Abstract
The high conductance voltage- and Ca2+-activated K+ channel is one of the most broadly expressed channels in mammals. This channel is named BK for ‘big K’ because of its single-channel conductance that can be as large as 250 pS in 100 mm symmetrical K+. BK channels increase their activity by membrane depolarization or an increase in cytosolic Ca2+. One of the key features that defines the behaviour of BK channels is that neither Ca2+ nor voltage is strictly necessary for channel activation. This and several other observations led to the idea that both Ca2+ and voltage increase the open probability by an allosteric mechanism. In this type of mechanism, the processes of voltage sensor displacement, Ca2+ binding and pore opening are independent equilibria that interact allosterically with each other. These allosteric interactions in BK channels reside in the structural characteristics of the BK channel in the sense that voltage and Ca2+ sensors and the pore need to be contained in different structures or ‘modules’. Through electrophysiological, mutagenesis, biochemical and fluorescence studies these modules have been identified and, more important, some of the interactions between them have been unveiled. In this review, we have covered the main advances achieved during the last few years in the elucidation of the structure of the BK channel and how this is related with its function as an allosteric protein.
Introduction
Since the first characterization of its single channel properties in rat muscle cells and rabbit skeletal muscle (Marty, 1981; Pallotta et al. 1981; Latorre et al. 1982), the large-conductance voltage- and Ca2+-activated K+ (BK) channel has held a fascination for many ion channel biophysicists and physiologists on account of its unusual characteristics. The BK channel has a very large single-channel conductance (250–300 pS in symmetrical 150 mm KCl; Pallotta et al. 1981; Latorre et al. 1982, 1989), its open probability increases on membrane depolarization or an increase in intracellular calcium concentration (Pallotta et al. 1981; Cui et al. 2009), and it is ubiquitously expressed among mammalian tissues (Toro et al. 1998). As its activation leads to membrane hyperpolarization, it serves as a negative-feedback mechanism for the excitatory events that lead to increases in calcium concentration or membrane depolarizaton. In vascular smooth muscle cells, BK channels play a key role in regulating the contractile tone (Nelson & Quayle, 1995; Brenner et al. 2000); in chromaffin cells they help to terminate the action potential and thus modulate secretion (Neely & Lingle, 1992; Solaro et al. 1995), and in neurons they colocalize with voltage-dependent calcium channels and are involved in the control of neurosecretion (Sah & Davies, 2000).
The BK channel is a homotetramer of its pore-forming α-subunit, which is encoded by the gene Slo1 (KNCMA1) and is a member of the voltage-dependent potassium (Kv) channel superfamily. As in all other Kv channels, the S4 transmembrane segment is part of an intrinsic voltage sensor (Diaz et al. 1998; Cui & Aldrich, 2000, Ma et al. 2006). Gating and ionic currents in BK channels can be elicited by membrane depolarization in the absence of calcium, suggesting that this is a voltage-dependent channel (Stefani et al. 1997; Horrigan & Aldrich, 2002). This divalent cation, by binding to sites contained in the BK C-terminus, acts as a modulator able to decrease the necessary energy to open the channel, promoting a leftward shift in the open probability (PO) vs. voltage relationships (reviewed in Magleby, 2003).
The BK α-subunit can be divided into two parts: the transmembrane region and the intracellular C-terminus region (Fig. 1). The transmembrane region is formed by seven TM segments (S0–S6): an extra hydrophobic segment at the NH2 terminus (S0) compared with the Kv channels (Meera et al. 1997), the voltage sensor domain (VSD) (S1–S4), and the pore domain (S5–S6). Thus, the NH2 terminus of the protein is placed at the extracellular side of the membrane. The intracellular C-terminal comprises about two-thirds of the protein containing four hydrophobic segments, several alternative splicing sites, and a stretch of negatively charged amino acids (aspartates) dubbed the ‘Ca2+ bowl’. The cytoplasmic domain of each α-subunit can be divided into two separate structures, both with sequence homology to the ‘regulator of K+ conductance’ (RCK). These two tandem C-terminal RCK domains form a gating ring that comprises the eight RCK domains from the four BK α-subunits (Yuan et al. 2010; see below).
Figure 1. α- and β1- subunit of the BK channel.
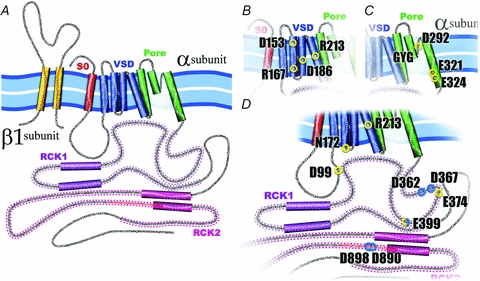
A, the α-subunit of the BK channel is formed by 7 transmembrane (S0–S6) segments and a large C-terminus. Segments S1–S4 have been implicated in voltage sensing whereas S5 and S6 form the conduction pore. S0 plays an important role in the functional coupling between α- and β1-subunit. The C-terminus contains two putative RCK domains where the Ca2+ and Mg2+ binding sites are located. All BK β subunits contain two transmembrane segments leaving their N- and C-terminus oriented towards the interior of the cell. B, voltage sensor domain (VSD). BK's VSD is positioned between the TM segments S1–S4, and four charged residues contribute to the channel voltage membrane sensitivity, D153 and R167 in the S2, D186 in the S3 and R213 in the S4. C, the pore. Three residues have been identified in BK pore as partially responsible for the channel high conductance, D292, E321 and E324. Additionally, in the pore, as in the majority of K+ channels, is located the signature sequence GYG, responsible for the high K+ selectivity of this channels. D, Ca2+ and Mg2+ sites. Spread throughout in the BK cytoplasmatic domain are located the high affinity Ca2+ sites, D362, D367 (in the RCK1), D898 and D890 (in the denominated calcium bowl in the RCK2), and the low affinity Ca2+ site, E399 (RCK1), which is shared with the site that coordinate Mg2+, D99 and N172 in the VSD, and E372 and E399 in the RCK1. The residue R213 showed here is electrostatically affected by the Mg2+ binding.
Transmembrane segments: S0, the voltage sensor and the pore
The minimal molecular component necessary and sufficient for BK activity is its pore-forming α-subunit, and functional channels are formed as tetramers of this protein (Shen et al. 1994). Tetramerization is driven by an association segment (BK-T1 segment) located between the S6 and the α-helix denominated S7 in the intracellular C-terminal region (Quirk & Reinhart, 2001). The additional TM segment S0 places the N-terminus towards the extracellular side and adds an extra intracellular linker between the S0 and the S1 compared to Kv channels (Fig. 1; Meera et al. 1997). The exact position of S0 is unknown, but the experiments of Liu et al. (2008), using disulfide cross-linking, indicate that S0 lies in close proximity to the S3–S4 loop. S0 is centrally positioned among the extracellular ends of S1–S4 transmembrane segments (Fig. 2A). A recent report using the disulfide crosslinking technique locates S0 next to S3 and S4 but not to S1 and S2 (Liu et al. 2010), an observation in agreement with a tryptophan scanning mutagenesis study of S0 that suggests that the middle and the N-terminus of this TM is in direct contact with the voltage sensor domain (S1–S4) (Koval et al. 2007; Semenova et al. 2009). The proximity of S0 to the voltage sensor has led to speculation that this transmembrane domain contributes to the stabilization of the voltage sensor in its resting state compared to Kv channels that lack this extra transmembrane segment.
Figure 2. Current structural status of the BK channel.
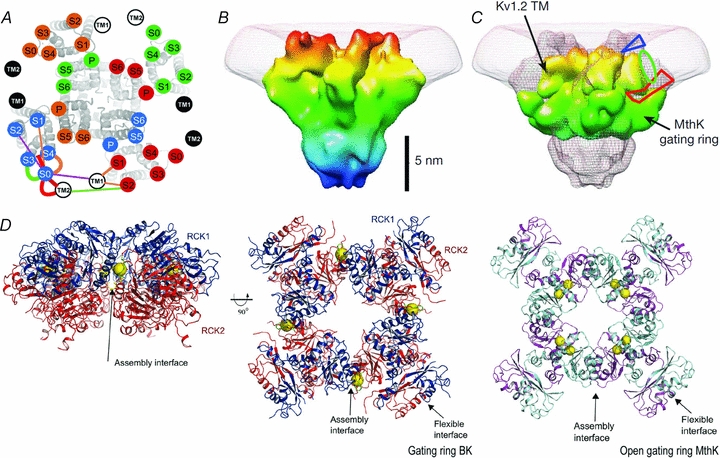
A, the transmembrane segments location using the cysteine cross-linking technique (Liu et al. 2008). Kv1.2/Kv2.1 chimera S1–S6 with superimposed, labelled circles, uniquely coloured for each subunit. TM1 and TM2 of β1 subunit are in white or black circles. B, BK 20 Å structure. BK structure resolved by electron cryomicroscopy at 20 Å shows a large protrusion at the periphery of the VSD, which has been suggested to correspond to the additional helix S0 and the extracellular 40 N-terminal residues of BK α-subunit. C, a view of the BK map (mesh) with Kv1.2 transmembrane region and MthK gating ring docked. The blue triangle and the red polygon indicate possible locations for the N-terminal region and the S0–S1 linker, respectively. The green oval is the proposed location of the S0 helix (Wang & Sigworth, 2009). D, left and centre panels, the BK gating ring solution at 6.0 Å. The centre panel is viewed down fourfold symmetry axis with RCK1 in blue and RCK2 in red. Calcium ions are shown as yellow spheres. Right panel, the open gating ring structure from the MthK channel viewed down the fourfold axis. Notice that a Ca2+ binds to the assembly interface in the BK gating ring whereas two Ca2+ ions bind to the flexible interface in the MthK gating ring (Yuan et al. 2010).
The BK structure recently resolved by electron cryomicroscopy at 17–20 Å shows a large protrusion at the periphery of the voltage sensing domain (VSD) which, as has been suggested, should correspond with the additional helix S0 and the extracellular ∼40 N-terminal residues of the BK α-subunit (Wang & Sigworth, 2009) (cf. Fig. 2B and C). Note that Fig. 2A is based on the model of the structure of Kv1.2 in the closed state (Yarov-Yarovoy et al. 2006) and the S0 position is one consistent with the experimental results.
As with other Kvs, the α-subunit contains a positively charged TM domain (S4); however, only one, R213 (Fig. 1), of the three positively charged residues present in S4 has been directly implicated in the voltage sensing (Diaz et al. 1998; Ma et al. 2006). Also different from other Kvs, a large portion of the gating charge movement is contributed by residues D153, R167 in S2 and D186 in S3 (Ma et al. 2006). Voltage clamp fluorometry, on the other hand, revealed voltage-dependent conformational changes of the S3–S4 region of R207Q mutant of the BK channel (Savalli et al. 2006) and Pantazis et al. (2010) using the same technique found evidence of cooperativity between S2 and S3. Charge neutralization in one segment modifies the effective valence of the other and vice versa, a result explained by Pantazis et al. assuming changes in the dielectric (dynamic field focusing) induced by the creation of aqueous crevices. In Kv channels the VSD is defined as the structure comprising S1–S4 (Jiang et al. 2003). Most probably, the architecture of the BK VSD would be affected by the presence of the S0 segment, which is something that can be confirmed once the crystal structure has been solved.
The pore domain of the channel is assigned to the region contained between the S5 and S6 segments, which includes the signature sequence of K+ channel TVGYG (Heginbotham et al. 1994). The external aspect of the pore determines the block properties of charybdtoxin (ChTx), iberiotoxin (IbTx) and tetraethylammonium (TEA) (Vergara et al. 1984; Miller et al. 1985; MacKinnon & Miller, 1989; Shen et al. 1994; Mullmann et al. 1999). In general, the architecture of the conduction machinery of BK channels is not different from that of other K+ channels but multiple alignments show that the external loop (turret) between S5 and the pore helix of BK channels contains several residues more than the other K+ channels analysed (Carvacho et al. 2008; Giangiacomo et al. 2008). This difference in the turret length between BK and Kv channels determines the specificity for only one subfamily of K+ channel toxins, α-KTx1.x, of the BK channel (Giangiacomo et al. 2008). Experiments to determine the structural motifs by the high conductance in BK channels (∼250 pS) has shown a ring of residues in the inner vestibule entrance in the pore (E321 and E324) that allow the concentrating of K+ ions in the inner vestibule through electrostatic mechanisms that double the conductance for outward currents with respect to low conductance Kv channels (Brelidze et al. 2003). Also, in the external vestibule near to the selectivity filter, the residue D292 would contribute to concentrate K+ ions, increasing the conductance of inward currents (Haug et al. 2004).
In Kv channels, voltage sensor activation leads to the opening of the bundle crossing formed by the S6 segments. In BK channels, on the other hand, the coupling mechanism between voltage sensor and pore opening is unknown, yet Wu et al. (2010) found that replacement of the S6 residue L312 or F315 with polar amino acids of smaller side chains strongly favoured the open-state. Their homology modelling and molecular dynamics simulations support the notion that L312 of one subunit strongly interacts with the aromatic ring of F315 in a neighbouring subunit, coupling that greatly stabilizes the closed configuration of the BK channel. It is still unclear, however, if activation in BK channels is mediated by the opening of the bundle crossing. The only evidence we have so far is that a synthetic inactivation peptide applied internally acts as a state dependent blocker (Li & Aldrich, 2006). This result implies that there is a considerable widening of the pore during the transition from closed to open but it does not demonstrate that the bundle crossing is the rate limiting step to small cations when the channel is closed.
A digression on the BK β1-subunit
In many tissues and in particular in smooth muscle, the BK channel α-subunit is associated with a β1-subunit. The β1-subunit is an intrinsic membrane protein consisting of two transmembrane domains (TM1 and TM2) and a large (∼120 residues) external loop and the functional coupling between these two subunits is manifested as an apparent increase in the BK Ca2+ sensitivity and a slowing of both the activation and the deactivation kinetics of the channel (reviewed in Orio et al. 2002). This functional coupling requires the S0 segment (Wallner et al. 1996). However, Morrow et al. (2006) reported that the physical association between the α-subunit and the β1-subunit requires the presence of S1, S2 and S3 segments (Morrow et al. 2006). More recently Liu et al. (2010) found that TM1 is in close proximity to S1 and S2 and TM2 is in the neighbourhood of S0 but of the adjacent α-subunit (Fig. 2A; Liu et al. 2010).
C-terminus domain: RCK domains, Ca2+ sensors and Ca2+ activation
The intracellular C-terminus domain comprising two-thirds of the protein contains four hydrophobic segments (Fig. 1) and Salkoff's group identified two molecular domains, S0–S8 (‘core’) and S9–S10 (‘tail’ or distal part of C-terminus domain), that when expressed together were able to produce functional channels (Wei et al. 1994). Closer inspection of the tail shows the presence of a domain consisting of 28 amino acids containing nine acidic residues including a string of five aspartate residues dubbed the ‘calcium bowl’.
The sequence and predicted secondary structure of the cytoplasmic domain comprising amino acid residues 339–516 in hSlo1 that includes the S7 domain are homologous to a regulatory domain for K+ conductance (RCK1 domain) that is found in a number of K+ channels (Jiang et al. 2001, 2002; Lingle, 2007). Homology models based on the E. coli six transmembrane K+ channel and the Ca2+-activated channel, MthK, have been proposed for the BK RCK1 (Jiang et al. 2001, 2002; Shi et al. 2002; Xia et al. 2002; Latorre & Brauchi, 2006). The existence of a second RCK domain (RCK2) contained in the distal part of the C-terminus has recently received support in a structure-based alignment study of the C-terminus of the BK and prokaryotic RCK domains (for a review see Cui et al. 2009). Circular dichroism experiments, on the other hand, support a structural homology of 90% between the MthK RCK2 crystal and the proposed one for BK. The structure proposed for BK RCK2 (residues 712–999) includes the high affinity calcium bowl, and has the characteristic Rossman-fold topology of RCK domains (Yusifov et al. 2008). In addition, the putative RCK2 undergoes a Ca2+-induced change in conformation associated with an α-helix to β-folded structural transition; deletion of the calcium bowl impairs this transition (Yusifov et al. 2008).
This year, the MacKinnon group determined the X-ray structure of the human BK C-terminus at a 3 Å resolution (Yuan et al. 2010). This structure confirmed two tandem RCK domains in each BK α-subunit and the deduced tetrameric structure indicated that the BK C-terminus forms a 350 kDa gating ring at the intracellular membrane surface. This study also confirmed that the Ca2+ bowl located within the second of the tandem RCK domains forms a Ca2+ binding site. This site is located on the outer perimeter of the gating ring, in a region denominated the assembly interface (Fig. 2D). However, Yuan et al. (2010) were unable to locate a second Ca2+ binding site as predicted by the biophysical studies (Bao et al. 2002: Xia et al. 2002; see below). Thus, as is the case for the MthK channel, the resulting structure of the BK gating ring is an octamer of RCK domains but different from the MthK channel (Jiang et al. 2002) where each subunit of the tetramer contributes one RCK domain and another is assembled from solution, and both RCK domains in the BK channel are intrinsic to the α-subunit. Yuan et al. (2010) speculate that this structural difference may be the origin of the different places to which Ca2+ is bound in these two gating rings. In the MthK, Ca2+ binds on the ‘flexible’ interface where a cleft between two RCK domains creates the Ca2+ binding site (Fig. 2D).
The structures of the BK gating ring, the MthK channel (Jiang et al. 2002), and the chimeric Kv1.2/Kv2.1 channel (Long et al. 2007) were used to build a complete BK channel model. In this model some of mutations of functional importance clustered towards the N-terminus of RCK1. These amino acids reside on the surface of the gating ring that faces the VSD indicating that Ca2+ binding, besides contributing directly to the energy necessary to open the pore, may modulate the voltage sensor, an observation that can give physical reality to the allosteric factor describing the interaction between the voltage sensor activation and Ca2+ binding in the general allosteric gating mechanism proposed by Horrigan & Aldrich (2002).
The problem of how the gating ring extracts the free energy of Ca2+ binding to open the BK channel gates was tackled by Niu et al. (2004) who proposed that the RCK1–S6 coupling system is contained in the linkers which behave as a passive spring that applies force to the gates in the absence of cytoplasmic Ca2+ to modulate voltage-dependent gating. An internal Ca2+ increase changes the force and increases further the BK channel open probability. We notice here that the crystal structure of the MthK gating ring obtained in the absence of Ca2+ and comparison with the structure of the open MthK (Jiang et al. 2002) channel allowed the visualization of the open and closed conformations of the Ca2+ binding-domain (Ye et al. 2006). Calcium binding to each of the RCK domains induces an expansion of the gating ring that in turn can exert a lateral force on the pore opening the channel.
Partial deletion or point mutations of the aspartates contained in the calcium bowl produced BK channels that were less sensitive to Ca2+ (reviewed in Latorre & Brauchi, 2006 and Cui et al. 2009). The fact that disruption of the Ca2+ bowl did not completely eliminate the BK channel sensitivity was a clear demonstration that there was more than one high Ca2+ affinity site. The second high-affinity Ca2+ sensor was identified on the proximal tail region embedded within the upstream RCK1 domain (Bao et al. 2002; Xia et al. 2002). Additional research identified a third lower affinity divalent cation sensing domain in the RCK1 able to bind Mg2+ as well as Ca2+ (Shi et al. 2002; Xia et al. 2002). Zeng et al. (2005) concluded that the Ca2+ bowl speeds up channel activation at low Ca2+ concentrations whereas the second high affinity site (D362/D367) modulates both activation and deactivation at [Ca2+] >10 μm. Surprisingly, the binding of Ca2+ to the high affinity site contained in the RCK1 domain is voltage dependent whereas at the Ca2+ bowl is not (Sweet & Cox, 2008). RCK1 also contains a high affinity Zn2+ binding site and BK channel activation by this divalent cation is reduced when H365, D367 and E399 are mutated (Hou et al. 2010).
Recently Yang et al. (2008b) found that Mg2+ is coordinated at the interface between the voltage sensor domain and the RCK1 domain to activate BK channels. Side chains of amino acid residues Asp99 (intracellular S0–S1 linker) and Asn172 (intracellular S2–S3 linker) in the VSD and Glu374 and Glu399 in the RCK1 domain of a different subunit domain form the Mg2+ binding site, suggesting a close proximity between these two domains. This proximity, on the other hand, enables the electrostatic interaction between the bound Mg2+ and Arg213 contained in the S4 segment which in turn affects the displacement of the voltage sensor giving an elegant mechanistic explanation to the Mg2+ BK channel activation. The average distance between R213 and R397 in the resting state is 9.1 Å (Yang et al. 2007, 2008a). The data of Yang et al. (2008b) suggested that the VSD domain of one subunit is located on top of the RCK1 domain from the neighbouring subunit, implying that the packing of the VSD domain or the cytoplasmic domain relative to the pore domain in BK channels may differ from that of MthK channels (cf. Yuan et al. 2010). This discrepancy may be due to the unique structural features of BK channels in particular to (a) the presence of S0, (b) the long cytoplasmic loop connecting S1 and S2, and (c) the interaction and packing of the VSD with the RCK1 domain.
Conclusion
Due to its important physiological roles, malfunction of BK channels can lead to hypertension and asthma and induce hearing loss and epilepsy (reviewed in Cui et al. 2009). Because of the profound involvement of BK in the health problems described above, activators and blockers of these channels have potential therapeutic implications and the development of new drugs targeting BK channels will be possible if the detailed relationships between channel structure and function are known. It is clear at present that voltage and Ca2+ sensors are located in different structures leading to pore opening via independent equilibria that interact allosterically with each other (see Fig. 3).
Figure 3. Allosteric model for BK activation by voltage and internal Ca2+.
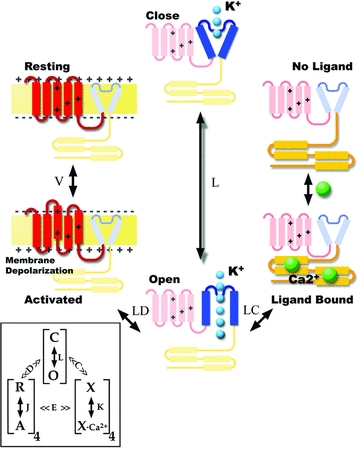
The cartoon is the representation of one α-subunit that can undergo a direct transition from the closed to the open configuration, a transition that is determined by the equilibrium constant L. Depolarization activates voltage sensors and in this case the transition from closed to open is determined by LD where D is an allosteric factor >1. Activation of voltage sensors makes it a factor of D times more easy to open the channel. Ca2+ binding also facilitates the channel opening. In this case the transition from closed to open is determined by LC (C > 1). Notice that if both voltage and Ca2+ sensors are ‘activated’ channel opening is determined by LDC. Inset, the complete allosteric model (Horrigan & Aldrich, 2002) taking into consideration that BK channels are tetramers and including some interaction between voltage sensor activation and Ca2+ binding. In this type of mechanism, neither voltage nor Ca2+ binding is strictly coupled to channel opening; these three processes are independent equilibria that interact allosterically with each other.
Acknowledgments
We thank Dr Neil A. O’Brien for comments on the manuscript. This work was supported by Fondecyt Grants 1070049 and 1090493 (to R.L.).
References
- Bao L, Rapin AM, Holmstrand EC, Cox DH. Elimination of the BKCa channels high-affinity Ca2+ sensitivity. J Gen Physiol. 2002;120:173–189. doi: 10.1085/jgp.20028627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelidze T, Niu IX, Magleby KL. A ring of eight conserved negatively charged amino acids doubles the conductance of BK channels and prevents inward rectification. Proc Natl Acad Sci U S A. 2003;100:9017–9022. doi: 10.1073/pnas.1532257100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- Carvacho I, Gonzalez W, Torres YP, Brauchi S, Alvarez O, Gonzalez-Nilo FD, Latorre R. Intrinsic electrostatic potential in the BK channel pore: role in determining single channel conductance and block. J Gen Physiol. 2008;131:147–161. doi: 10.1085/jgp.200709862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Aldrich RW. Allosteric linkage between voltage and Ca2+-dependent activation of BK-type mslo1 K+ channels. Biochemistry. 2000;39:15612–15619. doi: 10.1021/bi001509+. [DOI] [PubMed] [Google Scholar]
- Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci. 2009;66:852–875. doi: 10.1007/s00018-008-8609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz L, Meera P, Amigo J, Stefani E, Alvarez O, Toro L, Latorre R. Role of the S4 segment in a voltage-dependent calcium-sensitive potassium (hSlo) channel. J Biol Chem. 1998;273:32430–32436. doi: 10.1074/jbc.273.49.32430. [DOI] [PubMed] [Google Scholar]
- Giangiacomo KM, Becker J, Garsky C, Schmalhofer W, Garcia ML, Mullmann TJ. Novel α-KTx sites in the BK channel and comparative sequence analysis reveal distinguishing features of the BK and KV channel outer pore. Cell Biochem Biophys. 2008;52:47–58. doi: 10.1007/s12013-008-9026-3. [DOI] [PubMed] [Google Scholar]
- Haug T, Sigg D, Ciani S, Toro L, Stefani E, Olcese R. Regulation of K+ flow by a ring of negative charges in the outer pore of BKCa channels. Part I: Aspartate 292 modulates K+ conduction by external surface charge effect. J Gen Physiol. 2004;124:173–184. doi: 10.1085/jgp.200308949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L, Lu Z, Abramson T, MacKinnon R. Mutations in the K+ channel signature sequence. Biophys J. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J Gen Physiol. 2002;120:267–305. doi: 10.1085/jgp.20028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Vigeland LE, Zhang G, Xu R, Li M, Heinemann SH, Hoshi T. Zn2+ activates large conductance Ca2+-activated K+ channel via an intracellular domain. J Biol Chem. 2010;285:6434–6442. doi: 10.1074/jbc.M109.069211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Pico A, Cadene M, Chait BT, MacKinnon R. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron. 2001;29:593–601. doi: 10.1016/s0896-6273(01)00236-7. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- Koval OM, Fan Y, Rothberg BS. A role for the S0 transmembrane segment in voltage-dependent gating of BK channels. J Gen Physiol. 2007;129:209–220. doi: 10.1085/jgp.200609662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R, Brauchi S. Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage. Biol Res. 2006;39:385–401. doi: 10.4067/s0716-97602006000300003. [DOI] [PubMed] [Google Scholar]
- Latorre R, Oberhauser A, Labarca P, Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Latorre R, Vergara C, Hidalgo C. Reconstitution in planar lipid bilayers of a Ca2+-dependent K+ channel from transverse tubule membranes isolated from rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1982;79:805–809. doi: 10.1073/pnas.79.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Aldrich RW. State-dependent block of BK channels by synthesized Shaker ball peptides. J Gen Physiol. 2006;128:423–441. doi: 10.1085/jgp.200609521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle CJ. Gating rings formed by RCK domains: keys to gate opening. J Gen Physiol. 2007;129:101–107. doi: 10.1085/jgp.200709739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Niu X, Wu RS, Chudasama N, Yao Y, Jin X, Weinberg R, Zakharov SI, Motoike H, Marx SO, Karlin A. Location of modulatory beta subunits in BK potassium channels. J Gen Physiol. 2010;135:449–459. doi: 10.1085/jgp.201010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zakharov SI, Yang L, Deng SX, Landry DW, Karlin A, Marx SO. Position and role of the BK channel alpha subunit S0 helix inferred from disulfide crosslinking. J Gen Physiol. 2008;131:537–548. doi: 10.1085/jgp.200809968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- Ma Z, Lou XJ, Horrigan FT. Role of charged residues in the S1-S4 voltage sensor of BK channels. J Gen Physiol. 2006;127:309–328. doi: 10.1085/jgp.200509421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R, Miller C. Mutant potassium channels with altered binding of charybdotoxin, a pore-blocking peptide inhibitor. Science. 1989;245:1382–1385. doi: 10.1126/science.2476850. [DOI] [PubMed] [Google Scholar]
- Magleby KL. Gating mechanism of BK (Slo1) channels: so near, yet so far. J Gen Physiol. 2003;121:81–96. doi: 10.1085/jgp.20028721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A. Ca2+-dependent K+ channels with large unitary conductance in chromaffin cell membranes. Nature. 1981;291:497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Song M, Toro L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0-S6), an extracellular N terminus, and an intracellular (S9-S10) C terminus. Proc Natl Acad Sci U S A. 1997;94:14066–14071. doi: 10.1073/pnas.94.25.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C, Moczydlowski E, Latorre R, Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985;313:316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Morrow JP, Zakharov SI, Liu G, Yang L, Sok AJ, Marx SO. Defining the BK channel domains required for β1-subunit modulation. Proc Natl Acad Sci U S A. 2006;103:5096–5101. doi: 10.1073/pnas.0600907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullmann TJ, Munujos P, Garcia ML, Giangiacomo KM. Electrostatic mutations in iberiotoxin as a unique tool for probing the electrostatic structure of the maxi-K channel outer vestibule. Biochemistry. 1999;38:2395–2402. doi: 10.1021/bi982040+. [DOI] [PubMed] [Google Scholar]
- Neely A, Lingle CJ. Effects of muscarine on single rat adrenal chromaffin cells. J Physiol. 1992;453:133–166. doi: 10.1113/jphysiol.1992.sp019221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Niu X, Qian X, Magleby KL. Linker-gating ring complex as passive spring and Ca2+-dependent machine for a voltage- and Ca2+-activated potassium channel. Neuron. 2004;42:745–756. doi: 10.1016/j.neuron.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel β-subunits. News Physiol Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- Pallotta BS, Magleby KL, Barrett JN. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981;293:471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- Pantazis A, Gudzenko V, Savalli N, Sigg D, Olcese R. Operation of the voltage sensor of a human voltage- and Ca2+-activated K+ channel. Proc Natl Acad Sci U S A. 2010;107:4459–4464. doi: 10.1073/pnas.0911959107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk JC, Reinhart PH. Identification of a novel tetramerization domain in large conductance KCa channels. Neuron. 2001;32:13–23. doi: 10.1016/s0896-6273(01)00444-5. [DOI] [PubMed] [Google Scholar]
- Sah P, Davies P. Calcium-activated potassium currents in mammalian neurons. Clin Exp Pharmacol Physiol. 2000;27:657–663. doi: 10.1046/j.1440-1681.2000.03317.x. [DOI] [PubMed] [Google Scholar]
- Savalli N, Kondratiev A, Toro L, Olcese R. Voltage-dependent conformational changes in human Ca2+- and voltage-activated K+ channel revealed by voltage-clamp fluorometry. Proc Natl Acad Sci U S A. 2006;103:12619–12624. doi: 10.1073/pnas.0601176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova NP, Abarca-Heidemann K, Loranc E, Rothberg BS. Bimane fluorescence scanning suggests secondary structure near the S3-S4 linker of BK channels. J Biol Chem. 2009;284:10684–10693. doi: 10.1074/jbc.M808891200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KZ, Lagrutta A, Davies NW, Standen NB, Adelman JP, North RA. Tetraethylammonium block of Slowpoke calcium-activated potassium channels expressed in Xenopus oocytes: evidence for tetrameric channel formation. Pflugers Arch. 1994;426:440–445. doi: 10.1007/BF00388308. [DOI] [PubMed] [Google Scholar]
- Shi J, Krishnamoorthy G, Yang Y, Hu L, Chaturvedi N, Harilal D, Qin J, Cui J. Mechanism of magnesium activation of calcium-activated potassium channels. Nature. 2002;418:876–880. doi: 10.1038/nature00941. [DOI] [PubMed] [Google Scholar]
- Solaro CR, Prakriya M, Ding JP, Lingle CJ. Inactivating and noninactivating Ca2+- and voltage-dependent K+ current in rat adrenal chromaffin cells. J Neurosci. 1995;15:6110–6123. doi: 10.1523/JNEUROSCI.15-09-06110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani E, Ottolia M, Noceti F, Olcese R, Wallner M, Latorre R, Toro L. Voltage-controlled gating in a large conductance Ca2+-sensitive K+ channel (hslo) Proc Natl Acad Sci U S A. 1997;94:5427–5431. doi: 10.1073/pnas.94.10.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet TB, Cox DH. Measurements of the BKCa channel's high-affinity Ca2+ binding constants: effects of membrane voltage. J Gen Physiol. 2008;132:491–505. doi: 10.1085/jgp.200810094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro L, Wallner M, Meera P, Tanaka Y. Maxi-KCa, a unique member of the voltage-gated K+ channel superfamily. News Physiol Sci. 1998;13:112–117. doi: 10.1152/physiologyonline.1998.13.3.112. [DOI] [PubMed] [Google Scholar]
- Vergara C, Moczydlowski E, Latorre R. Conduction, blockade and gating in a Ca2+-activated K+ channel incorporated into planar lipid bilayers. Biophys J. 1984;45:73–76. doi: 10.1016/S0006-3495(84)84114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Meera P, Toro L. Determinant for β-subunit regulation in high-conductance voltage-activated and Ca2+-sensitive K+ channels: an additional transmembrane region at the N terminus. Proc Natl Acad Sci U S A. 1996;93:14922–14927. doi: 10.1073/pnas.93.25.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Sigworth FJ. Structure of the BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature. 2009;461:292–295. doi: 10.1038/nature08291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei A, Solaro C, Lingle C, Salkoff L. Calcium sensitivity of BK-type KCa channels determined by a separable domain. Neuron. 1994;13:671–681. doi: 10.1016/0896-6273(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Wu D, Delaloye K, Zaydman MA, Nekouzadeh A, Rudy Y, Cui J. State-dependent electrostatic interactions of S4 arginines with E1 in S2 during Kv7.1 activation. J Gen Physiol. 2010;135:595–606. doi: 10.1085/jgp.201010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 2002;418:880–884. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- Yang H, Hu L, Shi J, Delaloye K, Horrigan FT, Cui J. Mg2+ mediates interaction between the voltage sensor and cytosolic domain to activate BK channels. Proc Natl Acad Sci U S A. 2007;104:18270–18275. doi: 10.1073/pnas.0705873104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Shi J, Zhang G, Yang J, Delaloye K, Cui J. Activation of Slo1 BK channels by Mg2+ coordinated between the voltage sensor and RCK1 domains. Nat Struct Mol Biol. 2008a;15:1152–1159. doi: 10.1038/nsmb.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhang G, Shi J, Lee US, Delaloye K, Cui J. Subunit-specific effect of the voltage sensor domain on Ca2+ sensitivity of BK channels. Biophys J. 2008b;94:4678–4687. doi: 10.1529/biophysj.107.121590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, Baker D, Catterall WA. Voltage sensor conformations in the open and closed states in ROSETTA structural models of K+ channels. Proc Natl Acad Sci U S A. 2006;103:7292–7297. doi: 10.1073/pnas.0602350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Leonetti MD, Pico AR, Hsiung Y, MacKinnon R. Structure of the human BK channel Ca2+-activation apparatus at 3.0 Å resolution. Science. 2010;329:182–186. doi: 10.1126/science.1190414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Li Y, Chen L, Jiang Y. Crystal structures of a ligand free MthK gatin ring: insights into the ligand gating mechanisms of K+ channels. Cell. 2006;126:1161–1173. doi: 10.1016/j.cell.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Yusifov T, Savalli N, Gandhi CS, Ottolia M, Olcese R. The RCK2 domain of the human BKCa channel is a calcium sensor. Proc Natl Acad Sci U S A. 2008;105:376–381. doi: 10.1073/pnas.0705261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XH, Xia XM, Lingle CJ. Divalent cation sensitivity of BK channel activation supports the existence of three distinct binding sites. J Gen Physiol. 2005;125:273–286. doi: 10.1085/jgp.200409239. [DOI] [PMC free article] [PubMed] [Google Scholar]


