Abstract
Fifteen-year-old adolescents (N=109) in a longitudinal study of child development were recruited to examine differences in DNA methylation in relation to parent reports of adversity during the adolescents’ infancy and preschool periods. Microarray technology applied to 28,000 cytosine-guanine dinucleotide (CpG) sites within DNA derived from buccal epithelial cells showed differential methylation among adolescents whose parents reported high levels of stress during their children’s early lives. Maternal stressors in infancy and paternal stressors in the preschool years were most strongly predictive of differential methylation, and the patterning of such epigenetic marks varied by children’s gender. To the authors’ knowledge, this is the first report of prospective associations between adversities in early childhood and the epigenetic conformation of adolescents’ genomic DNA.
Keywords: epigenetics, DNA methylation, parental stress, stress exposure, infancy, preschool, adolescence
Epigenetic Vestiges of Early Developmental Adversity: Childhood Stress Exposure and DNA Methylation in Adolescence
Experience “gets under the skin” early in life, and does so in ways that affect the course of human development. Heart disease, diabetes, obesity, depression, substance abuse, school success, premature mortality, disability at retirement, and accelerated aging and memory loss all have determinants in early life (Harkonmaki, et al., 2007). In particular, childhood psychosocial adversity affects multiple outcomes, including smoking, suicide, depression, obesity, illicit drug use, alcoholism, teen pregnancy, sexual risk behaviors and sexually transmitted diseases (Felitti, et al., 1998; Thomas, Hypponen, & Power, 2008). Although physical and emotional abuse and other dramatic adverse childhood events have health and developmental consequences (Heim, Newport, Mletzko, Miller, & Nemeroff, 2008), it is often the less memorable yet prevalent, co-occurring, day-to-day misfortunes of early childhood (e.g., impoverished parent-child interactions) that have a lasting influence on the subsequent life course (Kishiyama, Boyce, Jimenez, Perry, & Knight, 2009).
The neurobiology of early experience
The biological plausibility of this scenario is well established. Experience plays a crucial role in the early phases of conception, prenatal, and post-natal periods of children's development (Swain, Lorberbaum, Kose, & Strathearn, 2007; Talge, Neal, & Glover, 2007). Sensitive periods in brain and biological development start in the prenatal period, reach a peak in the first few years of life, and continue at a declining rate throughout childhood and adolescence, such that the brain does not reach its "adult" state until the end of the second or beginning of the third decade of life (Fox, Levitt, & Nelson, 2010). Early sensory stimulation activates specific genes in different parts of the brain to differentiate neuronal function and establish sensory pathways. Sensory pathways, in turn, influence the development of neural pathways to other parts of the brain involved in coping, movement, language, cognition, and biological pathways, including the immune and hormone systems (McEwen, 2008). For example, key executive functions, regarding how an individual responds to social and emotional stimuli, develop in the prefrontal cortex from approximately age three to nine years, whereas neural connections to the prefrontal cortex, from centers in the midbrain that sense environmental threats, develop earlier (Gunnar & Nelson, 1994; Knudsen, Heckman, Cameron, & Shonkoff, 2006). Most importantly, early environments are mediated through relationships with primary caregivers. A prime example is infant attachment to a parental figure (usually the mother) in both higher primates and humans. Attachment, in turn, drives the development of neural pathways that help the developing child's brain become attuned to the immediate environment (Bowlby, 1969; Sroufe, 2005).
There has been substantial theoretical work on the multiple ecological factors associated with child development (Bronfenbrenner & Morris, 2006) and seminal empirical work on the cumulative risks these factors can pose (Rutter, 1979; Sameroff, 1998). Additional longitudinal studies have demonstrated the deleterious effects of accumulated risk on child outcomes (e.g., (Evans, Kim, Ting, Tesher, & Shannis, 2007)). Other researchers have focused on the child and adolescent sequelae of specific aspects of stressful family environments. For example, parental depression (Goodman & Gotlib, 1999; Halligan, Murray, Martins, & Cooper, 2007), marital conflict (Cummings & Davies, 2002), and parenting stress (Bayer, Hiscock, Ukoumunne, Price, & Wake, 2008) have been shown individually and jointly to predict child health and functioning. Research also suggests that the effects of children’s exposures to parents’ stress may vary depending on the timing of exposure (Essex, Klein, Cho, & Kalin, 2002; Lupien, McEwen, Gunnar, & Heim, 2009). The research base further leads to an expectation that mothers will contribute in different ways – and disproportionately at earlier stages – than fathers, although each may be expected to contribute to the child's development (Bornstein, 2002; Keller, Cummings, Peterson, & Davies, 2009; Ramchandani, et al., 2008). Furthermore, fathers and mothers may influence boys' and girls' development differently and to different degrees (Morrell & Murray, 2003).
In our own previous work, we have found that children’s exposure to parental stress factors, including parental depression, marital conflict, and family negative emotional climate, can contribute to children’s later mental health symptoms (e.g., (Essex, Klein, Cho, & Kraemer, 2003; Essex, Klein, Miech, & Smider, 2001)). We have shown that these effects sometimes vary as a function of child gender (Essex, et al., 2003; Essex, et al., 2001) and that fathers can make unique, significant contributions to children’s risk and well-being in the early years of development (Boyce, et al., 2005). We have also demonstrated the predictive utility of broader parental stress composites that incorporate the risk factors noted above plus others, including parenting stress and financial stress, for understanding children’s later mental health (Essex, et al., 2002; Essex, et al., 2006), as well as autonomic and adrenocortical reactivity to stress (Ellis, Essex, & Boyce, 2005).
Biological embedding and the epigenome
Here, we seek to understand the ”biological embedding” of early experience (Hertzman, 1999). Biological embedding is said to occur when: experience gets under the skin and alters human biological processes; systematic differences in experience, under different nurturant conditions, lead to different bio-developmental states; the differences are stable and long-term; and these differences influence health, well-being, learning, or behavior over the life course. The underlying mechanisms of biological embedding have not yet been fully demonstrated, but interest is now turning to the prospect that epigenetic processes are involved.
It is now well established that information contained in the genome’s inherited nucleotide sequence is complemented by other, more modifiable information inherent in the chromatin structure, or epigenome. At a molecular level, the chromatin material that constitutes human (and all eukaryotic) chromosomes consists of strands of DNA wrapped around octamers of eight histone proteins, resembling beads on a string. Chromatin modifications, which include DNA methylation, histone acetylation, and a variety of other chemical ‘marks’, render the protein-encoding portions of a given gene more or less accessible to the molecular transcriptional apparatus that decodes the DNA sequence into messenger RNA and then specific protein products. The epigenetically mediated, differential expression of such protein products in turn guides or changes the function of the cell and the tissue or organ of which it is a part. Emerging epigenetic science is thus “the study of stable alterations in gene expression by non-genomic mechanisms, resulting in stable alterations in phenotypes” (Kramer, 2005). Although DNA methylation is just one of an array of epigenetic mechanisms, it is the one regarded as most stable and most representative of the broader epigenetic modification of a given locus.(Hochberg, et al.). For comprehensive reviews of this rapidly expanding field of study, readers are referred to: (Champagne & Mashoodh, 2009; Cole, 2009; Curley, Jensen, Mashoodh, & Champagne; Hochberg, et al.; Meaney, 2010).
Our working hypothesis in the current study was that early experience leaves a mark on the epigenome that, in turn, leads to stable changes in expression of genes critical for human development and health (Szyf, McGowan, & Meaney, 2008). It is known that the enzymatic machinery responsible for creating and maintaining DNA methylation is responsive to environmental exposures during both intra-uterine development and after birth in animals (e.g., (Jirtle & Skinner, 2007; Weaver, Cervoni, et al., 2004)). Most notably, epigenetic changes in expression of the gene encoding the glucocorticoid receptor (GR) are mediated, during an early sensitive period, by naturally occurring differences in maternal behavior, specifically the intensity and frequency of licking, grooming and arched-back nursing of rat pups (Meaney, 2010; Weaver, Diorio, Seckl, Szyf, & Meaney, 2004). Parallel evidence is now accumulating in humans (e.g., (Heijmans, et al., 2008; McGowan, et al., 2009; Oberlander, et al., 2008; Terry, et al., 2008)). The GR gene has shown increased methylation in human cord-blood DNA from newborns of depressed or anxious mothers (Oberlander, et al., 2008). In other work, the imprinted IGF2 gene has shown reduced methylation in blood DNA of sixty year-old individuals who had been prenatally exposed to famine (Heijmans, et al., 2008). It is speculated that epigenetic processes, including methylation, transduce early environmental signals into conditionally adaptive changes in metabolic, endocrine and neuroregulatory pathways. These changes, in turn, are responsible for systematic developmental biases toward distinct profiles of growth, metabolism, immune responsiveness, developmental pace, and behavior.
Epigenetic methylation has the capacity to modify gene expression, although it does not always do so. This capacity is nonetheless one piece of evidence making plausible the proposition that DNA methylation is important for human developmental plasticity (Gluckman, Hanson, Buklijas, Low, & Beedle, 2009). In this report, we ask a logical next question: are there lasting DNA methylation differences in the tissues of adolescent children who spent their infant or preschool years in environments of maternal and paternal stress? In other words, do typical differences in daily experience during the early years influence DNA methylation over developmental time?
Method
Participants
The current study is based on a sub-sample of 109 children and their parents who participated in the Wisconsin Study of Families and Work (WSFW). The WSFW originally comprised 570 pregnant women and their partners who were recruited from prenatal clinics in and around Madison and Milwaukee, Wisconsin for a study of maternity leave and health outcomes. Eligible female participants were over the age of 18 years, in the second trimester of pregnancy, living with the baby’s biological father, and either employed or a full-time homemaker (see (Hyde, Klein, Essex, & Clark, 1995) for details). Of those eligible, 75% agreed to participate. Because the children were born over a 16-month period, they entered primary school in two cohorts (1996 and 1997). The present analyses are based on data from the 249 families in Cohort 1 who participated at the time of school entry. Parental stress data were obtained during the infancy and preschool periods, and child DNA samples were collected at age 15 years (M = 15.1 years, SD = 0.5). At age 15, 201 (81%) of Cohort 1 participants continued in the study; of these, 110 agreed to participate in the DNA collection and provided a sample sufficient for analysis. Methylation results for one participant were dropped as outliers. A total of 109 participants were thus included in analyses presented here.
At recruitment, the 109 mothers ranged in age from 20–41 years (Mdn = 29). Four percent of mothers had less than a high school education; 13% were high school graduates or equivalent; 31% had some post-secondary education or training; 38% were college graduates; and 14% had post-baccalaureate schooling or a degree; fathers’ education levels were comparable. Nearly all couples were married (95%); 44% were first-time parents; and 11% were ethnic minorities. Family incomes ranged from less than $10,000 per annum to $120,000 (Mdn = $45,000). There were no statistically significant differences between the demographic characteristics of the 109 families and the remainder of the 570 original families. There was a significant difference in the percentage of girls in the 109 sub-study families compared to that in the remainder (61% versus 49%, respectively; χ2 = 4.32, df = 1, p = .04). The 109 and remaining families did not significantly differ on measures of parental stress.
Measures of Parental Stress
Measures of parental stress were obtained separately from mothers and fathers at multiple times during their children’s infancy and preschool periods. For each parent, stress scores were composites of five domains: 1) depression symptoms, 2) family expressed anger, 3) parenting stress, 4) role overload, and 5) financial stress. Except where noted, scores for the infancy period were averaged over the three assessments conducted at 1, 4, and 12 months post-delivery; scores for the preschool period were averaged over the two assessments conducted at children’s ages 3.5 and 4.5 years. Parental depression symptoms were assessed by the Center for Epidemiologic Studies-Depression scale (CES-D; (Radloff, 1977)). Across all assessments, αs were ≥ .85 for both mothers and fathers. Family expressed anger in infancy was assessed with the average of three items from the Partner Role Quality scale tapping overt marital conflict (e.g., concerned about “arguing or fighting”; (Barnett & Marshall, 1989)); for the preschool assessments, the marital conflict scores were combined, using principal components analysis (PCA), with scores from the Anger Expression Inventory (Spielberger, 1988) and the Negative subscale of the Family Expressiveness Questionnaire (Halberstadt, 1986). Across all assessments, for both mothers and fathers, αs were ≥ .78 for marital conflict, .68 for anger expression, and .85 for negative family expressiveness. The first component of the PCA for each preschool assessment accounted for more than 50% of the variance. Parenting stress was calculated (PCA weights) from scores on the Competence and Child Reinforces Parent subscales of the Parenting Stress Inventory (PSI; (Abidin, 1986)) and the average of three negative items (e.g., “I often feel angry with my child”) from the Childrearing Practices Report (CRPR; (Block, 1965)); in the infancy period, the CRPR was assessed only at 12 months. All αs were ≥ .76 for PSI Competence, .64 for PSI Child Reinforces Parent, and .62 (except at age 12 months, where α = .49) for parent-child negativity. The first component of each PCA accounted for more than 50% of the variance. Parental role overload was calculated (z scores, averaged; r range = .43 – .50, ps < .001) from the Role Restriction subscale of the PSI (Abidin, 1986) and the average of five items from the Role Overload scale (e.g., feeling “pulled apart by conflicting obligations”; (Barnett & Marshall, 1989)); in the infancy period, the Role Overload scale was assessed only at 12 months. For the two scales, all αs were ≥ .77. Financial stress was the average of four items (e.g., “how much difficulty making monthly payments”); all αs were ≥ .74. Finally, separately for the infancy and preschool periods and for mothers and fathers, scores for the five stress domains were combined using PCA. The first component of each of the four PCAs accounted for more than 50% of the variance, and all stress domains had factor loadings greater than .50 for each period and for each parent. Four parental stress variables were thus employed in subsequent analyses: mother and father stress scores for both the infancy and preschool periods. Due to parental non-participation at some time points, the sample size was somewhat different for the analysis of each specific parental stress variable: thus, for infancy, n = 106 mothers and 100 fathers, and for preschool, n = 106 mothers and 85 fathers. For each parent, the infancy and preschool stress scores were strongly correlated (r = .74 for mothers, .73 for fathers, ps < .001), whereas there were only very modest correlations between the mother- and father-report scores within and across the two developmental periods (r range = .26 – .34, ps < .001). To deal with skewness, log-transformed variables were used in analyses.
Collection, Preparation and Analysis of Genomic DNA from Buccal Epithelial Cells
Before collection, participants rinsed their mouths with water twice for 15 s each. Buccal epithelial cells were collected using MasterAmpBuccal Swabs (Epicentre Biotechnologies), which were stored at −80°C prior to analysis. Genomic DNA was isolated using IsohelixBuccal DNA Isolation Kits (Cell Projects) and was purified and concentrated using DNA Clean & Concentrator (Zymo Research). Sample yield and purity were assessed spectrophotometrically using a NanoDrop ND-1000 (Thermo Scientific).
Quantitative DNA methylation measurements of purified genomic DNA were performed with the Infinium Human Methylation 27 BeadChip assay (Illumina). This microarray platform allows for the simultaneous measurement of the DNA methylation status within 27,578 cytosine-guanine dinucleotide (CpG) sites, covering more than 13,500 promoters, first exons of the coding region, or occasionally first intron of well-annotated genes, thereby representing more than half of all human genes. Each array also contains 254 CpG sites located in approximately 110 micro RNA loci. After random ordering of samples, 750ng of genomic DNA from each sample was bisulfite-converted using the EZ DNA Methylation Kit (Zymo Research). Bisulfite deaminates unmethylated cytosine to uracil, while methylated cytosine is protected from deamination. Bisulfite treatment thus converts epigenetic information to sequence-based information, which can be measured with methods similar to those used to distinguish single nucleotide polymorphisms. A sample of 160ng of bisulfite-converted DNA was whole-genome amplified, fragmented by an enzymatic process and hybridized to BeadChip arrays. Two oligonucleotide probes interrogated each CpG site, one probe with sequences targeting methylated DNA and the other containing sequences targeting unmethylated DNA. After extension with DNP-labeled and biotin-labeled dNTP, each array was stained with Cy5 labeled anti-DNP antibodies and Cy3 labeled streptavidin and scanned with the IlluminaiScanon a two-color channel to detect Cy3 labeled probes on the green channel and Cy5 labeled probes on the red channel. Using the IlluminaGenomeStudio software package, average beta values were calculated by dividing the methylated probe signal intensity by the sum of methylated and unmethylated probe signal intensities. Average beta values range from 0 (completely unmethylated) to 1 (fully methylated) and provide a quantitative readout of relative DNA methylation for each CpG site within the cell population interrogated. This method was highly reproducible, with replicates across runs achieving r > .99.
Normalization and Statistical Analysis of DNA Methylation Data
All normalization, calculations and statistical analysis was done using R 2.11.0 (http://www.R-project.org/). Average background intensity, measured by negative background probes present on the array, was subtracted from raw intensities to adjust for varying background signals across samples. Background adjustment was completed separately for raw data from the green and red channels to adjust for Cy3 and Cy5 differences. To control for batch effects across different sets of arrays, background adjusted raw data from both channels were quantile normalized separately (Bolstad, Irizarry, Astrand, & Speed, 2003). Average beta values were then recalculated per the following Illumina formula: background subtracted and quantile normalized intensities of methylated probes divided by the sum of normalized intensities from unmethylated and methylated probes. All CpG probes with a detection p > .05 were removed to ensure that only high-confidence probes were included in the subsequent analysis. The detection p-value assesses whether the signal intensity for the average beta value is significantly different from the negative probes, as well as consistent across all oligonucleotides interrogating a given CpG site on the array. CpG sites on the X-chromosome were removed to prevent confounding by gender. To increase analytic power, CpG sites whose average beta values across subjects were < 0.05 or > 0.95 were excluded, so that all sites would have between-sample differences in methylation > 5% of the average beta value.
This left a total of 18,231 CpG sites to be tested for associations with the four parental stress measures. Significance Analysis of Microarray (SAM, in the samr package of Bioconductor (Tibshirani, Chu, Hastie, & Narasimhan, 2010; Tusher, Tibshirani, & Chu, 2001)), using a ranked linear regression analyses, identified CpG sites with DNA methylation patterns associated with any of the stress variables. SAM assigns a score to each CpG site on the basis of differential DNA methylation relative to the standard deviation of repeated measurements. To account for multiple testing, the procedure uses permutations of repeated measures to estimate the false discovery rate (FDR), defined as the expected proportion of false positives among a ‘family’ of hypothesis tests. This allows the identification of a set of ‘candidate positives’ and is useful in statistical settings where priority is placed on the discernment of mostly true findings, rather than the strict avoidance of single false positives (Storey, 2011). The computed FDR thus measures the likelihood of a Type I error within a particular association, in a manner parallel to the p-value, but the background distribution to estimate the Type I error is generated from random assignment of stress variables to DNA methylation data. The q-value is the minimum FDR at which the test may be designated significant. In all cases, 1000 permutations were run and included FDRs up to 20%. Separate SAM analyses were run for the entire group of 109 subjects where stress data were available and for gender-specific subsamples. Reported results show differences in DNA methylation between the low and the high end of the regression line defined by regressing DNA methylation level on a parental stress measure.
Biological Commonalities among Differentially Methylated Genes
Finally, the functional annotation tool from the DAVID bioinformatics resource (Huang da, Sherman, & Lempicki, 2009) was used to identify common biological processes and pathways, molecular functions, and cellular components, as defined by Gene Ontology (GO) criteria. For this analysis, and using the default, “medium stringency” setting of the DAVID instrument, the enrichment of GO terms in the target gene list was compared, using Fisher’s Exact test, to a background list containing all genes analyzed. The Functional Annotation Cluster tool aggregates enriched GO terms based on their co-occurrence in the same gene; the tool also computes an enrichment score, the minus log transformed geometric mean of p-values from GO terms within each cluster. We report the single cluster from the DAVID analysis of 139 methylation sites associated with maternal stress at infancy that had an enrichment score > 1.3, corresponding to a p of 0.05.
Results
Characterization of Early Adversity
High levels of early adversity were defined as the upper 20% of PCA-derived scores for parent reported stress, for each reporter and time period. Among mothers reporting high stress during infancy, for example, at baby’s age 12 months the average CES-D score was 15 (with 43% ≥ the clinical threshold of 16 and 76% ≥ the clinical sub-threshold of 12), 67% reported marital conflict, 95% felt overloaded “occasionally” to “very often,” and 81% felt financially stressed. Among fathers reporting high stress in preschool, at child’s age 4½ years, the average CES-D score was 20 (with 59% ≥ 16 and 76% ≥ 12), 71% reported marital conflict, 88% felt overloaded “occasionally” to “very often,” and 65% felt financially stressed.
Differential Methylation Associated with Early Adversity
Raw data from the scanned microarrays are available to readers in the gene expression omnibus (GEO) database, a repository of high throughput gene expression data and hybridization arrays, which can be accessed under accession number GSE25892 at: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE25892.
Differences in DNA methylation were significantly associated with both maternal and paternal stressors reported 10–15 years prior to the collection of the children’s DNA. Table 1 is a summary of these results for both the full sample and the child gender-specfic subsamples. Because of the large number of associations identified, the chance of reporting false positive associations is reduced by focusing only on CpG sites with FDRs < 20%. Sites are further differentiated as high confidence sites (HCSs) when FDRs were < 5% and medium confidence sites (MCSs) when FDRs were between 5% and 20%. Finally, the number of sites with > 5% difference in methylation between low and high stress conditions is indicated in parentheses in the Table, and the range of site-specific Spearman rho correlation coefficients is displayed for associations between stress and site methylation. Figure 1 is a visual representation of the same data for HCSs only. Readers interested in a more detailed rendition of these results are referred to a web site, http://www.cmmt.ubc.ca/research/investigators/kobor/lab/childstress, where supplementary tables list methylation status for all specific CpG loci associated with parental stress. The posted tables display, for each CpG site, the specific gene symbol, the site interrogated, the slope of the regression line relating parental stress to DNA methylation level, the range of relative DNA methylation, the percent differential DNA methylation between low and high stress children, Spearman rho correlation coefficients, and the FDR. Shaded rows identify sites with DNA methylation differences exceeding 5%.
Table 1.
Summary of DNA Methylation Results for False Discovery Rate (FDR) < 20%
| Full Sample | Girls | Boys | ||||
|---|---|---|---|---|---|---|
| n=109 | rho range | n=60 | rho range | n=49 | rho range | |
| Infancy | ||||||
| Maternal Stress | HCS: ↑139 (37) | 0.21 to 0.4 (0.21 to 0.4) | HCS: ↑1(1) | 0.5 (0.5) | HCS: ↑3 (1) | 0.5 to 0.58 (0.53) |
| Paternal Stress | –– | HCS: ↓3(1) | −0.45 to −0.52 (−0.52) | –– | ||
| Preschool | ||||||
| Maternal Stress | –– | –– | –– | |||
| Paternal Stress | HCS: ↑2 (0) | 0.42 to 0.45 | HCS: ↑6(5) | 0.52 to 0.56 (0.52 to 0.56) | –– | |
| HCS: ↓9(3) | −0.51 to −0.61 (−0.54 to −0.61) | |||||
| MCS: ↑29 (12) | 0.34 to 0.39 (0.34 to 0.39) | MCS: ↑314 (151) | 0.34 to 0.51 (0.34 to 0.51) | –– | ||
| MCS: ↓1057(128) | −0.28 to −0.50 (−0.28 to −0.50) |
High Confidence Sites (HCS): FDR <5%
Medium Confidence Sites (MCS): FDR 5%–2%
(>5% differential methylation)
↑ = Increased Methylation
↓ = Decreased Methylation
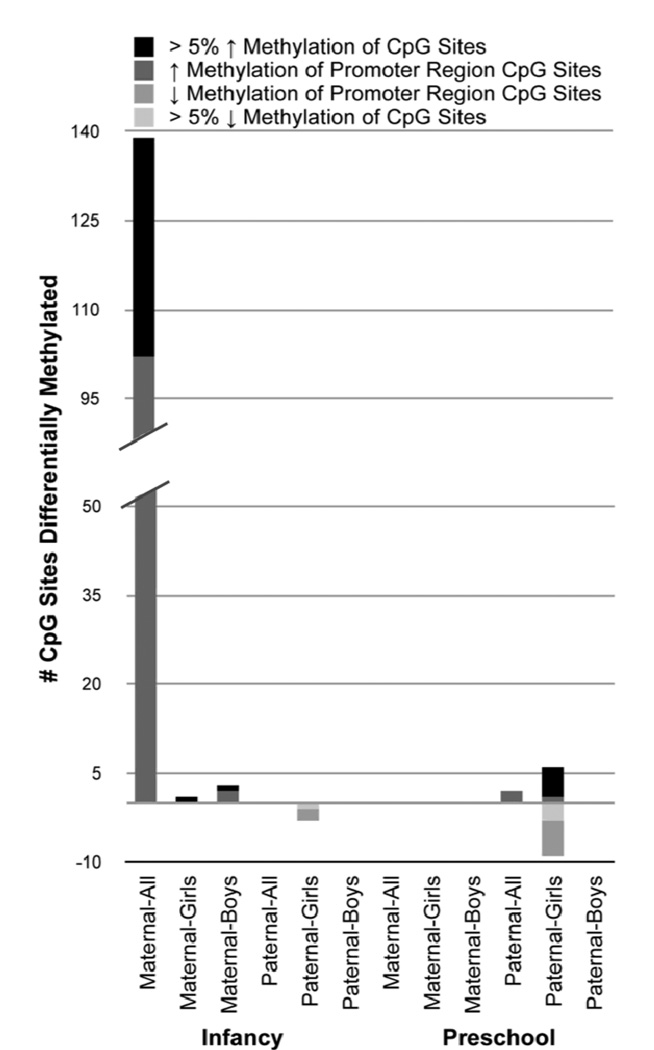
Figure 1. Number of differentially methylated CpG (cytosine-guanine dinucleotide) sites by child’s gender, maternal v paternal report of stressors, and during the infancy v preschool period.
The Figure is a visual display of data summarized in Table 1 for HCSs with increased methylation by parental stress (shown above the horizontal zero line) and with decreased methylation by parental stress (shown below the line). The figure also distinguishes sites with greater than 5% difference in methylation comparing low and high stress groups.
In the full sample, associations of DNA methylation were found with two of the four stress variables. First, maternal stress in infancy bore high confidence associations, with increased DNA methylation in 139 CpG sites, all of which had high confidence FDRs of 0% and 37 of which had more than a 5% difference in DNA methylation between children exposed to low and high maternal stress in their first year of life (Suppl Table 1). Further, rank order correlations between maternal stress in infancy and increased methylation of CpG sites in adolescence ranged from .21 to .40. Second, although the association of maternal stress in infancy with DNA methylation did not extend into the preschool years, paternal stress during this period was associated with increased DNA methylation in 31 CpG sites, of which 2 were HCSs and 12 had methylation differences > 5% (Suppl Table 2). Rank order correlation magnitudes for paternal stress in the preschool period ranged from .34 to .45.
Gene-Specific Analyses
The microarray contained a limited number of CpG sites located in the gene regulatory regions for several candidate genes in which DNA sequence polymorphisms or differential DNA methylation have known associations with environmental stress or behavior. These included genes for the glucocorticoid receptor (NR3C1), dopamine receptor (DRD4), serotonin transporter (5 HTT), brain-derived neurotrophic factor (BDNF), catechol-O-methyltransferase (COMT), and dopamine transporter (DAT1). No significant associations were identified between DNA methylation in these genes and the measures of parental stressors; Spearman rho correlations ranged from −.39 to .26 but failed multiple testing correction (FDR > 20% in cases; detailed data are shown in Suppl Table 3).
Gender-Specific Analyses
Repeat analyses for male and female children produced patterns of association distinctive from but commensurate with those observed in the full sample. In the infancy period, for example, increased exposures to maternal stress were associated with differential DNA methylation in both genders, consistent with findings from the full sample. In contrast to the large number of sites identified in the full sample, however, far fewer emerged in gender-specific analyses. The fewer differentially methylated sites are likely due, at least in part, to reductions in statistical power among the gender sub-samples, but all identified associations had substantially stronger correlations. The 3 HCSs with increased DNA methylation identified in boys had rank order correlation coefficients in the range of .50 – .58 (Suppl Table 4), and the single HCS found in girls had rho = .50 (Suppl Table 5). Further, differential methylation among CpG sites in girls was related to both maternal and paternal stressors in infancy, but in opposing directions. That is, maternal stress in infancy was associated with increased methylation of one CpG site (Suppl Table 5), whereas paternal stress was linked to decreased methylation at three CpG sites, with the latter having rank order correlation coefficients in the range of −.45 – −.52 (Suppl Table 6). In boys, on the other hand, only maternal stress in infancy was associated with differential DNA methylation, in the direction of increased methylation among stress-exposed boys.
Gender-specific analyses of parental stress in the preschool period indicated that differential methylation was uniquely associated with exposure to paternal stress and was specific to girls. In these findings, analyses identified distinctive sets of CpG loci that were either more methylated or less methylated with increased exposure to paternal stress. Specifically, 320 CpG sites were found with increased paternal stress-related DNA methylation: 6 belonging to the high confidence group (rhos= .52 – .56) and 314 to the medium confidence group (rhos = .34 – .51; Suppl Table 7). These 320 CpG sites included 21 out of the 31 sites identified in the full sample, with rank order correlations becoming stronger in each case (Suppl Table 9). Gender subset analyses also identified 1066 CpG sites that were less methylated with higher paternal stress: 9 classified as HCSs (rhos = −.51 – −.61) and 1057 as MCSs (rhos = −.28 – −.50). Paralleling results for the full sample, there was no differential DNA methylation associated with exposure to maternal stress during the preschool period for either gender.
Taken together, results summarized in Table 1 and Figure 1 indicate that differences in DNA methylation were associated with three contrasting predictors of epigenetic modification: a) the gender of the parent reporting the stressors (mother v father), b) the developmental period in which the stressors were reported (infancy v preschool), and c) the gender of the child (male v female).
Identification of Robust DNA Methylation Differences
To further evaluate these associations, an additional level of stringency was next applied to the analyses. Specifically, we focused on CpG sites within sets of HCSs that had more than a 5% difference in absolute methylation from the lowest to the highest point on the regression ‘fit’ line (calculated as the slope × range of the stress variable). Although the 5% cut-off is arbitrary, it was reasoned that this added stringency would highlight sites with the most robust differentials in DNA methylation and therefore those most likely to have a possible functional impact. The numbers of robust (> 5% methylation difference) CpG loci are indicated in parentheses for each set of associations in Table 1, along with their correlation range. These ranges of rank order correlation coefficients were quite similar to those of the entire set, suggesting that applying the more stringent filter did not bias toward higher correlations but merely removed sites with less pronounced DNA methylation changes.
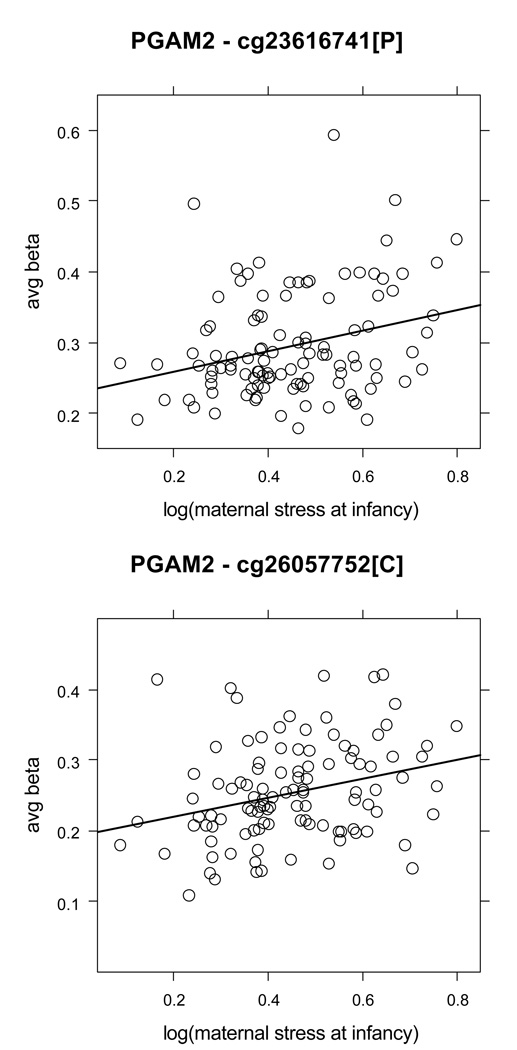
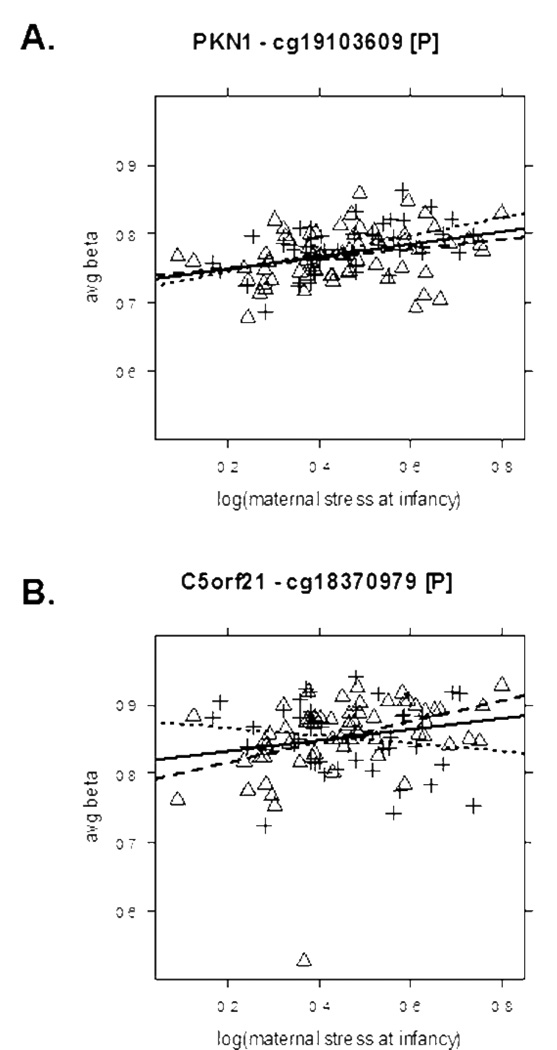
As noted above, of the 139 HCS CpG loci identified in analyses for exposure to maternal stress during the infancy period in the full sample, 37 had DNA methylation changes exceeding 5% (Suppl Table 1). We noted that, whereas almost all of the 37 CpG loci were from different genes, two belonged to the PGAM2 gene, which encodes phosphoglyceratemutase 2. These two associations are presented as scatterplots in Figure 2. As before, analysis by gender reduced the number of CpG sites associated with maternal stress in infancy. There were only 2 sites in the gender-specific analysis exceeding 5% methylation difference, but both were also identified for the full sample. Specifically, these sites were: a) a CpG site in the PKN1 gene promoter for boys, and b) a site in the C5orf21 promoter for girls. Scatterplots for these associations are presented in Figures 3A and 3B. As shown, the rank order correlations became stronger and their differences in DNA methylation more pronounced when analyzed by gender.
Figure 2. One CpG (cytosine-guanine dinucleotide) site in the promoter and one CpG site in the first exon of the PGAM2 gene were associated with maternal stress at infancy in the full group.
In the set of 37 high confidence sites associated with maternal stress at infancy and having more than 5% change in DNA methylation, two CpG loci belonged to the same gene, PGAM2, which encodes phosphoglycerate mutase 2. These are the cg26057752 CpG site (rho = .31, 9.6% DNA methylation change, slope = .14 (95% CI = .05 – .22), FDR = 0%) and the cg23616741 CpG site (rho = .25, 10.4% DNA methylation change, slope = .15 (95% CI = .05 – .24), FDR = 0%). No statistically significant associations for either of these 2 CpG sites were found in the gender-specific analysis. The average beta scale was restricted to show values between 0.15–0.65, rather than the full range of 0–1.0. Each circle represents one subject and the regression line for the full group is shown in solid. Note that, in each figure, [P] designates CpG sites from promoter regions; [C] = sites from the first exon of the coding region; and [I] = sites from the first intron.
Figure 3. Representative examples of CpG (cytosine-guanine dinucleotide) sites associated with maternal stress in infancy dependent on gender.
A. A specific CpG site in the promoter of the PKN1 gene (encoding protein kinase N1) was associated with maternal stress in the full group (rho = .40, 6.5% DNA methylation change, slope = .09 (95% CI = .05 – .14), FDR = 0%) and in boys only (rho = .50, 7.5% methylation change, slope = .13 (95% CI = .06 – .20), FDR=0%) but not in girls (rho = .33, 4.5% DNA methylation change, slope = .07 (95% CI = .01 – .13), FDR = 52%).
B. In contrast, while a CpG site in the promoter of the C5orf21 locus was weakly associated with maternal stress in the full group (rho = .23, 5.5% DNA methylation change, slope = .08 (95% CI = .01 – .15), FDR = 0%) it became much stronger in girls only (rho = .50, 10.7% DNA methylation change, slope = .15 (95% CI = .06 – .24), FDR = 0%). In boys, this CpG site tended to be negatively associated with maternal stress, although this was not statistically significant by our criteria (rho = − .17, −3.2% DNA methylation change, slope = −.06 (95% CI = −.17 – .06), FDR = 81%) The average beta scale was restricted to show values between 0.5–1.0 rather than the full range of 0–1.0. Girls are represented as triangles and boys as crosses. Regression lines for the full group are solid, wide-dashed for girls only, and narrow-dashed for boys only.
Filtering for greater robustness removed 2 of the 3 HCS CpG loci associated with paternal stress during infancy in girls. The remaining CpG site in the promoter of MGMT gene, which encodes O-6-methylguanine-DNA methyltransferase, had more than a 20% difference in methylation, the most pronounced difference in the study (Suppl Figure 1). Results for this site should be considered with caution, however, as our array contained 25 additional CpG sites in the MGMT gene, none of which were associated with parental stress.
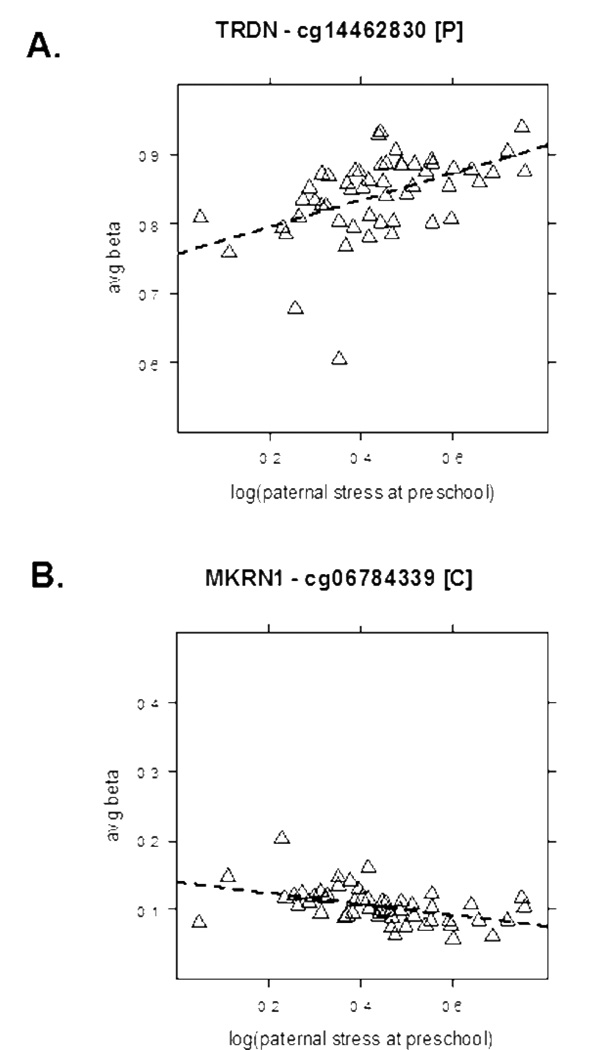
Application of the filtering procedure to the analysis of paternal stress during the preschool period in the full sample showed that the 2 HCSs had less than 5% DNA methylation difference. For 12 of the 29 MCSs, however, the differential DNA methylation exceeded 5%, ranging up to 14% (Suppl Table 2). Of the 15 HCSs identified in gender-specific analyses as being either more or less methylated following paternal stress in girls, 8 had DNA methylation changes exceeding 5% (Suppl Tables 7 and 9). This relation between differential DNA methylation and preschool paternal stress in girls is illustrated in Figure 4. A CpG site in the TRDN gene promoter showed increased methylation in girls as paternal stress increased (Fig 4A), whereas a CpG site in the MKRN1 first coding exon was less methylated under the same conditions (Fig. 4B). Notably, the C19orf30 first coding exon contained 2 CpG sites that had more DNA methylation with increasing paternal stress in girls (Suppl Figure 2). One additional CpG in the C19orf30 gene belonged to the set of MCSs that were less methylated as paternal stress increased, while 5 others in the same gene were not associated with parental stress according to our criteria.
Figure 4. Representative examples CpG (cytosine-guanine dinucleotide) sites positively and negatively associated with paternal stress at preschool in girls.
A. A CpG site in the promoter of the TRDN gene (encoding triadin, a possible regulator of calcium release in cardiac and skeletal muscle) was positively associated in girls with paternal stress at preschool ((rho = .55, 13.7% DNA methylation change, slope = .19 (95% CI = .10 – .29), FDR = 0%). No statistically significant association was found in the full group or boys-only analysis.
B. A CpG site in the first exon of the MKRN1 (makorin ring finger protein 1, a ubiquitin E3 ligase with roles in the regulation of telomere length) was negatively associated in girls with paternal stress at preschool (rho = − .54, −5.6% DNA methylation change, slope = −.08 (95% CI = −.12 – −.04), FDR = 0%). No statistically significant association was found in the full group or boys-only analysis. The average beta scale was restricted to show values between 0.5–1.0 (A) or 0 – 0.5 (B) rather than the full range of 0–1.0. Girls are represented as triangles with the regression line being wide-dashed.
Extracting Functional Biological Information from Sets of Methylation Loci
The above analyses focused principally on HCS sites, which had less than 5% FDRs, as a means of minimizing the likelihood of reporting false positive associations. Nonetheless, we also sought for ways to test both HCS and MCS loci for functional biological information. To do this, we searched the combined HCS and MCS datasets for increased and decreased DNA methylation of paternal stress at preschool for genes with more than one CpG site having DNA methylation change going in the same direction. This was motivated by our finding that some genes (e.g., PGAM2, c19orf30) had 2 associated CpG sites and by the underlying assumption that identification of several associated CpG sites in the same gene might reflect greater likelihood of a significant association. A large number of genes were identified using this approach (n=71 genes), prompting a reapplication of the 5% DNA methylation change screen, as described above (Suppl Table 10). This approach reduced the number of genes to 28, including several genes of potential functional interest. IGF2AS (3 out of 11 CpGs on the array with FDR < 20%; 1 out of 3 with > 5% change in DNA methylation), for example, is a gene that expresses a paternally imprinted antisense transcript of the insulin-like growth factor 2 gene. Another example, the TGFB2 gene (2 out of 2 CpGs on the array with FDR < 20%; 1 out of 2 with > 5% change in methylation) encodes a member of the transforming growth factor beta (TGFB) family of cytokines. A final example, the NNAT neuronatin gene (2 out of 7 CpGs on the array with FDR < 20%; both with > 5% change in methylation), is an imprinted gene encoding a proteolipid possibly involved in the regulation of ion channels during brain development.
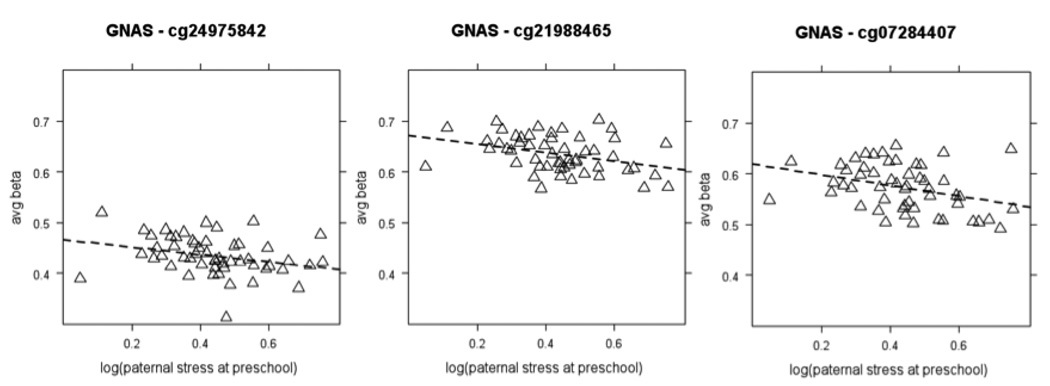
Further, 2 genes in the reduced set of 28 had 3 CpG sites with > 5% change. These were the GNAS gene cluster (6 out of 29 CpGs with FDR < 20%; 3 out of the 6 with > 5% difference in DNA methylation, the latter shown in Figure 5) and the MGC33302 gene, also known as MFSD8/CLN7 (3 of 6 CpGs with FDR < 20%; all with > 5% change in methylation and all shown in Supp Figure 3). Although it is not clear whether the DNA methylation pattern measured in buccal epithelial cells is conserved in more central tissues, both of these loci are of potential interest for brain development and behavior. GNAS is an imprinted locus consisting of a complex arrangement of several protein-coding and non-coding transcripts. In mice, deletion of Nesp55, one of the protein-coding genes located in the GNAS locus and under the control of its imprinting region, influences behavioral reactivity to novel environments (Plagge, et al., 2005). Genetic variation in MFSD8/CLN7 is associated with the variant late-infantile onset neuronal ceroid lipofuscinoses, the most common neurodegenerative disorders of childhood (Siintola, et al., 2007).
Figure 5. Representative example of a gene of potential functional interest associated in girls with paternal stress during preschool uncovered by focused examination of the medium-confidence CpG (cytosine-guanine dinucleotide) site set.
Three CpG sites in the imprinted GNAS locus were negatively associated in girls with paternal stress at preschool. Specifically, these were cg24975842 (rho = − .38, −5.2% DNA methylation change, slope = −.07 (95% CI = −.14 – −.01), FDR = 9.1%), cg21988465 (rho = − .37, −5.9% DNA methylation change, slope = −.08 (95% CI = −.15 – −.02), FDR = 11.7%) and cg07284407 (rho = − .36, −7.5% DNA methylation change, slope = −.11 (95% CI = −.11 – −.02), FDR = 11.7%). No statistically significant association was found in the full group or boys-only analysis The average beta scale was restricted to show values between 0 – 0.5 (A) or 0.3–0.8 (B) rather than the full range of 0–1.0. Girls are represented as triangles with the regression line being wide-dashed.
In addition to gene-specific analyses, it is often informative to look at broader patterns of genes with DNA methylation differences to identify cellular or biological process commonalities. To do this, we focused on the set of stress-associated CpG sites containing only HCSs, as this minimized the chances of reporting commonalities of lesser significance. The web-based DAVID platform (see: http://david.abcc.ncifcrf.gov/) is a project of the National Institute of Allergy and Infectious Diseases that was designed and published to foster functional annotation analyses in microarray and proteomics studies (Huang da, et al., 2009). Because the algorithm is optimized for gene lists containing 100–1000 genes, the 139 HCSs that were associated in the present study with maternal stress in infancy were selected as the set of genes on which to perform the DAVID analysis and illustrate its potential utility. The functional annotation cluster that was identified using this approach had an enrichment score of 1.45, which is above the 1.3 value that corresponds to p<0.05 and represents the minimal score considered significant (Suppl Table 11). This cluster contained GO terms centered on biosynthetic and metabolic processes.
Discussion
The study reported here had four principal findings of relevance to genetic research on child development. First, maternal stress during the infancy of a child—long suspected and more recently documented as a contributor to maladaptive developmental outcomes in children—was associated with a broad and substantial array of epigenetic marks assessed a decade and a half later, during children’s mid-adolescence. Interrogating nearly 28,000 CpG sites in the regulatory regions of 14,000 genes, a large number of DNA methylation sites were identified for which children whose mothers who were highly stressed in the child’s first year of life showed significantly higher levels of methylation than those less exposed, even after controlling for false discovery rates related to multiple comparisons. Such genomic sites with increased DNA methylation occurred both in genes with plausible linkages to family experiences of stress and adversity (e.g., NEUROG1, a bHLH transcription factor gene that is involved in neuronal differentiation and cell-type specification in distinct regions of the developing nervous system) and in genes with no such linkages (e.g., PGAM2.). To our knowledge, this is the first report of a long-term, prospective association between early childhood parental adversities and the epigenetic status of specific, regulatory sites in children’s genomic DNA.
Second, the presented data showed that experiences of adversity among both mothers and fathers bore reliable linkages to epigenetic profiles within specific developmental timeframes, with maternal stressors during the infancy period more potently predictive and paternal stressors during the preschool years more predictive. Fathers’ reports of high levels of adversity during their children’s preschool years were significantly associated with altered methylation status in a smaller, but still significant, number of regulatory loci. Findings therefore offer evidence for epigenomic correlates of biparental adversities, with sequenced, period-specific sensitivity to the stressors of one parent over the other.
Third, the patterning of epigenetic marks associated with early adversity varied by the gender of both parent and child, with fathers’ reported stressors more strongly associated with girls’ epigenetic modifications and mothers’ more related to those of both sexes. Such findings corroborate, at an epigenetic level of analysis, past observations of gender-specific influences of parenting on child development and well being. Father absence and non-participation in parenting has been linked, for example, to an earlier timing of puberty onset (Ellis, McFadyen-Ketchum, Dodge, Pettit, & Bates, 1999) and to the emergence of difficult temperamental characteristics in daughters, rather than in sons (Bezirganian & Cohen, 1992). The current study provides provisional evidence that sex-specific influences of parent-child relationships on maladaptive developmental processes may be subserved, in part, by epigenetic modifications capable of guiding such processes.
Fourth, the noted associations were identified epigenetically within buccal epithelial cells, a histological cell type not previously examined as a target tissue for social contextual, exposure-related epigenetic changes. Such DNA methylation differences in epithelial chromatin are likely not paralleled by similar marks in neural tissues, which are generally assumed to be the biological substrate for developmental events and processes. Nonetheless, the adversity-related epigenetic marks reported here constitute measurable epigenomic change that is associated with early life experience within proximal social contexts and has the potential for altering gene expression. Further, the epigenetic profiling of even readily accessible epithelial cells can advance understanding of issues such as the timing and persistence of early exposures’ biological correlates and the consistency of profiles across populations, geographies and time.
Gene-environment interplay
Taken together, these findings offer novel evidence for a biological embedding of early experience, or more specifically, the temporally remote correlates of early adverse experiences on the human epigenome and its regulatory role in the expression of specific genes, including genes that guide neurodevelopment. Both relative increases and decreases in promoter region methylation were detected in the genomes of adolescents whose parents had reported significant adversity in past years. Although DNA methylation is generally associated with a down-regulation in gene expression, the epigenetic control of differential transcription is almost certainly far more complex and involves a broad and diverse array of chromatin modifications (see, e.g., (Mehler, 2008)). As a consequence, discerning a coherent and functional ‘meaning’ of the reported findings lies beyond the present state of epigenetic science. We note, however, that to our knowledge these data constitute only the second report of altered DNA methylation in buccal epithelial cells associated with environmental exposure, following a recent study that reported DNA methylation marks in such cells from 5-year-old children associated with maternal smoking during the intra-uterine period (Breton, et al., 2009).
Importantly, our findings may be correctly viewed as an instantiation of ‘gene-environment interplay’ and the capacity for experience and genomic variation—allelic or epigenetic—to conjointly influence salient developmental endpoints (Gilbert & Epel, 2009; Rutter, Moffitt, & Caspi, 2006). One variety of such interplay is the moderation of early experiential effects by single nucleotide polymorphisms within genes affecting key neural circuitry and neurotransmission pathways (Caspi, et al., 2002). The most broadly recognized examples of epigenetic regulatory processes are those described within the caregiving behavior of the mother rat and within the dietary influences on coat color in mice. Studies by Meaney, Szyf and colleagues (Meaney & Szyf, 2005; Weaver, Cervoni, et al., 2004) have shown how phenotypic differences in the reactivity of the HPA system arise from the mother rat’s licking and grooming behavior in her pups’ first several post-natal days. Such behavior changes reactivity phenotypes by demethylating the binding site for transcription factor Egr1 in the enhancer region of the pups’ glucocorticoid receptor gene. Waterland and Jirtle (Waterland & Jirtle, 2003) supplemented the diets of pregnant, viable yellow Agouti mice with methyl-donors, such as folate, choline and betaine, and created dramatic differences in offspring coat color. Pups of mothers fed methyl-donor supplements had increased methylation of the Agouti allele that guides yellow fur development, resulting in suppressed gene expression and phenotypic reversion to a brown coat color. Far more limited examples of epigenetic processes in humans have included research by McGowan and colleagues (McGowan, et al., 2009; McGowan, et al., 2008) in the Meaney laboratory, revealing DNA methylation changes in promoter regions of the ribosomal RNA and glucocorticoid receptor genes among individuals with a history of childhood abuse and suicide. A recent report by van IJzendoorn and colleagues found higher levels of promoter methylation in the serotonin transporter gene among adult adoptees with unresolved trauma (van IJzendoorn, Caspers, Bakermans-Kranenburg, Beach, & Philibert, 2010). No prior work, however, has provided prospective human evidence of sustained epigenetic marks related to early life experience. The novelty of the current findings lies in the long-term association of family adversity over the first five years of life with epigenetic changes in a broad variety of methylation sites in adolescence and the identification of such changes in non-invasively accessible epithelial cells.
Convergence with past findings
These findings, however novel, are also usefully commensurate with several past sets of observations in the developmental and biological sciences. A now expansive literature documents, with increasing methodological strengths, the epidemiologic associations between experiences of early adversity in the life a young family and their long term consequences for health and development (Hertzman & Boyce, 2010; Shonkoff, Boyce, & McEwen, 2009). Such literature includes evidence that adverse childhood events are associated with a variety of mental and physical health outcomes in mid-life (e.g., (Felitti, et al., 1998)), that adult chronic diseases are associated with nutritional and growth deficits in very early, even prenatal development (e.g., (Barker, 1990)), that maternal depression during infancy is associated with systematic differences in the regulation of children’s stress responsive biological systems (e.g., (Essex, et al., 2002)), and that growing up in conditions of poverty is linked to proinflammatory shifts in cytokine expression, which is related in turn to risk for chronic cardiovascular disease in adult life (e.g., (Miller, Maletic, & Raison, 2009)). A recent report relating early life stress to differences in both whole genome and candidate gene methylation in bonnet macaques extends such findings to the epigenome (Kinnally, et al., 2011), and the current study adds new evidence that chromatin structure may bear the ‘mark’ of early life stressors, even in human children. Epigenetic variation may thus constitute a biological ‘memory’ of early life experience, perhaps especially experiences of adversity, stress and misfortune.
The study findings also substantiate prior observations that, although maternal stressors are preeminent in their potential influence on the developmental outcomes of infants and young children, such influence involves both parents, with fathers’ effects on adaptation and risk becoming especially salient beyond infancy. Past research has shown, for example, that fathers’ supportive presence has key influences on biological processes, from pubertal timing (Ellis, 2004) to the regulation of stress reactivity (Roubinov & Luecken, 2010), and on developmental processes, from school readiness (Martin, Ryan, & Brooks-Gunn, 2010) to the acquisition of significant, presyndromal behavior problems (Boyce, et al., 2005; Lamb, 1997). Again, at an especially fundamental level of biological impact, current findings add to the body of evidence implicating biparental experiences of adversity and depression in the advent of maladaptive development and disorders of mental and physical health.
Increasingly, studies of human development also identify important gender differences in both trajectories of normative development and in the determinants of deviation from such trajectories (Essex, et al., 2003; Landman-Peeters, et al., 2008). These gender differences in developmental course may be traceable, in part, to the roles of mothers and fathers in the care of and attention to male and female children, a differential involvement that may also differ by socioeconomic status (Roopnarine, Fouts, Lamb, & Lewis-Elligan, 2005). Similarly, the findings of the current study suggest significant differences between boys and girls in the parental-adversity-associated differential methylation of gene promoter regions. Such differences occurred both in the magnitude of changes in DNA site methylation, as well as in the specific epigenetic sites involved.
Among the biological and methodological issues involved in studying chromatin structure are the profound epigenetic differences between cells of differing histological origin. Indeed, since all cells in the body contain the identical DNA sequence, it is the epigenetic differentiation of primordial, germ layer cells that allows and guides the embryogenesis of different tissues and organs. Thus, although the neural tissue epigenome is closest to the neurobiological processes that drive development, human epigenetic research is necessarily relegated to the examination of peripheral, accessible tissues, such as immune cells, gametes, and biopsy materials. A recent report by Talens et al, however, offers methodological assurances of meaningful variation in the human BEC epigenome, stability in such variation over years of time, and substantial overlap between BEC and leukocyte epigenetic marks (Talens, et al., 2010). The present study offers additional evidence that experience-related changes in the epigenome are detectable in the DNA of epithelial cells, which are readily accessible to sampling and study.
Limitations
Interpretation of the results presented here should be informed by an awareness of specific weaknesses in the design and methods of the reported study. First, the current study sample comprised a subset of children from a larger, longitudinal study of child development and mental health. Although the examined subsample did not differ substantially in a variety of demographic descriptors, there remains the possibility that the children studied differed systematically from the larger cohort from which they were drawn. Second, while representative of the specific population from which participants were identified, findings may not be replicable within samples derived from more racially or socioeconomically diverse groups. Third, despite extensive efforts to minimize the likelihood of Type I errors, the search for differences among 28,000 potential methylation sites involved issues of multiple comparison, to say the least, and it is possible that false positive findings escaped efforts to preclude such errors. Fourth, the current study did not consider the epigenetic changes associated with prenatal versus infancy and preschool stress exposures. Replication and extension of the reported findings will thus be essential. Finally, useful extensions of the reported analyses would examine behavioral and psychological phenotypes that may be associated with both early parental stressors and differential methylation of gene promoters. Such analyses are now within reach of several research teams, and the co-authors of the current report look forward to pursuing linkages among early exposures, epigenetic marks and phenotypic variation in these and other, related data.
Even taking into account these limitations in design and methods, however, the reported research yielded singular observations linking parental adversity in early childhood to differences in chromatin structure a decade and a half later, in the mid-adolescence of the children studied. Such epigenetic differences may constitute a fundamental biological vestige of such early exposures, potentially altering the expression of genes affecting metabolic and physiologic pathways and changing trajectories of individual phenotypic development. As such, the observed epigenetic modifications could plausibly represent a ‘fundamental cause’ of the risk-enhancing trajectories leading to disease, disorder and troubled developmental outcomes.
Supplementary Material
Acknowledgments
This work was funded by the National Institute of Mental Health grants R01-MH044340, P50-MH069315, P50-MH084051, and R24-MH081797 and by research support to WTB, CH and MSK from the Canadian Institute for Advanced Research. MSK is a Scholar of the Mowafaghian Foundation through the Human Early Learning Partnership of the University of British Columbia. WTB holds the Sunny Hill Health Centre-BC Leadership Chair in Child Development. The authors wish to express their appreciation to the participating families who generously committed their time to the project and to Dr. Hunter Fraser (Stanford University), who provided important statistical assistance in the interpretation of data.
Contributor Information
Marilyn J. Essex, University of Wisconsin School of Medicine and Public Health
W. Thomas Boyce, University of British Columbia.
Clyde Hertzman, University of British Columbia.
Lucia L. Lam, University of British Columbia
Jeffrey M. Armstrong, University of Wisconsin School of Medicine and Public Health
Sarah M.A. Neumann, University of British Columbia
Michael S. Kobor, University of British Columbia
Bibliography
- Abidin RR. Parenting Stress Index. 2nd ed. Charlottesville, VA: Pediatric Psychology Press; 1986. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The fetal and infant origins of adult disease. Bmj. 1990;301(6761):1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett RC, Marshall NL. Preliminary Manual for the Role-Quality Scales: Wellesley College Center for Research on Women. 1989 [Google Scholar]
- Bayer JK, Hiscock H, Ukoumunne OC, Price A, Wake M. Early childhood aetiology of mental health problems: a longitudinal population-based study. J Child Psychol Psychiatry. 2008;49(11):1166–1174. doi: 10.1111/j.1469-7610.2008.01943.x. [DOI] [PubMed] [Google Scholar]
- Bezirganian S, Cohen P. Sex differences in the interaction between temperament and parenting. J Am Acad Child Adolesc Psychiatry. 1992;31(5):790–801. doi: 10.1097/00004583-199209000-00004. [DOI] [PubMed] [Google Scholar]
- Block JH. Berkeley, CA: University of California, Institute of Human Development; 1965. The Child-Rearing Practices Report (CRPR) [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on bias and variance. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, editor. Handbook of Parenting: Biology and Ecology of Parenting. Vol. 2. Mahwah, NJ: Erlbaum; 2002. [Google Scholar]
- Bowlby J. Attachment and Loss. London: Hogarth; 1969. [Google Scholar]
- Boyce WT, Essex MJ, Alkon A, Goldsmith HH, Kraemer HC, Kupfer D. Early father involvement moderates biobehavioral susceptibility to mental health problems in middle childhood. J Am Acad Child Adolesc Psychiatry. 2005;45(12):1510–1520. doi: 10.1097/01.chi.0000237706.50884.8b. [DOI] [PubMed] [Google Scholar]
- Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180(5):462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U, Morris PA. The bioecological model of human development. In: Lerner RM, Damon W, editors. Handbook of Child Psychology (6th ed ed., Vol. Vol. 1: Theoretical Models of Human Development. Hoboken, N.J.: John Wiley and Sons, Inc.; 2006. pp. 793–828. [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Champagne F, Mashoodh R. Genes in context: Geneñenvironment interplay and the origins of individual differences in behavior. Current Directions in Psychological Science. 2009;18(3):127–131. [Google Scholar]
- Cole SW. Social regulation of human gene expression. Curr Dir Psychol Sci. 2009;18(3):132–137. doi: 10.1111/j.1467-8721.2009.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings EM, Davies PT. Effects of marital conflict on children: recent advances and emerging themes in process-oriented research. J Child Psychol Psychiatry. 2002;43(1):31–63. doi: 10.1111/1469-7610.00003. [DOI] [PubMed] [Google Scholar]
- Curley J, Jensen C, Mashoodh R, Champagne F. Social influences on neurobiology and behavior: Epigenetic effects during development. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ. Timing of pubertal maturation in girls: an integrated life history approach. Psychol Bull. 2004;130(6):920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II.Empirical explorations of an evolutionary-developmental theory. Dev Psychopathol. 2005;17(2):303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, McFadyen-Ketchum S, Dodge KA, Pettit GS, Bates JE. Quality of early family relationships and individual differences in the timing of pubertal maturation in girls: A longitudinal test of an evolutionary model. Journal of Personality & Social Psychology. 1999;77(2):387–401. doi: 10.1037//0022-3514.77.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biol Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kraemer HC. Exposure to maternal depression and marital conflict: Gender differences in children's later mental health symptoms. J Am Acad Child Adol Psychiatry. 2003;42(6):728–737. doi: 10.1097/01.CHI.0000046849.56865.1D. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Miech R, Smider NA. Timing of initial exposure to maternal major depression and children's mental health symptoms in kindergarten. Brit J Psychiatry. 2001;179:151–156. doi: 10.1192/bjp.179.2.151. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Kraemer HC, Armstrong JM, Boyce WT, Goldsmith HH, Klein MH, et al. Exploring risk factors for the emergence of children's mental health problems. Arch Gen Psychiatry. 2006;63(11):1246–1256. doi: 10.1001/archpsyc.63.11.1246. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology. 2007;43(2):341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fox SE, Levitt P, Nelson CA., 3rd How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81(1):28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SF, Epel D. Ecological Developmental Biology: Integrating Epigenetics, Medicine, and Evolution. Sunderland, MA: Sinauer Associates; 2009. How agents in the environment effect molecular changes in development; pp. 37–78. [Google Scholar]
- Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009;5(7):401–408. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychol Rev. 1999;106(3):458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Nelson CA. Event-related potentials in year-old infants: relations with emotionality and cortisol. Child Dev. 1994;65(1):80–94. [PubMed] [Google Scholar]
- Halberstadt AG. Family socialization of emotional expression and non-verbal communication style and skills. J Pers Soc Psychology. 1986;51:827–836. [Google Scholar]
- Halligan SL, Murray L, Martins C, Cooper PJ. Maternal depression and psychiatric outcomes in adolescent offspring: a 13-year longitudinal study. J Affect Disord. 2007;97(1–3):145–154. doi: 10.1016/j.jad.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Harkonmaki K, Korkeila K, Vahtera J, Kivimaki M, Suominen S, Sillanmaki L, et al. Childhood adversities as a predictor of disability retirement. J Epidemiol Community Health. 2007;61(6):479–484. doi: 10.1136/jech.2006.052670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105(44):17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Ann N Y Acad Sci. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Boyce WT. How experience gets under the skin to create gradients in developmental health. Annu Rev Public Health. 2010;31:329–347. doi: 10.1146/annurev.publhealth.012809.103538. 323p following 347. [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Feil R, Constancia M, Fraga M, Junien C, Carel JC, et al. Child health, developmental plasticity, and epigenetic programming. Endocr Rev. 2010;32(2):159–224. doi: 10.1210/er.2009-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Klein MH, Essex MJ, Clark R. Maternity leave and women's mental health. Psychol Women Quarterly. 1995;19:257–285. [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8(4):253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller PS, Cummings EM, Peterson KM, Davies PT. Marital Conflict in the Context of Parental Depressive Symptoms: Implications for the Development of Children's Adjustment Problems. Soc Dev. 2009;18(3):536–555. doi: 10.1111/j.1467-9507.2008.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally EL, Feinberg C, Kim D, Ferguson K, Leibel R, Coplan JD, et al. DNA methylation as a risk factor in the effects of early life stress. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishiyama MM, Boyce WT, Jimenez AM, Perry LM, Knight RT. Socioeconomic disparities affect prefrontal function in children. J Cogn Neurosci. 2009;21(6):1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Heckman JJ, Cameron JL, Shonkoff JP. Economic, neurobiological, and behavioral perspectives on building America's future workforce. Proc Natl Acad Sci U S A. 2006;103(27):10155–10162. doi: 10.1073/pnas.0600888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer DA. Commentary: Gene-environment interplay in the context of genetics, epigenetics, and gene expression. J Am Acad Child Adolesc Psychiatry. 2005;44(1):19–27. doi: 10.1097/01.chi.0000145804.30112.6b. [DOI] [PubMed] [Google Scholar]
- Lamb ME. The Role of the Father in Child Development. 3rd ed. New York: Wiley; 1997. [Google Scholar]
- Landman-Peeters KM, Ormel J, Van Sonderen EL, Den Boer JA, Minderaa RB, Hartman CA. Risk of emotional disorder in offspring of depressed parents: gender differences in the effect of a second emotionally affected parent. Depress Anxiety. 2008;25(8):653–660. doi: 10.1002/da.20350. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Martin A, Ryan RM, Brooks-Gunn J. When fathers' supportiveness matters most: maternal and paternal parenting and children's school readiness. J Fam Psychol. 2010;24(2):145–155. doi: 10.1037/a0018073. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57(Suppl 2):S11–S15. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, Huang TC, Unterberger A, Suderman M, Ernst C, et al. Promoter-wide hypermethylation of the ribosomal RNA gene promoter in the suicide brain. PLoS ONE. 2008;3(5):e2085. doi: 10.1371/journal.pone.0002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Dev. 2010;81(1):41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7(2):103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF. Epigenetics and the nervous system. Annals of neurology. 2008;64(6):602–617. doi: 10.1002/ana.21595. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell J, Murray L. Parenting and the development of conduct disorder and hyperactive symptoms in childhood: a prospective longitudinal study from 2 months to 8 years. J Child Psychol Psychiatry. 2003;44(4):489–508. doi: 10.1111/1469-7610.t01-1-00139. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress response. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Plagge A, Isles AR, Gordon E, Humby T, Dean W, Gritsch S, et al. Imprinted Nesp55 influences behavioral reactivity to novel environments. Mol Cell Biol. 2005;25(8):3019–3026. doi: 10.1128/MCB.25.8.3019-3026.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ramchandani PG, Stein A, O'Connor TG, Heron J, Murray L, Evans J. Depression in men in the postnatal period and later child psychopathology: a population cohort study. J Am Acad Child Adolesc Psychiatry. 2008;47(4):390–398. doi: 10.1097/CHI.0b013e31816429c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopnarine JL, Fouts HN, Lamb ME, Lewis-Elligan TY. Mothers' and fathers' behaviors toward their 3- to 4-month-old infants in lower, middle, and upper socioeconomic African American families. Developmental Psychology. 2005;41(5):723–732. doi: 10.1037/0012-1649.41.5.723. [DOI] [PubMed] [Google Scholar]
- Roubinov DS, Luecken LJ. Father bonding and blood pressure in young adults from intact and divorced families. J Psychosom Res. 2010;69(2):161–168. doi: 10.1016/j.jpsychores.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Rutter M. Protective factors in children's responses to stress and disadvantage. In: Kent MW, Rolf JE, editors. Primary Prevention of Psychopathology. Vol. III. Hanover, New Hampshire: University Press of New England; 1979. [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry. 2006;47(3–4):226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ. Environmental risk factors in infancy. Pediatrics. 1998;102:1287–1292. [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, Molecular Biology, and the Childhood Roots of Health Disparities: Building a New Framework for Health Promotion and Disease Prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Siintola E, Topcu M, Aula N, Lohi H, Minassian BA, Paterson AD, et al. The novel neuronal ceroid lipofuscinosis gene MFSD8 encodes a putative lysosomal transporter. Am J Hum Genet. 2007;81(1):136–146. doi: 10.1086/518902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anger Expression Inventory (STAXI) Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Sroufe LA. Attachment and development: a prospective, longitudinal study from birth to adulthood. Attach Hum Dev. 2005;7(4):349–367. doi: 10.1080/14616730500365928. [DOI] [PubMed] [Google Scholar]
- Storey JD. False discovery rates. In: Lovric M, editor. International Encyclopedia of Statistical Science. 1st ed. Springer: 2011. [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48(3–4):262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M, McGowan P, Meaney MJ. The social environment and the epigenome. Environ Mol Mutagen. 2008;49(1):46–60. doi: 10.1002/em.20357. [DOI] [PubMed] [Google Scholar]
- Talens RP, Boomsma DI, Tobi EW, Kremer D, Jukema JW, Willemsen G, et al. Variation, patterns, and temporal stability of DNA methylation: considerations for epigenetic epidemiology. FASEB J. 2010;24(9):3135–3144. doi: 10.1096/fj.09-150490. [DOI] [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48(3–4):245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MB, Ferris JS, Pilsner R, Flom JD, Tehranifar P, Santella RM, et al. Genomic DNA methylation among women in a multiethnic New York City birth cohort. Cancer Epidemiol Biomarkers Prev. 2008;17(9):2306–2310. doi: 10.1158/1055-9965.EPI-08-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Hypponen E, Power C. Obesity and type 2 diabetes risk in midadult life: the role of childhood adversity. Pediatrics. 2008;121(5):e1240–e1249. doi: 10.1542/peds.2007-2403. [DOI] [PubMed] [Google Scholar]
- Tibshirani R, Chu G, Hastie T, Narasimhan B. samr: SAM: Significance Analysis of Microarrays. R package version 1.28. 2010 from http://CRAN.R-project.org/package=samr. [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proceedings of the National Academy of Sciences. 2001;98(9):5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn MH, Caspers K, Bakermans-Kranenburg MJ, Beach SR, Philibert R. Methylation matters: interaction between methylation density and serotonin transporter genotype predicts unresolved loss or trauma. Biol Psychiatry. 2010;68(5):405–407. doi: 10.1016/j.biopsych.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23(15):5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Diorio J, Seckl JR, Szyf M, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann N Y Acad Sci. 2004;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.