Abstract
Plus-strand (+)RNA viruses co-opt host RNA-binding proteins (RBPs) to perform many functions during viral replication. A few host RBPs have been identified that affect the recruitment of viral (+)RNAs for replication. Other subverted host RBPs help the assembly of the membrane-bound replicase complexes, regulate the activity of the replicase and control minus- or plus-strand RNA synthesis. Host RBPs also affect the stability of viral RNAs, which have to escape cellular RNA degradation pathways. While many host RBPs seem to have specialized functions, others participate in multiple events during infection. Several conserved RBPs, such as eEF1A, hnRNP proteins and the Lsm 1–7 complex, are co-opted by evolutionarily diverse (+)RNA viruses, underscoring some common themes in virus-host interactions. On the other hand, viruses also hijack unique RBPs, suggesting that (+)RNA viruses could utilize different RBPs to perform similar functions. Moreover, different (+) RNA viruses have adapted distinctive strategies for co-opting unique RBPs. Altogether, a deeper understanding of the functions of the host RBPs subverted for viral replication will help development of novel antiviral strategies and give new insights into host RNA biology.
Key words: Plus-strand RNA virus, replication, virus-host interaction, host factor, RNA-binding proteins, RNA-dependent RNA polymerase, viral replicase complex
Introduction
There are several hundreds of different RNA viruses that cause wide spread diseases in plants and animals. Due to the RNA nature of their genomes, these viruses have to utilize many RNA-binding proteins of both viral and host origin, for their replication. Since replication of viral genomic RNA takes place in the cytosol of the infected cells, viruses must be capable of recognizing and amplifying only their own genomes while discriminating against the numerous cellular RNAs present in cells. Overall, the efficiency and specificity of viral RNA genome amplification is quite remarkable since the host itself lacks pre-existing RNA replication machinery and RNA viruses have very limited-coding capacity. Therefore, it is predictable that RNA viruses would co-opt host RNA-binding proteins (RBPs) to facilitate their replication or evade host RNA degradation pathways.
The genome of each positive-strand (+)RNA virus codes for an RNA-dependent RNA polymerase (RdRp) that, often together with a few other replication ancillary proteins, provides the core enzymatic activity to amplify the viral RNA genome using the original viral (+)RNA as template. Despite the diverse genome expression strategies, (+)RNA viruses employ a common approach for replication. After entry into host cells, the genome of (+)RNA virus is released from the virion and serves as mRNA exploiting the host translation machinery. Once sufficient amounts of viral RdRp protein and other ancillary replication proteins are synthesized, the viral (+)RNA is rescued from translation into genome replication. Replication produces negative-strand (−)RNA, which, in turn, serves as template for the synthesis of new (+)RNA progeny. In the case of several groups of (+)RNA viruses, replication also leads to the generation of subgenomic (sg) mRNAs. Replication of (+)RNA viruses is an asymmetric process, resulting in a 10- to 100-fold excess of the positive- over negative-strand RNAs. An intriguing feature of virus replication is that the newly made viral (+)RNAs participate in several competing processes, such as new rounds of translation, replication or encapsidation.1,2 For plant (+)RNA viruses, the new (+)RNA progeny also participate in cell-to-cell movement. It is likely that RBPs play critical roles in determining the localization and selected function of viral (+)RNAs during any given period of infection.
Similar to cellular mRNAs, viral (+)RNAs also contain both protein-coding and untranslated (UTR) regions. Interestingly, the UTRs are usually highly structured and predicted to be involved in long-range interactions, often involving 5′ UTR-3′ UTR interactions, resulting in genome “circularization”. Also, the genomic RNAs of several (+)RNA viruses harbor conserved secondary and tertiary structural elements within their coding regions. Altogether, there are specific RNA structures, called cis-acting elements, within the genomic (+)RNA, which provide diverse functions. These elements include promoters that regulate the site of initiation, polarity and timing of RNA synthesis;3 enhancers and silencers that control the efficiency of RNA synthesis;4–8 stabilizing elements that protect viral RNA from degradation;9 replicase assembly elements that serve as platforms for the assembly process;10,11 and recognition elements that confer binding specificity to the viral replication machinery.12–18 Thus, replication of viral RNA is a highly regulated, efficient process that is specified by structural features of the viral RNA and the dynamic interaction of viral RNA with RBPs leads to the formation of functional ribonucleoprotein (RNP) complexes.
Since the host mRNAs and other cellular RNAs are always associated with RBPs, often forming stable and functional RNP complexes, it is likely that viral (+)RNAs are also bound by viral or host RBPs and not present as “naked” (+)RNAs in infected cells. Many of the host RBPs are conserved, abundant and play essential roles in many aspects of host RNA biogenesis ranging from RNA processing, splicing, posttranscriptional modifications, RNA transport, subcellular localization and translation to RNA decay.19 The RBPs have diverse functions, including RNA polymerases, RNA chaperones, helicases, specific RNA-binding factors, scaffold proteins or RNA modifying enzymes. Altogether, identification of those host RBPs that affect (+)RNA virus infections is a major area in current virology research as described in the following chapters.
Recent evidence suggests that cellular RBPs likely play essential and regulatory roles in many steps of (+)RNA virus genome amplification.20–23 The ability of viral RNAs to co-opt host RBPs could determine the efficiency of replication, the permissiveness of certain cell types, the host range of a given virus, the pathology of viral infection and virus evolution. Identification of co-opted host RBPs and elucidation of their functions in viral replication may provide new ways to control viral diseases and advance our understanding of cell biology. This review provides an overview on the currently defined roles of cellular RBPs during (+)RNA virus replication, whereas RBPs involved in other steps of the infection process will not be discussed here.
Identification of Host RBPs Affecting (+)RNA Virus Replication
Traditionally, host RBPs interacting with the viral RNA were identified using co-purification or pull-down experiments with immobilized viral RNAs, followed by protein determination with mass spectrometry or other methods.24–28 Several host RBPs have been shown to co-localize with the viral replicase complex (RC), suggesting that they are integral components of viral RCs.29–36 Recently, high throughput approaches have been developed to identify viral RNA-binding host proteins using yeast protein microarrays carrying thousands of purified recombinant host proteins, as demonstrated for Brome mosaic virus (BMV) and Tomato bushy stunt virus (TBSV).37,38 The various screens with the BMV and TBSV RNAs reveal that as many as ∼50 host proteins could interact with viral RNAs during infection.
Host RBPs have also been identified in the purified viral replicases. For example, proteomic analysis of the affinity purified tombusvirus RC from yeast led to the identification of several host proteins, including RBPs, such as Tdh2/3 (a yeast homologue of mammalian glyceraldehyde-3-phosphate dehydrogenase, GAPDH) and eukaryotic translation elongation factor 1A (eEF1A).34 Similarly, the purified replicase complexes from Tobacco mosaic virus (TMV) and Turnip mosaic virus also contain eEF1A.39,40
Another popular approach to identify host genes affecting (+)RNA virus replication is systematic genome-wide approaches using yeast single-gene deletion (YKO) and the essential gene (yTHC) libraries or RNAi screens in Drosophila or mammalian cell cultures.41–47 These screens have led to the identification of many host RBPs as well. For example, the screens covering ∼95% of all yeast genes, have led to the identification of ∼130 genes affecting TBSV replication, of which 25 genes are known RBPs.46,47 To date only a few of these RBPs, such as Nsr1 (nucleolin), Bud21, Npl3 and Xrn1 5′-3′ exoribonuclease have been shown to bind to the TBSV RNA.38,48 Nevertheless, the identified RBPs will be useful in formulating hypotheses that can be tested to determine the specific functions of these host RBPs during (+)RNA virus infections. Overall, the currently characterized host RBPs constitute only a small fraction of those identified in the genome-wide screens, suggesting that many more important RBPs are awaiting to be further characterized to gain deeper insights into the roles of host RBPs in (+)RNA virus replication.
Host RBPs Affect the Process of Viral (+)RNA Recognition and Recruitment for Replication
Replication of (+)RNA viruses can be divided to several sequential steps.49 Events during the early steps affect the subsequent steps, allowing (+)RNA viruses to regulate the replication process. Replication starts with selective recognition of the cognate (+)RNA genome. The template selection is tightly connected with recruitment of the (+)RNA template and viral replication proteins to the sites of replication, which are specific subcellular membranes in infected cells. Template selection for replication likely involves the switch of the genomic (+)RNA from translation to replication, since the same (+)RNA is used for both processes. Recruitment is followed by the assembly of viral RC, which contains the (+)RNA template, viral replication proteins, subverted host proteins and host membranes. The fully assembled and activated viral RC then will synthesize the complementary (−)RNA, which subsequently serves as a template for the synthesis of new (+)RNA progeny. The newly made (+)RNAs are then released from replication. This cascade of events demonstrates the sophisticated organization of efficiency of (+)RNA virus replication.
RBPs facilitate viral (+)RNA template selection.
Viral (+) RNA replication is a selective process since viruses are known to replicate only the cognate or very closely related (+)RNA genomes, but discriminate against heterologous viruses and the abundant cellular RNAs. The current models predict the selectivity of (+)RNA replication is due to specific template selection by dedicated viral replication proteins and less frequently by the viral-coded RdRp proteins, such as protein A for Flock house virus.50 Accordingly, the partially or fully purified RdRp preparations from virus infected cells often display limited template specificity.21 The promiscuity of viral RdRp in vitro is in contrast with high selectivity of viral replication in vivo. How viral replicase discriminates its cognate template from numerous cellular RNAs in the infected cells remains an enigma for many viruses. The emerging evidence suggests that viral replication proteins can bind selectively to specific cis-acting elements, called viral recognition elements, in the viral (+)RNA genomes. Indeed, mutations of these RNA elements severely impair virus replication as shown for several (+)RNA viruses.12,13,15–18,51,52 For instance, the auxiliary replication protein p33 of TBSV forms a homodimer or a heterodimer with p92pol RdRp and specifically recognizes a C•C mismatch present within a stem-loop structure located in an internal replication element (RE) within the RdRp coding sequence in the (+)RNA genome. The binding of p33 to RE is absolutely required for selective recruitment of the TBSV (+)RNA into replication.16,53,54 However, it is highly possible that host RBPs could also contribute to the recruitment of TBSV (+)RNA. Accordingly, eEF1A has been found to bind specifically to the stem-loop 3 (SL3) in the 3′ UTR of TBSV,38 which stimulates the recruitment of (+)RNA template to cellular membranes. Chemical inhibitors of eEF1A, which block eEF1A-RNA binding, inhibits membrane association of TBSV (+)RNA and represses viral RNA synthesis in vitro.55 For BMV, the helicase-like replication protein 1a mediates the recruitment of genomic RNAs to replication. The recruitment also requires the binding of host deadenylation complex Lsm1-7 to the tRNA-like structure (TLSs) within the 3′-UTR of BMV RNAs.56,57
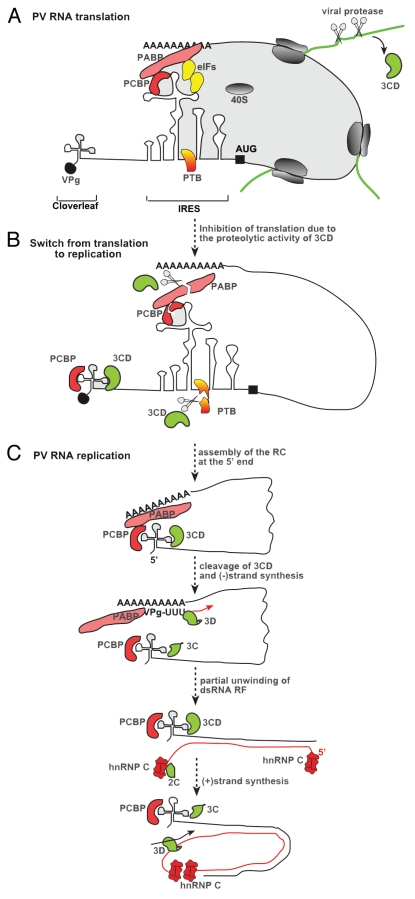
Another degree of replication specificity may be conferred by cis-preferential replication mechanism of viral genome. Studies with several viruses indicate that genome translation and replication are coupled.58–64 Those genomic RNAs, which undergo translation and produce functional replication proteins, will be preferentially subjected to replication due to binding of the newly made viral replication proteins in cis to the same genomic (+) RNA used for translation. In addition to the viral replication proteins, host factors could also contribute to cis-preferential replication. For example, in the case of poliovirus (PV), it has been suggested that the host poly(rC)-binding protein (PCBP), also known as heterogeneous nuclear ribonuclear protein E (hnRNP E), plays a pivotal role in template selection.65 PCBP interacts with two RNA structures within the 5′-UTR of PV genome that are important for both translation and replication. The binding of PCBP to stem-loop IV of internal ribosomal entry site (IRES) is essential for cap-independent translation of PV genome (Fig. 1A).65–68 PCBP also interacts with an upstream cloverleaf structure and forms RNP with polymerase precursor 3CD protein to promote initiation of (−)RNA synthesis (Fig. 1C).13,68–70
Figure 1.
A model of the roles of host RBPs in PV replication. (A) The roles of host RBPs in PV translation. The binding of host RBPs to stem-loop IV of internal ribosomal entry site (IRES, shown as stem-loop structures) is essential for cap-independent translation of PV genome. (B) The roles of host RBPs in a switch by the PV RNA from translation to replication. The dual roles of PCBP2 in PV translation and replication place PCBP2 in an ideal position to mediate the switch from translation to replication. The viral 3CD likely serves as a “sensor” to measure viral protein translation, while PCBP2 acts as a “molecular switch”. The involvement of PCBP2 in PV replication is facilitated by the proteolytic cleavage of PCBP2 by the viral protease 3C/3CD during the mid-to-late phase of infection. (C) The roles of host RBPs in PV RNA replication. PCBP interacts with the 5′ cloverleaf structure and forms RNP with polymerase precursor 3CD protein to promote initiation of (−)RNA synthesis. The replication initiation complex formed at 5′-UTR, which contains the viral 3Dpol RdRp (either activated via a cleavage of the 3CD precursor to yield the matured 3Dpol or via direct recruitment of 3Dpol), is brought to 3′-end by genome circularization via interaction of PCBP with PABP bound to the poly(A)-sequence in the 3′-UTR. hnRNP C has been proposed to maintain the single-stranded form of the 3′-end of PV (−)RNA via its RNA chaperone activity, and then, recruit viral 3CD replication protein to form an initiation complex for (+)RNA synthesis. Since hnRNP C interacts with both termini of PV (−)RNA, hnRNP oligomerization could bring the 5′- and 3′-end sequences into proximity, thus facilitating initiation of (+)RNA synthesis. The detailed functions of the RBPs and viral factors are described in the text.
The dependence of translation of BMV genomic (+)RNAs, but not sgRNA, on Lsm1-7 complex might also facilitate the selection of legitimate RNA templates for replication versus illegitimate (heterologous or defective) RNAs prior to the 1a-mediated recruitment of viral RNAs into replication.71 Similarly, Hepatitis C virus (HCV) also utilizes Lsm1-7 complex and polypyrimidine tract-binding protein (PTB) for both translation and replication,29,72 indicating these cellular factors could be involved in template selection for several (+)RNA viruses.
Intriguingly, not only eukaryotic (+)RNA viruses, but bacteriophage Qβ also takes advantage of host RBPs for its (+)RNA template recruitment. The binding of the Qβ replicase to the viral (+)RNA is mediated via the interactions of ribosomal protein S1 with two internally located recognition elements (S site and M site) within the Qβ genome.73,74
Host RBPs inhibiting recruitment of viral (+)RNA templates for replication.
(+)RNA viruses are able to hijack cellular RBPs for their genome translation and replication, but the host cell may also utilize an arsenal of RBPs to interfere with viral processes. For example, antiviral effect could be manifested by a host protein via specific binding to the viral (+)RNA followed by RNA degradation or redirecting/sequestering the viral (+)RNA to particular cellular compartments (away from the regular replication sites), preventing the viral (+)RNA from executing its normal functions. This strategy could be especially potent at the early stages of virus infection, when only single or a few copies of viral (+)RNAs are present in the infected cells, possibly leading to a basic resistance mechanism against viruses. Such scenario has been shown for the host nucleolin protein and TBSV. Nucleolin is a nucleolus-localized protein, but it also shuttles between the cytoplasm and the nucleus. It specifically binds to a stem-loop region within the 3′-UTR of TBSV genomic RNA and inhibits TBSV replication when it is present at the beginning, but not at the late stage of virus infection.48 Deletion of nucleolin binding sites from TBSV genome renders the replication of mutant RNA independent of the presence of nucleolin. Based on in vitro assay using cell-free extract from yeast model host, nucleolin has been found to inhibit TBSV replication likely by interfering with the recruitment of the viral (+)RNA for replication.48 Similarly, Arabidopsis NTR1 protein with three hnRNP K-homology (KH) RNA-binding domains has been shown to specifically bind to both 5′- and 3′-UTR of Tomato mosaic virus (ToMV) genome and to inhibit ToMV multiplication. BTR1 inhibits the translation of a reporter mRNA harboring a ToMV BTR1-binding site, suggesting that it may inhibit ToMV replication at the early stage of infection that might include (+)RNA recruitment.75
The Roles of Host RBPs during the Switch from Translation to Replication
Upon infection of cells by (+)RNA viruses, the viral genomic (+) RNA serves as template for both translation and genome replication, albeit the two essential processes are in conflict with each other as they utilize the same (+)RNA template but proceed in opposite directions. Indeed, when ribosome-bound bacteriophage Qβ RNA is used as template for replication, the Qβ replicase is not able to complete the (−)RNA synthesis efficiently.2 Similarly, translating ribosome also inhibits PV RNA synthesis.76 Treatment of cell-free translation and replication-compatible lysate with puromycin after translation, which induces dissociation of translating ribosome from mRNA, stimulates genome replication. On the other hand, additional translation inhibitors such as cycloheximide, which “freeze” the ribosome on mRNA, have inhibitory effect on viral RNA synthesis highlighting the necessity of clearance of ribosome from viral (+)RNA prior to replication.1 Therefore, (+)RNA viruses need to temporally coordinate these two processes to allow sufficient production of viral proteins before switching to replication. Such translation-replication balance has also been shown to be important for efficient amplification of Qβ in an in vitro reconstituted system.77 In addition, viral genomic (+)RNAs need to avoid degradation by mRNA decay pathways after translation in order to be recruited to subsequent replication.
Increasing evidence shows that some viral replication proteins facilitate repression of translation and switch to viral RNA synthesis.2,78–81 Host RBPs also play important roles in regulation of this event through their interactions with various cis-elements in the viral (+)RNA. Indeed, interaction of PV 3CD protein with stem-loop D of the cloverleaf structure at the 5′ end of PV (+)RNA greatly increases the binding affinity of PCBP2 to stem-loop B, resulting in repositioning of PCBP2 from the translation element (IRES) to the replication element (i.e., the cloverleaf structure).65 These events lead to inhibition of translation and promotion of RNA synthesis65,76 (Fig. 1B). The dual roles of PCBP2 in PV translation and replication place PCBP2 in an ideal position to mediate the switch from translation to replication. It appears that viral 3CD serves as a “sensor” to measure viral protein translation, while PCBP2 acts as a “molecular switch”. The involvement of PCBP2 in PV replication is facilitated by the proteolytic cleavage of PCBP2 by the viral protease 3C/3CD during the mid-to-late phase of infection.82 While the full-length PCBP2 with three RNA-binding (KH) domains binds selectively to the stem-loop IV of IRES,83 the proteolytic cleavage within a linker sequence between KH2 and KH3 renders the truncated PCBP2 protein (which lacks the KH3 domain) unable to bind to IRES. This leads to inhibition of PV translation.82 Interestingly, the truncated form of PCBP2 still maintains the activity to rescue PV (+)RNA replication in a PCBP2-depleted extract. This led the authors to propose an elegant model for translation-replication switch based on viral protease-mediated cleavage of PCBP2 (Fig. 1B).
Apart from PCBP2, other cellular factors, such as polypyrimidine tract-binding protein (PTB), poly(A)-binding protein (PABP) and lupus autoantigen (La), are also required for efficient PV IRES-dependent translation.84 It is conceivable that proteolytic cleavage of these factors may also contribute to inhibition of PV translation and the switch to (+)RNA replication.85,86 Overall, it appears that the combination of mechanisms may act in concert to trigger the switch from translation to PV RNA replication (Fig. 1B).
Studies with BMV and yeast model host revealed that the mRNA decapping activation complex Lsm1-7 and Pat1, Dhh1 might participate in facilitating the switch from translation of the BMV RNAs to viral replication. The purified Lsm1-7 complex binds to the tRNA-like structure (TLS) within the 3′ UTR and intergenic region (IR) of BMV RNA3.87 Lsm 1–7-dependent RNA3 translation and replication require TLS and substitution of TLS with poly(A) tail was found to circumvent the requirement of Lsm1-7 in translation of BMV RNA3 and its recruitment into replication. Efficient Lsm1-7-mediated translation also required specific RNA structures in the IR, whose deletion resulted in strong inhibition of RNA3 translation, while increased the recruitment of RNA3 into replication.87 The interaction of BMV RNA3 with Lsm1-7 might lead to clearance of translation initiation factors as described for cellular mRNAs with shortened poly(A) tail.88,89 Then, the formed mRNP complex is recruited to processing body (P-body),88,89 where the P-body components might facilitate replicase assembly by concentrating viral RNAs and replication protein in discrete compartment without the competition of translating polyribosome. Remarkably, the human homolog of yeast Lsm1-7 and Pat1, Dhh1 complex is also important for HCV translation and RNA accumulation.72,90 The reconstituted Lsm1-7 heptameric ring-shape complex specifically binds to HCV 5′- and 3′-UTR which contains important cis-element for translation and replication.72
For bovine viral diarrhea virus (BVDV),91 and HCV,92 host RPBs NF90/NFAR have been shown to circularize the genome through interactions with both 5′- and 3′-UTRs and the interaction appears to inhibit translation but promote RNA replication.93 It seems that the circular conformation of the viral genome is different during translation and replication. Thus, the switch from translation to replication could be mediated by viral replication proteins and host RPBs via architectural remodeling of the viral genomic RNA.
RBPs and Viral RNA Synthesis
Regulation of the assembly of the viral RC.
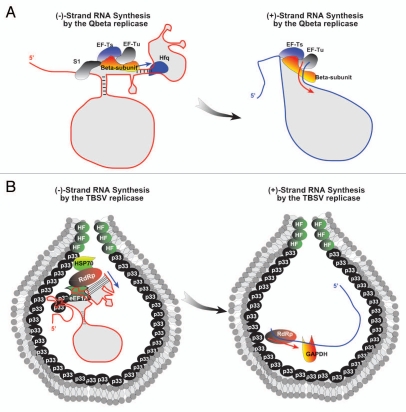
It is becoming evident that the assembly of functional viral RCs is a highly regulated process, which involves complex interactions among viral (+)RNA, the viral replication proteins and co-opted host proteins and lipids. The viral genomic (+)RNA can serve as a platform for the assembly of the viral RC. For example, purified preparations of TBSV p33/p92pol, BMV 1a/2apol and Alfalfa mosaic virus (AlMV) P1/P2 are nonfunctional in vitro when purified from yeast or plant cells in which these replication proteins are expressed in the absence of cognate viral RNAs.94–97 In contrast, when the above viral proteins are co-expressed with the cognate (+)RNAs, then the obtained viral replicases become active and are able to utilize added RNA templates in vitro. To explain the replicase “activation” phenomenon, it has been suggested that the complexes of these replication proteins must be assembled via the help of the viral RNAs that likely serve as “assembly platforms”. Detailed works defined that only short regions within the viral (+) RNAs are required for the assembly/activation of the BMV and TBSV RdRps.10,11 Although the mechanism of viral RdRp activation by (+)RNA template has not yet been determined, it is possible that the presence of viral (+)RNA may trigger conformation changes in the viral RdRps. Alternatively, viral RNA could be essential for recruitment of cellular RBPs to form functional viral RC. Indeed, the roles of host RBPs in replicase assembly and function has been documented for Qβ bacteriophage (Fig. 2A). In addition to virus-encoded polymerase β-subunit, functional Qβ replicase holoenzyme also contains three host translation factors: ribosomal protein S1 and elongation factors Tu (EF-Tu) and EF-Ts. β-subunit is the catalytic core of the replicase and the interaction of β-subunit with EF-Tu and EF-Ts is essential to maintain its polymerization activity (Fig. 2A).98 The replicase binding to the (+)RNA template is mediated by S1, which recognizes two internal sites (M and S site), while EF-Tu is involved in binding to the (−)RNA template.74 Crystal structure of β subunit in complex with EF-Tu and EF-Ts shows that the co-opted translation elongation factors have chaperone-like function to maintain the folding of the active β-subunit. EF-Tu:Ts may also regulate RNA folding and contribute to separation of RNA duplex formed between the template and nascent strands.99,100 An additional Qβ host factor, known as Hfq, is recruited by binding to 3′-end of Qβ genome and is required for (−)RNA synthesis101 (Fig. 2A).
Figure 2.
Comparison of the co-opted host factors for Qbeta bacteriophage and TBSV replication. (A) The long-range interactions between regions in the Qbeta (+)RNA genome are depicted by multiple lines. The detailed functions of the RBPs and viral factors are described in the text. (B) The long-range base-pairing in the TBSV (+)RNA genome brings recruitment element bound by p33 into the vicinity of the 3′-UTR, which also has base-pairing between the silencer element and the 3′ end as depicted by multiple lines. The shown RBPs facilitate TBSV RNA synthesis as described in the text.
Notably, several host proteins have been shown to interact with both viral proteins and the viral RNA. For example, hnRNP A1 interacts with HCV NS5b and both ends of the genomic (+) RNA,102 while hnRNP C binds to PV (+)RNA and 3CD replication protein.103,104 Also, eEF1A binds to viral replication proteins and viral (+)RNAs of several viruses, such as West Nile virus (WNV),105,106 TBSV54,55 and TMV.107,108 Proteome-wide screens also revealed significant overlap of cellular factors that bind to TBSV (+)RNA and replication proteins.54,109 These data suggest that viruses may utilize multiple molecular functions of subverted host factors to regulate RNA synthesis in the tightly organized viral replication compartments.
Regulation of negative-strand RNA synthesis by host RPBs.
During viral RNA replication, (−)RNA synthesis initiates from the 3′-terminal promoter sequence present in the viral (+)RNA.110 Unexpectedly, many viral RdRps bind to 5′ or internal sites in the (+)RNA genome, suggesting that additional factors could facilitate the re-positioning of the RdRp over the 3′ terminal promoter sequence prior to initiation.15,16,73,111 Accordingly, increasing amounts of evidence suggest that long-distance interactions within the (+)RNA genome mediated either by protein-RNA or RNA-RNA interactions could bring the 5′ terminal or internal cis-elements into proximity of the 3′-terminal sequences in the genomic RNA. For example, Qβ replicase is thought to bind to an internal recognition site (M site), which is about 1,450 nt away from the 3′-end of the genome.111 A long-range RNA-RNA base-pairing that forms between the loop of the 3′ terminal hairpin and a 1,200 nt upstream sequences serves to bring the Qβ replicase in close proximity to the 3′-terminus prior to initiation.112,113 Similarly, in the case of TBSV, a long-distance base-pairing between internal replication protein binding site and 3′-UTR is essential for (−)RNA synthesis and replicase assembly.114 Genome cyclization of Dengue virus (DENV) is mediated via long-distance RNA-RNA interaction of complementary sequences in the 5′- and 3′-UTR, including the cyclization sequences (CS) and upstream AUG region (UAR), which is required for genome replication.115–117 Also, a kissing-loop interaction between a sequence within the HCV NS5b coding region and the 3′-UTR is essential for HCV replication and is proposed to facilitate the coordination of the viral polymerase to the very 3′-end of the viral genome.118 The above-mentioned long-distance RNA-RNA interaction has been observed in the absence of protein, however, it is possible that host RBPs may facilitate or stabilize these RNA structures.
The cloverleaf structure in the 5′-UTR of PV (+)RNA serves as a platform for formation of the essential RNP complex consisting of PV 3CD replication protein and the co-opted PCBP (Fig. 1C).119 Then, the replication initiation complex formed at 5′-UTR, which contains the viral 3Dpol RdRp (activated via a cleavage of the 3CD precursor to yield the matured 3Dpol) is brought to 3′-end by genome circularization via interaction of PCBP with PABP bound to the poly(A)-sequence in the 3′-UTR (Fig. 1C).120 In the case of mouse hepatitis coronavirus (MHV), communication between the viral UTRs is facilitated by interactions between the PTB protein bound to 5′-UTR and hnRNP A1, which is bound to 3′-UTR of the viral genome. Mutations that impair binding of host factors to the UTRs inhibit replication and transcription of a MHV RNA, confirming the significance of host RPBs in coronavirus replication.121
eEF1A is one of the most common RBPs that has been identified in association with 3′-UTR of a wide variety of (+) RNA viruses.54,105–107,122 Mutation analysis of eEF1A-binding sites within the 3′-UTR of WNV revealed correlation between eEF1A binding and (−)RNA synthesis. eEF1A also interacts with WNV NS5 protein and is co-localized with the WNV RC in the infected cells, suggesting that it facilitates the interaction between the viral replicase and the 3′-UTR of the viral gRNA.105 Similarly, eEF1A has been shown to interact with TBSV replication proteins and 3′-UTR of viral RNA and is an integral component of the viral RC (Fig. 2B).54 A set of mutants of eEF1A was found to enhance TBSV RNA replication in yeast and increase (−)RNA synthesis in a cell-free TBSV replicase assay. Moreover, eEF1A was shown to stimulate initiation of (−)RNA synthesis in vitro by a closely related recombinant viral RdRp, suggesting a direct role of eEF1A in (+)RNA virus replication.55 This function of eEF1A could be facilitated by binding of eEF1A to a regulatory RNA element, termed replication silencer, which base-pairs with the extreme 3′-terminus of the viral RNA and downregulates (−)RNA synthesis in vitro.123 The binding of eEF1A to p92pol,38 might facilitate the proper positioning of the viral RdRp over the 3′-promoter to enhance the initiation events (Fig. 2B).55 Similar to TBSV replication silencer-promoter base-pairing, the 3′-terminal sequence of Qβ (+)RNA also forms a pseudoknot structure with an adjacent sequence that buries the promoter. Opening up this structured sequence prior to initiation of RNA synthesis is likely due to the Hfq host factor, an Sm-like protein, which forms hexameric complexes and harbors RNA chaperone activity. Hfq is required for efficient synthesis of Qβ (−)RNA both in vivo and in vitro.98 However, Qβ RNAs carrying mutations at the 3′-terminus, which disrupt the pseudoknot formation, are able to replicate independent of Hfq, suggesting that the RNA chaperone function of Hfq is to “unmask” the 3′-end and facilitate the access of the replicase to the 3′-terminus of the genome during replication101 (Fig. 2A). Another similarity between tombusvirus and Qβ RNA replication is the interaction of eEF1A with tombusviruses replication proteins p33 and p92 RdRp, which leads to stabilization of p33 and stimulation of tombusvirus replicase activity,54,55 reminiscent of the functions described for EF-Tu:Ts in Qβ RC99,100 (Fig. 2A and B).
Regulation of plus-strand RNA synthesis by RBPs.
Similar to (−)RNA synthesis, initiation of (+)RNA synthesis is likely facilitated by co-opted host RBPs. Brunner and co-workers identified the cellular hnRNP C, which binds specifically to the 3′-end of the PV (−)RNA (Fig. 1C).103,124 hnRNP C, which is redistributed to the cytoplasm from nucleus upon PV infection, also interacts with the PV 3CD precursor protein. It has been suggested that hnRNP C functions to maintain the single-stranded form of the 3′-end of PV (−)RNA via its RNA chaperone activity and then, recruit viral 3CD replication protein to form an initiation complex for (+)RNA synthesis.103 Accordingly, depletion of hnRNP C from a PV replication-competent Hela extract inhibited (+)RNA synthesis, which could be rescued by addition of wt recombinant hnRNP C protein, but not by a truncated form deficient in binding to 3CD. Since hnRNP C interacts with both termini of PV (−)RNA, hnRNP oligomerization could bring the 5′- and 3′-end sequences into proximity, thus facilitating initiation of (+) RNA synthesis (Fig. 1C).104
For WNV, the 3′-terminal 96 nt of (−)RNA containing a conserved stem-loop structure is proposed to function as promoter for (+)RNA synthesis.125 Interestingly, the stress granule proteins, termed the T-cell intracellular antigen-1 (TIA-1) and the TIA-1-related protein (TIAR) bind specifically to the 3′-SL of WNV (−)RNA.126 Mutations in 3′-SL, which reduce TIA1/TIAR binding, greatly decreases genomic RNA amplification, suggesting that specific binding of TIA1/TIAR facilitates efficient (+)RNA synthesis.127
In addition to the host RBPs, which bind to (−)RNA, those RBPs that bind to the 5′-terminus of (+)RNA might also affect (+) RNA synthesis. For example, the 5′-terminal cloverleaf structure present in PV (+)RNA is also required for initiation of (+)RNA synthesis, in addition to its established role as a promoter for (−)RNA synthesis.68–70 By duplication of the 5′-terminal cloverleaf structure, which allows the separation of its potential functions in initiation of RNA synthesis of both polarities, Dorothee and Andino have shown that, it is the cloverleaf structure formed in the (+)RNA, not the corresponding one in the (−)RNA, that is required for initiation of new (+)RNA synthesis. The binding of PCBP and 3CD to cloverleaf of (+)RNA is also required for (+)RNA synthesis. These findings led the authors to propose a trans-initiation model for PV (+)RNA synthesis, in which the PCBP and 3CD first bind to cloverleaf in (+)RNA to form an RNP complex that functions to keep the 3′-end of (−)RNA single stranded (by preventing the base-pairing of the 5′-end of the (+)RNA with the complementary minus-strand sequence) and facilitate the delivery of 3D polymerase to the (−)RNA template in trans (Fig. 1C).128
Regulation of asymmetrical RNA synthesis by host RPBs.
A characteristic feature of (+)RNA viruses is the asymmetric nature of viral RNA synthesis, which leads to 10:1 to 100:1 ratio of (+) versus (−)RNA progeny. The asymmetric replication could be attributed to differences in intrinsic promoter strength, as well as the presence of various regulatory RNA elements that function to enhance or repress RNA synthesis. Host RBP factors are also likely involved in regulating this process by interacting with viral replication proteins and cis-acting RNA elements. Indeed, the ratio of Qβ (+) versus (−)RNA is determined by the concentration of the co-opted hfq in vitro. In the presence of abundant amount of Hfq protein, equal quantities of (+) and (−)RNA is synthesized, while limiting amount of Hfq protein results in excess of (+) versus (−)RNA.98
The mechanism of asymmetric replication for eukaryotic (+)RNA viruses might be different from that of Qβ bacteriophage, since replication of eukaryotic RNA viruses is associated with virus-induced membrane invaginations that are derived from subcellular membrane surfaces in the infected host cells. Since the (+)RNA progeny must be released from the membrane-bound replicase to the cytoplasm, while (−)RNA remains inside the replication compartment,129 this step could also serve to regulate (+) versus (−)RNA levels. The mechanism underlying the asymmetric replication and release of (+)RNA progeny is largely unknown. An abundant metabolic enzyme GAPDH, which is recruited into the tombusvirus RC,34 has been shown to regulate asymmetrical TBSV RNA synthesis (Fig. 2B).35 Interestingly, depletion of GAPDH preferentially inhibits the accumulation of (+)RNA, resulting in a 1:1 ratio of RNA progeny of both polarities in yeast and in a natural plant host. It has been shown that GAPDH selectively binds to an AU pentamer sequence (AUUUA) present in the vicinity of the 3′-terminus of TBSV (−)RNA. Deletion of the AUUUA sequence also led to the production of (+) versus (−)RNA RNAs in a 1:1 ratio, even in the presence of wild-type levels of GAPDH. Based on these data, it has been proposed that GAPDH binds to and retains TBSV (−)RNA for multiple rounds of (+)RNA synthesis inside of the spherules containing the membrane-bound tombusviral RC (Fig. 2B). The (+)RNA progeny, which are not bound by GAPDH, then become released from the RC into the cytosol.35 It is also possible that GAPDH could play a direct role in (+)RNA synthesis, similar to that proposed for hnRNP C during PV replication.
Regulation of subgenomic RNA synthesis by host RPBs.
Many (+)RNA viruses with multiple open reading frames (ORFs) in the genomic (+)RNA express the 3′ proximal ORFs through the production of sgRNAs.130 The synthesis of sgRNAs is coordinated by cis- or trans-acting transcription elements.130–132 The viral replicase, possibly together with host factors, is involved in recognition of subgenomic promoter(s), followed by initiation and termination of transcription. For example, sgRNA transcription in coronaviruses requires the interaction between 5′-terminal leader sequence and an intergenic (IG) sequence immediately upstream of the initiating AUG of each ORF.133 In an attempt to identify host RBPs regulating MHV sgRNA transcription, Li et al. has found that one of the host proteins, hnRNP A1, specifically binds to the leader and IG sequences in MHV (−)RNA.134 Interestingly, the binding affinity of IG to hnRNP A1 correlates with the efficiency of MHV sgRNA transcription.134,135 Overexpression of wt hnRNP A1 has been shown to stimulate MHV RNA synthesis, while expression of dominant-negative mutant of hnRNP A1 inhibited replication.136 In vitro experiments indicate that the interaction of hnRNP A1 with the leader and IG sequences is essential for sgRNA transcription.137 Furthermore, hnRNP A1 also interacts with MHV N protein,138 suggesting that hnRNP A1 could recruit the viral N protein to MHV transcription sites. Another host protein implicated in regulating MHV sgRNA transcription is PTB (hnRNP I). PTB specifically interacts with positive-strand leader sequence, as well as with the 5′-UTR in (−)RNA. Deletion of PTB-binding sites in MHV defective interfering RNA significantly inhibits sgRNA transcription, with less effect on (−)RNA synthesis.139,140
Another host RPB implicated in viral sgRNA synthesis is hnRNP-K, a predominantly nuclear poly(C)-binding protein. Hardy and colleagues have found that hnRNP-K co-immunoprecipitates with the Sindbis virus (SINV) nsP1, nsP2 and nsP3 proteins. Moreover, hnRNP-K is redirected to viral RC in infected cells. hnRNP K is also co-precipitated with the SINV sgRNA, but not with the gRNA, suggesting that it could participate in sgRNA transcription.141
The production of noncoding sgRNAs with regulatory roles also requires host RBPs, namely Xrn1 5′-3′ exoribonuclease, as shown for Yellow fever virus and other flaviviruses.142,143 Interestingly, the host Xrn1 also affects tombusviral RNA recombination and RNA stability.144
Host RBPs and Stability of Viral RNA
Viruses must interact with cellular factors or pathways to maintain the integrity of viral RNAs and to suppress RNA degradation pathways. Relevant for this review is the role of host RBPs in stabilizing the viral RNAs. For example, HuR, which is a regulator of cellular mRNA stability, binds specifically to the U-rich element (URE) in the 3′ UTR of SINV and Venezuelan equine encephalitis virus (VEEV). The binding of the SINV RNA to HuR redirects the otherwise nuclear-localized HuR to cytoplasm upon SINV infection.9 Downregulation of HuR expression leads to accelerated decay of SINV mRNA and reduced virus production, indicating that HuR inhibits deadenylation-mediated degradation of SINV RNA through its binding to URE.145 Similarly, the 3′ UTR of the MHV coronavirus binds to the mitochondrial aconitase, a metabolic enzyme involved in citric acid cycle, which likely increases the stability and hence increases translation of viral proteins.146
Other viruses whose genomes lack either 5′-cap or 3′-poly(A), or both, may recruit host RBPs through various RNA elements to stabilize their genomes. The genome of PV contains a 5′ clover-leaf structure, which binds to PCBP that stabilizes PV (+)RNA in HeLa S10 extract. A mutant PCBP protein, which cannot bind to the cloverleaf structure, causes an accelerated degradation of PV (+)RNA. The interaction of PCBP with the PV (+)RNA likely blocks the Xrn1 5′-to-3′ exonuclease-mediated RNA decay, since in vitro capped PV (+)RNA could bypass the requirement of PCBP in stability assays.147
The 3′-UTR of HCV contains a number of stem-loop structures and a large U-rich tract, which interact with the La auto-antigen to prevent HCV degradation in HeLa S100 extract.148 HuR is also showed to bind to the U-rich tract in the 3′-UTR of HCV149 and knock-down of HuR expression by siRNA has been shown to inhibit HCV IRES-mediated translation and replicon RNA replication.150 However, the relevance of HuR in relation to HCV (+)RNA stability is not yet clear. Proteomics analysis have led to the identification of more than 70 human proteins associated with the HCV 3′-UTR and several of the identified host RPBs, such as hnRNP C and D, HuR, FBP, FBP2, YB-1 and NF90, have been implicated in controlling cellular mRNA stability.151 It will be interesting to find out if these host RPBs indeed participate in protection of the labile HCV (+)RNA from degradation.
The tRNA-like structure (TLS) in the 3′ end of turnip yellow mosaic virus (TYMV) becomes aminoacylated and interacts with host tRNA-binding proteins, such as eEF1A, which protects the viral RNA from RNase A digestion in vitro.152 Overall, how efficiently viruses can circumvent the viral RNA degradation may determine the outcome of infections. The above mentioned examples highlight the notion that the stability of viral RNA play an important role in successful infection and different viruses may evolve different mechanisms to protect their genomes, which likely involves subverted host RBPs.
Conclusion and Future Perspectives
Over the past 10 years, a great deal of knowledge has been accumulated on the host RBPs co-opted for viral (+)RNA replication. It is becoming evident that multiple steps in (+)RNA virus replication require coordination of (+)RNA elements viral factors and host-encoded RBPs. Indeed, recent genome- and proteome-wide screens have identified several hundred host factors affecting (+)RNA replication and 10–20% of these factors are RBPs.37,38,45,96,109 However, the functions of most of these RBPs in virus replication have yet to be determined.
The remarkable magnitude and diversity of host RBPs subverted for (+)RNA virus replication highlights that RBPs are major players determining virus-cell interactions. Several conserved RBPs, such as eEF1A, hnRNP proteins and Lsm 1–7 complex, have been identified in association with evolutionarily diverse (+)RNA viruses, underlying some common themes in virus-host interactions. However, there are unique RBPs identified as well, suggesting that either (+)RNA viruses could utilize different RBPs to perform similar functions or different (+)RNA viruses adapted unique strategies by co-opting specialized RBPs.
Due to the interdependent and sequential nature of many processes during (+)RNA virus infection, for instance, translation, replicase assembly, negative- and positive-strand RNA synthesis, pinpointing the exact step in which host RPBs are involved remains challenging. Therefore, the use of tractable model replication systems, such as TBSV, BMV and FHV in yeast, together with the fast-maturing RNAi technology, as well as structural and cell biology approaches, should facilitate rapid advance in our understanding of the roles of RPBs in (+)RNA virus replication. Knowledge of the functions of the subverted host RBPs in virus replication may not only lead to the development of novel antiviral strategies, but also facilitate our understanding of host RNA biology.
Acknowledgements
The authors thank Drs. Daniel Barajas, Tyng-Shyan Huang, Kunj Pathak and Judit Pogany for valuable comments. This work was supported by the National Science Foundation (IOB-0517218) and NIH-NIAID to P.D.N.
References
- 1.Barton DJ, Morasco BJ, Flanegan JB. Translating ribosomes inhibit poliovirus negative-strand RNA synthesis. J Virol. 1999;73:10104–10112. doi: 10.1128/jvi.73.12.10104-10112.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolakofsky D, Weissmann C. Q[beta] replicase as repressor of Q[beta] RNA-directed protein synthesis. Biochimica et Biophysica Acta (BBA) - Nucleic Acids and Protein Synthesis. 1971;246:596–599. doi: 10.1016/0005-2787(71)90799-4. [DOI] [PubMed] [Google Scholar]
- 3.Dreher TW. Functions of the 3′-untranslated regions of positive strand RNA viral genomes. Annu Rev Phytopathol. 1999;37:151–174. doi: 10.1146/annurev.phyto.37.1.151. [DOI] [PubMed] [Google Scholar]
- 4.Ranjith-Kumar CT, Zhang X, Kao CC. Enhancer-like activity of a Brome mosaic virus RNA promoter. J Virol. 2003;77:1830–1839. doi: 10.1128/JVI.77.3.1830-1839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panavas T, Nagy PD. The RNA replication enhancer element of tombusviruses contains two interchangeable hairpins that are functional during plus-strand synthesis. J Virol. 2003;77:258–269. doi: 10.1128/JVI.77.1.258-269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray D, Na H, White KA. Structural properties of a multifunctional T-shaped RNA domain that mediate efficient Tomato bushy stunt virus RNA replication. J Virol. 2004;78:10490–10500. doi: 10.1128/JVI.78.19.10490-10500.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panavas T, Nagy PD. Mechanism of stimulation of plus-strand synthesis by an RNA replication enhancer in a tombusvirus. J Virol. 2005;79:9777–9785. doi: 10.1128/JVI.79.15.9777-9785.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray D, White KA. An internally located RNA hairpin enhances replication of Tomato bushy stunt virus RNAs. J Virol. 2003;77:245–257. doi: 10.1128/JVI.77.1.245-257.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garneau NL, Sokoloski KJ, Opyrchal M, Neff CP, Wilusz CJ, Wilusz J. The 3′ untranslated region of Sindbis virus represses deadenylation of viral transcripts in mosquito and mammalian cells. J Virol. 2008;82:880–892. doi: 10.1128/JVI.01205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quadt R, Ishikawa M, Janda M, Ahlquist P. Formation of brome mosaic virus RNA-dependent RNA polymerase in yeast requires coexpression of viral proteins and viral RNA. Proc Natl Acad Sci USA. 1995;92:4892–4896. doi: 10.1073/pnas.92.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panaviene Z, Panavas T, Nagy PD. Role of an internal and two 3′-terminal RNA elements in assembly of tombusvirus replicase. J Virol. 2005;79:10608–10618. doi: 10.1128/JVI.79.16.10608-10618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahola T, den Boon JA, Ahlquist P. Helicase and capping enzyme active site mutations in brome mosaic virus protein 1a cause defects in template recruitment, negative-strand RNA synthesis, and viral RNA capping. J Virol. 2000;74:8803–8811. doi: 10.1128/jvi.74.19.8803-8811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andino R, Rieckhof GE, Achacoso PL, Baltimore D. Poliovirus RNA-synthesis utilizes an RNP complex formed around the 5′-end of viral RNA. EMBO J. 1993;12:3587–3598. doi: 10.1002/j.1460-2075.1993.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filomatori CV, Lodeiro MF, Alvarez DE, Samsa MM, Pietrasanta L, Gamarnik AV. A 5′ RNA element promotes dengue virus RNA synthesis on a circular genome. Genes & Dev. 2006;20:2238–2249. doi: 10.1101/gad.1444206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monkewich S, Lin H-X, Fabian MR, Xu W, Na H, Ray D, et al. The p92 polymerase coding region contains an internal RNA element required at an early step in tombusvirus genome replication. J Virol. 2005;79:4848–4858. doi: 10.1128/JVI.79.8.4848-4858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pogany J, White KA, Nagy PD. Specific binding of tombusvirus replication protein p33 to an internal replication element in the viral RNA is essential for replication. J Virol. 2005;79:4859–4869. doi: 10.1128/JVI.79.8.4859-4869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Wynsberghe PM, Ahlquist P. 5′ cis elements direct nodavirus RNA1 recruitment to mitochondrial sites of replication complex formation. J Virol. 2009;83:2976–2988. doi: 10.1128/JVI.02040-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Lee W-M, Watanabe T, Schwartz M, Janda M, Ahlquist P. Brome mosaic virus 1a nucleoside triphosphatase/helicase domain plays crucial roles in recruiting RNA replication templates. J Virol. 2005;79:13747–13758. doi: 10.1128/JVI.79.21.13747-13758.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glisovic T, Bachorik JL, Yong J, Dreyfuss G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008;582:1977–1986. doi: 10.1016/j.febslet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boguszewska-Chachulska AM, Haenni A. RNA Viruses redirect host factors to better amplify their genome. In: Karl M, Aaron JS, editors. Adv Virus Res. Academic Press; 2005. pp. 29–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai MM. Cellular factors in the transcription and replication of viral RNA genomes: a parallel to DNA-dependent RNA transcription. Virology. 1998;244:1–12. doi: 10.1006/viro.1998.9098. [DOI] [PubMed] [Google Scholar]
- 22.Ortín J, Parra F. Structure and function of RNA replication. Annu Rev Microbiol. 2006;60:305–326. doi: 10.1146/annurev.micro.60.080805.142248. [DOI] [PubMed] [Google Scholar]
- 23.Ahlquist P, Noueiry AO, Lee W-M, Kushner DB, Dye BT. Host factors in positive-strand RNA virus genome replication. J Virol. 2003;77:8181–8186. doi: 10.1128/JVI.77.15.8181-8186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi ST, Lai MMC. Viral and cellular proteins involved in coronavirus replication. Curr Top Microbiol Immunol. 2005;287:95–131. doi: 10.1007/3-540-26765-4_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li HP, Zhang X, Duncan R, Comai L, Lai MM. Heterogeneous nuclear ribonucleoprotein A1 binds to the transcription-regulatory region of mouse hepatitis virus RNA. Proc Natl Acad Sci USA. 1997;94:9544–9549. doi: 10.1073/pnas.94.18.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanda SK, Leibowitz JL. Mitochondrial aconitase binds to the 3′ untranslated region of the mouse hepatitis virus genome. J Virol. 2001;75:3352–3362. doi: 10.1128/JVI.75.7.3352-3362.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spagnolo JF, Hogue BG. Host protein interactions with the 3′ end of bovine coronavirus RNA and the requirement of the poly(A) tail for coronavirus defective genome replication. J Virol. 2000;74:5053–5065. doi: 10.1128/jvi.74.11.5053-5065.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Li Y, Kedersha N, Anderson P, Emara M, Swiderek KM, et al. Cell proteins TIA-1 and TIAR interact with the 3′ stem-loop of the West Nile virus complementary minus-strand RNA and facilitate virus replication. J Virol. 2002;76:11989–12000. doi: 10.1128/JVI.76.23.11989-12000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aizaki H, Choi K, Liu M, Li Y-j, Lai MM. Polypyrimidine-tract-binding protein is a component of the HCV RNA replication complex and necessary for RNA synthesis. J Biomed Sci. 2006;13:469–480. doi: 10.1007/s11373-006-9088-4. [DOI] [PubMed] [Google Scholar]
- 30.Huang T-S, Wei T, Laliberte J-F, Wang A. A host RNA helicase-like protein, AtRH8, interacts with the potyviral genome-linked protein, VPg, associates with the virus accumulation complex, and is essential for infection. Plant Physiol. 2010;152:255–266. doi: 10.1104/pp.109.147983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishikiori M, Dohi K, Mori M, Meshi T, Naito S, Ishikawa M. Membrane-bound Tomato mosaic virus replication proteins participate in RNA synthesis and are associated with host proteins in a pattern distinct from those that are not membrane bound. J Virol. 2006;80:8459–8468. doi: 10.1128/JVI.00545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thivierge K, Cotton S, Dufresne PJ, Mathieu I, Beauchemin C, Ide C, et al. Eukaryotic elongation factor 1A interacts with Turnip mosaic virus RNA-dependent RNA polymerase and VPg-Pro in virus-induced vesicles. Virology. 2008;377:216–225. doi: 10.1016/j.virol.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 33.You S, Rice CM. 3′ RNA elements in Hepatitis C virus replication: kissing partners and long poly(U) J Virol. 2008;82:184–195. doi: 10.1128/JVI.01796-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serva S, Nagy PD. Proteomics analysis of the tombusvirus replicase: Hsp70 molecular chaperone is associated with the replicase and enhances viral RNA replication. J Virol. 2006;80:2162–2169. doi: 10.1128/JVI.80.5.2162-2169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang RYL, Nagy PD. Tomato bushy stunt virus coopts the RNA-binding function of a host metabolic enzyme for viral genomic RNA synthesis. Cell Host & Microbe. 2008;3:178–187. doi: 10.1016/j.chom.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Emara MM, Brinton MA. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc Natl Acad Sci USA. 2007;104:9041–9046. doi: 10.1073/pnas.0703348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J, Gopinath K, Murali A, Yi G, Hayward SD, Zhu H, et al. RNA-binding proteins that inhibit RNA virus infection. Proc Natl Acad Sci USA. 2007;104:3129–3134. doi: 10.1073/pnas.0611617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z, Pogany J, Panavas T, Xu K, Esposito AM, Kinzy TG, et al. Translation elongation factor 1A is a component of the tombusvirus replicase complex and affects the stability of the p33 replication co-factor. Virology. 2009;385:245–260. doi: 10.1016/j.virol.2008.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thivierge K, Cotton S, Dufresne PJ, Mathieu I, Beauchemin C, Ide C, et al. Eukaryotic elongation factor 1A interacts with Turnip mosaic virus RNA-dependent RNA polymerase and VPg-Pro in virus-induced vesicles. Virology. 2008;377:216–225. doi: 10.1016/j.virol.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Nishikiori M, Dohi K, Mori M, Meshi T, Naito S, Ishikawa M. Membrane-bound tomato mosaic virus replication proteins participate in RNA synthesis and are associated with host proteins in a pattern distinct from those that are not membrane bound. J Virol. 2006;80:8459–8468. doi: 10.1128/JVI.00545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cherry S, Doukas T, Armknecht S, Whelan S, Wang H, Sarnow P, et al. Genome-wide RNAi screen reveals a specific sensitivity of IRES-containing RNA viruses to host translation inhibition. Genes Dev. 2005;19:445–452. doi: 10.1101/gad.1267905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez-Garcia MD, Mazzon M, Jacobs M, Amara A. Pathogenesis of flavivirus infections: using and abusing the host cell. Cell Host & Microbe. 2009;5:318–328. doi: 10.1016/j.chom.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Krishnan MN, Ng A, Sukumaran B, Gilfoy FD, Uchil PD, Sultana H, et al. RNA interference screen for human genes associated with West Nile virus infection. Nature. 2008;455:242–245. doi: 10.1038/nature07207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randall G, Panis M, Cooper JD, Tellinghuisen TL, Sukhodolets KE, Pfeffer S, et al. Cellular cofactors affecting hepatitis C virus infection and replication. Proc Natl Acad Sci USA. 2007;104:12884–12889. doi: 10.1073/pnas.0704894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kushner DB, Lindenbach BD, Grdzelishvili VZ, Noueiry AO, Paul SM, Ahlquist P. Systematic, genome-wide identification of host genes affecting replication of a positive-strand RNA virus. Proc Natl Acad Sci USA. 2003;100:15764–15769. doi: 10.1073/pnas.2536857100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panavas T, Serviene E, Brasher J, Nagy PD. Yeast genome-wide screen reveals dissimilar sets of host genes affecting replication of RNA viruses. Proc Natl Acad Sci USA. 2005;102:7326–7331. doi: 10.1073/pnas.0502604102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang Y, Serviene E, Gal J, Panavas T, Nagy PD. Identification of essential host factors affecting tombusvirus RNA replication based on the yeast Tet promoters Hughes Collection. J Virol. 2006;80:7394–7404. doi: 10.1128/JVI.02686-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang Y, Li Z, Nagy PD. Nucleolin/Nsr1p binds to the 3′ noncoding region of the tombusvirus RNA and inhibits replication. Virology. 2010;396:10–20. doi: 10.1016/j.virol.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagy PD, Pogany J. Global genomics and proteomics approaches to identify host factors as targets to induce resistance against tomato bushy stunt virus. Adv Virus Res. 2010;76:123–177. doi: 10.1016/S0065-3527(10)76004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Wynsberghe PM, Chen HR, Ahlquist P. Nodavirus RNA replication protein A induces membrane association of genomic RNA. J Virol. 2007;81:4633–4644. doi: 10.1128/JVI.02267-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwakawa HO, Mine A, Hyodo K, An M, Kaido M, Mise K, et al. Template recognition mechanisms by replicase proteins differ between bipartite positive-strand genomic RNAs of a plant virus. J Virol. 2011;85:497–509. doi: 10.1128/JVI.01754-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andino R, Rieckhof GE, Trono D, Baltimore D. Substitutions in the protease (3Cpro) gene of poliovirus can suppress a mutation in the 5′ noncoding region. J Virol. 1990;64:607–612. doi: 10.1128/jvi.64.2.607-612.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panavas T, Hawkins CM, Panaviene Z, Nagy PD. The role of the p33 : p33/p92 interaction domain in RNA replication and intracellular localization of p33 and p92 proteins of Cucumber necrosis tombusvirus. Virology. 2005;338:81–95. doi: 10.1016/j.virol.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 54.Pogany J, Stork J, Li Z, Nagy PD. In vitro assembly of the Tomato bushy stunt virus replicase requires the host Heat shock protein 70. Proc Natl Acad Sci USA. 2008;105:19956–19961. doi: 10.1073/pnas.0810851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z, Pogany J, Tupman S, Esposito AM, Kinzy TG, Nagy PD. Translation elongation factor 1A facilitates the assembly of the tombusvirus replicase and stimulates minus-strand synthsis. PLoS Pathog. 2010;6(11):e1001175. doi: 10.1371/journal.ppat.1001175. 10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mas A, Alves-Rodrigues I, Noueiry A, Ahlquist P, Diez J. Host deadenylation-dependent mRNA decapping factors are required for a key step in brome mosaic virus RNA replication. J Virol. 2006;80:246–251. doi: 10.1128/JVI.80.1.246-251.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diez J, Ishikawa M, Kaido M, Ahlquist P. Identification and characterization of a host protein required for efficient template selection in viral RNA replication. Proc Natl Acad Sci USA. 2000;97:3913–3918. doi: 10.1073/pnas.080072997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oster SK, Wu B, White KA. Uncoupled expression of p33 and p92 permits amplification of Tomato bushy stunt virus RNAs. J Virol. 1998;72:5845–5851. doi: 10.1128/jvi.72.7.5845-5851.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vlot AC, Laros SM, Bol JF. Coordinate replication of Alfalfa mosaic virus RNAs 1 and 2 involves cis- and trans-acting functions of the encoded helicase-like and polymerase-like domains. J Virol. 2003;77:10790–10798. doi: 10.1128/JVI.77.20.10790-10798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yi GG, Kao C. cis- and trans-acting functions of Brome mosaic virus protein 1a in genomic RNA1 replication. J Virol. 2008;82:3045–3053. doi: 10.1128/JVI.02390-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okamoto K, Nagano H, Iwakawa H, Mizumoto H, Takeda A, Kaido M, et al. cis-preferential requirement of a-1 frameshift product p88 for the replication of Red clover necrotic mosaic virus RNA1. Virology. 2008;375:205–212. doi: 10.1016/j.virol.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Novak JE, Kirkegaard K. Coupling between genome translation and replication in an RNA virus. Genes & Dev. 1994;8:1726–1737. doi: 10.1101/gad.8.14.1726. [DOI] [PubMed] [Google Scholar]
- 63.Weiland JJ, Dreher TW. Cis-preferential replication of the Turnip yellow mosaic virus RNA genome. Proc Natl Acad Sci USA. 1993;90:6095–6099. doi: 10.1073/pnas.90.13.6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J, Yeh H-H, Falk BW. cis preferential replication of Lettuce infectious yellows virus (LIYV) RNA 1: The initial step in the asynchronous replication of the LIYV genomic RNAs. Virology. 2009;386:217–223. doi: 10.1016/j.virol.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 65.Gamarnik AV, Andino R. Interactions of viral protein 3CD and poly(rC) binding protein with the 5′ untranslated region of the poliovirus genome. J Virol. 2000;74:2219–2226. doi: 10.1128/jvi.74.5.2219-2226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blyn LB, Swiderek KM, Richards O, Stahl DC, Semler BL, Ehrenfeld E. Poly(rC) binding protein 2 binds to stem-loop IV of the poliovirus RNA 5′ noncoding region: identification by automated liquid chromatography tandem mass spectrometry. Proc Natl Acad Sci USA. 1996;93:11115–11120. doi: 10.1073/pnas.93.20.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blyn LB, Towner JS, Semler BL, Ehrenfeld E. Requirement of poly(rC) binding protein 2 for translation of poliovirus RNA. J Virol. 1997;71:6243–6246. doi: 10.1128/jvi.71.8.6243-6246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gamarnik AV, Andino R. Two functional complexes formed by KH domain containing proteins with the 5′ noncoding region of poliovirus RNA. RNA. 1997;3:882–892. [PMC free article] [PubMed] [Google Scholar]
- 69.Andino R, Rieckhof GE, Baltimore D. A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell. 1990;63:369–380. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- 70.Parsley TB, Towner JS, Blyn LB, Ehrenfeld E, Semler BL. Poly (rC) binding protein 2 forms a ternary complex with the 5′-terminal sequences of poliovirus RNA and the viral 3CD proteinase. RNA. 1997;3:1124–1134. [PMC free article] [PubMed] [Google Scholar]
- 71.Noueiry AO, Diez J, Falk SP, Chen JB, Ahlquist P. Yeast Lsm1p-7p/Pat1p deadenylation-dependent mRNA-decapping factors are required for brome mosaic virus genomic RNA translation. Mol Cell Biol. 2003;23:4094–4106. doi: 10.1128/MCB.23.12.4094-4106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scheller N, Mina LB, Galão RP, Chari A, Giménez-Barcons M, Noueiry A, et al. Translation and replication of hepatitis C virus genomic RNA depends on ancient cellular proteins that control mRNA fates. Proc Natl Acad Sci USA. 2009;106:13517–13522. doi: 10.1073/pnas.0906413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miranda G, Schuppli D, Barrera I, Hausherr C, Sogo JM, Weber H. Recognition of bacteriophage Q[beta] plus strand RNA as a template by Q[beta] replicase: role of RNA interactions mediated by ribosomal proteins S1 and host factor. J Mol Biol. 1997;267:1089–1103. doi: 10.1006/jmbi.1997.0939. [DOI] [PubMed] [Google Scholar]
- 74.Brown D, Gold L. RNA replication by Q beta replicase: a working model. Proc Natl Acad Sci USA. 1996;93:11558–11562. doi: 10.1073/pnas.93.21.11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujisaki K, Ishikawa M. Identification of an Arabidopsis thaliana protein that binds to tomato mosaic virus genomic RNA and inhibits its multiplication. Virology. 2008;380:402–411. doi: 10.1016/j.virol.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 76.Gamarnik AV, Andino R. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes & Dev. 1998;12:2293–2304. doi: 10.1101/gad.12.15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ichihashi N, Matsuura T, Kita H, Hosoda K, Sunami T, Tsukada K, et al. Importance of translation-replication balance for efficient replication by the self-encoded replicase. ChemBioChem. 2008;9:3023–3028. doi: 10.1002/cbic.200800518. [DOI] [PubMed] [Google Scholar]
- 78.Weber H, Weissman C, Billeter MA, Kahane S, Hindley J, Porter A. Molecular basis for repressor activity of Q beta replicase. Nat New Biol. 1972;237:166–170. doi: 10.1038/newbio237166a0. [DOI] [PubMed] [Google Scholar]
- 79.Kolakofs D, Weissman C. Possible mechanism for transition of viral RNA from polysome to replication complex. Nat New Biol. 1971;231:42–46. doi: 10.1038/newbio231042a0. [DOI] [PubMed] [Google Scholar]
- 80.Stupina VA, Meskauskas A, McCormack JC, Yingling YG, Shapiro BA, Dinman JD, et al. The 3′ proximal translational enhancer of Turnip crinkle virus binds to 60S ribosomal subunits. RNA. 2008;14:2379–2393. doi: 10.1261/rna.1227808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zuo X, Wang J, Yu P, Eyler D, Xu H, Starich MR, et al. Solution structure of the cap-independent translational enhancer and ribosome-binding element in the 3′ UTR of turnip crinkle virus. Proc Natl Acad Sci USA. 2010;107:1385–1390. doi: 10.1073/pnas.0908140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perera R, Daijogo S, Walter BL, Nguyen JH, Semler BL. Cellular protein modification by poliovirus: the two faces of poly(rC)-binding protein. J Virol. 2007;81:8919–8932. doi: 10.1128/JVI.01013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sean P, Nguyen JH, Semler BL. The linker domain of poly(rC) binding protein 2 is a major determinant in poliovirus cap-independent translation. Virology. 2008;378:243–253. doi: 10.1016/j.virol.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Belsham GJ, Sonenberg N. Picornavirus RNA translation: roles for cellular proteins. Trends Microbiol. 2000;8:330–335. doi: 10.1016/s0966-842x(00)01788-1. [DOI] [PubMed] [Google Scholar]
- 85.Back SH, Kim YK, Kim WJ, Cho S, Oh HR, Kim J-E, et al. Translation of polioviral mRNA is inhibited by cleavage of polypyrimidine tract-binding proteins executed by polioviral 3Cpro. J Virol. 2002;76:2529–2542. doi: 10.1128/jvi.76.5.2529-2542.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bonderoff JM, LaRey JL, Lloyd RE. Cleavage of poly(A)-binding protein by poliovirus 3C proteinase inhibits viral internal ribosome entry site-mediated translation. J Virol. 2008;82:9389–9399. doi: 10.1128/JVI.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galao RP, Chari A, Alves-Rodrigues I, Lobao D, Mas A, Kambach C, et al. Lsm1-7 complexes bind to specific sites in viral RNA genomes and regulate their translation and replication. RNA. 2010;16:817–827. doi: 10.1261/rna.1712910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol. 2007;8:113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- 89.Parker R, Song H. The enzymes and control of eukaryotic mRNA turnover. Nat Struct Mol Biol. 2004;11:121–127. doi: 10.1038/nsmb724. [DOI] [PubMed] [Google Scholar]
- 90.Jangra RK, Yi M, Lemon SM. DDX6 (Rck/p54) is required for efficient hepatitis C virus replication but not for internal ribosome entry site-directed translation. J Virol. 2010;84:6810–6824. doi: 10.1128/JVI.00397-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Isken O, Grassmann CW, Sarisky RT, Kann M, Zhang S, Grosse F, et al. Members of the NF90/NFAR protein group are involved in the life cycle of a positive-strand RNA virus. EMBO J. 2003;22:5655–5665. doi: 10.1093/emboj/cdg562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Isken O, Baroth M, Grassmann CW, Weinlich S, Ostareck DH, Ostareck-Lederer A, et al. Nuclear factors are involved in hepatitis C virus RNA replication. RNA. 2007;13:1675–1692. doi: 10.1261/rna.594207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Isken O, Grassmann CW, Yu HY, Behrens SE. Complex signals in the genomic 3′ nontranslated region of bovine viral diarrhea virus coordinate translation and replication of the viral RNA. RNA. 2004;10:1637–1652. doi: 10.1261/rna.7290904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vlot AC, Neeleman L, Linthorst HJM, Bol JF. Role of the 3′-untranslated regions of Alfalfa mosaic virus RNAs in the formation of a transiently expressed replicase in plants and in the assembly of virions. J Virol. 2001;75:6440–6449. doi: 10.1128/JVI.75.14.6440-6449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Panaviene Z, Panavas T, Serva S, Nagy PD. Purification of the cucumber necrosis virus replicase from yeast cells: role of coexpressed viral RNA in stimulation of replicase activity. J Virol. 2004;78:8254–8263. doi: 10.1128/JVI.78.15.8254-8263.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blumenthal T, Landers TA, Weber K. Bacteriophage Qβ replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci USA. 1972;69:1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wahba AJ, Miller MJ, Niveleau A, Landers TA, Carmichael GG, Weber K, et al. Subunit I of Qβ Replicase and 30S Ribosomal Protein Sl of Escherichia coli. J Biol Chem. 1974;249:3314–3316. [PubMed] [Google Scholar]
- 98.Blumenthal T, Carmichael GG. RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- 99.Takeshita D, Tomita K. Assembly of Qβ viral RNA polymerase with host translational elongation factors EF-Tu and −Ts. Proc Natl Acad Sci USA. 2010;107:15733–15738. doi: 10.1073/pnas.1006559107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kidmose RT, Vasiliev NN, Chetverin AB, Andersen GR, Knudsen CR. Structure of the Qβ replicase, an RNA-dependent RNA polymerase consisting of viral and host proteins. Proc Natl Acad Sci USA. 2010;107:10884–10889. doi: 10.1073/pnas.1003015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schuppli D, Miranda G, Tsui H-CT, Winkler ME, Sogo JM, Weber H. Altered 3′-terminal RNA structure in phage Qβ adapted to host factor-less Escherichia coli. Proc Natl Acad Sci USA. 1997;94:10239–10242. doi: 10.1073/pnas.94.19.10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim CS, Seol SK, Song O-K, Park JH, Jang SK. An RNA-binding protein, hnRNP A1, and a scaffold protein, septin 6, facilitate hepatitis C virus replication. J Virol. 2007;81:3852–3865. doi: 10.1128/JVI.01311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brunner JE, Nguyen JH, Roehl HH, Ho TV, Swiderek KM, Semler BL. Functional interaction of heterogeneous nuclear ribonucleoprotein C with poliovirus RNA synthesis initiation complexes. J Virol. 2005;79:3254–3266. doi: 10.1128/JVI.79.6.3254-3266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ertel KJ, Brunner JE, Semler BL. Mechanistic consequences of hnRNP C binding to both RNA termini of poliovirus negative-strand RNA intermediates. J Virol. 2010;84:4229–4242. doi: 10.1128/JVI.02198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davis WG, Blackwell JL, Shi P-Y, Brinton MA. Interaction between the cellular protein eEF1A and the 3′-terminal stem-loop of West Nile virus genomic RNA facilitates viral minus-strand RNA synthesis. J Virol. 2007;81:10172–10187. doi: 10.1128/JVI.00531-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blackwell J, Brinton M. Translation elongation factor-1 alpha interacts with the 3′ stem-loop region of West Nile virus genomic RNA. J Virol. 1997;71:6433–6444. doi: 10.1128/jvi.71.9.6433-6444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zeenko VV, Ryabova LA, Spirin AS, Rothnie HM, Hess D, Browning KS, et al. Eukaryotic elongation factor 1A interacts with the upstream pseudoknot domain in the 3′ untranslated region of Tobacco mosaic virus RNA. J Virol. 2002;76:5678–5691. doi: 10.1128/JVI.76.11.5678-5691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamaji Y, Kobayashi T, Hamada K, Sakurai K, Yoshii A, Suzuki M, et al. In vivo interaction between Tobacco mosaic virus RNA-dependent RNA polymerase and host translation elongation factor 1A. Virology. 2006;347:100–108. doi: 10.1016/j.virol.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 109.Li Z, Barajas D, Panavas T, Herbst DA, Nagy PD. Cdc34p ubiquitin-conjugating enzyme is a component of the tombusvirus replicase complex and ubiquitinates p33 replication protein. J Virol. 2008;82:6911–6926. doi: 10.1128/JVI.00702-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kao CC, Singh P, Ecker DJ. De novo initiation of viral RNA-dependent RNA synthesis. Virology. 2001;287:251–260. doi: 10.1006/viro.2001.1039. [DOI] [PubMed] [Google Scholar]
- 111.Brown D, Gold L. Template recognition by an RNA-Dependent RNA-polymerase: identification and characterization of two RNA binding sites on Qbeta. replicase. Biochemistry. 1995;34:14765–14774. doi: 10.1021/bi00045a018. [DOI] [PubMed] [Google Scholar]
- 112.Klovins J, Berzins V, van Duin J. A long-range interaction in Qbeta RNA that bridges the thousand nucleotides between the M-site and the 3′ end is required for replication. RNA. 1998;4:948–957. doi: 10.1017/s1355838298980177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klovins J, van Duin J. A long-range pseudoknot in Qβ RNA is essential for replication. J Mol Biol. 1999;294:875–884. doi: 10.1006/jmbi.1999.3274. [DOI] [PubMed] [Google Scholar]
- 114.Wu BD, Pogany J, Na H, Nicholson BL, Nagy PD, White KA. A discontinuous RNA platform mediates RNA virus replication: building an integrated model for RNA-based regulation of viral processes. Plos Pathog. 2009;5(3):e1000323. doi: 10.1371/journal.ppat.1000323. 10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Villordo SM, Alvarez DE, Gamarnik AV. A balance between circular and linear forms of the dengue virus genome is crucial for viral replication. RNA. 2010;16:2325–2335. doi: 10.1261/rna.2120410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lodeiro MF, Filomatori CV, Gamarnik AV. Structural and functional studies of the promoter element for dengue virus RNA replication. J Virol. 2009;83:993–1008. doi: 10.1128/JVI.01647-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Filomatori CV, Lodeiro MF, Alvarez DE, Samsa MM, Pietrasanta L, Gamarnik AV. A 5′ RNA element promotes dengue virus RNA synthesis on a circular genome. Genes Dev. 2006;20:2238–2249. doi: 10.1101/gad.1444206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Friebe P, Boudet J, Simorre J-P, Bartenschlager R. Kissing-loop interaction in the 3′ end of the hepatitis C virus genome essential for RNA replication. J Virol. 2005;79:380–392. doi: 10.1128/JVI.79.1.380-392.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barton DJ, O'Donnell BJ, Flanegan JB. 5′ cloverleaf in poliovirus RNA is a cis-acting replication element required for negative-strand synthesis. EMBO J. 2001;20:1439–1448. doi: 10.1093/emboj/20.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Herold J, Andino R. Poliovirus RNA replication requires genome circularization through a protein-protein bridge. Mol Cell. 2001;7:581–591. doi: 10.1016/S1097-2765(01)00205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang P, Lai MM. Heterogeneous nuclear ribonucleoprotein A1 binds to the 3′-untranslated region and mediates potential 5′-3′-end cross talks of mouse hepatitis virus RNA. J Virol. 2001;75:5009–5017. doi: 10.1128/JVI.75.11.5009-5017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matsuda D, Yoshinari S, Dreher TW. eEF1A binding to aminoacylated viral RNA represses minus strand synthesis by TYMV RNA-dependent RNA polymerase. Virology. 2004;321:47–56. doi: 10.1016/j.virol.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 123.Pogany J, Fabian MR, White KA, Nagy PD. A replication silencer element in a plus-strand RNA virus. EMBO J. 2003;22:5602–5611. doi: 10.1093/emboj/cdg523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roehl H, Semler B. Poliovirus infection enhances the formation of two ribonucleoprotein complexes at the 3′ end of viral negative-strand RNA. J Virol. 1995;69:2954–2961. doi: 10.1128/jvi.69.5.2954-2961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chambers TJ, Hahn CS, Galler R, Rice CM. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 126.Li W, Li Y, Kedersha N, Anderson P, Emara M, Swiderek KM, et al. Cell proteins TIA-1 and TIAR interact with the 3′ stem-loop of the West Nile virus complementary minus-strand RNA and facilitate virus replication. J Virol. 2002;76:11989–12000. doi: 10.1128/JVI.76.23.11989-12000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Emara MM, Liu H, Davis WG, Brinton MA. Mutation of mapped TIA-1/TIAR binding sites in the 3′ terminal stem-loop of West Nile virus minus-strand RNA in an infectious clone negatively affects genomic RNA amplification. J Virol. 2008;82:10657–10670. doi: 10.1128/JVI.00991-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vogt DA, Andino R. An RNA element at the 5′-end of the poliovirus genome functions as a general promoter for RNA synthesis. Plos Pathog. 2010;6(6):e1000936. doi: 10.1371/journal.ppat.1000936. 10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.den Boon JA, Ahlquist P. Organelle-Like Membrane compartmentalization of positive-strand RNA virus replication factories. Annu Rev Microbiol. 2010;64:241–256. doi: 10.1146/annurev.micro.112408.134012. [DOI] [PubMed] [Google Scholar]
- 130.Miller WA, Koev G. Synthesis of subgenomic RNAs by positive-strand RNA viruses. Virology. 2000;273:1–8. doi: 10.1006/viro.2000.0421. [DOI] [PubMed] [Google Scholar]
- 131.White KA. The Premature Termination Model: A possible third mechanism for subgenomic mRNA transcription in (+)-strand RNA viruses. Virology. 2002;304:147–154. doi: 10.1006/viro.2002.1732. [DOI] [PubMed] [Google Scholar]
- 132.Sit TL, Vaewhongs AA, Lommel SA. RNA-mediated trans-activation of transcription from a viral RNA. Science. 1998;281:829–832. doi: 10.1126/science.281.5378.829. [DOI] [PubMed] [Google Scholar]
- 133.Shi S, Lai MMC. Viral and cellular proteins involved in coronavirus replication. In: Enjuanes L, editor. Coronavirus replication and reverse genetics. Berlin Heidelberg: Springer; 2005. pp. 95–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li H-P, Zhang X, Duncan R, Comai L, Lai MM. Heterogeneous nuclear ribonucleoprotein A1 binds to the transcription-regulatory region of mouse hepatitis virus RNA. Proc Natl Acad Sci USA. 1997;94:9544–9549. doi: 10.1073/pnas.94.18.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang X, Lai MM. Interactions between the cytoplasmic proteins and the intergenic (promoter) sequence of mouse hepatitis virus RNA: correlation with the amounts of subgenomic mRNA transcribed. J Virol. 1995;69:1637–1644. doi: 10.1128/jvi.69.3.1637-1644.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shi ST, Huang P, Li HP, Lai MM. Heterogeneous nuclear ribonucleoprotein A1 regulates RNA synthesis of a cytoplasmic virus. EMBO J. 2000;19:4701–4711. doi: 10.1093/emboj/19.17.4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang X, Li HP, Xue W, Lai MM. Formation of a ribonucleoprotein complex of mouse hepatitis virus involving heterogeneous nuclear ribonucleoprotein A1 and transcription-regulatory elements of viral RNA. Virology. 1999;264:115–124. doi: 10.1006/viro.1999.9970. [DOI] [PubMed] [Google Scholar]
- 138.Wang Y, Zhang X. The nucleocapsid protein of coronavirus mouse hepatitis virus interacts with the cellular heterogeneous nuclear ribonucleoprotein A1 in vitro and in vivo. Virology. 1999;265:96–109. doi: 10.1006/viro.1999.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li HP, Huang P, Park S, Lai MM. Polypyrimidine tract-binding protein binds to the leader RNA of mouse hepatitis virus and serves as a regulator of viral transcription. J Virol. 1999;73:772–777. doi: 10.1128/jvi.73.1.772-777.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huang P, Lai MM. Polypyrimidine tract-binding protein binds to the complementary strand of the mouse hepatitis virus 3′ untranslated region, thereby altering RNA conformation. J Virol. 1999;73:9110–9116. doi: 10.1128/jvi.73.11.9110-9116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Burnham AJ, Gong L, Hardy RW. Heterogeneous nuclear ribonuclear protein K interacts with Sindbis virus nonstructural proteins and viral subgenomic mRNA. Virology. 2007;367:212–221. doi: 10.1016/j.virol.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 142.Silva PA, Pereira CF, Dalebout TJ, Spaan WJ, Bredenbeek PJ. An RNA pseudoknot is required for production of yellow fever virus subgenomic RNA by the host nuclease XRN1. J Virol. 2010;84:11395–11406. doi: 10.1128/JVI.01047-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Funk A, Truong K, Nagasaki T, Torres S, Floden N, Balmori Melian E, et al. RNA structures required for production of subgenomic flavivirus RNA. J Virol. 2010;84:11407–11417. doi: 10.1128/JVI.01159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cheng CP, Serviene E, Nagy PD. Suppression of viral RNA recombination by a host exoribonuclease. J Virol. 2006;80:2631–2640. doi: 10.1128/JVI.80.6.2631-2640.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sokoloski KJ, Dickson AM, Chaskey EL, Garneau NL, Wilusz CJ, Wilusz J. Sindbis virus usurps the cellular HuR protein to stabilize its transcripts and promote productive infections in mammalian and mosquito cells. Cell Host & Microbe. 2010;8:196–207. doi: 10.1016/j.chom.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nanda SK, Leibowitz JL. Mitochondrial aconitase binds to the 3′ untranslated region of the mouse hepatitis virus genome. J Virol. 2001;75:3352–3362. doi: 10.1128/JVI.75.7.3352-3362.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murray KE, Roberts AW, Barton DJ. Poly(rC) binding proteins mediate poliovirus mRNA stability. RNA. 2001;7:1126–1141. doi: 10.1017/s1355838201010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Spangberg K, Wiklund L, Schwartz S. Binding of the La autoantigen to the hepatitis C virus 3′ untranslated region protects the RNA from rapid degradation in vitro. J Gen Virol. 2001;82:113–120. doi: 10.1099/0022-1317-82-1-113. [DOI] [PubMed] [Google Scholar]
- 149.Spångberg K, Wiklund L, Schwartz S. HuR, a protein implicated in oncogene and growth factor mRNA decay, binds to the 3′ ends of hepatitis C virus RNA of both polarities. Virology. 2000;274:378–390. doi: 10.1006/viro.2000.0461. [DOI] [PubMed] [Google Scholar]
- 150.Korf M, Jarczak D, Beger C, Manns MP, Kruger M. Inhibition of hepatitis C virus translation and subgenomic replication by siRNAs directed against highly conserved HCV sequence and cellular HCV cofactors. J Hepatol. 2005;43:225–234. doi: 10.1016/j.jhep.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 151.Harris D, Zhang Z, Chaubey B, Pandey VN. Identification of cellular factors associated with the 3′-nontranslated region of the hepatitis C virus genome. Mol Cell Proteomics. 2006;5:1006–1018. doi: 10.1074/mcp.M500429-MCP200. [DOI] [PubMed] [Google Scholar]
- 152.Joshi RL, Ravel JM, Haenni AL. Interaction of turnip yellow mosaic virus Val-RNA with eukaryotic elongation-factor EF-1[alpha]. Search for a function. EMBO J. 1986;5:1143–1148. doi: 10.1002/j.1460-2075.1986.tb04339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]