Abstract
The neuropeptide galanin regulates numerous physiological activities in the body, including feeding and metabolism, learning and memory, nociception and spinal reflexes, and anxiety and related behaviors. Modulation of blood glucose levels by suppressing insulin release was the first reported activity for galanin. This inhibition was mediated by one or more pertussis toxin-sensitive G proteins of the Gi/o subfamily. However, the molecular identities of the specific G protein(s) and intracellular effectors have not been fully revealed. Recently, we demonstrated that mice lacking Go2, but not other members of the Gi/o protein family, secrete more insulin than controls upon glucose challenge, indicating that Go2 is a major transducer for the inhibitory regulation of insulin secretion. In this study, we investigated galanin signaling mechanisms in β cells using cell biological and electrophysiological approaches. We found that islets lacking Go2, but not other Gi/o proteins, lose the inhibitory effect of galanin on insulin release. Potentiation of ATP-sensitive potassium (KATP) and inhibition of calcium currents by galanin were disrupted by anti-Go2α antibodies. Galanin actions on KATP and calcium currents were completely lost in Go2−/− β cells. Furthermore, the hyperglycemic effect of galanin is also blunted in Go2−/− mice. Our results demonstrate that Go2 mediates the inhibition of insulin release by galanin by regulating both KATP and Ca2+ channels in mice. Our findings provide insight into galanin's action in glucose homeostasis. The results may also be relevant to the understanding of galanin signaling in other biological systems, especially the central nervous system.
Keywords: glycemia, GTP-binding protein-coupled receptor, heterotrimeric, signal transduction, Alzheimer's disease
Galanin, a 29- to 30-residue neuropeptide hormone initially discovered in porcine intestine (1), is distributed throughout the central and peripheral nervous systems and the intestinal neuroendocrine system of many mammalian species (2, 3). The first 15 N-terminal amino acids, which retain the biological activity of the full-length peptide hormone, are highly conserved, underscoring a physiological importance across species. Galanin coexpresses and colocalizes with many neurotransmitters (4) and functions as an inhibitory modulator.
The biological effects of galanin are mediated by galanin receptors. Three types of galanin receptors (GalR1, GalR2, and GalR3) have been identified by molecular cloning and characterized pharmacologically in various species (5–8). All three subtypes of galanin receptors are members of the GTP-binding protein-coupled receptor (GPCR) superfamily. The three galanin receptors exhibit overlapping but distinctive patterns of expression in the central nervous system and periphery. The distinct distribution patterns of receptors support the notion that each receptor mediates some unique physiological function in the body.
Physiological Effects of Galanin.
The biological activity of galanin has been studied intensively in the central and peripheral nervous systems, as well as in the pituitary and the endocrine system. Galanin signaling is involved in feeding and metabolism, learning and memory, nociception and spinal reflexes, anxiety, neuron regeneration, and the pathogenesis of Alzheimer's disease (9–12). The precise role of galanin signaling in these states has not been elucidated. The first reported biological activity of galanin was its effect on plasma glucose levels in dogs and rats (1). The pancreas is highly innervated, and galanin localizes to autonomic nerve terminals in the endocrine pancreas (3) as well as to nerve cell bodies in the celiac ganglion (13). Infusion of galanin into animals results in a significant increase of blood glucose levels by inhibition of insulin secretion from pancreatic β cells (1, 14). Infusion of galanin directly into animals through the pancreatic artery at a concentration that is similar to that released from stimulated pancreatic nerve termini is sufficient to inhibit insulin secretion (15). Conversely, a galanin antagonist can block galanin-mediated inhibition of insulin release from islets (16). In galanin-deficient animals, the inhibition of insulin secretion induced by the chemical activation of sympathetic nerves was observed to be impaired (17). Interestingly, genetically obese ob/ob mice have severely decreased pancreatic content of galanin of less than 10% of levels found in control animals (18). In addition, the number of galanin immunoreactive cells is dramatically reduced in diabetic animals (19). Reduced islet innervation has been associated with impaired insulin secretion in type II diabetic hamsters (20). These results suggest that pancreatic galaninergic nerve dysfunction may contribute to the development of type II diabetes, which is an increasing worldwide public health problem.
Galanin-Receptor Signal Transduction.
The signaling mechanisms of GalR1 and GalR3, which are coupled to their effectors by pertussis toxin (PTX)-sensitive Gi/o G proteins, have been pharmacologically studied. Stimulation of receptors expressed in transfected cell lines or oocytes can inhibit forskolin-stimulated cAMP production, or activate G protein-regulated inwardly rectifying K+ channels in a PTX-sensitive manner (5, 7, 21, 22). Multiple subclasses of G proteins may be involved in GalR2 signaling (6, 22–24). GalR2 can activate phospholipase C and protein kinase C via Gq/11 and activate MAPK and/or inhibit forskolin-stimulated cAMP production through PTX-sensitive Gi/o G proteins. Like the galanin receptors, all five nonsensory PTX-sensitive Gi/o members are expressed in neurons and endocrine tissues. The signal transduction mechanisms mediating galanin's physiological effects have not been studied in native tissue or primary cells. Our previous studies demonstrated that all nonsensory members of the PTX-sensitive Gi/Go protein family, namely Gi1, Gi2, Gi3, Go1, and Go2, are expressed in pancreatic islets, and that only Go2, and not Gi1–3 or Go1, G protein-deficient mice show an “improved” glucose tolerance test and enhanced insulin release from pancreatic β cells (25). This suggests that Go2 is the major transducer mediating inhibitory effects on insulin release that prevent oversecretion. In this study, using Gi/Go α-subunit gene knockout animals established in our laboratory (26, 27), we demonstrate that the Go2 G protein mediates galanin's inhibitory effect on insulin release. We also identify potentiation of ATP-sensitive potassium (KATP) currents and inhibition of Ca2+ channels as possible molecular mechanisms mediating galanin-GalR-Go2 signaling.
Results and Discussion
Inhibition of Insulin Release by Galanin Is Lost in Go2α−/− Islets.
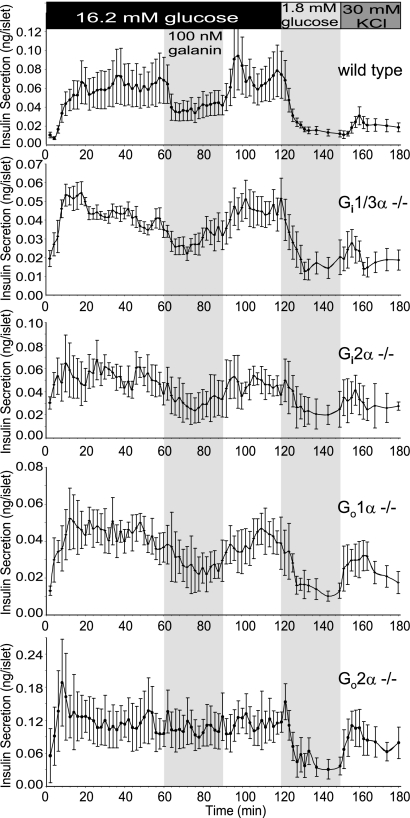
In the present study, we addressed the possible molecular mechanisms by which galanin inhibits insulin release. Galanin has been shown to elevate blood glucose levels by inhibiting insulin release. PTX blocks the inhibitory effects of galanin, suggesting the involvement of Gi/Go proteins in the process. PTX ADP ribosylates a cysteine at the carboxyl termini (−4 position) of the α subunits of Gi/o G proteins and collectively blocks all Gi/Go signaling. Therefore, PTX assays cannot delineate through which specific G protein(s) galanin signals. To investigate the mechanism and to screen the responsible Gi/Go protein(s) for mediating the inhibition of insulin release by galanin, we established a perifusion assay of isolated islets from Gi/Go-deficient animals aimed at identifying which Gi/Go protein(s) mediates galanin's inhibitory effects. A pool of 30–50 islets isolated shortly after a recovery period were placed onto a nylon membrane in a perifusion chamber. After 60 min of perifusion with 1.8 mM glucose in Krebs Ringer bicarbonate (KRB) solution to establish a stable rate of insulin secretion, the glucose concentration in the buffer was elevated to 16.2 mM and islets were continually perifused for 120 min at this high-glucose concentration. The perifusion solution was then switched back to 1.8 mM (low) glucose for 30 min, followed by perifusion with KCl (30 mM) for an additional 30 min. During the high-glucose (16.2 mM) perifusion period, galanin (100 nM) was included transiently (from minute 60 to 90) in the buffer. Fig. 1 shows insulin secretion profiles of islets isolated from WT and knockout mice lacking Gi2α, Gi1/Gi3α, Go1α, and Go2α. High glucose (16.2 mM) induced insulin release from pancreatic islets effectively from all of the genotypes. When the lower-glucose (1.8 mM) buffer was applied, insulin release from islets declined to basal levels. KCl (30 mM), which directly depolarizes the β-cell membrane, triggered insulin release from islets. Galanin (perifused together with high glucose) suppressed insulin secretion in islets from WT, Gi1α−/− Gi3α−/− double KO, Gi2α−/−, and Go1α−/− mice. Upon removal of galanin from the buffer, insulin secretion resumed at the elevated rates. In contrast, the inhibitory effect of galanin on insulin release was absent in islets from Go2α−/− mice. The results indicate that Go2 mediates the inhibitory effect of galanin on insulin release in pancreatic β cells. The levels of insulin released under basal, glucose-stimulated, and K+-induced conditions were comparable for islets from WT, Gi2α−/−, Gi1α−/− Gi3α−/− double knockout, and Go1α−/− mice, but were significantly higher for islets from Go2α−/− (P < 0.01). This suggests that, in addition to mediating galanin inhibition of insulin release, Go2 modulates the sensitivity of islets to glucose and maybe directly or indirectly regulates secretory machinery.
Fig. 1.
Lack of Go2α in the islets impairs the inhibitory effect of galanin on insulin secretion. Insulin release profiles of perifusion assays on islets from Go2α−/−, WT, and other Gi/Goα knockout mice are shown. Hand-picked islets (30–50) were packed in a perifusion chamber and perifused with low (1.8 mM) and high (16.2 mM) concentrations of glucose solutions and KCl as indicated. Galanin was applied during a 60–90 min period as indicated in the first shaded area in the charts. Insulin contents were measured by ELISA with a mouse insulin standard. Perifusion rate was 0.25 mL/min; fractions were collected every 2 min. Shown are traces of mean values ± SEM of insulin secretion from WT, Go2−/−, Go1−/−, Gi1/3 double knockout, and Gi2−/− islets’ response to glucose, galanin, and KCl.
KATP Channels in Go2α−/− Islets Show Normal Glucose Sensitivity.
It is well-established that glucose and other nutrients promote the depolarization of the β-cell membrane and Ca2+ influx through voltage-dependent channels. This constitutes the principal stimulus for insulin exocytosis (28). A glucose transporter (Glut-2) in the β cell facilitates entry of glucose into the cell (29). The enzyme glucokinase phosphorylates glucose to glucose-6-phosphate. Glucokinase appears to function as the fundamental glucose sensor that controls the subsequent β-cell response (30). Glucose is oxidized, leading to a rapid increase of the concentration of intracellular ATP and a decrease of the concentration of ADP. This in turn changes the ATP:ADP ratio in the β cell. ATP is a potent inhibitor of the ATP-sensitive K+ channel, whereas ADP is a stimulator of the channel (31, 32). Inhibition of K+ channels by ATP decreases K+ efflux from the β cell, thus leading to membrane depolarization (33). Depolarization opens voltage-dependent Ca2+ channels, causing a rapid increase in the concentration of intracellular Ca2+. This triggers exocytosis of pancreatic β-cell insulin granules (34). As observed, galanin can inhibit insulin secretion from pancreatic β cells in a PTX-sensitive manner.
To investigate whether the Go2-mediated signaling pathway regulates KATP and Ca2+ channels, we studied channel properties via patch-clamp recording. First, the integrity of KATP channels was investigated in β cells from Go2α knockout mice. Specifically, KATP channels were studied in response to glucose (Fig. S1). Freshly isolated islets were digested briefly with trypsin to generate single β cells. Short-term cultured β cells were used in the studies. Glucose inhibited KATP currents in β cells from both wild-type and Go2α−/− (Fig. S1 A–C). High glucose (16.2 mM) inhibited KATP currents in isolated β cells from −58.0 ± 15.6 to −22 ± 6.1 pA/pF for WT (n = 10, P < 0.01) and −49.8 ± 13.3 to −27.1 ± 5.1 pA/pF for Go2α−/− (n = 12, P < 0.05) mice (Fig. S1D). Furthermore, glucose deprivation stimulated KATP currents in both WT (−26.5 ± 5.0 to −62.6 ± 13.5 pA/pF; n = 9, P < 0.01) and Go2α−/− mice (−22.26 ± 4.0 to −65.34 ± 14 pA/pF; n = 6, P < 0.01). This result demonstrates that KATP channels in β cells isolated from Go2α−/− mice respond to glucose similarly to WT controls and suggests that lack of Go2α does not alter the responsiveness of KATP channels to glucose in β cells.
Galanin Fails to Potentiate KATP Channels in Go2α−/− Mice.
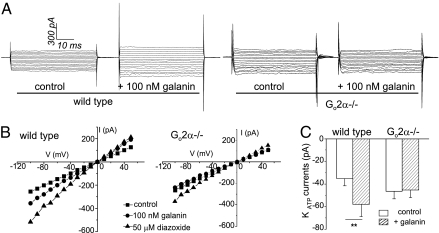
We next investigated the effects of galanin on the modulation of KATP channel activity by glucose. KATP channel currents in the β cells of WT and Go2α−/− mice were recorded in the absence of galanin, presence of 100 nM galanin, or 50 μM diazoxide. As shown in Fig. 2, the averages of glucose-sensitive KATP currents in β cells from WT mice were −35.4 ± 6.3 and −58.0 ± 10.7 pA/pF (n = 12) before and after application of 100 nM galanin to the bath solution, respectively. The averages of KATP currents in Go2α−/− β cells were −46.5 ± 6.7 and −45.3 ± 6.9 pA/pF (n = 11) before and after application of 100 nM galanin to the bath solution, respectively (Fig. 2). Diazoxide, a selective KATP channel opener, was able to further potentiate the channels in both WT and Go2α−/− β cells in the presence of galanin. Galanin significantly enhanced (P < 0.01) KATP currents in β cells from WT control mice; however, this current augmentation was completely absent in the β cells from Go2α−/− mice. The results suggest that Go2 protein mediates the inhibitory effect of galanin on KATP channels in mouse β cells, and that the potentiation of KATP by diazoxide is independent of the Go2-mediated pathway. These data are consistent with the existence of a specific signaling mechanism whereby the inhibitory neuropeptide galanin binds to its receptors and activates KATP channels through Go2 to inhibit insulin secretion from β cells.
Fig. 2.
Galanin-stimulated KATP channel activity is missing in Go2−/− β cells. (A) Representative KATP current traces recorded from β cells potentiated by 100 nM galanin. Testing potential (TP) = −100 to +50 mV, holding potential (HP) = −20 mV. (B) I–V (current-voltage) relationship curves show the effect of galanin and diazoxide (50 μM) on KATP current from WT and Go2−/− β cells. (C) Summary of KATP currents in β cells stimulated by galanin in WT and the diminished galanin stimulation in Go2−/− in the presence of 10 mM glucose (in normal culture medium-glucose condition) (n = 11–12, **P < 0.01, TP = −100 mV to +50 mV, HP = −20 mV).
Anti-Go2α Antibodies Suppress Basal KATP Currents and Abolish the Potentiating Effects of Galanin on KATP in WT β Cells.
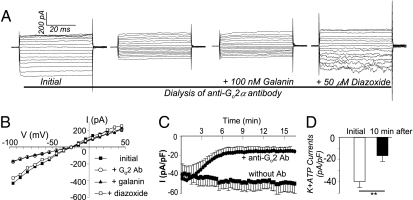
To further validate that Go2 G protein is a critical component in the inhibitory pathway mediating galanin signaling, we used affinity-purified anti-Go2α antibodies to titrate the endogenous Go2α protein and tested KATP current activity in islet β cells. WT β cells dialyzed with anti-Go2α antibodies showed a reduced basal KATP channel activity. After rupture of the patch, antibodies diffused into the cells from the holding pipettes, and the KATP current decreased from −43.7 ± 0.7 to −15.7 ± 0.1 pA/pF (n = 5, P < 0.01) within 10 min and reached a new steady state. KATP currents were stable when the holding pipettes contained nonspecific IgG or no antibodies. Galanin (100 nM) was perifused into the cell chamber during the steady phase. As expected, KATP currents were not enhanced by galanin in β cells dialyzed with anti-Go2α antibodies. Notably, the antibodies had no effects of the KATP channel opener diazoxide on β cells (Fig. 3). These findings are in agreement with studies on isolated β cells from Go2α−/− mice (Fig. 2), and confirm that Go2 is a critical downstream signal transducer of galanin receptors in β cells. Through modulation of KATP channel activity, Go2 regulates insulin secretion. The stimulation of KATP channels by galanin was abolished by dialyzing anti-Go2α antibodies into β cells (Fig. 3 A and B). Thus, antibodies dialyzed into the cytoplasm appear to titrate Go2 away from their effectors and change the dynamic regulation of effectors. The reduction of the basal KATP channel activity by anti-Go2α antibodies suggests that under basal conditions, Go2 potentiates KATP channel activity in β cells. The KATP channels in β cells are involved in maintaining membrane potential, and activation of KATP channels is required for retaining β cells in the hyperpolarized state to suppress insulin secretion. In addition, the observation of dynamic changes in KATP activity by dialyzed anti-Go2α antibodies suggests that the α subunit may be involved directly. Our results from native channel studies are also in agreement with previous findings that demonstrated that the activity of cloned SUR1-Kir6.2 channels (pancreatic form) can be enhanced by a Gi/oα protein during inside-out patch recordings in a reconstitution assay (35). In addition, recombinant Goα protein can potentiate neuronal (brain) KATP channel activity in inside-out patches (36). Taken together, these results support that the α subunit of Go also participates in the regulation of KATP channel activity.
Fig. 3.
Anti-Go2 antibodies reduce basal KATP currents and abolish galanin-potentiated KATP currents in WT β cells. (A) Representative KATP current traces in WT β cells dialyzed with anti-Go2α antibody and perifused with 100 nM galanin or 50 μM diazoxide. TP = −100 to +50 mV, HP = −20 mV. Basal KATP currents were blocked by the dialyzed anti-Go2 antibodies, and the anti-Go2 antibodies abolished galanin-potentiated KATP currents but not diazoxide-stimulated KATP currents. (B) Corresponding I–V (current-voltage) relationship curves. (C) Summary of time course of basal KATP currents blocked by anti-Go2 antibodies (n = 5, TP = −100 mV, HP = −20 mV). (D) Summary of abolishment of galanin-potentiated KATP currents by anti-Go2 antibody dialysis. Compare KATP currents before and 10 min after dialysis of anti-Go2 antibodies (n = 5, **P < 0.01, TP = −100 mV, HP = −20 mV).
Inhibitory Effect of Galanin on Ca2+ Currents Is Missing in Pancreatic β Cells from Go2α−/− Mice.
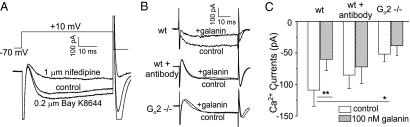
The L-type Ca2+ channel is a major route for elevating intracellular Ca2+ concentration, which in turn triggers insulin exocytosis. Modulation of Ca2+ channel activity would effectively regulate intracellular Ca2+ concentrations and insulin release in β cells. It has been shown that mouse β cells express L-type Ca2+ channels (both Cav1.2 and Cav1.3) (37, 38). To address whether the L-type Ca2+ channel is another intracellular effector in the galanin Go2-mediated pathway, we investigated galanin's effect on the regulation of Ca2+ currents. First, we verified the nature of the recorded Ca2+ currents in β cells using a Ca2+ channel potentiator and blocker. As shown in Fig. 4A, Bay K-8644 (0.2 μM) potentiated current, whereas nifedipine (1 μM) inhibited current, confirming the existence of classical L-type Ca2+ channels in β cells. The low-voltage T-type Ca2+ channels were not detectable in our mouse pancreatic β cells. We next tested the effect of galanin on regulating Ca2+ currents in β cells. Galanin caused a reduction in Ca2+ flow into β cells by inhibiting Ca2+ currents. Galanin (100 nM) significantly reduced Ca2+ current in β cells isolated from WT mice, with a decrease of 55% of peak currents from −108.93 ± 25.68 to −60.26 ± 17.96 pA/pF (n = 8, P < 0.01) (Fig. 4 B and C). When the cells were dialyzed with anti-Go2α antibodies, the average peak currents were −85.13 ± 21.58 and −72.07 ± 26.41 pA/pF (n = 8) before and after galanin (100 nM) application, respectively. No significant difference was observed before and after application of galanin in the presence of anti-Go2α antibodies. This demonstrates that anti-Go2α antibodies can block the inhibitory effect of galanin on Ca2+ currents. These observations suggest that in addition to modulating KATP channel activities, Go2 protein could also mediate effects of galanin by regulating the activity of the pancreatic L-type Ca2+ channel. To confirm this hypothesis, we performed patch-clamp recordings of Ca2+ channel activity of β cells isolated from Go2α−/− animals. In Go2α−/− β cells, average peak currents before and after application of galanin were −51.87 ± 11.9 and −38.54 ± 15.6 pA/pF (n = 6), respectively. There was also no statistically significant difference before and after application of galanin. These results show that the lack of Go2α in β cells by gene knockout or titration of Go2α by antibodies leads to the loss of galanin's inhibitory effect on Ca2+ channels. It is noteworthy that there is a statistically significant difference (P < 0.05) in the Ca2+ currents in WT and Go2α−/− β cells. The cause for this reduction of Ca2+ channel activity in Go2α knockout is currently unknown. Taken together, our results suggest that Go2 mediates the potentiation of KATP channels and inhibition of Ca2+ channels induced by galanin. Hence, Go2 is a major transducer for galanin receptor signaling in the pancreatic β cell.
Fig. 4.
The inhibition of Ca2+ current by galanin is missing in Go2α−/− β cells. (A) Current traces of whole-cell Ca2+ currents recorded from WT β cells under a control condition or the presence of 1 μM nifedipine or 0.2 μM Bay K-8644. TP = 0 mV, HP = −70 mV. (B) Representative Ca2+ current traces before and after 100 nM galanin application to WT β cells without or with dialyzed anti-Go2 antibodies and to Go2α−/− β cells (TP = 0 mV, HP = −70 mV). (C) Summary of galanin effects on Ca2+ currents in β cells from WT without or with dialyzed anti-Go2α antibodies and from Go2α−/−. The results suggest that Go2 mediates the inhibition of Ca2+ currents by galanin (TP = 0 mV, HP = −70 mV, *P < 0.05, **P < 0.01, n = 5–8). Values are means ± SEM.
Loss of Go2α Blunts the Hyperglycemic Effect of Galanin in Vivo.
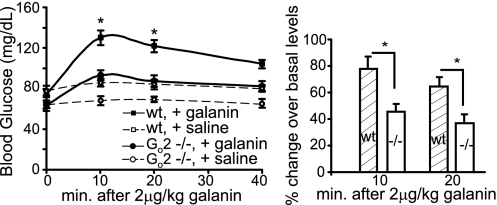
To confirm our findings that Go2 mediates galanin receptor signaling in pancreatic β cells and galanin's inhibitory effect on insulin secretion, we tested the effect of galanin administration to Go2α−/− and WT mice on their blood glucose levels. Galanin can effectively suppress insulin release from β cells; therefore, administration of galanin into the bloodstream will suppress insulin levels, and in turn increase blood glucose levels. Galanin (2 μg/kg) was delivered via the retroorbital vein in overnight-fasted mice. Blood glucose levels were monitored before and after administration of galanin. As shown in Fig. 5, galanin elevates blood glucose levels rapidly, reaching a maximum after 10 min in WT mice and declining slowly thereafter. In WT mice, blood glucose levels were raised from a basal level of 74.5 ± 3.4 mg/dL to 130.1 ± 7.4 mg/dL 10 min postinjection and 121.8 ± 5.7 mg/dL 20 min postinjection. These correspond to a 77.8 and 64.5% increase after administration of galanin, respectively. In contrast, galanin only elevated glucose levels slightly over basal levels in Go2α-null mice. The basal blood glucose level of Go2-null mice was 60.4 ± 1.7 mg/dL. The glucose levels were raised moderately after galanin injection to 85.5 ± 3.1 mg/dL 10 min postinjection and 81.8 ± 3.7 mg/dL 20 min postinjection, corresponding to a 45.7 and 36.9% increase over basal, respectively. The results demonstrate that galanin can effectively elevate blood glucose levels in WT mice, and that this effect is significantly reduced (P < 0.01) in mice lacking Go2α. This suggests that Go2 mediates a critical signaling pathway for galanin in the modulation of glucose homeostasis in the body. We did not observe a complete loss of galanin's hyperglycemic effect in Go2α-null mice, suggesting that galanin may also use other, Go2-independent mechanisms to augment glucose levels in β cells. In fact, galanin has been shown to enhance plasma glucagon levels secreted from pancreatic α cells. During the systemic infusion of galanin, pancreatic glucagon output rapidly doubled in dogs and mice (3, 39). Therefore, galanin-induced hyperglycemia is the additive action of lowering basal plasma insulin levels, increasing basal plasma glucagon levels, and possibly other mechanisms. Increasing plasma glucagon by galanin is believed to be mediated by G11/q proteins, but not by Gi/o proteins. Therefore, elevation of blood glucose levels by the action of glucagon should not be affected in Go2α−/− mice. Physiologically, the body responds to hypoglycemia by increasing glucose production and decreasing glucose sequestration (by reducing insulin levels) simultaneously.
Fig. 5.
Effects of galanin on blood glucose levels in Go2 knockout mice. Galanin at 2 μg/kg bodyweight was given at time 0. Blood glucose levels were monitored. (Left) Actual glucose levels. (Right) Percentage changes over the basal. WT: n = 9, 74.5 ± 3.4 (basal at 0 min), 130.1 ± 7.4 (10 min, increased 77.8% ± 14.3), 121.8 ± 5.7 (20 min, increased 64.5% ± 8.1); Go2α−/−: n = 18, 60.4 ± 1.7 (basal at 0 min), 85.5 ± 3.1 (10 min, increased 45.7% ± 5.7), 81.8 ± 3.7 (20 min, increased 36.9% ± 6.7). For the control no-galanin injection experiment, n = 6 and 8 for WT and Go2α−/− mice, respectively. *P < 0.01; statistical significance was determined using the Student's t test from unpaired data or analysis of variance.
The distribution of galanin suggests that it is a neuropeptide. Its activity is exerted locally, rather than systemically as a humoral agent (40). Pancreatic islets are highly innervated, and activation of pancreatic nerves is sufficient to influence islet function (15). Go was originally identified as the “other” PTX-sensitive G protein in the brain and is highly expressed in the CNS and endocrine cells (41). Logically, antagonizing the Gs pathway by suppressing adenylyl cyclase (AC) activity might be a mechanism for galanin receptor-Go protein-mediated signaling in cells. Galanin can inhibit AC activity through Gi3 (42) or Gi2/Gi3 proteins in the RINm5F cell line (43). However, galanin can still suppress insulin release from insulin-secreting cells in the presence of cAMP in the media (44). Using static islet incubation assays, we also confirmed that the suppression of insulin release by galanin is not dependent on the AC-cAMP pathway. Islets were incubated with or without galanin in the presence of 0.1 mM dibutyryl-cAMP and 16.2 mM glucose, and released insulin was measured. Galanin can inhibit insulin release from wild-type islets even in the presence of cAMP, as observed previously (45). This observation suggests that inhibiting AC and reducing cAMP levels are unlikely to be a mode for suppressing insulin release by galanin. In fact, Go is at best a poor AC inhibitor in vitro compared with Gi proteins (46). In addition, cellular cAMP levels are not elevated in Go−/− islets (47). Taken together, reducing cAMP levels by inhibiting AC activity in β cells does not appear to constitute a major route for the inhibitory effect of galanin on insulin release.
Our findings can be summarized as follows: (i) The inhibitory effects of galanin on insulin release are lost in β cells from Go2α−/− mice, but are intact in the other Gi/o knockouts; (ii) the potentiation by galanin of KATP currents is missing in Go2α−/− β cells; (iii) the inhibition of β-cell L-type Ca2+ currents by galanin is lost in Go2−/− β cells; (iv) antibodies specific against Go2α abolish galanin-mediated potentiation of KATP and inhibition of Ca2+ currents in WT β cells; and (v) in vivo, the galanin-induced hyperglycemic effect is blunted in mice lacking Go2α.
Galanin inhibits Ca2+-induced insulin release from permeabilized RINm5F cells in a PTX-sensitive manner (48), suggesting that direct inhibition of exocytosis is one mechanism of action for galanin. Constitutively active α subunits of Gi/o protein can inhibit the exocytosis process, suggesting α subunits can directly influence the process (49). In addition, enhanced potassium-induced insulin release in Go2−/− islets shown in Fig. 1 and our previous study (25) supports the notion that Go2 may act on the insulin exocytosis process directly. Interestingly, Gβγ can also regulate the exocytosis process. Gβγ can bind SNAP-25 directly, as demonstrated in in vitro assays (50). The C terminus of SNAP-25 may be a target of Gβγ for presynaptic inhibition (51). Recently, it has been reported that norepinephrine can block the exocytosis process via the Gβγ subunit, and that this process is mediated specifically by Gαi1/2 (52). Through study of G-protein knockout mice, we and others have observed that the expression levels of Gβγ correlate well with α-subunit levels in cells; Gβγ are down-regulated in α-subunit knockout and heterozygous mice (53). This suggests that signaling through Gα and Gβγ is well-coordinated in the cells. The mechanism for G-protein inhibition of the exocytosis process remains to be further elucidated.
Galanin has been shown to be an important neuropeptide that regulates glucose homeostasis in the body (17). Our study demonstrates that galanin inhibition of insulin release is mediated mainly by Go2, not other Gi/Go proteins in pancreatic β cells. Other Gi or Go proteins cannot compensate for the loss of Go2 in mediating galanin's modulation of insulin secretion. This indicates that galanin receptors preferentially couple to effectors by Go2 protein in β cells in vivo. At the molecular level, loss of Go2 protein in β cells results in impairment of galanin's modulation of KATP and Ca2+ channels. This suggests in the insulin secretion pathway that galanin achieves its inhibitory effects on insulin release by at least at three different action sites: by potentiating KATP channels, inhibiting L-type Ca2+ currents, and regulating the exocytosis process. Inhibitory peptide hormones play a critical role in maintaining euglycemia by preventing oversecretion of insulin into the bloodstream. Oversecretion of insulin could result in hypoglycemia that may impair the normal biological function of the endocrine and nervous systems. Furthermore, prolonged oversecretion of insulin may desensitize peripheral tissues and lead to the development of insulin resistance characteristic of type II diabetes.
Materials and Methods
All procedures were designed and performed in accordance with the generally accepted ethical standards for animal experimentation and approved by the Chancellor's Animal Research Committee of the University of California, Los Angeles. Detailed methods for islets and β-cell isolations, islet perifusion, electrophysiological recordings, and in vivo tests for galanin effects on glucose levels are described in SI Materials and Methods. Statistical analyses were done using paired and unpaired Student's t tests and analyses of variance in conjunction with Newman–Keuls tests where appropriate. Group differences at the level of P < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
This work is supported by National Institutes of Health Grants DK069771 (to M.J.) and DK19318 (to L.B.) and in part by Intramural Research Program of the NIH Project Z01-ES101643 (to L.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200100109/-/DCSupplemental.
References
- 1.Tatemoto K, Rökaeus A, Jörnvall H, McDonald TJ, Mutt V. Galanin—A novel biologically active peptide from porcine intestine. FEBS Lett. 1983;164(1):124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 2.Skofitsch G, Jacobowitz DM. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985;6:509–546. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- 3.Dunning BE, et al. Galanin: A novel pancreatic neuropeptide. Am J Physiol. 1986;251(1 Pt 1):E127–E133. doi: 10.1152/ajpendo.1986.251.1.E127. [DOI] [PubMed] [Google Scholar]
- 4.Melander T, et al. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. J Neurosci. 1986;6:3640–3654. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habert-Ortoli E, Amiranoff B, Loquet I, Laburthe M, Mayaux JF. Molecular cloning of a functional human galanin receptor. Proc Natl Acad Sci USA. 1994;91:9780–9783. doi: 10.1073/pnas.91.21.9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith KE, et al. Expression cloning of a rat hypothalamic galanin receptor coupled to phosphoinositide turnover. J Biol Chem. 1997;272:24612–24616. doi: 10.1074/jbc.272.39.24612. [DOI] [PubMed] [Google Scholar]
- 7.Smith KE, et al. Cloned human and rat galanin GALR3 receptors. Pharmacology and activation of G-protein inwardly rectifying K+ channels. J Biol Chem. 1998;273:23321–23326. doi: 10.1074/jbc.273.36.23321. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, He C, Hashemi T, Bayne M. Cloning and expressional characterization of a novel galanin receptor. Identification of different pharmacophores within galanin for the three galanin receptor subtypes. J Biol Chem. 1997;272:31949–31952. doi: 10.1074/jbc.272.51.31949. [DOI] [PubMed] [Google Scholar]
- 9.Counts SE, Perez SE, Ginsberg SD, De Lacalle S, Mufson EJ. Galanin in Alzheimer disease. Mol Interv. 2003;3(3):137–156. doi: 10.1124/mi.3.3.137. [DOI] [PubMed] [Google Scholar]
- 10.Steiner RA, et al. Galanin transgenic mice display cognitive and neurochemical deficits characteristic of Alzheimer's disease. Proc Natl Acad Sci USA. 2001;98:4184–4189. doi: 10.1073/pnas.061445598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wrenn CC, et al. Learning and memory performance in mice lacking the GAL-R1 subtype of galanin receptor. Eur J Neurosci. 2004;19:1384–1396. doi: 10.1111/j.1460-9568.2004.03214.x. [DOI] [PubMed] [Google Scholar]
- 12.Zorrilla EP, Brennan M, Sabino V, Lu X, Bartfai T. Galanin type 1 receptor knockout mice show altered responses to high-fat diet and glucose challenge. Physiol Behav. 2007;91:479–485. doi: 10.1016/j.physbeh.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahrén B, Lindskog S. Galanin and the regulation of islet hormone secretion. Int J Pancreatol. 1992;11(3):147–160. doi: 10.1007/BF02924180. [DOI] [PubMed] [Google Scholar]
- 14.McDonald TJ, et al. Galanin inhibits insulin secretion and induces hyperglycemia in dogs. Diabetes. 1985;34(2):192–196. doi: 10.2337/diab.34.2.192. [DOI] [PubMed] [Google Scholar]
- 15.Dunning BE, Taborsky GJ., Jr Galanin release during pancreatic nerve stimulation is sufficient to influence islet function. Am J Physiol. 1989;256(1 Pt 1):E191–E198. doi: 10.1152/ajpendo.1989.256.1.E191. [DOI] [PubMed] [Google Scholar]
- 16.Gregersen S, et al. Blockade of galanin-induced inhibition of insulin secretion from isolated mouse islets by the non-methionine containing antagonist M35. Eur J Pharmacol. 1993;232(1):35–39. doi: 10.1016/0014-2999(93)90725-w. [DOI] [PubMed] [Google Scholar]
- 17.Ahrén B, Pacini G, Wynick D, Wierup N, Sundler F. Loss-of-function mutation of the galanin gene is associated with perturbed islet function in mice. Endocrinology. 2004;145:3190–3196. doi: 10.1210/en.2003-1700. [DOI] [PubMed] [Google Scholar]
- 18.Dunning BE, Ahrén B. Reduced pancreatic content of the inhibitory neurotransmitter galanin in genetically obese, hyperinsulinemic mice. Pancreas. 1992;7:233–239. doi: 10.1097/00006676-199203000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Adeghate E, Ponery AS. Large reduction in the number of galanin-immunoreactive cells in pancreatic islets of diabetic rats. J Neuroendocrinol. 2001;13:706–710. doi: 10.1046/j.1365-2826.2001.00682.x. [DOI] [PubMed] [Google Scholar]
- 20.Kohnert KD, et al. Islet neuronal abnormalities associated with impaired insulin secretion in type 2 diabetes in the Chinese hamster. Regul Pept. 1999;82(1–3):71–79. doi: 10.1016/s0167-0115(99)00044-0. [DOI] [PubMed] [Google Scholar]
- 21.Parker EM, et al. Cloning and characterization of the rat GALR1 galanin receptor from Rin14B insulinoma cells. Brain Res Mol Brain Res. 1995;34(2):179–189. doi: 10.1016/0169-328x(95)00159-p. [DOI] [PubMed] [Google Scholar]
- 22.Kolakowski LF, Jr, et al. Molecular characterization and expression of cloned human galanin receptors GALR2 and GALR3. J Neurochem. 1998;71:2239–2251. doi: 10.1046/j.1471-4159.1998.71062239.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Hashemi T, He C, Strader C, Bayne M. Molecular cloning and pharmacological characterization of a new galanin receptor subtype. Mol Pharmacol. 1997;52:337–343. doi: 10.1124/mol.52.3.337. [DOI] [PubMed] [Google Scholar]
- 24.Fathi Z, et al. Molecular characterization, pharmacological properties and chromosomal localization of the human GALR2 galanin receptor. Brain Res Mol Brain Res. 1998;58(1-2):156–169. doi: 10.1016/s0169-328x(98)00116-8. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, et al. Augmented glucose-induced insulin release in mice lacking G(o2), but not G(o1) or G(i) proteins. Proc Natl Acad Sci USA. 2011;108:1693–1698. doi: 10.1073/pnas.1018903108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang M, et al. Multiple neurological abnormalities in mice deficient in the G protein Go. Proc Natl Acad Sci USA. 1998;95:3269–3274. doi: 10.1073/pnas.95.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudolph U, et al. Ulcerative colitis and adenocarcinoma of the colon in G α i2-deficient mice. Nat Genet. 1995;10(2):143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 28.Ashcroft FM, Gribble FM. ATP-sensitive K+ channels and insulin secretion: Their role in health and disease. Diabetologia. 1999;42:903–919. doi: 10.1007/s001250051247. [DOI] [PubMed] [Google Scholar]
- 29.Guillam MT, et al. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet. 1997;17:327–330. doi: 10.1038/ng1197-327. [DOI] [PubMed] [Google Scholar]
- 30.Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 31.Aguilar-Bryan L, et al. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78:227–245. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- 32.Babenko AP, Gonzalez G, Bryan J. Two regions of sulfonylurea receptor specify the spontaneous bursting and ATP inhibition of KATP channel isoforms. J Biol Chem. 1999;274:11587–11592. doi: 10.1074/jbc.274.17.11587. [DOI] [PubMed] [Google Scholar]
- 33.Ashcroft FM, Rorsman P. ATP-sensitive K+ channels: A link between B-cell metabolism and insulin secretion. Biochem Soc Trans. 1990;18(1):109–111. doi: 10.1042/bst0180109. [DOI] [PubMed] [Google Scholar]
- 34.Ashcroft FM, et al. Stimulus-secretion coupling in pancreatic β cells. J Cell Biochem. 1994;55(Suppl):54–65. doi: 10.1002/jcb.240550007. [DOI] [PubMed] [Google Scholar]
- 35.Sánchez JA, Gonoi T, Inagaki N, Katada T, Seino S. Modulation of reconstituted ATP-sensitive K(+)-channels by GTP-binding proteins in a mammalian cell line. J Physiol. 1998;507:315–324. doi: 10.1111/j.1469-7793.1998.315bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VanDongen AM, et al. Newly identified brain potassium channels gated by the guanine nucleotide binding protein Go. Science. 1988;242:1433–1437. doi: 10.1126/science.3144040. [DOI] [PubMed] [Google Scholar]
- 37.Namkung Y, et al. Requirement for the L-type Ca(2+) channel α(1D) subunit in postnatal pancreatic β cell generation. J Clin Invest. 2001;108:1015–1022. doi: 10.1172/JCI13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulla V, et al. Impaired insulin secretion and glucose tolerance in β cell-selective Ca(v)1.2 Ca2+ channel null mice. EMBO J. 2003;22:3844–3854. doi: 10.1093/emboj/cdg389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindskog S, Ahrén B. Galanin: Effects on basal and stimulated insulin and glucagon secretion in the mouse. Acta Physiol Scand. 1987;129:305–309. doi: 10.1111/j.1748-1716.1987.tb08073.x. [DOI] [PubMed] [Google Scholar]
- 40.Dupré J. Galanin: A selective inhibitor of insulin secretion? Pancreas. 1988;3(2):119–121. [PubMed] [Google Scholar]
- 41.Jiang M, Bajpayee NS. Molecular mechanisms of Go signaling. Neurosignals. 2009;17(1):23–41. doi: 10.1159/000186688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Mazancourt P, Goldsmith PK, Weinstein LS. Inhibition of adenylate cyclase activity by galanin in rat insulinoma cells is mediated by the G-protein Gi3. Biochem J. 1994;303:369–375. doi: 10.1042/bj3030369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDermott AM, Sharp GW. Gi2 and Gi3 proteins mediate the inhibition of adenylyl cyclase by galanin in the RINm5F cell. Diabetes. 1995;44:453–459. doi: 10.2337/diab.44.4.453. [DOI] [PubMed] [Google Scholar]
- 44.Sharp GW, et al. Galanin can inhibit insulin release by a mechanism other than membrane hyperpolarization or inhibition of adenylate cyclase. J Biol Chem. 1989;264:7302–7309. [PubMed] [Google Scholar]
- 45.Drews G, Debuyser A, Henquin JC. Significance of membrane repolarization and cyclic AMP changes in mouse pancreatic B-cells for the inhibition of insulin release by galanin. Mol Cell Endocrinol. 1994;105(1):97–102. doi: 10.1016/0303-7207(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 46.Taussig R, Iñiguez-Lluhi JA, Gilman AG. Inhibition of adenylyl cyclase by Gi α. Science. 1993;261:218–221. doi: 10.1126/science.8327893. [DOI] [PubMed] [Google Scholar]
- 47.Zhao A, et al. Gαo represses insulin secretion by reducing vesicular docking in pancreatic β-cells. Diabetes. 2010;59:2522–2529. doi: 10.2337/db09-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ullrich S, Wollheim CB. Galanin inhibits insulin secretion by direct interference with exocytosis. FEBS Lett. 1989;247:401–404. doi: 10.1016/0014-5793(89)81379-1. [DOI] [PubMed] [Google Scholar]
- 49.Lang J, et al. Direct control of exocytosis by receptor-mediated activation of the heterotrimeric GTPases Gi and G(o) or by the expression of their active G α subunits. EMBO J. 1995;14:3635–3644. doi: 10.1002/j.1460-2075.1995.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blackmer T, et al. G protein βγ subunit-mediated presynaptic inhibition: Regulation of exocytotic fusion downstream of Ca2+ entry. Science. 2001;292:293–297. doi: 10.1126/science.1058803. [DOI] [PubMed] [Google Scholar]
- 51.Gerachshenko T, et al. Gβγ acts at the C terminus of SNAP-25 to mediate presynaptic inhibition. Nat Neurosci. 2005;8:597–605. doi: 10.1038/nn1439. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Y, Fang Q, Straub SG, Lindau M, Sharp GW. Noradrenaline inhibits exocytosis via the G protein βγ subunit and refilling of the readily releasable granule pool via the α(i1/2) subunit. J Physiol. 2010;588:3485–3498. doi: 10.1113/jphysiol.2010.190090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valenzuela D, et al. G α(o) is necessary for muscarinic regulation of Ca2+ channels in mouse heart. Proc Natl Acad Sci USA. 1997;94:1727–1732. doi: 10.1073/pnas.94.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.