Abstract
Mammalian airways are protected from infection by a thin film of airway surface liquid (ASL) which covers airway epithelial surfaces and acts as a lubricant to keep mucus from adhering to the epithelial surface. Precise regulation of ASL volume is essential for efficient mucus clearance and too great a reduction in ASL volume causes mucus dehydration and mucus stasis which contributes to chronic airway infection. The epithelial Na+ channel (ENaC) is the rate-limiting step that governs Na+ absorption in the airways. Recent in vitro and in vivo data have demonstrated that ENaC is a critical determinant of ASL volume and hence mucus clearance. ENaC must be cleaved by either intracellular furin-type proteases or extracellular serine proteases to be active and conduct Na+, and this process can be inhibited by protease inhibitors. ENaC can be regulated by multiple pathways, and once proteolytically cleaved ENaC may then be inhibited by intracellular second messengers such as cAMP and PIP2. In the airways, however, regulation of ENaC by proteases seems to be the predominant mode of regulation since knockdown of either endogenous serine proteases such as prostasin, or inhibitors of ENaC proteolysis such as SPLUNC1, has large effects on ENaC activity in airway epithelia. In this review, we shall discuss how ENaC is proteolytically cleaved, how this process can regulate ASL volume, and how its failure to operate correctly may contribute to chronic airway disease.
Keywords: Na+ channel, Cystic fibrosis, Epithelial transport, Fluid absorption, Lung liquid
Introduction
The epithelial Na+ channel (ENaC) is expressed in the apical plasma membrane of several tissues including the colon, kidney, and lung and constitutes the rate-limiting step for Na+ absorption [84, 122]. ENaC in the airways is distinguished from ENaC expressed in other tissues by insensitivity to aldosterone [136]. Instead, ENaC in airway epithelia is regulated by glucocorticoids and so its expression is linked to changes in inflammation rather than changes in blood pressure/volume [136, 151]. ENaC can also be controlled by intracellular factors including pH, lipids (PIP2 and PIP3), and protein kinase A [91, 112, 166] which may affect the number of ENaCs inserted into the plasma membrane as well as affect gating [91, 156, 166]. The conducting airways are the primary interface between the extracellular environment and the lung and play a major role in host defense [9, 34, 48]. Mucus clearance is a central component of the innate defense system of the lung [35, 76]. During normal mucus clearance, inhaled pathogens and particles become trapped in the mucus layer and are then expelled before they can colonize the airways [30]. The mucus layer is of variable height, and mucus is kept away from the predominantly ciliated airway epithelia by the presence of a ~7 µm periciliary liquid layer which, as the name suggests, surrounds the beating cilia and acts as a lubricant to keep mucus away from the epithelial cell surface. Together, these layers make up the airway surface liquid (ASL) [14]. The rate of mucociliary clearance is strongly influenced by the hydration state of the ASL and mucus layers [142]. Mucus hydration is set by the volume of liquid present on airway surfaces, which in turn may be modified by active ion transport processes in both the superficial epithelium and glands [7, 14, 157, 159]. Secretion is mediated by the apical membrane Cl− channels TMEM16A and CFTR [3, 47] whilst absorption requires ENaC [123]. Intracellular second messengers play a large role in regulating these channels [3, 47, 63, 85, 90, 112]. However, in the last decade, soluble and epithelial membrane bound proteases have been found to regulate ENaC, which has opened up a whole new avenue of research [122, 123]. Channel-activating protease 1 (CAP1; prostasin) was first identified in Xenopus laevis oocytes [150] and, subsequently, CAP2 (transmembrane protease serine 4 [TMPRSS4]), CAP3 (matriptase or epithin), and human neutrophil elastase [22] as well as trypsin [150] were all found to activate ENaC. This review will focus on recent discoveries regarding the ability of these proteases and their inhibitors to affect ASL volume by varying the ENaC activity and on their potential clinical significance.
Structure of ENaC and non-proteolytic regulation
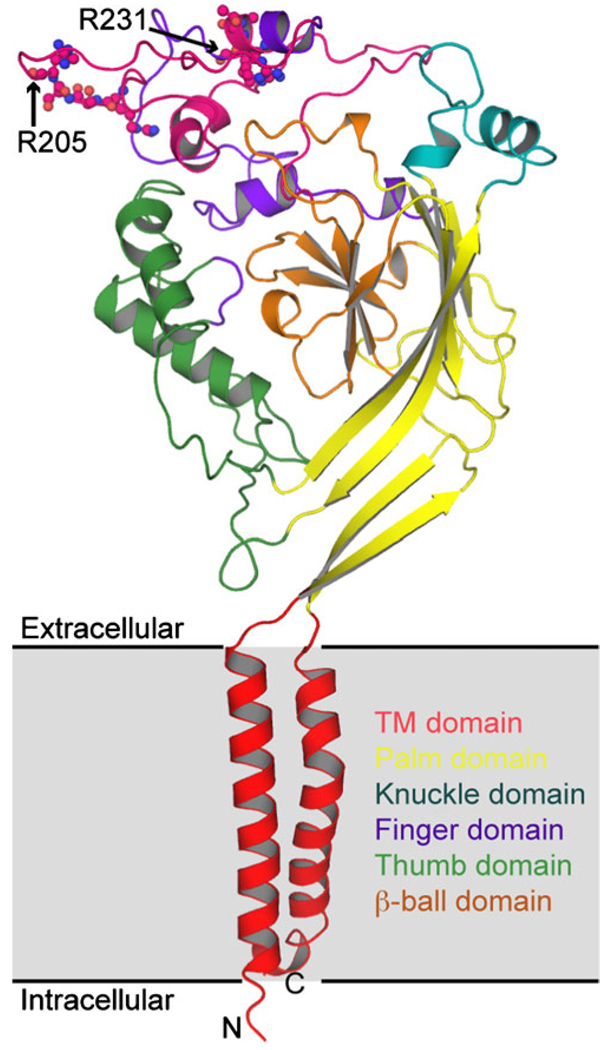
ENaC consists of three separate subunits termed α, β, and γ that are ~30% homologous to each other. These subunits form a channel that is highly selective for Na+ over K+ and is characterized by sensitivity to amiloride in the submicromolar range [122, 123]. Each subunit is composed of a large extracellular loop, which has been likened to a hand (Fig. 1). In addition, ENaC also has two transmembrane domains termed M1 and M2, respectively, and two short intracellular tails (Fig. 1). Site-directed mutagenesis has revealed that residues in the pre-M2 region of α, β, and γENaC subunits affect the channel conductance and amiloride binding [126]. Based on the recent crystal structure of the related acid-sensing channel (ASIC), the issue of ENaC subunit stoichiometry appears to be settled with ENaC presumably consisting of a 1α:1β:1γ complex [69]. However, ASIC is a homotrimer, and previous functional studies with ENaC subunits have suggested a heterotetrameric or even variable stoichiometry [2, 41, 80, 133]. When the αENaC subunit is injected into Xenopus oocytes alone, ~2% of current is seen relative to α, β, γENaC co-expression. In contrast, when the α, β or α, γ subunit combinations are co-injected, up to 15% of full activity is seen. The β or γ subunits do not produce currents when expressed alone and β, γ together induce ~2% of full activity, but only at several days post-injection [12]. Mueller et al. have reported that the C-terminus of αENaC plays a critical role in permitting exit of this channel from the endoplasmic reticulum [97]. Thus, αENaC may have an essential role in bringing the β and γ subunits to the plasma membrane, while the β and γ subunits are essential for stabilization of the multimer in the plasma membrane.
Fig. 1.
Cartoon representation of the structural model of the rat αENaC subunit. Each ENaC subunit has two transmembrane regions, a large extracellular loop and a small “Pre-TM2” region (not represented in the model) that may form part of the selectivity filter [126]. For all the subunits, the C- and N-termini are intracellular. The sequence alignment for modeling the structure of rat αENaC was generated by extending the one provided by Stockand et al. [135]. A portion of the ‘hypervariable region’ was included in our alignment to account for the furin sites in this model [135]. The structural model was generated using Medusa [42, 163] with the crystal structure of cASIC as the template [69]. Regions that do not have a template were modeled as loops using Modeller [46]
In addition to the well-characterized α, β, and γ subunits, a fourth ENaC subunit, termed δ, has been described [18, 155]. δENaC is highly expressed in the testis, ovary, pancreas, and brain and is also expressed in the nasal epithelium. The δENaC subunit shares considerable sequence similarity with the α subunit and forms functional ion channels together with the β and γ subunits in pancreas, testes, ovaries, and brain and in smaller amounts in heart, placenta, lung, liver, kidney, thymus, prostate, colon, and lymphocytes [8, 18, 71, 155, 161]. In Xenopus oocytes, expression of δ, β, γENaC results in an approximately tenfold increase in amiloride-sensitive current compared to α, β, γENaC. Consequently, differential expression of the α and δ subunits may provide an additional way of regulating ENaC activity and the δ subunit seems to promote constitutive cleavage of the γ subunit, which may favor the presence of active channels at the cell surface [56]. Thus, replacement of the α subunit with the δ subunit in the heterotrimeric channel may favor the constitutive activation of ENaC and may reduce the pool of near-silent plasma membrane channels that can be activated by extracellular proteases [56].
ENaC gating kinetics are characterized by long opening and closing times, [113] and structural studies have revealed two regions that are important for gating. The HG motif, so named because it possesses a glycine and a histidine in the amino terminus, is present in every ENaC/degenerin family member known to conduct ions and is involved in channel opening. Mutation of this residue in the βENaC subunit causes pseudohypoaldosteronism, a disease characterized by inactivity of ENaC [73, 134]. The DEG gating domain in the extracellular loop, close to the M2 region, was first described in degenerins channels of Caenorhabditis elegans, which are related to ENaC, and involves a critical serine residue [126]. Mutations in this region cause an increase in the open probability [72, 129].
Regulation of ENaC by intracellular 2nd messengers
Although it is perhaps surprising that ENaC is the sole ion channel responsible for apical Na+ entry into airway epithelial cells, it is clear that this channel can undergo multiple modes of regulation. For example, in addition to being cleaved and activated by several different types of proteases, ENaC can also be regulated by intracellular second messengers. In non-cystic fibrosis (CF) human airways, ENaC is inactivated by agonists that raise cAMP, such as adenosine, forskolin, or isoproterenol [16, 75, 78]. In CF airways, which lack functional CFTR, increases in cAMP stimulate ENaC [16, 75, 78], suggesting that CFTR acts to limit ENaC activity in the airways. Using the patch clamp technique, Stutts et al. demonstrated that CFTR expression directly altered ENaC sensitivity to forskolin-induced changes in intracellular cAMP/PKA [137, 138]. Using the Xenopus oocyte expression system, it was reported that the intracellular domains of CFTR directly interacted with ENaC sufficiently to alter ENaC’s sensitivity to cAMP [70, 86, 127]. However, since CFTR and ENaC are linked to the cytoskeleton, there may also be indirect interactions between these two ion channels [10, 68, 102].
A reduction in the intracellular Cl− concentration following stimulation of either CFTR or ClC Cl− channels has also been reported to lower ENaC activity, suggesting that ENaC is regulated by the intracellular Cl− concentration [6, 70, 96]. Despite a precedence for this type of regulation of ENaC in mouse salivary duct epithelia [32], this hypothesis remains to be tested in the airways, and other investigators using Xenopus oocytes have reported that ENaC is still regulated appropriately following co-injection of αβγENaC and a non-Cl-conducting CFTR mutant [139]. The interaction between these two ion channels may be variable, and in the sweat glands, where Cl− absorption rather than Cl− secretion occurs under basal conditions, ENaC is activated in parallel with CFTR rather than being inactivated [115]. Surprisingly, more than 20 years after the initial discovery that ENaC’s sensitivity to cAMP is CFTR dependent, the molecular basis underlying this phenomenon has not been fully understood.
Activation of P2Y2 receptors on the apical membrane of airway epithelial cells with ATP or UTP raises intracellular IP3 and Ca2+, in parallel with a fall in intracellular PIP2 [36]. These changes then stimulate Ca2+-mediated Cl− secretion and inhibit ENaC-mediated Na+ absorption [39, 67, 95]. The underlying Cl− channel has only recently been cloned and is likely to be TMEM16A/Ano1 [25, 60, 128, 162]. TMEM16A is sensitive to changes in intracellular Ca2+ levels [25, 128, 162]. However, ENaC is not directly Ca2+ sensitive and instead is activated by anionic lipids [91, 165]. Phosphatidylinositol 4,5-bisphosphate (PIP2), phosphatidylinositol 3,4,5-trisphosphate (PIP3), and phosphatidylserine were shown to prevent ENaC run-down in excised patches of plasma membrane [91] and PIP2 was shown to bind to all the three ENaC subunits [165], resulting in ENaC activation. Tong and Stockland demonstrated that the epidermal growth factor receptor decreases plasma membrane PIP2 levels to inhibit ENaC [148], whilst Kunzelmann et al. demonstrated that the stimulation of purinergic receptors decreases PIP2 levels to inhibit ENaC [82]. PIP2 is the substrate that is used by phospholipase C to form IP3 following stimulation of P2Y2-R [152]. In addition, numerous other regulators of ENaC exist, including, but not limited to, the epidermal growth factor receptor [148], endothelin receptors [52], epinephrine [110], as well as bacterial proteins and respiratory viruses [83, 87, 132].
Types of proteases that can cleave ENaC
In contrast to intracellular 2nd messengers, which can affect either the number of ENaC channels in the plasma membrane (n) or their open probability (Po), proteolytic cleavage of ENaC is thought to have a greater effect on Po. Three different types of proteases have been shown to cleave ENaC: (1) intracellular convertase-type proteases such as furin [64], (2) extracellular, cell-attached proteases, which can either be membrane spanning (with either the C or N terminus located intracellularly) [110] or glycosylphosphatidylinositol (GPI)-anchored [150], and (3) soluble proteases including trypsin and neutrophil elastase [22, 150]. A schema of how proteases may cleave ENaC to increase Po is shown in Fig. 2, whilst an overview of how ENaC may be cleaved by different proteases is shown in Fig. 3. Extracellular proteases may be more influenced by changes in the ASL microenvironment, especially during chronic airway disease, since the activity of these proteases can be affected by changes in ASL pH and/or the presence of extracellular protease inhibitors. However, intracellular proteases may also be affected by such changes as the lack of CFTR [103].
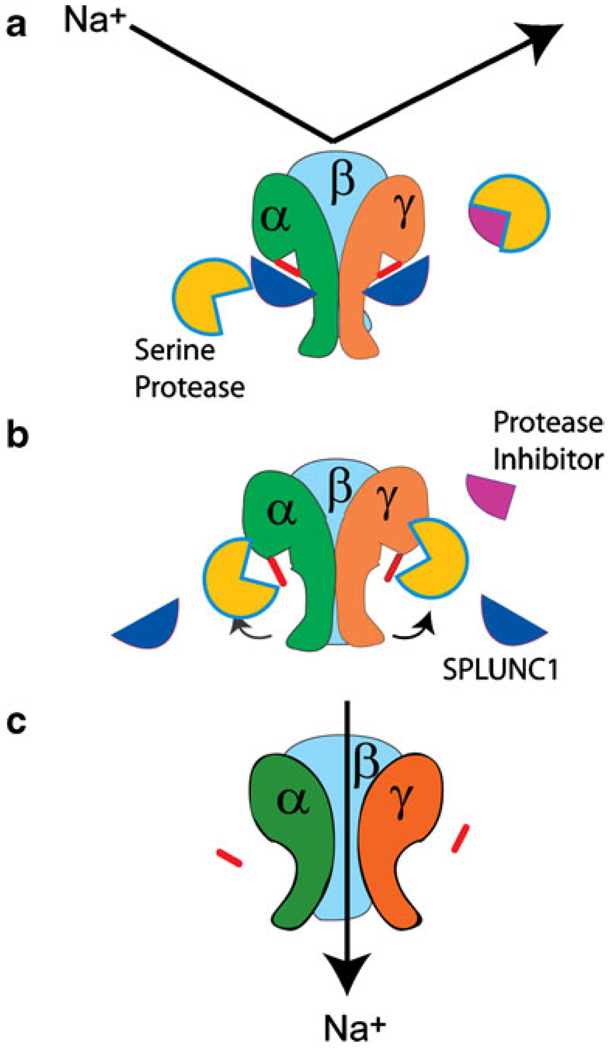
Fig. 2.
Proteolytic cleavage allows Na+ conduction through ENaC. ENaC is a heterotrimer consisting of α, β, and γ subunits that together form the functional channel. a In the absence of proteolysis, ENaC is uncleaved and cannot conduct Na+. b After proteolytic cleavage, the “inhibitory” tracts from the extracellular loops of the α and γ subunits are removed, possibly leading to a conformational change in the channel, which can now conduct Na+ (c)
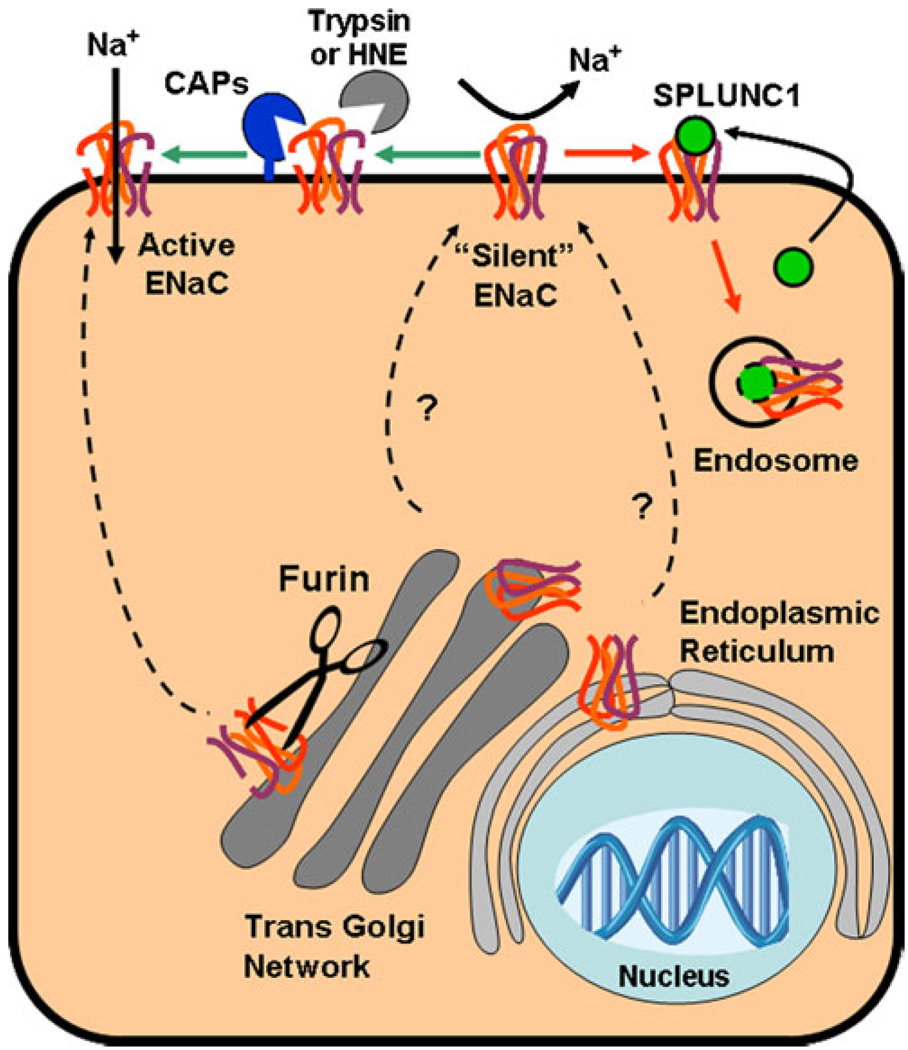
Fig. 3.
Speculation on ENaC proteolysis and how SPLUNC1 may inhibit this process. Uncleaved ENaCs can be cleaved in the trans-Golgi network by furin. These cleaved ENaCs are then transported to the plasma membrane in a highly regulated fashion (not shown, see [20, 131]) where they are ready to conduct Na+. An additional pool of newly formed ENaCs are able to bypass the furin cleavage step through an as-yet undetermined mechanism and directly inserted into the plasma membrane as uncleaved, inactive “silent” channels. These silent ENaCs can then be protetolytically cleaved by cell-attached, extracellular, channel-activating proteases (CAPS) or by soluble proteases such as trypsin or human neutrophil elastase and converted into active ENaC channels. Alternatively, silent ENaCs may bind to SPLUNC1, which is secreted from some epithelial cells in an autocrine/paracrine fashion. SPLUNC1 then triggers the internalization of ENaC through an unknown process, making ENaC unavailable for cleavage by CAPs or soluble proteases. Whether these internalized ENaCs continue to bind to SPLUNC1 when internalized and whether they can be recycled to the plasma membrane or are targeted for lysosomal degradation is not known
Furin-type proteases
Convertases are so named since they process precursor proteins into biologically active forms. Furin is a Ca2+-dependent serine endoprotease that cleaves precursor proteins at their paired basic amino acid processing sites [104]. The minimal consensus sequence for furin cleavage is R/K-X-X-R where X is any amino acid [65]. Endogenous, furin-dependent intracellular cleavage of ENaC was first suggested by Hughey et al. [64]. These investigators demonstrated that expression of α, β, or γENaC alone in MDCK cells showed full-length subunits by Western blot (~93–96 kDa). In contrast, co-expression of these three subunits resulted in the appearance of smaller ~65-kDa bands of α and γENaC subunits, but not the βENaC subunit, as well as the full-length bands [64]. The mass of the α and γ fragments matched the predictions for cleavage at two recognized furin sites in the α subunit and a single site in the γ subunit (see Fig. 1 for the location of the furin cleavage sites). These investigators then demonstrated by mutagenesis that cleavage of the α subunit had the greatest effects on macroscopic ENaC currents [65]. Paradoxically, cleavage of primarily the γ subunit by extracellular serine proteases also exerts large effects on ENaC function, and the difference between these two findings has yet to be explained [59]. Furin is predominantly located in the Golgi apparatus (Fig. 2). However, furin family convertases may also be active at the cell surface and could theoretically cleave ENaC whilst it is in or near the plasma membrane [147]. Thus, furin-dependent activation of ENaC has been suggested to provide a pool of ENaCs, whose activity can be further modified by membrane-associated CAPs and/or by intracellular second messengers such as PIP2 and PKA [109].
GPI-anchored proteases
Serine proteases are named because at least one of the amino acids in their active site is a serine and this does not refer to their target amino acid sequence, which can vary considerably depending on the serine protease in question. The first channel-activating protease (CAP1) discovered to activate ENaC was ultimately identified as murine prostasin (TMPRSS8) [154], and human prostasin was found to similarly activate ENaC [44]. Co-expression of the serine protease CAP1/prostasin, cloned from the Xenopus kidney epithelial cell line (A6 cells), with ENaC in Xenopus oocytes, resulted in several fold increases in ENaC-mediated whole cell currents [150]. Subsequently, prostasin has been soundly implicated by siRNA-mediated knockdown [149] or by gene disruption in mice [111] in the regulation of ENaC in the lung. Interestingly, this effect may not rely on extracellular CAP1 activity, because in other studies catalytically inactive prostasin mutants fully stimulated ENaC [153]. However, incubation in media containing the Kunitz-type serine protease inhibitor aprotinin prevented ENaC activation by CAP1, perhaps indicating that CAP1 plays a noncatalytic role in a proteolytic cascade that targets ENaC (see below) [150]. This study found no effect of CAP1 on the number of channels at the plasma membrane, suggesting that CAPs increase ENaC activity by changing the open probability (PO). Exogenous application of purified trypsin can mimic the actions of CAPs by activating ENaC in the oocyte expression model [150].
The human ortholog of CAP1 was found to be prostasin [44], which was originally cloned from bicarbonate-rich seminal fluid [164]. Prostasin is present in normal and CF airway epithelia [44], and Tong et al. demonstrated that knockdown of prostasin in a CF airway epithelial cell line lowered basal amiloride-sensitive current [149]. Since prostasin is GPI-anchored, it is cleaved by GPI-specific phospholipase C and can be secreted in addition to being membrane bound [29]. Thus, the (possibly indirect) activation of ENaC by prostasin may be relieved if prostasin is itself cleaved by phospholipase C and removed from the airway surface. While such a mode of regulation has been demonstrated in renal epithelia [140], it remains to be tested in the airways. If this happens in the airways, PLC-dependent regulation of ENaC may provide for an additional mode of regulation.
A polybasic stretch of residues in γ-ENaC was recently identified by mutagenesis as being required for CAP1/prostasin stimulation of ENaC [19]. Interestingly, Bruns et al. [19] reported that γ-ENaC processing occurs in oocytes expressing catalytically “dead” prostasin that could not directly cleave proteins following site-directed mutagenesis. Furthermore, this protein is maximally active at pH 9 and essentially inactive at pH 7 [164]. However, ASL pH is generally thought to be ≤pH 7, suggesting that prostasin may not be proteolytically active in the airway lumen [31]. Taken together, these data suggest that either (1) the catalytic mutant prostasin retains a portion of its protease activity or (2) prostasin may facilitate the activation of other channel-activating protease(s) by means other than its proteolytic capability. What is left unresolved from these studies, however, is the mechanism by which CAP1/prostasin stimulates ENaC. Bruns et al. proposed that prostasin cleaves γ-ENaC at a polybasic tract (RKRK) downstream from the γ-furin site and that prostasin worked in conjunction with furin to remove an inhibitory peptide from the γ-ENaC extracellular domain [19]. Interestingly, it has now been recognized that CAP1/prostasin stimulates ENaC independently of its own catalytic activity [150, 153]. Questions have been raised, but not settled, about the effectiveness of mutagenesis of the prostasin catalytic triad as a maneuver to completely eliminate prostasin catalytic activity [19], which would influence the interpretation of the earlier experiments. Most recently, Svenningsen and colleagues found that plasmin binds to prostasin and that this interaction is important for ENaC activation [140]. Although not tested or discussed by these authors, perhaps this role of prostasin does not require its catalytic activity and could reveal that prostasin plays multiple roles in proteolytic regulation of ENaC, either as a protease targeting ENaC or as an anchor in a complex of ENaC and other proteases.
Trans-membrane serine proteases
ENaC is also regulated by a variety of type II membrane-bound serine proteases whose N-termini are intracellularly located. CAP2 (TMPRSS4), CAP3 (matriptase), and human airway trypsin-like protease are all expressed in the airways [146]. CAPs 2 and 3 are known to regulate ENaC [153]. In contrast to CAP1, both CAP2 and CAP3 must possess catalytic activity to stimulate ENaC [4]. CAP2 cleaves ENaC at multiple sites in all three subunits, including cleavage at a conserved basic residue located in the vicinity of the degenerin site (α-K561, β-R503, and γ-R515) [49]. Multiple sites including the furin consensus sites in αENaC (R205/R231) and γENaC (R138) are responsible for the ENaC fragments observed in Xenopus oocytes coexpressing CAP2 [49]. However, the only cleavage site absolutely required for channel activation by CAP2 is the γENaC site R138 [49].
Soluble proteases
The first soluble serine protease shown to affect ENaC was trypsin [150]. Pretreatment with this enzyme diminished the size of γ-ENaC from 87 kDa (full length) or 76 kDa (furin-cleaved) to 67 kDa in Xenopus oocytes. Diakov et al. [40] found that cleavage of the γENaC subunit was important for changes in overall ENaC conductance and that a single-point mutation at position 181 (γK181A) removed the stimulatory effect of serine proteases [40]. Interestingly, in oocytes expressing α, β, and γK181A ENaC, the 67-kDa band was present even in the absence of extracellular trypsin, suggesting that this mutant was spontaneously cleaved and it is likely that this mutation increases the specificity of γENaC for endogenous proteases. However, when the furin-insensitive mutation was introduced into γENaC along with the γK181Amutation (α, β, γP138A, K181AENaC), the gain of function associated with the γK181A mutation alone was absent, indicating that cleavage at the furin site must occur before cleavage at position 181 by serine proteases [40].
In Xenopus oocytes expressing α, β, γENaC or in airway epithelia natively expressing ENaC, a brief exposure to the soluble serine protease neutrophil elastase increased amiloride-sensitive currents approximately fivefold [22, 58]. The effects of neutrophil elastase were not potentiated by trypsin, suggesting that ENaC is maximally activated by either serine protease. Harris et al. found that brief exposure of ENaC expressing Xenopus oocytes to neutrophil elastase generated a new fragment of γ-ENaC in the surface pool, consistent with cleavage downstream of the γ-ENaC furin site [58]. Elastase inhibitors prevented this effect and the elastase-induced fragment appeared only in the pool of proteins biotinylated at the cell surface [58]. However, a contemporaneous study identified the specific residues V182 and V193 in human γENaC as being essential for ENaC stimulation by human neutrophil elastase, a location consistent with the γENaC fragment generated by elastase in ENaC-expressing Xenopus oocytes [49].
Mast cells can release soluble, chymotrypsin-like serine proteases [141], which may also be able to activate ENaC [40]. However, little is known about the effect of this protease on ENaC activity in the airways. In Xenopus oocytes expressing δ, β, γENaC, exposure to chymotrypsin resulted in a twofold increase in whole cell current, which was less than the effect on α, β, γENaC (fivefold), consistent with the hypothesis that δENaC is more likely to be constitutively cleaved than αENaC [56]. The increase in whole-cell current following chymotrypsin application coincided with the appearance of an approximately 20-kDa δENaC cleavage fragment. Similar to αENaC, the δ subunit requires co-expression with the γENaC subunit for activation by external proteases.
Proteolytic activation of near silent ENaC channels
It has been suggested that a subpopulation of ENaCs somehow bypass furin cleavage in the Golgi apparatus, and following their insertion into the plasma membrane these channels display very little or no activity. Accordingly, these channels have been termed ‘near silent’ ENaCs (Fig. 3) [21]. Distinct, basally active, silent and/or near-silent ENaC populations were distinguished by patch clamp [22]. The application of exogenous trypsin increased the open probability of near-silent ENaC more than 50-fold in outside-out patch recordings, increasing their activity towards that of basally active ENaCs [21]. Extensive washing did not reverse this process, suggesting that the activation was irreversible, as one would expect after proteolytic cleavage [21]. The increase in open probability occurred within seconds to several minutes after application of trypsin and could be inhibited by soybean trypsin inhibitor [21]. Blockade of trypsin-sensitive G proteincoupled proteinase-activated receptors did not affect the increase in open probability of ENaC. Taken together, these results suggested a direct effect of trypsin on Po [21, 40].
In similar experiments, concentrations of neutrophil elastase aimed to reprise those seen in diseased lungs increased the activity of near-silent ENaCs approximately 100-fold in patches from mammalian NIH-3T3 fibroblast cells [22]. No significant effects of neutrophil elastase were observed on basally active ENaCs, again suggesting that neutrophil elastase only activates ENaCs which have not been cleaved. Trypsin exposure following neutrophil elastase did not increase the amiloride-sensitive shortcircuit current further, suggesting that these proteases share a common mode of action for increasing Na+ transport, likely through proteolytic activation of ENaC. Great variability in the extent of ENaC stimulation by application of exogenous proteases has been reported and partly reflects different extents of proteolytic stimulation of ENaC existing in different experimental systems. In addition, varying extents of proteolytic stimulation of ENaC or of Na+ absorption are consistent with the hypothesis that both intracellular, aprotinin-insensitive activation of ENaC prior to membrane insertion and extracellular activation of ENaC by serine proteases can occur. Since acute neutrophil elastase exposure rapidly diminishes mucus clearance rates in sheep [124], it is possible that a reserve of silent ENaCs exist in the plasma membrane of superficial airway epithelia under normal physiological conditions.
The relative contributions of α vs. γ subunit cleavage to macroscopic currents
The initial work of Hughey and Kleyman detected fragments of ENaC that matched the size of fragments predicted to arise from cleavage at furin consensus sequences in α and γENaC [64, 66]. Moreover, mutagenesis of these sites to prevent cleavage by furin reduced basal ENaC-mediated current which was then recovered by exogenous trypsin. The contributions of α and γ sites to this effect were disproportionate, with mutation of the cleavage sites in αENaC having the greatest effect on macroscopic currents. Although the validity of these observations has not been challenged (our unpublished data is the same), they are nonetheless difficult to reconcile with the overall body of subsequent work on non-furin type serine proteases that activate ENaC. For example, neutrophil elastase fully stimulates quiescent ENaC [22], and in two independent studies cleavage of γENaC at specific residues was found to be the requisite cleavage event for this activation [1, 58]. Prostasin-mediated stimulation of ENaC was also traced to cleavage of the γENaC subunit [19]. In addition, we reported that although CAP2 co-expression with ENaC generated fragments of α-ENaC predicted by cleavage at its two furin consensus sequences, mutation of those sites and prevention of cleavage was without effect on CAP2 stimulation of ENaC. In contrast, preventing cleavage of γENaC at its consensus furin sequence eliminated CAP2 activation of ENaC. Subsequently, much, if not all of the ability of trypsin [40] and plasmin [105] to stimulate ENaC have been traced to cleavage of γENaC. These findings with multiple non-furin serine proteases, each of which likely cleave ENaC only at the cell surface, are difficult to reconcile with the demonstrated importance of the α-subunit furin sites in earlier studies [26]. These findings clearly indicate that broad gaps in our knowledge exist, and the data may indicate as-yet, poorly understood contributions of αENaC to overall ENaC activity, perhaps relating to channel assembly, trafficking, or surface dwell time.
Protease inhibitors
Kunitz-type inhibitors
In their seminal 1997 Nature paper, Vallet et al. [150] demonstrated that CAP1-dependent activation of ENaC could be prevented by pretreatment with the 6.5-kDa protease inhibitor aprotinin [150]. Bridges et al. found that, out of several serine protease inhibitors tested in human airway epithelial cultures, only inhibitors containing naturally occurring Kunitz domains inhibited ENaC currents [17]. Aprotinin, also known as bovine pancreatic trypsin inhibitor, is a reversible Kunitz-type inhibitor of several serine proteases including trypsin and CAPs 1–3 [123]. Using single-channel noise analysis, Adebamiro et al. found that aprotinin caused an apparent decrease in the number of ENaC channels in the plasma membrane, which accounted for the decrease in macroscopic ENaC currents [1]. In fact, the noise technique correctly detected a decrease in the number of open (active) channels but could not recognize that they were replaced by near-silent channels. The rates of onset and offset for inhibition of ENaC by aprotinin and other protease inhibitors is much slower than for pore-blocking small molecules such as amiloride. The reason for this difference is that protease inhibitors act to prevent the conversion by proteolysis of uncleaved, near-silent ENaC to actively gating channels. In this schema, the slow onset of aprotinin inhibition of ENaC-mediated current reflects active channels being replaced by near-silent channels. The rate of onset of ENaC current inhibition by aprotinin or other broad-specificity inhibitors is expected to approximate the rate of retrieval of ENaC from the surface. Similarly, the gradual recovery from inhibition by aprotinin likely reflects the rate of insertion of new ENaC channels into the plasma membrane [123].
The Bayer pharmaceutical compound BAY 39–9437 is also a Kunitz-containing serine protease inhibitor that is similar to placental bikunin. BAY 39–9437 is considered a potential therapeutic agent for CF lung disease that would act by ameliorating the Na+ hyperabsorption in CF airways. EPI-hNE4 (DX-890) is a low molecular mass (6.2 kDa) inhibitor of neutrophil elastase that was modified from the second Künitz domain of inter-α-trypsin inhibitor [55, 118]. Replacement of the critical arginine residue at position 297 with an isoleucine makes this domain a potent neutrophil elastase inhibitor that has been demonstrated to protect the lungs of rats against neutrophil elastase and from CF sputum [38]. This compound also prevents ENaC from being activated by neutrophil elastase, but not by trypsin or CAP1 [58].
Serpins
Serpins are a group of proteins first identified as protease inhibitors. The acronym serpin was originally coined because many family members inhibited chymotrypsin-like serine proteases [27]. The first members of the serpin superfamily to be extensively studied were the human plasma proteins anti-thrombin and anti-trypsin, which play key roles in controlling blood coagulation and inflammation, respectively [53]. SerpinE2, also known as protease nexin 1 (PN-1), has been shown to regulate Na+ absorption in airway epithelia [99]. Myerberg et al. reported that PN-1 complexed with and inhibited prostasin on the apical surface of cultured airway epithelia, leading to inhibition of Na+ absorption. Although PN-1 has been referred to as the “cognate prostasin inhibitor”, Myerberg et al. discovered that PN-1 also inhibited matriptase on the surface of airway epithelia [46]. Since matriptase can activate both prostasin and ENaC, PN-1, and very likely other soluble, serpins are predicted to influence the proteolytic regulation of ENaC.
Small molecule inhibitors
In addition to protein serine protease inhibitors, low molecular weight chemically synthesized serine protease inhibitors have also been developed (125). Some of these inhibitors, including camostat, have been shown to inhibit proteolytic activation of ENaC in vitro and in vivo [33]. Many covalent and noncovalent protease inhibitors have been developed as anticoagulators [125]. While the effect of these compounds on ENaC activity is not currently known, they may represent a library of potentially useful compounds that could inhibit ENaC in vivo.
Non-classical inhibitors of ENaC proteolysis
Short palate lung and nasal epithelial clone 1 (SPLUNC1) was recently identified as a regulator of ENaC using a non-biased, proteomic screen designed to find ASL proteins capable of interacting with serine proteases [50]. SPLUNC1 is a secreted protein of ~28 kDa which shares homology with antibacterial proteins but has no obvious homology with Kunitz-type inhibitors or serpins [11]. SPLUNC1 has previously been identified in human airway epithelial cultures and this protein comprises up to 10% of the total protein content in their ASL [23]. SPLUNC1 was found to prevent ENaC proteolysis and inhibited ENaC-mediated currents in both Xenopus oocytes and in human bronchial epithelial cultures [50]. In addition to being structurally different from traditional protease inhibitors, which typically prevent cleavage of ENaC, its mode of action also appears to differ. For example, despite preventing ENaC proteolysis and activation by serine proteases such as trypsin, CAP1, and CAP2, SPLUNC1 does not inhibit protease activity directly and rather binds to ENaC, preventing it from being cleaved [50]. SPLUNC1 also reduces basal ENaC currents in Xenopus oocytes [50], and SPLUNC1 may actually reduce the number of ENaCs that are inserted into the plasma membrane, thus reducing both basal ENaC activity and the number of ENaCs that are available for proteolytic cleavage [121]. Whilst the nature of this internalization remains to be elucidated, SPLUNC1 has several predicted adaptor protein binding-domains. Such adaptor proteins have previously been shown to play a role in the internalization of ENaC [130, 158] and may be for a macromolecular complex with SPLUNC1 and α,β,γENaC to facilitate ENaC’s internalization.
Regulation of ASL volume by ENaC, extracellular proteases, and anti-proteases
Airway epithelial cells constitutively release extracellular purine nucleotides including ATP and UTP at levels sufficient to activate P2Y2 receptors on the apical membrane, which stimulate rises in intracellular IP3 and Ca2+, in parallel with a fall in intracellular PIP2 [36]. ATP can also be broken down to adenosine (ADO) by a series of extracellular enzymes including the 5’ ectonucleotidase CD73, and ADO can then stimulate A2B–R to raise cAMP which can activate CFTR and also regulate ENaC [88, 89, 106–108]. However, based on the observation that ENaC is basally active, airway epithelia were thought to be primarily absorptive, with little or no gradient for Cl− secretion, even when CFTR was activated [13]. Furthermore, Cl− secretion was only predicted to occur when ENaC was inhibited by molecules such as amiloride, which would hyperpolarize the apical membrane and provide an electrical gradient for Cl− secretion against the unfavorable chemical gradient [Clo− ~130 mM; Cli− ~40 mM] [13]. These initial studies were performed under “thick film conditions” where ASL was washed away and replaced with unphysiologically large volumes of Ringer solution. Thus, whilst the native ASL volume on a 1 cm2 airway epithelial culture is measured in microliters, such a culture may be bathed in up to 10 ml of Ringer solution during the course of an Ussing chamber-type experiment. However, studies of ASL volume regulation under thin film conditions, where native ASL is preserved, have demonstrated that ENaC can be spontaneously inactivated, permitting Cl− (and liquid) secretion to occur. For example, Widdicombe et al. demonstrated that ASL secretion could be induced in freshly excised bovine trachea [160]. Similarly, we have shown that purine nucleotides and nucleosides induced ASL secretion in the absence of pre-inhibition of ENaC with amiloride [142, 145]. These nucleotides and nucleosides may act as soluble volume sensors whose concentration or dilution can directly regulate ASL volume by changing the rates of Cl− secretion [120, 143, 145]. Whilst changes in nucleotides/nucleosides have been shown to directly affect ENaC under closed-circuit conditions, when these compounds are directly applied by the investigator at relatively high micromolar concentrations, it seems under open circuit/thin film conditions when endogenous ATP and ADO are much lower, and closer to concentrations seen in vivo (i.e., nanomolar levels) that ENaC is primarily under the control of the protease/protease inhibitor system despite the presence of endogenous ATP and ADO [50, 144, 146].
Human airway epithelial cultures were found to shift their phenotype from predominantly absorptive when excess solution was added to the culture surface to predominantly secretory when ASL height was similar to ciliary height (i.e., ~7 µm) [144]. Building upon this observation, further research indicated that ENaC was acutely sensitive to the overlying ASL volume and was activated when ASL volume was raised [98, 146], a termed coined “ASL volume expansion” [98]. Following ASL volume expansion, it is likely that silent ENaCs become rapidly activated, allowing for a rapid return to a 7 µm ASL height. Whilst ENaC is not itself likely to be volume sensitive, washing cultures with Ringer solution resulted in a brisk activation of ENaC, whilst allowing ASL to accumulate resulted in ENaC inhibition [98, 146]. Airway cultures express (1) membrane-attached serine proteases that are not affected by washing and/or dilution and (2) soluble protease inhibitors that could be removed/diluted [98, 146]. Thus, based on these observations, it was proposed that when ASL volume is high, soluble protease inhibitors are removed/diluted and ENaC is activated by cell-attached proteases (Fig. 4a) [28]. This is analogous to the situation seen in Ussing chambers when ASL is washed away or diluted by the Ringer solution which bathes the mucosal membrane. A physiological parallel may occur following glandular secretions onto airway surfaces. In contrast, when ASL is undisturbed, protease inhibitors are present in sufficient concentrations to inhibit ENaC (Fig. 4b).
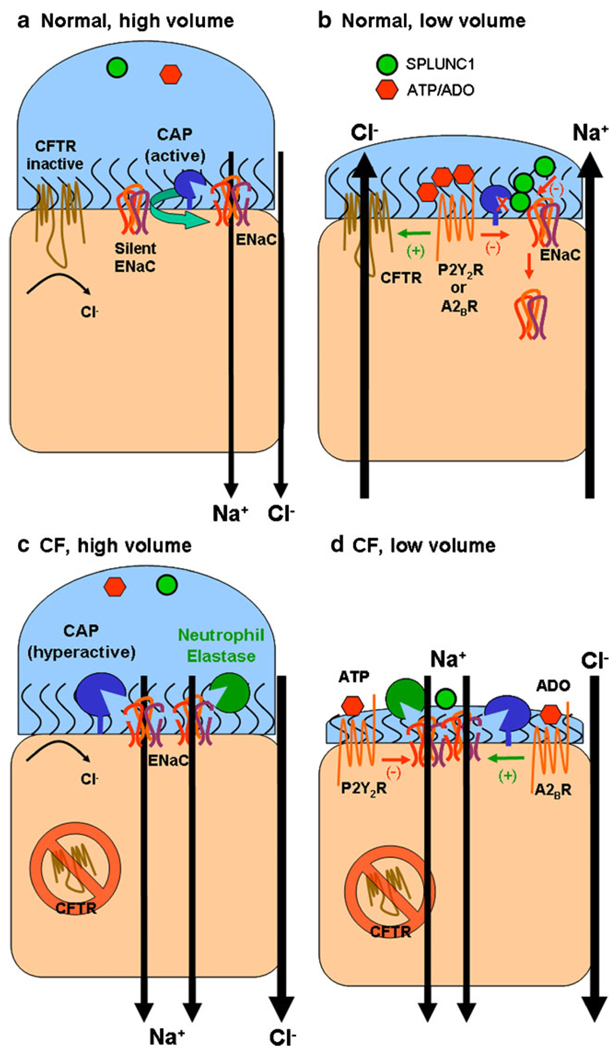
Fig. 4.
ENaC and ASL volume regulation. a In normal airways under high volume conditions, which may occur following glandular secretions, soluble inhibitors of ENaC proteolysis such as SPLUNC1 or purinergic inhibitors such as adenosine or ATP may be diluted to such an extent that ENaC is fully active, leading to Na+-led isotonic ASL absorption with Cl following through the paracellular pathway. Most non-cleaved, silent ENaCs are likely to be converted to cleaved active ENaCs by endogenous cell surface CAPs. Due to low levels of ATP and adenosine, CFTR and TMEM16A (not shown) are inactive and no Cl secretion occurs. b As ASL volume returns to steady-state levels, SPLUNC1 accumulates in the ASL in sufficient quantities to prevent the conversion of silent ENaCs to active ENaCs and SPLUNC1 may also reduce surface ENaC levels. Adenosine also accumulates to sufficient levels to activate CFTR (likely by stimulation of A2B adenosine receptors). This leads to a steady-state ASL height which approximates the height of outstretched cilia (7 µm). c High ASL volume conditions may occur in CF airways after inhalation of hypertonic saline. Under these conditions, Na+ absorption dominates, as in normal airways. d However, as ASL volume is reduced in CF airways, regulatory molecules such as SPLUNC1 are predicted to be ineffective at inhibiting ENaC, leading to an inappropriate reduction in ASL volume. In the absence of CFTR, active A2BR increases cAMP levels to stimulate rather than inactivate ENaC, further driving the reduction in ASL volume. Thus, unlike NL airways, CF airways are unable to make the switch from an absorbing to a secreting epithelia and ASL volume depletion results
The importance of soluble protease inhibitors as volumesensing regulators of ENaC has recently been confirmed and knockdown of the soluble ENaC inhibitor SPLUNC1 prevented spontaneous, ASL volume-dependent inactivation of ENaC in human airway cultures [50]. In the absence of SPLUNC1, ASL volume continued to be absorbed following the addition of a test solution and was not slowed as ASL height approached 7 µm, resulting in ASL volume collapse to CF-like levels [50]. These data indicate that SPLUNC1 plays a crucial role in ASL volume regulation [50]. SPLUNC1 is most highly expressed in glands of the proximal airways in vivo [11] which may reflect a more secretory phenotype of these regions. SPLUNC1 is less well expressed in the distal airways, consistent with Kilburn’s hypothesis that converging airways need to absorb excess solution to maintain constant ASL height [74]. Importantly, when soluble inhibitors or proteins such as SPLUNC1 increase their concentration in the ASL sufficiently to inhibit ENaC, this will facilitate Cl− secretion by hyperpolarizing the apical membrane, providing an electrical gradient for Cl− exit into the lumen [13]. Whether or not other highly expressed protease inhibitors such as the secretory leukocyte peptidase inhibitor can affect ENaC activity remains to be tested. However, other PLUNC family members that are expressed in the airways, including SPLUNC2 and LPLUNC1, do not appear to inhibit ENaC to the same degree as SPLUNC1 [121].
The majority of protease/protease inhibitor regulation dependent of ENaC occurs, by its nature, at the non-genomic level. However, normal airway epithelia exposed to prolonged 24 h ASL volume expansion showed increased prostasin expression [99]. This increase in prostasin expression was more pronounced after 12 h of ASL volume expansion whereas the effect of excess ASL on ENaC activation is immediate [98, 146]. It is likely therefore that, whilst the bulk of volume-induced ENaC regulation happens non-genomically, the epithelia also has an extensive reserve capacity and can increase expression of proteins that activate ENaC if required to do so.
ENaC and mucus clearance in vivo
The alveolar region of the lung has a surface area that is approximately the size of one side of a tennis court (i.e., ~75 m2) vs. the proximal airways, which have a surface area comparable to a tea towel (i.e., ~30 cm2). During this transition, there are 23 generations of airways, from the respiratory bronchioles to the trachea. As ASL and mucus move up the respiratory tract, excess liquid must be absorbed in a regulated fashion as two airways converge to keep ASL height/volume relatively constant. Failure to absorb excess ASL would result in an increase in ASL height with every generation of airways that would become greater than the diameter of the airways within a few generations, something that is clearly not seen in vivo. This model of regulated absorption was first proposed over 40 years ago by Kay Kilburn [74]. Kilburn’s hypothesis predicts that regulated absorption is active throughout the airways to help remove the excess liquid which occurs as two airways converge into one, and thus far is the only proposed model of mucus clearance that accounts for the movement of liquid up the airways vs. the huge reduction in surface area presented to this anterograde-moving liquid. Recently, in vivo experiments have been performed that support this hypothesis. For example, acute inhalation of the ENaC antagonists amiloride or Parion552, a highly specific ENaC antagonist with a Kd 100 times greater than amiloride (~7 nM), acutely increased mucus clearance rates in unanesthetized sheep [61, 62]. The inhibition of ENaC with either of these compounds in vivo likely results in an increase in mucus hydration followed by a speeding up of mucus clearance rates. A similar correlation between mucus hydration and mucus transport rates has previously been reported in vitro [144]. Protease inhibitors do not affect ENaC directly but block activation of near-silent channels arriving at the cell surface leading to a decrease in Na+ absorption. Inhalation of the serine protease inhibitor camostat decreases Na+ absorption and increases mucus clearance rates in vivo, suggesting that ENaC is likely to be active and proteolytically cleaved under normal physiological conditions in vivo [33]. As will be discussed later, neutrophil elastase, which also cleaves and activates ENaC, acutely decreases mucus clearance rates when inhaled, suggesting that ENaC can also be further activated beyond normal levels to increase fluid absorption and decrease mucus clearance rates [124]. This data also indicates that there is a pool of silent ENaCs that are present in the airways that are ready to be activated by this enzyme. Since neutrophil elastase levels are elevated in both CF and chronic obstructive pulmonary disease (COPD), an understanding of how this protease can affect ENaC levels and mucus clearance rates is of particular interest in the treatment of these diseases.
The contribution of ENaC to airway disease
CF reflects a spectrum of more than 1,600 mutations in the cystic fibrosis conductance regulator (CFTR) gene [37]. The CF gene product, CFTR, is an ATP-binding cassette family member [117]. The most common mutation, which occurs on 70% of all CF chromosomes, is the deletion of phenylalanine at position 508 of the CFTR protein, which is designated as ΔF508. The ΔF508 CFTR protein has a folding defect and is retained by the quality control system in the endoplasmic reticulum, falls short of the processing pathways, and is degraded by endoplasmic reticulumassociated degradation. CFTR functions as an apical, cAMP-activated chloride channel and thus the molecular pathogenesis of ΔF508 CFTR is reflected by the absence of functioning CFTR proteins in the apical plasma membrane of airway epithelia. Other mutations can produce a nonfunctioning CFTR Cl− channel at the apical membrane and/or a mutant CFTR with abnormal ion permeation characteristics. Despite relatively simple genetics, multiple mechanisms have been evoked to describe CF pathogenesis, beyond the lack of the apical cAMP-regulated Cl− conductance. For example, defective HCO3− secretion through CFTR has been suggested to cause abnormal mucin release, which may lead to mucus obstruction in CF airways [51, 114]. Furthermore, persistent airway inflammation and infection may be caused by a lack of CFTR [57, 92, 100]. However, although not universally accepted, an increasing body of evidence suggests that ASL volume regulation and mucociliary clearance are abnormal in CF due to defective ion transport processes [15] (Fig. 4c, d). Thus, CF patients, who exhibit Cl− hyposecretion, Na+ hyperabsorption, and reduced glandular secretions, have reduced mucus clearance [119], which can be partially restored by inhalation of nebulized hypertonic saline [43, 45] or by inhalation of the ENaC antagonist amiloride [5, 77]. In contrast, adult patients with pseudohypoaldosteronism have downregulated rates of Na+ transport and, consequently, an abundance of ASL which results in clearance rates greater than those seen in either normal controls or CF patients [73]. Unlike CF patients, who lack ASL volume, it seems that too much ASL does not compromise lung defense and pseudohypoaldosteronism patients are infection-free in adulthood [73]. The importance of ENaC in maintaining mucus clearance has recently been demonstrated (1) in a murine model overexpressing βENaC, which results in a decrease in ASL volume and mucus clearance and the emergence of chronic inflammation and lung disease [94], and (2) in sheep acutely exposed to neutrophil elastase, which results in a rapid decrease in mucus clearance rates [124]. Perhaps surprisingly, patients with Kartagener’s syndrome, who have immotile cila, exhibit near-normal rates of mucus clearance [101, 116]. This likely occurs in the face of an absence of ciliary beating due to increased rates of cough clearance in these patients as compared to normal subjects. Airway epithelial cultures derived from these patients undergo normal ASL volume homeostasis, suggesting that their mucus is normally hydrated [146]. Taken together, these data suggest that, even in the absence of beating cilia, mucus clearance can be preserved if the mucus layer is appropriately hydrated.
Boucher et al. first demonstrated that ENaC hyperactivity was at least in part due to abnormal stimulation of Na+ absorption by cAMP/PKA in CF airway epithelia [16], something that was subsequently confirmed by Mall et al. [93]. However, it is also possible that abnormal proteolytic cleavage of ENaC contributes to Na+ hyper-absorption. Due to secondary defects, which may include abnormal local pH environments and/or chronic inflammation, furin, prostasin, and CAP2 have been shown to be upregulated in the absence of CFTR [99, 103, 146]. This upregulation may contribute to a failure of CF airways to affect suitable ASL volume regulation, leading to ASL volume collapse which is indicative of a failure of mucus clearance in vivo. Evidence for this failure comes from studies performed under thin film conditions. For example, the ENaC-mediated transepithelial potential difference in airway epithelial cultures derived from non-CF donors was trypsin insensitive when ASL volume was high and ENaC-led absorption was underway, indicating that ENaC was fully active. The potential difference then spontaneously underwent a shift and became trypsin sensitive when ASL volume was low, suggesting that ENaC had been inactivated by the cultures [146]. In contrast, ENaC in CF-derived airway cultures remained trypsin insensitive, irrespective of ASL volume status, indicating a failure to regulate ENaC proteolysis. Interestingly, ENaCs in CF airway cultures remained aprotinin sensitive, suggesting that the defect lies at the level of the protease/anti-protease balance in the ASL and that CF ENaCs are inherently normal in their ability to be proteolytically cleaved [146].
As mentioned above, ENaC retains the ability to be cleaved in CF airway epithelia in an identical fashion to non-CF ENaCs. However, protease levels are elevated in diseased lungs, which may lead to abnormal ENaC activity. For example, whilst healthy individuals do not have detectable levels of neutrophil elastase in the airways [79], concentrations of active neutrophil elastase in excess of 1 µM or 200 µg/ml have been reported in CF lungs [38, 79]. Such an increase may occur not only in CF airway disease but also in COPD. Cigarette smoke exposure can induce downregulation of CFTR [24, 81], but as yet there is no evidence that ENaC is hyperactive in COPD airways. However, the high concentrations of neutrophil elastase measured in COPD airways make this a likely symptom of the disease. In addition, neutrophil elastase may overwhelm the ability of endogenous Kunitz-type anti-proteases or SPLUNC1 to inhibit ENaC. It should also be noted that since Kunitz-type proteases inhibitors do not inhibit neutrophil elastase and vice versa, therapies based around Kunitz-type anti-proteases may not be fully effective at inhibiting ENaC in inflamed/diseased airways which have elevated levels of neutrophil elastase.
ENaC-related therapies and clinical trials
In keeping with the hypothesis that abnormal CF airway ion transport results in mucus dehydration, improving the hydration of CF airway surfaces with inhaled hypertonic saline has been shown to improve lung function and decreases the number of acute exacerbations [43, 45]. However, since Na+ is the natural substrate for ENaC, these therapies have a relatively short duration of action [145]. To improve hydration, either alone or in combination with hypertonic saline, drugs directly blocking ENaC-mediated Na+ absorption are undergoing clinical evaluation. Early trials using nebulized amiloride revealed mixed benefits for CF patients [54, 77], and it was suggested that a weak affinity of amiloride for its receptor and the short half-life of amiloride (~10 min) [145] contributed to amiloride’s failure to consistently improve lung function in the clinic. It is also possible that the therapeutic benefit of amiloride was impeded by factors related to the chronic disease process such as the systematic destruction of CF small airways and that preventive amiloride therapy initiated shortly after birth, when CF lungs are structurally normal, may be the most effective treatment of CF lung disease [54, 77]. Support for this hypothesis was recently obtained in preclinical studies in βENaC-overexpressing mice by comparing therapeutic effects of preventive versus late amiloride treatment in this model of CF lung disease [167]. More recently, however, long-acting and highly potent ENaC blockers have been developed which may circumvent the pharmacokinetic limitations of amiloride [62]. As such, the compound Gilead GS9411, which is 100 times more potent than amiloride, has completed a phase 1 CF clinical trial, although the results have yet to be published (http://www.cff.org/treatments/Pipeline/).
In addition to ENaC antagonists, protease inhibitors may also be clinically effective. In a proof of concept study, Coote et al. reported that inhibition of ENaC function by the Kunitz-type serine protease inhibitor camostat increased mucus clearance [33]. This has led to a search for low molecular weight protease inhibitors as potential pharmacological agents to modulate ENaC function and improve mucociliary clearance and it may not be long before low molecular weight Kunitz-type serine protease inhibitors enter phase 1 clinical trials. Neutrophil elastase inhibitors have also been developed with a view towards inhibiting neutrophil elastase-induced ENaC hyperactivity in the lung [58]. Inhibition of neutrophil elastase by specific inhibitors may have additional beneficial effects on CF airways as this treatment will not only block ENaC activity but also prevent the detrimental effects of neutrophil elastase as mediator of tissue inflammation and destruction. Future clinical trials may soon reveal the benefits of elastase inhibitors in the treatment of CF patients.
Conclusions
Proteolytic cleavage is required for ENaC activation and controlled inhibition of this process is vital for limiting Na+ absorption rates and for efficient ASL volume homeostasis (Fig. 4). However, little is known about the physiological regulation of proteases and their inhibitors in the airways, and their contribution to ENaC disregulation in chronic lung disease is only now being fully appreciated. For example, unchecked activation of ENaC in CF airway epithelia likely contributes to the depletion of ASL volume and impaired mucociliary clearance in CF airways, yet the mechanism responsible for the protease– protease inhibitor imbalance in CF airways remains uncertain (Fig. 4). Thus, a better understanding of this complex regulatory process is warranted before we can develop effective treatments against ENaC hyperabsorption in chronic lung disease.
Acknowledgment
Funded by the CFF and by NIH P50 HL084934, P01 HL034322 and R01 HL080561.
Contributor Information
Erol A. Gaillard, Present Address: Department of Infection, Immunity and Inflammation, University of Leicester, PO Box 65, Robert Kilpatrick Clinical Sciences Building, Leicester Royal Infirmary, Leicester LE2 7LX, UK
Pradeep Kota, Department of Biochemistry and Biophysics, University of North Carolina, Chapel Hill, NC 27599, USA.
Martina Gentzsch, Cystic Fibrosis/Pulmonary Research and Treatment Center, The University of North Carolina, Thurston-Bowles Building, Chapel Hill, NC 27599-7248, USA.
Nikolay V. Dokholyan, Department of Biochemistry and Biophysics, University of North Carolina, Chapel Hill, NC 27599, USA
M. Jackson Stutts, Cystic Fibrosis/Pulmonary Research and Treatment Center, The University of North Carolina, Thurston-Bowles Building, Chapel Hill, NC 27599-7248, USA.
Robert Tarran, Email: robert_tarran@med.unc.edu, Cystic Fibrosis/Pulmonary Research and Treatment Center, The University of North Carolina, Thurston-Bowles Building, Chapel Hill, NC 27599-7248, USA.
References
- 1.Adebamiro A, Cheng Y, Rao US, Danahay H, Bridges RJ. A segment of gamma ENaC mediates elastase activation of Na+ transport. J Gen Physiol. 2007;130:611–629. doi: 10.1085/jgp.200709781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anantharam A, Palmer LG. Determination of epithelial Na+ channel subunit stoichiometry from single-channel conductances. J Gen Physiol. 2007;130:55–70. doi: 10.1085/jgp.200609716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson MP, Sheppard DN, Berger HA, Welsh MJ. Chloride channels in the apical membrane of normal and cystic fibrosis airway and intestinal epithelia. Am J Physiol. 1992;263:L1–L14. doi: 10.1152/ajplung.1992.263.1.L1. [DOI] [PubMed] [Google Scholar]
- 4.Andreasen D, Vuagniaux G, Fowler-Jaeger N, Hummler E, Rossier BC. Activation of epithelial sodium channels by mouse channel activating proteases (mCAP) expressed in Xenopus oocytes requires catalytic activity of mCAP3 and mCAP2 but not mCAP1. J Am Soc Nephrol. 2006;17:968–976. doi: 10.1681/ASN.2005060637. [DOI] [PubMed] [Google Scholar]
- 5.App EM, King M, Helfesrieder R, Kohler D, Matthys H. Acute and long-term amiloride inhalation in cystic fibrosis lung disease. A rational approach to cystic fibrosis therapy. Am Rev Respir Dis. 1990;141:605–612. doi: 10.1164/ajrccm/141.3.605. [DOI] [PubMed] [Google Scholar]
- 6.Bachhuber T, Konig J, Voelcker T, Murle B, Schreiber R, Kunzelmann K. Cl interference with the epithelial Na+ channel ENaC. J Biol Chem. 2005;280:31587–31594. doi: 10.1074/jbc.M504347200. [DOI] [PubMed] [Google Scholar]
- 7.Ballard ST, Spadafora D. Fluid secretion by submucosal glands of the tracheobronchial airways. Respir Physiol Neurobiol. 2007;159:271–277. doi: 10.1016/j.resp.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bangel-Ruland N, Sobczak K, Christmann T, Kentrup D, Langhorst H, Kusche-Vihrog K, Weber WM. Characterization of the epithelial sodium channel delta subunit in human nasal epithelium. Am J Respir Cell Mol Biol. 2009;42:498–505. doi: 10.1165/rcmb.2009-0053OC. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett JA, Fischer AJ, McCray PB., Jr Innate immune functions of the airway epithelium. Contrib Microbiol. 2008;15:147–163. doi: 10.1159/000136349. [DOI] [PubMed] [Google Scholar]
- 10.Berdiev BK, Latorre R, Benos DJ, Ismailov II. Actin modifies Ca2+ block of epithelial Na+ channels in planar lipid bilayers. Biophys J. 2001;80:2176–2186. doi: 10.1016/S0006-3495(01)76190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bingle CD, Craven CJ. PLUNC: a novel family of candidate host defence proteins expressed in the upper airways and nasopharynx. Hum Mol Genet. 2002;11:937–943. doi: 10.1093/hmg/11.8.937. [DOI] [PubMed] [Google Scholar]
- 12.Bonny O, Chraibi A, Loffing J, Jaeger NF, Grunder S, Horisberger JD, Rossier BC. Functional expression of a pseudohypoaldosteronism type I mutated epithelial Na+ channel lacking the pore-forming region of its alpha subunit. J Clin Invest. 1999;104:967–974. doi: 10.1172/JCI6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boucher RC. Human airway ion transport. Part one. Am J Respir Crit Care Med. 1994;150:271–281. doi: 10.1164/ajrccm.150.1.8025763. [DOI] [PubMed] [Google Scholar]
- 14.Boucher RC. Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch. 2003;445:495–498. doi: 10.1007/s00424-002-0955-1. [DOI] [PubMed] [Google Scholar]
- 15.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007;261:5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 16.Boucher RC, Stutts MJ, Knowles MR, Cantley L, Gatzy JT. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest. 1986;78:1245–1252. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bridges RJ, Newton BB, Pilewski JM, Devor DC, Poll CT, Hall RL. Na+ transport in normal and CF human bronchial epithelial cells is inhibited by BAY 39–9437. Am J Physiol Lung Cell Mol Physiol. 2001;281:L16–L23. doi: 10.1152/ajplung.2001.281.1.L16. [DOI] [PubMed] [Google Scholar]
- 18.Brockway LM, Zhou ZH, Bubien JK, Jovov B, Benos DJ, Keyser KT. Rabbit retinal neurons and glia express a variety of ENaC/DEG subunits. Am J Physiol Cell Physiol. 2002;283:C126–C134. doi: 10.1152/ajpcell.00457.2001. [DOI] [PubMed] [Google Scholar]
- 19.Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J Biol Chem. 2007;282:6153–6160. doi: 10.1074/jbc.M610636200. [DOI] [PubMed] [Google Scholar]
- 20.Butterworth MB, Edinger RS, Frizzell RA, Johnson JP. Regulation of the epithelial sodium channel by membrane trafficking. Am J Physiol Renal Physiol. 2009;296:F10–F24. doi: 10.1152/ajprenal.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol. 2004;286:C190–C194. doi: 10.1152/ajpcell.00342.2003. [DOI] [PubMed] [Google Scholar]
- 22.Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol. 2005;288:L813–L819. doi: 10.1152/ajplung.00435.2004. [DOI] [PubMed] [Google Scholar]
- 23.Campos MA, Abreu AR, Nlend MC, Cobas MA, Conner GE, Whitney PL. Purification and characterization of PLUNC from human tracheobronchial secretions. Am J Respir Cell Mol Biol. 2004;30:184–192. doi: 10.1165/rcmb.2003-0142OC. [DOI] [PubMed] [Google Scholar]
- 24.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 25.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 26.Carattino MD, Passero CJ, Steren CA, Maarouf AB, Pilewski JM, Myerburg MM, Hughey RP, Kleyman TR. Defining an inhibitory domain in the alpha-subunit of the epithelial sodium channel. Am J Physiol Renal Physiol. 2008;294:F47–F52. doi: 10.1152/ajprenal.00399.2007. [DOI] [PubMed] [Google Scholar]
- 27.Carrell RW, Owen MC. Plakalbumin, alpha 1-antitrypsin, antithrombin and the mechanism of inflammatory thrombosis. Nature. 1985;317:730–732. doi: 10.1038/317730a0. [DOI] [PubMed] [Google Scholar]
- 28.Chambers LA, Rollins BM, Tarran R. Liquid movement across the surface epithelium of large airways. Respir Physiol Neurobiol. 2007;159:256–270. doi: 10.1016/j.resp.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen LM, Skinner ML, Kauffman SW, Chao J, Chao L, Thaler CD, Chai KX. Prostasin is a glycosylphosphatidylinositolanchored active serine protease. J Biol Chem. 2001;276:21434–21442. doi: 10.1074/jbc.M011423200. [DOI] [PubMed] [Google Scholar]
- 30.Chmiel JF, Davis PB. State of the art: why do the lungs of patients with cystic fibrosis become infected and why can't they clear the infection? Respir Res. 2003;4:8. doi: 10.1186/1465-9921-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, O’Neal WK, Boucher RC. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci U S A. 2003;100:16083–16088. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook DI, Dinudom A, Komwatana P, Young JA. Control of Na+ transport in salivary duct epithelial cells by cytosolic Cland Na+ Eur J Morphol. 1998;36 Suppl:67–73. [PubMed] [Google Scholar]
- 33.Coote K, Atherton-Watson HC, Sugar R, Young A, MacKenzie-Beevor A, Gosling M, Bhalay G, Bloomfield G, Dunstan A, Bridges RJ, Sabater JR, Abraham WM, Tully D, Pacoma R, Schumacher A, Harris J, Danahay H. Camostat attenuates airway epithelial sodium channel function in vivo through the inhibition of a channel-activating protease. J Pharmacol Exp Ther. 2009;329:764–774. doi: 10.1124/jpet.108.148155. [DOI] [PubMed] [Google Scholar]
- 34.Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc. 2008;5:772–777. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danahay H, Jackson AD. Epithelial mucus-hypersecretion and respiratory disease. Curr Drug Targets Inflamm Allergy. 2005;4:651–664. doi: 10.2174/156801005774912851. [DOI] [PubMed] [Google Scholar]
- 36.Davis CW, Lazarowski E. Coupling of airway ciliary activity and mucin secretion to mechanical stresses by purinergic signaling. Respir Physiol Neurobiol. 2008;163:208–213. doi: 10.1016/j.resp.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173:475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 38.Delacourt C, Herigault S, Delclaux C, Poncin A, Levame M, Harf A, Saudubray F, Lafuma C. Protection against acute lung injury by intravenous or intratracheal pretreatment with EPI-HNE-4, a new potent neutrophil elastase inhibitor. Am J Respir Cell Mol Biol. 2002;26:290–297. doi: 10.1165/ajrcmb.26.3.4611. [DOI] [PubMed] [Google Scholar]
- 39.Devor DC, Pilewski JM. UTP inhibits Na+ absorption in wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol. 1999;276:C827–C837. doi: 10.1152/ajpcell.1999.276.4.C827. [DOI] [PubMed] [Google Scholar]
- 40.Diakov A, Bera K, Mokrushina M, Krueger B, Korbmacher C. Cleavage in the {gamma}-subunit of the epithelial sodium channel (ENaC) plays an important role in the proteolytic activation of near-silent channels. J Physiol. 2008;586:4587–4608. doi: 10.1113/jphysiol.2008.154435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dijkink L, Hartog A, van Os CH, Bindels RJ. The epithelial sodium channel (ENaC) is intracellularly located as a tetramer. Pflugers Arch. 2002;444:549–555. doi: 10.1007/s00424-002-0855-4. [DOI] [PubMed] [Google Scholar]
- 42.Ding F, Dokholyan NV. Emergence of protein fold families through rational design. PLoS Comput Biol. 2006;2:e85. doi: 10.1371/journal.pcbi.0020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 44.Donaldson SH, Hirsh A, Li DC, Holloway G, Chao J, Boucher RC, Gabriel SE. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem. 2002;277:8338–8345. doi: 10.1074/jbc.M105044200. [DOI] [PubMed] [Google Scholar]
- 45.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, Bye PT. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 46.Fiser A, Do RK, Sali A. Modeling of loops in protein structures. Protein Sci. 2000;9:1753–1773. doi: 10.1110/ps.9.9.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galietta LJ. The TMEM16 protein family: a new class of chloride channels? Biophys J. 2009;97:3047–3053. doi: 10.1016/j.bpj.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ganz T. Antimicrobial polypeptides. J Leukoc Biol. 2004;75:34–38. doi: 10.1189/jlb.0403150. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Caballero A, Dang Y, He H, Stutts MJ. ENaC proteolytic regulation by channel-activating protease 2. J Gen Physiol. 2008;132:521–535. doi: 10.1085/jgp.200810030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia-Caballero A, Rasmussen JE, Gaillard E, Watson MJ, Olsen JC, Donaldson SH, Stutts MJ, Tarran R. SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proc Natl Acad Sci U S A. 2009;106:11412–11417. doi: 10.1073/pnas.0903609106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest. 2009;119:2613–2622. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilmore ES, Stutts MJ, Milgram SL. SRC family kinases mediate epithelial Na+ channel inhibition by endothelin. J Biol Chem. 2001;276:42610–42617. doi: 10.1074/jbc.M106919200. [DOI] [PubMed] [Google Scholar]
- 53.Gooptu B, Lomas DA. Polymers and inflammation: disease mechanisms of the serpinopathies. J Exp Med. 2008;205:1529–1534. doi: 10.1084/jem.20072080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graham A, Hasani A, Alton EW, Martin GP, Marriott C, Hodson ME, Clarke SW, Geddes DM. No added benefit from nebulized amiloride in patients with cystic fibrosis. Eur Respir J. 1993;6:1243–1248. [PubMed] [Google Scholar]
- 55.Grimbert D, Vecellio L, Delepine P, Attucci S, Boissinot E, Poncin A, Gauthier F, Valat C, Saudubray F, Antonioz P, Diot P. Characteristics of EPI-hNE4 aerosol: a new elastase inhibitor for treatment of cystic fibrosis. J Aerosol Med. 2003;16:121–129. doi: 10.1089/089426803321919889. [DOI] [PubMed] [Google Scholar]
- 56.Haerteis S, Krueger B, Korbmacher C, Rauh R. The delta-subunit of the epithelial sodium channel (ENaC) enhances channel activity and alters proteolytic ENaC activation. J Biol Chem. 2009;284:29024–29040. doi: 10.1074/jbc.M109.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hampton TH, Stanton BA. A novel approach to analyze gene expression data demonstrates that the {Delta}F508 mutation in CFTR down regulates the antigen presentation pathway. Am J Physiol Lung Cell Mol Physiol. 2009;298:L473–L482. doi: 10.1152/ajplung.00379.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harris M, Firsov D, Vuagniaux G, Stutts MJ, Rossier BC. A novel neutrophil elastase inhibitor prevents elastase activation and surface cleavage of the epithelial sodium channel expressed in Xenopus laevis oocytes. J Biol Chem. 2007;282:58–64. doi: 10.1074/jbc.M605125200. [DOI] [PubMed] [Google Scholar]
- 59.Harris M, Garcia-Caballero A, Stutts MJ, Firsov D, Rossier BC. Preferential assembly of epithelial sodium channel (ENaC) subunits in Xenopus oocytes: role of furin-mediated endogenous proteolysis. J Biol Chem. 2008;283:7455–7463. doi: 10.1074/jbc.M707399200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartzell HC, Yu K, Xiao Q, Chien LT, Qu Z. Anoctamin / TMEM16 family members are Ca2+-activated Cl− channels. J Physiol. 2008;587:2127–2139. doi: 10.1113/jphysiol.2008.163709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirsh AJ, Sabater JR, Zamurs A, Smith RT, Paradiso AM, Hopkins S, Abraham WM, Boucher RC. Evaluation of second generation amiloride analogs as therapy for cystic fibrosis lung disease. J Pharmacol Exp Ther. 2004;311:929–938. doi: 10.1124/jpet.104.071886. [DOI] [PubMed] [Google Scholar]
- 62.Hirsh AJ, Zhang J, Zamurs A, Fleegle J, Thelin WR, Caldwell RA, Sabater JR, Abraham WM, Donowitz M, Cha B, Johnson KB, St George JA, Johnson MR, Boucher RC. Pharmacological properties of N-(3, 5-diamino-6-chloropyrazine-2-carbonyl)-N′-4-[4-(2, 3-dihydroxypropoxy) phenyl]butyl-guanidine methanesulfonate (552-02), a novel epithelial sodium channel blocker with potential clinical efficacy for cystic fibrosis lung disease. J Pharmacol Exp Ther. 2008;325:77–88. doi: 10.1124/jpet.107.130443. [DOI] [PubMed] [Google Scholar]
- 63.Huang P, Gilmore E, Kultgen P, Barnes P, Milgram S, Stutts MJ. Local regulation of cystic fibrosis transmembrane regulator and epithelial sodium channel in airway epithelium. Proc Am Thorac Soc. 2004;1:33–37. doi: 10.1513/pats.2306012. [DOI] [PubMed] [Google Scholar]
- 64.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem. 2004;279:18111–18114. doi: 10.1074/jbc.C400080200. [DOI] [PubMed] [Google Scholar]
- 65.Hughey RP, Carattino MD, Kleyman TR. Role of proteolysis in the activation of epithelial sodium channels. Curr Opin Nephrol Hypertens. 2007;16:444–450. doi: 10.1097/MNH.0b013e32821f6072. [DOI] [PubMed] [Google Scholar]
- 66.Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J Biol Chem. 2003;278:37073–37082. doi: 10.1074/jbc.M307003200. [DOI] [PubMed] [Google Scholar]
- 67.Inglis SK, Olver RE, Wilson SM. Differential effects of UTP and ATP on ion transport in porcine tracheal epithelium. Br J Pharmacol. 2000;130:367–374. doi: 10.1038/sj.bjp.0703324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ismailov II, Berdiev BK, Shlyonsky VG, Fuller CM, Prat AG, Jovov B, Cantiello HF, Ausiello DA, Benos DJ. Role of actin in regulation of epithelial sodium channels by CFTR. Am J Physiol. 1997;272:C1077–C1086. doi: 10.1152/ajpcell.1997.272.4.C1077. [DOI] [PubMed] [Google Scholar]
- 69.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 70.Ji HL, Chalfant ML, Jovov B, Lockhart JP, Parker SB, Fuller CM, Stanton BA, Benos DJ. The cytosolic termini of the beta- and gamma-ENaC subunits are involved in the functional interactions between cystic fibrosis transmembrane conductance regulator and epithelial sodium channel. J Biol Chem. 2000;275:27947–27956. doi: 10.1074/jbc.M002848200. [DOI] [PubMed] [Google Scholar]
- 71.Ji HL, Su XF, Kedar S, Li J, Barbry P, Smith PR, Matalon S, Benos DJ. Delta-subunit confers novel biophysical features to alpha beta gamma-human epithelial sodium channel (ENaC) via a physical interaction. J Biol Chem. 2006;281:8233–8241. doi: 10.1074/jbc.M512293200. [DOI] [PubMed] [Google Scholar]
- 72.Kellenberger S, Gautschi I, Schild L. An external site controls closing of the epithelial Na+ channel ENaC. J Physiol. 2002;543:413–424. doi: 10.1113/jphysiol.2002.022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kerem E, Bistritzer T, Hanukoglu A, Hofmann T, Zhou Z, Bennett W, MacLaughlin E, Barker P, Nash M, Quittell L, Boucher R, Knowles MR. Pulmonary epithelial sodiumchannel dysfunction and excess airway liquid in pseudohypoaldosteronism. N Engl J Med. 1999;341:156–162. doi: 10.1056/NEJM199907153410304. [DOI] [PubMed] [Google Scholar]
- 74.Kilburn KH. A hypothesis for pulmonary clearance and its implications. Am Rev Respir Dis. 1968;98:449–463. doi: 10.1164/arrd.1968.98.3.449. [DOI] [PubMed] [Google Scholar]
- 75.Knowles M, Gatzy J, Boucher R. Relative ion permeability of normal and cystic fibrosis nasal epithelium. J Clin Invest. 1983;71:1410–1417. doi: 10.1172/JCI110894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Knowles MR, Church NL, Waltner WE, Yankaskas JR, Gilligan P, King M, Edwards LJ, Helms RW, Boucher RC. A pilot study of aerosolized amiloride for the treatment of lung disease in cystic fibrosis. N Engl J Med. 1990;322:1189–1194. doi: 10.1056/NEJM199004263221704. [DOI] [PubMed] [Google Scholar]
- 78.Knowles MR, Stutts MJ, Spock A, Fischer N, Gatzy JT, Boucher RC. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983;221:1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- 79.Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med. 1994;150:448–454. doi: 10.1164/ajrccm.150.2.8049828. [DOI] [PubMed] [Google Scholar]
- 80.Kosari F, Sheng S, Li J, Mak DO, Foskett JK, Kleyman TR. Subunit stoichiometry of the epithelial sodium channel. J Biol Chem. 1998;273:13469–13474. doi: 10.1074/jbc.273.22.13469. [DOI] [PubMed] [Google Scholar]
- 81.Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol. 2005;288:L894–L902. doi: 10.1152/ajplung.00376.2004. [DOI] [PubMed] [Google Scholar]
- 82.Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J. 2005;19:142–143. doi: 10.1096/fj.04-2314fje. [DOI] [PubMed] [Google Scholar]
- 83.Kunzelmann K, Beesley AH, King NJ, Karupiah G, Young JA, Cook DI. Influenza virus inhibits amiloride-sensitive Na+ channels in respiratory epithelia. Proc Natl Acad Sci U S A. 2000;97:10282–10287. doi: 10.1073/pnas.160041997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- 85.Kunzelmann K, Mall M. Pharmacotherapy of the ion transport defect in cystic fibrosis: role of purinergic receptor agonists and other potential therapeutics. Am J Respir Med. 2003;2:299–309. doi: 10.1007/BF03256658. [DOI] [PubMed] [Google Scholar]
- 86.Kunzelmann K, Mall M, Briel M, Hipper A, Nitschke R, Ricken S, Greger R. The cystic fibrosis transmembrane conductance regulator attenuates the endogenous Ca2+ activated Cl− conductance of Xenopus oocytes. Pflugers Arch. 1997;435:178–181. doi: 10.1007/s004240050498. [DOI] [PubMed] [Google Scholar]
- 87.Kunzelmann K, Sun J, Meanger J, King NJ, Cook DI. Inhibition of airway Na+ transport by respiratory syncytial virus. J Virol. 2007;81:3714–3720. doi: 10.1128/JVI.02621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lazarowski ER, Boucher RC. Purinergic receptors in airway epithelia. Curr Opin Pharmacol. 2009;9:262–267. doi: 10.1016/j.coph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem. 2004;279:36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma HP, Chou CF, Wei SP, Eaton DC. Regulation of the epithelial sodium channel by phosphatidylinositides: experiments, implications, and speculations. Pflugers Arch. 2007;455:169–180. doi: 10.1007/s00424-007-0294-3. [DOI] [PubMed] [Google Scholar]
- 91.Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC) J Biol Chem. 2002;277:7641–7644. doi: 10.1074/jbc.C100737200. [DOI] [PubMed] [Google Scholar]
- 92.Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol. 2006;291:C218–C230. doi: 10.1152/ajpcell.00605.2005. [DOI] [PubMed] [Google Scholar]
- 93.Mall M, Bleich M, Greger R, Schreiber R, Kunzelmann K. The amiloride-inhibitable Na+ conductance is reduced by the cystic fibrosis transmembrane conductance regulator in normal but not in cystic fibrosis airways. J Clin Invest. 1998;102:15–21. doi: 10.1172/JCI2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 95.Mason SJ, Paradiso AM, Boucher RC. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. Br J Pharmacol. 1991;103:1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mo L, Wills NK. ClC-5 chloride channel alters expression of the epithelial sodium channel (ENaC) J Membr Biol. 2004;202:21–37. doi: 10.1007/s00232-004-0717-4. [DOI] [PubMed] [Google Scholar]
- 97.Mueller GM, Kashlan OB, Bruns JB, Maarouf AB, Aridor M, Kleyman TR, Hughey RP. Epithelial sodium channel exit from the endoplasmic reticulum is regulated by a signal within the carboxyl cytoplasmic domain of the alpha subunit. J Biol Chem. 2007;282:33475–33483. doi: 10.1074/jbc.M707339200. [DOI] [PubMed] [Google Scholar]
- 98.Myerburg MM, Butterworth MB, McKenna EE, Peters KW, Frizzell RA, Kleyman TR, Pilewski JM. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: a mechanism for sodium hypersabsorption in cystic fibrosis. J Biol Chem. 2006;281:27942–27949. doi: 10.1074/jbc.M606449200. [DOI] [PubMed] [Google Scholar]
- 99.Myerburg MM, McKenna EE, Luke CJ, Frizzell RA, Kleyman TR, Pilewski JM. Prostasin expression is regulated by airway surface liquid volume and is increased in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L932–L941. doi: 10.1152/ajplung.00437.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nichols D, Chmiel J, Berger M. Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signaling. Clin Rev Allergy Immunol. 2008;34:146–162. doi: 10.1007/s12016-007-8039-9. [DOI] [PubMed] [Google Scholar]
- 101.Noone PG, Bennett WD, Regnis JA, Zeman KL, Carson JL, King M, Boucher RC, Knowles MR. Effect of aerosolized uridine-5′-triphosphate on airway clearance with cough in patients with primary ciliary dyskinesia. Am J Respir Crit Care Med. 1999;160:144–149. doi: 10.1164/ajrccm.160.1.9806146. [DOI] [PubMed] [Google Scholar]
- 102.Okiyoneda T, Lukacs GL. Cell surface dynamics of CFTR: the ins and outs. Biochim Biophys Acta. 2007;1773:476–479. doi: 10.1016/j.bbamcr.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 103.Ornatowski W, Poschet JF, Perkett E, Taylor-Cousar JL, Deretic V. Elevated furin levels in human cystic fibrosis cells result in hypersusceptibility to exotoxin A-induced cytotoxicity. J Clin Invest. 2007;117:3489–3497. doi: 10.1172/JCI31499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ovaere P, Lippens S, Vandenabeele P, Declercq W. The emerging roles of serine protease cascades in the epidermis. Trends Biochem Sci. 2009;34:453–463. doi: 10.1016/j.tibs.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 105.Passero CJ, Mueller GM, Rondon-Berrios H, Tofovic SP, Hughey RP, Kleyman TR. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J Biol Chem. 2008;283:36586–36591. doi: 10.1074/jbc.M805676200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Picher M, Boucher RC. Human airway ecto-adenylate kinase. A mechanism to propagate ATP signaling on airway surfaces. J Biol Chem. 2003;278:11256–11264. doi: 10.1074/jbc.M208071200. [DOI] [PubMed] [Google Scholar]
- 107.Picher M, Burch LH, Boucher RC. Metabolism of P2 receptor agonists in human airways: implications for mucociliary clearance and cystic fibrosis. J Biol Chem. 2004;279:20234–20241. doi: 10.1074/jbc.M400305200. [DOI] [PubMed] [Google Scholar]
- 108.Picher M, Burch LH, Hirsh AJ, Spychala J, Boucher RC. Ecto 5′-nucleotidase and nonspecific alkaline phosphatase. Two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J Biol Chem. 2003;278:13468–13479. doi: 10.1074/jbc.M300569200. [DOI] [PubMed] [Google Scholar]
- 109.Planes C, Caughey GH. Regulation of the epithelial Na+ channel by peptidases. Curr Top Dev Biol. 2007;78:23–46. doi: 10.1016/S0070-2153(06)78002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Planes C, Leyvraz C, Uchida T, Angelova MA, Vuagniaux G, Hummler E, Matthay M, Clerici C, Rossier B. In vitro and in vivo regulation of transepithelial lung alveolar sodium transport by serine proteases. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1099–L1109. doi: 10.1152/ajplung.00332.2004. [DOI] [PubMed] [Google Scholar]
- 111.Planes C, Randrianarison NH, Charles RP, Frateschi S, Cluzeaud F, Vuagniaux G, Soler P, Clerici C, Rossier BC, Hummler E. ENaC-mediated alveolar fluid clearance and lung fluid balance depend on the channel-activating protease 1. EMBO Mol Med. 2009;2:26–37. doi: 10.1002/emmm.200900050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pochynyuk O, Bugaj V, Stockand JD. Physiologic regulation of the epithelial sodium channel by phosphatidylinositides. Curr Opin Nephrol Hypertens. 2008;17:533–540. doi: 10.1097/MNH.0b013e328308fff3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Puoti A, May A, Canessa CM, Horisberger JD, Schild L, Rossier BC. The highly selective low-conductance epithelial Na channel of Xenopus laevis A6 kidney cells. Am J Physiol. 1995;269:C188–C197. doi: 10.1152/ajpcell.1995.269.1.C188. [DOI] [PubMed] [Google Scholar]
- 114.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008;372:415–417. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 115.Reddy MM, Light MJ, Quinton PM. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl channel function. Nature. 1999;402:301–304. doi: 10.1038/46297. [DOI] [PubMed] [Google Scholar]
- 116.Regnis JA, Zeman KL, Noone PG, Knowles MR, Bennett WD. Prolonged airway retention of insoluble particles in cystic fibrosis versus primary ciliary dyskinesia. Exp Lung Res. 2000;26:149–162. doi: 10.1080/019021400269844. [DOI] [PubMed] [Google Scholar]
- 117.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 118.Roberts BL, Markland W, Ley AC, Kent RB, White DW, Guterman SK, Ladner RC. Directed evolution of a protein: selection of potent neutrophil elastase inhibitors displayed on M13 fusion phage. Proc Natl Acad Sci USA. 1992;89:2429–2433. doi: 10.1073/pnas.89.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robinson M, Eberl S, Tomlinson C, Daviskas E, Regnis JA, Bailey DL, Torzillo PJ, Menache M, Bye PT. Regional mucociliary clearance in patients with cystic fibrosis. J Aerosol Med. 2000;13:73–86. doi: 10.1089/089426800418604. [DOI] [PubMed] [Google Scholar]
- 120.Rollins BM, Burn M, Coakley RD, Chambers LA, Hirsh AJ, Clunes MT, Lethem MI, Donaldson SH, Tarran R. A2B adenosine receptors regulate the mucus clearance component of the lung’s innate defense system. Am J Respir Cell Mol Biol. 2008;39:190–197. doi: 10.1165/rcmb.2007-0450OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rollins BM, Garcia-Caballero A, Stutts MJ, Tarran R. SPLUNC1 expression reduces surface levels of the epithelial sodium channel (ENaC) in Xenopus laevis oocytes. Channels. 2010 doi: 10.4161/chan.4.4.12255. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rossier BC. The epithelial sodium channel: activation by membrane-bound serine proteases. Proc Am Thorac Soc. 2004;1:4–9. doi: 10.1513/pats.2306007. [DOI] [PubMed] [Google Scholar]
- 123.Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol. 2008;71:361–379. doi: 10.1146/annurev.physiol.010908.163108. [DOI] [PubMed] [Google Scholar]
- 124.Sabater JR, Lee TA, Abraham WM. Comparative effects of salmeterol, albuterol, and ipratropium on normal and impaired mucociliary function in sheep. Chest. 2005;128:3743–3749. doi: 10.1378/chest.128.5.3743. [DOI] [PubMed] [Google Scholar]
- 125.Sanderson PE. Small, noncovalent serine protease inhibitors. Med Res Rev. 1999;19:179–197. doi: 10.1002/(sici)1098-1128(199903)19:2<179::aid-med4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 126.Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the alpha, beta, and gamma subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. J Gen Physiol. 1997;109:15–26. doi: 10.1085/jgp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schreiber R, Hopf A, Mall M, Greger R, Kunzelmann K. The first-nucleotide binding domain of the cystic-fibrosis transmembrane conductance regulator is important for inhibition of the epithelial Na+ channel. Proc Natl Acad Sci U S A. 1999;96:5310–5315. doi: 10.1073/pnas.96.9.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sheng S, Li J, McNulty KA, Kieber-Emmons T, Kleyman TR. Epithelial sodium channel pore region. Structure and role in gating. J Biol Chem. 2001;276:1326–1334. doi: 10.1074/jbc.M008117200. [DOI] [PubMed] [Google Scholar]
- 130.Shimkets RA, Lifton RP, Canessa CM. The activity of the epithelial sodium channel is regulated by clathrin-mediated endocytosis. J Biol Chem. 1997;272:25537–25541. doi: 10.1074/jbc.272.41.25537. [DOI] [PubMed] [Google Scholar]
- 131.Snyder PM. Minireview: regulation of epithelial Na+ channel trafficking. Endocrinology. 2005;146:5079–5085. doi: 10.1210/en.2005-0894. [DOI] [PubMed] [Google Scholar]
- 132.Song W, Liu G, Bosworth CA, Walker JR, Megaw GA, Lazrak A, Abraham E, Sullender WM, Matalon S. Respiratory syncytial virus inhibits lung epithelial Na+ channels by up-regulating inducible nitric-oxide synthase. J Biol Chem. 2009;284:7294–7306. doi: 10.1074/jbc.M806816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Staruschenko A, Medina JL, Patel P, Shapiro MS, Booth RE, Stockand JD. Fluorescence resonance energy transfer analysis of subunit stoichiometry of the epithelial Na+ channel. J Biol Chem. 2004;279:27729–27734. doi: 10.1074/jbc.M404169200. [DOI] [PubMed] [Google Scholar]
- 134.Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciechanover A, Schild L, Rotin D. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. Embo J. 1997;16:6325–6336. doi: 10.1093/emboj/16.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stockand JD, Staruschenko A, Pochynyuk O, Booth RE, Silverthorn DU. Insight toward epithelial Na+ channel mechanism revealed by the acid-sensing ion channel 1 structure. IUBMB Life. 2008;60:620–628. doi: 10.1002/iub.89. [DOI] [PubMed] [Google Scholar]
- 136.Stokes JB, Sigmund RD. Regulation of rENaC mRNA by dietary NaCl and steroids: organ, tissue, and steroid heterogeneity. Am J Physiol. 1998;274:C1699–C1707. doi: 10.1152/ajpcell.1998.274.6.C1699. [DOI] [PubMed] [Google Scholar]
- 137.Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 138.Stutts MJ, Rossier BC, Boucher RC. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. J Biol Chem. 1997;272:14037–14040. doi: 10.1074/jbc.272.22.14037. [DOI] [PubMed] [Google Scholar]
- 139.Suaud L, Yan W, Carattino MD, Robay A, Kleyman TR, Rubenstein RC. Regulatory interactions of N1303K–CFTR and ENaC in Xenopus oocytes: evidence that chloride transport is not necessary for inhibition of ENaC. Am J Physiol Cell Physiol. 2007;292:C1553–C1561. doi: 10.1152/ajpcell.00064.2006. [DOI] [PubMed] [Google Scholar]
- 140.Svenningsen P, Uhrenholt TR, Palarasah Y, Skjoedt K, Jensen BL, Skott O. Prostasin-dependent activation of epithelial Na+ channels by low plasmin concentrations. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1733–R1741. doi: 10.1152/ajpregu.00321.2009. [DOI] [PubMed] [Google Scholar]
- 141.Tam EK, Aufderheide J, Hua XY. Chymotryptic activity in perfusates of isolated rat trachea: correlation with mucosal and connective tissue mast cell secretion. Am J Respir Cell Mol Biol. 1994;11:321–328. doi: 10.1165/ajrcmb.11.3.7522016. [DOI] [PubMed] [Google Scholar]
- 142.Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol. 2006;68:543–561. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- 143.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, Boucher RC. Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J Biol Chem. 2005;280:35751–35759. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J Gen Physiol. 2001;118:223–236. doi: 10.1085/jgp.118.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tarran R, Grubb BR, Parsons D, Picher M, Hirsh AJ, Davis CW, Boucher RC. The CF salt controversy: in vivo observations and therapeutic approaches. Mol Cell. 2001;8:149–158. doi: 10.1016/s1097-2765(01)00286-6. [DOI] [PubMed] [Google Scholar]
- 146.Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol. 2006;127:591–604. doi: 10.1085/jgp.200509468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tong Q, Stockand JD. Receptor tyrosine kinases mediate epithelial Na(+) channel inhibition by epidermal growth factor. Am J Physiol Renal Physiol. 2005;288:F150–F161. doi: 10.1152/ajprenal.00261.2004. [DOI] [PubMed] [Google Scholar]
- 149.Tong Z, Illek B, Bhagwandin VJ, Verghese GM, Caughey GH. Prostasin, a membrane-anchored serine peptidase, regulates sodium currents in JME/CF15 cells, a cystic fibrosis airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2004;287:L928–L935. doi: 10.1152/ajplung.00160.2004. [DOI] [PubMed] [Google Scholar]
- 150.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloridesensitive sodium channel. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- 151.Venkatesh VC, Katzberg HD. Glucocorticoid regulation of epithelial sodium channel genes in human fetal lung. Am J Physiol. 1997;273:L227–L233. doi: 10.1152/ajplung.1997.273.1.L227. [DOI] [PubMed] [Google Scholar]
- 152.von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y–receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 153.Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC. Synergistic activation of ENaC by three membrane-bound channel-activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus oocytes. J Gen Physiol. 2002;120:191–201. doi: 10.1085/jgp.20028598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vuagniaux G, Vallet V, Jaeger NF, Pfister C, Bens M, Farman N, Courtois-Coutry N, Vandewalle A, Rossier BC, Hummler E. Activation of the amiloride-sensitive epithelial sodium channel by the serine protease mCAP1 expressed in a mouse cortical collecting duct cell line. J Am Soc Nephrol. 2000;11:828–834. doi: 10.1681/ASN.V115828. [DOI] [PubMed] [Google Scholar]
- 155.Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+ channel. J Biol Chem. 1995;270:27411–27414. doi: 10.1074/jbc.270.46.27411. [DOI] [PubMed] [Google Scholar]
- 156.Weixel KM, Edinger RS, Kester L, Guerriero CJ, Wang H, Fang L, Kleyman TR, Welling PA, Weisz OA, Johnson JP. Phosphatidylinositol 4-phosphate 5-kinase reduces cell surface expression of the epithelial sodium channel (ENaC) in cultured collecting duct cells. J Biol Chem. 2007;282:36534–36542. doi: 10.1074/jbc.M703970200. [DOI] [PubMed] [Google Scholar]
- 157.Widdicombe JH. Regulation of the depth and composition of airway surface liquid. J Anat. 2002;201:313–318. doi: 10.1046/j.1469-7580.2002.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wiemuth D, Ke Y, Rohlfs M, McDonald FJ. Epithelial sodium channel (ENaC) is multi-ubiquitinated at the cell surface. Biochem J. 2007;405:147–155. doi: 10.1042/BJ20060747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wine JJ, Joo NS. Submucosal glands and airway defense. Proc Am Thorac Soc. 2004;1:47–53. doi: 10.1513/pats.2306015. [DOI] [PubMed] [Google Scholar]
- 160.Wu DX, Lee CY, Uyekubo SN, Choi HK, Bastacky SJ, Widdicombe JH. Regulation of the depth of surface liquid in bovine trachea. Am J Physiol. 1998;274:L388–L395. doi: 10.1152/ajplung.1998.274.3.L388. [DOI] [PubMed] [Google Scholar]
- 161.Yamamura H, Ugawa S, Ueda T, Nagao M, Shimada S. Protons activate the delta-subunit of the epithelial Na+ channel in humans. J Biol Chem. 2004;279:12529–12534. doi: 10.1074/jbc.M400274200. [DOI] [PubMed] [Google Scholar]
- 162.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 163.Yin S, Ding F, Dokholyan NV. Modeling backbone flexibility improves protein stability estimation. Structure. 2007;15:1567–1576. doi: 10.1016/j.str.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 164.Yu JX, Chao L, Chao J. Prostasin is a novel human serine proteinase from seminal fluid. Purification, tissue distribution, and localization in prostate gland. J Biol Chem. 1994;269:18843–18848. [PubMed] [Google Scholar]
- 165.Yue G, Malik B, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem. 2002;277:11965–11969. doi: 10.1074/jbc.M108951200. [DOI] [PubMed] [Google Scholar]
- 166.Yue G, Merlin D, Selsted ME, Lencer WI, Madara JL, Eaton DC. Cryptdin 3 forms anion selective channels in cytoplasmic membranes of human embryonic kidney cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G757–G765. doi: 10.1152/ajpgi.00152.2001. [DOI] [PubMed] [Google Scholar]
- 167.Zhou Z, Treis D, Schubert SC, Harm M, Schatterny J, Hirtz S, Duerr J, Boucher RC, Mall MA. Preventive but not late amiloride therapy reduces morbidity and mortality of lung disease in betaENaC-overexpressing mice. Am J Respir Crit Care Med. 2008;178:1245–1256. doi: 10.1164/rccm.200803-442OC. [DOI] [PubMed] [Google Scholar]