Background: The identity and immunogenicity of wcjE-mediated capsule features in Streptococcus pneumoniae serotype 9V are unclear.
Results: Isogenic serotype 9A lacks 6-O-acetylation of βManNAc present in the 9V capsule.
Conclusion: wcjE mediates βManNAc-6-O-acetylation, a modification that is preferentially targeted by anti-serotype 9V antibodies.
Significance: Elucidating the role of the widely conserved capsule biosynthesis gene wcjE aids in understanding pneumococcal-host interactions.
Keywords: Bacterial Pathogenesis, Cell Surface, Glycobiology, Polysaccharide, Streptococcus, O-Acetylation, S. pneumoniae, Polysaccharide Capsule, Serotype 9A, Serotype 9V
Abstract
The putative capsule O-acetyltransferase gene wcjE is highly conserved across various Streptococcus pneumoniae serotypes, but the role of the gene in capsule biosynthesis and bacterial fitness remains largely unclear. Isolates expressing pneumococcal serotype 9A arise from precursors expressing wcjE-associated serotype 9V through loss-of-function mutation to wcjE. To define the biosynthetic role of 9V wcjE, we characterized the structure and serological properties of serotype 9V and 9A capsule polysaccharide (PS). NMR data revealed that both 9V and 9A PS are composed of an identical pentasaccharide repeat unit, as reported previously. However, in sharp contrast to previous studies on 9A PS being devoid of any O-acetylation, we identified O-acetylation of α-glucuronic acid and α-glucose in 9A PS. In addition, 9V PS also contained –CH2 O-acetylation of β-N-acetylmannosamine, a modification that disappeared following in vitro recombinatorial deletion of wcjE. We also show that serotyping sera and monoclonal antibodies specific for 9V and 9A bound capsule PS in an O-acetate-dependent manner. Furthermore, IgG and to a lesser extent IgM from human donors immunized with serotype 9V PS displayed stronger binding to 9V compared with 9A PS. We conclude that serotype 9V wcjE mediates 6-O-acetylation of β-N-acetylmannosamine. This PS modification can be selectively targeted by antibodies in immunized individuals, identifying a potential selective advantage for wcjE inactivation and serotype 9A emergence.
Introduction
The Gram-positive diplococcus Streptococcus pneumoniae is an obligate colonizer of the human nasopharynx that can subsequently spread to normally sterile sites (e.g. blood, middle ear, etc.) to cause a wide spectrum of diseases. Pneumococcal survival in hosts is facilitated by expression of a polysaccharide (PS)2 capsule that prevents recognition and clearance of the bacterium by the host immune system. To date, 93 capsule types (commonly referred to as serotypes) have been identified according to their reactivity with reference antisera (e.g. group antisera, factor sera, etc.) or, more recently, according to their reactivity with monoclonal antibodies (mAb) (1–3). Hosts can produce serotype-specific antibodies that can mediate bacterial clearance and prevent colonization and disease. Thus, expression of heterogeneous capsule PS may help S. pneumoniae avoid humoral immunity in host.
Genes in the capsule synthesis (cps) locus of S. pneumoniae mediate synthesis of the repeating unit of the capsule PS, as well as export and polymerization of the repeating units. The cps locus has been sequenced for all known serotypes (1, 4, 5). Comparative analysis of cps genes and capsule structures has led to the assignment of biosynthetic roles to many S. pneumoniae cps gene products (6, 7). However, because of the incomplete description of various capsule structures, the role of many cps genes remains undefined. One example of a highly conserved cps gene with undefined enzymatic activity is wcjE, which can be found in the cps loci of 14 serotypes: 9V, 11A, 11D, 11F, 15F, 20, 31, 33A, 35A, 35C, 42, 43, 47A, and 47F (5) (9V, 11A and 20 are included in the current 23-valent pneumococcal capsule PS vaccine, PPV-23 (8)). The gene encodes a putative transmembrane O-acetyltransferase homologous to Pfam family PF01757, which includes peptidoglycan and lipopolysaccharide O-acetyltransferases (9–12). Accordingly, molecular, serological, and structural analyses revealed that wcjE in 11A, 11F, and possibly 11D mediates –CH2 O-acetylation of β-galactose (Gal) of the PS capsule (1, 13). However, 9V PS has no βGal in its capsule PS, and the enzymatic target of the wcjE gene product in serotype 9V and all other wcjE-associated serotypes is unclear.
Serotype 9A arises from serotype 9V following loss-of-function mutations to wcjE (14), providing a suitable model for studying the role of 9V wcjE in capsule synthesis and pneumococcal fitness. Serotypes 9V and 9A cross-react with the factor serum 9d, but only serotype 9V reacts with factor serum 9g and the monoclonal antibody Hyp9VG2 (14), suggesting that the latter two antibodies recognize wcjE-mediated epitopes. Although serotype 9V wcjE shares over 90% amino acid identity with serotype 11A wcjE (5), no O-acetylation site is conserved between 9V and 11A PS. The 9V pentasaccharide repeat unit contains six sites of O-acetylation of which the 6-position of β-N-acetylmannosamine (ManNAc) and the 3-position of α-glucuronic acid (GlcUA) are O-acetylated at frequencies greater than 0.25 m equivalents (Fig. 1 and Table 1) (15). Congruous with two major sites of O-acetylation, the 9V cps locus contains a second putative O-acetyltransferase gene wcjD, whose biosynthetic target is also poorly understood (5). In disagreement with the identification of intact alleles of wcjD in the cps loci of multiple 9A strains (5, 14), the reported structure of 9A PS is identical to the completely de-O-acetylated glycosidic backbone of 9V PS (Fig. 1 and Table 1) (15). Here, we characterize the structure and antigenicity of serotype 9V and 9A PS, identify the biosynthetic role of wcjE, and describe the contribution of different O-acetate substitutions to the recognition of these capsule PS by antibodies from immunized humans.
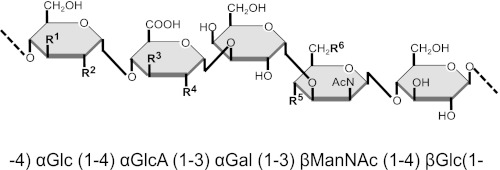
FIGURE 1.
Schematic and written representations of the capsule pentasaccharide repeat unit of serotypes 9V and 9A according to previous reports and this study. Lines represent inter-subunit glycosidic linkages. O-Acetylation sites are denoted by R followed by a superscript number. Rates of O-acetylation at each site are listed in Table 1.
TABLE 1.
Previously reported and currently proposed estimated rates of O-acetylation of corresponding sites in Fig. 1
| Previous study (15) |
Current study |
|||
|---|---|---|---|---|
| 9V | 9A | 9V | 9A | |
| R1 | 0.04a | 0 | 0.004 | 0.005 |
| R2 | 0.03 | 0 | 0.001 | 0.002 |
| R3 | 0.25 | 0 | 0.55 | 0.61 |
| R4 | 0.17 | 0 | 0.25 | 0.27 |
| R5 | 0.06 | 0 | 0.09 | 0.03 |
| R6 | 0.55 | 0 | 1.04 | 0 |
| Sumb | 1.1 | 0 | 1.95 | 0.92 |
| OAc:NAcc | 1.19:1 | 0:1 | 1.97:1 | 1.03:1 |
a Values are expressed as molar equivalents.
b Sum of all O-acetylation sites according to integration of resolved peaks in 1H NMR spectra.
c Molar ratio of O-acetate to N-acetate according to integration of acetate proton signals in 1H NMR spectra.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Culture Conditions
The strain SSISP 9V/4 is a serotype 9V reference strain purchased from Statens Seruminstitut (SSI, Copenhagen, Denmark). Strain MNZ869 is a clinical isolate obtained from the Centers for Disease Control (Atlanta, GA) and was previously serotyped as 9A (14). Strains JC01 and JC02 are isogenic strains expressing serotypes 9V and 9A, respectively. JC01 was derived by transforming an allele of the ribosomal protein gene rpsL that contained a polymorphism conferring streptomycin resistance into a clinical serotype 9V strain. JC02 was created by recombinatorial deletion of wcjE in JC01 as described (14). All strains were cultured on tryptic soy agar plates containing sheep blood or in a chemically defined medium (JRH Biosciences, Lenexa, KS) (16) supplemented with choline chloride (1 g/liter), sodium bicarbonate (2.5 g/liter), and cysteine-HCl (0.73 g/liter).
Preparation of Purified Polysaccharide Capsule Samples
Capsule PS was purified from the four strains mentioned above, as described (13, 17). Briefly, nucleic acids and proteins were removed from bacterial lysates by ethanol precipitation. Subsequently, PS was precipitated from these lysates. PS was further purified by ion exchange and size exclusion chromatography. The cell wall PS content of these samples was determined to be low as judged by NMR data (phosphocholine peak at 3.22 ppm, see supplemental Fig. S1). To de-O-acetylate samples, PS was incubated in 0.2 m sodium hydroxide for 3 h at room temperature. Equal mass of purified PS was incubated in water as “native PS” controls.
NMR Spectroscopy
NMR spectra were obtained on a Bruker Avance II (700 MHz 1H) spectrometer equipped with a cryoprobe, processed with NMRPIPE (18), and analyzed with NMRVIEW (19) or ACD/NMR Processor Academic Edition (Advanced Chemistry Development, Inc., Toronto, Canada). The 1H one-dimensional and decoupled 1H-13C two-dimensional heteronuclear multiple quantum coherence (HMQC) data were collected at 45 °C. Chemical shifts were assigned according to the previously reported assignments of 9V capsule PS (15). The molar ratio of βManNAc-6-O-acetate was determined by integrating the 1H peaks between 4.40 and 4.55 ppm and subtracting the estimated integral of βGlc H1, as determined by the βManNAc:βGlc ratio in de-O-acetylated samples. Molar ratios of all other O-acetate substitutions were calculated by integrating resolved 1H signals corresponding to O-acetylated residues (see below) compared with the integration of all β-ManNAc anomeric signals between 4.85 and 5.00 ppm.
Antibodies and Serum Samples
The serotyping factor sera 9d (cross-reacts with 9A and 9V), 9g (reacts only with 9V), and group 9 antiserum (cross-reacts with 9A, 9V, 9N, and 9L) were purchased from SSI (Copenhagen, Denmark). Monoclonal antibodies (mAb) were produced against 9V PS as described (20). Hybridoma culture supernatants were used as a source for the 9V-specific mAb Hyp9VG2 (14) and 9A/9V cross-reactive mAb Hyp9VM7 (see below). Pool 32 is a pool of equal volume of sera from 100 anonymous elderly donors vaccinated with PPV-23. The remaining serum samples used in this study are from healthy single donors that received PPV-23 vaccination and are described elsewhere (21).
Flow Cytometric Serotyping Assay (FCSA)
Bacteria concentrations were normalized according to A630 and incubated for 10 min at 4 °C with 1:50,000 dilution of factor sera 9d or 9g, 1:200 dilution of Hyp9VG2 culture supernatant, or 1:50 dilution of Hyp9VM7 culture supernatant. As a negative control, bacteria were incubated with serotype 11A specific Hyp11AG2 and Hyp11AM1 mAb. Surface-bound antibody was detected with goat anti-rabbit Ig conjugated to fluorescein isothiocyanate, rabbit anti-mouse IgG-conjugated phycoerythrin, or rabbit anti-mouse IgM phycoerythrin-Cy7-conjugated antibodies (Southern Biotechnology Associates, Inc., Birmingham, AL). Stained bacteria were analyzed using a FACSCalibur (BD Biosciences). Data analysis was performed with FCS Express (De Novo Software, Los Angeles, CA).
ELISA
ELISA plates were prepared by incubating wells with 100 μl of native or de-O-acetylated PS dissolved in phosphate-buffered saline (PBS) with 0.02% sodium azide (2.5 μg/ml according to PS weight prior to mild hydrolysis) at 37 °C for 4 h. Plates were then stored at 4 °C in a humidified chamber until needed. Plates were washed with PBS containing 0.1% Brij-35 (Sigma). 100 μl of antibody samples, which were serially diluted 5-fold in PBS containing 0.05% Tween 20, were added to the wells and incubated for 45 min at 37 °C. Following washing, bound antibodies were detected using alkaline phosphatase-conjugated anti-mouse, anti-rabbit, or anti-human (IgG, IgA, or IgM) antibodies. A405 was recorded for each well, and each sample was tested in duplicate. To exclude the role of common contaminants in mediating binding by serum antibodies, some serum samples were retested after absorption with 5 μg/ml purified serotype 22F PS and 5 μg/ml pneumococcal cell wall PS.
RESULTS
NMR Characterization Reveals O-Acetate Substitutions in 9A PS
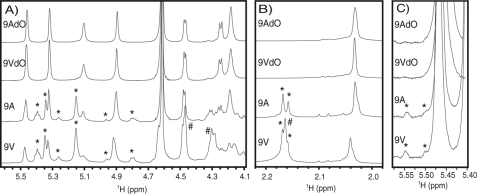
The wcjD gene conserved in the 9A and 9V cps loci encodes a putative O-acetyltransferase (5), which led us to hypothesize that O-acetate (OAc) substitutions are probably present in both 9A and 9V capsule PS. To examine this hypothesis, we purified capsule PS from strain SSISP 9V/4 (herein referred to as 9V PS) and from strain MNZ869 (herein referred to as 9A PS) and obtained 1H NMR data for both PS samples before and after de-O-acetylation (dO) by mild alkali hydrolysis (Fig. 2 and supplemental Fig. S1). The 1H NMR spectrum of 9AdO PS was indistinguishable from the spectrum of 9VdO (supplemental Fig. S1), suggesting that both PS share identical PS backbone structures, as reported previously (15). Furthermore, the 1H NMR spectrum of the native 9V PS was indistinguishable from the spectrum previously published for 9V (15). Multiple signals within the anomeric region of the 9V spectrum (Fig. 2, A and C) correspond to OAc-mediated PS features, as reflected by the disappearance of these peaks following mild hydrolysis (Fig. 2). We observed loss of unresolved 1H signals between 2.14 and 2.18 ppm in 9VdO PS (Fig. 2B). Signals in this region typically correspond to OAc groups, whereas signals between 2.02 and 2.06 ppm correspond to the N-acetate groups on βManNAc, which are resistant to mild hydrolysis.
FIGURE 2.
1H NMR spectra of native and de-O-acetylated (dO) 9V and 9A PS. Selected views of the anomeric (A) and acetate (B) regions and a magnified portion of the anomeric region (C) of the 1H NMR spectra in supplemental Fig. S1. Asterisks denote hydrolysis-sensitive signals shared by native 9A and 9V PS, and pound signs denote hydrolysis-sensitive signals observed only in 9V PS.
In contrast to previous studies, which have shown that 9A PS is identical to 9VdO PS (15), we show that native 9A PS also contains hydrolysis-sensitive signals in the anomeric region (Fig. 2A) and between 2.14 and 2.16 ppm (Fig. 2B). By integrating the signals between 2.14 and 2.18 ppm (OAc) and signals between 2.02 and 2.06 ppm (NAc), we estimate that 9V PS contains OAc/NAc ratio of ∼2:1, whereas 9A PS contains an OAc/NAc ratio of ∼1:1. It is very clear that 9A PS contained OAc substitutions, albeit at a lower rate relative to 9V PS.
9A PS Lacks the –CH2 O-Acetylation of βManNAc Present in 9V PS
1H-13C HMQC data have been obtained for 9A and 9V PS samples to unambiguously identify the position of the O-acetate substitutions. Guided by previously reported chemical shift assignments (15), we were able to assign most signals in the 9VdO spectra (supplemental Table S1 and supplemental Fig. S2) confirming that the glycosidic backbone of the PS samples analyzed in this study is also composed of the (−4)αGlc(1-4)αGlcUA(1–3)αGal(1–3)βManNAc(1–4)βGlc(1-pentasaccharide repeat unit (Fig. 1).
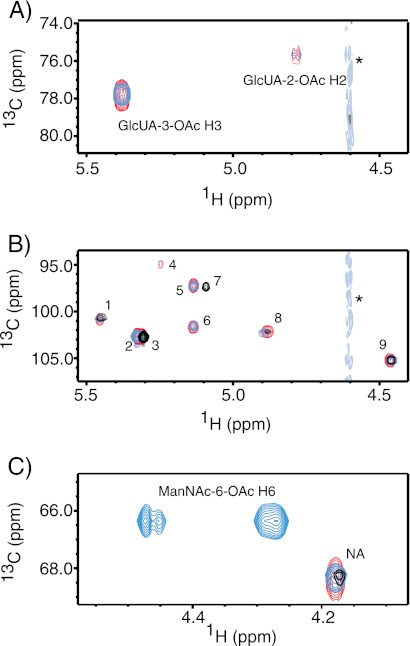
Next, we examined the presence or absence of key hydrolysis-sensitive peaks previously assigned to 9V O-acetate substitutions (15). As shown in Fig. 3A, HMQC spectra of 9V and 9A PS samples both show cross-peaks at 4.77/75.46 and 5.37/77.61 ppm (1H/13C). These signals correspond to H2 and H3 groups of αGlcUA, respectively, in the presence of geminal O-acetylation. Accordingly, we observed hydrolysis-sensitive anomeric signals shared by 9V and 9A PS (Fig. 3B) corresponding to αGlcUA-2-OAc, αGlcUA-3-OAc, and αGal adjacent to αGlcUA-3-OAc (1H/13C: 5.34/94.68; 5.13/97.08; and 5.33/102.44 ppm, respectively). Together, these findings indicate O-acetylation of αGlcUA in both 9A and 9V PS. Some hydrolysis-sensitive signals shared by the 9A and 9V 1H NMR spectra were not readily detectable in the HMQC spectra. As shown in the 1H spectra of both 9V and 9A (and not 9VdO or 9AdO), signals assigned to the anomeric protons of αGlc-2-OAc, αGlc-3-OAc, and βManNAc-4-OAc (15) are observed at 5.54, 5.50, and 4.95 ppm, respectively (Fig. 2, A and C).
FIGURE 3.
Overlay of two-dimensional 1H-13C HMQC spectra of native 9V (blue), native 9A (red), and 9VdO (black) PS. A, signals indicate GlcUA O-acetylation. B, anomeric signals are assigned according to Rutherford et al. (15) as follows: 1, αGlc H1; 2, αGal next to αGlcUA-3-OAc H1; 3, α-Gal H1; 4, αGlcUA-2-OAc H1; 5, αGlcUA-3-OAc H1; 6, αGlc next to αGlcUA-3-OAc H1; 7, αGlcUA H1; 8, βManNAc H1; and 9, βGlc H1. C, signals of the –CH2 group of βManNAc-6-OAc. Cross-peaks corresponding to protons with geminal O-acetylation are labeled in A and C. Asterisks in A and B denote HDO signal. NA, not assigned.
A major difference observed in the 9V and 9A HMQC spectra is the presence of a strong pair of 1H signals at 4.46 and 4.27 ppm (13C = 66.1 ppm) in 9V PS but not in 9A and 9VdO PS samples (Fig. 3C). These signals correspond to the –CH2 protons of βManNAc with geminal O-acetylation (15). In addition, the loss of signals at 3.46/79.13 ppm and the doublet signals at 3.89,3.91/63.13 and 3.79,3.80/63.12 ppm (data not shown), assigned to the H5 and H6 chemical shifts of βManNAc in 9A and 9VdO, indicates the presence of βManNAc-6-OAc in 9V but not 9A PS. To quantify the degree of O-acetylation at each identified site on 9V and 9A PS, we followed previous strategies (15). We determined the molar ratios of most O-acetate substitutions to be comparable between 9V and 9A (Fig. 1B). Total O-acetylation rates obtained with this strategy (i.e. 1.96 and 0.92 for 9V and 9A PS, respectively) were comparable with molar content calculated using acetate signals (1.97 versus 1.03, see above). Therefore, we propose revised structures for 9A and 9V PS as shown in Fig. 1 and Table 1.
βManNAc-6 O-Acetylation Is Mediated by wcjE
We recently demonstrated that genetic inactivation of wcjE alone was enough to effect seroswitching of serotype 9V to 9A (14). Because βManNAc –CH2 O-acetylation was associated with the 9V capsule PS structure, we hypothesized that wcjE mediates this O-acetate substitution. To test this hypothesis, we purified and analyzed the capsule PS of the isogenic strains JC01 (9V) and JC02 (9A). One-dimensional 1H NMR spectra were indistinguishable from the spectra of PS purified from clinical counterparts (supplemental Figs. S1 and S3A). Notably, both spectra show signals at 5.55, 5.50, 5.39, 4.96, and 4.80 ppm, indicating the presence of αGlc-2-OAc, αGlc-3-OAc, αGlcUA-3-OAc, βManNAc-4-OAc, and αGlcUA-2-OAc, respectively. Only the 1H spectrum of JC01, however, displayed strong signals at 4.47 and 4.28 ppm. Accordingly, the acetate region of JC01 contained three distinct peaks between 2.15 and 2.18 ppm, although the spectrum for JC02 only contained two discernable signals in this region (supplemental Fig. S3B). In the 1H-13C HMQC spectra, cross-peaks corresponding to H2 and H3 O-acetylation of αGlcUA are observed at 4.77/75.46 and 5.37/77.61 ppm for both JC01 and JC02 (data not shown). However, only JC01 shows strong signals corresponding to H6 O-acetylation of βManNAc at 4.46/66.14 and 4.27/66.15 ppm (supplemental Fig. S3C). Thus, we conclude that inactivation of wcjE activity results in the loss of βManNAc-6-O-acetylation characteristic of serotype 9V.
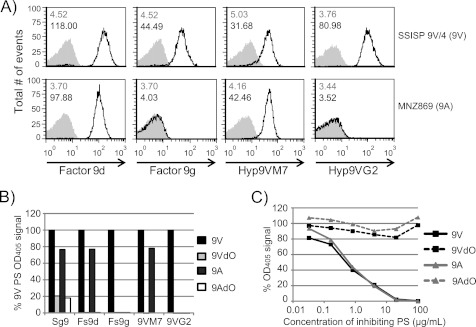
9A- and 9V-specific Factor Sera and mAbs Do Not Bind de-O-Acetylated PS
O-Acetylation of 9A PS contradicts the widely accepted structure of this PS (15, 22). We investigated the possibility that bacteria expressing two different capsule types (i.e. 9A with or without O-acetylation) could be serologically identical by testing factor sera (Fs) and mAb binding to bacteria expressing 9V and 9A PS by FCSA and purified preparations of these PS by ELISA. As expected, FCSA revealed that Fs 9d antibodies and Hyp9VM7 mAb comparably bound to strains SSISP 9V/4 (9V) and MNZ869 (9A), but Fs 9g antibodies and Hyp9VG2 mAb only bound to strain SSISP 9V/4 (Fig. 4A). None of these antibodies bound to bacteria expressing serotype 9N, 9L, or 11A (data not shown). Consistent with FCSA results, Fs 9d antibodies and Hyp9VM7 mAb bound ELISA wells coated with purified native 9A and 9V PS, although Fs 9g antibodies and Hyp11AG2 mAb bound only 9V-coated plates (Fig. 4B and supplemental Fig. S4). Plates coated with 9AdO or 9VdO PS, however, were not bound by any of these serotyping antibodies. We also examined the binding of antibodies in serogroup 9 (Sg9) antiserum, which, in addition to 9A and 9V PS, bind the non-O-acetylated PS of serotypes 9N and 9L. Binding of Sg9 antiserum antibodies was indistinguishable between 9VdO and 9AdO (Fig. 4B), although ∼10-fold reduced concentrations of antisera achieved comparable signals in 9A and 9V PS coated plates (supplemental Fig. S4).
FIGURE 4.
Serotyping antibody binding to 9A and 9V PS is O-acetate-dependent. A, binding of polyclonal antibodies in factor sera 9d and 9g (left two columns) and the monoclonal antibodies Hyp9VM7 and Hyp9VG2 (right two columns) to bacterial strains expressing serotype 9V (SSISP 9V/4, top row) and serotype 9A (MNZ869, bottom row) was examined by FCSA. Each histogram box contains the geometric mean fluorescence intensities of negative controls (top) and test samples (bottom). B, relative binding of antibodies to ELISA wells coated with 9V PS (black bars), 9VdO PS (light gray bars), 9A PS (dark gray bars), and 9AdO (white bars). Antibodies are indicated at the bottom of the graph as follows: Sg9, antiserum specific for serogroup 9 at a 1:1000 dilution; Fs9d, factor serum 9d at a 1:1000 dilution; Fs9g, factor serum 9g at a 1:1000 dilution; 9VM7 and 9VG2 are tissue culture supernatants of monoclonal antibodies Hyp9VM7 and Hyp9VG2. C, inhibition of factor serum 9d antibodies binding to 9A PS-coated plates was examined by inhibition ELISA and is expressed as a percentage of the A405 of negative control (i.e. wells that did not receive inhibitor PS). Values in B and C are averages of duplicate runs.
To further confirm OAc-mediated binding by typing serum, we tested the inhibition of antibody binding to 9A-coated plates using native and de-O-acetylated 9V and 9A PS. Native 9V and 9A PS inhibited factor serum 9d antibodies (Fig. 4C) and Hyp9VM7 mAb (data not shown) binding in a dose-dependent manner. Neither de-O-acetylated PS sample was able to inhibit antibody binding. We concluded that PS O-acetylation mediates 9A and 9V epitopes targeted by typing antibodies.
Serum from Human Donors Immunized with 9V PS Display O-Acetate-mediated Binding to Capsule PS
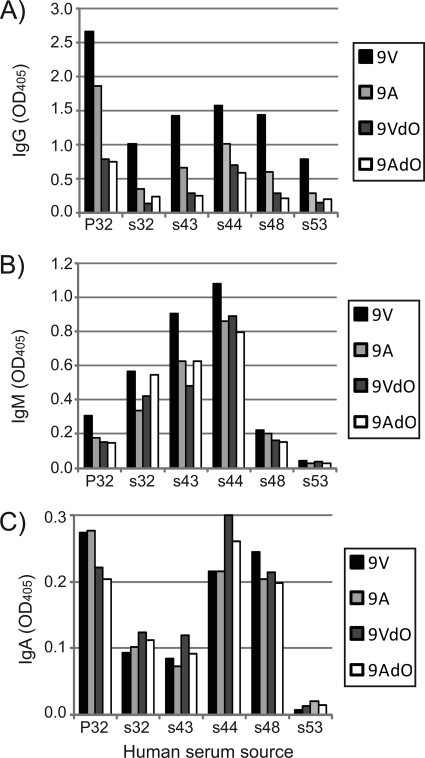
We observed that serotyping antibodies can selectively bind 9V PS in an O-acetate-dependent manner, suggesting that human antibodies may also selectively bind wcjE-mediated epitopes. We examined the binding of IgG, IgA, and IgM antibodies in serum of human donors vaccinated with 9V PS to purified 9V, 9A, 9AdO, and 9VdO PS (Fig. 5). IgG antibodies in a human serum pool (P32) yielded stronger signals in 9V PS-coated plates, followed by 9A PS and then 9VdO and 9AdO plates (the latter two yielded a comparable signal). A similar trend was observed when examining IgG of five single donors, where almost all serum (n = 4) samples displayed greater than 2-fold increased IgG signal to 9V PS plates compared with 9A (Fig. 5A). In all samples, IgG signal was the least in 9VdO and 9AdO PS plates. When examining IgM signals, 9V PS-associated signals was clearly higher for only P32, s43, and s44, although the difference between 9V PS-coated plates and plates coated with other PS preparations was not as marked as that observed for IgG (Fig. 5B). IgA binding was low for all samples, and no PS preparation was clearly associated with a greater signal compared with other PS (Fig. 5C).
FIGURE 5.
Binding of human serum antibodies to 9A and 9V capsule PS. Plates coated with 9V, 9A, 9VdO, and 9AdO PS were incubated with 1:50 dilutions of human serum samples, and the amount of bound IgG (A), IgA (B), and IgM (C) were compared according to A405. Values are averages of duplicate runs. P32 is a pool of 100 serum samples from individuals immunized with 9V PS. s32, s43, s44, s48, and s53 are serum samples from single healthy donors immunized with 9V PS.
The stronger IgG signal in 9V- and 9A-coated plates is not likely due to an increased amount of native PS coated on ELISA wells relative to the de-O-acetylated sample, as some serum samples (e.g. s32 and s44) displayed equal or greater IgM and IgA signals in 9VdO- and 9AdO-coated plates (Fig. 5 and supplemental Fig. S5). Relative binding of IgM and IgA among all plates remained indistinguishable even after absorption with purified serotype 22F PS and cell wall PS (data not shown), supporting that antibody binding to capsule PS is mediating A405 signal and that all PS preparations comparably adhered to ELISA wells.
DISCUSSION
Previous studies comparing 9V and 9A capsule properties were performed under the supposition that 9A PS contained no O-acetylation and thus used de-O-acetylated 9V PS samples as a surrogate for 9A PS (15, 23, 24). In contrast to the widely accepted view, we present compelling NMR evidence that 9A PS is extensively O-acetylated on its αGlcUA and to a much smaller extent on it αGlc and βManNAc moieties. Why previous structures of 9A PS were devoid of O-acetylation (15) remains unclear, although it is possible that the relatively labile O-acetate groups may have become fully hydrolyzed in prior purification or handling procedures, e.g. phenol-mediated bacteria lysis, enzymatic removal of nucleic and amino acids during purification, etc. This possibility is supported by the detection of higher 9V PS O-acetylation rates in this study compared with prior reports, despite using similar strategies to quantify these rates (15). Our revised structure for 9A PS (Fig. 1) is supported by the fact that O-acetate integrity was required to mediate characteristic binding by both factor serum 9d and the mAb Hyp9VM7 to PS. It should be noted that comparisons of antisera binding to PS expressed on the bacterial surface and purified PS was not reported in prior studies. Therefore, it is possible that the surface-associated PS and the purified PS used for prior analyses were structurally different.
The presence of O-acetylation on 9A PS is also consistent with genetic studies showing that both 9A and 9V cps loci contain identical alleles of the putative O-acetyltransferase gene wcjD (5, 14). wcjD encodes a soluble O-acetyltransferase homologous to Pfam family PF00132 (7), which includes the putative 11A-associated αGlc-2,3-O-acetyltransferase wcwT and βGal-4-O-acetyltransferase wcwC (13). Given that the O-acetylation sites are shared by both 9V and 9A PS, we propose that wcjD mediates O-acetylation of the 2- and 3-positions of αGlcUA and, to a smaller extent, O-acetylation of the structurally similar 2- and 3-positions of αGlc. We cannot readily explain the mechanism of βManNAc-4-OAc substitution found in 9A PS. It is possible that wcjD or a conserved O-acetyltransferase encoded outside the cps locus mediates this O-acetate substitution. To investigate these possibilities and fully define the role of wcjD, further biochemical studies are necessary.
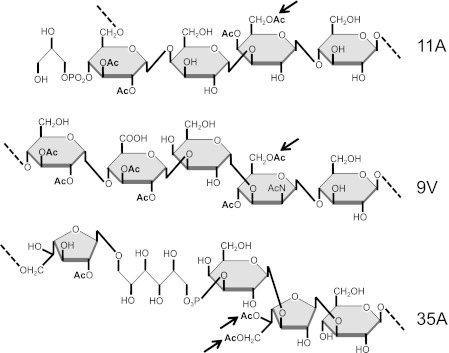
We identified βManNAc-6-O-acetylation in 9V but not 9A PS. Furthermore, this O-acetate substitution is lost following recombinatorial deletion of wcjE in strain JC02, highlighting the enzymatic target of the putative transmembrane O-acetyltransferase encoded by wcjE. The putative 9V wcjE gene product shares 93 and 91% amino acid sequence identity with the wcjE gene products of serogroup 11 and serotype 15F, respectively. Consistent with enzyme homology, the 6-βManNAc(1–4)βGlc target residues of serotype 9V wcjE resembles the 6-βGal(1–4)βGlc target of wcjE in 11A, 11F (Fig. 6) (13), and 11D.3 The reported PS structure of serotype 15F capsule contains 2 m equivalents of unplaced O-acetate groups (25). As 15F capsule contains βGal(1–4)βGlc identical to 11A, we hypothesize that 15F wcjE also mediates βGal 6-O-acetylation, although further work is necessary to examine this proposal. Taken together, the wcjE gene product of these serotypes may mediate hexopyranose-6-O-acetylation.
FIGURE 6.
Proposed targets of wcjE-mediated O-acetylation. The reported structures of serotypes 11A, 9V, and 35 capsule PS repeat units (13, 26) are shown. Inter-subunit glycosidic linkages are represented by dotted lines. Arrows point to O-acetate substitutions proposed to be mediated by wcjE in each serotype (see text). Ac, acetate.
The cps loci of the remaining nine serotypes that contain wcjE (i.e. serotypes 20, 31, 33A, 35A, 35C, 42, 43, 47A, and 47F) conserve a galactofuranose (Galf) mutase gene (glf) and galactofuronosyltransferase gene (wciB), the genes putatively necessary to integrate βGalf into the capsule repeat unit (6, 7). The fully elucidated PS structure of 35A contains βGalf-2-OAc and βGalf-5,6-OAc (Fig. 6) (26), in congruence with the 35A cps locus containing two putative O-acetyltransferase genes, wcjE and wciG (5). The reported capsule PS structure of the wcjE-associated serotype 20 also contains βGalf-5,6-OAc (26). The serotype 33F cps locus contains a frameshifted pseudogene of wcjE and a putatively intact allele of wciG (5). Although 33F cps locus contains glf and wciB, and 33F PS contains two potential βGalf enzymatic targets, 33F PS has βGalf-2-OAc but not βGalf-5,6-OAc (27). Thus, we propose that in glf/wciB-associated serotypes, wcjE may mediate βGalf-5,6-OAc substitution.
Although wcjE is conserved across multiple pneumococcal serogroups, the gene appears to be biochemically expendable as reflected by the unconventional emergence of wcjE-null serovariants. Serotypes 9A and 11E arise from strains expressing serotypes 9V and 11A, respectively, following loss-of-function mutations to wcjE (1, 14). Given the complex interaction of cps genes, it is commonly thought that pneumococcal serotypes evolve once and that all the cps loci of a clinically relevant serotype can be genetically traced back to a single founding event. However, each clinical isolate expressing serotypes 9A and 11E contain unique and unrelated mutations to wcjE, revealing the recurrent and independent evolution of these serotypes (1, 14).
Serotypes 9A and 11E are significantly more likely to occur among strains isolated from bacteremia cases than asymptomatic nasopharyngeal colonization (28, 29), suggesting a link between wcjE inactivation and bacterial survival during invasive disease. The host factor selecting for wcjE loss-of-function mutations may be antibodies to the capsule. We observed that human subjects immunized with 9V PS produce IgM and IgG antibodies that bind more strongly to 9V PS than 9A PS, in agreement with the observation that human antibodies can selectively target serogroup 9 epitopes mediated by O-acetylation (23). Our observations are also consistent with previous reports that highlight the important role of O-acetylation in shaping the humoral response to PS antigens (13, 30–32). As the IgG isotype is predominant in blood, 9V-specific antibodies that target βManNAc-6-OAc-associated epitopes may provide a selective advantage for serotype 9A and explain why this serotype is strongly associated with bacteremia isolates. Studies are underway to determine whether antibodies that strongly bind to 9V PS also mediate preferential opsonophagocytosis of strains expressing serotype 9V over those expressing 9A.
Actively circulating wcjE-null variants are expected to share a common loss-of-function mutation, so the lack of two isogenic wcjE-null 9A or 11E isolates (1, 14) suggests wcjE facilitates host-to-host transmission of 9V and 11A. A wcjE-associated benefit in relation to transmission is a possible reason why this gene is conserved across various serotypes. Perhaps wcjE-mediated –CH2 O-acetylation of capsule PS prevents recognition by host lectins encountered during nasopharyngeal colonization or promotes better adhesion to the mucosal surface. Another possibility is that wcjE-mediated extracytoplasmic O-acetylation may target other PS targets in addition to capsule, which in turn provides an advantage over wcjE-null counterparts. Fully elucidating the enzymatic targets of wcjE may lead to the discovery of key host-microbe interactions that mediate serotype-associated prevalence and pathology.
Supplementary Material
Acknowledgment
We thank Dr. Neal Ravenscroft in Cape Town, South Africa, for critical reading of this manuscript and experimental support.
This work was supported, in whole or in part, by National Institutes of Health Grants AI-93103 (to J. J. C.) and AI-31473 (to M. H. N.). This work was also supported by the Department of Microbiology at the University of Alabama at Birmingham (to J. S. S.). The University of Alabama at Birmingham has intellectual property rights for some of the reagents used in this study, and all authors are University of Alabama at Birmingham. MHN serves as a consultant to Merck Inc. for S. pneumoniae diagnosis assays.

This article contains supplemental Table S1 and Figs. S1–S5.
M. H. Nahm, unpublished data.
- PS
- polysaccharide
- ManNAc
- N-acetylmannosamine
- OAc
- O-acetate
- FCSA
- flow cytometric serotyping assay
- dO
- de-O-acetylation
- Fs
- factor sera
- HMQC
- heteronuclear multiple quantum coherence.
REFERENCES
- 1. Calix J. J., Nahm M. H. (2010) A new pneumococcal serotype, 11E, has a variably inactivated wcjE gene. J. Infect. Dis. 202, 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bratcher P. E., Kim K. H., Kang J. H., Hong J. Y., Nahm M. H. (2010) Identification of natural pneumococcal isolates expressing serotype 6D by genetic, biochemical, and serological characterization. Microbiology 156, 555–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Park I. H., Pritchard D. G., Cartee R., Brandao A., Brandileone M. C., Nahm M. H. (2007) Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 45, 1225–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park I. H., Park S., Hollingshead S. K., Nahm M. H. (2007) Genetic basis for the new pneumococcal serotype, 6C. Infect. Immun. 75, 4482–4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bentley S. D., Aanensen D. M., Mavroidi A., Saunders D., Rabbinowitsch E., Collins M., Donohoe K., Harris D., Murphy L., Quail M. A., Samuel G., Skovsted I. C., Kaltoft M. S., Barrell B., Reeves P. R., Parkhill J., Spratt B. G. (2006) Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2, e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mavroidi A., Aanensen D. M., Godoy D., Skovsted I. C., Kaltoft M. S., Reeves P. R., Bentley S. D., Spratt B. G. (2007) Genetic relatedness of the Streptococcus pneumoniae capsular biosynthetic loci. J. Bacteriol. 189, 7841–7855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aanensen D. M., Mavroidi A., Bentley S. D., Reeves P. R., Spratt B. G. (2007) Predicted functions and linkage specificities of the products of the Streptococcus pneumoniae capsular biosynthetic loci. J. Bacteriol. 189, 7856–7876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robbins J. B., Austrian R., Lee C. J., Rastogi S. C., Schiffman G., Henrichsen J., Mäkelä P. H., Broome C. V., Facklam R. R., Tiesjema R. H. (1983) Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J. Infect. Dis. 148, 1136–1159 [DOI] [PubMed] [Google Scholar]
- 9. Skinner J. M., Indrawati L., Cannon J., Blue J., Winters M., Macnair J., Pujar N., Manger W., Zhang Y., Antonello J., Shiver J., Caulfield M., Heinrichs J. H. (2011) Pre-clinical evaluation of a 15-valent pneumococcal conjugate vaccine (PCV15-CRM197) in an infant-rhesus monkey immunogenicity model. Vaccine 29, 8870–8876 [DOI] [PubMed] [Google Scholar]
- 10. Aubry C., Goulard C., Nahori M. A., Cayet N., Decalf J., Sachse M., Boneca I. G., Cossart P., Dussurget O. (2011) OatA, a peptidoglycan O-acetyltransferase involved in Listeria monocytogenes immune escape, is critical for virulence. J. Infect. Dis. 204, 731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verma N. K., Brandt J. M., Verma D. J., Lindberg A. A. (1991) Molecular characterization of the O-acetyltransferase gene of converting bacteriophage SF6 that adds group antigen 6 to Shigella flexneri. Mol. Microbiol. 5, 71–75 [DOI] [PubMed] [Google Scholar]
- 12. Fox K. L., Yildirim H. H., Deadman M. E., Schweda E. K., Moxon E. R., Hood D. W. (2005) Novel lipopolysaccharide biosynthetic genes containing tetranucleotide repeats in Haemophilus influenzae, identification of a gene for adding O-acetyl groups. Mol. Microbiol. 58, 207–216 [DOI] [PubMed] [Google Scholar]
- 13. Calix J. J., Nahm M. H., Zartler E. R. (2011) Elucidation of structural and antigenic properties of pneumococcal serotype 11A, 11B, 11C, and 11F polysaccharide capsules. J. Bacteriol. 193, 5271–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calix J. J., Oliver M. B., Sherwood L. K., Beall B. W., Hollingshead S. K., Nahm M. H. (2011) Streptococcus pneumoniae serotype 9A isolates contain diverse mutations to wcjE that result in variable expression of serotype 9V-specific epitope. J. Infect. Dis. 204, 1585–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rutherford T. J., Jones C., Davies D. B., Elliott A. C. (1991) Location and quantitation of the sites of O-acetylation on the capsular polysaccharide from Streptococcus pneumoniae type 9V by 1H nmr spectroscopy. Comparison with type 9A. Carbohydr. Res. 218, 175–184 [DOI] [PubMed] [Google Scholar]
- 16. van de Rijn I., Kessler R. E. (1980) Growth characteristics of group A streptococci in a new chemically defined medium. Infect. Immun. 27, 444–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zartler E. R., Porambo R. J., Anderson C. L., Chen L. H., Yu J., Nahm M. H. (2009) Structure of the capsular polysaccharide of pneumococcal serotype 11A reveals a novel acetylglycerol that is the structural basis for 11A subtypes. J. Biol. Chem. 284, 7318–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) NMRPipe. A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 19. Johnson B. A., Blevins R. A. (1994) NMR view. A Computer program for the visualization and analysis of NMR data J. Biomol. NMR 4, 603–614 [DOI] [PubMed] [Google Scholar]
- 20. Lin J., Kaltoft M. S., Brandao A. P., Echaniz-Aviles G., Brandileone M. C., Hollingshead S. K., Benjamin W. H., Nahm M. H. (2006) Validation of a multiplex pneumococcal serotyping assay with clinical samples. J. Clin. Microbiol. 44, 383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun Y., Park M. K., Kim J., Diamond B., Solomon A., Nahm M. H. (1999) Repertoire of human antibodies against the polysaccharide capsule of Streptococcus pneumoniae serotype 6B. Infect. Immun. 67, 1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamerling J. P. (2000) in Streptococcus pneumoniae Molecular Biology & Mechanisms of Disease (Tomasz A., ed) pp. 81–114, Mary Ann Liebert, Inc., Larchmont, NY [Google Scholar]
- 23. McNeely T. B., Staub J. M., Rusk C. M., Blum M. J., Donnelly J. J. (1998) Antibody responses to capsular polysaccharide backbone and O-acetate side groups of Streptococcus pneumoniae type 9V in humans and rhesus macaques. Infect. Immun. 66, 3705–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abeygunawardana C., Williams T. C., Sumner J. S., Hennessey J. P., Jr. (2000) Development and validation of an NMR-based identity assay for bacterial polysaccharides. Anal. Biochem. 279, 226–240 [DOI] [PubMed] [Google Scholar]
- 25. Perry M. B., Bundle D. R., Daoust V., Carlo D. J. (1982) The specific capsular polysaccharide of Streptococcus pneumoniae type 15F. Mol. Immunol. 19, 235–246 [DOI] [PubMed] [Google Scholar]
- 26. Beynon L. M., Richards J. C., Perry M. B. (1997) Identification of the common antigenic determinant shared by Streptococcus pneumoniae serotypes 35A and 20 capsular polysaccharides. Structural analysis of the Streptococcus pneumoniae serotype 35A capsular polysaccharide. Eur. J. Biochem. 250, 163–167 [DOI] [PubMed] [Google Scholar]
- 27. Lemercinier X., Jones C. (2006) Full assignment of the 1H and 13C spectra and revision of the O-acetylation site of the capsular polysaccharide of Streptococcus pneumoniae Type 33F, a component of the current pneumococcal polysaccharide vaccine. Carbohydr. Res. 341, 68–74 [DOI] [PubMed] [Google Scholar]
- 28. Calix J. J., Dagan R., Pelton S. I., Porat N., Nahm M. H. (2012) Clin. Infect. Dis. 54, 794–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sleeman K. L., Griffiths D., Shackley F., Diggle L., Gupta S., Maiden M. C., Moxon E. R., Crook D. W., Peto T. E. (2006) Capsular serotype-specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J. Infect. Dis. 194, 682–688 [DOI] [PubMed] [Google Scholar]
- 30. Berry D. S., Lynn F., Lee C. H., Frasch C. E., Bash M. C. (2002) Effect of O-acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune responses. Infect. Immun. 70, 3707–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Theilacker C., Coleman F. T., Mueschenborn S., Llosa N., Grout M., Pier G. B. (2003) Construction and characterization of a Pseudomonas aeruginosa mucoid exopolysaccharide-alginate conjugate vaccine. Infect. Immun. 71, 3875–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fattom A. I., Sarwar J., Basham L., Ennifar S., Naso R. (1998) Antigenic determinants of Staphylococcus aureus type 5 and type 8 capsular polysaccharide vaccines. Infect. Immun. 66, 4588–4592 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.