Abstract
The plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria utilizes a type III secretion (T3S) system to inject effector proteins into eukaryotic cells. T3S substrate specificity is controlled by HpaC, which promotes secretion of translocon and effector proteins but prevents efficient secretion of the early substrate HrpB2. HpaC and HrpB2 interact with the C-terminal domain (HrcUC) of the FlhB/YscU homologue HrcU. Here, we provide experimental evidence that HrcU is proteolytically cleaved at the conserved NPTH motif, which is required for binding of both HpaC and HrpB2 to HrcUC. The results of mutant studies showed that cleavage of HrcU contributes to pathogenicity and secretion of late substrates but is dispensable for secretion of HrpB2, which is presumably secreted prior to HrcU cleavage. The introduction of a point mutation (Y318D) into HrcUC activated secretion of late substrates in the absence of HpaC and suppressed the hpaC mutant phenotype. However, secretion of HrpB2 was unaffected by HrcUY318D, suggesting that the export of early and late substrates is controlled by independent mechanisms that can be uncoupled. As HrcUY318D did not interact with HrpB2 and HpaC, we propose that the substrate specificity switch leads to the release of HrcUC-bound HrpB2 and HpaC.
Introduction
Gram-negative plant and animal pathogenic bacteria often employ type III secretion (T3S) systems to deliver bacterial effector proteins directly into eukaryotic cells, a process referred to as translocation (Ghosh, 2004). Type III effector proteins manipulate host cellular pathways to the benefit of the bacteria and thus allow successful multiplication of the bacteria in the host tissue (Block et al., 2008; Galan, 2009). Translocation-associated T3S systems are evolutionarily related to flagellar T3S systems, which are the key bacterial motility organelles (Desvaux et al., 2006). Both T3S systems consist of a membrane-spanning secretion apparatus (basal body) but differ in their extracellular appendages. The flagellar basal body is linked via an extracellular hook to the flagellar filament, whereas the translocation-associated basal body is connected to a pilus (plant pathogens) or needle (animal pathogens) that serve as protein transport devices to the host–pathogen interface (Ghosh, 2004; Macnab, 2004). The T3S pilus from plant pathogenic bacteria is considerably longer (up to 2 µm) than the T3S needle (40–80 nm) and presumably spans the plant cell wall (Jin and He, 2001; Koebnik, 2001; Li et al., 2002; Ghosh, 2004). Needle and pilus are directly or indirectly connected to the bacterial channel-like T3S translocon in the host plasma membrane, which mediates effector protein translocation (Büttner and Bonas, 2002a; Coombes and Finlay, 2005; Mueller et al., 2008).
Translocation-associated T3S systems from plant and animal pathogenic bacteria secrete at least three different sets of substrates, i.e. (i) proteins involved in the assembly of the extracellular needle or pilus, (ii) components of the T3S translocon and (iii) effector proteins. Efficient secretion and/or translocation of T3S substrates depends on a signal that is often located in the N-terminal protein region and is not conserved on the amino acid level (Anderson and Schneewind, 1997; Lloyd et al., 2001; Petnicki-Ocwieja et al., 2002; Arnold et al., 2009; Samudrala et al., 2009). Furthermore, in some cases bacterial cytoplasmic T3S chaperones are involved that bind to secreted substrates and promote their stability and/or secretion (Parsot et al., 2003; Ghosh, 2004).
It is postulated that the secretion of extracellular components of the secretion apparatus precedes effector protein translocation. This implies that the substrate specificity of the T3S system switches from ‘early’ to ‘late’ substrates. In animal pathogenic bacteria the T3S substrate specificity is controlled by so-called T3S substrate specificity switch (T3S4) proteins that are themselves secreted. Examples are YscP from Yersinia spp. that switches the T3S substrate specificity from needle to translocon and effector proteins, and FliK from flagellar T3S systems that promotes secretion of filament proteins after hook assembly (Minamino et al., 1999a,b; Journet et al., 2003; Agrain et al., 2005; Sorg et al., 2007). The T3S substrate specificity switch depends on the interactions between T3S4 proteins and the cytoplasmic domains of conserved inner membrane proteins that belong to the FlhB/YscU family (Minamino and Macnab, 2000a; Ferris and Minamino, 2006; Waters et al., 2007; Botteaux et al., 2008). Members of this family contain four transmembrane helices and a C-terminal cytoplasmic domain that is proteolytically cleaved off but probably associates with the remaining part of the protein and was proposed to act as a substrate acceptor site (Allaoui et al., 1994; Minamino and MacNab, 2000a,b; Fraser et al., 2003; Deane et al., 2008; Berger et al., 2010). Cleavage of FlhB/YscU family members occurs autocatalytically between the asparagine and proline residues of a conserved NPTH (letters refer to amino acids) motif and results in a reorientation of the PTH loop (Minamino and MacNab, 2000a; Lavander et al., 2002; Ferris et al., 2005; Sorg et al., 2007; Deane et al., 2008; Zarivach et al., 2008; Björnfot et al., 2009; Lountos et al., 2009; Wiesand et al., 2009). It was proposed that the cleavage and presumably a conformational change in the C-terminal domain of FlhB/YscU family members that is induced upon binding of T3S4 proteins contribute to the T3S substrate specificity switch (Williams et al., 1996; Edqvist et al., 2003; Ferris et al., 2005; Cornelis et al., 2006; Deane et al., 2008; Minamino et al., 2008; Zarivach et al., 2008; Björnfot et al., 2009; Lountos et al., 2009; Wiesand et al., 2009). This model is corroborated by the finding that the wild-type phenotype in T3S4 mutants from Salmonella typhimurium, Yersinia pseudotuberculosis and enteropathogenic Escherichia coli can be restored by extragenic suppressor mutations in the C-terminal regions of FlhB, YscU and the homologous EscU protein respectively (Kutsukake et al., 1994; Williams et al., 1996; Edqvist et al., 2003; Zarivach et al., 2008).
While the molecular mechanisms underlying control of T3S substrate specificity have intensively been studied in animal pathogenic bacteria, little is known about the mechanisms in plant pathogens. In our laboratory, we study Xanthomonas campestris pv. vesicatoria, which is the causal agent of bacterial spot disease in pepper and tomato plants and one of the model systems for the analysis of T3S. The T3S system from X. campestris pv. vesicatoria is encoded by the chromosomal hrp (hypersensitive response and pathogenicity) gene cluster, which contains 25 genes that are organized in eight transcriptional units (Bonas et al., 1991; Büttner et al., 2007; Weber et al., 2007). Comparative sequence analysis of hrp gene products revealed that eleven proteins (referred to as Hrc for Hrp conserved) are conserved among plant and/or animal pathogenic bacteria (Büttner and Bonas, 2002b; He et al., 2004). They probably constitute the core components of the membrane-spanning secretion apparatus. Mutant studies revealed that hrc and most hrp genes are essential for pathogenicity (Fenselau et al., 1992; Fenselau and Bonas, 1995; Wengelnik et al., 1996; Huguet and Bonas, 1997; Rossier et al., 2000). Only in a few cases, mutations of individual genes of the hrp gene cluster do not completely abolish the bacteria–plant interaction. The corresponding gene products were therefore designated Hpa (Hrp associated) and proposed to be involved in the control of T3S (Huguet et al., 1998; Büttner et al., 2004; 2006; Lorenz et al., 2008a,b;). We have previously shown that the efficient secretion and translocation of effector proteins such as AvrBs1, AvrBs3, AvrBsT, XopC, XopJ and XopF1 depend on the T3S chaperone HpaB, which interacts with effector proteins and presumably targets them to the T3S system-associated ATPase HrcN (Büttner et al., 2004; 2006; Lorenz et al., 2008b). HpaB binds to HpaC, an additional cytoplasmic control protein that promotes secretion of translocon and effector proteins but prevents efficient secretion of HrpB2, which is required for pilus assembly and is therefore presumably one of the first substrates that travels the secretion apparatus (Rossier et al., 2000; Weber et al., 2005; Lorenz et al., 2008b). As HpaC differentially regulates the secretion of early (HrpB2) and late (effector and translocon proteins) T3S substrates, it likely acts a cytoplasmic T3S4 protein. This hypothesis is corroborated by the finding that HpaC interacts with the C-terminal domain of HrcU, which is a member of the FlhB/YscU family of inner membrane proteins (Lorenz et al., 2008b). Interestingly, however, HpaC does not interact with the full-length HrcU protein, suggesting that the interaction with the C-terminal domain of HrcU depends on a certain protein conformation that is altered in the context of the full-length HrcU protein (Lorenz et al., 2008b). In addition to HpaC, the C-terminal domain of HrcU was shown to interact with HrpB2 but not with other T3S substrates and is therefore presumably not a general T3S substrate acceptor site (Lorenz et al., 2008b).
In this study, we investigated the contribution of the T3S4 protein HpaC and the C-terminal cytoplasmic domain of HrcU (HrcUC) to T3S of early and late substrates from X. campestris pv. vesicatoria. The analysis of HrcU derivatives mutated in the NPTH motif suggests that the efficient cleavage of HrcU but not the cleavage event per se is required for pathogenicity and T3S of late substrates whereas HrpB2 is presumably secreted prior to HrcU cleavage. The results of protein–protein interaction studies revealed that the NPTH motif of HrcU is required for binding of both HrpB2 and HpaC to HrcUC. Notably, the introduction of a P265G mutation into HrcU abolished the HrcUC–HrpB2 interaction and also the efficient secretion of HrpB2. In contrast, secretion of HrpB2 was unaffected upon introduction of a point mutation (Y318D) into HrcUC, which suppressed the hpaC mutant phenotype with respect to pathogenicity and T3S of translocon and effector proteins. We therefore assume that the control mechanisms underlying secretion of early and late substrates can be uncoupled. Given the finding that HrcUY318D did not interact with HrpB2 and HpaC, the substrate specificity switch in X. campestris pv. vesicatoria likely leads to the release of HrcUC-bound HrpB2 and HpaC.
Results
Efficient proteolytic cleavage of HrcU depends on the conserved NPTH amino acid motif
The FlhB/YscU homologue HrcU from X. campestris pv. vesicatoria strain 85-10 contains four transmembrane helices and a C-terminal cytoplasmic region that is proteolytically cleaved in both E. coli and X. campestris pv. vesicatoria (Fig. 1A; Lorenz et al., 2008b; Berger et al., 2010). Cleavage of HrcU presumably occurs at the conserved NPTH motif (amino acids 264–267) as was described for HrcU homologues from animal pathogenic bacteria. To study the contribution of the NPTH motif of HrcU to protein cleavage and function, we introduced point mutations that led to an exchange of each amino acid residue of the NPTH motif by alanine respectively. The resulting HrcU mutant derivatives were analysed as C-terminally c-Myc epitope-tagged proteins in E. coli and X. campestris pv. vesicatoria strain 85-10ΔhrcU by immunoblotting. Using a c-Myc epitope-specific antibody, we detected the full-length HrcU-c-Myc, HrcUT266A-c-Myc and HrcUH267A-c-Myc proteins and/or corresponding cleavage products (Fig. 1B). As full-length HrcU-c-Myc was only detectable in E. coli but not in X. campestris pv. vesicatoria, we assume that the proteolytic cleavage of HrcU-c-Myc in X. campestris pv. vesicatoria was nearly complete (Fig. 1B). We detected increased levels of uncleaved HrcUT266A-c-Myc and HrcUH267A-c-Myc when compared with HrcU-c-Myc, suggesting that mutations of amino acids T266 and H267 of HrcU affect the efficiency of the proteolytic cleavage. The C-terminal HrcU cleavage product was not observed for HrcUN264A-c-Myc and only in significantly reduced amounts for HrcUP265A-c-Myc (upon overexposure of the blot; Fig. 1B and C). We also introduced an additional mutation into HrcU that led to an exchange of the proline residue at position 265 by a glycine. Notably, the P265G exchange resulted in a complete loss of detectable HrcU cleavage (Fig. 1C).
Fig. 1.
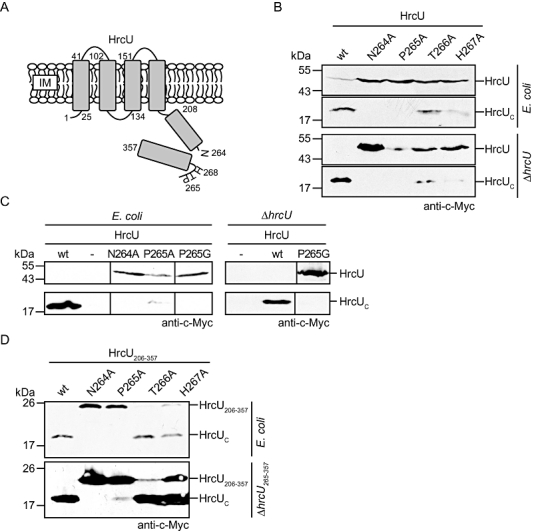
Proteolytic cleavage of HrcU depends on the NPTH motif.
A. Schematic representation of HrcU. HrcU contains four transmembrane helices and a C-terminal cytoplasmic region that is proteolytically cleaved. Cleavage presumably occurs at the NPTH motif and results in a conformational change of the PTH loop as was shown for HrcU homologues from animal pathogenic bacteria. Numbers refer to amino acid positions. IM, inner membrane.
B. Proteolytic cleavage of HrcU and point mutant derivatives. Equal amounts of total-cell extracts from E. coli and X. campestris pv. vesicatoria strain 85-10ΔhrcU (ΔhrcU) encoding HrcU-c-Myc (wt), HrcUN264A-c-Myc (N264A), HrcUP265A-c-Myc (P265A), HrcUT266A-c-Myc (T266A) and HrcUH267A-c-Myc (H267A), respectively, from corresponding expression constructs were analysed by immunoblotting using a c-Myc epitope-specific antibody.
C. HrcUP265A-c-Myc is partially cleaved. Equal amounts of total-cell extracts from E. coli and X. campestris pv. vesicatoria strain 85-10ΔhrcU (ΔhrcU) carrying the empty vector (−) or encoding HrcU-c-Myc (wt), HrcUN264A-c-Myc (N264A), HrcUP265A-c-Myc (P265A) and HrcUP265G-c-Myc (P265G), respectively, from corresponding expression constructs were analysed as described in (B). For the better visualization of the HrcU cleavage product, the blot was overexposed.
D. Mutations in the NPTH motif of HrcU206–357-c-Myc affect proteolytic cleavage. Equal amounts of total-cell extracts from E. coli and X. campestris pv. vesicatoria strain 85-10ΔhrcU265–357 (ΔhrcU265–357) encoding HrcU206–357-c-Myc (wt), HrcU206–357/N264A-c-Myc (N264A), HrcU206–357/P265A-c-Myc (P265A), HrcU206–357/T266A-c-Myc (T266A) and HrcU206–357/H267A-c-Myc (H267A), respectively, from corresponding expression constructs were analysed as described in (B).
In addition to the full-length proteins, we generated HrcU derivatives lacking the N-terminal 205 amino acids (HrcU206–357-c-Myc). HrcU206–357-c-Myc was expressed at higher levels than HrcU-c-Myc, which facilitated the detection of the C-terminal cleavage product. Immunoblot analysis revealed the presence of cleavage products for HrcU206–357-c-Myc and corresponding T266A and H267A mutants in both E. coli and X. campestris pv. vesicatoria (Fig. 1D). Cleavage was not observed for HrcU206–357/N264A-c-Myc; however, small amounts of the cleavage product were detectable for HrcU206–357/P265A-c-Myc, which supports the above finding that proteolytic cleavage is not completely abolished by the P265A mutation (Fig. 1D).
Mutations in the NPTH motif of HrcU interfere with protein function
To analyse whether HrcU mutant derivatives complement the hrcU mutant phenotype, X. campestris pv. vesicatoria strains 85-10 and 85-10ΔhrcU carrying the empty vector or hrcU expression constructs were inoculated into leaves of susceptible Early Cal Wonder (ECW) and resistant ECW-10R pepper plants. ECW-10R plants carry the Bs1 resistance (R) gene and induce the hypersensitive response (HR) upon recognition of the type III effector AvrBs1 that is delivered by strain 85-10 (Ronald and Staskawicz, 1988; Escolar et al., 2001). The HR is a rapid local plant cell death at the infection site that is activated by a plant R gene upon recognition of an individual type III effector [also termed avirulence (Avr) protein; Jones and Dangl, 2006].
As expected, strain 85-10 induced water-soaked lesions in ECW and the HR in ECW-10R plants whereas no plant reactions were observed after inoculation of strain 85-10ΔhrcU (Fig. 2A). The hrcU mutant phenotype was complemented by construct pBRMhrcU, which encodes a C-terminally c-Myc epitope-tagged HrcU derivative under control of the lac promoter (Fig. 2A). Partial complementation was observed for HrcUT266A-c-Myc and HrcUH267A-c-Myc, whereas strain 85-10ΔhrcU carrying HrcUN264A-c-Myc, HrcUP265A-c-Myc and HrcUP265G-c-Myc, respectively, did not cause visible plant reactions (Fig. 2A). We also performed infection assays with hrpG* strains that carry a mutated version of the key regulator HrpG and thus constitutively express the T3S genes (Rossier et al., 1999; Wengelnik et al., 1999). Notably, we observed a partial complementation of the hrcU mutant phenotype by HrcUP265A-c-Myc but not by HrcUP265G-c-Myc in the presence of hrpG* (Fig. 2A). We have previously observed that constitutive expression of the T3S genes promotes in planta symptom formation (Büttner et al., 2004; 2007; Lorenz and Büttner, 2009). The partial complementation of the hrcU mutant phenotype by HrcUP265A-c-Myc is in agreement with the finding that this HrcU mutant derivative is partially cleaved (see Fig. 1C).
Fig. 2.
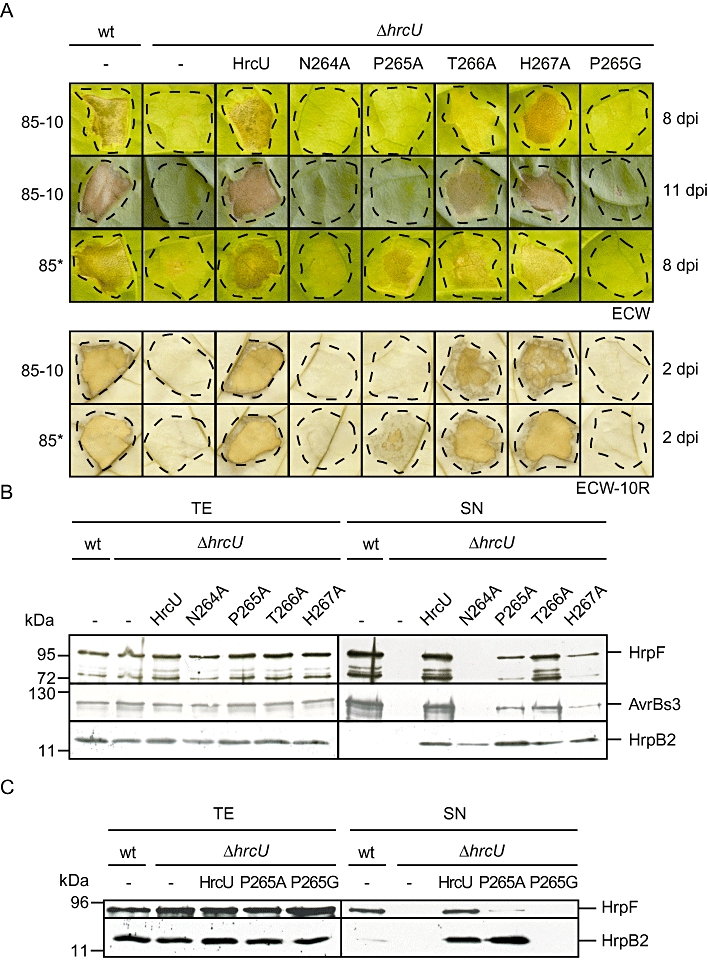
Complementation studies with HrcU mutant derivatives.
A. The conserved asparagine residue of the NPTH motif of HrcU is essential for pathogenicity. X. campestris pv. vesicatoria strains 85-10 (wt), 85* (wt), 85-10ΔhrcU (ΔhrcU) and 85*ΔhrcU (ΔhrcU) carrying the empty vector (−) or encoding HrcU-c-Myc (wt), HrcUN264A-c-Myc (N264A), HrcUP265A-c-Myc (P265A), HrcUT266A-c-Myc (T266A), HrcUH267A-c-Myc (H267A) and HrcUP265G-c-Myc (P265G), respectively, from corresponding expression constructs were inoculated into leaves of susceptible ECW and resistant ECW-10R pepper plants. Disease symptoms were photographed 8 and 11 dpi as indicated. For the better visualization of the HR, leaves were bleached in ethanol 2 dpi. Dashed lines mark the infiltrated areas.
B. The N264A mutation abolishes T3S of translocon and effector proteins but does not affect secretion of the pilus assembly protein HrpB2. X. campestris pv. vesicatoria strains 85* (wt) and 85*ΔhrcU (ΔhrcU) carrying the empty vector (−) or encoding HrcU-c-Myc (wt), HrcUN264A-c-Myc (N264A), HrcUP265A-c-Myc (P265A), HrcUT266A-c-Myc (T266A) and HrcUH267A-c-Myc (H267A), respectively, were incubated in secretion medium. Total-cell extracts (TE) and culture supernatants (SN) were analysed by immunoblotting using antibodies specific for the translocon protein HrpF, the effector protein AvrBs3 (ectopically expressed from construct pDSF300) and HrpB2.
C. HrcUP265G does not promote secretion of HrpB2. X. campestris pv. vesicatoria strains 85* (wt) and 85*ΔhrcU (ΔhrcU) carrying the empty vector (−), HrcU-c-Myc (HrcU), HrcUP265A-c-Myc (P265A) and HrcUP265G-c-Myc (P265G), respectively, were incubated in secretion medium. TE and SN were analysed by immunoblotting using HrpF- and HrpB2-specific antibodies respectively.
HrcU cleavage is required for T3S of late substrates
Next, we analysed T3S in strains 85-10hrpG* (85*) and 85*ΔhrcU carrying HrcU-c-Myc or derivatives mutated in the NPTH motif. For this, bacteria were incubated in secretion medium and total-cell extracts and culture supernatants were analysed by immunoblotting. The translocon protein HrpF and the effector protein AvrBs3 (ectopically expressed from construct pDSF300) were detected in the culture supernatants of strains 85* and 85*ΔhrcU carrying HrcU-c-Myc or the mutant derivatives HrcUP265A-c-Myc, HrcUT266A-c-Myc and HrcUH267A-c-Myc respectively. However, the secretion efficiency in the presence of HrcUP265A-c-Myc and HrcUH267A-c-Myc was reduced when compared with HrcUT266A-c-Myc (Fig. 2B). This is in agreement with the finding that HrcUP265A-c-Myc and HrcUH267A-c-Myc were less efficiently cleaved than HrcUT266A-c-Myc (see above). No secretion of HrpF and AvrBs3 was observed for strains 85*ΔhrcU and 85*ΔhrcU carrying HrcUN264A-c-Myc (Fig. 2B).
We also analysed secretion of the early substrate HrpB2. When compared with strain 85*, increased amounts of HrpB2 were present in the culture supernatant of strain 85*ΔhrcU carrying HrcU-c-Myc, suggesting that ectopic expression of hrcU-c-myc positively affects HrpB2 secretion (Fig. 2B). Notably, HrpB2 was also present in the culture supernatant of strain 85*ΔhrcU carrying HrcUN264A-c-Myc, HrcUP265A-c-Myc, HrcUT266A-c-Myc and HrcUH267A-c-Myc respectively (Fig. 2B). This finding was unexpected and suggests that HrpB2 secretion can occur in the absence of efficient HrcU cleavage. Interestingly, however, HrpB2 was not detected in the culture supernatant of strain 85*ΔhrcU containing HrcUP265G-c-Myc (Fig. 2C). As we observed a similar finding for the translocon protein HrpF, we assume that the P265G exchange abolishes secretion of both early and late substrates (Fig. 2C).
To confirm these results we introduced the hrcUP265G mutation into the genome of X. campestris pv. vesicatoria strains 85-10 and 85* respectively. The resulting mutant strains 85-10hrcUP265G and 85*hrcUP265G did not elicit visible disease symptoms and the HR when inoculated into leaves of susceptible and resistant pepper plants respectively (Fig. 3A). Furthermore, T3S of the translocon protein HrpF, the effector proteins AvrBs3, XopJ-c-Myc and XopE2-c-Myc (ectopically expressed from corresponding expression constructs) and HrpB2 was abolished in strain 85*hrcUP265G, which supports the finding that the P265G mutation in HrcU leads to a loss of protein function (Fig. 3B). Loss of efficient HrpB2 secretion was also observed in strain 85*hrcUP265GΔhpaC, suggesting that HrpB2 oversecretion in the hpaC deletion mutant is suppressed in the presence of HrcUP265G (Fig. 3C). The hrcUP265G mutant phenotype was restored with respect to virulence and T3S (shown for HrpF secretion) upon ectopic expression of hrcU-c-myc (Fig. 3A and D).
Fig. 3.
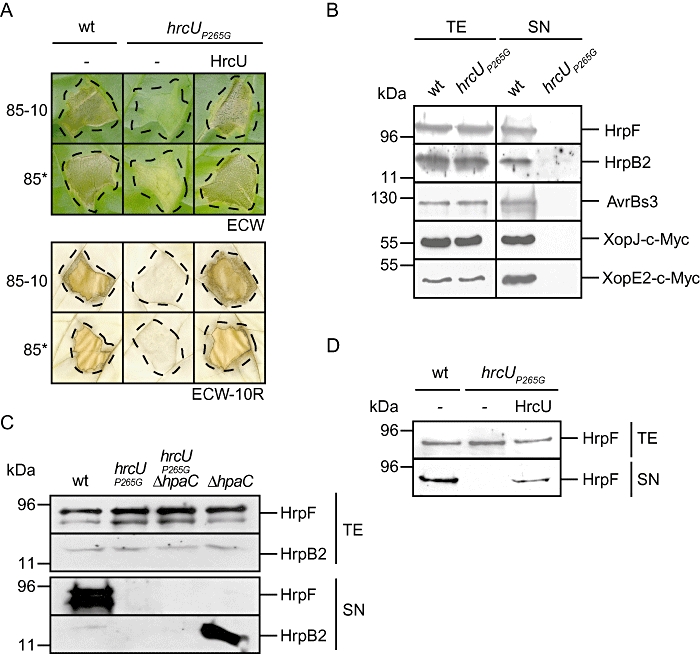
Characterization of a genomic hrcUP265G mutant.
A. The P265G mutation in HrcU abolishes bacterial pathogenicity. X. campestris pv. vesicatoria strains 85-10 (wt), 85* (wt), 85-10hrcUP265G (hrcUP265G) and 85*hrcUP265G (hrcUP265G) carrying the empty vector (−) or HrcU-c-Myc (HrcU) as indicated were inoculated into leaves of susceptible ECW and resistant ECW-10R pepper plants. Disease symptoms were photographed 7 dpi. For the better visualization of the HR, leaves were bleached in ethanol 2 dpi. Dashed lines indicate the infiltrated areas.
B. T3S of early and late substrates is abolished in the presence of hrcUP265G. X. campestris pv. vesicatoria strains 85* (wt) and 85*hrcUP265G (hrcUP265G) were incubated in secretion medium and total-cell extracts (TE) and culture supernatants (SN) were analysed by immunoblotting using antibodies specific for HrpF, HrpB2, AvrBs3 and the c-Myc epitope respectively. AvrBs3, XopJ-c-Myc and XopE2-c-Myc were encoded by corresponding expression constructs.
C. HrpB2 oversecretion in the hpaC deletion mutant is suppressed by the genomic hrcUP265G mutation. X. campestris pv. vesicatoria strains 85* (wt), 85*hrcUP265G (P265G), 85*hrcUP265GΔhpaC (hrcUP265GΔhpaC) and 85*ΔhpaC (ΔhpaC) were incubated in secretion medium. TE and SN were analysed by immunoblotting using HrpF- and HrpB2-specific antibodies.
D. HrpF secretion by strain 85*hrcUP265G is restored upon ectopic expression of hrcU-c-myc. X. campestris pv. vesicatoria strains 85* (wt) and 85*hrcUP265G (hrcUP265G) carrying the empty vector (−) or encoding HrcU-c-Myc (HrcU) as indicated were incubated in secretion medium. TE and SN were analysed by immunoblotting using a HrpF-specific antibody.
The C-terminal domain of HrcU is essential for pathogenicity and functions in trans
In addition to the NPTH motif, we studied the contribution of the C-terminal domain of HrcU (HrcUC, amino acids 265–357, which correspond to the predicted C-terminal HrcU cleavage product) to bacterial pathogenicity and T3S. For this, we deleted codons 265–357 of the chromosomal hrcU gene in X. campestris pv. vesicatoria strain 85-10. The resulting deletion mutant strain 85-10ΔhrcU265–357 did not elicit disease symptoms and the HR in susceptible and resistant pepper plants, respectively, suggesting that HrcUC is essential for pathogenicity (Fig. 4A). The mutant phenotype was complemented by HrcU-c-Myc whereas a partial complementation was observed when we provided a c-Myc epitope-tagged derivative of HrcUCin trans (HrcU265–357-c-Myc; Fig. 4A). However, HrcU265–357-c-Myc complemented the ΔhrcU265–357 mutant phenotype in the presence of hrpG* (Fig. 4A). Immunoblot analyses of total-cell extracts from X. campestris pv. vesicatoria confirmed that HrcU-c-Myc and HrcU265–357-c-Myc were synthesized (Fig. 4B). As described above, we did not detect the full-length HrcU-c-Myc protein in cell extracts of X. campestris pv. vesicatoria. Furthermore, the amounts of HrcU265–357-c-Myc were increased when compared with the amounts of the cleavage product of HrcU-c-Myc and presumably do not reflect native protein levels (Fig. 4B). The analysis of additional expression constructs encoding HrcU265–357-c-Myc under control of an alternative promoter (e.g. the native hrcU promoter) should clarify whether the expression level of hrcU265–357-c-myc influences its ability to complement the ΔhrcU265–357 mutant phenotype.
Fig. 4.
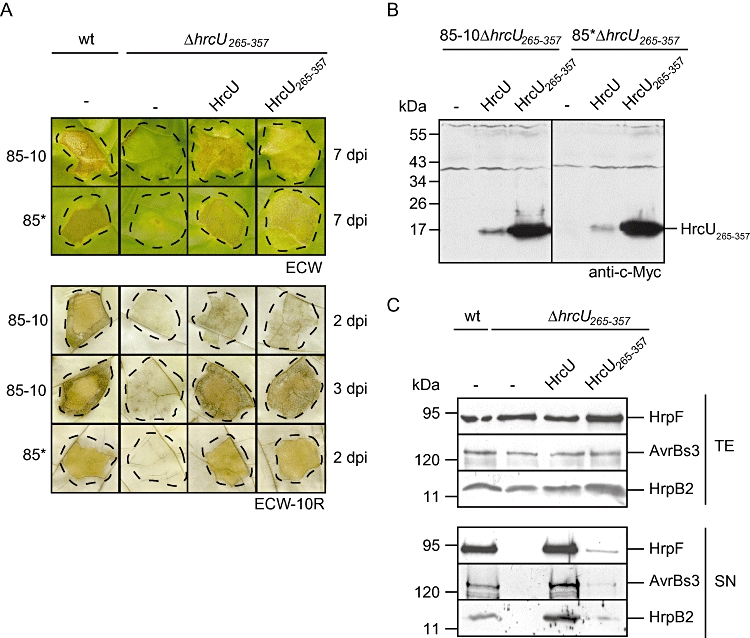
The C-terminal domain of HrcU is essential for pathogenicity.
A. The ΔhrcU265–357 mutant phenotype can be complemented in trans. X. campestris pv. vesicatoria strains 85-10 (wt), 85-10ΔhrcU265–357 (ΔhrcU265–357), 85* (wt) and 85*ΔhrcU265–357 (ΔhrcU265–357) carrying the empty vector (−) or expression constructs encoding HrcU-c-Myc (HrcU) and HrcU265–357-c-Myc (HrcU265–357), respectively, as indicated were inoculated into leaves of susceptible ECW and resistant ECW-10R pepper plants. Disease symptoms were photographed 7 dpi. For the better visualization of the HR, leaves were bleached in ethanol 2 or 3 dpi as indicated. Dashed lines mark the infiltrated areas.
B. Protein studies with HrcU-c-Myc and HrcU265–357-c-Myc. X. campestris pv. vesicatoria strains 85-10ΔhrcU265–357 and 85*ΔhrcU265–357 carrying the empty vector (−) or expression constructs encoding HrcU-c-Myc (HrcU) and HrcU265–357-c-Myc (HrcU265–357), respectively, as indicated were grown in minimal medium A. Equal amounts of total-cell extracts were analysed by immunoblotting, using a c-Myc epitope-specific antibody. The full-length HrcU-c-Myc protein is not detectable. The dominant signal corresponds to HrcU265–357-c-Myc; additional signals result from unspecific binding of the antibody.
C. T3S in the hrcU265–357 deletion mutant. X. campestris pv. vesicatoria strains 85* (wt) and 85*ΔhrcU265–357 (ΔhrcU265–357) carrying the empty vector (−) or expression constructs encoding HrcU-c-Myc (HrcU) and HrcU265–357-c-Myc (HrcU265–357), respectively, as indicated were incubated in secretion medium. Total-cell extracts (TE) and culture supernatants (SN) were analysed by immunoblotting, using antibodies specific for the translocon protein HrpF, the effector protein AvrBs3 (ectopically expressed from construct pDSF300) and HrpB2.
To investigate the contribution of HrcUC to T3S, strains 85* and 85*ΔhrcU265–357 were incubated in secretion medium and total-cell extracts and culture supernatants were analysed by immunoblotting. The translocon protein HrpF, the effector protein AvrBs3 (ectopically expressed from construct pDSF300) and the pilus assembly protein HrpB2 were detected in the culture supernatant of strain 85* but not of strain 85*ΔhrcU265–357 (Fig. 4C). Wild-type levels of secretion were restored by HrcU-c-Myc, whereas HrcU265–357-c-Myc only partially complemented the secretion deficiency (Fig. 4C). However, as HrcU265–357-c-Myc restored the in planta phenotype of strain 85*ΔhrcU265–357, reduced levels of T3S in strain 85*ΔhrcU265–357 were presumably sufficient for plant infection phenotypes (see Fig. 4A). We conclude from these findings that HrcUC is crucial for T3S and pathogenicity and functions in trans.
The NPTH motif of HrcU is required for the interaction with the T3S4 protein HpaC
We have previously shown that the T3S4 protein HpaC interacts with a GST–HrcU255–357 fusion protein (Lorenz et al., 2008b). To investigate whether the interaction depends on the NPTH motif (amino acids 264–268) of HrcU, we generated additional expression constructs encoding GST–HrcU265–357, which lacks the conserved asparagine residue, and GST–HrcU268–357, which is deprived of the complete NPTH motif. For protein–protein interaction studies, GST, GST–HrcU255–357, GST–HrcU265–357 and GST–HrcU268–357 were synthesized in E. coli, immobilized on glutathione sepharose and incubated with an E. coli lysate containing HpaC-c-Myc. Eluted proteins were analysed by immunoblotting using a c-Myc epitope-specific antibody. Figure 5A shows that HpaC-c-Myc was detected in the eluate of GST–HrcU255–357 as expected but not of GST, GST–HrcU265–357 and GST–HrcU268–357. We also performed interaction studies with GST–HrcU255–357 derivatives carrying single amino acid substitutions of the conserved asparagine and proline residues (N264A, P265A and P265G) of the NPTH motif. When GST–HrcU255–357/N264A, GST–HrcU255–357/P265A and GST–HrcU255–357/P265G were immobilized on glutathione sepharose and incubated with HpaC-c-Myc, HpaC-c-Myc was not detected in the eluates, suggesting that mutations of N264 and P265 abolish the efficient binding of HpaC to HrcUC (Fig. 5B).
Fig. 5.
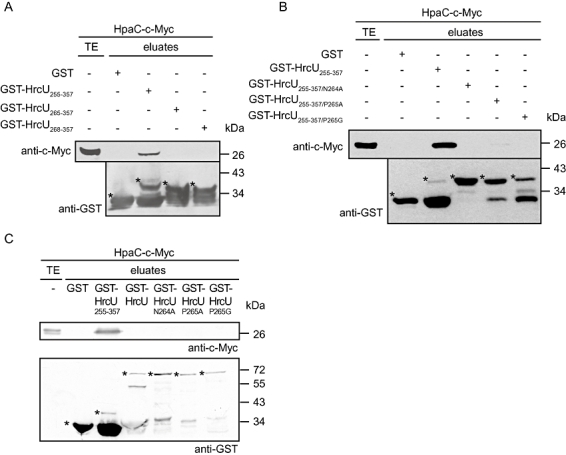
The NPTH motif of HrcU is required for the interaction with the T3S4 protein HpaC.
A. Amino acids 265–357 of HrcU are not sufficient for the interaction with HpaC. GST, GST–HrcU255–357, GST–HrcU265–357 and GST–HrcU268–357 were immobilized on glutathione sepharose and incubated with an E. coli lysate containing HpaC-c-Myc. The total-cell extract (TE) and eluted proteins (eluates) were analysed by immunoblotting using c-Myc epitope- and GST-specific antibodies respectively. GST and GST fusion proteins are marked by asterisks; lower bands correspond to degradation products.
B. Mutations within the NPTH motif abolish the interaction between HrcUC and HpaC. GST, GST–HrcU255–357, GST–HrcU255–357/N264A, GST–HrcU255–357/P265A and GST–HrcU255–357/P265G were immobilized on glutathione sepharose and incubated with an E. coli lysate containing HpaC-c-Myc. TE and eluates were analysed as described in (A). GST and GST fusion proteins are marked by asterisks; lower bands correspond to degradation products. N264A, P265A and P265G mutations led to significantly reduced cleavage of GST–HrcU255–357 and thus to enhanced amounts of the full-length fusion proteins.
C. HpaC-c-Myc does not bind to the full-length HrcU protein carrying mutations within the NPTH motif. GST, GST–HrcU255–357, GST–HrcUN264A, GST–HrcUP265A and GST–HrcUP265G were immobilized on glutathione sepharose and incubated with an E. coli lysate containing HpaC-c-Myc. TE and eluates were analysed as described in (A). GST and GST fusion proteins are marked by asterisks; lower bands correspond to degradation products.
We also analysed the influence of N264A, P265A and P265G mutations on the HrcUC–HpaC interaction in the context of the full-length HrcU protein. As described above, HpaC does not interact with the full-length HrcU protein, yet, it could not be excluded that the N264A, P265A and P265G mutations in HrcU lead to an alteration of the protein conformation that is permissive for binding of HpaC. However, when GST–HrcU255–357, GST–HrcU, GST–HrcUN264A, GST–HrcUP265A and GST–HrcUP265G were immobilized on glutathione sepharose and incubated with HpaC-c-Myc, we detected HpaC-c-Myc in the eluate of GST–HrcU255–357 as expected but not of GST–HrcU and mutant derivatives thereof (Fig. 5C). We have previously reported that GST–HrcU can be stably synthesized in E. coli and that sufficient amounts of the protein are present in the soluble fraction (Lorenz et al., 2008b). We observed similar findings for GST–HrcU255–357 and GST–HrcU derivatives carrying point mutations in the NPTH motif (Fig. S1; data not shown).
The NPTH motif contributes to the interaction between HrcUC and HrpB2
In addition to HpaC, GST–HrcU255–357 also interacts with a C-terminally c-Myc epitope-tagged derivative of the early T3S substrate HrpB2 (Lorenz et al., 2008b). To investigate whether the NPTH motif of HrcU contributes to the interaction between HrcUC and HrpB2, we performed additional pull-down assays with GST or GST–HrcU derivatives as described above. When GST–HrcU fusion proteins were immobilized on glutathione sepharose and incubated with HrpB2-c-Myc, we detected HrpB2-c-Myc in the eluate of GST–HrcU255–357 as expected but not in the eluates of GST–HrcU265–357 and GST–HrcU268–357 (Fig. 6A; Lorenz et al., 2008b). The presence of N264A and P265A point mutations in GST–HrcU255–357, respectively, did not significantly affect the binding of HrpB2-c-Myc (Fig. 6B). In contrast, HrpB2-c-Myc was not detected in the eluate of GST–HrcU255–357/P265G indicating that the P265G exchange abolishes the stable interaction between GST–HrcU255–357 and HrpB2 (Fig. 6B).
Fig. 6.
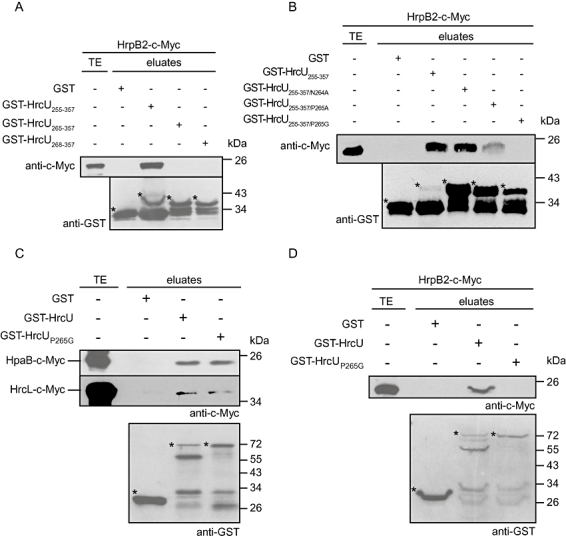
The NPTH motif of HrcU contributes to the interaction between HrcUC and HrpB2.
A. Amino acids 265–357 of HrcU are not sufficient for the interaction with HrpB2. GST, GST–HrcU255–357, GST–HrcU265–357 and GST–HrcU268–357 were immobilized on glutathione sepharose and incubated with an E. coli lysate containing HrpB2-c-Myc. The total-cell extract (TE) and eluted proteins (eluates) were analysed by immunoblotting using c-Myc epitope- and GST-specific antibodies respectively. GST and GST fusion proteins are marked by asterisks; lower bands correspond to degradation products.
B. The P265G exchange abolishes the interaction between HrcUC and HrpB2. GST, GST–HrcU255–357, GST–HrcU255–357/N264A, GST–HrcU255–357/P265A and GST–HrcU255–357/P265G were immobilized on glutathione sepharose and incubated with an E. coli lysate containing HrpB2-c-Myc. TE and eluates were analysed as described in (A). GST and GST fusion proteins are marked by asterisks; lower bands correspond to degradation products. N264A, P265A and P265G mutations led to significantly reduced cleavage of GST–HrcU255–357 and thus to enhanced amounts of the full-length fusion proteins.
C. The P265G exchange in HrcU does not affect binding of both HpaB and HrcL to HrcU. GST, GST–HrcU and GST–HrcUP265G were immobilized on glutathione sepharose and incubated with E. coli lysates containing HpaB-c-Myc and HrcL-c-Myc respectively. TE and eluates were analysed as described in (A). GST and GST fusion proteins are marked by asterisks; lower bands correspond to degradation products. One representative blot probed with the GST-specific antibody is shown.
D. GST–HrcUP265G does not interact with HrpB2. GST, GST–HrcU and GST–HrcUP265G were immobilized on glutathione sepharose and incubated with an E. coli lysate containing HrpB2-c-Myc. TE and eluates were analysed as described in (A). GST and GST fusion proteins are marked by asterisks; lower bands correspond to degradation products.
To investigate whether the P265G mutation also prevents binding of additional interaction partners of HrcU, we performed interaction studies with GST–HrcUP265G and C-terminally c-Myc epitope-tagged derivatives of the general T3S chaperone HpaB and the predicted regulator of the ATPase, HrcL. HpaB and HrcL were previously shown to interact with GST–HrcU but not with GST–HrcU255–357 (Lorenz and Büttner, 2009). HpaB-c-Myc and HrcL-c-Myc were detected in the eluates of GST–HrcU and GST–HrcUP265G but not of GST alone, suggesting that the P265G exchange in HrcU did not affect the interaction with both HpaB and HrcL (Fig. 6C). As an additional control, we incubated immobilized GST–HrcUP265G with HrpB2-c-Myc. Figure 6D shows that HrpB2-c-Myc was not detectable in the eluate of GST–HrcUP265G, which confirms the above finding that the P265G mutation abolishes the interaction between HrcUC and HrpB2. In this context it is of interest to note that HrcUP265G did not promote secretion of HrpB2. It is therefore conceivable that the interaction between HrcUC and HrpB2 is required for efficient HrpB2 secretion (see above, Fig. 2).
A point mutation (Y318D) in HrcUC suppresses the hpaC mutant phenotype
It was previously reported that the phenotype of T3S4 mutants from animal pathogenic bacteria can be suppressed upon introduction of point mutations into the C-terminal domain of FlhB/YscU family members (Kutsukake et al., 1994; Williams et al., 1996; Edqvist et al., 2003; Wood et al., 2008; Zarivach et al., 2008). To test this for X. campestris pv. vesicatoria, we introduced a point mutation (Y318D) into the chromosomal hrcU genes of strains 85-10 and 85-10ΔhpaC, respectively, which led to an exchange of the tyrosine residue at amino acid position 318 of HrcU by aspartic acid. Equivalent mutations in the C-terminal domains of YscU (YscUY317D) and FlhB (FlhBY323D) were shown to suppress the phenotypes of mutants deleted in the T3S4 genes yscP and fliK respectively (Kutsukake et al., 1994; Minamino and MacNab, 2000a; Edqvist et al., 2003; Wood et al., 2008). When X. campestris pv. vesicatoria hrcU wild-type and hrcUY318D mutant strains were inoculated into leaves of susceptible ECW and resistant ECW-10R pepper plants, respectively, strain 85-10hrcUY318D induced disease symptoms and the HR similarly to the wild type whereas strain 85-10ΔhpaC led to significantly reduced symptoms as expected (Fig. 7A; Büttner et al., 2006). The double mutant 85-10hrcUY318DΔhpaC induced wild-type disease symptoms, suggesting that HrcUY318D suppresses the hpaC mutant phenotype in susceptible plants (Fig. 7A). Furthermore, HrcUY318D partially restored the HR induction by strain 85-10hrcUY318DΔhpaC in resistant ECW-10R plants. However, a wild-type HR was observed for the hrpG* derivative 85*hrcUY318DΔhpaC (Fig. 7A).
Fig. 7.
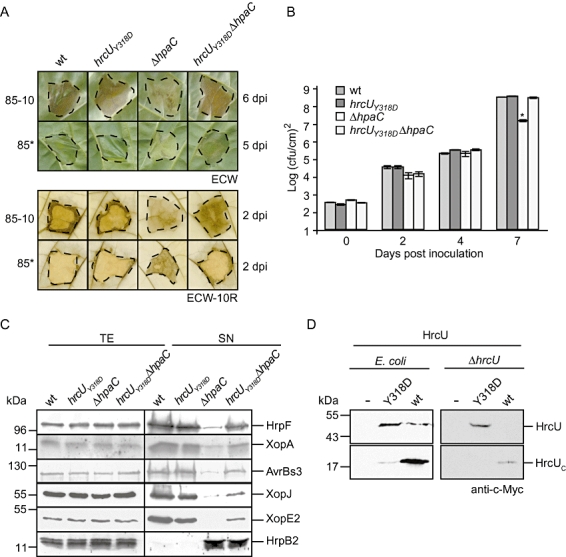
The Y318D mutation in HrcU suppresses the hpaC mutant phenotype and affects HrcU cleavage.
A. Infection studies with hrcU wild-type and hrcUY318D mutant strains. X. campestris pv. vesicatoria strains 85-10 (wt), 85* (wt), 85-10hrcUY318D (hrcUY318D), 85*hrcUY318D (hrcUY318D), 85-10ΔhpaC (ΔhpaC), 85*ΔhpaC (ΔhpaC), 85-10hrcUY318DΔhpaC (hrcUY318DΔhpaC) and 85*hrcUY318DΔhpaC (hrcUY318DΔhpaC) were inoculated into leaves of susceptible ECW and resistant ECW-10R pepper plants. Disease symptoms were photographed 5 and 6 dpi as indicated. For the better visualization of the HR, leaves were bleached in ethanol 2 dpi. Dashed lines mark the infiltrated areas.
B. In planta growth of hrcUY318D mutants. X. campestris pv. vesicatoria strains 85-10 (wt), 85-10hrcUY318D (hrcUY318D), 85-10ΔhpaC (ΔhpaC) and 85-10hrcUY318DΔhpaC (hrcUY318DΔhpaC) were inoculated into leaves of susceptible ECW pepper plants and bacterial growth was analysed over a period of 8 days. Values are the mean of three samples from three plants. Error bars represent standard deviations. The asterisk indicates a significant difference to the wild-type strain with P < 0.005 based on the results of an unpaired Student's t-test.
C. T3S assays with hrcUY318D mutants. Strains 85* (wt), 85*hrcUY318D (hrcUY318D), 85*ΔhpaC (ΔhpaC) and 85*hrcUY318DΔhpaC (hrcUY318DΔhpaC) were incubated in secretion medium. Total-cell extracts (TE) and culture supernatants (SN) were analysed by immunoblotting using antibodies specific for the putative translocon proteins HrpF and XopA, the effector protein AvrBs3, the pilus assembly protein HrpB2 and the c-Myc epitope. AvrBs3, XopJ-c-Myc and XopE2-c-Myc were encoded by corresponding expression constructs.
D. The Y318D mutation in HrcU affects proteolytic cleavage. Equal amounts of total-cell extracts from X. campestris pv. vesicatoria strain 85*ΔhrcU (ΔhrcU) and E. coli carrying the empty vector (−) or encoding HrcU-c-Myc (wt) and HrcUY318D-c-Myc (Y318D), respectively, as indicated were analysed by immunoblotting using a c-Myc epitope-specific antibody.
We also analysed in planta bacterial growth of strains 85-10, 85-10ΔhpaC, 85-10hrcUY318D and 85-10hrcUY318DΔhpaC in susceptible ECW pepper plants. As described earlier, bacterial counts of strain 85-10ΔhpaC were significantly reduced 8 days post inoculation (dpi) when compared with the wild-type strain, suggesting that HpaC contributes to bacterial multiplication at later stages of the infection (Fig. 7B; Büttner et al., 2006). Strain 85-10hrcUY318DΔhpaC grew similarly to strain 85-10, which is in agreement with the observation that HrcUY318D suppresses the hpaC mutant phenotype with respect to disease symptoms (Fig. 7B).
HrcUY318D restores secretion of translocon and effector proteins but does not affect HrpB2 oversecretion in the hpaC deletion mutant
In addition to infection experiments, we performed T3S assays with strains 85*, 85*hrcUY318D, 85*ΔhpaC and 85*hrcUY318DΔhpaC. Figure 7C shows that comparable amounts of the putative translocon proteins HrpF and XopA and the effector proteins AvrBs3, XopJ-c-Myc and XopE2-c-Myc (encoded by corresponding expression constructs) were secreted by strains 85* and 85*hrcUY318D, respectively, whereas secretion of these proteins by strain 85*ΔhpaC was severely reduced as expected (Büttner et al., 2006). Efficient secretion was restored in strain 85*hrcUY318DΔhpaC, suggesting that HrcUY318D activates secretion of late T3S substrates including translocon and effector proteins in the absence of HpaC (Fig. 7C).
In addition to translocon and effector proteins, we analysed secretion of HrpB2, which is secreted in small amounts (at the detection limit of the HrpB2-specific antibody) by the wild-type strain and oversecreted by the hpaC deletion mutant (Fig. 7C; Rossier et al., 2000; Lorenz et al., 2008b). Interestingly, oversecretion of HrpB2 was also observed for strain 85*hrcUY318DΔhpaC. Thus, HrcUY318D suppresses the hpaC mutant phenotype with respect to disease symptoms and T3S of late substrates but does not affect secretion of HrpB2 (Fig. 7C). This finding was unexpected and implies that secretion of early (HrpB2) and late (translocon and effector proteins) T3S substrates in X. campestris pv. vesicatoria is controlled by different mechanisms that can be uncoupled.
We also investigated whether the Y318D mutation affects HrcU cleavage. For this, we generated an expression construct encoding HrcUY318D-c-Myc and analysed the protein in X. campestris pv. vesicatoria strain 85*ΔhrcU by immunoblotting. We detected the full-length HrcUY318D-c-Myc protein and the C-terminal cleavage product; however, the amounts of the cleavage product were significantly reduced when compared with the wild-type HrcU-c-Myc (Fig. 7D). A similar difference in proteolytic cleavage was observed in E. coli (Fig. 7D). As both c-Myc epitope-tagged HrcU derivatives were only synthesized at low levels in E. coli, we did not detect full-length HrcU-c-Myc and the C-terminal cleavage product of HrcUY318D-c-Myc (Fig. 7D). Taken together, we conclude from these findings that the Y318D exchange in HrcU prevents efficient HrcU cleavage but activates secretion of late substrates in the absence of HpaC.
The Y318D mutation affects binding of both HrpB2 and HpaC to HrcUC
As HrcUY318D presumably mimics a protein conformation that is permissive for the secretion of late substrates, we investigated a possible influence of the Y318D mutation on the interaction of HrcUC with HrpB2 and HpaC. For this, GST, GST–HrcU255–357 and GST–HrcU255–357/Y318D were immobilized on glutathione sepharose and incubated with HrpB2-c-Myc and HpaC-c-Myc respectively. Figure 8A shows that HrpB2-c-Myc and HpaC-c-Myc co-eluted with GST–HrcU255–357 as expected but were not detectable in the eluate of GST–HrcU255–357/Y318D, suggesting that the Y318D mutation prevents the stable binding of both HrpB2 and HpaC to HrcUC. Given the finding that HrpB2 is oversecreted by strain 85*hrcUY318DΔhpaC, it is conceivable that the interaction of HrcUC and HrpB2 is not required for efficient HrpB2 secretion after the substrate specificity switch.
Fig. 8.
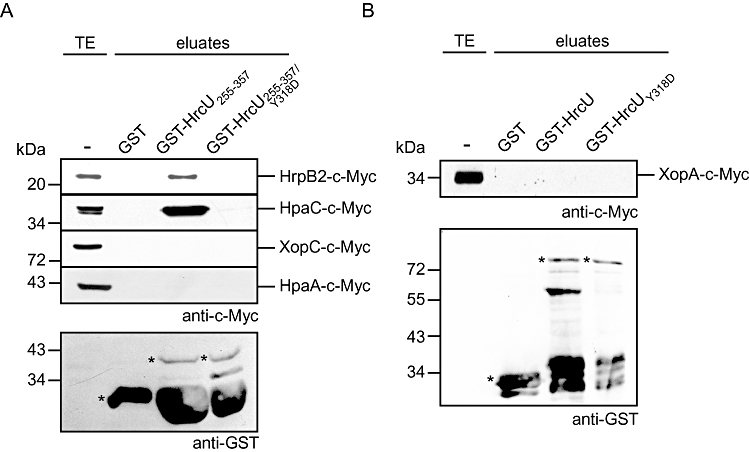
The Y318D mutation abolishes the interaction between the C-terminal region of HrcU and both HrpB2 and HpaC.
A. GST–HrcU255–357/Y318D does not interact with HrpB2, HpaC and T3S substrates. GST, GST–HrcU255–357 and GST–HrcU255–357/Y318D were immobilized on glutathione sepharose and incubated with E. coli lysates containing HrpB2-c-Myc, HpaC-c-Myc, XopC-c-Myc and HpaA-c-Myc respectively. Total-cell extracts (TE) and eluted proteins (eluates) were analysed by immunoblotting, using c-Myc- and GST-specific antibodies. Asterisks mark GST and GST fusion proteins; lower bands correspond to degradation products. One representative blot probed with the GST-specific antibody is shown.
B. HrcUY318D does not interact with the putative translocon protein XopA. GST, GST–HrcU and GST–HrcUY318D were immobilized on glutathione sepharose and incubated with XopA-c-Myc. TE and eluates were analysed as described in (A). Asterisks mark GST and GST fusion proteins; lower bands correspond to degradation products.
To date, HrpB2 is the only known T3S substrate that was shown to interact with HrcUC (Lorenz et al., 2008b). To investigate whether a potential binding of T3S substrates to HrcU is restricted to a certain protein conformation that is mimicked in the presence of HrcUY318D, we immobilized GST–HrcU255–357 and GST–HrcU255–357/Y318D on glutathione sepharose and incubated both proteins with C-terminally c-Myc epitope-tagged derivatives of the effector proteins HpaA and XopC respectively. Immunoblot analyses revealed that HpaA-c-Myc and XopC-c-Myc were not detectable in the eluates, suggesting that they did not stably interact with HrcU and HrcUY318D (Fig. 8A). Similarly, we did not detect a c-Myc epitope-tagged derivative of the putative translocon protein XopA in the eluates of GST–HrcU and GST–HrcUY318D (Fig. 8B).
Discussion
In this study, we describe novel mechanisms underlying the orchestration of T3S substrate specificity switching in a plant pathogenic bacterium. We investigated the role of the inner membrane protein HrcU and the T3S4 protein HpaC from X. campestris pv. vesicatoria during T3S and provide experimental evidence that HpaC binds to the conserved NPTH motif of HrcU, which is the predicted cleavage site. The analysis of HrcU mutant derivatives carrying single amino acid exchanges within the NPTH motif revealed that mutations of T266 and H267, respectively, only slightly affect HrcU cleavage whereas the N264A exchange abolishes detectable cleavage. For FlhB/YscU family members from animal pathogenic bacteria it was previously reported that cleavage is an autocatalytic process that involves cyclization of the conserved asparagine residue of the NPTH motif (Ferris et al., 2005; Deane et al., 2008; Zarivach et al., 2008; Lountos et al., 2009; Wiesand et al., 2009). In agreement with this model, mutation of the asparagine residue of the NPTH motif prevents cleavage not only of HrcU from X. campestris pv. vesicatoria but also of the homologous YscU, EscU and FlhB proteins from animal pathogenic bacteria (Lavander et al., 2002; Fraser et al., 2003; Sorg et al., 2007; Riordan and Schneewind, 2008; Zarivach et al., 2008; Björnfot et al., 2009; Smith et al., 2009; Wiesand et al., 2009). Exchange of the conserved proline residue P265 of HrcU by alanine led to a significant reduction of HrcU cleavage whereas the P265G mutation resulted in a complete loss of detectable cleavage (Fig. 1). A similar difference in cleavage was described for P264A and P264G mutant derivatives of the HrcU homologue YscU from Yersinia (Wiesand et al., 2009). Because autocatalytic cleavage of YscU depends on the positioning of the carbonyl group of the asparagine residue at position 263, the efficiency of the cleavage is presumably influenced by the amino acid residue at position 264 (Wiesand et al., 2009). Given the finding that YscU homologues share significant structural similarities (Deane et al., 2008; Zarivach et al., 2008; Lountos et al., 2009; Wiesand et al., 2009), a similar scenario might explain the difference in proteolytic cleavage of HrcUP265A and HrcUP265G.
Complementation studies with HrcU point mutants from X. campestris pv. vesicatoria revealed that loss of detectable HrcU cleavage correlates with a loss of bacterial pathogenicity (Fig. 2), which was confirmed for HrcUP265G by the analysis of a genomic hrcUP265G mutation (Fig. 3). This is an important experimental control because it was previously observed that the effects of point mutations in YscU from Yersinia might vary depending on whether YscU derivatives are provided in cis or in trans (Sorg et al., 2007; Björnfot et al., 2009). Taken together, we conclude from the analysis of HrcU point mutant derivatives that cleavage of HrcU is essential for the interaction of the bacteria with the plant. Notably, however, we also observed that HrcUC can function in trans, suggesting that it is not the cleavage event per se but rather the result of the cleavage which is required for pathogenicity (Fig. 4). A similar finding was previously reported for a HrcU homologue from Helicobacter pylori (Wand et al., 2006).
The N264A and P265A mutations in HrcU did not only lead to a loss of pathogenicity but also to a reduction in T3S of translocon and effector proteins. This is in contrast to the finding that the equivalent N263A exchange in the HrcU homologue YscU from Yersinia spp. abolished secretion of translocon but not of effector proteins (Sorg et al., 2007). It was therefore proposed that YscU cleavage is required to switch the T3S substrate specificity to translocon but not to effector proteins. The mechanisms underlying control of T3S substrate specificity switching might therefore vary in Yersinia spp. and X. campestris pv. vesicatoria.
In contrast to translocon and effector proteins, HrpB2 was efficiently secreted by HrcU cleavage mutants carrying alanine substitutions within the NPTH motif (Fig. 2). For yet unknown reasons, ectopic expression of hrcU under control of the lac promoter in a hrcU deletion mutant background led to increased HrpB2 secretion that was independent of HrcU cleavage. This implies that HrpB2 secretion is controlled by the amounts of HrcU and occurs prior to HrcU cleavage, which is in agreement with the notion that HrpB2 is an early substrate of the T3S system (Fig. 9). We previously reported that HrpB2 interacts with the C-terminal domain of HrcU (Lorenz et al., 2008b). Here, we show that HrpB2 does not stably interact with GST–HrcU deletion derivatives lacking the NPTH motif or carrying a P265G mutation (shown in the context of both GST–HrcU255–357 and GST–HrcU; Fig. 6). In contrast, N264A and P265A mutations in GST–HrcU255–357 did not significantly affect the interaction between HrcUC and HrpB2. It is conceivable that binding of HrpB2 depends on a certain conformation of HrcUC in or around the NPTH motif that is altered in P265G but not in N264A or P265A HrcU mutant derivatives. However, the P265G mutation presumably did not lead to a complete misfolding of HrcU because the interaction with the putative ATPase regulator HrcL and the general T3S chaperone HpaB was not affected. Notably, HrcUP265G did not promote secretion of HrpB2, which is in contrast to the mutant derivatives HrcUN264A and HrcUP265A (Fig. 2). It is therefore possible that the interaction between HrpB2 and HrcUC is required for the efficient secretion of HrpB2 during the early stage of the T3S process, i.e. prior to HrcU cleavage (Fig. 9).
Fig. 9.
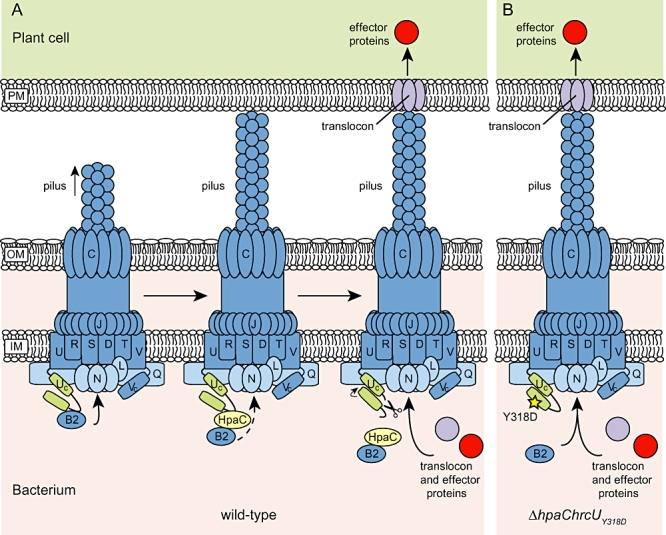
Model of the molecular mechanisms underlying the HpaC-HrcUC-mediated substrate specificity switch in X. campestris pv. vesicatoria.
A. HpaC controls secretion of early and late T3S substrates. The T3S system of X. campestris pv. vesicatoria consists of approximately 20 components, eleven of which (abbreviated with single letters) are designated Hrc (Hrp conserved) and presumably constitute the core components of the membrane-spanning secretion apparatus. Cytoplasmic components of the T3S apparatus are shown in light blue, the C-terminal domain of HrcU in green. During the initial step of T3S the early T3S substrate HrpB2 (abbreviated B2), which is required for pilus assembly, interacts with the C-terminal cytoplasmic domain of HrcU and is secreted. The efficient secretion of HrpB2 is inhibited upon binding of the T3S4 protein HpaC to HrpB2 and/or to HrcUC. The cleavage of HrcU at the conserved NPTH motif and a conformational change in HrcUC lead to the release of HrcUC-bound HpaC and HrpB2 and activate the secretion of late substrates including translocon and effector proteins. Dashed lines refer to reduced secretion of HrpB2, the arrow next to HrcUC to the predicted conformational change.
B. The Y318D mutation in HrcUC activates secretion of late substrates in the absence of HpaC. The Y318D mutation presumably leads to a conformational change in HrcUC, which allows the efficient secretion of late substrates but leads to reduced cleavage of HrcU and also abolishes the interaction between HrcUC and HrpB2. Secretion of HrpB2 is not affected by the Y318D exchange in HrcU.IM, inner membrane; OM, outer membrane; PM, plasma membrane of the host cell.
The results of our protein–protein interaction studies showed that mutations in the NPTH motif of HrcU did not only affect the HrcUC–HrpB2 interaction but also the binding of HpaC to HrcUC (Fig. 5). To our knowledge, this is the first experimental evidence that T3S4 proteins and T3S substrates compete for the same binding site in the C-terminal domains of FlhB/YscU family members. It remains to be investigated whether HpaC from X. campestris pv. vesicatoria prevents the efficient secretion of HrpB2 by blocking its access to HrcUC. Alternatively, given the finding that HpaC interacts with HrpB2 (Lorenz et al., 2008b), a direct interaction of both proteins in the bacterial cytoplasm might interfere with efficient HrpB2 secretion (Fig. 9). The latter hypothesis would explain the inability of HrcUY318D to restore wild-type levels of HrpB2 secretion in the absence of HpaC (see below; Figs 7 and 9). As the Y318D mutation in HrcU suppressed the hpaC mutant phenotype with respect to secretion of late substrates and disease symptom formation (Fig. 7), we conclude that the elevated levels of secreted HrpB2 in the hrcUY318DΔhpaC double mutant are not detrimental for bacterial pathogenicity.
HrpB2 shares limited sequence similarity with predicted inner rod proteins from animal pathogenic bacteria that presumably assemble at the base of the needle (Sukhan et al., 2003; Marlovits et al., 2006). It was previously reported that the inner rod protein YscI from Yersinia spp. is oversecreted in the absence of the T3S4 protein YscP (Wood et al., 2008). This is reminiscent of our finding that HrpB2 is oversecreted in the hpaC deletion mutant. Notably, however, wild-type secretion levels of YscI in the yscP mutant can be restored upon introduction of the point mutation Y317D into YscU (equivalent to mutation Y318D in HrcU from X. campestris pv. vesicatoria). As YscUY317D also restores the wild-type phenotype in the yscP mutant (Edqvist et al., 2003), the control mechanisms underlying secretion of YscI and late T3S substrates from Yersinia spp. are presumably linked. Notably, this is in contrast to our finding that HrcUY318D activates secretion of late substrates without affecting HrpB2 secretion. Secretion of HrpB2 and late substrates from X. campestris pv. vesicatoria is therefore presumably controlled by independent mechanisms that can be uncoupled. Given the possibility that HrpB2 secretion is directly controlled by HpaC, we will localize HpaC binding sites in HrpB2 and analyse their contribution to the control of HrpB2 secretion in future studies.
The identification of the Y318D exchange in HrcUC as an extragenic suppressor mutation of the hpaC mutant phenotype is reminiscent of the finding that point mutations in the C-terminal domains of FlhB and YscU suppress the phenotypes of T3S4 mutants from S. typhimurium and Y. pseudotuberculosis, respectively (Williams et al., 1996; Edqvist et al., 2003; Wood et al., 2008), and confirms the predicted role of the C-terminal domain of HrcU during the substrate specificity switch. It is tempting to speculate that HrcUY318D mimics a protein conformation of HrcU that allows the efficient secretion of late substrates including translocon and effector proteins in the absence of the T3S4 protein HpaC. Interestingly, the Y318D mutation in HrcU did not only suppress the hpaC mutant phenotype but also led to a significant reduction in proteolytic cleavage of HrcU and abolished stable binding of both HpaC and HrpB2 to HrcUC. Comparative sequence and crystal structure analyses of HrcU homologues from animal pathogenic bacteria revealed that the tyrosine residues corresponding to Y318 of HrcU are part of a conserved LARXLY amino acid motif, which is positioned in the vicinity of the PTH loop (Deane et al., 2008; Zarivach et al., 2008). Mutations in the LARXLY motif can therefore alter the orientation of the PTH loop, which might explain the reduced proteolytic cleavage and impaired binding of HrcUY318D to HpaC and HrpB2. The finding that HrcUY318D is less efficiently cleaved but suppresses the hpaC mutant phenotype suggests that secretion of late substrates can occur in the absence of efficient cleavage of HrcU and thus supports the notion that the T3S substrate specificity switch rather depends on a certain protein conformation of HrcU than on the cleavage event itself. Furthermore, we conclude from our data that the T3S substrate specificity switch that is mimicked in the presence of HrcUY318D leads to the release of HrcUC-bound HpaC and HrpB2 (Fig. 9). As the Y318D exchange did not alter HrpB2 oversecretion in the hpaC deletion mutant, we assume that the interaction between HrcUC and HrpB2 is dispensable for efficient HrpB2 secretion during later stages of the T3S process, i.e. after the T3S substrate specificity switch (Fig. 9). It remains to be investigated whether the switch and thus the predicted conformational change in HrcUC expose additional substrate acceptor sites at the inner membrane that could promote the entry of HrpB2 into the T3S channel in the absence of the HrcUC–HrpB2 interaction. In future studies, we therefore aim at the identification of T3S substrate docking sites in conserved components of the T3S system that are associated with the inner bacterial membrane.
Experimental procedures
Bacterial strains and growth conditions
Bacterial strains and plasmids used in this study are listed in Table 1. E. coli cells were grown at 37°C in lysogeny broth (LB) or Super medium (Qiagen, Hilden, Germany). X. campestris pv. vesicatoria strains were cultivated at 30°C in nutrient-yeast-glycerol (NYG) medium (Daniels et al., 1984) or in minimal medium A (Ausubel et al., 1996) supplemented with sucrose (10 mM) and casamino acids (0.3%). Plasmids were introduced into E. coli by electroporation and into X. campestris pv. vesicatoria by conjugation, using pRK2013 as a helper plasmid in triparental matings (Figurski and Helinski, 1979). Antibiotics were added to the media at the following final concentrations: ampicillin, 100 µg ml−1; kanamycin, 25 µg ml−1; rifampicin, 100 µg ml−1; spectinomycin, 100 µg ml−1; gentamicin, 7.5 µg ml−1.
Table 1.
Bacterial strains and plasmids used in this study
| Relevant characteristics | Reference or source | |
|---|---|---|
| X. campestris pv. vesicatoria | ||
| 85-10 | Pepper-race 2; wild type; Rifr | Canteros (1990); Kousik and Ritchie (1998) |
| 85-10ΔhrcU | 85-10 derivative deleted in codons 3–351 of hrcU | This study |
| 85-10ΔhrcU265–357 | 85-10 derivative deleted in codons 265–357 of hrcU | This study |
| 85-10hrcUY318D | hrcUY318D mutant derivative of strain 85-10 | This study |
| 85-10hrcUP265G | hrcUP265G mutant derivative of strain 85-10 | This study |
| 85-10ΔhpaC | hpaC deletion mutant of strain 85-10 | Büttner et al. (2006) |
| 85-10hrcUY318DΔhpaC | hrcUY318D mutant derivative of strain 85-10ΔhpaC | This study |
| 85* | 85-10 derivative containing the hrpG* mutation | Wengelnik et al. (1999) |
| 85*ΔhrcU | 85* derivative deleted in codons 3–351 of hrcU | This study |
| 85*ΔhrcU265–357 | 85* derivative deleted in codons 265–357 of hrcU | This study |
| 85*hrcUY318D | hrcUY318D mutant derivative of strain 85* | This study |
| 85*hrcUP265G | hrcUP265G mutant derivative of strain 85* | This study |
| 85*ΔhpaC | hpaC deletion mutant of strain 85* | Büttner et al. (2006) |
| 85*hrcUY318DΔhpaC | hrcUY318D mutant derivative of strain 85*ΔhpaC | This study |
| 85*hrcUP265GΔhpaC | hrcUP265G mutant derivative of strain 85*ΔhpaC | This study |
| E. coli | ||
| BL21 (DE3) | F-ompT hsdSB (rB- mB-) gal dcm (DE3) | Stratagene, Heidelberg, Germany |
| DH5α | F-recA hsdR17(rk-,mk+) Φ80dlacZΔM15 | Bethesda Research Laboratories, Bethesda, MD |
| DH5αλpir | F-recA hsdR17(rk-,mk+) Φ80dlacZΔM15 [λpir] | Ménard et al. (1993) |
| Plasmids | ||
| pBlueskript(II) KS | Phagemid, pUC derivative; Apr | Stratagene |
| pBRM | Golden Gate-compatible derivative of pBBR1MCS-5 | Szczesny et al. (2010) |
| pBRMhrcU | pBRM derivative encoding HrcU-c-Myc | This study |
| pBRMhrcUY318D | pBRM derivative encoding HrcUY318D-c-Myc | This study |
| pBRMhrcUN264A | pBRM derivative encoding HrcUN264A-c-Myc | This study |
| pBRMhrcUP265A | pBRM derivative encoding HrcUP265A-c-Myc | This study |
| pBRMhrcUT266A | pBRM derivative encoding HrcUT266A-c-Myc | This study |
| pBRMhrcUH267A | pBRM derivative encoding HrcUH267A-c-Myc | This study |
| pBRMhrcUP265G | pBRM derivative encoding HrcUP265G-c-Myc | This study |
| pBRMhrcU265–357 | pBRM derivative encoding HrcU265–357-c-Myc | This study |
| pBRMhrcU206–357 | pBRM derivative encoding HrcU206–357-c-Myc | This study |
| pBRMhrcU206–357/N264A | pBRM derivative encoding HrcU206–357/N264A-c-Myc | This study |
| pBRMhrcU206–357/P265A | pBRM derivative encoding HrcU206–357/P265A-c-Myc | This study |
| pBRMhrcU206–357/T266A | pBRM derivative encoding HrcU206–357/T266A-c-Myc | This study |
| pBRMhrcU206–357/H267A | pBRM derivative encoding HrcU206–357/H267A-c-Myc | This study |
| pBRMxopA | pBRM derivative encoding XopA-c-Myc | This study |
| pBRMxopE2 | pBRM derivative encoding XopE2-c-Myc | This study |
| pBRMxopJ | pBRM derivative encoding XopJ-c-Myc | This study |
| pDSK602 | Broad-host-range vector; contains triple lacUV5 promoter; Smr | Murillo et al. (1994) |
| pDSK604 | Derivative of pDSK602 with modified polylinker | Escolar et al. (2001) |
| pDMhpaA | pDSK604 derivative encoding HpaA-c-Myc | K. Hahn and U. Bonas (unpublished) |
| pDMhpaB | pDSK604 derivative encoding HpaB-c-Myc | Büttner et al. (2004) |
| pDMhpaC | pDSK604 derivative encoding HpaC-c-Myc | Büttner et al. (2006) |
| pDMhrcL | pDSK604 derivative encoding HrcL-c-Myc | Lorenz and Büttner (2009) |
| pDMhrpB2 | pDSK602 derivative encoding HrpB2-c-Myc | Lorenz et al. (2008b) |
| pDMxopC | pDSK602 derivative encoding XopC-c-Myc | Büttner et al. (2007) |
| pDSF300 | pDSK602 derivative encoding AvrBs3-FLAG | Van den Ackerveken et al. (1996) |
| pGEX-2TKM | GST expression vector; ptac GST lacIq pBR322 ori; Apr, derivative of pGEX-2TK with polylinker of pDSK604 | Stratagene; Escolar et al. (2001) |
| pGhrcU | pGEX-2TKM derivative encoding GST–HrcU | Lorenz et al. (2008b) |
| pGhrcUY318D | pGEX-2TKM derivative encoding GST–HrcUY318D | This study |
| pGhrcUN264A | pGEX-2TKM derivative encoding GST–HrcUN264A | This study |
| pGhrcUP265A | pGEX-2TKM derivative encoding GST–HrcUP265A | This study |
| pGhrcUP265G | pGEX-2TKM derivative encoding GST–HrcUP265G | This study |
| pGhrcU255–357 | pGEX-2TKM derivative encoding GST–HrcU255–357 | Lorenz et al. (2008b) |
| pGhrcU255–357/Y318D | pGEX-2TKM derivative encoding GST–HrcU255–357/Y318D | This study |
| pGhrcU255–357/N264A | pGEX-2TKM derivative encoding GST–HrcU255–357/N264A | This study |
| pGhrcU255–357/P265A | pGEX-2TKM derivative encoding GST–HrcU255–357/P265A | This study |
| pGhrcU255–357/P265G | pGEX-2TKM derivative encoding GST–HrcU255–357/P265G | This study |
| pOK1 | Suicide vector; sacB sacQ mobRK2 oriR6K; Smr | Huguet et al. (1998) |
| pOKΔhrcU | Derivative of pOK carrying the flanking regions of hrcU | This study |
| pOKΔhrcUC | Derivative of pOK carrying the flanking regions of hrcU265–357 | This study |
| pOKhrcUY318D | Derivative of pOK carrying hrcUY318D | This study |
| pOKhrcUP265G | Derivative of pOK carrying hrcUP265G | This study |
| pRK2013 | ColE1 replicon, TraRK+ Mob+; Kmr | Figurski and Helinski (1979) |
| pUC119 | ColE1 replicon; Apr | Vieira and Messing (1987) |
Ap, ampicillin; Km, kanamycin; Rif, rifampicin; Sm, spectinomycin; Gm, gentamicin; r, resistant.
Plant material and plant inoculations
The near-isogenic pepper cultivars Early Cal Wonder (ECW) and ECW-10R (Kousik and Ritchie, 1998; Astua-Monge et al., 2000) were grown and inoculated with X. campestris pv. vesicatoria as described previously (Bonas et al., 1991). Briefly, bacteria were inoculated into the intercellular spaces of leaves with a needle-less syringe at concentrations of 2 × 108 colony-forming units (cfu) ml−1 in 1 mM MgCl2 if not stated otherwise. The appearance of disease symptoms and the HR were scored over a period of one to eleven dpi. For the better visualization of the HR, leaves were bleached in 70% ethanol. Experiments were repeated at least three times. For in planta growth curves, bacteria were inoculated at a density of 104 cfu ml−1 into leaves of susceptible ECW plants. Bacterial counts were determined over a period of 7–10 dpi as described (Bonas et al., 1991).
Generation of X. campestris pv. vesicatoria hrcU mutants
To create a 1047 bp in-frame deletion of hrcU (deletion of codons 3–351), we amplified the flanking regions of hrcU including the first 6 and the last 23 bp of the gene by PCR and cloned the PCR products into the ApaI/SalI sites of the suicide plasmid pOK1. The resulting construct pOKΔhrcU was conjugated into X. campestris pv. vesicatoria strains 85-10 and 85*. Double cross-overs resulted in hrcU deletion mutants that were selected as described previously (Huguet et al., 1998). Sequences of primers used in this study are available upon request.
For the generation of a hrcU265–357 deletion mutant (deletion of codons 265–357), 750 bp of both flanking regions were amplified by PCR and cloned into the ApaI/SalI sites of pOK1. The resulting construct pOKΔhrcUC was conjugated into strains 85-10 and 85*. Double cross-overs resulted in strains 85-10ΔhrcU265–357 and 85*ΔhrcU265–357 respectively.
For the introduction of the Y318D mutation into genomic hrcU, we amplified 800 bp fragments flanking codon 318 of hrcU with a 9 bp overlap that spans codons 317–319 of hrcU. Both amplicons contained a CTG to CTT exchange (silent mutation of codon 317) which creates a BclI site and a TAT to GAT exchange which leads to an exchange of Y318 by D318. PCR products were digested with XbaI/BclI and BclI/SalI, respectively, and cloned into the XbaI/SalI sites of plasmid pOK1. The resulting construct pOKhrcUY318D was conjugated into strain 85-10, 85-10ΔhpaC, 85* and 85*ΔhpaC. Double cross-overs resulted in strains 85-10hrcUY318D, 85-10hrcUY318DΔhpaC, 85*hrcUY318D and 85*hrcUY318DΔhpaC respectively.
To introduce a point mutation into genomic hrcU leading to the P265G exchange, we amplified 750 bp fragments flanking codon 265 of hrcU with a 9 bp overlap that spans codons 265–267. Both amplicons contained a CCG to GGT exchange (mutation of codon 265) which creates a KpnI site and leads to the P265G mutation. PCR products were digested with XbaI/KpnI and KpnI/BamHI, respectively, and cloned into the XbaI/SalI sites of plasmid pOK1. The resulting construct pOKhrcUP265G was conjugated into strains 85-10, 85* and 85*ΔhpaC. Double cross-overs resulted in strains 85-10hrcUP265G, 85*hrcUP265G and 85*hrcUP265GΔhpaC respectively.
Generation of Golden Gate-expression constructs
For the generation of expression constructs encoding c-Myc epitope-tagged HrcU derivatives, hrcU or hrcU fragments encoding amino acids 265–357 and 206–357, respectively, were amplified by PCR and cloned into the Golden Gate-compatible expression vector pBRM in a one step restriction–ligation reaction as described (Engler et al., 2008). pBRM contains a single lac promoter and allows expression of genes in fusion with a C-terminal c-Myc epitope-encoding sequence (Szczesny et al., 2010). The Golden Gate system is based on type IIs restriction enzymes (e.g. BsaI) that cut DNA outside of the enzyme's recognition site. For the generation of hrcUY318D-c-myc and hrcUP265G-c-myc expression constructs, hrcUY318D and hrcUP265G were amplified by PCR from strains 85-10hrcUY318D and 85-10hrcUP265G, respectively, and cloned into pBRM. Furthermore, xopA, xopE2 and xopJ were amplified from X. campestris pv. vesicatoria strain 85-10 and cloned into pBRM. Expression constructs are listed in Table 1.
To generate HrcU point mutant derivatives with amino acid exchanges within the NPTH motif, codons 1–271 and 271–357 of hrcU were amplified by PCR. Both amplicons contained a 4 bp overlap and were cloned into pBRM in a single restriction–ligation reaction, generating pBRMhrcU. Individual mutations of codons 264–268, which replaced the amino acids N, P, T and H by A, respectively, were introduced by primer sequences (codon 264: AAC exchanged by GCC, codon 265: CCG exchanged by GCG, codon 266: ACC exchanged by GCC and codon 267: CAT exchanged by GCT). Similarly, for the introduction of point mutations into the NPTH motif of HrcU206–357-c-Myc, codons 206–271 and 271–357 of hrcU, respectively, were amplified by PCR and amplicons were cloned into pBRM as described above.
Generation of pGEX constructs
To construct GST–HrcU265–357 and GST–HrcU268–357 fusion proteins, corresponding hrcU fragments were amplified by PCR and cloned into the EcoRI/XhoI sites of pGEX, downstream and in frame with the GST-encoding sequence. For the generation of GST–HrcUY318D and GST–HrcU255–357/Y318D fusion proteins, hrcUY318D and hrcU255–357/Y318D were amplified by PCR from strain 85-10hrcUY318D and cloned into the EcoRI/XhoI sites of pGEX. To construct expression constructs encoding GST–HrcUN264A, GST–HrcUP265A, GST–HrcUP265G, GST–HrcU255–357(N264A), GST–HrcU255–357(P265A) and GST–HrcU255–357(P265G), respectively, corresponding hrcU fragments were amplified from pBRMhrcUN264A, pBRMhrcUP265A and pBRMhrcUP265G and cloned into pGEX as described above.
T3S assays and immunoblot analyses
Type III secretion assays were performed as described previously (Rossier et al., 1999). Briefly, bacteria were incubated in minimal medium A at pH 5.3 and equal amounts of bacterial total-cell extracts and culture supernatants were analysed by SDS-PAGE and immunoblotting (Rossier et al., 1999). In this study, we used polyclonal antibodies specific for HrpF (Büttner et al., 2002), XopA (Noël et al., 2002), AvrBs3 (Knoop et al., 1991) and HrpB2 (Rossier et al., 2000), respectively, and monoclonal anti-c-Myc (Roche Applied Science, Mannheim, Germany) and anti-GST antibodies (GE Healthcare, Munich, Germany). Horseradish peroxidase-labelled anti-rabbit, anti-mouse and anti-goat antibodies (GE Healthcare) were used as secondary antibodies. Antibody reactions were visualized by enhanced chemiluminescence (GE Healthcare). Experiments were repeated at least two times. Blots were routinely reacted with an antibody specific for the intracellular protein HrcN (Rossier et al., 2000) to ensure that no bacterial lysis had occurred (data not shown).
GST pull-down assays
For GST pull-down assays, GST and GST fusion proteins were synthesized in E. coli BL21(DE3). Bacterial cells from 50 ml of cultures were resuspended in phosphate-buffered saline (PBS) and broken with a French press. Insoluble cell debris was removed by centrifugation and soluble GST and GST fusion proteins were immobilized on a glutathione sepharose matrix according to the manufacturer's instructions (GE Healthcare). Unbound proteins were removed by washing twice with PBS and the glutathione sepharose matrix was incubated with 600 µl of E. coli cell lysates containing c-Myc epitope-tagged derivatives of the putative interaction partners for 2 h at 4°C. Unbound proteins were removed by washing four times with PBS and bound proteins were eluted with 10 mM reduced glutathione at room temperature for 2 h. Ten microlitres of total protein lysates and 20 µl eluted proteins were analysed by SDS-PAGE and immunoblotting.
Acknowledgments
We are grateful to M. Jordan for technical assistance and to U. Bonas for critical comments on the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (BU2145/1-1) and the Sonderforschungsbereich SFB 648 ‘Molekulare Mechanismen der Informationsverarbeitung in Pflanzen’ to D.B.
Supporting information
Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Agrain C, Callebaut I, Journet L, Sorg I, Paroz C, Mota LJ, Cornelis GR. Characterization of a type III secretion substrate specificity switch (T3S4) domain in YscP from Yersinia enterocolitica. Mol Microbiol. 2005;56:54–67. doi: 10.1111/j.1365-2958.2005.04534.x. [DOI] [PubMed] [Google Scholar]
- Allaoui A, Woestyn S, Sluiters C, Cornelis GR. YscU, a Yersinia enterocolitica inner membrane protein involved in Yop secretion. J Bacteriol. 1994;176:4534–4542. doi: 10.1128/jb.176.15.4534-4542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- Arnold R, Brandmaier S, Kleine F, Tischler P, Heinz E, Behrens S, et al. Sequence-based prediction of type III secreted proteins. PLoS Pathog. 2009;5:e1000376. doi: 10.1371/journal.ppat.1000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astua-Monge G, Minsavage GV, Stall RE, Davis MJ, Bonas U, Jones JB. Resistance of tomato and pepper to T3 strains of Xanthomonas campestris pv. vesicatoria is specified by a plant-inducible avirulence gene. Mol Plant Microbe Interact. 2000;13:911–921. doi: 10.1094/MPMI.2000.13.9.911. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1996. [Google Scholar]
- Berger C, Robin GP, Bonas U, Koebnik R. Membrane topology of conserved components of the type III secretion system from the plant pathogen Xanthomonas campestris pv. vesicatoria. Microbiology. 2010;156:1963–1974. doi: 10.1099/mic.0.039248-0. [DOI] [PubMed] [Google Scholar]
- Björnfot AC, Lavander M, Forsberg A, Wolf-Watz H. Auto-proteolysis of YscU of Yersinia pseudotuberculosis is important for regulation of expression and secretion of Yop proteins. J Bacteriol. 2009;191:4259–4267. doi: 10.1128/JB.01730-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block A, Li G, Fu ZQ, Alfano JR. Phytopathogen type III effector weaponry and their plant targets. Curr Opin Plant Biol. 2008;11:396–403. doi: 10.1016/j.jbi.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonas U, Schulte R, Fenselau S, Minsavage GV, Staskawicz BJ, Stall RE. Isolation of a gene-cluster from Xanthomonas campestris pv. vesicatoria that determines pathogenicity and the hypersensitive response on pepper and tomato. Mol Plant Microbe Interact. 1991;4:81–88. [Google Scholar]
- Botteaux A, Sani M, Kayath CA, Boekema EJ, Allaoui A. Spa32 interaction with the inner-membrane Spa40 component of the type III secretion system of Shigella flexneri is required for the control of the needle length by a molecular tape measure mechanism. Mol Microbiol. 2008;70:1515–1528. doi: 10.1111/j.1365-2958.2008.06499.x. [DOI] [PubMed] [Google Scholar]
- Büttner D, Bonas U. Port of entry – the type III secretion translocon. Trends Microbiol. 2002a;10:186–192. doi: 10.1016/s0966-842x(02)02331-4. [DOI] [PubMed] [Google Scholar]
- Büttner D, Bonas U. Getting across-bacterial type III effector proteins on their way to the plant cell. EMBO J. 2002b;21:5313–5322. doi: 10.1093/emboj/cdf536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner D, Nennstiel D, Klüsener B, Bonas U. Functional analysis of HrpF, a putative type III translocon protein from Xanthomonas campestris pv. vesicatoria. J Bacteriol. 2002;184:2389–2398. doi: 10.1128/JB.184.9.2389-2398.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner D, Gürlebeck D, Noel LD, Bonas U. HpaB from Xanthomonas campestris pv. vesicatoria acts as an exit control protein in type III-dependent protein secretion. Mol Microbiol. 2004;54:755–768. doi: 10.1111/j.1365-2958.2004.04302.x. [DOI] [PubMed] [Google Scholar]
- Büttner D, Lorenz C, Weber E, Bonas U. Targeting of two effector protein classes to the type III secretion system by a HpaC- and HpaB-dependent protein complex from Xanthomonas campestris pv. vesicatoria. Mol Microbiol. 2006;59:513–527. doi: 10.1111/j.1365-2958.2005.04924.x. [DOI] [PubMed] [Google Scholar]
- Büttner D, Noël L, Stuttmann J, Bonas U. Characterization of the non-conserved hpaB–hrpF region in the hrp pathogenicity island from Xanthomonas campestris pv. vesicatoria. Mol Plant Microbe Interact. 2007;20:1063–1074. doi: 10.1094/MPMI-20-9-1063. [DOI] [PubMed] [Google Scholar]
- Canteros BI. FL, USA: University of Florida; 1990. Diversity of plasmids and plasmid-encoded phenotypic traits in Xanthomonas campestris pv. vesicatoria. PhD thesis. [Google Scholar]
- Coombes BK, Finlay BB. Insertion of the bacterial type III translocon: not your average needle stick. Trends Microbiol. 2005;13:92–95. doi: 10.1016/j.tim.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Cornelis GR, Agrain C, Sorg I. Length control of extended protein structures in bacteria and bacteriophages. Curr Opin Microbiol. 2006;9:201–206. doi: 10.1016/j.mib.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Daniels MJ, Barber CE, Turner PC, Sawczyc MK, Byrde RJW, Fielding AH. Cloning of genes involved in pathogenicity of Xanthomonas campestris pv. campestris using the broad host range cosmid pLAFR1. EMBO J. 1984;3:3323–3328. doi: 10.1002/j.1460-2075.1984.tb02298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane JE, Graham SC, Mitchell EP, Flot D, Johnson S, Lea SM. Crystal structure of Spa40, the specificity switch for the Shigella flexneri type III secretion system. Mol Microbiol. 2008;69:267–276. doi: 10.1111/j.1365-2958.2008.06293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvaux M, Hebraud M, Henderson IR, Pallen MJ. Type III secretion: what's in a name? Trends Microbiol. 2006;14:157–160. doi: 10.1016/j.tim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Edqvist PJ, Olsson J, Lavander M, Sundberg L, Forsberg A, Wolf-Watz H, Lloyd SA. YscP and YscU regulate substrate specificity of the Yersinia type III secretion system. J Bacteriol. 2003;185:2259–2266. doi: 10.1128/JB.185.7.2259-2266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C, Kandzia R, Marillonnet S. A one pot, one step, precision cloning method with high throughput capability. PLoS ONE. 2008;3:e3647. doi: 10.1371/journal.pone.0003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escolar L, Van den Ackerveken G, Pieplow S, Rossier O, Bonas U. Type III secretion and in planta recognition of the Xanthomonas avirulence proteins AvrBs1 and AvrBsT. Mol Plant Pathol. 2001;2:287–296. doi: 10.1046/j.1464-6722.2001.00077.x. [DOI] [PubMed] [Google Scholar]
- Fenselau S, Bonas U. Sequence and expression analysis of the hrpB pathogenicity operon of Xanthomonas campestris pv. vesicatoria which encodes eight proteins with similarity to components of the Hrp, Ysc, Spa, and Fli secretion systems. Mol Plant Microbe Interact. 1995;8:845–854. doi: 10.1094/mpmi-8-0845. [DOI] [PubMed] [Google Scholar]
- Fenselau S, Balbo I, Bonas U. Determinants of pathogenicity in Xanthomonas campestris pv. vesicatoria are related to proteins involved in secretion in bacterial pathogens of animals. Mol Plant Microbe Interact. 1992;5:390–396. doi: 10.1094/mpmi-5-390. [DOI] [PubMed] [Google Scholar]
- Ferris HU, Minamino T. Flipping the switch: bringing order to flagellar assembly. Trends Microbiol. 2006;14:519–526. doi: 10.1016/j.tim.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Ferris HU, Furukawa Y, Minamino T, Kroetz MB, Kihara M, Namba K, Macnab RM. FlhB regulates ordered export of flagellar components via autocleavage mechanism. J Biol Chem. 2005;280:41236–41242. doi: 10.1074/jbc.M509438200. [DOI] [PubMed] [Google Scholar]
- Figurski D, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GM, Hirano T, Ferris HU, Devgan LL, Kihara M, Macnab RM. Substrate specificity of type III flagellar protein export in Salmonella is controlled by subdomain interactions in FlhB. Mol Microbiol. 2003;48:1043–1057. doi: 10.1046/j.1365-2958.2003.03487.x. [DOI] [PubMed] [Google Scholar]
- Galan JE. Common themes in the design and function of bacterial effectors. Cell Host Microbe. 2009;5:571–579. doi: 10.1016/j.chom.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. Process of protein transport by the type III secretion system. Microbiol Mol Biol Rev. 2004;68:771–795. doi: 10.1128/MMBR.68.4.771-795.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SY, Nomura K, Whittam TS. Type III protein secretion mechanism in mammalian and plant pathogens. Biochim Biophys Acta. 2004;1694:181–206. doi: 10.1016/j.bbamcr.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Huguet E, Bonas U. hrpF of Xanthomonas campestris pv. vesicatoria encodes an 87-kDa protein with homology to NolX of Rhizobium fredii. Mol Plant Microbe Interact. 1997;10:488–498. doi: 10.1094/MPMI.1997.10.4.488. [DOI] [PubMed] [Google Scholar]
- Huguet E, Hahn K, Wengelnik K, Bonas U. hpaA mutants of Xanthomonas campestris pv. vesicatoria are affected in pathogenicity but retain the ability to induce host-specific hypersensitive reaction. Mol Microbiol. 1998;29:1379–1390. doi: 10.1046/j.1365-2958.1998.01019.x. [DOI] [PubMed] [Google Scholar]
- Jin Q, He SY. Role of the Hrp Pilus in type III protein secretion in Pseudomonas syringae. Science. 2001;294:2556–2558. doi: 10.1126/science.1066397. [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Journet L, Agrain C, Broz P, Cornelis GR. The needle length of bacterial injectisomes is determined by a molecular ruler. Science. 2003;302:1757–1760. doi: 10.1126/science.1091422. [DOI] [PubMed] [Google Scholar]
- Knoop V, Staskawicz B, Bonas U. Expression of the avirulence gene avrBs3 from Xanthomonas campestris pv. vesicatoria is not under the control of hrp genes and is independent of plant factors. J Bacteriol. 1991;173:7142–7150. doi: 10.1128/jb.173.22.7142-7150.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebnik R. The role of bacterial pili in protein and DNA translocation. Trends Microbiol. 2001;9:586–590. doi: 10.1016/s0966-842x(01)02255-7. [DOI] [PubMed] [Google Scholar]
- Kousik CS, Ritchie DF. Response of bell pepper cultivars to bacterial spot pathogen races that individually overcome major resistance genes. Plant Dis. 1998;82:181–186. doi: 10.1094/PDIS.1998.82.2.181. [DOI] [PubMed] [Google Scholar]
- Kutsukake K, Minamino T, Yokoseki T. Isolation and characterization of FliK-independent flagellation mutants from Salmonella typhimurium. J Bacteriol. 1994;176:7625–7629. doi: 10.1128/jb.176.24.7625-7629.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavander M, Sundberg L, Edqvist PJ, Lloyd SA, Wolf-Watz H, Forsberg A. Proteolytic cleavage of the FlhB homologue YscU of Yersinia pseudotuberculosis is essential for bacterial survival but not for type III secretion. J Bacteriol. 2002;184:4500–4509. doi: 10.1128/JB.184.16.4500-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CM, Brown I, Mansfield J, Stevens C, Boureau T, Romantschuk M, Taira S. The Hrp pilus of Pseudomonas syringae elongates from its tip and acts as a conduit for translocation of the effector protein HrpZ. EMBO J. 2002;21:1909–1915. doi: 10.1093/emboj/21.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd SA, Norman M, Rosqvist R, Wolf-Watz H. Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol Microbiol. 2001;39:520–532. doi: 10.1046/j.1365-2958.2001.02271.x. [DOI] [PubMed] [Google Scholar]
- Lorenz C, Büttner D. Functional characterization of the type III secretion ATPase HrcN from the plant pathogen Xanthomonas campestris pv. vesicatoria. J Bacteriol. 2009;191:1414–1428. doi: 10.1128/JB.01446-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz C, Kirchner O, Egler M, Stuttmann J, Bonas U, Büttner D. HpaA from Xanthomonas is a regulator of type III secretion. Mol Microbiol. 2008a;69:344–360. doi: 10.1111/j.1365-2958.2008.06280.x. [DOI] [PubMed] [Google Scholar]
- Lorenz C, Schulz S, Wolsch T, Rossier O, Bonas U, Büttner D. HpaC controls substrate specificity of the Xanthomonas type III secretion system. PLoS Pathog. 2008b;4:e1000094. doi: 10.1371/journal.ppat.1000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lountos GT, Austin BP, Nallamsetty S, Waugh DS. Atomic resolution structure of the cytoplasmic domain of Yersinia pestis YscU, a regulatory switch involved in type III secretion. Protein Sci. 2009;18:467–474. doi: 10.1002/pro.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab RM. Type III flagellar protein export and flagellar assembly. Biochim Biophys Acta. 2004;1694:207–217. doi: 10.1016/j.bbamcr.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Marlovits TC, Kubori T, Lara-Tejero M, Thomas D, Unger VM, Galan JE. Assembly of the inner rod determines needle length in the type III secretion injectisome. Nature. 2006;441:637–640. doi: 10.1038/nature04822. [DOI] [PubMed] [Google Scholar]
- Ménard R, Sansonetti PJ, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Macnab RM. Domain structure of Salmonella FlhB, a flagellar export component responsible for substrate specificity switching. J Bacteriol. 2000a;182:4906–4914. doi: 10.1128/jb.182.17.4906-4914.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, MacNab RM. Interactions among components of the Salmonella flagellar export apparatus and its substrates. Mol Microbiol. 2000b;35:1052–1064. doi: 10.1046/j.1365-2958.2000.01771.x. [DOI] [PubMed] [Google Scholar]
- Minamino T, Doi H, Kutsukake K. Substrate specificity switching of the flagellum-specific export apparatus during flagellar morphogenesis in Salmonella typhimurium. Biosci Biotechnol Biochem. 1999a;63:1301–1303. doi: 10.1271/bbb.63.1301. [DOI] [PubMed] [Google Scholar]
- Minamino T, Gonzalez-Pedrajo B, Yamaguchi K, Aizawa SI, Macnab RM. FliK, the protein responsible for flagellar hook length control in Salmonella, is exported during hook assembly. Mol Microbiol. 1999b;34:295–304. doi: 10.1046/j.1365-2958.1999.01597.x. [DOI] [PubMed] [Google Scholar]
- Minamino T, Imada K, Namba K. Mechanisms of type III protein export for bacterial flagellar assembly. Mol Biosyst. 2008;4:1105–1115. doi: 10.1039/b808065h. [DOI] [PubMed] [Google Scholar]
- Mueller CA, Broz P, Cornelis GR. The type III secretion system tip complex and translocon. Mol Microbiol. 2008;68:1085–1095. doi: 10.1111/j.1365-2958.2008.06237.x. [DOI] [PubMed] [Google Scholar]
- Murillo J, Shen H, Gerhold D, Sharma A, Cooksey DA, Keen NT. Characterization of pPT23B, the plasmid involved in syringolide production by Pseudomonas syringae pv. tomato PT23. Plasmid. 1994;31:275–287. doi: 10.1006/plas.1994.1029. [DOI] [PubMed] [Google Scholar]
- Noël L, Thieme F, Nennstiel D, Bonas U. Two novel type III system-secreted proteins of Xanthomonas campestris pv. vesicatoria are encoded within the hrp pathogenicity island. J Bacteriol. 2002;184:1340–1348. doi: 10.1128/JB.184.5.1340-1348.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsot C, Hamiaux C, Page AL. The various and varying roles of specific chaperones in type III secretion systems. Curr Opin Microbiol. 2003;6:7–14. doi: 10.1016/s1369-5274(02)00002-4. [DOI] [PubMed] [Google Scholar]
- Petnicki-Ocwieja T, Schneider DJ, Tam VC, Chancey ST, Shan L, Jamir Y, et al. Genome-wide identification of proteins secreted by the Hrp type III protein secretion system of Pseudomonas syringae pv. tomato DC3000. Proc Natl Acad Sci USA. 2002;99:7652–7657. doi: 10.1073/pnas.112183899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan KE, Schneewind O. YscU cleavage and the assembly of Yersinia type III secretion machine complexes. Mol Microbiol. 2008;68:1485–1501. doi: 10.1111/j.1365-2958.2008.06247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald PC, Staskawicz BJ. The avirulence gene avrBs1 from Xanthomonas campestris pv. vesicatoria encodes a 50-kDa protein. Mol Plant Microbe Interact. 1988;1:191–198. [PubMed] [Google Scholar]
- Rossier O, Wengelnik K, Hahn K, Bonas U. The Xanthomonas Hrp type III system secretes proteins from plant and mammalian pathogens. Proc Natl Acad Sci USA. 1999;96:9368–9373. doi: 10.1073/pnas.96.16.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier O, Van den Ackerveken G, Bonas U. HrpB2 and HrpF from Xanthomonas are type III-secreted proteins and essential for pathogenicity and recognition by the host plant. Mol Microbiol. 2000;38:828–838. doi: 10.1046/j.1365-2958.2000.02173.x. [DOI] [PubMed] [Google Scholar]
- Samudrala R, Heffron F, McDermott JE. Accurate prediction of secreted substrates and identification of a conserved putative secretion signal for type III secretion systems. PLoS Pathog. 2009;5:e1000375. doi: 10.1371/journal.ppat.1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TG, Pereira L, Hoover TR. Helicobacter pylori FlhB processing-deficient variants affect flagellar assembly but not flagellar gene expression. Microbiology. 2009;155:1170–1180. doi: 10.1099/mic.0.022806-0. [DOI] [PubMed] [Google Scholar]
- Sorg I, Wagner S, Amstutz M, Muller SA, Broz P, Lussi Y, et al. YscU recognizes translocators as export substrates of the Yersinia injectisome. EMBO J. 2007;26:3015–3024. doi: 10.1038/sj.emboj.7601731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhan A, Kubori T, Galan JE. Synthesis and localization of the Salmonella SPI-1 type III secretion needle complex proteins PrgI and PrgJ. J Bacteriol. 2003;185:3480–3483. doi: 10.1128/JB.185.11.3480-3483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesny R, Jordan M, Schramm C, Schulz S, Cogez V, Bonas U, Büttner D. Functional characterization of the Xps and Xcs type II secretion systems from the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria. New Phytol. 2010;187:983–1002. doi: 10.1111/j.1469-8137.2010.03312.x. [DOI] [PubMed] [Google Scholar]
- Van den Ackerveken G, Marois E, Bonas U. Recognition of the bacterial avirulence protein AvrBs3 occurs inside the host plant cell. Cell. 1996;87:1307–1316. doi: 10.1016/s0092-8674(00)81825-5. [DOI] [PubMed] [Google Scholar]
- Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wand ME, Sockett RE, Evans KJ, Doherty N, Sharp PM, Hardie KR, Winzer K. Helicobacter pylori FlhB function: the FlhB C-terminal homologue HP1575 acts as a ‘spare part’ to permit flagellar export when the HP0770 FlhBCC domain is deleted. J Bacteriol. 2006;188:7531–7541. doi: 10.1128/JB.00263-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters RC, O'Toole PW, Ryan KA. The FliK protein and flagellar hook-length control. Protein Sci. 2007;16:769–780. doi: 10.1110/ps.072785407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Ojanen-Reuhs T, Huguet E, Hause G, Romantschuk M, Korhonen TK, et al. The type III-dependent Hrp pilus is required for productive interaction of Xanthomonas campestris pv. vesicatoria with pepper host plants. J Bacteriol. 2005;187:2458–2468. doi: 10.1128/JB.187.7.2458-2468.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Berger C, Bonas U, Koebnik R. Refinement of the Xanthomonas campestris pv. vesicatoria hrpD and hrpE operon structure. Mol Plant Microbe Interact. 2007;20:559–567. doi: 10.1094/MPMI-20-5-0559. [DOI] [PubMed] [Google Scholar]
- Wengelnik K, Marie C, Russel M, Bonas U. Expression and localization of HrpA1, a protein of Xanthomonas campestris pv. vesicatoria essential for pathogenicity and induction of the hypersensitive reaction. J Bacteriol. 1996;178:1061–1069. doi: 10.1128/jb.178.4.1061-1069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengelnik K, Rossier O, Bonas U. Mutations in the regulatory gene hrpG of Xanthomonas campestris pv. vesicatoria result in constitutive expression of all hrp genes. J Bacteriol. 1999;181:6828–6831. doi: 10.1128/jb.181.21.6828-6831.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesand U, Sorg I, Amstutz M, Wagner S, van den Heuvel J, Luhrs T, et al. Structure of the type III secretion recognition protein YscU from Yersinia enterocolitica. J Mol Biol. 2009;385:854–866. doi: 10.1016/j.jmb.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Williams AW, Yamaguchi S, Togashi F, Aizawa SI, Kawagishi I, Macnab RM. Mutations in fliK and flhB affecting flagellar hook and filament assembly in Salmonella typhimurium. J Bacteriol. 1996;178:2960–2970. doi: 10.1128/jb.178.10.2960-2970.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S, Jin J, Lloyd SA. YscP and YscU switch the substrate specificity of the Yersinia type III secretion system by regulating export of the inner rod protein YscI. J Bacteriol. 2008;190:4252–4262. doi: 10.1128/JB.00328-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarivach R, Deng W, Vuckovic M, Felise HB, Nguyen HV, Miller SI, et al. Structural analysis of the essential self-cleaving type III secretion proteins EscU and SpaS. Nature. 2008;453:124–127. doi: 10.1038/nature06832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


