Abstract
Aortic aneurysms (AA) are characterized by structural deterioration leading to progressive dilation. During the development of AA, two key structural changes are pronounced, one being degradation of extracellular matrix and the other loss of smooth muscle cells (SMCs) through apoptosis. Reactive oxygen species (ROS) are produced above physiological levels in dilated (aneurismal) part of the aorta compared to the nondilated part and they are known to be associated with both the extracellular matrix degradation and the loss of SMCs. In this study, we hypothesized that aneurismal SMCs are more prone to apoptosis and that at least some cells undergo apoptosis due to elevated ROS in the aortic wall. To test this hypothesis, we first isolated SMCs from thoracic aneurismal tissue and compared their apoptotic tendency with normal SMCs in response to H2O2, oxidized sterol, or UV treatment. Exposed cells exhibited morphological changes characteristic of apoptosis, such as cell shrinkage, membrane blebbing, chromatin condensation, and DNA fragmentation. Terminal deoxynucleotidyl transferased UTP nick end labeling (TUNEL) further confirmed the fragmentation of nuclear DNA in these cells. Vascular SMCs were analyzed for their micronuclei (MN) and binucleate (BN) frequency as indicators of genomic abnormality. These data were then compared to patient parameters, including age, gender, hypertension, or aortic diameter for existing correlations. While the tendency for apoptosis was not significantly different compared to normal cells, both the %MN and %BN were higher in aneurismal SMCs. The data suggest that there is increased DNA damage in TAA samples, which might play a pivotal role in disease development.
Data in this manuscript are consistent with oxidative stress-related damage of smooth muscle cells in blood vessels at the site of aneurysms.
Introduction
Aortic aneurysms (AA) are characterized as local inflammation with degeneration around the aorta leading to weakening and widening of the vessel. As predicted, the most dangerous complication associated with formation of an aneurysm is its rupture as a result of advanced weakening, a clinical condition presenting with high mortality rates, 31.7/100.000 individuals above the age 65 in USA (Booher and Eagle, 2011; CDC Database, 1999–2008).
AAs can occur at different locations of the aorta, thoracic, or abdominal, and due to their developmental variations and structural roles, the etiologies underlying enlargement appear to be diverse. While abdominal aortic aneurysms (AAA) are strongly associated with atherosclerosis and inflammation (Forsdahl et al., 2009), thoracic aortic aneurysms (TAA) can be a consequence of degenerative or hypertension associated aortic enlargement or based on genetic mutations in rare disorders, such as Ehler's Danlos or Loey's Dietz Syndrome (El-Hamamsy and Yacoub, 2009; Booher and Eagle, 2011). The pathology of TAAs can be characterized as (1) loss of smooth muscle cells (SMCs) through apoptosis (Boddy et al., 2008), (2) degradation of the extracellular matrix (ECM) proteins, or decrease in the formation of new ECM proteins (Barbour et al., 2007) and (3) accumulation of proteoglycans in areas where cells are depleted (Tang et al., 2005), a triad of events all noninflammatory in origin. During the pathogenesis of aneurysms, activated immune cells have been proposed to contribute to elimination of SMCs, which are a source of ECM proteins, in both AAAs (Henderson et al., 1999) and TAAs (He et al., 2006). Yet, in contrast to AAAs (Paravastu et al., 2009), inflammation is documented in only a few cases in TAAs (Biddinger et al., 1997; Girardi and Coselli, 1997; Roth et al., 2002).
The involvement of reactive oxygen species (ROS) is also proposed to be a part of AA development, as ROS are involved in both the degradation of ECM proteins (Rajagopalan et al., 1996) and act to trigger apoptosis of thoracic SMCs in vitro (Li et al., 1997b). ROS can be produced by various sources within the aortic wall, such as SMCs, fibroblasts, or endothelial cells, through several mechanisms (Griendling and FitzGerald, 2003). Interestingly, there is substantial evidence showing increased oxidative stress in aneurysms, which could potentially be responsible for apoptosis of SMCs during disease development. For instance, a striking increase in ROS production was detected in dilated aorta as compared to nondilated aorta of the same patient in both AAAs (Miller et al., 2002) and TAAs (Ejiri et al., 2003). Furthermore, consistently decreased superoxide dismutase activity and increased lipid peroxidation products were reported in thoracic aortic dissections as compared to normal aorta (Liao et al., 2008). Some more causal data for ROS in AA has come from the studies, where the gene expression profiles indicated induction of oxidative stress during experimental AAA formation in rats (Yajima et al., 2002) and differential expression of oxidative stress genes in dilated patient aortas in TAAs (Taketani et al., 2005). Recently, ROS has also been implied in changes in mechanical behavior as H2O2 was involved in remodeling across the vessel wall at the early stages of AAA in an apolipoprotein E deficient mice model (Maiellaro-Rafferty et al., 2011). Moreover, when pathways involved in ROS production were suppressed by knockout mice models, reduced TAA and AAA formation was observed, strongly suggesting that ROS is an important factor in the development of aneurysms (Thomas et al., 2006; Xiong et al., 2009).
Interestingly, the reduction of ROS production suppressed both ECM degradation and aneurysm formation, yet the level of apoptosis was not reported in the above studies (although predicatively a reduction would be expected). In brief, both the level of ROS and apoptosis are elevated in aneurismal tissues. However, do aneurismal SMCs undergo apoptosis in response to ROS and more specifically, are they more sensitive to oxidative stress?
To address this question, we hypothesized that SMCs isolated from TAA tissue will undergo apoptosis more efficiently or with lower doses of ROS under the same conditions. We also predicted that elevated levels of ROS within the tissue could have damaged the DNA of these SMCs and determined the genomic damage with their micronucleus (MN) frequency in the patient SMCs in comparison to their age-matched controls. Our analyses showed that while the degree of ROS sensitivity varied between cells from different patients, there did not appear to be a significant difference between the patient and the control group. Yet, patient cells exhibited increased genomic abnormalities and aneuploidy indicating genotoxic stress, which could have been due to elevated ROS exposure.
Materials and Methods
Aortic tissue and cell culture
The study was approved by the ethics committee of the Turkish Ministry of Health, Kartal Kosuyolu, Advanced Training and Research Hospital (ethics report number: 23, dated 21-03-2008 and protocol number: 184-04). Informed consents were signed by all participants.
SMCs used in this experiment were isolated from 37 individuals who underwent TAA surgery (27 males, 10 females) and 16 individuals without aneurysm (14 males, 2 females). Normal SMCs were obtained from patients without aneurysms undergoing by-pass surgery, from organ donor cadavers, or purchased commercially (Cell Applications, Inc.). A summary of the origins of individual samples are given in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/dna).
TAA specimens removed during surgery were transferred to the laboratory in a cold phosphate-buffered saline (PBS) solution with penicillin (10.000 U/mL) and streptomycin (10 mg/mL) and were processed within a few hours of operation. SMCs were isolated using the explant method (Leik et al., 2004). After the adventitia and endothelial layer of the vessel were removed, the tissue was cut into small pieces (∼2mm2), which were kept at %5 CO2, 37°C for 0.5-17 h on gelatin-coated plates for attachment before the addition of culture medium. The medium (DMEM/F12, Gibco Cat # 32500-035+10% fetal bovine serum, Biochrom AG Cat # S0115) was changed every 2–3 days, outgrowth of cells from the explants occurred in 7–10 days. Cells were passaged once ∼70%–80% confluency was reached. For all experiments, cells from passages 4–7 were used.
Immunohistochemistry staining
About 5000 cells (passage 4–7) were seeded on 18-mm coverslips and grown in culture for 48 h. The cells were then fixed in −20°C methanol (10 min) and washed with PBS. A ready-to-use detection system was used for immunohistochemistry (UltraVision Anti-Polyvalent, HRP, Thermo Scientific). Briefly, cells were incubated with H2O2 (room temperature (RT), 10–15 min), and blocking solution (Thermo Scientific ultra v Block, RT, 5 min). The polyclonal primary antibodies (α-actin, Abcam, ab5694, and Von Willebrand, Thermo Scientific, RB-281) were incubated for 30 min. The immunohistochemistry was completed with the DAB (diaminobenzidine) substrate and DAB chromogen mixture (RT, 5–10 min) incubation and finally Hematoxylin Mayer staining.
Induction of oxidative stress and measure of cell viability
SMCs (passage 4–7) were seeded at a ∼80%–90% confluency (7000–10000 cells/well) in 96-well plates. Cells were then treated with various concentrations of H2O2 in 70 μl/well volume. H2O2 was prepared from a 30% stock solution (Merck) and serially diluted in 1%FBS + DMEM/F12 culture medium. 7-ketocholesterol was diluted in ethanol and the treatment was performed as described in Lizard et al. (1998). The BioRad GeneLinker UV box (UVB lamp) was used as the UV source. After 4 h of incubation, 7 μL of WST-1 (Roche, Cat # 11644807001) was added to each well and incubated (2.5 h, 37°C). The plates were read at 455 nm with a 655 nm reference wavelength.
Annexin V staining
Cells were seeded in 6-well plates at ∼90%–95% confluency and treated with different H2O2 concentrations as described. Following treatment, cells were collected by tripsinization, washed in PBS, and stained with Annexin V in 100 μL of labeling mix (10 mM HEPES/NaOH, pH:7.4, 140 mM NaCl, 5 mM CaCl2) (10–15 min, RT). After the addition of 350 μL of labeling mix, 10000 cells were measured at 515 nm for Annexin V using a flow cytometer (BD, FACScan).
MN test, Hoechst staining, and scoring criteria
SMCs (passage 4–7) were seeded on 18-mm coverslips, grown to near confluency, fixed in −20°C methanol (10 min), and stained with 5–10 μL Hoechst 33342 dye (100 μg/mL). Micronuclei (MN) were scored using a 40×objective, A4 filter with a Leica DMI 6000 fluorescence microscope. Since the in vivo spontaneous MN frequency was under study, and no cytotoxic agent was used in MN experiments, cytokinesis block was not performed. A MN was scored if it appeared separately with no connecting DNA piece to the main nucleus, in size less than 1/3 of the main nucleus and existed within the borders of the same cell. For binucleates (BNs), cell borders were confirmed with the DIC filter. For each sample, at least 3000 cells were scored (>1000 cells×3 experiments).
Live cell microscopy
Cells were grown in 24- or 6-well glass bottom plates (Mattek Inc.) and treated with H2O2 as described and started imaging immediately using the Leica DMI 6000 microscope at 37°C, 5% CO2 with differential interference contrast microscopy and 20×objective. Images were taken over 24 h or 48 h (1 image/5 min for 24 h and 1 image/10 min for the 2nd 24-h period adding up to 48 h) and short movies were made at 25 images/second.
DNA condensation analysis and terminal deoxynucleotidyl transferase dUTP nick end labeling staining
Cells were seeded on 18-mm cover slips and treated with H2O2 as indicated in the figure. Cells were immediately fixed in 4% paraformaldehyde 9 h after treatment and stained with Hoechst 33342 dye for DNA staining and with in situ cell death detection kit for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. The protocol suggested by the manufacturer (Roche Applied Science, TUNEL-POD) was followed without modification.
Results
SMCs were obtained and cultured using the explant technique from the aneurismal tissue of patients who had undergone surgery for TAA and did not possess atheroschlerosis (Supplementary Fig. S1A). The cells were tested for smooth muscle α-actin positivity and Von Willebrand factor negativity (Supplementary Fig. S1B) to confirm smooth muscle origin. We assumed that cultured SMCs retain their in vivo properties in terms of sensitivity to ROS and genomic anomalies that they possess following their isolation by explant culture.
In an attempt to determine the apoptotic tendency of vascular SMCs induced by ROS, cells were treated with various doses of H2O2 (0 μM, 220 μM, 440 μM, 880 μM, and 1780 μM H2O2) and cell viability was measured at different time points using a colorimetric assay, based on cleavage of the tetrazolium salt, WST-1, to formazan, which is a measurable dye. Based on this assay, it was observed that cell death occurred as early as 4 h (Supplementary Fig. S2A) and the following experiments were continued with that time point as later time points, such as 24 h, could allow cells to recover from H2O2-induced cell death or adapt to oxidative stress by proliferation (Li et al., 1997a), resulting in data variations due to further cell division.
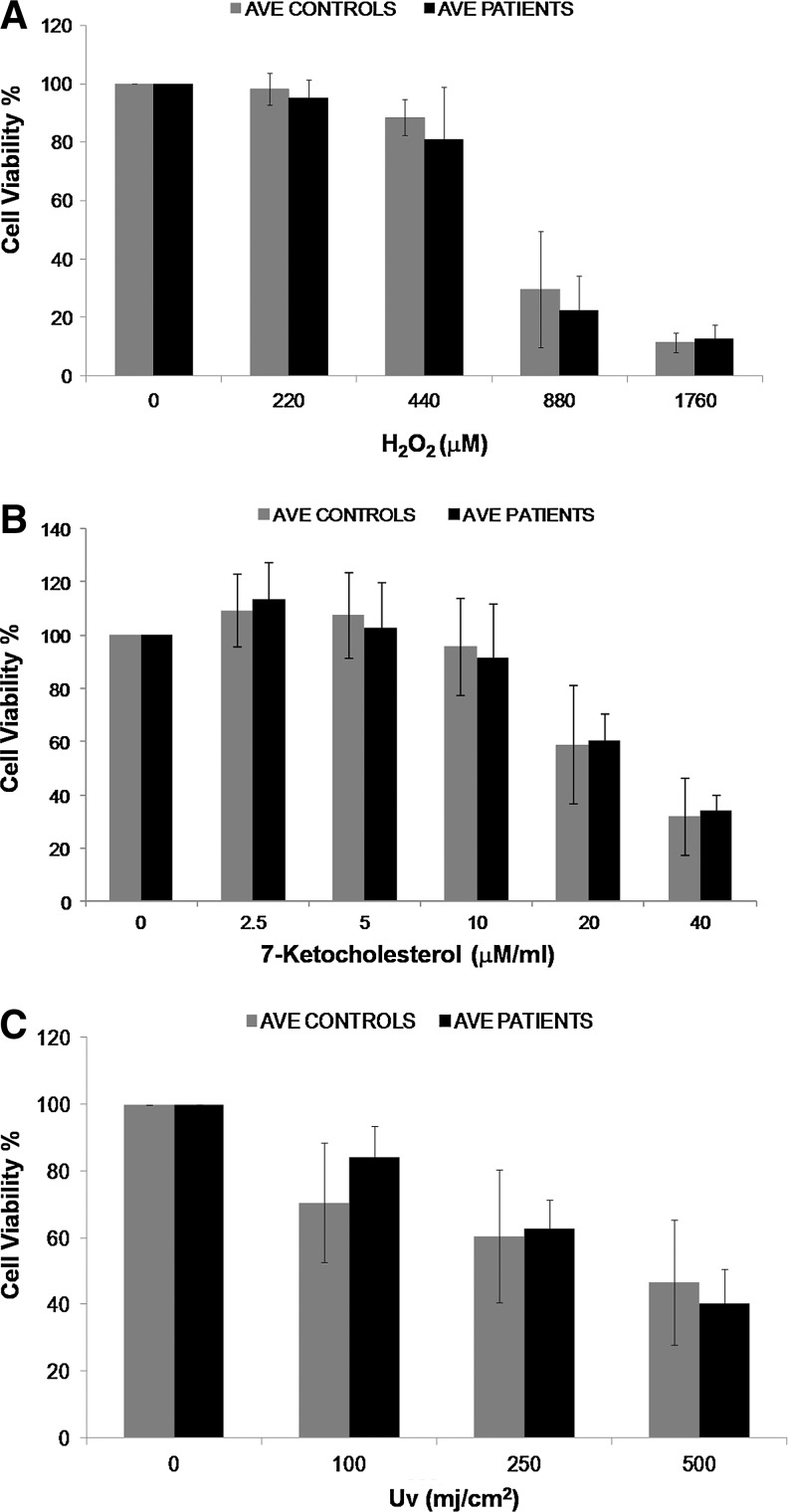
Using the qualifying SMCs, we treated 13 patients and 7 control SMCs with H2O2 and measured cell viability. There was no significant difference in the mean age of patient and control groups (Student's t-Test for independent samples for age, p=0.29). Interestingly, while different patients exhibited different levels of sensitivity to ROS, no significant difference was found between control versus patient mean values (Analysis based on Area Under Curve, Mann–Whitney U test, p=0.115) (Fig. 1A and Supplementary Fig. S3A). This result was surprising, as SMCs isolated from coronary atherosclerotic plaques were reported to undergo markedly higher frequencies of apoptosis both spontaneously and under low serum conditions compared to normal SMCs (Bennett et al., 1995). Hence, we investigated cell death through different means of cell injury, namely, 7-keto-cholesterol (7-KC) and UV treatments, with a subgroup of cells (6 patient and 8 control SMCs for 7-KC, 6 patient and 5 control SMCs for UV). 7-KC is an oxidized low-density lipoprotein and has been shown to lead both to induction of ROS in cells (Jang and Lee, 2011) and to apoptosis in SMCs, probably due to increased oxidative stress (Nishio et al., 1996; Lee and Chau, 2001). UV is known to adversely affect cells and induce apoptosis by various mechanisms one of which is ROS production (Valencia and Kochevar, 2006). Different concentrations/doses and incubation times were studied for both of these agents and 2.5–40 μM of 7-KC for 24 h and 100-500 mJ/cm2 exposure to UV and 4-h incubation was determined to be a good range for detecting differences (Supplementary Fig. S2B, C). In accordance with the results of H2O2-induced cell death, no significant change in cell death induced by either of these agents between control and aneurismal tissue- derived SMCs (Analysis based on Area Under Curve, Mann–Whitney U test, p=0.181 for UV and p=for 7-KC) (Fig. 1B, C and Supplementary Fig. S3B, C). Therefore, while increase in ROS can cause loss of SMCs, the disease state (aneurismal or normal tissue origin) does not explain the different levels of cell death in response to oxidative stress.
FIG. 1.
Comparison of cell viability profiles of smooth muscle cells (SMCs) extracted from different individuals in response to H2O2 (A), 7-Ketocholesterol (7-KC) (B), and uv (C) treatment. The graphs show the averages for 13 patients and 7 control SMCs for H2O2, 6 patients and 8 control SMCs for 7-KC, and 6 patients and 5 control SMCs for UV. Each sample was studied in triplicate, with at least three biological repeats. Cell viability curves for individual samples can be seen in Supplementary Figure S3. No significant difference in cell viability in response to oxidative stress was found between patient and control SMCs for all the agents tested.
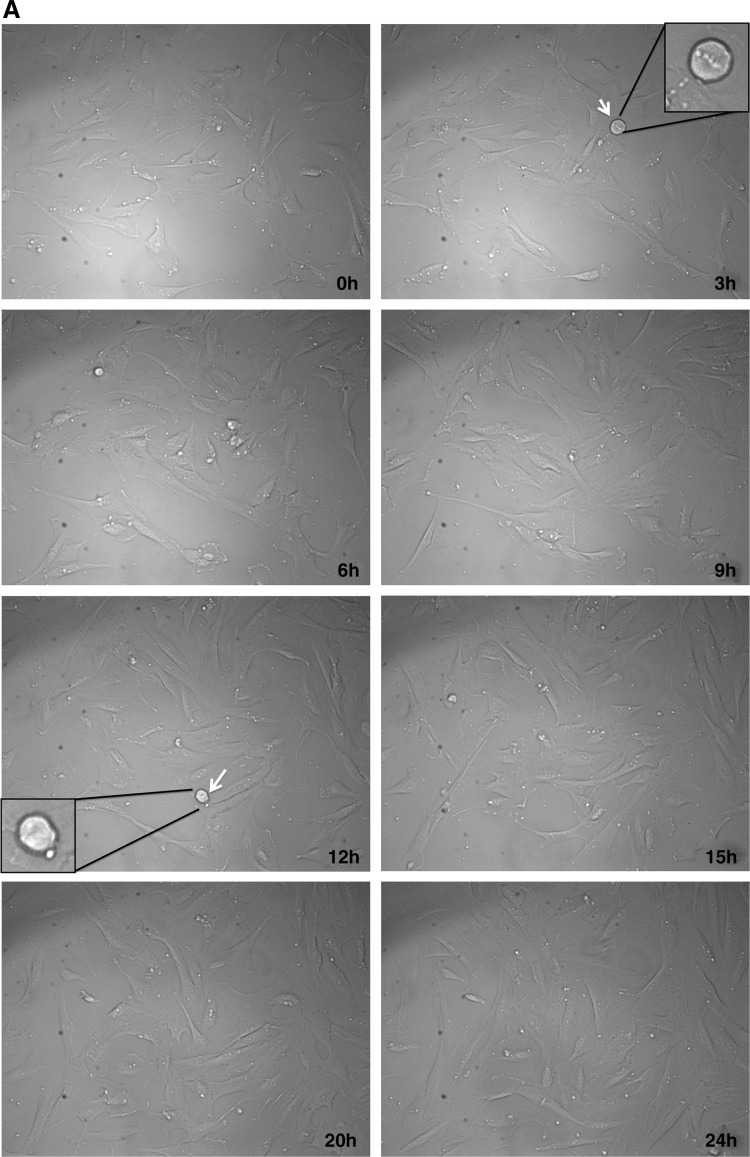
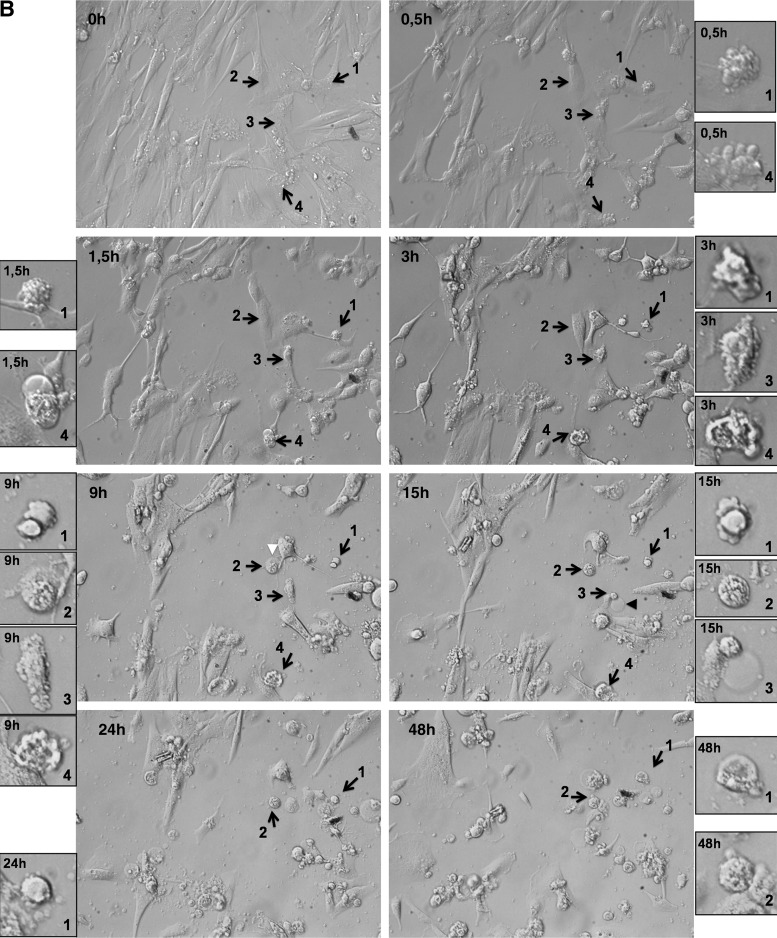
To determine whether cell death after H2O2 treatment is through apoptotic pathways, SMCs were initially analyzed with a live-cell imaging microscope and morphological changes were observed. As seen in Figure 2, while untreated cells migrated randomly and occasionally divided (Fig. 2A and Supplementary Movie S1A, Supplementary Movie S1A is available online at www.liebertpub.com/dna), majority of H2O2-treated cells shrunk, got smaller in size, and rounded up, which are characteristics of apoptosis (Fig. 2B and Supplementary Movie S1B, Supplementary Movie S1B is available online at www.liebertpub.com/dna). Blebbing was also observed and some cells finally underwent secondary necrosis after apoptosis, forming membrane blisters (Fig. 2B, arrowheads). We also observed that a few cells underwent necrosis (Fig. 2B, blue arrows). Hence, measured morphologically, H2O2 treatment induced both necrosis and apoptosis in SMCs derived from aortic tissue.
FIG. 2.
Morphological changes as a result of H2O2 treatment in normal SMCs followed by a live‐cell imaging microscope. (A) Untreated cells appeared healthy, evenly spread, and well attached over a period of 24 h. Arrows indicate dividing cells at different time points and the cell number increases by 24 h. Selected cells are enlarged in figure insets. (B) Treatment of H2O2 severely affected cell growth and morphology. Arrows numbered 1, 3, and 4 indicate cells that undergo apoptosis and show typical characteristics, such as detachment of the membrane, cell shrinkage, and blebbing. The cell indicated with the 3rd arrow underwent secondary necrosis after apoptosis and formed a blister (black arrowhead). The cell indicated with the 2nd arrow initially shrunk and probably started apoptosis, but underwent secondary necrosis and formed a blister (white arowhead). Although there are only few examples indicated with the arrows, the images show more cells undergoing apoptosis within the same field. Apoptosis started to occur as early as 0.5h after treatment and took place at different times. There were no cell divisions over a period of 48h. Enlarged views of selected cells can be seen for each time point as indicated. (20× magnification, DIC microscopy, movie at 25 frames/second). Similar results were obtained for both control and aneurismal SMCs.
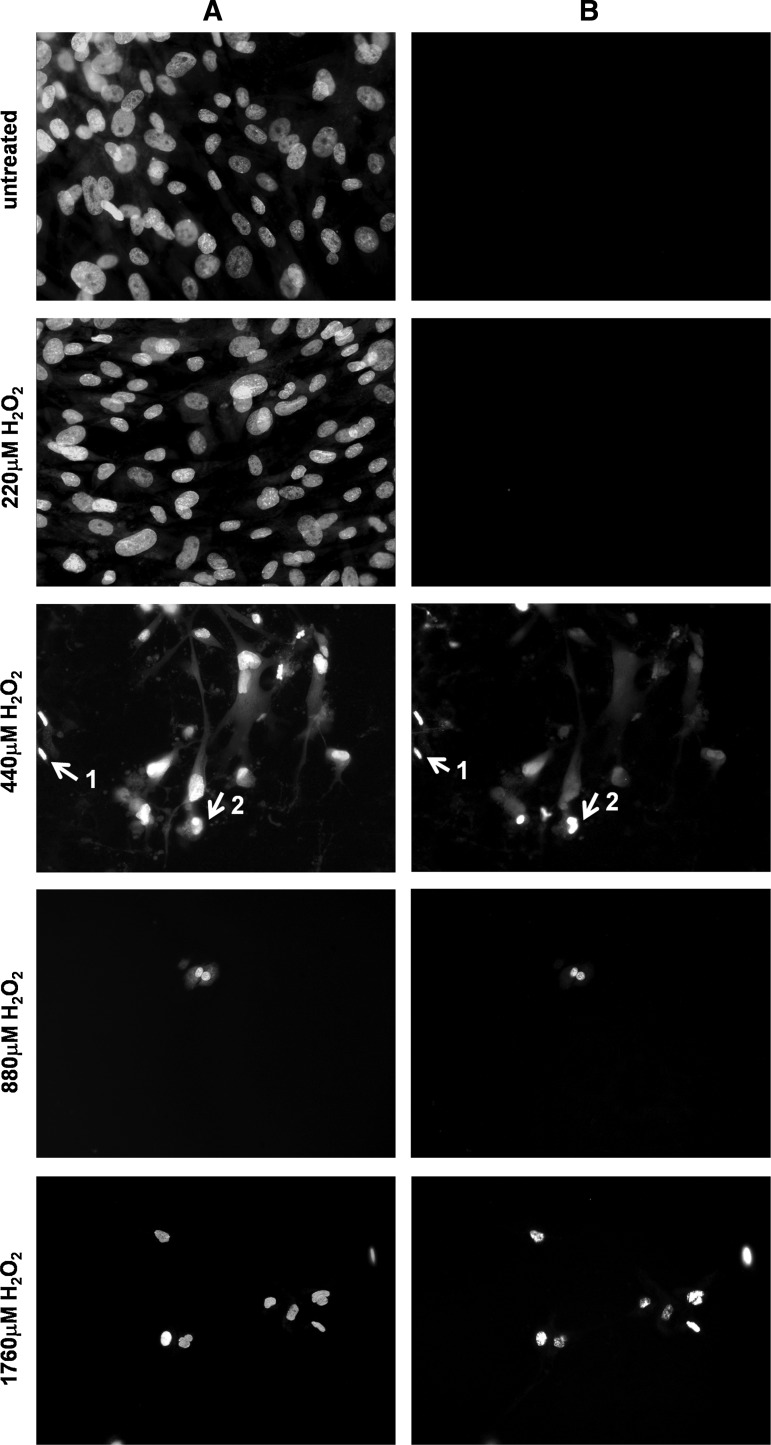
To further evaluate apoptosis in SMCs, DNA condensation was measured and cells were stained with Hoechst 33342 dye (Fig. 3A). H2O2 treatment resulted in shrinkage of nuclei, DNA condensation, and fragmentation as expected from cells that undergo apoptosis. The fragmentation of genomic DNA in these cells was further confirmed by in situ 3′ end labeling of DNA fragments with terminal transferase (Fig. 3B). Finally, Annexin V levels were found to be increased after H2O2 as determined by flow cytometry (Fig. 4). Similar results were obtained for both the patient and control cells and apoptotic features were observed. Thus, we concluded that SMCs die predominantly as a result of apoptosis in response to 440–1760 μM H2O2 treatment.
FIG. 3.
Representative images of double-stained terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) positive cells. Cells were treated with increasing doses of H2O2, fixed 9 h after treatment, and stained for (A) DNA (Hoechst dye) and (B) end labeled by TUNEL assay. It was possible to observe both condensed nuclei (arrow 1), and fragmented nuclei (arrow 2) in response to both treatments. The highest dose of exposure (1760 μM H2O2) resulted in shrinkage of nuclei and some condensation, but no fragmentation. Majority of cells were lost during staining procedure and floated away, hence, were not scored for TUNEL positivity.
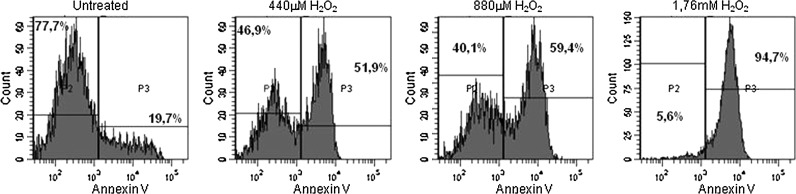
FIG. 4.
H2O2-treated cells exhibit increased Annexin V staining: Cells were treated with H2O2 at indicated doses, stained with Annexin V, and 10000 cells were scored per tube. Percentages in the graph indicate the frequency of Annexin V-positive or -negative cells with the drawn threshold. SMCs from P22 are shown in graph and similar results were obtained for both control and aneurismal SMCs.
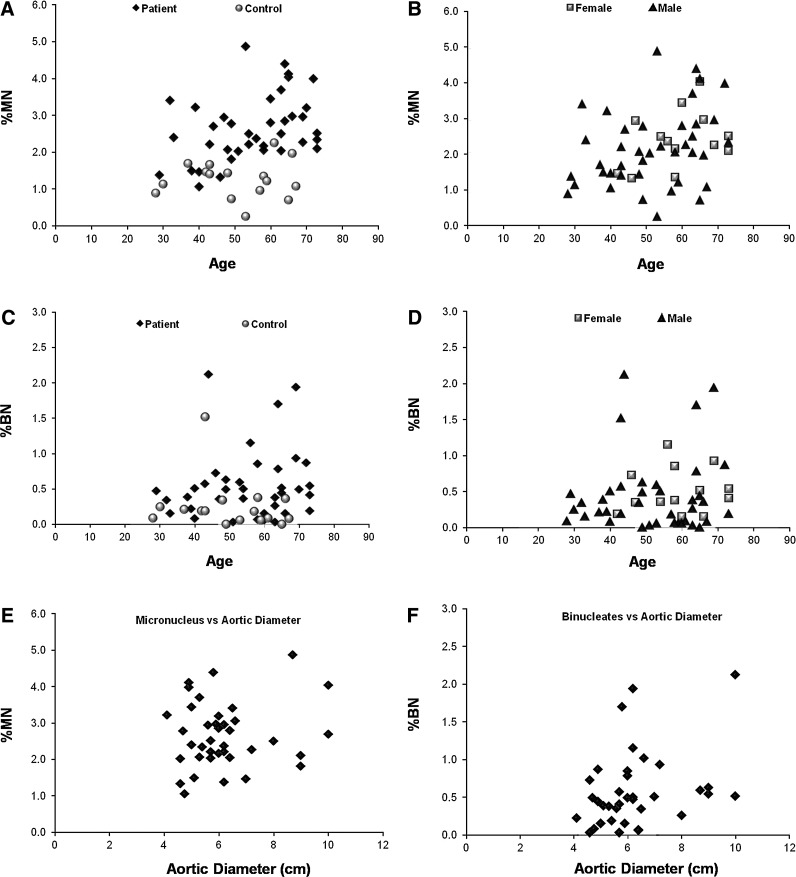
Because the aneurismal SMCs were reported to be exposed to more oxidative stress than normal cells, we wanted to test if this stress resulted in elevated genomic damage, which could explain the differences in cell death observed between different patient cells. The damage was determined via the MN assay, which is considered as a standard measure of the amount of genomic insult the cell had been exposed to (Dearfield et al., 1991). MN can be described as a whole or piece of a chromosome, which is enveloped by a separate nuclear membrane (Supplementary Fig. S4A). This piece of DNA material is mostly transcriptionally inactive and carries the risk of being lost in the next cell cycle (Hoffelder et al., 2004, Leach and Jackson-Cook, 2004). While MN can be seen in normal cells (Heddle, 1990), the frequency that they appear is strongly correlated with the amount of genotoxic insult (Heddle et al., 1991). ROS is known to induce both double- or single-stranded DNA breaks (Dahm-Daphi et al., 2000) and is also shown to result in elevated MN levels in cells in vitro (Hoffelder et al., 2004). We hypothesized that aneurismal SMCs would show elevated frequencies of MN compared to their age-matched controls and that the presence of MN could partly account for ROS sensitivity. Representative images of cells with MN are shown in Supplementary Figure S4A. MN were not distinguishable on tissue sections, as nuclei appeared of various sizes and shapes, and it was not possible to differentiate between a MN and a normal nucleus of small size or shaped differently (Supplementary Fig. S5). Studying with stacked images or increasing the width of the tissue section did not improve to properly distinguish MN in vivo. While the cells were scored for %MN, they were also analyzed for spontaneous apoptotic frequency (Supplementary Fig. S4B) and formation of BNs, another genomic anomaly, which is the state of having two nuclei in a single cell, mostly occurring as result of failure of cytokinesis (Supplementary Fig. S4C). Interestingly, the level of spontaneous apoptosis cultured SMCs was very low, either 0% or close to 0% in many cells, hence, was not analyzed for biological difference (Supplementary Fig. S4D). As expected, we observed varying degrees of MN in SMCs isolated from different individuals (Supplementary Fig. S4D and Fig. 5A) and in accordance with the literature, the frequency correlated with the age of the individuals (Pearson's correlation, p=0.017, r=0.386) (Fenech, 1998; Bolognesi et al., 1999). [Elder individuals tend to accumulate more damage and show higher levels of MN (Bolognesi et al., 1999).] We failed to confirm this correlation for control individuals, which could be due to smaller sample size (Pearson's correlation, p=0.823, r=0.061). In contrast to age, the aortic diameter did not seem to be correlated to the MN frequency (Spearman's correlation, p=0.86, r=0.03) (Fig. 5E).
FIG. 5.
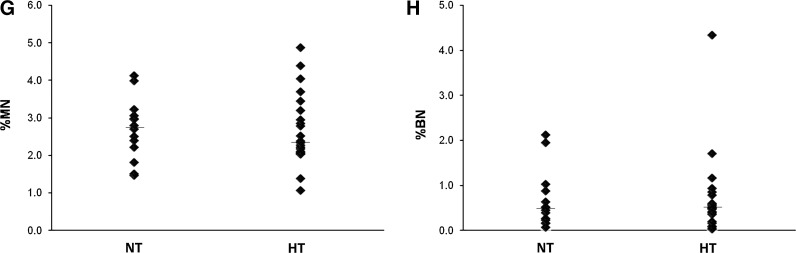
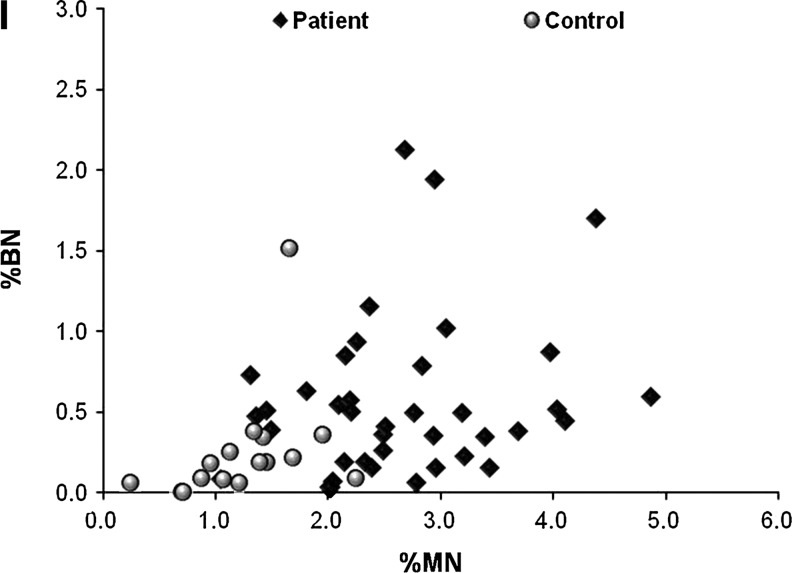
The comparison of criteria under study to patient clinical parameters. %MN (A) and %BN (C) are significantly higher in patient cells compared to their age matched controls, and the difference is not gender specific (B and D). The aortic diameter and blood pressure of control individuals were not measured exactly (yet, were reported to be smaller than 3 cm), hence, the data for controls are missing from (E) to (H). The correlation of the aortic diameter at the time of operation in TAA patients and %MN (E) and %BN (F) in SMCs isolated from these patients are shown in graphs. The bars drawn in (G) and (H) indicate the median value for the distribution of %MN and %BN in normotensive and hypertensive patients. The correlation of %MN and %BN is drawn in (I). (Black diamonds: Patients, Grey circles: Control individuals) MN, micronucleus; BN, binucleate; HT, hypertensive; NT, normotensive.
Interestingly, the mean for the frequency of MN was found to be significantly higher in patients than controls after age correction (ANCOVA, p<0.001) (Fig. 5A), indicating that SMCs of these individuals were exposed to more genotoxic stress. Consistently, previous observations had reported elevated ROS in aneurismal tissue (Miller et al., 2002; Liao et al., 2008). These observations do not appear to be gender- specific (Student's t-test for independent samples, for MN% p=0.46, for BN% p=0.92) (Fig. 5B, D). Furthermore, while there was no correlation between the frequency of BN and age (Spearman's correlation, p=0.53, r=0.087) (Fig. 5C), the occurrence of BN was significantly higher in aneurismal SMCs (Mann–Whitney U test, p=0.002), indicating elevated cytokinesis defects in these cells (Fig. 5C). Moreover, despite the correlation with age, neither of these variables was correlated with elevated blood pressure (hypertension) (Mann–Whitney U test, for MN%, p=0.79 and for BN%, p=0.85) (Fig. 5G, H). Interestingly, while the aortic diameter did not seem to have an impact on MN% (Spearman's correlation, r=0.03, p=0.86), it showed a weak, but close to significant correlation with the frequency of BNs (Spearman's correlation, r=0.311, p=0.06) (Fig. 5E, F). Finally, we also found a positive correlation between the occurrence of MN and BN (Spearman's correlation, r=0.42, p=0.002) (Fig. 5I). Table 1 summarizes the demographic data and mean values for these variables for patient and control samples involved in this analysis.
Table 1.
The Characteristics of Patient (n=37) and Control (n=16) Individuals
| Age | Aortic diameter (cm) | Micronucleus (%) | Binucleates (%) | Hypertension (% positive) | |
|---|---|---|---|---|---|
| Control (M, 87.5%) | 50.4±13.5 | <3 | 1.24±0.54 | 0.24±0.38 | N.A. |
| Control (F, 12.5%) | 50.0±11.3 | <3 | 1.41±0.08 | 0.29±0.13 | N.A. |
| TOTAL (M+F, 100%) | 50.4±12.4a | <3 | 1.26±0.51b | 0.25±0.36c | N.A. |
| Patient (M, 73%) | 52.8±13.1 | 6.07±1.42 | 2.66±0.96 | 0.69±0.91 | 52% |
| Patient (F, 27%) | 61.3±9.7 | 6.52±1.73 | 2.65±0.77 | 0.58±0.33 | 80% |
| TOTAL (M+F, 100%) | 55.1±12.8a | 6.19±1.50 | 2.65±0.90b | 0.66±0.79c | 59% |
p=0,736 (Student's t-test).
p<0.001 (ANCOVA).
p=0.002 (Mann–Whitney U test).
M, male; F, female; N.A., not available;±, plus or minus.
So, based on the fact that different individuals exhibit different levels of MN, BN, and different sensitivities to H2O2, 7-KC, or UV-induced cell death, we explored if MN or BN frequency assist to predict the cell viability after stress. Interestingly, cell death was not significantly correlated with either of these factors in response to H2O2 (Spearman's correlation, r=−0,188, p=0.43 for MN and r=0.32, p=0.17 for BN) or in response to UV (Spearman's correlation, r=−0.064, p=0,85 for MN and r=0.296, p=0.38 for BN) or in response to 7KC (Spearman's correlation, r=−0.099, p=0.75 for MN and r=−0.160, p=0.60 for BN). Hence, the degree of existing genomic abnormalities does not seem to have an additional impact on the cell's ability to withstand external stress at least for the factors analyzed in this study.
Discussion and Conclusions
In this study, we analyzed the effect of oxidative stress on aneurismal SMCs both with regard to stress-induced cell death and to the genomic damage the cells possess in comparison to patient parameters, such as age, gender, hypertension, or aortic diameter. Our data showed that while there is not a significant difference in response to ROS treatment between patient and control cells, the aneurismal SMCs exhibit elevated genomic abnormalities as determined by formation of MN and presence of BNs. To our knowledge, this is the first report demonstrating the increased genomic damage of thoracic aneurismal SMCs, and is important to show as genomic destabilization might have a pivotal role in the disease development.
The role of oxidative stress on the pathogenesis of aneurysms has long been established (McCormick et al., 2007). Furthermore, the local elevation in the level of ROS at the dilated aorta has been proposed as a potential factor for the loss of SMCs through apoptosis (McCormick et al., 2007). This hypothesis was tested in vitro using vascular SMCs isolated from atherosclerotic or normal tissue, and SMCs were shown to undergo apoptosis in response to oxidative stress or serum starvation indicating that these cells have active molecular machinery for apoptosis (Bennett et al., 1995; Li et al., 1997b; Li and Li, 1999; Brunt et al., 2006). In accordance, we showed that the same is true for SMCs isolated from TAA tissue in response to pro-oxidant species, such as H2O2 and UV. H2O2, can lead to different end points in cell culture, such as cell proliferation, activation, and apoptosis, in a dose-dependent manner (Irani, 2000). Lower levels of H2O2 have been previously proposed to be important for normal physiological functioning and signaling, while higher doses are associated with disease states (Schroder and Eaton, 2008). Analyses of kinetic constants and presence of peroxidases revealed that H2O2 concentrations up to 1 mM can be met in tissue (Schroder and Eaton, 2008). Furthermore intracellular H2O2 levels can only reach 1%–15% of the applied exogenous dose (Schroder and Eaton, 2008). Therefore, the doses applied in this study (maximum 1760 μM) can be considered to correspond to plausible intracellular concentrations in the diseased state. In contrast to the previously mentioned literature (Irani, 2000), a proliferation effect was not observed after low dose of H2O2 exposure, which could be due to measurement of cell viability shortly after (4 h) treatment. In our study, we observed that both normal and aneurismal SMCs can undergo apoptosis in culture after ROS treatment, suggesting that at least some of the apoptosis observed in the vessel could be due to the elevated ROS levels.
Interestingly, it appears that although there is variation in cell death in response to oxidative stress between cells isolated from different individuals, the aneurismal SMCs do not seem to have an increased tendency for apoptosis. In the literature, controversial results have been reported about the proliferation of SMCs derived from different sources. For instance, while cultured plaque-derived SMCs were vulnerable to apoptosis both spontaneously and under low serum conditions (Bennett et al., 1995), no difference in mean cell number was found between AAA-derived and control SMCs indicating similar proliferation rates in culture (Crowther et al., 2000). There are multiple explanations for this discrepancy. First, plaque-derived SMCs may exhibit different characteristics than aneurismal SMCs about this feature. Second, different protocols were used for SMC isolation, and apoptosis-resistant cells could have been selected during SMC extraction from tissue or in cell culture. Third, a real difference might have been missed due to a relatively small sample size. Indeed, the results need to be confirmed with in vivo studies for further verification. Interestingly, a study using an in vivo system where it is possible to induce apoptosis of vascular SMCs alone exogenously showed that while apoptosis is induced in vascular SMCs of both control and ApoE-/- deficient mice, SMCs are cleared efficiently in the control mice and do not cause inflammation, remodeling, calcification, or aneurysm formation (Clarke and Bennett, 2006, Clarke et al., 2006). On the other hand, apoptosis alone is sufficient to induce plaque vulnerability in the ApoE−/− deficient mice. Similarly, it could be speculated that SMCs in aneurismal tissue are vulnerable to ROS-induced apoptosis to a similar extent in vivo, but are not cleared well enough, resulting in immune triggers, vessel remodeling, and aneurysm formation. Likewise, based on our cell culture system aneurismal SMCs appear to tolerate the damage as efficiently as the control samples as similar levels of cell death were observed.
In our study, we also examined the level of genomic damage by scoring the level of MN and BN. We hypothesized that since ROS can cause genotoxicity (Beckman and Ames, 1997), and since there is increased ROS within the dilated vessel, SMCs from aneurismal tissue could exhibit increased damage. As expected, we observed significantly higher levels of MN and BN in aneurismal SMCs. Interestingly, while it is clear how ROS can lead to higher MN (Hoffelder et al., 2004), the correlation between BN and ROS has only recently been discussed in a few studies. For instance, it has been shown that cytokinesis failure leading to binucleated cell formation was associated with oxidative stress in CHO-K1 cells (Lin et al., 2009). In another study, scavenging of ROS during fetal–neonatal transition resulted in a decrease in the number of binucleated cardiomyocytes (Matsuyama and Kawahara, 2011). A correlative in vivo study was performed in mice, where the authors showed that vitamin C deficiency induces elevated ROS production, which causes oxidative liver injury and the increase of BN hepatocytes (Park et al., 2010). These data indicate a correlation between ROS and BN formation although it is still not known whether there is a direct causal effect. Regardless of the mechanism, both MN and BN are significantly elevated in aneurismal SMCs, indicating higher insult in the genome of these cells. We speculated that one of the means that an increase in genomic anomalies could lead to would be higher sensitivity to damaging agents, resulting in elevated levels of apoptosis. Yet, we failed to find such a correlation at least for the conditions tested in our laboratory. Hence, it appears that the accumulated genomic insult in the aneurismal SMCs, possibly caused by ROS, does not seem to explicate the tendency for apoptosis in TAAs.
Is there a cause and effect relationship between the increase in MN and BN and aneurysm development or is this simply a by stander phenomena? It is really hard to extrapolate an answer based on our current data, yet, it could be speculated that at least some of the changes involving either down or upregulation of genes can be explained by gross chromosomal changes that can be caused by MN and/or BN. In accordance with our findings, previous findings have indicated elevated oxidative DNA damage in other cardiovascular diseases, such as in the vessel wall of atherosclerotic plaques (Martinet et al., 2002), as well as in the endothelial cells and SMCs from AAAs (Cafueri et al., 2012). Hence, elevated genomic damage can be a common denominator in cardiovascular diseases and ROS appears to be a strong candidate responsible of this damage.
Supplementary Material
Acknowledgments
This work was supported by the 7th European framework program: Health-2007-2.4.2-2, entitled Fighting Aneurysmal Disease (FAD), Grant agreement no: 200647. We also thank Dr. Batu Erman (Sabanci University) for his help with the initial flow cytometry experiments.
Disclosure Statement
No competing financial interests exist.
References
- Barbour J.R. Spinale F.G. Ikonomidis J.S. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res. 2007;139:292–307. doi: 10.1016/j.jss.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Beckman K.B. Ames B.N. Oxidative decay of DNA. J Biol Chem. 1997;272:19633–19636. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- Bennett M.R. Evan G.I. Schwartz S.M. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J Clin Invest. 1995;95:2266–2274. doi: 10.1172/JCI117917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger A. Rocklin M. Coselli J. Milewicz D.M. Familial thoracic aortic dilatations and dissections: a case control study. J Vasc Surg. 1997;25:506–511. doi: 10.1016/s0741-5214(97)70261-1. [DOI] [PubMed] [Google Scholar]
- Boddy A.M. Lenk G.M. Lillvis J.H. Nischan J. Kyo Y. Kuivaniemi H. Basic research studies to understand aneurysm disease. Drug News Perspect. 2008;21:142–148. [PubMed] [Google Scholar]
- Bolognesi C. Lando C. Forni A. Landini E. Scarpato R. Migliore L., et al. Chromosomal damage and ageing: effect on micronuclei frequency in peripheral blood lymphocytes. Age Ageing. 1999;28:393–397. doi: 10.1093/ageing/28.4.393. [DOI] [PubMed] [Google Scholar]
- Booher A.M. Eagle K.A. Diagnosis and management issues in thoracic aortic aneurysm. Am Heart J. 2011;162:38–46. doi: 10.1016/j.ahj.2011.04.010. e1. [DOI] [PubMed] [Google Scholar]
- Brunt K.R. Fenrich K.K. Kiani G. Tse M.Y. Pang S.C. Ward C.A., et al. Protection of human vascular smooth muscle cells from H2O2-induced apoptosis through functional codependence between HO-1 and AKT. Arterioscler Thromb Vasc Biol. 2006;26:2027–2034. doi: 10.1161/01.ATV.0000236204.37119.8d. [DOI] [PubMed] [Google Scholar]
- Cafueri G. Parodi F. Pistorio A. Bertolotto M. Ventura F. Gambini C., et al. Endothelial and smooth muscle cells from abdominal aortic aneurysm have increased oxidative stress and telomere attrition. PLoS One. 2012;7:e35312. doi: 10.1371/journal.pone.0035312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [CDC] Centers for Disease Control Prevention. National Center for Health Statistics. Compressed Mortality File 1999–2008. 1999–2008. http://wonder.cdc.gov/cmf-icd10.html http://wonder.cdc.gov/cmf-icd10.html CDC WONDER Online Database, compiled from Compressed Mortality File 1999–2008 Series 20 No. 2N, 2011.
- Clarke M. Bennett M. Defining the role of vascular smooth muscle cell apoptosis in atherosclerosis. Cell Cycle. 2006;5:2329–2331. doi: 10.4161/cc.5.20.3383. [DOI] [PubMed] [Google Scholar]
- Clarke M.C. Figg N. Maguire J.J. Davenport A.P. Goddard M. Littlewood T.D., et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- Crowther M. Goodall S. Jones J.L. Bell P.R. Thompson M.M. Increased matrix metalloproteinase 2 expression in vascular smooth muscle cells cultured from abdominal aortic aneurysms. J Vasc Surg. 2000;32:575–583. doi: 10.1067/mva.2000.108010. [DOI] [PubMed] [Google Scholar]
- Dahm-Daphi J. Sass C. Alberti W. Comparison of biological effects of DNA damage induced by ionizing radiation and hydrogen peroxide in CHO cells. Int J Radiat Biol. 2000;76:67–75. doi: 10.1080/095530000139023. [DOI] [PubMed] [Google Scholar]
- Dearfield K.L. Auletta A.E. Cimino M.C. Moore M.M. Considerations in the U.S. Environmental Protection Agency's testing approach for mutagenicity. Mutat Res. 1991;258:259–283. doi: 10.1016/0165-1110(91)90012-k. [DOI] [PubMed] [Google Scholar]
- Ejiri J. Inoue N. Tsukube T. Munezane T. Hino Y. Kobayashi S., et al. Oxidative stress in the pathogenesis of thoracic aortic aneurysm: protective role of statin and angiotensin II type 1 receptor blocker. Cardiovasc Res. 2003;59:988–996. doi: 10.1016/s0008-6363(03)00523-6. [DOI] [PubMed] [Google Scholar]
- El-Hamamsy I. Yacoub M.H. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat Rev Cardiol. 2009;6:771–786. doi: 10.1038/nrcardio.2009.191. [DOI] [PubMed] [Google Scholar]
- Fenech M. Chromosomal damage rate, aging, and diet. Ann N Y Acad Sci. 1998;854:23–36. doi: 10.1111/j.1749-6632.1998.tb09889.x. [DOI] [PubMed] [Google Scholar]
- Forsdahl S.H. Singh K. Solberg S. Jacobsen B.K. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromso Study, 1994–2001. Circulation. 2009;119:2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- Girardi L.N. Coselli J.S. Inflammatory aneurysm of the ascending aorta and aortic arch. Ann Thorac Surg. 1997;64:251–253. doi: 10.1016/s0003-4975(97)00458-x. [DOI] [PubMed] [Google Scholar]
- Griendling K.K. FitzGerald G.A. Oxidative stress and cardiovascular injury: part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- He R. Guo D.C. Estrera A.L. Safi H.J. Huynh T.T. Yin Z., et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thorac Cardiovasc Surg. 2006;131:671–678. doi: 10.1016/j.jtcvs.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Heddle J.A. Micronuclei in vivo. Prog Clin Biol Res. 1990;340B:185–194. [PubMed] [Google Scholar]
- Heddle J.A. Cimino M.C. Hayashi M. Romagna F. Shelby M.D. Tucker J.D., et al. Micronuclei as an index of cytogenetic damage: past, present, and future. Environ Mol Mutagen. 1991;18:277–291. doi: 10.1002/em.2850180414. [DOI] [PubMed] [Google Scholar]
- Henderson E.L. Geng Y.J. Sukhova G.K. Whittemore A.D. Knox J. Libby P. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99:96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- Hoffelder D.R. Luo L. Burke N.A. Watkins S.C. Gollin S.M. Saunders W.S. Resolution of anaphase bridges in cancer cells. Chromosoma. 2004;112:389–397. doi: 10.1007/s00412-004-0284-6. [DOI] [PubMed] [Google Scholar]
- Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 2000;87:179–183. doi: 10.1161/01.res.87.3.179. [DOI] [PubMed] [Google Scholar]
- Jang E.R. Lee C.S. 7-ketocholesterol induces apoptosis in differentiated PC12 cells via reactive oxygen species-dependent activation of NF-kappaB and Akt pathways. Neurochem Int. 2011;58:52–59. doi: 10.1016/j.neuint.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Leach N.T. Jackson-Cook C. Micronuclei with multiple copies of the X chromosome: do chromosomes replicate in micronuclei? Mutat Res. 2004;554:89–94. doi: 10.1016/j.mrfmmm.2004.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. Chau L. Fas/Fas ligand-mediated death pathway is involved in oxLDL-induced apoptosis in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2001;280:C709–C718. doi: 10.1152/ajpcell.2001.280.3.C709. [DOI] [PubMed] [Google Scholar]
- Leik C.E. Willey A. Graham M.F. Walsh S.W. Isolation and culture of arterial smooth muscle cells from human placenta. Hypertension. 2004;43:837–840. doi: 10.1161/01.HYP.0000119191.33112.9c. [DOI] [PubMed] [Google Scholar]
- Li P.F. Dietz R. von Harsdorf R. Differential effect of hydrogen peroxide and superoxide anion on apoptosis and proliferation of vascular smooth muscle cells. Circulation. 1997a;96:3602–3609. doi: 10.1161/01.cir.96.10.3602. [DOI] [PubMed] [Google Scholar]
- Li P.F. Dietz R. von Harsdorf R. Reactive oxygen species induce apoptosis of vascular smooth muscle cell. FEBS Lett. 1997b;404:249–252. doi: 10.1016/s0014-5793(97)00093-8. [DOI] [PubMed] [Google Scholar]
- Li X.D. Li J. [Establishment of an apoptosis model of vascular smooth muscle cells induced by ultraviolet-C radiation in vitro] Sheng Li Xue Bao. 1999;51:234–239. [PubMed] [Google Scholar]
- Liao M. Liu Z. Bao J. Zhao Z. Hu J. Feng X., et al. A proteomic study of the aortic media in human thoracic aortic dissection: implication for oxidative stress. J Thorac Cardiovasc Surg. 2008;136:65–72. doi: 10.1016/j.jtcvs.2007.11.017. e1–e3. [DOI] [PubMed] [Google Scholar]
- Lin C.C. Chang M.C. Chang H.H. Wang T.M. Tseng W.Y. Tai T.F., et al. Areca nut-induced micronuclei and cytokinesis failure in Chinese hamster ovary cells is related to reactive oxygen species production and actin filament deregulation. Environ Mol Mutagen. 2009;50:367–374. doi: 10.1002/em.20463. [DOI] [PubMed] [Google Scholar]
- Lizard G. Gueldry S. Sordet O. Monier S. Athias A. Miguet C., et al. Glutathione is implied in the control of 7-ketocholesterol-induced apoptosis, which is associated with radical oxygen species production. FASEB J. 1998;12:1651–1663. doi: 10.1096/fasebj.12.15.1651. [DOI] [PubMed] [Google Scholar]
- Maiellaro-Rafferty K. Weiss D. Joseph G. Wan W. Gleason R.L., Jr. Taylor W.R. Catalase overexpression in aortic smooth muscle prevents pathological mechanical changes underlying abdominal aortic aneurysm formation. Am J Physiol Heart Circ Physiol. 2011;301:H355–H362. doi: 10.1152/ajpheart.00040.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet W. Knaapen M.W. De Meyer G.R. Herman A.G. Kockx M.M. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927–932. doi: 10.1161/01.cir.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- Matsuyama D. Kawahara K. Oxidative stress-induced formation of a positive-feedback loop for the sustained activation of p38 MAPK leading to the loss of cell division in cardiomyocytes soon after birth. Basic Res Cardiol. 2011;106:815–828. doi: 10.1007/s00395-011-0178-8. [DOI] [PubMed] [Google Scholar]
- McCormick M.L. Gavrila D. Weintraub N.L. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:461–469. doi: 10.1161/01.ATV.0000257552.94483.14. [DOI] [PubMed] [Google Scholar]
- Miller F.J., Jr. Sharp W.J. Fang X. Oberley L.W. Oberley T.D. Weintraub N.L. Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arterioscler Thromb Vasc Biol. 2002;22:560–565. doi: 10.1161/01.atv.0000013778.72404.30. [DOI] [PubMed] [Google Scholar]
- Nishio E. Arimura S. Watanabe Y. Oxidized LDL induces apoptosis in cultured smooth muscle cells: a possible role for 7-ketocholesterol. Biochem Biophys Res Commun. 1996;223:413–418. doi: 10.1006/bbrc.1996.0907. [DOI] [PubMed] [Google Scholar]
- Paravastu S.C. Murray D. Ghosh J. Serracino-Inglott F. Smyth J.V. Walker M.G. Inflammatory abdominal aortic aneurysms (IAAA): past and present. Vasc Endovascular Surg. 2009;43:360–363. doi: 10.1177/1538574409335915. [DOI] [PubMed] [Google Scholar]
- Park J.K. Hong I.H. Ki M.R. Chung H.Y. Ishigami A. Ji A.R., et al. Vitamin C deficiency increases the binucleation of hepatocytes in SMP30 knock-out mice. J Gastroenterol Hepatol. 2010;25:1769–1776. doi: 10.1111/j.1440-1746.2010.06239.x. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S. Meng X.P. Ramasamy S. Harrison D.G. Galis Z.S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. Lemke P. Bohle R.M. Klovekorn W.P. Bauer E.P. Inflammatory aneurysm of the ascending thoracic aorta. J Thorac Cardiovasc Surg. 2002;123:822–824. doi: 10.1067/mtc.2002.121291. [DOI] [PubMed] [Google Scholar]
- Schroder E. Eaton P. Hydrogen peroxide as an endogenous mediator and exogenous tool in cardiovascular research: issues and considerations. Curr Opin Pharmacol. 2008;8:153–159. doi: 10.1016/j.coph.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Taketani T. Imai Y. Morota T. Maemura K. Morita H. Hayashi D., et al. Altered patterns of gene expression specific to thoracic aortic aneurysms: microarray analysis of surgically resected specimens. Int Heart J. 2005;46:265–277. doi: 10.1536/ihj.46.265. [DOI] [PubMed] [Google Scholar]
- Tang P.C. Coady M.A. Lovoulos C. Dardik A. Aslan M. Elefteriades J.A., et al. Hyperplastic cellular remodeling of the media in ascending thoracic aortic aneurysms. Circulation. 2005;112:1098–1105. doi: 10.1161/CIRCULATIONAHA.104.511717. [DOI] [PubMed] [Google Scholar]
- Thomas M. Gavrila D. McCormick M.L. Miller F.J., Jr. Daugherty A. Cassis L.A., et al. Deletion of p47phox attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circulation. 2006;114:404–413. doi: 10.1161/CIRCULATIONAHA.105.607168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia A. Kochevar I.E. Ultraviolet A induces apoptosis via reactive oxygen species in a model for Smith-Lemli-Opitz syndrome. Free Radic Biol Med. 2006;40:641–650. doi: 10.1016/j.freeradbiomed.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Xiong W. Mactaggart J. Knispel R. Worth J. Zhu Z. Li Y., et al. Inhibition of reactive oxygen species attenuates aneurysm formation in a murine model. Atherosclerosis. 2009;202:128–134. doi: 10.1016/j.atherosclerosis.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima N. Masuda M. Miyazaki M. Nakajima N. Chien S. Shyy J.Y. Oxidative stress is involved in the development of experimental abdominal aortic aneurysm: a study of the transcription profile with complementary DNA microarray. J Vasc Surg. 2002;36:379–385. doi: 10.1067/mva.2002.124366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.