Abstract
The continually increasing rate of myocardial infarction (MI) in the Western world at least partly can be explained by a poor diet lacking in green vegetables, fruits, and fish, and enriched in food that contains saturated fat. In contrast, a number of epidemiological studies provide strong evidence highlighting the cardioprotective benefits of the Mediterranean diet enriched in green vegetables, fruits, fish and grape wine. Regular consumption of these products leads to an accumulation of nitrate/nitrite/NO•, polyunsaturated fatty acids (PUFA), and polyphenolic compounds, such as resveratrol, in the human body. Studies have confirmed that these constituents are bioactive exogenous mediators, which induce strong protection against MI. The aim of this review is to provide a critical, in-depth analysis of the cardioprotective pathways mediated by nitrite/NO•, PUFA, and phenolic compounds of grape wines discovered in the recent years, including cross-talk between different mechanisms and compounds. Overall, these findings may facilitate the design and synthesis of novel therapeutic tools for the treatment of MI.
1. Introduction
Myocardial infarction (MI) remains a major clinical problem in the western world. Acute MI takes about 140,000 lives every year in the USA alone [1]. In general, MI is a consequence of a long ischemic insult, which initiates irreversible intracellular events including shortage of ATP supply, collapse of ionic homeostasis, and massive cardiomyocyte death. Although restoration of the blood flow through the ischemic zone is absolutely required for survival, most of the intracellular damage occurs during reperfusion. Thus, the problems associated with MI are largely attributed to ischemic-reperfusion (IR) injury [2]. IR injury results in impaired contractile function and depression of mitochondrial bioenergetics, as the consequences of imbalanced ionic homeostasis [3] and functional disturbances due to protein/lipid modifications [4].
Ischemic preconditioning (IPC) is an effective strategy to protect the model heart from MI. IPC usually comprises one or more short ischemic insults intercepted with reperfusion prior to a prolonged period of IR [4,5]. While the signaling mechanisms of IPC are still unclear, important roles for a number of associated events have been proposed; this includes, but is not limited to, modifications and translocations of intracellular kinases [6], activation of mitochondrial ATP sensitive potassium (mKATP) channels [6], and mild mitochondrial uncoupling [7]. However, clinical application of IPC is difficult. For this reason, another strategy in the development of MI treatments entails investigation of the mechanisms of IPC and development of pharmacological tools to mimic these signaling pathways. Unfortunately, today not even a single FDA approved drug exists for the lowering of cardiac infarct size [8].
Based on several epidemiological, clinical and experimental studies, it has been established that certain types of diet may have beneficial effects for the cardiovascular (CV) system in general and be effective therapeutic tools for the prevention or treatment of MI [9,10,11,12]. For example, the Mediterranean diet, which typically includes a bolus of green vegetables, fruits, fish and grape wines, is associated with decreased concentrations of inflammatory markers such as C-reactive protein and interleukin-6 in MI survivors [13]. Although great success has been made in the accumulation of solid scientific background underlying these phenomena, the full mechanisms of protection are far from being elucidated. Recent studies show several components of the Mediterranean diet can trigger cardioprotection. This review focuses on a number of these components that have attracted attention over the past few years: (a) nitrite, (b) polyunsaturated fatty acids (PUFA), and (c) wine polyphenols. The interplay between these components is also discussed.
2. Nitrite as a promising therapeutic tool against MI
2.1. Accumulation of nitrate/nitrite in the human body: impact of the Mediterranean diet
Although 80% of the basal plasma nitrite (NO2−) level derives from oxidation of NO• [14], although reduction of nitrate (NO3−) may also contribute to elevation of NO2− [15]. It has been reported that exogenous NO3− intake (10mg/kg in humans) may increase -plasma NO2− concentration up to 4–5 fold in 30 min [16]. Notably, plasma NO2 concentration was decreased by 50% in mice that were placed on a dieting lacking in both NO3−/NO2− [17].
The largest dietary sources of NO3− for the human body include green vegetables such as spinach, lettuce, and collard greens and also radishes, beets [18,19], and meat [20]. Furthermore, NO2− itself can be found in cured meats [21]. Raat et al meta-analyzed a number of studies and based on the approximate amount of consumed vegetables reported average daily NO3− ingestion as 77 mg for US diet and 400 mg for Mediterranean diet [17]. A different study revealed that an exemplary Mediterranean meal contains approximately 325mg/serving of combined NO3−/NO2−, significantly higher in comparison to Western meals (only ~20mg/serving) [22]. These discrepancies indicate that the actual amount of daily NO3−/NO2− intake should be considered with caution because the data may vary depending on study design or targeted population [19,23]. Nevertheless, despite slight variation in the magnitude of the difference, the Mediterranean diet appears to provide greater NO3− supplementation than a regular Western diet.
2.2. NO2− as a stable precursor of NO• in the body
After being accumulated in the saliva, NO3− can be reduced to NO2− by several bacterial organisms including S. epidermidis, Veillonella, Nocardia, and S. aureus [24,25]. Among other functions, NO2− can be converted into NO• gas, which mediates numerous signaling events in the cell [26]. Accordingly, the following sections will be mostly devoted to the cardioprotective effects of NO2−/NO•.
The bio-efficacy of NO2− has been demonstrated in many studies, for example where consumption of nitrate-enriched beetroot resulted in a 2-fold elevation of the plasma NO2− level, which was also correlated with a reduction in blood pressure [27]. Further reduction of NO2− to NO• may occur under acidic conditions both in the oral cavity and in the stomach [28,29,30]. Additionally, several studies have indicated that bioconversion of NO2−-to-NO• is stimulated by polyphenols [29,31,32].
Until recently, NO2− had been considered primarily as a stable precursor of NO•, and thus, investigation of the NO•-dependent activity of NO2− dominated the field. In recent years, several studies have shown that NO2− itself can be recruited by cells and reduced to NO• when the latter is depleted [33,34,35]. Since NOS function requires O2 to make NO•, but the reduction of NO2− to NO• is not O2-dependent, this process may replace regular NOS function under ischemic conditions. This reduction can be mediated by several different proteins including hemo- and myoglobins [33,34], the mitochondrial respiratory chain [36], and xanthine oxidoreductase [37].
Both recent and ongoing studies have revealed significant therapeutic potential regarding the use of NO2−/NO• in the treatment of MI [35,38,39,40,41,42,43]. The mechanisms of cardioprotection by NO2− have been extensively investigated. Its cardioprotective efficacy has been confirmed at subcellular, cellular, organ and organism levels and in different models of IR injury, including isolated cardiac mitochondria [41], isolated cardiomyocytes [40], perfused heart [35] and in vivo left coronary artery occlusion [41,44].
2.3. Cardioprotection via nitrosylation: role of NO2− reduction
It is widely accepted that acute effects of NO2−/NO• may be mediated via post-translational modifications of proteins (for review see [45]). Nitrosylation is the reaction of NO• with metal centers such as heme. Classic NO•-dependent vasorelaxation is mediated via nitrosylation of soluble guanylate cyclase (sGC), causing subsequent regulation of vascular tone and blood flow [46]. Consistent with a role for sGC signaling in cardioprotection, pharmacological activation of sGC elicits protection against IR injury [47,48,49]. However, it was reported that NO2− caused hypoxic vasodilation via both sGC-dependent and independent pathways [50]. On the organ level, NO2−-dependent vasorelaxation may play a role in hypoxic blood flow regulation [51]. Mechanisms of NO2− reduction to NO• in the vessels have not been fully elucidated, but several studies demonstrated that this might include deoxygenated hemoglobin [52]. Importantly, Gladwin’s group found that deoxymyoglobin was a far more efficient NO2− reductase in comparison to deoxyhemoglobin, which indicated that myocardium contains a powerful NO2− reductase system [34]. The ability of NO2− to prevent myocardial infarction was completely abolished in myoglobin−/− mice [40]. It is odd, however, that other NO2− reductase systems, such as xanthine oxidoreductase, did not partake in the reduction of NO2− in this case, as even partial myoglobin-independent cardioprotection was not observed [40]. Nevertheless, this signifies that the existence of a deoxymyoglobin/NO2− reductase system may be exceptionally important for the cardioprotection afforded by NO2−.
While the role of myoglobin as an oxygen transporter in cardiomyocytes is widely acknowledged [53,54], novel evidence has uncovered deoxymyoglobin’s important role in the regulation of mitochondrial function, in which it facilitates NO2− reduction to NO• [34].
2.4. NO• signaling in mitochondria and cardioprotection
It is well-established that direct interaction of NO• with the electron-transport chain causes reversible inhibition of mitochondrial respiration [55]. Primarily, this effect may be associated with nitrosylation of complex IV or S-nitrosation of complex I (a reversible NO•-dependent modification of thiols) [56]. The mechanisms and consequences of NO• interaction with mitochondria have been extensively studied [45], especially in the context of the complex roles of both NO• and mitochondria in cardiac pathophysiology.
As a note, the mitochondrial bioenergetic machinery is a primary target for damage during IR injury [4]. Mitochondrial damage due to reactive oxygen species (ROS) generation and Ca2+ overload and its consequences, particularly the opening of the permeability transition (PT) pore, are key features of IR injury [57]. Effects of the PT pore opening include massive mitochondrial swelling, inner membrane disruption and loss of mitochondrial function [58]. In addition, IR results in irreversible damage of the mitochondrial respiratory chain primarily because of multiple structural modifications of proteins and lipids via oxidation [59], carbonylation [60] or alkylation [61].
It is widely accepted now that NO2−/NO• may preserve mitochondrial function during IR injury, eliciting strong cardioprotective effects [42]. In general, reversible inhibition of mitochondrial respiration has been considered as a potential therapeutic strategy for treatment of MI [55], and has been successfully tested in varied models of IR injury [55,62,63,64]. Reversible inhibition facilitates the slow re-introduction of electron flow through the respiratory chain at the beginning of reperfusion, which substantially prevents the burst of ROS and delays full restoration of the membrane potential, a driving force for Ca2+ uptake [55]. Hence, ROS generation and Ca2+ overload, the key factors in cardiac IR injury, are avoided [55].
A substantial amount of experimental evidence has revealed that reversible S-nitrosation of complex I may inhibit ROS generation and Ca2+ overload by mitochondria during early reperfusion, which is critical for cardioprotection [41,42,62,63,65,66]. We demonstrated that low-molecular weight S-nitrosothiols (e.g. S-nitroso-mercaptopropionyl-glycine) protected isolated cardiomyocytes and perfused rat hearts against IR injury and that this effect was accompanied by S-nitrosation and inhibition of complex I [62,63]. These results were recapitulated in an in-vivo mouse model of MI [63].
There is consensus that the mitochondrion is a central focus for IPC signaling, and the most commonly recognized mitochondrial signaling mechanisms in IPC are mediated via NO• [26]. We reported that endogenous S-nitrosation of mitochondrial proteins was detected during IPC [62,66]; furthermore Sun et al. found that a number of mitochondrial proteins were S-nitrosated during IPC, including the 75kDa subunit of complex I [62,66]. Reversible inhibition of mitochondrial respiration due to S-nitrosation of complex I may serve as a protective endogenouse mechamism during IPC [55,56,62].
Perhaps the best evidence for the mitochondrion as a terminal site for the cardioprotective effects of NO, is that a mitochondrially-targeted NO donor (which accumulates inside mitochondria at several-hundred-fold greater concentration relative to the cytosol), was cardioprotective at a concentration of only 100 nM in isolated perfused hearts, and at 100 ng/kg in vivo [65,67].
Further establishing a connection between NO2−, S-nitrosation and cardioprotection, it should be noted that administration of exogenous NO2− caused S-nitrosation and inhibition of complex I, and resulted in protection against MI [41]. Reversible inhibition of complex I has also been claimed to be a mechanism of NO2−-induced ardioprotection in a model of cardiac arrest [39]. It is important to note that intravenous injection of NO2− during the last 5 minutes of ischemia significantly reduced MI injury [68], which makes NO2− therapy clinically relevant. In this study S-nitrosothiols and iron-nitrosyl-protein complexes were not elevated in the whole blood or plasma after NO2− administration. This led the authors to conclude that NO2− itself likely accounted for the cardioprotection observed; however, the question remains whether S-nitrosation occurred in the myocardial tissue where protection actually took place [68].
Additionally, Hogg’s group found that NO2− may mediate protection against IR injury via mitochondrial ATP sensitive potassium (mKATP) channels [69]. Although the mechanism of NO2− interaction with mKATP channels was not revealed, several other groups have demonstrated that mKATP channels can be activated by S-nitrosothiols [70,71,72].
2.5. NO•-independent mechanisms of cardioprotection
Although NO•-independent mechanisms of cardioprotection by NO2− are less investigated, several important effects have recently been discovered. The discovery of nitrated lipids (or nitroalkenes) is one of the most novel and exciting findings in the field of reactive lipid research [73]. Nitrated lipids can be formed by several different mechanisms, such as through a reaction of NO2− with unsaturated fatty acid derivatives at low pH (≤4) [74]. Nitroalkenes are a novel class of cell-signaling molecules that possess potent anti-inflammatory properties via inhibition of both NF-kB activity and cytokine secretion [75]. As potent peroxisome proliferator-activated receptor-γ (PPARγ) ligands, nitroalkenes regulate gene expression and cell metabolism [76]. Due to their electrophilic nature, nitroalkenes may cause post-translational modifications of proteins that contain nucleophilic residues (Cys, Lys, His) [77]. Although the full therapeutic potential of nitroalkenes has not been fully elucidated thus far, strong evidence has emerged that low concentrations of nitrated linoleic acid elicit potent acute protection in isolated cardiomyocytes as a model of IR injury [78]. Further, Freeman’s group demonstrated that nitrated oleic acid induces significant protection against MI in the in-vivo IR injury model of left anterior descending (LAD) coronary artery occlusion [79]. Notably, we have found that different nitroalkenes possess varying efficacies in the reduction of infarct size (unpublished data). Although the mechanism underlying this has not yet been evaluated, it might be due to the inherent stability of nitroalkenes [76] or to the properties of their parent fatty acids, which are discussed below. Markedly, the Mediteranian diet rich in NO2− and PUFA, and supplemented with acidic vinegar, may favor intra-gastric generation of nitrated lipids. Indeed, d’Ischia’s group has shown nitration of unsaturated fatty acids from extra virgin olive oil under exposure to NO2− in mild acidic conditions [80].
In summary, in the past several years a number of fundamental studies have revealed NO2− as a bioactive molecule that may possess the capacity to diminish or even prevent the detrimental consequences of MI in diverse animal models. Moreover, promising preclinical results anticipate forthcoming clinical trials (www.clinicaltrials.gov/ct2/show/NCT00924118) of nitrite therapy for patients with anterior ST-segment elevation MI [81].
3. PUFA and their derivatives against MI
3.1. Cardioprotective efficacies of PUFA
Mediterranean diet traditionally includes an abundance of vegetables and fish, both of which contain a substantial amount of diverse PUFA (ω-3, 6, 9). Epidemiological studies demonstrate that there is a direct correlation between PUFA consumption (especiallyω-3) and low levels of CV diseases in some populations [82,83]. The scientific nomenclature of PUFA is well described (see for review [84]), and will not be discussed in detail herein. Briefly, PUFA are divided into 3 classes based on the position of the first double bond from the methyl carbon, labeled “ω”: (1) ω-3, i.e. DHA-docosahexaenoic, EPA-eicosapentaenoic, ALA-α-linolenic; (2) ω-6, i.e. LA-linoleic, GLA-γ-linolenic, AA-arachidonic; and (3) ω-9, i.e. OA-oleic. Several large studies [9,11] have drawn a correlation between regular PUFA consumption and reduced risk of CV diseases. Extending this knowledge, comparative studies have revealed that mixed ω-3s have a greater cardioprotective effect than any other PUFA [85]. It was shown that ω-3 [86,87,88], and to a lesser extent ω-6 [89,90], PUFA protected the heart from MI, while ω-9 and saturated FA have minor or no effect [85,91]. Although a recent study demonstrated that ALA offers significant protection against acute MI in humans [92], further investigations clarified that EPA and DHA take the most responsibility for ω-3-mediated cardioprotection [93]. Moreover, it has been discussed that the EPA-to-DHA ratio is a significant factor in determining the degree of protection (for review see [94]). Thus, the cardioprotective efficacies of PUFA may be determined by at least two factors: (i) structural specificities, i.e. position of the first double bond, and (ii) the ratio of acids within one individual “ω” group.
Until recently, much of the scientific research and even clinical trials were conducted without a clear understanding of the molecular mechanisms of cardioprotection afforded by ω-3. Early studies suggested that this cardioprotection was mediated via replacement of ω-6 with ω-3. Thereby an increased ratio of ω-3/ω-6 in cell membranes diminished the detrimental effects of pro-inflammatory AA-derived eicosanoids, prostaglandins and leukotrienes [95,96,97].
3.2. Cardioprotection by EPA and DHA derivatives
Extensive studies have revealed that the protective effects of EPA and DHA may be mediated through the formation of reactive lipid molecules called resolvins [98]. Biosynthesis of these molecules is a multi-step process and involves participation of several enzymes, such as acetylated cyclooxygenase-2, cytochrome P450, and lypoxygenases [98,99,100,101]. A more detailed description of the synthetic process, nomenclature and generic properties of resolvins is beyond the scope of this review and can be found elsewhere [101,102,103,104]. Due to their potent anti-inflammatory properties [105,106,107,108] these molecules may serve as promising therapeutic tools for MI treatment. Indeed, resolvins (E1, D1) prevent polymorphonuclear neutrophil (PMN) activation and translocation into the tissue [99,101], which may reduce ROS production and inflammation during reperfusion. Resolvin E1 regulates cytokine/chemokine production [108] and inhibits TNFα-induced nuclear translocation of NF-kB [109]. One can predict that based on their anti-inflammatory properties, resolvins might be particularly effective in the prevention of secondary inflammation initiated by PMN infiltration into ischemic tissue. Consequently, an ideal model to test these molecules would be an in vivo model of IR injury. Indeed, Keyes et al highlighted that administering resolvin E1 robustly decreased infarct size in the model of left coronary artery occlusion in rats [110]. Notably, in this study resolvin E1 was able to prevent IR injury when added 2 min before reperfusion at a concentration of 100nM, suggesting that this protocol might be clinically relevant [110]. Future studies will uncover the ability of other resolvins to prevent MI. In addition to resolvins, there are two other major classes of anti-inflammatory DHA-derivatives called protectins [111,112] and maresins [113]. Protectins are principally associated with anti-apoptotic effects in the brain and neuro-protection [114], while maresins’ function remains to be elucidated.
Recently, Freeman et al. characterized several electrophilic oxo-derivatives of DHA and EPA, which were synthesized in activated macrophages via the cyclooxygenase-2 dependent pathway. Similar to resolvins, these also were found to possess strong anti-inflammatory properties [115]. Furthermore, at physiological intracellular concentrations (28 –190nM), it has been shown that these molecules are potent PPARγ activators [115]. Therefore, it would be reasonable to propose that these electrophiles may elicit protection against MI by a means comparable to those of nitroalkenes and other PPAR ligands [76,116,117]. Nevertheless, these results should be tempered with recent findings that commercial PPARγ ligands (thiazolidinediones) are associated with increased adverse cardiac events in humans (discussed below).
3.3. Cardioprotection by ω-6 derivatives
Although investigation of the cardioprotective events of PUFAs has been considerably shifted toward ω-3, it should be noted that several ω-6 AA derivatives, including 12(S)-hydroperoxyeicosatetraenoic acid [118], epoxyeicosatrienoic acids [119,120] and 15-deoxy-Δ12,14-prostaglandin J2 [116,117], have already been shown to induce protection against MI. It was reported that one of the mechanisms against MI afforded by 12(S)-hydroperoxyeicosatetraenoic acid may be through the activation of transient receptor potential (TRP) channels (e.g. vanilloid receptor 1) [118]. These channels can be activated by electrophiles through covalent modification of critical Cys residues [121], and may be responsible for activation of cardiac nociceptors in response to myocardial ischemia [122]. Notably, capsaicin (an endogenous ligand of these receptors) is also cardioprotective against IR injury [123].
The significance of epoxyeicosatrienoic acids for cardioprotection was demonstrated in hearts overexpressing human cytochrome P450 AA epoxygenase CYP2J2 [124]. Transgenic mice elicited greater endogenous protection against IR injury compared with wild-type hearts [124]. Cardioprotection provided by epoxyeicosatrienoic acids may be mediated via activation of mKATP channels or p42/p44 mitogen-activated protein kinase (MAPK) [119,124]. Furthermore, since epoxyeicosatrienoic acid administration significantly prevented MI in dogs when administered at the time of reperfusion [120], these molecules may be effective, clinically-relevant therapeutic tools.
15-deoxy-Δ12,14-prostaglandin J2 provides cardioprotection by several independent mechanisms. For example, the cardioprotective effects of this molecule can be mediated by activation of heme-oxygenase-1, regulation of heat shock protein 70, or reduction of the expression of adhesion molecules ICAM-1 and P-selectin [116,117]. In addition, similar to nitroalkenes and oxo-derivatives of ω-3, 15-deoxy-Δ12,14-prostaglandin J2 is a potent activator of PPARγ [125], and thereby possesses metabolic and anti-inflammatory properties which might contribute to protection against MI [76,116,117].
Arachidonic acid is a precursor for another class of anti-inflammatory molecules called lipoxins [126]. Biosynthesis of these molecules is achieved by 15-lypoxygenase at the moment of inflammation, with ensuing regulation of PMN infiltration into the tissue [127]. Gavins et al. demonstrated a potential role of lipoxin A4 receptors in cardioprotection against MI in mice [128]. Prior to this, the protective role of lipoxin A4 had already been reported in cerebral IR injury [129], IR-induced gastric mucosal damage [130], and kidney IR [131]. Anti-inflammatory properties of lipoxin A4 may be mediated via PPARγ activation [132] suggesting that this mechanism may be common for many ω-3 and ω-6 lipid derivatives.
3.4. Potential pitfalls and new directions with regard to the clinical application of PUFA and their derivatives
Upon translating these findings into humans, several important limitations should be mentioned regarding PPARγ activators and reactive lipids. Despite their cardioprotective efficacy, some PPARγ ligands, including rosiglitazone, may increase the risk of acute MI, stroke, heart failure and mortality in elder patients [133]. Regarding reactive lipids, it should be noted that the desired cardioprotection afforded by them is strictly dose-dependent. For example, while high concentrations (>20μM) of α,β-unsaturated aldehyde 4-hydroxy-2-nonenal caused cardiomyocyte death, low dosage initiated Nrf2-Keap1 (nuclear factor erythroid-2 related factor 2 - Kelch-like ECH - associated protein 1)-mediated stimulation of antioxidant machinery and subsequent cardioprotection against IR injury [134]. Similar to this, high dosages (>20μM) of nitrated linoleic acid induced mitochondrial swelling and PT pore formation, however low concentrations (<1μM) elicited strong cardioprotection [78].
In addition, a major limiting factor which may determine the clinical applicability of molecules such as nitroalkenes, lipoxins, resolvins, and other electrophilic species, is their stability and chemical reactivity. Virtually most of these compounds are light sensitive and decompose rapidly in aqueous media. Thus, their “druggability” will depend on successful formulation for human clinical administration (e.g. lipid emulsions, liposomal ecapsulation, or stabilization in a suitable vehicle).
Overall, intensive studies of PUFA derivatives in recent years have revealed several classes of reactive lipid molecules possessing potent anti-inflammatory cardioprotective properties. Moreover, these findings have made possible a reconsideration of some existing postulates and the drawing of future perspectives for the field. For example, while regular OA (ω-9) and LA (ω-6) were not protective, the corresponding nitrated PUFA robustly prevented MI injury in mice [79] and IR injury in cardiomyocytes [78]. Likewise, Trostchansky et al. were able to synthesize several isomers of nitrated arachidonic acid and showed they possessed strong anti-inflammatory properties that have potential cardioprotective effects [135]. These studies demonstrate that nitroalkenes are a superb example of how structural manipulations of certain PUFA may dramatically enhance or create de-novo cardioprotective properties. Furthermore, it is possible that these structural and functional metamorphoses may be facilitated as a part of the Mediterranean diet, in which cells procure an additional supply of PUFAs and nitrite.
4. Cardioprotection by phenolic components of red and white wines: cross-talk with Sirtuins, caloric restriction and IPC
About 500 years ago Alvise Cornaro, who died at age 102, explained his longevity and good health in the book “THE ART OF LIVING LONG”. Based on his experiences, regular wine consumption and caloric restriction (CR) were two critical components of a long, healthy life.
Regular consumption of grape wine is an integral element of the Mediterranean diet. Epidemiological studies have shown the beneficial effects of moderate consumption of wine on the CV system [10,12]. Although the cardioprotective effects of red and white wines against IR injury have been known for some time and have been well described [136,137,138], it has not been until recently that the actual protection mechanisms have been extensively studied.
The cardioprotective benefits of grape wine are usually attributed to their phenolic components. In mammalian hearts, red wine polyphenols have been shown to elicit strong cardioprotective effects against IR injury [139,140,141,142]. Polyphenolic compounds such as quercetin, resveratrol, or catechins are potent antioxidants [141,142,143,144]; thus, one of the mechanism of protection they provide might be the inhibition of oxidative stress upon reperfusion. Brookes et al. demonstrated that quercetin-mediated protection against IR injury was associated with activation of manganese superoxide dismutase (MnSOD) and preservation of mitochondrial function [141]. The most studied polyphenol, resveratrol, may trigger a broad spectrum of cardioprotective pathways, including inhibition of glycogen synthase kinase-3β (GSK-3β) [145], activation of mKATP and large conductance Ca2+ -activated K+ channel (BKCa) channels [143], induction of autophagy via the mammalian target of rapamycin (mTOR) pathway [146], activation of transcription factor Nrf2 [147], expression of heme oxygenase 1 [148], and upregulation of eNOS [149]. In addition, polyphenols stimulate in vivo NO2− reduction to NO• [31,32], providing cardioprotection via NO•-mediated pathways. Interestingly, resveratrol is synthesized by plants in response to fungal infection [150], and so the cardioprotective efficacy of red wines may vary based upon external influences on the grape berries during vegetation.
Extensive studies of the phenolic components of white wine (n-tyrosol and hydroxytyrosol) revealed that they also possessed strong protection against IR injury [151,152]. Although cardioprotective mechanisms of white wine constituents are less studied in comparison to red wine polyphenols, several pathways have been revealed. Thus, in addition to being potent antioxidants [153], n-tyrosol and hydroxytyrosol activate eNOS [151], replicating one of the cardioprotective pathways described above for resveratrol.
Further elucidating the cardioprotective mechanisms of phenolic compounds derived from grape wine, herein we draw attention to the possible role of the sirtuin family of proteins. Sirtuins (SIRTs 1–7) are a family of mammalian NAD+-dependent lysine deacetylases, which are orthologs of yeast silent information regulator (Sir2P) histone deacetylase. In model organisms, SIRTs may replicate some signaling events triggered by CR [154] and can be activated by several components of red and white wine [137,154,155]. Although direct implication of SIRTs in the treatment of MI have not been fully elucidated, recent studies revealed that SIRTs activation may be a promising strategy for the treatment of several CV diseases, including hypertrophy [156] and chronic heart failure [157].
SIRTs have attracted much attention since the discovery that SIRT1 may be activated by resveratrol [155]. Sinclair and co-authors found that resveratrol stimulated Sir2 (an analog of human SIRT1) in S. cerevisiae and extended their lifespan [155]. In mammals, regulation of SIRT1 function by resveratrol was confirmed in many tissues [158], including myocardium [137]. Several other polyphenols were shown to stimulate SIRT1 activity, although to a lesser extent than resveratrol [155].
Having established the cardioprotective efficacy of n-tyrosol and hydroxytyrosol against IR injury, Das and colleagues found that these two constituents of white wine also activated SIRT1 in rat myocardium [137,152]. Furthermore, white wine was found to be an even more potent activator of SIRT1 than the resveratrol in red wine, although resveratrol showed greater cardioprotective efficacy [137]. These results indicated that the magnitude of cardioprotection provided by white wine or resveratrol does not exactly correlate with the degree of SIRT1 activity [137]. Therefore activation of SIRT1 may be necessary, but alone is not sufficient in providing the observed cardioprotection against IR injury. Nevertheless, these observations enable the conclusion to be drawn that the cardioprotection rendered by the components of grape wine is principally accompanied by myocardial SIRT1 activation.
In light of these findings, a number of important limitations should be mentioned. Foremost, since SIRT1 is the best-characterized of the sirtuin family proteins, most studies are focused on its activation, while information about the other 6 members of this family is very limited. Even so, activation of SIRTs 3, 4, and 7 has been demonstrated in rat cardiac H9C2 cells after exposure to resveratrol [159]. Second, phenolic compounds mediate multiple cardioprotective pathways (see above). Consequently, SIRT1 is not the only target [160], and thus at least a portion of the cardioprotective effect afforded by these compounds may, in reality, be SIRT1-independent. Because of this, significant efforts have been recently made toward the synthesis and testing of resveratrol analogs, with the hope that they might be more selective activators of SIRT1. Already, several novel compounds have been generated [154,161]. It was demonstrated that the synthetic SIRT1 activator SRT1720 was 1000 times more potent than resveratrol and could suppress aged-related disturbances in model animals [162]. However, these results concerning the ability of SIRT1720 to directly activate SIRT1 were recently questioned because of methodological artifacts [163]. Most of these inconsistencies between different laboratories are due to the lack of precise methods to measure SIRT activity. In the future, both the synthesis of specific activators and development of reliable methods for determination of SIRT1 activity will clarify the role of these proteins in cardiac pathologies, including MI.
In addition to exogenous activators, SIRTs can be upregulated and activated by several endogenous mechanisms initiated by CR [164], IPC [165,166] and mild stress [157,165]. One study found that CR upregulated and activated SIRT1 in various types of tissues, including myocardium [167]. Concurrently, CR reduced infarct size, improved left ventricular function and attenuated inflammatory response after cardiac ischemic insult [168,169,170,171]. Moreover, it is well-documented that CR has the ability to prevent aged-related intolerance to cardiac ischemia in model animals [167,169, 172,173]. It was demonstrated that phosphorylated AMP-activated protein kinase and elevated serum level of adiponectin were critical for CR-mediated cardioprotection [170,171]. Intriguingly, AMP-activated protein kinase regulates SIRT1 activity via modulation of NAD+ level, and thus observed protection may be regulated via SIRT1-dependent mechanism [174]. Investigating this apparent connection between CR and SIRT1, Bolli’s group has reported that 6 months of CR caused upregulation of nuclear SIRT1 and improved cardiac recovery after myocardial ischemia in middle-aged rats [167].
It has been shown that cardioprotection afforded by IPC may be associated with upregulation or activation of SIRT1 [165,175]. Recently, a role for SIRT1 in IPC was demonstrated in the brain [176]. Although direct involvement of SIRTs in cardiac IPC has not been completely proven, SIRT1 protein expression was found to be increased in porcine myocardium after IPC [165]. In isolated perfused and in vivo mouse hearts, we demonstrated that IPC stimulated SIRT1 activity and caused deacetylation of cytosolic proteins without increasing the level of SIRT1 protein [166]. Furthermore, SIRT1 inhibition blocked the cardioprotection afforded by IPC [166]. Alcendor et al. demonstrated that cardiac-specific over-expression of SIRT1 elicited resistance to oxidative stress, and that SIRT1 was upregulated in response to mild oxidative stress [157]. Therefore it seems reasonable to propose that SIRT1 might be activated in response to IPC mediated non-lethal oxidative stress. These findings support the idea that SIRT1 may regulate endogenous protective mechanisms against myocardial stress and may be involved in IPC signaling.
Intriguingly, it has been demonstrated that aging hearts fail to undergo IPC cardioprotection [177,178,179]. The mechanism of this resistance is yet to be elucidated, but it may include chronic oxidative stress, DNA mutations, or loss of protein function. Fortunately, CR preserves the ability of aging heart to be preconditioned [172,180,181]. Having established that CR stimulates SIRT1 activity, it is possible that CR can reverse the loss of IPC efficacy with aging via mechanisms involving SIRT1. Future studies should clarify if these events are mechanistically related.
5. Downstream mechanisms for SIRTs mediated cardioprotection
Because SIRT1 has been shown to be activated by phenolic compounds [137, 154,167], the downstream signaling cascades with account for the effects of this protein in cardioprotection are of great interest. SIRT1 deacetylates and activates eNOS, thereby increasing NO• production [182] which is known to be protective in several CV diseases, including MI [26]. Furthermore, SIRT1 can be upregulated and activated by NO• [183], forming a positive feed-back loop which enhances NO• availability. Thus, consumption of green vegetables enriched with nitrite may indirectly cause SIRT1 upregulation.
SIRT1 mediated deacetylation can inhibit several potentially detrimental mechanisms associated with MI. One of these mechanisms of SIRT1 protection may be inhibition of poly (ADP) ribose polymerase-1 (PARP1) activity, and subsequent prevention of PARP-dependent cell death [184]. This inhibition may occur either directly via deacetylation and deactivation of PARP1 [185], or indirectly via concurrent binding to the common substrate NAD+ [186]. In addition, autophagy, an essential process in self-cleaning and cardioprotection [187], is regulated by SIRT1 via deacetylation of Atg-5, 7 and 8 [188]. Indeed, SIRT1−/− embryonic heart tissue accumulates damaged mitochondria, indicating failure of autophagy [188]. Stimulation of autophagy has been shown to enhance anti-apoptotic mechanisms in cardiomyocytes after acute MI [189].
Furthermore, SIRT1 is known to regulate the activity of several important transcription factors and coactivators that are essential for the protection of numerous types of CV disturbances. For example, SIRT1 is able to induce the deacetylation and activation of Forkhead box O (FOXO1, 3 &4) [190,191], which regulates the expression of antioxidant enzymes and induces cardiac resistance to oxidative stress [192]. SIRT1 also deacetylates and stimulates Hif-2α [193], which is known to regulate cardiomyocyte resistance to ischemia [194] and PGC-1α [195], which promotes mitochondrial biogenesis and may be beneficial for recovery after MI [196]. Furthermore, SIRT1 inhibits the transcriptional activity of NF-kB via deacetylation of subunit RelA/p65 [197], which may prevent inflammatory responses during MI [198].
Although the cardiac expression and activation of SIRTs 2 to 7 by CR and exogenous nutrients have not been fully elucidated, recent studies have shown that several SIRTs are critical for heart development and normal cardiac function [199,200,201]. Among them SIRT3 is one of the best-characterized proteins, after SIRT1, and is the major mitochondrial class III deacetylase [202]. SIRT3 may be exceptionally important for cardiac tissue because one important downstream target of SIRT3 signaling is the regulation of mitochondrial metabolism [203]. For instance, SIRT3 deacetylates and activates isocitrate dehydrogenase 2 [204], increasing the oxidative decarboxylation of isocitrate and elevating the NADPH level, which may keep antioxidant enzymes in reduced states and provides efficient machinery for ROS scavenging upon reperfusion [205]. Furthermore, SIRT3 regulates OX-Phos by interacting with the 39kDa subunit of complex I (NADH dehydrogenase (ubiquinone) 1 alpha subcomplex 9) and modulating complex I activity through deacetylation [200]. Importantly, complex I activity in SRT3−/− mice is endogenously inhibited, and we found that intact, functional complex I is required for the cardioprotection afforded by IPC [63].
A number of seminal studies have demonstrated the direct involvement of SIRT3 in several anti-apoptotic mechanistic pathways. It has been shown that SIRT3 may elicit anti-apoptotic functions by deacetylating Ku70 and promoting its interaction with BAX [206]. This prevents BAX translocation into mitochondria and consequent cell death [207]. As mentioned above (section 2.4), another mechanism that mediates cell death during myocardial reperfusion is activation of the mitochondrial PT pore [58]. Cyclophilin D is a structural component of the PT pore, and deleting this enzyme or preventing it from binding to ANT significantly reduces IR injury [208,209]. Shulga et al. demonstrated that SIRT3 deacetylated cyclophilin D and prevented its interaction with ANT in HeLa cells [210].
Overall, despite the fact that SIRTs have not been shown to function directly in cardioprotection against IR injury it is likely that SIRTs may orchestrate the regulation of multiple signaling mechanisms responsible for downstream protective events. Therefore, the role of SIRTs in Mediterranean diet-mediated cardioprotection remains to be more fully elucidated.
5. Concluding remarks: possible interplay between NO2−/NO•, PUFAs and polyphenols
The amount of food and the type of food we consume are two important issues that have a great impact on normal CV function. They may also determine the level of risk for CV pathology, including MI. Large portions of green fruits, vegetables, fish and grape wine enrich the Mediterranean diet with nitrite, ω-3/ω-6 PUFAs, and polyphenols. This review describes the potential cardioprotective roles of these substances against MI, as well as a discussion of protective mechanisms. As highlighted in multiple parts of this review, it is exceptionally worthwhile to propose that some sort of cross linking interactions exist between NO2−/NO•, PUFAs and polyphenols. In fact, having established that grape wine stimulates SIRTs activity, several research projects involving human volunteers revealed that wine consumption may also increase the level of PUFAs. For example, a study of a cohort of 1604 volunteers revealed that moderate wine consumption was associated with increased blood concentration of ω-3 PUFAs (EPA and DHA) [10]. Additionally, in concurrence with this study, red wine supplementation improved Mediterranean diet by increasing PUFAs and balancing ω-3/ω-6 ratio [12]. Wine-dependent reduction of NO2− to NO• both in the stomach and the oral cavity [29,31] was demonstrated as well.
Further supporting a possible interplay between NO2− and PUFAs, it should be noted that PUFAs enhanced endothelial NO• generation in human endothelial cells [211]. As was mentioned above (section 2), the interaction of NO2−/NO• and PUFAs yields the formation of nitroalkenes [76,212]. Besides being potent cardioprotective mediators, nitroalkenes may also stimulate NO• production via expression of eNOS [213], forming a positive feed back loop. Further including SIRT1 in this chain of events, NO• stimulates SIRT1 activity [182] and vice versa, SIRT1 stimulates NO• production [183]. Therefore, aside from its exogenous supplementation, NO2−/NO• may also be produced endogenously, recruiting PUFAs- and SIRT1-dependent pathways (see figure 1).
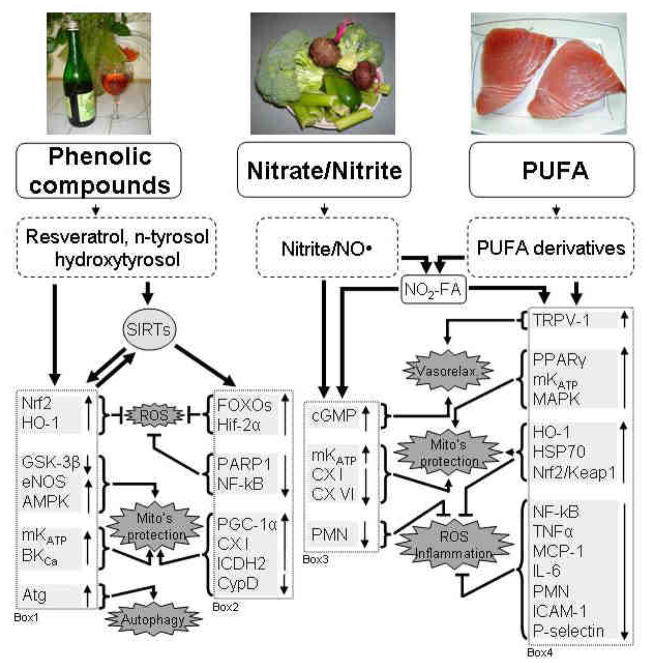
Figure 1. Mechanisms of cardioprotection provided by the components of the Mediterranean diet.
Phenolic compounds, nitrite/NO•, and PUFA are originated from the components of the Mediterranean diet. Mechanisms triggered by polyphenols and shown in box 1 & 2 can be mediated via SIRTs dependent (box 2) and independent (box 1) pathways (see section 3). The backward arrow from box 1 indicates that SIRTs can be activated by some of these events (for example cross talk between SIRTs and NO• [182,183]; AMPK [174] and autophagy [188]). Nitrite/NO•· are bioactive molecules, which provide cardioprotection via mechanisms described in section 2 and shown in box 3. PUFAs’ cardioprotective pathway involves biosynthesis of several classes of anti-inflammatory lipid derivatives (see section 3). Interaction of Nitrite/NO• with PUFA or its derivatives generates nitroalkenes, cell-signaling molecules, that possess strong protection against MI. Box 4 shows the mechanisms that mediate PUFAs’ derivatives’ cardioprotection. Nitroalkenes possess some NO•-dependent and independent properties (from box 3 & 4; see explanations in the text).
Abbreviations: PUFA – polyunsaturated fatty acids; NO• – nitric oxide; NO2-FA – nitroalkenes; ROS – reactive oxygen species; Nrf2 - Nuclear factor erythroid 2-related factor 2;HO-1 – heme oxygenase 1; GSK-3β - glycogen synthase kinase-3beta; eNOS - endothelial nitric oxide synthase; AMPK - AMP-activated protein kinase; mKATP - mitochondrial ATP-sensitive K+ channel; BKCa - large conductance Ca2+ -activated K+ channel; Atg – autophagy proteins; FOXOs - forkhead box O transcription factors; Hif-2α - hypoxia-inducible factor 2-alpha; PARP1 - Poly (ADP-ribose) polymerase 1; NF-kB - nuclear factor-kappa B; PGC-1α - peroxisome proliferator-activated receptor-γ coactivator 1alpha; CX I – complex I; ICDH2 - isocitrate dehydrogenase 2; CypD – cyclophilin D; cGMP - cyclic guanosine monophosphate; CX VI – complex IV; PMN - polymorphonuclear neutrophil; TRPV-1 - transient receptor potential cation channel, subfamily V, member 1; PPARγ - Peroxisome proliferator-activated receptor γ; MAPK - mitogen-activated protein kinase; HSP70 – heat shock protein 70; Nrf2/Keap1 - nuclear factor erythroid-2 related factor 2 - Kelch-like ECH - associated protein 1; TNFα - Tumor necrosis factor-alpha; MCP-1 - monocyte chemoattractant protein-1; IL-6 - interleukin-6; ICAM-1 - Inter-Cellular Adhesion Molecule 1.
In conclusion, the cardioprotective effects of the Mediterranean diet may arise through a variety of both distinct and convergent mechanisms summarized in figure 1. Interestingly, several of the events described above for the Mediterranean diet and illustrated in Figure 1 may also be implicated in the endogenous protection afforded by IPC. Indeed, IPC may be triggered via NO2−/NO• generation [214], PUFAs elevation [215], endogenous synthesis of electrophilic lipid derivatives [78,79], or SIRTs activation [165,175]. Taken as a whole, although IPC itself is impractical to administer in humans, through the Mediterranean diet it may be possible to obtain similar benefits and build up strong endogenous protection in an effort to prevent MI.
Acknowledgments
We thank Paul S. Brookes, Keith W. Nehrke, Andrew P. Wojtovich and William R. Urciuoli (Rochester) for stimulating discussions. This work was funded by a grant from National Institutes of Health to PSB (RO1-HL-071158).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De SG, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106:360–368. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Opie LH. Role of calcium and other ions in reperfusion injury. Cardiovasc Drugs Ther. 1991;5:237–247. doi: 10.1007/BF00054746. [DOI] [PubMed] [Google Scholar]
- 4.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 6.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–188. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 7.Nadtochiy SM, Tompkins AJ, Brookes PS. Different mechanisms of mitochondrial proton leak in ischaemia/reperfusion injury and preconditioning: implications for pathology and cardioprotection. Biochem J. 2006;395:611–618. doi: 10.1042/BJ20051927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res. 2004;95:125–134. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 9.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 10.Di GR, De LM, Salen P, Laporte F, Di CA, Krogh V, et al. Alcohol consumption and n-3 polyunsaturated fatty acids in healthy men and women from 3 European populations. Am J Clin Nutr. 2009;89:354–362. doi: 10.3945/ajcn.2008.26661. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 12.Urquiaga I, Guasch V, Marshall G, San MA, Castillo O, Rozowski J, et al. Effect of Mediterranean and Occidental diets, and red wine, on plasma fatty acids in humans. An intervention study. Biol Res. 2004;37:253–261. doi: 10.4067/s0716-97602004000200012. [DOI] [PubMed] [Google Scholar]
- 13.Panagiotakos DB, Dimakopoulou K, Katsouyanni K, Bellander T, Grau M, Koenig W, et al. Mediterranean diet and inflammatory response in myocardial infarction survivors. Int J Epidemiol. 2009;38:856–866. doi: 10.1093/ije/dyp142. [DOI] [PubMed] [Google Scholar]
- 14.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 15.Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Nitrate, bacteria and human health. Nat Rev Microbiol. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 16.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 17.Raat NJ, Noguchi AC, Liu VB, Raghavachari N, Liu D, Xu X, et al. Dietary nitrate and nitrite modulate blood and organ nitrite and the cellular ischemic stress response. Free Radic Biol Med. 2009;47:510–517. doi: 10.1016/j.freeradbiomed.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozdestan O, Uren A. Development of a cost-effective method for nitrate and nitrite determination in leafy plants and nitrate and nitrite contents of some green leafy vegetables grown in the Aegean region of Turkey. J Agric Food Chem. 2010;58:5235–5240. doi: 10.1021/jf904558c. [DOI] [PubMed] [Google Scholar]
- 19.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1–10. doi: 10.3945/ajcn.2008.27131. [DOI] [PubMed] [Google Scholar]
- 20.Ologhobo AD, Adegede HI, Maduagiwu EN. Occurrence of nitrate, nitrite and volatile nitrosamines in certain feedstuffs and animal products. Nutr Health. 1996;11:109–114. doi: 10.1177/026010609601100203. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Conca KR. Determination of nitrite in cured meats by ion-exclusion chromatography with electrochemical detection. J Assoc Off Anal Chem. 1990;73:561–564. [PubMed] [Google Scholar]
- 22.Bryant JL. Food, Nutrition and the Nitric Oxide Pathway: Biochemistry and Bioactivity. Lancaster: DEStech Publications, Inc; 2010. [Google Scholar]
- 23.Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc Res. 2007;75:327–338. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tannenbaum SR, Sinskey AJ, Weisman M, Bishop W. Nitrite in human saliva. Its possible relationship to nitrosamine formation. J Natl Cancer Inst. 1974;53:79–84. [PubMed] [Google Scholar]
- 25.Tannenbaum SR, Weisman M, Fett D. The effect of nitrate intake on nitrite formation in human saliva. Food Cosmet Toxicol. 1976;14:549–552. doi: 10.1016/s0015-6264(76)80006-5. [DOI] [PubMed] [Google Scholar]
- 26.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, et al. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 29.Takahama U, Tanaka M, Hirota S. Formation of nitric oxide, ethyl nitrite and an oxathiolone derivative of caffeic acid in a mixture of saliva and white wine. Free Radic Res. 2010;44:293–303. doi: 10.3109/10715760903486057. [DOI] [PubMed] [Google Scholar]
- 30.Takahama U, Hirota S, Oniki T. Production of nitric oxide-derived reactive nitrogen species in human oral cavity and their scavenging by salivary redox components. Free Radic Res. 2005;39:737–745. doi: 10.1080/10715760500043561. [DOI] [PubMed] [Google Scholar]
- 31.Gago B, Lundberg JO, Barbosa RM, Laranjinha J. Red wine-dependent reduction of nitrite to nitric oxide in the stomach. Free Radic Biol Med. 2007;43:1233–1242. doi: 10.1016/j.freeradbiomed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Rocha BS, Gago B, Barbosa RM, Laranjinha J. Dietary polyphenols generate nitric oxide from nitrite in the stomach and induce smooth muscle relaxation. Toxicology. 2009;265:41–48. doi: 10.1016/j.tox.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 34.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 35.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basu S, Azarova NA, Font MD, King SB, Hogg N, Gladwin MT, et al. Nitrite reductase activity of cytochrome c. J Biol Chem. 2008;283:32590–32597. doi: 10.1074/jbc.M806934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, et al. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation. 2010;121:98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 38.Calvert JW, Lefer DJ. Myocardial protection by nitrite. Cardiovasc Res. 2009;83:195–203. doi: 10.1093/cvr/cvp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP, et al. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiva S, Gladwin MT. Nitrite mediates cytoprotection after ischemia/reperfusion by modulating mitochondrial function. Basic Res Cardiol. 2009;104:113–119. doi: 10.1007/s00395-009-0009-3. [DOI] [PubMed] [Google Scholar]
- 43.Pabla R, Buda AJ, Flynn DM, Blesse SA, Shin AM, Curtis MJ, et al. Nitric oxide attenuates neutrophil-mediated myocardial contractile dysfunction after ischemia and reperfusion. Circ Res. 1996;78:65–72. doi: 10.1161/01.res.78.1.65. [DOI] [PubMed] [Google Scholar]
- 44.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:579–599. doi: 10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- 46.Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 47.Radovits T, Korkmaz S, Miesel-Groschel C, Seidel B, Stasch JP, Merkely B, et al. Pre-conditioning with the soluble guanylate cyclase activator Cinaciguat reduces ischaemia-reperfusion injury after cardiopulmonary bypass. Eur J Cardiothorac Surg. 2010 doi: 10.1016/j.ejcts.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 48.Korkmaz S, Radovits T, Barnucz E, Hirschberg K, Neugebauer P, Loganathan S, et al. Pharmacological activation of soluble guanylate cyclase protects the heart against ischemic injury. Circulation. 2009;120:677–686. doi: 10.1161/CIRCULATIONAHA.109.870774. [DOI] [PubMed] [Google Scholar]
- 49.Hamid SA, Totzeck M, Drexhage C, Thompson I, Fowkes RC, Rassaf T, et al. Nitric oxide/cGMP signalling mediates the cardioprotective action of adrenomedullin in reperfused myocardium. Basic Res Cardiol. 2010;105:257–266. doi: 10.1007/s00395-009-0058-7. [DOI] [PubMed] [Google Scholar]
- 50.Pinder AG, Pittaway E, Morris K, James PE. Nitrite directly vasodilates hypoxic vasculature via nitric oxide-dependent and -independent pathways. Br J Pharmacol. 2009;157:1523–1530. doi: 10.1111/j.1476-5381.2009.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Endeward V, Gros G, Jurgens KD. Significance of myoglobin as an oxygen store and oxygen transporter in the intermittently perfused human heart: a model study. Cardiovasc Res. 2010;87:22–29. doi: 10.1093/cvr/cvq036. [DOI] [PubMed] [Google Scholar]
- 54.Wittenberg JB, Wittenberg BA. Myoglobin-enhanced oxygen delivery to isolated cardiac mitochondria. J Exp Biol. 2007;210:2082–2090. doi: 10.1242/jeb.003947. [DOI] [PubMed] [Google Scholar]
- 55.Burwell LS, Nadtochiy SM, Brookes PS. Cardioprotection by metabolic shut-down and gradual wake-up. J Mol Cell Cardiol. 2009;46:804–810. doi: 10.1016/j.yjmcc.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 58.Halestrap AP. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans. 2010;38:841–860. doi: 10.1042/BST0380841. [DOI] [PubMed] [Google Scholar]
- 59.Misra MK, Sarwat M, Bhakuni P, Tuteja R, Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15:RA209–RA219. [PubMed] [Google Scholar]
- 60.Clarke SJ, Khaliulin I, Das M, Parker JE, Heesom KJ, Halestrap AP. Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circ Res. 2008;102:1082–1090. doi: 10.1161/CIRCRESAHA.107.167072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nadtochiy SM, Burwell LS, Brookes PS. Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J Mol Cell Cardiol. 2007;42:812–825. doi: 10.1016/j.yjmcc.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nadtochiy SM, Burwell LS, Ingraham CA, Spencer CM, Friedman AE, Pinkert CA, et al. In vivo cardioprotection by S-nitroso-2-mercaptopropionyl glycine. J Mol Cell Cardiol. 2009;46:960–968. doi: 10.1016/j.yjmcc.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stewart S, Lesnefsky EJ, Chen Q. Reversible blockade of electron transport with amobarbital at the onset of reperfusion attenuates cardiac injury. Transl Res. 2009;153:224–231. doi: 10.1016/j.trsl.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 65.Prime TA, Blaikie FH, Evans C, Nadtochiy SM, James AM, Dahm CC, et al. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2009;106:10764–10769. doi: 10.1073/pnas.0903250106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res. 2007;101:1155–1163. doi: 10.1161/CIRCRESAHA.107.155879. [DOI] [PubMed] [Google Scholar]
- 67.Chouchani ET, Hurd TR, Nadtochiy SM, Brookes PS, Fearnley IM, Lilley KS, et al. Identification of S-nitrosated mitochondrial proteins by S-nitrosothiol difference in gel electrophoresis (SNO-DIGE): implications for the regulation of mitochondrial function by reversible S-nitrosation. Biochem J. 2010;430:49–59. doi: 10.1042/BJ20100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, et al. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baker JE, Su J, Fu X, Hsu A, Gross GJ, Tweddell JS, et al. Nitrite confers protection against myocardial infarction: role of xanthine oxidoreductase, NADPH oxidase and K(ATP) channels. J Mol Cell Cardiol. 2007;43:437–444. doi: 10.1016/j.yjmcc.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Costa AD, Garlid KD. Intramitochondrial signaling: interactions among mitoKATP, PKCepsilon, ROS, and MPT. Am J Physiol Heart Circ Physiol. 2008;295:H874–H882. doi: 10.1152/ajpheart.01189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin Q, Yang XM, Cui L, Critz SD, Cohen MV, Browner NC, et al. Exogenous NO triggers preconditioning via a cGMP- and mitoKATP-dependent mechanism. Am J Physiol Heart Circ Physiol. 2004;287:H712–H718. doi: 10.1152/ajpheart.00954.2003. [DOI] [PubMed] [Google Scholar]
- 72.Sasaki N, Sato T, Ohler A, O’Rourke B, Marban E. Activation of mitochondrial ATP-dependent potassium channels by nitric oxide. Circulation. 2000;101:439–445. doi: 10.1161/01.cir.101.4.439. [DOI] [PubMed] [Google Scholar]
- 73.Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d’Ischia M. Nitro-fatty acid formation and signaling. J Biol Chem. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Napolitano A, Camera E, Picardo M, d’Ischia M. Acid-promoted reactions of ethyl linoleate with nitrite ions: formation and structural characterization of isomeric nitroalkene, nitrohydroxy, and novel 3-nitro-1,5-hexadiene and 1,5-dinitro-1, 3-pentadiene products. J Org Chem. 2000;65:4853–4860. doi: 10.1021/jo000090q. [DOI] [PubMed] [Google Scholar]
- 75.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, et al. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, et al. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280:42464–42475. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Batthyany C, Schopfer FJ, Baker PR, Duran R, Baker LM, Huang Y, et al. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J Biol Chem. 2006;281:20450–20463. doi: 10.1074/jbc.M602814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nadtochiy SM, Baker PR, Freeman BA, Brookes PS. Mitochondrial nitroalkene formation and mild uncoupling in ischaemic preconditioning: implications for cardioprotection. Cardiovasc Res. 2009;82:333–340. doi: 10.1093/cvr/cvn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rudolph V, Rudolph TK, Schopfer FJ, Bonacci G, Woodcock SR, Cole MP, et al. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc Res. 2010;85:155–166. doi: 10.1093/cvr/cvp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Napolitano A, Panzella L, Savarese M, Sacchi R, Giudicianni I, Paolillo L, et al. Acid-induced structural modifications of unsaturated Fatty acids and phenolic olive oil constituents by nitrite ions: a chemical assessment. Chem Res Toxicol. 2004;17:1329–1337. doi: 10.1021/tx049880b. [DOI] [PubMed] [Google Scholar]
- 81.Sinha SS, Shiva S, Gladwin MT. Myocardial protection by nitrite: evidence that this reperfusion therapeutic will not be lost in translation. Trends Cardiovasc Med. 2008;18:163–172. doi: 10.1016/j.tcm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 82.Yamagishi K, Iso H, Date C, Fukui M, Wakai K, Kikuchi S, et al. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol. 2008;52:988–996. doi: 10.1016/j.jacc.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 83.Davidson M, Bulkow LR, Gellin BG. Cardiac mortality in Alaska’s indigenous and non-Native residents. Int J Epidemiol. 1993;22:62–71. doi: 10.1093/ije/22.1.62. [DOI] [PubMed] [Google Scholar]
- 84.Calder PC. Polyunsaturated fatty acids and inflammation. Biochem Soc Trans. 2005;33:423–427. doi: 10.1042/BST0330423. [DOI] [PubMed] [Google Scholar]
- 85.Nageswari K, Banerjee R, Menon VP. Effect of saturated, omega-3 and omega-6 polyunsaturated fatty acids on myocardial infarction. J Nutr Biochem. 1999;10:338–344. doi: 10.1016/s0955-2863(99)00007-8. [DOI] [PubMed] [Google Scholar]
- 86.Mancardi D, Tullio F, Crisafulli A, Rastaldo R, Folino A, Penna C, et al. Omega 3 has a beneficial effect on ischemia/reperfusion injury, but cannot reverse the effect of stressful forced exercise. Nutr Metab Cardiovasc Dis. 2009;19:20–26. doi: 10.1016/j.numecd.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 87.Xiao YF, Sigg DC, Ujhelyi MR, Wilhelm JJ, Richardson ES, Iaizzo PA. Pericardial delivery of omega-3 fatty acid: a novel approach to reducing myocardial infarct sizes and arrhythmias. Am J Physiol Heart Circ Physiol. 2008;294:H2212–H2218. doi: 10.1152/ajpheart.91502.2007. [DOI] [PubMed] [Google Scholar]
- 88.McGuinness J, Neilan TG, Sharkasi A, Bouchier-Hayes D, Redmond JM. Myocardial protection using an omega-3 fatty acid infusion: quantification and mechanism of action. J Thorac Cardiovasc Surg. 2006;132:72–79. doi: 10.1016/j.jtcvs.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 89.Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation. 2002;105:2303–2308. doi: 10.1161/01.cir.0000015604.88808.74. [DOI] [PubMed] [Google Scholar]
- 90.Demaison L, Sergiel JP, Moreau D, Grynberg A. Influence of the phospholipid n-6/n-3 polyunsaturated fatty acid ratio on the mitochondrial oxidative metabolism before and after myocardial ischemia. Biochim Biophys Acta. 1994;1227:53–59. doi: 10.1016/0925-4439(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 91.Abdukeyum GG, Owen AJ, McLennan PL. Dietary (n-3) long-chain polyunsaturated fatty acids inhibit ischemia and reperfusion arrhythmias and infarction in rat heart not enhanced by ischemic preconditioning. J Nutr. 2008;138:1902–1909. doi: 10.1093/jn/138.10.1902. [DOI] [PubMed] [Google Scholar]
- 92.Campos H, Baylin A, Willett WC. Alpha-linolenic acid and risk of nonfatal acute myocardial infarction. Circulation. 2008;118:339–345. doi: 10.1161/CIRCULATIONAHA.107.762419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, et al. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 94.Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54:585–594. doi: 10.1016/j.jacc.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 95.Yang BC, Saldeen TG, Bryant JL, Nichols WW, Mehta JL. Long-term dietary fish oil supplementation protects against ischemia-reperfusion-induced myocardial dysfunction in isolated rat hearts. Am Heart J. 1993;126:1287–1292. doi: 10.1016/0002-8703(93)90524-d. [DOI] [PubMed] [Google Scholar]
- 96.Yanagisawa A, Matsukura T, Aoki N, Miyagawa M, Satoh K, Metori K, et al. Protection of the rat myocardium from ischemic injury by dietary lamprey oil. Eicosanoids. 1988;1:93–100. [PubMed] [Google Scholar]
- 97.Schror K. Eicosanoids and myocardial ischaemia. Basic Res Cardiol. 1987;82 (Suppl 1):235–243. doi: 10.1007/978-3-662-08390-1_27. [DOI] [PubMed] [Google Scholar]
- 98.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 101.Serhan CN, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004;73:155–172. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 102.Stables MJ, Gilroy DW. Old and new generation lipid mediators in acute inflammation and resolution. Prog Lipid Res. 2010 doi: 10.1016/j.plipres.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 103.Massaro M, Scoditti E, Carluccio MA, De CR. Basic mechanisms behind the effects of n-3 fatty acids on cardiovascular disease. Prostaglandins Leukot Essent Fatty Acids. 2008;79:109–115. doi: 10.1016/j.plefa.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 104.Das UN. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis. 2008;7:37. doi: 10.1186/1476-511X-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spite M, Summers L, Porter TF, Srivastava S, Bhatnagar A, Serhan CN. Resolvin D1 controls inflammation initiated by glutathione-lipid conjugates formed during oxidative stress. Br J Pharmacol. 2009;158:1062–1073. doi: 10.1111/j.1476-5381.2009.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 108.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 109.Ishida T, Yoshida M, Arita M, Nishitani Y, Nishiumi S, Masuda A, et al. Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflamm Bowel Dis. 2010;16:87–95. doi: 10.1002/ibd.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Keyes KT, Ye Y, Lin Y, Zhang C, Perez-Polo JR, Gjorstrup P, et al. Resolvin E1 protects the rat heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;299:H153–H164. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- 111.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 112.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, et al. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wayman NS, Hattori Y, McDonald MC, Mota-Filipe H, Cuzzocrea S, Pisano B, et al. Ligands of the peroxisome proliferator-activated receptors (PPAR-gamma and PPAR-alpha) reduce myocardial infarct size. FASEB J. 2002;16:1027–1040. doi: 10.1096/fj.01-0793com. [DOI] [PubMed] [Google Scholar]
- 117.Zingarelli B, Hake PW, Mangeshkar P, O’Connor M, Burroughs TJ, Piraino G, et al. Diverse cardioprotective signaling mechanisms of peroxisome proliferator-activated receptor-gamma ligands, 15-deoxy-Delta12,14-prostaglandin J2 and ciglitazone, in reperfusion injury: role of nuclear factor-kappaB, heat shock factor 1, and Akt. Shock. 2007;28:554–563. doi: 10.1097/shk.0b013e31804f56b9. [DOI] [PubMed] [Google Scholar]
- 118.Sexton A, McDonald M, Cayla C, Thiemermann C, Ahluwalia A. 12-Lipoxygenase-derived eicosanoids protect against myocardial ischemia/reperfusion injury via activation of neuronal TRPV1. FASEB J. 2007;21:2695–2703. doi: 10.1096/fj.06-7828com. [DOI] [PubMed] [Google Scholar]
- 119.Gross GJ, Hsu A, Falck JR, Nithipatikom K. Mechanisms by which epoxyeicosatrienoic acids (EETs) elicit cardioprotection in rat hearts. J Mol Cell Cardiol. 2007;42:687–691. doi: 10.1016/j.yjmcc.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nithipatikom K, Moore JM, Isbell MA, Falck JR, Gross GJ. Epoxyeicosatrienoic acids in cardioprotection: ischemic versus reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H537–H542. doi: 10.1152/ajpheart.00071.2006. [DOI] [PubMed] [Google Scholar]
- 121.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 122.Pan HL, Chen SR. Sensing tissue ischemia: another new function for capsaicin receptors? Circulation. 2004;110:1826–1831. doi: 10.1161/01.CIR.0000142618.20278.7A. [DOI] [PubMed] [Google Scholar]
- 123.Jones WK, Fan GC, Liao S, Zhang JM, Wang Y, Weintraub NL, et al. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation. 2009;120:S1–S9. doi: 10.1161/CIRCULATIONAHA.108.843938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seubert J, Yang B, Bradbury JA, Graves J, DeGraff LM, Gabel S, et al. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res. 2004;95:506–514. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- 125.Shiraki T, Kamiya N, Shiki S, Kodama TS, Kakizuka A, Jingami H. Alpha, beta-unsaturated ketone is a core moiety of natural ligands for covalent binding to peroxisome proliferator-activated receptor gamma. J Biol Chem. 2005;280:14145–14153. doi: 10.1074/jbc.M500901200. [DOI] [PubMed] [Google Scholar]
- 126.Serhan CN, Levy BD, Clish CB, Gronert K, Chiang N. Lipoxins, aspirin-triggered 15-epi-lipoxin stable analogs and their receptors in anti-inflammation: a window for therapeutic opportunity. Ernst Schering Res Found Workshop. 2000:143–185. doi: 10.1007/978-3-662-04047-8_8. [DOI] [PubMed] [Google Scholar]
- 127.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 128.Gavins FN, Kamal AM, D’Amico M, Oliani SM, Perretti M. Formyl-peptide receptor is not involved in the protection afforded by annexin 1 in murine acute myocardial infarct. FASEB J. 2005;19:100–102. doi: 10.1096/fj.04-2178fje. [DOI] [PubMed] [Google Scholar]
- 129.Ye XH, Wu Y, Guo PP, Wang J, Yuan SY, Shang Y, et al. Lipoxin A(4) analogue protects brain and reduces inflammation in a rat model of focal cerebral ischemia reperfusion. Brain Res. 2010 doi: 10.1016/j.brainres.2010.01.079. [DOI] [PubMed] [Google Scholar]
- 130.Peskar BM, Ehrlich K, Schuligoi R, Peskar BA. Role of lipoxygenases and the lipoxin A(4)/annexin 1 receptor in ischemia-reperfusion-induced gastric mucosal damage in rats. Pharmacology. 2009;84:294–299. doi: 10.1159/000244017. [DOI] [PubMed] [Google Scholar]
- 131.Kieran NE, Doran PP, Connolly SB, Greenan MC, Higgins DF, Leonard M, et al. Modification of the transcriptomic response to renal ischemia/reperfusion injury by lipoxin analog. Kidney Int. 2003;64:480–492. doi: 10.1046/j.1523-1755.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 132.Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernandez-Lopez D, Pradillo JM, et al. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci. 2009;29:3875–3884. doi: 10.1523/JNEUROSCI.5529-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Y, Sano M, Shinmura K, Tamaki K, Katsumata Y, Matsuhashi T, et al. 4-Hydroxy-2-nonenal protects against cardiac ischemia-reperfusion injury via the Nrf2-dependent pathway. J Mol Cell Cardiol. 2010 doi: 10.1016/j.yjmcc.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 135.Trostchansky A, Souza JM, Ferreira A, Ferrari M, Blanco F, Trujillo M, et al. Synthesis, isomer characterization, and anti-inflammatory properties of nitroarachidonate. Biochemistry. 2007;46:4645–4653. doi: 10.1021/bi602652j. [DOI] [PubMed] [Google Scholar]
- 136.Cui J, Tosaki A, Bertelli AA, Bertelli A, Maulik N, Das DK. Cardioprotection with white wine. Drugs Exp Clin Res. 2002;28:1–10. [PubMed] [Google Scholar]
- 137.Mukherjee S, Lekli I, Gurusamy N, Bertelli AA, Das DK. Expression of the longevity proteins by both red and white wines and their cardioprotective components, resveratrol, tyrosol, and hydroxytyrosol. Free Radic Biol Med. 2009;46:573–578. doi: 10.1016/j.freeradbiomed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 138.Dudley JI, Lekli I, Mukherjee S, Das M, Bertelli AA, Das DK. Does white wine qualify for French paradox? Comparison of the cardioprotective effects of red and white wines and their constituents: resveratrol, tyrosol, and hydroxytyrosol. J Agric Food Chem. 2008;56:9362–9373. doi: 10.1021/jf801791d. [DOI] [PubMed] [Google Scholar]
- 139.Lin JF, Lin SM, Chih CL, Nien MW, Su HH, Hu BR, et al. Resveratrol reduces infarct size and improves ventricular function after myocardial ischemia in rats. Life Sci. 2008;83:313–317. doi: 10.1016/j.lfs.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 140.Bartekova M, Carnicka S, Pancza D, Ondrejcakova M, Breier A, Ravingerova T. Acute treatment with polyphenol quercetin improves postischemic recovery of isolated perfused rat hearts after global ischemia. Can J Physiol Pharmacol. 2010;88:465–471. doi: 10.1139/y10-025. [DOI] [PubMed] [Google Scholar]
- 141.Brookes PS, Digerness SB, Parks DA, rley-Usmar V. Mitochondrial function in response to cardiac ischemia-reperfusion after oral treatment with quercetin. Free Radic Biol Med. 2002;32:1220–1228. doi: 10.1016/s0891-5849(02)00839-0. [DOI] [PubMed] [Google Scholar]
- 142.Yamazaki KG, Romero-Perez D, Barraza-Hidalgo M, Cruz M, Rivas M, Cortez-Gomez B, et al. Short- and long-term effects of (−)-epicatechin on myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2008;295:H761–H767. doi: 10.1152/ajpheart.00413.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Goh SS, Woodman OL, Pepe S, Cao AH, Qin C, Ritchie RH. The red wine antioxidant resveratrol prevents cardiomyocyte injury following ischemia-reperfusion via multiple sites and mechanisms. Antioxid Redox Signal. 2007;9:101–113. doi: 10.1089/ars.2007.9.101. [DOI] [PubMed] [Google Scholar]
- 144.Micallef M, Lexis L, Lewandowski P. Red wine consumption increases antioxidant status and decreases oxidative stress in the circulation of both young and old humans. Nutr J. 2007;6:27. doi: 10.1186/1475-2891-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xi J, Wang H, Mueller RA, Norfleet EA, Xu Z. Mechanism for resveratrol-induced cardioprotection against reperfusion injury involves glycogen synthase kinase 3beta and mitochondrial permeability transition pore. Eur J Pharmacol. 2009;604:111–116. doi: 10.1016/j.ejphar.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gurusamy N, Lekli I, Mukherjee S, Ray D, Ahsan MK, Gherghiceanu M, et al. Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc Res. 2010;86:103–112. doi: 10.1093/cvr/cvp384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Das S, Fraga CG, Das DK. Cardioprotective effect of resveratrol via HO-1 expression involves p38 map kinase and PI-3-kinase signaling, but does not involve NFkappaB. Free Radic Res. 2006;40:1066–1075. doi: 10.1080/10715760600833085. [DOI] [PubMed] [Google Scholar]
- 149.Hung LM, Su MJ, Chen JK. Resveratrol protects myocardial ischemia-reperfusion injury through both NO-dependent and NO-independent mechanisms. Free Radic Biol Med. 2004;36:774–781. doi: 10.1016/j.freeradbiomed.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 150.Roldan A, Palacios V, Caro I, Perez L. Resveratrol content of Palomino fino grapes: influence of vintage and fungal infection. J Agric Food Chem. 2003;51:1464–1468. doi: 10.1021/jf020774u. [DOI] [PubMed] [Google Scholar]
- 151.Thirunavukkarasu M, Penumathsa SV, Samuel SM, Akita Y, Zhan L, Bertelli AA, et al. White wine induced cardioprotection against ischemia-reperfusion injury is mediated by life extending Akt/FOXO3a/NFkappaB survival pathway. J Agric Food Chem. 2008;56:6733–6739. doi: 10.1021/jf801473v. [DOI] [PubMed] [Google Scholar]
- 152.Samuel SM, Thirunavukkarasu M, Penumathsa SV, Paul D, Maulik N. Akt/FOXO3a/SIRT1-mediated cardioprotection by n-tyrosol against ischemic stress in rat in vivo model of myocardial infarction: switching gears toward survival and longevity. J Agric Food Chem. 2008;56:9692–9698. doi: 10.1021/jf802050h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Oliveira CM, Ferreira AC, de Pinho PG, Silva AM. New qualitative approach in the characterization of antioxidants in white wines by antioxidant free radical scavenging and NMR techniques. J Agric Food Chem. 2008;56:10326–10331. doi: 10.1021/jf8013662. [DOI] [PubMed] [Google Scholar]
- 154.Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, et al. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 156.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 158.Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch Biochem Biophys. 2010 doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yu W, Fu YC, Zhou XH, Chen CJ, Wang X, Lin RB, et al. Effects of resveratrol on H(2)O(2)-induced apoptosis and expression of SIRTs in H9c2 cells. J Cell Biochem. 2009;107:741–747. doi: 10.1002/jcb.22169. [DOI] [PubMed] [Google Scholar]
- 160.Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 161.Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, et al. SIRT1 activation by small molecules - kinetic and biophysical evidence for direct interaction of enzyme and activator. J Biol Chem. 2010 doi: 10.1074/jbc.M110.133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pacholec M, Chrunyk BA, Cunningham D, Flynn D, Griffith DA, Griffor M, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010 doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kwon HS, Ott M. The ups and downs of SIRT1. Trends Biochem Sci. 2008;33:517–525. doi: 10.1016/j.tibs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 165.Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, et al. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879–886. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nadtochiy SM, Redman E, Rahman I, Brookes PS. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovasc Res. 2010 doi: 10.1093/cvr/cvq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295:H2348–H2355. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chandrasekar B, Nelson JF, Colston JT, Freeman GL. Calorie restriction attenuates inflammatory responses to myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2001;280:H2094–H2102. doi: 10.1152/ajpheart.2001.280.5.H2094. [DOI] [PubMed] [Google Scholar]
- 169.Shinmura K, Tamaki K, Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J Mol Cell Cardiol. 2005;39:285–296. doi: 10.1016/j.yjmcc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 170.Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 171.Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M, et al. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J Nutr Biochem. 2010;21:413–417. doi: 10.1016/j.jnutbio.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mitchell JR, Verweij M, Brand K, van d V, Goemaere N, van den ES, et al. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell. 2010;9:40–53. doi: 10.1111/j.1474-9726.2009.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Broderick TL, Belke T, Driedzic WR. Effects of chronic caloric restriction on mitochondrial respiration in the ischemic reperfused rat heart. Mol Cell Biochem. 2002;233:119–125. doi: 10.1023/a:1015506327849. [DOI] [PubMed] [Google Scholar]
- 174.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rane S, He M, Sayed D, Yan L, Vatner D, Abdellatif M. An antagonism between the AKT and beta-adrenergic signaling pathways mediated through their reciprocal effects on miR-199a-5p. Cell Signal. 2010;22:1054–1062. doi: 10.1016/j.cellsig.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Raval AP, Dave KR, Perez-Pinzon MA. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2006;26:1141–1147. doi: 10.1038/sj.jcbfm.9600262. [DOI] [PubMed] [Google Scholar]
- 177.Abete P, Ferrara N, Cioppa A, Ferrara P, Bianco S, Calabrese C, et al. Preconditioning does not prevent postischemic dysfunction in aging heart. J Am Coll Cardiol. 1996;27:1777–1786. doi: 10.1016/0735-1097(96)00070-8. [DOI] [PubMed] [Google Scholar]
- 178.Abete P, Cacciatore F, Testa G, la-Morte D, Galizia G, De SD, et al. Ischemic preconditioning in the aging heart: from bench to bedside. Ageing Res Rev. 2010;9:153–162. doi: 10.1016/j.arr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 179.Pepe S. Dysfunctional ischemic preconditioning mechanisms in aging. Cardiovasc Res. 2001;49:11–14. doi: 10.1016/s0008-6363(00)00283-2. [DOI] [PubMed] [Google Scholar]
- 180.Long P, Nguyen Q, Thurow C, Broderick TL. Caloric restriction restores the cardioprotective effect of preconditioning in the rat heart. Mech Ageing Dev. 2002;123:1411–1413. doi: 10.1016/s0047-6374(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 181.Abete P, Testa G, Ferrara N, De SD, Capaccio P, Viati L, et al. Cardioprotective effect of ischemic preconditioning is preserved in food-restricted senescent rats. Am J Physiol Heart Circ Physiol. 2002;282:H1978–H1987. doi: 10.1152/ajpheart.00929.2001. [DOI] [PubMed] [Google Scholar]
- 182.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 184.Moubarak RS, Yuste VJ, Artus C, Bouharrour A, Greer PA, Menissier-de MJ, et al. Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol Cell Biol. 2007;27:4844–4862. doi: 10.1128/MCB.02141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rajamohan SB, Pillai VB, Gupta M, Sundaresan NR, Birukov KG, Samant S, et al. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol Cell Biol. 2009;29:4116–4129. doi: 10.1128/MCB.00121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kolthur-Seetharam U, Dantzer F, McBurney MW, de MG, Sassone-Corsi P. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle. 2006;5:873–877. doi: 10.4161/cc.5.8.2690. [DOI] [PubMed] [Google Scholar]
- 187.Yan L, Sadoshima J, Vatner DE, Vatner SF. Autophagy in ischemic preconditioning and hibernating myocardium. Autophagy. 2009;5:709–712. doi: 10.4161/auto.5.5.8510. [DOI] [PubMed] [Google Scholar]
- 188.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang JL, Lu JK, Chen D, Cai Q, Li TX, Wu LS, et al. Myocardial autophagy variation during acute myocardial infarction in rats: the effects of carvedilol. Chin Med J (Engl) 2009;122:2372–2379. [PubMed] [Google Scholar]
- 190.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 191.Van der HA, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1) J Biol Chem. 2004;279:28873–28879. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- 192.Wong A, Woodcock EA. FoxO proteins and cardiac pathology. Adv Exp Med Biol. 2009;665:78–89. doi: 10.1007/978-1-4419-1599-3_6. [DOI] [PubMed] [Google Scholar]
- 193.Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, et al. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 194.Bautista L, Castro MJ, Lopez-Barneo J, Castellano A. Hypoxia inducible factor-2alpha stabilization and maxi-K+ channel beta1-subunit gene repression by hypoxia in cardiac myocytes: role in preconditioning. Circ Res. 2009;104:1364–1372. doi: 10.1161/CIRCRESAHA.108.190645. [DOI] [PubMed] [Google Scholar]
- 195.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 196.Rasbach KA, Schnellmann RG. PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun. 2007;355:734–739. doi: 10.1016/j.bbrc.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 197.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Van der HK, Cuhlmann S, Luong lA, Zakkar M, Evans PC. Role of nuclear factor kappaB in cardiovascular health and disease. Clin Sci (Lond) 2010;118:593–605. doi: 10.1042/CS20090557. [DOI] [PubMed] [Google Scholar]
- 199.Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, et al. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- 200.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem. 2005;280:21313–21320. doi: 10.1074/jbc.M413296200. [DOI] [PubMed] [Google Scholar]
- 202.Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci U S A. 2002;99:13653–13658. doi: 10.1073/pnas.222538099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382:790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 205.Kim SY, Park JW. Cellular defense against singlet oxygen-induced oxidative damage by cytosolic NADP+-dependent isocitrate dehydrogenase. Free Radic Res. 2003;37:309–316. doi: 10.1080/1071576021000050429. [DOI] [PubMed] [Google Scholar]
- 206.Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol. 2008;28:6384–6401. doi: 10.1128/MCB.00426-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax andBcl-X(L) during apoptosis. Proc Natl Acad Sci U S A. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 209.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 210.Shulga N, Wilson-Smith R, Pastorino JG. Sirtuin-3 deacetylation of cyclophilin D induces dissociation of hexokinase II from the mitochondria. J Cell Sci. 2010;123:894–902. doi: 10.1242/jcs.061846. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 211.Okuda Y, Kawashima K, Sawada T, Tsurumaru K, Asano M, Suzuki S, et al. Eicosapentaenoic acid enhances nitric oxide production by cultured human endothelial cells. Biochem Biophys Res Commun. 1997;232:487–491. doi: 10.1006/bbrc.1997.6328. [DOI] [PubMed] [Google Scholar]
- 212.Koenitzer JR, Freeman BA. Redox signaling in inflammation: interactions of endogenous electrophiles and mitochondria in cardiovascular disease. Ann N Y Acad Sci. 2010;1203:45–52. doi: 10.1111/j.1749-6632.2010.05559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Khoo NK, Rudolph V, Cole MP, Golin-Bisello F, Schopfer FJ, Woodcock SR, et al. Activation of vascular endothelial nitric oxide synthase and heme oxygenase-1 expression by electrophilic nitro-fatty acids. Free Radic Biol Med. 2010;48:230–239. doi: 10.1016/j.freeradbiomed.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Muscari C, Bonafe’ F, Gamberini C, Giordano E, Tantini B, Fattori M, et al. Early preconditioning prevents the loss of endothelial nitric oxide synthase and enhances its activity in the ischemic/reperfused rat heart. Life Sci. 2004;74:1127–1137. doi: 10.1016/j.lfs.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 215.Starkopf J, Andreasen TV, Bugge E, Ytrehus K. Lipid peroxidation, arachidonic acid and products of the lipoxygenase pathway in ischaemic preconditioning of rat heart. Cardiovasc Res. 1998;37:66–75. doi: 10.1016/s0008-6363(97)00240-x. [DOI] [PubMed] [Google Scholar]