Abstract
Purpose
Adoptive transfer of tumor infiltrating lymphocytes (TIL) can mediate regression of metastatic melanoma. However, many patients with cancer are ineligible for such treatment because their TIL do not expand sufficiently or because their tumors have lost expression of antigens and/or MHC molecules. Natural killer (NK) cells are large granular lymphocytes that lyse tumor cells in a non-MHC restricted manner. Therefore, we initiated in a clinical trial to evaluate the efficacy of adoptively transferred autologous NK cells to treat patients with cancers who were ineligible for treatment with TIL.
Experimental Design
Patients with metastatic melanoma or renal cell carcinoma were treated with adoptively transferred in vitro activated autologous NK cells after the patients received a lymphodepleting but non-myeloablative chemotherapy regimen. Clinical responses and persistence of the adoptively transferred cells were evaluated.
Results
Eight patients were treated with an average of 4.7×1010 (± 2.1×1010) NK cells. The infused cells exhibited high levels of lytic activity in vitro. Although no clinical responses were observed, the adoptively transferred NK cells appeared to persist in the peripheral circulation of patients for at least one week post-transfer, and in some patients for several months. However, the persistent NK cells in the circulation expressed significantly lower levels of the key activating receptor NKG2D and could not lyse tumor cell targets in vitro unless reactivated with IL-2.
Conclusions
The persistent NK cells could mediate antibody dependent cell-mediated cytotoxicity without cytokine reactivation in vitro which suggests that coupling adoptive NK cell transfer with monoclonal antibody administration deserves evaluation.
Introduction
Adoptive transfer of lymphocytes with anti-tumor reactivity can mediate the regression of metastatic melanoma (1–3). In a series of trials conducted in the Surgery Branch of the National Cancer Institute (1, 2), tumor reactive T lymphocyte populations were isolated from tumor infiltrating lymphocytes (TIL), expanded to large numbers (i.e. ~1010 cells) ex vivo, and adoptively transferred to autologous patients with interleukin 2 (IL-2) after the patients had received one of several lymphodepleting preparative regimens. Of 93 patients, 56% experienced objective clinical responses including 20 patients with durable complete regressions. However, not all patients with cancer are eligible for this type of immunotherapy because TIL from some melanoma patients may not expand sufficiently, and TIL with anti-tumor reactivity are rarely found in patients with cancers other than melanoma. In addition, tumors can lose surface expression of class I MHC molecules and thus not be susceptible to MHC restricted recognition by conventional T cells. In order to extend current adoptive cell transfer therapies to patients from whom tumor reactive T cell populations can not be generated and to patients with MHC-negative tumors, we initiated an investigation to isolate and expand ex vivo, natural killer cells that are capable of mediating tumor destruction in a non-MHC restricted manner.
Natural killer (NK) cells are large granular lymphocytes that are critical effector cells in the early innate immune response to pathogens and cancer (4–6). These cells account for 10–15% of peripheral blood lymphocytes and are phenotypically characterized by expression of CD56 and absence of CD3. NK cells can directly lyse virally infected cells and tumor cells without prior sensitization and provide immunoregulatory cytokines that shape the adaptive immune response. Unlike T lymphocytes, NK cells do not express specific antigen receptors. Instead NK cell function is mediated through a complex balance of activating and inhibitory signals delivered through a variety of different surface receptors (4, 6–8). Three predominant superfamilies of NK cell receptors (NKRs) have been identified that can either inhibit or activate NK cell function: (i) killer immunoglobulin (Ig)-like receptors (KIRs) that bind to classical class I MHC molecules; (ii) C-type lectin receptors that bind to non-classical class I MHC molecules or “class I – like” molecules; and (iii) natural cytotoxicity receptors for which ligands are currently not well defined (5). The most well-characterized activating NKR, NKG2D, is a C-type lectin receptor that binds to stess-inducible ligands, including the MHC class I chain-related (MIC) peptides, MICA and MICB, and the human cytomegalovirus UL16 binding proteins (ULBPs). These proteins are often up-regulated during the process of neoplastic transformation, which at least in part, explains why tumor cells are often susceptible NK cell targets (9). In contrast, many NKRs that inhibit NK cell function belong to the KIR superfamily. To date, at least 4 different inhibitory KIRs have been identified that bind to different allelic groups of HLA-A, HLA-B, or HLA-C molecules, although the HLA-C molecules predominate. For example, KIR2DL1 binds to HLA-C molecules that have a lysine residue at amino acid position 80 (eg. HLA-Cw2, -Cw4, -Cw5, -Cw6) whereas KIR2DL2 binds to HLA-C molecules that have an asparagine at position 80 (eg. HLA-Cw1, -Cw3, -Cw7, -Cw8). Unlike TCRs, NKR genes do not undergo somatic diversification, and therefore, the expression of inhibitory receptors specific for class I MHC molecules is largely random. Nonetheless, circulating NK cells usually do not lyse normal autologous tissues, and multiple mechanisms for this self-tolerance have been proposed (10). However, after in vitro activation with cytokines like IL-2, NK cells readily lyse tumor cells that express self-MHC molecules (11, 12).
Adoptive transfer of autologous NK cells for the treatment of patients with melanoma, renal cell carcinoma, lymphoma, and breast cancer has been evaluated in several previously described clinical trials using ex vivo generated lymphokine activated killer (LAK) cells (11, 13). No clear clinical benefit was observed in these trials. However, these results do not allow for a conclusion to be drawn related to the efficacy of purified autologous NK cell adoptive transfer because LAK cells consist predominantly of T lymphocytes (>90%), and only contain a small fraction (<10%) of cells having the phenotypic characteristics of classical NK cells (i.e. CD56+ CD3−). In addition, studies in several murine models suggest means for improving the efficacy of adoptive autologous NK cell transfer immunotherapies for the treatment of patients with cancer. In one study, syngeneic NK cells were transferred into NK-deficient Rag−/−γC−/− mice in comparison to NK-replete Rag−/− mice (14), and in another study, syngeneic NK cells were transferred into irradiated mice in comparison to normal mice (15). In both studies NK cells underwent homeostatic proliferation in the lymphopenic environment. Finally, in another more recent investigation, the observation was made that CD4+CD25+ regulatory T cells (Treg) inhibited NKG2D-mediated NK cell cytotoxicity in vitro, and depletion of Tregs in vivo significantly enhanced NK cell-mediated tumor rejection (16). All of these studies suggested that autologous NK cells may be efficacious for the treatment of patients with cancer, particularly if the patients are lymphodepleted prior to adoptive cell transfer.
Based on these studies and preclinical work conducted in our laboratory, we initiated a clinical study in which patients with metastatic cancers were treated with adoptively transferred, autologous, in vitro activated NK cells after the patients received a lymphodepleting chemotherapy regimen. For this trial, we selected patients with cancers that historically have been shown to be responsive to immunotherapy with IL-2, namely melanoma and renal cell carcinoma. We and others have observed that both of these types of tumor cells are susceptible to lysis by NK cells, even without complete loss of class I MHC, possibly related to upregulation of several ligands for activating NKRs (17) and lowered expression of HLA-B and –C molecules.
Materials and Methods
Cell lines and fresh tumor digests
Human melanoma cell lines, fibroblasts, EBV-transformed B cell lines, SKOv3 ovarian cancer cells, and MDA-MB-468 breast cancer cells were routinely cultured in RPMI 1640 supplemented with 10% FBS, 2 mM L-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin (Invitrogen). Renal cell carcinoma cell lines were routinely cultured in high-glucose DMEM supplemented with 10% FBS, 2 mM L-glutamine, and 25 mM HEPES buffer (Invitrogen). Prior to the initiation of this clinical trial, for authentication purposes, cell lines were characterized for expression of HLA molecules either by HLA typing conducted by HLA typing laboratory at the National Institutes of Health or by FACS analyses using anti-HLA antibodies (W6/32, Dako North America, Carpinteria, CA; anti-HLA-A2, One Lambda Inc., Canoga Park, CA).
Small scale pre-clinical expansion of NK cells in vitro
In preliminary experiments, NK cells were enriched from PBMCs from leukaphereses using an NK cell isolation kit (Milltenyi Biotech) in which NK cells were isolated via negative selection. In particular, non-NK cells were labeled with biotinylated antibodies against CD3, CD4, CD14, CD15, CD19, CD36, CD123, glycophorin A, and anti-biotin magnetic beads, and were then depleted by passage over a paramagnetic column. The enriched NK cells were then expanded in the presence of a 10 fold excess of allogeneic irradiated PBMCs as feeder cells in media containing 5 μg/ml PHA-L (Sigma-Aldrich) and 1200 IU/ml IL-2.
Clinical expansion of NK cells in vitro
PBMCs from leukaphereses were depleted of CD3+ cells using a Clinimacs machine and anti-CD3 reagent (Miltenyi Biotech). This depletion process consistently resulted in a CD3-depleted cell population that contained fewer than 1% CD3+ cells. Multiple T175 flasks were set up, each of which contained 107 CD3 depleted cells and 108 irradiated (3000 rad) autolous PBMCs as feeder cells in 100 ml AIMV media (Invitrogen) containing 10% heat inactivated human AB serum (Gemini Bio-Products, West Sacramento, CA) in the presence of 600 IU/ml IL-2 (Novartis) and 30 ng/ml OKT3 (Orthoclone). On average, 51 flasks were set up for each patient. 600 IU/ml IL-2 was added every 3–4 days, and on day 7–8, 100 ml of fresh media containing 5% hu AB serum were added to each flask. On or about day 10, the contents of three flasks were transferred to a single 3 liter LifeCell culture bag (Baxter), and the cell concentration was adjusted to ~0.5×106 cells/ml with AIMV media containing 5% hu AB serum containing 600 IU/ml IL-2. The average volume transferred to each 3 L bag was 600 ml, and the average cell count of the transferred population was 0.8×106 cells/ml. Also, on average, for each patient, the contents of 51 flasks were transferred to 17 3 L bags. Cells were then maintained as needed by adding fresh serum-free AIMV and IL-2 and/or splitting to maintain cell concentrations between 1–3×106 cells/ml. The strategy used to maintain this cell concentration varied significantly between patients, but on average, the total volume of serum-free AIMV added during the final stages of cell preparation was 30 L. Also, the average overall time to complete the NK cell expansions was 21 days.
Clinical trial
Patients older than 18 years with progressive stage IV melanoma or renal cell cancer who were negative for hepatitis B and C and HIV infection and had a good performance status and a life expectancy of at least three months were eligible for treatment on this protocol. All patients signed an institutional review board–approved consent and had melanoma or renal cell carcinoma that was histologically confirmed by pathologists at the Clinical Center, National Institutes of Health (Bethesda, MD). All patients had measurable disease on computed tomography scan or by physical exam and were refractory to standard treatments including high-dose IL-2 therapy (except patient 7, who did not receive IL-2 before entry into this protocol). Patients received nonmyeloablative lymphodepleting chemotherapy as previously described (18) consisting of 2 days of cyclophosphamide (60 mg/kg) followed by 5 days of fludarabine (25 mg/m2). On the day following the final dose of fludarabine, patients received the infusion of in vitro expanded NK cells and high-dose IL-2 therapy consisting of 720,000 IU/kg intravenously every 8 hours to tolerance as previously described (18).
Lysis assays
Standard 4-hour 51Cr release assays were performed as previously described (19). In some experiments, tumor cells were incubated with 10 μg of anti-HER2/neu (Herceptin® trastuzumab; Genetech) or anti-CD20 (Rituxan® rituximab; Genentech) monoclonal antibodies per 106 cells during the 51Cr labeling step.
Cytokine measurement assays
Serum was collected from patients and frozen before initiation of therapy and after lymphodepletion but before cell infusion. Serum samples were thawed, and the concentration of IL-15 in each sample was determined using a commercially available enzyme-linked immunosorbent assay kits (R & D Systems).
FACS analyses
Tumor cell lines, PBMCs, CD3 depleted PBMCs, and NK cell populations were stained with propidium iodide, and various antibodies as indicated throughout the text (BD Biosciences and eBioscience). The cells were then analyzed by FACS using a FACSCalibur flow cytometer (BD Biosciences), and data were analyzed using Cellquest (Becton Dickenson) or FlowJo software (Tree Star, Ashland, OR) after gating for live (propidium iodide- negative) cells.
Statistical analyses
Statistical comparisons between phenotypes of adoptively transferred cells and NK cells in peripheral blood were made using 2-tailed paired T tests.
Results
Preclinical Studies
In vitro activated NK cells efficiently lyse autologous tumor cells
In a previous clinical trial, three patients treated with adoptively transferred TIL recurred with tumors that had lost surface expression of class I MHC molecules. Therefore, we began to develop a method for expanding NK cells in vitro to provide an alternate treatment modality for these patients. In preclinical studies, we purified NK cells (CD56+ CD3− cells) from whole PBMC using a negative isolation kit (Miltenyi Biotech) and subsequently expanded these cells in vitro with PHA-L and IL-2. Fold expansions on day 24 ranged from 50 to 200, and the resulting cell populations were usually greater than 70% CD56+ CD3− NK cells (data not shown). We evaluated lysis of autologous tumor cells by the in vitro activated NK cells (Supplemental Figure 1), and noted that melanoma cell lines were effectively lysed by the NK cells regardless of class I MHC expression. We also evaluated the expression of a variety of activating and inhibitory NKRs on these cells after a 21 day culture period (one example is presented in Supplemental Figure 2). These cells expressed phenotypic markers consistent with activated NK cells. Namely, the majority of the cells expressed the activating receptors NKG2D, CD16, CD94, and NKp46. These cells also expressed a variety of inhibitory receptors including KIR2DL1 (CD158a), KIR2DL2/3 (CD158b), and KIR3DL1 (CD158e1). As has been previously reported, since NK cell receptor genes do not undergo somatic diversification like TCRs, the expression of inhibitory receptors specific for class I MHC molecules is largely random, and that phenomenon was observed in our in vitro expanded NK cell populations as well. For example (Supplemental Figure 2), Patient 888 expressed “Group I” HLA-C alleles (Ser 77 Asn 80) which binds the inhibitory receptor KIR2DL2/3 (CD158b), and nearly 60% of the NK cells from this patient expressed this receptor. However, 26% of the NK cells isolated from this patient expressed KIR2DL1 (CD158a) which would not be inhibited by these self MHC molecules.
Development of a GMP protocol for expanding large numbers of NK cells for clinical use
Based on our preclinical studies, we decided to pursue a clinical trial to treat patients with adoptively transferred autologous NK cells regardless of MHC expression on tumors. To generate large numbers of NK cells under GMP conditions suitable for adoptive transfer, we evaluated many protocols incorporating the following modifications: initiation of cultures with CD3-depleted PBMCs instead of NK cells initially purified from whole PBMCs using the Milltenyi negative isolation kit; elimination of PHA; the use of autologous PBMCs as feeder cells instead of allogeneic PBMCs; addition of anti-CD3 (OKT3) to stimulate feeder cells; initiation of cultures in the presence of 10% human AB serum with latter additions of serum-free media to minimize serum usage throughout the culture period; and initiation of cultures in flasks with transfer to large-scale cell-culture bags (LifeCell bags; Baxter) instead of maintaining the cells in flasks throughout the culture period. We arrived at the methodology described in the Materials and Methods section in which CD3 depleted PBMCs were stimulated to proliferate with OKT3-loaded autologous PBMC feeder cells in the presence of IL-2.
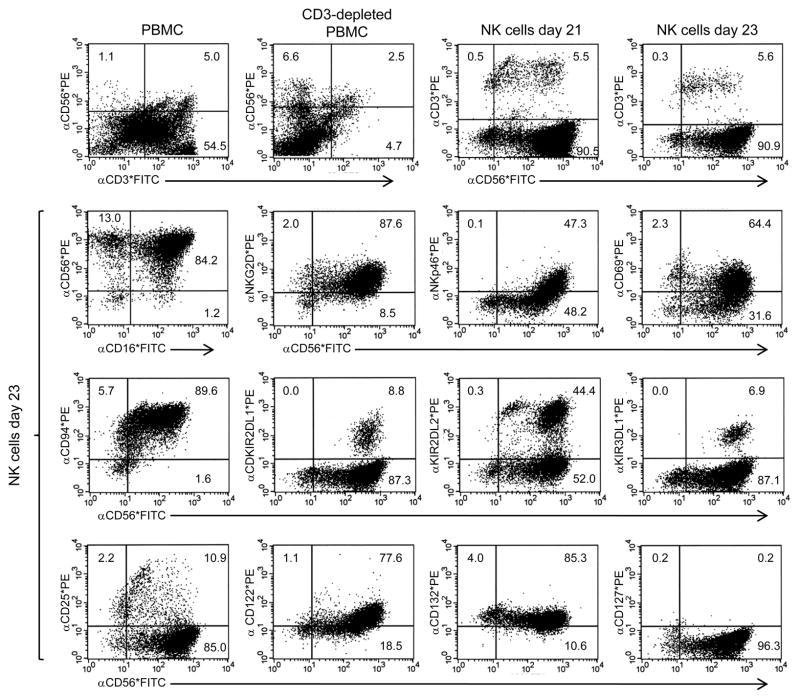
Using this methodology, prior to initiating a clinical trial, we evaluated the expansion, phenotype, and function of NK cells from three patients expanded at near clinical scale beginning with either fresh or cryopreserved PBMCs. Using this in vitro activation protocol, overall fold expansions between days 21 and 26 were 80, 79, and 322 from patients 1, 2, and 3 respectively. From all three donors, the cell populations that expanded within approximately 3 weeks were >90% CD56+ CD3- NK cells (one example is presented in Figure 1). Since the initial percentages of CD56+ CD3- NK cells in the CD3 depleted PBMC populations were 6.6, 25.9, and 30.0 from patients 1, 2, and 3 respectively, and the final percentages of NK cells in the expanded populations were 90.5, 91.1, and 96.5, the overall NK cell expansions were 1097, 278, and 942 respectively. The cells appeared to be highly activated NK cells expressing NKG2D, CD16, NKp46, and CD94, but they also expressed a variety of inhibitory receptors including KIR2DL1 (CD158a), KIR2DL2/3 (CD158b), and KIR3DL1 (CD158e1) (Figure 1). In addition, in vitro activated NK cells expressed the cytokine receptor common gamma chain (γc; CD132), as well as the β chain common to the IL2 and IL15 receptors (CD122), suggesting these cells could respond to intermediate doses of these cytokines. However, the majority of the cells did not express the IL2R α chain (CD25) or the IL7R α chain (CD127). We also evaluated the lytic function of NK cells expanded using this modified, clinically-applicable method. In vitro expanded and activated NK cells efficiently lysed melanoma (mel) and renal cell carcinoma (RCC) cell lines, but not autologous or allogeneic PBMCs (Supplemental Figure 3).
Figure 1.
Phenotype of NK cells activated in vitro with OKT3 and IL-2 at clinical scale. CD3+ T cells were depleted from whole PBMC using a Clinimacs machine and anti-CD3 reagent (Miltenyi Biotech) and were subsequently expanded in vitro with OKT3 and IL-2. On days 21 and 23, the resulting cell populations were evaluated by FACS for expression of a variety of activating and inhibitory NKRs and cytokine receptors.
Clinical Trial
We initiated a clinical trial to treat patients with metastatic cancers with adoptively transferred, in vitro activated, autologous NK cells after receiving a lymphodepleting chemotherapy regimen consisting of cyclophosphamide and fludarabine. Our goal was to treat each patient with at least 2.5×1010 functional NK cells. Based on preclinical studies, we believed it was possible to achieve at least a 50 fold total cell expansion during a 3 week culture period. Therefore, for each patient, cultures were initiated with at least 5×108 CD3 depleted cells and 5×109 irradiated autologous PBMCs as feeder cells distributed equally amongst 50 T175 flasks.
Patient and cell characteristics
Eight patients were enrolled in this clinical trial, seven with metastatic melanoma, and one with metastatic renal cell carcinoma. Patient characteristics and properties of the adoptively transferred cell products are presented in Table 1. Patients received an average of 4.7×1010 (± 2.1×1010) cells consisting of 96% (± 2%) NK cells defined by the CD3- CD56+ phenotype by FACS. The NK cells were infused over a period of 20–30 minutes. Adoptively transferred cells efficiently lysed allogeneic melanoma cells (888mel) with an average specific lysis of 82% (± 12%) at an E:T ratio of 10:1. All patients received at least six doses of high dose I.V. IL-2 (720,000 IU/kg/dose), and six of the eight patients received second cycles of IL-2, 20 to 27 days after the first treatment. One patient had transient shortness of breath requiring temporary supplemental oxygen following the cell infusion. There were no other toxicities related to the cell infusion.
Table 1.
Patient and adoptively transferred cell characteristics
| Patient | Age/Sex | Dxa | Sites of Diseaseb | Prior Immunotherapyc | Treatment
|
|||
|---|---|---|---|---|---|---|---|---|
| Cell number infused (×10−10) | % NK cellsd | % lysis 888mele | # IL-2 dosesf | |||||
| 1 | 21/F | MM | Breast, Skin | IL2, IFN | 6.45 | 97 | 88 | 6 |
| 2 | 34/F | MM | Hilum | IL2 | 7.60 | 96 | 64 | 7 |
| 3 | 31/F | MM | Pancreas | IL2 | 4.20 | 96 | 89 | 7 + 3 |
| 4 | 43/M | MM | LN, Chest Wall, Liver | IL2, IFN, GMCSF | 1.88 | 98 | 85 | 6 + 3 |
| 5 | 34/M | MM | Hilum, LN, Lungs | IL2, IFN | 3.46 | 97 | 67 | 8 + 6 |
| 6 | 51/M | RCC | Hilum, LN, Lungs | IL2, Sorafenib | 6.33 | 93 | 100 | 5 + 5 |
| 7 | 56/M | MM | LN, Lungs | DC vaccine | 2.38 | 97 | 87 | 12 + 8 |
| 8 | 42/F | MM | Muscle, LN, SQ, Lungs, Panc. | IL2, DTIC | 5.14 | 95 | 77 | 12 + 5 |
“Dx” stands for “diagnosis”; MM indicates metastatic melanoma; RCC indicates renal cell carcinoma.
“LN” indicated lymph nodes; “SQ” indicates sub-cutaneous; “Panc.” indicates pancreas.
“DC vaccine” indicates dendritic cell vaccine; “DTIC” indicates Dacarbazine
% NK cells in the infused cell population was determined by FACS as the % PI- CD3- CD56+ cells.
Specific lysis of 888 melanoma cells by the adoptively transferred cell population was measured using a 4-hour 51Cr release assays, and the value presented indicates the % specific lysis at a E:T ratio of 10:1.
Patients received the indicated number of doses of I.V. IL-2 (720,000 IU/dose). Some patients received a second cycle of IL-2 doses 20 to 27 days after the first treatment as indicated by the “+” sign.
Persistence, function, and phenotype of adoptively transferred NK cells
Although no objective clinical responses were observed in this trial, the adoptively transferred NK cells appeared to persist in the peripheral circulation of these patients for multiple days post-treatment (Table 2 and Figure 2). At one week post-treatment, the average absolute lymphocyte count (ALC) in peripheral blood from these patients was 2498 cells/μl (± 2667 cells/μl) and consisted of an average of 83% (± 13%) NK cells. In previous trials in which patients received adoptively transferred TIL after lymphodepleting chemotherapy, the majority of PBLs one-week post-transfer were T lymphocytes (20), suggesting that in our NK cell protocol, the majority of the NK cells persisting one week post-treatment were the adoptively transferred cells rather than endogenously recovering cells. In some patients, adoptively transferred NK cells appeared to persist for much longer times. For example, PBLs from patient 1 collected 48 days post-treatment still consisted of 69% NK cells (Figure 2).
Table 2.
Phenotype of NK cells in pre- and post-treatment PBMC compared to the infused cell populations
| Patient | % NK cellsa |
% CD16+ NK cells (MFI)b |
% NKG2D+ NK cells (MFI)c |
|||||
|---|---|---|---|---|---|---|---|---|
| Infused cells | 1 week post-Rx PBMC | Pre-Rx PBMC | Infused cells | 1 week post-Rx PBMC | Pre-Rx PBMC | Infused cells | 1 week post-Rx PBMC | |
| 1 | 97 | 90 | 75 (534) | ntd | 37 (136) | 79 (214) | nt | 50 (130) |
| 2 | 96 | 91 | nt | nt | 45 (172) | nt | nt | 59 (149) |
| 3 | 96 | 86 | 68 (279) | 55 (125) | 42 (149) | 21 (79) | 100 (884) | 14 (66) |
| 4 | 98 | 71 | 76 (325) | 33 (64) | 26 (44) | 42 (100) | 97 (440) | 18 (64) |
| 5 | 97 | 55 | 77 (430) | 47 (129) | 16 (43) | 66 (149) | 100 (778) | 20 (65) |
| 6 | 93 | 93 | 54 (271) | nt | 35 (122) | 37 (96) | nt | 29 (80) |
| 7 | 97 | 93 | 91 (675) | 63 (213) | 65 (266)e | 53 (140) | 98 (540) | 36 (109)e |
| 8 | 95 | 82 | 77 (610) | 42 (135) | 35 (108) | 78 (248) | 100 (1572) | 62 (153) |
%NK cells in infused cell populations was determined by FACS as the % PI- CD3- CD56+ cells. %NK cells in post-treatment PBMC samples approximately 1 week after treatment (day 7 or 8) was determined by the Hematology and Immunology Flow Cytometry Laboratories within the Department of Laboratory Medicine at the NIH Clinical Center as the number of CD3- cells that expressed either CD56 or CD16 as a percentage of the absolute lymphocyte count (ALC).
CD16 expression on the indicated cell populations was evaluated by FACS after gating on CD3- CD56+ cells.
NKG2D expression on the indicated cell populations was evaluated by FACS after gating on CD3- CD56+ cells.
“nt” indicated not tested due to limited sample availability.
For Patient 7, the 1 week post-treatment PBMC sample analyzed for CD16 and NKG2D expression was obtained 10 days after treatment since 7 or 8 day samples were not available.
Figure 2.
Adoptively transferred NK cells persist in the peripheral circulation of patients. Blood was drawn from patients at multiple time points throughout treatment and was analyzed by the Hematology and Immunology Flow Cytometry Laboratories within the Department of Laboratory Medicine at the NIH Clinical Center to determine the absolute lymphocyte count (ALC) and absolute NK cell count (ANK). Normal NK cell counts range from 100 – 500 cells/μl as noted by the dashed lines on the graphs. Arrows indicate initiation of high dose IL-2 administration.
All patients received lymphodepleting but non-myeloablative chemotherapy prior to adoptive NK cell transfer. As has been previously reported (2, 21), in all patients, serum IL-15 levels increased after receiving this regimen (Supplemental Figure 4) which may have supported the persistence and expansion of the adoptively transferred NK cells.
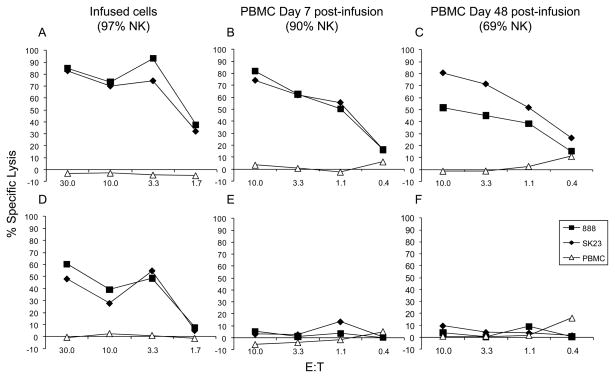
Although there was robust reconstitution and persistence of the transferred NK cells, one potential explanation for the lack of clinical responses may have been that the adoptively transferred NK cells became quiescent and lost their lytic capacity in vivo. To test this hypothesis, PBMCs from patient 1 were collected 7 and 48 days post-treatment and were cryopreserved. These samples and one from the population of infused cells were thawed and cultured for two days in the presence or absence of IL-2. Although the infused cells efficiently lysed melanoma cells whether or not they were cultured with IL-2, the 7- and 48-day post infusion cells only lysed melanomas if they were first reactivated in vitro with IL-2, despite the fact that 90% and 69% of the PBLs in these samples were NK cells respectively (Figure 3). Similar results were observed in tests of cells from four additional patients. To determine whether IL-2 could reactivate the adoptively transferred NK cells in vivo, we evaluated the lytic capacity of PBMCs from two patients collected 3 or 4 days after the initiation of the second dose of IL-2. These cells appeared similar to those collected at other time points in that they did not mediate lysis of tumor cells unless stimulated with IL-2 in vitro prior to the assay (data not shown).
Figure 3.
Adoptively transferred NK cells that persist in the peripheral circulation lose the ability to lyse tumor cells unless reactivated in vitro. PBMCs from patient 1 were collected at various time points after adoptive NK cell transfer as indicated. These cells and NK cells from the infused cell population were cultured for 2 days in the presence (A, B, and C) or absence (D, E, and F) of IL-2 and then evaluated for the ability to lyse two melanoma cell lines (888 and Sk23) in comparison to autologous PBMCs using standard 4-hour 51Cr release assays. Each symbol represents the average of 3 data points.
We also evaluated the phenotypes of CD56+ CD3- NK cells in pre- and post-infusion PBMCs from all patients to determine expression levels of a variety of activating NKRs (NKG2D, CD16, NK46, and 2B4) and cytokine receptors (CD132, CD122, CD25, and the IL12R α chain, CD215). No significant differences were observed for any of these markers between the infused NK cells and those present in the peripheral circulation pre- or post-treatment except for CD16 and NKG2D (Table 2). On average, 74% (± 11%) of pre-treatment circulating NK cells expressed high levels of CD16 (MFI = 446 ± 164), whereas fewer NK cells in the infused samples or one-week post-treatment PBMC samples expressed CD16 and at lower levels (48% ± 12%, p<0.01, MFI = 133 ± 53, p<0.01 for the infused NK cells; 38% ± 14%, p<0.01, MFI = 130 ± 72, p<0.01 for one-week post-treatment NK cells). More notably, on average, 97% (± 6%) of the infused NK cells expressed high levels of NKG2D (MFI = 843 ± 445) whereas many fewer NK cells in PBMC one week post-treatment expressed NKG2D and at lower levels (36% ± 19%, p<0.01; MFI = 102 ± 38; p=0.02). In fact, NK cells in post- one week treatment samples expressed lower levels of NKG2D than those in PBMC prior to therapy (54% ± 22%, p<0.01; MFI = 147 ± 63, p<0.01). These observations may partially explain the inability of the circulating NK cells post-treatment to lyse tumor cell targets without in vitro reactivation with IL-2.
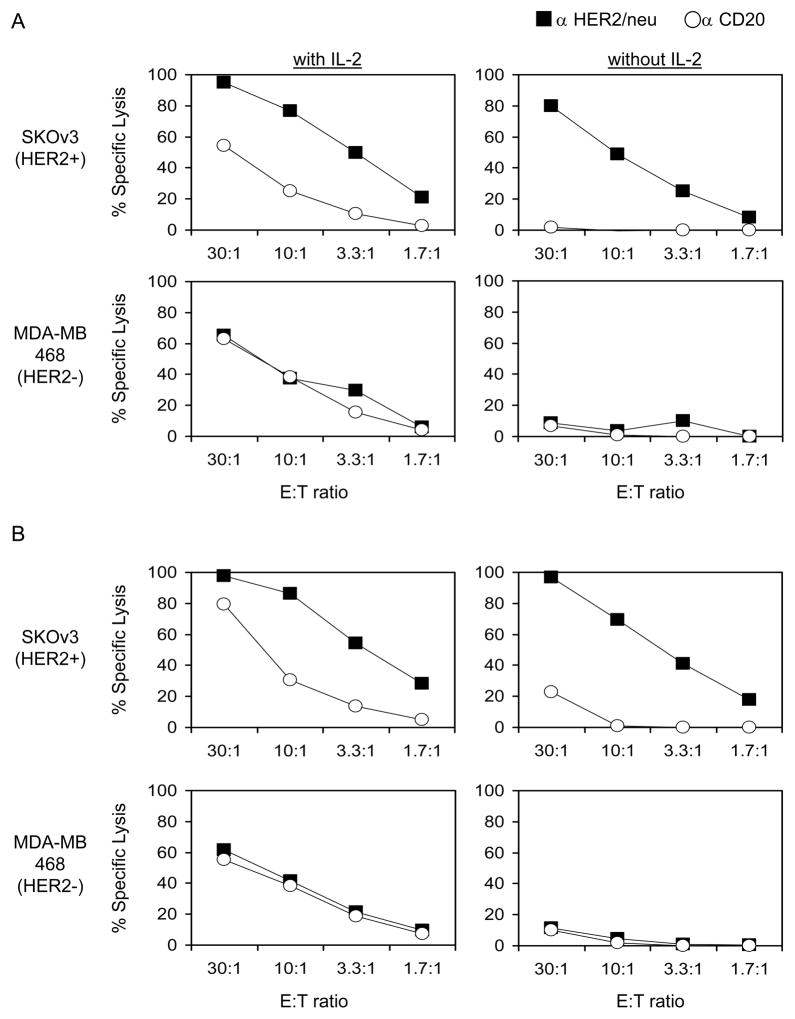
Since the adoptively transferred NK cells appeared to persist for long times in the peripheral circulation of patients, we began to consider coupling this therapy with other reagents that might improve the function of the adoptively transferred NK cells. Since some of the persistent cells retained expression of CD16, the low affinity Fc receptor for IgG, we evaluated the ability of these cells to mediate antibody dependent cell-mediated cytotoxicity (ADCC). Namely, we pre-incubated HER2/neu expressing tumor cell lines with an anti-HER2/neu antibody or a control antibody (anti-CD20) and performed lysis assays using post-treatment PBMCs, which were primarily NK cells, from patients as the effector cells. Cryopreserved PBMCs were thawed and cultured for 2 days in the presence or absence of IL-2 prior to lysis assays. One example of this is presented in Figure 4. 10-day (90% NK cells; Figure 4A) and 48-day (69% NK cells; Figure 4B) post-treatment PBMCs from patient 1 did not lyse HER2/neu+ SKOv3 cells or HER2/neu-MDA-MB-468 cells unless reactivated in vitro with IL-2. However, in the absence of IL-2, these cells could mediate ADCC and lyse HER2/neu+ SKOv3 cells when the cells were pre-incubated with anti-HER2/neu monoclonal antibody. In fact, the overall level of ADCC of the HER2/neu+ SKOv3 cell line as presented in both panels A and B of Figure 4 was similar whether or not the NK cells were stimulated with IL-2. Similar results were observed in tests of cells from two additional patients. This suggests that combining adoptive NK cell transfer with monoclonal antibody administration in vivo deserves evaluation.
Figure 4.
Adoptively transferred NK cells that persist in the peripheral circulation can mediate ADCC without in vitro reactivation. PBMCs from patient 1 were collected at 10 days (A) and 48 days (B) after adoptive NK cell transfer. At 10 days post-treatment, PBMCs contained 90% CD3-CD56+ NK cells, and at 48 days, PBMCs were 69% NK cells. These cells were cultured for 2 days in the presence or absence of IL-2 as noted and then evaluated for the ability to lyse antibody-coated tumor cells using standard 4-hour 51Cr release assays. Each symbol represents the average of 3 data points.
Discussion
In the work described here, we evaluated the treatment of cancer patients with large numbers (>1010) of adoptively transferred, in vitro activated, autologous NK cells after the patients received lymphodepleting but non-myeloablative chemotherapy. The infused NK cell populations were highly purified (96% ± 2% CD3- CD56+) and efficiently lysed melanoma cells in vitro (82% ± 12% specific lysis of 888mel at an E:T ratio of 10:1). Out of eight patients treated with adoptively transferred NK cells, zero experienced a clinical response. The upper 95% one-sided confidence interval on 0/8 is 31%; thus, from this limited number of subjects, we can be 95% confident that the true response rate is less than 31%. Although no clinical responses were observed in the eight patients treated, the adoptively transferred NK cells appeared to persist in the peripheral circulation of patients for at least one week post-transfer, and in some patients for several months. In vivo, NK cells are self-tolerant (10, 22, 23), and one of multiple proposed mechanisms for this is through decreased expression of stimulatory receptors. In our study, the persistent NK cells expressed significantly lower levels of the key activating receptor NKG2D and were quiescent in that they could not lyse tumor cell targets in vitro unless reactivated with IL-2, which may in part explain why we did not observe any clinical responses or autoimmunity.
Due to the self-tolerance associated with autologous NK cells, several investigators have explored the use of adoptively transferred allogeneic NK cells for treating patients with cancer. In a prior study in which 93 patients with acute myeloid leukemia (AML) were treated with haploidentical allogeneic stem cell transplants depleted of T cells (24), recipients that were class I MHC mismatched from their donors in a way that allowed for the development of “alloreactive” NK cells in the graft-vs.-host direction were significantly less likely to relapse than those who received stem cells that were not capable of mounting such an “alloreactive” NK cell response. In a separate investigation (25), 19 patients with poor-prognosis AML were treated with haploidentical PBMCs depleted of CD3+ T cells after a lymphodepleting chemotherapy regimen. Four of these patients were KIR ligand mismatched in the graft-vs.-host direction, and three of those 4 (75%) achieved a complete remission. In contrast, without this “alloreactivity”, only 2 of 15 (13%) achieved remission (p=0.04). In addition, the number of circulating NK cells was significantly greater in patients who achieved remission than those that did not. However, these results are controversial, and several other similar studies found no correlation between the risk of relapse and KIR ligand mismatching between donor and recipient in the context of AML (23, 26–29).
More recently, several investigators have explored the use of adoptively transferred allogeneic NK cells for the treatment of patients with solid tumors (21, 30, 31). In one study, 20 patients with breast or ovarian cancers received adoptively transferred allogeneic NK cells and IL-2 after lymphodepleting chemotherapy with or without additional total body irradiation (21). In this study, the authors stated that four patients with ovarian cancer had some tumor reduction; however, the duration of responses was not stated, and it is not clear whether these responses met RECIST criteria. The adoptively transferred cells were detected in the peripheral circulation of most patients one week after treatment but not at later time points, and the authors suggested that the transient donor chimerism may have been hampered due to reconstitution by recipients’ endogeneous regulatory T cells. In two separate studies, patients with melanoma, renal cell cancer, sarcoma, medulloblastoma, or PNET received adoptively transferred NK-92 cells (30, 31). The NK-92 cell line was originally established from a non-Hodgkin’s lymphoma that had NK cell-like morphology and expressed CD56 but not CD3 or CD16. These cells lack expression of inhibitory receptors and are highly cytolytic against many different types of tumor cells (32). In addition, NK-92 cells do not attack non-transformed cells and do not cause malignancies. In these trials, two mixed responses were observed, but no objective clinical responses according to RECIST criteria were reported; however, the NK-92 cells were irradiated prior to adoptive transfer and were given to lymphoreplete patients.
Thus far, in ours as well as published studies, adoptive transfer of NK cells, either autologous or allogeneic, have been largely unsuccessful for treating patients with solid tumors. However, multiple strategies for improving NK cell function in vivo are currently being pursued. As observed in our study, adoptively transferred NK cells that persisted in the peripheral circulation of patients retained some expression of CD16 and could mediate ADCC in vitro without in vitro reactivation with IL-2. Therefore, coupling of adoptive NK cell transfer with monoclonal antibody therapy is attractive, and clinical trials combining these treatment modalities are currently being conducted. Several other strategies to improve NK cell function have been suggested including blocking inhibitory signals with anti-KIR monoclonal antibodies or small interfering RNAs, genetic manipulation of NK cells to overexpress activating receptors or to introduce chimeric activating receptors, administration of chemotherapy, irradiation, or histone deacetylase inhibitors to upregulate NKG2D ligands on tumor cells, administration of proteasome or histone deacetylase inhibitors to upregulate TRAIL receptor expression on NK cells, and administration of other drugs such as thalidomide or Imatinib that promote survival, proliferation, and/or activation of NK cells (33, 34).
Supplementary Material
Translational Relevance.
Adoptively transferred tumor reactive T lymphocytes can mediate regression of metastatic cancers. However, many patients are ineligible for this type of treatment. Here we treated patients with adoptively transferred natural killer (NK) cells which can lyse tumor cells in a non-MHC restricted manner and independent of expression of tumor-associated antigens. Patients with metastatic melanoma or renal cell carcinoma were treated with large numbers (>1010) of adoptively transferred in vitro activated autologous NK cells after the patients received a lymphodepleting but non-myeloablative chemotherapy regimen. The infused cells were highly lytic in vitro, and although no clinical responses were observed, the adoptively transferred NK cells appeared to persist in the peripheral circulation of patients for weeks to months post-transfer. The persistent NK cells could not lyse tumor cell targets in vitro with cytokine reactivation. However, they could mediate antibody dependent cell-mediated cytotoxicity suggesting that coupling adoptive NK cell transfer with monoclonal antibody administration deserves evaluation.
Acknowledgments
This work was funded through the NCI intramural program. The authors thank Arnold Mixon and Shawn Farid for performing FACS analyses and all the clinical fellows and nursing staff in the Clinical Center of The National Institutes of Health who provided these patientswith outstanding care.
Footnotes
Authorship
M.R.P. and S.A.R. planned the initial experiments; J.P.R. and M.R.P. performed experiments and analyzed data; J.P.R. expanded all of the cells in vitro for patient treatments; M.E.D. aided in the preparation of cells for patient treatment; S.A.R. supervised the clinical trial; M.R.P. wrote the first draft of the manuscript which was revised in cooperation with all other authors.
Conflict of Interest Disclosure
The authors have no financial conflicts of interest to declare.
Reference List
- 1.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–40. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parham P. Immunology. NK cells lose their inhibition. Science. 2004;305:786–7. doi: 10.1126/science.1102025. [DOI] [PubMed] [Google Scholar]
- 5.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2005 doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valiante NM, Uhrberg M, Shilling HG, Lienert-Weidenbach K, Arnett KL, D’Andrea A, et al. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 1997;7:739–51. doi: 10.1016/s1074-7613(00)80393-3. [DOI] [PubMed] [Google Scholar]
- 8.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long EO. Tumor cell recognition by natural killer cells. Semin Cancer Biol. 2002;12:57–61. doi: 10.1006/scbi.2001.0398. [DOI] [PubMed] [Google Scholar]
- 10.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6:520–31. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–92. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 12.Hiserodt JC. Lymphokine-activated killer cells: biology and relevance to disease. Cancer Invest. 1993;11:420–39. doi: 10.3109/07357909309018874. [DOI] [PubMed] [Google Scholar]
- 13.Burns LJ, Weisdorf DJ, Defor TE, Vesole DH, Repka TL, Blazar BR, et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 2003;32:177–86. doi: 10.1038/sj.bmt.1704086. [DOI] [PubMed] [Google Scholar]
- 14.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–76. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172:864–70. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 16.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+CD25+ T Regulatory Cells Suppress NK Cell-Mediated Immunotherapy of Cancer. J Immunol. 2006;176:1582–7. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 17.Igarashi T, Wynberg J, Srinivasan R, Becknell B, McCoy JP, Jr, Takahashi Y, et al. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood. 2004;104:170–7. doi: 10.1182/blood-2003-12-4438. [DOI] [PubMed] [Google Scholar]
- 18.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parkhurst MR, Fitzgerald EB, Southwood S, Sette A, Rosenberg SA, Kawakami Y. Identification of a shared HLA-A*0201-restricted T-cell epitope from the melanoma antigen tyrosinase-related protein 2 (TRP2) Cancer Res. 1998;58:4895–901. [PubMed] [Google Scholar]
- 20.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–42. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–23. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruggeri L, Mancusi A, Capanni M, Martelli MF, Velardi A. Exploitation of alloreactive NK cells in adoptive immunotherapy of cancer. Curr Opin Immunol. 2005;17:211–7. doi: 10.1016/j.coi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 26.Malmberg KJ, Schaffer M, Ringden O, Remberger M, Ljunggren HG. KIR-ligand mismatch in allogeneic hematopoietic stem cell transplantation. Mol Immunol. 2005;42:531–4. doi: 10.1016/j.molimm.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 27.Davies SM, Ruggieri L, DeFor T, Wagner JE, Weisdorf DJ, Miller JS, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood. 2002;100:3825–7. doi: 10.1182/blood-2002-04-1197. [DOI] [PubMed] [Google Scholar]
- 28.Bornhauser M, Schwerdtfeger R, Martin H, Frank KH, Theuser C, Ehninger G. Role of KIR ligand incompatibility in hematopoietic stem cell transplantation using unrelated donors. Blood. 2004;103:2860–1. doi: 10.1182/blood-2003-11-3893. [DOI] [PubMed] [Google Scholar]
- 29.Farag SS, Bacigalupo A, Eapen M, Hurley C, Dupont B, Caligiuri MA, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12:876–84. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Tonn T, Becker S, Esser R, Schwabe D, Seifried E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res. 2001;10:535–44. doi: 10.1089/15258160152509145. [DOI] [PubMed] [Google Scholar]
- 31.Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–32. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- 32.Maki G, Klingemann HG, Martinson JA, Tam YK. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res. 2001;10:369–83. doi: 10.1089/152581601750288975. [DOI] [PubMed] [Google Scholar]
- 33.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–39. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 34.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–94. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.