Abstract
Maintenance of proper “labile iron” levels is a critical component in preserving homeostasis. Iron is a vital element that is a constituent of a number of important macromolecules, including those involved in energy production, respiration, DNA synthesis, and metabolism; however, excess “labile iron” is potentially detrimental to the cell or organism or both because of its propensity to participate in oxidation–reduction reactions that generate harmful free radicals. Because of this dual nature, elaborate systems tightly control the concentration of available iron. Perturbation of normal physiologic iron concentrations may be both a cause and a consequence of cellular damage and disease states. This review highlights the molecular mechanisms responsible for regulation of iron absorption, transport, and storage through the roles of key regulatory proteins, including ferroportin, hepcidin, ferritin, and frataxin. In addition, we present an overview of the relation between iron regulation and oxidative stress and we discuss the role of functional iron overload in the pathogenesis of hemochromatosis, neurodegeneration, and inflammation. Antioxid. Redox Signal. 10, 997–1030.
-
Iron-bound transferrin binds the transferrin receptor for cellular iron uptake
Regulation of transferrin receptor 1 by iron regulatory element–iron regulatory protein system
Differential regulation of transferrin receptor 1 and transferrin receptor 2
Transferrin receptor 1 is regulated by hereditary hemochromatosis protein
Ferroportin and hephaestin cooperate in iron efflux from intestinal cells
I. Introduction
Iron is a trace element of crucial importance to living cells that exists in a divalent state. Because of its divalent nature, iron may act as a redox component of proteins, and therefore is integral to vital biologic processes that require the transfer of electrons. It is intimately involved in numerous vital biologic processes, including oxygen transport, oxidative phosphorylation, DNA biosynthesis, and xenobiotic metabolism (131). Iron is a constituent of such important proteins as hemoglobin, cytochromes, oxygenases, flavoproteins, and redoxins. The transition metal participates in the transfer of electrons via oxidation-reduction reactions that result in the fluctuation of iron between its ferric (3+) and ferrous (2+) states (229). This character is largely responsible for the biologic significance of iron.
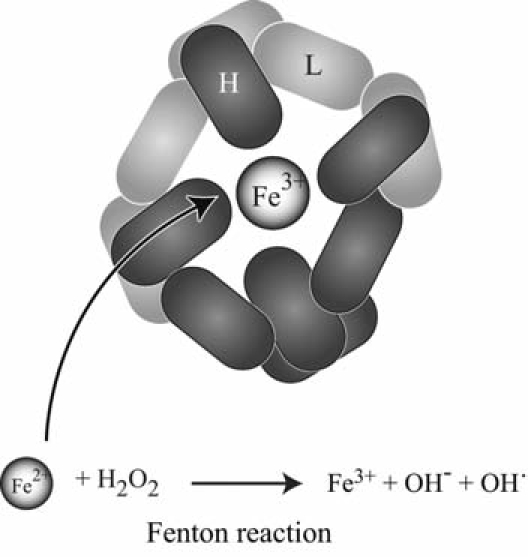
The same character that allows iron to participate in energy production by electron transfer also causes the toxicity resulting from an excess of “labile iron.” This propensity to undergo oxidation-reduction reactions is also responsible for the toxicity of iron (229). Most cytoplasmic iron is in its reduced form, meaning that it is an excellent substrate for oxidation. Donation of electrons leads to the formation of reactive free radicals; when ferrous iron interacts with H2O2, it undergoes the Fenton reaction (229). The Fenton reaction produces ferric iron, −OH, and the hydroxyl radical. It may also result in the peroxidation of adjacent lipids and lead to oxidative damage of DNA and other macromolecules.
In conjunction with this dichromatic nature, both severe iron overload and iron deficiency may be deleterious. Because iron is intimately involved in the production of energy and oxygen transport, iron deficiency is a serious problem that causes cell damage, reduction of cell growth and proliferation, hypoxia, and death. Each day ∼ 25 mg of iron is needed for erythropoiesis and other vital functions. Only 1 to 2 mg of iron comes from intestinal iron sources; thus, other mechanisms for iron regulation, including release of iron from cellular storage depots and recycling of iron from protein sources, are critically important to provide for organismal iron requirements. Likewise, an excess of iron systemically and at the cellular level leads to deleterious effects including free radical-induced damage to cells, cellular components, tissues, and organs. Deviations from normal iron levels have been indicated in the pathogenesis of aging, neurodegenerative disease, cancer, and infection (18, 23, 156, 180, 299, 334).
The duality of iron, being both essential and toxic, led to the evolution of elaborate systems to ensure adequate iron levels while preventing iron overload. In this review, we provide a comprehensive overview of the systems that maintain iron homeostasis. We focus on the critical components of the systems that control and provide iron storage, as well as the consequences resulting from disruption of the pathways that control iron concentrations at the organismal and cellular levels.
II. Iron Transport
A. Nonintestinal iron transport by transferrin
All cells require iron to maintain normal function. In nonintestinal cells, circulating iron is bound to transferrin (Tf) and is imported via receptor-mediated endocytosis after binding to the transferrin receptor (TfR) (249). Because Tf and TfR are absent from enterocytes, Tf binds iron and plays an essential role in the transport of iron only once it is exported from duodenal enterocytes into the bloodstream. Tf is also involved in the transport of iron from reticuloendocytic cells [red blood cell (RBC) recycling] and the liver to proliferative cells throughout the body, thereby controlling the levels of “labile iron” (293). In this sense, Tf serves as a storage sink for sequestering iron extracellularly until iron is needed, and then allowing it to reach target tissues. The human Tf gene is located at chromosome 3q21-q25 (330). The gene encodes a glycoprotein of ∼65,000 kDa. Human serum Tf is synthesized in hepatocytes and secreted by the liver to the plasma (4). Tf is composed of single chains that are bilobal, containing N- and C-lobes, each with two domains, referred to as the N1, N2, C1, and C2 domains. The lobes are connected by a hinge, which creates a cleft that contains the iron-binding domains. Iron binding and release are coordinated by a conformational change in which the two subdomains of each lobe open, and the N1, N2, C1, and C2 domains twist (114, 115). Each of the homologous amino domains binds one atom of Fe3+. Tf is an insulin-like growth factor-binding protein 3 (IGFBP3)-binding protein (318). IGFBP3 binds to circulating insulin-like growth factors (IGFs) and has growth-enhancing or inhibitory effects on cells, which are modulated by IGFBP3-binding protein. Although Tf has been shown to bind IGFs and IGFBP3, it does not contain the conserved GCGCCXXC-motif found in other IGFBPs (282). The role of Tf in the IGF-IGFBP pathway is unclear; however, treatment with exogenous Tf abrogated IGFBP3-mediated proliferation, and in prostate cancer cells, Tf inhibited apoptosis caused by IGFBP3 action (318). Tf bound with iron releases iron at acidic pH because of major conformational changes including a 54- to 63-degree rotation between the two domains on each lobe (103).
B. Iron-bound transferrin binds the transferrin receptor for cellular iron uptake
Two different TfRs, TfR1 and TfR2, are expressed and serve as gatekeepers for iron-bound Tf (Fe-Tf) (Fig. 1). TfR1 has three main domains per polypeptide chain: a protease-like domain, an apical domain, and a helical domain (176). The helical domain is important for homodimerization. TfR1 consists of a stalk, transmembrane, and cytoplasmic domain. Two molecules of Fe-Tf bind to the TfR homodimer on a conserved argi-nine-glycine-aspartate sequence located in the helical domain of TfR (75). The most important residues for Fe-Tf binding are located within the TfR1 helical domain; however, residues in the protease-like domain also are involved in Fe-Tf binding (105). A study using cryoelectron microscopy showed that Tf binds across the TfR1 dimer and stretches into the space between the receptor ectodomain terminus and the cell membrane (49). As shown in Fig. 1, once Fe-Tf is bound to TfR1, the complex is endocytosed into the cell in an acidic endosome. The endocytosis is stimulated by an internalization signal from a tyrosine located in the N-terminal portion of TfR in the cytoplasm (51). The change in pH in the endosome causes the iron to disassociate from Tf, and it is then exported from the endosome into the cytosol by a divalent metal transporter after reduction of iron by a ferric reductase (see section, Intestinal Iron Absorption, and Fig. 3 for a complete discussion).
FIG. 1.
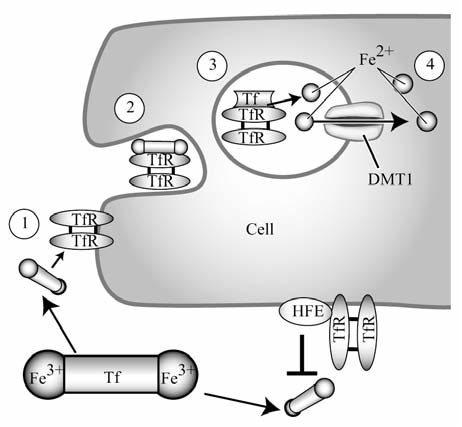
Cellular uptake of Tf-bound iron. Transferrin (Tf) is bound by two atoms of Fe3+ and circulates in plasma until reaching a target cell. It binds the transferrin receptor (TfR) on the plasma membrane of the cell, and then the Fe-Tf and TfR complex is endocytosed by the cell. In the acidic pH of the endosome, Fe3+ dissociates from Tf and is exported from the endosome by divalent metal transporter 1 (DMT1). The Tf-TfR complex is then recycled to the cell surface. Hereditary hemochromatosis protein (HFE) interacts with TfR in the Fe-Tf binding region and may thereby block the binding of Fe-Tf to TfR, preventing uptake of Fe-Tf, and negatively regulating cellular uptake of Tf-bound iron.
FIG. 3.
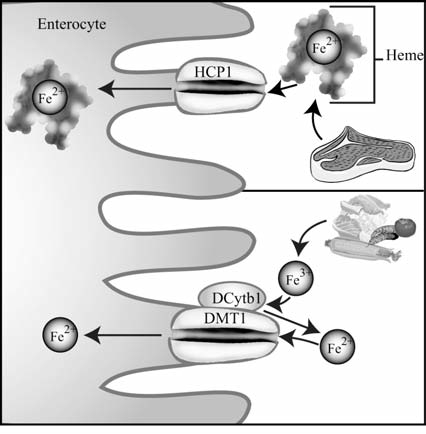
Intestinal absorption of dietary iron. Heme is primarily obtained from meat sources and is taken up by enterocytes by heme carrier protein 1 (HCP1) (upper), whereas inorganic iron (Fe3+) is reduced to Fe2+ by duodenal cytochrome b1 (DCytb1) and is subsequently transported into the enterocyte by divalent metal transporter 1 (DMT1) (lower).
A second TfR, TfR2, was identified more recently and shown to be involved in cellular iron import, but to have an expression pattern distinct from that of TfR1 (160). TfR2 is primarily expressed in hepatocytes and in the iron-absorbing cells of the intestine and duodenum (35). TfR2 has a lower affinity for Fe-Tf than does TfR1 (160, 320). Furthermore, TfR2 is not redundant for TfR1, for TfR1 knockout is embryonically lethal (184), despite the presence of TfR2. Deficiencies in TfR1 and TfR2 have different phenotypic outcomes: TfR1 deficiency results in low tissue iron levels, whereas TfR2 inadequacy leads to the development of hemochromatosis (34, 89).
C. Regulation of transferrin receptor 1 by iron regulatory element–iron regulatory protein system
TfR1 expression is controlled by a variety of cellular conditions, including iron and oxygen status. TfR1 mRNA contains five iron-regulatory elements (IREs), which convey posttran-scriptional regulation of expression by cellular iron concentration (41). IREs are structures located in untranslated regions (UTR) of mRNAs that are regulated by iron levels (130). The canonic IRE is composed of a stem-loop structure. The upper stem is 5 bp with variable nucleotide identity, and the bottom part of the stem is similarly structured. The upper and lower parts of the stem are separated by a variable bulge, which results from an unpaired cytosine residue. The loop consists of the six nucleotide consensus sequence 5′-CAGUGN-3′ (128). IRE elements are recognized by specific proteins, which are termed iron-regulatory proteins (IRPs). The activity of the two IRPs, IRP1 and IRP2, is controlled by iron levels in the cell. Both IRP1 and IRP2 are activated by iron-deficient conditions, leading them to bind the target IREs (130). Activated IRPs bind the hairpin loop and thereby modulate translation of the mRNA. The location of the IRE in the 5′ or 3′ end of the UTR of the target mRNA determines whether the regulation is positive or negative (130). Five IREs are located in the 3′ UTR of TfR1, allowing the binding of IRP during iron-deficient conditions to stabilize the mRNA, thereby causing upregulation of TfR1 (41, 211). IRP binding to an IRE in the 5′ UTR blocks the assembly of the 43S translation preinitiation complex (112). IRE-IRP effects on translation and stability of various target mRNAs, including TfR, ferritin, eLAS, Fpn, and DMT1, are summarized in Fig. 2. When iron concentrations are high, IRPs are degraded or inactivated, causing the downregulation of TfR1. In iron-replete cells, IRP1, which has ∼30% homology to mitochondrial aconitase based on amino acid alignments, loses its affinity for the IRE, and converts to a cytosolic aconitase (127, 263). Once iron is depleted, iron is removed from the iron–sulfur cluster (ISC) of IRP1, and mRNA-binding activity returns. IRP2 is degraded in conditions of iron excess, via a 73–amino acid domain called the iron-dependent degradation domain, which seems to be required for IRP2 degradation after protein oxidation by iron (140, 141). Degradation of IRP2 is mediated by the ubiquitin-proteasome pathway. For example, during heme-mediated gene regulation, heme induces IRP2 oxidation on Cys201, which is followed by enhanced IRP2 degradation (328). In this process, attachment of ubiquitin to oxidized IRP2 is mediated by the RING finger protein HOIL-1, which functions as an E3 ligase for oxidized IRP2 (328).
FIG. 2.

The IRP-IRE regulatory system. When iron regulatory proteins (IRPs) are activated by such conditions as low cellular iron, hypoxia, or H2O2, IRPs are able to bind to the iron-responsive element (IRE) of target mRNAs. When an IRP binds to an IRE located in the 5′-untranslated region (UTR) of target mRNA (ferritin H, ferritin L, eALAS, Fpn), it decreases translation by inhibiting the formation of the translation preinitiation complex (upper left panel). When an active IRP binds an IRE located in the 3′ UTR of target mRNA (TfR, DMT1), it increases mRNA stability (upper right panel). When the concentration of iron is high, IRP1 is deactivated by formation of an iron-sulfur cluster (ISC) and switches to cytosolic aconitase, whereas IRP2 is degraded. Under such conditions, IRP does not bind to IREs; therefore, mRNAs with IREs in the 5′ UTR are no longer blocked from translating (lower left panel), and those with IREs in the 3′ UTR of mRNA are destabilized (lower right panel).
D. Transcriptional regulation of transferrin receptor 1
TfR1 is also regulated at the transcriptional level. The promoter of TfR1 contains a hypoxia-responsive element (HRE) within a 100-bp sequence located upstream of the transcription start site (190). An HRE is a consensus sequence that dimerized hypoxia inducible factor (HIF-1α and HIF-1β) binds to in hypoxic conditions (319). In in vitro studies in hepatoma, K562, and HeLa cells, hypoxic conditions resulted in twofold to threefold increased TfR1 mRNA expression (190, 295). This effect was abrogated by almost 80% by mutation of the HIF-1 binding site within the putative HRE, whereas overexpression of the HIF-1α and HIF-1β subunits enhanced TfR1 promoter activity (190, 295). Treatment of HepB3 human hepatoma cells with an iron chelator, desferrioxamine, increased transcription of TfR1 (21). This study used regions of the TfR1 promoter, including that containing the putative HRE, cloned into a luciferase reporter, demonstrated that the transcriptional activation of the luciferase reporter in response to iron chelation was mediated by the HRE element of the TfR1 promoter. Furthermore, mutation of this element abrogated the induction, and HIF-1α binding to this sequence was induced by iron chelation. Cells with dysfunctional HIF-1α did not display transcriptional activation of TfR1 in response to iron chelation (21). Recently, low levels of H2O2 were shown to increase TfR1 expression (8). This response was demonstrated to be independent of protein stabilization, mRNA stabilization by activated IRP1, or transcriptional activation due to HIF-1α. In vitro 35S-methionine/cysteine labeling revealed that TfR1 protein synthesis was increased in response to long-term low-level H2O2 (8).
E. Differential regulation of transferrin receptor 1 and transferrin receptor 2
Unlike TfR1, TfR2 does not contain an IRE in its mRNA and does not appear to be regulated in an iron-dependent manner (90, 315). Studies of TfR2 expression in a mouse model for hemochromatosis revealed that TfR2 was not downregulated in conditions of iron overload (90), supporting the absence of an iron-mediated posttranscriptional regulatory mechanism. In addition, TfR2 was not upregulated in response to iron deficiency (90). The time- and dose-dependent increase in TfR2 expression in hepatocytes in response to the addition of exogenous Fe-Tf was observed only at the protein level, suggesting that such upregulation may be a result of protein stabilization (149). These results suggest that TfR1 and TfR2 are differentially regulated and thereby may serve diverse roles in terms of iron sensing and regulation.
F. Transferrin receptor 1 is regulated by hereditary hemochromatosis protein
Hereditary hemochromatosis protein (HFE) is implicated in hereditary hemochromatosis (HH) and belongs to the family of major histocompatibility complex class I molecules (82). HFE was linked to HH through studies of HFE-knockout mice. HFE-deficient mice developed severe iron overload and iron overload-related pathophysiology similar to that of humans with HH (336). HFE is expressed in epithelial cells involved in intestinal iron absorption, notably the crypt cells of the intestine (315). In the human placenta, HFE was shown to be associated with TfR1 (235). The exact function of HFE in terms of TfR1 regulation has not been fully elucidated and appears to be complex. The crystal structure of HFE and soluble TfR1 form a tight union in a basic pH solution, mimicking the conditions on the cell surface (179). Stoichiometric analysis of HFE binding to TfR showed that HFE binds to the TfR1 homodimer (179). Tf, conversely, binds 2:2 with TfR1, suggesting that HFE, Tf, and TfR1 may make a ternary complex that may be involved in the functional regulation of TfR (179). It has been hypothesized that in this ternary complex of HFE, TfR, and Tf, HFE may reduce the affinity of TfR and Tf. Stable overexpression of HFE in Chinese hamster ovary cells resulted in lower levels of TfR-mediated cellular iron uptake and decreased intracellular iron concentration (314). Interestingly, this effect was reversed by high levels of β2-microglobulin, which associates with HFE and stabilizes it (314). HFE-β2-microglobulin appeared to increase TfR recycling to the cell surface. HFE binds to TfR through the same helical domain as Fe-Tf (314). In this manner, HFE appears to block Tf binding to TfR through direct competition for the same binding site (179). This conclusion is supported by a more recent study, which used quantitative surface plasmon resonance assay to establish values for binding affinity of a group of mutant TfR. The critical residues for HFE binding to the TfR were spatially close to those identified as important for Tf binding (105). In addition, binding studies, which used a mutant heterodimer of TfR that conferred HFE binding to one TfR and Fe-Tf binding to the other, suggested that the effect of HFE on iron regulation by TfR may be exclusively related to HFE competing with Tf for binding (104).
G. Transferrin-independent cellular iron uptake
When iron levels exceed the binding capacity of the available Tf, free circulating iron may be available as non-Tf-bound iron (NTBI). Individuals with iron-overload disorders such as HH or β-thalassemia may have plasma NTBI in the micromolar range (144). This NTBI appears to be primarily absorbed by the liver and may contribute to cellular iron loading. A recent study suggests that the SLC39A zinc transporter, Zip14, may be involved in uptake of NTBI (188). Zip14 is highly expressed in the duodenum and jejunum and also expressed abundantly in the liver and heart (188). Overexpression of murine Zip14 resulted in increased uptake of Fe2+ by HEK293 cells, whereas decreased Zip14 expression by siRNA decreased iron accumulation in murine hepatocyte, AML12 cells (188).
H. Intestinal iron absorption
Dietary iron comes from two sources, heme and nonheme. Heme iron is obtained from meat sources and is more readily absorbed than nonheme iron obtained from consumption of grains and vegetables. The primary sites of heme transport are in the duodenum and in the liver. Heme is hydrophobic and thus has been proposed to diffuse passively through plasma membranes; however, recently an intestinal heme transporter was identified (277). This protein, named heme carrier protein 1 (HCP1), is abundantly expressed on the brush border of enterocytes in the duodenum, the primary location of intestinal iron absorption, and it appears to mediate cellular heme uptake (Fig. 3). In this study, heme uptake by HCP1 was temperature dependent and could be saturated. In addition, HCP1 seemed to be regulated by iron levels (277).
Although nonheme iron, such as that used in iron therapy, has been demonstrated to enter the intestines via passive diffusion between 100 and 200 mg of Fe2+ (169), at normal physiologic concentrations, nonheme iron does not readily cross a plasma membrane via passive diffusion; instead, it must be actively transported. Very little was known about metal ion transport until the simultaneous identification of DMT1 by two groups (88, 117). DMT1 is a metal-ion transporter with a uniquely broad range of divalent substrates such as Cd2+, Pb2+, Zn2+, Mn2+, Cu2+, and Co2+. Its expression was increased by an iron-deficient diet, and it was highly expressed in the rat duodenum. It also was shown to be highly homologous to the natural resistance-associated macrophage protein (Nramp) family of proteins and stimulated iron uptake (117). DMT1 (also known as Nramp2) was also originally identified as a gene mutated in a mouse model of severe iron deficiency called the mutant microcytic anemia (mk) mouse (88). The same mutation, Gly185Arg, in a transmembrane domain of the predicted protein, was identified in mk mouse, and another anemic strain, the Belgrade rat (87). DMT1 is a glycoprotein consisting of 12 transmembrane domains that is characterized by a high degree of hydrophobicity and ion channel transporter characteristics. A study of the expression and localization of DMT1 demonstrated that under normal conditions, DMT1 is expressed in the intestines at low levels; however, dietary iron deficiency resulted in a striking increase in DMT1 in the enterocytes of the duodenum (1, 38). DMT1 not only is involved in iron absorption in the intestine, but it also has been demonstrated to be involved in the process of iron recovery after endocytosis of Fe-Tf through the TfR. DMT1 is critical to the export of iron released from Tf in the acidic endosome into the cytosol (see Fig. 1) (117). It is important to note that nonheme iron is predominantly insoluble Fe3+. It must be reduced before transport by DMT1. Recently, an endosomal ferric reductase, Steap3, was identified (222). It was shown to colocalize with TfR1 and DMT1, and furthermore, its deletion was associated with the nm1054 mouse, which has hypochromic microcytic anemia caused by a defect in Fe-Tf cycle (221, 222). In addition, the recent identification of the ferric reductase, DCytb1, in areas of the intestine where DMT1 is concurrently expressed, shed some light on this process in intestinal iron absorption (197). DCytb is a homologue of cytochrome b561, with partial conservation of putative binding sites for the cytochrome b561 substrates ascorbic acid and semidehydroascorbic acid, suggesting that it is an ascorbate-dependent reductase (197). DCytb appears to be involved in the reduction of Fe3+ in the intestine, allowing it to be transported by DMT1 (197). A model depicting the function of DMT1 and DCytb1 in intestinal iron absorption into en-terocytes is depicted in Fig. 3.
I. Regulation of divalent metal transporter 1
DMT1 products include four different transcripts that occur as a result of alternative splicing at either the 3′ or 5′ end. The four different DMT1 isoforms produced in this manner are DMT1A, DMT1B, DMT+IRE, and DMT-IRE (137). In total, 543 amino acids are conserved in the region between the splice sites, and thus are found in all isoforms. Alternative splicing in the 5′ end of the gene leads to two distinct transcription start sites at exon 1A or 1B (137). DMT1A and DMT1B variants have different N-termini, with use of the exon 1A start site resulting in an additional 29 amino acids in humans (137). At the 3′ end, the main difference in the C-terminus splicing is the presence or absence of the IRE (DMT+IRE or DMT-IRE). The four isoforms of DMT1 displayed unique tissue expression patterns and subcellular locations. DMT1A is expressed mainly in the duodenum and in epithelial cells, whereas DMT1B is expressed predominantly in erythroid cells (38, 39). Additionally, both DMT isoforms are found both on the plasma membrane and in endosomes. The apical plasma membrane distribution appears to be controlled, at least in part, by N-glycosylation sites. Disruption of the sequence Y555XLXX in the cytoplasmic tail of DMT1 inhibited the localization of both DMT1A and DMT1B isoforms on the plasma membrane, but not in the endosome (286). The specific tissue and cellular localization of the different isoforms may be related to a differential function. DMT1A appears to be mostly localized to the apical membrane of polarized intestinal epithelial cells, and thus may play an important role in systemic iron absorption through the intestine, whereas DMT1B is more broadly expressed and also localized to erythroid cells (38, 39), suggesting that it may play a more widespread role in iron transport; perhaps, as indicated by its presence in the endosome, it may be involved in Tf-TfR iron import.
Regulation of DMT1 by the IRE-IRP system is complex, for the single IRE located in the 3′ UTR appears to have limited ability to influence DMT1 mRNA levels through an unclear mechanism in cultured cells (116). Further study revealed that regulation of DMT1 by iron is largely dependent on the cell line. DMT1A was regulated by iron in human Caco-2 cells, a model system for intestinal iron absorption (see Fig. 2) (137). A recent study demonstrated a distinct mechanism for regulation of expression of the DMT1B isoform. Increased nitric oxide levels during retinoic acid-induced differentiation of P19 cells resulted in the downregulation of both DMT+IRE and DMT-IRE isoforms as well as DMT1B, but not DMT1A (231). The DMT1B promoter contains a putative NF-κB element, and NF-κB bound to this response element during differentiation (231).
J. Ferroportin is responsible for cellular iron efflux
The transmembrane protein ferroportin (Fpn) is the only known iron exporter to date (Fig. 4). Fpn (also termed Ireg1, MTP1) was originally identified by positional cloning to identify the causative gene for hypochromic anemia of “weissherbst” mutant zebrafish (72). In zebrafish, Fpn encodes a protein with multiple-transmembrane domains and is expressed in the yolk sac, where it is required for transport of iron stored in the yolk to circulation. Exogenous Fpn expressed in Xenopus oocytes served as a ferrous iron exporter (72). Subsequent studies identified mouse and human Fpn in abundance in human placenta, liver, spleen, and kidney (73). Human Fpn is 571 amino acids long, and like both mouse and zebrafish Fpn, it contains a conserved hairpin loop sequence. Simultaneous studies characterized Fpn, which is abundantly expressed on the basolateral membrane of polarized enterocytes in the duodenum, in the cytosol of reticuloendothelial cells, and on the basal surface of syncytiotrophoblasts of the placenta (1, 72, 198). Expression of exogenous Fpn stimulates iron efflux (198). Fpn is also specifically expressed in reticuloendothelial macrophages, which play a critical role in iron reutilization through phagocytosis of erythrocytes (329). After erythrophagocytosis, Fpn is upregulated in cultured macrophages (164). In further studies, stable overexpression of Fpn by viral transduction in a mouse macrophage cell line resulted in a 70% increase in 59Fe release after phagocytosis of 59Fe-labeled erythrocytes (163).
FIG. 4.
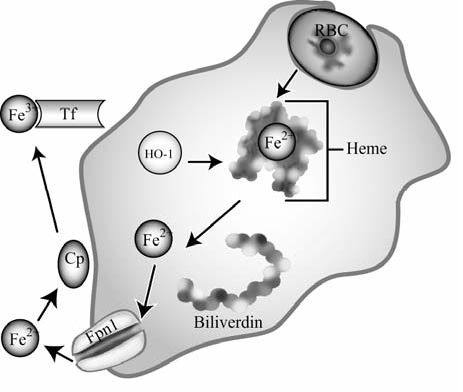
Iron transport in reticuloendocytic macrophages. Senescent red blood cells (RBCs) are phagocytosed and degraded. Their heme is broken down into biliverdin and Fe2+ by heme oxygenase 1 (HO-1), allowing Fe2+ to be exported from the macrophage by ferroportin1 (Fpn1). Once exported, Fe2+ is oxidized by ceruloplasmin (Cp) and binds to transferrin (Tf), where it may be delivered to target cells to fulfill cellular iron requirements for erythropoiesis or other processes.
Fpn is encoded by a gene on chromosome 2q in humans, which is larger than 20 kb in length, and is composed of eight exons (1, 72, 198). It also contains an IRE in its 5′ UTR (1, 72, 198). Mutations in this gene may cause autosomal dominant hemochromatosis; a missense mutation (A77D), which was proposed to lead to Fpn haploinsufficiency, was shown to be related to an iron-overloaded phenotype (205). Patients with the A77D mutation exhibit mainly early iron overload in the reticuloendocytic macrophages (205). In a different pedigree of autosomal dominant hemochromatosis, another missense mutation of Fpn (N144H) was identified (218). The authors concluded that the iron overload was a result of a gain-of-function mutation that would augment the iron-transport function of Fpn in enterocytes, thereby causing increased efflux of iron by the intestines (218). This result is consistent with the typical phenotype of HH, in which excess absorption of dietary iron in the intestines foments iron overload and excess storage in the cells of the reticuloendothelial system.
K. Ferroportin associates and cooperates with ceruloplasmin
Because Fpn transports Fe2+ into the plasma, and iron must be oxidized to be incorporated into transferrin (Tf), it is believed that Fpn cooperates with proteins that act as ferroxidases. In glioma cells, astrocytes, and macrophages, Fpn associates with ceruloplasmin (Cp) (123, 148). Cp is a multicopper oxidase that was originally isolated from pig serum in 1948 and was named “blue sky” protein because of its unique hue caused by the incorporation of six atoms of copper during its synthesis. Years later, it was revealed that Cp has ferroxidase activity and catalyzes the oxidation of Fe2+ to Fe3+ (228) (see Fig. 4). Studies of a yeast homologue of Cp, Fet3p, demonstrated its essential role in Fe2+ uptake (12, 63). The majority of Cp is expressed in the liver and secreted into the serum by hepatocytes, although it is also found in the brain and lung. This secreted form is unable to cross the blood-brain barrier. In the brain, a glycosylphosphatidylinositol (GPI)-anchored form of Cp is predominantly expressed by astrocytes (237, 238). Alternative splicing of Cp determines its form as a secreted or membrane-bound protein. Cp also functions as an antioxidant, because it converts Fe2+ to Fe3+, thereby decreasing the potential formation of reactive oxygen species (ROS) via Fenton chemistry (118).
In humans, deficiency in Cp results in a disease called aceruloplasminemia, which leads to late-onset degeneration of the retina and basal ganglia because of the pathologic accumulation of iron in various tissues, including the aforementioned affected tissues as well as the liver, spleen, and pancreas (106). Cp-knockout mice are normal at birth, but progressively accumulate iron, leading to a prominent elevation of iron content in the liver and spleen (123). They also have high levels of iron in cells of the reticuloendothelial system as well as in hepatocytes, as a result of impaired iron efflux (123). Conflicting evidence exists about the effect of disruption of murine Cp on brain iron homeostasis. In one characterization of Cp-knockout mice, central nervous system (CNS) iron overload was not observed (123), whereas in another study, Cp-knockout mice exhibited a modest iron overload of the CNS, and mouse embryonic fibroblasts (MEFs) from these lines were sensitive to oxidative stress (123, 239). Studies in mice with aceruloplasminemia demonstrated that injection of exogenous Fet3p increased iron saturation of Tf, suggesting that Cp may facilitate iron binding to Tf (122).
L. Ferroportin and hephaestin cooperate in iron efflux from intestinal cells
In the intestine, where Cp is not expressed, Fpn couples with another multicopper oxidase, hephaestin (Heph) (313). Heph is a transmembrane-bound homologue of Cp that is primarily expressed in the villi of the intestine, whereas Cp is not. Fpn and Heph colocalize on the basolateral membrane of absorptive enterocytes of the duodenum and intestines (121). A model depicting the function and association of Fpn and Heph in exportation of intestine-derived iron from enterocytes into the plasma is shown in Fig. 5. Heph was identified through study of sex-linked anemia (Sla) mice, which are deficient in transfer of iron absorbed in the intestines into the plasma (313). In this study, 582 nucleotides deleted in the Heph gene in Sla mice, which would lead to a 194 amino acid deletion in the gene product, were identified. Heph has 50% sequence similarity with Cp, including types I through III copper-binding domains and the cysteine residues in the disulfide bonds. The predicted structure of Heph includes a C-terminal transmembrane domain and a Cp-like extra cytosolic/extracellular component (313). Disruption of murine Heph and Cp genes resulted in increasing retinal iron concentrations with age that caused pathologic changes reminiscent of those seen in human aceruloplasminemia (119).
FIG. 5.

Transport of intestinal iron. Once iron is taken up by intestinal enterocytes by divalent metal transporter 1 (DMT1), it is then transported out of the enterocyte and into plasma by ferroportin1 (Fpn1). Fpn1 couples with hephaestin (Heph) in intestinal cells (in other cell types, it couples with ceruloplasmin), whose ferroxidase activity converts Fe2+ into Fe3+. This oxidation is necessary to aid in the binding of Fe3+ to apo-Tf in the plasma.
Several lines of evidence suggest that Fpn may be regulated by iron levels. The 5′ UTR of Fpn mRNA contains an IRE (198). As mentioned earlier, the presence of an IRE in the 5′ UTR of mRNA acts as a negative regulator of translation. Binding of IRP in this region sterically inhibits the stable formation of the 43S translation preinitiation complex (112). Thus, in conditions of iron depletion, the translation of Fpn is decreased, resulting in a decrease in iron export. Such an IRE in the 5′ UTR of mRNA is also located in other genes involved in the regulation of iron levels in the cell, including ferritin (heavy and light chains) and eALAS, which is involved in heme synthesis in erythroid cells (see Fig. 2) (131).
M. Hepcidin
Fpn is posttranslationally regulated by a peptide hormone, hepcidin (215). Hepcidin is synthesized by the liver as an 84–amino acid precursor, which is processed and then secreted as a final 25–amino acid peptide (232). Its expression is increased in response to iron and inflammation (247) and decreased by hypoxia and anemia (216). Hepcidin was originally identified as a peptide with a hairpin structure containing four stabilizing disulfide bonds possessing antimicrobial properties. Hepcidin was isolated independently by three groups, identifying it from plasma ultrafiltrate (168), urine (232), and livers (247). Because of its origin and antimicrobial property, it was originally termed hepcidin or LEAP-1 (liver-expressed antimicrobial peptide) (168, 232). In humans, hepcidin is encoded by 2.5-kb gene located on chromosome 19 (232). It has recently been proposed that hepcidin may be a master iron-regulatory hormone (98). Analysis of its structure revealed that hepcidin is a simple molecule containing a hairpin loop with two arms linked by disulfide bridges (139). The hydrophilic and hydrophobic side chains are separated in space, which is a trait in many antimicrobial peptides that disrupt cell walls of bacteria (139).
Several lines of evidence indicate that hepcidin is a negative regulator of plasma iron. Tissue-specific overexpression of hepcidin in murine liver resulted in body iron deficiency and severe microcytic hypochromic anemia of mice at birth (216). Administration of synthetic hepcidin induced rapid decline in serum iron levels (hypoferremia) in mice (258). It negatively regulates iron efflux by binding Fpn and triggering its internalization and subsequent degradation (215). The N-terminus of hepcidin is critical to the negative regulation of Fpn; deletion of all five of the amino acids of the N-terminus completely abrogated the hypoferremia (213). After hepcidin binding, Fpn is tyrosine phosphorylated on the plasma membrane (66). Mutation of two adjacent tyrosine residues at the phosphorylation site blocks internalization. Once inside the cell, Fpn is ubiquitinated after dephosphorylation, and ultimately, Fpn undergoes degradation in the lysosome (Fig. 6) (66). Ubiquitination appears to be dependent on the presence of bound Fe2+ on Fpn. A recent study revealed the functional significance of the coupling of Fpn with the GPI-linked form of Cp in glioma cells: when Cp was absent, Fe2+ remained bound to Fpn, leading to ubiquitination on Fpn Lys253 (65). This ubiquitination results in the subsequent degradation of Fpn. Fpn degradation is abrogated by oxidation, depletion of extracellular Fe2+, or mutation of Lys253 to Ala (65). This finding suggests a possible linkage between Cp and iron export and the iron accumulation exhibited in patients with aceruloplasminemia.
FIG. 6.
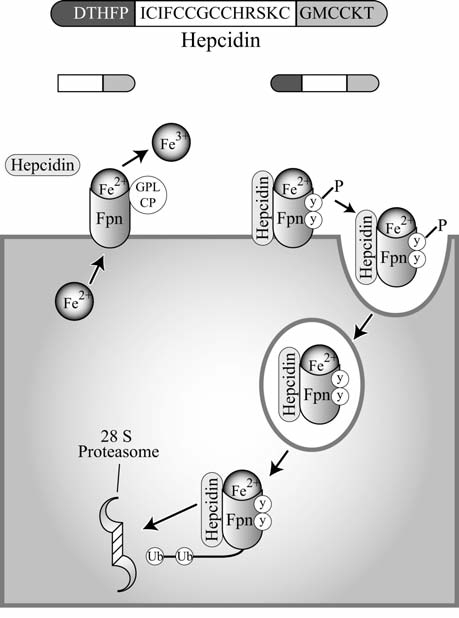
Hepcidin negatively regulates ferroportin. A truncated version of hepcidin is unable to bind to ferroportin (Fpn). Fpn is coupled with anchored ceruloplasmin (Cp), and exported iron is released and oxidized (left). On binding of wild-type hepcidin, Fpn is phosphorylated and subsequently endocytosed, ubiquitinated, and degraded by the 28S proteasome (right).
Iron overload induces hepcidin mRNA in hepatocytes (247); however, this mechanism has not been fully elucidated. Many proteins involved in iron transport and storage contain IRE in their mRNA that confers translational responsiveness to iron levels (see Fig. 2). The mRNA of hepcidin does not contain this element (99), suggesting that a sensor other than the IRE-IRP system may be necessary to regulate hepcidin translation in response to iron concentrations. The hepcidin promoter contains several CCAAT/enhancer-binding protein (C/EBP) consensus sequences. In addition, hepatocyte nuclear factor 4 and signal transducer and activator of transcription (STAT) motifs have also been identified in DNA encoding hepcidin (58). Thus, transcriptional regulation of hepcidin in response to iron may be one mechanism by which its expression is modulated. Studies in the liver demonstrated that C/EBPα plays a role in the iron-mediated regulation of hepcidin mRNA (58). Furthermore, levels of C/EBPα transcription factor in murine liver are doubled in response to iron loading, and mice deficient in C/EBPα exhibited lower levels of hepcidin mRNA, suggesting that C/EBPα modulates hepcidin transcription (58). It remains unclear, however, exactly how C/EBPα is involved in global iron sensing and hepcidin regulation in response to physiologic iron signals.
An alternative pathway for regulation of hepcidin expression during inflammation has also been suggested. Because hepcidin was originally identified as an antimicrobial peptide, investigators proposed that hepcidin may be regulated by mediators of inflammation. Indeed, hepcidin mRNA was increased in the murine liver and in hepatocytes in response to stimulation with LPS (247). Hypoferremia can develop during periods of chronic generalized inflammation. In mice, hepcidin was required for the development of anemia during inflammation (214). It was revealed that the upregulation of hepcidin during inflammation (a precipitating event in the development of hypoferremia) occurs, at least in part, through the action of IL-6 (214). The researchers treated primary human hepatocytes with LPS, observed an increase in hepcidin mRNA, and were able to abrogate the induction of hepcidin mRNA by treatment with anti-IL-6 antibodies. Furthermore, IL-6-knockout mice did not increase hepcidin mRNA in response to turpentine, another stimulus causing inflammation, and correspondingly, they did not show a decrease in serum iron (214). The mechanism by which IL-6 induces hepcidin mRNA involves the activation of STAT3 (324). Activation of STAT3 transcription factor by IL-6 results in the transactivation of a STAT binding sequence in the hepcidin promoter (245, 310).
An alternative mechanism of hepcidin regulation through a signaling pathway involving hemojuvelin (HFE2, also known as HJV) and bone morphogenetic proteins (BMP) was recently identified (14). BMPs are cytokines of the TGF-β superfamily that are intimately involved in development and cell fate, differentiation, growth, and apoptosis [reviewed in (219)]. The BMPs control these effects by binding BMP serine/threonine kinase receptors, which leads to the subsequent phosphorylation and activation of downstream second messengers including Smad proteins and mitogen-activated protein (MAP) kinases (219). HFE2 is a BMP coreceptor, and its mutation suppresses BMP signaling (14). This study showed that BMP could upregulate hepcidin in hepatocytes. Overexpression of HFE2 enhanced hepcidin production. Conversely, hepatocytes deficient in HFE2 had impaired hepcidin induction by BMP signaling (14). In this pathway, downstream activation of Smad4 was required for BMP-mediated hepcidin upregulation, for Smad4-knockout mice had lower levels of hepcidin and did not induce hepcidin in response to iron loading or IL-6 stimulation (14). More recently, BMP9, BMP4, and BMP2 were identified as inducers of hepcidin (300). Induction by administration of exogenous BMPs was greater than that caused by treatment with LPS (300). In addition, BMP-mediated induction of hepcidin was similar in wild-type congenic strains, and IL-6 knockout, HFE knockout, and TfR2 mutant mice, suggesting that BMP-mediated activation of hepcidin expression may not require these proteins (300). Also, in vitro studies show that addition of BMP-2 resulted in increased expression of hepcidin and decreased serum iron levels in vivo. Likewise, administration of soluble HJV inhibited BMP-mediated induction of hepcidin and resulted in increased levels of Fpn in vivo (15).
In addition, HIF-1 has been implicated in the transcriptional regulation of hepcidin in response to hypoxia (242). HIF-1 is responsible for activating a battery of genes in response to reduced oxygen levels. Under hypoxic conditions, HIF-1β (identical to the aryl hydrocarbon receptor nuclear translocator) binds to stabilized HIF-1α, and the heterodimeric HIF-1 then binds to a consensus DNA sequence termed HRE and activates transcription of the target gene (272). HIF-1 appears to function as a negative regulator of hepcidin expression: hepatocytes and liver samples from mice with deleted von Hip-pel-Lindau (VHL), which is the negative regulator of HIF-1, were iron deficient and showed increased expression of Fpn (Fig. 7) (242). In addition, in hepatocytes derived from HIF-1α-knockout mice, hepcidin expression in response to iron deficiency was increased (242). In this study, HIF-1α was shown to bind to TfR1 HRE. Regulation of hepcidin is complex and requires more study. In addition, hepcidin appears to be involved in the modulation of other important proteins in iron regulation.
FIG. 7.
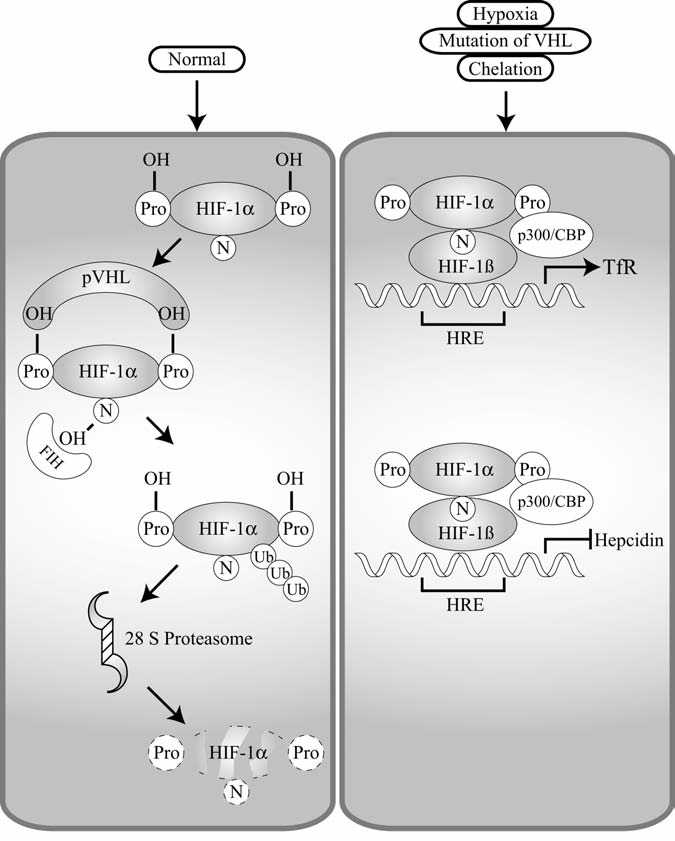
HIF-1α is involved in transcriptional regulation of hepcidin and Tf. Under normal conditions, hypoxia-inducible factor 1α (HIF-1α) is negatively regulated (left panel). Normally, HIF-1α is hydroxylated on proline 402 and proline 564. Tight control of HIF-1α is mediated by the tumor-suppressor protein von Hippel–Lindau (VHL), which is an E3 ligase. VHL targets hydroxylated HIF-1α for degradation via the ubiquitin–proteasomal pathway. In addition, hydroxylation of asparagines (N) by FIH hydroxylase prevents binding of HIF-1α and p300. Under conditions of hypoxia, iron chelation, or mutation of VHL, HIF-1α is positively regulated (right panel). The hydroxylases are not active, and therefore it can bind to p300 and also not targeted for degradation. Because it is not targeted for degradation, it is capable of binding to the hypoxia-responsive element (HRE) of target genes in a heterodimer with HIF-1β. Once bound to the HRE, it may positively or negatively regulate the transcription of the target genes. In the case of transferrin receptor (TfR), it activates transcription; however, it represses transcription of hepcidin.
III. Iron Storage and Ferritin
Ferritin is the major iron-storage protein at the cellular and organismal level. It is responsible for the sequestration of potentially harmful, reactive iron. Ferritin stores iron in its unreactive Fe3+ form inside its shell as a result of a strong equilibrium between ferritin-bound iron (Fe3+) and the labile iron pool in the cells (Fe2+), by which ferritin prevents the formation of ROS mediated by Fenton reaction. Because of its important function in the storage of iron, ferritin is ubiquitous in tissues, serum, and in other multiple locations within the cell. It is regulated at the transcriptional and posttranscriptional level by various pathways in response to diverse stimuli.
A. Structure, tissue distribution, and importance of cytoplasmic ferritin
Ferritin is found in the cytoplasm, nucleus, and mitochondria of cells (summarized in Table 1). In vertebrates, cytoplasmic ferritin is expressed in almost all tissues. This ubiquitous protein consists of 24 subunits of heavy (H) and light (L) chains in various ratios and can sequester 4,500 iron atoms (125). The H subunit has ferroxidase activity, which converts Fe2+ to Fe3+ for storage inside the shell (178) (Fig. 8). In contrast, the ferritin L subunit stabilizes ferritin structure and facilitates the uptake of iron into the shell (9). Ferritin H and L subunits are encoded by two different genes (322). The ratio of H and L subunits in the ferritin protein is not fixed and is tissue dependent (10). For example, H expression is abundant in the heart, whereas the L subunit is predominant in the liver and spleen (125). In the brain, the oligodendrocytes, microglia, and neurons express ferritin (53). Oligodendrocytes have equal amounts of both H and L subunits, whereas microglia express L-rich ferritin, and neurons have H-subunit abundant ferritin (206, 333).
Table 1.
Summary of Different Types of Ferritin
| Type of ferritin | Feature and function | Reference |
|---|---|---|
| Cytoplasmic ferritin | Iron store | 125 |
| Mitochondrial ferritin | Iron store/H-like encoded by different gene from ferritin H | 182 |
| Nuclear ferritin | Iron store/possess DNA binding/repress hemoglobin transcription | 32 |
| Serum ferritin | Iron store/L-rich ferritin/marker of several diseases | 250 |
FIG. 8.
Ferritin and iron storage. Ferritin is composed of 24 subunits, heavy (H) and light (L), with varied ratios of H to L in different cell types and physiologic conditions. Ferritin H has ferroxidase activity to convert Fe2+ to Fe3+ inside ferritin shell. Iron is imported and exported through the channels constructed by ferritin H or L subunits.
Several structures of apoferritin composed of either ferritin H or L subunits were determined by x-ray crystallographic analysis (177, 296). Twenty-four subunits are assembled into a hollow globular shell (177). Two types of channel-like pores exist in the globular shell, which were formed at the intersection of three or four subunits (177). Detailed structural information revealed that the iron-incorporation channel is formed by three intersecting subunits, whereas the ferroxidase activity center was composed of four H subunits (296).
Deletion of ferritin H in mice is embryonically lethal between 3.5 and 9.5 days of gestation (85). Because ferritin H is completely disrupted in these mice, the synthesized ferritins consist of only L-subunit homopolymers (85), which lack ferroxidase activity and has lower iron-incorporation capability (183). None of the ferritin H-deficient mice survived past embryonic day 9.5 (85). Accumulating evidence shows that ferritin acts as an antioxidant protein (59, 60, 78, 159, 227, 243). Ferritin H is induced by NF-κB in response to TNF-α treatment, resulting in suppression of ROS and inhibition of apoptosis (243). When ferritin H was transgenically overexpressed into Parkinson disease (PD) model mice, reduced oxidative stress was found (159). In addition, when HeLa cells stably transfected with tetracycline-inducible ferritin H and L were forced to overexpress ferritin, the accumulation of H2O2-induced ROS was reduced (59). Murine erythroleukemia cells that overexpress ferritin H displayed lower levels of the labile iron pool and ROS (78). In contrast, ferritin H disruption by siRNA sensitized the cells to H2O2-induced oxidative stress (60) because an excess amount Fe2+ causes ROS via the Fenton reaction. These observations support the concept that ferritin may act as an antioxidant gene in the body.
B. Iron efflux and ferritin degradation
To maintain cellular homeostasis, protein synthesis and degradation should be balanced, and in this respect, autophagy and ubiquitin-proteasome pathways play a central role in this process. Autophagy is a mechanism by which proteins are collectively and nonselectively degraded via a lysosomal and vacuolar system (264). Autophagy contributes to maintaining overall protein quality of the cytoplasm, and inactivation of autophagy has been implicated in the progress of cancer and neuromuscular diseases (264).
Lysosome-mediated ferritin degradation uses the autophagy system under particular conditions (173, 226) and iron chelator treatment (165) (Fig. 9). Amino acid and serum depletion activate autophagy, resulting in ferritin degradation, which sensitized cells to H2O2-induced oxidative stress because of an increased “labile iron” pool, which caused ROS production via the Fenton reaction (226). The gram-negative diplococcus, Neisseria meningitidis, rapidly induced ferritin degradation by the activation of the autophagy system, which allows meningococci replication in infected epithelial cells (173). Iron-mediated ferritin regulation is achieved mainly by posttranslational mechanisms; however, iron depletion by iron chelators was also shown to enhance lysosome-mediated ferritin degradation (165).
FIG. 9.
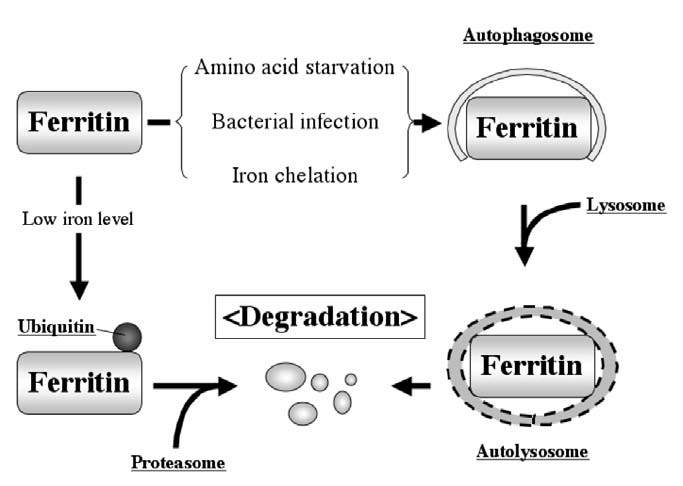
Mechanisms of ferritin degradation. Ferritin degradation occurs through either lysosomal or proteasomal degradation pathways. Amino acid starvation, bacterial infection, or iron chelation triggers navigation of ferritin into the autophagy-lysosome pathway, in which ferritin is trapped by autophagosomes, followed by lysosome fusion, resulted in hydrolase-mediated degradation. In the proteasomal degradation pathway, lower iron concentration induces monoubiquitination of ferritin, resulting in proteasomal degradation. Polyubiquitination of ferritin has not been observed.
Proteasome-mediated ferritin degradation has also been reported (199, 255). Proteasomal degradation commonly requires the attachment of polyubiquitin to a target protein that is misfolded, in most cases. However, polyubiquitination of ferritin protein has not been reported. Only monoubiquitinated ferritin was reported in an Fpn-overexpression experiment (64). Over-expression of Fpn accelerates export of cytosolic iron (215) and enhances ferritin degradation (64). Because proteasomal degradation has been believed to require polyubiquitination, and only monoubiquitinated ferritin has been observed, monoubiquitinated ferritin may disrupt ferritin assembly or may stimulate the signal for proteasomal degradation. However, a number of recent significant researches demonstrated that monoubiquitinated proteins are subjected to proteasomal degradation. For example, the Pax3 protein, which plays a key role in myogenesis during development, is monoubiquitinated during adult muscle stem cell activation (26). Monoubiquitinated Pax3 is associated with the Rad23B ubiquitin-binding protein in a ubiquitination-dependent manner, and this association results in the recruitment of proteasomes and eventual proteasomal degradation (26). It appears that Fpn expression enhances ferritin degradation via a proteasomal degradation-dependent pathway, because proteasome inhibitors treatment prevented ferritin degradation (64). Thus, an unknown but important proteasome-mediated ferritin-degradation mechanism may exist (Fig. 9). For the last decade, lysosome-mediated ferritin degradation has been thought to be the major mechanism of degradation in response to environmental stimuli or iron levels in the cells, but several recent studies examining proteasome-mediated ferritin degradation shed light on new ferritin regulation in cells. More research must be completed to understand the complexity of the crosstalk between lysosome- and proteasome-mediated ferritin degradation.
C. Serum ferritin and ferritin receptor
Ferritin has also been found circulating in the serum. Serum ferritin is identical to cytoplasmic ferritin, but the primary component of serum ferritin is the ferritin L subunit, which contains little iron (323). Serum ferritin has been thought to reflect the iron stores in the body and to increase as a secreted byproduct of intracellular ferritin synthesis (250, 297). Serum ferritin levels are often measured in patient screening for several diseases related to iron levels (11, 275, 309). Several lines of evidence suggested that ferritin may bind to the surface of certain cells and may be endocytosed. Elevated serum ferritin H levels were shown to be correlated with increased CD4+, CD25+, CD69− regulatory T cells (110). In addition, the recombinant ferritin H protein was reported to activate T cells (111). Given that ferritin is a circulating protein that binds to the cell surface, ferritin may have its own receptor (3, 192).
Mouse T-cell immunoglobulin-domain and mucin-domain (TIM)-2 was identified as a ferritin H subunit receptor (48) (Fig. 10). Chen et al. (2005) screened for soluble ligands for TIM-2 by transfecting a cDNA expression library from a macrophage into HEK 293 cells. The resulting supernatants were collected from the medium and screened for stimulatory effects on TIM-2-expressing B cells, by which ferritin H was identified as the protein responsible for the stimulation (48). Ferritin H incorporation was not observed in the absence of TIM-2 (48). Additional experiments also showed that ferritin L did not interact with TIM-2 (48). TIM-2 is a 305–amino acid protein with a molecular mass of 33.5 kDa. It is a member of the TIM gene family, located within the T-cell and airway phenotype regulator gene locus (196). Eight TIM gene families (TIM-1 to TIM-8) have been identified on mouse chromosome 11B1.1, and three genes (TIM-1, TIM-3, TIM-4) on human chromosome 5q33.2 (170). These families of proteins have a novel six-cysteine immunoglobulin-like domain, a mucin threonine/serine/proline–rich domain, and cytoplasmic tail. Human TIM-1 has the highest homology with mouse TIM-2. Although it has not been tested, however, human TIM-1 protein may have a function similar to that mouse TIM-2, including the ferritin H-receptor function.
FIG. 10.
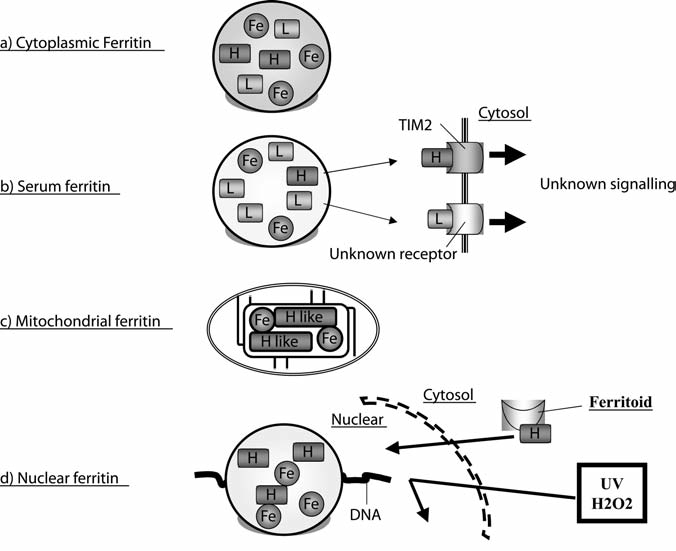
Different types of ferritin. (A) Cytoplasmic ferritin is composed of heteropolymers of H and L subunits and stores iron. The H subunit has ferroxidase activity to oxidize Fe2+ to Fe3+ for the iron storage. (B) Serum ferritin is L rich; however, only a T-cell immunoglobulin domain and mucin domain 2 (TIM-2) was identified as a receptor of ferritin H on B-cell surfaces. Downstream signaling after the association of ferritin H with TIM-2 is still largely unknown. (C) Mitochondrial ferritin is unique in that it is composed of ferritin H-like subunits. It has ferroxidase activity and maintains iron homeostasis in mitochondria. (D) Nuclear ferritin is composed of only H subunits and protects DNA from DNA-damaging agents, such as UV and H2O2. Ferritoid enhances ferritin translocation from cytosol to nucleus.
Murine TIM-2 is expressed in splenic B cells, in liver, and in renal tubule cells. TIM-2-deficient mice showed increased levels of cytokines IL-4, IL-5, IL-6, and IL-10, which may result in increased inflammation (256). Because increased serum and tissue ferritin expression have been observed in inflammation, the identification of a ferritin receptor and clarification of the interaction between ferritin H and TIM-2 highlight the possibility of a new role for ferritin in cells. Several studies showed that ferritin also binds to erythroid precursors (100), brain tissue (138), and placental microvilli membranes (186). In addition, binding of ferritin L to the cell surface of liver, T-, and B-lymphoid cells was demonstrated (7, 208), suggesting that the uptake of circulating ferritin into cells may be more widespread. Further investigation will help clarify the mechanism of this phenomenon and its importance in iron homeostasis and other biologic processes.
D. Mitochondrial ferritin
Even though cellular iron is stored primarily in the cytoplasm, mitochondria use most of the metabolically active iron. However, it remains unknown how these organelles maintain iron homeostasis and suppress iron-mediated toxicity. Mitochondrial ferritin was originally reported as an unusual intronless gene on chromosome 5q23.1 that encodes a 242–amino acid precursor of a ferritin H-like protein, which has a long N-terminal leader sequence with 60 amino acids for mitochondrial import (182). Mitochondrial ferritin is highly conserved among a wide variety of living creatures, including Drosophila (204, 332) and plants (204, 332), which supports the importance of this iron-storage molecule. The expression pattern of mitochondrial ferritin is very specific in human cells. The highest expression level was observed in testis; other tissue has very little expression (74, 182). In addition, mitochondrial ferritin genes do not have IRE in both the human and the mouse (74, 182). The structure of mitochondrial ferritin was determined at 1.38 angstrom resolution and was very similar to that of ferritin H, with ∼80% homology in the amino acid sequences (172). The functional elements of mitochondrial ferritin are similar to those of ferritin H, including ferroxidase activity and metal-binding sites (172) (Fig. 10). Forced expression of mitochondrial ferritin showed that the protein is functionally active in incorporating iron and apparently even more efficient than the cytoplasmic ferritin H (56). Interestingly, increased expression of mitochondrial ferritin in refractory anemia with ringed sideroblastic erythroblasts has been described at a very early stage of erythroid differentiation and is correlated with β-globin and GATA-1 induction involved in this process (288). This suggests that mitochondrial ferritin may also be able to control cell-wide iron metabolism as well as mitochondrial iron balance.
E. Nuclear ferritin
Nuclear ferritin is produced from the same mRNA as cytosolic ferritin and is composed predominantly of ferritin H, suggesting that it may serve to store iron in the nucleus (31, 32). Its cellular distribution was responsive to environmental situations or nutritional effects (285). Corneal epithelial cells are constantly exposed to UV that may cause DNA damage. In these cells, nuclear ferritin acts like cytoplasmic ferritin, in that it prevents iron-mediated ROS production, thereby protecting DNA from strand damage (32). Consistently, the inhibition of nuclear ferritin expression by the iron chelator deferoxamine (this may also decrease cytoplasmic ferritin expression) in corneal epithelial cells resulted in the sensitization of the cells to UV-induced DNA damage (32). Ferritin-mediated DNA protection from external stimuli was also investigated in SW1088 human astrocytoma cells. Ferric ammonium citrate, cytokines, and H2O2 exposure resulted in a change in ferritin localization from the cytosol to the nucleus (292). In response to these stimulations, ferritin translocated into the nucleus and bound to DNA without the requirement of a particular DNA-binding sequence (292). This association between ferritin and DNA may be a mechanism to prevent iron-induced oxidative damage (Fig. 10). Because mutant ferritin H (with amino acid substitutions E62K and H65G that destroyed the ferroxidase activity) could still translocate into the nucleus, ferritin H translocation into the nucleus does not require ferroxidase activity (292). However, the binding of this mutant ferritin H to DNA was lower and less effective for the protection of DNA from UV damage, suggesting that the ferroxidase activity may be an important function of nuclear ferritin (292).
Because ferritin is normally expressed in the cytoplasm and ferritin does not possess a nuclear localization signal, it has been speculated that an alternative mechanism induces nuclear translocation. One such mechanism is ferritoid-mediated ferritin translocation (Fig. 10). Ferritoid is a 30-kDa protein consisting of 270 amino acids specifically expressed in corneal epithelial cells (201). Multiple domains compose ferritoid, including a functional SV40-type nuclear localization signal and a ferritin-like region with 50% structural homology to ferritin H (201). When ferritin H was overexpressed in COS-7 cells, a majority of the expressed ferritin H protein was in the cytoplasm. Overexpression of ferritoid resulted in a shift in ferritin H distribution from cytoplasm to nucleus (201). Although a computational analysis suggested that the ferritin-like region of ferritoid might be important for interaction with ferritin and that this interaction might be necessary to transport ferritin into the nucleus (201), the detailed mechanism is still largely unknown.
Another proposed mechanism of ferritin translocation is O-linked glycosylation of the ferritin protein. Glycosylation has been shown to be either a process or the result of the addition of saccharide to protein and lipids. O-linked glycosylation is one type of glycosylation in which saccharide is attached to a target protein through its serine and threonine side chains. Nuclear ferritin was found to be glycosylated, and three potential glycosylation sites were identified in the N-terminal region (Thr1, Thr2, and Ser4) of ferritin H, whereas the ferritin L subunit appeared to contain only one (Ser2) (285). Moreover, the glycosylation inhibitor, alloxan, prevented translocation of ferritin into the nucleus, which suggests that O-linked glycosylation may be an indispensable posttranslational modification for ferritin H translocation (285). However, because both cytoplasmic and nuclear ferritin glycosylation were observed, it remains unclear whether ferritin glycosylation is crucial for its translocation or if another unidentified glycosylated protein may contribute to ferritin translocation.
IV. Regulation of Ferritin
A. Iron-mediated ferritin regulation
Iron-mediated ferritin regulation has been elegantly described over the last 20 years. The regulation of ferritin synthesis by iron is mainly due to posttranscriptional regulation (107, 129, 262) through the binding of IRP1 and IRP2 to an IRE located in the 5′ UTR of ferritin mRNA (41) (see Fig. 2). The interaction region of IRP1 with ferritin H IRE is partially overlapped with the ISC domain of IRP1, which supports the evidence that iron binding to IRP1 competes with IRP1–IRE interaction, so that increased iron levels in cells enhances translation of ferritin proteins (317).
Both IRP1 and IRP2 are expressed ubiquitously in most tissues. These IRP proteins have been believed to be requisite for maintenance of iron homeostasis in cells. However, IRP1-knockout mice survived with insignificant effects on iron homeostasis in kidney and brown fat that highly express IRP1 (55, 200). In contrast, IRP2-knockout mice showed abnormal induction of ferritin expression, resulting in iron misregulation in intestinal mucosa and the CNS (55, 200). These facts suggest that although both IRPs control ferritin expression in vitro (161), IRP2 may have the primary role in ferritin regulation in response to iron.
In addition to iron-mediated regulation, H2O2-induced oxidative stress and phorbol 12-myristate 13-acetate (PMA) have been shown to modulate the activity of IRP1 and IRP2 (269). PMA induces IRP1 and IRP2 phosphorylation and enhances this binding to ferritin IRE, resulting in decreased ferritin expression during PMA induced HL-60 differentiation (269). Ferritin H translation was also shown to be transiently repressed by H2O2 treatment, in which IRP1 binding to the ferritin H IRE is increased (33, 303).
B. Ferritin regulation by reactive oxygen species
Organisms face an onslaught of oxidant radicals induced by environmental stresses, such as ultraviolet radiation, xenobiotics, dietary components, pesticides, and chemotherapeutic drugs. Conversely, exposure of cells to these agents leads to the induction of phase II genes, including NAD(P)H quinone oxidoreductase 1 (NQO1) (81, 185), glutathione-S-transferase (GST) (225, 265), and heme oxygenase 1 (HO-1) (6), which protect cells from chemical and oxidative stress. Because the iron-storage function of ferritin prevents excess iron from taking part in the Fenton reaction that would cause ROS production, ferritin may serve as an antioxidant protein. Indeed, overexpression or knockdown of ferritin expression in mammalian cells has proven the cytoprotective role of ferritin under prooxidative conditions (59, 60, 78, 159, 227, 243). Consistently, induction of ferritin mRNA was observed in mouse liver (303) and erythroid cells (78) treated with oxidative stress–inducing compounds. This is regulated at the transcriptional level through an antioxidant-responsive element (ARE) (265) (also termed electrophile responsive element) (95) in the 5′-region of ferritin genes (133, 301, 302). The ARE sequence was also found in such phase II genes as NQO1 (185), GST (265), and HO-1 (6).
The ferritin H ARE was originally identified in studies of the molecular mechanism by which mouse NIH-3T3 fibroblasts transformed with the adenovirus E1A oncogene displayed reduced ferritin H mRNA expression (304). Reporter analysis, using a series of deletions in the 5′ flanking region of the mouse ferritin H gene, revealed that the E1A-mediated transcriptional repression was mediated via a 75-bp region located 4.1 kb upstream from the transcription initiation site of the mouse ferritin H gene (Fig. 11). This region contained the unique bidirectional ARE motifs composed of an AP1-like, an SP1-like, and an AP1/NF-E2 sequence (302). Subsequent investigation revealed that Nrf2 and AP1 family transcription factors, in conjunction with transcriptional coactivator p300/CBP, are involved in the activation of the ARE (246, 302, 306, 307) (Fig. 11).
FIG. 11.

Transcriptional regulation of ferritin H. (A) The mouse and human ferritin genes have a similar 5′-region that regulates transcription in response to external stimuli. TNF-α activates the mouse ferritin H gene through 4.8 kb upstream from the start codon, in which NF-κB is involved in this activation mechanism. Human ferritin H gene is also activated by TNF-α, but the responsible region has not been identified, although NF-κB participation was observed. Chemical and oxidative stress (such as H2O2, t-BHQ, hemin) activate human and mouse ferritin H gene through an antioxidant-responsive element (ARE). The ARE activation is achieved by Nrf2 and AP-1 family transcription factors synergized with p300/CBP histone acetyl transferases. Among the AP-1 family transcription factors, ATF1 serves as a repressor of ferritin H gene. Hemin and cAMP were also shown to induce ferritin genes through the proximal region, termed A- or B-box, by NF-Y transcription factors, which recruit coactivator p300/CBP proteins. Adenovirus E1A oncogene represses ferritin H transcription by inhibiting p300/CBP function. (B) Both human and mouse ferritin H genes have bidirectional ARE sequences (AP1-like and AP1/NFE2). Ferritin L has a single ARE (AP1/NFE2). The core ferritin ARE sequences are completely conserved.
The activation mechanism of an ARE by Nrf2 has been most studied and characterized. Nrf2-knockout mice developed normally and survived to adult stage, suggesting that Nrf2 is not an essential transcription factor for embryonic development or growth (45). However, Nrf2 disruption in mice enhanced susceptibility to severe airway inflammation, asthma, and cigarette smoke–induced emphysema (253, 254), indicating that Nrf2 may be involved in protection against environmental chemical stress. In accordance with the results showing the regulation of the ferritin H ARE by Nrf2 (143), decreased ferritin H expression was observed in Nrf2-knockout mice (290).
After the identification and characterization of the mouse ferritin H ARE, a conserved ARE sequence in the human ferritin L (133) and ferritin H gene (143, 301) was recently identified. The human ferritin H ARE is a 55-bp bidirectional ARE composed of an AP-1-like and an AP-1/NF-E2 sequence located 4.5 kb upstream from the transcription start site (301). Nrf2 was shown to bind and activate the human ferritin H ARE (143, 301). JunD was also involved in the transcriptional activation of the human ferritin H gene via the ARE during treatment of HepG2 hepatocarcinoma cells with H2O2 or tert-butylhydroquinone (tBHQ), in which JunD phosphorylation at Ser-100 was induced (301). Recently, JunD was reported as an important transcription factor that reduces tumor angiogenesis caused by Ras-produced ROS (101). In addition, JunD deficiency induced ROS accumulation in the cells (101). Collectively, these results suggest that JunD-mediated ferritin H induction may be an indispensable defense mechanism in a manner similar to that of Nrf2.
More recently, activating transcription factor 1 (ATF1) was shown to act as a repressor of the human ferritin H gene through the ARE (142) (Fig. 11). ATF1, as well as CREB (cAMP-responsive element–binding protein), was originally identified as a regulator of the cAMP response element (195). In the ATF1-mediated ferritin regulation, protein inhibitor of activated STAT3 (PIAS3) was identified as an ATF1-binding partner and was shown to reverse ATF1-mediated ferritin H ARE repression by blocking ATF1 binding to the ARE (142). This result suggests that PIAS3 is an activator of the human ferritin H transcription through the ARE by inhibiting the transcription-repression function of ATF1. Inhibition of PIAS3 expression by siRNA resulted in diminished activation of the ferritin H transcription in response to ARE-activating agents (142).
The human ferritin L ARE was recently identified; it contains only one consensus ARE sequence located at 1.35 kb from the transcription start site (133). Furthermore, tBHQ, sulforaphane, hemin, and high levels of iron activated this element (133); however, transcription factors and co-regulators responsible for ferritin L ARE regulation have not been characterized.
C. Ferritin transcriptional regulation by cytokines
Ferritin H was found as a TNF-α– or IL-1–inducible gene (171, 243, 294). In addition to ferritin H mRNA induction by TNF-α or IL-1, TfR induction was also observed in TNF-α– or IL-1–treated MRC5 human fibroblasts (305), which suggests that these cytokines may play a role in controlling iron homeostasis through modulating the expression of ferritin and TfR.
Rel/NF-κB has been described as a very important transcription factor that regulates several cellular events including cell death, cell proliferation, the innate and adaptive immune responses, inflammation, and the stress response (240). Recent numerous efforts have contributed to understanding the complexity of the apoptotic pathway induced by various stimuli, including TNF-α, in the immune, hepatic, epidermal, and nervous systems. NF-κB is activated by TNF-α and has been shown to induce mouse and human ferritin H (171, 243). This activation of mouse ferritin H occurred through two of the multiple NF-κB consensus sequences located 4.8 kb upstream from the transcription start site (171). Apoptosis induced by TNF-α was inhibited by NF-κB, which suggests that NF-κB-mediated ferritin H induction in response to TNF-α may be a mechanism to protect cells from ROS (243).
D. Ferritin regulation in erythroleukemic cells
Ferritin expression is induced during erythroid cell differentiation and may be involved in ensuring that enough iron is available for hemoglobin synthesis and maturation of the cells (271). Ferritin mRNA induction was observed during DMSO-or hemin-induced differentiation of mouse Friend leukemia cells (FLC) and hemin-mediated differentiation of K562 human chronic myelogenous leukemia cells (17, 143, 194). Recently, hemin-mediated transcriptional regulation of human ferritin H and L was reported. The proximal region of the human ferritin H gene was activated by NF-Y and p300 during hemin-induced FLC differentiation, and this proximal region was also responsible for cAMP (80, 194, 248). The NF-Y transcription factor was bound to a 100-bp region upstream from the transcription start site of the human ferritin H gene (194). All NF-Y subunits, A, B, and C, were required for binding to DNA; then at least the NF-YA subunit was required to activate the ferritin H gene in response to hemin treatment during differentiation of monocytes to macrophages (194). This activation mechanism was achieved by interaction of the NF-Y B subunit with p300/CBP; formation of this protein complex was enhanced by cAMP treatment (80) (Fig. 11).
Conversely, a far-upstream region was shown to be the important responsive element that activates ferritin transcription during erythroid differentiation (20, 143). An upstream 180-bp region of the mouse ferritin H gene was characterized as serving as an inducible enhancer during N,N’-hexamethylene-bis-acetamide–induced differentiation of mouse erythroleukemia cells (20). It should be noted that the 180-bp region (20) actually contains the mouse ferritin H ARE sequence identified as the responsive enhancer to electrophilic chemical stress (302, 303). The hemin-responsive element in the ferritin promoter region was also shown to overlap with the ARE of each human ferritin H and L gene (133, 143). In the case of human ferritin H transcription in K562 erythroid cells, hemin activates ferritin H ARE by recruiting several activators, such as JunD and Nrf2 (143). Moreover, redox factor 1, which was shown to enhance DNA binding of b-zip transcription factors through reduction of conserved cysteine residues (325), was involved in the ferritin ARE activation by Nrf2 during K562 differentiation (143). The mechanisms of transcription of ferritin H gene in response to multiple stimuli are summarized in Fig. 11.
V. Frataxin and Iron Homeostasis
A. Frataxin and Friedreich ataxia
Friedreich ataxia (FRDA) is an inherited cardio- and neurodegenerative disease (with an estimated prevalence of 1 in 50,000 among whites) characterized by progressive gait and limb ataxia, loss of position sense, and cardiomyopathy. FRDA is most commonly (96% to 98%) caused by the homozygous hyperexpansion of a GAA trinucleotide repeat in the first intron of the frataxin gene, which results in a marked repression of frataxin transcription (37). The GAA repeat in FRDA patients ranges from 100 to >1,000 repeats, whereas normal individuals have fewer than 35 repeats (37). A correlation between the length of the GAA repeat and deficiency of frataxin along with severity or onset of FRDA symptoms have been observed (76). Point mutations in the frataxin gene were also found in a small group (2% to 4%) of FRDA patients. Frataxin is a nuclear-encoded mitochondrial protein conserved from yeast to human (166), but with no homology with proteins of known functional domains. Homozygous deletions in the frataxin gene in mice caused embryonic lethality, indicating an important role of frataxin in mouse development (57). Although the primary function of frataxin is still elusive, it has been unveiled by various biochemical and genetic approaches that frataxin is involved in iron–sulfur cluster (ISC) biosynthesis and mitochondrial iron homeostasis.
The first evidence of frataxin's role in mitochondrial iron regulation was obtained by deletion of yeast frataxin homologue, YFH1, and characterization of the yeast phenotype, which revealed that the deletion of YFH1 caused mitochondrial iron accumulation and increased sensitivity to H2O2 toxicity (13). This observation was supported by mouse models for FRDA through a conditional deletion of the mouse frataxin gene (251, 279) or a GAA repeat expansion mutation (5), in which frataxin deficiency induced oxidative stress and led to cardiomyopathy, cerebellar and sensory ataxia, decreased activities of mitochondrial respiratory chain, decreased aconitase activity, and subsequent accumulation of iron in mitochondria (251). Furthermore, targeted disruption of hepatic frataxin expression caused increased oxidative stress, impaired mitochondrial function of respiration and ATP synthesis, along with decreased activity of ISC-containing proteins in liver (289). These mice exhibited a reduced life span and development of multiple hepatic tumor growths (289).
In contrast to these observations, homozygous insertion of a (GAA)230 repeat into the mouse frataxin gene (averaging 75% of wild-type frataxin expression levels) or heterozygous frataxin(-)/(GAA)230 repeat (reduction to 25% to 36% of wild-type mice frataxin levels that are compatible with frataxin expression level in mild but clinically evident FRDA patients) showed no obvious pathologic phenotype, with normal gait and limb movement as well as normal serum and tissue iron levels (202). The same research group generated frataxin-overexpressing mice, which also showed normal iron metabolism with no signs of abnormalities (203). In both wild-type and frataxin transgenic mice, 5 mg/kg of doxorubicin challenge increased similar levels of serum creatine kinase and lactate dehydrogenase, markers for heart and skeletal muscle damage, suggesting that overexpression of frataxin did not protect from doxorubicin-induced cardiotoxicity (203). These results suggest that, in mouse in vivo models, 25% to 30% of wild-type frataxin levels might be sufficient to maintain the normal frataxin function, and the surplus of frataxin may not give additional advantage to the cells in cytoprotection from oxidative stress.
Compared with yeast and mouse model systems, studies in human FRDA patients or in patient-derived tissues and cells are very limited. It was demonstrated that the skeletal muscle of FRDA patients had impaired mitochondrial respiration and a profound deficit of ATP production (189), which is consistent with the results in targeted disruption of frataxin in a mouse hepatocyte model (289). Oligomycin-mediated oxidative stress induced both superoxide dismutases (SODs) and iron import in normal fibroblasts, whereas these responses were attenuated in FRDA patients' fibroblasts, suggesting that frataxin deficiency impairs an antioxidant defense mechanism (46). As a potential pathogenesis of FRDA, increase in glutathionylation of total proteins was demonstrated in fibroblasts of FRDA patients, probably because of accumulation of iron and ROS. Treatment of control fibroblasts with FeSO4 increased protein glutathionylation (236). In this study, increased actin glutathionylation was observed in fibroblasts of FRDA patients, which leads to impaired or decreased actin polymerization and microfilament organization (236).
Several mechanisms of FRDA pathogenesis due to frataxin deficiency have been proposed, largely through studies in yeast and mouse model systems, which suggest that frataxin is involved in mitochondrial iron storage, iron transport, heme and ISC formation, antioxidant defense, and oxidative phosphorylation/ATP production. For instance, the disruption of the yeast frataxin homologue (YFH1) in yeast strain (Δyfh1) induced an accumulation of iron in mitochondria and enhanced their sensitivity to oxidative stress (by H2O2 and iron overload) (13, 93). Furthermore, yeast frataxin mutations that impair its ferroxidation or mineralization activity increased sensitivity to oxidative stress, suggesting the role of frataxin in iron detoxification and antioxidant defense (97). However, in FRDA patient lymphoblast and fibroblast cell lines, no difference was found between control and FRDA patient cells in the concentration of mitochondrial chelatable iron or TfR-mediated iron transport (283), although FRDA patient cells showed higher sensitivity to H2O2 toxicity in a ubiquitously distributed iron-dependent manner (283). Furthermore, in a mouse FRDA model, antioxidants (MnSOD mimetic or CuZnSOD overexpression) had no beneficial effect on the FRDA cardiomyopathy, along with the evidence for the absence of increased oxidative stress or decreased SOD expression in FRDA mouse tissues (273). It appears to be still a matter of debate as to the tight association of mitochondrial oxidative cell damage with the pathophysiology of FRDA.
B. Frataxin and mitochondrial iron traffic
Frataxin is a highly conserved mitochondrial protein in mammals; for instance, human and mouse frataxin proteins have 73% identity in their amino acid sequences, and the C-terminal 122 amino acids of the frataxin proteins are almost identical. Yeast frataxin (Yfh1, 174 amino acids) and human frataxin (210 amino acids) have a high homology at the C-terminus (Fig. 12). It was shown that both yeast and human frataxin are processed to a mature form in two sequential cleavage steps with a mitochondrial processing peptidase (MPP) (28, 43, 167) (Fig. 12). In yeast frataxin, the MPP cleaves the N-terminal 20 amino acids in the first step, followed by the second cleavage that gives rise to the mature yeast frataxin, consisting of 123 amino acids (aa 52 to 174) (28) (Fig. 12). Two molecular chaperones of the heat-shock protein 70 class, ssc1 and ssc2, were shown to facilitate mitochondrial frataxin import and maturation in yeast (162, 312). Similarly, the two-step processing of human frataxin was shown to give rise to an intermediate (aa 42 to 210) and mature (aa 56 to 210) frataxin (43) (Fig. 12). Thus, the mature human and yeast frataxin proteins have 35% identity and 65% similarity in their amino acid sequences. In addition to a one-step processing of human frataxin proposed by another group (108), it was recently shown by overexpression of frataxin in human cells that the main form of mature frataxin is a 130–amino acid protein (aa 81 to 210) after a proteolytic cleavage between Lys80 and Ser81 (Fig. 12), which is another potential MPP cleavage site (52). Importantly, the mature human frataxin 81 to 210 was shown to be active enough to rescue frataxin deficiency in cells of FRDA patients (52).
FIG. 12.
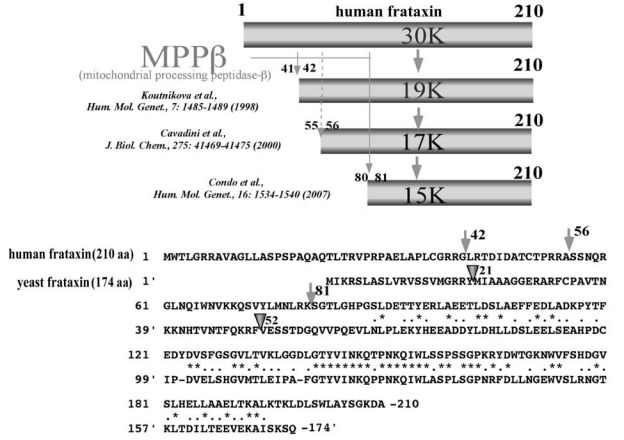
Maturation of frataxin proteins. Protein processing of human frataxin by mitochondrial protein peptidase-β (MPP-β) is summarized (top). The comparison of amino acid sequences between human and yeast frataxin and their cleavage sites by MPP-β is shown (bottom).
Frataxin amino acid sequences do not contain any known features of functional domains that suggest the biologic function of frataxin. Thus, understanding the solution and crystal structure of frataxin is a reasonable approach to gain insight into frataxin biology. Solution and crystal structures of yeast and human frataxin revealed a compact αβ sandwich motif consisting of α1, β1, β2, β3, β4, β5, β6, β7, and α2 (71). It was demonstrated that yeast mature frataxin directly binds iron (2), in which iron triggers assembly of frataxin oligomerization. This frataxin oligomerization reminds us of the assemblage of 24 subunits of the iron-storage protein ferritin, although ferritin assembly does not depend on the presence of iron (125). The same group demonstrated the ferroxidase activity of yeast frataxin (233) [similar to that of ferritin H, which oxidizes Fe2+ to Fe3+ (178)]. The recent crystal-structure study of apo- or iron-loaded frataxin trimer and 24 subunit homo-oligomer formation demonstrated the architectural similarity between frataxin and ferritin (151). The trimer of yeast frataxin was shown to bind one atom of iron that constitutes the basic 24-subunit oligomer (151). In human mature frataxin, the structure is compact, with no signature of iron binding or oligomerization in the presence of iron (212), whereas the mature human frataxin was reported to assemble in >600-kDa homopolymers and bind ∼10 atoms of iron per frataxin oligomer (44).
The proposed function of frataxin is mitochondrial iron homeostasis, including iron storage and transport, heme synthesis, and ISC formation, as well as antioxidant defense. In yeast, it was demonstrated that frataxin is involved in mitochondrial iron efflux (252) or iron storage (96, 234). Turning off the yeast frataxin promoter in an inducible system caused accumulation of iron in mitochondria and resulted in mitochondrial and nuclear oxidative DNA damage (153). The frataxin-deficient yeast also showed more production of hydrogen peroxide, higher sensitivity to DNA-damaging agents, and increased chromosomal instability (152), suggesting that detoxification of mitochondrial iron is a primary function of frataxin (97). Similarly, cells derived from FRDA patients contained 40% increase in mitochondrial iron content and were more susceptible to iron-mediated cytotoxicity and hydrogen peroxide (287, 321), which was rescued by treatment with the iron chelator deferoxamine (321) or by near-physiologic levels of frataxin expression (287). These results support frataxin's antioxidant function through iron storage or iron transport or both. Interestingly, expression of human mitochondrial ferritin (ferritin H-like protein) or human ferritin L-chain rescued frataxin-deficient yeast (36, 70), suggesting functional homology between iron-storage protein ferritin and frataxin.
C. Frataxin, heme synthesis, and iron–sulfur cluster biogenesis
Growing evidence indicates that frataxin is involved in heme (ferrous-protoporphyrin IX) synthesis and ISC formation through interactions with proteins involved in these biosynthesis pathways (Table 2). Heme is ubiquitous and a constituent of heme proteins such as hemoglobins and cytochromes involved in cellular oxygen utilization and endobiotics/xenobiotics metabolism. It was demonstrated that Saccharomyces cerevisiae cells lacking the YFH1 gene, the yeast homologue of the human frataxin gene, showed low levels of cytochrome and ferrochelatase (181). Ferrochelatase is involved in the last step of the heme-synthesis pathway that inserts Fe2+ into protoporphyrin IX. In the same study, YFH1 was shown to interact with the yeast ferrochelatase (Hem15p) in vitro, and that the YFH1 deficiency resulted in the defect of iron use in ferrochelatase activity (181). Yeast Mrs3 and Mrs4 were shown to serve as mitochondrial iron-transport proteins (94) and to cooperate with YFH1 in providing iron for heme synthesis (335). Similar results were reported in mammalian cells in which human frataxin interacted with human ferrochelatase (331), and microarray analysis of frataxin-deficient mouse heart cells revealed downregulation of the iron-sulfur scaffold protein Isu1, mitochondrial coproporphyrinogen oxidase, and ferrochelatase, which are all involved in the heme pathway (270).
Table 2.
Frataxin-Binding Proteins and Their Functions
| Frataxin | Binding protein | Function | Reference |
|---|---|---|---|
| Mouse frataxin | Mitochondrial processing peptidase beta | Protein processing | 167 |
| Rat frataxin | Aconitase | Citric cycle | 29 |
| Human frataxin | Ferrochelatase | Heme synthesis | 331 |
| Human frataxin | Succinate dehydrogenase | Electron transport chain | 106a |
| Human frataxin | Mortalin/GRP45 | Iron-sulfur cluster biogenesis | 275a |
| Human frataxin | ISD 11(Nfs1/Isul) | Iron-sulfur cluster biogenesis | 275a |
| Yeast frataxin | (Hem15P) ferrochelatase | Heme biogenesis | 181 |
| Yeast frataxin | Succinate dehydrogenase | Electron transport chain | 106a |
| Yeast frataxin | Isul/Nfs1 | Iron-sulfur cluster biogenesis | 102 |
| Yeast frataxin | yfh1 (yeast frataxin) | Frataxin oligomerization | 2 |
Accumulating evidence reported by several different research groups also indicates that frataxin plays a role in ISC biosynthesis. ISCs are important cofactors of proteins that play crucial roles in biosynthesis of amino acids and heme, purine metabolism, electron transport, iron homeostasis, and maturation of ISC proteins [reviewed in (187)]. It was reported that aconitase (an ISC-containing citric cycle enzyme that converts citrate to isocitrate) and mitochondrial ISC respiratory complexes I, II, and III are deficient in YFH1-deleted yeast, as well as FRDA patient cells (261), resulting in defects in ISC protein maturation, respiratory deficiency, and mitochondrial iron accumulation (47, 209). Yeast frataxin YFH1 interacts with the ISC-assembly complex composed of the scaffold protein Isu1 and the cysteine desulfurase Nfs1 in a ferrous iron–dependent manner (102), suggesting a role of YFH1 in ISC synthesis (Table 2). Frataxin was also found to interact with aconitase in a citrate-dependent manner and protects or restores the aconitase activity by converting the inactive [3Fe-4S]1+ form to the active [4Fe-4S]2+ form of aconitase (29). Knocking down frataxin with siRNA in human culture cells (HeLa and 293 cells) caused a defect in ISC proteins, including aconitase (191, 281). The proposed role of frataxin in heme synthesis and ISC formation is summarized in Fig. 13.
FIG. 13.
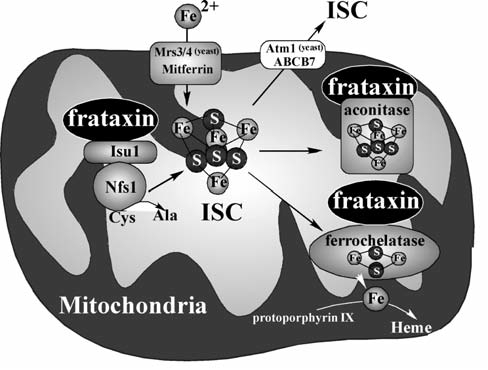
The role of frataxin in iron–sulfur cluster (ISC) formation and heme synthesis. Cytoplasmic iron is imported into mitochondria by Mrs3/4 (in yeast) or mitoferrin (in vertebrates). Frataxin interacts with a complex of the ISC scaffold protein Isu1and the cysteine desulfurase Nfs1, which release sulfur from cysteine. Frataxin also interacts with ferrochelatase and facilitates the heme synthesis through ferrochelatase-mediated insertion of ferrous iron into protoporphyrin IX. Frataxin protects aconitase activity by restoring the aconitase 4Fe-4S cluster. ISCs in mitochondria are exported into cytoplasm by Atm1 (ABC transporter of the mitochondrion 1 protein) in yeast or ABCB7, the human and mouse homologues of yeast Atm1.
D. Frataxin gene regulation
The human frataxin gene is located on chromosome 9 (q13-q21.1) consisting of seven exons spanning >95 kb of the genomic DNA (37). The most abundant frataxin mRNA is a transcript from exon 1 to exon 5a, with a size of 1.3 kb (37). The frataxin gene exhibits tissue-specific expression, with its mRNA most abundant in the heart, followed by the liver and skeletal muscle (166). The disease phenotype is prominent in the heart tissue and nervous system, suggesting that the required amount of frataxin in each tissue may be variable. It has been more than a decade since the FRDA gene was identified and characterized as a GAA repeat expansion of the frataxin gene (37); however, the molecular mechanisms that regulate transcription of frataxin and its tissue-specific expression remain largely unknown.
The GAA triplet-repeat expansion in the frataxin gene was shown to interfere with frataxin gene transcription (223) because of a self-associated triplex formation at long GAA:TTC repeats (sticky DNA) (22, 193, 267, 268). The attempt to restore frataxin expression by specifically targeting expanded GAA:TTC repeat demonstrated that β-alanine–linked polyamides bind the GAA:TTC repeat, resulting in disruption of the sticky-DNA conformation and alleviation of transcriptional repression of the frataxin gene in the FRDA lymphoid cell line (30). Similarly, by using stable HeLa cell lines transfected with a GFP reporter containing a GAA:TTC repeat expansion (GAA-148 repeats), 5 to 50 μM of small molecules such as diminazene, distamycin, pentamidine, DAPI, and Hoescht 33258 have been shown to increase GFP expression by >20-fold (109).
To understand the molecular basis of the transcription of the frataxin gene, 5′-promoter/enhancer elements of the human frataxin gene were investigated, in which a 1,255-bp 5′-region of the frataxin gene was shown to contain the minimal promoter activity (113). 3-nitropropionic acid (2 to 8 mM) and erythropoietin (1.1 to 9.9 unit/ml) appear to stimulate frataxin expression in lymphocytes derived from normal and FRDA patients (284, 308), although the mechanism of increased frataxin protein expression remains uncharacterized. Recently, HIF-2α knockout mice were shown to have impaired mitochondrial respiration, sensitized mitochondrial permeability transition pore opening, and reduced activity of aconitase, because of marked reduction of frataxin mRNA expression in HIF-2α−/−livers (224). Indeed, HIF-2α, but not HIF-1α, specifically activated an HRE that is located ∼2 kb upstream from the transcription-initiation site of the mouse frataxin gene (224). It is not known whether HIF-2α also regulates transcription of the human frataxin gene.
E. Treatments
Because decreased expression of frataxin (ranging from 5% to 30% of normal frataxin expression level) causes FRDA, strategies to enhance expression of frataxin will be a promising approach to treating the disease. As described earlier, one approach is to use DNA sequence-specific polyamides and small chemicals that can specifically bind to the GAA:TTC repeat and disrupt the triplex formation, leading to increased frataxin transcription (30, 109). Another trial for restoring frataxin expression is to use histone deacetylase inhibitors such as BML-210 (1 to 5 μM) that reverse frataxin-gene silencing through chromatin relaxation by inducing lysine acetylation of histone H3 and H4 (132). In addition, frataxin-encoding adeno-associated virus and lentivirus vectors increased frataxin expression and decreased sensitivity to oxidant stress in primary FRDA patient fibroblasts (86).
The second strategy to treat FRDA is to use iron chelators that decrease accumulated iron levels in tissues. The iron chelator deferoxamine was shown to protect FRDA fibroblasts from H2O2-induced cytotoxicity (321). Given the evidence for mitochondrial iron overload in patients with FRDA (27, 69), iron chelators that preferentially mobilize iron from mitochondria, such as pyridoxal isonicotinoyl hydrazone and 2-pyridylcarboxaldehyde isonicotinoyl hydrazone derivatives, were developed for the treatment of FRDA (257). It was recently reported that treatment of FRDA patients with 20 to 30 mg/kg of deferiprone (3-hydroxy-1,2-dimethylpyridine-4-one), an iron chelator that is orally active, membrane permeable, blood–brain barrier crossing, and that transfers chelated iron to transferrin, efficiently decreased brain iron accumulation without apparent hematologic or neurologic adverse effects (25).
The third strategy to treat FRDA is to use mitochondriatargeted antioxidants. The membrane-permeable antioxidant, Idebenone [2,3-dimethoxy-5-methyl-6-(10-hydroxydecyl)-1,4-benzoquinone], as well as its derivative decylubiquinone, was shown to protect FRDA fibroblasts from toxicity caused by glutathione-depletion conditions (146). Likewise, analogues of the electron-transport chain component coenzyme Q10 and small-molecule glutathione peroxidase mimetics such as ebselen [2-phenyl-1, 2-benzisoselenazol-3 (2H)-one] showed similar cytoprotective effects (146). Furthermore, the mitochondria-targeted antioxidants MitoQ (coenzyme Q10 derivative) and MitoVitE (vitamin E derivative) exhibited a significant increase in the efficacy of cytoprotection of FRDA fibroblasts from oxidative stress–mediated toxicity when compared with Idebenone (145). In a mouse model for FRDA, the antioxidant Idebenone delayed the onset of cardiac dysfunction without improvement of deficiency in ISC proteins (274).
VI. Functional Iron Overload and Human Health
A. Hereditary hemochromatosis
Hereditary hemochromatosis (HH) is the most common form of iron-overload disorder in individuals of northern European descent (299). HH is classified on a genetic, biochemical, and clinical basis as types 1 through 4. The phenotype/clinical outcomes are used to determine the type (1 to 4) of hemochromatosis (210). The types along with their associated genotype, mapped location, inheritance, and clinical manifestations are summarized in Table 3. HH is a heterogeneous group of disorders of normal iron metabolism caused by genetic factors that alter iron homeostasis. Resulting iron overload leads to a number of different pathologies, including structural damage from physical iron accumulation and oxidative damage to tissue, protein, lipids, and DNA resulting from iron-induced oxidative stress (229).
Table 3.
Types of Hereditary Hemochromatosis
| HH type | Genotype | Gene map | Inheritance | Phenotype/clinical outcome |
|---|---|---|---|---|
| 1 | HFE C282Y C282Y/H63D hetero | 6p21.3 | Autosomal recessive | Adult onset; Tf saturation, high serum ferritin, high liver iron, treat with phlebotomy |
| 2 | Hepcidin HFE2 | 19q13 1q21 | Autosomal recessive | Juvenile onset, severe iron overload, treat with phlebotomy |
| 3 | TfR2 | 7q22 | Autosomal recessive | Elevated total body iron, normal transferrin saturation and serum ferritin |
| 4 | Fpn | 2q32 | Autosomal dominant | High serum ferritin and transferrin saturation, slight elevation in total body iron |
The four types of hereditary hemochromatosis (HH) are summarized in this table, including the causative mutation or linked gene, the mapped location of the affected gene, and the inheritance pattern and phenotype of each HH type.
Prototypical HH is characterized by progressive iron loading, particularly in the liver, heart, and pancreas (131). Initially, the deleterious effects of iron loading are not clinically significant, and manifest as discomfort in hand joints, increased skin pigmentation, and fatigue (16). In later stages as tissue iron accumulates, liver damage ranging from fibrosis to cirrhosis develops in affected individuals. Those with HH have markedly increased risk for developing hepatocellular carcinoma (77). It is also common for endocrine disorders, such as diabetes, to develop, as well as cardiac pathologies. Increased incidence of arthritis also is observed in HH (54). Important animal models for HH and other iron-associated disease are summarized in Table 4.
Table 4.
Animal Models of Iron Regulation
| Mutant | Phenotype | Human disease | Reference |
|---|---|---|---|
| Hfe−/− | Hepatocyte iron overloading | HH Type 1 | 184, 336 |
| Hfe C28Y/C282Y | Macrophage iron overloading | ||
| Increased Tf saturation | |||
| Usf2−/− (Hepcidin) | Hepatocyte iron overloading | HH Type 2 | 215a |
| Macrophage iron overloading | |||
| Increased Tf saturation | |||
| TfR1−/− splicing | Tissue iron accumulation | Atransferrinemia | 20a |
| Microcytic hypochromic anemia | |||
| TfR1−/− | Embryonic lethal (GD 12.5) | Not applicable | 184 |
| TfR1+/− | Small RBCs; low hemoglobin | Anemia | 184 |
| Increased total iron stores | |||
| TfR2245X/245X | Hepatocyte iron overloading | HH Type 3 | 89 |
| Macrophage iron overloading | |||
| Increased Tf saturation | |||
| TfR2−/− | Iron overload | HH Type 3 | 317a |
| Ferritin H−/− | Embryonic lethal (early) | Not described | 85 |
| Ferritin H+/− | Elevated tissue and serum ferritin L | Not described | 85a |
| Cp−/− | Hepatocyte iron overloading | Acerulo-plasminemia | 123 |
| Macrophage iron overloading | |||
| Accelerated iron export | |||
| *DMTG185R | System iron disorder | Not described | 88 |
| Low iron uptake | |||
| *Heph(deletion) | Microcytic hypochromic anemia | Not described | 313 |
| Poor intestinal iron transport | |||
| IRP1−/− | Iron misregulation in kidney and brown fat | Not described | 200 |
| IRP2−/− | Iron accumulation in enterocytes, neurons, and oligodendrocytes | Not described | 175 |
| Neurodegeneration |
Spontaneous mouse mutation in hypotransferrinemia (hpx), microcytic anemia (mk) and sla mice, respectively. References cited identified causative mutation.
Animal models for iron regulation and disease associated with iron misregulation are summarized, including mutant genotype, phenotype, and associated human disease, as well as the reference that originally characterized the animal model.
Type 1 HH is associated with several mutations of the HFE gene, which was originally identified during an analysis of 178 individuals with HH (82). Of these, 83% were homozygous for a missense mutation to a gene resembling MHC class I genes, located on chromosome 6 (82). Later reports of HH pedigrees also observed this mutation in both alleles of the HFE gene that encodes a tyrosine instead of the normal cysteine at codon 282 (C282Y), as having a prevalence of between 60% and 100% (40, 147, 150). This mutation disrupts the formation of the disulfide bridge, which appears to be necessary for proper protein formation, causing the aberrant protein remains in the Golgi (24, 83). Normal HFE protein associates with TfR, usually in conjunction with β2-microglobulin, and competes with Tf for TfR binding, thereby inhibiting Fe-Tf uptake via the TfR in cells (83, 179). Disruption of the disulfide bridge results in decreased binding affinity for both β2-microglobulin and TfR (84). Further studies demonstrated that the C282Y HFE mutant is retained in the ER and Golgi and is rapidly degraded (316). Later, a mouse model for type 1 HH was identified; the HFE mutant mouse manifested the same set of alterations in systemic iron regulation and subsequent iron overload as observed in humans (336). Another mutation (H63D) in HFE is commonly observed, but does not lead to the same HH phenotype, probably because it is processed and expressed in the same manner as wild-type HFE (316).
Type 2 HH, also known as juvenile hemochromatosis, exhibits an increased rate of iron accumulation and an earlier onset than type 1. Individuals with juvenile hemochromatosis incur cardiomyopathies, liver cirrhosis, and diabetes, and have increased morbidity associated with a decreased life expectancy because of systemic organ failure (244). This particular type of HH is associated with a mutation that was mapped via positional cloning to chromosome 1q (230). The mutation occurs in a gene encoding hemojuvelin (HFE2). HFE2 is expressed mainly in skeletal muscle, cardiac tissue, and liver. HFE2 is a GPI-anchored protein that appears to be a sensor of dietary iron levels (230). In mice, HFE2 is localized to the surface of periportal hepatocytes, and HFE2-mutant mice display iron loading and a striking decrease in hepcidin upregulation in response to increased iron from exogenous sources, compared with controls (217). Thus, mutation of HFE2 appears to impede normal iron sensing, thus possibly allowing increased iron uptake through decreased expression of hepcidin, the negative regulator of Fpn. Individuals with HFE2 mutations have low levels of hepcidin in their urine, suggesting decreased excretion of secreted hepcidin. Studies of several mutant isoforms of HFE2 in HeLa and HepG2 cells revealed that several of the mutations resulted in the loss of the GPI anchor and were primarily retained in the ER or secreted as soluble protein (278). In addition, in contrast to wild-type HFE2, the mutant isoforms were not regulated by iron levels. Hepcidin is also directly implicated in the pathogenesis of HH type 2. Mutations in hepcidin that introduced either a frameshift or premature stop codon have been identified in individuals with HH type 2 (259).
Type 3 HH is pathophysiologically similar to type 1; however, it occurs as a result of a mutation in TfR2 (34). The role of TfR2 in the uptake of Fe-Tf is unclear, as it binds Fe-Tf with ∼30-fold less affinity than TfR1 (298). In addition, TfR2 does not appear to be regulated by iron concentrations, for it lacks IRE in its mRNA and is not inducible by iron deficiency (90). Studies have shown that TfR2 does not associate with HFE, suggesting an alternative role for TfR2 mutation in HH (320). Interestingly, iron accumulation in the liver in type 1 HH results in the downregulation of TfR1, but in the case of TfR2, it is continually expressed even during HH-mediated iron loading (90). In a study of a mouse model of type 3 HH, a premature stop codon (Y245X) was introduced in TfR2 to create an orthologous mutation to the Y250X observed in some individuals with type 3 HH (89). The resulting mutant mice showed aberrant iron homeostasis, including early hepatocellular iron overload coupled with decreased splenic iron and elevated transferrin saturation (89). Thus, TfR2 is important in iron homeostasis, and its absence leads to clinically significant disruption of iron homeostasis. The mechanism of mutant TfR2-mediated pathogenesis of type 3 HH needs further investigation.
Type 4 HH is caused by a mutation in the Fpn gene (205, 218). Individuals afflicted with this form of HH exhibit iron loading in macrophages, without elevated serum iron. This form of HH is autosomal dominant in nature, and various missense mutations lead to altered Fpn (92, 205, 218). Disruption of normal Fpn function may lead to a number of different iron-loading conditions because of its function as an iron exporter and its important role in recycling of iron during erythrophagocytosis (67, 68). Along with other conditions, this type of HH is rare, but clinically significant.
B. Mutant iron-responsive element–mediated iron overload
Another form of hereditary iron overload results from mutation of the IRE in ferritin H mRNA and results in an autosomal-dominant iron-overload disorder. It was revealed that a point mutation in the IRE consensus sequence is in ferritin H mRNA in a family with dominant iron-loading disorder (154). This A to U transition at position 49 was associated with an increased affinity for IRP compared with the wild type. Expression of the IRE mutant enhanced translational repression of ferritin H (154). In hyperferritinemia-cataract syndrome, mutation of the ferritin L IRE leads to constitutive expression of ferritin L. Although not associated with iron overloading, this condition results in the accumulation and aggregation of ferritin L in the lens, leading to the formation of cataracts (19). Interestingly, disruption of Cp and Heph in mice, which are necessary for the ferroxidase activity associated with Fpn activity and iron efflux, resulted in iron accumulation in the retina and subsequent retinal degeneration (119). These outcomes resulting from aberrant iron regulation indicate that either iron accumulation or oxidative damage may be involved in damage to sensitive systems such as the eye.
C. Iron regulation and neurodegeneration
Appropriate iron levels are especially important in the brain. Adequate iron levels are required for the increased respiration, neurotransmitter production, and myelinogenesis. Iron deficiency may have deleterious effects on neurotransmission, myelination, and dopamine receptor and transporter functions (18). At the other end of the spectrum, excess iron concentrations are particularly harmful because the highly oxidative microenvironment of the brain lends itself to the production of ROS (180). Brain iron regulation and uptake is not fully understood. It is apparent that many of the proteins involved in iron homeostasis throughout the body are also present in the brain (206). A number of studies have observed increased brain iron content that correlates directly with age. Furthermore, aberrant levels of increased brain iron have been observed in Alzheimer disease (AD) and Parkinson disease (PD) (23, 156). A study that examined postmortem unfixed frozen brain sections from elderly normal or PD-affected individuals showed that individual neurons from brains of individuals with PD had significantly elevated iron concentrations (220). Recent evidence has implicated abnormal iron metabolism in the pathogenesis of neurodegeneration (120, 241). Levels of accumulation of iron in the striatum of rhesus monkeys were positively correlated with aging-related motor deficits (42). Monkeys exhibited age-related declines in motor function and dopamine release in the striatum, whereas iron concentration in the striatum increased. Iron content was the best predictor of motor deficit (42). Befitting a model of altered iron-mediated mechanisms of damage in neurodegeneration, markers of oxidative stress, including lipid and protein oxidation, have been observed in PD and AD (334).
PD is the second most prevalent neurodegenerative disorder, for according to the National Institute of Neurological Disease and Stroke, PD affects ∼500,000 people in the United States, with ∼50,000 new cases reported annually. PD is caused by the degeneration of dopaminergic neurons of the nigrostriatal pathway in the brain. This pathway produces dopamine, an important neurotransmitter in movement, and thus is responsible for the initiation of signals necessary to produce voluntary movement. Individuals with PD typically have bradykinesias, dyskinesias, resting tremors, loss of reflexes, and inability to initiate movement, which are ameliorated by the administration of the dopamine precursor, levodopa (79). Studies have indicated that iron and oxidative stress may be involved in the pathogenesis of PD. Several studies have observed colocalization of such oxidative-damage indicators with regions of iron accumulation (334).
The iron-storage protein, ferritin, has been studied in PD because of the increased iron content in the afflicted regions in PD and the increase in evidence of oxidative stress. Many studies set a precedent of ferritin in cytoprotection against free-iron–catalyzed production of ROS (227, 243, 301). Ferritin H was transcriptionally activated by JunD through an ARE in the ferritin H promoter in response to oxidative stressors, including H2O2 (301). Ferritin was identified as the downstream mediator of the antioxidant and protective activities of NF-κB in response to TNF-α signaling in inflammation and apoptosis (243, 294). In addition, overexpression of ferritin H protected cells from the toxicity of MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, a drug used to create experimental models of PD) (159). Chelation of “labile iron” also protected cells from this toxicity, suggesting that iron may play a role in the pathogenesis of PD, at least in the MPTP model (159). Furthermore, generation of heterozygous ferritin H–null mutants shows brain iron levels similar to those of the wild type, despite a 50% expression of ferritin H that corresponded with increases in proteins involved in iron regulation, such as Tf, TfR, ferritin L, DMT1, and Cp (291). These mice also demonstrated an increase in markers of oxidative stress, including SOD activity, oxidative protein modifications, and apoptotic indicators (291).
Recently, results indicating a more complex, and perhaps detrimental, role for ferritin in neurodegeneration have been reported. Prolonged elevation of ferritin levels in dopaminergic neurons of the midbrain resulted in a progressive age-related neurodegeneration of these cells, suggesting that increased ferritin may not necessarily be cytoprotective and may play a role in neurodegeneration (158). Iron sequestered by ferritin can be made available for cellular use or exportation. Interestingly, Fpn expression was recently shown to result in cytoplasmic iron exportation, iron release from ferritin, and subsequent ubiquitination of ferritin, targeting it for degradation by the proteasome (64). Depletion of ferritin iron induces the monoubiquitination of ferritin subunits. Conditions of iron chelation may lead to ferritin being degraded through a lysosomal pathway (64). Aberrant release of ferritin iron may lead to the production of ROS and may thereby serve as a mechanism by which altered iron management may lead to cellular damage. More study is needed to elucidate the role of ferritin in regulation of brain iron under conditions of aging, increased iron content, and oxidative stress that compose the milieu of the brain in many neurodegenerative diseases. Evidence for a dual role of cytoprotective agent and mediator of iron-catalyzed ROS–mediated damage exists and warrants further examination.
Mutation of Cp has also been linked to several incidents of PD. A study of 176 individuals with PD and 180 control subjects who underwent transcranial ultrasound to reveal elevated substantia nigra iron levels, subsequently were evaluated for mutations in the Cp gene (134). In this study, five unique missense variations were identified including, a single incidence of I63T, D554E, which correlated with PD and positive ultrasound for increased substantia nigra iron levels, and R793H, which was linked to increased levels of iron levels in individuals with PD and their age/ethnic-matched individuals. This study also showed that Cp localizes with the Lewy bodies associated with the pathology of PD (134). In further investigation, the I63T mutant Cp isoform was expressed at half the normal level and displayed a distinct decrease in ferroxidase activity in vivo. In HEK293 cells, the I63T GPI-linked isoform of Cp remained in the endoplasmic reticulum. The D544E Cp was expressed at lower levels and exhibited decreased ferroxidase activity (135). Decreased expression of Cp and deficient ferroxidase activity may result in “free iron” accumulation and decreased antioxidant potential. In addition, retention of the I63T mutant Cp isoform in the endoplasmic reticulum (ER) may lead to cell death. An unfolded protein response (UPR) triggered by the accumulation of misfolded proteins in the ER has been shown to induce a signaling pathway that alleviates ER stress or apoptosis (155, 207, 327). It may be noted that chemicals used to model PD, such as MPTP, 6-hydroxydopamine (6-OHDA), and rotenone, were shown to increase expression of key members of the signaling pathway associated with the UPR in ER stress (136, 327). Cultured neurons from mice deficient in PERK (PKR-like ER kinase), which have an impaired UPR, exhibited enhanced sensitivity to 6-OHDA treatment (266).
IRP has an important role in the sensing of iron levels and transmission of signals to regulate posttranscriptionally a number of important factors involved in iron management (131). Another hallmark of PD is the aggregation of the protein α-synuclein. Such clustered α-synuclein has been shown, along with iron, to be a constituent of Lewy bodies in PD (157). Through an IRP1-dependent mechanism, MPTP administration caused upregulation of TfR, which preceded increased iron and oxidative stress (276). Coexposure to iron and H2O2 were shown to enhance α-synuclein aggregation in vitro (126). An antibody for the TfR, which blocked iron uptake, abrogated up-regulation of α-synuclein after MPP+ treatment. Global deletion of IRP2 in mice manifested in aberrant accumulation of iron within the brain that correlated with tremor, and movement disorders such as bradykinesia and ataxia (175) (Table 4). IRP1-knockout mice misregulate iron in specific tissues, namely in brown fat and kidney, whereas IRP2-knockout mice exhibit aberrant management of iron in multiple tissues. IRP2 was also sensitive to iron levels, whereas IRP1 was not, suggesting that IRP2 is the predominant posttranscriptional regulator of key genes involved iron homeostasis (200). A study of brain tissue from control and AD-afflicted subjects demonstrated that IRP1 expression was similar in both groups, whereas IRP2 expression was altered in AD. In AD, IRP2 was localized in regions with redox active iron and was associated with such pathologic features as neurofibrillary tangles and senile plaques (280). An IRE was recently identified at +51 to +94 from the 5′ cap in the 5′-UTR of Alzheimer amyloid precursor protein (260), a critical mediator of the formation of plaques involved in the pathogenesis of AD. This IRE was functional, as demonstrated by specific binding to IRP that was destroyed by functional mutation, and decreased translation in response to intracellular iron chelation (260).
In addition to increases in brain iron with age and in various pathologic conditions, altered brain iron metabolism is implicated in the pathogenesis of neurodegeneration by animal models involving mutation or deficiency in various proteins that regulate iron metabolism, or both, as well as by the neurodegenerative conditions caused by mutations in such proteins. Two different mutations in the ferritin L gene have been linked to neurodegenerative conditions (50, 62). Linkage studies identified a one-nucleotide insertion of adenine at position 460–461, which alters the last 20 amino acids of the C-terminal of ferritin L associated with the development of a dominant adult-onset basal ganglia disease with symptoms similar to those of Huntington disease (HD) or parkinsonism (50). Histochemical analysis revealed abnormal ferritin and iron aggregates (62). Studies with recombinant mutant ferritin L showed that, although it was able to assemble into 24-subunit ferritin shells, the efficiency of assembly and capacity to incorporate iron were both decreased in vitro (61). In addition, HeLa cells that express mutant ferritin L chains exhibited a phenotype of iron loading (61). Another and less frequent mutation on ferritin L is a two-nucleotide insertion (498–499InsTC), which changes the final nine amino acids of the C-terminus (311). Shells formed with mutant L chains, which are normally involved in stability of assembled ferritin, may confer instability or enhanced degradation.
Cp is similar to ferritin H in that it also has ferroxidase activity and thereby may serve an antioxidant role in addition to its role in iron regulation. In the CNS, Cp is found in its GPI-anchored form on cellular membranes where it is associated with Fpn (65). The total absence of Cp results in an autosomal-recessive disorder, aceruloplasminemia, in which afflicted individuals have adult-onset neurodegeneration (124). Mutations identified in aceruloplasminemia are nonsense mutations that result in the early termination of translation, causing serum Cp deficiency. The absence of serum Cp results in low serum iron concentrations, anemia, and increased serum ferritin levels, whereas tissues, especially the retina and the basal ganglia, exhibit iron overload. Over time, dystonias and dementia may develop (326). Cp-knockout mice exhibit severe defects in iron egress from astrocytes, probably resulting from the lack of ferroxidase activity, which is necessary for the exporter function and stability of Fpn. Inability to export iron may explain tissue iron accumulation. GPI-anchored Cp was required for export of iron from astrocytes (148) (Table 4). In addition, the absence of ferroxidase may lead to higher levels of Fe2+, which has an increased propensity to react and form ROS. Adult Cp-knockout mice exhibited accumulation of iron in the cerebellum and brainstem that correlated with the development of deficits in coordination of movement (148). Cells derived from the cerebellum of neonatal Cp-knockout mice were more sensitive to oxidative stress in vitro (239). Taken together, these results suggest a role for Cp in the regulation of cellular iron efflux and resistance to oxidative stress, and implicate Cp in the pathogenesis of neurodegeneration involving increased iron and oxidative damage, such as PD and AD.
VII. Conclusions and Future Directions
Dysregulation of iron metabolism causes numerous health problems including inflammatory, metabolic, hematologic, and neurodegenerative diseases. In the last several years, new proteins involved in iron homeostasis, such as DMT1 and hepcidin, have been discovered, and their roles in the maintenance of iron homeostasis and its disorders have been elucidated. Heme (Feprotoporphyrin IX) metabolism has not been discussed in depth in this review; however, heme is an essential component of various cellular key proteins, and its metabolism significantly affects the iron status systemically and at cellular levels. Heme-transport proteins have recently been identified in several different cell types [reviewed in (174)]. Further characterization of function/regulation of these heme-transport proteins as well as identification of new members of heme-transport and heme-binding proteins will enhance our comprehensive understanding of the iron metabolism network in normal and disease conditions. As represented in frataxin deficiency and cardio-and neurodegeneration, regulation of mitochondrial iron homeostasis is another important avenue of the research for understanding molecular pathogenesis of not only Friedreich ataxia, but also Parkinson, Alzheimer, and many other neurologic diseases. Characterization of functional similarities and differences between frataxin and mitochondrial ferritin will help us better understand the diseases associated with mitochondrial iron homeostasis. Finding the regulatory mechanism of frataxin and mitochondrial ferritin genes will allow us to develop a strategy of chemoprevention or complementation of mitochondrial iron overload and the deficiency of frataxin in FRDA patients. Furthermore, mitoferrin, a recently discovered mitochondrial iron importer in zebrafish and mouse (orthologues of yeast Mrs3 and Mrs4) may cooperate with frataxin and mitochondrial ferritin to maintain mitochondrial iron homeostasis. Further investigation into the regulation and the physiologic function of nuclear ferritin may advance the research of nuclear iron homeostasis including iron trafficking, subsequent iron utilization in many iron-containing nuclear proteins, and oxidative DNA damage. Finally, the sources of serum ferritin and its physiologic function and regulation have not been well defined. The presence of ferritin-binding proteins on the cell surface (ferritin receptors) was previously demonstrated; however, identification of the ferritin receptors was not reported until the recent identification of TIM-2 as a ferritin H receptor. Identification and characterization of serum ferritins and additional members of ferritin receptors will shed light into the roles of these proteins in iron homeostasis and network.
Footnotes
Reviewing Editors: Peter Geisser, Jay Hanas, Hideo Kimura, Torben Moos, and Sundararaman Swaminathan
Abbreviations
AD, Alzheimer disease; ARE, antioxidant responsive element; ATF1, activating transcription factor 1; BMP, bone morphogenetic protein; C/EBP, CCAAT/enhancer-binding proteins; CNS, central nervous system; Cp, ceruloplasmin; DMT1, divalent metal transporter 1; ER, endoplasmic reticulum; FLC, Friend leukemia cells; FRDA, Friedreich ataxia; Fpn, ferroportin; GPI, glycosylphosphatidylinositol; GST, glutathione-S-transferase; HCP1, heme carrier protein 1; HD, Huntington disease; Heph, hephaestin; HFE, hereditary hemochromatosis protein; HFE2, hemojuvelin; HH, hereditary hemochromatosis; HIF, hypoxia-inducible factor; HO-1, heme oxygenase 1; HRE, hypoxia-responsive element; IGFBP3, insulin-like growth factor-binding protein 3; IRE, iron-regulatory element; IRPs, iron-regulatory proteins; ISC, iron–sulfur cluster; MPP, mitochondrial processing peptidase; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NQO1, NAD(P)H quinone oxidoreductase 1; Nramp, natural resistance-associated macrophage protein; NTBI, non-Tf-bound iron; 6-OHDA, 6-hydroxy-dopamine; PD, Parkinson disease; PIAS3, protein inhibitor of activated STAT3; PMA, phorbol 12-myristate 13-acetate; ROS, reactive oxygen species; Sla, sex-linked anemia; SOD, superoxide dismutase; STAT, signal transducer and activator of transcription; tBHQ, tert-butylhydroquinone; Tf, transferrin; TfR, transferrin receptor; TIM, T-cell immunoglobulin domain and mucin domain; UPR, unfolded protein response; UTR, untranslated region; VHL, von Hippel–Lindau.
Acknowledgments
We are grateful to Steve House for his contribution to the preparation of illustrations. This work was supported in part by the National Institutes of Health research grant DK-60007 to Y. Tsuji.
References
- 1.Abboud S. Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 2.Adamec J. Rusnak F. Owen WG. Naylor S. Benson LM. Gacy AM. Isaya G. Iron-dependent self-assembly of recombinant yeast frataxin: implications for Friedreich ataxia. Am J Hum Genet. 2000;67:549–562. doi: 10.1086/303056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams PC. Powell LW. Halliday JW. Isolation of a human hepatic ferritin receptor. Hepatology. 1988;8:719–721. doi: 10.1002/hep.1840080402. [DOI] [PubMed] [Google Scholar]
- 4.Aisen P. Leibman A. Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem. 1978;253:1930–1937. [PubMed] [Google Scholar]
- 5.Al-Mahdawi S. Pinto RM. Varshney D. Lawrence L. Lowrie MB. Hughes S. Webster Z. Blake J. Cooper JM. King R. Pook MA. GAA repeat expansion mutation mouse models of Friedreich ataxia exhibit oxidative stress leading to progressive neuronal and cardiac pathology. Genomics. 2006;88:580–590. doi: 10.1016/j.ygeno.2006.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alam J. Camhi S. Choi AM. Identification of a second region upstream of the mouse heme oxygenase-1 gene that functions as a basal level and inducer-dependent transcription enhancer. J Biol Chem. 1995;270:11977–11984. doi: 10.1074/jbc.270.20.11977. [DOI] [PubMed] [Google Scholar]
- 7.Anderson GJ. Faulk WP. Arosio P. Moss D. Powell LW. Halliday JW. Identification of H- and L-ferritin subunit binding sites on human T and B lymphoid cells. Br J Haematol. 1989;73:260–264. doi: 10.1111/j.1365-2141.1989.tb00262.x. [DOI] [PubMed] [Google Scholar]
- 8.Andriopoulos B. Hegedusch S. Mangin J. Riedel HD. Hebling U. Wang J. Pantopoulos K. Mueller S. Sustained hydrogen peroxide induces iron uptake by transferrin receptor-1 independent of the iron regulatory protein/iron-responsive element network. J Biol Chem. 2007;282:20301–20308. doi: 10.1074/jbc.M702463200. [DOI] [PubMed] [Google Scholar]
- 9.Arosio P. Levi S. Ferritin, iron homeostasis, and oxidative damage. Free Radic Biol Med. 2002;33:457–463. doi: 10.1016/s0891-5849(02)00842-0. [DOI] [PubMed] [Google Scholar]
- 10.Arosio P. Yokota M. Drysdale JW. Structural and immunological relationships of isoferritins in normal and malignant cells. Cancer Res. 1976;36:1735–1739. [PubMed] [Google Scholar]
- 11.Arranz Caso JA. Garcia Tena J. Llorens MM. Moreno R. High serum ferritin concentration in an AIDS patient with miliary tuberculosis. Clin Infect Dis. 1997;25:1263–1264. doi: 10.1086/516972. [DOI] [PubMed] [Google Scholar]
- 12.Askwith C. Eide D. Van Ho A. Bernard PS. Li L. Davis-Kaplan S. Sipe DM. Kaplan J. The FET3 gene of S cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell. 1994;76:403–410. doi: 10.1016/0092-8674(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 13.Babcock M. de Silva D. Oaks R. Davis-Kaplan S. Jiralerspong S. Montermini L. Pandolfo M. Kaplan J. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science. 1997;276:1709–1712. doi: 10.1126/science.276.5319.1709. [DOI] [PubMed] [Google Scholar]
- 14.Babitt JL. Huang FW. Wrighting DM. Xia Y. Sidis Y. Samad TA. Campagna JA. Chung RT. Schneyer AL. Woolf CJ. Andrews NC. Lin HY. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 15.Babitt JL. Huang FW. Xia Y. Sidis Y. Andrews NC. Lin HY. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. 2007;117:1933–1939. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barton JC. Bertoli LF. Hemochromatosis: the genetic disorder of the twenty-first century. Nat Med. 1996;2:394–395. doi: 10.1038/nm0496-394. [DOI] [PubMed] [Google Scholar]
- 17.Battistini A. Coccia EM. Marziali G. Bulgarini D. Scalzo S. Fiorucci G. Romeo G. Affabris E. Testa U. Rossi GB. Peschle C. Intracellular heme coordinately modulates globin chain synthesis, transferrin receptor number, and ferritin content in differentiating Friend erythroleukemia cells. Blood. 1991;78:2098–2103. [PubMed] [Google Scholar]
- 18.Beard J. Iron deficiency alters brain development and functioning. J Nutr. 2003;133:1468S–1472S. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- 19.Beaumont C. Leneuve P. Devaux I. Scoazec JY. Berthier M. Loiseau MN. Grandchamp B. Bonneau D. Mutation in the iron responsive element of the L ferritin mRNA in a family with dominant hyperferritinaemia and cataract. Nat Genet. 1995;11:444–446. doi: 10.1038/ng1295-444. [DOI] [PubMed] [Google Scholar]
- 20.Beaumont C. Seyhan A. Yachou AK. Grandchamp B. Jones R. Mouse ferritin H subunit gene: functional analysis of the promoter and identification of an upstream regulatory element active in erythroid cells. J Biol Chem. 1994;269:20281–20288. [PubMed] [Google Scholar]
- 20a.Bernstein SE. J Lab Clin Med. 1987;110:690–705. [PubMed] [Google Scholar]
- 21.Bianchi L. Tacchini L. Cairo G. HIF-1-mediated activation of transferrin receptor gene transcription by iron chelation. Nucleic Acids Res. 1999;27:4223–4227. doi: 10.1093/nar/27.21.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bidichandani SI. Ashizawa T. Patel PI. The GAA triplet-repeat expansion in Friedreich ataxia interferes with transcription and may be associated with an unusual DNA structure. Am J Hum Genet. 1998;62:111–121. doi: 10.1086/301680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bishop GM. Robinson SR. Liu Q. Perry G. Atwood CS. Smith MA. Iron: a pathological mediator of Alzheimer disease? Dev Neurosci. 2002;24:184–187. doi: 10.1159/000065696. [DOI] [PubMed] [Google Scholar]
- 24.Bjorkman PJ. Parham P. Structure, function, and diversity of class I major histocompatibility complex molecules. Annu Rev Biochem. 1990;59:253–288. doi: 10.1146/annurev.bi.59.070190.001345. [DOI] [PubMed] [Google Scholar]
- 25.Boddaert N. Le Quan Sang KH. Rotig A. Leroy-Willig A. Gallet S. Brunelle F. Sidi D. Thalabard JC. Munnich A. Cabantchik ZI. Selective iron chelation in Friedreich ataxia: biologic and clinical implications. Blood. 2007;110:401–408. doi: 10.1182/blood-2006-12-065433. [DOI] [PubMed] [Google Scholar]
- 26.Boutet SC. Disatnik MH. Chan LS. Iori K. Rando TA. Regulation of pax3 by proteasomal degradation of monoubiquitinated protein in skeletal muscle progenitors. Cell. 2007;130:349–362. doi: 10.1016/j.cell.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 27.Bradley JL. Blake JC. Chamberlain S. Thomas PK. Cooper JM. Schapira AH. Clinical, biochemical and molecular genetic correlations in Friedreich's ataxia. Hum Mol Genet. 2000;9:275–282. doi: 10.1093/hmg/9.2.275. [DOI] [PubMed] [Google Scholar]
- 28.Branda SS. Cavadini P. Adamec J. Kalousek F. Taroni F. Isaya G. Yeast and human frataxin are processed to mature form in two sequential steps by the mitochondrial processing peptidase. J Biol Chem. 1999;274:22763–22769. doi: 10.1074/jbc.274.32.22763. [DOI] [PubMed] [Google Scholar]
- 29.Bulteau AL. O'Neill HA. Kennedy MC. Ikeda-Saito M. Isaya G. Szweda LI. Frataxin acts as an iron chaperone protein to modulate mitochondrial aconitase activity. Science. 2004;305:242–245. doi: 10.1126/science.1098991. [DOI] [PubMed] [Google Scholar]
- 30.Burnett R. Melander C. Puckett JW. Son LS. Wells RD. Dervan PB. Gottesfeld JM. DNA sequence-specific polyamides alleviate transcription inhibition associated with long GAA.TTC repeats in Friedreich's ataxia. Proc Natl Acad Sci USA. 2006;103:11497–11502. doi: 10.1073/pnas.0604939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai CX. Birk DE. Linsenmayer TF. Ferritin is a developmentally regulated nuclear protein of avian corneal epithelial cells. J Biol Chem. 1997;272:12831–12839. doi: 10.1074/jbc.272.19.12831. [DOI] [PubMed] [Google Scholar]
- 32.Cai CX. Birk DE. Linsenmayer TF. Nuclear ferritin protects DNA from UV damage in corneal epithelial cells. Mol Biol Cell. 1998;9:1037–1051. doi: 10.1091/mbc.9.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caltagirone A. Weiss G. Pantopoulos K. Modulation of cellular iron metabolism by hydrogen peroxide: effects of H2O2 on the expression and function of iron-responsive element-containing mRNAs in B6 fibroblasts. J Biol Chem. 2001;276:19738–19745. doi: 10.1074/jbc.M100245200. [DOI] [PubMed] [Google Scholar]
- 34.Camaschella C. Roetto A. Cali A. De Gobbi M. Garozzo G. Carella M. Majorano N. Totaro A. Gasparini P. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet. 2000;25:14–15. doi: 10.1038/75534. [DOI] [PubMed] [Google Scholar]
- 35.Camaschella C. Roetto A. De Gobbi M. Genetic haemochromatosis: genes and mutations associated with iron loading. Best Pract Res Clin Haematol. 2002;15:261–276. doi: 10.1016/s1521-6926(02)90207-0. [DOI] [PubMed] [Google Scholar]
- 36.Campanella A. Isaya G. O'Neill HA. Santambrogio P. Cozzi A. Arosio P. Levi S. The expression of human mitochondrial ferritin rescues respiratory function in frataxin-deficient yeast. Hum Mol Genet. 2004;13:2279–2288. doi: 10.1093/hmg/ddh232. [DOI] [PubMed] [Google Scholar]
- 37.Campuzano V. Montermini L. Molto MD. Pianese L. Cossee M. Cavalcanti F. Monros E. Rodius F. Duclos F. Monticelli A. Zara F. Canizares J. Koutnikova H. Bidichandani SI. Gellera C. Brice A. Trouillas P. De Michele G. Filla A. De Frutos R. Palau F. Patel PI. Di Donato S. Mandel JL. Cocozza S. Koenig M. Pandolfo M. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- 38.Canonne-Hergaux F. Gruenheid S. Ponka P. Gros P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood. 1999;93:4406–4417. [PubMed] [Google Scholar]
- 39.Canonne-Hergaux F. Zhang AS. Ponka P. Gros P. Characterization of the iron transporter DMT1 (NRAMP2/DCT1) in red blood cells of normal and anemic mk/mk mice. Blood. 2001;98:3823–3830. doi: 10.1182/blood.v98.13.3823. [DOI] [PubMed] [Google Scholar]
- 40.Carella M. D'Ambrosio L. Totaro A. Grifa A. Valentino MA. Piperno A. Girelli D. Roetto A. Franco B. Gasparini P. Camaschella C. Mutation analysis of the HLA-H gene in Italian hemochromatosis patients. Am J Hum Genet. 1997;60:828–832. [PMC free article] [PubMed] [Google Scholar]
- 41.Casey JL. Hentze MW. Koeller DM. Caughman SW. Rouault TA. Klausner RD. Harford JB. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science. 1988;240:924–928. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- 42.Cass WA. Grondin R. Andersen AH. Zhang Z. Hardy PA. Hussey-Andersen LK. Rayens WS. Gerhardt GA. Gash DM. Iron accumulation in the striatum predicts aging-related decline in motor function in rhesus monkeys. Neurobiol Aging. 2007;28:258–271. doi: 10.1016/j.neurobiolaging.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Cavadini P. Adamec J. Taroni F. Gakh O. Isaya G. Two-step processing of human frataxin by mitochondrial processing peptidase: precursor and intermediate forms are cleaved at different rates. J Biol Chem. 2000;275:41469–41475. doi: 10.1074/jbc.M006539200. [DOI] [PubMed] [Google Scholar]
- 44.Cavadini P. O'Neill HA. Benada O. Isaya G. Assembly and iron-binding properties of human frataxin, the protein deficient in Friedreich ataxia. Hum Mol Genet. 2002;11:217–227. doi: 10.1093/hmg/11.3.217. [DOI] [PubMed] [Google Scholar]
- 45.Chan K. Lu R. Chang JC. Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci U S A. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chantrel-Groussard K. Geromel V. Puccio H. Koenig M. Munnich A. Rotig A. Rustin P. Disabled early recruitment of antioxidant defenses in Friedreich's ataxia. Hum Mol Genet. 2001;10:2061–2067. doi: 10.1093/hmg/10.19.2061. [DOI] [PubMed] [Google Scholar]
- 47.Chen OS. Hemenway S. Kaplan J. Inhibition of Fe-S cluster biosynthesis decreases mitochondrial iron export: evidence that Yfh1p affects Fe-S cluster synthesis. Proc Natl Acad Sci U S A. 2002;99:12321–12326. doi: 10.1073/pnas.192449599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen TT. Li L. Chung DH. Allen CD. Torti SV. Torti FM. Cyster JG. Chen CY. Brodsky FM. Niemi EC. Nakamura MC. Seaman WE. Daws MR. TIM-2 is expressed on B cells and in liver and kidney and is a receptor for H-ferritin endocytosis. J Exp Med. 2005;202:955–965. doi: 10.1084/jem.20042433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng Y. Zak O. Aisen P. Harrison SC. Walz T. Structure of the human transferrin receptor-transferrin complex. Cell. 2004;116:565–576. doi: 10.1016/s0092-8674(04)00130-8. [DOI] [PubMed] [Google Scholar]
- 50.Chinnery PF. Crompton DE. Birchall D. Jackson MJ. Coulthard A. Lombes A. Quinn N. Wills A. Fletcher N. Mottershead JP. Cooper P. Kellett M. Bates D. Burn J. Clinical features and natural history of neuroferritinopathy caused by the FTL1 460InsA mutation. Brain. 2007;130:110–119. doi: 10.1093/brain/awl319. [DOI] [PubMed] [Google Scholar]
- 51.Collawn JF. Stangel M. Kuhn LA. Esekogwu V. Jing SQ. Trowbridge IS. Tainer JA. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990;63:1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- 52.Condo I. Ventura N. Malisan F. Rufini A. Tomassini B. Testi R. In vivo maturation of human frataxin. Hum Mol Genet. 2007;16:1534–1540. doi: 10.1093/hmg/ddm102. [DOI] [PubMed] [Google Scholar]
- 53.Connor JR. Boeshore KL. Benkovic SA. Menzies SL. Isoforms of ferritin have a specific cellular distribution in the brain. J Neurosci Res. 1994;37:461–465. doi: 10.1002/jnr.490370405. [DOI] [PubMed] [Google Scholar]
- 54.Conrad ME. Umbreit JN. Moore EG. Parmley RT. Hereditary hemochromatosis: a prevalent disorder of iron metabolism with an elusive etiology. Am J Hematol. 1994;47:218–224. doi: 10.1002/ajh.2830470313. [DOI] [PubMed] [Google Scholar]
- 55.Cooperman SS. Meyron-Holtz EG. Olivierre-Wilson H. Ghosh MC. McConnell JP. Rouault TA. Microcytic anemia, erythropoietic protoporphyria, and neurodegeneration in mice with targeted deletion of iron-regulatory protein 2. Blood. 2005;106:1084–1091. doi: 10.1182/blood-2004-12-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corsi B. Cozzi A. Arosio P. Drysdale J. Santambrogio P. Campanella A. Biasiotto G. Albertini A. Levi S. Human mitochondrial ferritin expressed in HeLa cells incorporates iron and affects cellular iron metabolism. J Biol Chem. 2002;277:22430–22437. doi: 10.1074/jbc.M105372200. [DOI] [PubMed] [Google Scholar]
- 57.Cossee M. Puccio H. Gansmuller A. Koutnikova H. Dierich A. LeMeur M. Fischbeck K. Dolle P. Koenig M. Inactivation of the Friedreich ataxia mouse gene leads to early embryonic lethality without iron accumulation. Hum Mol Genet. 2000;9:1219–1226. doi: 10.1093/hmg/9.8.1219. [DOI] [PubMed] [Google Scholar]
- 58.Courselaud B. Pigeon C. Inoue Y. Inoue J. Gonzalez FJ. Leroyer P. Gilot D. Boudjema K. Guguen-Guillouzo C. Brissot P. Loreal O. Ilyin G. C/EBPalpha regulates hepatic transcription of hepcidin, an antimicrobial peptide and regulator of iron metabolism: cross-talk between C/EBP pathway and iron metabolism. J Biol Chem. 2002;277:41163–41170. doi: 10.1074/jbc.M202653200. [DOI] [PubMed] [Google Scholar]
- 59.Cozzi A. Corsi B. Levi S. Santambrogio P. Albertini A. Arosio P. Overexpression of wild type and mutated human ferritin H-chain in HeLa cells: in vivo role of ferritin ferroxidase activity. J Biol Chem. 2000;275:25122–25129. doi: 10.1074/jbc.M003797200. [DOI] [PubMed] [Google Scholar]
- 60.Cozzi A. Corsi B. Levi S. Santambrogio P. Biasiotto G. Arosio P. Analysis of the biologic functions of H- and L-ferritins in HeLa cells by transfection with siRNAs and cDNAs: evidence for a proliferative role of L-ferritin. Blood. 2004;103:2377–2383. doi: 10.1182/blood-2003-06-1842. [DOI] [PubMed] [Google Scholar]
- 61.Cozzi A. Santambrogio P. Corsi B. Campanella A. Arosio P. Levi S. Characterization of the l-ferritin variant 460InsA responsible of a hereditary ferritinopathy disorder. Neurobiol Dis. 2006;23:644–652. doi: 10.1016/j.nbd.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Curtis AR. Fey C. Morris CM. Bindoff LA. Ince PG. Chinnery PF. Coulthard A. Jackson MJ. Jackson AP. McHale DP. Hay D. Barker WA. Markham AF. Bates D. Curtis A. Burn J. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat Genet. 2001;28:350–354. doi: 10.1038/ng571. [DOI] [PubMed] [Google Scholar]
- 63.Dancis A. Yuan DS. Haile D. Askwith C. Eide D. Moehle C. Kaplan J. Klausner RD. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell. 1994;76:393–402. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 64.De Domenico I. Vaughn MB. Li L. Bagley D. Musci G. Ward DM. Kaplan J. Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome. EMBO J. 2006;25:5396–5404. doi: 10.1038/sj.emboj.7601409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Domenico I. Ward DM. di Patti MC. Jeong SY. David S. Musci G. Kaplan J. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J. 2007;26:2823–2831. doi: 10.1038/sj.emboj.7601735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Domenico I. Ward DM. Langelier C. Vaughn MB. Nemeth E. Sundquist WI. Ganz T. Musci G. Kaplan J. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18:2569–2578. doi: 10.1091/mbc.E07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Domenico I. Ward DM. Musci G. Kaplan J. Iron overload due to mutations in ferroportin. Haematologica. 2006;91:92–95. [PMC free article] [PubMed] [Google Scholar]
- 68.De Domenico I. Ward DM. Nemeth E. Vaughn MB. Musci G. Ganz T. Kaplan J. The molecular basis of ferroportin-linked hemochromatosis. Proc Natl Acad Sci U S A. 2005;102:8955–8960. doi: 10.1073/pnas.0503804102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delatycki MB. Camakaris J. Brooks H. Evans-Whipp T. Thorburn DR. Williamson R. Forrest SM. Direct evidence that mitochondrial iron accumulation occurs in Friedreich ataxia. Ann Neurol. 1999;45:673–675. [PubMed] [Google Scholar]
- 70.Desmyter L. Dewaele S. Reekmans R. Nystrom T. Contreras R. Chen C. Expression of the human ferritin light chain in a frataxin mutant yeast affects ageing and cell death. Exp Gerontol. 2004;39:707–715. doi: 10.1016/j.exger.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 71.Dhe-Paganon S. Shigeta R. Chi YI. Ristow M. Shoelson SE. Crystal structure of human frataxin. J Biol Chem. 2000;275:30753–30756. doi: 10.1074/jbc.C000407200. [DOI] [PubMed] [Google Scholar]
- 72.Donovan A. Brownlie A. Zhou Y. Shepard J. Pratt SJ. Moynihan J. Paw BH. Drejer A. Barut B. Zapata A. Law TC. Brugnara C. Lux SE. Pinkus GS. Pinkus JL. Kingsley PD. Palis J. Fleming MD. Andrews NC. Zon LI. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 73.Donovan A. Lima CA. Pinkus JL. Pinkus GS. Zon LI. Robine S. Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Drysdale J. Arosio P. Invernizzi R. Cazzola M. Volz A. Corsi B. Biasiotto G. Levi S. Mitochondrial ferritin: a new player in iron metabolism. Blood Cells Mol Dis. 2002;29:376–383. doi: 10.1006/bcmd.2002.0577. [DOI] [PubMed] [Google Scholar]
- 75.Dubljevic V. Sali A. Goding JW. A conserved RGD (Arg-Gly-Asp) motif in the transferrin receptor is required for binding to transferrin. Biochem J. 1999;341:11–14. [PMC free article] [PubMed] [Google Scholar]
- 76.Durr A. Cossee M. Agid Y. Campuzano V. Mignard C. Penet C. Mandel JL. Brice A. Koenig M. Clinical and genetic abnormalities in patients with Friedreich's ataxia. N Engl J Med. 1996;335:1169–1175. doi: 10.1056/NEJM199610173351601. [DOI] [PubMed] [Google Scholar]
- 77.Elmberg M. Hultcrantz R. Ekbom A. Brandt L. Olsson S. Olsson R. Lindgren S. Loof L. Stal P. Wallerstedt S. Almer S. Sandberg-Gertzen H. Askling J. Cancer risk in patients with hereditary hemochromatosis and in their first-degree relatives. Gastroenterology. 2003;125:1733–1741. doi: 10.1053/j.gastro.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 78.Epsztejn S. Glickstein H. Picard V. Slotki IN. Breuer W. Beaumont C. Cabantchik ZI. H-ferritin subunit overexpression in erythroid cells reduces the oxidative stress response and induces multidrug resistance properties. Blood. 1999;94:3593–3603. [PubMed] [Google Scholar]
- 79.Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 80.Faniello MC. Bevilacqua MA. Condorelli G. de Crombrugghe B. Maity SN. Avvedimento VE. Cimino F. Costanzo F. The B subunit of the CAAT-binding factor NFY binds the central segment of the Co-activator p300. J Biol Chem. 1999;274:7623–7626. doi: 10.1074/jbc.274.12.7623. [DOI] [PubMed] [Google Scholar]
- 81.Favreau LV. Pickett CB. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene: identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- 82.Feder JN. Gnirke A. Thomas W. Tsuchihashi Z. Ruddy DA. Basava A. Dormishian F. Domingo R Jr. Ellis MC. Fullan A. Hinton LM. Jones NL. Kimmel BE. Kronmal GS. Lauer P. Lee VK. Loeb DB. Mapa FA. McClelland E. Meyer NC. Mintier GA. Moeller N. Moore T. Morikang E. Prass CE. Quintana L. Starnes SM. Schatzman RC. Brunke KJ. Drayna DT. Risch NJ. Bacon BR. Wolff RK. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 83.Feder JN. Penny DM. Irrinki A. Lee VK. Lebron JA. Watson N. Tsuchihashi Z. Sigal E. Bjorkman PJ. Schatzman RC. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc Natl Acad Sci U S A. 1998;95:1472–1477. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feder JN. Tsuchihashi Z. Irrinki A. Lee VK. Mapa FA. Morikang E. Prass CE. Starnes SM. Wolff RK. Parkkila S. Sly WS. Schatzman RC. The hemochromatosis founder mutation in HLA-H disrupts beta2-microglobulin interaction and cell surface expression. J Biol Chem. 1997;272:14025–14028. doi: 10.1074/jbc.272.22.14025. [DOI] [PubMed] [Google Scholar]
- 85.Ferreira C. Bucchini D. Martin ME. Levi S. Arosio P. Grand-champ B. Beaumont C. Early embryonic lethality of H ferritin gene deletion in mice. J Biol Chem. 2000;275:3021–3024. doi: 10.1074/jbc.275.5.3021. [DOI] [PubMed] [Google Scholar]
- 85a.Ferreira C, Santambrogio P, Martin ME, Andrieu V, Feldmann G, Henin D, and Beaumont C. Blood. 2001;98:525–532. doi: 10.1182/blood.v98.3.525. [DOI] [PubMed] [Google Scholar]
- 86.Fleming J. Spinoulas A. Zheng M. Cunningham SC. Ginn SL. McQuilty RC. Rowe PB. Alexander IE. Partial correction of sensitivity to oxidant stress in Friedreich ataxia patient fibroblasts by frataxin-encoding adeno-associated virus and lentivirus vectors. Hum Gene Ther. 2005;16:947–956. doi: 10.1089/hum.2005.16.947. [DOI] [PubMed] [Google Scholar]
- 87.Fleming MD. Romano MA. Su MA. Garrick LM. Garrick MD. Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fleming MD. Trenor CC., 3rd Su MA. Foernzler D. Beier DR. Dietrich WF. Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 89.Fleming RE. Ahmann JR. Migas MC. Waheed A. Koeffler HP. Kawabata H. Britton RS. Bacon BR. Sly WS. Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis. Proc Natl Acad Sci U S A. 2002;99:10653–10658. doi: 10.1073/pnas.162360699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fleming RE. Migas MC. Holden CC. Waheed A. Britton RS. Tomatsu S. Bacon BR. Sly WS. Transferrin receptor 2: continued expression in mouse liver in the face of iron overload and in hereditary hemochromatosis. Proc Natl Acad Sci U S A. 2000;97:2214–2219. doi: 10.1073/pnas.040548097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fleming RE. Migas MC. Zhou X. Jiang J. Britton RS. Brunt EM. Tomatsu S. Waheed A. Bacon BR. Sly WS. Mechanism of increased iron absorption in murine model of hereditary hemochromatosis: increased duodenal expression of the iron transporter DMT1. Proc Natl Acad Sci U S A. 1999;96:3143–3148. doi: 10.1073/pnas.96.6.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fleming RE. Sly WS. Ferroportin mutation in autosomal dominant hemochromatosis: loss of function, gain in understanding. J Clin Invest. 2001;108:521–522. doi: 10.1172/JCI13739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Foury F. Cazzalini O. Deletion of the yeast homologue of the human gene associated with Friedreich's ataxia elicits iron accumulation in mitochondria. FEBS Lett. 1997;411:373–377. doi: 10.1016/s0014-5793(97)00734-5. [DOI] [PubMed] [Google Scholar]
- 94.Foury F. Roganti T. Deletion of the mitochondrial carrier genes MRS3 and MRS4 suppresses mitochondrial iron accumulation in a yeast frataxin-deficient strain. J Biol Chem. 2002;277:24475–24483. doi: 10.1074/jbc.M111789200. [DOI] [PubMed] [Google Scholar]
- 95.Friling RS. Bensimon A. Tichauer Y. Daniel V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc Natl Acad Sci U S A. 1990;87:6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gakh O. Adamec J. Gacy AM. Twesten RD. Owen WG. Isaya G. Physical evidence that yeast frataxin is an iron storage protein. Biochemistry. 2002;41:6798–6804. doi: 10.1021/bi025566+. [DOI] [PubMed] [Google Scholar]
- 97.Gakh O. Park S. Liu G. Macomber L. Imlay JA. Ferreira GC. Isaya G. Mitochondrial iron detoxification is a primary function of frataxin that limits oxidative damage and preserves cell longevity. Hum Mol Genet. 2006;15:467–479. doi: 10.1093/hmg/ddi461. [DOI] [PubMed] [Google Scholar]
- 98.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 99.Ganz T. Nemeth E. Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol. 2006;290:G199–G203. doi: 10.1152/ajpgi.00412.2005. [DOI] [PubMed] [Google Scholar]
- 100.Gelvan D. Fibach E. Meyron-Holtz EG. Konijn AM. Ferritin uptake by human erythroid precursors is a regulated iron uptake pathway. Blood. 1996;88:3200–3207. [PubMed] [Google Scholar]
- 101.Gerald D. Berra E. Frapart YM. Chan DA. Giaccia AJ. Mansuy D. Pouyssegur J. Yaniv M. Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 102.Gerber J. Muhlenhoff U. Lill R. An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1. EMBO Rep. 2003;4:906–911. doi: 10.1038/sj.embor.embor918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gerstein M. Anderson BF. Norris GE. Baker EN. Lesk AM. Chothia C. Domain closure in lactoferrin: two hinges produce a see-saw motion between alternative close-packed interfaces. J Mol Biol. 1993;234:357–372. doi: 10.1006/jmbi.1993.1592. [DOI] [PubMed] [Google Scholar]
- 104.Giannetti AM. Bjorkman PJ. HFE and transferrin directly compete for transferrin receptor in solution and at the cell surface. J Biol Chem. 2004;279:25866–25875. doi: 10.1074/jbc.M401467200. [DOI] [PubMed] [Google Scholar]
- 105.Giannetti AM. Snow PM. Zak O. Bjorkman PJ. Mechanism for multiple ligand recognition by the human transferrin receptor. PLoS Biol. 2003;1:E51. doi: 10.1371/journal.pbio.0000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gitlin JD. Aceruloplasminemia. Pediatr Res. 1998;44:271–276. doi: 10.1203/00006450-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 106a.Gonzalez-Cabo P, Vazquez-Manrique RP, Garcia-Gimeno MA, Sanz P, and Palau F. Hum Mol Genet. 2005;14:2091–2098. doi: 10.1093/hmg/ddi214. [DOI] [PubMed] [Google Scholar]
- 107.Goossen B. Caughman SW. Harford JB. Klausner RD. Hentze MW. Translational repression by a complex between the iron-responsive element of ferritin mRNA and its specific cytoplasmic binding protein is position-dependent in vivo. EMBO J. 1990;9:4127–4133. doi: 10.1002/j.1460-2075.1990.tb07635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gordon DM. Shi Q. Dancis A. Pain D. Maturation of frataxin within mammalian and yeast mitochondria: one-step processing by matrix processing peptidase. Hum Mol Genet. 1999;8:2255–2262. doi: 10.1093/hmg/8.12.2255. [DOI] [PubMed] [Google Scholar]
- 109.Grant L. Sun J. Xu H. Subramony SH. Chaires JB. Hebert MD. Rational selection of small molecules that increase transcription through the GAA repeats found in Friedreich's ataxia. FEBS Lett. 2006;580:5399–5405. doi: 10.1016/j.febslet.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gray CP. Arosio P. Hersey P. Association of increased levels of heavy-chain ferritin with increased CD4+ CD25+ regulatory T-cell levels in patients with melanoma. Clin Cancer Res. 2003;9:2551–2559. [PubMed] [Google Scholar]
- 111.Gray CP. Arosio P. Hersey P. Heavy chain ferritin activates regulatory T cells by induction of changes in dendritic cells. Blood. 2002;99:3326–3334. doi: 10.1182/blood.v99.9.3326. [DOI] [PubMed] [Google Scholar]
- 112.Gray NK. Hentze MW. Iron regulatory protein prevents binding of the 43S translation pre-initiation complex to ferritin and eALAS mRNAs. EMBO J. 1994;13:3882–3891. doi: 10.1002/j.1460-2075.1994.tb06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Greene E. Entezam A. Kumari D. Usdin K. Ancient repeated DNA elements and the regulation of the human frataxin promoter. Genomics. 2005;85:221–230. doi: 10.1016/j.ygeno.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 114.Grossmann JG. Neu M. Evans RW. Lindley PF. Appel H. Hasnain SS. Metal-induced conformational changes in transferrins. J Mol Biol. 1993;229:585–590. doi: 10.1006/jmbi.1993.1063. [DOI] [PubMed] [Google Scholar]
- 115.Grossmann JG. Neu M. Pantos E. Schwab FJ. Evans RW. Townes-Andrews E. Lindley PF. Appel H. Thies WG. Hasnain SS. X-ray solution scattering reveals conformational changes upon iron uptake in lactoferrin, serum and ovo-transferrins. J Mol Biol. 1992;225:811–819. doi: 10.1016/0022-2836(92)90402-6. [DOI] [PubMed] [Google Scholar]
- 116.Gunshin H. Allerson CR. Polycarpou-Schwarz M. Rofts A. Rogers JT. Kishi F. Hentze MW. Rouault TA. Andrews NC. Hediger MA. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett. 2001;509:309–316. doi: 10.1016/s0014-5793(01)03189-1. [DOI] [PubMed] [Google Scholar]
- 117.Gunshin H. Mackenzie B. Berger UV. Gunshin Y. Romero MF. Boron WF. Nussberger S. Gollan JL. Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 118.Gutteridge JM. Quinlan GJ. Antioxidant protection against organic and inorganic oxygen radicals by normal human plasma: the important primary role for iron-binding and iron-oxidising proteins. Biochim Biophys Acta. 1992;1159:248–254. doi: 10.1016/0167-4838(92)90052-f. [DOI] [PubMed] [Google Scholar]
- 119.Hahn P. Qian Y. Dentchev T. Chen L. Beard J. Harris ZL. Dunaief JL. Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc Natl Acad Sci U S A. 2004;101:13850–13855. doi: 10.1073/pnas.0405146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 121.Han O. Kim EY. Colocalization of ferroportin-1 with hephaestin on the basolateral membrane of human intestinal absorptive cells. J Cell Biochem. 2007;101:1000–1010. doi: 10.1002/jcb.21392. [DOI] [PubMed] [Google Scholar]
- 122.Harris ZL. Davis-Kaplan SR. Gitlin JD. Kaplan J. A fungal multicopper oxidase restores iron homeostasis in aceruloplasminemia. Blood. 2004;103:4672–4673. doi: 10.1182/blood-2003-11-4060. [DOI] [PubMed] [Google Scholar]
- 123.Harris ZL. Durley AP. Man TK. Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci U S A. 1999;96:10812–10817. doi: 10.1073/pnas.96.19.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Harris ZL. Takahashi Y. Miyajima H. Serizawa M. MacGillivray RT. Gitlin JD. Aceruloplasminemia: molecular characterization of this disorder of iron metabolism. Proc Natl Acad Sci U S A. 1995;92:2539–2543. doi: 10.1073/pnas.92.7.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Harrison PM. Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 126.Hashimoto M. Hsu LJ. Xia Y. Takeda A. Sisk A. Sundsmo M. Masliah E. Oxidative stress induces amyloid-like aggregate formation of NACP/alpha-synuclein in vitro. Neuroreport. 1999;10:717–721. doi: 10.1097/00001756-199903170-00011. [DOI] [PubMed] [Google Scholar]
- 127.Hentze MW. Argos P. Homology between IRE-BP, a regulatory RNA-binding protein, aconitase, and isopropylmalate isomerase. Nucleic Acids Res. 1991;19:1739–1740. doi: 10.1093/nar/19.8.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hentze MW. Caughman SW. Casey JL. Koeller DM. Rouault TA. Harford JB. Klausner RD. A model for the structure and functions of iron-responsive elements. Gene. 1988;72:201–208. doi: 10.1016/0378-1119(88)90145-x. [DOI] [PubMed] [Google Scholar]
- 129.Hentze MW. Caughman SW. Rouault TA. Barriocanal JG. Dancis A. Harford JB. Klausner RD. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987;238:1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- 130.Hentze MW. Kuhn LC. Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci U S A. 1996;93:8175–8182. doi: 10.1073/pnas.93.16.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hentze MW. Muckenthaler MU. Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 132.Herman D. Jenssen K. Burnett R. Soragni E. Perlman SL. Gottesfeld JM. Histone deacetylase inhibitors reverse gene silencing in Friedreich's ataxia. Nat Chem Biol. 2006;2:551–558. doi: 10.1038/nchembio815. [DOI] [PubMed] [Google Scholar]
- 133.Hintze KJ. Theil EC. DNA and mRNA elements with complementary responses to hemin, antioxidant inducers, and iron control ferritin-L expression. Proc Natl Acad Sci U S A. 2005;102:15048–15052. doi: 10.1073/pnas.0505148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hochstrasser H. Bauer P. Walter U. Behnke S. Spiegel J. Csoti I. Zeiler B. Bornemann A. Pahnke J. Becker G. Riess O. Berg D. Ceruloplasmin gene variations and substantia nigra hyperechogenicity in Parkinson disease. Neurology. 2004;63:1912–1917. doi: 10.1212/01.wnl.0000144276.29988.c3. [DOI] [PubMed] [Google Scholar]
- 135.Hochstrasser H. Tomiuk J. Walter U. Behnke S. Spiegel J. Kruger R. Becker G. Riess O. Berg D. Functional relevance of ceruloplasmin mutations in Parkinson's disease. FASEB J. 2005;19:1851–1853. doi: 10.1096/fj.04-3486fje. [DOI] [PubMed] [Google Scholar]
- 136.Holtz WA. O'Malley KL. Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons. J Biol Chem. 2003;278:19367–19377. doi: 10.1074/jbc.M211821200. [DOI] [PubMed] [Google Scholar]
- 137.Hubert N. Hentze MW. Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: implications for regulation and cellular function. Proc Natl Acad Sci U S A. 2002;99:12345–12350. doi: 10.1073/pnas.192423399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hulet SW. Powers S. Connor JR. Distribution of transferrin and ferritin binding in normal and multiple sclerotic human brains. J Neurol Sci. 1999;165:48–55. doi: 10.1016/s0022-510x(99)00077-5. [DOI] [PubMed] [Google Scholar]
- 139.Hunter HN. Fulton DB. Ganz T. Vogel HJ. The solution structure of human hepcidin, a peptide hormone with antimicrobial activity that is involved in iron uptake and hereditary hemochromatosis. J Biol Chem. 2002;277:37597–37603. doi: 10.1074/jbc.M205305200. [DOI] [PubMed] [Google Scholar]
- 140.Iwai K. Drake SK. Wehr NB. Weissman AM. LaVaute T. Minato N. Klausner RD. Levine RL. Rouault TA. Iron-dependent oxidation, ubiquitination, and degradation of iron regulatory protein 2: implications for degradation of oxidized proteins. Proc Natl Acad Sci U S A. 1998;95:4924–4928. doi: 10.1073/pnas.95.9.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Iwai K. Klausner RD. Rouault TA. Requirements for iron-regulated degradation of the RNA binding protein, iron regulatory protein 2. EMBO J. 1995;14:5350–5357. doi: 10.1002/j.1460-2075.1995.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Iwasaki K. Hailemariam K. Tsuji Y. PIAS3 interacts with ATF1 and regulates the human ferritin H gene through an antioxidant-responsive element. J Biol Chem. 2007;282:22335–22343. doi: 10.1074/jbc.M701477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Iwasaki K. Mackenzie EL. Hailemariam K. Sakamoto K. Tsuji Y. Hemin-mediated regulation of an antioxidant-responsive element of the human ferritin H gene and role of Ref-1 during erythroid differentiation of K562 cells. Mol Cell Biol. 2006;26:2845–2856. doi: 10.1128/MCB.26.7.2845-2856.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jacobs EM. Hendriks JC. van Tits BL. Evans PJ. Breuer W. Liu DY. Jansen EH. Jauhiainen K. Sturm B. Porter JB. Scheiber-Mo-jdehkar B. von Bonsdorff L. Cabantchik ZI. Hider RC. Swinkels DW. Results of an international round robin for the quantification of serum non-transferrin-bound iron: need for defining standardization and a clinically relevant isoform. Anal Biochem. 2005;341:241–250. doi: 10.1016/j.ab.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 145.Jauslin ML. Meier T. Smith RA. Murphy MP. Mitochondriatargeted antioxidants protect Friedreich ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003;17:1972–1974. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- 146.Jauslin ML. Wirth T. Meier T. Schoumacher F. A cellular model for Friedreich ataxia reveals small-molecule glutathione peroxidase mimetics as novel treatment strategy. Hum Mol Genet. 2002;11:3055–3063. doi: 10.1093/hmg/11.24.3055. [DOI] [PubMed] [Google Scholar]
- 147.Jazwinska EC. Cullen LM. Busfield F. Pyper WR. Webb SI. Powell LW. Morris CP. Walsh TP. Haemochromatosis and HLA-H. Nat Genet. 1996;14:249–251. doi: 10.1038/ng1196-249. [DOI] [PubMed] [Google Scholar]
- 148.Jeong SY. David S. Glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. J Biol Chem. 2003;278:27144–27148. doi: 10.1074/jbc.M301988200. [DOI] [PubMed] [Google Scholar]
- 149.Johnson MB. Enns CA. Diferric transferrin regulates transferrin receptor 2 protein stability. Blood. 2004;104:4287–4293. doi: 10.1182/blood-2004-06-2477. [DOI] [PubMed] [Google Scholar]
- 150.Jouanolle AM. Gandon G. Jezequel P. Blayau M. Campion ML. Yaouanq J. Mosser J. Fergelot P. Chauvel B. Bouric P. Carn G. Andrieux N. Gicquel I. Le Gall JY. David V. Haemochromatosis and HLA-H. Nat Genet. 1996;14:251–252. doi: 10.1038/ng1196-251. [DOI] [PubMed] [Google Scholar]
- 151.Karlberg T. Schagerlof U. Gakh O. Park S. Ryde U. Lindahl M. Leath K. Garman E. Isaya G. Al-Karadaghi S. The structures of frataxin oligomers reveal the mechanism for the delivery and detoxification of iron. Structure. 2006;14:1535–1546. doi: 10.1016/j.str.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 152.Karthikeyan G. Lewis LK. Resnick MA. The mitochondrial protein frataxin prevents nuclear damage. Hum Mol Genet. 2002;11:1351–1362. doi: 10.1093/hmg/11.11.1351. [DOI] [PubMed] [Google Scholar]
- 153.Karthikeyan G. Santos JH. Graziewicz MA. Copeland WC. Isaya G. Van Houten B. Resnick MA. Reduction in frataxin causes progressive accumulation of mitochondrial damage. Hum Mol Genet. 2003;12:3331–3342. doi: 10.1093/hmg/ddg349. [DOI] [PubMed] [Google Scholar]
- 154.Kato J. Fujikawa K. Kanda M. Fukuda N. Sasaki K. Takayama T. Kobune M. Takada K. Takimoto R. Hamada H. Ikeda T. Niitsu Y. A mutation, in the iron-responsive element of H ferritin mRNA, causing autosomal dominant iron overload. Am J Hum Genet. 2001;69:191–197. doi: 10.1086/321261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kaur D. Andersen J. Does cellular iron dysregulation play a causative role in Parkinson's disease? Ageing Res Rev. 2004;3:327–343. doi: 10.1016/j.arr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 157.Kaur D. Andersen JK. Ironing out Parkinson's disease: Is therapeutic treatment with iron chelators a real possibility? Aging Cell. 2002;1:17–21. doi: 10.1046/j.1474-9728.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- 158.Kaur D. Rajagopalan S. Chinta S. Kumar J. Di Monte D. Cherny RA. Andersen JK. Chronic ferritin expression within murine dopaminergic midbrain neurons results in a progressive age-related neurodegeneration. Brain Res. 2007;1140:188–194. doi: 10.1016/j.brainres.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 159.Kaur D. Yantiri F. Rajagopalan S. Kumar J. Mo JQ. Boonplueang R. Viswanath V. Jacobs R. Yang L. Beal MF. DiMonte D. Volitaskis I. Ellerby L. Cherny RA. Bush AI. Andersen JK. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson's disease. Neuron. 2003;37:899–909. doi: 10.1016/s0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 160.Kawabata H. Yang R. Hirama T. Vuong PT. Kawano S. Gombart AF. Koeffler HP. Molecular cloning of transferrin receptor 2: a new member of the transferrin receptor-like family. J Biol Chem. 1999;274:20826–20832. doi: 10.1074/jbc.274.30.20826. [DOI] [PubMed] [Google Scholar]
- 161.Kim HY. Klausner RD. Rouault TA. Translational repressor activity is equivalent and is quantitatively predicted by in vitro RNA binding for two iron-responsive element-binding proteins, IRP1 and IRP2. J Biol Chem. 1995;270:4983–4986. doi: 10.1074/jbc.270.10.4983. [DOI] [PubMed] [Google Scholar]
- 162.Knight SA. Sepuri NB. Pain D. Dancis A. Mt-Hsp70 homolog, Ssc2p, required for maturation of yeast frataxin and mitochondrial iron homeostasis. J Biol Chem. 1998;273:18389–18393. doi: 10.1074/jbc.273.29.18389. [DOI] [PubMed] [Google Scholar]
- 163.Knutson MD. Oukka M. Koss LM. Aydemir F. Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and downregulated by hepcidin. Proc Natl Acad Sci U S A. 2005;102:1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Knutson MD. Vafa MR. Haile DJ. Wessling-Resnick M. Iron loading and erythrophagocytosis increase ferroportin 1 (FPN1) expression in J774 macrophages. Blood. 2003;102:4191–4197. doi: 10.1182/blood-2003-04-1250. [DOI] [PubMed] [Google Scholar]
- 165.Konijn AM. Glickstein H. Vaisman B. Meyron-Holtz EG. Slotki IN. Cabantchik ZI. The cellular labile iron pool and intracellular ferritin in K562 cells. Blood. 1999;94:2128–2134. [PubMed] [Google Scholar]
- 166.Koutnikova H. Campuzano V. Foury F. Dolle P. Cazzalini O. Koenig M. Studies of human, mouse and yeast homologues indicate a mitochondrial function for frataxin. Nat Genet. 1997;16:345–351. doi: 10.1038/ng0897-345. [DOI] [PubMed] [Google Scholar]
- 167.Koutnikova H. Campuzano V. Koenig M. Maturation of wild-type and mutated frataxin by the mitochondrial processing peptidase. Hum Mol Genet. 1998;7:1485–1489. doi: 10.1093/hmg/7.9.1485. [DOI] [PubMed] [Google Scholar]
- 168.Krause A. Neitz S. Magert HJ. Schulz A. Forssmann WG. Schulz-Knappe P. Adermann K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–150. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 169.Kreuzer M. Kirchgessner M. Endogenous iron excretion: a quantitative means to control iron metabolism? Biol Trace Elem Res. 1991;29:77–92. doi: 10.1007/BF03032686. [DOI] [PubMed] [Google Scholar]
- 170.Kuchroo VK. Umetsu DT. DeKruyff RH. Freeman GJ. The TIM gene family: emerging roles in immunity and disease. Nat Rev Immunol. 2003;3:454–462. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- 171.Kwak EL. Larochelle DA. Beaumont C. Torti SV. Torti FM. Role for NF-kappa B in the regulation of ferritin H by tumor necrosis factor-alpha. J Biol Chem. 1995;270:15285–15293. doi: 10.1074/jbc.270.25.15285. [DOI] [PubMed] [Google Scholar]
- 172.Langlois d'Estaintot B. Santambrogio P. Granier T. Gallois B. Chevalier JM. Precigoux G. Levi S. Arosio P. Crystal structure and biochemical properties of the human mitochondrial ferritin and its mutant Ser144Ala. J Mol Biol. 2004;340:277–293. doi: 10.1016/j.jmb.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 173.Larson JA. Howie HL. So M. Neisseria meningitidis accelerates ferritin degradation in host epithelial cells to yield an essential iron source. Mol Microbiol. 2004;53:807–820. doi: 10.1111/j.1365-2958.2004.04169.x. [DOI] [PubMed] [Google Scholar]
- 174.Latunde-Dada GO. Simpson RJ. McKie AT. Recent advances in mammalian haem transport. Trends Biochem Sci. 2006;31:182–188. doi: 10.1016/j.tibs.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 175.LaVaute T. Smith S. Cooperman S. Iwai K. Land W. Meyron-Holtz E. Drake SK. Miller G. Abu-Asab M. Tsokos M. Switzer R., 3rd Grinberg A. Love P. Tresser N. Rouault TA. Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat Genet. 2001;27:209–214. doi: 10.1038/84859. [DOI] [PubMed] [Google Scholar]
- 176.Lawrence CM. Ray S. Babyonyshev M. Galluser R. Borhani DW. Harrison SC. Crystal structure of the ectodomain of human transferrin receptor. Science. 1999;286:779–782. doi: 10.1126/science.286.5440.779. [DOI] [PubMed] [Google Scholar]
- 177.Lawson DM. Artymiuk PJ. Yewdall SJ. Smith JM. Livingstone JC. Treffry A. Luzzago A. Levi S. Arosio P. Cesareni G. Thomas CD. Shaw WV. Harrison PM. Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nature. 1991;349:541–544. doi: 10.1038/349541a0. [DOI] [PubMed] [Google Scholar]
- 178.Lawson DM. Treffry A. Artymiuk PJ. Harrison PM. Yewdall SJ. Luzzago A. Cesareni G. Levi S. Arosio P. Identification of the ferroxidase centre in ferritin. FEBS Lett. 1989;254:207–210. doi: 10.1016/0014-5793(89)81040-3. [DOI] [PubMed] [Google Scholar]
- 179.Lebron JA. West AP Jr. Bjorkman PJ. The hemochromatosis protein HFE competes with transferrin for binding to the transferrin receptor. J Mol Biol. 1999;294:239–245. doi: 10.1006/jmbi.1999.3252. [DOI] [PubMed] [Google Scholar]
- 180.Lee DW. Andersen JK. Kaur D. Iron dysregulation and neurodegeneration: the molecular connection. Mol Interv. 2006;6:89–97. doi: 10.1124/mi.6.2.6. [DOI] [PubMed] [Google Scholar]
- 181.Lesuisse E. Santos R. Matzanke BF. Knight SA. Camadro JM. Dancis A. Iron use for haeme synthesis is under control of the yeast frataxin homologue (Yfh1) Hum Mol Genet. 2003;12:879–889. doi: 10.1093/hmg/ddg096. [DOI] [PubMed] [Google Scholar]
- 182.Levi S. Corsi B. Bosisio M. Invernizzi R. Volz A. Sanford D. Arosio P. Drysdale J. A human mitochondrial ferritin encoded by an intronless gene. J Biol Chem. 2001;276:24437–24440. doi: 10.1074/jbc.C100141200. [DOI] [PubMed] [Google Scholar]
- 183.Levi S. Girelli D. Perrone F. Pasti M. Beaumont C. Corrocher R. Albertini A. Arosio P. Analysis of ferritins in lymphoblastoid cell lines and in the lens of subjects with hereditary hyperferritinemia-cataract syndrome. Blood. 1998;91:4180–4187. [PubMed] [Google Scholar]
- 184.Levy JE. Jin O. Fujiwara Y. Kuo F. Andrews NC. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet. 1999;21:396–399. doi: 10.1038/7727. [DOI] [PubMed] [Google Scholar]
- 185.Li Y. Jaiswal AK. Regulation of human NAD(P)H:quinone oxidoreductase gene. Role of AP1 binding site contained within human antioxidant response element. J Biol Chem. 1992;267:15097–15104. [PubMed] [Google Scholar]
- 186.Liao QK. Kong PA. Gao J. Li FY. Qian ZM. Expression of ferritin receptor in placental microvilli membrane in pregnant women with different iron status at mid-term gestation. Eur J Clin Nutr. 2001;55:651–656. doi: 10.1038/sj.ejcn.1601195. [DOI] [PubMed] [Google Scholar]
- 187.Lill R. Muhlenhoff U. Iron-sulfur protein biogenesis in eukaryotes: components and mechanisms. Annu Rev Cell Dev Biol. 2006;22:457–486. doi: 10.1146/annurev.cellbio.22.010305.104538. [DOI] [PubMed] [Google Scholar]
- 188.Liuzzi JP. Aydemir F. Nam H. Knutson MD. Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci U S A. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lodi R. Cooper JM. Bradley JL. Manners D. Styles P. Taylor DJ. Schapira AH. Deficit of in vivo mitochondrial ATP production in patients with Friedreich ataxia. Proc Natl Acad Sci U S A. 1999;96:11492–11495. doi: 10.1073/pnas.96.20.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lok CN. Ponka P. Identification of a hypoxia response element in the transferrin receptor gene. J Biol Chem. 1999;274:24147–24152. doi: 10.1074/jbc.274.34.24147. [DOI] [PubMed] [Google Scholar]
- 191.Lu C. Cortopassi G. Frataxin knockdown causes loss of cytoplasmic iron-sulfur cluster functions, redox alterations and induction of heme transcripts. Arch Biochem Biophys. 2007;457:111–122. doi: 10.1016/j.abb.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mack U. Powell LW. Halliday JW. Detection and isolation of a hepatic membrane receptor for ferritin. J Biol Chem. 1983;258:4672–4675. [PubMed] [Google Scholar]
- 193.Mariappan SV. Catasti P. Silks LA., 3rd Bradbury EM. Gupta G. The high-resolution structure of the triplex formed by the GAA/TTC triplet repeat associated with Friedreich's ataxia. J Mol Biol. 1999;285:2035–2052. doi: 10.1006/jmbi.1998.2435. [DOI] [PubMed] [Google Scholar]
- 194.Marziali G. Perrotti E. Ilari R. Testa U. Coccia EM. Battistini A. Transcriptional regulation of the ferritin heavy-chain gene: the activity of the CCAAT binding factor NF-Y is modulated in heme-treated Friend leukemia cells and during monocyte-to-macrophage differentiation. Mol Cell Biol. 1997;17:1387–1395. doi: 10.1128/mcb.17.3.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mayr B. Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 196.McIntire JJ. Umetsu SE. Akbari O. Potter M. Kuchroo VK. Barsh GS. Freeman GJ. Umetsu DT. DeKruyff RH. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol. 2001;2:1109–1116. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 197.McKie AT. Barrow D. Latunde-Dada GO. Rolfs A. Sager G. Mudaly E. Mudaly M. Richardson C. Barlow D. Bomford A. Peters TJ. Raja KB. Shirali S. Hediger MA. Farzaneh F. Simpson RJ. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291:1755–1759. doi: 10.1126/science.1057206. [DOI] [PubMed] [Google Scholar]
- 198.McKie AT. Marciani P. Rolfs A. Brennan K. Wehr K. Barrow D. Miret S. Bomford A. Peters TJ. Farzaneh F. Hediger MA. Hentze MW. Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 199.Mehlhase J. Sandig G. Pantopoulos K. Grune T. Oxidation-induced ferritin turnover in microglial cells: role of proteasome. Free Radic Biol Med. 2005;38:276–285. doi: 10.1016/j.freeradbiomed.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 200.Meyron-Holtz EG. Ghosh MC. Iwai K. LaVaute T. Brazzolotto X. Berger UV. Land W. Ollivierre-Wilson H. Grinberg A. Love P. Rouault TA. Genetic ablations of iron regulatory proteins 1 and 2 reveal why iron regulatory protein 2 dominates iron homeostasis. EMBO J. 2004;23:386–395. doi: 10.1038/sj.emboj.7600041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Millholland JM. Fitch JM. Cai CX. Gibney EP. Beazley KE. Linsenmayer TF. Ferritoid, a tissue-specific nuclear transport protein for ferritin in corneal epithelial cells. J Biol Chem. 2003;278:23963–23970. doi: 10.1074/jbc.M210050200. [DOI] [PubMed] [Google Scholar]
- 202.Miranda CJ. Santos MM. Ohshima K. Smith J. Li L. Bunting M. Cossee M. Koenig M. Sequeiros J. Kaplan J. Pandolfo M. Frataxin knockin mouse. FEBS Lett. 2002;512:291–297. doi: 10.1016/s0014-5793(02)02251-2. [DOI] [PubMed] [Google Scholar]
- 203.Miranda CJ. Santos MM. Ohshima K. Tessaro M. Sequeiros J. Pandolfo M. Frataxin overexpressing mice. FEBS Lett. 2004;572:281–288. doi: 10.1016/j.febslet.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 204.Missirlis F. Holmberg S. Georgieva T. Dunkov BC. Rouault TA. Law JH. Characterization of mitochondrial ferritin in Drosophila. Proc Natl Acad Sci U S A. 2006;103:5893–5898. doi: 10.1073/pnas.0601471103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Montosi G. Donovan A. Totaro A. Garuti C. Pignatti E. Cassanelli S. Trenor CC. Gasparini P. Andrews NC. Pietrangelo A. Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J Clin Invest. 2001;108:619–623. doi: 10.1172/JCI13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Moos T. Morgan EH. The metabolism of neuronal iron and its pathogenic role in neurological disease: review. Ann N Y Acad Sci. 2004;1012:14–26. doi: 10.1196/annals.1306.002. [DOI] [PubMed] [Google Scholar]
- 207.Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 208.Moss D. Fargion S. Fracanzani AL. Levi S. Cappellini MD. Arosio P. Powell LW. Halliday JW. Functional roles of the ferritin receptors of human liver, hepatoma, lymphoid and erythroid cells. J Inorg Biochem. 1992;47:219–227. doi: 10.1016/0162-0134(92)84067-w. [DOI] [PubMed] [Google Scholar]
- 209.Muhlenhoff U. Richhardt N. Ristow M. Kispal G. Lill R. The yeast frataxin homolog Yfh1p plays a specific role in the maturation of cellular Fe/S proteins. Hum Mol Genet. 2002;11:2025–2036. doi: 10.1093/hmg/11.17.2025. [DOI] [PubMed] [Google Scholar]
- 210.Muir WA. McLaren GD. Braun W. Askari A. Evidence for heterogeneity in hereditary hemochromatosis: evaluation of 174 persons in nine families. Am J Med. 1984;76:806–814. doi: 10.1016/0002-9343(84)90991-4. [DOI] [PubMed] [Google Scholar]
- 211.Mullner EW. Kuhn LC. A stem-loop in the 3’ untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell. 1988;53:815–825. doi: 10.1016/0092-8674(88)90098-0. [DOI] [PubMed] [Google Scholar]
- 212.Musco G. Stier G. Kolmerer B. Adinolfi S. Martin S. Frenkiel T. Gibson T. Pastore A. Towards a structural understanding of Friedreich's ataxia: the solution structure of frataxin. Structure. 2000;8:695–707. doi: 10.1016/s0969-2126(00)00158-1. [DOI] [PubMed] [Google Scholar]
- 213.Nemeth E. Preza GC. Jung CL. Kaplan J. Waring AJ. Ganz T. The N-terminus of hepcidin is essential for its interaction with ferroportin: structure-function study. Blood. 2006;107:328–333. doi: 10.1182/blood-2005-05-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nemeth E. Rivera S. Gabayan V. Keller C. Taudorf S. Pedersen BK. Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nemeth E. Tuttle MS. Powelson J. Vaughn MB. Donovan A. Ward DM. Ganz T. Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 215a.Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, and Vaulont S. Proc Natl Acad Sci USA. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nicolas G. Chauvet C. Viatte L. Danan JL. Bigard X. Devaux I. Beaumont C. Kahn A. Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Niederkofler V. Salie R. Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest. 2005;115:2180–2186. doi: 10.1172/JCI25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Njajou OT. Vaessen N. Joosse M. Berghuis B. van Dongen JW. Breuning MH. Snijders PJ. Rutten WP. Sandkuijl LA. Oostra BA. van Duijn CM. Heutink P. A mutation in SLC11A3 is associated with autosomal dominant hemochromatosis. Nat Genet. 2001;28:213–214. doi: 10.1038/90038. [DOI] [PubMed] [Google Scholar]
- 219.Nohe A. Keating E. Knaus P. Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16:291–299. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 220.Oakley AE. Collingwood JF. Dobson J. Love G. Perrott HR. Edwardson JA. Elstner M. Morris CM. Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurology. 2007;68:1820–1825. doi: 10.1212/01.wnl.0000262033.01945.9a. [DOI] [PubMed] [Google Scholar]
- 221.Ohgami RS. Campagna DR. Antiochos B. Wood EB. Sharp JJ. Barker JE. Fleming MD. nm1054: a spontaneous, recessive, hypochromic, microcytic anemia mutation in the mouse. Blood. 2005;106:3625–3631. doi: 10.1182/blood-2005-01-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ohgami RS. Campagna DR. Greer EL. Antiochos B. McDonald A. Chen J. Sharp JJ. Fujiwara Y. Barker JE. Fleming MD. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ohshima K. Montermini L. Wells RD. Pandolfo M. Inhibitory effects of expanded GAA.TTC triplet repeats from intron I of the Friedreich ataxia gene on transcription and replication in vivo. J Biol Chem. 1998;273:14588–14595. doi: 10.1074/jbc.273.23.14588. [DOI] [PubMed] [Google Scholar]
- 224.Oktay Y. Dioum E. Matsuzaki S. Ding K. Yan LJ. Haller RG. Szweda LI. Garcia JA. Hypoxia-inducible factor 2alpha regulates expression of the mitochondrial aconitase chaperone protein frataxin. J Biol Chem. 2007;282:11750–11756. doi: 10.1074/jbc.M611133200. [DOI] [PubMed] [Google Scholar]
- 225.Okuda A. Imagawa M. Maeda Y. Sakai M. Muramatsu M. Structural and functional analysis of an enhancer GPEI having a phorbol 12-O-tetradecanoate 13-acetate responsive element-like sequence found in the rat glutathione transferase P gene. J Biol Chem. 1989;264:16919–16926. [PubMed] [Google Scholar]
- 226.Ollinger K. Roberg K. Nutrient deprivation of cultured rat hepatocytes increases the desferrioxamine-available iron pool and augments the sensitivity to hydrogen peroxide. J Biol Chem. 1997;272:23707–23711. doi: 10.1074/jbc.272.38.23707. [DOI] [PubMed] [Google Scholar]
- 227.Orino K. Lehman L. Tsuji Y. Ayaki H. Torti SV. Torti FM. Ferritin and the response to oxidative stress. Biochem J. 2001;357:241–247. doi: 10.1042/0264-6021:3570241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Osaki S. Kinetic studies of ferrous ion oxidation with crystalline human ferroxidase (ceruloplasmin) J Biol Chem. 1966;241:5053–5059. [PubMed] [Google Scholar]
- 229.Papanikolaou G. Pantopoulos K. Iron metabolism and toxicity. Toxicol Appl Pharmacol. 2005;202:199–211. doi: 10.1016/j.taap.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 230.Papanikolaou G. Samuels ME. Ludwig EH. MacDonald ML. Franchini PL. Dube MP. Andres L. MacFarlane J. Sakellaropoulos N. Politou M. Nemeth E. Thompson J. Risler JK. Zaborowska C. Babakaiff R. Radomski CC. Pape TD. Davidas O. Christakis J. Brissot P. Lockitch G. Ganz T. Hayden MR. Goldberg YP. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. 2004;36:77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 231.Paradkar PN. Roth JA. Post-translational and transcriptional regulation of DMT1 during P19 embryonic carcinoma cell differentiation by retinoic acid. Biochem J. 2006;394:173–183. doi: 10.1042/BJ20051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Park CH. Valore EV. Waring AJ. Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 233.Park S. Gakh O. Mooney SM. Isaya G. The ferroxidase activity of yeast frataxin. J Biol Chem. 2002;277:38589–38595. doi: 10.1074/jbc.M206711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Park S. Gakh O. O'Neill HA. Mangravita A. Nichol H. Ferreira GC. Isaya G. Yeast frataxin sequentially chaperones and stores iron by coupling protein assembly with iron oxidation. J Biol Chem. 2003;278:31340–31351. doi: 10.1074/jbc.M303158200. [DOI] [PubMed] [Google Scholar]
- 235.Parkkila S. Waheed A. Britton RS. Bacon BR. Zhou XY. Tomatsu S. Fleming RE. Sly WS. Association of the transferrin receptor in human placenta with HFE, the protein defective in hereditary hemochromatosis. Proc Natl Acad Sci U S A. 1997;94:13198–13202. doi: 10.1073/pnas.94.24.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pastore A. Tozzi G. Gaeta LM. Bertini E. Serafini V. Di Cesare S. Bonetto V. Casoni F. Carrozzo R. Federici G. Piemonte F. Actin glutathionylation increases in fibroblasts of patients with Friedreich's ataxia: a potential role in the pathogenesis of the disease. J Biol Chem. 2003;278:42588–42595. doi: 10.1074/jbc.M301872200. [DOI] [PubMed] [Google Scholar]
- 237.Patel BN. David S. A novel glycosylphosphatidylinositol-an-chored form of ceruloplasmin is expressed by mammalian astrocytes. J Biol Chem. 1997;272:20185–20190. doi: 10.1074/jbc.272.32.20185. [DOI] [PubMed] [Google Scholar]
- 238.Patel BN. Dunn RJ. David S. Alternative RNA splicing generates a glycosylphosphatidylinositol-anchored form of ceruloplasmin in mammalian brain. J Biol Chem. 2000;275:4305–4310. doi: 10.1074/jbc.275.6.4305. [DOI] [PubMed] [Google Scholar]
- 239.Patel BN. Dunn RJ. Jeong SY. Zhu Q. Julien JP. David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J Neurosci. 2002;22:6578–6586. doi: 10.1523/JNEUROSCI.22-15-06578.2002. , [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Perkins ND. Integrating cell-signalling pathways with NF-kap-paB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 241.Perry G. Cash AD. Smith MA. Alzheimer disease and oxidative stress. J Biomed Biotechnol. 2002;2:120–123. doi: 10.1155/S1110724302203010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Peyssonnaux C. Zinkernagel AS. Schuepbach RA. Rankin E. Vaulont S. Haase VH. Nizet V. Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Pham CG. Bubici C. Zazzeroni F. Papa S. Jones J. Alvarez K. Jayawardena S. De Smaele E. Cong R. Beaumont C. Torti FM. Torti SV. Franzoso G. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 244.Pietrangelo A. Hereditary hemochromatosis: a new look at an old disease. N Engl J Med. 2004;350:2383–2397. doi: 10.1056/NEJMra031573. [DOI] [PubMed] [Google Scholar]
- 245.Pietrangelo A. Dierssen U. Valli L. Garuti C. Rump A. Corradini E. Ernst M. Klein C. Trautwein C. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132:294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 246.Pietsch EC. Chan JY. Torti FM. Torti SV. Nrf2 mediates the induction of ferritin H in response to xenobiotics and cancer chemopreventive dithiolethiones. J Biol Chem. 2003;278:2361–2369. doi: 10.1074/jbc.M210664200. [DOI] [PubMed] [Google Scholar]
- 247.Pigeon C. Ilyin G. Courselaud B. Leroyer P. Turlin B. Brissot P. Loreal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 248.Pilz RB. Impaired erythroid-specific gene expression in cAMP-dependent protein kinase-deficient murine erythroleukemia cells. J Biol Chem. 1993;268:20252–20258. [PubMed] [Google Scholar]
- 249.Ponka P. Beaumont C. Richardson DR. Function and regulation of transferrin and ferritin. Semin Hematol. 1998;35:35–54. [PubMed] [Google Scholar]
- 250.Prieto J. Barry M. Sherlock S. Serum ferritin in patients with iron overload and with acute and chronic liver diseases. Gastroenterology. 1975;68:525–533. [PubMed] [Google Scholar]
- 251.Puccio H. Simon D. Cossee M. Criqui-Filipe P. Tiziano F. Melki J. Hindelang C. Matyas R. Rustin P. Koenig M. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat Genet. 2001;27:181–186. doi: 10.1038/84818. [DOI] [PubMed] [Google Scholar]
- 252.Radisky DC. Babcock MC. Kaplan J. The yeast frataxin homologue mediates mitochondrial iron efflux: evidence for a mitochondrial iron cycle. J Biol Chem. 1999;274:4497–4499. doi: 10.1074/jbc.274.8.4497. [DOI] [PubMed] [Google Scholar]
- 253.Rangasamy T. Cho CY. Thimmulappa RK. Zhen L. Srisuma SS. Kensler TW. Yamamoto M. Petrache I. Tuder RM. Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Rangasamy T. Guo J. Mitzner WA. Roman J. Singh A. Fryer AD. Yamamoto M. Kensler TW. Tuder RM. Georas SN. Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Reinheckel T. Sitte N. Ullrich O. Kuckelkorn U. Davies KJ. Grune T. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem J. 1998;335:637–642. doi: 10.1042/bj3350637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Rennert PD. Ichimura T. Sizing ID. Bailly V. Li Z. Rennard R. McCoon P. Pablo L. Miklasz S. Tarilonte L. Bonventre JV. T cell, Ig domain, mucin domain-2 gene-deficient mice reveal a novel mechanism for the regulation of Th2 immune responses and airway inflammation. J Immunol. 2006;177:4311–4321. doi: 10.4049/jimmunol.177.7.4311. [DOI] [PubMed] [Google Scholar]
- 257.Richardson DR. Mouralian C. Ponka P. Becker E. Development of potential iron chelators for the treatment of Friedreich's ataxia: ligands that mobilize mitochondrial iron. Biochim Biophys Acta. 2001;1536:133–140. doi: 10.1016/s0925-4439(01)00041-2. [DOI] [PubMed] [Google Scholar]
- 258.Rivera S. Nemeth E. Gabayan V. Lopez MA. Farshidi D. Ganz T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. 2005;106:2196–2199. doi: 10.1182/blood-2005-04-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Roetto A. Papanikolaou G. Politou M. Alberti F. Girelli D. Christakis J. Loukopoulos D. Camaschella C. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33:21–22. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 260.Rogers JT. Randall JD. Cahill CM. Eder PS. Huang X. Gunshin H. Leiter L. McPhee J. Sarang SS. Utsuki T. Greig NH. Lahiri DK. Tanzi RE. Bush AI. Giordano T. Gullans SR. An iron-responsive element type II in the 5′-untranslated region of the Alzheimer's amyloid precursor protein transcript. J Biol Chem. 2002;277:45518–45528. doi: 10.1074/jbc.M207435200. [DOI] [PubMed] [Google Scholar]
- 261.Rotig A. de Lonlay P. Chretien D. Foury F. Koenig M. Sidi D. Munnich A. Rustin P. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Genet. 1997;17:215–217. doi: 10.1038/ng1097-215. [DOI] [PubMed] [Google Scholar]
- 262.Rouault TA. Hentze MW. Caughman SW. Harford JB. Klausner RD. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988;241:1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- 263.Rouault TA. Stout CD. Kaptain S. Harford JB. Klausner RD. Structural relationship between an iron-regulated RNA-binding protein (IRE-BP) and aconitase: functional implications. Cell. 1991;64:881–883. doi: 10.1016/0092-8674(91)90312-m. [DOI] [PubMed] [Google Scholar]
- 264.Rubinsztein DC. Gestwicki JE. Murphy LO. Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 265.Rushmore TH. Morton MR. Pickett CB. The antioxidant responsive element: activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 266.Ryu EJ. Harding HP. Angelastro JM. Vitolo OV. Ron D. Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson's disease. J Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Sakamoto N. Chastain PD. Parniewski P. Ohshima K. Pandolfo M. Griffith JD. Wells RD. Sticky DNA: self-association properties of long GAA.TTC repeats in R.R.Y triplex structures from Friedreich's ataxia. Mol Cell. 1999;3:465–475. doi: 10.1016/s1097-2765(00)80474-8. [DOI] [PubMed] [Google Scholar]
- 268.Sakamoto N. Ohshima K. Montermini L. Pandolfo M. Wells RD. Sticky DNA, a self-associated complex formed at long GAA*TTC repeats in intron 1 of the frataxin gene, inhibits transcription. J Biol Chem. 2001;276:27171–27177. doi: 10.1074/jbc.M101879200. [DOI] [PubMed] [Google Scholar]
- 269.Schalinske KL. Eisenstein RS. Phosphorylation and activation of both iron regulatory proteins 1 and 2 in HL-60 cells. J Biol Chem. 1996;271:7168–7176. doi: 10.1074/jbc.271.12.7168. [DOI] [PubMed] [Google Scholar]
- 270.Schoenfeld RA. Napoli E. Wong A. Zhan S. Reutenauer L. Morin D. Buckpitt AR. Taroni F. Lonnerdal B. Ristow M. Puccio H. Cortopassi GA. Frataxin deficiency alters heme pathway transcripts and decreases mitochondrial heme metabolites in mammalian cells. Hum Mol Genet. 2005;14:3787–3799. doi: 10.1093/hmg/ddi393. [DOI] [PubMed] [Google Scholar]
- 271.Schranzhofer M. Schifrer M. Cabrera JA. Kopp S. Chiba P. Beug H. Mullner EW. Remodeling the regulation of iron metabolism during erythroid differentiation to ensure efficient heme biosynthesis. Blood. 2006;107:4159–4167. doi: 10.1182/blood-2005-05-1809. [DOI] [PubMed] [Google Scholar]
- 272.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 273.Seznec H. Simon D. Bouton C. Reutenauer L. Hertzog A. Golik P. Procaccio V. Patel M. Drapier JC. Koenig M. Puccio H. Friedreich ataxia: the oxidative stress paradox. Hum Mol Genet. 2005;14:463–474. doi: 10.1093/hmg/ddi042. [DOI] [PubMed] [Google Scholar]
- 274.Seznec H. Simon D. Monassier L. Criqui-Filipe P. Gansmuller A. Rustin P. Koenig M. Puccio H. Idebenone delays the onset of cardiac functional alteration without correction of Fe-S enzymes deficit in a mouse model for Friedreich ataxia. Hum Mol Genet. 2004;13:1017–1024. doi: 10.1093/hmg/ddh114. [DOI] [PubMed] [Google Scholar]
- 275.Shalitin S. Carmi D. Weintrob N. Phillip M. Miskin H. Kornreich L. Zilber R. Yaniv I. Tamary H. Serum ferritin level as a predictor of impaired growth and puberty in thalassemia major patients. Eur J Haematol. 2005;74:93–100. doi: 10.1111/j.1600-0609.2004.00371.x. [DOI] [PubMed] [Google Scholar]
- 275a.Shan Y, Napoli E, and Cortopassi G. Hum Mol Genet. 2007;16:929–941. doi: 10.1093/hmg/ddm038. [DOI] [PubMed] [Google Scholar]
- 276.Shang T. Kotamraju S. Kalivendi SV. Hillard CJ. Kalyanaraman B. 1-Methyl-4-phenylpyridinium-induced apoptosis in cerebellar granule neurons is mediated by transferrin receptor iron-dependent depletion of tetrahydrobiopterin and neuronal nitric-oxide synthase-derived superoxide. J Biol Chem. 2004;279:19099–19112. doi: 10.1074/jbc.M400101200. [DOI] [PubMed] [Google Scholar]
- 277.Shayeghi M. Latunde-Dada GO. Oakhill JS. Laftah AH. Takeuchi K. Halliday N. Khan Y. Warley A. McCann FE. Hider RC. Frazer DM. Anderson GJ. Vulpe CD. Simpson RJ. McKie AT. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 278.Silvestri L. Pagani A. Fazi C. Gerardi G. Levi S. Arosio P. Camaschella C. Defective targeting of hemojuvelin to plasma membrane is a common pathogenetic mechanism in juvenile hemochromatosis. Blood. 2007;109:4503–4510. doi: 10.1182/blood-2006-08-041004. [DOI] [PubMed] [Google Scholar]
- 279.Simon D. Seznec H. Gansmuller A. Carelle N. Weber P. Metzger D. Rustin P. Koenig M. Puccio H. Friedreich ataxia mouse models with progressive cerebellar and sensory ataxia reveal autophagic neurodegeneration in dorsal root ganglia. J Neurosci. 2004;24:1987–1995. doi: 10.1523/JNEUROSCI.4549-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Smith MA. Wehr K. Harris PL. Siedlak SL. Connor JR. Perry G. Abnormal localization of iron regulatory protein in Alzheimer's disease. Brain Res. 1998;788:232–236. doi: 10.1016/s0006-8993(98)00002-x. [DOI] [PubMed] [Google Scholar]
- 281.Stehling O. Elsasser HP. Bruckel B. Muhlenhoff U. Lill R. Iron-sulfur protein maturation in human cells: evidence for a function of frataxin. Hum Mol Genet. 2004;13:3007–3015. doi: 10.1093/hmg/ddh324. [DOI] [PubMed] [Google Scholar]
- 282.Storch S. Kubler B. Honing S. Ackmann M. Zapf J. Blum W. Braulke T. Transferrin binds insulin-like growth factors and affects binding properties of insulin-like growth factor binding protein-3. FEBS Lett. 2001;509:395–398. doi: 10.1016/s0014-5793(01)03204-5. [DOI] [PubMed] [Google Scholar]
- 283.Sturm B. Bistrich U. Schranzhofer M. Sarsero JP. Rauen U. Scheiber-Mojdehkar B. de Groot H. Ioannou P. Petrat F. Friedreich's ataxia, no changes in mitochondrial labile iron in human lymphoblasts and fibroblasts: a decrease in antioxidative capacity? J Biol Chem. 2005;280:6701–6708. doi: 10.1074/jbc.M408717200. [DOI] [PubMed] [Google Scholar]
- 284.Sturm B. Stupphann D. Kaun C. Boesch S. Schranzhofer M. Wojta J. Goldenberg H. Scheiber-Mojdehkar B. Recombinant human erythropoietin: effects on frataxin expression in vitro. Eur J Clin Invest. 2005;35:711–717. doi: 10.1111/j.1365-2362.2005.01568.x. [DOI] [PubMed] [Google Scholar]
- 285.Surguladze N. Patton S. Cozzi A. Fried MG. Connor JR. Characterization of nuclear ferritin and mechanism of translocation. Biochem J. 2005;388:731–740. doi: 10.1042/BJ20041853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Tabuchi M. Tanaka N. Nishida-Kitayama J. Ohno H. Kishi F. Alternative splicing regulates the subcellular localization of divalent metal transporter 1 isoforms. Mol Biol Cell. 2002;13:4371–4387. doi: 10.1091/mbc.E02-03-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Tan G. Chen LS. Lonnerdal B. Gellera C. Taroni FA. Cortopassi GA. Frataxin expression rescues mitochondrial dysfunctions in FRDA cells. Hum Mol Genet. 2001;10:2099–2107. doi: 10.1093/hmg/10.19.2099. [DOI] [PubMed] [Google Scholar]
- 288.Tehranchi R. Invernizzi R. Grandien A. Zhivotovsky B. Fadeel B. Forsblom AM. Travaglino E. Samuelsson J. Hast R. Nilsson L. Cazzola M. Wibom R. Hellstrom-Lindberg E. Aberrant mitochondrial iron distribution and maturation arrest characterize early erythroid precursors in low-risk myelodysplastic syndromes. Blood. 2005;106:247–253. doi: 10.1182/blood-2004-12-4649. [DOI] [PubMed] [Google Scholar]
- 289.Thierbach R. Schulz TJ. Isken F. Voigt A. Mietzner B. Drewes G. von Kleist-Retzow JC. Wiesner RJ. Magnuson MA. Puccio H. Pfeiffer AF. Steinberg P. Ristow M. Targeted disruption of hepatic frataxin expression causes impaired mitochondrial function, decreased life span and tumor growth in mice. Hum Mol Genet. 2005;14:3857–3864. doi: 10.1093/hmg/ddi410. [DOI] [PubMed] [Google Scholar]
- 290.Thimmulappa RK. Mai KH. Srisuma S. Kensler TW. Yamamoto M. Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 291.Thompson K. Menzies S. Muckenthaler M. Torti FM. Wood T. Torti SV. Hentze MW. Beard J. Connor J. Mouse brains deficient in H-ferritin have normal iron concentration but a protein profile of iron deficiency and increased evidence of oxidative stress. J Neurosci Res. 2003;71:46–63. doi: 10.1002/jnr.10463. [DOI] [PubMed] [Google Scholar]
- 292.Thompson KJ. Fried MG. Ye Z. Boyer P. Connor JR. Regulation, mechanisms and proposed function of ferritin translocation to cell nuclei. J Cell Sci. 2002;115:2165–2177. doi: 10.1242/jcs.115.10.2165. [DOI] [PubMed] [Google Scholar]
- 293.Thorstensen K. Romslo I. The role of transferrin in the mechanism of cellular iron uptake. Biochem J. 1990;271:1–9. doi: 10.1042/bj2710001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Torti SV. Kwak EL. Miller SC. Miller LL. Ringold GM. Myambo KB. Young AP. Torti FM. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638–12644. [PubMed] [Google Scholar]
- 295.Toth I. Yuan L. Rogers JT. Boyce H. Bridges KR. Hypoxia alters iron-regulatory protein-1 binding capacity and modulates cellular iron homeostasis in human hepatoma and erythroleukemia cells. J Biol Chem. 1999;274:4467–4473. doi: 10.1074/jbc.274.7.4467. [DOI] [PubMed] [Google Scholar]
- 296.Toussaint L. Bertrand L. Hue L. Crichton RR. Declercq JP. High-resolution X-ray structures of human apoferritin H-chain mutants correlated with their activity and metal-binding sites. J Mol Biol. 2007;365:440–452. doi: 10.1016/j.jmb.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 297.Tran TN. Eubanks SK. Schaffer KJ. Zhou CY. Linder MC. Secretion of ferritin by rat hepatoma cells and its regulation by inflammatory cytokines and iron. Blood. 1997;90:4979–4986. [PubMed] [Google Scholar]
- 298.Trinder D. Baker E. Transferrin receptor 2: a new molecule in iron metabolism. Int J Biochem Cell Biol. 2003;35:292–296. doi: 10.1016/s1357-2725(02)00258-3. [DOI] [PubMed] [Google Scholar]
- 299.Trinder D. Fox C. Vautier G. Olynyk JK. Molecular pathogenesis of iron overload. Gut. 2002;51:290–295. doi: 10.1136/gut.51.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Truksa J. Peng H. Lee P. Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci U S A. 2006;103:10289–10293. doi: 10.1073/pnas.0603124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Tsuji Y. JunD activates transcription of the human ferritin H gene through an antioxidant response element during oxidative stress. Oncogene. 2005;24:7567–7578. doi: 10.1038/sj.onc.1208901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tsuji Y. Akebi N. Lam TK. Nakabeppu Y. Torti SV. Torti FM. FER-1, an enhancer of the ferritin H gene and a target of E1A-mediated transcriptional repression. Mol Cell Biol. 1995;15:5152–5164. doi: 10.1128/mcb.15.9.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Tsuji Y. Ayaki H. Whitman SP. Morrow CS. Torti SV. Torti FM. Coordinate transcriptional and translational regulation of ferritin in response to oxidative stress. Mol Cell Biol. 2000;20:5818–5827. doi: 10.1128/mcb.20.16.5818-5827.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Tsuji Y. Kwak E. Saika T. Torti SV. Torti FM. Preferential repression of the H subunit of ferritin by adenovirus E1A in NIH-3T3 mouse fibroblasts. J Biol Chem. 1993;268:7270–7275. [PubMed] [Google Scholar]
- 305.Tsuji Y. Miller LL. Miller SC. Torti SV. Torti FM. Tumor necrosis factor-alpha and interleukin 1-alpha regulate transferrin receptor in human diploid fibroblasts: relationship to the induction of ferritin heavy chain. J Biol Chem. 1991;266:7257–7261. [PubMed] [Google Scholar]
- 306.Tsuji Y. Moran E. Torti SV. Torti FM. Transcriptional regulation of the mouse ferritin H gene: involvement of p300/CBP adaptor proteins in FER-1 enhancer activity. J Biol Chem. 1999;274:7501–7507. doi: 10.1074/jbc.274.11.7501. [DOI] [PubMed] [Google Scholar]
- 307.Tsuji Y. Torti SV. Torti FM. Activation of the ferritin H enhancer, FER-1, by the cooperative action of members of the AP1 and Sp1 transcription factor families. J Biol Chem. 1998;273:2984–2992. doi: 10.1074/jbc.273.5.2984. [DOI] [PubMed] [Google Scholar]
- 308.Turano M. Tammaro A. De Biase I. Lo Casale MS. Ruggiero G. Monticelli A. Cocozza S. Pianese L. 3-Nitropropionic acid increases frataxin expression in human lymphoblasts and in transgenic rat PC12 cells. Neurosci Lett. 2003;350:184–186. doi: 10.1016/s0304-3940(03)00906-6. [DOI] [PubMed] [Google Scholar]
- 309.Vahdat Shariatpanaahi M. Vahdat Shariatpanaahi Z. Moshtaaghi M. Shahbaazi SH. Abadi A. The relationship between depression and serum ferritin level. Eur J Clin Nutr. 2007;61:532–535. doi: 10.1038/sj.ejcn.1602542. [DOI] [PubMed] [Google Scholar]
- 310.Verga Falzacappa MV. Vujic Spasic M. Kessler R. Stolte J. Hentze MW. Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 311.Vidal R. Ghetti B. Takao M. Brefel-Courbon C. Uro-Coste E. Glazier BS. Siani V. Benson MD. Calvas P. Miravalle L. Rascol O. Delisle MB. Intracellular ferritin accumulation in neural and extraneural tissue characterizes a neurodegenerative disease associated with a mutation in the ferritin light polypeptide gene. J Neuropathol Exp Neurol. 2004;63:363–380. doi: 10.1093/jnen/63.4.363. [DOI] [PubMed] [Google Scholar]
- 312.Voisine C. Schilke B. Ohlson M. Beinert H. Marszalek J. Craig EA. Role of the mitochondrial Hsp70s, Ssc1 and Ssq1, in the maturation of Yfh1. Mol Cell Biol. 2000;20:3677–3684. doi: 10.1128/mcb.20.10.3677-3684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Vulpe CD. Kuo YM. Murphy TL. Cowley L. Askwith C. Libina N. Gitschier J. Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999;21:195–199. doi: 10.1038/5979. [DOI] [PubMed] [Google Scholar]
- 314.Waheed A. Grubb JH. Zhou XY. Tomatsu S. Fleming RE. Costaldi ME. Britton RS. Bacon BR. Sly WS. Regulation of transferrin-mediated iron uptake by HFE, the protein defective in hereditary hemochromatosis. Proc Natl Acad Sci U S A. 2002;99:3117–3122. doi: 10.1073/pnas.042701499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Waheed A. Parkkila S. Saarnio J. Fleming RE. Zhou XY. Tomatsu S. Britton RS. Bacon BR. Sly WS. Association of HFE protein with transferrin receptor in crypt enterocytes of human duodenum. Proc Natl Acad Sci U S A. 1999;96:1579–1584. doi: 10.1073/pnas.96.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Waheed A. Parkkila S. Zhou XY. Tomatsu S. Tsuchihashi Z. Feder JN. Schatzman RC. Britton RS. Bacon BR. Sly WS. Hereditary hemochromatosis: effects of C282Y and H63D mutations on association with beta2-microglobulin, intracellular processing, and cell surface expression of the HFE protein in COS-7 cells. Proc Natl Acad Sci U S A. 1997;94:12384–12389. doi: 10.1073/pnas.94.23.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Walden WE. Selezneva AI. Dupuy J. Volbeda A. Fontecilla-Camps JC. Theil EC. Volz K. Structure of dual function iron regulatory protein 1 complexed with ferritin IRE-RNA. Science. 2006;314:1903–1908. doi: 10.1126/science.1133116. [DOI] [PubMed] [Google Scholar]
- 317a.Wallace DF. Summerville L. Lusby PE. Subramaniam VN. Gut. 2005;54:980–986. doi: 10.1136/gut.2004.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Weinzimer SA. Gibson TB. Collett-Solberg PF. Khare A. Liu B. Cohen P. Transferrin is an insulin-like growth factor-binding protein-3 binding protein. J Clin Endocrinol Metab. 2001;86:1806–1813. doi: 10.1210/jcem.86.4.7380. [DOI] [PubMed] [Google Scholar]
- 319.Wenger RH. Stiehl DP. Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 320.West AP Jr. Bennett MJ. Sellers VM. Andrews NC. Enns CA. Bjorkman PJ. Comparison of the interactions of transferrin receptor and transferrin receptor 2 with transferrin and the hereditary hemochromatosis protein HFE. J Biol Chem. 2000;275:38135–38138. doi: 10.1074/jbc.C000664200. [DOI] [PubMed] [Google Scholar]
- 321.Wong A. Yang J. Cavadini P. Gellera C. Lonnerdal B. Taroni F. Cortopassi G. The Friedreich's ataxia mutation confers cellular sensitivity to oxidant stress which is rescued by chelators of iron and calcium and inhibitors of apoptosis. Hum Mol Genet. 1999;8:425–430. doi: 10.1093/hmg/8.3.425. [DOI] [PubMed] [Google Scholar]
- 322.Worwood M. Brook JD. Cragg SJ. Hellkuhl B. Jones BM. Perera P. Roberts SH. Shaw DJ. Assignment of human ferritin genes to chromosomes 11 and 19q13.3–––19qter. Hum Genet. 1985;69:371–374. doi: 10.1007/BF00291657. [DOI] [PubMed] [Google Scholar]
- 323.Worwood M. Dawkins S. Wagstaff M. Jacobs A. The purification and properties of ferritin from human serum. Biochem J. 1976;157:97–103. doi: 10.1042/bj1570097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Wrighting DM. Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Xanthoudakis S. Curran T. Redox regulation of AP-1: a link between transcription factor signaling and DNA repair. Adv Exp Med Biol. 1996;387:69–75. [PubMed] [Google Scholar]
- 326.Xu X. Pin S. Gathinji M. Fuchs R. Harris ZL. Aceruloplasminemia: an inherited neurodegenerative disease with impairment of iron homeostasis. Ann N Y Acad Sci. 2004;1012:299–305. doi: 10.1196/annals.1306.024. [DOI] [PubMed] [Google Scholar]
- 327.Yamamuro A. Yoshioka Y. Ogita K. Maeda S. Involvement of endoplasmic reticulum stress on the cell death induced by 6-hydroxydopamine in human neuroblastoma SH-SY5Y cells. Neurochem Res. 2006;31:657–664. doi: 10.1007/s11064-006-9062-6. [DOI] [PubMed] [Google Scholar]
- 328.Yamanaka K. Ishikawa H. Megumi Y. Tokunaga F. Kanie M. Rouault TA. Morishima I. Minato N. Ishimori K. Iwai K. Identification of the ubiquitin-protein ligase that recognizes oxidized IRP2. Nat Cell Biol. 2003;5:336–340. doi: 10.1038/ncb952. [DOI] [PubMed] [Google Scholar]
- 329.Yang F. Liu XB. Quinones M. Melby PC. Ghio A. Haile DJ. Regulation of reticuloendothelial iron transporter MTP1 (Slc11a3) by inflammation. J Biol Chem. 2002;277:39786–39791. doi: 10.1074/jbc.M201485200. [DOI] [PubMed] [Google Scholar]
- 330.Yang F. Lum JB. McGill JR. Moore CM. Naylor SL. van Bragt PH. Baldwin WD. Bowman BH. Human transferrin: cDNA characterization and chromosomal localization. Proc Natl Acad Sci U S A. 1984;81:2752–2756. doi: 10.1073/pnas.81.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Yoon T. Cowan JA. Frataxin-mediated iron delivery to ferrochelatase in the final step of heme biosynthesis. J Biol Chem. 2004;279:25943–25946. doi: 10.1074/jbc.C400107200. [DOI] [PubMed] [Google Scholar]
- 332.Zancani M. Peresson C. Biroccio A. Federici G. Urbani A. Murgia I. Soave C. Micali F. Vianello A. Macri F. Evidence for the presence of ferritin in plant mitochondria. Eur J Biochem. 2004;271:3657–3664. doi: 10.1111/j.1432-1033.2004.04300.x. [DOI] [PubMed] [Google Scholar]
- 333.Zecca L. Stroppolo A. Gatti A. Tampellini D. Toscani M. Gallorini M. Giaveri G. Arosio P. Santambrogio P. Fariello RG. Karatekin E. Kleinman MH. Turro N. Hornykiewicz O. Zucca FA. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc Natl Acad Sci U S A. 2004;101:9843–9848. doi: 10.1073/pnas.0403495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Zecca L. Youdim MB. Riederer P. Connor JR. Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 335.Zhang Y. Lyver ER. Knight SA. Lesuisse E. Dancis A. Frataxin and mitochondrial carrier proteins, Mrs3p and Mrs4p, cooperate in providing iron for heme synthesis. J Biol Chem. 2005;280:19794–19807. doi: 10.1074/jbc.M500397200. [DOI] [PubMed] [Google Scholar]
- 336.Zhou XY. Tomatsu S. Fleming RE. Parkkila S. Waheed A. Jiang J. Fei Y. Brunt EM. Ruddy DA. Prass CE. Schatzman RC. O'Neill R. Britton RS. Bacon BR. Sly WS. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proc Natl Acad Sci U S A. 1998;95:2492–2497. doi: 10.1073/pnas.95.5.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]


