Abstract
Alzheimer's disease susceptibility genes, APP and gamma-secretase, are involved in the herpes simplex life cycle, and that of other suspect pathogens (C. pneumoniae, H. pylori, C. neoformans, B. burgdorferri, P. gingivalis) or immune defence. Such pathogens promote beta-amyloid deposition and tau phosphorylation and may thus be causative agents, whose effects are conditioned by genes. The antimicrobial effects of beta-amyloid, the localisation of APP/gamma-secretase in immunocompetent dendritic cells, and gamma secretase cleavage of numerous pathogen receptors suggest that this network is concerned with pathogen disposal, effects which may be abrogated by the presence of beta-amyloid autoantibodies in the elderly. These autoantibodies, as well as those to nerve growth factor and tau, also observed in Alzheimer's disease, may well be antibodies to pathogens, due to homology between human autoantigens and pathogen proteins. NGF or tau antibodies promote beta-amyloid deposition, neurofibrillary tangles, or cholinergic neuronal loss, and, with other autoantibodies, such as anti-ATPase, are potential agents of destruction, whose formation is dictated by sequence homology between pathogen and human proteins, and thus by pathogen strain and human genes. Pathogen elimination in the ageing population and removal of culpable autoantibodies might reduce the incidence and offer hope for a cure in this affliction.
1. Introduction
Hundreds of genes have been implicated in Alzheimer's disease, many of which can be grouped into discrete signalling networks and pathways relevant to the various subpathologies, risk factors, and biochemistry of Alzheimer's disease. Many of the environmental risk factors associated with Alzheimer's disease, including infectious agents (herpes simplex, chlamydia pneumonia, and Borrelia burgdorferi) as well as Vitamin A deficiency, hypercholesterolaemia, hyperhomocysteinaemia or folate deficiency, oestrogen depletion, cerebral nerve growth factor (NGF) deprivation, diabetes, cerebral hypoperfusion (leading to hypoxia and hypoglycaemia) or are able to promote cerebral beta-amyloid deposition (in the absence of any particular gene variant) in animal models [1]. KEGG pathway and other analyses of the multiple genes implicated in Alzheimer's disease have shown that subsets of susceptibility genes can be grouped into networks that are relevant to each of these amyloidogenic pathways (e.g., bacterial and viral entry pathways [1, 2], cholesterol/lipoprotein function [3, 4], growth factor signalling [5], folate and homocysteine pathways [6], insulin signalling [7], and steroid or Vitamin A metabolism [8, 9]). A large number of genes are also related to the immune network [10] (see http://www.polygenicpathways.co.uk/alzkegg.htm and a recent review for further details [1]). These gene subsets are thus related to multiple external factors that are each able to promote beta-amyloid deposition, suggesting that certain genes are related to the causes of Alzheimer's disease, (agents able to provoke beta-amyloid deposition) rather than (and as well as) to the underlying pathology of the disease itself.
Several studies have implicated the herpes simplex virus in the aetiology of Alzheimer's disease [11–13]. Viral DNA is found in amyloid plaques [14], which are also heavily enriched in proteins used by the virus during its life cycle, as well as in proteins related to the immune network [15], and Immunoglobulin IgM, but not IgG seropositivity for herpes simplex is predictive of the subsequent development of Alzheimer's disease [16]. IgM seropositivity is indicative of viral reactivation which again can be induced by several of the risk factors relevant to Alzheimer's disease and its underlying genetic pathways (e.g., NGF deprivation, 17-beta oestradiol, hypoxia, or fever and interleukin 6 activation, with the latter being common and general consequences of infection [1]).
Along with herpes simplex, a number of other pathogens have been implicated in Alzheimer's disease and its associated pathologies. The viral, bacterial, spirochete, and fungal pathogens implicated in dementia or Alzheimer's disease are referenced at (http://www.polygenicpathways.co.uk/alzenvrisk.htm) and include HHV-6, Chlamydia pneumoniae, Helicobacter pylori, periodontal pathogens involved in gum disease [17], Borrelia burgdorferi, and Cryptococcus neoformans. HIV-1 is also able to provoke dementia with Alzheimer's disease pathology [18]. Of these, H. pylori eradication has been reported to improve performance and increase lifespan in Alzheimer's disease patients [19], while two case reports indicated virtually complete recovery from long-term (3 years) misdiagnosed dementia/Alzheimer's disease following antifungal treatment for C. neoformans infection [20, 21]. Many of these pathogens including herpes simplex, HHV-6, C. Pneumoniae, H. pylori and the periodontal pathogen, P. Gingivalis, have also been implicated in atherosclerosis [22–25], while C. neoformans infection in rabbits induces an increase in neutrophil superoxide production, plasma lipid peroxidation, and an increase in inflammatory cells, forerunners of atherosclerosis [26]. Atherosclerosis of the carotid arteries, or of the circle of Willis and leptomeningeal arteries, is a significant predictor of risk in dementia or Alzheimer's disease and correlates with Alzheimer's disease pathology [27, 28]. Cerebral hypoperfusion (hypoglycaemia, hypoxia, ischaemia, or carotid occlusion) or other factors linked to atherosclerosis (e.g., high cholesterol or homocysteine levels) are also able, per se, to induce cerebral beta-amyloid deposition in animal models (see above).
Genomewide association studies (GWAS) have now identified a subset of genes which, along with APOE4 [29], contribute a high proportion of genetic risk. These include clusterin (CLU), phosphatidylinositol-binding clathrin assembly protein (PICALM) and complement receptor 1 (CR1) as well as the ATP cassette transporter ABCA7, Bridging integrator BIN1, a CD2-associated protein (CD2AP), CD33, ephrin A1 (EPHA1), and a membrane-spanning 4-domains, subfamily A (MS4A) cluster recently honed down to MS4A2, although other genes within this cluster may also be relevant [30, 31].
As discussed below, the major Alzheimer's disease genes implicated by the recent GWAS data, as well as APP and gamma secretase, and previous GWAS results are majoritarily involved in pathogen entry and defence, particularly in relation to herpes simplex, but also to other relevant pathogens, and in the immune network. This suggests that genes, pathogens, and the immune system act together to cause Alzheimer's disease, and that a focus on pathogen detection and elimination should be a priority in the ageing at risk population.
2. Methods
The genes identified in a number of recent genomewide association studies are available at the GWAS repository at the National Human Genome Research Institute http://www.genome.gov/gwastudies/ [32] and, along with pre-GWAS genes and environmental risk factors, at http://www.polygenicpathways.co.uk/alzenvrisk.htm. The genes returned from very large sample sets (N > 10,000) include ABCA7, APOE, BIN1, CD2AP, CD33, CLU, CR1, EPHA1, MS4A2, MS4A4A, MS4A4E, MS4A6A, and PICALM whose properties in relation to diverse pathogens were identified by literature survey. While it is recognised that such genes, particularly APOE, ABCA7, CR1, and clusterin, which are involved in lipoprotein function and/or amyloid processing (see below), may exert effects on other relevant branches of Alzheimer's disease pathophysiology, the focus of this paper is on pathogens and the immune system, which appear to be the common factors integrating this network. Throughout the text, these and other genes implicated in Alzheimer's disease from the GWAS and pre-GWAS era are highlighted in bold and appended to the various processes in which they are involved (derived from a KEGG pathway analysis of these genes http://www.polygenicpathways.co.uk/alzkegg.htm) Herpes simplex binding proteins, and key interactors, currently numbering over 450, are stocked and referenced at http://www.polygenicpathways.co.uk/herpeshost.html. KEGG pathway analysis of this interactome is provided at http://www.polygenicpathways.co.uk//HERPESKEGG.htm. Expression data are provided in Figure 1 and are also hyperlinked to the BioGPS webserver http://www.biogps.gnf.org/, which provides general gene information and mRNA expression profiles for most human genes, based on custom arrays from 79 human issues [33, 34]. Predicted B-cell epitopes from human beta-amyloid (1–42), nerve growth factor (NP_002497.2), or the microtubule protein, tau (NP_001116538.2) were identified using the BepiPred server http://www.cbs.dtu.dk/services/BepiPred/ [35] and their sequences compared with pathogen proteomes (Borrelia burgdorferi, C. neoformans, Helicobacter pylori, herpes viruses HSV-1, HSV-2, HHV-6, and the cytomegalovirus (HHV-5)) using the NCBI BLAST server (Protein versus protein: BlastP) [36].
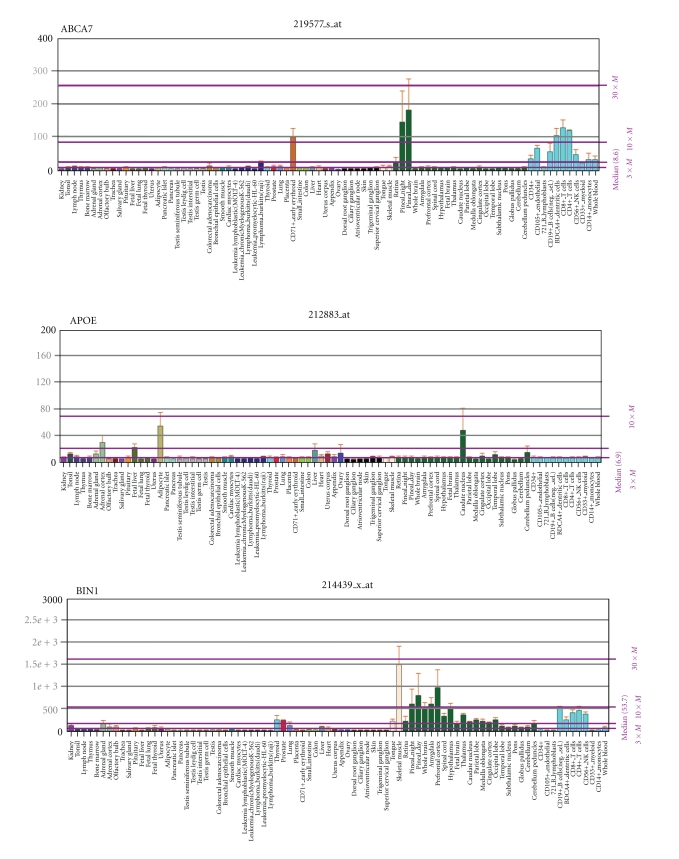
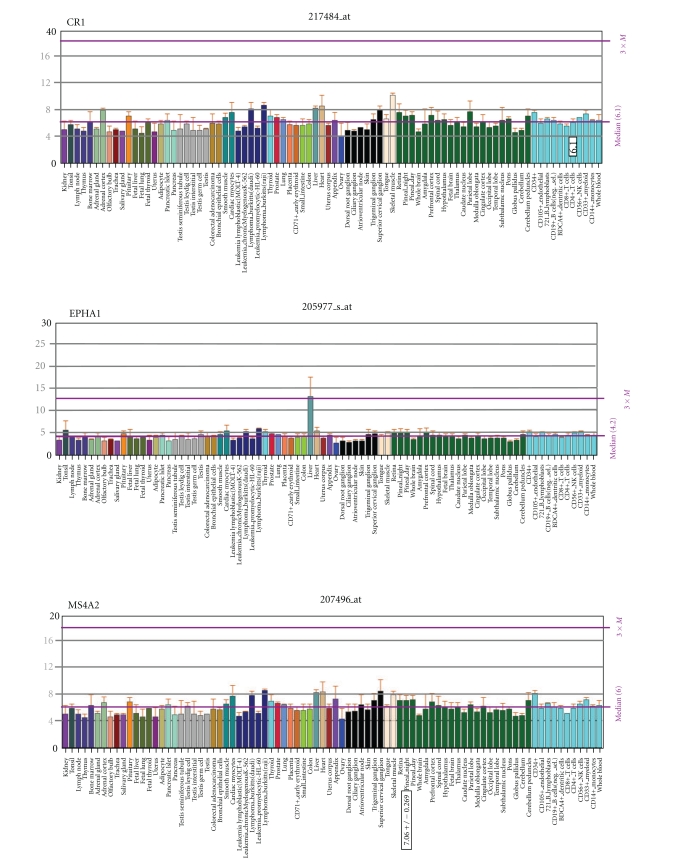
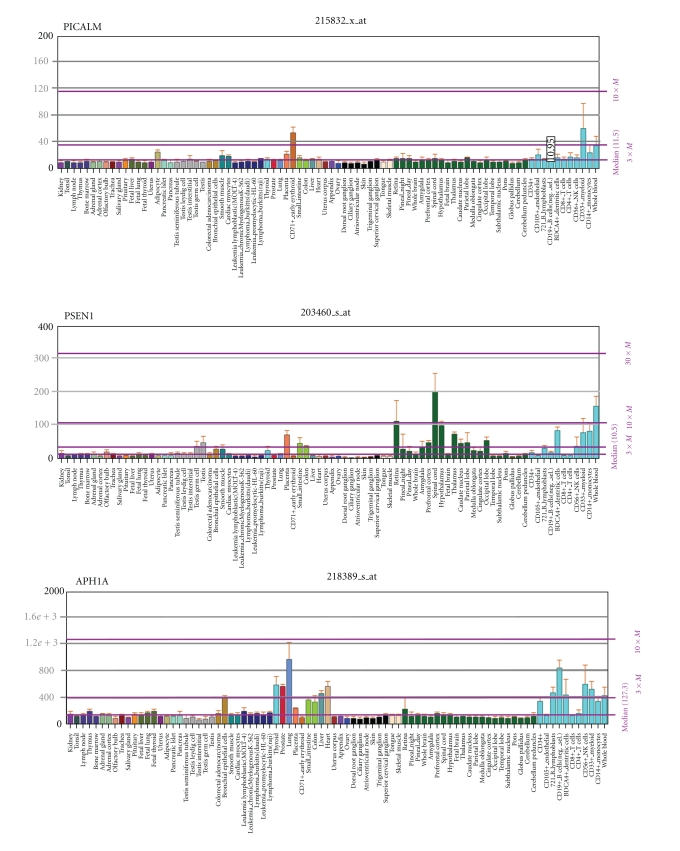
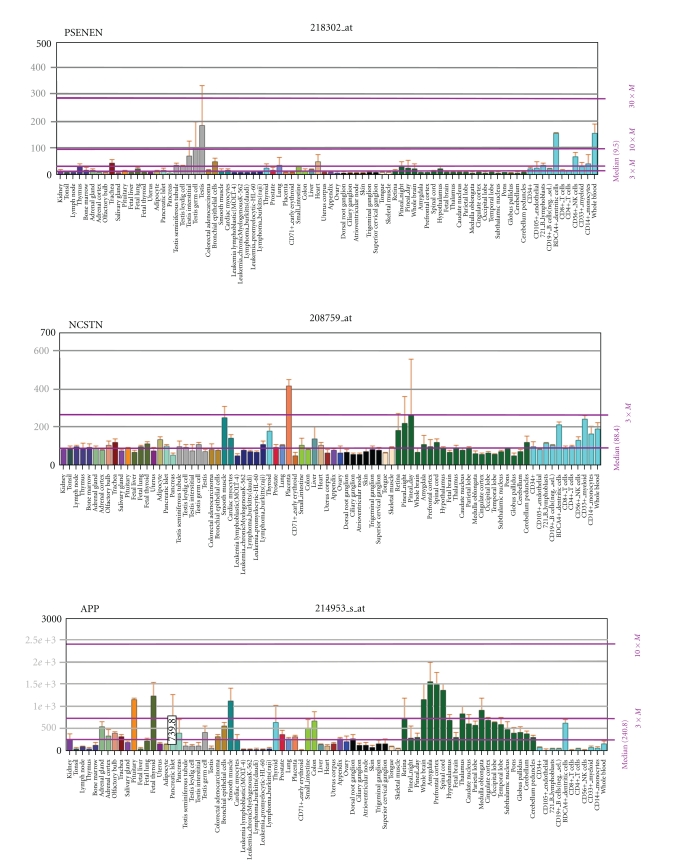
Figure 1.
The mRNA distribution of the major genes derived from GWAS in Alzheimer's disease, as well as that of APP and gamma-secretase components. Data are from the BioGps website.
3. Results
3.1. The Complement System (ABCA7, CR1, CLU, CD2AP, and Beta-Amyloid) Figure 2
Figure 2.
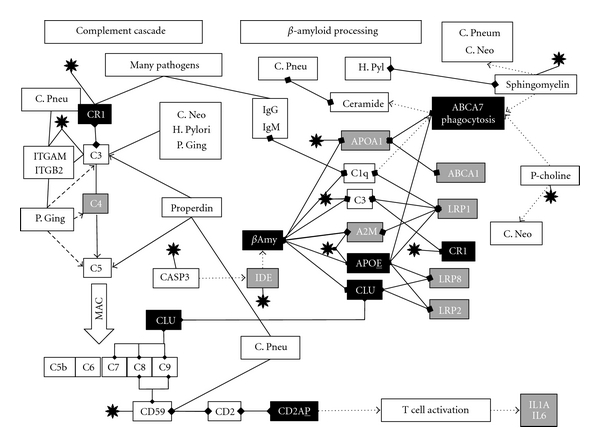
Portions of the complement cascade in relation to beta-amyloid processing: Alzheimer's disease susceptibility genes returned from very large genomewide association studies are in black, and those from the pre-GWAS era in grey. Binding interactions are indicated by linked diamonds and other effects by arrows. C. Neo: Cryptococcus neoformans; C. Pneu: chlamydia pneumoniae; H. Pyl: Helicobacter pylori; P. Ging: Porphyromonas gingivalis; C1–C9: complement components. MAC: membrane attack complex. P-choline: phosphatidylcholine; black star: Herpes simplex: see text for details.
Complement receptor 1 (highly expressed in myeloid CD33+ cells (bone marrow) http://www.biogps.org/#goto=genereport&id=1378/) is a receptor for herpes simplex, adenovirus 5, the influenza virus and HIV-1, as well as for a number of other pathogens, including P. gingivalis, C. neoformans, Streptococcus pneumoniae, Staphylococcus aureus, and the malaria parasite, Plasmodium falciparum [37–43] and is a general clearance receptor for complement opsonised pathogens [44]. Clusterin, predominantly expressed in brain, liver, and testis, (http://www.biogps.org/#goto=genereport&id=1191/) is a ligand for the lipoprotein receptor, megalin (LRP2) that is involved in beta-amyloid clearance, and also a complement inhibitor that prevents the formation of the membrane attack complex, a channel that is inserted into pathogen membranes, killing them by lysis [45]. This complex is also seen in Alzheimer's disease neurones [46, 47]. The herpes simplex virus interacts with other members of the complement cascade, by binding to the complement component and CR1 ligand, C3 and its derivatives and to CD59, a further inhibitor of the formation of the complement membrane attack complex (see review) [48]. C. pneumoniae interacts with this pathway by binding to properdin (CFP), a protein that stabilises the complement C3 and C5 convertase and contributes to the formation of the membrane attack complex [49]. CD59 is also incorporated into chlamydial inclusion bodies [50]. Complement component C3 binds to melanins derived from C. neoformans [51] and cryptococcal capsules bind to C3 and activate the alternative complement pathway [52]. Complement component C3 also binds to the bacterial surface of H. pylori, and the complement pathway is involved in bactericidal effects against this pathogen [53]. P. gingivalis also uses complement receptor 3 (an integrin complex of integrin, alpha M/integrin, beta 2 (ITGAM/ITGB2)) for entry [54], and herpes simplex glycoprotein C also binds to this complex [55] as does C. neoformans [56], while ITGB2 is involved in C. pneumoniae entry in human coronary artery endothelial cells [57]. This macrophage complement receptor, also known as MAC-1, generally mediates the phagocytosis of pathogens coated with complement C3 derivatives [58]. T. C3 also binds to P. gingivalis although the pathogen has devised an elegant escape strategy involving digestion of complement components C3, C4, and C5 by bacterial secreted proteases, known as gingipains [59].
The complement inhibitor CD59 is also a ligand for CD2, and CD59 activation of this receptor, presumably involving CD2AP, activates T cell receptor signalling resulting in the secretion of interleukins (IL1A, IL2 and IL6) and granulocyte macrophage colony stimulating factor (CSF2) [60, 61].
ABCA7 plays a role in the complement-mediated activation of phagocytosis in macrophages. Complement component C1q, which binds to IgM or IgG complexed antigens (relevant to most pathogens), binds to macrophage calreticulin and LRP1 and C1q binding to macrophages markedly increased the expression of both LRP1 and ABCA7, effects which enhance the phagocytic abilities of macrophages [62]. C1q also binds to complement receptor CR1, an effect involved in the immune clearance of opsonised pathogens [63]. C1q also binds to beta-amyloid and is involved in amyloid-related complement activation [64].
3.2. Clathrin-Mediated Endocytosis (BIN1, CLU, CD2AP, PICALM) Figure 3
Figure 3.
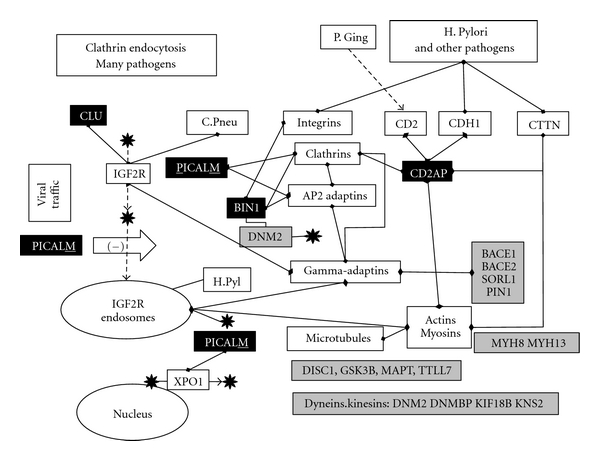
A schematic representation of clathrin-mediated endocytosis and intracellular transport pathways. Alzheimer's disease susceptibility genes returned from very large genomewide association studies are in black, and those from the pre-GWAS era in grey. Binding interactions are indicated by linked diamonds and other effects by arrows. C. Pneu: chlamydia pneumoniae; H. Pyl: Helicobacter pylori; P. Ging: Porphyromonas gingivalis; black star: Herpes simplex: see text for details.
Mammalian surface receptors are endocytosed, via clathrin-dependent or independent processes (KEGG: ADRB1, ADRB2, BIN1, CAV1, CD2AP, CLU, DNM2, HLA-A, HSPA1B, LDLR, NTRK1, PICALM) and either recycled or tagged for destruction by the ubiquitin/proteasome system (KEGG: UBD, UBE2I UBQLN1, UCHL1) or by lysosomes (KEGG: ABCA2, ARSA, ARSB, CTSD, CTSS, NPC1, NPC2, LIPA). Early endosomes receive traffic from the cell surface, which is transferred to late endosomes for traffic to lysosomes. Late endosomes also receive traffic from the trans-Golgi network used to synthesise proteins and from phagocytic pathways (KEGG: CTSS, DLD, DLST, DNM2, GAB2, HLA-A, HLA-DRB1, MPO, NOS1, OLR1, PIK3R1, PSK1, TAP2, TLR2, TLR4). Endosomal traffic moves along the microtubule (GSK3B, MAPT, TTLL7) or actin/myosin (MYH8, MYH13) networks via dynein/dynactin (DM2, DNMBP) or kinesin (KIF18B, KIF20B, KNS2), related motors and Rho GTPases, and vacuolar sorting proteins (SORCS1, SORCS2, SORCS3, SORL1) inter alia [65]. These processes are usurped by many viruses and other pathogens to gain access to cells and to various intracellular compartments, while the lysosomal or proteasomal pathways may be used to destroy pathogen proteins [66].
Clathrin-mediated endocytosis is one of several processes used by Helicobacter pylori, herpes simplex, and many other viral, bacterial and fungal pathogens to gain entry to cells [67–69].
PICALM, expressed primarily in myeloid and dendritic cells of the immune network http://www.biogps.org/#goto=genereport&id=8301/, plays a key role in clathrin-related endocytosis, binding to clathrin heavy chains (CLTC and CLTCL1), and recruiting the clathrin and adaptor protein 2 (AP-2) to the plasma membrane. The AP-2 complex is a heterotetramer consisting of permutations of two large adaptins (alpha (AP2A1, AP2A2)) or beta (AP2B1), a medium adaptin (AP1M1, AP1M2), and a small adaptin (sigma AP2S1). PICALM controls the endocytosis of the cation-independent mannose-6-phosphate IGF2 receptor (IGF2R) [70], one used by Herpes simplex for entry and cell-to-cell transmission [71] and by C. pneumoniae for cellular entry [57]. IGF2R is also a component of late endosomes disrupted by the Helicobacter pylori VacA cytotoxin [72]. The mannose-6-phosphate receptor binds to clusterin. PICALM also binds to a nuclear exportin crm-1 (XPO1) used by the herpes simplex virus during its life cycle [48].
Gamma-adaptins (GGA, GGA2, GGA3) bind to clathrins and mannose-6-phosphate receptors and regulate protein traffic between the Golgi network and the lysosome and the sorting of mannose-6-phosphate receptors (IGF2R and M6PR) at the trans-Golgi network [73]. This network is also related to important Alzheimer's disease susceptibility genes as the interactions culled from NCBI gene show that GGA1 binds to the sortilin-related receptor, SORL1, and the APP cleaving beta-secretase BACE2, while GGA2 binds to the beta-secretases BACE1 and BACE2, SORL1 and the prolyl-isomerase PIN1.
CD2AP, primarily expressed in dendritic cells and B lymphoblasts http://www.biogps.org/#goto=genereport&id=23607/, is a scaffolding molecule that regulates the actin cytoskeleton and is primarily associated with the T-lymphocyte marker protein CD2. CD2 stimulates T cell activation and is involved in the creation of contacts between antigen presenting cells and T cells (the immunological synapse), effects mediated via CD2AP and clathrin [74]. CD2AP is also involved in the entry of the helicobacter vacuolating toxin VacA and connects the actin cytoskeleton to early endosomes containing VacA [75]. CD2 is cleaved by gingipain proteases from P. gingivalis [76].
CD2AP also binds to the actin-bonding protein, cortactin (CTTN), a protein that is exploited by several bacteria (Escherichia coli, Shigella, Neisseria, Rickettsia, Chlamydia, Staphylococcus, Cryptosporidium, and Helicobacter pylori), fungi (Candida Albicans), and viruses (Vaccinia) enabling them to modify the actin cytoskeleton, which they use for transport [77–79]. CD2AP has not been specifically associated with herpes simplex, although the actin cytoskeleton is exploited by this and many other viruses [80].
CD2AP also associated with E-Cadherin, (CDH1) [81]. The ectodomain of E-cadherin is involved in bacterial adherence to mammalian cells [82]. E-Cadherin binds to the H. pylori toxin CagA [83] and is also cleaved by the Helicobacter pylori protein HtRA allowing the pathogen to invade the intracellular compartment [84]. CDH1 and CDH5 expressions are increased by C. pneumoniae infection of human brain microvascular endothelial cells, contributing to vascular permeability changes and atherosclerosis [85].
Bridging integrator 1 (BIN1), also known as amphiphysin 2, is primarily expressed in the pineal and skeletal muscle, or otherwise ubiquitously http://www.biogps.org/#goto=genereport&id=274/. It is also involved in the clathrin-mediated endocytosis machinery [86] and binds to dynamins that regulate the clathrin network [87] including DNM1 and the herpes simplex binding partner DNM2 [88] and to clathrins and the alpha adaptins, AP2A1 and AP2A2 [89]. BIN1 also participates in phagocytosis in macrophages and is associated, but only transiently, with early phagosomes; however, it is retained on vacuoles containing Chlamydia pneumoniae, an effect that reduces the ability of the macrophage system to kill the bacteria via nitric oxide generation. Macrophages expressing a dominant negative BIN1 internalise C. pneumoniae, but do not allow their killing [90]. BIN1 also binds to a number of alpha integrins (ITGA1, ITGA3, and ITGA6) [91]: integrins are used for attachment by many viruses, bacteria, and fungi and may serve as pattern recognition receptors regulating the immune response [92]. Individual integrins bind to many others, forming heteromeric complexes; for example, ITGA1 binds to ITGA3 or ITGA6, while ITGA3 binds to ITGB1 (a receptor for the H. pylori protein CagA [93]), ITGB4, or ITGB5, and ITGA6 binds to ITGB1 and ITGB4 (data from NCBI gene).
3.3. The Immune Network (APOE, BIN1, CD2AP, CD33, MS4A2) (Figure 4)
Figure 4.
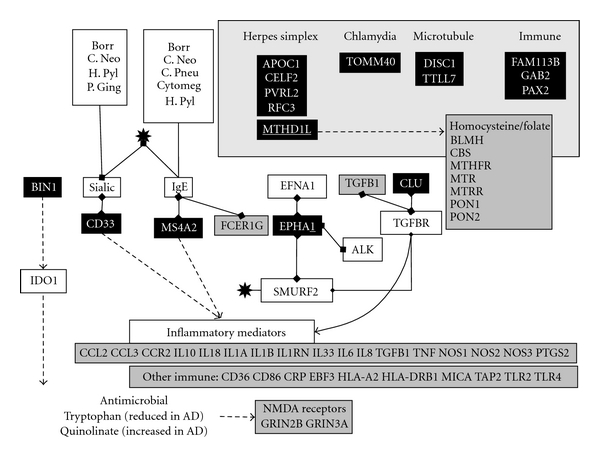
Immune-related genes: Alzheimer's disease susceptibility genes returned from very large genomewide association studies are in black, and those from the pre-GWAS era in grey. Binding interactions are indicated by linked diamonds and other effects by arrows. Borr: Borrelia burgdorferi; C. Pneu: chlamydia pneumoniae; Cytomeg: cytomegalovirus; H. Pyl: Helicobacter pylori; P. Ging: Porphyromonas gingivalis; IgE: immunoglobulin E, Sialic: alpha2-3- or alpha2-6-linked sialic acids: the genes in the top right square were returned from GWAS prior to the very large studies. black star: Herpes simplex: see text for details.
CD33, mainly expressed in myeloid cells, monocytes, and dendritic cells (http://www.biogps.org/#goto=genereport&id=945/), is a member of the sialic acid binding Immunoglobulin g-like lectin (SIGLEC) family. CD33-related SIGLECs regulate adaptive immune responses and are also important as macrophage pattern recognition receptors for sialylated pathogens, including enveloped viruses [94]. CD33 binds to alpha2-3- or alpha2-6-linked sialic acids (N-acetyl neuraminic acid) [95]. These particular sialic acids are expressed on the surface envelope glycoproteins (B, D, and H) of the herpes simplex virion, and these residues are required for viral entry into cells [96]. N-acetyl neuraminic acid is expressed by C. neoformans, is involved in fungal adhesion to macrophages [97], and is also a component of the cell wall of B. burgdorferi [98], while Helicobacter pylori adhesins also bind to this particular form of sialic acid [99, 100] as does P. gingivalis [101].
BIN1, as well as its relationship to the clathrin mediated endocytosis machinery, also regulates the expression of indoleamine 2,3-dioxygenase (IDO1), an enzyme that catalyzes the first rate-limiting step in tryptophan metabolism to N-formyl-kynurenine [102]. IDO1 upregulation is an important defence mechanism against pathogenic bacteria, many of which are unable to synthesise tryptophan. Their survival is compromised by the diversion of tryptophan metabolism to kynurenines [103]. This IDO1 response is also deleterious to other pathogens and parasites, including T. gondii, and to a number of viruses, including herpes simplex and other herpes viruses [104]. IDO1 protein expression is localised to plaques and tangles in the Alzheimer's disease brain. IDO1 activation can lead to the production of toxic tryptophan derivatives such as 3-hydroxyanthranilic acid or the N-methyl-D aspartate receptor agonist and excitatory neurotoxin, quinolinic acid [105] (GRIN2B, GRIN3A). Plasma tryptophan levels are also lower in the ageing population and in Alzheimer's disease, a pattern accompanied by immune activation, and by increased concentrations of quinolinic acid [106, 107].
MS4A2, expressed mainly in the tonsils, lymph nodes, B cells, and dendritic cells http://www.biogps.org/#goto=genereport&id=931/, is a component of the immunoglobulin E (IgE) receptor, which is involved in allergic responses in which allergens bound to receptor bound IgE result in the activation of allergic mediators such as histamine [108]. Mice immunised with inactivated herpes simplex develop IgE-specific antibodies to the virus [109]. High levels of IgE are also observed in man following recurrent herpes simplex infection [110] and human IgE antibodies are also known to interact with herpes family viruses including HSV-1 and 2 and the Epstein-Barr and cytomegalovirus [111] and also to C. pneumoniae, H. pylori, and B. burgdorferi [112–115]. IgE-related allergic responses are also involved in C. neoformans infection [116]. Other members of this gene cluster (including MS4A4A, MS4A4E, and MS4A6A) are also structurally related to the immunoglobulin E receptor and to CD20 (MS4A1) and also regulate B cell and T cell proliferation and/or differentiation [117, 118].
EPHA1 is an ephrin receptor, primarily expressed in the liver and otherwise ubiquitously (http://www.biogps.org/#goto=genereport&id=2041/). Only three protein/protein interactions for EPHA1 are reported in the NCBI gene interaction section, including its ligand EFNA1, the anaplastic lymphoma receptor tyrosine kinase (ALK), and a SMAD-specific E3 ubiquitin protein ligase 2 (SMURF2). EFNA1 is one of several proteins identified as being important in the entry of C. pneumoniae into human coronary artery endothelial cells [57]. SMURF2 is known to bind to the VP22 tegument protein of herpes simplex [119] and plays a role in clathrin-mediated endocytosis and the subsequent ubiquitin-related proteasomal degradation of TGF beta receptors, to which it binds [120]. Clusterin is a ligand for TGF beta receptors (TGFBR1/TGFBR2) [121]. TGF beta signalling exerts immunosuppressive effects and inhibits host immunosurveillance and the recruitment of immunocompetent cells by chemokines [122]. ALK is ubiquitously expressed (http://www.biogps.org/#goto=genereport&id=238/). It plays a role in neural development, and its expression decreases with age [123]. ALK is best characterised via its relationship with lymphomas, caused by ALK gene fusion with any of several other housekeeping genes [124]. Its key involvement in lymphoma suggests a role in the immune network although the function of the normal ALK protein is poorly understood.
3.4. Lipoprotein Related (APOE, ABCA7, CLU) (Figures 2 and 5)
Figure 5.
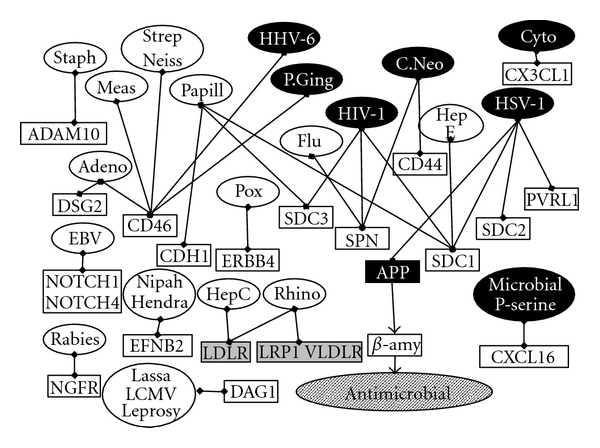
Gamma-secretase-mediated cleavage of viral and pathogen receptors. Gamma secretase substrates are indicated by the square boxes and their pathogen ligands in the oval boxes. Cyto: cytomegalovirus; C. Neo: Cryptococcus neoformans; Adeno: adenovirus; EBV: Epstein-Barr virus; Flu-influenza A.; HepC: hepatitis C; HepE: hepatitis E; HHV-6: human herpesvirus 6; HIV-1: human immunodeficiency virus; HSV-1: herpes simplex; Lassa: Lassa fever virus; LCMV: Lymphocytic choriomeningitis virus; Leprosy: mycobacterium leprae; Meas: measles; Neiss: Neisseria; Papill: papillomavirus; P. Ging: Porphyromonas gingivalis; Pox: Vaccinia and other pox viruses, Rhino: rhinoviruses; Staph: Staphylococcus aureus,Strep: streptococcus; P serine: phosphatidylserine: Alzheimer's disease susceptibility genes returned from very large genomewide association studies, and APP, are in black, as are the pathogens implicated in Alzheimer's disease Genes from the pre-GWAS era are in grey.
ABCA7 is an ATP-binding cassette transporter, predominantly localised in the pineal gland and cells of the immune network (T cells, natural killer cells, and dendritic cells http://www.biogps.org/#goto=genereport&id=10347/). The lipoproteins APOA1 and APOE are substrates for ABCA7, and in cultured HEK-293 cells, plasma membrane-situated ABCA7 increases the efflux of phosphatidylcholine and sphingomyelin efflux to APOA1 and APOE, with no effect on cholesterol efflux [125]. However, cholesterol efflux to lipid-laden APOE, but not to lipid free APOE, is increased by ABCA7 expression in HEK-293 cells [126]. Sphingomyelin is enriched in extracellular herpes simplex viral membranes: this sphingomyelin, together with phosphatidylserine, is collected by the viral envelope during viral passage from the nuclear membrane to the exocytosis pathway [127]. Herpes viral infection leads to an increased incorporation of phosphate into membrane sphingomyelin of the host [128]. Inhibition of sphingomyelinase has also been shown to markedly reduce herpes simplex viral reproduction [129] and also inhibits the antifungal effects of neutrophils against C. neoformans infection. Sphingomyelin is a receptor for the Helicobacter toxin VacA [130] and is also incorporated into inclusion bodies in C. pneumoniae-infected cells [131]. Phosphatidylcholine plays an important role in the fusion of herpes simplex glycoproteins B and H with the host cell lipid membrane, a process used in viral entry [132]. Phosphatidylcholine is also able to trigger capsular enlargement in C. neoformans infection [133].
ABCA7 expression increases the extracellular surface deposition of ceramide (derived from sphingomyelin) [134]. Ceramide, a potent activator of apoptosis, as well as its downstream target, caspase 3 (CASP3) are both able to reactivate the herpes simplex virus from latency [135]. Ceramide is also incorporated into C. pneumoniae inclusions, an effect that may play a role in the antiapoptotic effects of this bacterium [136]. APOA1 exerts antiviral effects against herpes simplex and inhibits viral entry into cells as well as viral-induced cell fusion and intercellular spread [137]. In macrophages, ABCA7 is expressed intracellularly and does not participate in cholesterol or phospholipid efflux, instead playing a role in the phagocytosis of apoptotic cells, an important general defence mechanism against invading pathogens [62, 138].
3.4.1. Apolipoprotein E
Possession of the APOE4 allele facilitates the entry and transmission of herpes simplex in mice models [139]. In man, APOE is also involved in hepatitis C, HIV-1, and herpes simplex infectivity [140–143], and APOE4 facilitates the binding of C. pneumoniae elementary bodies to host cells [144].
APOE mRNA is primarily expressed in the liver, adipocytes; kidney and brain, with very low expression in the peripheral immune network (http://www.biogps.org/#goto=genereport&id=348/) but nevertheless plays an important role in the immune system. For example, the presence of the APOE4 allele is associated with an enhanced macrophage inflammatory response, and cytokine responses to the intracerebral injection of lipopolysaccharide are increased in APOE4 transgenic mice, which also exhibit increased microglial activation. The anti-inflammatory effects of 17-beta-oeastradiol on microglia are also reduced in such animals [145, 146]. C-reactive protein (CRP) levels are also decreased in APOE4 carriers [145–147]. CRP is an acute phase protein that binds to phosphocholine on dead or dying cells and on bacteria, subsequently activating the complement pathway [148]. Resistance to infection (Klebsiella pneumoniae) or endotoxaemia is also decreased in APOE knockout mice [149].
In addition, atherosclerosis is induced or worsened by infection with a number of relevant pathogens (Cytomegalovirus, herpes simplex, Helicobacter pylori, influenza, C. pneumoniae or P. gingivalis) in APOE knockout mice [150–156]. Helicobacter pylori is able to promote atherosclerosis in heterozygous APOE (+/−) LDLR (+/−) mice, which is associated with an immune response to the bacterial heat shock protein hsp60 [157].
3.5. Other GWAS Genes (Figure 4)
Prior to the very large GWAS collaboration, several other genes had been identified in smaller genomewide studies (APOC1, CELF2, DISC1, FAM113B, GAB2, MTHFD1L, PAX2, PCDH11X, PVRL2 RFC3, SASH1, TOMM40, TTLL7, and ZNF224). PVRL2 is a receptor for herpes simplex (HSV-1 and HSV-2) [158], and the mitochondrial translocator, TOMM40, a receptor for certain chlamydial species [159]. The replication factor RFC3 is part of a complex necessary for human DNA polymerase activity, a process exploited by many viruses including herpes simplex, whose virion component ICP34.5 binds to proliferating cell nuclear antigen (PCNA), an RFC3 binding partner and also a cofactor for DNA polymerase [160]. ZNF224 is a transcriptional repressor binding to the protein arginine methyltransferase, PRMT5 [161]. Protein arginine methylation is important in viral infection and replication, as well as in cytokine signalling, and a related arginine methyltransferase, PRMT1, regulates herpes simplex replication via methylation of the ICP27 viral gene [162].
DISC1 is a component of the microtubule-associated dynein motor complex used in viral traffic [163]; TTLL7 (tubulin tyrosine ligase-like family, member 7) also regulates tubulin phosphorylation [164] and can again be related to viral traffic along the microtubule network (see below). CELF2 (also known as CUGBP2) is a member of the APOBEC1 cytidine deaminase mRNA editing complex that also controls herpes simplex viral replication [165]. GAB2 is a member of the GRB2-associated binding protein family which act as adapter hubs transmitting signalling via cytokine and growth factor receptors, and T- and B-cell antigen receptors (definition from NCBI gene), while PAX2 inhibits the expression of the antimicrobial peptide beta defensin (DEFB1) [166], a gene associated with HSV-1 and cytomegalovirus seropositivity in children with acute lymphoblastic leukaemia [167], as well as with H. pylori or chlamydial infections [168, 169], also endowed with antimicrobial activity against C. neoformans and other pathogens [170]. MTHD1L (methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 1-like) is involved in the mitochondrial synthesis of tetrahydrofolate which in turn is important in the de novo synthesis of purines and thymidylate and in the regeneration of methionine from homocysteine (definition from NCBI gene). Many pathogens, including herpes simplex, express thymidylate kinases, which are important for viral replication and a target for acyclovir [171]. Hyperhomocysteinaemia correlates with C. pneumoniae IgG immunoreactivity in carotid artery atherosclerosis [172] and is also associated with H. pylori infection in the context of atherosclerosis [173]. The apolipoprotein APOC1 is a component of high-density lipoprotein: herpes simplex is present in all lipoprotein blood fractions in blood (VLDL, LDL and HDL) and the lipid component of these lipoproteins binds to viral glycoprotein B [174] (c.f. APOA1, APOA4, APOA5, APOC1, APOC2, APOC3, APOC4, APOD, and APOE). No immediately apparent pathogen-relevant interactions were found for FAM113B (expressed exclusively in T cells, dendritic cells, and natural killer cells http://www.biogps.org/#goto=genereport&id=91523/), PCDH11X (which is ubiquitously expressed http://www.biogps.org/#goto=genereport&id=27328/), or SASH1 (primarily expressed in the brain and lung although also in other tissues, including the immune network http://www.biogps.org/#goto=genereport&id=23328/) although the pathogen/immune theme is clearly carried through, particularly in relation to herpes simplex, in this second rank of Alzheimer's disease susceptibility genes.
3.6. Beta Amyloid Processing (Figure 2)
APOE, clusterin, and complement receptor 1 play key roles in beta amyloid clearance as do two further herpes simplex binding proteins APOA1, and alpha-2 macroglobulin (A2M). This is primarily mediated via lipoprotein receptors. A2M, or APOE-bound Aβ, is cleared by the lipoprotein receptor LRP1, while LRP2 (megalin) clears clusterin-bound Aβ. LRP8 is a receptor for both APOE and clusterin. APOA1 is also involved in beta-amyloid clearance via its transporter ABCA1. The role of ABCA7 has not been examined, although APOA1 is also a ligand for this transporter (see above). The Varicella Zoster and herpes simplex glycoprotein E binding protein, insulin-degrading enzyme, are also involved in beta-amyloid degradation, as is caspase-3 which is activated by the herpes simplex viral US3 kinase. The HSV-1 binding protein, complement C3 is also a ligand for LRP1 and LRP8, both of which play a role in C3 cellular uptake. Beta amyloid in the bloodstream is processed by its binding to complement C3, which subsequently binds to complement receptor 1 on erythrocytes. The effects above are referenced in a recent review [48].
Clathrin-dependent endocytosis is also involved in the internalisation and recycling of neuronal APP, a procedure necessary for the subsequent cleavage of APP and the generation of beta-amyloid [175, 176], and in the neuronal [177], but not the microglial uptake of both soluble and aggregated beta-amyloids, the latter representing an important rout of disposal [178]. However, while knockdown of the clathrin assembly protein AP180 in a neuronal cell line does reduce beta-amyloid generation, PICALM knockdown does not [179]. The accumulation of beta-amyloid in the brain interstitial space, related to the prior endocytosis of APP, is clathrin dependent [180]. Clathrin-mediated endocytosis is relevant to many receptors, including members of the lipoprotein family (LRP1, LRP2, LRP8, LDLR, VLDLR) [181], all of which are involved in beta-amyloid clearance, as well as in cholesterol and lipoprotein physiology [182].
ABCA7 plays a role in beta-amyloid secretion, which is increased in Chinese hamster ovary cells expressing APP and ABCA7. This was related to an effect on APP intracellular retention, rather than on secretase-mediated proteolysis of APP [126].
Gamma-secretase cleaves APP, and other gamma-secretase substrates also play key roles in APP processing (ADAM10) [183], lipid and cholesterol function (LRP1, LDLR, VLDLR), and other processes relevant to Alzheimer's disease, for example, NOTCH signalling [184].
No other immediately apparent relationships with beta-amyloid could be found by literature survey for CD2AP, CD33, and EPHA1 or for MS4A-related proteins, although such are not precluded.
3.6.1. The Microtubule Network and Tau Phosphorylation
Many pathogens, including herpes simplex, helicobacter, chlamydiae, P. gingivalis, and C. neoformans [185–188], use the microtubule network that serves as a useful railway track between various cellular compartments, and may hijack dynein and kinesin motors for this purpose (see http://www.polygenicpathways.co.uk/herpeshost.html for herpes simplex). Tau (MAPT) stabilises microtubules by interacting with tubulins and promoting microtubule assembly [189]. When tau is phosphorylated, by any of several kinases, microtubules become disorganised. Tau hyperphosphorylation and neurofibrillary tangles are among the core pathologies of Alzheimer's disease [190] and can be promoted by herpes simplex infection [191]. In relation to viral/human protein homology, herpes simplex proteins are homologous to a number of kinases known to phosphorylate tau (GSK3A, GSK3B, MAPK1, and CAMK2B) suggesting that tau phosphorylation could be a direct result of a viral kinase [192].
3.6.2. APP and Gamma Secretase
APP plays a key role in the herpes simplex life cycle and is involved in its intracellular transport [193], an effect likely related to the ability of both APP and the herpes simplex protein, US11, to bind to the APP and kinesin binding protein APPBP2 (also known as pat1) [194, 195].
The AntiMicrobial Effects of Beta-Amyloid —
Beta-amyloid is an antimicrobial peptide with broad spectrum activity against a variety of yeasts and bacteria, effects that were attenuated by anti-Aβ antibodies [196], Beta-amyloid also has antiviral effects and, like acyclovir, attenuates the stimulatory effects of herpes simplex on miRNA-146a levels in neuronal cells [197]. Beta-amyloid also activates innate immune responses via the activation of pattern recognition receptors, such as Toll receptors (TLR2, TLR4), which are also involved in beta-amyloid clearance [198, 199]. The antimicrobial, antiviral, and immunostimulant properties of beta amyloid are, however, likely to be abrogated by the presence of beta-amyloid autoantibodies in the sera of the ageing population and in Alzheimer's disease [200]. As immunogenic regions of beta-amyloid are homologous to similar regions within proteins expressed by all of the principal pathogens discussed in this paper, such antibodies are likely to be derived from antibodies raised to numerous pathogens (see below).
Gamma Secretase: Localisation to Dendritic Cells and Cleavage of Pathogen Receptors —
Gamma secretase is constituted of four components: the presenilins (PSEN1 or PSEN2), anterior pharynx-defective-1 (APH1A), the Presenilin enhancer-2 (PSENEN), and nicastrin (NCSTN) [201]. While all components are expressed in cerebral tissue, the major focus of distribution is within cells of the immune network; dendritic cells, myeloid cells, and monocytes for PSEN1 http://www.biogps.org/#goto=genereport&id=5663/; dendritic cells and natural killer cells for PSENEN http://www.biogps.org/#goto=genereport&id=55851/, dendritic cells and myeloid cells for nicastrin http://www.biogps.org/#goto=genereport&id=23385/ and B cells, dendritic cells, natural killer cells, and myeloid cells for APH1A. The substrate, APP, is the only gene in this set that appears to be preferentially distributed in brain compartments, but, as with gamma-secretase components, it is also highly expressed in dendritic cells of the immune system (http://www.biogps.org/#goto=genereport&id=351/). The primary function of such cells is to process antigens and present them to B cells and T cells. They scout for and recognise pathogens via the agency of numerous pattern recognition receptors, for example, Toll receptors (TLR2, TLR4), or viral DNA sensors, expressed on their surface [202, 203].
As well as cleaving APP, gamma secretase is involved in the proteolysis of at least three herpes simplex receptors, nectin 1 alpha (PVRL1) [204], and syndecans (SDC1, SDC2) [205]. SDC1 is also a receptor for HIV-1, Hepatitis E, and the human papillomavirus [206–209], while SDC3, also a gamma secretase substrate, is an HIV-1 and papillomavirus receptor [210, 211].
Several other gamma secretase substrates (reviewed by Lleó and Saura [201]) also function as viral/pathogen receptors, including ADAM10, a receptor for the cytotoxin Staphylococcus aureus alpha-haemolysin [212], CD44, an entry receptor for C. neoformans [213], CD46, a receptor for adenoviruses, measles virus, human herpes virus 6 (HHV-6), Streptococci, and Neisseria [214]: CD46 is also cleaved by a protease secreted by P. gingivalis [215]. Desmoglein-2 is an adenovirus receptor [216], while rhinovirus receptors include the lipoprotein receptors LRP1, LDLR, and VLDLR [217, 218]. LDLR is also a hepatitis C receptor [219]. NOTCH1 and NOTCH4 are activated by the Epstein-Barr virus [220, 221], while ERBB4 is a receptor for vaccinia and other pox viruses [222]. The low affinity nerve growth factor (NGFR) is a rabies virus receptor, [223], Ephrin B2, (EFNB2) a Nipah virus and Hendra virus receptor [224] and sialophorin (SPN), a receptor for the influenza A, and both human and simian immunodeficiency viruses [225, 226] and for the C. neoformans virulence factor, galactoxylomannan [227]. Fractalkine (CX3CL1) binds to the cytomegalovirus chemokine receptor, US28 [228], while the chemokine CXCL16 is a scavenger receptor for phosphatidylserine and oxidized low density lipoprotein [229]: phosphatidylserine is the major lipid membrane component in most bacteria [230]. Dystroglycan is a receptor for the Lymphocytic choriomeningitis and Lassa fever viruses [231, 232] and also for mycobacterium leprae (the Leprosy pathogen) [233].
The antimicrobial and immunostimulant effects of beta-amyloid, the cleavage of a number of pathogen receptors by gamma secretase, and the concentration of both APP and gamma-secretase components in dendritic cells suggest that a major function of this key group, implicated in both familial and late onset Alzheimer's disease, is dedicated to pathogen defence, and that increased beta-amyloid generation is primarily a defence mechanism to rid the body (and brain) of invading pathogens: This scenario is supported by the ability of herpes simplex, C. pneumoniae, and B. burgdorferi to increase beta-amyloid deposition [234–236]. One might expect many other pathogens to increase beta-amyloid deposition, and that, as has been noted in atherosclerosis, (a component of Alzheimer's disease pathology), the final extent of risk may depend upon the overall pathogen burden, rather than upon any specific pathogen [237].
3.6.3. Autoantibodies Derived from Pathogens as Contributory Causative Agents
Viruses and bacteria express proteins containing short contiguous amino acid stretches (pentapeptides or more, or longer gapped consensi) that are identical to those in human proteins: these pathogen/human consensi number in millions and concern all human proteins [238–241].
Autoantibodies, which are observed in many, if not most human diseases, are often regarded as an epiphenomenon of little consequence. However, they can traverse the blood brain barrier [242] (which is compromised in Alzheimer's disease [243]) and are also able to enter cells, essentially by hitching a ride on viruses, via high affinity IgG receptors (Fc gamma receptors) (FCER1G) in the case of the rhinovirus, or the SARS coronavirus, or via the tripartite motif protein, TRIM21, in the case of adenoviruses, where they are able to activate an intracellular immune attack. It would appear that the cellular entry of antibody laden viruses is diverted from their usually preferred receptors towards those used by antibodies [244–246]. This may be relevant to the MS4A family. Fc gamma receptors are localised in microglia and astrocytes in the brain and their expression is upregulated by blood brain barrier disruption [247], while TRIM21 appears to be exclusively localised in peripheral immunocompetent cells (http://www.biogps.org/#goto=genereport&id=6737/).
This ability places autoantibodies in a rather more sinister context, as their targeting of extracellular and intracellular human proteins would be expected to effect protein knockdown, a strictly immunopharmacological effect, as well as immune attack.
In multiple sclerosis, schizophrenia, and cystic fibrosis, as well as in Alzheimer's disease, numerous autoantigens targeted by the autoantibodies reported in these conditions, contain peptide sequences identical to those in the pathogens also implicated in the disease. Such regions of homology are focalised within epitope regions of the human autoantigen [192, 248–250]. In Parkinson's disease, antibodies to the Epstein-Barr virus, which has been implicated in postencephalitic adult and juvenile Parkinsonism, are also known to cross-react with synuclein, a key protein involved in neurodegeneration in this disorder [251–253]. In addition, 22 autoantigens reported in HIV-1/AIDS contain HIV-1/human matching sequences [254], supporting the contention that autoantibodies are in many cases antibodies initially raised to pathogens, which because of this homology, then target their human homologues. It has been argued that slightly dissimilar, rather than exact matches, are the more malignant in terms of autoimmunity, being less likely to be regarded as self, while the antibodies would retain low affinity for human counterparts [254, 255].
Autoantibody production would also be sustained, even after pathogen elimination, by continued encounter of the human homologue. The production of autoantibodies must be dependent upon the extent of pathogen/human matching, and thus by genes which determine human protein sequences. These pathogen/human matches are also highly and significantly enriched in the products of susceptibility genes implicated in Alzheimer's disease, multiple sclerosis, and schizophrenia [192, 248, 250]. Many genes related to Alzheimer's disease, including those described above, are involved in the immune network [10, 256], and the propensity for developing autoantibodies to particular proteins is also genetically determined and inherited [257]. Thus, despite the fact that all human proteins likely possess pathogen homologues, whether or not autoantibodies will be produced will depend on the extent of human/pathogen matching (determined by human genes and the strain of pathogen encountered), on whether the pathogen protein is deemed as self or nonself (a factor determined soon after birth) and on other genetic factors related to the immune network, and autoimmunity. Somatic hypermutation, that drives the creation of multiple antibodies and which selects against those reacting to self, is disrupted in autoimmune disorders [258]. These links suggest an interplay, applicable to many diseases, where susceptibility gene products, risk promoting pathogens and autoimmunity can all be related via protein sequence homology.
It has also been noted that autoantigens have a tendency to relate to proteins known to bind to dermatan sulphate, a component of dead cells [259] and a constituent of glycosaminoglycan receptors for many bacteria and viruses [260].
3.6.4. Sequence Comparisons: Beta-Amyloid, NGF, and Tau versus Pathogen Proteins
All three of these proteins are autoantigens in Alzheimer's disease and were chosen for analysis because of the ability of their antibodies to promote features of Alzheimer's disease, in vivo. In mice, immunisation with neuronal tau produces neurofibrillary tangle-like structures, axonal damage, and gliosis, as in Alzheimer's disease [261]. In addition, in transgenic mice, initially expressing NGF antibodies only in lymphocytes, NGF antibodies subsequently enter the brain provoking extensive cortical degeneration, cholinergic neuronal loss, tau hyperphosphorylation, and beta-amyloid deposition [262]. Beta-amyloid autoantibodies are also able to promote meningoencephalitis, both in laboratory models and in clinical trials [263, 264]. Beta-amyloid plaques contain numerous inflammatory proteins, and even without meningoencephalitis, these are commonly found within the walls of meningeal and medium-sized cortical arteries in Alzheimer's disease [265].
Almost the entire length of the tau protein (638/776 amino acids = 82.2%) was predicted as immunogenic (B cell epitope), as defined by the server set cutoff index of 0.35. For the analysis in Table 1, only regions of the tau protein with an immunogenicity index >2.5 were examined for homology. For other proteins (NGF and beta-amyloid), the analysis concerned immunogenic regions above the cutoff value of 0.35.
Table 1.
Viral, bacterial, and fungal protein homology with beta-amyloid (top), NGF (middle), or tau (bottom); Predicted B cell epitope segments were compared with Borrelia burgdorferi, C. neoformans, C. pneumoniae, H. pylori, P. gingivalis and herpes viruses (HSV1, HHV6, and cytomegalovirus) proteomes by BLAST analysis. The B cell antigenicity indices are shown, and those above the server set threshold of 0.35 are in italics. The first column shows the amino acid number within the peptide or protein sequence, and the second shows the amino acid pertaining to that position. The alignments with pathogen proteins are shown. Spaces represent nonidentical amino acids and + signs amino acids with similar physicochemical properties. Only highly antigenic regions of pentapeptides or more were processed. The VGGVV sequence, antibodies to which label beta-amyloid in brain tissue, despite relatively low antigenicity, has already been reported to be identical to proteins expressed by 69 viruses including HSV-1, HSV-2, and HHV6.
(a)
| Position B-Amy | Amino acid | B-cell index | Alignments |
|---|---|---|---|
| 1 | D | 0.41 | C. neoformans +AE HDSG+ Borrelia burgdorferi DAE F H+SG EV H. pylori DA FRH HSV-1 +AE RH HHV-6 D FR DS P. gingivalis +AEFR C. pneumoniae DA EFRHD and +AEFR +SG |
| 2 | A | 0.35 | C. neoformans AEFR D GY+V H. pylori AEF D S YE and AE+RH+ Borrelia burgdorferi AEF H+ Cytomagalovirus AEFR HD HSV-1 AE R SG HHV-6 AE+ HD P. gingivalis A+F H+S and AEFR C. pneumoniae AEF DSG |
| 3 | E | 0.62 | H. pylori EFRHD HHV-6 EF DSG Borrelia Burgdorferi EFR DS C. neoformans EF R DS YE P. gingivalis E R DSGY V C. pneumoniae EF SGYEV |
| 4 | F | 0.73 | C. neoformans FRHDS Borrelia burgdorferi +RH SGY++ and F H+SG H. pylori F HD EV Cytomegalovirus FR SGY P. gingivalis +RHDS C. Pneumonia F H+SGY |
| 5 | R | 0.85 | C. neoformans R D GYEV H. pylori RHDS Y V and R SGYE Borrelia burgdorferi RH+ GY Cytomegalovirus RHD YE and RHDSG HSV-1 RH SG HHV-6 RHDS P. gingivalis R+DS Y+ |
| 6 | H | 0.57 | C. neoformans HDSGY H. pylori H. pylori +DSGY and HD G EV and HD EV Borrelia burgdorferi H+SG Y+V HSV-1 HDSG P. gingivalis HDSG C. pneumoniae ++SGY+V |
| 7 | D | 0.69 | C. neoformans DSGY+V H. pylori +SG+EV HSV-1 DSGY P. gingivalis DSG+EV C. pneumoniae DSGY V |
| 8 | S | 0.38 | P. gingivalis SGYEV H. pylori SGYE C. neoformans SGY++ C. pneumoniae SG+EV |
| 9 | G | 0.63 | H. pylori GYEVH Borrelia burgdorferi GYE V KL+ C. neoformans GYE LV and GY++ + LV P. gingivalis GYEV C. pneumoniae GYEV and GY HH |
| 10 | Y | 0.56 | H. pylori YE HH and YE+ HQ and Y++H Q and YE HHQ Cytomegalovirus YEVH Borrelia Burgdorferi YE+ KL C. neoformans YE + QK FC P. gingivalis Y++H H+K C. pneumoniae Y+V +Q LV |
| 11 | E | 0.58 | H. pylori EV +QK Cytomegalovirus EV HQ L Borrelia Burgdorferi EV +KL C. neoformans EV Q LV P. gingivalis EV KLV C. pneumoniae EV QKLV |
| 12 | V | 0.35 | H. pylori. +H QK Cytomegalovirus V HQ LV HHV-6 VH QK+V Borrelia Burgdorferi VH KL C. neoformans +HH LV P. gingivalis VH + LV C. pneumoniae V HQKL |
| 13 | H | −0.17 | H. pylori and C. pneumoniae HHQK Cytomegalovirus HH KL P. gingivalis HH KL |
| 14 | H | −0.66 | Borreli Burgdorferi HQKL+ C. pneumoniae and HSV-1 HQKL P. gingivalis +QKLV |
| 15 | Q | −1.03 | C. neoformans and P. gingivalis and C. pneumoniae QKLV |
| 16 | K | −1.47 | H. pylori: Cryptococcus neoformans Borrelia burgdorferi Chlamydophila pneumoniae KLVFF Human herpesvirus 1 KLVF |
| 17 | L | −1.34 | Human herpesvirus 5: Human herpesvirus 6 LVFF |
| 18 | V | −1.20 | |
| 19 | F | −0.93 | |
| 20 | F | −0.98 | |
| 21 | A | −0.82 | |
| 22 | E | −0.31 | |
| 23 | D | 0.23 | |
| 24 | V | 0.81 | Borrelia burgdorferi Cryptococcus neoformans Porphyromonas gingivalis VGSNK Cytomegalovirus +GSNK Helicobacter pylori Chlamydophila pneumoniae VGSN |
| 25 | G | 1.24 | H. pylori Chlamydophila pneumoniae GSNK |
| 26 | S | 1.22 | |
| 27 | N | 0.90 | |
| 28 | K | 0.36 | |
| 29 | G | 0.30 | |
| 30 | A | −0.24 | |
| 31 | I | −0.58 | |
| 32 | I | −1.00 | |
| 33 | G | −1.14 | |
| 34 | L | −1.19 | |
| 35 | M | −1.23 | |
| 36 | V | −1.16 | 69 viruses/phages VGGVV |
| 37 | G | −0.97 | |
| 38 | G | −1.02 | |
| 39 | V | −0.63 | |
| 40 | V | −0.45 | |
| 41 | I | −0.80 | |
| 42 | A | −1.06 |
(b)
| NGF position | Amino acid | B-cell index | Alignment |
|---|---|---|---|
| 18 | A | 0.59 | C. neoformans AEPHS |
| 19 | E | 1.16 | P. gingivalis EPHSES—NVP Cytomegalovirus EPHS+S |
| 20 | P | 1.32 | C. neoformans P+S NVPAG and PHSES and P SESNV |
| 21 | H | 1.78 | Borrelia burgdorferi HSESN C. neoformans HSES VP and HSESN P H +P+ |
| 22 | S | 1.64 | C. neoformans S+S VPAG T P Borrelia burgdorferi burgdorferi C. neoformans Chlamydophila pneumoniae P. gingivalis SESNV P. gingivalis S+SNVP C. pneumoniae SESNV A |
| 23 | E | 1.68 | C. neoformans ESNVP and ESNV AG |
| 24 | S | 1.51 | C. neoformans SNVPA |
| 25 | N | 1.61 | C. neoformans Cytomegalovirus P. gingivalis NVPAG C. neoformans NV TIPQA P. gingivalis +VPAG HT |
| 26 | V | 1.45 | C. neoformans P. gingivalis VPAGH C. neoformans VP AGHT C. neoformans VPAG TI and V AGHT+ |
| 27 | P | 1.36 | C. neoformans HSV-1 PAGHT C. neoformans PAGHT P C. neoformans PAG TIP |
| 28 | A | 1.04 | C. neoformans H. pylori AGHTI C. neoformans AG H IPQA and AGHT PQ and AGHT+P and AG TIP HSV-1 HSV-2 AGH PQ and AGHT QA |
| 29 | G | 1.00 | C. neoformans GHTI Q and GHT PQ and G TIPQ |
| 30 | H | 0.93 | C. pneumoniae HTI QA C. neoformans HT PQA and HTIP A |
| 31 | T | 1.05 | C. neoformans P. gingivalis TIPQA |
| 32 | I | 0.95 | |
| 33 | P | 0.64 | |
| 34 | Q | 0.41 | |
| 35 | A | 0.46 | |
| 51 | A | 0.38 | C. neoformans AR SAPA and AR APAA and A SAPAA and ARSA AA and ARSAP A and ARS PAA and AR—SAPAA |
| 52 | R | 0.54 | HSV-1 RSAPAA Borrelia burgdorferi burgdorferri RSA AA |
| 53 | S | 0.91 | C. neoformans C. pneumoniae Cytomegalovirus HSV-1 HSV-2 P. gingivalis SAPAA |
| 54 | A | 0.89 | |
| 55 | P | 0.72 | |
| 56 | A | 0.69 | |
| 57 | A | 0.53 | |
| 64 | A | 0.46 | C. neoformans AG TRNI and AGQT RN and AGQTR P. gingivalis AGQTR |
| 65 | G | 0.59 | C. neoformans + RNITV and GQTRN |
| 66 | Q | 0.42 | P. gingivalis Borrelia burgdorferi QTRNI Cytomegalovirus QTRN—IT |
| 67 | T | 0.43 | C. neoformans P. gingivalis TRNIT |
| 68 | R | 0.49 | C. neoformans RNIT DP and RNITV |
| 69 | N | 0.76 | C. neoformans C. pneumoniae H. pylori NITVD |
| 70 | I | 0.66 | |
| 71 | T | 0.56 | |
| 90 | S | 0.40 | C. neoformans STQPPR and STQPP AA and STQPP EA C. pneumoniae STQ PRE C. pneumoniae Cytomegalovirus STQPP HSV-1 STQ PR |
| 91 | T | 0.63 | C. neoformans TQP REA and TQPPR |
| 92 | Q | 1.06 | C. neoformans QPPRE |
| 93 | P | 1.49 | C. neoformans PP REAA C Neoformans P. gingivalis PPREA |
| 94 | P | 1.91 | C. neoformans HSV-2 H. pylori PREAA |
| 95 | R | 2.13 | C. neoformans P. gingivalis REAAD and REAA TQ and RE ADT C. neoformans REAA DT and R AADT and R+AADT and READD HSV-1 REAA T Borrelia burgdorferi burgdorferi Cytomagalovirus RE ADT Cytomagalovirus REAA TQ P. gingivalis REA +TQ |
| 96 | E | 2.13 | P. gingivalis EAADTQ and EA TQDLD C. neoformans EAADT+ and EAAD QD and EAAD Q and EAA TQ |
| 97 | A | 1.92 | Borrelia burgdorferi burgdorferi C. neoformans AA TQD Borrelia burgdorferi burgdorferi P. gingivalis AAD QD HSV-2 AADT D HHV-6 AAD +DL C. neoformans AADT D and AA TQD and A DTQD and A TQDL and AADTQ and AADT+ D+D P. gingivalis ADTQ L H. pylori AADTQ |
| 98 | A | 1.9 | Borrelia burgdorferi ADT DLD C. neoformans AD QDLD and AD QDL P. gingivalis ADT DL and AD QDL H. pylori +TQDLD |
| 99 | D | 1.39 | C. neoformans DT DLD and D QDLD C. pneumoniae DTQDL |
| 100 | T | 1.25 | C. neoformans H. pylori P. gingivalis TQDLD |
| 101 | Q | 0.66 | Borrelia burgdorferi burgdorferi C. neoformans H. pylori P. gingivalis QDLDF H. pylori QD DFEV |
| 102 | D | 0.73 | Borrelia burgdorferi burgdorferi C. neoformans H. pylori DLDFE C. neoformans DLD EVG and DLDF VG |
| 103 | L | 0.54 | C. neoformans LD EVGG and LDFE GG and LDFEV and LDF VGG H. pylori LDF EVGG HSV-1 HSV-2 L+ EVGG |
| 104 | D | 0.45 | Borrelia burgdorferi burgdorferi DFEVG C. neoformans DF VGGA and D EVGGA |
| 105 | F | 0.42 | C. neoformans FEVGG Cytomegalovirus FE GGAA |
| 106 | E | 0.49 | C. neoformans EVGGAA and EVGGA P and EVGGA and EV GAAP and E+GGAAP H. pylori EVGGA Borrelia burgdorferi burgdorferi E+GG A PF+ |
| 107 | V | 0.39 | P. gingivalis VGGAAP and VGGAA C. neoformans VGGAA PF and VGGAA NR and VGGAA and VG GAAP C. pneumoniae VGGA AP H. pylori VG GAAP and VGG APF and VGGAA P. gingivalis VGGAA |
| 108 | G | 0.74 | C. neoformans C. pneumoniae HSV-2 GGAAP |
| 109 | G | 0.44 | C. neoformans GA APFN T and GAAPF |
| 110 | A | 0.95 | C. pneumoniae AAPFN Borrelia burgdorferi AAP+NR HSV-1 AAP RT |
| 111 | A | 0.83 | C. neoformans APFN T RS C. neoformans H. pylori APFN TH C. pneumoniae AP N RT R RSSS |
| 112 | P | 0.99 | C. neoformans PFNRT and PF RTH Borrelia burgdorferi burgdorferi PF NRT |
| 113 | F | 0.84 | C. neoformans FNRT SKR |
| 114 | N | 0.75 | C. neoformans NR RSKRS S and NRT R RSSS Cytomegalovirus N T RSKRS |
| 115 | R | 0.63 | C. neoformans RT R SKRS and RTHRS Borrelia burgdorferi burgdorferi RTHRS |
| 116 | T | 0.63 | C. neoformans THRS RS and THRSK |
| 117 | H | 0.64 | C. neoformans HRS RSS and HRSKR C. Pneumonie HRSKR |
| 118 | R | 0.94 | C. neoformans RSKRSS and RS RSSS and RS KRSS and RS KRSS and RSK SSS and RSKRS S P. gingivalis RSKR S and RSKRS Borrelia burgdorferi burgdorferi RSKRS |
| 119 | S | 0.88 | C. neoformans SKRSSS and SKRSS C. pneumoniae H. pylori HSV-1 HSV-2 SKRSS |
| 120 | K | 1.12 | C. neoformans Cytomegalovirus HHV-6 H. pylori KRSSS |
| 121 | R | 1.13 | |
| 122 | S | 1.12 | |
| 123 | S | 0.83 | |
| 124 | S | 0.50 | |
| 144 | G | 0.52 | C. neoformans GDKTTA and GDKTT |
| 145 | D | 0.54 | H. pylori DK TATDI Borrelia burgdorferi burgdorferi C. neoformans C. pneumoniae H. pylori DKTTA HSV-1 +KTT TD |
| 146 | K | 0.84 | CF. Pneumoniae KTTAT+ C. neoformans H. pylori KTTAT |
| 147 | T | 1.27 | C. neoformans HSV-1 TTATDI C. neoformans P. gingivalis TTATD |
| 148 | T | 1.22 | C. neoformans TATDIK Borrelia burgdorferi burgdorferi C. neoformans C. pneumoniae H. pylori TATDI |
| 149 | A | 1.21 | C. neoformans HHV-6 HHV-6B P. gingivalis ATDIK |
| 150 | T | 1.08 | |
| 151 | D | 1.01 | |
| 152 | I | 0.97 | |
| 153 | K | 0.61 | |
| 179 | C | 0.87 | C. neoformans CR PNPV C. pneumoniae CRDPN P+ S RGI |
| 180 | R | 1.40 | C. pneumoniae RDPNPV Borrelia burgdorferi burgdorferi RD NP VDS C. neoformans RDP PVDS and RDPNP+ and RDPNP HHV-6 HHV-6B RDPNP HSV-1 HSV-2 RDPN V |
| 181 | D | 1.36 | Borrelia burgdorferi burgdorferi D NPVD and DPN VD C. neoformans DPNPV and DP PVD and DP PVDS and DPN VDS C. pneumoniae DPN VD HSV-1 DPNP S HSV-2 +P PVDS |
| 182 | P | 1.69 | C. neoformans PN VDSG and PNP DSG and PNPVD P. gingivalis PNPV+S and P PVDS |
| 183 | N | 1.72 | H. pylori NPVD G |
| 184 | P | 1.85 | C. neoformans PV DSGCR and P PVDS RG and PVDSG Cytomagalovirus P DSG RGI P. gingivalis PV DSGC |
| 185 | V | 1.74 | HSV-2 VDSG RG HSV-1 +DSG RG C. neoformans VDSG R |
| 186 | D | 1.51 | C. neoformans DSGC GI and DSG RG |
| 187 | S | 1.28 | Borrelia burgdorferi burgdorferi C. neoformans SGCRG and SG RGI and S CRGI |
| 188 | G | 0.79 | C. neoformans GC IDSKH W and GCRG D P. gingivalis GCRGI and GCRG+ |
| 189 | C | 0.79 | P. gingivalis CRGID C. neoformans C GIDS |
| 190 | R | 0.85 | C. neoformans R IDSK and RGIDS and RGID K H. pylori RGIDS HSV-1 HSV-2 RG+DS H. pylori RG DSK Borrelia burgdorferi burgdorferi RGID K |
| 191 | G | 0.65 | C. neoformans GIDS HW and GIDSK and GIDS H Borrelia burgdorferi burgdorferi H. pylori P. gingivalis GIDSK P. gingivalis GID KH and G DSKH H. pylori GI SKH |
| 192 | I | 0.60 | Borrelia burgdorferi burgdorferi IDSKH C. neoformans P. gingivalis IDSK W |
| 193 | D | 0.3 | C. neoformans DSKH W Borrelia burgdorferi burgdorferi DSK WN |
| 194 | S | 0.40 | C. neoformans SKHW+ |
| 195 | K | 0.44 | |
| 197 | W | 0.41 | |
| 212 | T | 0.33 | C. neoformans TMDGKQ and TMDGK |
| 213 | M | 0.31 | P. gingivalis MDGKQ |
| 214 | D | 0.39 | C. neoformans DGKQAA and DGKQA C. pneumoniae DGK AA |
| 215 | G | 0.48 | C. neoformans GKQAA |
| 216 | K | 0.53 | |
| 217 | Q | 0.45 |
(c)
| Tau position | Amino acid | B-cell index | Alignment |
|---|---|---|---|
| 53 | E | 2.516 | C. neoformans (GSK3)EDGSEEP S and E+G EEPG and +DGS+EP S and ED GSEE GS and EDGS++ GS and EDGSE and +D EEPG and EDG S PGS Cytomagalovirus EDG EEP and +DG EE and ED GSEE P. gingivalis EDGSEE and E+G SEEP and EDGS EE HHV-6 HHV-6B EDGS EE C. pneumoniae E GSEE Borrelia burgdorferi E GSEE HHV-6 E GSEE |
| 54 | D | 2.568 | C. neoformans DGSEEP and DGS+EPG and DGSEE G P. gingivalis DGS EPGS and DGSEE and DGS EP and DGSE EP and DGSE P Cytomegalovirus DGS EP and DG EE G C. pneumoniae DGSE GS and +G EE GS Borrelia burgdorferi DGSE+ P. gingivalis +GSEE |
| 55 | G | 2.621 | C. neoformans GSEEP and G EEPG and G +EPGS and GS EEPG and GSEE G Cytomegalovirus GS+EP S P. gingivalis GS EPG H. pylori GSEE G C. pneumoniae GSEE GS |
| 56 | S | 2.732 | C. neoformans SEEPG and SEE GS and S EPGS and SEEP S C. pneumoniae SEEPG and SEE GS and SE PGS P. gingivalis SEE GS and S EPGS |
| 57 | E | 2.774 | C. neoformans EEPGS and +EPGS and EEPG E and EE GSE C. pneumoniae EEPGS HSV-1 HSV-2 EEPG |
| 58 | E | 2.678 | HHV-6 HHV-6B EPGSE C. neoformans EPGSE and EPGS+ P. gingivalis EPGS+ |
| 59 | P | 2.564 | |
| 60 | G | 2.544 | |
| 61 | S | 2.556 | |
| 62 | E | 2.287 | |
| 171 | S | 2.315 | Borrelia burgdorferi SG GPED C. neoformans SG GPEDT and SGT PE and SGTGP and SG GPE and SGTG E and SG GP+ P. gingivalis SGTG E C. pneumoniae SGTG PE Cytomegalovirus SGTGP+ |
| 172 | G | 2.54 | C. neoformans GT PED and GTG ED and GTGPE and G GPED and GTGP D and G EDTE HSV-1 GTGPE and GTGP D and GTGP+D HSV-2 GTGP D and G GPED C. pneumoniae GTGPE H. pylori GTGP D |
| 173 | T | 2.731 | HHV-6 TGPEDT and TG PEDT C. neoformans TG PEDT and TG EDT and T PEDT and TGPED C. pneumoniae TGPED H. pylori TG EDT Borrelia burgdorferi TGPE T+ |
| 174 | G | 2.709 | C. neoformans GPEDT and GPED TE and GP DTE and GPED E HHV-6 HHV-6B TG PEDT |
| 175 | P | 2.807 | C. neoformans H. pylori P. gingivalis PEDTE |
| 176 | E | 2.7 | |
| 177 | D | 2.563 | |
| 178 | T | 2.397 | |
| 179 | E | 2.225 | |
| 228 | S | 2.396 | HSV-1 SP DSPP and SPQ SP C. neoformans SPQDS and S QDSP and SP DSPP and SP DSPP and SPQD PP and PQ DSPPS and SPQ PPSK and SPQ SP and SP DSP and SPQ SP Cytomegalovirus SP DSPP P. gingivalis SP DSP Borrelia burgdorferi SP++SPP and SP D PSK |
| 229 | P | 2.744 | C. neoformans PQ SPPS and P DSPPS and PQDSP and P+DSPP and PQ S PPSK and PQ SPP and PQD PP and P DSPP P. gingivalis PQDS P HSV-2 P DSPP HHV-6 PQ+ PP and PQ PP K Borrelia burgdorferi PQ+ P SK |
| 230 | Q | 2.763 | C. neoformans QDSPP and ++ PPSK and QDS PS and Q SPPS P. gingivalis QD PPS C. pneumoniae QDS PS |
| 231 | D | 2.929 | C. neoformans DSP PSK and DSPPS and DSP SK and D PPSK and +SPPS P. gingivalis DSP SK |
| 232 | S | 2.882 | C. neoformans SPPSK |
| 233 | P | 2.75 | |
| 234 | P | 2.794 | |
| 235 | S | 2.68 | |
| 242 | D | 2.387 | C. neoformans DGRPP C. pneumoniae DG PPQ HSV-1 DG PPQ HSV-2 DGRPP and DG PP+ Cytomegalovirus DG R PQ and DG PP and +GRPP Borrelia burgdorferi DG PP |
| 243 | G | 2.49 | C. neoformans GRPPQ |
| 244 | R | 2.56 | |
| 245 | P | 2.42 | |
| 246 | P | 2.164 | |
| 247 | Q | 2.004 | |
| 331 | P | 2.259 | C. neoformans PGEG PE and P EGPEA and PGEGP and PGEG EA and P EGPEA and PGEG E Cytomegalovirus PGEGP EA and PG GPE C. pneumoniae PGE PEA and PGEGP H. pylori PG GPE HSV-1 HHV-6 PG GP A |
| 332 | G | 2.681 | C. neoformans GEGPE and GEG EA and GE PEA and G GPEA and GEGP A and GEG EA and G+GPEA C. pneumoniae GE PEA P. gingivalis GEGPE and GEG EA |
| 333 | E | 2.715 | C. neoformans P. gingivalis EGPEA HSV-2 +GPEA |
| 334 | G | 2.634 | |
| 335 | P | 2.592 | |
| 336 | E | 2.463 | |
| 337 | A | 2.405 | |
| 414 | H | 1.819 | HSV-1 HSV-2 HPTPG C. neoformans HPT GSS and HP PGSS and HPTP SS and HP P SS C. pneumoniae HP PGS and +PT SS |
| 415 | P | 2.274 | Cytomegalovirus P PGSS and PTPG SS C. pneumoniae PT GSS and PTPG SS |
| 416 | T | 2.503 | |
| 417 | P | 2.717 | |
| 418 | G | 2.469 | |
| 419 | S | 2.054 | |
| 420 | S | 1.76 | |
| 493 | P | 2.43 | HSV-1 P APKTP and P APKTP and PPAP PP and PPA PP HSV-2 PPAP TPP Cytomegalovirus P APKT and PPAP PP and PP P PPS and PP KTPP and PP A TPPS and PPA TPP C. neoformans PPAP TPPS and PPAP KTP S and PP PKTP and PP PKTP and PPAPKT and PPAP KT P. gingivalis PPAPK P C. pneumoniae PPAPK and PP PKT and PP AP PP and PP TPP Borrelia burgdorferi PAP T PS and PPA KTP and PP P TPP |
| 494 | P | 2.77 | HSV-1 PAP KTP and PA KTPP HSV-2 PAPK PP HHV-6 PAP PPS C. neoformans PAPKTP S and PAPK PP P. gingivalis PAPKT C. pneumoniae PAP TP and PAPK P |
| 495 | A | 2.94 | C. neoformans AP PPS P. gingivalis A KTPPS HHV-6 APKTP |
| 496 | P | 2.89 | C. neoformans PKTPPS and PKTP PS and PK TPPS P. gingivalis PKT PS |
| 497 | K | 2.95 | C. neoformans KTPPS |
| 498 | T | 2.98 | |
| 499 | P | 2.69 | |
| 500 | P | 2.51 | |
| 501 | S | 2.343 | |
| 502 | S | 2.123 |
All pathogens express proteins with homology to each autoantigen, specifically within predicted B-cell epitope regions of the human autoantigen (Table 1), suggesting that antibodies raised to any could be responsible for targeting these human proteins, under the appropriate circumstances. Perversely, the successful elimination of the pathogen via antibody production could set in motion the very autoimmune responses that may be crucial to the development of Alzheimer's disease, a Pyrrhic victory, which would also be promulgated by any further encounter with these very common pathogens, or by structurally related proteins from other pathogens, as well as by continual encounter of the human autoantigen homologue (see Section 3.6.5).
As well as autoantibodies to these three proteins, a number of autoantibodies targeting highly relevant proteins have been reported in Alzheimer's disease. These have functional effects on their target proteins and include antibodies that block the activity of ATP synthase, induce apoptosis, and increase the cellular uptake of high density lipoprotein [266, 267], antibodies to cholinergic neurones that cause immunalysis of brain synaptosomes [268], antibrain antibodies that enhance intraneuronal beta-amyloid deposition [269] as well as antibodies to the nicotinic receptor CHRNA7, that displace its ligand alpha-bungarotoxin [270]: autoantibodies to the receptor for advanced glycation products (AGER) [271], to the antimicrobial peptide S100B, have also been reported [272].
3.6.5. Population Genetics: Susceptibility Genes Related to the Cause of the Disease, rather than to the Disease Itself
Using a classical example from the field of population genetics and Darwinism, the light coloured genes of the peppered moth favour its selective predation by many different birds when it alights on dark trees covered with soot pollution, while darker melanised forms are selectively targeted on lighter coloured trees [273]. The coloration susceptibility genes, or the variety of tree (risk factors), do not kill the moth but allow several causes to do so. The causes can hide in plain sight, as epidemiological studies, as applied to human diseases, could conclude that the birds are not killing the moths, as they are always present, on both sets of trees, in both genetic conditions, whether the moths are alive or dead (c.f. the ubiquitous C. neoformans and many other common pathogens). Many of the pathogens implicated in Alzheimer's disease (herpes simplex, Borrelia burgdorferi and C. pneumoniae), and several other risk factors (cholesterol, homocysteine, diabetes, or vitamin A or nerve growth factor deficiency) are able to promote cerebral beta-amyloid deposition in animal models, without the aid of any gene variant. Subsets of susceptibility genes can be related to each of these amyloidogenic pathways [1]. In the case of genomewide association studies, the genes returned, as well as APP, beta amyloid, and gamma secretase, seem intimately concerned with pathogen life cycles and defence and the immune network. This suggests that the diverse microbial risk factors as well as other dietary and environmental factors implicated in Alzheimer's disease are in fact causative agents, whose deleterious effects are conditioned by susceptibility genes. As the environmental risk factors are amenable to therapy, while the susceptibility gene products have so far proved largely resistant, this suggests numerous ways with which to tackle the problem of Alzheimer's disease.
Herpes Simplex Reactivation —
Alzheimer's disease plaques and tangles are highly enriched in human proteins used the herpes simplex during its life cycle, as well as in many immune-related proteins, suggesting that immune attack on a reactivated virus, diverted to neurones, which contain the complement membrane attack complex, may be ultimately responsible for the extensive neuronal destruction seen in Alzheimer's disease [15]. The herpes simplex virus establishes latency in neurones, existing as a dormant form where only the latency transcript is expressed. A number of factors again related to susceptibility genes and environmental risk factors in Alzheimer's disease can be related to herpes simplex latency and reactivation. The cerebral herpes simplex viral load is decreased in APOE knockout mice, while the viral load is much increased in APOE4 transgenic mice, compared to APOE3 mice, suggesting that APOE4 favours the establishment of a greater number of latent viruses in cerebral tissue [274]. In neuroblastoma cells, this latent form may even exert beneficial effects, blocking apoptosis and promoting neurite extension via AKT1 upregulation [275]. However, this latent form can be reactivated by NGF deprivation [276], and NGF promotes viral latency via the TrkA receptor NTRK1 [277], the expression of which is reduced in the Alzheimer's disease brain [278]. (Relevant pathways and genes: neurotrophin signalling (GSK3B, NTRK1, NTRK2, PIK3R1, and SOS2). Vitamin A supplementation in rats increases the cerebral levels of both NGF and BDNF [279], while oestrogen deficiency lowers cerebral NGF levels, an effect reversed by 17-beta oestradiol [280], which is, however, also able to reactivate the virus, via oestrogen receptor alpha (ESR1) [281]. High levels of total oestradiol have been reported as a risk factor for Alzheimer's disease in both women and men [282, 283]. Vitamin A related gens include APOE4, the isoform least able to transport the vitamin A precursor retinyl palmitate, A2M ABCA1 ALB ALDH2 APOA1 CHD4 CLU CYP46A1 ESR1 GSTM1 GSTP1 HSPG2 KLF5 LIPA LPL LRAT LRP2 LRPAP1 MEF2A NPAS2 NR1H2 PARP1 PIN1 POU2F1 PPARA PPARG RXRA THRA TTR UBQLN1 VDR and many others controlled by retinoid receptor element (reviewed in [126]).
The virus can also be reactivated by heat [284], IL6 [285], or TNF [286]. IL6 plasma and CSF levels have been reported to be increased in Alzheimer's disease and the secretion of IL6 from monocytes is increased [287–289]. IL6 plasma levels are raised by infection with C. pneumoniae [290] or Helicobacter pylori [291], and IL6 production in monocytes is stimulated by C. neoformans [292]. Stress-activated corticosterone is also able to reactivate the virus [293] (pathway = steroid hormone biosynthesis: COMT, CYP19A1, HSD11B1). Cortisol levels are increased in the ageing population and in Alzheimer's disease [294, 295]. A number of related stressors including adrenaline [296], or downstream effectors such as cyclic AMP, protein kinase A, or C activation [297] are also able to reactivate the virus. Herpes simplex reactivation is blocked by beta-receptor antagonism (ADRB1) [296, 298]. Glucocorticoids and prostaglandins are also able to reactivate the virus [299]: cyclooxygenase inhibitors (PTGS2), celecoxib and indomethacin block viral reactivation and nonsteroidal anti-inflammatory use has been associated with a lower incidence of Alzheimer's disease. Lysophosphatidic acid is also able to reactivate the virus [300] (LPAR5). Hypoxia also increases the replication of herpes simplex [301]: transient cerebral ischaemia also lowers NGF levels [302], and these effects are relevant to the cerebral hypoperfusion seen in ageing and in Alzheimer's disease [303]. Vitamin A and the retinoic acid isomers all-trans-, 9-cis-, and 13-cis-Retinoic Acid are all able to reduce viral replication [304]. Low Vitamin A levels are a problem in the ageing population and even in successfully ageing persons can be observed in 50% of the population over the age of 80–85 [305]. Low vitamin A levels are also a risk factor for Alzheimer's disease [306]. Mice deficient in the nitric oxide synthase NOS2 are also more susceptible to herpes virus infection, and reactivation occurs is stimulated by caspase 3 activation (CASP3) [135].
Thus, fever or cytokine release induced by diverse infections might be expected to reactivate herpes simplex as would several of the conditions associated with Alzheimer's disease (cerebral hypoperfusion, low cerebral NGF levels, vitamin A deficiency, or high oestradiol levels).
3.6.6. The Microbiome in Alzheimer's Disease
These pathogens form a very small proportion of an extensive human microbiome comprising of trillions of bacteria, viruses, and other pathogens, whose influence on diseases is increasingly recognised [307]. Individual species may exert benevolent or malevolent effects which may well be dictated by the very extensive sequence homology between human and pathogen proteins which enables pathogens to intercalate with numerous important signalling networks, via competition with their human counterparts [239, 241, 248, 250, 254]. These networks are implicated in diseases, and their efficiency, as well as pathogen/host homology, is dictated by susceptibility genes. This host/pathogen matching covers the entire human proteome and is represented by millions of short consensi of pentapeptides or more, not counting the longer gapped consensi. Clearly, powerful algorithms are needed to trawl human and pathogen proteomes and to link these homologies to susceptibility genes and to epitopes and autoantigens.
The principles discussed here may apply to many other, if not most, human diseases.
Protective Agents in Alzheimer's Disease —
Statins [308], fish consumption [309], omega-3 polyunsaturated fatty acids [310], the Mediterranean diet [311], nonsteroidal anti-inflammatories [312], or the generally healthy life-style of nuns living in convents [313] have all been associated with a reduced incidence or severity of Alzheimer's disease, while homocysteine lowering B vitamins and folate have been shown to slow the rate of brain atrophy in cognitively impaired elderly patients [314]. A rather pessimistic study [315] recently stated that no firm conclusions could be drawn on the association of any modifiable factors with risk of Alzheimer's disease. While this may be a justifiable statistical conclusion from meta-analysis, this is not to reckon with the diversity of the underlying genomic platform of each individual or with the profusion of diverse interacting risk and protective factors (epistasis, gene/environment, and environment/environment interactions).
As with the risk factors, protective agents can be linked to genetic and pathological pathways involved in cholesterol and lipid function (statins, fish, and diet, e.g., ABCA1, ABCA2, ABCA7, ABCG1, ACADS, ALDH2, APP, APOA1, APOA4, APOA5, APOC1-4, APOE, CH25H, CYP46A1, DHCR24, FDPS, HMGCR, HMGCS2, HSD11B1, LIPC, LRP1, LRP2, LRP8, LDLR, LIPA, LPL, OLR1, PPARA, PPARG, PTPLA, VLDLR, NPC1, NPC2, SOAT1, SREBF1), homocysteine, methionine, and folate metabolism (folate and vitamins, e.g., BLMH, CBS, COMT, NAT2, MTHFD1L, MTHFR,MTR, MTRR, PON1, PON2, PON3, VDR) and inflammatory pathways (NSAID'S e.g., C4A, C4B, CD2AP, CD33, CFH, CCL2, CCL3, CCR2, CSF1, CLU, CR1, FAS, GSK3B, IL1A, IL1B, IL6, IL8, IL10, IL18, PLAU, SERPINA1, SERPINE1, SERPINF2, PTGS2, TGFB1, TNF). As with the risk factors, the success of such protective agents is likely to be determined by genes and other confounding factors. In this genomic era, affordable whole genome sequencing will soon be achieved, ushering in an age of more effective treatments tailored to individual genetic profiles. Alzheimer's disease is clearly multifactorial with many related genetic and environmental risk factors, several underlying pathologies, and several available protective strategies. A recent study has also shown that many other seemingly benign factors (eyesight, hearing, denture wearing, stomach, kidney, bladder or bowel problems, coughs, and colds) as well as high blood pressure and diabetes, constituting a frailty index, combine to markedly increase both the incidence and severity of Alzheimer's disease [316]. A further study, identifying physical inactivity, depression, smoking, mid-life hypertension or obesity, low education, and diabetes as key risk factors, has estimated that ~50% of Alzheimer's disease cases may be preventable [317].
Elimination of the risk factors, including the regular detection and elimination of pathogens in the elderly, adherence to sensible dietary and vitamin supplement recommendations, and the genetically tailored use of certain drug regimens are together likely to be able to markedly reduce the incidence of Alzheimer's disease. Using phage display, it is now possible to express peptide fragments of the entire human proteome in a phage library, and to use this to trap autoantibodies in blood or other bodily fluid samples. The antigen expressed by the labelled phage can be identified by high throughput sequencing [318]. Such technology is likely to be extremely useful in characterising biomarkers and pathological immune processes as well as potential pathogen/human cross-reactivity.
Proteome-wide characterisation of the autoantibodies relevant to Alzheimer's disease (a move from GWAS to PWAS) would also be very informative as selective autoantibody removal via affinity dialysis might well be expected to influence the severity and progression of Alzheimer's disease.
Since the submission of this paper, Miklossy has reported a highly significant association between spirochete infection and Alzheimer's disease. As well as B. burgdorferi, several periodontal pathogen treponemas species were detected in brain samples and the pathological features of Alzheimer's disease were reproduced by infection in vitro [319]. In addition, two groups have reported a very extensive repertoire of autoantigens in Alzheimer's disease, which can be characterised with a high degree of accuracy by a definitive immunosignature [320, 321]. The principles outlined above could thus be tested by analysis of pathogen/autoantigen cross-reactivity.
References
- 1.Carter CJ. The fox and the rabbits, environmental variables and population genetics. 1: replication problems in association studies and the untapped power of GWAS. 2: vitamin A deficiency, herpes simplex reactivation and other causes of Alzheimer's disease. ISRN Neurology. 2011;2011:29 pages. doi: 10.5402/2011/394678. Article ID 394678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter CJ. Interactions between the products of the Herpes simplex genome and Alzheimer's disease susceptibility genes: relevance to pathological-signalling cascades. Neurochemistry International. 2008;52(6):920–934. doi: 10.1016/j.neuint.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Carter CJ. Convergence of genes implicated in Alzheimer's disease on the cerebral cholesterol shuttle: APP, cholesterol, lipoproteins, and atherosclerosis. Neurochemistry International. 2007;50(1):12–38. doi: 10.1016/j.neuint.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Papassotiropoulos A, Wollmer MA, Tsolaki M, et al. A cluster of cholesterol-related genes confers susceptibility for Alzheimer's disease. Journal of Clinical Psychiatry. 2005;66(7):940–947. [PubMed] [Google Scholar]
- 5.Menon R, Farina C. Shared molecular and functional frameworks among five complex human disorders: a comparative study on interactomes linked to susceptibility genes. PLoS ONE. 2011;6(4) doi: 10.1371/journal.pone.0018660. Article ID e18660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naj AC, Beecham GW, Martin ER, et al. Dementia revealed: novel chromosome 6 locus for Late-onset alzheimer disease provides genetic evidence for Folate-pathway abnormalities. PLoS Genetics. 2010;6(9) doi: 10.1371/journal.pgen.1001130. Article ID e1001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liolitsa D, Powell J, Lovestone S. Genetic variability in the insulin signalling pathway may contribute to the risk of late onset Alzheimer's disease. Journal of Neurology Neurosurgery and Psychiatry. 2002;73(3):261–266. doi: 10.1136/jnnp.73.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodman AB, Pardee AB. Evidence for defective retinoid transport and function in late onset Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(5):2901–2905. doi: 10.1073/pnas.0437937100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseveleki V, Rubio R, Vamvakas SS, et al. Comparative gene expression analysis in mouse models for multiple sclerosis, Alzheimer's disease and stroke for identifying commonly regulated and disease-specific gene changes. Genomics. 2010;96(2):82–91. doi: 10.1016/j.ygeno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert JC, Grenier-Boley B, Chouraki V, et al. Implication of the immune system in Alzheimer's disease: evidence from genome-wide pathway analysis. Journal of Alzheimer's Disease. 2010;20(4):1107–1118. doi: 10.3233/JAD-2010-100018. [DOI] [PubMed] [Google Scholar]
- 11.Hill JM, Zhao Y, Clement C, Neumann DM, Lukiw WJ. HSV-1 infection of human brain cells induces miRNA-146a and Alzheimer-type inflammatory signaling. NeuroReport. 2009;20(16):1500–1505. doi: 10.1097/WNR.0b013e3283329c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itzhaki RF, Wozniak MA. Herpes simplex virus type 1 in Alzheimer's disease: the enemy within. Journal of Alzheimer's Disease. 2008;13(4):393–405. doi: 10.3233/jad-2008-13405. [DOI] [PubMed] [Google Scholar]
- 13.Pyles RB. The association of herpes simplex virus and Alzheimer's disease: a potential synthesis of genetic and environmental factors. Herpes. 2001;8(3):64–68. [PubMed] [Google Scholar]
- 14.Wozniak MA, Mee AP, Itzhaki RF. Herpes simplex virus type 1 DNA is located within Alzheimer's disease amyloid plaques. Journal of Pathology. 2009;217(1):131–138. doi: 10.1002/path.2449. [DOI] [PubMed] [Google Scholar]
- 15.Carter CJ. Alzheimer's disease plaques and tangles: cemeteries of a Pyrrhic victory of the immune defence network against herpes simplex infection at the expense of complement and inflammation-mediated neuronal destruction. Neurochemistry International. 2011;58(3):301–320. doi: 10.1016/j.neuint.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Letenneur L, Pérès K, Fleury H, et al. Seropositivity to Herpes Simplex Virus antibodies and risk of Alzheimer's disease: a population-based cohort study. PLoS ONE. 2008;3(11) doi: 10.1371/journal.pone.0003637. Article ID e3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ. Inflammation and Alzheimer's disease: possible role of periodontal diseases. Alzheimer's and Dementia. 2008;4(4):242–250. doi: 10.1016/j.jalz.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. Journal of Neurology Neurosurgery and Psychiatry. 1998;65(1):29–33. doi: 10.1136/jnnp.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kountouras J, Boziki M, Gavalas E, et al. Five-year survival after Helicobacter pylori eradication in Alzheimer disease patients. Cognitive and Behavioral Neurology. 2010;23(3):199–204. doi: 10.1097/WNN.0b013e3181df3034. [DOI] [PubMed] [Google Scholar]
- 20.Ala TA, Doss RC, Sullivan CJ. Reversible dementia: a case of cryptococcal meningitis masquerading as Alzheimer's disease. Journal of Alzheimer's Disease. 2004;6(5):503–508. doi: 10.3233/jad-2004-6507. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann M, Muniz J, Carroll E, de Villasante J. Cryptococcal meningitis misdiagnosed as alzheimer's disease: complete neurological and cognitive recovery with treatment. Journal of Alzheimer's Disease. 2009;16(3):517–520. doi: 10.3233/JAD-2009-0985. [DOI] [PubMed] [Google Scholar]
- 22.Kaklikkaya I, Kaklikkaya N, Birincioglu I, Buruk K, Turan N. Detection of human herpesvirus 6 DNA but not human herpesvirus 7 or 8 DNA in atherosclerotic and nonatherosclerotic vascular tissues. Heart Surgery Forum. 2010;13(5):E345–E349. doi: 10.1532/HSF98.20101023. [DOI] [PubMed] [Google Scholar]
- 23.Makris GC, Makris MC, Wilmot VV, Geroulakos G, Falagas ME. The role of infection in carotid plaque pathogenesis and stability: the clinical evidence. Current Vascular Pharmacology. 2010;8(6):861–872. doi: 10.2174/157016110793563889. [DOI] [PubMed] [Google Scholar]
- 24.Nazmi A, Diez-Roux AV, Jenny NS, Tsai MY, Szklo M, Aiello AE. The influence of persistent pathogens on circulating levels of inflammatory markers: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis. BMC Public Health. 2010;10, article 706 doi: 10.1186/1471-2458-10-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taniguchi A, Nishimura F, Murayama Y, et al. Porphyromonas gingivalis infection is associated with carotid atherosclerosis in non-obese Japanese type 2 diabetic patients. Metabolism. 2003;52(2):142–145. doi: 10.1053/meta.2003.50001. [DOI] [PubMed] [Google Scholar]
- 26.Hall CJ, Bouhafs L, Dizcfalusy U, Sandstedt K. Cryptococcus neoformans causes lipid peroxidation; therefore it is a potential inducer of atherogenesis. Mycologia. 2010;102(3):546–551. doi: 10.3852/08-110. [DOI] [PubMed] [Google Scholar]
- 27.Roher AE, Esh C, Rahman A, Kokjohn TA, Beach TG. Atherosclerosis of cerebral arteries in Alzheimer disease. Stroke. 2004;35(11):2623–2627. doi: 10.1161/01.STR.0000143317.70478.b3. [DOI] [PubMed] [Google Scholar]
- 28.van Oijen M, de Jong FJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MMB. Atherosclerosis and risk for dementia. Annals of Neurology. 2007;61(5):403–410. doi: 10.1002/ana.21073. [DOI] [PubMed] [Google Scholar]
- 29.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 30.Antúnez C, Boada M, González-Pérez A, et al. The membrane-spanning 4-domains, subfamily A (MS4A) gene cluster contains a common variant associated with Alzheimer's disease. Genome Medicine. 2011;3(5, article 33) doi: 10.1186/gm249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan K. The three new pathways leading to Alzheimer's disease. Neuropathology and Applied Neurobiology. 2011;37(4):353–357. doi: 10.1111/j.1365-2990.2011.01181.x. [DOI] [PubMed] [Google Scholar]
- 32.Hindorff LA, Sethupathy P, Junkins HA, et al. A Catalog of Published Genome-Wide Association Studies. http://www.genome.gov/gwastudies/. Accessed [June 2011]
- 33.Su AI, Wiltshire T, Batalov S, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(16):6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C, Orozco C, Boyer J, et al. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biology. 2009;10(11, article R130) doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsen JE, Lund O, Nielsen M. Improved method for predicting linear B-cell epitopes. Immunome Research. 2006;2(2) doi: 10.1186/1745-7580-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altschul SF, Madden TL, Schäffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck Z, Brown BK, Matyas GR, Polonis VR, Rao M, Alving CR. Infection of human peripheral blood mononuclear cells by erythrocyte-bound HIV-1: effects of antibodies and complement. Virology. 2011;412(2):441–447. doi: 10.1016/j.virol.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 38.Carlisle RC, Di Y, Cerny AM, et al. Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1. Blood. 2009;113(9):1909–1918. doi: 10.1182/blood-2008-09-178459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gyimesi E, Bankovich AJ, Schuman TA, Goldberg JB, Lindorfer MA, Taylor RP. Staphylococcus aureus bound to complement receptor 1 on human erythrocytes by bispecific monoclonal antibodies is phagocytosed by acceptor macrophages. Immunology Letters. 2004;95(2):185–192. doi: 10.1016/j.imlet.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Hatano Y, Taniuchi S, Masuda M, et al. Phagocytosis of heat-killed Staphylococcus aureus by eosinophils: comparison with neutrophils. APMIS. 2009;117(2):115–123. doi: 10.1111/j.1600-0463.2008.00022.x. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Wang JP, Ghiran I, et al. Complement receptor 1 expression on mouse erythrocytes mediates clearance of Streptococcus pneumoniae by immune adherence. Infection and Immunity. 2010;78(7):3129–3135. doi: 10.1128/IAI.01263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spadafora C, Awandare GA, Kopydlowski KM, et al. Complement receptor 1 is a sialic acid-independent erythrocyte receptor of Plasmodium falciparum. PLoS Pathogens. 2010;6(6) doi: 10.1371/journal.ppat.1000968. Article ID e1000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powers JH, Buster BL, Reist CJ, et al. Complement-independent binding of microorganisms to primate erythrocytes in vitro by cross-linked monoclonal antibodies via complement receptor 1. Infection and Immunity. 1995;63(4):1329–1335. doi: 10.1128/iai.63.4.1329-1335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Repik A, Pincus SE, Ghiran I, et al. A transgenic mouse model for studying the clearance of blood-borne pathogens via human complement receptor 1 (CR1) Clinical and Experimental Immunology. 2005;140(2):230–240. doi: 10.1111/j.1365-2249.2005.02764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calero M, Tokuda T, Rostagno A, et al. Functional and structural properties of lipid-associated apolipoprotein J (clusterin) Biochemical Journal. 1999;344(2):375–383. [PMC free article] [PubMed] [Google Scholar]
- 46.Itagaki S, Akiyama H, Saito H, McGeer PL. Ultrastructural localization of complement membrane attack complex (MAC)-like immunoreactivity in brains of patients with Alzheimer's disease. Brain Research. 1994;645(1-2):78–84. doi: 10.1016/0006-8993(94)91640-3. [DOI] [PubMed] [Google Scholar]
- 47.McGeer PL, Akiyama H, Itagaki S, McGeer EG. Activation of the classical complement pathway in brain tissue of Alzheimer patients. Neuroscience Letters. 1989;107(1–3):341–346. doi: 10.1016/0304-3940(89)90843-4. [DOI] [PubMed] [Google Scholar]
- 48.Carter CJ. APP, APOE, complement receptor 1, clusterin and PICALM and their involvement in the herpes simplex life cycle. Neuroscience Letters. 2010;483(2):96–100. doi: 10.1016/j.neulet.2010.07.066. [DOI] [PubMed] [Google Scholar]
- 49.Cortes C, Ferreira VP, Pangburn MK. Native properdin binds to Chlamydia pneumoniae and promotes complement activation. Infection and Immunity. 2011;79(2):724–731. doi: 10.1128/IAI.00980-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasegawa A, Sogo LF, Tan M, Sütterlin C. Host complement regulatory protein CD59 is transported to the chlamydial inclusion by a golgi apparatus-independent pathway. Infection and Immunity. 2009;77(4):1285–1292. doi: 10.1128/IAI.01062-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosas ÁL, MacGill RS, Nosanchuk JD, Kozel TR, Casadevall A. Activation of the alternative complement pathway by fungal melanins. Clinical and Diagnostic Laboratory Immunology. 2002;9(1):144–148. doi: 10.1128/CDLI.9.1.144-148.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gates-Hollingsworth MA, Kozel TR. Phenotypic heterogeneity in expression of epitopes in the Cryptococcus neoformans capsule. Molecular Microbiology. 2009;74(1):126–138. doi: 10.1111/j.1365-2958.2009.06855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez-Valencia G, Perez-Perez GI, Washburn RG, Blaser MJ. Susceptibility of Helicobacter pylori to the bactericidal activity of human serum. Helicobacter. 1996;1(1):28–33. doi: 10.1111/j.1523-5378.1996.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 54.Hajishengallis G, Wang M, Liang S, et al. Subversion of innate immunity by periodontopathic bacteria via exploitation of complement receptor-3. Advances in Experimental Medicine and Biology. 2008;632:203–219. doi: 10.1007/978-0-387-78952-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Altieri DC, Etingin OR, Fair DS, et al. Structurally homologous ligand binding of integrin Mac-1 and viral glycoprotein C receptors. Science. 1991;254(5035):1200–1202. doi: 10.1126/science.1957171. [DOI] [PubMed] [Google Scholar]
- 56.Jones GJ, Wiseman JC, Marr KJ, Wei S, Djeu JY, Mody CH. In contrast to anti-tumor activity, YT cell and primary NK cell cytotoxicity for Cryptococcus neoformans bypasses LFA-1. International Immunology. 2009;21(4):423–432. doi: 10.1093/intimm/dxp010. [DOI] [PubMed] [Google Scholar]
- 57.Wang A, Johnston SC, Chou J, Dean D. A systemic network for Chlamydia pneumoniae entry into human cells. Journal of Bacteriology. 2010;192(11):2809–2815. doi: 10.1128/JB.01462-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang ZY, Hunter S, Chien P, et al. Interaction of two phagocytic host defense systems: Fcγ receptors and complement receptor 3. Journal of Biological Chemistry. 2011;286(1):160–168. doi: 10.1074/jbc.M110.163030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. Journal of Immunology. 2007;178(11):7242–7250. doi: 10.4049/jimmunol.178.11.7242. [DOI] [PubMed] [Google Scholar]
- 60.Deckert M, Ticchioni M, Mari B, Mary D, Bernard A. The glycosylphosphatidylinositol-anchored CD59 protein stimulates both T cell receptor ζ/ZAP-70-dependent and -independent signaling pathways in T cells. European Journal of Immunology. 1995;25(7):1815–1822. doi: 10.1002/eji.1830250704. [DOI] [PubMed] [Google Scholar]
- 61.Naderi S, Hofmann P, Seiter S, Tilgen W, Abken H, Reinhold U. CD2-mediated CD59 stimulation in keratinocytes results in secretion of IL-1α, IL-6, and GM-CSF: implications for the interaction of keratinocytes with intraepidermal T lymphocytes. International Journal of Molecular Medicine. 1999;3(6):609–614. doi: 10.3892/ijmm.3.6.609. [DOI] [PubMed] [Google Scholar]
- 62.Jehle AW, Gardai SJ, Li S, et al. ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. Journal of Cell Biology. 2006;174(4):547–556. doi: 10.1083/jcb.200601030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tas SW, Klickstein LB, Barbashov SF, Nicholson-Weller A. C1q and C4b bind simultaneously to CR1 and additively support erythrocyte adhesion. Journal of Immunology. 1999;163(9):5056–5063. [PubMed] [Google Scholar]
- 64.Sarvari M, Vago I, Weber CS, et al. Inhibition of C1q-beta-amyloid binding protects hippocampal cells against complement mediated toxicity. Journal of Neuroimmunology. 2003;137(1-2):12–18. doi: 10.1016/s0165-5728(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 65.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walker P. Molecular Biology of the Cell. New York, NY, USA: Garland Science; 2007. [Google Scholar]
- 66.Flint SJ, Enquist LW, Racaniello VR, Skalka AM. Principles of Virology. Herndon, Va, USA: ASM Press; 2008. [Google Scholar]
- 67.Gianni T, Campadelli-Fiume G, Menotti L. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. Journal of Virology. 2004;78(22):12268–12276. doi: 10.1128/JVI.78.22.12268-12276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parker H, Chitcholtan K, Hampton MB, Keenan JI. Uptake of Helicobacter pylori outer membrane vesicles by gastric epithelial cells. Infection and Immunity. 2010;78(12):5054–5061. doi: 10.1128/IAI.00299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pizarro-Cerdá J, Bonazzi M, Cossart P. Clathrin-mediated endocytosis: what works for small, also works for big. BioEssays. 2010;32(6):496–504. doi: 10.1002/bies.200900172. [DOI] [PubMed] [Google Scholar]
- 70.Tebar F, Bohlander SK, Sorkin A. Clathrin assembly lymphoid myeloid leukemia (CALM) protein: localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic. Molecular Biology of the Cell. 1999;10(8):2687–2702. doi: 10.1091/mbc.10.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brunetti CR, Burke RL, Hoflack B, Ludwig T, Dingwell KS, Johnson DC. Role of mannose-6-phosphate receptors in herpes simplex virus entry into cells and cell-to-cell transmission. Journal of Virology. 1995;69(6):3517–3528. doi: 10.1128/jvi.69.6.3517-3528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Molinari M, Galli C, Norais N, et al. Vacuoles induced by Helicobacter pylori toxin contain both late endosomal and lysosomal markers. Journal of Biological Chemistry. 1997;272(40):25339–25344. doi: 10.1074/jbc.272.40.25339. [DOI] [PubMed] [Google Scholar]
- 73.Nakayama K, Wakatsuki S. The structure and function of GGAs, the traffic controllers at the TGN sorting crossroads. Cell Structure and Function. 2003;28(5):431–442. doi: 10.1247/csf.28.431. [DOI] [PubMed] [Google Scholar]
- 74.Rabin EM, Gordon K, Knoppers MH, et al. Inhibition of T cell activation and adhesion functions by soluble CD2 protein. Cellular Immunology. 1993;149(1):24–38. doi: 10.1006/cimm.1993.1133. [DOI] [PubMed] [Google Scholar]
- 75.Gauthier NC, Monzo P, Gonzalez T, et al. Early endosomes associated with dynamic F-actin structures are required for late trafficking of H. pylori VacA toxin. Journal of Cell Biology. 2007;177(2):343–354. doi: 10.1083/jcb.200609061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yun PLW, Decarlo AA, Chapple CC, Collyer CA, Hunter N. Binding of Porphyromonas gingivalis gingipains to human CD4+ T cells preferentially down-regulates surface CD2 and CD4 with little affect on co-stimulatory molecule expression. Microbial Pathogenesis. 2005;38(2-3):85–96. doi: 10.1016/j.micpath.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 77.Moreno-Ruiz E, Galán-Díez M, Zhu W, et al. Candida albicans internalization by host cells is mediated by a clathrin-dependent mechanism. Cellular Microbiology. 2009;11(8):1179–1189. doi: 10.1111/j.1462-5822.2009.01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Selbach M, Backert S. Cortactin: an Achilles' heel of the actin cytoskeleton targeted by pathogens. Trends in Microbiology. 2005;13(4):181–189. doi: 10.1016/j.tim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 79.Tegtmeyer N, Wittelsberger R, Hartig R, Wessler S, Martinez-Quiles N, Backert S. Serine phosphorylation of cortactin controls focal adhesion kinase activity and cell scattering induced by Helicobacter pylori. Cell Host and Microbe. 2011;9(6):520–531. doi: 10.1016/j.chom.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 80.Cudmore S, Reckmann I, Way M. Viral manipulations of the actin cytoskeleton. Trends in Microbiology. 1997;5(4):142–148. doi: 10.1016/S0966-842X(97)01011-1. [DOI] [PubMed] [Google Scholar]
- 81.Mustonen H, Lepistö A, Lehtonen S, Lehtonen E, Puolakkainen P, Kivilaakso E. CD2AP contributes to cell migration and adhesion in cultured gastric epithelium. Biochemical and Biophysical Research Communications. 2005;332(2):426–432. doi: 10.1016/j.bbrc.2005.04.140. [DOI] [PubMed] [Google Scholar]
- 82.Lecuit M, Hurme R, Pizarro-Cerda J, Ohayon H, Geiger B, Cossart P. A role for α-and β-catenins in bacterial uptake. Proceedings of the National Academy of Sciences. 2000;97(18):10008–10013. doi: 10.1073/pnas.97.18.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Backert S, Tegtmeyer N, Selbach M. The versatility of helicobacter pylori caga effector protein functions: the master key hypothesis. Helicobacter. 2010;15(3):163–176. doi: 10.1111/j.1523-5378.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 84.Hoy B, Löwer M, Weydig C, et al. Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Reports. 2010;11(10):798–804. doi: 10.1038/embor.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.MacIntyre A, Hammond CJ, Little CS, Appelt DM, Balin BJ. Chlamydia pneumoniae infection alters the junctional complex proteins of human brain microvascular endothelial cells. FEMS Microbiology Letters. 2002;217(2):167–172. doi: 10.1111/j.1574-6968.2002.tb11470.x. [DOI] [PubMed] [Google Scholar]
- 86.Taylor MJ, Perrais D, Merrifield CJ. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biology. 2011;9(3) doi: 10.1371/journal.pbio.1000604. Article ID e1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nicot AS, Toussaint A, Tosch V, et al. Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nature Genetics. 2007;39(9):1134–1139. doi: 10.1038/ng2086. [DOI] [PubMed] [Google Scholar]
- 88.Nishi K, Saigo K. Cellular internalization of green fluorescent protein fused with herpes simplex virus protein VP22 via a lipid raft-mediated endocytic pathway independent of caveolae and Rho family GTPases but dependent on dynamin and Arf6. Journal of Biological Chemistry. 2007;282(37):27503–27517. doi: 10.1074/jbc.M703810200. [DOI] [PubMed] [Google Scholar]
- 89.McMahon HT, Wigge P, Smith C. Clathrin interacts specifically with amphiphysin and is displaced by dynamin. FEBS Letters. 1997;413(2):319–322. doi: 10.1016/s0014-5793(97)00928-9. [DOI] [PubMed] [Google Scholar]
- 90.Gold ES, Simmons RM, Petersen TW, Campbell LA, Kuo CC, Aderem A. Amphiphysin IIm is required for survival of Chlamydia pneumoniae in macrophages. Journal of Experimental Medicine. 2004;200(5):581–586. doi: 10.1084/jem.20040546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wixler V, Laplantine E, Geerts D, et al. Identification of novel interaction partners for the conserved membrane proximal region of α-integrin cytoplasmic domains. FEBS Letters. 1999;445(2-3):351–355. doi: 10.1016/s0014-5793(99)00151-9. [DOI] [PubMed] [Google Scholar]
- 92.Ulanova M, Gravelle S, Barnes R. The role of epithelial integrin receptors in recognition of pulmonary pathogens. Journal of Innate Immunity. 2008;1(1):4–17. doi: 10.1159/000141865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tegtmeyer N, Wessler S, Backert S. Role of the cag-pathogenicity island encoded type IV secretion system in Helicobacter pylori pathogenesis. FEBS Journal. 2011;278(8):1190–1202. doi: 10.1111/j.1742-4658.2011.08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Crocker PR, Redelinghuys P. Siglecs as positive and negative regulators of the immune system. Biochemical Society Transactions. 2008;36(6):1467–1471. doi: 10.1042/BST0361467. [DOI] [PubMed] [Google Scholar]
- 95.Brinkman-Van der Linden EC, Varki A. New aspects of siglec binding specificities, including the significance of fucosylation and of the sialyl-Tn epitope. Sialic acid-binding immunoglobulin superfamily lectins. Journal of Biological Chemistry. 2000;275(12):8625–8632. doi: 10.1074/jbc.275.12.8625. [DOI] [PubMed] [Google Scholar]
- 96.Teuton JR, Brandt CR. Sialic acid on herpes simplex virus type 1 envelope glycoproteins is required for efficient infection of cells. Journal of Virology. 2007;81(8):3731–3739. doi: 10.1128/JVI.02250-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rodrigues ML, Rozental S, Couceiro JN, Angluster J, Alviano CS, Travassos LR. Identification of N-Acetylneuraminic acid and its 9-O-acetylated derivative on the cell surface of Cryptococcus neoformans. Influence on fungal phagocytosis. Infection and Immunity. 1997;65(12):4937–4942. doi: 10.1128/iai.65.12.4937-4942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hulínská D, Volf P, Grubhoffer L. Characterization of Borrelia burgdorferi glycoconjugates and surface carbohydrates. Zentralblatt fur Bakteriologie. 1992;276(4):473–480. doi: 10.1016/s0934-8840(11)80672-9. [DOI] [PubMed] [Google Scholar]
- 99.Aspholm M, Olfat FO, Nordén J, et al. SabA is the H. pylori hemagglutinin and is polymorphic in binding to sialylated glycans. PLoS Pathogens. 2006;2(10) doi: 10.1371/journal.ppat.0020110. Article ID e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bennett HJ, Roberts IS. Identification of a new sialic acid-binding protein in Helicobacter pylori. FEMS Immunology and Medical Microbiology. 2005;44(2):163–169. doi: 10.1016/j.femsim.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 101.Hallén U, Björkner AE, Hallberg EC. Binding of the periodontitis associated bacterium Porphyromonas gingivalis to glycoproteins from human epithelial cells. Oral Microbiology and Immunology. 2008;23(5):367–371. doi: 10.1111/j.1399-302X.2008.00437.x. [DOI] [PubMed] [Google Scholar]
- 102.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nature Medicine. 2005;11(3):312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 103.Müller A, Heseler K, Schmidt SK, Spekker K, MacKenzie CR, Däubener W. The missing link between indoleamine 2,3-dioxygenase mediated antibacterial and immunoregulatory effects. Journal of Cellular and Molecular Medicine. 2009;13(6):1125–1135. doi: 10.1111/j.1582-4934.2008.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.MacKenzie CR, Heseler K, Müller A, Däubener W. Role of indoleamine 2,3-dioxygenase in antimicrobial defence and immuno-regulation: tryptophan depletion versus production of toxic kynurenines. Current Drug Metabolism. 2007;8(3):237–244. doi: 10.2174/138920007780362518. [DOI] [PubMed] [Google Scholar]
- 105.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacology and Therapeutics. 2011;130(2):226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gulaj E, Pawlak K, Bien B, Pawlak D. Kynurenine and its metabolites in Alzheimer's disease patients. Advances in Medical Sciences. 2010;55(2):204–211. doi: 10.2478/v10039-010-0023-6. [DOI] [PubMed] [Google Scholar]
- 107.Widner B, Leblhuber F, Walli J, Tilz GP, Demel U, Fuchs D. Tryptophan degradation and immune activation in Alzheimer's disease. Journal of Neural Transmission. 2000;107(3):343–353. doi: 10.1007/s007020050029. [DOI] [PubMed] [Google Scholar]
- 108.Hamilton RG, MacGlashan DW, Jr., Saini SS. IgE antibody-specific activity in human allergic disease. Immunologic Research. 2010;47(1–3):273–284. doi: 10.1007/s12026-009-8160-3. [DOI] [PubMed] [Google Scholar]
- 109.Ida S, Siraganian RP, Notkins AL. Cell-bound and circulating IgE antibody to herpes simplex virus. Journal of General Virology. 1983;64(3):533–537. doi: 10.1099/0022-1317-64-3-533. [DOI] [PubMed] [Google Scholar]
- 110.Lagace-Simard J, Portnoy JD, Wainberg MA. High levels of IgE in patients suffering from frequent recurrent herpes simplex lesions. Journal of Allergy and Clinical Immunology. 1986;77(4):582–585. doi: 10.1016/0091-6749(86)90349-0. [DOI] [PubMed] [Google Scholar]
- 111.Calenoff E, Zhao JC, Derlacki EL, et al. Patients with Meniere's disease possess IgE reacting with herpes family viruses. Archives of Otolaryngology: Head and Neck Surgery. 1995;121(8):861–864. doi: 10.1001/archotol.1995.01890080029005. [DOI] [PubMed] [Google Scholar]
- 112.Aceti A, Celestino D, Caferro M, et al. Basophil-bound and serum immunoglobulin E directed against Helicobacter pylori in patients with chronic gastritis. Gastroenterology. 1991;101(1):131–137. doi: 10.1016/0016-5085(91)90469-2. [DOI] [PubMed] [Google Scholar]
- 113.Bluth MH, Robin J, Ruditsky M, et al. IgE anti-Borrelia burgdorferi components (p18, p31, p34, p41, p45, p60) and increased blood CD8+CD60+ T cells in children with Lyme disease. Scandinavian Journal of Immunology. 2007;65(4):376–382. doi: 10.1111/j.1365-3083.2007.01904.x. [DOI] [PubMed] [Google Scholar]
- 114.Emre U, Sokolovskaya N, Roblin PM, Schachter J, Hammerschlag MR. Detection of anti-Chlamydia pneumoniae IgE in children with reactive airway disease. Journal of Infectious Diseases. 1995;172(1):265–267. doi: 10.1093/infdis/172.1.265. [DOI] [PubMed] [Google Scholar]
- 115.Liutu M, Kalimo K, Uksila J, Savolainen J. Extraction of ige-binding components of helicobacter pylori by immunoblotting analysis in chronic urticaria patients. International Archives of Allergy and Immunology. 2001;126(3):213–217. doi: 10.1159/000049516. [DOI] [PubMed] [Google Scholar]
- 116.Feldmesser M, Casadevall A, Kress Y, Spira G, Orlofsky A. Eosinophil-Cryptococcus neoformans interactions in vivo and in vitro. Infection and Immunity. 1997;65(5):1899–1907. doi: 10.1128/iai.65.5.1899-1907.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ishibashi K, Suzuki M, Sasaki S, Imai M. Identification of a new multigene four-transmembrane family (MS4A) related to CD20, HTm4 and β subunit of the high-affinity IgE receptor. Gene. 2001;264(1):87–93. doi: 10.1016/s0378-1119(00)00598-9. [DOI] [PubMed] [Google Scholar]
- 118.Xu H, Yan Y, Williams MS, et al. MS4a4B, a CD20 Homologue in T Cells, Inhibits T Cell Propagation by Modulation of Cell Cycle. PLoS ONE. 2010;5(11) doi: 10.1371/journal.pone.0013780. Article ID e13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li M, Wang L, Ren X, Zheng C. Host cell targets of tegument protein VP22 of herpes simplex virus 1. Archives of Virology. 2011;156(6):1079–1084. doi: 10.1007/s00705-011-0960-9. [DOI] [PubMed] [Google Scholar]
- 120.di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nature Cell Biology. 2003;5(5):410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 121.Reddy KB, Karode MC, Harmony AK, Howe PH. Interaction of transforming growth factor β receptors with apolipoprotein J/clusterin. Biochemistry. 1996;35(1):309–314. doi: 10.1021/bi951880a. [DOI] [PubMed] [Google Scholar]
- 122.Yang L, Pang Y, Moses HL. TGF-β and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends in Immunology. 2010;31(6):220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tartari CJ, Scapozza L, Gambacorti-Passerini C. The ALK gene, an attractive target for inhibitor development. Current Topics in Medicinal Chemistry. 2011;11(11):1406–1419. doi: 10.2174/156802611795589593. [DOI] [PubMed] [Google Scholar]
- 124.Lamant L, Gascoyne RD, Duplantier MM, et al. Non-muscle myosin heavy chain (MYH9): a new partner fused to ALK in anaplastic large cell lymphoma. Genes Chromosomes and Cancer. 2003;37(4):427–432. doi: 10.1002/gcc.10232. [DOI] [PubMed] [Google Scholar]
- 125.Wang N, Lan D, Gerbod-Giannone M, et al. ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. Journal of Biological Chemistry. 2003;278(44):42906–42912. doi: 10.1074/jbc.M307831200. [DOI] [PubMed] [Google Scholar]
- 126.Chan SL, Kim WS, Kwok JB, et al. ATP-binding cassette transporter A7 regulates processing of amyloid precursor protein in vitro. Journal of Neurochemistry. 2008;106(2):793–804. doi: 10.1111/j.1471-4159.2008.05433.x. [DOI] [PubMed] [Google Scholar]
- 127.Van Genderen IL, Brandimarti R, Torrisi MR, Campadelli G, Van Meer G. The phospholipid composition of extracellular herpes simplex virions differs from that of host cell nuclei. Virology. 1994;200(2):831–836. doi: 10.1006/viro.1994.1252. [DOI] [PubMed] [Google Scholar]
- 128.Steinhart WL, Nicolet CM, Howland JL. Incorporation of 32P-phosphate into membrane phospholipids during infection of cultured human fibroblasts by herpes simplex virus type 1. Intervirology. 1981;16(2):80–85. doi: 10.1159/000149251. [DOI] [PubMed] [Google Scholar]
- 129.Steinhart WL, Busch JS, Oettgen JP, Howland JL. Sphingolipid metabolism during infection of human fibroblasts by herpes simplex virus type 1. Intervirology. 1984;21(2):70–76. doi: 10.1159/000149504. [DOI] [PubMed] [Google Scholar]
- 130.Gupta VR, Wilson BA, Blanke SR. Sphingomyelin is important for the cellular entry and intracellular localization of Helicobacter pylori VacA. Cellular Microbiology. 2010;12(10):1517–1533. doi: 10.1111/j.1462-5822.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wolf K, Hackstadt T. Sphingomyelin trafficking in Chlamydia pneumoniae-infected cells. Cellular Microbiology. 2001;3(3):145–152. doi: 10.1046/j.1462-5822.2001.00098.x. [DOI] [PubMed] [Google Scholar]
- 132.Galdiero S, Falanga A, Vitiello G, et al. Role of membranotropic sequences from herpes simplex virus type I glycoproteins B and H in the fusion process. Biochimica et Biophysica Acta. 2010;1798(3):579–591. doi: 10.1016/j.bbamem.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 133.Chrisman CJ, Albuquerque P, Guimaraes AJ, Nieves E, Casadevall A. Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages. PLoS Pathogens. 2011;7(5) doi: 10.1371/journal.ppat.1002047. Article ID e1002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kielar D, Kaminski WE, Liebisch G, et al. Adenosine triphosphate binding cassette (ABC) transporters are expressed and regulated during terminal keratinocyte differentiation: a potential role for ABCA7 in epidermal lipid reorganization. Journal of Investigative Dermatology. 2003;121(3):465–474. doi: 10.1046/j.1523-1747.2003.12404.x. [DOI] [PubMed] [Google Scholar]
- 135.Hunsperger EA, Wilcox CL. Caspase-3-dependent reactivation of latent herpes simplez virus type 1 in sensory neuronal cultures. Journal of NeuroVirology. 2003;9(3):390–398. doi: 10.1080/13550280390201678. [DOI] [PubMed] [Google Scholar]
- 136.Marino J, Stoeckli I, Walch M, et al. Chlamydophila pneumoniae derived from inclusions late in the infectious cycle induce aponecrosis in human aortic endothelial cells. BMC Microbiology. 2008;8, article 32 doi: 10.1186/1471-2180-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Srinivas RV, Venkatachalapathi YV, Rui Z, et al. Inhibition of virus-induced cell fusion by apolipoprotein A-I and its amphipathic peptide analogs. Journal of Cellular Biochemistry. 1991;45(2):224–237. doi: 10.1002/jcb.240450214. [DOI] [PubMed] [Google Scholar]
- 138.Tanaka N, Abe-Dohmae S, Iwamoto N, Yokoyama S. Roles of ATP-binding cassette transporter A7 in cholesterol homeostasis and host defense system. Journal of Atherosclerosis and Thrombosis. 2011;18:274–281. doi: 10.5551/jat.6726. [DOI] [PubMed] [Google Scholar]
- 139.Burgos JS, Ramirez C, Sastre I, Valdivieso F. Apolipoprotein E genotype influences vertical transmission of herpes simplex virus type 1 in a gender specific manner. Aging Cell. 2007;6(6):841–842. doi: 10.1111/j.1474-9726.2007.00332.x. [DOI] [PubMed] [Google Scholar]
- 140.Cun W, Jiang J, Luo G. The C-terminal α-helix domain of apolipoprotein E is required for interaction with nonstructural protein 5A and assembly of hepatitis C virus. Journal of Virology. 2010;84(21):11532–11541. doi: 10.1128/JVI.01021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hishiki T, Shimizu Y, Tobita R, et al. Infectivity of hepatitis C virus is influenced by association with apolipoprotein E isoforms. Journal of Virology. 2010;84(22):12048–12057. doi: 10.1128/JVI.01063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Turchan-Cholewo J, Liu Y, Gartner S, et al. Increased vulnerability of ApoE4 neurons to HIV proteins and opiates: protection by diosgenin and l-deprenyl. Neurobiology of Disease. 2006;23(1):109–119. doi: 10.1016/j.nbd.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 143.Lin WR, Wozniak MA, Esiri MM, Klenerman P, Itzhaki RF. Herpes simplex encephalitis: involvement of apolipoprotein E genotype. Journal of Neurology Neurosurgery and Psychiatry. 2001;70(1):117–119. doi: 10.1136/jnnp.70.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gérard HC, Fomicheva E, Whittum-Hudson JA, Hudson AP. Apolipoprotein E4 enhances attachment of Chlamydophila (Chlamydia) pneumoniae elementary bodies to host cells. Microbial Pathogenesis. 2008;44(4):279–285. doi: 10.1016/j.micpath.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 145.Brown CM, Choi E, Xu Q, Vitek MP, Colton CA. The APOE4 genotype alters the response of microglia and macrophages to 17β-estradiol. Neurobiology of Aging. 2008;29(12):1783–1794. doi: 10.1016/j.neurobiolaging.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vitek MP, Brown CM, Colton CA. APOE genotype-specific differences in the innate immune response. Neurobiology of Aging. 2009;30(9):1350–1360. doi: 10.1016/j.neurobiolaging.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Haan MN, Aiello AE, West NA, Jagust WJ. C-reactive protein and rate of dementia in carriers and non carriers of Apolipoprotein APOE4 genotype. Neurobiology of Aging. 2008;29(12):1774–1782. doi: 10.1016/j.neurobiolaging.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thompson D, Pepys MB, Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure. 1999;7(2):169–177. doi: 10.1016/S0969-2126(99)80023-9. [DOI] [PubMed] [Google Scholar]
- 149.de Bont N, Netea MG, Demacker PN, et al. Apolipoprotein E knock-out mice are highly susceptible to endotoxemia and Klebsiella pneumoniae infection. Journal of Lipid Research. 1999;40(4):680–685. [PubMed] [Google Scholar]
- 150.Alber DG, Powell KL, Vallance P, Goodwin DA, Grahame-Clarke C. Herpesvirus infection accelerates atherosclerosis in the apolipoprotein E-deficient mouse. Circulation. 2000;102(7):779–785. doi: 10.1161/01.cir.102.7.779. [DOI] [PubMed] [Google Scholar]
- 151.Ayada K, Yokota K, Kobayashi K, Shoenfeld Y, Matsuura E, Oguma K. Chronic infections and atherosclerosis. Annals of the New York Academy of Sciences. 2007;1108:594–602. doi: 10.1196/annals.1422.062. [DOI] [PubMed] [Google Scholar]
- 152.Ezzahiri R, Stassen FR, Kurvers HA, van Pul MM, Kitslaar PJ, Bruggeman CA. Chlamydia pneumoniae infection induces an unstable atherosclerotic plaque phenotype in LDL-receptor, ApoE double knockout mice. European Journal of Vascular and Endovascular Surgery. 2003;26(1):88–95. doi: 10.1053/ejvs.2002.1913. [DOI] [PubMed] [Google Scholar]
- 153.Hsich E, Zhou YF, Paigen B, Johnson TM, Burnett MS, Epstein SE. Cytomegalovirus infection increases development of atherosclerosis in Apolipoprotein-E knockout mice. Atherosclerosis. 2001;156(1):23–28. doi: 10.1016/s0021-9150(00)00608-0. [DOI] [PubMed] [Google Scholar]
- 154.Li L, Messas E, Batista EL, Jr., Levine RA, Amar S. Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model. Circulation. 2002;105(7):861–867. doi: 10.1161/hc0702.104178. [DOI] [PubMed] [Google Scholar]
- 155.Naghavi M, Wyde P, Litovsky S, et al. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation. 2003;107(5):762–768. doi: 10.1161/01.cir.0000048190.68071.2b. [DOI] [PubMed] [Google Scholar]
- 156.Portugal LR, Fernandes LR, Cesar GC, et al. Infection with Toxoplasma gondii increases atherosclerotic lesion in ApoE-deficient mice. Infection and Immunity. 2004;72(6):3571–3576. doi: 10.1128/IAI.72.6.3571-3576.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ayada K, Yokota K, Hirai K, et al. Regulation of cellular immunity prevents Helicobacter pylori-induced atherosclerosis. Lupus. 2009;18(13):1154–1168. doi: 10.1177/0961203309106600. [DOI] [PubMed] [Google Scholar]
- 158.Warner MS, Geraghty RJ, Martinez WM, et al. A cell surface protein with herpesvirus entry activity (Hveb) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology. 1998;246(1):179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 159.Derré I, Pypaert M, Dautry-Varsat A, Agaisse H. RNAi screen in Drosophila cells reveals the involvement of the tom complex in Chlamydia infection. PLoS Pathogens. 2007;3(10):1446–1458. doi: 10.1371/journal.ppat.0030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Harland J, Dunn P, Cameron E, Conner J, Brown SM. The herpes simplex virus (HSV) protein ICP34.5 is a virion component that forms a DNA-binding complex with proliferating cell nuclear antigen and HSV replication proteins. Journal of NeuroVirology. 2003;9(4):477–488. doi: 10.1080/13550280390218788. [DOI] [PubMed] [Google Scholar]
- 161.Lupo A, Cesaro E, Montano G, Izzo P, Costanzo P. ZNF224: structure and role of a multifunctional KRAB-ZFP protein. International Journal of Biochemistry and Cell Biology. 2011;43(4):470–473. doi: 10.1016/j.biocel.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 162.Yu J, Shin B, Park ES, et al. Protein arginine methyltransferase 1 regulates herpes simplex virus replication through ICP27 RGG-box methylation. Biochemical and Biophysical Research Communications. 2010;391(1):322–328. doi: 10.1016/j.bbrc.2009.11.057. [DOI] [PubMed] [Google Scholar]
- 163.Kamiya A, Kubo K, Tomoda T, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nature Cell Biology. 2005;7(12):1067–1078. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- 164.Janke C, Rogowski K, Wloga D, et al. Biochemistry: tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science. 2005;308(5729):1758–1762. doi: 10.1126/science.1113010. [DOI] [PubMed] [Google Scholar]
- 165.Gee P, Ando Y, Kitayama H, et al. APOBEC1-mediated editing and attenuation of HSV-1 DNA implicates an antiviral role in neurons during encephalitis. The Journal of Virology. 2011;85(19):9726–9736. doi: 10.1128/JVI.05288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bose SK, Gibson W, Bullard RS, Donald CD. PAX2 oncogene negatively regulates the expression of the host defense peptide human beta defensin-1 in prostate cancer. Molecular Immunology. 2009;46(6):1140–1148. doi: 10.1016/j.molimm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tesse R, Santoro N, Giordano P, Cardinale F, Mattia DD, Armenio L. Association between defb1 gene haplotype and herpes viruses seroprevalence in children with acute lymphoblastic leukemia. Pediatric Hematology and Oncology. 2009;26(8):573–582. doi: 10.3109/08880010903271705. [DOI] [PubMed] [Google Scholar]
- 168.Kocsis AK, Kiss ZF, Tiszlavicz L, Tiszlavicz Z, Mandi Y. Potential role of human beta-defensin 1 in Helicobacter pylori-induced gastritis. Scandinavian Journal of Gastroenterology. 2009;44:289–295. doi: 10.1080/00365520802530879. [DOI] [PubMed] [Google Scholar]
- 169.Wiechula B, Cholewa K, Ekiel A, Romanik M, Dolezych H, Martirosian G. HBD-1 and hBD-2 are expressed in cervico-vaginal lavage in female genital tract due to microbial infections. Ginekologia Polska. 2010;81(4):268–271. [PubMed] [Google Scholar]
- 170.Circo R, Skerlavaj B, Gennaro R, Amoroso A, Zanetti M. Structural and functional characterization of hBD-1(Ser35), a peptide deduced from a DEFB1 polymorphism. Biochemical and Biophysical Research Communications. 2002;293(1):586–592. doi: 10.1016/S0006-291X(02)00267-X. [DOI] [PubMed] [Google Scholar]
- 171.Meyer P, Caillat C, Topalis D, Balzarini J, Deville-Bonne D. Structural basis for the specificity of thymidylate kinases from human pathogens: implications for nucleotide analogues activation. Nucleic Acids Symposium Series. 2009;(53):p. 41. doi: 10.1093/nass/nrp021. [DOI] [PubMed] [Google Scholar]
- 172.Sawayama Y, Tatsukawa M, Maeda S, Ohnishi H, Furusyo N, Hayashi J. Association of hyperhomocysteinemia and Chlamydia pneumoniae infection with carotid atherosclerosis and coronary artery disease in Japanese patients. Journal of Infection and Chemotherapy. 2008;14(3):232–237. doi: 10.1007/s10156-008-0607-2. [DOI] [PubMed] [Google Scholar]
- 173.Matsui T. Helicobacter pylori and Arteriosclerosis. Gan To Kagaku Ryoho. 2011;38(3):365–369. [PubMed] [Google Scholar]
- 174.Huemer HP, Menzel HJ, Potratz D, et al. Herpes simplex virus binds to human serum lipoprotein. Intervirology. 1988;29(2):68–76. doi: 10.1159/000150031. [DOI] [PubMed] [Google Scholar]
- 175.Vieira SI, Rebelo S, Esselmann H, et al. Retrieval of the Alzheimer's amyloid precursor protein from the endosome to the TGN is S655 phosphorylation state-dependent and retromer-mediated. Molecular Neurodegeneration. 2010;5(1, article 40) doi: 10.1186/1750-1326-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marquez-Sterling NR, Lo AC, Sisodia SS, Koo EH. Trafficking of cell-surface β-amyloid precursor protein: evidence that a sorting intermediate participates in synaptic vesicle recycling. Journal of Neuroscience. 1997;17(1):140–151. doi: 10.1523/JNEUROSCI.17-01-00140.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Song MS, Baker GB, Todd KG, Kar S. Inhibition of beta-amyloid1-42 internalization attenuates neuronal death by stabilizing the endosomal-lysosomal system in rat cortical cultured neurons. Neuroscience. 2011;178:181–188. doi: 10.1016/j.neuroscience.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 178.Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble aβ through fluid phase macropinocytosis. Journal of Neuroscience. 2009;29(13):4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wu F, Matsuoka Y, Mattson MP, Yao PJ. The clathrin assembly protein AP180 regulates the generation of amyloid-β peptide. Biochemical and Biophysical Research Communications. 2009;385(2):247–250. doi: 10.1016/j.bbrc.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cirrito JR, Kang JE, Lee J, et al. Endocytosis is required for synaptic activity-dependent release of amyloid-β in vivo. Neuron. 2008;58(1):42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hussain MM. Structural, biochemical and signaling properties of the low-density lipoprotein receptor gene family. Frontiers in Bioscience. 2001;6:D417–D428. doi: 10.2741/hussain1. [DOI] [PubMed] [Google Scholar]
- 182.Dieckmann M, Dietrich MF, Herz J. Lipoprotein receptors-an evolutionarily ancient multifunctional receptor family. Biological Chemistry. 2010;391(11):1341–1363. doi: 10.1515/BC.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Deuss M, Reiss K, Hartmann D. Part-time α-secretases: the functional biology of ADAM 9, 10 and 17. Current Alzheimer Research. 2008;5(2):187–201. doi: 10.2174/156720508783954686. [DOI] [PubMed] [Google Scholar]
- 184.Sato C, Zhao G, Ilagan MX. An overview of notch signaling in adult tissue renewal and maintenance. doi: 10.2174/156720512799361600. Current Alzheimer Research. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fuchs BB, Tang RJ, Mylonakis E. The temperature-sensitive role of Cryptococcus neoformans ROM2 in cell morphogenesis. PLoS ONE. 2007;2(4, article e368) doi: 10.1371/journal.pone.0000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mital J, Hackstadt T. Diverse requirements for SRC-family tyrosine kinases distinguish chlamydial species. MBio. 2011;2(2) doi: 10.1128/mBio.00031-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pai R, Cover TL, Tarnawski AS. Helicobacter pylori vacuolating cytotoxin (VacA) disorganizes the cytoskeletal architecture of gastric epithelial cells. Biochemical and Biophysical Research Communications. 1999;262(1):245–250. doi: 10.1006/bbrc.1999.1194. [DOI] [PubMed] [Google Scholar]
- 188.Takeuchi H, Furuta N, Morisaki I, Amano A. Exit of intracellular Porphyromonas gingivalis from gingival epithelial cells is mediated by endocytic recycling pathway. Cellular Microbiology. 2011;13(5):677–691. doi: 10.1111/j.1462-5822.2010.01564.x. [DOI] [PubMed] [Google Scholar]
- 189.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proceedings of the National Academy of Sciences of the United States of America. 1975;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dolan PJ, Johnson GV. The role of tau kinases in Alzheimer's disease. Current Opinion in Drug Discovery and Development. 2010;13(5):595–603. [PMC free article] [PubMed] [Google Scholar]
- 191.Zambrano Á, Solis L, Salvadores N, Cortés M, Lerchundi R, Otth C. Neuronal cytoskeletal dynamic modification and neurodegeneration induced by infection with herpes simplex virus type 1. Journal of Alzheimer's Disease. 2008;14(3):259–269. doi: 10.3233/jad-2008-14301. [DOI] [PubMed] [Google Scholar]
- 192.Carter CJ. Alzheimer's disease: a pathogenetic autoimmune disorder caused by herpes simplex in a gene-dependent manner. International Journal of Alzheimer's Disease. 2010, article 140539 doi: 10.4061/2010/140539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cheng SB, Ferland P, Webster P, Bearer EL. Herpes simplex virus dances with amyloid precursor protein while exiting the cell. PLoS ONE. 2011;6(3) doi: 10.1371/journal.pone.0017966. Article ID e17966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Benboudjema L, Mulvey M, Gao Y, Pimplikar SW, Mohr I. Association of the herpes simplex virus type 1 Us11 gene product with the cellular kinesin light-chain-related protein PAT1 results in the redistribution of both polypeptides. Journal of Virology. 2003;77(17):9192–9203. doi: 10.1128/JVI.77.17.9192-9203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zheng P, Eastman J, Vande PS, Pimplikar SW. PAT1, a microtubule-interacting protein, recognizes the basolateral sorting signal of amyloid precursor protein. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(25):14745–14750. doi: 10.1073/pnas.95.25.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Soscia SJ, Kirby JE, Washicosky KJ, et al. The Alzheimer's disease-associated amyloid β-protein is an antimicrobial peptide. PLoS ONE. 2010;5(3) doi: 10.1371/journal.pone.0009505. Article ID e9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lukiw WJ, Cui JG, Yuan LY, et al. Acyclovir or Aβ42 peptides attenuate HSV-1-induced miRNA-146a levels in human primary brain cells. NeuroReport. 2010;21(14):922–927. doi: 10.1097/WNR.0b013e32833da51a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer's disease: amyloid-β oligomers trigger innate immunity defence via pattern recognition receptors. Progress in Neurobiology. 2009;87(3):181–194. doi: 10.1016/j.pneurobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 199.Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Aβ uptake and clearance. Brain. 2006;129(11):3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sohn J-H, So JO, Hong HJ, et al. Identification of autoantibody against beta-amyloid peptide in the serum of elderly. Frontiers in Bioscience. 2009;14(10):3879–3883. doi: 10.2741/3496. [DOI] [PubMed] [Google Scholar]
- 201.Lleó A, Saura CA. γ-secretase substrates and their implications for drug development in Alzheimer's disease. Current Topics in Medicinal Chemistry. 2011;11(12):1513–1527. doi: 10.2174/156802611795861004. [DOI] [PubMed] [Google Scholar]
- 202.Takeuchi O, Akira S. Innate immunity to virus infection. Immunological Reviews. 2009;227(1):75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zanoni I, Granucci F. Regulation of antigen uptake, migration, and lifespan of dendritic cell by Toll-like receptors. Journal of Molecular Medicine. 2010;88(9):873–880. doi: 10.1007/s00109-010-0638-x. [DOI] [PubMed] [Google Scholar]
- 204.Kim DY, Ingano LA, Kovacs DM. Nectin-1α, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/γ-secretase-like cleavage. Journal of Biological Chemistry. 2002;277(51):49976–49981. doi: 10.1074/jbc.M210179200. [DOI] [PubMed] [Google Scholar]
- 205.Hemming ML, Elias JE, Gygi SP, Selkoe DJ. Proteomic profiling of gamma-secretase substrates and mapping of substrate requirements. PLoS Biology. 2008;6(10):p. e257. doi: 10.1371/journal.pbio.0060257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bacsa S, Karasneh G, Dosa S, Liu J, Valyi-Nagy T, Shukla D. Syndecan-1 and syndecan-2 play key roles in herpes simplex virus type-1 infection. Journal of General Virology. 2011;92(4):733–743. doi: 10.1099/vir.0.027052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kalia M, Chandra V, Rahman SA, Sehgal D, Jameel S. Heparan sulfate proteoglycans are required for cellular binding of the hepatitis E virus ORF2 capsid protein and for viral infection. Journal of Virology. 2009;83(24):12714–12724. doi: 10.1128/JVI.00717-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shafti-Keramat S, Handisurya A, Kriehuber E, Meneguzzi G, Slupetzky K, Kirnbauer R. Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. Journal of Virology. 2003;77(24):13125–13135. doi: 10.1128/JVI.77.24.13125-13135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Smith AJ, Schacker TW, Reilly CS, Haase AT. A role for syndecan-1 and claudin-2 in microbial translocation during HIV-1 infection. Journal of Acquired Immune Deficiency Syndromes. 2010;55(3):306–315. doi: 10.1097/QAI.0b013e3181ecfeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.de Witte L, Bobardt M, Chatterji U, et al. Syndecan-3 is a dendritic cell-specific attachment receptor for HIV-1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(49):19464–19469. doi: 10.1073/pnas.0703747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.de Witte L, Zoughlami Y, Aengeneyndt B, et al. Binding of human papilloma virus L1 virus-like particles to dendritic cells is mediated through heparan sulfates and induces immune activation. Immunobiology. 2008;212(9-10):679–691. doi: 10.1016/j.imbio.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 212.Wilke GA, Bubeck WJ. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus α-hemolysin—mediated cellular injury. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(30):13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jong A, Wu CH, Shackleford GM, et al. Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells. Cellular Microbiology. 2008;10(6):1313–1326. doi: 10.1111/j.1462-5822.2008.01128.x. [DOI] [PubMed] [Google Scholar]
- 214.Persson BD, Schmitz NB, Santiago C, et al. Structure of the extracellular portion of CD46 provides insights into its interactions with complement proteins and pathogens. PLoS Pathogens. 2010;6(9) doi: 10.1371/journal.ppat.1001122. Article ID e01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mahtout H, Chandad F, Rojo JM, Grenier D. Porphyromonas gingivalis mediates the shedding and proteolysis of complement regulatory protein CD46 expressed by oral epithelial cells. Oral Microbiology and Immunology. 2009;24(5):396–400. doi: 10.1111/j.1399-302X.2009.00532.x. [DOI] [PubMed] [Google Scholar]
- 216.Wang H, Li Z-Y, Liu Y, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nature Medicine. 2011;17(1):96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hofer F, Gruenberger M, Kowalski H, et al. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(5):1839–1842. doi: 10.1073/pnas.91.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ronacher B, Marlovits TC, Moser R, Blaas D. Expression and folding of human very-low-density lipoprotein receptor fragments: neutralization capacity toward human rhinovirus HRV2. Virology. 2000;278(2):541–550. doi: 10.1006/viro.2000.0636. [DOI] [PubMed] [Google Scholar]
- 219.Jahan S, Khaliq S, Samreen B, et al. Effect of combined siRNA of HCV E2 gene and HCV receptors against HCV. Virology Journal. 2011;8, article 295 doi: 10.1186/1743-422X-8-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Anderson LJ, Longnecker R. Epstein-Barr virus latent membrane protein 2A exploits Notch1 to alter B-cell identity in vivo. Blood. 2009;113(1):108–116. doi: 10.1182/blood-2008-06-160937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kusano S, Raab-Traub N. An Epstein-Barr virus protein interacts with Notch. Journal of Virology. 2001;75(1):384–395. doi: 10.1128/JVI.75.1.384-395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tzahar E, Moyer JD, Waterman H, et al. Pathogenic poxviruses reveal viral strategies to exploit the ErbB signaling network. EMBO Journal. 1998;17(20):5948–5963. doi: 10.1093/emboj/17.20.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tuffereau C, Bénéjean J, Blondel D, Kieffer B, Flamand A. Low-affinity nerve-growth factor receptor (P75NTR) can serve as a receptor for rabies virus. EMBO Journal. 1998;17(24):7250–7259. doi: 10.1093/emboj/17.24.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bowden TA, Aricescu AR, Gilbert RJ, Grimes JM, Jones EY, Stuart DI. Structural basis of Nipah and Hendra virus attachment to their cell-surface receptor ephrin-B2. Nature Structural and Molecular Biology. 2008;15(6):567–572. doi: 10.1038/nsmb.1435. [DOI] [PubMed] [Google Scholar]
- 225.Orentas RJ, Hildreth JE. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Research and Human Retroviruses. 1993;9(11):1157–1165. doi: 10.1089/aid.1993.9.1157. [DOI] [PubMed] [Google Scholar]
- 226.Rothwell SW, Wright DG. Characterization of influenza A virus binding sites on human neutrophils. Journal of Immunology. 1994;152(5):2358–2367. [PubMed] [Google Scholar]
- 227.Pericolini E, Gabrielli E, Cenci E, et al. Involvement of glycoreceptors in galactoxylomannan-induced T cell death. Journal of Immunology. 2009;182(10):6003–6010. doi: 10.4049/jimmunol.0803833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Vomaske J, Melnychuk RM, Smith PP, et al. Differential ligand binding to a human cytomegalovirus chemokine receptor determines cell type-specific motility. PLoS Pathogens. 2009;5(2) doi: 10.1371/journal.ppat.1000304. Article ID e1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Minami M, Kume N, Shimaoka T, et al. Expression of SR-PSOX, a novel cell-surface scavenger receptor for phosphatidylserine and oxidized LDL in human atherosclerotic lesions. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(11):1796–1800. doi: 10.1161/hq1001.096652. [DOI] [PubMed] [Google Scholar]
- 230.Geiger O, González-Silva N, López-Lara IM, Sohlenkamp C. Amino acid-containing membrane lipids in bacteria. Progress in Lipid Research. 2010;49(1):46–60. doi: 10.1016/j.plipres.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 231.Cao W, Henry MD, Borrow P, et al. Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282(5396):2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 232.Sevilla N, Kunz S, Holz A, et al. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. Journal of Experimental Medicine. 2000;192(9):1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sasaki S, Takeshita F, Okuda K, Ishii N. Mycobacterium leprae and leprosy: a compendium. Microbiology and Immunology. 2001;45(11):729–736. doi: 10.1111/j.1348-0421.2001.tb01308.x. [DOI] [PubMed] [Google Scholar]
- 234.Boelen E, Stassen FR, van der Ven AJ, et al. Detection of amyloid beta aggregates in the brain of BALB/c mice after Chlamydia pneumoniae infection. Acta Neuropathologica. 2007;114(3):255–261. doi: 10.1007/s00401-007-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Miklossy J, Kis A, Radenovic A, et al. β-amyloid deposition and Alzheimer's type changes induced by Borrelia spirochetes. Neurobiology of Aging. 2006;27(2):228–236. doi: 10.1016/j.neurobiolaging.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 236.Piacentini R, Civitelli L, Ripoli C, et al. HSV-1 promotes Ca2+-mediated APP phosphorylation and Aβ accumulation in rat cortical neurons. Neurobiology of Aging. 2011;32(12):2323.e13–2323.e26. doi: 10.1016/j.neurobiolaging.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 237.Elkind MS. Infectious burden: a new risk factor and treatment target for atherosclerosis. Infectious Disorders. 2010;10(2):84–90. doi: 10.2174/187152610790963519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kanduc D. Describing the hexapeptide identity platform between the influenza A H5N1 and Homo sapiens proteomes. Biologicals. 2010;4:245–261. doi: 10.2147/btt.s12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kanduc D, Stufano A, Lucchese G, Kusalik A. Massive peptide sharing between viral and human proteomes. Peptides. 2008;29(10):1755–1766. doi: 10.1016/j.peptides.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ricco R, Kanduc D. Hepatitis B virus and Homo sapiens proteome-wide analysis: a profusion of viral peptide overlaps in neuron-specific human proteins. Biologics. 2010;(4):75–81. doi: 10.2147/btt.s8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Trost B, Lucchese G, Stufano A, Bickis M, Kusalik A, Kanduc D. No human protein is exempt from bacterial motifs, not even one. Self/Nonself. 2010;1(4):328–334. doi: 10.4161/self.1.4.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pardridge WM. Re-engineering biopharmaceuticals for delivery to brain with molecular Trojan horses. Bioconjugate Chemistry. 2008;19(7):1327–1338. doi: 10.1021/bc800148t. [DOI] [PubMed] [Google Scholar]
- 243.Grammas P, Martinez J, Miller B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Reviews in Molecular Medicine. 2011;13 doi: 10.1017/S1462399411001918. Article ID e19. [DOI] [PubMed] [Google Scholar]
- 244.Baravalle G, Brabec M, Snyers L, Blaas D, Fuchs R. Human Rhinovirus Type 2-Antibody Complexes Enter and Infect Cells via Fc-γ Receptor IIB1. Journal of Virology. 2004;78(6):2729–2737. doi: 10.1128/JVI.78.6.2729-2737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21) Proceedings of the National Academy of Sciences of the United States of America. 2010;107(46):19985–19990. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jaume M, Yip MS, Cheung CY, et al. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway. The Journal of Virology. 2011;85(20):10582–10597. doi: 10.1128/JVI.00671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Li YN, Qin XJ, Kuang F, et al. Alterations of Fc γ receptor I and Toll-like receptor 4 mediate the antiinflammatory actions of microglia and astrocytes after adrenaline-induced blood-brain barrier opening in rats. Journal of Neuroscience Research. 2008;86(16):3556–3565. doi: 10.1002/jnr.21810. [DOI] [PubMed] [Google Scholar]
- 248.Carter CJ. Epstein-Barr and other viral mimicry of autoantigens, myelin and vitamin D related proteins, and of EIF2B, the cause of vanishing white matter disease: massive mimicry of multiple sclerosis relevant proteins by the Synechococcus phage. doi: 10.3109/08923973.2011.572262. Immunopharmacology and Immunotoxicology. In press. [DOI] [PubMed] [Google Scholar]
- 249.Carter CJ. Pathogen and autoantigen homologous regions within the cystic fibrosis transmembrane conductance regulator (CFTR) protein suggest an autoimmune treatable component of cystic fibrosis. FEMS Immunology & Medical Microbiology. 2011;62(2):197–214. doi: 10.1111/j.1574-695X.2011.00803.x. [DOI] [PubMed] [Google Scholar]
- 250.Carter CJ. Schizophrenia: a pathogenetic autoimmune disease caused by viruses and pathogens and dependent on genes. Journal of Pathogens. 2011;2011:37 pages. doi: 10.4061/2011/128318. Article ID 128318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Espay AJ, Henderson KK. Postencephalitic parkinsonism and basal ganglia necrosis due to Epstein-Barr virus infection. Neurology. 2011;76(17):1529–1530. doi: 10.1212/WNL.0b013e318217e7dd. [DOI] [PubMed] [Google Scholar]
- 252.Roselli F, Russo I, Fraddosio A, et al. Reversible Parkinsonian syndrome associated with anti-neuronal antibodies in acute EBV encephalitis: a case report. Parkinsonism and Related Disorders. 2006;12(4):257–260. doi: 10.1016/j.parkreldis.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 253.Woulfe J, Hoogendoorn H, Tarnopolsky M, Munoz DG. Monoclonal antibodies against Epstein-Barr virus cross-react with alpha-synuclein in human brain. Neurology. 2000;55(9):1398–1401. doi: 10.1212/wnl.55.9.1398. [DOI] [PubMed] [Google Scholar]
- 254.Carter CJ. Extensive viral mimicry of 22 AIDS-related autoantigens by HIV-1 proteins and pathway analysis of 561 viral/human homologues suggest an initial treatable autoimmune component of AIDS. FEMS Immunology & Medical Microbiology. 2011;63(2):254–268. doi: 10.1111/j.1574-695X.2011.00848.x. [DOI] [PubMed] [Google Scholar]
- 255.Kanduc D. The self/nonself issue a confrontation between proteomes. Self/Nonself. 2010;1(3):255–258. doi: 10.4161/self.1.3.11897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Jones L, Holmans PA, Hamshere ML, et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer's disease. PLoS ONE. 2010;5(11) doi: 10.1371/journal.pone.0013950. Article ID e13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Phillips D, Prentice L, Upadhyaya M, et al. Autosomal dominant inheritance of autoantibodies to thyroid peroxidase and thyroglobulin—studies in families not selected for autoimmune thyroid disease. Journal of Clinical Endocrinology and Metabolism. 1991;72(5):973–975. doi: 10.1210/jcem-72-5-973. [DOI] [PubMed] [Google Scholar]
- 258.Zuckerman NS, Hazanov H, Barak M, et al. Somatic hypermutation and antigen-driven selection of B cells are altered in autoimmune diseases. Journal of Autoimmunity. 2010;35(4):325–335. doi: 10.1016/j.jaut.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 259.Rho JH, Zhang W, Murali M, Roehrl MH, Wang JY. Human proteins with affinity for dermatan sulfate have the propensity to become autoantigens. American Journal of Pathology. 2011;178(5):2177–2190. doi: 10.1016/j.ajpath.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wadström T, Ljungh Å. Glycosaminoglycan-binding microbial proteins in tissue adhesion and invasion: key events in microbial pathogenicity. Journal of Medical Microbiology. 1999;48(3):223–233. doi: 10.1099/00222615-48-3-223. [DOI] [PubMed] [Google Scholar]
- 261.Rosenmann H, Grigoriadis N, Karussis D, et al. Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Archives of Neurology. 2006;63(10):1459–1467. doi: 10.1001/archneur.63.10.1459. [DOI] [PubMed] [Google Scholar]
- 262.Capsoni S, Ugolini G, Comparini A, Ruberti F, Berardi N, Cattaneo A. Alzheimer-like neurodegeneration in aged antinerve growth factor transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(12):6826–6831. doi: 10.1073/pnas.97.12.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ferrer I, Boada RM, Sánchez Guerra ML, Rey MJ, Costa-Jussà F. Neuropathology and pathogenesis of encephalitis following amyloid-β immunization in Alzheimer's disease. Brain Pathology. 2004;14(1):11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Furlan R, Brambilla E, Sanvito F, et al. Vaccination with amyloid-β peptide induces autoimmune encephalomyelitis in C57/BL6 mice. Brain. 2003;126(2):285–291. doi: 10.1093/brain/awg031. [DOI] [PubMed] [Google Scholar]
- 265.Rozemuller AJ, van Gool WA, Eikelenboom P. The neuroinflammatory response in plaques and amyloid angiopathy in Alzheimer's disease: therapeutic implications. Current Drug Targets. 2005;4(3):223–233. doi: 10.2174/1568007054038229. [DOI] [PubMed] [Google Scholar]
- 266.Vacirca D, Barbati C, Scazzocchio B, et al. Anti-ATP synthase autoantibodies from patients with Alzheimer's disease reduce extracellular HDL level. Journal of Alzheimer's Disease. 2011;26(3):441–445. doi: 10.3233/JAD-2011-110350. [DOI] [PubMed] [Google Scholar]
- 267.Vacirca D, Delunardo F, Matarrese P, et al. Autoantibodies to the adenosine triphosphate synthase play a pathogenetic role in Alzheimer's disease. doi: 10.1016/j.neurobiolaging.2010.05.013. Neurobiology of Aging. In press. [DOI] [PubMed] [Google Scholar]
- 268.Foley P, Bradford HF, Docherty M, et al. Evidence for the presence of antibodies to cholinergic neurons in the serum of patients with Alzheimer's disease. Journal of Neurology. 1988;235(8):466–471. doi: 10.1007/BF00314249. [DOI] [PubMed] [Google Scholar]
- 269.Nagele RG, Clifford PM, Siu G, et al. Brain-reactive autoantibodies prevalent in human sera increase intraneuronal amyloid-γ1-42 deposition. Journal of Alzheimer's Disease. 2011;25(4):605–622. doi: 10.3233/JAD-2011-110098. [DOI] [PubMed] [Google Scholar]
- 270.Koval L, Lykhmus O, Kalashnyk O, et al. The presence and origin of autoantibodies against α4 and α7 nicotinic acetylcholine receptors in the human blood: possible relevance to Alzheimer's pathology. Journal of Alzheimer's Disease. 2011;25(4):747–761. doi: 10.3233/JAD-2011-101845. [DOI] [PubMed] [Google Scholar]
- 271.Mruthinti S, Buccafusco JJ, Hill WD, et al. Autoimmunity in Alzheimer's disease: increased levels of circulating IgGs binding Aβ and RAGE peptides. Neurobiology of Aging. 2004;25(8):1023–1032. doi: 10.1016/j.neurobiolaging.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 272.Gruden MA, Davidova TB, Mališauskas M, et al. Differential neuroimmune markers to the onset of Alzheimer's disease neurodegeneration and dementia: autoantibodies to Aβ(25-35) oligomers, S100b and neurotransmitters. Journal of Neuroimmunology. 2007;186(1-2):181–192. doi: 10.1016/j.jneuroim.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 273.Kettlewell HBD. Selection experimants on industrial melanism in the Lepidoptera . Heredity. 1955;9:323–342. [Google Scholar]
- 274.Burgos JS, Ramirez C, Sastre I, Valdivieso F. Effect of apolipoprotein E on the cerebral load of latent herpes simplex virus type 1 DNA. Journal of Virology. 2006;80(11):5383–5387. doi: 10.1128/JVI.00006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Li S, Carpenter D, Hsiang C, Wechsler SL, Jones C. Herpes simplex virus type 1 latency-associated transcript inhibits apoptosis and promotes neurite sprouting in neuroblastoma cells following serum starvation by maintaining protein kinase B (AKT) levels. Journal of General Virology. 2010;91(4):858–866. doi: 10.1099/vir.0.015719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Wilcox CL, Johnson EM., Jr. Nerve growth factor deprivation results in the reactivation of latent herpes simplex virus in vitro. Journal of Virology. 1987;61(7):2311–2315. doi: 10.1128/jvi.61.7.2311-2315.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Camarena V, Kobayashi M, Kim JY, et al. Nature and duration of growth factor signaling through receptor tyrosine kinases regulates HSV-1 latency in neurons. Cell Host and Microbe. 2010;8(4):320–330. doi: 10.1016/j.chom.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Counts SE, Nadeem M, Wuu J, Ginsberg SD, Saragovi HU, Mufson EJ. Reduction of cortical TrkA but not p75NTR protein in early-stage Alzheimer's disease. Annals of Neurology. 2004;56(4):520–531. doi: 10.1002/ana.20233. [DOI] [PubMed] [Google Scholar]
- 279.Kheirvari S, Uezu K, Yamamoto S, Nakaya Y. High-dose dietary supplementation of vitamin A induces brain-derived neurotrophic factor and nerve growth factor production in mice with simultaneous deficiency of vitamin A and zinc. Nutritional Neuroscience. 2008;11(5):228–234. doi: 10.1179/147683008X301603. [DOI] [PubMed] [Google Scholar]
- 280.Jiang B, Liao EY, Tan LM, Dai RC, Xiao ZJ, Liao HJ. Effects of long-term replacement therapy of compound nylestriol tablet or low-dose 17 beta-estradiol on the expression of nerve growth factor in OVX rat hippocampal formation. Zhong Nan Da Xue Xue Bao. Yi Xue Ban. 2004;29(5):529–533. [PubMed] [Google Scholar]
- 281.Vicetti Miguel RD, Sheridan BS, Harvey SAK, Schreiner RS, Hendricks RL, Cherpes TL. 17-β estradiol promotion of herpes simplex virus type 1 reactivation is estrogen receptor dependent. Journal of Virology. 2010;84(1):565–572. doi: 10.1128/JVI.01374-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Geerlings MI, Launer LJ, de Jong FH, et al. Endogenous estradiol and risk of dementia in women and men: the Rotterdam study. Annals of Neurology. 2003;53(5):607–615. doi: 10.1002/ana.10521. [DOI] [PubMed] [Google Scholar]
- 283.Ravaglia G, Forti P, Maioli F, et al. Endogenous sex hormones as risk factors for dementia in elderly men and women. Journals of Gerontology. 2007;62(9):1035–1041. doi: 10.1093/gerona/62.9.1035. [DOI] [PubMed] [Google Scholar]
- 284.Clement C, Bhattacharjee PS, Kaufman HE, Hill JM. Heat-induced reactivation of HSV-1 in latent mice: upregulation in the TG of CD83 and other immune response genes and their LAT-ICP0 locus. Investigative Ophthalmology and Visual Science. 2009;50(6):2855–2861. doi: 10.1167/iovs.08-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Kriesel JD, Ricigliano J, Spruance SL, Garza HH, Jr., Hill JM. Neuronal reactivation of herpes simplex virus may involve interleukin-6. Journal of NeuroVirology. 1997;3(6):441–448. doi: 10.3109/13550289709031190. [DOI] [PubMed] [Google Scholar]
- 286.Walev I, Podlech J, Falke D. Enhancement by TNF-alpha of reactivation and replication of latent herpes simplex virus from trigeminal ganglia of mice. Archives of Virology. 1995;140(6):987–992. doi: 10.1007/BF01315409. [DOI] [PubMed] [Google Scholar]
- 287.Kálmán J, Juhász A, Laird G, et al. Serum interleukin-6 levels correlate with the severity of dementia in down syndrome and in Alzheimer's disease. Acta Neurologica Scandinavica. 1997;96(4):236–240. doi: 10.1111/j.1600-0404.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 288.Shalit F, Sredni B, Stern L, Kott E, Huberman M. Elevated interleukin-6 secretion levels by mononuclear cells of Alzheimer's patients. Neuroscience Letters. 1994;174(2):130–132. doi: 10.1016/0304-3940(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 289.Sun YX, Minthon L, Wallmark A, Warkentin S, Blennow K, Janciauskiene S. Inflammatory markers in matched plasma and cerebrospinal fluid from patients with Alzheimer's disease. Dementia and Geriatric Cognitive Disorders. 2003;16(3):136–144. doi: 10.1159/000071001. [DOI] [PubMed] [Google Scholar]
- 290.Kragsbjerg P, Vikerfors T, Holmberg H. Cytokine responses in patients with pneumonia caused by Chlamydia or Mycoplasma. Respiration. 1998;65(4):299–303. doi: 10.1159/000029280. [DOI] [PubMed] [Google Scholar]
- 291.Mehmet N, Refik M, Harputluoglu M, Ersoy Y, Aydin NE, Yildirim B. Serum and gastric fluid levels of cytokines and nitrates in gastric diseases infected with Helicobacter pylori. New Microbiologica. 2004;27(2):139–148. [PubMed] [Google Scholar]
- 292.Delfino D, Cianci L, Lupis E, et al. Interleukin-6 production by human monocytes stimulated with Cryptococcus neoformans components. Infection and Immunity. 1997;65(6):2454–2456. doi: 10.1128/iai.65.6.2454-2456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Noisakran S, Halford WP, Veress L, Carr DJ. Role of the hypothalamic pituitary adrenal axis and IL-6 in stress- induced reactivation of latent herpes simplex virus type 1. Journal of Immunology. 1998;160(11):5441–5447. [PubMed] [Google Scholar]
- 294.Davis KL, Davis BM, Greenwald BS, et al. Cortisol and Alzheimer's disease. I: basal studies. American Journal of Psychiatry. 1986;143(3):300–305. doi: 10.1176/ajp.143.3.300. [DOI] [PubMed] [Google Scholar]
- 295.Huang CW, Lui CC, Chang WN, Lu CH, Wang YL, Chang CC. Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer's disease. Journal of Clinical Neuroscience. 2009;16(10):1283–1286. doi: 10.1016/j.jocn.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 296.Rodriguez A, Sainz DLM, Missry J, Foster CS. The role of cyclic nucleotide mediators in latency and reactivation of HSV-1 infected neuroblastoma cells. Eye. 1991;5(5):627–635. doi: 10.1038/eye.1991.109. [DOI] [PubMed] [Google Scholar]
- 297.Smith RL, Pizer LI, Johnson EM, Jr., Wilcox CL. Activation of second-messenger pathways reactivates latent herpes simplex virus in neuronal cultures. Virology. 1992;188(1):311–318. doi: 10.1016/0042-6822(92)90760-m. [DOI] [PubMed] [Google Scholar]
- 298.Gebhardt BM, Kaufman HE. Propranolol suppresses reactivation of herpesvirus. Antiviral Research. 1995;27(3):255–261. doi: 10.1016/0166-3542(95)00009-b. [DOI] [PubMed] [Google Scholar]
- 299.Sainz B, Loutsch JM, Marquart ME, Hill JM. Stress-associated immunomodulation and herpes simplex virus infections. Medical Hypotheses. 2001;56(3):348–356. doi: 10.1054/mehy.2000.1219. [DOI] [PubMed] [Google Scholar]
- 300.Martin RE, Loutsch JM, Garza HH, Boedeker DJ, Hill JM. Iontophoresis of lysophosphatidic acid into rabbit cornea induces HSV-1 reactivation: evidence that neuronal signaling changes after infection. Molecular Vision. 1999;5:p. 36. [PubMed] [Google Scholar]
- 301.Aghi MK, Liu TC, Rabkin S, Martuza RL. Hypoxia enhances the replication of oncolytic herpes simplex virus. Molecular Therapy. 2009;17(1):51–56. doi: 10.1038/mt.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Hashimoto Y, Kawatsura H, Shiga Y, Furukawa S, Shigeno T. Significance of nerve growth factor content levels after transient forebrain ischemia in gerbils. Neuroscience Letters. 1992;139(1):45–46. doi: 10.1016/0304-3940(92)90853-y. [DOI] [PubMed] [Google Scholar]
- 303.de la Torre JC. The vascular hypothesis of Alzheimer's disease: bench to bedside and beyond. Neurodegenerative Diseases. 2010;7(1–3):116–121. doi: 10.1159/000285520. [DOI] [PubMed] [Google Scholar]
- 304.Isaacs CE, Kascsak R, Pullarkat RK, Xu W, Schneidman K. Inhibition of herpes simplex virus replication by retinoic acid. Antiviral Research. 1997;33(2):117–127. doi: 10.1016/s0166-3542(96)01009-1. [DOI] [PubMed] [Google Scholar]
- 305.Toffanello ED, Inelmen EM, Minicuci N, et al. Ten-year trends in vitamin intake in free-living healthy elderly people: the risk of subclinical malnutrition. Journal of Nutrition, Health and Aging. 2010;15:99–103. doi: 10.1007/s12603-011-0020-x. [DOI] [PubMed] [Google Scholar]
- 306.Jimenez-Jimenez FJ, Molina JA, de Bustos F, et al. Serum levels of β-carotene, α-carotene and vitamin A in patients with Alzheimer's disease. European Journal of Neurology. 1999;6:495–497. doi: 10.1046/j.1468-1331.1999.640495.x. [DOI] [PubMed] [Google Scholar]
- 307.Frank DN, Zhu W, Sartor RB, Li E. Investigating the biological and clinical significance of human dysbioses. Trends in Microbiology. 2011;19(9):427–434. doi: 10.1016/j.tim.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Archives of Neurology. 2000;57(10):1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 309.Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Archives of Neurology. 2003;60(7):940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 310.Lopez LB, Kritz-Silverstein D, Barrett-Connor CE. High dietary and plasma levels of the omega-3 fatty acid docosahexaenoic acid are associated with decreased dementia risk: the Rancho Bernardo study. Journal of Nutrition, Health and Aging. 2010;15:25–31. doi: 10.1007/s12603-011-0009-5. [DOI] [PubMed] [Google Scholar]
- 311.Solfrizzi V, Panza F, Capurso A. The role of diet in cognitive decline. Journal of Neural Transmission. 2003;110(1):95–110. doi: 10.1007/s00702-002-0766-8. [DOI] [PubMed] [Google Scholar]
- 312.McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996;47(2):425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- 313.Snowdon DA. Healthy aging and dementia: findings from the nun study. Annals of Internal Medicine. 2003;139(5):450–454. doi: 10.7326/0003-4819-139-5_part_2-200309021-00014. [DOI] [PubMed] [Google Scholar]
- 314.Smith AD, Smith SM, de Jager CA, et al. Homocysteine-lowering by b vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS ONE. 2010;5(9):1–10. doi: 10.1371/journal.pone.0012244. Article ID e12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Daviglus ML, Plassman BL, Pirzada A, et al. Risk factors and preventive interventions for Alzheimer disease: state of the science. Archives of Neurology. 2011;68(9):1185–1190. doi: 10.1001/archneurol.2011.100. [DOI] [PubMed] [Google Scholar]
- 316.Song X, Mitnitski A, Rockwood K. Nontraditional risk factors combine to predict Alzheimer disease and dementia. Neurology. 2011;77(3):227–234. doi: 10.1212/WNL.0b013e318225c6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. The Lancet Neurology. 2011;10(9):819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Larman HB, Zhao Z, Laserson U, et al. Autoantigen discovery with a synthetic human peptidome. Nature Biotechnology. 2011;29(6):535–541. doi: 10.1038/nbt.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Miklossy J. Alzheimer's disease—a neurospirochetosis. Analysis of the evidence following Koch's and Hill's criteria. Journal of Neuroinflammation. 2011;8:90–96. doi: 10.1186/1742-2094-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Nagele E, Han M, Demarshall C, Belinka B, Nagele R. Diagnosis of Alzheimer's disease based on disease-specific autoantibody profiles in human sera. PLoS ONE. 2011;6(8) doi: 10.1371/journal.pone.0023112. Article ID e23112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Restrepo L, Stafford P, Magee DM, Johnston SA. Application of immunosignatures to the assessment of Alzheimer's disease. Annals of Neurology. 2011;70(2):286–295. doi: 10.1002/ana.22405. [DOI] [PubMed] [Google Scholar]