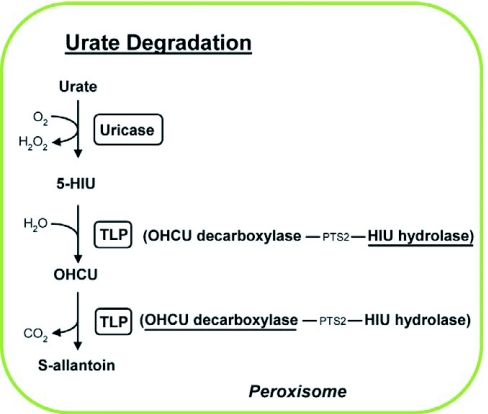
Abstract
Peroxisomes are small and single membrane-delimited organelles that execute numerous metabolic reactions and have pivotal roles in plant growth and development. In recent years, forward and reverse genetic studies along with biochemical and cell biological analyses in Arabidopsis have enabled researchers to identify many peroxisome proteins and elucidate their functions. This review focuses on the advances in our understanding of peroxisome biogenesis and metabolism, and further explores the contribution of large-scale analysis, such as in sillco predictions and proteomics, in augmenting our knowledge of peroxisome function In Arabidopsis.
1. INTRODUCTION: A HISTORICAL OVERVIEW
Following their first discovery in mouse kidney through microscopy, peroxisomes underwent extensive studies by Christian de Duve and Pierre Baudhuin. These researchers used rat liver cells to provide biochemical evidence for the presence of hydrogen peroxide (H2O2)-producing and -degrading activities in these sub-cellular structures, which led to their being named peroxisomes (De Duve and Baudhuin, 1966). Peroxisomes are small eukaryotic organelles surrounded by a single membrane and specialized in oxidative metabolic reactions. They are devoid of nucleic acids and ribosomes and import their complement of proteins post-translationally from the cytosol. The physiological significance of peroxisomes is underscored by the occurrence of devastating human genetic diseases such as the Zellweger syndrome, neonatal adrenoleukodystrophy, and infantile refsum disease, which result from defects in peroxisome function or formation. These diseases are accompanied by severe symptoms characterized by mental retardation, abnormalities in neurological, hepatic, and renal functions, and ultimately prove to be fatal (Schrader and Fahimi, 2008).
Plant peroxisomes are believed to be plastic in physiological functions, modulating their enzymatic constituents depending on the cell type, plant organ, developmental stage, and prevailing environmental conditions (Johnson and Olsen, 2001; Hayashi and Nishimura, 2006). Until recently, plant peroxisomes had been classified as (1) glyoxysomes in germinating seedlings, (2) gerontosomes in senescing tissue, (3) leaf peroxisomes, and (4) unspecialized peroxisomes (Johnson and Olsen, 2001; Kamada et al., 2003; Hayashi and Nishimura, 2006). However, it was suggested that the differences among these subtypes are subtle and that the term “peroxisome” should be applied to all the peroxisomal variants in plants to avoid confusions (Pracharoenwattana and Smith, 2008). Plant peroxisomes are multi faceted and perform a plethora of functions including lipid metabolism, photorespiration, nitrogen metabolism, detoxification, and synthesis of some plant hormones (Beevers, 1979; Olsen and Harada, 1995; Hayashi and Nishimura, 2003). Recent findings also ascribe roles for peroxisomes in photomorphogenesis and plant-pathogen interactions (Hu et al., 2002; Lipka et al., 2005; McCartney et al., 2005; Bednarek et al., 2009). Peroxisome functions are essential for embryogenesis and subsequent seedling germination, resulting in lethal plant phenotypes when absent (Hu et al., 2002; Rylott et al., 2003; Schumann et al., 2003; Sparkes et al., 2003; Fan et al., 2005). Numerous metabolic processes in the peroxisome are dependent on the cooperative exchange of metabolites with other subcellular compartments, most notably plastids and mitochondria, as evidenced by the intimate physical association of peroxisomes with these organelles (Fig. 1). Taken together, plant peroxisomes make invaluable contributions toward many metabolic processes that have potential ramifications in agriculture and economics.
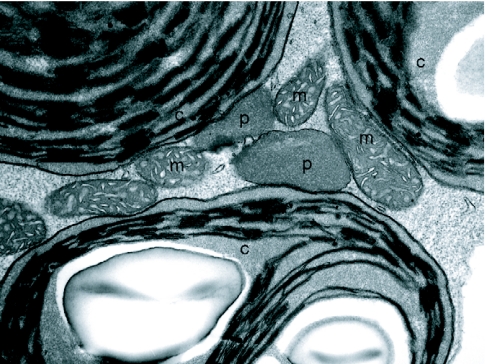
Figure 1.
Transmission electron micrograph of organelles from a green cotyledon cell in a 7d wild-type Arabidopsis seedling.
The association of peroxisomes (p) with chloroplasts (c) and mitochondria (m) are shown.
Early studies on plant peroxisomes were initiated in the laboratories of Harry Beevers and Edward Tolbert, using castor bean endosperm and spinach leaves, respectively. It was discovered that glyoxylate cycle enzymes are localized in peroxisomes (hence the name glyoxysomes) and responsible for the spurt of gluconeogenesis during germination of oil seeds, and that peroxisomes are the exclusive site for fatty acid β-oxidation in plants. These discoveries consequently led to the use of a variety of “fatty seeds”, such as pumpkin, cucumber, sunflower and castor bean, in subsequent investigations of peroxisomes (Beevers, 1993).
The first Arabidopsis peroxisome mutant, sat (serine:glyoxylate aminotransferase), was isolated in a pioneering screen for photorespiratory mutants, which presaged the use of Arabidopsis in genetic screens and established the species as a model plant (Somerville and Ogren, 1980). Its amenability to molecular genetic manipulations and the availability of the complete genome sequence in recent years have since paved the way for Arabidopsis to become the favored organism for plant peroxisome research. More recently, various groups have successfully employed innovative genetic screens to identify peroxisomal proteins and characterize their functions. One peroxisome-specific screen was based on the premise that, since proto-auxins are activated by β-oxidation, mutants that survive in higher concentrations of the synthetic auxin 2,4-dichlorophenoxybutyric acid (2,4-DB) and exhibit elongated roots should have defects in peroxisomal β-oxidation. This hypothesis was ratified by the subsequent isolation of the ped1 mutant defective in the gene encoding 3-ketoacyl CoA thiolase, a key enzyme in the β-oxidation pathway, along with other peroxisomal proteins that indirectly participate in 2,4-DB metabolism, such as those involved in protein import and fatty acid transport (Hayashi et al., 1998; Hayashi et al., 2000; Hayashi et al., 2002). A similar screen was performed using indole-3-butyric acid (IBA, a precursor of the biologically active auxin, IAA) and identified several classes of mutants, some of which have since been demonstrated to be mutated in genes encoding peroxisome biogenesis factors and enzymes in IBA metabolism (Zolman et al., 2000; Zolman et al., 2001 b; Zolman et al., 2001 a; Zolman and Bartel, 2004; Zolman et al., 2005; Zolman et al., 2007; Zolman et al., 2008). Further, mutants compromised in peroxisome division or matrix protein import have also been identified from screens scoring for abnormal morphology of fluorescently tagged peroxisomes or mis-targeting of peroxisomal marker proteins (Mano et al., 2004; Mano et al., 2006; Zhang and Hu, 2009).
Complete sequencing of the Arabidopsis genome has additionally facilitated the identification of numerous peroxisomal proteins through homology-based cloning of plant homologs of known yeast and mammalian peroxisomal proteins. Of late, in silico and proteomic analyses have expedited the discovery of novel Arabidopsis peroxisome proteins hitherto unassociated with these organelles.
2. PEROXISOME BIOGENESIS
2.1. Peroxisome Biogenesis: The ER Connection
Peroxisome biogenesis encompasses the processes of peroxisome membrane formation, import of matrix proteins, and proliferation and inheritance of the organelle (Eckert and Erdmann, 2003). Proteins responsible for peroxisome biogenesis have been designated peroxins, with PEX representing the gene acronym (Distel et al., 1996). Here, Arabidopsis peroxins are represented as AtPEX to avoid confusion with yeast and mammalian peroxins.
One contentious issue in peroxisome biology has been the de novo formation of peroxisomes in the cell, a subject that has been studied most comprehensively in fungi. While the ER is unambiguously credited with supplying the requisite membrane lipids for peroxisomes, there has been no consensus on whether peroxisomes originate de novo from the ER or are autonomously formed within the cell. Three peroxisome membrane proteins (PMPs)—PEX3, PEX16 and PEX19—are vital for early events in peroxisome membrane assembly and maintenance in many organisms; their absence results in complete loss of detectable peroxisomes (South and Gould, 1999; Hettema et al., 2000; Heiland and Erdmann, 2005). Reintroducing the wild-type proteins into their respective mutant cells can restore peroxisome formation, thus favoring the theory for de novo origin of peroxisomes (Matsuzono et al., 1999; South and Gould, 1999; Muntau et al., 2000; Titorenko and Rachubinski, 2001; Faber et al., 2002; Geuze et al., 2003; Hoepfner et al., 2005; Kragt et al., 2005; Haan et al., 2006; Kim et al., 2006). The observations that some membrane proteins initially localize in the ER gave impetus to the arguments that the ER is the source for de novo peroxisome formation (Hoepfner et al., 2005; Kragt et al., 2005; Tam et al., 2005; Haan et al., 2006; Kim et al., 2006; Mullen and Trelease, 2006; Motley and Hettema, 2007). Recently, research in yeast demonstrated convincingly that although peroxisomes are capable of forming de novo from the ER in cells deprived of peroxisomes, they routinely multiply by growth and division from pre-existing peroxisomes (Motley and Hettema, 2007). Yeasts are ideal systems to study peroxisome formation, because peroxisomes are dispensable for yeast cells and only become essential under certain nutritional conditions (van der Klei and Veenhuis, 2006). However, peroxisomes are essential to plants and viable plant mutants lacking peroxisomes are unavailable, thus making it challenging to address the question of ER contribution to the de novo formation of plant peroxisomes.
PEX3, PEX16, PEX19—three PMPs implicated in early events of peroxisome biogenesis, have Arabidopsis homologs, with At-PEX3 and AtPEX19 each being encoded by two genes. However, it is not known if these PMPs have a role in the ER origin of plant peroxisomes. Depending on the sorting pathway utilized, Arabidopsis PMPs have been categorized into two groups. Group I PMPs are inserted post-translationally into the ER membrane after being synthesized on free cytosolic ribosomes, and then traffic to the peroxisome via specific ER vesicles, whereas group II PMPs sort to peroxisomes directly from the cytosol (Mullen and Trelease, 2006). AtPEX3 and AtPEX19 appear to be Group II PMPs (Hunt and Trelease, 2004; Hadden et al., 2006), whereas AtPEX16 and AtPEX10 belong to Group I because of their dual ER-peroxisome steady-state localizations (Flynn et al., 2005; Karnik and Trelease, 2005, 2007).
Studies on the peroxisome membrane-bound enzyme ascorbate peroxidase (APX) have shed more light on the relationship between ER and peroxisomes in plants. Cottonseed APX expressed in tobacco BY-2 cells localizes to peroxisomes and network structures resembling the ER; treatment of the BY-2 cells with Brefeldin A (BFA), a fungal metabolite that reversibly blocks transport from the ER, led to the ER retention of this protein (Mullen et al., 1999). Subcellular fractionation and immunogold localization studies in Arabidopsis cell cultures further confirmed APX3 to target to a specific subdomain of the ER, designated peroxisomal ER (pER), prompting the hypothesis that APX3 marks the sites for possible export of PMPs in pER vesicles destined for peroxisomes (Lisenbee et al., 2003). More recent studies on AtPEX16 found that trafficking from the ER to peroxisomes is routed through a non-peroxisomal, non-Golgi structure named ER-peroxisome intermediate compartment (ERPIC). BFA or cold treatment (15° C), which disrupts egress of ER proteins, results in accumulation of AtPEX16 in the ER, whereas removal of the blockage leads to resumption of AtPEX16 trafficking to peroxisomes through the ERPIC (Karnik and Trelease, 2007). These data collectively spawned the ER semi-autonomous peroxisome maturation and replication model. Because peroxisome multiplication is dealt with later in this review, only the ER semi-autonomous peroxisome maturation aspect is discussed here. Briefly, the model proposes that Group I PMPs, after being translated in the cytosol, are sorted to the ER and later exit the ER through the blebbing of ER to a nascent vesicle, which matures into an intermediate sorting compartment, the ERPIC. Presumably, the ERPIC fuses with pre-existing peroxisomes and delivers the PMPs and membrane lipids to the organelle. The model also depicts possible targeting of Group II PMPs and matrix proteins to the ERPIC prior to fusion of the ERPIC vesicle with pre-existing peroxisomes. Thus, according to this model, the growth and maturation of pre-existing plant peroxisomes rely on the ER as a primary source for membrane lipids and a subgroup of PMPs (Mullen and Trelease, 2006).
An interesting caveat to this model is the existence of a retrograde, peroxisome-pER pathway in plant cells infected by the positive strand RNA virus, tomato bushy stunt virus (McCartney et al., 2005; Mullen and Trelease, 2006). The virus co-opts the host peroxisomes by inducing proliferative vesiculation of the peroxisome to facilitate the replication and pervasive spreading of the virus within the host cells (Appiano et al., 1983; Appiano et al., 1986; Scholthof et al., 1995). A viral protein, P33, was implicated in causing peroxisome aggregation and, along with ADP-ribosylation factorl (ARF1), promoting reverse trafficking of host cell PMPs to the pER. This process is reminiscent of the COPI-dependent, vesicle-mediated retrieval of escaped ER membrane proteins from the Golgi (McCartney et al., 2005). Although direct supporting evidence has not been procured for uninfected cells, it is possible that normal cells also adopt this mechanism to retrieve escaped/mistargeted ER resident proteins (Mullen and Trelease, 2006).
2.2. Peroxisome Protein Import
2.2.1. Matrix protein import
2.2.1.1. Peroxisomal Targeting Signals (PTSs) and receptors for matrix proteins.
The elucidation of peroxisome protein import pathways in yeast has been of paramount importance in guiding the study of these pathways in Arabidopsis. Peroxisomal proteins are nuclear encoded and translated in the cytosol by polyribosomes before being imported into the organelle. The targeting of peroxisomal matrix proteins is determined by the presence of peroxisome targeting signals (PTSs) on these proteins. PTS1, possessed by a majority of known peroxisomal matrix proteins, is a C-terminal conserved tripeptide with SKL as the canonical sequence. PTS2 is a nonapeptide with RLx5HL as the prototype sequence and typically located within 30 amino acids of the N terminus. Arabidopsis catalase and sarcosine oxidase are the only characterized peroxisomal matrix proteins lacking recognizable PTSs. Readers are referred to section 4.3 of this chapter for more detailed information regarding peroxisome targeting signals.
PEX5 and PEX7 are receptors for matrix proteins containing PTS1 and PTS2, respectively, whereby these receptors bind to the cargo synthesized in the cytosol and escort the proteins to peroxisome membranes. PEX5 contains a tetratricopeptide repeat (TPR) domain, which interacts with the PTS1 peptide, whereas PEX7 is a WD40 repeat protein that binds to both PTS2 and PEX5. In plants and mammals, the PTS2 protein-bound PEX7 interacts with PEX5 before the whole complex is transported to peroxisomes. PEX5 from tobacco and watermelon can complement the pex5 mutants of the yeasts Saccharomyces cerevisiae and Hansenula polymorpha, respectively (Brickner and Olsen, 1998; Kragler et al., 1998; Wimmer et al., 1998; Schumann et al., 1999; Nito et al., 2002).
Two groups independently addressed the role of PEX5 and PEX7 in Arabidopsis (Hayashi et al., 2005; Woodward and Bartel, 2005). Mutants of AtPEX5 and AtPEX7 were recovered from the aforementioned IBA resistance screen and display characteristic peroxisome defective phenotypes, including resistance to root growth inhibition by IBA and reduction in the import rate of PTS2 proteins. The pex5 mutant isolated from the IBA reistance screen has no defects in PTS1 protein import, which was perplexing until the point mutation was mapped to the PEX7-interaction domain on AtPEX5. pex5 pex7 double mutants show enhanced PTS2-import defects, an absolute dependence on exogenous sucrose for germination, and reduced stature and seed viability, indicating that some PTS2 proteins are required for seedling establishment, vegetative growth, and seed development (Woodward and Bartel, 2005). Gene silencing studies on AtPEX5 and AtPEX7 using RNAi also corroborated the role for AtPEX5 in mediating the import of both PTS1- and PTS2-containing proteins. It was concluded that AIPEX7 has an important role in seedling establishment by virtue of its function as the PTS2 receptor, while PEX5 additionally influences photorespiration in leaf peroxisomes (Hayashi et al., 2005).
2.2.1.2. Docking events.
In yeast, docking of the cargo-bound receptors at the peroxisomal membrane is facilitated by PEX13, PEX14 and PEX17; the interactions of PEX14 with PEX17 and the SH3 (Src Homology 3) domain of PEX13 are deemed vital for protein import (Eckert and Erdmann, 2003). Arabidopsis only contains apparent orthologs for PEX13 and PEX14. The function of PEX14 in protein import was elucidated through the genetic screen for mutants resistant to 2,4-DB, in which the pex14 mutant was isolated and found to be impaired in both PTS1 and PTS2 protein import and consequently exhibits pleiotropic defects in peroxisomal processes such as fatty acid catabolism during germination, and photorespiration, and in peroxisome morphology. These results suggest that PEX14 is required for the transport of both PTS1 and PTS2 proteins in plants (Hayashi et al., 2000). Further corroboration of the role for PEX14 in import came from a study showing that Arabidopsis PEX14 and PEX5 interact in a yeast two-hybrid system (Nito et al., 2002). Because PEX5 also binds to PEX7, these proteins might assemble into a ternary complex at the peroxisome membranes in planta. The pex13 mutants identified from a GFP-PTS1-based peroxisome morphology screen and RNAi lines of AtPEX13 are defective in both PTS1 and PTS2 import and accumulate PEX5 on the peroxisomal membranes, indicating a role for AtPEX13 in protein import in Arabidopsis (Mano et al., 2006; Nito et al., 2007). Furthermore, the characterization of another AtPEX13 mutant allele, amc (abstinence by mutual consent), showed that peroxisome function is critical for male-female gametophyte recognition and that AMC/PEX13 is essential for protein import into gametophytic peroxisomes (Boisson-Dernier et al., 2008). These results thus reinforce the idea that in Arabidopsis, AtPEX14 and AtPEX13 may mediate docking of the receptor-cargo complex, although it is unknown whether the two proteins also interact at the peroxisome membrane during the docking process.
2.2.1.3. Post-docking events.
Events taking place after docking are not very well understood. Current knowledge derived cumulatively from yeast and mammalian systems suggests that the RING domain-containing peroxins (PEX2, PEX10, PEX12) facilitate translocation of the receptor cargo complex (Platta and Erdmann, 2007). Although several hypotheses, including the simple shuttle, extended shuttle, and transient pore theory, have been postulated for the receptor-cargo translocation, none have been proved conclusively. The shuttle hypotheses revolve around the tenet that either part (simple shuttle) or the entirety (extended shuttle) of the receptor-cargo complex reaches into the peroxisomal lumen, whereas proponents of the transient pore model champion the cause of a dynamic pore formed by the receptor itself (Dammai and Subramani, 2001; Kunau, 2001; Erdmann and Schliebs, 2005). At some point post translocation, the cargo proteins are released into the peroxisome matrix by an unknown mechanism and the receptors are returned to the cytosol. In yeast, the release of cargo might be performed by a bridging protein, PEX8, and receptor recycling and dislocation require monoubiquitination of the receptors by the putative ubiquitin-conjugating enzyme PEX4 and the AAA ATPases (AAA+ adenosine triphosphatases) PEX1 and PEX6 (Platta et al., 2004; Kiel et al., 2005b; Kragt et al., 2005; Platta et al., 2007). Recently, mammalian PEX5 was shown to be ubiquitinated, but the protein responsible remains mysterious because PEX4 homologs have not been detected in mammals (Carvalho et al., 2007). It has also been demonstrated that as a quality control response, PEX5 is polyubiquitinated upon recycling, and that yeast mutants deficient for PEX5-recycling exhibit a higher rate of PEX5 protein degradation (Platta et al., 2004; Kiel et al., 2005a).
Homologs of the three RING domain peroxins have been characterized in Arabidopsis. Null mutants for all three are embryo lethal, indicating their obligatory requirement for plant development (Hu et al., 2002; Schumann et al., 2003; Sparkes et al., 2003; Fan et al., 2005). Further, RNAi lines of all three RING peroxins exhibit defects in β-oxidation and peroxisomal protein import (Fan et al., 2005; Nito et al., 2007). Other approaches to probe the physiological functions of these genes revealed new phenotypes that implicate the execution of plant-specific functions by these peroxins. For instance, a photomorphogenic phenotype was found for the AtPEX2 dominant mutant, ted3 (reverse of de-etiolation). The Arabidopsis DET/COP/FUS proteins repress photomorphogenesis in the absence of light; their loss-of-function mutants are de-etiolated even in the dark. The dominant ted3 mutation partially suppresses the mutant phenotypes of both det1 and cop 1 mutants, indicating a novel function for PEX2/peroxisomes in light signaling (Hu et al., 2002). In addition, pleiotropic phenotypes were determined for Atpex10 mutants, whereby silencing the gene results in variegated leaves and reduced root cell size (Nito et al., 2007) and overexpressing a dysfunctional RING finger domain of PEX10 leads to multilobed and clustered peroxisomes with diminished association with chloroplasts (Schumann et al., 2007). These studies together reiterate and accentuate the non-redundant contributions by each of the RING peroxins in Arabidopsis.
The genetic screen for IBA-resistant mutants identifed a pex4 allele, which displays sucrose-dependent seedling development and reduced lateral root production. A subsequent yeast twohybrid screen using AIPEX4 as bait retrieved AIPEX22, whose putative ortholog in yeast is implicated in tethering PEX4 to the peroxisome. Despite the weak sequence similarity, AIPEX22 contains structural and topological similarities with the yeast PEX22, suggesting that the identified protein is a bona fide PEX22 ortholog. Generation of the pex4 pex22 double mutant compounded defects exhibited by pex4, strengthening the notion that AIPEX22 is a genuine peroxin. Isocitrate lyase (ICL), a glyoxysomal enzyme transiently expressed during germination in wild-type plants, is persistently present in the double mutant, prompting the hypothesis that AtPEX4-mediated ubiquitination could potentiate the degradation or retrotranslocation of peroxisome matrix proteins (Zolman et al., 2005). RNAi lines of AtPEX4 display sucrose dependence in germination, 2,4-DB resistance in root elongation, and defects in peroxisomal localization of PTS1 and PTS2 proteins (Nito et al., 2007). Further investigation of AtPEX4 might be instrumental in identifying other possible ubiquitination targets and thus clarifying the function this protein imparts in Arabidopsis.
Two AAA ATPases, PEX1 and PEX6, comprise part of the peroxisome protein import machinery and are believed to play important roles in the retrotranslocation of the receptor proteins to the cytosol, a process driven by ATP hydrolysis (Thoms and Erdmann, 2006). An Atpex6 mutant isolated from the IBA-resistance screen has abnormal peroxisome number and morphology and significantly reduced levels of AtPEX5, supporting the role for AtPEX6 in receptor recycling (Zolman and Bartel, 2004). RNAi plants of AtPEX1 have β-oxidation malfunction and mis-distribution of peroxisome matrix proteins in the cytosol, suggesting that AtPEX1 plays a role in matrix protein import (Nito et al., 2007). One might expect that AtPEX1, similar to its yeast orthologs, also performs dislocation functions in plant peroxisomes.
A recent study points to roles for AIPEX4, AIPEX22, AIPEX6 and AtPEX5 in the degradation of damaged or obsolete peroxisome matrix proteins (Lingard et al., 2009). During the transition of seedling peroxisomes to leaf peroxisomes, mutants of these peroxins have stabilized glyoxylate cycle enzymes, i.e., ICL and MLS (malate synthase). In addition, elevated or diminished levels of peroxisome-generated H2O2 expedite or impede the degradation of ICL and MLS. Collectively, the study suggests that these four Arabidopsis peroxins have novel roles in the removal of peroxisome matrix proteins, a process termed PexAD (Peroxisome- associated protein degradation).
2.2.2. Membrane protein import.
Group I and Group II PMPs show distinct targeting sequences, known as membrane PTSs (mPTSs). Group II PMPs employ mPTS1, a stretch of positively charged residues in proximity to a transmembrane domain (TMD), whereas Group I PMPs use mPTS2, which bears an additional ER sorting signal that either overlaps or is adjacent to the mPTS1 (Mullen and Trelease, 2006; Van Ael and Fransen, 2006). In yeasts and humans, PEX19, a versatile peroxin with multiple binding domains that bind to a number of PMPs, acts as a cytosolic receptor for PMPs. It is believed that PEX19 binds to nascent PMPs, stabilizes them, and transports them to the peroxisome membrane, where it anchors by binding to its membrane-bound receptor, PEX3 (Fang et al., 2004; Shibata et al., 2004; Fujiki et al., 2006). It is unknown what triggers the subsequent insertion of PMPs into the peroxisome membrane. Additionally, PEX19-independent pathways for PMP targeting are present in mammals and hinted at for yeasts (Fransen et al., 2004; Otzen et al., 2004). Arabidopsis has two PEX19 isoforms, AtPEX19-1 and AtPEX19-2, with AtPEX19-1 existing as dimeric species in vivo and binding to AtPEX10 in pulldown assays (Hadden et al., 2006). RNAi lines of AtPEX19-1 or AtPEX19-2 contain enlarged peroxisomes, suggesting non-redundant functions of the two proteins. Knocking down either of the two AtPEX3 isoforms had no effect, whereas double knockdown AtPEX3 plants display peroxisome elongation. The phenotypes of these RNAi lines led to the conclusion that AtPEX3 and AtPEX19 influence peroxisome morphology but do not contribute to peroxisome protein import (Nito et al., 2007). Mechanisms by which plant peroxisomes recruit PMPs remain to be elucidated.
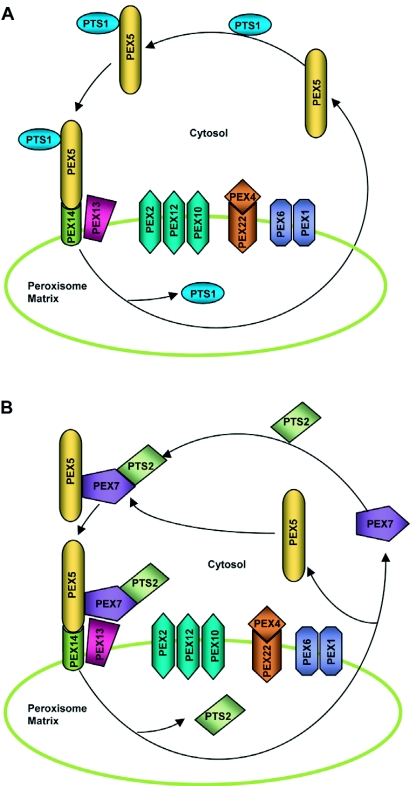
Fig. 2 depicts current models for PTS1 and PTS2 import pathways in plants. SupplementalTable I (92.5KB, xls) summarizes the characterized Arabidopsis PEX proteins and their mutant phenotypes. Although many facets of peroxisome protein import, especially the components involved and their impacts on plant development and physiology, have been elucidated, our knowledge of this process is still hazy. Are there other unidentified peroxins in plants? Do functional homologs of PEX17 or PEX8 exist? What is the significance of having two isoforms for PEX19 and PEX3 and have they acquired plant-specific functions? Does AtPEX4 also function in receptor recycling? Are any of the other Arabidopsis peroxins involved in PexAD? These are some of the questions that merit further investigations.
Figure 2.
Speculative model for matrix protein import.
(A) PTS1 import. PEX5 recognizes and binds PTS1-containing proteins in the cytosol. The receptor-PTS1 protein complex then traffics to the peroxisome where it associates with the docking complex on the peroxisome membrane. The docking complex in Arabidopsis probably comprises PEX14 and PEX13 and is believed to tether the receptor-protein complex to the peroxisome membrane through the interaction of PEX5 with PEX14. Subsequently, the PTS1 protein is dissociated from PEX5 and released into the peroxisomal matrix by an unknown mechanism. The export of the receptor to the cytosol might be facilitated by its possible ubiquitination (not shown) by the PEX22-anchored PEX4, a putative ubiquitin-conjugating enzyme, followed by ATP-driven dislocation mediated by the peroxisomal AAA ATPases PEX1 and PEX6. The RING complex, which is composed of the PEX2, PEX10 and PEX12 RING peroxins, plausibly plays a role in the import and export processes, although the exact function(s) of this complex are not well understood.
(B) PTS2 import. PEX7 recognizes and binds PTS2-containing proteins in the cytosol. The receptor-PTS2 protein complex binds coordinately with PEX5 and is ferried to the peroxisome docking complex. The subsequent steps of import are assumed to be similar to PTS1 import. The events facilitating the release of PEX7 and PTS2 protein are not well known.
2.2.3. Post-import process: heat shock proteins (Hsps) and endoproteinases
Most subcellular organelles host a battery of Hsps and molecular chaperones in their lumen to assist in the folding of newly imported proteins or refolding of denatured polypeptides (reviewed in (Leidhold and Voos, 2007). Peroxisomes are distinguished from other organelles by their capacity to import fully folded proteins, as indicated by the phenomena of piggybacking (Lee et al., 1997). This import of folded substrates seems to preclude the need for chaperone activity within the organelle. Although in vitro import assays have shown that cytosolic Hsp70 and Hsp90 chaperone activities are associated with and enhance peroxisomal protein import (Crookes and Olsen, 1998), little is known about protein (re-)folding within peroxisomes. Considering the high production rate of reactive oxygen species in peroxisomes, it is likely that peroxisomes have evolved a mechanism to safeguard matrix proteins from denaturation.
Although mammals and yeast do not seem to have any chaperones in their peroxisomes, two Arabidopsis small Hsps in the peroxisome matrix complemented the yeast mutant, hinting at the functional conservation of this type of Hsps as chaperones across kingdoms (Ma et al., 2006). Moreover, one of the sHsps is heat and oxidative stress inducible, further cementing the notion that peroxisomes can implement stress responses (Ma et al., 2006). Analysis of loss-of-function mutants of the sHsps will be instrumental in defining the physiological function of these proteins in greater details. Small Hsps lack ATP-hydrolyzing activity and need other Hsps for protein renaturation. Various Hsp70 isoforms and DnaJ homologues have been reported to be present in peroxisomes in other plant species (Preisig-Muller et al., 1994; Corpas and Trelease, 1997; Wimmer et al., 1997; Diefenbach and Kindl, 2000); their orthologs could conceivably be present in Arabidopsis. These proteins, in conjunction with the identified sHsps, may help to alleviate stress-induced protein aggregation and denaturation in the peroxisome matrix.
One prominent feature for PTS2 protein import is the cleavage of the N-terminal signal peptide after import. In mitochondria and chloroplasts, cleavage is mandatory for folding or further targeting to sub-structures (Adam and Clarke, 2002; Gakh et al., 2002). However, the activity of the peroxisomal protein malate dehydrogenase (MDH) was shown to be unaffected in the absence of PTS2 cleavage (Helm et al., 2007). PTS2 protein processing seems to be restricted to higher eukaryotes, as PTS2-containing proteins in yeasts do not undergo any cleavage. Arabidopsis encodes a significant number of PTS2 proteins, and in silico and proteomic analyses also indicate the presence of several proteases in Arabidopsis peroxisomes (Reumann et al., 2004; Reumann et al., 2007; Reumann et al., 2009). Recently, an Arabidopsis DEG protease (DEG15) was characterized as a serine endopeptidase and implicated in the processing of PTS2 peroxisomal proteins such as MDH, KAT2 and long-chain acyl-CoA synthetase isoform 6 (LACS6; Schuhmann et al., 2008). Studies in pea have found up to seven endoprotease activities associated with senescent peroxisomes, and these activities were speculated to be involved in turning over peroxisomal proteins during early senescence, in addition to their role in degrading proteins from multiple subcellular compartments during advanced stages of senescence (Distefano et al., 1997; Distefano et al., 1999).
An unanticipated role has been determined for the mammalian ortholog of AtDEGI5, Tysnd1, which not only cleaves PTS2 off of PTS2 proteins but also cleaves three PTS1 proteins involved in the β-oxidation pathway internally (Kurochkin et al., 2007). It was speculated that this processing facilitates the arrangement of the enzymes into multienzyme complexes, resulting in effective metabolic channeling and consequently higher enzymatic efficiencies (Wouters et al., 1998). Plant peroxisomes accommodate enzymes for various metabolic processes and are also purported to have similar multienzyme complex arrangements in their matrix (Heupel et al., 1991; Heupel and Heldt, 1994). Several prospective peroxisome proteases were identified in an Arabidopsis leaf proteomic experiment (Reumann et al., 2009). Validation and characterization of these putative peroxisomal proteases, as well as identification of processing-competent PTS1 proteins, may shed light on the precise nature of peroxisomal endoproteolytic activity and its possible contribution to efficient assembly of multienzyme complexes or other processes in the Arabidopsis peroxisome.
2.3. Peroxisome Division and Movement
2.3.1. Peroxisome division
2.3.1.1. Introduction.
In addition to arising de novo from the ER, peroxisomes are also known to multiply via division, a process encompassing several partially overlapping steps, i.e., peroxisome elongation/enlargement, membrane constriction, and fission (Hoepfner et al., 2005; Titorenko and Mullen, 2006; Fagarasanu et al., 2007; Motley and Hettema, 2007). Peroxisome division occurs constitutively and under inducible conditions, resulting in the formation of two or multiple daughter peroxisomes from a pre-existing peroxisome (Yan et al., 2005). Several core components of the peroxisome division apparatus are conserved across the fungal, animal, and plant kingdoms (Fig. 3).
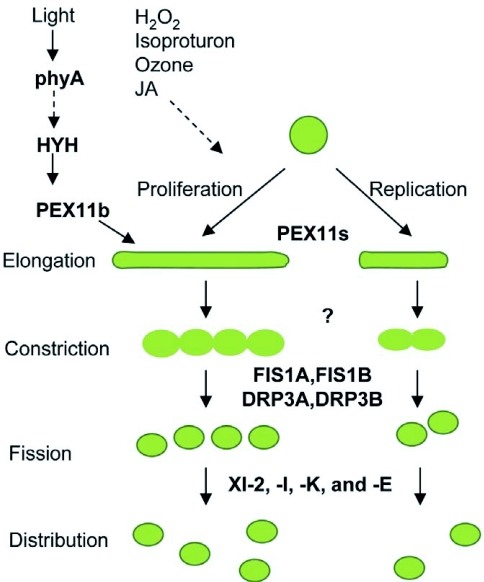
Figure 3.
Model for peroxisome proliferation and replication in Arabidopsis.
The PEX11 family of proteins (PEX11a-e) acts in the initial steps of peroxisome proliferation and replication, resulting in pronounced elongation or expansion of peroxisomes. Proteins overseeing the subsequent membrane constriction are not known. Fission of the constricted peroxisomes is enabled by the scission activities of dynamin-related proteins DRP3A and DRP3B, which are tethered to the peroxisome membrane by FIS1A and FIS1B. The divided peroxisomes are then transported to various parts of the cell by the indicated myosin proteins (XI-2, XI-I, XI-E, XI-K) along actin cables. The transcription factor HYH has been implicated in the phyA-mediated light regulation of peroxisome elongation via activation of the PEX11b gene. Hydrogen peroxide (H2O2), isoproturon, ozone and Jasmonates (JA) are other cues that induce or repress peroxisome proliferation through factors currently unknown, indicated by dotted arrow.
2.3.1.2. Early steps: the PEROXIN11 (PEX11) protein family.
Seminal work carried out with yeast peroxisome mutants has provided critical insights into the molecular players involved in peroxisome division. Factors involved in peroxisome division have been classified into four families of peroxisome membrane proteins, namely, Pex11p, Pex25p/Pex27p, Pex28p/Pex29p, and Pex30p/Pex31p/Pex32p (Rottensteiner et al., 2003; Tam et al., 2003; Vizeacoumar et al., 2003; Vizeacoumar et al., 2004). Amongst these proteins, Pex11p was the first to be identified and is believed to mediate the initial step of peroxisome division, i.e., peroxisome elongation/tubulation (Fagarasanu et al., 2007). The S. cerevisiae Pex11p protein was shown to be a peripheral membrane protein located in the inner surface of the peroxisome membrane. The pex11 mutant embodies prototypical division mutant phenotypes, with loss-of-function mutation resulting in peroxisomes enlarged in size and reduced in number and overexpression conferring an increase in peroxisome abundance (Erdmann and Blobel, 1995; Marshall et al., 1995). Pex11p also comprises the only PMP family exclusively involved in peroxisome division that has known counterparts in mammals and plants. Phylogenetic analysis suggested that PEX11 genes have a single origin and evolved and diversified independently in plant, fungal, and animal lineages (Orth et al., 2007). PEX11 in mammals have three isoforms (PEX11α, -β, and -γ), each exerting varying contributions to peroxisome division (Schrader et al., 1998; Li et al., 2002b; Li et al., 2002a). In S. cerevisiae, Pex11p shares weak sequence similarity with two homologous proteins, Pex25p and Pex27p; partial redundancy was found among these three proteins (Rottensteiner et al., 2003).
The Arabidopsis PEX11 family has further expanded into five isoforms grouped into three subfamilies: AtPEX11a, AtPEX11b, and AtPEX11c-e, all of which are integral membrane proteins of the peroxisome (Lingard and Trelease, 2006; Orth et al., 2007). Protein expression and topology studies using Arabidopsis suspension cell cultures confirmed that all AtPEX11s are Group II PMPs that target directly to peroxisomes without trafficking via the ER. Furthermore, AtPEX11b-e, like mammalian PEX11 homologs, have both the N- and C-terminal tails exposed to the cytosol, whereas AtPEX11a has a distinctive C terminus that protrudes into the peroxisomal matrix (Lingard and Trelease, 2006). Overexpression of individual AtPEX11 isoforms variedly promoted peroxisome elongation and/or increase in the number of peroxisomes (Lingard and Trelease, 2006; Orth et al., 2007). Decreasing the expression of individual PEX11 or a subfamily of PEX11 genes using RNAi led to reduction in the total number of peroxisomes (Orth et al., 2007) or slightly enlarged peroxisomes (Nito et al., 2007). No physiological or growth phenotypes were observed in the RNAi plants, possibly reflecting functional redundancy among isoforms (Orth et al., 2007). Further, AtPEX11e and -c partially compensated for the lack of Pex11p in the yeast mutant (Orth et al., 2007). Taken together, all these data point toward a conserved and positive role for PEX11 in regulating peroxisome proliferation across kingdoms.
Among the three mammalian PEX11 proteins, PEX11β is essential for survival, leading to embryo lethality when its function is disruped (Li et al., 2002a). In contrast, none of the AtPEX11 family members are vital for survival. Drawing from the observations that null mutants for some Arabidopsis peroxisome proteins are embryo lethal (see 2.2.1), it is possible that peroxisome proliferation is essential to plant survival and hence plants have evolved functionally redundant PEX11 isoforms that perform in specific peroxisome subtypes and respond to diverse environmental cues. Evidence supporting these notions includes specific transcriptional induction of particular isoforms (Orth et al., 2007; Desai and Hu, 2008) and distinct peroxisome morphologies caused by over-expressing different family members (Lingard and Trelease, 2006; Orth et al., 2007). Alternatively, based on the facts that (i) only a subset of the AtPEX11 proteins complemented the yeast mutant, (ii) AtPEX11a carries a distinctive membrane topology, and (iii) a C-terminal dilysine motif is restricted to the PEX11c-e subfamily in Arabidopsis, it is speculated that PEX11 proteins may perform discrete biochemical functions (Hu, 2007).
Although PEX11 has been the best-characterized protein in peroxisome division and is known to be primarily responsible for initiating the process, there are gaps in our knowledge of its precise mode of action. Functions proposed for PEX11 include membrane modification through phospholipid binding, transport of metabolites, and recruitment of downstream effector proteins (Thoms and Erdmann, 2005; Fagarasanu et al., 2007). Some PEX11 proteins, including AtPEX11c-e, contain a C-terminal dilysine motif. This motif was shown in rat to bind to coat protein 1 (COP1) and recruit (ADP)-ribosylation factor (ARF1), suggesting that PEX11 and coatamers coordinately execute peroxisome division by promoting membrane vesiculation (Passreiter et al., 1998; Anton et al., 2000). However, this motif is dispensable for promoting peroxisome division in Arabidopsis cell cultures (Lingard and Trelease, 2006), indicating that species-specific factors may be involved in facilitating the function of PEX11.
2.3.1.3. Late steps: dynamin-related proteins (DRPs) and FISSION1 (FIS1) proteins.
Although proteins that specifically control the step following peroxisome elongation, i.e., membrane constriction, are still elusive, several elements involved in the fission of peroxisomes are characterized. Dynamins and DRPs are large GTPases in eukaryotic cells that participate in intracellular vesicle trafficking, cell division, endocytosis, and organelle division. They serve as mechanochemical enzymes or signaling GTPases by self-assembling into spirals surrounding membranes and powering their fission and fusion (Osteryoung and Nunnari, 2003; Koch et al., 2004; Praefcke and McMahon, 2004; Hoppins et al., 2007). Some DRPs from yeasts and mammals have been shown to be required for peroxisome division (Wilsbach and Payne, 1993; Hoepfner et al., 2001; Koch et al., 2003; Li and Gould, 2003; Koch et al., 2004; Kuravi et al., 2006; Schrader, 2006).
The Arabidopsis DRP family is composed of 16 members classified into six subfamilies based on sequence similarity, with DRP3A and DRP3B constituting subfamily 3 (Hong et al., 2003). Numerous alleles of DRP3A, isolated as the aberrant peroxisome morphology1 (apm1) mutants from a genetic screen performed in the GFP-PTS1 background, exhibit abnormally enlarged peroxisomes with extended tails in addition to defects in mitchondrial divisions and plant growth (Mano et al., 2004). Previous investigations of mutants for both DRP3A and DRP3B found an altered mitochondrial morphology, prompting the suggestion that both proteins are required for mitochondrial division in plants (Arimura and Tsutsumi, 2002; Arimura et al., 2004; Logan et al., 2004). A recent re-examinination of the DRP3 subfamily revealed that, like DRP3A, DRP3B also targets to both peroxisomes and mitochondria and causes defects in peroxisome fission when the gene function is disrupted. The drp3A drp3B double mutants display a compounded phenotype, with highly clumped and enlarged peroxisomes that were noticeably reduced in number per cell. The defects in peroxisomal and mitochondrial division also translate into defects in plant size and pigmentation (Zhang and Hu, 2009). DRP3A and DRP3B are functionally redundant in mitochondrial division, whereas their roles in peroxisome division are somewhat disctinct. Specifically, drp3A mutants display stronger peroxisomal phenotypes than drp3B (Fujimoto et al., 2009; Zhang and Hu, 2009), and DRP3B was unable to complement the peroxisomal phenotype of drp3A (Fujimoto et al., 2009). Lastly, the not-so-strong phenotype of the drp3A drp3B double mutants indicates that additional members of the DRP family may be involved in peroxisome fission.
Another shared component of the peroxisomal and mitochondrial division machineries in diverse eukaryotic species is FIS1 (Koch et al., 2003; Koch et al., 2005; Kuravi et al., 2006; Kobayashi et al., 2007). Mammalian and yeast FIS1 proteins are C-terminal tail-anchored membrane proteins of peroxisomes and mitochondria. Mammalian FIS1 tethers DRP to the membrane by interacting with DRP via its cytoplasmic N-terminal region harboring the tetratricopeptide repeat (TPR) domain (Mozdy et al., 2000; James et al., 2003; Koch et al., 2003; Yoon et al., 2003; Stojanovski et al., 2004; Koch et al., 2005; Kuravi et al., 2006; Kobayashi et al., 2007). Arabidopsis contains two isoforms of FIS1, FIS1A and FIS1B, both of which are dual targeted to, and facilitate the division of, peroxisomes and mitochondria (Zhang and Hu, 2008, 2009). Single and double mutants of FIS1A and FIS1B display plant growth inhibition and organelle defects, with peroxisomes and mitochondria incomplete in fission, enlarged in size, and decreased in total abundance (Zhang and Hu, 2008, 2009). However, in Arabidopsis cell cultures the roles for FIS1A and FIS1B seem to differ, as cell cycle-associated replication of peroxisomes requires FIS1B but not FIS1A (Lingard et al., 2008).
Similar to results obtained from human COS-7 cells, which show that overexpressing myc-hFIS1 causes dramatic increases in peroxisomal number and mitochondrial fragmentation (Yoon et al., 2003; Koch et al., 2005), ectopic expression of FIS1A or FIS1B also significantly increases the fission of these two types of organelles in plants (Zhang and Hu, 2008, 2009). These observations reinforced the notion that FIS1 is a limiting factor for the fission step in diverse species. The targeting mechanism for FIS1 has some unique features in plants. Although the C-terminal half of FIS1 is both necessary and sufficient for targeting to peroxisomes in both mammals and plants, specific regions to which the targeting signals are restricted seem to differ in different species. For example, the C-terminal end segment adjacent to the transmembrane domain is required for peroxisome targeting in mammalian cells yet dispensable in plants (Zhang and Hu, 2008).
Studies from plants and other organisms collectively suggest that, whereas the initial steps of peroxisome division are controlled by the peroxisome-specific PEX11 proteins, late stages of peroxisome division share common regulators (e.g., DRP and FIS1) with those of the division/vesiculation of other subcellular compartments such as mitochondria (Delille et al., 2009). Despite their distinct evolutionary histories, organelle structures, and many biochemical functions, peroxisomes and mitochondria function cooperatively in some major metabolic pathways, such as fatty acid metabolism and photorespiration in plants (Beevers, 1979). Thus, it is possible that the divisions of peroxisomes and mitochondria are coordinated at some level to ensure efficient completion of these collaborative pathways.
Studies in mammalian cells showed that PEX11β forms a ternary complex with DLP1 (a DRP) and FIS1 on peroxisomal membranes, and that FIS1 anchors the DLP1 protein (Kobayashi et al., 2007), suggesting coordination between components of the peroxisome division machinery. Bimolecular fluorescence complementation (BiFC) experiments with Arabidopsis cell cultures demonstrated interaction between all five PEX11 isoforms with FIS1B (Lingard et al., 2008), supporting the existence of a similar protein complex on plant peroxisomes. More detailed genetic and biochemical analysis of PEX11, DRP3 and FIS1 in Arabidopsis should be instrumental in discerning the contribution and necessity of each component toward divison and the interplay between components.
2.3.1.4. Other peroxisomal proteins in division.
Other proteins and accessory factors involved in peroxisome division in yeasts and mammals include the yeast peroxisomal membrane proteins Pex28p, Pex29p, Pex30p, Pex31p, and Pex32p (Thoms and Erdmann, 2005). In addition, Mff, a tail-anchored membrane protein in mammalian cells, which does not have apparent orthologs in yeasts, promotes the fission of both mitochondria and peroxisomes in a FIS1-independent fashion (Gandre-Babbe and van der Bliek, 2008). Moreover, Caf4p and Mdv1p, yeast proteins functioning as molecular linkers in the mitochondrial division apparatus, also play a role in Dnmp1 (a DRP)-mediated peroxisomal fission (Motley et al., 2008). Further studies need to address whether Pex28p-32p, Mff, and Caf4p/Mdv1p have cognate orthologs in plants. Analysis of additional Arabidopsis mutants isolated from fluorescent protein-based peroxisome morphological mutant screens and proteomic analysis of the peroxisome membrane should also assist in identifying additional proteins in peroxisome division, including those that are plant- and peroxisome-specific and those acting independently from PEX11, FIS1, and DRPs.
2.3.2. Peroxisome movement and distribution
After multiplication, peroxisomes are separated and move to different locations within a cell, and partition into daughter cells in approximately equal numbers upon cell division or budding (Fagarasanu et al., 2007). In animal cells, peroxisomes primarily travel along microtubules, facilitated by microtubule-based motor molecules such as kinesin and dynein proteins (Rapp et al., 1996; Schrader et al., 1996b; Schrader et al., 1996a; Wiemer et al., 1997). In contrast, yeast and plant peroxisomes employ the actin-based cytoskeleton for intracellular trafficking and their movement is powered by actin-associated molecular motors named myosins (Hoepfner et al., 2001; Jedd and Chua, 2002; Mano et al., 2002; Mathur et al., 2002). Co-visualization studies using fluorescent protein-tagged peroxisomes and microfilaments revealed that plant peroxisomes associate with actin microfilaments in the cell, and that the application of the actin inhibitor latrunculin B arrests peroxisome movement (Jedd and Chua, 2002; Mano et al., 2002; Mathur et al., 2002). Individual peroxisome movement was found to be independent and highly dynamic with frequent changes in the state of motility, velocity and direction (Mathur et al., 2002). Velocity of the movement was variously reported as 2–10 µm s-1 (Jedd and Chua, 2002; Mano et al., 2002), much higher than the known motility rates for mammalian peroxisomes on the microtubules, which were recorded to be 0.75–2 µm s-1 and 0.2–0.5 µm s-1 (Rapp et al., 1996; Wiemer et al., 1997).
The myosin motor proteins are classified into 24 classes; however, plant myosins are restricted to class VIII and XI (Reddy and Day, 2001; Jiang and Ramachandran, 2004). Myosins utilize ATP hydrolysis via their N-terminal ATP-binding domains to shuttle cargo molecules on actin tracks. These proteins share a conserved motor/head for actin and ATP binding, a neck region consisting of IQ motifs that bind to calmodulin or calmodulin-like light chains, a coiled-coil domain in the stalk, and a highly variable tail that binds to cargos and determines functional specificity (Foth et al., 2006). Several Arabidopsis class XI myosins, including XI-2, XI-I, XI-K, and XI-E, have been shown to partially associate with peroxisomes and play roles in the movement of peroxisomes (Fig. 3). Subcellular targeting can be accomplished using merely the globular tail domain, indicating that the cargo binding and targeting information is present in the globular tails (Hashimoto et al., 2005; Li and Nebenfuhr, 2007; Reisen and Hanson, 2007; Sparkes et al., 2008). A model was proposed for assembly of myosin XI-cargo complex on the actin filament, wherein coordinated dimerization, organelle targeting and actin-based processive movement by the myosin XI facilitates efficient regulation of the motor activity (Li and Nebenfuhr, 2008).
Studies from Arabidopsis and yeast have shown that multiple isoforms of myosins are normally associated with an organelle to facilitate its movement. Likewise, each myosin can participate in the movement of several types of organelles, because knockout mutants for a single myosin can slow down the velocity of several subcellular compartments and sometimes lead to developmental defects (Hashimoto et al., 2005; Fagarasanu et al., 2007; Li and Nebenfuhr, 2007; Reisen and Hanson, 2007; Peremyslov et al., 2008; Sparkes et al., 2008). How is each myosin recognized by a particular organelle? It turns out that in budding yeast, a Class V myosin named Myo2p, which is associated with at least six types of cargos such as the Golgi vesicles, vacuoles, secretory vesicles, mitochondria, mitotic spindle, and peroxisomes, bind to specific receptors on the respective organelles via distinct domains in the myosin globular tail (Fagarasanu et al., 2007; Li and Nebenfuhr, 2008). Inp2p, an integral membrane protein with a cytoplasmically exposed coiled-coil domain, is the peroxisomespecific receptor for Myo2p (Fagarasanu et al., 2005; Fagarasanu et al., 2006). It remains to be elucidated in plants whether such organelle-specific receptors exist and how exactly peroxisome transport and inheritance is achieved.
Peroxisomes are often found to be intimately associated with chloroplasts and mitochondria. Thus, chloroplast and mitochondrial components or their overall homeostasis might dictate peroxisome distribution in the cell. Peroxisomes in the Arabidopsis conditional mutants of PEX10 were found to have lost their physical contacts with chloroplasts, indicating that PEX10 might be a peroxisomal protein mediating the association of peroxisomes with chloroplasts (Schumann et al., 2007).
2.3.3. Environmental, metabolic, and nuclear regulation of peroxisome proliferation
A wide range of endogenous and exogenous cues are reported to have an impact on peroxisome abundance. Peroxisome proliferation, or induced division, refers specifically to increases in peroxisome abundance or volume in response to environmental and metabolic stimuli (Yan et al., 2005). Oleates and hypolipidemic ligands (e.g., clofibrate) induce peroxisome proliferation in yeast and mammals, respectively. Transcription factors responsible for implementing peroxisome proliferation in both yeasts (Oaf1p, Pip2p, and Adr1p) and mammals (PPARα and RXR) and the responsive promoter elements in the target genes have been identified (Desvergne and Wahli, 1999; Gurvitz and Rottensteiner, 2006). Reports of equivalent proteins in Arabidopsis are yet lacking, although introduction of Xenopus PPARα into tobacco plants was found to induce acyl-CoA oxidase (ACX) gene expression along with a concomitant increase in peroxisome number (Nila et al., 2006). There are some hints that plants synthesize analogs to hypolipidemic drugs, as extracts from bitter gourd, plant isoprenols (farnesol and geranylgeraniol), and soybean isoflavones were found to activate PPARα and -γ (Takahashi et al., 2002; Chao and Huang, 2003; Shay and Banz, 2005). Treatment of pea and Arabidopsis with clofibrate results in an increase in peroxisome number and an induction in the transcription of Arabidopsis PEX1, PEX14, and KAT2 genes (Palma et al., 1991; Castillo et al., 2008). Collectively these results can be interpreted to indicate some degree of conservation between pathways regulating metabolic and nuclear control of peroxisome proliferation in plants and mammals (Hu, 2007).
Various treatments such as ozone, high light, isoproturon, and jasmonate have been observed to cause change in peroxisome number in plants (de Felipe et al., 1988; Ferreira et al., 1989; Ulloa et al., 2002; Oksanen et al., 2003; Castillo et al., 2008). Recent observations also support the hypothesis that metabolic status of the peroxisome might modulate peroxisome morphology and division decisions as well (Titorenko and Rachubinski, 2004; Quo et al., 2007). Studies in plants showed that elevated levels of acyl-CoAs within the peroxisome matrix appear to drive peroxisome enlargement (reviewed in Baker et al., 2006). Another study conducted in Vigna nodules found retarded peroxisome development in the absence of uricase, an enzyme involved in the purine oxidation pathway (Lee et al., 1993). In addition, the size and morphology of plant peroxisomes change between developmental stages (Mano et al., 2002). Lastly, in Arabidopsis, exposure to H2O2 and UV light-induced hydroxyl radicals causes peroxisomes to produce dramatic extensions termed peroxules, followed by fission of the elongated peroxisomes (Sinclair et al., 2009). Thus, peroxisomes seem to be sensitive to a multitude of signals and rapidly incorporate and manifest these signals by integrative changes in their abundance and morphology. However, despite the numerous tabulated cues for peroxisome proliferation, the underlying molecular mechanisms are largely unknown in plants.
A recent work has partially dissected the mechanism by which light regulates plant peroxisome abundance (Desai and Hu, 2008). It was observed that light treatment results in an increase in AtPEX11b transcript levels, which parallels the increase in peroxisome abundance during dark-light transition in Arabidopsis cotyledons and hypocotyls. In agreement with this observation, AtPEX11b RNAi lines exhibit decreased peroxisome proliferation under light treatment. Subsequent screening of various light signaling mutants for changes in AtPEX11b expression resulted in the identification of the photoreceptor phyA and a bZIP transcription factor, HYH, to be mediators of the light-induced expression of AtPEX11b. Both phyA and hyh mutants exhibit reduced AtPEX11b expression in the light and contain drastically reduced peroxisome numbers, a phenotype that can be rescued by over-expression of AtPEX11b. The promoter of AtPEX11b contains several light responsive elements and interacts with the HYH protein, suggesting that AtPEX11b is a direct transcriptional target of HYH. The response to light appears preparative in nature, compelled by the need for efficient photosynthesis and photorespiration with resultant peroxisome proliferation during seedling photomorphogenesis. The authors proposed the existence of a novel branch of the phyA-mediated light signaling network, which consists of the downstream effector HYH and the AtPEX11b gene, and suggested that peroxisome proliferation is an outcome of HYH binding and activation of AtPEX11b (Desai and Hu, 2008). This model does not rule out possible regulation of the AtPEX11b gene by other signaling intermediates in the phyA network. Future work should define this circuitry with higher precision and identify additional nuclear factors supervising peroxisome proliferation in plants. It would also be interesting to investigate whether various cues for proliferation are channeled through discrete or overlapping pathways and whether they converge at a probable master regulator of peroxisome proliferation.
Signals for changes in peroxisome abundance can also be emitted from other organelles. In budding yeast, respiratory deficiency in mitochondria results in retrograde regulation of nuclear genes, turning on a suite of genes associated with peroxisome biogenesis and proliferation (Hallstrom and Moye-Rowley, 2000; Epstein et al., 2001; Butow and Avadhani, 2004). Such retrograde signaling pathways have not been identified for plant peroxisomes.
3. METABOLIC FUNCTIONS
3.1. Introduction
Plant peroxisomes participate in numerous metabolic functions. One important facet of peroxisome functions is their integration into several metabolic networks compartmentalized across different subcellular organelles (Igamberdiev and Lea, 2002). This particular property led to the term “organelles at crossroads” for peroxisomes (Erdmann et al., 1997). In this section, we elaborate on the currently known biochemical processes associated with plant peroxisomes and speculate on some other reactions that, as of yet, lack direct evidence but have a strong possibility of being catalyzed, at least in part, in peroxisomes.
3.2 and 3.3 only give brief overviews of the Arabidopsis β-oxidation and glyoxylate pathways and emphasize recent work not summarized in other reviews. Readers are referred to the chapter on storage reserve mobilization in this book for a more comprehensive review on the subjects (Penfield et al., 2006). It is also worth noting that new proteins with novel metabolic functions are still being discovered for plant peroxisomes. For example, a recent leaf peroxisome proteomic analysis identified three members of the histidine triad (HIT) protein family, all of which contain PTS1 or PTS2 and reside in the peroxisome matrix (Reumann et al., 2009). Proteins with the HIT domain are nucleotide-binding, -hydrolyzing, and -transferring enzymes in other species (Brenner, 2002; Huber and Weiske, 2008), but their functions have not been elucidated in plants. Further biochemical analysis of this group of proteins will possibly reveal new metabolic functions of the plant peroxisome.
3.2. Fatty Acid β-oxidation
Fatty acid (FA) degradation in plants occurs in peroxisomes through the β-oxidation pathway, β-oxidation is known to be important for FA catabolism, turnover of membrane lipids, and hormone synthesis and metabolism; lately, it has also been recognized to be involved in the control of germination potential (Baker et al., 2006). FAs must be imported into peroxisomes and activated to their CoA-esters prior to entering the β-oxidation cycle. The peroxisome ABC transporter PXA1/CTS/PED3 has been implicated in the transport of FAs across the peroxisomal membrane (Zolman et al., 2001 a; Footitt et al., 2002; Hayashi et al., 2002). Two members of the acyl-activating enzyme (AAE) protein family, LACS6 and LACS7, are among the proteins responsible for the activation of FAs (Fulda et al., 2004). The ATP required for the activation is supplied, at least in part, by the two recently identified peroxisomal ATP transporters that are responsible for importing cytosolic ATP into the peroxisome (Arai et al., 2008b; Linka et al., 2008). Suppression of the expression of both genes using RNAi results in plant phenotypes such as sucrose-dependent germination, inhibition of fatty acid breakdown during germination, and resistance to proto-auxins (Arai et al., 2008b; Linka et al., 2008). These observations highlight the significant contribution of peroxisomal ATP transporters to seedling development through their essential role in ATP generation in the β-oxidation reactions
Fatty acid β-oxidation results in the sequential cleavage of an acetyl-CoA from the substrate FA-CoA in each repetitive cycle. Each cycle comprises four steps accomplished by the successive enzymatic activities of acyl-CoA oxidase (ACX), multifunctional protein (MFP), and 3-ketoacyl-CoA thiolase (KAT) that catalyze FA-CoA oxidation, hydration and dehydrogenation, and thiolysis, respectively (reviewed in Graham, 2008). Most of the enzymes participating in the β-oxidation spiral in Arabidopsis peroxisomes belong to multigene families; there are six ACXs (ACX1–6), two MFPs (AIM1 and MFP2), and three KATs (KAT2/PED1, KAT1, and KAT5 (reviewed in Goepfert and Poirier, 2007).
The degradation of cis-unsaturated fatty acids requires auxiliary enzymatic activities, due to the generation of enoyl-CoA intermediates that resist metabolism by the core β-oxidation enzymes. Two of these auxiliary activities are known to be associated with the cucumber MFP, which possesses Δ3- Δ2-enoyl-CoA-isomerase (ECI) and 3-hydroxylacyl-CoA epimerase activities (Behrends et al., 1988; Guhnemann-Schaferand Kindl, 1995b, a). Arabidopsis MFP contains an epimerase domain but the status of isomerase activity is unclear (reviewed in Goepfert and Poirier, 2007). The ECI along with 2,4-dienoyl-CoA reductase (DECR) and Δ3,5- Δ2,4-enoyl-CoA-isomerase (DCI) are necessary for the degradation of fatty acids unsaturated on odd-numbered carbons. The cDNA encoding DCI has been cloned and the enzyme was found to localize in peroxisomes (Goepfert et al., 2005). Recently, the ECI activity was shown to be conferred independently of the MFP by two peroxisomal proteins, ECI1 and ECI2 (Goepfert et al., 2008). Proteomic analysis also unveiled short chain dehydrogenase/reductase isoform b (SDRb), which displays high sequence similarity to yeast and mammalian DECRs, to be a promising candidate for the DECR activity (Reumann et al., 2007). Further, a peroxisome localized monofunctional type 2 enoyl-CoA hydratase (ECH2) has been determined to be involved in the degradation of enoyl-CoA intermediates that are unsaturated on even-numbered carbons (Goepfert et al., 2006). Several novel proteins with potential β-oxidation links have been discovered in the course of proteomics experiments (Reumann et al., 2007; Reumann et al., 2009). Functional analysis of these proteins would validate the predicted functions and possibly unravel new roles for β-oxidation events in the peroxisome.
3.3. The Glyoxylate Cycle
The acetyl-CoA produced by the β-oxidation pathway is converted via the glyoxylate cycle to 4-carbon compounds, which can be further metabolized to hexoses by gluconeogenesis or used as substrates for respiration. Thus, the operation of the glyoxylate cycle in peroxisomes is credited with fuelling the growth spurt during seedling germination by converting fatty acid-derived acetyl-CoAs into consumable sugar moieties. Additionally, free acetate can be imported into peroxisomes, where it is esterified by AAE7/ACN1, a short-chain acetyl-CoA synthetase (Turner et al., 2005). Furthermore, mutant screens aimed at identifying genes responsible for import of free acetate into the peroxisomes revealed that the ABC transporter PXA1/CTS/PED3 is involved in acetate utilization (Hooks et al., 2007).
The glyoxylate cycle utilizes activities of aconitase (ACO), malate dehydrogenase (MDH), citrate synthase (CSY), isocitrate lyase (ICL), and malate synthase (MLS). CSY, ICL, and MLS are localized in peroxisomes, with ICL and MLS functioning exclusively in the glyoxylate cycle (reviewed in Penfield et al., 2006; Graham, 2008). However, known Arabidopsis peroxisomal MDHs seem to be explicitly associated with the β-oxidation process, instead of directly participating in the glyoxylate cycle (Pracharoenwattana et al., 2007).
As in the tricarboxylic acid (TCA) cycle, the glyoxylate cycle initiates with CSY transferring an acetyl-CoA to oxaloacetate to form citrate, which is then isomerized to isocitrate by a cytosolic ACO. Isocitrate is then cleaved by ICL, yielding succinate and glyoxylate. MLS catalyzes the subsequent condensation of glyoxylate and acetyl-CoA to form malate, which may be oxidized by a cytosolic MDH to oxaloacetate that in turn cycles back to CSY to reinitiate the cycle (reviewed in Graham, 2008). The glyoxylate cycle is not believed to be vital during Arabidopsis seed germination, since mutants of ICL and MLS are only partially impaired in seedling establishment. Both icl and mls mutants are capable of respiring the acetyl-CoAs through the TCA cycle, while the glyoxylate produced in the mls mutant is directed into the photorespiratory pathway (Eastmond et al., 2000; Cornah et al., 2004). The csy2 csy3 (two peroxisomal CSYs) double mutant displays characteristics of mutants disrupted in β-oxidation, such as defects in storage oil catabolism, seedling establishment, and 2,4-DB resistance. Further, it was shown that CSY activities are required for both the glyoxylate cycle and the β-oxidation pathway in Arabidopsis (Pracharoenwattana et al., 2005).
3.4. Photorespiration
Photorespiration accompanies photosynthesis and is necessitated by the promiscuous activity of RubisCO towards molecular oxygen. This pathway uptakes O2 and releases CO2 in the light, salvaging and recycling phosphoglycolate back to the chloroplast. Although often regarded as wasteful due to the loss of fixed carbon, photorespiration is also thought to buffer the adverse effects of stresses such as high light intensity, drought, and salinity. Photorespiration is coordinated across three compartments, chloroplasts, peroxisomes, and mitochondria (Fig. 4).
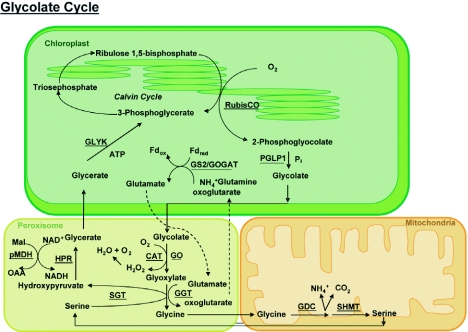
Figure 4.
Schematic of the photorespiratory pathway (for details see text in section 3.4).
Photorespiration initiates with RubisCO accepting oxygen as a substrate, resulting in the formation of phosphoglycolate, which is then dephosphorylated by the chloroplastic phosphoglycolate phosphatase (PGLP1). Glycolate then undergoes oxidation in the peroxisome matrix by glycolate oxidase (GO) to form glyoxylate, with the simultaneous production of H2O2, which is subsequently degraded by catalase. The glyoxylate is then transaminated to glycine by two peroxisomal aminotransferases, ser:glyoxylate and glu:glyoxylate aminotransferase (SGT and GGT). Glycine thus formed is subsequently decarboxylated and converted to serine in mitochondria by the consecutive activities of glycine decarboxylase (GDC) and serine hydroxymethyl transferase (SHMT). Ammonia released as a by-product of the GDC catalysis is likely reassimilated by the combined activity of glutamine synthase (GS2) and a ferredoxin (Fd) dependent glutamate synthase (Fd-GO-GAT) in the plastids to generate glutamate. Serine then reenters the peroxisome to be transaminated by SGT to yield hydroxypyruvate, which is reduced by NADH to glycerate in a reaction catalyzed by hydroxypyruvate reductase (HPR). The reduced cofactor is provided by peroxisomal malate dehydrogenase (pMDH). Glycerate is phosphorylated in the chloroplast by a stromal glycerate kinase (GLYK) to produce 3-phosphoglycerate, which feeds into the Calvin cycle (reviewed in Reumann and Weber, 2006; Foyer et al., 2009). Thus, leaf peroxisomes house six of the eleven enzymes directly involved in the photorespiratory pathway. Further, all of the photorespiratory enzymes in peroxisomes are believed to be arranged into multiprotein complexes to facilitate efficient substrate channeling and minimize leakage of intermediary metabolites. The occurrence of multienzyme complexes was demonstrated using isolated membraneless peroxisomes, which could process intermediate metabolites and produce glycerate at the same rate as intact leaf peroxisomes (Heupel et al., 1991; Heupel and Heldt, 1994).
Recently, a second HPR activity has been identified in Arabidopsis and characterized to represent a cytosolic bypass for the conventional photorespiratory cycle (Timm et al., 2008). Though the hpr double mutants show compromised growth in ambient air, they are not fatal. Additionally, it has also been found that the pMDHs are not absolutely essential for photorespiration (Cousins et al., 2008). These observations suggest that additional enzymes contribute to generating reductant for the peroxisomal HPR activity and further prompt the speculation that insufficient reductant in the peroxisomes induces the cytosolic bypass pathway (Cousins et al., 2008; Timm et al., 2008).
Most of the enzymes active in photosynthesis and photorespiration were originally identified in plant species other than Arabidopsis, with the exception of both known peroxisomal aminotransferases. Mutants in photorespiration often display chlorotic phenotypes in ambient air, which can be rescued by transferring the plants to high CO2 conditions. This conspicuous characteristic was exploited in a mutant screen to identify Arabidopsis mutants in photorespiratory pathways. Although the screen identified the sat mutant, which has defects in aminotransferase activity, the responsible gene was not mapped (Somerville and Ogren, 1980). The AtSGT was finally identified by querying the EST database with sequence of the human ala:glyoxylate aminotransferase. The AtSGT protein targets to peroxisomes and is capable of effectively utilizing both glyoxylate and serine as substrates. Further, the sat mutant was found to carry a point mutation in the SGT gene and to be specifically defective in SGT activity (Liepman and Olsen, 2001). A biochemical approach identified the peroxisomal GGT, which is encoded by two genes, GGT1 and GG72 (Liepman and Olsen, 2003). GGT1 is peroxisome localized, with its loss-of-function mutant displaying typical photorespiratory defects and inhibited growth in the absence of sucrose or under high light conditions, indicating the non-overlapping roles between GGT1 and GGT2 for efficient photorespiration (Igarashi et al., 2003). Further, GGT1 was also identifed from a mutant screen for altered ABA sensitivity. The increased H2O2 concentration in ggt1 was shown to have an impact on ABA-induced proline accumulation, ABA induced gene expression, and ABA accumulation in response to stress and ABA treatment (Verslues et al., 2007). Bioinformatic searches revealed that peroxisomes might have three additional aminotransferases (Reumann et al., 2004), one of which (encoded by At4g39660) was recently demonstrated to be dual targeted to mitochondria and peroxisomes by an N-terminal mitochondrial presequence and a C-terminal PTS1, respectively (Carrie et al., 2009). Aspartate aminotransferase isoform 3 (ASP3) is dual-targeted to chloroplasts and peroxisomes (L.J. Olsen, pers. comm.; Reumann et al., 2007) and implicated in β-oxidation-related redox shuttle (Mettler and Beevers, 1980).
Peroxisomes perform various metabolic reactions in concert with chloroplasts and mitochondria, which necessitates efficient exchange of reaction intermediates. Peroxisome membranes are thought to be permeable to small metabolites but not to larger cofactors such as NADH and CoA (van Roermund et al., 1995; Antonenkov et al., 2004). Electrophysiological experiments conducted on purified peroxisome membranes demonstrated the presence of an anion-selective porin-like channel in spinach and Ricinus peroxisomes (Reumann et al., 1995, 1996; Reumann et al., 1997). Further permeability experiments with leaf peroxisomes determined that the channel is capable of mediating passage of photorespiratory intermediates such as malate, OAA, and α-ketoglutarate (Reumann et al., 1998). In addition, immunoelectron microscopy studies from cucumber reported the presence of a structural homolog of the mitochondrial voltage-dependent anion selective channel (VDAC) on the peroxisome membrane (Corpas et al., 2000). Recent proteomic analysis of peroxisomes from etiolated soybean also identified a specific VDAC homolog, which was subsequently shown to be localized to peroxisomes in onion epidermal cells as RFP fusions (Arai et al., 2008a). Finally, a peroxisome channel protein homologous to putative membrane channel proteins in mitochondria (Tim17/22/23) and chloroplasts (OEP16) has been identified in bromegrass cell culture, immunolocalized to the peroxisomal membrane, and found to be inducible by cold, drought and abscisic acid (Wu et al., 2005). Although Arabidopsis peroxisome proteomic studies have not identified any porin channels, it is possible that some candidate proteins might be dual targeted, thus leading to their being categorized as contaminants from other organelles (Millar et al., 2006).
3.5. Jasmonate Biosynthesis
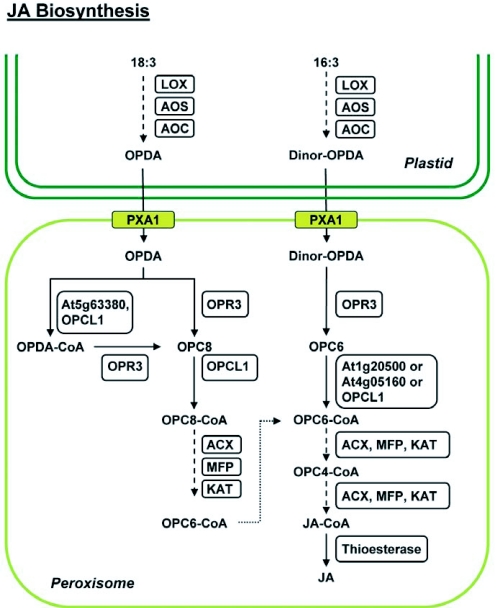
Jasmonates (JAs) are a family of plant hormones including jasmonic acid, methyl jasmonate (MeJA), and other bioactive oxylipins that regulate diverse aspects of plant growth, development, and defense (Wasternack, 2007; Katsir et al., 2008). JA biosynthesis spans two cellular compartments, commencing in the chloroplast and completing in the peroxisome (Fig. 5). Experiments with labeled 12-oxo-phytodienoic acid (OPDA) revealed that JA biosynthesis involves oxidative shortening of the 8-carbon side chain, leading to the inference that three rounds of peroxisomal β-oxidation are required for the conversion of OPDA to JA (Vick and Zimmerman, 1984). OPDA is synthesized in the chloroplast from trienoic precursors through a series of consecutive reactions catalyzed by lipoxygenase (LOX), allene oxide synthase (AOS), and allene oxide cyclase (AOC). Subsequent conversion of OPDA to JA occurs in the peroxisome matrix (Fig. 5). The peroxisome ABC transporter CTS/PXA1/PED3 is believed to be partially responsible for the transport of chloroplast-derived cyclopentenone intermediates (e.g., OPDA) to the peroxisomes. However, it remains unknown whether CTS/PXA1/PED3 exerts its functions directly as a transporter or indirectly via modulating the activity of other transporters or proteins. The cts mutant shows lower levels of basal JA and reduced JA accumulation in response to wounding, leading to the conclusion that OPDA or OPDA-CoA is transported into the peroxisomal lumen in an ATP-dependent manner by the CTS protein (Theodoulou et al., 2005). The high residual level of JA in cts plants explains why this mutant does not exhibit male sterility or other JA-deficient symptoms, suggesting the presence of alternate transport routes. One hypothesis is that OPDA can traverse the peroxisome membrane via an ion-trapping mechanism that accounts for a basal level of JA synthesis in the cts mutant. Alternatively, these two routes of transport could be responsible for the biosynthesis of JA at different developmental stages or in response to varied stimuli (Theodoulou et al., 2005).
Figure 5.
JA biosynthesis pathway.
Biosynthesis of (+)-7-iso-jasmonic acid (JA) initiates in the chloroplast with the sequential action of lipoxygenase (LOX), allene oxide synthase (AOS), and allene oxide cyclase (AOC) on linolenic acid (C18:3) or hexadeca-trienoic acid (C16:3) to generate 12-oxophytodienoic acid (OPDA) and dinor-OPDA, respectively. Transport of OPDA and dinor-OPDA into peroxisomes is facilitated either by the ABC transporter (PXA1) or by an uncharacterized pathway (not shown). Oxophytodienoic acid reductase 3 (OPR3) reduces OPDA to 3-oxo-2-(2′-[Z]-pentenyl) cyclopentane-1-octanoic acid (OPC8) and dnOPDA to 3-oxo-2-(2′-pentenyl)-cyclopentane-1-hexanoic acid (OPC6), with the resultant compounds being activated to their corresponding CoA esters by OPC:8 CoA ligasel (OPCL1) or the indicated acyl-CoA synthetases (At1g20500, At4g05160, At5g63380). The CoA derivatives undergo β-oxidation by the consecutive activities of acyl-CoA oxidase (ACX), multifunctional protein (MFP) and 3-ketoacyl-CoA thiolase (KAT), eventually generating JA-CoA after being subjected to the requisite number of β-oxidation cycles (3 for OPCS-CoA and 2 for OPC6-CoA). A putative thioesterase cleaves the CoA moiety, releasing JA. Dotted arrows depict catalysis of substrate(s) by the consecutive actions of the indicated enzymes.
Upon import into the peroxisome, OPDA is reduced to 3-oxo-2-(2′-[Z]-pentenyl) cyclopentane-1-octanoic acid (OPC:8) by an OPDA reductase, OPR3. Arabidopsis contains at least three OPR isoforms, but genetic and biochemical experiments determined that only OPR3 acts specifically in the JA biosynthesis pathway (Sanders et al., 2000; Schaller et al., 2000; Stintzi and Browse, 2000). opr3 mutants have a male sterile phenotype that can be rescued by exogenous JA. Reduced accumulation of free OPDA in the opr3 mutant can likely be explained by disruption of the JA-mediated positive feedback loop that results in upregulation of the JA biosynthetic capacity (Stintzi and Browse, 2000; Wasternack, 2007).
OPC:8 is activated to its corresponding CoA ester prior to its entry into the β-oxidation cycle. Co-expression analysis revealed OPC:8 CoA ligase1 (OPCL1), a member of the 4-coumarate-CoA ligase-like (4CL) family of the acyl-activating enzyme (AAE) super family, to be the likely candidate enzyme for activation of OPC:8 (Koo et al., 2006). Wounded leaves of opcl1 null mutants contain reduced levels of JA and increased accumulation of OPC:8. In vitro activation assays revealed that OPCL1 also activates OPDA and medium- and long-chain fatty acids (Schneider et al., 2005; Koo et al., 2006; Kienow et al., 2008). This lack of strict substrate specificity suggests that the initial steps in the peroxisomal pathway for JA synthesis may involve substrate channeling into multienzyme complexes. The absence of a malesterile phenotype and persistence of ∼50% of wild-type JA levels in opcl1 mutants implies that other members of the 4CL family can contribute to OPDA activation. A recent study showed that three 4CL-like enzymes, designated OPDA-CL (At5g63380) and OPC:6-CL (At1 g20500, At4g05160), exhibit activity toward OPDA and OPC:6, respectively. However, double and triple mutants of OPDA-CL and OPC:6-CLs do not display male sterility or any further reduction in JA content, implying the existence of alternative routes of JA biosynthesis that would bypass these enzymes (Kienow et al., 2008).
OPCL-activated intermediates then feed into the peroxisomal β-oxidation cycle catalyzed by the subsequent activities of ACX1/ACX5, AIM1, KAT2, and an unknown thioesterase, to yield JA (Cruz Castillo et al., 2004; Afitlhile et al., 2005; Li et al., 2005; Pinfield-Wells et al., 2005; Schilmiller et al., 2007). The involvement of ACX1 and ACX5 in JA biosynthesis has been underpinned by genetic studies utilizing acx1 acx5 double mutants, which show male sterility, reduced JA accumulation upon wounding, and decreased resistance to chewing insects (Schilmiller et al., 2007). However, the observation that JA synthesis is unhindered in the acx1 acx5 mutant upon infection by the pathogenic fungus, Alternaria brassicicola but inhibited during herbivory by leaf-eating insect Trichoplusia ni, indicates that depending on the stress condition, other ACXs could also be involved in JA biosynthesis (Schilmiller et al., 2007). AIM1 encodes one of the two isoforms of the multifunctional protein (MFP), with its mutant having a decreased level of wound-induced JA (Richmond and Bleecker, 1999; Delker et al., 2007). Further transcriptional profiling and genetic analysis also indicated that the thiolase KAT2 plays a role in JA biosynthesis (Cruz Castillo et al., 2004; Afitlhile et al., 2005; Pinfield-Wells et al., 2005).
JA biosynthesis is regulated by a positive feedback loop, wherein the expression of genes encoding JA biosynthetic enzymes is induced by JA and MeJA. Interestingly, phytochrome chromophore mutants show overproduction of JA and higher expression of JA biosynthetic genes such as OPR3, LOX1, and AOS, indicating a role for light in regulating JA synthesis (Zhai et al., 2007). The crystal structure of tomato OPR3 provides evidence that (i) the protein undergoes dimerization in vivo, which would serve to inhibit enzyme activity and thus exert a regulatory impact on JA biosynthesis, and (ii) a possible phosphorylation event may control this dimerization (Breithaupt et al., 2006). The crystal structure of AtACX1 also predicts this enzyme to function as a dimer (Pedersen and Henriksen, 2004). There are hints that ACX5 might inhibit JA biosynthesis, as the acx5 mutant has higher levels of JA (Schilmiller et al., 2007). Considering the dimerization of ACX1, it is speculated that ACX1-ACX5 heterodimerization might repress ACX1 activity and thus serves to regulate JA biosynthesis (Schilmiller et al., 2007).
In summary, studies of the JA biosynthetic pathway suggest that there are other unidentified proteins that play a role in JA biosynthesis, and that various stimuli such as fungi and insects might lead to JA production channeled through different isoforms of biosynthetic enzymes. The involvement of multigene families and shared components between fatty acid β-oxidation and JA biosynthesis suggests that, in addition to utilizing discrete sets of regulons for each process, the regulation of these pathways could also overlap at certain biosynthetic steps.
3.6. Indole-3-butyric Acid (IBA) Metabolism
IBA is an endogenous auxin with a specific role in lateral root formation and has been suggested to act as a reservoir of auxin by being metabolized into the bioactive auxin, indole-3-acetic acid (IAA) (Woodward and Bartel, 2005; Zolman et al., 2008). Experiments using leaf extracts demonstrated that IBA and its chemical analog, 2,4-DB, undergo a two-carbon shortening (a hallmark of β-oxidation reactions) to release IAA or 2,4-D, leading to the hypothesis that the bioactivation of proto-auxins such as IBA and 2,4-DB occurs through β-oxidation (Fawcett et al., 1960). This hypothesis was supported by the isolation of numerous mutants of β-oxidation enzymes using the 2,4DB/IBA-resistance genetic screens (Hayashi et al., 1998; Zolman et al., 2000); however, specific components acting in the IBA oxidation pathway are still elusive. A working model of enzymes involved in this pathway, summarized from cumulative evidence, is depicted in Fig. 6.
Figure 6.
IBA metabolism pathway in the peroxisome.
An uncharacterized acyl-activating enzyme (AAE) activates IBA by the addition of a CoA moiety. The IBA-CoA derivative goes through a single β-oxidation cycle, resulting in its sequential oxidation, hydration, oxidation, and thiolysis to yield IAA-CoA and an acetyl-CoA. The putative enzymes involved in each of these catalytic steps are indicated. The IAA-CoA thus generated is hydrolysed by the activity of an unknown thioesterase to release the active auxin IAA. The conversion of 2,4-DB to 2,4-D most likely follows the same cascade of enzymatic reactions differing only in the initial step, where AAE18 probably catalyzes the activation of 2,4-DB. For further details see text in section 3.6.
Recently, a candidate protein acting specifically in the IBA pathway has surfaced through characterization of the ibr3 mutant identified from the IBA resistant mutant screen (Zolman et al., 2007). Unlike other peroxisomal targeted proteins identified from the 2,4DB/IBA-resistance screens, ibr3 is neither sucrose dependent nor impaired in peroxisome protein import, suggesting that the mutated protein is not a β-oxidation enzyme or peroxisome biogenesis factor but rather might be an enzyme specific to IBA metabolism. Subsequent positional cloning revealed that IBR3 encodes a putative acyl-CoA dehydrogenase, presumably acting in the first step of IBA β-oxidation and yielding the corresponding CoA ester (Zolman et al., 2007). Previous reports indicate that ACX3 also catalyzes the same step as IBR3 and that the acx3 ibr3 double mutant displays enhanced resistance to IBA (Adham et al., 2005). Although IBR3 and ACX3 carry out analogous reactions, it is unclear whether IBR3 is a genuine dehydrogenase. IBR3 possesses an N-terminal aminoglycoside phosphotransferase (APH) domain, which is commonly found in bacterial enzymes that resemble eukaryotic kinases by utilizing phosphorylation to impart antibiotic resistance. Although the exact contribution of the APH domain is not known, a truncated IBR3 protein deleted for APH was unable to rescue ibr3, emphasizing the importance of this domain in IBR3 function (Zolman et al., 2007).
Two other loci, IBR1 and IBR10, have also been implicated to be specifically involved in the conversion of IBA to IAA (Zolman et al., 2008). Akin to ibr3, ibr1 and ibr10 mutants were identified from an IBA-resistant root growth screen and show attenuated IBA response while maintaining normal fatty acid β-oxidation. Higherorder mutants of ibr1, ibr3 and ibr10 do not display aggravated IBA-resistant phenotype, further suggesting that these genes possibly act linearly in the same pathway. IBR1 encodes a protein in the short-chain dehydrogenase/reductase family (SDR), and IBR10 is an enoyl-CoA hydratase named EC12 (Zolman et al., 2008). IBR10 and IBR1 were suggested to act consecutively on the IBR3 product, α,β-unsaturated thioester, by catalyzing hydration and subsequently a dehydrogenation step to eventually yield a keto-IBA-CoA (Zolman et al., 2008). AIM1 might also be involved in the IBA-to-IAA conversion, given that its mutant is resistant to IBA (Richmond and Bleecker, 1999). The presence of additional enzymes overseeing the conversion of IBA to IAA can also be inferred from the ibr1 ibr10 double mutant, which displays a wild-type IBA response under higher concentrations of IBA.
All three peroxisomal 3-ketoacyl-thiolases seem to be capable of catalyzing the conversion of keto-IBA-CoA to IAA-CoA (Hayashi et al., 1998; Zolman et al., 2000). The subsequent cleavage of IAA-CoA to IAA is probably mediated by a hitherto unidentified thioesterase. The IBA- activating enzymes have not been identified, as the known acyl-CoA synthetases LACS6 and LACS7 do not seem to be involved in IBA metabolism (Fulda et al., 2004). The AAE superfamily has several putative peroxisome-targeting members, i.e., eight 4CL-like and 6 AAEs (Kienow et al., 2008), some of which could possibly activate IBA. This possibility is further supported by the finding that several members of the same superfamily are responsible for the ATP-dependent adenylation of IAA (Staswick et al., 2002; Shockey et al., 2003; Staswick et al., 2005). In addition, putative AAEs have also been identified by proteome analysis of Arabidopsis peroxisomes (Reumann et al., 2007; Eubel et al., 2008; Reumann et al., 2009). Eventually, all the genetic evidence needs to be supplemented by vigorous biochemical assays to firmly establish a consolidated picture of IBA β-oxidation.
It had been believed that IBA and 2,4-DB are processed in similar manners in the peroxisomes. However, a genetic screen aimed at identifying auxiliary enzymes of β—oxidation has uncovered a putative acyl-activating enzyme, AAE18, that seems to be specifically involved in 2,4-DB activation. The aae18 mutant has longer roots on 2,4-DB media, but displays wild-type responses to IBA and does not exhibit sucrose dependence (Wiszniewski et al., 2009). These results imply that AAE18 has a direct role in 2,4-DB metabolism and is not involved in the fatty acid or IBA activation process. Additionally, IBR1 was also identified as having a role in the oxidation of 2,4-DB (Wiszniewski et al., 2009). It has been proposed that the β-oxidation enzymes ACX3, ACX4, AIM1, and KAT2 are also likely involved in the first, second, and fourth steps, respectively, in the β-oxidation of 2,4-DB (Zolman et al., 2000; Rylott et al., 2003; Wiszniewski et al., 2009).
3.7. Detoxification
3.7.1. Catalase
Oxidative reactions are at the core of peroxisome metabolism. One common by-product generated by such reactions is hydrogen peroxide (H2O2), which is scavenged primarily by catalase in the peroxisome matrix. Catalase is one of the most abundant peroxisomal proteins and forms a tetramer containing four porphyrin heme (iron) groups. It is also one of the very few matrix proteins that carry internal PTS1-like tripeptides located approx. 10 amino acids upstream of the C terminus (Kamigaki et al., 2003). Despite being the key enzyme in H2O2 metabolism, catalase has a very high Km, which renders the enzyme an inefficient scavenger at low concentrations. Moreover, catalase is light sensitive, undergoing photoinactivation followed by degradation in high light conditions (Feierabend and Engel, 1986; Tel-Or et al., 1986; Volk and Feierabend, 1989; Feierabend et al., 1992; Hertwig et al., 1992). Initial studies on catalase, which were mostly conducted in tobacco and focused on the association of this enzyme with the photorespiratory cycle, found that elevated catalase levels can reduce photorespiratory carbon losses and increase net photosynthesis under conditions that favor photorespiration (Zelitch, 1989; Zelitch et al., 1991; Zelitch, 1992; Brisson et al., 1998). Depletion of CAT1 activity in tobacco results in necrotic lesions in high light, hypersensitive response to bacterial infection, and increased susceptibility to paraquat, salt and ozone (Willekens et al., 1997; Mittler et al., 1999).
Arabidopsis expresses three peroxisome-targeted catalase isoforms, among which CAT2 has been the best characterized for its paramount role in photorespiration. CAT2 is maximally expressed in leaves, induced by cold and light, and regulated by the circadian clock (McClung, 1997). Reduction of CAT2 activity increases plant sensitivity towards ozone and photorespiratory H2O2-induced cell death; microarray analysis of the mutant also revealed that photorespiration-generated H2O2 influences transcriptional programs in plants (Vandenabeele et al., 2004). Moreover, CAT2 antisense plants show reduced activities of peroxisomal enzymes such as ICL and MLS during seedling germination (Vandenabeele et al., 2004; Eastmond, 2007). In concurrence with this observation, ICL activity can be inactivated by H2O2, whereas catalase associates with ICL to prevent this inactivation (Vandenabeele et al., 2004; Nguyen and Donaldson, 2005; Yanik and Donaldson, 2005). Another study found the cat2 null mutant to be highly compromised in growth in ambient air, with decreased rosette biomass, redox perturbations, and increased oxidative stress responses (Queval et al., 2007). Additionally, the cat2 mutant has been used as a tool to investigate effects of increased H2O2 on ion homeostasis, whereby the mutant is pale green and hypersensitive to assorted stresses including H2O2, salt, norspermidine, and cold, but surprisingly tolerant to lithium. It was subsequently deduced that the mutant is defective in ethylene production and perception and confers lithium tolerance, thus revealing novel cross talks between oxidative stress, cation homeostasis, and ethylene (Bueso et al., 2007). Consistent with the predominant expression of AtCAT3 in non-photosynthetic vasculature, the cat3 mutant does not display any evident phenotypes (Zimmermann et al., 2006). Lastly, CAT1 expression is induced by ABA via the mitogen associated protein kinase (MARK) signaling module (Xing et al., 2008).
3.7.2. APX, MDAR, DHAR, and GR
Besides catalase, plant peroxisomes utilize the cooperative activities of ascorbate peroxidase (APX), monohydroascorbate reductase (MDAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) as part of the ascorbate-glutathione cycle to purge the peroxisome-generated H2O2 (Yamaguchi et al., 1995; Bunkelmann and Trelease, 1996; Mullen et al., 1999; Karyotou and Donaldson, 2005; Lisenbee et al., 2005). The system operates with APX catalyzing the oxidation of ascorbate to monohydroascorbate and the simultaneous reduction of H2O2 to water (Asada, 1992). Next, the monohydroascorbate is converted back into ascorbate by MDAR in an NADH-dependent manner (Hossain and Asada, 1984; Asada and Takahashi, 1987). Ascorbate is also capable of reacting with either superoxide or H2O2 nonenzymatically to produce dehydroascorbate or monodehydroascorbate. Spontaneous disproportionation of monohydroascorbate also occurs, whereas dehydroascrobate can be reduced to ascorbate by DHAR using glutathione as a reductant (Hossain and Asada, 1984). The glutathione oxidized in this process is reduced by GR using the cofactor NADPH. Overall, the cycle has no net consumption of the antioxidants, ascorbate or glutathione, while reducing H2O2 and generating oxidized cofactor NAD+ and NADP+.
The actions of the membrane-associated APX/MDAR system have been proposed to be a preventive measure, barricading the seepage of H2O2 and avoiding oxidative damage to membrane lipids and integral proteins (Yamaguchi et al., 1995; Mullen and Trelease, 1996). Senescence-associated degradation of proteins, lipid peroxidation, and general disintegration of organellar structures are believed to be incurred due to the high concentration of H2O2 , which is accumulated partly from the loss of catalase and diminished activities of APX/MDAR (del Rio et al., 1998; Zimmermann et al., 2006). Furthermore, these scavenging activities for ROS are regulated. For example, plant APX and CAT activities are suppressed during plant pathogen interactions, thereby increasing the incidence of H2O2-triggered defense responses (Dorey et al., 1998; Mittler et al., 1998; Leterrier et al., 2005).
APXs have been characterized to show higher affinity for H2O2 than catalase (Mittler and Zilinskas, 1991; Ishikawa et al., 1998). Arabidopsis is predicted to have eight APXs, among them APX3 is localized at the cytosolic side of the peroxisome membrane (Jespersen et al., 1997; Panchuk et al., 2002; Narendra et al., 2006). Based on N- and C-terminal sequence similarity to APX3, APX5 was postulated to be peroxisomal (Panchuk et al., 2002) but has not yet been experimentally associated with the organelle. Numerous studies report varying degrees of benefits to plants from overexpressing peroxisomal APX proteins, such as enhanced tolerance to high temperature, increased protection against oxidative stress caused by inhibition of catalase, and higher seed production under drought conditions (Wang et al., 1999; Shi et al., 2001; Yan et al., 2003). However, an analysis of the Arabidopsis apx3T-DNA insertion mutant indicated that APX3 is dispensable for growth and development, although this lack of phenotype could also be attributed to compensatory activities by other enzymes such as catalase and other APXs in the peroxisome (Narendra et al., 2006).
Arabidopsis peroxisomes have been catalogued to possess two MDARs with possibly redundant roles in ascorbate recycling. MDAR1 is a PTS1-containing matrix enzyme, whereas MDAR4 is an integral membrane protein with its catalytic domain facing the matrix side (Lisenbee et al., 2005). The functional importance of MDAR4 has been elucidated using a combination of forward genetic screens and extensive biochemical characterizations (Eastmond, 2007). MDAR4 activity is essential in detoxifying H2O2, which could otherwise leak and cause oxidative damage to the peroxisome-neighboring oil bodies. Because the TAG lipase present in the oil bodies is inhibited by H2O2, the leakage directly affects lipid metabolism by causing an effective block of TAG hydrolysis and the subsequent fatty acid β-oxidation steps, thus rendering sucrose dependence to post-germinative growth of the plant. However, peroxisome functions in the mdar4 mutant are unaffected, implying that other antioxidant enzymes may compensate for MDAR inactivity within the peroxisome. The primary physiological role of MDAR4 was proposed to be preventing the escape of H2O2 beyond the peroxisomal membrane (Eastmond, 2007).
DHAR1 (At1g19570) was detected in Arabidopsis leaf peroxisomes by proteomics and confirmed to be peroxisome localized (Reumann et al., 2009). Proteomic experiments also identified GR1 (At3g24170) in Arabidopsis peroxisomes (Eubel et al., 2008; Reumann et al., 2009), and its peroxisomal targeting was confirmed in vivo (A. Kataya and S. Reumann, unpublished data). The oxidized cofactors generated during the operation of the ascorbate-glutathione cycle are most likely reduced by the activities of several dehydrogenases documented to be present in the peroxisomes, i.e., malate dehydrogenases, NADP isocitrate dehydrogenase (At1g54340), 6-phosphogluconate dehydrogenase (At3g02360), and 6-phosphoglucono-lactonase (At5g24400) (Nyathi and Baker, 2006; Reumann et al., 2007; Eubel et al., 2008; Reumann et al., 2009).
3.7.3. Other antioxidant enzymes
Glutathione S-transferases (GST), peroxiredoxins (Prx), and superoxide dismutase are other antioxidant enzymes reported to exist in plant peroxisomes (del Rio et al., 2006). GSTs are involved in detoxification by virtue of their ability to conjugate glutathione to various electrophilic metabolites, tagging them for vacuolar sequestration (Edwards et al., 2000). All three members of the theta (T) family of GST, namely, GSTT1, 2, and 3, localize to peroxisomes and preferentially act as glutathione peroxidases, reducing toxic hydroperoxides to monohydroxy alcohols (Reumann et al., 2007; Dixon et al., 2009). Glutathione peroxidase activity has been implicated in conferring oxidative stress tolerance, thus it is likely that these enzymes contribute actively to nullifying the threat posed by the high ROS flux and phytotoxic fatty acid hydroperoxides in peroxisomes (Cummins et al., 1999; Dixon et al., 2009). A Myb-like DNA-binding domain is located at the C termini of GSTT2 and GSTT3, masking a predicted PTS1; it was postulated that alternative splicing leads to the omission of the 3′ Myb domain and consequently peroxisome localization of the PTS1-containing protein (Dixon et al., 2009). Members of the phi (F) and tau (U) family were also detected by a proteome analysis of Arabidopsis leaf peroxisomes (Reumann et al., 2009).
Prxs are a group of H2O2-decomposing enzymes acting upon alkyl hydroperoxides and peroxinitrites. They are often associated with thioredoxins and cyclophilins, both of which have been detected in peroxisome proteome studies (Dietz et al., 2006; Reumann et al., 2009). Although reported in pea peroxisomes, to date none of the 10 Arabidopsis Prxs have been demonstrated to be peroxisomal (Horling and Dietz, 2002; Dietz et al., 2006). Finally, Arabidopsis peroxisomes also harbor a specific isoform of Cu-Zn superoxide dismutase (SOD3), which counters the threat posed by O2-; further information about this protein can be found in the chapter devoted to oxidative stress in this book (Grene, 2002).
Taken together, Arabidopsis peroxisomes contain a comprehensive set of antioxidant enzymes that act coordinately to fortify the plant against H2O2-imposed oxidative stresses. A model summarizing the reactions catalyzed by this group of enzymes is depicted in Fig. 7.
Figure 7.
Model for detoxification reactions in plant peroxisomes.
H2O2 is a potent ROS generated as a by-product of various metabolic reactions occurring within the peroxisome. It has the potential to inflict severe damage to enzymes and membrane lipids, thus necessitating its rapid detoxification. H2O2 is scavenged either by the catalases (CAT1, -2, and -3) in the matrix or through the activity of the ascorbate (ASC)-glutathione (GSH) cycle. In the latter case, H2O2 is reduced to H2O by ascorbate peroxidase (APX), producing monodehydroascorbate (MDA). MDA can either be reduced to ASC by monodehydroascorbate reductase (MDAR) with the simultaneous oxidation of NADH to NAD+ or, alternatively, MDA undergoes disproportionation to yield ASC and dehydroascorbate (DA). MDAR isoforms are associated both with the peroxisomal matrix (MDAR1) and the peroxisome membrane (MDAR4). Dehydroascorbate reductase (DHAR) acts on DA, utilizing reduced glutathione (GSH) to reduce DA to ASC; GSH is oxidized to GSSG in this reaction. Glutathione reductase (GR) regenerates GSH by using the reduced cofactor NADPH. Peroxisomal isocitrate dehydrogenase and 6-phosphogluconate dehydrogenase probably catalyze the reduction of NADP+ to NADPH, regenerating the reduced cofactor for the operation of GR (not shown). Three members of the theta family of glutathione S-transferases (GSTT) function mainly as glutathione peroxidases (GP), reducing fatty acid hydroperoxides (R-OOH) and H2O2 at the expense of GSH, although they also show limited transferase activity in conjugating GSH to metabolite (R).
3.8. Pathogen Response
The strongest evidence supporting an important role of peroxisomes in pathogen responses comes from analysis of the pen2 mutant. This mutant, which is susceptible to a host of fungal pathogens, was identified from a mutant screen aimed at isolating proteins responsible for resistance to non-invasive pathogens in Arabidopsis (Lipka et al., 2005). PEN2 encodes a glycosyl hydrolase, whose catalytic activity and peroxisome localization are absolutely essential to maintaining resistance. It was postulated that PEN2 functions are indispensable for resistance against a broad spectrum of non-adapted pathogens (Lipka et al., 2005). Additional confirmation for the role of PEN2 in non-host resistance came from a discovery that the coil-16 (coronatine insensitive 1–16) mutant, which is insensitive to JA, harbors a second mutation in the PEN2 gene and exhibits the previously reported pen2 mutant phenotypes. This new pen2 allele has greatly reduced levels of the PEN2 protein, prompting the hypothesis that the mutation possibly destabilizes the protein (Westphal et al., 2008).
Treatment of plants with microbe-associated molecular pattern (MAMP) molecules such as bacterial flagellin elicits basal defense responses, including callose deposition at sites of pathogen perception, production of ROS, and induction of secondary metabolites such as glucosinolates (Gomez-Gomez and Boiler, 2002; Nurnberger and Kemmerling, 2006). Recent work has demonstrated that PEN2 is a critical part of the Arabidopsis innate immune response and required for the microbe-triggered callose deposition as well as glucosinolate activation (Clay et al., 2009). Consistent with the observation that PEN2 gene expression is inducible upon treatment with Flg22 (synthetic 22 aminoacid peptide corresponding to most conserved domain of eubacterial flagellin), the pen2 mutants fail to display Flg22-induced callose deposition. Furthermore, Flg22-induced callose response requires indoleglucosinolate (IGS) biosynthesis; metabolite profiling in the Flg22-treated pen2 mutants revealed accumulation of a particular IGS species, 4-methoxy-indole 3-ylmethylglucosinolate (4MI3G). These results suggest that PEN2 possibly acts as a myrosinase to hydrolyze 4MI3G, and the products of this reaction most likely function as signaling molecules or coactivators in Flg22-induced callose deposition (Clay et al., 2009).
A comparative metabolite profiling experiment conducted on pen2 mutants infected by the non-adapted fungus Blumeria graminis hordei also found differences in accumulated metabolites compared with wild-type plants (Bednarek et al., 2009). Notably, the mutants contain higher concentrations of 4MI3G and I3G, and a loss of accumulation of fungal induced raphanusamic acid (RA) and a cysteine derivative, indole 3-yl methylamine (13A). This result prompted the hypothesis that PEN2 functions as a myrosinase catalyzing the hydrolysis of a specific glucosinolate (13G) to yield 13A and RA, which act as toxic compounds inhibiting fungal growth. Subsequent experiments confirmed that PEN2 has both glycosyl hydrolase and myrosinase activities and that the latter is responsible for generating defense-related metabolites. Due to structural similarity between the predicted 4MI3G hydrolysis product and phytoalexins, it was further suggested that this specific end-product might be the signaling molecule or a callose deposition coactivator responsible for the PEN2-mediated innate immune response mentioned earlier (Bednarek et al., 2009).
Studies to visualize subcellular events in plant response to the fungal pathogen Erysiphe cichoracearum found preferential accumulation of peroxisomes at penetration sites (Koh et al., 2005). Peroxisomes tagged by PEN2-GFP also exhibit focal accumulation at incipient entry sites, upon inoculation with the grass powdery mildew fungus Blumeria graminis f. sp. Hordei (Lipka et al., 2005). Another study of Arabidopsis epidermal cells following mechanical stimulation by a fine needle found that organelles including peroxisomes converge at needle contact sites, leading to the conclusion that mechanical pressure simulates penetration by a pathogen in inducing plant basal defense (Hardham et al., 2008).
What is the role for organelles in this basal defense response? Studies have also found a role for the photorespiratory enzyme SGT in pathogen resistance in melon (Taler et al., 2004). Enhanced expression of SGT was shown to be the basis of downy mildew resistance displayed by an ecotype of wild melon, a result that was further corroborated by the finding that transgenic melons overexpressing SGT are resistant to the disease. Glycolate oxidase (GO) activity in these transgenic plants also increased many-fold. The reaction catalyzed by GO generates glyoxylate and H2O2, with the latter inducing the plant to mount a basal defense response. Thus, it is believed that the elevated H2O2 production in the peroxisomes is fundamentally responsible for conferring resistance (Taler et al., 2004). However, it has not been shown if these enzymes also confer pathogen resistance in Arabidopsis.
3.9. Polyamine Catabolism
Polyamines are a group of polycationic compounds with multiple roles in plant development and defense (Alcazar et al., 2006). These compounds are catabolized by oxidative deamination through the activities of either flavin-containing polyamine oxidases (PAOs) or copper-containing amine oxidases (CuAOs; Cona et al., 2006).
In mammals, polyamine degradation occurs through either an interconversion pathway that results in the conversion of spermine and spermidine to putrescine or a terminal, irreversible catabolism pathway that oxidizes polyamine to their consequent aminoaldehydes (Seiler, 2004). It was believed that plants do not back-convert polyamines; however, the isolation of a cytosolic spermine-to-spermidine converting enzyme, AtPAO1, reinforced the notion that plants are also capable of back-conversions (Tavladoraki et al., 2006). Recently, two independent studies revealed the involvement of peroxisomal PAOs (Fig. 8) in executing polyamine interconversions (Kamada-Nobusada et al., 2008; Moschou et al., 2008). Biochemical characterization of AtPAO3 demonstrated that it catalyzes the sequential conversion of spermine to spermidine and spermidine to putrescine, although spermidine was determined to be the best substrate for the enzyme. In marked contrast to mammalian PAOs, AtPAO3 has preference for non-acetylated substrates and, surprisingly, only generates H2O2 as the reaction by-product (Moschou et al., 2008). AtPA04 preferentially catalyzes the conversion of spermine to spermidine, with its loss-of-function mutant containing increased levels of spermine along with a concurrent decrease of spermidine. The high expression of AtPAO4 in roots also suggests that this enzyme plays a particularly important role in establishing polyamine homeostasis in root tissue (Kamada-Nobusada et al., 2008).
Figure 8.
Polyamine oxidation pathways in peroxisomes.
(A) Interconversion pathway. Spermine and spermidine are substrates for peroxisomal polyamine oxidases (PAO2-4). PAO4 acts specifically on spermine to oxidize it to spermidine. PAO3 catalyses the sequential back-conversion of spermine to spermidine and spermidine to putrescine, and is subjected to feedback inhibition by its end product, putrescine. The specific substrate for PAO2 has not been established.
(B) Speculative pathway for terminal catabolism. Peroxisomal copper amine oxidase (CuAO) oxidatively deaminates putrescine to produce ammonia and H2O2. The reaction product 4-aminobutyraldehyde spontaneously isomerizes to Δ1-pyrroline, which is a substrate for peroxisomal betaine aldehyde dehydrogenase (BADH) that catalyzes its conversion to γ-aminobutyric acid (GABA) and releases H2O2 as a by-product.
CuAOs are homodimers with each subunit containing two cofactors, a copper ion and a 2,4,5-trihydroxyphenylalanine quinone (TPQ; Cona et al., 2006). One Arabidopsis CuAO isoform, the apoplastic ATAO1, catalyzes the oxidation of putrescine to yield 4-aminobutyraldehyde, which undergoes spontaneous cyclization to generate Δ1-pyrroline, ammonia and H2O2 (Moller and McPherson, 1998). Recently, an AtCuAO was identified by two independent peroxisome proteomic studies (Eubel et al., 2008; Reumann et al., 2009) and confirmed to be peroxisome localized using in vivo targeting analysis of YFP-fusion proteins (Reumann et al., 2009). Two previous papers report that the tobacco counterpart of the AtCuAO function is methylputrescine oxidase (MPO), an enzyme essential in the process of tropane alkaloid biosynthesis (Heim et al., 2007; Katoh et al., 2007). However, considering that Arabidopsis and other Brassicaceae (except for Cochlearia officinalis) do not contain tropane alkaloids, it is probable that, like ATAO1, AtCuAO also catalyzes the deamination of putrescine. If this holds true, it raises more interesting possibilities that the CuAO reaction product, 4-aminobutyraldehyde/Δ1-pyrroline, can be further metabolized to γ-aminobutyric acid (GABA, a plant signaling molecule) by the action of an aldehyde dehydrogenase (Cona et al., 2006). A plausible candidate for the GABA-forming aldehyde dehydrogenase is the peroxisomal betaine aldehyde dehydrogenase (pBADH; Reumann et al., 2007). Although believed to catalyze the oxidation of choline to glycine betaine, several studies report that plant BADHs are capable of metabolizing a range of substrates including 4-aminobutyraldehyde/Δ1-pyrroline (Trossat et al., 1997; Incharoensakdi et al., 2000; Hibino et al., 2002; Livingstone et al., 2003; Oishi and Ebina, 2005; Bradbury et al., 2008). Moreover, rice BADHs function as aldehyde dehydrogenases, demonstrating the highest affinity for 4-aminobutyraldehyde/Δ1-pyrroline as substrates (Bradbury et al., 2008; Chen et al., 2008). Considering the high sequence similarity with the rice BADHs, presence of PTS1, and the availability of substrates in Arabidopsis, it seems reasonable to presume that pBADH, like rice BADHs, catalyzes the formation of GABA in the peroxisomes. Further work on CuAO and pBADH will be crucial in defining their precise functions in the Arabidopsis peroxisome.
3.10. Sulfite Oxidation
Assimilated in the chloroplast, sulfur is an essential macronutrient in plants. In chloroplasts, sulfate is either reduced and incorporated into cysteine or attached covalently to a variety of compounds such as glucosinolates. Reduced sulfur (sulfite) is potentially damaging because it causes disulfide bridges to break (sulfitolysis), rendering proteins inactive. The detrimental effects of excess sulfur are countered by the activity of sulfite oxidase [SO; (Hansch and Mendel, 2005)]. immunogold labeling experiments and localization of reporter protein fusion established AtSO to be peroxisomal (Nakamura et al., 2002; Nowak et al., 2004). Biochemically, AtSO is a molybdenum enzyme that, in contrast to the mammalian SO, lacks a heme domain and utilizes oxygen (intead of cytochrome c) as the terminal electron acceptor (Eilers et al., 2001; Hansch et al., 2006). Thus, sulfite is oxidized to sulfate, forming H2O2 as a reaction by-product, which further oxidizes another sulfite (Hansch et al., 2006). Interestingly, increasing concentrations of sulfite inhibit catalase activity, leading to the hypothesis that under conditions of high sulfite the inhibited catalase would render greater production of H2O2 that would propel the non-enzymatic oxidation of sulfite (Hansch et al., 2006). The mutants of AtSO are more sensitive to SO2 fumigation and display phenotypes such as bleaching, necrosis, loss of turgor, and increased amounts of sulfur metabolites (Lang et al., 2007). As this gene is inducible by SO2 exposure, the protein is believed to be a key factor in protecting plants from excessive sulfite derived from the atmosphere (e.g., acid rain) or produced during sulfur assimilation, by annulling the toxic sulfite to sulfate (Hansch et al., 2006; Lang et al., 2007).
3.11. Other Functions
3.11.1. Branched-chain amino acid metabolism
Peroxisomes in isolated mungbean hypocotyls could completely catabolize branched-chain amino acids (BCAA) such as valine, leucine, and isoleucine (Gerbling and Gerhardt, 1988, 1989). However, based on knowledge derived from mammalian systems and subsequent analysis in Arabidopsis, mitochondria harbor all enzymes required for catabolism of BCAAs to propionyl-CoA, with the fate of the propionyl-CoA unresolved [reviewed in (Graham and Eastmond, 2002)].
The basis for the peroxisomal involvement in BCAA metabolism came from the isolation of a peroxisomal β-hydroxyisobutyryl (HIBYL) -CoA hydrolase from the IBA-resistant mutant screen. The mutant, chy1, displays IBA resistance in root elongation and lateral root induction, and sucrose-dependent germination. The recombinant protein can hydrolyze HIBYL-CoA in vitro, and the chy1 mutant phenotype was complemented by the closest mammalian homolog, the mitochondrial CoA thioester hydrolase. These observations led to the proposition that CHY1 catabolizes valine in Arabidopsis and that the block in CHY1 activity causes accumulation of a toxic intermediate, methacrylyl-CoA, which would sequester free CoA and Cys-containing proteins, thus impairing β-oxidation activities (Zolman et al., 2001 b). A genetic screen for 2,4-DB-resistant (dbr) mutants identified the same gene (DBR5) and subsequent analysis demonstrated that a decrease in KAT activity, possibly caused by inhibition of the enzyme by the accumulated methacrylyl-CoA, was the cause for IBA resistance and sucrose dependence in the dbr5 mutant (Lange et al., 2004). 14C feeding assays showed that both dbr5 and chy1 mutants have significantly reduced valine catabolism (Lange et al., 2004).
A subsequent study found that the chy1 mutant was sensitive to increasing concentrations of isobutyrate and propionate but not valine. These results led to the suggestion that CHY1 likely functions in the metabolism of isobutyryl-CoA and propionyl-CoA, which are derived from branched-chain fatty acids such as phytanic acid, instead of valine (Lucas et al., 2007). However, results from this study seem difficult to reconcile with the reduction in valine catabolism observed previously in the dbr5 and chy1 mutants (Lange et al., 2004). A recent work has also implicated CHY1 in cold stress signaling, where the mutant showed altered expression of CSFs [C-repeat (CRT) dehydration responsive element (DRE)-binding factor], increased accumulation of ROS, higher sensitivity to freezing treatment post cold acclimation, and darkness-induced starvation (Dong et al., 2009). The exact function and biochemical role of CHY1 in the peroxisome still remains to be established.
Completion of the Arabidopsis genome sequencing led to the discovery of the monomeric sarcosine oxidase (SOX), which belongs to a family of flavin-containing enzymes whose homologs in bacteria and mammals catalyze oxidative reactions using secondary or tertiary amino acids as substrates (Reuber et al., 1997; Trickey et al., 1999). SOX not only acts on sarcosine, a glycine derivative and a key metabolite in mammals, but also utilizes L-pipecolate as substrate. Although there is no evidence that sarcosine occurs in plants, pipecolate is a known catabolite of lysine in plants (Galili et al., 2001; Farres et al., 2002; Rontein et al., 2002). The recombinant AtSOX catalyzes the oxidation of sarcosine and N-methyl amino acids, and the oxidation of L-pipecolate to Δ1-piperideine-6-carboxylate (P6C). In vitro import assays established the peroxisomal localization of this protein. Suppression of AtSOX through RNAi resulted in hyperaccumulation of pipecolate and reduction in α-aminoadipate, indicating that AtSOX metabolizes pipecolate in planta. It was proposed that AtSOX serves as a salvage enzyme, recycling pipecolate back to P6C to feed into the lysine catabolic pathway. Based on the activity of recombinant AtSOX against sarcosine and N-methyl amino acids, both of which are widespread in nature, and the fact that AtSOX could also detoxify antimetabolites, structural analogs of N-methyl aminoacids, it was speculated that this enzyme enables the plant to opportunistically utilize the two natural substrates (Goyer et al., 2004).
3.11.2. The ureide pathway
The ureide pathway funnels the nitrogen released from the purine bases into amino acid backbones and assumes additional importance in leguminous plants, which transport fixed nitrogen in the form of ureides from the nodules to the aerial portions of the plant (Todd et al., 2006). The pathway constitutes three enzymes that act sequentially in the conversion of uric acid to the ureide, S-allantoin (Fig. 9). Uricase oxidizes uric acid to produce H2O2 and 5-hydroxyisourate (5-HIU), which is then hydrolyzed by HIU hydrolase to 2-oxo-4-hydroxy-4-carboxy-5-ureidoimidazoline (OHCU). Then, OHCU is decarboxylated by OHCU decarboxylase to yield S-allantoin (Todd et al., 2006).
Figure 9.
Urate degradation
The oxidation of urate is catalyzed by uricase to generate 5-Hydroxyisourate (5-HIU). 5-HIU is subsequently hydrolysed by HIU hydrolase (HIUase) to yield 2-oxo-4-hydroxy-4-carboxy-5-ureidoimidazoline (OHCU), which undergoes decarboxylation by OHCU decarboxylase to release carbon dioxide and produce S-allantoin. The latter two activities are imparted by the bifunctional transthyretin-like protein (TLP) in Arabidopsis.
Uricase has been extensively studied as a peroxisomal enzyme in nodules of leguminous species and detected in Arabidopsis peroxisomes by proteomic experiments (Reumann et al., 2007; Eubel et al., 2008; Reumann et al., 2009). HIU hydrolase activity was first found to be associated with a soybean β-glucosidase-like protein in the peroxisome; however, none of its closest homologs in Arabidopsis are predicted to target to peroxisomes, suggesting that the peroxisomal isoform of the β-glucosidase-like protein might be restricted to leguminous species (Sarma et al., 1999; Raychaudhuri and Tipton, 2002). On the other hand, recombinant transthyretin-like proteins (TLPs or TTLs) from various non-plant species contain HIUase activity; the high sequence conservation among TLPs and 3D structure predictions suggested that the Arabidopsis TLP may possess similar activity (Hennebry et al., 2006; Ramazzina et al., 2006). Indeed, the Arabidopsis TLP, a bifunctional protein with a PTS2 sequence lodged between the HIU hydrolase and OHCU decarboxylase domains, was confirmed to be peroxisomal in a leaf peroxisome proteome analysis (Hennebry et al., 2006; Reumann et al., 2007). HIU hydrolase is catalytically active as a tetrameric protein, whereas OHCU decarboxylase processes its substrate as a protein dimer, prompting the hypothesis that the bifunctional Arabidopsis protein possibly undergoes post-import cleavage to facilitate efficient catalysis (Hennebry et al., 2006). However, the detection of full-length TLP in a 2-DE-based proteome study strongly argues against this idea, but instead favors a 1:1 stoichiometry of HIU hydrolase and OHCU decarboxylase in the plant peroxisomal HIU hydrolase/OHCU decarboxylase complex. S-allantoin is subsequently processed to ammonia and carbon dioxide in the ER (Werner et al., 2008). In addition to its function in the ureide pathway, Arabidopsis TLP may play other physiological roles. For example, two alternative splice variants of TLP lack the internal PTS2 and are likely to represent non-peroxisomal isoforms performing other functions (Reumann et al., 2007). In addition, full-length TLP was also shown to be plasma membrane-associated and interacting with the brassinosteroid receptor BRI1 (Nam and Li, 2004).
Thus, in all likelihood the ureide pathway is compartmentalized in the peroxisome (Fig. 9) and executed by the consecutive actions of uricase, HIU hydrolase, and OHCU carboxylase to release S-allantoin, whose subsequent processing occurs in the ER to liberate ammonia and carbon dioxide.
3.11.3. Salicylic acid biosynthesis
Salicylic acid (SA) is an important signaling molecule in inducing plant response to pathogens (Metraux, 2002). It is believed that SA is generated either through chorismate via the shikimate pathway or alternatively, via processing of cinnamic acid derived from phenylalanine (Sticher et al., 1997; Wildermuth et al., 2001). Because the processing of cinnamate to SA ultimately involves reduction by two carbon moieties, it has been suggested that this reduction occurs through β-oxidation in peroxisomes (Reumann, 2004). It was predicted that peroxisomal 4CL-like AAE isoforms might possibly be involved in the CoA activation of a small subset of structurally related compounds such as cinnamic and benzoic acids (Shockey et al., 2003). It is worth noting that, in an in vitro enzyme assay, three putative peroxisomal 4CL-like proteins activated cinnamic acid and derivatives thereof to their corresponding CoA esters (Kienow et al., 2008). Further in-depth characterization of these isoforms may help expanding our knowledge of SA biosynthesis. Currently, the specific contribution of the cinnamate-SA pathway in modulating SA-induced responses is unclear, thus warranting further work to establish its relevance in Arabidopsis (Metraux, 2002).
3.11.4. Isoprenoid mevalonic acid pathway
Isoprenoids are naturally occurring compounds found in all organisms and are widespread in plants in various forms, spanning from photosynthetic pigments, redox cofactors, hormones and sterols to a multitude of secondary metabolites (Seemann et al., 2006). All isoprenoids are synthesized via condensation of isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP; McGarvey and Croteau, 1995). In mammals, enzymes in the mevalonic acid (MVA) pathway are localized in peroxisomes (Kovacs et al., 2002; Kovacs et al., 2007). In plants, IPP/DMAPP biosynthesis occurs through the cytosolic MVA pathway or the plastidic methylerythritol phosphate pathway (MEP; McGarvey and Croteau, 1995; Rodriguez-Concepcion and Boronat, 2002). However, recent studies suggest that at least part of the MVA pathway may have been duplicated to localize in the peroxisome.
The first enzyme of the cytosolic MVA pathway, acetoacetyl-CoA thiolase (ACAT), seems to be dual located. A specific transcriptional variant of Arabidopsis ACAT isoform 1 (ACAT1.3) targets to peroxisomes in vivo as a C-terminal GFP fusion, whereas the best predicted peroxisomal candidate, ACAT1.2, which carries a PTS1-related tripeptide SAL>, remains cytosolic as an N-terminal fusion (Carrie et al., 2007). The second ACAT isoform, ACAT2, which lacks a PTS-related sequence, was identified from Arabidopsis leaf peroxisomes by proteomics (Reumann et al., 2007), but its localization has not been authenticated.
Sapir-Mir et al. (2008) analyzed the localization of the two known Arabidopsis IPP isomerases (IPI1 and IPI2), which catalyze the seventh reaction of the MVA biosynthesis in both cytosol and plastids by reversibly isomerizing IPP to DMAPP. The two IPP isomerases each have two transcriptional isoforms, with the full-length version containing N-terminal targeting signals for the plastid and mitochondria and the shorter isoform lacking the targeting signals. Both longer variants are dual targeted to plastids and mitochondria as C-terminal GFP fusions (IPIlong-GFP); the shorter variants either remain cytosolic as N-terminal fusions (GFP-IPI1short) or target to peroxisomes with GFP inserted 10 residues upstream of the C-terminus (IPIshort-GFP-10aa). The C-terminal tripeptide (HKL>) of IPI1 and IPI2 has not been characterized as a PTS1 in any plant study nor is H known to be tolerated at pos. -3 in any plant PTS1 (see Fig. 10). It is possible that the enzyme adopts a default confirmation in the cytosol, in which the C terminus is internally hidden, and that the novel PTS1 HKL> becomes functionally active in peroxisome targeting only under specific yet unknown conditions.
Figure 10.
Summary of known plant PTS1 tripeptides.
The PTS1 tripeptides that have been experimentally verified as functional PTS or found in significant numbers (≥2) in datasets of homologous sequences (Reumann, 2004) are in bold. All other amino acid combinations of amino acid residues present in PTS1 tripeptides are in grey. SQL> and SAL>, both of which have been characterized only in PEX5 protein-peptide interaction studies (Kragler et al., 1998) and carry amino acid residues (Q and A at pos. -2) that have not been described in plant in vivo studies are not shown. References 1–9 are: Hayashi et al., 1997, Mullen et al., 1997, Kragler et al., 1998, Reumann, 2004, Lisenbee et al., 2005, Goepfert et al., 2006, Reumann et al., 2007, Ma and Reumann, 2008, and Reumann et al., 2009.
Three other enzymes of the cytosolic mevalonate pathway, i.e., HMG-CoA synthase, mevalonate kinase, and mevalonate-PP decarboxylase, were proposed to carry PTS2-related nonapeptides (Sapir-Mir et al., 2008). However, one or even both of the two invariant residues of the plant PTS2 nonapeptides (Rx6Hx) defined by Reumann (2004) are altered in all three enzymes, bringing their peroxisome targeting into question. Further work to determine the subcellular localization of these enzymes will be important before the association of the MVA pathway with plant peroxisomes can be firmly established.
4. LARGE-SCALE PROTEOME ANALYSES
4.1. Introduction
Peroxisomal proteins have been discovered either on the basis of inferred functions or sequence similarity, or by classical genetic screens. These methods, despite being powerful, are limited to small-scale protein identifications. Furthermore, genetic screens are not only time consuming and labor intensive, but also encounter insurmountable problems of functional redundancy and embryo/seedling lethality. Completion of the Arabidopsis genome sequencing, knowledge of the conserved targeting signals, and many technological advances have facilitated more comprehensive and highthroughput studies of the peroxisome proteome.
4.2. Phylogenetic Analysis
Like mitochondria and chloroplasts, peroxisomes have been touted to have an endosymbiotic origin (Cavalier-Smith, 1987, 1990). Arguments in favor of this view cite the facts that peroxisomes import their proteins post-translationally, possess their own protein import machinery, and undergo binary fission-like divisions (Lazarow and Fujiki, 1985). Further, it was postulated that the lack of DNA could be indicative of an endosymbiotic event predating the appearance of plastids and mitochondria, followed by the transfer of genes to the host cell's nucleus (Latruffe and Vamecq, 2000). It was also suggested that peroxisomes may have appeared as hydrogenosomes while the primitive earth's atmosphere was still reducing (Stabenau et al., 1998). There is considerable dissent over the placement of peroxisomes in the evolutionary history of the eukaryotic cell. Some argue that peroxisomes appeared after mitochondria, whereas others believe that peroxisomes arose before, or almost simultaneously with, mitochondria (de Duve, 2007; Cavalier-Smith, 2009).
Two groups conducted large-scale phylogenetic analyses of the peroxisomal proteome to determine the origin of peroxisomes (Gabaldon et al., 2006; Schluter et al., 2006). Comparing the rat and yeast peroxisomal proteins, it was determined that a significant fraction of peroxisomal enzymes have an α-proteobacterial origin suggestive of subversive recruitment of mitochondrial proteins (Gabaldon et al., 2006). Further, it was found that the majority of peroxins have a eukaryotic origin and that the matrix import system bears striking resemblance to an ER quality control pathway termed endoplasmic reticulum associated degradation (ERAD; Gabaldon et al., 2006; Schluter et al., 2006). ERAD involves the retrograde translocation of misfolded proteins from the ER lumen to the cytosol for subsequent proteasome-mediated degradation (Meusser et al., 2005). Proteins mediating the process include a TPR-containing integral membrane protein, HRD3, three peripheral Ub-conjugating enzymes (UBC1, -6, and -7), two multispanning integral membrane E3 RING ligases (HRD1 and DOA10), and an AAA ATPase, Cdc48. The peroxisome counterparts for these proteins, despite limited sequence similarities between them, are believed to be PEX5 (TPR), PEX4 (UBC), the three RING peroxins (PEX2, PEX10, and PEX12), and the AAA ATPases PEX1 and PEX6 (Gabaldon et al., 2006; Schluter et al., 2006). That PEX5 recycling in yeasts involves ubiquitination of the PEX5 protein (Platta et al., 2004; Kiel et al., 2005b; Kiel et al., 2005a) was taken as strong evidence for the evolutionary relatedness of the peroxisome protein import and the ERAD pathways. Collectively, these analyses suggest that peroxisomes do not have an endosymbiotic origin but likely arose from the ER (Gabaldon et al., 2006; Schluter et al., 2006). However, there are still speculations that the peroxisome is not an ER offshoot, evidenced by the different protein import systems employed by the two organelles, the α-proteobacterial origin of peroxisomal enzymes, and the evolution of both N- and C-terminal targeting signals by peroxisomal proteins, all of which are difficult to reconcile with an ER origin (de Duve, 2007). The shared characteristic of importing folded proteins by the peroxisomal matrix protein import system and the eubacterial twin-arginine translocase (TAT) system has led to the theory that peroxisomes most likely originated from the protoendomembrane system (Cavalier-Smith, 2009). The lack of a peroxisome genome precludes a definitive answer to the question regarding evolutionary origin of peroxisomes, which remains a matter of considerable debate.
4.3. Computational Prediction of PTS1/2 Proteins
4.3.1. Overview
Computational prediction uses mathematical algorithms (models) to predict organelle targeted proteins from genome sequences. Localization of the predicted proteins can then be verified in transient in vivo systems using fluorescent protein fusions. Computational prediction is particularly important in discovering low abundance peroxisomal proteins, and proteins whose genes are specifically expressed in organs inaccessible to peroxisomal proteomic analyses or induced only under specific developmental or environmental conditions.
Successful prediction algorithms need to meet two criteria, high prediction sensitivity and high prediction specificity. Proteins targeted to an organelle by a specific type of targeting signal such as PTS1 may be predictable if the primary structure of the signals is sufficiently specific and conserved. For peroxisomes, only matrix proteins carrying C-terminal PTS1 or N-terminal PTS2 peptides can be predicted. Proteins that cannot be predicted include those that are imported into the matrix either by internally located PTS1/2 peptides or by alternative targeting signals or pathways, proteins possibly imported by “piggy-backing”, and peripheral and integral membrane proteins (Saleem et al., 2006).
Although mechanisms of the PTS1 and PTS2 protein import pathways are largely conserved across evolutionary lineages (see 2.2), they vary in some details in each lineage. Biochemically, the three amino acid residues in PTS1 tripeptides are generally small-basic-hydrophobic, but significant differences in the ability to tolerate certain amino acid changes within PTS1s have been found between kingdoms (Kragler et al., 1998; Lamet-schwandtner et al., 1998). For example, histidine frequently occurs at position (pos.) -2 in PTS1s in fungi (Lametschwandtner et al., 1998), but it is rarely present at the same position in plant PTS1s (Reumann, 2004). Plant PTS1s also differ from those of fungi by frequently tolerating non-basic residues such as S and L at pos. -2 (Reumann et al., 2007; Reumann et al., 2009). Thus, PTS peptides need to be defined specifically for each taxon (e.g., higher plants, spermatophyta) before taxon-specific genome screens are conducted. Likewise, general prediction algorithms developed primarily for specific taxa should be applied to other organisms with caution (Emanuelsson et al., 2003; Neuberger et al., 2003b; Hawkins et al., 2007).
Although the C-terminal PTS1 tripeptide is considered the major determinant for the PTS1 protein import pathway, approx. seven to nine amino acid residues upstream of the tripeptide may also be pivotal in enhancing or reducing the efficiency of peroxisomal protein targeting (Neuberger et al., 2003b). This upstream region and the C-terminal PTS1 tripeptide are together referred to as the PTS1 domain. Therefore, PTS1 protein prediction is generally a two-step procedure whereby (i) the probability for the C-terminal tripeptide to represent a functional PTS1 (PTS1 tripeptide filter) and (N) the degree to which the upstream region (pos. -4 to -10 or -12) matches consensus PTS1 domains, are sequentially evaluated.
4.3.2. Plant PTS1 tripeptides
Comprehensive identification of PTS1 tripeptides in higher plants (spermatophyta) is necessary to ensure a high prediction and identification rate of plant peroxisomal proteins. Plant PTS1 tripeptides have been defined by both large-scale experimental and computational analyses.
Experimental studies include (i) in vivo subcellular targeting of reporter proteins fused to short peptides containing PTS1 (Hayashi et al., 1996; Hayashi et al., 1997; Mullen et al., 1997), and (ii) yeast two-hybrid analysis of the interaction between these small peptides with the PTS1 receptor PEX5 (Kragler et al., 1998). A number of PTS1 tripeptides were confirmed by independent studies, whereas other tripeptides (e.g., SKI>, SSL>, SHL>, and GRL>) yielded discrepant subcellular targeting results, possibly due to differences in analytical sensitivity, primary structure of the reporter protein, or structure of the spacer region upstream of PTS1 (Fig. 11, also see summary in Reumann, 2004).
Figure 11.
Sequence logos for plant proteins with major or minor PTS1 tripeptides.
Position-specific amino acid compositions of the C-terminal 10 amino acid residues of true peroxisomal PTS1-containing proteins, which carry either one of the nine major PTS1s (A) or one of the eleven minor PTS1s (B) as defined by Reumann (2004), are shown. The sequence logo was generated by WebLogo 3 (weblogo.berkeley.edu/).
For computational prediction, a training set of true positive examples is generally assembled by searching well-annotated protein databases for known PTS1 proteins, followed by a homology-based search for the orthologs of the identified proteins (Neuberger et al., 2003a; Hawkins et al., 2007). Due to the relatively small number of known PTS1 proteins, model organisms for peroxisome research, and fully sequenced genomes, previous sequence datasets for mammals, fungi, and plants were rather limited in size. Expressed sequence tag (EST) databases are a valuable resource in identifying orthologous PTS1 proteins from a wide variety of organisms. The usage of EST databases was first applied to Arabidopsis, which led to a 5-fold extension of the full-length plant PTS1 protein dataset and the identification of novel, non-canonical PTS1 tripeptides (Reumann, 2004).
Despite valuable PTS1 predictions, the computational approach has been severely limited by the low number of known plant PTS1 proteins available. For example, only 13 orthologous groups could be used as queries for the identification of homologous plant ESTs (Reumann, 2004). Furthermore, plant homologs of most known PTS1 enzymes, such as those involved in photorespiration, ROS metabolism and fatty acid β-oxidation, usually carry well-known major PTS1s and thus hardly contribute to the identification of novel PTS1 tripeptides. In contrast, plant homologs of a few other enzymes such as sulfite oxidase preferentially carry non-canonical PTS1 tripeptides (e.g., SNL>, ANL>, SSM>, SNM>), which allowed the identification of a large number of previously unrecognized, minor PTS1s (Reumann, 2004). Why plant PTS1 proteins display such a great variety in PTS1 tripeptides remains largely speculative. However, limited experimental and computational data suggest that (i) low-abundance PTS1 proteins with reduced expression levels can tolerate less efficient PTS1 tripeptides for quantitative import into peroxisomes, and (ii) sequences upstream of the C-terminal tripeptides in some orthologous groups of PTS1 proteins are particularly enriched in conserved targeting-enhancing elements that may compensate for the lower peroxisome targeting efficiency of non-canonical, minor PTS1s (see below).
Proteome analysis of Arabidopsis peroxisomes has emerged as a particularly successful approach, both directly and indirectly (after retrieval of homologous ESTs), in identifying novel PTS1 proteins/tripeptides. Two recent proteome analyses of leaf peroxisomes, combined with in vivo protein targeting studies, together established five new tripeptides as novel functional plant PTS1s (Reumann et al., 2007; Reumann et al., 2009). In addition, the C-terminal tripeptide AKI> in monodehydroascorbate reductase was shown to be another likely PTS1 (Lisenbee et al., 2005), and SHL> in a peroxisomal protein kinase was characterized as a functional PTS1 (Ma and Reumann, 2008). In total, 35 tripeptides have been confirmed or are predicted from reliable datasets to represent functional plant PTS1 tripeptides (Fig. 10).
Six to seven amino acid residues appear to be allowed at each position in plant PTS1 tripeptides (pos. -3: S, A, P, C, G, T; pos. -2: R, K, N, S, M, H, L; pos. -1: L, M, I, V, F, Y; Fig. 10). Although the current list of functional PTS1s is certainly incomplete due to training dataset limitations, it seems that only a subset of all possible 252 amino acid combinations of the principally allowed amino acid residues are functional plant PTS1s. As a general rule, a plant PTS1 tripeptide is only functional in peroxisome targeting if it carries at least two of the six most abundant position-specific amino acid residues (i.e., S, A, R, K, L, M) in the form of [SA][RK]x>, [SA]y[LM]>, or z[RK][LM]> (Reumann, 2004). To date only one PTS1 tripeptide, SSI>, slightly diverges from this rule (Fig. 10).
4.3.3. Effects of upstream sequences on PTS1 targeting
Peroxisome targeting efficiency differs among distinct PTS1 tripeptides, several of which are sufficient to target reporter proteins to peroxisomes irrespective of upstream residues (Hayashi et al., 1997; Mullen et al., 1997; Kragler et al., 1998). These “stand-alone” signals, such as SKL> and SRL>, are the prototypical “strong” PTS1 tripeptides. The function of other so-called weak PTS1 tripeptides often depends on some basic amino acid residues located in close proximity to the C-terminal tripeptide (Fig. 11). Experimentally defined weak PTS1 tripeptides include ANL> and SHL> (Mullen et al., 1997; Ma and Reumann, 2008).
Given the lack of systematic experimental data and large sequence datasets, the current classification of strong and weak PTS1 tripeptides is only arbitrary. Plant PTS1s have been categorized into nine major and eleven minor PTS1 tripeptides based on their abundance in the dataset, which is thought to roughly correlate with the targeting strength of the tripeptides (Reumann, 2004). This classification is further supported by statistically significant differences in position-specific amino acid composition between plant proteins carrying major and minor PTS1s. Upstream domains in proteins terminating with a minor PTS1 are significantly enriched in basic residues, prolines, and/ or hydrophobic residues (A, L, V, I), whereas these regions in proteins with a major PTS1 are on average only weakly enriched in such residues (Fig. 11). Except for the well-documented function of basic residues (R, K), the predicted physiological function of these enriched residues as enhancing elements for targeting remains to be verified experimentally. The predicted inhibitory effect of residues underrepresented in PTS1 domains (e.g. E, D) has been largely untested. However, a striking example for such putative inhibitory elements comes from the study of an Arabidopsis protein kinase, AtPK1, which contains several acidic residues upstream from the C-terminal prototypical PTS1 (SKL>) that appeared to inhibit peroxisome targeting. Such upstream acidic residues can inhibit and even prevent peroxisome targeting despite the presence of strong PTS1 tripeptides, and thus also need to be considered in prediction algorithms (Ma and Reumann, 2008).
4.3.4. Definition of PTS2 peptides and domains
About one-third of plant peroxisomal matrix proteins appear to use the PTS2 import pathway (Reumann, 2004). The corresponding targeting signal PTS2, with RLx5HL as the prototype, is a nonapeptide located near the N terminus and consisting of four conserved residues split into two parts by a stretch of five relatively flexible residues. More restrictive PTS2s such as R[ILQx5HL] (Kato et al., 1996; Kato et al., 1998) or permissive PTS2s such as [RK]X6[HQ][ALF] (Flynn et al., 1998) have been defined. Twelve functional PTS2 nonapeptides, all of which carry R at pos. 1 and H at pos. 8, were defined from a plant-specific EST training dataset of PTS2 proteins. Pos. 2 was found to be the most variable position of the nonapeptide, allowing L, I, Q, T, M, A, and V; pos. 9 is the second variable, allowing L, I, and F (Reumann, 2004). The up- and downstream regions of PTS2 and the internal x5-region are enriched in basic amino acid residues, such as R and P, and scarce in acidic residues.
PTS2 nonapeptides were believed to be confined to the first 30 amino acid residues at the N terminus. This general rule has been challenged by the proteomic (followed by in vivo targeting) identification of the Arabidopsis transthyretin-like protein (TLP), a bifunctional enzyme involved in purine metabolism and carrying an internal PTS2 (RLx5HL) in a linker region connecting the decarboxylase and hydrolase domains (Reumann et al., 2007). This atypical PTS2 appears to have evolved in TLP by fusion of a decarboxylase gene with a hydrolase gene containing the PTS2 (see 3.11.2). Thus, functional PTS2 nonapeptides are not restricted to N-terminal protein domains. Genome screens should be extended to proteins with internally located PTS2s, giving specific consideration to bifunctional proteins.
4.3.5. Prediction of PTS1/2 proteins in Arabidopsis
Moderate prediction accuracy is generally sufficient in predicting the localization of specific proteins. However, large-scale genomic screens require high prediction specificity to keep the number of false positive predictions to a minimum. In general, PTS1 tripeptide-based search algorithms or machine learning techniques have been applied to develop prediction tools (Emanuelsson et al., 2003; Neuberger et al., 2003a, b; Hawkins et al., 2007). Current PTS1 prediction servers include PEROXIP (www.bioinfo.se/PeroxiP: Emanuelsson et al., 2003), the PTS1 PREDICTOR (mendel.imp.ac.at/mendeljsp/sat/pts1/PTS1 predictor.jsp; Neuberger et al., 2003a), and PProwler (pprowler.itee. uq.edu.au; Hawkins et al., 2007). Plant-specific predictions by machine learning techniques are still in their infancy because of small-sized and non-representative datasets (Emanuelsson et al., 2003; Neuberger et al., 2003a).
Public databases allow the scientific community to gain access to genome-wide predicted PTS1 proteins. Genetic and functional information about known peroxisomal proteins of Homo sapiens (85 proteins) and Saccharomyces (61 proteins) can be found in the database PeroxisomeDB (www.peroxisomedb.org; Schluter et al., 2007). The knowledge of higher plant PTS1 and PTS2 peptides combined with improved ORF prediction and large-scale full-length cDNA sequencing for Arabidopsis, led to the prediction of approx. 320 PTS1 and 60 PTS2 proteins in the Arabidopsis genome (Kamada et al., 2003; Reumann et al., 2004); this information is summarized in the database AraPerox 1.2 (www3.uis.no/araperoxV1). Many predicted PTS1 proteins have been verified as truly peroxisome targeted. The nearly 30 novel Arabidopsis PTS1 proteins identified since 2004 should significantly improve the size and quality (i.e. coverage of natural variability) of the EST sequence training dataset and assist with the development of plant-specific PTS1 prediction algorithms. Future challenges include the prediction of surface-exposed PTSs and dual/multiple subcellular protein targeting and transfer of prediction methodology established for PTS1 proteins to the more difficult prediction of PTS2 proteins.
Dual targeting of plant peroxisomal proteins has been addressed in two recent studies (Carrie et al., 2008; Carrie et al., 2009) but remains difficult to predict, if native folding of full-length proteins and competition of multiple targeting signals are to be considered. In recent years, complexity of the Arabidopsis proteome has further increased with the characterization of additional protein variants, many of which differ in the presence or absence of predicted PTSs. The existence of these variants indicates that subcellular targeting is often regulated at the transcriptional, translational, and even post-translational levels. Thus, dual or multiple protein localization is much more common than previously anticipated.
4.4. Experimental Proteome Analysis
4.4.1. Methodology and dynamic range of protein identification
Most proteomic studies of plant peroxisomes have emerged only recently. There are several inherent problems for plant peroxisomal proteome analyses. First, genomic sequence information is lacking for established model plant species (e.g., pea, pumpkin, and spinach) used in peroxisome research, which prompted the need to establish peroxisome isolation protocols specifically for Arabidopsis. Second, Brassicaceae are known to contain the highest numbers and concentrations of organelle-destabilizing secondary metabolites, making peroxisomes particularly fragile during isolation. Third, tight physical interaction between peroxisomes, mitochondria, and chloroplasts in Brassicaceae reduces the purity of leaf peroxisome isolates. Here, we summarize and compare all six proteome studies of plant peroxisomes published to date, with respect to methodologies in organelle isolation, protein and peptide separation, dynamic range of protein identification, and peroxisome targeting verification.
Two pioneering proteomic studies in Arabidopsis were performed on peroxisomes from greening and etiolated cotyledons, respectively (Fukao et al., 2002; Fukao et al., 2003). Peroxisomes were purified by a single density gradient, and the purity was analyzed by immunoblotting of the gradient fractions. After reduction of sample complexity by 2D-gel electrophoresis (2DE), the proteins were visualized by silver staining and the tryptic peptides were analyzed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Protein identification was solely based on peptide mass fingerprinting (PMF) data, which bears some risk in identifying false positives. Several enzymes involved in ROS metabolism were identified from both studies, whereas known proteins of photorespiration and fatty acid β-oxidation were only specifically identified in green or etiolated cotyledons. Twenty of the 29 proteins identified from green cotyledons and 13 of the 19 proteins identified from etiolated cotyledons were described as putative novel proteins of peroxisomes.
A later study focusing on peroxisomes from mature Arabidopsis leaves used a Percoll density gradient followed by sucrose density gradient centrifugation to enrich the organelles (Reumann et al., 2007). For comparison of peroxisome isolates, this work used the specific activity of a marker enzyme, hydroxypyruvate reductase (HPR), as an indication of organelle purity. Sample complexity was reduced by 2DE and proteins were identified by MALDI-TOF tandem MS, or by gel-free shotgun proteomics using LC-MS/MS. This study detected 36 established peroxisomal proteins, including many proteins with roles in so-called minor functions of leaf peroxisomes such as fatty acid β-oxidation, S and N metabolism, and JA biosynthesis. Forty-two proteins were considered putative novel proteins, indicating a higher level of sensitivity achieved in protein identification.
The dynamic range of protein identification was further increased in a study carried out by the Arabidopsis 2010 Peroxisome Project (www.peroxisome.msu.edu) using mature leaves (Reumann et al., 2009). Peroxisome enrichment was further improved through post-preparative immunoblotting analysis and by application of a 1DE shotgun LC-MS/MS approach. Among the 150 peroxisome-associated proteins identified, 65 represent established peroxisomal proteins including nearly all known matrix proteins of plant peroxisomes and several membrane proteins such as PXA1, PMP38, and all five PEX11 isoforms. Using stringent selection criteria, 85 proteins were assigned as putatively novel, among which 30 proteins had also been identified by previous proteome analysis but not yet verified by independent methods, and 55 proteins were discovered for the first time in the proteome of plant peroxisomes.
Using Arabidopsis suspension-cultured cells, Eubel et al. (2008) purified peroxisomes with a free-flow electrophoresis method established earlier for plant mitochondrial enrichment (Eubel et al., 2007). This study applied two quantitative proteomic methodologies to identify true peroxisomal proteins: (i) differential in-gel electrophoresis (DIGE) of enriched peroxisomes and mitochondria, and (N) normalized spectral count analysis of shotgun proteome data from peroxisome fractions differing in their degree of purity. The identification of membrane proteins was optimized by sodium carbonate treatment of peroxisomes. In total, eighty-nine proteins were assigned as peroxisomal; among them about 20 proteins had not been associated with peroxisomes before. Phosphopeptide enrichment also allowed the detection of a peptide from PMP38 (At2g39970), which was phosphorylated at Ser-155.
Only a single peroxisome proteome study has been reported for plant species other than Arabidopsis, mostly due to difficulties in protein identification that generally requires de novo sequencing of tryptic peptides. Arai et al. (2008a) purified peroxisomes from etiolated soybean (Glycine max) cotyledons, using Percoll followed by iodixanol density gradient centrifugation. Immunoblotting was applied for purity monitoring and protocol optimization. Following protein separation by 2DE, protein identification by PMF was made possible by data searches against a soybean database (DFCI Soybean Gene Index) consisting of non-redundant soybean tentative consensus sequences created by soybean EST assemblies. Thirty proteins were assigned to peroxisomes, including 26 orthologs of established plant peroxisomal proteins, three orthologs of proteins already identified by former proteome studies of Arabidopsis peroxisomes, and one putative novel protein, i.e., a homolog of the mitochondrial VDAC family. In a follow-up study, Arai et al. (2008b) separated protein complexes by blue native/SDS-PAGE and identified one membrane protein (Gm PNC1) that was later characterized as a member of the peroxisomal adenine nucleotide carrier family.
4.4.2. Verification of peroxisome targeting by in vivo subcellular targeting analysis
Peroxisomes can be enriched over other organelles, but they cannot be isolated to absolute purity. For this reason, the distinction between true peroxisomal proteins and proteins originating from minor contaminations by other organelles is a serious concern for peroxisome proteome studies in all organisms. With an increasing dynamic range of protein identification, the annotation of unknown proteins becomes more challenging. Several methods have been applied to verify the postulated peroxisome association of unknown proteins detected in peroxisomal proteome studies: (i) analysis of predicted PTSs, (ii) analysis of PTS peptide conservation in orthologous proteins and homologous EST sequences (Reumann et al., 2007; Reumann et al., 2009), (iii) immunobiochemical analysis of density gradient fractions for protein co-migration with markers (Fukao et al., 2003), (iv) semi-quantitative proteome analysis of peroxisome isolates with different degrees of purity (Eubel et al., 2008), and (v) in vivo subcellular targeting analysis of fluorescent protein fusions. This last method has emerged as the most efficient way to provide conclusive evidence for peroxisomal targeting and is nowadays widely applied as an extension to proteome analysis of peroxisomes from fungi, mammals, and plants (Marelli et al., 2004; Reumann et al., 2007; Wiese et al., 2007; Arai et al., 2008a; Eubel et al., 2008; Reumann et al., 2009).
In the two initial proteomic studies, the number of putative novel proteins carrying known or PTS1/2-related peptides was low (Fukao et al., 2002; Fukao et al., 2003). In the green cotyledon peroxisomal analysis, for instance, two putative novel proteins carry C-terminal PTS1s (the protein kinase PK2, At4g31230, PKL>; and a putative beta glucosidase, At4g27820, SSL>) and two additional proteins contain PTS1-related peptides, SKD> and IRL> (Fukao et al., 2002). The protein kinase PK2 has been verified to carry a functional PTS1 domain that is sufficient to direct GFP to peroxisomes; however, the full-length protein remains cytosolic when expressed in onion epidermal cells, indicating that the kinase is likely dual-targeted to both the cytosol and peroxisomes in a transient and regulated manner (Ma and Reumann, 2008). Three proteins identified from the peroxisome proteome of etiolated cotyledons (Fukao et al., 2003) carry predicted PTS1 tripeptides (isocitrate dehydrogenase, IDH, At1g54340, SRL>; small thioesterase 3, sT3, At3g61200, SKL>; and glyoxysomal protein kinase 1, GPK1/PK7, At3g17420, AKI>). Both IDH and sT3 have been repeatedly identified in later proteome analyses of Arabidopsis peroxisomes and were confirmed to be peroxisome-targeted as fluorescent protein fusions (Reumann et al., 2007; Eubel et al., 2008; Reumann et al., 2009). Peroxisome targeting of glyoxysomal protein kinase 1 (GPK1/PK7) was supported in the original proteome study by immunobiochemical analysis of the density gradient fractions (Fukao et al., 2003). However, organelle-like but non-peroxisomal targeting of full-length EYFP-GPK1/PK7, EYFP-GPK1/PK7ΔAKI, and EYFP fused with the putative PTS1 domain of GPK1/PK7 raised some doubts on its localization in peroxisomes (Ma and Reumann, 2008).
Of the 42 putative novel proteins identified by the first proteome study of Arabidopsis peroxisomes from mature leaves, 28 carry known or PTS-related targeting signals, many of which are conserved among homologous ESTs. Eleven proteins were confirmed to be peroxisomal as GFP fusions in the same study (Reumann et al., 2007). Five additional proteins (BSMDR, At1g49670; ECHIa, At4g16210; ECHIc, At1g65520; SDRa, At4g05530; and NBP/HIT1, At4g16566) from this list have subsequently been verified as peroxisomal by in vivo targeting analyses (Eubel et al., 2008; Goepfert et al., 2008; Reumann et al., 2009; Wiszniewski et al., 2009).
Eubel et al. (2008) detected about 20 putative novel proteins of peroxisomes; one of them, which is homologous to the yeast peroxisomal adenine nucleotide transporter ANT1 (Palmieri et al., 2001) and encoded by At5g27520, was confirmed to be peroxisomal by in vivo subcellular targeting analysis in the same study. This protein and its paralog encoded by At3g05290 have been functionally characterized as plant peroxisomal adenine nucleotide carriers, with PNC1 assigned to At3g05290 and PNC2 to At5g27520 (Arai et al., 2008b; Linka et al., 2008). Five of the 20 putative novel proteins identified by this study, i.e., acyl-activating enzyme 1 (AAE1), zinc-binding dehydrogenase (ZnDH), malonyl-CoA decarboxylase (MCD), indigoidine synthase A (IndA), and copper amine oxidase (CuAO), were later conclusively demonstrated to be peroxisome targeted (Reumann et al., 2009).
The most comprehensive in vivo subcellular localization study of peroxisomal proteins was carried out in an in-depth proteome analysis of Arabidopsis leaf peroxisomes (Reumann et al., 2009). For medium throughput cloning of candidate genes, Gateway®-compatible vectors were created for fusing the coding region of the candidate cDNAs to the N (for PTS2-containing proteins) or C terminus (for PTS1-containing proteins) of YFP. Among the 55 putative novel peroxisomal proteins assigned in this study, nine proteins carry predicted plant PTS peptides and seven contain PTS1/2-related sequences such as STL>, SLL>, SQV>, SLM>, and RVx5HF. Peroxisomal localization was confirmed for 13 proteins, including six with known PTSs, three carrying PTS-related peptides and four proteins that lack conventional targeting signals. This study also validated the peroxisomal targeting for five proteins that had independently been detected by Eubel et al. (2008). Although the 14 proteins that failed to show peroxisome targeting in the transient assay are possible contaminants from other cell compartments, several of them were expected to be truly associated with peroxisomes in plant cells under specific physiological conditions.
In the soybean peroxisome proteome study, all three PTS1 proteins (GmSDR, GmECHI, and GmHCDH) non-homologous to established plant peroxisomal proteins were verified as peroxisomal by in vivo subcellular targeting analysis (Arai et al., 2008a). Arabidopsis orthologs for these three proteins (AtSDRa, At4g05530; AtECHIa, At4g16210; AtHCDH, At3g15290) were also identified and proven to be peroxisomal targeted in vivo (Reumann et al., 2007; Eubel et al., 2008; Wiszniewski et al., 2009). Surprisingly, a specific homolog of the mitochondrial superfamily of voltage-dependent anion-selective channels (VDAC) was shown in vivo to be targeted exclusively to peroxisomes in both N- and C-terminal GFP orientations (Arai et al., 2008a). Functional characterization of the conductance properties of this VDAC homolog is required to determine whether it is similar to the well-characterized mitochondrial VDAC homologs or the porin-like channel characterized in spinach leaf peroxisomes and castor bean glyoxysomes (see 3.4).
4.4.3. Novel targeting signals
The identification of novel peroxisomal matrix proteins by proteome approaches is not only essential to discovering unexpected functions of plant peroxisomes, but also important in improving the prediction of PTS1/2 proteins from plant genome sequences. For several novel peroxisomal proteins containing PTS1 or PTS2, more than 50 homologous plant sequences can be retrieved from EST databases for each protein. Novel matrix proteins can thus substantially contribute to the extension of the training dataset needed for developing high accuracy prediction algorithms. To this end, in vivo subcellular targeting analyses should be performed for each novel protein to show that (i) the full-length protein is peroxisomal, (ii) removal of the corresponding PTS leads to mistargeting of the protein to other locations, and (iii) fusion of the predicted PTS domain to a fluorescent protein directs the protein to peroxisomes. Using these methods, several novel PTS1 tripeptides (SSL>, SSI>, ASL>, SLM>, and SKV>) were conclusively identified in extended proteome studies (Reumann et al., 2007; Reumann et al., 2009). These novel PTS1 s can be directly applied to genome-wide searches for novel PTS1 proteins, with the note of caution that peroxisome targeting by these presumably weak PTS1 tripeptides is likely to depend on the presence of auxiliary targeting-enhancing elements in the upstream region. Additionally, the nonapeptide RVx5HF also represents a new PTS2 variant (Reumann et al., 2009; S. Quan and J. Hu, unpublished data).
With the increasing sensitivity of proteome studies, more peroxisomal proteins possessing PTS-related peptides or even lacking recognizable PTSs have been identified. This suggests that low-abundance proteins of plant peroxisomes mostly carry non-canonical and atypical PTS peptides and that yet unrecognized PTS1/2-independent targeting pathways exist for some peroxisomal matrix proteins.
4.4.4. Future challenges and opportunities
With elevated sensitivity in proteomics and MS methodology, quantification techniques become increasingly important to differentiate between peroxisomal proteins and low-abundance contaminants. Mass spectrometry is inherently poorly quantitative, but gel-based relative quantification techniques have been developed for quantification at the protein level (e.g., DIGE, differential in-gel electrophoresis) and successfully applied to plant peroxisomes (Eubel et al., 2008). In shotgun MS experiments, peptides can be quantified by spectral counts, i.e., integrated spectrum peak (Ong and Mann, 2005) or spectral abundance factors normalized to the protein size, NSAF (Paoletti et al., 2006). For the assignment of membrane proteins to specific organelles, the LOPIT (localization of organelle proteins by isotope tagging) method was developed for proteome studies of endomembranes (Dunkley et al., 2004; Dunkley et al., 2006); a related approach was also successfully applied to mouse peroxisomes (Wiese et al., 2007). However, these methods have not been employed to plant peroxisomes.
The identification of peroxisomal membrane proteins remains most challenging. Forty grams of yeast cells or Arabidopsis leaves, after labor-intensive enrichment procedures, generally yield less than 100 µg of purified peroxisomes. As such, the isolation of 50 µ9 of peroxisomal membrane proteins, which comprise less than 5% of total peroxisomal proteins, would require more than 10 high-purity organelle isolations. Moreover, membrane proteome analyses of peroxisomes require even higher peroxisome purity as compared to whole organelle proteome studies, because the membrane protein content of contaminating mitochondria and chloroplasts exceeds that of peroxisomes and leads to an apparent increase in non-peroxisomal proteins in the sample. Affinity-tagging of peroxisomal membrane proteins, a method used for S. cerevisiae (Marelli et al., 2004) and rat liver (Kikuchi et al., 2004) peroxisomes may help to alleviate this problem and even allow proteome characterization of peroxisomal sub-populations in plants.
In addition to reliable assignment of low-abundance proteins to peroxisomes by quantitative proteomics methods, it will soon be desirable to identify proteins transiently associated with peroxisomes and to monitor peroxisomal proteome alterations in Arabidopsis mutants. A widely applied alternative method for achieving higher sensitivity in protein identifications is chemical labelling of peptides derived from two different biological samples. One such example is isotope-coded affinity tagging (ICAT), which has been applied to Saccharomyces peroxisomes (Marelli et al., 2004). Its variant, referred to as isobaric Tag for Relative and Absolute Quantitation (iTRAQ), is increasingly applied. An alternative to chemical labelling is metabolic labelling of proteins with stable heavy isotopes. SILAC (stable isotope labelling by amino acids in cell culture) was particularly developed for cell cultures, in which 15N-L-lysine was provided to the cells. The relative protein abundance is determined by comparing lysine- or N-containing differentially labelled peptide pairs (Conrads et al., 2001). For proteome analysis of whole plants, hydroponic isotope labeling of entire plants (HILEP) seems to be a cost-effective method that enables metabolic labeling of whole and mature plants with a stable isotope such as 15N, which is applied in the form of nitrate as the sole nitrogen source (Bindschedler et al., 2008). The application of these quantitative MS methods will allow us to gain deeper insights into the dynamics of the peroxisome proteome and the characterization of peroxisomal subpopulations.
PERSPECTIVES
The post-genomic era has seen a rapid surge in the discovery and functional analysis of peroxisomal proteins. Collectively, these studies have clearly illustrated the complexity and functional diversity of plant peroxisomes. However, as alluded to in the text, there are several aspects of peroxisomes that are yet unresolved and merit further investigation. Moreover, our knowledge of the protein composition and metabolic and regulatory networks and fluxes within the peroxisome is still far from complete. We also know little of the mechanics and signals for inter-organellar coordination. Future studies might involve undertaking/exploiting interdisciplinary approaches to define the peroxisome in a systems biology context. The challenges then would be to assimilate the data generated from the various interdisciplinary approaches into building comprehensive and high-definition models for peroxisome biogenesis and function. These would be vital in garnering a better understanding of peroxisomes and their interplay with various organelles in plants.
ACKNOWLEDGMENTS
We are grateful to Gregg Howe for critical reading of the JA biosynthesis section, and Karen Bird for editorial assistance.
Footnotes
Citation: Kaur N., Reumann S., and Hu J.(2009) Peroxisome Biogenesis and Function. The Arabidopsis Book 7:e0123. doi:10.1199/tab.0123
elocation-id: e0123
First published on September 11, 2009
REFERENCES CITED
- Adam Z., Clarke A.K. Cutting edge of chloroplast proteolysis. Trends Plant Sci. 2002;77(1):451–456. doi: 10.1016/s1360-1385(02)02326-9. [DOI] [PubMed] [Google Scholar]
- Adham A.R., Zolman B.K., Millius A., Bartel B. Mutations in Arabidopsis acyl-CoA oxidase genes reveal distinct and overlapping roles in beta-oxidation. Plant J. 2005;417(1):859–874. doi: 10.1111/j.1365-313X.2005.02343.x. [DOI] [PubMed] [Google Scholar]
- Afitlhile M.M., Fukushige H., Nishimura M., Hildebrand D.F. A defect in glyoxysomal fatty acid beta-oxidation reduces jasmonic acid accumulation in Arabidopsis. Plant Physiol. Biochem. 2005;437(1):603–609. doi: 10.1016/j.plaphy.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Alcazar R., Marco F., Cuevas J.C., Patron M., Ferrando A., Carrasco P., Tiburcio A.F., Altabella T. Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 2006;287(1):1867–1876. doi: 10.1007/s10529-006-9179-3. [DOI] [PubMed] [Google Scholar]
- Anton M., Passreiter M., Lay D., Thai T.P., Gorgas K., Just W.W. ARF- and coatomer-mediated peroxisomal vesiculation. Cell Biochem. Biophys. 2000;327(1):27–36. doi: 10.1385/cbb:32:1-3:27. Spring. [DOI] [PubMed] [Google Scholar]
- Antonenkov V.D., Sormunen R.T., Hiltunen U.K. The rat liver peroxisomal membrane forms a permeability barrier for cofactors but not for small metabolites in vitro. J. Cell Sci. 2004;1177(1):5633–5642. doi: 10.1242/jcs.01485. [DOI] [PubMed] [Google Scholar]
- Appiano A., Bassi M., D'Agostino G. Cytochemical and autoradiographic observations on tomato bushy stunt virus-induced multivesicular bodies. Ultramicroscopy. 1983;127(1):162. [Google Scholar]
- Appiano A., D'Agostino G., Bassi M., Barbieri N., Viale G., Dell'Orto P. Origin and function of tomato busy stunt virus-induced inclusion bodies. II. Quantitative electron microscope autoradiography and immunogold cytochemistry. Ultrastruct. Mol. Struct. Res. 1986;977(1):31–38. [Google Scholar]
- Arai Y., Hayashi M., Nishimura M. Proteomic analysis of highly purified peroxisomes from etiolated soybean cotyledons. Plant Cell Physiol. 2008a;497(1):526–539. doi: 10.1093/pcp/pcn027. [DOI] [PubMed] [Google Scholar]
- Arai Y., Hayashi M., Nishimura M. Proteomic identification and characterization of a novel peroxisomal adenine nucleotide transporter supplying ATP for fatty acid beta-oxidation in soybean and Arabidopsis. Plant Cell. 2008b;207(1):3227–3240. doi: 10.1105/tpc.108.062877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura S., Tsutsumi N. A dynamin-like protein (ADL2b), rather than FtsZ, is involved in Arabidopsis mitochondrial division. Proc Natl. Acad. Sci. USA. 2002;997(1):5727–5731. doi: 10.1073/pnas.082663299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura S., Aida G.P., Fujimoto M., Nakazono M., Tsutsumi N. Arabidopsis dynamin-like protein 2a (ADL2a), like ADL2b, is involved in plant mitochondrial division. Plant Cell Physiol. 2004;457(1):236–242. doi: 10.1093/pcp/pch024. [DOI] [PubMed] [Google Scholar]
- Asada K. Ascorbate peroxidase: a hydrogen peroxide-scavenging enzyme in plants. Physiologia Plantarum. 1992;857(1):235–241. [Google Scholar]
- Asada K., Takahashi M. Production and scavenging of active oxygen in photosynthesis. Kyle DL, Osmond CB, Arntzen CJ, editors. Photoinhibition. 1987;7(1):227–287. [Google Scholar]
- Baker A., Graham I.A., Holdsworth M., Smith S.M., Theodoulou F.L. Chewing the fat: beta-oxidation in signalling and development. Trends Plant Sci. 2006;117(1):124–132. doi: 10.1016/j.tplants.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Bednarek P., Pislewska-Bednarek M., Svatos A., Schnelder B., Doubsky J., Mansurova M., Humphry M., Consonni C., Panstruga R., Sanchez-Vallet A., Molina A., Schulze-Lefert P. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science. 2009;3237(1):101–106. doi: 10.1126/science.1163732. [DOI] [PubMed] [Google Scholar]
- Beevers H. Microbodies in higher plants. Annu. Rev. Plant Physiol. 1979;307(1):159–193. [Google Scholar]
- Beevers H. Forty years in the new world. Annu. Rev. Plant Physiol. 1993;447(1):1–13. [Google Scholar]
- Behrends W., Engeland K., Kindl H. Characterization of two forms of the multifunctional protein acting in fatty acid beta-oxidation. Arch. Biochem. Biophys. 1988;2637(1):161–169. doi: 10.1016/0003-9861(88)90624-8. [DOI] [PubMed] [Google Scholar]
- Bindschedler L.V., Palmblad M., Cramer R. Hydroponic isotope labelling of entire plants (HILEP) for quantitative plant proteomics; an oxidative stress case study. Phytochemistry. 2008;697(1):1962–1972. doi: 10.1016/j.phytochem.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A., Frietsch S., Kim T.H., Dizon M.B., Schroeder J.I. The peroxin loss-of-function mutation abstinence by mutual consent disrupts male-female gametophyte recognition. Curr. Biol. 2008;187(1):63–68. doi: 10.1016/j.cub.2007.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury L.M., Gillies S.A., Brushett D.J., Waters D.L., Henry R.J. Inactivation of an aminoaldehyde dehydrogenase is responsible for fragrance in rice. Plant Mol. Biol. 2008;687(1):439–449. doi: 10.1007/s11103-008-9381-x. [DOI] [PubMed] [Google Scholar]
- Breithaupt C., Kurzbauer R., Lilie H., Schaller A., Strassner J., Huber R., Macheroux P., Clausen T. Crystal structure of 12-oxophytodienoate reductase 3 from tomato: self-inhibition by dimerization. Proc. Natl. Acad. Sci. USA. 2006;1037(1):14337–14342. doi: 10.1073/pnas.0606603103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner C. Hint, Fhit, and GalT: function, structure, evolution, and mechanism of three branches of the histidine triad superfamily of nucleotide hydrolases and transferases. Biochemistry. 2002;417(1):9003–9014. doi: 10.1021/bi025942q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickner D.G., Olsen L.J. Nucleotide triphosphates are required for the transport of glycolate oxidase into peroxisomes. Plant Physiol. 1998;1167(1):309–317. doi: 10.1104/pp.116.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson L.F., Zelitch I., Havir E.A. Manipulation of catalase levels produces altered photosynthesis in transgenic tobacco plants. Plant Physiol. 1998;1167(1):259–269. doi: 10.1104/pp.116.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueso E., Alejandro S., Carbonell P., Perez-Amador M.A., Fayos J., Belles J.M., Rodriguez P.L., Serrano R. The lithium tolerance of the Arabidopsis cat2 mutant reveals a cross-talk between oxidative stress and ethylene. Plant J. 2007;527(1):1052–1065. doi: 10.1111/j.1365-313X.2007.03305.x. [DOI] [PubMed] [Google Scholar]
- Bunkelmann J.R., Trelease R.N. Ascorbate peroxidase. A prominent membrane protein in oilseed glyoxysomes. Plant Physiol. 1996;1107(1):589–598. doi: 10.1104/pp.110.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butow R.A., Avadhani N.G. Mitochondrial signaling: the retrograde response. Mol. Cell. 2004;147(1):1–15. doi: 10.1016/s1097-2765(04)00179-0. [DOI] [PubMed] [Google Scholar]
- Carrie C., Murcha M.W., Millar A.H., Smith S.M., Whelan J. Nine 3-ketoacyl-CoA thiolases (KATs) and acetoacetyl-CoA thiolases (ACATs) encoded by five genes in Arabidopsis thaliana are targeted either to peroxisomes or cytosol but not to mitochondria. Plant Mol. Biol. 2007;637(1):97–108. doi: 10.1007/s11103-006-9075-1. [DOI] [PubMed] [Google Scholar]
- Carrie C., Kuhn K., Murcha M.W., Duncan O., Small I.D., O'Toole N., Whelan J. Approaches to defining dual-targeted proteins in Arabidopsis. Plant J. 2009;577(1):1128–1139. doi: 10.1111/j.1365-313X.2008.03745.x. [DOI] [PubMed] [Google Scholar]
- Carrie C., Murcha M.W., Kuehn K., Duncan O., Barthet M., Smith P.M., Eubel H., Meyer E., Day D.A., Millar A.H., Whelan J. Type II NAD(P)H dehydrogenases are targeted to mitochondria and chloroplasts or peroxisomes in Arabidopsis thaliana. FEBS Lett. 2008;5827(1):3073–3079. doi: 10.1016/j.febslet.2008.07.061. [DOI] [PubMed] [Google Scholar]
- Carvalho A.F., Pinto M.P., Grou C.P., Alencastre I.S., Fransen M., Sa-Miranda C., Azevedo J.E. Ubiquitination of mammalian Pex5p, the peroxisomal import receptor. J. Biol. Chem. 2007;2827(1):31267–31272. doi: 10.1074/jbc.M706325200. [DOI] [PubMed] [Google Scholar]
- Castillo M.C., Sandalio L.M., Del Rio L.A., Leon J. Peroxisome proliferation, wound-activated responses and expression of peroxisome-associated genes are cross-regulated but uncoupled in Arabidopsis thaliana. Plant Cell Environ. 2008;317(1):492–505. doi: 10.1111/j.1365-3040.2008.01780.x. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. The simultaneous symbiotic origin of mitochondria, chloroplasts, and microbodies. Ann. New York Acad. Sci. 1987;5037(1):55–71. doi: 10.1111/j.1749-6632.1987.tb40597.x. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Symbiotic origin of peroxisomes. (Paris: Institut National de la Recherche Agronomique); 1990. [Google Scholar]
- Cavalier-Smith T. Predation and eukaryote cell origins: a coevolutionary perspective. Int. J. Biochem. Cell Biol. 2009;417(1):307–322. doi: 10.1016/j.biocel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Chao C.Y., Huang C.J. Bitter gourd (Momordica charantia) extract activates peroxisome proliferator-activated receptors and upregulates the expression of the acyl CoA oxidase gene in H4IIEC3 hepatoma cells. J. Biomed. Sci. 2003;107(1):782–791. doi: 10.1159/000073966. [DOI] [PubMed] [Google Scholar]
- Chen S., Yang Y., Shi W., Ji Q., He F., Zhang Z., Cheng Z., Liu X., Xu M. Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell. 2008;207(1):1850–1861. doi: 10.1105/tpc.108.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay N.K., Adio A.M., Denoux C., Jander G., Ausubel F.M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science. 2009;3237(1):95–101. doi: 10.1126/science.1164627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cona A., Rea G., Angelini R., Federico R., Tavladoraki P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006;117(1):80–88. doi: 10.1016/j.tplants.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Conrads T.P., Alving K., Veenstra T.D., Belov M.E., Andersen G.A., Anderson D.J., Lipton M.S., Pasa-Tolic L., Udseth H.R., Chrisler W.B., Thrall B.D., Smith R.D. Quantitative analysis of bacterial and mammalian proteomes using a combination of cysteine affinity tags and 15N-metabolic labeling. Anal. Chem. 2001;737(1):2132–2139. doi: 10.1021/ac001487x. [DOI] [PubMed] [Google Scholar]
- Cornah J.E., Germain V., Ward J.L., Beale M.H., Smith S.M. Lipid utilization, gluconeogenesis, and seedling growth in Arabidopsis mutants lacking the glyoxylate cycle enzyme malate synthase. J. Biol. Chem. 2004;2797(1):42916–42923. doi: 10.1074/jbc.M407380200. [DOI] [PubMed] [Google Scholar]
- Corpas F.J., Trelease R.N. The plant 73 kDa peroxisomal membrane protein (PMP73) is immunorelated to molecular chaperones. Eur. J. Cell Biol. 1997;737(1):49–57. [PubMed] [Google Scholar]
- Corpas F.J., Sandalio L.M., Brown M.J., del Rio L.A., Trelease R.N. Identification of porin-like polypeptide(s) in the boundary membrane of oilseed glyoxysomes. Plant Cell Physiol. 2000;417(1):1218–1228. doi: 10.1093/pcp/pcd054. [DOI] [PubMed] [Google Scholar]
- Cousins A.B., Pracharoenwattana I., Zhou W., Smith S.M., Badger M.R. Peroxisomal malate dehydrogenase is not essential for photorespiration in Arabidopsis but its absence causes an increase in the stoichiometry of photorespiratory CO2 release. Plant Physiol. 2008;1487(1):786–795. doi: 10.1104/pp.108.122622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crookes W.J., Olsen L.J. The effects of chaperones and the influence of protein assembly on peroxisomal protein import. J. Biol. Chem. 1998;2737(1):17236–17242. doi: 10.1074/jbc.273.27.17236. [DOI] [PubMed] [Google Scholar]
- Cruz Castillo M., Martinez C., Buchala A., Metraux J.P., Leon J. Gene-specific involvement of beta-oxidation in wound-activated responses in Arabidopsis. Plant Physiol. 2004;1357(1):85–94. doi: 10.1104/pp.104.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins I., Cole D.J., Edwards R. A role for glutathione transferases functioning as glutathione peroxidases in resistance to multiple herbicides in black-grass. Plant J. 1999;187(1):285–292. doi: 10.1046/j.1365-313x.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- Dammal V., Subramani S. The human peroxisomal targeting signal receptor, Pex5p, is translocated into the peroxisomal matrix and recycled to the cytosol. Cell. 2001;1057(1):187–196. doi: 10.1016/s0092-8674(01)00310-5. [DOI] [PubMed] [Google Scholar]
- de Duve C. The origin of eukaryotes: a reappraisal. Nat. Rev. Genet. 2007;87(1):395–403. doi: 10.1038/nrg2071. [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966;467(1):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- de Felipe M.R., Lucas M.M., Pozuelo J.M. Cytochemical study of catalase and peroxidase in the mesophyll of Lollium rigidum plants treated with isoproturon. J. Plant Physiol. 1988;1327(1):67–73. [Google Scholar]
- del Rio L.A., Sandalio L.M., Corpas F.J., Palma J.M., Barroso J.B. Reactive oxygen species and reactive nitrogen species in peroxisomes: production, scavenging, and role in cell signaling. Plant Physiol. 2006;1417(1):330–335. doi: 10.1104/pp.106.078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio L.A., Pastori G.M., Palma J.M., Sandalio L.M., Sevilla F., Corpas F.J., Jimenez A., Lopez-Huertas E., Hernandez J.A. The activated oxygen role of peroxisomes in senescence. Plant Physiol. 1998;1167(1):1195–1200. doi: 10.1104/pp.116.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delker C., Zolman B.K., Miersch O., Wasternack C. Jasmonate biosynthesis in Arabidopsis thaliana requires peroxisomal betaoxidation enzymes--additional proof by properties of pex6 and aim1. Phytochemistry. 2007;687(1):1642–1650. doi: 10.1016/j.phytochem.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Desai M., Hu J. Light induces peroxisome proliferation in Arabidopsis seedlings through the photoreceptor phytochrome A, the transcription factor HY5 HOMOLOG, and the peroxisomal protein PEROXIN11b. Plant Physiol. 2008;1467(1):1117–1127. doi: 10.1104/pp.107.113555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvergne B., Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 1999;207(1):649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Diefenbach J., Kindl H. The membrane-bound DnaJ protein located at the cytosolic site of glyoxysomes specifically binds the cytosolic isoform 1 of Hsp70 but not other Hsp70 species. Eur. J. Biochem. 2000;2677(1):746–754. doi: 10.1046/j.1432-1327.2000.01053.x. [DOI] [PubMed] [Google Scholar]
- Dietz K.J., Jacob S., Oelze M.L., Laxa M., Tognetti V., de Miranda S.M., Baler M., Finkemeier I. The function of peroxiredoxins in plant organelle redox metabolism. J. Exp. Bot. 2006;577(1):1697–1709. doi: 10.1093/jxb/erj160. [DOI] [PubMed] [Google Scholar]
- Distefano S., Palma J.M., Gomez M., Rio L.A. Characterization of endoproteases from plant peroxisomes. Biochem. J. 1997;3277(1):399–405. doi: 10.1042/bj3270399. (Pt2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distefano S., Palma J.M., McCarthy I.I., del Rio L.A. Proteolytic cleavage of plant proteins by peroxisomal endoproteases from senescent pea leaves. Planta. 1999;2097(1):308–313. doi: 10.1007/s004250050637. [DOI] [PubMed] [Google Scholar]
- Distel B., Erdmann R., Gould S.J., Blobel G., Crane D.I., Cregg J.M., Dodt G., Fujikl Y., Goodman J.M., Just W.W., Kiel J.A., Kunau W.H., Lazarow P.B., Mannaerts G.P., Moser H.W., Osumi T., Rachubinski R.A., Roscher A., Subramani S., Tabak H.F., Tsukamoto T., Valle D., van der Klei I., van Veldhoven P.P., Veenhuis M. A unified nomenclature for peroxisome biogenesis factors. J. Cell Biol. 1996;1357(1):1–3. doi: 10.1083/jcb.135.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon O.P., Hawkins T., Hussey P.J., Edwards R. Enzyme activities and subcellular localization of members of the Arabidopsis glutathione transferase superfamily. J. Exp. Bot. 2009;607(1):1207–1218. doi: 10.1093/jxb/ern365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C.H., Zolman B.K., Bartel B., Lee B.H., Stevenson B., Agarwal M., Zhu J.K. Disruption of Arabidopsis CHY1 reveals an important role of metabolic status in plant cold stress signaling. Mol. Plant. 2009;27(1):59–72. doi: 10.1093/mp/ssn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey S., Baillieul F., Saindrenan P., Fritig B., Kauffmann S. Tobacco class I and class II catalases are differentially expressed during elicitor-induced hypersensitive cell death and localized acquired resistance. Mol. Plant—Microbe Interact. 1998;117(1):1102–1109. [Google Scholar]
- Dunkley T.P., Watson R., Griffin J.L., Dupree P., Lilley K.S. Localization of organelle proteins by isotope tagging (LOPIT). Mol. Cell Proteomics. 2004;37(1):1128–1134. doi: 10.1074/mcp.T400009-MCP200. [DOI] [PubMed] [Google Scholar]
- Dunkley T.P., Hester S., Shadforth I.P., Runions J., Weimar T., Hanton S.L., Griffin J.L., Bessant C., Brandizzi F., Hawes C., Watson R.B., Dupree P., Lilley K.S. Mapping the Arabidopsis organelle proteome. Proc. Natl. Acad. Sci. USA. 2006;1037(1):6518–6523. doi: 10.1073/pnas.0506958103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond P.J. MONODEHYROASCORBATE REDUCTASE4 is required for seed storage oil hydrolysis and postgerminative growth in Arabidopsis. Plant Cell. 2007;197(1):1376–1387. doi: 10.1105/tpc.106.043992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond P.J., Germain V., Lange P.R., Bryce J.H., Smith S.M., Graham I.A. Postgerminative growth and lipid catabolism in oilseeds lacking the glyoxylate cycle. Proc. Natl. Acad. Sci. USA. 2000;977(1):5669–5674. doi: 10.1073/pnas.97.10.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert J.H., Erdmann R. Peroxisome biogenesis. Rev Physiol Biochem Pharmacol. 2003;1477(1):75–121. doi: 10.1007/s10254-003-0007-z. [DOI] [PubMed] [Google Scholar]
- Edwards R., Dixon D.P., Walbot V. Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci. 2000;57(1):193–198. doi: 10.1016/s1360-1385(00)01601-0. [DOI] [PubMed] [Google Scholar]
- Eilers T., Schwarz G., Brinkmann H., Witt C., Richter T., Nieder J., Koch B., Hille R., Hansch R., Mendel R.R. Identification and biochemical characterization of Arabidopsis thaliana sulfite oxidase. A new player in plant sulfur metabolism. J. Biol. Chem. 2001;2767(1):46989–46994. doi: 10.1074/jbc.M108078200. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O., Elofsson A., von Heijne G., Cristobal S. In silico prediction of the peroxisomal proteome in fungi, plants and animals. J. Mol. Biol. 2003;3307(1):443–456. doi: 10.1016/s0022-2836(03)00553-9. [DOI] [PubMed] [Google Scholar]
- Epstein C.B., Waddle J.A., Hale W.t., Dave V., Thornton J., Macatee T.L., Garner H.R., Butow R.A. Genome-wide responses to mitochondrial dysfunction. Mol. Biol. Cell. 2001;127(1):297–308. doi: 10.1091/mbc.12.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann R., Blobel G. Giant peroxisomes in oleic acid-induced Saccharomyces cerevisiae lacking the peroxisomal membrane protein Pmp27p. J. Cell Biol. 1995;1287(1):509–523. doi: 10.1083/jcb.128.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann R., Schliebs W. Peroxisomal matrix protein import: the transient pore model. Nat. Rev. Mol. Cell Biol. 2005;67(1):738–742. doi: 10.1038/nrm1710. [DOI] [PubMed] [Google Scholar]
- Erdmann R., Veenhuis M., Kunau W.H. Peroxisomes: Organelles at the crossroads. Trends Cell Biol. 1997;77(1):400–407. doi: 10.1016/S0962-8924(97)01126-4. [DOI] [PubMed] [Google Scholar]
- Eubel H., Lee C.P., Kuo J., Meyer E.H., Taylor N.L., Millar A.M. Free-flow electrophoresis for purification of plant mitochondria by surface charge. Plant J. 2007;527(1):583–594. doi: 10.1111/j.1365-313X.2007.03253.x. [DOI] [PubMed] [Google Scholar]
- Eubel H., Meyer E.H., Taylor N.L., Bussell J.D., O'Toole N., Heazlewood J.L., Castleden I., Small I.D., Smith S.M., Millar A.M. Novel proteins, putative membrane transporters, and an integrated metabolic network are revealed by quantitative proteomic analysis of Arabidopsis cell culture peroxisomes. Plant Physiol. 2008;1487(1):1809–1829. doi: 10.1104/pp.108.129999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber K.N., Haan G.J., Baerends R.J., Kram A.M., Veenhuis M. Normal peroxisome development from vesicles induced by truncated Hansenula polymorpha Pex3p. J. Biol. Chem. 2002;2777(1):11026–11033. doi: 10.1074/jbc.M112347200. [DOI] [PubMed] [Google Scholar]
- Fagarasanu A., Fagarasanu M., Rachubinski R.A. Maintaining peroxisome populations: a story of division and inheritance. Annu. Rev. Cell Dev. Biol. 2007;237(1):321–344. doi: 10.1146/annurev.cellbio.23.090506.123456. [DOI] [PubMed] [Google Scholar]
- Fagarasanu A., Fagarasanu M., Eitzen G.A., Aitchison J.D., Rachubinski R.A. The peroxisomal membrane protein Inp2p is the peroxisome-specific receptor for the myosin V motor Myo2p of Saccharomyces cerevisiae. Dev. Cell. 2006;107(1):587–600. doi: 10.1016/j.devcel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Fagarasanu M., Fagarasanu A., Tam Y.Y., Aitchison J.D., Rachubinski R.A. Inp1p is a peroxisomal membrane protein required for peroxisome inheritance in Saccharomyces cerevisiae. J. Cell Biol. 2005;1697(1):765–775. doi: 10.1083/jcb.200503083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Quan S., Orth T., Awai C., Chory J., Hu J. The Arabidopsis PEX12 gene is required for peroxisome biogenesis and is essential for development. Plant Physiol. 2005;1397(1):231–239. doi: 10.1104/pp.105.066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Morrell J.C., Jones J.M., Gould S.J. PEX3 functions as a PEX19 docking factor in the import of class I peroxisomal membrane proteins. J. Cell Biol. 2004;1647(1):863–875. doi: 10.1083/jcb.200311131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farres J., Holmberg N., Schlattner U., Bailey J.E., Walllmann T., Kallio P.T. Expressing creatine kinase in transgenic tobacco-a first step towards introducing an energy buffering system in plants. Transgenic Res. 2002;117(1):49–59. doi: 10.1023/a:1013957819596. [DOI] [PubMed] [Google Scholar]
- Fawcett C.H., Wain R.L., Wightman F. The metabolism of 3-indolylalkanecarboxylic acids, and their amides, nitriles and methyl esters in plant tissues. Proc. Royal Soc. London Ser B. 1960;1527(1):231–254. doi: 10.1098/rspb.1960.0035. [DOI] [PubMed] [Google Scholar]
- Feierabend J., Engel S. Photoinactivation of catalase in vitro and in leaves. Arch. Biochem. Biophys. 1986;2517(1):567–576. doi: 10.1016/0003-9861(86)90365-6. [DOI] [PubMed] [Google Scholar]
- Feierabend J., Schaan C., Hertwig B. Photoinactivation of Catalase Occurs under Both High- and Low-Temperature Stress Conditions and Accompanies Photoinhibition of Photosystem II. Plant Physiol. 1992;1007(1):1554–1561. doi: 10.1104/pp.100.3.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R.M.B., Bird B., Davies D.D. The effect of light on the ultrastructure and organisation of Lemna peroxisomes. J. Exp. Bot. 1989;407(1):1029–1035. [Google Scholar]
- Flynn C.R., Mullen R.T., Trelease R.N. Mutational analyses of a type 2 peroxisomal targeting signal that is capable of directing oligomeric protein import into tobacco BY-2 glyoxysomes. Plant J. 1998;167(1):709–720. doi: 10.1046/j.1365-313x.1998.00344.x. [DOI] [PubMed] [Google Scholar]
- Flynn C.R., Heinze M., Schumann U., Gietl C., Trelease N.R. Compartmentation of the plant peroxin, AtPex10p, within subdomain(s) of ER. Plant Science. 2005;1687(1):635–652. [Google Scholar]
- Footitt S., Slocombe S.P., Larner V., Kurup S., Wu Y., T Larson., Graham I., Baker A., Holdsworth M. Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. Embo J. 2002;217(1):2912–2922. doi: 10.1093/emboj/cdf300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foth B.J., Goedecke M.C., Soldati D. New insights into myosin evolution and classification. Proc. Nat. Acad. Sci. USA. 2006;1037(1):3681–3686. doi: 10.1073/pnas.0506307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C.H., Bloom A.J., Queval G., Noctor G. Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annu. Rev. Plant Biol. 2009;607(1):455–484. doi: 10.1146/annurev.arplant.043008.091948. [DOI] [PubMed] [Google Scholar]
- Fransen M., Vastiau I., Brees C., Brys V., Mannaerts G.P., Van Veldhoven P.P. Potential role for Pex19p in assembly of PTS-receptor docking complexes. J. Biol. Chem. 2004;2797(1):12615–12624. doi: 10.1074/jbc.M304941200. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Matsuzono Y., Matsuzaki T., Fransen M. Import of peroxisomal membrane proteins: the interplay of Pex3p- and Pex19p-mediated interactions. Biochim. Biophys. Acta. 2006;17637(1):1639–1646. doi: 10.1016/j.bbamcr.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Fujimoto M., Arimura S.I., Mano S., Kondo M., Saito C., Ueda T., Nakazono M., Nakano A., Nishimura M., Tsutsumi N. Arabidopsis dynamin-related proteins DRP3A and DRP3B are functionally redundant in mitochondrial fission, but have distinct roles in peroxisomal fission. Plant J. 2009;587(1):388–400. doi: 10.1111/j.1365-313X.2009.03786.x. [DOI] [PubMed] [Google Scholar]
- Fukao Y., Hayashi M., Nishimura M. Proteomic analysis of leaf peroxisomal proteins in greening cotyledons of Arabidopsis thaliana. Plant Cell Physiol. 2002;437(1):689–696. doi: 10.1093/pcp/pcf101. [DOI] [PubMed] [Google Scholar]
- Fukao Y., Hayashi M., Hara-Nishimura I., Nishimura M. Novel glyoxysomal protein kinase, GPK1, identified by proteomic analysis of glyoxysomes in etiolated cotyledons of Arabidopsis thaliana. Plant Cell Physiol. 2003;447(1):1002–1012. doi: 10.1093/pcp/pcg145. [DOI] [PubMed] [Google Scholar]
- Fulda M., Schnurr J., Abbadl A., Heinz E., Browse J. Peroxisomal Acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana. Plant Cell. 2004;167(1):394–405. doi: 10.1105/tpc.019646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaldon T., Snel B., van Zimmeren F., Hemrika W., Tabak H., Huynen M.A. Origin and evolution of the peroxisomal proteome. Biol. Direct. 2006;17(1):8. doi: 10.1186/1745-6150-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakh O., Cavadini P., lsaya G. Mitochondrial processing peptidases. Biochim. Biophys. Acta. 2002;15927(1):63–77. doi: 10.1016/s0167-4889(02)00265-3. [DOI] [PubMed] [Google Scholar]
- Galili G., Tang G., Zhu X., Gakiere B. Lysine catabolism: a stress and development super-regulated metabolic pathway. Curr. Opin. Plant Biol. 2001;47(1):261–266. doi: 10.1016/s1369-5266(00)00170-9. [DOI] [PubMed] [Google Scholar]
- Gandre-Babbe S., van der Bliek A.M. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol. Biol. Cell. 2008;197(1):2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbling H., Gerhardt B. Oxidative decarboxylation of branched-chain 2-oxo fatty acids by higher plant peroxisomes. Plant Physiol. 1988;887(1):13–15. doi: 10.1104/pp.88.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbling H., Gerhardt B. Peroxisomal degradation of branched-chain 2-oxo acids. Plant Physiol. 1989;917(1):1387–1392. doi: 10.1104/pp.91.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H.J., Murk J.L., Stroobants A.K., Griffith J.M., Kleijmeer M.J., Koster A.J., Verkleij A.J., Distel B., Tabak H.F. Involvement of the endoplasmic reticulum in peroxisome formation. Mol. Biol. Cell. 2003;147(1):2900–2907. doi: 10.1091/mbc.E02-11-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepfert S., Poirier Y. Beta-oxidation in fatty acid degradation and beyond. Curr. Opin. Plant Biol. 2007;107(1):245–251. doi: 10.1016/j.pbi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Goepfert S., Hiltunen U.K., Poirier Y. Identification and functional characterization of a monofunctional peroxisomal enoyl-CoA hydratase 2 that participates in the degradation of even cis-unsaturated fatty acids in Arabidopsis thaliana. J. Biol. Chem. 2006;2817(1):35894–35903. doi: 10.1074/jbc.M606383200. [DOI] [PubMed] [Google Scholar]
- Goepfert S., Vidoudez C., Rezzonico E., Hiltunen U.K., Poirier Y. Molecular identification and characterization of the Arabidopsis delta(3,5), delta(2,4)-dienoyl-coenzyme A isomerase, a peroxisomal enzyme participating in the beta-oxidation cycle of unsaturated fatty acids. Plant Physiol. 2005;1387(1):1947–1956. doi: 10.1104/pp.105.064311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepfert S., Vidoudez C., Tellgren-Roth C., Delessert S., Hiltunen J.K., Poirier Y. Peroxisomal delta(3), delta(2)-enoyl CoA isomerases and evolution of cytosolic paralogues in embrophytes. Plant J. 2008;567(1):728–742. doi: 10.1111/j.1365-313X.2008.03635.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Gomez L., Boiler T. Flagellin perception: a paradigm for innate immunity. Trends Plant Sci. 2002;77(1):251–256. doi: 10.1016/s1360-1385(02)02261-6. [DOI] [PubMed] [Google Scholar]
- Goyer A., Johnson T.L., Olsen L.J., Collakova E., Shachar-Hill Y., Rhodes D., Hanson A.D. Characterization and metabolic function of a peroxisomal sarcosine and pipecolate oxidase from Arabidopsis. J. Biol. Chem. 2004;2797(1):16947–16953. doi: 10.1074/jbc.M400071200. [DOI] [PubMed] [Google Scholar]
- Graham I.A. Seed storage oil mobilization. Annu. Rev. Plant. Biol. 2008;597(1):115–142. doi: 10.1146/annurev.arplant.59.032607.092938. [DOI] [PubMed] [Google Scholar]
- Graham I.A., Eastmond P.J. Pathways of straight and branched chain fatty acid catabolism in higher plants. Prog. Lipid Res. 2002;417(1):156–181. doi: 10.1016/s0163-7827(01)00022-4. [DOI] [PubMed] [Google Scholar]
- Grene R. Oxidative Stress and Acclimation Mechanisms in Plants :April 4, 2002. The Arabidopsis Book. Rockville, MD: American Society of Plant Biologists; 2002. doi: 10.1199/tab.0036.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guhnemann-Schafer K., Kindl H. The leaf peroxisomal form (MFP IV) of multifunctional protein functioning in fatty acid betaoxidation. Planta. 1995a;1967(1):642–646. [Google Scholar]
- Guhnemann-Schafer K., Kindl H. Fatty acid beta-oxidation in glyoxysomes. Characterization of a new tetrafunotional protein (MFP III). Biochim. Biophys. Acta. 1995b;12567(1):181–186. doi: 10.1016/0005-2760(95)00020-d. [DOI] [PubMed] [Google Scholar]
- Guo T., Gregg C., Boukh-Viner T., Kyryakov P., Goldberg A., Bourque S., Banu F., Haile S., Milijevic S., San K.H., Solomon J., Wong V., Titorenko V.I. A signal from inside the peroxisome initiates its division by promoting the remodeling of the peroxisomal membrane. J. Cell Biol. 2007;1777(1):289–303. doi: 10.1083/jcb.200609072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvitz A., Rottensteiner H. The biochemistry of oleate induction: Transcriptional upregulation and peroxisome proliferation. Biochim. Biophys. Acta. 2006;17637(1):1392–1402. doi: 10.1016/j.bbamcr.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Haan G.J., Baerends R.J., Krikken A.M., Otzen M., Veenhuis M., van der Klei I.J. Reassembly of peroxisomes in Hansenula polymorpha pex3 cells on reintroduction of Pex3p involves the nuclear envelope. FEMS Yeast Res. 2006;67(1):186–194. doi: 10.1111/j.1567-1364.2006.00037.x. [DOI] [PubMed] [Google Scholar]
- Hadden D.A., Phillipson B.A., Johnston K.A., Brown L.A., Manfield I.W., El-Shami M., Sparkes I.A., Baker A. Arabidopsis PEX19 is a dimeric protein that binds the peroxin PEX10. Mol. Membr. Biol. 2006;237(1):325–336. doi: 10.1080/09687860600738221. [DOI] [PubMed] [Google Scholar]
- Hallstrom T.C., Moye-Rowley W.S. Multiple signals from dysfunctional mitochondria activate the pleiotropic drug resistance pathway in Saccharomyces cerevisiae. J. Biol. Chem. 2000;2757(1):37347–37356. doi: 10.1074/jbc.M007338200. [DOI] [PubMed] [Google Scholar]
- Hansch R., Mendel R.R. Sulfite oxidation in plant peroxisomes. Photosynth. Res. 2005;867(1):337–343. doi: 10.1007/s11120-005-5221-x. [DOI] [PubMed] [Google Scholar]
- Hansch R., Lang C., Riebeseel E., Lindigkeit R., Gessler A., Rennenberg H., Mendel R.R. Plant sulfite oxidase as novel producer of H2O2: combination of enzyme catalysis with a subsequent non-enzymatic reaction step. J. Biol. Chem. 2006;2817(1):6884–6888. doi: 10.1074/jbc.M513054200. [DOI] [PubMed] [Google Scholar]
- Hardham A.R., Takemoto D., White R.G. Rapid and dynamic subcellular reorganization following mechanical stimulation of Arabidopsis epidermal cells mimics responses to fungal and oomycete attack. BMC Plant Biol. 2008;87(1):63. doi: 10.1186/1471-2229-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Igarashi H., Mano S., Nishimura M., Shimmen T., Yokota E. Peroxisomal localization of a myosin XI isoform in Arabidopsis thaliana. Plant Cell Physiol. 2005;467(1):782–789. doi: 10.1093/pcp/pci085. [DOI] [PubMed] [Google Scholar]
- Hawkins J., Mahony D., Maetschke S., Wakabayashi M., Teasdale R.D., Boden M. Identifying novel peroxisomal proteins. Proteins. 2007;697(1):606–616. doi: 10.1002/prot.21420. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Nishimura M. Entering a new era of research on plant peroxisomes. Curr. Opin. Plant Biol. 2003;67(1):577–582. doi: 10.1016/j.pbi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Nishimura M. Arabidopsis thaliana—a model organism to study plant peroxisomes. Biochim. Biophys. Acta. 2006;17637(1):1382–1391. doi: 10.1016/j.bbamcr.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Aoki M., Kondo M., Nishimura M. Changes in targeting efficiencies of proteins to plant microbodies caused by amino acid substitutions in the carboxy-terminal tripeptide. Plant Cell Physiol. 1997;387(1):759–768. doi: 10.1093/oxfordjournals.pcp.a029233. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Toriyama K., Kondo M., Nishimura M. 2,4-Dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid beta-oxidation. Plant Cell. 1998;107(1):183–195. doi: 10.1105/tpc.10.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Aoki M., Kato A., Kondo M., Nishimura M. Transport of chimeric proteins that contain a carboxy-terminal targeting signal into plant microbodies. Plant J. 1996;107(1):225–234. doi: 10.1046/j.1365-313x.1996.10020225.x. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Yagi M., Nito K., Kamada T., Nishimura M. Differential contribution of two peroxisomal protein receptors to the maintenance of peroxisomal functions in Arabidopsis. J. Biol. Chem. 2005;2807(1):14829–14835. doi: 10.1074/jbc.M411005200. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Nito K., Toriyama-Kato K., Kondo M., Yamaya T., Nishimura M. AtPex14p maintains peroxisomal functions by determining protein targeting to three kinds of plant peroxisomes. EMBO J. 2000;197(1):5701–5710. doi: 10.1093/emboj/19.21.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Nito K., Takei-Hoshi R., Yagi M., Kondo M., Suenaga A., Yamaya T., Nishimura M. Ped3p is a peroxisomal ATP-binding cassette transporter that might supply substrates for fatty acid beta-oxidation. Plant Cell Physiol. 2002;437(1):1–11. doi: 10.1093/pcp/pcf023. [DOI] [PubMed] [Google Scholar]
- Heiland I., Erdmann R. Biogenesis of peroxisomes. Topogenesis of the peroxisomal membrane and matrix proteins. FEBS J. 2005;2727(1):2362–2372. doi: 10.1111/j.1742-4658.2005.04690.x. [DOI] [PubMed] [Google Scholar]
- Heim W.G., Sykes K.A., Hildreth S.B., Sun J., Lu R.H., Jelesko J.G. Cloning and characterization of a Nicotiana tabacum methylputrescine oxidase transcript. Phytochemistry. 2007;687(1):454–463. doi: 10.1016/j.phytochem.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Helm M., Luck C., Prestele J., Hierl G., Huesgen P.F., Frohlich T., Arnold G.J., Adamska I., Gorg A., Lottspeich F., Gietl C. Dual specificities of the glyoxysomal/peroxisomal processing protease Deg15 in higher plants. Proc. Natl. Acad. Sci. USA. 2007;1047(1):11501–11506. doi: 10.1073/pnas.0704733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennebry S.C., Wright H.M., Likic V.A., Richardson S.J. Structural and functional evolution of transthyretin and transthyretin-like proteins. Proteins. 2006;647(1):1024–1045. doi: 10.1002/prot.21033. [DOI] [PubMed] [Google Scholar]
- Hertwig B., Streb P., Feierabend J. Light dependence of catalase synthesis and degradation in leaves and the influence of interfering stress conditions. Plant Physiol. 1992;1007(1):1547–1553. doi: 10.1104/pp.100.3.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema E.H., Girzalsky W., van Den Berg M., Erdmann R., Distel B. Saccharomyces cerevisiae pex3p and pex19p are required for proper localization and stability of peroxisomal membrane proteins. EMBO J. 2000;197(1):223–233. doi: 10.1093/emboj/19.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heupel R., Heldt H.W. Protein organization in the matrix of leaf peroxisomes. A multi-enzyme complex involved in photorespiratory metabolism. Eur. J. Biochem. 1994;2207(1):165–172. doi: 10.1111/j.1432-1033.1994.tb18611.x. [DOI] [PubMed] [Google Scholar]
- Heupel R., Markgraf T., Robinson D.G., Heldt H.W. Compartmentation Studies on Spinach Leaf Peroxisomes : Evidence for Channeling of Photorespiratory Metabolites in Peroxisomes Devoid of Intact Boundary Membrane. Plant Physiol. 1991;967(1):971–979. doi: 10.1104/pp.96.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino T., Waditee R., Araki E., Ishikawa H., Aoki K., Tanaka Y., Takabe T. Functional characterization of choline monooxygenase, an enzyme for betaine synthesis in plants. J. Biol. Chem. 2002;2777(1):41352–41360. doi: 10.1074/jbc.M205965200. [DOI] [PubMed] [Google Scholar]
- Hoepfner D., van den Berg M., Philippsen P., Tabak H.F., Hettema E.H. A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J. Cell Biol. 2001;1557(1):979–990. doi: 10.1083/jcb.200107028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepfner D., Schildknegt D., Braakman I., Philippsen P., Tabak H.F. Contribution of the endoplasmic reticulum to peroxisome formation. Cell. 2005;1227(1):85–95. doi: 10.1016/j.cell.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Hong Z., Bednarek S.Y., Blumwald E., Hwang I., Jurgens G., Menzel D., Osteryoung K.W., Raikhel N.V., Shinozaki K., Tsutsumi N., Verma D.P. A unified nomenclature for Arabidopsis dynaminrelated large GTPases based on homology and possible functions. Plant Mol. Biol. 2003;537(1):261–265. doi: 10.1023/b:plan.0000007000.29697.81. [DOI] [PubMed] [Google Scholar]
- Hooks M.A., Turner J.E., Murphy E.G., Johnston K.A., Burr S., Jaroslawski S. The Arabidopsis ALDP protein homologue COMATOSE is instrumental in peroxisomal acetate metabolism. Biochem. J. 2007;4067(1):399–406. doi: 10.1042/BJ20070258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppins S., Lackner L., Nunnari J. The machines that divide and fuse mitochondria. Annu. Rev. Biochem. 2007;767(1):751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- Horling F., Dietz K.J. Type II peroxiredoxin C, a member of the peroxiredoxin family of Arabidopsis thaliana; its expression and activity in comparison with other peroxiredoxins. Plant Physiol. Biochem. 2002;407(1):491–499. [Google Scholar]
- Hossain M.A., Asada K. Purification of dehydroascorbate reductase from spinach and its characterization as a thiol enzyme. Plant Cell Physiol. 1984;257(1):85–92. [Google Scholar]
- Hu J. Plant peroxisome multiplication: highly regulated and still enigmatic. J. Integr. Plant Biol. 2007;497(1):1112–1118. [Google Scholar]
- Hu J., Aguirre M., Peto C., Alonso J., Ecker J., Chory J. A role for peroxisomes in photomorphogenesis and development of Arabidopsis. Science. 2002;2977(1):405–409. doi: 10.1126/science.1073633. [DOI] [PubMed] [Google Scholar]
- Huber O., Weiske J. Beta-catenin takes a HIT. Cell cycle (Georgetown, Tex) 2008;77(1):1326–1331. doi: 10.4161/cc.7.10.5926. [DOI] [PubMed] [Google Scholar]
- Hunt J.E., Trelease R.N. Sorting pathway and molecular targeting signals for the Arabidopsis peroxin 3. Biochem. Biophys. Res. Commun. 2004;3147(1):586–596. doi: 10.1016/j.bbrc.2003.12.123. [DOI] [PubMed] [Google Scholar]
- Igamberdiev A.U., Lea P.J. The role of peroxisomes in the integration of metabolism and evolutionary diversity of photosynthetic organisms. Phytochemistry. 2002;607(1):651–674. doi: 10.1016/s0031-9422(02)00179-6. [DOI] [PubMed] [Google Scholar]
- Igarashi D., Miwa T., Seki M., Kobayashi M., Kato T., Tabata S., Shinozaki K., Ohsumi C. Identification of photorespiratory glutamate:glyoxylate aminotransferase (GGAT) gene in Arabidopsis. Plant J. 2003;337(1):975–987. doi: 10.1046/j.1365-313x.2003.01688.x. [DOI] [PubMed] [Google Scholar]
- Incharoensakdi A., Matsuda N., Hibino T., Meng Y.L., Ishikawa H., Hara A., Funaguma T., Takabe T., Takabe T. Overproduction of spinach betaine aldehyde dehydrogenase in Escherichia coli. Structural and functional properties of wild-type, mutants and E. coli enzymes. Eur. J. Biochem. 2000;2677(1):7015–7023. doi: 10.1046/j.1432-1327.2000.01797.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Yoshimura K., Sakai K., Tamoi M., Takeda T., Shigeoka S. Molecular characterization and physiological role of a glyoxysome-bound ascorbate peroxidase from spinach. Plant Cell Physiol. 1998;397(1):23–34. doi: 10.1093/oxfordjournals.pcp.a029285. [DOI] [PubMed] [Google Scholar]
- James D.I., Parone P.A., Mattenberger Y., Martinou J.C. hFis1, a novel component of the mammalian mitochondrial fission machinery. J. Biol. Chem. 2003;2787(1):36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- Jedd G., Chua N.H. Visualization of peroxisomes in living plant cells reveals acto-myosin-dependent cytoplasmic streaming and peroxisome budding. Plant Cell Physiol. 2002;437(1):384–392. doi: 10.1093/pcp/pcf045. [DOI] [PubMed] [Google Scholar]
- Jespersen H.M., Kjaersgard I.V., Ostergaard L., Welinder K.G. From sequence analysis of three novel ascorbate peroxidases from Arabidopsis thaliana to structure, function and evolution of seven types of ascorbate peroxidase. Biochem J. 1997;3267(1):305–310. doi: 10.1042/bj3260305. (Pt2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Ramachandran S. Identification and molecular characterization of myosin gene family in Oryza sativa genome. Plant Cell Physiol. 2004;457(1):590–599. doi: 10.1093/pcp/pch061. [DOI] [PubMed] [Google Scholar]
- Johnson T.L., Olsen L.J. Building new models for peroxisome biogenesis. Plant Physiol. 2001;1277(1):731–739. [PMC free article] [PubMed] [Google Scholar]
- Kamada-Nobusada T., Hayashi M., Fukazawa M., Sakakibara H., Nishimura M. A putative peroxisomal polyamine oxidase, AtPAO4, is involved in polyamine catabolism in Arabidopsis thaliana. Plant Cell Physiol. 2008;497(1):1272–1282. doi: 10.1093/pcp/pcn114. [DOI] [PubMed] [Google Scholar]
- Kamada T., Nito K., Hayashi H., Mano S., Hayashi M., Nishimura M. Functional differentiation of peroxisomes revealed by expression profiles of peroxisomal genes in Arabidopsis thaliana. Plant Cell Physiol. 2003;447(1):1275–1289. doi: 10.1093/pcp/pcg173. [DOI] [PubMed] [Google Scholar]
- Kamigaki A., Mano S., Terauchi K., Nishi Y., Tachibe-Kinoshita Y., Nito K., Kondo M., Hayashi M., Nishimura M., Esaka M. Identification of peroxisomal targeting signal of pumpkin catalase and the binding analysis with PTS1 receptor. Plant J. 2003;337(1):161–175. doi: 10.1046/j.0960-7412.2003.001605.x. [DOI] [PubMed] [Google Scholar]
- Karnik S.K., Trelease R.N. Arabidopsis peroxin 16 coexists at steady state in peroxisomes and endoplasmic reticulum. Plant Physiol. 2005;1387(1):1967–1981. doi: 10.1104/pp.105.061291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik S.K., Trelease R.N. Arabidopsis peroxin 16 trafficks through the ER and an intermediate compartment to pre-existing peroxisomes via overlapping molecular targeting signals. J. Exp. Bot. 2007;587(1):1677–1693. doi: 10.1093/jxb/erm018. [DOI] [PubMed] [Google Scholar]
- Karyotou K., Donaldson R.P. Ascorbate peroxidase, a scavenger of hydrogen peroxide in glyoxysomal membranes. Arch. Biochem. Biophys. 2005;4347(1):248–257. doi: 10.1016/j.abb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Kato A., Hayashi M., Kondo M., Nishimura M. Targeting and processing of a chimeric protein with the N-terminal presequence of the precursor to glyoxysomal citrate synthase. Plant Cell. 1996;87(1):1601–1611. doi: 10.1105/tpc.8.9.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A., Takeda-Yoshikawa Y., Hayashi M., Kondo M., Hara-Nishimura I., Nishimura M. Glyoxysomal malate dehydrogenase in pumpkin: cloning of a cDNA and functional analysis of its presequence. Plant Cell Physiol. 1998;397(1):186–195. doi: 10.1093/oxfordjournals.pcp.a029356. [DOI] [PubMed] [Google Scholar]
- Katoh A., Shoji T., Hashimoto T. Molecular cloning of N-methylputrescine oxidase from tobacco. Plant Cell Physiol. 2007;487(1):550–554. doi: 10.1093/pcp/pcm018. [DOI] [PubMed] [Google Scholar]
- Katsir L., Chung H.S., Koo A.J., Howe G.A. Jasmonate signaling: a conserved mechanism of hormone sensing. Curr. Opin. Plant Biol. 2008;117(1):428–435. doi: 10.1016/j.pbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel J.A., Otzen M., Veenhuis M., van der Kiel I.J. Obstruction of polyubiquitination affects PTS1 peroxisomal matrix protein import. Biochim. Biophys. Acta. 2005a;17457(1):176–186. doi: 10.1016/j.bbamcr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Kiel J.A., Emmrich K., Meyer H.E., Kunau W.H. Ubiquitination of the peroxisomal targeting signal type 1 receptor, Pex5p, suggests the presence of a quality control mechanism during peroxisomal matrix protein import. J. Biol. Chem. 2005b;2807(1):1921–1930. doi: 10.1074/jbc.M403632200. [DOI] [PubMed] [Google Scholar]
- Kienow L., Schneider K., Bartsch M., Stuible H.P., Weng H., Miersch O., Wasternack C., Kombrink E. Jasmonates meet fatty acids: functional analysis of a new acyl-coenzyme A synthetase family from Arabidopsis thaliana. J. Exp. Bot. 2008;597(1):403–419. doi: 10.1093/jxb/erm325. [DOI] [PubMed] [Google Scholar]
- Kikuchi M., Hatano N., Yokota S., Shimozawa N., Imanaka T., Taniguchi H. Proteomic analysis of rat liver peroxisome: presence of peroxisome-specific isozyme of Lon protease. J. Biol. Chem. 2004;2797(1):421–428. doi: 10.1074/jbc.M305623200. [DOI] [PubMed] [Google Scholar]
- Kim P.K., Mullen R.T., Schumann U., Lippincott-Schwartz J. The origin and maintenance of mammalian peroxisomes involves a de novo PEX16-dependent pathway from the ER. J. Cell Biol. 2006;1737(1):521–532. doi: 10.1083/jcb.200601036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Tanaka A., Fujiki Y. Fis1, DLP1, and Pex11p coordinately regulate peroxisome morphogenesis. Exp. Cell Res. 2007;3137(1):1675–1686. doi: 10.1016/j.yexcr.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Koch A., Schneider G., Luers G.H., Schrader M. Peroxisome elongation and constriction but not fission can occur independently of dynamin-like protein 1. J. Cell Sci. 2004;1177(1):3995–4006. doi: 10.1242/jcs.01268. [DOI] [PubMed] [Google Scholar]
- Koch A., Yoon Y., Bonekamp N.A., McNiven M.A., Schrader M. A role for Fis1 in both mitochondrial and peroxisomal fission in mammalian cells. Mol. Biol. Cell. 2005;167(1):5077–5086. doi: 10.1091/mbc.E05-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A., Thiemann M., Grabenbauer M., Yoon Y., McNiven M.A., Schrader M. Dynamin-like protein 1 is involved in peroxisomal fission. J. Biol. Chem. 2003;2787(1):8597–8605. doi: 10.1074/jbc.M211761200. [DOI] [PubMed] [Google Scholar]
- Koh S., Andre A., Edwards H., Ehrhardt D., Somerville S. Arabidopsis thaliana subcellular responses to compatible Erysiphe cichoracearum infections. Plant J. 2005;447(1):516–529. doi: 10.1111/j.1365-313X.2005.02545.x. [DOI] [PubMed] [Google Scholar]
- Koo A.J., Chung H.S., Kobayashi Y., Howe G.A. Identification of a peroxisomal acyl-activating enzyme involved in the biosynthesis of jasmonic acid in Arabidopsis. J. Biol. Chem. 2006;2817(1):33511–33520. doi: 10.1074/jbc.M607854200. [DOI] [PubMed] [Google Scholar]
- Kovacs W.J., Olivier L.M., Krisans S.K. Central role of peroxisomes in isoprenoid biosynthesis. Prog. Lipid Res. 2002;417(1):369–391. doi: 10.1016/s0163-7827(02)00002-4. [DOI] [PubMed] [Google Scholar]
- Kovacs W.J., Tape K.N., Shackelford J.E., Duan X., Kasumov T., Kelleher U.K., Brunengraber H., Krisans S.K. Localization of the pre-squalene segment of the isoprenoid biosynthetic pathway in mammalian peroxisomes. Histochem. Cell Biol. 2007;1277(1):273–290. doi: 10.1007/s00418-006-0254-6. [DOI] [PubMed] [Google Scholar]
- Kragler F., Lametschwandtner G., Christmann J., Hartig A., Harada J.J. Identification and analysis of the plant peroxisomal targeting signal 1 receptor NtPEX5. Proc. Natl. Acad. Sci. USA. 1998;957(1):13336–13341. doi: 10.1073/pnas.95.22.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragt A., Voorn-Brouwer T., van den Berg M., Distel B. Endoplasmic reticulum-directed Pex3p routes to peroxisomes and restores peroxisome formation in a Saccharomyces cerevisiae pex3Delta strain. J. Biol. Chem. 2005;2807(1):34350–34357. doi: 10.1074/jbc.M505432200. [DOI] [PubMed] [Google Scholar]
- Kunau W.H. Peroxisomes: the extended shuttle to the peroxisome matrix. Curr. Biol. 2001;117(1):R659–662. doi: 10.1016/s0960-9822(01)00386-4. [DOI] [PubMed] [Google Scholar]
- Kuravi K., Nagotu S., Krikken A.M., Sjollema K., Deckers M., Erdmann R., Veenhuis M., van der Klei IJ. Dynamin-related proteins Vps1p and Dnm1p control peroxisome abundance in Saccharomyces cerevisiae. J. Cell Sci. 2006;1197(1):3994–4001. doi: 10.1242/jcs.03166. [DOI] [PubMed] [Google Scholar]
- Kurochkin I.V., Mizuno Y., Konagaya A., Sakaki Y., Schonbach C., Okazaki Y. Novel peroxisomal proteaselysndl processes PTS1- and PTS2-containing enzymes involved in beta-oxidation of fatty acids. EMBO J. 2007;267(1):835–845. doi: 10.1038/sj.emboj.7601525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lametschwandtner G., Brocard C., Fransen M., Van Veldhoven P., Berger J., Hartig A. The difference in recognition of terminal tripeptides as peroxisomal targeting signal 1 between yeast and human is due to different affinities of their receptor Pex5p to the cognate signal and to residues adjacent to it. J. Biol. Chem. 1998;2737(1):33635–33643. doi: 10.1074/jbc.273.50.33635. [DOI] [PubMed] [Google Scholar]
- Lang C., Popko J., Wirtz M., Hell R., Herschbach C., Kreuzwieser J., Rennenberg H., Mendel R.R., Hansch R. Sulphite oxidase as key enzyme for protecting plants against sulphur dioxide. Plant Cell Environ. 2007;307(1):447–455. doi: 10.1111/j.1365-3040.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- Lange P.R., Eastmond P.J., Madagan K., Graham I.A. An Arabidopsis mutant disrupted in valine catabolism is also compromised in peroxisomal fatty acid beta-oxidation. FEBS Lett. 2004;5717(1):147–153. doi: 10.1016/j.febslet.2004.06.071. [DOI] [PubMed] [Google Scholar]
- Latruffe N., Vamecq J. Evolutionary aspects of peroxisomes as cell organelles, and of genes encoding peroxisomal proteins. Biol. Cell. 2000;927(1):389–395. doi: 10.1016/s0248-4900(00)01083-2. [DOI] [PubMed] [Google Scholar]
- Lazarow P.B., Fujiki Y. Biogenesis of peroxisomes. Annu. Rev. Cell Biol. 1985;17(1):489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Lee M.S., Mullen R.T., Trelease R.N. Oilseed isocitrate lyases lacking their essential type 1 peroxisomal targeting signal are piggybacked to glyoxysomes. Plant Cell. 1997;97(1):185–197. doi: 10.1105/tpc.9.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N.G., Stein B., Suzuki H., Verma D.P. Expression of antisense nodulin-35 RNA in Vigna aconitifolia transgenic root nodules retards peroxisome development and affects nitrogen availability to the plant. Plant J. 1993;37(1):599–606. doi: 10.1046/j.1365-313x.1993.03040599.x. [DOI] [PubMed] [Google Scholar]
- Leidhold C., Voos W. Chaperones and proteases-guardians of protein integrity in eukaryotic organelles. Ann. New York Acad. Sci. 2007;11137(1):72–86. doi: 10.1196/annals.1391.011. [DOI] [PubMed] [Google Scholar]
- Leterrier M., Corpas F.J., Barroso J.B., Sandalio L.M., del Rio L.A. Peroxisomal monodehydroascorbate reductase. Genomic clone characterization and functional analysis under environmental stress conditions. Plant Physiol. 2005;1387(1):2111–2123. doi: 10.1104/pp.105.066225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Schilmiller A.L., Liu G., Lee G.I., Jayanty S., Sageman C., Vrebalov J., Giovannoni J.J., Yagi K., Kobayashi Y., Howe G.A. Role of beta-oxidation in jasmonate biosynthesis and systemic wound signaling in tomato. Plant Cell. 2005;177(1):971–986. doi: 10.1105/tpc.104.029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.F., Nebenfuhr A. The tail that wags the dog: The globular tail domain defines the function of myosin V/XI. Traffic. 2008;97(1):290–298. doi: 10.1111/j.1600-0854.2007.00687.x. [DOI] [PubMed] [Google Scholar]
- Li J.F., Nebenfuhr A. Organelle targeting of myosin XI is mediated by two globular tail subdomains with separate cargo binding sites. J. Biol. Chem. 2007;2827(1):20593–20602. doi: 10.1074/jbc.M700645200. [DOI] [PubMed] [Google Scholar]
- Li X., Gould S.J. The dynamin-like GTPase DLP1 is essential for peroxisome division and is recruited to peroxisomes in part by PEX11. J. Biol. Chem. 2003;2787(1):17012–17020. doi: 10.1074/jbc.M212031200. [DOI] [PubMed] [Google Scholar]
- Li X., Baumgart E., Morrell J.C., Jimenez-Sanchez G., Valle D., Gould S.J. PEX11 beta deficiency is lethal and impairs neuronal migration but does not abrogate peroxisome function. Mol. Mol. Cell Biol. 2002a;227(1):4358–4365. doi: 10.1128/MCB.22.12.4358-4365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Baumgart E., Dong G.X., Morrell J.C., Jimenez-Sanchez G., Valle D., Smith K.D., Gould S.J. PEX11alpha is required for peroxisome proliferation in response to 4-phenylbutyrate but is dispensable for peroxisome proliferator-activated receptor alpha-mediated peroxisome proliferation. Mol. Cell Biol. 2002b;227(1):8226–8240. doi: 10.1128/MCB.22.23.8226-8240.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepman A.M., Olsen L.J. Peroxisomal alanine : glyoxylate aminotransferase (AGT1) is a photorespiratory enzyme with multiple substrates in Arabidopsis thaliana. Plant J. 2001;257(1):487–498. doi: 10.1046/j.1365-313x.2001.00961.x. [DOI] [PubMed] [Google Scholar]
- Liepman A.M., Olsen L.J. Alanine aminotransferase homologs catalyze the glutamate:glyoxylate aminotransferase reaction in peroxisomes of Arabidopsis. Plant Physiol. 2003;1317(1):215–227. doi: 10.1104/pp.011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingard M.J., Trelease R.N. Five Arabidopsis peroxin 11 homologs individually promote peroxisome elongation, duplication or aggregation. J. Cell Sci. 2006;1197(1):1961–1972. doi: 10.1242/jcs.02904. [DOI] [PubMed] [Google Scholar]
- Lingard M.J., Monroe-Augustus M., Bartel B. Peroxisome-associated matrix protein degradation in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2009;1067(1):4561–4566. doi: 10.1073/pnas.0811329106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingard M.J., Gidda S.K., Bingham S., Rothstein SJ., Mullen R.T., Trelease R.N. Arabidopsis PEROXIN11c-e, FISSION1b, and DYNAMIN-RELATED PROTEIN3A cooperate in cell cycle-associated replication of peroxisomes. Plant Cell. 2008;207(1):1567–1585. doi: 10.1105/tpc.107.057679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linka N., Theodoulou F.L., Haslam R.P., Linka M., Napier J.A., Neuhaus H.E., Weber A.P. Peroxisomal ATP import is essential for seedling development in Arabidopsis thaliana. Plant Cell. 2008;207(1):3241–3257. doi: 10.1105/tpc.108.062042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka V., Dittgen J., Bednarek P., Bhat R., Wiermer M., Stein M., Landtag J., Brandt W., Rosahl S., Scheel D., Llorente F., Molina A., Parker J., Somerville S., Schulze-Lefert P. Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science. 2005;3107(1):1180–1183. doi: 10.1126/science.1119409. [DOI] [PubMed] [Google Scholar]
- Lisenbee C.S., Heinze M., Trelease R.N. Peroxisomal ascorbate peroxidase resides within a subdomain of rough endoplasmic reticulum in wild-type Arabidopsis cells. Plant Physiol. 2003;1327(1):870–882. doi: 10.1104/pp.103.019976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisenbee C.S., Lingard M.J., Trelease R.N. Arabidopsis peroxisomes possess functionally redundant membrane and matrix isoforms of monodehydroascorbate reductase. Plant J. 2005;437(1):900–914. doi: 10.1111/j.1365-313X.2005.02503.x. [DOI] [PubMed] [Google Scholar]
- Livingstone J.R., Maruo T., Yoshida I., Tarui Y., Hirooka K., Yamamoto Y., Tsutul N., Hirasawa E. Purification and properties of betaine aldehyde dehydrogenase from Avena sativa. J. Plant Res. 2003;1167(1):133–140. doi: 10.1007/s10265-003-0077-7. [DOI] [PubMed] [Google Scholar]
- Logan D.C., Scott I., Tobin A.K. ADL2a, like ADL2b. is involved in the control of higher plant mitochondrial morphology. J. Exp. Bot. 2004;557(1):783–785. doi: 10.1093/jxb/erh073. [DOI] [PubMed] [Google Scholar]
- Lucas K.A., Filley J.R., Erb J.M., Graybill E.R., Hawes J.W. Peroxisomal metabolism of propionic acid and isobutyric acid in plants. J. Biol. Chem. 2007;2827(1):24980–24989. doi: 10.1074/jbc.M701028200. [DOI] [PubMed] [Google Scholar]
- Ma C., Reumann S. Improved prediction of peroxisomal PTS1 proteins from genome sequences based on experimental subcellular targeting analyses as exemplified for protein kinases from Arabidopsis. J. Exp. Bot. 2008;597(1):3767–3779. doi: 10.1093/jxb/ern221. [DOI] [PubMed] [Google Scholar]
- Ma C., Haslbeck M., Babujee L., Jahn O., Reumann S. Identification and characterization of a stress-inducible and a constitutive small heat-shock protein targeted to the matrix of plant peroxisomes. Plant Physiol. 2006;1417(1):47–60. doi: 10.1104/pp.105.073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano S., Nakamori C., Kondo M., Hayashi M., Nishimura M. An Arabidopsis dynamin-related protein, DRP3A, controls both peroxisomal and mitochondrial division. Plant J. 2004;387(1):487–498. doi: 10.1111/j.1365-313X.2004.02063.x. [DOI] [PubMed] [Google Scholar]
- Mano S., Nakamori C., Nito K., Kondo M., Nishimura M. The Arabidopsis pex12 and pex13 mutants are defective in both PTS1-and PTS2-dependent protein transport to peroxisomes. Plant J. 2006;477(1):604–618. doi: 10.1111/j.1365-313X.2006.02809.x. [DOI] [PubMed] [Google Scholar]
- Mano S., Nakamori C., Hayashi M., Kato A., Kondo M., Nishimura M. Distribution and characterization of peroxisomes in Arabidopsis by visualization with GFP: dynamic morphology and actin-dependent movement. Plant Cell Physiol. 2002;437(1):331–341. doi: 10.1093/pcp/pcf037. [DOI] [PubMed] [Google Scholar]
- Marelli M., Smith J.J., Jung S., Yi E., Nesvizhskii A.I., Christmas R.H., Saleem R.A., Tam Y.Y., Fagarasanu A., Goodlett D.R., Aebersold R., Rachubinski R.A., Aitchison J.D. Quantitative mass spectrometry reveals a role for the GTPase Rho1p in actin organization on the peroxisome membrane. J. Cell Biol. 2004;1677(1):1099–1112. doi: 10.1083/jcb.200404119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P.A., Krimkevich Y.I., Lark R.H., Dyer J.M., Veenhuis M., Goodman J.M. Pmp27 promotes peroxisomal proliferation. J. Cell Biol. 1995;1297(1):345–355. doi: 10.1083/jcb.129.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur J., Mathur N., Hulskamp M. Simultaneous visualization of peroxisomes and cytoskeletal elements reveals actin and not microtubule-based peroxisome motility in plants. Plant Physiol. 2002;1287(1):1031–1045. doi: 10.1104/pp.011018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzono Y., Kinoshita N., Tamura S., Shimozawa N., Hamasaki M., Ghaedi K., Wanders R.J., Suzuki Y., Kondo N., Fujiki Y. Human PEX19: cDNA cloning by functional complementation, mutation analysis in a patient with Zellweger syndrome, and potential role in peroxisomal membrane assembly. Proc. Natl. Acad. Sci. USA. 1999;967(1):2116–2121. doi: 10.1073/pnas.96.5.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney A.W., Greenwood U.S., Fabian M.R., White K.A., Mullen R.T. Localization of the tomato bushy stunt virus replication protein p33 reveals a peroxisome-to-endoplasmic reticulum sorting pathway. Plant Cell. 2005;177(1):3513–3531. doi: 10.1105/tpc.105.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung C.R. Regulation of catalases in Arabidopsis. Free Radic. Biol. Med. 1997;237(1):489–496. doi: 10.1016/s0891-5849(97)00109-3. [DOI] [PubMed] [Google Scholar]
- McGarvey D.J., Croteau R. Terpenoid metabolism. Plant Cell. 1995;77(1):1015–1026. doi: 10.1105/tpc.7.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metraux J.P. Recent breakthroughs in the study of salicylic acid biosynthesis. Trends Plant Sci. 2002;77(1):332–334. doi: 10.1016/s1360-1385(02)02313-0. [DOI] [PubMed] [Google Scholar]
- Mettler I.J., Beevers H. Oxidation of NADH in glyoxysomes by a malate-aspartate shuttle. Plant Physiol. 1980;667(1):555–560. doi: 10.1104/pp.66.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusser B., Hirsch C., Jarosch E., Sommer T. ERAD: the long road to destruction. Nat. Cell Biol. 2005;77(1):766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- Millar A.H., Whelan J., Small I. Recent surprises in protein targeting to mitochondria and plastids. Curr. Opin. Plant Biol. 2006;97(1):610–615. doi: 10.1016/j.pbi.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Mittler R., Zilinskas B.A. Purification and characterization of pea cytosolic ascorbate peroxidase. Plant Physiol. 1991;977(1):962–968. doi: 10.1104/pp.97.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R., Feng X., Cohen M. Post-transcriptional suppression of cytosolic ascorbate peroxidase expression during pathogen-induced programmed cell death in tobacco. Plant Cell. 1998;107(1):461–473. doi: 10.1105/tpc.10.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R., Herr E.H., Orvar B.L., van Camp W., Willekens H., Inze D., Ellis B.E. Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hyperresponsive to pathogen infection. Proc. Natl. Acad. Sci. USA. 1999;967(1):14165–14170. doi: 10.1073/pnas.96.24.14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller S.G., McPherson M.J. Developmental expression and biochemical analysis of the Arabidopsis atao1 gene encoding an H2O2-generating diamine oxidase. Plant J. 1998;137(1):781–791. doi: 10.1046/j.1365-313x.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- Moschou P.N., Sanmartin M., Andriopoulou A.M., Rojo E., Sanchez-Serrano J.J., Roubelakis-Angelakis K.A. Bridging the gap between plant and mammalian polyamine catabolism: a novel peroxisomal polyamine oxidase responsible for a full back-conversion pathway in Arabidopsis. Plant Physiol. 2008;1477(1):1845–1857. doi: 10.1104/pp.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley A.M., Hettema E.H. Yeast peroxisomes multiply by growth and division. J. Cell Biol. 2007;1787(1):399–410. doi: 10.1083/jcb.200702167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley A.M., Ward G.P., Hettema E.H. Dnm1p-dependent peroxisome fission requires Caf4p, Mdv1p and Fis1p. J. Cell Sci. 2008;1217(1):1633–1640. doi: 10.1242/jcs.026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozdy A.D., McCaffery J.M., Shaw J.M. Dnm1p GTPasemediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fislp. J. Cell Biol. 2000;1517(1):367–380. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen R.T., Trelease R.N. Biogenesis and membrane properties of peroxisomes: does the boundary membrane serve and protect? Trends Plant Sci. 1996;17(1):389–394. [Google Scholar]
- Mullen R.T., Trelease R.N. The ER-peroxisome connection in plants: development of the “ER semi-autonomous peroxisome maturation and replication” model for plant peroxisome biogenesis. Biochim. Biophys. Acta. 2006;17637(1):1655–1668. doi: 10.1016/j.bbamcr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Mullen R.T., Lee M.S., Flynn C.R., Trelease R.N. Diverse amino acid residues function within the type 1 peroxisomal targeting signal. Implications for the role of accessory residues upstream of the type 1 peroxisomal targeting signal. Plant Physiol. 1997;1157(1):881–889. doi: 10.1104/pp.115.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen R.T., Lisenbee C.S., Miernyk J.A., Trelease R.N. Peroxisomal membrane ascorbate peroxidase is sorted to a membranous network that resembles a subdomain of the endoplasmic reticulum. Plant Cell. 1999;117(1):2167–2185. doi: 10.1105/tpc.11.11.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntau A.C., Mayerhofer P.U., Paton B.C., Kammerer S., Roscher A.A. Defective peroxisome membrane synthesis due to mutations in human PEX3 causes Zellweger syndrome, complementation group G. Am. J. Hum. Genet. 2000;677(1):967–975. doi: 10.1086/303071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Meyer C., Sano H. Molecular cloning and characterization of plant genes encoding novel peroxisomal molybdoenzymes of the sulphite oxidase family. J Exp Bot. 2002;537(1):1833–1836. doi: 10.1093/jxb/erf042. [DOI] [PubMed] [Google Scholar]
- Nam K.H., Li J. The Arabidopsis transthyretin-like protein is a potential substrate of BRASSINOSTEROID-INSENSITIVE 1. Plant Cell. 2004;167(1):2406–2417. doi: 10.1105/tpc.104.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra S., Venkataramani S., Shen G., Wang J., Pasapula V., Lin Y., Kornyeyev D., Holaday A.S., Zhang H. The Arabidopsis ascorbate peroxidase 3 is a peroxisomal membrane-bound antioxidant enzyme and is dispensable for Arabidopsis growth and development. J. Exp. Bot. 2006;577(1):3033–3042. doi: 10.1093/jxb/erl060. [DOI] [PubMed] [Google Scholar]
- Neuberger G., Maurer-Stroh S., Eisenhaber B., Hartig A., Eisenhaber F. Prediction of peroxisomal targeting signal 1 containing proteins from amino acid sequence. J. Mol. Biol. 2003a;3287(1):581–592. doi: 10.1016/s0022-2836(03)00319-x. [DOI] [PubMed] [Google Scholar]
- Neuberger G., Maurer-Stroh S., Eisenhaber B., Hartig A., Eisenhaber F. Motif refinement of the peroxisomal targeting signal 1 and evaluation of taxon-specific differences. J. Mol. Biol. 2003b;3287(1):567–579. doi: 10.1016/s0022-2836(03)00318-8. [DOI] [PubMed] [Google Scholar]
- Nguyen A.T., Donaldson R.P. Metal-catalyzed oxidation induces carbonylation of peroxisomal proteins and loss of enzymatic activities. Arch. Biochem. Biophys. 2005;4397(1):25–31. doi: 10.1016/j.abb.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Nila A.G., Sandalio L.M., Lopez M.G., Gomez M., del Rio L.A., Gomez-Lim M.A. Expression of a peroxisome proliferator-activated receptor gene (xPPARalpha) from Xenopus laevis in tobacco (Nicotiana tabacum) plants. Planta. 2006;2247(1):569–581. doi: 10.1007/s00425-006-0246-8. [DOI] [PubMed] [Google Scholar]
- Nito K., Hayashi M., Nishimura M. Direct interaction and determination of binding domains among peroxisomal import factors in Arabidopsis thaliana. Plant Cell Physiol. 2002;437(1):355–366. doi: 10.1093/pcp/pcf057. [DOI] [PubMed] [Google Scholar]
- Nito K., Kamigaki A., Kondo M., Hayashi M., Nishimura M. Functional classification of Arabidopsis peroxisome biogenesis factors proposed from analyses of knockdown mutants. Plant Cell Physiol. 2007;487(1):763–774. doi: 10.1093/pcp/pcm053. [DOI] [PubMed] [Google Scholar]
- Nowak K., Luniak N., Witt C., Wustefeld Y., Wachter A., Mendel R.R., Hansch R. Peroxisomal localization of sulfite oxidase separates it from chloroplast-based sulfur assimilation. Plant Cell Physiol. 2004;457(1):1889–1894. doi: 10.1093/pcp/pch212. [DOI] [PubMed] [Google Scholar]
- Nurnberger T., Kemmerling B. Receptor protein kinases-pattern recognition receptors in plant immunity. Trends Plant Sci. 2006;117(1):519–522. doi: 10.1016/j.tplants.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Nyathi Y., Baker A. Plant peroxisomes as a source of signalling molecules. Biochim. Biophys. Acta. 2006;17637(1):1478–1495. doi: 10.1016/j.bbamcr.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Oishi H., Ebina M. Isolation of cDNA and enzymatic properties of betaine aldehyde dehydrogenase from Zoysia tenuifolia. J. Plant Physiol. 2005;1627(1):1077–1086. doi: 10.1016/j.jplph.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Oksanen E., Kaikio E., Sober J., Karnosky D.F. Ozone-induced H2O2 accumulation in field-grown aspen and birch is linked to foliar structure and peroxisomal activity. New Phytologists. 2003;1617(1):791–799. doi: 10.1111/j.1469-8137.2003.00981.x. [DOI] [PubMed] [Google Scholar]
- Olsen L.J., Harada J. Peroxisomes and their assembly in higher plants. Annu. Rev Plant Biol. 1995;467(1):123–146. [Google Scholar]
- Ong S.E., Mann M. Mass spectrometry-based proteomics turns quantitative. Nat. Chem. Biol. 2005;17(1):252–262. doi: 10.1038/nchembio736. [DOI] [PubMed] [Google Scholar]
- Orth T., Reumann S., Zhang X., Fan J., Wenzel D., Quan S., Hu J. The PEROXIN11 protein family controls peroxisome proliferation in Arabidopsis. Plant Cell. 2007;197(1):333–350. doi: 10.1105/tpc.106.045831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteryoung K.W., Nunnari J. The division of endosymbiotic organelles. Science. 2003;3027(1):1698–1704. doi: 10.1126/science.1082192. [DOI] [PubMed] [Google Scholar]
- Otzen M., Perband U., Wang D., Baerends R.J., Kunau W.H., Veenhuis M., Van der Klei I.J. Hansenula polymorpha Pex19p is essential for the formation of functional peroxisomal membranes. J. Biol. Chem. 2004;2797(1):19181–19190. doi: 10.1074/jbc.M314275200. [DOI] [PubMed] [Google Scholar]
- Palma J.M., Garrido M., Rodriguez-Garcia M.I., del Rio L.A. Peroxisome proliferation and oxidative stress mediated by activated oxygen species in plant peroxisomes. Arch Biochem. Biophys. 1991;2877(1):68–74. doi: 10.1016/0003-9861(91)90389-z. [DOI] [PubMed] [Google Scholar]
- Palmieri L., Rottensteiner H., Girzalsky W., Scarcia P., Palmieri F., Erdmann R. Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. EMBO J. 2001;207(1):5049–5059. doi: 10.1093/emboj/20.18.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchuk II, Volkov R.A., Schoffl F. Heat stress- and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis. Plant Physiol. 2002;1297(1):838–853. doi: 10.1104/pp.001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti A.C., Parmely T.J., Tomomori-Sato C., Sato S., Zhu D., Conaway R.C., Conaway J.W., Florens L., Washburn M.P. Quantitative proteomic analysis of distinct mammalian Mediator complexes using normalized spectral abundance factors. Proc. Natl. Acad. Sci. USA. 2006;1037(1):18928–18933. doi: 10.1073/pnas.0606379103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passreiter M., Anton M., Lay D., Frank R., Harter C., Wieland F.T., Gorgas K., Just W.W. Peroxisome biogenesis: involvement of ARF and coatomer. J. Cell Biol. 1998;1417(1):373–383. doi: 10.1083/jcb.141.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen L., Henriksen A. Expression, purification and crystallization of two peroxisomal acyl-CoA oxidases from Arabidopsis thaliana. Acta Crystallogr. D. Biol. Crystallogr. 2004;607(1):1125–1128. doi: 10.1107/S0907444904007577. [DOI] [PubMed] [Google Scholar]
- Penfield S., Pinfield-Wells H.M., Graham I.A. Storage Reserve Mobilisation and Seedling Establishment in Arabidopsis: October 4, 2006. The Arabidopsis Book. Rockville, MD: American Society of Plant Biologists; 2006. doi: 10.1199/tab.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peremyslov V.V., Prokhnevsky A.I., Avisar D., Dolja V.V. Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis thaliana. Plant Physiol. 2008;1467(1):1109–1116. doi: 10.1104/pp.107.113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinfield-Wells H., Rylott E.L., Gilday A.D., Graham S., Job K., Larson T.R., Graham I.A. Sucrose rescues seedling establishment but not germination of Arabidopsis mutants disrupted in peroxisomal fatty acid catabolism. Plant J. 2005;437(1):861–872. doi: 10.1111/j.1365-313X.2005.02498.x. [DOI] [PubMed] [Google Scholar]
- Platta H.W., Erdmann R. Peroxisomal dynamics. Trends Cell Biol. 2007;177(1):474–484. doi: 10.1016/j.tcb.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Platta H.W., Girzalsky W., Erdmann R. Ubiquitination of the peroxisomal import receptor Pex5p. Biochem J. 2004;3847(1):37–45. doi: 10.1042/BJ20040572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platta H.W., El Magraoui F., Schlee D., Grunau S., Girzalsky W., Erdmann R. Ubiquitination of the peroxisomal import receptor Pex5p is required for its recycling. J. Cell Biol. 2007;1777(1):197–204. doi: 10.1083/jcb.200611012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pracharoenwattana I., Smith S.M. When is a peroxisome not a peroxisome? Trends Plant Sci. 2008;137(1):522–525. doi: 10.1016/j.tplants.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Pracharoenwattana I., Cornah J.E., Smith S.M. Arabidopsis peroxisomal citrate synthase is required for fatty acid respiration and seed germination. Plant Cell. 2005;177(1):2037–2048. doi: 10.1105/tpc.105.031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pracharoenwattana I., Cornah J.E., Smith S.M. Arabidopsis peroxisomal malate dehydrogenase functions in beta-oxidation but not in the glyoxylate cycle. Plant J. 2007;507(1):381–390. doi: 10.1111/j.1365-313X.2007.03055.x. [DOI] [PubMed] [Google Scholar]
- Praefcke G.J., McMahon H.T. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol. 2004;57(1):133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- Preisig-Muller R., Muster G., Kindl H. Heat shock enhances the amount of prenylated Dnaj protein at membranes of glyoxysomes. Eur. J. Biochem. 1994;2197(1):57–63. doi: 10.1111/j.1432-1033.1994.tb19914.x. [DOI] [PubMed] [Google Scholar]
- Queval G., Issakidis-Bourguet E., Hoeberichts F.A., Vandorpe M., Gakiere B., Vanacker H., Miginiac-Maslow M., Van Breusegem F., Noctor G. Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J. 2007;527(1):640–657. doi: 10.1111/j.1365-313X.2007.03263.x. [DOI] [PubMed] [Google Scholar]
- Ramazzina I., Folli C., Secchi A., Berni R., Percudani R. Completing the uric acid degradation pathway through phylogenetic comparison of whole genomes. Nat. Chem. Biol. 2006;27(1):144–148. doi: 10.1038/nchembio768. [DOI] [PubMed] [Google Scholar]
- Rapp S., Saffrich R., Anton M., Jakle U., Ansorge W., Gorgas K., Just W.W. Microtubule-based peroxisome movement. J. Cell Sci. 1996;1097(1):837–849. doi: 10.1242/jcs.109.4.837. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri A., Tipton P.A. Cloning and expression of the gene for soybean hydroxyisourate hydrolase. Localization and implications for function and mechanism. Plant Physiol. 2002;1307(1):2061–2068. doi: 10.1104/pp.011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A.S., Day I.S. Analysis of the myosins encoded in the recently completed Arabidopsis thaliana genome sequence. Genome Biol. 2001;27(1):RESEARCH0024. doi: 10.1186/gb-2001-2-7-research0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen D., Hanson M.R. Association of six YFP-myosin XI-tail fusions with mobile plant cell organelles. BMC Plant Biol. 2007;77(1):6. doi: 10.1186/1471-2229-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuber B.E., Karl C., Relmann S.A., Mihalik S.J., Dodt G. Cloning and functional expression of a mammalian gene for a peroxisomal sarcosine oxidase. J. Biol. Chem. 1997;2727(1):6766–6776. doi: 10.1074/jbc.272.10.6766. [DOI] [PubMed] [Google Scholar]
- Reumann S. Specification of the peroxisome targeting signals type 1 and type 2 of plant peroxisomes by bioinformatics analyses. Plant Physiol. 2004;1357(1):783–800. doi: 10.1104/pp.103.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann S., Weber A.P. Plant peroxisomes respire in the light: some gaps of the photorespiratory C2 cycle have become filled--others remain. Biochim. Biophys. Acta. 2006;17637(1):1496–1510. doi: 10.1016/j.bbamcr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Reumann S., Maier E., Benz R., Heldt H.W. The membrane of leaf peroxisomes contains a porin-like channel. J. Biol. Chem. 1995;2707(1):17559–17565. doi: 10.1074/jbc.270.29.17559. [DOI] [PubMed] [Google Scholar]
- Reumann S., Maier E., Benz R., Heldt H.W. A specific porin is involved in the malate shuttle of leaf peroxisomes. Biochem. Soc. Trans. 1996;247(1):754–757. doi: 10.1042/bst0240754. [DOI] [PubMed] [Google Scholar]
- Reumann S., Bettermann M., Benz R., Heldt H.W. Evidence for the Presence of a Porin in the Membrane of Glyoxysomes of Castor Bean. Plant Physiol. 1997;1157(1):891–899. doi: 10.1104/pp.115.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann S., Maier E., Heldt H.W., Benz R. Permeability properties of the porin of spinach leaf peroxisomes. Eur. J. Biochem. 1998;2517(1):359–366. doi: 10.1046/j.1432-1327.1998.2510359.x. [DOI] [PubMed] [Google Scholar]
- Reumann S., Ma C., Lemke S., Babujee L. AraPerox. A database of putative Arabidopsis proteins from plant peroxisomes. Plant Physiol. 2004;1367(1):2587–2608. doi: 10.1104/pp.104.043695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann S., Babujee L., Ma C., Wienkoop S., Siemsen T., Antonicelll G.E., Rasche N., Luder F., Weckwerth W., Jahn O. Proteome analysis of Arabidopsis leaf peroxisomes reveals novel targeting peptides, metabolic pathways, and defense mechanisms. Plant Cell. 2007;197(1):3170–3193. doi: 10.1105/tpc.107.050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann S., Quan S., Aung K., Yang P., Manandhar-Shrestha K., Holbrook D., Linka N., Switzenberg R., Wilkerson C.G., Weber A.P., Olsen L.J., Hu J. In-depth proteome analysis of Arabidopsis leaf peroxisomes combined with in vivo subcellular targeting verification indicates novel metabolic and regulatory functions of peroxisomes. Plant Physiol. 2009;1507(1):125–143. doi: 10.1104/pp.109.137703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond T.A., Bleecker A.B. A defect in beta-oxidation causes abnormal inflorescence development in Arabidopsis. Plant Cell. 1999;117(1):1911–1924. doi: 10.1105/tpc.11.10.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Concepcion M., Boronat A. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol. 2002;1307(1):1079–1089. doi: 10.1104/pp.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rontein D., Basset G., Hanson A.D. Metabolic engineering of osmoprotectant accumulation in plants. Metab. Eng. 2002;47(1):49–56. doi: 10.1006/mben.2001.0208. [DOI] [PubMed] [Google Scholar]
- Rottensteiner H., Stein K., Sonnenhol E., Erdmann R. Conserved function of pex11p and the novel pex25p and pex27p in peroxisome biogenesis. Mol. Biol. Cell. 2003;147(1):4316–4328. doi: 10.1091/mbc.E03-03-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylott E.L., Rogers C.A., Gilday A.D., Edgell T., Larson T.R., Graham I.A. Arabidopsis mutants in short- and medium-chain acyl-CoA oxidase activities accumulate acyl-CoAs and reveal that fatty acid beta-oxidation is essential for embryo development. J. Biol. Chem. 2003;2787(1):21370–21377. doi: 10.1074/jbc.M300826200. [DOI] [PubMed] [Google Scholar]
- Saleem R.A., Smith J.J., Aitchison J.D. Proteomics of the peroxisome. Biochim Biophys Acta. 2006;17637(1):1541–1551. doi: 10.1016/j.bbamcr.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders P.M., Lee P.Y., Biesgen C., Boone J.D., Beals T.P., Weiler E.W., Goldberg R.B. The arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell. 2000;127(1):1041–1061. doi: 10.1105/tpc.12.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir-Mir M., Mett A., Belausov E., Tal-Meshulam S., Frydman A., Gidoni D., Eyal Y. Peroxisomal localization of Arabidopsis isopentenyl diphosphate isomerases suggests that part of the plant isoprenoid mevalonic acid pathway is compartmentalized to peroxisomes. Plant Physiol. 2008;1487(1):1219–1228. doi: 10.1104/pp.108.127951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma A.D., Serfozo P., Kahn K., Tipton P.A. Identification and purification of hydroxyisourate hydrolase, a novel ureide-metabolizing enzyme. J. Biol. Chem. 1999;2747(1):33863–33865. doi: 10.1074/jbc.274.48.33863. [DOI] [PubMed] [Google Scholar]
- Schaller F., Biesgen C., Mussig C., Altmann T., Weiler E.W. 12-Oxophytodienoate reductase 3 (OPR3) is the isoenzyme involved in jasmonate biosynthesis. Planta. 2000;2107(1):979–984. doi: 10.1007/s004250050706. [DOI] [PubMed] [Google Scholar]
- Schilmiller A.L., Koo A.J., Howe G.A. Functional diversification of acyl-coenzyme A oxidases in jasmonic acid biosynthesis and action. Plant Physiol. 2007;1437(1):812–824. doi: 10.1104/pp.106.092916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter A., Fourcade S., Ripp R., Mandel J.L., Poch O., Pujol A. The evolutionary origin of peroxisomes: an ER-peroxisome connection. Mol. Biol. Evol. 2006;237(1):838–845. doi: 10.1093/molbev/msj103. [DOI] [PubMed] [Google Scholar]
- Schluter A., Fourcade S., Domenech-Estevez E., Gabaldon T., Huerta-Cepas J., Berthommier G., Ripp R., Wanders R.J., Poch O., Pujol A. PeroxisomeDB: a database for the peroxisomal proteome, functional genomics and disease. Nucleic Acids Res. 2007;357(1):D815–822. doi: 10.1093/nar/gkl935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K., Kienow L., Schmelzer E., Colby T., Bartsch M., Miersch O., Wasternack C., Kombrink E., Stuible H.P. A new type of peroxisomal acyl-coenzyme A synthetase from Arabidopsis thaliana has the catalytic capacity to activate biosynthetic precursors of jasmonic acid. J. Biol. Chem. 2005;2807(1):13962–13972. doi: 10.1074/jbc.M413578200. [DOI] [PubMed] [Google Scholar]
- Scholthof H.B., Scholthof K.B., Kikkert M., Jackson A.O. Tomato bushy stunt virus spread is regulated by two nested genes that function in cell-to-cell movement and host-dependent systemic invasion. Virology. 1995;2137(1):425–438. doi: 10.1006/viro.1995.0015. [DOI] [PubMed] [Google Scholar]
- Schrader M. Shared components of mitochondrial and peroxisomal division. Biochimica Biophys. Acta. 2006;17637(1):531–541. doi: 10.1016/j.bbamcr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Schrader M., Fahimi H.D. The peroxisome: still a mysterious organelle. Histochem. Cell Biol. 2008;1297(1):421–440. doi: 10.1007/s00418-008-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M., Burkhardt U.K., Baumgart E., Luers G., Volkl A., Fahimi H.D. The importance of microtubules in determination of shape and intracellular distribution of peroxisomes. Ann. New York Acad. Sci. 1996a;8047(1):669–671. doi: 10.1111/j.1749-6632.1996.tb18660.x. [DOI] [PubMed] [Google Scholar]
- Schrader M., Burkhardt U.K., Baumgart E., Luers G., Spring H., Volkl A., Fahimi H.D. Interaction of microtubules with peroxisomes. Tubular and spherical peroxisomes in HepG2 cells and their alterations induced by microtubule-active drugs. Eur. J. Cell Biol. 1996b;697(1):24–35. [PubMed] [Google Scholar]
- Schrader M., Reuber B.E., Morrell J.C., Jimenez-Sanchez G., Obie C., Stroh T.A., Valle D., Schroer T.A., Gould S.J. Expression of PEX11beta mediates peroxisome proliferation in the absence of extracellular stimuli. J. Biol. Chem. 1998;2737(1):29607–29614. doi: 10.1074/jbc.273.45.29607. [DOI] [PubMed] [Google Scholar]
- Schuhmann H., Huesgen P.F., Gietl C., Adamska I. The DEG15 serine protease cleaves peroxisomal targeting signal 2-containing proteins in Arabidopsis. Plant Physiol. 2008;1487(1):1847–1856. doi: 10.1104/pp.108.125377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann U., Gietl C., Schmid M. Sequence analysis of a cDNA encoding Pex7p, a peroxisomal targeting signal 2 receptor from Arabidopsis thaliana. Plant Physiol. 1999;1207(1):339. [Google Scholar]
- Schumann U., Wanner G., Veenhuis M., Schmid M., Gietl C. AthPEX10, a nuclear gene essential for peroxisome and storage organelle formation during Arabidopsis embryogenesis. Proc. Natl. Acad. Sci. USA. 2003;1007(1):9626–9631. doi: 10.1073/pnas.1633697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann U., Prestele J., O'Geen H., Brueggeman R., Wanner G., Gietl C. Requirement of the C3HC4 zinc RING finger of the Arabidopsis PEX10 for photorespiration and leaf peroxisome contact with chloroplasts. Proc. Natl. Acad. Sci. USA. 2007;1047(1):1069–1074. doi: 10.1073/pnas.0610402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann M., Tse Sum Bul B., Wolff M., Miginiac-Maslow M., Rohmer M. Isoprenoid biosynthesis in plant chloroplasts via the MEP pathway: direct thylakoid/ferredoxin-dependent photoreduction of GcpE/IspG. FEBS Lett. 2006;5807(1):1547–1552. doi: 10.1016/j.febslet.2006.01.082. [DOI] [PubMed] [Google Scholar]
- Seiler N. Catabolism of polyamines. Amino Acids. 2004;267(1):217–233. doi: 10.1007/s00726-004-0070-z. [DOI] [PubMed] [Google Scholar]
- Shay N.F., Banz W.J. Regulation of gene transcription by botanicals: novel regulatory mechanisms. Annu. Rev. Nutr. 2005;257(1):297–315. doi: 10.1146/annurev.nutr.25.050304.092639. [DOI] [PubMed] [Google Scholar]
- Shi W.M., Muramoto Y., Ueda A., Takabe T. Cloning of peroxisomal ascorbate peroxidase gene from barley and enhanced thermotolerance by overexpressing in Arabidopsis thaliana. Gene. 2001;2737(1):23–27. doi: 10.1016/s0378-1119(01)00566-2. [DOI] [PubMed] [Google Scholar]
- Shibata H., Kashiwayama Y., Imanaka T., Kato H. Domain architecture and activity of human Pex19p, a chaperone-like protein for intracellular trafficking of peroxisomal membrane proteins. J. Biol. Chem. 2004;2797(1):38486–38494. doi: 10.1074/jbc.M402204200. [DOI] [PubMed] [Google Scholar]
- Shockey J.M., Fulda M.S., Browse J. Arabidopsis contains a large superfamily of acyl-activating enzymes. Phylogenetic and biochemical analysis reveals a new class of acyl-coenzyme a synthetases. Plant Physiol. 2003;1327(1):1065–1076. doi: 10.1104/pp.103.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair A.M., Trobacher C.P., Mathur N., Greenwood U.S., Mathur J. Peroxule extension over ER defined paths constitutes a rapid subcellular response to hydroxyl stress. Plant J. 2009;597(1):231–242. doi: 10.1111/j.1365-313X.2009.03863.x. [DOI] [PubMed] [Google Scholar]
- Somerville C.R., Ogren W.L. Photorespiration mutants of Arabidopsis thaliana deficient in serine-glyoxylate aminotransferase activity. Proc. Natl. Acad. Sci. USA. 1980;777(1):2684–2687. doi: 10.1073/pnas.77.5.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South S.T., Gould S.J. Peroxisome synthesis in the absence of preexisting peroxisomes. J. Cell Biol. 1999;1447(1):255–266. doi: 10.1083/jcb.144.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes I.A., Teanby N.A., Hawes C. Truncated myosin XI tail fusions inhibit peroxisome, golgi, and mitochondrial movement in tobacco leaf epidermal cells: a genetic tool for the next generation. J. Exp. Bot. 2008;597(1):2499–2512. doi: 10.1093/jxb/ern114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes I.A., Brandizzi F., Slocombe S.P., El-Shami M., Hawes C., Baker A. An Arabidopsis pex10 null mutant is embryo lethal, implicating peroxisomes in an essential role during plant embryogenesis. Plant Physiol. 2003;1337(1):1809–1819. doi: 10.1104/pp.103.031252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabenau H., Winkler U., Saftel W. On the origin of microbodies in plants. 1. Vol. 7. University of Oldenburg; Oldenburg, Germany: 1998. pp. 3–8. [Google Scholar]
- Staswick P.E., Tiryaki I., Rowe M.L. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell. 2002;147(1):1405–1415. doi: 10.1105/tpc.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P.E., Serban B., Rowe M., Tiryaki I., Maldonado M.T., Maldonado M.C., Suza W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell. 2005;177(1):616–627. doi: 10.1105/tpc.104.026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticher L., Mauch-Mani B., Metraux J.P. Systemic acquired resistance. Annu. Rev. Phytopathol. 1997;357(1):235–270. doi: 10.1146/annurev.phyto.35.1.235. [DOI] [PubMed] [Google Scholar]
- Stintzi A., Browse J. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl. Acad. Sci. USA. 2000;977(1):10625–10630. doi: 10.1073/pnas.190264497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovski D., Koutsopoulos O.S., Okamoto K., Ryan M.T. Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J. Cell Sci. 2004;1177(1):1201–1210. doi: 10.1242/jcs.01058. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Kawada T., Goto T., Yamamoto T., Taimatsu A., Matsui N., Kimura K., Saito M., Hosokawa M., Miyashita K., Fushiki T. Dual action of isoprenols from herbal medicines on both PPARgamma and PPARalpha in 3T3-L1 adipocytes and HepG2 hepatocytes. FEBS Lett. 2002;5147(1):315–322. doi: 10.1016/s0014-5793(02)02390-6. [DOI] [PubMed] [Google Scholar]
- Taler D., Galperin M., Benjamin I., Cohen Y., Kenigsbuch D. Plant eR genes that encode photorespiratory enzymes confer resistance against disease. Plant Cell. 2004;167(1):172–184. doi: 10.1105/tpc.016352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam Y.Y., Fagarasanu A., Fagarasanu M., Rachubinski R.A. Pex3p initiates the formation of a preperoxisomal compartment from a subdomain of the endoplasmic reticulum in Saccharomyces cerevisiae. J. Biol. Chem. 2005;2807(1):34933–34939. doi: 10.1074/jbc.M506208200. [DOI] [PubMed] [Google Scholar]
- Tam Y.Y., Torres-Guzman J.C., Vizeacoumar F.J., Smith J.J., Marelli M., Aitchison J.D., Rachubinski R.A. Pex11-related proteins in peroxisome dynamics: a role for the novel peroxin Pex27p in controlling peroxisome size and number in Saccharomyces cerevisiae. Mol. Biol. Cell. 2003;147(1):4089–4102. doi: 10.1091/mbc.E03-03-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavladoraki P., Rossi M.N., Saccuti G., Perez-Amador M.A., Polticelli F., Angelini R., Federico R. Heterologous expression and biochemical characterization of a polyamine oxidase from Arabidopsis involved in polyamine back conversion. Plant Physiol. 2006;1417(1):1519–1532. doi: 10.1104/pp.106.080911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tel-Or E., Huflejt M.E., Packer L. Hydroperoxide metabolism in cyanobacteria. Arch Biochem. Biophys. 1986;2467(1):396–402. doi: 10.1016/0003-9861(86)90485-6. [DOI] [PubMed] [Google Scholar]
- Theodoulou F.L., Job K., Slocombe S.P., Footitt S., Holdsworth M., Baker A., Larson T.R., Graham I.A. Jasmonic acid levels are reduced in COMATOSE ATP-binding cassette transporter mutants. Implications for transport of jasmonate precursors into peroxisomes. Plant Physiol. 2005;1377(1):835–840. doi: 10.1104/pp.105.059352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoms S., Erdmann R. Dynamin-related proteins and Pex11 proteins in peroxisome division and proliferation. FEBS J. 2005;2727(1):5169–5181. doi: 10.1111/j.1742-4658.2005.04939.x. [DOI] [PubMed] [Google Scholar]
- Thoms S., Erdmann R. Peroxisomal matrix protein receptor ubiquitination and recycling. Biochim. Biophys. Acta. 2006;17637(1):1620–1628. doi: 10.1016/j.bbamcr.2006.08.046. [DOI] [PubMed] [Google Scholar]
- Timm S., Nunes-Nesi A., Parnik T., Morgenthal K., Wienkoop S., Keerberg O., Weckwerth W., Kleczkowski L.A., Fernie A.R., Bauwe H. A cytosolic pathway for the conversion of hydroxypyruvate to glycerate during photorespiration in Arabidopsis. Plant Cell. 2008;207(1):2848–2859. doi: 10.1105/tpc.108.062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titorenko V.I., Rachubinski R.A. Dynamics of peroxisome assembly and function. Trends Cell Biol. 2001;117(1):22–29. doi: 10.1016/s0962-8924(00)01865-1. [DOI] [PubMed] [Google Scholar]
- Titorenko V.I., Rachubinski R.A. The peroxisome: orchestrating important developmental decisions from inside the cell. J. Cell Biol. 2004;1647(1):641–645. doi: 10.1083/jcb.200312081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titorenko V.I., Mullen R.T. Peroxisome biogenesis: the peroxisomal endomembrane system and the role of the ER. J. Cell Biol. 2006;1747(1):11–17. doi: 10.1083/jcb.200604036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd C.D., Tipton P.A., Blevins D.G., Piedras P., Pineda M., Polacco J.C. Update on ureide degradation in legumes. J. Exp. Bot. 2006;577(1):5–12. doi: 10.1093/jxb/erj013. [DOI] [PubMed] [Google Scholar]
- Trickey P., Wagner M.A., Jorns M.S., Mathews F.S. Monomeric sarcosine oxidase: structure of a covalently flavinylated amine oxidizing enzyme. Structure. 1999;77(1):331–345. doi: 10.1016/s0969-2126(99)80043-4. [DOI] [PubMed] [Google Scholar]
- Trossat C., Rathinasabapathi B., Hanson A.D. Transgenically expressed betaine aldehyde dehydrogenase efficiently catalyzes oxidation of dimethylsulfoniopropionaldehyde and [omega]-aminoaldehydes. Plant Physiol. 1997;1137(1):1457–1461. doi: 10.1104/pp.113.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.E., Greville K., Murphy E.G., Hooks M.A. Characterization of Arabidopsis fluoroacetate-resistant mutants reveals the principal mechanism of acetate activation for entry into the glyoxylate cycle. J. Biol. Chem. 2005;2807(1):2780–2787. doi: 10.1074/jbc.M407291200. [DOI] [PubMed] [Google Scholar]
- Ulloa R.M., Raices M., Macintosh G.C., Maldonado S., Tellezlnon M.T. Jasmonic acid affects plant morphology and calcium-dependent protein kinase expression and activity in Solanum tuberosum. Physiol. Plant. 2002;1157(1):417–427. doi: 10.1034/j.1399-3054.2002.1150312.x. [DOI] [PubMed] [Google Scholar]
- Van Ael E., Fransen M. Targeting signals in peroxisomal membrane proteins. Biochim. Biophys. Acta. 2006;17637(1):1629–1638. doi: 10.1016/j.bbamcr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- van der Kiel I.J., Veenhuis M. Yeast and filamentous fungi as model organisms in microbody research. Biochim. Biophys. Acta. 2006;17637(1):1364–1373. doi: 10.1016/j.bbamcr.2006.09.014. [DOI] [PubMed] [Google Scholar]
- van Roermund C.W., Elgersma Y., Singh N., Wanders R.J., Tabak H.F. The membrane of peroxisomes in Saccharomyces cerevisiae is impermeable to NAD(H) and acetyl-CoA under in vivo conditions. EMBO J. 1995;147(1):3480–3486. doi: 10.1002/j.1460-2075.1995.tb07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele S., Vanderauwera S., Vuylsteke M., Rombauts S., Langebartels C., Seidlitz H.K., Zabeau M., Van Montagu M., Inze D., Van Breusegem F. Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J. 2004;397(1):45–58. doi: 10.1111/j.1365-313X.2004.02105.x. [DOI] [PubMed] [Google Scholar]
- Verslues P.E., Kim Y.S., Zhu U.K. Altered ABA, proline and hydrogen peroxide in an Arabidopsis glutamate:glyoxylate aminotransferase mutant. Plant Mol. Biol. 2007;647(1):205–217. doi: 10.1007/s11103-007-9145-z. [DOI] [PubMed] [Google Scholar]
- Vick B.A., Zimmerman D.C. Biosynthesis of Jasmonic acid by several plant species. Plant Physiol. 1984;757(1):458–461. doi: 10.1104/pp.75.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizeacoumar F.J., Torres-Guzman J.C., Tam Y.Y., Aitchison J.D., Rachubinski R.A. YHR150w and YDR479c encode peroxisomal integral membrane proteins involved in the regulation of peroxisome number, size, and distribution in Saccharomyces cerevisiae. J. Cell Biol. 2003;1617(1):321–332. doi: 10.1083/jcb.200210130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizeacoumar F.J., Torres-Guzman J.C., Bouard D., Aitchison J.D., Rachubinski R.A. Pex30p, Pex31p, and Pex32p form a family of peroxisomal integral membrane proteins regulating peroxisome size and number in Saccharomyces cerevisiae. Mol. Biol. Cell. 2004;157(1):665–677. doi: 10.1091/mbc.E03-09-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk S., Feierabend J. Photoinactivation of catalase at low temperature and its relevance to photosynthetic and peroxide metabolism in leaves. Plant Cell Environ. 1989;127(1):701–712. [Google Scholar]
- Wang J., Zhang H., Allen R.D. Overexpression of an Arabidopsis peroxisomal ascorbate peroxidase gene in tobacco increases protection against oxidative stress. Plant Cell Physiol. 1999;407(1):725–732. doi: 10.1093/oxfordjournals.pcp.a029599. [DOI] [PubMed] [Google Scholar]
- Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. (Lond) 2007;1007(1):681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A.K., Sparkes I.A., Romels T., Witte C.P. Identification, biochemical characterization, and subcellular localization of allantoate amidohydrolases from Arabidopsis and soybean. Plant Physiol. 2008;1467(1):418–430. doi: 10.1104/pp.107.110809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal L., Scheel D., Rosahl S. The coil -16 Mutant Harbors a Second Site Mutation Rendering PEN2 Nonfunctional. Plant Cell. 2008;207(1):824–826. doi: 10.1105/tpc.107.056895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemer E.A., Wenzel T., Deerinck T.J., Ellisman M.H., Subramani S. Visualization of the peroxisomal compartment in living mammalian cells: dynamic behavior and association with microtubules. J. Cell Biol. 1997;1367(1):71–80. doi: 10.1083/jcb.136.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese S., Gronemeyer T., Ofman R., Kunze M., Grou O.P., Almeida J.A., Eisenacher M., Stephen C., Hayen H., Schollenberger L., Korosec T., Waterham H.R., Schliebs W., Erdmann R., Berger J., Meyer H.E., Just W., Azevedo J.E., Wanders R.J., Warscheid B. Proteomics characterization of mouse kidney peroxisomes by tandem mass spectrometry and protein correlation profiling. Mol. Cell. Proteomics. 2007;67(1):2045–2057. doi: 10.1074/mcp.M700169-MCP200. [DOI] [PubMed] [Google Scholar]
- Wildermuth M.C., Dewdney J., Wu G., Ausubel F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;4147(1):562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- Willekens H., Chamnongpol S., Davey M., Schraudner M., Langebartels C., Van Montagu M., Inze D., Van Camp W. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 1997;167(1):4806–4816. doi: 10.1093/emboj/16.16.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsbach K., Payne G.S. Vps1p, a member of the dynamin GTPase family, is necessary for Golgi membrane protein retention in Saccharomyces cerevisiae. EMBO J. 1993;127(1):3049–3059. doi: 10.1002/j.1460-2075.1993.tb05974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer B., Lottspeich F., van der Kiel I., Veenhuis M., Gietl C. The glyoxysomal and plastid molecular chaperones (70-kDa heat shock protein) of watermelon cotyledons are encoded by a single gene. Proc. Natl. Acad. Sci. USA. 1997;947(1):13624–13629. doi: 10.1073/pnas.94.25.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer C., Schmid M., Veenhuis M., Gietl C. The plant PTS1 receptor: similarities and differences to its human and yeast counterparts. Plant J. 1998;167(1):453–464. doi: 10.1046/j.1365-313x.1998.00320.x. [DOI] [PubMed] [Google Scholar]
- Wiszniewski A.A., Zhou W., Smith S.M., Bussell J.D. Identification of two Arabidopsis genes encoding a peroxisomal oxidoreductase-like protein and an acyl-CoA synthetase-like protein that are required for responses to pro-auxins. Plant Mol. Biol. 2009;697(1):503–515. doi: 10.1007/s11103-008-9431-4. [DOI] [PubMed] [Google Scholar]
- Woodward A.W., Bartel B. The Arabidopsis peroxisomal targeting signal type 2 receptor PEX7 is necessary for peroxisome function and dependent on PEX5. Mol. Biol. Cell. 2005;167(1):573–583. doi: 10.1091/mbc.E04-05-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters F.S., Bastiaens P.I., Wirtz K.W., J.M Jovin. FRET microscopy demonstrates molecular association of non-specific lipid transfer protein (nsL-TP) with fatty acid oxidation enzymes in peroxisomes. EMBO J. 1998;177(1):7179–7189. doi: 10.1093/emboj/17.24.7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Robertson A.J., Zheng P., Liu X., Gusta L.V. Identification and immunogold localization of a novel bromegrass (Bromus inermis Leyss) peroxisome channel protein induced by ABA, cold and drought stresses, and late embryogenesis. Gene. 2005;3637(1):77–84. doi: 10.1016/j.gene.2005.06.046. [DOI] [PubMed] [Google Scholar]
- Xing Y., Jia W., Zhang J. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J. 2008;547(1):440–451. doi: 10.1111/j.1365-313X.2008.03433.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Mori H., Nishimura M. A novel isoenzyme of ascorbate peroxidase localized on glyoxysomal and leaf peroxisomal membranes in pumpkin. Plant Cell Physiol. 1995;367(1):1157–1162. doi: 10.1093/oxfordjournals.pcp.a078862. [DOI] [PubMed] [Google Scholar]
- Yan J., Wang J., Tissue D., Holaday A.S., Allen R., Zhang H. Photosynthesis and seed production under water-deficit conditions in transgenic tobacco plants that overexpress an Arabidopsis ascorbate peroxidase gene. Crop Science. 2003;437(1):1477–1483. [Google Scholar]
- Yan M., Rayapuram N., Subramani S. The control of peroxisome number and size during division and proliferation. Curr. Opin. Cell Biol. 2005;177(1):376–383. doi: 10.1016/j.ceb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Yanik T., Donaldson R.P. A protective association between catalase and isocitrate lyase in peroxisomes. Arch. Biochem. Biophys. 2005;4357(1):243–252. doi: 10.1016/j.abb.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Yoon Y., Krueger E.W., Oswald B.J., McNiven M.A. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol. Cell. Biol. 2003;237(1):5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Selection and characterization of tobacco plants with novel O(2)-resistant photosynthesis. Plant Physiol. 1989;907(1):1457–1464. doi: 10.1104/pp.90.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Factors affecting expression of enhanced catalase activity in a tobacco mutant with O(2)-resistant photosynthesis. Plant Physiol. 1992;987(1):1330–1335. doi: 10.1104/pp.98.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I., Havir E.A., McGonigle B., McHale N.A., Nelson T. Leaf catalase mRNA and catalase-protein levels in a high-catalase tobacco mutant with O(2)-resistant photosynthesis. Plant Physiol. 1991;977(1):1592–1595. doi: 10.1104/pp.97.4.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Q., Li C.B., Zheng W., Wu X., Zhao J., Zhou G., Jiang H., Sun J., Lou Y., Li C. Phytochrome chromophore deficiency leads to overproduction of jasmonic acid and elevated expression of jasmonate-responsive genes in Arabidopsis. Plant Cell Physiol. 2007;487(1):1061–1071. doi: 10.1093/pcp/pcm076. [DOI] [PubMed] [Google Scholar]
- Zhang X., Hu J. FISSION1A and FISSION1B proteins mediate the fission of peroxisomes and mitochondria in Arabidopsis. Mol. Plant. 2008;17(1):1036–1047. doi: 10.1093/mp/ssn056. [DOI] [PubMed] [Google Scholar]
- Zhang X., Hu J. Two small protein families, DYNAMIN-RELATED PROTEIN3 and FISSION1, are required for peroxisome fission in Arabidopsis. Plant J. 2009;577(1):146–159. doi: 10.1111/j.1365-313X.2008.03677.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann P., Heinlein C., Orendi G., Zentgraf U. Senescence-specific regulation of catalases in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ. 2006;297(1):1049–1060. doi: 10.1111/j.1365-3040.2005.01459.x. [DOI] [PubMed] [Google Scholar]
- Zolman B.K., Bartel B. An Arabidopsis indole-3-butyric acid-response mutant defective in PEROXIN6, an apparent ATPase implicated in peroxisomal function. Proc. Natl. Acad. Sci. USA. 2004;1017(1):1786–1791. doi: 10.1073/pnas.0304368101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman B.K., Yoder A., Bartel B. Genetic analysis of Indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics. 2000;1567(1):1323–1337. doi: 10.1093/genetics/156.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman B.K., Silva I.D., Bartel B. The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid beta-oxidation. Plant Physiol. 2001a;1277(1):1266–1278. [PMC free article] [PubMed] [Google Scholar]
- Zolman B.K., Nyberg M., Bartel B. IBR3, a novel peroxisomal acyl-CoA dehydrogenase-like protein required for indole-3-butyric acid response. Plant Mol. Biol. 2007;647(1):59–72. doi: 10.1007/s11103-007-9134-2. [DOI] [PubMed] [Google Scholar]
- Zolman B.K., Monroe-Augustus M., Silva I.D., Bartel B. Identification and functional characterization of Arabidopsis PEROXIN4 and the interacting protein PEROXIN22. Plant Cell. 2005;177(1):3422–3435. doi: 10.1105/tpc.105.035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman B.K., Martinez N., Millius A., Adham A.R., Bartel B. Identification and characterization of Arabidopsis indole-3-butyric Acid response mutants defective in novel peroxisomal enzymes. Genetics. 2008;1807(1):237–251. doi: 10.1534/genetics.108.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman B.K., Monroe-Augustus M., Thompson B., Hawes J.W., Krukenberg K.A., Matsuda S.P., Bartel B. chy1, an Arabidopsis mutant with impaired beta-oxidation, is defective in a peroxisomal beta-hydroxyisobutyryl-CoA hydrolase. J. Biol. Chem. 2001b;2767(1):31037–31046. doi: 10.1074/jbc.M104679200. [DOI] [PubMed] [Google Scholar]