Abstract
Plant trypsin proteinase inhibitors (TPIs) are potent herbivore- and jasmonate (JA)-induced defenses, but support for the commonly invoked explanation for their inducible expression, namely their associated fitness costs, has been elusive. To determine whether the expression of TPIs incurs fitness costs, we expressed 175 bp of the seven-domain pi from Nicotiana attenuata in an antisense orientation in a TPI-producing genotype (WT) of N. attenuata to reduce TPI expression. Moreover, we expressed the full-length seven-domain pi in a sense orientation under control of a constitutive promoter to restore TPI activity in a natural genotype unable to produce TPIs because of a mutation in its endogenous pi gene. Lifetime reproductive output was determined from high and low TPI-producing plants of the same genetic background with and without JA elicitation and grown in the same pot to simulate natural competitive and nutrient regimes. Transformants with either low or no TPI activity grew faster and taller, flowered earlier, and produced more seed capsules (25-53%) than did neighboring TPI-producing genotypes, and JA elicitation increased TPI production and decreased seed capsule production further. Growth under high light levels only marginally reduced these fitness costs. Results were similar regardless of whether TPI activity was suppressed or restored by transformation: the larger the difference in TPI activity between neighbors, the larger the difference in seed capsule production (R2 = 0.57). TPI production is costly for a plant's components of fitness when grown under realistic competitive regimes and is consistent with the hypothesis that inducibility evolved as a cost-saving mechanism.
Although defenses might benefit plants in the presence of herbivores, plant resistance to herbivores can be costly in the absence of plant enemies (1-4). This cost-benefit paradigm has motivated most of the theory about the evolution of plant defenses against herbivores (5-8). Conclusive evidence attributing fitness costs to a particular defense trait has been elusive, but recent studies have made significant advances (9, 10). The paradigm has been difficult to test for two reasons: (i) fitness costs, which can be measured as reductions in either male (11) or female (1, 9, 12) reproductive function, arise from many different types of compromises that could result from the expression of defense traits; and (ii) the fitness costs of a defense trait must be disentangled from the costs of genetically correlated traits (13-15).
The fitness costs of defense can arise from both internal processes to the plant, such as when fitness-limiting resources are allocated to defenses that cannot be rapidly reallocated to growth and reproduction (16) or autotoxicity (2, 17, 18), and external processes (ecological costs) that occur when defense expression results in reduced pollination, attracts enemies, or impairs the expression of other resistance traits (3, 19, 20). Fitness measures integrate a plant's performance in a given environment and consequently should be measured under conditions commonly found in the plant's natural environment (20, 21). For example, the large reductions in lifetime seed production associated with jasmonate (JA)-elicited herbivore resistance in Nicotiana attenuata were found only when plants were grown with competitors (22-24), one of the dominant selective factors for this species, which synchronizes its germination from long-lived seed banks after fires in the Great Basin Desert in the United States (12). Hence costs may not be apparent in experiments on isolated plants grown under optimized conditions; this contingency makes negative evidence for fitness costs difficult to evaluate.
Although experimental work with natural populations ensures realism in the measurement of potential costs, demonstrating that a fitness cost can be attributed to the expression of a defense is difficult in genetically heterogeneous natural populations (2). Ideally, one should determine the cost of defenses in plants that differ only in the gene that controls the expression of a resistance trait but are otherwise genetically identical (25). Many defense traits are elicited after herbivore attack, and inducible expression is thought to allow plants to forgo the costs of defense when they are not needed, namely in environments without pests or pathogens. Numerous studies (reviewed in refs. 2 and 3) have exploited inducible expression as a means of controlling for, or homogenizing, the genetic background of plants and have measured plant fitness before and after eliciting resistance in plants in herbivore-free environments. The discovery that herbivore attack elicits the JA cascade in many species, and that exogenous JA treatments elicits induced resistance without the wounding that normally accompanies herbivore attack, has motivated studies to measure the fitness costs of JA-induced responses (1, 10, 26-29). However, because of pleiotropic effects of the elicitors, the observed fitness differences do not arise solely from the expression of the resistant trait (12, 30), and therefore these studies are likely to overestimate the fitness costs of resistance.
These experimental difficulties can be addressed with mutants defective in the endogenous production of the defense elicitors, but most studies focusing on molecular aspects of resistance signaling do not report factors such as growth rate or seed set (20). A recent exception to this trend is a study that used the jar1-1 mutant in Arabidopsis thaliana, which is deficient in JA signaling and expression of proteinase inhibitors (PIs), but surprisingly found greater reductions in seed production after JA elicitation in the mutant line than in the Col WT lines (10). Transformation technology provides a novel approach to manipulating plant resistance traits. Recently, an elegant study that rigorously controlled for potential differences in genetic background demonstrated that the presence of a particular R gene (RPM1) that confers resistance against particular strains of Pseudomonas syringae pathogens decreased reproductive output by 9% in A. thaliana (9). The R gene protein functions as the receptor for the pathogen elicitors, the AvrRpm1 or AvrB proteins, but the responses elicited by this pathogen recognition system responsible for the decrease in reproductive output are unknown. The A. thaliana genome contains >100 R genes, and it is unlikely that the expression of each results in a 9% fitness reduction.
Here, we used N. attenuata to examine the fitness consequences of trypsin PI (TPI) production, an established defense against a variety of different herbivores (24, 31). We compared the components of fitness of N. attenuata genotypes with either low or no TPI production with that of TPI-producing genotypes in competitive experiments in which plants were either elicited or not with methyl JA (MeJA) applications to increase TPI production and other insect resistance traits. We compared two independently transformed N. attenuata lines in which the expression of the pi gene was down-regulated by antisense expression of a 175-bp fragment of the N. attenuata pi gene with two lines independently transformed with empty vector constructs, which had fitness and PI production not distinguishable from untransformed WT plants of the same genetic background (an inbred line collected from Utah). We additionally compared the fitness of an untransformed N. attenuata genotype collected from Arizona (A), which has a mutation in the endogenous seven-domain pi gene and does not produce pi transcripts or TPI activity, with A plants transformed with the full-length cDNA of the seven-domain pi gene in a sense orientation under control of a constitutive promotor, which produced TPIs at 60% of the level found in MeJA-elicited WT Utah genotype plants. These constructs allowed us to compare the fitness consequences of TPI expression both by silencing endogenous TPI production in TPI-producing genotypes and restoring TPI production in the mutant A genotype by expressing a functional pi. Our data demonstrate that constitutive and inducible TPI production incurs large fitness costs when plants compete against plants of the same genetic background that lack the ability to produce TPIs. Previous work with the A genotype (24) and ongoing research with all genotypes used in this study (32) demonstrates that TPI expression profoundly determines herbivore resistance.
Materials and Methods
Plant Material and Transformation, DNA Isolation, and Southern Hybridization. For details see Supporting Text, which is published as supporting information on the PNAS web site.
Fitness Consequences of TPI Expression. We used a competition design optimized to detect the fitness consequences of JA-induced resistance in N. attenuata and simulate the soil mineral nutrition and competition levels that typify this plant's natural habitat (12). In this experimental setup, plants compete for below-ground resources (23) as they do in their natural habitat, and are synchronized in their germination and early growth, so that all fitness-based differences result from differences in performance during competitive growth (refs. 22-24 and Supporting Text). Seeds were germinated in diluted liquid smoke solutions as described (33). Two seedlings of similar size and appearance were transplanted 7 cm apart in 2-liter pots in a glasshouse in the conditions as described (ref. 24 and Supporting Text) with a minimum 800-900 μmol·m-2·s-1 photosynthetic photon flux density supplied by 450-W Na-vapor high-intensity discharge bulbs.
Genotypes (C1 and C2, empty vector transformed WT; AS-- and AS-, WT transformed with a construct containing the pi gene in an antisense orientation; A, Arizona genotype that completely lacks the ability to produce TPIs; S++, A transformed to express the functional pi) were grown in three different combinations that represented three separate experiments: AS-- competing with C1 (AS-- vs. C1), AS- competing with C2 (AS- vs. C2), and A competing with S++ (A vs. S++). The choice of particular AS-C pairs was randomly chosen, as informed by the results of a preliminarily experiment.
A preliminary competition experiment was conducted to determine whether the two empty vector transformed lines (C1 and C2) differed from each other in lifetime seed production: nine replicate pairs of C1-C2 plants were grown in a glasshouse under a minimum 1,000-1,300 μmol·m-2·s-1 photosynthetic photon flux density irradiance. No significant differences in seed capsule production were found in the experiment: C1 (94 ± 7.1) competing with C2 (103 ± 10.6; paired t test, t8-C1-C2 = 0.585; P = 0.57).
In each of the three experiments, individual plants were either uninduced (CON) or elicited with 150 μg of MeJA (*) and pairs of plants were assigned to the following three treatment groups: (i) CON-CON (AS-- vs. C1; AS- vs. C2; A vs. S++), (ii) MeJA-MeJA (AS--* vs. C1*; AS-* vs. C2*; A* vs. S++*), and (iii) CON-MeJA (AS-- vs. C1*; AS- vs. C2*; A vs. S++*). Either pure lanolin paste (20 μl) or lanolin paste containing 150 μg of MeJA in 20 μl was applied to the node +1 (one position older than the source-sink transition leaf) leaf of each plant as described (34) 11 days after transplanting to 2-liter pots. Each experiment had 14 replicate pairs for each of the three treatment groups: four randomly selected pairs were destructively harvested for chemical characterization, whereas the remaining 10 were used for growth and fitness characterization. The entire AS- vs. C2 experiment was replicated under the same growth conditions, whereas the entire AS-- vs. C1 experiment was replicated under higher irradiance (minimum of 1,000-1,300 μmol·m-2·s-1 photosynthetic photon flux density) supplied by 600-W Na-vapor high-intensity discharge bulbs. As an additional check on the choice of pairing, seven replicate pots of AS- vs. C1 for the CON-CON treatment were established.
Treated leaves were harvested 24 h after induction for Northern blot analysis of TPI mRNA accumulation as described (24, 35) in four replicate plants from each genotype and treatment and pooled. These plants were excluded from subsequent analysis. Constitutive and MeJA-induced TPI activity and nicotine were determined from all remaining replicates at the rosette stage. Leaves growing at node 0 (source-sink transition leaf) were harvested 3 days after elicitation, and protein concentrations and TPI activity were measured by radial diffusion assay and expressed as nmol·mg-1 as described (34). Nicotine concentrations were measured by HPLC as described (36) and expressed as mg·g-1 of fresh mass.
To compare the lifetime reproductive performance among genotypes, we recorded for each plant: (i) stalk length starting on the day with measurable stalk growth (14 days after transplanting) for 22 subsequent days, (ii) the day of first flowering (when the first flower had fully opened), and (iii) the number of seed capsules 51 d after transplanting. Watering was stopped 15 d before mimicking the typically growth period in the plant's natural environment. The number of capsules per plant reflects the lifetime reproductive output in N. attenuata under natural or glasshouse conditions (1, 26).
Statistical Analysis. Data were analyzed with statview (SAS Institute, Cary, NC). The TPI and nicotine were analyzed by ANOVAs followed by Fisher's protected least significant difference post hoc comparisons in all experiments. Data of reproductive output from the competition experiments were analyzed by paired t tests for all comparisons of competing plant pairs in one pot. Differences in stalk elongation between competitors were analyzed with repeated-measures ANOVA. Data of the mean differences and the percentage of mean differences in seed capsule number (all proportions were arcsine square root transformed before statistical analysis to correct non-normality) between the different plant pairs were analyzed by ANOVA. The difference in capsule production between plants in each pot was calculated as x - y (capsule production from the plant with higher seed capsules is considered as x and capsule with lower seed capsule production as y). The percentage differences between plants in seed capsule production were calculated as 100% - (y/x*100%). These values were averaged per treatment combination to obtain the mean differences and percentage mean differences.
Results
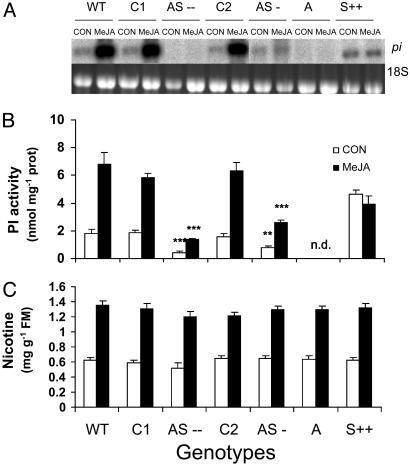
Characterization of Transgenic Plants. To silence the expression of N. attenuata's pi gene, WT was transformed with pNATPI1 (see Supporting Text) containing 175 bp of N. attenuata's pi gene in an antisense orientation under the control of cauliflower mosaic virus (CaMV) 35S promoter. Two initial transformants (T0), AS-- and AS-, were selected for low and intermediate TPI expression as determined from an activity assay (34). Southern analysis (Fig. 5, which is published as supporting information on the PNAS web site) and segregation ratios (3:1 for both lines) for nourseothricin resistance revealed that both AS-- and AS- contained one copy of T-DNA at one locus. A genotype of N. attenuata collected from Arizona (A), which completely lacks the ability to produce TPI (24), was transformed with pRESC2PIA2 (see Supporting Text and Fig. 6, which is published as supporting information on the PNAS web site) containing the full-length N. attenuata pi gene in the sense orientation under the control of CaMV 35S promoter. Southern analysis (Fig. 5) and segregation ratios (3:1) of hygromycin resistance revealed one copy of T-DNA present at one locus. To examine the constitutive and inducible levels of TPI mRNA of the transformed lines, Northern blot analysis was performed on total RNA from transformed lines (AS--, AS-, S++, C1, and C2) and untransformed genotypes (WT and A). Unelicited untransformed WT and lines transformed with empty vector constructs (C1 and C2) revealed a 1.4-kb TPI transcript that increased 4-fold 24 h after elicitation with 150 μg of MeJA (Fig. 1A). Although TPI transcripts were not detectable in AS-- plants, not even after MeJA elicitation, intermediate levels were found in the AS- line (Fig. 1 A). The difference in TPI expression between the two lines is likely caused by differences in transgene insertion sites. TPI mRNA in A genotype, which lack the ability to produce TPIs (24), was not detectable, and constitutive and inducible levels in A genotype plants that were transformed with the full-length N. attenuata pi gene in the sense orientation (S++) were similar to the constitutive levels found in WT plants (Fig. 1 A). TPI mRNA accumulation correlated well with TPI activity levels.
Fig. 1.
Northern blot analysis of TPI mRNA and concentrations of two direct defenses (nicotine and TPI activity) in untransformed WT N. attenuata plants of the Utah genotype; two homozygous T3 independently transformed lines of the Utah genotype that had been transformed either with a construct containing a 175-bp pi gene fragment in an antisense orientation (AS--, AS-) or an empty vector construct (C1, C2); and untransformed plants of the Arizona (A) genotype and plants of a homozygous T3 transformed line of the Arizona genotype transformed with a construct containing the full-length pi gene in a sense (S++) orientation. MeJA (150 μg) in a lanolin paste or pure lanoline (CON) was applied to leaves growing at node +1 (one position older than the source-sink transition leaf: node 0) at the rosette stage 11 days after transplanting. Asterisks indicate the level of significant differences between members of pairs (*, P < 0.05; **, P < 0.001; ***, P < 0.0001). (A) RNA gel blot analysis of pi gene transcripts in +1 leaves of unelicited control (CON) and MeJA-elicited plants 24 h after elicitation [TPI mRNA, 1.4 kb (Upper) and 18S rRNA, 3.4 kb (Lower)]. (B) TPI activity (mean ± SEM) in leaves at node 0 in CON and MeJA-elicited plants 3 d after elicitation. n.d., not detectable. (C) Nicotine concentrations (mean ± SEM) in leaves at node 0 in control (CON) and MeJA-elicited plants 3 d after elicitation. FM, fresh mass.
Leaf TPI activity was determined before and3dafterelicitation with 150 μg of MeJA in transformed and untransformed genotypes. Compared to the constitutive levels of TPI activity in the WT, C1, and C2 plants (which did no differ significantly; F2,92 = 0.593; P = 0.55), levels in AS-- and AS- plants were 77% and 50% lower, respectively (Fig. 1B; F4,166 = 14.397; P < 0.0001). Elicitation with MeJA increased TPI activity 3.6-fold in WT, C1, and C2 plants, whereas AS-- and AS- TPI levels were 22% and 40% of their respective controls (Fig. 1B; F4,166 = 38.851; P < 0.0001). MeJA elicitation did not alter (F1,55 = 1.007; P = 0.31) TPI activity in S++ plants, which remained at ≈61% of the induced WT plants (Fig. 1B; F1,55 = 7.742; P = 0.007). As expected, the untransformed A genotype showed no TPI activity even after induction with MeJA (Fig. 1B).
To facilitate comparison between constitutive and inducible TPI activity measures between members of competitor pairs and the fitness differences between competitors, we calculated the TPI activity difference between pairs. Although MeJA elicitation increased the difference of TPI activity between pairs from 1.4 ± 0.2 to 4.5 ± 0.7 nmol·protein-1 in AS-- vs. C1 (3.2-fold) and from 0.7 ± 0.2 to 3.7 ± 0.5 nmol·protein-1 in AS- vs. C2 (5.1-fold), no difference was found in the A vs. S++ pair (from 4.6 ± 0.8 to 3.9 ± 1.6 nmol·protein-1). The difference of TPI activity at the constitutive level in the AS-- vs. C1 pair was higher (2-fold) than in the AS- vs. C2 pair (F1,74 = 5.025; P = 0.02), whereas after elicitation the difference of TPI activity in the AS-- vs. C1 pair was only 20% higher than that of AS- vs. C2 (F1,74 = 1.156; P = 0.28). Transformation with pi genes did not affect constitutive and inducible nicotine production, another nitrogen-intensive direct defense of N. attenuata (12). No significant differences were found in either constitutive or MeJA-induced nicotine content among genotypes (Fig. 1C; F6,98-CON = 0.925, P = 0.48; F6.98-MeJA = 0.59, P = 0.73).
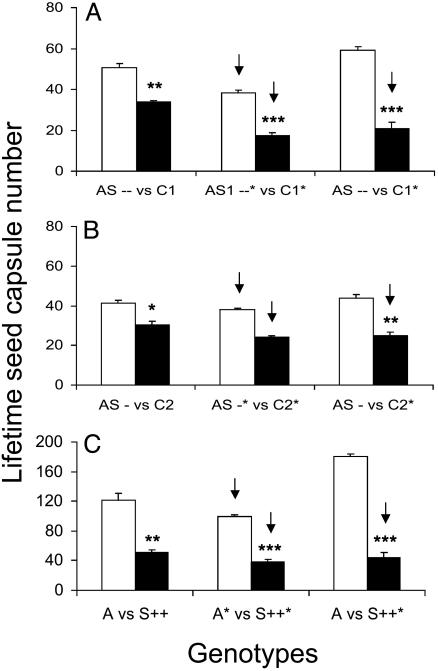
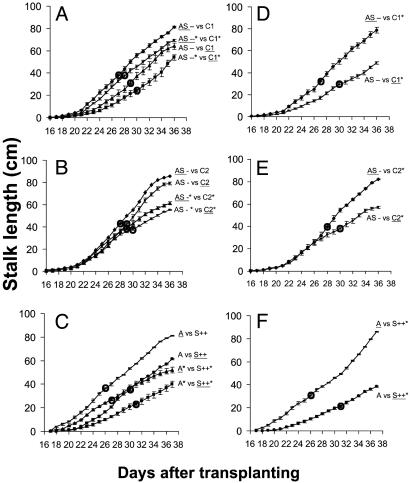
Fitness Consequences of Differential TPI Expression. We measured stalk lengths during elongation, seed capsule number, and the day of first flowering and calculated the mean differences and the percentage mean differences in seed capsule production for pairs with the same elicitation to estimate consequences of constitutive and inducible TPI production for components of fitness. Growth under higher irradiance levels increased the number of seed capsules produced and the difference in capsule production between pairs (by 68-51 capsules in AS-- vs. C1, with a 17-capsule increase in the difference), but the relative difference in seed capsule production was similar between different irradiance environments (see Fig. 3 and Table 1; see also Fig. 7 and Table 2, which are published as supporting information on the PNAS web site). When untreated AS--, AS- lines, and A genotype competed with uninduced C1, C2, and S++ neighbors, respectively, they not only grew faster and produced significantly taller stalks, but flowered earlier (Fig. 2 A-C; repeated measures ANOVA, F1,18-AS---C1 = 4.686, P = 0.04; F1,18-AS--C2 = 9.175, P = 0.007; F1,18-A-S++ = 256.227, P < 0.0001) and produced significantly more seed capsules (Figs. 3 and 7) than their neighbors. The choice of C and AS pairs did not influence the results. Similar results were found when the AS- competed with C1; AS- plants produced more seed capsules (39.2 ± 2.6) than C1 lines (29.8 ± 1.3) with a 9.4-capsule difference between neighbors (paired t test, t6 = 2.361, P = 0.056).
Fig. 3.
Mean (± SEM) lifetime seed capsule number produced by N. attenuata genotypes differing in TPI production (see Fig. 1 for abbreviations) were grown in competition with each other and were either uninduced or elicited with 150 μg (*) of MeJA. Arrows depict the genotype that was elicited with MeJA, and asterisks indicate the level of significant differences between members of pairs (*, P < 0.05; **, P < 0.001; ***, P < 0.0001). (A) Mean (± SEM) capsule number of AS-- (open bars) and C1 (solid bars) genotypes. (B) Mean (± SEM) capsule number of AS- (open bars) and C2 (solid bars) genotypes. (C) Mean (± SEM) capsule number of A (open bars) and S++ (solid bars) genotypes.
Table 1. Absolute and relative mean differences in lifetime seed capsule production from pairs of developmentally synchronized plants from homozygous T3 independently transformed lines of a WT genotype of N. attenuata, which had been transformed with constructs containing a pi gene fragment in an antisense orientation (AS--, AS-), or an empty vector construct (C1, C2); untransformed plants of the Arizona (A) genotype and plants of the Arizona genotype transformed with constructs containing the full-length pi gene in a sense (S++) orientation.
| Competitors | Mean difference in capsule no. | P | % mean difference in capsule no. | P |
|---|---|---|---|---|
| AS-- vs. C1 | 16.8 ± 2.9 | 0.0003 | 33.2 ± 4.3 | 0.0001 |
| AS--* vs. C1* | 20.8 ± 0.9 | <0.0001 | 54.2 ± 1.8 | <0.0001 |
| AS1-- vs. C1* | 38.4 ± 2.5 | <0.0001 | 64.9 ± 4.5 | <0.0001 |
| AS- vs. C2 | 10.6 ± 1.8 | 0.0032 | 25.7 ± 3.0 | 0.0063 |
| AS-* vs. C2* | 14.0 ± 1.0 | <0.0001 | 36.8 ± 2.8 | <0.0001 |
| AS- vs. C2* | 19.0 ± 3.2 | 0.0005 | 43.3 ± 5.4 | <0.0001 |
| A vs. S++ | 70.2 ± 10.7 | 0.0001 | 53.4 ± 5.3 | <0.0001 |
| A* vs. S++* | 60.3 ± 3.8 | <0.0001 | 60.7 ± 3.5 | <0.0001 |
| A vs. S++* | 136 ± 5.5 | <0.0001 | 75.5 ± 3.2 | <0.0001 |
Two plants were grown in the same pot in three different pair combinations (AS-- vs C1, AS- vs C2, and A vs S++). Plants were either treated with 150 μg of MeJA in 20 μl of lanolin paste (*) to elicit JA-induced defenses or treated with 20 μl of pure lanolin paste as controls; n = 10 per treatment and pair combination. P values are from paired t test comparisons; relative values were arsine square root transformed before analysis.
Fig. 2.
Growth and flowering time of N. attenuata genotypes differing in TPI production (see Fig. 1 for abbreviations) grown in competition with each other and either uninduced or elicited with 150 μg (*) of MeJA. Data shown stalk lengths (mean ± SEM) of the genotype (underlined) starting on the day with measurable stalk growth for 22 subsequent days; circles depict the mean day of first flowering. (A-C) Both competitors from the same pot had the same treatment: either MeJA (*) or control. (D-F) Competitors from the same pot were either treated with MeJA (*) or untreated. Stalk length (mean ± SEM) is shown for AS-- and C1 genotypes (A and D), AS- and C2 genotypes (B and E), and A and S++ genotypes (C and F).
The highest absolute and relative difference of seed capsule production was observed in the A vs. S++ pair (70.2 capsules, 53.4%) and the lowest in the AS- vs. C2 (10.6 capsules, 25.7%; and 9.5 capsules, 25.0%) pair (Tables 1 and 2). Delay in first flower production within pairs was 1 d in AS-- vs. C1, 2 d in AS- vs. C2, and 3 d in A vs. S++ (Fig. 2 A-C; ANOVA, F5,54-Flower = 8.879, P < 0.0001).
When both competitors were elicited with MeJA, stalk length (Fig. 2 A-C; repeated measures ANOVA, F1,18-AS---C1 = 10.411, P = 0.004; F1,18-AS--C2 = 2.661, P = 0.1; F1,18-A-S++ = 36.751, P < 0.0001) and seed capsule production (Figs. 3 and 7) decreased in both competitors, and the relative difference between competitors in seed capsule production was amplified in comparison to the differences observed when neither competitor was elicited: 21.0% and 20.2% increases in the medium and high light replicates of AS--* vs. C1*; 11.1% and 16.1% increases for the two replicate experiments of AS-* vs. C2* (Tables 1 and 2). These increases in fitness costs are commensurate with MeJA-elicited increases in TPI production (Fig. 1B). The smallest increases in MeJA-elicited fitness costs were observed in S++ genotypes (ANOVA, F2,27 = 2.027, P = 0.15; a 7.3% increase in A* vs. S++*; Fig. 3C). MeJA elicitation did not increase TPI production in S++ plants, because pi is under control of a constitutive promoter in these plants. After MeJA elicitation, flowering was delayed by 1 d in all cases (Fig. 2 A-C).
Elicitation of only the control plants (C) from either AS-- vs. C1 or AS- vs. C2 or the A plants of the A vs. S++ pairs reduced stalk length and seed capsule production of the elicited plant and resulted in the greatest fitness differentials between competitors (Fig. 2 D and E; repeated measures ANOVA, F1,18-AS---C1 = 26.13, P < 0.0001; F1,18-AS--C2 = 100.098, P < 0.0001; Figs. 3 A and B and 7). These large differences in lifetime seed production were caused by both the costs of TPI production and the opportunity benefit realized by the unelicited neighbor. Unelicited plants growing adjacent to induced plants produce more seed than do uninduced plants growing adjacent similarly uninduced plants because of greater resource acquisition of the uninduced plants (22). When unelicited A competed with elicited S++ plants, the absolute difference of seed capsule production (136 total) was 2-fold greater than when competing with unelicited S++ (70.2), and this difference arose from an increase in 60 seed capsules (a 33% increase) in the A genotype, rather than a decrease in the output of the S++ genotype, representing an opportunity benefit (Fig. 3C and Table 1).
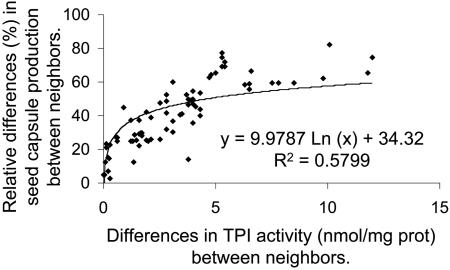
To determine the effect of TPI production on seed capsule production, we regressed the differences in TPI activity between neighbors against the relative differences in seed capsule production between neighbors and found that a logarithmic function [Y = 9.9787 Ln (PI) + 34.32; R2 = 0.5799; Fig. 4; P < 0.01] represented the best fit. The relationship suggests that the higher the difference in TPI activity between neighbors, the higher the relative differences in seed capsule production. For this analysis we only included plants from pots where both competitors were either unelicited or elicited with 150 μg of MeJA.
Fig. 4.
Relative differences in seed capsule production (as percentages) between neighbors of N. attenuata genotypes that had been transformed with constructs containing the pi gene in an antisense orientation or an empty vector construct, untransformed plants of the Arizona genotype, and plants of the Arizona genotype transformed with constructs containing the full-length pi gene in a sense orientation, regressed against the differences in TPI activity (nmol/mg of protein) between neighbors. The analysis included only plant pairs in which both competitors received the same treatment, either uninduced or induced with 150 μg of MeJA. The line represent a regression fitted to the points [Y = 9.9787 Ln (PI) + 34.32; R2 = 0.5799].
Discussion
Our experiments demonstrate that TPI is directly responsible for the observed fitness differences between neighbors. Across all experiments, the larger the difference in TPI activity between neighbors, the larger was the difference in seed capsule production (Fig. 4). Plants with high constitutive TPI levels (C1, C2, and S++) growing next to plants with low TPI levels (AS--, AS-, and A), realized a large reduction in lifetime seed production: 26% and 25% in AS- vs. C2, 33% and 28% in AS-- vs. C1, and 53% in A vs. S++ (Fig. 4 and Tables 1 and 2). Not only were the qualitative results entirely reproducible across all replicates of the experiments, but the quantitative measures of the relative fitness consequences of TPI production were also remarkably similar between experiments, regardless of whether pi expression was silenced or restored. For example, when both competitors were elicited in the AS-- vs. C1 experiment, which resulted in an average difference in TPI activity between neighbors equivalent to the difference in TPI activity measured between the A vs. S++ pairs (4.6 nmol·protein-1; Fig. 1B), the relative fitness differences were the same (53-54% differences; Table 1). The rank order of fitness differences within the different silencing experiments tracked the differences in TPI production; the fitness differences in the AS-- experiments with low TPI production (16 and 33 capsules) were greater than those observed in the AS- experiments that had intermediate TPI production (from 9 to 10 capsules; Fig. 3 and Tables 1 and 2). Elicitation of both competitors with MeJA increased the differences in TPI production and consistently increased the realized fitness differences between competitors (AS- vs. C2, 4 and 1 capsules; AS-- vs. C1, 4 and 13 capsules; Tables 1 and 2). Because seed capsule production did not differ between controls (C1 vs. C2) and WT (C1 vs. WT) and the fitness differences between AS- competing with either C1 or C2 were not different (10 capsules), the observed fitness differences cannot be attributed to particular pairing combinations. We conclude from these results that TPI production is intrinsically costly when plants compete for below-ground resources with conspecifics, as they commonly do in nature.
Why TPI production is so costly for the reproductive performance of competitively growing plants remains an open question. TPI production may make demands on a plant's nitrogen (N) budget that a plant could otherwise allocate to growth and reproduction. In addition, such demands might decrease the allocation of N to other N-intensive defenses. However, we found no evidence that TPI expression had any effect on either constitutive or inducible nicotine production (Fig. 1C), a N-intensive defense that can use 6% of N. attenuata's whole-plant N budget (26). Whole-plant nicotine pools in N. attenuata are stable, increase under N-limited growth, and are not metabolized and reused for growth. In contrast, PIs are thought to be metabolized and decrease under competitive- and N-limited growth in other plant systems (37), suggesting that an investment of fitness-limiting resources to PIs can be adjusted to internal resource levels. We found no evidence that competitive growth decreased TPI activity in any line (Fig. 1B and J.A.Z. and I.T.B., unpublished data). Similarly, increases in the irradiation levels to the competing plants did not alleviate the fitness differences associated with differential TPI production. Growth under high light levels increased reproductive output of competing plants but the fitness and growth differentials were retained (Tables 1 and 2). In an earlier experiment that examined the mechanisms responsible for the fitness costs of MeJA elicitation in WT N. attenuata plants, it was found that increases in below-ground N supply accentuated fitness consequences of elicitation rather than decreasing them (23). These results suggest that the simple allocation of fitness-limiting resources to TPI production cannot directly account for their costs. However, the changes in metabolism associated with or required to support increases in TPI production may contribute to the observed fitness differences.
Growth and fitness differences between competing plants in our experimental design result in part from differences in the rate of harvesting below-ground resources. Slow-growing plants do not harvest resources as rapidly as fast-growing plants, providing an opportunity benefit for the fast-growing plants (22, 23, 27). Unelicited WT plants growing next to MeJA-elicited WT neighbors acquire more 15NO3 from the soil, grow faster, and allocate more 15N to seed production than do unelicited plants growing next to competitively similar, unelicited plants (23). Interestingly, such opportunity benefits were not observed in the experiments with plants with silenced TPI production, but were clearly apparent in the A vs. S++ experiment, in which A plants growing next to elicited S++ plants realized an opportunity benefit of 60 capsules, an 33% increase in reproductive output, over A plants growing next to unelicited S++ plants (Fig. 3C). These results suggest that production of TPIs does not directly interfere with a plant's ability to take up soil N (as MeJA elicitation clearly does), because if it did, the neighboring plant would be able to capitalize on this unclaimed soil resource and increase growth and reproductive output. Alternatively, the silencing of TPI production may somehow interfere with a plant's ability to capitalize on this opportunity benefit, but this idea seems unlikely given the large fitness increases associated with the silencing of TPI production. MeJA elicitation decreases the transcription of photosynthetic-related genes (30, 38, 39), and this down-regulation may be required to free up resources for defense-related processes. Transcriptional analysis of the AS lines will be required to determine whether the down-regulation of growth-related transcripts after elicitation are comparable to those of TPI-producing lines.
Hence alternative physiological explanations for the costs associated with TPI production are needed. PIs have been suggested to play an endogenous regulatory role to protect cells from proteinase activity in unwanted locations (40). It is possible that the large quantities of TPI required for defense also inhibit enzyme activities that support rapid growth. However, until the physiological under-pinnings of rapid growth required for competitive prowess are better understood, this hypothesis will be difficult to test.
The fitness costs of TPI production measured in this laboratory study included only one of the plant's natural ecological interactions: intraspecific competition. When plants grow with their full complement of natural ecological interactions, these costs are likely to be balanced by the fitness benefits resulting from the defensive utility of TPI expression (24). However, ecological costs might also be incurred that result from the complicated interactions with other species (2, 16), such as the decrease the attractiveness of the pollinators (2, 41). Because TPI expression frequently slows the grow rate of insect herbivores by making their digestive processes less efficient (40, 42), the fitness benefits of TPI expression may result from extending the period during which larvae can be successfully attacked by natural enemies. Hence without the attraction of natural enemies, TPI expression may not increase plant fitness in environments with herbivores. Interestingly, the most important natural enemy of N. attenuata herbivores, Georcoris pallens, is attracted to herbivore-attacked plants by volatile signals released from the plant (43), which are elicited by the same signals that elicit TPI production (refs. 34, 44, and 45 and A. Roda and I.T.B., unpublished results), and hence these direct and indirect defense traits are coordinately expressed in WT plants. The A genotype, in addition to not producing TPIs, does not release an important predator-attracting component of the herbivore-induced volatile blend: cis-α-bergamotene (24). Because volatile release may also attract other herbivores, it is possible that AS plants, with their down-regulated TPI production but intact volatile release (J.A.Z. and I.T.B., unpublished results) may incur additional fitness costs beyond those attributable to the down-regulation of PI-based defenses. These results underscore the relevance of an important assumption in life history theory: that defense expression incurs fitness costs.
Supplementary Material
Acknowledgments
We thank the Max Planck Society for funding and two anonymous reviewers for improving the manuscript.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PI, proteinase inhibitor; TPI, trypsin PI; JA, jasmonate; MeJA, methyl JA.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY184823 and AF234297).
References
- 1.Baldwin, I. T. (1998) Proc. Natl. Acad. Sci. USA 95, 8113-8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strauss, S. Y., Rudgers, J. A., Lau, J. A. & Irwin, R. E. (2002) Trends Ecol. Evol. 17, 278-285. [Google Scholar]
- 3.Cipollini, D. F., Purrington, C. B. & Bergelson, J. (2003) Basic Appl. Ecol. 4, 79-85. [Google Scholar]
- 4.Hare, D. J., Elle, E. & van Dam, N. M. (2003) Evolution 57, 793-805. [DOI] [PubMed] [Google Scholar]
- 5.Feeny, P. P. (1976) Recent Adv. Phytochem. 10, 1-40. [Google Scholar]
- 6.Rhoades, D. F. & Cates, R. G. (1976) Recent Adv. Phytochem. 10, 168-213. [Google Scholar]
- 7.Coley, P. D., Bryant, J. P. & Chapin, F. S. I. (1985) Science 230, 895-899. [DOI] [PubMed] [Google Scholar]
- 8.Herms, D. A. & Mattson, W. J. (1992) Q. Rev. Biol. 67, 283-335. [Google Scholar]
- 9.Tian, D., Traw, M. B., Chen, J. Q., Kreitman, M. & Bergelson, J. (2003) Nature 423, 74-77. [DOI] [PubMed] [Google Scholar]
- 10.Cipollini, D. F. (2002) Oecologia 131, 514-520. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal, A. A., Strauss, S. Y. & Stout, M. J. (1999) Evolution 53, 1093-1104. [DOI] [PubMed] [Google Scholar]
- 12.Baldwin, I. T. (2001) Plant Physiol. 127, 1449-1458. [PMC free article] [PubMed] [Google Scholar]
- 13.Berembaum, M. E., Zangerl, A. R. & Nitao, J. K. (1986) Evolution 40, 1215-1228. [DOI] [PubMed] [Google Scholar]
- 14.Coley, P. D. (1986) Oecologia 70, 238-241. [DOI] [PubMed] [Google Scholar]
- 15.Briggs, M. A. & Schultz, J. C. (1990) Oecologia 83, 32-37. [DOI] [PubMed] [Google Scholar]
- 16.Karban, R. & Baldwin, I. T. (1997) Induced Responses to Herbivory (Univ. of Chicago Press, Chicago).
- 17.Heil, M. (2001) Eur. J. Plant Pathol. 107, 137-146. [Google Scholar]
- 18.Baldwin, I. T. & Callahan, P. (1993) Oecologia 94, 534-541. [DOI] [PubMed] [Google Scholar]
- 19.Euler, M. & Baldwin, I. T. (1996) Oecologia 107, 102-112. [DOI] [PubMed] [Google Scholar]
- 20.Heil, M. & Baldwin, I. T. (2002) Trends Plant Sci. 7, 61-67. [DOI] [PubMed] [Google Scholar]
- 21.Koricheva, J. (2002) Ecology 83, 176-190. [Google Scholar]
- 22.van Dam, N. M. & Baldwin, I. T. (1998) Ecol. Lett. 1, 30-33. [Google Scholar]
- 23.van Dam, N. M. & Baldwin, I. T. (2001) Funct. Ecol. 15, 406-415. [Google Scholar]
- 24.Glawe, A. G., Zavala, J. A., Kessler, A., van Dam, N. M. & Baldwin, I. T. (2003) Ecology 84, 79-90. [Google Scholar]
- 25.Bergelson, J. & Purrington, C. B. (1996) Am. Nat. 148, 536-558. [Google Scholar]
- 26.Baldwin, I. T., Gorham, D., Schmelz, E. A., Lewandowski, C. A. & Lynds, G. Y. (1998) Oecologia 115, 541-552. [DOI] [PubMed] [Google Scholar]
- 27.Baldwin, I. T. & Hamilton, W. (2000) J. Chem. Ecol. 26, 915-952. [Google Scholar]
- 28.Thaler, J. S. (1999) Nature 399, 686-688. [Google Scholar]
- 29.Thaler, J. S., Stout, M. J., Karban, R. & Duffey, S. S. (1999) J. Chem. Ecol. 22, 1767-1781. [DOI] [PubMed] [Google Scholar]
- 30.Hermsmeier, D., Schittko, U. & Baldwin, I. T. (2001) Plant Physiol. 125, 683-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan, C. A. (1990) Annu. Rev. Phytopathol. 28, 425-449. [Google Scholar]
- 32.Zavala, J. A., Patankar, A. P., Gase, K., Hui, D. & Baldwin, I. T. (2004) Plant Physiol., in press. [DOI] [PMC free article] [PubMed]
- 33.Baldwin, I. T., Staszakkozinki, L. & Davidson, R. (1994) J. Chem. Ecol. 20, 2345-2371. [DOI] [PubMed] [Google Scholar]
- 34.van Dam, N. M., Horn, M., Mares, M. & Baldwin, I. T. (2001) J. Chem. Ecol. 27, 547-568. [DOI] [PubMed] [Google Scholar]
- 35.Winz, R. A. & Baldwin, I. T. (2001) Plant Physiol. 125, 2189-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keinänen, M., Oldham, N. J. & Baldwin, I. T. (2001) J. Agric. Food Chem. 49, 3553-3358. [DOI] [PubMed] [Google Scholar]
- 37.Cipollini, D. F. & Bergelson, J. (2001) J. Chem. Ecol. 27, 593-610. [DOI] [PubMed] [Google Scholar]
- 38.Halitschke, R., Gase, K., Hui, D., Schmidt, D. & Baldwin, I. T. (2003) Plant Physiol. 131, 1894-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hui, D., Iqbal, J., Lehmann, K., Gase, K., Saluz, H. P. & Baldwin, I. T. (2003) Plant Physiol. 131, 1877-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laing, W. & McManus, M. T. (2002) in Protein-Protein Interactions in Plant Biology, eds. McManus, M. T., Laing, W. A. & Allan, A. C. (CRC, Boca Raton, FL), pp. 77-119.
- 41.Burgess, E. P. J., Malone, L. A. & Christeller, J. T. (1996) J. Insect. Physiol. 42, 823-828. [DOI] [PubMed] [Google Scholar]
- 42.Winterer, J. & Bergelson, J. (2001) Mol. Ecol. 10, 1069-1074. [DOI] [PubMed] [Google Scholar]
- 43.Kessler, A. & Baldwin, I. T. (2001) Science 291, 2141-2144. [DOI] [PubMed] [Google Scholar]
- 44.Halitschke, R., Keβler, A., Kahl, J., Lorenz, A. & Baldwin, I. T. (2000) Oecologia 124, 408-417. [DOI] [PubMed] [Google Scholar]
- 45.Halitschke, R., Schittko, U., Pohnert, G., Boland, W. & Baldwin, I. T. (2001) Plant Physiol. 125, 711-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.