Background: We have analyzed the role of flotillins during EGF receptor signaling.
Results: Flotillin-1 knockdown results in impaired activation of EGFR and MAP kinases, with which flotillin-1 interacts.
Conclusion: Flotillin-1 may be a novel scaffolding protein in MAP kinase signaling.
Significance: Flotillin-1 is essential for proper MAP kinase signaling.
Keywords: Epidermal Growth Factor Receptor (EGFR), Lipid Raft, MAP Kinases (MAPKs), Raf, Signal Transduction
Abstract
Our previous work has shown that the membrane microdomain-associated flotillin proteins are potentially involved in epidermal growth factor (EGF) receptor signaling. Here we show that knockdown of flotillin-1/reggie-2 results in reduced EGF-induced phosphorylation of specific tyrosines in the EGF receptor (EGFR) and in inefficient activation of the downstream mitogen-activated protein (MAP) kinase and Akt signaling. Although flotillin-1 has been implicated in endocytosis, its depletion affects neither the endocytosis nor the ubiquitination of the EGFR. However, EGF-induced clustering of EGFR at the cell surface is altered in cells lacking flotillin-1. Furthermore, we show that flotillins form molecular complexes with EGFR in an EGF/EGFR kinase-independent manner. However, knockdown of flotillin-1 appears to affect the activation of the downstream MAP kinase signaling more directly. We here show that flotillin-1 forms a complex with CRAF, MEK1, ERK, and KSR1 (kinase suppressor of RAS) and that flotillin-1 knockdown leads to a direct inactivation of ERK1/2. Thus, flotillin-1 plays a direct role during both the early phase (activation of the receptor) and late (activation of MAP kinases) phase of growth factor signaling. Our results here unveil a novel role for flotillin-1 as a scaffolding factor in the regulation of classical MAP kinase signaling. Furthermore, our results imply that other receptor-tyrosine kinases may also rely on flotillin-1 upon activation, thus suggesting a general role for flotillin-1 as a novel factor in receptor-tyrosine kinase/MAP kinase signaling.
Introduction
Epidermal growth factor receptor (EGFR)3 is a transmembrane receptor belonging to the receptor-tyrosine kinase family. Ligand binding results in dimerization and activation of the EGFR. Within the dimerized EGFR, several Tyr residues are autophosphorylated in trans, whereas others can be phosphorylated e.g. by Src kinases. These events result in recruitment of signaling partners of EGFR and activation of downstream signaling pathways, especially the MAP kinase pathway. In addition, the activated EGFR is ubiquitinated by Cbl, facilitating EGFR endocytosis and degradation in lysosomes (1). EGFR has been shown to be endocytosed mainly by means of clathrin-dependent endocytosis through coated pits (2–4). However, recent findings have indicated that cholesterol-rich membrane microdomains (also known as rafts) are involved in the signaling and/or trafficking of the EGFR (5–7).
The flotillin/reggie protein family contains two members, flotillin-1/reggie-2 (flot-1) and flotillin-2/reggie-1 (flot-2), which are highly conserved through species. Flotillins were discovered as neuronal regeneration proteins in the goldfish optic nerve (8) and shown to be associated with membrane rafts by means of myristoylation and/or palmitoylation (9–11). Despite the fact that flotillins were discovered already more than a decade ago, their true molecular function has remained enigmatic (for a review, see Refs. 12 and 13). Flotillins have been shown to be involved in signaling processes, phagocytosis, non-clathrin endocytosis, cell adhesion, and organization of the actin cytoskeleton (14–20). In addition, they have been suggested to function as scaffolding proteins for a specific type of lipid rafts in some cell types (21, 22).
Flotillins have been implicated in the signaling processes of several membrane receptors, including insulin receptor, IgE receptor, G protein-coupled receptors, and the neurotrophin receptor TrkA (16, 17, 19, 23, 24). Earlier findings from us and others suggest that flotillins may also be involved in signaling through the EGFR. We have shown that flot-2 becomes Tyr-phosphorylated by Src kinase upon EGFR activation (18). In addition, EGF stimulation results in uptake of flotillins from the plasma membrane into late endosomes (18). Flotillins are capable of forming hetero-oligomers, which already exist in unstimulated cells (25, 26). In fact, the major pool of cellular flotillins appears to be bound in hetero-oligomers (27). Upon EGF stimulation, these oligomers coalesce together, forming larger oligomers, which is likely to result in immobilization and subsequent endocytosis of flotillins (26). Phosphorylation by Src kinase does not appear to be the driving force for EGF-induced endocytosis of flotillins (26), and a role for Fyn kinase has recently been suggested (28).
Previous findings have suggested that flotillins are modified by EGFR signaling. This study was carried out to see how the depletion of flotillins affects the activation and endocytosis of the EGFR and the downstream signaling to MAP kinases.
EXPERIMENTAL PROCEDURES
Antibodies and Constructs
Rabbit polyclonal antibody against EGFR and antibodies against phospho-EGFR (Tyr(P)-1068 and Tyr(P)-1173), Akt, phospho-Akt (Ser-473), phospho-CRAF (Ser-338), MEK1/2, and phospho-MEK and phospho-Tyr were purchased from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal antibody against extracellularly regulated kinases 1 and 2 (ERK1/2), CRAF, and growth factor receptor-associated protein 2 (Grb2) and mouse monoclonal antibodies against EGFR, ubiquitin, and pERK1/2 were from Santa Cruz Biotechnology (Santa Cruz, CA). A mouse monoclonal antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was obtained from Biozol (Eching, Germany). Rabbit polyclonal antibodies against flot-1 and flot-2 were purchased from Sigma. For detection of flot-1 and flot-2 in Western blots, monoclonal antibodies from Transduction Laboratories were used. Rabbit monoclonal antibody against KSR1 was purchased from Millipore. The primary antibodies used for immunofluorescence were detected with a Cy3-conjugated goat anti-mouse antibody (Jackson ImmunoResearch, West Grove, PA) and with an Alexa Fluor 488 donkey anti-rabbit antibody (Molecular Probes, Karlsruhe, Germany). Constitutive activation of CRAF with two Asp substitutions (RAF-DD; pCDNA3-FLAG-CRAF-Y340DY341D) has been described earlier (29). Rat flotillin-1 coding region was cloned into pET-41a vector (Novagen/Merck), whereas the coding regions of human MEK1 and rat ERK2 were cloned into pGEX-4T-1 (GE Healthcare). Human CRAF-GST in pGEX-2T was a kind gift of Ulf Rapp.
Cell Culture, siRNA, and shRNA Knockdown
HeLa cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) with high glucose and 10% fetal calf serum (Invitrogen), 100 units/ml penicillin, and 100 μg/ml streptomycin (Sigma) at 8% CO2 and 37 °C. Knockdown of flot-1 was performed as described previously (26) using the StealthTM siRNA system (Invitrogen). As a control, an oligo which does not target any human sequence (StealthTM RNAi Negative Control, medium GC content) was used. The cells were used for the assays 3 days post-transfection. Stable HeLa cells depleted of KSR1 were produced using lentiviral infection with Mission shRNA (Sigma) viruses. Control cells were infected with a virus carrying an shRNA that does not recognize any human sequence. Infected cells were selected in the growth medium containing puromycin, and depletion of KSR1 was verified by Western blot.
Growth Factor Treatment
HeLa cells were serum-starved for 16 h before treatment with 100 or 10 ng/ml EGF (Sigma). In some experiments the cells were treated for 10 min with 25 ng/ml basic fibroblast growth factor (bFGF; Peprotech, Germany) or 50 ng/ml phorbol myristate acetate (PMA) for 20 min.
Reporter Gene Assays
The PathDetect® pSRE-Luc Cis-Reporter Plasmid and the pRL-SV40 control vector were from Stratagene (Waldbronn, Germany) and Promega (Mannheim, Germany). 24 h after siRNA knockdown, each well of a 12-well plate was transfected with 500 ng of pSRE-Luc and 7.5 ng pRL-SV40 using Lipofectamine 2000 and after 6 h transferred onto 24-well plates. The cells were serum-starved for 16 h, treated with 10 or 100 ng/ml EGF for 8 h, and harvested in lysis buffer. Luciferase assays were performed in triplicate using the Dual Luciferase® Reporter Assay System (Promega). The activity of firefly luciferase was normalized to renilla luciferase activity.
Immunofluorescence Analysis
HeLa cells grown on coverslips were treated with EGF for the indicated times after starvation and fixed with 4% paraformaldehyde or methanol. Permeabilization after paraformaldehyde fixation was carried out with digitonin (50 μg/ml). The cells were labeled with primary antibodies and Cy3 and/or Alexa Fluor 488-conjugated secondary antibodies and then embedded in Gelmount (Biomeda, Foster City, CA) supplemented with 50 mg/ml 1,4-diazadicyclo(2,2,2)octane (Fluka, Neu-Ulm, Germany). The specimens were examined using a Zeiss LSM510 Meta confocal laser scanning microscope (Carl Zeiss, Jena, Germany).
Total Internal Reflection (TIRF) Microscopy
HeLa cells transfected with siRNA oligos were starved overnight on coverslips. The cells were either stimulated for 2 min with 100 ng/ml EGF or not, fixed, and labeled with an anti-EGFR antibody (1:100, Santa Cruz) and subsequently with a Cy3-conjugated secondary antibody. Coverslips were stored in PBS until TIRF microscopy was performed using an Olympus BX50 microscope with a 561-nm diode laser line and an MT-20 illumination system. Images were acquired with the Cell̂R Software (Olympus) and analyzed with Image J Software (National Institutes of Health).
Cell Lysis for Western Blotting
The cells were lysed in lysis buffer (50 mm Tris, pH 7.4, 0.15 m NaCl, 2 mm EDTA, 1% Nonidet P-40) supplemented with Protease Inhibitor Mixture (Sigma) and 1 mm sodium orthovanadate (for bFGF treatment) on ice for 30 min. Protein concentrations were determined, and equal protein amounts were analyzed by 10% SDS-polyacrylamide gel electrophoresis and Western blot. Alternatively, the cell pellets were lysed directly in 1× SDS-PAGE loading buffer supplemented with 5% β-mercaptoethanol and 25 mm dithiothreitol (DTT) followed by a brief sonication.
Internalization of 125I-EGF
For internalization assays, control and flotillin-1 knockdown (F1-KD) HeLa cells grown on 24-well plates (100,000 cells/well, triplicates) were starved for 18 h. The cells were stimulated with 1.5 ng/ml 125I-EGF (PerkinElmer Life Sciences) in a binding buffer (DMEM medium, 0.1% BSA, 20 mm HEPES, pH 7.4) for 2, 4, and 6 min at 37 °C. Surface-bound EGF was removed with an acid wash solution (0.2 m acetic acid, 0.5 m NaCl, pH 2.5). Internalized EGF was extracted by lysing the cells in 1 m NaOH. Radioactivity of surface-bound EGF fractions and internalized EGF fractions was measured in a gamma counter (Wallac 1480 Wizard). The ratio of internalized to surface-bound EGF was plotted against the time. The endocytic rate constant Ke (30) was determined as the slope of the trendline.
Immunoprecipitation
Cells previously transfected with siRNA oligos were lysed as described above. Unspecific binding material was removed with 50 μl of Pansorbin beads (Calbiochem). The lysates were incubated for 16 h at 4 °C with 30 μl of magnetic Dynabeads Protein G (Invitrogen) coupled with 1.6 μg of mouse anti-EGFR antibody. The beads were washed with 1 ml each of Neufeld buffer (10 mm Tris, 600 mm NaCl, 0.1% SDS, 0.05% Nonidet P40, pH 8.5), immunomix (IMM) buffer (1% Triton X-100 and 0.5% sodium desoxycholate in PBS), IMM with 2 m KCl, and 0.1× PBS. The precipitated proteins were solubilized into 1× SDS sample buffer containing 25 mm DTT by boiling and subjected to SDS-PAGE and Western blotting. In most cases, 10% SDS-PAGE gels were used. For the analysis of EGFR ubiquitination, 4–12% NuPage (Invitrogen) Bis-Tris gradient gels were used.
Co-immunoprecipitation of EGFR and Flotillins
HeLa cells were either starved or kept in serum-containing medium. Starved cells were then either stimulated with 100 ng/ml EGF for 5 or 15 min or left untreated and lysed in co-immunoprecipitation buffer (10 mm Tris-HCl, pH 8.0, 150 mm NaCl, 5 mm EDTA, 0.5% Triton X-100, 1 mm vanadate, 5 mm NaF, 60 mm n-octyl-β-d-glucopyranoside (AppliChem) and protease inhibitor mixture. In some experiments the cells were treated with an EGFR kinase inhibitor PD153035, a non-inhibiting control compound AG9 (both at 20 nm; Calbiochem) or DMSO for 5 min before EGF stimulation. Antibodies against flot-1, flot-2 (rabbit, Sigma), or myc (rabbit, Santa Cruz) for control were coupled on Protein A Dynabeads (Invitrogen). The lysates and beads were rolled overnight at 4 °C, and the beads were then washed 3 times in 1 ml of co-immunoprecipitation buffer. The beads and aliquots of lysates (=input) were supplemented with SDS-PAGE loading buffer and cooked for 5 min at 94 °C.
GST Protein Expression and Purification
Bacterial expression vectors encoding for flotillin-1-GST, CRAF-GST, ERK2-GST, MEK1-GST, or GST were transformed into a bacterial expression strain, grown in a liquid culture at 37 °C to reach A600 0.4–0.6. Bacteria transformed with GST were then induced with 1 mm isopropyl 1-thio-β-d-galactopyranoside (IPTG) for 5 h at 37 °C, whereas those transformed with the other GST fusions were induced with 50–300 μm IPTG overnight at 19 °C. The cells were pelleted and lysed in GST lysis buffer (50 mm Hepes, pH 7.5, 150 mm NaCl, 1 mm EDTA, 5% glycerol, 0.1% Nonidet P-40) supplemented with 100 μg/ml lysozyme, 1 mm PMSF, 1 mm DTT, and 1 mm protease inhibitors (aprotinin, leupeptin, pepstatin). The GST proteins were bound to glutathione-Sepharose beads (GE Healthcare). For the direct pulldown assays with GST-RAF, -MEK1, or -ERK2, flot-1-GST was cleaved with thrombin to remove the GST tag. Cleavage was performed with 20 μg of purified flot-1-GST bound to glutathione-Sepharose beads. The beads were first washed 3 times with cleavage buffer (20 mm Tris-HCl, pH 8.4, 0.15 mm NaCl, 2.5 mm CaCl2) freshly supplemented with 1 mm DTT. The reaction was carried out using 1 unit of thrombin in 200 μl of the cleavage buffer overnight at 19 °C. The next day thrombin was inactivated with 1 mm PMSF, and the GST-bound beads were removed by centrifugation. The supernatant containing flot-1 was used for pulldown assays.
GST Pulldown Assays
HeLa cells were lysed in lysis buffer for GST pulldown (100 mm Tris pH 8.0, 150 mm NaCl, 1 mm MgCl2, 1% Triton X-100) supplemented with Protease Inhibitor Mixture (Sigma) and 60 mm n-octyl-β-d-glucopyranoside. The cell lysate was incubated overnight at 4 °C with either GST or flotillin-1-GST immobilized on the beads. The beads were washed 3 times with 1 ml of lysis buffer. The samples were then prepared for SDS-PAGE.
GST Pulldown Using Purified Proteins
Direct GST pulldown experiments were performed on ice for 3 h with 5 μg of each of the purified proteins (GST, CRAF-GST, MEK1-GST, ERK2-GST, and Flot1). The beads were washed 3 times with washing buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm DTT, 0.01% Triton X-100), resuspended in 2× SDS sample buffer containing 50 mm DTT, boiled for 5 min at 94 °C, and separated by SDS-PAGE.
Detergent-free Preparation of Lipid Rafts
After knocking down flot-1 with siRNA, cells were transferred in 15-cm dishes for 48 h. Thereafter, they were starved overnight and treated or not with EGF (100 ng/ml) for 30 min on ice. Lipid rafts were prepared according to Macdonald and Pike (31), with a modification (sonication step included after mechanical homogenization to facilitate proper membrane shearing). After collecting the fractions, 400 μl of each fraction were precipitated with acetone, and the pellet was resuspended in 70 μl of 2× loading buffer containing 50 mm DTT. Equal volumes were analyzed by SDS-PAGE and Western blotting. For the detection of the ganglioside GM1, 5 μl of the fractions were slot-blotted onto nitrocellulose and incubated with 0.5 μg/ml cholera toxin subunit B (Invitrogen) conjugated with HRP.
RNA Isolation and Quantitative PCR
Total RNA was isolated using peqGold Trifast (Peqlab, Erlangen, Germany). RNA (3 μg) was reverse-transcribed with 150 fmol of oligo(dT)15 primers and 180 units of Moloney murine leukemia virus reverse transcriptase (Promega) in a total volume of 45 μl. Real time PCRs (iQ™5 QPCR System, Bio-Rad) were performed in triplicate with 1 μl of 5-fold diluted cDNA in 25-μl reaction mixtures using Absolute QPCR SYBR Green Mix (Thermo Fisher Scientific, Bonn, Germany). The annealing temperature was 60 °C for all PCR reactions. Primers were designed to be specific for cDNA with PerlPrimer. Primer sequences were: cyclin D1 forward, 5′-TCGTGGCCTCTAAGATGAAGGA-3′; cyclin D1 reverse, 5′-CAGCTCCATTTGCAGCAGCTC-3′; Rpl13a forward, 5′-CCTGGAGGAGAAGAGGAAAGAGA-3′; Rpl13a reverse, 5′-TTGAGGACCTCTGTGTATTTGTCAA-3′; GAPDH forward, 5′-CATCTTCCAGGAGCGAGATCCC-3′; GAPDH reverse, 5′-CCAGCCTTCTCCATGGTGGT-3′.
PCR products were quantified with a standard curve. The mean of the reference genes Rpl13a and GAPDH was used for normalization.
Statistical Analysis
Unless otherwise stated, all experiments were performed at least three times. For the statistical analysis, Western blot bands of phosphorylated proteins were quantified by scanning densitometry using Quantity One Software (Bio-Rad) and normalized against the total amount of the respective protein. Data are shown as the mean ± S.D. Statistical comparisons between groups were made using Student's t tests or 2-way analysis of variance as appropriate using GraphPad Prism 4 software. Values of p < 0.05 were considered significant (*), whereas values of p < 0.01 and p < 0.001 were defined very significant (**) and highly significant (***), respectively.
Electronic Manipulation of Images
The images shown have in some cases as a whole been subjected to contrast or brightness adjustments. No other manipulations have been performed unless otherwise stated.
RESULTS
Phosphorylation of EGFR Is Reduced in Cells Depleted of Flot-1
We have previously shown that flotillins get phosphorylated and endocytosed upon EGFR signaling (9, 18, 26). The primary aim of this study was to investigate if flotillins play a role in the activation and signaling of the EGFR. Because flot-2 knockdown results in destabilization of flot-1 (25, 26) and, thus, it is not possible to assign any of the observed effects to a single flotillin protein after flot-2 depletion, we focused our analysis on flot-1. Using transient transfection of siRNAs, we generally obtained a knockdown efficiency of about 85–90% in more than 95% of the cells (data not shown and Figs. 1, 3, 7, and 8). Most experiments were performed using two different siRNAs directed against flot-1, with highly similar results. For the sake of brevity, only the results with the siRNA-B are shown below. For some experiments, the data are shown in the supplemental Fig. S4.
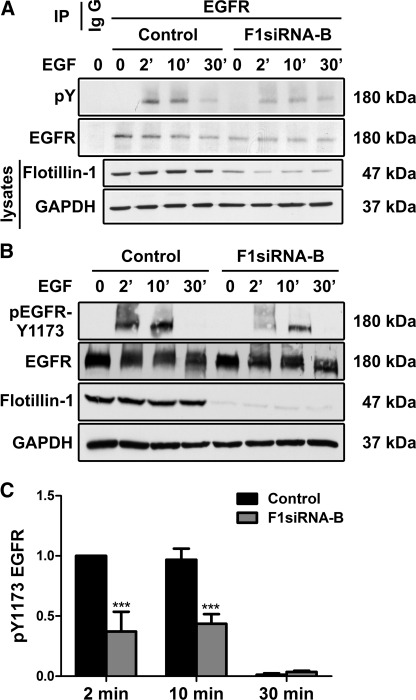
FIGURE 1.
Knockdown of flot-1 results in reduced Tyr phosphorylation of EGFR. Flot-1 was depleted by means of siRNAs. The cells were subsequently starved and stimulated or not with EGF for 2, 10, or 30 min, and EGFR was immunoprecipitated (IP). Precipitated proteins were separated by SDS-PAGE and probed with the indicated antibodies. The cell lysates were analyzed with the monoclonal antibody against flot-1 to verify its depletion, and GAPDH was used as an equal loading control. Effect of flotillin knockdown on total tyrosine phosphorylation (pY; A) and Tyr-1173 (B) was measured. C, the bar graph show a densitometric quantification of the phosphorylation of Tyr-1173. The signals of phosphorylated Tyr-1173 were normalized to the amount of total EGFR. Bars represent the mean ± S.D. of at least three individual experiments. ***, p < 0.001.
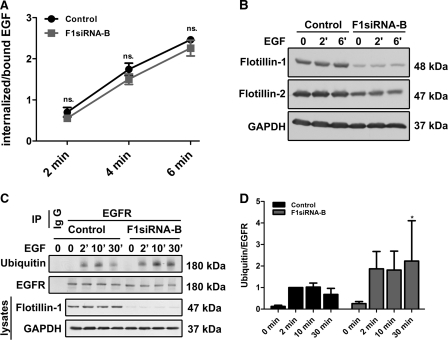
FIGURE 3.
Flot-1 depletion does not inhibit EGFR endocytosis and ubiquitination. F1-KD and control cells were incubated with 125I-EGF (1.5 ng/ml) for the indicated time points, and the internalized versus cell surface-bound EGFs were measured. The data are a summary of three independent experiments, each with triplicate samples for each time point. A, the graph shows the ratio of internalized to surface bound EGF as a function of endocytosis time, including the S.D. The difference between control cells and F1-KD cells was not significant (ns.). B, F1-KD efficiency in the cells used for the experiment is shown. C, Flot-1 was depleted by means of RNAi in HeLa cells that were subsequently starved and stimulated or not with EGF for 2, 10, or 30 min. EGFR was immunoprecipitated (IP) from the cells, and the precipitates were probed with an anti-ubiquitin antibody. IgG, an isotype matched control antibody was used to show the specificity. D, quantification of EGFR ubiquitination from four individual experiments is shown. Ubiquitin signals were normalized to total EGFR. *, p < 0.05.
FIGURE 7.
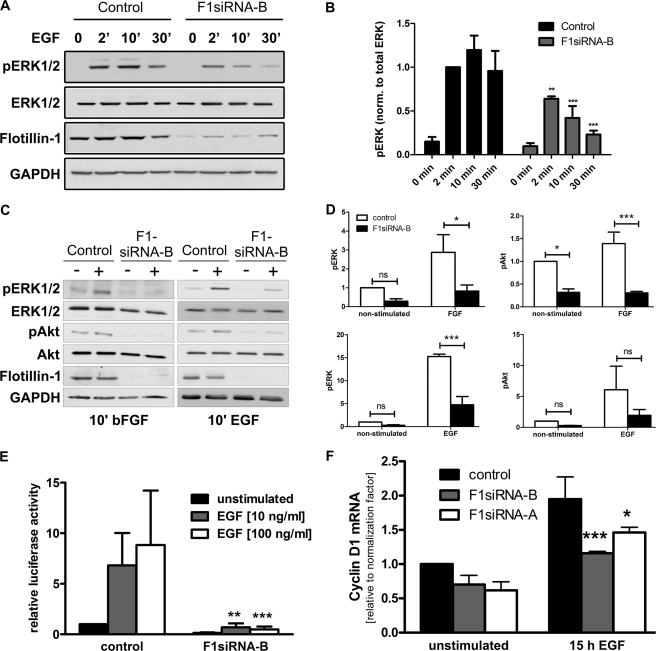
Activation of MAP kinase pathway is reduced after flot-1 knockdown. Flot-1 was knocked down in HeLa cells. The cells were starved and stimulated or not with EGF for 2, 10, or 30 min. Cell lysates were separated by SDS-PAGE and probed with the indicated antibodies. GAPDH was used as an equal loading control. A, knockdown of flot-1 resulted in severe reduction of activation of the MAP kinases ERK1/2 (41/42 kDa). B, densitometric quantification of the pERK1/2 (42/42 kDa) is shown. The values of phosphorylated ERK1/2 were normalized to total ERK. C, in F1-KD cells, activation of ERK1/2 and Akt were measured after a 10-min treatment with bFGF or EGF. D, shown is quantification of the results in C. ns, not significant. E, transcriptional activation after EGF stimulation was measured using a serum-responsive element-based luciferase reporter gene assay. Transcriptional activation in F1-KD cells was severely impaired irrespective of the EGF dose used. F, quantitative real-time PCR was used to measure the mRNA for cyclin D, which was less induced after EGF stimulation of F1-KD cells. B and D–F, bars represent the means of at least three individual experiments with standard deviation. **, p < 0.01; ***, p < 0.001.
FIGURE 8.
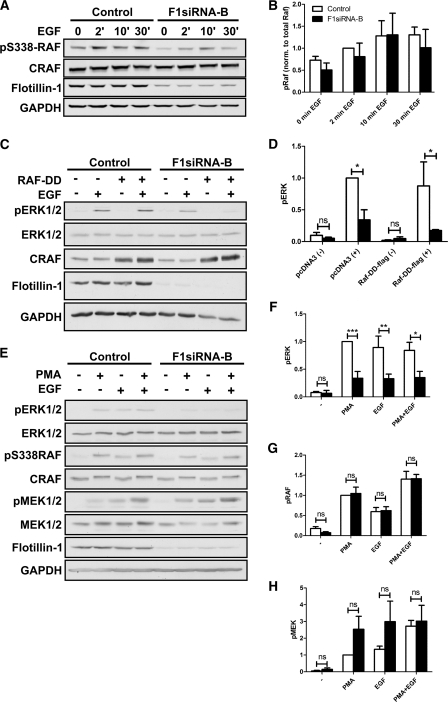
Forced activation of CRAF does not result in improved ERK phosphorylation. A, phosphorylation of CRAF on Ser-338 was studied after EGF stimulation in F1-KD and control cells. B, quantification of the phosphorylation of CRAF p338 after normalization to total CRAF (n = 3). C, transfection of F1-KD cells with an active CRAF mutant (RAF-DD) was not able to rescue the reduced phosphorylation of ERK1/2. D, quantification of the data in C (n = 3). E, stimulation of the cells with PMA did not result in improved ERK1/2 phosphorylation in F1-KD cells. F–H, shown is quantification of the phosphorylation data shown in E. F = pERK. G = p338 CRAF. H = pMEK (n = 3). ns, not significant. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
When Tyr phosphorylation of EGFR was analyzed with a non-site-specific anti-phospho-Tyr antibody after immunoprecipitation from control or flot-1 siRNA-transfected cells, we observed a marked reduction of EGFR phosphorylation in F1-KD cells (Fig. 1A). These results prompted us to analyze the phosphorylation of EGFR by means of antibodies detecting the phosphorylation status of specific Tyr residues. Several Tyr residues in the cytoplasmic domain of EGFR become phosphorylated during EGF stimulation. One of the first ones is Tyr-1173, which is autophosphorylated in trans by the dimerized EGFR. We observed a statistically highly significant (p < 0.001) reduction of phosphorylation of Tyr-1173 of about 50% in F1-KD cells after EGF stimulation (Fig. 1, B and C). Furthermore, the kinetics of the activation was delayed as compared with control cells. Likewise, the phosphorylation of Tyr-1068 (supplemental Fig. S1) showed the same tendency, although the results were not statistically significant. We attempted to analyze Tyr-1045, -992, and -845 but were unable to obtain quantitative results due to weak signals. Taken together, these results indicate that knockdown of flot-1 significantly affects the activation/phosphorylation of EGFR at least in Tyr-1173, which is an important autophosphorylation site.
F1-KD Impairs EGF-induced Clustering of EGFR at Cell Surface
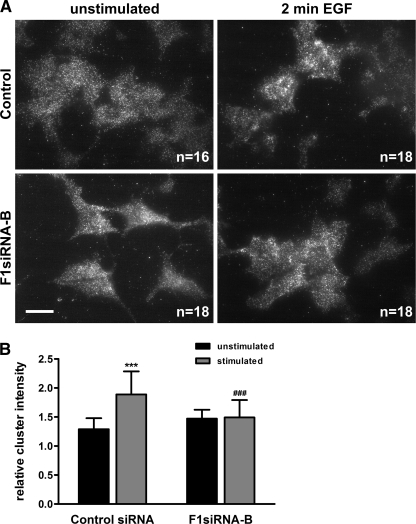
Because the reduced phosphorylation of EGFR was pronounced already after a short (2-min) EGF stimulation, we postulated that the absence of flot-1 affects an early step during EGFR activation, such as formation of receptor clusters at the cell surface. To study this, we used total TIRF microscopy, which can specifically and sensitively measure events that take place at or very close (about 200 nm) to the plasma membrane without interference of organelles residing deeper in the cells, such as endosomes. In addition, we used only a very short EGF stimulation of 2 min, during which only limited endocytosis takes place. Cells were plated on coverslips, starved, and either stimulated or not with EGF. After fixation, EGFR was detected with antibodies and their fluorescent conjugates and observed under a TIRF microscope (Fig. 2A). Images of the cells were analyzed for the clustering behavior (integrated fluorescence intensity of each cluster) of EGFR at the cell surface in starved versus EGF-stimulated cells (Fig. 2B). In control siRNA cells, EGFR underwent a statistically highly significant (p < 0.001) EGF-induced increase in clustering. In the F1-KD cells, EGFR showed a slight but statistically insignificant increase in clustering already in unstimulated cells without any further change upon EGF stimulation. However, the difference in the fluorescence intensity of the clusters in EGF-stimulated control versus F1-KD cells was statistically highly significant. These results suggest that upon ligand-mediated activation, EGFR forms clusters at the cell surface and that in the absence of flot-1 this clustering is impaired. This is in line with our previous data showing that flotillin hetero-oligomers increase in size upon EGF stimulation (26).
FIGURE 2.
EGF-induced clustering of EGFR at the cell surface is impaired by flot-1 knockdown. Flot-1 was knocked down in HeLa cells by siRNA. Knockdown and control cells were starved and stimulated or not for 2 min with EGF. The cells were fixed and immunostained for EGF receptor. Cell surface associated EGFR was detected by total internal reflection microscopy. The images (A) were analyzed for the mean EGFR cluster intensity. B, shown is quantification of the EGFR cluster intensity upon EGF stimulation in control and F1-KD cells (n = 3). ***, p < 0.001 compared to unstimulated control; ###, p > 0.001 compared to stimulated control.
Knockdown of Flot-1 Does Not Inhibit EGFR Endocytosis or Ubiquitination
Our earlier findings would suggest that flotillins are not directly involved in the endocytosis of EGFR (26). However, some colocalization of EGFR with flotillins can be observed in late endosomes after longer time points of EGF stimulation (18). Because both clathrin-mediated and clathrin-independent endocytosis routes for EGFR have been described (32, 33), the possibility exists that flotillins might still play a role in the latter. Thus, it was important to measure the early endocytosis kinetics of EGFR internalization in the F1-KD cells. For this we used an assay measuring the rate of endocytosis of the radiolabeled ligand EGF for 2–6 min. There was no significant difference in the amount of radioactive ligand bound to the cell surface of control versus F1-KD cells. The ratio of the internalized versus surface bound EGF was determined for each time point (2, 4, and 6 min) in three independent experiments (each with three parallel samples for each time point) and plotted for control and F1-KD cells (Fig. 3A). The rate constant of the endocytosis was calculated and found to be only slightly changed (Ke = 0.88) for F1-KD cells as compared with control siRNA cells (Ke = 0.85). However, this difference was statistically not significant (Fig. 3), indicating that the early endocytosis kinetics of EGFR is not significantly altered after flot-1 depletion. Fig. 3B shows the successful knockdown of flot-1 in the cells used for this assay.
After longer time points of endocytosis, EGFR localization and endocytosis were not visibly affected in F1-KD cells as compared with cells transfected with the control siRNA (10 min endocytosis, supplemental Fig. S2A). Furthermore, EGFR was able to reach late endosomes in F1-KD cells, as evidenced by its colocalization with LAMP3/CD63 after 15 min of EGF stimulation (supplemental Fig. S2B).
EGFR endocytosis and degradation have been shown to be regulated e.g. by ubiquitination of the cytoplasmic domain of EGFR (34, 35). We immunoprecipitated EGFR from starved or EGF-treated control or flot-1 siRNA-transfected cells and used ubiquitin-specific antibodies to detect receptor ubiquitination in the immunoprecipitates. As shown in Fig. 3C, a robust signal for ubiquitinated EGFR was detected already after 2 min of EGF stimulation in both control and F1-KD cells. In fact, the ubiquitination of EGFR appeared to be even slightly higher in the cells lacking flot-1 expression. However, quantification of the results from four independent experiments showed that the difference was not significant. Only after a longer stimulation of 30 min did the difference became slightly significant, but the standard deviation was very large due to considerable variation between the experiments. Taken together, the data from our EGF uptake, immunofluorescence, and ubiquitination assays suggest that the observed inhibition of EGFR activation in F1-KD cells is not due to impairment of EGFR endocytosis but must rely on some other molecular mechanism.
EGF Receptor and Flotillins Form Constitutive Complex
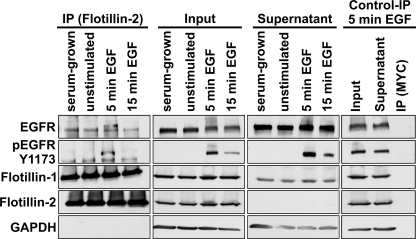
Because F1-KD affected the clustering of EGFR, we performed co-immunoprecipitation experiments to analyze if flotillins are found in complex with EGFR. We immunoprecipitated flot-2 from serum-cultured, -starved, and EGF-stimulated (5 and 15 min) cells. EGFR was coprecipitated with flot-2 under all conditions, suggesting that the complexes are constitutive (Fig. 4, left). In the 5-min EGF samples, a slower migrating band appeared in the immunoprecipitates, apparently representing Tyr-1173-phosphorylated EGFR, which was precipitated with flot-2 (Fig. 4, left, second panel from top). No co-precipitation was detected in control immunoprecipitation samples (stimulated 5 min EGF). As shown by us before (26), flot-1 heavily coprecipitated with flot-2 at all time points. Western blots of the supernatant fractions show that only a fraction of EGFR/Tyr(P)-1173 is associated with flotillins.
FIGURE 4.
EGFR co-immunoprecipitates with flotillins. HeLa cells (serum-grown, unstimulated, or EGF stimulated) were lysed and immunoprecipitated with antibodies against flot-2. Co-immunoprecipitation of EGFR with flotillins was detected in all samples. After 5 min of EGF, a precipitated band (185 kDa) migrated slightly higher than EGFR (180 kDa) in unstimulated samples and corresponded to the Tyr-1173-phosphorylated EGFR, as evidenced by detection with an anti-Tyr(P)-1173 antibody. Flot-1 (47 kDa) coprecipitated with flot-2 (48 kDa) in all samples, whereas no precipitation of EGFR or flotillins was detected in the control immunoprecipitation carried out with an anti-myc antibody (Control IP). Equal loading was verified with GAPDH (37 kDa).
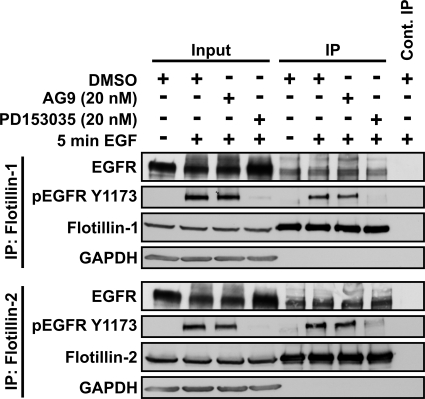
To test if the complex of EGFR could be disrupted by inhibiting the EGFR kinase activity, the cells were preincubated with the EGFR kinase inhibitor PD153035 or a non-inhibiting compound AG9 or DMSO as controls before EGF stimulation. Immunoprecipitation was performed either for flot-1 (Fig. 5, upper part) or flot-2 (lower part). EGFR co-immunoprecipitated with both flotillins despite inhibition of its kinase activity. Successful inhibition of EGFR was demonstrated by reduced phosphorylation on Tyr-1173 (Fig. 5, second panel from top). Thus, the flotillin/EGFR complexes appear to be independent of EGFR kinase activity and phosphorylation. However, on the basis of these data, we cannot say if there is a direct interaction between flotillins and EGFR or if the complex formation is mediated by bridging proteins/adapters.
FIGURE 5.
Co-precipitation of EGFR and flotillins is not dependent on EGFR kinase activity. Starved HeLa cells were treated with an EGFR kinase inhibitor PD153035, non-inhibiting analog AG9 (each 20 nm), or DMSO and then stimulated for 5 min with EGF. Immunoprecipitation (IP) was performed with anti-flot-1 or flot-2 antibodies. Co-precipitation of EGFR with both flotillins was detected also in samples treated with PD153035, and Tyr-1173-phosphorylated EGFR was coprecipitated in the samples not treated with the inhibitor but treated with EGF. For the molecular masses of the proteins, please refer to the legend of Fig. 4.
Association of EGFR with Light Membranes Requires Flot-1
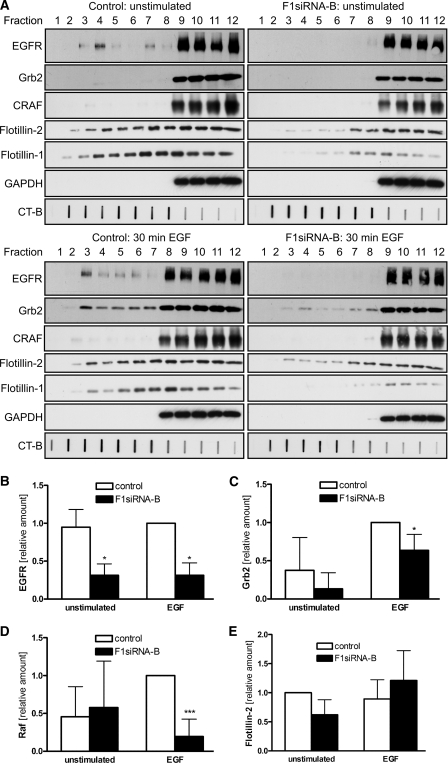
EGFR has been reported to associate with membrane rafts during signaling and to undergo a dynamic inclusion into rafts (6, 7). However, some discrepancy exists as to whether the non-activated or activated form of EGFR is a raft resident and if the receptor moves in or out of rafts upon activation. To study if the depletion of flot-1 affects the raft association of EGFR during signaling, we isolated rafts from non-stimulated and EGF-treated cells by means of a modification of a detergent-free fractionation method (31). We included a sonication step after the mechanical homogenization to facilitate proper shearing of the membrane fragments. The detergent-free method was used because we were unable to detect any significant fraction of EGFR in detergent-insoluble membrane fractions isolated after extraction with several different detergents (data not shown). Stimulation was performed for 30 min on ice, as described previously (36), to prevent endocytosis of EGFR during the stimulation. In starved cells transfected with the control siRNA, EGFR was mainly distributed in fractions 9–12, and a fraction of the total EGFR was found associated with the light fractions 3 and 4 (Fig. 6A, left, upper part). The distribution of EGFR was only moderately changed after EGF (Fig. 6A, left, lower part). However, in F1-KD cells, EGFR was only found in the heavy fractions 9–12, indicating that in the absence of flot-1, EGFR is not capable of associating with the light membranes/rafts either in starved or stimulated cells (Fig. 6A, right). Densitometric quantification of the signals in the light fractions 1–5 (4 independent experiments) showed that there is a statistically significant difference in the amount of EGFR present in these fractions in both EGF stimulated and unstimulated control versus F1-KD cells (Fig. 6B).
FIGURE 6.
EGFR and Grb2 do not translocate into light fractions in F1-KD cells. Flot-1 was knocked down in HeLa cells by siRNA. The cells were starved and stimulated with 100 ng/ml EGF for 30 min on ice or left untreated. A, rafts were isolated from the cells using a detergent-free method. Fractions were collected from the top (fraction 1 = the lightest fraction), run on SDS-PAGE, and analyzed by Western blotting. The localization of EGFR (180 kDa), Grb2 (25 kDa), CRAF (74 kDa), flotillins (47/48 kDa), and GAPDH (37 kDa) was studied. The raft marker GM1 was detected with HRP-coupled cholera toxin in a slot blot. Quantification of the relative distribution in the light fractions: EGFR (B); Grb2 (C); CRAF (D); flotillin-2 (E) (summary of four independent experiments). CT-B , cholera toxin B subunit. *, p < 0.05; ***, p < 0.001.
We also studied the location of the MAP kinase pathway signaling partners of EGFR, Grb2, and CRAF, in our gradients. In control cells, Grb2 shifted into the light fractions (up to fraction 3) upon EGF treatment (Fig. 6A, left), and this shift was also observed in the absence of flot-1 (Fig. 6A, right). However, there was a statistically significant reduction in the amount of Grb2 present in the light fractions of stimulated flot-1 knockdown cells (Fig. 6C). No major difference in the location of CRAF was observed between control and F1-KD cells in unstimulated cells. However, after EGF stimulation, significantly less CRAF was found in the light fractions in F1-KD cells (Fig. 6D), although this only represents a minor fraction of the total CRAF signal in the gradient. Surprisingly, flot-2 was found to display a very broad distribution throughout fractions 2–12 in control cells, without any clear change upon EGF stimulation (Fig. 6A, left). In F1-KD cells, flot-2 was found mainly in fractions 7–12, with only a minor amount present in fractions 3–6. Again, the distribution of flot-2 did not change upon EGF incubation (Fig. 6A, right). However, this difference turned out to be statistically nonsignificant (Fig. 6E). We observed no difference in the distribution of the GM1 ganglioside binding cholera toxin B subunit (CT-B) between control and F1-KD cells, suggesting that the formation of rafts as such is not impaired in the absence of flot-1 (Fig. 6A). Similar results were obtained when EGF stimulation was performed for 2 min at 37 °C, where we observed a shift of Grb2 into raft fractions in the control but far less in F1-KD cells (supplemental Fig. S3). In those experiments a fraction of CRAF was detectable in the light fractions also without stimulation, with an increase after EGF, indicating recruitment into rafts. Taken together, these results show that in HeLa cells, a fraction of EGFR is constitutively localized in rafts, consistent with our results from co-precipitation experiments, whereas Grb2 is recruited into membrane rafts upon EGF stimulation. In both cases flot-1 expression is necessary for the localization of EGFR, Grb2 and CRAF into rafts.
Knockdown of flot-1 Impairs MAP Kinase Signaling
EGFR signaling results in activation of various downstream pathways, e.g. the MAP kinase pathway, and impaired activation of EGFR should also inhibit the activation of these downstream signaling events. We thus studied the activation of MAP kinases ERK1/2 after flot-1 depletion. Consistent with the low degree of activation of EGFR in flot-1-depleted cells, we observed a highly significantly (p < 0.001) reduced phosphorylation of ERK1/2 in the absence of flot-1 expression (Fig. 7, A and B), which again was evident already after a short stimulation and persisted after longer times (30 min) of stimulation. Please refer to supplemental Fig. S4, A and B for the data with a second siRNA against flot-1.
We next tested if the activation of the signaling pathways of other receptor Tyr kinases might also be impaired upon flot-1 depletion. In cells stimulated with bFGF (Fig. 7C, left), we also observed a significantly reduced activation of MAP kinase (measured as phospho-ERK1/2, quantification of three experiments in Fig. 7D, upper left). The phosphatidylinositol 3-kinase pathway (measured as activation of protein kinase B/Akt) showed a basal activation that was only mildly (1.5 times) stimulated by FGF. However, both the basal activity and the stimulation response were significantly reduced upon F1-KD (Fig. 7D, upper right). Significantly reduced ERK activation was also detected in F1-KD cells after EGF treatment (Fig. 7, C, right, and D, lower left), whereas the mild reduction in Akt activation was statistically not significant (Fig. 7D, lower right). The data for the second flot-1 siRNA are shown in supplemental Fig. S4C. These results show that the activation of the MAP kinase pathway and the Akt signaling is inhibited in flot-1-depleted cells stimulated by two different growth factors.
Activation of MAP kinase signaling culminates into a transcriptional response during which transcription of specific genes is enhanced. To study the transcriptional activation by MAP kinases in EGF-stimulated control and F1-KD cells, we used a reporter gene assay with a plasmid containing five copies of the serum responsive element in front of the luciferase gene (Fig. 7E). Control cells showed a clear increase in luciferase activity after EGF treatment, whereas in F1-KD cells barely any luciferase activity was detected. We next used quantitative real-time PCR to measure the induction of cyclin D after EGF stimulation. Again, a significantly reduced induction of cyclin D mRNA was seen in F1-KD cells with two siRNAs (Fig. 7F). These data show that the EGF-induced transcriptional activation mediated by MAP kinases is inhibited in the absence of flot-1.
Consistent with an upstream failure to activate MAPK pathway, we observed a tendency to a reduced phosphorylation of CRAF on Ser-338 in F1-KD cells after EGF stimulation (Fig. 8A). However, due to the large variation, these results were statistically not significant (Fig. 8B). We postulated that because failure to activate EGFR in F1-KD cells results in reduced phosphorylation of MAP kinase pathway components, transfection with an active CRAF mutant should result in normal MAPK activation. Control and F1-KD cells were transfected with a mutant CRAF (RAF-DD) in which two Tyr residues in the catalytic domain have been exchanged into aspartic acid (29). Surprisingly, RAF-DD was not able to rescue ERK phosphorylation in F1-KD cells (Fig. 8C, quantification is shown in Fig. 8D). On the contrary, overexpression of RAF-DD resulted in even further diminished ERK phosphorylation upon flot-1 knockdown. Similar results were obtained with another RAF mutant, which contains a S259A mutation (data not shown).
Although the RAF mutants above display an increased activity, they are still dependent on the activation of Ras. However, in the F1-KD cells, the upstream activation signals coming from the EGFR are reduced. Thus, to overcome the problem with the reduced upstream activity, we next attempted to rescue the MAPK activation with PMA, which activates protein kinase C (PKC). PKC has been shown to mediate the activation of CRAF in a manner that bypasses Ras (37, 38). Thus, if F1-KD only affected Ras activation or steps upstream of it, PMA should result in a normal activation of CRAF, MEK1/2, and ERK. PMA and EGF resulted in robust activation of CRAF, MEK1/2, and ERK1/2 in control cells, whereas the diminished activation of ERK1/2 was still evident in F1-KD cells (Fig. 8, E and F). However, the PMA-induced activation of CRAF and MEK1/2 in F1-KD cells were comparable with control cells (Fig. 8, G and H). These results strongly suggest that the EGF-induced activation of MAPK signaling is impaired both upstream (at the level of EGFR) and downstream of Ras, as bypassing Ras (by PMA) could not rescue ERK1/2 activation in F1-KD cells. This regulation appears to take place downstream of CRAF and MEK1/2.
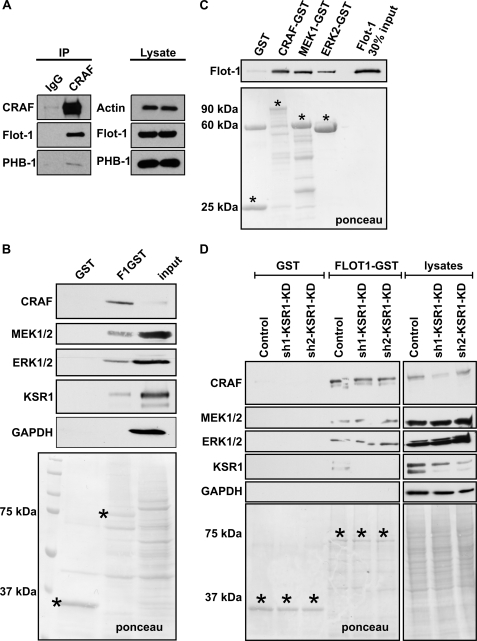
To test if CRAF activation might be directly modulated by flot-1, we analyzed if CRAF would be found in a complex with flot-1. Indeed, flot-1 was detected in CRAF immunoprecipitates from HeLa cells (Fig. 9A), as was prohibitin-1 (PHB-1), another member of the PHB protein family that flotillins also belong to and that has previously been shown to interact with CRAF (13, 39). Because overexpression of CRAF mutants in F1-KD resulted in reduced ERK activity, this might imply that flot-1 functions as a scaffold for MAPK signaling. To test this, we performed a GST pulldown assay using flot-1-GST (Fig. 9B). We successfully pulled down CRAF, MEK1/2, and ERK1/2 from serum-grown HeLa cells. KSR1 (kinase suppressor of RAS), a well characterized scaffold of MAP kinases, was also found to be precipitated in this assay. Because KSR1 is capable of binding CRAF, MEK, and ERK, it was important to test if KSR1 mediates the interaction of flot-1 with the MAPK cascade proteins. In direct pulldown experiments, CRAF-GST, MEK1-GST, and ERK2-GST all bound recombinant, purified flot-1 directly (Fig. 9C). Furthermore, all three proteins from cell lysates of HeLa cells depleted of KSR1 bound to flot1-GST (Fig. 9D). Thus, the binding of CRAF, MEK1, and ERK2 to flot-1 does not require KSR1.
FIGURE 9.
Flotillin-1 functions as a MAP kinase scaffold. A, CRAF was immunoprecipitated (IP) from HeLa cells, and the immunoprecipitates were probed for flot-1 (47 kDa) and prohibitin-1 (PHB-1; 30 kDa), which both coprecipitated with CRAF. B, in flotillin-1-GST (75 kDa) pulldown assays, CRAF (74 kDa), MEK1/2 (45/47 kDa), ERK1/2 (42 kDa), and KSR1 (110 kDa) interacted with flot-1. C, purified recombinant flot-1 directly interacted with GST-tagged CRAF, MEK1, and ERK2. D, KSR1 was knocked down with two different shRNAs in HeLa cells. Pulldown experiments with flotillin-1-GST show that CRAF, MEK1/2, and ERK1/2 are capable of binding to flot-1 in the absence of KSR1. In addition, KSR1 was also found in flot-1 pulldown assays from control cells. B–D, Ponceau staining was used to visualize the fusion proteins (marked by an asterisk) on the blot membranes.
Taken together, our results show that flot-1 is directly involved in the regulation of MAPK pathway activation, first at the level of EGFR and later on by regulating the ERK activation, whereupon flot-1 may function as a novel MAPK scaffold.
DISCUSSION
Flot-1 Depletion Results in Signaling Defects of Receptor-tyrosine Kinases
Our earlier studies show that flotillin proteins are phosphorylated and endocytosed from the plasma membrane upon EGF stimulation of the cells (18, 26). These findings raised the question if flotillins are also directly involved in the signaling and/or trafficking of EGFR. Here we could show that the activation of EGFR, measured as phosphorylation of specific Tyr residues, and the downstream signaling by MAP kinases ERK1/2 was severely compromised in cells depleted of flot-1. These results suggest that flot-1 and possibly flot-2 indeed play a role in the activation of EGFR and its downstream signaling pathways. However, these effects are not prominent in cells highly expressing EGFR receptor, such as A431 cells, suggesting that pathological levels of EGFR might overcome flot-1 deficiency by other mechanisms (data not shown). Nevertheless, impairment of signaling of membrane receptors upon F1-KD seems not to be restricted to EGFR. Here, we observed a reduced activation of ERK1/2 and Akt after bFGF stimulation in the absence of flot-1, indicating that flot-1 is also important for FGF receptor signaling. Flot-1 has previously been shown to be involved in signaling induced by several membrane receptors such as the neurotrophin receptor TrkA, chemokine receptor CXCR4, and IgE receptor (17, 24, 40). Knockdown of flot-1 was shown to result in reduced Ca2+ mobilization and phosphorylation of ERK1/2 upon IgE receptor stimulation. Furthermore, phosphorylation of IgE receptor, which is mediated by the Src family kinase Lyn, was also impaired as a result of flot-1 depletion (17). However, the molecular mechanism of impairment of the IgE receptor signaling by F1-KD is different from that observed in our study for EGFR.
Flot-1 Is Not Essential for EGFR Endocytosis
Some studies have suggested that flot-1 is involved in endocytosis and even defines a novel non-clathrin, non-caveolin endocytic pathway (15, 27). Recent findings have suggested that basolateral endocytosis of glycosylphosphatidylinositol-anchored proteins (41) and the protein kinase C-induced endocytosis of the dopamine transporter DAT (20) might rely on flotillins. However, to date there is no direct evidence for the involvement of flotillins in EGFR endocytosis! EGFR can be endocytosed by means of both a clathrin-dependent and a non-clathrin, raft-mediated endocytosis. When low concentrations of EGF are used for the stimulation, the clathrin pathway is the predominant means of endocytosis, whereas higher EGF concentrations (>20 ng/ml) direct EGFR more toward the raft pathway (33, 42). Because EGFR and flotillins colocalize after endocytosis (26), it would be reasonable to assume that flot-1 might be involved in the raft-mediated endocytosis of EGFR. However, we did not observe any significant difference in the endocytosis of EGFR between control and F1-KD cells either in the radioligand uptake assay or by means of immunofluorescence staining. Furthermore, our unpublished data show that after simultaneous knockdown of flot-1 and clathrin heavy chain, EGFR endocytosis still occurs. Although the necessity of Tyr phosphorylation of EGFR for its endocytosis is still a matter of debate (see an excellent review Sorkin and Goh (43)), ubiquitination of the cytoplasmic domain of EGFR has been suggested to be sufficient for the endocytic uptake to take place (34, 35). In F1-KD cells, ubiquitination of EGFR was comparable or even slightly increased as compared with the control cells, thus facilitating normal endocytic uptake. Thus, our results demonstrate that flot-1 is not directly involved in the endocytosis of EGFR in HeLa cells.
Flot-1 Regulates Clustering of Membrane Proteins at Cell Surface and Recruitment of Signaling Proteins into Rafts
Endocytosis of EGFR was not affected by F1-KD, and our biochemical results suggested that flot-1 is necessary for early events that take place at the plasma membrane during EGFR activation and signaling, such as EGFR autophosphorylation and activation of downstream signaling toward MAP kinases. In line with this, our TIRF data demonstrated that knockdown of flot-1 alters the clustering behavior of EGFR at the plasma membrane upon EGF stimulation. Intriguingly, previous findings have shown that flot-2 plays a role in the clustering of the Alzheimer amyloid precursor protein at the cell surface, thereby affecting its proteolytic processing and generation of the pathogenic amyloid β peptide (44). Thus, one important function of flotillins might be to regulate the formation of macromolecular complexes/clusters at the plasma membrane that are necessary for initiating cellular processes such as signal transduction or, in the case of amyloid precursor protein, endocytosis and proteolytic processing. Consistent with this, we were able to co-immunoprecipitate flotillins together with the EGFR, suggesting that a fraction of EGFR forms a constitutive complex with flotillins, and flot-1 may be required for the localization of EGFR into rafts. However, at this point we are not able to say if there is a direct interaction between flotillins and EGFR or if further proteins are required to mediate the complex formation.
Membrane rafts are known to participate in the regulation of EGFR signaling, and in some cell types, a significant portion of non-activated EGFR is localized in non-caveolar rafts (7, 45, 46). However, recent findings have suggested that EGF stimulation may cause different kinds of rafts carrying different molecules to coalesce (47). Consistently, we found that a fraction of EGFR was localized in rafts in both unstimulated and EGF-treated cells and that this localization was lost upon knockdown of flot-1. Furthermore, early signaling partners of EGFR, such as Grb2, were found to translocate toward light membrane fractions upon EGF stimulation. We showed earlier that flot-1/flot-2 hetero-oligomers exist already in unstimulated cells, but their size increases upon EGF stimulation (26). Thus, our results here would be consistent with the model postulating that the increase in the oligomer size would result from coalescence of different subpopulations of flotillin-containing rafts, which each may contain their own EGFR/MAPK signaling constituents in complex with flotillins.
Flotillins are clearly interdependent, and flot-2 depletion results in profound loss of flot-1, whereas F1-KD reduces the amount of flot-2 by about 50%. Thus, it is also possible that the effect on EGFR clustering is partly due to the severe reduction of flot-1/flot-2 hetero-oligomers or even a dominant negative effect of flot-2 homo-oligomers. However, the impairment of the downstream signaling seen at the level of ERK is most likely a direct consequence of flot-1 depletion due to the direct interaction of flot-1 with the MAPK cascade proteins.
Our data would support the view that MAP kinase signaling cascade is at least partly localized in rafts. Our results also suggest that rafts and flotillins, especially flot-1, are actively involved in the early signaling events that lead to activation of EGFR and downstream signaling pathways, and flotillin rafts might actually represent the domains in which the signaling is initiated.
Is Flot-1 MAP Kinase Scaffold?
In addition to its direct effect on EGFR activation, we discovered a novel, unexpected function of flot-1 in the regulation of further signaling steps downstream of Ras. In F1-KD cells, ERK1/2 was found to be poorly phosphorylated, consistent with an upstream activation defect at the level of EGFR. However, PMA, which induces a PKC mediated activation of CRAF independently of Ras, failed to enhance ERK activation in F1-KD cells. However, PMA resulted in fairly normal phosphorylation of CRAF on Ser-338 and activation of MEK1/2. These data suggest that the defect caused by flot-1 depletion must reside even downstream of CRAF/MEK1/2. On the other hand, CRAF was found to be present in a complex with flot-1, which also contained MEK-1/2, KSR1, and ERK1/2. However, the multimeric protein complexes containing flot-1 and EGFR, shown by co-immunoprecipitation, versus those containing flot-1 and MAPKs and KSR are likely to be separated spatially and by the presence of their individual constituents. Thus, it is possible that EGF stimulation results in coalescence of different kind of microdomains, as suggested by Hofman et al. (47), and these microdomains may contain flotillins. Flotillin-dependent clustering of EGFR molecules at the cell surface, as evidenced by our TIRF data, might bring together essential signaling elements for MAP kinase activation. This is supported by the observation that recruitment of Grb2 to the raft fractions was very much impaired in flot-1-depleted cells. The role of flotillin complexes might be either to act as scaffolds for MAP kinase signaling proteins, as suggested by our data, or to protect EGFR from dephosphorylation by protein phosphatases, which negatively regulate signaling. The former function is also supported by our findings that we have recently identified a direct interaction between flot-1 and fibroblast growth factor receptor substrate 2 (52), which has been suggested to function as a MAP kinase regulator/scaffold during signaling and to bind to ERK (48–51). Although FRS2 mainly functions during FGF signaling, our observations that F1-KD also impairs FGF receptor signaling not only toward MAP kinases but also phosphatidylinositol 3-kinase/Akt signaling are consistent with the model in which flot-1 may play a more general role in regulating receptor-tyrosine kinase signaling in rafts. Thus, here we have revealed a previously undetected function for flotillin-1 as a MAP kinase scaffold that is capable of uniting several MAPK pathway proteins and is required for productive growth factor signaling. Because flot-1 directly interacts with the MAPK cascade proteins, it appears to represent a novel MAPK scaffold. In future studies, it will be interesting to unveil the functional details of the direct interaction of the MAPK signaling proteins with flot-1 and to characterize the putative interplay of flot-1 with KSR1. In addition, it will be important to characterize if these flot-1 scaffolds are active at the plasma membrane or in endosomal membranes to which flotillins relocate after EGF stimulation (18).
Supplementary Material
Acknowledgments
We acknowledge Nina Kurrle for critical reading of the manuscript and Petra Janson for skilled technical support. We are grateful to Dr. R. Schmidt, D. Hinchliffe, and G. Weigand (Central Biotechnical Unit, University of Giessen) for use of the excellent isotope laboratory facility and friendly supervision. We thank Dr. Ivan Dikic, head of the Institute of Biochemistry II, Frankfurt, for allowing us continued use of the microscopic facilities of the institute even after our move to Giessen.
This work was supported by German Research Council (Deutsche Forschungsgemeinschaft) Grants Ti291/6-1 and Ti291/6-2 (to R. T.) and Emmy Noether Program Grant RA1739/1-1 (to K. R.).

This article contains supplemental Figs. S1–S4.
- EGFR
- EGF receptor
- bFGF
- basic FGF
- Grb2
- growth factor receptor-associated protein 2
- KSR
- kinase suppressor of RAS
- TIRF
- total internal reflection of fluorescence
- flot
- flotillin
- F1-KD
- flotillin-1 knockdown
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- PMA
- phorbol 12-myristate 13-acetate
- GM1
- galactosyl-N-acetylgalactosaminyl-sialyl-galactosylglucosylceramide.
REFERENCES
- 1. Levkowitz G., Waterman H., Zamir E., Kam Z., Oved S., Langdon W. Y., Beguinot L., Geiger B., Yarden Y. (1998) c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 12, 3663–3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carpentier J. L., Gorden P., Anderson R. G., Goldstein J. L., Brown M. S., Cohen S., Orci L. (1982) Co-localization of 125I-epidermal growth factor and ferritin low density lipoprotein in coated pits. A quantitative electron microscopic study in normal and mutant human fibroblasts. J. Cell Biol. 95, 73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gorden P., Carpentier J. L., Cohen S., Orci L. (1978) Epidermal growth factor. Morphological demonstration of binding, internalization, and lysosomal association in human fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 75, 5025–5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanover J. A., Willingham M. C., Pastan I. (1984) Kinetics of transit of transferrin and epidermal growth factor through clathrin-coated membranes. Cell 39, 283–293 [DOI] [PubMed] [Google Scholar]
- 5. Waugh M. G., Lawson D., Hsuan J. J. (1999) Epidermal growth factor receptor activation is localized within low buoyant density, non-caveolar membrane domains. Biochem. J. 337, 591–597 [PMC free article] [PubMed] [Google Scholar]
- 6. Pike L. J., Han X., Gross R. W. (2005) Epidermal growth factor receptors are localized to lipid rafts that contain a balance of inner and outer leaflet lipids. A shotgun lipidomics study. J. Biol. Chem. 280, 26796–26804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roepstorff K., Thomsen P., Sandvig K., van Deurs B. (2002) Sequestration of epidermal growth factor receptors in non-caveolar lipid rafts inhibits ligand binding. J. Biol. Chem. 277, 18954–18960 [DOI] [PubMed] [Google Scholar]
- 8. Schulte T., Paschke K. A., Laessing U., Lottspeich F., Stuermer C. A. (1997) Reggie-1 and reggie-2, two cell surface proteins expressed by retinal ganglion cells during axon regeneration. Development 124, 577–587 [DOI] [PubMed] [Google Scholar]
- 9. Neumann-Giesen C., Falkenbach B., Beicht P., Claasen S., Lüers G., Stuermer C. A., Herzog V., Tikkanen R. (2004) Membrane and raft association of reggie-1/flotillin-2. Role of myristoylation, palmitoylation, and oligomerization and induction of filopodia by overexpression. Biochem. J. 378, 509–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu J., Deyoung S. M., Zhang M., Dold L. H., Saltiel A. R. (2005) The stomatin/prohibitin/flotillin/HflK/C domain of flotillin-1 contains distinct sequences that direct plasma membrane localization and protein interactions in 3T3-L1 adipocytes. J. Biol. Chem. 280, 16125–16134 [DOI] [PubMed] [Google Scholar]
- 11. Morrow I. C., Rea S., Martin S., Prior I. A., Prohaska R., Hancock J. F., James D. E., Parton R. G. (2002) Flotillin-1/reggie-2 traffics to surface raft domains via a novel Golgi-independent pathway. Identification of a novel membrane targeting domain and a role for palmitoylation. J. Biol. Chem. 277, 48834–48841 [DOI] [PubMed] [Google Scholar]
- 12. Babuke T., Tikkanen R. (2007) Dissecting the molecular function of reggie/flotillin proteins. Eur. J. Cell Biol. 86, 525–532 [DOI] [PubMed] [Google Scholar]
- 13. Morrow I. C., Parton R. G. (2005) Flotillins and the PHB domain protein family. Rafts, worms, and anaesthetics. Traffic 6, 725–740 [DOI] [PubMed] [Google Scholar]
- 14. Dermine J. F., Duclos S., Garin J., St-Louis F., Rea S., Parton R. G., Desjardins M. (2001) Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J. Biol. Chem. 276, 18507–18512 [DOI] [PubMed] [Google Scholar]
- 15. Glebov O. O., Bright N. A., Nichols B. J. (2006) Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol. 8, 46–54 [DOI] [PubMed] [Google Scholar]
- 16. Hazarika P., McCarty M. F., Prieto V. G., George S., Babu D., Koul D., Bar-Eli M., Duvic M. (2004) Up-regulation of flotillin-2 is associated with melanoma progression and modulates expression of the thrombin receptor protease-activated receptor 1. Cancer Res. 64, 7361–7369 [DOI] [PubMed] [Google Scholar]
- 17. Kato N., Nakanishi M., Hirashima N. (2006) Flotillin-1 regulates IgE receptor-mediated signaling in rat basophilic leukemia (RBL-2H3) cells. J. Immunol. 177, 147–154 [DOI] [PubMed] [Google Scholar]
- 18. Neumann-Giesen C., Fernow I., Amaddii M., Tikkanen R. (2007) Role of EGF-induced tyrosine phosphorylation of reggie-1/flotillin-2 in cell spreading and signaling to the actin cytoskeleton. J. Cell Sci. 120, 395–406 [DOI] [PubMed] [Google Scholar]
- 19. Sugawara Y., Nishii H., Takahashi T., Yamauchi J., Mizuno N., Tago K., Itoh H. (2007) The lipid raft proteins flotillins/reggies interact with Galphaq and are involved in Gq-mediated p38 mitogen-activated protein kinase activation through tyrosine kinase. Cell. Signal. 19, 1301–1308 [DOI] [PubMed] [Google Scholar]
- 20. Cremona M. L., Matthies H. J., Pau K., Bowton E., Speed N., Lute B. J., Anderson M., Sen N., Robertson S. D., Vaughan R. A., Rothman J. E., Galli A., Javitch J. A., Yamamoto A. (2011) Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat. Neurosci. 14, 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Langhorst M. F., Reuter A., Luxenhofer G., Boneberg E. M., Legler D. F., Plattner H., Stuermer C. A. (2006) Preformed reggie/flotillin caps. Stable priming platforms for macrodomain assembly in T cells. FASEB J. 20, 711–713 [DOI] [PubMed] [Google Scholar]
- 22. Rajendran L., Masilamani M., Solomon S., Tikkanen R., Stuermer C. A., Plattner H., Illges H. (2003) Asymmetric localization of flotillins/reggies in preassembled platforms confers inherent polarity to hematopoietic cells. Proc. Natl. Acad. Sci. U.S.A. 100, 8241–8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baumann C. A., Ribon V., Kanzaki M., Thurmond D. C., Mora S., Shigematsu S., Bickel P. E., Pessin J. E., Saltiel A. R. (2000) CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 407, 202–207 [DOI] [PubMed] [Google Scholar]
- 24. Limpert A. S., Karlo J. C., Landreth G. E. (2007) Nerve growth factor stimulates the concentration of TrkA within lipid rafts and extracellular signal-regulated kinase activation through c-Cbl-associated protein. Mol. Cell. Biol. 27, 5686–5698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Solis G. P., Hoegg M., Munderloh C., Schrock Y., Malaga-Trillo E., Rivera-Milla E., Stuermer C. A. (2007) Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem. J. 403, 313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Babuke T., Ruonala M., Meister M., Amaddii M., Genzler C., Esposito A., Tikkanen R. (2009) Hetero-oligomerization of reggie-1/flotillin-2 and reggie-2/flotillin-1 is required for their endocytosis. Cell. Signal. 21, 1287–1297 [DOI] [PubMed] [Google Scholar]
- 27. Frick M., Bright N. A., Riento K., Bray A., Merrified C., Nichols B. J. (2007) Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr. Biol. 17, 1151–1156 [DOI] [PubMed] [Google Scholar]
- 28. Riento K., Frick M., Schafer I., Nichols B. J. (2009) Endocytosis of flotillin-1 and flotillin-2 is regulated by Fyn kinase. J. Cell Sci. 122, 912–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marais R., Light Y., Paterson H. F., Marshall C. J. (1995) Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 14, 3136–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wiley H. S., Cunningham D. D. (1982) The endocytotic rate constant. A cellular parameter for quantitating receptor-mediated endocytosis. J. Biol. Chem. 257, 4222–4229 [PubMed] [Google Scholar]
- 31. Macdonald J. L., Pike L. J. (2005) A simplified method for the preparation of detergent-free lipid rafts. J. Lipid Res. 46, 1061–1067 [DOI] [PubMed] [Google Scholar]
- 32. Orth J. D., Krueger E. W., Weller S. G., McNiven M. A. (2006) A novel endocytic mechanism of epidermal growth factor receptor sequestration and internalization. Cancer Res. 66, 3603–3610 [DOI] [PubMed] [Google Scholar]
- 33. Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., Di Fiore P. P., Polo S. (2005) Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. U.S.A. 102, 2760–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haglund K., Sigismund S., Polo S., Szymkiewicz I., Di Fiore P. P., Dikic I. (2003) Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 5, 461–466 [DOI] [PubMed] [Google Scholar]
- 35. Mosesson Y., Shtiegman K., Katz M., Zwang Y., Vereb G., Szollosi J., Yarden Y. (2003) Endocytosis of receptor-tyrosine kinases is driven by monoubiquitylation, not polyubiquitylation. J. Biol. Chem. 278, 21323–21326 [DOI] [PubMed] [Google Scholar]
- 36. Puri C., Tosoni D., Comai R., Rabellino A., Segat D., Caneva F., Luzzi P., Di Fiore P. P., Tacchetti C. (2005) Relationships between EGFR signaling-competent and endocytosis-competent membrane microdomains. Mol. Biol. Cell 16, 2704–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ueda Y., Hirai S., Osada S., Suzuki A., Mizuno K., Ohno S. (1996) Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J. Biol. Chem. 271, 23512–23519 [DOI] [PubMed] [Google Scholar]
- 38. Zou Y., Komuro I., Yamazaki T., Aikawa R., Kudoh S., Shiojima I., Hiroi Y., Mizuno T., Yazaki Y. (1996) Protein kinase C, but not tyrosine kinases or Ras, plays a critical role in angiotensin II-induced activation of Raf-1 kinase and extracellular signal-regulated protein kinases in cardiac myocytes. J. Biol. Chem. 271, 33592–33597 [DOI] [PubMed] [Google Scholar]
- 39. Rajalingam K., Wunder C., Brinkmann V., Churin Y., Hekman M., Sievers C., Rapp U. R., Rudel T. (2005) Prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat. Cell Biol. 7, 837–843 [DOI] [PubMed] [Google Scholar]
- 40. Giri B., Dixit V. D., Ghosh M. C., Collins G. D., Khan I. U., Madara K., Weeraratna A. T., Taub D. D. (2007) CXCL12-induced partitioning of flotillin-1 with lipid rafts plays a role in CXCR4 function. Eur. J. Immunol. 37, 2104–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aït-Slimane T., Galmes R., Trugnan G., Maurice M. (2009) Basolateral internalization of GPI-anchored proteins occurs via a clathrin-independent flotillin-dependent pathway in polarized hepatic cells. Mol. Biol. Cell 20, 3792–3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sigismund S., Argenzio E., Tosoni D., Cavallaro E., Polo S., Di Fiore P. P. (2008) Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev. Cell 15, 209–219 [DOI] [PubMed] [Google Scholar]
- 43. Sorkin A., Goh L. K. (2009) Endocytosis and intracellular trafficking of ErbBs. Exp. Cell Res. 315, 683–696 [DOI] [PubMed] [Google Scholar]
- 44. Schneider A., Rajendran L., Honsho M., Gralle M., Donnert G., Wouters F., Hell S. W., Simons M. (2008) Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J. Neurosci. 28, 2874–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ringerike T., Blystad F. D., Levy F. O., Madshus I. H., Stang E. (2002) Cholesterol is important in control of EGF receptor kinase activity but EGF receptors are not concentrated in caveolae. J. Cell Sci. 115, 1331–1340 [DOI] [PubMed] [Google Scholar]
- 46. Waugh M. G., Lawson D., Tan S. K., Hsuan J. J. (1998) Phosphatidylinositol 4-phosphate synthesis in immunoisolated caveolae-like vesicles and low buoyant density non-caveolar membranes. J. Biol. Chem. 273, 17115–17121 [DOI] [PubMed] [Google Scholar]
- 47. Hofman E. G., Ruonala M. O., Bader A. N., van den Heuvel D., Voortman J., Roovers R. C., Verkleij A. J., Gerritsen H. C., van Bergen En Henegouwen P. M. (2008) EGF induces coalescence of different lipid rafts. J. Cell Sci. 121, 2519–2528 [DOI] [PubMed] [Google Scholar]
- 48. Lax I., Wong A., Lamothe B., Lee A., Frost A., Hawes J., Schlessinger J. (2002) The docking protein FRS2alpha controls a MAP kinase-mediated negative feedback mechanism for signaling by FGF receptors. Mol. Cell 10, 709–719 [DOI] [PubMed] [Google Scholar]
- 49. Melillo R. M., Santoro M., Ong S. H., Billaud M., Fusco A., Hadari Y. R., Schlessinger J., Lax I. (2001) Docking protein FRS2 links the protein-tyrosine kinase RET and its oncogenic forms with the mitogen-activated protein kinase signaling cascade. Mol. Cell. Biol. 21, 4177–4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xu H., Goldfarb M. (2001) Multiple effector domains within SNT1 coordinate ERK activation and neuronal differentiation of PC12 cells. J. Biol. Chem. 276, 13049–13056 [DOI] [PubMed] [Google Scholar]
- 51. Wu Y., Chen Z., Ullrich A. (2003) EGFR and FGFR signaling through FRS2 is subject to negative feedback control by ERK1/2. Biol. Chem. 384, 1215–1226 [DOI] [PubMed] [Google Scholar]
- 52. Tomasovic A., Traub S., Tikkanen R. (2012) Molecular networks in FGF signaling. Flotillin-1 and Cbl-associated protein compete for the binding to fibroblast growth factor receptor substrate 2. PLoS ONE 7, e29739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.