Abstract
We present a transcriptomic analysis aimed at investigating whether the changes in gene expression that occur under inbreeding generally reduce or enhance inbreeding depression. Discerning between these two alternatives can be addressed only when both changes in expression due to inbreeding and to inbreeding depression are estimated simultaneously. We used Affymetrix 2.0 arrays to study the changes in gene expression associated with both inbreeding and inbreeding depression for fitness in four sets of inbred sublines of Drosophila melanogaster. We found that for most genes showing changes in expression associated with inbreeding, the least depressed sublines were those showing the largest departures in expression from that of the outbred control. This suggests a pattern consistent with a protective role of expression changes against inbreeding effects, and would reveal a new dimension of the transcriptomics of inbreeding. The variation in depression observed could then be due not only to the genetic damages primarily originating that depression, but also possibly to differences in the ability to carry out the appropriate adjustments in gene expression to cope with the inbreeding. We also found that these expression changes with a putative protective role against inbreeding effects show a clear specificity on RNA synthesis and splicing and energy derivation functions.
Keywords: microarrays, inbreeding depression, transcriptomics
INBREEDING depression, the reduction in fitness observed in the progeny of genetically related individuals, plays a key role in population biology, affecting processes as diverse as the management of livestock and endangered wild species (Keller and Waller 2002; Koenig and Simianer 2006), the evolution of mating systems (Kelly 2005), and the dispersal strategies (Motro 1991; Gandon 1999). The population genetics of this depression is well understood (Lynch 1991; Charlesworth and Charlesworth 1999; Charlesworth and Willis 2009), but the genomic details about the mechanisms causing it are just starting to be unveiled (Paige 2010). In particular, it would be important to determine the relationship between gene regulation and inbreeding, as regulatory variation underlies much of phenotypic diversity (Wilson et al. 1974; Carroll 2005; Ranz and Machado 2006). Evidence has been provided for significant intraspecific variation in transcript abundance for a large fraction of the genome (Primig et al. 2000; Sandberg et al. 2000) and to show that much of such variation is heritable (Cavalieri et al. 2000; Karp et al. 2000; Jin et al. 2001), suggesting that regulatory variation is likely the main mediator of phenotypic divergence in evolution (King and Wilson 1975; Wray et al. 2003; Hoekstra and Coyne 2007). Therefore, genetic correlations between expression phenotypes and organismal phenotypes point to the molecular pathways that underlie the organismal phenotypes (Rockman and Kruglyak 2006). Thus, an understanding of the molecular basis of inbreeding depression requires knowledge of variation at the whole-genome level (Ayroles et al. 2009).
Using whole-genome cDNA microarrays, which make it possible to study the gene-expression changes associated with inbreeding, Kristensen et al. (2005) compared the expression profiles of Drosophila melanogaster virgin males taken from inbred and control outbred lines and discovered that many different genes were differentially expressed with inbreeding and that genes involved in metabolism, biological defense, and stress responses were overrepresented among them. Ayroles et al. (2009) carried out a comparison between the expressions of inbred lines of D. melanogaster differing in their magnitude of inbreeding depression. They set up lines completely homozygous for different third chromosomes derived from a wild population and compared the male gene expression in lines showing strong and weak depression for male competitive reproductive success. In their analysis there was not an evaluation of the expression of an outbred control. Again, many genes showed differences in expression between depression levels, those related with metabolism, and stress and defense responses being overrepresented. A comparison of the gene lists showing inbreeding and inbreeding depression effects in both studies found a significant overlap between the two, although there was also a great effect of genetic background on transcriptome patterns (Sarup et al. 2011).
The above studies, however, did not allow ascertaining whether the observed expression changes were either a functional, fitness-increasing response to compensate for some physiological inefficiency caused by inbreeding or just a consequence of defective gene regulation (Girardot et al. 2004; Kristensen et al. 2010). Discerning the changes that alleviate the depression from those that generate it can be addressed only when both the changes in expression due to inbreeding and to inbreeding depression are estimated simultaneously. Here we present a study focused on this objective.
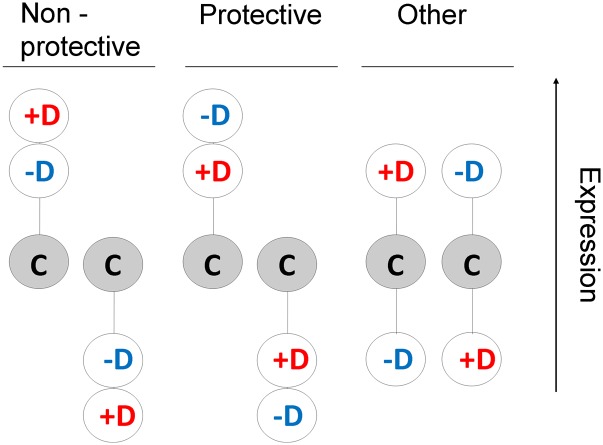
The alternative hypotheses that we try to test are shown in Figure 1. For those genes where inbred lines’ expression differs from outbred lines’ expression, two patterns are possible. In the first, the most depressed individuals (+D) deviate more from the outbred controls (C) than the least depressed ones (–D). These expression changes could be either dysfunctional (the most depressed individuals change most) or protective (changes are needed to be larger in the individuals suffering most depression). Because of this ambiguity and to be conservative, we denote this pattern as nonprotective. For the alternative pattern (protective), in which the most depressed individuals deviate less from the outbred control than the least depressed ones, the interpretation is less ambiguous. Genes showing this kind of response could have a role in protecting individuals against the effects of inbreeding, those failing to complete the appropriate changes in expression contributing to the depression.
Figure 1 .
Alternative patterns of expression for genes showing a differential expression under inbreeding. Circles represent the level of expression of genes in an outbred control set (C, shaded circles), in an inbred highly depressed set (+D, in red), and in an inbred lowly depressed set (–D, in blue). Three gene-expression configurations are possible. In the nonprotective configuration, the most depressed set shows an expression that deviates from the outbred control expression more than that for the least depressed set. The opposite occurs in the protective configuration.
In this work, we analyzed changes in gene expression in different D. melanogaster inbred sublines differing in their magnitudes of inbreeding depression relative to the expression in an outbred control to evaluate the relative importance of protective and deleterious gene-expression changes associated with inbreeding. Our results suggest that most changes in gene expression associated with inbreeding are compatible with a protection against the effects of inbreeding.
Materials and Methods
Base population and culture conditions
The D. melanogaster base population was founded in November 2006 from a sample of more than 1000 individuals collected in a wine cellar close to Vigo (Galicia, northwest Spain). The population was maintained in ∼30 bottles (30–60 individuals per bottle) with circular mating until the start of the experiment in July 2008. Flies were reared in a culture medium composed of 1 liter water, 100 g brewer’s yeast, 100 g sucrose, 12 g agar, 2.5 g NaCl, and 5 ml propionic acid and were handled at room temperature under CO2 anesthesia. All cultures were incubated in a chamber at 25 ± 1°, 65 ± 5% relative humidity, and maintained under continuous lighting. Virgin males and females were used for mating across the entire experiment.
Inbred lines and sublines
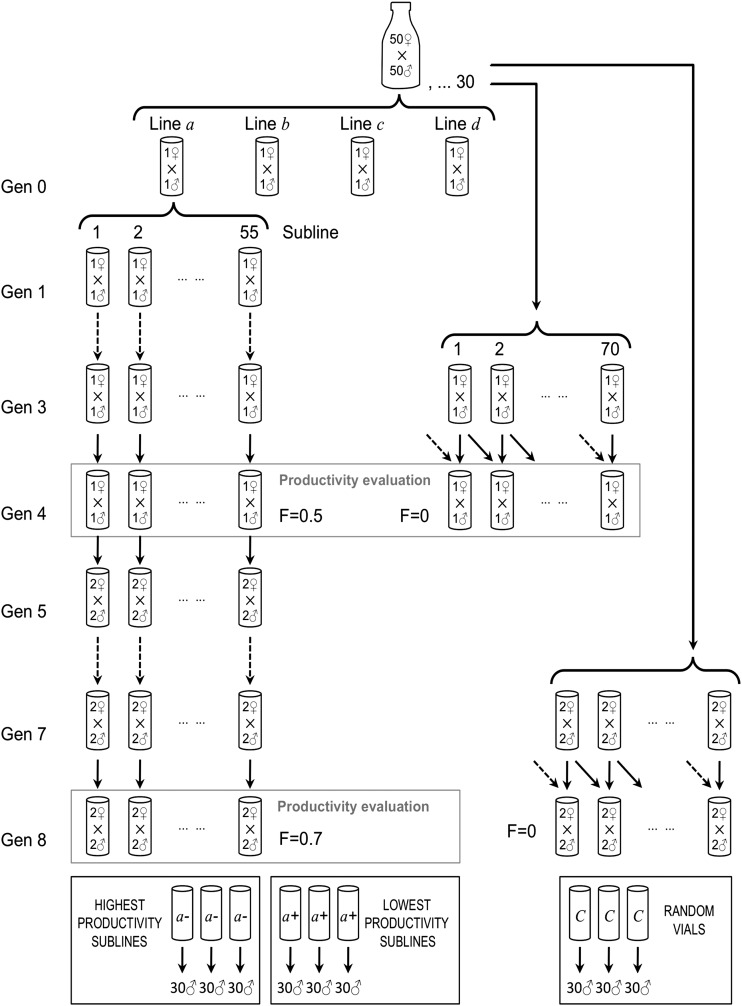
Four couples were randomly collected from the base population and placed into separate vials to generate four independent lines (Figure 2). From their progeny, 55 full-sib sublines (single brother–sister mating) were initiated for each line. At generation 4, when the expected inbreeding coefficient achieved was 0.5, the number of surviving sublines was 38, 43, 27, and 39 for each of the 4 lines, respectively. To minimize further line losses, from generation 5 to generation 8 the sublines were maintained with two males and two females (full sibs) placed into the same vial. Because of this, the expected inbreeding coefficient at generation 8 (F = 0.709) would present a small variance among sublines (0.000512; value obtained by simulating 1000 times the pedigree data of the mating system carried out until generation 8). The numbers of surviving sublines available for analysis in generation 8 in the four lines were 25, 31, 17, and 27.
Figure 2 .
Experimental design to produce four sets of sublines with the highest inbreeding depression (lowest productivity, sublines labeled with +) and sublines with the lowest inbreeding depression (highest productivity, sublines labeled with –). Expression analysis was carried out for these sublines’ sets as well as for an outbred control obtained from the base population.
Productivity evaluation
The character measured to select the most and least depressed sublines was pupa productivity, a composite trait including fecundity and offspring–pupa viability. The trait behaves as a typical fitness trait, showing substantial inbreeding depression (1.2% per 1% increase in inbreeding coefficient) and asymmetrical response to artificial selection (realized heritability 0.05 for upward selection and 0.4 for downward selection) (unpublished data obtained from the same base population). The productivity of the sublines was evaluated at generations 4 and 8. From each subline, three evaluation replicate vials were established using a single virgin couple per vial (generation 4) or two virgin couples per vial (generation 8). At generation 4 an evaluation of the productivity was also carried out for the base population (used as a noninbred control). Seventy virgin couples were randomly collected from the base population and put in single vials simultaneously with the inbred sublines. To evaluate the total productivity of each vial, we counted the number of pupa present after a 14-day incubation period (so that most life-time pupa production was considered), and the productivity of each subline was computed as the mean value of the three replicates. Since we had only one (at generation 4) or two (at generation 8) laying females per vial, population density was uniformly low in all vials. Mean productivities for each line at generations 4 and 8 as well as for the control base population at generation 4 are shown in Supporting Information, Figure S1. At generation 4, the rate of inbreeding depression was 1.14, 1.02, 0.97, and 1.12% per 1% increase in inbreeding for lines a, b, c, and d, respectively.
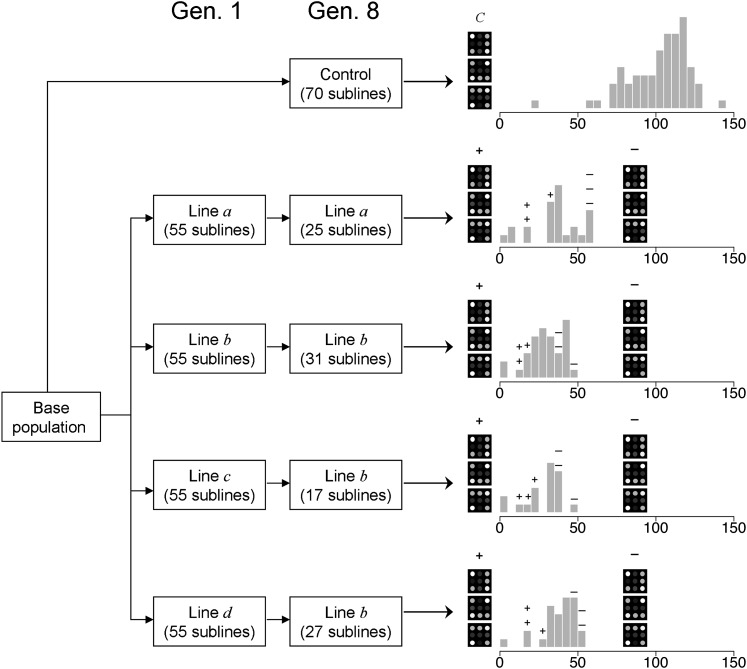
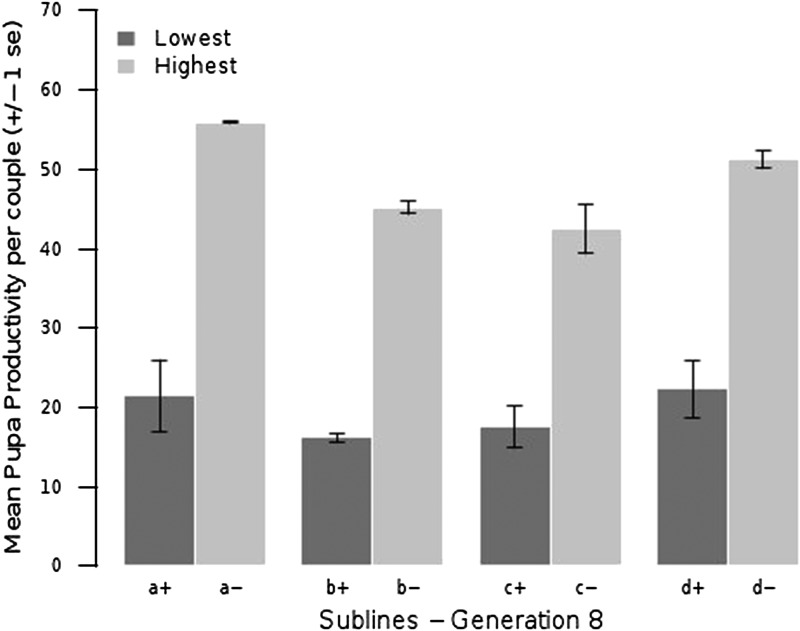
For each line, the three sublines showing the lowest productivity and the three sublines showing the highest productivity at generation 8 were chosen for expression analysis, along with the outbred control base population (Figure 3). The number of arrays analyzed was thus 27. The distributions of the productivities of all sublines are shown in Figure 3, and the average productivity of the chosen sublines are shown in Figure 4. Note that the average productivity of the highest productivity sublines was about three times larger than that of the lowest productivity sublines.
Figure 3 .
Design for the analysis of arrays. From each full-sib line (a, b, c, d), sets of full-sib sublines were established and analyzed for productivity. The distribution of these productivities is shown. Samples from the three sublines with the largest inbreeding depression (with symbol +) and those with the lowest inbreeding depression (with symbol –) with at least 30 males, were analyzed along with three samples from an outbred control. Thus, a total of 27 arrays were analyzed.
Figure 4 .
Mean pupa productivity for sublines with the highest and lowest productivity. Mean pupa productivity for the three sublines showing the lowest productivity and the three sublines showing the highest productivity at generation 8, which were chosen for expression analysis. Letters a, b, c, and d denote the inbred line code, and + and – denote most depressed (lowest productivity) and least depressed (highest productivity), respectively.
Expression arrays
For the expression analysis, 30 males from each selected subline and 3 groups of 30 males from the control (in all cases between 1 and 6 days after adult emergence) were collected at generation 8. These flies were anesthetized with CO2, frozen in liquid nitrogen, and stored at −80° prior to RNA extraction. Total RNA purification was performed with the RNeasy Mini kit (QIAGEN, Valencia, CA). RNA concentration was determined using a Thermo Fisher Scientific NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). RNA integrity was assessed on an Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA). cDNA was synthesized with One-Cycle cDNA synthesis kit (Affymetrix) following the Affymetrix Expression Analysis Technical Manual protocol. cRNA was synthesized from this cDNA using the Affymetrix IVT labeling kit and purified with the Affymetrix GeneChip Sample Cleanup Module kit, being eluted in a total volume of 22 μl of RNase-free water. Purified cRNA was quantified spectrophotometrically, and 15 μg of each biotinylated cRNA sample was fragmented and mixed with hybridization buffer (100 mM MES, 1 M NaCl, 20 mM EDTA, 0.01% Tween 20) and loaded into the Affymetrix Drosophila GeneChip Array (v. 2.0). This single array contains 18,500 transcripts with 14 probes per transcript. After hybridization, arrays were washed and stained using Affymetrix fluidic station. Arrays were then scanned using the Affymetrix GeneChip Scanner 3000. The robust multichip average (RMA) method (Bolstad et al. 2003) was used for background adjustment, quantile normalization, and probe-level summarization of the microarray samples. RMA expression summary was computed using Partek Genomics Suite v. 7.3.3 (Partek) and the affy package in Bioconductor (Gentleman et al. 2004). To exclude genes that were not accurately detected in the data analysis probe, sets with less than one present call within at least one of the samples were disregarded.
We used two normalized sets of expression data. The first set includes data of the three control samples and all inbred sublines, to study the effect of inbreeding. The second set includes the inbred sublines only, to study the effect of the line, depression importance, and their interaction. The number of probe sets with some evidence of expression was 9133 in the inbred + control sublines set and 9113 in the only inbred sublines set.
Probe sets associated with phenotypic variation
We used statistic analysis for microarrays (SAM; Tusher et al. 2001) to analyze the variation in expression. SAM is a permutation-based multitest statistical procedure that takes into account the correlations between probe sets in the calculation of false discovery rates (FDRs; Storey 2002). In the case of the comparisons between the expression levels in inbred lines and the outbred control (i.e., the analysis of inbreeding effects, Inb, calculated as inbreds’ minus controls’ average), based on Student’s t-test, we used the Bioconductor R package siggenes (Schwender et al. 2006). For the more complex comparisons between the inbred sublines we used a normalized data set including only these sublines’ data and applied a mixed model factorial analysis of variance (ANOVA) according to the model Y = µ + Lin + Dep + Lin × Dep + W, where µ is the overall mean, Lin the random main effect of line, Dep the fixed main effect of the magnitude of inbreeding depression (calculated as most depressed minus least depressed lines’ average), Lin × Dep is the random effect of the line by depression interaction, and W is the within-replicate variance. The FDR values for the ANOVA’s F’s were obtained following Storey and Tibshirani (2003), in a SAM procedure in which the permutation pattern was adapted to each studied factor following the guidelines for mixed models in Anderson and Ter Braak (2003): for the fixed Dep, the Lin residuals were permuted as Dep × Lin units (approximate test). For the random Lin, raw observations were randomized within levels of Dep (exact test). For the interactions, the residuals left after adjusting for the Dep and Lin effects were permuted (approximate test). The R code used for these analyses is available from the authors on request. In every probe set, the fixed Dep mean squares were tested against the Lin × Dep mean squares, and the random Lin, against the error mean squares.
Analysis of gene functions
We used the DAVID Bioinformatics Resources 6.7 (http://david.abcc.ncifcrf.gov/home.jsp; Dennis et al. 2003). These programs can use lists of probe sets as input and provide results in terms of the corresponding genes. Our basic tool was the functional annotation clustering, which clusters functionally similar terms in the user’s gene list into groups. In all cases, we chose the medium classification stringency and, as recommended by the authors, retained clusters having enrichment scores >1.3. The enrichment score is the geometric mean of all expression analysis systematic explorer (EASE; Hosack et al. 2003) scores (P values) of each annotation term in the group. Enrichment score 1.3 is equivalent to nonlogarithmic scale 0.05. The DAVID cluster annotation tool enables the user to specify a background list of genes against which to look for relative functional enrichment in a given gene list. We applied this procedure to compare the most significant genes in a gene-expression category against the whole of genes in that category. Because the functional annotation clustering tool has a limit of 3000 genes for the input lists, we used instead the less restrictive functional annotation chart tool (we kept functional terms with FDR < 0.1) for the analysis of the longer gene lists in the more general gene categories in our results. In addition to this large-scale exploratory analysis, we made direct significance tests for genes pinpointed as candidates by different kinds of a priori information.
Results
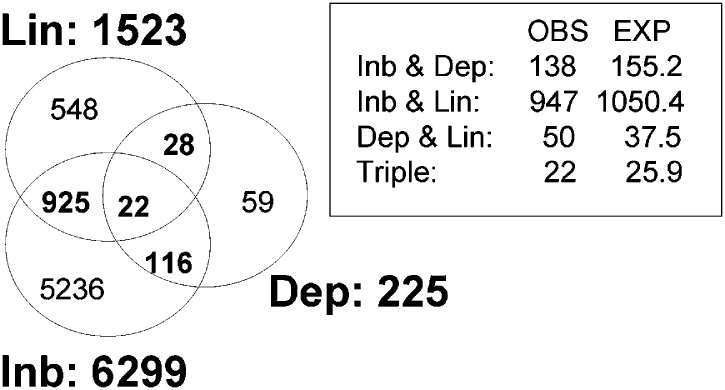
The number of significant probe sets resulting in FDR < 0.1 in the inbreds vs. control contrasts (Inb effects) was 6299 (4535 of them upregulated and 1764 downregulated in the inbred lines), 1523 in the variance between lines analyses (Lin effects), 225 in the most depressed vs. less depressed contrasts (Dep effects; 95 of them upregulated and 130 downregulated in the most depressed lines), and 2 in the Lin × Dep analysis. The lists of SAM selected probe sets for Inb, Lin, and Dep effects are in File S1.
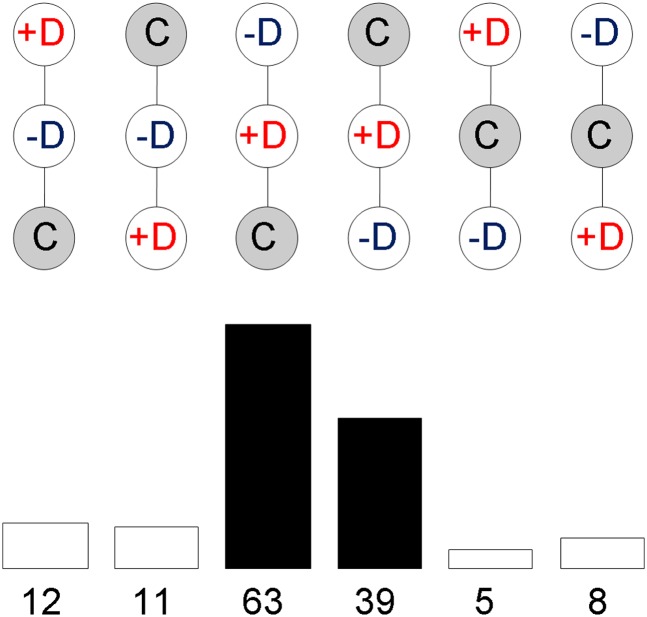
The lists of SAM-selected probe sets for Inb, Lin, and Dep effects did not show clear nonrandom overlaps. Interestingly, having marked Inb effects did not increase the probability of having Dep effects (Figure 5). However, the distribution of up- and downregulation for the two effects was not independent. In most probe sets showing Inb and Dep effects (138 probes; listed along with their functional annotations in File S2), the most depressed sublines were intermediate in expression between the controls and the least depressed sublines (Figure 6), corresponding to the protective configuration of Figure 1. The analysis of the corresponding Inb × Dep contingency table resulted in a = 37.008, P = 10−9. However, this test does not consider the lack of independence between the gene expressions in the lists. We complemented it with a randomization test (Westfall and Young 1993) that takes into account the correlations between observations. We randomized the positions of the inbred samples (10000 permutations) in these 12 + 11 + 63 + 39 + 5 + 8 = 138 probe sets. Only 1.94% of randomizations had as many or more than 63 + 39 = 102 probe sets with the most depressed samples in intermediate positions. Thus, the Dep effects were not in general mere extensions of the Inb effects, as would be the case if the most depressed sublines simply showed an intensification of the Inb effects.
Figure 5 .
Numbers of probes differentially expressed in the experiment. Overlap in the lists of probe sets affected by inbreeding (Inb), line (Lin) and depression (Dep) effects. The total numbers of probe sets are shown besides the circles (size not proportional to list length). The inlaid box compares the number of probe sets shared between lists (OBS) with that expected if the overall proportions of probe sets showing a given effect were conserved in the other lists (EXP). No significance tests were applied to these comparisons because the expressions in the probe sets were not independent. The numbers of expressed probe sets were 9133 (in the analysis of Inb effects) and 9113 (in that of Dep and Lin).
Figure 6 .
Number of probes SAM-selected for Inb and Dep effects for different patterns of expression. Relative rankings of sublines (C, outbred controls; +D, most depressed; –D, least depressed) expression levels in probe sets showing both inbreeding (Inb) and depression (Dep) effects (FDR < 0.1 in both cases) and their frequencies. The black bars correspond to probes with the most depressed samples in the intermediate positions, corresponding to the protective configuration given in Figure 1.
The power of the functional enrichment analysis is low when the gene lists used are short (Huang et al. 2009), as was the case with the categories shown in Figure 6. For the 39 probes downregulated in the inbreds and having a protective configuration we found two functional clusters with enrichment scores >1.3. The first included GO:0044242, cellular lipid catabolic process; GO:0016042, lipid catabolic process, and GO:0005811, lipid particle, and the second, SP PIR Keywords ank repeat; Interpro ankyrin, and Smart ANK.
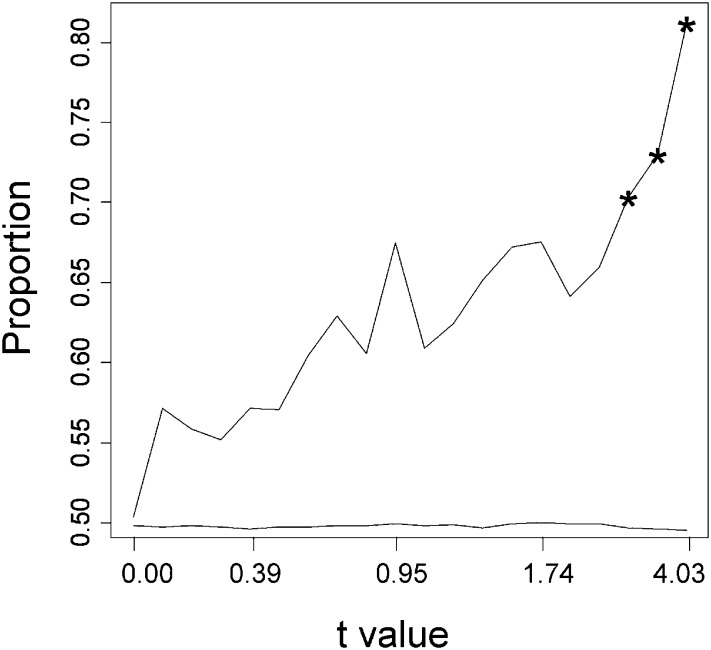
We next investigated whether the prevalence of the protective pattern found in the 138 probes significant for Inb and Dep effects (Figure 6) was also apparent for the whole set of analyzed probes. Thus, we carried out a randomization test for the proportion of probes showing a protective vs. nonprotective pattern (we excluded from this analysis the pattern called “other” in Figure 1, as these were not relevant for the intended test). Thus, we carried out 1000 permutations of the positions of the inbred samples for all probes. Figure 7 shows the observed proportion of protective patterns found (between 50% and >80%) in comparison with the expected random proportion (50% of each pattern). In this figure, probes are represented according to the absolute value of the Student’s t-value for the comparison between the most depressed and the least depressed contrasts (Dep effects). Thus, in the left-hand side of the x-axis are represented those probes with little difference in expression between the most depressed and the least depressed sublines, whereas in the right-hand side of the x-axis are represented those probes with the largest difference in expression between the most depressed and the least depressed sublines. The figure shows that the prevalence of the protective pattern is a general observation. However, only the three right-hand points had a proportion of protective patterns significantly larger than those obtained at random after randomization (P = 0.00, 0.01 and 0.02, respectively).
Figure 7 .
Proportion of probes showing a protective pattern of expression (see Figure 1) after ranking the 9133 expressed probe sets according to their Student’s t absolute value in the most depressed vs. least depressed test and dividing the rank in 20 tiers of size 456 (except the last one, of size 469). Thus, the right-hand part of the figure indicates probes where there is a large difference in expression between the most depressed and the least depressed sublines, and those on the left-hand side are probes where this difference is low. The lower line (without symbols) corresponds to the average proportion after 1000 randomizations of the expressions for the inbred sublines. Asterisks indicate a significant difference (P < 0.05) between the observed proportion and that obtained after randomization.
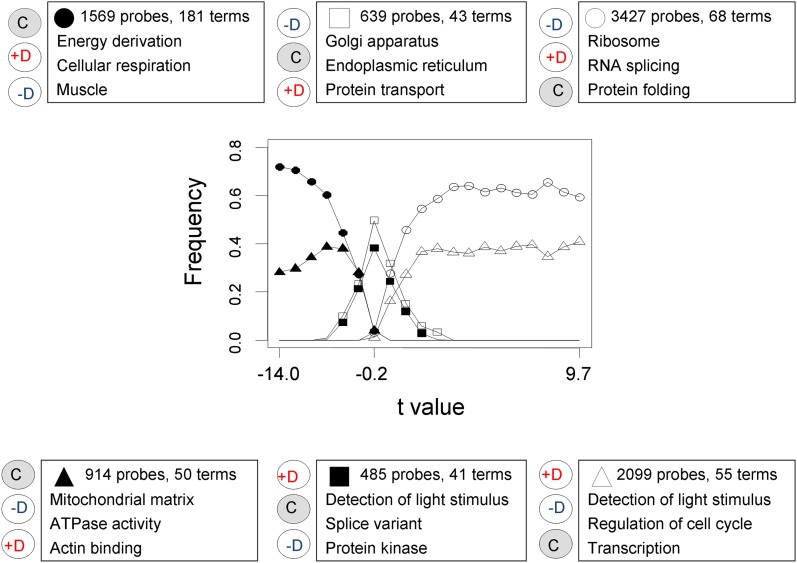
We further investigated if a higher proportion of probes with the protective configuration was apparent when all 9133 were ranked according to the Student’s t-value for the comparison between inbred and outbred contrasts. This is shown in Figure 8. It is clear that, for probes with large absolute values of t, there is again an overrepresentation of the protective configuration (circles) with respect to the nonprotective one (triangles). The same pattern was dominant when the four experimental lines were analyzed separately (Figure S2). However, no significant differences between the observed proportion of protective probes and a random proportion were found in the randomizations for this case (not shown), likely because these differences were less associated with the proportion of protective patterns (compare these proportions’ extreme values in Figure 7 and Figure 8).
Figure 8 .
Relative frequencies of probe sets in each gene-expression profile of Figure 5. We ranked the 9133 expressed probe sets according to their Student’s t-value in the inbreds vs. control test, and divided the rank in 20 tiers of size 456 (except the last one, of size 469). Thus, the right-hand part of the figure indicates probes upregulated with inbreeding, and those in the left-hand side are probes downregulated with inbreeding. We show the frequencies of the different expression profiles in each tier. The most relevant functional terms enriching the genes in each profile are shown, giving preference to those most informative, significant, and nonredundant.
The probes’ classification in Figure 8 enabled us to make a larger, more powerful functional analysis distinguishing genes up- and downregulated after inbreeding (see File S3). Genes downregulated in the inbred sublines (solid circles and triangles) were enriched in energy-related terms, those upregulated (opened circles and triangles) in transcription and translation-related terms, and those having intermediate expression in the control lines (squares), in terms related with the production of functional versions of proteins and RNA. It is apparent from the figure that the overrepresentation of probe sets consistent with protective effects (circles) tended to increase as the Student’s t-values testing the inbreeding effects became more extreme, especially in the case of the inbreeding downregulated probe sets. This supports the interpretation that protective profiles are associated with real changes in gene expression. Furthermore, some of the functional associations seen in Figure 8 are reinforced in the probe sets with extreme t-values, especially in those showing protective expression profiles (Table S1). When the analysis was restricted to the probe sets upregulated and with protective expression profiles (open circles), those showing the most extreme upregulation were relatively more enriched in RNA splicing functions. Moreover, when the analysis was restricted to the probe sets downregulated and with protective expression profiles (solid circles), those showing the most extreme downregulation were relatively more enriched in energy derivation related terms.
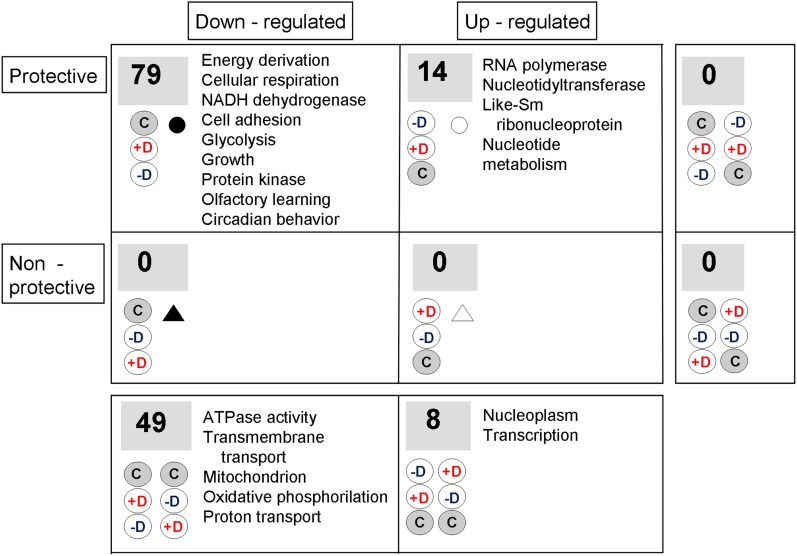
To conduct a more conservative and robust functional analysis, we looked for enriching terms that appeared for a particular profile (protective, nonprotective, upregulated, or downregulated) in all four inbred lines and did not appear in any line for the other profiles. These are the results given in Figure 9. The inbreeding downregulated genes with protective profiles had a clearly higher degree of similarity and specificity between lines, the number of functional terms coincident in the four lines was as large as 79. These results suggest a precisely regulated transcriptomic response to inbreeding. The other category of protective profiles, that involving upregulated probes, had 14 specific functional terms. No specific terms were found for the nonprotective categories, which suggest a more heterogeneous causation for these expression differences. There were no common functional terms for both protective and nonprotective extreme probe sets (right-hand marginal zero values), but the upregulated categories, and also the downregulated ones, shared some (lower marginal figures), very general functional terms. The inbreeding upregulated probes tended to be related with transcription, and the inbreeding downregulated ones, with energy. The terms in the corresponding protective categories involved similar concepts, but were more restricted, and in the case of the protective downregulation, much more diverse.
Figure 9 .
Number of functional terms enriching exclusively each expression profile. Analysis of the functional terms enriching the gene-expression profiles in the four most extreme tiers in each direction in Figure 8 (1824 probe sets in the case of the most negative t-values and 1837 in that of the most positive ones). As a conservative analysis, we show the number of terms enriching a particular profile in all four inbred lines and not found in any line for the other profiles. The marginal cells correspond to the terms exclusive to the whole set of protective probes, to the whole set of downregulated ones, etc. The most informative, significant, and nonredundant terms in each cell are shown. See File S4 for complete lists of terms. Triangles and circles help to relate these categories with their values in Figure 8.
Discussion
Inbreeding seems to produce large-scale transcriptomic changes in Drosophila melanogaster. About one-half of the expressed genes in our experiment had differences (FDR < 0.1) in expression between inbred lines and outbred controls. Upregulation arising from inbreeding was more frequent than downregulation, and a majority of changes corresponded to expression profiles consistent with protective responses, where the least depressed lines changed in expression more than the most depressed ones. The high frequency of protective-consistent patterns of expression seems to be a robust observation, made in the four independent lines analyzed. According to this interpretation, inbreeding would therefore constitute an organismal challenge requiring the activation or deactivation of a large number of genes to cope with it. For many genes, this change would not be fulfilled, leading to a higher inbreeding depression for individual fitness.
A possible problem with gene-expression measurements using microarray data are sequence mismatches due to sequence heterogeneity among target DNA at many base pairs. In the presence of such sequence mismatches, relative hybridization intensities could reflect both differences in transcript abundance and differences in hybridization kinetics. However, the impact of sequence heterogeneity on the main results of our article is expected, a priori, to be modest, because we aimed at highly inbred lines from a single population and a single species. Gene-expression measurements in different species (D. melanogaster and Drosophila simulans) using single-species Affymetrix arrays did not reveal a consistent variation in signal intensity due to sequence mismatches (Nuzhdin et al. 2004), despite that these two species split 2.3 ± 0.3 MYA ago (Li et al. 1999).
In any case, the hypothesis that the pervasive protective pattern observed from our data is a consequence of sequence mismatches is difficult to defend because it would imply two contrasting assumptions. We observed that, for a majority of genes, the expressions of the most depressed sublines were intermediate between the expressions of the control and the least depressed sublines, and this happened both for up- and downregulated genes under inbreeding. It could be hypothesized that the largest heterogeneity in probe mismatch sequence occurred in the most depressed sublines, leading to higher mismatch rates and weaker hybridization signals (i.e., closer to the control ones), as observed in the upregulated genes. But then, to explain the same protective pattern in the downregulated genes, it should be necessary to assume that the largest heterogeneity and weaker hybridization signals (i.e., farther away from the control ones) occurred this time in the least depressed sublines. Thus, it would be hard to explain why mismatches should be more frequent on the most depressed sublines than in the least depressed sublines in the case of upregulated genes and the opposite in the case of downregulated genes.
Nevertheless, a way to obtain indirect information on the effects of sequence variation and other confounding factors on the results of our experiment is to ascertain the agreement between probes of the same gene. We identified the 300 expressed genes displaying more than one probe set in the array. Probes attributed to the same gene would be subject to the same regulation and would be expected to have consistent expression levels. The average Pearson correlation of hybridization signals for the 248 genes with only two probe sets across the 27 microarrays assayed in this experiment was 0.58, a positive value consistent with regulation-caused variation in signal intensity, but not with independent changes in sequence in the two probe sets from the same gene. This large but nonunity correlation would reflect experimental noise, possible sequence mismatches, and alternative splicing. To do a test directly related with the protective variation patterns, we took each of the above 300 genes and assigned values of 1 or 0 to the two probes of each gene depending on whether they showed protective patterns or not, respectively. Thus, for each gene, the two probes could show protective effects (1, 1), one protective and one nonprotective (1, 0), or both nonprotective (0, 0). The F-value for the gene effect in an ANOVA was 2.168, with a probability as low as P = 1.15E-12. The calculated intraclass correlation coefficient between the probe values was t = 0.344, whereas a test randomizing the probe sets’ gene identities found a maximum value of t = 0.150 for the coefficient among 999 random replicates. These results therefore suggest a clear hybridization protective variation signal common to both probe sets from the same gene.
The high frequency of genes with protective-consistent expression changes, i.e., those changes most extreme in the least depressed sublines, was not related with an increase in variance in these sublines within probe set. The variance between lines within probe sets was on average (for the 9133 probe sets) very similar for the most (0.043) and the least (0.044) depressed groups of sublines. Moreover, the average variance between sublines within lines was 0.028 for the control sublines, 0.044 for the least depressed sublines, and 0.086 for the most depressed sublines. A larger variance among inbred lines expressions than among outbred control expressions was also found by Kristensen et al. (2005).
As explained in Materials and Methods, we had to pool the extracts from 30 adult males in every analyzed sample to obtain the mRNA quantities required to run each microarray in the experiment. Appropriate pooling has been shown to improve efficiency for microarray experiments (Peng et al. 2003). One study, however (Shih et al. 2004), found discordances between the average expression of individual samples and that of a corresponding pool sample, the magnitude of the discordance in each probe set increasing monotonically with its signal in the microarray. In any case, this effect was not associated with our results. When the list of expressed probe sets was divided in 20 intervals according to their mean microarray signal, the correlation between the intervals’ median of the averages and their proportion of protective expression patterns (the relationship studied in Shih et al. 2004) was 0.188 (P = 0.427).
Another concern that may be raised regarding our main observation of a pervasive protective-consistent pattern is that the normalization process performed on the data (RMA) could generate some sort of bias in the relative expression changes of the least and most depressed sublines, so that the main result of this study is an artifact of the normalization. To address this concern we analyzed directly the raw data without any adjustment or normalization step, simply averaging in each individual array the perfect match probes in each probe set. In this case, the number of probes with protective, nonprotective, and other patterns (following Figure 1) are 4830, 3045, and 1258, respectively. When only the first step in the RMA sequence of adjustment steps (the probe-position background correction) is made, the numbers are 4993, 2918, and 1222, respectively. These numbers can be compared with the corresponding ones using RMA normalized data, which are 4996, 3013, and 1124, respectively. It is clear that the preponderance of protective patterns over nonprotective ones is equally observed in the raw data. This is also evident by looking at Figure S3, which is equivalent to Figure 8, but considering raw expression data rather than normalized data. Therefore, normalization is not responsible for any artifact causing the observed general patterns.
Protective-consistent changes in expression were more specific in function than nonprotective ones. This would be expected if the first corresponded to functional, established pathways coping with alterations in body condition, whereas the second corresponded to contingent, heterogeneous disruptions in development and metabolism. The functions of protective-consistent genes were diverse but a clear specificity on RNA splicing functions for upregulated probes and on energy generation for downregulated probes was apparent.
The enrichment in energy derivation functions among the downregulated protective-consistent genes suggests physiological measures to save energy. There are to our knowledge no clear expectations about energy use changes in inbred D. melanogaster. It has been found (Ketola and Kotiaho 2009) that inbreeding in the insect Gryllus firmus had, relative to controls, increased CO2 production (used as a proxy of respiration and energy use) at rest and decreased under forced exercise (the signs of these differences implied a depression in the capacity to mobilize body resources), but the extrapolation to gene expression at the moment of sampling in our fly populations is not straightforward. In any case, the observation that the reductions in expression were largest in the least depressed sublines suggests that they had a fitness-enhancing role. An inspection of functional annotations for genes involved in these reductions could throw some light on their physiological implications. Among the genes showing the strongest evidence for protective reductions in expression (File S2), in addition to Dmel\CG6020 and Dmel\CG12400, directly involved in energy derivation and respiration, we found Toll and Relish, involved in the Toll pathway, the major regulator of innate immune response in Drosophila (De Gregorio et al. 2002). The repression of these genes and pathway leads to both the repression of immunity and the activation of insulin signaling, which increases nutrient reserves and growth (Di Angelo et al. 2009). In the same list of genes with strong protective reductions in expression is Ankyrin, which communicates with the Toll pathway (Lemaitre 2004).
These results suggest that inbreeding resulted in a change in the optimum resource allocation to different metabolic pathways. The most productive–least depressed individuals would have improved their energy balance and approached that new optimum not only reducing the expression of both energy derivation and growth regulating genes, but also trading immune competence for growth, which could increase fitness in the isolated laboratory environment. Another interesting gene among the downregulated protective ones is SNF1A/AMP-activated protein kinase, which works as an energy sensor, being activated at low levels of the ratio AMP/ATP. It represses the raptor branch of the mTOR pathway, a key positive regulator of cell growth and proliferation (Sarbassov et al. 2005). Thus, the stability of the laboratory environment might have favored genotypes less sensitive to energy availability. All these expression changes in the least depressed sublines suggest that reducing depression was not simply a question of maintaining as much as possible the expression patterns observed in the outbred individuals. Specific adjustments in gene expression were required to maintain individuals functional in an inbreeding situation.
Protective-consistent increases in expression were more numerous and heterogeneous in function than the corresponding decreases. They were enriched in functional terms related with the synthesis of proteins, but also with RNA polymerase, mRNA splicing, and the spliceosome. The latter relationship is specially interesting because it raises the possibility that changes in splicing may be a relevant mechanism of response to inbreeding. Recent studies have shown that alternative splicing determines the binding properties, intracellular localization, enzymatic activity, protein stability, and post-translational modifications of a large number of proteins. The magnitude of the effects ranges from a complete loss of function or acquisition of a new function to very subtle modulations (Stamm et al. 2005). Changes in splicing have already been found to contribute to the response to the stress generated by pathogens in Citrus trees (Del Carratore et al. 2011) and exposition to heavy metals in animals (Jeong et al. 2011).
We defined the protective pattern as that in which the expression of the least depressed sublines is more extreme than that of the most depressed sublines and the nonprotective one as that in which the opposite occurs. This is a conservative view though, because changes in expression with a protective role against inbreeding may occur also under the remaining scenarios, as is the case with genes showing more expression in the least depressed sublines than in the controls and in the controls more expression than in the most depressed sublines (open rectangles in Figure 8 and functional enrichment analysis in File S3). These genes tended to be associated with the endoplasmic reticulum and Golgi apparatus, and their functions of protein synthesis, maturation, transport, and localization, and with the synthesis of carbohydrates and their addition to proteins in Glycosylation reactions. The upregulation of these genes in the least depressed sublines and their downregulation in the most depressed ones suggests a special role of this process in the generation of inbreeding depression. Homozygosis could result in polypeptides with abnormal conformations that prevent normal folding or their associations with other subunits or cofactors (Sherman and Goldberg 2001; Pedersen et al. 2005; Kristensen et al. 2010). Disruptions in cellular homeostasis could be also involved. Successful protein folding requires a tightly controlled environment of substrates that include glucose, calcium, and redox buffers (Sherman and Goldberg 2001). Kristensen et al. (2005) had already found that inbreeding affected the expression of protein Hsp70, involved in protein quality control, and interestingly, the protein Hsp70-annotated gene Dmel\CG2918 was one of the eight genes included in our Figure 6 that were upregulated in the least depressed and downregulated in the most depressed sublines (listed in File S2). More evidence for a relationship between inbreeding and protein folding can be found in the lists of SAM-selected probe sets related with inbreeding in Kristensen et al. (2005). These show that six upregulated probes corresponded to genes in the protein-processing functional category GO:0006511, ubiquitin-dependent protein catabolic process. Ubiquitines are small proteins binding to unneeded proteins and labeling them for destruction in the proteasome (Hochstrasser 2009). Only one of the probe sets in our platform corresponding to these six was significant (P < 0.05; 1633951_at, for the gene Drosophila damage-specific DNA-binding protein-piccolo-; upregulated in inbreds, nonsignificant for Dep).
Two of the eight genes upregulated in the least depressed and downregulated in the most depressed sublines were related in particular with protein targeting and localization. This function is very stringently regulated. Reumers et al. (2005) found that <1% human SNPs changed the subcellular localization of proteins, whereas >50% affected protein folding and stability, indicating that the first changes were far less benign (see also Kohn et al. 2006). One of these two genes, Protein transport protein Sec61 gamma-2 subunit-Sec61γ-, was already annotated as having cytoprotective effects in D. melanogaster (Arsham and Neufeld 2009). A simple and tentative interpretation of the above observations would be that inbreeding reduces the efficiency in the production of functionally matured and located proteins and that the reduction was partially compensated in sublines that increased the expression of genes related with this production.
While both Kristensen et al.’s (2005, 2006) and the present study found inbreeding affecting the expression of genes involved in protein processing, the overlap in results was modest at most when considering specific genes (Table S2). Ayroles et al. (2009) listed the probe codes of three genes differentially expressed in their three highly depressed lines; Cecropin B, AFFYID: 1626530_at; Hsp70Bc, AFFYID: 1632841_x_at; and one gluthatione transferase, Dmel\CG6673, AFFYID: 1638074_at. The first was not expressed in our experiment, and the other two showed no significant Dep effects (the corresponding F-values in our analysis were 0.270 and 0.848, respectively). Such limited overlap between the lists of gene expressions affected by inbreeding in different populations of this species might be the most common situation, as was found also by Sarup et al. (2011) in their comparison of published studies. Instead, they found a high degree of overlap between studies made in samples from the same genetic background, even when they involved different sexes and traits. These effects of genetic background suggest that interpretations based on expression changes in individual genes or very specific functional categories might be inappropriately simplistic. Expression traits consistently show complex inheritance, explicable only by multiple underlying loci and interactions among them (Rockman and Kruglyak 2006; Nègre et al. 2011) and may be heavily dependent on sex and chromosome context even within the same population (Wayne et al. 2007). The total numbers of genes showing changes in expression were also lower in the above experiments. Differences could be related to the culture environment, which is known to affect gene expression (see, for example, Kristensen et al. 2006) and the numbers of arrays analyzed and of flies pooled per sample.
Comparisons of our results with those of Kristensen et al. (2005, 2006) and Ayroles et al. (2009) are restricted to contrast of inbreds vs. outbreds in one case and more and less depressed samples in the other. As explained in the introduction, the existence of protective responses could not be detected in these experiments because they did not consider separately the effects of inbreeding and depression. The microarray analysis by Sorensen’s et al. (2007) of D. melanogaster populations artificially selected to withstand different kinds of stress is a valuable source of information about gene-expression protective responses, as changes observed in these selected lines may be considered as adaptations protecting the organism against stressful situations. Similarity between experiment lists of Sorensen et al. and our study of Inb and Dep SAM selected genes would indicate that similar expression changes are involved in inbreeding and response to stress. However, the number of coincidences in the corresponding gene lists was very similar to those expected after random sampling of terms (Table S3).
Inbreeding is a whole-organism phenomenon, and it would not be surprising if it were related with genome-wide transcription alterations. Studies like this one, analyzing broad patterns of gene expression and gene function categories, give a picture of the overall consequences of inbreeding that cannot be achieved when searching individual genes contributing to inbreeding depression. Our results seem to provide a new view on the genetics of inbreeding and are compatible with the hypothesis that inbreeding induces large-scale changes in gene regulation that would alleviate the ensuing depression. The between individuals variation in depression would be due not only to differences in the severity of the particular genetic alterations originating that depression, but also to differences in the ability to carry out depression-alleviating adjustments. Nevertheless, further evidence will be needed to confirm the prevalence of a pattern of expression compatible with the protection against inbreeding depression effects. In addition, a more complete understanding of the consequences of inbreeding would require research on the two mechanisms involved: those causing the depression and those reducing its magnitude.
Supplementary Material
Acknowledgments
We thank Lucía Alvariño, Raquel Sampedro, Nieves Santamaría, and Pilar Alvariño for technical help. This work was funded by Xunta de Galicia (INCITE08PXIB200119PR Grupos de Referencia Competitiva, 2010/80), Ministerio de Ciencia y Tecnología (CGL2009-13278-C02), and Fondos Feder.
Footnotes
Communicating editor: J. A. Birchler
Literature Cited
- Anderson M. J., Ter Braak C. J. F., 2003. Permutation tests for multi-factorial analyses of variance. J. Statist. Comput. Simulation 73: 85–113 [Google Scholar]
- Arsham A. M., Neufeld T. P., 2009. A genetic screen in Drosophila reveals novel cytoprotective functions of the autophagy-lysosome pathway. PLoS ONE 4: e6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayroles J. F., Hughes K. A., Rowe K. C., Reedy M. M., Rodríguez-Zas S. L., et al. , 2009. A genomewide assessment of inbreeding depression: gene number, function, and mode of action. Conserv. Biol. 23: 920–930 [DOI] [PubMed] [Google Scholar]
- Bolstad B. M., Irizarry A., Astrand M., Speed T. P., 2003. A comparison of normalization methods for high density oligonucleotide array data based on bias and variance. Bioinformatics 19: 185–193 [DOI] [PubMed] [Google Scholar]
- Carroll S. B., 2005. Evolution at two levels: on genes and form. PLoS Biol. 3: e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri D., Townsend J. P., Hartl D. L., 2000. Manifold anomalies in gene expression in a vineyard isolate of Saccharomyces cerevisiae revealed by DNA microarray analysis. Proc. Natl. Acad. Sci. USA 97: 12369–12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B., Charlesworth D., 1999. The genetic basis of inbreeding depression. Genet. Res. 74: 329–340 [DOI] [PubMed] [Google Scholar]
- Charlesworth D., Willis J. H., 2009. The genetics of inbreeding depression. Nat. Rev. Genet. 10: 783–796 [DOI] [PubMed] [Google Scholar]
- De Gregorio E., Spellman P. T., Tzou P., Rubin G. M., Lemaitre B., 2002. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 21: 2568–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Carratore R. D., Magaldi E., Podda A., Beffy P., Migheli Q., et al. , 2011. A stress responsive alternative splicing mechanism in Citrus clementina leaves. J. Plant Physiol. 168: 952–959 [DOI] [PubMed] [Google Scholar]
- Dennis G., Jr., Sherman B. T., Hosack D. A., Yang J., Gao W., et al. , 2003. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 4: 3. [PubMed] [Google Scholar]
- Di Angelo J. R., Bland M. L., Bambina S., Cherry S., Birnbaum M. J., 2009. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc. Natl. Acad. Sci. USA 106: 20853–20858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandon S., 1999. Kin competition, the cost of inbreeding and the evolution of dispersal. J. Theor. Biol. 200: 345–364 [DOI] [PubMed] [Google Scholar]
- Gentleman R., Carey V. J., Bates D. M., Bolstad B., Dettling M., et al. , 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5: R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardot F., Monnier V., Tricore H., 2004. Genome wide analysis of common and specific stress responses in adult Drosophila melanogaster. BMC Genomics 5: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser M., 2009. Origin and function of ubiquitin-like proteins. Nature 458: 422–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra H. E., Coyne J. A., 2007. The locus of evolution: Evo Devo and the genetics of adaptation. Evolution 61: 995–1016 [DOI] [PubMed] [Google Scholar]
- Hosack G. D., Jr., Sherman B. T., Lane H. C., Lempicki R. A., 2003. Identifying biological themes within lists of genes with EASE. Genome Biol. 4: R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D. W., Sherman B. T., Lempicki R. A., 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4: 44–57 [DOI] [PubMed] [Google Scholar]
- Jeong S. W., Kim H. L., Seo Y. R., 2011. Microarray analysis of global gene expression in Caenorhabditis elegans exposed to potassium dichromate. BioChip J 5: 151–157 [Google Scholar]
- Jin W., Riley R. M., Wolfinger R. D., White K. P., Passador-Gurgel G., et al. , 2001. The contributions of sex, genotype and age to transcriptional variance in Drosophila melanogaster. Nat. Genet. 29: 389–395 [DOI] [PubMed] [Google Scholar]
- Karp C. L., Grupe A., Schadt E., Ewart S. L., Keane-Moore M., et al. , 2000. Identification of complement factor 5 as a susceptibility locus for experimental allergic asthma. Nat. Immunol. 1: 221–226 [DOI] [PubMed] [Google Scholar]
- Keller L. F., Waller D. M., 2002. Inbreeding effects in wild populations. Trends Ecol. Evol. 17: 230–241 [Google Scholar]
- Kelly J. K., 2005. Family level inbreeding depression and the evolution of plant mating systems. New Phytol. 165: 55–62 [DOI] [PubMed] [Google Scholar]
- Ketola T., Kotiaho J. S., 2009. Inbreeding, energy use and condition. J. Evol. Biol. 22: 770–781 [DOI] [PubMed] [Google Scholar]
- Koenig S., Simianer H., 2006. Approaches to the management of inbreeding and relationship in the German Holstein dairy cattle population. Livestock Sci. 103: 40–53 [Google Scholar]
- Kohn M. H., Murphy W. J., Ostrander E. A., Wayne R. K., 2006. Genomics and conservation genetics. Trends Ecol. Evol. 21: 629–637 [DOI] [PubMed] [Google Scholar]
- King M. C., Wilson A. C., 1975. Evolution at two levels in humans and chimpanzees. Science 188: 107–116 [DOI] [PubMed] [Google Scholar]
- Kristensen T. N., Sørensen P., Kruh M., Pedersen K. S., Loeschcke V., 2005. Genome-wide analysis on inbreeding effects on gene expression in Drosophila melanogaste. Genetics 171: 157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen T. N., Sorensen P., Pedersen K. S., Kruhoffer M., Loeschcke V., 2006. Inbreeding by environmental interactions affect gene expression in Drosophila melanogaster. Genetics 173: 1329–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen T. N., Pedersen K. S., Vermeulen C. J., Loeschcke V., 2010. Research on inbreeding in the ’omic’ era. Trends Ecol. Evol. 25: 44–52 [DOI] [PubMed] [Google Scholar]
- Lemaitre B., 2004. The road to Toll. Nat. Rev. Immunol. 4: 521–527 [DOI] [PubMed] [Google Scholar]
- Li Y. L., Sata Y., Takahata N., 1999. Paleo-demography of the Drosophila melanogaster subgroup: application of the maximum likelihood method. Genes Genet. Syst. 74: 117–127 [DOI] [PubMed] [Google Scholar]
- Lynch M., 1991. The genetic interpretation of inbreeding depression and outbreeding depression. Evolution 45: 622–629 [DOI] [PubMed] [Google Scholar]
- Motro U., 1991. Avoiding inbreeding and sibling competition: the evolution of sexual dimorphism for dispersal. Am. Nat. 137: 108–115 [Google Scholar]
- Nègre N., Brown C. D., Ma L., Bristow C. A., Miller S. W., et al. , 2011. A cis-regulatory map of the Drosophila genome. Nature 471: 527–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzhdin S. V., Wayne M. L., Harmon K. L., McIntyre L. M., 2004. Common pattern of evolution of gene expression level and protein sequence in Drosophila. Mol. Biol. Evol. 21: 1308–1317 [DOI] [PubMed] [Google Scholar]
- Paige K. N., 2010. The functional genomics of inbreeding depression: a new approach to an old problem. Bioscience 60: 267–277 [Google Scholar]
- Pedersen K. S., Kristensen T. N., Loeschcke V., 2005. Effects of inbreeding and rate of inbreeding in Drosophila melanogaster: Hsp70 expression and fitness. J. Evol. Biol. 18: 756–762 [DOI] [PubMed] [Google Scholar]
- Peng X., Wood C. L., Blalock E. M., Chen K. C., Landfield P. W., et al. , 2003. Statistical implications of pooling RNA samples for microarray experiments. BMC Bioinformatics 4: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primig M., Williams R. M., Winzeler E. A., Tevzadze G. G., Conway A. R., et al. , 2000. The core meiotic transcriptome in budding yeasts. Nat. Genet. 26: 415–423 [DOI] [PubMed] [Google Scholar]
- Ranz J. M., Machado C. A., 2006. Uncovering evolutionary patterns of gene expression using microarrays. Trends Ecol. Evol. 21: 29–37 [DOI] [PubMed] [Google Scholar]
- Reumers J., Schymkowitz J., Ferkinghoff-Borg J., Stricher F., Serrano L., et al. , 2005. SNPeffect v2.0: a new step in investigating the molecular phenotypic effects of human non-synonymous SNPs. Nucleic Acids Res. 33: D527–D532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman M. V., Kruglyak L., 2006. Genetics of global gene expression. Nat. Rev. Genet. 7: 862–872 [DOI] [PubMed] [Google Scholar]
- Sandberg R., Yasuda R., Pankratz D. G., Carter T. A., Del Río J. A., et al. , 2000. Regional and strain-specific gene expression mapping in the adult mouse brain. Proc. Natl. Acad. Sci. USA 97: 11038–11043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov D. D., Ali S. M., Sabatini D. M., 2005. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 17: 596–603 [DOI] [PubMed] [Google Scholar]
- Sarup P., Sørensen J. G., Kristensen T. N., Hoffmann A. A., Loeschcke V., et al. , 2011. Candidate genes detected in transcriptome studies are strongly dependent on genetic background. PLoS ONE 6: e15644 .10.1371/journal.pone.0015644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwender H., Krause A., Ickstadt K., 2006. Identifying interesting genes with siggenes. RNews 6: 45–50 [Google Scholar]
- Sherman M. Y., Goldberg A. L., 2001. Cellular defences against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron 29: 15–32 [DOI] [PubMed] [Google Scholar]
- Shih J. H., Michalowska A. M., Dobbin K., Ye Y., Qiu T. H., et al. , 2004. Effects of pooling mRNA in microarray class comparisons. Bioinformatics 20: 3318–3325 [DOI] [PubMed] [Google Scholar]
- Sorensen J. G., Nielsen M. M., Loeschcke V., 2007. Gene expression profile analysis of Drosophila melanogaster selected for resistance to environmental stressors. J. Evol. Biol. 20: 1624–1636 [DOI] [PubMed] [Google Scholar]
- Stamm S., Ben-Ari S., Rafalska I., Tang Y., Zhang Z., et al. , 2005. Function of alternative splicing. Gene 344C: 1–20 [DOI] [PubMed] [Google Scholar]
- Storey J. D., 2002. A direct approach to false discovery rates. J. R. Stat. Soc. B 64: 479–498 [Google Scholar]
- Storey J. D., Tibshirani R., 2003. SAM thresholding and false discovery rates for detecting differential gene expression in DNA microarrays, pp. 272–312 in The Analysis of Gene Expression Data: Methods and Software, edited by Parmigiani G., Garett E. S., Irizarry R. A., Zeger S. L. Springer-Verlag, New York [Google Scholar]
- Tusher V. G., Tibshirani R., Chu G., 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98: 5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne M. L., Telonis-Scott M., Bono L., Harshman L., Kopp A., et al. , 2007. Simpler mode of inheritance of transcriptional variation in male Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 104: 18577–18582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall P. H., Young S. S., 1993. Resampling-Based Multiple Testing: Examples and Methods for P-Value Adjustment. Wiley, New York [Google Scholar]
- Wilson A. C., Maxson L. R., Sarich V. M., 1974. Two types of molecular evolution: evidence from studies of interspecific hybridization. Proc. Natl. Acad. Sci. USA 71: 2843–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray G. A., Hahn M. W., Abouheif E., Balhoff J. P., Pizer M., et al. , 2003. The evolution of transcriptional regulation in eukaryotes. Mol. Biol. Evol. 20: 1377–1419 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.