Abstract
Epstein–Barr virus (EBV) latent infection membrane protein 1 (LMP1)-induced NF-κB activation is important for infected cell survival. LMP1 activates NF-κB, in part, by engaging tumor necrosis factor (TNF) receptor-associated factors (TRAFs), which also mediate NF-κB activation from LTβR and CD40. LTβR and CD40 activation of p100/NF-κB2 is now known to be NIK/IKKα-dependent and IKKβ/IKKγ independent. In the experiments described here, we found that EBV LMP1 induced p100/NF-κB2 processing in human lymphoblasts and HEK293 cells. LMP1-induced p100 processing was NIK/IKKα dependent and IKKβ/IKKγ independent. Furthermore, the LMP1 TRAF-binding site was required for p100 processing and p52 nuclear localization, whereas the LMP1 death domain-binding site was not. Moreover, the LMP1 TRAF-binding site preferentially caused RelB nuclear accumulation. In murine embryo fibroblasts (MEFs), IKKβ was essential for LMP1 up-regulation of macrophage inflammatory protein (MIP)-2, TNFα, I-TAC, ELC, MIG, and CXCR4 RNAs. Interestingly, in IKKα knockout MEFs, LMP1 hyperinduced MIP-2, TNFα, and I-TAC expression, consistent with a role for IKKα in down-modulating canonical IKKβ activation or its effects. In contrast, LMP1 failed to up-regulate CXCR4 and MIG RNA in IKKα knockout MEFs, indicating a dependence on noncanonical IKKα activation. Furthermore, LMP1 up-regulation of MIP-2 RNA in MEFs was both IKKβ- and IKKγ-dependent, whereas LMP1 upregulation of MIG and I-TAC RNA was fully IKKγ independent. Thus, LMP1 induces typical canonical IKKβ/IKKγ-dependent, atypical canonical IKKβ-dependent/IKKγ-independent, and noncanonical NIK/IKKα-dependent NF-κB activations; NIK/IKKα-dependent NF-κB activation is principally mediated by the LMP1 TRAF-binding site.
Tumor necrosis factor (TNF) and Toll/IL-1 receptor (TIR)-mediated NF-κB activation is required for innate and adaptive immune responses to pathogens, secondary lymphoid organ development, lymphocyte maturation, and inflammatory responses (1–4). TIRs and TNF receptors (TNFRs) signal through adaptor molecules and kinases to activate the IKKα and IKKβ kinases, which regulate the proteolysis of IκBα, IκBβ, or IκBε and enable NF-κB heterodimers to translocate to the nucleus and activate transcription (5–12). Most TIR and TNFR NF-κB activation is mediated by a complex of IKKα/IKKβ/IKKγ, which is referred to as canonical NF-κB activation. Frequently, canonical NF-κB activation initially results in translocation of p50/RelA heterodimers, which then up-regulate transcription of other NF-κB components. Canonical NF-κB activation depends on IKKβ and IKKγ, but can be independent of IKKα (9, 12–14).
BAFF, LTβ, CD40L, and lipopolysaccharide (LPS) also induce noncanonical NF-κB activation (15–22). Noncanonical NF-κB activation is mediated by IKKα and the upstream kinase NIK. BAFF, LTβ, CD40L, or LPS receptors engage cytoplasmic signaling molecules such as TNFR-associated factors (TRAFs), which can activate NIK, which then activates IKKα to phosphorylate the p100/NF-κB2 precursor, leading to ubiquitylation and proteasome processing of the p100 C terminus and nuclear translocation of p52-RelB heterodimers to activate transcription. In contrast to canonical NF-κB activation, LTβ-, BAFF-, CD40L-, and LPS-induced noncanonical NF-κB activation does not require IKKβ or IKKγ (15–17, 23–26). Noncanonical signaling is crucial to secondary lymphoid organ development as is evident in LTβR, NIK, IKKα, RelB, and p52 knockout (KO) mice (16, 18, 24, 25, 27–33).
Epstein–Barr virus (EBV) latent infection membrane protein 1 (LMP1) mimics a constitutively activated TNFR (34). LMP1 is essential for EBV conversion of infected B lymphocytes into perpetually proliferating lymphoblasts (35, 36) and is expressed in EBV-associated lymphoproliferative disease, Hodgkin's disease, and nasopharyngeal carcinoma (for review, see ref. 37). LMP1 has six hydrophobic transmembrane domains that constitutively self aggregate (38, 39). Aggregated LMP1 signals through two C-terminal cytoplasmic domains, an essential domain that engages TRAF3, TRAF1, TRAF2, and TRAF5 and a critical domain that engages TNFR-associated death domain proteins including TRADD and RIP (34, 40–42). Both domains activate NF-κB (43–45). LMP1 induces p100 and p105 NF-κB precursors and up-regulates their processing into p52 and p50, respectively (46), implicating LMP1 in both canonical NF-κB up-regulation and noncanonical p100 processing into p52. Furthermore, LMP1 engages TRAFs, including TRAF3 through a site similar to that of LTβR and CD40 (34, 47, 48) and the same site specifically activates TRAF1 and epidermal growth factor receptor expression (40, 49), compatible with the notion that the LMP1 TRAF-binding site might mediate an element of noncanonical as well as canonical NF-κB activation. These experiments were therefore undertaken to investigate whether the LMP1 TRAF-binding site has a particularly important role in noncanonical NF-κB activation.
Materials and Methods
Plasmids. pSG5-FLMP1 W T, P204A/Q206A (A A), and YYD384–386ID (ID), pCDNA3-FIKKβ KM (Δ34), pRK5-mycIKKα KM (K44A), pCDNA3-wt or aly DN-NIK, 3XκBL, and pGK-β-galactosidase (β-gal) have been described (41, 48, 50, 51). LMP1 double mutant (DM) has both the AA and ID mutations. GFP, LMP1 WT, and LMP1 DM retroviral expression constructs were created by PCR-cloning of the cDNAs as HindIII–NotI fragments into pEAK12-MMP (52). Retroviral stocks were made by harvesting supernatants 72 h after transfection of HEK293T cells with pEAK12-MMP, gag-pol, and vesicular stomatitis virus (VSV)-G expression constructs.
Cell Lines, Transductions, and Reporter Gene Assays. IKKα, IKKβ, and IKKγ KO murine embryonic fibroblasts (MEFs) were kindly provided by M. Karin (IKKα and IKKβ) and by M. Parsparakis and K. Rajewsky (IKKγ) (9, 13, 53). MEFs were maintained in DMEM supplemented with 10% FBS (D10) (Invitrogen), 2 mM l-glutamine, and antibiotics. Parental (10.10.3) or IKKγ-deficient (A45) Jurkat cells were maintained in RPMI medium 1640 supplemented with 10% FBS (Invitrogen), 2 mM lglutamine, and antibiotics. Jurkat cells and MEFs were transduced with retroviral supernatants in the presence of 1–4 μg/ml polybrene.
Stable BJAB cells expressing FLMP1 WT, AA, and ID were previously described (54), as were stable LMP1 expressing C33A cells (40). HEK293T cells were maintained in D10 with supplements as above. Transient transfections used Lipofectamine 2000 (Invitrogen). Luciferase reporter assays were normalized for transfection efficiency by cotransfecting a β-gal expression plasmid and dividing luciferase by β-gal activities at 20–24 h after transfection. Luciferase activity in the presence of LMP1 was plotted relative to luciferase reporter (3XκBL) in the absence of LMP1. Luciferase assays (Promega) and β-gal activities (TROPIX) were measured by using manufacturers protocols.
Quantitative RT-PCR (QPCR). Total RNA was extracted from MEFs by using the RNeasy kit according to the manufacturer's protocol (Qiagen, Valencia, CA). Briefly, after DNase I (Invitrogen) treatment, 1 μg of total RNA from each sample was used as template for reverse transcription in a 100-μl reaction with oligo(dT)15, random hexamers, and multiscribe reverse transcriptase (Applied Biosystems) at 25°C for 10 min followed by 48°C for 30 min. QPCR reactions were in 25 μl with 2 μl of cDNA, 12.5 μlof2× SYBR Green master mix (Stratagene), and 250 nmol of sense and antisense primer. The QPCR reactions were performed by using the MX4000 Multiplex quantitative PCR system (Stratagene). The reaction conditions were 50°C for 2 min, 95°C for 10 min, then 40 cycles of 95°C for 15 sec and 60°C for 1 min. Emitted fluorescence for each reaction was measured three times during the annealing/extension phase, and amplification plots were analyzed by using the MX4000 software version 3.0 (Stratagene). Analysis was performed on the data output from the MX4000 software by using Microsoft excel. Subsequently, the threshold cycle (Ct) was determined by using the adaptive baseline algorithm in MX4000. Quantity values (copies) for gene expression were generated by comparing the fluorescence of each sample with known quantities. The calculated number of copies was divided by the number of copies of β2-microglobulin.
Results
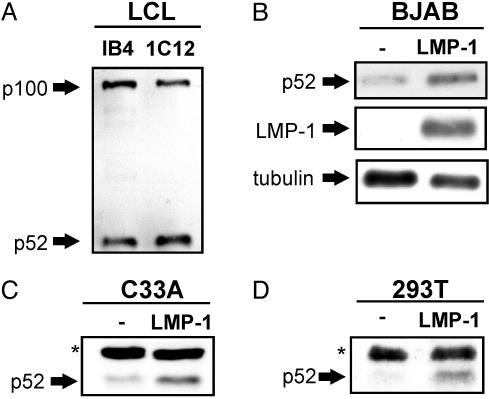
LMP1 Induces p100 Processing to p52 in B Lymphocytes and Epithelial Cells. EBV-transformed lymphoblastoid cell lines (LCLs) exhibit constitutive p100 processing as evidenced by p52 detection in gel shifts (55) and in immunoblots of cell lysates; p52 and p100 levels are similar in established and recently derived IB4 and 1C12 LCLs, respectively (Fig. 1A). Furthermore, LMP1 converted BJAB lymphoblasts, and C33A epithelial cells had higher p52 levels than expression vector converted nonexpressing control cells (Fig. 1 B and C). Moreover, LMP1 expression in HEK293T cells also induced higher p52 levels than control vector transfection (Fig. 1D and data not shown).
Fig. 1.
LMP1 induces p100 processing to p52 in B lymphocytes and epithelial cells. (A) Lysates of EBV growth transformed lymphoblastoid cell lines, IB4 and 1C12, have high levels of p100 and p52 as detected by SDS/PAGE and immunoblot using p52-specific antibody (Upstate Biotechnology, catalog no. 06-413). (B and C) Lysates of EBV uninfected BJAB B lymphoma or C33A carcinoma cells that stably express LMP1 have higher levels of p52 than BJAB or C33A cells that do not express LMP1 as detected by SDS/PAGE and immunoblot using p52, LMP1 (S12 monoclonal), and tubulin control (Sigma B-5–1-2) antibodies. (D) Lysates of HEK293T cells 24 h after transfection with 1 μg of pSG5-FLMP1 (FLAG tagged) have higher levels of p52 than lysates of cells transfected with vector control plasmid as detected by SDS/PAGE and p52 immunoblot. Asterisks indicate nonspecific bands. The results shown are representative of findings from multiple experiments.
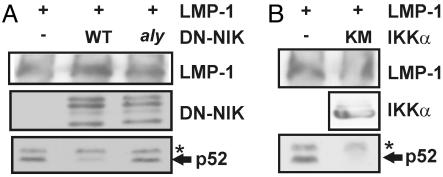
LMP1-Induced p100 Processing Depends on NIK and IKKα. The role of NIK and IKKα in LMP1-induced p100 processing was evaluated by expression of dominant negative NIK624–947 (DN-NIK), aly point mutant DN-NIK (DN-NIKaly), or IKKα K44A kinase point mutant (KM-IKKα) alleles with LMP1 in HEK293T cells. DN-NIK or KM-IKKα mutant alleles inhibited LMP1-induced p100 processing to p52 without affecting LMP1 or p100 expression levels (Fig. 2 A and B and data not shown). However, DN-NIK with the aly point mutation, which is unable to associate with IKKα (29, 51) and likely cannot block endogenous NIK association with IKKα, did not inhibit LMP1-induced p100 processing to p52, despite similar expression levels (Fig. 2 A). Thus, NIK association with IKKα has a critical role in LMP1-induced p52 processing from p100.
Fig. 2.
LMP1-induced p100 processing is specifically blocked by NIK or IKKα dominant negative mutant alleles but not by the NIK aly dominant negative allele. HEK293T cells were cotransfected with 1 μg of pSG5-FLMP1 and 1 μgof expression plasmid for WT or aly NIK624–947 (A) or myc-tagged kinase negative IKKα K44A (IKKα KM) (B). After 24 h, cells were harvested, lysed, and analyzed by SDS/PAGE and immunoblot for p52, LMP1, NIK, and myc-tagged IKKα levels. Asterisks indicate nonspecific bands. The results shown are representative of at least two similar experiments for each transfection.
LMP1-Induced p100 Processing Is Independent of IKKβ and IKKγ. The role of IKKβ in LMP1-induced p100 processing was evaluated by expressing a dominant negative kinase inactive (KM) IKKβ allele along with LMP1 in HEK293T cells and examining the effect on LMP1-induced p100 processing to p52. In contrast to NIK and IKKα dominant negative alleles, LMP1-induced p52 levels were minimally affected by expression of the IKKβ KM dominant negative (Fig. 3A). The minimal effect of the IKKβ KM allele on LMP1-induced p100 processing contrasted with the potent inhibition of LMP1-mediated activation of a canonical NF-κB-dependent reporter by the IKKβ KM allele (ref. 50 and data not shown).
Fig. 3.
LMP1-induced p100 processing is not IKKβ- or IKKγ-dependent. (A) HEK293T cells were cotransfected with 1 μg of pSG5-FLMP1 and with 1 μgof pCDNA3 or pCDNA3-FIKKβΔ34, which is a kinase mutant (KM) allele. After 24 h, cells were harvested, lysed, and analyzed by SDS/PAGE and immunoblot for p52, LMP1, and F-IKKβ using M2 antibody (Sigma). Results shown are representative of several independent transfections with LMP1 and IKKβΔ34. (B) Jurkat cells (IKKγ WT) and IKKγ-deficient Jurkat cells (IKKγ KO) were lysed and analyzed by SDS/PAGE and immunoblot with IKKγ (kindly provided by M. Horwitz; ref. 64) and tubulin control antibodies or incubated for the indicated times (in minutes) with 100 ng/ml human TNF-α and analyzed by SDS/PAGE and immunoblot for endogenous IκBα (Santa Cruz Biotechnology C-21). (C) Jurkat or IKKγ-deficient Jurkat cells were transduced with a retrovirus expressing WT LMP1 (WT) or DM LMP1 (DM), which is mutated at the TRAF- and death domain protein-binding sites. After 24 h, cells were harvested and immunoblotted for endogenous p52. Asterisks indicate nonspecific bands. Experiments in IKKγ-deficient Jurkat cells were repeated three times with similar results.
To assess the role of IKKγ in LMP1-induced p100 processing to p52, the effect of LMP1 expression on p52 induction was compared in IKKγ deficient and WT Jurkat cells (Fig. 3 B and C). As expected (12, 53), TNF-α induced IκBα degradation in Jurkat cells and had no affect on IκBα in Jurkat cells that specifically lacked IKKγ expression (Fig. 3B). In contrast, LMP1-induced p100 processing was similar in WT and IKKγ-negative Jurkat cells, whereas an LMP1 allele that is DM for the TRAF and death domain-binding sites failed to induce p52 (Fig. 3C). Thus, IKKβ and IKKγ are not essential for LMP1 induction of p52 in HEK293 or Jurkat cells, consistent with this induction being mediated by an IKKα complex independent of IKKβ and IKKγ, as has been described for CD40L, LTβ, LPS, and BAFF (15–17, 23–26).
LMP1-Induced p100 Processing Requires the TRAF-Binding Site but Not the Death Domain-Binding Site. To assess the relative roles of the LMP1 TRAF and death domain-binding sites in LMP1-induced p100 processing, HEK293T cells were transfected with expression plasmids for WT LMP1, LMP1 with null mutations (AA in the TRAF or ID in the death domain-binding site), or LMP1 DM, which is doubly mutant with AA and ID. LMP1 activated a minimal promoter with three upstream repeats of the MHC class I NF-κB site and a luciferase reporter ≈15-fold, whereas LMP1 DM was similar to vector control and LMP1AA and LMP1ID each activated 6- to 7-fold (Fig. 4A and refs. 40 and 45). When extracts from these cells were immunoblotted for p52, LMP1 and LMP1ID had induced p52 processing from p100, whereas LMP1AA was consistently less active and LMP1DM failed to induce p52 processing (Fig. 4A). By immunoblot, LMP1 and LMP1 mutants were expressed at similar levels (data not shown). In stably transfected BJAB B lymphoblasts, LMP1 WT and LMP1ID also induced p100 processing to p52, and LMP1AA was substantially less active (Fig. 4B). Thus, the LMP1 TRAF-binding domain has a uniquely critical role in p100 processing to p52 in human epithelial cells and B lymphoblasts.
Fig. 4.
LMP1-induced p100 processing depends on the TRAF-binding site but not the death domain-binding site. (A) HEK293T cells were transfected with 1 μg of pSG5-FLAG, pSG5-FLMP1 WT, pSG5-FLMP1 AA that is mutated for the TRAF-binding site, pSG5-FLMP1 ID that is mutated for the death domain protein-binding site, or pSG5-FLMP1 DM that is mutant at both sites along with 0.35 μg of a NF-κB-dependent luciferase reporter construct (3xκBL) and 0.35 μg of a constitutive β-gal expression construct (pGK-βgal). After 24 h, cells were harvested and luciferase and β-gal values were determined and are reported as fold increased luciferase divided by β-gal activity relative to vector transfected cells. Below the luciferase reporter values is an immunoblot of the transfected cell total lysates with anti-p52 antibody. The asterisk indicates a nonspecific band. WT and mutant LMP1 expression levels were similar as determined by immunoblot of whole cell lysates with M2 or S12 antibodies (data not shown). Results shown are representative of three independent experiments performed with these mutants. (B) BJAB cells that had been stably transfected with pSG5-FLAG or pSG5-FLMP1, pSG5-FLMP1 AA, or pSG5-FLMP1 ID or were lysed, and proteins were analyzed by SDS/PAGE and immunoblot for p52 and tubulin. WT and mutant LMP1 expression levels were similar (data not shown). Similar results were obtained in multiple experiments with these and other clones stably transfected with LMP1 WT and mutant alleles.
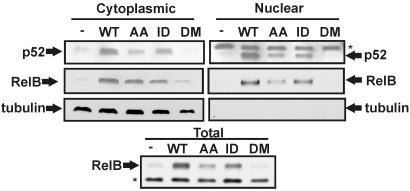
LMP1-Induced p52 and RelB Nuclear Localization and RelB Expression Requires the TRAF-Binding Site but Not the Death Domain-Binding Site. To assess the role of the LMP1 TRAF and death domain-binding sites in p52 and RelB nuclear translocation, cytoplasmic and nuclear fractions of WT or mutant LMP1-transfected HEK293T cells were separated and immunoblotted for p52 and RelB. RelB and p52 were not detected or barely detected in empty vector or LMP1DM transfected cell total, cytoplasmic, or nuclear extracts, and were readily detected in LMP1 and LMP1ID cytoplasmic and nuclear immunoblots (Fig. 5). LMP1AA transfected cells consistently had substantially less cytoplasmic and nuclear p52 and less nuclear and total RelB, whereas cytoplasmic RelB levels were similar in LMP1AA and LMP1ID transfectants (Fig. 5). Expression levels of WT and mutant LMP1s were similar (data not shown). Multiple experiments similar to these and those in Fig. 4 indicate that the LMP1 TRAF-binding site has most of the WT LMP1 activity in p52 processing from p100 and in p52 and RelB nuclear translocation. Thus, the LMP1 TRAF-binding site is the principal mediator of p100 processing and of p52 and RelB nuclear localization.
Fig. 5.
LMP1 and LMP1 ID induce nuclear p52 and RelB accumulation; LMP1 AA is less active. HEK293T were transfected with expression vectors for WT and mutant LMP1 as in Fig. 4A. Nuclear and cytoplasmic fractions were analyzed by SDS/PAGE and immunoblot for p52, RelB (Santa Cruz Biotechnology), and tubulin as a control for fractionation and cytoplasmic protein levels. Total cell lysates (Lower) were also immunoblotted for RelB. Asterisks indicate nonspecific bands. The results shown in these blots were consistent with those found in similar repeat experiments.
LMP1-Induced Canonical IKKβ and Noncanonical IKKα Mediated RNA Regulation. To identify LMP1 regulated genes that are induced by canonical IKKβ versus noncanonical IKKα NF-κB activation, we investigated the expression of 30 chemokines, cytokines, and receptors by quantitative real-time PCR in WT versus IKKα, IKKβ, or IKKγ KO MEFs (9, 13, 53). LMP1 reproducibly up-regulated the expression of 20 genes at least 2-fold in WT MEFs; TLR9, Fractalkine, IL-1β, CXCR5, ELC, IP-10, MIP-1α, CXCR4, MCP-6, MCP-2, TNF-α, MIP-2, I-TAC, and MIG RNAs were 2.1-, 2.8-, 2.8-, 2.8-, 3.3-, 3.6-, 4.4-, 5.9-, 6.0-, 6.0-, 12-, 14-, 15-, and 56-fold up-regulated, respectively. LMP1 did not alter MIP-1β and SDF-1 RNAs and repressed LARC, TLR4, TLR2, BRAC, I-309, and MCP-3 RNAs 59-, 34-, 6.5-, 2.9-, 2.4-, and 2.1-fold, repectively. SLC and BLC were not detected in WT MEFs.
NF-κB was critical for LMP1-induced RNA up-regulation. Six of the 10 RNAs that were up-regulated at least 3-fold by LMP1 were tested for IKKβ dependence. All were at least 3-fold IKKβ dependent, as was evident from lower level LMP1 induction in IKKβ KO MEFs versus WT controls (Table 1). These RNAs fell into two patterns with regard to IKKα dependence (Table 1 and Fig. 6). MIP-2, TNF-α, and I-TAC were 3- to 6-fold more LMP1 responsive in IKKα KO MEF than in WT MEFs. Clearly, IKKα has a prominent down-modulatory role in LMP1-mediated MIP-2, TNF-α, and I-TAC RNA regulation. In contrast to MIP-2, TNF-α, and I-TAC RNAs, LMP1 induced similar ELC RNA levels in IKKα KO and WT MEFs and 3- to 8-fold less MIG and CXCR4 RNA in IKKα KO MEFs than in WT MEFs (Table 1). The ≈3-fold decrease in LMP1-induced MIG RNA up-regulation and the complete loss of LMP1-induced CXCR4 RNA up-regulation in IKKα KO MEFs indicate that MIG and CXCR4 RNAs are substantially IKKα dependent for their induction. The IKKα dependence of LMP1-induced MIG and CXCR4 RNA levels may be greater and may extend to ELC if we impute an underlying hyper-IKKβ activity to the IKKα KO state as was evident with LMP1-induced MIP-2, TNF-α, and I-TAC RNA up-regulation in IKKα KO MEFs. Indeed, LMP1-induced CXCR4 expression was strictly dependent on both IKKα and IKKβ, and LMP1 did not induce CXCR4 expression in aly/aly or p52 KO MEF (data not shown).
Table 1. LMP1-induced gene expression in MEFs.
| WT | IKKα-/- | IKKβ-/- | |
|---|---|---|---|
| MIP-2 | 13.9 ± 0.2 | 81.9 ± 14.1 | 4.2 ± 0.5 |
| TNF-α | 12.4 ± 1.1 | 73.2 ± 22.1 | 0.6 ± 0.1 |
| I-TAC | 15.4 ± 0.5 | 42.1 ± 4.5 | 1.9 ± 0.2 |
| ELC | 3.3 ± 0.1 | 4.7 ± 1.8 | 0.6 ± 0.1 |
| MIG | 56.4 ± 14.9 | 21.1 ± 0.3 | 0.9 ± 0.4 |
| CXCR4 | 5.9 ± 2.1 | 0.7 ± 0.1 | 0.2 ± 0.02 |
Fig. 6.
LMP1 induces canonical, noncanonical, and atypical NF-κB-dependent gene expression. WT, IKKα, IKKβ, and IKKγ KO MEFs were transduced with GFP (G)-, LMP1 WT (L)-, or LMP1 DM (M)-expressing retrovirus. After 24 h, RNA was harvested, reverse transcribed, and quantitated by using MIP-2-specific (A), MIG-specific(B), or I-TAC-specific(C) primers (available upon request). cDNA copy number was normalized to β-2 microglobulin cDNA as an internal control and is shown as fold induction relative to GFP-expressing retrovirus for each MEF ± standard error. QPCR was performed in duplicate for each gene, and experiments were repeated at least twice.
Comparison among LMP1-induced RNAs in IKKγ KO MEFs revealed a third category of NF-κB activation. As shown in Fig. 6, LMP1-induced MIP-2 RNA up-regulation was both IKKβ and IKKγ dependent, consistent with typical canonical NF-κB activation. However, LMP1-induced MIG and I-TAC RNA levels were stringently IKKβ dependent, but were IKKγ independent (Fig. 6). Indeed, LMP1-induced MIG and I-TAC RNA levels were similar in IKKγ KO and WT MEFs. Thus, LMP1 has an unusual ability to induce IKKβ-mediated NF-κB activation, which is completely IKKγ independent as with MIG and I-TAC or is IKKγ dependent as with MIP-2. Interestingly, IKKβ-dependent and IKKγ-independent transcription can be IKKα dependent as with MIG or can be potentiated in an IKKα KO as with I-TAC.
Discussion
The experiments reported here investigate the role of NIK, IKKα, IKKβ, and IKKγ in LMP1-induced p100 processing, the relative contributions of the LMP1 TRAF and death domain-binding sites to p100 processing and p52/RelB nuclear translocation, and the dependence of LMP1-induced gene expression on IKKα, IKKβ, and IKKγ. We now find that LMP1 induces p100 processing in a NIK/IKKα-dependent, IKKβ/IKKγ-independent manner similar to CD40, LTβR, TIRs, and BAFF-R (15–18, 20, 21, 24–26). Because EBV transformed LCLs have sustained p52 levels as well as RelA containing NF-κB complexes, LMP1-induced NF-κB activation likely has substantial canonical and noncanonical components. Because NF-κB is required for LCL survival (56), NIK and IKKα may have a role in LCL survival, and inhibitors of their activity may precipitate cell death or arrest cell growth.
Another important aspect of these data are the identification of the dominant role of the LMP1 TRAF1/2 and TRAF3/5-binding site in p100 processing and p52 and RelB nuclear translocation. CD40 also depends on its TRAF2/3/5-binding site for p100 processing (16). The LMP1 TRAF-binding site has a dominant role in epidermal growth factor receptor, TRAF1, and EBI3 transcriptional up-regulation and in primary B lymphocyte immortalization (40, 57). The importance of the LMP1 TRAF-binding site in p100 processing and p52/RelB nuclear translocation together with LMP1-induced IKKα-dependent expression of MIG and CXCR4 now genetically link LMP1-induced p100 processing and p52/RelB nuclear translocation to epidermal growth factor receptor, TRAF1, EBI3, MIG, and CXCR4 transcriptional upregulation and B lymphocyte immortalization. Indeed, lymphoma-genic alleles of p100 undergo constitutive processing to p52, indicating a role for noncanonical NF-κB activation in lymphoma genesis (21, 58).
The induction of p100 processing by LMP1, CD40, BAFF-R, and LTβR suggests a hypothesis where TRAF3, a major common binding protein of these receptors, is an important mediator of noncanonical signaling. However, TRAF3 KO mice do not exhibit the expected defects in lymph node or B cell development (59), whereas TRAF6 KO mice display several defects similar to aly/aly mice, including lack of lymph nodes and low B cell levels in the spleen (60). Thus, although signaling through TRAFs 1, 2, 3, and 5 may be important in initiating noncanonical signals, TRAF6 could be an essential mediator of NIK and IKKα activation. Indeed, evidence from CD40 signaling suggests that TRAF2/3/5-binding site-dependent NF-κB activation is TRAF6 dependent in B cells (61, 62). Thus, a molecular bridge between TRAFs 2, 3, and 5 and TRAF6, such as TANK (63), may be a critical link in these pathways.
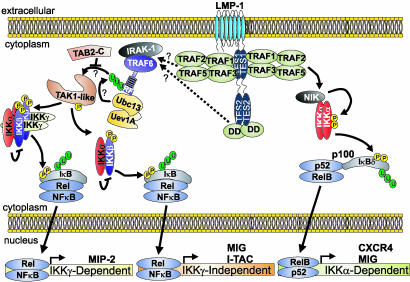
Finally, in this study we identify three pathways of LMP1-mediated NF-κB activation; typical canonical IKKβ/IKKγ-dependent, atypical canonical IKKβ-dependent/IKKγ-independent, and noncanonical NIK/IKKα-dependent (Fig. 7). Although all LMP1-induced gene expression tested was IKKβ-dependent, we find that LMP1 is unique in its ability to activate certain genes, such as MIP-2, in an IKKγ-dependent manner and others, such as I-TAC, in an IKKγ-independent manner. Because IKKγ is critical for IKKα/IKKβ/IKKγ complex assembly and LMP1 differs from natural receptors in constitutively forming unusually large aggregates in the plasma membrane, LMP1 complexes may induce aggregates of TRAFs, death domain proteins, or other intermediate signaling molecules and thereby substitute for IKKγ in assembling IKKα and IKKβ. Alternatively, LMP1 may preferentially engage an IKKγ-like protein such as FIP-2 (64).
Fig. 7.
Model for LMP1-induced canonical and noncanonical NF-κB activation. LMP1 can induce canonical IKKβ-dependent NF-κB activation through both TRAF-binding (TES1) and death domain (DD)-binding (TES2) sites. This pathway depends on IRAK1 and TRAF6 and is inhibited by dominant negative forms of TAB2 and Ubc13 (65). The activation of an unknown IKK kinase similar to TAK-1 downstream of TRAF6 is illustrated. All LMP1-induced NF-κB activation is IKKβ-dependent; thus, NIK/IKKα-induced p100 processing likely requires the IKKβ-dependent expression of a critical signaling factor. MIP-2 RNA induction by LMP1 depends on IKKγ, whereas MIG and I-TAC RNA inductions are IKKγ-independent and therefore atypical. In addition to canonical NF-κB activation, the LMP1 TRAF-binding site is the primary mediator of noncanonical NIK/IKKα activation leading to p100 processing to p52 and p52/RelB nuclear translocation. ELC, MIG, and CXCR4 RNA induction likely depend on this pathway. Essential molecules in each pathway are labeled in white.
Our analysis of LMP1-induced gene expression further indicates that LMP1 is similar to activated LTβR (15) in that IKKβ-mediated NF-κB activation can be potentiated in cells lacking IKKα activity, consistent with the notion that IKKα can have a role in down-regulating the extent or duration of LMP1-induced IKKα–IKKβ complex signaling, whether the complex is IKKγ dependent as with MIP-2 or IKKγ independent as with I-TAC. However, LMP1 induction of MIG and CXCR4, similar to LTβR induced SLC, ELC, and BLC expression, depended on IKKα, indicating a critical role for this kinase in transcriptional up-regulation downstream of LMP1 and LTβR.
Acknowledgments
Drs. Seddon Thomas, Terry Means, and Andrew Luster contributed advice, technical assistance, and access to a Stratagene MX-4000 QPCR machine. Drs. Tasuku Honjo and Reiko Shinkura or Dr. Ulrich Siebenlist kindly provided aly/aly or p52 KO MEFs. George Mosialos and members of the Kieff, Wang, and Kaye laboratories contributed helpful suggestions. This work was supported by National Cancer Institute (U.S. Public Health Service) Grants CA47006 and CA85180.
Abbreviations: EBV, Epstein–Barr virus; TNF, tumor necrosis factor; TNFR, TNF receptor; KO, knockout; TIR, Toll/IL-1 receptor; β-gal, β-galactosidase; MEF, murine embryonic fibroblast; LCL, lymphoblastoid cell line; DM, double mutant; QPCR, quantitative PCR; MIP, macrophage inflammatory protein.
References
- 1.Suzuki, N., Suzuki, S. & Yeh, W. C. (2002) Trends Immunol. 23, 503–506. [DOI] [PubMed] [Google Scholar]
- 2.Caamano, J. & Hunter, C. A. (2002) Clin. Microbiol. Rev. 15, 414–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcamo, E., Hacohen, N., Schulte, L. C., Rennert, P. D., Hynes, R. O. & Baltimore, D. (2002) J. Exp. Med. 195, 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kontgen, F., Grumont, R. J., Strasser, A., Metcalf, D., Li, R., Tarlinton, D. & Gerondakis, S. (1995) Genes Dev. 9, 1965–1977. [DOI] [PubMed] [Google Scholar]
- 5.Mercurio, F., Zhu, H., Murray, B. W., Shevchenko, A., Bennett, B. L., Li, J., Young, D. B., Barbosa, M., Mann, M., Manning, A. & Rao, A. (1997) Science 278, 860–866. [DOI] [PubMed] [Google Scholar]
- 6.Zandi, E., Rothwarf, D. M., Delhase, M., Hayakawa, M. & Karin, M. (1997) Cell 91, 243–252. [DOI] [PubMed] [Google Scholar]
- 7.DiDonato, J. A., Hayakawa, M., Rothwarf, D. M., Zandi, E. & Karin, M. (1997) Nature 388, 548–554. [DOI] [PubMed] [Google Scholar]
- 8.Rothwarf, D. M., Zandi, E., Natoli, G. & Karin, M. (1998) Nature 395, 297–300. [DOI] [PubMed] [Google Scholar]
- 9.Li, Z. W., Chu, W., Hu, Y., Delhase, M., Deerinck, T., Ellisman, M., Johnson, R. & Karin, M. (1999) J. Exp. Med. 189, 1839–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, Q., Van Antwerp, D., Mercurio, F., Lee, K. F. & Verma, I. M. (1999) Science 284, 321–325. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka, M., Fuentes, M. E., Yamaguchi, K., Durnin, M. H., Dalrymple, S. A., Hardy, K. L. & Goeddel, D. V. (1999) Immunity 10, 421–429. [DOI] [PubMed] [Google Scholar]
- 12.Rudolph, D., Yeh, W. C., Wakeham, A., Rudolph, B., Nallainathan, D., Potter, J., Elia, A. J. & Mak, T. W. (2000) Genes Dev. 14, 854–862. [PMC free article] [PubMed] [Google Scholar]
- 13.Hu, Y., Baud, V., Delhase, M., Zhang, P., Deerinck, T., Ellisman, M., Johnson, R. & Karin, M. (1999) Science 284, 316–320. [DOI] [PubMed] [Google Scholar]
- 14.Takeda, K., Takeuchi, O., Tsujimura, T., Itami, S., Adachi, O., Kawai, T., Sanjo, H., Yoshikawa, K., Terada, N. & Akira, S. (1999) Science 284, 313–316. [DOI] [PubMed] [Google Scholar]
- 15.Dejardin, E., Droin, N. M., Delhase, M., Haas, E., Cao, Y., Makris, C., Li, Z. W., Karin, M., Ware, C. F. & Green, D. R. (2002) Immunity 17, 525–535. [DOI] [PubMed] [Google Scholar]
- 16.Coope, H. J., Atkinson, P. G., Huhse, B., Belich, M., Janzen, J., Holman, M. J., Klaus, G. G., Johnston, L. H. & Ley, S. C. (2002) EMBO J. 21, 5375–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller, J. R. & Siebenlist, U. (2003) J. Biol. Chem. 278, 12006–12012. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz, Z. B., Weih, D. S., Sivakumar, V. & Weih, F. (2003) EMBO J. 22, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saccani, S., Pantano, S. & Natoli, G. (2003) Mol. Cell 11, 1563–1574. [DOI] [PubMed] [Google Scholar]
- 20.Senftleben, U., Cao, Y., Xiao, G., Greten, F. R., Krahn, G., Bonizzi, G., Chen, Y., Hu, Y., Fong, A., Sun, S. C. & Karin, M. (2001) Science 293, 1495–1499. [DOI] [PubMed] [Google Scholar]
- 21.Xiao, G., Harhaj, E. W. & Sun, S. C. (2001) Mol. Cell 7, 401–409. [DOI] [PubMed] [Google Scholar]
- 22.Derudder, E., Dejardin, E., Pritchard, L. L., Green, D. R., Korner, M. & Baud, V. (2003) J. Biol. Chem. 278, 23278–23284. [DOI] [PubMed] [Google Scholar]
- 23.Saitoh, T., Nakano, H., Yamamoto, N. & Yamaoka, S. (2002) FEBS Lett. 532, 45–51. [DOI] [PubMed] [Google Scholar]
- 24.Claudio, E., Brown, K., Park, S., Wang, H. & Siebenlist, U. (2002) Nat. Immunol. 3, 958–965. [DOI] [PubMed] [Google Scholar]
- 25.Kayagaki, N., Yan, M., Seshasayee, D., Wang, H., Lee, W., French, D. M., Grewal, I. S., Cochran, A. G., Gordon, N. C., Yin, J., et al. (2002) Immunity 17, 515–524. [DOI] [PubMed] [Google Scholar]
- 26.Mordmuller, B., Krappmann, D., Esen, M., Wegener, E. & Scheidereit, C. (2003) EMBO Rep. 4, 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinkura, R., Kitada, K., Matsuda, F., Tashiro, K., Ikuta, K., Suzuki, M., Kogishi, K., Serikawa, T. & Honjo, T. (1999) Nat. Genet. 22, 74–77. [DOI] [PubMed] [Google Scholar]
- 28.Yamada, T., Mitani, T., Yorita, K., Uchida, D., Matsushima, A., Iwamasa, K., Fujita, S. & Matsumoto, M. (2000) J. Immunol. 165, 804–812. [DOI] [PubMed] [Google Scholar]
- 29.Matsushima, A., Kaisho, T., Rennert, P. D., Nakano, H., Kurosawa, K., Uchida, D., Takeda, K., Akira, S. & Matsumoto, M. (2001) J. Exp. Med. 193, 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caamano, J. H., Rizzo, C. A., Durham, S. K., Barton, D. S., Raventos-Suarez, C., Snapper, C. M. & Bravo, R. (1998) J. Exp. Med. 187, 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Futterer, A., Mink, K., Luz, A., Kosco-Vilbois, M. H. & Pfeffer, K. (1998) Immunity 9, 59–70. [DOI] [PubMed] [Google Scholar]
- 32.Wu, Q., Sun, Y., Wang, J., Lin, X., Wang, Y., Pegg, L. E., Futterer, A., Pfeffer, K. & Fu, Y. X. (2001) J. Immunol. 166, 1684–1689. [DOI] [PubMed] [Google Scholar]
- 33.Kang, H. S., Chin, R. K., Wang, Y., Yu, P., Wang, J., Newell, K. A. & Fu, Y. X. (2002) Nat. Immunol. 3, 576–582. [DOI] [PubMed] [Google Scholar]
- 34.Mosialos, G., Birkenbach, M., Yalamanchili, R., VanArsdale, T., Ware, C. & Kieff, E. (1995) Cell 80, 389–399. [DOI] [PubMed] [Google Scholar]
- 35.Kaye, K. M., Izumi, K. M. & Kieff, E. (1993) Proc. Natl. Acad. Sci. USA 90, 9150–9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaye, K. M., Izumi, K. M., Mosialos, G. & Kieff, E. (1995) J. Virol. 69, 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rickinson, A. & Kieff, E. (2001) in Fields Virology, eds. Howley, P. M. & Knipe, D. (Lippincott Williams and Wilkins, Philadelphia), pp. 2575–2628.
- 38.Wang, D., Liebowitz, D., Wang, F., Gregory, C., Rickinson, A., Larson, R., Springer, T. & Kieff, E. (1988) J. Virol. 62, 4173–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liebowitz, D., Wang, D. & Kieff, E. (1986) J. Virol. 58, 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Devergne, O., Cahir McFarland, E. D., Mosialos, G., Izumi, K. M., Ware, C. F. & Kieff, E. (1998) J. Virol. 72, 7900–7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Izumi, K. M. & Kieff, E. D. (1997) Proc. Natl. Acad. Sci. USA 94, 12592–12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izumi, K. M., McFarland, E. C., Ting, A. T., Riley, E. A., Seed, B. & Kieff, E. D. (1999) Mol. Cell. Biol. 19, 5759–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laherty, C. D., Hu, H. M., Opipari, A. W., Wang, F. & Dixit, V. M. (1992) J. Biol. Chem. 267, 24157–24160. [PubMed] [Google Scholar]
- 44.Mitchell, T. & Sugden, B. (1995) J. Virol. 69, 2968–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huen, D. S., Henderson, S. A., Croom-Carter, D. & Rowe, M. (1995) Oncogene 10, 549–560. [PubMed] [Google Scholar]
- 46.Paine, E., Scheinman, R. I., Baldwin, A. S., Jr., & Raab-Traub, N. (1995) J. Virol. 69, 4572–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.VanArsdale, T. L., VanArsdale, S. L., Force, W. R., Walter, B. N., Mosialos, G., Kieff, E., Reed, J. C. & Ware, C. F. (1997) Proc. Natl. Acad. Sci. USA 94, 2460–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Devergne, O., Hatzivassiliou, E., Izumi, K. M., Kaye, K. M., Kleijnen, M. F., Kieff, E. & Mosialos, G. (1996) Mol. Cell. Biol. 16, 7098–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller, W. E., Earp, H. S. & Raab-Traub, N. (1995) J. Virol. 69, 4390–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sylla, B. S., Hung, S. C., Davidson, D. M., Hatzivassiliou, E., Malinin, N. L., Wallach, D., Gilmore, T. D., Kieff, E. & Mosialos, G. (1998) Proc. Natl. Acad. Sci. USA 95, 10106–10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luftig, M. A., Cahir-McFarland, E., Mosialos, G. & Kieff, E. (2001) J. Biol. Chem. 276, 14602–14606. [DOI] [PubMed] [Google Scholar]
- 52.Randow, F. & Seed, B. (2001) Nat. Cell Biol. 3, 891–896. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt-Supprian, M., Bloch, W., Courtois, G., Addicks, K., Israel, A., Rajewsky, K. & Pasparakis, M. (2000) Mol. Cell 5, 981–992. [DOI] [PubMed] [Google Scholar]
- 54.Higuchi, M., Izumi, K. M. & Kieff, E. (2001) Proc. Natl. Acad. Sci. USA 98, 4675–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaye, K. M., Izumi, K. M., Li, H., Johannsen, E., Davidson, D., Longnecker, R. & Kieff, E. (1999) J. Virol. 73, 10525–10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cahir-McFarland, E. D., Davidson, D. M., Schauer, S. L., Duong, J. & Kieff, E. (2000) Proc. Natl. Acad. Sci. USA 97, 6055–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller, W. E., Cheshire, J. L. & Raab-Traub, N. (1998) Mol. Cell. Biol. 18, 2835–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao, G. & Sun, S. C. (2003) Oncogene 22, 4868–4874. [DOI] [PubMed] [Google Scholar]
- 59.Xu, Y., Cheng, G. & Baltimore, D. (1996) Immunity 5, 407–415. [DOI] [PubMed] [Google Scholar]
- 60.Naito, A., Azuma, S., Tanaka, S., Miyazaki, T., Takaki, S., Takatsu, K., Nakao, K., Nakamura, K., Katsuki, M., Yamamoto, T. & Inoue, J. (1999) Genes Cells 4, 353–362. [DOI] [PubMed] [Google Scholar]
- 61.Jabara, H., Laouini, D., Tsitsikov, E., Mizoguchi, E., Bhan, A., Castigli, E., Dedeoglu, F., Pivniouk, V., Brodeur, S. & Geha, R. (2002) Immunity 17, 265–276. [DOI] [PubMed] [Google Scholar]
- 62.Lomaga, M. A., Yeh, W. C., Sarosi, I., Duncan, G. S., Furlonger, C., Ho, A., Morony, S., Capparelli, C., Van, G., Kaufman, S., et al. (1999) Genes Dev. 13, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng, G. & Baltimore, D. (1996) Genes Dev. 10, 963–973. [DOI] [PubMed] [Google Scholar]
- 64.Li, Y., Kang, J., Friedman, J., Tarassishin, L., Ye, J., Kovalenko, A., Wallach, D. & Horwitz, M. S. (1999) Proc. Natl. Acad. Sci. USA 96, 1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luftig, M. & Kieff, E. (2003) Proc. Natl. Acad. Sci. USA 100, 15595–15600. [DOI] [PMC free article] [PubMed] [Google Scholar]