Abstract
Exposure to genotoxins may compromise DNA integrity in male reproductive cells, putting future progeny at risk for developmental defects and diseases. To study the usefulness of sperm DNA damage as a biomarker for genotoxic exposure, we have investigated cellular and molecular changes induced by benzo[a]pyrene (B[a]P) in human sperm in vitro, and results have been compared for smokers and non-smokers. Sperm DNA obtained from five smokers was indeed more fragmented than sperm of six non-smokers (mean % Tail DNA 26.5 and 48.8, respectively), as assessed by the alkaline comet assay (P < 0.05). B[a]P-related DNA adducts were detected at increased levels in smokers as determined by immunostaining. Direct exposure of mature sperm cells to B[a]P (10 or 25 μM) caused moderate increases in DNA fragmentation which was independent of addition of human liver S9 mix for enzymatic activation of B[a]P, suggesting some unknown metabolism of B[a]P in ejaculates. In vitro exposure of samples to various doses of B[a]P (with or without S9) did not reveal any significant differences in sensitivity to DNA fragmentation between smokers and non-smokers. Incubations with the proximate metabolite benzo[a]pyrene-r-7,t-8-dihydrodiol-t9,10-epoxide (BPDE) produced DNA fragmentation in a dose-dependent manner (20 or 50 μM), but only when formamidopyrimidine DNA glycosylase treatment was included in the comet assay. These levels of DNA fragmentation were, however, low in relation to very high amounts of BPDE–DNA adducts as measured with 32P postlabelling. We conclude that sperm DNA damage may be useful as a biomarker of direct exposure of sperm using the comet assay adapted to sperm, and as such the method may be applicable to cohort studies. Although the sensitivity is relatively low, DNA damage induced in earlier stages of spermatogenesis may be detected with higher efficiencies.
Introduction
Environmental pollutants such as products of fossil fuel combustion and contaminants in food and water are—together with lifestyle factors—unquestionably linked to human health. One such compound is the polycyclic aromatic hydrocarbon (PAH), benzo[a]pyrene (B[a]P), an ubiquitous environmental pollutant found in, e.g., cigarette smoke and grilled food. When ingested or inhaled, B[a]P can be transformed by phase I enzymes such as the CYP450 enzymes into several DNA-reactive metabolites, including the directly acting carcinogen benzo[a]pyrene-7,8-diol-9,10-epoxide (BPDE) [reviewed in refs (1,2)], which binds covalently to DNA (3). BPDE can also act in synergy with other exposures e.g. ultraviolet light (4), ultimately increasing the risk of mutation, cancer or possibly other adverse effects (5–7). B[a]P can also contribute to the genotoxic burden in cells by reaction via the aldo-keto reductase superfamily leading to oxidative stress [reviewed in ref. (8)]. The oxidized quinones produced via this pathway may in addition revert to catechols, which can react with DNA and other biomolecules to form covalent adducts.
When mutations occur in male or female reproductive cells, future progeny may be at risk of hereditary predispositions to various developmental defects or disease including cancer. Studies suggest that exposure to PAHs is correlated with reduced semen quality and higher risk of infertility; men with idiopathic infertility and also abnormal sperm parameters [World Health Organization (WHO) criteria (1999)] were shown to have increased levels of urinary PAH metabolites compared to control groups (9). Moreover, studies have shown that smoking men have higher levels of BPDE–DNA adducts than non-smokers and that the level of adducts in embryos from smoking couples seems to depend more on the smoking habit of the father than that of the mother, implying that the adducts in embryos could be more of paternal origin (10,11). Sperm is a useful human biomaterial, being available from volunteering individuals and from sperm banks; sperm has a well-studied physiology and there are established methods for their characterization (WHO). For tissues such as white blood cells and mouth mucous cells, the application of the comet assay (12) has been useful in biomonitoring studies (13). This assay traditionally detects DNA strand breaks and alkali-labile sites (e.g. baseless sites in DNA), is fast and sensitive and can readily be modified to achieve higher specificity for the detection of specific types of DNA lesions (14–18). Analysis of human sperm using the comet assay [reviewed in (19) and (20)] provides information on DNA integrity in relevance to male fertility, and the method may supplement established techniques e.g. sperm chromatin structure assay and terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labelling (TUNEL) assay. In addition to issues relating to fertility, DNA damage in the mature sperm may provide information about the genotoxic burden of the germ line. In vivo, such DNA lesions may arise in dividing testicular stem cells or at varying stages during spermatogenesis and spermiogenesis. The susceptibility at the various stages to chemicals will depend on the ability of the cells to remove the damage by DNA repair (21–26), but it also relates to the packaging and restructuring of the chromatin at the various stages, including replacement of histones with protamines which occurs progressively during spermiogenesis [reviewed in refs (27,28)]. Mature sperm could potentially have a great potential as biomarker for genotoxic exposure in vivo when assayed with the comet assay. This, however, requires that induced lesions are measurable in a sensitive and robust manner. If human sperm collected from healthy men is susceptible to DNA damage induction after chemical treatment in vitro, it is likely that mature sperm may also be damaged in situ at a late stage of maturation, i.e. in the epididymis.
The present study was undertaken to evaluate the use of sperm DNA damage as a biomarker in population studies. Using B[a]P as an example of environmental exposure, semen samples were exposed to this compound or its proximate metabolite BPDE in vitro. Samples were obtained repetitively from one donor or from groups of donors representing smokers and non-smokers. The experiments were carried out in three laboratories, using the comet assay adapted to sperm; in addition, DNA adducts were analysed in some samples after exposure to B[a]P or BPDE in vitro, using 32P postlabelling and immunostaining.
Materials and methods
Chemicals
Benzo[a]pyrene-r-7,t-8-dihydrodiol-t9,10-epoxide [(±)anti-BPDE] was obtained from the National Cancer Institute (NCI), Chemical Carcinogen Reference Standard Repository at the Midwest Research Institute, Kansas City, MO, USA (0477 NCI#: L0137# Batch: 42 Lot.: 01262007-PSU). B[a]P (48564-SUPELCO) was obtained from Sigma-Aldrich®, Munich, Germany. SYBRGold™ was from Molecular Probes, Invitrogen, Paisley, UK; ethidium bromide was from Sigma, Gillingham, Dorset, UK. Proteinase K was from Hoffman-La Roche, Basel, Switzerland. Other chemicals were pro analysi grade from commercial suppliers.
Human liver fraction S9-mix and essential reagents
Pooled human liver S9-mix (10 individuals, mixed gender) was obtained from In vitro Technologies, Cat. no. X008023—LOT RTO. Glucose-6-phosphate (G-6-P), glucose-6-phosphate dehydrogenase (G-6-P DH), grade I from yeast and nicotinamide adenine dinucleotide phosphate (NADP) were obtained from Roche Diagnostics, Mannheim, Germany.
Semen samples
Semen samples were obtained from healthy volunteers after informed consent, at the University of Bradford after 3–5 days of sexual abstinence. Detailed information on age, ethnicity, sexual activity and children (and also other factors e.g. occupation, smoking and drinking habits, vitamin intake, existing urogenital pathologies, X-ray exposure, chemotherapy) was collected for other purposes but was not used for further analysis because of the small sample size. Smoking habits were confirmed by cotinine levels measured in seminal plasma (data not shown). The samples were labelled with date and time of collection and given an anonymous donor code. Routine analysis of semen quality was conducted within 2 h of collection using WHO criteria (1999) to provide details on colour, volume, viscosity, sperm concentration, pH, motility and morphology; all samples were found to be normospermic. All samples were aliquoted, snap frozen in liquid nitrogen and then kept at −80°C. Treated samples (5, 10 and 25 μM B[a]P ± S9 mix or 5, 20 and 50 μM BPDE) from three separate experiments were frozen and shipped to the participating laboratories for 32P postlabelling analysis. Ethical approval was obtained from the Research Ethics Sub-committee for Human Subjects at Bradford University in June 2005.
Cell treatments
Aliquots of 2–5 μl from a thawed sperm sample were incubated for 1 h in 1 ml solutions of phosphate-buffered saline (PBS), pH 7.4, with either 1, 5, 10 or 25 μM of B[a]P dissolved in dimethyl sulfoxide (DMSO; end concentration 1%), ±1% human liver fraction S9-mix or 5, 20 and 50 μM BPDE (DMSO 1%), at 32°C. X irradiation was used as positive control and was calibrated using Fricke's ferrous sulphate chemical dosimetry. The reduced nicotinamide adenine dinucleotide phosphate regenerating system (NRS) and the S9-mix (S9 + NRS) were freshly prepared before each experiment as previously described (29). When mixing the ingredients, the S9 fraction and the G-6-P DH were added at last to the mix, immediately before addition to the cells for exposure (NRS end concentrations: 33 mM potassium chloride (KCl), 8 mM magnesium chloride (MgCl2*6H2O), 5 mM G-6-P, 3,75 U/ml G-6-P DH, 4 mM NADP, 0.3 × PBS). For immunostaining analysis, sperm aliquots were incubated as described above but with 40 μM of B[a]P + S9-mix or with vehicle alone.
Alkaline comet assay
Two different methods were applied to study comet formation in mature sperm after incubation with B[a]P, and essential differences between protocols are mentioned in Table I. The two protocols were initially established to obtain relatively stable baseline levels of DNA damage of donor samples. They were then used as part of a large European Commission (EC) project (NewGeneris) to study various genotoxic and immunotoxic compounds using various end points including human sperm.
Table I.
Differences in comet assay Protocols 1 and 2
| Experimental conditions | Protocol 1 | Protocol 2 |
| Layers | 1. Layer: normal slide with 1% NMP | 1. Layer: GelBond® films using a 12-well format (4 × 3 wells) |
| 2. Layer: 1:1 cells with 2.0% LMP (end concentration 1%) | 2. Layer: 1:10 cells with 1.7% LMP (end concentration 1.53%) | |
| 3. Layer: 0.5% LMP | ||
| Lysis | 1. Lysis: slides in lysis buffer with 10 mM DTT, at 4°C, for 60 min | 1. Lysis: gel bond films in lysis buffer at 4°C, over night |
| 2. Lysis: slides in lysis stock with 0.05 mg/ml proteinase K, at 4°C, for 60 min | 2. Lysis: gel bond films in lysis buffer with 10 mM DTT, at 4°C, for 60 min | |
| 3. Lysis: gel bond films in 100 mM Trizma® buffer with 4 mM lithium diiodosalicyclate, pH 7.6, at ambient temperature, for 90 min | ||
| Enzyme treatment | 1 μg/ml FPG | |
| DNA unwinding | Electrophoresis buffer pH 13.2, 20 min | Electrophoresis buffer pH 13.2, 20 min |
| Electrophoresis | Electrophoresis: 25 V (0.78 V/cm) and not exceeding 300 mA at 4°C for 20 min | Electrophoresis: 20 V (0.8 V/cm) and 300 mA at 8°C for 20 min |
| After electrophoresis | pH neutralization | pH neutralization and fixation in ethanol (>70%) for 90 min, dried and stored |
| Staining: ethidium bromide | Staining: SYBRGold™ |
LMP, low melting point agarose; NMP, normal melting point agarose.
Comet assay Protocol 1
This protocol is based on Singh et al. (30), with modifications. Following exposure, sperm samples were centrifuged for 10 min at 450×g at room temperature, resuspended in cold PBS and kept on ice until mixed 1:1 with 2% low melting point agarose. The samples were cast onto standard glass slides pre-coated with 1% normal melting point agarose. Cell lysis was achieved through submerging the slides in two consecutive lysis solutions: (i) lysis buffer (2.5 M NaCl, 100 mM Na2 ethylenediaminetetraacetic acid (EDTA), 10 mM Trizma® base, 1% Triton X-100, pH 10) containing 10 mM dithiothreitol (DTT) for 60 min at 4°C and (ii) lysis buffer containing 0.05 mg/ml proteinase K for 60 min at 4°C. After lysis, the slides were incubated in electrophoresis buffer (300 mM NaOH, 1 mM Na2EDTA, pH 13.2) at 4°C for 20 min to unwind DNA and then subjected to electrophoresis at 25 V (0.8 V/cm on platform, 300 mA) for 20 min at 4°C. Prior to staining with ethidium bromide (20 μg/ml) for 10 min, the slides were neutralized (0.4 M Trizma® base, pH 7.5) three times for 5 min each. Comet visualization and scoring was performed with a Leica fluorescence microscope equipped with a Charge Coupled Device (CCD) camera using the image analysis software ‘Komet 4’ from Kinetic Imaging Ltd.
Comet assay Protocol 2
The general comet assay protocol is also based on Singh et al. (30) but was performed as adapted for sperm (31), with some modifications: following exposure, sperm samples were centrifuged for 8 min at 700×g at room temperature to avoid precipitation of B[a]P, resuspended in 1 ml of cold PBS and kept on ice until mixed 1:10 with 1.7% low melting point agarose. Samples (60 μl) were cast onto 8 × 11 cm GelBond® films using a 12-well format. Cell lysis was achieved through submerging the films in three consecutive lysis solutions: (i) lysis buffer: 2.5 M NaCl, 100 mM Na2EDTA, 10 mM Trizma® base, 1% Triton X-100, pH 10, at 4°C, overnight; (ii) lysis buffer with 10 mM DTT, pH 10, at 4°C, for 60 min and (iii) 100 mM Trizma® buffer with 4 mM lithium diiodosalicylate, pH 7.6, at ambient temperature, for 90 min. After lysis, all films were equilibrated in enzyme buffer (40 mM HEPES, 100 mM KCl, 0.5 mM Na2EDTA, pH 8) at 4°C for 60 min, after which the films were incubated in pre-warmed enzyme buffer with 0.2 mg/ml bovine serum albumin at 37°C for 60 min with or without 1 μg/ml (sufficient for saturating cleavage of oxidative lesions induced in human lymphocytes by Ro 12-9786 + visible light; data not shown) formamidopyrimidine DNA glycosylase (FPG) crude extract (A.K. Olsen, personal communication). Enzyme treatment was followed by unwinding in electrophoresis buffer (300 mM NaOH, 1 mM Na2EDTA, adjusted to pH 13.2 with HCl) at 4°C for 20 min, and electrophoresis at 20 V (0.8 V/cm on platform, 300 mA) at 8°C for 20 min. After 3 × 5 minutes of neutralization in 0.4 M Trizma® base, pH 7.5, films were rinsed in water and fixed in ethanol (>70%) for 90 min and dried. Gels were rehydrated and stained in Tris-EDTA buffer (1 mM Na2EDTA, 10 mM Trizma® HCl, pH 8) with SYBERGold® (1:12 000 dilution) for 20 min. Comet visualization and scoring were performed using the image analysis software ‘Comet assay IV’ from Perceptive Instruments Ltd, Haverhill, Suffolk, UK, and an Olympus BX51 fluorescence microscope (Japan), with an A312f camera (Basler Vision Technologies, Ahrensburg, Germany).
Immunodetection of monoclonal antibodies
Semen aliquots were centrifuged for 10 min at 450×g, the supernatant was carefully discarded and the pellet was dissolved in 300 μl PBS and again spun at 450×g for 10 min. This step was repeated twice to wash the sperm pellet thoroughly. The pellet was then resuspended in a 3:1 methanol–acetic acid solution (fixative); during continuous mixing on a vortex, the fixative was slowly dropped into the tube in 30 μl steps to avoid clumping of the sperm followed by incubation for 30 min. The fixed sperm were then applied in 50 μl drops to glass slides and air-dried for 3 h. The sperm were decondensed by incubating slides in ice-cold 10 mM DTT in a Coplin jar for 30 min, washing twice in 2× saline-sodium citrate buffer for 10 min each and dehydration in an ethanol series (70, 90 and 100%) for 3 min each. Finally, slides were air-dried. The decondensed sperm were permeabilized with 0.5% Triton X-100 and then analysed under a light microscope to check the density and possible over-decondensation. With a hydrophobic PapPen©, an area with an optimal density and quality was then marked for hybridization (10,11).
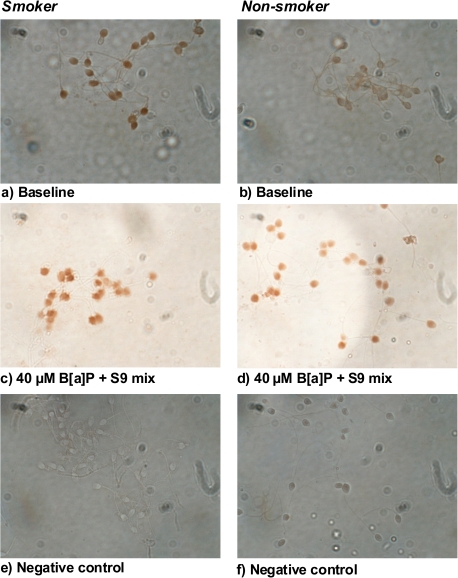
Unspecific binding sites were first blocked with 10% goat serum in PBS, followed by application of 100 μl 1:50 5D11 monoclonal antibody (Amersham, Piscataway, NJ, USA) in 1% goat serum. Clone 5D11 was reported to react directly with BPDE–DNA adducts as well as with diol epoxide–DNA adducts of different PAHs in a cross-reaction (32). The slides were incubated in a moist chamber at 4°C for 16 h to allow slow and thorough recognition of DNA adducts by the antibody. After hybridization, the slides were washed three times in PBS for 10 min each, followed by adding 100 μl of a solution containing the secondary goat anti-mouse antibody conjugated with biotin (DakoCytomation, Cambridge, UK). After incubation for 40 min at room temperature in a humid chamber, the slides were washed again three times in PBS for 10 min each, followed by application of 100 μl of a solution containing Streptavidin conjugated to horseradish peroxidase (DakoCytomation) and incubation at room temperature for 40 min in a moist chamber. Slides were washed twice in PBS for 10 min and finally for 10 min in double distilled H2O. The peroxidase reaction was developed with the 3.3′-diaminobenzidine enhanced liquid system (Sigma-Aldrich®). As a negative control, sperm from smokers were treated with 10% goat serum instead of the primary antibody, followed by application of the secondary antibodies and peroxidise reaction system. All slides were coded by co-workers and scored blindly. For the evaluation of staining intensity (STI), each sperm cell was scored as negative (STI 0), weak (STI 1), moderate (STI 2) or strong (STI 3) for a total of 100 sperm per semen donor. A staining intensity score (e.g. the sum of the products for the STI levels 0–3, with STI 0 = 1 points, STI 1 = 2 points, STI 2 = 3 points, STI 3 = 4 points) was calculated for each successfully hybridized sperm sample (10,11). Images (Figure 4) were obtained using a 100× Pan-NeoFluar objective and a CCD-camera.
Fig. 4.
In situ immunodetection with a monoclonal BPDE–DNA antibody, in sperm from smokers and non-smokers treated with 40μM B[a]P (in 1% DMSO) and human S9-mix. (a) Smoker sperm cells, untreated; (b) non-smoker sperm cells, untreated; (c) smoker sperm cells, treated with 40 μM B[a]P and human S9-mix; (d) non-smoker sperm cells, treated with 40 μM B[a]P and human S9-mix; (e) control for smoker sperm cells, no primary antibody applied and (f) control for non-smoker sperm cells, no primary antibody applied.
32P postlabelling
Spermatozoa obtained from frozen ejaculates were lysed overnight at 55°C using a buffer containing 20 mM Tris–HCl, 20 mM EDTA, 200 mM NaCl, 80 mM DTT, 4% sodium dodecyl sulphate and 250 μg/ml proteinase K. DNA from the lysed spermatozoa was isolated using the DNeasy Tissue Kit (Qiagen Westburg bv., Leusden, The Netherlands) according to manufacturers’ instructions with minor modifications: after the lysis of the cells, Buffer AL and 100% ethanol (EtOH) were added. This mixture was brought onto DNeasy Spin Columns and centrifuged. Thereafter, Buffer AW1 was added to the column and centrifuged and next, the column was washed with Buffer AW2. After centrifuging the column, the DNA was eluted in distilled water and stored at −20°C until use. The 32P postlabelling assay was subsequently performed as described (33). Briefly, DNA was digested using micrococcal endonuclease and spleen phosphodiesterase. Unadducted nucleotides were dephosphorylated by Nuclease P1 treatment and the modified nucleotides were subsequently radiolabelled (50 μCi per sample) by incubation with T4 polynucleotide kinase and γ-32P-ATP. Radiolabelled adduct nucleotide biphosphates were separated by chromatography on polyethylenimine-cellulose sheets from Machery-Nagel (Düren, Germany). In each experiment, three standards of BPDE-modified DNA with known modification levels (1 adduct per 107, 108 and 109 nucleotides) were run in parallel for quantification purposes using phosphor-imaging technology (Molecular Dynamics™, Sunnyvale, CA, USA).
High-performance liquid chromatography analysis of metabolites after exposure of mature sperm to B[a]P
Sperm sample aliquots of 250 μl were exposed under two different conditions, either unwashed in the presence of seminal plasma or washed in PBS. Washed samples were prepared by centrifugation at 9300×g for 5 min; the plasma above the cell pellets was removed and the pellets were resuspended and mixed thoroughly in 250 μl PBS and centrifuged another 5 min. The supernatant was then removed and the cell pellets were resuspended in a new 250 μl PBS. A volume of 250 μl ejaculate or washed sperm cells was exposed to 10 μM B[a]P dissolved in DMSO. All incubations were performed at 32°C for 1 or 24 h. After incubation, the samples were extracted three times with 1 ml ethyl acetate, which was subsequently evaporated under a continuous nitrogen flow. The residues were redissolved in 100% methanol and analysed by reversed phase high-performance liquid chromatography (HPLC); the samples were injected onto a Supelco Hypersil 5 column (250 × 3 mm). Elution was performed by using two mobile phases: Mobile phase A contained 100% methanol and Mobile phase B contained 40% methanol and 60% (v/v) water. A gradient elution programme, with a flow rate of 0.5 ml/min, started with 30% A and 70% B for 5 min, then gradually changed to 90% A in 25 min, held at 90% for 5 min, then back to 30% A in 2 min and held at 30% A for 3 min before the next injection. Detection was performed by a Perkin Elmer model LS 30 fluorescence spectrometer with 257 and >350 nm as excitation and emission wavelengths, respectively.
Statistics
The comet assay sample differences were determined by one-way analysis of variance with a Dunnett's or Tukey's post-hoc test, using SPSS 17©. For immunostaining, all generated data were processed with Metlab© software for the median and standard error. SPSS 15.0© was used to measure asymmetry of the probability distribution and skewness and to measure if the results were peaked or flat relative to a normal distribution and kurtosis. All the immunostaining data were found to violate normality and therefore, non-parametric Mann–Whitney was used to test for significant differences. If not specified, the level of significance is P < 0.05.
Results
Comet assay analysis of sperm from non-smoking volunteers
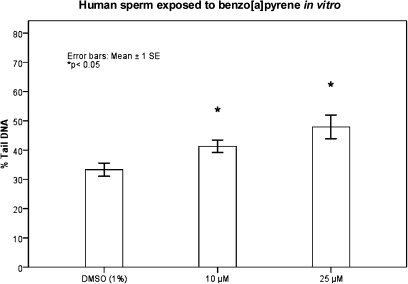
Comet assay experiments using Protocol 1 showed moderate but consistent increases in DNA strand breaks in sperm from three donors, exposed to 10 or 25 μM of B[a]P (P < 0.05; Figure 1). These increases were found without addition of liver S9-mix.
Fig. 1.
DNA damage in sperm from three donors, exposed to 10 or 25 μM B[a]P (without human S9-mix). Data were assessed by one-way analysis of variance with a Dunnett's post-hoc test. The dose response is presented as mean % Tail DNA ± 1SE. Each treatment group was compared to the control group (1% DMSO); *P < 0.05. Experiments were repeated several times for each donor (unexposed controls, n = 10, 10 or 25 μM B[a]P, n = 4). Comet assay Protocol 1.
In the in vitro experiments analysed by the comet Protocol 2, sperm aliquots from only one donor were used. Ejaculates were divided into several 50 μl aliquots and frozen at −80°C so that cells from the same ejaculate could be used repeatedly. During the 2-year course of the project, about 10 ejaculates from the same person were collected and used. No major difference in response to chemical treatment in vitro was observed between ejaculates, suggesting minor intra-individual differences during the project period.
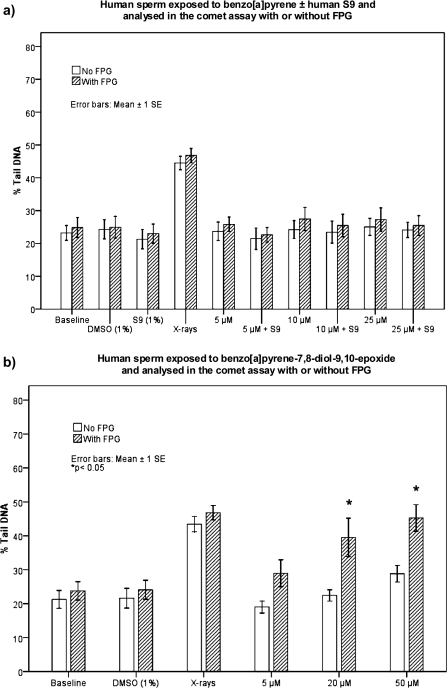
Exposure to either B[a]P or B[a]P plus S9-mix showed no statistically significant increase in DNA strand breaks, and the inclusion of FPG treatment did not lead to B[a]P-associated DNA damage (Figure 2a). BPDE produced weakly increased levels of DNA damage at 20 and 50 μM, as compared to the control (Figure 2b). These increases were significant only with FPG treatment (P < 0.05). These exposures were also repeated with samples from a second donor, giving highly similar results (data not shown).
Fig. 2.
(a) DNA damage in sperm from one donor exposed to 0, 5, 10 or 25 μM B[a]P (in 1% DMSO) ± human S9-mix. Comet analysis with or without FPG. Data represent the mean % Tail DNA ± 1SE, of three to six individual experiments, and were assessed by one-way analysis of variance (ANOVA) with a Tukeys's post-hoc test. No significant differences were observed for the various treatments (except for the positive control, X-rays 200 Gy; P < 0.05) compared to baseline and vehicle control samples. (b) DNA damage in sperm exposed to 0, 5, 20 or 50 μM BPDE in 1% DMSO. Comet analysis with or without FPG. Data are represented as mean % Tail DNA from four to six individual experiments ± 1SE. Assessed by one-way ANOVA with a Tukeys's post-hoc test. Only exposure to 20 or 50 μM BPDE combined with FPG treatment induced significantly (*P < 0.05) higher DNA damage levels compared to control samples. Positive control: X-rays 200 Gy. Comet assay Protocol 2.
Comet assay analysis comparing sperm from non-smokers and smokers
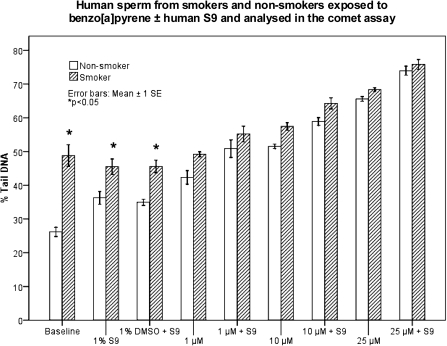
On the basis of the differences observed between the responses with the two protocols, Protocol 1 was chosen to study sperm from five smokers and six non-smokers. These groups showed significant differences in their baseline DNA damage levels and also differences in the levels obtained in samples when treated in vitro with DMSO plus S9 or S9 alone; samples from the smokers had higher levels of DNA damage than non-smokers (P < 0.001; P = 0.003; P = 0.023, respectively) (Figure 3). When the samples were treated with B[a]P in vitro, there were dose-dependent increases in DNA damage, in both groups (except non-significant differences of smoker's baseline levels versus treatment of the same samples with 1 μM B[a]P ± S9). However, a further increase with S9 was only observed in non-smoker samples exposed to either 1 or 25 μM B[a]P (P = 0.03; P = 0.04, respectively).
Fig. 3.
DNA damage in sperm from five smokers and six non-smokers. DNA damage levels are shown [baseline and in vitro exposure to 0, 1, 10 and 25 μM of B[a]P (in 1% DMSO) ± human S9-mix]. Data represent the mean % Tail DNA ± 1SE, from three individual experiments repeated for all treatments and samples. Sperm from the smokers had significantly higher levels of DNA damage compared to non-smokers (baseline, S9, S9 + DMSO). For both groups, in vitro exposure to B[a]P gave a dose-dependent increase in DNA damage. Comet assay Protocol 1.
In situ immunodetection in sperm cells from smokers and non-smokers using a B[a]P monoclonal antibody
Table II summarises staining intensity levels using the BPDE–DNA antibody, with samples from three smokers and three non-smokers. The data are derived from typical images shown in Figure 4; baseline values are shown as well as responses to 40 μM B[a]P activated with 1% S9-mix. The STI score was significantly increased in smokers as compared to non-smokers.
Table II.
Mean values for staining intensity levels in sperm cells of non-smokers and smokers
| STI score | STI 0 | STI 1 | STI 2 | STI 3 | |
| Non-smokers, vehicle only (n = 3, mean ± SE) | 69.0 ± 2.3 | 36.0 ± 3.74 | 59.0 ± 4.8 | 5.0 ± 1.4 | 0.0 ± 0 |
| Smokers, vehicle only (n = 3, mean ± SE) | 182.0 ± 15.2 | 3.0 ± 1.87 | 37.0 ± 5.3 | 35.0 ± 3.0 | 25.0 ± 10.0 |
| P-value | 0.050 | 0.050 | 0.046 | 0.050 | 0.037 |
| Non-smokers, B[a]P treated (n = 3, mean ± SE) | 186.0 ± 5.1 | 0.0 ± 0 | 34.0 ± 3.0 | 46.0 ± 3.9 | 20.0 ± 4.3 |
| Smokers, B[a]P treated (n = 3, mean ± SE) | 216.0 ± 9.4 | 0.0 ± 0 | 22.0 ± 3.2 | 40.0 ± 5.0 | 38.0 ± 8.3 |
| P-value | 0.050 | 1.000 | 0.050 | 0.275 | 0.127 |
Staining intensity levels (STI ± SE) and statistical differences (P-values) are shown for sperm samples treated for 1 h with 40 μM B[a]P plus S9 mix, or vehicle alone and analysed with a monoclonal antibody to detect DNA adducts.
BPDE adducts analysed with 32P postlabelling in sperm exposed in vitro
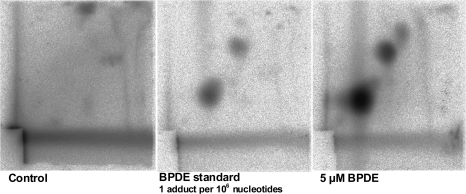
Sperm samples were treated in vitro with 5, 10 or 25 μM B[a]P ± S9 mix or 5, 20 or 50 μM BPDE. DNA was extracted and analysed with the 32P postlabelling technique. No B[a]P-induced adducts were detected by 32P postlabelling in samples exposed to either B[a]P or B[a]P plus S9-mix (data not shown). However, BPDE exposure led to a dose-dependent increase in adduct levels; these levels were extremely high and far beyond what is considered to be physiologically relevant (Figure 5).
Fig. 5.
32P postlabelling analysis of BPDE adducts in sperm cells. Results for control (DMSO 1%), BPDE standard and sperm cells exposed to 5 μM BPDE are shown.
HPLC analysis of metabolites after exposure of mature sperm to B[a]P
Mature sperm obtained from an ejaculate is not expected to be able to transform B[a]P into reactive metabolites, but the positive results in the comet assay with B[a]P in our study prompted a metabolite analysis by HPLC. Three B[a]P-related metabolites were indeed detectable after 24 h of incubation of sperm with 10 μM B[a]P (data not shown). These derivatives could not be chemically identified since they had different retention times as compared to several known metabolites (including 7,8-diol and 9,10-diol B[a]P). The unidentified B[a]P metabolites had retention times very close, but not similar to several known quinones (including 1,6-; 3,6- and 6,12-B[a]P-dione). Although the measured metabolites could not be identified, these results suggest that mature human sperm—or the seminal fluid—is capable of some form of oxidative metabolism of B[a]P.
Discussion
B[a]P has been extensively tested for its adverse effects in a large number of in vitro and in vivo studies and much of its toxicity is well documented. In general, metabolic activation by endogenous enzymes is required for B[a]P to be genotoxic; in particular, the metabolite BPDE is known to interact with biomolecules such as proteins and DNA (34), with new reactive B[a]P metabolites still being discovered (35). In vivo, some B[a]P activation products probably have sufficient stability to be transported from cells or organs possessing the ability to metabolize B[a]P, to cells or tissues lacking such activity (36). We recently detected adducts in sperm from mice exposed to B[a]P in vivo, strongly suggesting that B[a]P activation products had diffused from metabolically competent surrounding tissues (37). In view of the current understanding of the metabolism of B[a]P, it is expected that there would be little genotoxicity of this compound in various cell types, such as sperm, assumed to be deficient in metabolizing activity when incubated in vitro. However, Anderson et al. have shown genotoxic activities in both lymphocytes as well as sperm, of various compounds generally assumed to require enzymatic activation to be genotoxic (38–43).
DNA damage levels were assessed in B[a]P-exposed samples using the alkaline comet assay. Two protocols were employed to validate sperm DNA damage detection in two different laboratories using similar chemical exposure regimens. Contrary to what was expected, we observed a moderate but significantly increased level of DNA damage induced by B[a]P. This effect of B[a]P was observed when using Protocol 1, but not with Protocol 2. Protocol 2 was able to detect DNA damage after treatment with BPDE combined with FPG treatment and also X-ray-induced lesions (at the high doses required when used as a positive control for DNA damage induction in sperm). It is not likely that the different results with the two protocols are related to inter-individual differences, since the response with Protocol 1 was highly uniform for the 11 donors examined. Furthermore, the Protocol 2 experiments included a second donor (data not shown) showing a very similar response to the other donor, to the various treatments (negative to B[a]P ± S9). Russo et al. (44)—using a protocol similar to Protocol 1 to analyse human sperm—reported DNA damage in the absence of S9 (at very high concentrations, 500 μM), and increased levels of cellular oxidants were measured. It was suggested that peroxidases present in sperm are important for the oxidative stress induced by B[a]P. A possible factor of importance for the two protocols is the presence of light during in vitro cell exposure. Platt et al. (45)—using the Comet Assay—reported that several PAHs (including B[a]P) were found to induce DNA damage in V79 lung fibroblasts when co-exposed to visible light, apparently without a need for metabolic activation. We observed no significant effect of co-exposure of B[a]P and visible light (data not shown).
Using the 32P postlabelling assay, extremely high levels of BPDE–DNA adducts were measured in BPDE-exposed sperm samples (Figure 5). By means of in situ immunodetection using a monoclonal BPDE–DNA antibody, adducts were readily detected also in samples exposed to B[a]P plus S9. This increased staining was not paralleled by 32P postlabelling (data not shown), suggesting the existence of B[a]P-related modifications that were recognized by the antibody but could not be detected by 32P postlabelling. The higher immunostaining in smokers versus non-smokers (Figure 4) is in correspondence with the data from Zenzes et al. (10,11,46). Similarly, the observed higher baseline levels of DNA damage in sperm from smokers compared to non-smokers in the comet assay is in correspondence with the findings of Fraga et al. (47) (Figure 3).
Most bulky DNA adducts induced by B[a]P/BPDE are stable, and they are not detected as DNA breaks in the comet assay in the absence of DNA repair (48,49). In a cell-free system, BPDE reacts with not only DNA forming stable adducts (notably, BPDE–N2–Gua) but also unstable ones including BPDE–N7–guanine, N6–adenine and N3–cytosine (50,51). Unstable lesions caused by chemicals in cells may lead to the formation of ring-opened bases which may in turn be substrates for glycosylases such as FPG (52,53), as has been demonstrated for methyl methanesulfonate (54). The ultimate level of lesions that are detectable in the comet assay will also be largely affected by the tight packaging of DNA in sperm cells—even after extensive lysis and unwinding, as demonstrated with X-rays.
Taken together, our findings with the different end points seem to mostly correspond with the occurrence and the nature of the various types of lesions induced by BPDE (or B[a]P plus S9). However, the observed effects of B[a]P alone on sperm DNA in vitro using Protocol 1 require alternative explanations. The formation of unstable adducts from B[a]P is known to occur via a one-electron oxidation; however, both CYP enzymes and aldo-keto reductase are needed also for this pathway (34). An ‘untraditional’ form of activation of B[a]P therefore seems to be taking place, either via extracellularly or via mitochondria. Some metabolism of B[a]P, possibly by mature sperm, was confirmed by the observation of B[a]P-related metabolites after an in vitro incubation of sperm with B[a]P, and the retention times of these unknown derivatives indicated that they behaved like B[a]P quinones. Quinones are known to generate reactive oxidative species and they have both spermicidal and spermostatic effects (55). Anderson et al. (56) suggested—based on experiments using the comet assay—that the DNA damage induced by oestrogens in human sperm in vitro may be due to quinone formation. Rivrud (57) described an increased mutagenic activity of B[a]P in the Ames test when seminal fluid was included, suggesting that enzymes in the seminal fluid might be responsible for B[a]P activation. In our comet experiments, the seminal fluid was present during chemical exposures but it was diluted more than 100-fold.
Conclusions
The presence of DNA adducts in sperm after in vitro B[a]P exposure, as demonstrated with 32P postlabelling or immunostaining, suggests that mature sperm is susceptible to B[a]P adduct induction also when exposed to this compound in vivo. Correspondingly, sperm could possibly be used as a biomarker for exposure to environmental chemicals, both for the individual and at the group level. Differences in biomarker levels may then be attributed to environmental and/or lifestyle factors, which could ultimately be related to fertility and the genetic integrity of sperm.
Despite the very high sensitivity of 32P postlabelling and immunostaining, these techniques are time consuming. The comet assay is quick and low cost and has been made more suitable for cohort studies through the EC FP6 NewGeneris project (http://www.newgeneris.org/). The differing results obtained using the two separate comet assay protocols emphasize the need for a standardized experimental procedure when applied to sperm (19). The observation of higher levels of baseline sperm DNA damage in smokers versus non-smokers suggests that lesions induced during spermatogenesis may be specifically measured using the comet assay.
Funding
Funding to pay the Open Access publication charges for this article was provided by the European Union 6th Framework Programme, Priority 5: ‘Food Quality and Safety’ [NewGeneris (Newborns and Genotoxic exposure risks; Contract No 016320-2].
Acknowledgments
Thanks to Ann-Karin Olsen PhD for critical reading of the manuscript.
References
- 1.Pelkonen O, Nebert DW. Metabolism of polycyclic aromatic hydrocarbons: etiologic role in carcinogenesis. Pharmacol. Rev. 1982;34:189–222. [PubMed] [Google Scholar]
- 2.Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol. Appl. Pharmacol. 2005;206:73–93. doi: 10.1016/j.taap.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Jeffrey AM, Weinstein IB, Jennette KW, et al. Structures of benzo(a)pyrene—nucleic acid adducts formed in human and bovine bronchial explants. Nature. 1977;269:348–350. doi: 10.1038/269348a0. [DOI] [PubMed] [Google Scholar]
- 4.Gao D, Luo Y, Guevara D, et al. Benzo[a]pyrene and its metabolites combined with ultraviolet A synergistically induce 8-hydroxy-2'-deoxyguanosine via reactive oxygen species. Free Radic. Biol. Med. 2005;39:1177–1183. doi: 10.1016/j.freeradbiomed.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Denissenko MF, Pao A, Tang M, et al. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274:430–432. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- 6.Eastman A, Barry MA. The origins of DNA breaks: a consequence of DNA damage, DNA repair, or apoptosis? Cancer Invest. 1992;10:229–240. doi: 10.3109/07357909209032765. [DOI] [PubMed] [Google Scholar]
- 7.Li D, Wang M, Dhingra K, et al. Aromatic DNA adducts in adjacent tissues of breast cancer patients: clues to breast cancer etiology. Cancer Res. 1996;56:287–293. [PubMed] [Google Scholar]
- 8.Penning TM. Aldo-keto reductases and formation of polycyclic aromatic hydrocarbon o-quinones. Methods Enzymol. 2004;378:31–67. doi: 10.1016/S0076-6879(04)78003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia Y, Zhu P, Han Y, et al. Urinary metabolites of polycyclic aromatic hydrocarbons in relation to idiopathic male infertility. Hum. Reprod. 2009;24:1067–1074. doi: 10.1093/humrep/dep006. [DOI] [PubMed] [Google Scholar]
- 10.Zenzes MT, Puy LA, Bielecki R, et al. Detection of benzo[a]pyrene diol epoxide-DNA adducts in embryos from smoking couples: evidence for transmission by spermatozoa. Mol. Hum. Reprod. 1999;5:125–131. doi: 10.1093/molehr/5.2.125. [DOI] [PubMed] [Google Scholar]
- 11.Zenzes MT, Bielecki R, Reed TE. Detection of benzo(a)pyrene diol epoxide-DNA adducts in sperm of men exposed to cigarette smoke. Fertil. Steril. 1999;72:330–335. doi: 10.1016/s0015-0282(99)00230-7. [DOI] [PubMed] [Google Scholar]
- 12.Tice RR, Agurell E, Anderson D, et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000;35:206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 13.Dusinska M, Collins AR. The comet assay in human biomonitoring: gene-environment interactions. Mutagenesis. 2008;23:191–205. doi: 10.1093/mutage/gen007. [DOI] [PubMed] [Google Scholar]
- 14.Cordelli E, Fresegna AM, D'Alessio A, et al. ReProComet: a new in vitro method to assess DNA damage in mammalian sperm. Toxicol. Sci. 2007;99:545–552. doi: 10.1093/toxsci/kfm191. [DOI] [PubMed] [Google Scholar]
- 15.Thorne D, Wilson J, Kumaravel TS, et al. Measurement of oxidative DNA damage induced by mainstream cigarette smoke in cultured NCI-H292 human pulmonary carcinoma cells. Mutat. Res. 2009;673:3–8. doi: 10.1016/j.mrgentox.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Collins AR, Duthie SJ, Dobson VL. Direct enzymic detection of endogenous oxidative base damage in human lymphocyte DNA. Carcinogenesis. 1993;14:1733–1735. doi: 10.1093/carcin/14.9.1733. [DOI] [PubMed] [Google Scholar]
- 17.Collins AR, Dusinska M, Gedik CM, et al. Oxidative damage to DNA: do we have a reliable biomarker? Environ. Health Perspect. 1996;104(Suppl. 3):465–469. doi: 10.1289/ehp.96104s3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins A, Dusinska M, Franklin M, et al. Comet assay in human biomonitoring studies: reliability, validation, and applications. Environ. Mol. Mutagen. 1997;30:139–146. doi: 10.1002/(sici)1098-2280(1997)30:2<139::aid-em6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner A, Cemeli E, Anderson D. The comet assay in male reproductive toxicology. Cell Biol. Toxicol. 2009;25:81–98. doi: 10.1007/s10565-007-9041-y. [DOI] [PubMed] [Google Scholar]
- 20.Lewis SE, Agbaje IM. Using the alkaline comet assay in prognostic tests for male infertility and assisted reproductive technology outcomes. Mutagenesis. 2008;23:163–170. doi: 10.1093/mutage/gem052. [DOI] [PubMed] [Google Scholar]
- 21.Chandley AC. On the parental origin of Denovo mutation in man. Journal of Medical Genetics. 1991;28:217–223. doi: 10.1136/jmg.28.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen AK, Bjortuft H, Wiger R, et al. Highly efficient base excision repair (BER) in human and rat male germ cells. Nucleic Acids Res. 2001;29:1781–1790. doi: 10.1093/nar/29.8.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen AK, Duale N, Bjoras M, et al. Limited repair of 8-hydroxy-7,8-dihydroguanine residues in human testicular cells. Nucleic Acids Res. 2003;31:1351–1363. doi: 10.1093/nar/gkg216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsen AK, Lindeman B, Wiger R, et al. How do male germ cells handle DNA damage? Toxicol. Appl. Pharmacol. 2005;207:521–531. doi: 10.1016/j.taap.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 25.Holstein AF, Schulze W, Davidoff M. Understanding spermatogenesis is a prerequisite for treatment. Reprod. Biol. Endocrinol. 2003;1:107. doi: 10.1186/1477-7827-1-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansen J, Olsen AK, Wiger R, et al. Nucleotide excision repair in rat male germ cells: low level of repair in intact cells contrasts with high dual incision activity in vitro. Nucleic Acids Res. 2001;29:1791–1800. doi: 10.1093/nar/29.8.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchetti F, Wyrobek AJ. Mechanisms and consequences of paternally-transmitted chromosomal abnormalities. Birth Defects Res. C Embryo Today. 2005;75:112–129. doi: 10.1002/bdrc.20040. [DOI] [PubMed] [Google Scholar]
- 28.Ward WS, Coffey DS. DNA packaging and organization in mammalian spermatozoa: comparison with somatic cells. Biol. Reprod. 1991;44:569–574. doi: 10.1095/biolreprod44.4.569. [DOI] [PubMed] [Google Scholar]
- 29.van Leeuwen DM, Gottschalk RW, van Herwijnen MH, et al. Differential gene expression in human peripheral blood mononuclear cells induced by cigarette smoke and its constituents. Toxicol. Sci. 2005;86:200–210. doi: 10.1093/toxsci/kfi168. [DOI] [PubMed] [Google Scholar]
- 30.Singh NP, McCoy MT, Tice RR, et al. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 31.Agbaje IM, Rogers DA, McVicar CM, et al. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum. Reprod. 2007;22:1871–1877. doi: 10.1093/humrep/dem077. [DOI] [PubMed] [Google Scholar]
- 32.Santella RM, Lin CD, Cleveland WL, et al. Monoclonal antibodies to DNA modified by a benzo[a]pyrene diol epoxide. Carcinogenesis. 1984;5:373–377. doi: 10.1093/carcin/5.3.373. [DOI] [PubMed] [Google Scholar]
- 33.Godschalk RW, Ostertag JU, Moonen EJ, et al. Aromatic DNA adducts in human white blood cells and skin after dermal application of coal tar. Cancer Epidemiol. Biomarkers Prev. 1998;7:767–773. [PubMed] [Google Scholar]
- 34.Godschalk RW, van Schooten FJ, Bartsch H. A critical evaluation of DNA adducts as biological markers for human exposure to polycyclic aromatic compounds. J Biochem. Mol. Biol. 2003;36:1–11. doi: 10.5483/bmbrep.2003.36.1.001. [DOI] [PubMed] [Google Scholar]
- 35.Sagredo C, Olsen R, Greibrokk T, et al. Epimerization and stability of two new cis-benzo[a]pyrene tetrols by the use of liquid chromatography-fluorescence and mass spectrometry. Chem. Res. Toxicol. 2006;19:392–398. doi: 10.1021/tx0502746. [DOI] [PubMed] [Google Scholar]
- 36.Ginsberg GL, Atherholt TB. Transport of DNA-adducting metabolites in mouse serum following benzo[a]pyrene administration. Carcinogenesis. 1989;10:673–679. doi: 10.1093/carcin/10.4.673. [DOI] [PubMed] [Google Scholar]
- 37.Verhofstad N, van Oostrom CT, van BJ, et al. DNA adduct kinetics in reproductive tissues of DNA repair proficient and deficient male mice after oral exposure to benzo(a)pyrene. Environ. Mol. Mutagen. 2010;51:123–129. doi: 10.1002/em.20516. [DOI] [PubMed] [Google Scholar]
- 38.Anderson D, Dobrzynska MM, Yu TW, et al. DNA integrity in human sperm. Teratog. Carcinog. Mutagen. 1997;17:97–102. doi: 10.1002/(sici)1520-6866(1997)17:3<97::aid-tcm1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 39.Anderson D, Basaran N, Dobrzynska MM, et al. Modulating effects of flavonoids on food mutagens in human blood and sperm samples in the comet assay. Teratog. Carcinog. Mutagen. 1997;17:45–58. [PubMed] [Google Scholar]
- 40.Anderson D, Dobrzynska MM, Basaran N. Effect of various genotoxins and reproductive toxins in human lymphocytes and sperm in the Comet assay. Teratog. Carcinog. Mutagen. 1997;17:29–43. doi: 10.1002/(sici)1520-6866(1997)17:1<29::aid-tcm5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 41.Najafzadeh M, Reynolds PD, Baumgartner A, et al. Flavonoids inhibit the genotoxicity of hydrogen peroxide (H(2)O(2)) and of the food mutagen 2-amino-3-methylimadazo[4,5-f]-quinoline (IQ) in lymphocytes from patients with inflammatory bowel disease (IBD) Mutagenesis. 2009;24:405–411. doi: 10.1093/mutage/gep016. [DOI] [PubMed] [Google Scholar]
- 42.Ruf AA, Webb J, Anderson D. Modulation by flavonoids of the effects of a food mutagen in different thalassaemia genotypes in the Comet assay. Teratog. Carcinog. Mutagen. 2003;(Suppl. 2):93–102. doi: 10.1002/tcm.10083. [DOI] [PubMed] [Google Scholar]
- 43.Ruf AA, Jerwood D, Webb J, et al. Sensitivity of different thalassaemia genotypes to food mutagens in the Comet assay. Teratog. Carcinog. Mutagen. 2003;(Suppl. 2):83–91. doi: 10.1002/tcm.10078. [DOI] [PubMed] [Google Scholar]
- 44.Russo A, Troncoso N, Sanchez F, et al. Propolis protects human spermatozoa from DNA damage caused by benzo[a]pyrene and exogenous reactive oxygen species. Life Sci. 2006;78:1401–1406. doi: 10.1016/j.lfs.2004.10.085. [DOI] [PubMed] [Google Scholar]
- 45.Platt KL, Aderhold S, Kulpe K, et al. Unexpected DNA damage caused by polycyclic aromatic hydrocarbons under standard laboratory conditions. Mutat. Res. 2008;650:96–103. doi: 10.1016/j.mrgentox.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Zenzes MT. Smoking and reproduction: gene damage to human gametes and embryos. Hum. Reprod. Update. 2000;6:122–131. doi: 10.1093/humupd/6.2.122. [DOI] [PubMed] [Google Scholar]
- 47.Fraga CG, Motchnik PA, Wyrobek AJ, et al. Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutat. Res. 1996;351:199–203. doi: 10.1016/0027-5107(95)00251-0. [DOI] [PubMed] [Google Scholar]
- 48.Speit G, Schutz P, Hoffmann H. Enhancement of genotoxic effects in the comet assay with human blood samples by aphidicolin. Toxicol. Lett. 2004;153:303–310. doi: 10.1016/j.toxlet.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 49.Speit G, Hartmann A. The contribution of excision repair to the DNA effects seen in the alkaline single cell gel test (comet assay) Mutagenesis. 1995;10:555–559. doi: 10.1093/mutage/10.6.555. [DOI] [PubMed] [Google Scholar]
- 50.Osborne MR, Harvey RG, Brookes P. The reaction of trans-7,8-dihydroxy-anti-9,10-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene with DNA involves attack at the N7-position of guanine moieties. Chem. Biol. Interact. 1978;20:123–130. doi: 10.1016/0009-2797(78)90087-x. [DOI] [PubMed] [Google Scholar]
- 51.Osborne MR, Jacobs S, Harvey RG, et al. Minor products from the reaction of (+) and (-) benzo[a]-pyrene-anti-diolepoxide with DNA. Carcinogenesis. 1981;2:553–558. doi: 10.1093/carcin/2.6.553. [DOI] [PubMed] [Google Scholar]
- 52.Chetsanga CJ, Frenette GP. Excision of aflatoxin B1-imidazole ring opened guanine adducts from DNA by formamidopyrimidine-DNA glycosylase. Carcinogenesis. 1983;4:997–1000. doi: 10.1093/carcin/4.8.997. [DOI] [PubMed] [Google Scholar]
- 53.Tudek B. Imidazole ring-opened DNA purines and their biological significance. J. Biochem. Mol. Biol. 2003;36:12–19. doi: 10.5483/bmbrep.2003.36.1.012. [DOI] [PubMed] [Google Scholar]
- 54.Speit G, Schutz P, Bonzheim I, et al. Sensitivity of the FPG protein towards alkylation damage in the comet assay. Toxicol. Lett. 2004;146:151–158. doi: 10.1016/j.toxlet.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 55.Hughes LM, Griffith R, Carey A, et al. The spermostatic and microbicidal actions of quinones and maleimides: toward a dual-purpose contraceptive agent. Mol. Pharmacol. 2009;76:113–124. doi: 10.1124/mol.108.053645. [DOI] [PubMed] [Google Scholar]
- 56.Anderson D, Schmid TE, Baumgartner A, et al. Oestrogenic compounds and oxidative stress (in human sperm and lymphocytes in the Comet assay) Mutat. Res. 2003;544:173–178. doi: 10.1016/j.mrrev.2003.06.016. [DOI] [PubMed] [Google Scholar]
- 57.Rivrud GN. Mutagenicity testing of seminal fluid: seminal fluid increases the mutagenicity of the precursor mutagen benzo[a]pyrene in the presence of S9 mix. Mutat. Res. 1988;208:195–200. doi: 10.1016/0165-7992(88)90060-7. [DOI] [PubMed] [Google Scholar]