Abstract
The vacuolar-ATPase (v-ATPase) is a proton transporter found on many intra-cellular organelles and the plasma membrane (PM). The v-ATPase on PMs of cancer cells may contribute to their invasive properties in vitro. Its relevance to human cancer tissues remains unclear. We investigated whether the expression and cellular localization of v-ATPase corresponded to the stage of human pancreatic cancer, and its effect on matrix metalloproteinase (MMP) activation in vitro. The intensity of v-ATPase staining increased significantly across the range of pancreatic histology from normal ducts to pancreatic intra-epithelial neoplasms (PanIN) and finally pancreatic ductal adenocarcinoma (PDAC). Low-grade PanIN lesions displayed polarized staining confined to the basal aspect of the cell in the majority (86%) of fields examined. High-grade PanIN lesions and PDAC demonstrated intense and diffuse v-ATPase localization. In pancreatic cancer cells, PM-associated v-ATPase co-localized with cortactin, a component of the leading edge that helps direct MMP release. Blockade of the v-ATPase with concanamycin or shRNA targeting the V1E subunit reduced MMP-9 activity; this effect was greatest in cells with prominent PM-associated v-ATPase. In cells with detectable MMP-2 activities, however, treatment with concanamycin markedly increased MMP-2’s most activated forms. V-ATPase blockade inhibited functional migration and invasion in those cells with predominantly MMP-9 activity. These results indicate that human PDAC specimens demonstrate loss of v-ATPase polarity and increased expression that correlates with increasing invasive potential. Thus, v-ATPase selectively modulates specific MMPs that may be linked to an invasive cancer phenotype.
Keywords: MMP, PanIN, pancreatic cancer, v-ATPase
Cancer cells often exist in hypoxic conditions due to unchecked growth and a deficient blood supply.1 The acidic tumor microenvironment that results from hypoxia evokes compensatory changes in the cancer cell that confer survival and growth advantages. One such mechanism is the activity of the vacuolar-ATPase (v-ATPase).2, 3 This ubiquitous proton transporter mediates multiple pH-dependent intracellular processes including vesicular trafficking, coupled ion gradient-molecular transport, and protease activation.4-7 The v-ATPase was originally characterized in association with intracellular organelles, but is now also recognized as a component of plasma membranes that mediates proton extrusion in some normal and malignant cells.8–11
In breast cancer cells, the abundance of v-ATPases on the plasma membrane correlates with an invasive phenotype.11 Further, v-ATPase inhibitors reduce cell migration in cancer cells with high levels of plasma membrane v-ATPase.11 It has been postulated that cell surface v-ATPase activity can provide a localized proton flux creating an acidic extracellular microenvironment.3 One potential effect of this activity is to optimize pH for extracellular protease activation, thereby facilitating matrix degradation and cellular invasion.
The v-ATPase is comprised of two large, multi-subunit complexes organized into a soluble domain (V1) responsible for ATP hydrolysis and a membrane-bound domain (V0) that forms the pore allowing for proton transport across membranes.12 Multiple isoforms of the “a” subunit that comprise the V0 complex exist. Distinct V0a isoforms are thought to be critical for targeting the V0 complex to distinct cellular membranes. For example, the V0a3 subunit on apical plasma membranes of osteoclasts generates the acidic extracellular microenvironment required for bone resorption.13, 14 Mutations in V0a3 result in the human autosomal recessive disease, osteopetrosis, which is characterized by excess bone and severely impaired osteoclast activity.15, 16 A mouse model of V0a3 deletion results in severe skeletal deformities and early death, and can be averted by V0a3 gene restoration.17, 18 In breast cancer cells, the V0a3 and V0a4 isoforms were also recently shown to be essential for cellular invasion.19 These findings demonstrate that specific V0a isoforms provide a mechanism to target v-ATPase activities to the cell surface and affect cell function. These findings suggest that differences in the v-ATPase localization assessed by immuno-labeling could be used to predict the behavior of cancer types, and that its use in clinical specimens could serve as a histological marker of aggressiveness.
Expression and localization of v-ATPase in human cancer specimens has not been extensively explored. To determine whether elevated v-ATPase staining corresponds to the invasive phentoype in human pancreatic cancer, we assessed the range of tissues from non-invasive Pancreatic Intraepithelial Neoplasia (PanIN) lesions to pancreatic ductal adenocarcinoma (PDAC). Here, we report that the v-ATPase in human PDAC loses its polarity with increasing invasive potential. Furthermore, we observed that select v-ATPase isoforms are found on human pancreatic cancer cells, and that the v-ATPase localizes with known components of the cellular invasion apparatus and has functional effects on matrix metalloproteinase (MMP) activation.
MATERIALS AND METHODS
Human Tissue
Archival specimens were obtained from patients who underwent surgery for a diagnosis of PDAC. The pathological diagnosis confirmed PDAC in all cases (n=16). Fifty random normal ducts, PanIN lesions and PDAC lesions were evaluated independently by two pathologists (SEC, PF). Intensity of staining was scored as 1+ (mild), 2+ (moderate), or 3+ (intense). Immuno-labeling was characterized as basal, mixed basal/apical or mixed basal/diffuse. The Institutional Review Board of the VA CT Healthcare System approved the study.
Antibodies and Reagents
Antibodies to V1E (Genway), V0a2 and V0a3 (gift of Dr. Beth S. Lee, Ohio State School of Medicine) were used to assess v-ATPase isoform specificity. Antibodies to cell surface markers E-cadherin (BD Biosciences) and epidermal growth factor receptor (Cell Signaling) were used to delineate localization of v-ATPase on plasma membranes. An anti-cortactin antibody (AbCam) was used to mark cellular invasive fronts.20, 21 Secondary fluorescent antibodies were purchased from Invitrogen. Chemical reagents were purchased from Sigma.
Cell Culture
The human pancreatic cancer cell lines Panc-1, MiaPaCa, and BXPC3 were maintained according to ATCC guidelines. Since v-ATPase assembly is glucose-dependent,22, 23 DMEM with low (1 g/L) and high (4.5 g/L) glucose were used to assess the role of v-ATPase on protease activation. To obtain conditioned medium (CM), cells were grown to 80% confluence, washed twice with serum-free media, and then incubated with serum-free media overnight. CM was obtained after 18–20 hours and concentrated approximately 40-fold using Amicon Ultra centrifugal filters (Millipore) with a 10 kDa cutoff.
Short-Hairpin RNA Knockdown of V-ATPase Subunit, V1E
Oligonucleotide targeting sequences corresponding to the coding regions of human V1E were annealed and ligated into pSuper.retro.puro (Oligoengine) (Supplementary Table 1). Panc-1 cells were transfected with adeno-associated viral vector and transfected clones selected with puromycin (1–2.5 μg/ml). Surviving clones were maintained in puromycin 2.0 μg/ml. After immunoblotting V1E, percent knockdown was assessed by densitometry using NIH Image J software.
Immunohistochemistry & Immunofluorescence
Immunohistochemistry was performed as described.24 Sections were deparaffinized, treated to inhibit endogenous peroxidase and subjected to antigen retrieval. Slides were washed in tris-buffered saline and incubated with primary antibodies. Sections were washed, incubated with biotinylated anti-serum, and then with streptavidin complexed with horseradish peroxidase followed by diaminobenzidine. Sections were then counter-stained with hematoxylin & eosin.
For immunofluorescence (IF) labeling, pancreatic cancer cells were grown on methanol-treated coverslips. Cells were rinsed with phosphate-buffered saline, permeabilized with 0.05% saponin for 15 minutes, and blocked in 3% BSA. Coverslips were incubated with primary antibody, and then corresponding secondary antibodies. Slides were mounted with Prolong Gold with DAPI (Invitrogen). Control slides were incubated in secondary antibody only. Slides were examined with a Zeiss Axiophot immunofluorescence microscope. Images were obtained with SPOT software and overlay images obtained using Adobe Photoshop, version 9.0.
Zymography and Immunoblotting
Matrix metalloproteinases (MMPs) were first cloned as cancer-specific genes and play a critical role in tumor invasion and metastases.25 To detect MMP-2/9 activities in pancreatic cancer secretions, zymography was performed using commercial (Invitrogen) 10% gelatin-containing gels. Briefly, 10–20 μg of cellular proteins were subject to non-denaturing electrophoresis as described.26 Gels were washed in 2.5% Triton X-100 and then incubated in developing buffer. After Coomassie blue staining, gels were destained and the amount of MMP activity detected as clear bands was analyzed by densitometry. The purified intermediately active form (66 kDa) of MMP-2, (5–10 ng; Calbiochem), was used as a positive control. Optimal incubation times were: Panc-1 (12–16hrs); MiaPaCa (18–24hrs); and BXPC3 (6–8hrs), n=4 experiments per group. The protease activity was estimated using NIH ImageJ software as follows: a measurement box was placed over cleared areas of degradation and the immediate non-degraded background. Values were normalized to the highest density and comparisons were made within low and high glucose conditions, and then across the different glucose concentrations.
Immunoblotting was performed as described.24 Protein content was determined by Bradford assay. After blocking in a 5% milk solution, membranes were incubated overnight with antibodies to PEDF. After washing in TBS and 0.05% Tween, the primary antibody was labeled using a peroxidase-conjugated goat anti-rabbit IgG. Peroxidase was detected by a chemiluminescence assay (Pierce). Equivalence of loading was confirmed using β-actin (Sigma) or Coomassie stain of conditioned medium (CM).
Invasion & Migration Assays
An agarose-spot assay assessing cellular invasion number and depth was performed as described.27 Agarose spots were plated on recessed collagen-coated cell culture plates in the presence of epidermal growth factor (EGF, R&D Systems) at a final concentration of 30–100 ng/μl. Cells were plated for four to six hours and allowed to adhere in the presence of normal growth medium. Medium was then changed to serum-free media for 12–24 hours in the absence and presence of concanamycin 50 nM. Cells that were able to invade across the outer rim of the agarose spot were counted as positive. Ten randomly selected fields under 10× for each condition were visualized.
Wound migration assays were performed as described.11 In brief, a 2 mm horizontal wound was created after cells were grown to confluence. The cells were washed and placed under serum-free conditions in the absence and presence of concanamycin [50 nM]. Cells were evaluated at 24–48 hrs for migration across the wound. Cells were fixed and 10 random images were captured. In each image, three random measurements of the diameter were done using NIH Image J software, averaged, and values normalized based on the largest diameter. Assays were done in triplicate.
Statistical Analysis
P-values were calculated using two-tailed Student’s t-tests on Prism software Version 4.0, with P<0.05 deemed statistically significant. For differences in immunohistochemistry density, one-way analysis of variance was used. Values were stated as mean ± s.e.m. or s.d.
RESULTS
Human PDAC Tissues Demonstrate Increased V-ATPase Levels and Loss of Polarity with Advancing Neoplastic Features
Histological analysis from normal ducts through PanIN lesions and finally to PDAC demonstrated distinct patterns of V1E subunit staining that differed in intensity and distribution. In the absence of a primary antibody, there was little labeling (Figure 1a). Using an antibody targeting the V1E subunit, normal ducts (Figure 1b) displayed weak staining with a mixed basal/apical (estimated avg ratio intensity to negative control–1.24±0.43) pattern. Low-grade PanIN (Figure 1c) lesions demonstrated a typical columnar appearance with basally oriented nuclei, an increased N/C ratio, and the presence of mild nuclear atypia. These lesions showed more intense v-ATPase immunoreactivity than normal ducts (intensity: 1.72±0.45). Further, low-grade PanIN lesions revealed striking differences in the distribution of v-ATPase E subunit compared to apical and basal staining in normal ducts. Thus, in 86% of the fields, labeling in low-grade PanIN lesions was basally-oriented with the remaining 14% showing mixed basal/apical distribution. High-grade PanIN (Figure 1d) lesions displayed even more intense cellular labeling than the lower grade lesions (intensity: 2.56±0.50), but its distribution was more diffuse than observed with lower grade lesions. (Additional images contrasting low grade from high grade PanIN are provided in Supplementary Figure 1.) PDAC, (Figures 1e-g) whether from the primary tumor (Figure 1e), or metastatic to lymph nodes (Figure 1g) showed uniformly intense staining and diffuse labeling. Finally, since endothelial and stromal cells release proteases and contribute to tumor spread, areas of stroma were also examined for v-ATPase labeling. Positive V1E labeling that corresponded to vascular structures (Figure 1h, circular areas) or spindle-shaped cells that likely reflect fibroblasts (Figure 1h, arrows) was present in tumor-associated stroma but not in stroma adjacent to normal ducts (Figure 1b). Findings from our histological analysis are summarized in Table 1. These results indicate a significantly increased intensity (P<0.001) and a loss of v-ATPase polarity that occurs from low-grade PanIN to invasive PDAC. The findings are consistent with in vitro studies that illustrate v-ATPase function is necessary for cancer cell homeostatic and invasive properties.11, 19
Figure 1.
Immuno-labeling of v-ATPase shows increased intensity and loss of polarity that corresponds to the grade of human pancreatic cancer. Labeling of the V1E subunit in: (a) Negative control. (b) A normal pancreatic duct shows faint staining of the v-ATPase with a mixed basal/apical distribution. (c) Low grade PanINs display a more intense and polarized (basal) distribution of v-ATPase than normal cells. (d) High grade PanIN lesions demonstrate increased irregularity in the distribution of v-ATPase within cells compared to low-grade PanIN lesions. (e) PDAC (low power) and (f) PDAC (high power) show loss of polarization, diffuse but heterogenous distribution of v-ATPase reactivity and more intense staining than normal cells or PanIN lesions. (g) PDAC within a lymph node shows diffuse and robust staining within the cancer cells. (h) Tumor-associated stroma reveals v-ATPase labeling within endothelial cells (circular structures) and spindle-shaped cells (arrows) that likely represent tumor-associated fibroblasts. Scale Bar: a, e, g, 200 μm; b, c, d, f, h, 80 μm.
Table 1.
V-ATPase immuno-labeling demonstrates a progressive increase in intensity and loss of polarity from early PanIN lesions to pancreatic ductal adenocarcinoma (PDAC). Immunohistochemical staining of v-ATPase was graded by two independent pathologists based on intensity (upper panel) and distribution within the cells (lower panel) in randomly selected fields. The intensity score for normal ducts (1.24±0.43), low grade PanINs (1.72±0.45), high grade PanINs (2.56±0.50) and PDAC (all 3+) were significantly different among the groups, P<0.001. While the majority of normal pancreatic ducts showed a faint mixed basal/apical v-ATPase pattern, low grade PanINs mostly (86%) had a basal v-ATPase distribution within cells. The majority (94%) of high grade PanINs and all the PDAC had a diffuse distribution of v-ATPase compared to low grade PanIN lesions, P<0.001.
| Histology | 1+ | 2+ | 3+ | Intensity score (mean ± SD) |
|---|---|---|---|---|
| Normal ducts | 38/50 (76) | 12/50 (24) | 0/50 | 1.24 ± 0.43 |
| Low grade PanIN | 14/50 (28) | 36/50 (72) | 0/50 | 1.72 ± 0.45 |
| High grade PanIN | 0/50 | 22/50 (44) | 28/50 (56) | 2.56 ± 0.50 |
| PDAC | 0/50 | 0/50 | 50/50 (100) | 3.00 ±0.00 |
| Histology | Basal | Mixed basal/apical | Diffuse |
|---|---|---|---|
| Normal ducts | 0/50 | 50/50 (100) | 0/50 |
| Low grade PanIN | 43/50 (86) | 5/50 (10) | 2/50(4) |
| High grade PanIN | 3/50(6) | 0/50 | 47/50 (94) |
| PDAC | 0/50 | 0/50 | 50/50 (100) |
Pancreatic Cancer Cells Display V-ATPase PM Localization and Co-localize with Components of Cellular Invasion
Analogous to studies in other cancer cells,11, 19 v-ATPase isoforms were evident on the plasma membranes of some pancreatic cancer cells while others displayed minimal localization on or near the plasma membranes. Well-differentiated BXPC3 cells which have been described as the least invasive of the three cell lines used,28 displayed far less prominent plasma membrane staining of the v-ATPase a3 isoform (Figure 2a) than that found on Panc-1 cells (Figure 2b). Similar to the a3 isoform, the soluble V1E isoform demonstrated little plasma membrane localization (Figure 2c) in BXPC3 cells. To further characterize these differences, co-localization with E-cadherin was performed. BXPC3 cells, as previously described, demonstrated faint E-cadherin expression when plated at low density (data not shown). In culture conditions approaching confluence, E-cadherin was discernible at sites of cell-to-cell contact (Figure 2d, arrows), and demonstrated no discernible overlap with V1E. In contrast, the leading edge of Panc-1 cells exhibited plasma membrane V1E staining (Figure 2e) that overlapped with E-cadherin labeling on plasma membranes (Figure 2f, arrows) but not at cellular junctions (Figure 2f, arrowheads). (Additional high magnification images of V1E and E-cadherin labeling in Panc-1 cells are included in Supplementary Figure 2.) Staining for V1E and epidermal growth factor receptor (EGFR) in Panc-1 cells (Figure 2g and h) further demonstrated that the v-ATPase is present on plasma membranes (arrows). The hypoxia mimetic cobalt chloride 200 μM did not appear to increase the degree of v-ATPase labeling on plasma membranes in Panc-1 cells (data not shown). Thus, the localization of the v-ATPase on PMs may reflect the functional ability to acidify local peri-cellular domains and contribute to the invasive phenotype of pancreatic cancer cells.
Figure 2.
Pancreatic cancer cells display plasma membrane localization of v-ATPase isoforms and show co-localization with cell surface markers. Immunofluorescent (IF) images of V0a3 subunit labeling in (a) BXPC3 and (b) Panc-1 cells reveals that localization to plasma membranes is greater in some pancreatic cancer cells than others. Labeling of the V1E subunit (c) and the cell surface marker E-cadherin (d, arrows) in BXPC3 cells demonstrates minimal colocalization. Panc-1 cells display cell surface labeling for V1E (e) and E-cadherin with areas of colocalization (f, arrows) on the leading edge of cells while cell to cell adhesions stained only for E-cadherin (f, arrowheads). Colocalization of V1E (g, arrows) in Panc-1 cells was also seen with other cell surface markers such as EGFR-1 (h). Scale Bar: 10μm.
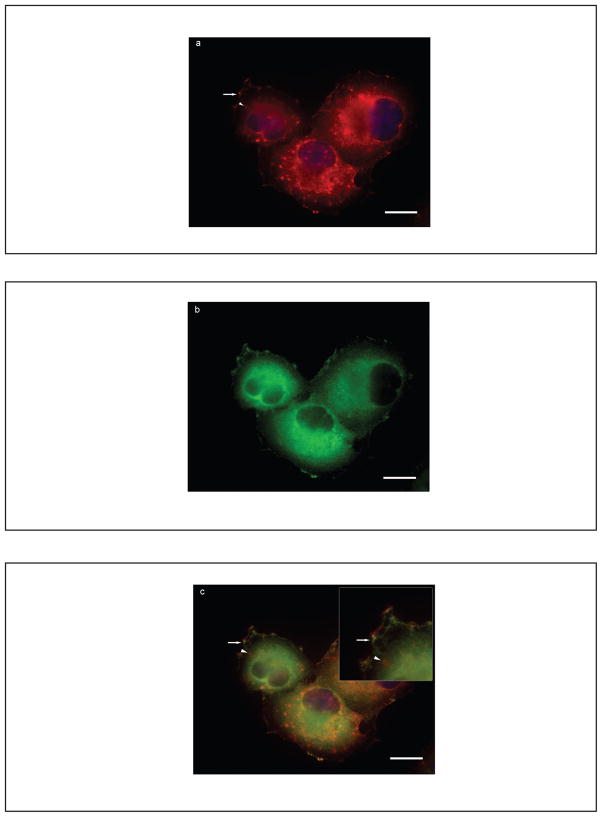
Specific regions of cancer cells reflecting the leading edge or invasive front are responsible for focal protease release required for matrix degradation and cell invasion.29 We investigated whether the v-ATPase co-localizes with other proteins that are know to reside in the leading edge. Cortactin is a prominent tyrosine kinase substrate that produces an actin assembly complex required for actin-based protrusions at the leading edge and directs MMP release at invasion sites.20, 21, 30 In Panc-1 cells, V1E labeling (Figure 3a) occurred on plasma membranes (arrow) and intracellular organelles (arrowhead). Cortactin localized to well-defined patches at the plasma membrane (Figure 3b). Double-labeling studies demonstrated that the v-ATPase V1E subunit was located at or adjacent to regions of cortactin immunoreactivity (Figure 3c, arrow) with no overlap with cortactin staining on intracellular organelles (Figure 3c, inset, arrowhead). These results indicate that the v-ATPase on plasma membranes co-distributes with known components of the cellular invasion apparatus. (Cortactin co-localization with another v-ATPase subunit, V0a3, is shown in Supplementary Figure 3.)
Figure 3.
Panc-1 cells demonstrate co-localization of v-ATPase with cortactin, a component of the cellular invasion apparatus. Immunofluorescent (IF) images of the V1E isoform (a, red) and cortactin (b, green) in Panc-1 cells reveal that in those cells with plasma membrane v-ATPase localization proximity to known components of the cellular invasion apparatus such as cortactin was observed. Non-overlapping intracellular areas of V1E localization are shown (inset, red circular structures, arrowhead) adjacent to merged areas (c, inset, yellow).
Pancreatic Cancer Cell-Derived MMP-9, but not MMP-2, Activities are Decreased with V-ATPase Blockade
To examine the functional effect of v-ATPases on MMP activities, zymography of conditioned medium (CM) was performed with chemical inhibitors and shRNA-mediated knockdown of the v-ATPase. MMP-9 activity was present in the three pancreatic cancer cell lines examined. Incubation with concanamycin [50 nM] led to significantly decreased MMP-9 activity in each cell line; this effect was most evident in Panc-1 and MiaPaCa cells. For Panc-1 and MiaPaCa cells, MMP-9 activities were decreased under both low and high glucose conditions (Figure 4a, P<0.05; Figure 4b, P<0.01). V-ATPase inhibition had modest effects on BXPC3 cells (Figure 4c) under low glucose conditions only. These findings were corroborated in Panc-1 cells with shRNA knockdown of the V1E subunit (Figure 4d). These cells demonstrated significantly decreased MMP-9 activity that corresponded to the extent of V1E subunit knockdown. These results indicate pancreatic cancer cells with plasma membrane localization of the v-ATPase are susceptible to MMP-9 activities that depend on v-ATPase function.
Figure 4.
Pancreatic cancer cell-derived MMP-9, but not MMP-2, display reduced activities after v-ATPase blockade. Effects of v-ATPase inhibition on MMP-9 activity using concanamycin in (a) Panc-1, (b) MiaPaCa, and (c) BXPC3 cells are shown. In low glucose conditions, concanamycin reduced MMP-9 activity more than 2-fold in Panc-1 and MiaPaCa cells and to a lesser extent in BXPC3 cells. In high glucose conditions concanamycin also reduced MMP-9 activity in Panc-1 and MiaPaCa cells but did not have a significant effect on BXPC3 cells. (d) Targeted knockdown of V1E by shRNA constructs E1 and E2 results in decreased MMP-9 activities. (e) V-ATPase inhibition with concanamycin results in increased activity of the fully activated (62 kDa) MMP-2 isoform. Representative zymogram of Panc-1 CM shown (top panel). Higher MMP-2 activity of the intermediately active (66 kDa) form under high glucose conditions was seen (middle panel), while the 62 kDa form was significantly higher in the presence of concanamycin under both low and high glucose conditions, (upper panel).
V-ATPase inhibition yielded different effects on MMP-2 activity. MMP-2 activity was evident in the CM of Panc-1 cells but barely detectable in MiaPaCa and BXPC3 lines (data not shown). In Panc-1 cells the intermediately active (66 kDa) zymogen and the fully active (62 kDa) protease forms (Figure 4e) could be differentiated. V-ATPase inhibition increased the most active forms of MMP-2 significantly more than under control conditions (P<0.001). Similar findings on MMP-2 activation were obtained using the related v-ATPase inhibitor, bafilomycin [50 nM], (Supplementary Figure 4). These results point to the different regulation and activation of MMP-2 versus MMP-9 precursor forms, and are consistent with a previous report on enhanced MMP-2 activities in melanoma cells treated with bafilomycin.31
Invasion and Cellular Degradation are V-ATPase-Dependent
The functional effects of v-ATPase inhibition on cell invasion and migration were assessed. An agar-based assay showed that MiaPaCa cells were able to invade and this was inhibited in the presence of concanamycin (Figure 5a). Furthermore, concanamycin treatments also inhibited migration across a wound (Figure 5b, P<0.05), with 21.9% and 35.1% greater diameter compared to controls under low and high glucose conditions, respectively. Panc-1 cells demonstrated no differences in invasion in the presence of concanamycin (data not shown), a finding that may reflect the complex effects of concanamycin treatment on MMP activities: decreased MMP-9 but increased MMP-2 active isoforms.
Figure 5.
V-ATPase function is necessary for pancreatic cancer cell migration and invasion. (a) Control MiaPaCa cells (right) migrate into an agar disk, while adding the v-ATPase inhibitor concanamycin to the medium inhibits their migration. (b) Scratch wound migration assay shows larger relative wound diameter after concanamycin compared to control MiaPaCa cells, demonstrating that v-ATPase blockade inhibits migration.
DISCUSSION
This study illustrated that the cellular distribution of v-ATPase in human pancreatic cancer tissues may influence cancer cell activity since polarity is lost and expression was increased with advancing malignant features. In fact, striking differences in v-ATPase polarity and staining intensity distinguished early from advanced PanIN lesions. Invasive pancreatic cancers and metastatic lesions meanwhile demonstrated uniformly diffuse and intense v-ATPase staining. These findings indicate that elevated expression and loss of v-ATPase polarity may be key steps in modulating the tumor microenvironment, thereby providing a clinical correlate to previous in vitro work in breast cancer cells that identified v-ATPase expression as a marker of cancer cell aggressiveness.11
A previous study in human pancreatic cancer specimens compared mRNA levels and immuno-labeling of the v-ATPase V0c subunit in PDAC in relation to precursor lesions and benign cystic tumors.32 This study indicated that the mRNA levels of this subunit were increased in PDAC eight-fold over normal pancreas. Similar to the results reported here, the intensity of v-ATPase expression was highest in PDAC. The absence of positive staining in non-invasive cancers or in benign cystic neoplasms in this previous study is a notable difference from our results. The current study found that PanIN lesions demonstrated prominent v-ATPase labeling with a clear loss of polarity that coincided with increasing malignant features. Our current results demonstrate a unique pattern to v-ATPase (V1E subunit) labeling within PDAC precursors, which suggests an early role for v-ATPase function in cancer cell homeostasis and invasive capacity as corroborated by previous literature.11, 19, 33 Future evaluation of v-ATPase expression in human cancer sections using standardized antibodies and techniques may be necessary to resolve these differences.
Other studies have demonstrated that the v-ATPase on plasma membranes contributes to acidification of the extracellular space which promotes invasive properties.11, 34 Targeted inhibition of the V0c subunit led to diminished MMP-2 expression and reduced hepatocellular carcinoma growth in an animal model indicating that the therapeutic potential of inhibiting the v-ATPase may be due in part to reducing MMP-2 activity.34, 35 However, whether these findings are relevant to other types of cancers and other MMPs is unclear. We demonstrated that the v-ATPase is present on plasma membranes of Panc-1 cells; these cell lines have been described to have invasive potential in vivo.28 In Panc-1 cells, the v-ATPase co-localizes with cortactin, a component of the cellular invasion apparatus implicated in focal MMP-9 release.20, 21 Significantly, MMP-9 activity was reduced with v-ATPase blockade in three pancreatic cancer cell lines, but was least affected in BXPC3 cells which demonstrated little v-ATPase PM localization. Targeted inhibition of the V1E subunit using shRNA constructs confirmed these findings in Panc-1 cells. Thus, specific pancreatic cancer cells display v-ATPase plasma membrane localization and MMP-9 activities that are v-ATPase dependent.
In contrast to MMP-9, concanamycin and bafilomycin inhibition of the v-ATPase increased the fully active MMP-2 isoform. A potential explanation for this difference may be the differential regulation and activation of MMP-2 and -9 precursors. MT1-MMP, a cell surface activator of MMP-2, is rapidly and constitutively down-regulated through a v-ATPase-dependent degradation process.31, 36 V-ATPase blockade results in increased levels of active MT1-MMP on the cell surface thereby amplifying MMP-2 activities, which is consistent with our findings.31, 37 These findings indicate that potential targeting of the v-ATPase in cancer studies may have indirect (and unintended) effects on regulators of MMP activation that could enhance, rather than block, specific protease activities.
Since MMP-2 activation with concanamycin treatments reflects the ability of intact intracellular degradation pathways that are v-ATPase dependent, the current studies using chemical v-ATPase inhibitors do not allow us to discern the relative contributions of extracellular versus intracellular proton flux. Future experiments targeting the specific subunits associated with plasma membrane localization may help to provide additional evidence supporting a role for v-ATPase-mediated extracellular acidification.
Although we have shown that the v-ATPase can modulate MMP activities in cancer cells, this transporter can affect other cellular responses that may be relevant to cancer biology. For instance, v-ATPases may mediate resistance to chemotherapeutic agents. Tumor cells, when exposed to chemotherapeutic drugs, demonstrate transcriptional promoter activity that leads to the induction of specific v-ATPase levels.38, 39 This induction precludes cancer cell apoptosis in response to chemotherapy demonstrating that v-ATPase expression may be a protective mechanism against chemically-induced apoptosis. Combined application of chemotherapy and a v-ATPase inhibitor restored the ability of these agents to cause cancer cell apoptosis.39 Thus, in addition to effects on MMP activities, targeting v-ATPases could also enhance the sensitivity of cancer cells to chemotherapeutics.
Accumulating evidence also points to the importance of cell surface v-ATPase function in non-malignant processes. In renal tubular epithelium, the v-ATPase is vital for urine acidification. Osteoclasts are enriched with specific v-ATPase isoforms at the ruffled border, which enables an optimal pH environment for proteases to degrade matrix. Endothelial cells adjacent to an induced wound display inducible and prominent plasma membrane v-ATPase localization and activity.10 Endothelial cells obtained from diabetic rat models, however, display significantly reduced cell surface v-ATPase activity and show a functional impairment in migratory behavior.40 These findings indicate that physiological and wound-repair processes likely require plasma membrane v-ATPase activity, possibly due to the effects on MMPs.
In summary, v-ATPase staining of human pancreatic malignancy, ranging from PanIN lesions to PDAC, demonstrated a marked loss of polarity and increased intensity with increasing tumor invasiveness. These changes were seen in PanIN lesions suggesting a role for the v-ATPase in early stages of malignant transformation. Inhibiting v-ATPase function decreased MMP-9 activities, but increased MMP-2 activation in vitro. These results indicate that the v-ATPase plays a complex role in regulating MMPs and the pancreatic cancer phenotype.
Supplementary Material
Acknowledgments
This work was supported by a VA CDA Award, National Pancreas Foundation (CC); NIH CA133346 (AJK); NSF Graduate Research Fellowship (CCM); R01 CA064238 (SEC); RO1 DK54021 (FSG)
ABBREVIATIONS
- MMP
matrix metalloproteinase
- PanIN
pancreatic intraepithelial neoplasms
- PDAC
pancreatic ductal adenocarcinoma
- shRNA
short hairpin RNA
- v-ATPase
vacuolar-ATPase
Footnotes
Supplementary information accompanies the paper on the Laboratory Investigation website (http://www.laboratoryinvestigation.org)
DISCLOSURE/CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–75. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinton A, Bond S, Forgac M. V-ATPase functions in normal and disease processes. Pflugers Arch. 2009;457:589–98. doi: 10.1007/s00424-007-0382-4. [DOI] [PubMed] [Google Scholar]
- 3.Sennoune SR, Martinez-Zaguilan R. Plasmalemmal vacuolar H+-ATPases in angiogenesis, diabetes and cancer. J Bioenerg Biomembr. 2007;39:427–33. doi: 10.1007/s10863-007-9108-8. [DOI] [PubMed] [Google Scholar]
- 4.Nelson N. Structure, molecular genetics, and evolution of vacuolar H+-ATPases. J Bioenerg Biomembr. 1989;21:553–71. doi: 10.1007/BF00808113. [DOI] [PubMed] [Google Scholar]
- 5.Arvan P, Castle JD. Isolated secretion granules from parotid glands of chronically stimulated rats possess an alkaline internal pH and inward-directed H+ pump activity. J Cell Biol. 1986;103:1257–67. doi: 10.1083/jcb.103.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orci L, Ravazzola M, Amherdt M, et al. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J Cell Biol. 1986;103:2273–81. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyenbach KW, Wieczorek H. The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J Exp Biol. 2006;209:577–89. doi: 10.1242/jeb.02014. [DOI] [PubMed] [Google Scholar]
- 8.Wagner CA, Finberg KE, Breton S, et al. Renal vacuolar H+-ATPase. Physiol Rev. 2004;84:1263–314. doi: 10.1152/physrev.00045.2003. [DOI] [PubMed] [Google Scholar]
- 9.Nanda A, Brumell JH, Nordstrom T, et al. Activation of proton pumping in human neutrophils occurs by exocytosis of vesicles bearing vacuolar-type H+-ATPases. J Biol Chem. 1996;271:15963–70. doi: 10.1074/jbc.271.27.15963. [DOI] [PubMed] [Google Scholar]
- 10.Rojas JD, Sennoune SR, Maiti D, et al. Vacuolar-type H+-ATPases at the plasma membrane regulate pH and cell migration in microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2006;291:H1147–57. doi: 10.1152/ajpheart.00166.2006. [DOI] [PubMed] [Google Scholar]
- 11.Sennoune SR, Bakunts K, Martinez GM, et al. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol. 2004;286:C1443–52. doi: 10.1152/ajpcell.00407.2003. [DOI] [PubMed] [Google Scholar]
- 12.Nishi T, Forgac M. The vacuolar (H+)-ATPases--nature’s most versatile proton pumps. Nat Rev Mol Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- 13.Toyomura T, Oka T, Yamaguchi C, et al. Three subunit a isoforms of mouse vacuolar H(+)-ATPase. Preferential expression of the a3 isoform during osteoclast differentiation. J Biol Chem. 2000;275:8760–5. doi: 10.1074/jbc.275.12.8760. [DOI] [PubMed] [Google Scholar]
- 14.Toyomura T, Murata Y, Yamamoto A, et al. From lysosomes to the plasma membrane: localization of vacuolar-type H+-ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem. 2003;278:22023–30. doi: 10.1074/jbc.M302436200. [DOI] [PubMed] [Google Scholar]
- 15.Frattini A, Orchard PJ, Sobacchi C, et al. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet. 2000;25:343–6. doi: 10.1038/77131. [DOI] [PubMed] [Google Scholar]
- 16.Kornak U, Schulz A, Friedrich W, et al. Mutations in the a3 subunit of the vacuolar H(+)-ATPase cause infantile malignant osteopetrosis. Hum Mol Genet. 2000;9:2059–63. doi: 10.1093/hmg/9.13.2059. [DOI] [PubMed] [Google Scholar]
- 17.Li YP, Chen W, Liang Y, et al. Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat Genet. 1999;23:447–51. doi: 10.1038/70563. [DOI] [PubMed] [Google Scholar]
- 18.Frattini A, Blair HC, Sacco MG, et al. Rescue of ATPa3-deficient murine malignant osteopetrosis by hematopoietic stem cell transplantation in utero. Proc Natl Acad Sci U S A. 2005;102:14629–34. doi: 10.1073/pnas.0507637102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinton A, Sennoune SR, Bond S, et al. Function of a subunit isoforms of the V-ATPase in pH homeostasis and in vitro invasion of MDA-MB231 human breast cancer cells. J Biol Chem. 2009;284:16400–8. doi: 10.1074/jbc.M901201200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark ES, Weaver AM. A new role for cortactin in invadopodia: regulation of protease secretion. Eur J Cell Biol. 2008;87:581–90. doi: 10.1016/j.ejcb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark ES, Whigham AS, Yarbrough WG, et al. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–35. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 22.Sautin YY, Lu M, Gaugler A, et al. Phosphatidylinositol 3-kinase-mediated effects of glucose on vacuolar H+-ATPase assembly, translocation, and acidification of intracellular compartments in renal epithelial cells. Mol Cell Biol. 2005;25:575–89. doi: 10.1128/MCB.25.2.575-589.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumner JP, Dow JA, Earley FG, et al. Regulation of plasma membrane V-ATPase activity by dissociation of peripheral subunits. J Biol Chem. 1995;270:5649–53. doi: 10.1074/jbc.270.10.5649. [DOI] [PubMed] [Google Scholar]
- 24.Chung C, Shugrue C, Nagar A, et al. Ethanol exposure depletes hepatic pigment epithelium-derived factor, a novel lipid regulator. Gastroenterology. 2009;136:331–40. e2. doi: 10.1053/j.gastro.2008.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 26.Theret N, Musso O, L’Helgoualc’h A, et al. Activation of matrix metalloproteinase-2 from hepatic stellate cells requires interactions with hepatocytes. Am J Pathol. 1997;150:51–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Wiggins H, Rappoport J. An agarose spot assay for chemotactic invasion. Biotechniques. 48:121–4. doi: 10.2144/000113353. [DOI] [PubMed] [Google Scholar]
- 28.Suemizu H, Monnai M, Ohnishi Y, et al. Identification of a key molecular regulator of liver metastasis in human pancreatic carcinoma using a novel quantitative model of metastasis in NOD/SCID/gammacnull (NOG) mice. Int J Oncol. 2007;31:741–51. [PubMed] [Google Scholar]
- 29.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 30.Clark ES, Brown B, Whigham AS, et al. Aggressiveness of HNSCC tumors depends on expression levels of cortactin, a gene in the 11q13 amplicon. Oncogene. 2009;28:431–44. doi: 10.1038/onc.2008.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maquoi E, Peyrollier K, Noel A, et al. Regulation of membrane-type 1 matrix metalloproteinase activity by vacuolar H+-ATPases. Biochem J. 2003;373:19–24. doi: 10.1042/BJ20030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohta T, Numata M, Yagishita H, et al. Expression of 16 kDa proteolipid of vacuolar-type H(+)-ATPase in human pancreatic cancer. Br J Cancer. 1996;73:1511–7. doi: 10.1038/bjc.1996.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fais S, De Milito A, You H, Qin W. Targeting vacuolar H+-ATPases as a new strategy against cancer. Cancer Res. 2007;67:10627–30. doi: 10.1158/0008-5472.CAN-07-1805. [DOI] [PubMed] [Google Scholar]
- 34.Lu X, Qin W, Li J, et al. The growth and metastasis of human hepatocellular carcinoma xenografts are inhibited by small interfering RNA targeting to the subunit ATP6L of proton pump. Cancer Res. 2005;65:6843–9. doi: 10.1158/0008-5472.CAN-04-3822. [DOI] [PubMed] [Google Scholar]
- 35.Kato Y, Lambert CA, Colige AC, et al. Acidic extracellular pH induces matrix metalloproteinase-9 expression in mouse metastatic melanoma cells through the phospholipase D-mitogen-activated protein kinase signaling. J Biol Chem. 2005;280:10938–44. doi: 10.1074/jbc.M411313200. [DOI] [PubMed] [Google Scholar]
- 36.Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122:3015–24. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- 37.Zucker S, Hymowitz M, Conner CE, et al. Rapid trafficking of membrane type 1-matrix metalloproteinase to the cell surface regulates progelatinase a activation. Lab Invest. 2002;82:1673–84. doi: 10.1097/01.lab.0000041713.74852.2a. [DOI] [PubMed] [Google Scholar]
- 38.Murakami T, Shibuya I, Ise T, et al. Elevated expression of vacuolar proton pump genes and cellular PH in cisplatin resistance. Int J Cancer. 2001;93:869–74. doi: 10.1002/ijc.1418. [DOI] [PubMed] [Google Scholar]
- 39.Torigoe T, Izumi H, Ishiguchi H, et al. Enhanced expression of the human vacuolar H+-ATPase c subunit gene (ATP6L) in response to anticancer agents. J Biol Chem. 2002;277:36534–43. doi: 10.1074/jbc.M202605200. [DOI] [PubMed] [Google Scholar]
- 40.Rojas JD, Sennoune SR, Martinez GM, et al. Plasmalemmal vacuolar H+-ATPase is decreased in microvascular endothelial cells from a diabetic model. J Cell Physiol. 2004;201:190–200. doi: 10.1002/jcp.20059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.