Abstract
The discovery of infectious proteins, denoted prions, was unexpected. After much debate over the chemical basis of heredity, resolution of this issue began with the discovery that DNA, not protein, from pneumococcus was capable of genetically transforming bacteria (Avery et al. 1944). Four decades later, the discovery that a protein could mimic viral and bacterial pathogens with respect to the transmission of some nervous system diseases (Prusiner 1982) met with great resistance. Overwhelming evidence now shows that Creutzfeldt–Jakob disease (CJD) and related disorders are caused by prions. The prion diseases are characterized by neurodegeneration and lethality. In mammals, prions reproduce by recruiting the normal, cellular isoform of the prion protein (PrPC) and stimulating its conversion into the disease-causing isoform (PrPSc). PrPC and PrPSc have distinct conformations: PrPC is rich in α-helical content and has little β-sheet structure, whereas PrPSc has less α-helical content and is rich in β-sheet structure (Pan et al. 1993). The conformational conversion of PrPC to PrPSc is the fundamental event underlying prion diseases. In this article, we provide an introduction to prions and the diseases they cause.
Prions are aberrantly folded proteins that self-propagate through recruitment of their normally folded counterparts. The conformational conversion of cellular PrPC to infectious PrPSc underlies prion diseases.
PRION PROTEIN ISOFORMS
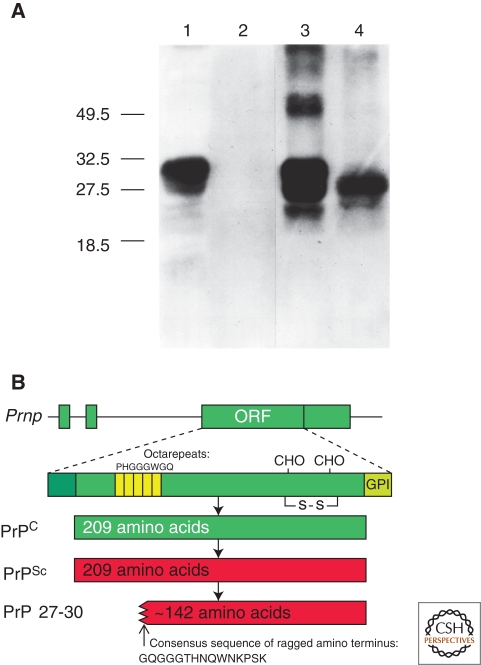
PrPSc, an alternative or abnormal isoform of PrP, stimulates the conversion of PrPC into nascent PrPSc; in the brain, accumulation of PrPSc causes neurodegeneration. In Syrian hamsters, PrPC and PrPSc are both 209-residue proteins with two glycosylation sites and a glycosylphosphatidyl inositol (GPI) anchor (Fig. 1). PrP is posttranslationally processed to remove a 22-amino-acid, amino-terminal signal peptide and a 23-amino-acid, carboxy-terminal peptide, which directs addition of the GPI anchor that tethers the protein to the cell membrane. No posttranslational modifications to the primary structure differentiate PrPC from PrPSc (Stahl et al. 1993). Limited protease digestion of PrPSc often produces a smaller, protease-resistant molecule of approximately 142 amino acids, referred to as PrP 27–30 (Fig. 1). Under the same conditions, PrPC and some forms of PrPSc are completely hydrolyzed. Although resistance to limited proteolysis has proved to be a convenient tool for detecting PrPSc, not all PrPSc molecules are resistant to protease digestion (Hsiao et al. 1994; Telling et al. 1996; Safar et al. 1998; Gambetti et al. 2008; Colby et al. 2010); these protease-sensitive PrPSc forms are denoted sPrPSc. Furthermore, PrPSc from different species or prion strains may show different degrees of protease resistance.
Figure 1.
Prion protein isoforms. (A) Western immunoblot of brain homogenates from uninfected (lanes 1 and 2) and prion-infected (lanes 3 and 4) Syrian hamsters. Samples in lanes 2 and 4 were digested with 50 µg/µl proteinase K for 30 min at 37°C, completely hydrolyzing PrPC. Proteinase digestion cleaves ∼67 amino acids from the amino terminus of PrPSc to generate PrP 27–30 (lane 4). Blot developed with anti-PrP polyclonal antiserum R073 (Serban et al. 1990). (B) Bar diagrams of the hamster Prnp gene and PrP isoforms. The Prnp ORF encodes a protein of 254 residues, which is shortened to 209 residues during posttranslational processing. PrPSc is an alternate conformation of PrPC with identical primary structure. Limited proteolysis of PrPSc cleaves the amino terminus and produces PrP 27-30, composed of approximately 142 residues. Panel A, reprinted with permission, from Prusiner 2004.
In the presence of detergent, PrP 27–30 polymerizes into amyloid (McKinley et al. 1991). The tendency of prions to form amyloids has also provided a useful means of prion detection (Colby et al. 2007); however, amyloid formation is a nonobligatory feature of prion disease (Wille et al. 2000). Prion rods formed by limited proteolysis and detergent extraction are indistinguishable from the filaments that aggregate to form PrP amyloid plaques in the CNS (DeArmond et al. 1985). Both the rods and the PrP amyloid filaments found in brain tissue show similar ultrastructural morphology and green-gold birefringence after staining with Congo red dye (Prusiner et al. 1983).
As in mammals, proteins with self-propagating conformations have been found in fungi; these fungal prions share many similarities with mammalian prions (Chien et al. 2004). Because of the ease of genetic manipulation and fast growth rates of fungi, fungal prion research has progressed at a rapid pace, often presaging discoveries in mammalian prion research. In yeast, alternative conformational states of the Ure2p and Sup35 proteins encipher the [URE3] and [PSI] phenotypes (Wickner 1994; Patino et al. 1996), respectively, whereas the Het-s protein enciphers the [HET-s] phenotype in Podospora anserine (Coustou et al. 1997). However, it is important to note that there are also many differences between yeast and mammalian prions—for example, yeast prions do not cause disease nor do they transmit from one mature cell to another.
THE PrP GENE
A chromosomal gene encodes PrP and is denoted Prnp, which is a member of the Prn gene family that also includes Prnd, encoding the doppel protein (Moore et al. 1999), and Sprn, encoding shadoo (Watts and Westaway 2007). In all known PrP genes from various species, the PrP open reading frame (ORF) is encoded within a single exon although the gene itself comprises two to three exons (Basler et al. 1986; Westaway et al. 1987; Hsiao et al. 1989; Gabriel et al. 1992). The other exons contain untranslated sequences including the promoter and termination sites. The PrP promoter contains multiple copies of GC-rich repeats—a canonical binding site for the transcription factor Sp1 (McKnight and Tjian 1986), driving expression in many different tissues.
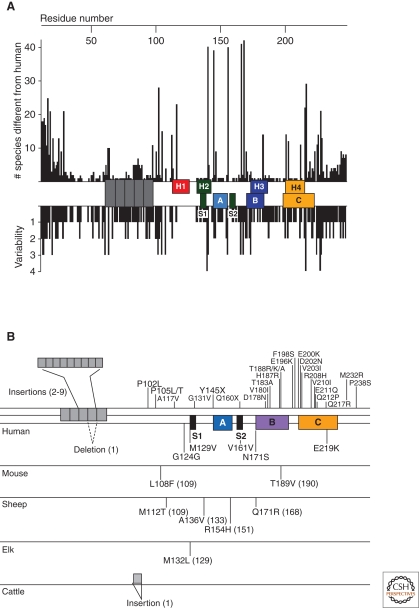
The alignment of the translated sequences from more than 40 PrP genes shows a striking degree of conservation between the mammalian sequences, suggesting the retention of some important function for PrP through evolution. However, variations in PrP sequences exist both between species and between individuals within species (Fig. 2), greatly affecting susceptibility to prion infection.
Figure 2.
Variation of in the prion protein gene. (A) Species variations of the prion protein gene. The x-axis represents the human PrP sequence, with the five octarepeats and H1–H4 regions of the putative secondary structure shown, as well as the three α-helices A, B, and C and the two β-strands S1 and S2 as determined by NMR. Vertical bars above the axis indicate the number of species that differ from the human sequence at each position. Below the axis, the length of the bars indicates the number of alternative amino acids at each position in the alignment. (B) PrP mutations causing inherited human prion disease (above the line) and PrP polymorphisms (below the line) found in humans, mice, sheep, elk, and cattle. Residue numbers in parentheses correspond to the human codons. Data in Panel A compiled by P. Bamborough and F.E. Cohen and reprinted, with permission, from Prusiner 2004.
The shortest incubation times, or the interval between inoculation and clinical signs of disease, are achieved with intracerebral inoculation of prions with a sequence identical to that of the host animal; under these conditions, all animals develop prion disease within a narrow interval for a particular dose. When the donor prion originates from a species different from the host animal, and thus, the sequences differ between infecting PrPSc and host PrPC, the incubation time can be prolonged and vary substantially between individual animals inoculated; often, many of the inoculated animals do not develop disease (Carlson et al. 1989; Telling et al. 1994; Telling et al. 1995; Tateishi et al. 1996). This phenomenon is referred to as the species barrier that was first noted by Ian Pattison (Pattison 1965).
HUMAN PRION DISEASES
Prion diseases occur as sporadic, genetic, and transmissible disease in humans (Table 1). Although infectious forms of prion disease are most well known to the general public, sporadic and heritable forms of the disease occur much more frequently in humans, with sporadic (s) CJD accounting for approximately 85% of cases. sCJD has no known cause although spontaneous misfolding of PrPC into PrPSc is a leading hypothesis (Prusiner 1989; Hsiao et al. 1991a). Alternate hypotheses include somatic mutation of PRNP, undetected horizontal transmission (Gajdusek 1977), and infrequent amplification of low levels of PrPSc that are part of “normal” protein homeostasis. The brains of sCJD patients harbor infectious prions that are transmissible to experimental animals (Gibbs et al. 1968; Brown et al. 1994). In humans, virtually all forms of prion disease feature neuropathological changes including vacuolation (resulting in the spongiform appearance of brain tissue), astrocytic gliosis, and PrP deposition. The morphology of vacuoles and PrP deposits varies depending on the prion strain and host, as do the regions of the brain affected.
Table 1.
Prion diseases in humans and animals.
| Disease | Host | Mechanism of pathogenesis |
|---|---|---|
| Kuru | humans (Fore people) | infection through ritualistic cannibalism |
| Iatrogenic CJD | humans | infection from prion-contaminated HGH, medical equipment, etc. |
| Variant CJD | humans | infection from bovine prions |
| Familial CJD | humans | germline mutations in the PRNP gene |
| GSS | humans | germline mutations in the PRNP gene |
| FFI | humans | germline mutations in the PRNP gene |
| Sporadic CJD | humans | somatic mutation or spontaneous conversion of PrPC to PrPSc |
| sFI | humans | somatic mutation or spontaneous conversion of PrPC to PrPSc |
| Scrapie | sheep | infection |
| BSE | cattle | infection or sporadic |
| TME | mink | infection with prions from sheep or cattle |
| CWD | deer, elk | infection |
| FSE | cats | infection with prion-contaminated bovine tissues or MBM |
| Exotic ungulate encephalopathy | greater kudu, nyala, oryx | infection with prion-contaminated MBM |
To date, over 40 different mutations of the PrP gene have been shown to segregate with the heritable human prion diseases (Fig. 2). The resulting diseases have been classified as Gerstmann–Sträussler–Scheinker syndrome (GSS), familial (f) CJD, or fatal familial insomnia (FFI) according to the clinical symptoms, although all result from PRNP mutations. At the time when the discoveries were reported that fCJD and GSS could be transmitted to apes and monkeys, many still thought that scrapie, CJD, and related disorders were caused by slow viruses (Roos et al. 1973; Masters et al. 1981). Only the discovery that a proline-to-leucine mutation at codon 102 of the human PrP gene was genetically linked to some GSS pedigrees permitted the unprecedented conclusion that prion disease can have both genetic and infectious etiologies (Hsiao et al. 1989; Prusiner 1989). This mutation has been found in unrelated families from several countries (Doh-ura et al. 1989; Goldgaber et al. 1989; Kretzschmar et al. 1991), and other mutations causing GSS have since been identified (Dlouhy et al. 1992; Petersen et al. 1992; Poulter et al. 1992; Rosenmann et al. 1998).
Likewise, several different mutations have also been discovered to cause fCJD. A repeat expansion in the amino-terminal region of PrP, which in the healthy population contains five repetitive sequences of eight residues each (octarepeats), has been genetically linked to fCJD. Insertions of two to nine additional octarepeats have been found in individuals within fCJD pedigrees (Owen et al. 1989; Goldfarb et al. 1991a). Molecular genetic investigations have revealed that Libyan and Tunisian Jews with fCJD have a PrP gene point mutation at codon 200, resulting in a glutamic acid-to-lysine substitution (Goldfarb et al. 1990a; Hsiao et al. 1991b), a mutation that has since been identified in fCJD pedigrees in many locations (Goldfarb et al. 1990a; Goldfarb et al. 1990b; Bertoni et al. 1992).
The D178N mutation can cause either fCJD or FFI, depending on the polymorphism present at codon 129, where both methionine and valine are commonly found. D178N coupled with V129 produces fCJD, in which patients present with dementia and widespread deposition of PrPSc (Goldfarb et al. 1991c). If the disease mutation is coupled with M129, however, FFI results and patients present with a progressive sleep disorder that is ultimately fatal. Postmortem analysis of FFI brains revealed deposition of PrPSc confined largely to specific regions of the thalamus (Lugaresi et al. 1986; Gambetti et al. 1995).
Infectious forms of prion diseases include kuru, iatrogenic (i) CJD, and variant (v) CJD. Kuru in the highlands of New Guinea was transmitted by ritualistic cannibalism, as people in the region ate the brains of their dead relatives in an attempt to immortalize them (Glasse 1967; Alpers 1968; Gajdusek 1977). Iatrogenic transmissions include prion-tainted human growth hormone and gonadotropin, dura mater grafts, and transplants of corneas obtained from people who died of CJD (Koch et al. 1985; PHS 1997). In addition, CJD cases have been recorded after neurosurgical procedures in which ineffectively sterilized depth electrodes or instruments were used.
More than 200 teenagers and young adults have died of vCJD, mostly in Britain (Spencer et al. 2002; Will 2003). Both epidemiologic and experimental studies have built a convincing case that vCJD resulted from prions being transmitted from cattle with bovine spongiform encephalopathy (BSE, or “mad cow” disease) to humans through consumption of contaminated beef products (Chazot et al. 1996; Will et al. 1996; Cousens et al. 1997). Until recently, all of the vCJD-affected individuals were identified to express methionine homozygously at codon 129. A single case of vCJD in a patient heterozygous at codon 129 has been reported, raising the possibility of a second wave of “mad cow”–related deaths (Kaski et al. 2009).
PRION DISEASES OF ANIMALS
Prion diseases occur naturally in many mammals, including scrapie of sheep and goats, BSE, transmissible mink encephalopathy (TME), chronic wasting disease (CWD) of mule deer and elk, feline spongiform encephalopathy, and exotic ungulate encephalopathy (Table 1). Unlike in humans, prion diseases in animals mainly occur as infectious disorders. As in humans, prion disease in animals is characterized by neuropathologic changes, including vacuolation, astrocytic gliosis, and PrP deposition.
Scrapie of sheep has been documented in Europe for hundreds of years. Despite efforts attempting to link scrapie to CJD, no evidence exists to establish a relationship (Chatelain et al. 1981). Polymorphisms in sheep PrP modulate susceptibility to scrapie, rendering some breeds more resistant to infection than others (Goldmann et al. 1991). As scrapie prions can persist in soil for years (Palsson 1979; Brown and Gajdusek 1991), selective breeding programs may be the most effective means to eradicate scrapie. In part because scrapie is not infectious for humans, hamster- and mouse-adapted scrapie strains, such as Sc237 and RML, are important laboratory tools for studying prions.
During the BSE epidemic in Britain, it was estimated that nearly one million cattle were infected with prions (Anderson et al. 1996; Nathanson et al. 1997). The mean incubation time for BSE is approximately 5 years. Most cattle were slaughtered between 2 and 3 years of age, and therefore, in a presymptomatic phase of infection (Stekel et al. 1996). BSE is a massive common-source epidemic caused by meat and bone meal (MBM) fed primarily to dairy cows (Wilesmith et al. 1991; Nathanson et al. 1997). MBM was prepared from the offal of sheep, cattle, pigs, and chickens as a high-protein nutritional supplement. In the late 1970s, the hydrocarbon-solvent extraction method used in the rendering of offal began to be abandoned, resulting in MBM with a much higher fat content (Wilesmith et al. 1991; Muller et al. 2007). It is now thought that this change allowed scrapie prions from sheep or low levels of bovine prions generated sporadically to survive the rendering process, resulting in the widespread infection of cattle. Changes in the methods used for feeding cattle have since eliminated the epidemic, although sporadic BSE cases arise occasionally.
Mule deer, white-tailed deer, and elk have been reported to develop CWD. As the only prion disease identified in free-ranging animals, CWD appears to be far more communicable than other forms of prion disease. CWD was first described in 1967 and was reported to be a spongiform encephalopathy in 1978 on the basis of histopathology of the brain. Originally detected in the American West, CWD has spread across much of North America and has been reported also in South Korea. In captive populations, up to 90% of mule deer have been reported to be positive for prions (Williams and Young 1980). The incidence of CWD in cervids living in the wild has been estimated to be as high as 15% (Miller et al. 2000). The development of transgenic (Tg) mice expressing cervid PrP, and thus susceptible to CWD, has enhanced detection of CWD and the estimation of prion titers (Browning et al. 2004; Tamgüney et al. 2006). Shedding of prions in the feces, even in presymptomatic deer, has been identified as a likely source of infection for these grazing animals (Williams and Miller 2002; Tamgüney et al. 2009b). CWD has been transmitted to cattle after intracerebral inoculation, although the infection rate was low (4 of 13 animals [Hamir et al. 2001]). This finding raised concerns that CWD prions might be transmitted to cattle grazing in contaminated pastures.
TRANSGENIC MICE
The development of various lines of Tg mice has provided valuable insight on these disorders. Altering the expression level of PrP in Tg mice can lead to abnormalities in uninfected mice and strongly affects incubation times in prion-infected mice. Tg mice expressing different levels of wild-type (wt) PrP of the Syrian hamster (SHa) sequence showed incubation times following prion inoculation that were inversely proportional to the level of PrP expression (Prusiner et al. 1990). Older, uninoculated mice expressing high levels of SHaPrP, ovine PrP, or mouse PrP(F108,V189) developed neurological dysfunction that was distinct from prion disease (Westaway et al. 1994).
Mice with the Prnp gene knocked out, termed Prnp0/0 mice, have also been created; ablation of Prnp does not affect normal development but renders mice resistant to prion disease (Büeler et al. 1992; Büeler et al. 1993; Prusiner et al. 1993; Manson et al. 1994). Altered synaptic behavior in the brains of Prnp0/0 mice was found in some studies (Collinge et al. 1994; Whittington et al. 1995) but not others (Herms et al. 1995; Lledo et al. 1996). Some early findings of dysfunction in Prnp0/0 mice were later attributed to abnormal expression of the doppel protein, which resulted from the technique used to ablate Prnp gene expression (Sakaguchi et al. 1996; Moore et al. 1999).
Tg mouse models of genetic forms of prion disease have been constructed, and several recapitulate key features of prion disease. Tg mice overexpressing high levels of mouse (Mo) PrP with a P→L substitution at position 101, which corresponds to the mutation causing GSS in humans, spontaneously develop neuropathology characteristic of prion disease and accumulate an abnormal isoform of PrP (Hsiao et al. 1990; Tremblay et al. 2004). Serial passage of brain homogenates to Tg mice expressing lower levels of the same transgene accelerated the onset of disease (Hsiao et al. 1994). As models of FFI and fCJD in humans, Tg mice expressing D178N coupled with M129 and V129, respectively, show behavioral abnormalities, misfolded PrP, and neuropathological changes (Dossena et al. 2008; Jackson et al. 2009). Mice overexpressing a novel set of mutations (S170N/N174T) engineered to alter the structure of PrP also developed disease, which transmitted to animals expressing wt PrP (Sigurdson et al. 2009).
PRION REPLICATION
Prion propagation requires conversion of PrPC to PrPSc, thought to occur by a template-assisted process in which PrPSc acts as a template onto which PrPC is refolded into the infectious conformation. The faithful replication of prion strains supports this theory. Evidence for this theory also comes from investigations of Tg mice expressing both SHaPrP and MoPrP, designated Tg(SHaPrP)Prnp+/+ mice (Prusiner et al. 1990). When these mice were inoculated with prions originating from mice, MoPrPC was recruited and converted into MoPrPSc. Inoculation of these mice with hamster prions resulted in the conversion of SHaPrPC into SHaPrPSc. These findings indicate that molecules with the PrP sequence that is most well suited to adapt to the PrPSc template are selected for conversion.
PrPC may need to enter a partially unfolded, intermediate state to interact with PrPSc and undergo conversion; this intermediate state is referred to as PrP* (Cohen et al. 1994). During in vitro conversion, PrPC must be denatured either by GdnHCl (Kocisko et al. 1994; Kaneko et al. 1997b) or by sonication (Castilla et al. 2005). This denaturation is presumed to convert PrPC into a PrP*-like molecule. The conversion of PrPC to PrPSc may also require the assistance of one or more as-yet-unidentified cofactors, provisionally designated protein X. Presumably, protein X binds to PrPC and enables it to interact with PrPSc for conversion. Overexpression of protein X would thus shorten incubation times for disease, whereas ablation of protein X would prolong or abolish prion disease. Many putative protein X genes have been identified, but transgenic knockouts for these genes have failed to alter incubation times substantially (Tamgüney et al. 2008). Several in vitro investigations have suggested that polyanions, including nucleic acids, may accelerate prion formation (Deleault et al. 2007; Wang et al. 2010) although this has not been shown in animals. For yeast prions, several protein chaperones that modulate prion states have been identified (Paushkin et al. 1997; Shorter and Lindquist 2008).
In mammalian cell cultures, prion accumulation was determined by the interplay between de novo prion formation, catabolism, cell division, and horizontal cell-to-cell transmission. Using a subline of neuroblastoma (N2a) cells, we studied the kinetics of prion propagation and found that cell division led to a predictable reduction in steady-state prion levels but not to complete clearance (Ghaemmaghami et al. 2007). Scrapie-infected N2a cells were capable of accumulating different steady-state levels of prions, dictated partly by the rate of cell division. We also observed that prions in this subline of N2a cells were transmitted primarily from mother to daughter cells, rather than horizontal cell-to-cell transmission. Our kinetic results were modeled based on a mechanism that assumed a subpopulation of prions is capable of self-catalysis, and the levels of this subpopulation reached saturation in fully infected cells.
BIOLUMINESCENCE IMAGING
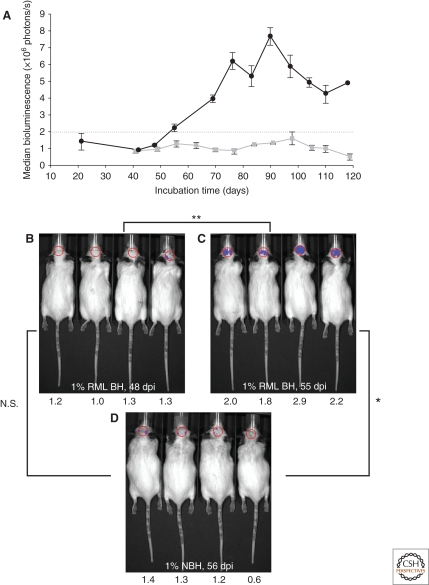
Because astrocytic gliosis marked by the deposition of fibrils composed of GFAP is a prominent feature of prion disease (DeArmond et al. 1987; Hwang et al. 2009), we investigated whether GFAP might be used as a surrogate marker for prions. To interrogate this posit, we inoculated prions into Tg mice expressing luciferase (luc) under the GFAP gene (Gfap) promoter, denoted Tg(Gfap-luc) mice (Tamgüney et al. 2009a). Weekly noninvasive, bioluminescence imaging (BLI) detected an increase in light emitted from the brains of Tg(Gfap-luc) mice at ∼55 d after inoculation and ∼62 d before neurologic deficits appeared (Fig. 3). To determine whether BLI could be used as a proxy bioassay for prion infectivity, we performed endpoint titrations of prions in Tg(Gfap-luc) mice. BLI bioassays were as or more sensitive than those determined by the onset of neurological dysfunction, and were completed in approximately half the time. These findings indicate that BLI is likely to be a suitable surrogate for measuring prion infectivity, and might be useful in the study of Tg mouse models for other neurodegenerative illnesses.
Figure 3.
Bioluminescence in Tg(Gfap-luc) mice inoculated intracerebrally with RML prions (n = 12) indicated a reactive astrocytic gliosis. (A) Bioluminescence measured from the brains of prion-inoculated mice (black circles) began to increase at 55 d postinoculation (dpi). Bioluminescence in control Tg(Gfap-luc) mice inoculated with 1% normal brain homogenate (NBH) (n = 4, gray squares) remained low throughout the incubation period. (B–D) Photos of representative Tg(Gfap-luc) mice, with overlays of the circular area above the brain from which bioluminescence was quantified. Bioluminescence measured, ×106 photons/s, from each mouse brain is shown below each image. The bioluminescence measured from the brains of prion-infected mice significantly increased (**, P < 0.001, Bonferroni t test) from 48 dpi (B) to 55 dpi (C). Similarly, bioluminescence measured from infected mice at 55 dpi (C) was also significantly (*, P < 0.005) greater than in control mice inoculated with NBH and imaged at 56 dpi (D). No significant difference (N.S., P < 0.5) was measured between RML-inoculated mice at 48 dpi (B) and control mice at 56 dpi (D). Based on this result, astrocytic gliosis was detectable at bioluminescence measurements >2.0 × 106 photons/s. Reprinted, with permission, from Tamgüney et al. 2009a.
PrP AMYLOID
As mentioned earlier, amyloid plaques are a nonobligatory feature of prion diseases. Approximately 10% of sCJD cases whereas 70% of kuru cases show amyloid plaques; all vCJD cases show amyloid plaques surrounded by a halo of spongiform degeneration—such structures are called florid plaques (Klatzo et al. 1959; Will et al. 1996). In Tg(SHaPrP)Prnp+/+ mice expressing both MoPrP and SHaPrP, amyloid plaques were found when hamster prions replicated but not when mouse prions replicated (Prusiner et al. 1990). These experimental studies showed unequivocally that amyloid plaques need not accompany prion replication. In earlier studies, the 87V prion strain that produced numerous amyloid plaques was isolated from Cheviot sheep with scrapie and resulted in amyloid when passaged in Prnpb/b mice (Bruce et al. 1976; Jeffrey et al. 1994).
Importantly, ionizing radiation studies showed the target size for scrapie prions was ∼55,000 Da regardless of the preparation (Bellinger-Kawahara et al. 1988). Fractions containing purified PrP 27–30 amyloid rods showed the same resistance to inactivation by X-rays as crude brain homogenates or PrP 27–30 dispersed into liposomes. Electron crystallography of purified PrP 27–30 amyloid rods identified two-dimensional (2D) crystals with a unit cell of 70 Å, which allowed sufficient space for a PrP 27–30 trimer assuming each protein contained a β-helix (Wille et al. 2002; Govaerts et al. 2004b; Wille et al. 2009b). Because each PrP 27–30 molecule is composed of approximately 140 amino acids, an infectious trimer is readily accommodated by the putative target size.
Although some investigators argue that mammalian prions multiply by a seeded polymerization process during which PrPC is transformed into PrPSc, there is little evidence for such a process. More likely it is a template-assisted replication mechanism whereby the conformation of PrPSc is copied with a high degree of fidelity. As noted earlier, it seems likely that chaperone proteins feature in the formation of mammalian prions but none have been identified to date. Some investigators argue that yeast prions replicate through polymerization into amyloid fibers (Wickner et al. 1995; Speransky et al. 2001). The chaperone protein Hsp104 appears to enhance fungal prion replication by breaking the amyloid fibers to create more seeds for polymerization; in addition, there is evidence that other chaperones, including Hsp 40 and Hsp 70, participate in yeast prion replication (Shorter and Lindquist 2008).
CELL BIOLOGY OF PrPSc FORMATION
Prion-infected cell lines, including scrapie-infected neuroblastoma (ScN2a) cells, have been used to investigate the subcellular localization of PrP conversion. In scrapie-infected cells, PrPC molecules are trafficked to the cell surface via their GPI anchor before conversion into PrPSc (Stahl et al. 1987; Borchelt et al. 1990; Caughey and Raymond 1991). PrPC then appears to re-enter the cell through subcellular compartments, which are likely cholesterol-rich, detergent-insoluble membranes called caveolae-like domains (Gorodinsky and Harris 1995; Taraboulos et al. 1995; Vey et al. 1996; Kaneko et al. 1997a; Naslavsky et al. 1997). Within this cholesterol-rich, nonacidic compartment, GPI-anchored PrPC can be either converted into PrPSc or partially degraded (Taraboulos et al. 1995; Peters et al. 2003). Subsequently, PrPSc is trimmed at the amino terminus in an acidic compartment in scrapie-infected cultured cells to form PrP 27–30 (Caughey et al. 1991a). In contrast, amino-terminal trimming of PrPSc is minimal in the brain, where little PrP 27–30 is found (McKinley et al. 1991).
STRUCTURAL FEATURES OF PrPC AND PrPSc
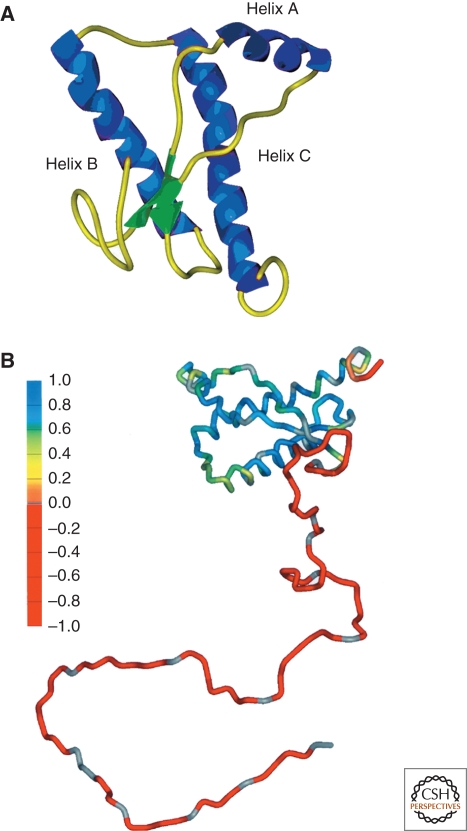
Determining the structural features that differ between PrPC and PrPSc will likely provide important insight into the pathogenic conversion of PrPC into PrPSc. NMR structures of recombinant PrP from many different species have been solved over the past 15 years, representing the best estimate of the structure of PrPC. All reveal a three alpha-helix bundle protein with two short antiparallel β-strands (Riek et al. 1996; James et al. 1997; Riek et al. 1998; Zahn et al. 2000) (Fig. 4). These well-folded structural elements are composed of the carboxyl terminus of the protein; the amino-terminal domain is highly flexible and lacks identifiable secondary structure under the experimental conditions employed (Donne et al. 1997). More recently, a crystal structure of PrP has been obtained, largely in agreement with the NMR structures (Antonyuk et al. 2009).
Figure 4.
Structures of PrPC. (A) NMR structure of Syrian hamster (SHa) recombinant (rec) PrP(90–231), which presumably resembles PrPC. Blue, α-helices; yellow, loops; green, β-strands (James et al. 1997). (B) Schematic diagram showing degree of structure for entire PrP polypeptide chain based on {1H}-15N NOE data. Red, most flexible regions of the protein; blue, least flexible regions (James et al. 1997). Arbitrary structure is shown for residues 23–89. Reprinted, with permission, from Prusiner 2004.
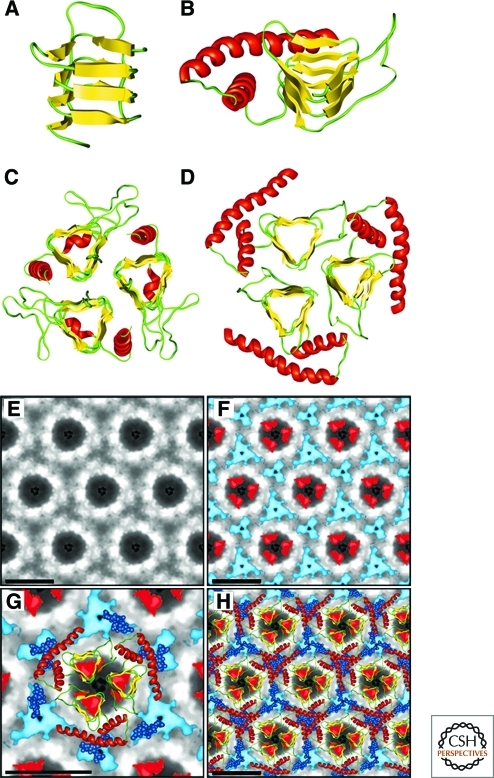
Because PrPSc is insoluble and forms aggregates with some degree of disorder, no successful attempts at crystallization or solution-based NMR have been reported. Investigations using solid-state NMR have been limited by the ability to produce labeled PrPSc and by the molecular size of PrP. However, key insights into the structure of PrPSc have been obtained through electron crystallography coupled with computational modeling (Govaerts et al. 2004a; Wille et al. 2009b) (Fig. 5). Isomorphous, 2D crystals were discovered by negative-stain electron microscopy. Such crystals were found both in preparations of PrP 27–30 and in preparations of a “miniprion” composed of 106 residues formed from discontinuous PrP segments (termed PrPSc106). Image processing allowed the extraction of limited structural information to 7-Å resolution. Models were generated based on known protein folds, constrained by space filling of the 2D crystals, the amount of β-sheet content measure by FTIR (Caughey et al. 1991b; Pan et al. 1993), the locations of the glycosylation sites, and the location of the deleted protein segments in PrPSc106 (Supattapone et al. 1999a). Only models including parallel β-helices as the key element could satisfy the constraints (Wille et al. 2002). Subsequent computational modeling identified trimeric, left-handed β-helices as the most likely substructure for PrPSc (Govaerts et al. 2004a). X-ray diffraction patterns obtained from PrP 27–30 fibers were consistent with this model (Wille et al. 2009a).
Figure 5.
Structural models of PrPSc. (A) Residues 89–174 of PrP threaded into a left-handed β-helix based on UDP N-acetylglucosamine O-acyltransferase from Escherichia coli (PDB ID code 1LXA). (B) Model of the monomer of PrP 27–30 with the α-helical region (residues 177–227) as determined by NMR spectroscopy shown in red. (C) The crystal structure of the trimeric carbonic anhydrase from Methanosarcina thermophila. (D) Trimeric model of PrP 27–30 built by superimposing three monomeric models onto the structure shown in C. (E) Projection map of PrP 27–30 obtained by processing and averaging three independent 2D crystals of PrP 27–30. (F) Statistically significant differences between PrP 27–30 and PrPSc106 overlaid onto the projection map of PrP 27–30. The differences attributed to the internal deletion of PrPSc106 (residues 141–176) are shown in red; the differences in glycosylation between PrP 27–30 and PrPSc106 are shown in blue. (G) Superimposition of the trimeric left-handed model onto the EM maps. The trimeric left-handed α-helical model of PrP 27–30 is superimposed on a 1:1 scale with the electron crystallographic maps of PrP 27–30. (H) The scaled trimeric model was copied onto the neighboring units of the crystals to show the crystallographic packing suggested by the model. Bars in panels E–H represent 50 Å. Reprinted with permission, from Govaerts et al. 2004a.
Given the evidence that distinct conformations of PrP result in different prion strains (see sections “De novo Generation of Prions” and “Prion Strains” below), it is perhaps better to speak of prion structures rather than a single structure. Whether the structural differences that encipher prion strains are subtle or more substantial remains to be determined.
DE NOVO GENERATION OF PRIONS
Refolding PrP into an infectious conformation in vitro has been considered by many to be final proof of the protein-only hypothesis. Many studies have advanced knowledge toward achieving this goal. In Tg(PrP,P101L) mice, an experimental model of human GSS, prion disease was transmitted from high-expressing Tg(PrP,P101L) mice to Tg mice expressing low levels of MoPrP(P101L), which are far less susceptible to spontaneous prion disease (Hsiao et al. 1990; Hsiao et al. 1994; Nazor et al. 2005). Similar transmissions were later accomplished with a synthetic, 55-residue peptide carrying the same P→L mutation and folded into a β-rich structure (Kaneko et al. 2000; Tremblay et al. 2004).
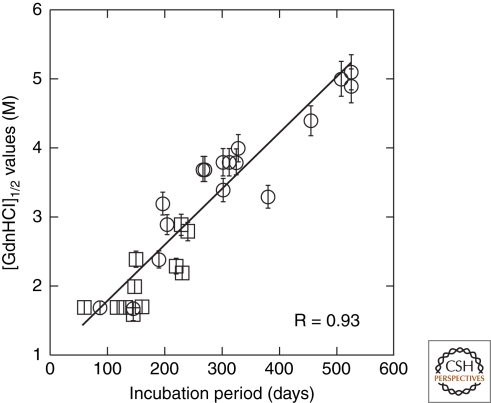
Synthetic prions were formed by polymerization of recombinant MoPrP into amyloid fibers (Legname et al. 2004). Inoculation of PrP amyloid fibers into Tg9949 mice, which overexpress amino-terminally truncated PrP at 16–32× levels, led to the recovery of prions containing protease-resistant (r) PrPSc and to neuropathological changes typical of prion disease. The conformational stability of the resulting prion isolate, as measured by the GdnHCl concentration required to denature half of the sample ([GdnHCl]1/2), was unusually high (∼4.5 M), confirming the novelty of the prion strain generated (Legname et al. 2005). Subsequent serial passage of this isolate led to shortened incubation periods and a decrease in the conformational stability of the resulting prion isolate. Combining these data with those available for naturally occurring prion strains, it was found that the conformational stability of prions was directly proportional to the incubation period (Fig. 6) (Legname et al. 2006).
Figure 6.
The conformational stability of prions is directly proportional to the length of the incubation time in mice. The [GdnHCl]1/2 values for prions were plotted as a function of the incubation times. Synthetic prions (circles) in the brains of Tg9949, Tg4053, and non-Tg FVB mice were plotted with many naturally occurring prions passaged (squares) in both non-Tg and Tg mice. R = 0.93. Reprinted, with permission, from Legname et al. 2006.
Based on the relationship between conformational stability and incubation period (Legname et al. 2006), the conditions used to refold recombinant PrP were altered to generate a spectrum of amyloids with different conformational stabilities. The amyloids were inoculated into mice that moderately overexpress full-length PrP (8×), resulting in distinguishable prion strains with incubation periods and conformational stabilities dictated by the stability of the recombinant PrP amyloid fibers (Colby et al. 2009). Amyloids with higher conformational stability resulted in prions with longer incubation periods, whereas amyloids of low conformational stability caused prion disease in shorter durations. Amyloids of intermediate stability enciphered intermediate incubation periods. This direct demonstration of the conformational basis of prion strain diversity provided further evidence that synthetic prions arise from the recombinant amyloid preparations, and not from the host or from contamination. If prions were arising spontaneously in the host, one would expect the strain properties to be independent of the amyloid properties. Exhaustive negative controls also excluded spontaneous prion generation and contamination.
In other work, amyloid inoculation of Tg9949 mice overexpressing an amino-terminally truncated PrP resulted in novel, protease-sensitive, synthetic prions (Colby et al. 2010). Although these strains lacked protease resistance, they caused severe neuropathology and were serially transmissible both in Tg9949 mice and in Tg mice moderately overexpressing full-length PrP. Most, if not all, naturally occurring prions contain some fraction of PrPSc in a conformation that resists protease digestion (McKinley et al. 1983). This observation has led some researchers to equate protease resistance with prion infectivity and pathogenesis. However, many naturally occurring prion strains also contain PrPSc in a conformation that is sensitive to protease digestion (Safar et al. 1998). The novel, protease-sensitive, synthetic prion strains showed that sPrPSc is both transmissible and pathogenic.
Synthetic prions have also been generated using sonication (Deleault et al. 2007; Barria et al. 2009; Wang et al. 2010). Infectivity was spontaneously generated in sonicated mixtures of polyanions combined with PrPC, which was accompanied by copurified lipids (Deleault et al. 2007). Prions were generated in a similar fashion using brain homogenate as the substrate, rather than minimal components described earlier (Barria et al. 2009). Prions created in these studies using PrPC or normal brain homogenate had titers that were sufficient to infect hamsters with prolonged incubation periods of 113 to 168 days, compared to incubation periods of approximately 70 days with some naturally occurring prion strains (Kimberlin and Walker 1977). Synthesis of high-titer prions from recombinant PrP was reported using sonication in the presence of lipids and RNA (Wang et al. 2010); the infectivity of these prions was comparable to naturally occurring strains.
Synthetic yeast prions have also been constructed. A recombinant fragment of the Sup35 NM protein fragment was polymerized into amyloid fibrils and introduced into yeast (Sparrer et al. 2000). Similar studies have also been performed for the [HET-s] and [URE3] fungal prions (Maddelein et al. 2002; Brachmann et al. 2005).
PRION STRAINS
Naturally occurring prion strains have been isolated, each with a distinct incubation period and characteristic pathology; these traits are often conserved on serial transmission (Dickinson and Meikle 1969; Fraser and Dickinson 1973). Because prions are composed only of protein and replicate using the PrP substrate present in the host, differences in prion strains cannot be attributed to genetic variability, which accounts for the existence of viral strains. Rather, prion strains arise from conformational variability—that is, PrP can assume several different, self-propagating conformations, each of which enciphers a distinct prion strain. Biochemical evidence (Bessen and Marsh 1994; Collinge et al. 1996; Telling et al. 1996; Peretz et al. 2001a) and recent studies with synthetic prions support this theory (Colby et al. 2009).
Studies with synthetic prions showed that the mouse synthetic prion (MoSP) strain 1 gradually adopted properties associated with naturally occurring prion strains such as RML, including short incubation times and low conformational stabilities (Ghaemmaghami et al., in prep.). These changes were accompanied by a structural transformation, as indicated by a shift in the molecular mass of the protease-resistant core of MoSP1 from approximately 19 kDa [MoSP1(2)] to 21 kDa [MoSP1(1)]. We found that MoSP1(1) and MoSP1(2) could be bred with fidelity when cloned in N2a cells but when present as a mixture, MoSP1(1) propagation led to the disappearance of MoSP1(2). In culture, the rate of this transformation could be modified by the culture media and the presence of polyamidoamines. These findings showed that prions exist as conformationally diverse populations and each strain can replicate with high fidelity. Competition and selection among the pool of strains provide a mechanism for prion transformation and adaptation (Li et al. 2010).
Yeast also show multiple prion strains. A recombinant Sup35 protein fragment refolded into two different conformations was shown to initiate two distinct [PSI+] strain phenotypes on transduction into yeast (King and Diaz-Avalos 2004; Tanaka et al. 2004). The propagation rates for these synthetic yeast prion strains were coupled to their conformational stability (Tanaka et al. 2004), a finding that was later extended to mammalian prion strains (Legname et al. 2006; Colby et al. 2009).
ENLARGING SPECTRUM OF PRION-LIKE DISEASES
The discovery that prions form amyloid prompted one of us to suggest that the common neurodegenerative diseases are also caused by prions (Prusiner 1984; Prusiner 2001) despite the inability to transmit such illnesses to monkeys and apes (Goudsmit et al. 1980). Brain extracts from either Alzheimer's patients or aged Tg mice expressing mutant APP injected into the brains of Tg mice expressing the amyloid precursor protein (APP) carrying the Swedish point mutation (Haass et al. 1995) accelerated the formation of Aβ amyloid plaques (Meyer-Luehmann et al. 2006; Eisele et al. 2009). Brain extracts from Tg mice expressing mutant tau injected into the brains of Tg mice expressing human wt tau produced aggregates of human tau (Clavaguera et al. 2009). Similar results were found for aggregated tau protein added to cultured cells, which induced the aggregation of nascent tau (Frost et al. 2009). These findings suggest that the tauopathies result from a prion-like process that induces hyperphosphorylation of tau followed by polymerization into filamentous aggregates. The production of hyperphosphorylated tau also appears to be stimulated by oligomers of the Aβ peptide, whereas amyloid fibrils comprised of Aβ are a much less efficient stimulus (Lambert et al. 1998). An expanded 44-mer polyglutamine repeat of a truncated huntingtin protein was found to stimulate aggregation of a “normal” 25 mer; this aggregated state could be maintained in cell culture over many generations, arguing for prion-like propagation of huntingtin aggregates (Ren et al. 2009). Patients suffering from Parkinson's disease who received fetal grafts of substantia nigral cells later showed aberrantly folded α-synuclein in Lewy bodies within the transplanted grafts, arguing that α-synuclein acted like a prion (Kordower et al. 2008; Li et al. 2008; Olanow and Prusiner 2009). Taken together, these findings argue that prion-like, self-propagating states feature in many different, if not all, neurodegenerative diseases.
A general model of propagation of mammalian prion-like conformational states should include the following considerations (Table 2): First, when the precursor protein is converted to a prion, it undergoes posttranslational modification. Such changes generally result in the acquisition of a high β-sheet content. Proteolytic cleavage features in Alzheimer's disease (AD) (Glenner and Wong 1984; Masters et al. 1985) and hyperphosphorylation occurs in both AD and the tauopathies (Grundke-Iqbal et al. 1986; Lee et al. 1991). Second, the β-sheet–rich conformers form oligomers that are toxic to cells (Walsh and Selkoe 2007). Third, such oligomers are generally rendered less toxic when they polymerize into amyloid fibrils. Fourth, amyloid fibrils are sequestered into biological wastebaskets in the CNS where they are designated “plaques” in the extracellular space, and “tangles” or “bodies” within the cytoplasm of neurons. Inert PrP amyloid fibrils coalesce to form plaques in prion diseases whereas fibrils composed of the Aβ peptide form plaques in AD. Paired-helical filaments composed of hyperphosphorylated tau form neurofibrillary tangles in AD, whereas tau fibrils coalesce into deposits called Pick bodies in one of the frontotemporal dementias generally labeled Pick's disease. In other tauopathies, less well-formed tau aggregates have been identified inside cells. After α-synuclein acquires a high β-sheet content, it polymerizes into amyloid fibrils that coalesce in neurons to form Lewy bodies. Fifth, mutations in the corresponding proteins cause familial neurodegenerative diseases and facilitate conversion of the protein to its prion state. For example, over 40 mutations in PrP have been identified that cause GGS, fCJD, and FFI (Hsiao et al. 1989; Goldfarb et al. 1991b; Medori et al. 1992). Mutations in APP or presenilin (γ-secretase) that cleaves APP into Aβ cause familial AD (Goate et al. 1991), and duplication of the APP gene in Down's syndrome invariably causes AD (Goldgaber et al. 1987). Mutations in tau cause tauopathies (Hutton et al. 1998). Mutations in α-synuclein cause familial Parkinson's disease (Polymeropoulos et al. 1997); duplication or triplication of the α-synuclein gene also causes Parkinson's disease (Singleton et al. 2003).
Table 2.
Some characteristics of mammalian prions.
| • When the precursor protein is converted to a prion, it undergoes posttranslational modification during which it becomes enriched in β-sheet structure. |
| • β-sheet–rich conformers form oligomers that are toxic to cells. |
| • Prion oligomers are generally rendered less toxic when they polymerize into amyloid fibrils. |
| • Amyloid fibrils are sequestered in biological wastebaskets such as plaques, tangles, or inclusion bodies. |
| • Mutations in specific proteins cause familial neurodegenerative diseases by facilitating conversion of the protein into the prion state. |
Prions need not cause disease but may function as regulators of cell metabolism. In yeast, all of the prion proteins found to date have a CG-rich domain that adopts a β-sheet–rich conformation that polymerizes into amyloid. The Sup35 protein in the prion state causes a reduction in the fidelity of polypeptide chain termination during protein synthesis (Wickner et al. 2007). The Aplysia prion comprised of the cytoplasmic polyadenylation element binding (CPEB) protein appears to facilitate polyadenylation within limited regions of neuronal cells, such as dendrites, and has been suggested to function in long-term memory (Si et al. 2010).
TOWARD THERAPEUTICS FOR PRION DISEASES
Despite these advances in understanding prions and many of the neurodegenerative diseases, no treatment is currently available to halt the progression of any of these illnesses. Studies of prions in mice have elucidated several aspects of neurodegeneration that may prove useful in developing effective therapeutics. First, reduction of the precursor protein PrPC prolongs the incubation time (Büeler et al. 1993; Prusiner et al. 1993; Safar et al. 2005). Second, slowing prion formation by inhibiting of the formation of nascent PrPSc prolongs the incubation time (Kawasaki et al. 2007). Third, reducing the availability of PrPC in cells or mice where prion infection has already been established allows for existing prions to be cleared (Enari et al. 2001; Peretz et al. 2001b; Safar et al. 2005). Fourth, enhancing the clearance of PrPSc provides an alternative route of action for therapeutic intervention (Supattapone et al. 1999b; Supattapone et al. 2001).
Blocking conversion of PrPC to PrPSc would seem to be the most practical therapeutic approach, as the cellular pathogenesis of prion disease is downstream of this event and not well understood. Many compounds that inhibit conversion have been identified, including polysulfated anions, dextrans, Congo red dye, oligonucleotides, and cyclic tetrapyrroles (for reviews, see Trevitt and Collinge [2006]; Sim and Caughey [2009]; Silber [2010]). Effective treatment for prion disease is hampered by the difficulty of these and other putative therapeutics to access the CNS, and by the difficulty of identifying small molecules that can prevent the protein–protein interactions that result in propagation of alternatively folded protein isoforms. Studies with a phenylhydrazone revealed restricted efficacy for specific prion strains (Kawasaki et al. 2007) whereas studies with the drug quinacrine revealed the development of drug-resistant prions (Ghaemmaghami et al. 2009).
It seems likely that studies on therapeutics for prion diseases will inform the development of drugs that halt AD, the frontotemporal dementias, or Parkinson's disease; moreover, the lack of success in treating such diseases argues for new paradigms. Work on the prion diseases suggests that treatment for a limited time that reduces or interrupts the formation of nascent prions may be sufficient for the normal cellular clearance mechanisms to overtake the synthesis of new prions. Such an approach would argue for the development of drugs that can be administered for a short period of time instead of many years, which is the commonly held supposition.
Footnotes
Current address: Department of Chemical Engineering, University of Delaware, Newark, Delaware 19716
Editors: Richard Morimoto, Jeffrey Kelly, and Dennis Selkoe
Additional Perspectives on Protein Homeostasis available at www.cshperspectives.org
REFERENCES
- Alpers MP 1968. Kuru: implications of its transmissibility for the interpretation of its changing epidemiological pattern. In The central nervous system: some experimental models of neurological diseases. (ed. Bailey OT, Smith DE), pp. 234–251 Williams and Wilkins Company, Baltimore [Google Scholar]
- Anderson RM, Donnelly CA, Ferguson NM, Woolhouse MEJ, Watt CJ, Udy HJ, MaWhinney S, Dunstan SP, Southwood TRE, Wilesmith JW, et al. 1996. Transmission dynamics and epidemiology of BSE in British cattle. Nature 382: 779–788 [DOI] [PubMed] [Google Scholar]
- Antonyuk SV, Trevitt CR, Strange RW, Jackson GS, Sangar D, Batchelor M, Cooper S, Fraser C, Jones S, Georgiou T, et al. 2009. Crystal structure of human prion protein bound to a therapeutic antibody. Proc Natl Acad Sci 106: 2554–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery OT, MacLeod CM, McCarty M 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a deoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med 79: 137–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria MA, Mukherjee A, Gonzalez-Romero D, Morales R, Soto C 2009. De novo generation of infectious prions in vitro produces a new disease phenotype. PLoS Pathog 5: e1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler K, Oesch B, Scott M, Westaway D, Wälchli M, Groth DF, McKinley MP, Prusiner SB, Weissmann C 1986. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell 46: 417–428 [DOI] [PubMed] [Google Scholar]
- Bellinger-Kawahara CG, Kempner E, Groth DF, Gabizon R, Prusiner SB 1988. Scrapie prion liposomes and rods exhibit target sizes of 55,000 Da. Virology 164: 537–541 [DOI] [PubMed] [Google Scholar]
- Bertoni JM, Brown P, Goldfarb LG, Rubenstein R, Gajdusek DC 1992. Familial Creutzfeldt-Jakob disease (codon 200 mutation) with supranuclear palsy. JAMA 268: 2413–2415 [PubMed] [Google Scholar]
- Bessen RA, Marsh RF 1994. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J Virol 68: 7859–7868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt DR, Scott M, Taraboulos A, Stahl N, Prusiner SB 1990. Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J Cell Biol 110: 743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann A, Baxa U, Wickner RB 2005. Prion generation in vitro: amyloid of Ure2p is infectious. EMBO J 24: 3082–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Gajdusek DC 1991. Survival of scrapie virus after 3 years' interment. Lancet 337: 269–270 [DOI] [PubMed] [Google Scholar]
- Brown P, Gibbs CJ Jr, Rodgers-Johnson P, Asher DM, Sulima MP, Bacote A, Goldfarb LG, Gajdusek DC 1994. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol 35: 513–529 [DOI] [PubMed] [Google Scholar]
- Browning SR, Mason GL, Seward T, Green M, Eliason GA, Mathiason C, Miller MW, Williams ES, Hoover E, Telling GC 2004. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J Virol 78: 13345–13350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce ME, Dickinson AG, Fraser H 1976. Cerebral amyloidosis in scrapie in the mouse: Effect of agent strain and mouse genotype. Neuropathol Appl Neurobiol 2: 471–478 [Google Scholar]
- Büeler H, Aguzzi A, Sailer A, Greiner R-A, Autenried P, Aguet M, Weissmann C 1993. Mice devoid of PrP are resistant to scrapie. Cell 73: 1339–1347 [DOI] [PubMed] [Google Scholar]
- Büeler H, Fisher M, Lang Y, Bluethmann H, Lipp H-P, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C 1992. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356: 577–582 [DOI] [PubMed] [Google Scholar]
- Carlson GA, Westaway D, DeArmond SJ, Peterson-Torchia M, Prusiner SB 1989. Primary structure of prion protein may modify scrapie isolate properties. Proc Natl Acad Sci 86: 7475–7479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla J, Saa P, Hetz C, Soto C 2005. In vitro generation of infectious scrapie prions. Cell 121: 195–206 [DOI] [PubMed] [Google Scholar]
- Caughey B, Raymond GJ 1991. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J Biol Chem 266: 18217–18223 [PubMed] [Google Scholar]
- Caughey BW, Dong A, Bhat KS, Ernst D, Hayes SF, Caughey WS 1991b. Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochemistry 30: 7672–7680 [DOI] [PubMed] [Google Scholar]
- Caughey B, Raymond GJ, Ernst D, Race RE 1991a. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): Implications regarding the site of conversion of PrP to the protease-resistant state. J Virol 65: 6597–6603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelain J, Cathala F, Brown P, Raharison S, Court L, Gajdusek DC 1981. Epidemiologic comparisons between Creutzfeldt-Jakob disease and scrapie in France during the 12-year period 1968-1979. J Neurol Sci 51: 329–337 [DOI] [PubMed] [Google Scholar]
- Chazot G, Broussolle E, Lapras CI, Blättler T, Aguzzi A, Kopp N 1996. New variant of Creutzfeldt-Jakob disease in a 26-year-old French man. Lancet 347: 1181. [DOI] [PubMed] [Google Scholar]
- Chien P, Weissman JS, DePace AH 2004. Emerging principles of conformation-based prion inheritance. Annu Rev Biochem 73: 617–656 [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, et al. 2009. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11: 909–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen FE, Pan K-M, Huang Z, Baldwin M, Fletterick RJ, Prusiner SB 1994. Structural clues to prion replication. Science 264: 530–531 [DOI] [PubMed] [Google Scholar]
- Colby DW, Giles K, Legname G, Wille H, Baskakov IV, DeArmond SJ, Prusiner SB 2009. Design and construction of diverse mammalian prion strains. Proc Natl Acad Sci 106: 20417–20422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby DW, Wain R, Baskakov IV, Legname G, Palmer CG, Nguyen H-OB, Lemus A, Cohen FE, DeArmond SJ, Prusiner SB 2010. Protease-sensitive synthetic prions. PLoS Pathog 6: e1000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby DW, Zhang Q, Wang S, Groth D, Legname G, Riesner D, Prusiner SB 2007. Prion detection by an amyloid seeding assay. Proc Natl Acad Sci 104: 20914–20919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge J, Sidle KCL, Meads J, Ironside J, Hill AF 1996. Molecular analysis of prion strain variation and the aetiology of “new variant” CJD. Nature 383: 685–690 [DOI] [PubMed] [Google Scholar]
- Collinge J, Whittington MA, Sidle KC, Smith CJ, Palmer MS, Clarke AR, Jefferys JGR 1994. Prion protein is necessary for normal synaptic function. Nature 370: 295–297 [DOI] [PubMed] [Google Scholar]
- Cousens SN, Vynnycky E, Zeidler M, Will RG, Smith PG 1997. Predicting the CJD epidemic in humans. Nature 385: 197–198 [DOI] [PubMed] [Google Scholar]
- Coustou V, Deleu C, Saupe S, Begueret J 1997. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc Natl Acad Sci 94: 9773–9778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeArmond SJ, McKinley MP, Barry RA, Braunfeld MB, McColloch JR, Prusiner SB 1985. Identification of prion amyloid filaments in scrapie-infected brain. Cell 41: 221–235 [DOI] [PubMed] [Google Scholar]
- DeArmond SJ, Mobley WC, DeMott DL, Barry RA, Beckstead JH, Prusiner SB 1987. Changes in the localization of brain prion proteins during scrapie infection. Neurology 37: 1271–1280 [DOI] [PubMed] [Google Scholar]
- Deleault NR, Harris BT, Rees JR, Supattapone S 2007. Formation of native prions from minimal components in vitro. Proc Natl Acad Sci 104: 9741–9746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson AG, Meikle VMH 1969. A comparison of some biological characteristics of the mouse-passaged scrapie agents, 22A and ME7. Genet Res 13: 213–225 [DOI] [PubMed] [Google Scholar]
- Dlouhy SR, Hsiao K, Farlow MR, Foroud T, Conneally PM, Johnson P, Prusiner SB, Hodes ME, Ghetti B 1992. Linkage of the Indiana kindred of Gerstmann-Sträussler-Scheinker disease to the prion protein gene. Nat Genet 1: 64–67 [DOI] [PubMed] [Google Scholar]
- Doh-ura K, Tateishi J, Sasaki H, Kitamoto T, Sakaki Y 1989. Pro->Leu change at position 102 of prion protein is the most common but not the sole mutation related to Gerstmann-Sträussler syndrome. Biochem Biophys Res Commun 163: 974–979 [DOI] [PubMed] [Google Scholar]
- Donne DG, Viles JH, Groth D, Mehlhorn I, James TL, Cohen FE, Prusiner SB, Wright PE, Dyson HJ 1997. Structure of the recombinant full-length hamster prion protein PrP(29-231): the N terminus is highly flexible. Proc Natl Acad Sci 94: 13452–13457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossena S, Imeri L, Mangieri M, Garofoli A, Ferrari L, Senatore A, Restelli E, Balducci C, Fiordaliso F, Salio M, et al. 2008. Mutant prion protein expression causes motor and memory deficits and abnormal sleep patterns in a transgenic mouse model. Neuron 60: 598–609 [DOI] [PubMed] [Google Scholar]
- Eisele YS, Bolmont T, Heikenwalder M, Langer F, Jacobson LH, Yan ZX, Roth K, Aguzzi A, Staufenbiel M, Walker LC, et al. 2009. Induction of cerebral β-amyloidosis: Intracerebral versus systemic Aβ inoculation. Proc Natl Acad Sci 106: 12926–12931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M, Flechsig E, Weissmann C 2001. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc Natl Acad Sci 98: 9295–9299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser H, Dickinson AG 1973. Scrapie in mice. Agent-strain differences in the distribution and intensity of grey matter vacuolation. J Comp Pathol 83: 29–40 [DOI] [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI 2009. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem 284: 12845–12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel J-M, Oesch B, Kretzschmar H, Scott M, Prusiner SB 1992. Molecular cloning of a candidate chicken prion protein. Proc Natl Acad Sci 89: 9097–9101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek DC 1977. Unconventional viruses and the origin and disappearance of kuru. Science 197: 943–960 [DOI] [PubMed] [Google Scholar]
- Gambetti P, Dong Z, Yuan J, Xiao X, Zheng M, Alshekhlee A, Castellani R, Cohen M, Barria MA, Gonzalez-Romero D, et al. 2008. A novel human disease with abnormal prion protein sensitive to protease. Ann Neurol 63: 697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambetti P, Parchi P, Petersen RB, Chen SG, Lugaresi E 1995. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: Clinical, pathological and molecular features. Brain Pathol 5: 43–51 [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami S, Ahn M, Lessard P, Giles K, Legname G, DeArmond SJ, Prusiner SB 2009. Continuous quinacrine treatment results in the formation of drug-resistant prions. PLoS Pathog 5: e1000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami S, Phuan PW, Perkins B, Ullman J, May BC, Cohen FE, Prusiner SB 2007. Cell division modulates prion accumulation in cultured cells. Proc Natl Acad Sci 104: 17971–17976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs CJ Jr, Gajdusek DC, Asher DM, Alpers MP, Beck E, Daniel PM, Matthews WB 1968. Creutzfeldt-Jakob disease (spongiform encephalopathy): Transmission to the chimpanzee. Science 161: 388–389 [DOI] [PubMed] [Google Scholar]
- Glasse R 1967. Cannibalism in the kuru region of New Guinea. Trans NY Acad Sci [Ser 2] 29: 748–754 [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW 1984. Alzheimer's disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120: 885–890 [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin M-C, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. 1991. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 349: 704–706 [DOI] [PubMed] [Google Scholar]
- Goldfarb LG, Brown P, McCombie WR, Goldgaber D, Swergold GD, Wills PR, Cervenakova L, Baron H, Gibbs CJJ, Gajdusek DC 1991a. Transmissible familial Creutzfeldt-Jakob disease associated with five, seven, and eight extra octapeptide coding repeats in the Prnp gene. Proc Natl Acad Sci 88: 10926–10930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb LG, Brown P, Mitrova E, Cervenakova L, Goldin L, Korczyn AD, Chapman J, Galvez S, Cartier L, Rubenstein R, et al. 1991b. Creutzfeldt-Jacob disease associated with the Prnp codon 200Lys mutation: An analysis of 45 families. Eur J Epidemiol 7: 477–486 [DOI] [PubMed] [Google Scholar]
- Goldfarb LG, Haltia M, Brown P, Nieto A, Kovanen J, McCombie WR, Trapp S, Gajdusek DC 1991c. New mutation in scrapie amyloid precursor gene (at codon 178) in Finnish Creutzfeldt-Jakob kindred. Lancet 337: 425. [DOI] [PubMed] [Google Scholar]
- Goldfarb LG, Korczyn AD, Brown P, Chapman J, Gajdusek DC 1990a. Mutation in codon 200 of scrapie amyloid precursor gene linked to Creutzfeldt-Jakob disease in Sephardic Jews of Libyan and non-Libyan origin. Lancet 336: 637–638 [DOI] [PubMed] [Google Scholar]
- Goldfarb LG, Mitrova E, Brown P, Toh BH, Gajdusek DC 1990b. Mutation in codon 200 of scrapie amyloid protein gene in two clusters of Creutzfeldt-Jakob disease in Slovakia. Lancet 336: 514–515 [DOI] [PubMed] [Google Scholar]
- Goldgaber D, Goldfarb LG, Brown P, Asher DM, Brown WT, Lin S, Teener JW, Feinstone SM, Rubenstein R, Kascsak RJ, et al. 1989. Mutations in familial Creutzfeldt-Jakob disease and Gerstmann-Sträussler-Scheinker's syndrome. Exp Neurol 106: 204–206 [DOI] [PubMed] [Google Scholar]
- Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC 1987. Charaterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science 235: 877–880 [DOI] [PubMed] [Google Scholar]
- Goldmann W, Hunter N, Benson G, Foster JD, Hope J 1991. Different scrapie-associated fibril proteins (PrP) are encoded by lines of sheep selected for different alleles of the Sip gene. J Gen Virol 72: 2411–2417 [DOI] [PubMed] [Google Scholar]
- Gorodinsky A, Harris DA 1995. Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin. J Cell Biol 129: 619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit J, Morrow CH, Asher DM, Yanagihara RT, Masters CL, Gibbs CJ Jr, Gajdusek DC 1980. Evidence for and against the transmissibility of Alzheimer's disease. Neurology 30: 945–950 [DOI] [PubMed] [Google Scholar]
- Govaerts C, Wille H, Prusiner SB, Cohen FE 2004a. Evidence for assembly of prions with left-handed β-helices into trimers. Proc Natl Acad Sci 101: 8342–8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govaerts C, Wille H, Prusiner SB, Cohen FE 2004b. Structural studies of prion proteins. In Prion biology and diseases (ed. Prusiner SB), pp. 243–282 Cold Spring Harbor Laboratory Press, Cold Spring Harbor [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung Y-C, Quinlan M, Wisniewski HM, Binder LI 1986. Abnormal phosphorylation of the microtubule-associated protein (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci 83: 4913–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Lemere CA, Capell A, Citron M, Seubert P, Schenk D, Lannfelt L, Selkoe DJ 1995. The Swedish mutation causes early-onset Alzheimer's disease by β-secretase cleavage within the secretory pathway. Nat Med 1: 1291–1296 [DOI] [PubMed] [Google Scholar]
- Hamir AN, Cutlip RC, Miller JM, Williams ES, Stack MJ, Miller MW, O'Rourke KI, Chaplin MJ 2001. Preliminary findings on the experimental transmission of chronic wasting disease agent of mule deer to cattle. J Vet Diagn Invest 13: 91–96 [DOI] [PubMed] [Google Scholar]
- Herms JW, Kretzschmar HA, Titz S, Keller BU 1995. Patch-clamp analysis of synaptic transmission to cerebellar Purkinje cells of prion protein knockout mice. Eur J Neurosci 7: 2508–2512 [DOI] [PubMed] [Google Scholar]
- Hsiao K, Baker HF, Crow TJ, Poulter M, Owen F, Terwilliger JD, Westaway D, Ott J, Prusiner SB 1989. Linkage of a prion protein missense variant to Gerstmann-Sträussler syndrome. Nature 338: 342–345 [DOI] [PubMed] [Google Scholar]
- Hsiao KK, Cass C, Schellenberg GD, Bird T, Devine-Gage E, Wisniewski H, Prusiner SB 1991b. A prion protein variant in a family with the telencephalic form of Gerstmann-Sträussler-Scheinker syndrome. Neurology 41: 681–684 [DOI] [PubMed] [Google Scholar]
- Hsiao KK, Groth D, Scott M, Yang S-L, Serban H, Rapp D, Foster D, Torchia M, DeArmond SJ, Prusiner SB 1994. Serial transmission in rodents of neurodegeneration from transgenic mice expressing mutant prion protein. Proc Natl Acad Sci 91: 9126–9130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Meiner Z, Kahana E, Cass C, Kahana I, Avrahami D, Scarlato G, Abramsky O, Prusiner SB, Gabizon R 1991a. Mutation of the prion protein in Libyan Jews with Creutzfeldt-Jakob disease. N Engl J Med 324: 1091–1097 [DOI] [PubMed] [Google Scholar]
- Hsiao KK, Scott M, Foster D, Groth DF, DeArmond SJ, Prusiner SB 1990. Spontaneous neurodegeneration in transgenic mice with mutant prion protein. Science 250: 1587–1590 [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, et al. 1998. Association of missense and 5'-splice-site mutations in τ with the inherited dementia FTDP-17. Nature 393: 702–705 [DOI] [PubMed] [Google Scholar]
- Hwang D, Lee IY, Yoo H, Gehlenborg N, Cho JH, Petritis B, Baxter D, Pitstick R, Young R, Spicer D, et al. 2009. A systems approach to prion disease. Mol Syst Biol 5: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WS, Borkowski AW, Faas H, Steele AD, King OD, Watson N, Jasanoff A, Lindquist S 2009. Spontaneous generation of prion infectivity in fatal familial insomnia knockin mice. Neuron 63: 438–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TL, Liu H, Ulyanov NB, Farr-Jones S, Zhang H, Donne DG, Kaneko K, Groth D, Mehlhorn I, Prusiner SB, et al. 1997. Solution structure of a 142-residue recombinant prion protein corresponding to the infectious fragment of the scrapie isoform. Proc Natl Acad Sci 94: 10086–10091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey M, Goodsir CM, Bruce M, McBride PA, Scott JR, Halliday WG 1994. Correlative light and electron microscopy studies of PrP localisation in 87V scrapie. Brain Res 656: 329–343 [DOI] [PubMed] [Google Scholar]
- Kaneko K, Ball HL, Wille H, Zhang H, Groth D, Torchia M, Tremblay P, Safar J, Prusiner SB, DeArmond SJ, et al. 2000. A synthetic peptide initiates Gerstmann-Sträussler-Scheinker (GSS) disease in transgenic mice. J Mol Biol 295: 997–1007 [DOI] [PubMed] [Google Scholar]
- Kaneko K, Vey M, Scott M, Pilkuhn S, Cohen FE, Prusiner SB 1997a. COOH-terminal sequence of the cellular prion protein directs subcellular trafficking and controls conversion into the scrapie isoform. Proc Natl Acad Sci 94: 2333–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Wille H, Mehlhorn I, Zhang H, Ball H, Cohen FE, Baldwin MA, Prusiner SB 1997b. Molecular properties of complexes formed between the prion protein and synthetic peptides. J Mol Biol 270: 574–586 [DOI] [PubMed] [Google Scholar]
- Kaski D, Mead S, Hyare H, Cooper S, Jampana R, Overell J, Knight R, Collinge J, Rudge P 2009. Variant CJD in an individual heterozygous for PRNP codon 129. Lancet 374: 2128. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Kawagoe K, Chen CJ, Teruya K, Sakasegawa Y, Doh-ura K 2007. Orally administered amyloidophilic compound is effective in prolonging the incubation periods of animals cerebrally infected with prion diseases in a prion strain-dependent manner. J Virol 81: 12889–12898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin R, Walker C 1977. Characteristics of a short incubation model of scrapie in the golden hamster. J Gen Virol 34: 295–304 [DOI] [PubMed] [Google Scholar]
- King CY, Diaz-Avalos R 2004. Protein-only transmission of three yeast prion strains. Nature 428: 319–323 [DOI] [PubMed] [Google Scholar]
- Klatzo I, Gajdusek DC, Zigas V 1959. Pathology of kuru. Lab Invest 8: 799–847 [PubMed] [Google Scholar]
- Koch TK, Berg BO, DeArmond SJ, Gravina RF 1985. Creutzfeldt-Jakob disease in a young adult with idiopathic hypopituitarism. Possible relation to the administration of cadaveric human growth hormone. N Engl J Med 313: 731–733 [DOI] [PubMed] [Google Scholar]
- Kocisko DA, Come JH, Priola SA, Chesebro B, Raymond GJ, Lansbury PT Jr, Caughey B 1994. Cell-free formation of protease-resistant prion protein. Nature 370: 471–474 [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW 2008. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med 14: 504–506 [DOI] [PubMed] [Google Scholar]
- Kretzschmar HA, Honold G, Seitelberger F, Feucht M, Wessely P, Mehraein P, Budka H 1991. Prion protein mutation in family first reported by Gerstmann, Sträussler, and Scheinker. Lancet 337: 1160. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, et al. 1998. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci 95: 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM-Y, Balin BJ, Orvos IJ, Trojanowksi JQ 1991. A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science 251: 645–678 [DOI] [PubMed] [Google Scholar]
- Legname G, Baskakov IV, Nguyen H-OB, Riesner D, Cohen FE, DeArmond SJ, Prusiner SB 2004. Synthetic mammalian prions. Science 305: 673–676 [DOI] [PubMed] [Google Scholar]
- Legname G, Nguyen H-OB, Baskakov IV, Cohen FE, DeArmond SJ, Prusiner SB 2005. Strain-specified characteristics of mouse synthetic prions. Proc Natl Acad Sci 102: 2168–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legname G, Nguyen H-OB, Peretz D, Cohen FE, DeArmond SJ, Prusiner SB 2006. Continuum of prion protein structures enciphers a multitude of prion isolate-specified phenotypes. Proc Natl Acad Sci 103: 19105–19110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Browning S, Mahal SP, Oelschlegel AM, Weissmann C 2010. Darwinian evolution of prions in cell culture. Science 327: 869–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, et al. 2008. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med 14: 501–503 [DOI] [PubMed] [Google Scholar]
- Lledo P-M, Tremblay P, DeArmond SJ, Prusiner SB, Nicoll RA 1996. Mice deficient for prion protein exhibit normal neuronal excitability and synaptic transmission in the hippocampus. Proc Natl Acad Sci 93: 2403–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugaresi E, Medori R, Montagna P, Baruzzi A, Cortelli P, Lugaresi A, Tinuper P, Zucconi M, Gambetti P 1986. Fatal familial insomnia and dysautonomia with selective degeneration of thalamic nuclei. N Engl J Med 315: 997–1003 [DOI] [PubMed] [Google Scholar]
- Maddelein ML, Dos Reis S, Duvezin-Caubet S, Coulary-Salin B, Saupe SJ 2002. Amyloid aggregates of the HET-s prion protein are infectious. Proc Natl Acad Sci 99: 7402–7407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JC, Clarke AR, Hooper ML, Aitchison L, McConnell I, Hope J 1994. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol Neurobiol 8: 121–127 [DOI] [PubMed] [Google Scholar]
- Masters CL, Gajdusek DC, Gibbs CJ Jr, 1981. Creutzfeldt-Jakob disease virus isolations from the Gerstmann-Sträussler syndrome. Brain 104: 559–588 [DOI] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K 1985. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci 82: 4245–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MP, Bolton DC, Prusiner SB 1983. A protease-resistant protein is a structural component of the scrapie prion. Cell 35: 57–62 [DOI] [PubMed] [Google Scholar]
- McKinley MP, Meyer RK, Kenaga L, Rahbar F, Cotter R, Serban A, Prusiner SB 1991. Scrapie prion rod formation in vitro requires both detergent extraction and limited proteolysis. J Virol 65: 1340–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S, Tjian R 1986. Transcriptional selectivity of viral genes in mammalian cells. Cell 46: 795–805 [DOI] [PubMed] [Google Scholar]
- Medori R, Tritschler H-J, LeBlanc A, Villare F, Manetto V, Chen HY, Xue R, Leal S, Montagna P, Cortelli P, et al. 1992. Fatal familial insomnia, a prion disease with a mutation at codon 178 of the prion protein gene. N Engl J Med 326: 444–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, Neuenschwander A, Abramowski D, Frey P, Jaton AL, et al. 2006. Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science 313: 1781–1784 [DOI] [PubMed] [Google Scholar]
- Miller MW, Williams ES, McCarty CW, Spraker TR, Kreeger TJ, Larsen CT, Thorne ET 2000. Epizootiology of chronic wasting disease in free-ranging cervids in Colorado and Wyoming. J Wildl Dis 36: 676–690 [DOI] [PubMed] [Google Scholar]
- Moore RC, Lee IY, Silverman GL, Harrison PM, Strome R, Heinrich C, Karunaratne A, Pasternak SH, Chishti MA, Liang Y, et al. 1999. Ataxia in prion protein (PrP)-deficient mice is associated with upregulation of the novel PrP-like protein doppel. J Mol Biol 292: 797–817 [DOI] [PubMed] [Google Scholar]
- Muller H, Stitz L, Wille H, Prusiner SB, Riesner D 2007. Influence of water, fat, and glycerol on the mechanism of thermal prion inactivation. J Biol Chem 282: 35855–35867 [DOI] [PubMed] [Google Scholar]
- Naslavsky N, Stein R, Yanai A, Friedlander G, Taraboulos A 1997. Characterization of detergent-insoluble complexes containing the cellular prion protein and its scrapie isoform. J Biol Chem 272: 6324–6331 [DOI] [PubMed] [Google Scholar]
- Nathanson N, Wilesmith J, Griot C 1997. Bovine spongiform encephalopathy (BSE): cause and consequences of a common source epidemic. Am J Epidemiol 145: 959–969 [DOI] [PubMed] [Google Scholar]
- Nazor KE, Kuhn F, Seward T, Green M, Zwald D, Purro M, Schmid J, Biffiger K, Power AM, Oesch B, et al. 2005. Immunodetection of disease-associated mutant PrP, which accelerates disease in GSS transgenic mice. EMBO J 24: 2472–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Prusiner SB 2009. Is Parkinson's disease a prion disorder? Proc Natl Acad Sci 106: 12571–12572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen F, Poulter M, Lofthouse R, Collinge J, Crow TJ, Risby D, Baker HF, Ridley RM, Hsiao K, Prusiner SB 1989. Insertion in prion protein gene in familial Creutzfeldt-Jakob disease. Lancet 333: 51–52 [DOI] [PubMed] [Google Scholar]
- Palsson PA 1979. Rida (scrapie) in Iceland and its epidemiology. In Slow transmissible diseases of the nervous system, Vol. 1 (ed. Prusiner SB, Hadlow WJ), pp. 357–366 Academic Press, New York [Google Scholar]
- Pan K-M, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick RJ, Cohen FE, et al. 1993. Conversion of α-helices into β-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci 90: 10962–10966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino MM, Liu J-J, Glover JR, Lindquist S 1996. Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science 273: 622–626 [DOI] [PubMed] [Google Scholar]
- Pattison IH 1965. Experiments with scrapie with special reference to the nature of the agent and the pathology of the disease. In Slow, latent and temperate virus infections, NINDB Monograph 2, (ed. Gajdusek DC, Gibbs CJ Jr, Alpers MP), pp. 249–257 U.S. Government Printing, Washington, D.C. [Google Scholar]
- Paushkin SV, Kushnirov VV, Smirnov VN, Ter-Avanesyan MD 1997. In vitro propagation of the prion-like state of yeast Sup35 protein. Science 277: 381–383 [DOI] [PubMed] [Google Scholar]
- Peretz D, Scott M, Groth D, Williamson A, Burton D, Cohen FE, Prusiner SB 2001a. Strain-specified relative conformational stability of the scrapie prion protein. Protein Sci 10: 854–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz D, Williamson RA, Kaneko K, Vergara J, Leclerc E, Schmitt-Ulms G, Mehlhorn IR, Legname G, Wormald MR, Rudd PM, et al. 2001b. Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature 412: 739–743 [DOI] [PubMed] [Google Scholar]
- Peters PJ, Mironov A, Peretz D, van Donselaar E, Leclerc E, Erpel S, DeArmond SJ, Burton DR, Williamson RA, Vey M, et al. 2003. Trafficking of prion proteins through a caveolae-mediated endosomal pathway. J Cell Biol 162: 703–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RB, Tabaton M, Berg L, Schrank B, Torack RM, Leal S, Julien J, Vital C, Deleplanque B, Pendlebury WW, et al. 1992. Analysis of the prion protein gene in thalamic dementia. Neurology 42: 1859–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Service 1997. Report on human growth hormone and Creutzfeldt-Jakob disease. Public Health Service Interagency Coordinating Committee, Washington, DC: 14: 1–11 [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. 1997. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276: 2045–2047 [DOI] [PubMed] [Google Scholar]
- Poulter M, Baker HF, Frith CD, Leach M, Lofthouse R, Ridley RM, Shah T, Owen F, Collinge J, Brown G, et al. 1992. Inherited prion disease with 144 base pair gene insertion. 1. Genealogical and molecular studies. Brain 115: 675–685 [DOI] [PubMed] [Google Scholar]
- Prusiner SB 1982. Novel proteinaceous infectious particles cause scrapie. Science 216: 136–144 [DOI] [PubMed] [Google Scholar]
- Prusiner SB 1984. Some speculations about prions, amyloid, and Alzheimer's disease. N Engl J Med 310: 661–663 [DOI] [PubMed] [Google Scholar]
- Prusiner SB 1989. Creutzfeldt-Jakob disease and scrapie prions. Alzheimer Dis Assoc Disord 3: 52–78 [DOI] [PubMed] [Google Scholar]
- Prusiner SB 2001. Shattuck Lecture—Neurodegenerative diseases and prions. N Engl J Med 344: 1516–1526 [DOI] [PubMed] [Google Scholar]
- Prusiner SB 2004. An introduction to prion biology and diseases. In Prion Biology and Diseases, (ed. Prusiner SB), pp. 1–87 Cold Spring Harbor Laboratory Press, Cold Spring Harbor [Google Scholar]
- Prusiner SB, Groth D, Serban A, Koehler R, Foster D, Torchia M, Burton D, Yang S-L, DeArmond SJ 1993. Ablation of the prion protein (PrP) gene in mice prevents scrapie and facilitates production of anti-PrP antibodies. Proc Natl Acad Sci 90: 10608–10612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB, McKinley MP, Bowman KA, Bolton DC, Bendheim PE, Groth DF, Glenner GG 1983. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell 35: 349–358 [DOI] [PubMed] [Google Scholar]
- Prusiner SB, Scott M, Foster D, Pan K-M, Groth D, Mirenda C, Torchia M, Yang S-L, Serban D, Carlson GA, et al. 1990. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 63: 673–686 [DOI] [PubMed] [Google Scholar]
- Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR 2009. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol 11: 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riek R, Hornemann S, Wider G, Billeter M, Glockshuber R, Wüthrich K 1996. NMR structure of the mouse prion protein domain PrP(121–231). Nature 382: 180–182 [DOI] [PubMed] [Google Scholar]
- Riek R, Wider G, Billeter M, Hornemann S, Glockshuber R, Wüthrich K 1998. Prion protein NMR structure and familial human spongiform encephalopathies. Proc Natl Acad Sci 95: 11667–11672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos R, Gajdusek DC, Gibbs CJ Jr 1973. The clinical characteristics of transmissible Creutzfeldt-Jakob disease. Brain 96: 1–20 [DOI] [PubMed] [Google Scholar]
- Rosenmann H, Vardi J, Finkelstein Y, Chapman J, Gabizon R 1998. Identification in Israel of 2 Jewish Creutzfeldt-Jakob disease patients with a 178 mutation at their PrP gene. Acta Neurol Scand 97: 184–187 [DOI] [PubMed] [Google Scholar]
- Safar JG, DeArmond SJ, Kociuba K, Deering C, Didorenko S, Bouzamondo-Bernstein E, Prusiner SB, Tremblay P 2005. Prion clearance in bigenic mice. J Gen Virol 86: 2913–2923 [DOI] [PubMed] [Google Scholar]
- Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB 1998. Eight prion strains have PrPSc molecules with different conformations. Nat Med 4: 1157–1165 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Katamine S, Nishida N, Moriuchi R, Shigematsu K, Sugimoto T, Nakatani A, Kataoka Y, Houtani T, Shirabe S, et al. 1996. Loss of cerebellar Purkinje cells in aged mice homozygous for a disrupted PrP gene. Nature 380: 528–531 [DOI] [PubMed] [Google Scholar]
- Serban D, Taraboulos A, DeArmond SJ, Prusiner SB 1990. Rapid detection of Creutzfeldt-Jakob disease and scrapie prion proteins. Neurology 40: 110–117 [DOI] [PubMed] [Google Scholar]
- Shorter J, Lindquist S 2008. Hsp104, Hsp70 and Hsp40 interplay regulates formation, growth and elimination of Sup35 prions. EMBO J 27: 2712–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si K, Choi YB, White-Grindley E, Majumdar A, Kandel ER 2010. Aplysia CPEB can form prion-like multimers in sensory neurons that contribute to long-term facilitation. Cell 140: 421–435 [DOI] [PubMed] [Google Scholar]
- Sigurdson CJ, Nilsson KP, Hornemann S, Heikenwalder M, Manco G, Schwarz P, Ott D, Rulicke T, Liberski PP, Julius C, et al. 2009. De novo generation of a transmissible spongiform encephalopathy by mouse transgenesis. Proc Natl Acad Sci 106: 304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber BM 2010. Driving drug discovery: the fundamental role of academic labs. Sci Transl Med 2: 30cm16. [DOI] [PubMed] [Google Scholar]
- Sim VL, Caughey B 2009. Recent advances in prion chemotherapeutics. Infect Disord Drug Targets 9: 81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, et al. 2003. α-Synuclein locus triplication causes Parkinson's disease. Science 302: 841. [DOI] [PubMed] [Google Scholar]
- Sparrer HE, Santoso A, Szoka FC Jr, Weissman JS 2000. Evidence for the prion hypothesis: Induction of the yeast [PSI+] factor by in vitro-converted Sup35 protein. Science 289: 595–599 [DOI] [PubMed] [Google Scholar]
- Spencer MD, Knight RS, Will RG 2002. First hundred cases of variant Creutzfeldt-Jakob disease: Retrospective case note review of early psychiatric and neurological features. BMJ 324: 1479–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speransky VV, Taylor KL, Edskes HK, Wickner RB, Steven AC 2001. Prion filament networks in [URE3] cells of Saccharomyces cerevisiae. J Cell Biol 153: 1327–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl N, Baldwin MA, Teplow DB, Hood L, Gibson BW, Burlingame AL, Prusiner SB 1993. Structural analysis of the scrapie prion protein using mass spectrometry and amino acid sequencing. Biochemistry 32: 1991–2002 [DOI] [PubMed] [Google Scholar]
- Stahl N, Borchelt DR, Hsiao K, Prusiner SB 1987. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell 51: 229–240 [DOI] [PubMed] [Google Scholar]
- Stekel DJ, Nowak MA, Southwood TRE 1996. Prediction of future BSE spread. Nature 381: 119. [DOI] [PubMed] [Google Scholar]
- Supattapone S, Bosque P, Muramoto T, Wille H, Aagaard C, Peretz D, Nguyen H-OB, Heinrich C, Torchia M, Safar J, et al. 1999a. Prion protein of 106 residues creates an artificial transmission barrier for prion replication in transgenic mice. Cell 96: 869–878 [DOI] [PubMed] [Google Scholar]
- Supattapone S, Nguyen H-OB, Cohen FE, Prusiner SB, Scott MR 1999b. Elimination of prions by branched polyamines and implications for therapeutics. Proc Natl Acad Sci 9614529–14534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supattapone S, Wille H, Uyechi L, Safar J, Tremblay P, Szoka FC, Cohen FE, Prusiner SB, Scott MR 2001. Branched polyamines cure prion-infected neuroblastoma cells. J Virol 75: 3453–3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamgüney G, Francis KP, Giles K, Lemus A, DeArmond SJ, Prusiner SB 2009a. Measuring prions by bioluminescence imaging. Proc Natl Acad Sci 106: 15002–15006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamgüney G, Giles K, Bouzamondo-Bernstein E, Bosque PJ, Miller MW, Safar J, DeArmond SJ, Prusiner SB 2006. Transmission of elk and deer prions to transgenic mice. J Virol 80: 9104–9114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamgüney G, Giles K, Glidden DV, Lessard P, Wille H, Tremblay P, Groth DF, Yehiely F, Korth C, Moore RC, et al. 2008. Genes contributing to prion pathogenesis. J Gen Virol 89: 1777–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamgüney G, Miller MW, Wolfe LL, Sirochman TM, Glidden DV, Palmer C, Lemus A, DeArmond SJ, Prusiner SB 2009b. Asymptomatic deer excrete infectious prions in faeces. Nature 461: 529–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Chien P, Naber N, Cooke R, Weissman JS 2004. Conformational variations in an infectious protein determine prion strain differences. Nature 428: 323–328 [DOI] [PubMed] [Google Scholar]
- Taraboulos A, Scott M, Semenov A, Avrahami D, Laszlo L, Prusiner SB 1995. Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibits formation of the scrapie isoform. J Cell Biol 129: 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi J, Kitamoto T, Hoque MZ, Furukawa H 1996. Experimental transmission of Creutzfeldt-Jakob disease and related diseases to rodents. Neurology 46: 532–537 [DOI] [PubMed] [Google Scholar]
- Telling GC, Parchi P, DeArmond SJ, Cortelli P, Montagna P, Gabizon R, Mastrianni J, Lugaresi E, Gambetti P, Prusiner SB 1996. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274: 2079–2082 [DOI] [PubMed] [Google Scholar]
- Telling GC, Scott M, Hsiao KK, Foster D, Yang S-L, Torchia M, Sidle KCL, Collinge J, DeArmond SJ, Prusiner SB 1994. Transmission of Creutzfeldt-Jakob disease from humans to transgenic mice expressing chimeric human-mouse prion protein. Proc Natl Acad Sci 91: 9936–9940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telling GC, Scott M, Mastrianni J, Gabizon R, Torchia M, Cohen FE, DeArmond SJ, Prusiner SB 1995. Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell 83: 79–90 [DOI] [PubMed] [Google Scholar]
- Tremblay P, Ball HL, Kaneko K, Groth D, Hegde RS, Cohen FE, DeArmond SJ, Prusiner SB, Safar JG 2004. Mutant PrPSc conformers induced by a synthetic peptide and several prion strains. J Virol 78: 2088–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevitt CR, Collinge J 2006. A systematic review of prion therapeutics in experimental models. Brain 129: 2241–2265 [DOI] [PubMed] [Google Scholar]
- Vey M, Pilkuhn S, Wille H, Nixon R, DeArmond SJ, Smart EJ, Anderson RG, Taraboulos A, Prusiner SB 1996. Subcellular colocalization of the cellular and scrapie prion proteins in caveolae-like membranous domains. Proc Natl Acad Sci 93: 14945–14949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ 2007. Aβ oligomers - a decade of discovery. J Neurochem 101: 1172–1184 [DOI] [PubMed] [Google Scholar]
- Wang F, Wang X, Yuan C-G, Ma J 2010. Generating a prion with bacterially expressed recombinant prion protein. Science 327: 1132–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Westaway D 2007. The prion protein family: diversity, rivalry, and dysfunction. Biochim Biophys Acta 1772: 654–672 [DOI] [PubMed] [Google Scholar]
- Westaway D, DeArmond SJ, Cayetano-Canlas J, Groth D, Foster D, Yang S-L, Torchia M, Carlson GA, Prusiner SB 1994. Degeneration of skeletal muscle, peripheral nerves, and the central nervous system in transgenic mice overexpressing wild-type prion proteins. Cell 76: 117–129 [DOI] [PubMed] [Google Scholar]
- Westaway D, Goodman PA, Mirenda CA, McKinley MP, Carlson GA, Prusiner SB 1987. Distinct prion proteins in short and long scrapie incubation period mice. Cell 51: 651–662 [DOI] [PubMed] [Google Scholar]
- Whittington MA, Sidle KCL, Gowland I, Meads J, Hill AF, Palmer MS, Jefferys JGR, Collinge J 1995. Rescue of neurophysiological phenotype seen in PrP null mice by transgene encoding human prion protein. Nat Genet 9: 197–201 [DOI] [PubMed] [Google Scholar]
- Wickner RB 1994. [URE3] as an altered URE2 protein: Evidence for a prion analog in Saccharomyces cerevisiae. Science 264: 566–569 [DOI] [PubMed] [Google Scholar]
- Wickner RB, Masison DC, Edskes HK 1995. [PSI] and [URE3] as yeast prions. Yeast 11: 1671–1685 [DOI] [PubMed] [Google Scholar]
- Wickner RB, Edskes HK, Shewmaker F, Nakayashiki T 2007. Prions of fungi: Inherited structures and biological roles. Nat Rev Microbiol 5: 611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilesmith JW, Ryan JBM, Atkinson MJ 1991. Bovine spongiform encephalopathy: Epidemiologic studies on the origin. Vet Rec 128: 199–203 [DOI] [PubMed] [Google Scholar]
- Will RG 2003. Acquired prion disease: Iatrogenic CJD, variant CJD, kuru. Br Med Bull 66: 255–265 [DOI] [PubMed] [Google Scholar]
- Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith PG 1996. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347: 921–925 [DOI] [PubMed] [Google Scholar]
- Wille H, Prusiner SB, Cohen FE 2000. Scrapie infectivity is independent of amyloid staining properties of the N-terminally truncated prion protein. J Struct Biol 130: 323–338 [DOI] [PubMed] [Google Scholar]
- Wille H, Bian W, McDonald M, Kendall A, Colby DW, Bloch L, Ollesch J, Boronvinskiy AL, Cohen FE, Prusiner SB, et al. 2009a. Natural and synthetic prion structure from X-ray fiber diffraction. Proc Natl Acad Sci 106: 16990–16995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille H, Michelitsch MD, Guénebaut V, Supattapone S, Serban A, Cohen FE, Agard DA, Prusiner SB 2002. Structural studies of the scrapie prion protein by electron crystallography. Proc Natl Acad Sci 99: 3563–3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille H, Shanmugam M, Murugesu M, Ollesch J, Stubbs G, Long JR, Safar JG, Prusiner SB 2009b. Surface charge of polyoxometalates modulates polymerization of the scrapie prion protein. Proc Natl Acad Sci 106: 3740–3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ES, Young S 1980. Chronic wasting disease of captive mule deer: A spongiform encephalopathy. J Wildl Dis 16: 89–98 [DOI] [PubMed] [Google Scholar]
- Williams ES, Miller MW 2002. Chronic wasting disease in deer and elk in North America. Rev Sci Tech 21: 305–316 [DOI] [PubMed] [Google Scholar]
- Zahn R, Liu A, Lührs T, Riek R, von Schroetter C, López García F, Billeter M, Calzolai L, Wider G, Wüthrich K 2000. NMR solution structure of the human prion protein. Proc Natl Acad Sci 97: 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]