Abstract
Women with schizophrenia have later onset and better response to antipsychotic drugs (APDs) than men during reproductive years, but the menopausal period is associated with increased symptom severity and reduced treatment response. Estrogen replacement therapy has been suggested as beneficial but clinical data are inconsistent. Latent inhibition (LI), the capacity to ignore irrelevant stimuli, is a measure of selective attention that is disrupted in acute schizophrenia patients and in rats and humans treated with the psychosis-inducing drug amphetamine and can be reversed by typical and atypical APDs. Here we used amphetamine (1 mg/kg)-induced disrupted LI in ovariectomized rats to model low levels of estrogen along with hyperfunction of the dopaminergic system that may be occurring in menopausal psychosis, and tested the efficacy of APDs and estrogen in reversing disrupted LI. 17β-Estradiol (50, 150 μg/kg), clozapine (atypical APD; 5, 10 mg/kg), and haloperidol (typical APD; 0.1, 0.3 mg/kg) effectively reversed amphetamine-induced LI disruption in sham rats, but were much less effective in ovariectomized rats; 17β-estradiol and clozapine were effective only at high doses (150 μg/kg and 10 mg/kg, respectively), whereas haloperidol failed at both doses. Haloperidol and clozapine regained efficacy if coadministered with 17β-estradiol (50 μg/kg, an ineffective dose). Reduced sensitivity to dopamine (DA) blockade coupled with spared/potentiated sensitivity to DA stimulation after ovariectomy may provide a novel model recapitulating the combination of increased vulnerability to psychosis with reduced response to APD treatment in female patients during menopause. In addition, our data show that 17β-estradiol exerts antipsychotic activity.
Keywords: amphetamine, antipsychotic drugs, estrogen, latent inhibition, menopausal psychosis, schizophrenia
INTRODUCTION
Since description of schizophrenia as ‘men illness' by Kraepeline (1919), scores of epidemiological and clinical studies have documented gender differences in schizophrenia. Women have a more favorable illness course during the reproductive years, characterized by later onset of symptoms, lower symptom severity, and better response to antipsychotic drug (APD) treatment (Agius et al, 2009; Angermeyer and Kuhn, 1988; Hafner, 2003; Hafner et al, 1989; Iacono and Beiser, 1992; Lindamer et al, 1997; Mortimer, 2007; Pregelj, 2009; Riecher-Rossler and Hafner, 2000; Salem and Kring, 1998; Seeman, 1982, 1986; Szymanski et al, 1995; Tamminga, 1997). In contrast, menopause is associated with increased vulnerability to illness, elevated symptom severity, and reduced response to treatment (Horacek et al, 2006; Kulkarni et al, 1996, 2008b; Lane et al, 1999; Salokangas, 1995; Saugstad, 1989; Seeman and Lang, 1990). Increased symptom severity and reduced treatment response are associated also with low-estrogen phases of the menstrual cycle (Ereshefsky et al, 1991; Farina et al, 1981; Lane et al, 1999; Salokangas, 1995; Seeman, 1989; Simpson et al, 1990; Tamminga, 1997). These data have been captured in the estrogen hypothesis of schizophrenia that posits exacerbations of illness manifestations in women are related to low levels of estrogen (Hafner et al, 1989; Huber et al, 2004; Seeman and Lang, 1990). Accordingly, it has been suggested that exogenous estrogen on its own or combined with APDs may have therapeutic potential in schizophrenia, although this notion has been challenged (for recent reviews see Agius et al, 2009; Mortimer, 2007).
Ovariectomy (OVX)-induced hormonal decline in rats is considered to model decreased gonadal function during menopause (Adam et al, 2009; Daniel et al, 2006; Gurkan et al, 1986; LeBlanc et al, 2009; Rogers et al, 2009; Walf et al, 2009), and has been proposed specifically to model the hormonal state associated with predisposition to schizophrenia during menopause (Bosse and Di Paolo, 1995). Although many studies showed that OVX is associated with poorer behavioral and cognitive performance, including tasks considered relevant to schizophrenia such as social interaction and object recognition (Barnes et al, 2006; Frye, 2001; Frye et al, 2006b, 2007; Frye and Rhodes, 2006a; Paris and Frye, 2008), the study of the estrogen hypothesis using animal models of schizophrenia has been limited. Some support derives from studies showing that estrogen affects prepulse inhibition (PPI) (Gogos et al, 2009; Gogos and Van den Buuse, 2004; Koch, 1998; Vaillancourt et al, 2002; Van den Buuse and Eikelis, 2001), a measure of sensorimotor gating whose disruption is considered to model sensorimotor deficits in schizophrenia, as well as the response to the pro-psychotic drugs amphetamine and cocaine (Becker and Beer, 1986; Becker and Rudick, 1999; Earley and Leonard, 1978; Gibbs et al, 1998; Naik et al, 1978; Segarra et al, 2009).
We have recently tested the estrogen hypothesis using the latent inhibition (LI) model of schizophrenia (Arad and Weiner, 2008, 2009). LI is a cross-species selective attention phenomenon manifested as retarded conditioning to a stimulus that was pre-exposed (PE) without consequence before conditioning, and is commonly considered to index the ability to ignore irrelevant stimuli. Loss of LI induced in the rat by the psychotomimetic dopamine (DA) releaser amphetamine and its reversal by APDs is a well-established model of positive symptoms of schizophrenia (Lipska and Weinberger, 2002; Moser et al, 2000; Weiner, 2003; Weiner and Arad, 2009). We (Arad and Weiner, 2009) have shown that hormonal cessation after OVX led to loss of LI, which was restored after the administration of 17β-estradiol. Most interestingly, we found that OVX interfered with the efficacy of the typical APD haloperidol to restore LI, mimicking the reduced sensitivity to APD treatment seen in schizophrenic women during menopause. Furthermore, haloperidol regained efficacy when coadministered with a behaviorally inactive dose of 17β-estradiol, indirectly supporting an antipsychotic action of 17β-estradiol.
Here we sought to expand the OVX/LI model by testing the efficacy of 17β-estradiol, haloperidol, and clozapine to reverse amphetamine-induced disruption of LI in OVX rats. Given our previous finding that OVX reduced the efficacy of APDs, one major question of interest here was whether OVX would also reduce the efficacy of amphetamine. We hypothesized that amphetamine would not lose its efficacy to disrupt LI in OVX compared to sham rats. Conversely, APDs would be less effective in reversing amphetamine-induced LI disruption in OVX rats, but their action would be potentiated by concurrent 17β-estradiol treatment, which would also block amphetamine effect on its own. These outcomes would capture the combination of increased vulnerability to psychosis with reduced response to APD treatment that may be occurring during menopause in women vulnerable to psychosis, and support a direct antipsychotic (anti-amphetamine) action of 17β-estradiol.
MATERIALS AND METHODS
Animals
Female Wistar rats bred in our laboratory were housed 3–4 per cage under reversed cycle lighting (lights on 0700–1900 hours) with ad lib access to food and water. They were about 7 weeks old and weighing 155–267 g when submitted to OVX and approximately 3 months old and weighing 248–469 g when behavioral testing begun. All experimental protocols conformed to the guidelines of the Institutional Animal Care and Use Committee of Tel Aviv University, Israel, and to the guidelines of the NIH (animal welfare assurance number A5010–01, expires on 30 September 2011). All efforts were made to minimize the number of animals used and their suffering.
Ovariectomy
Rats were bilaterally ovariectomized under isoflurane (Nicholas Piramal, UK) anesthesia. After shaving the abdominal area, we made a midline incision through the skin and muscle layer. Fallopian tubes were ligated by a nylon thread, after which the ovaries were carefully removed. Sutures of muscle layer and skin were removed 10 days later. Rats were allowed additional 3 weeks of recovery after removal of the sutures, before the beginning of water restriction (see below). Within the 3-week recovery period, about a week after removal of sutures, vaginal smears were collected daily in the morning for 8 days in sham and OVX rats to confirm regular or discontinuation of estrous cycle. Phases of the estrous cycle were determined by the morphology of cells in the vaginal smear under a light microscope (Marcondes et al, 2002). Sham-operated controls underwent an identical surgical procedure without ovaries' removal. Only sham females with regular 4-day cycles in succession and OVX rats without estrous cycle were used for behavioral testing.
Latent Inhibition
LI was measured in a thirst-motivated conditioned emotional response procedure as described previously (Arad and Weiner, 2009). Water-restricted (23 h) rats were trained to drink in the experimental chambers for 15–20 min per day for 5 days. Water in the chambers was given in addition to the 1 h water in home cages. The LI procedure consisted of four stages given 24 h apart. Pre-exposure: with the bottle removed, PE rats received 40 or 50 tones (10 s, 80 dB, 2.8 kHz) 40 s apart, whereas non-pre-exposed (NPE) rats were confined to the chamber. Conditioning: with the bottle removed, all rats received one or two tone-shock (tone: 10 s, 80 dB, 2.8 kHz; shock: 1 s, 0.5 mA) pairings given 5 min apart. Lick retraining: rats were given a 15 min drinking session as in initial training. Data of rats that failed to complete 600 licks were dropped from the analysis. Test: rats were placed in the chambers with access to the bottle. When the rat completed 75 licks, the tone was presented for 5 min. Times to complete 25 licks before and after tone onset were recorded. Times to complete licks 76–100 were submitted to logarithmic transformation to allow parametric analysis of variance (ANOVA). Longer log times indicate stronger suppression of drinking. LI is defined as shorter times to complete licks 76–100 after tone onset (weaker fear conditioning) of the PE compared to NPE rats.
Drug and Hormone Administration
Amphetamine and APDs were administered intraperitoneally and 17β-estradiol was administered subcutaneously, all in a volume of 1 ml/kg, 30 (amphetamine and clozapine), 60 (haloperidol), or 120 (17β-estradiol) min before pre-exposure and conditioning stages. Amphetamine (Sigma, Israel) was dissolved in saline and administered at a dose of 1 mg/kg. Haloperidol (Johnson&Johnson, Belgium) was prepared from an ampoule containing 5 mg haloperidol in 1 ml solvent containing 6 mg lactic acid, diluted with saline, and administered at doses of 0.1 or 0.3 mg/kg. Clozapine (Novartis, Switzerland) was dissolved in 1 N acetic acid (1.5 ml/10 mg), diluted with saline, and administered at doses of 5 or 10 mg/kg. The doses of haloperidol and clozapine are routinely used in our LI studies (Arad and Weiner, 2009; Weiner et al, 1996b). 17β-Estradiol (Sigma) was dissolved in corn oil and administered at doses of 10, 50, and 150 μg/kg. These doses were chosen on the basis of behavioral literature and used in our previous study in OVX rats (Arad and Weiner, 2009; Galea et al, 2001; Gibbs et al, 1998; Nofrey et al, 2008; Van den Buuse and Eikelis, 2001; Walf and Frye, 2010). No-drug controls received the corresponding vehicle/s as follows: saline as amphetamine vehicle, saline solution containing 1% of lactic acid as haloperidol vehicle, saline solution containing 7.5% acetic acid as clozapine vehicle, and oil as 17β-estradiol vehicle. In all experiments, lick retraining and test sessions were conducted in a drug-free state.
Assessment of Estradiol Serum Levels
Serum estradiol levels produced by the four 17β-estradiol conditions were determined in separate groups of sham and OVX rats (n per group 7–8). Rats were given two injections 24 h apart (mimicking the injection protocol of LI).
Blood was taken by cardiac puncture (1 ml, without preservative). The blood was allowed to clot for 20–40 min and was centrifuged at 930 g (2000 r.p.m.) for 20 min. Afterward serum was collected and was assayed immediately.
17β-Estradiol serum levels were measured using an enzyme-linked immunosorbent assay kits (Cayman, Michigan, USA), based on manufacturer's instructions. Table 1 presents mean serum levels of estradiol following our administration regime in comparison to levels reported for different stages of the estrous cycle and pregnancy (Nequin et al, 1979; Shaikh, 1971).
Table 1. Serum Levels of Estradiol in Sham and OVX Rats After Administration of 17β-Estradiol at Three Doses.
|
Gonads |
E state |
||||||
|---|---|---|---|---|---|---|---|
| Control | Low E (10 μg/kg) | Metestrus | Medium E (50 μg/kg) | Proestrus | High E (150 μg/kg) | Pregnancy (GD 20–22) | |
| Sham | 48.7±12.7 | 20.2±3.2 | 20.6±1.6 | 120.6±14.0 | 142.2±45.8 | 628.4±95.0 | 628±210 |
| OVX | 5.5±0.6 | 17.9±3.5 | — | 125.8±15.7 | — | 660.4±59.4 | — |
Mean (±SEM) of serum level of estradiol 2 h after second injection (given 24 h apart) of 0, 10, 50, or 150 μg/kg of 17β-estradiol, in sham and OVX rats.
For comparison, levels found by Nequin et al (1979) along the cycle (metestrus and proestrus) and by Shaikh (1971) on gestation days (GD) 20–22 are provided.
Experimental Design
Experiment 1. In our previous study we showed that LI was absent in OVX rats (Arad and Weiner, 2009). Because here we intended to test whether amphetamine disrupts LI in OVX rats, we needed to create conditions under which OVX no longer disrupts LI, so that the effect of amphetamine could be manifested. As detailed by us elsewhere (Weiner, 1990, 2003; Weiner and Arad, 2009), the expression of LI is a function of the balance between the strength of pre-exposure and the strength of conditioning, so that increasing the strength of pre-exposure (eg, by increasing the number of stimulus pre-exposures) and/or decreasing the strength of conditioning (eg, by reducing the number of conditioning trials) are expected to promote the expression of LI. The aim of experiment 1 was to test whether such manipulations of pre-exposure and conditioning parameters would restore LI in OVX rats. In our previous study, OVX-induced disruption of LI was obtained using 40 pre-exposures and 2 conditioning trials. Here, we either increased the number of pre-exposures from 40 to 50 while keeping 2 conditioning trials or reduced the number of conditioning trials from 2 to 1 while keeping 40 pre-exposures, and compared LI under these two combinations to the previous combination of 40 pre-exposures and 2 conditioning trials. The experiment included 36 OVX rats divided into six experimental groups (n per group 6) in a 2 × 3 design with main factors of pre-exposure (NPE, PE) and pre-exposure-conditioning combination (40 pre-exposures and 1 conditioning trial; 40 pre-exposures and 2 conditioning trials; 50 pre-exposures and 2 conditioning trials). The latter combination that yielded LI in OVX rats was used in experiments 2–7.
Experiment 2 tested the capacity of amphetamine to disrupt LI in OVX rats. The experiment included 62 rats (32 sham, 30 OVX) divided into eight experimental groups (n per group 6–8) in a 2 × 2 × 2 design with main factors of pre-exposure (0, 50), gonadal status (sham, OVX), and treatment (0, 1 mg/kg amphetamine).
Experiment 3 tested the capacity of 17β-estradiol to reverse amphetamine-induced LI disruption in OVX rats. The experiment included 236 rats (119 sham, 117 OVX) divided into 32 experimental groups (n per group 6–8) in a 2 × 2 × 2 × 4 design with main factors of pre-exposure (0, 50), gonadal status (sham, OVX), treatment (0, 1 mg/kg amphetamine), and pretreatment (0, 10, 50, or 150 μg/kg 17β-estradiol).
Experiments 4 and 5 tested the capacity of the typical and atypical APDs, haloperidol (0.1, 0.3 mg/kg) and clozapine (5, 10 mg/kg), respectively, to reverse amphetamine-induced LI disruption in OVX rats. Because it is well documented that haloperidol and clozapine at the doses and injection protocol used here do not affect LI (Weiner and Feldon, 1987; Weiner et al, 1997; Weiner et al, 1996b), we did not use separate control (sham and OVX) groups for each APD dose but instead injected each dose to half of the controls. Both experiments included 20 experimental groups (n per group 6–8) in a 2 × 2 × 5 design with main factors of pre-exposure (0, 50), gonadal status (sham, OVX), and treatment (vehicle, APD, amphetamine, amphetamine+low APD dose, amphetamine+high APD dose). Experiment 4 included 141 rats (71 sham, 70 OVX), whereas experiment 5 included 151 rats (77 sham, 74 OVX).
Experiments 6 and 7. Because amphetamine-induced disruption of LI in OVX rats was resistant to haloperidol (experiment 4) and showed reduced response to clozapine (experiment 5), here we tested whether the efficacy of haloperidol (0.1 mg/kg; experiment 6) and clozapine (5 mg/kg; experiment 7) in reversing amphetamine-induced LI disruption would be restored by their coadministration with an ineffective dose of 17β-estradiol (50 μg/kg). The experiments included 115 and 116, respectively, OVX rats divided into 16 experimental groups (n per group 7–8) in a 2 × 2 × 4 design with main factors of pre-exposure (0, 50), treatment (saline, amphetamine), and pretreatment (vehicle, APD, 17β-estradiol, APD+17β-estradiol).
Statistical Analysis
Times to complete licks 51–75 (before tone onset) and logarithmically transformed mean times to complete licks 76–100 (after tone onset) were analyzed with two-way ANOVA with main factors of pre-exposure and gonadal status (experiment 1); three-way ANOVAs with main factors of pre-exposure, gonadal status, and treatment (experiment 2, 4, and 5) or pre-exposure, treatment, and pretreatment (experiment 6 and 7); and a four-way ANOVA with main factors of pre-exposure, gonadal status, treatment, and pretreatment (experiment 3). In cases of significant interactions involving the factor of pre-exposure, LSD post hoc comparisons were used to assess the difference between the PE and NPE groups within each treatment condition.
RESULTS
There were no differences between the experimental groups in the times to complete licks 51–75 (A period; all p's>0.05) in any of the seven experiments (overall mean A periods were 8.19, 9.58, 7.92, 10.77, 10.04, 9.66, and 7.13 for experiments 1–7, respectively).
Experiment 1: LI in OVX Rats
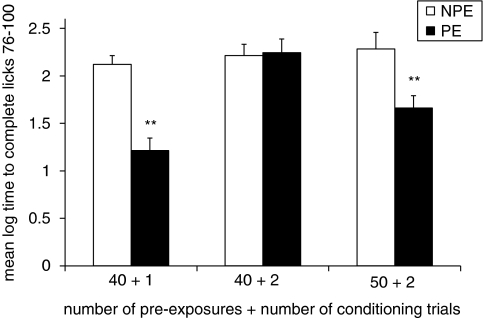
Figure 1 presents the mean log times to complete licks 76–100 (after tone onset) of the PE and NPE OVX rats in the three experimental conditions. As shown by us previously, when 40 pre-exposures were followed by 2 conditioning trials (40+2), there was no difference in suppression between the PE and NPE groups, ie, no LI. In contrast, rats exhibited LI, ie, lower suppression of the PE as compared to the NPE group, when 40 pre-exposures were followed by 1 conditioning trial (40+1) or when 50 pre-exposures were followed by 2 conditioning trials (50+1). ANOVA yielded significant main effects of pre-exposure (F(1,30)=20.88, p<0.01) and pre-exposure/conditioning combination (F(2,30)=8.85, p<0.01), as well as their interaction (F(2,30)=6.45, p<0.01). Post hoc comparisons confirmed the presence of LI in the 40+1 and 50+2 conditions (p<0.01), but not in the 40+2 condition.
Figure 1.
LI in OVX rats. Mean (±SEM) log times to complete licks 76–100 (after tone onset) of the pre-exposed (PE) and the non-pre-exposed (NPE) OVX rats in three pre-exposure/conditioning combinations (number of pre-exposures+number of conditioning trials): 40 pre-exposures and 1 conditioning trial (40+1); 40 pre-exposures and 2 conditioning trials (40+2); and 50 pre-exposures and 2 conditioning trials (50+2). Asterisks (**) indicate significant difference between the PE and NPE groups, namely, presence of LI (p<0.01).
On the basis of these results in all the following experiments (2–7), we used 50 pre-exposures and 2 conditioning trials.
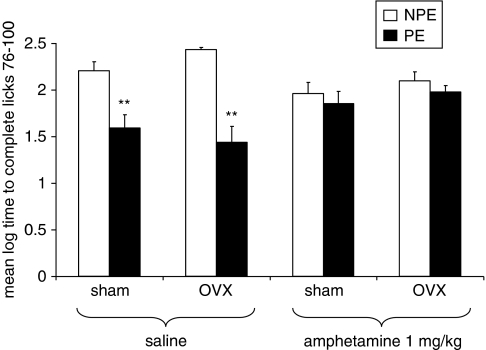
Experiment 2: Effects of Amphetamine (1 mg/kg) on LI in Sham and OVX Rats
Figure 2 presents the mean log times to complete licks 76–100 (after tone onset) of the PE and NPE sham and OVX rats injected with amphetamine (0, 1 mg/kg). As can be seen, saline-injected sham and OVX rats exhibited LI, but LI was lost in both groups after amphetamine injection. ANOVA yielded a significant effect of pre-exposure (F(1,54)=34.08, p<0.001) and a significant pre-exposure × treatment interaction (F(1,54)=19.57, p<0.001). Post hoc comparisons confirmed the presence of LI in saline (p<0.001), but not in amphetamine conditions.
Figure 2.
Effects of amphetamine (1 mg/kg) on LI in sham and OVX rats. Mean (±SEM) log times to complete licks 76–100 (after tone onset) of the pre-exposed (PE) and the non-pre-exposed (NPE) sham and OVX rats injected with saline or 1 mg/kg amphetamine. Asterisks (**) indicate significant difference between the PE and NPE groups, namely, presence of LI (p<0.01).
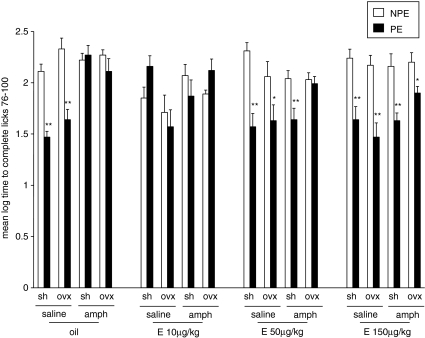
Experiment 3: Effects of 17β-Estradiol (10, 50, or 150 μg/kg) on Amphetamine-Induced LI Disruption in Sham and OVX Rats
Figure 3 presents the mean log times to complete licks 76–100 (after tone onset) of the PE and NPE saline- or amphetamine-injected sham and OVX rats pretreated with 0, 10, 50, or 150 μg/kg of 17β-estradiol. As can be seen, saline-injected sham and OVX rats exhibited LI whereas amphetamine-injected sham and OVX rats did not exhibit LI. LI was restored in amphetamine-injected sham rats given 50 or 150 μg/kg of 17β-estradiol, whereas in OVX rats only the high 17β-estradiol dose reversed amphetamine-induced disruption of LI. On its own, 17β-estradiol disrupted LI at the low dose of 10 μg/kg in both sham and OVX rats but spared LI at the two higher doses. ANOVA yielded main effects of pre-exposure (F(1,204)=55.024, p<0.0001), treatment (F(1,204)=11.296, p<0.001), and pretreatment (F(3,204)=2.74, p<0.05), as well as a significant pre-exposure × gonadal status × treatment × pretreatment interaction (F(3,204)=3.023, p<0.05). Post hoc comparisons confirmed the presence of LI in saline-injected sham and OVX rats given 0, 50, or 150 μg/kg 17β-estradiol; in amphetamine-injected sham rats given 50 or 150 μg/kg 17β-estradiol; and in OVX rats given 150 μg/kg 17β-estradiol (p's<0.05), but not in the other conditions.
Figure 3.
Effects of 17β-estradiol (10, 50, or 150 μg/kg) on amphetamine-induced LI disruption in sham and OVX rats. Mean (±SEM) log times to complete licks 76–100 (after tone onset) of the pre-exposed (PE) and the non-pre-exposed (NPE) saline- or amphetamine (amph)-injected sham (sh) and OVX (ovx) females, administered with 0, 10, 50, or 150 μg/kg of 17β-estradiol (oil, E 10 μg/kg, E 50 μg/kg, or E 150 μg/kg, respectively). Asterisks indicate a significant difference between the PE and NPE groups, namely, presence of LI (*p<0.05; **p<0.01).
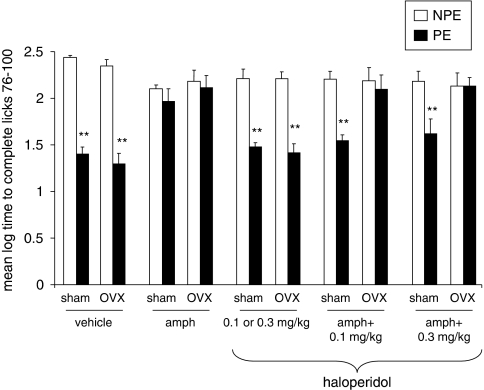
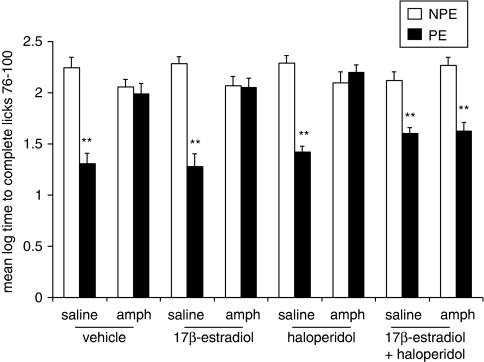
Experiment 4: Effects of Haloperidol (0.1, 0.3 mg/kg) on Amphetamine-Induced LI Disruption in Sham and OVX Rats
Figure 4 presents the mean log times to complete licks 76–100 (after tone onset) of the PE and NPE saline- or amphetamine-injected sham and OVX rats pretreated with 0, 0.1, or 0.3 mg/kg of haloperidol. As can be seen, saline-injected sham and OVX rats exhibited LI whereas amphetamine-injected sham and OVX rats did not exhibit LI. LI was restored in amphetamine-injected sham rats given both doses of haloperidol, whereas both doses were ineffective in amphetamine-injected OVX rats. On its own, haloperidol spared LI. ANOVA yielded significant main effects of pre-exposure (F(1,121)=119.42, p<0.001), gonadal status (F(1,121)=5.32, p<0.05), and treatment (F(4,121)=4.99, p<0.05), as well as a significant pre-exposure × gonadal status × treatment interaction (F(4,121)=2.75, p<0.05). Post hoc comparisons confirmed the presence of LI in saline-injected sham and OVX rats that received vehicle or haloperidol and in amphetamine-injected sham rats that received both doses of haloperidol (p's<0.01), but not in the other conditions.
Figure 4.
Effects of haloperidol (0.1, 0.3 mg/kg) on amphetamine-induced LI disruption in sham and OVX rats. Mean (±SEM) log times to complete licks 76–100 (after tone onset) of the pre-exposed (PE) and the non-pre-exposed (NPE) saline- or amphetamine (amph)-injected sham and OVX rats that received haloperidol (0.1 or 0.3 mg/kg to half of the group), amphetamine+0.1 mg/kg haloperidol, or amphetamine+0.3 mg/kg haloperidol. Asterisks (**) indicate significant difference between the PE and NPE groups, namely, presence of LI (p<0.01).
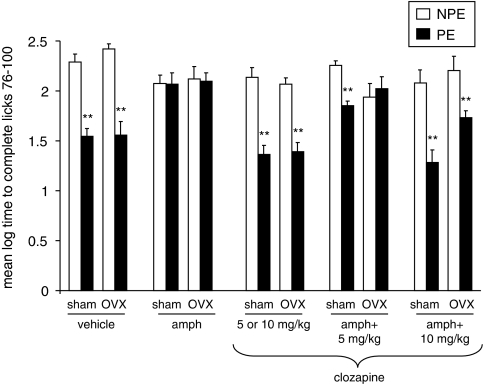
Experiment 5: Effects of Clozapine (5, 10 mg/kg) on Amphetamine-Induced LI Disruption in Sham and OVX Rats
Figure 5 presents the mean log times to complete licks 76–100 (after tone onset) of the PE and NPE saline- or amphetamine-injected sham and OVX rats pretreated with 0, 5, or 10 mg/kg of clozapine. As can be seen, saline-injected sham and OVX rats exhibited LI whereas amphetamine-injected sham and OVX rats did not exhibit LI. LI was restored in amphetamine-injected sham rats given both doses of clozapine, whereas only the high dose was effective in amphetamine-injected OVX rats. On its own, clozapine spared LI.
Figure 5.
Effects of clozapine (5, 10 mg/kg) on amphetamine-induced LI disruption in sham and OVX rats. Mean (±SEM) log times to complete licks 76–100 (after tone onset) of the pre-exposed (PE) and the non-pre-exposed (NPE) saline- or amphetamine (amph)-injected sham and OVX rats that received clozapine (5 or 10 mg/kg to half of the group), amphetamine+5 mg/kg clozapine, or amphetamine+10 mg/kg clozapine. Asterisks (**) indicate significant difference between the PE and NPE groups, namely, presence of LI (p<0.01).
ANOVA yielded significant main effects of pre-exposure (F(1,131)=143.36, p<0.001), gonadal status (F(1,131)=3.96, p<0.05), and treatment (F(4,131)=13.84, p<0.001), as well as a significant pre-exposure × gonadal status × treatment interaction (F(4,131)=2.93, p<0.05). Post hoc comparisons confirmed the presence of LI in saline-injected sham and OVX rats that received vehicle or clozapine, in amphetamine-injected sham rats that received both doses of clozapine, and in amphetamine-injected OVX rats that received the high dose of clozapine (p's<0.01), but not in the other conditions.
Experiment 6: Effects of Coadministration of Haloperidol (0.1 mg/kg) and 17β-Estradiol (50 μg/kg) on Amphetamine-Induced LI Disruption in OVX Rats
Figure 6 presents the mean log times to complete licks 76–100 (after tone onset) of the PE and NPE saline- or amphetamine-injected OVX rats pretreated with vehicle, 0.1 mg/kg haloperidol, 50 μg/kg 17β-estradiol or haloperidol+17β-estradiol. As can be seen, vehicle-injected OVX rats exhibited LI whereas amphetamine-injected OVX rats did not exhibit LI. Administration of haloperidol or 17β-estradiol alone failed to restore LI in amphetamine-injected OVX rats, but LI was restored in rats that were coadministered with haloperidol and 17β-estradiol. On their own, haloperidol and 17β-estradiol spared LI. ANOVA yielded significant main effects of pre-exposure (F(1,99)=126.53, p<0.001) and treatment (F(1,99)=26.45, p<0.001), as well as a significant pre-exposure × treatment × pretreatment interaction (F(3,99)=9.39, p<0.001). Post hoc comparisons confirmed the presence of LI in all four groups of saline-injected OVX rats, as well as in amphetamine-injected rats that received haloperidol+17β-estradiol (p's<0.01), but not in the other conditions.
Figure 6.
Effects of coadministration of haloperidol (0.1 mg/kg) and 17β-estradiol (50 μg/kg) on amphetamine-induced LI disruption in OVX rats. Mean (±SEM) log times to complete licks 76–100 (after tone onset) of the pre-exposed (PE) and the non-pre-exposed (NPE) saline- or amphetamine (amph)-injected OVX rats administered with vehicle (vehicle), 50 μg/kg 17β-estradiol (17β-estradiol), 0.1 mg/kg haloperidol (haloperidol), or 17β-estradiol+haloperidol. Asterisks (**) indicate significant difference between the PE and NPE groups, namely, presence of LI (p<0.01).
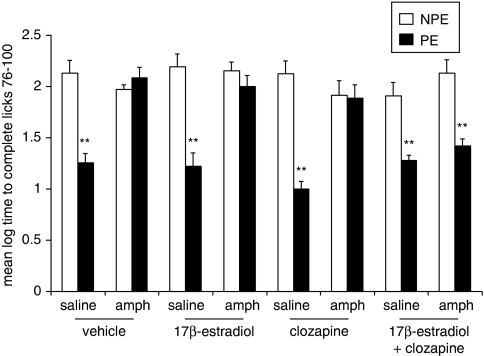
Experiment 7: Effects of Coadministration of Clozapine (5 mg/kg) and 17β-Estradiol (50 μg/kg) on Amphetamine-Induced LI Disruption in OVX Rats
Figure 7 presents the mean log times to complete licks 76–100 (after tone onset) of the PE and NPE saline- or amphetamine-injected OVX rats pretreated with vehicle, 5 mg/kg clozapine, 50 μg/kg 17β-estradiol or clozapine+17β-estradiol. As can be seen, vehicle-injected OVX rats exhibited LI whereas amphetamine-injected OVX rats did not exhibit LI. Administration of clozapine or 17β-estradiol alone failed to restore LI in amphetamine-injected OVX rats, but LI was restored in rats that were coadministered with clozapine and 17β-estradiol. On their own, clozapine and 17β-estradiol spared LI. ANOVA yielded significant main effects of pre-exposure (F(1,100)=102.39, p<0.001), treatment (F(1,99)=31.76, p<0.001), and pretreatment (F(3,100)=3.38, p<0.05), as well as a significant pre-exposure × treatment × pretreatment interaction (F(3,100)=6.21, p<0.001). Post hoc comparisons confirmed the presence of LI in all four groups of saline-injected OVX rats, as well as in amphetamine-injected rats that received clozapine+17β-estradiol (p's<0.01), but not in the other conditions.
Figure 7.
Effects of coadministration of clozapine (5 mg/kg) and 17β-estradiol (50 μg/kg) on amphetamine-induced LI disruption in OVX rats. Mean (±SEM) log times to complete licks 76–100 (after tone onset) of the pre-exposed (PE) and the non-pre-exposed (NPE) saline- or amphetamine (amph)-injected OVX rats administered with vehicle (vehicle), 50 μg/kg (17β-estradiol), 5 mg/kg (clozapine), or 17β-estradiol+clozapine. Asterisks (**) indicate significant difference between the PE and NPE groups, namely, presence of LI p<0.01).
DISCUSSION
In this study, we showed that (1) amphetamine disrupted LI in OVX rats as it did in sham-operated controls; (2) compared to sham controls, reversal of amphetamine-induced LI disruption in OVX rats required higher doses of 17β-estradiol and clozapine and was resistant to haloperidol; (3) coadministration of low dose of clozapine and haloperidol with an ineffective dose of 17β-estradiol restored LI in amphetamine-injected OVX rats; and (4) 17β-estradiol at medium and high doses reversed amphetamine-induced LI disruption in sham rats.
Compared to our previous study centered on OVX-induced disruption of LI (Arad and Weiner, 2009), in this work the LI protocol has been changed so that OVX no longer disrupted LI and an effect of amphetamine could be shown. In experiment 1, as in the work by Arad and Weiner (2009), no LI was evident in OVX rats if 40 pre-exposures were followed by 2 conditioning trials. However, raising the number of pre-exposures to 50 or reducing the number of conditioning trials to 1 led to emergence of LI in these rats. Nofrey et al (2008) have also found LI in OVX rats. The fact that OVX effects on LI can be influenced by changing procedural parameters indicates that hormonal level has no role in the acquisition of LI, but exerts a modulatory influence on its expression. Given the well-documented capacity of estrogen to modulate DA neurotransmission (Becker, 1999; Bourque et al, 2009; Chavez et al, 2010; Dluzen and Horstink, 2003; Hughes et al, 2009; Morissette et al, 2008), it is noteworthy that DA manipulations also exert only a modulatory influence on the expression of LI (Weiner, 2003).
Under conditions yielding LI in OVX rats, amphetamine disrupted LI as it did in sham controls. Disruption of LI reflects a selective attention deficit, whereby animals lose the capacity to ignore the irrelevant stimulus, and is also observed in amphetamine-treated humans, as well as in high-schizotypal humans (Braunstein-Bercovitz et al, 2002; Gray et al, 1992b; Salgado et al, 2000; Swerdlow et al, 2003; Thornton et al, 1996) and in acutely psychotic schizophrenia patients (Baruch et al, 1988; Gray et al, 1992a, 1995b; Rascle et al, 2001; but also see Swerdlow et al, 2005). A failure to inhibit attention to irrelevant stimuli is likely to give rise to aberrantly increased salience perception and distractibility that are associated with psychotic symptoms (Kapur et al, 2005; Weiner and Arad, 2009). These results show that unlike the reduced efficacy of APDs in OVX rats, the efficacy of amphetamine is not compromised by OVX. The latter suggests that the pro-psychotic action of amphetamine, and by extension, of increased dopaminergic function does not require estrogen. Similar results were reported with PPI. OVX spared PPI, which was disrupted by the DA agonist apomorphine (Gogos et al, 2009; Van den Buuse and Eikelis, 2001). PPI in OVX rats was disrupted also by the pro-psychotic NMDA antagonist MK-801. We have also found that MK-801 exerts the same effect on LI in OVX and sham rats (M Arad and I Weiner, unpublished data). Taken together, these results indicate that pro-psychotic drugs from different classes remain effective in OVX rats.
As shown repeatedly in the past using males (for review see Weiner, 2003; Weiner and Arad, 2009), amphetamine-induced LI disruption in sham female rats was reversed here by both clozapine and haloperidol, at both doses used. However, both drugs were less effective in OVX rats. Clozapine was effective only at the higher 10 mg/kg dose, and haloperidol failed to restore LI at both the 0.1 and 0.3 mg/kg doses. These outcomes extend our previous demonstration of reduced APD efficacy in reversing LI disruption in pharmacologically nontreated OVX rats (Arad and Weiner, 2009). Moreover, LI disruption in amphetamine-treated OVX rats is more resistant to APDs because in the previous study, clozapine was effective at the 5 mg/kg dose. These results support our previous conclusion that OVX-induced loss of estrogen reduces the potency of APDs, and further indicate that loss of estrogen coupled with hyperdopaminergia aggravates the loss of APDs efficacy so that not only typical but also atypical APDs, which are considered more effective antipsychotics in general (Horacek et al, 2006; Stone and Pilowsky, 2006) and in women in particular (de Leon et al, 2004), lose their efficacy.
Failure of APDs to restore disrupted LI, and in particular the different efficacies of haloperidol and clozapine, in OVX rats, are of particular interest given that in male rats, reversal by both classes of APDs has characterized to date all known instances of LI disruption, be they induced by pharmacological (Barak and Weiner, 2007; Russig et al, 2003), brain lesion (Coutureau et al, 1999; Weiner et al, 1996a), neurodevelopmental (Zuckerman et al, 2003), or parametric (Killcross et al, 1994b; Shadach et al, 1999; Weiner et al, 1996b) manipulations. Thus, loss of estrogen in female rats appears to exert a unique and powerful interference with APD action.
Following our previous demonstration that concurrent 17β-estradiol treatment potentiated the action of APDs (Arad and Weiner, 2009), here we postulated that ineffective APD doses would also regain their capacity to block amphetamine-induced disrupted LI in the presence of estrogen. Indeed we found that coadministration of low haloperidol and low clozapine doses with an ineffective dose of 17β-estradiol restored the efficacy of haloperidol and clozapine, although each of the drugs alone (in these doses) did not restore LI. These results indicate that 17β-estradiol restores specifically the anti-psychotic (anti-amphetamine) action of APDs, both typical and atypical. The latter outcome is consistent with other reports that the behavioral effects of D2 antagonists in OVX rats are potentiated by coadministration of 17β-estradiol (Bedard et al, 1982; Daniel, 2006; De Ryck et al, 1982; Di Paolo et al, 1979, 1984; Nicoletti et al, 1983; Palermo-Neto and Dorce, 1990).
17β-Estradiol not only potentiated the anti-amphetamine action of APDs but also prevented amphetamine from disrupting LI when given on its own. Gogos et al (2009) recently found that chronic estradiol blocked disruption of PPI induced by apomorphine. Importantly, here 17β-estradiol blocked amphetamine effects in both OVX and sham rats. Furthermore, although the anti-amphetamine action was exerted in sham rats by both 50 and 150 μg/kg 17β-estradiol, in OVX rats only the highest dose of 150 μg/kg exerted such action. The fact that a higher 17β-estradiol dose was needed to counteract amphetamine action in OVX rats suggests that low level of hormones and hyperdopaminergia are synergistic, supporting the notion that OVX-induced hormonal reduction is pro-psychotic.
The low dose of 17β-estradiol (10 μg/kg), although having no effect on amphetamine-induced disruption in both sham and OVX rats, disrupted LI in both sham and OVX rats. Nofrey et al (2008) have also reported that under conditions in which OVX spared LI, 10 μg/kg of 17β-estradiol disrupted LI. Taken together, results by Nofrey et al (2008) and by us suggest that high doses of estradiol exert an antipsychotic action whereas low doses exert a pro-psychotic action.
Amphetamine-induced LI disruption and its reversal by APDs are mediated by increased DA release and blockade of DA transmission, respectively, within the nucleus accumbens (Gray et al, 1995a; Warburton et al, 1996; Weiner, 2003). Consequently, our results imply that acute high dose of estradiol, which blocked the effects of amphetamine and potentiated the effects of haloperidol on LI, reduced mesolimbic DA function, whereas low dose, which disrupted LI, increased DA release within the nucleus accumbens. Results consistent with both reduction and increase of striatal dopaminergic function by estradiol have been reported for all indices of dopaminergic activity, including receptor levels/binding, membrane dopamine transporter levels, and release, depending on dose and treatment paradigm (Arvin et al, 2000; Bazzett and Becker, 1994; Becker and Beer, 1986; Becker and Rudick, 1999; Di Paolo, 1994, 1982, 1984, 1985; Disshon et al, 1998; Disshon and Dluzen, 2000; Dluzen, 1997; Landry et al, 2002; McDermott, 1993; McDermott et al, 1994; Morissette et al, 2008; Morissette and Di Paolo, 1993; Peris et al, 1991; Shieh and Yang, 2008; Thompson and Moss, 1994; Zhou et al, 2002). It has been suggested that antidopaminergic effects are primarily exerted by high doses of estrogen or chronic administration, whereas pro-dopaminergic actions are more associated with lower physiological levels of estrogen (Barber et al, 1976; Becker, 1999; Bedard et al, 1977; Cyr et al, 2002; Di Paolo, 1994; Di Paolo et al, 1981; Hruska and Silbergeld, 1980; McEwen and Alves, 1999; Riddoch et al, 1971). The specific mechanisms by which estradiol exerts the effects observed here remain to be elucidated.
OVX had been proposed to model gonadal hormone withdrawal occurring at menopause (Bosse and Di Paolo, 1995; Le Saux and Di Paolo, 2006; Vaillancourt et al, 2002). In support of this notion, we showed previously that OVX disrupts LI and impairs the efficacy of APDs to restore LI in OVX rats, in line with reduced APD efficacy reported in menopausal women with schizophrenia (Arad and Weiner, 2009). In this study, we used a combination of reduced hormonal level induced by OVX and increased dopaminergic activity induced by amphetamine to more closely model what may be occurring during menopause in women vulnerable to psychosis. Using disrupted LI and its restoration by 17β-estradiol and APDs as a behavioral readout for this biological constellation, our results have extended and strengthened our previous findings and their implications for the often-debated relationship between hormonal level and vulnerability to psychosis.
First, cessation of hormones interferes with the antipsychotic action of APDs as reflected in lowered efficacy to reverse amphetamine-induced LI disruption. Because the behavioral effects of amphetamine in general and amphetamine-induced LI disruption in particular are well-established models of psychosis, fortified by the capacity of amphetamine to induce and exacerbate psychosis in healthy and schizophrenic humans as well as enhance striatal DA release in schizophrenia patients (Laruelle et al, 1996, 1999), reduced efficacy of APDs in blocking amphetamine-induced behavioral abnormality allows a strong conclusion that loss of estrogen reduces specifically the antipsychotic potency of APDs. This reduced anti-amphetamine efficacy of APDs on the background of low hormonal level provides a close parallel to reduced efficacy of APD treatment in women with schizophrenia during periods associated with low levels of hormones (Kulkarni et al, 1996; Saugstad, 1989; Seeman, 1989; Seeman and Lang, 1990).
Second, cessation of hormones may be synergistic with hyperdopaminergia induced by amphetamine. Although our data do not show directly that amphetamine is more efficacious in disrupting LI in OVX rats, indirectly the data indicate that the effects of amphetamine on LI in OVX rats were more potent than in sham rats. Thus, a three times higher dose of 17β-estradiol was needed to block the LI disruptive effect of amphetamine in OVX than the dose needed in sham rats; in addition, both low and high doses of haloperidol and clozapine reversed disrupted LI in sham and OVX rats, but only high dose of clozapine was effective in OVX rats injected with amphetamine. It remains to be shown directly that OVX rats are more sensitive to LI disruptive effects of amphetamine. Such increased sensitivity could be shown by proving that amphetamine is effective in disrupting LI in OVX rats under conditions at which it loses capacity to disrupt LI in normal rats (De la Casa et al, 1993; Killcross et al, 1994a), or that LI disruption in OVX rats can be achieved by lower amphetamine doses than in control rats. However, be it normal or higher compared to sham, the potency of amphetamine in OVX rats sharply contrasts with the loss of potency of APDs after OVX. Reduced sensitivity to dopaminergic blockade coupled with intact/increased sensitivity to increased DA transmission in OVX rats may provide clues as to how loss of estrogen exacerbates or triggers psychosis in vulnerable women.
Finally, our results show that 17β-estradiol can exert antipsychotic activity as reflected in reversal of amphetamine-induced LI disruption in OVX and sham rats. As detailed in the Introduction, it has been suggested that exogenous estrogen may have antipsychotic properties or increase response to APDs in women with schizophrenia. Although the latter has been supported in several studies (Agius et al, 2009; Akhondzadeh et al, 2003; Cyr et al, 2002; Korhonen et al, 1995; Kulkarni, 2009; Kulkarni et al, 1996, 2001, 2008a,; Lindamer et al, 2001; Mortimer, 2007; Rao and Kolsch, 2003), other studies reported that estrogen treatment failed to improve or even worsened symptoms (Bergemann et al, 2005; Chua et al, 2005; Gattaz et al, 1994; Lindamer et al, 2001; for review see Mortimer, 2007). We previously provided support for antipsychotic capacity of 17β-estradiol by showing a synergistic effect of ineffective doses of 17β-estradiol and APDs in reversing OVX-induced disrupted LI (Arad and Weiner, 2009), and have replicated such a synergistic effect here for amphetamine-induced disrupted LI. However, although a synergistic action between APDs and 17β-estradiol implies that APDs require a certain level of estrogen to be effective, such dependence does not confer 17β-estradiol a direct antipsychotic action. In contrast, the capacity of 17β-estradiol given on its own to reverse amphetamine-induced disrupted LI in OVX rats as well as in sham rats strongly supports a direct antipsychotic action of 17β-estradiol. We have recently found that 17β-estradiol also reverses amphetamine-induced disrupted LI in male rats (M Arad and I Weiner, unpublished data).
In summary, the differential sensitivity to blockade and enhancement of dopaminergic transmission after OVX as seen here, with reduced sensitivity to DA blockade and spared or potentiated sensitivity to DA stimulation, may provide a novel model of menopausal psychosis that is associated with low levels of estrogen along with hyperfunction of the dopaminergic system. This constellation appears to rather accurately model the combination of increased vulnerability to psychosis with reduced response to APD treatment in female patients during menopause (Hafner, 2003; Seeman, 1989). Such a model may have important implications for the clinical progression and treatment of schizophrenia in women. Regarding the latter, our data are clear in showing that estrogen exerts antipsychotic activity. Unfortunately, in OVX rats and by extension in menopausal women, very high doses of 17β-estradiol would be needed. However, our data do suggest that using physiological 17β-estradiol doses as an add-on treatment may augment APD efficacy, and in fact may be more effective than raising the dose of APD.
A gender focus on mental disorders has been rapidly emerging in the last few years kindled by the acknowledgment of salient gender differences in all major psychiatric disorders. Much effort still needs to be invested by both clinical and basic research to study the biological and psychological causes and impacts of psychiatric disorders in the female gender. The present model is a step in this direction.
Acknowledgments
We acknowledge the Joseph Sagol Fellowship Program in Humanities and Social Sciences at Tel-Aviv University for the funding (MA). We also acknowledge Novartis, Switzerland, for its generous gift of clozapine.
The authors declare no conflict of interest.
References
- Adam SK, Das S, Jaarin K. A detailed microscopic study of the changes in the aorta of experimental model of postmenopausal rats fed with repeatedly heated palm oil. Int J Exp Pathol. 2009;90:321–327. doi: 10.1111/j.1365-2613.2009.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agius M, Hockings H, Wilson C, Lane D. Is oestrogen neuroprotective. Psychiatr Danub. 2009;21 (Suppl 1:120–127. [PubMed] [Google Scholar]
- Akhondzadeh S, Nejatisafa AA, Amini H, Mohammadi MR, Larijani B, Kashani L, et al. Adjunctive estrogen treatment in women with chronic schizophrenia: a double-blind, randomized, and placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1007–1012. doi: 10.1016/S0278-5846(03)00161-1. [DOI] [PubMed] [Google Scholar]
- Angermeyer MC, Kuhn L. Gender differences in age at onset of schizophrenia. An overview. Eur Arch Psychiatry Neurol Sci. 1988;237:351–364. doi: 10.1007/BF00380979. [DOI] [PubMed] [Google Scholar]
- Arad M, Weiner I. Fluctuation of latent inhibition along the estrous cycle in the rat: modeling the cyclicity of symptoms in schizophrenic women. Psychoneuroendocrinology. 2008;33:1401–1410. doi: 10.1016/j.psyneuen.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Arad M, Weiner I. Disruption of latent inhibition induced by ovariectomy can be reversed by estradiol and clozapine as well as by co-administration of haloperidol with estradiol but not by haloperidol alone. Psychopharmacology (Berl) 2009;206:731–740. doi: 10.1007/s00213-009-1464-0. [DOI] [PubMed] [Google Scholar]
- Arvin M, Fedorkova L, Disshon KA, Dluzen DE, Leipheimer RE. Estrogen modulates responses of striatal dopamine neurons to MPP(+): evaluations using in vitro and in vivo techniques. Brain Res. 2000;872:160–171. doi: 10.1016/s0006-8993(00)02511-7. [DOI] [PubMed] [Google Scholar]
- Barak S, Weiner I. Scopolamine induces disruption of latent inhibition which is prevented by antipsychotic drugs and an acetylcholinesterase inhibitor. Neuropsychopharmacology. 2007;32:989–999. doi: 10.1038/sj.npp.1301208. [DOI] [PubMed] [Google Scholar]
- Barber PV, Arnold AG, Evans G. Recurrent hormone dependent chorea: effects of oestrogens and progestogens. Clin Endocrinol (Oxf) 1976;5:291–293. doi: 10.1111/j.1365-2265.1976.tb01956.x. [DOI] [PubMed] [Google Scholar]
- Barnes P, Staal V, Muir J, Good MA. 17-Beta estradiol administration attenuates deficits in sustained and divided attention in young ovariectomized rats and aged acyclic female rats. Behav Neurosci. 2006;120:1225–1234. doi: 10.1037/0735-7044.120.6.1225. [DOI] [PubMed] [Google Scholar]
- Baruch I, Hemsley DR, Gray JA. Differential performance of acute and chronic schizophrenics in a latent inhibition task. J Nerv Ment Dis. 1988;176:598–606. doi: 10.1097/00005053-198810000-00004. [DOI] [PubMed] [Google Scholar]
- Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–172. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity: behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res. 1986;19:27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav. 1999;64:53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- Bedard P, Langelier P, Villeneuve A. Oestrogens and extrapyramidal system. Lancet. 1977;2:1367–1368. doi: 10.1016/s0140-6736(77)90429-9. [DOI] [PubMed] [Google Scholar]
- Bedard PJ, Malouin F, Dipaolo T, Labrie F. Estradiol, TRH and striatal dopaminergic mechanisms. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:555–561. doi: 10.1016/s0278-5846(82)80149-8. [DOI] [PubMed] [Google Scholar]
- Bergemann N, Mundt C, Parzer P, Pakrasi M, Eckstein-Mannsperger U, Haisch S, et al. Estrogen as an adjuvant therapy to antipsychotics does not prevent relapse in women suffering from schizophrenia: results of a placebo-controlled double-blind study. Schizophr Res. 2005;74:125–134. doi: 10.1016/j.schres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Bosse R, Di Paolo T. Dopamine and GABAA receptor imbalance after ovariectomy in rats: model of menopause. J Psychiatry Neurosci. 1995;20:364–371. [PMC free article] [PubMed] [Google Scholar]
- Bourque M, Dluzen DE, Di Paolo T. Neuroprotective actions of sex steroids in Parkinson's disease. Front Neuroendocrinol. 2009;30:142–157. doi: 10.1016/j.yfrne.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Braunstein-Bercovitz H, Rammsayer T, Gibbons H, Lubow RE. Latent inhibition deficits in high-schizotypal normals: symptom-specific or anxiety-related. Schizophr Res. 2002;53:109–121. doi: 10.1016/s0920-9964(01)00166-9. [DOI] [PubMed] [Google Scholar]
- Chavez C, Hollaus M, Scarr E, Pavey G, Gogos A, van den Buuse M.2010The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: an autoradiography study Brain Res(e-pub ahead of print) [DOI] [PubMed]
- Chua WL, de Izquierdo SA, Kulkarni J, Mortimer A.2005Estrogen for schizophrenia Cochrane Database Syst Rev 4Art. No. CD004719. [DOI] [PubMed] [Google Scholar]
- Coutureau E, Galani R, Gosselin O, Majchrzak M, Di Scala G. Entorhinal but not hippocampal or subicular lesions disrupt latent inhibition in rats. Neurobiol Learn Mem. 1999;72:143–157. doi: 10.1006/nlme.1998.3895. [DOI] [PubMed] [Google Scholar]
- Cyr M, Calon F, Morissette M, Di Paolo T. Estrogenic modulation of brain activity: implications for schizophrenia and Parkinson's disease. J Psychiatry Neurosci. 2002;27:12–27. [PMC free article] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research. J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- De la Casa LG, Ruiz G, Lubow RE. Amphetamine-produced attenuation of latent inhibition is modulated by stimulus preexposure duration: implications for schizophrenia. Biol Psychiatry. 1993;33:707–711. doi: 10.1016/0006-3223(93)90120-3. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ, Josiassen RC, Simpson GM. Possible individual and gender differences in the small increases in plasma prolactin levels seen during clozapine treatment. Eur Arch Psychiatry Clin Neurosci. 2004;254:318–325. doi: 10.1007/s00406-004-0505-2. [DOI] [PubMed] [Google Scholar]
- De Ryck M, Hruska RE, Silbergeld EK. Estrogen and haloperidol-induced versus handling-related catalepsy in male rats. Pharmacol Biochem Behav. 1982;17:1027–1035. doi: 10.1016/0091-3057(82)90489-0. [DOI] [PubMed] [Google Scholar]
- Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5:27–41. doi: 10.1515/revneuro.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Carmichael R, Labrie F, Raynaud JP. Effects of estrogens on the characteristics of [3H]spiroperidol and [3H]RU24213 binding in rat anterior pituitary gland and brain. Mol Cell Endocrinol. 1979;16:99–112. doi: 10.1016/0303-7207(79)90108-4. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Daigle M, Labrie F. Effect of estradiol and haloperidol on hypophysectomized rat brain dopamine receptors. Psychoneuroendocrinology. 1984;9:399–404. doi: 10.1016/0306-4530(84)90047-7. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Dupont A, Daigle M. Effect of chronic estradiol treatment on dopamine concentrations in discrete brain nuclei of hypophysectomized female rats. Neurosci Lett. 1982;32:295–300. doi: 10.1016/0304-3940(82)90310-x. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Poyet P, Labrie F. Effect of chronic estradiol and haloperidol treatment on striatal dopamine receptors. Eur J Pharmacol. 1981;73:105–106. doi: 10.1016/0014-2999(81)90153-9. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Rouillard C, Bedard P. 17Beta-estradiol at a physiological dose acutely increases dopamine turnover in rat brain. Eur J Pharmacol. 1985;117:197–203. doi: 10.1016/0014-2999(85)90604-1. [DOI] [PubMed] [Google Scholar]
- Disshon KA, Boja JW, Dluzen DE. Inhibition of striatal dopamine transporter activity by 17beta-estradiol. Eur J Pharmacol. 1998;345:207–211. doi: 10.1016/s0014-2999(98)00008-9. [DOI] [PubMed] [Google Scholar]
- Disshon KA, Dluzen DE. Estrogen reduces acute striatal dopamine responses in vivo to the neurotoxin MPP+ in female, but not male rats. Brain Res. 2000;868:95–104. doi: 10.1016/s0006-8993(00)02329-5. [DOI] [PubMed] [Google Scholar]
- Dluzen D. Estrogen decreases corpus striatal neurotoxicity in response to 6-hydroxydopamine. Brain Res. 1997;767:340–344. doi: 10.1016/s0006-8993(97)00630-6. [DOI] [PubMed] [Google Scholar]
- Dluzen D, Horstink M. Estrogen as neuroprotectant of nigrostriatal dopaminergic system: laboratory and clinical studies. Endocrine. 2003;21:67–75. doi: 10.1385/endo:21:1:67. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Leonard BE. Behavioural studies on the effects of d-amphetamine and estradiol benzoate alone and in combination. Psychopharmacology (Berl) 1978;56:179–183. doi: 10.1007/BF00431846. [DOI] [PubMed] [Google Scholar]
- Ereshefsky L, Saklad SR, Watanabe MD, Davis CM, Jann MW. Thiothixene pharmacokinetic interactions: a study of hepatic enzyme inducers, clearance inhibitors, and demographic variables. J Clin Psychopharmacol. 1991;11:296–301. [PubMed] [Google Scholar]
- Farina G, Moretti G, Crivelli G. [Psychopathology and clinical picture of symbiotic psychoses] Minerva Psichiatr. 1981;22:215–225. [PubMed] [Google Scholar]
- Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Brain Res Rev. 2001;37:201–222. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 5alpha-pregnan-3alpha-ol-20-one (3alpha,5alpha-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3alpha,5alpha-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol. 2006a;18:960–975. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3Alpha-hydroxy-5alpha-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience. 2006b;138:1007–1014. doi: 10.1016/j.neuroscience.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- Gattaz WF, Vogel P, Riecher-Rossler A, Soddu G. Influence of the menstrual cycle phase on the therapeutic response in schizophrenia. Biol Psychiatry. 1994;36:137–139. doi: 10.1016/0006-3223(94)91195-9. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Burke AM, Johnson DA. Estrogen replacement attenuates effects of scopolamine and lorazepam on memory acquisition and retention. Horm Behav. 1998;34:112–125. doi: 10.1006/hbeh.1998.1452. [DOI] [PubMed] [Google Scholar]
- Gogos A, Kwek P, Chavez CA, van den Buuse M.2009Estrogen treatment blocks 8-OH-DPAT- and apomorphine-induced disruptions of prepulse inhibition: involvement of dopamine D1 or D2, serotonin 5-HT1A, 5-HT2A or 5-HT7 receptors J Pharmacol Exp Ther(e-pub ahead of print) [DOI] [PubMed]
- Gogos A, Van den Buuse M. Estrogen and progesterone prevent disruption of prepulse inhibition by the serotonin-1A receptor agonist 8-hydroxy-2-dipropylaminotetralin. J Pharmacol Exp Ther. 2004;309:267–274. doi: 10.1124/jpet.103.061432. [DOI] [PubMed] [Google Scholar]
- Gray JA, Joseph MH, Hemsley DR, Young AM, Warburton EC, Boulenguez P, et al. The role of mesolimbic dopaminergic and retrohippocampal afferents to the nucleus accumbens in latent inhibition: implications for schizophrenia. Behav Brain Res. 1995a;71:19–31. doi: 10.1016/0166-4328(95)00154-9. [DOI] [PubMed] [Google Scholar]
- Gray NS, Hemsley DR, Gray JA. Abolition of latent inhibition in acute, but not chronic, schizophrenics. Neurol Psychiatry Brain Res. 1992a;1:83–89. [Google Scholar]
- Gray NS, Pickering AD, Hemsley DR, Dawling S, Gray JA. Abolition of latent inhibition by a single 5 mg dose of d-amphetamine in man. Psychopharmacology (Berl) 1992b;107:425–430. doi: 10.1007/BF02245170. [DOI] [PubMed] [Google Scholar]
- Gray NS, Pilowsky LS, Gray JA, Kerwin RW. Latent inhibition in drug naive schizophrenics: relationship to duration of illness and dopamine D2 binding using SPET. Schizophr Res. 1995b;17:95–107. doi: 10.1016/0920-9964(95)00034-j. [DOI] [PubMed] [Google Scholar]
- Gurkan L, Ekeland A, Gautvik KM, Langeland N, Ronningen H, Solheim LF. Bone changes after castration in rats. A model for osteoporosis. Acta Orthop Scand. 1986;57:67–70. doi: 10.3109/17453678608993219. [DOI] [PubMed] [Google Scholar]
- Hafner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28 (Suppl 2:17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Hafner H, Riecher A, Maurer K, Loffler W, Munk-Jorgensen P, Stromgren E. How does gender influence age at first hospitalization for schizophrenia? A transnational case register study. Psychol Med. 1989;19:903–918. doi: 10.1017/s0033291700005626. [DOI] [PubMed] [Google Scholar]
- Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, et al. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20:389–409. doi: 10.2165/00023210-200620050-00004. [DOI] [PubMed] [Google Scholar]
- Hruska RE, Silbergeld EK. Increased dopamine receptor sensitivity after estrogen treatment using the rat rotation model. Science. 1980;208:1466–1468. doi: 10.1126/science.7189902. [DOI] [PubMed] [Google Scholar]
- Huber TJ, Borsutzky M, Schneider U, Emrich HM. Psychotic disorders and gonadal function: evidence supporting the oestrogen hypothesis. Acta Psychiatr Scand. 2004;109:269–274. doi: 10.1046/j.1600-0447.2003.00251.x. [DOI] [PubMed] [Google Scholar]
- Hughes ZA, Liu F, Marquis K, Muniz L, Pangalos MN, Ring RH, et al. Estrogen receptor neurobiology and its potential for translation into broad spectrum therapeutics for CNS disorders. Curr Mol Pharmacol. 2009;2:215–236. doi: 10.2174/1874467210902030215. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Beiser M. Where are the women in first-episode studies of schizophrenia. Schizophr Bull. 1992;18:471–480. doi: 10.1093/schbul/18.3.471. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis—linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Killcross AS, Dickinson A, Robbins TW. Amphetamine-induced disruptions of latent inhibition are reinforcer mediated: implications for animal models of schizophrenic attentional dysfunction. Psychopharmacology (Berl) 1994a;115:185–195. doi: 10.1007/BF02244771. [DOI] [PubMed] [Google Scholar]
- Killcross AS, Dickinson A, Robbins TW. Effects of the neuroleptic alpha-flupenthixol on latent inhibition in aversively- and appetitively-motivated paradigms: evidence for dopamine-reinforcer interactions. Psychopharmacology (Berl) 1994b;115:196–205. doi: 10.1007/BF02244772. [DOI] [PubMed] [Google Scholar]
- Koch M. Sensorimotor gating changes across the estrous cycle in female rats. Physiol Behav. 1998;64:625–628. doi: 10.1016/s0031-9384(98)00098-5. [DOI] [PubMed] [Google Scholar]
- Korhonen S, Saarijarvi S, Aito M. Successful estradiol treatment of psychotic symptoms in the premenstrual phase: a case report. Acta Psychiatr Scand. 1995;92:237–238. doi: 10.1111/j.1600-0447.1995.tb09575.x. [DOI] [PubMed] [Google Scholar]
- Kraepeline E. Dementia Praecox and Paraphrenia. Krieger: New York, NY; 1919. [Google Scholar]
- Kulkarni J. Oestrogen—a new treatment approach for schizophrenia. Med J Aust. 2009;190 (4 Suppl:S37–S38. doi: 10.5694/j.1326-5377.2009.tb02373.x. [DOI] [PubMed] [Google Scholar]
- Kulkarni J, de Castella A, Fitzgerald PB, Gurvich CT, Bailey M, Bartholomeusz C, et al. Estrogen in severe mental illness: a potential new treatment approach. Arch Gen Psychiatry. 2008a;65:955–960. doi: 10.1001/archpsyc.65.8.955. [DOI] [PubMed] [Google Scholar]
- Kulkarni J, de Castella A, Smith D, Taffe J, Keks N, Copolov D. A clinical trial of the effects of estrogen in acutely psychotic women. Schizophr Res. 1996;20:247–252. doi: 10.1016/0920-9964(96)82949-5. [DOI] [PubMed] [Google Scholar]
- Kulkarni J, Gurvich C, Gilbert H, Mehmedbegovic F, Mu L, Marston N, et al. Hormone modulation: a novel therapeutic approach for women with severe mental illness. Aust N Z J Psychiatry. 2008b;42:83–88. doi: 10.1080/00048670701732715. [DOI] [PubMed] [Google Scholar]
- Kulkarni J, Riedel A, de Castella AR, Fitzgerald PB, Rolfe TJ, Taffe J, et al. Estrogen—a potential treatment for schizophrenia. Schizophr Res. 2001;48:137–144. doi: 10.1016/s0920-9964(00)00088-8. [DOI] [PubMed] [Google Scholar]
- Landry M, Levesque D, Di Paolo T. Estrogenic properties of raloxifene, but not tamoxifen, on D2 and D3 dopamine receptors in the rat forebrain. Neuroendocrinology. 2002;76:214–222. doi: 10.1159/000065951. [DOI] [PubMed] [Google Scholar]
- Lane HY, Chang YC, Chang WH, Lin SK, Tseng YT, Jann MW. Effects of gender and age on plasma levels of clozapine and its metabolites: analyzed by critical statistics. J Clin Psychiatry. 1999;60:36–40. doi: 10.4088/jcp.v60n0108. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux M, Di Paolo T. Influence of oestrogenic compounds on monoamine transporters in rat striatum. J Neuroendocrinol. 2006;18:25–32. doi: 10.1111/j.1365-2826.2005.01380.x. [DOI] [PubMed] [Google Scholar]
- LeBlanc AJ, Reyes R, Kang LS, Dailey RA, Stallone JN, Moningka NC, et al. Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1713–R1723. doi: 10.1152/ajpregu.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindamer LA, Buse DC, Lohr JB, Jeste DV. Hormone replacement therapy in postmenopausal women with schizophrenia: positive effect on negative symptoms. Biol Psychiatry. 2001;49:47–51. doi: 10.1016/s0006-3223(00)00995-1. [DOI] [PubMed] [Google Scholar]
- Lindamer LA, Lohr JB, Harris MJ, Jeste DV. Gender, estrogen, and schizophrenia. Psychopharmacol Bull. 1997;33:221–228. [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. A neurodevelopmental model of schizophrenia: neonatal disconnection of the hippocampus. Neurotox Res. 2002;4:469–475. doi: 10.1080/1029842021000022089. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62 (4A:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- McDermott JL. Effects of estrogen upon dopamine release from the corpus striatum of young and aged female rats. Brain Res. 1993;606:118–125. doi: 10.1016/0006-8993(93)91578-g. [DOI] [PubMed] [Google Scholar]
- McDermott JL, Liu B, Dluzen DE. Sex differences and effects of estrogen on dopamine and DOPAC release from the striatum of male and female CD-1 mice. Exp Neurol. 1994;125:306–311. doi: 10.1006/exnr.1994.1034. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Morissette M, Al Sweidi S, Callier S, Di Paolo T. Estrogen and SERM neuroprotection in animal models of Parkinson's disease. Mol Cell Endocrinol. 2008;290:60–69. doi: 10.1016/j.mce.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Morissette M, Di Paolo T. Effect of chronic estradiol and progesterone treatments of ovariectomized rats on brain dopamine uptake sites. J Neurochem. 1993;60:1876–1883. doi: 10.1111/j.1471-4159.1993.tb13415.x. [DOI] [PubMed] [Google Scholar]
- Mortimer AM. Relationship between estrogen and schizophrenia. Expert Rev Neurother. 2007;7:45–55. doi: 10.1586/14737175.7.1.45. [DOI] [PubMed] [Google Scholar]
- Moser PC, Hitchcock JM, Lister S, Moran PM. The pharmacology of latent inhibition as an animal model of schizophrenia. Brain Res Brain Res Rev. 2000;33:275–307. doi: 10.1016/s0165-0173(00)00026-6. [DOI] [PubMed] [Google Scholar]
- Naik SR, Kelkar MR, Sheth UK. Attenuation of stereotyped behaviour by sex steroids. Psychopharmacology (Berl) 1978;57:211–214. doi: 10.1007/BF00426890. [DOI] [PubMed] [Google Scholar]
- Nequin LG, Alvarez J, Schwartz NB. Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biol Reprod. 1979;20:659–670. doi: 10.1095/biolreprod20.3.659. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Ferrara N, Patti F, Viglianesi M, Rampello L, Bianchi A, et al. Influence of sex steroids and prolactin on haloperidol-induced catalepsy. Brain Res. 1983;279:352–358. doi: 10.1016/0006-8993(83)90209-3. [DOI] [PubMed] [Google Scholar]
- Nofrey BS, Ben-Shahar OM, Brake WG. Estrogen abolishes latent inhibition in ovariectomized female rats. Brain Cogn. 2008;66:156–160. doi: 10.1016/j.bandc.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Palermo-Neto J, Dorce VA. Influences of estrogen and/or progesterone on some dopamine related behavior in rats. Gen Pharmacol. 1990;21:83–87. doi: 10.1016/0306-3623(90)90600-q. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Frye CA. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105–115. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, Decambre N, Coleman-Hardee ML, Simpkins JW. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated striatal [3H]dopamine release. Brain Res. 1991;566:255–264. doi: 10.1016/0006-8993(91)91706-7. [DOI] [PubMed] [Google Scholar]
- Pregelj P. Neurobiological aspects of psychosis and gender. Psychiatr Danub. 2009;21 (Suppl 1:128–131. [PubMed] [Google Scholar]
- Rao ML, Kolsch H. Effects of estrogen on brain development and neuroprotection—implications for negative symptoms in schizophrenia. Psychoneuroendocrinology. 2003;28 (Suppl 2:83–96. doi: 10.1016/s0306-4530(02)00126-9. [DOI] [PubMed] [Google Scholar]
- Rascle C, Mazas O, Vaiva G, Tournant M, Raybois O, Goudemand M, et al. Clinical features of latent inhibition in schizophrenia. Schizophr Res. 2001;51:149–161. doi: 10.1016/s0920-9964(00)00162-6. [DOI] [PubMed] [Google Scholar]
- Riddoch D, Jefferson M, Bickerstaff ER. Chorea and the oral contraceptives. Br Med J. 1971;4:217–218. doi: 10.1136/bmj.4.5781.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecher-Rossler A, Hafner H. Gender aspects in schizophrenia: bridging the border between social and biological psychiatry. Acta Psychiatr Scand Suppl. 2000;407:58–62. doi: 10.1034/j.1600-0447.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russig H, Kovacevic A, Murphy CA, Feldon J. Haloperidol and clozapine antagonise amphetamine-induced disruption of latent inhibition of conditioned taste aversion. Psychopharmacology (Berl) 2003;170:263–270. doi: 10.1007/s00213-003-1544-5. [DOI] [PubMed] [Google Scholar]
- Salem JE, Kring AM. The role of gender differences in the reduction of etiologic heterogeneity in schizophrenia. Clin Psychol Rev. 1998;18:795–819. doi: 10.1016/s0272-7358(98)00008-7. [DOI] [PubMed] [Google Scholar]
- Salgado JV, Hetem LA, Vidal M, Graeff FG, Danion JM, Sandner G. Reduction of latent inhibition by D-amphetamine in a conditioned suppression paradigm in humans. Behav Brain Res. 2000;117:61–67. doi: 10.1016/s0166-4328(00)00279-5. [DOI] [PubMed] [Google Scholar]
- Salokangas RK. Gender and the use of neuroleptics in schizophrenia. Further testing of the oestrogen hypothesis. Schizophr Res. 1995;16:7–16. doi: 10.1016/0920-9964(94)00059-h. [DOI] [PubMed] [Google Scholar]
- Saugstad LF. Social class, marriage, and fertility in schizophrenia. Schizophr Bull. 1989;15:9–43. doi: 10.1093/schbul/15.1.9. [DOI] [PubMed] [Google Scholar]
- Seeman MV. Gender differences in schizophrenia. Can J Psychiatry. 1982;27:107–112. doi: 10.1177/070674378202700204. [DOI] [PubMed] [Google Scholar]
- Seeman MV. Current outcome in schizophrenia: women vs men. Acta Psychiatr Scand. 1986;73:609–617. doi: 10.1111/j.1600-0447.1986.tb02732.x. [DOI] [PubMed] [Google Scholar]
- Seeman MV. Prenatal gonadal hormones and schizophrenia in men and women. Psychiatr J Univ Ott. 1989;14:473–475. [PubMed] [Google Scholar]
- Seeman MV, Lang M. The role of estrogens in schizophrenia gender differences. Schizophr Bull. 1990;16:185–194. doi: 10.1093/schbul/16.2.185. [DOI] [PubMed] [Google Scholar]
- Segarra AC, Agosto-Rivera JL, Febo M, Lugo-Escobar N, Menéndez-Delmestre R, Puig-Ramos A, et al. 2009Estradiol: a key biological substrate mediating the response to cocaine in female rats Horm Behavdoi: 10.1016/j.yhbeh.2009.12.003(e-pub ahead of print) [DOI] [PMC free article] [PubMed]
- Shadach E, Feldon J, Weiner I. Clozapine-induced potentiation of latent inhibition is due to its action in the conditioning stage: implications for the mechanism of action of antipsychotic drugs. Int J Neuropsychopharmacol. 1999;2:283–291. doi: 10.1017/S1461145799001583. [DOI] [PubMed] [Google Scholar]
- Shaikh AA. Estrone and estradiol levels in the ovarian venous blood from rats during the estrous cycle and pregnancy. Biol Reprod. 1971;5:297–307. doi: 10.1093/biolreprod/5.3.297. [DOI] [PubMed] [Google Scholar]
- Shieh KR, Yang SC. Effects of estradiol on the stimulation of dopamine turnover in mesolimbic and nigrostriatal systems by cocaine- and amphetamine-regulated transcript peptide in female rats. Neuroscience. 2008;154:1589–1597. doi: 10.1016/j.neuroscience.2008.01.086. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Yadalam KG, Levinson DF, Stephanos MJ, Lo ES, Cooper TB. Single-dose pharmacokinetics of fluphenazine after fluphenazine decanoate administration. J Clin Psychopharmacol. 1990;10:417–421. doi: 10.1097/00004714-199010060-00007. [DOI] [PubMed] [Google Scholar]
- Stone JM, Pilowsky LS. Antipsychotic drug action: targets for drug discovery with neurochemical imaging. Expert Rev Neurother. 2006;6:57–64. doi: 10.1586/14737175.6.1.57. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Stephany N, Wasserman LC, Talledo J, Sharp R, Auerbach PP. Dopamine agonists disrupt visual latent inhibition in normal males using a within-subject paradigm. Psychopharmacology (Berl) 2003;169:314–320. doi: 10.1007/s00213-002-1325-6. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Stephany N, Wasserman LC, Talledo J, Sharp R, Minassian A, et al. Intact visual latent inhibition in schizophrenia patients in a within-subject paradigm. Schizophr Res. 2005;72:169–183. doi: 10.1016/j.schres.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Szymanski S, Lieberman JA, Alvir JM, Mayerhoff D, Loebel A, Geisler S, et al. Gender differences in onset of illness, treatment response, course, and biologic indexes in first-episode schizophrenic patients. Am J Psychiatry. 1995;152:698–703. doi: 10.1176/ajp.152.5.698. [DOI] [PubMed] [Google Scholar]
- Tamminga CA. Gender and schizophrenia. J Clin Psychiatry. 1997;58 (Suppl 15:33–37. [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem. 1994;62:1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Thornton JC, Dawe S, Lee C, Capstick C, Corr PJ, Cotter P, et al. Effects of nicotine and amphetamine on latent inhibition in human subjects. Psychopharmacology (Berl) 1996;127:164–173. doi: 10.1007/BF02805990. [DOI] [PubMed] [Google Scholar]
- Vaillancourt C, Cyr M, Rochford J, Boksa P, Di Paolo T. Effects of ovariectomy and estradiol on acoustic startle responses in rats. Pharmacol Biochem Behav. 2002;74:103–109. doi: 10.1016/s0091-3057(02)00967-x. [DOI] [PubMed] [Google Scholar]
- Van den Buuse M, Eikelis N. Estrogen increases prepulse inhibition of acoustic startle in rats. Eur J Pharmacol. 2001;425:33–41. doi: 10.1016/s0014-2999(01)01139-6. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Estradiol reduces anxiety- and depression-like behavior of aged female mice. Physiol Behav. 2010;99:169–174. doi: 10.1016/j.physbeh.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Paris JJ, Frye CA. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology. 2009;34:909–916. doi: 10.1016/j.psyneuen.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Mitchell SN, Joseph MH. Calcium dependence of sensitised dopamine release in rat nucleus accumbens following amphetamine challenge: implications for the disruption of latent inhibition. Behav Pharmacol. 1996;7:119–129. [PubMed] [Google Scholar]
- Weiner I. Neural substrates of latent inhibition: the switching model. Psychol Bull. 1990;108:442–461. doi: 10.1037/0033-2909.108.3.442. [DOI] [PubMed] [Google Scholar]
- Weiner I. The ‘two-headed' latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacology (Berl) 2003;169:257–297. doi: 10.1007/s00213-002-1313-x. [DOI] [PubMed] [Google Scholar]
- Weiner I, Arad M. Using the pharmacology of latent inhibition to model domains of pathology in schizophrenia and their treatment. Behav Brain Res. 2009;204:369–386. doi: 10.1016/j.bbr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Weiner I, Feldon J. Facilitation of latent inhibition by haloperidol in rats. Psychopharmacology (Berl) 1987;91:248–253. doi: 10.1007/BF00217073. [DOI] [PubMed] [Google Scholar]
- Weiner I, Gal G, Rawlins JN, Feldon J. Differential involvement of the shell and core subterritories of the nucleus accumbens in latent inhibition and amphetamine-induced activity. Behav Brain Res. 1996a;81:123–133. doi: 10.1016/s0166-4328(96)00051-4. [DOI] [PubMed] [Google Scholar]
- Weiner I, Shadach E, Barkai R, Feldon J. Haloperidol- and clozapine-induced enhancement of latent inhibition with extended conditioning: implications for the mechanism of action of neuroleptic drugs. Neuropsychopharmacology. 1997;16:42–50. doi: 10.1016/S0893-133X(96)00145-5. [DOI] [PubMed] [Google Scholar]
- Weiner I, Shadach E, Tarrasch R, Kidron R, Feldon J. The latent inhibition model of schizophrenia: further validation using the atypical neuroleptic, clozapine. Biol Psychiatry. 1996b;40:834–843. doi: 10.1016/0006-3223(95)00573-0. [DOI] [PubMed] [Google Scholar]
- Zhou W, Cunningham KA, Thomas ML. Estrogen regulation of gene expression in the brain: a possible mechanism altering the response to psychostimulants in female rats. Brain Res Mol Brain Res. 2002;100:75–83. doi: 10.1016/s0169-328x(02)00134-1. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]