Abstract
Biological protein pores and pore-forming peptides can generate a pathway for the flux of ions and other charged or polar molecules across cellular membranes. In nature, these nanopores have diverse and essential functions that range from maintaining cell homeostasis and participating in cell signaling to activating or killing cells. The combination of the nanoscale dimensions and sophisticated – often regulated – functionality of these biological pores make them particularly attractive for the growing field of nanobiotechnology. Applications range from single-molecule sensing to drug delivery and targeted killing of malignant cells. Potential future applications may include the use of nanopores for single strand DNA sequencing and for generating bio-inspired, and possibly, biocompatible visual detection systems and batteries. This article reviews the current state of applications of pore-forming peptides and proteins in nanomedicine, sensing, and nanoelectronics.
Introduction
Biological pores, which comprise proteins and peptides, provide nanoscopic pathways for the passage of ions and other charged or polar molecules across the hydrophobic barrier of cellular membranes. Pore-forming molecules range from short peptides that can self-assemble to pores with weak selectivity for specific ions (or other permeants) to large transmembrane ion channel proteins with exquisite selectivity for certain ions [1]. Table 1 summarizes various functions of biological nanopores in nature; these include sensing, signaling and communication, defense against pathogens, and transport of proteins and nucleotides across membranes [2,3••,4•,5]. Biological nanopores are, hence, essential for all living cells, and – owing to their functional sophistication and nanometer-scale dimensions – they offer intriguing possibilities for applications in nanobiotechnology [6].
Table 1.
Biological nanopores and their physiological functions
| Pore | Function |
|---|---|
| Antimicrobial and toxin peptides [7,8] | Lysis of microbial cells; disruption of homeostasis of intracellular ions; transport of proteins into target cells. |
| Porins [9] | Transport of water soluble molecules across membranes of bacteria or organelles. |
| Aquaporins [10] | Rapid transport of water across lipid membranes. |
| Membrane-attack complex [11] | Lysis of pathogen cells as a defense mechanism of the innate immune system. |
| Ion channel proteins [2] | Transport of ions across membranes, regulation of membrane potential, signal transduction and amplification, maintaining cell homeostasis of ion concentrations. |
| Nuclear pore complexes [2] | Transport of nucleotides, proteins, and other molecules across the nuclear envelope. |
| Translocator protein pores of the endoplasmic reticulum [2] | Transport of proteins across the membrane of the endoplasmic reticulum. |
| Viral pores [12] | Postulated transport of nucleocapsids of e.g. herpes simplex virus (HSV) across the nucleus membrane. |
| Amyloid pores [13–15] | Aberrant function of amyloidogenic proteins; possibly involved in pathogenic pathway of amyloidogenic diseases |
The field of nanobiotechnology strives to combine life sciences with physical sciences and nanosciences in order to advance technology. Proteins, in particular, are being increasingly employed for the development of novel, often hierarchical, materials and devices with nanoscale dimensions and desired functionalities [6]. Proteins are compelling, because their sophisticated three-dimensional structure on the nanoscale, their capability to be regulated, and their specificity allow them to carry out an impressive spectrum of complex tasks. Functional proteins, thus, represent an intriguing playground for exploration and imagination in nanobiotechnology. Among the different types of functional proteins, the class of ion channels, porins, and pore-forming peptides stands out for applications in nanobiotechnology [3••,16•,17]. These applications include detection of individual molecules [3••,16•,18] (both small molecules [19] and macromolecules [20]), monitoring chemical and biochemical reactions at a single-molecule level [17,21,22,23•,24], targeted cytolysis of cancer cells [4•,25], formation of bio-inspired batteries [26••], potential development of biocompatible nanotransistors [27,28•], and possibly sequencing of long strands of DNA or RNA [29•,30•]. Figure 1 shows examples of biological pores and lists some of their most important physiological functions as well as potential future applications. This article reviews a selection of the applications of pore-forming peptides and proteins in nanobiotechnology with a focus on nanomedicine, sensing, and nanoelectronics.
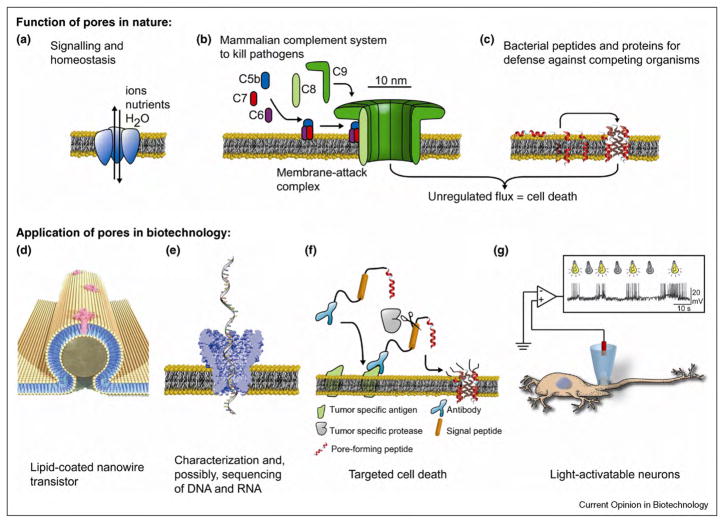
Figure 1.
Functions of biological nanopores in nature and applications of these pores in nanobiotechnology. (a) Ion channel proteins transport ions across the plasma membrane of a cell for maintaining homeostasis in the cell and for signaling purposes. (b) Membrane-attack complexes form a lytic pore with a diameter of ~10 nm in the plasma membrane of a pathogen by self-assembly of the complement proteins C5b to C9. (c) Antibiotic peptides (here alamethicin [31]) insert into the membrane of target microbes and form lytic pores. (d) Bionanoelectronic device consisting of a silicon nanowire coated with a lipid bilayer with peptide pores. Image by Scott Dougherty, Lawrence Livermore National Laboratory. (e) Translocation of a single-stranded DNA molecule through an engineered bacterial porin, MspA, leads to partial blockage of the pore; translocation can be monitored by the resulting fluctuations in the ionic current through the pore. (f) Activation of multimeric pores by a tumor specific protease targets and kills malignant cells. (g) Remotely activated firing of neurons by engineered, light-activated ion channel proteins.
Ion channel proteins are an intriguing choice for nano-biotechnological applications because they already fulfill key functions in signal transduction and amplification in living cells (Figure 1a) [3••,4•]. For instance, ion channel proteins can regulate ion flux by various gating mechanisms; they switch between closed and open states in response to specific stimuli such as ligand-binding (ligand-gated ion channels), change in transmembrane voltage (voltage-gated ion channels), or mechanical force (mechano-gated ion channels [32]). Ligand-gated ion channels are particularly impressive, since binding of one or a few ligand molecules to a channel protein can induce channel opening and facilitate the flux of millions of ions across the membrane per second. These ion channels are, thus, signal amplifiers with million-fold amplification. As a result, these proteins play a vital role in cell–cell signaling (for instance, in nerve transduction) and in biological processes that require rapid responses from cells (such as triggering muscle contraction). The ability to transport chemical charges across lipid membranes at fast rates, in combination with the exquisite signal amplification capability of ion channel proteins and ion channel-forming peptides, makes them particularly attractive for development of nanoscale sensors and electronics [28•,33•,34••,35].
One of the essential functions of ion channel proteins is to regulate the distribution and concentration of ions inside cells. Many of the natural antimicrobial toxins kill their target cells by disrupting their homeostasis of ions [36,37]. As illustrated in Figure 1c, these toxins form pores through the membranes of their target cells leading to uncontrolled transmembrane ion flux and eventually to death of these cells. Similarly, the complement system, a part of the innate immune response of mammals, employs pore-forming proteins to destroy invading pathogens [11]. In the complement system, binding of host antibodies to antigens on the surface of pathogens initiates a cascade of molecular events that leads to the self-assembly of a membrane-attack complex, as shown in Figure 1b. This complex forms a pore in the plasma membrane of the pathogens and leads to cell lysis. In nanomedicine, the ability of pore-forming peptides and proteins to target and kill cells is particularly attractive for the development of novel therapeutic approaches to kill cancer cells as depicted in Figure 1f. In addition, the ability to form pores in the plasma membrane of cells provides a unique tool to access the inside of cells both chemically and electrically. This capability is useful for delivering genes or drugs into the cell or for manipulating the membrane potential [38–40].
One important, recent development that is accelerating the pace of applications of biological pores in nanotechnology is the increasing availability of structural information on membrane proteins. Owing to the physiological and medical importance of biological pores, an enormous effort has been devoted to elucidate crystal structures and to reveal structure–function relationships. Relatively recent breakthroughs include the development of the single channel recording technique by Neher and Sakmann [41,42], the discovery of aquaporins by Agre [43–45], and findings from MacKinnon’s group on the structure and function of potassium channels [46–48], as well as crystal structures of other ion channels, porins, and assemblies of pore-forming peptides [49–62]. Other vibrant research fields are the de novo design of synthetic ion channels and computational approaches to study ion channel functions [63–67]. Together, these advances in the life sciences, combined with substantial progress in re-engineering or synthetic modification of ion channels and pores in order to tailor their properties, provide an inspiring playground for future applications. A first example of what might be possible is the recently presented sequencing of short DNAstrands with genetically engineered MspA pores [68].
Table 2 introduces some of the most frequently used biological nanopores for applications in three major areas of nanotechnology and biotechnology: nanomedicine, sensing, and nanoelectronics.
Table 2.
Selection of commonly applied biological pores in nanobiotechnology. Note, all illustrations of pores and lipids are drawn to scale to facilitate the comparison of their sizes
| Pore | Source | Pore assembly | La | Illustration | ø | |
|---|---|---|---|---|---|---|
| Large Proteins | α-hemolysin [69–71] | Staphylococcus aureus bacterium | Heptameric Pore |

|
||
| aerolysin [72] | Aeromonas hydrophila bacterium | Heptameric Pore |
|
|||
| anthrax toxin [73] | Bacillus anthracis bacterium | Heptameric Pore |
|
|||
| diphtheria toxin [4•] | Corynebacterium diphtherian bacterium | Monomeric | not shown | |||
|
| ||||||
| Small Peptides | gramicidin A [33•,74–76] | Bacillus brevis bacterium | Head-to-head dimerization |
|
||
| alamethicin [77,78] | Trichoderma viride fungus | Bundle of α-helices (4–11) |
|
|||
| melittin [79,80] | Apis mellifera bee venom | Bundle of α-helices | similar to alamethicin | |||
|
| ||||||
| Porins | MspA [30•] | Myobacterium smegmatis bacterium | Octameric Pore |

|
||
| OmpG [81] | Escherichia coli bacterium | Monomeric Pore |

|
|||
Length of the constriction zone within the lumen of the pore.
For their application in nanobiotechnology, ion channel proteins and pore-forming peptides typically have to be reconstituted into lipid membranes. Table 3 summarizes the most commonly applied model membranes for this purpose; these include supported lipid bilayers [82–86,87••,88–93], planar lipid bilayers (also called black lipid membranes, BLM) [94,95], liposomes [40,96–106], and droplet interface bilayer systems [107••,108–110]. So far, most of the applications of proteinaceous nanopores are based on current recordings through planar lipid bilayers [23•,94,111•,112]. This technique was developed in 1962 by Mueller et al. [113••,114] with modifications by Montal and Mueller in 1972 [115••]. Planar lipid bilayer recordings have the benefit of providing well-defined experimental conditions to study and apply biological pores. This technique is also more accessible to novices than other techniques such as patch clamp recordings for studying ion flux through protein pores. Despite their usefulness and popularity, planar bilayer recordings have serious limitations for real world applications in harsh environments. Planar lipid bilayers are mechanically and chemically fragile, have limited lifetime, and recordings across these membranes can be associated with significant electrical current noise [94,95,111•]. Several recent developments, including microsized and nanosized planar lipid bilayers as well as hydrogel-supported planar bilayers, improved the stability from hours to days and weeks [116–118,119•,120–126]. Unless lipid bilayers could be made on demand in an automated setup [127,128] that also reconstitutes biological pores, further improvements will be required in order to reach a stability of months to years and to advance nanopore-based bilayer recordings from research labs to real world applications. As a potentially more stable and practical alternative of traditional planar lipid bilayer systems, Babakov and co-workers introduced a different bilayer platform in 1966 [107••]. In this platform, two lipid monolayers, each assembled at an interface between an aqueous and an organic phase, are brought into contact to form a lipid bilayer. In 2006, Takeuchi’s group revisited this platform and, by using the same concept, developed droplet interface bilayer systems in which two aqueous droplets coated with a lipid monolayer formed a lipid bilayer at their interface [108]. Since then, several groups have employed this platform with slight modifications [26••,110,129].
Table 3.
Commonly applied model lipid membranes for reconstitution and application of biological pores in nanobiotechnology
| Platform | Description | Illustration | Typical Application |
|---|---|---|---|
| Supported Lipid Bilayer | Bilayer supported on a solid substrate |

|
Incorporation of biological pores changes the electrical impedance of the supported bilayers. Binding to these pores can be detected by additional changes in impedance. |
| Planar Lipid Bilayer | Bilayer spanning a small pore between two aqueous solutions |
|
Reconstitution of ion channel proteins or pore-forming peptides changes the ionic conductance across the bilayer. Ion currents through individual pores as well as changes in conductance due to the presence of analytes can be detected. |
| Liposomes | Lipid bilayer vesicles suspended in aqueous solutions |

|
Reconstitution of biological pores permeabilizes liposomal membranes; cargo molecules encapsulated inside the liposomes can be released through these pores. |
| Droplet Interface Bilayer | Lipid bilayer formed at the interface of two aqueous droplets that are coated with a monolayer of lipids within an oil phase |
|
Similar to planar lipid bilayers. Incorporation of biological pores changes the ionic conductance across the bilayer. Ionic currents through individual pores can be detected by inserting an electrode into each droplet. |
Applications of biological pores in nanomedicine
The field of nanomedicine applies nanotechnology to address medically relevant challenges. Biological nanopores attract increasing attention for applications in nanomedicine; these applications include cancer treatment, antimicrobial drug development, and drug delivery [4•,36,130,131]. This section highlights examples of applications of pore-forming peptides and proteins for cancer treatment, development of antimicrobial drugs, and targeted delivery in nanomedicine. Panchal et al. examined this topic in an excellent review in 2002 [4•].
Biological pores for cancer treatment
Current approaches for cancer treatment, including radiation and chemotherapy, face obstacles such as tumor metastasis and resistance [4•,25]. In addition, side effects of these therapeutic approaches have the drawback of damaging healthy cells when administrated at effective doses [4•,25]. Therapeutic strategies with improved specificity and efficacy as well as reduced toxicity are, therefore, still sought after. One potential novel strategy is the application of pore-forming antimicrobial peptides for killing cancer cells [4•,25,130,132]. The cytotoxicity of these peptides is exerted either through the formation of cytolytic pores in the membrane of the targeted cancer cells (this mechanism requires high concentrations of the peptide), or it is conferred by increasing the uptake of chemotherapeutic agents such as doxorubicin by permeabilizing the membrane of cancer cells, (this mechanism is achievable at low concentrations of the peptide) [132].
One of the challenges for the application of biological pores to kill cancer cells is to equip these peptides with targeting mechanisms that guide them specifically to malignant cells. Cancer cells typically overexpress specific antigens, carbohydrate moieties, or growth factor receptors on their surface that can be employed for targeting [4•,25]. To target these tumor-associated antigens and receptors, pore-forming peptides and proteins can either be genetically engineered or they can be chemically attached to appropriate ligands or antibodies. Figure 2 shows the concept of this targeting approach [4•]. Several studies have examined the potential of these approaches to kill cells that overexpress a specific receptor or antigen [4•,133–137]. Among the biological pores that have been examined for targeted cytolysis are δ-endotoxin from the Bacillus thuringiensis bacterium, equinatoxin II from the sea anemone Actinia equina, Sticholysin I from the sea anemone Stichodactyla helianthus, and diphtheria toxin from the bacterium Corynebacterium diphtheriae [132,134,138,139]. One of these studies applied a fusion protein composed of the pore-forming toxin Sticholysin I and a monoclonal antibody against the tumor-specific antigen ior C2. This work evaluated the binding and cytotoxic activity of this fusion protein against a colon cancer cell line [139]. In addition, Murphy and co-workers have reported the development of a number of fusion proteins with diphtheria toxin as pore former; these included conjugates of diphtheria toxin with interleukin 2 and conjugates of diphtheria toxin with epidermal growth factor. These studies demonstrated the intriguing potential of cytolytic fusion proteins to kill cells that overexpress the targeted receptor [140,141]. The conjugates of diphtheria toxin with interleukin 2 are currently undergoing clinical trials for the treatment of hematopoietic malignancies [132].
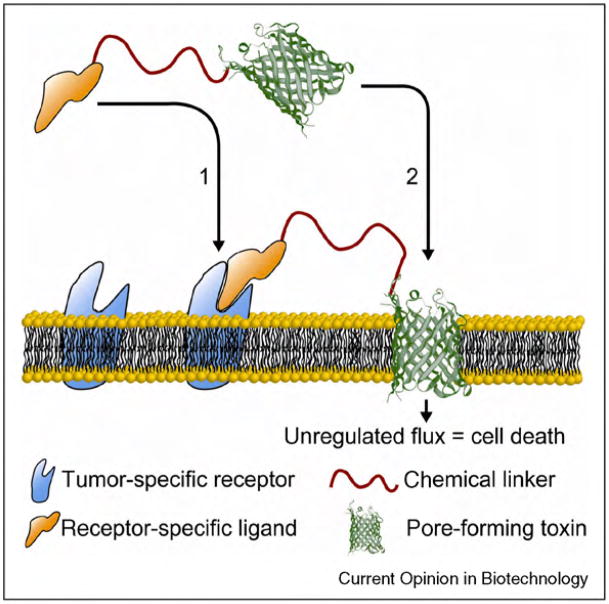
Figure 2.
Cartoon illustrating a simple approach employed for targeted cytolysis of cancer cells that uses biological pores. A pore-forming peptide or protein is attached to a ligand that recognizes tumor specific receptor proteins. Once the ligand binds on the surface of a cancer cell, the pore-forming peptide or protein inserts into the membrane of the cell and forms a lytic pore.
In order to improve the specificity for targeting beyond the level that can be achieved with these fusion proteins, inactive pore-forming peptides or proteins with built-in ‘switches’ have been explored. These ‘pro-drug’ proteins are activated in response to a biological stimulus [4•,25,142]. Figure 3 depicts the concept of this method. For example, malignant cells often overexpress and secrete proteases and the activity of these enzymes can act as a trigger to activate inactive toxins in the vicinity of malignant cells. Using this approach, Panchal et al. demonstrated that modification of α-hemolysin pores by including a protease-activated trigger made it possible to induce pore formation specifically in those cells that expressed the tumor-specific protease cathepsin B (this protease has been implicated in tumor invasion and metastasis) [143•]. The α-hemolysin trigger in this case included a peptide extension that was positioned at the midpoint of the central sequence of α-hemolysin and inhibited pore formation; cleavage of this inhibitory peptide by cathepsin B restored the pore-forming activity. In a recent study, Denmeade and co-workers used a different bacterial pore-forming protein, aerolysin, and coupled an inactive precursor of this protein to a peptide that could only be cleaved by a protease from prostate cancer cells [142]. Cleavage of the attached peptide produced active aerolysin proteins, which formed pores in the membrane of cancer cells and led to cell lysis. These examples of modified biological pores with built-in ‘triggers’ illustrate their exciting potential for cancer treatment. The number of reports on the modification of biological nanopores with incorporated triggering systems is growing, and examples of these systems will be discussed briefly in the section ‘Using biological pores to engineer light-activated ion channels’. In addition to their role in cell malignancy, proteases may play a role in other diseases such as Alzheimer’s disease (AD) and rheumatoid arthritis. Protease activation of pore-forming proteins and peptides may, therefore, also be beneficial for treatment of diseases other than cancer [25,143•]. One potential obstacle of these approaches could, however, be the question of possible immune responses against pore-forming peptides and protein therapeutics.
Figure 3.

Basic concept of using a multimeric pore, with a built-in ‘trigger’ system to target and kill cancer cells. Monomeric peptides are attached to monoclonal antibodies that recognize tumor specific antigens on targeted cancer cells. Upon this binding, tumor specific proteases secreted by these cancer cells recognize and cleave the peptide extensions on the monomers that inhibited their assembly to pores. The resulting active peptides can form cytolytic membrane pores and kill cancer cells.
Biological pores for delivery of macromolecules into cells
Biological pores are attractive for mediating transport of various molecules such as therapeutic agents into cells [4•,144]. Crossing the plasma membrane is one of the major challenges for the delivery of many therapeutic molecules including polar or charged drugs, proteins, or nucleic acids into cells. Owing to their inherent capability to permeabilize the plasma membrane of cells, pore-forming peptides and proteins can facilitate delivery of therapeutics into the cytosol. One biological pore that has been applied for delivery of macromolecules is the antibiotic peptide gramicidin. Legendre et al. reported its application to develop a non-viral gene delivery system. In this case, employing a gramicidin–lipid–DNA complex made it possible to deliver a plasmid DNA to a variety of mammalian cells in culture including human lung cells [145]. Lee and co-workers have explored the application of another biological pore, listeriolysin O (LLO), from the pathogenic bacterium Listeria monocytogenes, as a delivery vehicle for macromolecules such as proteins into cells both in vitro and in vivo [40,96–98,146–148]. These studies exploited that a relatively high proton concentration (pH range of 4.9–6.7) triggers the membrane insertion and pore-forming activity of LLO. They demonstrated that encapsulation of LLO inside pH-sensitive liposomes, along with other molecules to be delivered, enabled the cytosolic release of cargo molecules such as antigens. For instance, internalization by macrophages rapidly released the encapsulated cargo molecule from the liposomes, first into endosomes and then into the cytosol. This entire process happened without measurably harming the cells. A recent study by the Lee group employed these LLO liposomes as an efficient vaccine delivery system. In this case, the liposomes carried a viral antigenic protein to generate protective antiviral immunity [40]. Targeted delivery of antigens for generating protective antiviral immunity has also been achieved with anthrax toxin [39,149,150]; Goletz et al. employed this toxin to deliver a portion of the human immunodeficiency virus-1 (HIV-1) envelope protein to the cytosol of living cells [149].
Biological pores for development of antimicrobial drugs
Biological pores, including those formed by antimicrobial peptides, act as the first line of defense against invading microbes in living organisms [4•,151]. As a result, these membrane pores can serve as agents to treat infections. An example is the pore-forming peptide nystatin which is used to treat fungal infections [152]. Conventional antibiotics usually exert their effects by disrupting metabolic pathways of bacteria by targeting bacterial enzymes or membrane proteins [153]. As these antibiotics came into wide-spread use, natural selection and induced bacterial mutations led to the development of bacterial resistance against many of these drugs [153]. In order to overcome the growing issue of microbial resistance to conventional antibiotics, the pharmaceutical industry has become interested in the development of antimicrobial peptides as human therapeutics [131]. Compared to conventional antibiotics, these peptides exert their effect by damaging the integrity of bacterial cell membranes. As a result of this non-specific mechanism of action, the risk of development of bacterial resistance against these antimicrobial peptides is minimized [131,153]. Several natural antimicrobial peptides and their analogs have been employed as topically or systemically administered antibiotics for treatment of various infections including infections of the skin (e.g. acne) and wounds (e.g. wounds from burns and surgery-related wounds), infected diabetic foot ulcers, implant-related infections, catheter infections, and infections as a result of dental procedures or complications [37,131,153–157]. Among the antimicrobial peptides applied in this context are magainin, protegrin, lactoferricin, and defensin [37,131,153,157–159]. Several excellent reviews have previously examined this topic [131,153,158].
Antimicrobial peptides can also serve as antiviral agents. Several studies have investigated the potential of antimicrobial peptides, such as gramicidin, melittin, magainin, and cecropin, as antiviral agents. These trials demonstrated the ability of antimicrobial peptides to limit the transmission of pathogens such as Neisseria gonorrhoeae, Chlamydia trachomatis, human immunodeficiency virus (HIV), and herpes simplex virus (HSV) [131,159–163] that are responsible for sexually transmitted diseases..
Applications of biological pores for sensing
Detecting single molecules is an enabling capability for fundamental science in fields such as biophysics and chemistry [16•] and a useful advancement for applied fields such as medicine, environmental pollution monitoring, and defense [16•,164–166]. Developing sensors that are capable of detecting individual molecules has, therefore, become an important field of research [16•,18,167]. In this context, biological pores and channels stand out as relatively simple components of single-molecule sensors [3••,167]. In this section, we discuss the reasons for their popularity as well as applications of biological nanopores as sensing platforms.
In nature, detection at the single-molecule level is routinely achieved through ligand-gated ion channel proteins that open in response to binding of individual ligands [3••,168••]. Binding of these ligands, which can include simple ions (such as Ca2+) or neurotransmitters (such as acetylcholine), to the receptor site of the channel protein causes a transient conformational change that can lead to a physiologically significant, and measurable, change in ion permeability across the membrane [2]. A critical feature of this signaling mechanism is the strong molecular amplification that it entails; binding of a single ligand molecule typically leads to the passage of 104–107 ions through the membrane, often resulting in significant changes of the transmembrane potential [169]. Not surprisingly, these channel proteins are particularly appealing for sensing applications since the protein itself possesses not only a recognition element but also the signal transduction and amplification components. In addition, sensing platforms based on transmembrane channels offer high sensitivity, often require no labeling, and are relatively economical owing to the electrical nature of the resulting signal.
Several studies have exploited natural ligand-gated ion channels, [170,171•] such as the glutamate receptor [172,173] and nicotinic acetylcholine receptor, [174–177] as sensing elements. The intrinsic ligand recognition modality of these channels makes the resulting sensors very selective for specific ligands. Ion channel-based sensors are not, however, limited to ligand-gated ion channels. Many studies have explored other types of membrane pores – including those that lack a gating mechanism, such as gramicidin pores – for development of sensing platforms [178–180]. Here, we will briefly introduce the most common sensing mechanisms that are used in such platforms and review representative examples of these platforms.
Principal sensing mechanisms based on biological pores
Sensing by biological nanopores has been achieved through a number of approaches including single channel recordings, [181] impedance measurements [92,180,182, 183,184•,185–188], and resistive-pulse sensing [189,190••]. Table 4 provides a summary of the approaches that are most commonly employed.
Table 4.
Principal mechanisms of sensing by biological nanopores
| Mechanism of sensing | Examples |
|---|---|
| Resistive-pulse sensing; the translocation of an analyte molecule through a nanopore results in a partial pore blockage and a detectable change in ionic current passing through the pore | Translocation of a polynucleotide through a pore results in a detectable change in the ionic current [16•,29•]. |
| Change in the single channel conductance (induced by changes of residues of the pore itself or by changes of the environment of the pore) | Surface charge of a membrane surrounding a pore affects the conductance of the pore [23•,33•,191••]. Activation of a pore by light induces a chemical change, which affects conductance [192]. |
| Pore blockage (induced by binding of a molecule to the pore) | Binding of a protein (e.g. streptavidin) to a biotin-labeled pore limits the access of ions to the pore [193•]. |
| Pore opening | A physical or chemical stimulus (such as ligand-binding or light) leads to pore opening [194••]. |
| Change in the kinetics of pore formation | Binding of a protein (e.g. avidin) to ligand-labeled lipids in a membrane affects the kinetics (e.g. lifetime) of pore formation [195]. Changes in mechanical properties of the bilayer affect the lifetime of self-assembled pores [196]. |
| Disrupting pore assembly (in case of multimeric pores) | Binding of a protein (e.g. carbonic anhydrase) to ligand-presenting pore-forming peptides (e.g. sulfonamide-labeled alamethicin) disrupts their assembly to a pore [24]. |
Detection of small ions and organic molecules by biological pores
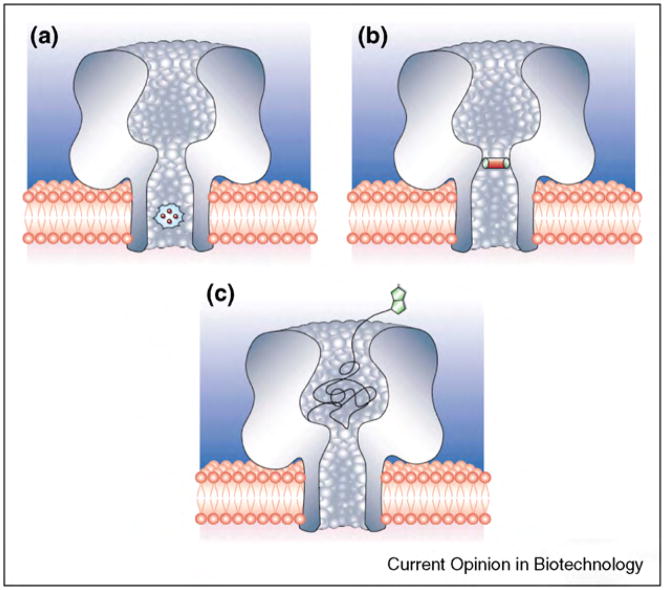
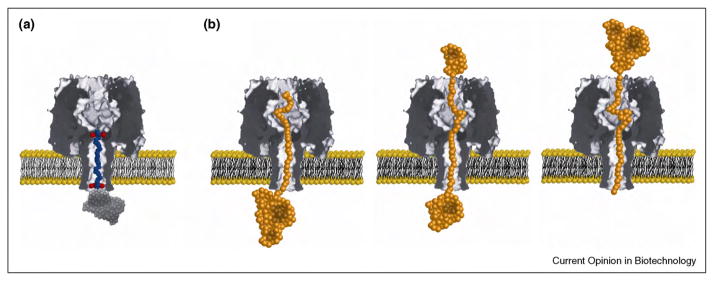
While natural pore-based sensory systems typically employ ion channel proteins that respond to physical stimuli such as binding of a ligand, approaches in nano-biotechnology make it possible to extend the repertoire of biological pores to porin proteins and pore-forming peptides. In particular the pores that are not gated (usually porins), enable an additional type of sensing compared to natural systems. This type of sensing is called resistive-pulse sensing and is illustrated in Figure 4. In resistive-pulse sensing, the passage of single analyte molecules through a nanopore results in partial blockage of the pore and, hence, a measurable change in the ionic current through the pore when a constant transmembrane potential difference is applied [190••]. This detection technique is attractive since it provides high sensitivity and can characterize single molecules with regard to the volume of the translocated molecule (based on the amplitude of the resistive current pulse) [197] and the concentration of the analyte in solution (based on the frequency of pore-blockage events) [198,199]. Another interesting parameter is the translocation time, which represents the time it takes for the analyte to pass through the detection zone of the nanopore. Determining these three parameters can make it possible to distinguish different molecules in a mixture on the basis of characteristic pore-blocking events. Despite these compelling attributes, the passage of most molecules and analytes through nanopores is so rapid that blockage events are often not completely time resolved [200•]. This issue has been addressed successfully in some cases by chemical or genetic modification of biological pores. In these cases, the pores harbored analyte-binding sites either near or in the pore as shown in Figure 4. For instance, α-hemolysin pores, which are the most commonly used biological pores for resistive-pulse sensing, can be modified by at least three approaches, as depicted in Figure 5: (i) genetic engineering to place desirable amino acids inside the β-barrel domain (Figure 5a), (ii) placement of ring-shaped molecular adaptors, such as cyclodextrins, inside the β-barrel (Figure 5b), and (iii) covalent attachment of a ligand-terminated poly (ethylene glycol) (PEG) polymer chain into the lumen of the pore (Figure 5c).
Figure 4.
Schematic drawing illustrating the principle of resistive-pulse sensing of analytes (green sphere) with α-hemolysin pores. The pores are engineered to contain an artificial binding site for the analyte in their lumen. In the presence of a transmembrane potential, binding of an analyte molecule results in a partial blockage of the pore; this modulation can be detected by the fluctuations in the ionic current passing through the pore. Figure reprinted from reference [3••] with permission.
Figure 5.
Schematic illustration of the three main approaches to engineer α-hemolysin pores for sensing. (a) Genetic modification of the pore makes it possible to position desirable amino acid residues inside the lumen of the pore. (b) Placement of ring-shaped molecular adaptors such as cyclodextrins, inside its lumen. (c) Covalent attachment of a ligand-terminated PEG polymer into the lumen of the pore. Figure adapted from reference [3••] with permission.
Using genetically engineered α-hemolysin pores, Bayley’s group developed sensors for the detection of ionic species and organic molecules in solution [16•,19,165,201,202]. In one of these studies, an α-hemolysin pore that contained a binding site for divalent metal ions (one of the seven subunits of α-hemolysin was a mutant with four histidine residues) detected metal ions including Zn2+ and Co2+ ions at nanomolar concentrations [19,167]. Characteristic signals produced by the binding of various metal ions made it possible to distinguish between different ions in a mixture of two or more ions. In another study, a genetically modified α-hemolysin pore with a ring-shaped arrangement of aromatic residues in its lumen was able to detect and distinguish 2,4,6-trinitrotoluene (TNT) from other nitroaromatics [165]. The same group reported the application of genetically engineered α-hemolysin pores for detection of phosphate anions [201] and nitrogen mustards that are relevant in chemical warfare [202].
The second modification, that is incorporation of non-covalent molecular adaptors such as cyclodextrins into the β-barrel of α-hemolysin pores, afforded the detection of organic molecules; these ring-shaped molecular adaptors fit inside the α-hemolysin pore and reduce its conductance while presenting a binding site for a variety of organic analytes, including therapeutic drugs [164]. Therefore, binding of an analyte to the adapter resulted in an additional and detectable decrease of the ionic current through the pore. For instance, Gu et al. employed cyclodextrins as adapters and demonstrated the ability of these modified pores to detect adamantane derivatives and therapeutic drugs in solution [164]. Searching for additional versatility with regard to molecular adapters for α-hemolysin pores, Braha and co-workers examined the effect of incorporating several cyclic peptides within the lumen of the pore [203]. Some of these peptides reduced the channel conductance upon partitioning into the pore; moreover, these positively charged peptides were able to act as binding sites for various small poly-anions.
The third modification, that is the attachment of ligand-terminated PEG into α-hemolysin pores, made it possible to detect protein–ligand interactions. These studies will be discussed in the section of protein-binding interactions below.
Intriguing work by Bezrukov and co-workers demonstrated the capability of resistive-pulse sensing to investigate interactions between various antibiotics and the bacterial outer membrane porin F (OmpF) [204] at a single-molecule level [205•]. On the basis of time-resolved interactions of single antibiotic molecules with OmpF pores, these authors concluded that ampicillin along with several other penicillins and cephalosporins strongly interacts with the residues of the constriction zone of OmpF pores and hypothesized that ‘in analogy to substrate-specific channels that evolved to bind certain metabolite molecules, antibiotics have evolved to be channel-specific’ [205•].
Using protein pores to probe the translocation and structure of polymers, polynucleotides, and polypeptides
Transport of polymers across membranes is essential to life. For instance, inside of living mammalian cells, nascent polypeptides and proteins are transported routinely across the membranes of the endoplasmic reticulum, [206,207] mitochondria [208,209], and choloroplast [210]. Other polypeptides cross membranes during infection, such as anthrax lethal factor and edema factors that are transported across cellular membranes through a pore formed by protective antigen PA7 of the anthrax toxin [211,212•,213] [214–216]. Moreover, protein pores are commonly involved in transport of polynucleotides across membranes in phage infection [217], bacterial conjugation [217], and uptake of polynucleotides in organs [218]. Considering the importance of the transport of biological polymers across membranes inside living cells, a thorough understanding of the mechanisms that govern this process may accelerate gene therapy and clinical applications of biologics [218,219]. Electrophoretic phenomena due to transmembrane potentials in vivo can drive the translocation of some biological polymers through protein pores [220,221]. Consequently, systematic in vitro studies that employ protein pores are well suited to investigate the transport of biological polymers across membranes as well as the properties of complex, long-chain polymers in aqueous solutions and in confined environments. Resistive-pulse sensing enables estimation of the hydrodynamic diameter of elongated polymer chains (assuming most polymers are longer than the length of the protein pore) from the magnitude of current blockages and estimation of the length of polymers from the translocation time [29•,222,223••,224••,225]. Resistive-pulse sensing can characterize an ensemble of polymer molecules rapidly because each current blockage is due to a single, contiguous polymer translocating through the pore and because individual blockages can be resolved at frequencies larger than 100 Hz. Here, we highlight some of the investigations that exploited resistive-pulse sensing for probing the size and transport of synthetic polymers, polynucleotides, and polypeptides.
Nanopore-based sensing of polymers
The first experiments involving the translocation of uncharged polymers through protein pores in vitro exploited well-defined polymers to interrogate the lumen of three different protein pores. These studies, which were performed in 1992 by Krasilnikov et al., analyzed current blockages due to translocating PEG polymers of various molecular weights to determine the volume of a lumen of the α-hemolysin pore, a cytolysin pore from non-Vibrio cholerae, and a pore formed by the β-subunits of Vibrio cholerae [223••]. Since then, this concept has been used to interrogate the volume of the lumen of the mitochondrial protein Toc75 (a polypeptide transporter), [210] the anthrax porin PA7, [73] the colicin 1a channel, [226], and connexon pores [227].
The translocation characteristics of PEG through protein pores can also yield information about the properties of amino acid residues that are exposed on the walls of the lumen of the pore. Nestorovich et al. employed PEG polymers to investigate the ionization of the interior of OmpF porins as a function of pH [228]. By translocating PEG polymers, the authors determined that pH-dependent protonation and deprotonation of amino acid side chains in the lumen could reduce or increase the conductance (based on electrostatic interactions with ions) while the size of the pore was not affected. Furthermore, Movileanu et al. and Merzlyak et al. used reactions between translocating PEG polymers and cysteine residues that were selectively inserted within the interior of modified α-hemolysin pores to probe the geometry of the lumen [229•,230]. In this case, the formation of disulfide linkages with the PEG at various locations in the lumen caused current blockages of different magnitudes, thus permitting estimates of the location of constrictions within the α-hemolysin pore.
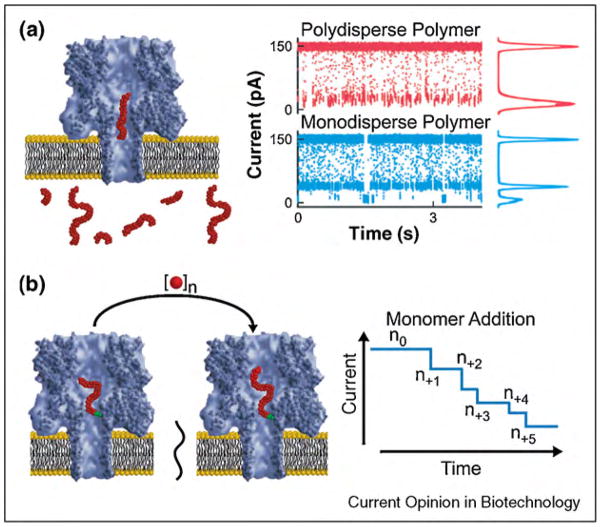
As opposed to using well-defined molecules to interrogate the properties of pores, well-defined pores make it possible to interrogate the size of polymers in solution (Figure 6a). In 1994, Bezrukov et al. employed pores formed from alamethicin peptides to determine the diffusion coefficient of PEG and the number of PEG monomers within the pore [231••]. More recently, the group of Kasianowicz used α-hemolysin pores to resolve current blockages due to the translocation of PEG. Remarkably, this study revealed the number of monomers within the polymer (Figure 6a) [232–236]. In another approach, Bayley and co-workers studied the kinetics of polymer elongation with a modified α-hemolysin pore (Figure 6b). In this case, chemical reactions led to the addition of monomers to a polymer that was linked covalently to residues within the pore; as a result the conductance of the pore decreased with each monomer addition (Figure 6b) [237]. Several groups have since exploited the translocation of polymers through protein pores to explore the energetic cost of confining the polymers in molecular-scale volumes [238–245]. Recently, complementary analytical models describing the kinetics of polymer transport through pores and the energy requirements of this process have made considerable progress [245,246,247,248,249•,250–254]. Without doubt, the combination of analytical models and computational approaches with nanopore recordings of polymer translocation will further increase the insight that can be gained from these experiments.
Figure 6.
Resistive-pulse sensing through protein pores (here α-hemolysin) makes it possible to determine the size of polymers in solution as well as to monitor the kinetics of polymer chain elongation. (a) Polymers of different molecular weight translocating through a protein pore result in transient current blockages of different magnitude. Figure adapted form reference [222] with permission. (b) Polymers that are linked covalently to the interior of a protein pore can be used to observe chemical reactions that lead to the addition of individual monomers; each added monomer decreases the current through the pore.
Nanopore-based sensing of polynucleotides
Motivated by the ultimate vision of ultrafast nanopore-based sequencing, the most studied biopolymers in biological nanopores are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). The Deamer group and the Church group proposed independently that strands of DNA or RNA could be driven electrophoretically through a small pore, and the resulting current fluctuations might enable real-time sequencing of polynucleotides [29•,224••,255]. Using α-hemolysin, Kasianowicz et al. later demonstrated that, as predicted, single-stranded DNA (ssDNA) and RNA could be detected as they passed through a pore [224••]. In a step toward sequencing, Akeson et al. and Meller et al. were able to distinguish different homopolymers of poly-DNA and poly-RNA (i.e. poly-A from poly-C) as well as the transition from poly-A to poly-C RNA within a diblock polymer [256,257•]. Using another approach, Szabo et al. and Rostovtseva et al. explored the translocation of double-stranded DNA (dsDNA) through a mitochondrial porin, the so-called voltage-dependent anion channel (VDAC) [258,259], and across bilayers containing ion channels from bacillus subtilus [258]. The size of these ion channels and their voltage-gated character, however, made them impractical for sequencing applications. While these early studies proved that long strands of DNA and RNA could be driven linearly through biological pores, they also revealed several challenges for achieving rapid DNA sequencing by resistive-pulse sensing.
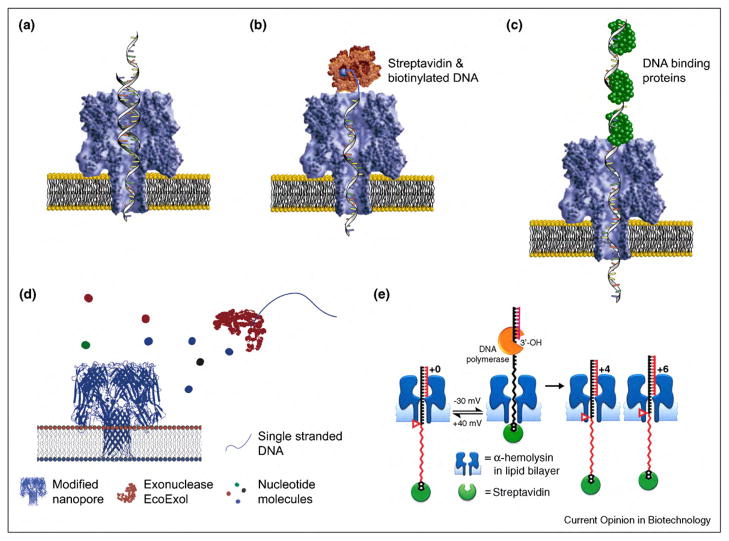
One challenge is spatial resolution; the ability to distinguish individual nucleotides within the lumen of the pore requires that the ionic current through the pore is influenced predominantly by one nucleotide at a time. The lumen of α-hemolysin cannot achieve this resolution because 10–15 nucleotides of DNA span the length of the pore [260]. To complicate matters, the convergence of electric field lines from the bulk solution to the entrance of a nanopore (the so-called convergence resistance) results in several nucleotides affecting the magnitude of the current blockade even in an infinitely short pore [249•,261–263]. Consequently, Bayley’s group and Schmidt’s group identified two specific regions within the lumen of α-hemolysin that could distinguish individual nucleotides within a single-stranded oligonucleotide sequence that was immobilized within the lumen. Figure 7b shows how the investigators took advantage of biotinylated ssDNA and streptavidin to immobilize single-stranded DNA. In these studies, individual bases at the position of each nucleotide were changed within the immobilized single-stranded DNA and the magnitude of the current blockages was measured. As a result, two locations within the β-barrel of the α-hemolysin pore emerged that generated different current blockages for each nucleotide [236,264,265•,266•]. Knowledge of these two zones with specificity toward the nucleotides may prove useful for further attempts conferring specificity for different nucleotide bases to α-hemolysin pores. In a recent, exciting development, the Gundlach group explored an alternative to α-hemolysin pores. These authors engineered the mycobacterium smegmatis porin A protein, named MspA, specifically for increased stability and capture of polynucleotides [30•]. These genetically optimized MspA pores are promising for sensing and sequencing applications owing to the short length (~1 nm) and small diameter (~1 nm) of their sensing zone [30•]. Further modifications of MspA may render this pore more responsive to individual nucleotides than the larger α-hemolysin pore. Guo’s group recently explored the pore of the phi29 motor protein from a bacteriophage for the detection of double-stranded DNA [267]. Owing to the ability to pass double-stranded DNA, this pore may have unique applications in micro-electromechanical sensing, gene delivery, and DNA sequencing [267].
Figure 7.
Overview of techniques that are being explored for sequencing single-stranded DNA or RNA with protein pores. (a) A segment of double-stranded DNA temporarily stops the translocation of long single-stranded DNA segments. This technique may be employed for de novo sequencing by hybridization [29•,268]. (b) Streptavidin bound to single-stranded, biotinylated DNA can immobilize the DNA fragment in the pore, permitting a sufficiently long residence time for identification of individual base mutations [236,264,265•,266•]. (c) Proteins bound to DNA can slow the translocation of single-stranded DNA through pores facilitating identification of nuleotides [269]. (d) The pore α-hemolysin with a cyclodextrin adapter can be employed to distinguish between different nucleosides based on the magnitude of current blockages. In combination with an exonuclease to digest single-stranded DNA, this approach might allow sequencing [270••]. Figure adapted form reference [270••] with permission. (e) Single-stranded DNA that is attached to a biotinylated PEG-polymer on one side of the pore and to a complimentary DNA segment on the other side can be trapped within α-hemolysin pores. The activity of DNA polymerase adds individual nucleotides which increases the conductance of the pore because the PEG polymer chain, which has a smaller diameter than DNA, occupies more of the α-hemolysin pore after the addition of each nucleotide [271•]. Figure adapted form reference [271•] with permission.
Another challenge for nanopore-based sequencing of polynucleotides is to achieve sufficient temporal resolution of the current recordings. Individual nucleotides within DNA and RNA polymers typically pass through protein pores at a rate of 0.5–1 nucleotides μs−1 (at a potential difference of ~120 mV across the pore); according to Kasianowicz et al., resolving single nucleotides requires reduction of translocation rates to ~1 nucleotide ms−1 [224••,257•,272]. As a result, reducing the temperature, reducing the applied voltage, or increasing viscosity to slow the electrophoretic drift velocity of polynucleotides through the pore has all been explored[224••,257•,273]. These techniques, however, decrease the steady-state flux of charge carriers (ions) through the pore, and hence, the magnitude of the current blockages. As a result, the capability to resolve amplitude differences between different nucleotides is compromised. One promising solution is illustrated in Figure 7c. It entails the use of proteins that bind single-stranded DNA; these interactions prohibit translocation of the single-stranded DNA until the proteins unbind and reduce the rate of translocation [269]. Other promising techniques include the use of organic salts that interact with DNA [274,275] and nanoparticles that partially obstruct the entrance to a pore [275]. These nanoparticles made it possible to decrease the translocation speed of single-stranded DNA through α-hemolysin pores by a factor of 10–100 [275]. Nevertheless, even with prolonged translocation times, single nucleotide resolution from a continuously translocating single-stranded DNA remains elusive to date.
Yet another challenge for nanopore-based sequencing that also entails temporal resolution, stems from the random motion of the polynucleotide and the potential for non-specific interactions within the pore. Both effects can result in translocation times that differ by two orders of magnitude for two identical molecules. As a result, the number of nucleotides passing through the pore can be uncertain [29•,256,257•,260,272]. On the other hand, the yet to be realized nanopore-based sequencing technique would ideally be able to count nucleotides and be able to distinguish between different nucleotides. The two recent approaches illustrated in Figure 7d and e achieved both single nucleotide resolution and, effectively, single nucleotide counting. Ghadiri’s group detected the addition of individual nucleotides to single-stranded DNA by DNA polymerase activity; this approach permitted sequencing of a segment of nine nucleotides [271•]. In this work, the authors threaded a complex of streptavidin–PEG–ssDNA through the lumen of α-hemolysin pores and trapped the single-stranded DNA within the pore via binding of a DNA primer sequence to the single-stranded DNA in the streptavidin–PEG–ssDNA complex (Figure 7e). Sequencing long strands of DNA with this method has not been demonstrated and will likely require further modifications. In the second approach, Bayley’s group attached a molecular adapter within the lumen of α-hemolysin. This modification enabled detection and identification of free nucleoside monophosphates (including 5′-methylcytosine, which is useful for studying methylation patterns and in the context of epigenetics) [270••,276]. The authors demonstrated that an exonuclease in a solution containing single-stranded DNA resulted in five species of 5′-mono-phasphates (G, A, T, C, and 5′-methylcytosine) and that all of the species could be distinguished while passing through the lumen of the modified α-hemolysin pore (Figure 7d) [270••]. Achieving nucleotide sequencing with this method will, however, require manipulation of the exonuclease such that each released monophosphate is forced to enter the α-hemolysin pore [270••]. In addition, the processivity of the enzyme may become a concern in the sense that the enzyme would have to act on the same single-stranded DNA continuously to enable sequencing long strands of DNA [270••].
An alternative strategy for sequencing with protein pores circumvents most of the challenges mentioned thus far; this approach entails the concept of de novo sequencing by hybridization [29•,277]. Sequencing by hybridization takes advantage of nucleotide fragments with a known sequence (the so-called probe sequence) to determine the sequence of a single-stranded DNA. The method requires determining the location of the probe on the single-stranded DNA (i.e. the sites of double-stranded DNA). Owing to the small pore diameter of α-hemolysin, only single-stranded DNA can translocate through the pore, and consequently, segments of DNA that are double-stranded stop the translocation of the entire DNA segment temporarily (Figure 7a) [268]. In an electric field, the electrophoretic force on the double-stranded DNA fragment can ‘unzip’ the double-stranded DNA segment, permitting translocation of the single-stranded DNA to continue [268]. Thus, if a biological pore makes it possible to determine the location of the hybridized fragments, it might be possible to sequence long fragments of DNA with this strategy. Similar to this method, Akeson’s group demonstrated that mismatches between individual base pairs within a hairpin turn of single-stranded DNA (resulting in a segment of double-stranded DNA) can be identified [278,279]. Additional variations of sequencing by hybridization include linking single-stranded DNA covalently to an α-hemolysin pore [71,280] or to a gramicidin peptide [281] combined with detecting the binding of complimentary DNA sequences. These approaches enabled detection of complimentary sequences of 10–23 base pairs in length [71,280,281]. While sequencing by hybridization may emerge as a practical method of sequencing oligonucleotides with protein pores, it is currently limited to relatively short-read lengths, relatively slow speed of sequencing, and difficulty in identifying the location of the hybridized segments on the DNA [29•].
Despite these significant challenges, nanopore-based sequencing remains compelling owing to the prospects of: (i) inexpensive sample preparation [29•,282], (ii) small sample requirement of only ~108 copies of DNA (a number that can be obtained without amplification) [29•,283], (iii) potentially long-read lengths of several kilobases [29•,222,282], and (iv) rapid sequencing speed. As a result, alternative protein pores are being explored as potential sensing elements, and novel strategies are being applied to identify individual nucleotides [30•,270••]. In addition, recent efforts to sequence single-stranded DNA with nanopores fabricated in synthetic substrates such as silicon nitride have been undertaken; [284••,285,286] these efforts are the subject of several excellent recent reviews [16•,29•,222,282,287–289].
Nanopore-based sensing of polypeptides
The transport of polypeptides is more complex than transport of uniform polymers and polynucleotides owing to the large variety of amino acid side chains, which can present positively charged, negatively charged, neutral, or hydrophobic residues [216]. To resolve the effect of these residues on the translocation of polypeptides through protein pores, several groups have performed systematic investigations with pores and polypeptides that contain segments of these residues. Bayley’s group, for instance, investigated the interaction of cationic polypeptides with the β-barrel of the α-hemolysin pore. In these experiments, increasing voltage and decreasing peptide length prolonged the duration of the interaction between the lumen of the pore and the peptide [290]. Recently, the Movileanu group determined that surface charges in the lumen of α-hemolysin pores can result in electrostatic traps that lower the free energy of translocation of cationic peptides [291•]. In addition, the same group demonstrated that attachment of a short polypeptide with positively charged residues to a large protein can selectively capture the protein at the entrance of a modified α-hemolysin pore with negatively charged residues in its lumen (Figure 8a) [292].
Figure 8.
Cartoon illustrating the concept of electrostatic traps to capture proteins in biological pores as well as the investigation of unfolding of a protein during translocation. (a) Negatively charged residues in the lumen of α-hemolysin pores can capture polypeptides that are positively charged. This effect can be used for selective capture of a large protein at the entrance of a pore [292]. (b) Cartoon illustrating the concept of unfolding of a protein at the entrance of a pore before translocation through the pore. Refolding of the protein on the other side of the pore may complete the process.
With regard to the translocation of natural peptides, the Collier group recently determined that the pore PA7 requires a phenylalanine-enriched region to facilitate the translocation of anthrax lethal factor across membranes. The authors proposed that these phenylalanine residues interact with the hydrophobic regions of anthrax lethal factor in order to denature the protein in a chaperon-like manner while facilitating the translocation of anthrax lethal factor through PA7 [211,212•]. Figure 8b illustrates how unfolding of a protein may permit its translocation through a pore [293•]. In support of this hypothesis, Lee and co-workers demonstrated that the histidine-containing protein Hpr from Escherichia coli unfolds as it translocates through α-hemolysin and aerolysin pores. They also showed that single amino acid mutations can dramatically change the magnitude and the duration of the resulting current blockage. These results emphasize the importance of interactions between the amino acids within the pore and the transmembrane protein [294].
Several groups have explored the potential of protein pores to determine the structure and, possibly, the primary sequence of polypeptides in a manner analogous to the attempts toward polynucleotide sequencing. For instance, the Lee group demonstrated that different α-helical structures of peptides can be distinguished with α-hemolysin and aerolysin pores. These authors distinguished between polypeptides with (gly-pro-gly) repeats that differed in the formation of single, double, or collagen-like triple helices [295•,296]. In an attempt to probe protein folding with biological nanopores, Auvray’s group examined the translocation of partially folded and unfolded maltoporin-binding protein through α-hemolysin pores in the presence of a chemical denaturant [293•]. Completely denatured maltoporin-binding protein was able to translocate through α-hemolysin [293•]. Goodrich et al. observed similar effects by showing that polypeptides with a β-hairpin structure resulted in longer current blockades than polypeptides without the hairpin. These results suggest that unfolding of a protein may be the rate-limiting step for translocation through certain pores (Figure 8b) [297]. For pores with internal diameters below the diameter of translocating proteins, a range of intramolecular interactions must be overcome in order to unfold proteins selectively at, or in, a biological pore to achieve translocation. A thorough understanding of these interactions and the resulting conformations may reveal insights in protein folding and could prove useful for the intracellular delivery of biologics for clinical applications.
Using biological nanopores to detect or monitor protein-binding interactions
A large number of cellular functions such as cell adhesion, cell signaling, or the action of therapeutic drugs depend on molecular binding interactions such as ligand–receptor interactions [182,184•,298,299]. Understanding these interactions is, therefore, important both for fundamental science and for the design of therapeutically effective modulators. Biological pores provide an excellent platform to study molecular binding interactions at a single-molecule level [86,182,183,184•,300,301]. Here, we highlight several different approaches that have been developed to apply pore-forming peptides and proteins for detection of molecular binding interactions.
One of the early approaches reported by Cornell et al. employed gramicidin derivatives [302] for detection of molecular binding interactions of antibodies with antigens [168••]. The sensor comprised a supported lipid bilayer that was tethered on a gold electrode and contained tethered gramicidin monomers in one leaflet and free monomers of gramicidin derivatives (gramicidin monomers with a biotin molecule attached) in the other leaflet (Figure 9a). A biotinylated antigen-binding fragment (Fab) of an antibody was attached to the biotinylated gramicidin monomer via streptavidin. The presence of a protein analyte deterred dimerization of free and tethered gramicidin monomers and led to a measurable decrease in bilayer conductance at picomolar concentrations of the protein analyte. Figure 9a depicts the concept of this sensor. While this method was applicable for a range of receptor types, including antibodies and nucleotides, it relied on switching of a population of ion channels to detect the binding event; it was not a single-molecule technique. Other approaches have demonstrated that single channel recordings can report molecular binding events. For instance, binding of proteins to lipids in a membrane typically affects the structure of the lipid bilayer and leads to changes in the kinetics of pore formation by biological pores such as gramicidin [195,303]. Using this effect, Hirano et al. detected antibody binding to antigen-decorated lipids (Figure 9b) [195]. Another study by Bennekou and co-workers monitored calcium-dependent binding of the peripheral membrane protein annexin [83,304] to lipid membranes; in this case, the resulting changes in the lifetime and single channel conductance of gramicidin pores provided the signal [303].
Figure 9.

Concept of detection of protein–ligand-binding interactions using the antibiotic peptide gramicidin A. (a) A lipid bilayer tethered on a gold electrode contains tethered gramicidin monomers in one leaflet of the bilayer and free monomers with attached antigen-binding fragments (Fab) of antibodies in the other leaflet of the bilayer. In the absence of an analyte, dimerization of gramicidin monomers in the two leaflets leads to the formation of gramicidin pores across the bilayer and to an increase in the ionic conductivity of the membrane. Binding of analyte to the antibodies on the gramicidin monomers crosslinks these Fab molecules and limits the diffusion of bound gramicidin monomers within the outer leaflet of the bilayer. This interaction slows the formation of channel dimers and lowers the electrical conductivity of the membrane. Figure reprinted from reference [168••] with permission. (b) Binding of a protein (in this case avidin) to lipids with covalently attached ligands (in this case biotin lipids) in a planar lipid membrane results in a local distortion of the bilayer structure leading to detectable changes in the kinetics of formation of gramicidin pores (i.e. changes in lifetime and opening frequency). Figure reprinted from reference [195] with permission.
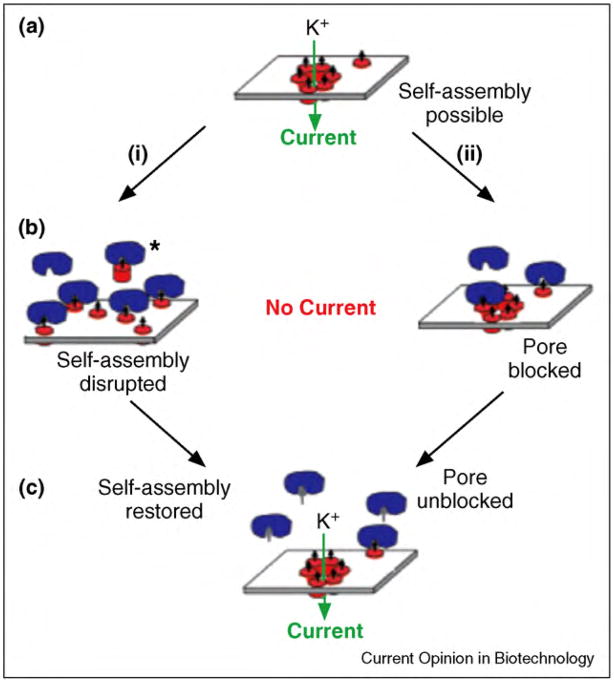
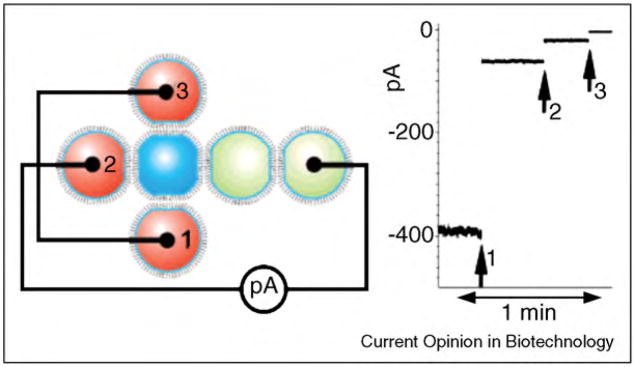
Ligand-decorated derivatives of antibiotic peptides such as gramicidin [302] and alamethicin have also provided platforms to detect [193•,305–309] or quantify [24] interactions between proteins and ligands. Antonenko and coworkers have explored the application of biotinylated gramicidin peptides for studying interactions between avidin or streptavidin with biotin. One of these studies examined the effect of monovalent and multivalent binding of streptavidin to biotinylated gramicidin on the dynamics of reversible channel formation [307–309]. The group of Sugiura reported the application of biotinylated derivatives of gramicidin A and alamethicin to detect and monitor streptavidin–biotin and antibody–biotin interactions [193•,305,306]. More recently, Mayer et al. applied an alamethicin derivative to detect and quantify protein–ligand interactions. In this case, binding of the protein carbonic anhydrase II to alamethicin monomers that carried sulfonamide ligands disrupted the self-assembly of alamethicin monomers. The resulting inhibition of pore formation in the lipid bilayer led to a detectable and quantifiable signal (Figure 10) [24].
Figure 10.
Basic concept of sensing protein–ligand interactions by disrupting the self-assembly of pore formers to a conducting pore. (a) Alamethicin monomers (red cylinders) with covalently attached ligands (small black arrows) self-assemble to form pores in a planar lipid bilayer as evident from single channel recordings. (b) Binding of a aprotein (here carbonic anhydrase II, shown in blue) to the ligand could have two consequences: (1) disruption of the pore, either by steric hindrance, or by removing the peptide from the bilayer, or (2) blockage of the mouth of the pore. In both cases, the binding interaction reduces the ionic current through the pore. (c) Addition of competitive ligand (small gray arrows) to the solution leads to binding of free ligand to the proteins and to the release of alamethicin peptides, which lead to pore formation. Figure reprinted from reference [24] with permission.
Bayley and co-workers pioneered the application α-hemolysin pores for detecting molecular binding interactions [241,310,311]. For example, an α-hemolysin pore with disaccharides tethered into its lumen made it possible to study the binding kinetics of lectins [311]. In another study, the attachment of a biotin-terminated polyethylene glycol (PEG) chain to the lumen of α-hemolysin pores allowed detection of nanomolar concentrations of streptavidin and antibodies; binding of these proteins to the biotin molecule at the end of the PEG chain resulted in detectable changes in ionic current through the pore [241,310]. More recently, the Bayley group applied α-hemolysin pores to probe reversible binding interactions between RNA and a viral motor protein for packaging RNA, the motor protein P4 from the bacteriophage φ8. Detection of these interactions was based on single channel current recordings; formation or dissociation of RNA–P4 complexes resulted in detectable changes in the amplitude and lifetime of current blockages. Such studies may open up a new means to examine the motor activity of RNA-processing or DNA-processing enzymes [20].
Using nanopore recordings to monitor enzyme activity
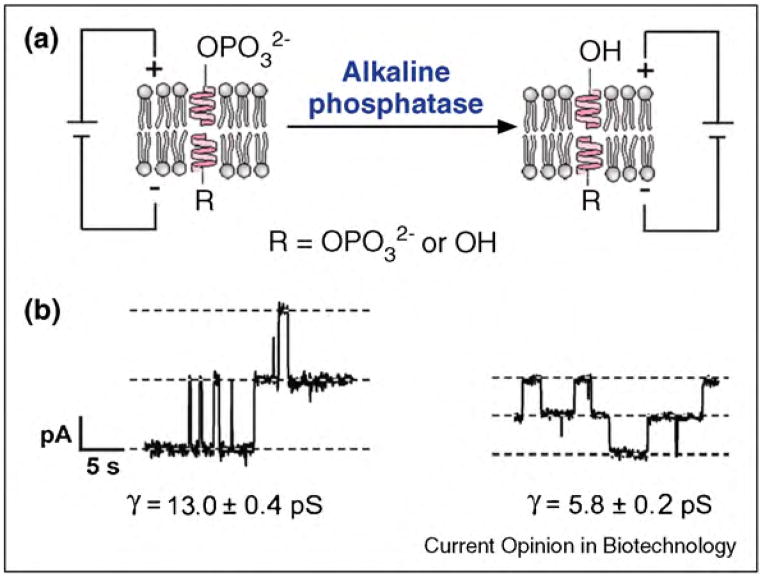
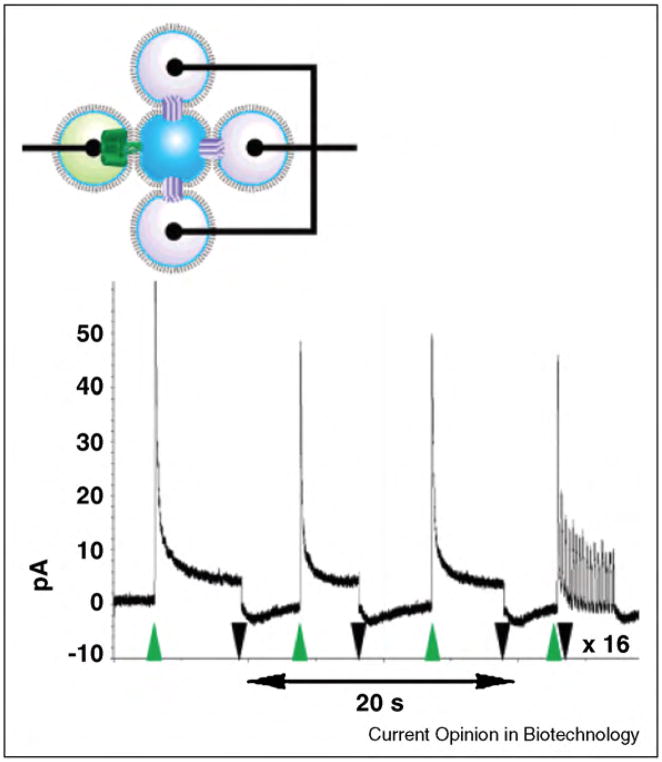
A relatively novel application of pore-forming peptides and proteins in nanobiotechnology is to detect and monitor the activity of enzymes in situ. Enzyme activities can be indicative of normal or abnormal cellular function and are often used for diagnostic purposes [22]. For instance, the activity of alkaline phosphatase in serum can indicate liver disease [22]. Sensitive methods to detect enzyme activity are, therefore, important for many clinical assays and for elucidating the role of these proteins in complex biochemical networks. While many of the current studies on enzymes require labeling the substrate (for instance with fluorescent tags), ion channel-based assays offer high sensitivity as well as rapid measurements and well-controlled experiments that require no substrate labeling. Within the past year, a few groups developed sensitive assays that employ ion channels to detect the activity of various enzymes [22,23•,112,271•]. In one of these assays, chemically modified gramicidin [312] peptides probed the enzymatic activity of picomolar concentrations of alkaline phosphatase and nanomolar concentrations of anthrax lethal factor in solution (Figure 11) [22]. In this assay, the enzyme-induced modification of individual gramicidin-derived substrates led to measurable changes in single-channel conductance through gramicidin pores.
Figure 11.
Concept of a gramicidin-based sensor for monitoring the enzymatic activity of alkaline phosphatase in situ. (a) Enzyme-catalyzed hydrolysis of a negatively charged phosphate group from gramicidin-phosphate to a gramicidin-derivative with a neutral alcohol group. (b) Corresponding current versus time recordings. At low ionic strength in the recording buffer, the single channel conductance, γ, through pores of the neutral gramicidin derivative is significantly smaller than the conductance through pores of the charged gramicidin-phosphate. This effect results from electrostatic accumulation of monovalent cations (which carry the charge through gramicidin pores) near the pore entrance of the negatively charged gramicidin-phosphate. Figure reprinted from reference [22] with permission.
In another study, Zhao et al. employed genetically modified α-hemolysin pores to detect the activity of a protease enzyme in solution [112]. This assay relied on detectable differences in channel blockages due to the translocation of enzyme substrates (a small peptide, in this case residues 10–20 of amyloid-β peptides) and products (peptide fragments) to detect protease activity. Figure 12 is a schematic illustration of this assay.
Figure 12.
Schematic illustration of an α-hemolysin-based platform for monitoring the cleavage of a peptide by a protease. Before addition of the protease, only substrate molecules (in this case, residues 10–20 of amyloid-β peptides) pass through the pore and produce characteristic blockage events as shown in pathway (a). Addition of the protease to the solution results in cleavage of the substrate peptides, producing smaller peptide fragments. Passage of the resulting fragments through the engineered α-hemolysin pore can be detected through blockage events that are significantly different in amplitude and length from those produced by the substrate peptide, as shown in pathway (b). Figure reprinted from reference [112] with permission.
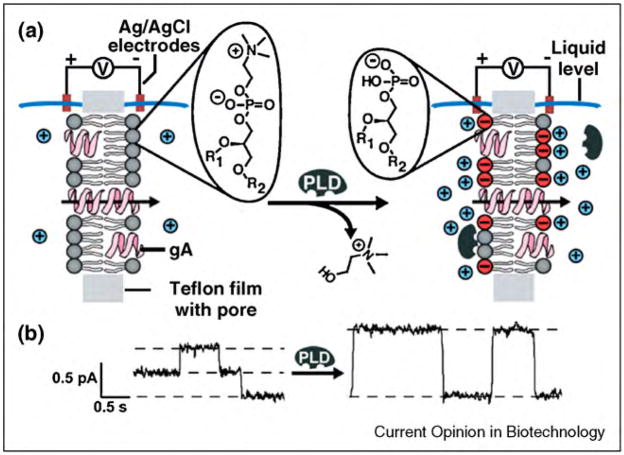
In a third approach, as illustrated in Figure 13, Majd et al. reported that native gramicidin peptides can be used to monitor the activity of the membrane-active enzymes phospholipase D (PLD) and phospholipase C (PLC) on lipid membranes [23•]. This assay took advantage of the dependence of the single-channel conductance of gramicidin channels on the presence of electrical charges at the lipid membrane that surrounded the gramicidin pores. Enzyme-induced modifications of electrical charges on lipid molecules were monitored within minutes, in situ, and on unmodified lipid substrates.
Figure 13.
Basic concept of monitoring the activity of a membrane-active enzyme, phospholipase D (PLD), on planar lipid bilayers. Enzyme activity is recorded by changes in single channel conductance of gramicidin pores. (a) As PLD hydrolyzes electrically neutral phosphatidylcholine (PC) lipids and produces negatively charged phosphatidic acid (PA) lipids, the electrostatic accumulation of cations close to the membrane surface leads to a significant increase in channel conductance of gramicidin pores. Negative charges are shown in red, and positive ions are shown in blue. (b) Corresponding current versus time recordings before and after addition of PLD. Figure adapted from reference [23•] with permission.
Using biological nanopores to monitor chemical reactions
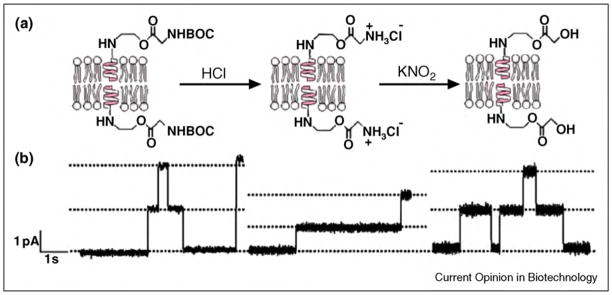
Examining chemical reactions at a single-molecule level is the ultimate goal of analytical techniques since it reveals details on the chemistry of molecules that are otherwise difficult to obtain (such as rapid intermediate steps in the reaction mechanism) [313•]. Most techniques that allow observation of chemical reactions at the single-molecule level rely on optical techniques, but methods based on electrical recordings coupled with nanopores are gaining momentum [314]. These nanopore-based approaches take advantage of chemical reactions within or near the entrance of a nanopore because they can affect the ionic current through the pore. As a result, the reaction can be monitored in situ and with a temporal resolution of 10–100 μs. Finkel-stein and co-workers pioneered the application of a biological pore, the diphtheria toxin (DT) pore, [315] to observe single-step chemical reactions [313•]. These investigators replaced several residues of the channel-forming domain of DT with cysteines and detected their reaction with sulfuhydryl-specific reagents by monitoring changes in conductance of the channel [313•]. Other biological pores that have been employed to monitor chemical reactions at the single-molecule level include gramicidin and α-hemolysin pores. Woolley and co-workers used a derivative of gramicidin that carried a carbamate group near its pore entrance to monitor the temperature dependence of the rate of transition between cis and trans isomers of carbamate. Transitions from the trans to the cis isomer resulted in positioning of a protonated, and hence positively charged, amino group on the C-terminal extension closer to the entrance of the gramicidin pore; this change resulted in a detectable decrease in the channel conductance [316]. More recently, the groups of Yang and Mayer have applied gramicidin peptides for detection of more complex chemical reactions than protonation [21,33•,317]. In one of these studies, a gramicidin derivative that carried a tert-butyloxycarbonyl-protected (Boc-protected) amine made it possible to monitor, in situ, the conversion of this group to a free amine and the subsequent diazotization/hydrodediazoniation of the amine to an alcohol group. The principle of detection relied on changes in the single channel conductance of the resulting gramicidin derivatives (Figure 14) [21].
Figure 14.
Monitoring chemical reactions on functional groups attached to gramicidin peptides through single channel recordings. (a) Illustration of the stepwise conversion of gramicidin carrying a Boc-protected glycine group (left) to gramicidin carrying a glycolic acid group (right) in the presence of different reagents. (b) Corresponding single channel recordings with characteristic conductance values of each derivative. Figure reprinted from reference [33•] with permission.
Finally, The Bayley group has made several contributions in this area on the basis of applications of α-hemolysin pores for detection of different chemical reactions [314,318•,319••]. For instance, an engineered α-hemolysin pore with thiol groups in its lumen enabled the observation of reversible formation of covalent bonds between thiols and organoarsenic (III) compounds in the solution [319••]. In another study, modified α-hemolysin pores with a cysteine residue inside their lumen monitored the formation and cleavage of a disulfide bond between the cysteine residue and 5,5′-dithiols (2-nitrobenzoic acid). This study demonstrated, for the first time, the observation of a short-lived intermediate in this reaction [314].
Using nanopores to probe the surface charge and the pH value near lipid membranes
For most natural antibiotic peptides, the kinetics of pore formation and the single-channel conductance depend on the properties of the target membrane. These properties include the surface charge or the elasticity of the membrane [320–322]. As a result, pore-forming peptides have the potential to serve as sensors for probing properties of their surrounding lipid environment. For instance, the surface charge of the lipid membrane surrounding a pore can influence its ionic conductance [23•,191••]. In this scenario, the presence of electrical charges on the lipid membrane leads to an accumulation of counterions near the pore entrance. This effect is particularly pronounced at low ionic strengths and, depending on the pore selectivity and the charge of accumulated ions, this effect can increase or decrease the channel conductance. This modulation, which follows predictions by the Gouy-Chapman theory, was experimentally demonstrated for the first time by Lauger and co-workers in 1979 [191••]. These authors investigated the effect of membrane surface charge on the conductance of gramicidin channels and demonstrated that at low ionic strength, the conductance of gramicidin pores embedded in a negatively charged membrane was significantly larger than the conductance of gramicidin pores embedded in a membrane composed of neutral lipids [191••]. Since then, this effect has been further investigated with a number of other pores and channels including gramicidin pores [33•,235,323], alamethicin pores [234,321,324], and cecropin pores [234,321,324]. For instance, several reports employed this local electrostatic effect on the conductance through nanopores to detect the surface charge of lipid membranes [235,323]. In one of these studies, Kell et al. probed the surface charge of cell-attached membrane patches in living cells by monitoring the single channel conductance of a cardiac inward-rectifier channel that was present in these patches [324].
More recently, the sensitivity of gramicidin pores to the electrostatic accumulation or repulsion of ions near its entrance has been employed to detect changes in pH near the membrane or even to detect individual chemical reactions and processes [22,23•,24,33•,317,325]. In order to probe the local pH near the membrane surface, Borisenko et al. employed a pH-sensitive derivative of gramicidin. The single channel conductance of gramicidin pores in this system reflected the degree of protonation of the chemical group appended to the peptide and, hence, reported the local pH near the membrane [325].
Biological nanopores for sensing mechanical properties of membranes
Biological membranes are complex mixtures of a wide variety of lipids, small non-polar molecules, an assortment of proteins, and a host of other lipophilic constituents [326]. Changes in the membrane composition and, therefore, in the physical properties of the bilayer, can regulate or alter the function or conformation of proteins embedded in the bilayer [327,328]. This interplay between the membrane and membrane proteins reflects a relationship between the free-energy difference between protein conformations and the energy required to deform the lipid bilayer in order to accommodate this change in protein conformation [328,329•].
Physical parameters of the bilayer that influence protein function include specific interactions between certain lipid molecules and membrane proteins as well as non-specific interactions that govern protein–bilayer interactions. These non-specific properties include influences from membrane thickness, viscosity, intrinsic curvature, elastic moduli, as well as compression and bending moduli of the bilayer [327,329•,330–332]. Figure 15 illustrates examples of conformational distortions caused, in this example, by a hydrophobic mismatch between lipids and an embedded protein. Changes in one or several of these intrinsic physical parameters of the bilayer can lead to significant changes in protein conformation, distribution, or function. The free-energy difference between two conformations of a typical membrane protein can amount to ±6 kcal mol−1 [329•], demonstrating the significant effects that bilayers can exert on protein conformation.
Figure 15.
Hydrophobic mismatch between a membrane and an embedded transmembrane protein or peptide. (a) Cartoon of a protein that has a transmembrane segment of length (h) that is shorter than the distance (d) across the hydrophobic core of the lipid bilayer, and the resulting compression of the bilayer. (b) The transmembrane segment of the protein has the same length as the hydrophobic core of the lipid bilayer. (c) The hydrophobic segment of the protein is longer than the membrane can accommodate without generating the energetic expense associated with stretching and bending forces.
The groups of Andersen and Koeppe introduced the use of gramicidin pores to study the underlying physical effects that determine this non-specific mechanism of protein regulation. They showed that gramicidin S can act as a molecular force probe to study various physical parameters of membranes [328,329•]. Knowledge inferred from these studies could be correlated with an understanding of the effects of membrane composition on proteins embedded in, or bound to, a lipid bilayer [196,329•]. For instance, throughout a wide variety of lipid compositions and acyl-chain lengths, the structure and conductance properties of gramicidin show little change, while the open channel lifetime and the frequency of pore formation are responsive to the lipid environment [333]. This energetic coupling between readily detectable physical parameters of gramicidin (i.e. channel-opening probability and channel lifetime) and differences in the membrane allows for in situ measurements of these parameters. Several excellent reviews on molecular force probes have been published previously [196,329•].
Using biological pores to engineer light-activated ion channels
Optical control of ion channels offers tremendous potential in a range of fields including non-invasive stimulation and control of biological cells as well as sensing technology and nanofluidic circuits [246,334]. Considering the key role of ion channels in physiological processes such as neuronal signaling, the ability to modulate the conductance of these proteins via light is appealing for remote and non-invasive control. For instance, an intriguing study by the groups of Kramer and Trauner recently demonstrated the potential of this approach to control neuronal firing in rat hippocampal neurons by the use of light-activated ion channels (Figure 16) [34••,335].
Figure 16.
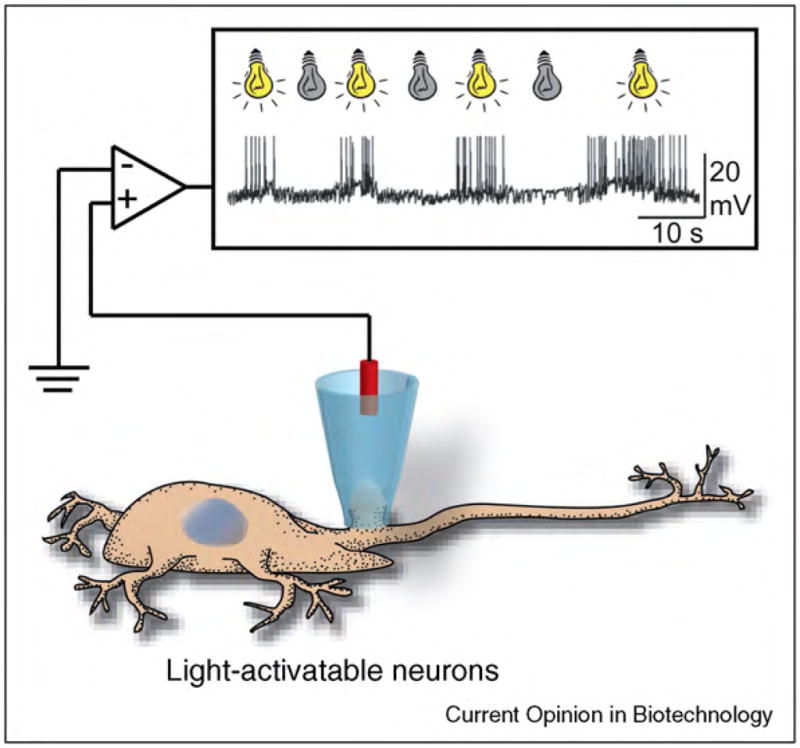
Cartoon illustrating the concept of remote control of neuronal firing by light. Expression of a light-activated ion channel (in this case, a modified potassium channel) in rat hippocampal neurons made these neurons sensitive to light as confirmed by current clamp recordings. Exposure to light with a wavelength of 500 nm resulted in spontaneous action potentials, while exposure to light with a wavelength of 380 nm silenced these action potentials. The inset shows a current versus time trace as adapted from reference [34••] with permission.
Light-controlled flow of ions through pores can be achieved via several distinct methods [336,337]. One approach to generate light-gated ion channels entails covalent conjugation of a pore-blocking moiety via photo-isomerizable linkers to the entrance or the exit of a channel [34••,35,338–340]. Upon exposure to specific wavelengths of light, the linkers undergo a change in effective length or spatial orientation, leading to blockage or reversal of blockage of the channel. In these systems, ions flow through the channel when the linkers are locked into a conformation that forces the blocking molecule away from the entrance or exit of the pore. Figure 17 illustrates an example of such a photo-gated channel as reported by the groups of Trauner and Kramer [34••,35,339]. In this example, a photoisomerizable azo-benzene group connected a positively charged quaternary amine group to the exit of a potassium channel. Exposure of the channel to ultraviolet light (380 nm) favored the cis-conformation of the azobenzene group, resulting in the motion of the quaternary amine blocking group away from the exit of the pore. By contrast, exposure of the channel to visible light (500 nm) induced isomerization of the azobenzene group to the trans-conformation. This reaction forced the quaternary amine blocking group into close proximity to the exit of the channel and blocked the channel conductance. These investigators employed light-activated potassium channels for remote control of neuronal firing [34••]. Expression of this modified potassium channel in rat hippocampal neurons made these neurons sensitive to light. Current clamp recordings confirmed that action potentials in these neurons could be silenced or evoked by simply exposing these cells to light with a wavelength of 380 or 500 nm. Figure 16 illustrates this intriguing concept.
Fig. 17.
Example of a light-gated potassium ion channel generated by covalent attachment of a pore-blocking group via a photoisomerizable linker to the exit of the channel protein. A covalently linked, positively charged group moved either close to, or away from, the exit of the channel depending on the light-induced configuration of the linker. Figure adapted from reference [34••] with permission.
A second strategy to engineer optical gating of ion channels is to disrupt the interactions that inherently hold the channel protein in a closed or open configuration. Feringa et al. demonstrated an example of such a light-gated channel through modification of a mechanosensitive ion channel from E. coli, MscL [194••]. Under physiologic conditions, the hydrophobic core of this channel is tightly closed and it opens only in response to high osmotic pressure in cells. This triggered conformational change leads to a pore with a diameter of ~3 nm. Engineering a polar residue into the hydrophobic lumen of the channel results in spontaneous opening of the pore. Feringa et al. manipulated MscL channels by attaching a photo-responsive spiropyran molecule to the interior of the pore (Figure 18a). When exposed to visible light (>460 nm), the spiropyran group remained in a non-polar conformation and the channel remained closed. Upon exposure to ultraviolet light (366 nm), the equilibrium of the spiropyran group shifted to the zwitterionic merocyanine state. The formation of this polar merocyanine species within the hydrophobic lumen of MscL destabilized the pore, resulting in an increased frequency of channel-opening events (Figure 18b). Exposure of the channel to visible light restored the non-polar spiropyran group and reduced the frequency of channel openings significantly. The authors demonstrated that this photoswitch-able gating mechanism was reversible over many cycles.
Figure 18.
Example of a photo-gated, MscL ion channel generated by incorporation of a photo-activatable spiropyran group into the lumen of the pore. (a) Chemical structure and reversible photo-induced conversion of a non-polar spiropyran molecule to a polar merocyanine conformational state. (b) Current versus time trace of a modified MscL channel carrying a covalently attached spiropyran group inside its lumen. The frequency of channel openings decreased significantly upon exposure of these channels to visible light compared to exposure to ultraviolet light. (c) Results of a leakage assay demonstrating the release profile of fluorescent calcein molecules from proteoliposomes that contained modified MscL pores when exposed to (■) ultraviolet, or (□) visible light. Figure adapted from reference [194••] with permission.
Owing to the large diameter of the MscL pore, a potentially interesting application for such a photo-gated ion channel would be light-controlled transport of drugs and proteins through MscL pores embedded within liposomal drug delivery vessels. Figure 18c shows that proteoliposomes comprising spiropyran-modified MscL pores released relatively little cargo (in this case, the membrane-impermeable fluorescent dye calcein) upon exposure to visible light compared to fast release kinetics upon exposure of the same proteoliposomes to ultraviolet light.
In addition to these two approaches, several light-modulated channels have been developed on the basis of derivatization of the antibiotic peptide gramicidin A. One strategy entailed covalent attachment of the N-termini of two gramicidin monomers via a diazobenzene linkage (Figure 19) [336]. The trans-conformation of the diazobenzene linker was dominant upon exposure to visible light. This conformation forced the two monomeric units of the gramicidin dimers apart and discouraged channel formation. By contrast, upon exposure to ultraviolet light, the diazobenzene linker predominantly existed in the cis-conformation, resulting in stable and open transmembrane ion channels.
Figure 19.
Concept of a light-gated gramicidin channel with a diazobenzene linker that was used to control the alignment of two gramicidin monomers within a membrane. Figure reprinted from reference [336] with permission.
Additional approaches to manipulate the conductance of gramicidin pores by light consist of: (i) light-induced changes of the dipole moment within the pore lumen; [337] (ii) controlling the distance of a covalently attached channel-blocking group from the entrance of the pore [338]; or (iii) changing the charge of functional groups presented near the opening of the pore [192]. Two recent reviews highlighted further examples for the design and implementation of light-gated ion channels [246,334].
Applications of biological nanopores in nanoelectronics
Living cells have evolved various mechanisms to transmit and receive information; one of these mechanisms employs ionic currents across membranes. Electronic devices, by contrast, rely on electronic currents typically through inorganic materials. One of the arising challenges in the field of neuroengineering entails the transfer of information between these two forms of charge transfer at the interface between biological systems and electronic devices [341–344]. Biological nanopores constitute potentially compelling components to bridge this gap [344]. Specifically, peptide or protein pores could act as key mediators in the bidirectional conversion between electron and ionic currents; in that case they may accelerate the development of bio-nanoelectronics [344,345].
Several research fields would benefit from the development of a reliable interface between integrated circuits and biological systems. In particular, neuroprosthetics may be advanced by extending the functionality, bio-compatibility, and possibly lifetime of implantable devices [341,342,346]. In addition, bio-nanoelectronics has potential applications in biosensing [168••], drug delivery [38], construction of artificial cells [347], development of bio-inspired batteries [26••], neuroscience, and medicine [28•].
Biological nanopores as nanofluidic diodes
Devices that rectify current allow the flow of charge carriers (ions or electrons) in predominantly one direction. Depending on the applied potential difference, these devices are either in a conducting or a non-conducting state. In semiconductor electronic devices, the elementary component for almost all devices is a p–n junction. A p–n junction refers to the interface between an n-type semiconductor, in which the majority of charge carriers are electrons, and a p-type semiconductor, in which the majority of charge carriers are holes. At a p–n junction, holes and electrons diffuse down their concentration gradients. The movement of these charge carriers leaves behind fixed charges that generate an electric field at the junction opposing the diffusion gradient until equilibrium is reached. This movement of charge carriers results in a charged region termed depletion zone. To allow current flow, an electric field has to be applied that is greater than, and opposite in charge to, the electric field generated in the depletion zone (forward bias potential). A potential that reinforces this internal electric field (reverse bias potential) results in no current flow (Figure 20a) [348]. Diodes (and other p–n junction devices such as transistors and solar cells) are commonly used in electronic devices to regulate voltage, emit light (light emitting diodes), tune receivers and transmitters, rectify current, and construct many other electronic building blocks. Moreover, combinations of p–n junctions in series to form p–n–p or n–p–n junctions are essential in electronic circuits, especially for forming bipolar junction transistors (BJTs), which are important for switching and amplification processes as well as high-frequency electronic circuits [349].
Figure 20.
Current rectification in man-made electronic circuits, in biology and nanobiotechnology. (a) Semiconductor diode with a p–n junction that allows electrons to flow only in one direction. (b) Inward-rectifying potassium channel (Kir2.2) that conducts potassium ions most efficiently in one direction. The current versus voltage graph is from reference [48] with permission. (c) Two different derivatives of gramicidin (one carrying a positive charge at its C-terminus and the other carrying a negative charge at its C-terminus) form a pore across a membrane. This heterodimeric gramicidin pore acts as the smallest nanofluidic diode and rectifies current. The current versus voltage graph is from reference [350•] with permission.
Interestingly, man-made electronic circuits are not the only signal processing devices that use diodes; nature also employs rectifiers. Recently, MacKinnon and co-workers were able to determine the crystal structure of an inward-rectifying potassium channel (Kir2.2) (Figure 20b) [48]. Inward-rectifying potassium channels play a vital role in regulating the resting potential across the plasma membrane of excitable cells [274]. These ion channels contain a potassium (K+) selectivity filter on their extracellular side and multiple ion binding sites with higher affinity for divalent ions compared with monovalent ions, on their intracellular side. When the membrane of these cells is depolarized (intracellular space polarized positively compared to the extracellular solution), divalent cations (such as Mg2+) from the cytoplasmic side bind to the ion binding sites within the channel. This binding blocks K+ conduction. By contrast, hyperpolarizing the membrane (i.e. when the polarity of the intracellular space is negative), the divalent blocking ions are cleared from the pore, leading to an inward conduction of K+ ions [48]. Figure 20b illustrates this mechanism of rectification of these channels.
In the field of nanotechnology, a number of different abiological [351–354] and biological pores have been developed that exhibit rectification properties [109,350•,355–357]. These pores have been used for generation of membrane potentials, [26••] sensing of enzymatic reactions, [350•], and building basic bioelectrical circuits [357,358].
In order to bias ion flow in one preferred direction, that is in order to achieve rectification in nanopores, an asymmetric electrical potential distribution is required [359]. This requirement may be satisfied through either an asymmetric charge distribution along the wall of the nanopore, an asymmetric pore geometry, or a combination of the two [354,359]. Interestingly, such an asymmetry was achieved with the wild-type outer membrane protein F (OmpF) from E. coli bacteria [355]. Although this bacterial porin does not naturally exhibit a rectifying behavior, Alacaraz et al. demonstrated that its reconstitution into a planar lipid bilayer, which separated solutions of different pH values, led to current rectification. Increasing the pH difference between the two sides of the bilayer caused an increase in electrostatic attraction between anions and the acidic side of the channel (which was positively charged) and an increase in electrostatic attraction between cations and the basic side of the channel (which was negatively charged). Under a non-conducting potential, the ions were attracted outward from the channel leaving a region depleted of charge carriers. Reversing the potential (to the conducting potential) resulted in an opposite effect; both effects incurred rectifying behavior to OmpF channels. Miedema et al. extended the scope of this work by engineering OmpF pores with diode behavior even in bilayers that separated solutions with symmetric pH values [356]. These genetically modified OmpF porins presented altered net charges in the selectivity filters of the pore compared to wild-type pores. The authors proposed that these engineered OmpF porins created a depletion zone under reverse biased potentials (in the sense that application of a potential with one polarity may have generated a depletion zone of charge carriers, which resulted in a reduction of conductance through the pore, while application of the other polarity supported the flux of ions), reminiscent of a p–n junction. On the basis of this study, it may be possible to develop biological p–n–p or n–p–n pores. For this functionality, a third selectivity filter would be required in the engineered pores. This idea appears plausible as p–n–p and n–p–n behavior has been demonstrated in artificial nanopores in three-layered membranes [360].
In a step toward electrically functional assemblies of biological nanopores, the Bayley group recently constructed simple electric circuits based on diode-like pores that were reconstituted in droplet interface bilayer systems [181,361]. The lipid bilayers between the droplets in these networks contained engineered α-hemolysin pores. These mutated α-hemolysin pores contained seven positively charged arginine side chains that faced into the lumen of the pore (7R-αHL) [109]. By controlling the incorporation of the engineered α-hemolysin into lipid bilayers between specific droplets, the authors formed droplet networks that acted like half-wave and full-wave rectifier circuits. Figure 21 shows an example of a full-wave rectifier circuit and the corresponding droplet network with similar characteristics [109]. This work is inspiring since it not only involved the application of biological pores as rectifiers but also integrated these rectifiers into basic electric circuits [361].
Figure 21.
Comparison between the performance of a full-wave bridge rectifier based on semiconductor diodes and a full-wave rectifier based on engineered biological pores. (a) Circuit diagram of a bridge rectifier with four diodes to achieve full-wave rectification. (b) Illustration of a four-droplet network to form a bridge rectifier with the mutant α-hemolysin protein 7R-αHL. (c) Photograph of the system in (b). (d) Electric properties of the droplet network circuit: input 0.1 Hz triangular wave (top); output current observed from a bridge rectifier using semiconductor diodes (middle); output current from a bridge rectifier system based on a droplet interface bilayer network (bottom). Figure reprinted from reference [109] with permission.
Recently the groups of Yang and Mayer reported one of the smallest feasible ionic diodes by self-assembly of chemically modified gramicidin pores [350•]. These gramicidin-derivatives carried a permanent positive charge at one end of the pore and a permanent negative charge at the other end (Figure 20c). By incorporating these oppositely charged gramicidin-derivatives in each leaflet of the lipid bilayers, the resulting assemblies generated gramicidin pores with charge asymmetry. These heterodimeric gramicidin pores rectified current as demonstrated in Figure 20c. An extension of this work introduced a novel enzyme-catalyzed approach to trigger the formation of a gramicidin-based nanodiode in situ. By adding alkaline phosphatase to only one side of a lipid bilayer that contained homodimeric channels composed of gramicidin-phosphate, the enzyme cleaved off the negatively charged phosphate group only on one side of the pores. This one-sided enzyme activity generated charge asymmetry, and hence the enzyme activity triggered the rectification properties of the pore in situ [350•]. This study demonstrated the possibility of developing nano-devices that are based on biological nanopores and that respond with a time resolution below milliseconds to external stimuli. Such platforms hold potential for various biosensing applications, including sensing of enzyme activity or chemical reactions on individual molecules. Interestingly, the gramicidin pores that were employed in this study constitute probably the smallest possible engineered nanofluidic diodes. The internal diameter of gramicidin pores is ~0.4 nm, a value that approaches the size of monovalent cations without their hydration shell. The diameter of Cs+ ions, for instance, is ~0.34 nm [362].
Using biological nanopores for the development of bio-functionalized nanowires and feedback systems
In addition to developing rectifiers, recent experiments with biological pores include the combination of nano-wires and transistors with lipid bilayers that harbor transmembrane pores. For instance, Bernards et al. developed a microscopic organic transistor that was controlled by biological nanopores [363]. Placement of a lipid bilayer on the gate of these organic electrochemical transistors blocked the gating of the transistors, while incorporation of gramicidin pores into the bilayer restored the gating mechanism [363]. By changing the density of the incorporated pores, the gate currents varied by six orders of magnitude. The same organic electrochemical transistor configuration could be used to distinguish between monovalent and divalent ions by exploiting the valence-dependent permeability of gramicidin pores (divalent cations have a significantly reduced permeability through gramicidin pores compared to monovalent cations) [363,364].
Owing to currently existing limitations in reducing the dimensions of organic electrochemical transistors below the micron-scale, their application in the field of nanotechnology is limited [365]. One example employing nanoscale transistors was work by Misra et al., who coated silicon nanowires with lipid bilayers that contained biological nanopores [28•]. In this investigation, the ion channel-forming peptides gramicidin or alamethicin were used as shown in Figure 22a. In the absence of a bilayer, the surrounding pH had a measurable effect on the protonation state and conductance of the SiO2 nanowires. This effect could be eliminated when the nanowires were coated with a lipid bilayer. Not surprisingly, the sensitivity of the nanowires to pH was recovered by incorporating the pore formers gramicidin or alamethicin into the membrane. Figure 22b demonstrates that calcium ions could be sensed by blocking the gramicidin pores. Incorporation of voltage-gated alamethicin channels into the bilayer resulted in voltage-controlled opening of these pores. The functionality of these nanowire-based pH sensors could therefore be switched on and off by voltage control. These first studies illustrate that biological pores can not only be used as functional elements, but their function can even be controlled or modified by external stimuli [28•].
Figure 22.
Design and function of bilayer-coated nanowires. (a) Schematic illustration of bilayer-coated nanowires that are connected to microfabricated electrodes, which constitute the source (S) and drain (D). The insets of this figure show a cartoon of the bilayer membranes with embedded ion channel-forming peptides. (b) Graph illustrating the change of conductance of bilayer-coated nanowires as the pH is altered from 5 to 7. Red curve: nanowire device without a lipid bilayer coating. Blue curve: nanowire device with a lipid bilayer coating and incorporated gramicidin pores. Black curve: nanowire device with a lipid bilayer coating and incorporated gramicidin pores in the presence of channel-blocking calcium ions. Figure adapted from reference [28•] with permission.
Recently, Martinez et al. explored the use of biological nanopores for the development of doped nanowire electrodes [27]. Similar to previous reports, the formation of a supported lipid bilayer to coat the nanowires not only conferred biocompatibility to the electrodes, but also resulted in their electrical insulation. Charge transfer was then partially recovered by the incorporation of α-hemolysin pores in the membrane. This work reinforces that encapsulation of non-electronic devices within lipid bilayers that contain protein pores provides a means to modulate the electrochemical responses of these hybrid devices [27].
Sarles and Leo recently used biological pores to explore the potential for developing controllable, active biosystems [357]. These authors tailored the properties of ion transport through lipid bilayers by incorporation of protein channels and by using external feedback loops in droplet interface bilayer systems. Using voltage-gated alamethicin channels, the conductance of a bilayer membrane could be increased by two to three orders of magnitude when an electrical potential was applied to activate the channels. Figure 23 shows a unique development by these investigators, namely the formation of feedback controlled droplet interface bilayer networks with two different modalities to control the transport dynamics through the lipid bilayers: current tracking (Figure 23) and voltage control (not shown). Using this feedback network, the integral compensator computed a deviation of the measured current from the desired current and applied a corrective control voltage to reduce the error. The system was efficient for controlling transport of ions across the membrane at frequencies below 10 mHz. This feedback control mechanism is encouraging with regard to the development of ‘bio-nanosystems’ for a range of possible applications from controlled nanoreactors and bio-communication systems to various techniques of protein investigations that use optical, chemical, or mechanical stimuli [357].
Figure 23.
Block diagram of a droplet interface bilayer network with feedback current control. Figure adapted from reference [357] with permission.
Biological nanopores for development of bio-inspired batteries
One intriguing development of bio-nanoelectronics is engineering of bio-inspired mechanisms for providing electrical power based on rectifying biological pores in membranes. In a mechanism similar to excitable cells, the formation of an ionic gradient across ion-specific channels in a lipid bilayer can generate membrane potentials. In live cells, these transmembrane potentials power processes that are directly or indirectly driven by transmembrane voltages [1]. Using this principle, Bayley’s group recently developed a bio-inspired battery that employed α-hemolysin pores to generate a membrane potential across a lipid bilayer [26••]. The authors genetically modified α-hemolysin pores to render them anion-selective and incorporated these pores into droplet interface bilayers. A three-droplet network was formed in a linear arrangement with the anion selective α-hemolysin pores in the first droplet. The droplet in the middle contained a high concentration of NaCl relative to the first. Consequently, the interface between the first and middle droplet resulted in a preferred flux of Cl− ions (relative to Na+) to generate a membrane potential of 30 mV. The interface between the middle and third droplet contained a different type of mutated α-hemolysin pore that was used to demonstrate the generation of a transmembrane voltage by measurable fluctuations in current due to the binding and unbinding of β-cyclodextrin (βCD) in the lumen of the pore. The investigators extended their work to a droplet interface bilayer network of six droplets (Figure 24) in order to generate ionic currents up to 0.4 nA. As shown in Figure 24, the red droplets labeled 1, 2, and 3, all contained the anion selective α-hemolysin pore and the central blue droplet contained a relatively high concentration of NaCl. The last two green droplets contained wild-type α-hemolysin pores. This network was able to generate currents for extended periods as shown in the current versus time trace on the right. Removal of droplets from this network illustrated one possible strategy to control the voltage and hence the current sustained by such a bio-inspired battery [26••].
Figure 24.
Schematic illustration of an experimental setup used to form a bio-inspired battery and current versus time trace recorded from this setup. Arrows indicate removal of red droplets one at a time. Figure adapted from reference [26••] with permission.
Additional work by these investigators shows that the incorporation of biological pores in combination with a light-driven proton pump, bacteriorhodopsin, has the potential of using biological pores to generate current by a light-driven mechanism [26••]. Figure 25 shows the experimental setup developed by Holden et al. The three purple droplets attached to the central blue droplet contained bacteriorhodopsin proteins. The central droplet was attached to the green droplet, which contained wild-type α-hemolysin. Illumination of the network with a green laser caused a sharp increase in current, while blocking the laser light caused a rapid decay in current. During illumination, this network was able to generate 5 pA of current at steady state. Such a light-sensitive system suggests that it may be possible to develop bio-inspired and possibly biocompatible image acquisition techniques with biological proteins and pores [26••]. Another intriguing futuristic vision would be to generate electrical power from sunlight with such bio-inspired systems. These devices might find applications for portable systems with low power requirements.
Figure 25.
Diagram of a droplet interface bilayer network that can sense light and current versus time trace showing a large upward spike when laser was switched on (green upward arrows) followed by a rapid decay to a steady-state current of ~5 pA. When the laser was turned off (black downward arrows), the current temporarily reached negative values before returning to baseline. Figure adapted from reference [26••] with permission.
LaVan’s group investigated the theoretical aspects of generating power using ionic gradients created by selective ion flux through ion channel proteins [366]. By modeling protocells (cells with the minimal set of elements required to maintain function), these authors investigated the upper limits of energy output from such systems. Using a two-droplet system separated by a lipid bilayer (analogous to droplet interface bilayer systems), they demonstrated that these bio-inspired batteries could yield energy densities of 6.9 MJ × m−3 and that this configuration at maximum power density could reach an energy conversion efficiency of 10% [366].
These examples of recent advances in the application of biological nanopores in nanoelectronics illustrate the potential of biological circuits. Future refinements might make it possible to interface semiconductors with biological cells; for instance, the development of bidirectional information transfer between neurons and semiconductors has already been observed [367]. It remains to be seen if such devices will mature to the level required for implantation and long-term functional use for biomedical applications.
Conclusion
Like all functional proteins, biological pores, and in particular gated biological pores, are intriguing nanostructures. Unlike most proteins, however, the function of biological pores can be readily investigated and exploited on a single-molecule level [368]. Anyone who has witnessed current recordings from opening and closing events of single ion channel proteins live on a computer screen cannot help but watch in amazement. It is remarkable that the activity of one – just one – protein can be monitored as it switches its conformation between an open and a closed state. In the context of nanobiotechnology, the fascination with biological pores includes their capability to detect single molecules, to sequence short strands of DNA, to elicit light-triggered action potentials, to rectify current, or to target and kill cancer cells. Given the impressive range of applications of biological pores covered in this review and the promise of these approaches for nanomedicine, sensing, and nanoelectronics, the question arises: what are, at present, the limitations for realizing the full potential of biological nanopores in nanobiotechnology?
In the context of nanomedicine, one complication is the high molecular weight of pores or pore formers with targeting functionality. Unlike most successful therapeutic drugs, these constructs do not obey Lipinski’s rule of five [369]. Challenges that will have to be met for developing therapeutics based on biological pores include: (i) appropriate circulation half-life and stability in the human body, (ii) effective distribution to the target organs, (iii) release in active form at targeted tissue at doses that are effective and elicit minimal side effects, (iv) possible adverse immune reactions against these constructs, and, possibly, (v) high costs of production. From an optimistic point of view, however, the membrane-attack complex of the complement system in the innate immune response [11] is an example of a selective, triggered, and effective pore-based ‘nanokiller’ that is not hampered by the formation of resistances in cells targeted for destruction.
In the context of sensing, the limitations of biological nanopores are mostly based on the poor stability of either the pore itself or the lipid membrane that supports it. Living cells are designed to be adaptive and their proteins are, therefore, not engineered to ‘last forever’. Membrane proteins, including ion channel proteins, are typically among the most fragile of proteins, while porins and pore-forming peptides can have reasonable stability. This stability is one of the reasons why porins and poreforming peptides currently dominate the field of sensing with biological pores. Biological pores also evolved to function under physiologic conditions in an aqueous environment and at moderate temperatures. The harsh conditions encountered by real world sensors such as extreme temperatures, pH values, and solvent composition will further limit the stability of these delicate nanostructures. For technical applications, most biological pores will, therefore, have to be replaced frequently. This requirement evokes three associated challenges: (1) functional reconstitution of ion channel proteins into bilayer lipid membranes is still rather an art than a science; (2) the availability of purified, functional biological pores is limited to a select few proteins; (3) the cost of available proteins is typically extremely high. Another challenge for using sensors based on biological pores is the limited stability of the lipid bilayer that supports the pore. Non-physiological conditions such as temperature extremes, presence of non-aqueous solvents or detergents, and mechanical agitation can break lipid bilayers or generate noise in current recordings [111•]. Stabilizing membranes by reducing their size, [121] embedding [116,124] and linking them to a supporting hydrogel, [119•] or forming them between water droplets in oil [107••,108] are promising developments but it is difficult to imagine that any of these strategies will lead to lifetimes that are commonly achieved with non-biologic sensors. Owing to the limited lifetime of bilayer membranes, successful technical applications will likely require the capability to reform membranes on demand in an automated fashion, followed by rapid and automated reconstitution of functional pores. Even in this scenario, all components will need to remain stable for extended periods until their use as well as during exposure to real world samples.
Together these limitations of biological pores have led to attempts to fabricate pores in synthetic materials. These materials include polymers, glass, silicon, silicon nitride, silicon oxide, metals and ceramics, which share the advantage of long-term stability and robustness even under harsh conditions. The advances in this field over the past decade alone are fascinating. For instance, it is now possible to fabricate stable nanopores with diameters as small as one nanometer in ‘membranes’ of silicon nitride, which can taper to thicknesses below 10 nm. For the first time, these man-made pores approach the dimensions of biological pores. Such synthetic pores can even distinguish between different macromolecules at a single-molecule level [29•,283]. With very few exceptions, [370••] these synthetic pores, are however passive structures. This aspect is one of the crucial differences between pores in synthetic materials and biological pores; the full potential of pore-based sensing can, however, only be reached with pores that are active structures. Such active pores would ideally have the capability to change their function in response to stimuli such as ligand-binding or in response to changes in the surrounding membrane or the applied potential difference, to name a few. These bioinspired, responsive, synthetic pores may also replicate some of the attributes of ion channel proteins: exquisite specificity for certain analytes, close to million-fold signal amplification, reproducible dimensions of the pore lumen on an atomic scale, amenability to regulating and fine-tuning the function of the pore. Before nanotechnology will make it possible to fabricate active synthetic nanopores with this level of functionality, perhaps one interesting development will be hybrid systems in which the functional biological components will be reduced to the absolute minimum, while as much as possible of the structure will be synthetic. One might imagine a biological pore fit neatly into a pore in a synthetic substrate that is just big enough to accommodate the protein and a few lipid molecules to sustain its function. Before such hybrid biologic-synthetic pores become reality, currently existing synthetic pores will require improvements in order to make the transition from academic research laboratories to real-world sensing applications. Specific challenges for sensing with synthetic pores include: non-specific binding of biomolecules (in particular proteins) to the walls of the pores, limited reproducibility of fabrication on the subnanometer and even nanometer scale, electrical breakdown of extremely thin synthetic membranes that are required to support short pores, lack of control of the surface chemistry of the pore walls, bubble formation in the pore, and pore clogging (interestingly, this set of challenges is unique to synthetic pores, natural selection already solved these issues in biological pores). Despite this list of shortcomings, recent advances with synthetic pores, in particular in the context of DNA sequencing, are very encouraging. Owing to their robustness, synthetic pores may therefore be closer to real-world applications than sensors based on biological pores. At present, applications of biological pores remain limited to well-controlled laboratory environments. Probably the most important established application of biological pores is using ion channel proteins for sensing drug candidates or toxins that can modulate channel function, while the most aggressively pursued, and yet to be realized, application is ultra-fast sequencing of long DNA strands with α-hemolysin or MspA pores [29•].
In the context of nanoelectronics, most of the challenges discussed for applications of biological pores in sensing apply as well. The functional incorporation and availability of the appropriate biological pore, and its stability or renewal during use, are factors that will have to be addressed for real world applications. In addition, components of conventional electronics can already be fabricated at scales below 100 nm with the exquisite performance of semiconductor electronics, therefore, in order to compete, the demands on technical applications of pore-based electronics will be high. Initial applications of biological pores may therefore be focused on devices that can act as interfaces between ionic currents from cells and electronic currents in man-made circuits. Other possible niche applications may be circuits with ultra-small footprints, low power requirements, or altogether novel functionality. An inspiring recent example of the last category is the bio-inspired generation of ‘bio-batteries’ [26••], that is the generation of transmembrane potentials based on chemiosmotic gradients and mutant versions of α-hemolysin pores. If technically viable, hybrid nanobioelectronic devices could have tremendous potential, in particular, in emerging fields such as neural engineering, functional prosthetics, and implantable power sources.
Acknowledgments
This work was supported by the NIH (M.M., 1R01GM081705), by Thermo Fisher Inc. (S.M.), a NSF CAREER Award (M.M. award no.: 449088), a NSF EFRI BSBA (Y.N.B., award no: 0937323), and a fellowship from the Graduate Assistance in Areas of National Need, GAANN (E.C.Y.). This work was also supported by the UCSD Center for AIDS Research (J.Y., NIAID 5 P30 AI36214), a UC TSR&TP graduate research fellowship (M.X.M.), and a California Space Grant Consortium graduate fellowship (M.X.M.).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Aidley DJ, Stanfield PR. Ion Channels: Molecules in Action. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- 2.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4. New York: Garland Science; 2002. [Google Scholar]
- 3••.Bayley H, Cremer PS. Stochastic sensors inspired by biology. Nature. 2001;413:226–230. doi: 10.1038/35093038. This article provides an insight to the application of engineered biological pores for development of rapid and sensitive biosensors with a focus on the application of these pores in stochastic sensing. [DOI] [PubMed] [Google Scholar]
- 4•.Panchal RG, Smart ML, Bowser DN, Williams DA, Petrou S. Pore-forming proteins and their application in biotechnology. Curr Pharm Biotechnol. 2002;3:99–115. doi: 10.2174/1389201023378418. This excellent review introduces a number of channel proteins and pore-forming peptides that have been used in biotechnology. It also provides a brief overview of the applications of these pores in medicine and sensing. [DOI] [PubMed] [Google Scholar]
- 5.Estes DJ, Memarsadeghi S, Lundy SK, Marti F, Mikol DD, Fox DA, Mayer M. High-throughput profiling of ion channel activity in primary human lymphocytes. Anal Chem. 2008;80:3728–3735. doi: 10.1021/ac800164v. [DOI] [PubMed] [Google Scholar]
- 6.Astier Y, Bayley H, Howorka S. Protein components for nanodevices. Curr Opin Chem Biol. 2005;9:576–584. doi: 10.1016/j.cbpa.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Melo MN, Ferre R, Castanho M. Antimicrobial peptides: linking partition, activity and high membrane-bound concentrations. Nat Rev Microbiol. 2009;7:245–250. doi: 10.1038/nrmicro2095. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert RJC. Pore-forming toxins. Cell Mol Life Sci. 2002;59:832–844. doi: 10.1007/s00018-002-8471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Achouak W, Heulin T, Pages JM. Multiple facets of bacterial porins. FEMS Microbiol Lett. 2001;199:1–7. doi: 10.1111/j.1574-6968.2001.tb10642.x. [DOI] [PubMed] [Google Scholar]
- 10.Kruse E, Uehlein N, Kaldenhoff R. The aquaporins. Genome Biol. 2006;7:206. doi: 10.1186/gb-2006-7-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbas AK, Lichtman AH. Cellular and Molecular Immunology. 5. Philadelphia: Elsevier Saunders; 2005. [Google Scholar]
- 12.Hofemeister H, O’Hare P. Nuclear pore composition and gating in herpes simplex virus-infected cells. J Virol. 2008;82:8392–8399. doi: 10.1128/JVI.00951-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lashuel HA, Lansbury PT. Are amyloid diseases caused by protein aggregates that mimic bacterial pore-forming toxins? Q Rev Biophys. 2006;39:167–201. doi: 10.1017/S0033583506004422. [DOI] [PubMed] [Google Scholar]
- 14.Arispe N, Rojas E, Pollard HB. Alzheimer-disease amyloid beta-protein forms calcium channels in bilayer-membranes—blockade by tromethamine and aluminum. Proc Natl Acad Sci U S A. 1993;90:567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capone R, Quiroz FG, Prangkio P, Saluja I, Sauer AM, Bautista MR, Turner RS, Yang J, Mayer M. Amyloid-beta-induced ion flux in artificial lipid bilayers and neuronal cells: resolving a controversy. Neurotox Res. 2009;16:1–13. doi: 10.1007/s12640-009-9033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Howorka S, Siwy Z. Nanopore analytics: sensing of single molecules. Chem Soc Rev. 2009;38:2360–2384. doi: 10.1039/b813796j. This recent comprehensive review describes the use of protein pores and pores in synthetic, solid-state materials for sensing molecules that range from low molecular weight drugs to proteins and long-chain polymers. [DOI] [PubMed] [Google Scholar]
- 17.Gu LQ, Shim JW. Single molecule sensing by nanopores and nanopore devices. Analyst. 2010;135:431–443. doi: 10.1039/b907735a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayley H, Martin CR. Resistive-pulse sensing—from microbes to molecules. Chem Rev. 2000;100:2575–2594. doi: 10.1021/cr980099g. [DOI] [PubMed] [Google Scholar]
- 19.Braha O, Gu LQ, Zhou L, Lu XF, Cheley S, Bayley H. Simultaneous stochastic sensing of divalent metal ions. Nat Biotechnol. 2000;18:1005–1007. doi: 10.1038/79275. [DOI] [PubMed] [Google Scholar]
- 20.Astier Y, Kainov DE, Bayley H, Tuma R, Howorka S. Stochastic detection of motor protein-RNA complexes by single-channel current recording. Chemphyschem. 2007;8:2189–2194. doi: 10.1002/cphc.200700179. [DOI] [PubMed] [Google Scholar]
- 21.Blake S, Mayer T, Mayer M, Yang J. Monitoring chemical reactions by using ion-channel-forming peptides. ChemBioChem. 2006;7:433–435. doi: 10.1002/cbic.200500532. [DOI] [PubMed] [Google Scholar]
- 22.Macrae MX, Blake S, Jiang XY, Capone R, Estes DJ, Mayer M, Yang J. A semi-synthetic ion channel platform for detection of phosphatase and protease activity. ACS Nano. 2009;3:3567–3580. doi: 10.1021/nn901231h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Majd S, Yusko EC, MacBriar AD, Yang J, Mayer M. Gramicidin pores report the activity of membrane-active enzymes. J Am Chem Soc. 2009;131:16119–16126. doi: 10.1021/ja904072s. This paper demonstrated the first application of gramicidin pores for monitoring the enzymatic activity of membrane-active enzymes on lipid bilayers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer M, Semetey V, Gitlin I, Yang J, Whitesides GM. Using ion channel-forming peptides to quantify protein–ligand interactions. J Am Chem Soc. 2008;130:1453–1465. doi: 10.1021/ja077555f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panchal RG. Novel therapeutic strategies to selectively kill cancer cells. Biochem Pharmacol. 1998;55:247–252. doi: 10.1016/s0006-2952(97)00240-2. [DOI] [PubMed] [Google Scholar]
- 26••.Holden MA, Needham D, Bayley H. Functional bionetworks from nanoliter water droplets. J Am Chem Soc. 2007;129:8650–8655. doi: 10.1021/ja072292a. This article was the first to apply a biological pore, α-hemolysin, to generate power and build a bio-inspired ‘battery’. [DOI] [PubMed] [Google Scholar]
- 27.Martinez JA, Misra N, Wang YM, Stroeve P, Grigoropoulos CP, Noy A. Highly efficient biocompatible single silicon nanowire electrodes with functional biological pore channels. Nano Lett. 2009;9:1121–1126. doi: 10.1021/nl8036504. [DOI] [PubMed] [Google Scholar]
- 28•.Misra N, Martinez JA, Huang SCJ, Wang YM, Stroeve P, Grigoropoulos CP, Noy A. Bioelectronic silicon nanowire devices using functional membrane proteins. Proc Natl Acad Sci U S A. 2009;106:13780–13784. doi: 10.1073/pnas.0904850106. Silicon nanowires were coated with lipid bilayers, and pore-forming peptides were incorporated into the bilayers. Ion transport through the peptide pores enabled signal transduction between ionic and electronic signals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Branton D, Deamer DW, Marziali A, Bayley H, Benner SA, Butler T, Di Ventra M, Garaj S, Hibbs A, Huang XH, et al. The potential and challenges of nanopore sequencing. Nat Biotechnol. 2008;26:1146–1153. doi: 10.1038/nbt.1495. This excellent review outlines progress made in sequencing polynucleotides with protein pores and synthetic, solid-state pores; it also describes the challenges that need to be overcome, in order to achieve sequencing of polynucleotides with nanopores. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Butler TZ, Pavlenok M, Derrington IM, Niederweis M, Gundlach JH. Single-molecule DNA detection with an engineered MspA protein nanopore. Proc Natl Acad Sci U S A. 2008;105:20647–20652. doi: 10.1073/pnas.0807514106. A new protein pore for sensing the translocation of polynucleotides is described. This pore, MspA, has the potential to achieve high resolution in sequencing of polynucleotides as well as in general sensing applications owing to its small sensing zone and ability to accommodate genetically engineered modifications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boheim G. Statistical-analysis of alamethicin channels in black lipid-membranes. J Membr Biol. 1974;19:277–303. doi: 10.1007/BF01869983. [DOI] [PubMed] [Google Scholar]
- 32.Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 33•.Capone R, Blake S, Restrepo MR, Yang J, Mayer M. Designing nanosensors based on charged derivatives of gramicidin A. J Am Chem Soc. 2007;129:9737–9745. doi: 10.1021/ja0711819. This paper outlined the key parameters for design and development of sensing platforms based on charged derivatives of gramicidin peptides. [DOI] [PubMed] [Google Scholar]
- 34••.Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7:1381–1386. doi: 10.1038/nn1356. This article presented an intriguing application of light-activated ion channels; an engineered potassium channel that opened and closed in response to different wavelengths of light made it possible to control neuronal firing in cultured rat hippocampal neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kramer RH, Chambers JJ, Trauner D. Photochemical tools for remote control of ion channels in excitable cells. Nat Chem Biol. 2005;1:360–365. doi: 10.1038/nchembio750. [DOI] [PubMed] [Google Scholar]
- 36.Ganz T, Lehrer RI. Antibiotic peptides from higher eukaryotes: biology and applications. Mol Med Today. 1999;5:292–297. doi: 10.1016/s1357-4310(99)01490-2. [DOI] [PubMed] [Google Scholar]
- 37.Miyasaki KT, Lehrer RI. Beta-sheet antibiotic peptides as potential dental therapeutics. Int J Antimicrob Agents. 1998;9:269–280. doi: 10.1016/s0924-8579(98)00006-5. [DOI] [PubMed] [Google Scholar]
- 38.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 39.Goletz TJ, Klimpel KR, Leppla SH, Keith JM, Berzofsky JA. Delivery of antigens to the MHC class I pathway using bacterial toxins. Human Immunol. 1997;54:129–136. doi: 10.1016/s0198-8859(97)00081-5. [DOI] [PubMed] [Google Scholar]
- 40.Mandal M, Kawamura KS, Wherry EJ, Ahmed R, Lee KD. Cytosolic delivery of viral nucleoprotein by listeriolysin O-liposome induces enhanced specific cytotoxic T lymphocyte response and protective immunity. Mol Pharm. 2004;1:2–8. doi: 10.1021/mp034021m. [DOI] [PubMed] [Google Scholar]
- 41.Neher E, Sakmann B, Steinbach JH. Extracellular patch clamp—method for resolving currents through individual open channels in biological-membranes. Pfluegers Arch Eur J Physiol. 1978;375:219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- 42.Sakmann B, Neher E. Patch clamp techniques for studying ionic channels in excitable-membranes. Annu Rev Physiol. 1984;46:455–472. doi: 10.1146/annurev.ph.46.030184.002323. [DOI] [PubMed] [Google Scholar]
- 43.Agre P. Aquaporin water channels. Biosci Rep. 2004;24:127–163. doi: 10.1007/s10540-005-2577-2. [DOI] [PubMed] [Google Scholar]
- 44.Agre P. Aquaporin water channels (Nobel lecture) Angew Chem Int Ed. 2004;43:4278–4290. doi: 10.1002/anie.200460804. [DOI] [PubMed] [Google Scholar]
- 45.Agre P, Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett. 2003;555:72–78. doi: 10.1016/s0014-5793(03)01083-4. [DOI] [PubMed] [Google Scholar]
- 46.Doyle DA, Cabral JM, Pfuetzner RA, Kuo AL, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 47.Jiang YX, Lee A, Chen JY, Cadene M, Chait BT, MacKinnon R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 48.Tao X, Avalos JL, Chen JY, MacKinnon R. Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 angstrom resolution. Science. 2009;326:1668–1674. doi: 10.1126/science.1180310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bechinger B. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J Membr Biol. 1997;156:197–211. doi: 10.1007/s002329900201. [DOI] [PubMed] [Google Scholar]
- 50.Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 51.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of 3 homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 52.Choe S. Potassium channel structures. Nat Rev Neurosci. 2002;3:115–121. doi: 10.1038/nrn727. [DOI] [PubMed] [Google Scholar]
- 53.Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- 54.Roosild TP, Greenwald J, Vega M, Castronovo S, Riek R, Choe S. NMR structure of Mistic, a membrane-integrating protein for membrane protein expression. Science. 2005;307:1317–1321. doi: 10.1126/science.1106392. [DOI] [PubMed] [Google Scholar]
- 55.Roosild TP, Le KT, Choe S. Cytoplasmic gatekeepers of K+-channel flux: a structural perspective. Trends Biochem Sci. 2004;29:39–45. doi: 10.1016/j.tibs.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Zhou W, Qian Y, Kunjilwar K, Pfaffinger PJ, Choe S. Structural insights into the functional interaction of KChIP1 with Shal-type K+ channels. Neuron. 2004;41:573–586. doi: 10.1016/s0896-6273(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 57.Cheng XL, Ivanov I, Wang HL, Sine SM, McCammon JA. Molecular-dynamics simulations of ELIC-a prokaryotic homologue of the nicotinic acetylcholine receptor. Biophys J. 2009;96:4502–4513. doi: 10.1016/j.bpj.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sigworth FJ. Sodium channels in nerve apparently have 2 conductance states. Nature. 1977;270:265–267. doi: 10.1038/270265a0. [DOI] [PubMed] [Google Scholar]
- 59.Mutter M, Vuilleumier S. A chemical approach to protein design - template-assembled synthetic proteins (TASP) Angew Chem Int Ed Engl. 1989;28:535–554. [Google Scholar]
- 60.Oiki S, Danho W, Montal M. Channel protein engineering-synthetic 22-mer peptide from the primary structure of the voltage-sensitive sodium-channel forms ionic channels in lipid bilayers. Proc Natl Acad Sci U S A. 1988;85:2393–2397. doi: 10.1073/pnas.85.7.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Degrado WF, Wasserman ZR, Lear JD. Protein design, a minimalist approach. Science. 1989;243:622–628. doi: 10.1126/science.2464850. [DOI] [PubMed] [Google Scholar]
- 62.Lear JD, Wasserman ZR, Degrado WF. Synthetic amphiphilic peptide models for protein ion channels. Science. 1988;240:1177–1181. doi: 10.1126/science.2453923. [DOI] [PubMed] [Google Scholar]
- 63.Sisson AL, Shah MR, Bhosale S, Matile S. Synthetic ion channels and pores (2004–2005) Chem Soc Rev. 2006;35:1269–1286. doi: 10.1039/b512423a. [DOI] [PubMed] [Google Scholar]
- 64.Matile S, Som A, Sorde N. Recent synthetic ion channels and pores. Tetrahedron. 2004;60:6405–6435. [Google Scholar]
- 65.Sakai N, Brennan KC, Weiss LA, Matile S. Toward biomimetic ion channels formed by rigid-rod molecules: length-dependent ion-transport activity of substituted oligo(p-phenylene)s. J Am Chem Soc. 1997;119:8726–8727. [Google Scholar]
- 66.Akerfeldt KS, Kim RM, Camac D, Groves JT, Lear JD, Degrado WF. Tetraphilin—A 4-helix proton channel built on a tetraphenylporphyrin framework. J Am Chem Soc. 1992;114:9656–9657. [Google Scholar]
- 67.Akerfeldt KS, Lear JD, Wasserman ZR, Chung LA, Degrado WF. Synthetic peptides as models for ion channel proteins. Accounts Chem Res. 1993;26:191–197. [Google Scholar]
- 68.Derrington IM, Collins MD, Pavlenok M, Niederweis M, Gundlach JH. A novel DNA sensing technique using the nanopore MspA. 54th Annual Meeting of the Biophysical-Society; San Francisco, CA. 2010. [Google Scholar]
- 69.Tobkes N, Wallace BA, Bayley H. Secondary structure and assembly mechanism of an oligomeric channel protein. Biochemistry. 1985;24:1915–1920. doi: 10.1021/bi00329a017. [DOI] [PubMed] [Google Scholar]
- 70.Song LZ, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 71.Howorka S, Cheley S, Bayley H. Sequence-specific detection of individual DNA strands using engineered nanopores. Nat Biotechnol. 2001;19:636–639. doi: 10.1038/90236. [DOI] [PubMed] [Google Scholar]
- 72.Tsitrin Y, Morton CJ, El Bez C, Paumard P, Velluz MC, Adrian M, Dubochet J, Parker MW, Lanzavecchia S, van der Goot FG. Conversion of a transmembrane to a watersoluble protein complex by a single point mutation. Nat Struct Biol. 2002;9:729–733. doi: 10.1038/nsb839. [DOI] [PubMed] [Google Scholar]
- 73.Nablo BJ, Halverson KM, Robertson JWF, Nguyen TL, Panchal RG, Gussio R, Bavari S, Krasilnikov OV, Kasianowicz JJ. Sizing the Bacillus anthracis PA(63) channel with nonelectrolyte poly(ethylene glycols) Biophys J. 2008;95:1157–1164. doi: 10.1529/biophysj.107.121715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urry DW. Gramicidin-A transmembrane channel—proposed PI (L,D) helix. Proc Natl Acad Sci U S A. 1971;68:672–676. doi: 10.1073/pnas.68.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stankovic CJ, Heinemann SH, Delfino JM, Sigworth FJ, Schreiber SL. Transmembrane channels based on tartaric acid gramicidin-A hybrids. Science. 1989;244:813–817. doi: 10.1126/science.2471263. [DOI] [PubMed] [Google Scholar]
- 76•.Hladky SB, Haydon DA. Discreteness of conductance change in bimolecular lipid membranes in presence of certain antibiotics. Nature. 1970;225:451–453. doi: 10.1038/225451a0. First single-molecule experiment with a biomolecule, here gramicidin. [DOI] [PubMed] [Google Scholar]
- 77.Fox RO, Richards FM. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-Å resolution. Nature. 1982;300:325–330. doi: 10.1038/300325a0. [DOI] [PubMed] [Google Scholar]
- 78.Li C, Salditt T. Structure of magainin and alamethicin in model membranes studied by X-ray reflectivity. Biophys J. 2006;91:3285–3300. doi: 10.1529/biophysj.106.090118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vogel H. Comparison of the conformation and orientation of alamethicin and melittin in lipid-membranes. Biochemistry. 1987;26:4562–4572. doi: 10.1021/bi00388a060. [DOI] [PubMed] [Google Scholar]
- 80.Pott T, Paternostre M, Dufourc EJ. A comparative study of the action of melittin on sphingomyelin and phosphatidylcholine bilayers. Eur Biophys J Biophys. 1998;27:237–245. doi: 10.1007/s002490050130. [DOI] [PubMed] [Google Scholar]
- 81.Chen M, Khalid S, Sansom MSP, Bayley H. Outer membrane protein G. engineering a quiet pore for biosensing. Proc Natl Acad Sci U S A. 2008;105:6272–6277. doi: 10.1073/pnas.0711561105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tamm LK, McConnell HM. Supported phospholipid-bilayers. Biophys J. 1985;47:105–113. doi: 10.1016/S0006-3495(85)83882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Majd S, Mayer M. Hydrogel stamping of arrays of supported lipid bilayers with various lipid compositions for the screening of drug-membrane and protein-membrane interactions. Angew Chem Int Ed. 2005;44:6697–6700. doi: 10.1002/anie.200502189. [DOI] [PubMed] [Google Scholar]
- 84.Cremer PS, Boxer SG. Formation and spreading of lipid bilayers on planar glass supports. J Phys Chem B. 1999;103:2554–2559. [Google Scholar]
- 85.Groves JT, Ulman N, Cremer PS, Boxer SG. Substrate-membrane interactions: mechanisms for imposing patterns on a fluid bilayer membrane. Langmuir. 1998;14:3347–3350. [Google Scholar]
- 86.Terrettaz S, Mayer M, Vogel H. Highly electrically insulating tethered lipid bilayers for probing the function of ion channel proteins. Langmuir. 2003;19:5567–5569. [Google Scholar]
- 87••.Schmidt C, Mayer M, Vogel H. A chip-based biosensor for the functional analysis of single ion channels. Angew Chem Int Ed. 2000;39:3137–3140. doi: 10.1002/1521-3773(20000901)39:17<3137::aid-anie3137>3.0.co;2-d. First demonstration of the application of silicon microchips with micron size pores for single ion channel recordings. [DOI] [PubMed] [Google Scholar]
- 88.Majd S, Mayer M. Generating arrays with high content and minimal consumption of functional membrane proteins. J Am Chem Soc. 2008;130:16060–16064. doi: 10.1021/ja8055485. [DOI] [PubMed] [Google Scholar]
- 89.Ling T, Majd S, Mayer M, Guo LJ. Detection and quantification of lipid membrane binding on silica micro-tube resonator sensor - art. no. 68620B. In: Enderlein J, Gryczynski ZK, Erdmann R, editors. Proceedings of the Society of Photo-Optical Instrumentation Engineers (SPIE); San Jose, CA. 2008. pp. B8620–B8620. [Google Scholar]
- 90.Sackmann E. Supported membranes: scientific and practical applications. Science. 1996;271:43–48. doi: 10.1126/science.271.5245.43. [DOI] [PubMed] [Google Scholar]
- 91.Tanaka M, Sackmann E. Supported membranes as biofunctional interfaces and smart biosensor platforms. Physica Status Solidi a—Appl Mat Sci. 2006;203:3452–3462. [Google Scholar]
- 92.Koper I. Insulating tethered bilayer lipid membranes to study membrane proteins. Mol BioSys. 2007;3:651–657. doi: 10.1039/b707168j. [DOI] [PubMed] [Google Scholar]
- 93.White RJ, Zhang B, Daniel S, Tang JM, Ervin EN, Cremer PS, White HS. Ionic conductivity of the aqueous layer separating a lipid bilayer membrane and a glass support. Langmuir. 2006;22:10777–10783. doi: 10.1021/la061457a. [DOI] [PubMed] [Google Scholar]
- 94.Winterhalter M. Black lipid membranes. Curr Opin Colloid Interface Sci. 2000;5:250–255. [Google Scholar]
- 95.Castellana ET, Cremer PS. Solid supported lipid bilayers: from biophysical studies to sensor design. Surf Sci Rep. 2006;61:429–444. doi: 10.1016/j.surfrep.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee KD, Oh YK, Portnoy DA, Swanson JA. Delivery of macromolecules into cytosol using liposomes containing hemolysin from Listeria monocytogenes. J Biol Chem. 1996;271:7249–7252. [PubMed] [Google Scholar]
- 97.Mathew E, Hardee GE, Bennett CF, Lee KD. Cytosolic delivery of antisense oligonucleotides by listeriolysin O-containing liposomes. Gene Therapy. 2003;10:1105–1115. doi: 10.1038/sj.gt.3301966. [DOI] [PubMed] [Google Scholar]
- 98.Provoda CJ, Stier EM, Lee KD. Tumor cell killing enabled by listeriolysin O-liposome-mediated delivery of the protein toxin gelonin. J Biol Chem. 2003;278:35102–35108. doi: 10.1074/jbc.M305411200. [DOI] [PubMed] [Google Scholar]
- 99.Gong Y, Luo YM, Bong D. Membrane activation: selective vesicle fusion via small molecule recognition. J Am Chem Soc. 2006;128:14430–14431. doi: 10.1021/ja0644576. [DOI] [PubMed] [Google Scholar]
- 100.Gong Y, Ma M, Luo Y, Bong D. Functional determinants of a synthetic vesicle fusion system. J Am Chem Soc. 2008;130:6196–6205. doi: 10.1021/ja711184u. [DOI] [PubMed] [Google Scholar]
- 101.Ma MM, Gong Y, Bong D. Lipid membrane adhesion and fusion driven by designed, minimally multivalent hydrogen-bonding lipids. J Am Chem Soc. 2009;131:16919–16926. doi: 10.1021/ja9072657. [DOI] [PubMed] [Google Scholar]
- 102.Estes DJ, Lopez SR, Fuller AO, Mayer M. Triggering and visualizing the aggregation and fusion of lipid membranes in microfluidic chambers. Biophys J. 2006;91:233–243. doi: 10.1529/biophysj.105.076398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Estes DJ, Mayer M. Giant liposomes in physiological buffer using electroformation in a flow chamber. Biochim Biophys Acta-Biomembr. 2005;1712:152–160. doi: 10.1016/j.bbamem.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 104.Estes DJ, Mayer M. Electroformation of giant liposomes from spin-coated films of lipids. Colloids Surf B Biointerfaces. 2005;42:115–123. doi: 10.1016/j.colsurfb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 105.Horger KS, Estes DJ, Capone R, Mayer M. Films of agarose enable rapid formation of giant liposomes in solutions of physiologic ionic strength. J Am Chem Soc. 2009;131:1810–1819. doi: 10.1021/ja805625u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stamou D, Duschl C, Delamarche E, Vogel H. Self-assembled microarrays of attoliter molecular vessels. Angew Chem Int Ed. 2003;42:5580–5583. doi: 10.1002/anie.200351866. [DOI] [PubMed] [Google Scholar]
- 107••.Tsofina LM, Liberman EA, Babakov AV. Production of bimolecular protein–lipid membranes in aqueous solution. Nature. 1966;212:681–683. This article introduced the basic concept of ‘droplet interface bilayers’ for the first time. A free standing bilayer formed when two lipid monolayers, each formed at an aqueous/organic interface, were brought together. [Google Scholar]
- 108.Funakoshi K, Suzuki H, Takeuchi S. Lipid bilayer formation by contacting monolayers in a microfluidic device for membrane protein analysis. Anal Chem. 2006;78:8169–8174. doi: 10.1021/ac0613479. [DOI] [PubMed] [Google Scholar]
- 109.Maglia G, Heron AJ, Hwang WL, Holden MA, Mikhailova E, Li QH, Cheley S, Bayley H. Droplet networks with incorporated protein diodes show collective properties. Nat Nanotechnol. 2009;4:437–440. doi: 10.1038/nnano.2009.121. [DOI] [PubMed] [Google Scholar]
- 110.Poulos JL, Jeon TJ, Damoiseaux R, Gillespie EJ, Bradley KA, Schmidt JJ. Ion channel and toxin measurement using a high throughput lipid membrane platform. Biosens Bioelectron. 2009;24:1806–1810. doi: 10.1016/j.bios.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 111•.Mayer M, Kriebel JK, Tosteson MT, Whitesides GM. Microfabricated teflon membranes for low-noise recordings of ion channels in planar lipid bilayers. Biophys J. 2003;85:2684–2695. doi: 10.1016/s0006-3495(03)74691-8. This article presents a thorough analysis of noise sources in bilayer recordings, as well as the relationship between bilayer diameter and bilayer stability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao QT, de Zoysa RSS, Wang DQ, Jayawardhana DA, Guan XY. Real-time monitoring of peptide cleavage using a nanopore probe. J Am Chem Soc. 2009;131:6324–6325. doi: 10.1021/ja9004893. [DOI] [PubMed] [Google Scholar]
- 113••.Mueller P, Rudin DO, Tien HT, Wescott WC. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature. 1962;194:979–980. doi: 10.1038/194979a0. This article introduced the “painting” method for formation of planar lipid bilayers. [DOI] [PubMed] [Google Scholar]
- 114.Mueller P, Wescott WC, Rudin DO, Tien HT. Methods for formation of single bimolecular lipid membranes in aqueous solution. J Phys Chem. 1963;67:534–535. [Google Scholar]
- 115••.Montal M, Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. Introduction of the ‘folding’ technique for formation of planar lipid bilayers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jeon TJ, Malmstadt N, Schmidt JJ. Hydrogel-encapsulated lipid membranes. J Am Chem Soc. 2006;128:42–43. doi: 10.1021/ja056901v. [DOI] [PubMed] [Google Scholar]
- 117.Jeon TJ, Malmstadt N, Poulos JL, Schmidt JJ. Black lipid membranes stabilized through substrate conjugation to a hydrogel. Biointerphases. 2008;3:FA96–FA100. doi: 10.1116/1.2948314. [DOI] [PubMed] [Google Scholar]
- 118.Jeon TJ, Poulos JL, Schmidt JJ. Long-term storable and shippable lipid bilayer membrane platform. Lab on a Chip. 2008;8:1742–1744. doi: 10.1039/b807932c. [DOI] [PubMed] [Google Scholar]
- 119•.Malmstadt N, Jeon LJ, Schmidt JJ. Long-lived planar lipid bilayer membranes anchored to an in situ polymerized hydrogel. Adv Mater. 2008;20:84–89. This article presented a new method to form mechanically stable planar lipid bilayers by anchoring bilayers to a hydrogel. [Google Scholar]
- 120.Jeon TJ, Malmstadt N, Poulos J, Schmidt JJ. Lipid bilayer membrane stabilization by hydrogel encapsulation. 51st Annual Meeting of the Biophysical-Society; Baltimore, MD. 2007. pp. 652A–652A. [Google Scholar]
- 121.Mach T, Chimerel C, Fritz J, Fertig N, Winterhalter M, Fuetterer C. Miniaturized planar lipid bilayer: increased stability, low electric noise and fast fluid perfusion. Anal Bioanal Chem. 2008;390:841–846. doi: 10.1007/s00216-007-1647-7. [DOI] [PubMed] [Google Scholar]
- 122.Sondermann M, George M, Fertig N, Behrends JC. High-resolution electrophysiology on a chip: transient dynamics of alamethicin channel formation. Biochim Biophys Acta-Biomembr. 2006;1758:545–551. doi: 10.1016/j.bbamem.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 123.Stoelzle S, Haythornthwaite AR, Farre C, Haarmann C, Brueggemann A, George M, Fertig N. Automated patch clamp for ion channel screening. J Physiol Sci. 2009;59:402–1402. [Google Scholar]
- 124.Costello RF, Peterson IR, Heptinstall J, Walton DJ. Improved gel-protected bilayers. Biosens Bioelectron. 1999;14:265–271. [Google Scholar]
- 125.Schmitt EK, Vrouenraets M, Steinem C. Channel activity of OmpF monitored in nano-BLMs. Biophys J. 2006;91:2163–2171. doi: 10.1529/biophysj.106.083592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wong D, Jeon TJ, Schmidt J. Single molecule measurements of channel proteins incorporated into biomimetic polymer membranes. Nanotechnology. 2006;17:3710–3717. [Google Scholar]
- 127.Malmstadt N, Nash MA, Purnell RF, Schmidt JJ. Automated formation of lipid-bilayer membranes in a microfluidic device. Nano Lett. 2006;6:1961–1965. doi: 10.1021/nl0611034. [DOI] [PubMed] [Google Scholar]
- 128.Baaken G, Sondermann M, Schlemmer C, Ruhe J, Behrends JC. Planar microelectrode-cavity array for high-resolution and parallel electrical recording of membrane ionic currents. Lab on a Chip. 2008;8:938–944. doi: 10.1039/b800431e. [DOI] [PubMed] [Google Scholar]
- 129.Poulos JL, Nelson WC, Jeon TJ, Kim CJ, Schmidt JJ. Electrowetting on dielectric-based microfluidics for integrated lipid bilayer formation and measurement. Appl Phys Lett. 2009:95. [Google Scholar]
- 130.Hoskin DW, Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. Biochim Biophys Acta-Biomembr. 2008;1778:357–375. doi: 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 132.Soletti RC, de Faria GP, Vernal J, Terenzi H, Anderluh G, Borges HL, Moura-Neto V, Gabilan NH. Potentiation of anticancer-drug cytotoxicity by sea anemone pore-forming proteins in human glioblastoma cells. Anti-Cancer Drugs. 2008;19:517–525. doi: 10.1097/CAD.0b013e3282faa704. [DOI] [PubMed] [Google Scholar]
- 133.Alyahyaee SAS, Ellar DJ. Cell targeting of a pore-forming toxin. CytA delta-endotoxin from Bacillus thuringiensis subspecies israelensis, by conjugating CytA with anti-Thy 1 monoclonal antibodies and insulin. Bioconjugate Chem. 1996;7:451–460. doi: 10.1021/bc960030k. [DOI] [PubMed] [Google Scholar]
- 134.Pederzolli C, Belmonte G, Dallaserra M, Macek P, Menestrina G. Biochemical and cytotoxic properties of conjugates of transferrin with equinatoxin-II, a cytolysin from a sea-anemone. Bioconjugate Chem. 1995;6:166–173. doi: 10.1021/bc00032a003. [DOI] [PubMed] [Google Scholar]
- 135.Avila AD, Deacosta CM, Lage A. A new immunotoxin built by linking a hemolytic toxin to a monoclonal-antibody specific for immature lymphocytes-T. Int J Cancer. 1988;42:568–571. doi: 10.1002/ijc.2910420417. [DOI] [PubMed] [Google Scholar]
- 136.vanderSpek JC, Murphy JR. Genetic construction, expression, and characterization of diphtheria toxin-based growth factor fusion proteins. Methods Mol Biol. 2000;145:89–99. doi: 10.1385/1-59259-052-7:89. [DOI] [PubMed] [Google Scholar]
- 137.Little RB, Dubos RJ, Hotchkiss RD. Action of gramicidin on streptococci of bovine mastitis. Proc Soc Exp Biol Med. 1940;44:444–445. [Google Scholar]
- 138.vanderSpek JC, Murphy JR. Fusion protein toxins based on diphtheria toxin: Selective targeting of growth factor receptors of eukaryotic cells. Appl Chimeric Genes Hybrid Proteins Pt B. 2000;327:239–249. doi: 10.1016/s0076-6879(00)27280-7. [DOI] [PubMed] [Google Scholar]
- 139.Tejuca M, Diaz I, Figueredo R, Roque L, Pazos F, Martinez D, Iznaga-Escobar N, Perez R, Alvarez C, Lanio ME. Construction of an immunotoxin with the pore forming protein St1 and ior C5, a monoclonal antibody against a colon cancer cell line. Int Immunopharmacol. 2004;4:731–744. doi: 10.1016/j.intimp.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 140.Murphy JR, Lakkis FG, Vanderspek JC, Anderson P, Strom TB. Protein engineering of diphtheria toxin development of receptor-specific cytotoxic agents for the treatment of human disease. Targeted Diagn and Ther. 1992;7:365–382. [PubMed] [Google Scholar]
- 141.Murphy JR, Vanderspek JC. Targeting diphtheria-toxin to growth-factor receptors. Semin Cancer Biol. 1995;6:259–267. doi: 10.1006/scbi.1995.0034. [DOI] [PubMed] [Google Scholar]
- 142.Williams SA, Merchant RF, Garrett-Mayer E, Isaacs JT, Buckley JT, Denmeade SR. A prostate-specific antigen-activated channel-forming toxin as therapy for prostatic disease. J Natl Cancer Inst. 2007;99:376–385. doi: 10.1093/jnci/djk065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143•.Panchal RG, Cusack E, Cheley S, Bayley H. Tumor protease-activated, pore-forming toxins from a combinatorial library. Nat Biotechnol. 1996;14:852–856. doi: 10.1038/nbt0796-852. This article presented mutants of the pore-forming toxin α-hemolysin that were activated by tumor-specific proteases for targeted lysis of cancer cells. [DOI] [PubMed] [Google Scholar]
- 144.Provoda CJ, Lee KD. Bacterial pore-forming hemolysins and their use in the cytosolic delivery of macromolecules. Adv Drug Del Rev. 2000;41:209–221. doi: 10.1016/s0169-409x(99)00067-8. [DOI] [PubMed] [Google Scholar]
- 145.Legendre JY, Szoka FC. Cyclic amphipathic peptide DNA complexes mediate high-efficiency transfection of adherent mammalian-cells. Proc Natl Acad Sci U S A. 1993;90:893–897. doi: 10.1073/pnas.90.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mandal M, Lee KD. Listeriolysin O-containing-liposome mediated cytosolic delivery of exogenous antigen. FASEB J. 2000;14:A1055–A11055. [Google Scholar]
- 147.Mandal M, Lee KD. Listeriolysin O-liposome-mediated cytosolic delivery of macromolecule antigen in vivo: enhancement of antigen-specific cytotoxic T lymphocyte frequency, activity, and tumor protection. Biochim Biophys Acta-Biomembr. 2002;1563:7–17. doi: 10.1016/s0005-2736(02)00368-1. [DOI] [PubMed] [Google Scholar]
- 148.Stier EM, Mandal M, Lee KD. Differential cytosolic delivery and presentation of antigen by listeriolysin O-liposomes to macrophages and dendritic cells. Mol Pharm. 2005;2:74–82. doi: 10.1021/mp049896v. [DOI] [PubMed] [Google Scholar]
- 149.Goletz TJ, Klimpel KR, Arora N, Leppla SH, Keith JM, Berzofsky JA. Targeting HIV proteins to the major histocompatibility complex class I processing pathway with a novel gp120-anthrax toxin fusion protein. Proc Natl Acad Sci U S A. 1997;94:12059–12064. doi: 10.1073/pnas.94.22.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Varughese M, Chi A, Teixeira AV, Nicholls PJ, Keith JM, Leppla SH. Internalization of a Bacillus anthracis protective antigen c-Myc fusion protein mediated by cell surface anti-c-Myc antibodies. Mol Med. 1998;4:87–95. [PMC free article] [PubMed] [Google Scholar]
- 151.Witkowska D, Bartys A, Gamian A. Defensins and cathelicidins as natural peptide antibiotics. Postepy Higieny I Medycyny Doswiadczalnej. 2008;62:694–707. [PubMed] [Google Scholar]
- 152.Ogawa Y, Mizumoto N, Tanaka H, Matsushima H, Takashima A. Identification of novel pharmacological activities of an antifungal agent, nystatin, to promote dendritic cell maturation. J Invest Dermatol. 2006;126:349–353. doi: 10.1038/sj.jid.5700081. [DOI] [PubMed] [Google Scholar]
- 153.Lockwood NA, Mayo KH. The future for antibiotics: bacterial membrane disintegrators. Drugs Future. 2003;28:911–923. [Google Scholar]
- 154.McKellar SH, Allred BD, Marks JD, Cowley CG, Classen DC, Gardner SC, Long JW. Treatment of infected left ventricular assist device using antibiotic-impregnated beads. Annals Thoracic Surg. 1999;67:554–555. doi: 10.1016/s0003-4975(98)01243-0. [DOI] [PubMed] [Google Scholar]
- 155.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 156.Steinberg DA, Hurst MA, Fujii CA, Kung AHC, Ho JF, Cheng FC, Loury DJ, Fiddes JC. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob Agents Chemother. 1997;41:1738–1742. doi: 10.1128/aac.41.8.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jacob L, Zasloff M. Potential therapeutic applications of magainins and other antimicrobial agents of animal origin. In: Marsh J, Goode JA, editors. Symposium on Antimicrobial Peptides; London, England. 1994. pp. 197–216. [DOI] [PubMed] [Google Scholar]
- 158.Koczulla AR, Bals R. Antimicrobial peptides—current status and therapeutic potential. Drugs. 2003;63:389–406. doi: 10.2165/00003495-200363040-00005. [DOI] [PubMed] [Google Scholar]
- 159.Zairi A, Tangy F, Bouassida K, Hani K. Dermaseptins and magainins: antimicrobial peptides from frogs’ skin-new sources for a promising spermicides microbicides—a mini review. J Biomed Biotechnol. 2009 doi: 10.1155/2009/452567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wachinger M, Kleinschmidt A, Winder D, von Pechmann N, Ludvigsen A, Neumann M, Holle R, Salmons B, Erfle V, Brack-Werner R. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J Gen Virol. 1998;79:731–740. doi: 10.1099/0022-1317-79-4-731. [DOI] [PubMed] [Google Scholar]
- 161.Yasin B, Pang M, Turner JS, Cho Y, Dinh NN, Waring AJ, Lehrer RI, Wagar EA. Evaluation of the inactivation of infectious herpes simplex virus by host-defense peptides. Eur J Clin Microbiol Infect Dis. 2000;19:187–194. doi: 10.1007/s100960050457. [DOI] [PubMed] [Google Scholar]
- 162.Bourinbaiar AS, Coleman CF. The effect of gramicidin, a topical contraceptive and antimicrobial agent with anti-HIV activity, against herpes simplex viruses type 1 and 2 in vitro. Arch Virol. 1997;142:2225–2236. doi: 10.1007/s007050050237. [DOI] [PubMed] [Google Scholar]
- 163.Bourinbaiar AS, Lee CH. Synergistic effect of gramicidin and EDTA in inhibiting sperm motility and cervical mucus penetration in vitro. Contraception. 1996;54:367–372. doi: 10.1016/s0010-7824(96)00205-3. [DOI] [PubMed] [Google Scholar]
- 164.Gu LQ, Braha O, Conlan S, Cheley S, Bayley H. Stochastic sensing of organic analytes by a pore-forming protein containing a molecular adapter. Nature. 1999;398:686–690. doi: 10.1038/19491. [DOI] [PubMed] [Google Scholar]
- 165.Guan XY, Gu LQ, Cheley S, Braha O, Bayley H. Stochastic sensing of TNT with a genetically engineered pore. ChemBioChem. 2005;6:1875–1881. doi: 10.1002/cbic.200500064. [DOI] [PubMed] [Google Scholar]
- 166.Smith CL, Kricka L, Krull UJ. The development of single molecule environmental sensors. Genet Anal—Biomol Eng. 1995;12:33–37. doi: 10.1016/1050-3862(95)00105-0. [DOI] [PubMed] [Google Scholar]
- 167.Braha O, Walker B, Cheley S, Kasianowicz JJ, Song LZ, Gouaux JE, Bayley H. Designed protein pores as components for biosensors. Chem Biol. 1997;4:497–505. doi: 10.1016/s1074-5521(97)90321-5. [DOI] [PubMed] [Google Scholar]
- 168••.Cornell BA, BraachMaksvytis VLB, King LG, Osman PDJ, Raguse B, Wieczorek L, Pace RJ. A biosensor that uses ion-channel switches. Nature. 1997;387:580–583. doi: 10.1038/42432. One of the first demonstrations of applying a pore-forming peptide, gramicidin, to monitor protein–ligand interactions with impedence spectroscopy. [DOI] [PubMed] [Google Scholar]
- 169.Pace RJ, Braach-Maksvytis VBL, King LG, Osman PDJ, Raguse B, Wieczorek L, Cornell BA. The gated ion channel biosensor - a functioning nano-machine. In: Fearey BL, editor. Proceedings of the Society of Photo-Optical Instrumentation Engineers (SPIE); San Jose, CA. 1998. pp. 50–59. [Google Scholar]
- 170.Minami H, Sugawara M, Odashima K, Umezawa Y, Uto M, Michaelis EK, Kuwana T. Ion channel sensors for glutamic-acid. Anal Chem. 1991;63:2787–2795. doi: 10.1021/ac00023a022. [DOI] [PubMed] [Google Scholar]
- 171•.Sugawara M, Kojima K, Sazawa H, Umezawa Y. Ion-channel sensors. Anal Chem. 1987;59:2842–2846. doi: 10.1021/ac00151a004. One of the first publications on ion channel-based sensors. [DOI] [PubMed] [Google Scholar]
- 172.Favero G, Campanella L, Cavallo S, D’Annibale A, Perrella M, Mattei E, Ferri T. Glutamate receptor incorporated in a mixed hybrid bilayer lipid membrane array, as a sensing element of a biosensor working under flowing conditions. J Am Chem Soc. 2005;127:8103–8111. doi: 10.1021/ja042904g. [DOI] [PubMed] [Google Scholar]
- 173.Uto M, Michaelis EK, Hu IF, Umezawa Y, Kuwana T. Biosensor development with a glutamate receptor ion-channel reconstituted in a lipid bilayer. Anal Sci. 1990;6:221–225. [Google Scholar]
- 174.Reiken SR, VanWie BJ, Sutisna H, Moffett DF, Koch AR, Silber M, Davis WC. Bispecific antibody modification of nicotinic acetylcholine receptors for biosensing. Biosens Bioelectron. 1996;11:91–102. doi: 10.1016/0956-5663(96)83716-x. [DOI] [PubMed] [Google Scholar]
- 175.Allen TGJ. The ‘sniffer-patch’ technique for detection of neurotransmitter release. Trends Neurosci. 1997;20:192–197. doi: 10.1016/s0166-2236(96)01039-9. [DOI] [PubMed] [Google Scholar]
- 176.Hume RI, Role LW, Fischbach GD. Acetylcholine-release from growth cones detected with patches of acetylcholine receptor-rich membranes. Nature. 1983;305:632–634. doi: 10.1038/305632a0. [DOI] [PubMed] [Google Scholar]
- 177.Young SH, Poo MM. Spontaneous release of transmitter from growth cones of embryonic neurons. Nature. 1983;305:634–637. doi: 10.1038/305634a0. [DOI] [PubMed] [Google Scholar]
- 178.Rokitskaya TI, Antonenko YN, Kotova EA. The interaction of phthalocyanine with planar lipid bilayers—photodynamic inactivation of gramicidin channels. FEBS Lett. 1993;329:332–335. doi: 10.1016/0014-5793(93)80248-s. [DOI] [PubMed] [Google Scholar]
- 179.Anrather D, Smetazko M, Saba M, Alguel Y, Schalkhammer T. Supported membrane nanodevices. J Nanosci Nanotechnol. 2004;4:1–22. doi: 10.1166/jnn.2004.226. [DOI] [PubMed] [Google Scholar]
- 180.Nikolelis DP, Siontorou CG, Krull UJ, Katrivanos PL. Ammonium ion minisensors from self-assembled bilayer lipid membranes using gramicidin as an ionophore. Modulation of ammonium selectivity by platelet-activating factor. Anal Chem. 1996;68:1735–1741. doi: 10.1021/ac950403v. [DOI] [PubMed] [Google Scholar]
- 181.Hwang WL, Holden MA, White S, Bayley H. Electrical behavior of droplet interface bilayer networks: experimental analysis and modeling. J Am Chem Soc. 2007;129:11854–11864. doi: 10.1021/ja074071a. [DOI] [PubMed] [Google Scholar]
- 182.Heyse S, Stora T, Schmid E, Lakey JH, Vogel H. Emerging techniques for investigating molecular interactions at lipid membranes. Biochimica Et Biophysica Acta-Rev Biomembranes. 1998;1376:319–338. doi: 10.1016/s0304-4157(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 183.Stora T, Lakey JH, Vogel H. Ion-channel gating in transmembrane receptor proteins: functional activity in tethered lipid membranes. Angew Chem Int Ed. 1999;38:389–392. doi: 10.1002/(SICI)1521-3773(19990201)38:3<389::AID-ANIE389>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 184•.Terrettaz S, Stora T, Duschl C, Vogel H. Protein-binding to supported lipid membranes—investigation of the cholera-toxin ganglioside interaction by simultaneous impedance spectroscopy and surface-plasmon resonance. Langmuir. 1993;9:1361–1369. Monitoring of protein–lipid interactions on supported lipid bilayer using impedance spectroscopy and surface-plamon resonance. [Google Scholar]
- 185.Miller C, Cuendet P, Gratzel M. K+ sensitive bilayer supporting electrodes. J Electroanal Chem. 1990;278:175–192. [Google Scholar]
- 186.Steinem C, Janshoff A, Galla HJ, Sieber M. Impedance analysis of ion transport through gramicidin channels incorporated in solid supported lipid bilayers. Bioelectrochem Bioenerg. 1997;42:213–220. [Google Scholar]
- 187.Naumann R, Schiller SM, Giess F, Grohe B, Hartman KB, Karcher I, Koper I, Lubben J, Vasilev K, Knoll W. Tethered lipid Bilayers on ultraflat gold surfaces. Langmuir. 2003;19:5435–5443. [Google Scholar]
- 188.Steinem C, Janshoff A, Ulrich WP, Sieber M, Galla HJ. Impedance analysis of supported lipid bilayer membranes: a scrutiny of different preparation techniques. Biochim Biophys Acta-Biomembr. 1996;1279:169–180. doi: 10.1016/0005-2736(95)00274-x. [DOI] [PubMed] [Google Scholar]
- 189.Magath TB, Berkson J. Electronic blood-cell counting. Am J Clin Pathol. 1960;34:203–213. doi: 10.1093/ajcp/34.3.203. [DOI] [PubMed] [Google Scholar]
- 190••.Coulter WH. Means for counting particles suspended in a fluid. 2656508. US Patent. 1953 Original Coulter-counting patent; forms the basis of resistive-pulse sensing with nanopores.
- 191••.Apell HJ, Bamberg E, Lauger P. Effects of surface-charge on the conductance of the gramicidin channel. Biochim Biophys Acta. 1979;552:369–378. doi: 10.1016/0005-2736(79)90181-0. First investigation on the effect of electrical charges near the entrance of gramicidin channels on the conductance of these pores. [DOI] [PubMed] [Google Scholar]
- 192.Macrae MX, Blake S, Mayer T, Mayer M, Yang J. Reactive derivatives of gramicidin enable light-and ion-modulated ion channels. Proceedings of the Society of Photo-Optical Instrumentation Engineers; 2009. p. 739709. [Google Scholar]
- 193•.Futaki S, Zhang YJ, Sugiura Y. Detecting a tag on a channel opening: blockage of the biotinylated channels by streptavidin. Tetrahedron Lett. 2001;42:1563–1565. This paper demonstrates that nanopores can be used to characterize interactions between DNA and proteins; moreover, this binding significantly reduced the translocation speed of polynucleotides that were bound to proteins. [Google Scholar]
- 194••.Kocer A, Walko M, Meijberg W, Feringa BL. A light-actuated nanovalve derived from a channel protein. Science. 2005;309:755–758. doi: 10.1126/science.1114760. This article reported the development of a molecular-scale valve that was controlled by light based on an engineered mechanosensitive channel protein, MscL. This work was an important step toward realization of nanoscale device control. [DOI] [PubMed] [Google Scholar]
- 195.Hirano A, Wakabayashi M, Matsuno Y, Sugawara M. A single-channel sensor based on gramicidin controlled by molecular recognition at bilayer lipid membranes containing receptor. Biosens Bioelectron. 2003;18:973–983. doi: 10.1016/s0956-5663(02)00219-1. [DOI] [PubMed] [Google Scholar]
- 196.Lundbaek JA, Collingwood SA, Ingolfsson HI, Kapoor R, Andersen OS. Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. J R Soc Interface. 2010;7:373–395. doi: 10.1098/rsif.2009.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Uram JD, Ke K, Hunt AJ, Mayer M. Label-free affinity assays by rapid detection of immune complexes in submicrometer pores. Angew Chem Int Ed. 2006;45:2281–2285. doi: 10.1002/anie.200502862. [DOI] [PubMed] [Google Scholar]
- 198.Uram JD, Ke K, Hunt AJ, Mayer M. Submicrometer pore-based characterization and quantification of antibody-virus interactions. Small. 2006;2:967–972. doi: 10.1002/smll.200600006. [DOI] [PubMed] [Google Scholar]
- 199.Uram JD, Mayer M. Estimation of solid phase affinity constants using resistive-pulses from functionalized nanoparticles. Biosens Bioelectron. 2007;22:1556–1560. doi: 10.1016/j.bios.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 200•.Uram JD, Ke K, Mayer M. Noise and bandwidth of current recordings from submicrometer pores and nanopores. ACS Nano. 2008;2:857–872. doi: 10.1021/nn700322m. This paper presents a comprehensive analysis of noise sources and bandwidth considerations for resistive-pulse recordings with nanopores. [DOI] [PubMed] [Google Scholar]
- 201.Cheley S, Gu LQ, Bayley H. Stochastic sensing of nanomolar inositol 1,4,5-trisphosphate with an engineered pore. Chem Biol. 2002;9:829–838. doi: 10.1016/s1074-5521(02)00172-2. [DOI] [PubMed] [Google Scholar]
- 202.Wu HC, Bayley H. Single-molecule detection of nitrogen mustards by covalent reaction within a protein nanopore. J Am Chem Soc. 2008;130:6813–6819. doi: 10.1021/ja8004607. [DOI] [PubMed] [Google Scholar]
- 203.Sanchez-Quesada J, Ghadiri MR, Bayley H, Braha O. Cyclic peptides as molecular adapters for a pore-forming protein. J Am Chem Soc. 2000;122:11757–11766. [Google Scholar]
- 204.Van Gelder P, Dumas F, Winterhalter M. Understanding the function of bacterial outer membrane channels by reconstitution into black lipid membranes. Biophys Chem. 2000;85:153–167. doi: 10.1016/s0301-4622(99)00153-2. [DOI] [PubMed] [Google Scholar]
- 205•.Nestorovich EM, Danelon C, Winterhalter M, Bezrukov SM. Designed to penetrate: time-resolved interaction of single antibiotic molecules with bacterial pores. Proc Natl Acad Sci U S A. 2002;99:9789–9794. doi: 10.1073/pnas.152206799. This paper applied the resistive-pulse-sensing concept to study the interactions between antibiotic molecules and the bacterial outer membrane porin F at a single-molecule level. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wirth A, Jung M, Bies C, Frien M, Tyedmers J, Zimmermann R, Wagner R. The Sec61p complex is a dynamic precursor activated channel. Mol Cell. 2003;12:261–268. doi: 10.1016/s1097-2765(03)00283-1. [DOI] [PubMed] [Google Scholar]
- 207.Hessa T, Kim H, Bihlmaier K, Lundin C, Boekel J, Andersson H, Nilsson I, White SH, von Heijne G. Recognition of transmembrane helices by the endoplasmic reticulum translocon. Nature. 2005;433:377–381. doi: 10.1038/nature03216. [DOI] [PubMed] [Google Scholar]
- 208.Muro C, Grigoriev SM, Pietkiewicz D, Kinnally KW, Campo ML. Comparison of the TIM and TOM channel activities of the mitochondrial protein import complexes. Biophys J. 2003;84:2981–2989. doi: 10.1016/S0006-3495(03)70024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rehling P, Model K, Brandner K, Kovermann P, Sickmann A, Meyer HE, Kuhlbrandt W, Wagner R, Truscott KN, Pfanner N. Protein insertion into the mitochondrial inner membrane by a twin-pore translocase. Science. 2003;299:1747–1751. doi: 10.1126/science.1080945. [DOI] [PubMed] [Google Scholar]
- 210.Hinnah SC, Wagner R, Sveshnikova N, Harrer R, Soll J. The chloroplast protein import channel Toc75: pore properties and interaction with transit peptides. Biophys J. 2002;83:899–911. doi: 10.1016/S0006-3495(02)75216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Krantz BA, Finkelstein A, Collier RJ. Protein translocation through the anthrax toxin transmembrane pore is driven by a proton gradient. J Mol Biol. 2006;355:968–979. doi: 10.1016/j.jmb.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 212•.Krantz BA, Melnyk RA, Zhang S, Juris SJ, Lacy DB, Wu ZY, Finkelstein A, Collier RJ. A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science. 2005;309:777–781. doi: 10.1126/science.1113380. This paper describes the features of the anthrax pore PA7 that are necessary to translocate proteins; as a result, evidence is provided for the concept that protein pores facilitate the denaturing of large proteins in order to translocate them through pores and across membranes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang S, Udho E, Wu ZY, Collier RJ, Finkelstein A. Protein translocation through anthrax toxin channels formed in planar lipid bilayers. Biophys J. 2004;87:3842–3849. doi: 10.1529/biophysj.104.050864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Schatz G, Dobberstein B. Common principles of protein translocation across membranes. Science. 1996;271:1519–1526. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- 215.Simon SM, Blobel G. Signal peptides open protein-conducting channels in Escherichia coli. Cell. 1992;69:677–684. doi: 10.1016/0092-8674(92)90231-z. [DOI] [PubMed] [Google Scholar]
- 216.Movileanu L. Squeezing a single polypeptide through a nanopore. Soft Matter. 2008;4:925–931. doi: 10.1039/b719850g. [DOI] [PubMed] [Google Scholar]
- 217.Dreiseikelmann B. Translocation of DNA across bacterial-membranes. Microbiol Rev. 1994;58:293–316. doi: 10.1128/mr.58.3.293-316.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hanss B, Leal-Pinto E, Bruggeman LA, Copeland TD, Klotman PE. Identification and characterization of a cell membrane nucleic acid channel. Proc Natl Acad Sci U S A. 1998;95:1921–1926. doi: 10.1073/pnas.95.4.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Szabo I, Bathori G, Tombola F, Coppola A, Schmehl I, Brini M, Ghazi A, De Pinto V, Zoratti M. Double-stranded DNA can be translocated across a planar membrane containing purified mitochondrial porin. FASEB J. 1998;12:495–502. doi: 10.1096/fasebj.12.6.495. [DOI] [PubMed] [Google Scholar]
- 220.Pfanner N, Truscott KN. Powering mitochondrial protein import. Nat Struct Biol. 2002;9:234–236. doi: 10.1038/nsb0402-234. [DOI] [PubMed] [Google Scholar]
- 221.Attardi G, Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- 222.Kasianowicz JJ, Robertson JWF, Chan ER, Reiner JE, Stanford VM. Nanoscopic porous sensors. Annu Rev Anal Chem. 2008;1:737–766. doi: 10.1146/annurev.anchem.1.031207.112818. [DOI] [PubMed] [Google Scholar]
- 223••.Krasilnikov OV, Sabirov RZ, Ternovsky VI, Merzliak PG, Muratkhodjaev JN. A simple method for the determination of the pore radius of ion channels in planar lipid bilayer-membranes. FEMS Microbiol Immunol. 1992;105:93–100. doi: 10.1111/j.1574-6968.1992.tb05891.x. This paper was the first to outline a simple method for estimating the smallest radius of ion channels from simple conductivity measurements through a pore. [DOI] [PubMed] [Google Scholar]
- 224••.Kasianowicz JJ, Brandin E, Branton D, Deamer DW. Characterization of individual polynucleotide molecules using a membrane channel. Proc Natl Acad Sci U S A. 1996;93:13770–13773. doi: 10.1073/pnas.93.24.13770. This work provided the first electrical recordings through nanopores that showed the translocation of individual polynucleotides. As a result, the concept of sequencing polynucleotides with nanopores gained momentum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bezrukov SM, Kasianowicz JJ. The charge state of an ion channel controls neutral polymer entry into its pore. Eur Biophys J Biophys. 1997;26:471–476. doi: 10.1007/s002490050101. [DOI] [PubMed] [Google Scholar]
- 226.Krasilnikov OV, Da Cruz JB, Yuldasheva LN, Varanda WA, Nogueira RA. A novel approach to study the geometry of the water lumen of ion channels: Colicin Ia channels in planar lipid bilayers. J Membr Biol. 1998;161:83–92. doi: 10.1007/s002329900316. [DOI] [PubMed] [Google Scholar]
- 227.Qu Y, Dahl G. Accessibility of cx46 hemichannels for uncharged molecules and its modulation by voltage. Biophys J. 2004;86:1502–1509. doi: 10.1016/S0006-3495(04)74218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nestorovich EM, Rostovtseva TK, Bezrukov SM. Residue ionization and ion transport through OmpF channels. Biophys J. 2003;85:3718–3729. doi: 10.1016/S0006-3495(03)74788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229•.Movileanu L, Cheley S, Howorka S, Braha O, Bayley H. Location of a constriction in the lumen of a transmembrane pore by targeted covalent attachment of polymer molecules. J Gen Physiol. 2001;117:239–251. doi: 10.1085/jgp.117.3.239. This paper describes a method for scanning dimensions of the interior of biological pores, which is particularly interesting because the three-dimensional structure of protein pores is difficult to obtain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Merzlyak PG, Capistrano MFP, Valeva A, Kasianowicz JJ, Krasilnikov OV. Conductance and ion selectivity of a mesoscopic protein nanopore probed with cysteine scanning mutagenesis. Biophys J. 2005;89:3059–3070. doi: 10.1529/biophysj.105.066472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231••.Bezrukov SM, Vodyanoy I, Parsegian VA. Counting polymers moving through a single-ion channel. Nature. 1994;370:279–281. doi: 10.1038/370279a0. This paper was the first to detect the translocation of polymers through single ion channels; consequently, the concept of employing resistive-pulse sensing at the nanoscale became reality. [DOI] [PubMed] [Google Scholar]
- 232.Robertson JWF, Rodrigues CG, Stanford VM, Rubinson KA, Krasilnikov OV, Kasianowicz JJ. Single-molecule mass spectrometry in solution using a solitary nanopore. Proc Natl Acad Sci U S A. 2007;104:8207–8211. doi: 10.1073/pnas.0611085104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ambjornsson T, Apell SP, Konkoli Z, Di Marzio EA, Kasianowicz JJ. Charged polymer membrane translocation. J Chem Phys. 2002;117:4063–4073. [Google Scholar]
- 234.Aguilella VM, Bezrukov SM. Alamethicin channel conductance modified by lipid charge. Eur Biophys J Biophys. 2001;30:233–241. doi: 10.1007/s002490100145. [DOI] [PubMed] [Google Scholar]
- 235.Aguilella V, Rostovtseva TK, Vodyanoy I, Bezrukov SM, Parsegian VA. Gramicidin A channel as a sensor of membrane lipid titration. Biophys J. 1997;72:TH275–TH1275. doi: 10.1016/S0006-3495(98)77620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kasianowicz JJ, Henrickson SE, Weetall HH, Robertson B. Simultaneous multianalyte detection with a nanometer-scale pore. Anal Chem. 2001;73:2268–2272. doi: 10.1021/ac000958c. [DOI] [PubMed] [Google Scholar]
- 237.Shin SH, Bayley H. Stepwise growth of a single polymer chain. J Am Chem Soc. 2005;127:10462–10463. doi: 10.1021/ja052194u. [DOI] [PubMed] [Google Scholar]
- 238.Bolitho P, Street SEA, Westwood JA, Edelmann W, MacGregor D, Waring P, Murray WK, Godfrey DI, Trapani JA, Johnstone RW, et al. Perforin-mediated suppression of B-cell lymphoma. Proc Natl Acad Sci U S A. 2009;106:2723–2728. doi: 10.1073/pnas.0809008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rodrigues CG, Machado DC, Chevtchenko SF, Krasilnikov OV. Mechanism of KCl enhancement in detection of nonionic polymers by nanopore sensors. Biophys J. 2008;95:5186–5192. doi: 10.1529/biophysj.108.140814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Huang L, Makarov DE. The rate constant of polymer reversal inside a pore. J Chem Phys. 2008;128:114903. doi: 10.1063/1.2890006. [DOI] [PubMed] [Google Scholar]
- 241.Howorka S, Movileanu L, Lu XF, Magnon M, Cheley S, Braha O, Bayley H. A protein pore with a single polymer chain tethered within the lumen. J Am Chem Soc. 2000;122:2411–2416. [Google Scholar]
- 242.Krasilnikov OV, Rodrigues CG, Bezrukov SM. Single polymer molecules in a protein nanopore in the limit of a strong polymer-pore attraction. Phys Rev Lett. 2006;97:018301. doi: 10.1103/PhysRevLett.97.018301. [DOI] [PubMed] [Google Scholar]
- 243.Movileanu L, Bayley H. Partitioning of a polymer into a nanoscopic protein pore obeys a simple scaling law. Proc Natl Acad Sci U S A. 2001;98:10137–10141. doi: 10.1073/pnas.181089798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Movileanu L, Cheley S, Bayley H. Partitioning of individual flexible polymers into a nanoscopic protein pore. Biophys J. 2003;85:897–910. doi: 10.1016/S0006-3495(03)74529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Muthukumar M. Polymer translocation through a hole. J Chem Phys. 1999;111:10371–10374. [Google Scholar]
- 246.Banghart MR, Volgraf M, Trauner D. Engineering light-gated ion channels. Biochemistry. 2006;45:15129–15141. doi: 10.1021/bi0618058. [DOI] [PubMed] [Google Scholar]
- 247.Ding KJ, Cai DQ, Zhan FR, Wu LJ, Wu YJ, Yu ZL. Single long-polymer translocation through a long pore. Chin Phys. 2006;15:940–946. [Google Scholar]
- 248.Slonkina E, Kolomeisky AB. Polymer translocation through a long nanopore. J Chem Phys. 2003;118:7112–7118. [Google Scholar]
- 249•.Muthukumar M, Kong CY. Simulation of polymer translocation through protein channels. Proc Natl Acad Sci U S A. 2006;103:5273–5278. doi: 10.1073/pnas.0510725103. This paper combines Langevin dynamics for polymers and Poisson–Nernst–Planck equations for ionic currents to simulate the translocation of polymers and subsequent ionic currents through protein pores. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kumar R, Muthukumar M. Origin of translocation barriers for polyelectrolyte chains. J Chem Phys. 2009;131:194903. doi: 10.1063/1.3264632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sung W, Park PJ. Polymer translocation through a pore in a membrane. Phys Rev Lett. 1996;77:783–786. doi: 10.1103/PhysRevLett.77.783. [DOI] [PubMed] [Google Scholar]
- 252.Lubensky DK, Nelson DR. Driven polymer translocation through a narrow pore. Biophys J. 1999;77:1824–1838. doi: 10.1016/S0006-3495(99)77027-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sebastian KL. Kink motion in the barrier crossing of a chain molecule. Phys Rev E. 2000;61:3245–3248. [Google Scholar]
- 254.Murphy RJ, Muthukumar M. Threading synthetic polyelectrolytes through protein pores. J Chem Phys. 2007;126:051101. doi: 10.1063/1.2435717. [DOI] [PubMed] [Google Scholar]
- 255.Nakane JJ, Akeson M, Marziali A. Nanopore sensors for nucleic acid analysis. J Phys Condes Matter. 2003;15:R1365–R1393. [Google Scholar]
- 256.Akeson M, Branton D, Kasianowicz JJ, Brandin E, Deamer DW. Microsecond time-scale discrimination among polycytidylic acid, polyadenylic acid, and polyuridylic acid as homopolymers or as segments within single RNA molecules. Biophys J. 1999;77:3227–3233. doi: 10.1016/S0006-3495(99)77153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257•.Meller A, Nivon L, Brandin E, Golovchenko J, Branton D. Rapid nanopore discrimination between single polynucleotide molecules. Proc Natl Acad Sci U S A. 2000;97:1079–1084. doi: 10.1073/pnas.97.3.1079. For the first time, electrical recordings across a nanopore enabled discrimination between different polynucleotide molecules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Szabo I, Bathori G, Tombola F, Brini M, Coppola A, Zoratti M. DNA translocation across planar bilayers containing Bacillus subtilis ion channels. J Biol Chem. 1997;272:25275–25282. doi: 10.1074/jbc.272.40.25275. [DOI] [PubMed] [Google Scholar]
- 259.Rostovtseva TK, Komarov A, Bezrukov SM, Colombini M. Dynamics of nucleotides in VDAC channels: structure-specific noise generation. Biophys J. 2002;82:193–205. doi: 10.1016/S0006-3495(02)75386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Meller A, Nivon L, Branton D. Voltage-driven DNA translocations through a nanopore. Phys Rev Lett. 2001;86:3435–3438. doi: 10.1103/PhysRevLett.86.3435. [DOI] [PubMed] [Google Scholar]
- 261.Aksimentiev A, Heng JB, Timp G, Schulten K. Microscopic kinetics of DNA translocation through synthetic nanopores. Biophys J. 2004;87:2086–2097. doi: 10.1529/biophysj.104.042960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Aksimentiev A, Schulten K. Imaging alpha-hemolysin with molecular dynamics: ionic conductance, osmotic permeability, and the electrostatic potential map. Biophys J. 2005;88:3745–3761. doi: 10.1529/biophysj.104.058727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Liu H, Qian SZ, Bau HH. The effect of translocating cylindrical particles on the ionic current through a nanopore. Biophys J. 2007;92:1164–1177. doi: 10.1529/biophysj.106.089268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Henrickson SE, Misakian M, Robertson B, Kasianowicz JJ. Driven DNA transport into an asymmetric nanometer-scale pore. Phys Rev Lett. 2000;85:3057–3060. doi: 10.1103/PhysRevLett.85.3057. [DOI] [PubMed] [Google Scholar]
- 265•.Stoddart D, Heron AJ, Mikhailova E, Maglia G, Bayley H. Single-nucleotide discrimination in immobilized DNA oligonucleotides with a biological nanopore. Proc Natl Acad Sci U S A. 2009;106:7702–7707. doi: 10.1073/pnas.0901054106. This paper identified two locations in the lumen of the α-hemolysin pore that, if a single nucleotide could be held at either location long enough, were capable of distinguishing between individual bases in polynucleotides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266•.Purnell RF, Schmidt JJ. Discrimination of single base substitutions in a DNA strand immobilized in a biological nanopore. ACS Nano. 2009;3:2533–2538. doi: 10.1021/nn900441x. In a complementary fashion to reference [265•], this paper identified the same two locations in the lumen of the α-hemolysin pore. [DOI] [PubMed] [Google Scholar]
- 267.Wendell D, Jing P, Geng J, Subramaniam V, Lee TJ, Montemagno C, Guo PX. Translocation of double-stranded DNA through membrane-adapted phi29 motor protein nanopores. Nat Nanotechnol. 2009;4:765–772. doi: 10.1038/nnano.2009.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sauer-Budge AF, Nyamwanda JA, Lubensky DK, Branton D. Unzipping kinetics of double-stranded DNA in a nanopore. Phys Rev Lett. 2003;90:238101. doi: 10.1103/PhysRevLett.90.238101. [DOI] [PubMed] [Google Scholar]
- 269.Hornblower B, Coombs A, Whitaker RD, Kolomeisky A, Picone SJ, Meller A, Akeson M. Single-molecule analysis of DNA-protein complexes using nanopores. Nat Methods. 2007;4:315–317. doi: 10.1038/nmeth1021. [DOI] [PubMed] [Google Scholar]
- 270••.Clarke J, Wu HC, Jayasinghe L, Patel A, Reid S, Bayley H. Continuous base identification for single-molecule nanopore DNA sequencing. Nat Nanotechnol. 2009;4:265–270. doi: 10.1038/nnano.2009.12. Individual nucleotide bases were detected translocating through a modified α-hemolysin pore. Furthermore, these bases were detected from polynucleotides that were digested by an exonuclease. [DOI] [PubMed] [Google Scholar]
- 271•.Cockroft SL, Chu J, Amorin M, Ghadiri MR. A single-molecule nanopore device detects DNA polymerase activity with single-nucleotide resolution. J Am Chem Soc. 2008;130:818–820. doi: 10.1021/ja077082c. Individual nucleotides were detected incorporating into a growing complementary DNA strand via DNA polymerase acitivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Deamer DW, Branton D. Characterization of nucleic acids by nanopore analysis. Accounts Chem Res. 2002;35:817–825. doi: 10.1021/ar000138m. [DOI] [PubMed] [Google Scholar]
- 273.Mathe J, Aksimentiev A, Nelson DR, Schulten K, Meller A. Orientation discrimination of single-stranded DNA inside the alpha-hemolysin membrane channel. Proc Natl Acad Sci U S A. 2005;102:12377–12382. doi: 10.1073/pnas.0502947102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.de Zoysa RSS, Jayawardhana DA, Zhao QT, Wang DQ, Armstrong DW, Guan XY. Slowing DNA translocation through nanopores using a solution containing organic salts. J Phys Chem B. 2009;113:13332–13336. doi: 10.1021/jp9040293. [DOI] [PubMed] [Google Scholar]
- 275.Astier Y, Uzun O, Stellacci F. Electrophysiological study of single gold nanoparticle/alpha-hemolysin complex formation: a nanotool to slow down ssDNA through the alpha-hemolysin nanopore. Small. 2009;5:1273–1278. doi: 10.1002/smll.200801779. [DOI] [PubMed] [Google Scholar]
- 276.Astier Y, Braha O, Bayley H. Toward single molecule DNA sequencing: Direct identification of ribonucleoside and deoxyribonucleoside 5′-monophosphates by using an engineered protein nanopore equipped with a molecular adapter. J Am Chem Soc. 2006;128:1705–1710. doi: 10.1021/ja057123+. [DOI] [PubMed] [Google Scholar]
- 277.Drmanac R, Drmanac S, Chui G, Diaz R, Hou A, Jin H, Jin P, Kwon S, Lacy S, Moeur B, et al. In: Advances in biochemical engineering/biotechnology: chip technology. Scheper T, editor. Springer-Verlag; 2002. [Google Scholar]
- 278.Vercoutere W, Winters-Hilt S, Olsen H, Deamer D, Haussler D, Akeson M. Rapid discrimination among individual DNA hairpin molecules at single-nucleotide resolution using an ion channel. Nat Biotechnol. 2001;19:248–252. doi: 10.1038/85696. [DOI] [PubMed] [Google Scholar]
- 279.Vercoutere WA, Winters-Hilt S, DeGuzman VS, Deamer D, Ridino SE, Rodgers JT, Olsen HE, Marziali A, Akeson M. Discrimination among individual Watson-Crick base pairs at the termini of single DNA hairpin molecules. Nucleic Acids Res. 2003;31:1311–1318. doi: 10.1093/nar/gkg218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Howorka S, Movileanu L, Braha O, Bayley H. Kinetics of duplex formation for individual DNA strands within a single protein nanopore. Proc Natl Acad Sci U S A. 2001;98:12996–13001. doi: 10.1073/pnas.231434698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Lucas SW, Harding MM. Detection of DNA via an ion channel switch biosensor. Anal Biochem. 2000;282:70–79. doi: 10.1006/abio.2000.4568. [DOI] [PubMed] [Google Scholar]
- 282.Rhee M, Burns MA. Nanopore sequencing technology: nanopore preparations. Trends Biotechnol. 2007;25:174–181. doi: 10.1016/j.tibtech.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 283.Martin CR, Siwy ZS. Learning nature’s way: Biosensing with synthetic nanopores. Science. 2007;317:331–332. doi: 10.1126/science.1146126. [DOI] [PubMed] [Google Scholar]
- 284••.Li J, Stein D, McMullan C, Branton D, Aziz MJ, Golovchenko JA. Ion-beam sculpting at nanometre length scales. Nature. 2001;412:166–169. doi: 10.1038/35084037. Fabrication of synthetic, solid-state pores on the scale of biological pores by using low-energy ion beams. [DOI] [PubMed] [Google Scholar]
- 285.Iqbal SM, Akin D, Bashir R. Solid-state nanopore channels with DNA selectivity. Nat Nanotechnol. 2007;2:243–248. doi: 10.1038/nnano.2007.78. [DOI] [PubMed] [Google Scholar]
- 286.Heng JB, Ho C, Kim T, Timp R, Aksimentiev A, Grinkova YV, Sligar S, Schulten K, Timp G. Sizing DNA using a nanometer-diameter pore. Biophys J. 2004;87:2905–2911. doi: 10.1529/biophysj.104.041814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Healy K. Nanopore-based single-molecule DNA analysis. Nanomedicine. 2007;2:459–481. doi: 10.2217/17435889.2.4.459. [DOI] [PubMed] [Google Scholar]
- 288.Ryan D, Rahimi M, Lund J, Mehta R, Parviz BA. Toward nanoscale genome sequencing. Trends Biotechnol. 2007;25:385–389. doi: 10.1016/j.tibtech.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 289.Healy K, Schiedt B, Morrison AP. Solid-state nanopore technologies for nanopore-based DNA analysis. Nanomedicine. 2007;2:875–897. doi: 10.2217/17435889.2.6.875. [DOI] [PubMed] [Google Scholar]
- 290.Movileanu L, Schmittschmitt JP, Scholtz JM, Bayley H. Interactions of peptides with a protein pore. Biophys J. 2005;89:1030–1045. doi: 10.1529/biophysj.104.057406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291•.Wolfe AJ, Mohammad MM, Cheley S, Bayley H, Movileanu L. Catalyzing the translocation of polypeptides through attractive interactions. J Am Chem Soc. 2007;129:14034–14041. doi: 10.1021/ja0749340. This paper demonstrates that electrostatic interactions between the lumen of the pore and polypeptides can catalyze the translocation of polypeptides, thus, providing insight into the mechanisms of translocation of proteins and polypeptides across membranes. [DOI] [PubMed] [Google Scholar]
- 292.Mohammad MM, Prakash S, Matouschek A, Movileanu L. Controlling a single protein in a nanopore through electrostatic traps. J Am Chem Soc. 2008;130:4081–4088. doi: 10.1021/ja710787a. [DOI] [PubMed] [Google Scholar]
- 293•.Oukhaled G, Mathe J, Biance AL, Bacri L, Betton JM, Lairez D, Pelta J, Auvray L. Unfolding of proteins and long transient conformations detected by single nanopore recording. Phys Rev Lett. 2007;98:158101. doi: 10.1103/PhysRevLett.98.158101. This work detects the translocation of proteins through protein pores under conditions where the protein must denature in order to pass through the pore. [DOI] [PubMed] [Google Scholar]
- 294.Stefureac R, Waldner L, Howard P, Lee JS. Nanopore analysis of a small 86-residue protein. Small. 2008;4:59–63. doi: 10.1002/smll.200700402. [DOI] [PubMed] [Google Scholar]
- 295•.Sutherland TC, Long YT, Stefureac RI, Bediako-Amoa I, Kraatz HB, Lee JS. Structure of peptides investigated by nanopore analysis. Nano Lett. 2004;4:1273–1277. This work demonstrated that the secondary and tertiary structure of polypeptides can be determined from the translocation signature of these peptides through protein pores. [Google Scholar]
- 296.Stefureac R, Long YT, Kraatz HB, Howard P, Lee JS. Transport of alpha-helical peptides through alpha-hemolysin and aerolysin pores. Biochemistry. 2006;45:9172–9179. doi: 10.1021/bi0604835. [DOI] [PubMed] [Google Scholar]
- 297.Goodrich CP, Kirmizialtin S, Huyghues-Despointes BM, Zhu AP, Scholtz JM, Makarov DE, Movileanu L. Single-molecule electrophoresis of beta-hairpin peptides by electrical recordings and Langevin dynamics simulations. J Phys Chem B. 2007;111:3332–3335. doi: 10.1021/jp071364h. [DOI] [PubMed] [Google Scholar]
- 298.Sanderson JM. Peptide lipid interactions: insights and perspectives. Org Biomol Chem. 2005;3:201–212. doi: 10.1039/b415499a. [DOI] [PubMed] [Google Scholar]
- 299.Lauffenburger DA, Linderman JJ. Receptors: Models for Binding,. Trafficking, and Signaling. New York: Oxford University Press; 1993. [Google Scholar]
- 300.Mayer M, Terrettaz S, Giovangrandi L, Vogel H. Functional analysis of ion channels: planar patch clamp and impedance spectroscopy of tethered lipid membranes. In: Cooper JM, Cass AEG, editors. Biosensors: A Practical Approach. 2. Vol. 1. Oxford University Press; 2003. [Google Scholar]
- 301.Pawlak M, Meseth U, Dhanapal B, Mutter M, Vogel H. Template-assembled melittin—structural and functional-characterization of a designed, synthetic channel-forming protein. Protein Sci. 1994;3:1788–1805. doi: 10.1002/pro.5560031019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Fraenkelconrat H, Lewis JC, Dimick KP, Edwards B, Reitz HC, Ferrel RE, Brandon BA, Olcott HS. Chemical derivatives of gramicidin. Proc Soc Exp Biol Med. 1946;63:302–308. doi: 10.3181/00379727-63-15581. [DOI] [PubMed] [Google Scholar]
- 303.Eskesen K, Kristensen BI, Jorgensen AJ, Kristensen P, Bennekou P. Calcium-dependent association of annexins with lipid bilayers modifies gramicidin A channel parameters. Eur Biophys J Biophys. 2001;30:27–33. doi: 10.1007/s002490000114. [DOI] [PubMed] [Google Scholar]
- 304.Majd S, Estes DJ, Mayer M. Assays for studying annexin binding to artificial lipid bilayers. Calcium Binding Proteins. 2006;1:26–29. [Google Scholar]
- 305.Futaki S, Zhang YJ, Kiwada T, Nakase I, Yagami T, Oiki S, Sugiura Y. Gramicidin-based channel systems for the detection of protein–ligand interaction. Biorg Med Chem. 2004;12:1343–1350. doi: 10.1016/j.bmc.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 306.Zhang Y, Futaki S, Kiwada T, Sugiura Y. Detection of protein–ligand interaction on the membranes using C-terminus biotin-tagged alamethicin. Biorg Med Chem. 2002;10:2635–2639. doi: 10.1016/s0968-0896(02)00105-0. [DOI] [PubMed] [Google Scholar]
- 307.Antonenko Y, Rokitskaya T, Kotova E. Monovalent and multivalent binding of streptavidin to biotinylated gramicidin determines kinetic properties of the ion channel. Biophys J. 2002;82:2720. doi: 10.1023/b:biry.0000018955.61289.c8. [DOI] [PubMed] [Google Scholar]
- 308.Antonenko YN, Rokitskaya TI, Kotova EA, Reznik GO, Sano T, Cantor CR. Effect of streptavidins with varying biotin binding affinities on the properties of biotinylated gramicidin channels. Biochemistry. 2004;43:4575–4582. doi: 10.1021/bi034984r. [DOI] [PubMed] [Google Scholar]
- 309.Rokitskaya TI, Antonenko YN, Kotova EA, Anastasiadis A, Separovic F. Effect of avidin on channel kinetics of biotinylated gramicidin. Biochemistry. 2000;39:13053–13058. doi: 10.1021/bi0007876. [DOI] [PubMed] [Google Scholar]
- 310.Movileanu L, Howorka S, Braha O, Bayley H. Detecting protein analytes that modulate transmembrane movement of a polymer chain within a single protein pore. Nat Biotechnol. 2000;18:1091–1095. doi: 10.1038/80295. [DOI] [PubMed] [Google Scholar]
- 311.Howorka S, Nam J, Bayley H, Kahne D. Stochastic detection of monovalent and bivalent protein–ligand interactions. Angew Chem Int Ed. 2004;43:842–846. doi: 10.1002/anie.200352614. [DOI] [PubMed] [Google Scholar]
- 312.Lewis JC, Dimick KP, Feustel IC, Fevold HL, Olcott HS, Fraenkelconrat H. Modification of gramicidin through reaction with formaldehyde. Science. 1945;102:274–275. doi: 10.1126/science.102.2646.274. [DOI] [PubMed] [Google Scholar]
- 313•.Mindell JA, Zhan HJ, Huynh PD, Collier RJ, Finkelstein A. Reaction of diphtheria-toxin channels with sulfhydryl-specific reagents - observation of chemical-reactions at the single-molecule level. Proc Natl Acad Sci U S A. 1994;91:5272–5276. doi: 10.1073/pnas.91.12.5272. This work presented the first application of a biological pore, the diphtheria toxin pore, for monitoring chemical reactions to achieve single-molecule resolution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Luchian T, Shin SH, Bayley H. Single-molecule covalent chemistry with spatially separated reactants. Angew Chem Int Ed. 2003;42:3766–3771. doi: 10.1002/anie.200351313. [DOI] [PubMed] [Google Scholar]
- 315.Donovan JJ, Simon MI, Draper RK, Montal M. Diphtheria-toxin forms transmembrane channels in planar lipid bilayers. Proc Natl Acad Sci U S A-Biol Sci. 1981;78:172–176. doi: 10.1073/pnas.78.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Jaikaran DCJ, Woolley GA. Characterization of thermal-isomerization at the single-molecule level. J Phys Chem. 1995;99:13352–13355. [Google Scholar]
- 317.Blake S, Capone R, Mayer M, Yang J. Chemically reactive derivatives of gramicidin A for developing ion channel-based nanoprobes. Bioconjugate Chem. 2008;19:1614–1624. doi: 10.1021/bc800180z. [DOI] [PubMed] [Google Scholar]
- 318•.Luchian T, Shin SH, Bayley H. Kinetics of a three-step reaction observed at the single-molecule level. Angew Chem Int Ed. 2003;42:1925–1929. doi: 10.1002/anie.200250666. This paper presented the first application of a protein pore, α-hemolysin, for monitoring a multistep chemical reaction at the single-molecule level. [DOI] [PubMed] [Google Scholar]
- 319••.Shin SH, Luchian T, Cheley S, Braha O, Bayley H. Kinetics of a reversible covalent-bond-forming reaction observed at the single-molecule level. Angew Chem Int Ed. 2002;41:3707–3709. doi: 10.1002/1521-3773(20021004)41:19<3707::AID-ANIE3707>3.0.CO;2-5. Monitoring the formation and cleavage of a disulfide bond by α-hemolysin pore resulted, for the first time, in the observation of a short-lived intermediate. [DOI] [PubMed] [Google Scholar]
- 320.Bezrukov SM, Rand RP, Vodyanoy I, Parsegian VA. Lipid packing stress and polypeptide aggregation: alamethicin channel probed by proton titration of lipid charge. Faraday Discuss. 1998;111:173–183. doi: 10.1039/a806579i. [DOI] [PubMed] [Google Scholar]
- 321.McHaourab HS, Hyde JS, Feix JB. Binding and state of aggregation of spin-labeled cecropin AD in phospholipid-bilayers—effects of surface-charge and fatty acyl-chain length. Biochemistry. 1994;33:6691–6699. doi: 10.1021/bi00187a040. [DOI] [PubMed] [Google Scholar]
- 322.Sobko AA, Kotova EA, Antonenko YN, Zakharov SD, Cramer WA. Lipid dependence of the channel properties of a colicin E1-lipid toroidal pore. J Biol Chem. 2006;281:14408–14416. doi: 10.1074/jbc.M513634200. [DOI] [PubMed] [Google Scholar]
- 323.Rostovtseva TK, Aguilella VM, Vodyanoy I, Bezrukov SM, Parsegian VA. Membrane surface-charge titration probed by gramicidin A channel conductance. Biophys J. 1998;75:1783–1792. doi: 10.1016/S0006-3495(98)77620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Kell MJ, Defelice LJ. Surface-charge near the cardiac inward-rectifier channel measured from single-channel conductance. J Membr Biol. 1988;102:1–10. doi: 10.1007/BF01875348. [DOI] [PubMed] [Google Scholar]
- 325.Borisenko V, Zhang ZH, Woolley GA. Gramicidin derivatives as membrane-based pH sensors. Biochim Biophys Acta-Biomembr. 2002;1558:26–33. doi: 10.1016/s0005-2736(01)00415-1. [DOI] [PubMed] [Google Scholar]
- 326.Myher JJ, Kuksis A, Pind S. Molecular species of glycerophospholipids and sphingomyelins of human erythrocytes: improved method of analysis. Lipids. 1989;24:396–407. doi: 10.1007/BF02535147. [DOI] [PubMed] [Google Scholar]
- 327.Lundbaek JA, Birn P, Hansen AJ, Sogaard R, Nielsen C, Girshman J, Bruno MJ, Tape SE, Egebjerg J, Greathouse DV. Regulation of sodium channel function by bilayer elasticity: the importance of hydrophobic coupling. Effects of micelle-forming amphiphiles and cholesterol. J Gen Physiol. 2004;123:599–621. doi: 10.1085/jgp.200308996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Lundbaek JA. Regulation of membrane protein function by lipid bilayer elasticity—a single molecule technology to measure the bilayer properties experienced by an embedded protein. J Phys: Condens Matter. 2006;18:S1305–S1344. doi: 10.1088/0953-8984/18/28/S13. [DOI] [PubMed] [Google Scholar]
- 329•.Andersen OS, Koeppe RE. Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. This excellent review describes, in details, the role of lipid bilayer on the distribution, organization, and function of integral proteins within cellular membranes. [DOI] [PubMed] [Google Scholar]
- 330.Nielsen C, Andersen OS. Inclusion-induced bilayer deformations: effects of monolayer equilibrium curvature. Biophys J. 2000;79:2583–2604. doi: 10.1016/S0006-3495(00)76498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Winterhalter M, Helfrich W. Effect of surface-charge on the curvature elasticity of membranes. J Phys Chem. 1988;92:6865–6867. [Google Scholar]
- 332.Nipper ME, Majd S, Mayer M, Lee JCM, Theodorakis EA, Haidekker MA. Characterization of changes in the viscosity of lipid membranes with the molecular rotor FCVJ. Biochim Biophys Acta-Biomembr. 2008;1778:1148–1153. doi: 10.1016/j.bbamem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 333.Arseniev AS, Lomize AL, Barsukov IL, Bystrov VF. Gramicidin A transmembrane ion-channel. Three-dimensional structure reconstruction based on NMR spectroscopy and energy refinement. Biol Membr. 1986;3:1077–1104. [Google Scholar]
- 334.Gorostiza P, Isacoff E. Optical switches and triggers for the manipulation of ion channels and pores. Mol BioSys. 2007;3:686–704. doi: 10.1039/b710287a. [DOI] [PubMed] [Google Scholar]
- 335.Kramer RH, Fortin DL, Trauner D. New photochemical tools for controlling neuronal activity. Curr Opin Neurobiol. 2009;19:544–552. doi: 10.1016/j.conb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Stankovic CJ, Heinemann SH, Schreiber SL. Photo-modulated ion channels based on covalently linked gramicidins. Biochim Biophys Acta. 1991;1061:163–170. doi: 10.1016/0005-2736(91)90281-c. [DOI] [PubMed] [Google Scholar]
- 337.Lougheed T, Borisenko V, Hennig T, Rück-Braun K, Woolley GA. Photomodulation of ionic current through hemithioindigo-modified gramicidin channels. Org Biomol Chem. 2004;2:2798–2801. doi: 10.1039/B408485C. [DOI] [PubMed] [Google Scholar]
- 338.Lien L, Jaikaran DCJ, Zhang Z, Woolley GA. Photomodulated blocking of gramicidin ion channels. J Am Chem Soc. 1996;118:12222–12223. [Google Scholar]
- 339.Fortin DL, Banghart MR, Dunn TW, Borges K, Wagenaar DA, Gaudry Q, Karakossian MH, Otis TS, Kristan WB, Trauner D. Photochemical control of endogenous ion channels and cellular excitability. Nat Methods. 2008;5:331–338. doi: 10.1038/nmeth.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol. 2006;2:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Cogan SF. Neural stimulation and recording electrodes. Annu Rev Biomed Eng. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- 342.Merrill DR, Bikson M, Jefferys JGR. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods. 2005;141:171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 343.Nilsson D, Chen MX, Kugler T, Remonen T, Armgarth M, Berggren M. Bi-stable and dynamic current modulation in electrochemical organic transistors. Adv Mater. 2002;14:51–54. [Google Scholar]
- 344.Fromherz P, Kiessling V, Kottig K, Zeck G. Membrane transistor with giant lipid vesicle touching a silicon chip. Appl Phys A-Mater Sci Process. 1999;69:571–576. [Google Scholar]
- 345.Simmel FC. Bioelectronics: wiring-up ion channels. Nat Phys. 2009;5:783–784. [Google Scholar]
- 346.Zeck G, Fromherz P. Noninvasive neuroelectronic interfacing with synaptically connected snail neurons immobilized on a semiconductor chip. Proc Natl Acad Sci U S A. 2001;98:10457–10462. doi: 10.1073/pnas.181348698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Xu J, Lavan DA. Designing artificial cells to harness the biological ion concentration gradient. Nat Nanotechnol. 2008;3:666–670. doi: 10.1038/nnano.2008.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Neudeck GW. In: The pn junction diode. 2. Neudeck GW, Pierret RF, editors. Vol. 2. Addison-Wesley Publishing Company, Inc; 1989. [Google Scholar]
- 349.Sedra AS, Smith KC. Microelectronic Circuits. Vol. 4. New York: Oxford University Press; 1998. [Google Scholar]
- 350•.Macrae MX, Blake S, Mayer M, Yang J. Nanoscale ionic diodes with tunable and switchable rectifying behavior. J Am Chem Soc. 2010;132:1766–1767. doi: 10.1021/ja909876h. This paper presents probably the smallest possible fluidic diode based on gramicidin peptides. [DOI] [PubMed] [Google Scholar]
- 351.He Y, Gillespie D, Boda D, Vlassiouk I, Eisenberg RS, Siwy ZS. Tuning transport properties of nanofluidic devices with local charge inversion. J Am Chem Soc. 2009;131:5194–5202. doi: 10.1021/ja808717u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Karnik R, Duan CH, Castelino K, Daiguji H, Majumdar A. Rectification of ionic current in a nanofluidic diode. Nano Lett. 2007;7:547–551. doi: 10.1021/nl062806o. [DOI] [PubMed] [Google Scholar]
- 353.Vlassiouk I, Siwy ZS. Nanofluidic diode. Nano Lett. 2007;7:552–556. doi: 10.1021/nl062924b. [DOI] [PubMed] [Google Scholar]
- 354.Yusko EC, An R, Mayer M. Electroosmotic flow can generate ion current rectification in nano- and micropores. ACS Nano. 2010;4:477–487. doi: 10.1021/nn9013438. [DOI] [PubMed] [Google Scholar]
- 355.Alcaraz A, Ramirez P, Garcia-Gimenez E, Lopez ML, Andrio A, Aguilella VM. A pH-tunable nanofluidic diode: Electrochemical rectification in a reconstituted single ion channel. J Phys Chem B. 2006;110:21205–21209. doi: 10.1021/jp063204w. [DOI] [PubMed] [Google Scholar]
- 356.Miedema H, Vrouenraets M, Wierenga J, Meijberg W, Robillard G, Eisenberg B. A biological porin engineered into a molecular, nanofluidic diode. Nano Lett. 2007;7:2886–2891. doi: 10.1021/nl0716808. [DOI] [PubMed] [Google Scholar]
- 357.Sarles SA, Leo DJ. Tailored current-voltage relationships of droplet-interface bilayers using biomolecules and external feedback control. J Intell Mater Syst Struct. 2009;20:1233–1247. [Google Scholar]
- 358.Maglia G, Henricus M, Wyss R, Li Q, Cheley S, Bayley H. DNA strands from denatured duplexes are translocated through engineered protein nanopores at alkaline pH. Nano Lett. 2009;9:3831–3836. doi: 10.1021/nl9020232. [DOI] [PubMed] [Google Scholar]
- 359.Gracheva ME, Vidal J, Leburton JP. p–n semiconductor membrane for electrically tunable ion current rectification and filtering. Nano Lett. 2007;7:1717–1722. doi: 10.1021/nl0707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Gracheva ME, Melnikov DV, Leburton JP. Multilayered semiconductor membranes for nanopore ionic conductance modulation. ACS Nano. 2008;2:2349–2355. doi: 10.1021/nn8004679. [DOI] [PubMed] [Google Scholar]
- 361.Aguilella VM, Alcaraz A. A fluid approach to simple circuits. Nat Nanotechnol. 2009;4:403–404. doi: 10.1038/nnano.2009.168. [DOI] [PubMed] [Google Scholar]
- 362.Hille B. Ion channels of excitable membranes. 3. Sunderland, Massachusetts, U.S.A: Sinauer Associates, Inc; 2001. [Google Scholar]
- 363.Bernards DA, Malliaras GG, Toombes GES, Gruner SM. Gating of an organic transistor through a bilayer lipid membrane with ion channels. Appl Phys Lett. 2006:89. [Google Scholar]
- 364.Andersen OS. Gramicidin channels. Annu Rev Physiol. 1984;46:531–548. doi: 10.1146/annurev.ph.46.030184.002531. [DOI] [PubMed] [Google Scholar]
- 365.Havener R, Boyea J, Malone E, Bernards D, DeFranco J, Malliaras G, Lipson H. Freeform fabrication of organic electrochemical transistors. Proceedings of the 18th Solid Freeform Fabrication Symposium; Austin TX. 2007. pp. 60–73. [Google Scholar]
- 366.Xu J, Sigworth FJ, LaVan DA. Synthetic protocells to mimic and test cell function. Adv Mater. 2010;22:120–127. doi: 10.1002/adma.200901945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Fromherz P, Offenhausser A, Vetter T, Weis J. A neuron-silicon junction—a retzius cell of the leech on an insulated-gate field-effect transistor. Science. 1991;252:1290–1293. doi: 10.1126/science.1925540. [DOI] [PubMed] [Google Scholar]
- 368.Sakmann B, Neher E, editors. Single-Channel Recording. 2. New York: Plenum Publishing Corporation; 1995. [Google Scholar]
- 369.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Del Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 370••.Jovanovic-Talisman T, Tetenbaum-Novatt J, McKenney AS, Zilman A, Peters R, Rout MP, Chait BT. Artificial nanopores that mimic the transport selectivity of the nuclear pore complex. Nature. 2009;457:1023–1027. doi: 10.1038/nature07600. This paper presented a unique application of synthetic nanopores; decorating synthetic pores with a lining of transport-factor-binding proteins made these pores able to mimic the transport selectivity of nuclear pore complexes. [DOI] [PMC free article] [PubMed] [Google Scholar]