Abstract
Serum opacity factor (SOF), a virulence determinant of Streptococcus pyogenes, converts plasma high density lipoproteins (HDL) to three distinct species: lipid-free apolipoprotein (apo) A-I, neo HDL, a small discoidal HDL-like particle, and a large cholesteryl ester-rich microemulsion (CERM), that contains the cholesterol esters (CE) of up to ~400,000 HDL particles and apo E as its major protein. Similar SOF reaction products are obtained with HDL, total plasma lipoproteins and whole plasma. We hypothesized that hepatic uptake of CERM-CE via multiple apo E dependent receptors would be faster than that of HDL-CE. We tested our hypothesis using human hepatoma cells and lipoprotein receptor-specific Chinese hamster ovary (CHO) cells. [3H]CE uptake by HepG2 and Huh7 cells from HDL after SOF treatment, which transfers >90% of HDL-CE to CERM, was respectively 2.4 and 4.5 times faster than from control HDL. CERM-[3H]CE uptake was inhibited by LDL and HDL, suggestive of uptake by both the LDL receptor (LDL-R) and scavenger receptor class B type I (SR-BI). Studies in CHO cells specifically expressing LDL-R and SR-BI confirmed CERM-[3H]CE uptake by both receptors. RAP and heparin inhibit CERM-[3H]CE but not HDL-[3H]CE uptake thereby implicating LRP-1 and cell surface proteoglycans in this process. These data demonstrate that SOF treatment of HDL increases CE uptake via multiple hepatic apo E receptors. In so doing, SOF might increase hepatic disposal of plasma cholesterol in a way that is therapeutically useful.
Keywords: HDL function, cholesteryl ester uptake, Huh7, HepG2, apo E, LDL-R, LRP, SR-BI, RAP, heparin
Serum opacity factor (SOF), a virulence determinant of Streptococcus pyogenes, converts HDL to lipid-free (LF) apo A-I, neo HDL, which is a small HDL-like particle, and a large cholesteryl ester-rich microemulsion (CERM) that contains the cholesteryl esters (CE) of ~400,000 HDL particles and monomeric apo E and its heterodimer with apo A-II as its sole apos (1-6). Recombinant SOF (rSOF) is potent and catalytic; rSOF (1 μg/mL) quantitatively converts HDL to CERM, neo HDL, and LF apo A-I with a halftime of ~30 min (4;5). Based on the reaction products and kinetics, we proposed a model for the rSOF reaction in which rSOF is a heterodivalent fusogenic protein that uses a docking site to displace apo A-I and bind to exposed CE surfaces on HDL (4). The initial rSOF-HDL complex recruits additional HDL with its binding-delipidation site and through multiple fusion steps forms large CERM and releases neo HDL and LF apo A-I (4). Importantly, CERM contain apo E. We hypothesized that with its high apo E and CE contents, CERM could transfer large amounts of cholesterol to the liver for disposal via LDL receptor (LDL-R) or other apo E receptors (4).
CERM and chylomicron remnants (CR) share some properties suggesting that they might be cleared via similar pathways. Both contain apo E as a major protein that could mediate cellular uptake via multiple apo E dependent receptors. Both have large neutral lipid cores that comprise a mixture of cholesteryl esters and triglycerides. Hepatic CR undergo sequestration within the perisinusoidal space, where locally secreted apo E enhances binding and uptake (7). Heparan sulfate proteoglycans (HSPG) within the hepatic perisinusoidal space can also bind apo E, which anchors the CR for hydrolysis by lipases, subsequently promoting its uptake. The final endocytic step is mediated by the low density lipoprotein receptor (LDL-R) and by the low density lipoprotein receptor-related protein (LRP). Cell-surface HSPG are important in the initial sequestration in the perisinusoidal space and integral to HSPG/LDL-R and LRP pathways. HSPG also function as independent receptors. Given its high CE content and complement of apo E, conversion of HDL to CERM via the rSOF reaction might enhance hepatic CE uptake via multiple apo E dependent receptors. In contrast, most hepatic HDL-CE uptake of CE occurs via the selective pathway mediated by SR-BI, the mouse ortholog of human CLA-1 (8;9). Here we show that rSOF converts human HDL to CERM in whole plasma and that uptake of CERM-CE by human hepatoma cells occurs via multiple apoE-dependent lipoprotein receptors and is faster than that of the HDL-CE from which the CERM were formed.
MATERIALS AND METHODS
Materials
HDL were isolated from normal human plasma obtained from The Methodist Hospital Blood Donor Center by sequential flotation at d = 1.063 and 1.21 g/mL. KBr was removed by dialysis against Tris-buffered saline (TBS = 10 mM Tris, 100 mM NaCl, 1 mM EDTA, pH = 7.4), which was used as the standard buffer except where noted otherwise. Lipoprotein purity was verified by SDS-PAGE and size exclusion chromatography (SEC) over two Superose HR 6 columns (GE Healthcare) in tandem (4). SEC was also used to obtain lipoprotein profiles according to size. HDL-[3H]CE was prepared from HDL and [3H]-cholesterol (Amersham/GE Healthcare) by biological labeling as described (4). Cholesteryl 4,4-difluoro-5,7-dimethyl-4-bora-3α,4α-diaza-s-indacene-3-docecanoate (Bodipy-CE), a fluorescent CE analog, was from Invitrogen/Molecular Probes (Seattle, WA). Buffer salts were from Thermo/Fisher Scientific, Inc. (Rockville, MD). A recombinant polyhistidine-tagged, truncated form of sof2, encoding amino acids 38-843 (rSOF) was cloned and expressed in Escherichia coli and purified by metal affinity chromatography as described (6). Protein concentration was determined using the DC Protein Kit (BioRad). Immunoblots were done as described (4) with the Amersham™ ECL Plus Western Blotting Detection System (GE Healthcare).
Cell Culture
HepG2 cells and wild-type CHO cells, CHO-K1, (LDL-R-positive) were from American Type Culture Collection (Manassas, VA); CHO-ldlA7 cells (LDL-R-negative) and the latter transfected to express high amounts of mouse SR-BI (CHO-SR-BI) were provided by Dr. Monty Krieger (10). Huh7 cells were provided by Drs. Yumin Xu and Boris Yoffe (11). Huh7 and HepG2 cells were cultured as described (12) in Minimal Essential Medium (MEM) with 10% fetal bovine serum (FBS), 1 mM sodium pyruvate and penicillin-streptomycin antibiotics (10 U/mL, 10 μg/mL respectively). CHO cell lines were cultured in Hams F-12 with 5% FBS, 1 mM sodium pyruvate and penicillin-streptomycin antibiotics (10 U/mL, 10 μg/mL respectively). The CHO-SR-BI medium also contained G418 (300 μg/mL). Tissue culture reagents were from InVitrogen (Carlsbad, CA).
CE uptake
The CE uptake was assayed as described (8). The day prior to the assay, HDL-[3H]CE (1 mg/mL) was incubated overnight at 37°C without (control) or with (+SOF) rSOF (2 μg/mL) in cell culture medium with 0.5% fatty-acid free bovine serum albumin (BSA), (Medium B, (8)). SEC analysis of the reaction mixtures confirmed that rSOF treatment transferred > 90% of the [3H]CE into CERM-[3H]CE. Uptake was initiated by adding 20 μL aliquots of control (HDL-[3H]CE) or CERM-[3H]CE (HDL-[3H]CE +rSOF) reaction mixture to cells in 1 mL of Medium B, cells were incubated for 0 – 3 hr at 37°C in a 5% CO2 incubator, uptake was stopped by placing cells on ice, cells were washed, and [3H]CE taken up by the cells determined by extraction with isopropanol and β-counting. Inhibitors of uptake, unlabelled HDL and LDL, RAP (Innovative Research Low Endotoxin Human RAP) (13-15) and heparin (Sigma Heparin Sodium from Porcine Intestinal Mucosa) (13;16), were diluted in Medium B, and added as 100 μL aliquots to washed cells in Medium B just prior to addition of the radiolabelled reaction mixture aliquots, to give a final volume of 1 mL. The effect of Simvastatin (Sigma, St. Louis) on CE uptake was determined as described (17;18). In brief, Simvastatin was activated to the acid form according to the Sigma protocol. Huh7 and HepG2 cells were pretreated with 0.01 – 10 μM Simvastatin for 18 hours in media with 10% lipoprotein deficient plasma prior to the start of the uptake assay.
Bodipy-CE Labeling of Lipoproteins
Bodipy-CE (1 mg) was dried from chloroform on to the bottom of a conical glass test tube under a stream of N2. Human plasma HDL (12.5 mg protein/2.5 mL) was added and sonicated (40-watts) on ice for 40 min. The labeled HDL was filtered (0.45 μm) giving HDL-Bodipy-CE. HDL-Bodipy-CE (5 mg/mL) was incubated overnight with rSOF (10 μg/mL) giving CERM-Bodipy-CE, which was isolated by SEC. To prepare LDL-Bodipy-CE, Bodipy-CE (1 mg) was dried on to the bottom of a glass test tube as above, human plasma LDL (~5mg/mL) was added in TBS, and the mixture incubated for 24 hrs at 37°C with continuous low speed vortexing. Unbound Bodipy-CE was removed by filtration (0.45 μm). Label incorporation into lipoproteins was determined fluorometrically.
Confocal microscopy
Cells grown in 35 mm Glass Bottom Dishes No. 1.5 (MatTek Corporation, Ashland, MA) were used for live cell imaging. Huh7 cells were incubated with HDL-, CERM- or LDL-Bodipy-CE (50 μg/mL in complete media) for 60 - 90 min at 37°C. Cells were washed 3 times with phosphate buffered saline (PBS) and placed on the microscope stage. In some experiments, after the lipoprotein uptake, cells were counterstained with Hoechst dye to label nuclei and Lysotracker DND-99 (Lysotracker Red) to label lysosomes (both from Molecular Probes/InVitrogen, Carlsbad, CA). For nuclear staining, cells were incubated for 5 min at 37°C with ~ 2mL of 2 μg/mL Hoechst dye in Hank’s balanced salt solution (HBSS), prepared from a 1mM stock, and then washed twice with HBSS. For lysosomal labeling cells were incubated for 2 min at 25°C with 2 mL of 100nM Lysotracker DND-99 in HBSS and washed twice with HBSS. A Zeiss LSM 510 laser scanning two photon confocal microscope was used to collect images, which were captured using a 63x oil immersion objective with 512×512 pixel (8 bit) image size and a scan rate of 26 μsec/image. Excitation was provided by an argon ion laser and the emission passed through a bandpass filter and a main dichroic filter to reduce excitation light. Detector gain was between 700 to 900 with an amplifier offset of 0.1 and an amplifier gain of 1. Images were analyzed with the Zeiss software package designed for the LSM 510.
RESULTS
rSOF is Active in the Presence of Total Lipoproteins In Vitro
In previous work we showed that rSOF opacifies HDL but not LDL nor VLDL (3). In the rSOF reaction with HDL, three products are formed, CERM, neo HDL and lipid-free apo A-I (4). We now report that these same products are formed in the presence of total lipoproteins. Total lipoproteins (TLP; 0.5 mg/mL protein) from a 1.21 g/mL KBr flotation of human plasma were incubated with rSOF (1 μg/mL) overnight at 37° C. As shown in Figure 1 A (grey fill), SEC separates the major lipoproteins. Moreover, all of the products of the rSOF reaction against HDL are formed in the presence of LDL and VLDL, i.e., CERM, neo HDL and LF apo A-I (Figure 1 A, black curve). There is a small shift in the LDL peak to a larger particle size. These data show that most of the observations made with isolated HDL for mechanistic studies (4;5) are preserved in the more physiological setting of TLP.
Figure 1.
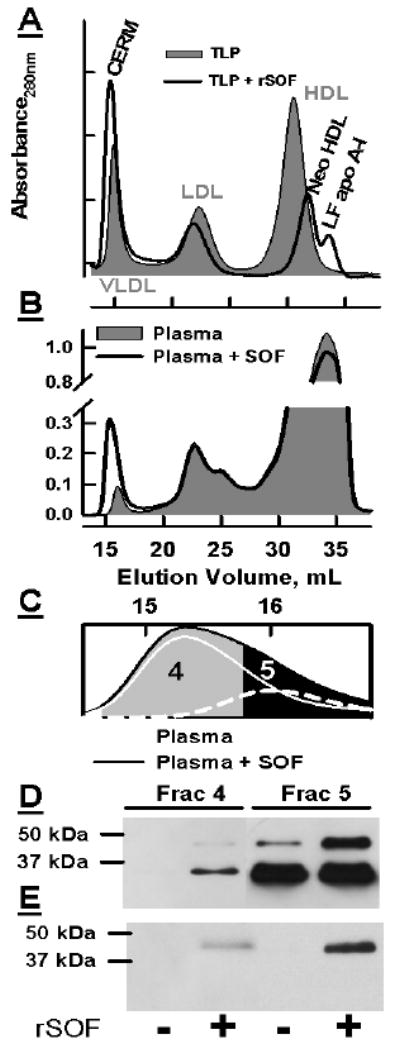
A and B, respectively: SEC of TLP and plasma before (grey fill) and after (black curve) incubation with rSOF. C: Expansion of the SEC profile to show separation of VLDL and CERM in rSOF-treated plasma. Dashed line, plasma VLDL; white line ,CERM, the difference between plasma and rSOF-treated plasma. D and E: Immunoblots of fractions 4 and 5 probed with anti apos E and A-II respectively.
rSOF Transfers Apo E to CERM in Whole Plasma
Although rSOF treatment of isolated HDL produces CERM that contain apo E (4), its effects on the distribution of apo E in whole plasma was not known. To address this, whole plasma was treated with rSOF. As found with TLP, rSOF treatment of whole plasma produces CERM; observation of neo HDL and LF apo A-I in the plasma SEC profile is obscured by other plasma proteins (Figure 1 B). VLDL elutes near the void volume (~ 16 mL), while the peak corresponding to CERM elutes at 15.3 mL. Figure 1 C shows an expansion of this region and the fractions that were collected for immunoblot analysis with anti apo E and anti apo A-II (Figure 1 D, E) according to Gillard et al. (4). Fraction 4 includes the peak tube for the CERM of the rSOF-treated plasma and only the leading edge of plasma VLDL in the untreated sample. Fraction 5 contains the peak for the VLDL of whole plasma and the trailing edge of the CERM. The reproducibility of the CERM elution volume in 14 consecutive injections was 15.30 ± 0.016 μL. Figure 1 D shows that Fraction 4 of the untreated plasma contains no detectable apo E whereas the same fraction for the treated plasma contains a strong apo E-positive band as well as a band with a molecular weight ~45 kDa, which corresponds to the apo E-apo A-II heterodimer (12). Although Fraction 5 of the untreated plasma contains the same pair of bands, they are much more intense for the rSOF-treated sample. Immunoblotting with anti-apo A-II supports our assignment of the 45 kDa band as the apo E-apo A-II heterodimer (Figure 1 E). The untreated samples contain no apo A-II whereas fractions 4 and 5 from the rSOF-treated samples contain an apo A-II-positive band with a migration distance identical to that of the higher molecular weight band visualized by anti-apo E. These data clearly show that apo E is present on CERM formed in whole plasma as it is on CERM obtained by rSOF treatment of isolated HDL (4). These data also show that the rSOF reaction in TLP and whole plasma emulates that of rSOF against isolated HDL.
rSOF Transfers HDL-CE to CERM
SEC analysis of HDL that was biologically labeled with [3H]CE according to Gillard et al. (4) showed that the HDL is a single radiolabled species (Figure 2). Treatment of the HDL-[3H]CE with rSOF transferred > 90% of the [3H]CE to CERM and the balance to neo HDL. We compared the uptake of [3H]CE from HDL-[3H]CE and from the rSOF product, CERM-[3H]CE, in hepatic cell lines and in CHO cells expressing specific lipoprotein receptors.
Figure 2.
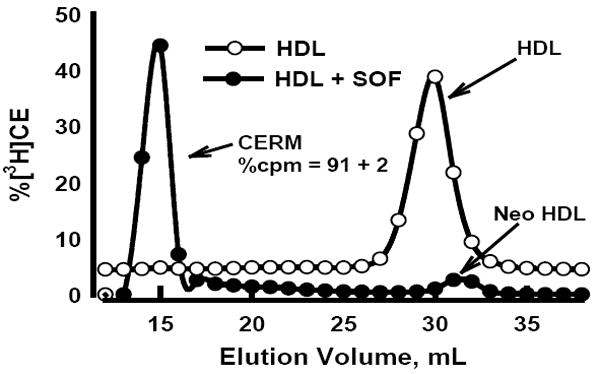
SEC profiles of control HDL-[3H]CE (○) and rSOF treated HDL-[3H]CE (●) reaction mixtures. rSOF treatment transfers (91 ± 2)% of the total [3H]CE to the CERM fraction. Aliquots of the control and rSOF reaction mix were used for [3H]CE uptake experiments. The SEC trace for the control HDL-[3H]CE begins at zero absorbance but in this figure is offset from that of HDL-[3H]CE + SOF to permit better comparison.
rSOF-Treatment Increases Hepatic HDL-CE Uptake
The kinetics of cellular uptake of HDL-[3H]CE and its product CERM-[3H]CE were tested in two human hepatoma lines, Huh7 and HepG2. In all cases, the rates were linear with time up to 3 h (Figure 3 A, B). Comparison of the slopes of the rate curves showed that rSOF treatment increased [3H]CE uptake to 4.5 and 2.4 fold greater than control HDL-[3H]CE uptake respectively for Huh7 and HepG2 cells (Table 1).
Figure 3.
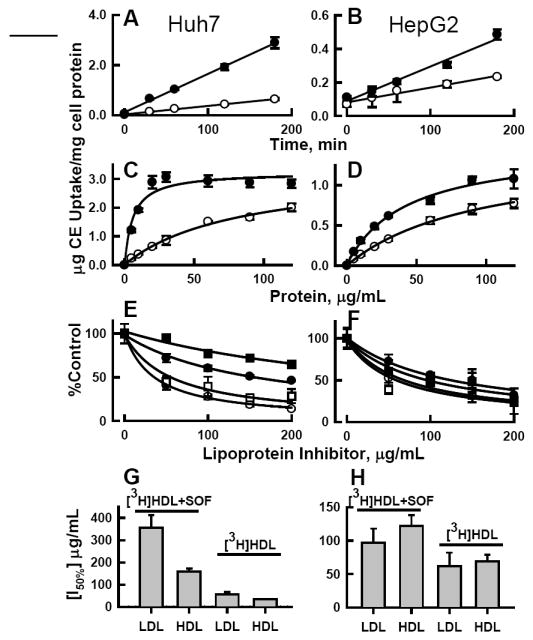
[3H]CE uptake from HDL ± rSOF by human hepatoma cells. Left panels, Huh7 cells; right panels, HepG2 cells. A, B: time course; C, D: dose response; open circles (○) uptake from HDL-[3H]CE; black circles (●) uptake from HDL-[3H]CE pretreated with rSOF to form CERM-[3H]CE. E, F: Inhibition of uptake by HDL (circles) or LDL (squares) from HDL-[3H]CE (open symbols) or CERM-[3H]CE (black symbols) at 20 μg/mL. G, H: Comparison of the concentration of LDL or HDL lipoprotein inhibitors (indicated on X-axis) needed to reduce CE uptake by 50%, calculated from panels E and F according to %Control = (a + [I50%])/([I50%] + [I]) where [I50%] is the concentration of lipoprotein inhibitor required for 50% inhibition, [I] is the inhibitor concentration (μg/mL), and a is the initial % uptake of radiolabeled CERM-CE (left two bars) or HDL-CE (right two bars).
Table 1.
Rates of Cholesterol Ester Uptake by Hepatoma and CHO Cell Lines.
| Cell Type | CE Uptake Ratea | r2 | |||
|---|---|---|---|---|---|
| Control HDL-CE | +SOF CERM-CE | ratio +SOF/Control | Control | +SOF | |
| Huh7 | 3.39 ± 0.10 | 15.32 ± 0.40 | 4.52 | 0.99 | 0.99 |
| HepG2 | 0.88 ± 0.09 | 2.07 ± 0.20 | 2.35 | 0.97 | 0.97 |
| CHO-K1 | 1.14 ± 0.09 | 3.03 ± 0.48 | 2.66 | 0.98 | 0.93 |
| ldl-A7 | 0.64 ± 0.22 | 1.64 ± 0.20 | 2.56 | 0.73 | 0.97 |
| SR-BI | 14.20 ± 1.20 | 14.10 ± 1.80 | 0.99 | 0.97 | 0.96 |
ng CE/ mg cell protein/ min, from Figures 3A, B and 4A, B.
Kinetic constants were also determined from [3H]CE uptake measured as a function of HDL-protein concentration (Figure 3 C, D). These data, summarized in Table 2, show that for hepatoma cells the maximum uptake Vmax was not changed by rSOF treatment. In contrast, the HDL protein concentration at which uptake was 50% of maximum (Bm) was reduced to 7% and 44% of control by rSOF treatment for Huh7 and HepG2 cells, respectively, indicating that uptake of CE from CERM occurs by higher affinity interaction than uptake from HDL. Based on the kinetic parameters of Table 2, rSOF treatment of HDL increased the catalytic efficiency Cateff for [3H]CE uptake by 14.4 and 2.4 fold for Huh7 and HepG2 cells, respectively.
Table 2.
Kinetic Parameters for CE Uptake from Control and rSOF-Treated HDL by Hepatoma and CHO Cell Linesa
| Cell Type | Vmax ng CE/mg cell protein/min |
Bm μg HDL protein/mL |
Cateff= V max/Bm | r2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control HDL-CE | +SOF CERM-CE | Control HDL-CE | +SOF CERM-CE | Control HDL-CE | +SOF CERM-CE | ratio +SOF/Control | Control | +SOF | |
| Huh7 | 3,300 ± 30 | 3,300 ± 40 | 82 ± 18 | 5.7 ± 2.0 | 40.2 | 579 | 14.4 | 0.92 | 0.99 |
| HepG2 | 1,400 ± 80 | 1,500 ± 80 | 96 ± 12 | 42 ± 8 | 14.6 | 35.7 | 2.44 | 0.99 | 0.99 |
| CHO K1 | 259 ± 62 | 526 ± 11 | 42 ± 21 | 6.2 ± 0.6 | 6.2 | 84.8 | 13.7 | 0.92 | 0.99 |
| ldl-A7 | 228 ± 21 | 183 ± 6 | 37 ± 7 | 4.5 ± 0.7 | 6.2 | 40.6 | 6.60 | 0.98 | 0.98 |
| SR-BI | 7,047 ± 316 | 11,866 ± 1,800 | 47 ± 5 | 79 ± 22 | 150 | 152 | 0.99 | 0.99 | 0.98 |
Based on data of Figures 3 C, D and 4 D, E and F.
To identify the receptors mediating uptake of CERM-CE, the effects of unlabeled HDL and LDL on CE uptake from HDL-[3H]CE and CERM-[3H]CE were determined (Figure 3 E, F) and the concentration of lipoprotein-inhibitor required to reduce [3H]CE uptake by 50% (I50%) was calculated. These data, summarized in Figure 3 G, H, showed the following: In Huh7 cells, CE uptake from HDL-[3H]CE was well inhibited by both HDL and LDL, while uptake from CERM-[3H]CE was poorly inhibited, requiring 5 and 6 times more HDL and LDL for 50% inhibition, and LDL was a much weaker inhibitor of uptake from CERM-[3H]CE. Similar studies in HepG2 cells showed smaller differences for HDL and LDL inhibition of uptake from CERM-[3H]CE vs HDL-[3H]CE, with ~ 2 times more HDL and LDL required for 50% inhibition of CERM-[3H]CE than HDL-[3H]CE uptake. For both cell types, CE uptake from CERM was less inhibited by HDL or LDL than was uptake from HDL.
rSOF Treatment of HDL Increases [3H]CE Uptake by Receptor-Specific CHO Cells
The results with Huh7 and HepG2 cells suggested that both LDL and HDL receptors mediate CERM-[3H]CE uptake. Thus, we compared the uptake of CE from control HDL with that of rSOF-treated HDL in CHO cells expressing different levels of lipoprotein receptors. These cells were CHO-K1, which express LDL-R and low levels of SR-BI, CHO-ldl-A7, in which LDL- R has been ablated, but which still have low level endogenous SR-BI, and CHO-SR-BI, CHO-ldl-A7 transfected to express high levels of SR-BI (8). rSOF treatment of HDL-[3H]CE increased rates of [3H]CE uptake by CHO-K1 and CHO-ldlA7 to 2.7 and 2.6 times control HDL-[3H]CE uptake respectively. With CHO-SR-BI cells, CE uptake rates were 5 – 20 times faster than with CHO-K1 and CHO-ldlA7 cells, whereas the rates were similar for HDL-[3H]CE and CERM-[3H]CE (Figure 4 A – C and Table 1).
Figure. 4.
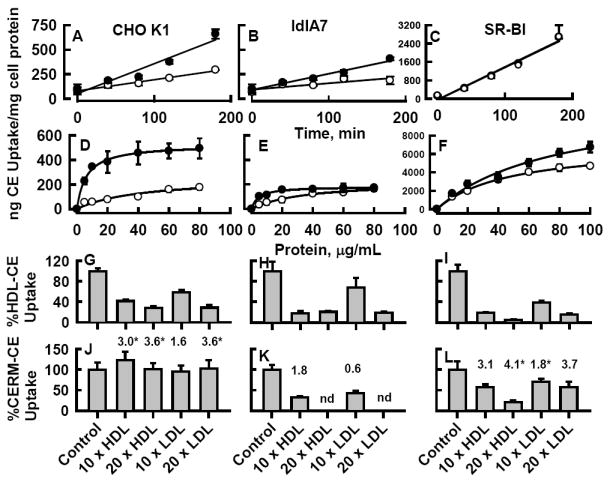
[3H]CE uptake from HDL ± rSOF treatment by CHO cell variants. Left panels, CHO-K1; middle panels, CHO-ldlA7; right panels, CHO-SR-BI. A – C: time course; D – F: dose response; (○) HDL-[3H]CE; (●) HDL-[3H]CE pretreated with rSOF to form CERM-[3H]CE. G –L: Inhibition of CHO cell [3H]CE Uptake from HDL ± rSOF treatment by unlabeled lipoproteins. CHO cell variants were incubated with 10 μg/mL HDL-[3H]CE ± rSOF pretreatment in the presence or absence of a 10- or 20-fold excess (100 or 200 μg/mL) human plasma HDL or LDL. *p < 0.05, +rSOF vs. control. Numbers above bars are the ratio of (%CERM-CE uptake/%HDL-CE uptake) in the presence of the various lipoprotein inhibitors.
Dose-response kinetic constants for HDL-[3H]CE ± rSOF were determined by measuring CE uptake as a function of lipoprotein concentration (Figure 4 D - F). In CHO-K1 cells, Vmax was higher and Bm lower leading to a 13.7 fold higher Cateff for uptake of CERM-[3H]CE than for HDL-[3H]CE (Table 2). In CHO-ldlA7 cells, rSOF treatment of HDL-[3H]CE decreased Vmax slightly and reduced Bm 8-fold, which led to a 6.6-fold higher Cateff . Although Vmax was higher for control vs. rSOF treated HDL-[3H]CE, the much lower Bm associated with rSOF treatment again led to an increase in Cateff with rSOF treatment. As expected, CE uptake from HDL-[3H]CE by SR-BI overexpressing cells was much higher than for CHO-K1 and CHO-ldlA7 cells (8). In contrast to the other cell lines, rSOF treatment did not have a profound effect on the kinetic parameters for CE uptake in cells overexpressing SR-BI; rSOF treatment increased both Vmax and Bm resulting in Cateff values that were the same with and without rSOF treatment (Table 2).
The effects of lipoprotein inhibitors on HDL-[3H]CE ± SOF uptake by the CHO cells were compared (Figure 4 G - L). For all three CHO cell lines, both LDL and HDL reduced [3H]CE uptake from HDL-[3H]CE (Figure 4 G - I). Lipoprotein inhibition of [3H]CE uptake from CERM-[3H]CE was less than from HDL-[3H]CE. In particular, in CHO-K1 cells, uptake from CERM-[3H]CE was not inhibited by 20-fold excess HDL or LDL (Figure 4J). The ratio of [3H]CE uptake from CERM-[3H]CE to that of HDL-[3H]CE in the presence of the lipoprotein inhibitors illustrates the magnitude of this effect for all three CHO cells (Figure 4 J - L; values above bars). Thus, the CERM-[3H]CE rSOF products have a higher binding affinity for cells than do the HDL-[3H]CE from which they were formed, especially for the CHO-K1 cells.
Inhibition of HDL-[3H]CE and CERM-[3H]CE Uptake by RAP and Heparin
The resistance of CERM-CE uptake to inhibition by HDL and especially LDL indicated that receptors in addition to LDL-R and SB-BI were involved in CE uptake from CERM. RAP (13-15) and heparin (13;16) inhibit lipid uptake mediated by the LRP receptor and by heparan sulfate proteoglycans (HSPG) respectively. Thus, we tested the effects of RAP and heparin on HDL-[3H]CE ± SOF uptake by Huh7 cells. Addition of RAP to cell media just prior to the start of the uptake assay had no effect on [3H]CE uptake from HDL-[3H]CE (Figure 5 A) but produced a dose-dependent reduction in [3H]CE uptake from rSOF-treated HDL-[3H]CE. The effects of heparin alone or in combination with RAP were also tested (Figure 5 B). Heparin alone did not significantly affect uptake from HDL-[3H]CE. However the combination of 100 U/mL heparin and 20 μg/mL RAP reduced [3H]CE uptake from HDL-[3H]CE by 22% (p= 0.018). Heparin alone reduced CE uptake from CERM-[3H]CE (28% inhibition at 100 U/mL, p=0.006), and the combination of 100 U/mL heparin and 20 μg/mL RAP produced a 32% inhibition (p=0.001), similar to RAP alone (36% inhibition, p=0.004). To compare RAP and heparin inhibition of uptake from HDL-[3H]CE and CERM-[3H]CE we calculated the ratios of the percent activity in the presence of the inhibitors vs. control (Figure 5 B, values above bars). These values show the greatest difference for RAP (0.58) and RAP plus high dose heparin (0.84). Thus both LRP and cell surface HSPG promote CE uptake from CERM.
Figure 5.
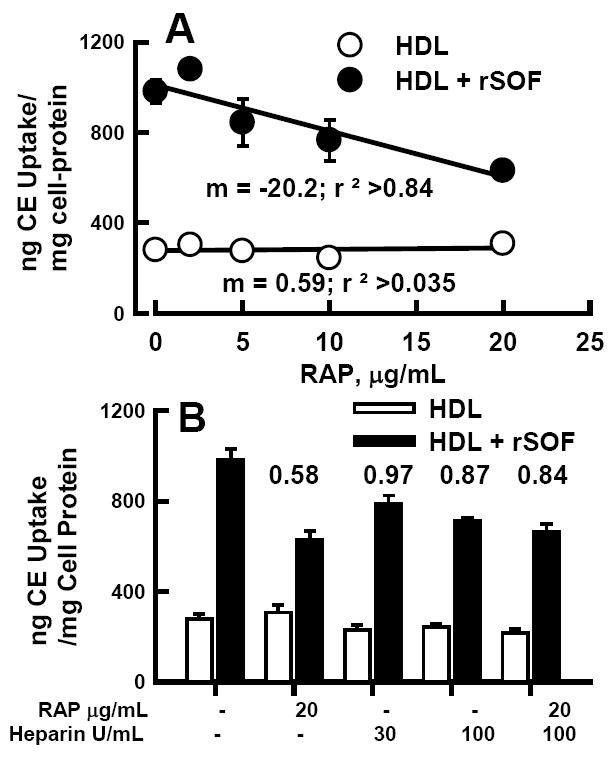
RAP and heparin inhibition of HDL-[3H]CE ± SOF uptake by Huh7 cells. A. Dose-dependent effect of RAP on [3H]CE uptake from HDL-[3H]CE (○) and HDL-[3H]CE pretreated with rSOF to form CERM-[3H]CE (●). B. Effects of heparin and RAP on [3H]CE uptake from HDL-[3H]CE (open bars) and CERM-[3H]CE (black bars). Numbers above bars are the ratio of (%CERM-CE uptake/%HDL-CE uptake) in the presence of the various inhibitors.
Statins upregulate LDL-R expression in hepatocytes cells (17;18). We tested the effect of simvastatin (0.01 – 1.0 μM) pretreatment of CE uptake by Huh7 cells and found only a slight effect, with CE uptake increased by 10 – 20% for HDL-[3H]CE and CERM-[3H]CE (data not shown).
Hepatic Uptake of Bodipy-CE
The subcellular localization of a fluorescent CE analog, Bodipy-CE, after uptake from CERM, was compared to subcellular localization after uptake from HDL or LDL by confocal fluorescence microscopy. Huh7 cells were incubated with Bodipy-CE-labeled CERM, HDL or LDL (Figure 6, green fluorescence). Cell lysosomes were visualized with LysoTracker Red (red fluorescence) and nuclei with Hoechst stain (blue fluorescence). Numerous perinuclear Bodipy-CE positive vesicles were seen after a 90 min incubation of cells with Bodipy-CE-labeled HDL (Figure 6, top green panel). Lysosomes were primarily perinuclear (Figure 6, top red panel), and merged images showed that a portion of the Bodipy-CE label co-localized with lysosomes (Figure 6, top left panel, yellow vesicles in the merged image). Thus by 90 min, some of the Bodipy-CE taken by up cells from HDL-Bodipy-CE had reached the lysosomes. rSOF treatment of HDL-Bodipy-CE formed CERM-Bodipy-CE. Incubation of cells with CERM-Bodipy-CE gave a similar pattern for Bodipy-CE fluorescence, a portion of which also co-localized with LysoTracker Red (Figure 6, middle panels). Uptake of LDL-Bodipy-CE showed numerous bright Bodipy-CE positive spherical bodies and some overlap with lysosomes (Figure 6, bottom panels). Many of these spherical bodies co-localize with phase dense lipid droplets in Huh7 cells (Supplementary Figure 1). Thus confocal microscopic imaging indicates vesicular CERM-CE uptake by hepatocytes and at least a portion of the CE traffics to lysosomes, as occurs for Bodipy-CE uptake from HDL and LDL.
Figure 6.
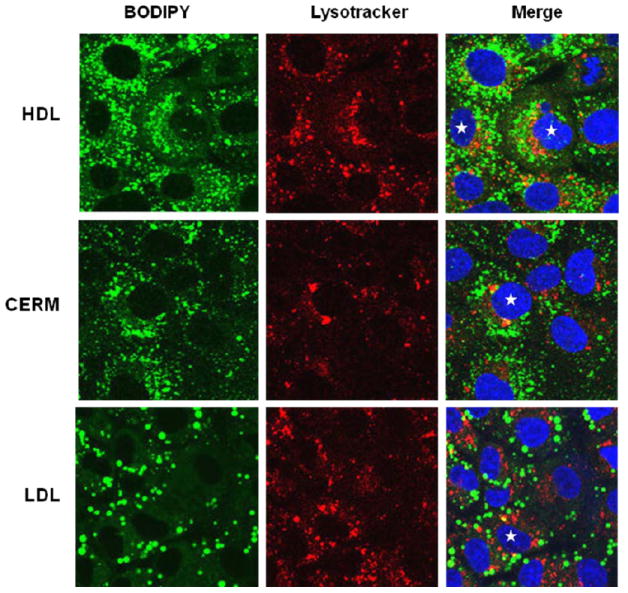
Confocal fluorescence microscopy of the accumulation of Bodipy CE in Huh7 cells incubated with Bodipy-CE labeled HDL, CERM and LDL (Left panels, green). Cells were also stained with LysoTracker Red to label lysosomes (Middle panels, red) and with Hoeschst (blue) to label nuclei. The merged images (Right panels) show distinct green labeling of intracellular vesicles, as well as some co-localization with lysomes (yellow) as seen especially in the cells noted with the stars.
DISCUSSION
SOF is produced by Streptococcus pyogenes, a human pathogen that causes a variety of diseases ranging from infections of the pharynx and skin to highly invasive infections that have high degrees of morbidity and mortality (1). This raises an interesting question of whether there is a reduction in plasma cholesterol during an infection by S. pyogenes. However, we do not know of any study in which cholesterol levels have been measured in patients in conjunction with S. pyogenes infection. This is not meant to suggest that an infection by S. pyogenes would be beneficial as the consequences of infection can be too severe. However, our recent studies support the concept that purified rSOF may have a beneficial effect of lowering plasma cholesterol in vivo (19) without the ramifications of an infection by a highly virulent organism. Towards establishing the efficacy of an rSOF based therapy to reduce plasma cholesterol, we report here that rSOF enhances hepatocyte uptake of HDL cholesterol and elsewhere (20) that rSOF treatment of HDL increases macrophage cholesterol efflux and reduces macrophage inflammatory response.
The data of Figure 1 show that rSOF acts on HDL in a mixture of total plasma lipoproteins and in whole plasma, as previously reported for HDL alone (4), to form an apo E containing-CERM that contains small amounts of an apo E heterodimer with apo A-II. These data suggest that conclusions based on the effects of rSOF treatment of HDL would be applicable to the more complex setting of whole plasma, perhaps even in vivo. Treatment of [3H]CE-labeled HDL with rSOF transfers nearly all of the [3H]CE to CERM (Figure 2) so that uptake of [3H]CE from labeled HDL after rSOF incubation is essentially CERM-[3H]CE uptake as discussed below.
Our data demonstrate that hepatocyte uptake of CE from CERM occurs faster than from the parent HDL and is inhibited by both HDL and LDL suggesting that both SR-BI and LDL-R mediate CERM-CE as well as HDL-CE uptake. However, inhibition of CERM-CE uptake is less profound than that of HDL-CE uptake indicating that CERM has a higher affinity interaction with these cell surface receptors. To demonstrate that LDL-R and SR-BI receptors are involved in CERM-CE uptake, we utilized CHO cells expressing various lipoprotein receptors. Ablation of LDL-R in CHO-ldlA7 cells compared to wild type CHO-K1 decreases the rate of CE uptake from both HDL and CERM, with a larger 66% reduction in Vmax of CERM-CE than HDL-CE uptake (Figure 4 A vs. B and D vs. E, Tables 1 and 2). Loss of the LDL-R does not affect the Cateff for HDL-CE but decreases that for CERM-CE uptake by half. Thus, LDL-R is a major receptor for CERM in these cells, but not the only one. A similar comparison of CE uptake by CHO-ldlA7 versus CHO-SR-BI cells shows that the SR-BI receptor mediates CE uptake from both HDL and CERM with essentially equal efficiency (Figure 4 B vs. C and E vs. F, Tables 1 and 2).
These data are complemented by studies of CE uptake inhibited with excess unlabeled HDL and LDL. As with hepatocyte uptake, CHO cell uptake of CERM-CE was more resistant to inhibition by HDL and LDL than was HDL-CE uptake. Even for the CHO-SR-BI cells, which showed similar rates of CE uptake from CERM and HDL (Figure 4 C), the uptake inhibition data (Figure 4 I and L) show that CERM has a higher affinity for SR-BI than does HDL. The most profound differences in HDL and LDL inhibition of CE uptake were seen in the CHO-K1 cells (Figure 4 G and J). While HDL-CE uptake was robustly inhibited by both HDL and LDL, neither lipoprotein inhibited CERM-CE uptake. This implicates other receptors in CERM-CE uptake.
In addition to LDL-R, apo E is a ligand for LRP-1 and cell surface heparan sulfate proteoglycans (7). Given the high apo E content of CERM, we evaluated the role of these receptors in CERM-CE uptake using their respective inhibitors, RAP and heparin (Figure 5) and found that both receptors promote hepatocyte CE uptake from CERM but not from HDL. Thus, hepatocyte uptake of CE from CERM occurs by interaction with the apoE receptors LDL-R and LRP-1, and is facilitated by binding to cell surface heparan sulfate proteoglycans, as well as via the HDL receptor SR-BI. As shown in Figure 6, CERM-CE is taken up and internalized by hepatocytes with a subcellular distribution similar to that for CE uptake from HDL and LDL. Importantly, CERM-CE traffics to lysosomes where it can be catabolized.
In summary, the uptake data and inhibition studies indicate that multiple apo E-dependent receptors are involved in the uptake of CERM-CE and that hepatocyte CERM-CE uptake is more robust than that of HDL-CE. This effect is likely mediated by apo E and not the apo E heterodimer with apo A-II because of the lower abundance of the latter in the CERM and the lower affinity of heterodimer for the LDL-R (21). Comparative affinities for LRP-1 and proteoglycans are not known.
Figure 7 is a model of how the rSOF reaction could improve RCT. rSOF activity converts HDL to neo HDL, lipid-free apo A-I, and CERM (2;4). With its low cholesterol content the neo HDL could remove additional cholesterol from macrophages, with a higher activity than the parent HDL from which neo HDL was formed (20), after which lecithin:cholesterol, acyltransferase activity (LCAT) esterifies and remodels the particle to its more mature form (22), which could enter another cycle of the rSOF reaction. Lipid-free apo A-I could interact with ABCA1 producing additional early forms of HDL that also mature to rSOF targets, although excess lipid-free apo A-I is removed by the kidney cubilin receptor (23). However, the key step for rSOF-driven RCT is the formation of CERM with apo E and the clearance of cholesterol via multiple hepatic apo E-dependent receptors in addition to the SR-BI receptor, a process that gives directionality to the overall rSOF reaction as an agent of enhanced RCT.
Figure 7.
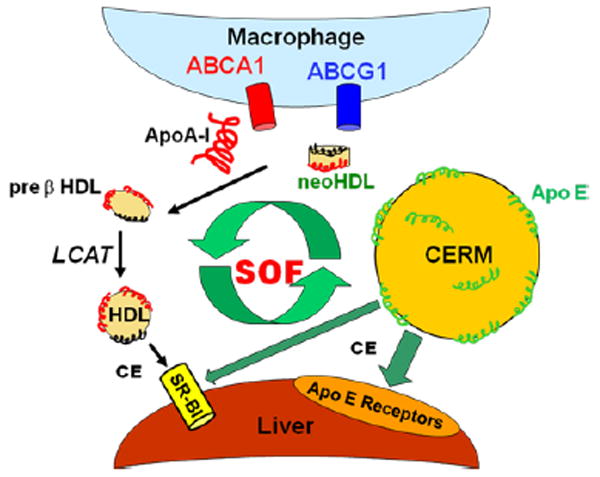
Model for promotion of RCT by the action of rSOF. rSOF activity transfers CE from HDL to form CERM, a cholesterol ester rich micoemulsion with surface apo E. CERM CE is taken up by liver cells via various apo E receptors, including LDL-R, LRP and HSPG as well as the HDL receptor SR-BI. rSOF activity also produces lipid-free apo A-I and neo HDL, both of which can remove additional cholesterol from macrophages to form pre-β HDL. After LCAT esterification and remodeling, the mature HDL can enter another cycle of the SOF reaction.
Mechanisms that enhance RCT are thought to be anti atherogenic (24). The conventional view is that high plasma levels of HDL-C correlate with efficient RCT. On the other hand, other evidence suggests that future therapies might better focus on enhancing RCT irrespective of the effects on HDL-C. Several observations support this view. First, a common CETP gene mutation that lowers plasma CETP activity is associated with high HDL-C and possibly with increased coronary heart disease in men with hypertriglyceridemia (25;26). Second, in murine models of atherosclerosis, hepatic overexpression of SR-BI, the HDL receptor that “pulls” cholesterol out of extrahepatic spaces, decreases plasma HDL-C (27-29), and increases HDL-CE clearance (28-30), biliary cholesterol, and its transport into bile (27;29;31), and reduces atherosclerosis (32-34). Conversely, ablated or attenuated hepatic SR-BI expression elevates plasma HDL-C, reduces selective HDL-CE clearance and is atherogenic (35-37). Thus, enhancement of the final RCT step, hepatic removal of lipoprotein-cholesterol and its conversion to bile salts that are excreted, is likely to have therapeutic value.
Like the genetic changes that lower HDL-C but decrease atherogenesis, our studies show that rSOF could potentially increase transfer of cholesterol to the liver via apo E dependent receptors even while likely reducing plasma HDL-C levels. Indeed, recent studies have shown that injection of rSOF in mice rapidly and profoundly reduces plasma cholesterol levels via hepatic uptake (19)so we are interested in identifying the mechanisms and receptors that mediate this process in vivo. Studies of cholesterol transfer between cells and rHDL containing various amounts of cholesterol have shown that above ~15 mol%, rHDL is a cholesterol donor rather than acceptor (38). Given that cholesterol constitutes ~14 mol% of the surface lipid of mature HDL (39), there is likely little reserve for accepting more cholesterol from cells, and thus for increasing the amount of RCT mediated directly by mature plasma HDL. Rather, transfer of the HDL-CE by action of rSOF to CERM, a new lipid particle that contains apo E, might enhance hepatocyte CE clearance and thus RCT. This activity is similar to that of CETP, which exchanges HDL-CE for TG of triglyceride-rich lipoproteins, with subsequent clearance by liver receptors (40-42). Our future studies of subcellular trafficking and metabolism of CERM-CE will determine whether the higher cholesterol uptake from CERM is followed by higher rates of cholesterol conversion to bile salts and their excretion. If successful, these studies would provide a compelling rationale for pursuing other tests of the rSOF reaction as a modality for promoting RCT and reversing atherosclerosis in mouse models of dyslipidemic atherosclerosis.
Supplementary Material
Acknowledgments
We thank Professor Robert Raphael, PhD, Department of Bioengineering, Rice University, for use of the LSM 510 confocal fluorescence microscope. This work was supported by grants-in-aid from the National Institutes of Health HL-30914 and HL-56865 (HJP) and the Department of Veterans Affairs (HSC).
Abbreviations
- ABCA1
ATP-binding cassette transporter A1
- ABCG1
ATP-binding cassette transporter G1
- apo
apolipoprotein
- Bodipy-CE
Cholesteryl 4,4-difluoro-5,7-dimethyl-4-bora-3α,4α-diaza-s-indacene-3-dodecanoate
- BSA
bovine serum albumin
- C
cholesterol
- CE
cholesteryl ester
- CERM
cholesteryl ester-rich microemulsion
- CETP
cholesteryl ester transfer protein
- CHO
Chinese hamster ovary
- CR
chylomicron remnants
- FBS
fetal bovine serum
- HBSS
Hank’s balanced salt solution
- HDL
high density lipoproteins
- HSPG
heparan sulfate proteoglycans
- LDL
low density lipoproteins
- LDL-R
low density lipoprotein receptor
- LF
lipid-free
- LRP
low density lipoprotein receptor-related protein
- MEM
minimum essential medium
- rSOF
recombinant serum opacity factor
- SEC
size exclusion chromatography
- SR-BI
scavenger receptor class B type I
- RAP
receptor associated protein
- RCT
reverse cholesterol transport
- TBS
Tris-buffered saline
- TLP
total lipoproteins
Footnotes
This work was supported by grants-in-aid from the National Institutes of Health (HL-30914 and HL-56865 to H.J.P.) and the Department of Veterans Affairs (HSC).
SUPPORTING INFORMATION AVAILABLE
Supplemental Figure 1. LDL-Bodipy-CE uptake labels phase dense lipid droplets of Huh7 cells. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Courtney HS, Pownall HJ. The structure and function of serum opacity factor: a unique streptococcal virulence determinant that targets high-density lipoproteins. J Biomed Biotechnol. 2010;2010:956071. doi: 10.1155/2010/956071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courtney HS, Zhang YM, Frank MW, Rock CO. Serum opacity factor, a streptococcal virulence factor that binds to apolipoproteins A-I and A-II and disrupts high density lipoprotein structure. J Biol Chem. 2006;281:5515–5521. doi: 10.1074/jbc.M512538200. [DOI] [PubMed] [Google Scholar]
- 4.Gillard BK, Courtney HS, Massey JB, Pownall HJ. Serum opacity factor unmasks human plasma high-density lipoprotein instability via selective delipidation and apolipoprotein A-I desorption. Biochemistry. 2007;46:12968–12978. doi: 10.1021/bi701525w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han M, Gillard BK, Courtney HS, Ward K, Rosales C, Khant H, Ludtke SJ, Pownall HJ. Disruption of human plasma high-density lipoproteins by streptococcal serum opacity factor requires labile apolipoprotein A-I. Biochemistry. 2009;48:1481–1487. doi: 10.1021/bi802287q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courtney HS, Hasty DL, Li Y, Chiang HC, Thacker JL, Dale JB. Serum opacity factor is a major fibronectin-binding protein and a virulence determinant of M type 2 Streptococcus pyogenes. Mol Microbiol. 1999;32:89–98. doi: 10.1046/j.1365-2958.1999.01328.x. [DOI] [PubMed] [Google Scholar]
- 7.Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40:1–16. [PubMed] [Google Scholar]
- 8.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 9.Krieger M. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J Clin Invest. 2001;108:793–797. doi: 10.1172/JCI14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieland TJ, Penman M, Dori L, Krieger M, Kirchhausen T. Discovery of chemical inhibitors of the selective transfer of lipids mediated by the HDL receptor SR-BI. Proc Natl Acad Sci U S A. 2002;99:15422–15427. doi: 10.1073/pnas.222421399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, Yoffe B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 2002;36:592–601. doi: 10.1053/jhep.2002.35441. [DOI] [PubMed] [Google Scholar]
- 12.Gillard BK, Lin HY, Massey JB, Pownall HJ. Apolipoproteins A-I, A-II and E are independently distributed among intracellular and newly secreted HDL of human hepatoma cells. Biochim Biophys Acta. 2009;1791:1125–1132. doi: 10.1016/j.bbalip.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gauthier A, Lau P, Zha X, Milne R, McPherson R. Cholesteryl ester transfer protein directly mediates selective uptake of high density lipoprotein cholesteryl esters by the liver. Arterioscler Thromb Vasc Biol. 2005;25:2177–2184. doi: 10.1161/01.ATV.0000183613.13929.13. [DOI] [PubMed] [Google Scholar]
- 14.Jensen JK, Dolmer K, Schar C, Gettins PG. Receptor-associated protein (RAP) has two high-affinity binding sites for the low-density lipoprotein receptor-related protein (LRP): consequences for the chaperone functions of RAP. Biochem J. 2009;421:273–282. doi: 10.1042/BJ20090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vassiliou G, Benoist F, Lau P, Kavaslar GN, McPherson R. The low density lipoprotein receptor-related protein contributes to selective uptake of high density lipoprotein cholesteryl esters by SW872 liposarcoma cells and primary human adipocytes. J Biol Chem. 2001;276:48823–48830. doi: 10.1074/jbc.M103954200. [DOI] [PubMed] [Google Scholar]
- 16.Huff MW, Miller DB, Wolfe BM, Connelly PW, Sawyez CG. Uptake of hypertriglyceridemic very low density lipoproteins and their remnants by HepG2 cells: the role of lipoprotein lipase, hepatic triglyceride lipase, and cell surface proteoglycans. J Lipid Res. 1997;38:1318–1333. [PubMed] [Google Scholar]
- 17.Scharnagl H, Schinker R, Gierens H, Nauck M, Wieland H, Marz W. Effect of atorvastatin, simvastatin, and lovastatin on the metabolism of cholesterol and triacylglycerides in HepG2 cells. Biochem Pharmacol. 2001;62:1545–1555. doi: 10.1016/s0006-2952(01)00790-0. [DOI] [PubMed] [Google Scholar]
- 18.Mullen PJ, Luscher B, Scharnagl H, Krahenbuhl S, Brecht K. Effect of simvastatin on cholesterol metabolism in C2C12 myotubes and HepG2 cells, and consequences for statin-induced myopathy. Biochem Pharmacol. 2010;79:1200–1209. doi: 10.1016/j.bcp.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Rosales C, Tang D, Gillard BK, Courtney HS, Pownall HJ. Low-dose Streptococcal serum opacity factor reduces plasma cholesterol in vivo. American Heart Association 11th Annual Conference on Arteriosclerosis, Thrombosis and Vascular Biology 2010. 2010:357. doi: 10.1161/ATVBAHA.111.224360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tchoua U, Rosales C, Tang D, Gillard BK, Vaughan A, Lin HY, Courtney HS, Pownall HJ. Serum opacity factor enhances HDL-mediated cholesterol efflux, esterification and anti-inflammatory effects. Lipids. 2010 doi: 10.1007/s11745-010-3484-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Innerarity TL, Mahley RW, Weisgraber KH, Bersot TP. Apoprotein (E--A-II) complex of human plasma lipoproteins. II. Receptor binding activity of a high density lipoprotein subfraction modulated by the apo(E--A-II) complex. J Biol Chem. 1978;253:6289–6295. [PubMed] [Google Scholar]
- 22.Clay MA, Pyle DH, Rye KA, Barter PJ. Formation of spherical, reconstituted high density lipoproteins containing both apolipoproteins A-I and A-II is mediated by lecithin:cholesterol acyltransferase. J Biol Chem. 2000;275:9019–9025. doi: 10.1074/jbc.275.12.9019. [DOI] [PubMed] [Google Scholar]
- 23.Hammad SM, Stefansson S, Twal WO, Drake CJ, Fleming P, Remaley A, Brewer HB, Jr, Argraves WS. Cubilin, the endocytic receptor for intrinsic factor-vitamin B(12) complex, mediates high-density lipoprotein holoparticle endocytosis. Proc Natl Acad Sci U S A. 1999;96:10158–10163. doi: 10.1073/pnas.96.18.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–2555. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 25.Zhong S, Sharp DS, Grove JS, Bruce C, Yano K, Curb JD, Tall AR. Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J Clin Invest. 1996;97:2917–2923. doi: 10.1172/JCI118751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruce C, Sharp DS, Tall AR. Relationship of HDL and coronary heart disease to a common amino acid polymorphism in the cholesteryl ester transfer protein in men with and without hypertriglyceridemia. J Lipid Res. 1998;39:1071–1078. [PubMed] [Google Scholar]
- 27.Kozarsky KF, Donahee MH, Rigotti A, Iqbal SN, Edelman ER, Krieger M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 1997;387:414–417. doi: 10.1038/387414a0. [DOI] [PubMed] [Google Scholar]
- 28.Wang N, Arai T, Ji Y, Rinninger F, Tall AR. Liver-specific overexpression of scavenger receptor BI decreases levels of very low density lipoprotein ApoB, low density lipoprotein ApoB, and high density lipoprotein in transgenic mice. J Biol Chem. 1998;273:32920–32926. doi: 10.1074/jbc.273.49.32920. [DOI] [PubMed] [Google Scholar]
- 29.Ueda Y, Royer L, Gong E, Zhang J, Cooper PN, Francone O, Rubin EM. Lower plasma levels and accelerated clearance of high density lipoprotein (HDL) and non-HDL cholesterol in scavenger receptor class B type I transgenic mice. J Biol Chem. 1999;274:7165–7171. doi: 10.1074/jbc.274.11.7165. [DOI] [PubMed] [Google Scholar]
- 30.Ji Y, Wang N, Ramakrishnan R, Sehayek E, Huszar D, Breslow JL, Tall AR. Hepatic scavenger receptor BI promotes rapid clearance of high density lipoprotein free cholesterol and its transport into bile. J Biol Chem. 1999;274:33398–33402. doi: 10.1074/jbc.274.47.33398. [DOI] [PubMed] [Google Scholar]
- 31.Sehayek E, Ono JG, Shefer S, Nguyen LB, Wang N, Batta AK, Salen G, Smith JD, Tall AR, Breslow JL. Biliary cholesterol excretion: a novel mechanism that regulates dietary cholesterol absorption. Proc Natl Acad Sci U S A. 1998;95:10194–10199. doi: 10.1073/pnas.95.17.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arai T, Wang N, Bezouevski M, Welch C, Tall AR. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor BI transgene. J Biol Chem. 1999;274:2366–2371. doi: 10.1074/jbc.274.4.2366. [DOI] [PubMed] [Google Scholar]
- 33.Ueda Y, Gong E, Royer L, Cooper PN, Francone OL, Rubin EM. Relationship between expression levels and atherogenesis in scavenger receptor class B, type I transgenics. J Biol Chem. 2000;275:20368–20373. doi: 10.1074/jbc.M000730200. [DOI] [PubMed] [Google Scholar]
- 34.Kozarsky KF, Donahee MH, Glick JM, Krieger M, Rader DJ. Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler Thromb Vasc Biol. 2000;20:721–727. doi: 10.1161/01.atv.20.3.721. [DOI] [PubMed] [Google Scholar]
- 35.Varban ML, Rinninger F, Wang N, Fairchild-Huntress V, Dunmore JH, Fang Q, Gosselin ML, Dixon KL, Deeds JD, Acton SL, Tall AR, Huszar D. Targeted mutation reveals a central role for SR-BI in hepatic selective uptake of high density lipoprotein cholesterol. Proc Natl Acad Sci U S A. 1998;95:4619–4624. doi: 10.1073/pnas.95.8.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Covey SD, Krieger M, Wang W, Penman M, Trigatti BL. Scavenger receptor class B type I-mediated protection against atherosclerosis in LDL receptor-negative mice involves its expression in bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2003;23:1589–1594. doi: 10.1161/01.ATV.0000083343.19940.A0. [DOI] [PubMed] [Google Scholar]
- 37.Huszar D, Varban ML, Rinninger F, Feeley R, Arai T, Fairchild-Huntress V, Donovan MJ, Tall AR. Increased LDL cholesterol and atherosclerosis in LDL receptor-deficient mice with attenuated expression of scavenger receptor B1. Arterioscler Thromb Vasc Biol. 2000;20:1068–1073. doi: 10.1161/01.atv.20.4.1068. [DOI] [PubMed] [Google Scholar]
- 38.Picardo M, Massey JB, Kuhn DE, Gotto AM, Jr, Gianturco SH, Pownall HJ. Partially reassembled high density lipoproteins. Effects on cholesterol flux, synthesis, and esterification in normal human skin fibroblasts. Arteriosclerosis. 1986;6:434–441. doi: 10.1161/01.atv.6.4.434. [DOI] [PubMed] [Google Scholar]
- 39.Havel RJ, Goldstein JL, Brown MS. Lipoproteins and Lipid Transport. In: Bondy PK, Rosenberg LE, editors. The Metabolic Control of Disease. Saunders Publishing; Philadelphia: 1980. pp. 393–494. [Google Scholar]
- 40.Morton RE, Zilversmit DB. Purification and characterization of lipid transfer protein(s) from human lipoprotein-deficient plasma. J Lipid Res. 1982;23:1058–1067. [PubMed] [Google Scholar]
- 41.Hesler CB, Swenson TL, Tall AR. Purification and characterization of a human plasma cholesteryl ester transfer protein. J Biol Chem. 1987;262:2275–2282. [PubMed] [Google Scholar]
- 42.Asztalos BF, Horvath KV, Kajinami K, Nartsupha C, Cox CE, Batista M, Schaefer EJ, Inazu A, Mabuchi H. Apolipoprotein composition of HDL in cholesteryl ester transfer protein deficiency. J Lipid Res. 2004;45:448–455. doi: 10.1194/jlr.M300198-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


