Abstract
The BK channel is one of the most broadly expressed ion channels in mammals. In many tissues, the BK channel pore-forming α-subunit is associated to an auxiliary β-subunit that modulates the voltage- and Ca2+-dependent activation of the channel. Structural components present in β-subunits that are important for the physical association with the α-subunit are yet unknown. Here, we show through co-immunoprecipitation that the intracellular C-terminus, the second transmembrane domain (TM2) and the extracellular loop of the β2-subunit are dispensable for association with the α-subunit pointing transmembrane domain 1 (TM1) as responsible for the interaction. Indeed, the TOXCAT assay for transmembrane protein–protein interactions demonstrated for the first time that TM1 of the β2-subunit physically binds to the transmembrane S1 domain of the α-subunit.
Keywords: BK channels, β2-Subunit, TOXCAT assay, Transmembrane domain TM1, BK α and β-subunits interactions
1. Introduction
The high-conductance voltage- and Ca2+-activated K+ channel is one of the most ubiquitously expressed potassium channels in mammals [1]. This channel is named BK for “big K”, given its single- channel conductance that can be as large as 250 pS in 100 mM, symmetrical KCl [2–4]. BK channels increase their activity by membrane depolarization or rises in cytosolic Ca2+ levels [2,5]. As its activation leads to membrane hyperpolarization, it serves as a negative-feedback mechanism for the excitatory events that lead to increases in calcium concentration or membrane depolarization.
The BK channel is a homotetramer of its pore-forming α-subunit, which is encoded by the gene Slo1 (kcnma1) and is a member of the voltage-dependent potassium (Kv) channels superfamily. As in all other Kv channels, the S4 transmembrane segment is part of an intrinsic voltage sensor [6,7]. Gating and ionic currents in BK channels can be elicited by membrane depolarization in the absence of calcium, suggesting that this is a voltage-dependent channel [8,9].
In many tissues, the BK channel α-subunit is associated to an auxiliary β-subunit. The β-subunits are intrinsic membrane proteins consisting of two transmembrane domains (TM1 and TM2) and a large (~120 residues) external loop. There are four types of β-subunits, β1–β4, that have tissue-specific distributions and impart unique effects on voltage- and Ca2+-dependent activation of BK channels (reviewed in [10]).
The functional coupling between α- and β1-subunits requires the transmembrane segment S0 of the α-subunit [11]. However, Morrow et al. (2006) reported that the physical association between the α-subunit and the β1-subunit does not require the S0 segment but rather the presence of S1, S2 and S3 segments [12]. More recently, using a disulfide cross-linking assay, it was found that the outer face of β1-subunit TM1 is in close proximity to the outer face of S1 and S2 domains in the α-subunit, and that the outer face of TM2 is in the neighborhood of the outer face of S0 in the adjacent α-subunit [13–15]. However, the structural components of β-subunits that are important for the physical association with the α-subunit are not known.
The conserved structural regions of β1–β4 subunit proteins are encoded by 3 exons, likely suggesting the selection of functional cassettes in each exon (Entrez Gene IDs, 3779, 10242, 27094, 27345; see Evidence Viewer) [16,17]. In this regard, the distinctive NH2-termini of β2 and β3 subunits that endow these β subunits with inactivation properties are encoded by additional exons. In this study, we took as framework the β2-subunit, and analyzed the coding sequence (additional exons may contain 5′ and 3′ UTR sequences) to determine the importance of corresponding structural regions in binding to the α subunit. We found, using Co-IP assays, that protein regions encoded by exon 5 (rest of the extracellular loop, TM2 and C-terminus) and by exon 4 (part of the extracellular loop) are not required for the physical association between β2 and the α-subunit. We also show the first experimental evidence of direct binding between the β2-subunit TM1 domain and the α-subunit S1 domain using the TOXCAT assay.
2. Materials and methods
2.1. Materials
Primary antibodies were polyclonal and monoclonal anti-FLAG (Sigma), and polyclonal or monoclonal anti-c-Myc (Sigma). Secondary antibodies (goat) for Western blot (WB) were Alexa Fluor 680 anti-rabbit (Invitrogen), and IRDye 800 anti-mouse (Rockland Immunochemicals).
2.2. Plasmid constructs
All plasmids were generated using standard recombinant DNA techniques. Human Slo1 (hSlo1) (U11058, KCNMA1) (with N-terminal c-Myc epitope) [18] was subcloned in pcDNA3 (Invitrogen), human β2 subunit was tagged with 3xFLAG epitope (DYKKDHD GDYKDHDIDYKDDDDK) by subcloning in p3×FLAG-CMV-14 (Sigma). The deletion mutants were generated through PCR-based overlapping extension mutagenesis technique [19], using PfuTurbo DNA Polymerase (Stratagene). Subsequent restriction mapping and sequencing confirmed the accuracy of all clones.
The vectors pML-27 and pML-28 were a gift from Dr. K. M. Blumenthal [20,21].
2.3. Cell culture and transfection
HEK293T cells were grown under 95% air, 5% CO2 atmosphere at 37 °C and transfected with LipofectAMINE 2000 (Invitrogen) according to the manufacturer instructions. Experiments involving transfected cells were performed 2 days post-transfection.
2.4. Co-Immunoprecipitation assays (Co-IP)
HEK293T cells co-transfected with α and β2 subunit constructs were lysed in (mM): 150 NaCl, 50 Tris–HCl, 5 EDTA, 10 HEPES, pH 7.4, 0.1% Nonidet P-40, 0.25% sodium deoxycholate, plus a cocktail of protease inhibitors (Protease Inhibitor Cocktails tablet, Roche) by shaking (1 h, 4 °C). Homogenates were centrifuged (3300×g, 10 min, 4 °C), and the supernatant was precleared with 50 μl of protein-G-Sepharose beads (1 h, 4 °C) followed by centrifugation (15000×g, 30 min, 4 °C). Antibodies were pre-bound to protein-G-Sepharose beads (2 μg of Ab/50 μl of beads, 3 h, 4 °C). Precleared lysates (~1.5 mg of protein) were incubated (overnight, 4 °C) with anti-FLAG or anti-c-Myc Abs (2 μg Abs pre-bound to protein-G-Sepharose beads), washed (10% glycerol/lysis buffer), eluted with loading buffer (plus 1.4 M β-mercaptoethanol), boiled, and subjected to Western Blotting (WB). WB signals were detected by infrared fluorescence imaging (Li-COR Biosciences).
2.5. TOXCAT assay
To perform the TOXCAT assay we used the vectors pML-27 and pML-28 [20,21]. pML-27 was derived from the original vector pcc-KAN [22] but the C-terminal maltose binding protein moiety is replaced by the beta-lactamase cDNA. TM1 (and part of NH2 terminus both encoded by exon 3) of β2, and BK transmembrane S1 segment cDNAs were cloned between NheI and BamHI restriction sites of pML-27 or pML-28 vectors. The cDNA of the respective TMs were generated by synthetic oligonucleotides, Exon3-β2 cDNA was from KCNMB2 cDNA (NM_181361): (5′ gctagcaatatttaccagaa aatcagggaccatgac ctcctggacaaaaggaaaacagtcacagcactgaaggcaggaga ggaccgagctattctcctgggactggctatgatggtgtgctccatcatgatgtattttctgctgg gaatcacactcctgcgctcatacatgcagagccggatcc-3′) and the S1 cDNA from KCNMA1 cDNA (U11058) 5′ gctagcggcagagtcctggttg tcttagtcttt gctctcagcatcggtgcacttgtaatatacttcatagattcacggat-3′. The synthetic oligonucleotides containing the overlapped and complementary NheI and BamHI restriction sequences were annealed using an equimolar ratio and 5 min incubation at 95 °C in ligation buffer followed by 1 h at room temperature. The double stranded DNA was then ligated to pML-27 or pML-28 vector. To confirm the in frame insertion of TMs cDNAs, all constructs were sequenced using the primer 5′-GAATACGCAGAATCAAG CAGTGTG-3′ located in the toxR cassette. Transformed bacteria were selected in spectinomycin (50 μg/ml). To perform the TOXCAT homodimerization and blank controls, the colonies were transformed with pML-27, Exon3β2-pML-27 or S1-pML-27 constructs and grown overnight in ampicillin (100 μg/ml). Only the colonies that grew well in ampicillin were used in the assay. Aliquots of these bacterial cultures were transferred to different tubes containing 10 ml of LB media plus ampicillin (100 μg/ml) and different concentrations of chloramphenicol (20–120 μg/ml) at an initial bacterial density of 0.025 (optical density, OD, measured at 600 nm). Then the cultures were grown 8–10 h and the cell density was quantified by measuring the final OD of the culture. For the heterodimerization TOXCAT assay, we followed the same procedure but bacteria were co-transformed with the constructs Exon3β2-pML-27 and S1-pML-28, and selected by resistance to spectinomycin and kanamycin, respectively.
2.6. Patch Clamp
Macroscopic currents were measured in cell-attached patches. Pipette and bath solutions were (mM): 105 Potassium methanesulfonate, 5 KCl, 10 HEPES, pH 7.0. To compare current densities, we used pipettes of similar resistances (~2MΩ) indicating similar diameters, and normalized the currents to the resistance of each patch pipette.
3. Results
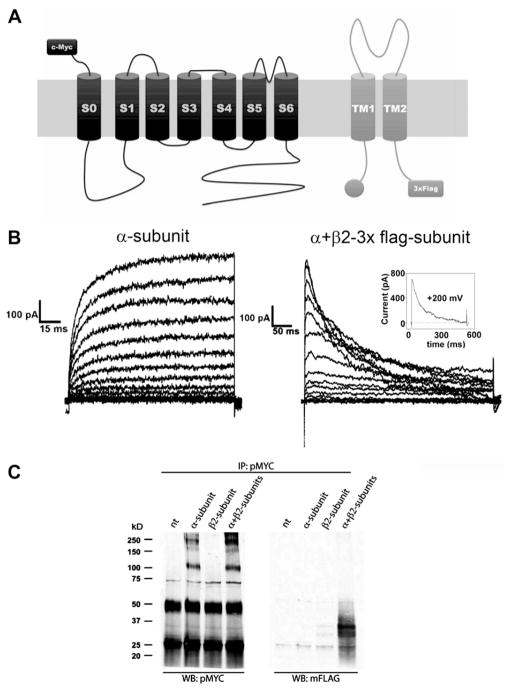
Our aim was to determine the role of different regions of β2 subunit in its binding to α-subunits in BK channels. As a first step, we used co-immunoprecipitation (co-IP) assays to circumscribe associating regions. To this end, we tagged the β2-subunit with a 3×FLAG epitope in the C-terminus (Fig. 1A), which allows straight-forward detection by WB using anti-FLAG antibodies. To evaluate the function of these β2-3×FLAG constructs, we recorded the effects of the tagged β2 on the BK α-subunit by co-expressing them in HEK293T cells. When BK α-subunit is co-transfected with β2-3×FLAG, the BK current shows inactivation, the signature effect of β2-subunit activity (Fig. 1B). The inactivation process induced by the β2-3×FLAG is complete at high applied voltages (Fig. 1B insert) and the time constant that describe the inactivation time course compares well with those reported in the literature [23]. It is important to note here that BK channels formed by the α subunit alone when detected using the cell-attached configuration do not inactivate [24] (Fig. 1B right). This result suggests that β-subunits tagged with 3×FLAG at the C-terminus are working similar to wild type β-subunits.
Fig. 1.
β2-Subunit with a C-terminal 3xFLAG tag is functional. (A) Schematic representation of BK α-subunit tagged with a c-Myc epitope at the N-terminus and β2-subunit tagged with 3xFLAG at the C-terminus. (B) Macroscopic currents of α-subunit with (right panel) or without (left panel) β2-3xFLAG subunit. Currents were elicited after a 100 ms prepulse to −60 mV by voltage steps from −100 to 200 mV, every 20 mV. Pulse duration was 150 ms (right) or 500 ms (left). Holding potential was 0 mV. Insert shows the current time course induced by a 200 mV voltage step. Current decay is well fitted using a single exponential with a time constant of 130 ms. (C) Co-IP assay in HEK 293T cells transfected with α-subunit tagged with c-Myc and β2-3xFLAG subunit. IP of α-subunits was carried out with polyclonal anti-c-myc antibody (pMYC). Western blot (WB) was done with pMYC to detect the α-subunit and with monoclonal anti-FLAG antibody (mFLAG) to detect the β2-3xFLAG subunit. The two strong bands at ~50 KDa and ~25 KDa in the left blot correspond to heavy and light immunoglobulin chains from antibodies used in IP.
We next set up Co-IP assays between β2-3×FLAG constructs and c-Myc tagged BK α-subunits (Fig. 1A). When we use a polyclonal anti-c-Myc antibody to immunoprecipitate the α-subunit (Fig. 1C), we detected this subunit with the same antibody as a ~125 KDa band for monomer and more than 200 KDa bands for oligomers, in extracts from cells transfected with α-subunit alone and in extracts from cells co-transfected with α- and β2-3×FLAG (Fig. 1C, left panel). However, we only detected co-immunoprecipitation of β2-3×FLAG (double band ~35 KDa) with a monoclonal anti-FLAG antibody in extracts from cells co-transfected with α- and β2-3×FLAG (Fig. 1C, right panel). This result was confirmed with the reverse Co-IP, when a polyclonal anti-FLAG antibody was used to immunoprecipitate (IP) the β2-3×FLAG subunit. Co-IP of α-subunit was found only in co-transfected cells (data not shown). The specificity of these assays was tested by performing a Co-IP assay using extract from cells co-transfected with β2-3×FLAG and deletion mutants of α-subunit tagged with c-Myc. All the constructs tested expressed well (Fig. S1A) and the Co-IP assay was only positive with deletion mutants of α-subunit that contained the transmembrane domains (Fig. S1B). These data are consistent with the results obtained by Morrow et al. (2006), who showed that only α-subunit’s constructs containing at least the transmembrane segments S1 to S3 can bind the β-subunits [12].
3.1. Deletion of exon 5 does not affect binding of β2-subunit to the α-subunit
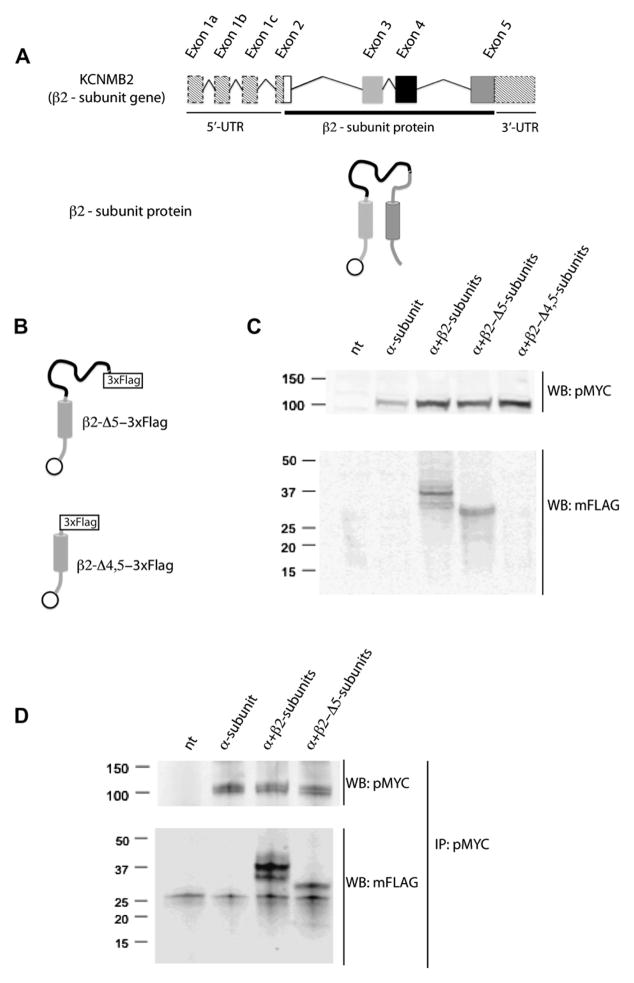
We designed a deletion strategy following the gene structure of the β2-subunit (Fig. 2A) where exon 2 encodes for the inactivation ball, exon 3 encodes for TM1, exon 4 encodes part of the extracellular loop, and exon 5 encodes for part of the extracellular loop, TM2 and C-terminus (Fig. 2A). To find the region of β2 subunit involved in the interaction with the α subunit, we made deletion mutants without the region encoded by one or two exons (Fig. 2B) and association to the α-subunit was tested by Co-IP. The mutant with exon 5 deleted (β2-Δ5-3×FLAG) was expressed in HEK 293T cells (Fig. 2C), but the mutant with exons 4 and 5 deleted (β2-Δ4,5-3×FLAG) was not detected by WB (Fig. 2C). The β2-Δ5-3×FLAG subunit retains its interaction with the α-subunit as detected in Co-IP assays (Fig. 2D). This result suggests that the intracellular C-terminus, TM2 and the region of the extracellular loop encoded by exon 5 are not essential for association to the α-subunit.
Fig. 2.
β2-Subunit without regions encoded by exon 5 retains α-subunit associating activity. (A) Schematic representations of β2-subunit gene and protein. The exons and the corresponding regions in the protein are represented by the same colors. Dashed boxes correspond to 5′ and 3′ UTR. (B) Schematic of β2-Δ5-3×FLAG and β2-Δ4,5-3×FLAG mutants. (C) HEK293T cells were non-transfected (nt) or transfected with α-subunit alone, or α-subunit together with β2-subunit, β2-Δ5-3×FLAG or β2-Δ4,5-3×FLAG. Proper expression of α-subunit was confirmed with a polyclonal anti-c-Myc antibody (pMYC). Anti-FLAG monoclonal antibody (mFLAG) was used to detect β2-subunit constructs. Both β2 and β2-Δ5-3×FLAG constructs were readily expressed while the β2-Δ4,5-3×FLAG mutant was not detected (N = 3). (D) β2-Δ5-3×FLAG mutant co-IP with the α-subunit similar to the wild type β2 subunit. The IP of α-subunit was carried out with pMYC antibody and β2 subunit mutants were detected with mFLAG antibody (N = 3).
3.2. Deletion of exon 4 does not affect binding of β2-subunit to the α-subunit
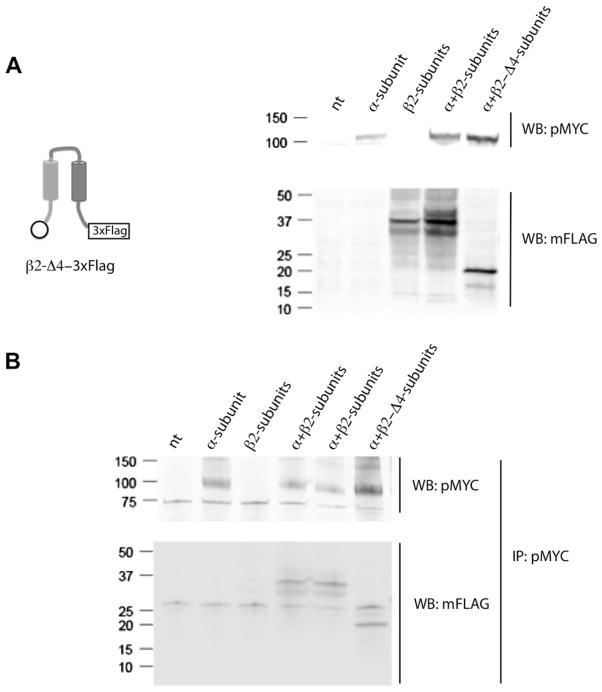
Next, we constructed a mutant with a deletion of exon 4 that encodes the N-terminal part of the extracellular loop (β2-Δ4-3×FLAG) and expressed it in HEK 293T cells. The resulting protein was detected in WB using an anti-FLAG monoclonal antibody (Fig. 3A). When the β2-Δ4-3×FLAG subunit was co-expressed with the α-subunit, an interaction between both subunits was still detected in the Co-IP assays (Fig. 3B). This result suggests that the region of the extracellular loop encoded by exon 4 is also not required for association to the α-subunit strongly supporting a role for sequences encoded by exon 3 including TM1.
Fig. 3.
β2-Subunit without regions encoded by exon 4 retains α-subunit associating activity. (A) β2-Δ4-3×FLAG mutant was expressed in HEK 293T cells and detected with anti-FLAG monoclonal antibody (mFLAG) by Western blot. The α-subunit was detected with a polyclonal anti c-myc antibody (pMYC) (N = 3). (B) β2-Δ4-3×FLAG mutant co-IP with α-subunit similar to the wild type β2 subunit. The IP of α-subunit was carried out with pMYC antibody and β2 subunit mutants were detected with mFLAG antibody by Western blot (N = 3).
3.3. The TM1 segment of the β2-subunit binds to the S1 domain of α-subunits
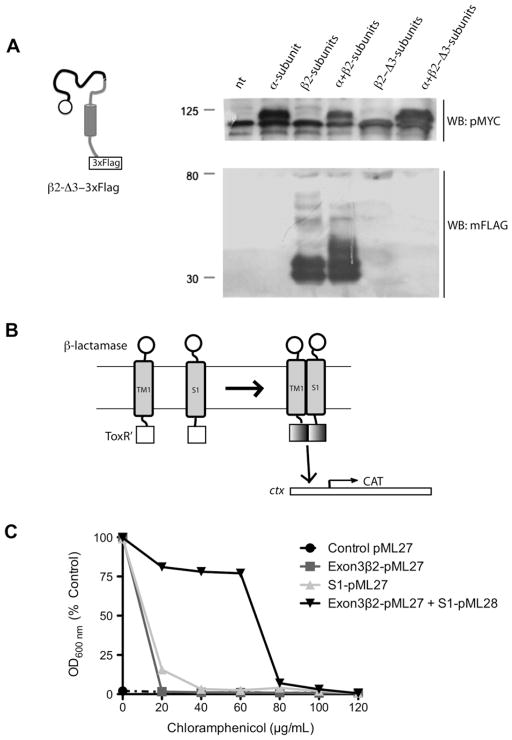
Finally, we deleted regions of the β2-subunit encoded by exon 3 (β2-Δ3-3×FLAG), but this mutant was not expressed at levels detectable by WB (Fig. 4A), which prevented us from getting useful information using a Co-IP assay as to whether TM1 (encode by exon 3) was involved in the interaction between β2- and the α-subunit. To investigate this potential interaction further, we used the TOXCAT assay system to study transmembrane association between the region encoded by exon 3 of the β2-subunit (TM1) and the α-subunit S1 domain in a membrane environment [22]. Bacteria co-transformed with TM1 (exon 3) of β2-subunit and the S1 domain of the α-subunit showed high resistance to chloramphenicol in comparison with bacteria transformed with either exon 3 or S1 constructs alone (Fig. 4C), a clear indication of TM-mediated dimerization.
Fig. 4.
The TM1 domain of β2-subunit binds to the S1 domain of α-subunit: (A) Schematic of the β2-Δ3-3×FLAG mutant and Western blot to detect its expression. β2-Δ3-3×FLAG was not detected (N = 2). (B) Schematic representation of TOXCAT assay system. TOXCAT has been designed to measure oligomerization of transmembrane segments of proteins within the context of the cytoplasmic membrane of living cells. In TOXCAT constructs, the transmembrane and periplasmic domains of ToxR were replaced by region coded by exon 3 of β2-subunit (that include TM1 and a short cytoplasmic tail) and S1 of α-subunit and β-lactamase sequences, respectively [21,22]. As a result, dimerization must be driven solely by interactions between the TM segments. Such dimerization allows the cytoplasmic transcriptional activation domains (ToxR′) to interact with the ctx promoter (cholera toxin promoter or ctx is positively regulated by ToxR [27]), thereby initiating transcription of a reporter gene encoding chloramphenicol acetyltransferase (CAT) [21]. TM-induced dimerization is observed as acquired resistance to the antibiotic chloramphenicol in vivo. (C) TOXCAT assay showing dimerization between TM1 (exon3-β2) and S1 (black inverted triangle). Note that bacteria co-transformed with TM1 (exon3-β2) and S1 acquired resistance to chloramphenicol in contrast to bacteria transformed with TM1 (dark gray square) or S1 alone (light gray triangle) (N = 2).
4. Discussion
The β2-subunit modulates BK α-subunit activity by increasing its Ca2+ sensitivity. Early studies indicated that in order to exert this modulatory effect, the β2-subunit has to be physically bound to α-subunit. There is evidence that this binding process occurs in intracellular compartments like the endoplasmic reticulum [25] and fully assembled channels composed of α- and β2-subunits reach the plasma membrane at the end of their biosynthetic sorting route [12]. In this study, we showed evidence that the extracellular loop; the TM2 domain and the intracellular C-terminus of the β2 subunit (encoded by exons 4 and 5) are not required for association with the α-subunit.
Early studies of the inactivation properties of β2-subunits showed that the region encoded by exon 2 is responsible for an N-type or fast inactivation of BK currents. The region is known as the “inactivation ball’ [23,26]. Besides this inactivation effect, the β2 subunit deletion mutants lacking the inactivation ball (β2-IR) still enhances BK channel’s calcium sensitivity, in a very similar way to that of the β1 subunit. Indirectly, these experiments also showed that the β2-IR mutant could indeed associate to the α-subunit.
These three mutants discussed thus far (β2-IR, β2-Δ4-3×FLAG and β2-Δ5-3×FLAG) have only one region in common: TM1 (encoded by exon 3). This led us to hypothesize that this domain could function as the α-subunit binding region of β2-subunit. However, we could not rule out the possibility that other regions of the β2-subunit may also contribute to α-subunit binding.
In support of this hypothesis, our experiments, using the TOX-CAT assay (Fig. 4C) show a direct physical interaction between transmembrane segments TM1 of β2 subunits and S1 of α-subunits. At present, we cannot rule out that TM1 could also bind to other transmembrane segments of the α-subunit. Collectively, our data suggest a model in which the TM1 of the β2 subunit is responsible for direct binding to S1 of α-subunit, and it is also consistent with disulfide cross-linking studies showing that, when β1 [13,15] and β4 [14] subunits are co-expressed with the α-subunit, TM1 is located close to S1 and S2.
Supplementary Material
Acknowledgments
This work was supported by FONDECYT Grants 1110430 (to R.L.), 1090573 (to A.D.M.) and 1120802 (to C.G.) DID-UACH Grant S-2012-13 (to F.J.M.), National Institutes of Health Grants HL54970 (to L.T.), HL088640 (to E.S.), and HL096740 (to E.S. and L.T.), CONICYT doctoral fellowships (To F.J.M. and M.S.) and Beca de Estadia en Centros Internacionales de Investigacion Cientifica, Direccion de Postgrado UACH (to F.J.M.). The Centro Interdisciplinario de Neurociencia de Valparaíso is a Millennium Institute supported by the Millennium Scientific Initiative of the Minesterio de Economía, Fomento y Turismo.
We thank Drs. Pablo Mardones for helpful comments on the manuscript and Oscar Jara, Rodrigo Acuna, Eduardo Rosenmann, Luisa Soto, Rodrigo Toro and Maria Jose Guerra for technical assistance.
Abbreviations
- BK
high-conductance voltage- and Ca2+-activated K+ channel
- TM
transmembrane
- WB
western blot
- Kv
voltage-dependent potassium channels
- HEK
human embryonic kidney cells
- Co-IP
co-immunoprecipitation
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.febslet.2012.05.066.
References
- 1.Toro L, Wallner M, Meera P, Tanaka Y. Maxi-K(Ca), a unique member of the voltage-gated K+ channel superfamily. News Physiol Sci. 1998;13:112–117. doi: 10.1152/physiologyonline.1998.13.3.112. [DOI] [PubMed] [Google Scholar]
- 2.Pallotta BS, Magleby KL, Barrett JN. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- 3.Latorre R, Vergara C, Hidalgo C. Reconstitution in planar lipid bilayers of a Ca2+-dependent K+ channel from transverse tubule membranes isolated from rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1982;79 (3):805– 809. doi: 10.1073/pnas.79.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latorre R, Oberhauser A, Labarca P, Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- 5.Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci. 2009;66 (5):852–875. doi: 10.1007/s00018-008-8609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Díaz L, Meera P, Amigo J, Stefani E, Alvarez O, Toro L, Latorre R. Role of the S4 segment in a voltage-dependent calcium-sensitive potassium (hSlo) channel. J Biol Chem. 1998;273 (49):32430–32436. doi: 10.1074/jbc.273.49.32430. [DOI] [PubMed] [Google Scholar]
- 7.Cui J, Aldrich RW. Allosteric linkage between voltage and Ca2+- dependent activation of BK-type mslo1 K+ channels. Biochemistry. 2000;39 (50):15612–15619. doi: 10.1021/bi001509+. [DOI] [PubMed] [Google Scholar]
- 8.Stefani E, Ottolia M, Noceti F, Olcese R, Wallner M, Latorre R, Toro L. Voltage-controlled gating in a large conductance Ca2+-sensitive K+ channel (hslo) Proc Natl Acad Sci U S A. 1997;94 (10):5427–5431. doi: 10.1073/pnas.94.10.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J Gen Physiol. 2002;120 (3):267–305. doi: 10.1085/jgp.20028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- 11.Wallner M, Meera P, Toro L. Determinant for beta-subunit regulation in high-conductance voltage-activated and Ca2+-sensitive K+ channels: an additional transmembrane region at the N terminus. Proc Natl Acad Sci U S A. 1996;93 (25):14922–14927. doi: 10.1073/pnas.93.25.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrow JP, Zakharov SI, Liu G, Yang L, Sok AJ, Marx SO. Defining the BK channel domains required for beta1-subunit modulation. Proc Natl Acad Sci U S A. 2006;103 (13):5096–5101. doi: 10.1073/pnas.0600907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu G, Zakharov SI, Yang L, Wu RS, Deng SX, Landry DW, Karlin A, Marx SO. Locations of the beta1 transmembrane helices in the BK potassium channel. Proc Natl Acad Sci U S A. 2008;105 (31):10727–10732. doi: 10.1073/pnas.0805212105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu RS, Chudasama N, Zakharov SI, Doshi D, Motoike H, Liu G, Yao Y, Niu X, Deng SX, Landry DW, Karlin A, Marx SO. Location of the beta 4 transmembrane helices in the BK potassium channel. J Neurosci. 2009;29 (26):8321–8328. doi: 10.1523/JNEUROSCI.6191-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu G, Niu X, Wu RS, Chudasama N, Yao Y, Jin X, Weinberg R, Zakharov SI, Motoike H, Marx SO, Karlin A. Location of modulatory beta subunits in BK potassium channels. J Gen Physiol. 2010;135 (5):449–459. doi: 10.1085/jgp.201010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Z, Wallner M, Meera P, Toro L. Human and rodent MaxiK channel beta-subunit genes: cloning and characterization. Genomics. 1999;55 (1):57–67. doi: 10.1006/geno.1998.5627. [DOI] [PubMed] [Google Scholar]
- 17.Uebele VN, Lagrutta A, Wade T, Figueroa DJ, Liu Y, McKenna E, Austin CP, Bennett PB, Swanson R. Cloning and functional expression of two families of beta-subunits of the large conductance calcium-activated K+ channel. J Biol Chem. 2000;275 (30):23211–23218. doi: 10.1074/jbc.M910187199. [DOI] [PubMed] [Google Scholar]
- 18.Meera P, Wallner M, Song M, Toro L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0–S6), an extracellular N terminus, and an intracellular (S9–S10) C terminus. Proc Natl Acad Sci U S A. 1997;94 (25):14066–14071. doi: 10.1073/pnas.94.25.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook J, Russell DW. Molecular Cloning a Laboratory Manual. 3. CSHL Press; New York: 2001. [Google Scholar]
- 20.Kashlan OB, Maarouf AB, Kussius C, Denshaw RM, Blumenthal KM, Kleyman TR. Distinct structural elements in the first membrane-spanning segment of the epithelial sodium channel. J Biol Chem. 2006;281 (41):30455–30462. doi: 10.1074/jbc.M604615200. [DOI] [PubMed] [Google Scholar]
- 21.Lis M, Blumenthal K. A modified, dual reporter TOXCAT system for monitoring homodimerization of transmembrane segments of proteins. Biochem Biophys Res Commun. 2006;339 (1):321–324. doi: 10.1016/j.bbrc.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Russ WP, Engelman DM. TOXCAT: a measure of transmembrane helix association in a biological membrane. Proc Natl Acad Sci U S A. 1999;96 (3):863–868. doi: 10.1073/pnas.96.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane β-subunit homolog. Proc Natl Acad Sci U S A. 1999;96:4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz F, Wallner M, Stefani E, Toro L, Latorre R. Interaction of internal Ba2+ with a cloned Ca2+-dependent K+ (hslo) channel from smooth muscle. J Gen Physiol. 1996;107 (3):399–407. doi: 10.1085/jgp.107.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7 (7):548–562. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- 26.Xia XM, Ding JP, Lingle CJ. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J Neurosci. 1999;19:5255–5264. doi: 10.1523/JNEUROSCI.19-13-05255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller VL, Mekalanos JJ. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc Natl Acad Sci U S A. 1984;81 (11):3471–3475. doi: 10.1073/pnas.81.11.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.