24070 | CslA10 | GT2 | 3 | At1g71220 | GT24 | 1 | At4g11600 | 1 | |||||
| At4g37450 | AGP18K | 4 | At1g70290 | GT20 | 3 | At1g74800 | GT31 | 1 | At4g35000 | APX3 | 1 | |||||
| At1g21310 | EXT3/5 | 3 | At1g73160 | GT4 | 3 | At2g25300 | GT31 | 1 | At5g24070 | 1 | ||||||
| At1g28290 | AGP31I | 3 | At2g22900 | GT34 | 3 | At2g31960 | Gsl03 | GT48 | 1 | At5g64120 | 1 | |||||
| At2g04780 | FLA7 | 3 | At2g30150 | GT1 | 3 | At2g32530 | CslB02 | GT2 | 1 | |||||||
| At3g45230 | AGP57C | 3 | At3g62720 | GT34 | 3 | At2g35610 | GT77 | 1 | ||||||||
| At4g16140 | EXT37 | 3 | At4g02130 | GT8 | 3 | At3g01180 | GT5 | 1 | ||||||||
| At4g31840 | PAG13 | 3 | At4g07960 | CslC12 | GT2 | 3 | At3g07020 | GT1 | 1 | |||||||
| At4g32710 | PERK14 | 3 | At5g19690 | GT66 | 3 | At3g10630 | GT4 | 1 | ||||||||
| At5g11740 | AGP15P | 3 | At5g39990 | GT14 | 3 | At3g15350 | GT14 | 1 | ||||||||
| At5g21160 | AGP32I | 3 | At5g65685 | GT5 | 3 | At3g21750 | GT1 | 1 | ||||||||
| At5g55730 | FLA1 | 3 | At5g66690 | GT1 | 3 | At3g28340 | GT8 | 1 | ||||||||
| At5g56540 | AGP14P | 3 | At1g06000 | GT1 | 2 | At3g45100 | GT4 | 1 | ||||||||
| At2g21140 | PRP2 | 2 | At1g07240 | GT1 | 2 | At3g55710 | GT1 | 1 | ||||||||
| At2g33790 | AGP30I | 2 | At1g10400 | GT1 | 2 | At3g59100 | Gsl11 | GT48 | 1 | |||||||
| At2g47930 | AGP26C | 2 | At1g30530 | GT1 | 2 | At4g01220 | GT77 | 1 | ||||||||
| At3g52370 | FLA15 | 2 | At1g43620 | GT1 | 2 | At4g04970 | Gsl01 | GT48 | 1 | |||||||
| At3g61640 | AGP20P | 2 | At1g53290 | GT31 | 2 | At4g09500 | GT1 | 1 | ||||||||
| At4g09030 | AGP10C | 2 | At1g71070 | GT14 | 2 | At4g15550 | GT1 | 1 | ||||||||
| At4g18670 | LRX5 | 2 | At1g78580 | GT20 | 2 | At4g16600 | GT8 | 1 | ||||||||
| At5g15780 | PRP11 | 2 | At2g15370 | FUT5 | GT37 | 2 | At4g18230 | GT1 | 1 | |||||||
| At5g40730 | AGP24P | 2 | At2g18700 | GT20 | 2 | At4g21060 | GT31 | 1 | ||||||||
| At1g26150 | PERK10 | 1 | At2g20370 | GT47 | 2 | At4g23490 | GT31 | 1 | ||||||||
| At1g26240 | EXT20 | 1 | At2g20810 | GT8 | 2 | At4g24000 | CslG2 | GT2 | 1 | |||||||
| At1g62500 | PRP14 | 1 | At2g24630 | CslC08 | GT2 | 2 | At4g31590 | CslC05 | GT2 | 1 | ||||||
| At1g70990 | EXT33 | 1 | At2g35650 | CslA07 | GT2 | 2 | At5g01220 | GT4 | 1 | |||||||
| At2g22470 | AGP2C | 1 | At2g37585 | GT14 | 2 | At5g11110 | GT4 | 1 | ||||||||
| At2g25060 | PAG2 | 1 | At2g44660 | GT57 | 2 | At5g12890 | GT1 | 1 | ||||||||
| At2g35860 | FLA16 | 1 | At3g02350 | GT8 | 2 | At5g15650 | GT75 | 1 | ||||||||
| At3g22120 | PRP16 | 1 | At3g16520 | GT1 | 2 | At5g22740 | CslA02 | GT2 | 1 | |||||||
| At3g22800 | LRX6 | 1 | At3g27540 | GT17 | 2 | At5g38460 | GT57 | 1 | ||||||||
| At3g24550 | PERK1 | 1 | At3g29320 | GT35 | 2 | At5g50420 | GT68 | 1 | ||||||||
| At3g60900 | FLA10 | 1 | At3g46970 | GT35 | 2 | At5g62220 | GT47 | 1 | ||||||||
| At5g14920 | PRP18 | 1 | At3g56000 | CslA14 | GT2 | 2 | ||||||||||
| At5g25090 | PAG15 | 1 | At4g02500 | GT34 | 2 | |||||||||||
| At5g53870 | PAG17 | 1 | At4g18240 | GT5 | 2 | |||||||||||
| At5g60490 | FLA12 | 1 | At4g31780 | GT28 | 2 | |||||||||||
| At5g64310 | AGP1C | 1 | At4g32120 | GT31 | 2 | |||||||||||
| At5g65390 | AGP7C | 1 | At4g32410 | CesA01 | GT2 | 2 |
For the GTs, the most coexpressed was CslB04, a member of the GT2 family, which was coexpressed with nine EXTs. Also highly coexpressed were At1g24170 (GT8), At1g74380 (GT34), At4g15290 (GT2), and At5g22940 (GT47), all of which were coexpressed with seven EXTs. Notably, several of the GTs that were coexpressed with EXTs were also coexpressed with AGPs. For example, one member of the GT8 family, At1g24170, was coexpressed with seven different EXTs and 13 different AGPs. For the P4Hs, four of 13 members of the P4H gene family were coexpressed with various EXTs. Among these, one P4H gene (At3g06300 or P4H2) was coexpressed with six different EXTs. As reported above, this P4H gene was also coexpressed with 10 different AGPs. Many peroxidase genes were coexpressed, but the greatest amount of coexpression was exhibited by At1g05240, At3g49960, At4g26010, At5g17820, and At5g67400, which were all coexpressed with eight different EXTs. Interestingly, these same peroxidase genes were coexpressed with the greatest number of AGP genes as well (Table III). Given that EXTs are known to be cross-linked at YXY sequence motifs by an EXT peroxidase with an acidic pI, it was interesting to observe that the At3g03670-encoded peroxidase, which had a predicted endomembrane localization and a predicted pI of 4.8, was coexpressed with two of the three EXTs containing the greatest numbers of YXY sequence repeats (i.e. EXT20 and EXT21).
EXT Gene Organization and Mutants
Information was extracted from the TAIR and SALK Web sites with regard to the gene structure and available genetic mutants for each of the predicted EXTs. With the exception of the PERK genes, EXT genes including the four HRGP hybrid genes contain few, if any, introns (Table V; Supplemental Table S6). Of the 46 non-PERK EXT genes, 36 had no introns and eight had only one or two introns. All four HAEs contained either zero or one intron. One chimeric EXT (At3g11030), however, was predicted to have four introns. In contrast, the PERK genes contained between six and eight introns.
Examination of the various mutant lines available for research showed that all of the EXT genes (including HAEs) had one or more mutants available. Of these mutants, 29% are in the promoter region, 17% are in the 5′ UTR, 30% are in an exon, 4% are in an intron, and 20% are in the 3′ UTR (Table V; Supplemental Table S7).
Finding and Classifying PRPs
The BIO OHIO program was used to identify potential PRPs primarily by searching for proteins with a biased amino acid composition of at least 45% PVKCYT. In addition, PRPs were identified by searching for KKPCPP and PPVX(K/T) sequences repeated two or more times (Fowler et al., 1999). The program initially identified 113 potential PRPs by searching for 45% PVKCYT and identified 13 and two potential PRPs by searching for the PPVX(K/T) and KKPCPP repeats, respectively. Eleven of these 13 potential PRPs and both of these two potential PRPs were also identified with the 45% PVKCYT search criteria (Table VII).
Table VII. PRPs identified from the Arabidopsis genome based on biased amino acid composition and repeat units.
The number in parentheses indicates the number of proteins that had a predicted signal peptide sequence.
| Search Criteria | Total | PRPs | AGP | EXT | Hybrid | Other |
| ≥45% PVKCYT | 113 (64) | 15 (14) | 10 (10) | 31 (26) | 3 (2) | 54 (12) |
| Two or more KKPCPP | 2 (2) | 2 (2) | 0 | 0 | 0 | 0 |
| Two or more PPVX[KT] | 13 (11) | 7 (7) | 2 (2) | 1 (1) | 1 (0) | 2 (1) |
The 113 proteins identified by the program were further examined individually to determine if they appeared to be PRPs. The presence of a signal peptide was one such factor, as was the presence and location of PPV repeats, since these peptide sequences are often present in known PRPs. The PRPs, like the EXTs, are not known to contain GPI anchor addition sequences, but the presence of such sequences was queried nonetheless. By these criteria, 15 of the 113 were classified as PRPs. The 45% PVKCYT search criteria failed to find all the potential PRP sequences and had a high rate of false positives. In addition to the 15 PRPs, nine AGPs (AGP45P, AGP56C, AGP9C, AGP7C, AGP4C, AGP18K, AGP19K, AGP30I, AGP33I), 31 EXTs (EXT40, EXT17, EXT32, EXT37, EXT41, LRX3, LRX1, EXT39, EXT20, EXT21, EXT3/5, EXT8, EXT7, EXT35, EXT9, EXT10, EXT2, EXT11, EXT13, EXT16, EXT15, EXT18, EXT1/4, EXT22, EXT19, EXT30, PEX3, EXT6, EXT12, EXT14, EXT51), and three hybrid HRGPs (HAE2, HAE3, HAE4) were found with the 45% PVKCYT search. In addition, two AGPs (AGP4C, AGP9C), one EXT (EXT1/4), and one hybrid HRGP (HAE3) were found with the two PPVX(K/T) repeat search; further information on these sequences was presented in the AGP and EXT sections above. Three additional PRPs (PRP8, PRP9, PRP11) did not pass the biased amino acid test but were found instead by a database annotation search. The locus identifiers of these sequences are indicated in italics in Table VIII. With these additional PRPs, 18 total PRPs were found and subjected to further analysis. Six of the 18 PRPs contained a non-HRGP domain along with a PRP domain and thus were classified as chimeric PRPs. The remaining 12 PRPs were not divided further into subclasses (Table VIII). Representative sequences of these two classes of PRPs are shown in Figure 4.
Table VIII. Identification, characterization, and classification of the PRP genes in Arabidopsis.
| Locus Identifiera | Nameb | Class | PPVX[KT]/KKPCPP/PPV Repeats | Amino Acids | SPc | GPI | Organ-Specific Expression | Introns | P/5/E/I/3 Mutantsd | Top Five BLAST Hits HRGPse |
| At1g15825 | PRP5 | PRP | 1/0/4 | 126 | No | No | 0 | 0/1/3/0/8 | AGP9C, PRP6, PRP11, PRP4, AGP19K | |
| At1g54970 | PRP1 | PRP | 13/0/2 | 335 | Yes | No | Roots | 1 | 0/0/1/1/0 | PRP3, PRP7, AGP31I, PRP6, PRP16 |
| At2g21140 | PRP2 | PRP | 0/4/7 | 321 | Yes | No | 1 | 6/1/0/0/0 | PRP4 | |
| At2g27380 | PRP6 | PRP | 22/0/24 | 761 | Yes | No | Endosperm | 0 | 6/1/1/0/2 | EXT6, EXT10, EXT9, PEX1, EXT2 |
| At2g47530 | PRP7 | PRP | 0/0/0 | 184 | Yes | No | Roots | 1 | 3/6/5/0/1 | PRP1, PRP3, AGP31I, AGP30I |
| At3g50570 | PRP8 | PRP | 0/0/0 | 189 | Yes | No | Stamen | 0 | 3/0/3/0/2 | HAE2 |
| At3g62680 | PRP3 | PRP | 14/0/0 | 313 | Yes | No | Roots | 1 | 2/3/7/0/5 | PRP1, PRP7, AGP30I, AGP31I, EXT1/4 |
| At4g38770 | PRP4 | PRP | 0/7/14 | 448 | Yes | No | 1 | 7/0/5/1/3 | PRP2, AGP55C | |
| At5g09520 | PRP9 | PRP | 0/0/0 | 130 | Yes | No | Radicle, root | 0 | 5/2/1/0/1 | PRP10, AGP1C |
| At5g09530 | PRP10 | PRP | 0/0/0 | 360 | Yes | No | Radicle, root | 0 | 3/0/7/0/7 | PRP9, PRP11, PRP4, PRP15, PRP16 |
| At5g15780 | PRP11 | PRP | 0/0/3 | 401 | Yes | No | 1 | 1/2/6/2/2 | AGP31I, AGP30I, PRP1 | |
| At5g59170 | PRP12 | PRP | 0/0/5 | 288 | Yes | No | Seeds | 0 | 5/4/5/0/0 | AGP55C |
| At1g09460 | PRP13 | Chimeric | 2/0/4 | 330 | Yes | Yes | 2 | 3/0/1/0/0 | EXT51, AGP9C, PRP18, PRP16, PERK8 | |
| At1g62500 | PRP14 | Chimeric | 4/0/4 | 297 | Yes | No | Shoot apex | 0 | 6/1/0/0/0 | PRP10, PRP9, PRP11, PRP4, PRP15 |
| At2g10940 | PRP15 | Chimeric | 0/0/11 | 291 | Yes | No | 1 | 2/2/1/0/2 | PRP14, PRP16, PRP17, HAE4, AGP2C | |
| At3g22120 | PRP16 | Chimeric | 7/0/0 | 334 | Yes | No | 0 | 2/0/1/0/0 | PRP17, PRP14, PRP15, HAE4 | |
| At4g15160 | PRP17 | Chimeric | 1/0/0 | 428 | Yes | No | 3 | 2/1/4/3/2 | PRP16, PRP14, PRP15 | |
| At5g14920 | PRP18 | Chimeric | 2/0/7 | 275 | Yes | No | Petiole | 3 | 2/1/4/1/1 | PRP6, AGP31I, PRP16, EXT51, PEX3 |
Italics indicates a protein found using the Arabidopsis database annotation search.
Boldface indicates a protein that was not previously identified in the primary literature.
Signal peptide.
Indicates the number of mutants available in each location: P, promoter; 5, 5′ UTR; E, exon; I, intron; 3, 3′ UTR.
Underline indicates the result of a BLAST search with filtering turned off.
Figure 4.
Protein sequences encoded by representative PRP gene classes in Arabidopsis. Colored sequences at the N terminus indicate predicted signal peptide (green). PPVX(K/T) (gray), KKPCPP (teal), and PPV (pink) repeats are also indicated.
BLAST analysis was conducted to identify other potential PRP sequences and to provide insight to PRP sequences with the greatest similarity (Table VIII; Supplemental Table S1). BLAST was somewhat successful in identifying other PRPs, but all PRPs cannot be found with a single BLAST search. Interestingly, the BLAST searches showed that six of the 18 PRPs are similar to AGP30, a nonclassical (chimeric) AGP. In fact, when AGP30 was used as the query sequence in a BLAST search, the top four hits were all PRPs rather than AGPs (Table II; Supplemental Table S1). Also consistent with these findings is the fact that AGP30 was not identified with the traditional 50% PAST search used for AGPs but was found with the 45% PVKCYT search used for PRPs.
The PRPs ranged in size from 126 to 761 amino acids (Table VIII; Supplemental Fig. S11). Eleven of the 12 PRPs were predicted to have a signal peptide, but none was predicted to have a GPI anchor. The six chimeric PRPs ranged in size from 275 to 428 amino acids. All six chimeric PRPs were predicted to have a signal peptide, and one was predicted to have a GPI anchor.
PRP Gene Expression and Coexpressed HRGPs, GTs, P4Hs, and Peroxidases
In order to elucidate patterns of gene expression for these predicted PRPs, the same three public databases were searched as with the AGPs and EXTs. While most PRPs had a broad range of expression throughout the plant, several of the PRP genes showed organ-specific expression. Notably, several PRPs were specifically or preferentially expressed in the roots, while other individual PRPs were expressed in the endosperm, shoot apex, and petiole (Table VIII; Supplemental Figs. S12–S15). Moreover, in examining the expression levels of all the PRP genes, endosperm-specific At2g27380 (PRP6) was the most highly expressed one, as indicated by its high relative signal intensity.
Unlike the AGPs and EXTs, the PRPs displayed some common and dramatic (i.e. approximately 8-fold or more) patterns of environmental stress-induced gene expression. For example, eight of the PRP genes (PRP1, -2, -8, -3, -4, -9, -10, and -15) were down-regulated by ABA, while two of the PRP genes (PRP6 and -14) were up-regulated by ABA. In addition, three PRPs (PRP2, -3, and -11) were up-regulated by zeatin, three PRPs (PRP 4, -11, and -16) were up-regulated by nematode infection, and two PRPs (PRP9 and -10) were up-regulated by Pseudomonas syringae infection.
Next, the PRP genes were examined with respect to coexpressed genes using The Arabidopsis Co-Response Database (Table IX; Supplemental Table S8). Twelve out of the 18 PRPs had data available. In analyzing the data, a focus was placed not only on other HRGPs but on GTs, P4Hs, and peroxidases, since these enzymes are responsible for posttranslational modification of PRPs; this approach represents one potential avenue to identify genes involved in the posttranslational modification of PRPs. In terms of PRPs being expressed with other HRGPs, 46 different HRGPs are coexpressed with at least one PRP. The HRGP showing greatest coexpression was FLA8, which was coexpressed with five PRPs; FLA8 was also coexpressed with 16 AGPs. FLA9 and FLA2, which were coexpressed with many AGPs and EXTs, were each coexpressed with three PRPs. For the GTs, At5g22940 of the GT47 family was coexpressed with six PRPs, twice as many as any other GT. Moreover, At1g24170, a GT8 family member that was coexpressed with many AGPs and EXTs, was not coexpressed with any PRPs. At3g14570 (Gsl04), a member of the GT family 48, was coexpressed with three PRPs; it was also coexpressed with four AGPs but no EXTs. For the P4Hs, two of 13 members of the P4H gene family, At3g06300 (P4H2) and At5g18900 (P4H4), were coexpressed with two and one PRPs, respectively, as well as with many AGPs and EXTs. For the peroxidases, some peroxidase genes were coexpressed. The greatest amount of coexpression was exhibited by At1g77490 (tAPX) and At2g22420 (PER17); each was coexpressed with two PRPs. Both of these peroxidases also were coexpressed with EXTs and AGPs.
Table IX. HRGPs, GTs, P4Hs, and peroxidases coexpressed with PRPs.
| HRGP Locus Identifier | Name | No. of Coexpressed PRPs | GT Locus Identifier | Name | Family | No. of Coexpressed PRPs | P4H Locus Identifier | Name | No. of Coexpressed PRPs | Peroxidase Locus Identifier | Name | No. of Coexpressed PRPs |
| At2g45470 | FLA8 | 5 | At5g22940 | GT47 | 6 | At3g06300 | P4H2 | 2 | At1g68850 | 2 | ||
| At4g16980 | AGP58C | 4 | At3g14570 | Gsl04 | GT48 | 3 | At5g18900 | P4H4 | 1 | At1g77490 | tAPX | 2 |
| At1g03870 | FLA9 | 3 | At1g07250 | GT1 | 2 | At2g22420 | PER17 | 2 | ||||
| At1g52290 | PERK15 | 3 | At1g08660 | GT29 | 2 | At1g05240 | 1 | |||||
| At2g47930 | AGP26C | 3 | At3g29320 | GT35 | 2 | At1g30870 | 1 | |||||
| At4g12730 | FLA2 | 3 | At3g46970 | GT35 | 2 | At1g71695 | PER12 | 1 | ||||
| At2g04780 | FLA7 | 2 | At4g02500 | GT34 | 2 | At2g25080 | GPX1 | 1 | ||||
| At3g06750 | EXT34 | 2 | At4g31780 | GT28 | 2 | At2g31570 | 1 | |||||
| At4g18670 | LRX5 | 2 | At4g39350 | CesA02 | GT2 | 2 | At3g21770 | PER30 | 1 | |||
| At4g26320 | AGP13P | 2 | At5g03760 | CslA09 | GT2 | 2 | At3g28200 | 1 | ||||
| At4g37450 | AGP18K | 2 | At5g05890 | GT1 | 2 | At3g49120 | 1 | |||||
| At5g55730 | FLA1 | 2 | At5g22740 | CslA02 | GT2 | 2 | At3g49960 | 1 | ||||
| At5g56540 | AGP14P | 2 | At5g50420 | GT68 | 2 | At4g08770 | 1 | |||||
| At1g09460 | PRP13 | 1 | At1g06780 | GT8 | 1 | At4g09010 | 1 | |||||
| At1g23720 | EXT6 | 1 | At1g11720 | GT5 | 1 | At4g26010 | ATP13a | 1 | ||||
| At1g26150 | PERK10 | 1 | At1g13250 | GT8 | 1 | At5g17820 | PER57 | 1 | ||||
| At1g28290 | AGP31I | 1 | At1g16570 | GT33 | 1 | At5g22410 | 1 | |||||
| At2g10940 | PRP15 | 1 | At1g19360 | GT77 | 1 | At5g67400 | PER73 | 1 | ||||
| At2g24980 | EXT7 | 1 | At1g21480 | GT47 | 1 | |||||||
| At2g33790 | AGP30I | 1 | At1g23480 | CslA03 | GT2 | 1 | ||||||
| At2g35860 | FLA16 | 1 | At1g27440 | GT47 | 1 | |||||||
| At3g11700 | FLA18 | 1 | At1g71220 | GT24 | 1 | |||||||
| At3g19430 | EXT51 | 1 | At1g78580 | GT20 | 1 | |||||||
| At3g22120 | PRP16 | 1 | At2g03220 | FUT1 | GT37 | 1 | ||||||
| At3g24480 | LRX4 | 1 | At2g22900 | GT34 | 1 | |||||||
| At3g52370 | FLA15 | 1 | At2g29750 | GT1 | 1 | |||||||
| At3g54590 | EXT2 | 1 | At2g31790 | GT1 | 1 | |||||||
| At3g60900 | FLA10 | 1 | At2g32620 | CslB04 | GT2 | 1 | ||||||
| At4g08410 | EXT10 | 1 | At2g35650 | CslA07 | GT2 | 1 | ||||||
| At4g09030 | AGP10C | 1 | At3g06440 | GT31 | 1 | |||||||
| At4g13340 | LRX3 | 1 | At3g18170 | GT61 | 1 | |||||||
| At4g15160 | PRP17 | 1 | At3g24040 | GT14 | 1 | |||||||
| At4g16140 | EXT37 | 1 | At3g45100 | GT4 | 1 | |||||||
| At4g27520 | PAG10 | 1 | At3g59100 | Gsl11 | GT48 | 1 | ||||||
| At5g06630 | EXT13 | 1 | At3g61130 | GT8 | 1 | |||||||
| At5g06640 | EXT14 | 1 | At4g02130 | GT8 | 1 | |||||||
| At5g09520 | PRP9 | 1 | At4g07960 | CslC12 | GT2 | 1 | ||||||
| At5g09530 | PRP10 | 1 | At4g15290 | CslB05 | GT2 | 1 | ||||||
| At5g10430 | AGP4C | 1 | At4g18240 | GT5 | 1 | |||||||
| At5g14920 | PRP18 | 1 | At4g38040 | GT47 | 1 | |||||||
| At5g15780 | PRP11 | 1 | At4g38270 | GT8 | 1 | |||||||
| At5g18690 | AGP25C | 1 | At5g05170 | CesA03 | GT2 | 1 | ||||||
| At5g21160 | AGP32I | 1 | At5g15650 | GT75 | 1 | |||||||
| At5g40730 | AGP24P | 1 | At5g16190 | CslA11 | GT2 | 1 | ||||||
| At5g53250 | AGP22P | 1 | At5g16510 | GT75 | 1 | |||||||
| At5g60490 | FLA12 | 1 | At5g17420 | CesA07 | GT2 | 1 | ||||||
| At5g19690 | GT66 | 1 | ||||||||||
| At5g24300 | GT5 | 1 | ||||||||||
| At5g41460 | GT31 | 1 | ||||||||||
| At5g47780 | GT8 | 1 | ||||||||||
| At5g53340 | GT31 | 1 | ||||||||||
| At5g54690 | GT8 | 1 |
PRP Gene Organization and Mutants
Information was extracted from the TAIR and SALK Web sites with regard to the gene structure and available genetic mutants for each of the predicted PRP genes. None of the 18 PRPs contained more than three introns, with most containing either zero (eight of 18) or one intron (seven of 18; Table VIII; Supplemental Table S9).
Examination of the various mutant lines available for research showed that all of the PRP genes have one or more mutants available. Of these mutants, 32% were in the promoter region, 14% were in the 5′ UTR, 30% were in an exon, 4% were in an intron, and 20% were in the 3′ UTR (Table VIII; Supplemental Table S10).
DISCUSSION
The BIO OHIO Program for Finding and Analyzing HRGP Genes Based on Biased Amino Acid Compositions and Amino Acid Sequence Motifs
As genomes are sequenced, bioinformatic tools need to be developed to analyze such data efficiently and accurately. Here, we describe one such tool for the purpose of identifying and analyzing HRGPs encoded by nucleic acid sequences. The BIO OHIO software has the ability to identify AGPs, EXTs, and PRPs as well as hybrid and chimeric HRGPs. This program requires only that the protein sequence data be available as a data file, which is routinely generated in a completed genome sequencing project. Here, the BIO OHIO program was used to search the 28,952 protein sequences encoded by the Arabidopsis genome. Several different strategies were used by the program to identify candidate HRGPs. Specifically, the program has the ability to identify proteins meeting a user-defined amino acid composition in full-length proteins or proteins of some defined size. This strategy was effective in identifying candidate classical AGPs, Lys-rich AGPs, AG peptides, and certain PRPs. The program can also be used to identify proteins containing specific, user-defined peptide sequences repeated any number of times. This strategy was used to identify candidate FLAs, EXTs, and certain PRPs. Both strategies were able to identify candidate hybrid and chimeric HRGPs. Another search strategy built into the program is to search for keywords within the annotated Arabidopsis protein database. This approach proved useful in finding some chimeric AGPs and PRPs not identified by the above approaches. In addition, the program can search for signal peptide sequences, GPI anchor addition sequences, and repeating sequences within proteins; such additional information in conjunction with careful examination of the protein sequence was used to manually identify candidate proteins as HRGPs. In total, this bioinformatics approach identified 166 candidate HRGPs, including 85 AGPs (22 classical AGPs, three Lys-rich AGPs, 16 AG peptides, 21 [chimeric] FLAs, 17 [chimeric] PAGs, and six other chimeric AGPs), 59 EXTs (three SP5 EXTs, two SP5/SP4 EXTs, 12 SP4 EXTs, two SP4/SP3 EXTs, one SP3 EXT, 12 short EXTs, 11 [chimeric] LRXs, 13 [chimeric] PERKs, and three other chimeric EXTs),18 PRPs (12 PRPs and six chimeric PRPs), and four AGP/EXT HAEs (Table X).
Table X. A summary of the HRGP superfamily in Arabidopsis.
Boldface entries are subtotals for the various HRGP families.
| HRGP Family | HRGP Subfamily | Predicted No. of: |
||
| Genes | Signal Peptides | GPI Anchors | ||
| AGPs | Classical AGPs | 22 | 19 | 14 |
| AGPs | Lys-rich classical AGPs | 3 | 3 | 2 |
| AGPs | AG peptides | 16 | 16 | 12 |
| AGPs | (Chimeric) FLAs | 21 | 20 | 10 |
| AGPs | (Chimeric) PAGs | 17 | 17 | 16 |
| AGPs | Other chimeric AGPs | 6 | 5 | 1 |
| AGPs | All AGP subfamilies | 85 | 80 | 55 |
| EXTs | SP5 EXTs | 3 | 3 | 0 |
| EXTs | SP5/SP4 EXTs | 2 | 2 | 0 |
| EXTs | SP4 EXTs | 12 | 9 | 0 |
| EXTs | SP4/SP3 EXTs | 2 | 2 | 0 |
| EXTs | SP3 EXT | 1 | 1 | 0 |
| EXTs | Short EXTs | 12 | 11 | 7 |
| EXTs | (Chimeric) LRXs | 11 | 9 | 0 |
| EXTs | (Chimeric) PERKs | 13 | 0 | 0 |
| EXTs | Other chimeric EXTs | 3 | 2 | 0 |
| EXTs | All EXT subfamilies | 59 | 39 | 7 |
| Hybrid | HAE (AGP/EXT) | 4 | 3 | 0 |
| Hybrid | All hybrid HRGPs | 4 | 3 | 0 |
| PRPs | PRPs | 12 | 11 | 0 |
| PRPs | Chimeric PRPs | 6 | 6 | 1 |
| PRPs | All PRP subfamilies | 18 | 17 | 1 |
| Total | All AGPs, EXTs, and PRPs | 166 | 139 | 63 |
This bioinformatics approach has advantages over conventional BLAST searches in terms of speed and accuracy. BLAST searches are time-consuming, requiring much postanalysis data acquisition and analysis after a list of “hits” to a query sequence is obtained. Furthermore, BLAST analyses fail to identify all members of an AGP, EXT, or PRP subfamily, since many of the subfamily members have limited amino acid sequence similarities and/or have various repeated amino acid sequence modules within a given sequence, complicating the alignment process. Nonetheless, BLAST analysis was used here to identify the most closely related sequences to a given HRGP, and by playing a version of the six degrees of separation game, it could be used to identify many, but not all, HRGP members in a time-consuming, convoluted, and laborious endeavor.
Schultz et al. (2002) previously utilized a bioinformatics approach to identify candidate AGP genes from Arabidopsis. In contrast to this study, only 52 AGPs (14 classical AGPs, three Lys-rich AGPs, 10 AG peptides, 21 [chimeric] FLAs, and four other chimeric AGPs) were identified. The additional AGPs found in this study are largely attributed to using an updated Arabidopsis protein database, altering the definition of an AG peptide to include up to 90 amino acids (compared with 75), and analyzing HRGP-related sequences based on annotations in the database. In addition, Schultz et al. (2002) also identified 19 candidate EXT genes as a by-product of searching for AGPs using the greater than 50% PAST amino acid bias. As explained by Johnson et al. (2003b), these 19 genes were subsequently examined for the presence of a signal peptide and SP3 and SP4 repeat units. In contrast, the additional EXTs found in this study are largely attributed to using an updated protein database, to searching for SP3 and SP4 repeats in all the proteins encoded by the genome (not just those proteins passing the 50% PAST test), and to analyzing HRGP-related sequences based on annotations in the database and literature. Johnson et al. (2003b) also reported the existence of 17 PRPs based on searching for proteins with greater than 49% PKVY and greater than 47% PKVL amino acid biases, similar to the findings obtained in this study.
While most of the AGP, EXT, and PRP genes fitting canonical sequencing parameters are now identified, identifying chimeric HRGPs, particularly chimeric AGPs, remains a challenge, given that no clear consensus sequence exists as for the AGPs. Thus, while we have identified six chimeric AGPs in addition to the FLAs and PAGs, it is likely that other proteins contain AGP modules. For instance, two homologous Arabidopsis genes, At5g64080 and At2g13820, designated Arabidopsis XYLOGEN PROTEIN1 (AtXYP1) and AtXYP2, respectively, are known to contain AGP-like regions, but they were not identified in our searches. A glimpse of other such chimeric AGPs was provided in a previous study, where putative GPI-anchored proteins were identified by bioinformatics to reveal not only numerous GPI-anchored AGPs but also approximately 50 other proteins containing AGP sequence modules, but annotated as phytocyanins, stellacyanin-like, uclacyanin-like, early nodulin-like, COBRA, β-(1,3)-glucanases, aspartyl proteases, LTPL, SKU5, receptor-like kinases, and other unknown or hypothetical proteins (Borner et al., 2003).
In order to identify such chimeric AGPs, the sliding windows feature of the BIO OHIO program was utilized. Specifically, the Arabidopsis protein database was searched using windows of 10, 20, and 30 amino acids and searching for greater than 80%, 90%, and 95% PAST. In order to find all 85 AGPs identified in our searches with a sliding windows approach, an amino acid composition of greater than 60% PAST is required with a window size of 10 amino acids. While this approach finds all of the AGPs predicted by our searches, it produces many false positives in the process, making this approach of limited usefulness in initial searches on its own. However, the sliding windows feature is especially useful to identify single or multiple AGP modules in chimeric AGPs when identified by other approaches.
Laboratory experimentation has verified and validated this in silico approach to identifying HRGPs. With respect to the AGPs, reports on several cloned AGP genes and/or characterized AGP glycoproteins in Arabidopsis exist and substantiate predictions made by the program (Schultz et al., 2000, 2004; Johnson et al., 2003a; van Hengel and Roberts, 2003; Sun et al., 2005; Liu and Mehdy, 2007; Yang et al., 2007). Moreover, at the protein level, several of the AGPs predicted here to have signal peptides and GPI anchors are substantiated in these reports. With respect to the EXTs, only three nonchimeric EXT genes (EXT1/4, EXT2, EXT3/5) and several LRXs and PERKs are cloned (Merkouropoulos et al., 1999; Yoshiba et al., 2001; Baumberger et al., 2003b; Nakhamchik et al., 2004). Moreover, both the LRXs and PERKs were previously examined using BLAST and other homology-based genomic tools to identify members of these two chimeric EXT classes, in agreement with the bioinformatics findings presented here (Baumberger et al., 2003a; Nakhamchik et al., 2004). In contrast to the AGPs, there is little information on the EXTs at the glycoprotein level in Arabidopsis. With respect to the PRPs, only four PRPs are cloned in Arabidopsis, namely PRP1, -2, -3, and -4, and little is known about any of the Arabidopsis PRPs from glycoprotein studies (Fowler et al., 1999). Thus, this work extends and consolidates the experimental inventory of HRGPs and makes testable predictions with respect to the presence (or absence) of signal peptides and GPI anchor addition sequences. Although the majority of HRGPs identified by this bioinformatics approach contain signal peptides, several HRGPs do not. It is unknown whether this represents limitations to the predictive power of the program or is due to the possibility that HRGPs lacking such a sequence remain inside the cells or are secreted by an alternative secretory pathway, as reported in some cases (Nickel, 2003; Lee et al., 2004). For instance, all PERKs lack a signal peptide but are localized to the plasma membrane, with the EXT region extending into the cell wall (Nakhamchik et al., 2004). Similarly, while GPI anchors predicted for many AGPs are experimentally verified in several instances, including in Arabidopsis, it was surprising to observe here and elsewhere that several EXTs and one PRP also have predicted GPI anchor addition sequences (Borner et al., 2003), which await biochemical and functional verification at the protein and cell biology levels, respectively.
Four hybrid HRGPs containing AGP and EXT sequence motifs also are encoded by the Arabidopsis genome. These hybrids, like the chimeric HRGPs, complicate the classification system. Indeed, it is human nature to classify things into discrete categories, but the chimeric and hybrid HRGPs remind us that nature cares little for the organizational principles coveted by the human mind. Consequently, it is perhaps best to view the HRGPs as a spectrum of molecules composed of some combination of hyperglycosylated AGP modules, moderately glycosylated EXT modules, lightly glycosylated or nonglycosylated PRP modules, and, in the case of chimeric HRGPs, other non-HRGP modules.
HRGP Gene Expression in Development and in Response to Biotic and Abiotic Stress
Microarray as well as MPSS data are valuable, publicly available genetic resources for the Arabidopsis community, effectively revealing developmental, organ-specific, and stress-specific patterns of gene expression for nearly all of the Arabidopsis genes. These resources can thus provide clues to possible HRGP functions and/or allow researchers to focus their research projects. For example, in looking for phenotypic alterations in a HRGP mutant plant, microarray or MSPP data can guide the researcher in terms of the particular developmental times, organs, or conditions to examine in order to reveal a phenotype. Microarray and MPSS data are available for all but a few HRGPs. The majority of the AGP and EXT genes demonstrate organ-specific expression, while the remaining genes are expressed in multiple organs. Many AGPs, including classical AGPs, AG peptides, and at least one FLA, show pollen-specific expression. Likewise, root-specific AGPs are found in each AGP class. In contrast, pollen-specific expression of the EXT genes is restricted to the chimeric EXTs, most notably to certain LRXs (i.e. PEXs) and PERKs. Root-specific expression is exhibited by certain members of virtually all EXT classes. Approximately half of the PRPs show organ-specific expression, mostly in roots, while the rest are more widely expressed. Clearly, the notion that HRGPs in a particular class have some common organ-specific function appears unlikely, although the idea that certain AGPs are markers of cellular identity is supported by the organ-specific expression patterns revealed here (Knox et al., 1989). Comparing published northern and reverse transcription-PCR data on selected HRGP genes in studies conducted by various researchers with the microarray and MPSS data has consistently resulted in good agreement between these various methods to determine patterns of gene expression.
The recently updated Genevestigator Web site has considerably simplified the process of examining stress-induced gene expression in Arabidopsis microarrays. Virtually all HRGP genes are up- and down-regulated by various abiotic and biotic stress conditions. With the exception of some of the PRP genes, which exhibit common regulatory responses to auxin, zeatin, and infection by nematodes and P. syringae, it is difficult to summarize the diverse array of responses exhibited by the various HRGP genes. However, the coexpression database analysis takes into account these data, making common patterns of regulation much easier to recognize and examine. Nonetheless, if one is interested in a particular HRGP gene or in regulation by a particular stress condition, the data collected here constitute an ideal starting point for verification of this stress-induced gene regulation and for formulating functional hypotheses for particular HRGP genes.
HRGP Networks and Genes Involved in Posttranslational Modification
One unique genetic resource available to Arabidopsis researchers is the coexpression database. This database reports genes that are coexpressed with a gene of interest based on hundreds of different microarray gene analyses experiments. For HRGPs, this coexpression database offers the opportunity to reveal networks of genes associated with a given HRGP gene. In this study, the focus was placed on elucidating HRGP gene networks and in identifying candidate genes involved with the posttranslational modification of HRGPs, including genes involved with prolyl hydroxylation, glycosylation, and cross-linking. With regard to HRGP networks, it was remarkable that certain FLAs, namely FLA2, -7, -8, and -9, were coexpressed with so many different AGPs, EXTs, and PRPs. One interpretation of this result is that these FLAs play important roles in coordinating activities among various HRGP molecules; however, this and other interpretations must await functional characterization of these FLAs. Clearly, HRGP gene networks likely exist, given that sets of HRGP genes appear to be coregulated by a variety of conditions. It is possible that such regulatory networks are controlled by common regulatory sequences found in the HRGP genes. Efforts are currently under way as an extension of this work to identify such sequences using bioinformatics to allow for subsequent experimental testing of these elements and the transcription factors that bind to them.
It was hypothesized that a number of GT genes are expressed in conjunction with various HRGP genes to allow for the coordinated glycosylation of the encoded core protein. Furthermore, it was hypothesized that particular GTs would be responsible for synthesis of the various sugar linkages associated with the arabinogalactan polysaccharides attached to noncontiguous Hyp residues in AGPs, while other GTs would be associated with synthesis of the short arabinoside oligosaccharide chains attached to contiguous Hyp residues in EXTs and PRPs according to the Hyp continuity hypothesis (Tan et al., 2003). It was also hypothesized that GTs responsible for the addition of single Gal units to Ser residues in EXTs would be found. Moreover, based on the elucidated structures of dicot EXTs (Akiyama et al., 1980) and a well-characterized Hyp-AG isolated from transgenic tobacco (Nicotiana tabacum; Tan et al., 2004), and knowing the specificity of GTs, a minimum of 20 transferase activities are likely to be involved in the O-linked glycosylation of HRGPs. Specifically, for EXTs and PRPs, we predict one Ser-α-galactosyltransferase, at least one Hyp-β-arabinosyltransferase, one α-(1,2)arabinosyltransferase, and two β-(1,2)arabinosyltransferases, while for AGPs, we predict one Hyp-β-galactosyltransferase, one α-(1,5)arabinosyltransferase, at least four α-(1,3)arabinosyltransferases, at least three β-(1,3)galactosyltransferases, three β-(1,6)galactosyltransferases that add the three branch sites on the AG core, at least two β-(1,6)glucuronyltransferases, one α-(1,4)rhamnosyltransferase, and at least two α-(1,2)fucosyltransferases. Indeed, many GT genes are coexpressed with AGPs, EXTs, and PRPs. In fact, 36 different GTs representing 19 families were coexpressed with all three HRGP subfamilies, while some GTs are expressed only with two subfamilies or are restricted to one particular HRGP subfamily. While it is possible to speculate on the activities of these various GTs with respect to HRGPs based on their annotations and proposed mechanisms (i.e. inverting or retaining) in the CAZY database, such speculations would have to be tested by developing appropriate biochemical assays and/or obtaining and biochemically characterizing GT mutants. Indeed, such research is currently under way in a number of cell wall laboratories and is beginning to yield results. For example, it was recently shown that a mutant in the At2g35610 gene, encoding a GT77 family member, results in the production of underarabinosylated EXTs (Gille et al., 2009). Thus, the At2g35610 gene likely encodes one of the arabinosyltransferases required for EXT glycosylation and possibly for clustered Hyp residues in certain AGPs, consistent with the identification of this gene in the coexpression data presented here in Tables VI and III, respectively.
Although only four plant P4Hs are cloned and characterized to date (two [P4H1 and P4H2] from Arabidopsis [Hieta and Myllyharju, 2002; Tiainen et al., 2005], one from tobacco [Yuasa et al., 2005], and one from Chlamydomonas [Keskiaho et al., 2007]), 13 P4H genes are predicted to exist for Arabidopsis (Vlad et al., 2007). The coexpression analysis performed here shows that only one of these P4H genes, namely P4H2, was consistently coexpressed with numerous HRGPs. This indicates that this P4H likely acts on AGPs, EXTs, and PRPs and is not restricted to a particular HRGP subfamily. Unfortunately, no published reports on P4H-2 mutants, or any P4H mutants in Arabidopsis, exist at present. However, the genetic redundancy in the P4H family may make such mutant work difficult. Nonetheless, a report that a P4H gene silenced by RNA interference in Chlamydomonas has an altered wall phenotype should bolster similar work in Arabidopsis (Keskiaho et al., 2007).
An acidic EXT peroxidase was isolated from tomato (Solanum lycopersicum) with EXT cross-linking activity (Schnabelrauch et al., 1996). It is also likely that PRPs and possibly AGPs undergo similar peroxidase-catalyzed cross-linking. In an effort to identify potential peroxidases involved with HRGP cross-linking, the coexpression database was used. Indeed, an acidic peroxidase (At3g03670) was identified using this approach and was coexpressed with the two most Tyr-rich EXTs. It will now be interesting to overexpress this enzyme for use in the EXT cross-linking assay and/or to obtain mutants in this gene and observe whether EXT is altered in these mutant plants in terms of more soluble EXTs, less cross-linked EXTs, or reduced amounts of the diisodityrosine/puchrescein cross-linking agent. It should be noted that several other peroxidase genes are also coexpressed and are worthy candidates for similar types of analysis.
HRGP Mutants Are Genetic Tools to Uncover HRGP Function
Genetic mutants are one of the most valuable resources available to the Arabidopsis community, as they provide insight to protein function and facilitate further research to elucidate the mechanism of action. This is clearly the case with HRGP research, where several genetic mutants in AGPs, EXTs, and PRPs are serving as useful tools to elucidate function. It should also be noted that for each informative HRGP mutant, there are many HRGP mutants that fail to reveal a phenotype. There are many potential reasons for such failure, including but not limited to one or more of the following: the existence of genetic redundancy or other genetic backup systems, the inability of certain mutants to adequately reduce mRNA or protein levels to reveal a phenotype, and the inability to examine the mutant under the proper environmental conditions to reveal its phenotype.
At present, several reports on HRGP mutants exist in Arabidopsis, including agp17 (Gaspar et al., 2004), agp18 (Acosta-Garcia and Vielle-Calzada, 2004), agp19 (Yang et al., 2007), sos5 (fla4; Shi et al., 2003), agp30 (van Hengel and Roberts, 2003; van Hengel et al., 2004), rsh-ext3 (Hall and Cannon, 2002), lrx1 (Baumberger et al., 2001), and perk13 (Humphrey et al., 2007). All these mutants have provided functional insights to the role of various AGPs and EXTs. The agp17 mutant displays resistance to Agrobacterium tumefaciens transformation with reduced levels of AtAGP17 in the roots. An RNA interference approach was used to silence the AGP18 and reveal its role in female gametogenesis. An agp19 mutant revealed that AGP19 plays a role in plant growth and development, specifically in cell division and expansion. Studies with the transposon-insertion mutant agp30 suggest that AGP30I has a role in root regeneration and seed germination. The sos5 mutant study indicates that FLA4 plays a role in cell expansion. The rsh-ext3 mutant shows that EXT3 plays an important role in embryo development and cell plate formation, while the lrx1 and perk13 mutants indicate roles for LRX1 and PERK13 in root hair formation and root cell elongation, respectively.
There are currently 1,442 mutant lines available for nearly every HRGP gene, as shown in Tables II, V, and VIII and in Supplemental Tables S4, S7, and S10. While this list is now current, new mutant lines are continually being added to the collection, some of which are now being made available as homozygous knockout lines, saving the researcher valuable time and effort. In any event, once the mutant seed lines are received, they must be planted and verified by PCR analysis to confirm the presence of the mutation in the gene of interest. Mutations existing in the exon regions generally offer the highest probability of obtaining a null mutant and when available should probably be examined first. If a phenotype is observed in the mutant, it is important to confirm that the mutant phenotype is caused by the mutated gene of interest and not by another mutation elsewhere in the genome. Such confirmation can be achieved by studying other mutant lines (i.e. allelic mutants) for a gene of interest and observing the same mutant phenotype or by complementing the original mutant with the wild-type version of the gene of interest. Although mutants affecting the HRGP core proteins allow for the assessment of a particular HRGP's functional role, obtaining mutants in the genes responsible for HRGP posttranslational modification (i.e. GTs, P4Hs, peroxidases) offers perhaps even greater opportunities to address and reveal HRGP function, as multiple HRGPs would be affected by such a mutation.
CONCLUSION
The BIO OHIO bioinformatics program reported here represents a valuable tool to mine genomic databases for HRGP genes, including AGPs, EXTs, PRPs, chimeric HRGPs, and hybrid HRGPs. While this program was utilized to mine the Arabidopsis proteome, it can now be utilized to examine proteomes resulting from other plant genome projects, namely poplar (Populus species), rice (Oryza sativa), Physcomitrella, and Chlamydomonas. Preliminary evidence indicates, not surprisingly, that poplar is most similar to Arabidopsis in terms of its HRGP inventory, while the other species have considerable differences from the dicot HRGP inventory. In Arabidopsis, there are many surprises with respect to the HRGP family members beyond just finding new putative HRGPs, including finding HRGPs that apparently lack signal peptides, the predicted existence of GPI anchor addition sequences in certain EXTs, the numerous HRGPs that show organ-specific expression, and the likely existence of coregulated HRGP networks. Depending upon an investigator's interest, there is now a wealth of information provided to guide future HRGP research. Many of these predictions will require verification or confirmation, but hypotheses can now be formed and specific experiments designed based on the information presented here to facilitate future HRGP research.
Refinements to the BIO OHIO program are possible. In particular, reducing the number of false positives during a search and improving or developing search strategies to identify the chimeric HRGPs, particularly chimeric AGPs and chimeric PRPs, represent two of the most challenging areas for improving the predictive power of the program. In addition to the sliding windows approach, other more novel approaches are being examined to improve the predictive power of the program, including using hidden Markov models, neural networks, as well as supervised and unsupervised learning approaches.
Finally, while the program was specifically developed to identify HRGPs from plant genomic data, it can be readily adapted to identify other proteins or protein families. The ability to select any amino acid bias or sequence motif of interest should make this program attractive to other researchers, including those outside of the plant community, who wish to screen whole genome protein sequences meeting their desired criteria. In addition, this program can be used to screen virtually any protein database, including those created manually or from EST databases.
MATERIALS AND METHODS
Development and Basic Operation of the BIO OHIO Bioinformatics Program
A Perl program, named BIO OHIO, was written that analyzes each predicted protein sequence in the Arabidopsis (Arabidopsis thaliana) genome. This program is available upon request along with a user manual describing the use and operation of this program; however, an abbreviated version of the program is accessible at http://132.235.14.51/functional_genomics.html. The database used (i.e. ATH1.pep) was dated June 10, 2004, and downloaded from The Institute for Genomic Research (ftp.tigr.org/pub/data/a_thaliana/ath1/SEQUENCES/). The program is able to categorize proteins based on various characteristics and patterns of amino acids as specified by the user/researcher. For each identified protein or “hit,” the following information was provided: (1) the Arabidopsis Genome Initiative locus identifier and sequence name; (2) the entire protein sequence; (3) the length of the protein; (4) the total PAST percentage for each protein; (5) analysis for the presence of a signal peptide within the first 50 amino acid residues; and (6) analysis for the presence of a GPI anchor addition sequence. In addition, the program provided analysis of repeated sequences within the proteins. In particular, the presence of AP, PA, SP, and TP dipeptide repeats were noted, as these sequences are typically associated with known AGPs. Protein hits were classified as AGPs if they did not contain repeats associated with EXTs or PRPs (e.g. multiple SP4, SP3, or PPV repeats) but contained predominantly AP, PA, SP, or TP repeats. In order to verify the predictions easily, the program predicted signal peptides and GPI anchor addition sequences and also allowed direct connection to the SignalP Web site (http://www.cbs.dtu.dk/services/SignalP/) to verify signal peptides, the Plant big-PI predictor Web site (http://mendel.imp.ac.at/gpi/plant_server.html) to verify GPI anchor predictions, and the TAIR Web site (http://arabidopsis.org/) for gene and protein information. When conflicts arose between BIO OHIO and the SignalP Web site or the Plant big-PI predictor Web site, data from the SignalP Web site or the Plant big-PI predictor Web site were used.
Finding Classical AGPs and AG Peptides Using Biased Amino Acid Compositions and Finding FLAs by Searching for Fasciclin Motifs
Classical AGPs were identified as proteins of any length that consisted of 50% or greater of the amino acids P, A, S, and T (PAST). AG peptides were identified as proteins of 50 to 90 amino acids in length consisting of 35% or greater PAST. A reduced PAST level was used, since AG peptides usually contain an N-terminal signal peptide and possibly a C-terminal GPI anchor addition signal sequence, which can make up about half of the peptide and contain little PAST. FLAs were designated as proteins containing the consensus motif [MALIT]T[VILS][FLCM][CAVT][PVLIS][GSTKRNDPEIV]+[DNS][DSENAGE]+[ASQM]. This motif was constructed by comparison of all known Arabidopsis FLAs as reported by Johnson et al. (2003a).
Finding EXTs by Searching for SP4 and SP3 Repeat Motifs
The program allowed for searches of any given amino acid string written as a regular expression. Thus, EXTs were identified by searching for the occurrence of two or more SP4 (or SP3) repeats in the protein. Since some of these hits were already annotated as PERKs in the TAIR database, we also manually included other known members of this family from the published literature (Baumberger et al., 2003a; Nakhamchik et al., 2004). Hits were examined for the location and distribution of SP4 and SP3 repeats as well as for the occurrence of other repeating sequences, including YXY. In addition, these sequences were examined for potential signal peptides and GPI anchor addition sequences as described above.
Finding PRPs by Using Biased Amino Acid Compositions and by Searching for PPVX(K/T) and KKPCPP Repeat Motifs
PRPs were first identified by searching for a biased amino acid composition of greater then 45% PVKCYT (Fowler et al., 1999). PRPs were also identified by searching for the occurrence of two or more PPVX(K/T) (where X represents any amino acid) and KKPCPP motifs (Fowler et al., 1999). Hits were examined for the location and distribution of these repeats as well as PPV repeat units. In addition, these sequences were examined for potential signal peptides and GPI anchor addition sequences as described above.
Finding Amino Acid Sequence Repeats in a Protein Sequence
Operating on a Bio::Perl sequence object, a frequency function determines the repeating elements in a given protein sequence. The length of the repeating elements is a parameter that can be set by specifying a minimum length of an element and a maximum length of an element. This variability allows a very thorough examination of the sequence. For each length that lies between the minimum and maximum length, set in the parameters, a sliding window of that length is used and shifted across the sequence, in increments of one amino acid, starting at position 1 and ending at the last position: the length of the sliding window + 1. The discovered elements are stored in a hash structure, with the subsequence of the sliding window as the key and the number of occurrences as the entry. Upon this hash structure, the percentages are computed and stored. This extended hash is then passed onto a visualization function that adds html tags around a currently highlighted pattern and thus allows the analysis of pattern distribution among the complete amino acid sequence.
Searching User-Defined Regions (Sliding Windows) to Find HRGP Domains in a Protein Sequence
The sliding window is a feature built into the BIO OHIO program that can be used for looking at small sections of a protein rather than the protein as a whole. The sliding window starts at the beginning of the protein and slides along the sequence, searching for a biased amino acid composition in a user-designated window size. The sliding windows feature is most useful to find chimeric HRGPs, since only small sections of these proteins contain HRGP motifs. The sliding window can also be used to visualize HRGP regions in proteins found using other searches, as with FLAs or PAGs.
Annotation of Examined Sequences following Our Analysis
Another feature of the program is the ability to create custom annotations for genes identified following a search. This option takes the form of a box into which one types particular keywords about the identified gene. Once the keywords are entered for a particular gene, that gene will appear with an asterisk in all future searches as an indicator that it was identified previously. The keywords are also searchable so that the custom-annotated genes can easily be found at a later time.
Finding Potential HRGPs by Searching Annotations in the Arabidopsis Database
In addition to using biased amino acid composition and repeat searches, an annotation search feature built into the BIO OHIO program was also utilized. Keywords, including extensin, Pro-rich, arabinogalactan, plastocyanin, and Hyp, were entered to see if any additional proteins in the database were already annotated with these keywords. These proteins were then examined as described above to determine whether they were indeed likely AGPs, EXTs, or PRPs.
BLAST Analysis
BLAST analysis was performed on each identified HRGP using TAIR WU-Blast 2.0 (http://www.arabidopsis.org/wublast/index2.jsp) to identify other potential HRGP sequences and to provide insight to HRGP sequences with the greatest similarity. Specifically, the BLASTX: NT query to AA db was used along with the AGI Proteins (Protein) database. BLAST searches were conducted with the “filter query” option both on and off.
Elucidation of Expression Patterns of HRGP Genes Using Public Databases
In order to elucidate patterns of gene expression for the predicted HRGPs, three public databases were searched: Genevestigator (https://www.genevestigator.ethz.ch/), Arabidopsis Membrane Protein Library (http://www.cbs.umn.edu/arabidopsis/), and Arabidopsis MPSS Plus Database (http://mpss.udel.edu/at/).
Identification of HRGP, GT, P4H, and Peroxidase Genes Coexpressed with the Predicted HRGP Genes in Arabidopsis
All HRGP genes were examined with respect to coexpressed genes using The Arabidopsis Co-Response Database (http://csbdb.mpimp-golm.mpg.de/csbdb/dbcor/ath.html). At this site, “single gene query” was selected. Each of the HRGPs was searched using the four different matrices: nasc0271, atge0100, atge0200, and atge0250. The default settings for coefficient and output were used. These results were examined, and only GTs, P4Hs, peroxidases, and other HRGPs that were coexpressed with a given HRGP were selected.
Identification of Gene Structure and Genetic Mutants for the Identified HRGP Genes
Information on HRGP gene structures was obtained from the TAIR database (http://www.arabidopsis.org). In order to determine if genetic mutants exist in each of these predicted HRGP genes, T-DNAexpress: The SIGnAL Arabidopsis Gene Mapping Tool (http://signal.salk.edu/cgi-bin/tdnaexpress) was utilized. All reported mutant lines were documented following the search.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Amino acid sequences of AGPs.
Supplemental Figure S2. Genevestigator anatomy expression for AGPs.
Supplemental Figure S3. Genevestigator stimulus expression for AGPs.
Supplemental Figure S4. Arabidopsis Membrane Protein Library data for AGPs.
Supplemental Figure S5. MPSS data for AGPs.
Supplemental Figure S6. Amino acid sequences of EXTs.
Supplemental Figure S7. Genevestigator anatomy expression for EXTs and hybrid HRGPs.
Supplemental Figure S8. Genevestigator stimulus expression for EXTs and hybrid HRGPs.
Supplemental Figure S9. Arabidopsis Membrane Protein Library data for EXTs and hybrid HRGPs.
Supplemental Figure S10. MPSS data for EXTs and hybrid HRGPs.
Supplemental Figure S11. Amino acid sequences of PRPs.
Supplemental Figure S12. Genevestigator anatomy expression for PRPs.
Supplemental Figure S13. Genevestigator stimulus expression for PRPs.
Supplemental Figure S14. Arabidopsis Membrane Protein Library data for PRPs.
Supplemental Figure S15. MPSS data for PRPs.
Supplemental Table S1. Results of HRGP BLAST searches with filter on (worksheet A) and off (worksheet B).
Supplemental Table S2. HRGPs, GTs, P4Hs, and peroxidases coexpressed with AGPs.
Supplemental Table S3. Locations of introns and exons in AGPs.
Supplemental Table S4. Locations of available T-DNA mutant lines for AGPs.
Supplemental Table S5. HRGPs, GTs, P4Hs, and peroxidases coexpressed with EXTs.
Supplemental Table S6. Locations of introns and exons in EXTs and hybrid HRGPs.
Supplemental Table S7. Locations of available T-DNA mutant lines for EXTs and hybrid HRGPs.
Supplemental Table S8. HRGPs, GTs, P4Hs, and peroxidases coexpressed with PRPs.
Supplemental Table S9. Locations of introns and exons in PRPs.
Supplemental Table S10. Locations of available T-DNA mutant lines for PRPs.
Supplementary Material
References
- Acosta-Garcia G, Vielle-Calzada JP. (2004) A classical arabinogalactan protein is essential for the initiation of female gametogenesis in Arabidopsis. Plant Cell 16: 2614–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama Y, Mori M, Kato K. (1980) 13C-NMR analysis of hydroxyproline arabinosides from Nicotiana tabacum. Agric Biol Chem 44: 2487–2489 [Google Scholar]
- Baldwin TC, Domingo C, Schindler T, Seetharaman G, Stacey N, Roberts K. (2001) DcAGP1, a secreted arabinogalactan protein, is related to a family of basic proline-rich proteins. Plant Mol Biol 45: 421–435 [DOI] [PubMed] [Google Scholar]
- Baumberger N, Doesseger B, Guyot R, Diet A, Parsons R, Clark M, Simmons MP, Bedinger P, Goff S, Ringli C, et al. (2003a) Whole-genome comparison of leucine-rich repeat extensins in Arabidopsis and rice: a conserved family of cell wall proteins form a vegetative and a reproductive clade. Plant Physiol 131: 1313–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberger N, Ringli C, Keller B. (2001) The chimeric leucine-rich repeat/extensin cell wall protein LRX1 is required for root hair morphogenesis in Arabidopsis thaliana. Genes Dev 15: 1128–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberger N, Steiner M, Ryser U, Keller B, Ringli C. (2003b) Synergistic interaction of the two paralogous Arabidopsis genes LRX1 and LRX2 in cell wall formation during root hair development. Plant J 35: 71–81 [DOI] [PubMed] [Google Scholar]
- Borner GHH, Lilley KS, Stevens TJ, Dupree P. (2003) Identification of glycosylphosphatidylinositol-anchored proteins in Arabidopsis: a proteomic and genomic analysis. Plant Physiol 132: 568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JD, Sadler IH, Fry SC. (1996) Di-isodityrosine, a novel tetrameric derivative of tyrosine in plant cell wall proteins: a new potential cross-link. Biochem J 315: 323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JD, Sadler IH, Fry SC. (1998) Pulcherosine, an oxidatively coupled trimer of tyrosine in plant cell walls: its role in cross-link formation. Phytochemistry 47: 349–353 [DOI] [PubMed] [Google Scholar]
- Cannon MC, Terneus K, Hall Q, Tan L, Wang Y, Wegenhart BL, Chen L, Lamport DTA, Chen Y, Kieliszewski MJ. (2008) Self-assembly of the plant cell wall requires an extensin scaffold. Proc Natl Acad Sci USA 105: 2226–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassab GI. (1998) Plant cell wall proteins. Annu Rev Plant Physiol Plant Mol Biol 49: 281–309 [DOI] [PubMed] [Google Scholar]
- Fowler TJ, Bernhardt C, Tierney ML. (1999) Characterization and expression of four proline-rich cell wall protein genes in Arabidopsis encoding two distinct subsets of multiple domain proteins. Plant Physiol 121: 1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar YM, Nam J, Schultz CJ, Lee LY, Gilson PR, Gelvin SB, Bacic A. (2004) Characterization of the Arabidopsis lysine-rich arabinogalactan-protein AtAGP17 mutant (rat1) that results in a decreased efficiency of Agrobacterium transformation. Plant Physiol 135: 2162–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille S, Hänsel U, Ziemann M, Pauly M. (2009) Identification of plant cell wall mutants by means of a forward chemical genetic approach using hydrolases. Proc Natl Acad Sci USA 106: 14699–14704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Q, Cannon M. (2002) The cell wall hydroxyproline-rich glycoprotein RSH is essential for normal embryo development in Arabidopsis. Plant Cell 14: 1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held MA, Tan L, Kamyab A, Hare M, Shpak E, Kieliszewski MJ. (2004) Di-isodityrosine is the intermolecular cross-link of isodityrosine-rich extensin analogs cross-linked in vitro. J Biol Chem 279: 55474–55482 [DOI] [PubMed] [Google Scholar]
- Hieta R, Myllyharju J. (2002) Cloning and characterization of a low molecular weight prolyl 4-hydroxylase from Arabidopsis thaliana: effective hydroxylation of proline-rich, collagen-like, and hypoxia-inducible transcription factor alpha-like peptides. J Biol Chem 277: 23965–23971 [DOI] [PubMed] [Google Scholar]
- Humphrey TV, Bonetta DT, Goring DR. (2007) Sentinels at the wall: cell wall receptors and sensors. New Phytol 176: 7–21 [DOI] [PubMed] [Google Scholar]
- Johnson KL, Jones BJ, Bacic A, Schultz CJ. (2003a) The fasciclin-like arabinogalactan proteins of Arabidopsis: a multigene family of putative cell adhesion molecules. Plant Physiol 133: 1911–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KL, Jones BJ, Schultz CJ, Bacic A. (2003b) Non-enzymic cell wall (glyco)proteins. Rose JKC, , The Plant Cell Wall. Blackwell Publishers, Oxford, pp 111–154 [Google Scholar]
- José-Estanyol M, Puigdomènech P. (2000) Plant cell wall glycoproteins and their genes. Plant Physiol Biochem 38: 97–108 [Google Scholar]
- Keskiaho K, Hieta R, Sormunen R, Myllyharju J. (2007) Chlamydomonas reinhardtii has multiple prolyl 4-hydroxylases, one of which is essential for proper cell wall assembly. Plant Cell 19: 256–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszewski MJ, Lamport DTA. (1994) Extensin: repetitive motifs, functional sites, posttranslational codes and phylogeny. Plant J 5: 157–172 [DOI] [PubMed] [Google Scholar]
- Kjellbom P, Snogerup L, Stohr C, Reuzeau C, McCabe PF, Pennell RI. (1997) Oxidative cross-linking of plasma membrane arabinogalactan proteins. Plant J 12: 1189–1196 [DOI] [PubMed] [Google Scholar]
- Knox J, Day S, Roberts K. (1989) A set of cell surface glycoproteins forms an early position, but not cell type, in the root apical carota L. Development 106: 47–56 [Google Scholar]
- Lee S, Saravanan RS, Damasceno CMB, Yamane H, Kim B, Rose JKC. (2004) Digging deeper into the plant cell wall proteome. Plant Physiol Biochem 42: 979–988 [DOI] [PubMed] [Google Scholar]
- Liu C, Mehdy M. (2007) A nonclassical arabinogalactan protein gene highly expressed in vascular tissues, AGP31, is transcriptionally repressed by methyl jasmonic acid in Arabidopsis. Plant Physiol 145: 863–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkouropoulos G, Barnett DC, Shirsat AH. (1999) The Arabidopsis extensin gene is developmentally regulated, is induced by wounding, methyl jasmonate, abscisic, and salicylic acid and codes for a protein with unusual motifs. Planta 208: 212–219 [DOI] [PubMed] [Google Scholar]
- Nakhamchik A, Zhao Z, Provart NJ, Shiu S, Keatley SK, Cameron RK, Goring DR. (2004) A comprehensive expression analysis of the Arabidopsis proline-rich extensin-like receptor kinase gene family using bioinformatic and experimental approaches. Plant Cell Physiol 45: 1875–1881 [DOI] [PubMed] [Google Scholar]
- Nickel W. (2003) The mystery of nonclassical protein secretion: a current view on cargo proteins and potential export routes. Eur J Biochem 270: 2109–2119 [DOI] [PubMed] [Google Scholar]
- Nothnagel EA. (1997) Proteoglycans and related components in plant cells. Int Rev Cytol 174: 195–291 [DOI] [PubMed] [Google Scholar]
- Schnabelrauch LS, Kieliszewski MJ, Upham BL, Alizedeh H, Lamport DTA. (1996) Isolation of pI 4.6 extensin peroxidase from tomato cell suspension cultures and identification of Val-Tyr-Lys as putative intermolecular cross-link site. Plant J 9: 477–489 [DOI] [PubMed] [Google Scholar]
- Schultz CJ, Ferguson KL, Lahnstein J, Bacic A. (2004) Post-translational modifications of arabinogalactan-peptides of Arabidopsis thaliana: endoplasmic reticulum and glycosylphosphatidylinositol-anchor signal cleavage sites and hydroxylation of proline. J Biol Chem 279: 45503–45511 [DOI] [PubMed] [Google Scholar]
- Schultz CJ, Johnson KL, Currie G, Bacic A. (2000) The classical arabinogalactan protein gene family of Arabidopsis. Plant Cell 12: 1751–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz CJ, Rumsewicz MP, Johnson KL, Jones BJ, Gaspar YM, Bacic A. (2002) Using genomic resources to guide research directions: the arabinogalactan protein gene family as a test case. Plant Physiol 129: 1448–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert GJ, Roberts K. (2007) The biology of arabinogalactan proteins. Annu Rev Plant Biol 58: 137–161 [DOI] [PubMed] [Google Scholar]
- Sherrier DJ, Prime TA, Dupree P. (1999) Glycosylphosphatidylinositol-anchored cell-surface proteins from Arabidopsis. Electrophoresis 20: 2027–2035 [DOI] [PubMed] [Google Scholar]
- Shi H, Kim Y, Guo Y, Stevenson B, Zhu JK. (2003) The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant Cell 15: 19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter AM. (1993) Structure and function of plant cell wall proteins. Plant Cell 5: 9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter AM. (2001) Arabinogalactan-proteins: structure, expression and function. Cell Mol Life Sci 58: 1399–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Xu J, Yang J, Kieliszewski MJ, Showalter AM. (2005) The lysine-rich arabinogalactan-protein subfamily in Arabidopsis: gene expression, glycoprotein purification and biochemical characterization. Plant Cell Physiol 46: 975–984 [DOI] [PubMed] [Google Scholar]
- Svetek J, Yadav MP, Nothnagel EA. (1999) Presence of a glycosylphosphatidylinositol lipid anchor on rose arabinogalactan proteins. J Biol Chem 274: 14724–14733 [DOI] [PubMed] [Google Scholar]
- Tan L, Leykam JF, Kieliszewski MJ. (2003) Glycosylation motifs that direct arabinogalactan addition to arabinogalactan proteins. Plant Physiol 132: 1362–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Qiu F, Lamport DTA, Kieliszewski MJ. (2004) Structure of a hydroxyproline (Hyp)-arabinogalactan polysaccharide from repetitive Ala-Hyp expressed in transgenic Nicotiana tabacum. J Biol Chem 279: 13156–13165 [DOI] [PubMed] [Google Scholar]
- Tiainen P, Myllyharju J, Koivunen P. (2005) Characterization of a second Arabidopsis thaliana prolyl 4-hydroxylase with distinct substrate specificity. J Biol Chem 280: 1142–1148 [DOI] [PubMed] [Google Scholar]
- van Hengel AJ, Barber C, Roberts K. (2004) The expression patterns of arabinogalactan-protein AtAGP30 and GLABRA2 reveal a role for abscisic acid in the early stages of root epidermal patterning. Plant J 39: 70–83 [DOI] [PubMed] [Google Scholar]
- van Hengel AJ, Roberts K. (2003) AtAGP30, an arabinogalactan-protein in the cell walls of the primary root, plays a role in root regeneration and seed germination. Plant J 36: 256–270 [DOI] [PubMed] [Google Scholar]
- Vlad F, Spano T, Vlad D, Daher FB, Ouelhadj A, Kalaitzis P. (2007) Arabidopsis prolyl 4-hydroxylases are differentially expressed in response to hypoxia, anoxia and mechanical wounding. Physiol Plant 130: 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Sardar HS, McGovern KR, Zhang Y, Showalter AM. (2007) A lysine-rich arabinogalactan protein in Arabidopsis is essential for plant growth and development, including cell division and expansion. Plant J 49: 629–640 [DOI] [PubMed] [Google Scholar]
- Yoshiba Y, Aoki C, Iuchi S, Nanjo T, Seki M, Sekiguchi F, Yamaguchi-Shinozaki K, Shinozaki K. (2001) Characterization of four extensin genes in Arabidopsis thaliana by differential gene expression under stress and non-stress conditions. DNA Res 8: 115–122 [DOI] [PubMed] [Google Scholar]
- Youl JJ, Bacic A, Oxley D. (1998) Arabinogalactan-proteins from Nicotiana alata and Pyrus communis contain glycosylphosphatidylinositol membrane anchors. Proc Natl Acad Sci USA 95: 7921–7926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa K, Toyooka K, Fukuda H, Matsuoka K. (2005) Membrane-anchored prolyl hydroxylase with an export signal from the endoplasmic reticulum. Plant J 41: 81–94 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.