Abstract
The mammalian ionotropic glutamate receptor family encodes 18 gene products that coassemble to form ligand-gated ion channels containing an agonist recognition site, a transmembrane ion permeation pathway, and gating elements that couple agonist-induced conformational changes to the opening or closing of the permeation pore. Glutamate receptors mediate fast excitatory synaptic transmission in the central nervous system and are localized on neuronal and non-neuronal cells. These receptors regulate a broad spectrum of processes in the brain, spinal cord, retina, and peripheral nervous system. Glutamate receptors are postulated to play important roles in numerous neurological diseases and have attracted intense scrutiny. The description of glutamate receptor structure, including its transmembrane elements, reveals a complex assembly of multiple semiautonomous extracellular domains linked to a pore-forming element with striking resemblance to an inverted potassium channel. In this review we discuss International Union of Basic and Clinical Pharmacology glutamate receptor nomenclature, structure, assembly, accessory subunits, interacting proteins, gene expression and translation, post-translational modifications, agonist and antagonist pharmacology, allosteric modulation, mechanisms of gating and permeation, roles in normal physiological function, as well as the potential therapeutic use of pharmacological agents acting at glutamate receptors.
I. Introduction and Nomenclature
The past decade has revealed both breathtaking advances in our understanding of structure and function and a growing sophistication at virtually all levels of experimental design. The structure of a membrane-spanning tetrameric glutamate receptor has been described, revealing unprecedented features of channel structure together with long-awaited details on the pore-forming elements and the channel gate, subunit arrangement, and the nature of linkers connecting multiple semiautonomous domains that comprise the extracellular portion of the receptor. These compelling data have set the stage for a predictably explosive increase in work on all aspects of function and hold the promise of catalyzing timely breakthroughs in therapeutic strategies.
Assembling this review was an exciting yet daunting task. A staggering volume of literature has been published over the last 11 years, the period this review most seeks to summarize. We have focused primarily on the pharmacology of glutamate receptors, the structural basis of receptor function as it relates to neuronal function and neurological disease, and the regulation of receptor function by phosphorylation. We only touch upon the anatomical distribution of glutamate receptors, their role in behavior and cognition, their role in nervous system development, and the means by which the myriad of proteins that bind to glutamate receptors regulate receptor trafficking. We focus on mammalian receptors, with an emphasis on their relation to potential therapies now under development. In selecting the necessarily limited number of references used to illustrate advances, we have sought to recognize principle, precedent, perspective, and (importantly) to acknowledge the full spectrum of talented individuals and productive laboratories engaged in this field. We regret that space does not allow a complete listing of relevant work related to each point made; many fine articles simply could not be cited.
After the first report appeared in December 1989 of the cloning of a glutamate receptor subunit (Hollmann et al., 1989), the early 1990s witnessed a flurry of activity, resulting in reports of more than a dozen glutamate receptor clones in various species within the subsequent 6 months. As might be expected, the nomenclature was uncoordinated, with species-or laboratory-specific names for the same transcript being promoted in the literature. This situation has resolved slowly. An excellent history of glutamate receptor cloning and nomenclature has appeared (Lodge, 2009). Glutamate receptor nomenclature has recently undergone a needed and systematic revision, the International Union of Basic and Clinical Pharmacology name replacing the common names (Collingridge et al., 2009) (see http://www.iuphar-db.org/LGICNomenclature.jsp). Table 1 summarizes the nomenclature used throughout this review for both genes and gene products.
TABLE 1.
Glutamate receptor subunits
Nonhuman genes are represented by lowercase HUGO symbols (e.g., Gria1).
| IUPHAR Name | HUGO Symbol | Common Names | Human Chromosome | Amino Acids in Longest Splice Variant |
|---|---|---|---|---|
| GluA1 | GRIA1 | GluR1, GluRA | 5q31.1 | 906 |
| GluA2 | GRIA2 | GluR2. GluRB | 4q32-q33 | 901 |
| GluA3 | GRIA3 | GluR3, GluRC | Xq25-q26 | 894 |
| GluA4 | GRIA4 | GluR4, GluRD | 11q22 | 902 |
| GluK1 | GRIK1 | GluR5 | 21q22.11 | 918 |
| GluK2 | GRIK2 | GluR6 | 6q16.3-q21 | 908 |
| GluK3 | GRIK3 | GluR7 | 1p34-p33 | 919 |
| GluK4 | GRIK4 | KA1 | 11q22.3 | 956 |
| GluK5 | GRIK5 | KA2 | 19q13.2 | 981 |
| GluN1 | GRIN1 | NMDAR1, NR1, GluRξ1 | 9q34.3 | 938 |
| GluN2A | GRIN2A | NMDAR2A, NR2A, GluRϵ1 | 16p13.2 | 1464 |
| GluN2B | GRIN2B | NMDAR2B, NR2B, GluRϵ2 | 12p12 | 1484 |
| GluN2C | GRIN2C | NMDAR2C, NR2C, GluRϵ3 | 17q25 | 1236 |
| GluN2D | GRIN2D | NMDAR2D, NR2D, GluRϵ4 | 19q13.1-qter | 1336 |
| GluN3A | GRIN3A | NR3A | 9q31.1 | 1115 |
| GluN3B | GRIN3B | NR3B | 19p13.3 | 1043 |
| GluD1 | GRID1 | δ1, GluR delta-1 | 10q22 | 1009 |
| GluD2 | GRID2 | δ2, GluR delta-2 | 4q22 | 1007 |
II. Structure
A. Subunit Organization and Quaternary Structure
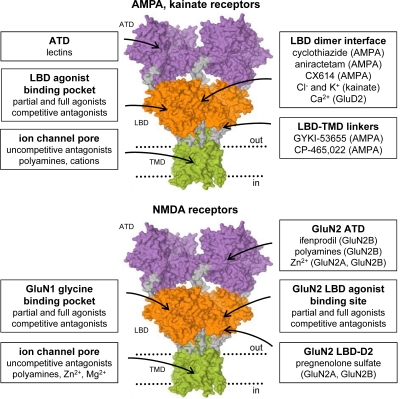
Ionotropic glutamate receptors are integral membrane proteins composed of four large subunits (>900 residues) that form a central ion channel pore. Sequence similarity among all known glutamate receptor subunits, including the AMPA,1 kainate, NMDA, and δ receptors, suggests they share a similar architecture (Table 2). Glutamate receptor subunits are modular structures that contain four discrete semiautonomous domains: the extracellular amino-terminal domain (ATD), the extracellular ligand-binding domain (LBD), the transmembrane domain (TMD), and an intracellular carboxyl-terminal domain (CTD) (Fig. 1A). Apart from the CTD and the M4 segment, each of the individual domains exhibits low sequence homology to bacterial proteins with known structures and, in some instances, a related function (O'Hara et al., 1993; Wo and Oswald, 1995; Wood et al., 1995; Paas, 1998; Kuner et al., 2003). Detailed crystallographic structures have been described for a membrane-spanning tetrameric glutamate receptor (Sobolevsky et al., 2009) as well as the isolated ATDs and LBDs in complex with various agonists, antagonists, and modulators (discussed in section VI). These data, along with functional and biochemical experiments, have begun to define the relationship between receptor structure and function.
TABLE 2.
Sequence identity and conservation of residues in glutamate receptor subunits
Numbers are the percentage of residues in the regions that are identical in all subunits within the group. Numbers in parenthesis are the percentage of residues that are identical in 50% of the subunits in the group (i.e., conserved). ATD includes the signal peptide, M1M2M3 includes pre-M1 and intracellular loops, LBD is S1 and S2. TMD is M1M2M3 and M4. In GluA2, the regions were defined as amino acids 1–397 (signal peptide and ATD), 398–414 (ATD-S1 linker), 415–527 (S1), 528–534 (S1-M1 linker), 535–647 (M1M2M3), 648–652 (M3-S2 linker), 653–794 (S2), 795–809 (S2-M4 linker), 810–838 (M4) and 839–884 (CTD) using the structures of the isolated GluA2 LBD (Armstrong and Gouaux, 2000) and the membrane-spanning tetrameric GluA2 (Sobolevsky et al., 2009) as guides.
| Receptor Subunits | ATD | S1 | S2 | LBD | M1M2M3 | M4 | TMD | CTD | All |
|---|---|---|---|---|---|---|---|---|---|
| GluA1–4 | 35 (89) | 74 (99) | 84 (100) | 80 (100) | 84 (97) | 93 (100) | 87 (98) | 9 (60) | 54 (90) |
| GluK1–5 | 16 (67) | 54 (89) | 53 (94) | 53 (92) | 60 (96) | 41 (97) | 56 (96) | 0.0 (13) | 29 (70) |
| GluA1–4, GluK1–5 | 6 (36) | 37 (81) | 33 (77) | 34 (79) | 45 (78) | 28 (62) | 42 (77) | 0.0 (3) | 17 (48) |
| GluN1, GluN2A-D, GluN3A-B | 1 (24) | 19 (61) | 18 (76) | 19 (68) | 16 (81) | 10 (83) | 14 (81) | 0.0 (2.9) | 5 (29) |
| GluN2A-D | 19 (76) | 60 (94) | 66 (99) | 63 (96) | 75 (99) | 69 (100) | 73 (99) | 2 (47) | 25 (70) |
| GluD1–2 | 60 (60) | 67 (67) | 57 (57) | 62 (62) | 51 (51) | 62 (62) | 54 (54) | 34 (34) | 54 (54) |
| All subunits | 0.2 (15) | 7 (49) | 6 (48) | 6 (48) | 10 (55) | 10 (52) | 10 (55) | 0.0 (0.2) | 2 (19) |
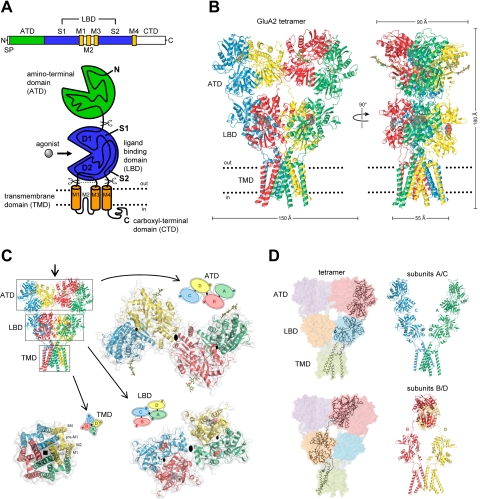
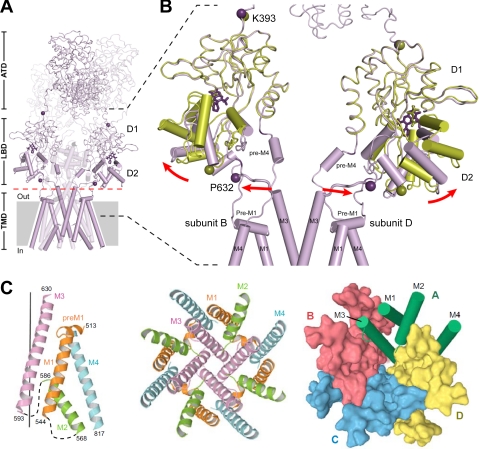
Fig. 1.
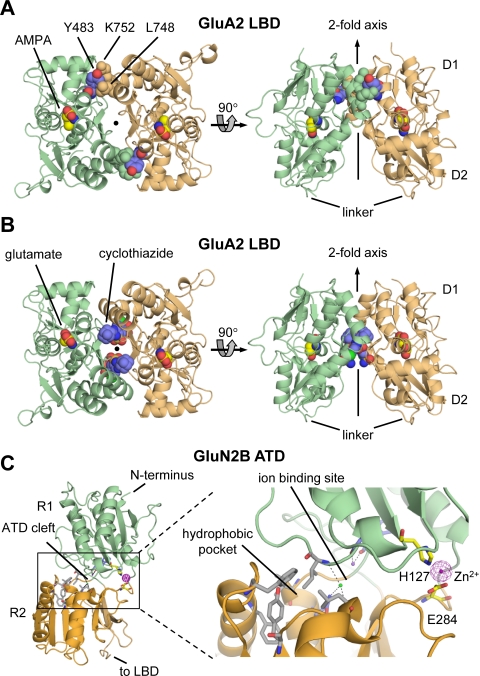
Structure and domain organization of glutamate receptors. A, linear representation of the subunit polypeptide chain and schematic illustration of the subunit topology. Glutamate receptor subunits have a modular structure composed of two large extracellular domains [the ATD (green) and the LBD (blue)]; a TMD (orange) that forms part of the ion channel pore; and an intracellular CTD. The LBD is defined by two segments of amino acids termed S1 and S2. The TMD contains three membrane-spanning helices (M1, M3, and M4) and a membrane re-entrant loop (M2). The isolated S1 and S2 segments have been constructed by deleting the ATD along with the TMD and joining S1 and S2 with a hydrophilic linker (dotted line). SP, signal peptide. B, crystal structure at 3.6 Å of the membrane-spanning tetrameric GluA2 AMPA receptor (PDB code 3KG2). C, subunit interfaces between the ATD, LBD, and TMD of the four subunits in the membrane-spanning tetrameric GluA2 AMPA receptor. The subunits are viewed from top down the 2-fold axis of symmetry. The ATDs and LBDs have a 2-fold axis of symmetry, whereas the TMDs have 4-fold axis of symmetry. D, the symmetry mismatch between the TMDs and the extracellular domains (ATDs and LBDs) as well as the subunit crossover (or domain swapping) from the LBD to the ATD give rise to two distinct types of subunits in the homotetrameric GluA2 receptor with two distinct conformations. The subunits are referred to as the A/C and B/D subunits. [Adapted from Sobolevsky AI, Rosconi MP, and Gouaux E (2009) X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462:745–756. Copyright © 2009 Nature Publishing Group. Used with permission.]
The first views of the quaternary glutamate receptor structure were provided by single particle images of recombinant and native AMPA receptors obtained by electron microscopy (Safferling et al., 2001; Tichelaar et al., 2004; Nakagawa et al., 2005, 2006; Midgett and Madden, 2008). Although these images show the receptors at lower resolution (∼40–20 Å), some structural features could be extracted. For example, an internal 2-fold rotational symmetry was observed for some of these receptor structures (Tichelaar et al., 2004; Midgett and Madden, 2008), consistent with indications that glutamate receptors assemble as a dimer of dimers. This proposed 2-fold rotational symmetry for glutamate receptors is in contrast to the symmetry observed in structures of other ion channels, such as tetrameric K+-channels and the pentameric nicotinic acetylcholine receptor, in which the quaternary subunit arrangement leads to rotational symmetries that correlate with subunit-number (MacKinnon, 2003; Miyazawa et al., 2003; Sobolevsky et al., 2004; Wollmuth and Sobolevsky, 2004).
Crystallographic studies have provided the first detailed structure of a membrane-spanning glutamate receptor (3.6 Å) (Fig. 1B). This structure of an antagonist-bound tetrameric rat GluA2 demonstrates that the receptor has an overall 2-fold symmetry perpendicular to the membrane plane; the extracellular ATDs and LBDs are organized as dimers of dimers, and the ion channel domain exhibits a 4-fold symmetry (Sobolevsky et al., 2009). This subunit arrangement relates one ATD dimer to another and one LBD dimer to the second, and half of the pore-forming TMDs to the other half. The symmetry mismatch between the ATDs and LBDs arises because the receptor contains two conformationally distinct subunits, which can be denoted A/C and B/D subunits (Fig. 1, C and D). Consequently, the A/C subunits will couple differently to the ion channel gate than will the B/D subunits, which may have important implications for the function of the glutamate receptors.
In the tetrameric structure (Sobolevsky et al., 2009), the ATD forms two distinct types of subunit-subunit contacts. The most extensive contact is formed between A/B and C/D subunits, and this contact is identical to that observed between subunits in the structure of the isolated GluA2 and GluK2 ATD dimer (Clayton et al., 2009; Jin et al., 2009; Kumar et al., 2009). The other contact is located on the 2-fold symmetry axis and is formed between B and D subunits of the A/B and C/D dimers (Fig. 1C). In addition, at the level of the LBD, two distinct types of subunit-subunit contacts are formed. The LBDs are arranged as A/D and B/C dimers with contacts between the A and C subunits (Fig. 1C). The domain swapping or subunit crossover causes a different subunit arrangement at the levels of the ATD and the LBD. As predicted by topology studies (Hollmann et al., 1994; Bennett and Dingledine, 1995) and the homology to the tetrameric K+-channels (Wo and Oswald, 1995; Wood et al., 1995; Kuner et al., 2003), the glutamate receptor TMD consists of three transmembrane helices (M1, M3, and M4) and a membrane re-entrant loop (M2) (Sobolevsky et al., 2009). In addition, the subunits have a short helix (pre-M1) that is oriented parallel to the membrane. M1, M2, and M3 form a structure that closely resembles that of an inverted K+-channel pore, and M4 primarily makes contacts with the TMD of an adjacent subunit.
The observation that subunits with the same polypeptide sequence adopt two distinct conformations in the tetrameric receptor complex is without precedent in an ion channel (Sobolevsky et al., 2009). The subunit crossover between the ATD and LBD levels of the tetramer (Fig. 1D) is primarily mediated by the ATD-S1 amino acid linkers that connect the ATD with the LBD. The ATD-S1 linkers of the A/C subunits adopt a compact conformation, whereas the ATD-S1 linkers of the B/D subunits have an extended conformation. This structural role of the ATD-S1 linker is intriguing, because previous studies have implicated this segment in the control of the open probability of NMDA receptors (Gielen et al., 2009; Yuan et al., 2009a). The symmetry mismatch between the LBD and the TMD levels also is mediated primarily by the linkers connecting the two domains (S1-M1, M3-S2, and S2-M4 linkers). Also here, the linkers adopt two different conformations corresponding to the A/C subunits and the B/D subunits. The involvement of the TMD-LBD linkers in the function of glutamate receptors has been extensively studied (Krupp et al., 1998; Villarroel et al., 1998; Sobolevsky et al., 2002a,b; Watanabe et al., 2002; Yelshansky et al., 2004; Balannik et al., 2005; Schmid et al., 2007), and the tetrameric structure provides an excellent opportunity to interpret these and other results in a structural context. Whereas tetrameric kainate receptors appear to have the same extracellular architecture as AMPA receptors (Das et al., 2010), it remains to be shown how well the tetrameric AMPA receptor structure corresponds to structures for NMDA receptors.
B. Subunit Stoichiometry
The glutamate receptors assemble as tetrameric complexes of subunits (Laube et al., 1998; Mano and Teichberg, 1998; Rosenmund et al., 1998; Greene, 2001; Matsuda et al., 2005; Nakagawa et al., 2005; Sobolevsky et al., 2009), and functional receptors are formed exclusively by assembly of subunits within the same functional receptor class (Partin et al., 1993; Kuusinen et al., 1999; Leuschner and Hoch, 1999; Ayalon and Stern-Bach, 2001; Ayalon et al., 2005). Glutamate receptors are grouped into four distinct classes based on pharmacology and structural homology, including the AMPA receptors (GluA1–GluA4), the kainate receptors (GluK1–GluK5), the NMDA receptors (GluN1, GluN2A–GluN2D, GluN3A, and GluN3B), and the δ receptors (GluD1 and GluD2). The AMPA receptor subunits GluA1 to GluA4 can form both homo- and heteromers. The kainate receptor subunits GluK1 to GluK3 also form both homo- and heteromers, but GluK4 and GluK5 form functional receptors only when coexpressed with GluK1 to GluK3. The δ receptors GluD1 and GluD2 are capable of forming homomeric receptors yet seem incapable of forming heteromers with AMPA, kainate, and NMDA receptor subunits, both in native cells and in heterologous expression systems (Partin et al., 1993, 1995; Mayat et al., 1995; Zuo et al., 1997; Kohda et al., 2000; Ikeno et al., 2001; Naur et al., 2007). In addition, GluD1 and GluD2 seem incapable of forming receptors that can be activated by any known agonists (see section V.A). Whether GluD1 and GluD2 can form heteromeric receptors is unresolved.
Functional NMDA receptors require assembly of two GluN1 subunits together with either two GluN2 subunits or a combination of GluN2 and GluN3 subunits (Monyer et al., 1992; Schorge and Colquhoun, 2003; Ulbrich and Isacoff, 2007, 2008). NMDA receptors further require simultaneous binding of both glutamate and glycine for activation (Johnson and Ascher, 1987; Kleckner and Dingledine, 1988; Lerma et al., 1990). The GluN1 and GluN3 subunits provide the glycine binding sites (Furukawa and Gouaux, 2003; Furukawa et al., 2005; Yao et al., 2008), and the GluN2 subunits form the glutamate binding sites (Furukawa et al., 2005). The GluN1 subunit expressed alone in Xenopus laevis oocytes responded weakly to coapplication of glutamate and glycine (Moriyoshi et al., 1991; Nakanishi et al., 1992; Yamazaki et al., 1992). These responses have been proposed to arise because X. laevis oocytes express low levels of endogenous NMDA receptor subunits (XenGluN1 and XenGluN2) that under some circumstances functionally assemble with GluN1 (Green et al., 2002; Schmidt et al., 2006, 2009; Schmidt and Hollmann, 2008, 2009), which can complicate studies on NMDA receptors using the X. laevis expression system. No responses are observed from GluN1 expressed alone in mammalian cells.
GluN1 also can combine with two different GluN2 subunits to form triheteromeric receptors. Numerous studies support the formation of GluN1/GluN2A/GluN2B, GluN1/GluN2A/GluN2C, GluN1/GluN2B/GluN2D, GluN1/GluN2A/GluN2D receptors in different brain regions and in specific neuronal subpopulations (Chazot et al., 1994; Sheng et al., 1994; Chazot and Stephenson, 1997; Luo et al., 1997; Sundström et al., 1997; Dunah et al., 1998a; Cathala et al., 2000; Green and Gibb, 2001; Piña-Crespo and Gibb, 2002; Brickley et al., 2003; Dunah and Standaert, 2003; Fu et al., 2005; Jones and Gibb, 2005; Lu et al., 2006; Brothwell et al., 2008). Few studies have addressed the functional implications of the presence of two different GluN2 subunits in the NMDA receptor complex (Brimecombe et al., 1997; Cheffings and Colquhoun, 2000; Hatton and Paoletti, 2005).
The GluN3 subunits bind glycine and do not form functional receptors alone (Chatterton et al., 2002; Yao and Mayer, 2006). When coexpressed with GluN1 in X. laevis oocytes, GluN1/GluN3 receptors can form receptors that are activated by glycine alone (Chatterton et al., 2002), but these excitatory glycine receptors have not yet been observed in GluN3-expressing neurons (Matsuda et al., 2003). At present, surface expression of glycine-activated GluN1/GluN3A or GluN1/GluN3B receptors in HEK293 cells is unresolved, but GluN1/GluN3A/GluN3B shows some functional expression (Smothers and Woodward, 2007). When GluN3 is coexpressed with GluN1 and GluN2 in X. laevis oocytes, NMDA- and glutamate-activated current amplitudes are reduced compared with current from GluN1/GluN2, suggesting that either triheteromeric GluN1/GluN2/GluN3 receptors form that have a lower conductance, or GluN3 expression reduces trafficking or assembly of GluN1/GluN2 (Das et al., 1998; Perez-Otano et al., 2001; Ulbrich and Isacoff, 2007, 2008). Triheteromeric GluN1/GluN2/GluN3 receptors presumably form in cortical neurons based on the observation of single-channel currents with properties that could not be attributed to either GluN1/GluN2 or GluN1/GluN3 receptors (Sasaki et al., 2002). The subunit stoichiometry and surface expression of GluN3-containing NMDA receptors and the physiological relevance of triheteromeric GluN1/GluN2/GluN3 receptors are not fully resolved.
C. Receptor Assembly and Trafficking
AMPA receptors assemble as dimers of dimers with ATD interactions presumably mediating the initial dimer formation. Subsequent tetramerization (i.e., assembly of two subunit dimers) occurs through interactions of the LBDs and the TMDs (Ayalon and Stern-Bach, 2001; Mansour et al., 2001; Ayalon et al., 2005). Receptor assembly occurs in the endoplasmic reticulum (ER), where quality control mechanisms ensure correct subunit folding and assembly. Data suggests that conformational changes associated with the normal function of glutamate receptors, such as ligand binding, activation, and desensitization, take place in the ER lumen, and these conformational changes may influence trafficking (Greger et al., 2002; Fleck et al., 2003; Grunwald and Kaplan, 2003; Mah et al., 2005; Valluru et al., 2005; Greger et al., 2006; Priel et al., 2006; Penn et al., 2008). Consequently, glutamate receptors may require ligands or “chemical chaperones” for efficient folding and export from the ER. This is evident when the conformational changes associated with the normal function are modified by mutagenesis. Nondesensitizing GluA2(L483Y) mutants exit from the ER inefficiently, whereas GluA2 (N754D), which has increased desensitization, exits efficiently from the ER (Greger et al., 2006). Block of desensitization has been shown to similarly influence kainate receptor trafficking (Priel et al., 2006; Nayeem et al., 2009). The mechanisms are unclear, but block of desensitization could interfere with association and/or dissociation of chaperones and/or transport proteins, with potential candidates being TARPs or CNIHs that are thought to be auxiliary subunits (see section II.H).
Data suggest that the ATD plays a crucial role in receptor oligomerization and perhaps trafficking (Kuusinen et al., 1999; Leuschner and Hoch, 1999; Ayalon and Stern-Bach, 2001; Ayalon et al., 2005; Qiu et al., 2009). The interaction between the ATDs is sufficient to allow isolated ATDs to form stable dimers in solution (Clayton et al., 2009; Jin et al., 2009; Kumar et al., 2009). A key role of the AMPA receptor ATD may be to direct assembly of the tetrameric receptor and to prevent kainate or NMDA receptor subunits from entering the tetramer (Kuusinen et al., 1999; Leuschner and Hoch, 1999; Ayalon et al., 2005), and some segments of the AMPA receptor ATD have been implicated in subtype-specific assembly (Leuschner and Hoch, 1999; Ayalon et al., 2005). In addition, AMPA receptor subunit stoichiometry is controlled by RNA editing, which precedes mRNA splicing and protein synthesis at two sites that modulate function: the RG site within the GluA2 to GluA4 LBD, and the QRN site at tip of the reentrant pore loop. Editing switches the codon at the QRN site from Gln to Arg in a majority of GluA2 RNA. These sites are located within subunit interfaces and are thought to affect receptor assembly by favoring heterodimerization over homodimerization, which partly explains why GluA2-containing AMPA receptors are mostly heteromers (Mansour et al., 2001; Greger et al., 2002, 2003, 2006). In addition, GluA2 subunits edited at the QRN site have increased dwell time in the ER compared with other AMPA receptor subunits, thereby increasing their availability for assembly with other subunits (Greger et al., 2002).
Three models have been suggested for assembly of NMDA receptors. The first model suggests that GluN1-GluN1 and GluN2-GluN2 homodimers initially form and subsequently coassemble to form the tetrameric receptor (Meddows et al., 2001; Schorge and Colquhoun, 2003; Papadakis et al., 2004; Qiu et al., 2005). The second model proposes that a GluN1-GluN1 homodimer forms a correctly folded stable complex to which two GluN2 monomers are added sequentially to form the NMDA receptor tetramer (Atlason et al., 2007). The third model suggests initial GluN1-GluN2 heterodimer formation and subsequent tetramerization (Schüler et al., 2008). At present, there are insufficient data to distinguish between the different models. However, the two conformationally distinct subunits with two types of subunit-subunit contacts observed in the GluA2 AMPA receptor structure (Sobolevsky et al., 2009) might provide the structural framework needed to design experiments to resolve this issue. Similar to AMPA receptors, the ATD is thought to mediate initial dimer formation of NMDA receptor subunits (Meddows et al., 2001; Papadakis et al., 2004).
D. The Extracellular Ligand Binding Domain
The LBD is highly conserved within the different glutamate classes (Table 2) and is formed by two extracellular stretches of amino acids historically referred to as S1 and S2 (Stern-Bach et al., 1994) (Fig. 1A). The structures of excised S1 and S2 amino acid sequences joined by an artificial polypeptide linker to form the LBDs have been described both with agonist and antagonist bound. All LBD structures adopt a clamshell-like conformation, where the polypeptide segment S1, located on the amino-terminal side of membrane helix M1, forms most of one half of the clamshell (D1), and the segment S2 between the M3 and M4 membrane helices forms most of the opposite half of the clamshell (D2) (Fig. 1A). The agonist binding pocket is located within the cleft between these two lobes. Several lines of experimental work have validated that the agonist-binding site in the soluble LBDs used for crystallization faithfully resembles the binding sites in intact receptors (Armstrong and Gouaux, 2000; Furukawa and Gouaux, 2003; Du et al., 2005; Gonzalez et al., 2008; Sobolevsky et al., 2009). In addition, comparison of UV absorption spectra that probe the molecular configuration of the AMPA receptor antagonist CNQX bound to either the isolated GluA2 LBD or the full-length GluA2 suggests that the structure of the soluble LBD resembles that within the full-length receptor (Deming et al., 2003).
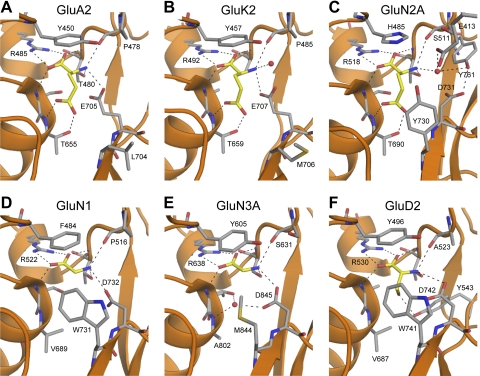
The initial step in glutamate receptor activation is binding of the agonist to the LBD. Glycine, d-serine, aspartate, and glutamate analogs are agonists and uniformly contain moieties that correspond to the α-amino and α-carboxyl groups. The regions of the binding pocket that form atomic interactions with the α-carboxyl and the α-amino groups are similar in all LBD structures and are composed primarily of residues from D1 (Fig. 2; see also section V.A). Crystallography studies together with homology modeling of the AMPA receptor subunits GluA1 to GluA4 show that residues that directly interact with α-carboxyl and α-amino groups of glutamate, AMPA, and kainate are conserved (Armstrong et al., 1998; Armstrong and Gouaux, 2000; Bjerrum et al., 2003, Pentikäinen et al., 2003; Gill et al., 2008) (Fig. 2). Greater variation is observed for the binding mode of the γ-positioned groups among AMPA receptor agonists, with a variety of atomic contacts being made for different agonists. Residues lining the agonist binding cavities of kainate receptor subunits are not fully conserved, providing opportunities for the development of subunit-selective agonists (Mayer, 2005) (Fig. 2; discussed in section V). Residues that interact with agonists in the NMDA receptor GluN2 subunits are fully conserved (Anson et al., 1998; Laube et al., 2004; Chen et al., 2005; Hansen et al., 2005a; Kinarsky et al., 2005; Erreger et al., 2007). As expected from sequence alignments, the agonist binding pockets of GluN1 and GluN3 are similar to those of GluA2 and GluN2A, but several key differences suggest how these subunits discriminate between glutamate and glycine (discussed in section V). Glutamate receptor activation involves a conformational change of the LBD upon binding of the agonist. Direct structural evidence for this idea arose from comparison of GluA2 LBD structures with and without agonist bound, as well as structures with bound competitive antagonists (Armstrong and Gouaux, 2000). In the antagonist-bound and the unbound apo structures, D1 and D2 are separated and adopt a more open conformation than in the agonist-bound structure, where D1 and D2 adopt a closed conformation (see also section VII.B for more detail). This mechanism is likely to be conserved in all glutamate receptor subunits, because all agonist-bound LBDs examined so far adopt conformations that are closed to different degrees relative to the apo structure.
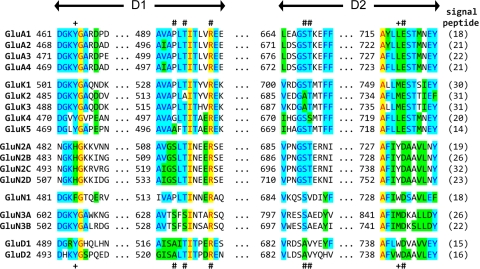
Fig. 2.
Alignments of agonist-binding residues of glutamate receptor subunits. Residue numbering is according to the total protein including the signal peptide (initiating methionine is 1). For reference, the predicted size of the signal peptide (SP) is included in parenthesis at the end of the alignment. Amino acid numbering in AMPA and kainate receptor subunits has historically been for the mature protein without the signal peptide, whereas amino acid numbering of NMDA and GluD receptor subunits has started with the initiating methionine as 1. Fully conserved residues are yellow, conserved residues are blue, and similar residues are green. # denotes residues capable of forming hydrogen bonds or electrostatic interactions with the agonist; + denotes residues capable of forming van der Waals contacts with the agonist.
Agonist-induced cleft closure within the LBD dimer, arranged with 2-fold symmetry in a back-to-back fashion, is an early conformational event that triggers the subsequent transition of the ion channel domain into an open state (see section VII). The intersubunit D1-D1 contacts formed across the dimer interface create both monovalent and divalent ion binding sites as well as sites for drug-like allosteric modulators (see section VI). In brief, upon agonist binding, the D2 lobes move and probably trigger rearrangement of the short segments that link the ion channel-containing TMD to the LBD, which drive rearrangement of M3 and subsequent channel opening (Erreger et al., 2004; Mayer, 2006; Hansen et al., 2007) (Fig. 3 discussed in section VII). The movement of D1 and D2 relative to each other results in instability at the TMD and at the LBD dimer interface. Stability can be restored by LBD reopening, which is the first step in the process of agonist dissociation, and we assume that it must be preceded by channel closure (or a change in subconductance state). Alternatively, the reduced stability of the interactions at the LBD dimer interface upon agonist binding can lead to a rearrangement of the dimer interface, allowing the receptor to enter a desensitized state (Sun et al., 2002; Jin et al., 2003., 2005; Horning and Mayer, 2004; Armstrong et al., 2006; Weston et al., 2006b) (Fig. 3; see section VII).
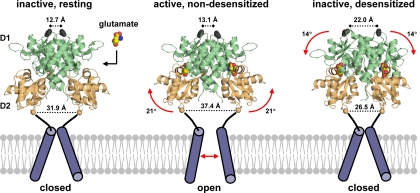
Fig. 3.
Conformational changes in the functioning AMPA receptor. Ribbon diagrams of the crystal structures of the GluA2 LBD dimer in conformations that correspond to the resting state (apo form; PDB code 1FT0), active state (glutamate-bound; PDB code 1FTJ) and desensitized state (glutamate-bound; PDB code 2I3V). In these structures, the LBD exists in a bilobed clamshell-like arrangement with the agonist-binding pocket located deep within the cleft between the two lobes referred to as D1 and D2. Binding of glutamate induces a transition of D2 that leads to separation of the linker segments that replace the TMDs in the full-length subunits (represented here by cylinders). The NTD and CTD are omitted for clarity. Distances between the linkers that face the TMD and distances between a glycine residue (Gly739) at the top of the dimer are taken from Armstrong et al. (2006). Upon glutamate binding and domain closure, separation of the linkers can result in reorientation of the transmembrane helices and opening of the ion channel. The active, nondesensitized receptor conformation is unstable, and stability can be restored either by reopening of the ABD or by rearrangement at the dimer interface. Rearrangement at the dimer interface results in desensitization by repositioning the transmembrane helices such that the ion channel is closed.
Alternative splicing of the AMPA receptor subunits generates two isoforms of the LBD termed flip and flop (Sommer et al., 1990), which control desensitization and deactivation as well as sensitivity to allosteric modulators (Mosbacher et al., 1994; Partin et al., 1994, 1995). The growing list of structures for LBDs from all subfamilies in complex with different agonists provides a firm basis for understanding agonist selectivity. For several of these ligands, structural studies in combination with site-directed mutagenesis and homology modeling have provided the structural determinants within the binding pocket that guide subunit selectivity (see section V).
E. The Extracellular Amino-Terminal Domain
Beginning at the extracellular ATD, all glutamate receptors contain a short signal peptide (14–33 residues) that targets the protein to the membrane and is removed by proteolysis after membrane insertion. Subsequent to the signal sequence, the first ∼400 to 450 residues in all glutamate receptor subunits (except bacterial GluR0, which lacks the ATD) fold into a semiautonomous domain. Glutamate receptor ATDs have sequence homology and are structurally similar to the LBD of the metabotropic glutamate receptor mGluR1a and a group of soluble bacterial periplasmatic amino acid binding proteins, such as the leucine/isoleucine/valine binding protein (O'Hara et al., 1993; Paas et al., 1996; Paas, 1998; Masuko et al., 1999a; Paoletti et al., 2000; Clayton et al., 2009; Jin et al., 2009; Karakas et al., 2009; Kumar et al., 2009). However, the similarity is confounded by numerous structural differences, such as the different locations of disulfide bonds, as well as inserts and deletions. Nonetheless, the similarity between the glutamate receptor ATD and these proteins suggests that the function of the ATD could be to bind endogenous ligands, perhaps within a putative pocket located between the lobes. Numerous mutant subunits have been created that lack the entire ATD (Fayyazuddin et al., 2000; Pasternack et al., 2002; Horning and Mayer, 2004; Matsuda et al., 2005; Rachline et al., 2005; Gielen et al., 2009; Yuan et al., 2009a), and these truncated subunits seem to assemble into receptors that are functionally similar to wild-type receptors. The nonessential nature of the ATD for the core function of the glutamate receptors is consistent with a regulatory role for this domain. Truncations of the ATD have been found to influence open probability, deactivation, desensitization, and regulation of subunit-specific assembly (Kuusinen et al., 1999; Leuschner and Hoch, 1999; Ayalon and Stern-Bach, 2001; Meddows et al., 2001; Ayalon et al., 2005; Gielen et al., 2009; Yuan et al., 2009a). The ATD also harbors binding sites for divalent cations, such as Zn2+, and subunit-selective negative allosteric modulators, such as the phenylethanolamine ifenprodil (see sections V and VI). In addition, the ATD may contain binding sites for extracellular proteins, such as N-cadherin (Saglietti et al., 2007) and neuronal pentraxins (NARP and NP1) for AMPA receptors (O'Brien et al., 1999; Sia et al., 2007) the ephrin receptor for NMDA receptors (Dalva et al., 2000; Takasu et al., 2002); cerebellin1 precursor protein for GluD2 receptors (Matsuda et al., 2010; Uemura et al., 2010; see also Uemura and Mishina, 2008; Kakegawa et al., 2009).
The glutamate receptors are glycosylated during their passage through the endoplasmic reticulum and Golgi. The consensus sites for N-linked glycosylation primarily are located in the ATD, but a few are located in the LBD (Hollmann et al., 1994; Standley and Baudry, 2000). It is not clear how many of these consensus sites are glycosylated, but cell-specific differences in glycosylation of the glutamate receptor subunits might contribute to the differences in ligand affinities, trafficking, and molecular weights observed between different native receptors and those expressed in heterologous systems (Chazot et al., 1995; Sydow et al., 1996; Everts et al., 1997; Standley et al., 1998; Standley and Baudry, 2000; Clayton et al., 2009: Kumar et al., 2009). Although the effects of glycosylation on glutamate receptor function have not been studied in detail, glycosylation can affect desensitization and maximal currents of AMPA and kainate receptors (Hollmann et al., 1994; Everts et al., 1997). In addition, the lectin concanavalin A (con A) inhibits desensitization of kainate receptors in a manner that involves association of con A with the N-linked oligosaccharides (Partin et al., 1993; Everts et al., 1997, 1999) (see section VI).
Like the glutamate receptor LBDs, the GluN2B ATD is a clamshell-like structure, roughly composed of two halves (R1 and R2) tethered together by loops (Karakas et al., 2009). The N terminus is located at the top of R1, and the linker to the LBD is located at the bottom of R2. Overall, the GluN2B ATD structure resembles the ligand binding domain of the metabotropic glutamate receptor mGluR1 (Kunishima et al., 2000), although the position of R1 is 50° twisted relative to R2 in GluN2B ATD compared with mGluR1. The cleft between R1 and R2 can be divided into three sites: 1) the hydrophilic pocket at the outer end of the cleft, which contains polar residues involved in Zn2+ binding; 2) the hydrophobic pocket deep inside the cleft, which contains residues that seem to affect ifenprodil binding; and 3) the ion-binding site that accommodates Na+ and Cl− ions with unknown physiological relevance. Binding of ifenprodil to GluN2B and Zn2+ to GluN2A or GluN2B has been proposed to stabilize a closed-cleft conformation of the ATD (see section VI; Karakas et al., 2009), although structural data in support of the hypothesized intracleft binding site is lacking. Nevertheless, the proposed cleft-closure has been speculated to lead to separation of the two R2 lobes in the ATD dimer (Gielen et al., 2008, 2009).
In contrast to NMDA receptors, no ions or small molecules are known to bind to the AMPA or kainate receptor ATD. Crystal structures of the GluA2 and the GluK2 ATDs show that these AMPA and kainate receptor ATDs adopt an overall structure similar to that of the ATD from the NMDA receptor subunit GluN2B, but the twist between R1 and R2 in GluN2B ATD was less pronounced in GluA2 and GluK2 ATDs (Clayton et al., 2009; Jin et al., 2009; Kumar et al., 2009). Unlike GluN2B, the isolated GluA2 and the GluK2 ATDs form dimers in solution and in the crystal lattice. Likewise, the ATDs of GluA1 and GluA4 also form dimers in solution (Kuusinen et al., 1999; Wells et al., 2001b; Jin et al., 2009).
Comparison of the R1 and R2 lobes of GluA2 and GluK2 ATDs with the corresponding domains of mGluR1 shows that the GluA2 and GluK2 ATDs adopt a conformation that is intermediate between the canonical open-cleft and closed-cleft states of mGluR1. In addition, there are extensive interactions between the two ATD subunits of the dimer for both GluA2 and GluK2 that involve multiple R1-R1 and R2-R2 domain contacts (Clayton et al., 2009; Jin et al., 2009; Kumar et al., 2009). The extensive interactions between the R2 lobes are mostly hydrophobic contacts situated in a large patch that is buried after dimerization of the ATD. The residues in this hydrophobic patch are conserved or conservatively substituted between AMPA and kainate receptors. In NMDA receptors, the sequence conservation is lower at the R2-R2 interface, consistent with the idea that binding of modulators to the NMDA receptor ATD could stabilize ATD cleft closure and separation at the R2-R2 interface (Gielen et al., 2008, 2009). A separation at the R2-R2 interface in the non-NMDA receptor ATD dimer would expose the large hydrophobic patch on the R2 lobe to the solvent, which would be energetically unfavorable. The “weak” R2-R2 interface in the NMDA receptor could better allow closure of the R1-R2 clamshell and separation at the R2-R2 interface, thereby triggering allosteric modulation of the ion channel. Solution of dimeric forms of the ATD will help clarify these ideas.
F. The Transmembrane Domain
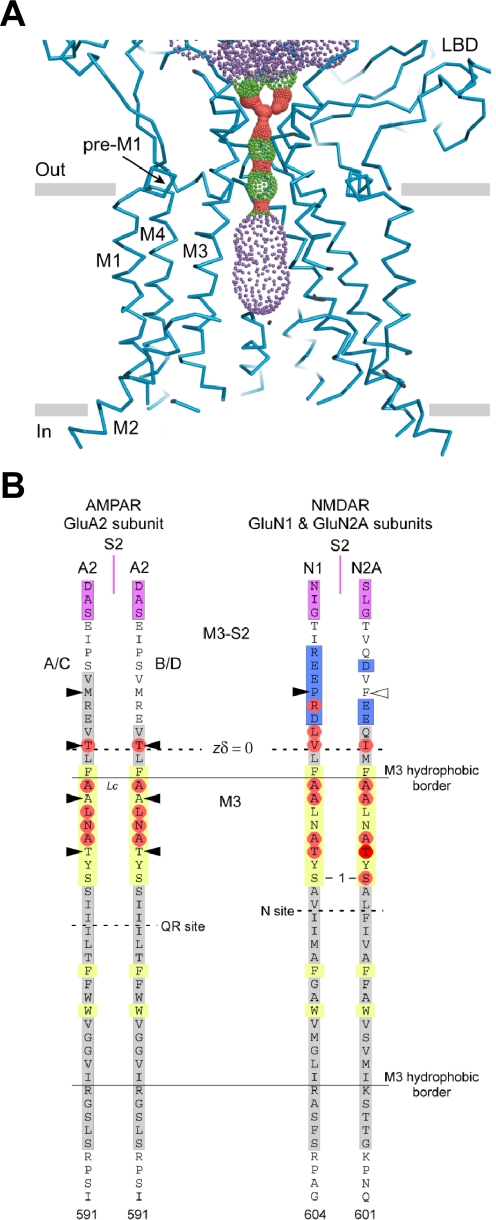
In all glutamate receptors, the LBD is connected to the conserved TMD through three short linkers (Fig. 1A). The transmembrane helices M1, M3, and M4 from each of the four subunits contribute to formation of the core of the ion channel and have a small but significant sequence homology with the inverted ion channel domain of K+ channels (Wo and Oswald, 1995; Kuner et al., 2003). This similarity is further highlighted by the bacterial glutamate receptor, GluR0, which shares strong functional and structural homology with the mammalian glutamate receptors and is a potassium-selective channel with inverted topology compared with the mammalian glutamate receptors (Chen et al., 1999a). The permeation properties of GluA2-containing AMPA receptors and GluK1 and GluK2 kainate receptors are modified post-transcriptionally by RNA editing at the Gln codon that resides at the apex of the re-entrant M2 loop (QRN site). The glutamine within the QRN site is converted to arginine by adenosine deaminase (Sommer et al., 1991; Bass, 2002). For GluA2, the overwhelming majority of RNA is edited. AMPA or kainate receptors that contain the unedited form of GluA2 (Q) have high permeability to Ca2+ and are insensitive to extracellular and intracellular polyamine channel blockers, whereas AMPA receptors containing the edited form of GluA2 (R) have low Ca2+ permeability and are insensitive to polyamine channel blockers (see section VIII.C). It is noteworthy that the extended region of the M2 loop in the new GluA2 structure that encompasses the QRN site is disordered. It is unclear whether this reflects crystallization conditions or a native conformation, which might have significant functional consequences for ion permeation and block.
The structure of the antagonist-bound tetrameric rat GluA2 shows that the four subunits arrange their TMDs in a 4-fold axis of symmetry with the core of the ion channel (M1–M3), strikingly similar to K+ channels (Sobolevsky et al., 2009) (Fig. 1C). The M2 loop lines the inner cavity of the pore, whereas the M3 helices line the outer cavity, with positions at the apex tightly opposed, presumably forming the gate that occludes the flux of ions in the closed state (see sections VII and VIII). The M1 helix is positioned on the exterior of M2 and M3. It is noteworthy that the M4 segment from one subunit is associated with the ion channel core (M1-M3) of an adjacent subunit. In addition, the linker region preceding M1 (pre-M1) makes a short helix that is oriented parallel to the plane of the membrane, making contacts with carboxyl- and amino-terminal ends of transmembrane helices M3 and M4, respectively. The pre-M1s from the four subunits resemble a cuff around the external surface of the ion channel pore that could be an important determinant for channel gating (see section VII).
G. The Intracellular Carboxyl-Terminal Domain and Protein Binding Partners
The CTD is the most diverse domain in terms of amino acid sequence (Table 2), varying in sequence and in length among the glutamate receptor subunits (Figs. 5–7). It shows no sequence homology to any known proteins but encodes short docking motifs for intracellular binding proteins. No structural details exist for this domain except for part of the GluN1 CTD with bound Ca2+/calmodulin (Ataman et al., 2007). The CTD is thought to influence membrane targeting, stabilization, post-translational modifications (see section IV), and targeting for degradation. For some glutamate receptor subunits (e.g., GluN1, GluN2A), deletion of this domain does not abolish function but does alter regulation (Köhr and Seeburg, 1996; Ehlers et al., 1998; Krupp et al., 1998; Vissel et al., 2001), because the CTDs contain different phosphorylation sites (see section IV) and binding sites for intracellular proteins important for regulation of membrane trafficking and receptor function. Several ER retention signals reside in alternatively spliced exons of GluN1, as well as in GluN2B (Horak and Wenthold, 2009). It is noteworthy that there is also a short span of sequence immediately C-terminal to M4 in GluN2 that also participates in trafficking (Hawkins et al., 2004).
Fig. 5.
Post-translational modifications of AMPA and kainate receptor C-terminal domains. Multiple forms of post-translational modifications (including palmitoylation, phosphorylation, and SUMOylation) that influence receptor trafficking, channel activity, and interactions with other proteins are shown. The C-terminal domains of GluA1–4 and GluK1–5 given in the center column. The left column contains the receptor subunit with the UniProt-SwissProt human accession number. The length of the subunit, including the signal peptide, is given in the column at right, with residue numbering beginning with the initiating methionine. The beginning of the CTD is defined by hydrophobicity analyses. Modified residues are in red, with the enzyme (if known) indicated by a symbol above the residue. When no enzyme is given, the modification has been identified through fragmentation and mass spectrometry (Munton et al., 2007; Ballif et al., 2008; Trinidad et al., 2008).
Fig. 6.
Post-translational modifications of GluN1 and GluN2A NMDA receptor C-terminal domains. The GluN1 and GluN2A NMDA receptor subunits undergo the indicated post-translational modification. The left column is the NMDA receptor subunit with the UniProt-SwissProt human accession number. The C-terminal domains of the GluN1 and GluN2A subunits are listed in the center column. The length of the receptor subunit is given in the right column, the numbering beginning with the initiating methionine. The beginning of the CTD is defined by hydrophobicity analyses. Modified residues are in red, with the enzyme (when known) indicated by a symbol above.
Fig. 7.
Post-translational modifications of the GluN2B-D NMDA and GluD1–2 δ receptor C-terminal domains. The post-translational modifications of the NMDA and δ receptors are shown in red. The enzymes mediating the modifications are identified (when known) by a symbol above. When no enzyme is designated, the modification has been identified by fragmentation and mass spectrometry. The left column is the receptor subunit and the UniProt-SwissProt human accession number. The C-terminal domains are shown in the center column, and the length of the subunit, beginning with the initiating methionine, is in the right column. The beginning of the CTD is defined by hydrophobicity analyses.
Virtually all members of the glutamate receptor family bind to a variety of intracellular proteins, which fall into several classes. Tables 3 and 4 contain noncomprehensive lists that summarize some of the better known interactions between glutamate receptor C-terminal and PDZ, cytoskeletal, scaffolding, adaptor, anchoring, structural, signaling, and other proteins. In addition to these interactions, several glutamate receptor subunits bind directly to signaling proteins, including GluA1 and cGMP-dependent protein kinase II (Serulle et al., 2007), GluA4 and PKC (Correia et al., 2003), multiple NMDA receptor subunits and Ca2+/calmodulin-dependent protein kinase (CamK) II (Gardoni et al., 1998; Strack and Colbran, 1998; Leonard et al.,, 1999, 2002), as well as tyrosine phosphatase and GluD2 (Hironaka et al., 2000). These interactions allow local signaling to proceed, providing the possibility of spatial and temporal specificity to receptor regulation. Additional localization of signaling molecules can be mediated by adjacent proteins, and the glutamate receptors are embedded into a rich complex of signaling molecules that are localized by a myriad of adaptor and scaffolding proteins within the post synaptic density (Husi et al., 2000). Further enhancing the complexity among different subunits, alternative RNA splicing of several AMPA and kainate receptor subunits as well as the NMDA receptor subunit GluN1 causes variation in the CTD that also will affect binding sites for intracellular proteins.
TABLE 3.
Carboxyl-terminal protein binding partners for AMPA and kainate receptor subunits
Entries indicate data supporting direct interactions between the indicated protein and glutamate receptor subunit.
| Protein | Class | GluA1 | GluA2 | GluA3 | GluA4 | GluK1 | GluK2 | GluK5 |
|---|---|---|---|---|---|---|---|---|
| CASK | PDZ | Y2H,coIP,EP1 | ||||||
| GRIP | PDZ | Y2H,coIP,IHC2,3,4,5 | Y2H,coIP,IHC2,3,4,6 | Y2H,coIP,IHC7 | Y2H,coIP,EP8 | |||
| GRIP2 | PDZ | Y2H,coIP,IHC4,5,7 | Y2H,coIP,IHC4,7 | Y2H,coIP,IHC7 | Y2H,coIP,EP8 | |||
| mLIN-10 | PDZ | coIP9 | coIP9 | |||||
| PICK1 | PDZ | Y2H,coIP,IHC2,6 | Y2H,coIP,IHC2,6 | Y2H,coIP,IHC2,6 | Y2H,coIP,EP8 | Y2H,coIP,EP8 | ||
| PSD95 | PDZ | Y2H,coIP,EP8 | Y2H,coIP,IHC,EP8,10 | coIP,IHC,EP10 | ||||
| SAP97 | PDZ | coIP,IHC11,12 | Y2H,coIP,EP10 | |||||
| SAP102 | PDZ | Y2H,coIP,EP10 | ||||||
| Shank3 | PDZ | Y2H,coIP,IHC13 | ||||||
| Syntenin | PDZ | Y2H14 | Y2H14 | Y2H14 | Y2H14 | Y2H,coIP,EP8 | Y2H,coIP,EP8 | |
| RIL | PDZ / LIM | Y2H,coIP,IHC,EP15 | ||||||
| 4.1 | Cytoskeletal | Y2H,coIP,IHC16,17 | coIP,IHC18 | |||||
| α-Actinin-1 | Cytoskeletal | Y2H,coIP,IHC19 | ||||||
| Actinfilin | Cytoskeletal | Y2H,coIP,IHC20 | Y2H,coIP,IHC20 | |||||
| Contactin | Cytoskeletal | coIP21 | ||||||
| Dynamin-1 | Cytoskeletal | coIP21 | ||||||
| Dynamitin | Cytoskeletal | coIP21 | ||||||
| Profilin | Scaffold | coIP21 | ||||||
| Spectrin | Scaffold | coIP21 | ||||||
| AP2 | Adaptor | coIP,IHC,EP22,23 | ||||||
| NSF | ATPase | Y2H,coIP,IHC,EP24,25,26 | coIP21 | |||||
| IQGAP1 | GTPase | Y2H,coIP,IHC19 | ||||||
| Calmodulin | Ca2+ sensor | coIP21 | ||||||
| VILIP1 | Ca2+ sensor | coIP21 | ||||||
| VILIP3 | Ca2+ sensor | coIP21 | ||||||
| 14-3-3 | Other27 | coIP21 | coIP28 | |||||
| COPI | Other27 | coIP28 | ||||||
| G-α(q/11) | Other27 | coIP,EP29 | ||||||
| SUMO | Other27 | Y2H,coIP,IHC,EP30 |
coIP, coimmunoprecipitation or pull-down assay; EP, electrophysiology; IHC, immunohistochemistry; Y2H, yeast two-hybrid.
Synaptic transmembrane proteins, signaling proteins, ubiquitin-like proteins, or transport regulatory proteins.
TABLE 4.
Carboxyl-terminal protein binding partners for NMDA and delta receptor subunits
Entries indicate data supporting direct interactions between the indicated protein and glutamate receptor subunit.
| Protein | GluN1 | GluN1 | GluN2A | GluN2B | GluN2C,D | GluN3A | GluD1 | GluD2 |
|---|---|---|---|---|---|---|---|---|
| Delphilin | PDZ | Y2H,coIP,IHC1 | ||||||
| LIN7 | PDZ | coIP,IHC2 | ||||||
| nPIST | PDZ | Y2H,coIP,IHC3 | Y2H,coIP,IHC3 | |||||
| PICOIPK1 | PDZ | Y2H,coIP,IHC4 | ||||||
| PSD93 | PDZ | Y2H,coIP5 | Y2H,coIP5 | |||||
| PSD95 | PDZ | Y2H6 | Y2H6,7 | Y2H6,7 | Y2H,coIP5 | Y2H,coIP5 | ||
| SAP97 | PDZ | Y2H7 | Y2H7 | Y2H6 | Y2H,coIP5 | Y2H,coIP5 | ||
| SAP102 | PDZ | coIP8,9 | ||||||
| Shank1 | PDZ | Y2H,coIP,IHC10 | ||||||
| Shank2 | PDZ | Y2H,coIP,IHC10 | ||||||
| S-SCAM | PDZ | Y2H,coIP,IHC11 | Y2H,coIP,IHC11 | Y2H,coIP,IHC12 | ||||
| α-actinin-2 | Cytoskeletal | Y2H,coIP,IHC13 | Y2H,coIP,IHC13 | |||||
| EMAP | Cytoskeletal | Y2H14 | Y2H14 | |||||
| MAP1S | Cytoskeletal | Y2H,coIP,IHC15 | ||||||
| Plectin | Scaffold | Y2H16 | ||||||
| RACK1 | Scaffold | coIP,EP17 | ||||||
| Spectrin | Scaffold | Y2H,coIP,IHC18 | ||||||
| AP2 | Adaptor | Y2H,IHC,EP19,20 | Y2H,coIP,EP19,21 | |||||
| AP4 | Adaptor | Y2H,coIP,IHC22 | ||||||
| PACSIN1 | Adaptor | Y2H,coIP,EP23 | ||||||
| Calmodulin | Ca2+ sensor | coIP,EP24 | ||||||
| CARP1 | Other25 | Y2H16 | ||||||
| COPII | Other25 | coIP,IHC26 | ||||||
| GPS2 | Other25 | Y2H16 | ||||||
| SALM1 | Other25 | Y2H,coIP,IHC27 |
coIP, coimmunoprecipitation or pull-down assay; EP, electrophysiology; IHC, immunohistochemistry; Y2H, yeast two-hybrid.
Synaptic transmembrane proteins, signaling proteins, ubiquitin-like proteins, or transport regulatory proteins.
H. Transmembrane α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid Receptor Regulatory Proteins and other Auxiliary Subunits
A confound in the study of AMPA receptor biophysical properties has been the occasional lack of congruence between the properties of recombinant receptors expressed in heterologous systems and those of native receptors studied in isolated tissue. This mismatch suggests that heterologously expressed receptors lack a modulatory component that can influence essential properties. The discovery of the interaction between AMPA receptor subunits and the transmembrane AMPA receptor regulatory proteins (TARPs) has solved many of these discrepancies. TARPs are integral membrane proteins with four transmembrane domains (Letts et al., 1998; Hashimoto et al., 1999; Chen et al., 2000; Tomita et al., 2003; Coombs and Cull-Candy, 2009) that selectively interact with AMPA receptors early in synthesis and trafficking and direct proper expression and localization of the receptor at the cell surface (Hashimoto et al., 1999; Chen et al., 2000; Schnell et al., 2002; Tomita et al., 2004, 2005a; Vandenberghe et al., 2005). TARPs are present in the majority of AMPA receptor complexes in the brain, suggesting that TARPs are auxiliary subunits for native AMPA receptors (Fukata et al., 2005; Nakagawa et al., 2005, 2006; Vandenberghe et al., 2005). It has been suggested that two or four TARPs can associate with the AMPA receptor tetramer, depending on availability (Vandenberghe et al., 2005; Milstein et al., 2007; Shi et al., 2009). The interaction sites between TARPs and AMPA receptors involve intracellular, transmembrane, and extracellular regions of both proteins (Tomita et al., 2005a, 2007; Bedoukian et al., 2006; Milstein and Nicoll, 2009; Sager et al., 2009).
The classic members of TARPs, γ-2, γ-3, γ-4, and γ-8, interact with all four AMPA receptor subunits. The prototypical TARP (γ-2 or stargazin) originally was identified in the cerebellum as a protein essential for delivery of AMPA receptors to the plasma membrane (Letts et al., 1998; Chen et al., 2000). The functional properties of AMPA receptors associated with the TARP subtypes γ-2, γ-3, γ-4, and γ-8 are different from those of AMPA receptors devoid of auxiliary subunits. In addition to their trafficking capabilities, TARPs increase single channel conductance, increase open probability, increase the activation rate, slow the deactivation time course, and reduce desensitization (Yamazaki et al., 2004; Priel et al., 2005; Tomita et al., 2005a; Turetsky et al., 2005; Zhang et al., 2006b; Kato et al., 2007; Soto et al., 2007, 2009). Prolonged activation of AMPA receptors triggers a form of desensitization that results from dissociation of the TARP, potentially providing a novel mechanism for receptor tuning (Morimoto-Tomita et al., 2009). Finally, γ-2 reduces GluA2-lacking AMPA receptor affinity for polyamine block, resulting in receptors with weak inward-rectification (Soto et al., 2007).
The TARP γ-5 increases glutamate potency and glutamate-evoked peak currents, reduces steady-state currents, and accelerates the time course of deactivation and desensitization only in GluA2-containing receptors, but this modulation does not involve regulation of GluA2 surface expression (Kato et al., 2008). The TARP γ-7 shares many of these properties but is not selective for receptors comprising GluA2-containing subunits, enhancing peak currents in channels containing GluA1 or GluA2 (Kato et al., 2007). In contrast, other studies show that γ-5 interacts with all AMPA receptor subunits and modifies their behavior (Soto et al., 2009).
Proteins with homology to TARP (STG-1 and STG-2) and with similar functional roles have been discovered in Caenorhabditis elegans, Apis mellifera, and Drosophila melanogaster (Walker et al., 2006a; Wang et al., 2008). However, an important difference between TARPs and STG-1 and STG-2 is the obligatory requirement of an additional transmembrane auxiliary subunit (SOL-1) that is structurally unrelated to TARPs and interacts directly with invertebrate AMPA receptor subunits (GLR-1 and GLR-2) to slow and reduce the extent of receptor desensitization (Zheng et al., 2006; Walker et al., 2006a, b).
Another distinct class of transmembrane proteins has been shown to assemble with and regulate AMPA receptors (Schwenk et al., 2009). These proteins, CNIH-2 and CNIH-3, are members of the mammalian CNIH family and are homologous to the cornichon proteins from flies and yeast (Roth et al., 1995; Bökel et al., 2006; Castro et al., 2007). CNIH proteins are necessary for the export of a number of proteins from the endoplasmic reticulum, including the epidermal growth factor receptor ligands. The role of the CNIH proteins in AMPA receptor regulation is not yet fully understood.
Accessory proteins for NMDA and kainate receptors have also recently been described. Neto1 possesses a single transmembrane domain containing two complement C1r/C1s, Uegf, Bmp1 domains and is a component of the NMDA receptor complex (Ng et al., 2009). Neto1 interacts with an extracellular domain of GluN2 as well as through an intracellular interaction with PSD95. Loss of Neto1 in transgenic mice preferentially results in a loss of synaptic GluN2A expression, with only a modest impact on GluN2B expression, which leads to impaired hippocampal LTP and hippocampal-dependent learning and memory (Ng et al., 2009). A second C1r/C1s, Uegf, Bmp1 domain-containing protein, Neto2, interacts with GluK2 to increase peak amplitude and open probability and to slow the decay time course of both GluK2 recombinant receptors and kainate receptor-mediated mEPSCs in cerebellar granule cells (Zhang et al., 2009c). In recombinant expression systems, Neto2 had no impact on GluK2 surface expression.
III. Regulation of Transcription and Translation
The level of expressed glutamate receptors reflects a balance of transcription, translation, mRNA level, protein stability, receptor assembly, and presentation at the cell surface, all of which are integrated through numerous environmental stimuli. Therefore, the particular subunits that each neuron chooses to express are strong determinants of synaptic phenotype, and this is the rationale for understanding how the genetic cis elements and trans factors regulate gene transcription in neural cells. Over the past decade steady work toward understanding the control of ionotropic glutamate expression in neuronal and non-neuronal cells has occurred, roughly doubling both the number of subunits studied and the identification of promoter elements controlling expression in neuronal cells. Furthermore, how chromatin remodeling affects glutamate receptor expression in both neurons and non-neuronal cells has been identified after, for example, status epilepticus or transient ischemia. Most studies have employed a combination of protein-DNA binding assays with functional analysis of native and mutant promoter constructs driving a reporter gene, overexpression of candidate transcription factors in cultured cells or in vivo, occupancy of cis elements by transcription factors in vivo using chromatin immunoprecipitation (ChIP) assays, and the use of real-time quantitative polymerase chain reaction experiments. The use of ChIP assays and real-time quantitative polymerase chain reaction on endogenous gene transcripts (and also on exogenously expressed constructs) have been particularly fruitful in helping to advance our understanding of how an acute stimulus causes a change in transcript level dependent on candidate promoter elements and trans-acting factors. Studies such as these have begun to tie neuronal activity, energy metabolism, and glutamate receptor expression together more coherently. Furthermore, an appreciation for the role of epigenetic modifications and chromatin remodeling at glutamate receptor promoters is also emerging and holds promise for new understanding of the neurobiology of glutamate receptors. Despite this progress, vexing questions still remain regarding the mechanisms that control cell-specific and developmental expression for glutamate receptor subunits.
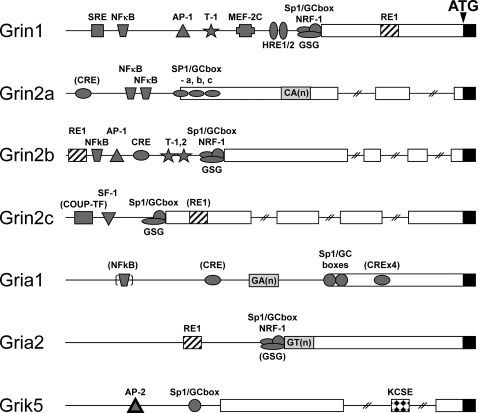
Glutamate receptor genes have a number of features in common, such as multiple transcriptional start sites in a TATAA-less promoter with high GC content. The 5′UTR ranges between 200 bp (Gria1) to over 1200 bp (Grin2a) and, in the case of Gria4, Grik5, Grin2a, Grin2b, and Grin2c, is formed from multiple exons. Finally, one or more Sp1 elements reside near the major transcriptional start site of all genes studied, several glutamate receptor promoters contain NFκB, CRE, AP1/2, Tbr-1, NRF-1, and RE1/NRSE sites, and gene expression of many is responsive to neuronal activity. The schematic organization of the promoter regions is presented in Fig. 4. It also should be noted that NMDA, AMPA, and kainate receptors are key mediators of signal transduction events that convert environmental stimuli into genetic changes through regulation of gene transcription and epigenetic chromatin remodeling in neural cells, an area of emerging interest (Carrasco and Hidalgo, 2006; Wang et al., 2007; Cohen and Greenberg, 2008; Lubin et al., 2008).
Fig. 4.
Schematic diagram of the proximal promoter regulatory regions of glutamate receptors. The proximal promoter regions of the GluN1, GluN2A, GluN2B, and GluN2C NMDA receptors, the GluA1 and GluA2 AMPA receptors, and the GluK5 kainate receptor are shown. Promoters are shown as thin lines and introns as thin lines with hashmarks. The 5′-untranslated exon sequences are represented by open bars; blackened bars designate the protein coding domains. Glutamate receptor regulatory elements are identified; those requiring further confirmation are in parentheses. The promoter regions are not drawn to scale.
A. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid Receptors
1. Gria1.
The rat Gria1 gene has been evaluated for transcription initiation and regulation of promoter function by transfection of constructs into primary mixed neuronal cultures. Gria1 promoter activity was stronger in neurons, neuronal specificity being primarily dependent on sequences lying within the regions −1395 to −743 and −253 to −48. Although five CRE sites were able to bind recombinant CRE binding-bZIP proteins, conditions under which these CRE sites come into play in native neurons are unknown. GluA1 receptors (like all AMPA receptor subunits) are expressed by neurons and glial cells in vivo and in vitro (Gallo and Ghiani, 2000), but the density of functional receptors is much lower in astrocytes than neurons. In oligodendrocyte progenitor O-2A cells, the transcriptional rate of GluA1 is increased by platelet-derived growth factor and basic fibroblast growth factor (Chew et al., 1997). Regulation of Gria1 transcription occurs via acid sphingomyelinase and NFκB sites in the Gria1 promoter (Borges and Dingledine, 2001), which was found to both account for the elevation of GluA1 by tumor necrosis factor-α (Yu et al., 2002) and contribute to the sensitization by tumor necrosis factor-α of NT2-N cells to kainate-induced cell death.
2. Gria2.
The relative use of 5′ transcriptional start sites in Gria2 is different in cortex and cerebellum, longer transcripts being more dominant in cerebellum (Myers et al., 1998). Analysis of Gria2 promoter constructs in cultured forebrain neurons and glia revealed that the Gria2 promoter was 30-fold neuronal selective. In this study, Sp1 and NRF-1 positive regulatory elements, nestled at the 5′ end of a 141-bp transcription initiation region, and an RE1/NRSE proximal promoter silencer element were important for neuron-selective expression. The RE1/NRSE repressed expression 2- to 3-fold in non-neuronal cells compared with neuronal cells (Myers et al., 1998). Suppression of GluA2 expression in glia occurs by occupancy of the Gria2 RE1/NRSE element by the REST/NRSF repressor, which in turn recruits histone deacetylase (HDAC) complexes to the Gria2 promoter, resulting in chromatin remodeling and decreased expression (Huang et al., 1999). In neurons, the Gria2 promoter is associated with acetylated H3 and H4 histones, whereas in C6 glioma cells, there is little to no association with acetylated histones, consistent with active and inactive gene expression, respectively (Huang et al., 1999). After induction of status epilepticus by pilocarpine, the acetylation of histone H4 bound to the Gria2 promoter was reduced before GluA2 expression became down-regulated in rat hippocampal CA3 neurons. Seizure-induced GluA2 mRNA down-regulation was reversed by the HDAC inhibitor trichostatin A (Huang et al., 2002a). Likewise, Calderone et al. (2003) showed that global ischemia triggers expression of the repressor REST and reduces GluA2 expression in CA1 neurons destined to die. Moreover, kainate reduces activity of the neuronal Gria2 promoter in a manner consistent with REST occupancy of the RE1 element, recruitment of HDAC to the promoter, and reduced histone acetylation (Jia et al., 2006). It is noteworthy that a preconditioning sublethal ischemic episode can prevent subsequent ischemia-induced down-regulation of GluA2 in CA1 neurons (Tanaka et al., 2002) by preventing an increase in REST expression in these same neurons (Calderone et al., 2003).
TTX reduces GluA2 expression in visual cortical neurons, suggesting that expression is linked to neuronal activity (Wong-Riley and Jacobs 2002; Bai and Wong-Riley 2003). The transcription factor NRF-1 binds to the Gria2 promoter (Dhar et al., 2009), confirming earlier studies identifying the NRF-1 element as a critical feature for neuronal Gria2 transcription (Myers et al., 1998). NRF-1 is a nuclear transcription factor important for regulating multiple cytochrome oxidase (COX) genes. Reduction of NRF-1 with small hairpin RNA prevented the depolarization-stimulated increase of GluA2 expression, whereas overexpression of NRF-1 restored GluA2 expression in the face of TTX treatment. Changes in GluA1, GluA3, and GluA4 expression were not observed, rendering NRF-1 control specific to GluA2 (Dhar et al., 2009). Thus, neuronal activity is tightly coupled at the molecular level to GluA2 expression by a process that involves NRF-1.
3. Gria3 and Gria4.
The human GRIA3 gene is on the long arm of the X chromosome and is subject to X inactivation through methylation (Gécz et al., 1999). The Gria3 transcript contains a TC repeat in the 5′-UTR that is polymorphic and is also present in rodent transcripts. The 5′-UTR and ATG codon are contained in a large 1102-bp exon 1 and are conserved in rodent and human sequences. Neither an RE1 repressor element nor an NRF-1 site was identified.
GluA4 is widely expressed in brain; however, its abundance is less than GluA1–3 (Petralia and Wenthold, 1992). In transfected mixed cortical cultures, Gria4 promoter constructs drove luciferase expression predominantly in neurons, indicating a 6- to 12-fold neuronal preference (Borges et al., 2003). Deletion of the Gria4 transcriptional initiation region decreased luciferase activity in neurons, but increased activity in C6 cells, suggesting that neuronal regulatory elements reside in this region. Sp1, Ikaros, and basic helix-loop-helix binding element sites are conserved in rat, mouse, and human genes within ±150 bp of transcription initiation sites; however, specific evaluation of these elements requires further investigation. A distal region of Gria4, −4427 to −4885, is in a long interspersed element sequence that has been suggested to recruit chromatin remodeling enzymes to the Gria4 gene (Borges et al., 2003).
B. Kainate Receptors Grik1 to Grik5
Regulatory cis elements have been computationally predicted in promoter regions of human GRIK1 and GRIK2 genes but not functionally evaluated (Barbon and Barlati, 2000; Barbon et al., 2001). However, GRIK2 was identified as a novel epigenetic target in gastric cancer as a potential tumor suppressor gene (Wu et al., 2010). There are no reports on the functional evaluation of transcriptional regulation of the Grik3 and Grik4 genes.
Initial studies of the rat Grik5 gene identified a negative regulatory sequence in the first intron that binds nuclear orphan receptors such as chicken ovalbumin upstream promoter transcription factor I in both neural and non-neural cells (Huang and Gallo, 1997; Chew et al., 1999). Transgenic mouse lines carrying 4 kb of the 5′-flanking sequence showed lacZ reporter expression predominantly in the nervous system. Reporter assays in central glial (CG-4) and non-neural cells indicated that a 1200-bp 5′-flanking region could sustain neural cell-specific promoter activity (Chew et al., 2001). Sp1 binding suggests a functional role for Sp1 in initiator-mediated activation of Grik5 transcription that involves transcription factor II D-mediated basal activity (Chew et al., 2001). Removal of two putative AP2 sequences reduced promoter activity in both neural and non-neural cells, suggesting that these sites are also important for basal transcription. Furthermore, a 77-bp sequence termed the kainate cell-specific enhancer region, involved in cell-specific expression, includes a functional Sp1 site that when placed downstream of the Grik5 promoter, silenced reporter expression in NIH3T3 fibroblasts and attenuated activity in CG-4 cells. These studies show that elements contributing to tissue-specific expression are contained within the first exon (Chew et al., 2001).
C. N-Methyl-d-aspartate Receptors
1. Grin1.
Rat, human, and chicken Grin1 promoter regions have been cloned and characterized (Bai and Kusiak, 1993, 1995; Zimmer et al., 1995; Moreno-González et al., 2008). Transcription of the Grin1 gene is controlled by both positive and negative regulatory elements. A consensus RE1/NRSE silencer in exon1 contributes to neuronal-specific expression (Bai et al., 1998; Okamoto et al., 1999). Ablation of the RE1/NRSE site elevated GluN1 expression in non-neural cell lines and undifferentiated P19 cells (Bai et al., 1998, 2003; Okamoto et al., 1999, 2002). Likewise, during differentiation of P19 cells, REST/NRSF is down-regulated, resulting in de-repression of the Grin1 promoter (Okamoto et al., 1999). De-repression of the Grin1 promoter by absence of REST/NRSF occurs before subsequent expression of positive acting trans factors required for full Grin1 promoter activity (Bai et al., 2003). A 27-bp GC-rich region (GC-box) proximal to the transcription start sites has been identified that controls induction of the Grin1 gene upon differentiation of P19 cells, and this site is recognized by Sp1 and myc-associated zinc finger protein transcription factors (Okamoto et al., 2002). These sites previously were known to respond to Sp1, -3, and -4 transcription factors (Bai and Kusiak, 1995, 1997; Bai et al., 1998; Liu et al., 2001) and interact with an element further 5′ in the promoter (−520/−529) that is recognized by myocyte enhancer factor 2C (Krainc et al., 1998). Studies with Grin1 promoter constructs in PC12 cells suggest that NGF uses both the Ras/extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase pathways to up-regulate Grin1 promoter activity through Sp1 (Liu et al., 2001). Activation of serum glucocorticoid kinase 1, a downstream target of phosphatidylinositol 3-kinase, increases Grin1 promoter activity in PC12 cells and hippocampal neurons in an NFκB-independent manner (Tai et al., 2009). This finding is consistent with a previous report that the Sp-related factors regulate Grin1 promoter activity through occupancy of a putative NFκB consensus element ∼3 kb upstream of the GC box in neurons and in cell lines (Liu et al., 2004a).
AP1 protein complexes containing ΔFosB bind the rat Grin1 promoter at the AP1 consensus element, and AP1 binding is up-regulated after electroconvulsive shocks. Furthermore, an increase in Fos-like immunoreactivity was observed in the same cortical neurons that showed an increase in GluN1 immunoreactivity. Accordingly, up-regulation of GluN1 did not occur after seizures in fosB(−/−) mice (Hiroi et al., 1998).
GluN1 expression might be coupled to energy metabolism based on evidence that both the Grin1 and mitochondrial COX genes are under control of the NRF-1 transcription factor via binding elements in their respective proximal promoter regions (Dhar et al., 2008, Dhar and Wong-Riley, 2009). Grin1 and Grin2b, but not Grin2a or Grin3a, are positively regulated by the NRF-1 transcription factor through NRF-1 promoter elements. Furthermore, control of Grin1 and Grin2b by NRF-1 was activity-dependent. KCl up-regulated and TTX down-regulated expression in cultured rat cortical neurons, and NRF-1 itself is up-regulated at both protein and mRNA levels by depolarization (Yang et al., 2006; Dhar and Wong-Riley, 2009). Thus, NRF-1 is an essential transcription factor in the coregulation of Grin1, Grin2b, Gria2, and COX genes, coupling coordinated expression of glutamate receptors and energy metabolism at the transcriptional level (Wong-Riley et al., 1998a,b; Dhar et al., 2008, 2009; Dhar and Wong-Riley, 2009).
A nonpalindromic T-box element in the Grin1 promoter is likely to be recognized and regulated by Tbr-1/CASK protein complexes in vivo because GluN1 expression was reduced in Tbr-1(−/−) (T-brain-1) mice by ∼ 50% (Wang et al., 2004b). Tbr-1 is a neuron-specific T-box factor (Hsueh et al., 2000) that may play a role in neurogenesis and induction of GluN1. GluN1 expression is subject to control by hypoxia-inducible factors that function under stress conditions, especially during hypoxia (Yeh et al., 2008). Based on GluN1 up-regulation after lipopolysaccharide injection into the prefrontal cortex and in cultured neurons, the predicted cis hypoxia response elements were localized within the Grin1 promoter. However, 1 h of ischemia produced by middle cerebral artery occlusion decreased GluN1 (Gascón et al., 2005), possibly as a result of activation of the RE1 silencer (Fig. 4).
2. Grin2a.
Regulation of the rat Grin2a promoter was explored with a series of 3′- and 5′-truncated constructs in primary neurons, primary glia, and non-neuronal cell lines (Desai et al., 2002; Richter et al., 2002; Liu et al., 2003). The core promoter resides in exon 1, prefers neurons but also requires downstream sequences for full activity, and does not use a consensus TATA box. On the basis of overexpression studies and gel shift assays in stable cell lines, three GC-boxes (A, B, and C) seem to regulate Sp1 and Sp4 but not Sp3 transactivation (Liu et al., 2003).
Two regions of the mouse Grin2a promoter, from −9.2kb/−210 or −1253/−210, were able to confer nervous system expression of a transgene reporter. Based on primary cultures prepared from a −9.2kb/−210 Grin2a luciferase mouse, there was ∼700-fold selective expression in neuronal enriched cultures compared with glial cultures. Two RE1/NRSE-like sequences that contain key mismatches in the consensus sequences were identified at −989 and −427 and do not seem to act as silencers of the Grin2a gene. Thus, neuronal specificity for GluN2A expression seems to result from transcriptional activation selectively in neurons rather than by non-neuronal silencing. Furthermore, three regions were identified (−1253/−1079, −486/−447, 8 kb 5′ of −1253) that are important for maximal neuron-selective expression. Sequences between −9.2 kb and −1253 bp contribute to the maturational increase of Grin2a expression in cultured neurons and elements residing between −1253 and −1180 bp are crucial for this up-regulation (Desai et al., 2002).
Two NFκB sites were identified in the Grin2a promoter, which, when removed by mutation, resulted in loss of modification of transactivation by constitutively active SGK (SGK-S422D) that activates NFκB (Tai et al., 2009). Furthermore, the transactivation of a Grin2a construct was sensitive to the NFκB inhibitor peptide SN50 (Lin et al., 1995). A putative CRE element variant found in numerous promoters was identified at −1195 in mouse and −1215 in rat and raises interest in activity-dependent elevation of Grin2a in vivo. The putative CRE site resides in a region important for positive neuronal expression in both rat and mouse promoters (Desai et al., 2002; Richter et al., 2002; Liu et al., 2003).
3. Grin2b.
Initial promoter analysis using transgene constructs in mice (Sasner and Buonanno, 1996) revealed that the proximal promoter region and exon 1 (−550/+255 relative to the 5′-most transcription site) were sufficient to restrict tissue specificity to brain. However, inclusion of intron 1 and exon 2 in the transgene (−550/+1627) were required both to restrict expression to brain and to recapitulate the proper developmental profile of GluN2B expression in cerebellar granule cells. The presence of an RE1/NRSE-like element at the end of exon 1 was not responsible for conferring neural-selective expression in the mouse transgenes (Sasner and Buonanno, 1996). Of several putative RE1/NRSE elements in the more distal Grin2b promoter, the −2029/−2049 NRSE element bound REST/NRSF and repressed expression of Grin2b reporter constructs transfected into cultured neurons. Moreover, ethanol treatment of cortical cultures reduced REST/NRSF expression, resulting in GluN2B derepression (Qiang et al., 2005). Analysis of the Grin2b promoter identified Sp1 and CRE elements (Klein et al., 1998), and the CRE site was later confirmed to bind to phospho-CREB in a gel-shift assay. Mutation of the CRE motif in the Grin2b promoter region significantly decreased promoter activity in transfected cortical cells and also abolished ethanol-induced increase in promoter activity (Rani et al., 2005). Likewise, an AP-1 site was active in cultured neurons and responsive to ethanol treatment (Qiang and Ticku, 2005). Furthermore, it was found that long-term ethanol exposure promoted demethylation of CpG islands in the Grin2b promoter region that could result in up-regulation of the gene in mouse cortical neurons (Ravindran and Ticku, 2005).
Two nonpalindromic T-box elements in the Grin2b promoter were identified that are conserved across rat, human, and mouse (Wang et al., 2004b), and these elements are recognized by the Tbr-1 protein (Wang et al., 2004b). Functional and mutational studies in rat hippocampal cultures showed that overexpression of Tbr-1 alone and in combination with CASK elevated Grin2b promoter-driven luciferase activity by up to 120-fold dependent on each T-box element with the upstream T-element dominant. Recognition of the Grin2b T-box elements by a Tbr-1–CASK complex in vivo was demonstrated by ChIP analysis using rat hippocampal cultured neurons (Wang et al., 2004a). In Tbr-1–null mice, GluN2B expression was decreased up to 60%, and GluN2B expression was down-regulated in brain regions where Tbr-1 immunoreactivity was lost in the mutant mice. These two T-box elements reside within the minimal transgene construct sufficient for neuron-specific expression of the Grin2b gene (Sasner and Buonanno, 1996). A point mutation in CASK that disrupts CASK–Tbr-1–CASK-interacting nucleosome assembly protein complexes down-regulates Grin2b promoter activity (Huang and Hsueh, 2009). CASK interacts with transcription factor Tbr-1 and CASK-interacting nucleosome assembly protein–cell division autoantigen-1–differentially expressed nucleolar transforming growth factor-β1 target in the nuclei of neurons, which may remodel the chromatin structure flanking Tbr-1 binding sites (Hsueh et al., 2000; Wang et al., 2004a).
As shown for the Grin2a promoter, activation of serum glucocorticoid kinase 1 pathway elevates Grin2b gene expression in hippocampal neurons and Neuro2A cells in an NFκB-dependent manner. The site of NFκB binding in the Grin2b promoter was not specifically identified but was proposed to reside between −1480 and −2020 bp from the rat transcription start site (Tai et al., 2009).
Stimulation of cortical cultures with bicuculline elevated GluN2B expression in a transcription- and calcineurin-dependent manner (Qiu and Ghosh, 2008) and revealed association of the Grin2b promoter with CREST, brahma-related gene 1, CRE binding protein, and HDAC-1. Bicuculline stimulation increased CRE binding protein, decreased HDAC1, and increased the association of the Grin2b promoter with acetylated histones. The increase in GluN2B expression also required NMDA receptor activation and was shown to depend on CREST in vivo because a bicuculline-induced increase in GluN2B expression was absent in CREST-null neurons. These findings suggest that the activity-dependent increase in GluN2B expression involves a switch from a repressor to activator complex and requires CREST function that may involve CRE and Sp1 sites in the promoter in a manner similar to regulation of the immediate early gene c-fos.
Evidence for the coupling of GluN2B expression to energy metabolism has also been described through a series of electrophoretic mobility shift assay, supershift, and ChIP assays and promoter mutations (Yang et al., 2006; Dhar et al., 2009). GluN2B was shown to be regulated in cultured neurons by NRF-1 transcription factor via an NRF-1 element in the proximal promoter region. GluN1, GluN2B, and NRF-1 transcripts are up-regulated by KCl and down-regulated by TTX in cultured primary neurons. Thus, NRF-1 coordinates the coregulation of Grin1, Grin2b, and COX genes (Dhar et al., 2008; Dhar and Wong-Riley, 2009).
4. Grin2c, Grin2d, Grin3a, and Grin3b.
Elements within Grin2c exon 1 and intron 1 in transgenic mouse lines selectively drive expression of β-galactosidase in cerebellar granule cells (Suchanek et al., 1995, 1997). This region contains a consensus RE1/NRSE silencer, but the role of this element has not been fully evaluated. Furthermore, Sp1 and COUP-TF consensus elements (Nagasawa et al., 1996; Pieri et al., 1999) bound Sp1 and fushi tarazu factor 1/COUP-TF protein in gel shift assays, but mutations did not modify promoter function in a transfected neuronal cell line (Pieri et al., 1999). The COUP-TF site seems to require other elements for function. Coexpression of steroidogenic factor-1 elevated Grin2c promoter activity modestly in a neuronal cell line dependent on a promoter region centered −250 from transcription + 1 site (Pieri et al., 1999); however, direct expression in neurons was not examined.
The developmental up-regulation of GluN2C in the adult cerebellum upon innervation of mossy fibers onto granule cells has been reported to be due to neuregulin-β (Ozaki et al., 1997). Neuregulin-β potently up-regulated GluN2C with no change in GluN2B expression in cultured mouse cerebellar slices; up-regulation was sensitive to block by TTX or the NMDA antagonist 2-amino-5-phosphonopentanoate, suggesting that synaptically activated NMDA receptors are involved (Ozaki et al., 1997). Activity regulates GluN2B expression in cerebellar granule cells (Vallano et al., 1996), and both GluN2B and GluN2C in organotypic cultures (Audinat et al., 1994), although the stimulatory effect of neuregulin-β on GluN2C was not recapitulated in cultures of dissociated granule cells (Rieff et al., 1999). For granule cells cultured in low (5 mM) KCl, BDNF up-regulated GluN2C mRNA via the tyrosine kinase receptor ERK1/2 cascade, whereas under 25 mM KCl, depolarization stimulated Ca2+ entry through voltage-sensitive Ca2+ channels and activated Ca2+/calmodulin-dependent calcineurin phosphatase, which opposed GluN2C mRNA up-regulation (Suzuki et al., 2005). However, the depolarization-induced Ca2+ increases simultaneously up-regulated BDNF mRNA via CaMK. Thus, convergent mechanisms of the BDNF and Ca2+ signaling cascades are important for GluN2C induction in granule cells during development (Suzuki et al., 2005). NMDA receptor activation was shown to coordinate both the up-regulation of GluN2C and the down-regulation of GluN2B mRNA, including a switch of GluN2 subunit associated with cell surface NMDA receptors in cultured mouse granule cells (Iijima et al., 2008). Although much has been learned about the signal transduction pathways leading to receptor subunit changes in granule cells, the promoter control elements responsible for transcription subunit switching remain to be identified.
In the human GRIN2D gene, the 3′-UTR contains four half-palindromic estrogen responsive elements within a 0.2-kb region that are highly preserved in the rat, suggesting that the GluN2D subunit may be up-regulated in vivo via neuroendocrine control. In ovariectomized rats, up-regulation of GluN2D mRNA in the hypothalamus upon 17β-estradiol treatment was observed (Watanabe et al., 1999), and the Grin2d half-palindromic estrogen responsive elements, placed in a 5′ or 3′-UTR position in a chloramphenicol acetyltransferase promoter construct, were responsive to estrogen and thyroid hormone exposure in an orientation- and hormone receptor-dependent manner (Watanabe et al., 1999; Vasudevan et al., 2002).
The amino acid sequences and expression profile for Grin3a and Grin3b have been reported (Ciabarra et al., 1995; Sucher et al., 1995; Andersson et al., 2001; Nishi et al., 2001; Chatterton et al., 2002; Eriksson et al., 2002; Matsuda et al., 2002; Bendel et al., 2005). Studies describing control of transcription with respect to cis regulatory elements and trans factors have not been reported.
D. Translational Control of Glutamate Receptors
The translation of mRNA to protein is regulated by mechanisms that control 5′ capping, 3′ polyadenylation, splicing, RNA editing, mRNA transport, stability, and initiation and elongation (VanDongen and VanDongen, 2004; Coyle, 2009). The 5′-UTR of most glutamate receptor mRNAs is unusually long. These long 5′-UTRs often exhibit stretches of high GC content and sometimes contain multiple out-of-frame AUG codons that could act as decoys for scanning ribosomes, reducing or preventing translation initiation at the true glutamate receptor AUG (Myers et al., 1999; VanDongen and VanDongen, 2004). Translational suppression has been inferred for GluN1 mRNA natively expressed in PC12 cells because no GluN1 protein can be detected despite a moderately high mRNA level (Sucher et al., 1993). On the basis of that study (Sucher et al., 1993), it was proposed that translation of GluN1 message may be suppressed, perhaps by an unidentified motif in the 5′- or 3′-UTR. Two pools of GluN1 mRNA with different translational activities have been identified in neonate and adult brain, further implicating potential translation control mechanisms at work in neurons (Awobuluyi et al., 2003). Whether GluN1 translation rate was determined by alternative 3′UTRs was not explored. Visual deprivation in juvenile mice reduced the GluN2A/GluN2B ratio in the deprived cortex with the finding that translation of GluN2B is probably a major regulatory mechanism (Chen and Bear, 2007).
The efficiency of translation of another subunit, GluN2A, also depends upon features of the 5′-UTR. Here, in both in vitro translation in rabbit reticulocyte lysate and the X. laevis oocyte expression system, removal of most of the 282 bases of the 5′-UTR from the cDNA increased GluN1/GluN2A protein expression by more than 100-fold. Mutation of three of the five upstream AUG codons modestly increased translation; however, disruption of a proposed GC-rich stem-loop structure 170 bases upstream of the AUG increased GluN2A translation by 40-fold (Wood et al., 1996). Translation suppression of GluN2A transcripts in vivo is one interpretation of the finding that high GluN2A mRNA levels were measured in the inferior and superior colliculus and striatum of adult rats (Goebel and Poosch, 1999), whereas immunocytochemical studies showed staining for the GluN2A protein to be light in these areas (Petralia et al., 1994).
The translation efficiencies of several GluA2 5′-UTR transcripts in rabbit reticulocyte lysates, X. laevis oocytes, and primary cultured neurons has been investigated (Myers et al., 2004). Transcripts containing long 5′ leaders were translated poorly compared with those with shorter leaders, and short transcripts were preferentially associated with polyribosomes in vivo. Suppression of GluA2 translation was dominated by a 34- to 42-nucleotide imperfect GU repeat sequence in the 5′-UTR predicted to form a secondary structure. It is noteworthy that the GU repeat domain is polymorphic in man and is included in a subset of rat and human GluA2 transcripts based on the site of transcription initiation (Myers et al., 1998, 2004). GluA2 translation was not modified significantly by deletion of any or all of the five upstream AUG codons. An interpretation of both the GluN2A and GluA2 studies is that a scanning ribosome encounters the proposed stem-loop and stalls because of an inability to “melt” the stem-loop structure; alternatively, a ribosome may encounter a blocking protein bound at the stem-loop motif. Either case could result in dissociation of the ribosome from the mRNA.
GluA2 transcripts are processed to form either a short or a long 3′-UTR giving rise to two pools of GluA2 mRNAs of 4 and 6 kb in length in brain. In the hippocampus, long 3′-UTR GluA2 transcripts are retained primarily in translationally dormant complexes of ribosome-free messenger ribonucleoprotein, whereas GluA2 transcripts bearing the short 3′UTR are associated mostly with actively translating ribosomes (Irier et al., 2009b). After pilocarpine-induced status epilepticus, selective translational derepression of GluA2 mRNA mediated by the long 3′-UTR transcripts was observed, suggesting that the long 3′-UTR of GluA2 mRNA alone is sufficient to suppress translation and that an activity-dependent regulatory signaling mechanism exists that differentially targets GluA2 transcripts with alternative 3′-UTRs (Irier et al., 2009b). Differential effects of antibiotics that target translational initiation and elongation suggest that the long 3′-UTR suppresses GluA2 at the initiation step, implying a loop-back mechanism (Irier et al., 2009a). The mechanism of translation is not known but could involve binding of a cytoplasmic polyadenylation element binding protein (CPEB). Among the CPEB proteins, CPEB3 is expressed specifically in neurons (Theis et al., 2003) and seems to bind to GluA2 long 3′-UTR. RNA interference knockdown of CPEB3 mRNA induces GluA2 protein expression in cultured hippocampal neurons (Huang et al., 2006). CPEB protein regulates translation initiation (Richter and Sonenberg, 2005), facilitates targeting of mRNAs to dendrites (Huang et al., 2003), and has been implicated in control of GluN1 translation (Wells et al., 2001a). A related report identified a deletion allele in the Grik4 gene 3′-UTR that was negatively associated with bipolar disorder, and it was proposed, on the basis of expression data, that RNA secondary structure modified mRNA stability to enhance protein expression (Pickard et al., 2008).
The localization of translation machinery near postsynaptic sites (Steward and Levy, 1982; Steward and Reeves, 1988) and differential distribution of mRNAs to dendrites, including those for GluA1, GluA2, and GluN1, have been investigated (Steward and Schuman, 2001; Schratt et al., 2004; Grooms et al., 2006). It is becoming clear that these dendritic mRNAs may form a pool poised for translation to modify neuronal plasticity. Other studies have demonstrated that protein synthesis in dendrites is critical for long-term potentiation (LTP) and long-term depression (LTD) (Kang and Schuman, 1996; Huber et al., 2000; Tang and Schuman, 2002; Bradshaw et al., 2003; Cracco et al., 2005; Mameli et al., 2007). The induction of protein synthesis is, not unexpectedly, dependent upon NMDA receptor activation (Scheetz et al., 2000; Huang et al., 2002b; Gong et al., 2006; Tran et al., 2007).
IV. Post-Translational Regulation
A. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid and Kainate Receptor Phosphorylation
Considerable advances have been made to identify dynamically regulated post-translational phosphorylation sites on the C-terminal domains of most of the glutamate receptor subunits and to understand the functions of these modifications. Phosphorylation has been shown to regulate glutamate receptor trafficking from the ER, insertion into the plasma membrane, endocytosis, synaptic localization, and binding to other proteins (Malinow and Malenka, 2002). In a few cases, phosphorylation seems to regulate the relative frequency of opening for different subconductance levels of the channels in a manner independent of trafficking. However, more work is needed to understand the mechanisms by which phosphorylation can control glutamate receptor function. In some cases, the strategic combination of specific phosphoprotein antibodies, site-directed mutagenesis, and chromophore-tagged receptors has made it possible to associate the functional consequences of kinase activation with phosphorylation of specific amino acid residues. However, the specific functional roles of many phosphorylation sites have simply not yet been explored, and in nearly all cases, the molecular mechanisms by which phosphorylation changes receptor function (membrane insertion, open probability, etc.) are unknown. These topics represent excellent opportunities for future progress.
The C-terminal domains are shown for each of the subunits in Figs. 5–7, with residues that have been shown to be post-translationally modified in living cells in red; residues identified only in cell-free kinase assays are omitted. Authors have variably included the signal sequence in the numbering schemes and studied various C-terminal splice variants. Here, sites will be referred to by residue position in the literature. To minimize confusion with numbering schemes, we simply include the surrounding sequences in the text together with the most commonly used residue number. This number diverges from the numbering in Figs. 5 to 7 when authors indexed the predicted signal peptide cleavage site as 1.
The GluA1 C terminus has multiple phosphorylation sites, including four for PKC, one for PKA, and one for CAMKII. Phosphorylation of the two membrane-proximal PKC sites (SRS816ES818KR) enhances interaction between the actin-binding 4.1N protein and the GluA1 C-terminal domain, which facilitates insertion of this subunit into the plasma membrane (Boehm et al., 2006; Lin et al., 2009), a mechanism involved in long-term potentiation. Phosphorylation of TST840LPR by PKC has been suggested to influence synaptic transmission in an age-dependent fashion (Lee et al., 2007b). Two other GluA1 phosphorylation sites control functional properties of AMPA receptor channels. Phosphorylation of RNS845GA by PKA (Roche et al., 1996) increases the open probability of homomeric GluA1 channels studied in outside-out patches (Banke et al., 2000), which has been proposed to reflect a change in the equilibrium of GluA1 with an inactive state, perhaps relating to phosphodependent binding of intracellular regulatory proteins. PKA phosphorylation additionally drives GluA1 subunits into synaptic membranes (Esteban et al., 2003; Man et al., 2007). Phosphorylation of QQS831IN by CAMKII or PKC (Barria et al., 1997; Mammen et al., 1997) increases single channel conductance (Derkach et al., 1999; Oh and Derkach, 2005). In vivo evidence from transgenic animals suggests that GluA1 phosphorylation is critical for synaptic plasticity (Lee et al., 2000, 2003; Whitlock et al., 2006; Tsui and Malenka, 2006). Considerably more work will be required before we achieve adequate understanding of the relative contribution of phosphorylation-linked changes in trafficking and channel function to changes in synaptic strength (Song and Huganir, 2002; Derkach et al., 2007; Kessel and Malinow., 2009) (see sections IX.E and IX.F).
GluA2 splice variants create short and long C termini (Fig. 5), phosphorylation of which influences receptor trafficking, synaptic plasticity, and several receptor-protein interactions. The long tail has a phosphorylation site at VMT874PE that is a Jun kinase target (Thomas et al., 2008). Dephosphorylation at this site is activity-dependent and promotes reinsertion of internalized GluA2 back into the plasma membrane. Targeted disruption of the PKC recognition sequence around IES880VK eliminated LTD in mouse cerebellum (Steinberg et al., 2006), confirming a major role for this modification in synaptic plasticity. The activity-dependent phosphorylation of GluA2-short by PKC on IES880VK weakens its binding to GRIP but improves binding to protein interacting with C kinase 1, which slows recycling of GluA2-containing AMPA receptors back to the plasma membrane after internalization (Matsuda et al., 1999; Chung et al., 2000; Seidenman et al., 2003; Lin and Huganir, 2007; Park et al., 2009). The situation is complex, however, because States et al. (2008) identified a large population of synaptic GluA2 receptors bearing phospho-Ser880, which presumably had secured synaptic anchors other than GRIP. Finally, phosphorylation of the nearby NVY876GI by src family kinases also seems to weaken association with GRIP (Hayashi and Huganir, 2004).
Three phosphorylation sites have been identified by mass spectrometry (Munton et al., 2007; Ballif et al., 2008; Trinidad et al., 2008) on the GluA3 C-tail but have not yet been studied functionally. The TES891VK site near the terminus is surrounded by sequences homologous with those of the other AMPA receptor subunits and may be a PKC target.
GluA4 has alternate C-tails, with the short tail terminated by a PKC target at TES899IK (Esteban et al., 2003). The long GluA4 C-tail harbors a combined PKC/PKA target at RLS842IT (Carvalho et al., 1999; Gomes et al., 2007) and may bind directly to some PKC isoforms (Correia et al., 2003). Phosphorylation is enhanced by synaptic activity and promotes surface expression of GluA4 by disrupting its association with α-actinin-1 (Nuriya et al., 2005). Similar to GluA2, the Jun kinase site on GluA4 (VLT855PD) is phosphorylated at rest but rapidly dephosphorylated within minutes of synaptic activity and presumably functions, as in GluA2-long, to enhance surface expression of GluA4 (Thomas et al., 2008). The functional consequence of GluA4 phosphorylation by c-Jun NH2-terminal kinase has not yet been examined.
Most studies have suggested a trafficking function for phosphorylation of specific residues in AMPA receptor C termini, and a similar pattern seems to exist for kainate receptor phosphorylation. Serine residues KKS879RT and GKS885SF of GluK1 are phosphorylated by PKC, resulting in internalization (Rivera et al., 2007). These sites, previously identified by Hirbec et al. (2003) on the basis of in vitro kinase assays, may be involved in autoregulation by kainate receptor activation (Rivera et al., 2007). The long C-tail of GluK2 is phosphorylated by PKA on serine residues KFS825FC and RMS837LK, which potentiates receptor activation in whole-cell patch studies (Kornreich et al., 2007), apparently through an increase in receptor open probability (Traynelis and Wahl, 1997). There are no reported modification sites in the C-tail of GluK3 or GluK5, whereas GluK4 has four phosphorylation sites identified by mass spectrometry.
B. N-Methyl-d-aspartate and δ Receptor Phosphorylation
Given the function of NMDA receptors in synaptic plasticity (see section IX), a wealth of studies exist describing the consequences of modification of specific residues on its C termini. GluN1 has four different C-terminal tails created by alternative splicing (Fig. 6), only the longest of which has been shown to be influenced by phosphorylation. Disrupting a ring of tyrosine residues adjacent to the M4 domain by site-directed mutagenesis of IAY837KR on GluN1 and LFY842WK on GluN2A prevented use-dependent desensitization of GluN1/GluN2A receptors (Vissel et al., 2001). It is noteworthy that tyrosine phosphorylation of GluN2A but not GluN1 was detected with phosphotyrosine antibodies (Lau and Huganir, 1995). This same ring of tyrosines is present in all NMDA receptor subunits as well as all AMPA and kainate receptor subunits except GluK4 and GluK5 (Figs. 5 to 7), but whether each of these tyrosines is subject to phosphorylation by src family kinases with resulting functional consequences has yet to be explored. PKC targets serine residues ASS890FK and RRS896SK on GluN1, and PKA targets the adjacent RSS897KD. Within minutes of PKC activation, phosphorylation of ASS890FK disrupts surface clusters of NMDA receptors (Tingley et al., 1997). The dual PKC-PKA phosphorylation of RRS896S897K, on the other hand, promotes exit of the subunit from the endoplasmic reticulum and transit to the surface membrane (Scott et al., 2001, 2003). The phosphorylation of GluN1 ASS890FK and RRS896SK is achieved by PKCγ and PKCα, respectively (Sanchez-Perez and Felipo, 2005), potentially contributing to the selective modulation of NMDA receptor function and/or intracellular localization. Prolonged synaptic activation during status epilepticus initially causes dephosphorylation of ASS890FK, then hyperphosphorylation that develops over hours and subsides within a day (Niimura et al., 2005). The PKA site RSS897KD behaves similarly, but the adjacent PKC site RRS896SK seems untouched by status epilepticus, demonstrating a remarkable specificity.
The GluN2A subunit also occurs with alternative C-tails (Fig. 6) (444 and 626 amino acids), and each has phosphorylation sites that can modify function. The consequence of phosphorylation of LFY842WK, which is present on both C-tails, was described above. Both C-tails also harbor MRS1232PF; phosphorylation of this serine by CDK5 is associated with increased NMDA-evoked currents (Li et al., 2001), and excitotoxic death of hippocampal CA1 pyramidal cells (Wang et al., 2003). Krupp et al. (2002) demonstrated that glycine-independent desensitization of GluN1/GluN2A receptors could be eliminated by mutation of either of two serine residues proximal to the fourth transmembrane domain, LRS900AK and RGS932LI, but the kinases acting on these serines have not been identified. Nine additional serines or tyrosines have been reported by mass spectroscopy as phosphorylated on both long and short C-tails, but phosphorylation of exclusive residues near the end of the longer C-tail regulates NMDA receptor modulation. For example, src kinase weakens high-affinity zinc inhibition of recombinant GluN1/GluN2A receptors, an effect that is eliminated by mutation of DPY1387KH (Zheng et al., 1998; but see Xiong et al., 1999); this same tyrosine was shown to account for approximately 30% of the src-induced phosphorylation of GluN2A (Yang and Leonard, 2001). Src also phosphorylated HSY1292DN (Yang and Leonard, 2001), but this had no effect on zinc inhibition (Zheng et al., 1998). By contrast, mutation of QVY1267QQ blocked the src effect on zinc inhibition, but this residue does not seem to be directly phosphorylated by src (Yang and Leonard, 2001). These results, considered together, raise the possibility that the electrophysiological consequences of mutating Tyr1267 are due to an allosteric effect on receptor function.
Insulin potentiates the activation of GluN2A-containing NMDA receptors, an effect traced to two serines phosphorylated by PKC, QHS1291YD, and SIS1312LK (Jones and Leonard, 2005). It is noteworthy that Ser1291 is immediately adjacent to the src target Tyr1292, yielding high potential for cross-talk between these kinases; one wonders, for example, whether the lack of effect of Tyr1292 on zinc inhibition (Zheng et al., 1998) was due to obstructive phosphorylation of the adjacent Ser1291 by PKC.
The GluN2B C-tail (Fig. 7) contains a prominent src target very near the C terminus on HVY1472EK, which may regulate endocytosis in some conditions (Cheung and Gurd, 2001; Nakazawa et al., 2001; Snyder et al., 2005). Phosphorylation of this tyrosine is eliminated in a fyn knockout (Abe et al., 2005) and is increased in hippocampal CA1 during LTP (Nakazawa et al., 2001) as well as in a chronic neuropathic pain model (Abe et al., 2005). Although regulation of this tyrosine by fyn kinase is well understood, the immediate functional consequence of phosphorylation has not been studied. This tyrosine is also phosphorylated in an activity-dependent manner by the transmembrane tyrosine kinase EphrinB2 (Nateri et al., 2007), which is activated by upstream ERK pathways. Therefore, multiple kinases target Tyr1472 in an activity-dependent manner.
Another major modification of GluN2B in the postsynaptic density fraction of the forebrain occurs by phosphorylation of QHS1303YD by CaMKII (Omkumar et al., 1996). The CaMKII system provides a mechanism by which GluN2B-containing NMDA receptors can be modified rapidly upon Ca2+ influx associated with NMDA receptor activation and in this sense may represent the prototype of a kinase activated by its target (Bayer et al., 2001), but the functional consequences of this modification have not been directly addressed. Finally, the C-terminal PDZ domain of GluN2B contains a site, IES1480DV, that, when phosphorylated by casein kinase II, disrupts the interaction of GluN2B with PSD-95 and SAP102, thereby decreasing surface expression of this receptor (Chung et al., 2004). This represents another activity-dependent phosphorylation that regulates trafficking of GluN2B in the plasma membrane. The association between active CaMKII and GluN2B seems to be required for LTP, although the phosphorylation target has not been identified (Barria and Malinow, 2005).
PKC and PKA both phosphorylate GluN2C RIS1230SL (Fig. 7) near the extreme carboxyl terminus but exert no apparent effect on surface expression of GluN2C or on its interaction with PDZ family proteins on the adjacent PDZ domain. However, a phosphomimetic GluN2C(S1230E) mutant exhibited faster activation and inactivation kinetics in outside-out patches (Chen et al., 2006). Thus, unlike other glutamate receptor subunits, phosphorylation of this serine near the PDZ domain does not affect trafficking but instead alters channel properties. A site in the C-terminal domain of GluN2C, HAS1096LP, is a unique (among the glutamate receptors) target for PKB/Akt; interestingly, phosphorylation of Ser1096 creates a binding site for 14-3-3 (Chen and Roche, 2009), which apparently assists in the trafficking of GluN2C to the surface membrane. Phosphorylation of Ser1096 is enhanced by insulin-like growth factor-1 stimulation or NMDA receptor activation, providing a link between hormonal state and NMDA receptor function. Three phosphorylated serines in GluN2D have been identified by mass spectroscopy (Fig. 7) but have not yet been studied functionally. Likewise, a phosphotyrosine antibody labels GluN2D immunoprecipitated from rat thalamus (Dunah et al., 1998b), but the targeted tyrosines have not been identified.
No modifications of GluN3A, GluN3B, or GluD1 have been described yet, but GluD2 has four phosphoserines, one of which (TLS945AK) was shown to be a PKC target (Fig. 7) (Kondo et al., 2005). The GluD2 subunit is expressed mainly by cerebellar Purkinje cells, and both it and PKC seem to be essential for long-term depression at the parallel fiber-Purkinje cell synapse (Kashiwabuchi et al., 1995; Kondo et al., 2005). However, LTD could be rescued in a GluD2-null mouse by a GluD2(S945A) transgene, demonstrating that phosphorylation of Ser945 plays no role in LTD (Nakagami et al., 2008). Indeed, a transgene lacking the transmembrane domains could rescue LTD (Kakegawa et al., 2007b), suggesting that GluD2 might function as a scaffolding protein rather than an ionotropic receptor.
C. Other Post-Translational Modifications of Glutamate Receptors
Additional post-translational modifications can affect glutamate receptor localization or activity. All ionotropic glutamate receptor subunits seem to be glycosylated (see section II), which seems to be involved in proper folding of the subunit during synthesis (Everts et al., 1997; Mah et al., 2005; Nanao et al., 2005; Gill et al., 2009). In addition, multiple glutamate receptor subunits undergo dynamic regulation by palmitoylation (Figs. 5–7). The AMPA receptors have two known palmitoylation sites in the membrane domain 2 and C-tail. All AMPA receptors have a conserved cysteine residue proximal to M4 that undergoes palmitoylation (GluA1-EFC811YK, GluA2-C836, GluA3-C841, GluA4-C817) (Hayashi et al., 2005). In GluA1 and GluA2, palmitoylation of the C-tail residue reduces insertion rate (Hayashi et al., 2005) and regulates phosphorylation of the two serines on the C-terminal tail by PKC (Lin et al., 2009). Thus, the interplay between palmitoylation and phosphorylation of residues on this membrane-proximal region of GluA1 influences membrane insertion and thus synaptic availability of this subunit. All AMPA receptors have another conserved cysteine residue just downstream of the QRN site (GluA1-QGC585DI, GluA2-C610, GluA3-C615, GluA4-C611) that, when palmitoylated, increases AMPA receptor surface expression (Hayashi et al., 2005). The homomeric kainate receptor GluK2 undergoes palmitoylation of the cysteines SFC858SA and LKC871QR, both of which have no apparent effect on basal receptor function. In addition to palmitoylation, GluK2 has been shown to be SUMOylated at lysine IVK896TE, a process that facilitates endocytosis after kainate receptor activation (Martin et al., 2007). GluK3 has been shown to be SUMOylated, although the site has not been identified (Wilkinson et al., 2008).
Like AMPA and kainate receptors, the GluN2A and GluN2B subunits also undergo palmitoylation at their C termini (Hayashi et al., 2009). Both of these subunits have two separate clusters of cysteine residues that are palmitoylated with distinct consequences on receptor expression and internalization. The first cluster of palmitoylated cysteine residues within GluN2A (RFC848FT, GVC853SD, YSC870IH) and the homologous cysteines in GluN2B (Fig. 7) increase nearby tyrosine phosphorylation of the C-tails by src family kinases, regulating surface expression and receptor internalization. Tyrosine phosphorylation of the C-tails eliminated receptor interaction with activator protein-2, decreasing clathrin-mediated endocytosis. Palmitoylation of the second cluster of cysteine residues within GluN2A (THC1214RSC1217LS, FKC1236DAC1239LR) and the homologous residues in GluN2B plus EAC1245KK leads to accumulation of the NMDA receptors in the Golgi apparatus and decreased surface expression (Hayashi et al., 2009). Thus, palmitoylation of GluN2A and GluN2B provides a dual mechanism by which post-translational modification controls NMDA receptor surface expression. It is unknown if GluN2C or GluN2D subunits undergo palmitoylation, but both have cysteine residues homologous to GluN2A and GluN2B within their C-terminal tails, leading to the possibility that palmitoylation also regulates their surface expression. Studies so far suggest that the GluN1 subunit does not undergo palmitoylation (Hayashi et al., 2005).
Both GluN1 and GluN2 subunits undergo S-nitrosylation on cysteine thiol groups by endogenous nitric oxide (NO) and exogenously applied S-nitrosothiols. The GluN1 subunit has two cysteine groups within the M3–M4 extracellular linker, QKC744DL and QEC798DS, and the GluN2A subunit has three cysteine groups within the ATD, HVC87DL, ASC320YG, and SDC399EP, that can be S-nitrosylated (Choi et al., 2000). The S-nitrosylated cysteine residues within the GluN1 and GluN2 subunits mainly fit the S-nitrosylation consensus motif of a cysteine residue preceded by an acidic or basic residue and followed by an acidic residue (Stamler et al., 1997). S-Nitrosylation of any of these cysteine residues within GluN1/GluN2A leads to moderate NMDA receptor inhibition, decreasing the response to agonist-evoked currents by approximately 20%, but a majority of the inhibition is due to Cys399 on the GluN2A subunit ATD (Choi et al., 2000). Inhibition of GluN2A current amplitude through S-nitrosylation of Cys399 is due to decreased channel opening, which may be caused by the increased affinity of the receptor for Zn2+ and glutamate, leading to receptor desensitization (Paoletti et al., 2000; Zheng et al., 2001; Lipton et al., 2002). The GluN2A subunit is sensitized to S-nitrosylation when Cys744 and Cys798 of the GluN1 subunit are S-nitrosylated (Takahashi et al., 2007). It is not known whether the remaining GluN2 subunits are S-nitrosylated or inhibited by this modification. The ability of NO to regulate NMDA receptors may provide a feedback mechanism to prevent excessive receptor activity, because NMDA receptor-catalyzed transmembrane currents increase the local Ca2+concentration, which activates neuronal nitric-oxide synthase through their mutual association with PSD-95 (Sattler et al., 1999; Rameau et al., 2003).
NMDA receptors are regulated by the extracellular redox state; sulfhydryl reducing agents such as dithiothreitol and dihydrolipoic acid potentiate NMDA-evoked currents through the formation of free thiol groups, whereas oxidizing agents such as 5,5′-dithio-bis(2-nitrobenzoic acid) and oxidized glutathione inhibit currents through the formation of disulfide bonds (Aizenman et al., 1989; Sucher and Lipton, 1991; Köhr et al., 1994; Choi and Lipton, 2000). Both GluN1 and GluN2 subunits are responsible for redox modulation. Disulfide bonds formed between two pairs of cysteine residues within GluN1 (SVC79ED and RGC308VG within the ATD and Cys744 and Cys798 in the LBD) cause the intermediate and slow components of redox modulation for all GluN2-containing NMDA receptors (Sullivan et al., 1994; Choi et al., 2001). A disulfide bond has been proposed to form in the GluN2A subunit between Cys87 and Cys320 of the GluN2A ATD and has been shown to form between Cys86 and Cys321 in the crystal structure of GluN2B ATD (Karakas and Furukawa, 2009). This disulfide bond has been suggested to mediate a fast-component of redox modulation, although this may also reflect modification of the Zn2+ binding site (Köhr et al., 1994; Choi et al., 2001). Reducing agents can chelate zinc, and transient potentiation that occurs only in the presence of agents such as dithiothreitol reflects in part the relief of zinc inhibition through zinc chelation (Paoletti et al., 1997). In addition, reduction of cysteine residues within GluN1/GluN2A NMDA receptors also reduces high-affinity voltage-independent Zn2+ inhibition of NMDA receptors (Choi et al., 2001).
D. Proteolysis of Glutamate Receptors
A number of proteases can cleave glutamate receptors. Principal among these are serine proteases, which can act on GluN1, GluN2A, and GluN2B. Tissue plasminogen activator can cleave GluN1 at ALR260YA, whereas plasmin and thrombin cleave at multiple sites within GluN1, presumably with functional consequences (Gingrich et al., 2000; Fernández-Monreal et al., 2004; Samson et al., 2008). A specific plasmin cleavage site (EAK317AS) has been described in GluN2A, proteolysis of which leads to removal of the ATD and the high-affinity Zn2+ binding site contained therein (Yuan et al., 2009b). Thrombin cleaves the analogous residue (Lys318) in GluN2B (Leung et al., 2007). In addition, the calcium-dependent nonlysosomal protease family calpains can cleave the C termini of GluA1, GluN2A, GluN2B, and GluN2C, resulting in receptor degradation and reduced synaptic activity (Bi et al., 1998a,b; Guttmann et al., 2001, 2002; Rong et al., 2001; Simpkins et al., 2003; Araújo et al., 2005). It is noteworthy that specific proteolytic cleavage has been proposed to occur in pathological situations such as ischemia (Yuen et al., 2007), blood-brain barrier breakdown (Yuan et al., 2009b), and status epilepticus (Araújo et al., 2005). In addition, matrix metalloproteinase-7 has been shown to cleave the LBDs of the GluN1 and GluN2A subunits, decreasing the NMDA receptor-mediated calcium influx and increasing the ratio of AMPA to NMDA receptors in cortical slices (Szklarczyk et al., 2008). Among the AMPA receptors, GluA3 has been reported to serve as a substrate for proteolysis by gamma secretase and granzyme B (Gahring et al., 2001; Meyer et al., 2002); glycosylation of ISN388DS protects GluA3 from cleavage by granzyme B during breakdown of the blood-brain barrier. GluA1 can be cleaved at SHD865FP through activation of calpain and caspase8-like activity (Bi et al., 1996; Meyer et al., 2002). AMPA receptor proteolysis may be common in neuropathological conditions (Bi et al., 1998a; Chan et al., 1999).
V. Agonist and Antagonist Pharmacology
Amino acid numbering in AMPA and kainate receptor subunits has historically been for the mature protein without the signal peptide, whereas amino acid numbering of NMDA and GluD2 receptor subunits has started with the initiating methionine as 1. To simplify comparison with the published literature, we will maintain this informal convention here.
A. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid, Kainate, and δ Receptor Agonists
Glutamate activates all AMPA and kainate receptors by binding within the cleft between domains D1 and D2 of the LBD to induce domain closure. Neither NMDA nor l-aspartate can activate the non-NMDA receptors, and d-aspartate acts as a low-affinity competitive antagonist for native AMPA receptors (Gong et al., 2005). In the structure of the glutamate-bound GluA2 LBD (Fig. 8A) (Armstrong and Gouaux, 2000), the α-amino group of glutamate forms a tetrahedral network of interactions with the backbone carbonyl oxygen of Pro478, the side chain hydroxyl of Thr480, and the carboxylate group of Glu705. The α-carboxyl group of glutamate forms a bidentate interaction with Arg485 and receives hydrogen bonds from the backbone amide nitrogens of Thr480 and Ser654. The glutamate γ-carboxyl group interacts with the hydroxyl group and backbone amide nitrogen of Thr655 (Fig. 8A), whereas the isoxazole hydroxyl group of AMPA interacts with the amide nitrogen of Thr655 via a water molecule. Furthermore, the side chain of Tyr450 forms an electron-dense ring structure above the glutamate α- and β-carbon atoms resembling a lid above the agonist binding pocket (Fig. 8A). Structures of the kainate receptor subunits GluK1 and GluK2 LBDs show similar atomic contacts for the α-carboxyl group, α-amino group, and γ-carboxyl groups of glutamate, although residues lining the GluK1 agonist binding pocket are smaller than GluK2 (Mayer, 2005; Nanao et al., 2005; Naur et al., 2005; Mayer et al., 2006) (Fig. 8B). There are several noteworthy differences among the kainate receptor LBDs, such as the loss of a hydrogen bond to the agonist α-amino group at GluK2 Ala487, which is equivalent to Thr480 in GluA2 and Thr503 in GluK1. This may contribute to the higher glutamate EC50 for GluK2 compared with GluK1 (Mayer, 2005). A central feature of AMPA and kainate receptor agonist binding is closure of the cleft in which the agonist binds, a conformational change mediated by movement of D2 relative to D1 within the bilobed LBD (Armstrong and Gouaux, 2000; Mayer, 2005) (see sections II.D, VII.B).
Fig. 8.
Agonist binding pockets of glutamate receptors. A, binding of glutamate (yellow) in the agonist binding pocket of GluA2 (PDB code 1FTJ). Only side chains of interacting residues are shown. Not all residues are labeled. B, binding of glutamate in GluK2 (PDB code 1S7Y). Compared with the glutamate-bound ligand binding pocket of GluA2, there is a loss of a direct hydrogen bond to the α-amino group of glutamate at position Ala487 in GluK2, which is the site equivalent to Thr480 in GluA2. An additional water molecule forms a hydrogen bond to the α-amino group of glutamate in GluK2. C, binding of glutamate in GluN2A (PDB code 2A5T). Compared with glutamate bound in GluA2, the salt bridge between Asp731 and the positively charged α-amino group of glutamate is absent. Instead, the α-amino group of glutamate forms water-mediated hydrogen bonds to Glu413 and Tyr761. D, binding of glycine in GluN1 (PDB code 2A5T). Specificity of GluN1 for glycine can be explained by the hydrophobic environment created by Val689 and the steric barrier formed by Trp731. E, binding of glycine in GluN3A (PDB code 2RC7). Trp731 of GluN1 is replaced by M844, allowing room for a water molecule in the pocket. F, binding of d-serine in GluD2 (PDB code 2V3U).
In addition to glutamate, a number of naturally occurring molecules, such as ibotenic acid and willardiine, plus an array of AMPA, ibotenic acid, and willardiine analogs, activate AMPA and kainate receptors (Table 5). It has been difficult to identify naturally occurring or synthetic agonists that discriminate well between all AMPA and kainate receptors. AMPA acts as a partial agonist at some kainate receptor subunit combinations (Herb et al., 1992; Swanson et al., 1996; Schiffer et al., 1997), and kainate can induce very rapid desensitization of neuronal AMPA receptors (Patneau et al., 1993). Nevertheless, there are some examples of kainate receptor-selective agonists, such as (2S,4R)-4-methylglutamic acid (SYM2081) and perhaps ATPA, a tert-butyl analog of AMPA (see Tables 5 and 6). Crystal structures of the GluK1 and GluK2 LBDs have revealed differences between kainate and AMPA receptors that partly explain these kainate receptor-selective actions. The agonist-binding cavities of GluK1 and GluK2 are 40 and 16% larger, respectively, than GluA2, allowing GluK1 and GluK2 to accommodate larger ligands (Mayer, 2005; Naur et al., 2005). Indeed, steric occlusion between the 4-methyl group of SYM2081 and GluA2 Leu650 contributes to its selectivity for GluK1 and GluK2, both of which have a smaller valine in the corresponding positions (GluK1, Val670; GluK2, Val654) (Armstrong et al., 1998, 2003; Mayer, 2005). Likewise, the isoprenyl group of kainate shows reduced steric occlusion in GluK1 and GluK2 compared with GluA2 because of the smaller valine residues. Steric occlusion also may explain selectivity of ATPA for GluK1, because its bulky tert-butyl group interacts with Leu650, Thr686, and Met708 in GluA2, which are replaced by the smaller amino acids Val670, Ser706, and Ser726, respectively, in GluK1 (Lunn et al., 2003). The availability of crystallographic data for multiple kainate receptor subunits emphasizes how useful structural information can be across each subunit family. Thus, there remains the need for new crystallographic data for additional members of each glutamate receptor subtype, as opposed to the reliance on homology modeling and molecular dynamics simulations. Although useful, molecular dynamics simulations of homology models carry significant caveats, including uncertainty associated with representation of amino acid insertions, placement of new ligands, approximations of force fields for membrane-spanning elements, and computational limitations in simulating movement of large multisubunit protein assemblies. At the same time, crystals are rarely formed on demand, low resolution can create ambiguities in protein threading and details of ligand binding pose, and dynamic aspects ranging from ligand interactions to domain coupling are not revealed by a static X-ray structure. Early in a project, in the absence of a three-dimensional structure, a homology model can offer unique insights, whereas in late phases of a project, possession of a crystal structure can benefit significantly from dynamic refinement and trajectory analysis. The approaches are complementary both in terms of a project's evolution and the information content provided by the two structural perspectives.
TABLE 5.
AMPA receptor agonist EC50 values in micromolar
EC50 values for GluA1, GluA3, or GluA4 coexpressed with GluA2 can be found in Stein et al. (1992), Coquelle et al. (2000), Nakanishi et al. (1990), and Vogensen et al. (2000).
| Agonist | GluA1 | GluA2 | GluA3 | GluA4 |
|---|---|---|---|---|
| μM | ||||
| l-Glutamate | 3.4–22a–d | 6.2–296a,e,f | 1.3–35a–c | 560g |
| AMPA | 1.3–8.7c,h,i | 66f | 1.4–130c,h,i | 1.3i |
| Kainate | 32–34d,h | 130–170j | 31–36c,h | |
| Willardiine | 11.5k | 6.3l | ||
| F-Willardiine | 0.47k | 0.2–0.5l,m | 20.9m | 11.9m |
| Br-Willardiine | 2.8k | 0.84l | ||
| I-Willardiine | 33.6k | 1.5l | ||
| Br-HIBO | 14a | 5.4a | 202a | 39a |
| Cl-HIBO | 4.7n | 1.7n | 2700n | 1300n |
| (S)-CPW399 | 24.9o | 13.9o | 224o | 34.3o |
| (S)-ATPA | 22p | 7.9p | 7.6p | |
| ACPA | 1.1–11c,q | 15q | 0.1–5c,q | 1.1q |
| (S)-4-AHCP | 4.5r | 7.2r | 15r | |
| (S)-Thio-ATPA | 5.2s | 13–40s | 32s | 20s |
| 2-Et-Tet-AMPA | 42t | 52t | 18t | 4t |
| (S)-2-Me-Tet-AMPA | 0.16i | 3.4f | 0.014i | 0.009i |
| SYM2081 | 132b | 453b | ||
| Domoic Acid | 1.3d 0.97b | 21b | ||
ACPA, (R,S)-2-amino-3-(3-carboxy-5-methyl-4-isoxazolyl)propionic acid; (S)-4-AHCP, (R,S)-2-amino-3-(3-hydroxy-7,8-dihydro-6H-cyclohepta[d]isoxazol-4-yl)propionic acid; 2-Et-Tet-AMPA, (R,S)-2-amino-3-[3-hydroxy-5-(2-ethyl-2H-5-tetrazolyl)-4-isoxazolyl]propionic acid; (S)-2-Me-Tet-AMPA, (S)-2-amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5-yl)isoxazol-4-yl]propionic acid; (S)-ATPA, (S)-2-amino-3-(5-tert-butyl-3-hydroxyisoxazol-4-yl)propionic acid; (S)-thio-ATPA, (S)-2-amino-3-(5-tert-butyl-3-hydroxy-4-isothiazolyl)propionic acid; (S)-CPW399, (S)-1-(2-amino-2-carboxyethyl)-6,7-dihydro-1H-cyclopentapyrimidine-2,4(1H,3H)dione; Br-HIBO, (R,S)-4-bromo-HIBO; Cl-HIBO, (R,S)-4-chloro-HIBO.
Coquelle et al. (2000). (S)-Br-HIBO is active; however, the racemic mixture was used for the determination of EC50.
Stensbol et al. (2001).
TABLE 6.
Kainate receptor agonist EC50 values in micromolar
| Agonist | GluK1 | GluK2 | GluK3 | GluK1/GluK2 | GluK1/GluK5 | GluK2/GluK5 |
|---|---|---|---|---|---|---|
| μM | ||||||
| l-Glutamate | 47a | 9a | 5900b | 48a | 19a | 8a |
| AMPA | 208a | N.E.c | N.E.d | 154a | 123a | 137a |
| Kainate | 4.9a | 1.1a | 7.4a | 1.5a | 0.6a | |
| Willardiine | 28.9e | 127f | ||||
| F-Willardiine | 1.8e | |||||
| Cl-Willardiine | 0.057e | |||||
| Br-Willardiine | 0.0091e | |||||
| I-Willardiine | 0.21a | N.E.a | 0.47a | 0.06a | 30a | |
| (S)-ATPA | 0.33a | N.E.a | 0.8a | 0.38a | 106a | |
| SYM2081 | 0.18a | 0.29a | 0.38a | 0.06a | 0.34a | |
| Domoic acid | 0.36a | 0.07a | 0.19a | 0.05a | 0.12a | |
| LY339434 | 2.5g | >100g | - | |||
| Dysiherbaine | 0.0005h | 0.0013h | N.D.i | |||
| neoDH | 0.008h | 0.03h | ||||
| ACPA | 22j | 101j | ||||
| (S)-4-AHCP | 0.13k | N.E.k | 6.4k | |||
| (S)-Thio-ATPA | 0.1l | N.E.l | 4.9l | |||
| 2-Me-Tet-AMPA | 8.7m | 15.3m | ||||
| 8-Deoxy-neoDH | 0.0015n | 48n | 2.9n | |||
| 9-Deoxy-neoDH | 0.169n | >100n | >100n | |||
| MSVIII-19 | 3.6o | N.E. (>100)o | ||||
ACPA, (R,S)-2-amino-3-(3-carboxy-5-methyl-4-isoxazolyl)propionic acid; (S)-4-AHCP, (R,S)-2-amino-3-(3-hydroxy-7,8-dihydro-6H-cyclohepta[d]isoxazol-4-yl)propionic acid; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; 2-Me-Tet-AMPA, 2-amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5-yl)isoxazol-4-yl]propionic acid; (S)-ATPA, (S)-2-amino-3-(5-tert-butyl-3-hydroxyisoxazol-4-yl)propionic acid; (S)-thio-ATPA, (S)-2-amino-3-(5-tert-butyl-3-hydroxy-4-isothiazolyl)propionic acid; N.D., not determined; N.E., no effect; neoDH, neodysiherbaine.
Data from calcium influx (fluorometric imaging plate reader) in HEK293 cells stably transfected with human receptors and treated with con A (Alt et al., 2004).
Data from patch-clamp recordings in HEK293 cells transfected with rat receptors (Schiffer et al., 1997).
Ki values from displacement of [3H]kainate at human receptors (Jane et al., 1997).
Data from willardiine-evoked currents from HEK293 cells expressing GluK2/GluK5 (Fukushima et al., 2001).
Data from patch-clamp recordings in HEK293 cells stably transfected with human receptors and treated with con A. EC50 of LY339434 at isolated dorsal root ganglion, cerebellar Purkinje cells and cultured hippocampal neurons was 0.8, 362, and 2.5 μM, respectively. The EC50 of LY339434 at GluA1, GluA2, and GluA4 receptors was greater than 10,000 μM (Small et al., 1998).
Data for dysiherbaine and neodysiherbaine are Ki values based on inhibition of [3H]kainate binding to receptors expressed in HEK293 cells from Sakai et al. (2001b) and Sanders et al. (2005), respectively. The Ki for dysiherbaine binding to neuronal AMPA receptors was 26–153 μM (Sakai et al., 2001b).
GluK1/GluK5 receptors were proposed to have a high-affinity dysiherbaine binding site at GluK1 and a low-affinity site at GluK5. Supporting this, dysiherbaine bound to homomeric GluK5 receptors with a Ki of 4.9 μM (Swanson et al. 2002).
Data from X. laevis oocytes treated with con A (Brehm et al., 2003).
Data from Stensbøl et al. (2001).
Data from X. laevis oocytes (Vogensen et al., 2000).
Although both 8-deoxy-neoDH and 9-deoxy-neoDH elicited current from GluK1 receptors, their potencies were reported as Ki values calculated from IC50 values for displacing [3H]kainate at recombinant kainate receptors (Lash et al., 2008).
EC50 for MSVIII-19 at GluK1 recombinant receptors expressed in HEK293 cells treated with Con A. MSVIII-19 failed to displace kainate from recombinant GluK2 expressed in HEK293 cells (Frydenvang et al., 2009).
A few agonists show useful selectivity between the GluA1/GluA2 and GluA3/GluA4 subunits (Table 5). Br-HIBO, an analog of ibotenic acid, preferentially activates GluA1 and GluA2 versus GluA3 and GluA4 receptors (Coquelle et al., 2000) through involvement of water-mediated hydrogen bonding to Tyr702 in GluA1 and GluA2, which is Phe in GluA3 and GluA4. Thus, ordered water molecules within the agonist binding site interact with the ligand to influence specificities among GluA subunits (Banke et al., 2001; Hogner et al., 2002; Pentikäinen et al., 2003; Frandsen et al., 2005). Cl-HIBO was synthesized after molecular modeling predicted that the exchange of bromine for chlorine would improve selectivity (Bjerrum et al., 2003). Cl-HIBO activates GluA1 and GluA2 with 275- to 1600-fold selectivity over GluA3 or GluA4, respectively. The agonist 2-benzyl-tetrazol-AMPA shows 40-fold selectivity for GluA4 over GluA1 (Jensen et al., 2007). Crystal structures of 2-benzyl-tetrazol-AMPA bound to the GluA2 LBD reveal that the benzyl group occupies a novel cavity opened up by movement of Met708 in GluA2, and the selectivity of 2-benzyl-tetrazol-AMPA is due to residues adjacent to this cavity (Val690 and Ala691), which are conserved in GluA2 to GluA4 but correspond to Met686 and Ile687 in GluA1 (Vogensen et al., 2007).
Although some agonists discriminate between GluA1/GluA2 and GluA3/GluA4 subunits, it remains to be shown whether agonists that act selectively at an individual GluA subunit can be developed. The amino acid sequences for the LBDs of the four GluA subunits are 80% identical (Table 2). Ligands with agonist activity at AMPA receptors contain a chemical moiety equivalent to the α-amino and α-carboxyl groups of glutamate, and the binding of this moiety is conserved for the agonists crystallized thus far. Moreover, crystal structures and homology models show the residues in direct contact with agonists such as glutamate, AMPA, and kainate are fully conserved across all GluA subunits. Crystallization of LBDs bound to additional agonists, however, could allow identification of novel binding modes or differences in agonist binding that could be exploited through medicinal chemistry efforts to achieve greater subunit selectivity. Alternatively, molecular modeling, which can account for important motions within the receptor, could allow for analysis of binding to subunits that have not been crystallized and has been used successfully, for example, to predict the activity of Cl-HIBO.
The development of useful subunit-selective compounds is further complicated by the formation of heteromeric receptors in native tissue, which contain two different GluA subunits, and by the association of AMPA receptors with interacting partners. Association of the TARP auxiliary subunits with AMPA receptors (see section II.H) increases efficacy and affinity for a range of AMPA receptor agonists, including kainate (Turetsky et al., 2005; Kott et al., 2007). Because agonist pharmacology has been studied extensively in recombinant systems without coexpression of TARPs, the caveat exists as to whether agonist properties (Table 5) will remain the same in the presence of TARPs.
Kainate has a 2-carboxypyrrolidine-3-acetic acid backbone, and analogs containing this backbone, known as kainoids (Sonnenberg et al., 1996; Hodgson et al., 2005; Sagot et al., 2008; Bunch and Krogsgaard-Larsen, 2009), include domoic acid (Hampson et al., 1992; Alt et al., 2004) and acromelic acid (Kwak et al., 1992; Smith and McIlhinney, 1992). Agonist potency and efficacy are subunit-specific, because kainate and domoic acid are potent agonists of GluK1 and GluK2 but show low potency at GluK3 receptors (Table 6; Jane et al., 2009). Agonists acting preferentially at kainate receptors over AMPA receptors include the glutamate analogs SYM2081 (Zhou et al., 1997), dysiherbaine (Sakai et al., 1997), and neodysiherbaine (Sakai et al., 2001a). SYM2081 has similar potencies for GluK1 and GluK2 and causes pronounced desensitization (Jane et al., 2009). Dysiherbaine has nanomolar affinity for GluK1 and GluK2 and micromolar affinity for GluK5 (Sakai et al., 2001b; Swanson et al., 2002; Sanders et al., 2005). Because of its high affinity for GluK1, dysiherbaine promotes a desensitized state of the receptor that persists for at least 20 to 45 min after removal. This unique activity of dysiherbaine was used to block GluK1 subunits in GluK1/GluK5 diheteromeric receptors, which revealed that glutamate evokes a desensitizing response from the remaining GluK5 subunits (Swanson et al., 2002). This finding suggests that kainate receptors undergo subunit-specific gating similar to AMPA receptors (Jin et al., 2003).
Multiple agonists act selectively at GluK1 over the other kainate receptor subunits (Table 6). The neodysiherbaine analogs 8-deoxy-neodysiherbaine, 9-deoxy-neodysiherbaine, and MSVIII-19 show nanomolar affinity for GluK1 and >1000-fold selectivity over GluK2, GluK3, and GluK5, with 8- and 9-deoxy-neodysiherbaine acting as a partial and full agonists, respectively (Lash et al., 2008). MSVIII-19 was originally reported as a GluK1 antagonist (Sanders et al., 2005), but crystallographic studies revealed that it induces full domain closure of the GluK1 LBD, prompting further functional studies that showed it to be an agonist of extremely low efficacy (Frydenvang et al., 2009). (2S,4R,6E)-2-Amino-4-carboxy-7-(2-naphthyl)hept-6-enoic acid (LY339434), ATPA, (S)-2-amino-3-(3-hydroxy-7,8-dihydro-6H-cyclohepta[d]isoxazol-4-yl) propionic acid, (S)-5-iodowillardiine, and (4R)-isopentyl glutamate are potent agonists at homomeric GluK1 receptors but have little to no activity at GluK2 receptors (Clarke et al., 1997; Jane et al., 1997; Zhou et al., 1997; Small et al., 1998; Brehm et al., 2003; Bunch et al., 2009). GluK1 selectivity arises from the larger GluK1 binding cavity, which relieves steric occlusion of the bulky tert-butyl group of ATPA and halogen atom of (S)-5-iodowillardiine (Mayer, 2005). Likewise, steric occlusion explains why AMPA binds GluK1 but not GluK2, because the isoxazole ring cannot make key contacts with D2 (Mayer, 2005). Mutagenesis studies have confirmed the importance of cavity size, and the exchange of Ser706 in GluK1 to the larger Asn690 in GluK2 predictably alters potency of AMPA, iodowillardiine, and ATPA toward GluK1 (Swanson et al., 1997a, 1998; Nielsen et al., 2003).
The LBD of the GluD2 (δ2) family of glutamate receptor subunits binds d-serine, sharing some but not all features of d-serine binding to GluN1 (Fig. 8F) (Naur et al., 2007). However, GluD2 receptor function remains poorly understood. Transgenic experiments show that insertion of a mutant GluD2 into GluD2(−/−) mice can rescue these mice from neurological deficits even when the inserted GluD2 has a mutation in the ion channel pore that either disrupts Ca2+ permeability (Kakegawa et al., 2007a) or abolishes current flow through the ion channel (Kakegawa et al., 2007b). In addition, GluD2 can induce presynaptic terminal differentiation even without its LBD, which contains the d-serine binding site (Kuroyanagi et al., 2009; Torashima et al., 2009). These data suggest that GluD2 does not influence cerebellar function through actions as a ligand-gated ion channel. No data has yet shown functional ionic currents in wild-type GluD2 receptors. However, a mutation in M3, GluD2(A654T), within the highly conserved SYTANLAAF gating motif causes spontaneously active receptors (Zuo et al., 1997; Kohda et al., 2000), and these receptors are inhibited by the binding of d-serine, perhaps through destabilization of the dimer interface and desensitization (Naur et al., 2007; Hansen et al., 2009). It remains to be determined whether d-serine has functional effects on neuronal GluD2.
B. N-Methyl-d-aspartate Receptor Agonists
NMDA receptors are unique among the glutamate receptor family in that the simultaneous binding of glycine to GluN1 and glutamate to GluN2 is required for activation (Kleckner and Dingledine, 1988). Crystal structures of the bilobed GluN1 LBD show that glycine and related agonists (Table 7) bind within the cleft between the D1 and D2 domains. The α-carboxyl group of glycine forms hydrogen bonds within the binding pocket with Arg522, Thr518, and Ser688, whereas the amino group of glycine interacts with the carbonyl oxygen of Pro516, the hydroxyl group of Thr518, and the carboxylate oxygen of Asp732 (Fig. 8D) (Furukawa and Gouaux, 2003). Trp731 within the GluN1 binding pocket has been proposed to hinder glutamate binding because of steric clash between Trp731 and the glutamate γ-carboxyl group. In GluN2A, the smaller side chain of Tyr730 is in van der Waals contact with the γ-carboxylate of glutamate (Fig. 8C). GluN1 has Val689 corresponding to GluN2A Thr690, which leads to loss of a hydrogen bond donor that stabilizes the glutamate γ-carboxyl group in GluN2A (Fig. 8, C and D). Comparison between crystallographic structures of the GluN1 subunit bound to full and partial agonists indicates that the degree of closure of the LBDs' D1 and D2 domains is not correlated with relative agonist efficacy, as has been demonstrated with the GluA2 LBD. Partial agonists 1-aminocyclobutane-1-carboxylic acid and 1-aminocyclopropane-1-carboxylic acid (Priestley et al., 1995) induce a similar degree of domain closure as glycine, differing by less than 0.5° (Inanobe et al., 2005).
TABLE 7.
EC50 values in micromolar for agonists binding to the GluN1 subunit of the NMDA receptor
Percentage relative efficacy (in parentheses) is the current response to a maximally effective concentration of agonist relative to the response to a maximally effective concentration of glycine. All values are from recombinant rat NMDA receptors expressed in X. laevis oocytes and coactivated by glutamate (Chen et al., 2008; Dravid et al., 2010) except HA 966 and ACBC (Priestley et al., 1995). Values are given to two significant digits.
| Glycine-Site Agonist | GluN2A | GluN2B | GluN2C | GluN2D |
|---|---|---|---|---|
| μM (%) | ||||
| Glycine | 1.1 (100) | 0.72 (100) | 0.34 (100) | 0.13 (100) |
| l-Serine | 212 (95) | 77 (98) | 27 (110) | 15 (98) |
| d-Serine | 1.3 (98) | 0.65 (96) | 0.32 (110) | 0.16 (93) |
| l-Alanine | 96 (79) | 36 (65) | 28 (92) | 13 (97) |
| d-Alanine | 3.1 (96) | 0.89 (84) | 0.56 (96) | 0.22 (99) |
| d-Cycloserine | 19 (90) | 8.2 (65) | 3.3 (190) | 2.9 (94) |
| HA 966 | 12 (12) | 4.6 (14) | ||
| β-Cl-d-Alanine | 21 (84) | 9.9 (88) | 3.7 (79) | 1.7 (81) |
| β-F-dl-Alanine | 11 (92) | 0.98 (88) | 0.40 (84) | 0.40 (91) |
| tri-F-dl-Alanine | 1.3 (130) | 0.65 (64) | 0.32 (110) | 0.16 (93) |
| ACPC | 1.3 (79) | 0.65 (89) | 0.35 (88) | 0.083 (89) |
| ACBC | 45 (13) | 6.6 (33) | ||
ACBC, 1-aminocyclobutane-1-carboxylic acid; ACPC, 1-aminocyclopropane-1-carboxylic acid; HA 966, (+)-(1-hydroxy-3-aminopyrrolidine-2-one).
In addition to glycine, the d- and l-isomers of serine and alanine are agonists at the GluN1 subunit (Pullan et al., 1987; McBain et al., 1989) (Table 7). d-Serine is more potent than l-serine and may be the primary ligand for GluN1 in regions such as the supraoptic nucleus (e.g., Panatier et al., 2006). d-Serine is synthesized from l-serine by serine racemase in both astrocytes (Wolosker et al., 1999) and neurons (Mustafa et al., 2004; Miya et al., 2008; Wolosker et al., 2008). It is noteworthy that serine racemase is regulated by NMDA receptor activity, mGluR5 activation, nitrosylation, divalent cations, and nucleotides (Shoji et al., 2006; Baumgart and Rodríguez-Crespo, 2008; Balan et al., 2009; Mustafa et al., 2009), and deletion of serine racemase alters glutamatergic synaptic transmission and produces behavioral phenotypes (Basu et al., 2009).
Cyclic and halogenated analogs of glycine, including d-cycloserine, act as GluN1 partial agonists (Hood et al., 1989; Priestley and Kemp, 1994; Sheinin et al., 2001; Dravid et al., 2010) (Table 7). Although d-cycloserine is a partial agonist of GluN2A-, GluN2B-, and GluN2D-containing NMDA receptors, the responses of GluN2C-containing NMDA receptors are greater in d-cycloserine than those evoked by glycine (Sheinin et al., 2001; Dravid et al., 2010). This raises the possibility that potentiation of GluN2C-containing NMDA receptors could underlie the positive effects of d-cycloserine on cognition, fear extinction, and motor dysfunction (Kalia et al., 2008; Norberg et al., 2008) through action on GluN2C-expressing neurons (Monyer et al., 1994). It is noteworthy that the identity of the GluN2 subunit within the NMDA receptor determines the potencies of GluN1 agonists, which are least potent (highest EC50) for GluN1/GluN2A and most potent (lowest EC50) for GluN1/GluN2D (Kuryatov et al., 1994; Wafford et al., 1995; Furukawa and Gouaux, 2003; Chen et al., 2008) (Table 7).
Glutamate binding to GluN2A involves interactions of the agonist α-carboxylate group with Arg518 and the agonist γ-carboxylate group with Tyr730 within the binding pocket, together with an interdomain hydrogen bond formed between Tyr730 and Glu413 (Fig. 8C; Furukawa et al., 2005). The crystal structure of the agonist-bound GluN1-GluN2A LBD heterodimer suggests a mechanism for selectivity for NMDA over AMPA receptors (Furukawa et al., 2005). The GluN2A subunit has Asp731 within the binding pocket, whereas the GluA2, GluK1, and GluK2 receptor subunits have a glutamate residue at the corresponding position that interacts with the agonist amino group (Fig. 8, A–C). Because the aspartate residue within the GluN2A subunit is a methylene group shorter than the glutamate residue found in GluA and GluK subunits, it cannot interact with the agonist α-amino group of glutamate, which instead forms water-mediated hydrogen bonds to GluN2A Glu413 and Tyr761. Surprisingly, the charge-conserving substitution GluN2A(D731E) and GluN2B(D732E) renders the receptor nonfunctional (Williams et al., 1996; Laube et al., 2004; Chen et al., 2005), perhaps a result of interference with the water-mediated interactions at the α-amino group of glutamate and/or a disruption of the binding pocket. The reduced side-chain length of Asp731 also creates space for NMDA to fit within the GluN2A binding pocket. Modeling of NMDA into the GluN2A LBD suggests that the N-methyl group of NMDA is accommodated in the binding pocket by displacement of the water molecule that binds the α-amino group of glutamate (Furukawa et al., 2005). Other studies using mutagenesis and homology modeling of GluN2A and GluN2B LBDs have suggested that NMDA cannot bind to GluA subunits because of steric clash between the N-methyl group of NMDA and Met708 in GluA2, which is conserved among all AMPA receptors (Laube et al., 2004; Chen et al., 2005).
GluN2 endogenous agonists include glutamate, d- and l-aspartate (Benveniste, 1989; Nicholls, 1989; Fleck et al., 1993; Schell et al., 1997; Wang and Nadler, 2007; Errico et al., 2008; Zhang and Nadler, 2009), homocysteate, and cysteinesulfinate (Do et al., 1986, 1988; Olney et al., 1987; Yuzaki and Connor, 1999) (Table 8). Cyclic analogs with conformationally constrained rings also act as potent GluN2 agonists, in some cases with EC50 values lower than glutamate (Shinozaki et al., 1989; Schoepp et al., 1991; Erreger et al., 2007) (Table 8). The potencies and relative agonist efficacies of GluN2 ligands generally display a graded variation among GluN2A- through GluN2D-containing NMDA receptors, with the lowest potency at GluN2A-containing NMDA receptors and the highest potency at GluN2D-containing receptors (Kutsuwada et al., 1992; Monyer et al., 1992; Erreger et al., 2007) (Table 8). Selective agonists that distinguish between the GluN2 subunits have not been developed, although series such as the N-hydroxypyrazol-5-yl glycine derivatives show promising selectivity for some GluN2 subunits (Clausen et al., 2008) (Table 8). In addition, SYM2081 has a 46-fold lower EC50 value for GluN2D-containing receptors compared with GluN2A-containing NMDA receptors (Table 8). The modest subunit selectivity of SYM2081 may be due to steric clash of the 4-methyl substituent with the Tyr730, which in molecular dynamics simulations resides within the cleft of GluN2A but makes a hydrogen bond with the γ-carboxyl of glutamate in GluN2D (Erreger et al., 2007).
TABLE 8.
EC50 values in micromolar for agonists binding to the GluN2 subunit of the NMDA receptor
Percentage relative efficacy (in parentheses) is the current response to a maximally effective concentration of agonist relative to the response to a maximally effective concentration of glutamate. All values are from recombinant rat NMDA (GluN1 plus the indicated GluN2) receptors expressed in X. laevis oocytes activated by agonist plus maximally effective concentration of glycine. NHP5G, ethyl-NHP5G, and propyl-NHP5G values are from Clausen et al. (2008). l-trans-ADC values are from Sivaprakasam et al. (2009). cis-2,3-Piperidinedicarboxylic acid are from Priestley et al. (1995). All remaining values are from Erreger et al. (2007). Values are given to two significant digits.
| Glutamate-Site Agonist | GluN2A | GluN2B | GluN2C | GluN2D |
|---|---|---|---|---|
| μM (%) | ||||
| l-Glutamate | 3.3 (100) | 2.9 (100) | 1.7 (100) | 0.51 (100) |
| d-Glutamate | 250 (99) | 160 (120) | 110 (100) | 42 (110) |
| l-Aspartate | 48 (99) | 14 (78) | 41 (110) | 12 (91) |
| d-Aspartate | 30 (103) | 10 (91) | 9.3 (99) | 2.1 (90) |
| N-Methyl-l-aspartate | 580 (99) | 130 (69) | 150 (66) | 40 (75) |
| N-Methyl-d-aspartate | 94 (93) | 30 (78) | 22 (86) | 7.3 (92) |
| SYM2081 | 140 (72) | 25 (89) | 18 (71) | 3.2 (75) |
| l-Homocysteinsulfinate | 73 (91) | 18 (94) | 14 (70) | 6.2 (84) |
| d-Homocysteinsulfinate | 36 (89) | 14 (99) | 7.9 (92) | 3.0 (100) |
| l-Homocysteate | 34 (86) | 8.1 (90) | 12 (53) | 3.4 (69) |
| d-Homocysteate | 180 (92) | 86 (94) | 110 (74) | 22 (87) |
| l-Cysteinesulfinate | 140 (110) | 100 (81) | 22 (100) | 9.2 (98) |
| l-Cysteate | 560 (95) | 180 (77) | 80 (82) | 30 (83) |
| d-Cysteate | 220 (31) | 210 (67) | 580 (110) | 100 (97) |
| Homoquinolinate | 22 (75) | 16 (96) | 81 (51) | 32 (88) |
| Ibotenate | 160 (99) | 26 (89) | 40 (72) | 13 (78) |
| (R,S)-(Tetrazol-5-yl)glycine | 1.7 (98) | 0.52 (97) | 0.49 (89) | 0.099 (78) |
| L-CCG-IV | 0.26 (99) | 0.083 (120) | 0.11 (90) | 0.036 (110) |
| trans-ACBD | 3.1 (91) | 0.99 (81) | 1.2 (67) | 0.51 (81) |
| cis-ADA | 890 (100) | 220 (95) | 80 (81) | 32 (140) |
| trans-ADC | 470 (38) | 170 (48) | 95 (73) | 50 (80) |
| cis-ACPD | 61 (76) | 21 (75) | 22 (49) | 11 (77) |
| cis-2,3-Piperidinedicarboxylic acid | 21 (3) | 38 (7) | ||
| (R)-NHP4G | 150 (33) | 61 (76) | 120 (54) | 48 (77) |
| (R,S)-Ethyl-NHP5G | 47 (5) | 68 (45) | 91 (52) | 43 (70) |
| (R)-Propyl-NHP5G | N.E. | 105 (6) | 429 (22) | 153 (37) |
trans-ACBD, trans-1-aminocyclobutane-1,3-dicarboxylate; cis-ACPD, (1R,3R)-aminocyclopentane-cis-dicarboxylate; ADC, azetidine-2,4-dicarboxylic acid; cis-ADA, cis-azetidine-2,4-dicarboxylic acid; L-CCG-IV, (2S,3R,4S)-2-(carboxycyclopropyl)glycine; N.E., no effect; NHP4G, 2-(N-hydroxylpyrazol-4-yl)glycine; NHP5G, 2-(N-hydroxypyrazol-5-yl)glycine.
GluN3, like GluN1, binds glycine and can coassemble with other NMDA receptor subunits. Several differences exist between the manner by which GluN3 and GluN1 subunits bind glycine (Yao et al., 2008). Glycine and d-serine bind within the GluN3 cleft of the clamshell formed by domains D1 and D2, which causes a degree of closure similar to that caused by glycine when bound to GluN1. In GluN3A, the α-carboxyl group interacts with Arg638, Ser633, and Ser801, and the α-amino group interacts with Asp845, Ser631, and Ser633 (Fig. 8E) (Yao et al., 2008). Although both GluN1 and GluN3 subunits bind glycine, their LBDs are only ∼30% homologous, and glycine affinity for the isolated GluN3 LBD is more than 600-fold higher than that for the isolated GluN1 LBD (Yao and Mayer, 2006). The ligand binding site of GluN3A is capped by Tyr605, which hydrogen bonds with the side chains of Ser631 and Glu522. These interactions are not present in GluN1 because all three amino acids differ. The differences in GluN1 and GluN3 residues within the binding pocket markedly alter the water molecule organization. Eight interdomain interactions between domains D1 and D2 of the GluN3 subunit are unique for the GluN3 subunit when glycine is bound and may contribute to the differences in ligand affinity (Yao and Mayer, 2006; Yao et al., 2008).
The GluN3 subunit forms functional diheteromeric cation-permeable GluN1/GluN3 receptors in X. laevis oocytes, and GluN3 may be incorporated into GluN1/GluN2/GluN3 receptors (Cavara and Hollmann, 2008; Schüler et al., 2008; Ulbrich and Isacoff, 2008). The exact nature and extent of GluN3 involvement in neuronal and glial receptors is an area of active study (Stys and Lipton, 2007). Within recombinant GluN1/GluN3 receptors expressed in heterologous systems, binding of glycine to GluN3 alone seems permissive for channel activation, in contrast to GluN1/GluN2 receptors, which require simultaneous binding of glycine to GluN1 and glutamate to GluN2. Occupancy of the GluN1 subunit by ligand within GluN1/GluN3 receptors seems to facilitate desensitization. In GluN1/GluN3 receptors, GluN3 is activated fully by glycine and partially by d-serine and 1-aminocyclopropane-1-carboxylic acid (Chatterton et al., 2002; Smothers and Woodward, 2007), which may account for observations that d-serine antagonized GluN1/GluN3 receptors (Awobuluyi et al., 2007).
C. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid and Kainate Receptor Competitive Antagonists
AMPA and kainate antagonists typically possess an α-amino group connected by a heterocyclic ring system to an acidic moiety (Szymańska et al., 2009). The first widely used competitive AMPA receptor antagonists were quinoxalinediones (CNQX, DNQX, NBQX), which were highly selective over NMDA receptors but antagonized kainate receptors. NBQX seems to be more selective for AMPA receptors (Wilding and Huettner, 1996) and is thus commonly used to block AMPA receptor-mediated currents (Table 9). Surprisingly, association of AMPA receptors with TARPs converts CNQX and DNQX, but not NBQX, from antagonists to weak partial agonists. CNQX and DNQX induce partial domain closure, consistent with the activity of a partial agonist (Armstrong and Gouaux, 2000). Although it is not known how interaction with TARPs results in the partial agonist activity of CNQX and DNQX, it is possible that the auxiliary subunits alter the extent of domain closure necessary for channel opening (Menuz et al., 2007; Kott et al., 2009).
TABLE 9.
Equilibrium dissociation constants in micromolar for AMPA receptor competitive antagonists
For all antagonist tables, Ki is the equilibrium dissociation constant calculated from radioligand binding studies, and KB is the equilibrium dissociation constant calculated from functional assays using either a Schild analysis or a Cheng-Prusoff correction of IC50 values. IC50 values are the concentration of drug required to produce half-maximal inhibition. Data for CNQX and NBQX inhibition of GluA1 or GluA4 coexpressed with GluA2 can be found in Stein et al. (1992).
| Antagonist | GluA1 | GluA2 | GluA3 | GluA4 |
|---|---|---|---|---|
| μM | ||||
| CNQX | 0.6a | 0.18b | 2.11c | |
| DNQX | 0.25d | 0.45b | 1.66c | 0.19–0.49d |
| NBQX | 0.4e | 0.59f | 0.31–0.63c,f | 0.1e |
| ATPO | 38f | 65f | 110f | 150f |
| YM90K | 1.96c | |||
| NS102 | N.E.c | |||
| NS1209 | 0.12g | 0.13g | 0.11g | 0.06g |
| Kynurenic acid | 1900h | |||
| LY293558 | 9.2i | 0.4–3.2i,j | 32k | 51i |
| LY377770 | >100j | |||
| LY382884 | N.E.l | N.E.j,l | N.E.l | N.E.l |
| LY466195 | 75m | 270m N.E.j | 312m | 432m |
| UBP296 | N.E.n | |||
| UBP310 | >100o | |||
| ACET | >100o | |||
ACET, (S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxy-5-phenylthiophene-3-yl-methyl)-5-methylpyrimidine-2,4-dione; LY377770, (3S,4aR,6S,8aR)-6-(((1H- tetrazol-5-ylmethyl)oxy)methyl)-1,2,3,4,4a,5,6,7,8,8a-decahydroisoquinoline-3-carboxylic acid; N.E., no effect; NS102, 5-nitro-6,7,8,9-tetrahydrobenzo[g]indole-2,3-dione-3-oxime; UBP296, (R,S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxybenzyl)pyrimidine-2,4-dione; YM90K, 6-(1H-imidazol-1-yl)-7-nitro-2,3-(1H,4H)-quinoxalinedione.
KB values are from Schild analysis of responses from rat receptors expressed in X. laevis oocytes and activated by kainate (Dawson et al., 1990).
Ki values are for displacing [3H]AMPA binding to BHK cells transfected with GluA2(Q) (Tygesen et al., 1995).
KB values are from the Cheng-Prusoff correction of IC50 values for inhibition of Ca2+ influx evoked by 30 μM glutamate in HEK293 cells transfected with human GluA3 (Varney et al., 1998).
Ki values are for displacing [3H]AMPA binding to BHK cells transfected with rat cDNA (Andersen et al., 1996).
KB values are from Schild analysis of responses from recombinant receptors expressed in X. laevis oocytes activated by glutamate (Stein et al., 1992).
KB values are from the Cheng-Prusoff correction of IC50 values for inhibition of glutamate-activated Ca2+ influx in HEK293 cells stably transfected with rat recombinant receptors (Strange et al., 2006).
Data for GluA1 and GluA4 are Ki values for displacement of [3H]AMPA from Sf9 cells. GluA4 exhibited low- and high-affinity binding; the high-affinity Ki is reported here. Data for GluA2 and GluA3 are KB values from the Cheng-Prusoff correction of IC50 values for inhibition of Ca2+ influx stimulated by glutamate (250 μM) in HEK293 cells stably expressing GluA2 or GluA3 (Kasper et al., 2006).
IC50 values are for inhibition of GluA2 expressed in X. laevis oocytes and activated by 100 μM glutamate (Prescott et al., 2006).
Ki values are for displacing [3H]AMPA at human receptors expressed in HEK293 cells (Simmons et al., 1998).
KB values are from the Cheng-Prusoff correction of IC50 values from inhibition of glutamate (200 μM)-evoked Ca2+ influx at HEK293 cells expressing GluA2. LY377770 caused a 35% inhibition at 100 μM, whereas LY382884 and LY466195 (called compound 5 in this reference) had no effect at 100 μM (Jones et al., 2006).
Ki values are for displacing [3H]AMPA at human GluA3 expressed in HEK293 cells (Bleakman et al., 1999).
Ki values are for displacing [3H]AMPA at human receptors expressed in HEK293 cells. N.E. indicates LY382884 displaced less than 30% at 100 μM (Bortolotto et al., 1999).
Ki values are for displacing [3H]AMPA at human receptors expressed in HEK293 cells (Weiss et al., 2006).
KB values are from the Cheng-Prusoff corrected IC50 values for inhibition of Ca2+ influx evoked by glutamate (100 μM) at human receptors expressed in HEK293 cells (Dolman et al., 2005).
IC50 values are for inhibition of Ca2+ influx activated by 100 μM glutamate at HEK293 cells expressing human GluA2 (Dolman et al., 2007).
Crystal structures of DNQX and the structurally distinct competitive AMPA receptor antagonist ATPO in complex with the GluA2 LBD showed that both ligands induce only a small degree of domain closure and that their binding to the receptor is different. DNQX interacts with residues primarily within domain D1 of the clamshell, thereby depriving agonist of its initial contact sites with the open cleft of the binding pocket (Armstrong and Gouaux, 2000). By contrast, ATPO binds in a manner similar to agonists, contacting residues in domains D1 and D2, but the bulky γ-substituents prevent cleft closure (Hogner et al., 2003; Hald et al., 2007). In contrast to ATPO and DNQX, the AMPA receptor competitive antagonists 8-methyl-5-(4-(N,N-dimethylsulfamoyl)phenyl)-6,7,8,9-tetrahydro-1H-pyrrolo(3,2-h)-isoquinoline-2,3-dione-3-O-(4-hydroxybutyric acid-2-yl)oxime (NS1209) and (αS)-α-amino-3-[(4-carboxyphenyl)methyl]-3,4-dihydro-2,4-dioxo-1(2H)-pyrimidinepropanoic acid (UBP282) stabilize the LBD in a hyperextended conformation compared with the apo state (Kasper et al., 2006; Ahmed et al., 2009), suggesting that the LBD has more flexibility than previously thought.
Several classes of GluK1-selective antagonists designed from different templates include the decahydroisoquinolines (3S,4aR,6S,8aR)-6-((4-carboxyphenyl)methyl)-1,2,3, 4,4a,5,6,7,8,8a-decahydroisoquinoline-3-carboxylic acid (LY382884) (Bortolotto et al., 1999) and 6-((2-carboxy-4, 4-difluoro-1-pyrrolidinyl)methyl)decahydro-3-isoquinolinecarboxylic acid (LY466195) (Weiss et al., 2006), and the 3-substituted phenylalanine analogs (Szymańska et al., 2009) (Table 10). LY382884 is a competitive antagonist of heteromeric kainate receptors, including GluK1/GluK2 and GluK1/GluK5, with similar potencies as at homomeric GluK1 (Bortolotto et al., 1999; Alt et al., 2004). The willardiine analogs UBP302 (More et al., 2004), (S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxythiophene-3-ylmethyl)-5-methylpyrimidine-2,4-dione (UBP310) (Mayer et al., 2006), and (S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxy-5-phenylthiophene-3-yl-methyl)-5-methylpyrimidine-2,4-dione (Dolman et al., 2007; Dargan et al., 2009) also are selective for GluK1- or GluK3-containing kainate receptors over GluK2 and AMPA receptors (Perrais et al., 2009). Crystal structures of UBP302 and UBP310 bound to the GluK1 LBD indicate that the antagonists force the LBD of GluK1 to adopt a conformation that is more open than for quinoxalinediones. In contrast to other agonists and antagonists, the α-amino group of these ligands does not directly interact with the carboxyl group of Glu723 in GluK5 (Glu705 in GluA2); instead, Glu723 adopts a conformation similar to that of the corresponding Glu705 in the apo structure of the GluA2 LBD. GluK1 selectivity is achieved as a result of steric clash between the antagonist and residues lining the GluA2 and GluK2 binding pockets.
TABLE 10.
Equilibrium dissociation constants in micromolar for kainate receptor competitive antagonists
| Antagonist | GluK1 | GluK2 | GluK3 | GluK1/GluK2 | GluK1/GluK5 | GluK2/GluK5 |
|---|---|---|---|---|---|---|
| μM | ||||||
| CNQXa | 2.6 | 1.5 | 1.3 | 1.6 | 5.3 | |
| DNQX | 0.35b | |||||
| NBQXa | 8.0 | 1.7 | 3c | 6.2 | 4.2 | 6.4 |
| ATPO | 54d | >340d | ||||
| NS102 | 1.4e | |||||
| NS1209 | 0.62f | 13f | ||||
| LU97175g | 0.088 | 0.31 | 0.022 | |||
| LU115455g | 1.3 | 0.37 | 0.21 | |||
| LU136541g | 1.1 | 1.2 | 0.26 | |||
| Kynurenic acida | 130 | 33 | 160 | 99 | N.E. | |
| LY293558a | 0.22 | N.E. | N.E. | 0.65 | 0.80 | N.E. |
| LY377770a | 0.064 | N.E. | 0.13 | 0.16 | N.E. | |
| LY382884a | 0.64 | N.E. | N.E. | 1.3 | 0.64 | N.E. |
| LY466195h | 0.024 | N.E. | 8.9 | 0.024 | 0.054 | |
| UBP296 | 0.6i | N.E.i | 374j | 0.8i | 1.0i | N.E.i |
| UBP302k | 0.6l | N.E.m | 4.0m | 0.8l | 1.0l | N.E.l |
| UBP304 | 0.12n | N.E.n | 111o | 0.12n | 0.18n | N.E.n |
| UBP310 | 0.010l | N.E.l | 0.023m | 0.008l | N.E.l | |
| ACETp | 0.007 | N.E. | 0.092m | 0.005 | N.E. | |
| 2,4-Epi-neoDHq | 7.5 (2.4) | 74 (7.7) | ||||
2,4-Epi-neoDH, 2,4-epi-neodysiherbaine; ACET, (S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxy-5-phenylthiophene-3-yl-methyl)-5-methylpyrimidine-2,4-dione; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; LU115455, N-(1-(1-carboxymethyl-5,6,7,8-tetrahydro-benzo[f]quinoxaline-2,3-(1H,4H)-dion-9-yl)pyrrol-3-yl)methyl-N0-(4-carboxyphenyl)-urea; LU136541, N′-(4-carboxyphenyl)-N-(1-(1-hydroxy-5,6,7,8-tetrahydrobenzo[f]quinoxaline-2,3-(1H,4H)-dion-9-yl)pyrrol-3-yl)methyl-urea; LU97175, 1-benzamido-7-pyrrol-1-yl-6-trifluoromethylquinoxaline-2,3-(1H,4H)-dione; LY377770, (3S,4aR,6S,8aR)-6-(((1H-tetrazol-5-ylmethyl)oxy)methyl)-1,2,3,4,4a,5,6,7,8,8a-decahydroisoquinoline-3-carboxylic acid; N.E., no effect; NS102, 5-nitro-6,7,8,9-tetrahydrobenzo[g]indole-2,3-dione-3-oxime; UBP296, (R,S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxybenzyl)pyrimidine-2,4-dione; UBP302, 1-(2-amino-2-carboxyethyl)-3-(2-carboxybenzyl)pyrimidine-2,4-dione; UBP304, 1-(2-amino-2-carboxyethyl)-3-(2-carboxythiophene-3-ylmethyl)pyrimidine-2,4-dione.
KB values for NBQX, CNQX, kynurenic acid, LY293558, LY377770, and LY382884 are calculated using the Cheng-Prusoff correction with the IC50 values reported in Alt et al. (2004).
Ki values are for displacing [3H]AMPA binding to BHK cells stably transfected with GluK2 (Tygesen et al., 1995).
Ki values are calculated from the Cheng-Prusoff correction using IC50 values for displacement of [3H]kainate in GluK3 receptors expressed in HEK (Löscher et al., 1999).
KB values are from the Cheng-Prusoff correction of IC50 values for inhibition of glutamate-activated Ca2+ influx in HEK293 cells stably transfected with rat recombinant receptors (Strange et al., 2006).
Ki values are from inhibition of [3H]kainate binding to homomeric GluK2 expressed in HEK293 cells (Verdoorn et al, 1994). In functional studies, however, 10 μM NS102 caused only 50% inhibition of currents evoked from GluK2 by 300 μM glutamate and inhibited GluA2/GluA4 receptors 20%. In functional studies at rat cerebral cortical neurons or dorsal root ganglion neurons, taken to be AMPA receptors and kainate receptors, respectively, NS102 had a KB of 114 μM and 6 μM, respectively (Wilding and Huettner, 1996).
Ki values are for inhibition of [3H]AMPA and [3H]kainate binding at recombinant human GluK1 and GluK2, respectively, expressed in HEK293 cells (Christensen et al., 2004b).
Ki for displacement of [3H]kainate to recombinant receptors expressed in HEK293 cells. LU 97175 had a Ki of 1.3 μM and LU 115455 had a Ki of 0.018 μM for displacement of [3H]AMPA at rat brain membranes (Löscher et al., 1999).
Ki values are for displacement of [3H]kainate at human receptors expressed in HEK293 cells (Weiss et al., 2006).
KB values are from the Cheng-Prusoff corrected IC50 values for inhibition of Ca2+ influx evoked by glutamate (100 μM) at recombinant human receptors expressed in HEK293 cells (More et al., 2004).
Ki value are for displacement of [3H]kainate from human GluK3 expressed in HEK293 cells (Dolman et al., 2005).
UBP302 is the active enantiomer of UBP296.
KB values are for inhibition of Ca2+ influx evoked by glutamate (100 μM) at human recombinant receptors expressed in HEK293 cells (Dolman et al., 2007).
IC50 values are for inhibition of currents activated by glutamate (30 mM) at HEK293 cells expressing recombinant receptors (Perrais et al., 2009).
KB values were calculated using the Cheng-Prusoff correction for inhibition of Ca2+ influx in HEK293 cells expressing human recombinant receptors (Dolman et al., 2006).
Ki values were calculated from displacement of [3H]kainate from human GluK3 expressed in HEK293 cells (Dolman et al., 2006).
Data for ACET at GluK1, GluK2, GluK1/GluK5, and GluK2/GluK5 are from Dolman et al. (2007).
IC50 values are for inhibition of currents activated by glutamate (10 mM) from HEK293-T/17 cells expressing recombinant receptors (Lash et al., 2008). In the same assay, 300 μM 2,4-epi-neoDH failed to inhibit GluA4 receptors. Ki values for displacement of [3H]kainate from HEK293-T/17 cells expressing recombinant receptors are given in parentheses.
D. N-Methyl-d-aspartate Receptor Competitive Antagonists
Many competitive antagonists of the GluN1 subunits have been identified, including 7-chlorokynurenic acid and its analog 5,7-dichlorokynurenic acid (5,7-DCKA) (Birch et al., 1988; Kemp et al., 1988; Mayer et al., 1988; Kessler et al., 1989; Kleckner and Dingledine, 1989; McNamara et al., 1990) (Table 11). The GluN1 LBD shows 24° less domain closure when 5,7-DCKA is bound compared with when glycine is bound, suggesting that the antagonist stabilizes an open-cleft conformation (Furukawa and Gouaux, 2003). A majority of the contacts 5,7-DCKA forms with residues within the binding pocket are with domain D1 of the LBD. The carboxylate of 5,7-DCKA forms a hydrogen bond with Thr518 and interacts with Arg523, and the amino group forms a hydrogen bond with Pro516. Van der Waals interactions are formed between the aromatic rings of Phe408 and Trp731 and the chlorine atoms of 5,7-DCKA. Although GluN1 agonists have interdomain contacts formed between Gln405 and Trp731/Asp732, these interactions are disrupted by 5,7-DCKA (Furukawa and Gouaux, 2003). Like the GluN1 agonists, the GluN2 composition of the NMDA receptor influences the potencies of these antagonists by less than 10-fold (Ikeda et al., 1992; Kutsuwada et al., 1992; Buller et al., 1994; Priestley et al., 1995). The noble gas xenon exerts anesthetic actions independent of effects on GABAergic transmission and has been proposed to inhibit the NMDA receptor (Franks et al., 1998; de Sousa et al., 2000) through a direct interaction within the glycine binding site (Dickinson et al., 2007). Thus, xenon, like ketamine, may target the glutamatergic system to provide anesthetic effects.
TABLE 11.
Equilibrium dissociation constants in micromolar for NMDA receptor competitive antagonists
Data presented as Ki except where indicated as KB or Kd.
| Competitive Antagonist | Site | GluN2A | GluN2B | GluN2C | GluN2D |
|---|---|---|---|---|---|
| μM | |||||
| 7-CKAa | GluN1 | 0.6 | 0.2 | ||
| 5,7-DCKAb | GluN1 | 0.03 | 0.05 | 0.17 | 0.09 |
| CGP-61594 (KB)b | GluN1 | 0.43 | 0.045 | 0.16 | 0.34 |
| CGP-58411 (KB)c | GluN1 | 0.24 | 0.13 | ||
| GV150,526Ad | GluN1 | 0.08 | 0.08 | 0.11 | 0.05 |
| GV196,771Ad | GluN1 | 0.48 | 0.22 | 0.18 | 0.15 |
| MDL105,519d | GluN1 | 0.012 | 0.015 | 0.012 | 0.018 |
| ACEA-1011 (KB)e | GluN1 | 0.33 | 0.46 | 0.21 | 0.74 |
| ACEA-1021f | GluN1 | 0.004 | 0.004 | 0.003 | 0.011 |
| L-689,560 (KB)c | GluN1 | 0.004 | 0.02 | ||
| L-701,324a | GluN1 | 0.005 | 0.005 | ||
| (R)-AP5g | GluN2 | 0.28 | 0.46 | 1.6 | 3.7 |
| (R)-AP7g | GluN2 | 0.49 | 4.1 | 6.4 | 17 |
| PMPAg | GluN2 | 0.84 | 2.7 | 3.5 | 4.2 |
| (R)-CPPg | GluN2 | 0.041 | 0.27 | 0.63 | 1.99 |
| NVP-AAM077 (KB)h | GluN2 | 0.015 | 0.078 | ||
| PPDAi | GluN2 | 0.55 | 0.31 | 0.096 | 0.13 |
| (R)-α-AAi | GluN2 | 6.5 | 25 | 44 | 110 |
| PBPDi | GluN2 | 16 | 5.0 | 8.9 | 4.3 |
| UBP141j | GluN2 | 14 | 19 | 4.2 | 2.8 |
| CGS-19755 (selfotel)g | GluN2 | 0.15 | 0.58 | 0.58 | 1.1 |
| CGP-43487 (KB)c | GluN2 | 0.28 | 1.6 | ||
| CGP-40116 (KB)c | GluN2 | 0.04 | 0.03 | ||
| Con-Brk | GluN2 | 0.68 | 0.14 | 4.9 | 0.31 |
| Con-Gl | GluN2 | >10 | 0.1 | 1 | 1 |
| Con-Pr1l | GluN2 | >10 | 0.2 | >10 | 1 |
| Con-Pr2l | GluN2 | >10 | 0.5 | >10 | 1 |
| Con-Pr3l | GluN2 | >10 | 0.5 | >10 | 8 |
| Con-Rl | GluN2 | 1 | 1 | 7 | >10 |
| Con-T (Kd)m | GluN2 | 3.2 | 2.9 | ||
α-AA, α-aminoadipate; 5,7-DCKA, 5,7-dichlorokynurenic acid; 7-CKA, 7-chlorokynurenic acid; ACEA-1011, 5-chloro-7-trifluoromethyl-1,4-dihydro-2,3-quinoxalinedione; ACEA-1021, licostinel; AP5, 2-amino-5-phosphonopentanoate; AP7, 2-amino-7-phosphonopentanoate; CGP-61594, (±)-trans-4-[2-(4-azidophyenyl)acetylamino]-5,7-dichloro-1,2,3,4-tetrahydroquinoline-2-carboxylic acid; CGP-40116, d-(E)-2-amino-4-methyl-5-phosphono-3-pentenoic acid; CGP-43487, d-(E)-2-amino-4-methyl-5-phosphono-3-pentenoic acid methyl ester; CGP-58411, 7-chloro-4-hydroxy-3-phenyl-1H-quinolin-2-one. CGS-19755, (2R,4S)-4-(phosphonomethyl)piperidine-2-carboxylic acid; CPP, 4-(3-phosphonopropyl) pizerazine-2-carboxylic acid; GV150,526A, gavestinel; GV196,771A, (E)-4,6-dichloro-3-[(2-oxo-1-phenyl-3-pyrrolidinylidene)methyl]-1H-indole-2-carboxylic acid; L-689,560, 4-trans-2-carboxy-5,7-dichloro-4-phenylaminocarbonylamino-1,2,3,4-tetrahydroquinoline; L-701,324, 7-chloro-4-hydroxy-3-(3-phenoxy)phenyl-2(1H)-quinolone; MDL105,519, (E)-3-(2-phenyl-2-carboxyethenyl)-4, 6-dichloro-1H-indole-2-carboxylic acid; PBPD, (2S,3R)-1-(biphenyl-4-carbonyl)piperazine-2,3-dicarboxylic acid; PMPA, (R,S)-4-(phosphonomethyl)-piperazine-2-carboxylic acid; PPDA, (2S,3R)-1-(phenanthren-2-carbonyl)piperazine-2,3-dicarboxylic acid.
KB values are from the Cheng-Prusoff correction of IC50 values measured for inhibition of glycine-activated currents in mouse L(tk−) cells (Priestley et al., 1995).
Kd was calculated from on and off rates (Sheng et al., 2007).
The GluN2 competitive antagonist (R)-2-amino-5-phosphonopentanoate and its analogs are used widely as pharmacological tools to distinguish NMDA receptor-mediated activity from AMPA and kainate receptor activity (Davies et al., 1981, 1986; Evans et al., 1981; Lester et al., 1990) (Table 11). Unfortunately, GluN2 subunit selectivity has been difficult to achieve for competitive antagonists. For example, the antagonist 3-((R)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid shows ∼50-fold preference for GluN2A over GluN2D but has intermediate affinities at GluN2B and GluN2C (Ikeda et al., 1992; Kutsuwada et al., 1992; Feng et al., 2005) (Table 11). The competitive antagonist (R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquinoxalin-5-yl)-methyl-phosphonic acid (NVP-AAM077), originally reported to have more than 100-fold selectivity for GluN2A-containing receptors over GluN2B-containing receptors, was used to evaluate physiological roles of GluN2A and GluN2B in rodent models of synaptic plasticity and neurotoxicity before a full pharmacological characterization was completed (Liu et al., 2004b; Massey et al., 2004; Zhou and Baudry, 2006). Subsequent determination of KB from Schild analysis suggested that the selectivity was only ∼5-fold, precluding its use as a selective tool (Frizelle et al., 2006; Neyton and Paoletti, 2006). Antagonists with bulky, hydrophobic substituents, such as phenanthrene-piperazine dicarboxylic acid analogs (2S,3R)-1-(phenanthren-2-carbonyl)piperazine-2,3-dicarboxylic acid and (2R,3S)-1-(phenanthrenyl-3-carbonyl)piperazine-2,3-dicarboxylic acid (UBP141), show only modest 10-fold higher affinity for GluN2C- and GluN2D-containing receptors over GluN2A and GluN2B (Feng et al., 2004, 2005; Morley et al., 2005; Costa et al., 2009) (Table 11). Subunit selectivity for competitive antagonists may be difficult to achieve because of high homology among GluN2 subunit LBDs. Of the 39 residues lining the binding pocket, only 8 are divergent in GluN2A to GluN2D. All 10 residues that come into direct contact with glutamate are conserved between GluN2 subunits (Furukawa et al., 2005; Kinarsky et al., 2005).
Conantokins, peptides of 17 to 27 amino acids that lack disulfide bonds and are rich in γ-carboxyglutamate residues, have both competitive and noncompetitive antagonist activity on NMDA receptors (Prorok and Castellino, 2007). Conantokin-G is 20-fold more potent on GluN2A and GluN2B-containing NMDA receptors than on GluN2C and GluN2D-containing receptors (Wittekindt et al., 2001; Sheng et al., 2007; Teichert et al., 2007), and conantokin-Br is more potent for the GluN2D-containing NMDA receptors than other conantokins (Table 11) (Twede et al., 2009). Although conantokin-G binds within the GluN2 ligand binding pocket, one molecular determinant of specificity of conantokin-G for GluN2B has been identified as a Met739 residue outside of the binding pocket within the D2 domain of the LBD. This residue is not conserved in GluN2A receptors but is present in GluN2C and GluN2D subunits (Teichert et al., 2007). Conantokin-R and conantokin-T are antagonists of NMDA receptors, but antagonism is not GluN2 subunit-specific (Klein et al., 2001; Sheng et al., 2007; Teichert et al., 2007). Conantokins R and G have been studied preclinically for their effectiveness in the treatment of chronic pain, stroke, and seizure (White et al., 2000; Layer et al., 2004; Hama and Sagen, 2009).
E. Noncompetitive Antagonists
Several classes of noncompetitive antagonists at AMPA receptors have been used to selectively block AMPA but not kainate receptors, including 2,3-benzodiazepines and 1,2-dihydrophthalazines, as well as tetrahydroisoquinolines (Gitto et al., 2003). However, the most potent of the 2,3-benzodiazepines, 1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine (GYKI-53655) (Table 12), blocks GluK2/GluK3 heteromeric and GluK3 homomeric receptors with an IC50 that is approximately 10-fold higher than at AMPA receptors (Bleakman et al., 1996; Perrais et al., 2009). In contrast to the competitive antagonists CNQX and DNQX, GYKI-53655 remains an effective AMPA receptor antagonist in the presence of TARPs, with the potency of the antagonist increased (Menuz et al., 2007; Cokić and Stein, 2008). (S)-3-(2-Chlorophenyl)-2-[2-(6-diethylaminomethyl-pyridin-2-yl)-vinyl]-6-fluoro-3H-quinazolin-4-one (CP-465,022) (Menniti et al., 2000), an analog of quinazolinone, is approximately 100-fold more potent than GYKI-53655 at neuronal AMPA receptors (Bleakman et al., 1996; Menniti et al., 2000; Lazzaro et al., 2002) and seems 100-fold selective for AMPA over kainate receptors (Lazzaro et al., 2002). Mutagenesis studies suggest that GYKI-53655 and CP-465,022 share overlapping molecular determinants of action, requiring both S2–M4 and the S1–M1 linkers (Fig. 9; Balannik et al., 2005), the latter of which may be a critical element in gating (Sobolevsky et al., 2009) (see section VII.B and VII.D). The mechanism of block remains unclear but seems not to involve desensitization (Donevan and Rogawski, 1998).
TABLE 12.
IC50 values in micromolar for noncompetitive AMPA and kainate receptor antagonists
| Noncompetitive Antagonist | GluA1 | GluA2 | GluA3 | GluA4 | GluK1 | GluK2 | GluK3 | GluK2/GluK5 |
|---|---|---|---|---|---|---|---|---|
| μM | ||||||||
| GYKI 52466 | 18–117a,b | 34c | 22–66a,b | >100a | >100a | |||
| GYKI 53405 (LY 293606) | 24a | 28a | >100a | >100a | ||||
| GYKI 53773 (LY 300164)d | 21e | 18e | 19e | 18e | >100a | |||
| GYKI 53655 (LY 300168) | 6a | 5a | >100a | 198f | 63f | >100a | ||
| GYKI 53784 (LY 303070)d | 3a | 3a | >100a | >100a | >100a | |||
| CP-465,022 | 0.5g | 0.5g | 0.3g | >100g | >1h | |||
| NS-3763 | 1.6i | >30i | N.E.j | |||||
GYKI 52466, 1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine; GYKI 53405, 1-(4-aminophenyl)-3-acetyl-4-methyl-3,4-dihydro-7,8-methylenedioxy-5H-2,3-benzodiazepine; GYKI 53655, 1-(4-aminophenyl)-3-methylcarbamoyl-4-methyl-3,4-dihydro-7,8-methylenedioxy-5H-2,3 benzodiazepine; GYKI 53784, (−)-1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-4,5-dihydro-3-methylcarbamoyl-2,3-benzodiazepine.
IC50 value was determined from inhibition of Ca2+ influx activated by 30 μM glutamate at human GluA3 expressed in HEK69–8 cells (Varney et al., 1998).
This compound is the active enantiomer of the compound directly above it.
Values are for inhibition of Ca2+ influx evoked by domoate (2 μM for GluK1 and 0.2 μM for GluK2) in HEK293 cells expressing recombinant human receptors (Christensen et al., 2004b).
Values are for inhibition of glutamate-evoked currents at HEK293 cells expressing recombinant receptors (Christensen et al., 2004b).
Fig. 9.
Binding sites for the agonists, antagonists, and modulators described in sections V and VI are shown for the glutamate receptor. The receptor targets of ligands selective for one or several subunits are listed in parenthesis. AMPA and kainate indicates that the ligand selectively targets GluA or GluK receptor subunits, respectively. The ATDs, LBDs, TMDs, and linkers are shown in purple, orange, green, and gray, respectively. [Adapted from Sobolevsky AI, Rosconi MP, and Gouaux E (2009) X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462:745–756. Copyright © 2009 Nature Publishing Group. Used with permission.]
A major advance in kainate receptor pharmacology was made with the identification of arylureidobenzoic acids as noncompetitive GluK1 receptor antagonists (Valgeirsson et al., 2003, 2004). The most potent of these 2-arylureidobenzoic acids is 4,6-bis(benzoylamino)-1,3-benzenedicarboxylic acid (NS-3763), a selective antagonist of homomeric GluK1 (Table 12) that has no activity at heteromeric GluK1/GluK2 or GluK1/GluK5, AMPA receptors, or NMDA receptors. NS-3763 exhibits 10-fold greater potency in inhibiting glutamate-evoked currents from GluK1–2b versus GluK1–1a, two splice variants differing in 15 extracellular amino acid residues in the ATD and a cytoplasmic domain (Christensen et al., 2004b). Because NS-3763 inhibits only homomeric GluK1 receptors, it has been useful in exploring native kainate receptor stoichiometry (Christensen et al., 2004a).
The first subunit-selective NMDA receptor antagonist was the phenylethanolamine ifenprodil, which defined a new class of noncompetitive, voltage-independent partial antagonists (maximal inhibition ∼90%) of GluN2B-containing NMDA receptors (Table 13). Ifenprodil inhibits GluN2B-containing receptors with high affinity and is 200 to 400-fold more potent for GluN1/GluN2B receptors than for GluN1/GluN2A, GluN1/GluN2C, or GluN1/GluN2D receptors (Williams, 1993; Hess et al., 1998; IC50 ∼150 nM). GluN1 splice variants do not influence the ability of ifenprodil to inhibit GluN2B-containing receptors (Gallagher et al., 1996), but triheteromeric receptors containing GluN1/GluN2A/GluN2B are proposed to be less sensitive to ifenprodil (Hatton and Paoletti, 2005), and insensitive to CP-101,606 (Brimecombe et al., 1997; Chazot et al., 2002). The kinetic properties of ifenprodil also are altered in triheteromeric receptors; the macroscopic association rate of ifenprodil in GluN1/GluN2A/GluN2B receptors was slower, whereas the dissociation rate was faster than in GluN1/GluN2B receptors (Hatton and Paoletti, 2005). Ifenprodil and its more potent derivatives, including α-(4-hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1-piperidine propanol (Ro 25-6981), 1-[2-(4-hydroxy-phenoxy)-ethyl]-4-(4-methyl-benzyl)-piperidin-4-ol (Ro 63-1908), besonprodil (CI-1041), and traxoprodil mesylate (CP-101,606) (Gotti et al., 1988; Williams, 1993; Chenard et al., 1995; Fischer et al., 1997; Mott et al., 1998; Gill et al., 2002; Barton and White, 2004) (Table 13) are thought to interact with the GluN2B ATD and have been proposed to act by stabilizing an agonist-bound state in which the receptor has a low open probability (Kew et al., 1996, 1998; Fischer et al., 1997; Perin-Dureau et al., 2002) (Fig. 9). Ifenprodil inhibition is incomplete at saturating concentrations, and ifenprodil binds to a known modulatory domain, suggesting its actions might also be considered negative allosteric modulation, although it is widely referred to in the literature as a noncompetitive antagonist. Similar to Zn2+ binding to the ATD of GluN2A (see section VI.E), ifenprodil increases the potency of proton inhibition of NMDA receptors (Pahk and Williams, 1997; Mott et al., 1998). Rich pharmacology exists for this site, with nearly a dozen structural classes described, including oxamides (Barta-Szalai et al., 2004), 4-(3,4-dihydro-1H-isoquinolin-2-yl)-quinolines (Büttelmann et al., 2003), benzamidines (Claiborne et al., 2003), 5-substituted benzimidazoles (McCauley et al., 2004), indole-2-carboxamides (Borza et al., 2006, 2007), benzyl cinnamamidines (Tamiz et al., 1999; Curtis et al., 2003), and other biaryl analogs (Tamiz et al., 1998; Wright et al., 2000; Tahirovic et al., 2008; Mosley et al., 2009).
TABLE 13.
IC50 values in micromolar for noncompetitive GluN2B-selective NMDA receptor antagonists
Data are for rat recombinant receptors, except for ifenprodil, which was characterized on human recombinant receptors. Data for ifenprodil are from Hess et al. (1998). Values for Ro 25-6981 are from Fischer et al. (1997) and values for CP 101,606 are from Mott et al. (1998). Values for eliprodil are from Avenet et al. (1997), and values for felbamate are from Harty and Rogawski (2000). Values for haloperidol are from Ilyin et al. (1996).
| Noncompetitive Antagonist | GluN2A | GluN2B | GluN2C | GluN2D |
|---|---|---|---|---|
| μM | ||||
| Ifenprodil | 39 | 0.15 | 29 | 76 |
| Ro 25-6981 | 52 | 0.0090 | ||
| CP-101,606 | >100 | 0.039 | >100 | >100 |
| Eliprodil | >100 | 3.02 | ||
| Felbamate | 2600 | 520 | 2400 | |
| Haloperidol | N.E. | 3 | N.E. | N.E. |
CP-101,606, traxoprodil mesylate; N.E., <10% inhibition at 300 μM.
GluN2B-selective antagonists have been implicated in the treatment of a variety of diseases and neurological disorders (see section X). However, development of GluN2B-selective antagonists has been hindered by their activity at α1-adrenergic receptors, serotonin receptors, calcium channels, and hERG potassium channels (Lynch and Gallagher, 1996). The problem of target selectivity has been partially overcome by potent ifenprodil analogs such as Ro 25-6981 and CP-101,606 (Fischer et al., 1997; Taniguchi et al., 1997; Tahirovic et al., 2008). Off-target activity at hERG channels by GluN2B antagonists is decreased by eliminating basic nitrogen atoms; increasing the number of oxygen atoms within the linker of GluN2B antagonists also decreases affinity for hERG channels and α1-adrenergic receptors (Kawai et al., 2007; Mosley et al., 2009). The GluN2B selective antagonist (3S,4R)-4-methylbenzyl 3-fluoro-4-((pyrimidin-2-ylamino)methyl)piperidine-1-carboxylate (MK-0657) unexpectedly increased both systolic and diastolic blood pressure. It is unclear whether these effects occur peripherally or centrally (Addy et al., 2009; Mony et al., 2009).
Certain quinazolin-4-one analogs of the noncompetitive AMPA receptor antagonist CP-465,022 can also inhibit recombinant NMDA receptors in a voltage-independent and noncompetitive fashion (Mosley et al., 2010). These data suggest that a new site for noncompetitive antagonism may exist on recombinant NMDA receptors. Some quinazolin-4-ones are greater than 50-fold more potent at recombinant NMDA receptors that contain GluN2C/GluN2D subunits over NMDA receptors that contain GluN2A/GluN2B subunits or AMPA/kainate receptors. These compounds could provide a starting point for the development of new classes of subunit-selective antagonists for NMDA receptors that contain GluN2C or GluN2D subunits.
Ethanol has been proposed to be a noncompetitive antagonist of NMDA receptors, with sensitivity dependent upon the GluN2 subunit (Lovinger et al., 1989, 1990; Kuner et al., 1993; Masood et al., 1994; Mirshahi and Woodward, 1995; Kash et al., 2008; Nagy, 2008) but independent of GluN1 splice variant, pH, Zn2+, or redox state (Kuner et al., 1993; Chu et al., 1995; Peoples and Weight, 1999). A wide range of studies has suggested that actions at native NMDA receptors contribute to the central effects of alcohol, which may involve perturbation of the interaction between glutamate and dopamine (Lovinger, 2002; Maldve et al., 2002). However, recent studies suggest that recombinant NMDA receptor inhibition is modest at the U.S./U.K. drink-drive limit as well as at intoxicating levels (Otton et al., 2009). Dynorphin peptides have been reported to have a variety of actions on NMDA receptors, acting as noncompetitive antagonists (Chen and Huang, 1998; Kanemitsu et al., 2003; Oz et al., 2004) as well as potentiators under some circumstances (Zhang et al., 1997; Caudle and Dubner, 1998; Lai et al., 1998). Dynorphin A (1–17) and (2–17) can displace glycine at GluN1 (Voorn et al., 2007) and can bind to an anionic intracellular epitope on GluN1 that may interact with dopamine receptors (Jackson et al., 2006; Woods et al., 2006). Potency for Dynorphin A (1–13) is dependent upon GluN2 subunit, with the peptide being most potent for GluN2A-containing NMDA receptors (Brauneis et al., 1996). Longer dynorphin peptides are more potent inhibitors [e.g., Dynorphin A (1–32); Chen and Huang, 1998]. Inhibition by dynorphin A is pH sensitive in that inhibition increases at lower pH (Kanemitsu et al., 2003; Oz et al., 2004).
F. Uncompetitive Antagonists
Open channel blockers require that the receptor pore be open to allow access to the blockers' binding site to cause subsequent block of receptor activity (Neely and Lingle, 1986; Huettner and Bean, 1988; Kiskin et al., 1989) (Fig. 9). Because of this requirement, the onset of inhibition is use-dependent and increases with increasing channel open probability. After channel closure, some blockers can become trapped in the pore, and these antagonists are called trapping blockers [PCP, dizocilpine maleate (MK-801), ketamine at NMDA receptors, 1-trimethylammonio-5-(1-adamantane-methylammoniopentane dibromide) (IEM-1460) at AMPA receptors] and partially trapping blockers (memantine at NMDA receptors). Channel block by trapping blockers is slow to reverse and requires channel reactivation by agonists before the blocker can dissociate (Brackley et al., 1993; Parsons et al., 1995; Blanpied et al., 1997; Magazanik et al., 1997).
A large number of naturally occurring AMPA and kainate receptor channel blockers, as well as a host of synthetic analogs, have been identified (Table 14), including argiotoxin-636 (Herlitze et al., 1993), Joro spider toxin (Blaschke et al., 1993), Ageltoxin-489 (Washburn and Dingledine, 1996), philanthotoxin-433 (Jones et al., 1990), IEM-1460 (Magazanik et al., 1997), and N1-naphthylacetylspermine (Koike et al., 1997), which also blocks mutant Lurcher GluD2 channels. Some of these compounds have nonspecific actions at other ion channels (Welch et al., 2008). All of these uncompetitive antagonists have structural similarity (i.e., a polyamine moiety) and, when applied extracellularly, exhibit voltage-dependent block. These compounds act primarily on GluA2-lacking Ca2+-permeable AMPA receptors, although Joro spider toxin and philanthotoxin also block unedited GluK2 channels (Blaschke et al., 1993; Bähring and Mayer, 1998). The QRN site at the apex of the M2 reentrant pore-lining loop is a key structural determinant of polyamine block (see sections II.E and VIII), with receptors lacking the edited GluA2(R) subunits showing strong block by polyamines and toxins. Thus, these channel blockers have been useful pharmacological tools to probe the subunit composition of AMPA receptors (Laezza et al., 1999; Liu and Cull-Candy, 2000; Plant et al., 2006), although many also show actions at kainate receptors. The amino groups of these compounds interact with residues that reside deeper in the pore than the QRN site, including the main-chain oxygen atom from the QRN + 2 site (Tikhonov et al., 2002), and at least two amino groups are required for potent antagonism at AMPA receptors (Bolshakov et al., 2005). Some compounds (e.g., phenylcyclohexyl derivative IEM-1925) can permeate the channel, allowing closed channels to escape from block (Tikhonova et al., 2008). Other blockers [e.g., adamantane derivative IEM-1676 (Tikhonova et al., 2008)] produce a voltage-dependent closed channel block from the intracellular compartment in addition to open channel block from the extracellular compartment (Tikhonova et al., 2009). Association of AMPA receptors with TARPs γ2, γ3, and γ8 reduces channel block by N1-naphthylacetylspermine (Kott et al., 2009), an intriguing finding because TARPs also increase channel opening frequency (Tomita et al., 2005a) (see section II.H).
TABLE 14.
IC50 values in micromolar for uncompetitive AMPA receptor antagonists
All data from GluA2 are from the edited form [GluA2(R)].
| Uncompetitive Antagonist | GluA1 | GluA2 | GluA3 | GluA4 | GluA1/GluA2 |
|---|---|---|---|---|---|
| μM | |||||
| Argiotoxin 636 | 0.35–3.4a,b | N.E.a | 0.23a | 0.43a | 300b |
| Joro spider toxin | 0.04c | N.E.c | 0.03c | N.E.c | |
| Philanthotoxin 433d | 0.8 | N.E. | |||
| Philanthotoxin 343 | 2.8b | 270b | |||
| Philanthotoxin 56 | 3.3 pMe | 5.2e | |||
| Philanthotoxin 74 | 0.17e | 1.6e | |||
| IEM-1460 | 1.6f | N.E.g | 1.6f | ||
| IEM-1754 | 6.0f | 6.0f | |||
| HPP-spermined | 0.5 | 0.08 | 0.5 | N.E. | |
HPP-spermine, N-(4-hydroxyphenylpropanoyl)spermine trihydrochloride; IEM-1754, 1-ammonio-5-(1-adamantane-methylammoniopentane dibromide); N.E., no effect.
Herlitze et al. (1993); holding membrane potential was −70 mV.
Brackley et al. (1993); holding membrane potential, −80 mV.
Blaschke et al. (1993); holding membrane potential, −100 mV.
Washburn and Dingledine (1996); holding membrane potential, −70 mV. Although IC50 values were not calculated, initial experiments suggested that philanthotoxin-433 had a lower affinity for GluA1 and GluA4 receptors compared with GluA3.
Philanthotoxin 56 has an IC50 of 3.3 pM for recombinant GluA1 (Kromann et al., 2002); holding membrane potential, −80 mV.
Magazanik et al. (1997); holding membrane potential, −80 mV.
Schlesinger et al. (2005); holding membrane potential, −60 mV, recombinant human GluA2(R).
Structure-activity relationships of philanthotoxins have highlighted the importance of the polyamine moiety and led to potent and selective AMPA receptor blockers. Shortening the polyamine chain of PhTX-343 caused a marked decrease in potency at AMPA receptors (Mellor et al., 2003). Moreover, replacing the two secondary amines in the polyamine moiety with either oxygen or methylene resulted in a complete loss of activity, whereas replacing only one with methylene improved potency 15-fold and increased selectivity for AMPA versus NMDA receptors to 100-fold (Mellor et al., 2003). Further modification of the polyamine tail of PhTX-343 resulted in PhTX-56 and PhTX-74, which differ in the number of amines and intervening methylenes (Kromann et al., 2002). PhTX-56 is highly selective for Ca2+-permeable AMPA receptors, being 1000-fold more potent at GluA2-lacking receptors. PhTX-56 is 500-fold selective for AMPA over kainate receptors (Kromann et al., 2002). PhTX-74 inhibits GluA1/GluA2 but not GluA2/GluA3 receptors (Nilsen and England, 2007).
Uncompetitive NMDA receptor antagonists include Mg2+, polyamines (see section VIII.C), dissociative anesthetics phencyclidine and ketamine, MK-801, amino-adamantane derivatives memantine and amantadine, pentamidine, 9-tetrahydroaminoacridine, dextromethorphan, and its metabolite dextrorphan (Table 15). It is noteworthy that pentamidine and 9-tetrahydroaminoacridine also inhibit currents of the mutant Lurcher GluD2 receptors (Williams et al., 2003). The structure-activity relationship underlying the trapping nature of blockers is unrelated to lipophilicity, and thus blockers are not capable of escaping through the membrane (Mealing et al., 2001; Bolshakov et al., 2005). Partially trapping blockers, such as memantine and amantadine, bind after channel opening. However, these drugs also unbind rapidly (Blanpied et al., 1997, 2005; Chen and Lipton, 1997; Mealing et al., 1999), which has been proposed to be therapeutically beneficial, because normal synaptic transmission may not be influenced by the drug, but overactivation of NMDA receptors should be decreased (Chen and Lipton, 2006) (see section X.G). Memantine may access multiple binding sites, one of which has been proposed to reside superficially near the extracellular end of the pore in GluN1/GluN2A. Occupancy of this superficial site may prevent full channel closure (Sobolevsky and Koshelev, 1998; Sobolevsky et al., 1998; Bolshakov et al., 2003); however, more work is needed to determine the exact location with respect to the gate of this superficial site. The presence of the superficial binding site also could be relevant for improved side effect profile for memantine (Kotermanski et al., 2009).
TABLE 15.
IC50 values in micromolar for uncompetitive NMDA receptor antagonists
All values were measured in 0 Mg2+, unless otherwise indicated. Values for memantine and (±)-ketamine are from Kotermanski and Johnson (2009) with membrane potential held at −66 mV. All remaining values are from Dravid et al. (2007) with membrane potential held at −40 mV.
| Uncompetitive Antagonist | GluN2A | GluN2B | GluN2C | GluN2D |
|---|---|---|---|---|
| μM | ||||
| (+)-MK-801 | 0.015 | 0.009 | 0.024 | 0.038 |
| (−)-MK-801 | 0.35 | 0.32 | 0.038 | 0.17 |
| (−)-Ketamine | 16 | 1.6 | 1.1 | 1.5 |
| (±)-Norketamine | 51 | 8.7 | 5.6 | 7.5 |
| Dextromethorphan | 11 | 3.7 | 1.1 | 5.4 |
| Levomethorphan | 13 | 2.2 | 1.1 | 2.6 |
| Dextrorphan | 1.3 | 0.33 | 0.15 | 0.74 |
| Levorphanol | 1.8 | 1.2 | 0.58 | 2.1 |
| Phencyclidine | 0.82 | 0.16 | 0.16 | 0.22 |
| PCA | 19 | 3.9 | 1.6 | 1.7 |
| CNS-1102 | 0.13 | 0.068 | 0.087 | 0.14 |
| Amantadine | 130 | 70 | 35 | 38 |
| Remacemide | 81 | 35 | 92 | 63 |
| Pentamidine | 0.72 | 1.5 | 10 | 9.1 |
| 9-aminoacridine | 7.8 | 7.5 | 29 | 38 |
| Memantine | 0.80 | 0.57 | 0.52 | 0.54 |
| Memantine–1 mM Mg2+ | 13 | 10 | 1.6 | 1.8 |
| (±)-Ketamine | 0.33 | 0.31 | 0.51 | 0.83 |
| (±)-Ketamine–1 mM Mg2+ | 5.4 | 5.08 | 1.2 | 2.9 |
CNS-1102, aptiganel; PCA, 1-phenylcyclohexylamine.
Most NMDA receptor channel blockers are either nonselective or at best weakly selective (<10-fold) for specific GluN2 subtypes (Yamakura et al., 1993). For example, MK-801 is ∼10-fold more potent for GluN2A- and GluN2B-containing receptors than GluN2C- and GluN2D-containing receptors (Bresink et al., 1996; Dravid et al., 2007). However, aryl-polyamine derivatives show high potency as well as slightly better subunit selectivity, with N1-dansyl-spermine and the tribenzyltriamine TB-3-4 showing approximately 40-fold lower IC50 values at GluN2A- than GluN2D-containing NMDA receptors (Chao et al., 1997; Igarashi et al., 1997; Jin et al., 2007). The molecular determinants of activity of channel blockers are not identical but overlap, with dependence on residues within the M2 reentrant pore region and residues in other pore-forming elements as well as the pre-M1 region (Yamakura et al., 1993; Yamakura and Shimoji, 1999; Kashiwagi et al., 2002; LePage et al., 2005; Jin et al., 2007). The sequence similarity within the pore-forming elements of NMDA receptors may constitute a challenge in the future development of subunit-selective compounds. It is noteworthy that the potencies of channel blocking compounds are sensitive to pH. Although acidic pH reduces NMDA receptor open probability (Traynelis and Cull-Candy, 1990), acidic pH paradoxically increases the association rate of the trapping blocker MK-801, which may reflect an interaction of MK-801 with the structural elements forming the gate of the receptor (Dravid et al., 2007). Several reports raise the possibility that trapping blockers do not passively reside in the pore when their exit path is removed as a result of channel closure, but rather stabilize the closed state, thereby promoting channel closure and their own subsequent trapping (Blanpied et al., 2005; Yuan et al., 2005). Recent work conducted on ketamine and memantine indicate that these antagonists may be more selective at physiological conditions than previously reported, because 1 mM Mg2+ produced a 5- to 10-fold selectivity for GluN2C- and GluN2D-containing receptors over GluN2A and GluN2B (Table 15; Kotermanski and Johnson, 2009). These findings may be clinically and functionally significant, depending on concentrations of memantine reached in brain. These results also emphasize the need to study pharmacology under physiological conditions.
VI. Allosteric Regulation
Allosteric modulators for glutamate receptors have attracted attention because of their ability to fine-tune normal receptor function as well as for their potential utility as therapeutic agents. Drug-like positive or negative allosteric modulators have distinct therapeutic advantages over agonists and competitive antagonists, including higher potential for receptor subtype selectivity, because they often target less conserved regions than the agonist binding site (Figs. 2 and 9). Clinically, modulators are generally thought to be better tolerated because they modify existing levels and patterns of receptor activation, rather than constitutively blocking (as with antagonists) or overactivating (as with agonists) all receptors.
A. Positive and Negative Allosteric Modulators
A growing range of compounds potentiate AMPA receptor activity through modification of the deactivation and/or desensitization time course (Arai et al., 1994; Stäubli et al., 1994; Lauterborn et al., 2000). Binding of AMPA receptor agonists within the cleft of the LBD induces closure around the ligand, which has been proposed to create strain within the receptor complex that can be relieved by channel opening or relaxation of the LBD dimer interface to a desensitized state, in which agonist remains bound but the channel is closed (Sun et al., 2002; Sobolevsky et al., 2009) (Fig. 3). The receptor deactivates as the channel closes and the ligand fully unbinds, exiting the binding cleft. Positive AMPA receptor modulators bind at the LBD dimer interface, making a number of intraprotomer atomic contacts that stabilize the dimerized configuration and prevent transition to the relaxed state after agonist binding, thus preventing desensitization (Figs. 3 and 10B). Some of these compounds also can slow the rate of agonist exit, thus preventing deactivation.
Fig. 10.
Allosteric regulation of glutamate receptors. A, the structure of the dimer formed between LBDs of the L483Y mutated GluA2 (PDB code 1LB8) is shown from the top (left) and perpendicular (right) to the 2-fold axis. Mutation of residue 483 (blue) located on D1 from Leu to Tyr attenuates desensitization and stabilizes the dimer interface by interactions with Leu748 and Lys752 on the opposing protomer. B, the LBD dimer interface contains two binding sites for the positive AMPA receptor modulator cyclothiazide (blue) that inhibits receptor desensitization (PDB code 1LBC). Cyclothiazide stabilizes the dimer interface by forming additional intersubunit interactions in the dimer interface. C, structure of the GluN2B ATD with bound Zn2+ (PDB code 3JPY). The cleft formed by the upper R1 and the lower R2 lobes can be divided into three pockets: the hydrophobic pocket (gray carbon atoms), the ion binding site with Na+ and Cl−, and the hydrophilic pocket with the Zn2+ binding site. The hydrophobic pocket is thought to bind ifenprodil and its analogs.
At present, there are three structural classes of positive AMPA receptor modulators: 1) pyrrolidinone and related piperidine compounds [e.g., aniracetam, piracetam, oxiracetam, and the ampakines 2H,3H,6aH-pyrrolidino(2′,1′-3′, 2′)1,3-oxazino(6′,5′-5,4)benzo(e)1,4-dioxan-10-one (CX614), ampalex (CX516), and 1-(1,4-benzodioxan-6-ylcarbonyl) piperidine (CX546)], 2) benzothiadiazide compounds [e.g., cyclothiazide, diazoxide, (S)-2,3-dihydro-(3,4)cyclopentano-1,2,4-benzothiadiazine-1,1-dioxide (S18986), and 7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-S,S-dioxide (IDRA-21)], and 3) biarylpropylsulfonamide compounds [e.g., PEPA, N-[2-(4′-cyanobiphenyl-4-yl)propyl]propane-2-sulfonamide (LY404187), N-(2-(4-(thiophen-3-yl)phenyl)propyl)propane-2-sulfonamide (LY392098), (R)-2-(4-(3,5-difluorobenzoylamino)phenyl)-1-(2-propanesulfonamido)-propane (LY450108), LY451395, and LY503430] (Ito et al., 1990; Copani et al., 1992; Arai et al., 1996, 2000; Baumbarger et al., 2001a,b). Although positive AMPA receptor modulators fall into three structural classes, the most important difference between them is their mechanisms of action.
Aniracetam primarily slows the deactivation rate without changing agonist potency. In contrast, PEPA potentiates AMPA receptor function by attenuating the extent of receptor desensitization without any effect on deactivation (Sekiguchi et al., 2002). Cyclothiazide seems to slow the onset of desensitization and indirectly slow the channel closure rate by increasing agonist potency (Yamada and Tang, 1993; Partin et al., 1994, 1996; Sekiguchi et al., 2002; Arai and Kessler, 2007). LY404187 stabilizes the open state of AMPA receptors in the presence of agonist without altering the rate at which channels desensitize, thereby permitting desensitized AMPA receptors to make a transition to an open state either directly or through intermediate desensitized and/or closed states (Baumbarger et al., 2001b). Some compounds (e.g., CX614) inhibit both desensitization and deactivation; the mechanism underlying this is not well understood (Arai et al., 2000).
Further complexity arises from differences in the potencies of positive allosteric modulators for RNA splice variants of the AMPA receptors, particularly the flip/flop region within the LBD. Cyclothiazide almost completely eliminates desensitization of flip splice variants but only slows the entry into desensitized state for the flop splice variant (Johansen et al., 1995; Partin et al., 1996; Hennegriff et al., 1997; Sekiguchi et al., 2002). The specificity of cyclothiazide for flip splice variants arises from its interaction with Ser754, which is a larger Asn in flop variants and precludes tight cyclothiazide binding (Sun et al., 2002). Aniracetam has a similar potency at flip and flop splice forms, but the efficacy is greater for the flop form (Johansen et al., 1995). PEPA and CX614 are selective for the flop variants (Hennegriff et al., 1997; Sekiguchi et al., 1997, 1998). LY404187 and LY503430 suppress receptor desensitization with a distinct time-dependence in the presence of agonist; these compounds show the highest potency for flip variants of GluA2 and GluA4 (Miu et al., 2001; Murray et al., 2003).
The functional effects of the different positive allosteric modulators are complex because multiple partially overlapping binding sites exist (Partin et al., 1996; Yamada and Turetsky, 1996; Lindén et al., 2001; Arai et al., 2002; Sun et al., 2002). The binding sites of compounds that affect desensitization reside within the dimer interface between the ligand binding domains, which supports the interpretation that desensitization involves rearrangement of the dimer interface. Crystallographic studies indicate that cyclothiazide, which mainly modulates desensitization, has two binding sites within the dimer interface, whereas CX614 and aniracetam, which control desensitization and deactivation time course, bind at a different site at the hinge of the dimer interface in two alternate orientations (Sun et al., 2002; Jin et al., 2005) (Fig. 10, A and B). Aniracetam and CX614 bind to the center of the dimer interface and do not penetrate the ligand binding domain clamshell. The binding sites for aniracetam/CX614 and cyclothiazide do overlap, with the endocyclic sulfonamide group from cyclothiazide overlapping with the site recognizing the five-member rings of CX614/aniracetam. Aniracetam and CX614 stabilize the closed-cleft conformation of the ligand binding domain, slowing the deactivation time course. Cyclothiazide, which does not significantly stabilize the closed-cleft conformation of the LBD, instead stabilizes the dimer assembly, leading to an attenuation of desensitization. These data indicate that the molecular and structural determinants of deactivation and desensitization are separable (Sun et al., 2002; Jin et al., 2005). The challenge is now to understand how these mechanistically distinct compounds differentially affect brain circuitry to produce effects on behavior that are therapeutically meaningful (Lynch, 2006).
Con A, a plant lectin from Canavalia ensiformis, irreversibly potentiates agonist-evoked currents from most kainate receptors by apparently reducing receptor desensitization and increasing agonist affinity (Huettner, 1990; Partin et al., 1993; Wong et al., 1994; Bleakman et al., 2002). Lectins seem to bind to N-linked glycosylation within the ATD (Everts et al., 1997, 1999). The action of con A is state-dependent, because agonist-induced desensitization before application of con A eliminates potentiation (Fay and Bowie, 2006). Con A has been suggested to keep the activated channel in the open state and inhibit conformational changes leading to the desensitized state (Partin et al., 1993; Wong and Mayer 1993; Yue et al., 1995). Alternatively, con A may shift the contribution of different kainate receptor open states (Bowie et al., 2003). Although con A has proven experimentally useful for characterizing kainate receptor pharmacology, it does not alter synaptic kainate receptor currents and only weakly potentiates whole-cell currents from cultured hippocampal neurons (Wilding and Huettner, 1997). Other lectins, however, including wheat germ agglutinin, soybean agglutinin, and succinyl-concanavalin A, potentiate native kainate receptor function (Thio et al., 1993; Yue et al., 1995).
B. Divalent Ions
Multiple divalent cations influence glutamate receptor inactivation, voltage-dependent channel block, and agonist dissociation rates, in addition to potentiating and inhibiting receptor responses. Among divalent cations, extracellular Zn2+ most potently inhibits native (Peters et al., 1987; Westbrook and Mayer, 1987) and recombinant NMDA receptors (Williams et al., 1996; Chen et al., 1997; Paoletti et al., 1997; Traynelis et al., 1998). The endogenous ion zinc is packaged into synaptic vesicles in axons terminating in the hippocampus, striatum, amygdala, neocortex, and cortex (Pérez-Clausell and Danscher, 1985; Frederickson, 1989; Valente et al., 2002; Danscher and Stoltenberg, 2005; Paoletti et al., 2009) and may be coreleased with glutamate from the vesicles into the synaptic cleft during neuronal activity. The expression of GluN2A- and GluN2B-containing NMDA receptors may allow zinc to act as an endogenous modulator of the NMDA receptor, depending on its synaptic concentration. Zn2+ inhibition curves for GluN1/GluN2A receptors are biphasic, revealing high-affinity voltage-independent inhibition (IC50 10–30 nM) and low-affinity voltage-dependent channel block (IC50 20–100 μM). In the absence of voltage-dependent channel block, Zn2+ inhibition is incomplete even at saturating concentrations. The high-affinity Zn2+ binding site resides within the cleft of the GluN2A bilobed ATD and most likely involves Zn2+coordination by a series of histidine residues (Choi and Lipton, 1999; Fayyazuddin et al., 2000; Low et al., 2000). Removal of the GluN2A ATD by mutagenesis or alteration of this domain after cleavage by the endogenous serine protease plasmin at Lys317 eliminates or reduces voltage-independent high-affinity Zn2+ inhibition, respectively (Gielen et al., 2009; Yuan et al., 2009a,b). Low-affinity voltage-dependent Zn2+ inhibition involves residues inside the reentrant M2 pore loop similar to the Mg2+ blocking site (Legendre and Westbrook, 1990; Paoletti et al., 2000).
Micromolar concentrations of Zn2+ inhibit GluN2B-containing receptors in both a voltage-independent and voltage-dependent manner (Williams, 1996; Traynelis et al., 1998; Choi and Lipton, 1999; Rachline et al., 2005). Crystallographic coordinates of the GluN2B ATD show that a Zn2+ binding site exists within the cleft of the clamshell of the ATD (Fig. 9C) (Karakas et al., 2009), and mutagenesis suggests that this site can account for voltage-independent inhibition. As hypothesized for high-affinity binding of Zn2+ to the GluN2A ATD (Paoletti et al., 2000), crystallographic studies of GluN2B show Zn2+ binding stabilizes a closed-cleft conformation within the ATD through direct contact with residues His127 and Glu284. Mutation of these residues does not influence inhibition by the GluN2B-selective antagonist ifenprodil, suggesting that Zn2+ and ifenprodil bind at unique sites within the GluN2B ATD. Residues Glu47 and Asp265, although not directly involved in Zn2+ binding, influence Zn2+ sensitivity, perhaps through coordination of water molecules that can interact with Zn2+ (Karakas et al., 2009). It is noteworthy that mutations at GluN2B His127, one of the residues in contact with Zn2+, also block potentiation of GluN2B receptors by Ni2+ (Marchetti and Gavazzo, 2005; Gavazzo et al., 2009).
Studies with covalent modification of mutant GluN2A receptors that harbor a cysteine residue within the ATD cleft raise the idea that the extent of ATD closure around the Zn2+ binding cleft negatively correlates with open probability, perhaps through actions at the LBD dimer interface (Gielen et al., 2009). Specifically, high-affinity Zn2+ inhibition of NMDA receptors has been proposed to involve domain closure within the bilobed ATD around Zn2+, and this has been suggested to destabilize the dimer interface of the LBD (Mayer, 2006; Gielen et al., 2008) analogous to desensitization of AMPA and kainate receptors (Armstrong et al., 2006; Weston et al., 2006b; Plested and Mayer, 2007) (see sections II.D and VII.E). In support of this idea, mutations that destabilize the LBD dimer interface enhance Zn2+ sensitivity, whereas cysteine cross-linking of the dimer interface of mutant GluN1 and GluN2A receptors reduce Zn2+ inhibition (Gielen et al., 2008). In addition to effects at the dimer interface, Zn2+ shifts the proton inhibition curve leftward, suggesting that Zn2+ binding to the ATD increases proton sensitivity (discussed below in section VI.D) and consequently increases the proportion of protonated, nonfunctional receptors at physiological pH (Choi and Lipton, 1999; Low et al., 2000). Fitting both macroscopic and single-channel data with kinetic models containing explicit Zn2+ and proton binding steps suggests that association of Zn2+ with its GluN2A binding site enhances proton binding (Erreger and Traynelis, 2005, 2008). Mutations at the same LBD dimer interface residues that alter Zn2+ inhibition also strongly influence proton inhibition (Gielen et al., 2008), supporting a functional link between these two forms of modulation and confirming the hypothesis that Zn2+ binding enhances proton inhibition. The potential link between proton inhibition and NMDA receptor LBD dimer interface stability fits well with previous observations that protons modify GluA receptor desensitization (Ihle and Patneau, 2000; Lei et al., 2001), a process unequivocally shown to involve rearrangement at the LBD dimer interface (see sections II.D and VII.E). Chen et al. (1997) first described a rapid component of desensitization in the presence of extracellular Zn2+ and postulated that Zn2+ accelerated receptor desensitization. Subsequent studies, drawing from the mechanism of glycine-dependent desensitization (see section VII.E), showed that a positive intrasubunit interaction occurs between glutamate binding to the LBD and Zn2+ binding to the ATD (Zheng et al., 2001). The working hypothesis, supported by multiple lines of evidence, suggests that binding of glutamate enhances Zn2+ binding, which at subsaturating concentrations of Zn2+ causes a relaxation to a new equilibrium as Zn2+ binds to the receptor in a concentration-dependent fashion (Zheng et al., 2001; Erreger and Traynelis, 2005). Thus, the Zn2+-dependent desensitization time course reflects the time course for Zn2+ association with GluN2A receptors after a shift of the Zn2+ binding site into a high-affinity state.
Calcium, barium, magnesium, and zinc ions can inhibit or potentiate current responses of recombinant and native AMPA receptors (Rassendren et al., 1990; Bresink et al., 1996; Dreixler and Leonard, 1997; Shen and Yang, 1999; Zhang et al., 2002; Blakemore and Trombley, 2004; Kreitzer et al., 2009) and kainate receptors (Hoo et al., 1994; Fukushima et al., 2003; Mott et al., 2008). Zn2+ and Ca2+ can permeate through AMPA/kainate receptors (Sensi et al., 1999; Weiss and Sensi, 2000) (see section VIII.B). Of these various actions, kainate receptors are most sensitive to inhibition by Zn2+, which exerts voltage-independent inhibition, suggesting it acts as a noncompetitive antagonist (Fukushima et al., 2003). Zinc inhibition of kainate receptors is subunit-dependent, because GluK4- and GluK5-containing receptors have lower IC50 values (1–2 μM) than GluK1–3 receptors (∼70 μM). Inhibition by Zn2+ is also dependent upon pH, because increasing the proton concentration decreases Zn2+ potency by 2- to 9-fold for recombinant receptors and 15-fold for synaptic receptors (Mott et al., 2008). One possible mechanism for proton-dependent zinc inhibition of kainate receptors is that receptor protonation diminishes the ability of Zn2+ to bind the receptor, but this has not been tested.
Although the GluD2 glutamate receptor has no known agonists or modulators, the spontaneously active Lurcher mutant GluD2 receptor is positively modulated by Ca2+ (Wollmuth et al., 2000). The current amplitude of Lurcher GluD2 more than doubles in the presence of Ca2+ in a voltage-independent manner unaffected by editing of the QRN site within the pore (Wollmuth et al., 2000). Potentiation by Ca2+ reflects stabilization of the LBD dimer interface, which decreases desensitization of the active receptor (Hansen et al., 2009). Ca2+ decreases the potency of d-serine, a ligand that inhibits Lurcher GluD2 currents, and crystallographic data of the GluD2 LBD show that Ca2+ binds at the interface and is coordinated by residues Glu531, Asp535, and Asp782 (Naur et al., 2007; Hansen et al., 2009). Taken together, these results suggest that Ca2+ binding stabilizes the LBD dimer interface, thereby reducing the ability of d-serine to cause dimer interface breakdown and desensitization (Hansen et al., 2009).
C. Monovalent Ions
External monovalent ions, such as Na+ and Cl−, regulate the gating of kainate receptors, but not AMPA or NMDA receptors, through an allosteric mechanism involving both anions and cations (Bowie, 2002; Wong et al., 2007). Increased concentrations of external Na+ and Cl− potentiate the amplitude and prolong deactivation of kainate receptor responses. Structural studies indicate that two Na+ ions flank a single Cl− ion and bind in a charged pocket of the LBD dimer interface between two subunits, leading to a 50-fold increase in dimer affinity and a decrease in the rate of receptor desensitization (Plested and Mayer, 2007; Chaudhry et al., 2009). Other monovalent cations, including Li+, K+, Rb+, and Cs+, are capable of binding to kainate receptors, but these ions bind at lower affinity and are less efficacious than Na+ (Plested et al., 2008). Mutation of a Met770 in the D1 region of the GluK2 LBD dimer interface, although not directly involved in cation binding, can perturb the site (Paternain et al., 2003; Plested and Mayer, 2009).
Neuronal NMDA receptor function is potentiated up to 2-fold by increases in intracellular Na+ to 40 mM, with Na+ sensitivity set by the nonreceptor tyrosine kinase src (Yu and Salter, 1998, 1999). The enhancement of action by intracellular Na+ interacts with Ca2+-dependent inactivation (Xin et al., 2005). Increases in intracellular Na+ concentration peak during high frequency firing coincident with src activation (Yu and Salter, 1999), suggesting that this effect could be relevant to synaptic plasticity and cell death caused by overactivation of NMDA receptors (Yu, 2006).
D. Protons
Protons inhibit all glutamate receptors without changing ionization of the agonist (Christensen and Hida, 1990; Giffard et al., 1990; Tang et al., 1990; Traynelis and Cull-Candy, 1990; Vyklický et al., 1990; Ihle and Patneau, 2000; Lei et al., 2001; Mott et al., 2003). Among NMDA receptors, proton IC50 for inhibition varies with the GluN2 subunit, with IC50 values near physiological pH for GluN2A, GluN2B, and GluN2D (7.0–7.4), leading to the idea that these receptors are under tonic inhibition (Traynelis et al., 1995; Gielen et al., 2009). Proton inhibition depends on alternative RNA splicing of the GluN1 subunit within the ATD (Traynelis et al., 1995), a region that controls proton sensitivity on its own (Gielen et al., 2009) and through association with Zn2+ (see section VI.B) or ifenprodil (Mott et al., 1998) (see section V.E). Proton inhibition is independent of voltage and ligand binding (Banke et al., 2005). At the single channel level, protons reduce open probability of GluN2B subunit-containing NMDA receptors, with modest effects on open duration and single channel conductance (Traynelis and Cull-Candy, 1991; Banke et al., 2005). Protons inhibit GluN1/GluN2A receptors somewhat differently than GluN1/GluN2B, reducing mean channel open time and open probability (Dravid et al., 2007; Erreger and Traynelis, 2008). Mutagenesis data show a cluster of residues that mediate pH sensitivity located near the gate and the LBD dimer interface (Low et al., 2003; Gielen et al., 2008; Sobolevsky et al., 2009), and it seems likely that the NMDA receptor gating elements are tightly coupled to the proton sensor. Evidence supporting tight coupling between protons and gating includes the ability of channel blockers to sense the protonation state of the receptor while entering the pore (see section V.F), and the observation that the proton sensor is a common downstream substrate for modulators binding to the ATD.
Extracellular protons inhibit AMPA receptors in a voltage-independent manner by enhancing receptor desensitization and lowering channel open probability (Christensen and Hida, 1990; Ihle and Patneau, 2000; Lei et al., 2001). Proton inhibition varies with receptor subunit and flip/flop isoform, because GluA4 flop receptors are most sensitive to proton inhibition (Ihle and Patneau, 2000). Extracellular protons also inhibit recombinant and native kainate receptors in a voltage-independent manner without altering the desensitization time course (Mott et al., 2003). The IC50 values for GluK1 and GluK2 correspond to pH 6.9, indicating that receptors may be partially inhibited by protons at physiological pH. Like AMPA receptors, inhibition of kainate receptors by protons is subunit-dependent, because heteromeric receptors containing GluK5 are less sensitive to inhibition by protons, and GluK2/GluK4 heteromeric receptors are potentiated by protons (Mott et al., 2003). Mutant Lurcher GluD2 receptors are incompletely inhibited by protons in a voltage-independent manner but with an IC50 corresponding to pH ∼7.5 (Williams et al., 2003). Thus, pH sensitivity is a shared feature across the entire glutamate receptor family and could be important during processes that alter extracellular pH such as normal synaptic activity, glutamate release, glutamate uptake (Siesjö, 1985; Chesler and Kaila, 1992), and neuropathological conditions including stroke and seizure (Balestrino and Somjen, 1988; Giffard et al., 1990; Nedergaard et al., 1991; Kaku et al., 1993; Hirano et al., 2003).
E. Polyamines
Voltage-dependent block of glutamate receptors by polyamines and their analogs is described in section VIII.C. In addition, extracellular polyamines such as spermine and spermidine enhance NMDA receptor responses in a voltage-independent manner by both glycine-dependent and -independent mechanisms. Glycine-dependent potentiation occurs in GluN2A- and GluN2B-containing NMDA receptors through enhancement of glycine binding (Ransom and Deschenes, 1990; Ransom, 1991; Williams et al., 1994; Zheng et al., 1994; Dingledine et al., 1999) as a result of an allosteric interaction between polyamine binding to the ATD and glycine binding to the LBD (Masuko et al., 1999a; Han et al., 2008). Glycine-independent polyamine potentiation of NMDA receptor function occurs in saturating glycine concentrations only for GluN2B-containing NMDA receptors (for review, see Johnson 1996; Williams, 1997; Dingledine et al., 1999). Polyamine binding shifts the pKa of the proton sensor to reduce tonic inhibition at physiological pH in NMDA receptors that lack the highly charged GluN1 exon5 (Durand et al., 1993; Traynelis et al., 1995; Williams et al., 1995; Kashiwagi et al., 1996, 1997; Kumamoto, 1996; Masuko et al., 1999a).
Similar to its action on NMDA receptors, the endogenous polyamine spermine potentiates edited GluK2(R) receptor current response, apparently by relieving proton inhibition (Mott et al., 2003). Spermine potentiation is voltage-independent and affects neither the time course of desensitization nor agonist EC50 (Mott et al., 2003). However, unedited GluK2(Q) is inhibited by spermine and spermidine in a manner similar to AMPA receptors (see section VIII.C). Polyamines accelerate the deactivation of GluK2(Q), possibly through increased closing rate and stabilization of closed states (Bowie and Mayer, 1995; Bowie et al., 1998). Studies on the effects of polyamines on the Lurcher GluD2 receptors indicate that they undergo voltage-dependent channel block by endogenous and synthetic polyamines (Wollmuth et al., 2000; Williams et al., 2003).
F. Neurosteroids
NMDA receptors can be positively or negatively modulated by endogenous sulfated neurosteroids, the sulfate or negatively charged group at the C3 carbon being essential for activity (Wu et al., 1991; Park-Chung et al., 1997; Weaver et al., 2000). Unsaturated sulfated neurosteroids act as potentiators of NMDA receptors, whereas planar, saturated neurosteroids act as inhibitors (Weaver et al., 2000). Neurosteroid potentiation is subunit-dependent, because pregnenolone sulfate significantly potentiates GluN2A- and GluN2B-containing NMDA receptors but has much lower efficacy at GluN2C- and GluN2D-containing NMDA receptors (Malayev et al., 2002; Jang et al., 2004; Horak et al., 2006). This subunit-selectivity was used to identify the molecular determinants of potentiation for pregnenolone sulfate, which reside on the D2 domain of the GluN2 LBD (Jang et al., 2004; Horak et al., 2006; Stoll et al., 2007). Pregnenolone sulfate causes potentiation by increasing channel open probability, but no consensus has developed regarding the underlying mechanism (Bowlby, 1993; Ceccon et al., 2001; Malayev et al., 2002). Other saturated sulfated steroids, including pregnanolone sulfate, are use-dependent yet voltage-independent NMDA receptor inhibitors (Park-Chung et al., 1997; Petrovic et al., 2005). Pregnanolone sulfate reduces open probability, reduces channel open time, and increases receptor desensitization (Park-Chung et al., 1997; Petrovic et al., 2005; Kussius et al., 2009). Inhibition by pregnanolone sulfate is weakly subunit-dependent, being 2-fold more potent at GluN2C- and GluN2D-containing NMDA receptors than GluN2A- and GluN2B-containing receptors (Petrovic et al., 2005).
Although less work has been conducted on neurosteroid activity at AMPA and kainate receptors compared with NMDA receptors, studies have shown that sulfated steroids, such as pregnenolone sulfate, pregnanolone sulfate, and pregnenolone hemisuccinate inhibit GluA1 and GluA3 AMPA receptors and GluK2 kainate receptors (Yaghoubi et al., 1998; Shirakawa et al., 2005; Sedlácek et al., 2008). Sulfated steroid inhibition is voltage-independent and noncompetitive, reducing agonist efficacy but not potency. Steroids may bind AMPA receptors at the LBD (Spivak et al., 2004).
G. Fatty Acids
NMDA receptors are positively modulated through a direct interaction with polyunsaturated fatty acids, including arachidonic acid, oleic acid, and docosahexaenoic acid (Miller et al., 1992; Nishikawa et al., 1994). Potentiation by arachidonic acid occurs through an increase in open probability with no change in channel conductance (Nishikawa et al., 1994; Tabuchi et al., 1997; Casado and Ascher, 1998). The fatty acid binding site is not known. It is noteworthy that lysophospholipids reversibly inhibited the NMDA receptor in a voltage-independent manner (Casado and Ascher, 1998).
Although saturated fatty acids, such as myristic, palmitic, and stearic acids have no effect on kainate receptors, unsaturated fatty acids inhibit AMPA and kainate receptors in a voltage-independent and use-independent manner (Kovalchuk et al., 1994; Wilding et al., 1998). Inhibition of kainate receptors by fatty acids depends on the residue at the QRN site within the reentrant M2 loop (Wilding et al., 2008). GluK2(R) homomers and GluK1(R)/GluK2(R) heteromers are inhibited by docosahexaenoic acid, arachidonic acid, and linolenic acid (Wilding et al., 1998, 2005). However, GluK1(Q), GluK2(Q), and GluK1(Q)/GluK2(Q) heteromers containing at least one unedited subunit with Q at the QRN site are less sensitive to unsaturated fatty acids (Wilding et al., 2005).
H. Other Allosteric Modulators
In addition to the modulators discussed above, NMDA receptor function also is sensitive to osmotic pressure, with open probability reduced by compression and increased by stretch (Paoletti and Ascher, 1994; Casado and Ascher, 1998). Pb2+ is a voltage-independent antagonist of NMDA receptors that has been proposed to share a partially overlapping binding site with Zn2+ (Guilarte and Miceli, 1992; Büsselberg et al., 1994; Guilarte and McGlothan, 2003; Marchetti and Gavazzo, 2005; Gavazzo et al., 2008). The neuropeptide N-acetylaspartylglutamate is an inhibitor of NMDA receptor function, although some studies suggest that it may also act as a potentiator (Westbrook et al., 1986; Coyle, 1997; Greene, 2001; Bergeron et al., 2005, 2007; Fricker et al., 2009). Another peptide, the pituitary adenylate cyclase-activating polypeptide, increases NMDA receptor opening frequency (Wu and Dun, 1997; Liu and Madsen, 1998; Yaka et al., 2003; Yang et al., 2009). In addition, ATP may have actions on NMDA receptors (Kloda et al., 2004). It is noteworthy that aminoglycoside antibiotics (Masuko et al., 1999b) and histamine (Bekkers, 1993; Vorobjev et al., 1993; Williams, 1994; Burban et al., 2010) have been suggested to selectively potentiate the function of GluN2B-containing NMDA receptors. By contrast, some H3-histamine receptor competitive antagonists also bind to the GluN2B ATD to inhibit receptor function, perhaps at a site overlapping the histamine binding site (Hansen et al., 2010).
VII. Molecular Determinants of Gating
A. Time Course of Glutamate Receptor Activation and Deactivation
One of the most prominent features of glutamate receptors is their diversity in gating kinetics, which defines the time course of synaptic currents (Lester et al., 1990) and their role in synaptic physiology and plasticity. The response time course varies among receptor subtypes, specific subunits within each subtype, alternative RNA splicing, posttranslational modifications, and accessory subunits. Recombinant AMPA receptor subtypes show fast activation and deactivation rates in addition to both rapid and strong desensitization (Table 16; Fig. 11), which restrict signaling to the millisecond time scale (Mosbacher et al., 1994; Edmonds et al., 1995; Erreger et al., 2004). In contrast, NMDA receptors show much slower gating kinetics, activating in milliseconds, deactivating between tens and thousands of milliseconds, with relatively weak or no desensitization (Monyer et al., 1992; Vicini et al., 1998; Wyllie et al., 1998) (Table 16; Fig. 11). One idea advanced to explain the slow deactivation time course of the NMDA receptor was derived from structural data showing that GluN1 Tyr535 occupies a position in the GluN1/GluN2A LBD heterodimer analogous to that of the AMPA receptor potentiator aniracetam in GluA2 LBD homodimer (see section VI.A). Because Tyr535 is complexed at the protomer interface in a manner analogous to that of aniracetam, it may similarly modulate deactivation of NMDA receptors, assuming the two receptors share similar mechanisms underlying deactivation (Furukawa et al., 2005). The time course of deactivation of virtually all GluN1/GluN2 receptors can be described by multiple exponential components, which for GluN2A may reflect complex channel behavior (e.g., Erreger et al., 2005b; Zhang et al., 2008b). Heterologously expressed recombinant kainate receptors, like AMPA receptors, can show relatively fast gating kinetics and strong desensitization (Table 16). At synapses, the time course of a single evoked excitatory postsynaptic current mediated by AMPA receptors is faster than that mediated by NMDA receptors (Fig. 12). Native kainate receptors mediate a slower synaptic current than AMPA receptors (Castillo et al., 1997; Kidd and Isaac, 1999, 2001) (Fig. 12A).
TABLE 16.
Kinetic parameters describing glutamate activation of AMPA, kainate and NMDA receptors
| EC501 | τ-Deactivation2 | τ-Desensitization | τ-Recovery3,4 | SS/Peak Ratio | |
|---|---|---|---|---|---|
| μM | ms | ms | ms | ||
| GluA1-flip | 500–7005,6 | 0.7–1.27–9 | 2.5–4.17–10 | 111–1476,7 | 0.002–0.0327,11,12 |
| GluA1-flop | 45013 | 0.86–1.37–9,14 | 3.2–4.27–10,14 | 147–1557,14 | 0.023–0.0807,14,15 |
| GluA2-flipQ16 | 139017 | 0.62–1.19,17 | 5.9–9.99,10,17 | 11.717 | 0.06817 |
| GluA2-flopQ16 | 1140–138013,17 | 0.54–0.99,17 | 1.2–1.99,10,17 | 31.317 | 0.01117 |
| GluA3-flip | 1000–197015,18,19 | 0.5615 | 3.0–5.18,10,15,20 | 15–7015,21 | 0.024–0.05415,21 |
| GluA3-flop | 1100–178015,18,19 | 0.63–1.0514,15 | 1.1–2.88–10,14,15,20 | 55–14214,15,22 | 0.0115 |
| GluA4-flip | 181012,18 | 0.68 | 3.6–5.18,10 | 6–216,21 | 0.006–0.0421 |
| GluA4-flop | 44.323–25 | 0.68 | 0.98,10 | 31–4321 | 0.00321 |
| GluA1-flip/GluA2-flip | 5.126 | 28–6726 | 0.00926 | ||
| GluA3-flip/GluA2-flip | 4.926 | 15–2626 | 0.015–0.02226 | ||
| GluK1a Q16 | 63027 | 4.1–8.9,6927,28 | 50,510029,30 | 0.0129 | |
| GluK2 Q16 | 427–104031–34 | 1.6–2.529,32,33 | 3.4–5.331–36 | 1900–302031–33,35,37 | 0.008–0.0431,35,36 |
| GluK3a38 | 590039 | 8.4–939 | 0.0439 | ||
| GluK1 Q/GluK540 | 1941 | 2.1–1542 | |||
| GluK2 Q/GluK5 | 15–3134,41 | 0.5, 4634 | 4.734 | 2400–270034 | |
| GluK3a/GluK4 | 7.639 | 0.0339 | |||
| GluK3a/GluK5 | 5.339 | 0.01839 | |||
| GluN1/GluN2A | 1.8–7.743,44 | 22–23045–47 | 386–750, 200045,47 | 61845 | 0.28–4248,49 |
| GluN1/GluN2B | 0.9–443,44,50 | 71–95, 538–61745,50 | 100, 49550 | 1014–210045,50 | 0.027–0.5318,50,51 |
| GluN1/GluN2C | 1.024,52,53 | 260–38245–47 | 59–71954 | 1.047 | |
| GluN1/GluN2D | 0.424,53,55 | 1700–440845,56 | N.A. | N.A. | 1.056 |
N.A., not applicable; i.e., GluN1/GluN2C and GluN1/GluN2D receptors show no or minimal desensitization in the continued presence of agonist.
Determined from the peak response to rapid glutamate application.
Measurements are from outside out-patches; two time constants can be detected for many receptors.
See Lomeli et al. (1994) for RNA editing control of τ recovery.
The rate of recovery from desensitization is more complex; see Bowie (2002), Robert and Howe (2003).
Edited receptors or mutant receptors had a glutamine at the Q/R/N site.
Values predicted from simulations using rate constants.
Determined in X. laevis oocytes.
Determined for activation by kainate.
Onset and recovery from desensitization is variable from cell to cell.
Splice variants 7a and 7b have similar rates.
Determined by rapid application of glutamate; kainate-evoked currents desensitized with a dual exponential time course, with the fastest time constant being 15 ms (Herb et al., 1992).
SS/Peak current ratio is typically higher in whole-cell recordings.
Fig. 11.
Desensitization of recombinant AMPA, kainate, and NMDA receptors expressed in the absence of accessory proteins, which can alter response time course (section II). AMPA and kainate receptors activated by l-glutamate undergo pronounced and rapid desensitization that occurs within milliseconds after activation and results in steady-state currents less than 5% of the peak response. A and B, voltage-clamp recordings are shown from outside-out patches excised from human embryonic kidney 293 cells expressing recombinant rat GluA1 AMPA receptors (A) or recombinant rat GluK2 kainate receptors (B). Receptors are activated by saturating glutamate (10 mM) for 75 ms. C, voltage-clamp recordings from excised outside-out patches for GluN1/GluN2A-C and a whole-cell voltage-clamp recording of GluN1/GluN2D are given in which the receptors are activated for 1 s by saturating l-glutamate and glycine. The degree and time course of desensitization is subunit-dependent. GluN2A-containing receptors desensitize rapidly, GluN2B-containg receptors show slower desensitization, and GluN2C- and GluN2D-containing receptors undergo little to no desensitization. All traces are shown with the peak amplitude normalized to 1. Bottom, steady-state single-channel recordings of GluA1, GluK2, GluN1/GluN2A and GluN1/GluN2D are shown beneath appropriate panels, and illustrate qualitative differences in unitary currents exhibited by AMPA, kainate, and NMDA receptors. Unpublished data for GluK2, GluN2A, GluN2C, and GluN2D, from S. M. Dravid, K. M. Vance, and S. F. Traynelis. Data for GluA1 single-channel recordings were from Banke et al. (2000), GluK2 single channel recordings were from Zhang et al. (2009c), and GluN2B macroscopic current recordings were from Banke et al. (2005).
Fig. 12.
Contribution of glutamate receptor subtypes to synaptic activity. A, a recording of spontaneous mEPSCs in the presence of 100 μM (R)-2-amino-5-phosphonopentanoate (d-APV), 100 μM bicuculline, and 1 μM tetrodotoxin from a CA3 pyramidal cell shows the contribution of AMPA and kainate receptors to synaptic activity. Fast mEPSCs mediated by synaptic AMPA receptors and slower mEPSCs mediated by synaptic kainate receptors were both present before application of 100 μM benzenamine (GYKI-52466), but only the slower kainate receptor-mediated mEPSC persisted in the presence of GYKI-52466. CNQX blocks both fast AMPA-mediated and slow kainate receptor-mediated mEPSCs. B, AMPA and NMDA receptor-mediated EPSCs at the pyramidal to multipolar interneuron synapse in the visual cortex. The contributions of AMPA and NMDA receptors to synaptic time course are contrasted using evoked responses from a synaptically connected pair of neurons (pyramidal-to-interneuron). In the presence of the AMPA receptor antagonist CNQX and the absence of Mg2+, the NMDA receptor activity is isolated, highlighting the slow rise time and deactivation time course. There is no significant kainate receptor component at this synapse. Data in A is from Mott et al., 2008; unpublished data in B is from L. P. Wollmuth.
B. Mechanisms Linking Agonist Binding to Channel Gating
Many studies using varied approaches have addressed how ligand binding can lead to opening of the glutamate receptor pore. Glutamate receptors have, as core structural/functional elements, an LBD and an ion channel (see section II). Our current understanding of how agonist binding leads to channel opening has so far largely been driven by structural (e.g., crystallography or NMR) and functional (e.g., UV and IR spectrometric measurements) studies of the water-soluble LBD (S1S2 construct) combined with functional studies of the intact receptor. Structures of the LBD are available for representatives of all major GluR subtypes, including the unbound or apo state, the agonist-bound state, and with various competitive antagonists (Madden, 2002; Gouaux, 2004; Mayer, 2006; Oswald et al., 2007). The principles of agonist binding and channel gating seem to be common across all glutamate receptor subtypes and involve at least three sequential steps: 1) initial agonist association or binding, 2) a conformational change—so-called clam shell closure—that prevents agonist dissociation (Armstrong et al., 1998; Abele et al., 2000; Armstrong and Gouaux, 2000; Cheng et al., 2005), and 3) a conformational change in the ion channel that is tightly coupled to that in the LBD (Jin et al., 2003; Zhang et al., 2008a). The slower activation kinetics of NMDA receptors allows additional kinetically distinct conformational changes to be observed that have yet to be clearly linked to structural elements (see section VII.D).
Crystallographic studies unequivocally indicate that the agonist binding site is in the cleft of a clamshell-like structure (Armstrong et al., 1998; Armstrong and Gouaux, 2000; Sobolevsky et al., 2009). The D1 portion of the LBD clamshell (Figs. 1 and 3) forms an interface between the LBD of adjacent dimerized subunits, and the second lobe (D2) moves as γ-carboxylate atomic interactions are satisfied to “close the clamshell” and prevent glutamate dissociation during subsequent gating steps (Fig. 13). Considerable evidence (with a few exceptions) supports the idea that movement of D2, the lobe proximal to the ion channel with connections to M1 and M3, enhances the probability of channel opening. This combination of agonist binding and clamshell closure provides the energy to drive channel opening in NMDA, AMPA, and kainate receptors.
Fig. 13.
A, the structure of GluA2 with two subunits (A and C) transparent. Red dashed line indicates interface between LBD and TMD. B, expanded view of the LBD-TMD regions of subunits B and D. The structure of the water-soluble GluA2 LBD (S1S2) crystallized in complex with glutamate has been superimposed, using the D1 domain, on the corresponding region of GluA2cryst and is shown in green. Helical regions of the ion channel as well as parts of LBD that are proposed to move upon activation are shown as cylinders. Purple and green spheres indicate positions of the α-carbons for the residues Lys393 and Pro632. Stick models of ZK200775 and glutamate are shown in purple and green, respectively. Red arrows indicate proposed movement during receptor activation. C, the transmembrane domain architecture is shown for subunit A parallel to the channel pore as a ribbon structure (left). The transmembrane domains for all four subunits are shown viewed from the intracellular side down the axis of the pore (center), and as a surface representation for subunits B, C, and D with subunit A membrane-associated helices shown as green cylinders (right). [Adapted from Sobolevsky AI, Rosconi MP, and Gouaux E (2009) X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462:745–756. Copyright © 2009 Nature Publishing Group. Used with permission.]
Evidence supporting the idea that binding proceeds via a two-step process comes in part from time-resolved monitoring of agonist binding to LBD in solution using UV and IR spectrometric measurements with ultrafast application of agonists (Abele et al., 2000; Cheng et al., 2005). These results show that the ligand initially makes contact with residues on D1, inducing a relatively slower second step during which D2 undergoes a transition to form further ligand-protein and D1-D2 interactions. This leads to closure of the clamshell and locking of the ligand into the agonist-bound conformation (Armstrong and Gouaux, 2000). Similar dock-and-lock mechanisms are well described for the LBD of the homologous G-protein coupled mGluRs (Lampinen et al., 1998; Salopek-Sondi et al., 2003).
Dimerization of the LBD of adjacent subunits is a key structural/functional feature that underlies coupling of cleft closure to conformational changes in the ion channel (Sun et al., 2002). Within the receptor, the four LBDs are arranged as 2-fold symmetric pairs, each pair composed of a back-to-back dimer interface that includes both hydrophobic and nonhydrophobic surface regions on the D1 domain (Armstrong and Gouaux, 2000; Sun et al., 2002; Sobolevsky et al., 2009) (see Fig. 3). The intersubunit D1-D1 contacts formed across the dimer interface constrain D1 movement (Horning and Mayer, 2004; Furukawa et al., 2005; Mayer, 2005; Naur et al., 2007; Sobolevsky et al., 2009). In contrast, the D2 domains appear relatively free to move, which is of particular interest because this part of the LBD contains the anchor points that, in full-length receptors, extend to the ion channel-containing transmembrane segments (Fig. 1). Superposition of apo and agonist-bound structures reveals a striking difference in the relative position of residues that in the intact receptor would be connected to the transmembrane segments. These structures suggest that the distance between the linkers on the bottom of D2 in the LBD dimer is increased after agonist binding, consistent with a structural model for AMPA receptor activation in which cleft closure involves movement of D2, whereas D1 and the dimer interface remain relatively fixed. The D2 transition leads to displacement of the linker regions, which would reposition transmembrane segments (such as M3) in the intact subunit and drive channel opening (Erreger et al., 2004; Mayer, 2006; Hansen et al., 2007) (discussed further in section VII.D).
Crystal structures, although valuable, are inherently static in nature and do not provide details of permitted motions that underlie the protein transitions under physiological conditions—a problem highlighted by findings that some functionally distinct partial agonists induce a similar degree of cleft closure in GluA2 (e.g., Holm et al., 2005) and by functional and crystallographic studies of GluN1 and GluN3 (Inanobe et al., 2005; Yao et al., 2008) (see section V.D). Computer-aided molecular dynamics (MD) simulations that are based on the growing number of LBD structures can be a useful tool to estimate protein fluctuations at physiological temperatures, provided the appropriate caveats are recognized (see section V.A). Such studies have provided hints about permissible intraprotein motions within the LBD during the cleft closure transition as well as interactions at the domain interface (Arinaminpathy et al., 2002; Kaye et al., 2006; Lau and Roux, 2007; Dravid et al., 2010). MD simulations can provide estimates of the stability of the ligand-protein interactions in the binding pocket, adding further detail to our understanding of ligand selectivity and efficacy (Mendieta et al., 2001, 2005; Erreger et al., 2005b; Kaye et al., 2006; Pentikäinen et al., 2006; Erreger et al., 2007). Additional experimental techniques that can test predictions obtained from MD simulations or crystal structures include NMR, ultraviolet or infrared spectroscopy, and fluorescence resonance energy transfer (McFeeters and Oswald, 2002, 2004; Cheng et al., 2005; Du et al., 2005; Madden et al., 2005; Valentine and Palmer, 2005).
C. Molecular Determinants and Mechanisms of Partial Agonism
Structural data have stimulated new work addressing the mechanism by which different ligands can occupy the same binding site yet have different relative efficacies. Partial agonists exist for all glutamate receptor subtypes (see sections V.A and V.B) and show different effects on the LBDs. A series of AMPA receptor partial agonists, the 5-substituted willardiines (Table 5), vary by a single atom and induce a differential degree of cleft closure that correlates with their relative efficacy (Partin et al., 1994; Jin et al., 2003). Single-channel studies show that willardiines with different relative efficacies activate different proportions of the same set of conductance levels. Building on previous studies (Rosenmund et al., 1998; Smith and Howe, 2000), AMPA receptors have been proposed to ratchet open their channel as a function of the agonist-induced conformational changes within each subunit, with the probability that each subunit can partially open the gate increasing with the degree of agonist-induced cleft closure (Jin and Gouaux, 2003; Jin et al., 2003). This finding provides a structural mechanism underlying partial agonism at AMPA receptors in which the relative effectiveness or efficiency with which each agonist can activate a single subunit accounts for observed changes in single channel conductances (Jin et al., 2003). This mechanism has recently been extended to show that AMPA receptor subunit gating displays cooperativity, a property most notable at low agonist concentrations (Prieto and Wollmuth, 2010). Comparison of the structures of the GluK2 kainate receptor subunit in complex with full agonists (glutamate, SYM2081, quisqualate) and a partial agonist (kainate) reveals that the latter induces a lesser degree of cleft closure, consistent with the idea that the degree of cleft closure can influence agonist efficacy for GluK2 (Mayer, 2005).
The first structures available for the LBD of GluN1 complexed with ligands exhibited cleft closure that paralleled agonist- or antagonist-bound GluA2 structures (Furukawa and Gouaux, 2003; Furukawa et al., 2005; Inanobe et al., 2005). However, in contrast to GluA2, no substantial difference in the degree of cleft closure exists between the full agonist glycine, the partial agonist d-cycloserine, and a series of structurally related partial agonists. Thus, the isolated LBD of the GluN1 subunit shows no relationship between the degree of agonist-induced cleft closure and agonist efficacy. This finding suggests that important differences exist between AMPA and NMDA receptor subunits with respect to how intraprotein conformational changes are transmitted from the LBD to the channel gate.
D. Molecular Determinants and Mechanisms of Gating
The three transmembrane helical segments (M1, M3, M4) are directly coupled to the LBD in all glutamate receptor subunits (Fig. 1). Not surprisingly, point mutations in all of these transmembrane helices, in the linkers that couple them to the LBD, and in the pore lining reentrant M2 loop can affect gating (Schneggenburger and Ascher, 1997; Zuo et al., 1997; Krupp et al., 1998; Villarroel et al., 1998; Ren et al., 2003). Hence, multiple structural elements contribute to the free energy of any state-dependent conformation. Understanding how multiple structural determinants of closed and open conformations relate to atomic structure presents a daunting challenge.
The M3 segment is a key determinant of gating in glutamate receptors. The initial evidence for this came from the Lurcher M3 mutant GluD2(A654T), which produced constitutively active channels (Zuo et al., 1997). This led to a number of mutagenesis studies that described striking effects of M3 mutations on glutamate receptor function (Kohda et al., 2000; Jones et al., 2002; Yuan et al., 2005). This region in M3 contains the most highly conserved motif (SYTANLAAF) among the mammalian glutamate receptor subunits (Kuner et al., 2003). Several activity-dependent changes in the accessibility and reactivity of substituted cysteines have been identified in this region (Sobolevsky et al., 2002a; Chang and Kuo, 2008). The M3 segment in glutamate receptors is homologous to the major gating domain (the inner helix) in K+ channels (Doyle et al., 1998; Jiang et al., 2002; Yellen, 2002; Swartz, 2004).
An essential determinant of function in all channels is the activation gate—the structure that occludes the flux of ions in the closed state. Numerous mechanisms can be envisioned to account for an activation gate, including local potential changes, electrostatic repulsion, and steric hindrance (Hille, 2001). In certain K+ channel subtypes, the activation gate arises from tight steric closure (del Camino and Yellen, 2001) at the bundle helical crossing of the inner helix located at the end of the internal cavity (Swartz, 2004). In other K+ channels and the related cyclic nucleotide-gated channels, the activation gate is formed at the P-loop (Flynn and Zagotta, 2001; Bruening-Wright et al., 2002). The AMPA receptor structure, obtained in the antagonist-bound (closed) state, showed that positions (underlined residues) in the highly conserved gating motif as well as those located C-terminal to it in tetramer subunits A/C (SYTANLAAFLTVERM) and tetramer subunits B/D (SYTANLAAFLTVERM) are located near each other (Fig. 14). Given this finding, the high overall structural similarity to inverted K+ channels, and existing functional data, Sobolevsky et al., (2009) persuasively argue that this region represents the activation gate in the glutamate receptor family.
Fig. 14.
A, surface representation of a closed ion conduction pathway and the pore diameter as a function of distance along the central axis of the channel (red < 1.4 Å < green < 2.8 Å < purple). The residues most proximal to each other that form the activation gate in the closed state are located at the top of the ion channel pore. B, AMPA receptor subunit, GluA2 (left). Subunits A/C and B/D are indicated (see section II). Sequence of the M3 segment (α-helical portion highlighted in gray), the M3–S2 linker, and the S2 lobe (highlighted in magenta). Positions highlighted in yellow are conserved across all mammalian glutamate receptor subunits, including the most highly conserved sequence SYTANLAAF. Also indicated is the border for the M3 segment defined from hydropathy plots (M3 hydrophobic border). Black triangles indicate positions that are located in proximity to each other in the structure of the closed state (red representation in A) and presumably reflect the activation gate. Positions below the dashed line (zδ = 0) show voltage-dependent reactivity to cysteine-reactive reagents, whereas those above do not (Sobolevsky et al., 2003). The Lurcher (Lc) position is highlighted (Zuo et al., 1997; Kohda et al., 2000). Mutations of positions highlighted red have been identified to increase leak current or potentiate glutamate-activated current when modified by cysteine-reactive reagents (Sobolevsky et al., 2003), suggesting that they alter gating. The approximate location of the QRN site is indicated, because this region is disordered in the crystal structure (see sections II.F and VIII.A) (Sobolevsky et al., 2009). NMDA receptors subunits GluN1 and GluN2A (right). Arrangement is the same as in A except that triangles refer to positions that when mutated to cysteine formed cross-linked dimers (black triangle) or did not form dimers (white triangle). Based on these results, the GluN1 subunits are presumed to adopt the A/C conformation and the GluN2 subunits to adopt the B/D conformation (Sobolevsky et al., 2009). Mutations of positions highlighted red either alter leak currents or potentiate glutamate-activated currents when modified by cysteine-reactive reagents (Beck et al., 1999; Jones et al., 2002; Sobolevsky et al., 2002a,b, 2007; Yuan et al., 2005), show increases in leak current with single amino acid substitutions (Yuan et al., 2005; Chang and Kuo, 2008), alter channel block (Kashiwagi et al., 2002), and/or alter proton sensitivity (Low et al., 2003). The DRPEER motif in the GluN1 subunit that affects Ca2+ permeability (Watanabe et al., 2002) is highlighted blue, as are corresponding negative charges in the GluN2A subunit that do not affect Ca2+ permeability. Data in A are from Sobolevsky et al. (2009).
The idea that the activation gate was located at the external entrance to the pore was originally based on experiments, as in K+ channels, describing the state-dependent block by organic blockers and Mg2+ (Qian and Johnson, 2002). Most experiments have been carried out in NMDA receptors (but see Tikhonova et al., 2008) because of the diversity of channel blockers both in terms of size and kinetic properties. Uncompetitive NMDA receptor antagonists such as memantine and amantadine act as “trapping” blockers (see section V.F) (Blanpied et al., 1997). That is, the channel gate can close and agonist can unbind with the blocking molecule bound within the pore. The most likely explanation for this result is that the blocker is trapped in a cavity located internal to the activation gate but external to the apex of the reentrant M2 loop, an idea supported by the full-length GluA2 structure (Sobolevsky et al., 2009). Additional support for this model is provided by sequential blockers such as 9-aminoacridine (Benveniste and Mayer, 1995), larger amantadine derivatives (Antonov and Johnson, 1996; Antonov et al., 1998), and tetrapentylammonium (Sobolevsky et al., 1999). These molecules apparently bind in the same cavity as trapping blockers, but their large size interferes with the gating machinery located more externally, thus preventing channel closure.
Experiments evaluating accessibility of substituted cysteines to covalent modifying reagents found that the external entrance of the pore undergoes considerable rearrangements, becoming more restricted with channel closure (Beck et al., 1999; Sobolevsky et al., 2002a, 2003). Experiments by Chang and Kuo (2008) taking advantage of substituted cysteines, charge substitutions, and mutant cycle analysis with the GluN1 and GluN2B receptor subunits demonstrated that the activation gate was located within the SYTANLAAF motif, the underlined alanine representing the point of the bundle helical crossing.
At present, the mechanism by which the conformation change induced by ligand binding leads to opening of the activation gate is unknown, in part because of the absence of a structure of the intact receptor in the presence of glutamate. Nevertheless, given the structural similarity of GluA2 to known structures of open and closed K+-selective and nonselective cation channels (Doyle et al., 1998; Jiang et al., 2002; Alam and Jiang, 2009) and the available structures of the water-soluble LBDs in the antagonist- (closed) and agonist-bound states (see above), Sobolevsky et al. (2009) propose that the gate opens via a rotation of the M3 helices away from the central axis of the pore, comparable with that proposed for K+ channels (Doyle et al., 1998; Jiang et al., 2002). However, given that glutamate receptors lack a glycine hinge within their M3 transmembrane helix, a structural element proposed to be essential for K+ channel opening (Doyle et al., 1998; Jiang et al., 2002), there will be differences in detail of how the conformational changes in M3 occur.
The contribution of M1 and M4 transmembrane helices to channel function are unknown, other than data suggesting that mutations in M1 (Krupp et al., 1998; Villarroel et al., 1998) and M4 (Ren et al., 2003) can alter gating. M1 and M4 could act as anchors for the LBD, and the external location of M4 relative to the M3 gating element could serve to reduce M3 interactions with the bilayer (Fig. 13C). It is noteworthy that channels such as KcsA and inward rectifiers with only two transmembrane elements per subunit (eight per channel) can function independently of any structural elements with similarity to M4, as can the two transmembrane prokaryotic glutamate receptor subunit GluR0 (Chen et al., 1999a). Yet the M4 segment appears to be required at least for NMDA receptor function, because truncated NMDA receptor subunits lacking M4 do not show detectable glutamate-activated currents (Schorge and Colquhoun, 2003). However, function can be restored if these truncated subunits were coexpressed with an independently encoded M4 segment. It is noteworthy that the M4 segment, which is found in all eukaryotic glutamate receptors subunits, is associated with the ion channel core (M1–M3) of an adjacent subunit (Fig. 13C).
Certain noncompetitive AMPA receptor antagonists (GYKI-53655, CP-465,022) interact with the external ends of the M1 and M4 transmembrane helices (Balannik et al., 2005). The linker region preceding the M1 transmembrane helix (the pre-M1 region; Fig. 13) makes a short helix that is oriented parallel to the plane of the membrane, making contacts with carboxyl and amino terminal ends of transmembrane helices M3 and M4, respectively. The M3 helices cross relative to each other at this level along the permeation path for the antagonist-bound closed GluA2 structure, raising the intriguing possibility that this pre-M1 helix may restrain M3 mobility in the closed state, yet with domain closure, promote channel opening in the ligand-bound state (Sobolevsky et al., 2009). If this structural feature were shared by NMDA receptors, and four pre-M1 helices were to move independently in the agonist-bound receptor, one might imagine these conformational changes could constitute rate limiting and kinetically distinct steps that precede concerted channel opening involving simultaneous rearrangement of all four M3 helices. Single channel studies of agonist-bound NMDA receptors have identified multiple kinetically distinguishable steps that precede channel opening via a concerted pore dilation involving all or no repositioning of M3 transmembrane helices (Banke and Traynelis, 2003; Popescu and Auerbach, 2003; Auerbach and Zhou, 2005; Erreger et al., 2005a; Schorge et al., 2005; Dravid et al., 2008). These rate-limiting and kinetically distinct steps must reflect rate limiting conformational changes that occur before rapid pore dilation (Schorge et al., 2005). It is noteworthy that the role for the pre-M1 linker proposed by Sobolevsky et al. (2009) provides an example of a potential structural rearrangement that could occur in a subunit-dependent fashion (Banke and Traynelis, 2003) and necessarily precede simultaneous rearrangement of all four M3 helices as the pore opens. Moreover, the mechanisms underlying multiple conductance levels for AMPA receptors and gating to the subconductance and main conductance states for NMDA receptors could lie in how this pre-M1 region interacts with the each M3 helix that contributes to the gate. Perhaps M3 rearrangement within individual subunits is permitted for AMPA receptors, giving rise to as many as four detectable conductance levels (Rosenmund et al., 1998; Banke et al., 2000; Smith and Howe, 2000; Jin et al., 2003; Prieto and Wollmuth, 2010), in contrast to GluN2A/B-containing NMDA receptors, for which all four M3 helices might move at once, giving rise primarily to a single conductance level (Table 17).
TABLE 17.
Single channel properties of AMPA, kainate, and NMDA receptors
| POPEN | Open Time | Conductance | |
|---|---|---|---|
| ms | pS | ||
| GluA1-flip | 0.4–1.01–3 | 0.2–0.91 | 8, 15, 23, 311,4–6 |
| GluA2-flip Q7 | 0.618 | 0.32, 1.479 | 7, 15, 24, 369,10 |
| GluA3-flip | 0.8211,12 | ||
| GluA4-flip | 0.772,12 | 0.14, 3.313 | 9, 20, 31, 4513,14 |
| GluK1 Q7 | 0.3, 0.615 | 5, 9, 1415 | |
| GluK2 Q7 | 0.5–1.03,16 | 0.6, 2.315 | 8, 15, 2515 |
| GluK1 Q/GluK5 | 0.315 | 5, 9, 1715 | |
| GluK2 Q/GluK5 | 0.4, 2.115 | 7, 13, 2015 | |
| GluN1/GluN2A | 0.36–0.5017,18,19 | 0.06, 1.3, 3.620–22 | 51, 3822 |
| GluN1/GluN2B | 0.07–0.1717,19,23 | 0.6, 2–3.223 | 51, 3922 |
| GluN1/GluN2C | 0.024 | 0.622 | 36, 1922 |
| GluN1/GluN2D | 0.01–0.0420,25 | 0.1, 0.9, 2.620 | 35, 1726 |
| GluN1/GluN2A/GluN2D | 0.08–0.2427 | 0.07, 1.25, 3.027 | 30, 40, 5027 |
| GluN1/GluN2B/GluN2D | 0.02–0.0328,29 | 2.4–2.828,29 | 18, 30, 41, 5428,29 |
| GluN1/GluN3B | 12, 3730 | ||
| GluN1/GluN2A/GluN3B | 4.431 | 26, 4831 | |
| GluN1/GluN2A/GluN3A | 0.1, 4.732 | 35, 7533/29, 4732 |
POPEN depends on PKA phosphorylation of Ser845.
Measurements in cell-attached patches were similar to those in outside-out patches.
Edited receptors or mutant receptors have a Gln at the QRN site; editing to Arg decreases conductance (Howe, 1996; Swanson et al., 1996).
Predicted from simulations using rate constants; GluA3-flop was predicted to have a POPEN value of 0.42.
E. Molecular Determinants of Desensitization
All glutamate receptors undergo desensitization, which by definition is a reduction in response in the presence of a sustained stimulus. This process is fast in AMPA and kainate receptors, occurring within 20 ms and generating greater than 90% decrease in current amplitudes at steady state (Table 16). However, the onset of desensitization is slower and less extensive in NMDA receptors and is virtually absent for GluN2C- and GluN2D-containing NMDA receptors (Monyer et al., 1994; Krupp et al., 1996; Wyllie et al., 1998; Dravid et al., 2008). Our understanding of the structural mechanisms underlying rapid desensitization, particularly for AMPA and kainate receptors, has been dramatically accelerated through a broad range of structural studies on the isolated AMPA and kainate receptor LBDs in combination with extensive functional studies on the full-length receptor (Sun et al., 2002; Robert and Howe, 2003; Horning and Mayer, 2004; Klein and Howe, 2004; Robert et al., 2005; Armstrong et al., 2006; Weston et al., 2006a; Zhang et al., 2006a; Nayeem et al., 2009).
For GluA2, the initial step of desensitization is a rearrangement of the D1-D1 dimer interface between LBDs of adjacent subunits (Sun et al., 2002; Horning and Mayer, 2004). This idea was driven by LBD structures of wild type GluA2 and GluA2 containing a leucine-to-tyrosine substitution GluA2(L483Y), a mutation that in the intact receptor blocks desensitization (Stern-Bach et al., 1998), and structures of GluA2 bound to cyclothiazide, an inhibitor of AMPA receptor desensitization (Partin et al., 1995; Sun et al., 2002). Ultracentrifugation studies, which showed that both the mutation and cyclothiazide increased dimer stability (Sun et al., 2002), suggested a role for the dimer interface in desensitization (see section VI.A). The mutation GluA2(L483Y) or cyclothiazide stabilized the dimer formation, further suggesting that stabilization of the LBD dimer interface in full-length functional AMPA receptors can reduce desensitization (Sun et al., 2002). On the atomic level, Leu483 is located in the dimer interface, and mutation to tyrosine promotes formation of additional interactions with residues located on the opposite LBD (Fig. 10A).
The rate at which cysteine residues introduced at the GluA2 dimer interface are modified by (2-sulfonatoethyl)methane thiosulfonate (MTSES) depends on whether the receptor is resting, active or desensitized (Armstrong et al., 2006). The mutation GluA2(E486C) displays pronounced state-dependent modification by (2-sulfonatoethyl)methane thiosulfonate, being more accessible in the desensitized state than in the resting or active states. Crystallographic data show that GluA2 Glu486 participates in interactions at the dimer interface and is largely inaccessible to solvent when the receptor is in the active state. However, in the desensitized state, Glu486 becomes solvent accessible, providing direct evidence that desensitization in full-length AMPA receptors is accompanied by a rearrangement at the dimer interface (Armstrong et al., 2006). The same study also used variable length cross-linkers as molecular rulers to restrict motion at the dimer interface and thus report on the magnitude of the rearrangement. The structure of an additional cross-linked mutant, GluA2(S729C), possessed a conformation with the dimer interface separated by distances that agreed well with those estimated from cross-linking experiments at different residues. These data suggest that the structure of cross-linked GluA2(S729C) reveals a conformation similar to full-length desensitized AMPA receptors (Armstrong et al., 2006). Furthermore, imaging studies suggest that the separation of the dimer interface in the desensitized state is not accompanied by significant changes in the degree of domain closure in the isolated LBD (Du et al., 2005). Thus, during receptor desensitization, the separation of the dimer interface enables the linkers that replace the transmembrane segments to move closer together by 10 Å relative to the agonist-bound nondesensitized conformation, thereby preventing opening of the ion channel (Fig. 3).
Mutations in the AMPA receptor binding pocket that reduce steric collision between the agonist and the LBD can increase agonist efficacy and desensitization (Madden et al., 2004; Holm et al., 2005). Furthermore, there is a direct correlation between the degree of domain closure with full and partial AMPA receptor agonists and the degree of desensitization produced by those agonists (Jin and Gouaux, 2003; Jin et al., 2003; Frandsen et al., 2005). Thus, the degree of agonist-induced LBD closure seems to be the primary determinant of tension at the LBD dimer interface and thus governs transition into both the open and desensitized state.
Using the LBD crystal structure of the nondesensitizing GluA2(L483Y) as a guide, Weston et al. (2006b) introduced cysteine residues in kainate receptor subunits at positions where cross-linking should stabilize the dimer interface. This yielded nondesensitizing GluK1, GluK2, and GluK3 responses. The AMPA receptor subunit GluA2 was rendered nondesensitizing when cross-linked at homologous positions, thereby establishing that the dimer interface rearrangement is necessary for kainate receptor desensitization (Weston et al., 2006b). However, not all interactions predicted on the basis of AMPA receptor studies are necessary for kainate receptor desensitization (Fleck et al., 2003; Nanao et al., 2005).
Desensitization of NMDA receptors has been studied extensively (reviewed by Dingledine et al., 1999) and involves a number of pathways, including glycine-dependent desensitization (Mayer et al., 1989; Benveniste et al., 1990; Lester et al., 1993; Nahum-Levy et al., 2001; Regalado et al., 2001), Ca2+-dependent inactivation (Clark et al., 1990; Legendre et al., 1993, Vyklický, 1993; Rosenmund and Westbrook, 1993; Medina et al., 1995; Vissel et al., 2002), Zn2+-dependent desensitization (see section VI), and glycine/Ca2+-independent desensitization (Chen et al., 2004; Hu and Zheng, 2005; Sessoms-Sikes et al., 2005). The molecular mechanisms for these forms of desensitization are poorly understood.
VIII. Molecular Determinants of Ion Permeation and Block
A. Nature of the Ion Permeation Pathway
The ion channel associated with all glutamate receptors consists of a water-filled pore divided into external and internal cavities separated by a narrow constriction at which the pore reaches its smallest dimension (see section II). The residues lining the pore, including those positioned at the external and internal entrances, form the permeation pathway and are the primary determinants of ion selectivity and unitary conductance. With only rare exceptions, the conduction pathway is cation-selective. Glutamate receptors show a wide range of single channel conductances that vary from <1 to ∼30 pS for AMPA and kainate receptors and from 20 to 60 pS for NMDA receptors (Table 17). AMPA receptors show up to four different conductance levels, the presence of which has been explained by their mechanism of activation (see section VII). NMDA receptors show a pair of conductance states, the amplitude and relative frequency of which depends on the GluN2 subunit present and ionic conditions.
The pore of the glutamate receptor family is formed by three transmembrane helices and a reentrant loop, arranged with striking similarity to an inverted potassium channel (Doyle et al., 1998; Sobolevsky et al., 2009); this similarity had been predicted to exist on the basis of sequence similarity and from shared functional properties of a bacterial glutamate receptor and potassium channels (Wo and Oswald, 1995; Wood et al., 1995; Chen et al., 1999a; Panchenko et al., 2001; Kuner et al., 2003). Functional and biophysical experiments corroborate the available structure. For example, studies examining the channel block of NMDA receptors by organic cations and Mg2+ (Villarroel et al., 1995; Zarei and Dani, 1995; Antonov and Johnson, 1999), and the voltage-dependence of the reactivity rate for substituted cysteines (Sobolevsky et al., 2002a, 2003) suggested that the narrow constriction is located approximately halfway across the transmembrane electric field. Scanning mutagenesis studies correctly predicted that the re-entrant M2 pore loop lines the inner cavity with the QRN site located at the tip of this loop (Kuner et al., 1996, 2001; Wollmuth et al., 1996).
Immediately external to the tip of the reentrant pore loop lies a central cavity (Fig. 14A) (Sobolevsky et al., 2009), similar to that of potassium channels, lined primarily by M3 (Beck et al., 1999; Kashiwagi et al., 2002; Sobolevsky et al., 2003). All glutamate receptor subtypes share a common transmembrane arrangement, but there are differences in permeation and block properties between subtypes. These differences imply some degree of molecular heterogeneity within the pore, most likely involving the narrowest constriction that sets the maximum rate of ion permeation either through steric or electrostatic effects.
A key determinant of the conductance and permeation properties of all glutamate receptors is the identity of the residue occupying a functionally critical position at the apex of the M2 loop, the QRN site. This site harbors either Gln (Q, unedited) or Arg (R, edited) in AMPA and kainate receptors (Hume et al., 1991; Verdoorn et al., 1991), Asn (N) in GluN1 and GluN2 receptors (Burnashev et al., 1992b; Mori et al., 1992), Gly (G) in GluN3 subunits, and Gln (Q) in GluD2 subunits. Not surprisingly, the key structural positioning of the QRN site allows this residue to influence single-channel conductance (Swanson et al., 1996, 1997b; Traynelis and Wahl, 1997), Ca2+ permeability (Burnashev et al., 1992a,b; Egebjerg and Heinemann, 1993), channel block by polyamines (Bowie and Mayer, 1995; Kamboj et al., 1995; Washburn et al., 1997), Mg2+ sensitivity (Burnashev et al., 1992b; Dingledine et al., 1992; Mori et al., 1992), channel block by organic compounds (Kashiwagi et al., 2002; Chen and Lipton, 2005), and assembly into heteromeric complexes (Greger et al., 2003). In addition, homomeric edited R-forms of kainate receptor channels are no longer purely cation-selective, but are also permeable to Cl− (Burnashev et al., 1996) (Table 18). Replacement of the QRN Asn with Gln in GluN1 or GluN2B yields channels with multiple conductance levels (Premkumar et al., 1997; Schneggenburger and Ascher, 1997). The functional effects of TARPs on AMPA receptors (Körber et al., 2007) and of membrane lipids on kainate receptors (Wilding et al., 2005) are influenced by editing at the QRN site. It is noteworthy that Sobolevsky et al. (2009) observed gaps between the transmembrane helices, which they predict may be filled by residues projecting from TARPs (see section II.H). This potential association with the pore could provide a means by which TARPs influence ion permeation, conductance, and polyamine block.
TABLE 18.
Pore properties of AMPA, kainate, and NMDA receptors
The relative permeability of Ca2+ to monovalent ions (PCa/PX) and the fractional Ca2+ current (Pf) values shown are not necessarily those given in the original manuscript but rather have been standardized to common parameters for direct comparison. The values shown are not corrected for activities. Pore dimensions are derived from the permeability of differently sized organic cations. Only values derived from recombinant channels are shown; see Dingledine et al. (1999) for more details and examples of native channels. PCa/PX values are derived from the Lewis equation (see Jatzke et al., 2002) based on changes in reversal potentials given in articles typically measured under bionic conditions where a reference solution (X, usually Cs+ or Na+) is replaced with a solution containing high Ca2+ (typically ≈100 mM). PCa/PX (from Pf) is derived from Pf measurements using the GHK equation (Burnashev et al., 1995). Fractional Ca2+ currents (Pf) are the percentage of the total current carried by Ca2+ and are measured using whole-cell currents and high concentrations of fura-2 to ensure that all incoming Ca2+ is captured by dye. Pf values are concentration- and voltage-dependent with the magnitude of these effects dependent on specific subtypes (Burnashev et al., 1995; Jatzke et al., 2002). The values shown were either recorded at −60 mV and 1.8 mM Ca2+ or adjusted to these conditions assuming GHK (see Jatzke et al., 2002).
| Subunit Combination | Pore Diameter | PCa/PX | PCa/PX (from Pf) | Pf | PCl/PCs |
|---|---|---|---|---|---|
| nm | % | ||||
| GluA1a,b | 0.78c | 1.6 | ≈0.7 | 3.2–3.6 | |
| GluA2 (Arg)c | 0.14 | ||||
| GluA1/GluA2 (Arg)c | 0.7 | 0.03 | 0.11 | 0.54 | ≈0 |
| GluA4 | 3.9 | ||||
| GluK2 (Gln)a,b,d | 0.75g | 0.14–0.17 | 0.30–0.48 | 1.55–2.4 | ≈0 |
| GluK2 (Arg)a,c,d | 0.76 | <0.04 | <0.2 | 0.74 | |
| GluK2 (Gln)/GluK2 (Arg)a,c,d | 0.74 | 0.58 | ≈0 | ||
| GluN1-GluN2Aa,b,e,f,g | 0.55h,i | 2.8–4.5 | 3.0–3.6 | 11.0–15.9 | |
| GluN1-GluN2Bf | 3.6 | 3.6 | 15.9 | ||
| GluN1-GluN2Ca | 1.8 | 1.73 | 8.2 | ||
| GluN1-GluN3Aj | 0.6 | ||||
| GluD2Lc | 2.6k,l |
The kainate receptor GluK2 is edited in three different locations: two in M1 (Ile, Tyr fully unedited and Val, Cys fully edited) and one in M2 (Gln in unedited, Arg in edited). All values shown are for receptors that are fully edited in M1.
GluD2Lc is a constitutively active form (Lurcher) of the GluD2 subunit.
NMDA receptors are obligate heteromultimers likely composed of 2 GluN1 and 2 GluN2 subunits in a 1-2-1-2 arrangement (Sobolevsky et al., 2009). The narrow constriction in NMDA receptors is formed by, at minimum, the QRN site asparagine of GluN1 and an asparagine adjacent to the QRN site (i.e., QRN + 1 site) in GluN2 (Fig. 14A) (Wollmuth et al., 1996). It is noteworthy that there is also a functional asymmetry between QRN Asn residues in the various NMDA receptor subunits: substitutions at the GluN1 QRN site have strong effects on Ca2+ permeability but only weak effects on Mg2+ block, and equivalent substitutions of the QRN site in GluN2 produce opposite effects (Burnashev et al., 1992b). This functional and presumed structural asymmetry between subunits most likely reflects modest differences at the atomic scale. A highly conserved GluN2B tryptophan residue in the M2 loop also has been proposed to contribute to the narrow constriction (Williams et al., 1998), and the homologous tryptophan in GluN1 can influence channel block (e.g., Jin et al., 2007).
B. Mechanisms of Ion Permeation
A major determinant of ion selectivity arises from the physical or electrical interactions of permeating ions with the pore wall within the narrow region of a channel in which ions and side chains or main-chain atoms of residues come close enough to create a permeation barrier. The dimensions of the smallest diameters of various glutamate receptor channels have been estimated from the permeability of differently sized organic cations (Table 18). The finding that channels containing one or more arginine residues at the QRN site have roughly the same dimension as channels containing the smaller glutamine residues suggests that the side chain of the QRN site does not define the dimensions of the narrow constriction and that its effect on permeation may be influenced by electrostatics (Kuner et al., 2001).
All mammalian glutamate receptor subunits are cation-selective and lack the highly conserved TVGYG sequence that defines selectivity in K+ channels. The prokaryotic GluR0 does have the TVGYG sequence and is K+-selective (Chen et al., 1999a), but introduction of this sequence into mammalian glutamate receptor subunits does not confer K+ selectivity (Hoffmann et al., 2006a), probably because of different interactions of this region within the mammalian glutamate receptor compared with K+ channels. Indeed, in the membrane-spanning GluA2 structure, the extended region in the M2 loop is disordered, and this may reflect the absence of TVGYG and stabilizing interactions that underlie K+ selectivity in K+ channels. Moreover, under certain nonphysiological conditions, edited (R) forms of homomeric GluA2 and GluK2 subunits are permeable to Cl− ions (Table 18). The central location of the QRN site and the fact that these homomeric receptor channels show mixed cation/anion permeability suggests other structural elements such as M2 loop dipole, carbonyl side chains in M2, and/or elements in the outer cavity may contribute to cation selectivity. Indeed, in K+ channels, the pore loop dipole alters the environment in the inner cavity (corresponding to the outer cavity in glutamate receptors) to allow a high flux while maintaining ion selectivity (Morais-Cabral et al., 2001). It is noteworthy that the GluA2 structure reveals various charged residues positioned near the apex of the M3 segment that could influence conductance. Consistent with this idea, mutations of homologous positions in GluN1 M3 reduce single channel conductance and Ca2+ permeability (Watanabe et al., 2002).
Selectivity, unitary conductance, and channel block can all be influenced by the number of ions that reside in the pore at any one time. Potassium channels can accommodate multiple permeating ions, and this is a key component of their high selectivity and transport rates (Doyle et al., 1998; Morais-Cabral et al., 2001; Zhou et al., 2001). Studies of NMDA receptors suggest that the pore contains only a single permeating ion (Zarei and Dani, 1994; Schneggenburger, 1996; Iino et al., 1997), which may reflect the nonselective nature of the pore. In K+ channels as well as in other highly selective channels, the interaction between the narrow constriction and permeating ions is tight and this reduces their free energy in this region. Allowing multiple permeating ions creates ion-ion interactions that are critical to facilitating high conductance rates (Morais-Cabral et al., 2001). In contrast, in the larger glutamate receptor channel, the interaction between the pore walls and permeating ions may be weaker (i.e., fewer waters of hydration are removed), which could prevent occupancy by multiple ions. The pore of NMDA receptors can energetically accommodate a blocking particle such as Mg2+ and additional permeant ions (Antonov et al., 1998; Antonov and Johnson, 1999), and these additional permeant ion-binding sites may make a significant contribution to ion selectivity and conductance in addition to effects on channel block.
Glutamate receptors show limited selectivity for alkali metal cations, which for GluN1/GluN2A is Cs+ > Rb+ ≈ K+ > Na+ > Li+, for GluA1 is K+ ≈ Rb+ > Cs+ > Na+ ≈ Li+, and for GluK2(Q) is K+ ≈ Cs+ ≈ Rb+ > Na+ ≈ Li+ (Tsuzuki et al., 1994; Jatzke et al., 2002). In terms of Eisenman sequences (Hille, 2001), NMDA receptors display type I weak-field-strength sites, whereas non-NMDA receptors display type IV sites. In terms of selectivity at a synapse (K+ versus Na+), the differences are minimal (PK/PNa ≈ 1.14 for NMDA receptors and PK/PNa ≈ 1.25 for AMPA and kainate receptors). Thus, the current carried through most glutamate receptor channels is a mixture of monovalent cations (K+ and Na+) plus Ca2+ (MacDermott et al., 1986; Mayer and Westbrook, 1987). Receptors containing the edited form of GluA2, GluK1, or GluK2 (Hume et al., 1991; Burnashev et al., 1992a), however, are impermeable to Ca2+ (Table 18). The flux of Ca2+ through NMDA and Ca2+-permeable AMPA receptor channels is a key factor contributing to various forms of synaptic plasticity (Gu et al., 1996; Bloodgood and Sabatini, 2007; Citri and Malenka, 2008) (sections IV and IX), gene regulation (section III), neuropathology (Choi, 1995; Kalia et al., 2008) (section X), and fast transmitter release (see section IX) (Chávez et al., 2006).
Three general approaches have been used to study the magnitude and mechanism of Ca2+ permeation in glutamate receptors, and include measurement of 1) relative Ca2+ permeability (PCa/PMonovalent), 2) channel block by Ca2+, and 3) fractional Ca2+ currents (Pf), which are determined from whole-cell currents recorded in the presence of high concentrations of intracellular Fura-2 (Burnashev et al., 1995; Premkumar and Auerbach, 1996; Schneggenburger, 1996; Sharma and Stevens, 1996). The use of fractional Ca2+ currents is advantageous over the other methods for channels with a mixed Ca2+ and monovalent permeability because it has fewer assumptions about flux properties, directly quantifies the Ca2+ flux under physiological conditions, and allows the fraction of the total current carried by Ca2+ to be quantified over a wide voltage range (Neher, 1995).
NMDA receptors are approximately 3 to 4 times more permeable to Ca2+ than unedited Ca2+-permeable AMPA or kainate receptors (Table 18). For NMDA receptors, there are subunit-specific differences, dictated by the GluN2 and GluN3 subunits, with receptors containing GluN2A/GluN2B showing the highest Ca2+ permeability, GluN2C showing a somewhat reduced Ca2+ permeability, and GluN3 showing the lowest permeability. Ca2+-permeable AMPA receptors show a slightly greater Ca2+ permeability than kainate receptors. Mutant Lurcher GluD2 channels are Ca2+-permeable (Table 18). The magnitude of Ca2+ influx through at least NMDA receptors can be regulated by phosphorylation (Skeberdis et al., 2006) and synaptic activity (Sobczyk and Svoboda, 2007).
It is noteworthy that NMDA receptors are highly permeable to Ca2+ yet show the peculiar property of being blocked by external Ca2+ in a largely voltage-independent manner (Ascher and Nowak, 1988; Premkumar and Auerbach, 1996; Sharma and Stevens, 1996), which manifests as a reduction in single channel conductance (Gibb and Colquhoun, 1991; Stern et al., 1994; Premkumar and Auerbach, 1996). These results lead to the suggestion of multiple Ca2+ binding sites within the pore (Premkumar and Auerbach, 1996; Sharma and Stevens, 1996), including the QRN site as well as an external site for Ca2+. Moreover, NMDA receptors show an interaction between Ca2+ and monovalent ions in the pore, concentration-dependent PCa/PMonovalent (Wollmuth and Sakmann, 1998), and block by Ca2+. However, the PCa/PMonovalent value for NMDA receptors derived from the Goldman-Hodgkin-Katz equation, which assumes no ion-ion interactions in the pore (Hille, 2001), describes fractional Ca2+ currents over a wide voltage and concentration range (Burnashev et al., 1995; Schneggenburger, 1996, 1998; Jatzke et al., 2002). These differences could be reconciled if the pore, which is occupied by a single ion, largely existed in two states, both of which individually follow Goldman-Hodgkin-Katz, one with the pore occupied by Ca2+ and the other occupied by a monovalent ion. In this model, a competition for the pore occurs between Ca2+ and monovalent cations (Schneggenburger, 1998).
The process of Ca2+ influx in Ca2+-permeable AMPA receptors might be less complex than that in NMDA receptors, because Ca2+-permeable AMPA receptors do not select for or against divalent cations. PCa/PMonovalent in Ca2+-permeable AMPA receptors is approximately unity (not taking into account ion activities; Table 18). Ca2+-permeable AMPA receptors are not blocked by Ca2+, and the predicted fractional Ca2+ current at −60 mV in ∼150 mM monovalent ions and 1.8 mM Ca2+ is ∼4%, in agreement with a channel that is nonselective for Ca2+ (PCa/PMonovalent = 1; Table 18; see Jatzke et al., 2002).
Ca2+ permeability is influenced by residues occupying the QRN site residing at the channel's narrow constriction (see section VIII.A). NMDA receptors in which the QRN site asparagine is mutated to various other residues, including the glutamine found in AMPA receptors, show reduced Ca2+ permeability (Burnashev et al., 1992b) (Table 18). In addition to residues at or near the QRN sites within the narrow constriction, other elements in the inner cavity may also influence Ca2+ permeability, either directly or by altering the structure of the M2 loop (Ferrer-Montiel et al., 1996). One such residue is a negatively charged Glu in the M2 loop, located five positions C-terminal to the QRN site in GluN1. Mutation of this Glu to the positively charged Lys reduces Ca2+ permeability (Schneggenburger, 1998). In addition, in the GluN2A subunit, an adjacent position is occupied by the polar glutamine, and replacing it with a Glu (as in GluN2C subunits that have reduced Ca2+ permeability; Table 18) yields channels that show a reduced Ca2+ permeability (Vissel et al., 2002), suggesting that this position may underlie GluN2 subunit-specific differences in Ca2+ permeability. Nevertheless, it is unclear whether Ca2+ directly interacts with the side chain at this position or whether mutations at this position alter the structure of the QRN site, because this position is not water accessible (Kuner et al., 1996).
The high Ca2+ influx in NMDA receptors has been proposed to be due, at least in part, to an external Ca2+ binding site (Premkumar and Auerbach, 1996; Sharma and Stevens, 1996). The most likely determinant of this Ca2+ binding site is a cluster of charged residues, the DRPEER motif (Fig. 14), located C-terminal to the M3 segment in GluN1 but absent in all GluN2, GluA, GluD, and GluK subunits (Watanabe et al., 2002). This unique motif is positioned at the external entrance to the channel (Beck et al., 1999), and possesses a net negative charge, predicted from the three potentially (depending on local pKa values) negative and one positive water-accessible residues. In mutant channels with the negative charges in DRPEER neutralized (ARPAAR), Pf values are reduced by approximately half at −60 mV (Watanabe et al., 2002), suggesting that the negatively charged side chains represent an important determinant of high fractional Ca2+ currents in NMDA receptors. It is noteworthy that the GluN2A subunit has a net negative charge at positions homologous to the DRPEER motif, but mutation of these residues has no effect on Ca2+ permeability (Watanabe et al., 2002). One potential explanation that is consistent with structural data placing elements of the GluN1 M3-S2 linker (tetramer A/C subunits) in the vicinity of each other (Sobolevsky et al., 2009) is that the DRPEER motif lies closer to the permeation pathway than homologous positions in GluN2 (tetramer B/D subunits). It is noteworthy that substituting an Asn at the QRN site of AMPA receptors, which should make the narrow constriction more NMDA receptor-like, results in only a modest increase in fractional Ca2+ current from 4% to 5% (Wollmuth and Sakmann, 1998). Part of the difference in Ca2+ permeability between AMPA and NMDA receptors may reflect the absence of the DRPEER motif in non-NMDA receptors (Fig. 14; Jatzke et al., 2003).
For NMDA receptors, coexpression of GluN3 subunits (which contain a Gly at the QRN site) with GluN1 or GluN1/GluN2 results in channels with reduced single channel conductance, decreased Ca2+ permeability, and reduced Mg2+ block (Perez-Otano et al., 2001; Matsuda et al., 2003) (Table 18), suggesting that GluN3 directly influences the structure of the permeation path. Additional evidence for this idea was provided by Wada et al. (2006), who showed that cysteine-substitutions in the M3 segment of GluN3A were modified by extracellular cysteine-reactive reagents and suggested that some residues lining the pore may differ from GluN1 and GluN2 subunits. Nevertheless, molecular mechanisms regulating conductance and permeability in GluN3-containing receptors are poorly understood.
C. Voltage-Dependent Channel Block by Endogenous Ions
A number of endogenous ions can regulate glutamate receptor function, including protons, Zn2+, Mg2+, and polyamines (see section VI). Of these, the multivalent ions Zn2+, Mg2+, and polyamines have voltage-dependent block mechanisms that have important implications for neuronal function independent of the dynamic regulation of their concentrations. We will consider the mechanism and implications of block of the pore by Mg2+ and polyamines, both of which endow receptors with the ability to detect previous neuronal activity, and thus have roles in short- and long-term plasticity. Although channel block by Zn2+ may be important in some situations (Vogt et al., 2000), Zn2+ channel block is relatively low affinity, rapidly reversing, and will not be considered further.
Mg2+ block represents perhaps the most distinctive feature of the NMDA receptor and was first described in a now-classic series of studies from multiple laboratories (Mayer et al., 1984; Nowak et al., 1984; Ascher et al., 1988; Dingledine et al., 1999). Because of the strong voltage-dependence of block, NMDA receptors act as coincident detectors, sensing postsynaptic depolarization at the same time as or shortly after release of glutamate or other excitatory amino acids. This block allows NMDA receptors to mediate cellular mechanisms of learning and memory (see section IX). The most prominent biophysical feature of extracellular Mg2+ block is its strong voltage-dependence. Indeed, the major blocking site for Mg2+ is at or near the narrow constriction (for review, see Dingledine et al., 1999), located approximately 50% across the channel. Part of the additional voltage-dependence is due to the presence of permeant ion-binding sites in the external and internal cavities (Antonov and Johnson, 1999; Zhu and Auerbach, 2001a,b; Qian et al., 2002). Occupancy of these ion binding sites by Na+ or K+ alters the association and dissociation rates of Mg2+ from its blocking site. The nature of these permeant ion-binding sites differs between GluN2 subunits (Qian and Johnson, 2006) and hence may underlie some GluN2-specific effects on Mg2+ block (Monyer et al., 1994; Kuner and Schoepfer, 1996).
An advance in our mechanistic understanding of extracellular Mg2+ block has come from studies of the rate of Mg2+ block and unblock, which provides a physiological view of the actions of Mg2+. As a first approximation, the block and unblock by extracellular Mg2+ was considered instantaneous; however, this simplifying assumption would limit the role of NMDA receptors in dendritic integration. A variety of studies show that the rate of unblock and reblock of the channel is not instantaneous, showing both fast and slow components (Nowak et al., 1984; Vargas-Caballero and Robinson, 2003, 2004; Kampa et al., 2004; Clarke and Johnson, 2006) with rates depending on the GluN2 subunit (Clarke and Johnson, 2006). For GluN2B, the slow component of Mg2+ unblock arises from inherent voltage dependence of NMDA receptor gating (Clarke and Johnson, 2008).
These rates for Mg2+ unblock and reblock at the synapse will alter the response time and can lengthen the window of opportunity for back-propagating action potentials and local synaptic activity to modulate current flux (including Ca2+) through NMDA receptors. It is noteworthy that receptors containing GluN2C or GluN2D unblock more rapidly—almost instantaneously—than those containing GluN2A or GluN2B, the latter of which shows the slowest unblock (Clarke and Johnson, 2006). Given the possible regulation of Mg2+ block by membrane lipids (Parnas et al., 2009), perturbations in ion concentrations during activity, and GluN2 subunit-specific differences in rates of unblock and reblock, mechanisms regulating NMDA receptor block by Mg2+ at a synapse are likely to be diverse.
Regulation of NMDA receptor activity by extracellular Mg2+ is well known for its contribution to synaptic plasticity and associative learning. Less well known is the potential role in short-term synaptic plasticity played by the block of Ca2+-permeable non-NMDA receptors by internal polyamines such as spermine, spermidine, and putrescine. Polyamine block of AMPA and kainate receptors is dependent on membrane potential, which may reflect interaction in the pore between monovalent ions and polyamines, rather than membrane voltage itself (Bowie et al., 1998). Polyamines interact with the pore at various sites in the M2 loop including the QRN site and a negatively charged residue (Asp in AMPA receptors and Glu in kainate receptors) located C-terminal to the QRN site (for review, see Dingledine et al., 1999; Panchenko et al., 1999). Polyamines can dissociate from open channels to either the internal or external solution and thus permeate the channel (Bähring et al., 1997). Under physiological conditions, unedited AMPA and kainate receptors have doubly rectifying current-voltage relations reflecting block by internal polyamines at depolarized membrane potentials and permeation of internal polyamines at even more positive membrane potentials. Surprisingly, polyamine block is also state-dependent. The affinity for polyamines is higher in closed than in open channels, but the kinetics of polyamine block are slower in closed channels (Bowie et al., 1998; Rozov et al., 1998). As a result, synaptic Ca2+-permeable AMPA receptors can be blocked by polyamines in resting synapses. Synaptic activity partially relieves this polyamine block, and block recovers slowly, so that the response to subsequent synaptic events can be potentiated (Rozov and Burnashev, 1999; Shin et al., 2005). The degree of potentiation depends on the frequency of synaptic events. If the frequency is too low, block of closed channels returns to its equilibrium level between the first and second synaptic events, and no potentiation occurs. Alternatively, if the frequency is sufficiently high, block does not re-equilibrate during the brief interval between synaptic events, and the response to the second synaptic event is potentiated. Thus, state-dependent polyamine block of postsynaptic AMPA receptors could act as a high-pass filter for synaptic activity and may be an important component of circuit function in which information is encoded by synaptic timing (Abbott and Regehr, 2004).
IX. Role in Synaptic Function and Plasticity
A. Synaptic α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid Receptors
Within the mammalian CNS, the vast majority of fast excitatory synaptic transmission is mediated by heteromeric AMPA receptors assembled from differing combinations of the four subunits, GluA1 to GluA4. Expression of all subunits is developmentally regulated, region and cell-type specific, and activity-dependent (Ashby et al., 2008). Within adult forebrain principal neurons, including hippocampus and cortex, the predominant GluA subtype comprises GluA1 and GluA2 with a secondary minor role played by GluA2/3 receptors (Lu et al., 2009). By virtue of its edited QRN site, the presence or absence of GluA2 is a critical determinant of GluA function and dictates the single channel conductance and Ca2+-permeability of the native AMPA receptor (see section VIII.B). An absence of GluA2 also renders the channel pore sensitive to extra- and intracellular polyamine block, endowing GluA receptors with an inwardly rectifying current-voltage relationship and a novel postsynaptic mechanism of short-term plasticity involving the use-dependent unblock of polyamines (Isaac et al., 2007) (see section VIII.C). In many neurons, GluA2 expression is developmentally regulated, with low early postnatal expression levels, which, coupled with the transient high expression of GluA4, renders many neonatal forebrain receptors Ca2+-permeable (Pickard et al., 2000; Zhu et al., 2000). This Ca2+-permeability may play a role in synapse maturation and circuit development (Aizenman et al., 2002; Kumar et al., 2002; Eybalin et al., 2004; Ho et al., 2007; Migues et al., 2007). In the forebrain, as GluA2 expression increases throughout the early postnatal period, GluA4 is down-regulated. In adult, GluA4 expression remains highly enriched primarily in cerebellar tissue, where it exists as heteromeric GluA2/GluA4 in granule cells and in non-neuronal Bergmann glia as homomeric GluA4 (Petralia and Wenthold, 1992; Martin et al., 1993).
At a prototypical synapse, binding of glutamate to synaptic AMPA receptors triggers a brief, rapidly rising conductance that decays rapidly (1–2 ms; Fig. 12) as a result of the deactivation of the agonist-receptor complex. The kinetics and amplitude of the excitatory synaptic response are determined by the biophysical properties of the receptor subunit combination (Table 17; Fig. 12; see section VII.A) and the density of receptor expression, convolved with the time course of glutamate release and uptake. Receptor subunit composition and the kinetics of the synaptic response are tailored to the role played by the synapse and cell type in the circuit in which they are embedded. For example, many cortical local circuit inhibitory interneurons express AMPA receptors that comprise homomeric GluA1 (Isaac et al., 2007). These receptors have rapid kinetics such that the overall synaptic conductance time course is complete in less than a millisecond. The subsequent excitatory postsynaptic potential (EPSP) is large, rises and decays rapidly, and is capable of triggering action potentials within a narrow temporal window (Geiger et al., 1997). Such receptor assemblies are typical in cells involved in the timing of oscillatory activity (Lawrence and McBain, 2003; Jonas et al., 2004; Isaac et al., 2007).
Although the subunit composition is a major determinant of synaptic conductance kinetics, synapses typically are distributed across often elaborate dendritic trees. The resulting EPSP can be subject to extensive cable attenuation as it travels from its source (e.g., spines or smooth portions of narrow dendrites) to the site of action potential initiation. Consequently, in many neurons, synaptic potentials are often capable of triggering appreciable intrinsic voltage-dependent sodium, calcium, and/or potassium conductances that can normalize the impact of dendritic location or alter the temporal voltage window initiated by the synaptic conductance (Bloodgood and Sabatini, 2008).
Systematic mRNA and immunohistochemical profiling of GluA subunit expression in individual neurons has provided a detailed picture showing how receptor types are linked to the functional properties of the cell. However, such profiling is complicated by the observation that single neurons often manufacture AMPA receptors of different subunit compositions and target them to different synaptic inputs across the somatodendritic axis (Tóth and McBain, 2000). How individual neurons differentially target such receptor assemblies to often overlapping afferent inputs remains to be determined. However, in neurons of the lateral geniculate nucleus, Ras and Rap2 drive bidirectional trafficking of GluA1 between the deliverable and synaptic pools at retinogeniculate synapses (GluA1 dominant) but not at corticogeniculate synapses (GluA4 dominant), despite the existence of intracellular pools of GluA1 at both synapses (Kielland et al., 2009). This suggests that intrinsic activity within the vision-dependent pathway preferentially drives GluA1 between deliverable and synaptic pools at retinogeniculate synapses. Furthermore, in cortical principal cells, where the dominant AMPA receptor configuration is composed of GluA1 and GluA2, significant intracellular pools of GluA2-lacking Ca2+-permeable receptors exist that can reach the surface under certain conditions (Ju et al., 2004; Clem and Barth, 2006; Plant et al., 2006; Sutton et al., 2006), suggesting that AMPA receptor expression is a dynamic and highly regulated process.
B. Synaptic Kainate Receptors
Since the binding studies of Monaghan and Cotman (1982), it has been clear that high-affinity kainate receptor binding sites are prevalent throughout the CNS. Translating early binding studies into a function for all kainate receptor subunits in synaptic transmission has proved harder than would have been expected. In recombinant systems, GluK1 to GluK3 form functional homomeric receptors, whereas GluK4 and GluK5 do not. These latter two subunits are believed to play a supporting role by modifying both the pharmacological and biophysical receptor properties (Contractor and Swanson, 2008). Unlike AMPA and NMDA receptors (although exceptions exist), kainate receptors can play prominent roles at both pre- and postsynaptic sites. The rules of subunit assembly and combination remain unclear, and kainate receptors show strong developmental and regional regulation (Contractor and Swanson, 2008). During development, synaptic transmission at thalamocortical synapses is initially mediated by slow kainate receptors but switches during the first postnatal week to AMPA-receptor mediated transmission, representing a novel developmental form of LTP (Kidd and Isaac, 1999). This switch from kainate receptor to AMPA receptor subunits as the primary mediators of synaptic transmission shortens the window for coincidence detection and output timing in this circuit (Bannister et al., 2005; Daw et al., 2006). A similar activity-dependent switch from kainate to AMPA receptor-mediated transmission also has been observed at synapses onto perirhinal cortex Layer I/II neurons (Park et al., 2006).
Unlike currents through recombinant kainate receptors (see section VII.A), synaptic kainate receptors often possess a slower time course (decay time constants often >100 ms) and typically are activated only after short bursts of presynaptic activity (Contractor and Swanson, 2008). The best studied site of kainate receptor-mediated synaptic transmission is at the mossy fiber-CA3 pyramidal cell synapse. There, heterotetramers formed by GluK2 and GluK5 are found postsynaptically, whereas receptors formed from combinations of GluK1 to GluK3 are located presynaptically. At mossy fiber synapses, kainate receptors act in a concerted manner to amplify synaptic integration and frequency-dependent spike transmission (but see Kwon and Castillo, 2008; Sachidhanandam et al., 2009). Long-term depression of the kainate receptor component of the mossy fiber EPSC was traced to internalization of kainate receptors, mediated by a protein interacting with C kinase/synaptosome-associated protein 25/PKC complex (Selak et al., 2009). In contrast, kainate receptors are absent at associational/commissural synapses also made onto CA3 pyramidal cells, indicating another example of target-specific localization of glutamate receptors (Tóth and McBain, 2000). Elsewhere in cortical circuits, GluK2 is widely expressed at synapses on local circuit inhibitory interneurons (Cossart et al., 1998, 2002; Frerking et al., 1998; Mulle et al., 2000), where they have been implicated in the generation of θ and γ oscillatory activity (Fisahn et al., 2004; Goldin et al., 2007). GluK1-containing receptors have been described on inhibitory axons presynaptic to principal cells and inhibitory interneurons, where they function to set the inhibitory tone of the hippocampal network (Cossart et al., 1998; Semyanov and Kullmann, 2001; Fisahn et al., 2004).
In addition to their role as ionotropic receptors, kainate receptors also signal via G-protein-coupled second-messenger cascades to downstream effectors (Rodríguez-Moreno and Lerma, 1998). By triggering a PKC-signaling cascade, GluK2-containing receptors modulate the slow- and medium-duration afterhyperpolarization conductance in CA1 and CA3 pyramidal cells (Melyan et al., 2002; Fisahn et al., 2005). Moreover, different subunits within the receptor complex have been proposed to independently control the ionotropic (GluK2) and metabotropic (GluK5) actions of kainate (Ruiz et al., 2005). However, knockout of GluK4 and GluK5 eliminated kainate-mediated EPSCs at mossy fiber-CA3 synapses but failed to eliminate the kainate-mediated modulation of the slow afterhyperpolarization, demonstrating that GluK4 and GluK5 are essential for normal ionotropic function but are not linked to the proposed metabotropic function of native kainate receptors (Fernandes et al., 2009).
C. Synaptic and Extrasynaptic N-Methyl-d-aspartate Receptors
At virtually all central synapses, NMDA receptors colocalize with AMPA receptors to form the functional synaptic unit, such that presynaptically released glutamate typically coactivates both NMDA and AMPA receptors (Petralia and Wenthold, 2008). The ratio of AMPA receptor- to NMDA receptor-mediated synaptic current varies across a wide range. Synaptic NMDA receptors are thought to be heterotetramers comprising two GluN1 subunits and two GluN2 or GluN3 subunits (Wenthold et al., 2003); evidence also exists for native triheteromeric receptors composed of GluN1/GluN2A/GluN2B, GluN1/GluN2A/GluN2C, and GluN1/GluN2B/GluN2D (see section II.B). Like AMPA receptor subunits, GluN subunit expression is under developmental and regional control during the early postnatal life. Because GluN1 is an obligate receptor subunit for channel function, development is targeted primarily toward GluN2 and GluN3 expression. The best example of this developmental expression is the switch from GluN1/GluN2B receptors in cortex, hippocampus, and cerebellum, which predominate in the first weeks of postnatal life, to GluN1/GluN2A-containing receptors (van Zundert et al., 2004). GluN1/GluN2B receptors are important for circuit formation and development, and this switch to GluN1/GluN2A results in NMDA receptor-mediated EPSCs with a more rapid decay. In hippocampal principal cells, this subunit switch is triggered by agonist binding (Barria and Malinow, 2002) and can be driven by stimulus protocols that produce long-lasting potentiation (Bellone and Nicoll, 2007). Likewise, sensory experience drives this subunit switch at thalamocortical synapses in primary sensory neocortex (Quinlan et al., 1999; Barth and Malenka, 2001; Lu et al., 2001). In mossy fiber-cerebellar granule cell synapses, a further subunit switch to GluN1/GluN2C is observed around P40 and is accompanied by a slowing of current kinetics and a reduction in Mg2+ sensitivity (Cathala et al., 2000). NMDA receptors containing GluN2D are most common in early postnatal life in neurons of the diencephalon, brainstem, and cerebellum; mRNA expression levels persist in subpopulations of interneurons in, for example, the hippocampus (Monyer et al., 1994). The signaling mechanism(s) underlying these regulatory steps are unknown. GluN3A is expressed primarily in early postnatal life, whereas GluN3B is found in adult brainstem and spinal cord (Petralia and Wenthold, 2008). GluN3A expression seems to be a critical step in synaptogenesis and spine formation, and it maintains receptors in an immature state (Pérez-Otaño et al., 2006), perhaps with enhanced neuroprotection (Nakanishi et al., 2009). Subsequent down-regulation of GluN3A expression is important for synapse maturation and the emergence of a competent state for synaptic plasticity (Roberts et al., 2009). The potential roles for the myriad of glutamate receptor compositions have been discussed elsewhere (Petralia and Wenthold, 2008). Although exceptions exist, GluN2B-, GluN2D-, and GluN3A-containing receptors generally predominate early in postnatal life, whereas GluN2A- and GluN2C-containing receptors become more abundant in adult brain (Watanabe et al., 1992, 1993, 1994a,b,c).
At some synapses, there may be a correlation between the types of AMPA- and NMDA-receptors expressed. Synapses between mossy fiber axons of the dentate gyrus granule cells and stratum lucidum interneurons are composed of two AMPA receptor types. At GluA2-lacking AMPA receptor synapses, the NMDA to AMPA receptor ratio is low compared with that observed at GluA2-containing AMPA receptor synapses (Lei and McBain, 2002). At GluA2-lacking AMPA receptor synapses, NMDA receptors comprise GluN2B subunits that have a low open probability and contribute currents of small amplitude and long duration. By contrast, synapses with GluA2-containing AMPA receptors typically use GluN2A-containing NMDA receptors (Bischofberger and Jonas, 2002; Lei and McBain, 2002). This coregulation may reflect gene expression, receptor assembly, or trafficking (Barria and Malinow, 2002). Like AMPA receptor subunits, examples exist of cells targeting NMDA receptors of distinct subunit composition to different locations across their somatodendritic axis (Köhr, 2006). Thus, NMDA receptor subunit composition and location are major determinants of glutamatergic postsynaptic properties. Moreover, NMDA receptors spatially interact with voltage-dependent conductances in spines or dendrites to shape the synaptic signal (Bloodgood and Sabatini, 2008) (see section VIII.C).
Although the synaptic composition of NMDA receptors varies throughout the brain, most mature cortical synapses contain GluN2A, whereas GluN2B-containing receptors are often extrasynaptic. Although there is pharmacological evidence that extrasynaptic receptors composed of GluN2B can participate in the induction of long-term depression, data suggesting synaptically located receptors comprising GluN2A can induce long-lasting potentiation (Liu et al., 2004b; Massey et al., 2004) have been compromised by poor selectivity of the competitive antagonist NVP-AAM077 (see section V.D). Moreover, GluN2A-containing subunits also have been observed extrasynaptically (Rumbaugh and Vicini, 1999; Thomas et al., 2006), in addition to evidence for GluN2B at adult synapses (Petralia et al., 2005). Recent studies suggest that astrocytic release of glutamate can influence nonsynaptic NMDA receptors, which can alter function of postsynaptic NMDA receptors (Lee et al., 2007a; Hermann et al., 2009).
D. Synaptic Plasticity
1. N-Methyl-d-aspartate Receptors.
The enormous volume of work exploring mechanisms for postsynaptic expression of NMDA-dependent LTP and LTD brought an unexpected bonus. The combination of electrophysiological, biochemical, and cell biological approaches used to dissect potential presynaptic and postsynaptic mechanisms involved in LTP and LTD also advanced our understanding of receptor assembly and trafficking, providing the basis for a careful dissection of the myriad proteins that interact with AMPA and NMDA receptors (see section II.G). Several excellent reviews on these protein-protein interactions exist (Collingridge et al., 2004; Malenka and Bear, 2004; Shepherd and Huganir, 2007; Ashby et al., 2008; Pelkey and McBain, 2008).
Within the hippocampus, a brief high-frequency train of stimuli delivered to the Schaffer collateral input to CA1 pyramidal cells initiates a long-lasting strengthening of synaptic transmission. Induction of this form of LTP is unambiguously NMDA receptor-dependent at most synapses onto principal neurons. When NMDA receptors embedded in the postsynaptic grid are activated, the subsequent increase in intracellular Ca2+ (see section VIII.B) triggers a long-lasting change in AMPA receptor-mediated synaptic transmission. This plasticity has been the most widely studied form of LTP and has been posited as a cellular correlate underlying learning and memory (Kessels and Malinow, 2009). NMDA receptors, by virtue of their voltage-dependent Mg2+ block, function as coincidence detectors whose participation satisfies the requirement for synapse specificity, associativity, and cooperativity. Within hippocampal CA1 pyramidal cells, NMDA receptors are assemblies of GluN1 in combination with GluN2A or GluN2B, with GluN2A-containing receptors being predominant in the adult. Overexpression of GluN2B enhances LTP and shifts the frequency-dependence of induction to lower frequencies, consistent with the longer synaptic conductance time course mediated by GluN2B (Tang et al., 1999; Erreger et al., 2005a).
Downstream of the NMDA receptor-associated increase in intracellular Ca2+, a number of effector mechanisms, such as CaMKII, PKC, and PKA are thought to be involved in LTP induction. CaMKII is the principal resident of the postsynaptic density, optimally positioned to sense the transient Ca2+ elevation. PKA seems to be a key mediator of LTP induction in young animals, whereas CaMKII is necessary and sufficient for early-phase NMDA receptor-dependent LTP in older animals. Roles for the atypical PKC isoform PKMζ, the nonreceptor tyrosine kinase src, and nitric-oxide synthase have been described previously (Pelkey and McBain, 2008).
The original observation of “silent” synapses (i.e., synapses that contain functional NMDA receptors but lack functional AMPA receptors) paved the way for the subsequent deluge of studies that demonstrated AMPA receptor delivery and retrieval as a mechanism underlying long-lasting plasticity (Kerchner and Nicoll, 2008). In the original studies (Isaac et al., 1995; Liao et al., 1995), silent synapses were subjected to an LTP-induction protocol, which then triggered the appearance of AMPA receptor-mediated currents with no change in NMDA receptor-mediated events. This emergence of AMPA receptor-mediated currents suggested that the Ca2+ entry after NMDA receptor activation triggered an insertion of nascent AMPA receptors into the postsynaptic density, a hypothesis that has received strong experimental support (Collingridge et al., 2004; Ashby et al., 2008; Pelkey and McBain, 2008). To summarize, this model suggests that LTP arises from the interplay between the trafficking of two distinct AMPA receptor species in hippocampal principal neurons. GluA1/GluA2 heteromers are typically excluded from synaptic sites in the absence of appropriate LTP conditioning stimuli. After LTP induction, GluA1/GluA2 receptors are rapidly incorporated into either silent or active synapses. In the original model, GluA2/GluA3 receptors, which were active in constitutive cycling, then replaced GluA1/GluA2 receptors at recently potentiated or unsilenced synapses. However, emerging evidence suggests only a minor role for GluA2/GluA3 receptors at these synapses (Lu et al., 2009). The original evidence that GluA1/GluA2 were inserted was based on the observation that the vast majority of AMPA receptors in pyramidal cells contain GluA2. However, homomeric GluA1 receptors are preferentially incorporated into synapses after LTP induction protocols or by active CaMKII (Shi et al., 2001). In addition, principal neurons contain substantial reserve pools of GluA2-lacking AMPA receptors, and evidence suggests that GluA2-lacking Ca2+-permeable AMPA receptors, presumably GluA1 homomers, are inserted immediately after LTP induction, only to be replaced by GluA2-containing receptors 20 min after induction (Plant et al., 2006; but see Adesnik and Nicoll, 2007). The role for GluA1 in LTP is strengthened by the observed defects of conventional high frequency-induced NMDA receptor-dependent LTP in adult mice lacking the GluA1 subunit; however, a slowly developing form of θ burst-induced LTP remains (Zamanillo et al., 1999; Mack et al., 2001). In contrast, LTP is enhanced in GluA2 and GluA2/GluA3 knockout mice, which may reflect the increased Ca2+ influx through the remaining Ca2+-permeable AMPA receptors (Jia et al., 1996; Meng et al., 2003).
2. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid Receptors.
Native GluA1 subunits undergo regulated phosphorylation at two sites (Ser831 and Ser845) in their C-terminal tails (see section IV.A). Ser831 is a substrate for both CaMKII- and PKC-dependent phosphorylation, whereas Ser845 is a substrate for PKA phosphorylation (Roche et al., 1996; Mammen et al., 1997). LTP is markedly diminished in adult mice bearing a GluA1 knock-in phosphomutant that lacks both serine residues (Lee et al., 2003). However, the incomplete block of LTP, especially at the earliest time points after induction, argues against a role for either of these sites in high-frequency stimulation-induced NMDA receptor-dependent LTP. Moreover, recombinant mutant GluA1(S831A) receptors are still driven into synapses after an LTP induction protocol or active CaMKII treatment (Esteban et al., 2003). In contrast, the mutant GluA1(S845A) cannot be driven into synapses by constitutively active CaMKII. In addition, PKA phosphorylation of Ser845 is insufficient to drive GluA1 homomers into synapses, suggesting that the Ser845 site may be important for anchoring newly inserted receptors before their turnover to the stable population (Lee et al., 2003). Given that neither of these sites is a substrate for CaMKII-mediated receptor insertion suggests that other signaling cascades are also subject to CaMKII phosphorylation, such as the Ras family of GTPases and mitogen-activated protein kinases (Pelkey and McBain, 2008).
CaMKII- and PKA-dependent phosphorylation of recombinant GluA1 Ser831 and Ser845 also increases channel open probability and single-channel conductance (see section IV.A). Although evidence for a change in open probability after LTP is lacking, evidence supports an increase in AMPA receptor conductance as a mechanism for LTP expression (Benke et al., 1998; Lüthi et al., 2004; Palmer et al., 2004). CaMKII can still phosphorylate GluA1 Ser831 in recombinant GluA1/GluA2, but this does not increase the single-channel conductance, as observed in homomeric GluA1, unless a TARP such as stargazin is coexpressed (Oh and Derkach, 2005; Jenkins et al., 2010). Moreover, the potential insertion of GluA2-lacking receptors (which possess a higher conductance than GluA2-containing receptors) into the synapse after LTP could account in part for the conductance change (Plant et al., 2006). It is clear that more work is needed to untangle the overlapping roles of CaMKII and PKA phosphorylation.
A second mechanism of plasticity comes from the emerging role of TARPs in AMPA receptor trafficking (Tomita et al., 2005a,b). The original observation that overexpression of γ-2 could rescue AMPA receptor-mediated transmission in the stargazer mutant mouse suggested a role for TARPs in the biosynthetic and trafficking pathway of the AMPA receptors (Chen et al., 2000, 2003). Indeed, TARP association in the endoplasmic reticulum is essential for export to the Golgi apparatus and ultimate surface expression. An absence of TARP association renders receptors unstable and ultimately targets them for degradation (Tomita et al., 2003). TARP-associated AMPA receptors are inserted into the plasma membrane at extrasynaptic sites and subsequently incorporated at synapses via an interaction of the TARP C-terminal tail and PSD-95. This synaptic incorporation requires CaMKII/PKC-dependent serine phosphorylation of the TARP C-terminal tail, and this active incorporation differs from the constitutive insertion of AMPA receptors at extrasynaptic sites (Milstein and Nicoll, 2009). A critical role for TARP C-terminal phosphorylation in synaptic incorporation, but not delivery to the plasma membrane, was demonstrated using a truncated form of γ-2 in which substantial extrasynaptic receptors accumulated in the absence of significant synaptic incorporation. In this model, AMPA receptors ready for delivery to the plasma membrane are held in an extrasynaptic pool before being incorporated into synapses in an activity-dependent manner (Chetkovich et al., 2002; Bats et al., 2007). The relative simplicity of the TARP two-step model of AMPA receptor incorporation into synapses is an attractive hypothesis to explain many features of synaptic plasticity.
X. Therapeutic Potential
A. Glutamate Receptor Antagonists and the Prevention of Acute Neuronal Death
Cerebrovascular accident is projected to become the fourth most prevalent cause of disability by 2020 (Michaud et al., 2001) and remains a leading cause of death, which continues to fuel investigation of glutamate receptor ligands for therapeutic use in preventing acute neuronal death caused by cerebral ischemia and traumatic brain injury (TBI) (Kalia et al., 2008). The NMDA receptor is strongly implicated in this acute neurotoxicity, and NMDA receptor antagonists targeted at the glutamate binding site, the glycine binding site, the channel pore, and an allosteric site on the GluN2B subunit are all neuroprotective in multiple preclinical models (Green, 2002; Parsons et al., 2002; Lo et al., 2003; Hoyte et al., 2004; Small and Tauskela, 2005; Wang and Shuaib, 2005; Muir, 2006). These data supported the clinical investigation for utility in preventing death and long term disability after stroke and TBI in man. Several large clinical trials have been undertaken (Dyker et al., 1999; Lees et al., 2000; Albers et al., 2001; Sacco et al., 2001; Yurkewicz et al., 2005). Unfortunately, all clinical tests of glutamate antagonists for neuroprotection have failed.
The reasons for the failure of the NMDA antagonists in stroke and TBI trials have been extensively discussed (Dawson et al., 2001; Danysz and Parsons, 2002; Gladstone et al., 2002; Muir and Lees, 2003; Doppenberg et al., 2004; Hoyte et al., 2004; Small and Tauskela, 2005; Muir, 2006). In some cases, failure is attributable to class-specific factors. For the channel blockers and the glutamate site antagonists, the levels of occupancy needed for neuroprotection can also alter cardiovascular function and disrupt cognition, leading to psychotomimetic effects (Small and Tauskela, 2005; Muir, 2006). For the channel blocker aptiganel (Cerestat; CNS 1102), these side effects limited plasma concentrations tested in phase III trials to the minimum predicted for neuroprotection, thereby limiting chances for efficacy (Dyker et al., 1999; Albers et al., 2001). In contrast, the glycine site antagonist gavestinel (GV150526) was well tolerated and administered to plasma concentration and duration significantly above those predicted to be neuroprotective, yet gavestinel also failed to improve outcome in stroke (Lees et al., 2000; Sacco et al., 2001). In this case, the relative lack of CNS side effects raised the question of whether adequate brain exposure was achieved. The uniform clinical failures of the NMDA antagonists must also call into question the hypothesis that blockade of NMDA receptors alone is sufficient to yield improved clinical outcome. Stroke and TBI trigger a number of potentially neurotoxic cascades in addition to NMDA receptor-mediated toxicity (Lo et al., 2003, 2005; Rogalewski et al., 2006; Doyle et al., 2008; Green, 2008). In addition to neuron loss, white matter damage may be particularly significant to outcome in humans (Dewar et al., 1999). Unique NMDA receptors have been identified on oligodendrocytes (Káradóttir et al., 2005; Salter and Fern, 2005; Micu et al., 2006; Stys and Lipton, 2007), and it is unclear whether inhibition of these receptors is sufficient to prevent ischemia-induced white matter loss (Baltan et al., 2008; Baltan, 2009). There is also strong evidence indicating that NMDA receptor inhibition can impede recovery of function of neurons damaged but not killed during ischemia (Yu et al., 2001; Ikonomidou and Turski, 2002). Thus, the duration of NMDA receptor inhibition in relation to the phase of injury may be a critical variable (Ikonomidou and Turski, 2002). Another aspect of this dual role is that NMDA receptors in different subcellular compartments may mediate beneficial or deleterious effects (Hardingham, 2006; Papadia and Hardingham, 2007; Léveillé et al., 2008). In particular, deleterious effects can be mediated by extrasynaptic receptors containing GluN2B subunits (Tu et al., 2010). However, the GluN2B-selective antagonist CP-101,606 was apparently insufficient to achieve efficacy in severe TBI (Yurkewicz et al., 2005).
Many of the issues raised above are perhaps surmountable with different pharmacologies, pharmaceutics, and durations of treatment. However, a factor not easily addressed is the time between onset of brain injury and initiation of drug treatment. The preclinical literature indicates that efficacy with NMDA receptor antagonists can be realized with treatment initiated up to ∼2 h after injury (Dirnagl et al., 1999), although the optimal timing may be shorter (Hoyte et al., 2004). However, a 2-h time window is challenging in typical critical care units. Thus, out of necessity, treatment windows in previous clinical trials were extended to hours after the onset of injury, reducing potential neuroprotective effects of NMDA receptor antagonists. Despite the extended window, patients still were enrolled before the extent of injury was characterized, exacerbating heterogeneity in sampled populations. In sum, the narrow treatment window meant that trials had to capture small effects from a highly variable patient sample. This was probably a significant, if not principal, factor in the across-the-board failure of the NMDA antagonists as neuroprotectants. Nevertheless, NMDA receptor antagonists remain an attractive target for treatment of acute brain injury, and clinical trials such as the FAST-MAG trial have demonstrated the feasibility of initiating treatment within 2 h of the onset of injury (Saver et al., 2004), which should allow a more rigorous evaluation of the neuroprotective potential of NMDA receptor antagonists.
An alternative to preventing glutamate-induced neurotoxicity is to target overactivation of AMPA and kainate receptors. Interest in this mechanism arose from the neuroprotective efficacy of NBQX and analogs (Sheardown et al., 1990; Catarzi et al., 2007), as well as protective effects of AMPA receptor antagonists on white matter injury (McDonald et al., 1998; Follett et al., 2000, 2004). These compounds are competitive AMPA receptor antagonists that also inhibit NMDA and kainate receptors to varying degrees (Bleakman and Lodge, 1998). The efficacy of these compounds in a variety of preclinical models of neuronal injury is more robust than that of the NMDA receptor antagonists (Gill, 1994), perhaps with a longer therapeutic window (Xue et al., 1994; Hoyte et al., 2004). These compounds also protect against ischemia-induced white matter damage (Follett et al., 2000). However, the poor solubility of NBQX and another early clinical candidate [6-(1H-imidazol-1-yl)-7-nitro-2,3(1H,4H)-quinoxalinedione] prevented successful development (Akins and Atkinson, 2002). A second generation compound, (1,2,3,4-tetrahydro-7-morpholinyl-2,3-dioxo-6-(trifluoromethyl)quinoxalin-1-yl)methylphosphonate (ZK200775) (Turski et al., 1998), had improved pharmaceutical properties, but development was halted in phase II trials because of CNS depression and a resultant transient worsening in National Institutes of Health stroke scale score during treatment (Walters et al., 2005). Results of phase II trials with another second generation compound, [2,3-dioxo-7-(1H-imidazol-1-yl)-6-nitro-1,2,3,4-tetrahydroquinoxalin-1-yl]-acetic acid monohydrate (YM872) (Takahashi et al., 2002), in ischemic stroke are not yet available.
The CNS-depressant effects of AMPA receptor antagonists that led to the halting of the ZK20075 trial are not unexpected, given that AMPA receptors mediate fast excitatory synaptic transmission. However, it is unclear whether the remarkable neuroprotective efficacy of the quinoxalinediones in preclinical models actually requires AMPA receptor inhibition. Two findings prompt this question. First, a series of decahydroisoquinoline competitive antagonists that varied in specificity for AMPA and kainate receptors were evaluated in a global ischemia model and found to differ in neuroprotective efficacy (O'Neill et al., 1998). Significantly, efficacy was not correlated with either AMPA or kainate receptor inhibition. Second, the quinazolinone CP-465,022, a noncompetitive antagonist highly specific for AMPA receptors (Lazzaro et al., 2002; Balannik et al., 2005), failed to demonstrate efficacy in ischemia models under conditions identical to those in which NBQX was highly efficacious (Menniti et al., 2003). These findings challenge whether AMPA receptor inhibition per se can account for the efficacy of NBQX and related molecules. This line of inquiry may help identify new glutamate receptor targets to prevent neuronal death, perhaps while circumventing the CNS-depressant liability of AMPA receptor inhibition. There are a wealth of structurally diverse non-NMDA receptor antagonists that could be explored (Gill, 1994; Bleakman and Lodge, 1998; O'Neill et al., 1998; Elger et al., 2006; Catarzi et al., 2007).
Clinical study over the last decade has brought the future of glutamate receptor antagonists as neuroprotectants to an impasse. The Stroke Therapy Academic Industry Roundtable (STAIR) initiative continues to lead an effort to integrate preclinical and clinical research in stroke to guide development of new neuroprotective stroke therapies. Particularly relevant is the idea of a multimodal neuroprotective approach (Rogalewski et al., 2006; Fisher et al., 2007), which may include glutamate receptor antagonists. Extension of the treatment initiation time window also is a relevant concept (Saver et al., 2009). Success in these efforts, together with clinical strategies to administer treatment within two hours (Saver et al., 2004), may reinvigorate the study of glutamate receptor antagonists for acute brain injury.
B. The Next Generation α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid and Kainate Receptor Antagonists
There are two AMPA receptor antagonists in late stage clinical development (Swanson, 2009). Talampanel (GYKI 53773, LY300164; Howes and Bell, 2007) and perampanel (E2007) are noncompetitive antagonists that are highly selective for AMPA over other glutamate receptors. Talampanel demonstrated efficacy in phase II trials in patients with refractory partial seizures (Chappell et al., 2002), and perampanel is under study. There remains an interest in AMPA receptor inhibition as a neuroprotective strategy, and talampanel is currently in a phase II clinical trial for amyotrophic lateral sclerosis (Duncan, 2009). It has also been reported that parampanel had efficacy against neuropathic pain (Swanson, 2009).
Although kainate receptors are widely expressed in the brain, much less is known about their physiological function compared with AMPA and NMDA receptors. This is due in large part to the slow development of specific pharmacological tools (reviewed by Jane et al., 2009). To date, the most advanced of these are a series of decahydroisoquinolines that are competitive inhibitors of GluK1, with varying degrees of selectivity for competitive inhibition of AMPA receptors (Bortolotto et al., 1999; Bleakman et al., 2002). Localization of GluK1 to dorsal root ganglion neurons and the results of studies in preclinical models of neuropathic pain suggested that inhibitors of GluK1 may have therapeutic analgesic activity. This hypothesis received preliminary support in humans with the activity of LY293558 (tezampanel, prodrug NGX424) in a clinical model of dental pain (Gilron et al., 2000). The results of preclinical studies also suggest a utility in migraine, and again this hypothesis received preliminary support in a small study with this same compound in patients suffering acute migraine (Sang et al., 2004). Preclinical data suggest additional indications for GluK1 inhibitors, including in epilepsy and neuropsychiatric conditions (Jane et al., 2009).
C. The Next Generation N-methyl-d-aspartate Receptor Antagonists
The side effect liabilities of NMDA antagonists, although potentially manageable in life-threatening situations, pose significant problems in less acute neuropsychiatric conditions. However, the complex nature of NMDA receptor modulation and block offers the opportunity for pharmacological manipulations that may nonetheless provide an advantageous therapeutic benefit-to-side effect ratio. For example, the NMDA receptor antagonist memantine has achieved approval for treatment of moderate to severe Alzheimer's disease (Reisberg et al., 2003; Tariot, 2006; Winblad et al., 2007). Memantine also benefits patients with moderate to severe vascular dementia (Möbius and Stöffler, 2003) and the dementia of Parkinson's disease (Aarsland et al., 2009), although it is not yet approved for such use. Memantine primarily improves activities of daily living and reduces caregiver burden in these severely impaired patients (Doody et al., 2004). Significantly, memantine is well tolerated at clinically used doses in healthy persons and in patients with dementia.
The tolerability profile of memantine seems paradoxical, given the cognitive disruption and poor tolerability of other NMDA receptor channel blockers such as ketamine, phencyclidine, and MK-801 (Schmitt, 2005; Muir, 2006; Wolff and Winstock, 2006). However, memantine differs mechanistically from potent first generation channel blockers in its lower affinity, faster dissociation kinetics, the distinct mechanism by which it becomes trapped in the channel (Parsons et al., 1999; Danysz et al., 2000; Chen and Lipton, 2006; Kotermanski et al., 2009; Kotermanski and Johnson, 2009) (see section V.F), and its potent blocking action of α7-nicotinic receptors (Aracava et al., 2005). These properties may allow memantine to effectively re-establish activity-dependent NMDA receptor channel block disrupted by the pathological conditions of the Alzheimer brain (Danysz et al., 2000). It has been suggested that the affinity, kinetics, and mechanism of memantine block at the NMDA channel are such that the compound preferentially blocks aberrantly activated NMDA receptors that contribute to pathology but spares physiological levels of activity essential for tolerability (Chen and Lipton, 2006). Memantine may preferentially inhibit extrasynaptic NMDA receptors involved in triggering toxic signaling cascades (Léveillé et al., 2008). Emerging literature suggests NMDA receptor inhibition paradoxically augments the activity of glutamatergic pyramidal neurons through inhibition of GABAergic interneurons (Krystal et al., 2002). Whereas this “hyperglutamatergic” state results in cognitive and behavioral disruption in healthy persons who are administered high-affinity channel blockers, it possible that such an effect is beneficial in boosting the activity of underactive circuitry in mid- to late-stage Alzheimer's disease. It remains to be established whether memantine has longer-term effects on the neurodegenerative process (Chen and Lipton, 2006).
Memantine demonstrates that clinical success can be achieved with NMDA antagonists of unique pharmacological characteristics. Another class of compounds that has gained attention as having such potential is the GluN2B subunit-selective NMDA receptor antagonists (Chazot, 2004; Gogas, 2006; Mony et al., 2009), for which a rich pharmacology exists (Chenard and Menniti, 1999; Nikam and Meltzer, 2002; McCauley, 2005; Layton et al., 2006). By blocking only one NMDA receptor subtype, these compounds could have therapeutic benefits but minimize on-target side effects. GluN2B-selective antagonists act as negative modulators, and a small degree (∼10%) of receptor function remains even at saturating concentrations, which may also limit undesirable effects. GluN2B-selective antagonists have efficacy in a wide variety of preclinical models (Chizh et al., 2001; Chazot, 2004; Gogas, 2006; Mony et al., 2009; Wu and Zhuo, 2009). It is noteworthy that GluN2B antagonists do not cause behavioral disruption in preclinical species, suggesting that specifically targeting the GluN2B subunit may capture the efficacy afforded by pan-NMDA receptor inhibition and retain good tolerability. Clinical data in support of this hypothesis has emerged from recent studies with CP-101,606 (see section X.D). Although the clinical study of CP-101,606 has ended, several new GluN2B antagonists, including MK-0657 and radiprodil, are under consideration for clinical development.
D. N-Methyl-d-aspartate Antagonists
1. Neuropathic Pain.
Extensive preclinical literature indicates that NMDA receptor inhibition reduces or prevents the development of neuropathic pain (Petrenko et al., 2003). These data suggest that hyperalgesic states arise from NMDA receptor-dependent maladaptive plasticity in neuronal pain pathways (Woolf and Thompson, 1991; Woolf and Salter, 2000). Clinical support for this hypothesis is most clearly demonstrated for the channel blocker ketamine in short-term treatment of pain resulting from surgery, cancer, peripheral nerve disease, and spinal cord injury (Hocking and Cousins, 2003; Visser and Schug, 2006; Bell, 2009). However, psychotomimetic effects and a relatively short half-life relegate ketamine to second- or third-tier treatment (Hocking and Cousins, 2003). Cardiovascular side effects also have impeded the development of a newer high-affinity channel blocker, N-(2-chloro-5-(methylmercapto)phenyl)-N′-methylguanidine monohydrochloride (CNS 5161) (Forst et al., 2007). In search of better tolerability, lower affinity channel blockers have been investigated but with mixed results. Clinical studies of dextromethorphan, which is metabolized to the more potent antagonist dextrorphan (Wong et al., 1988), suggest efficacy for short-term treatment of postoperative pain, phantom limb pain, and diabetic neuropathy but not postherpetic neuralgia (Ben-Abraham and Weinbroum, 2000; Weinbroum et al., 2000, 2001, 2002, 2003, 2004; Sang et al., 2002; Ben Abraham et al., 2003; Siu and Drachtman, 2007). Memantine has modest or no efficacy for diabetic neuropathy and postherpetic neuralgia (Sang et al., 2002), pain after surgery (Nikolajsen et al., 2000), and phantom limb pain (Maier et al., 2003). Although positive results have been reported for complex regional pain syndrome (Sinis et al., 2007), more studies are needed (Rogers et al., 2009). Taken together, these clinical data suggest that NMDA receptor channel block may provide efficacy for the short-term treatment of neuropathic pain and reduce the development of hyperalgesic states, although higher affinity channel block seems to correlate with clinical efficacy, and treatments with channel blockers may be limited by side effects.
Preclinical pharmacological (Taniguchi et al., 1997; Boyce et al., 1999; Suetake-Koga et al., 2006) and genetic (Wei et al., 2001; Tan et al., 2005) studies indicate that GluN2B subunit-containing NMDA receptors may be specifically targeted to treat neuropathic pain (Chizh et al., 2001; Wu and Zhuo, 2009). Prompted by these data, the analgesic efficacy of a single dose of CP-101,606 was tested in a small number of patients suffering from pain due to spinal cord injury and monoradiculopathy, and a clinically meaningful reduction in reported pain was observed (Sang et al., 2003). CP-101,606 infusion was associated with cognitive adverse events, although these were insufficiently severe to halt infusions. Further study of dose response relationships for GluN2B antagonists in a variety of neuropathic pain conditions is needed to determine whether an acceptable therapeutic index can be achieved.
There also has been long-standing interest in the use of NMDA antagonists to modify the analgesic response to opiates and to block tolerance (Wiesenfeld-Hallin, 1998; Mao, 1999; Ko et al., 2008). Clinical support for such effects was evident in a meta-analysis of 40 small clinical trials (McCartney et al., 2004). Large scale clinical study of this concept has been conducted around Morphidex, a combination of morphine and dextromethorphan (Caruso and Goldblum, 2000). However, this combination did not prove to be advantageous over morphine in a series of late-stage clinical trials (Galer et al., 2005). Further work is needed to realize the benefit in this concept.
2. Major Depression.
Major depression is the most prevalent neuropsychiatric disorder and a major cause of disability (Kessler et al., 2003). Multiple classes of compounds are used to treat this disorder (Hansen et al., 2005b; Gartlehner et al., 2008; Cipriani et al., 2009). However, all treatments show poor efficacy in as many as half of the patients (Insel, 2006; Trivedi et al., 2006), and require multiple weeks of treatment before a substantial benefit is realized (Anderson et al., 2000; Mitchell, 2006). Recent clinical data suggest that short-term NMDA receptor inhibition may address these shortcomings.
Skolnick and coworkers (Paul and Skolnick, 2003; Skolnick et al., 2009) first suggested that NMDA antagonists may have antidepressant effects. Clinical proof-of-concept was established by Berman et al. (2000) in a small study using ketamine. Forty-minute infusion of ketamine produced clear antidepressant efficacy within 2 h, and this effect was maintained for 3 days. A similar effect was reported in treatment-resistant patients (Zarate et al., 2006a; Mathew et al., 2009). Furthermore, the prolonged antidepressant effect of brief ketamine infusion was sustained with repeated dosing when the drug was administered every 2 days over a 12-day period (aan het Rot et al., 2010). In these studies, ketamine infusion was accompanied by psychotomimetic effects. However, these were confined to the infusion period, in contrast to the antidepressant effects, which endured for days. By contrast, memantine failed to demonstrate antidepressant activity when administered at the well tolerated doses used to treat Alzheimer's disease (Zarate et al., 2006b).
More recently, the antidepressant effects of CP-101, 606 were investigated in treatment-resistant patients (Preskorn et al., 2008). In that trial, a small number of patients received a high dose of drug, and several suffered dissociative effects. When the dose was reduced, so were the incidence and severity of dissociative effects. Analysis of the combined dose groups indicated an antidepressant effect that was maintained in some patients for as long as 30 days. It is noteworthy that approximately half of the patients that responded to treatment experienced no dissociative episodes during drug infusion.
These clinical studies suggest that NMDA antagonists may provide antidepressant efficacy that is robust and of rapid onset in treatment-resistant patients. On the basis of the Preskorn et al. (2008) study with CP-101,606, it seems that antidepressant effects can be realized in the absence of significant psychotomimetic effects, at least with GluN2B antagonists. These results also indicate that episodic dosing is sufficient to provide long-lasting efficacy, which in some ways mirrors effects of electroconvulsive shock therapy (Fink and Taylor, 2007) and sleep deprivation (Giedke and Schwärzler, 2002). This raises the interesting possibility that the antidepressant effects observed with these latter treatments may stem from brief inhibition of NMDA receptors. Clinical trials in depression with the glycine-site partial agonists and the mixed GluN2A and GluN2B antagonists are ongoing.
3. Parkinson's Disease.
The glutamatergic system has long been considered a target for the treatment of Parkinson's disease (Greenamyre and O'Brien, 1991). NMDA receptor antagonists have multiple advantageous effects in animal models of Parkinson's disease, including on primary parkinsonian symptoms and on dopamine agonist-induced side effects (Hallett and Standaert, 2004). However, the clinical translation of these findings has been mixed. The low-affinity NMDA antagonist amantadine (Table 15) has been used as an adjunct to l-DOPA therapy, producing a modest potentiation of the antiparkinsonian effects and reducing dyskinesias (Crosby et al., 2003a,b). Memantine seems to have similar effects against primary symptoms but not for dyskinesias (Rabey et al., 1992). However, a meta-analysis found insufficient clinical data to definitively establish the therapeutic value of either compound (Lang and Lees, 2002). Remacemide, another low-affinity channel blocker (Table 15), demonstrated a trend toward improvement in primary motor symptoms in patients treated with l-DOPA in a 279 patient phase II trial (Shoulson et al., 2001). However, the results were insufficiently robust to underwrite further development. Recent phase II studies of GluN2B antagonists also provide mixed results. MK-0657, administered as a single dose in the absence of a dopamine agonist, failed to improve primary motor symptoms in Parkinson's patients (Addy et al., 2009). Infusion of CP-101,606 alone at two different rates (before initiating a coinfusion with l-DOPA) provided no effect on primary motor symptoms (Nutt et al., 2008), but was associated with a decrease in l-DOPA-induced dyskinesias. It is noteworthy that although both infusion rates had equivalent efficacy against dyskinesias, there was a clear dose response with regard to dissociative effects, suggesting that it may be possible to separate efficacy from side effects.
E. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid and Kainate Receptor Potentiation
Considerable therapeutic potential is associated with augmentation of synaptic AMPA receptor activity (Lynch and Gall, 2006; Lynch, 2006), and a diverse class of compounds that modulate AMPA receptor deactivation and desensitization have been discovered (see section VI.A). Extensive preclinical work with these compounds suggests that therapeutic benefit may be derived from effects on synaptic network dysfunction, synaptic plasticity, and activity-dependent production of the neurotrophin BDNF (Lynch and Gall, 2006). All of these functional effects may influence learning and memory (Lynch, 2002; O'Neill and Dix, 2007). In addition, the potential up-regulation of BDNF suggests utility in the treatment of depression, Parkinson's disease, Huntington's disease (O'Neill and Witkin, 2007; Simmons et al., 2009), and possibly neurodegenerative conditions (Destot-Wong et al., 2009).
The compound for which there is the most clinical data is CX516 (Danysz, 2002; Lynch, 2006), a low-affinity AMPA receptor modulator that primarily slows receptor deactivation (see section VI). Initial clinical studies in aged healthy volunteers found CX516 facilitates recognition memory performance (Ingvar et al., 1997; Lynch et al., 1997). This compound also facilitated attention and reduced memory deficits in schizophrenic patients taking clozapine (Goff et al., 2001), although it was without dramatic effect when used as sole treatment (Marenco et al., 2002). Unfortunately, the results of larger studies of patients with schizophrenia showed no effect of CX516 on cognitive dysfunction (Goff et al., 2008b). CX516 was without effect on cognitive dysfunction in patients with Fragile X syndrome (Berry-Kravis et al., 2006). CX717 is a second generation AMPA receptor potentiator with improved potency and pharmaceutical properties. Nonhuman primate and human studies suggest a beneficial effect on decreased vigilance and cognitive function caused by sleep deprivation. The compound failed to have an effect in a phase II study simulating shift work (Wesensten et al., 2007). Another AMPA potentiator of the same class, farampator, showed acute effects on short term memory in a preliminary study in aged healthy human volunteers (Wezenberg et al., 2007).
Another class of AMPA receptor modulators is represented by LY451395 (Chappell et al., 2007), which slows both desensitization and deactivation and has the potential to more robustly potentiate AMPA receptor responses. To date, LY451395 has been tested in a phase II trial for efficacy in patients with mild to moderate Alzheimer's disease but failed to improve cognitive function assessed by ADAS-Cog (Chappell et al., 2007). S18986 (Desos et al., 1996) represents a class of compounds with impact on AMPA receptor function intermediate between that of CX516 and LY451395. S18986, and N-((3R,4S)-3-(4-(5-cyanothiophen-2-yl)phenyl)-tetrahydro-2H-pyran-4-yl)propane-2-sulfonamide (PF-4778574) are all under consideration for clinical development.
F. N-Methyl-d-aspartate Receptor Potentiation
The majority of pharmaceutical development related to NMDA receptors has focused on antagonists. However, there is substantial therapeutic potential in augmentation of NMDA receptor activity (Lisman et al., 2008). Indeed, overexpression of some NMDA receptor subunits (GluN2B) can enhance learning and memory in model systems (Tang et al., 1999, 2001; Cao et al., 2007). As with antagonists, the complexity of these receptors offers opportunities for pharmacological manipulation in ways that may provide a therapeutic benefit-to-side effect ratio. The recognition that NMDA inhibitor-induced behavioral effects closely mimic the symptoms of schizophrenia (Luby et al., 1959; Javitt and Zukin, 1991) engendered the hypothesis that NMDA receptor dysfunction may be a causative factor in the disease (Olney et al., 1999; Krystal et al., 2002; Tsai and Coyle, 2002; Yamada et al., 2005; Javitt, 2007; Morita et al., 2007). This hypothesis directly led to the idea that NMDA receptor potentiation may have therapeutic benefit (Heresco-Levy, 2000), a strategy explored in clinical trials of agonists at the glycine site on the NMDA receptor (Coyle and Tsai, 2004; Shim et al., 2008; Labrie and Roder, 2010). A meta-analysis of seven small studies of glycine and d-serine as adjuncts to first-line therapy antipsychotics found evidence for a moderate reduction of negative symptoms and a trend toward a decrease in cognitive symptoms but no evidence for a beneficial effect on positive symptoms (Tuominen et al., 2005). A recent clinical trial indicated a beneficial effect of the glycine site agonist d-alanine on positive and negative symptoms (Tsai et al., 2006). The effect of glycine was examined in a larger patient population in the CONSIST trial (Buchanan et al., 2007). Surprisingly, there was no efficacy against any symptom domain. The majority of patients enrolled in CONSIST were taking second-generation antipsychotic medications, and this may account for the failure to replicate the earlier positive findings.
d-Cycloserine, an antibiotic and glycine site ligand, was the first synthetic compound examined for augmentation of primary antipsychotic therapy. This compound is a partial agonist at the glycine site and may preferentially activate NMDA receptors containing the GluN2C subunit (Sheinin et al., 2001; Dravid et al., 2010) expressed on interneurons and cerebellar granule cells (Monyer et al., 1994). Initial clinical studies of d-cycloserine indicated a beneficial effect on negative symptoms over a narrow dose range (Goff et al., 1999). However, in meta-analysis of five trials, evidence of benefit was not supported (Tuominen et al., 2005). d-Cycloserine was also examined in the CONSIST trial, and no evidence of efficacy was found (Buchanan et al., 2007). However, the preclinical data suggest the possibility of tachyphylaxis to glycine site ligands, leading to examination of the effects of d-cycloserine intermittently dosed, which improved negative symptoms compared with placebo in patients suffering schizophrenia (Goff et al., 2008a).
d-Cycloserine has been studied as an adjunct to behavioral therapy to promote the extinction of maladaptive associations. This therapy is based on the hypothesis that d-cycloserine will augment therapy-directed learning through potentiation of NMDA receptor-dependent learning. In clinical trials, d-cycloserine increased the efficacy of behavioral therapy in patients suffering acrophobia (Ressler et al., 2004), social anxiety disorder (Hofmann et al., 2006b), and obsessive compulsive disorder (Kushner et al., 2007; Wilhelm et al., 2008). In a meta-analysis of the preclinical and clinical data, Norberg et al. (2008) stress that the timing of d-cycloserine treatment relative to the period of learning consolidation is a key factor in attaining efficacy, in terms of both promoting learning consolidation and avoiding tachyphylaxis.
The work summarized above evidences both considerable progress and remaining hurdles in developing the therapeutic potential of NMDA receptor augmentation. The first and most obvious hurdle is the need for improved pharmaceutical agents. Problems inherent in the use of glycine, including the very high dose (60 g/day), poor brain penetration, and poor tolerability, make it difficult to use for detailed hypothesis testing. d-Serine and d-alanine suffer the same issues, albeit to a lesser degree. Fortunately, there are two new molecular targets being pursued that offer unique pharmacology and the promise of more attractive small molecules. The more advanced target is the inhibition of glycine reuptake by GlyT1 in the perisynaptic region (Javitt, 2008, 2009). Preclinical studies indicate GlyT1 inhibitors augment NMDA receptor-dependent processes in vitro and in vivo (Sur and Kinney, 2007; Bridges et al., 2008; Yang and Svensson, 2008). Clinical proof of concept has been obtained with sarcosine, a naturally occurring intermediate in glycine metabolism that is a low-affinity GlyT1 inhibitor (Zhang et al., 2009a,b). Sarcosine improves positive and negative symptoms in schizophrenic patients stabilized on antipsychotic medication (Tsai et al., 2004, see also Lane et al., 2006) or those suffering an acute exacerbation of symptoms (Lane et al., 2005), although it is only weakly efficacious in the absence of antipsychotic medication (Lane et al., 2008). There are now several synthetic GlyT1 inhibitors in early clinical development, including N-methyl-N-(6-methoxy-1-phenyl-1,2,3,4-tetrahydronaphthalen-2-ylmethyl)aminom ethylcarboxylic acid (SCH 900435 or Org 25935), and 1-methyl-1H-imidazole-4-carboxylic acid (3-chloro-4-fluoro-benzyl)-(3-methyl-3-aza-bicyclo[3.1.0]hex-6-yl methyl)-amide (PF-03463275). A second new approach to augmenting NMDA receptor activity targets d-serine metabolism. In the last decade, d-serine has been recognized as a principal physiological coagonist at the glycine binding site of the NMDA receptor (Mothet et al., 2000; Wolosker, 2007). In brain, d-serine is degraded by d-amino acid oxidase (DAAO) (Mothet et al., 2000; Wang and Zhu, 2003), and there is genetic evidence for an involvement of DAAO in schizophrenia (Chumakov et al., 2002, but see Williams, 2009). DAAO inhibitors may increase levels of endogenous d-amino acids or block the metabolism of exogenously administered compounds (Horio et al., 2009; Smith et al., 2009).
Despite the positive findings noted above, the fact remains that efficacy with glycine and related analogs in schizophrenia has been modest and primarily confined to negative symptoms. This is in contrast to the dramatic efficacy by which NMDA antagonists recapitulate positive, negative, and cognitive symptom domains. Improved efficacy may come with better pharmaceutical tools and better understanding of optimal dosing intervals. Nonetheless, the clinical data may also be read to suggest limited efficacy through targeting the glycine site. The physiological underpinnings of this limit may stem from the relative affinities of different GluN2-containing receptors for glycine. Specifically, the potency for glycine is higher for GluN2B-, GluN2C-, and GluN2D- compared with the GluN2A-containing receptors (see section V). Thus, if GluN2A were a key target, glycine therapy might be expected to yield only modest results. It is noteworthy that the clinical finding that the GluN2B selective antagonist CP-101,606 produces dissociative effects, at least at high doses, suggests that GluN2B subunit-containing receptors could be an important target. Several preclinical studies of mice overexpressing the GluN2B subunit suggest that GluN2B-selective potentiators may indeed have procognitive efficacy (Tang et al., 2001; Cao et al., 2007). More data on GluN2-selective potentiation could provide needed clarity on the therapeutic potential for NMDA receptor augmentation in schizophrenia.
XI. Conclusions
Without question, the field of glutamate receptors has entered a new, structural era. Crystallographic data sets have created new opportunities to design functional studies from a perspective that previously was largely conjecture. Such studies promise to achieve a new level of understanding on how glutamate receptors link agonist binding to channel gating and will aide in defining how channel activation is controlled by receptor subtypes, stoichiometry, post-translational modifications, and protein-protein/protein-lipid interactions. These studies are essential because the gating machinery at all glutamate receptors represents a promising target for modulating neuronal and glial function for therapeutic gain in an unusually wide range of neurological diseases. Emerging clarity in how cells regulate synaptic glutamate receptor function and control localization provide further understanding that aids in elucidating the role of glutamate receptors in normal functions such as learning and memory as well as in disease. Progress toward the initial promise of new glutamate receptor-based clinical agents is being made despite early setbacks. This progress is accompanied by the very real prospect of breakthrough medicines that expand treatment options for many patients. Indeed, rather than winding down as newer topics compete for investigator interest, glutamate receptor biology is instead poised for an increase in interest and activity in the coming decade, driven by emerging structural concepts.
Acknowledgments.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants NS065371, NS036654, NS068464 (all to S.F.T.), NS036604 (to R.D.)]; the National Institutes of Health National Institute of Mental Health [Grant MH066892 (to L.P.W.); the National Institutes of Health National Eye Institute [Grant EY01697905 (to L.P.W.); the National Institutes of Health National Institute of Child Health & Human Development [Intramural Research Award (to C.J.M.)]; the National Institutes of Health National Institute of General Medical Sciences [Grant T32-GM008602 (to K.K.O.)]; the National Institutes of Health National Institute on Drug Abuse [Grant T32-DA01504006 (to K.M.V.)]; the National Institutes of Health National Institute of Environmental Health Sciences [Grant T32-ES012870 (to K.M.V.)]; the Michael J. Fox Foundation (to S.F.T.); the Lundbeck Foundation (to K.B.H.); and the Villum Kann Rasmussen Foundation (to K.B.H.). We are grateful to Stefka Gyoneva, Randy Hall, Richard Huganir, Meag Jenkins, Jon Johnson, John Isaac, Mark Mayer, and Ken Pelkey for providing critical comments on portions of the manuscript, as well as the reviewers for their painstaking and careful reading of the manuscript. We also thank Drs. Howe, Furukawa, Mott, Rosconi, Sobolevsky, Gouaux, and others for sharing unpublished data and Polina Lyuboslavsky and Kimberly Vellano for excellent technical assistance.
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.109.002451.
- 5,7-DCKA
- 5,7-dichlorokynurenic acid
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP1
- activator protein-1
- ATD
- amino-terminal domain
- ATPA
- 2-amino-3-(5-tert-butyl-3-hydroxyisoxazol-4-yl)propionic acid
- ATPO
- (R,S)-2-amino-3-[5-tert-butyl-3-(phosphonomethoxy)-4-isoxazolyl] propionic acid
- BDNF
- brain-derived neurotrophic factor
- bp
- base pair(s)
- CA1
- cornu ammonis 1
- CamKII
- Ca2+/calmodulin-dependent protein kinase II
- CASK
- calcium/calmodulin-dependent serine protein kinase
- ChIP
- chromatin immunoprecipitation
- CI-1041
- besonprodil
- CNIH
- protein cornichon homolog
- CNQX
- 6-cyano-2,3-dihydroxy-7-nitroquinoxaline
- CNS
- central nervous system
- CNS 5161
- N-(2-chloro-5-(methylmercapto)phenyl)-N′-methylguanidine monohydrochloride
- con A
- concanavalin A
- COUP-TF
- chicken ovalbumin upstream promoter transcription factor
- COX
- cytochrome oxidase
- CP-101,606
- traxoprodil mesylate
- CP-465,022
- (S)-3-(2-Chlorophenyl)-2-[2-(6-diethylaminomethyl-pyridin-2-yl)-vinyl]-6- fluoro-3H-quinazolin-4-one
- CPEB
- cytoplasmic polyadenylation element binding protein
- CRE
- cAMP response element
- CREST
- calcium-responsive transactivator
- CTD
- carboxyl-terminal domain
- CX516
- ampalex
- CX546
- 1-(1,4-benzodioxan-6-ylcarbonyl)piperidine
- CX614
- 2H,3H,6aH-pyrrolidino(2′,1′-3′,2′)1,3-oxazino(6′,5′-5,4)benzo(e)1,4-dioxan-10-one
- DAAO
- d-amino acid oxidase
- DNQX
- 6,7-dinitroquinoxaline-2,3-dione
- EPSP
- excitatory postsynaptic potential
- ER
- endoplasmic reticulum
- ERK
- extracellular signal-regulated kinases
- GRIP
- glutamate receptor interacting protein
- GV150526
- gavestinel
- GYKI 53773
- talampanel
- GYKI-52466
- benzenamine
- GYKI-53655
- 1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine
- HDAC
- histone deacetylase
- hERG
- human ether-à-go-go–related gene
- HEK
- human embryonic kidney
- HIBO
- homoibotenic acid
- IDRA-21
- 7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine-S,S-dioxide
- IEM-1460
- 1-trimethylammonio-5-(1-adamantane-methylammoniopentane dibromide)
- kb
- kilobase(s)
- LBD
- ligand-binding domain
- LTD
- long-term depression
- LTP
- long-term potentiation
- LY293558
- tezampanel
- LY300164
- talampanel
- LY339434
- (2S,4R,6E)-2-amino-4-carboxy-7-(2-naphthyl)hept-6-enoic acid
- LY382884
- (3S,4aR,6S,8aR)-6-((4-carboxyphenyl)methyl)-1,2,3,4,4a,5,6,7,8,8a-decahydroisoquinoline-3-carboxylic acid
- LY392098
- N-(2-(4-(thiophen-3-yl)phenyl)propyl)propane-2-sulfonamide
- LY404187
- N-[2-(4′-cyanobiphenyl-4-yl)propyl]propane-2-sulfonamide
- LY450108
- (R)-2-(4-(3,5-difluorobenzoylamino)phenyl)-1-(2-propanesulfonamido)-propane
- LY451395
- N-((2-(4′-(2-(methylsulfonyl)amino)ethyl)(1,1′-biphenyl)-4-yl)propyl)-2-propanesulfonamide
- LY466195
- 6-((2-carboxy-4,4-difluoro-1-pyrrolidinyl)methyl)decahydro-3-isoquinolinecarboxylic acid
- LY503430
- (R)-4′-(1-fluoro-1-methyl-2-(propane-2-sulfonylamino)-ethyl)-biphenyl-4-carboxylic acid methylamide
- MD
- molecular dynamics
- mEPSC
- miniature excitatory postsynaptic current
- MK-0657
- (3S,4R)-4-methylbenzyl 3-fluoro-4-((pyrimidin-2-ylamino)methyl)piperidine-1-carboxylate
- MK-801
- dizocilpine maleate
- MSVIII-19
- 8,9-dideoxyneodysiherbaine
- NBQX
- 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(f)quinoxaline
- NFκB
- nuclear factor κB
- NMDA
- N-methyl-d-aspartate
- NRF-1
- nuclear respiratory factor 1
- NRSE
- neuron restrictive silencer element
- NRSF
- neuron restrictive silencing factor
- NS1209
- 8-methyl-5-(4-(N,N-dimethylsulfamoyl)phenyl)-6,7,8,9,-tetrahydro-1H-pyrrolo(3,2-h)-isoquinoline-2,3-dione-3-O-(4-hydroxybutyric acid-2-yl)oxime
- NS-3763
- 4,6-bis(benzoylamino)-1,3-benzenedicarboxylic acid
- NVP-AAM077
- (R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquinoxalin-5-yl)-methyl-phosphonic acid
- NGX424
- tezampanel
- Org 25935
- N-methyl-N-(6-methoxy-1-phenyl-1,2,3,4-tetrahydronaphthalen-2-ylmethyl)aminomethylcarboxylic acid
- PDZ
- postsynaptic density 95/disc-large/zona occludens
- PEPA
- 4-(2-(phenylsulfonylamino)ethylthio)-2,6-difluoro-phenoxyacetamide
- PF-03463275
- 1-methyl-1H-imidazole-4-carboxylic acid (3-chloro-4-fluoro-benzyl)-(3-methyl-3-aza-bicyclo[3.1.0]hex-6-ylmethyl)-amide
- PF-4778574
- N-((3R,4S)-3-(4-(5-cyanothiophen-2-yl)phenyl)-tetrahydro-2H-pyran-4-yl)propane-2-sulfonamide
- PhTX
- philanthotoxin
- PKA
- protein kinase A
- PKC
- protein kinase C
- RE1
- restriction element-1
- REST
- RE-1 silencing transcription factor
- Ro 25-6981
- α-(4-hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1-piperidine propanol
- Ro 63-1908
- 1-[2-(4-hydroxy-phenoxy)-ethyl]-4-(4-methyl-benzyl)-piperidin-4-ol
- S18986
- (S)-2,3-dihydro-(3,4)cyclopentano-1,2,4-benzothiadiazine-1,1-dioxide
- SCH 900435
- N-methyl-N-(6-methoxy-1-phenyl-1,2,3,4-tetrahydronaphthalen-2-ylmethyl)aminomethylcarboxylic acid
- SN50
- Ala-Ala-Val-Ala-Leu-Leu-Pro-Ala-Val-Leu-Leu-Ala-Leu-Leu-Ala-Pro-Val-Gln-Arg-Lys-Arg-Gln-Lys-Leu-Met-Pro
- Sp1
- specific transcription factor 1
- SYM2081
- (2S,4R)-4-methylglutamic acid
- TARP
- transmembrane AMPA receptor regulatory proteins
- TBI
- traumatic brain injury
- Tbr-1
- T-brain-1
- TMD
- transmembrane domain
- TTX
- tetrodotoxin
- UBP141
- (2R,3S)-1-(phenanthrenyl-3-carbonyl)piperazine-2,3-dicarboxylic acid
- UBP282
- (αS)-α-amino-3-[(4-carboxyphenyl)methyl]-3,4-dihydro-2,4-dioxo-1(2H)-pyrimidinepropanoic acid
- UBP310
- (S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxythiophene-3-ylmethyl)-5-methylpyrimidine-2,4-dione
- UTR
- untranslated region
- ZK200775
- (1,2,3,4-tetrahydro-7-morpholinyl-2,3-dioxo-6-(trifluoromethyl)quinoxalin-1-yl)methylphosphonate.
References
- aan het Rot et al., 2010.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145 [DOI] [PubMed] [Google Scholar]
- Aarsland et al., 2009.Aarsland D, Ballard C, Walker Z, Bostrom F, Alves G, Kossakowski K, Leroi I, Pozo-Rodriguez F, Minthon L, Londos E. (2009) Memantine in patients with Parkinson's disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol 8:613–618 [DOI] [PubMed] [Google Scholar]
- Abbott and Regehr, 2004.Abbott LF, Regehr WG. (2004) Synaptic computation. Nature 431:796–803 [DOI] [PubMed] [Google Scholar]
- Abe et al., 2005.Abe T, Matsumura S, Katano T, Mabuchi T, Takagi K, Xu L, Yamamoto A, Hattori K, Yagi T, Watanabe M, et al. (2005) Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci 22:1445–1454 [DOI] [PubMed] [Google Scholar]
- Abele et al., 2000.Abele R, Keinanen K, Madden DR. (2000) Agonist-induced isomerization in a glutamate receptor ligand-binding domain. A kinetic and mutagenetic analysis. J Biol Chem 275:21355–21363 [DOI] [PubMed] [Google Scholar]
- Addy et al., 2009.Addy C, Assaid C, Hreniuk D, Stroh M, Xu Y, Herring WJ, Ellenbogen A, Jinnah HA, Kirby L, Leibowitz MT, et al. (2009) Single-dose administration of MK-0657, an NR2B-selective NMDA antagonist, does not result in clinically meaningful improvement in motor function in patients with moderate Parkinson's disease. J Clin Pharmacol 49:856–864 [DOI] [PubMed] [Google Scholar]
- Adesnik and Nicoll, 2007.Adesnik H, Nicoll RA. (2007) Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci 27:4598–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed et al., 2009.Ahmed AH, Thompson MD, Fenwick MK, Romero B, Loh AP, Jane DE, Sondermann H, Oswald RE. (2009) Mechanisms of antagonism of the GluR2 AMPA receptor: structure and dynamics of the complex of two willardiine antagonists with the glutamate binding domain. Biochemistry 48:3894–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman et al., 2002.Aizenman CD, Muñoz-Elías G, Cline HT. (2002) Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron 34:623–634 [DOI] [PubMed] [Google Scholar]
- Aizenman et al., 1989.Aizenman E, Lipton SA, Loring RH. (1989) Selective modulation of NMDA responses by reduction and oxidation. Neuron 2:1257–1263 [DOI] [PubMed] [Google Scholar]
- Akins and Atkinson, 2002.Akins PT, Atkinson RP. (2002) Glutamate AMPA receptor antagonist treatment for ischaemic stroke. Curr Med Res Opin 18:s9–s13 [DOI] [PubMed] [Google Scholar]
- Alam and Jiang, 2009.Alam A, Jiang Y. (2009) High-resolution structure of the open NaK channel. Nat Struct Mol Biol 16:30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers et al., 2001.Albers GW, Goldstein LB, Hall D, Lesko LMAptiganel Acute Stroke Investigators (2001) Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. JAMA 286:2673–2682 [DOI] [PubMed] [Google Scholar]
- Alt et al., 2004.Alt A, Weiss B, Ogden AM, Knauss JL, Oler J, Ho K, Large TH, Bleakman D. (2004) Pharmacological characterization of glutamatergic agonists and antagonists at recombinant human homomeric and heteromeric kainate receptors in vitro. Neuropharmacology 46:793–806 [DOI] [PubMed] [Google Scholar]
- Andersen et al., 1996.Andersen PH, Tygesen CK, Rasmussen JS, Søegaard-Nielsen L, Hansen A, Hansen K, Kiemer A, Stidsen CE. (1996) Stable expression of homomeric AMPA-selective glutamate receptors in BHK cells. Eur J Pharmacol 311:95–100 [DOI] [PubMed] [Google Scholar]
- Anderson et al., 2000.Anderson IM, Nutt DJ, Deakin JF. (2000) Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 1993 British Association for Psychopharmacology guidelines. British Association for Psychopharmacology. J Psychopharmacol 14:3–20 [DOI] [PubMed] [Google Scholar]
- Andersson et al., 2001.Andersson O, Stenqvist A, Attersand A, von Euler G. (2001) Nucleotide sequence, genomic organization, and chromosomal localization of genes encoding the human NMDA receptor subunits NR3A and NR3B. Genomics 78:178–184 [DOI] [PubMed] [Google Scholar]
- Anson et al., 1998.Anson LC, Chen PE, Wyllie DJ, Colquhoun D, Schoepfer R. (1998) Identification of amino acid residues of the NR2A subunit that control glutamate potency in recombinant NR1/NR2A NMDA receptors. J Neurosci 18:581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov and Johnson, 1996.Antonov SM, Johnson JW. (1996) Voltage-dependent interaction of open-channel blocking molecules with gating of NMDA receptors in rat cortical neurons. J Physiol (Lond) 493:425–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov and Johnson, 1999.Antonov SM, Johnson JW. (1999) Permeant ion regulation of N-methyl-d-aspartate receptor channel block by Mg2+. Proc Natl Acad Sci USA 96:14571–14576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov et al., 1998.Antonov SM, Gmiro VE, Johnson JW. (1998) Binding sites for permeant ions in the channel of NMDA receptors and their effects on channel block. Nat Neurosci 1:451–461 [DOI] [PubMed] [Google Scholar]
- Aracava et al., 2005.Aracava Y, Pereira EF, Maelicke A, Albuquerque EX. (2005) Memantine blocks α7* nicotinic acetylcholine receptors more potently than N-methyl-d-aspartate receptors in rat hippocampal neurons. J Pharmacol Exp Ther 312:1195–1205 [DOI] [PubMed] [Google Scholar]
- Arai et al., 1996.Arai A, Kessler M, Ambros-Ingerson J, Quan A, Yigiter E, Rogers G, Lynch G. (1996) Effects of a centrally active benzoylpyrrolidine drug on AMPA receptor kinetics. Neuroscience 75:573–585 [DOI] [PubMed] [Google Scholar]
- Arai et al., 1994.Arai A, Kessler M, Xiao P, Ambros-Ingerson J, Rogers G, Lynch G. (1994) A centrally active drug that modulates AMPA receptor gated currents. Brain Res 638:343–346 [DOI] [PubMed] [Google Scholar]
- Arai and Kessler, 2007.Arai AC, Kessler M. (2007) Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets 8:583–602 [DOI] [PubMed] [Google Scholar]
- Arai et al., 2000.Arai AC, Kessler M, Rogers G, Lynch G. (2000) Effects of the potent ampakine CX614 on hippocampal and recombinant AMPA receptors: interactions with cyclothiazide and GYKI 52466. Mol Pharmacol 58:802–813 [DOI] [PubMed] [Google Scholar]
- Arai et al., 2002.Arai AC, Xia YF, Rogers G, Lynch G, Kessler M. (2002) Benzamide-type AMPA receptor modulators form two subfamilies with distinct modes of action. J Pharmacol Exp Ther 303:1075–1085 [DOI] [PubMed] [Google Scholar]
- Araújo et al., 2005.Araújo IM, Xapelli S, Gil JM, Mohapel P, Petersén A, Pinheiro PS, Malva JO, Bahr BA, Brundin P, Carvalho CM. (2005) Proteolysis of NR2B by calpain in the hippocampus of epileptic rats. Neuroreport 16:393–396 [DOI] [PubMed] [Google Scholar]
- Arinaminpathy et al., 2002.Arinaminpathy Y, Sansom MS, Biggin PC. (2002) Molecular dynamics simulations of the ligand-binding domain of the ionotropic glutamate receptor GluR2. Biophys J 82:676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong and Gouaux, 2000.Armstrong N, Gouaux E. (2000) Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron 28:165–181 [DOI] [PubMed] [Google Scholar]
- Armstrong et al., 2006.Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E. (2006) Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell 127:85–97 [DOI] [PubMed] [Google Scholar]
- Armstrong et al., 2003.Armstrong N, Mayer M, Gouaux E. (2003) Tuning activation of the AMPA-sensitive GluR2 ion channel by genetic adjustment of agonist-induced conformational changes. Proc Natl Acad Sci USA 100:5736–5741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong et al., 1998.Armstrong N, Sun Y, Chen GQ, Gouaux E. (1998) Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature 395:913–917 [DOI] [PubMed] [Google Scholar]
- Ascher et al., 1988.Ascher P, Bregestovski P, Nowak L. (1988) N-methyl-d-aspartate-activated channels of mouse central neurones in magnesium-free solutions. J Physiol 399:207–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher and Nowak, 1988.Ascher P, Nowak L. (1988) The role of divalent cations in the N-methyl-d-aspartate responses of mouse central neurones in culture. J Physiol (Lond) 399:247–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby et al., 2008.Ashby MC, Daw MI, Isaac JTR. (2008) AMPA receptors, in The Glutamate Receptors (Gereau RW, Swanson GT. eds) pp 1–44, Humana Press, Totowa, NJ [Google Scholar]
- Ataman et al., 2007.Ataman ZA, Gakhar L, Sorensen BR, Hell JW, Shea MA. (2007) The NMDA receptor NR1 C1 region bound to calmodulin: structural insights into functional differences between homologous domains. Structure 15:1603–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlason et al., 2007.Atlason PT, Garside ML, Meddows E, Whiting P, McIlhinney RA. (2007) N-methyl-d-aspartate (NMDA) receptor subunit NR1 forms the substrate for oligomeric assembly of the NMDA receptor. J Biol Chem 282:25299–25307 [DOI] [PubMed] [Google Scholar]
- Audinat et al., 1994.Audinat E, Lambolez B, Rossier J, Crépel F. (1994) Activity-dependent regulation of N-methyl-d-aspartate receptor subunit expression in rat cerebellar granule cells. Eur J Neurosci 6:1792–1800 [DOI] [PubMed] [Google Scholar]
- Auerbach and Zhou, 2005.Auerbach A, Zhou Y. (2005) Gating reaction mechanisms for NMDA receptor channels. J Neurosci 25:7914–7923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenet et al., 1997.Avenet P, Léonardon J, Besnard F, Graham D, Depoortere H, Scatton B. (1997) Antagonist properties of eliprodil and other NMDA receptor antagonists at rat NR1A/NR2A and NR1A/NR2B receptors expressed in Xenopus oocytes. Neurosci Lett 223:133–136 [DOI] [PubMed] [Google Scholar]
- Awobuluyi et al., 2003.Awobuluyi M, Lipton SA, Sucher NJ. (2003) Translationally distinct populations of NMDA receptor subunit NR1 mRNA in the developing rat brain. J Neurochem 87:1066–1075 [DOI] [PubMed] [Google Scholar]
- Awobuluyi et al., 2007.Awobuluyi M, Yang J, Ye Y, Chatterton JE, Godzik A, Lipton SA, Zhang D. (2007) Subunit-specific roles of glycine-binding domains in activation of NR1/NR3 N-methyl-d-aspartate receptors. Mol Pharmacol 71:112–122 [DOI] [PubMed] [Google Scholar]
- Ayalon and Stern-Bach, 2001.Ayalon G, Stern-Bach Y. (2001) Functional assembly of AMPA and kainate receptors is mediated by several discrete protein-protein interactions. Neuron 31:103–113 [DOI] [PubMed] [Google Scholar]
- Ayalon et al., 2005.Ayalon G, Segev E, Elgavish S, Stern-Bach Y. (2005) Two regions in the N-terminal domain of ionotropic glutamate receptor 3 form the subunit oligomerization interfaces that control subtype-specific receptor assembly. J Biol Chem 280:15053–15060 [DOI] [PubMed] [Google Scholar]
- Bähring et al., 1997.Bähring R, Bowie D, Benveniste M, Mayer ML. (1997) Permeation and block of rat GluR6 glutamate receptor channels by internal and external polyamines. J Physiol (Lond) 502:575–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähring and Mayer, 1998.Bähring R, Mayer ML. (1998) An analysis of philanthotoxin block for recombinant rat GluR6(Q) glutamate receptor channels. J Physiol (Lond) 509:635–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai and Kusiak, 1993.Bai G, Kusiak JW. (1993) Cloning and analysis of the 5′-flanking sequence of the rat N-methyl-d-aspartate receptor 1 (NMDAR1) gene. Biochim Biophys Acta 1152:197–200 [DOI] [PubMed] [Google Scholar]
- Bai and Kusiak, 1995.Bai G, Kusiak JW. (1995) Functional analysis of the proximal 5′-flanking region of the N-methyl-d-aspartate receptor subunit gene, NMDAR1. J Biol Chem 270:7737–7744 [DOI] [PubMed] [Google Scholar]
- Bai and Kusiak, 1997.Bai G, Kusiak JW. (1997) Nerve growth factor up-regulates the N-methyl-d-aspartate receptor subunit promoter in PC12 cells. J Biol Chem 272:5936–5942 [DOI] [PubMed] [Google Scholar]
- Bai et al., 1998.Bai G, Norton DD, Prenger MS, Kusiak JW. (1998) Single-stranded DNA-binding proteins and neuron-restrictive silencer factor participate in cell-specific transcriptional control of the NMDAR1 gene. J Biol Chem 273:1086–1091 [DOI] [PubMed] [Google Scholar]
- Bai et al., 2003.Bai G, Zhuang Z, Liu A, Chai Y, Hoffman PW. (2003) The role of the RE1 element in activation of the NR1 promoter during neuronal differentiation. J Neurochem 86:992–1005 [DOI] [PubMed] [Google Scholar]
- Bai and Wong-Riley, 2003.Bai X, Wong-Riley MT. (2003) Neuronal activity regulates protein and gene expressions of GluR2 in postnatal rat visual cortical neurons in culture. J Neurocytol 32:71–78 [DOI] [PubMed] [Google Scholar]
- Balan et al., 2009.Balan L, Foltyn VN, Zehl M, Dumin E, Dikopoltsev E, Knoh D, Ohno Y, Kihara A, Jensen ON, Radzishevsky IS, et al. (2009) Feedback inactivation of D-serine synthesis by NMDA receptor-elicited translocation of serine racemase to the membrane. Proc Natl Acad Sci USA 106:7589–7594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balannik et al., 2005.Balannik V, Menniti FS, Paternain AV, Lerma J, Stern-Bach Y. (2005) Molecular mechanism of AMPA receptor noncompetitive antagonism. Neuron 48:279–288 [DOI] [PubMed] [Google Scholar]
- Balestrino and Somjen, 1988.Balestrino M, Somjen GG. (1988) Concentration of carbon dioxide, interstitial pH and synaptic transmission in hippocampal formation of the rat. J Physiol (Lond) 396:247–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballif et al., 2008.Ballif BA, Carey GR, Sunyaev SR, Gygi SP. (2008) Large-scale identification and evolution indexing of tyrosine phosphorylation sites from murine brain. J Proteome Res 7:311–318 [DOI] [PubMed] [Google Scholar]
- Baltan, 2009.Baltan S. (2009) Ischemic injury to white matter: an age-dependent process. Neuroscientist 15:126–133 [DOI] [PubMed] [Google Scholar]
- Baltan et al., 2008.Baltan S, Besancon EF, Mbow B, Ye Z, Hamner MA, Ransom BR. (2008) White matter vulnerability to ischemic injury increases with age because of enhanced excitotoxicity. J Neurosci 28:1479–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke et al., 2000.Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. (2000) Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci 20:89–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke et al., 2005.Banke TG, Dravid SM, Traynelis SF. (2005) Protons trap NR1/NR2B NMDA receptors in a nonconducting state. J Neurosci 25:42–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke et al., 2001.Banke TG, Greenwood JR, Christensen JK, Liljefors T, Traynelis SF, Schousboe A, Pickering DS. (2001) Identification of amino acid residues in GluR1 responsible for ligand binding and desensitization. J Neurosci 21:3052–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke et al., 1997.Banke TG, Schousboe A, Pickering DS. (1997) Comparison of the agonist binding site of homomeric, heteromeric, and chimeric GluR1(o) and GluR3(o) AMPA receptors. J Neurosci Res 49:176–185 [DOI] [PubMed] [Google Scholar]
- Banke and Traynelis, 2003.Banke TG, Traynelis SF. (2003) Activation of NR1/NR2B NMDA receptors. Nat Neurosci 6:144–152 [DOI] [PubMed] [Google Scholar]
- Bannister et al., 2005.Bannister NJ, Benke TA, Mellor J, Scott H, Gürdal E, Crabtree JW, Isaac JT. (2005) Developmental changes in AMPA and kainate receptor-mediated quantal transmission at thalamocortical synapses in the barrel cortex. J Neurosci 25:5259–5271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis et al., 2008.Barberis A, Sachidhanandam S, Mulle C. (2008) GluR6/KA2 kainate receptors mediate slow-deactivating currents. J Neurosci 28:6402–6406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbon and Barlati, 2000.Barbon A, Barlati S. (2000) Genomic organization, proposed alternative splicing mechanisms, and RNA editing structure of GRIK1. Cytogenet Cell Genet 88:236–239 [DOI] [PubMed] [Google Scholar]
- Barbon et al., 2001.Barbon A, Vallini I, Barlati S. (2001) Genomic organization of the human GRIK2 gene and evidence for multiple splicing variants. Gene 274:187–197 [DOI] [PubMed] [Google Scholar]
- Barria et al., 1997.Barria A, Derkach V, Soderling T. (1997) Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem 272:32727–32730 [DOI] [PubMed] [Google Scholar]
- Barria and Malinow, 2002.Barria A, Malinow R. (2002) Subunit-specific NMDA receptor trafficking to synapses. Neuron 35:345–353 [DOI] [PubMed] [Google Scholar]
- Barria and Malinow, 2005.Barria A, Malinow R. (2005) NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48:289–301 [DOI] [PubMed] [Google Scholar]
- Barta-Szalai et al., 2004.Barta-Szalai G, Borza I, Bozó E, Kiss C, Agai B, Proszenyák A, Keseru GM, Gere A, Kolok S, Galgóczy K, et al. (2004) Oxamides as novel NR2B selective NMDA receptor antagonists. Bioorg Med Chem Lett 14:3953–3956 [DOI] [PubMed] [Google Scholar]
- Barth and Malenka, 2001.Barth AL, Malenka RC. (2001) NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci 4:235–236 [DOI] [PubMed] [Google Scholar]
- Barton and White, 2004.Barton ME, White HS. (2004) The effect of CGX-1007 and CI-1041, novel NMDA receptor antagonists, on kindling acquisition and expression. Epilepsy Res 59: 1–12 [DOI] [PubMed] [Google Scholar]
- Bass, 2002.Bass BL. (2002) RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem 71:817–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu et al., 2009.Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, et al. (2009) Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry 14:719–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bats et al., 2007.Bats C, Groc L, Choquet D. (2007) The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 53:719–734 [DOI] [PubMed] [Google Scholar]
- Baumbarger et al., 2001a.Baumbarger P, Muhlhauser M, Yang CR, Nisenbaum ES. (2001a) LY392098, a novel AMPA receptor potentiator: electrophysiological studies in prefrontal cortical neurons. Neuropharmacology 40:992–1002 [DOI] [PubMed] [Google Scholar]
- Baumbarger et al., 2001b.Baumbarger PJ, Muhlhauser M, Zhai J, Yang CR, Nisenbaum ES. (2001b) Positive modulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors in prefrontal cortical pyramidal neurons by a novel allosteric potentiator. J Pharmacol Exp Ther 298:86–102 [PubMed] [Google Scholar]
- Baumgart and Rodríguez-Crespo, 2008.Baumgart F, Rodríguez-Crespo I. (2008) d-amino acids in the brain: the biochemistry of brain serine racemase. FEBS J 275:3538–3545 [DOI] [PubMed] [Google Scholar]
- Bayer et al., 2001.Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. (2001) Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411:801–805 [DOI] [PubMed] [Google Scholar]
- Beck et al., 1999.Beck C, Wollmuth LP, Seeburg PH, Sakmann B, Kuner T. (1999) NMDAR channel segments forming the extracellular vestibule inferred from the accessibility of substituted cysteines. Neuron 22:559–570 [DOI] [PubMed] [Google Scholar]
- Bedoukian et al., 2006.Bedoukian MA, Weeks AM, Partin KM. (2006) Different domains of the AMPA receptor direct stargazin-mediated trafficking and stargazin-mediated modulation of kinetics. J Biol Chem 281:23908–23921 [DOI] [PubMed] [Google Scholar]
- Bekkers, 1993.Bekkers JM. (1993) Enhancement by histamine of NMDA-mediated synaptic transmission in the hippocampus. Science 261(5117):104–106 [DOI] [PubMed] [Google Scholar]
- Bell, 2009.Bell RF. (2009) Ketamine for chronic non-cancer pain. Pain 141:210–214 [DOI] [PubMed] [Google Scholar]
- Bellone and Nicoll, 2007.Bellone C, Nicoll RA. (2007) Rapid bidirectional switching of synaptic NMDA receptors. Neuron 55:779–785 [DOI] [PubMed] [Google Scholar]
- Ben Abraham et al., 2003.Ben Abraham R, Marouani N, Weinbroum AA. (2003) Dextromethorphan mitigates phantom pain in cancer amputees. Ann Surg Oncol 10:268–274 [DOI] [PubMed] [Google Scholar]
- Ben-Abraham and Weinbroum, 2000.Ben-Abraham R, Weinbroum AA. (2000) Dextromethorphan in chronic pain: a disappointing update. Isr Med Assoc J 2:708–710 [PubMed] [Google Scholar]
- Bendel et al., 2005.Bendel O, Meijer B, Hurd Y, von Euler G. (2005) Cloning and expression of the human NMDA receptor subunit NR3B in the adult human hippocampus. Neurosci Lett 377:31–36 [DOI] [PubMed] [Google Scholar]
- Benke et al., 1998.Benke TA, Lüthi A, Isaac JT, Collingridge GL. (1998) Modulation of AMPA receptor unitary conductance by synaptic activity. Nature 393:793–797 [DOI] [PubMed] [Google Scholar]
- Bennett and Dingledine, 1995.Bennett JA, Dingledine R. (1995) Topology profile for a glutamate receptor: three transmembrane domains and a channel-lining reentrant membrane loop. Neuron 14:373–384 [DOI] [PubMed] [Google Scholar]
- Benveniste, 1989.Benveniste H. (1989) Brain microdialysis. J Neurochem 52:1667–1679 [DOI] [PubMed] [Google Scholar]
- Benveniste et al., 1990.Benveniste M, Clements J, Vyklický L, Jr, Mayer ML. (1990) A kinetic analysis of the modulation of N-methyl-d-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol 428:333–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste and Mayer, 1995.Benveniste M, Mayer ML. (1995) Trapping of glutamate and glycine during open channel block of rat hippocampal neuron NMDA receptors by 9-aminoacridine. J Physiol 483:367–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron et al., 2005.Bergeron R, Coyle JT, Tsai G, Greene RW. (2005) NAAG reduces NMDA receptor current in CA1 hippocampal pyramidal neurons of acute slices and dissociated neurons. Neuropsychopharmacology 30:7–16 [DOI] [PubMed] [Google Scholar]
- Bergeron et al., 2007.Bergeron R, Imamura Y, Frangioni JV, Greene RW, Coyle JT. (2007) Endogenous N-acetylaspartylglutamate reduced NMDA receptor-dependent current neurotransmission in the CA1 area of the hippocampus. J Neurochem 100:346–357 [DOI] [PubMed] [Google Scholar]
- Berman et al., 2000.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354 [DOI] [PubMed] [Google Scholar]
- Berry-Kravis et al., 2006.Berry-Kravis E, Krause SE, Block SS, Guter S, Wuu J, Leurgans S, Decle P, Potanos K, Cook E, Salt J, et al. (2006) Effect of CX516, an AMPA-modulating compound, on cognition and behavior in fragile X syndrome: a controlled trial. J Child Adolesc Psychopharmacol 16:525–540 [DOI] [PubMed] [Google Scholar]
- Bi et al., 1996.Bi X, Chang V, Molnar E, McIlhinney RA, Baudry M. (1996) The C-terminal domain of glutamate receptor subunit 1 is a target for calpain-mediated proteolysis. Neuroscience 73:903–906 [DOI] [PubMed] [Google Scholar]
- Bi et al., 1998a.Bi X, Chen J, Baudry M. (1998a) Calpain-mediated proteolysis of GluR1 subunits in organotypic hippocampal cultures following kainic acid treatment. Brain Res 781:355–357 [DOI] [PubMed] [Google Scholar]
- Bi et al., 1998b.Bi X, Rong Y, Chen J, Dang S, Wang Z, Baudry M. (1998b) Calpain-mediated regulation of NMDA receptor structure and function. Brain Res 790:245–253 [DOI] [PubMed] [Google Scholar]
- Birch et al., 1988.Birch PJ, Grossman CJ, Hayes AG. (1988) Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol 154:85–87 [DOI] [PubMed] [Google Scholar]
- Bischofberger and Jonas, 2002.Bischofberger J, Jonas P. (2002) TwoB or not twoB: differential transmission at glutamatergic mossy fiber-interneuron synapses in the hippocampus. Trends Neurosci 25:600–603 [DOI] [PubMed] [Google Scholar]
- Bjerrum et al., 2003.Bjerrum EJ, Kristensen AS, Pickering DS, Greenwood JR, Nielsen B, Liljefors T, Schousboe A, Bräuner-Osborne H, Madsen U. (2003) Design, synthesis, and pharmacology of a highly subtype-selective GluR1/2 agonist, (RS)-2-amino-3-(4-chloro-3-hydroxy-5-isoxazolyl)propionic acid (Cl-HIBO). J Med Chem 46:2246–2249 [DOI] [PubMed] [Google Scholar]
- Blakemore and Trombley, 2004.Blakemore LJ, Trombley PQ. (2004) Diverse modulation of olfactory bulb AMPA receptors by zinc. Neuroreport 15:919–923 [DOI] [PubMed] [Google Scholar]
- Blanpied et al., 1997.Blanpied TA, Boeckman FA, Aizenman E, Johnson JW. (1997) Trapping channel block of NMDA-activated responses by amantadine and memantine. J Neurophysiol 77:309–323 [DOI] [PubMed] [Google Scholar]
- Blanpied et al., 2005.Blanpied TA, Clarke RJ, Johnson JW. (2005) Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J Neurosci 25:3312–3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke et al., 1993.Blaschke M, Keller BU, Rivosecchi R, Hollmann M, Heinemann S, Konnerth A. (1993) A single amino acid determines the subunit-specific spider toxin block of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptor channels. Proc Natl Acad Sci USA 90:6528–6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleakman et al., 1996.Bleakman D, Ballyk BA, Schoepp DD, Palmer AJ, Bath CP, Sharpe EF, Woolley ML, Bufton HR, Kamboj RK, Tarnawa I, et al. (1996) Activity of 2,3-benzodiazepines at native rat and recombinant human glutamate receptors in vitro: stereospecificity and selectivity profiles. Neuropharmacology 35:1689–1702 [DOI] [PubMed] [Google Scholar]
- Bleakman et al., 2002.Bleakman D, Gates MR, Ogden AM, Mackowiak M. (2002) Kainate receptor agonists, antagonists and allosteric modulators. Curr Pharm Des 8:873–885 [DOI] [PubMed] [Google Scholar]
- Bleakman and Lodge, 1998.Bleakman D, Lodge D. (1998) Neuropharmacology of AMPA and kainate receptors. Neuropharmacology 37:1187–1204 [DOI] [PubMed] [Google Scholar]
- Bleakman et al., 1999.Bleakman D, Ogden AM, Ornstein PL, Hoo K. (1999) Pharmacological characterization of a GluR6 kainate receptor in cultured hippocampal neurons. Eur J Pharmacol 378:331–337 [DOI] [PubMed] [Google Scholar]
- Bloodgood and Sabatini, 2007.Bloodgood BL, Sabatini BL. (2007) Ca2+ signaling in dendritic spines. Curr Opin Neurobiol 17:345–351 [DOI] [PubMed] [Google Scholar]
- Bloodgood and Sabatini, 2008.Bloodgood BL, Sabatini BL. (2008) Regulation of synaptic signalling by postsynaptic, non-glutamate receptor ion channels. J Physiol 586:1475–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm et al., 2006.Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. (2006) Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron 51:213–225 [DOI] [PubMed] [Google Scholar]
- Bökel et al., 2006.Bökel C, Dass S, Wilsch-Bräuninger M, Roth S. (2006) Drosophila Cornichon acts as cargo receptor for ER export of the TGFalpha-like growth factor Gurken. Development 133:459–470 [DOI] [PubMed] [Google Scholar]
- Bolshakov et al., 2003.Bolshakov KV, Gmiro VE, Tikhonov DB, Magazanik LG. (2003) Determinants of trapping block of N-methyl-d-aspartate receptor channels. J Neurochem 87:56–65 [DOI] [PubMed] [Google Scholar]
- Bolshakov et al., 2005.Bolshakov KV, Kim KH, Potapjeva NN, Gmiro VE, Tikhonov DB, Usherwood PN, Mellor IR, Magazanik LG. (2005) Design of antagonists for NMDA and AMPA receptors. Neuropharmacology 49:144–155 [DOI] [PubMed] [Google Scholar]
- Borges and Dingledine, 2001.Borges K, Dingledine R. (2001) Functional organization of the GluR1 glutamate receptor promoter. J Biol Chem 276:25929–25938 [DOI] [PubMed] [Google Scholar]
- Borges et al., 2003.Borges K, Myers SJ, Zhang S, Dingledine R. (2003) Activity of the rat GluR4 promoter in transfected cortical neurons and glia. J Neurochem 86:1162–1173 [DOI] [PubMed] [Google Scholar]
- Bortolotto et al., 1999.Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, et al. (1999) Kainate receptors are involved in synaptic plasticity. Nature 402:297–301 [DOI] [PubMed] [Google Scholar]
- Borza et al., 2007.Borza I, Bozó E, Barta-Szalai G, Kiss C, Tárkányi G, Demeter A, Gáti T, Háda V, Kolok S, Gere A, et al. (2007) Selective NR1/2B N-methyl-d-aspartate receptor antagonists among indole-2-carboxamides and benzimidazole-2-carboxamides. J Med Chem 50:901–914 [DOI] [PubMed] [Google Scholar]
- Borza et al., 2006.Borza I, Kolok S, Gere A, Nagy J, Fodor L, Galgóczy K, Fetter J, Bertha F, Agai B, Horváth C, et al. (2006) Benzimidazole-2-carboxamides as novel NR2B selective NMDA receptor antagonists. Bioorg Med Chem Lett 16:4638–4640 [DOI] [PubMed] [Google Scholar]
- Bowie, 2002.Bowie D. (2002) External anions and cations distinguish between AMPA and kainate receptor gating mechanisms. J Physiol (Lond) 539:725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie et al., 2003.Bowie D, Garcia EP, Marshall J, Traynelis SF, Lange GD. (2003) Allosteric regulation and spatial distribution of kainate receptors bound to ancillary proteins. J Physiol 547:373–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie et al., 1998.Bowie D, Lange GD, Mayer ML. (1998) Activity-dependent modulation of glutamate receptors by polyamines. J Neurosci 18:8175–8185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie and Mayer, 1995.Bowie D, Mayer ML. (1995) Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15:453–462 [DOI] [PubMed] [Google Scholar]
- Bowlby, 1993.Bowlby MR. (1993) Pregnenolone sulfate potentiation of N-methyl-d-aspartate receptor channels in hippocampal neurons. Mol Pharmacol 43:813–819 [PubMed] [Google Scholar]
- Boyce et al., 1999.Boyce S, Wyatt A, Webb JK, O'Donnell R, Mason G, Rigby M, Sirinathsinghji D, Hill RG, Rupniak NM. (1999) Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology 38:611–623 [DOI] [PubMed] [Google Scholar]
- Brackley et al., 1993.Brackley PT, Bell DR, Choi SK, Nakanishi K, Usherwood PN. (1993) Selective antagonism of native and cloned kainate and NMDA receptors by polyamine-containing toxins. J Pharmacol Exp Ther 266:1573–1580 [PubMed] [Google Scholar]
- Bradshaw et al., 2003.Bradshaw KD, Emptage NJ, Bliss TV. (2003) A role for dendritic protein synthesis in hippocampal late LTP. Eur J Neurosci 18:3150–3152 [DOI] [PubMed] [Google Scholar]
- Brauneis et al., 1996.Brauneis U, Oz M, Peoples RW, Weight FF, Zhang L. (1996) Differential sensitivity of recombinant N-methyl-d-aspartate receptor subunits to inhibition by dynorphin. J Pharmacol Exp Ther 279:1063–1068 [PubMed] [Google Scholar]
- Brehm et al., 2003.Brehm L, Greenwood JR, Hansen KB, Nielsen B, Egebjerg J, Stensbøl TB, Bräuner-Osborne H, Sløk FA, Kronborg TT, Krogsgaard-Larsen P. (2003) (S)-2-Amino-3-(3-hydroxy-7,8-dihydro-6H-cyclohepta[d]isoxazol-4-yl)propion ic acid, a potent and selective agonist at the GluR5 subtype of ionotropic glutamate receptors. Synthesis, modeling, and molecular pharmacology. J Med Chem 46:1350–1358 [DOI] [PubMed] [Google Scholar]
- Bresink et al., 1996.Bresink I, Benke TA, Collett VJ, Seal AJ, Parsons CG, Henley JM, Collingridge GL. (1996) Effects of memantine on recombinant rat NMDA receptors expressed in HEK 293 cells. Br J Pharmacol 119:195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley et al., 2003.Brickley SG, Misra C, Mok MH, Mishina M, Cull-Candy SG. (2003) NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J Neurosci 23:4958–4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges et al., 2008.Bridges TM, Williams R, Lindsley CW. (2008) Design of potent GlyT1 inhibitors: in vitro and in vivo profiles. Curr Opin Mol Ther 10:591–601 [PubMed] [Google Scholar]
- Brimecombe et al., 1997.Brimecombe JC, Boeckman FA, Aizenman E. (1997) Functional consequences of NR2 subunit composition in single recombinant N-methyl-d-aspartate receptors. Proc Natl Acad Sci USA 94:11019–11024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothwell et al., 2008.Brothwell SL, Barber JL, Monaghan DT, Jane DE, Gibb AJ, Jones S. (2008) NR2B- and NR2D-containing synaptic NMDA receptors in developing rat substantia nigra pars compacta dopaminergic neurones. J Physiol 586:739–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruening-Wright et al., 2002.Bruening-Wright A, Schumacher MA, Adelman JP, Maylie J. (2002) Localization of the activation gate for small conductance Ca2+-activated K+ channels. J Neurosci 22:6499–6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan et al., 2007.Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT. (2007) The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry 164:1593–1602 [DOI] [PubMed] [Google Scholar]
- Buller et al., 1994.Buller AL, Larson HC, Schneider BE, Beaton JA, Morrisett RA, Monaghan DT. (1994) The molecular basis of NMDA receptor subtypes: native receptor diversity is predicted by subunit composition. J Neurosci 14:5471–5484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunch et al., 2009.Bunch L, Gefflaut T, Alaux S, Sagot E, Nielsen B, Pickering DS. (2009) Pharmacological characterization of (4R)-alkyl glutamate analogues at the ionotropic glutamate receptors—focus on subtypes iGlu(5–7). Eur J Pharmacol 609:1–4 [DOI] [PubMed] [Google Scholar]
- Bunch and Krogsgaard-Larsen, 2009.Bunch L, Krogsgaard-Larsen P. (2009) Subtype selective kainic acid receptor agonists: discovery and approaches to rational design. Med Res Rev 29:3–28 [DOI] [PubMed] [Google Scholar]
- Burban et al., 2010.Burban A, Faucard R, Armand V, Bayard C, Vorobjev V, Arrang JM. (2010) Histamine potentiates N-methyl-d-aspartate receptors by interacting with an allosteric site distinct from the polyamine binding site. J Pharmacol Exp Ther 332:912–921 [DOI] [PubMed] [Google Scholar]
- Burnashev et al., 1992a.Burnashev N, Monyer H, Seeburg PH, Sakmann B. (1992a) Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron 8:189–198 [DOI] [PubMed] [Google Scholar]
- Burnashev et al., 1992b.Burnashev N, Schoepfer R, Monyer H, Ruppersberg JP, Günther W, Seeburg PH, Sakmann B. (1992b) Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science 257:1415–1419 [DOI] [PubMed] [Google Scholar]
- Burnashev et al., 1996.Burnashev N, Villarroel A, Sakmann B. (1996) Dimensions and ion selectivity of recombinant AMPA and kainate receptor channels and their dependence on Q/R site residues. J Physiol 496:165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev et al., 1995.Burnashev N, Zhou Z, Neher E, Sakmann B. (1995) Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol 485:403–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büsselberg et al., 1994.Büsselberg D, Michael D, Platt B. (1994) Pb2+ reduces voltage-activated and N-methyl-d-aspartate (NMDA)-activated calcium channel currents. Cell Mol Neurobiol 14:711–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttelmann et al., 2003.Büttelmann B, Alanine A, Bourson A, Gill R, Heitz MP, Mutel V, Pinard E, Trube G, Wyler R. (2003) 4-(3,4-Dihydro-1H-isoquinolin-2yl)-pyridines and 4-(3,4-dihydro-1H-isoquinolin-2-yl)-quinolines as potent NR1/2B subtype selective NMDA receptor antagonists. Bioorg Med Chem Lett 13:1759–1762 [DOI] [PubMed] [Google Scholar]
- Calderone et al., 2003.Calderone A, Jover T, Noh KM, Tanaka H, Yokota H, Lin Y, Grooms SY, Regis R, Bennett MV, Zukin RS. (2003) Ischemic insults derepress the gene silencer REST in neurons destined to die. J Neurosci 23:2112–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campiani et al., 2001.Campiani G, Morelli E, Nacci V, Fattorusso C, Ramunno A, Novellino E, Greenwood J, Liljefors T, Griffiths R, Sinclair C, et al. (2001) Characterization of the 1H-cyclopentapyrimidine-2,4(1H,3H)-dione derivative (S)-CPW399 as a novel, potent, and subtype-selective AMPA receptor full agonist with partial desensitization properties. J Med Chem 44:4501–4504 [DOI] [PubMed] [Google Scholar]
- Cao et al., 2007.Cao X, Cui Z, Feng R, Tang YP, Qin Z, Mei B, Tsien JZ. (2007) Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur J Neurosci 25:1815–1822 [DOI] [PubMed] [Google Scholar]
- Carrasco and Hidalgo, 2006.Carrasco MA, Hidalgo C. (2006) Calcium microdomains and gene expression in neurons and skeletal muscle cells. Cell Calcium 40:575–583 [DOI] [PubMed] [Google Scholar]
- Caruso and Goldblum, 2000.Caruso FS, Goldblum R. (2000) Dextromethorphan, an NMDA-receptor antagonist, enhances the analgesic properties of morphine. Inflammopharmacology 8:161–173 [Google Scholar]
- Carvalho et al., 1999.Carvalho AL, Kameyama K, Huganir RL. (1999) Characterization of phosphorylation sites on the glutamate receptor 4 subunit of the AMPA receptors. J Neurosci 19:4748–4754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado and Ascher, 1998.Casado M, Ascher P. (1998) Opposite modulation of NMDA receptors by lysophospholipids and arachidonic acid: common features with mechanosensitivity. J Physiol (Lond) 513:317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo et al., 1997.Castillo PE, Malenka RC, Nicoll RA. (1997) Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 388:182–186 [DOI] [PubMed] [Google Scholar]
- Castro et al., 2007.Castro CP, Piscopo D, Nakagawa T, Derynck R. (2007) Cornichon regulates transport and secretion of TGFalpha-related proteins in metazoan cells. J Cell Sci 120:2454–2466 [DOI] [PubMed] [Google Scholar]
- Catarzi et al., 2007.Catarzi D, Colotta V, Varano F. (2007) Competitive AMPA receptor antagonists. Med Res Rev 27:239–278 [DOI] [PubMed] [Google Scholar]
- Cathala et al., 2000.Cathala L, Misra C, Cull-Candy S. (2000) Developmental profile of the changing properties of NMDA receptors at cerebellar mossy fiber-granule cell synapses. J Neurosci 20:5899–5905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle and Dubner, 1998.Caudle RM, Dubner R. (1998) Ifenprodil blocks the excitatory effects of the opioid peptide dynorphin 1–17 on NMDA receptor-mediated currents in the CA3 region of the guinea pig hippocampus. Neuropeptides 32:87–95 [DOI] [PubMed] [Google Scholar]
- Cavara and Hollmann, 2008.Cavara NA, Hollmann M. (2008) Shuffling the deck anew: how NR3 tweaks NMDA receptor function. Mol Neurobiol 38:16–26 [DOI] [PubMed] [Google Scholar]
- Ceccon et al., 2001.Ceccon M, Rumbaugh G, Vicini S. (2001) Distinct effect of pregnenolone sulfate on NMDA receptor subtypes. Neuropharmacology 40:491–500 [DOI] [PubMed] [Google Scholar]
- Chan et al., 1999.Chan SL, Griffin WS, Mattson MP. (1999) Evidence for caspase-mediated cleavage of AMPA receptor subunits in neuronal apoptosis and Alzheimer's disease. J Neurosci Res 57:315–323 [DOI] [PubMed] [Google Scholar]
- Chang and Kuo, 2008.Chang HR, Kuo CC. (2008) The activation gate and gating mechanism of the NMDA receptor. J Neurosci 28:1546–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao et al., 1997.Chao J, Seiler N, Renault J, Kashiwagi K, Masuko T, Igarashi K, Williams K. (1997) N1-dansyl-spermine and N1-(n-octanesulfonyl)-spermine, novel glutamate receptor antagonists: block and permeation of N-methyl-d-aspartate receptors. Mol Pharmacol 51:861–871 [DOI] [PubMed] [Google Scholar]
- Chappell et al., 2007.Chappell AS, Gonzales C, Williams J, Witte MM, Mohs RC, Sperling R. (2007) AMPA potentiator treatment of cognitive deficits in Alzheimer disease. Neurology 68:1008–1012 [DOI] [PubMed] [Google Scholar]
- Chappell et al., 2002.Chappell AS, Sander JW, Brodie MJ, Chadwick D, Lledo A, Zhang D, Bjerke J, Kiesler GM, Arroyo S. (2002) A crossover, add-on trial of talampanel in patients with refractory partial seizures. Neurology 58:1680–1682 [DOI] [PubMed] [Google Scholar]
- Chatterton et al., 2002.Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, et al. (2002) Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature 415:793–798 [DOI] [PubMed] [Google Scholar]
- Chaudhry et al., 2009.Chaudhry C, Plested AJ, Schuck P, Mayer ML. (2009) Energetics of glutamate receptor ligand binding domain dimer assembly are modulated by allosteric ions. Proc Natl Acad Sci USA 106:12329–12334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez et al., 2006.Chávez AE, Singer JH, Diamond JS. (2006) Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature 443:705–708 [DOI] [PubMed] [Google Scholar]
- Chazot, 2004.Chazot PL. (2004) The NMDA receptor NR2B subunit: a valid therapeutic target for multiple CNS pathologies. Curr Med Chem 11:389–396 [DOI] [PubMed] [Google Scholar]
- Chazot et al., 1995.Chazot PL, Cik M, Stephenson FA. (1995) An investigation into the role of N-glycosylation in the functional expression of a recombinant heteromeric NMDA receptor. Mol Membr Biol 12:331–337 [DOI] [PubMed] [Google Scholar]
- Chazot et al., 1994.Chazot PL, Coleman SK, Cik M, Stephenson FA. (1994) Molecular characterization of N-methyl-d-aspartate receptors expressed in mammalian cells yields evidence for the coexistence of three subunit types within a discrete receptor molecule. J Biol Chem 269:24403–24409 [PubMed] [Google Scholar]
- Chazot et al., 2002.Chazot PL, Lawrence S, Thompson CL. (2002) Studies on the subtype selectivity of CP-101,606: evidence for two classes of NR2B-selective NMDA receptor antagonists. Neuropharmacology 42:319–324 [DOI] [PubMed] [Google Scholar]
- Chazot and Stephenson, 1997.Chazot PL, Stephenson FA. (1997) Molecular dissection of native mammalian forebrain NMDA receptors containing the NR1 C2 exon: direct demonstration of NMDA receptors comprising NR1, NR2A, and NR2B subunits within the same complex. J Neurochem 69:2138–2144 [DOI] [PubMed] [Google Scholar]
- Cheffings and Colquhoun, 2000.Cheffings CM, Colquhoun D. (2000) Single channel analysis of a novel NMDA channel from Xenopus oocytes expressing recombinant NR1a, NR2A and NR2D subunits. J Physiol 526:481–491 [PubMed] [Google Scholar]
- Chen et al., 2006.Chen BS, Braud S, Badger JD, 2nd, Isaac JT, Roche KW. (2006) Regulation of NR1/NR2C N-methyl-d-aspartate (NMDA) receptors by phosphorylation. J Biol Chem 281:16583–16590 [DOI] [PubMed] [Google Scholar]
- Chen and Roche, 2009.Chen BS, Roche KW. (2009) Growth factor-dependent trafficking of cerebellar NMDA receptors via protein kinase B/Akt phosphorylation of NR2C. Neuron 62:471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al., 1999a.Chen GQ, Cui C, Mayer ML, Gouaux E. (1999a) Functional characterization of a potassium-selective prokaryotic glutamate receptor. Nature 402:817–821 [DOI] [PubMed] [Google Scholar]
- Chen and Lipton, 1997.Chen HS, Lipton SA. (1997) Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism. J Physiol 499:27–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen and Lipton, 2005.Chen HS, Lipton SA. (2005) Pharmacological implications of two distinct mechanisms of interaction of memantine with N-methyl-d-aspartate-gated channels. J Pharmacol Exp Ther 314:961–971 [DOI] [PubMed] [Google Scholar]
- Chen and Lipton, 2006.Chen HS, Lipton SA. (2006) The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem 97:1611–1626 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2000.Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. (2000) Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 408:936–943 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2003.Chen L, El-Husseini A, Tomita S, Bredt DS, Nicoll RA. (2003) Stargazin differentially controls the trafficking of alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate and kainate receptors. Mol Pharmacol 64:703–706 [DOI] [PubMed] [Google Scholar]
- Chen and Huang, 1998.Chen L, Huang LY. (1998) Dynorphin block of N-methyl-d-aspartate channels increases with the peptide length. J Pharmacol Exp Ther 284:826–831 [PubMed] [Google Scholar]
- Chen et al., 2004.Chen N, Li B, Murphy TH, Raymond LA. (2004) Site within N-methyl-d-aspartate receptor pore modulates channel gating. Mol Pharmacol 65:157–164 [DOI] [PubMed] [Google Scholar]
- Chen et al., 1999b.Chen N, Luo T, Raymond LA. (1999b) Subtype-dependence of NMDA receptor channel open probability. J Neurosci 19:6844–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al., 1997.Chen N, Moshaver A, Raymond LA. (1997) Differential sensitivity of recombinant N-methyl-d-aspartate receptor subtypes to zinc inhibition. Mol Pharmacol 51:1015–1023 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2001.Chen N, Ren J, Raymond LA, Murphy TH. (2001) Changes in agonist concentration dependence that are a function of duration of exposure suggest N-methyl-d-aspartate receptor nonsaturation during synaptic stimulation. Mol Pharmacol 59:212–219 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2008.Chen PE, Geballe MT, Katz E, Erreger K, Livesey MR, O'Toole KK, Le P, Lee CJ, Snyder JP, Traynelis SF, et al. (2008) Modulation of glycine potency in rat recombinant NMDA receptors containing chimeric NR2A/2D subunits expressed in Xenopus laevis oocytes. J Physiol 586:227–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al., 2005.Chen PE, Geballe MT, Stansfeld PJ, Johnston AR, Yuan H, Jacob AL, Snyder JP, Traynelis SF, Wyllie DJ. (2005) Structural features of the glutamate binding site in recombinant NR1/NR2A N-methyl-d-Aspartate receptors determined by site-directed mutagenesis and molecular modeling. Mol Pharmacol 67:1470–1484 [DOI] [PubMed] [Google Scholar]
- Chen and Bear, 2007.Chen WS, Bear MF. (2007) Activity-dependent regulation of NR2B translation contributes to metaplasticity in mouse visual cortex. Neuropharmacology 52:200–214 [DOI] [PubMed] [Google Scholar]
- Chenard et al., 1995.Chenard BL, Bordner J, Butler TW, Chambers LK, Collins MA, De Costa DL, Ducat MF, Dumont ML, Fox CB, Mena EE. (1995) (1S,2S)-1-(4-hydroxyphenyl)-2-(4-hydroxy-4-phenylpiperidino)-1-propanol: a potent new neuroprotectant which blocks N-methyl-d-aspartate responses. J Med Chem 38:3138–3145 [DOI] [PubMed] [Google Scholar]
- Chenard and Menniti, 1999.Chenard BL, Menniti FS. (1999) Antagonists selective for NMDA receptors containing the NR2B subunit. Curr Pharm Des 5:381–404 [PubMed] [Google Scholar]
- Cheng et al., 2005.Cheng Q, Du M, Ramanoudjame G, Jayaraman V. (2005) Evolution of glutamate interactions during binding to a glutamate receptor. Nat Chem Biol 1:329–332 [DOI] [PubMed] [Google Scholar]
- Chesler and Kaila, 1992.Chesler M, Kaila K. (1992) Modulation of pH by neuronal activity. Trends Neurosci 15:396–402 [DOI] [PubMed] [Google Scholar]
- Chetkovich et al., 2002.Chetkovich DM, Chen L, Stocker TJ, Nicoll RA, Bredt DS. (2002) Phosphorylation of the postsynaptic density-95 (PSD-95)/discs large/zona occludens-1 binding site of stargazin regulates binding to PSD-95 and synaptic targeting of AMPA receptors. J Neurosci 22:5791–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung and Gurd, 2001.Cheung HH, Gurd JW. (2001) Tyrosine phosphorylation of the N-methyl-d-aspartate receptor by exogenous and postsynaptic density-associated Src-family kinases. J Neurochem 78:524–534 [DOI] [PubMed] [Google Scholar]
- Chew et al., 1997.Chew LJ, Fleck MW, Wright P, Scherer SE, Mayer ML, Gallo V. (1997) Growth factor-induced transcription of GluR1 increases functional AMPA receptor density in glial progenitor cells. J Neurosci 17:227–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew et al., 1999.Chew LJ, Huang F, Boutin JM, Gallo V. (1999) Identification of nuclear orphan receptors as regulators of expression of a neurotransmitter receptor gene. J Biol Chem 274:29366–29375 [DOI] [PubMed] [Google Scholar]
- Chew et al., 2001.Chew LJ, Yuan X, Scherer SE, Qie L, Huang F, Hayes WP, Gallo V. (2001) Characterization of the rat GRIK5 kainate receptor subunit gene promoter and its intragenic regions involved in neural cell specificity. J Biol Chem 276:42162–42171 [DOI] [PubMed] [Google Scholar]
- Chizh et al., 2001.Chizh BA, Headley PM, Tzschentke TM. (2001) NMDA receptor antagonists as analgesics: focus on the NR2B subtype. Trends Pharmacol Sci 22:636–642 [DOI] [PubMed] [Google Scholar]
- Choi, 1995.Choi DW. (1995) Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci 18:58–60 [PubMed] [Google Scholar]
- Choi et al., 2001.Choi Y, Chen HV, Lipton SA. (2001) Three pairs of cysteine residues mediate both redox and Zn2+ modulation of the NMDA receptor. J Neurosci 21:392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi and Lipton, 1999.Choi YB, Lipton SA. (1999) Identification and mechanism of action of two histidine residues underlying high-affinity Zn2+ inhibition of the NMDA receptor. Neuron 23:171–180 [DOI] [PubMed] [Google Scholar]
- Choi and Lipton, 2000.Choi YB, Lipton SA. (2000) Redox modulation of the NMDA receptor. Cell Mol Life Sci 57:1535–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi et al., 2000.Choi YB, Tenneti L, Le DA, Ortiz J, Bai G, Chen HS, Lipton SA. (2000) Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat Neurosci 3:15–21 [DOI] [PubMed] [Google Scholar]
- Chopra et al., 2000.Chopra B, Chazot PL, Stephenson FA. (2000) Characterization of the binding of two novel glycine site antagonists to cloned NMDA receptors: evidence for two pharmacological classes of antagonists. Br J Pharmacol 130:65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen and Hida, 1990.Christensen BN, Hida E. (1990) Protonation of histidine groups inhibits gating of the quisqualate kainate channel protein in isolated catfish cone horizontal cells. Neuron 5:471–478 [DOI] [PubMed] [Google Scholar]
- Christensen et al., 2004a.Christensen JK, Paternain AV, Selak S, Ahring PK, Lerma J. (2004a) A mosaic of functional kainate receptors in hippocampal interneurons. J Neurosci 24:8986–8993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen et al., 2004b.Christensen JK, Varming T, Ahring PK, Jørgensen TD, Nielsen EØ. (2004b) In vitro characterization of 5-carboxyl-2,4-di-benzamidobenzoic acid (NS3763), a noncompetitive antagonist of GLUK5 receptors. J Pharmacol Exp Ther 309:1003–1010 [DOI] [PubMed] [Google Scholar]
- Chu et al., 1995.Chu B, Anantharam V, Treistman SN. (1995) Ethanol inhibition of recombinant heteromeric NMDA channels in the presence and absence of modulators. J Neurochem 65:140–148 [DOI] [PubMed] [Google Scholar]
- Chumakov et al., 2002.Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, Bougueleret L, Barry C, Tanaka H, La Rosa P, et al. (2002) Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA 99:13675–13680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung et al., 2004.Chung HJ, Huang YH, Lau LF, Huganir RL. (2004) Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci 24:10248–10259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung et al., 2000.Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. (2000) Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci 20:7258–7267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciabarra et al., 1995.Ciabarra AM, Sullivan JM, Gahn LG, Pecht G, Heinemann S, Sevarino KA. (1995) Cloning and characterization of χ-1: a developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J Neurosci 15:6498–6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani et al., 2009.Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, et al. (2009) Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 373:746–758 [DOI] [PubMed] [Google Scholar]
- Citri and Malenka, 2008.Citri A, Malenka RC. (2008) Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33:18–41 [DOI] [PubMed] [Google Scholar]
- Claiborne et al., 2003.Claiborne CF, McCauley JA, Libby BE, Curtis NR, Diggle HJ, Kulagowski JJ, Michelson SR, Anderson KD, Claremon DA, Freidinger RM, et al. (2003) Orally efficacious NR2B-selective NMDA receptor antagonists. Bioorg Med Chem Lett 13:697–700 [DOI] [PubMed] [Google Scholar]
- Clark et al., 1990.Clark GD, Clifford DB, Zorumski CF. (1990) The effect of agonist concentration, membrane voltage and calcium on N-methyl-d-aspartate receptor desensitization. Neuroscience 39:787–797 [DOI] [PubMed] [Google Scholar]
- Clarke et al., 1997.Clarke VR, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas J, Sharpe EF, Davies CH, Ornstein PL, et al. (1997) A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature 389:599–603 [DOI] [PubMed] [Google Scholar]
- Clarke and Johnson, 2006.Clarke RJ, Johnson JW. (2006) NMDA receptor NR2 subunit dependence of the slow component of magnesium unblock. J Neurosci 26:5825–5834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke and Johnson, 2008.Clarke RJ, Johnson JW. (2008) Voltage dependent gating of NR1/2B NMDA receptors. J Physiol 586:5727–5741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen et al., 2008.Clausen RP, Christensen C, Hansen KB, Greenwood JR, Jørgensen L, Micale N, Madsen JC, Nielsen B, Egebjerg J, Bräuner-Osborne H, et al. (2008) N-Hydroxypyrazolyl glycine derivatives as selective N-methyl-d-aspartic acid receptor ligands. J Med Chem 51:4179–4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton et al., 2009.Clayton A, Siebold C, Gilbert RJ, Sutton GC, Harlos K, McIlhinney RA, Jones EY, Aricescu AR. (2009) Crystal structure of the GluR2 amino-terminal domain provides insights into the architecture and assembly of ionotropic glutamate receptors. J Mol Biol 392:1125–1132 [DOI] [PubMed] [Google Scholar]
- Clem and Barth, 2006.Clem RL, Barth A. (2006) Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron 49:663–670 [DOI] [PubMed] [Google Scholar]
- Cohen and Greenberg, 2008.Cohen S, Greenberg ME. (2008) Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol 24:183–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cokić and Stein, 2008.Cokić B, Stein V. (2008) Stargazin modulates AMPA receptor antagonism. Neuropharmacology 54:1062–1070 [DOI] [PubMed] [Google Scholar]
- Coleman et al., 2003.Coleman SK, Cai C, Mottershead DG, Haapalahti JP, Keinänen K. (2003) Surface expression of GluR-D AMPA receptor is dependent on an interaction between its C-terminal domain and a 4.1 protein. J Neurosci 23:798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge et al., 2004.Collingridge GL, Isaac JT, Wang YT. (2004) Receptor trafficking and synaptic plasticity. Nat Rev Neurosci 5:952–962 [DOI] [PubMed] [Google Scholar]
- Collingridge et al., 2009.Collingridge GL, Olsen RW, Peters J, Spedding M. (2009) A nomenclature for ligand-gated ion channels. Neuropharmacology 56:2–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor and Swanson, 2008.Contractor A, Swanson GT. (2008) Kainate receptors, in The Glutamate Receptors (Gereau RW, Swanson GT. eds) pp 99–158, Humana Press, Totowa, NJ [Google Scholar]
- Coombs and Cull-Candy, 2009.Coombs ID, Cull-Candy SG. (2009) Transmembrane AMPA receptor regulatory proteins and AMPA receptor function in the cerebellum. Neuroscience 162:656–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copani et al., 1992.Copani A, Genazzani AA, Aleppo G, Casabona G, Canonico PL, Scapagnini U, Nicoletti F. (1992) Nootropic drugs positively modulate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-sensitive glutamate receptors in neuronal cultures. J Neurochem 58:1199–1204 [DOI] [PubMed] [Google Scholar]
- Coquelle et al., 2000.Coquelle T, Christensen JK, Banke TG, Madsen U, Schousboe A, Pickering DS. (2000) Agonist discrimination between AMPA receptor subtypes. Neuroreport 11:2643–2648 [DOI] [PubMed] [Google Scholar]
- Correia et al., 2003.Correia SS, Duarte CB, Faro CJ, Pires EV, Carvalho AL. (2003) Protein kinase C gamma associates directly with the GluR4 alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subunit. Effect on receptor phosphorylation. J Biol Chem 278:6307–6313 [DOI] [PubMed] [Google Scholar]
- Cossart et al., 2002.Cossart R, Epsztein J, Tyzio R, Becq H, Hirsch J, Ben-Ari Y, Crépel V. (2002) Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron 35:147–159 [DOI] [PubMed] [Google Scholar]
- Cossart et al., 1998.Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. (1998) GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci 1:470–478 [DOI] [PubMed] [Google Scholar]
- Costa et al., 2009.Costa BM, Feng B, Tsintsadze TS, Morley RM, Irvine MW, Tsintsadze V, Lozovaya NA, Jane DE, Monaghan DT. (2009) N-methyl-d-aspartate (NMDA) receptor NR2 subunit selectivity of a series of novel piperazine-2,3-dicarboxylate derivatives: preferential blockade of extrasynaptic NMDA receptors in the rat hippocampal CA3-CA1 synapse. J Pharmacol Exp Ther 331:618–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton and Partin, 2000.Cotton JL, Partin KM. (2000) The contributions of GluR2 to allosteric modulation of AMPA receptors. Neuropharmacology 39:21–31 [DOI] [PubMed] [Google Scholar]
- Coussen et al., 2002.Coussen F, Normand E, Marchal C, Costet P, Choquet D, Lambert M, Mège RM, Mulle C. (2002) Recruitment of the kainate receptor subunit glutamate receptor 6 by cadherin/catenin complexes. J Neurosci 22:6426–6436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussen et al., 2005.Coussen F, Perrais D, Jaskolski F, Sachidhanandam S, Normand E, Bockaert J, Marin P, Mulle C. (2005) Co-assembly of two GluR6 kainate receptor splice variants within a functional protein complex. Neuron 47:555–566 [DOI] [PubMed] [Google Scholar]
- Coyle, 1997.Coyle JT. (1997) The nagging question of the function of N-acetylaspartylglutamate. Neurobiol Dis 4:231–238 [DOI] [PubMed] [Google Scholar]
- Coyle, 2009.Coyle JT. (2009) MicroRNAs suggest a new mechanism for altered brain gene expression in schizophrenia. Proc Natl Acad Sci USA 106:2975–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle and Tsai, 2004.Coyle JT, Tsai G. (2004) The NMDA receptor glycine modulatory site: a therapeutic target for improving cognition and reducing negative symptoms in schizophrenia. Psychopharmacology (Berl) 174:32–38 [DOI] [PubMed] [Google Scholar]
- Cracco et al., 2005.Cracco JB, Serrano P, Moskowitz SI, Bergold PJ, Sacktor TC. (2005) Protein synthesis-dependent LTP in isolated dendrites of CA1 pyramidal cells. Hippocampus 15:551–556 [DOI] [PubMed] [Google Scholar]
- Crosby et al., 2003a.Crosby NJ, Deane KH, Clarke CE. (2003a) Amantadine in Parkinson's disease. Cochrane Database Syst Rev 2003:CD003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby et al., 2003b.Crosby NJ, Deane KH, Clarke CE. (2003b) Amantadine for dyskinesia in Parkinson's disease. Cochrane Database Syst Rev 2003:CD003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis et al., 2003.Curtis NR, Diggle HJ, Kulagowski JJ, London C, Grimwood S, Hutson PH, Murray F, Richards P, Macaulay A, Wafford KA. (2003) Novel N1-(benzyl)cinnamamidine derived NR2B subtype-selective NMDA receptor antagonists. Bioorg Med Chem Lett 13:693–696 [DOI] [PubMed] [Google Scholar]
- Dalva et al., 2000.Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. (2000) EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell 103:945–956 [DOI] [PubMed] [Google Scholar]
- Danscher and Stoltenberg, 2005.Danscher G, Stoltenberg M. (2005) Zinc-specific autometallographic in vivo selenium methods: tracing of zinc-enriched (ZEN) terminals, ZEN pathways, and pools of zinc ions in a multitude of other ZEN cells. J Histochem Cytochem 53:141–153 [DOI] [PubMed] [Google Scholar]
- Danysz, 2002.Danysz W. (2002) CX-516 Cortex pharmaceuticals. Curr Opin Investig Drugs 3:1081–1088 [PubMed] [Google Scholar]
- Danysz and Parsons, 2002.Danysz W, Parsons CG. (2002) Neuroprotective potential of ionotropic glutamate receptor antagonists. Neurotox Res 4:119–126 [DOI] [PubMed] [Google Scholar]
- Danysz et al., 2000.Danysz W, Parsons CG, Mobius HJ, Stoffler A, Quack G. (2000) Neuroprotective and symptomatological action of memantine relevant for Alzheimer's disease—a unified glutamatergic hypothesis on the mechanism of action. Neurotox Res 2: 85–97 [DOI] [PubMed] [Google Scholar]
- Dargan et al., 2009.Dargan SL, Clarke VR, Alushin GM, Sherwood JL, Nisticò R, Bortolotto ZA, Ogden AM, Bleakman D, Doherty AJ, Lodge D, et al. (2009) ACET is a highly potent and specific kainate receptor antagonist: characterisation and effects on hippocampal mossy fibre function. Neuropharmacology 56:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das et al., 2010.Das U, Kumar J, Mayer ML, Plested AJ. (2010) Domain organization and function in GluK2 subtype kainate receptors. Proc Natl Acad Sci USA 107:8463–8468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das et al., 1998.Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, et al. (1998) Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature 393:377–381 [DOI] [PubMed] [Google Scholar]
- Davies et al., 1981.Davies J, Evans RH, Francis AA, Jones AW, Watkins JC. (1981) Antagonism of excitatory amino acid-induced and synaptic excitation of spinal neurones by cis-2,3-piperidine dicarboxylate. J Neurochem 36:1305–1307 [DOI] [PubMed] [Google Scholar]
- Davies et al., 1986.Davies J, Evans RH, Herrling PL, Jones AW, Olverman HJ, Pook P, Watkins JC. (1986) CPP, a new potent and selective NMDA antagonist. Depression of central neuron responses, affinity for [3H]d-AP5 binding sites on brain membranes and anticonvulsant activity. Brain Res 382:169–173 [DOI] [PubMed] [Google Scholar]
- Daw et al., 2006.Daw MI, Bannister NV, Isaac JT. (2006) Rapid, activity-dependent plasticity in timing precision in neonatal barrel cortex. J Neurosci 26:4178–4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson et al., 2001.Dawson DA, Wadsworth G, Palmer AM. (2001) A comparative assessment of the efficacy and side-effect liability of neuroprotective compounds in experimental stroke. Brain Res 892:344–350 [DOI] [PubMed] [Google Scholar]
- Dawson et al., 1990.Dawson TL, Nicholas RA, Dingledine R. (1990) Homomeric GluR1 excitatory amino acid receptors expressed in Xenopus oocytes. Mol Pharmacol 38:779–784 [PubMed] [Google Scholar]
- de Sousa et al., 2000.de Sousa SL, Dickinson R, Lieb WR, Franks NP. (2000) Contrasting synaptic actions of the inhalational general anesthetics isoflurane and xenon. Anesthesiology 92:1055–1066 [DOI] [PubMed] [Google Scholar]
- del Camino and Yellen, 2001.del Camino D, Yellen G. (2001) Tight steric closure at the intracellular activation gate of a voltage-gated K+ channel. Neuron 32:649–656 [DOI] [PubMed] [Google Scholar]
- Deming et al., 2003.Deming D, Cheng Q, Jayaraman V. (2003) Is the isolated ligand binding domain a good model of the domain in the native receptor? J Biol Chem 278:17589–17592 [DOI] [PubMed] [Google Scholar]
- Derkach et al., 1999.Derkach V, Barria A, Soderling TR. (1999) Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci USA 96:3269–3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach et al., 2007.Derkach VA, Oh MC, Guire ES, Soderling TR. (2007) Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci 8:101–113 [DOI] [PubMed] [Google Scholar]
- Desai et al., 2002.Desai A, Turetsky D, Vasudevan K, Buonanno A. (2002) Analysis of transcriptional regulatory sequences of the N-methyl-d-aspartate receptor 2A subunit gene in cultured cortical neurons and transgenic mice. J Biol Chem 277:46374–46384 [DOI] [PubMed] [Google Scholar]
- Desos et al., 1996.Desos P, Serkiz B, Morain P, Lepagnol J, Cordi A. (1996) Enantioselective synthesis of a pyrrolo-benzothiadiazine derivative S 18986, a new AMPA receptor positive modulator. Bioorg Med Chem Lett 6:3003–3008 [Google Scholar]
- Destot-Wong et al., 2009.Destot-Wong KD, Liang K, Gupta SK, Favrais G, Schwendimann L, Pansiot J, Baud O, Spedding M, Lelièvre V, Mani S, et al. (2009) The AMPA receptor positive allosteric modulator, S18986, is neuroprotective against neonatal excitotoxic and inflammatory brain damage through BDNF synthesis. Neuropharmacology 57:277–286 [DOI] [PubMed] [Google Scholar]
- Dev et al., 1999.Dev KK, Nishimune A, Henley JM, Nakanishi S. (1999) The protein kinase C alpha binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharmacology 38:635–644 [DOI] [PubMed] [Google Scholar]
- Dewar et al., 1999.Dewar D, Yam P, McCulloch J. (1999) Drug development for stroke: importance of protecting cerebral white matter. Eur J Pharmacol 375:41–50 [DOI] [PubMed] [Google Scholar]
- Dhar et al., 2009.Dhar SS, Liang HL, Wong-Riley MT. (2009) Nuclear respiratory factor 1 co-regulates AMPA glutamate receptor 2 and cytochrome c oxidase: tight coupling of glutamatergic transmission and energy metabolism in neurons. J Neurochem 108:1595–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar et al., 2008.Dhar SS, Ongwijitwat S, Wong-Riley MT. (2008) Nuclear respiratory factor 1 regulates all ten nuclear-encoded subunits of cytochrome c oxidase in neurons. J Biol Chem 283:3120–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar and Wong-Riley, 2009.Dhar SS, Wong-Riley MT. (2009) Coupling of energy metabolism and synaptic transmission at the transcriptional level: role of nuclear respiratory factor 1 in regulating both cytochrome c oxidase and NMDA glutamate receptor subunit genes. J Neurosci 29:483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson et al., 2007.Dickinson R, Peterson BK, Banks P, Simillis C, Martin JC, Valenzuela CA, Maze M, Franks NP. (2007) Competitive inhibition at the glycine site of the N-methyl-d-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology 107:756–767 [DOI] [PubMed] [Google Scholar]
- Dingledine et al., 1999.Dingledine R, Borges K, Bowie D, Traynelis SF. (1999) The glutamate receptor ion channels. Pharmacol Rev 51:7–61 [PubMed] [Google Scholar]
- Dingledine et al., 1992.Dingledine R, Hume RI, Heinemann SF. (1992) Structural determinants of barium permeation and rectification in non-NMDA glutamate receptor channels. J Neurosci 12:4080–4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl et al., 1999.Dirnagl U, Iadecola C, Moskowitz MA. (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22:391–397 [DOI] [PubMed] [Google Scholar]
- Do et al., 1988.Do KQ, Herrling PL, Streit P, Cuénod M. (1988) Release of neuroactive substances: homocysteic acid as an endogenous agonist of the NMDA receptor. J Neural Transm 72:185–190 [DOI] [PubMed] [Google Scholar]
- Do et al., 1986.Do KQ, Herrling PL, Streit P, Turski WA, Cuenod M. (1986) In vitro release and electrophysiological effects in situ of homocysteic acid, an endogenous N-methyl-(D)-aspartic acid agonist, in the mammalian striatum. J Neurosci 6:2226–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolman et al., 2007.Dolman NP, More JC, Alt A, Knauss JL, Pentikäinen OT, Glasser CR, Bleakman D, Mayer ML, Collingridge GL, Jane DE. (2007) Synthesis and pharmacological characterization of N3-substituted willardiine derivatives: role of the substituent at the 5-position of the uracil ring in the development of highly potent and selective GLUK5 kainate receptor antagonists. J Med Chem 50:1558–1570 [DOI] [PubMed] [Google Scholar]
- Dolman et al., 2006.Dolman NP, More JC, Alt A, Knauss JL, Troop HM, Bleakman D, Collingridge GL, Jane DE. (2006) Structure-activity relationship studies on N3-substituted willardiine derivatives acting as AMPA or kainate receptor antagonists. J Med Chem 49:2579–2592 [DOI] [PubMed] [Google Scholar]
- Dolman et al., 2005.Dolman NP, Troop HM, More JC, Alt A, Knauss JL, Nistico R, Jack S, Morley RM, Bortolotto ZA, Roberts PJ, et al. (2005) Synthesis and pharmacology of willardiine derivatives acting as antagonists of kainate receptors. J Med Chem 48:7867–7881 [DOI] [PubMed] [Google Scholar]
- Donevan et al., 1998.Donevan SD, Beg A, Gunther JM, Twyman RE. (1998) The methylglutamate, SYM 2081, is a potent and highly selective agonist at kainate receptors. J Pharmacol Exp Ther 285:539–545 [PubMed] [Google Scholar]
- Donevan and Rogawski, 1998.Donevan SD, Rogawski MA. (1998) Allosteric regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate receptors by thiocyanate and cyclothiazide at a common modulatory site distinct from that of 2,3-benzodiazepines. Neuroscience 87:615–629 [DOI] [PubMed] [Google Scholar]
- Dong et al., 1997.Dong H, O'Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. (1997) GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature 386:279–284 [DOI] [PubMed] [Google Scholar]
- Dong et al., 1999.Dong H, Zhang P, Song I, Petralia RS, Liao D, Huganir RL. (1999) Characterization of the glutamate receptor-interacting proteins GRIP1 and GRIP2. J Neurosci 19:6930–6941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody et al., 2004.Doody R, Wirth Y, Schmitt F, Möbius HJ. (2004) Specific functional effects of memantine treatment in patients with moderate to severe Alzheimer's disease. Dement Geriatr Cogn Disord 18:227–232 [DOI] [PubMed] [Google Scholar]
- Doppenberg et al., 2004.Doppenberg EM, Choi SC, Bullock R. (2004) Clinical trials in traumatic brain injury: lessons for the future. J Neurosurg Anesthesiol 16:87–94 [DOI] [PubMed] [Google Scholar]
- Doyle et al., 1998.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. (1998) The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280:69–77 [DOI] [PubMed] [Google Scholar]
- Doyle et al., 2008.Doyle KP, Simon RP, Stenzel-Poore MP. (2008) Mechanisms of ischemic brain damage. Neuropharmacology 55:310–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravid et al., 2010.Dravid SM, Burger PB, Prakash A, Geballe MT, Yadav R, Le P, Vellano K, Snyder JP, Traynelis SF. (2010) Structural determinants of d-cycloserine efficacy at the NR1/NR2C NMDA receptor. J Neurosci 30:2741–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravid et al., 2007.Dravid SM, Erreger K, Yuan H, Nicholson K, Le P, Lyuboslavsky P, Almonte A, Murray E, Mosely C, Barber J, et al. (2007) Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J Physiol 581:107–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravid et al., 2008.Dravid SM, Prakash A, Traynelis SF. (2008) Activation of recombinant NR1/NR2C NMDA receptors. J Physiol 586:4425–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreixler and Leonard, 1997.Dreixler JC, Leonard JP. (1997) Effects of external calcium on zinc modulation of AMPA receptors. Brain Res 752:170–174 [DOI] [PubMed] [Google Scholar]
- Du et al., 2005.Du M, Reid SA, Jayaraman V. (2005) Conformational changes in the ligand-binding domain of a functional ionotropic glutamate receptor. J Biol Chem 280:8633–8636 [DOI] [PubMed] [Google Scholar]
- Dunah et al., 1998a.Dunah AW, Luo J, Wang YH, Yasuda RP, Wolfe BB. (1998a) Subunit composition of N-methyl-d-aspartate receptors in the central nervous system that contain the NR2D subunit. Mol Pharmacol 53:429–437 [DOI] [PubMed] [Google Scholar]
- Dunah and Standaert, 2003.Dunah AW, Standaert DG. (2003) Subcellular segregation of distinct heteromeric NMDA glutamate receptors in the striatum. J Neurochem 85:935–943 [DOI] [PubMed] [Google Scholar]
- Dunah et al., 1998b.Dunah AW, Yasuda RP, Wolfe BB. (1998b) Developmental regulation of tyrosine phosphorylation of the NR2D NMDA glutamate receptor subunit in rat central nervous system. J Neurochem 71:1926–1934 [DOI] [PubMed] [Google Scholar]
- Duncan, 2009.Duncan K. (2009) The role of AMPA receptor-mediated excitotoxicity in ALS: is deficient RNA editing to blame? Curr Anaesth Crit Care 20:227–235 [Google Scholar]
- Durand et al., 1993.Durand GM, Bennett MV, Zukin RS. (1993) Splice variants of the N-methyl-d-aspartate receptor NR1 identify domains involved in regulation by polyamines and protein kinase C. Proc Natl Acad Sci USA 90:6731–6735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyker et al., 1999.Dyker AG, Edwards KR, Fayad PB, Hormes JT, Lees KR. (1999) Safety and tolerability study of aptiganel hydrochloride in patients with an acute ischemic stroke. Stroke 30:2038–2042 [DOI] [PubMed] [Google Scholar]
- Edmonds et al., 1995.Edmonds B, Gibb AJ, Colquhoun D. (1995) Mechanisms of activation of glutamate receptors and the time course of excitatory synaptic currents. Annu Rev Physiol 57:495–519 [DOI] [PubMed] [Google Scholar]
- Egebjerg et al., 1991.Egebjerg J, Bettler B, Hermans-Borgmeyer I, Heinemann S. (1991) Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature 351:745–748 [DOI] [PubMed] [Google Scholar]
- Egebjerg and Heinemann, 1993.Egebjerg J, Heinemann SF. (1993) Ca2+ permeability of unedited and edited versions of the kainate selective glutamate receptor GluR6. Proc Nat Acad Sci USA 90:755–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers et al., 1998.Ehlers MD, Fung ET, O'Brien RJ, Huganir RL. (1998) Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J Neurosci 18:720–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers et al., 1996.Ehlers MD, Zhang S, Bernhadt JP, Huganir RL. (1996) Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell 84:745–755 [DOI] [PubMed] [Google Scholar]
- Elger et al., 2006.Elger B, Gieseler M, Schmuecker O, Schumann I, Seltz A, Huth A. (2006) Extended therapeutic time window after focal cerebral ischemia by non-competitive inhibition of AMPA receptors. Brain Res 1085:189–194 [DOI] [PubMed] [Google Scholar]
- Eriksson et al., 2002.Eriksson M, Nilsson A, Froelich-Fabre S, Akesson E, Dunker J, Seiger A, Folkesson R, Benedikz E, Sundström E. (2002) Cloning and expression of the human N-methyl-d-aspartate receptor subunit NR3A. Neurosci Lett 321:177–181 [DOI] [PubMed] [Google Scholar]
- Eriksson et al., 2007a.Eriksson M, Nilsson A, Samuelsson H, Samuelsson EB, Mo L, Akesson E, Benedikz E, Sundström E. (2007a) On the role of NR3A in human NMDA receptors. Physiol Behav 92:54–59 [DOI] [PubMed] [Google Scholar]
- Eriksson et al., 2007b.Eriksson M, Samuelsson H, Samuelsson EB, Liu L, McKeehan WL, Benedikz E, Sundström E. (2007b) The NMDAR subunit NR3A interacts with microtubule-associated protein 1S in the brain. Biochem Biophys Res Commun 361:127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger et al., 2004.Erreger K, Chen PE, Wyllie DJ, Traynelis SF. (2004) Glutamate receptor gating. Crit Rev Neurobiol 16:187–224 [DOI] [PubMed] [Google Scholar]
- Erreger et al., 2005a.Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. (2005a) Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol 563:345–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger et al., 2005b.Erreger K, Geballe MT, Dravid SM, Snyder JP, Wyllie DJ, Traynelis SF. (2005b) Mechanism of partial agonism at NMDA receptors for a conformationally restricted glutamate analog. J Neurosci 25:7858–7866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger et al., 2007.Erreger K, Geballe MT, Kristensen A, Chen PE, Hansen KB, Lee CJ, Yuan H, Le P, Lyuboslavsky PN, Micale N, et al. (2007) Subunit-specific agonist activity at NR2A-, NR2B-, NR2C-, and NR2D-containing N-methyl-d-aspartate glutamate receptors. Mol Pharmacol 72:907–920 [DOI] [PubMed] [Google Scholar]
- Erreger and Traynelis, 2005.Erreger K, Traynelis SF. (2005) Allosteric interaction between zinc and glutamate binding domains on NR2A causes desensitization of NMDA receptors. J Physiol 569:381–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger and Traynelis, 2008.Erreger K, Traynelis SF. (2008) Zinc inhibition of rat NR1/NR2A N-methyl-d-aspartate receptors. J Physiol 586:763–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico et al., 2008.Errico F, Rossi S, Napolitano F, Catuogno V, Topo E, Fisone G, D'Aniello A, Centonze D, Usiello A. (2008) D-aspartate prevents corticostriatal long-term depression and attenuates schizophrenia-like symptoms induced by amphetamine and MK-801. J Neurosci 28:10404–10414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban et al., 2003.Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. (2003) PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6:136–143 [DOI] [PubMed] [Google Scholar]
- Evans et al., 1981.Evans RH, Francis AA, Jones AW, Smith DA, Watkins JC. (1981) The effects of a series of omega-phosphonic alpha-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations. Br J Pharmacol 75:65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts et al., 1999.Everts I, Petroski R, Kizelsztein P, Teichberg VI, Heinemann SF, Hollmann M. (1999) Lectin-induced inhibition of desensitization of the kainate receptor GluR6 depends on the activation state and can be mediated by a single native or ectopic N-linked carbohydrate side chain. J Neurosci 19:916–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts et al., 1997.Everts I, Villmann C, Hollmann M. (1997) N-Glycosylation is not a prerequisite for glutamate receptor function but is essential for lectin modulation. Mol Pharmacol 52:861–873 [DOI] [PubMed] [Google Scholar]
- Eybalin et al., 2004.Eybalin M, Caicedo A, Renard N, Ruel J, Puel JL. (2004) Transient Ca2+-permeable AMPA receptors in postnatal rat primary auditory neurons. Eur J Neurosci 20:2981–2989 [DOI] [PubMed] [Google Scholar]
- Fay and Bowie, 2006.Fay AM, Bowie D. (2006) Concanavalin-A reports agonist-induced conformational changes in the intact GluR6 kainate receptor. J Physiol 572:201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyazuddin et al., 2000.Fayyazuddin A, Villarroel A, Le Goff A, Lerma J, Neyton J. (2000) Four residues of the extracellular N-terminal domain of the NR2A subunit control high-affinity Zn2+ binding to NMDA receptors. Neuron 25:683–694 [DOI] [PubMed] [Google Scholar]
- Feng et al., 2005.Feng B, Morley RM, Jane DE, Monaghan DT. (2005) The effect of competitive antagonist chain length on NMDA receptor subunit selectivity. Neuropharmacology 48:354–359 [DOI] [PubMed] [Google Scholar]
- Feng et al., 2004.Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT. (2004) Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol 141:508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes et al., 2009.Fernandes HB, Catches JS, Petralia RS, Copits BA, Xu J, Russell TA, Swanson GT, Contractor A. (2009) High-affinity kainate receptor subunits are necessary for ionotropic but not metabotropic signaling. Neuron 63:818–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Monreal et al., 2004.Fernández-Monreal M, López-Atalaya JP, Benchenane K, Cacquevel M, Dulin F, Le Caer JP, Rossier J, Jarrige AC, Mackenzie ET, Colloc'h N, et al. (2004) Arginine 260 of the amino-terminal domain of NR1 subunit is critical for tissue-type plasminogen activator-mediated enhancement of N-methyl-d-aspartate receptor signaling. J Biol Chem 279:50850–50856 [DOI] [PubMed] [Google Scholar]
- Ferrer-Montiel et al., 1996.Ferrer-Montiel AV, Sun W, Montal M. (1996) A single tryptophan on M2 of glutamate receptor channels confers high permeability to divalent cations. Biophys J 71:749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink and Taylor, 2007.Fink M, Taylor MA. (2007) Electroconvulsive therapy: evidence and challenges. JAMA 298:330–332 [DOI] [PubMed] [Google Scholar]
- Fisahn et al., 2004.Fisahn A, Contractor A, Traub RD, Buhl EH, Heinemann SF, McBain CJ. (2004) Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate-induced hippocampal gamma oscillations. J Neurosci 24:9658–9668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn et al., 2005.Fisahn A, Heinemann SF, McBain CJ. (2005) The kainate receptor subunit GluR6 mediates metabotropic regulation of the slow and medium AHP currents in mouse hippocampal neurones. J Physiol 562:199–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer et al., 1997.Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, Heitz MP, Kemp JA. (1997) Ro 25-6981, a highly potent and selective blocker of N-methyl-d-aspartate receptors containing the NR2B subunit. J Pharmacol Exp Ther 283:1285–1292 [PubMed] [Google Scholar]
- Fisher et al., 2007.Fisher M, Hanley DF, Howard G, Jauch EC, Warach SSTAIR Group (2007) Recommendations from the STAIR V Meeting on acute stroke trials, technology and outcomes. Stroke 38:245–248 [DOI] [PubMed] [Google Scholar]
- Fleck et al., 2003.Fleck MW, Cornell E, Mah SJ. (2003) Amino-acid residues involved in glutamate receptor 6 kainate receptor gating and desensitization. J Neurosci 23:1219–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck et al., 1993.Fleck MW, Henze DA, Barrionuevo G, Palmer AM. (1993) Aspartate and glutamate mediate excitatory synaptic transmission in area CA1 of the hippocampus. J Neurosci 13:3944–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn and Zagotta, 2001.Flynn GE, Zagotta WN. (2001) Conformational changes in S6 coupled to the opening of cyclic nucleotide-gated channels. Neuron 30:689–698 [DOI] [PubMed] [Google Scholar]
- Follett et al., 2004.Follett PL, Deng W, Dai W, Talos DM, Massillon LJ, Rosenberg PA, Volpe JJ, Jensen FE. (2004) Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci 24:4412–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett et al., 2000.Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. (2000) NBQX attenuates excitotoxic injury in developing white matter. J Neurosci 20:9235–9241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst et al., 2007.Forst T, Smith T, Schütte K, Marcus P, Pfützner ACNS 5161 Study Group (2007) Dose escalating safety study of CNS 5161 HCl, a new neuronal glutamate receptor antagonist (NMDA) for the treatment of neuropathic pain. Br J Clin Pharmacol 64:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen et al., 2005.Frandsen A, Pickering DS, Vestergaard B, Kasper C, Nielsen BB, Greenwood JR, Campiani G, Fattorusso C, Gajhede M, Schousboe A, et al. (2005) Tyr702 is an important determinant of agonist binding and domain closure of the ligand-binding core of GluR2. Mol Pharmacol 67:703–713 [DOI] [PubMed] [Google Scholar]
- Franks et al., 1998.Franks NP, Dickinson R, de Sousa SL, Hall AC, Lieb WR. (1998) How does xenon produce anaesthesia? Nature 396:324. [DOI] [PubMed] [Google Scholar]
- Frederickson, 1989.Frederickson CJ. (1989) Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol 31:145–238 [DOI] [PubMed] [Google Scholar]
- Frerking et al., 1998.Frerking M, Malenka RC, Nicoll RA. (1998) Synaptic activation of kainate receptors on hippocampal interneurons. Nat Neurosci 1:479–486 [DOI] [PubMed] [Google Scholar]
- Fricker et al., 2009.Fricker AC, Mok MH, de la Flor R, Shah AJ, Woolley M, Dawson LA, Kew JN. (2009) Effects of N-acetylaspartylglutamate (NAAG) at group II mGluRs and NMDAR. Neuropharmacology 56:1060–1067 [DOI] [PubMed] [Google Scholar]
- Frizelle et al., 2006.Frizelle PA, Chen PE, Wyllie DJ. (2006) Equilibrium Constants for (R)-[(S)-1-(4-Bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquinoxalin-5-yl)-methyl]-phosphonic acid (NVP-AAM077) Acting at recombinant NR1/NR2A and NR1/NR2B N-methyl-d-aspartate receptors: implications for studies of synaptic transmission. Mol Pharmacol 70:1022–1032 [DOI] [PubMed] [Google Scholar]
- Frydenvang et al., 2009.Frydenvang K, Lash LL, Naur P, Postila PA, Pickering DS, Smith CM, Gajhede M, Sasaki M, Sakai R, Pentikaïnen OT, et al. (2009) Full domain closure of the ligand-binding core of the ionotropic glutamate receptor iGluR5 induced by the high affinity agonist dysiherbaine and the functional antagonist 8,9-dideoxyneodysiherbaine. J Biol Chem 284:14219–14229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu et al., 2005.Fu Z, Logan SM, Vicini S. (2005) Deletion of the NR2A subunit prevents developmental changes of NMDA-mEPSCs in cultured mouse cerebellar granule neurones. J Physiol 563:867–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata et al., 2005.Fukata Y, Tzingounis AV, Trinidad JC, Fukata M, Burlingame AL, Nicoll RA, Bredt DS. (2005) Molecular constituents of neuronal AMPA receptors. J Cell Biol 169:399–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima et al., 2001.Fukushima T, Marshall J, Sakata K, Shingai R. (2001) Calcium inhibits willardiine-induced responses in kainate receptor GluR6(Q)/KA-2. Neuroreport 12:163–167 [DOI] [PubMed] [Google Scholar]
- Fukushima et al., 2003.Fukushima T, Shingai R, Ogurusu T, Ichinose M. (2003) Inhibition of willardiine-induced currents through rat GluR6/KA-2 kainate receptor channels by Zinc and other divalent cations. Neurosci Lett 349:107–110 [DOI] [PubMed] [Google Scholar]
- Furukawa and Gouaux, 2003.Furukawa H, Gouaux E. (2003) Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO J 22:2873–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa et al., 2005.Furukawa H, Singh SK, Mancusso R, Gouaux E. (2005) Subunit arrangement and function in NMDA receptors. Nature 438:185–192 [DOI] [PubMed] [Google Scholar]
- Gahring et al., 2001.Gahring L, Carlson NG, Meyer EL, Rogers SW. (2001) Granzyme B proteolysis of a neuronal glutamate receptor generates an autoantigen and is modulated by glycosylation. J Immunol 166:1433–1438 [DOI] [PubMed] [Google Scholar]
- Galer et al., 2005.Galer BS, Lee D, Ma T, Nagle B, Schlagheck TG. (2005) MorphiDex (morphine sulfate/dextromethorphan hydrobromide combination) in the treatment of chronic pain: three multicenter, randomized, double-blind, controlled clinical trials fail to demonstrate enhanced opioid analgesia or reduction in tolerance. Pain 115:284–295 [DOI] [PubMed] [Google Scholar]
- Gallagher et al., 1996.Gallagher MJ, Huang H, Pritchett DB, Lynch DR. (1996) Interactions between Ifenprodil and the NR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem 271:9603–9611 [DOI] [PubMed] [Google Scholar]
- Gallo et al., 1992.Gallo V, Upson LM, Hayes WP, Vyklicky L, Jr, Winters CA, Buonanno A. (1992) Molecular cloning and development analysis of a new glutamate receptor subunit isoform in cerebellum. J Neurosci 12:1010–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo and Ghiani, 2000.Gallo V, Ghiani CA. (2000) Glutamate receptors in glia: new cells, new inputs and new functions. Trends Pharmacol Sci 21:252–258 [DOI] [PubMed] [Google Scholar]
- Garcia et al., 1998.Garcia EP, Mehta S, Blair LA, Wells DG, Shang J, Fukushima T, Fallon JR, Garner CC, Marshall J. (1998) SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron 21:727–739 [DOI] [PubMed] [Google Scholar]
- Gardoni et al., 1998.Gardoni F, Caputi A, Cimino M, Pastorino L, Cattabeni F, Di Luca M. (1998) Calcium/calmodulin-dependent protein kinase II is associated with NR2A/B subunits of NMDA receptor in postsynaptic densities. J Neurochem 71:1733–1741 [DOI] [PubMed] [Google Scholar]
- Gartlehner et al., 2008.Gartlehner G, Gaynes BN, Hansen RA, Thieda P, DeVeaugh-Geiss A, Krebs EE, Moore CG, Morgan L, Lohr KN. (2008) Comparative benefits and harms of second-generation antidepressants: background paper for the American College of Physicians. Ann Intern Med 149:734–750 [DOI] [PubMed] [Google Scholar]
- Gascón et al., 2005.Gascón S, Deogracias R, Sobrado M, Roda JM, Renart J, Rodríguez-Peña A, Díaz-Guerra M. (2005) Transcription of the NR1 subunit of the N-methyl-d-aspartate receptor is down-regulated by excitotoxic stimulation and cerebral ischemia. J Biol Chem 280:35018–35027 [DOI] [PubMed] [Google Scholar]
- Gavazzo et al., 2009.Gavazzo P, Guida P, Zanardi I, Marchetti C. (2009) Molecular determinants of multiple effects of nickel on NMDA receptor channels. Neurotox Res 15:38–48 [DOI] [PubMed] [Google Scholar]
- Gavazzo et al., 2008.Gavazzo P, Zanardi I, Baranowska-Bosiacka I, Marchetti C. (2008) Molecular determinants of Pb2+ interaction with NMDA receptor channels. Neurochem Int 52:329–337 [DOI] [PubMed] [Google Scholar]
- Gécz et al., 1999.Gécz J, Barnett S, Liu J, Hollway G, Donnelly A, Eyre H, Eshkevari HS, Baltazar R, Grunn A, Nagaraja R, et al. (1999) Characterization of the human glutamate receptor subunit 3 gene (GRIA3), a candidate for bipolar disorder and nonspecific X-linked mental retardation. Genomics 62:356–368 [DOI] [PubMed] [Google Scholar]
- Geiger et al., 1997.Geiger JR, Lübke J, Roth A, Frotscher M, Jonas P. (1997) Submillisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron 18:1009–1023 [DOI] [PubMed] [Google Scholar]
- Gibb and Colquhoun, 1991.Gibb AJ, Colquhoun D. (1991) Glutamate activation of a single NMDA receptor-channel produces a cluster of channel openings. Proc Biol Sci 243:39–45 [DOI] [PubMed] [Google Scholar]
- Giedke and Schwärzler, 2002.Giedke H, Schwärzler F. (2002) Therapeutic use of sleep deprivation in depression. Sleep Med Rev 6:361–377 [PubMed] [Google Scholar]
- Gielen et al., 2008.Gielen M, Le Goff A, Stroebel D, Johnson JW, Neyton J, Paoletti P. (2008) Structural rearrangements of NR1/NR2A NMDA receptors during allosteric inhibition. Neuron 57:80–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen et al., 2009.Gielen M, Siegler Retchless B, Mony L, Johnson JW, Paoletti P. (2009) Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature 459:703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffard et al., 1990.Giffard RG, Monyer H, Christine CW, Choi DW. (1990) Acidosis reduces NMDA receptor activation, glutamate neurotoxicity, and oxygen-glucose deprivation neuronal injury in cortical cultures. Brain Res 506:339–342 [DOI] [PubMed] [Google Scholar]
- Gill et al., 2008.Gill A, Birdsey-Benson A, Jones BL, Henderson LP, Madden DR. (2008) Correlating AMPA receptor activation and cleft closure across subunits: crystal structures of the GluR4 ligand-binding domain in complex with full and partial agonists. Biochemistry 47:13831–13841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill et al., 2009.Gill MB, Vivithanaporn P, Swanson GT. (2009) Glutamate binding and conformational flexibility of ligand-binding domains are critical early determinants of efficient kainate receptor biogenesis. J Biol Chem 284:14503–14512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, 1994.Gill R. (1994) The pharmacology of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate antagonists and their role in cerebral ischaemia. Cerebrovasc Brain Metab Rev 6:225–256 [PubMed] [Google Scholar]
- Gill et al., 2002.Gill R, Alanine A, Bourson A, Buttelmann B, Fischer G, Heitz MP, Kew JN, Levet-Trafit B, Lorez HP, Malherbe P, et al. (2002) Pharmacological characterization of Ro 63–1908 (1-[2-(4-hydroxy-phenoxy)-ethyl]-4-(4-methyl-benzyl)-piperidin-4-ol), a novel subtype-selective N-methyl-d-aspartate antagonist. J Pharmacol Exp Ther 302:940–948 [DOI] [PubMed] [Google Scholar]
- Gilron et al., 2000.Gilron I, Max MB, Lee G, Booher SL, Sang CN, Chappell AS, Dionne RA. (2000) Effects of the 2-amino-3-hydroxy-5-methyl-4-isoxazole-proprionic acid/kainate antagonist LY293558 on spontaneous and evoked postoperative pain. Clin Pharmacol Ther 68:320–327 [DOI] [PubMed] [Google Scholar]
- Gingrich et al., 2000.Gingrich MB, Junge CE, Lyuboslavsky P, Traynelis SF. (2000) Potentiation of NMDA receptor function by the serine protease thrombin. J Neurosci 20:4582–4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitto et al., 2003.Gitto R, Barreca ML, De Luca L, De Sarro G, Ferreri G, Quartarone S, Russo E, Constanti A, Chimirri A. (2003) Discovery of a novel and highly potent noncompetitive AMPA receptor antagonist. J Med Chem 46:197–200 [DOI] [PubMed] [Google Scholar]
- Gladstone et al., 2002.Gladstone DJ, Black SE, Hakim AMHeart and Stroke Foundation of Ontario Centre of Excellence in Stroke Recovery (2002) Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke 33:2123–2136 [DOI] [PubMed] [Google Scholar]
- Goebel and Poosch, 1999.Goebel DJ, Poosch MS. (1999) NMDA receptor subunit gene expression in the rat brain: a quantitative analysis of endogenous mRNA levels of NR1com, NR2A, NR2B, NR2C, NR2D, and NR3A. Mol Brain Res 69:164–170 [DOI] [PubMed] [Google Scholar]
- Goff et al., 2008a.Goff DC, Cather C, Gottlieb JD, Evins AE, Walsh J, Raeke L, Otto MW, Schoenfeld D, Green MF. (2008a) Once-weekly D-cycloserine effects on negative symptoms and cognition in schizophrenia: an exploratory study. Schizophr Res 106:320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff et al., 2008b.Goff DC, Lamberti JS, Leon AC, Green MF, Miller AL, Patel J, Manschreck T, Freudenreich O, Johnson SA. (2008b) A placebo-controlled add-on trial of the Ampakine, CX516, for cognitive deficits in schizophrenia. Neuropsychopharmacol 33:465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff et al., 2001.Goff DC, Leahy L, Berman I, Posever T, Herz L, Leon AC, Johnson SA, Lynch G. (2001) A placebo-controlled pilot study of the ampakine CX516 added to clozapine in schizophrenia. J Clin Psychopharmacol 21:484–487 [DOI] [PubMed] [Google Scholar]
- Goff et al., 1999.Goff DC, Tsai G, Levitt J, Amico E, Manoach D, Schoenfeld DA, Hayden DL, McCarley R, Coyle JT. (1999) A placebo-controlled trial of d-cycloserine added to conventional neuroleptics in patients with schizophrenia. Arch Gen Psychiatry 56:21–27 [DOI] [PubMed] [Google Scholar]
- Gogas, 2006.Gogas KR. (2006) Glutamate-based therapeutic approaches: NR2B receptor antagonists. Curr Opin Pharmacol 6:68–74 [DOI] [PubMed] [Google Scholar]
- Goldin et al., 2007.Goldin M, Epsztein J, Jorquera I, Represa A, Ben-Ari Y, Crépel V, Cossart R. (2007) Synaptic kainate receptors tune oriens-lacunosum moleculare interneurons to operate at theta frequency. J Neurosci 27:9560–9572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes et al., 2007.Gomes AR, Correia SS, Esteban JA, Duarte CB, Carvalho AL. (2007) PKC anchoring to GluR4 AMPA receptor subunit modulates PKC-driven receptor phosphorylation and surface expression. Traffic 8:259–269 [DOI] [PubMed] [Google Scholar]
- Gong et al., 2006.Gong R, Park CS, Abbassi NR, Tang SJ. (2006) Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem 281:18802–18815 [DOI] [PubMed] [Google Scholar]
- Gong et al., 2005.Gong XQ, Frandsen A, Lu WY, Wan Y, Zabek RL, Pickering DS, Bai D. (2005) D-aspartate and NMDA, but not L-aspartate, block AMPA receptors in rat hippocampal neurons. Br J Pharmacol 145:449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez et al., 2008.Gonzalez J, Rambhadran A, Du M, Jayaraman V. (2008) VLRET investigations of conformational changes in the ligand binding domain of a functional AMPA receptor. Biochemistry 47:10027–10032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti et al., 1988.Gotti B, Duverger D, Bertin J, Carter C, Dupont R, Frost J, Gaudilliere B, MacKenzie ET, Rousseau J, Scatton B. (1988) Ifenprodil and SL 82.0715 as cerebral anti-ischemic agents. I. Evidence for efficacy in models of focal cerebral ischemia. J Pharmacol Exp Ther 247:1211–1221 [PubMed] [Google Scholar]
- Gouaux, 2004.Gouaux E. (2004) Structure and function of AMPA receptors. J Physiol 554:249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, 2002.Green AR. (2002) Why do neuroprotective drugs that are so promising in animals fail in the clinic? An industry perspective. Clin Exp Pharmacol Physiol 29:1030–1034 [DOI] [PubMed] [Google Scholar]
- Green, 2008.Green AR. (2008) Pharmacological approaches to acute ischaemic stroke: reperfusion certainly, neuroprotection possibly. Br J Pharmacol 153 (Suppl 1):S325–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green and Gibb, 2001.Green GM, Gibb AJ. (2001) Characterization of the single-channel properties of NMDA receptors in laminae I and II of the dorsal horn of neonatal rat spinal cord. Eur J Neurosci 14:1590–1602 [DOI] [PubMed] [Google Scholar]
- Green et al., 2002.Green T, Rogers CA, Contractor A, Heinemann SF. (2002) NMDA receptors formed by NR1 in Xenopus laevis oocytes do not contain the endogenous subunit XenU1. Mol Pharmacol 61:326–333 [DOI] [PubMed] [Google Scholar]
- Greenamyre and O'Brien, 1991.Greenamyre JT, O'Brien CF. (1991) N-methyl-d-aspartate antagonists in the treatment of Parkinson's disease. Arch Neurol 48:977–981 [DOI] [PubMed] [Google Scholar]
- Greene, 2001.Greene R. (2001) Circuit analysis of NMDAR hypofunction in the hippocampus, in vitro, and psychosis of schizophrenia. Hippocampus 11:569–577 [DOI] [PubMed] [Google Scholar]
- Greenwood et al., 2006.Greenwood JR, Mewett KN, Allan RD, Martín BO, Pickering DS. (2006) 3-Hydroxypyridazine 1-oxides as carboxylate bioisosteres: a new series of subtype-selective AMPA receptor agonists. Neuropharmacology 51:52–59 [DOI] [PubMed] [Google Scholar]
- Greger et al., 2006.Greger IH, Akamine P, Khatri L, Ziff EB. (2006) Developmentally regulated, combinatorial RNA processing modulates AMPA receptor biogenesis. Neuron 51:85–97 [DOI] [PubMed] [Google Scholar]
- Greger et al., 2002.Greger IH, Khatri L, Ziff EB. (2002) RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron 34:759–772 [DOI] [PubMed] [Google Scholar]
- Greger et al., 2003.Greger IH, Khatri L, Kong X, Ziff EB. (2003) AMPA receptor tetramerization is mediated by Q/R editing. Neuron 40:763–774 [DOI] [PubMed] [Google Scholar]
- Grooms et al., 2006.Grooms SY, Noh KM, Regis R, Bassell GJ, Bryan MK, Carroll RC, Zukin RS. (2006) Activity bidirectionally regulates AMPA receptor mRNA abundance in dendrites of hippocampal neurons. J Neurosci 26:8339–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskreutz et al., 2003.Grosskreutz J, Zoerner A, Schlesinger F, Krampfl K, Dengler R, Bufler J. (2003) Kinetic properties of human AMPA-type glutamate receptors expressed in HEK293 cells. Eur J Neurosci 17:1173–1178 [DOI] [PubMed] [Google Scholar]
- Grunwald and Kaplan, 2003.Grunwald ME, Kaplan JM. (2003) Mutations in the ligand-binding and pore domains control exit of glutamate receptors from the endoplasmic reticulum in C. elegans. Neuropharmacology 45:768–776 [DOI] [PubMed] [Google Scholar]
- Gu et al., 1996.Gu JG, Albuquerque C, Lee CJ, MacDermott AB. (1996) Synaptic strengthening through activation of Ca2+-permeable AMPA receptors. Nature 381:793–796 [DOI] [PubMed] [Google Scholar]
- Guilarte and McGlothan, 2003.Guilarte TR, McGlothan JL. (2003) Selective decrease in NR1 subunit splice variant mRNA in the hippocampus of Pb2+-exposed rats: implications for synaptic targeting and cell surface expression of NMDAR complexes. Mol Brain Res 113:37–43 [DOI] [PubMed] [Google Scholar]
- Guilarte and Miceli, 1992.Guilarte TR, Miceli RC. (1992) Age-dependent effects of lead on [3H]MK-801 binding to the NMDA receptor-gated ionophore: in vitro and in vivo studies. Neurosci Lett 148:27–30 [DOI] [PubMed] [Google Scholar]
- Guttmann et al., 2001.Guttmann RP, Baker DL, Seifert KM, Cohen AS, Coulter DA, Lynch DR. (2001) Specific proteolysis of the NR2 subunit at multiple sites by calpain. J Neurochem 78:1083–1093 [DOI] [PubMed] [Google Scholar]
- Guttmann et al., 2002.Guttmann RP, Sokol S, Baker DL, Simpkins KL, Dong Y, Lynch DR. (2002) Proteolysis of the N-methyl-d-aspartate receptor by calpain in situ. J Pharmacol Exp Ther 302:1023–1030 [DOI] [PubMed] [Google Scholar]
- Hald et al., 2007.Hald H, Naur P, Pickering DS, Sprogøe D, Madsen U, Timmermann DB, Ahring PK, Liljefors T, Schousboe A, Egebjerg J, et al. (2007) Partial agonism and antagonism of the ionotropic glutamate receptor iGLuR5: structures of the ligand-binding core in complex with domoic acid and 2-amino-3-[5-tert-butyl-3-(phosphonomethoxy)-4-isoxazolyl]propionic acid. J Biol Chem 282:25726–25736 [DOI] [PubMed] [Google Scholar]
- Hallett and Standaert, 2004.Hallett PJ, Standaert DG. (2004) Rationale for and use of NMDA receptor antagonists in Parkinson's disease. Pharmacol Ther 102:155–174 [DOI] [PubMed] [Google Scholar]
- Hama and Sagen, 2009.Hama A, Sagen J. (2009) Antinociceptive effects of the marine snail peptides conantokin-G and conotoxin MVIIA alone and in combination in rat models of pain. Neuropharmacology 56:556–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson et al., 1992.Hampson DR, Huang XP, Wells JW, Walter JA, Wright JL. (1992) Interaction of domoic acid and several derivatives with kainic acid and AMPA binding sites in rat brain. Eur J Pharmacol 218:1–8 [DOI] [PubMed] [Google Scholar]
- Han et al., 2008.Han X, Tomitori H, Mizuno S, Higashi K, Füll C, Fukiwake T, Terui Y, Leewanich P, Nishimura K, Toida T, et al. (2008) Binding of spermine and ifenprodil to a purified, soluble regulatory domain of the N-methyl-d-aspartate receptor. J Neurochem 107:1566–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen et al., 2005a.Hansen KB, Clausen RP, Bjerrum EJ, Bechmann C, Greenwood JR, Christensen C, Kristensen JL, Egebjerg J, Bräuner-Osborne H. (2005a) Tweaking agonist efficacy at N-methyl-d-aspartate receptors by site-directed mutagenesis. Mol Pharmacol 68:1510–1523 [DOI] [PubMed] [Google Scholar]
- Hansen et al., 2010.Hansen KB, Mullasseril P, Dawit S, Kurtkaya NL, Yuan H, Vance KM, Orr AG, Kvist T, Ogden KK, Le P, et al. (2010) Implementation of a fluorescence-based screening assay identifies histamine H3 receptor antagonists clobenpropit and iodophenpropit as subunit-selective N-methyl-d-aspartate receptor antagonists. J Pharmacol Exp Ther 333:650–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen et al., 2009.Hansen KB, Naur P, Kurtkaya NL, Kristensen AS, Gajhede M, Kastrup JS, Traynelis SF. (2009) Modulation of the dimer interface at ionotropic glutamate-like receptor delta2 by d-serine and extracellular calcium. J Neurosci 29:907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen et al., 2007.Hansen KB, Yuan H, Traynelis SF. (2007) Structural aspects of AMPA receptor activation, desensitization and deactivation. Curr Opin Neurobiol 17:281–288 [DOI] [PubMed] [Google Scholar]
- Hansen et al., 2005b.Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS. (2005b) Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann Intern Med 143:415–426 [DOI] [PubMed] [Google Scholar]
- Hardingham, 2006.Hardingham GE. (2006) 2B synaptic or extrasynaptic determines signalling from the NMDA receptor. J Physiol 572:614–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty and Rogawski, 2000.Harty TP, Rogawski MA. (2000) Felbamate block of recombinant N-methyl -d-aspartate receptors: selectivity for the NR2B subunit. Epilepsy Res 39:47–55 [DOI] [PubMed] [Google Scholar]
- Hashimoto et al., 1999.Hashimoto K, Fukaya M, Qiao X, Sakimura K, Watanabe M, Kano M. (1999) Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J Neurosci 19:6027–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton and Paoletti, 2005.Hatton CJ, Paoletti P. (2005) Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron 46:261–274 [DOI] [PubMed] [Google Scholar]
- Hawkins et al., 2004.Hawkins LM, Prybylowski K, Chang K, Moussan C, Stephenson FA, Wenthold RJ. (2004) Export from the endoplasmic reticulum of assembled N-methyl-d-aspartic acid receptors is controlled by a motif in the c terminus of the NR2 subunit. J Biol Chem 279:28903–28910 [DOI] [PubMed] [Google Scholar]
- Hayashi and Huganir, 2004.Hayashi T, Huganir RL. (2004) Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J Neurosci 24:6152–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi et al., 2005.Hayashi T, Rumbaugh G, Huganir RL. (2005) Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron 47:709–723 [DOI] [PubMed] [Google Scholar]
- Hayashi et al., 2009.Hayashi T, Thomas GM, Huganir RL. (2009) Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron 64:213–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann et al., 1996.Heckmann M, Bufler J, Franke C, Dudel J. (1996) Kinetics of homomeric GluR6 glutamate receptor channels. Biophys J 71:1743–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennegriff et al., 1997.Hennegriff M, Arai A, Kessler M, Vanderklish P, Mutneja MS, Rogers G, Neve RL, Lynch G. (1997) Stable expression of recombinant AMPA receptor subunits: binding affinities and effects of allosteric modulators. J Neurochem 68:2424–2434 [DOI] [PubMed] [Google Scholar]
- Herb et al., 1992.Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg PH. (1992) The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron 8:775–785 [DOI] [PubMed] [Google Scholar]
- Heresco-Levy, 2000.Heresco-Levy U. (2000) N-Methyl-d-aspartate (NMDA) receptor-based treatment approaches in schizophrenia: the first decade. Int J Neuropsychopharmacol 3:243–258 [DOI] [PubMed] [Google Scholar]
- Herlitze et al., 1993.Herlitze S, Raditsch M, Ruppersberg JP, Jahn W, Monyer H, Schoepfer R, Witzemann V. (1993) Argiotoxin detects molecular differences in AMPA receptor channels. Neuron 10:1131–1140 [DOI] [PubMed] [Google Scholar]
- Hermann et al., 2009.Hermann GE, Van Meter MJ, Rood JC, Rogers RC. (2009) Proteinase-activated receptors in the nucleus of the solitary tract: evidence for glial-neural interactions in autonomic control of the stomach. J Neurosci 29:9292–9300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess et al., 1996.Hess SD, Daggett LP, Crona J, Deal C, Lu CC, Urrutia A, Chavez-Noriega L, Ellis SB, Johnson EC, Veliçelebi G. (1996) Cloning and functional characterization of human heteromeric N-methyl-d-aspartate receptors. J Pharmacol Exp Ther 278:808–816 [PubMed] [Google Scholar]
- Hess et al., 1998.Hess SD, Daggett LP, Deal C, Lu CC, Johnson EC, Veliçelebi G. (1998) Functional characterization of human N-methyl-d-aspartate subtype 1A/2D receptors. J Neurochem 70:1269–1279 [DOI] [PubMed] [Google Scholar]
- Hille, 2001.Hille B. (2001) Ion Channels of Excitable Membranes, 3rd ed Sinauer Associates, Inc., Sunderland, MA [Google Scholar]
- Hirai and Matsuda, 1999.Hirai H, Matsuda S. (1999) Interaction of the C-terminal domain of delta glutamate receptor with spectrin in the dendritic spines of cultured Purkinje cells. Neurosci Res 34:281–287 [DOI] [PubMed] [Google Scholar]
- Hirano et al., 2003.Hirano A, Moridera N, Akashi M, Saito M, Sugawara M. (2003) Imaging of L-glutamate fluxes in mouse brain slices based on an enzyme-based membrane combined with a difference-image analysis. Anal Chem 75:3775–3783 [DOI] [PubMed] [Google Scholar]
- Hirao et al., 1998.Hirao K, Hata Y, Ide N, Takeuchi M, Irie M, Yao I, Deguchi M, Toyoda A, Sudhof TC, Takai Y. (1998) A novel multiple PDZ domain-containing molecule interacting with N-methyl-d-aspartate receptors and neuronal cell adhesion proteins. J Biol Chem 273:21105–21110 [DOI] [PubMed] [Google Scholar]
- Hirbec et al., 2003.Hirbec H, Francis JC, Lauri SE, Braithwaite SP, Coussen F, Mulle C, Dev KK, Coutinho V, Meyer G, Isaac JT, et al. (2003) Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron 37:625–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirbec et al., 2002.Hirbec H, Perestenko O, Nishimune A, Meyer G, Nakanishi S, Henley JM, Dev KK. (2002) The PDZ proteins PICK1, GRIP, and syntenin bind multiple glutamate receptor subtypes. Analysis of PDZ binding motifs. J Biol Chem 277:15221–15224 [DOI] [PubMed] [Google Scholar]
- Hiroi et al., 1998.Hiroi N, Marek GJ, Brown JR, Ye H, Saudou F, Vaidya VA, Duman RS, Greenberg ME, Nestler EJ. (1998) Essential role of the fosB gene in molecular, cellular, and behavioral actions of chronic electroconvulsive seizures. J Neurosci 18:6952–6962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hironaka et al., 2000.Hironaka K, Umemori H, Tezuka T, Mishina M, Yamamoto T. (2000) The protein-tyrosine phosphatase PTPMEG interacts with glutamate receptor delta 2 and epsilon subunits. J Biol Chem 275:16167–16173 [DOI] [PubMed] [Google Scholar]
- Ho et al., 2007.Ho MT, Pelkey KA, Topolnik L, Petralia RS, Takamiya K, Xia J, Huganir RL, Lacaille JC, McBain CJ. (2007) Developmental expression of Ca2+-permeable AMPA receptors underlies depolarization-induced long term depression at mossy fiber CA3 pyramid synapses. J Neurosci 27:11651–11662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking and Cousins, 2003.Hocking G, Cousins MJ. (2003) Ketamine in chronic pain management: an evidence-based review. Anesth Analg 97:1730–1739 [DOI] [PubMed] [Google Scholar]
- Hodgson et al., 2005.Hodgson DM, Hachisu S, Andrews MD. (2005) Stereocontrolled syntheses of kainoid amino acids from 7-azabicyclo[2.2.1]heptadienes using tandem radical addition-homoallylic radical rearrangement. J Org Chem 70:8866–8876 [DOI] [PubMed] [Google Scholar]
- Hoffmann et al., 2006a.Hoffmann J, Gorodetskaia A, Hollmann M. (2006a) Ion pore properties of ionotropic glutamate receptors are modulated by a transplanted potassium channel selectivity filter. Mol Cell Neurosci 33:335–343 [DOI] [PubMed] [Google Scholar]
- Hofmann et al., 2006b.Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW. (2006b) Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry 63:298–304 [DOI] [PubMed] [Google Scholar]
- Hogner et al., 2003.Hogner A, Greenwood JR, Liljefors T, Lunn ML, Egebjerg J, Larsen IK, Gouaux E, Kastrup JS. (2003) Competitive antagonism of AMPA receptors by ligands of different classes: crystal structure of ATPO bound to the GluR2 ligand-binding core, in comparison with DNQX. J Med Chem 46:214–221 [DOI] [PubMed] [Google Scholar]
- Hogner et al., 2002.Hogner A, Kastrup JS, Jin R, Liljefors T, Mayer ML, Egebjerg J, Larsen IK, Gouaux E. (2002) Structural basis for AMPA receptor activation and ligand selectivity: crystal structures of five agonist complexes with the GluR2 ligand-binding core. J Mol Biol 322:93–109 [DOI] [PubMed] [Google Scholar]
- Hollmann et al., 1989.Hollmann M, O'Shea-Greenfield A, Rogers SW, Heinemann S. (1989) Cloning by functional expression of a member of the glutamate receptor family. Nature 342:643–648 [DOI] [PubMed] [Google Scholar]
- Hollmann et al., 1994.Hollmann M, Maron C, Heinemann S. (1994) N-glycosylation site tagging suggests a three transmembrane domain topology for the glutamate receptor GluRI. Neuron 13:1331–1343 [DOI] [PubMed] [Google Scholar]
- Holm et al., 2005.Holm MM, Lunn ML, Traynelis SF, Kastrup JS, Egebjerg J. (2005) Structural determinants of agonist-specific kinetics at the ionotropic glutamate receptor 2. Proc Natl Acad Sci USA 102:12053–12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoo et al., 1994.Hoo KH, Nutt SL, Fletcher EJ, Elliott CE, Korczak B, Deverill RM, Rampersad V, Fantaske RP, Kamboj RK. (1994) Functional expression and pharmacological characterization of the human EAA4 (GluR6) glutamate receptor: a kainate selective channel subunit. Recept Channels 2:327–337 [PubMed] [Google Scholar]
- Hood et al., 1989.Hood WF, Compton RP, Monahan JB. (1989) d-Cycloserine: a ligand for the N-methyl-d-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci Lett 98:91–95 [DOI] [PubMed] [Google Scholar]
- Horak et al., 2006.Horak M, Vlcek K, Chodounska H, Vyklicky L., Jr (2006) Subtype-dependence of N-methyl-d-aspartate receptor modulation by pregnenolone sulfate. Neuroscience 137:93–102 [DOI] [PubMed] [Google Scholar]
- Horak and Wenthold, 2009.Horak M, Wenthold RJ. (2009) Different roles of C-terminal cassettes in the trafficking of full-length NR1 subunits to the cell surface. J Biol Chem 284:9683–9691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio et al., 2009.Horio M, Fujita Y, Ishima T, Iyo M, Ferraris D, Tsukamoto T, Hashimoto K. (2009) Effects of d-amino acid oxidase inhibitor on the extracellular d-alanine levels and the efficacy of D-alanine on dizocilpine-induced prepulse inhibition deficits in mice. Open Clin Chem J 2:16–21 [Google Scholar]
- Horning and Mayer, 2004.Horning MS, Mayer ML. (2004) Regulation of AMPA receptor gating by ligand binding core dimers. Neuron 41:379–388 [DOI] [PubMed] [Google Scholar]
- Howe, 1996.Howe JR. (1996) Homomeric and heteromeric ion channels formed from the kainate-type subunits GluR6 and KA2 have very small, but different, unitary conductances. J Neurophysiol 76:510–519 [DOI] [PubMed] [Google Scholar]
- Howes and Bell, 2007.Howes JF, Bell C. (2007) Talampanel. Neurotherapeutics 4:126–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyte et al., 2004.Hoyte L, Barber PA, Buchan AM, Hill MD. (2004) The rise and fall of NMDA antagonists for ischemic stroke. Curr Mol Med 4:131–136 [DOI] [PubMed] [Google Scholar]
- Hsueh et al., 2000.Hsueh YP, Wang TF, Yang FC, Sheng M. (2000) Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2. Nature 404:298–302 [DOI] [PubMed] [Google Scholar]
- Hu and Zheng, 2005.Hu B, Zheng F. (2005) Molecular determinants of glycine-independent desensitization of NR1/NR2A receptors. J Pharmacol Exp Ther 313:563–569 [DOI] [PubMed] [Google Scholar]
- Huang and Gallo, 1997.Huang F, Gallo V. (1997) Gene structure of the rat kainate receptor subunit KA2 and characterization of an intronic negative regulatory region. J Biol Chem 272:8618–8627 [DOI] [PubMed] [Google Scholar]
- Huang and Hsueh, 2009.Huang TN, Hsueh YP. (2009) CASK point mutation regulates protein–protein interactions and NR2b promoter activity. Biochem Biophys Res Com 382:219–222 [DOI] [PubMed] [Google Scholar]
- Huang et al., 2002a.Huang Y, Doherty JJ, Dingledine R. (2002a) Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. J Neurosci 22:8422–8428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al., 1999.Huang Y, Myers SJ, Dingledine R. (1999) Transcriptional repression by REST: recruitment of Sin3A and histone deacetylase to neuronal genes. Nat Neurosci 2:867–872 [DOI] [PubMed] [Google Scholar]
- Huang et al., 2003.Huang YS, Carson JH, Barbarese E, Richter JD. (2003) Facilitation of dendritic mRNA transport by CPEB. Genes Dev 17:638–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al., 2002b.Huang YS, Jung MY, Sarkissian M, Richter JD. (2002b) N-methyl-d-aspartate receptor signaling results in Aurora kinase-catalyzed CPEB phosphorylation and alpha CaMKII mRNA polyadenylation at synapses. EMBO J 21:2139–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al., 2006.Huang YS, Kan MC, Lin CL, Richter JD. (2006) CPEB3 and CPEB4 in neurons: analysis of RNA-binding specificity and translational control of AMPA receptor GluR2 mRNA. EMBO J 25:4865–4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber et al., 2000.Huber KM, Kayser MS, Bear MF. (2000) Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288:1254–1257 [DOI] [PubMed] [Google Scholar]
- Huettner, 1990.Huettner JE. (1990) Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron 5:255–266 [DOI] [PubMed] [Google Scholar]
- Huettner and Bean, 1988.Huettner JE, Bean BP. (1988) Block of N-methyl-d-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci USA 85:1307–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume et al., 1991.Hume RI, Dingledine R, Heinemann SF. (1991) Identification of a site in glutamate receptor subunits that controls calcium permeability. Science 253:1028–1031 [DOI] [PubMed] [Google Scholar]
- Husi et al., 2000.Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. (2000) Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci 3:661–669 [DOI] [PubMed] [Google Scholar]
- Igarashi et al., 1997.Igarashi K, Shirahata A, Pahk AJ, Kashiwagi K, Williams K. (1997) Benzyl-polyamines: novel, potent N-methyl-d-aspartate receptor antagonists. J Pharmacol Exp Ther 283:533–540 [PubMed] [Google Scholar]
- Ihle and Patneau, 2000.Ihle EC, Patneau DK. (2000) Modulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor desensitization by extracellular protons. Mol Pharmacol 58:1204–1212 [DOI] [PubMed] [Google Scholar]
- Iijima et al., 2008.Iijima K, Abe H, Okazawa M, Moriyoshi K, Nakanishi S. (2008) Dual regulation of NR2B and NR2C expression by NMDA receptor activation in mouse cerebellar granule cell cultures. Proc Natl Acad Sci USA 105:12010–12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino et al., 1997.Iino M, Ciani S, Tsuzuki K, Ozawa S, Kidokoro Y. (1997) Permeation properties of Na+ and Ca2+ ions through the mouse ε2/ζ1 NMDA receptor channel expressed in Xenopus oocytes. J Membr Biol 155:143–156 [DOI] [PubMed] [Google Scholar]
- Ikeda et al., 1992.Ikeda K, Nagasawa M, Mori H, Araki K, Sakimura K, Watanabe M, Inoue Y, Mishina M. (1992) Cloning and expression of the epsilon 4 subunit of the NMDA receptor channel. FEBS Lett 313:34–38 [DOI] [PubMed] [Google Scholar]
- Ikeno et al., 2001.Ikeno K, Yamakura T, Yamazaki M, Sakimura K. (2001) The Lurcher mutation reveals Ca2+ permeability and PKC modification of the GluRdelta channels. Neurosci Res 41:193–200 [DOI] [PubMed] [Google Scholar]
- Ikonomidou and Turski, 2002.Ikonomidou C, Turski L. (2002) Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol 1:383–386 [DOI] [PubMed] [Google Scholar]
- Ilyin et al., 1996.Ilyin VI, Whittemore ER, Guastella J, Weber E, Woodward RM. (1996) Subtype-selective inhibition of N-methyl-d-aspartate receptors by haloperidol. Mol Pharmacol 50:1541–1550 [PubMed] [Google Scholar]
- Inanobe et al., 2005.Inanobe A, Furukawa H, Gouaux E. (2005) Mechanism of partial agonist action at the NR1 subunit of NMDA receptors. Neuron 47:71–84 [DOI] [PubMed] [Google Scholar]
- Ingvar et al., 1997.Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers GA, Schehr RS, Lynch G. (1997) Enhancement by an ampakine of memory encoding in humans. Exp Neurol 146:553–559 [DOI] [PubMed] [Google Scholar]
- Insel, 2006.Insel TR. (2006) Beyond efficacy: the STAR*D trial. Am J Psychiatry 163:5–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irier et al., 2009a.Irier HA, Quan Y, Yoo J, Dingledine R. (2009a) Control of glutamate receptor 2 (GluR2) translational initiation by its alternative 3′ untranslated regions. Mol Pharmacol 76:1145–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irier et al., 2009b.Irier HA, Shaw R, Lau A, Feng Y, Dingledine R. (2009b) Translational regulation of GluR2 mRNAs in rat hippocampus by alternative 3′ untranslated regions. J Neurochem 109:584–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac et al., 1995.Isaac JT, Nicoll RA, Malenka RC. (1995) Evidence for silent synapses: implications for the expression of LTP. Neuron 15:427–434 [DOI] [PubMed] [Google Scholar]
- Isaac et al., 2007.Isaac JT, Ashby M, McBain CJ. (2007) The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54:859–871 [DOI] [PubMed] [Google Scholar]
- Ishii et al., 1993.Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M. (1993) Molecular characterization of the family of the N-methyl-d-aspartate receptor subunits. J Biol Chem 268:2836–2843 [PubMed] [Google Scholar]
- Ito et al., 1990.Ito I, Tanabe S, Kohda A, Sugiyama H. (1990) Allosteric potentiation of quisqualate receptors by a nootropic drug aniracetam. J Physiol 424:533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson et al., 2006.Jackson SN, Wang HY, Yergey A, Woods AS. (2006) Phosphate stabilization of intermolecular interactions. J Proteome Res 5:122–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane et al., 1997.Jane DE, Hoo K, Kamboj R, Deverill M, Bleakman D, Mandelzys A. (1997) Synthesis of willardiine and 6-azawillardiine analogs: pharmacological characterization on cloned homomeric human AMPA and kainate receptor subtypes. J Med Chem 40:3645–3650 [DOI] [PubMed] [Google Scholar]
- Jane et al., 2009.Jane DE, Lodge D, Collingridge GL. (2009) Kainate receptors: pharmacology, function and therapeutic potential. Neuropharmacology 56:90–113 [DOI] [PubMed] [Google Scholar]
- Jang et al., 2004.Jang MK, Mierke DF, Russek SJ, Farb DH. (2004) A steroid modulatory domain on NR2B controls N-methyl-d-aspartate receptor proton sensitivity. Proc Natl Acad Sci USA 101:8198–8203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatzke et al., 2003.Jatzke C, Hernandez M, Wollmuth LP. (2003) Extracellular vestibule determinants of Ca2+ influx in Ca2+-permeable AMPA receptor channels. J Physiol 549:439–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatzke et al., 2002.Jatzke C, Watanabe J, Wollmuth LP. (2002) Voltage and concentration dependence of Ca2+ permeability in recombinant glutamate receptor subtypes. J Physiol 538:25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt, 2007.Javitt DC. (2007) Glutamate and schizophrenia: phencyclidine, N-methyl-d-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol 78:69–108 [DOI] [PubMed] [Google Scholar]
- Javitt, 2008.Javitt DC. (2008) Glycine transport inhibitors and the treatment of schizophrenia. Biol Psychiatry 63:6–8 [DOI] [PubMed] [Google Scholar]
- Javitt, 2009.Javitt DC. (2009) Glycine transport inhibitors for the treatment of schizophrenia: symptom and disease modification. Curr Opin Drug Discov Devel 12:468–478 [PubMed] [Google Scholar]
- Javitt and Zukin, 1991.Javitt DC, Zukin SR. (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148:1301–1308 [DOI] [PubMed] [Google Scholar]
- Jenkins et al., 2010.Jenkins MA, Kristensen AS, Traynelis SF. (2010) Stargazin and the GluA1 C-terminus influence the effects of CamKII-mediated phosphorylation on AMPA-R conductance, at the 54th Annual Meeting of the Biophysical Society; 2010 Feb 20–24; San Francisco, CA Poster L-220 Biophysical Society, Bethesda, MD [Google Scholar]
- Jensen et al., 2007.Jensen AA, Christesen T, Bølcho U, Greenwood JR, Postorino G, Vogensen SB, Johansen TN, Egebjerg J, Bräuner-Osborne H, Clausen RP. (2007) Functional characterization of Tet-AMPA [tetrazolyl-2-amino-3-(3-hydroxy-5-methyl- 4-isoxazolyl)propionic acid] analogues at ionotropic glutamate receptors GluR1-GluR4. The molecular basis for the functional selectivity profile of 2-Bn-Tet-AMPA. J Med Chem 50:4177–4185 [DOI] [PubMed] [Google Scholar]
- Jia et al., 2006.Jia YH, Zhu X, Li SY, Ni JH, Jia HT. (2006) Kainate exposure suppresses activation of GluR2 subunit promoter in primary cultured cerebral cortical neurons through induction of RE1-silencing transcription factor. Neurosci Lett 403:103–108 [DOI] [PubMed] [Google Scholar]
- Jia et al., 1996.Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna FA, Velumian A, MacDonald J, Carlen P, et al. (1996) Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron 17:945–956 [DOI] [PubMed] [Google Scholar]
- Jiang et al., 2002.Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. (2002) The open pore conformation of potassium channels. Nature 417:523–526 [DOI] [PubMed] [Google Scholar]
- Jin et al., 2007.Jin L, Sugiyama H, Takigawa M, Katagiri D, Tomitori H, Nishimura K, Kaur N, Phanstiel O, 4th, Kitajima M, Takayama H, et al. (2007) Comparative studies of anthraquinone- and anthracene-tetraamines as blockers of N-methyl-d-aspartate receptors. J Pharmacol Exp Ther 320:47–55 [DOI] [PubMed] [Google Scholar]
- Jin et al., 2003.Jin R, Banke TG, Mayer ML, Traynelis SF, Gouaux E. (2003) Structural basis for partial agonist action at ionotropic glutamate receptors. Nat Neurosci 6:803–810 [DOI] [PubMed] [Google Scholar]
- Jin et al., 2005.Jin R, Clark S, Weeks AM, Dudman JT, Gouaux E, Partin KM. (2005) Mechanism of positive allosteric modulators acting on AMPA receptors. J Neurosci 25:9027–9036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin and Gouaux, 2003.Jin R, Gouaux E. (2003) Probing the function, conformational plasticity, and dimer-dimer contacts of the GluR2 ligand-binding core: studies of 5-substituted willardiines and GluR2 S1S2 in the crystal. Biochemistry 42:5201–5213 [DOI] [PubMed] [Google Scholar]
- Jin et al., 2002.Jin R, Horning M, Mayer ML, Gouaux E. (2002) Mechanism of activation and selectivity in a ligand-gated ion channel: structural and functional studies of GluR2 and quisqualate. Biochemistry 41:15635–15643 [DOI] [PubMed] [Google Scholar]
- Jin et al., 2009.Jin R, Singh SK, Gu S, Furukawa H, Sobolevsky AI, Zhou J, Jin Y, Gouaux E. (2009) Crystal structure and association behaviour of the GluR2 amino-terminal domain. EMBO J 28:1812–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo et al., 1999.Jo K, Derin R, Li M, Bredt DS. (1999) Characterization of MALS/Velis-1, -2, and -3: a family of mammalian LIN-7 homologs enriched at brain synapses in association with the postsynaptic density-95/NMDA receptor postsynaptic complex. J Neurosci 19:4189–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen et al., 1995.Johansen TH, Chaudhary A, Verdoorn TA. (1995) Interactions among GYKI-52466, cyclothiazide, and aniracetam at recombinant AMPA and kainate receptors. Mol Pharmacol 48:946–955 [PubMed] [Google Scholar]
- Johnson and Ascher, 1987.Johnson JW, Ascher P. (1987) Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325:529–531 [DOI] [PubMed] [Google Scholar]
- Johnson, 1996.Johnson TD. (1996) Modulation of channel function by polyamines. Trends Pharmacol Sci 17:22–27 [DOI] [PubMed] [Google Scholar]
- Jonas et al., 2004.Jonas P, Bischofberger J, Fricker D, Miles R. (2004) Interneuron Diversity series: fast in, fast out—temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci 27:30–40 [DOI] [PubMed] [Google Scholar]
- Jones et al., 2006.Jones CK, Alt A, Ogden AM, Bleakman D, Simmons RM, Iyengar S, Dominguez E, Ornstein PL, Shannon HE. (2006) Antiallodynic and antihyperalgesic effects of selective competitive GLUK5 (GluR5) ionotropic glutamate receptor antagonists in the capsaicin and carrageenan models in rats. J Pharmacol Exp Ther 319:396–404 [DOI] [PubMed] [Google Scholar]
- Jones et al., 2002.Jones KS, VanDongen HM, VanDongen AM. (2002) The NMDA receptor M3 segment is a conserved transduction element coupling ligand binding to channel opening. J Neurosci 22:2044–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones et al., 1990.Jones MG, Anis NA, Lodge D. (1990) Philanthotoxin blocks quisqualate-, AMPA- and kainate-, but not NMDA-, induced excitation of rat brainstem neurones in vivo. Br J Pharmacol 101:968–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones and Leonard, 2005.Jones ML, Leonard JP. (2005) PKC site mutations reveal differential modulation by insulin of NMDA receptors containing NR2A or NR2B subunits. J Neurochem 92:1431–1438 [DOI] [PubMed] [Google Scholar]
- Jones and Gibb, 2005.Jones S, Gibb AJ. (2005) Functional NR2B- and NR2D-containing NMDA receptor channels in rat substantia nigra dopaminergic neurons. J Physiol 569:209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju et al., 2004.Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. (2004) Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci 7:244–253 [DOI] [PubMed] [Google Scholar]
- Kakegawa et al., 2007b.Kakegawa W, Kohda K, Yuzaki M. (2007b) The delta2 ‘ionotropic’ glutamate receptor functions as a non-ionotropic receptor to control cerebellar synaptic plasticity. J Physiol 584:89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa et al., 2007a.Kakegawa W, Miyazaki T, Hirai H, Motohashi J, Mishina M, Watanabe M, Yuzaki M. (2007a) Ca2+ permeability of the channel pore is not essential for the delta2 glutamate receptor to regulate synaptic plasticity and motor coordination. J Physiol 579:729–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa et al., 2009.Kakegawa W, Miyazaki T, Kohda K, Matsuda K, Emi K, Motohashi J, Watanabe M, Yuzaki M. (2009) The N-terminal domain of GluD2 (GluRdelta2) recruits presynaptic terminals and regulates synaptogenesis in the cerebellum in vivo. J Neurosci 29:5738–5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku et al., 1993.Kaku DA, Giffard RG, Choi DW. (1993) Neuroprotective effects of glutamate antagonists and extracellular acidity. Science 260:1516–1518 [DOI] [PubMed] [Google Scholar]
- Kalia et al., 2008.Kalia LV, Kalia SK, Salter MW. (2008) NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol 7:742–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboj et al., 1995.Kamboj SK, Swanson GT, Cull-Candy SG. (1995) Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol 486:297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa et al., 2004.Kampa BM, Clements J, Jonas P, Stuart GJ. (2004) Kinetics of Mg2+ unblock of NMDA receptors: implications for spike-timing dependent synaptic plasticity. J Physiol 556:337–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemitsu et al., 2003.Kanemitsu Y, Hosoi M, Zhu PJ, Weight FF, Peoples RW, McLaughlin JS, Zhang L. (2003) Dynorphin A inhibits NMDA receptors through a pH-dependent mechanism. Mol Cell Neurosci 24:525–537 [DOI] [PubMed] [Google Scholar]
- Kang and Schuman, 1996.Kang H, Schuman EM. (1996) A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science 273:1402–1406 [DOI] [PubMed] [Google Scholar]
- Káradóttir et al., 2005.Káradóttir R, Cavelier P, Bergersen LH, Attwell D. (2005) NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438:1162–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas et al., 2009.Karakas E, Simorowski N, Furukawa H. (2009) Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. EMBO J 28:3910–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash et al., 2008.Kash TL, Matthews RT, Winder DG. (2008) Alcohol inhibits NR2B-containing NMDA receptors in the ventral bed nucleus of the stria terminalis. Neuropsychopharmacology 33:1379–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwabuchi et al., 1995.Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Yagi T, Kang Y. (1995) Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell 81:245–252 [DOI] [PubMed] [Google Scholar]
- Kashiwagi et al., 1996.Kashiwagi K, Fukuchi J, Chao J, Igarashi K, Williams K. (1996) An aspartate residue in the extracellular loop of the N-methyl-d-aspartate receptor controls sensitivity to spermine and protons. Mol Pharmacol 49:1131–1141 [PubMed] [Google Scholar]
- Kashiwagi et al., 2002.Kashiwagi K, Masuko T, Nguyen CD, Kuno T, Tanaka I, Igarashi K, Williams K. (2002) Channel blockers acting at N-methyl-d-aspartate receptors: differential effects of mutations in the vestibule and ion channel pore. Mol Pharmacol 61:533–545 [DOI] [PubMed] [Google Scholar]
- Kashiwagi et al., 1997.Kashiwagi K, Pahk AJ, Masuko T, Igarashi K, Williams K. (1997) Block and modulation of N-methyl-d-aspartate receptors by polyamines and protons: role of amino acid residues in the transmembrane and pore-forming regions of NR1 and NR2 subunits. Mol Pharmacol 52:701–713 [DOI] [PubMed] [Google Scholar]
- Kasper et al., 2006.Kasper C, Pickering DS, Mirza O, Olsen L, Kristensen AS, Greenwood JR, Liljefors T, Schousboe A, Wätjen F, Gajhede M, et al. (2006) The structure of a mixed GluR2 ligand-binding core dimer in complex with (S)-glutamate and the antagonist (S)-NS1209. J Mol Biol 357:1184–1201 [DOI] [PubMed] [Google Scholar]
- Kastning et al., 2007.Kastning K, Kukhtina V, Kittler JT, Chen G, Pechstein A, Enders S, Lee SH, Sheng M, Yan Z, Haucke V. (2007) Molecular determinants for the interaction between AMPA receptors and the clathrin adaptor complex AP-2. Proc Natl Acad Sci USA 104:2991–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato et al., 2008.Kato AS, Siuda ER, Nisenbaum ES, Bredt DS. (2008) AMPA receptor subunit-specific regulation by a distinct family of type II TARPs. Neuron 59:986–996 [DOI] [PubMed] [Google Scholar]
- Kato et al., 2007.Kato AS, Zhou W, Milstein AD, Knierman MD, Siuda ER, Dotzlaf JE, Yu H, Hale JE, Nisenbaum ES, Nicoll RA, et al. (2007) New transmembrane AMPA receptor regulatory protein isoform, gamma-7, differentially regulates AMPA receptors. J Neurosci 27:4969–4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai et al., 2007.Kawai M, Ando K, Matsumoto Y, Sakurada I, Hirota M, Nakamura H, Ohta A, Sudo M, Hattori K, Takashima T, et al. (2007) Discovery of (−)-6-[2-[4-(3-fluorophenyl)-4-hydroxy-l-piperidinyl]-1-hydroxyethyl]-3, 4-dihydro-2(1H)-quinolinone—a potent NR2B-selective N-methyl d-aspartate (NMDA) antagonist for the treatment of pain. Bioorg Med Chem Lett 17:5558–5562 [DOI] [PubMed] [Google Scholar]
- Kaye et al., 2006.Kaye SL, Sansom MS, Biggin PC. (2006) Molecular dynamics simulations of the ligand-binding domain of an N-methyl-d-aspartate receptor. J Biol Chem 281:12736–12742 [DOI] [PubMed] [Google Scholar]
- Kemp et al., 1988.Kemp JA, Foster AC, Leeson PD, Priestley T, Tridgett R, Iversen LL, Woodruff GN. (1988) 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-d-aspartate receptor complex. Proc Natl Acad Sci USA 85:6547–6550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner and Nicoll, 2008.Kerchner GA, Nicoll RA. (2008) Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci 9:813–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels and Malinow, 2009.Kessels HW, Malinow R. (2009) Synaptic AMPA receptor plasticity and behavior. Neuron 61:340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler et al., 1989.Kessler M, Terramani T, Lynch G, Baudry M. (1989) A glycine site associated with N-methyl-d-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem 52:1319–1328 [DOI] [PubMed] [Google Scholar]
- Kessler et al., 2003.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PSNational Comorbidity Survey Replication (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289:3095–3105 [DOI] [PubMed] [Google Scholar]
- Kew et al., 1996.Kew JN, Trube G, Kemp JA. (1996) A novel mechanism of activity-dependent NMDA receptor antagonism describes the effect of ifenprodil in rat cultured cortical neurones. J Physiol 497:761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew et al., 1998.Kew JN, Trube G, Kemp JA. (1998) State-dependent NMDA receptor antagonism by Ro 8–4304, a novel NR2B selective, non-competitive, voltage-independent antagonist. Br J Pharmacol 123:463–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd and Isaac, 1999.Kidd FL, Isaac JT. (1999) Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature 400:569–573 [DOI] [PubMed] [Google Scholar]
- Kidd and Isaac, 2001.Kidd FL, Isaac JT. (2001) Kinetics and activation of postsynaptic kainate receptors at thalamocortical synapses: role of glutamate clearance. J Neurophysiol 86:1139–1148 [DOI] [PubMed] [Google Scholar]
- Kielland et al., 2009.Kielland A, Bochorishvili G, Corson J, Zhang L, Rosin DL, Heggelund P, Zhu JJ. (2009) Activity patterns govern synapse-specific AMPA receptor trafficking between deliverable and synaptic pools. Neuron 62:84–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinarsky et al., 2005.Kinarsky L, Feng B, Skifter DA, Morley RM, Sherman S, Jane DE, Monaghan DT. (2005) Identification of subunit- and antagonist-specific amino acid residues in the N-methyl-d-aspartate receptor glutamate-binding pocket. J Pharmacol Exp Ther 313:1066–1074 [DOI] [PubMed] [Google Scholar]
- Kiskin et al., 1989.Kiskin NI, Kryshtal' OA, Tsyndrenko AIa, Volkova TM, Grishin EV. (1989) [Argiopine, argiopinines and pseudoargiopinines—blockers of the glutamate receptors in hippocampal neurons]. Neirofiziologiia 21:748–756 [PubMed] [Google Scholar]
- Kistler and Fleck, 2007.Kistler T, Fleck MW. (2007) Functional consequences of natural substitutions in the GluR6 kainate receptor subunit ligand-binding site. Channels (Austin) 1:417–428 [DOI] [PubMed] [Google Scholar]
- Kizelsztein et al., 2000.Kizelsztein P, Eisenstein M, Strutz N, Hollmann M, Teichberg VI. (2000) Mutant cycle analysis of the active and desensitized states of an AMPA receptor induced by wllardiines. Biochemistry 39:12819–12827 [DOI] [PubMed] [Google Scholar]
- Kleckner and Dingledine, 1988.Kleckner NW, Dingledine R. (1988) Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science 241:835–837 [DOI] [PubMed] [Google Scholar]
- Kleckner and Dingledine, 1989.Kleckner NW, Dingledine R. (1989) Selectivity of quinoxalines and kynurenines as antagonists of the glycine site on N-methyl-d-aspartate receptors. Mol Pharmacol 36:430–436 [PubMed] [Google Scholar]
- Klein et al., 1998.Klein M, Pieri I, Uhlmann F, Pfizenmaier K, Eisel U. (1998) Cloning and characterization of promoter and 5′-UTR of the NMDA receptor subunit epsilon 2: evidence for alternative splicing of 5′-non-coding exon. Gene 208:259–269 [DOI] [PubMed] [Google Scholar]
- Klein et al., 2001.Klein RC, Prorok M, Galdzicki Z, Castellino FJ. (2001) The amino acid residue at sequence position 5 in the conantokin peptides partially governs subunit-selective antagonism of recombinant N-methyl-d-aspartate receptors. J Biol Chem 276:26860–26867 [DOI] [PubMed] [Google Scholar]
- Klein and Howe, 2004.Klein RM, Howe JR. (2004) Effects of the lurcher mutation on GluR1 desensitization and activation kinetics. J Neurosci 24:4941–4951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloda et al., 2004.Kloda A, Clements JD, Lewis RJ, Adams DJ. (2004) Adenosine triphosphate acts as both a competitive antagonist and a positive allosteric modulator at recombinant N-methyl-d-aspartate receptors. Mol Pharmacol 65:1386–1396 [DOI] [PubMed] [Google Scholar]
- Ko et al., 2008.Ko SW, Wu LJ, Shum F, Quan J, Zhuo M. (2008) Cingulate NMDA NR2B receptors contribute to morphine-induced analgesic tolerance. Mol Brain 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohda et al., 2000.Kohda K, Wang Y, Yuzaki M. (2000) Mutation of a glutamate receptor motif reveals its role in gating and delta2 receptor channel properties. Nat Neurosci 3:315–322 [DOI] [PubMed] [Google Scholar]
- Köhr, 2006.Köhr G. (2006) NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res 326:439–446 [DOI] [PubMed] [Google Scholar]
- Köhr et al., 1994.Köhr G, Eckardt S, Lüddens H, Monyer H, Seeburg PH. (1994) NMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron 12:1031–1040 [DOI] [PubMed] [Google Scholar]
- Köhr and Seeburg, 1996.Köhr G, Seeburg PH. (1996) Subtype-specific regulation of recombinant NMDA receptor-channels by protein tyrosine kinases of the src family. J Physiol 492:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike et al., 1997.Koike M, Iino M, Ozawa S. (1997) Blocking effect of 1-naphthyl acetyl spermine on Ca2+-permeable AMPA receptors in cultured rat hippocampal neurons. Neurosci Res 29:27–36 [DOI] [PubMed] [Google Scholar]
- Koike et al., 2000.Koike M, Tsukada S, Tsuzuki K, Kijima H, Ozawa S. (2000) Regulation of kinetic properties of GluR2 AMPA receptor channels by alternative splicing. J Neurosci 20:2166–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo et al., 2005.Kondo T, Kakegawa W, Yuzaki M. (2005) Induction of long-term depression and phosphorylation of the delta2 glutamate receptor by protein kinase C in cerebellar slices. Eur J Neurosci 22:1817–1820 [DOI] [PubMed] [Google Scholar]
- Körber et al., 2007.Körber C, Werner M, Hoffmann J, Sager C, Tietze M, Schmid SM, Kott S, Hollmann M. (2007) Stargazin interaction with alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptors is critically dependent on the amino acid at the narrow constriction of the ion channel. J Biol Chem 282:18758–18766 [DOI] [PubMed] [Google Scholar]
- Kornau et al., 1995.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. (1995) Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269:1737–1740 [DOI] [PubMed] [Google Scholar]
- Kornreich et al., 2007.Kornreich BG, Niu L, Roberson MS, Oswald RE. (2007) Identification of C-terminal domain residues involved in protein kinase A-mediated potentiation of kainate receptor subtype 6. Neuroscience 146:1158–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotermanski and Johnson, 2009.Kotermanski SE, Johnson JW. (2009) Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer's drug memantine. J Neurosci 29:2774–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotermanski et al., 2009.Kotermanski SE, Wood JT, Johnson JW. (2009) Memantine binding to a superficial site on NMDA receptors contributes to partial trapping. J Physiol 587:4589–4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kott et al., 2009.Kott S, Sager C, Tapken D, Werner M, Hollmann M. (2009) Comparative analysis of the pharmacology of GluR1 in complex with transmembrane AMPA receptor regulatory proteins gamma2, gamma3, gamma4, and gamma8. Neuroscience 158:78–88 [DOI] [PubMed] [Google Scholar]
- Kott et al., 2007.Kott S, Werner M, Körber C, Hollmann M. (2007) Electrophysiological properties of AMPA receptors are differentially modulated depending on the associated member of the TARP family. J Neurosci 27:3780–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk et al., 1994.Kovalchuk Y, Miller B, Sarantis M, Attwell D. (1994) Arachidonic acid depresses non-NMDA receptor currents. Brain Res 643:287–295 [DOI] [PubMed] [Google Scholar]
- Krainc et al., 1998.Krainc D, Bai G, Okamoto S, Carles M, Kusiak JW, Brent RN, Lipton SA. (1998) Synergistic activation of the N-methyl-d-aspartate receptor subunit 1 promoter by myocyte enhancer factor 2C and Sp1. J Biol Chem 273:26218–26224 [DOI] [PubMed] [Google Scholar]
- Krampfl et al., 2001.Krampfl K, Schlesinger F, Wolfes H, Dengler R, Bufler J. (2001) Functional diversity of recombinant human AMPA type glutamate receptors: possible implications for selective vulnerability of motor neurons. J Neurol Sci 191:19–23 [DOI] [PubMed] [Google Scholar]
- Kreitzer et al., 2009.Kreitzer MA, Birnbaum AD, Qian H, Malchow RP. (2009) Pharmacological characterization, localization, and regulation of ionotropic glutamate receptors in skate horizontal cells. Vis Neurosci 26:375–387 [DOI] [PubMed] [Google Scholar]
- Kromann et al., 2002.Kromann H, Krikstolaityte S, Andersen AJ, Andersen K, Krogsgaard-Larsen P, Jaroszewski JW, Egebjerg J, Strømgaard K. (2002) Solid-phase synthesis of polyamine toxin analogues: potent and selective antagonists of Ca2+-permeable AMPA receptors. J Med Chem 45:5745–5754 [DOI] [PubMed] [Google Scholar]
- Krupp et al., 1996.Krupp JJ, Vissel B, Heinemann SF, Westbrook GL. (1996) Calcium-dependent inactivation of recombinant N-methyl-d-aspartate receptors is NR2 subunit specific. Mol Pharmacol 50:1680–1688 [PubMed] [Google Scholar]
- Krupp et al., 1998.Krupp JJ, Vissel B, Heinemann SF, Westbrook GL. (1998) N-terminal domains in the NR2 subunit control desensitization of NMDA receptors. Neuron 20:317–327 [DOI] [PubMed] [Google Scholar]
- Krupp et al., 2002.Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. (2002) Calcineurin acts via the C-terminus of NR2A to modulate desensitization of NMDA receptors. Neuropharmacology 42:593–602 [DOI] [PubMed] [Google Scholar]
- Krystal et al., 2002.Krystal JH, Anand A, Moghaddam B. (2002) Effects of NMDA receptor antagonists: implications for the pathophysiology of schizophrenia. Arch Gen Psychiatry 59:663–664 [DOI] [PubMed] [Google Scholar]
- Kumamoto, 1996.Kumamoto E. (1996) Neuromodulation by Mg2+ and polyamines of excitatory amino acid currents in rodent neurones in culture. Magnes Res 9:317–327 [PubMed] [Google Scholar]
- Kumar et al., 2009.Kumar J, Schuck P, Jin R, Mayer ML. (2009) The N-terminal domain of GluR6-subtype glutamate receptor ion channels. Nat Struct Mol Biol 16:631–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar et al., 2002.Kumar SS, Bacci A, Kharazia V, Huguenard JR. (2002) A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci 22:3005–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner et al., 2001.Kuner T, Beck C, Sakmann B, Seeburg PH. (2001) Channel-lining residues of the AMPA receptor M2 segment: structural environment of the Q/R site and identification of the selectivity filter. J Neurosci 21:4162–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner and Schoepfer, 1996.Kuner T, Schoepfer R. (1996) Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. J Neurosci 16:3549–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner et al., 1993.Kuner T, Schoepfer R, Korpi ER. (1993) Ethanol inhibits glutamate-induced currents in heteromeric NMDA receptor subtypes. Neuroreport 5:297–300 [DOI] [PubMed] [Google Scholar]
- Kuner et al., 2003.Kuner T, Seeburg PH, Guy HR. (2003) A common architecture for K+ channels and ionotropic glutamate receptors? Trends Neurosci 26:27–32 [DOI] [PubMed] [Google Scholar]
- Kuner et al., 1996.Kuner T, Wollmuth LP, Karlin A, Seeburg PH, Sakmann B. (1996) Structure of the NMDA receptor channel M2 segment inferred from the accessibility of substituted cysteines. Neuron 17:343–352 [DOI] [PubMed] [Google Scholar]
- Kunishima et al., 2000.Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. (2000) Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407:971–977 [DOI] [PubMed] [Google Scholar]
- Kuroyanagi et al., 2009.Kuroyanagi T, Yokoyama M, Hirano T. (2009) Postsynaptic glutamate receptor delta family contributes to presynaptic terminal differentiation and establishment of synaptic transmission. Proc Natl Acad Sci USA 106:4912–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov et al., 1994.Kuryatov A, Laube B, Betz H, Kuhse J. (1994) Mutational analysis of the glycine-binding site of the NMDA receptor: structural similarity with bacterial amino acid-binding proteins. Neuron 12:1291–1300 [DOI] [PubMed] [Google Scholar]
- Kushner et al., 2007.Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, McCabe J, Peterson J, Foa EB. (2007) d-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry 62:835–838 [DOI] [PubMed] [Google Scholar]
- Kussius et al., 2009.Kussius CL, Kaur N, Popescu GK. (2009) Pregnanolone sulfate promotes desensitization of activated NMDA receptors. J Neurosci 29:6819–6827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuwada et al., 1992.Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M. (1992) Molecular diversity of the NMDA receptor channel. Nature 358:36–41 [DOI] [PubMed] [Google Scholar]
- Kuusinen et al., 1999.Kuusinen A, Abele R, Madden DR, Keinänen K. (1999) Oligomerization and ligand-binding properties of the ectodomain of the alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunit GluRD. J Biol Chem 274:28937–28943 [DOI] [PubMed] [Google Scholar]
- Kwak et al., 1992.Kwak S, Aizawa H, Ishida M, Shinozaki H. (1992) New, potent kainate derivatives: comparison of their affinity for [3H]kainate and [3H]AMPA binding sites. Neurosci Lett 139:114–117 [DOI] [PubMed] [Google Scholar]
- Kwon and Castillo, 2008.Kwon HB, Castillo PE. (2008) Role of glutamate autoreceptors at hippocampal mossy fiber synapses. Neuron 60:1082–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie and Roder, 2010.Labrie V, Roder JC. (2010) The involvement of the NMDA receptor d-serine/glycine site in the pathophysiology and treatment of schizophrenia. Neurosci Biobehav Rev 34:351–372 [DOI] [PubMed] [Google Scholar]
- Laezza et al., 1999.Laezza F, Doherty JJ, Dingledine R. (1999) Long-term depression in hippocampal interneurons: joint requirement for pre- and postsynaptic events. Science 285:1411–1414 [DOI] [PubMed] [Google Scholar]
- Lai et al., 1998.Lai SL, Gu Y, Huang LY. (1998) Dynorphin uses a non-opioid mechanism to potentiate N-methyl-d-aspartate currents in single rat periaqueductal gray neurons. Neurosci Lett 247:115–118 [DOI] [PubMed] [Google Scholar]
- Lampinen et al., 1998.Lampinen M, Pentikäinen O, Johnson MS, Keinänen K. (1998) AMPA receptors and bacterial periplasmic amino acid-binding proteins share the ionic mechanism of ligand recognition. EMBO J 17:4704–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane et al., 2005.Lane HY, Chang YC, Liu YC, Chiu CC, Tsai GE. (2005) Sarcosine or D-serine add-on treatment for acute exacerbation of schizophrenia: a randomized, double-blind, placebo-controlled study. Arch Gen Psychiatry 62:1196–1204 [DOI] [PubMed] [Google Scholar]
- Lane et al., 2006.Lane HY, Huang CL, Wu PL, Liu YC, Chang YC, Lin PY, Chen PW, Tsai G. (2006) Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to clozapine for the treatment of schizophrenia. Biol Psychiatry 60:645–649 [DOI] [PubMed] [Google Scholar]
- Lane et al., 2008.Lane HY, Liu YC, Huang CL, Chang YC, Liau CH, Perng CH, Tsai GE. (2008) Sarcosine (N-methylglycine) treatment for acute schizophrenia: a randomized, double-blind study. Biol Psychiatry 63:9–12 [DOI] [PubMed] [Google Scholar]
- Lang and Lees, 2002.Lang AE, Lees A. (2002) Management of Parkinson's disease: An evidence-based review. Mov Disord 17:S1–S166 [DOI] [PubMed] [Google Scholar]
- Lash et al., 2008.Lash LL, Sanders JM, Akiyama N, Shoji M, Postila P, Pentikäinen OT, Sasaki M, Sakai R, Swanson GT. (2008) Novel analogs and stereoisomers of the marine toxin neodysiherbaine with specificity for kainate receptors. J Pharmacol Exp Ther 324:484–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau and Roux, 2007.Lau AY, Roux B. (2007) The free energy landscapes governing conformational changes in a glutamate receptor ligand-binding domain. Structure 15:1203–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau and Huganir, 1995.Lau LF, Huganir RL. (1995) Differential tyrosine phosphorylation of N-methyl-d-aspartate receptor subunits. J Biol Chem 270:20036–20041 [DOI] [PubMed] [Google Scholar]
- Laube et al., 1998.Laube B, Kuhse J, Betz H. (1998) Evidence for a tetrameric structure of recombinant NMDA receptors. J Neurosci 18:2954–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube et al., 2004.Laube B, Schemm R, Betz H. (2004) Molecular determinants of ligand discrimination in the glutamate-binding pocket of the NMDA receptor. Neuropharmacology 47:994–1007 [DOI] [PubMed] [Google Scholar]
- Lauterborn et al., 2000.Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM. (2000) Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci 20:8–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzari et al., 2004.Lavezzari G, McCallum J, Dewey CM, Roche KW. (2004) Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci 24:6383–6391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence and McBain, 2003.Lawrence JJ, McBain CJ. (2003) Interneuron diversity series: containing the detonation—feedforward inhibition in the CA3 hippocampus. Trends Neurosci 26:631–640 [DOI] [PubMed] [Google Scholar]
- Layer et al., 2004.Layer RT, Wagstaff JD, White HS. (2004) Conantokins: peptide antagonists of NMDA receptors. Curr Med Chem 11:3073–3084 [DOI] [PubMed] [Google Scholar]
- Layton et al., 2006.Layton ME, Kelly MJ, 3rd, Rodzinak KJ. (2006) Recent advances in the development of NR2B subtype-selective NMDA receptor antagonists. Curr Top Med Chem 6:697–709 [DOI] [PubMed] [Google Scholar]
- Lazzaro et al., 2002.Lazzaro JT, Paternain AV, Lerma J, Chenard BL, Ewing FE, Huang J, Welch WM, Ganong AH, Menniti FS. (2002) Functional characterization of CP-465,022, a selective, noncompetitive AMPA receptor antagonist. Neuropharmacology 42:143–153 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2007a.Lee CJ, Mannaioni G, Yuan H, Woo DH, Gingrich MB, Traynelis SF. (2007a) Astrocytic control of synaptic NMDA receptors. J Physiol 581:1057–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al., 2000.Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. (2000) Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405:955–959 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2003.Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, et al. (2003) Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112:631–643 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2007b.Lee HK, Takamiya K, Kameyama K, He K, Yu S, Rossetti L, Wilen D, Huganir RL. (2007b) Identification and characterization of a novel phosphorylation site on the GluR1 subunit of AMPA receptors. Mol Cell Neurosci 36:86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al., 2002.Lee SH, Liu L, Wang YT, Sheng M. (2002) Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron 36:661–674 [DOI] [PubMed] [Google Scholar]
- Lees et al., 2000.Lees KR, Asplund K, Carolei A, Davis SM, Diener HC, Kaste M, Orgogozo JM, Whitehead J. (2000) Glycine antagonist (gavestinel) in neuroprotection (GAIN International) in patients with acute stroke: a randomised controlled trial. GAIN International Investigators. Lancet 355:1949–1954 [DOI] [PubMed] [Google Scholar]
- Legendre et al., 1993.Legendre P, Rosenmund C, Westbrook GL. (1993) Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J Neurosci 13:674–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre and Westbrook, 1990.Legendre P, Westbrook GL. (1990) The inhibition of single N-methyl-d-aspartate-activated channels by zinc ions on cultured rat neurones. J Physiol 429:429–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei and McBain, 2002.Lei S, McBain CJ. (2002) Distinct NMDA receptors provide differential modes of transmission at mossy fiber-interneuron synapses. Neuron 33:921–933 [DOI] [PubMed] [Google Scholar]
- Lei et al., 2001.Lei S, Orser BA, Thatcher GR, Reynolds JN, MacDonald JF. (2001) Positive allosteric modulators of AMPA receptors reduce proton-induced receptor desensitization in rat hippocampal neurons. J Neurophysiol 85:2030–2038 [DOI] [PubMed] [Google Scholar]
- Leonard et al., 2002.Leonard AS, Bayer KU, Merrill MA, Lim IA, Shea MA, Schulman H, Hell JW. (2002) Regulation of calcium/calmodulin-dependent protein kinase II docking to N-methyl-d-aspartate receptors by calcium/calmodulin and alpha-actinin. J Biol Chem 277:48441–48448 [DOI] [PubMed] [Google Scholar]
- Leonard et al., 1998.Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. (1998) SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem 273:19518–19524 [DOI] [PubMed] [Google Scholar]
- Leonard et al., 1999.Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW. (1999) Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-d-aspartate receptor. Proc Natl Acad Sci USA 96:3239–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePage et al., 2005.LePage KT, Ishmael JE, Low CM, Traynelis SF, Murray TF. (2005) Differential binding properties of [3H]dextrorphan and [3H]MK-801 in heterologously expressed NMDA receptors. Neuropharmacology 49:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma et al., 1990.Lerma J, Zukin RS, Bennett MV. (1990) Glycine decreases desensitization of N-methyl-d-aspartate (NMDA) receptors expressed in Xenopus oocytes and Is required for NMDA responses. Proc Natl Acad Sci USA 87:2354–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester et al., 1990.Lester RA, Clements JD, Westbrook GL, Jahr CE. (1990) Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature 346:565–567 [DOI] [PubMed] [Google Scholar]
- Lester et al., 1993.Lester RA, Tong G, Jahr CE. (1993) Interactions between the glycine and glutamate binding sites of the NMDA receptor. J Neurosci 13:1088–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letts et al., 1998.Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS, 2nd, Mori Y, Campbell KP, Frankel WN. (1998) The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat Genet 19:340–347 [DOI] [PubMed] [Google Scholar]
- Leung et al., 2007.Leung HW, Wong PT, Low CM. (2007) Regulation of NR1/NR2B NMDA receptor function by thrombin. Soc Neuosci Abstr 33:678.16/F28 [Google Scholar]
- Leuschner and Hoch, 1999.Leuschner WD, Hoch W. (1999) Subtype-specific assembly of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits is mediated by their N-terminal domains. J Biol Chem 274:16907–16916 [DOI] [PubMed] [Google Scholar]
- Léveillé et al., 2008.Léveillé F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. (2008) Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J 22:4258–4271 [DOI] [PubMed] [Google Scholar]
- Li et al., 2001.Li BS, Sun MK, Zhang L, Takahashi S, Ma W, Vinade L, Kulkarni AB, Brady RO, Pant HC. (2001) Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc Natl Acad Sci USA 98:12742–12747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao et al., 1995.Liao D, Hessler NA, Malinow R. (1995) Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375:400–404 [DOI] [PubMed] [Google Scholar]
- Lin and Huganir, 2007.Lin DT, Huganir RL. (2007) PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J Neurosci 27:13903–13908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al., 2009.Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL. (2009) Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci 12:879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al., 1995.Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. (1995) Inhibition of nuclear translocation of transcription factor NF-κB by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem 270:14255–14258 [DOI] [PubMed] [Google Scholar]
- Lindén et al., 2001.Lindén AM, Yu H, Zarrinmayeh H, Wheeler WJ, Skolnick P. (2001) Binding of an AMPA receptor potentiator ([3H]LY395153) to native and recombinant AMPA receptors. Neuropharmacology 40:1010–1018 [DOI] [PubMed] [Google Scholar]
- Lipton et al., 2002.Lipton SA, Choi YB, Takahashi H, Zhang D, Li W, Godzik A, Bankston LA. (2002) Cysteine regulation of protein function—as exemplified by NMDA-receptor modulation. Trends Neurosci 25:474–480 [DOI] [PubMed] [Google Scholar]
- Lisman et al., 2008.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. (2008) Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci 31:234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al., 2004a.Liu A, Hoffman PW, Lu W, Bai G. (2004a) NF-κB site interacts with Sp factors and up-regulates the NR1promoter during neuronal differentiation. J Biol Chem 279:17449–17458 [DOI] [PubMed] [Google Scholar]
- Liu et al., 2001.Liu A, Prenger MS, Norton DD, Mei L, Kusiak JW, Bai G. (2001) Nerve growth factor uses Ras/ERK and phosphatidylinositol 3-kinase cascades to up-regulate the N-methyl-d-aspartate receptor 1 promoter. J Biol Chem 276:45372–45379 [DOI] [PubMed] [Google Scholar]
- Liu et al., 2003.Liu A, Zhuang Z, Hoffman PW, Bai G. (2003) Functional analysis of the rat N-methyl-d-aspartate receptor 2A promoter: multiple transcription start points, positive regulation by Sp factors, and translational regulation. J Biol Chem 278:26423–26434 [DOI] [PubMed] [Google Scholar]
- Liu and Madsen, 1998.Liu GJ, Madsen BW. (1998) Modulatory action of PACAP27 on NMDA receptor channel activity in cultured chick cortical neurons. Brain Res 791:290–294 [DOI] [PubMed] [Google Scholar]
- Liu et al., 2004b.Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. (2004b) Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304:1021–1024 [DOI] [PubMed] [Google Scholar]
- Liu and Cull-Candy, 2000.Liu SQ, Cull-Candy SG. (2000) Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature 405:454–458 [DOI] [PubMed] [Google Scholar]
- Lo et al., 2003.Lo EH, Dalkara T, Moskowitz MA. (2003) Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 4:399–415 [DOI] [PubMed] [Google Scholar]
- Lo et al., 2005.Lo EH, Moskowitz MA, Jacobs TP. (2005) Exciting, radical, suicidal: how brain cells die after stroke. Stroke 36:189–192 [DOI] [PubMed] [Google Scholar]
- Lodge, 2009.Lodge D. (2009) The history of the pharmacology and cloning of ionotropic glutamate receptors and the development of idiosyncratic nomenclature. Neuropharmacology 56:6–21 [DOI] [PubMed] [Google Scholar]
- Lomeli et al., 1994.Lomeli H, Mosbacher J, Melcher T, Höger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A, Seeburg PH. (1994) Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science 266:1709–1713 [DOI] [PubMed] [Google Scholar]
- Löscher et al., 1999.Löscher W, Lehmann H, Behl B, Seemann D, Teschendorf HJ, Hofmann HP, Lubisch W, Höger T, Lemaire HG, Gross G. (1999) A new pyrrolyl-quinoxalinedione series of non-NMDA glutamate receptor antagonists: pharmacological characterization and comparison with NBQX and valproate in the kindling model of epilepsy. Eur J Neurosci 11:250–262 [DOI] [PubMed] [Google Scholar]
- Lovinger, 2002.Lovinger DM. (2002) NMDA receptors lose their inhibitions. Nat Neurosci 5:614–616 [DOI] [PubMed] [Google Scholar]
- Lovinger et al., 1989.Lovinger DM, White G, Weight FF. (1989) Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243:1721–1724 [DOI] [PubMed] [Google Scholar]
- Lovinger et al., 1990.Lovinger DM, White G, Weight FF. (1990) NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci 10:1372–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low et al., 2003.Low CM, Lyuboslavsky P, French A, Le P, Wyatte K, Thiel WH, Marchan EM, Igarashi K, Kashiwagi K, Gernert K, et al. (2003) Molecular determinants of proton-sensitive N-methyl-d-aspartate receptor gating. Mol Pharmacol 63:1212–1222 [DOI] [PubMed] [Google Scholar]
- Low et al., 2000.Low CM, Zheng F, Lyuboslavsky P, Traynelis SF. (2000) Molecular determinants of coordinated proton and zinc inhibition of N-methyl-d-aspartate NR1/NR2A receptors. Proc Natl Acad Sci USA 97:11062–11067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al., 2006.Lu C, Fu Z, Karavanov I, Yasuda RP, Wolfe BB, Buonanno A, Vicini S. (2006) NMDA receptor subtypes at autaptic synapses of cerebellar granule neurons. J Neurophysiol 96:2282–2294 [DOI] [PubMed] [Google Scholar]
- Lu et al., 2001.Lu HC, Gonzalez E, Crair MC. (2001) Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron 32:619–634 [DOI] [PubMed] [Google Scholar]
- Lu et al., 2009.Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. (2009) Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 62:254–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin et al., 2008.Lubin FD, Roth TL, Sweatt JD. (2008) Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci 28:10576–10586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby et al., 1959.Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. (1959) Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry 81:363–369 [DOI] [PubMed] [Google Scholar]
- Lunn et al., 2003.Lunn ML, Hogner A, Stensbøl TB, Gouaux E, Egebjerg J, Kastrup JS. (2003) Three-dimensional structure of the ligand-binding core of GluR2 in complex with the agonist (S)-ATPA: implications for receptor subunit selectivity. J Med Chem 46:872–875 [DOI] [PubMed] [Google Scholar]
- Luo et al., 1997.Luo J, Wang Y, Yasuda RP, Dunah AW, Wolfe BB. (1997) The majority of N-methyl-d-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B). Mol Pharmacol 51:79–86 [DOI] [PubMed] [Google Scholar]
- Lüthi et al., 2004.Lüthi A, Wikström MA, Palmer MJ, Matthews P, Benke TA, Isaac JT, Collingridge GL. (2004) Bi-directional modulation of AMPA receptor unitary conductance by synaptic activity. BMC Neurosci 5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly et al., 2002.Ly CD, Roche KW, Lee HK, Wenthold RJ. (2002) Identification of rat EMAP, a delta-glutamate receptor binding protein. Biochem Biophys Res Commun 291: 85–90 [DOI] [PubMed] [Google Scholar]
- Lynch and Gallagher, 1996.Lynch DR, Gallagher MJ. (1996) Inhibition of N-methyl-d-aspartate receptors by haloperidol: developmental and pharmacological characterization in native and recombinant receptors. J Pharmacol Exp Ther 279:154–161 [PubMed] [Google Scholar]
- Lynch, 2002.Lynch G. (2002) Memory enhancement: the search for mechanism-based drugs. Nat Neurosci 5:1035–1038 [DOI] [PubMed] [Google Scholar]
- Lynch, 2006.Lynch G. (2006) Glutamate-based therapeutic approaches: ampakines. Curr Opin Pharmacol 6:82–88 [DOI] [PubMed] [Google Scholar]
- Lynch and Gall, 2006.Lynch G, Gall CM. (2006) Ampakines and the threefold path to cognitive enhancement. Trends Neurosci 29:554–562 [DOI] [PubMed] [Google Scholar]
- Lynch et al., 1997.Lynch G, Granger R, Ambros-Ingerson J, Davis CM, Kessler M, Schehr R. (1997) Evidence that a positive modulator of AMPA-type glutamate receptors improves delayed recall in aged humans. Exp Neurol 145:89–92 [DOI] [PubMed] [Google Scholar]
- MacDermott et al., 1986.MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL. (1986) NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones [published erratum appears in Nature 321:888, 1986]. Nature 321:519–522 [DOI] [PubMed] [Google Scholar]
- Mack et al., 2001.Mack V, Burnashev N, Kaiser KM, Rozov A, Jensen V, Hvalby O, Seeburg PH, Sakmann B, Sprengel R. (2001) Conditional restoration of hippocampal synaptic potentiation in Glur-A-deficient mice. Science 292:2501–2504 [DOI] [PubMed] [Google Scholar]
- MacKinnon, 2003.MacKinnon R. (2003) Potassium channels. FEBS Lett 555:62–65 [DOI] [PubMed] [Google Scholar]
- Madden, 2002.Madden DR. (2002) The inner workings of the AMPA receptors. Curr Opin Drug Discov Devel 5:741–748 [PubMed] [Google Scholar]
- Madden et al., 2005.Madden DR, Armstrong N, Svergun D, Pérez J, Vachette P. (2005) Solution X-ray scattering evidence for agonist- and antagonist-induced modulation of cleft closure in a glutamate receptor ligand-binding domain. J Biol Chem 280:23637–23642 [DOI] [PubMed] [Google Scholar]
- Madden et al., 2004.Madden DR, Cheng Q, Thiran S, Rajan S, Rigo F, Keinänen K, Reinelt S, Zimmermann H, Jayaraman V. (2004) Stereochemistry of glutamate receptor agonist efficacy: engineering a dual-specificity AMPA/kainate receptor. Biochemistry 43:15838–15844 [DOI] [PubMed] [Google Scholar]
- Magazanik et al., 1997.Magazanik LG, Buldakova SL, Samoilova MV, Gmiro VE, Mellor IR, Usherwood PN. (1997) Block of open channels of recombinant AMPA receptors and native AMPA/kainate receptors by adamantane derivatives. J Physiol 505:655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah et al., 2005.Mah SJ, Cornell E, Mitchell NA, Fleck MW. (2005) Glutamate receptor trafficking: endoplasmic reticulum quality control involves ligand binding and receptor function. J Neurosci 25:2215–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier et al., 2003.Maier C, Dertwinkel R, Mansourian N, Hosbach I, Schwenkreis P, Senne I, Skipka G, Zenz M, Tegenthoff M. (2003) Efficacy of the NMDA-receptor antagonist memantine in patients with chronic phantom limb pain—results of a randomized double-blinded, placebo-controlled trial. Pain 103:277–283 [DOI] [PubMed] [Google Scholar]
- Malayev et al., 2002.Malayev A, Gibbs TT, Farb DH. (2002) Inhibition of the NMDA response by pregnenolone sulphate reveals subtype selective modulation of NMDA receptors by sulphated steroids. Br J Pharmacol 135:901–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldve et al., 2002.Maldve RE, Zhang TA, Ferrani-Kile K, Schreiber SS, Lippmann MJ, Snyder GL, Fienberg AA, Leslie SW, Gonzales RA, Morrisett RA. (2002) DARPP-32 and regulation of the ethanol sensitivity of NMDA receptors in the nucleus accumbens. Nat Neurosci 5:641–648 [DOI] [PubMed] [Google Scholar]
- Malenka and Bear, 2004.Malenka RC, Bear MF. (2004) LTP and LTD: an embarrassment of riches. Neuron 44:5–21 [DOI] [PubMed] [Google Scholar]
- Malinow and Malenka, 2002.Malinow R, Malenka RC. (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25:103–126 [DOI] [PubMed] [Google Scholar]
- Mameli et al., 2007.Mameli M, Balland B, Luján R, Lüscher C. (2007) Rapid synthesis and insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science 317:530–533 [DOI] [PubMed] [Google Scholar]
- Mammen et al., 1997.Mammen AL, Kameyama K, Roche KW, Huganir RL. (1997) Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem 272:32528–32533 [DOI] [PubMed] [Google Scholar]
- Man et al., 2007.Man HY, Sekine-Aizawa Y, Huganir RL. (2007) Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci USA 104:3579–3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano and Teichberg, 1998.Mano I, Teichberg VI. (1998) A tetrameric subunit stoichiometry for a glutamate receptor-channel complex. Neuroreport 9:327–331 [DOI] [PubMed] [Google Scholar]
- Mansour et al., 2001.Mansour M, Nagarajan N, Nehring RB, Clements JD, Rosenmund C. (2001) Heteromeric AMPA receptors assemble with a preferred subunit stoichiometry and spatial arrangement. Neuron 32:841–853 [DOI] [PubMed] [Google Scholar]
- Mao, 1999.Mao J. (1999) NMDA and opioid receptors: their interactions in antinociception, tolerance and neuroplasticity. Brain Res Brain Res Rev 30:289–304 [DOI] [PubMed] [Google Scholar]
- Marchetti and Gavazzo, 2005.Marchetti C, Gavazzo P. (2005) NMDA receptors as targets of heavy metal interaction and toxicity. Neurotox Res 8:245–258 [DOI] [PubMed] [Google Scholar]
- Marenco et al., 2002.Marenco S, Egan MF, Goldberg TE, Knable MB, McClure RK, Winterer G, Weinberger DR. (2002) Preliminary experience with an ampakine (CX516) as a single agent for the treatment of schizophrenia: a case series. Schizophr Res 57:221–226 [DOI] [PubMed] [Google Scholar]
- Martin et al., 1993.Martin LJ, Blackstone CD, Levey AI, Huganir RL, Price DL. (1993) AMPA glutamate receptor subunits are differentially distributed in the brain. Neuroscience 53:327–358 [DOI] [PubMed] [Google Scholar]
- Martin et al., 2007.Martin S, Nishimune A, Mellor JR, Henley JM. (2007) SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature 447:321–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood et al., 1994.Masood K, Wu C, Brauneis U, Weight FF. (1994) Differential ethanol sensitivity of recombinant N-methyl-d-aspartate receptor subunits. Mol Pharmacol 45:324–329 [PubMed] [Google Scholar]
- Massey et al., 2004.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. (2004) Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci 24:7821–7828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuko et al., 1999a.Masuko T, Kashiwagi K, Kuno T, Nguyen ND, Pahk AJ, Fukuchi J, Igarashi K, Williams K. (1999a) A regulatory domain (R1–R2) in the amino terminus of the N-methyl-d-aspartate receptor: effects of spermine, protons, and ifenprodil, and structural similarity to bacterial leucine/isoleucine/valine binding protein. Mol Pharmacol 55:957–969 [DOI] [PubMed] [Google Scholar]
- Masuko et al., 1999b.Masuko T, Kuno T, Kashiwagi K, Kusama T, Williams K, Igarashi K. (1999b) Stimulatory and inhibitory properties of aminoglycoside antibiotics at N-methyl-d-aspartate receptors. J Pharmacol Exp Ther 290:1026–1033 [PubMed] [Google Scholar]
- Mathew et al., 2009.Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. (2009) Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol 13:71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda et al., 2003.Matsuda K, Fletcher M, Kamiya Y, Yuzaki M. (2003) Specific assembly with the NMDA receptor 3B subunit controls surface expression and calcium permeability of NMDA receptors. J Neurosci 23:10064–10073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda et al., 2002.Matsuda K, Kamiya Y, Matsuda S, Yuzaki M. (2002) Cloning and characterization of a novel NMDA receptor subunit NR3B: a dominant subunit that reduces calcium permeability. Brain Res Mol Brain Res 100:43–52 [DOI] [PubMed] [Google Scholar]
- Matsuda et al., 2005.Matsuda S, Kamiya Y, Yuzaki M. (2005) Roles of the N-terminal domain on the function and quaternary structure of the ionotropic glutamate receptor. J Biol Chem 280:20021–20029 [DOI] [PubMed] [Google Scholar]
- Matsuda et al., 2010.Matsuda K, Miura E, Miyazaki T, Kakegawa W, Emi K, Narumi S, Fukazawa Y, Ito-Ishida A, Kondo T, Shigemoto R, et al. (2010) Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science 328:363–368 [DOI] [PubMed] [Google Scholar]
- Matsuda et al., 1999.Matsuda S, Mikawa S, Hirai H. (1999) Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J Neurochem 73:1765–1768 [DOI] [PubMed] [Google Scholar]
- Mayat et al., 1995.Mayat E, Petralia RS, Wang YX, Wenthold RJ. (1995) Immunoprecipitation, immunoblotting, and immunocytochemistry studies suggest that glutamate receptor delta subunits form novel postsynaptic receptor complexes. J Neurosci 15:2533–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, 2005.Mayer ML. (2005) Crystal structures of the GluR5 and GluR6 ligand binding cores: molecular mechanisms underlying kainate receptor selectivity. Neuron 45:539–552 [DOI] [PubMed] [Google Scholar]
- Mayer, 2006.Mayer ML. (2006) Glutamate receptors at atomic resolution. Nature 440:456–462 [DOI] [PubMed] [Google Scholar]
- Mayer et al., 2006.Mayer ML, Ghosal A, Dolman NP, Jane DE. (2006) Crystal structures of the kainate receptor GluR5 ligand binding core dimer with novel GluR5-selective antagonists. J Neurosci 26:2852–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer et al., 1989.Mayer ML, Vyklicky L, Jr, Clements J. (1989) Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature 338:425–427 [DOI] [PubMed] [Google Scholar]
- Mayer and Westbrook, 1987.Mayer ML, Westbrook GL. (1987) Permeation and block of N-methyl-d-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol 394:501–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer et al., 1984.Mayer ML, Westbrook GL, Guthrie PB. (1984) Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309:261–263 [DOI] [PubMed] [Google Scholar]
- Mayer et al., 1988.Mayer ML, Westbrook GL, Vyklický L., Jr (1988) Sites of antagonist action on N-methyl-D-aspartic acid receptors studied using fluctuation analysis and a rapid perfusion technique. J Neurophysiol 60:645–663 [DOI] [PubMed] [Google Scholar]
- McBain et al., 1989.McBain CJ, Kleckner NW, Wyrick S, Dingledine R. (1989) Structural requirements for activation of the glycine coagonist site of N-methyl-d-aspartate receptors expressed in Xenopus oocytes. Mol Pharmacol 36:556–565 [PubMed] [Google Scholar]
- McCartney et al., 2004.McCartney CJ, Sinha A, Katz J. (2004) A qualitative systematic review of the role of N-methyl-d-aspartate receptor antagonists in preventive analgesia. Anesth Analg 98:1385–1400 [DOI] [PubMed] [Google Scholar]
- McCauley, 2005.McCauley JA. (2005) NR2B subtype-selective NMDA receptor antagonists: 2001–2004. Expert Opin Ther Pat 15:389–407 [Google Scholar]
- McCauley et al., 2004.McCauley JA, Theberge CR, Romano JJ, Billings SB, Anderson KD, Claremon DA, Freidinger RM, Bednar RA, Mosser SD, Gaul SL, et al. (2004) NR2B-selective N-methyl-d-aspartate antagonists: synthesis and evaluation of 5-substituted benzimidazoles. J Med Chem 47:2089–2096 [DOI] [PubMed] [Google Scholar]
- McDonald et al., 1998.McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP. (1998) oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med 4:291–297 [DOI] [PubMed] [Google Scholar]
- McFeeters and Oswald, 2002.McFeeters RL, Oswald RE. (2002) Structural mobility of the extracellular ligand-binding core of an ionotropic glutamate receptor. Analysis of NMR relaxation dynamics. Biochemistry 41:10472–10481 [DOI] [PubMed] [Google Scholar]
- McFeeters and Oswald, 2004.McFeeters RL, Oswald RE. (2004) Emerging structural explanations of ionotropic glutamate receptor function. FASEB J 18:428–438 [DOI] [PubMed] [Google Scholar]
- McNamara et al., 1990.McNamara D, Smith EC, Calligaro DO, O'Malley PJ, McQuaid LA, Dingledine R. (1990) 5,7-Dichlorokynurenic acid, a potent and selective competitive antagonist of the glycine site on NMDA receptors. Neurosci Lett 120:17–20 [DOI] [PubMed] [Google Scholar]
- Mealing et al., 1999.Mealing GA, Lanthorn TH, Murray CL, Small DL, Morley P. (1999) Differences in degree of trapping of low-affinity uncompetitive N-methyl-D-aspartic acid receptor antagonists with similar kinetics of block. J Pharmacol Exp Ther 288:204–210 [PubMed] [Google Scholar]
- Mealing et al., 2001.Mealing GA, Lanthorn TH, Small DL, Murray RJ, Mattes KC, Comas TM, Morley P. (2001) Structural modifications to an N-methyl-d-aspartate receptor antagonist result in large differences in trapping block. J Pharmacol Exp Ther 297:906–914 [PubMed] [Google Scholar]
- Meddows et al., 2001.Meddows E, Le Bourdelles B, Grimwood S, Wafford K, Sandhu S, Whiting P, McIlhinney RA. (2001) Identification of molecular determinants that are important in the assembly of N-methyl-d-aspartate receptors. J Biol Chem 276:18795–18803 [DOI] [PubMed] [Google Scholar]
- Medina et al., 1995.Medina I, Filippova N, Charton G, Rougeole S, Ben-Ari Y, Khrestchatisky M, Bregestovski P. (1995) Calcium-dependent inactivation of heteromeric NMDA receptor-channels expressed in human embryonic kidney cells. J Physiol 482:567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor et al., 2003.Mellor IR, Brier TJ, Pluteanu F, Strømgaard K, Saghyan A, Eldursi N, Brierley MJ, Andersen K, Jaroszewski JW, Krogsgaard-Larsen P, et al. (2003) Modification of the philanthotoxin-343 polyamine moiety results in different structure-activity profiles at muscle nicotinic ACh, NMDA and AMPA receptors. Neuropharmacology 44:70–80 [DOI] [PubMed] [Google Scholar]
- Melyan et al., 2002.Melyan Z, Wheal HV, Lancaster B. (2002) Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron 34:107–114 [DOI] [PubMed] [Google Scholar]
- Mendieta et al., 2005.Mendieta J, Gago F, Ramírez G. (2005) Binding of 5′-GMP to the GluR2 AMPA receptor: insight from targeted molecular dynamics simulations. Biochemistry 44:14470–14476 [DOI] [PubMed] [Google Scholar]
- Mendieta et al., 2001.Mendieta J, Ramírez G, Gago F. (2001) Molecular dynamics simulations of the conformational changes of the glutamate receptor ligand-binding core in the presence of glutamate and kainate. Proteins 44:460–469 [DOI] [PubMed] [Google Scholar]
- Meng et al., 2003.Meng Y, Zhang Y, Jia Z. (2003) Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron 39:163–176 [DOI] [PubMed] [Google Scholar]
- Menniti et al., 2003.Menniti FS, Buchan AM, Chenard BL, Critchett DJ, Ganong AH, Guanowsky V, Seymour PA, Welch WM. (2003) CP-465,022, a selective noncompetitive AMPA receptor antagonist, blocks AMPA receptors but is not neuroprotective in vivo. Stroke 34:171–176 [DOI] [PubMed] [Google Scholar]
- Menniti et al., 2000.Menniti FS, Chenard BL, Collins MB, Ducat MF, Elliott ML, Ewing FE, Huang JI, Kelly KA, Lazzaro JT, Pagnozzi MJ, et al. (2000) Characterization of the binding site for a novel class of noncompetitive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonists. Mol Pharmacol 58:1310–1317 [DOI] [PubMed] [Google Scholar]
- Menuz et al., 2007.Menuz K, Stroud RM, Nicoll RA, Hays FA. (2007) TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists. Science 318:815–817 [DOI] [PubMed] [Google Scholar]
- Meyer et al., 2002.Meyer EL, Gahring LC, Rogers SW. (2002) Nicotine preconditioning antagonizes activity-dependent caspase proteolysis of a glutamate receptor. J Biol Chem 277:10869–10875 [DOI] [PubMed] [Google Scholar]
- Michaud et al., 2001.Michaud CM, Murray CJ, Bloom BR. (2001) Burden of disease—implications for future research. JAMA 285:535–539 [DOI] [PubMed] [Google Scholar]
- Micu et al., 2006.Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, et al. (2006) NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature 439:988–992 [DOI] [PubMed] [Google Scholar]
- Midgett and Madden, 2008.Midgett CR, Madden DR. (2008) The quaternary structure of a calcium-permeable AMPA receptor: conservation of shape and symmetry across functionally distinct subunit assemblies. J Mol Biol 382:578–584 [DOI] [PubMed] [Google Scholar]
- Migues et al., 2007.Migues PV, Cammarota M, Kavanagh J, Atkinson R, Powis DA, Rostas JA. (2007) Maturational changes in the subunit composition of AMPA receptors and the functional consequences of their activation in chicken forebrain. Dev Neurosci 29:232–240 [DOI] [PubMed] [Google Scholar]
- Miller et al., 1992.Miller B, Sarantis M, Traynelis SF, Attwell D. (1992) Potentiation of NMDA receptor currents by arachidonic acid. Nature 355:722–725 [DOI] [PubMed] [Google Scholar]
- Milstein and Nicoll, 2009.Milstein AD, Nicoll RA. (2009) TARP modulation of synaptic AMPA receptor trafficking and gating depends on multiple intracellular domains. Proc Natl Acad Sci USA 106:11348–11351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein et al., 2007.Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA. (2007) TARP subtypes differentially and dose-dependently control synaptic AMPA receptor gating. Neuron 55:905–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirshahi and Woodward, 1995.Mirshahi T, Woodward JJ. (1995) Ethanol sensitivity of heteromeric NMDA receptors: effects of subunit assembly, glycine and NMDAR1 Mg2+-insensitive mutants. Neuropharmacology 34:347–355 [DOI] [PubMed] [Google Scholar]
- Mitchell, 2006.Mitchell AJ. (2006) Two-week delay in onset of action of antidepressants: new evidence. Br J Psychiatry 188:105–106 [DOI] [PubMed] [Google Scholar]
- Miu et al., 2001.Miu P, Jarvie KR, Radhakrishnan V, Gates MR, Ogden A, Ornstein PL, Zarrinmayeh H, Ho K, Peters D, Grabell J, et al. (2001) Novel AMPA receptor potentiators LY392098 and LY404187: effects on recombinant human AMPA receptors in vitro. Neuropharmacology 40:976–983 [DOI] [PubMed] [Google Scholar]
- Miya et al., 2008.Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, Hongou K, Miyawaki T, Mori H. (2008) Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol 510:641–654 [DOI] [PubMed] [Google Scholar]
- Miyagi et al., 2002.Miyagi Y, Yamashita T, Fukaya M, Sonoda T, Okuno T, Yamada K, Watanabe M, Nagashima Y, Aoki I, Okuda K, et al. (2002) Delphilin: a novel PDZ and formin homology domain-containing protein that synaptically colocalizes and interacts with glutamate receptor delta 2 subunit. J Neurosci 22:803–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa et al., 2003.Miyazawa A, Fujiyoshi Y, Unwin N. (2003) Structure and gating mechanism of the acetylcholine receptor pore. Nature 423:949–955 [DOI] [PubMed] [Google Scholar]
- Möbius and Stöffler, 2003.Möbius HJ, Stöffler A. (2003) Memantine in vascular dementia. Int Psychogeriatr 15:207–213 [DOI] [PubMed] [Google Scholar]
- Monaghan and Cotman, 1982.Monaghan DT, Cotman CW. (1982) The distribution of [3H]kainic acid binding sites in rat CNS as determined by autoradiography. Brain Res 252:91–100 [DOI] [PubMed] [Google Scholar]
- Mony et al., 2009.Mony L, Kew JN, Gunthorpe MJ, Paoletti P. (2009) Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br J Pharmacol 157:1301–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer et al., 1994.Monyer H, Burnashev N, dzLaurie DJ, Sakmann B, Seeburg PH. (1994) Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12:529–540 [DOI] [PubMed] [Google Scholar]
- Monyer et al., 1992.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. (1992) Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 256:1217–1221 [DOI] [PubMed] [Google Scholar]
- Morais-Cabral et al., 2001.Morais-Cabral JH, Zhou Y, MacKinnon R. (2001) Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature 414:37–42 [DOI] [PubMed] [Google Scholar]
- More et al., 2004.More JC, Nistico R, Dolman NP, Clarke VR, Alt AJ, Ogden AM, Buelens FP, Troop HM, Kelland EE, Pilato F, et al. (2004) Characterisation of UBP296: a novel, potent and selective kainate receptor antagonist. Neuropharmacology 47:46–64 [DOI] [PubMed] [Google Scholar]
- Moreno-González et al., 2008.Moreno-González G, López-Colomé AM, Rodríguez G, Zarain-Herzberg A. (2008) Transcription of the chicken Grin1 gene is regulated by the activity of SP3 and NRSF in undifferentiated cells and neurons. Biosci Rep 28:177–188 [DOI] [PubMed] [Google Scholar]
- Mori et al., 1992.Mori H, Masaki H, Yamakura T, Mishina M. (1992) Identification by mutagenesis of a Mg2+-block site of the NMDA receptor channel. Nature 358:673–675 [DOI] [PubMed] [Google Scholar]
- Morimoto-Tomita et al., 2009.Morimoto-Tomita M, Zhang W, Straub C, Cho CH, Kim KS, Howe JR, Tomita S. (2009) Autoinactivation of neuronal AMPA receptors via glutamate-regulated TARP interaction. Neuron 61:101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita et al., 2007.Morita Y, Ujike H, Tanaka Y, Otani K, Kishimoto M, Morio A, Kotaka T, Okahisa Y, Matsushita M, Morikawa A, et al. (2007) A genetic variant of the serine racemase gene is associated with schizophrenia. Biol Psychiatry 61:1200–1203 [DOI] [PubMed] [Google Scholar]
- Moriyoshi et al., 1991.Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. (1991) Molecular cloning and characterization of the rat NMDA receptor. Nature 354: 31–37 [DOI] [PubMed] [Google Scholar]
- Morley et al., 2005.Morley RM, Tse HW, Feng B, Miller JC, Monaghan DT, Jane DE. (2005) Synthesis and pharmacology of N1-substituted piperazine-2,3-dicarboxylic acid derivatives acting as NMDA receptor antagonists. J Med Chem 48:2627–2637 [DOI] [PubMed] [Google Scholar]
- Mosbacher et al., 1994.Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH, Ruppersberg JP. (1994) A molecular determinant for submillisecond desensitization in glutamate receptors. Science 266:1059–1062 [DOI] [PubMed] [Google Scholar]
- Mosley et al., 2009.Mosley CA, Myers SJ, Murray EE, Santangelo R, Tahirovic YA, Kurtkaya N, Mullasseril P, Yuan H, Lyuboslavsky P, Le P, et al. (2009) Synthesis, structural activity-relationships, and biological evaluation of novel amide-based allosteric binding site antagonists in NR1A/NR2B N-methyl-d-aspartate receptors. Bioorg Med Chem 17:6463–6480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley et al., 2010.Mosley CA, Acker TM, Hansen KB, Mullasseril P, Andersen K, Le P, Vellano KM, Bräuner-Osborne H, Liotta DC, Traynelis SF. (2010) Quinazolin-4-one derivatives: A novel class of non-competitive NR2C/D subunit-selective N-methyl-D-aspartate receptor antagonists. J Med Chem, DOI: 10.1021/jm100027p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet et al., 2000.Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. (2000) D-serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor. Proc Natl Acad Sci USA 97:4926–4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott et al., 2008.Mott DD, Benveniste M, Dingledine RJ. (2008) pH-dependent inhibition of kainate receptors by zinc. J Neurosci 28:1659–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott et al., 1998.Mott DD, Doherty JJ, Zhang S, Washburn MS, Fendley MJ, Lyuboslavsky P, Traynelis SF, Dingledine R. (1998) Phenylethanolamines inhibit NMDA receptors by enhancing proton inhibition. Nat Neurosci 1:659–667 [DOI] [PubMed] [Google Scholar]
- Mott et al., 2003.Mott DD, Washburn MS, Zhang S, Dingledine RJ. (2003) Subunit-dependent modulation of kainate receptors by extracellular protons and polyamines. J Neurosci 23:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu et al., 2003.Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD. (2003) Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron 40:581–594 [DOI] [PubMed] [Google Scholar]
- Müller et al., 1996.Müller BM, Kistner U, Kindler S, Chung WJ, Kuhlendahl S, Fenster SD, Lau LF, Veh RW, Huganir RL, Gundelfinger ED, et al. (1996) SAP102, a novel postsynaptic protein that interacts with NMDA receptor complexes in vivo. Neuron 17:255–265 [DOI] [PubMed] [Google Scholar]
- Muir, 2006.Muir KW. (2006) Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol 6:53–60 [DOI] [PubMed] [Google Scholar]
- Muir and Lees, 2003.Muir KW, Lees KR. (2003) Excitatory amino acid antagonists for acute stroke. Cochrane Database Syst Rev:CD001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle et al., 2000.Mulle C, Sailer A, Swanson GT, Brana C, O'Gorman S, Bettler B, Heinemann SF. (2000) Subunit composition of kainate receptors in hippocampal interneurons. Neuron 28:475–484 [DOI] [PubMed] [Google Scholar]
- Munton et al., 2007.Munton RP, Tweedie-Cullen R, Livingstone-Zatchej M, Weinandy F, Waidelich M, Longo D, Gehrig P, Potthast F, Rutishauser D, Gerrits B, et al. (2007) Qualitative and quantitative analyses of protein phosphorylation in naive and stimulated mouse synaptosomal preparations. Mol Cell Proteomics 6:283–293 [DOI] [PubMed] [Google Scholar]
- Murray et al., 2003.Murray TK, Whalley K, Robinson CS, Ward MA, Hicks CA, Lodge D, Vandergriff JL, Baumbarger P, Siuda E, Gates M, et al. (2003) LY503430, a novel alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor potentiator with functional, neuroprotective and neurotrophic effects in rodent models of Parkinson's disease. J Pharmacol Exp Ther 306:752–762 [DOI] [PubMed] [Google Scholar]
- Mustafa et al., 2004.Mustafa AK, Kim PM, Snyder SH. (2004) D-Serine as a putative glial neurotransmitter. Neuron Glia Biol 1:275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa et al., 2009.Mustafa AK, van Rossum DB, Patterson RL, Maag D, Ehmsen JT, Gazi SK, Chakraborty A, Barrow RK, Amzel LM, Snyder SH. (2009) Glutamatergic regulation of serine racemase via reversal of PIP2 inhibition. Proc Natl Acad Sci USA 106:2921–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers et al., 1999.Myers SJ, Dingledine R, Borges K. (1999) Genetic regulation of glutamate receptor ion channels. Annu Rev Pharmacol Toxicol 39:221–241 [DOI] [PubMed] [Google Scholar]
- Myers et al., 2004.Myers SJ, Huang Y, Genetta T, Dingledine R. (2004) Inhibition of glutamate receptor 2 translation by a polymorphic repeat sequence in the 5′-untranslated leaders. J Neurosci 24:3489–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers et al., 1998.Myers SJ, Peters J, Huang Y, Comer MB, Barthel F, Dingledine R. (1998) Transcriptional regulation of the GluR2 gene: neural-specific expression, multiple promoters, and regulatory elements. J Neurosci 18:6723–6739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa et al., 1996.Nagasawa M, Sakimura K, Mori KJ, Bedell MA, Copeland NG, Jenkins NA, Mishina M. (1996) Gene structure and chromosomal localization of the mouse NMDA receptor channel subunits. Mol Brain Res 36:1–11 [DOI] [PubMed] [Google Scholar]
- Nagy, 2008.Nagy J. (2008) Alcohol related changes in regulation of NMDA receptor functions. Curr Neuropharmacol 6:39–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Levy et al., 2001.Nahum-Levy R, Lipinski D, Shavit S, Benveniste M. (2001) Desensitization of NMDA receptor channels is modulated by glutamate agonists. Biophys J 80:2152–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami et al., 2008.Nakagami R, Kohda K, Kakegawa W, Kondo T, Kato N, Yuzaki M. (2008) Phosphorylation of delta2 glutamate receptors at serine 945 is not required for cerebellar long-term depression. Keio J Med 57:105–110 [DOI] [PubMed] [Google Scholar]
- Nakagawa et al., 2005.Nakagawa T, Cheng Y, Ramm E, Sheng M, Walz T. (2005) Structure and different conformational states of native AMPA receptor complexes. Nature 433:545–549 [DOI] [PubMed] [Google Scholar]
- Nakagawa et al., 2006.Nakagawa T, Cheng Y, Sheng M, Walz T. (2006) Three-dimensional structure of an AMPA receptor without associated stargazin/TARP proteins. Biol Chem 387:179–187 [DOI] [PubMed] [Google Scholar]
- Nakanishi et al., 1990.Nakanishi N, Shneider NA, Axel R. (1990) A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron 5:569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi et al., 1992.Nakanishi N, Axel R, Shneider NA. (1992) Alternative splicing generates functionally distinct N-methyl-d-aspartate receptors. Proc Natl Acad Sci USA 89:8552–8556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi et al., 2009.Nakanishi N, Tu S, Shin Y, Cui J, Kurokawa T, Zhang D, Chen HS, Tong G, Lipton SA. (2009) Neuroprotection by the NR3A subunit of the NMDA receptor. J Neurosci 29:5260–5265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa et al., 2001.Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. (2001) Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-d-aspartate receptor. J Biol Chem 276:693–699 [DOI] [PubMed] [Google Scholar]
- Nanao et al., 2005.Nanao MH, Green T, Stern-Bach Y, Heinemann SF, Choe S. (2005) Structure of the kainate receptor subunit GluR6 agonist-binding domain complexed with domoic acid. Proc Natl Acad Sci USA 102:1708–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nateri et al., 2007.Nateri AS, Raivich G, Gebhardt C, Da Costa C, Naumann H, Vreugdenhil M, Makwana M, Brandner S, Adams RH, Jefferys JG, et al. (2007) ERK activation causes epilepsy by stimulating NMDA receptor activity. EMBO J 26:4891–4901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naur et al., 2007.Naur P, Hansen KB, Kristensen AS, Dravid SM, Pickering DS, Olsen L, Vestergaard B, Egebjerg J, Gajhede M, Traynelis SF, et al. (2007) Ionotropic glutamate-like receptor delta 2 binds d-serine and glycine. Proc Natl Acad Sci USA 104:14116–14121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naur et al., 2005.Naur P, Vestergaard B, Skov LK, Egebjerg J, Gajhede M, Kastrup JS. (2005) Crystal structure of the kainate receptor GluR5 ligand-binding core in complex with (S)-glutamate. FEBS Lett 579:1154–1160 [DOI] [PubMed] [Google Scholar]
- Nayeem et al., 2009.Nayeem N, Zhang Y, Schweppe DK, Madden DR, Green T. (2009) A nondesensitizing kainate receptor point mutant. Mol Pharmacol 76:534–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard et al., 1991.Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA. (1991) Dynamics of interstitial and intracellular pH in evolving brain infarct. Am J Physiol 260:R581–R588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely and Lingle, 1986.Neely A, Lingle CJ. (1986) Trapping of an open-channel blocker at the frog neuromuscular acetylcholine channel. Biophys J 50:981–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher, 1995.Neher E. (1995) The use of fura-2 for estimating Ca buffers and Ca fluxes. Neuropharmacology 34:1423–1442 [DOI] [PubMed] [Google Scholar]
- Neyton and Paoletti, 2006.Neyton J, Paoletti P. (2006) Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci 26:1331–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng et al., 2009.Ng D, Pitcher GM, Szilard RK, Sertié A, Kanisek M, Clapcote SJ, Lipina T, Kalia LV, Joo D, McKerlie C, et al. (2009) Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol 7:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls, 1989.Nicholls DG. (1989) Release of glutamate, aspartate, and gamma-aminobutyric acid from isolated nerve terminals. J Neurochem 52:331–341 [DOI] [PubMed] [Google Scholar]
- Nielsen et al., 2003.Nielsen MM, Liljefors T, Krogsgaard-Larsen P, Egebjerg J. (2003) The selective activation of the glutamate receptor GluR5 by ATPA is controlled by serine 741. Mol Pharmacol 63:19–25 [DOI] [PubMed] [Google Scholar]
- Niethammer et al., 1996.Niethammer M, Kim E, Sheng M. (1996) Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci 16:2157–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimura et al., 2005.Niimura M, Moussa R, Bissoon N, Ikeda-Douglas C, Milgram NW, Gurd JW. (2005) Changes in phosphorylation of the NMDA receptor in the rat hippocampus induced by status epilepticus. J Neurochem 92:1377–1385 [DOI] [PubMed] [Google Scholar]
- Nikam and Meltzer, 2002.Nikam SS, Meltzer LT. (2002) NR2B selective NMDA receptor antagonists. Curr Pharm Des 8:845–855 [DOI] [PubMed] [Google Scholar]
- Nikolajsen et al., 2000.Nikolajsen L, Gottrup H, Kristensen AG, Jensen TS. (2000) Memantine (a N-methyl-d-aspartate receptor antagonist) in the treatment of neuropathic pain after amputation or surgery: a randomized, double-blinded, cross-over study. Anesth Analg 91:960–966 [DOI] [PubMed] [Google Scholar]
- Nilsen and England, 2007.Nilsen A, England PM. (2007) A subtype-selective, use-dependent inhibitor of native AMPA receptors. J Am Chem Soc 129:4902–4903 [DOI] [PubMed] [Google Scholar]
- Nishi et al., 2001.Nishi M, Hinds H, Lu HP, Kawata M, Hayashi Y. (2001) Motoneuron-specific expression of NR3B, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J Neurosci 21:RC185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa et al., 1994.Nishikawa M, Kimura S, Akaike N. (1994) Facilitatory effect of docosahexaenoic acid on N-methyl-d-aspartate response in pyramidal neurons of rat cerebral cortex. J Physiol 475:83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune et al., 1998.Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. (1998) NSF binding to GluR2 regulates synaptic transmission. Neuron 21:87–97 [DOI] [PubMed] [Google Scholar]
- Norberg et al., 2008.Norberg MM, Krystal JH, Tolin DF. (2008) A meta-analysis of d-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry 63:1118–1126 [DOI] [PubMed] [Google Scholar]
- Nowak et al., 1984.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. (1984) Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307:462–465 [DOI] [PubMed] [Google Scholar]
- Nuriya et al., 2005.Nuriya M, Oh S, Huganir RL. (2005) Phosphorylation-dependent interactions of alpha-Actinin-1/IQGAP1 with the AMPA receptor subunit GluR4. J Neurochem 95:544–552 [DOI] [PubMed] [Google Scholar]
- Nutt et al., 2008.Nutt JG, Gunzler SA, Kirchhoff T, Hogarth P, Weaver JL, Krams M, Jamerson B, Menniti FS, Landen JW. (2008) Effects of a NR2B selective NMDA glutamate antagonist, CP-101,606, on dyskinesia and Parkinsonism. Mov Disord 23:1860–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien et al., 1999.O'Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. (1999) Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron 23:309–323 [DOI] [PubMed] [Google Scholar]
- Oh and Derkach, 2005.Oh MC, Derkach VA. (2005) Dominant role of the GluR2 subunit in regulation of AMPA receptors by CaMKII. Nat Neurosci 8:853–854 [DOI] [PubMed] [Google Scholar]
- O'Hara et al., 1993.O'Hara PJ, Sheppard PO, Thøgersen H, Venezia D, Haldeman BA, McGrane V, Houamed KM, Thomsen C, Gilbert TL, Mulvihill ER. (1993) The ligand-binding domain in metabotropic glutamate receptors is related to bacterial periplasmic binding proteins. Neuron 11:41–52 [DOI] [PubMed] [Google Scholar]
- Okamoto et al., 2002.Okamoto S, Sherman K, Bai G, Lipton SA. (2002) Effect of the ubiquitous transcription factors, SP1 and MAZ, on NMDA receptor subunit type 1 (NR1) expression during neuronal differentiation. Mol Brain Res 107:89–96 [DOI] [PubMed] [Google Scholar]
- Okamoto et al., 1999.Okamoto S, Sherman K, Lipton SA. (1999) Absence of binding activity of neuron-restrictive silencer factor is necessary, but not sufficient for transcription of NMDA receptor subunit type 1 in neuronal cells. Mol Brain Res 74:44–54 [DOI] [PubMed] [Google Scholar]
- Olney et al., 1999.Olney JW, Newcomer JW, Farber NB. (1999) NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res 33:523–533 [DOI] [PubMed] [Google Scholar]
- Olney et al., 1987.Olney JW, Price MT, Salles KS, Labruyere J, Ryerson R, Mahan K, Frierdich G, Samson L. (1987) l-homocysteic acid: an endogenous excitotoxic ligand of the NMDA receptor. Brain Res Bull 19:597–602 [DOI] [PubMed] [Google Scholar]
- Omkumar et al., 1996.Omkumar RV, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB. (1996) Identification of a phosphorylation site for calcium/calmodulin-dependent protein kinase II in the NR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem 271:31670–31678 [DOI] [PubMed] [Google Scholar]
- O'Neill and Dix, 2007.O'Neill MJ, Dix S. (2007) AMPA receptor potentiators as cognitive enhancers. IDrugs 10:185–192 [PubMed] [Google Scholar]
- O'Neill and Witkin, 2007.O'Neill MJ, Witkin JM. (2007) AMPA receptor potentiators: application for depression and Parkinson's disease. Curr Drug Targets 8:603–620 [DOI] [PubMed] [Google Scholar]
- O'Neill et al., 1998.O'Neill MJ, Bond A, Ornstein PL, Ward MA, Hicks CA, Hoo K, Bleakman D, Lodge D. (1998) Decahydroisoquinolines: novel competitive AMPA/kainate antagonists with neuroprotective effects in global cerebral ischaemia. Neuropharmacology 37:1211–1222 [DOI] [PubMed] [Google Scholar]
- Osten et al., 1998.Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, States BA, Einheber S, Milner TA, Hanson PI, et al. (1998) The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron 21:99–110 [DOI] [PubMed] [Google Scholar]
- Oswald et al., 2007.Oswald RE, Ahmed A, Fenwick MK, Loh AP. (2007) Structure of glutamate receptors. Curr Drug Targets 8:573–582 [DOI] [PubMed] [Google Scholar]
- Otton et al., 2009.Otton HJ, Janssen A, O'Leary T, Chen PE, Wyllie DJ. (2009) Inhibition of rat recombinant GluN1/GluN2A and GluN1/GluN2B NMDA receptors by ethanol at concentrations based on the US/UK drink-drive limit. Eur J Pharmacol 614: 14–21 [DOI] [PubMed] [Google Scholar]
- Oz et al., 2004.Oz M, Woods AS, Shippenberg T, Kaminski RM. (2004) Effects of extracellular pH on the dynorphin A inhibition of N-methyl-d-aspartate receptors expressed in Xenopus oocytes. Synapse 52:84–88 [DOI] [PubMed] [Google Scholar]
- Ozaki et al., 1997.Ozaki M, Sasner M, Yano R, Lu HS, Buonanno A. (1997) Neuregulin-β induces expression of an NMDA-receptor subunit. Nature 390:691–694 [DOI] [PubMed] [Google Scholar]
- Paas, 1998.Paas Y. (1998) The macro- and microarchitectures of the ligand-binding domain of glutamate receptors. Trends Neurosci 21:117–125 [DOI] [PubMed] [Google Scholar]
- Paas et al., 1996.Paas Y, Eisenstein M, Medevielle F, Teichberg VI, Devillers-Thiéry A. (1996) Identification of the amino acid subsets accounting for the ligand binding specificity of a glutamate receptor. Neuron 17:979–990 [DOI] [PubMed] [Google Scholar]
- Pahk and Williams, 1997.Pahk AJ, Williams K. (1997) Influence of extracellular pH on inhibition by ifenprodil at N-methyl-d-aspartate receptors in Xenopus oocytes. Neurosci Lett 225:29–32 [DOI] [PubMed] [Google Scholar]
- Palmer et al., 2004.Palmer MJ, Isaac JT, Collingridge GL. (2004) Multiple, developmentally regulated expression mechanisms of long-term potentiation at CA1 synapses. J Neurosci 24:4903–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier et al., 2006.Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. (2006) Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125:775–784 [DOI] [PubMed] [Google Scholar]
- Panchenko et al., 2001.Panchenko VA, Glasser CR, Mayer ML. (2001) Structural similarities between glutamate receptor channels and K+ channels examined by scanning mutagenesis. J Gen Physiol 117:345–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchenko et al., 1999.Panchenko VA, Glasser CR, Partin KM, Mayer ML. (1999) Amino acid substitutions in the pore of rat glutamate receptors at sites influencing block by polyamines. J Physiol 520:337–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti and Ascher, 1994.Paoletti P, Ascher P. (1994) Mechanosensitivity of NMDA receptors in cultured mouse central neurons. Neuron 13:645–655 [DOI] [PubMed] [Google Scholar]
- Paoletti et al., 1997.Paoletti P, Ascher P, Neyton J. (1997) High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci 17:5711–5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti et al., 2000.Paoletti P, Perin-Dureau F, Fayyazuddin A, Le Goff A, Callebaut I, Neyton J. (2000) Molecular organization of a zinc binding n-terminal modulatory domain in a NMDA receptor subunit. Neuron 28:911–925 [DOI] [PubMed] [Google Scholar]
- Paoletti et al., 2009.Paoletti P, Vergnano AM, Barbour B, Casado M. (2009) Zinc at glutamatergic synapses. Neuroscience 158:126–136 [DOI] [PubMed] [Google Scholar]
- Papadakis et al., 2004.Papadakis M, Hawkins LM, Stephenson FA. (2004) Appropriate NR1-NR1 disulfide-linked homodimer formation is requisite for efficient expression of functional, cell surface N-methyl-d-aspartate NR1/NR2 receptors. J Biol Chem 279:14703–14712 [DOI] [PubMed] [Google Scholar]
- Papadia and Hardingham, 2007.Papadia S, Hardingham GE. (2007) The dichotomy of NMDA receptor signaling. Neuroscientist 13:572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park et al., 2009.Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, et al. (2009) Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci 29:3206–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park et al., 2006.Park Y, Jo J, Isaac JT, Cho K. (2006) Long-term depression of kainate receptor-mediated synaptic transmission. Neuron 49:95–106 [DOI] [PubMed] [Google Scholar]
- Park-Chung et al., 1997.Park-Chung M, Wu FS, Purdy RH, Malayev AA, Gibbs TT, Farb DH. (1997) Distinct sites for inverse modulation of N-methyl-d-aspartate receptors by sulfated steroids. Mol Pharmacol 52:1113–1123 [DOI] [PubMed] [Google Scholar]
- Parnas et al., 2009.Parnas M, Katz B, Lev S, Tzarfaty V, Dadon D, Gordon-Shaag A, Metzner H, Yaka R, Minke B. (2009) Membrane lipid modulations remove divalent open channel block from TRP-like and NMDA channels. J Neurosci 29:2371–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons et al., 1995.Parsons CG, Quack G, Bresink I, Baran L, Przegalinski E, Kostowski W, Krzascik P, Hartmann S, Danysz W. (1995) Comparison of the potency, kinetics and voltage-dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology 34:1239–1258 [DOI] [PubMed] [Google Scholar]
- Parsons et al., 2002.Parsons CG, Danysz W, Lodge D. (2002) Introduction to glutamate receptors, their function and pharmacology, in Ionotropic Glutamate Receptors as Therapeutic Targets (Danysz W, Lodge D, Parsons CG. eds) pp 1–30, F. P. Graham Publishing Co., Johnson City, TN [Google Scholar]
- Parsons et al., 1999.Parsons CG, Danysz W, Quack G. (1999) Memantine is a clinically well tolerated N-methyl-d-aspartate (NMDA) receptor antagonist—a review of preclinical data. Neuropharmacology 38:735–767 [DOI] [PubMed] [Google Scholar]
- Partin et al., 1995.Partin KM, Bowie D, Mayer ML. (1995) Structural determinants of allosteric regulation in alternatively spliced AMPA receptors. Neuron 14:833–843 [DOI] [PubMed] [Google Scholar]
- Partin et al., 1996.Partin KM, Fleck MW, Mayer ML. (1996) AMPA receptor flip/flop mutants affecting deactivation, desensitization, and modulation by cyclothiazide, aniracetam, and thiocyanate. J Neurosci 16:6634–6647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin et al., 1994.Partin KM, Patneau DK, Mayer ML. (1994) Cyclothiazide differentially modulates desensitization of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor splice variants. Mol Pharmacol 46:129–138 [PubMed] [Google Scholar]
- Partin et al., 1993.Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. (1993) Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron 11:1069–1082 [DOI] [PubMed] [Google Scholar]
- Pasternack et al., 2002.Pasternack A, Coleman SK, Jouppila A, Mottershead DG, Lindfors M, Pasternack M, Keinänen K. (2002) Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor channels lacking the N-terminal domain. J Biol Chem 277:49662–49667 [DOI] [PubMed] [Google Scholar]
- Paternain et al., 2003.Paternain AV, Cohen A, Stern-Bach Y, Lerma J. (2003) A role for extracellular Na+ in the channel gating of native and recombinant kainate receptors. J Neurosci 23:8641–8648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patneau et al., 1993.Patneau DK, Vyklicky L, Jr, Mayer ML. (1993) Hippocampal neurons exhibit cyclothiazide-sensitive rapidly desensitizing responses to kainate. J Neurosci 13:3496–3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul and Skolnick, 2003.Paul IA, Skolnick P. (2003) Glutamate and depression: clinical and preclinical studies. Ann NY Acad Sci 1003:250–272 [DOI] [PubMed] [Google Scholar]
- Pei et al., 2007.Pei W, Huang Z, Niu L. (2007) GluR3 flip and flop: differences in channel opening kinetics. Biochemistry 46:2027–2036 [DOI] [PubMed] [Google Scholar]
- Pei et al., 2009.Pei W, Huang Z, Wang C, Han Y, Park JS, Niu L. (2009) Flip and flop: a molecular determinant for AMPA receptor channel opening. Biochemistry 48:3767–3777 [DOI] [PubMed] [Google Scholar]
- Pelkey and McBain, 2008.Pelkey KA, McBain CJ. (2008) Ionotropic glutamate receptors in synaptic plasticity, in The Glutamate Receptors (Gereau RW, Swanson GT. eds) pp 179–246, Humana Press, Totowa, NJ [Google Scholar]
- Penn et al., 2008.Penn AC, Williams SR, Greger IH. (2008) Gating motions underlie AMPA receptor secretion from the endoplasmic reticulum. EMBO J 27:3056–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentikäinen et al., 2003.Pentikäinen OT, Settimo L, Keinänen K, Johnson MS. (2003) Selective agonist binding of (S)-2-amino-3-(3-hydroxy-5-methyl-4-isoxazolyl)propionic acid (AMPA) and 2S-(2alpha,3beta,4beta)-2-carboxy-4-(1-methylethenyl)-3-pyrrolidineacetic acid (kainate) receptors: a molecular modeling study. Biochem Pharmacol 66:2413–2425 [DOI] [PubMed] [Google Scholar]
- Pentikäinen et al., 2006.Pentikäinen U, Settimo L, Johnson MS, Pentikäinen OT. (2006) Subtype selectivity and flexibility of ionotropic glutamate receptors upon antagonist ligand binding. Org Biomol Chem 4:1058–1070 [DOI] [PubMed] [Google Scholar]
- Peoples and Weight, 1999.Peoples RW, Weight FF. (1999) Differential alcohol modulation of GABA(A) and NMDA receptors. Neuroreport 10:97–101 [DOI] [PubMed] [Google Scholar]
- Pérez-Clausell and Danscher, 1985.Pérez-Clausell J, Danscher G. (1985) Intravesicular localization of zincin rat telencephalic boutons. A histochemical study. Brain Res 337:91–98 [DOI] [PubMed] [Google Scholar]
- Pérez-Otaño et al., 2006.Pérez-Otaño I, Luján R, Tavalin SJ, Plomann M, Modregger J, Liu XB, Jones EG, Heinemann SF, Lo DC, Ehlers MD. (2006) Endocytosis and synaptic removal of NR3A-containing NMDA receptors by PACSIN1/syndapin1. Nat Neurosci 9:611–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Otano et al., 2001.Perez-Otano I, Schulteis CT, Contractor A, Lipton SA, Trimmer JS, Sucher NJ, Heinemann SF. (2001) Assembly with the NR1 subunit is required for surface expression of NR3A-containing NMDA receptors. J Neurosci 21:1228–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin-Dureau et al., 2002.Perin-Dureau F, Rachline J, Neyton J, Paoletti P. (2002) Mapping the binding site of the neuroprotectant ifenprodil on NMDA receptors. J Neurosci 22:5955–5965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais et al., 2009.Perrais D, Pinheiro PS, Jane DE, Mulle C. (2009) Antagonism of recombinant and native GluK3-containing kainate receptors. Neuropharmacology 56:131–140 [DOI] [PubMed] [Google Scholar]
- Peters et al., 1987.Peters S, Koh J, Choi DW. (1987) Zinc selectively blocks the action of N-methyl-d-aspartate on cortical neurons. Science 236:589–593 [DOI] [PubMed] [Google Scholar]
- Petralia et al., 2005.Petralia RS, Sans N, Wang YX, Wenthold RJ. (2005) Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol Cell Neurosci 29:436–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia et al., 1994.Petralia RS, Wang YX, Wenthold RJ. (1994) The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J Neurosci 14:6102–6120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia and Wenthold, 1992.Petralia RS, Wenthold RJ. (1992) Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol 318:329–354 [DOI] [PubMed] [Google Scholar]
- Petralia and Wenthold, 2008.Petralia RS, Wenthold RJ. (2008) NMDA receptors, in The Glutamate Receptors (Gereau RW, Swanson GT. eds) pp 45–98, Humana Press, Totowa, NJ [Google Scholar]
- Petrenko et al., 2003.Petrenko AB, Yamakura T, Baba H, Shimoji K. (2003) The role of N-methyl-d-aspartate (NMDA) receptors in pain: a review. Anesth Analg 97:1108–1116 [DOI] [PubMed] [Google Scholar]
- Petrovic et al., 2005.Petrovic M, Sedlacek M, Horak M, Chodounska H, Vyklický L., Jr (2005) 20-Oxo-5beta-pregnan-3alpha-yl sulfate is a use-dependent NMDA receptor inhibitor. J Neurosci 25:8439–8450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard et al., 2008.Pickard BS, Knight HM, Hamilton RS, Soares DC, Walker R, Boyd JK, Machell J, Maclean A, McGhee KA, Condie A, Porteous DJ, St Clair D, Davis I, Blackwood DH, Muir WJ., Clair D, Davis I, Blackwood DHR, Muir WJ. (2008) A common variant in the 3′UTR of the GRIK4 glutamate receptor gene affects transcript abundance and protects against bipolar disorder. Proc Natl Acad Sci USA 105:14940–14945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard et al., 2000.Pickard L, Noël J, Henley JM, Collingridge GL, Molnar E. (2000) Developmental changes in synaptic AMPA and NMDA receptor distribution and AMPA receptor subunit composition in living hippocampal neurons. J Neurosci 20:7922–7931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieri et al., 1999.Pieri I, Klein M, Bayertz C, Gerspach J, van der Ploeg A, Pfizenmaier K, Eisel U. (1999) Regulation of the murine NMDA-receptor-subunit NR2C promoter by Sp1 and fushi tarazu factor1 (FTZ-F1) homologues. Eur J Neurosci 11:2083–2092 [DOI] [PubMed] [Google Scholar]
- Piña-Crespo and Gibb, 2002.Piña-Crespo JC, Gibb AJ. (2002) Subtypes of NMDA receptors in new-born rat hippocampal granule cells. J Physiol 541:41–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant et al., 2006.Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. (2006) Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci 9:602–604 [DOI] [PubMed] [Google Scholar]
- Plested et al., 2008.Plested AJ, Vijayan R, Biggin PC, Mayer ML. (2008) Molecular basis of kainate receptor modulation by sodium. Neuron 58:720–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plested and Mayer, 2007.Plested AJ, Mayer ML. (2007) Structure and mechanism of kainate receptor modulation by anions. Neuron 53:829–841 [DOI] [PubMed] [Google Scholar]
- Plested and Mayer, 2009.Plested AJ, Mayer ML. (2009) Engineering a high-affinity allosteric binding site for divalent cations in kainate receptors. Neuropharmacology 56:114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu and Auerbach, 2003.Popescu G, Auerbach A. (2003) Modal gating of NMDA receptors and the shape of their synaptic response. Nat Neurosci 6:476–483 [DOI] [PubMed] [Google Scholar]
- Popescu et al., 2004.Popescu G, Robert A, Howe JR, Auerbach A. (2004) Reaction mechanism determines NMDA receptor response to repetitive stimulation. Nature 430:790–793 [DOI] [PubMed] [Google Scholar]
- Premkumar and Auerbach, 1996.Premkumar LS, Auerbach A. (1996) Identification of a high affinity divalent cation binding site near the entrance of the NMDA receptor channel. Neuron 16:869–880 [DOI] [PubMed] [Google Scholar]
- Premkumar et al., 1997.Premkumar LS, Qin F, Auerbach A. (1997) Subconductance states of a mutant NMDA receptor channel kinetics, calcium, and voltage dependence. J Gen Physiol 109:181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott et al., 2006.Prescott C, Weeks AM, Staley KJ, Partin KM. (2006) Kynurenic acid has a dual action on AMPA receptor responses. Neurosci Lett 402:108–112 [DOI] [PubMed] [Google Scholar]
- Preskorn et al., 2008.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. (2008) An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-d-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 28:631–637 [DOI] [PubMed] [Google Scholar]
- Priel et al., 2005.Priel A, Kolleker A, Ayalon G, Gillor M, Osten P, Stern-Bach Y. (2005) Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J Neurosci 25:2682–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel et al., 2006.Priel A, Selak S, Lerma J, Stern-Bach Y. (2006) Block of kainate receptor desensitization uncovers a key trafficking checkpoint. Neuron 52:1037–1046 [DOI] [PubMed] [Google Scholar]
- Priestley and Kemp, 1994.Priestley T, Kemp JA. (1994) Kinetic study of the interactions between the glutamate and glycine recognition sites on the N-methyl-D-aspartic acid receptor complex. Mol Pharmacol 46:1191–1196 [PubMed] [Google Scholar]
- Priestley et al., 1995.Priestley T, Laughton P, Myers J, Le Bourdellés B, Kerby J, Whiting PJ. (1995) Pharmacological properties of recombinant human N-methyl-d-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol Pharmacol 48:841–848 [PubMed] [Google Scholar]
- Prieto and Wollmuth, 2010.Prieto ML, Wollmuth LP. (2010) Gating modes in AMPA receptors. J Neurosci 30:4449–4459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prorok and Castellino, 2007.Prorok M, Castellino FJ. (2007) The molecular basis of conantokin antagonism of NMDA receptor function. Current Drug Targets 8:633–642 [DOI] [PubMed] [Google Scholar]
- Prybylowski et al., 2005.Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. (2005) The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron 47:845–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan et al., 1987.Pullan LM, Olney JW, Price MT, Compton RP, Hood WF, Michel J, Monahan JB. (1987) Excitatory amino acid receptor potency and subclass specificity of sulfur-containing amino acids. J Neurochem 49:1301–1307 [DOI] [PubMed] [Google Scholar]
- Qian et al., 2002.Qian A, Antonov SM, Johnson JW. (2002) Modulation by permeant ions of Mg2+ inhibition of NMDA-activated whole-cell currents in rat cortical neurons. J Physiol 538:65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian and Johnson, 2002.Qian A, Johnson JW. (2002) Channel gating of NMDA receptors. Physiol Behav 77:577–582 [DOI] [PubMed] [Google Scholar]
- Qian and Johnson, 2006.Qian A, Johnson JW. (2006) Permeant ion effects on external Mg2+ block of NR1/2D NMDA receptors. J Neurosci 26:10899–10910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang et al., 2005.Qiang M, Rani CS, Ticku MK. (2005) Neuron-restrictive silencer factor regulates the N-methyl-d-aspartate receptor 2B subunit gene in basal and ethanol-induced gene expression in fetal cortical neurons. Mol Pharmacol 67:2115–2125 [DOI] [PubMed] [Google Scholar]
- Qiang and Ticku, 2005.Qiang M, Ticku MK. (2005) Role of AP-1 in ethanol-induced N-methyl-d-aspartate receptor 2B subunit gene up-regulation in mouse cortical neurons. J Neurochem 95:1332–1341 [DOI] [PubMed] [Google Scholar]
- Qiu et al., 2005.Qiu S, Hua YL, Yang F, Chen YZ, Luo JH. (2005) Subunit assembly of N-methyl-d-aspartate receptors analyzed by fluorescence resonance energy transfer. J Biol Chem 280:24923–24930 [DOI] [PubMed] [Google Scholar]
- Qiu et al., 2009.Qiu S, Zhang XM, Cao JY, Yang W, Yan YG, Shan L, Zheng J, Luo JH. (2009) An endoplasmic reticulum retention signal located in the extracellular amino-terminal domain of the NR2A subunit of N-methyl-d-aspartate receptors. J Biol Chem 284:20285–20298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu and Ghosh, 2008.Qiu Z, Ghosh A. (2008) A calcium-dependent switch in a CREST-BRG1 complex regulates activity-dependent gene expression. Neuron 60:775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan et al., 1999.Quinlan EM, Philpot BD, Huganir RL, Bear MF. (1999) Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci 2:352–357 [DOI] [PubMed] [Google Scholar]
- Quirk et al., 2004.Quirk JC, Siuda ER, Nisenbaum ES. (2004) Molecular determinants responsible for differences in desensitization kinetics of AMPA receptor splice variants. J Neurosci 24:11416–11420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabey et al., 1992.Rabey JM, Nissipeanu P, Korczyn AD. (1992) Efficacy of memantine, an NMDA receptor antagonist, in the treatment of Parkinson's disease. J Neural Transm Park Dis Dement Sect 4:277–282 [DOI] [PubMed] [Google Scholar]
- Rachline et al., 2005.Rachline J, Perin-Dureau F, Le Goff A, Neyton J, Paoletti P. (2005) The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J Neurosci 25:308–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameau et al., 2003.Rameau GA, Chiu LY, Ziff EB. (2003) NMDA receptor regulation of nNOS phosphorylation and induction of neuron death. Neurobiol Aging 24:1123–1133 [DOI] [PubMed] [Google Scholar]
- Rani et al., 2005.Rani CS, Qiang M, Ticku MK. (2005) Potential role of cAMP response element-binding protein in ethanol-induced N-methyl-d-aspartate receptor 2B subunit gene transcription in fetal mouse cortical cells. Mol Pharmacol 67:2126–2136 [DOI] [PubMed] [Google Scholar]
- Ransom, 1991.Ransom RW. (1991) Polyamine and ifenprodil interactions with the NMDA receptor's glycine site. Eur J Pharmacol 208:67–71 [DOI] [PubMed] [Google Scholar]
- Ransom and Deschenes, 1990.Ransom RW, Deschenes NL. (1990) Polyamines regulate glycine interaction with the N-methyl-d-aspartate receptor. Synapse 5:294–298 [DOI] [PubMed] [Google Scholar]
- Rassendren et al., 1990.Rassendren FA, Lory P, Pin JP, Nargeot J. (1990) Zinc has opposite effects on NMDA and non-NMDA receptors expressed in Xenopus oocytes. Neuron 4:733–740 [DOI] [PubMed] [Google Scholar]
- Ravindran and Ticku, 2005.Ravindran CRM, Ticku MK. (2005) Role of CpG islands in the up-regulation of NMDA receptor NR2B gene expression following chronic ethanol treatment of cultured cortical neurons of mice. Neurochem Intl 46:313–327 [DOI] [PubMed] [Google Scholar]
- Regalado et al., 2001.Regalado MP, Villarroel A, Lerma J. (2001) Intersubunit cooperativity in the NMDA receptor. Neuron 32:1085–1096 [DOI] [PubMed] [Google Scholar]
- Reisberg et al., 2003.Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJMemantine Study Group (2003) Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med 348:1333–1341 [DOI] [PubMed] [Google Scholar]
- Ren et al., 2003.Ren H, Honse Y, Karp BJ, Lipsky RH, Peoples RW. (2003) A site in the fourth membrane-associated domain of the N-methyl-d-aspartate receptor regulates desensitization and ion channel gating. J Biol Chem 278:276–283 [DOI] [PubMed] [Google Scholar]
- Ressler et al., 2004.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. (2004) Cognitive enhancers as adjuncts to psychotherapy: use of d-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 61:1136–1144 [DOI] [PubMed] [Google Scholar]
- Richter and Sonenberg, 2005.Richter JD, Sonenberg N. (2005) Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 433:477–480 [DOI] [PubMed] [Google Scholar]
- Richter et al., 2002.Richter M, Suau P, Ponte I. (2002) Sequence and analysis of the 5′ flanking and 5′ untranslated regions of the rat N-methyl-d-aspartate receptor 2A gene Gene 295:135–142 [DOI] [PubMed] [Google Scholar]
- Rieff et al., 1999.Rieff HI, Raetzman LT, Sapp DW, Yeh HH, Siegel RE, Corfas G. (1999) Neuregulin induces GABAA receptor subunit expression and neurite outgrowth in cerebellar granule cells. J Neurosci 19:10757–10766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera et al., 2007.Rivera R, Rozas JL, Lerma J. (2007) PKC-dependent autoregulation of membrane kainate receptors. EMBO J 26:4359–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert et al., 2005.Robert A, Armstrong N, Gouaux JE, Howe JR. (2005) AMPA receptor binding cleft mutations that alter affinity, efficacy, and recovery from desensitization. J Neurosci 25:3752–3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert and Howe, 2003.Robert A, Howe JR. (2003) How AMPA receptor desensitization depends on receptor occupancy. J Neurosci 23:847–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert et al., 2001.Robert A, Irizarry SN, Hughes TE, Howe JR. (2001) Subunit interactions and AMPA receptor desensitization. J Neurosci 21:5574–5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts et al., 2009.Roberts AC, Díez-García J, Rodriguiz RM, López IP, Luján R, Martínez-Turrillas R, Picó E, Henson MA, Bernardo DR, Jarrett TM, et al. (2009) Downregulation of NR3A-containing NMDARs is required for synapse maturation and memory consolidation. Neuron 63:342–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalewski et al., 2006.Rogalewski A, Schneider A, Ringelstein EB, Schäbitz WR. (2006) Toward a multimodal neuroprotective treatment of stroke. Stroke 37:1129–1136 [DOI] [PubMed] [Google Scholar]
- Roche et al., 1999.Roche KW, Ly CD, Petralia RS, Wang YX, McGee AW, Bredt DS, Wenthold RJ. (1999) Postsynaptic density-93 interacts with the delta2 glutamate receptor subunit at parallel fiber synapses. J Neurosci 19:3926–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche et al., 1996.Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. (1996) Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16:1179–1188 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moreno and Lerma, 1998.Rodríguez-Moreno A, Lerma J. (1998) Kainate receptor modulation of GABA release involves a metabotropic function. Neuron 20:1211–1218 [DOI] [PubMed] [Google Scholar]
- Rogers et al., 2009.Rogers M, Rasheed A, Moradimehr A, Baumrucker SJ. (2009) Memantine (Namenda) for neuropathic pain. Am J Hosp Palliat Care 26:57–59 [DOI] [PubMed] [Google Scholar]
- Rong et al., 2001.Rong Y, Lu X, Bernard A, Khrestchatisky M, Baudry M. (2001) Tyrosine phosphorylation of ionotropic glutamate receptors by Fyn or Src differentially modulates their susceptibility to calpain and enhances their binding to spectrin and PSD-95. J Neurochem 79:382–390 [DOI] [PubMed] [Google Scholar]
- Rosenmund et al., 1998.Rosenmund C, Stern-Bach Y, Stevens CF. (1998) The tetrameric structure of a glutamate receptor channel. Science 280:1596–1599 [DOI] [PubMed] [Google Scholar]
- Rosenmund and Westbrook, 1993.Rosenmund C, Westbrook GL. (1993) Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron 10:805–814 [DOI] [PubMed] [Google Scholar]
- Roth et al., 1995.Roth S, Neuman-Silberberg FS, Barcelo G, Schüpbach T. (1995) Cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell 81:967–978 [DOI] [PubMed] [Google Scholar]
- Rozov and Burnashev, 1999.Rozov A, Burnashev N. (1999) Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature 401:594–598 [DOI] [PubMed] [Google Scholar]
- Rozov et al., 1998.Rozov A, Zilberter Y, Wollmuth LP, Burnashev N. (1998) Facilitation of currents through rat Ca2+-permeable AMPA receptor channels by activity-dependent relief from polyamine block. J Physiol (Lond) 511:361–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz et al., 2005.Ruiz A, Sachidhanandam S, Utvik JK, Coussen F, Mulle C. (2005) Distinct subunits in heteromeric kainate receptors mediate ionotropic and metabotropic function at hippocampal mossy fiber synapses. J Neurosci 25:11710–11718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh and Vicini, 1999.Rumbaugh G, Vicini S. (1999) Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. J Neurosci 19:10603–10610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco et al., 2001.Sacco RL, DeRosa JT, Haley EC, Jr, Levin B, Ordronneau P, Phillips SJ, Rundek T, Snipes RG, Thompson JLGlycine Antagonist in Neuroprotection Americas Investigators (2001) Glycine antagonist in neuroprotection for patients with acute stroke: GAIN Americas: a randomized controlled trial. JAMA 285:1719–1728 [DOI] [PubMed] [Google Scholar]
- Sachidhanandam et al., 2009.Sachidhanandam S, Blanchet C, Jeantet Y, Cho YH, Mulle C. (2009) Kainate receptors act as conditional amplifiers of spike transmission at hippocampal mossy fiber synapses. J Neurosci 29:5000–5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safferling et al., 2001.Safferling M, Tichelaar W, Kümmerle G, Jouppila A, Kuusinen A, Keinänen K, Madden DR. (2001) First images of a glutamate receptor ion channel: oligomeric state and molecular dimensions of GluRB homomers. Biochemistry 40:13948–13953 [DOI] [PubMed] [Google Scholar]
- Sager et al., 2009.Sager C, Terhag J, Kott S, Hollmann M. (2009) C-terminal domains of transmembrane alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptor regulatory proteins not only facilitate trafficking but are major modulators of AMPA receptor function. J Biol Chem 284:32413–32424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglietti et al., 2007.Saglietti L, Dequidt C, Kamieniarz K, Rousset MC, Valnegri P, Thoumine O, Beretta F, Fagni L, Choquet D, Sala C, et al. (2007) Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron 54:461–477 [DOI] [PubMed] [Google Scholar]
- Sagot et al., 2008.Sagot E, Pickering DS, Pu X, Umberti M, Stensbøl TB, Nielsen B, Chapelet M, Bolte J, Gefflaut T, Bunch L. (2008) Chemo-enzymatic synthesis of a series of 2,4-syn-functionalized (S)-glutamate analogues: new insight into the structure-activity relation of ionotropic glutamate receptor subtypes 5, 6, and 7. J Med Chem 51:4093–4103 [DOI] [PubMed] [Google Scholar]
- Sakai et al., 1997.Sakai R, Kamiya H, Murata M, Shimamoto K. (1997) Dysiherbaine: a new neurotoxic amino acid from the micronesian marine sponge dysidea herbacea. J Am Chem Soc 119:4112–4116 [Google Scholar]
- Sakai et al., 2001a.Sakai R, Koike T, Sasaki M, Shimamoto K, Oiwa C, Yano A, Suzuki K, Tachibana K, Kamiya H. (2001a) Isolation, structure determination, and synthesis of neodysiherbaine A, a new excitatory amino acid from a marine sponge. Org Lett 3:1479–1482 [DOI] [PubMed] [Google Scholar]
- Sakai et al., 2001b.Sakai R, Swanson GT, Shimamoto K, Green T, Contractor A, Ghetti A, Tamura-Horikawa Y, Oiwa C, Kamiya H. (2001b) Pharmacological properties of the potent epileptogenic amino acid dysiherbaine, a novel glutamate receptor agonist isolated from the marine sponge Dysidea herbacea. J Pharmacol Exp Ther 296:650–658 [PubMed] [Google Scholar]
- Salinas et al., 2006.Salinas GD, Blair LA, Needleman LA, Gonzales JD, Chen Y, Li M, Singer JD, Marshall J. (2006) Actinfilin is a Cul3 substrate adaptor, linking GluR6 kainate receptor subunits to the ubiquitin-proteasome pathway. J Biol Chem 281:40164–40173 [DOI] [PubMed] [Google Scholar]
- Salopek-Sondi et al., 2003.Salopek-Sondi B, Vaughan MD, Skeels MC, Honek JF, Luck LA. (2003) (19)F NMR studies of the leucine-isoleucine-valine binding protein: evidence that a closed conformation exists in solution. J Biomol Struct Dyn 21:235–246 [DOI] [PubMed] [Google Scholar]
- Salter and Fern, 2005.Salter MG, Fern R. (2005) NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature 438:1167–1171 [DOI] [PubMed] [Google Scholar]
- Samson et al., 2008.Samson AL, Nevin ST, Croucher D, Niego B, Daniel PB, Weiss TW, Moreno E, Monard D, Lawrence DA, Medcalf RL. (2008) Tissue-type plasminogen activator requires a co-receptor to enhance NMDA receptor function. J Neurochem 107:1091–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Perez and Felipo, 2005.Sanchez-Perez AM, Felipo V. (2005) Serines 890 and 896 of the NMDA receptor subunit NR1 are differentially phosphorylated by protein kinase C isoforms. Neuroche Int 47:84–91 [DOI] [PubMed] [Google Scholar]
- Sanders et al., 2005.Sanders JM, Ito K, Settimo L, Pentikäinen OT, Shoji M, Sasaki M, Johnson MS, Sakai R, Swanson GT. (2005) Divergent pharmacological activity of novel marine-derived excitatory amino acids on glutamate receptors. J Pharmacol Exp Ther 314:1068–1078 [DOI] [PubMed] [Google Scholar]
- Sang et al., 2002.Sang CN, Booher S, Gilron I, Parada S, Max MB. (2002) Dextromethorphan and memantine in painful diabetic neuropathy and postherpetic neuralgia: efficacy and dose-response trials. Anesthesiology 96:1053–1061 [DOI] [PubMed] [Google Scholar]
- Sang et al., 2004.Sang CN, Ramadan NM, Wallihan RG, Chappell AS, Freitag FG, Smith TR, Silberstein SD, Johnson KW, Phebus LA, Bleakman D, et al. (2004) LY293558, a novel AMPA/GluR5 antagonist, is efficacious and well-tolerated in acute migraine. Cephalalgia 24:596–602 [DOI] [PubMed] [Google Scholar]
- Sang et al., 2003.Sang CN, Weaver JJ, Jinga L, Wouden J, Saltarelli MD. (2003) The NR2B subunit-selective NMDA receptor antagonist CP-101,606 reduces pain intensity in patients with central and peripheral neuropathic pain. Soc Neurosci Abstr 29:814.9 [Google Scholar]
- Sans et al., 2003.Sans N, Prybylowski K, Petralia RS, Chang K, Wang YX, Racca C, Vicini S, Wenthold RJ. (2003) NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat Cell Biol 5:520–530 [DOI] [PubMed] [Google Scholar]
- Sans et al., 2001.Sans N, Racca C, Petralia RS, Wang YX, McCallum J, Wenthold RJ. (2001) Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. J Neurosci 21:7506–7516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki et al., 2002.Sasaki YF, Rothe T, Premkumar LS, Das S, Cui J, Talantova MV, Wong HK, Gong X, Chan SF, Zhang D, et al. (2002) Characterization and comparison of the NR3A subunit of the NMDA receptor in recombinant systems and primary cortical neurons. J Neurophysiol 87:2052–2063 [DOI] [PubMed] [Google Scholar]
- Sasner and Buonanno, 1996.Sasner M, Buonanno A. (1996) Distinct N-methyl-d-aspartate receptor 2B subunit gene sequences confer neural and developmental specific expression. J Biol Chem 271:21316–21322 [DOI] [PubMed] [Google Scholar]
- Sattler et al., 1999.Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. (1999) Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science 284:1845–1848 [DOI] [PubMed] [Google Scholar]
- Saver et al., 2009.Saver JL, Albers GW, Dunn B, Johnston KC, Fisher MSTAIR VI Consortium (2009) Stroke Therapy Academic Industry Roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke 40:2594–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver et al., 2004.Saver JL, Kidwell C, Eckstein M, Starkman SFAST-MAG Pilot Trial Investigators (2004) Prehospital neuroprotective therapy for acute stroke: results of the Field Administration of Stroke Therapy-Magnesium (FAST-MAG) pilot trial. Stroke 35:e106–e108 [DOI] [PubMed] [Google Scholar]
- Scheetz et al., 2000.Scheetz AJ, Nairn AC, Constantine-Paton M. (2000) NMDA receptor-mediated control of protein synthesis at developing synapses. Nat Neurosci 3:211–216 [DOI] [PubMed] [Google Scholar]
- Schell et al., 1997.Schell MJ, Cooper OB, Snyder SH. (1997) d-aspartate localizations imply neuronal and neuroendocrine roles. Proc Natl Acad Sci USA 94:2013–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer et al., 1997.Schiffer HH, Swanson GT, Heinemann SF. (1997) Rat GluR7 and a carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron 19:1141–1146 [DOI] [PubMed] [Google Scholar]
- Schlesinger et al., 2005.Schlesinger F, Tammena D, Krampfl K, Bufler J. (2005) Two mechanisms of action of the adamantane derivative IEM-1460 at human AMPA-type glutamate receptors. Br J Pharmacol 145:656–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid et al., 2007.Schmid SM, Körber C, Herrmann S, Werner M, Hollmann M. (2007) A domain linking the AMPA receptor agonist binding site to the ion pore controls gating and causes lurcher properties when mutated. J Neurosci 27:12230–12241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt and Hollmann, 2008.Schmidt C, Hollmann M. (2008) Apparent homomeric NR1 currents observed in Xenopus oocytes are caused by an endogenous NR2 subunit. J Mol Biol 376:658–670 [DOI] [PubMed] [Google Scholar]
- Schmidt and Hollmann, 2009.Schmidt C, Hollmann M. (2009) Molecular and functional characterization of Xenopus laevis N-methyl-d-aspartate receptors. Mol Cell Neurosci 42:116–127 [DOI] [PubMed] [Google Scholar]
- Schmidt et al., 2009.Schmidt C, Klein C, Hollmann M. (2009) Xenopus Laevis oocytes endogenously express all subunits of the ionotropic glutamate receptor family. J Mol Biol 390:182–195 [DOI] [PubMed] [Google Scholar]
- Schmidt et al., 2006.Schmidt C, Werner M, Hollmann M. (2006) Revisiting the postulated “unitary glutamate receptor”: electrophysiological and pharmacological analysis in two heterologous expression systems fails to detect evidence for its existence. Mol Pharmacol 69:119–129 [DOI] [PubMed] [Google Scholar]
- Schmitt, 2005.Schmitt HP. (2005) Pouring oil into the fire? On the conundrum of the beneficial effects of NMDA receptor antagonists in Alzheimer disease. Psychopharmacology 179:151–153 [DOI] [PubMed] [Google Scholar]
- Schneggenburger, 1996.Schneggenburger R. (1996) Simultaneous measurement of Ca2+ influx and reversal potentials in recombinant N-methyl-d-aspartate receptor channels. Biophys J 70:2165–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger, 1998.Schneggenburger R. (1998) Altered voltage dependence of fractional Ca2+ current in N-methyl-d-aspartate channel pore mutants with a decreased Ca2+ permeability. Biophys J 74:1790–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger and Ascher, 1997.Schneggenburger R, Ascher P. (1997) Coupling of permeation and gating in an NMDA-channel pore mutant. Neuron 18:167–177 [DOI] [PubMed] [Google Scholar]
- Schnell et al., 2002.Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. (2002) Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA 99:13902–13907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp et al., 1991.Schoepp DD, Smith CL, Lodge D, Millar JD, Leander JD, Sacaan AI, Lunn WH. (1991) d,l-(tetrazol-5-yl) glycine: a novel and highly potent NMDA receptor agonist. Eur J Pharmacol 203:237–243 [DOI] [PubMed] [Google Scholar]
- Schorge and Colquhoun, 2003.Schorge S, Colquhoun D. (2003) Studies of NMDA receptor function and stoichiometry with truncated and tandem subunits. J Neurosci 23:1151–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorge et al., 2005.Schorge S, Elenes S, Colquhoun D. (2005) Maximum likelihood fitting of single channel NMDA activity with a mechanism composed of independent dimers of subunits. J Physiol 569:395–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt et al., 2004.Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. (2004) BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci 24:7366–7377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüler et al., 2008.Schüler T, Mesic I, Madry C, Bartholomäus I, Laube B. (2008) Formation of NR1/NR2 and NR1/NR3 heterodimers constitutes the initial step in N-methyl-d-aspartate receptor assembly. J Biol Chem 283:37–46 [DOI] [PubMed] [Google Scholar]
- Schulz et al., 2004.Schulz TW, Nakagawa T, Licznerski P, Pawlak V, Kolleker A, Rozov A, Kim J, Dittgen T, Köhr G, Sheng M, et al. (2004) Actin/alpha-actinin-dependent transport of AMPA receptors in dendritic spines: role of the PDZ-LIM protein RIL. J Neurosci 24:8584–8594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk et al., 2009.Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, et al. (2009) Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science 323:1313–1319 [DOI] [PubMed] [Google Scholar]
- Scott et al., 2003.Scott DB, Blanpied TA, Ehlers MD. (2003) Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology 45:755–767 [DOI] [PubMed] [Google Scholar]
- Scott et al., 2001.Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. (2001) An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci 21:3063–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlácek et al., 2008.Sedlácek M, Korínek M, Petrovic M, Cais O, Adamusova E, Chodounská H, Vyklický L. (2008) Neurosteroid modulation of ionotropic glutamate receptors and excitatory synaptic transmission. Physiol Res 57 (Suppl 3):S49–S57 [DOI] [PubMed] [Google Scholar]
- Seidenman et al., 2003.Seidenman KJ, Steinberg JP, Huganir R, Malinow R. (2003) Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci 23:9220–9228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi et al., 1997.Sekiguchi M, Fleck MW, Mayer ML, Takeo J, Chiba Y, Yamashita S, Wada K. (1997) A novel allosteric potentiator of AMPA receptors: 4–2-(phenylsulfonylamino)ethylthio-2,6-difluoro-phenoxyacetamide. J Neurosci 17:5760–5771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi et al., 2002.Sekiguchi M, Nishikawa K, Aoki S, Wada K. (2002) A desensitization-selective potentiator of AMPA-type glutamate receptors. Br J Pharmacol 136:1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi et al., 1998.Sekiguchi M, Takeo J, Harada T, Morimoto T, Kudo Y, Yamashita S, Kohsaka S, Wada K. (1998) Pharmacological detection of AMPA receptor heterogeneity by use of two allosteric potentiators in rat hippocampal cultures. Br J Pharmacol 123:1294–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak et al., 2009.Selak S, Paternain AV, Aller MI, Aller IM, Picó E, Rivera R, Lerma J. (2009) A role for SNAP25 in internalization of kainate receptors and synaptic plasticity. Neuron 63:357–371 [DOI] [PubMed] [Google Scholar]
- Semyanov and Kullmann, 2001.Semyanov A, Kullmann DM. (2001) Kainate receptor-dependent axonal depolarization and action potential initiation in interneurons. Nat Neurosci 4:718–723 [DOI] [PubMed] [Google Scholar]
- Sensi et al., 1999.Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. (1999) Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci USA 96:2414–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serulle et al., 2007.Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff EB. (2007) A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron 56:670–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessoms-Sikes et al., 2005.Sessoms-Sikes S, Honse Y, Lovinger DM, Colbran RJ. (2005) CaMKIIalpha enhances the desensitization of NR2B-containing NMDA receptors by an autophosphorylation-dependent mechanism. Mol Cell Neurosci 29:139–147 [DOI] [PubMed] [Google Scholar]
- Sharma and Stevens, 1996.Sharma G, Stevens CF. (1996) Interactions between two divalent ion binding sites in N-methyl-d-aspartate receptor channels. Proc Natl Acad Sci USA 93:14170–14175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheardown et al., 1990.Sheardown MJ, Nielsen EO, Hansen AJ, Jacobsen P, Honoré T. (1990) 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science 247:571–574 [DOI] [PubMed] [Google Scholar]
- Sheinin et al., 2001.Sheinin A, Shavit S, Benveniste M. (2001) Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology 41:151–158 [DOI] [PubMed] [Google Scholar]
- Shen et al., 2000.Shen L, Liang F, Walensky LD, Huganir RL. (2000) Regulation of AMPA receptor GluR1 subunit surface expression by a 4. 1N-linked actin cytoskeletal association. J Neurosci 20:7932–7940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen and Yang, 1999.Shen Y, Yang XL. (1999) Zinc modulation of AMPA receptors may be relevant to splice variants in carp retina. Neurosci Lett 259:177–180 [DOI] [PubMed] [Google Scholar]
- Sheng et al., 1994.Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. (1994) Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 368:144–147 [DOI] [PubMed] [Google Scholar]
- Sheng et al., 2007.Sheng Z, Dai Q, Prorok M, Castellino FJ. (2007) Subtype-selective antagonism of N-methyl-d-aspartate receptor ion channels by synthetic conantokin peptides. Neuropharmacology 53:145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd and Huganir, 2007.Shepherd JD, Huganir RL. (2007) The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol 23:613–643 [DOI] [PubMed] [Google Scholar]
- Shi et al., 2001.Shi S, Hayashi Y, Esteban JA, Malinow R. (2001) Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105:331–343 [DOI] [PubMed] [Google Scholar]
- Shi et al., 2009.Shi Y, Lu W, Milstein AD, Nicoll RA. (2009) The stoichiometry of AMPA receptors and TARPs varies by neuronal cell type. Neuron 62:633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim et al., 2008.Shim SS, Hammonds MD, Kee BS. (2008) Potentiation of the NMDA receptor in the treatment of schizophrenia: focused on the glycine site. Eur Arch Psychiatry Clin Neurosci 258:16–27 [DOI] [PubMed] [Google Scholar]
- Shin et al., 2005.Shin J, Shen F, Huguenard JR. (2005) Polyamines modulate AMPA receptor-dependent synaptic responses in immature layer v pyramidal neurons. J Neurophysiol 93:2634–2643 [DOI] [PubMed] [Google Scholar]
- Shinozaki et al., 1989.Shinozaki H, Ishida M, Shimamoto K, Ohfune Y. (1989) A conformationally restricted analogue of l-glutamate, the (2S,3R,4S) isomer of l-alpha-(carboxycyclopropyl)glycine, activates the NMDA-type receptor more markedly than NMDA in the isolated rat spinal cord. Brain Res 480:355–359 [DOI] [PubMed] [Google Scholar]
- Shirakawa et al., 2005.Shirakawa H, Katsuki H, Kume T, Kaneko S, Akaike A. (2005) Pregnenolone sulphate attenuates AMPA cytotoxicity on rat cortical neurons. Eur J Neurosci 21:2329–2335 [DOI] [PubMed] [Google Scholar]
- Shoji et al., 2006.Shoji K, Mariotto S, Ciampa AR, Suzuki H. (2006) Regulation of serine racemase activity by d-serine and nitric oxide in human glioblastoma cells. Neurosci Lett 392:75–78 [DOI] [PubMed] [Google Scholar]
- Shoulson et al., 2001.Shoulson I, Penney J, McDermott M, Schwid S, Kayson E, Chase T, Fahn S, Greenamyre JT, Lang A, Siderowf A, et al. (2001) A randomized, controlled trial of remacemide for motor fluctuations in Parkinson's disease. Neurology 56:455–462 [DOI] [PubMed] [Google Scholar]
- Sia et al., 2007.Sia GM, Béïque JC, Rumbaugh G, Cho R, Worley PF, Huganir RL. (2007) Interaction of the N-terminal domain of the AMPA receptor GluR4 subunit with the neuronal pentraxin NP1 mediates GluR4 synaptic recruitment. Neuron 55:87–102 [DOI] [PubMed] [Google Scholar]
- Siesjö, 1985.Siesjö BK. (1985) Acid-base homeostasis in the brain: physiology, chemistry, and neurochemical pathology. Prog Brain Res 63:121–154 [DOI] [PubMed] [Google Scholar]
- Simmons et al., 2009.Simmons DA, Rex CS, Palmer L, Pandyarajan V, Fedulov V, Gall CM, Lynch G. (2009) Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington's disease knockin mice. Proc Natl Acad Sci USA 106:4906–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons et al., 1998.Simmons RM, Li DL, Hoo KH, Deverill M, Ornstein PL, Iyengar S. (1998) Kainate GluR5 receptor subtype mediates the nociceptive response to formalin in the rat. Neuropharmacology 37:25–36 [DOI] [PubMed] [Google Scholar]
- Simpkins et al., 2003.Simpkins KL, Guttmann RP, Dong Y, Chen Z, Sokol S, Neumar RW, Lynch DR. (2003) Selective activation induced cleavage of the NR2B subunit by calpain. J Neurosci 23:11322–11331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinis et al., 2007.Sinis N, Birbaumer N, Gustin S, Schwarz A, Bredanger S, Becker ST, Unertl K, Schaller HE, Haerle M. (2007) Memantine treatment of complex regional pain syndrome: a preliminary report of six cases. Clin J Pain 23:237–243 [DOI] [PubMed] [Google Scholar]
- Siu and Drachtman, 2007.Siu A, Drachtman R. (2007) Dextromethorphan: a review of N-methyl-d-aspartate receptor antagonist in the management of pain. CNS Drug Rev 13:96–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaprakasam et al., 2009.Sivaprakasam M, Hansen KB, David O, Nielsen B, Traynelis SF, Clausen RP, Couty F, Bunch L. (2009) Stereocontrolled synthesis and pharmacological evaluation of azetidine-2,3-dicarboxylic acids at NMDA receptors. ChemMedChem 4:110–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeberdis et al., 2006.Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, et al. (2006) Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci 9:501–510 [DOI] [PubMed] [Google Scholar]
- Skolnick et al., 2009.Skolnick P, Popik P, Trullas R. (2009) Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci 30:563–569 [DOI] [PubMed] [Google Scholar]
- Smith and McIlhinney, 1992.Smith AL, McIlhinney RA. (1992) Effects of acromelic acid A on the binding of [3H]-kainic acid and [3H]-AMPA to rat brain synaptic plasma membranes. Br J Pharmacol 105:83–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small et al., 1998.Small B, Thomas J, Kemp M, Hoo K, Ballyk B, Deverill M, Ogden AM, Rubio A, Pedregal C, Bleakman D. (1998) LY339434, a GluR5 kainate receptor agonist. Neuropharmacology 37:1261–1267 [DOI] [PubMed] [Google Scholar]
- Small and Tauskela, 2005.Small DL, Tauskela JS. (2005) Glutamate receptor pharmacology: lessons learned from the last decade of stroke trials, in Glutamate Receptors in Peripheral Tissue: Excitatory Transmission Outside the CNS (Gill S, Pulido O. eds) pp 27–45, Kluwer Academic/Plenum Publishers, New York [Google Scholar]
- Smith et al., 2009.Smith SM, Uslaner JM, Yao L, Mullins CM, Surles NO, Huszar SL, McNaughton CH, Pascarella DM, Kandebo M, Hinchliffe RM, et al. (2009) The behavioral and neurochemical effects of a novel D-amino acid oxidase inhibitor compound 8 [4H-thieno[3,2-b]pyrrole-5-carboxylic acid] and d-serine. J Pharmacol Exp Ther 328:921–930 [DOI] [PubMed] [Google Scholar]
- Smith and Howe, 2000.Smith TC, Howe JR. (2000) Concentration-dependent substate behavior of native AMPA receptors. Nat Neurosci 3:992–997 [DOI] [PubMed] [Google Scholar]
- Smothers and Woodward, 2007.Smothers CT, Woodward JJ. (2007) Pharmacological characterization of glycine-activated currents in HEK 293 cells expressing N-methyl-d-aspartate NR1 and NR3 subunits. J Pharmacol Exp Ther 322:739–748 [DOI] [PubMed] [Google Scholar]
- Snyder et al., 2005.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, et al. (2005) Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci 8:1051–1058 [DOI] [PubMed] [Google Scholar]
- Sobczyk and Svoboda, 2007.Sobczyk A, Svoboda K. (2007) Activity-dependent plasticity of the NMDA-receptor fractional Ca2+ current. Neuron 53:17–24 [DOI] [PubMed] [Google Scholar]
- Sobolevsky and Koshelev, 1998.Sobolevsky A, Koshelev S. (1998) Two blocking sites of amino-adamantane derivatives in open N-methyl-d-aspartate channels. Biophys J 74:1305–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky et al., 2002a.Sobolevsky AI, Beck C, Wollmuth LP. (2002a) Molecular rearrangements of the extracellular vestibule in NMDAR channels during gating. Neuron 33:75–85 [DOI] [PubMed] [Google Scholar]
- Sobolevsky et al., 1998.Sobolevsky AI, Koshelev SG, Khodorov BI. (1998) Interaction of memantine and amantadine with agonist-unbound NMDA-receptor channels in acutely isolated rat hippocampal neurons. J Physiol 512:47–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky et al., 1999.Sobolevsky AI, Koshelev SG, Khodorov BI. (1999) Probing of NMDA channels with fast blockers. J Neurosci 19:10611–10626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky et al., 2007.Sobolevsky AI, Prodromou ML, Yelshansky MV, Wollmuth LP. (2007) Subunit-specific contribution of pore-forming domains to NMDA receptor channel structure and gating. J Gen Physiol 129:509–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky et al., 2002b.Sobolevsky AI, Rooney L, Wollmuth LP. (2002b) Staggering of subunits in NMDAR channels. Biophys J 83:3304–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky et al., 2009.Sobolevsky AI, Rosconi MP, Gouaux E. (2009) X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462:745–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky et al., 2003.Sobolevsky AI, Yelshansky MV, Wollmuth LP. (2003) Different gating mechanisms in glutamate receptor and K+ channels. J Neurosci 23:7559–7568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky et al., 2004.Sobolevsky AI, Yelshansky MV, Wollmuth LP. (2004) The outer pore of the glutamate receptor channel has 2-fold rotational symmetry. Neuron 41:367–378 [DOI] [PubMed] [Google Scholar]
- Sommer et al., 1992.Sommer B, Burnashev N, Verdoorn TA, Keinänen K, Sakmann B, Seeburg PH. (1992) A glutamate receptor channel with high affinity for domoate and kainate. EMBO J 11:1651–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer et al., 1990.Sommer B, Keinänen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Köhler M, Takagi T, Sakmann B, Seeburg PH. (1990) Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science 249:1580–1585 [DOI] [PubMed] [Google Scholar]
- Sommer et al., 1991.Sommer B, Köhler M, Sprengel R, Seeburg PH. (1991) RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell 67:11–19 [DOI] [PubMed] [Google Scholar]
- Song and Huganir, 2002.Song I, Huganir RL. (2002) Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci 25:578–588 [DOI] [PubMed] [Google Scholar]
- Song et al., 1998.Song I, Kamboj S, Xia J, Dong H, Liao D, Huganir RL. (1998) Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron 21:393–400 [DOI] [PubMed] [Google Scholar]
- Sonnenberg et al., 1996.Sonnenberg JD, Koch HP, Willis CL, Bradbury F, Dauenhauer D, Bridges RJ, Chamberlin AR. (1996) The role of the C-4 side chain of kainate and dihydrokainate in EAA receptor and transporter selectivity. Bioorg Med Chem Lett 6:1607–1612 [Google Scholar]
- Soto et al., 2007.Soto D, Coombs ID, Kelly L, Farrant M, Cull-Candy SG. (2007) Stargazin attenuates intracellular polyamine block of calcium-permeable AMPA receptors. Nat Neurosci 10:1260–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto et al., 2009.Soto D, Coombs ID, Renzi M, Zonouzi M, Farrant M, Cull-Candy SG. (2009) Selective regulation of long-form calcium-permeable AMPA receptors by an atypical TARP, gamma-5. Nat Neurosci 12:277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak et al., 2004.Spivak V, Lin A, Beebe P, Stoll L, Gentile L. (2004) Identification of a neurosteroid binding site contained within the GluR2–S1S2 domain, in 95th American Oil Chemists Society Annual Meeting, pp 811–819; Cincinnati, OH; 2004 May 9–12 American Oil Chemists Society, Urbana, IL: [DOI] [PubMed] [Google Scholar]
- Srivastava et al., 1998.Srivastava S, Osten P, Vilim FS, Khatri L, Inman G, States B, Daly C, DeSouza S, Abagyan R, Valtschanoff JG, et al. (1998) Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron 21:581–591 [DOI] [PubMed] [Google Scholar]
- Stamler et al., 1997.Stamler JS, Toone EJ, Lipton SA, Sucher NJ. (1997) (S)NO signals: translocation, regulation, and a consensus motif. Neuron 18:691–696 [DOI] [PubMed] [Google Scholar]
- Standley and Baudry, 2000.Standley S, Baudry M. (2000) The role of glycosylation in ionotropic glutamate receptor ligand binding, function, and trafficking. Cell Mol Life Sci 57:1508–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley et al., 1998.Standley S, Tocco G, Wagle N, Baudry M. (1998) High- and low-affinity alpha-[3H]amino-3-hydroxy-5-methylisoxazole-4-propionic acid ([3H]AMPA) binding sites represent immature and mature forms of AMPA receptors and are composed of differentially glycosylated subunits. J Neurochem 70:2434–2445 [DOI] [PubMed] [Google Scholar]
- States et al., 2008.States BA, Khatri L, Ziff EB. (2008) Stable synaptic retention of serine-880-phosphorylated GluR2 in hippocampal neurons. Mol Cell Neurosci 38:189–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stäubli et al., 1994.Stäubli U, Perez Y, Xu FB, Rogers G, Ingvar M, Stone-Elander S, Lynch G. (1994) Centrally active modulators of glutamate receptors facilitate the induction of long-term potentiation in vivo. Proc Natl Acad Sci USA 91:11158–11162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein et al., 1992.Stein E, Cox JA, Seeburg PH, Verdoorn TA. (1992) Complex pharmacological properties of recombinant alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subtypes. Mol Pharmacol 42:864–871 [PubMed] [Google Scholar]
- Steinberg et al., 2006.Steinberg JP, Takamiya K, Shen Y, Xia J, Rubio ME, Yu S, Jin W, Thomas GM, Linden DJ, Huganir RL. (2006) Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron 49:845–860 [DOI] [PubMed] [Google Scholar]
- Stensbøl et al., 1999.Stensbøl TB, Borre L, Johansen TN, Egebjerg J, Madsen U, Ebert B, Krogsgaard-Larsen P. (1999) Resolution, absolute stereochemistry and molecular pharmacology of the enantiomers of ATPA. Eur J Pharmacol 380:153–162 [DOI] [PubMed] [Google Scholar]
- Stensbøl et al., 2001.Stensbøl TB, Jensen HS, Nielsen B, Johansen TN, Egebjerg J, Frydenvang K, Krogsgaard-Larsen P. (2001) Stereochemistry and molecular pharmacology of (S)-thio-ATPA, a new potent and selective GluR5 agonist. Eur J Pharmacol 411:245–253 [DOI] [PubMed] [Google Scholar]
- Stern et al., 1992.Stern P, Béhé P, Schoepfer R, Colquhoun D. (1992) Single-channel conductances of NMDA receptors expressed from cloned cDNAs: comparison with native receptors. Proc Biol Sci 250:271–277 [DOI] [PubMed] [Google Scholar]
- Stern et al., 1994.Stern P, Cik M, Colquhoun D, Stephenson FA. (1994) Single channel properties of cloned NMDA receptors in a human cell line: comparison with results from Xenopus oocytes. J Physiol 476:391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern-Bach et al., 1994.Stern-Bach Y, Bettler B, Hartley M, Sheppard PO, O'Hara PJ, Heinemann SF. (1994) Agonist selectivity of glutamate receptors is specified by two domains structurally related to bacterial amino acid-binding proteins. Neuron 13:1345–1357 [DOI] [PubMed] [Google Scholar]
- Stern-Bach et al., 1998.Stern-Bach Y, Russo S, Neuman M, Rosenmund C. (1998) A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron 21:907–918 [DOI] [PubMed] [Google Scholar]
- Steward and Levy, 1982.Steward O, Levy WB. (1982) Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci 2:284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward and Reeves, 1988.Steward O, Reeves TM. (1988) Protein-synthetic machinery beneath postsynaptic sites on CNS neurons: association between polyribosomes and other organelles at the synaptic site. J Neurosci 8:176–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward and Schuman, 2001.Steward O, Schuman EM. (2001) Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci 24:299–325 [DOI] [PubMed] [Google Scholar]
- Stoll et al., 2007.Stoll L, Hall J, Van Buren N, Hall A, Knight L, Morgan A, Zuger S, Van Deusen H, Gentile L. (2007) Differential regulation of ionotropic glutamate receptors. Biophys J 92:1343–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack and Colbran, 1998.Strack S, Colbran RJ. (1998) Autophosphorylation-dependent targeting of calcium/ calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem 273:20689–20692 [DOI] [PubMed] [Google Scholar]
- Strange et al., 2006.Strange M, Bräuner-Osborne H, Jensen AA. (2006) Functional characterisation of homomeric ionotropic glutamate receptors GluR1-GluR6 in a fluorescence-based high throughput screening assay. Comb Chem High Throughput Screen 9:147–158 [DOI] [PubMed] [Google Scholar]
- Stricker and Huganir, 2003.Stricker NL, Huganir RL. (2003) The PDZ domains of mLin-10 regulate its trans-Golgi network targeting and the surface expression of AMPA receptors. Neuropharmacology 45:837–848 [DOI] [PubMed] [Google Scholar]
- Stys and Lipton, 2007.Stys PK, Lipton SA. (2007) White matter NMDA receptors: an unexpected new therapeutic target? Trends Pharmacol Sci 28:561–566 [DOI] [PubMed] [Google Scholar]
- Suchanek et al., 1995.Suchanek B, Seeburg PH, Sprengel R. (1995) Gene structure of the murine N-methyl-d-aspartate receptor subunit NR2C. J Biol Chem 270:41–44 [DOI] [PubMed] [Google Scholar]
- Suchanek et al., 1997.Suchanek B, Seeburg PH, Sprengel R. (1997) Tissue specific control regions of the N-methyl-d-aspartate receptor subunit NR2C promoter. Biol Chem 378:929–934 [PubMed] [Google Scholar]
- Sucher et al., 1995.Sucher NJ, Akbarian S, Chi CL, Leclerc CL, Awobuluyi M, Deitcher DL, Wu MK, Yuan JP, Jones EG, Lipton SA. (1995) Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDARL) in the rodent brain. J Neurosci 15:6509–6520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucher et al., 1993.Sucher NJ, Brose N, Deitcher DL, Awobuluyi M, Gasic GP, Bading H, Cepko CL, Greenberg ME, Jahn R, Heinemann SF. (1993) Expression of endogenous NMDAR1 transcripts without receptor protein suggests post-transcriptional control in PC12 cells. J Biol Chem 268:22299–22304 [PubMed] [Google Scholar]
- Sucher and Lipton, 1991.Sucher NJ, Lipton SA. (1991) Redox modulatory site of the NMDA receptor-channel complex: regulation by oxidized glutathione. J Neurosci Res 30:582–591 [DOI] [PubMed] [Google Scholar]
- Suetake-Koga et al., 2006.Suetake-Koga S, Shimazaki T, Takamori K, Chaki S, Kanuma K, Sekiguchi Y, Suzuki T, Kikuchi T, Matsui Y, Honda T. (2006) In vitro and antinociceptive profile of HON0001, an orally active NMDA receptor NR2B subunit antagonist. Pharmacol Biochem Behav 84:134–141 [DOI] [PubMed] [Google Scholar]
- Sullivan et al., 1994.Sullivan JM, Traynelis SF, Chen HS, Escobar W, Heinemann SF, Lipton SA. (1994) Identification of two cysteine residues that are required for redox modulation of the NMDA subtype of glutamate receptor. Neuron 13:929–936 [DOI] [PubMed] [Google Scholar]
- Sun et al., 2002.Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. (2002) Mechanism of glutamate receptor desensitization. Nature 417:245–253 [DOI] [PubMed] [Google Scholar]
- Sundström et al., 1997.Sundström E, Whittemore S, Mo LL, Seiger A. (1997) Analysis of NMDA receptors in the human spinal cord. Exp Neurol 148:407–413 [DOI] [PubMed] [Google Scholar]
- Sur and Kinney, 2007.Sur C, Kinney GG. (2007) Glycine transporter 1 inhibitors and modulation of NMDA receptor-mediated excitatory neurotransmission. Curr Drug Targets 8:643–649 [DOI] [PubMed] [Google Scholar]
- Sutton et al., 2006.Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. (2006) Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125:785–799 [DOI] [PubMed] [Google Scholar]
- Suzuki et al., 2005.Suzuki K, Sato M, Morishima Y, Nakanishi S. (2005) Neuronal depolarization controls brain-derived neurotrophic factor-induced upregulation of NR2C NMDA receptor via calcineurin signaling. J Neurosci 25:9535–9543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, 2009.Swanson GT. (2009) Targeting AMPA and kainate receptors in neurological disease: therapies on the horizon? Neuropsychopharmacology 34:249–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson et al., 1996.Swanson GT, Feldmeyer D, Kaneda M, Cull-Candy SG. (1996) Effect of RNA editing and subunit co-assembly single-channel properties of recombinant kainate receptors. J Physiol 492:129–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson et al., 1997a.Swanson GT, Gereau RW, 4th, Green T, Heinemann SF. (1997a) Identification of amino acid residues that control functional behavior in GluR5 and GluR6 kainate receptors. Neuron 19:913–926 [DOI] [PubMed] [Google Scholar]
- Swanson et al., 1998.Swanson GT, Green T, Heinemann SF. (1998) Kainate receptors exhibit differential sensitivities to (S)-5-iodowillardiine. Mol Pharmacol 53:942–949 [PubMed] [Google Scholar]
- Swanson et al., 2002.Swanson GT, Green T, Sakai R, Contractor A, Che W, Kamiya H, Heinemann SF. (2002) Differential activation of individual subunits in heteromeric kainate receptors. Neuron 34:589–598 [DOI] [PubMed] [Google Scholar]
- Swanson and Heinemann, 1998.Swanson GT, Heinemann SF. (1998) Heterogeneity of homomeric GluR5 kainate receptor desensitization expressed in HEK293 cells. J Physiol (Lond) 513:639–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson et al., 1997b.Swanson GT, Kamboj SK, Cull-Candy SG. (1997b) Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci 17:58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz, 2004.Swartz KJ. (2004) Towards a structural view of gating in potassium channels. Nat Rev Neurosci 5:905–916 [DOI] [PubMed] [Google Scholar]
- Sydow et al., 1996.Sydow S, Köpke AK, Blank T, Spiess J. (1996) Overexpression of a functional NMDA receptor subunit (NMDAR1) in baculovirus-infected Trichoplusia ni insect cells. Brain Res Mol Brain Res 41:228–240 [DOI] [PubMed] [Google Scholar]
- Szklarczyk et al., 2008.Szklarczyk A, Ewaleifoh O, Beique JC, Wang Y, Knorr D, Haughey N, Malpica T, Mattson MP, Huganir R, Conant K. (2008) MMP-7 cleaves the NR1 NMDA receptor subunit and modifies NMDA receptor function. Faseb J 22:3757–3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymańska et al., 2009.Szymańska E, Pickering DS, Nielsen B, Johansen TN. (2009) 3-Substituted phenylalanines as selective AMPA- and kainate receptor ligands. Bioorg Med Chem 17:6390–6401 [DOI] [PubMed] [Google Scholar]
- Tabuchi et al., 1997.Tabuchi S, Kume K, Aihara M, Ishii S, Mishina M, Shimizu T. (1997) Lipid mediators modulate NMDA receptor currents in a Xenopus oocyte expression system. Neurosci Lett 237:13–16 [DOI] [PubMed] [Google Scholar]
- Tahirovic et al., 2008.Tahirovic YA, Geballe M, Gruszecka-Kowalik E, Myers SJ, Lyuboslavsky P, Le P, French A, Irier H, Choi WB, Easterling K, et al. (2008) Enantiomeric propanolamines as selective N-methyl-d-aspartate 2B receptor antagonists. J Med Chem 51:5506–5521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai et al., 2009.Tai DJ, Su CC, Ma YL, Lee EH. (2009) SGK1 phosphorylation of IkappaB kinase alpha and p300 up-regulates NF-kappaB activity and increases N-methyl-d-aspartate receptor NR2A and NR2B expression. J Biol Chem 284:4073–4089 [DOI] [PubMed] [Google Scholar]
- Takahashi et al., 2007.Takahashi H, Shin Y, Cho SJ, Zago WM, Nakamura T, Gu Z, Ma Y, Furukawa H, Liddington R, Zhang D, et al. (2007) Hypoxia enhances S-nitrosylation-mediated NMDA receptor inhibition via a thiol oxygen sensor motif. Neuron 53:53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi et al., 2002.Takahashi M, Kohara A, Shishikura J, Kawasaki-Yatsugi S, Ni JW, Yatsugi S, Sakamoto S, Okada M, Shimizu-Sasamata M, Yamaguchi T. (2002) YM872: a selective, potent and highly water-soluble alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor antagonist. CNS Drug Rev 8:337–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasu et al., 2002.Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. (2002) Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science 295:491–495 [DOI] [PubMed] [Google Scholar]
- Tamiz et al., 1999.Tamiz AP, Cai SX, Zhou ZL, Yuen PW, Schelkun RM, Whittemore ER, Weber E, Woodward RM, Keana JF. (1999) Structure and activity relationship of N-(phenylalkyl)cinnamides as novel NR2B subtype-selective NMDA receptor antagonists. J Med Chem 42:3412–3420 [DOI] [PubMed] [Google Scholar]
- Tamiz et al., 1998.Tamiz AP, Whittemore ER, Zhou ZL, Huang JC, Drewe JA, Chen JC, Cai SX, Weber E, Woodward RM, Keana JF. (1998) Structure and activity relationships for a series of bis(phenylalkyl)amines: potent subtype-selective inhibitors of N-methyl-d-aspartate receptors. J Med Chem 41:3499–3506 [DOI] [PubMed] [Google Scholar]
- Tan et al., 2005.Tan PH, Yang LC, Shih HC, Lan KC, Cheng JT. (2005) Gene knockdown with intrathecal siRNA of NMDA receptor NR2B subunit reduces formalin-induced nociception in the rat. Gene Ther 12:59–66 [DOI] [PubMed] [Google Scholar]
- Tanaka et al., 2002.Tanaka H, Calderone A, Jover T, Grooms SY, Yokota H, Zukin RS, Bennett MV. (2002) Ischemic preconditioning acts upstream of GluR2 down-regulation to afford neuroprotection in the hippocampal CA1. Proc Natl Acad Sci USA 99:2362–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang et al., 1990.Tang CM, Dichter M, Morad M. (1990) Modulation of the N-methyl-d-aspartate channel by extracellular H+. Proc Natl Acad Sci USA 87:6445–6449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang and Schuman, 2002.Tang SJ, Schuman EM. (2002) Protein synthesis in the dendrite. Philos Trans R Soc Lond B Biol Sci 357:521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang et al., 1999.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. (1999) Genetic enhancement of learning and memory in mice. Nature 401: 63–69 [DOI] [PubMed] [Google Scholar]
- Tang et al., 2001.Tang YP, Wang H, Feng R, Kyin M, Tsien JZ. (2001) Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology 41:779–790 [DOI] [PubMed] [Google Scholar]
- Taniguchi et al., 1997.Taniguchi K, Shinjo K, Mizutani M, Shimada K, Ishikawa T, Menniti FS, Nagahisa A. (1997) Antinociceptive activity of CP-101,606, an NMDA receptor NR2B subunit antagonist. Br J Pharmacol 122:809–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariot, 2006.Tariot PN. (2006) Contemporary issues in the treatment of Alzheimer's disease: tangible benefits of current therapies. J Clin Psychiatry 67 (Suppl 3):15–22 [PubMed] [Google Scholar]
- Teichert et al., 2007.Teichert RW, Jimenez EC, Twede V, Watkins M, Hollmann M, Bulaj G, Olivera BM. (2007) Novel conantokins from Conus parius venom are specific antagonists of N-methyl-d-aspartate receptors. J Biol Chem 282:36905–36913 [DOI] [PubMed] [Google Scholar]
- Theis et al., 2003.Theis M, Si K, Kandel ER. (2003) Two previously undescribed members of the mouse CPEB family of genes and their inducible expression in the principal cell layers of the hippocampus. Proc Natl Acad Sci USA 100:9602–9607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thio et al., 1993.Thio LL, Clifford DB, Zorumski CF. (1993) Concanavalin A enhances excitatory synaptic transmission in cultured rat hippocampal neurons. Synapse 13:94–97 [DOI] [PubMed] [Google Scholar]
- Thomas et al., 2006.Thomas CG, Miller AJ, Westbrook GL. (2006) Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol 95:1727–1734 [DOI] [PubMed] [Google Scholar]
- Thomas et al., 2008.Thomas GM, Lin DT, Nuriya M, Huganir RL. (2008) Rapid and bi-directional regulation of AMPA receptor phosphorylation and trafficking by JNK. EMBO J 27:361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichelaar et al., 2004.Tichelaar W, Safferling M, Keinänen K, Stark H, Madden DR. (2004) The three-dimensional structure of an ionotropic glutamate receptor reveals a dimer-of-dimers assembly. J Mol Biol 344:435–442 [DOI] [PubMed] [Google Scholar]
- Tikhonov et al., 2002.Tikhonov DB, Mellor JR, Usherwood PN, Magazanik LG. (2002) Modeling of the pore domain of the GLUR1 channel: homology with K+ channel and binding of channel blockers. Biophys J 82:1884–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonova et al., 2008.Tikhonova TB, Barygin OI, Gmiro VE, Tikhonov DB, Magazanik LG. (2008) Organic blockers escape from trapping in the AMPA receptor channels by leaking into the cytoplasm. Neuropharmacology 54:653–664 [DOI] [PubMed] [Google Scholar]
- Tikhonova et al., 2009.Tikhonova TB, Tikhonov DB, Magazanik LG. (2009) Common binding site for externally and internally applied AMPA receptor channel blockers. J Mol Neurosci 39:169–174 [DOI] [PubMed] [Google Scholar]
- Tingley et al., 1997.Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. (1997) Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-d-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem 272:5157–5166 [DOI] [PubMed] [Google Scholar]
- Tomita et al., 2005a.Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Nicoll RA, Bredt DS. (2005a) Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature 435:1052–1058 [DOI] [PubMed] [Google Scholar]
- Tomita et al., 2003.Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. (2003) Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol 161:805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita et al., 2004.Tomita S, Fukata M, Nicoll RA, Bredt DS. (2004) Dynamic interaction of stargazin-like TARPs with cycling AMPA receptors at synapses. Science 303:1508–1511 [DOI] [PubMed] [Google Scholar]
- Tomita et al., 2007.Tomita S, Shenoy A, Fukata Y, Nicoll RA, Bredt DS. (2007) Stargazin interacts functionally with the AMPA receptor glutamate-binding module. Neuropharmacology 52:87–91 [DOI] [PubMed] [Google Scholar]
- Tomita et al., 2005b.Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. (2005b) Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron 45:269–277 [DOI] [PubMed] [Google Scholar]
- Torashima et al., 2009.Torashima T, Iizuka A, Horiuchi H, Mitsumura K, Yamasaki M, Koyama C, Takayama K, Iino M, Watanabe M, Hirai H. (2009) Rescue of abnormal phenotypes in delta2 glutamate receptor-deficient mice by the extracellular N-terminal and intracellular C-terminal domains of the delta2 glutamate receptor. Eur J Neurosci 30:355–365 [DOI] [PubMed] [Google Scholar]
- Tóth and McBain, 2000.Tóth K, McBain CJ. (2000) Target-specific expression of pre- and postsynaptic mechanisms. J Physiol 525:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran et al., 2007.Tran DH, Gong R, Tang SJ. (2007) Differential roles of NR2A and NR2B subtypes in NMDA receptor-dependent protein synthesis in dendrites. Neuropharmacology 53:252–256 [DOI] [PubMed] [Google Scholar]
- Traynelis et al., 1998.Traynelis SF, Burgess MF, Zheng F, Lyuboslavsky P, Powers JL. (1998) Control of voltage-independent zinc inhibition of NMDA receptors by the NR1 subunit. J Neurosci 18:6163–6175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis and Cull-Candy, 1990.Traynelis SF, Cull-Candy SG. (1990) Proton inhibition of N-methyl-d-aspartate receptors in cerebellar neurons. Nature 345:347–350 [DOI] [PubMed] [Google Scholar]
- Traynelis and Cull-Candy, 1991.Traynelis SF, Cull-Candy SG. (1991) Pharmacological properties and H+ sensitivity of excitatory amino acid receptor channels in rat cerebellar granule neurones. J Physiol 433:727–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis et al., 1995.Traynelis SF, Hartley M, Heinemann SF. (1995) Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science 268:873–876 [DOI] [PubMed] [Google Scholar]
- Traynelis and Wahl, 1997.Traynelis SF, Wahl P. (1997) Control of rat GluR6 glutamate receptor open probability by protein kinase A and calcineurin. J Physiol 503:513–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinidad et al., 2008.Trinidad JC, Thalhammer A, Specht CG, Lynn AJ, Baker PR, Schoepfer R, Burlingame AL. (2008) Quantitative analysis of synaptic phosphorylation and protein expression. Mol Cell Proteomics 7:684–696 [DOI] [PubMed] [Google Scholar]
- Trivedi et al., 2006.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, et al. (2006) Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163:28–40 [DOI] [PubMed] [Google Scholar]
- Tsai and Coyle, 2002.Tsai G, Coyle JT. (2002) Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol 42:165–179 [DOI] [PubMed] [Google Scholar]
- Tsai et al., 2004.Tsai G, Lane HY, Yang P, Chong MY, Lange N. (2004) Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry 55:452–456 [DOI] [PubMed] [Google Scholar]
- Tsai et al., 2006.Tsai GE, Yang P, Chang YC, Chong MY. (2006) D-alanine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry 59:230–234 [DOI] [PubMed] [Google Scholar]
- Tsui and Malenka, 2006.Tsui J, Malenka RC. (2006) Substrate localization creates specificity in calcium/calmodulin-dependent protein kinase II signaling at synapses. J Biol Chem 281:13794–13804 [DOI] [PubMed] [Google Scholar]
- Tsuzuki et al., 1994.Tsuzuki K, Mochizuki S, Iino M, Mori H, Mishina M, Ozawa S. (1994) Ion permeation properties of the cloned mouse epsilon 2/zeta 1 NMDA receptor channel. Mol Brain Res 26:37–46 [DOI] [PubMed] [Google Scholar]
- Tu et al., 2010.Tu W, Xu X, Peng L, Zhong X, Zhang W, Soundarapandian MM, Balel C, Wang M, Jia N, Zhang W, et al. (2010) DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell 140:222–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen et al., 2005.Tuominen HJ, Tiihonen J, Wahlbeck K. (2005) Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis. Schizophr Res 72:225–234 [DOI] [PubMed] [Google Scholar]
- Turetsky et al., 2005.Turetsky D, Garringer E, Patneau DK. (2005) Stargazin modulates native AMPA receptor functional properties by two distinct mechanisms. J Neurosci 25:7438–7448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski et al., 1998.Turski L, Huth A, Sheardown M, McDonald F, Neuhaus R, Schneider HH, Dirnagl U, Wiegand F, Jacobsen P, Ottow E. (1998) ZK200775: a phosphonate quinoxalinedione AMPA antagonist for neuroprotection in stroke and trauma. Proc Natl Acad Sci USA 95:10960–10965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twede et al., 2009.Twede VD, Teichert RW, Walker CS, Gruszczyński P, Kaźmierkiewicz R, Bulaj G, Olivera BM. (2009) Conantokin-Br from Conus brettinghami and selectivity determinants for the NR2D subunit of the NMDA receptor. Biochemistry 48:4063–4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tygesen et al., 1995.Tygesen CK, Jørgensen M, Andersen PH. (1995) The importance of two specific domains in ligand binding to the AMPA/kainate glutamate receptors GluR2 and GluR6. FEBS Lett 363:184–188 [DOI] [PubMed] [Google Scholar]
- Uchino et al., 2006.Uchino S, Wada H, Honda S, Nakamura Y, Ondo Y, Uchiyama T, Tsutsumi M, Suzuki E, Hirasawa T, Kohsaka S. (2006) Direct interaction of post-synaptic density-95/Dlg/ZO-1 domain-containing synaptic molecule Shank3 with GluR1 alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor. J Neurochem 97:1203–1214 [DOI] [PubMed] [Google Scholar]
- Uemura and Mishina, 2008.Uemura T, Mishina M. (2008) The amino-terminal domain of glutamate receptor delta2 triggers presynaptic differentiation. Biochem Biophys Res Commun 377:1315–1319 [DOI] [PubMed] [Google Scholar]
- Uemura et al., 2004.Uemura T, Mori H, Mishina M. (2004) Direct interaction of GluRdelta2 with Shank scaffold proteins in cerebellar Purkinje cells. Mol Cell Neurosci 26:330–341 [DOI] [PubMed] [Google Scholar]
- Uemura et al., 2010.Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M, Taguchi R, Sakimura K, Mishina M. (2010) Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell 141:1068–1079 [DOI] [PubMed] [Google Scholar]
- Ulbrich and Isacoff, 2007.Ulbrich MH, Isacoff EY. (2007) Subunit counting in membrane-bound proteins. Nat Methods 4:319–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrich and Isacoff, 2008.Ulbrich MH, Isacoff EY. (2008) Rules of engagement for NMDA receptor subunits. Proc Natl Acad Sci USA 105:14163–14168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente et al., 2002.Valente T, Auladell C, Pérez-Clausell J. (2002) Postnatal development of zinc-rich terminal fields in the brain of the rat. Exp Neurol 174:215–229 [DOI] [PubMed] [Google Scholar]
- Valentine and Palmer, 2005.Valentine ER, Palmer AG., 3rd (2005) Microsecond-to-millisecond conformational dynamics demarcate the GluR2 glutamate receptor bound to agonists glutamate, quisqualate, and AMPA. Biochemistry 44:3410–3417 [DOI] [PubMed] [Google Scholar]
- Valgeirsson et al., 2004.Valgeirsson J, Nielsen EO, Peters D, Mathiesen C, Kristensen AS, Madsen U. (2004) Bioisosteric modifications of 2-arylureidobenzoic acids: selective noncompetitive antagonists for the homomeric kainate receptor subtype GluR5. J Med Chem 47:6948–6957 [DOI] [PubMed] [Google Scholar]
- Valgeirsson et al., 2003.Valgeirsson J, Nielsen EØ, Peters D, Varming T, Mathiesen C, Kristensen AS, Madsen U. (2003) 2-arylureidobenzoic acids: selective noncompetitive antagonists for the homomeric kainate receptor subtype GluR5. J Med Chem 46:5834–5843 [DOI] [PubMed] [Google Scholar]
- Vallano et al., 1996.Vallano ML, Lambolez B, Audinat E, Rossier J. (1996) Neuronal activity differentially regulates NMDA receptor subunit expression in cerebellar granule cells. J Neurosci 16:631–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valluru et al., 2005.Valluru L, Xu J, Zhu Y, Yan S, Contractor A, Swanson GT. (2005) Ligand binding is a critical requirement for plasma membrane expression of heteromeric kainate receptors. J Biol Chem 280:6085–6093 [DOI] [PubMed] [Google Scholar]
- van Zundert et al., 2004.van Zundert B, Yoshii A, Constantine-Paton M. (2004) Receptor compartmentalization and trafficking at glutamate synapses: a developmental proposal. Trends Neurosci 27:428–437 [DOI] [PubMed] [Google Scholar]
- Vandenberghe et al., 2005.Vandenberghe W, Nicoll RA, Bredt DS. (2005) Stargazin is an AMPA receptor auxiliary subunit. Proc Natl Acad Sci USA 102:485–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDongen and VanDongen, 2004.VanDongen AM, VanDongen HM. (2004) Effects of mRNA untranslated regions on translational efficiency of NMDA receptor subunits. Neurosignals 13:194–206 [DOI] [PubMed] [Google Scholar]
- Vargas-Caballero and Robinson, 2003.Vargas-Caballero M, Robinson HP. (2003) A slow fraction of Mg2+ unblock of NMDA receptors limits their contribution to spike generation in cortical pyramidal neurons. J Neurophysiol 89:2778–2783 [DOI] [PubMed] [Google Scholar]
- Vargas-Caballero and Robinson, 2004.Vargas-Caballero M, Robinson HP. (2004) Fast and slow voltage-dependent dynamics of magnesium block in the NMDA receptor: the asymmetric trapping block model. J Neurosci 24:6171–6180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varney et al., 1996.Varney MA, Jachec C, Deal C, Hess SD, Daggett LP, Skvoretz R, Urcan M, Morrison JH, Moran T, Johnson EC, et al. (1996) Stable expression and characterization of recombinant human heteromeric N-methyl-d-aspartate receptor subtypes NMDAR1A/2A and NMDAR1A/2B in mammalian cells. J Pharmacol Exp Ther 279:367–378 [PubMed] [Google Scholar]
- Varney et al., 1998.Varney MA, Rao SP, Jachec C, Deal C, Hess SD, Daggett LP, Lin F, Johnson EC, Veliçelebi G. (1998) Pharmacological characterization of the human ionotropic glutamate receptor subtype GluR3 stably expressed in mammalian cells. J Pharmacol Exp Ther 285:358–370 [PubMed] [Google Scholar]
- Vasudevan et al., 2002.Vasudevan N, Kia HK, Inoue S, Muramatsu M, Pfaff D. (2002) Isoform specificity for oestrogen receptor and thyroid hormone receptor genes and their interactions on the NR2D gene promoter. J Neuroendocrinol 14:836–842 [DOI] [PubMed] [Google Scholar]
- Verdoorn et al., 1991.Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B. (1991) Structural determinants of ion flow through recombinant glutamate receptor channels. Science 252:1715–1718 [DOI] [PubMed] [Google Scholar]
- Verdoorn et al., 1994.Verdoorn TA, Johansen TH, Drejer J, Nielsen EO. (1994) Selective block of recombinant glur6 receptors by NS-102, a novel non-NMDA receptor antagonist. Eur J Pharmacol 269:43–49 [DOI] [PubMed] [Google Scholar]
- Vicini et al., 1998.Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. (1998) Functional and pharmacological differences between recombinant N-methyl-d-aspartate receptors. J Neurophysiol 79:555–566 [DOI] [PubMed] [Google Scholar]
- Villarroel et al., 1995.Villarroel A, Burnashev N, Sakmann B. (1995) Dimensions of the narrow portion of a recombinant NMDA receptor channel. Biophys J 68:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel et al., 1998.Villarroel A, Regalado MP, Lerma J. (1998) Glycine-independent NMDA receptor desensitization: localization of structural determinants. Neuron 20:329–339 [DOI] [PubMed] [Google Scholar]
- Vissel et al., 2001.Vissel B, Krupp JJ, Heinemann SF, Westbrook GL. (2001) A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat Neurosci 4:587–596 [DOI] [PubMed] [Google Scholar]
- Vissel et al., 2002.Vissel B, Krupp JJ, Heinemann SF, Westbrook GL. (2002) Intracellular domains of NR2 alter calcium-dependent inactivation of N-methyl-d-aspartate receptors. Mol Pharmacol 61:595–605 [DOI] [PubMed] [Google Scholar]
- Visser and Schug, 2006.Visser E, Schug SA. (2006) The role of ketamine in pain management. Biomed Pharmacother 60:341–348 [DOI] [PubMed] [Google Scholar]
- Vivithanaporn et al., 2006.Vivithanaporn P, Yan S, Swanson GT. (2006) Intracellular trafficking of KA2 kainate receptors mediated by interactions with coatomer protein complex I (COPI) and 14–3-3 chaperone systems. J Biol Chem 281:15475–15484 [DOI] [PubMed] [Google Scholar]
- Vogensen et al., 2007.Vogensen SB, Frydenvang K, Greenwood JR, Postorino G, Nielsen B, Pickering DS, Ebert B, Bølcho U, Egebjerg J, Gajhede M, et al. (2007) A tetrazolyl-substituted subtype-selective AMPA receptor agonist. J Med Chem 50:2408–2414 [DOI] [PubMed] [Google Scholar]
- Vogensen et al., 2000.Vogensen SB, Jensen HS, Stensbøl TB, Frydenvang K, Bang-Andersen B, Johansen TN, Egebjerg J, Krogsgaard-Larsen P. (2000) Resolution, configurational assignment, and enantiopharmacology of 2-amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5-yl)isoxazol-4-yl]propionic acid, a potent GluR3- and GluR4-preferring AMPA receptor agonist. Chirality 12:705–713 [DOI] [PubMed] [Google Scholar]
- Vogt et al., 2000.Vogt K, Mellor J, Tong G, Nicoll R. (2000) The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 26:187–196 [DOI] [PubMed] [Google Scholar]
- Voorn et al., 2007.Voorn P, van de Witte SV, Li K, Jonker AJ. (2007) Dynorphin displaces binding at the glycine site of the NMDA receptor in the rat striatum. Neurosci Lett 415:55–58 [DOI] [PubMed] [Google Scholar]
- Vorobjev et al., 1993.Vorobjev VS, Sharonova IN, Walsh IB, Haas HL. (1993) Histamine potentiates N-methyl-d-aspartate responses in acutely isolated hippocampal neurons. Neuron 11:837–844 [DOI] [PubMed] [Google Scholar]
- Vyklický, 1993.Vyklický L., Jr (1993) Calcium-mediated modulation of N-methyl-d-aspartate (NMDA) responses in cultured rat hippocampal neurons. J Physiol 470:575–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyklický et al., 1990.Vyklický L, Jr, Vlachová V, Krůsek J. (1990) The effect of external pH changes on responses to excitatory amino acids in mouse hippocampal neurones. J Physiol 430:497–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada et al., 2006.Wada A, Takahashi H, Lipton SA, Chen HS. (2006) NR3A modulates the outer vestibule of the “NMDA” receptor channel. J Neurosci 26:13156–13166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford et al., 1995.Wafford KA, Kathoria M, Bain CJ, Marshall G, Le Bourdellès B, Kemp JA, Whiting PJ. (1995) Identification of amino acids in the N-methyl-d-aspartate receptor NR1 subunit that contribute to the glycine binding site. Mol Pharmacol 47:374–380 [PubMed] [Google Scholar]
- Wahl et al., 1998.Wahl P, Anker C, Traynelis SF, Egebjerg J, Rasmussen JS, Krogsgaard-Larsen P, Madsen U. (1998) Antagonist properties of a phosphono isoxazole amino acid at glutamate R1–4 (R,S)-2-amino-3-(3-hydroxy-5-methyl-4-isoxazolyl)propionic acid receptor subtypes. Mol Pharmacol 53:590–596 [DOI] [PubMed] [Google Scholar]
- Walker et al., 2006a.Walker CS, Brockie PJ, Madsen DM, Francis MM, Zheng Y, Koduri S, Mellem JE, Strutz-Seebohm N, Maricq AV. (2006a) Reconstitution of invertebrate glutamate receptor function depends on stargazin-like proteins. Proc Natl Acad Sci USA 103:10781–10786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker et al., 2006b.Walker CS, Francis MM, Brockie PJ, Madsen DM, Zheng Y, Maricq AV. (2006b) Conserved SOL-1 proteins regulate ionotropic glutamate receptor desensitization. Proc Natl Acad Sci USA 103:10787–10792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters et al., 2005.Walters MR, Kaste M, Lees KR, Diener HC, Hommel M, De Keyser J, Steiner H, Versavel M. (2005) The AMPA antagonist ZK 200775 in patients with acute ischaemic stroke: a double-blind, multicentre, placebo-controlled safety and tolerability study. Cerebrovasc Dis 20:304–309 [DOI] [PubMed] [Google Scholar]
- Wang and Shuaib, 2005.Wang CX, Shuaib A. (2005) NMDA/NR2B selective antagonists in the treatment of ischemic brain injury. Curr Drug Targets CNS Neurol Disord 4:143–151 [DOI] [PubMed] [Google Scholar]
- Wang et al., 2006.Wang CY, Chang K, Petralia RS, Wang YX, Seabold GK, Wenthold RJ. (2006) A novel family of adhesion-like molecules that interacts with the NMDA receptor. J Neurosci 26:2174–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2004a.Wang GS, Hong CJ, Yen TY, Huang HY, Ou Y, Huang TN, Jung WG, Kuo TY, Sheng M, Wang TF, et al. (2004a) Transcriptional modification by a CASK-interacting nucleosome assembly protein. Neuron 42:113–128 [DOI] [PubMed] [Google Scholar]
- Wang et al., 2003.Wang J, Liu S, Fu Y, Wang JH, Lu Y. (2003) Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nat Neurosci 6:1039–1047 [DOI] [PubMed] [Google Scholar]
- Wang et al., 2007.Wang JQ, Fibuch EE, Mao L. (2007) Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem 100:1–11 [DOI] [PubMed] [Google Scholar]
- Wang and Nadler, 2007.Wang L, Nadler JV. (2007) Reduced aspartate release from rat hippocampal synaptosomes loaded with Clostridial toxin light chain by electroporation: evidence for an exocytotic mechanism. Neurosci Lett 412:239–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang and Zhu, 2003.Wang LZ, Zhu XZ. (2003) Spatiotemporal relationships among D-serine, serine racemase, and D-amino acid oxidase during mouse postnatal development. Acta Pharmacol Sin 24:965–974 [PubMed] [Google Scholar]
- Wang et al., 2008.Wang R, Walker CS, Brockie PJ, Francis MM, Mellem JE, Madsen DM, Maricq AV. (2008) Evolutionary conserved role for TARPs in the gating of glutamate receptors and tuning of synaptic function. Neuron 59:997–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2004b.Wang TF, Ding CN, Wang GS, Luo SC, Lin YL, Ruan Y, Hevner R, Rubenstein JL, Hsueh YP. (2004b) Identification of Tbr-1/CASK complex target genes in neurons. J Neurochem 91:1483–1492 [DOI] [PubMed] [Google Scholar]
- Washburn and Dingledine, 1996.Washburn MS, Dingledine R. (1996) Block of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by polyamines and polyamine toxins. J Pharmacol Exp Ther 278:669–678 [PubMed] [Google Scholar]
- Washburn et al., 1997.Washburn MS, Numberger M, Zhang S, Dingledine R. (1997) Differential dependence on GluR2 expression of three characteristic features of AMPA receptors. J Neurosci 17:9393–9406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe et al., 2002.Watanabe J, Beck C, Kuner T, Premkumar LS, Wollmuth LP. (2002) DRPEER: A motif in the extracellular vestibule conferring high Ca2+ flux rates in NMDA receptor channels. J Neurosci 22:10209–10216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe et al., 1992.Watanabe M, Inoue Y, Sakimura K, Mishina M. (1992) Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport 3:1138–1140 [DOI] [PubMed] [Google Scholar]
- Watanabe et al., 1993.Watanabe M, Inoue Y, Sakimura K, Mishina M. (1993) Distinct distributions of five N-methyl-d-aspartate receptor channel subunit mRNAs in the forebrain. J Comp Neurol 338:377–390 [DOI] [PubMed] [Google Scholar]
- Watanabe et al., 1994a.Watanabe M, Mishina M, Inoue Y. (1994a) Distinct distributions of five NMDA receptor channel subunit mRNAs in the brainstem. J Comp Neurol 343:520–531 [DOI] [PubMed] [Google Scholar]
- Watanabe et al., 1994b.Watanabe M, Mishina M, Inoue Y. (1994b) Distinct spatiotemporal distributions of the N-methyl-d-aspartate receptor channel subunit mRNAs in the mouse cervical cord. J Comp Neurol 345:314–319 [DOI] [PubMed] [Google Scholar]
- Watanabe et al., 1994c.Watanabe M, Mishina M, Inoue Y. (1994c) Distinct spatiotemporal expressions of five NMDA receptor channel subunit mRNAs in the cerebellum. J Comp Neurol 343:513–519 [DOI] [PubMed] [Google Scholar]
- Watanabe et al., 1999.Watanabe T, Inoue S, Hiroi H, Orimo A, Muramatsu M. (1999) NMDA receptor type 2D gene as target for estrogen receptor in the brain. Mol Brain Res 63:375–379 [DOI] [PubMed] [Google Scholar]
- Weaver et al., 2000.Weaver CE, Land MB, Purdy RH, Richards KG, Gibbs TT, Farb DH. (2000) Geometry and charge determine pharmacological effects of steroids on N-methyl-d-aspartate receptor-induced Ca2+ accumulation and cell death. J Pharmacol Exp Ther 293:747–754 [PubMed] [Google Scholar]
- Wei et al., 2001.Wei F, Wang GD, Kerchner GA, Kim SJ, Xu HM, Chen ZF, Zhuo M. (2001) Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nat Neurosci 4:164–169 [DOI] [PubMed] [Google Scholar]
- Weinbroum et al., 2003.Weinbroum AA, Bender B, Bickels J, Nirkin A, Marouani N, Chazam S, Meller I, Kollender Y. (2003) Preoperative and postoperative dextromethorphan provides sustained reduction in postoperative pain and patient-controlled epidural analgesia requirement: a randomized, placebo-controlled, double-blind study in lower-body bone malignancy-operated patients. Cancer 97:2334–2340 [DOI] [PubMed] [Google Scholar]
- Weinbroum et al., 2004.Weinbroum AA, Bender B, Nirkin A, Chazan S, Meller I, Kollender Y. (2004) Dextromethorphan-associated epidural patient-controlled analgesia provides better pain- and analgesics-sparing effects than dextromethorphan-associated intravenous patient-controlled analgesia after bone-malignancy resection: a randomized, placebo-controlled, double-blinded study. Anesth Analg 98:714–722 [DOI] [PubMed] [Google Scholar]
- Weinbroum et al., 2002.Weinbroum AA, Gorodetzky A, Nirkin A, Kollender Y, Bickels J, Marouani N, Rudick V, Meller I. (2002) Dextromethorphan for the reduction of immediate and late postoperative pain and morphine consumption in orthopedic oncology patients: a randomized, placebo-controlled, double-blind study. Cancer 95:1164–1170 [DOI] [PubMed] [Google Scholar]
- Weinbroum et al., 2001.Weinbroum AA, Gorodezky A, Niv D, Ben-Abraham R, Rudick V, Szold A. (2001) Dextromethorphan attenuation of postoperative pain and primary and secondary thermal hyperalgesia. Can J Anaesth 48:167–174 [DOI] [PubMed] [Google Scholar]
- Weinbroum et al., 2000.Weinbroum AA, Rudick V, Paret G, Ben-Abraham R. (2000) The role of dextromethorphan in pain control. Can J Anaesth 47:585–596 [DOI] [PubMed] [Google Scholar]
- Weiss et al., 2006.Weiss B, Alt A, Ogden AM, Gates M, Dieckman DK, Clemens-Smith A, Ho KH, Jarvie K, Rizkalla G, Wright RA, et al. (2006) Pharmacological characterization of the competitive GLUK5 receptor antagonist decahydroisoquinoline LY466195 in vitro and in vivo. J Pharmacol Exp Ther 318:772–781 [DOI] [PubMed] [Google Scholar]
- Weiss and Sensi, 2000.Weiss JH, Sensi SL. (2000) Ca2+-Zn2+ permeable AMPA or kainate receptors: possible key factors in selective neurodegeneration. Trends Neurosci 23:365–371 [DOI] [PubMed] [Google Scholar]
- Welch et al., 2008.Welch NC, Lin W, Juranka PF, Morris CE, Stys PK. (2008) Traditional AMPA receptor antagonists partially block Na v1.6-mediated persistent current. Neuropharmacology 55:1165–1171 [DOI] [PubMed] [Google Scholar]
- Wells et al., 2001a.Wells DG, Dong X, Quinlan EM, Huang YS, Bear MF, Richter JD, Fallon JR. (2001a) A role for the cytoplasmic polyadenylation element in NMDA receptor-A role for the cytoplasmic polyadenylation element in NMDA receptor-regulated mRNA translation in neurons. J Neurosci 21:9541–9548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells et al., 2001b.Wells GB, Lin L, Jeanclos EM, Anand R. (2001b) Assembly and ligand binding properties of the water-soluble extracellular domains of the glutamate receptor 1 subunit. J Biol Chem 276:3031–3036 [DOI] [PubMed] [Google Scholar]
- Wenthold et al., 2003.Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. (2003) Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol 43:335–358 [DOI] [PubMed] [Google Scholar]
- Wesensten et al., 2007.Wesensten NJ, Reichardt RM, Balkin TJ. (2007) Ampakine (CX717) effects on performance and alertness during simulated night shift work. Aviat Space Environ Med 78:937–943 [DOI] [PubMed] [Google Scholar]
- Westbrook and Mayer, 1987.Westbrook GL, Mayer ML. (1987) Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature 328:640–643 [DOI] [PubMed] [Google Scholar]
- Westbrook et al., 1986.Westbrook GL, Mayer ML, Namboodiri MA, Neale JH. (1986) High concentrations of N-acetylaspartylglutamate (NAAG) selectively activate NMDA receptors in mouse spinal-cord neurons in cell culture. J Neurosci 6:3385–3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston et al., 2006a.Weston MC, Gertler C, Mayer ML, Rosenmund C. (2006a) Interdomain interactions in AMPA and kainate receptors regulate affinity for glutamate. J Neurosci 26:7650–7658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston et al., 2006b.Weston MC, Schuck P, Ghosal A, Rosenmund C, Mayer ML. (2006b) Conformational restriction blocks glutamate receptor desensitization. Nat Struct Mol Biol 13:1120–1127 [DOI] [PubMed] [Google Scholar]
- Wezenberg et al., 2007.Wezenberg E, Verkes RJ, Ruigt GS, Hulstijn W, Sabbe BG. (2007) Acute effects of the ampakine farampator on memory and information processing in healthy elderly volunteers. Neuropsychopharmacology 32:1272–1283 [DOI] [PubMed] [Google Scholar]
- White et al., 2000.White HS, McCabe RT, Armstrong H, Donevan SD, Cruz LJ, Abogadie FC, Torres J, Rivier JE, Paarmann I, Hollmann M, et al. (2000) In vitro and in vivo characterization of conantokin-R, a selective NMDA receptor antagonist isolated from the venom of the fish-hunting snail Conus radiatus. J Pharmacol Exp Ther 292:425–432 [PubMed] [Google Scholar]
- Whitlock et al., 2006.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. (2006) Learning induces long-term potentiation in the hippocampus. Science 313:1093–1097 [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin, 1998.Wiesenfeld-Hallin Z. (1998) Combined opioid-NMDA antagonist therapies. What advantages do they offer for the control of pain syndromes? Drugs 55:1–4 [DOI] [PubMed] [Google Scholar]
- Wilding et al., 1998.Wilding TJ, Chai YH, Huettner JE. (1998) Inhibition of rat neuronal kainate receptors by cis-unsaturated fatty acids. J Physiol 513:331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding et al., 2008.Wilding TJ, Fulling E, Zhou Y, Huettner JE. (2008) Amino acid substitutions in the pore helix of GluR6 control inhibition by membrane fatty acids. J Gen Physiol 132:85–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding and Huettner, 1996.Wilding TJ, Huettner JE. (1996) Antagonist pharmacology of kainate- and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-preferring receptors. Mol Pharmacol 49:540–546 [PubMed] [Google Scholar]
- Wilding and Huettner, 1997.Wilding TJ, Huettner JE. (1997) Activation and desensitization of hippocampal kainate receptors. J Neurosci 17:2713–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding et al., 2005.Wilding TJ, Zhou Y, Huettner JE. (2005) Q/R site editing controls kainate receptor inhibition by membrane fatty acids. J Neurosci 25:9470–9478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm et al., 2008.Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, Cannistraro P, Jenike MA, Rauch SL. (2008) Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry 165:335–341 [DOI] [PubMed] [Google Scholar]
- Wilkinson et al., 2008.Wilkinson KA, Nishimune A, Henley JM. (2008) Analysis of SUMO-1 modification of neuronal proteins containing consensus SUMOylation motifs. Neurosci Lett 436:239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, 1993a.Williams K. (1993a) Ifenprodil discriminates subtypes of the N-methyl-d-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol 44:851–859 [PubMed] [Google Scholar]
- Williams, 1993b.Williams K. (1993b) Subunit-specific potentiation of recombinant N-methyl-d-aspartate receptors by histamine. Mol Pharmacol 46:531–541 [PubMed] [Google Scholar]
- Williams, 1996.Williams K. (1996) Separating dual effects of zinc at recombinant N-methyl-d-aspartate receptors. Neurosci Lett 215:9–12 [DOI] [PubMed] [Google Scholar]
- Williams, 1997.Williams K. (1997) Interactions of polyamines with ion channels. Biochem J 325:289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams et al., 1996.Williams K, Chao J, Kashiwagi K, Masuko T, Igarashi K. (1996) Activation of N-methyl-d-aspartate receptors by glycine: role of an aspartate residue in the M3–M4 loop of the NR1 subunit. Mol Pharmacol 50:701–708 [PubMed] [Google Scholar]
- Williams et al., 2003.Williams K, Dattilo M, Sabado TN, Kashiwagi K, Igarashi K. (2003) Pharmacology of delta2 glutamate receptors: effects of pentamidine and protons. J Pharmacol Exp Ther 305:740–748 [DOI] [PubMed] [Google Scholar]
- Williams et al., 1995.Williams K, Kashiwagi K, Fukuchi J, Igarashi K. (1995) An acidic amino acid in the N-methyl-d-aspartate receptor that is important for spermine stimulation. Mol Pharmacol 48:1087–1098 [PubMed] [Google Scholar]
- Williams et al., 1998.Williams K, Pahk AJ, Kashiwagi K, Masuko T, Nguyen ND, Igarashi K. (1998) The selectivity filter of the N-methyl-d-aspartate receptor: a tryptophan residue controls block and permeation of Mg2+. Mol Pharmacol 53:933–941 [PubMed] [Google Scholar]
- Williams et al., 1994.Williams K, Zappia AM, Pritchett DB, Shen YM, Molinoff PB. (1994) Sensitivity of the N-methyl-d-aspartate receptor to polyamines is controlled by NR2 subunits. Mol Pharmacol 45:803–809 [PubMed] [Google Scholar]
- Williams, 2009.Williams M. (2009) Commentary: genome-based CNS drug discovery: d-amino acid oxidase (DAAO) as a novel target for antipsychotic medications: progress and challenges. Biochem Pharmacol 78:1360–1365 [DOI] [PubMed] [Google Scholar]
- Winblad et al., 2007.Winblad B, Jones RW, Wirth Y, Stöffler A, Möbius HJ. (2007) Memantine in moderate to severe Alzheimer's disease: a meta-analysis of randomised clinical trials. Dement Geriatr Cogn Disord 24:20–27 [DOI] [PubMed] [Google Scholar]
- Wittekindt et al., 2001.Wittekindt B, Malany S, Schemm R, Otvos L, Maccecchini ML, Laube B, Betz H. (2001) Point mutations identify the glutamate binding pocket of the N-methyl-d-aspartate receptor as major site of conantokin-G inhibition. Neuropharmacology 41:753–761 [DOI] [PubMed] [Google Scholar]
- Wo and Oswald, 1995.Wo ZG, Oswald RE. (1995) Unraveling the modular design of glutamate-gated ion channels. Trends Neurosci 18:161–168 [DOI] [PubMed] [Google Scholar]
- Wolff and Winstock, 2006.Wolff K, Winstock AR. (2006) Ketamine : from medicine to misuse. CNS Drugs 20:199–218 [DOI] [PubMed] [Google Scholar]
- Wollmuth et al., 2000.Wollmuth LP, Kuner T, Jatzke C, Seeburg PH, Heintz N, Zuo J. (2000) The lurcher mutation identifies delta 2 as an AMPA/kainate receptor-like channel that is potentiated by Ca2+. J Neurosci 20:5973–5980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmuth et al., 1996.Wollmuth LP, Kuner T, Seeburg PH, Sakmann B. (1996) Differential contribution of the NR1- and NR2A-subunits to the selectivity filter of recombinant NMDA receptor channels. J Physiol 491:779–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmuth and Sakmann, 1998.Wollmuth LP, Sakmann B. (1998) Different mechanisms of Ca2+ transport in NMDA and Ca2+-permeable AMPA glutamate receptor channels. J Gen Physiol 112:623–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmuth and Sobolevsky, 2004.Wollmuth LP, Sobolevsky AI. (2004) Structure and gating of the glutamate receptor ion channel. Trends Neurosci 27:321–328 [DOI] [PubMed] [Google Scholar]
- Wolosker, 2007.Wolosker H. (2007) NMDA receptor regulation by d-serine: new findings and perspectives. Mol Neurobiol 36:152–164 [DOI] [PubMed] [Google Scholar]
- Wolosker et al., 1999.Wolosker H, Blackshaw S, Snyder SH. (1999) Serine racemase: a glial enzyme synthesizing d-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc Natl Acad Sci USA 96:13409–13414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker et al., 2008.Wolosker H, Dumin E, Balan L, Foltyn VN. (2008) d-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J 275:3514–3526 [DOI] [PubMed] [Google Scholar]
- Wong et al., 2007.Wong AY, MacLean DM, Bowie D. (2007) Na+/Cl− dipole couples agonist binding to kainate receptor activation. J Neurosci 27:6800–6809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong et al., 1988.Wong BY, Coulter DA, Choi DW, Prince DA. (1988) Dextrorphan and dextromethorphan, common antitussives, are antiepileptic and antagonize N-methyl-d-aspartate in brain slices. Neurosci Lett 85:261–266 [DOI] [PubMed] [Google Scholar]
- Wong and Mayer, 1993.Wong LA, Mayer ML. (1993) Differential modulation by cyclothiazide and concanavalin A of desensitization at native alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid- and kainate-preferring glutamate receptors. Mol Pharmacol 44:504–510 [PubMed] [Google Scholar]
- Wong et al., 1994.Wong LA, Mayer ML, Jane DE, Watkins JC. (1994) Willardiines differentiate agonist binding sites for kainate- versus AMPA-preferring glutamate receptors in DRG and hippocampal neurons. J Neurosci 14:3881–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley et al., 1998a.Wong-Riley M, Anderson B, Liebl W, Huang Z. (1998a) Neurochemical organization of the macaque striate cortex: correlation of cytochrome oxidase with Na+K+ATPase, NADPH-diaphorase, nitric oxide synthase, and N-methyl-d-aspartate receptor subunit 1. Neuroscience 83:1025–1045 [DOI] [PubMed] [Google Scholar]
- Wong-Riley et al., 1998b.Wong-Riley MT, Huang Z, Liebl W, Nie F, Xu H, Zhang C. (1998b) Neurochemical organization of the macaque retina: effect of TTX on levels and gene expression of cytochrome oxidase and nitric oxide synthase and on the immunoreactivity of Na+ K+ ATPase and NMDA receptor subunit I. Vision Res 38:1455–1477 [DOI] [PubMed] [Google Scholar]
- Wong-Riley and Jacobs, 2002.Wong-Riley MT, Jacobs P. (2002) AMPA glutamate receptor subunit 2 in normal and visually deprived macaque visual cortex. Vis Neurosci 19:563–573 [DOI] [PubMed] [Google Scholar]
- Wood et al., 1995.Wood MW, VanDongen HM, VanDongen AM. (1995) Structural conservation of ion conduction pathways in K channels and glutamate receptors. Proc Natl Acad Sci USA 92:4882–4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood et al., 1996.Wood MW, VanDongen HM, VanDongen AM. (1996) The 5′-untranslated region of the N-methyl-d-aspartate receptor NR2A subunit controls efficiency of translation. J Biol Chem 271:8115–8120 [DOI] [PubMed] [Google Scholar]
- Woods et al., 2006.Woods AS, Kaminski R, Oz M, Wang Y, Hauser K, Goody R, Wang HY, Jackson SN, Zeitz P, Zeitz KP, et al. (2006) Decoy peptides that bind dynorphin noncovalently prevent NMDA receptor-mediated neurotoxicity. J Proteome Res 5:1017–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward et al., 1995b.Woodward RM, Huettner JE, Guastella J, Keana JF, Weber E. (1995b) In vitro pharmacology of ACEA-1021 and ACEA-1031: systemically active quinoxalinediones with high affinity and selectivity for N-methyl-d-aspartate receptor glycine sites. Mol Pharmacol 47:568–581 [PubMed] [Google Scholar]
- Woodward et al., 1995a.Woodward RM, Huettner JE, Tran M, Guastella J, Keana JF, Weber E. (1995a) Pharmacology of 5-chloro-7-trifluoromethyl-1,4-dihydro-2,3-quinoxalinedione: a novel systemically active ionotropic glutamate receptor antagonist. J Pharmacol Exp Ther 275:1209–1218 [PubMed] [Google Scholar]
- Woolf and Salter, 2000.Woolf CJ, Salter MW. (2000) Neuronal plasticity: increasing the gain in pain. Science 288:1765–1769 [DOI] [PubMed] [Google Scholar]
- Woolf and Thompson, 1991.Woolf CJ, Thompson SW. (1991) The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain 44:293–299 [DOI] [PubMed] [Google Scholar]
- Wright et al., 2000.Wright JL, Gregory TF, Kesten SR, Boxer PA, Serpa KA, Meltzer LT, Wise LD, Espitia SA, Konkoy CS, Whittemore ER, et al. (2000) Subtype-selective N-methyl-d-aspartate receptor antagonists: synthesis and biological evaluation of 1-(Heteroarylalkynyl)-4-benzylpiperidines. J Med Chem 43:3408–3419 [DOI] [PubMed] [Google Scholar]
- Wu et al., 2010.Wu CS, Lu Y-J, Li H-P, Hsueh C, Lu C-Y, Leu Y-W, Liu H-P, Lin K-H, Huang TH-M, Chang Y-S. (2010) Glutamate receptor, ionotropic, kainate 2 silencing by DNA hypermethylation possesses tumor suppressor function in gastric cancer. Intl J Cancer in press [DOI] [PubMed] [Google Scholar]
- Wu et al., 1991.Wu FS, Gibbs TT, Farb DH. (1991) Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-d-aspartate receptor. Mol Pharmacol 40:333–336 [PubMed] [Google Scholar]
- Wu and Zhuo, 2009.Wu LJ, Zhuo M. (2009) Targeting the NMDA receptor subunit NR2B for the treatment of neuropathic pain. Neurotherapeutics 6:693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu and Dun, 1997.Wu SY, Dun NJ. (1997) Potentiation of NMDA currents by pituitary adenylate cyclase activating polypeptide in neonatal rat sympathetic preganglionic neurons. J Neurophysiol 78:1175–1179 [DOI] [PubMed] [Google Scholar]
- Wyllie et al., 1998.Wyllie DJ, Béhé P, Colquhoun D. (1998) Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J Physiol 510:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie et al., 1996.Wyllie DJ, Béhé P, Nassar M, Schoepfer R, Colquhoun D. (1996) Single-channel currents from recombinant NMDA NR1a/NR2D receptors expressed in Xenopus oocytes. Proc Biol Sci 263:1079–1086 [DOI] [PubMed] [Google Scholar]
- Wyszynski et al., 1997.Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, Sheng M. (1997) Competitive binding of alpha-actinin and calmodulin to the NMDA receptor. Nature 385:439–442 [DOI] [PubMed] [Google Scholar]
- Wyszynski et al., 1999.Wyszynski M, Valtschanoff JG, Naisbitt S, Dunah AW, Kim E, Standaert DG, Weinberg R, Sheng M. (1999) Association of AMPA receptors with a subset of glutamate receptor-interacting protein in vivo. J Neurosci 19:6528–6537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia et al., 1999.Xia J, Zhang X, Staudinger J, Huganir RL. (1999) Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron 22:179–187 [DOI] [PubMed] [Google Scholar]
- Xin et al., 2005.Xin WK, Zhao XH, Xu J, Lei G, Kwan CL, Zhu KM, Cho JS, Duff M, Ellen RP, McCulloch CA, et al. (2005) The removal of extracellular calcium: a novel mechanism underlying the recruitment of N-methyl-d-aspartate (NMDA) receptors in neurotoxicity. Eur J Neurosci 21:622–636 [DOI] [PubMed] [Google Scholar]
- Xiong et al., 1999.Xiong ZG, Pelkey KA, Lu WY, Lu YM, Roder JC, MacDonald JF, Salter MW. (1999) Src potentiation of NMDA receptors in hippocampal and spinal neurons is not mediated by reducing zinc inhibition. J Neurosci 19: RC37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue et al., 1994.Xue D, Huang ZG, Barnes K, Lesiuk HJ, Smith KE, Buchan AM. (1994) Delayed treatment with AMPA, but not NMDA, antagonists reduces neocortical infarction. J Cereb Blood Flow Metab 14:251–261 [DOI] [PubMed] [Google Scholar]
- Yaghoubi et al., 1998.Yaghoubi N, Malayev A, Russek SJ, Gibbs TT, Farb DH. (1998) Neurosteroid modulation of recombinant ionotropic glutamate receptors. Brain Res 803:153–160 [DOI] [PubMed] [Google Scholar]
- Yaka et al., 2003.Yaka R, He DY, Phamluong K, Ron D. (2003) Pituitary adenylate cyclase-activating polypeptide (PACAP(1–38)) enhances N-methyl-d-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem 278:9630–9638 [DOI] [PubMed] [Google Scholar]
- Yaka et al., 2002.Yaka R, Thornton C, Vagts AJ, Phamluong K, Bonci A, Ron D. (2002) NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc Natl Acad Sci USA 99:5710–5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura et al., 1993.Yamakura T, Mori H, Masaki H, Shimoji K, Mishina M. (1993) Different sensitivities of NMDA receptor channel subtypes to non-competitive antagonists. Neuroreport 4:687–690 [DOI] [PubMed] [Google Scholar]
- Yamakura and Shimoji, 1999.Yamakura T, Shimoji K. (1999) Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog Neurobiol 59:279–298 [DOI] [PubMed] [Google Scholar]
- Yamada et al., 2005.Yamada K, Ohnishi T, Hashimoto K, Ohba H, Iwayama-Shigeno Y, Toyoshima M, Okuno A, Takao H, Toyota T, Minabe Y, et al. (2005) Identification of multiple serine racemase (SRR) mRNA isoforms and genetic analyses of SRR and DAO in schizophrenia and D-serine levels. Biol Psychiatry 57:1493–1503 [DOI] [PubMed] [Google Scholar]
- Yamada and Tang, 1993.Yamada KA, Tang CM. (1993) Benzothiadiazides inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents. J Neurosci 13:3904–3915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada and Turetsky, 1996.Yamada KA, Turetsky DM. (1996) Allosteric interactions between cyclothiazide and AMPA/kainate receptor antagonists. Br J Pharmacol 117:1663–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki et al., 1992.Yamazaki M, Mori H, Araki K, Mori KJ, Mishina M. (1992) Cloning, expression and modulation of a mouse NMDA receptor subunit. FEBS Lett 300:39–45 [DOI] [PubMed] [Google Scholar]
- Yamazaki et al., 2004.Yamazaki M, Ohno-Shosaku T, Fukaya M, Kano M, Watanabe M, Sakimura K. (2004) A novel action of stargazin as an enhancer of AMPA receptor activity. Neurosci Res 50:369–374 [DOI] [PubMed] [Google Scholar]
- Yang and Svensson, 2008.Yang CR, Svensson KA. (2008) Allosteric modulation of NMDA receptor via elevation of brain glycine and d-serine: the therapeutic potentials for schizophrenia. Pharmacol Ther 120:317–332 [DOI] [PubMed] [Google Scholar]
- Yang et al., 2009.Yang K, Trepanier CH, Li H, Beazely MA, Lerner EA, Jackson MF, MacDonald JF. (2009) Vasoactive intestinal peptide acts via multiple signal pathways to regulate hippocampal NMDA receptors and synaptic transmission. Hippocampus 19:779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang and Leonard, 2001.Yang M, Leonard JP. (2001) Identification of mouse NMDA receptor subunit NR2A C-terminal tyrosine sites phosphorylated by coexpression with v-Src. J Neurochem 77:580–588 [DOI] [PubMed] [Google Scholar]
- Yang et al., 2006.Yang SJ, Liang HL, Wong-Riley MT. (2006) Activity-dependent transcriptional regulation of nuclear respiratory factor-1 in cultured rat visual cortical neurons. Neurosci 141:1181–1192 [DOI] [PubMed] [Google Scholar]
- Yao and Mayer, 2006.Yao Y, Mayer ML. (2006) Characterization of a soluble ligand binding domain of the NMDA receptor regulatory subunit NR3A. J Neurosci 26:4559–4566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao et al., 2008.Yao Y, Harrison CB, Freddolino PL, Schulten K, Mayer ML. (2008) Molecular mechanism of ligand recognition by NR3 subtype glutamate receptors. EMBO J 27:2158–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap et al., 2003a.Yap CC, Murate M, Kishigami S, Muto Y, Kishida H, Hashikawa T, Yano R. (2003a) Adaptor protein complex-4 (AP-4) is expressed in the central nervous system neurons and interacts with glutamate receptor delta2. Mol Cell Neurosci 24:283–295 [DOI] [PubMed] [Google Scholar]
- Yap et al., 2003b.Yap CC, Muto Y, Kishida H, Hashikawa T, Yano R. (2003b) PKC regulates the delta2 glutamate receptor interaction with S-SCAM/MAGI-2 protein. Biochem Biophys Res Commun 301:1122–1128 [DOI] [PubMed] [Google Scholar]
- Yawata et al., 2006.Yawata S, Tsuchida H, Kengaku M, Hirano T. (2006) Membrane-proximal region of glutamate receptor delta2 subunit is critical for long-term depression and interaction with protein interacting with C kinase 1 in a cerebellar Purkinje neuron. J Neurosci 26:3626–3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh et al., 2008.Yeh SH, Hung JJ, Gean PW, Chang WC. (2008) Hypoxia-inducible factor-1alpha protects cultured cortical neurons from lipopolysaccharide-induced cell death via regulation of NR1 expression. J Neurosci 28:14259–14270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen, 2002.Yellen G. (2002) The voltage-gated potassium channels and their relatives. Nature 419:35–42 [DOI] [PubMed] [Google Scholar]
- Yelshansky et al., 2004.Yelshansky MV, Sobolevsky AI, Jatzke C, Wollmuth LP. (2004) Block of AMPA receptor desensitization by a point mutation outside the ligand-binding domain. J Neurosci 24:4728–4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al., 2001.Yu SP, Canzoniero LM, Choi DW. (2001) Ion homeostasis and apoptosis. Curr Opin Cell Biol 13:405–411 [DOI] [PubMed] [Google Scholar]
- Yu, 2006.Yu XM. (2006) The role of intracellular sodium in the regulation of NMDA-receptor-mediated channel activity and toxicity. Mol Neurobiol 33:63–80 [DOI] [PubMed] [Google Scholar]
- Yu and Salter, 1998.Yu XM, Salter MW. (1998) Gain control of NMDA-receptor currents by intracellular sodium. Nature 396:469–474 [DOI] [PubMed] [Google Scholar]
- Yu and Salter, 1999.Yu XM, Salter MW. (1999) Src, a molecular switch governing gain control of synaptic transmission mediated by N-methyl-d-aspartate receptors. Proc Natl Acad Sci USA 96:7697–7704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al., 2002.Yu ZF, Cheng G, Wen X, Wu GD, Lee W-T, Pleasure D. (2002) Tumor necrosis factor a increases neuronal vulnerability to excitotoxic necrosis by inducing expression of the AMPA–glutamate receptor subunit GluR1 via an acid sphingomyelinase and NF-kB-dependent mechanism. Neurobiol Disease 11:199–213 [DOI] [PubMed] [Google Scholar]
- Yuan et al., 2005.Yuan H, Erreger K, Dravid SM, Traynelis SF. (2005) Conserved structural and functional control of N-methyl-d-aspartate receptor gating by transmembrane domain M3. J Biol Chem 280:29708–29716 [DOI] [PubMed] [Google Scholar]
- Yuan et al., 2009a.Yuan H, Hansen KB, Vance KM, Ogden KK, Traynelis SF. (2009a) Control of NMDA receptor function by the NR2 subunit amino-terminal domain. J Neurosci 29:12045–12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan et al., 2009b.Yuan H, Vance KM, Junge CE, Geballe MT, Snyder JP, Hepler JR, Yepes M, Low CM, Traynelis SF. (2009b) The serine protease plasmin cleaves the amino-terminal domain of the NR2A subunit to relieve zinc inhibition of the N-methyl-d-aspartate receptors. J Biol Chem 284:12862–12873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue et al., 1995.Yue KT, MacDonald JF, Pekhletski R, Hampson DR. (1995) Differential effects of lectins on recombinant glutamate receptors. Eur J Pharmacol 291:229–235 [DOI] [PubMed] [Google Scholar]
- Yue et al., 2002.Yue Z, Horton A, Bravin M, DeJager PL, Selimi F, Heintz N. (2002) A novel protein complex linking the delta 2 glutamate receptor and autophagy: implications for neurodegeneration in lurcher mice. Neuron 35:921–933 [DOI] [PubMed] [Google Scholar]
- Yuen et al., 2007.Yuen EY, Gu Z, Yan Z. (2007) Calpain regulation of AMPA receptor channels in cortical pyramidal neurons. J Physiol 580:241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkewicz et al., 2005.Yurkewicz L, Weaver J, Bullock MR, Marshall LF. (2005) The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J Neurotrauma 22:1428–1443 [DOI] [PubMed] [Google Scholar]
- Yuzaki and Connor, 1999.Yuzaki M, Connor JA. (1999) Characterization of L-homocysteate-induced currents in Purkinje cells from wild-type and NMDA receptor knockout mice. J Neurophysiol 82:2820–2826 [DOI] [PubMed] [Google Scholar]
- Zamanillo et al., 1999.Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Köster HJ, Borchardt T, Worley P, et al. (1999) Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284:1805–1811 [DOI] [PubMed] [Google Scholar]
- Zarate et al., 2006a.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. (2006a) A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864 [DOI] [PubMed] [Google Scholar]
- Zarate et al., 2006b.Zarate CA, Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, Manji HK, Charney DS. (2006b) A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry 163:153–155 [DOI] [PubMed] [Google Scholar]
- Zarei and Dani, 1994.Zarei MM, Dani JA. (1994) Ionic permeability characteristics of the N-methyl-d-aspartate receptor channel. J Gen Physiol 103:231–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei and Dani, 1995.Zarei MM, Dani JA. (1995) Structural basis for explaining open-channel blockade of the NMDA receptor. J Neurosci 15:1446–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2002.Zhang DQ, Ribelayga C, Mangel SC, McMahon DG. (2002) Suppression by zinc of AMPA receptor-mediated synaptic transmission in the retina. J Neurophysiol 88:1245–1251 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2009a.Zhang HX, Hyrc K, Thio LL. (2009a) The glycine transport inhibitor sarcosine is an NMDA receptor co-agonist that differs from glycine. J Physiol 587:3207–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2009b.Zhang HX, Lyons-Warren A, Thio LL. (2009b) The glycine transport inhibitor sarcosine is an inhibitory glycine receptor agonist. Neuropharmacology 57:551–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 1997.Zhang L, Peoples RW, Oz M, Harvey-White J, Weight FF, Brauneis U. (1997) Potentiation of NMDA receptor-mediated responses by dynorphin at low extracellular glycine concentrations. J Neurophysiol 78:582–590 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2008a.Zhang W, Cho Y, Lolis E, Howe JR. (2008a) Structural and single-channel results indicate that the rates of ligand binding domain closing and opening directly impact AMPA receptor gating. J Neurosci 28:932–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2008b.Zhang W, Howe JR, Popescu GK. (2008b) Distinct gating modes determine the biphasic relaxation of NMDA receptor currents. Nat Neurosci 11:1373–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2006b.Zhang W, Robert A, Vogensen SB, Howe JR. (2006b) The relationship between agonist potency and AMPA receptor kinetics. Biophys J 91:1336–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2009c.Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumioka A, Morimoto-Tomita M, Kim KS, Straub C, Burlingame AL, Howe JR, et al. (2009c) A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron 61:385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang and Nadler, 2009.Zhang X, Nadler JV. (2009) Postsynaptic response to stimulation of the Schaffer collaterals with properties similar to those of synaptosomal aspartate release. Brain Res 1295:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2008c.Zhang Y, Nayeem N, Green T. (2008c) Mutations to the kainate receptor subunit GluR6 binding pocket that selectively affect domoate binding. Mol Pharmacol 74:1163–1169 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2006a.Zhang Y, Nayeem N, Nanao MH, Green T. (2006a) Interface interactions modulating desensitization of the kainate-selective ionotropic glutamate receptor subunit GluR6. J Neurosci 26:10033–10042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng et al., 2001.Zheng F, Erreger K, Low CM, Banke T, Lee CJ, Conn PJ, Traynelis SF. (2001) Allosteric interaction between the amino terminal domain and the ligand binding domain of NR2A. Nat Neurosci 4:894–901 [DOI] [PubMed] [Google Scholar]
- Zheng et al., 1998.Zheng F, Gingrich MB, Traynelis SF, Conn PJ. (1998) Tyrosine kinase potentiates NMDA receptor currents by reducing tonic zinc inhibition. Nat Neurosci 1:185–191 [DOI] [PubMed] [Google Scholar]
- Zheng et al., 1994.Zheng X, Zhang L, Durand GM, Bennett MV, Zukin RS. (1994) Mutagenesis rescues spermine and Zn2+ potentiation of recombinant NMDA receptors. Neuron 12:811–818 [DOI] [PubMed] [Google Scholar]
- Zheng et al., 2006.Zheng Y, Brockie PJ, Mellem JE, Madsen DM, Walker CS, Francis MM, Maricq AV. (2006) SOL-1 is an auxiliary subunit that modulates the gating of GLR-1 glutamate receptors in Caenorhabditis elegans. Proc Natl Acad Sci USA 103:1100–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al., 1997.Zhou LM, Gu ZQ, Costa AM, Yamada KA, Mansson PE, Giordano T, Skolnick P, Jones KA. (1997) (2S,4R)-4-methylglutamic acid (SYM 2081): a selective, high-affinity ligand for kainate receptors. J Pharmacol Exp Ther 280:422–427 [PubMed] [Google Scholar]
- Zhou and Baudry, 2006.Zhou M, Baudry M. (2006) Developmental changes in NMDA neurotoxicity reflect developmental changes in subunit composition of NMDA receptors. J Neurosci 26:2956–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al., 2001.Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. (2001) Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature 414:43–48 [DOI] [PubMed] [Google Scholar]
- Zhu et al., 2000.Zhu JJ, Esteban JA, Hayashi Y, Malinow R. (2000) Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci 3:1098–1106 [DOI] [PubMed] [Google Scholar]
- Zhu and Auerbach, 2001a.Zhu Y, Auerbach A. (2001a) K+ occupancy of the N-methyl-d-aspartate receptor channel probed by Mg2+ block. J Gen Physiol 117:287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu and Auerbach, 2001b.Zhu Y, Auerbach A. (2001b) Na+ occupancy and Mg2+ block of the N-methyl-d-aspartate receptor channel. J Gen Physiol 117:275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer et al., 1995.Zimmer M, Fink TM, Franke Y, Lichter P, Spiess J. (1995) Cloning and structure of the gene encoding the human N-methyl-d-aspartate receptor (NMDAR1). Gene 159:219–223 [DOI] [PubMed] [Google Scholar]
- Zuo et al., 1997.Zuo J, De Jager PL, Takahashi KA, Jiang W, Linden DJ, Heintz N. (1997) Neurodegeneration in Lurcher mice caused by mutation in delta 2 glutamate receptor gene. Nature 388:769–773 [DOI] [PubMed] [Google Scholar]