Abstract
This review summarizes the brain mechanisms controlling sleep and wakefulness. Wakefulness promoting systems cause low-voltage, fast activity in the electroencephalogram (EEG). Multiple interacting neurotransmitter systems in the brain stem, hypothalamus, and basal forebrain converge onto common effector systems in the thalamus and cortex. Sleep results from the inhibition of wake-promoting systems by homeostatic sleep factors such as adenosine and nitric oxide and GABAergic neurons in the preoptic area of the hypothalamus, resulting in large-amplitude, slow EEG oscillations. Local, activity-dependent factors modulate the amplitude and frequency of cortical slow oscillations. Non-rapid-eye-movement (NREM) sleep results in conservation of brain energy and facilitates memory consolidation through the modulation of synaptic weights. Rapid-eye-movement (REM) sleep results from the interaction of brain stem cholinergic, aminergic, and GABAergic neurons which control the activity of glutamatergic reticular formation neurons leading to REM sleep phenomena such as muscle atonia, REMs, dreaming, and cortical activation. Strong activation of limbic regions during REM sleep suggests a role in regulation of emotion. Genetic studies suggest that brain mechanisms controlling waking and NREM sleep are strongly conserved throughout evolution, underscoring their enormous importance for brain function. Sleep disruption interferes with the normal restorative functions of NREM and REM sleep, resulting in disruptions of breathing and cardiovascular function, changes in emotional reactivity, and cognitive impairments in attention, memory, and decision making.
I. INTRODUCTION
The purpose of sleep is one of the great unsolved mysteries of biology and has fascinated people for millennia. Although the function or functions of sleep are still unresolved, great progress has been made in understanding the brain mechanisms that control sleep and wakefulness. An understanding of these mechanisms is of paramount importance to our society. Sleeping tablets are among the most widely prescribed medicines, and disturbances in sleep are associated with a wide range of medical and psychiatric conditions. Conversely, an increase in sleep is one important mechanism that the body uses to combat infection and maintain optimal health. Adequate sleep is also essential for optimal cognitive function; lack of sleep has been implicated in major industrial disasters as well as car and workplace accidents. In this unusually comprehensive review we summarize current knowledge regarding the brain mechanisms which control wakefulness, non-rapid-eye-movement (NREM) sleep, and rapid-eye-movement (REM) sleep.
A. Characteristics of Sleep-Wake States
Sleep is defined in the sleep laboratory, in both humans and animals, by recording the electrical field activity of large groups of cortical neurons and muscle cells. Thus scalp electrodes record the electroencephalogram (EEG), electrodes placed on or in skeletal muscles record the electromyogram (EMG), whereas electrodes placed over or near the muscles responsible for horizontal eye movement record the electro-oculogram (EOG). Deep brain electrodes are used to record the activity of individual brain areas or individual neurons. These so-called polysomnographic recordings are used to define the states of wakefulness and sleep as follows (FIGURE 1): wakefulness is defined by low-voltage fast EEG activity (LVFA) and high muscle tone, NREM sleep is characterized by high-amplitude low-frequency EEG and decreased muscle tone, whereas REM sleep has LVFA coupled with a complete loss of muscle tone (REM muscle atonia) and characteristic rapid eye movements which contrast with the slow rolling eye movements observed during NREM. Further characteristics of these three states and the brain circuitry which generates them are discussed in sections II–IV. A summary of studies involving inactivation of different parts of the brain controlling sleep and wake is provided in TABLE 1. The location of these brain regions is shown in FIGURE 2.
FIGURE 1.
Electroencephalographic (EEG) recordings in the human and rat capture differences between vigilance states (wakefulness, NREM sleep, and REM sleep). Wakefulness in both species is characterized by low-amplitude/high-frequency activity. Note that high-frequency beta and gamma activity is not easily visible at this slow timescale. In the human, NREM sleep begins in stage 1, the prevalent EEG frequency begins to slow, with strong alpha activity at posterior sites and theta activity at anterior sites. In NREM sleep stages 2/3, both sleep spindles (7–14 Hz) and K-complexes are seen, as the EEG amplitude increases and frequency further slows. In NREM sleep stage 4, also known as slow wave sleep, strong delta (0.5–4 Hz) activity is evident, accompanied by a large increase in amplitude. During REM sleep, the EEG returns to a profile similar to wakefulness, with low-amplitude and high-frequency activity. In the rodent, NREM sleep is usually not parsed into separate stages. NREM sleep exhibits a significant increase of delta range activity, as well as an increase in amplitude. REM sleep is defined by the strong synchronous theta range (7–9 Hz) activity, probably generated in the hippocampus. Human EEG recordings are adapted from Purves et al. (1028). Note the voltage scales are not matched between species.
Table 1.
Inactivation of brain regions/neurotransmitter systems and effects on sleep-wake behavior
| Brain Area/System Targeted | Inactivation Technique | Effect on Sleep-Wake and EEG |
|---|---|---|
| Brain stem | ||
| Midbrain/pontine reticular formation | Transections in cat (136–138, 580, 730, 1393). | Loss of cortical activation during waking and REM sleep. |
| Cerveau isole mesencephalic transaction immediately caudal to the third nerve nuclei (effects contrast with encephale isole intact sleep-wake with transaction at C1 level of spinal cord). | Loss of forebrain signs of REM sleep. | |
| Brain stem damage in human patients (980). | Coma. | |
| Transection at caudal pontine or prebulbar level (1341, 1393). | Loss of REM sleep and of the ability of pontine carbachol to elicit tonic and phasic REM components. | |
| Electrolytic lesions in cat (730). | Loss of cortical activation (not observed when lateral sensory pathways were interrupted). Coma like state. | |
| Neurotoxic lesions ibotenic acid lesions in cat (302) or rat (758). | Temporary increase in EEG slow waves but no long-term effects (cat). One week coma like state for combined PH/MPRF lesions. Recovery occurred. No effect (rat). | |
| Dorsolateral pons including the dorsal subcoeruleus (=sublaterodorsal nucleus or peri-locus coeruleus alpha) or dorsal pontine nucleus oralis (PnO) | Electrolytic (387, 388, 495, 498, 569, 893, 900, 1111, 1121, 1122). | Very large lesions: |
| Neurotoxic kainic acid (577, 1122, 1394), ibotenic acid (758), NMDA (682, 683), hypocretin 2-saporin (100), quisqualic acid (606). | Loss of REM sleep correlated with loss of cholinergic neurons. | |
| Acute brain stem encephalitis with isolated inflammatory lesion in dorsomedial pontine tegmentum (including DRN, MR, PnO, LC, SubC, LDT, PPT) (810). | Large lesions including the SubC and surrounding areas: | |
| Pharmacological tetrodotoxin (1111, 1114). | REM sleep behavior disorder (RBD) in humans, oneiric (dream-like) behavior in animals. | |
| Idiopathic degeneration in humans (400, 1133). | Smaller discrete lesions (focusing on SLD/SubC area): | |
| REM without atonia in animals, increased limb movements during sleep. | ||
| GABA or muscimol (183, 1112, 1440, 1441) | Wakefulness↑, NREM↓, REM sleep↓. | |
| Norepinephrine (262), noradrenergic α2 agonist clonidine (1300), or dopamine acting on α2 receptors(263). | Decreased REM and/or REM without atonia. | |
| Noradrenergic β antagonist, propanolol (1301). | REM↑ due to increased number of REM episodes. | |
| Inhibition of adenylyl cyclase (799). | REM sleep↑ (increased frequency of episodes). | |
| Peribrachial pons (surrounding brachium conjunctivum): includes PPT/LDT, cuneiform nucleus, subcoeruleus (FTG in cat), medial and lateral parabrachial nucleus. | Cooling, electrolytic or neurotoxic lesion in P-wave generation zone (277, 278, 604, 690, 691, 814, 1100). | Loss of pontine component of PGO waves (P-waves). Reduced expression of learning related genes and proteins following active avoidance training. Reduced frequency of hippocampal theta and reduced synchronization between hippocampus and amygdala. |
| M2 receptor antagonist, methoctramine (283). | Block of enhanced PGO-wave activity and REM-sleep like state induced by carbachol in the cat. | |
| Serotonin (279) | Inhibition of P-waves when injected into P-wave generator in dorsal subcoeruleus. | |
| Medial parabrachial nucleus | Neurotoxic: ibotenic acid (758). | Wake 21%↓ |
| Precoeruleus | Neurotoxic: ibotenic acid (758). | Loss of theta rhythm during REM |
| Ventral medulla (gigantocellular and magnocellular tegmental fields) | Neurotoxic quisqualic acid in cat (514) or neonatal rat (606). | Muscle tone↑ during NREM and REM sleep. Increased movements during REM sleep. Reduction in REM sleep and atonia duration during first postlesion week followed by recovery in weeks 2 and 3. Amount of remaining REM sleep correlated positively with ratio of remaining cholinergic or GABA neurons to serotonergic neurons. |
| Transection at ponto-medullary junction in decerebrate cat (1179) or injection of lidocaine into pontine reticular formation (652). | Abolition of muscle atonia produced by electrical stimulation of medial medulla. | |
| Brain stem cholinergic (PPT) | Ibotenic acid (758). | Wake 30%↓ |
| Locus coeruleus (LC) | Electrolytic lesion (576). | No effect on REM sleep generation. |
| Scopolamine, minipump perfusion (1168). | REM sleep↓ during the daytime (inactive period) in the rat. | |
| GABAA agonist muscimol (959, 1303); | Increased REM sleep with muscimol (due to increase in number of REM bouts) Possibly due to preferential inhibition of local GABAergic neurons. | |
| GABAB receptor agonist baclofen into PPT/DpMe (370). | Decreased REM sleep and memory consolidation with baclofen. | |
| α2 Agonist, clonidine, α1 antagonist prazosin or β antagonist (958). | REM sleep↑. | |
| Adenylyl cyclase or protein kinase A inhibition (67, 282). | REM sleep ↓ (due to decrease in number of episodes). | |
| Serotonin (279, 1113). | No effect on PGO waves. | |
| Brain stem cholinergic (LDT) | Ibotenic acid (758). | LDT: increased fragmentation but no effect on amount of sleep. |
| Neurotoxic lesion: kainic acid (1394), ibotenic acid (758), dopamine-β- hydroxylase saporin (100). | ||
| Cooling (201) | REM sleep ↑ | |
| RNAi knockdown of orexin 1 receptor (219). | REM sleep during dark period↑ | |
| Microdialysis of α2 agonist clonidine (1096). | Decreased activity of LC neurons. Waking ↓, NREM sleep ↑. | |
| DSP-4 lesion (238, 244, 881). | Reduced immediate-early and synaptic plasticity related gene expression. | |
| Either no change in baseline sleep-wake (238, 244) or increase in REM (881). | ||
| Dorsal raphe nucleus (DRN) | Electrolytic lesion (1451). | No effect. |
| Neurotoxic lesion: ibotenic acid (758), 5,7-dihydroxytryptamine (757). | No effect on amounts of sleep-wake. | |
| GABAA receptor agonist, muscimol (926). | REM sleep ↑. | |
| Median raphe (MR) | Electrolytic:(1451) | Hippocampal theta rhythm ↑. |
| Pharmacological glutamatergic antagonists (629); GABAA agonist, muscimol (630), 5-HT1A receptor agonists (1364). | ||
| Dopamine- vPAG/DRN | 6-OHDA or ibotenic acid (757). | Marked decrease in waking (>20%), concomitant increase in sleep. |
| Ventrolateral periaqueductal gray (vlPAG) | Electrolytic lesion in encephale isole cats (730). | Cortical activation preserved. |
| Neurotoxic lesion: orexin 2-Saporin (612, 758) | REM sleep ↑ in both normal and orexin KO animals. | |
| Pharmacological muscimol in the cat (1120), rat (1118), and guinea pig (1336). | Increased REM bouts and REM bout duration during dark period. | |
| Lateral pontine tegmentum (LPT) = deep mesencephalic nucleus (DpMe) | Electrolytic lesion in encephale isole cats (730). | Cortical activation preserved. |
| Neurotoxic lesion orexin 2-saporin in the rat (758) and mouse (612). | REM sleep ↑. Increased REM bouts during light period and occasional bouts of cataplexy. | |
| Pharmacological muscimol in the cat (1120), rat (1118), and guniea pig (1336). | ||
| Superior colliculus/pretectum | Aspiration (857) | Abolition of lights off-induced increase in REM sleep. |
| Hypothalamus | ||
| Preoptic area/anterior hypothalamus | Viral insult in humans (1378). | Prolonged (>3 wk) and large suppression of sleep (both NREM and REM). |
| Electrolytic lesions in the cat (761, 840, 911) and neonatal rat (873). | ||
| Neurotoxic lesions in the cat: ibotenic (1105) or kainic acid (1255). | ||
| Lateral preoptic area/bed nucleus of the stria terminalis (BNST) | Neurotoxic lesions in the rat. Ibotenic acid (1138) or NMDA (1201). | Reduction in number of erections during REM sleep (1138). NREM sleep ↓ (1201). |
| Ventrolateral preoptic area (VLPO, core) | Neurotoxic (rats). ibotenic acid (756). | NREM 50–60%↓, REM sleep 59%↓, EEG delta power 60–70% ↓lasting at least 3 wk. Extent of lesion correlated with loss of NREM sleep. Sleep-wake fragmentation. |
| GABA/galanin-positive neurons | ||
| Extended VLPO (dorsomedial) | Neurotoxic (rat). ibotenic acid (756). | REM sleep 35% ↓, NREM sleep,15%↓, 25% loss of delta mainly during light period. Extent of lesion correlated with loss of REM sleep. |
| Ventromedial preoptic area | Neurotoxic (rat). ibotenic acid (756), NMDA (563, 1201). | No effect on sleep-wake (756). Reduced NREM and REM sleep (563, 1201). Disrupted body temperature regulation. |
| Median preoptic area | Pharmacological: muscimol perfusion (1251) | Prolonged waking state |
| Suprachiasmatic nucleus (SCN) | Electrolytic: rat (1439). | Loss of circadian rhythms. Reduced REM sleep during the rest (light) phase. |
| Dorsomedial hypothalamus (DMH) | Neurotoxic: ibotenic acid (52, 224). | Loss of circadian rhythms of sleep-wakefulness. |
| Posterior/lateral hypothalamus (PH/LH) | Viral insult (1378) | Hypersomnolence in human patients following influenza pandemic. |
| Orexin/hypocretin (perifornical hypothalamus; PFH) | Damage to PH/LH including perifornical hypothalamus due to tumor (43) or stroke (1129). | Narcolepsy. |
| Electrolytic/transection: (730, 840, 911, 1046) | Reduction or abolition of cortical activation. Hypersomnolence for several days followed by recovery (cat). | |
| Neurotoxic: | PH/LH: no long-term effects (cat) | |
| LH: hypocretin 2-saporin (411, 413) | TMN area: minor changes. | |
| Orexin KO or orexin receptor doubleknockouts (29, 214, 598, 640). | Narcolepsy with cataplexy in mice. Unchanged 24 h wake amount but sleep-wake fragmentation, cataplexy, loss of circadian control of REM sleep. Reduced voluntary motor activity (wheel running). | |
| Orexin receptor 2 mutations (727). | Inherited narcolepsy in dogs. | |
| Loss (degeneration) of >90% of orexin neurons and reduced CSF orexin (999, 1284). | Idiopathic narcolepsy-cataplexy in humans. | |
| Partial (33%) loss of orexin neurons (1285). | Idiopathic narcolepsy without cataplexy in humans. | |
| Mutation in preproorexin (999). | Early onset narcolepsy in humans. | |
| Orexin postnatal genetic (ataxin-3) lesion (96, 479) | Narcolepsy with cataplexy in mice and rats. | |
| Knockdown of orexin in PFH with siRNA (221). | REM sleep during dark period in rats↑. | |
| Orexin receptor 1 KO mice (640). | Mild effects on sleep-wake. No cataplexy or sleep-onset REM episodes. | |
| Orexin receptor 2 KO mice (1415). | Milder form of narcolepsy-cataplexy (less cataplexy or sleep onset REM episodes). | |
| Orexin receptor (1 and 2) antagonist (140). | Increased sleep, especially REM sleep in rat, dogs, and humans. No cataplexy. | |
| Melanin concentrating hormone (MCH) | MCH knockout mice (5). | NREM and REM sleep ↓. |
| Perifornical hypothalamus | MCH 1 receptor antagonist (12) | |
| Histamine (tuberomammillary nucleus) | Reduced CSF histamine in narcolepsy and idiopathic hypersomnia (597). | Excessive daytime sleepiness in humans. |
| HDC (synthetic enzyme) knockout mice (29, 978). | No change in 24 h wake amount. Increased fragmentation. Decreased θ-power during waking increased δ during sleep. Increased REM during light period. Decreased sleep latency in novel environment. | |
| α-FMH (histamine decarboxylase inhibitor) in cat (724), mice (978), and rat (551, 1152). | Wake ↓ (cat, mice). No effect on 24 h values (rat). | |
| Systemic H1R antagonists crossing the blood-brain barrier: human (1402), cat (724), mice (528). | Reduced wakefulness and alertness ↓ (human, cat). No change (mice), decreased fragmentation. | |
| H1R knockout mice (528) | No change in 24 h wake amount or diurnal rhythms of sleep-wake. Decreased fragmentation. | |
| Systemic H3R (autoreceptor) agonist in cat (725), rat (686, 885) | Cat: wake ↓ NREM sleep↑ | |
| Rat: no effect. | ||
| Mammillary body (MB) | Pharmacological inhibition: local anesthetic procaine (639). | Abolition of hippocampal theta in urethane-anesthetized animals. Reduced frequency (1 Hz less) of hippocampal theta in awake animals. |
| Supramammillary nucleus (SuM)/posterior hypothalamus | Pharmacological inhibition local anesthetic procaine (639). | Abolition of hippocampal theta in urethane-anesthetized rats. Reduced frequency (1 Hz less) of hippocampal theta in awake rats. |
| Basal forebrain | ||
| Rostral basal forebrain (MS, vDB) | Electrolytic lesions rabbits (30, 1090). | Hippocampal theta rhythm reduced (neurotoxic) or abolished (electrolytic). |
| Neurotoxic lesions: rat: kainic acid (preferential loss of noncholinergic neurons) (1463), Orexin-saporin (415). | No other change in sleep-wake. | |
| Rostral BF cholinergic | Pharmacological: AP5 (NMDA receptor antagonist) (104); muscimol (106); procaine(945). | Reduced power of hippocampal theta. |
| Neurotoxic lesion IgG192 saporin or orexin-saporin (80, 415, 600, 697, 1463). | Reduced amplitude of hippocampal theta rhythm and theta-gamma coupling. No change in sleep-wake. | |
| Rostral BF GABAergic (mainly PV-Pos) | Pharmacological inhibition of H-current with ZD7288 in rat (1343, 1446). | Reduced hippocampal theta (1446) or minor effects (1343). |
| Caudal basal forebrain (SI, HDB, MCPO) | Neurotoxic ibotenic acid or quisqualate (177, 611, 1064). | No effect on 24 h sleep-wake. Increased delta power in all states. Reduced recovery sleep and delta power after ±SD. |
| Caudal BF cholinergic | Pharmacological procaine (190), adenosine (1247). | Increased sleep, delta wave activity. |
| IgG192-saporin (94, 102, 103, 592, 611). | No or minor effects on baseline sleep-wake. Reduced EEG gamma. Reduced recovery sleep and delta power after sleep deprivation (611). | |
| Caudal BF, TMN, LC | Triple lesions using saporin-conjugated neurotoxins (101). | No changes in daily amounts of wake. More sleep during light-to dark transition period. More stable sleep architecture. |
| Forebrain | ||
| Thalamus | Electrolytic: monkey (1046). | No effect on sleep-wake or EEG except abolition of high-voltage spindles (sharp-wave/ripples). |
| Neurotoxic ibotenic acid in rat (177, 397). | ||
| Basal ganglia | Ibotenic acid lesion (1029). | Rostral striatum: |
| Wake 15 %↓ | ||
| Sleep fragmentation, slowing of EEG during waking (theta→delta). | ||
| Globus pall.: wake 46 % ↑ Increased fragmentation. | ||
| Slowing of EEG. | ||
| NAcc core: wake 27 % ↑ NREM bout duration ↓. | ||
| Slowing of EEG. | ||
| STN: minor changes. | ||
| SNr: minor changes. | ||
| Substantia nigra | Hypocretin-2 saporin (412). | Insomnia. |
| SN/VTA | NMDA lesion (683). | No decrease in wakefulness. |
| Neurotransmitters/neuromodulators (Systemic or icv effects) | ||
| Acetylcholine | Systemic muscarinic antagonists (177, 555, 744). | Increase in EEG delta waves. Increased high-voltage spindles (sharp waves/ ripples). Block of PGO waves. |
| M2/M4 double knockouts (434). | No effect on sleep-wake | |
| M3 receptor knockouts (434). | REM 22% ↓ | |
| Rats reared on a diet lacking choline (1256). | Reduced NREM and REM sleep | |
| Serotonin | Depletion of serotonin (363, 555, 582, 1080). | Increased PGO waves in all states of sleep-wake. |
| Norepinephrine | Dopamine-β-hydroxylase knockout mice (539, 956). | Either no change in baseline sleep-wake or decrease in REM. Shorter sleep latency after mild stress. |
FIGURE 2.
Location of brain nuclei controlling the sleep-wake cycle (see sects. II–IV) in sagittal (A) and coronal (B) schematics of the rat brain. Location of sections in B are represented as vertical dashed lines in A. Medulla oblongata: DPGi, dorsal aspect of the paragigantocellular reticular nucleus; GiV, ventral gigantocellular nucleus. Pons/midbrain: DR, dorsal raphe nucleus; LC, locus coeruleus; LDT, laterodorsal tegmental nucleus; LPB, lateral parabrachial nucleus; LPT, lateral pontine tegmental region; MPB, medial parabrachial nucleus; MR, median raphe nucleus; PB, parabrachial nucleus; PnC, nucleus pontine caudalis; PnO, nucleus pontine oralis; PPT, pedunculopontine tegmental nucleus; SubCA, subcoeruleus nucleus, alpha; SubCD, subcoeruleus nucleus, dorsal; SubCV, subcoeruleus nucleus, ventral; SN, substantia nigra; vlPAG, ventrolateral aspect of the periaqueductal gray; VTA, ventral tegmental area; VTG, ventral tegmental nucleus of Gudden. Hypothalamus: DMH, dorsomedial nucleus of the hypothalamus; LH, lateral hypothalamus; MM, medial mammillary nucleus; MnPO, median preoptic nucleus; MPO, medial preoptic nucleus; PeF, perifornical region of the hypothalamus; PH, posterior hypothalamus; PO, preoptic region (including VLPO); SCN, suprachiasmatic nucleus; SUM, supramammillary nucleus; TMN, tuberomammillary nucleus; VLPO, ventrolateral preoptic nucleus. Forebrain: BF, basal forebrain (including HDB, horizontal limb of the diagonal band; MCPO, magnocellular preoptic nucleus; SI, substantia innominata; VP, ventral pallidum); AMY, amygdala; CPu, caudate putamen; GP, globus pallidus. Thalamus: CM, thalamic centromedial nucleus; LGN, lateral geniculate nucleus; PV, thalamic paraventricular nucleus; RE, nucleus reuniens; RT, thalamic reticular nucleus VL, thalamic ventrolateral nucleus; VM, thalamic ventromedial nucleus; VMPO, ventromedial preoptic nucleus. Hippocampus: CA1, CA1 region of the hippocampus; CA3, CA3 region of the hippocampus; DG, dentate gyrus of the hippocampus. Neocortex: AC, anterior cingulate cortex; IL, infralimbic cortex; PrL, prelimbic cortex. [Adapted from Paxinos and Watson (989), with permission from Elsevier.]
B. Control of Sleep Timing and Intensity
The timing, depth, and duration of sleep are controlled by the interaction of time of day (circadian control, process C) and by the duration of prior wakefulness (homeostatic control, process S) as proposed in the two-process model of Borbely (122). The cellular mechanisms in the suprachiasmatic nucleus (SCN) which generate circadian rhythms are not covered herein, since they have been reviewed extensively elsewhere (1263). The output pathways from the SCN that control the circadian timing of NREM and REM sleep are covered in sections III and IV. Homeostatic control of sleep is also covered in these sections.
C. Effects of Sleep Loss on Cognition
An important function ascribed to sleep is offline processing of information encountered during the day and consolidation of memory. Conversely, loss of sleep, either voluntarily or due to an underlying medical disorder, is associated with substantial impairments in cognitive function. The mechanisms underlying these impairments are discussed in Section V.
D. Sleep Ontogeny
Sleep is the predominant behavioral state in developing animals (645, 1070), and REM sleep is proportionally more abundant in young mammals (1070). As such, sleep, in particular REM sleep, has been suggested to play an important role in the elaboration of neuronal circuitry during development (1070). The circuitry controlling sleep and wakefulness appears to mature early in development (606), although cycling between states is more frequent in younger animals (111, 645). EEG signs of sleep and wakefulness do not become “adultlike” until the later full development of the cortex (110, 377, 585, 1144). In fact, in humans, the development of fast EEG synchrony typical of wakefulness continues through adolescence, reflecting the prolonged maturation of the cortex in higher primates (1318).
E. Sleep Phylogeny
A form of NREM sleep appears to be present in most animals investigated to date (185, 1478), which is one of the arguments in favor of sleep performing a vital function (1174). A distinct REM sleep state only appears in mammals, although a primitive form is evident in reptiles and birds (1175). Sleep physiology is adapted to the particular features of different animals. For example, dolphins and other cetaceans exhibit unihemispheric sleep (1174). The distribution of the durations of sleep bouts in mammals is exponential with time scales that vary across species from mice to humans that are proportional to body mass and metabolic rate, indicating a connection with energy metabolism (742, 1174; discussed more fully in sect. III). Whereas most early animal studies of sleep used cats, dogs, and rats as experimental subjects, more recently there has been an explosion of interest in using more genetically tractable organisms to identify and study the genes and proteins involved in controlling sleep (1478). This work is reviewed in section VI. Interestingly, this work suggests that even organisms such as the fly Drosophila melanogaster (492, 1159) and the worm Caenorhabditis elegans (1042) have a “rest state” with similarities to mammalian sleep. Furthermore, several homologs of genes controlling rest in these species play a role in the control of mammalian sleep (230).
F. Sleep Disorders
Polysomnographic recordings are used not only in experimental studies but also in clinical sleep laboratories to identify sleep disorders such as sleep apnea and narcolepsy which involve a dissociation and fragmentation of waking, NREM, and REM (780). Disorders of sleep and the brain mechanisms that underlie them are discussed in section VII.
II. WAKEFULNESS
A. Electrographic Signs of Wakefulness
Synchronized electrical activity in large numbers of cortical neurons provides the basis for observable extracellular field potential changes in the EEG. Summed synaptic currents from the apical dendrites of pyramidal neurons are the main contributors to these EEG waves, although intrinsic membrane properties and neuronal firing also contribute (178). Faster frequency EEG rhythms (LVFA) typical of wakefulness and REM sleep are of low amplitude and involve synchronized activity in small, functionally interrelated areas. Lower frequency rhythms such as the theta rhythm occur over more widespread areas and synchronize faster, locally generated fast rhythms (beta/gamma). These EEG rhythms are thought to provide a temporal framework for higher-order brain functions such as attention, memory formation, and conscious awareness by binding together the firing of neurons within cortical areas and by synchronizing cortical and subcortical sites (178, 1238). During quiet or drowsy wakefulness, the slower EEG frequencies become more prevalent. Alpha rhythms appear in posterior cortical recordings whilst theta rhythms increase in frontal cortical regions.
1. Gamma/beta rhythms (15–120 Hz)
Low-amplitude gamma (30–120 Hz) and beta (15–30 Hz) frequency rhythms are a prominent feature of the EEG during quiet waking (baseline or spontaneous gamma; FIGURE 1) and are enhanced in particular cortical areas following presentation of sensory stimuli (evoked or steady-state gamma). Gamma rhythms often occur concurrently with theta rhythms during active waking and during REM sleep (187, 739, 880), particularly following phasic REM periods with PGO wave activity (26). Gamma rhythms also occur during the brief upstate of the slow oscillation during NREM sleep (see sect. III) (1219). In some studies, gamma rhythms have been subdivided into two frequency bands: low gamma (30–70 Hz) and high gamma (7–120 Hz), which arise in different cortical layers and have different pharmacological modulation properties (35, 950). We here primarily discuss low gamma. Gamma rhythms are generated by cortical networks of fast-spiking [especially parvalbumin (PV)-Pos] interneurons targeting the cell bodies of glutamatergic neurons (FIGURE 3). Rhythmic inhibition and disinhibition of the pyramidal neurons are responsible for the observed field potentials with rate being set by the decay time of the inhibitory synaptic currents. In turn, the interneurons are driven by excitatory input from the pyramidal neurons. Synchrony is enhanced by electrical synapses mediated by gap junctions between interneuronal networks and between the axons of pyramidal neurons as well as by interneuron-interneuron chemical synapses (1405).
FIGURE 3.
Simplified model of cortical circuitry generating gamma oscillations. Cortical circuits consist primarily of excitatory pyramidal neurons and inhibitory GABAergic interneurons. Inhibitory drive generated by interneurons plays an important role in the generation of oscillatory output. Fast spiking interneurons containing parvalbumin (PV) that synapse onto the cell bodies of pyramidal neurons are particularly important in generating gamma oscillations. Recurrent glutamatergic synapses onto GABAergic interneurons provide excitatory drive to the fast spiking interneurons. Both chemical and electrical synapses between PV-positive interneurons enhance synchrony and the coupling of gamma rhythms to theta rhythms.
Gamma rhythms are generated locally in the neocortex but are modulated by subcortical inputs. The ability to elicit gamma rhythms in isolated brain slices in vitro (163, 366, 1407), together with current-source density and cross-correlational analysis in vivo (26, 1218), suggests that gamma rhythms are generated locally in the cortex. However, their dependence on behavioral state and stimulus presentation indicates that their occurrence is also dependent on subcortical inputs. In fact, gamma rhythms are enhanced by stimulation of the mesencephalic reticular formation, the origin of the ascending reticular activating system (502, 903). Further information on the subcortical control of gamma rhythms is provided in section IIC.
Fast-spiking interneurons containing PV generate gamma rhythms. Evidence supports the conclusion that beta and gamma rhythms are generated by GABAergic interneurons, in particular fast-spiking, PV GABAergic interneurons which synapse on the cell bodies and axon initial segments of pyramidal neurons. 1) In vivo, fast spiking interneurons discharge at gamma frequency and their firing is phase-locked to the extracellularly recorded oscillation (133, 1026, 1311). 2) In vitro, gamma and beta rhythms are completely blocked by GABAA receptor antagonists (366, 1407), and gamma frequency is inversely correlated with the decay time constant of inhibitory synaptic currents (1406). 3) Optogenetic stimulation of PV neocortical interneurons in vivo (via genetic introduction of bacterial light-activated ion channels) can elicit gamma rhythms, whereas optogenetic inhibition reduces gamma (193, 1194). 4) Wavelet analysis of local field potentials in the CA3 region of the hippocampus combined with simultaneous intracellular recordings from pyramidal neurons during cholinergically induced gamma rhythms revealed that perisomatic inhibitory currents generated the majority of the field potential (955). 5) In human visual cortex, GABA concentration measured by magnetic resonance spectroscopy predicts peak gamma frequency and orientation discrimination performance (331, 906). 6) Gene linkage analysis indicates significant linkage between the beta frequencies of the human EEG and GABAA receptor genes (1015).
PV knockout mice have enhanced gamma oscillations (1379), suggesting PV itself may not be required, although developmental compensation may have taken place. Alterations in PV neurons may be responsible for dysfunctional gamma rhythms in schizophrenia and other disorders that are associated with cognitive abnormalities (1319, 1428).
A) Beta Oscillations
Beta frequency EEG oscillations (15–30 Hz) are thought to represent one or more of the following: 1) a slow gamma oscillation, 2) a subharmonic of ongoing gamma whereby inhibitory neurons fire at gamma frequencies but some excitatory neurons remain refractory for longer periods so that they only fire on a proportion of the gamma cycles, or 3) a rhythm with its own distinct underlying properties (653). Computational modeling suggests that beta rhythms are more effective than gamma rhythms in synchronizing activity between spatially distant brain loci (653).
2. Alpha rhythms (8–14 Hz)
The two most well-known alpha rhythms in humans are the occipital alpha rhythm which dominates the EEG during relaxed wakefulness (FIGURE 1) and the Rolandic mu rhythm observed over somatosensory cortex in the absence of movement (536). Occipital α rhythms were one of the first described EEG rhythms (7, 92). They are commonly observed during relaxed wakefulness in parietal and occipital cortex areas including primary visual cortex and are suppressed by eye opening and visual stimuli (961). Alpha rhythms may play an important role in internally directed thought processes since they are strengthened during tasks requiring mental arithmetic and visual imagery (1052).
A) Thalamocortical Mechanisms Generating Alpha Rhythms
The mechanisms underlying the generation of alpha rhythms were little understood until recently (536). Alpha rhythms result from an interaction of thalamic and neocortical circuitry, together with a moderate level of brain stem cholinergic input. At the level of the visual cortex, alpha waves are due to a dipole located at the level of the cell bodies of pyramidal neurons in layer V and basal dendrites of pyramidal neurons in layers IV where thalamic input terminates (747, 748). At the level of the thalamus, the firing of two groups of thalamocortical relay neurons in the lateral geniculate nucleus are suppressed at either the positive or negative peak of the alpha rhythm through phasic inhibition. For the occipital alpha rhythm, local lateral geniculate GABAergic interneurons excited by high-threshold bursting thalamocortical neurons are important in periodically silencing thalamocortical neurons, whereas for the mu rhythm the GABAergic reticular nucleus may fulfill this role.
In vitro work in the cat thalamus suggests that alpha rhythms require stimulation of muscarinic cholinergic receptors (mimicking brain stem input) or stimulation of metabotropic glutamate receptor stimulation (mimicking cortical input). Stimulation of these receptors leads to depolarization and the generation of an after depolarizing potential (ADP) in the gap junction-coupled network of high-threshold bursting thalamocortical neurons (537, 751, 752), leading to synchronized firing. Concerning these two mechanisms, in vivo microdialysis experiments suggest that the brain stem muscarinic input is more important (752). Interestingly, the number of spikes in a burst and the interburst frequency (2–14 Hz) are dependent on the level of muscarinic receptor activation so that the transition from alpha to the slower theta frequency waves in early (light) sleep or drowsy wakefulness may reflect a gradual withdrawal of brain stem cholinergic input (536, 537).
3. Theta rhythms (4–8 Hz)
Theta-rhythms occur prominently during waking associated with movement in rodents, during tasks requiring attention/ memory in humans, and during REM sleep in all mammals (FIGURE 1). They provide a temporal code for pyramidal/granule cell firing important for spatial navigation and episodic memory formation and facilitate synaptic plasticity (178, 587, 1362). In rodents and other lower mammals, very regular theta rhythms, also called rhythmic slow activity, have been studied most closely in the hippocampus and related temporal lobe structures, which in these species are located close to the dorsal surface of the brain (FIGURE 2) and strongly influence the EEG signal during movement and REM sleep. In humans, where the temporal lobe is located ventrally, theta rhythms are recorded and studied mainly in frontal and midline cortices that are part of the default network. Interestingly, in both animals and humans, theta-band activity increases strongly in frontal-midline areas during the course of sleep deprivation and is correlated with sleep drive (365, 1386). However, this theta activity is less regular than that generated by the hippocampus and may result from different mechanisms. Here, the mechanisms underlying hippocampal theta are discussed first followed by mechanisms that may be involved in human frontal-midline theta.
The medial septum drives hippocampal theta. A major afferent input to the hippocampus arises in the rostral basal forebrain (medial septum, vertical limb of the diagonal band; MS/vDB, FIGURE 2) via the fimbria-fornix. Withdrawal of this input by lesions, pharmacological inactivation or transection completely abolishes hippocampal theta rhythm (30, 106, 445, 1090). MS/vDB neurons fire rhythmically in phase with theta rhythm (997, 998). Thus the MS/vDB is thought to be a pacemaker for hippocampal theta (FIGURE 4). Selective lesion of MS/vDB cholinergic neurons reduce the amplitude but do not change the frequency of hippocampal theta (697). In contrast, kainic acid lesion of the MS/vDB, which largely spares cholinergic neurons but kills PV GABAergic projection neurons, and likely other noncholinergic neurons, eliminated hippocampal theta (1463). In vivo, single-unit recordings from identified PV neurons reported bursts of action potentials at theta frequency which are synchronized with the ongoing hippocampal theta activity (125, 1182). Within the burst, action potential firing rates are at gamma frequencies, providing an explanation of the phase-locking of gamma rhythms to theta rhythms. Two populations of PV MS/vDB neurons fired anti-phasically, i.e., one population fired at the peak of hippocampal theta, whereas the other fired at the trough (125). In contrast to the GABAergic neurons, slow-firing cholinergic neurons fired only single action potentials in synchrony with theta rhythm (1182). Together these data suggest that PV GABAergic MS neurons are crucial pacemakers for hippocampal theta, whereas cholinergic neurons modulate the amplitude. MS/vDB PV projection neurons selectively innervate the PV hippocampal interneurons (basket and chandelier neurons) responsible for controlling firing of principal neurons (385). Thus hippocampal theta rhythm is (at least partially) generated by rhythmic inhibition and disinhibition of hippocampal pyramidal and dentate granule cells (176). In addition, rhythmic input from the entorhinal cortex plays a large role in the observed variation in extracellular potential (176).
FIGURE 4.
A simplified structural model of hippocampal theta rhythm control. Tonic neuronal activity of the reticular formation, largely originating in the nucleus pontine oralis (PnO), excites the supramammillary nucleus (SUM) by means of glutamatergic projections. Pontine tonic activity is converted to rhythmic firing in SUM, indicated by the wave symbol. Glutamatergic SUM output then excites GABAergic and cholinergic neurons of the medial septum/vertical limb of the diagonal band (MS/vDB), which serves as the pacemaker of the hippocampal theta rhythm.
A) Brain Stem Control of Theta Rhythm
The ascending pathways from the brain stem which generate theta rhythm are still an active area of investigation (1365). Precise mapping studies by Vertes and colleagues (1356, 1365) revealed that the most effective brain stem stimulation sites for theta generation are located within the nucleus pontis oralis (PnO) region of the brain stem reticular formation (FIGURE 2). Extracellular single- unit recordings in the PnO of freely moving rats identified cells that fire in association with states when theta rhythm is present. However, these cells did not fire rhythmically, but fired tonically at high rates (60–100 Hz) (935, 1355). Thus these neurons are unlikely to be involved in coding the frequency of hippocampal theta rhythm.
Tonic brain stem input is converted into rhythmic firing in the supramammillary nucleus (FIGURE 4). Although it was originally assumed that tonic firing in the reticular formation is translated into rhythmic firing in the MS/vDB, anatomical tracing studies revealed that few neurons in the reticular formation project directly to the MS/vDB (1357, 1359). Thus at least one additional nucleus is likely interposed between these two areas. Anatomical tracing and physiological mapping studies using the local anesthetic procaine suggested that MS/vDB projecting, glutamatergic neurons containing the calcium-binding protein calretinin in the supramammillary nucleus (SuM; FIGURE 2) may fulfill this role (638, 710, 1360, 1366) (FIGURE 4). Single-unit SuM recordings in urethane-anesthetized animals reported single spike or rhythmic burst firing phase locked with hippocampal theta (105, 637, 639, 649). Rhythmic SuM firing is not due to descending inputs from the septum or hippocampus since it was not altered by inactivation of the MS/vDB with the local anesthetic procaine (639). However, SuM procaine injection blocked the ability of PnO stimulation to elicit theta (638). Thus it was proposed that the SuM translates tonic firing of the reticular input into phasic bursting at the frequency of hippocampal theta (636). However, in contrast to experiments in urethane-anesthetized animals, in freely moving animals procaine causes only a small reduction in the frequency of hippocampal theta (845, 1286), suggesting that additional pathways are involved. The precoeruleus region of the pons (758), located just rostral to the locus coeruleus, provides the major brain stem glutamatergic input to the MS/vDB. In addition, the nucleus incertus of the medulla (933) projects to the MS/vDB and SuM regions. Although these areas have been implicated in brain stem theta generation, rhythmically firing neurons have not been recorded in these regions. Thus they may relay the activity of the reticular formation (especially PnO) to the MS/vDB and SuM. In contrast, the GABAergic ventral tegmental nucleus of Gudden, located just ventral to the dorsal raphe, contains intrinsically bursting neurons (155) which fire rhythmically at theta frequencies during waking and REM sleep and may generate theta rhythmicity in the limbic Papez circuit through their interconnections with the medial mammillary body (81, 648).
B) Theta Rhythms in Humans
In humans, where the hippocampal formation and temporal formation are located ventrally, theta oscillations are most commonly recorded just anterior to the Fz electrode site over frontal and midline cortices (prefrontal and anterior cingulate) (862). Mechanisms that may be responsible for generating this frontal-midline theta (FM-theta) are as follows: 1) FM-theta may be generated through direct or indirect projections from the hippocampal formation, synchronizing information flow between hippocampus and neocortex (1184). However, FM-theta is not always coherent with hippocampal theta (862). 2) FM-theta may represent a slow alpha-rhythm generated by thalamocortical loops during drowsiness (see preceding section). 3) FM-theta may be generated by pacemaker GABAergic (PV-Pos), cholinergic, and glutamatergic projections from the caudal basal forebrain (BF). GABAergic PV-Pos and cholinergic neurons in the caudal BF show similar firing patterns (321, 481, 698) and projections to interneurons and pyramidal neurons in the neocortex (386) as their counterparts in the rostral BF which project to the hippocampus. Furthermore, the firing of ensembles of noncholinergic BF neurons are correlated with prefrontal cortex field potentials (729). Further research is required to determine the contribution of these three mechanisms to FM-theta. They are not mutually exclusive, and any of them may contribute under different conditions.
B. Brain Stem Reticular and Basal Forebrain Activating Systems
The work of a handful of researchers in the first half of the 20th century allowed the development of current ideas of how LVFA typical of wakefulness and REM sleep is generated. Frederic Bremer (136) found that transection of the brain of cats at the midcollicular level (“cerveau isole” preparation) led to “sleeplike” behavior and slow waves in the cortex. In contrast, transection at the junction of the brain stem and spinal cord (“encephale isole” preparation) did not alter the normal cyclic alternation of sleep-wake states and demonstrated that sensory input from the spinal cord was not necessary for wakefulness to occur. Later work by Giuseppe Moruzzi and Horace Magoun showed that electrical stimulation of the midbrain reticular formation in anesthetized cats caused the appearance of an “activated” EEG similar to that seen during waking (898). Together these findings led to the important concept of the “ascending reticular activating system (ARAS),” a network (reticulum) of nerve fibers ascending from the brain stem, which through multiple intermediary sites causes activation of the forebrain during waking and REM sleep (FIGURE 5). The activity of brain stem reticular neurons, the origin of both of these pathways, reliably predicts the onset of changes in behavioral state (818, 1228, 1232, 1236).
FIGURE 5.
Dorsal and ventral pathways of the ascending reticular activating system (ARAS). The dorsal pathway (blue) originates in pontine and midbrain reticular formation, most prominently cholinergic (LDT/PPT) and glutamatergic neurons which project to the “nonspecific” intralaminar and midline thalamic nuclei which diffusely innervate many areas of the cerebral cortex as well as thalamic relay neurons with more selective projections patterns. The ventral pathway also originates in pontine/midbrain regions and projects to the lateral hypothalamic (LH) and tuberomammillary (TMN) nuclei of the hypothalamus, as well as the basal forebrain (BF). Output of LH and TMN also ascend to BF, which in turn projects to the cortex. Noradrenergic neurons of the locus coeruleus (LC) and serotonergic neurons in the dorsal raphe (DR) contribute to both pathways and send direct projections to the cortex as do histamine neurons of the TMN and orexinergic neurons in the LH. LDT, laterodorsal tegmental nucleus; PPT, pedunculopontine nucleus. [Adapted from Paxinos and Watson (989), with permission from Elsevier.]
The ARAS consists of dorsal and ventral pathways (FIGURE 5). Axonal tracing studies coupled with histochemical or immunohistochemical visualization of particular neurotransmitter systems revealed the anatomical pathways transferring brain stem activity to the cerebral cortex (573, 1228). Single-unit recordings and indirect measures of neuronal activity (using immunohistochemical detection of the immediate early gene product Fos) defined the neurons in these areas whose activity is correlated with wakefulness or sleep (575, 1228). Two main pathways have been identified (FIGURE 5).
1. The dorsal pathway of the ARAS (Figure 5)
This comprises midbrain, pontine, and medullary reticular formation glutamate neurons (261, 916, 1227, 1235) and cholinergic neurons in the pedunculopontine and laterodorsal tegmental nuclei (PPT/LDT) (473, 1235) which innervate the midline and intralaminar (nonspecific) thalamocortical projection system (paraventricular, parataenial, intermediodorsal, centrolateral, paracentral, centromedial, rhomboid, reuniens, centromedian and parafascicular thalamic nuclei). These thalamic nuclei project to widespread and overlapping neocortical areas (559, 578, 750, 970, 1203), although each nucleus has some selectivity in their density of projections to their neocortical targets (1327). Stimulation of the nonspecific nuclei yields widespread cortical responses and elicits fast cortical rhythms (478, 559, 891), while electrical stimulation in sensory relay nuclei elicits short responses in local areas of sensory cortex. In addition to thalamic projections, the brain stem cholinergic (LDT/PPT) neurons also innervate the dopaminergic and GABAergic neurons of the midbrain ventral tegmental area of Tsai (260), which are involved in reward processes and project prominently to the nucleus accumbens and prefrontal cortex. Surprisingly, experiments in rodents (177, 397) and cats (1370) showed that very large lesions of the thalamus appear to have very little effect on cortical activation and the sleep-wake cycle in general, aside from a loss of sleep spindles, suggesting that the dorsal pathway is not absolutely necessary. However, a complete and selective ablation of thalamus is hard to achieve with lesion techniques, and it remains possible that a small thalamic projection remained after these lesions which was sufficient to maintain function. These findings in animals are also seemingly at variance with human studies of coma patients (see sect. VII) and imaging studies of sleepwake and anesthesia which suggest that changes in reticular and thalamic function precede changes in the cortical EEG (66, 152, 928). At the very least, one can conclude that under normal conditions, the dorsal pathway is involved in and shapes cortical activation.
2. The ventral pathway of the ARAS (Figure 5)
This comprises fibers of the medial forebrain bundle which pass through and make contact with neurons in the midbrain, posterior/lateral hypothalamus, and basal forebrain (BF) on the way to the cortex. The ascending fibers from the brain stem include glutamatergic (parabrachial), noradrenergic (locus coeruleus, LC), serotonergic (dorsal and median raphe), and dopaminergic (periaqueductal gray) neurons. These systems synapse onto glutamatergic, histaminergic, and orexinergic/ hypocretin neurons in the posterior/lateral hypothalamus (575). All of these systems converge onto caudal BF cholinergic, GABAergic, and glutamatergic neurons which project to and activate the neocortex (305, 316, 573, 1148, 1228). A branch of this system innervates the rostral BF theta rhythm generator.
In contrast to the thalamic lesions discussed above, a recent study showed that large lesions of the BF, or of the brain stem parabrachial nucleus (PB), which provides the major brain stem glutamatergic input to the BF, led to a comatose state in rats (397), whereas, as discussed above, thalamic lesions had little effect. However, it is important to note that in this study, orexin-saporin was used to lesion the BF and PB, whereas ibotenate was used to lesion the thalamus; thus the two experiments are not directly comparable. Orexin-saporin is a relatively new lesioning tool that requires further study. In particular, it is important to determine if very large lesions, resulting in widespread neuronal death, also affect fibers of passage.
Direct projections to the cortex and the nonspecific thalamic nuclei also arise from brain stem noradrenergic and serotonergic neurons as well as the hypothalamic histaminergic and orexinergic/hypocretin neurons. In section IIC, we discuss the role of the different components of the ARAS, subdivided according to neurotransmitter phenotype.
3. Default network
One novel finding from human imaging studies is the existence of a so-called “default network” of functionally interconnected cortical regions that are active when individuals are left to think to themselves and are not involved in responding to the external environment (1040). Anatomically, the default network consists of regions along the anterior and posterior midline, the lateral parietal cortex, prefrontal cortex, and temporal lobe (33, 1040). Upon presentation of external stimuli requiring a response, the default network regions show a decrease in activity, in contrast to other cortical areas that show increases or no change. Thus, while animal studies have often considered cortical activation as being fairly uniform throughout cortical regions, human imaging studies show that this is not the case. Future studies should distinguish how the ascending systems controlling the default network differ from those affecting other cortical areas.
C. Neurotransmitter Systems Promoting Wakefulness
Multiple neurotransmitter systems contribute to the promotion of wakefulness. However, none of them appears to be absolutely essential. In this section we describe the effects of inactivation or stimulation of these systems, the mechanisms by which they act, and their possible function during wakefulness.
1. Acetylcholine
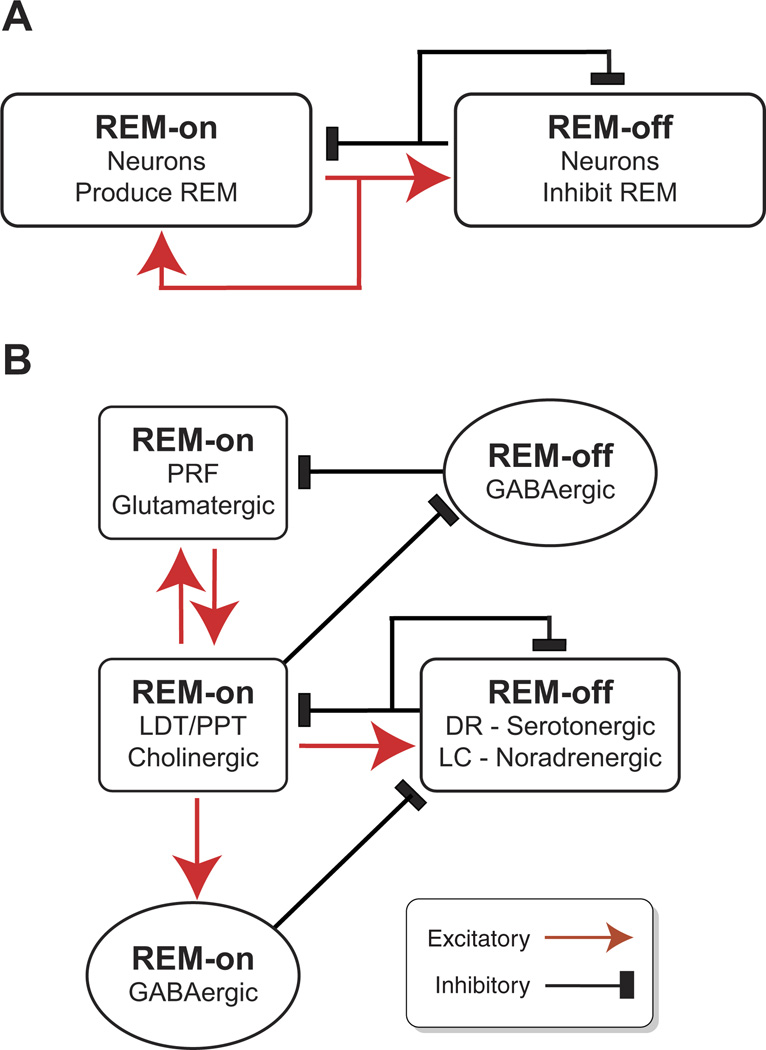
The cholinergic system promotes high-frequency oscillatory activity typical of wakefulness and REM sleep. The BF cholinergic system has an additional role in the homeostatic sleep response to prolonged waking (discussed more fully in sect. III). The important role of brain stem cholinergic neurons in REM sleep control is discussed in section IV.
Neurons involved in sleep-wake control that release acetylcholine are located in the BF and in the mesopontine tegmentum (LDT/PPT) of the brain stem (44, 849) (Figs. 2 and 6). Identified, cortically-projecting cholinergic neurons in the caudal BF (substantia innominata, horizontal limb of the diagonal band, magnocellular preoptic area, nucleus basalis) fire fastest during both wakefulness and REM sleep (481, 698), and their firing is correlated with cortical activation (321, 698, 789). In particular, caudal BF cholinergic neurons fire bursts of spikes in association with neocortical theta rhythms (698). Rostral BF (MS/ vDB) cholinergic neurons projecting to the hippocampus also fire in association with hippocampal theta rhythm but fire only single spikes per cycle (1182). Wake/ REM-on neurons have also been recorded in cholinergic brain stem areas although, to date, the firing of identified brain stem cholinergic (LDT/PPT) neurons projecting to the thalamus has not been recorded across the sleep-wake cycle. In urethane-anesthetized animals, identified brain stem cholinergic neurons fire in association with cortical activation produced by tail pinch (126). Consistent with the firing patterns of cortical and thalamic-projecting cholinergic neurons, acetylcholine levels are highest in these areas during wakefulness and REM sleep (200, 560, 1412). Thus increased activity of both brain stem and BF cholinergic systems is associated with states when cortical activation and conscious awareness occur (572, 993, 1429).
FIGURE 6.
Functional interactions between wake-promoting neuromodulatory systems projecting to the cortex. Wake-promoting neuromodulatory systems are interconnected mainly in a mutually excitatory network. Cholinergic (ACh) basal forebrain, orexinergic (OX) lateral hypothalamic, serotonergic (5–HT) raphe, noradrenergic (NA) locus coeruleus, and histaminergic (HA) tuberomammillary neurons all interact to promote wakefulness. Thus, if one region is experimentally lesioned, other systems remain and maintain cortical activation and wakefulness. The main exceptions to this pattern are inhibitory serotonergic and norepinephrine projections to cholinergic and orexin neurons. Cortically projecting glutamatergic and GABAergic systems are also important in cortical activation and wakefulness (see text). Note: adrenergic projections to the histamine neurons act by disinhibition (inhibition of GABAergic synaptic inputs), whereas other effects shown are postsynaptic. Receptors involved are as follows: α1, alpha adrenergic type 1; α2, alpha adrenergic type 2; H1, histaminergic type 1; M3, muscarinic cholinergic; OxR1, orexin receptor type 1; OxR2, orexin receptor type 2.
BF cholinergic neurons projecting to the neocortex promote LVFA. Caudal BF neurons affect electrographic activity via a direct projection to the cortex (305, 450, 500, 1115, 1148, 1431). Intracellular recordings from cortical neurons in vivo and in vitro have revealed a plethora of cholinergic effects that lead to increased excitability and a facilitation of fast EEG rhythms at the expense of slow oscillations typical of NREM sleep (827, 1216). Prominent muscarinic effects include the following: 1) a depolarization of pyramidal neurons via block of a leak potassium conductance (M-current) and activation of mixed cation channels; 2) facilitation of subthreshold oscillations in the beta/gamma range (20–40 Hz), and 3) blockade of slow after hyperpolarizations. Nicotinic actions include presynaptic facilitation of glutamate release (443) and depolarization of interneurons (20, 579). In vivo, application of agents which depolarize cholinergic neurons in vitro (22, 334, 375, 619) increases theta and gamma cortical activity, together with waking and REM sleep. In particular, the action of neurotensin is noteworthy, since it appears to be selective for cholinergic neurons (191). Conversely, application of serotonin, which hyperpolarizes BF cholinergic neurons (618), reduces gamma activity (189).
A) Cholinergic Elicitation of Fast Eeg Rhythms in vitro and in vivo
In vitro, application of cholinergic agonists causes theta and gamma/beta rhythms in isolated hippocampal (366, 534, 1165) or neocortical areas (107, 163, 950) and promote alpha or theta oscillations in thalamic relay nuclei such as the lateral geniculate nucleus (751, 752). The cholinergic neuromodulatory system is unique in this regard since only cholinergic or glutamatergic agonists have been shown to induce oscillatory activity in vitro. Early, in vivo studies in urethane or ether anaesthetized rats and rabbits established that one form of theta activity (type I theta, 4–7 Hz) was abolished by systemic administration of the muscarinic antagonist atropine sulfate (667). Both brain stem (LDT/PPT) cholinergic neurons projecting to the diencephalon and MS/vDB cholinergic neurons projecting to the hippocampus and neocortex promote theta activity. Infusion of the cholinergic agonist carbachol into the brain stem (PnO) or SuM/posterior hypothalamus increases hippocampal theta (635, 944, 1363) whilst selective lesion of MS/vDB cholinergic neurons reduces the amplitude of hippocampal theta (697). Muscarinic receptor blockade weakens the coupling between gamma and theta rhythms (501), suggesting that the enhanced acetylcholine release that occurs during waking and REM sleep promotes this coupling. Thus acetylcholine promotes the cortical rhythms typical of wakefulness and REM sleep and the coupling of gamma to theta rhythms.
B) Brain Stem Cholinergic Projections to the Thalamus
While BF cholinergic neurons promote cortical activation via a direct projection to the cortex, brain stem cholinergic neurons do so via their projections to the thalamus, comprising a major component of the dorsal ARAS pathway (FIGURE 5). Anterograde and retrograde tracing studies coupled with choline acetyltransferase immunohistochemistry revealed a massive cholinergic projection to the thalamus (291, 473, 474, 940, 974, 1193, 1235, 1430) which, depending on the thalamic region studied, make up 25–85% of the projection from all neurons in the pontine tegmentum. A minor cholinergic projection to the thalamus, especially the reticular nucleus and anterior nuclei, arises from BF (976).
Similar to BF cholinergic neurons, the firing of brain stem cholinergic neurons correlates with, and anticipates, cortical activation and deactivation (126, 336, 615, 1221). In vivo, electrical stimulation of brain stem areas containing cholinergic neurons enhances beta/gamma frequency firing in thalamocortical neurons and in the EEG (1226). In vitro, cholinergic agonists depolarize relay neurons via a muscarinic (M1/M3, Gαq G protein) receptor-mediated block of leak potassium conductance. This depolarization facilitates single-spike firing at the expense of the rhythmic bursting observed during NREM sleep (143, 826, 832). Acetylcholine directly depolarizes ventral tegmental area dopaminergic neurons via nicotinic receptors containing α4-α7 and α2 subunits (646, 1002), and by activation of muscarinic M1-like (probably M5) receptors (680, 1461), which increases burst firing (743) and facilitates dopamine release in target regions such as the nucleus accumbens (373).
In addition to acetylcholine, brain stem cholinergic neurons also release the gaseous neurotransmitter NO. In vitro, electrical stimulation of LDT produced NO (707), whereas in vivo studies showed that NO is released in the thalamus (1414) and medial pontine reticular formation (709) in relation to behavioral state. Administration of NO donors enhances neuronal activity in the thalamus and neocortex (265), while NOS inhibitors cause inhibition of thalamic cell activity. NO dampens the oscillatory activity of thalamocortical relay neurons by altering the voltage dependence of the hyperpolarization activated cation current, Ih (967).
C) Effects of Cholinergic Lesions
While electrical or pharmacological stimulation of cholinergic neurons is highly effective in stimulating LVFA, lesioning of brain stem or BF cholinergic neurons does not lead to pronounced changes in 24-h amounts of wakefulness. Selective lesioning of BF cholinergic neurons using the toxin 192IgG-saporin led to relatively minor changes in wakefulness (94, 611). However, high-frequency EEG power, especially gamma-activity, was strongly reduced with extensive lesions of caudal cholinergic BF neurons (94, 600) but was unchanged with less complete lesions (611, 1398, 1399). More consistently, IgG192-saporin lesions of MS/vDB cholinergic neurons reduced the amplitude of hippocampal theta rhythm (80, 415, 697, 1463). Lesioning of the cholinergic neurons reduced the homeostatic response to sleep deprivation, but again this required an extensive destruction of cholinergic neurons (102, 592, 611). Thus it appears that there is considerable redundancy in the cholinergic system, and effects are only seen with extensive lesions.
2. Serotonin
Overall, the evidence suggests that serotonin promotes a quiet waking state with reduced cortical activation. Serotonin also plays an important role in suppression of REM sleep (sect. IV) and in the response to stress, which may account for some aspects of stress-related sleep disorders (sect. VII).
Serotonin neurons are clustered in several nuclei along the midline of the brain stem in the raphe nuclei (FIGURE 2) (553). Early experiments where serotonin levels were depleted erroneously suggested that serotonin promotes sleep (581, 899). Recent experiments examining mice in which serotonin neurons are genetically deleted suggest that insomnia resulting from disruption of serotonin signaling was due to a disruption of thermoregulation, leading to an increase in motor activity to generate heat (162). In contrast to the early depletion experiments, recording of the electrical discharge of serotonin neurons (839, 1308) and measurements of serotonin release (1020, 1411) revealed that serotonin neurons are wake-active, suggesting that serotonin is wake-promoting. Neuronal firing decreased during NREM sleep and ceased during REM sleep. Accordingly, systemic application of serotonergic receptor agonists increases waking and reduces NREM and REM sleep (884). Serotonergic suppression of NREM sleep is likely due to a 5-HT1A receptor-mediated postsynaptic inhibition of sleep-active VLPO neurons (403), whereas the inhibition of REM sleep involves a postsynaptic inhibition of REM-on brain stem cholinergic neurons (130, 522).
A) Mechanisms by which Serotonin Promotes Wakefulness
Serotonin promotes waking via depolarization of histaminergic tuberomammillary neurons (344) and BF GABA neurons projecting to the hippocampus (23) and neocortex (154). Serotonin has complex effects on the thalamus. A direct depolarization of lateral geniculate neurons and other first-order thalamic relay neurons via a 5-HT7 receptor- mediated modulation of hyperpolarization-activated cation conductance was initially reported (205, 206, 829, 1342), an action which blocks spindle oscillations (696). However, most higher-order relay and nonspecific nuclei are inhibited by serotonin (877) via a combination of a direct 5-HT1A-mediated postsynaptic hyperpolarization and an indirect increase in inhibitory input due to depolarization of GABAergic thalamic reticular nucleus neurons (833). Sensory relay neurons may also be inhibited by serotonin (1071) through a depolarization of local interneurons (877, 969, 1109). However, serotonin also facilitates glutamate release from thalamocortical terminals via 5-HT2A receptors (10, 11), the main target of hallucinogenic drugs such as lysergic acid diethylamine (LSD) which act as partial agonists of this receptor (794). Serotonin blocks the slow after hyperpolarizations of intralaminar thalamic (430), hippocampal (1302), and neocortical pyramidal neurons (40, 1475) via activation of receptors coupled to stimulation of adenylyl cyclase (5–HT4/5-HT7), allowing the faster firing typical of wakefulness.
B) State-Dependent firing of Serotonin Neurons
Most serotonin neurons fire in a slow, tonic fashion across the sleep-wake cycle (839, 1308). However, a subpopulation also fires in bursts (471). In contrast to norepinephrine and histamine neurons, most serotonin neurons recorded in vitro do not fire action potentials spontaneously (1335). Thus afferent input from other wake-active systems is required to maintain their firing (158, 713, 1095, 1335). Serotonin neurons are depolarized by norepinephrine, histamine, and orexins via activation of a long-lasting inward current due to the opening of mixed cation channels (158, 735, 1335), likely of the transient receptor potential family (1151). Unlike the other wake-active neuromodulatory systems discussed here, serotonin neurons promote a state of quiet or relaxed waking; single-unit recordings report highest activity during feeding and decreased firing during active waking (554). Serotonin neurons are also activated by stress (476), and 5-HT1A knockout mice lack the rebound of REM sleep observed following the stress of immobilization (130).
C) Serotonin Inhibits Theta and Gamma Rhythms
Serotonin acts in opposition to the cholinergic system (FIGURE 6), inhibiting both BF (618) and brain stem cholinergic neurons (763, 1280), resulting in a blockade of fast rhythms (especially theta and gamma) promoted by activation of the cholinergic system. In particular, median raphe (MR) serotonergic neurons inhibit hippocampal theta rhythm (1365). Electrical or pharmacological stimulation of the MR abolishes theta rhythm in both anesthetized and unanesthetized rats (51, 629, 1356, 1451), whereas lesions or pharmacological inactivation of MR result in continuous theta (630, 1451). An involvement of serotonin in these effects was suggested by the following findings: 1) treating rats with p-chlorophenylalanine, resulting in a 60–80% depletion of forebrain serotonin, blocked the effects of MR electrical stimulation (51); 2) continuous theta in raphe lesioned animals could be interrupted by administration of the serotonin precursor l-5-hydroxytryptophan (1451); and 3) inhibition of MR serotonin neurons with 5-HT1A agonists generates theta rhythm in urethane-anesthetized rats (631, 1364). Similarly, serotonin inhibits caudal BF cholinergic neurons (618) and reduces EEG gamma activity (189).
3. Norepinephrine
Norepinephrine neurons are generally thought of as part of the central flight-or-fight response, being particularly important in waking associated with stressful situations. Norepinephrine also plays an important role in the maintenance of muscle tone during waking and suppression of REM sleep (see sects. IV and VII).
Norepinephrine neurons are located in small clusters throughout the brain stem (194). The most prominent noradrenergic innervation of the forebrain arises from the LC (FIGURE 2). It is this nucleus that has been studied most closely with respect to the sleep-wake cycle. LC neurons fire most rapidly during wakefulness and are activated further by stressful stimuli (1050), but their firing slows during NREM sleep and ceases prior to and during REM sleep (511). Norepinephrine strongly excites many neurons of the ARAS (FIGURE 6), mainly via α1 receptors, including thalamic relay neurons (829), serotonin dorsal raphe (DRN) neurons (9, 68, 158, 962, 1335), BF cortically-projecting cholinergic (375) and GABAergic neurons (154). Norepinephrine inhibits neurons in the sleep-active ventrolateral (403) and median preoptic nuclei (63), as well as REM-on brain stem cholinergic neurons (706, 1280), by acting on postsynaptic α2 receptors and activating an inwardly rectifying potassium conductance. β-Receptors inhibit slow calcium-dependent after hyperpolarizations of cortical pyramidal neurons, allowing the faster firing typical of wakefulness (463) and blocking the slow oscillations typical of NREM sleep (1220).
Studies utilizing neurotoxic or electrolytic lesions of the LC or norepinephrine system reported minor changes in the amount of wakefulness (100, 244, 576, 758, 881). However, depletion of norepinephrine using peripheral administration of the toxin DSP-4 reduced the expression of ~20% of waking-related gene transcripts, particularly those involved in synaptic plasticity and cellular stress responses (238, 241, 244). Studies of long-term potentiation implicate noradrenergic β-receptors in promotion of synaptic plasticity (519, 1202). Thus one important function of norepinephrine released during waking appears to be the promotion of synaptic plasticity required for memory formation, in particular emotional memory (1312).
4. Histamine
Histamine neurons were first implicated in wake promotion due to the sedative side effects of first-generation antihistamines (H1 receptor antagonists) that cross the blood-brain barrier and affect central histaminergic systems (159, 1402). More recent studies have clearly shown that histamine neurons in the tuberomammillary nucleus (TMN; FIGURE 2) are slow firing (<10 Hz) and have a wake-on, NREM-slow and REM-off firing pattern (564, 1264, 1338). In vitro, histamine neurons are spontaneously active (464, 1242) due to the activity of a persistent tetrodotoxin-sensitive sodium current (1257). They are excited directly by orexins (342) and serotonin [via 5-HT2C receptors (344)] and indirectly by norepinephrine [through inhibition of GABAergic inputs (1243)]. Histamine has excitatory effects on most nuclei of the ARAS (FIGURE 6; Refs. 159, 465) and, accordingly, injection of histamine into many nuclei of the ARAS promotes wakefulness (722). Conversely, histamine inhibits sleep-active projection neurons of the VLPO via excitation of local inhibitory interneurons, leading to a promotion of wakefulness (736).
Modest decreases/increases in waking have been observed following pharmacological suppression or activation, respectively, of the histamine system (159, 722). However, inactivation of the histamine system via lesions (302, 413), knockout of the histamine H1 receptor (528), administration of an irreversible inhibitor of the histamine synthesizing enzyme histidine decarboxylase (HDC; Refs. 551, 642, 1152) or knockout of HDC (29, 978) have relatively minor effects on 24-h amounts of waking or cortical activation suggesting that, similar to the other aminergic systems, the histamine system is not absolutely essential for wakefulness. Histamine neurons maintain their level of firing during cataplectic attacks in narcoleptic animals (in contrast to norepinephrine and serotonin neurons) implicating them in the preservation of consciousness which accompanies the cataplectic state (564). In addition, increased activation of histamine neurons as measured by Fos activity has been observed during feeding anticipatory behavior (851, 1323). More fine-grained analysis of sleep and wakefulness in HDC knockout animals revealed a deficit in wakefulness when placed in a novel, potentially dangerous environment (29, 978). This is consistent with a role for histamine in stress- or danger-induced arousal (159).
5. Orexins/hypocretins
Orexins/hypocretins were discovered relatively recently by two groups who gave them their two names (290, 1101). We will use the term orexins for these peptide neurotransmitters in this review. Orexins consolidate wakefulness (increase the duration of long waking bouts), suppress REM sleep (sect. IV), and enhance wakefulness in periods of starvation (1452). Considerable evidence links them to the sleep disorder narcolepsy (see sect. VII).
A) Orexins Promote Wakefulness
Early work showed that intracerebroventricular application of orexin A dose-dependently increases wakefulness in rats (1005). More recent work using light-activation of orexin neurons via viral vector- mediated introduction of channelrhodopsins (6) found that excitation of orexin neurons in the lateral hypothalamus at frequencies above 5 Hz increased the probability of a transition from sleep to wakefulness. Conversely, administration of recently developed orexin receptor antagonists increased both NREM and REM sleep in animals and humans at the expense of wakefulness (140).
B) Orexin Neurons Increase Waking in Response to Low Food Availability
One function of the orexin system may be to integrate nutritional state with arousal (4, 1416, 1452). Orexin neurons respond to a wide variety of peripheral and central signals indicating nutritional state (164, 268, 393, 1048, 1452). Several metabolic signals which increase with feeding, such as glucose, leptin, and neuropeptide Y, inhibit orexin neurons in vitro (164, 393, 1452). In contrast, orexin neurons are activated by fasting in non-human primates (308), and given their wake-promoting effects, they are likely to be primary mediators of the increase in waking and suppression of sleep caused by limited availability of food. In fact, orexin knockout mice fail to respond to fasting with an increase in waking and activity (1452).
C) Orexin Neurons are Wake-Active
Orexin neurons are most active during waking as assessed by Fos immunohistochemistry (351, 872) and measurements of peptide release (641). In the squirrel monkey, which has a sleep-wake cycle similar to that of humans, orexin levels peaked in the latter third of the day and remained elevated during 4 h of extended wakefulness, consistent with a role for orexins in consolidating wakefulness in opposition to accumulating sleep drive (1472). Single-unit recordings in the rat from the area where orexin neurons are located revealed one group of slow-firing neurons that were wake-active and REM-off (13, 666). Later recordings in freely moving rats confirmed that this population corresponds to orexin neurons, determined by electrophysiological criteria (856) or post hoc immunohistochemical staining (699, 1265). Orexin neurons fire fastest during active waking, decrease firing during quiet waking, and cease firing during sleep, except during microarousals or immediately preceding the arousal from sleep.
In vitro, intracellular recordings from identified orexin neurons revealed that they have a depolarized resting membrane potential (333, 715), leading to spontaneous firing in the absence of injected current or application of neurotransmitter agonists. In addition, they are excited by a positive feedback loop involving local orexin release, activation of orexin type 2 receptors (1455), and excitation of local glutamatergic inputs (715). This positive feedback loop may help to synchronize the firing of the whole orexin neuron population. Furthermore, glutamatergic inputs to orexin neurons are potentiated via a cAMP-dependent mechanism during prolonged waking (1047), which is a mechanism suggested to be important in the maintenance of wakefulness in the face of increased sleep pressure (1299). However, recent optogenetic stimulation experiments found that sleep deprivation blocks the ability of orexin to activate its downstream targets and enhance waking (195).
D) Control of Orexin Neurons by Afferent Inputs
Orexin neurons receive afferent inputs from other nodes of the sleep-wake circuitry (FIGURE 6) as well as from areas involved in emotional regulation such as the amygdala and lateral septum (1102, 1466). They are excited by acetylcholine via M3 muscarinic receptors (84, 947, 1454) but inhibited by serotonin via a postsynaptic activation of 5-HT1A receptors (715, 905). This inhibitory action is also observed in vivo since intracerebroventricular application of an 5-HT1A antagonist, WAY100635, increased locomotor activity during the dark (active) phase in wild-type mice, but not in orexin/ataxin-3 mice in which orexin neurons are ablated (905). Both inhibitory (715, 716, 1453) and excitatory (84, 1453, 1454) effects of norepinephrine on orexin neurons have been reported in recordings from mouse and rat brain slices. The inhibitory response is mediated by α2 receptors activating inwardly rectifying potassium conductance (716, 1453), whereas the excitatory action is due to activation of α1 receptors and activation of a nonselective cationic current (1453). In the rat, it has been suggested that the response to norepinephrine shifts from an excitation to an inhibition during a short period (2 h) of sleep deprivation (452). In addition, norepinephrine increases the frequency of inhibitory postsynaptic currents (IPSCs) via an effect on presynaptic GABAergic terminals (716, 1453). In vitro, dopamine inhibits orexin neurons via D2 receptors (716), whereas in vivo, systemic dopaminergic agonists increase their activity as assessed by Fos immunohistochemistry, likely by an indirect action (161). Orexin neurons are unaffected by histamine, which is somewhat surprising, considering the close proximity of histamine and orexin neurons in the hypothalamus (342).
E) Inhibition of Orexin Neurons During Sleep
The spontaneous activity of orexin neurons in vitro suggests that they must be actively inhibited during NREM and REM sleep when their activity level slows markedly. This inhibition likely arises from GABAergic neurons in the preoptic area and BF. Orexin neurons are postsynaptically inhibited by both GABAA and GABAB receptors (15, 715, 1150, 1445), and GABAB receptors also mediate a presynaptic inhibition of glutamatergic and GABAergic inputs (1445). In vivo, antagonism of GABAA receptors increases the firing rate of wake-on (presumed orexinergic) neurons in the perifornical hypothalamus during NREM sleep, indicating an inhibitory GABA receptor-mediated tone during this state (15). Mutant mice with a constitutive loss of GABAB receptors in orexin neurons (via knockout of the GABA-B1 gene) have fragmented sleep-wake cycles, due to an upregulation of inhibitory tone which shunts (short-circuits) excitatory and inhibitory inputs (811). Feedback control of orexin neurons may occur through the release of coexpressed dynorphin peptides (223), which cause a hyperpolarization, inhibition of calcium channels, and reduction of excitatory synaptic inputs (717), although direct evidence for feedback control by this mechanism is lacking at present. In addition, orexin neurons are inhibited by the sleep homeostatic factor adenosine via A1 receptors (14, 1277) (see sect. III).
F) Downstream Effectors of Orexin Promotion of Wakefulness
How do the orexins consolidate wakefulness? Anatomical studies demonstrated a strong innervation of sleepwake circuitry by the orexin neurons, particularly the aminergic nuclei (273, 524, 1000). The strongest projection was found to the LC which expresses exclusively the type I receptor, whereas most other sleep-wake nuclei express the type II receptor or both type I and II (249, 506, 793, 853, 1307). In vivo, injections of orexin A into the LC enhanced wakefulness at the expense of REM sleep (128, 467), whereas in vitro recordings revealed a postsynaptic excitation mediated by activation of nonselective cation channels and blockade of leak potassium channels (524, 552, 904, 1326). Similarly, in vitro studies showed that orexins had excitatory effects on serotonergic DRN neurons (157, 158, 651, 735), histaminergic tuberomammillary neurons (85, 342, 1456), BF (47, 334), and brain stem cholinergic neurons (169, 651) and ventral tegmental area dopamine and GABA neurons (660). Furthermore, orexins target neurons in the dorsal ARAS pathway, exciting neurons in the reticular formation (160), nonspecific thalamic nuclei (83, 312, 436, 527), thalamocortical terminals (685), and deep layer VI cortical neurons (86). In addition to the LC, in vivo studies showed wake-promoting effects of orexins in the BF (348, 1279), tuberomammillary nucleus (530), laterodorsal tegmentum (1442), and reticular formation (1392). Orexins also directly increase muscle tone via excitation of spinal cord motoneurons (1457).
It was proposed that orexins exert their wake-promoting action through stimulation of the histamine system since orexins excite histamine neurons in vitro (342), and the wake-promoting effect of intracerebroventricular orexin A is reduced/lost in HDC knockouts and histamine H1 receptor knockouts (530) or with application of a histamine H1 receptor antagonist (1163). Furthermore, low histamine levels have been reported in the brains of narcoleptic dogs (922) and in the cerebrospinal fluid (CSF) of human narcoleptics, particularly in unmedicated patients (597, 925). However, the dependence of the intracerebroventricular effect of orexin A application on the histamine system may simply reflect the close proximity of histamine neurons to the ventricular system, compared with other postsynaptic targets. In contrast to orexin knockout animals, HDC or histamine H1 receptor knockout animals do not have reduced duration of sleep-wake states (29), and optogenetic stimulation of orexin neurons is still able to increase the probability of awakening in HDC knockout animals. However, expression of the orexin type II receptor in histamine neurons and other areas surrounding the TMN in mice lacking type II receptors was sufficient to consolidate wakefulness, although sleep was still fragmented (869). Orexins actions at other sites are likely to be similarly important. For instance, optogenetic inhibition of LC norepinephrine neurons inhibited the wake-promoting effect resulting from optogenetic excitation of orexin neurons (196).
6. Neuropeptide S
Like the orexins, neuropeptide S (NPS) is a recently discovered peptide activating a previously “orphan” G proteincoupled receptor activating phospholipase C (1449). NPS is coexpressed in glutamate-producing neurons located just rostral to the LC (precoeruleus region) which project to widespread areas of the brain, including sleep-wake regulatory regions such as the midline thalamic nuclei, lateral hypothalamus, and preoptic area (1448, 1449). Intracerebroventricular application of NPS increased locomotor activity and decreased sleep in rats (1449), whereas NPS receptor knockout mice had reduced exploratory activity in a novel environment (317). In addition to its role in promoting wakefulness, recent experiments suggest a role for the peptide in controlling fear and anxiety (586, 1449).
7. Dopamine
Pharmacological agents increasing dopaminergic tone such as amphetamines and modafinil (Provigil) are the most potent wake-promoting substances currently known. As such, they are commonly prescribed to treat sleep disorders involving excessive daytime sleepiness (see sect. VII). Although these substances can enhance the release of other neuromodulators such as serotonin and norepinephrine, their effects are abolished in dopamine transporter (DAT) knockout animals (1425), confirming that their main effect is on dopaminergic systems (129). Additional evidence supporting a role for dopaminergic systems in promotion of wakefulness comes from analysis of D2 receptor knockout mice that exhibit a significant decrease in waking amounts due to a shorter wake bout duration and a concomitant increase in sleep (1032). One possible mechanism explaining this effect is a disinhibition of intralaminar thalamic neurons via indirect basal ganglia-thalamic pathways (1135). In Parkinson’s disease, where dopamine neurons in the substantia nigra degenerate, waking is interrupted by sleep episodes (1087). However, dopamine neurons are not the only neurons to be affected by this disease.
A) Ventral Tegmental Area Dopamine Neurons
While the average firing rate of dopamine neurons in the ventral tegmental area (VTA) and substantia nigra does not vary across sleep-wake states (858), VTA dopamine neurons fire more bursts during waking and REM sleep, resulting in increased release of dopamine in target areas such as the nucleus accumbens and prefrontal cortex (271). In particular, increased bursting is observed in the presence of rewarding or aversive stimuli requiring an alerting response (1209). VTA neurons are excited in vitro by several neuromodulators that promote arousal such as orexins, substance P, and corticotrophin releasing hormone (658, 660).
B) Ventral Periaqueductal Gray Dopamine Neurons
Dopaminergic neurons in the ventral periaqueductal gray (vPAG)/DRN (FIGURE 2) show Fos activity during waking but not during sleep (757). Selective lesioning of these neurons by injections of 6-hydroxydopamine (63% loss) or nonselective lesions with ibotenic acid (80% loss) resulted in a marked (>20%) reduction in 24-h amounts of wakefulness, one of the most pronounced effects of lesions on wakefulness reported to date (757). In contrast, lesions of the serotonergic neurons in this area were without effect on 24-h amounts of sleep and waking. Retrograde tracing studies showed that dopaminergic vPAG neurons project to other parts of the ARAS such as the BF and midline thalamus, and receive input from sleep-active VLPO neurons (757). These data all support a role for these neurons in control of wakefulness, but electrophysiological recordings from these neurons across behavioral state are lacking at present.
8. GABA
GABAergic neurons and glutamatergic neurons (reviewed below) are very abundant and widely distributed in the brain. Hence, it is not surprising that some populations of the neurons utilizing these two neurotransmitters are involved in promoting wakefulness, whereas others are associated with sleep. Thus, although pharmacological agents potentiating the activity of GABAergic systems have been most closely linked with sleep (see sects. III and IV), select GABAergic subpopulations in the cortex (especially PV interneurons, sect. IIA) and in subcortical sites, are thought to be critical in the production of cortical LVFA. Cortically-projecting GABA neurons are located in the BF (386, 450, 500), hypothalamus [colocalized in histamine (1371), and melanin-concentrating hormone neurons (60)] and in the VTA (1206). Hypothalamic melanin-concentrating hormone neurons fire predominantly during sleep (482) and so are unlikely to contribute to wakefulness. While the activity of histamine neurons is correlated with wakefulness, the function of GABA in histamine neurons is unclear, especially since it would be expected to counteract excitatory actions of histamine on target neurons. GABAergic neurons in the thalamic reticular nucleus play a crucial role in thalamocortical rhythms during sleep and wakefulness (see sect. III).
A) Bf and Vta Gaba Neurons
GABAergic neurons in the BF and VTA (FIGURE 2) in particular appear to be important for cortical LVFA since a fast-firing subpopulation of these neurons increases their activity during waking and REM sleep (481, 700). Many GABAergic BF neurons projecting to the cortex contain PV (451). Preliminary studies (154) showed that identified cortically-projecting BF GABA neurons are excited by neurotransmitters promoting cortical activation (acetylcholine, norepinephrine, histamine, orexins), likely accounting for their faster firing rate during waking and REM sleep (481). Rostral and caudal PV GABAergic projection neurons synapse onto hippocampal (385) and neocortical PV-positive neurons (386) which control hippocampal and cortical gamma rhythms, respectively (see sect. IIA). Other subpopulations of BF GABA neurons that are likely sleep related project to the thalamic reticular nucleus (49) and lateral hypothalamus (449). The firing of cortically projecting GABA neurons in the BF (481) but not VTA (700) was correlated with gamma activity in the EEG. However, VTA GABA neurons increased their firing prior to intracranial self-stimulation of the medial forebrain bundle, indicating that they may be involved in the attentive processes related to brain reward (1205). VTA GABA neurons are excited by the wake-promoting orexins (660) and by histamine (659). Ibotenic acid lesions of the rostral BF (MS/vDB), which preferentially affect noncholinergic neurons, abolish theta and gamma rhythms in the hippocampus (see sect. IIA). Similarly, chemical lesions of the caudal BF have dramatic effects on cortical LVFA and attention that are correlated with the loss of PV-positive GABA neurons (166, 397, 611).
B) Striatal Medium Spiny Neurons
GABAergic medium spiny neurons in the striatum receive a massive glutamatergic cortical input and control the activity of thalamocortical neurons. Transitions from NREM sleep to wakefulness convert the firing of striatal neurons from fast cyclic firing, synchronized with cortical field potentials, to an irregular pattern of action potentials triggered by disorganized depolarizing synaptic events of variable amplitude (777). Cell body specific lesions of the rostral striatum reduce waking by ~15% and produce cortical slowing of the EEG (1029). Conversely, lesions of the globus pallidus, the main recipient of inhibitory striatal projections, increase waking by 46%. Improved function in minimally conscious patients produced by stimulation of the nonspecific thalamic nuclei (1136) may be mediated by increased cortico-striatal-thalamic interplay (1135).
9. Glutamate
The vast majority (>90%) of glutamatergic projections to the cortex arise from the thalamic relay nuclei innervating cortical layers III and IV, and from nonspecific thalamic nuclei innervating layers I and VI (540, 750). In addition, the BF (500), claustrum, amygdala, VTA, laterodorsal tegmentum, and hypothalamus (540) provide minor glutamatergic projections to the cortex. Vesicular glutamate transporters are expressed in cortically projecting orexin neurons in the perifornical hypothalamus (1077) and serotonergic DRN neurons (439), suggesting that glutamate is a cotransmitter in these neurons. Furthermore, glutamate is the major neurotransmitter released from rostral midbrain brain stem reticular formation neurons projecting to the thalamus. Dissociative anesthetic agents such as ketamine inhibit glutamatergic NMDA receptors, whereas pharmacological agents that prolong the decay of AMPA receptor currents (AMPAkines) are proposed to enhance attention and cognition.
A) Thalamic Intralaminar and Relay Neurons
The thalamus is an important component of the dorsal branch of the ARAS involving the nonspecific thalamic nuclei (FIGURE 5), as well as the specific relay nuclei which convey external sensory information to the cortex. EEG rhythms typical of wakefulness are sculpted through interactions between the thalamocortical relay neurons, corticothalamic pyramidal neurons, and GABAergic neurons in the thalamic reticular nucleus. At the onset of conscious states (i.e., wakefulness and REM sleep), thalamic relay neurons are excited by the action of acetylcholine, norepinephrine, and histamine, leading to a switch in firing pattern from synchronized burst firing (typical of NREM sleep) to tonic firing able to faithfully transmit sensory information to the cortex (826, 828, 1228). This switch in firing pattern is due to a depolarization mediated by a block of leak potassium conductance by Gq-coupled receptors (muscarinic M1/3, norepinephrine α1, histamine H1) and a block of the pacemaker current Ih by Gs/adenylyl cyclase-coupled receptors (muscarinic M2/M4, norepinephrine β, serotonin 5-HT4,6, histamine H2). In a thalamocortical slice preparation, coincident stimulation of nonspecific thalamic nuclei (centrolateral intralaminar nucleus) or direct stimulation of layer I together with relay nucleus stimulation induced supralinear summation of the two inputs in cortical output layer V, providing a possible mechanism by which the nonspecific nuclei promote arousal (741).
10. Effector systems of neurotransmitters promoting wakefulness
The effector systems used by the neurotransmitter systems involved in generation of wakefulness have been studied by in vitro electrophysiology, pharmacology, and genetic methods (see sect. VI). The majority of the receptors implicated in cortical LVFA and wakefulness are either ionotropic (glutamatergic AMPA, kainate and NMDA receptors, GABAA receptors, nicotinic acetylcholine and serotonin 5-HT3 receptors) or metabotropic receptors coupled to Gq G proteins and the beta form of the enzyme phospholipase C (glutamatergic mGluR1 and mGluR5, cholinergic muscarinic M1, M3, M5, norepinephrine α1, histamine H1, serotonin 5-HT2, orexin type I and type II receptors). Phospholipase C (PLC)-β occurs in four isoforms. Mice lacking the β1 or β4 subunits of PLC have disrupted theta rhythms and other EEG abnormalities (595, 1166). Activation of these metabotropic receptors causes a depolarization in target neurons mediated by one or a combination of three mechanisms: 1) blockade of leak potassium conductances (two-pore potassium channels) (1348); similar to deletion of PLC-β isoforms, mice lacking the TASK3 two-pore potassium channel have deficient theta oscillations and altered sleep behavior (965); 2) activation of mixed cation channels [likely of the transient receptor potential (TRP) family] (1151); and 3) activation of electrogenic sodium-calcium exchangers (343, 1434). In addition, effects on other intrinsic membrane currents contribute to the activation of thalamocortical and limbic neurons (828, 918). For the most part, the role of individual subunits of these channels/transporters in the control of sleep-wake behavior remains to be determined.
D. Synthesis
Studies involving stimulation of the brain areas and neurotransmitter systems comprising the ARAS consistently report EEG activation and wakefulness as a result. These studies include both older techniques of electrical stimulation or infusion of pharmacological agents as well as state-of-the-art optogenetic techniques where light-activated ion channels are introduced into the desired neuronal population by genetic engineering techniques (6, 1473). In contrast to the stimulation experiments, studies where localized inactivation of individual neurotransmitter systems or nuclei of the ARAS have been performed (summarized in TABLE 1) generally produce relatively minor changes in cortical EEG or the amount of wakefulness in a 24-h period (see sect. IIC), with the possible exception of the parabrachial nucleus (see sect. IIB). There are several possible explanations for this dichotomy between stimulation and inactivation experiments. First, the ARAS systems are strongly interconnected, mutually excitatory to each other (FIGURE 6) and converge onto common effector systems at the level of thalamic and cortical neurons (826, 918). Thus there is considerable redundancy in the system, and inactivation of any individual component of the system is compensated for by the other systems. This is perhaps not surprising considering the enormous adaptive advantage of wakefulness! A second possibility for the mild effects of loss-of-function experiments is that the systems so far targeted are not absolutely required for wakefulness. The majority of studies have focused on neuromodulatory systems, whereas selective inactivation of glutamatergic and GABAergic systems projecting to the neocortex have not been tested due to technical difficulties in targeting these systems. The neuromodulatory systems are clearly able to generate cortical activation when stimulated but may only be required for specific aspects of wakefulness. Specific roles for these systems could be 1) facilitation of LVFA (acetylcholine); 2) inhibition of sleep-active neurons (norepinephrine, serotonin, acetylcholine; see sect. III); 3) maintenance of high muscle tone (norepinephrine) during waking (see sect. IVA); 4) consolidation of wake periods (orexins); 5) maintenance of waking in a novel environment (histamine); 6) enhanced arousal in the presence of rewarding stimuli (dopamine, acetylcholine); 7) enhanced arousal in the presence of aversive stimuli (norepinephrine, serotonin, histamine); and 8) consolidation of memories through enhancement of synaptic plasticity (acetylcholine, norepinephrine, serotonin, histamine, dopamine, orexins). Methods to selectively stimulate these systems (e.g., using optogenetic techniques) together with whole brain imaging will be helpful in further delineating their function.
III. NREM SLEEP
Subjectively experienced as a loss of conscious awareness, the onset of sleep is heralded in the EEG by the replacement of LVFA by large-amplitude, slow (<4 Hz) waves and the appearance of thalamocortical spindles (FIGURE 1). These EEG changes are due to the progressive reduction in firing of neurons in the ARAS. This section describes the mechanisms underlying the EEG signs of NREM sleep (also called slow-wave sleep) and the mechanisms that cause the circadian and homeostatic inhibition of wake-promoting ARAS neurons.
A. Electrographic Signs of NREM Sleep
In humans, the different stages of NREM sleep are classified according to the criteria established by Rechtschaffen and Kales (1053) (FIGURE 1). Stage 1 NREM sleep exhibits theta activity at frontal sites and alpha activity posteriorally, similar to drowsy waking (sect. II). Stage 2 NREM sleep is characterized by the appearance of sleep spindles (7–15 Hz) and K-complexes in the EEG. Stages 3 and 4 of NREM sleep (deep sleep) exhibit prominent, high-amplitude slow, delta waves (1–4 Hz). The cortical slow oscillation (0.5–1 Hz) discovered by Steriade and colleagues synchronizes the activity of cortical and thalamic neurons that generate spindle and delta waves throughout NREM sleep (1217). In animals, NREM sleep is not usually subdivided into these four stages, but deep (delta) sleep may be distinguished from light NREM sleep. NREM sleep is also characterized by low skeletal muscle tone and slow, rolling eye movements. Here we first describe phasic events occurring during NREM sleep in the thalamocortical system (spindles) and hippocampal formation (sharp wave-ripple complexes) and then discuss the delta and slow oscillations typical of deep NREM sleep.
1. Thalamocortical spindles
Spindles and K-complexes are the defining features of stage 2 NREM sleep in humans (FIGURE 1). K-complexes represent a combination of one cycle of the neocortical slow oscillation followed by a spindle in thalamocortical neurons (27, 28, 197). Several lines of evidence support the contention that spindles are generated in the thalamic GABAergic reticular and perigeniculate nuclei (394, 1228): 1) spindles occur even in the absence of the cerebral cortex (1215); 2) large thalamic lesions (177, 397, 1370) or specific lesions/deafferentation of the thalamic reticular nucleus abolish spindles in thalamocortical neurons (1223); 3) spindle activity can be recorded in the deafferented reticular nucleus (1224); 4) spindles are absent in the anterior part of the thalamus, which does not receive afferents from the thalamic reticular nucleus (i.e., the anterodorsal, anteromedial, and anteroventral nuclei; Ref. 975), and in their projection areas (cingulate cortex, habenular nucleus). Although the thalamic reticular nucleus is the generator of spindles, in intact animals spindles are initiated and terminated in concert with delta and slow oscillations in corticothalamic and thalamocortical neurons due to the extensive interconnections of these cells (828).
A) Cellular Mechanisms Underlying Spindles
As aminergic inputs are slowly withdrawn during early NREM sleep, long-lasting (50 ms) bursts of action potentials are generated in reticular nucleus neurons due to activation of low-threshold (T-type) calcium channels. These channels are of the Cav3.2 and CaV3.3 subtype (1269), which allow bursting at the resting membrane potential. Bursts at spindle frequencies lead to large and long-lasting inhibitory synaptic potentials (IPSPs) in thalamocortical neurons which remove the inactivation of T-type (Cav3.1) (1269) calcium channels. Thus, at the offset of the IPSPs, when the cell becomes more depolarized, the low-threshold calcium channels are activated, calcium enters the cell, resulting in a low-threshold calcium spike crowned by a short (5–15 ms) burst of sodium-dependent action potentials in the thalamocortical neurons. This burst in thalamocortical neurons leads to EPSPs in cortical neurons and to action potentials which together make up the spindle recorded in the EEG. Synchronization of spindles is achieved via recurrent inhibitory and electrical synaptic connections between thalamic reticular neurons (828). Spindles can also be recorded in cortical projection sites such as the basal ganglia (298), possibly providing a substrate for procedural learning during sleep. Spindles decline during deep sleep due to the increased hyperpolarization of thalamocortical relay neurons but may reappear just prior to the transition to REM sleep when thalamocortical relay neurons become more depolarized again (due to increased ascending brain stem excitation).
B) Inhibition of Spindles During Wakefulness and Rem
In vivo, extracellular and intracellular recording studies revealed that thalamic reticular neurons fire tonically during waking and switch to burst firing during NREM sleep, similar to thalamocortical relay neurons (802, 1222). Tonic firing and inhibition of bursts/spindles during waking is likely due to excitation of these thalamic reticular neurons by norepinephrine and serotonin (833) released from ascending projections arising in the LC and DRN. Norepinephrine, acting via α1 receptors and serotonin, acting via 5-HT2 receptors, causes a depolarization by block of a leak potassium conductance leading to an inactivation of low-threshold calcium channels responsible for bursting (833). Spindles can also be inhibited by input from other ARAS systems, in particular brain stem cholinergic (974) and BF cholinergic and GABAergic inputs (49, 976). The mechanism underlying inhibition of spindle activity during REM sleep is less clear but has been proposed to be due to input from REM-on cholinergic neurons which hyperpolarize thalamic reticular neurons via a muscarinic M2 receptor mediated inhibition of leak potassium conductance (192, 831).
2. Hippocampal sharp waves and high-frequency ripples
High-frequency (100–400 Hz) field potentials termed sharp wave-ripple complexes can be recorded in the hippocampus and associated areas during quiet wakefulness and NREM sleep in rodents (174, 180, 181) and in humans (132). Sharp-waves occur in the CA3 region of the hippocampus when the highly interconnected CA3 pyramidal network is released from the control exerted by subcortical inputs, especially the MS/vDB. Sharp-wave like epilepti-form waves occur in the hippocampus following transection of the fimbria-fornix, the main fiber bundle carrying ascending fibers from the MS/vDB and other ARAS systems (182). Ripple occurrence is also increased by pharmacological blockade of histamine H1 receptors, which mediate histaminergic excitation of MS/vDB neurons (647, 1012) or blockade of serotonin 5-HT3 receptors (1012), which excite inhibitory hippocampal/dentate interneurons. When released from inhibition, the synchronized firing of CA3 pyramidal neurons leads to a concerted activation of Schaffer-collateral synapses in the CA1 region and subsequently of subicular and downstream retrohippocampal cortical structures (227). Feed-forward and feedback activation of hippocampal GABAergic interneurons leads to a high-frequency oscillation in the membrane potential of pyramidal neurons due to IPSPs, reflected as a high-frequency ripple in the extracellular potential (1462). Phase-locked interneurons fire at high frequencies on every cycle of the extracellularly recorded oscillation and entrain the firing of pyramidal neurons, which fire at lower frequencies (643, 1462). Accordingly, ripple frequency is reduced by pharmacological prolongation of GABAA receptor-mediated currents (1013). Surprisingly, ripple amplitude and entrainment of pyramidal neurons were increased in mice lacking the GluR1 subunit of AMPA-type glutamatergic receptors specifically on PV-positive interneurons, possibly as a result of developmental compensation (1035). Although many pyramidal neurons contribute to each sharp wave/ripple, each wave of a ripple reflects the firing of a discrete subpopulation of these neurons (228, 1462). Modeling studies suggest that electrical coupling between the axons of pyramidal neurons is required to synchronize their activity (1306). In support of this idea, the occurrence of ripples in vitro was reduced in mice lacking one type of gap junction protein (connexin 36), and the intraripple frequency was reduced (781). However, in another report, the occurrence of in vitro kainate-induced sharp waves was actually increased in these mice (957).
3. Delta (1–4 Hz) and slow (<1 Hz) oscillations
Delta and slow oscillations in the cortex and thalamus are typical of the deeper stages of NREM sleep (FIGURE 1). They result from increased withdrawal of excitatory neuromodulatory inputs (primarily cholinergic and aminergic), resulting in a more hyperpolarized membrane potential of the pyramidal/thalamic relay neurons. Delta oscillations are best understood at the thalamic level. Recordings in vivo from thalamocortical neurons revealed that stereotyped high-frequency bursts of action potentials occur at delta frequencies interspersed with silent periods (25, 314, 817, 932, 1225), a pattern which can be abolished by brain stem cholinergic stimulation or by increases in ambient light (25, 932, 1225). The ability of thalamocortical neurons to generate burst firing in the delta frequency range is due to their intrinsic membrane properties (556, 557, 711, 830, 1199). Hyperpolarization resulting from the activation of calcium-dependent potassium conductances after a burst of action potentials or from inhibitory synaptic inputs leads to the opening of hyperpolarization-activated, cAMP-modulated cation (HCN) channels causing the so-called H-current (Ih). This slowly activating current provides a depolarizing drive towards the threshold for action potentials and is a major contributor to the duration of the interburst interval (830, 968). Ih is modulated during waking by activation of neurotransmitter receptors coupled to stimulation of cAMP (e.g., norepinephrine β, histamine H2) and by release of NO from cholinergic projections (967, 968) resulting in a shift in the activation curve to more positive membrane potentials and reducing the ability of the cells to generate intrinsic oscillations (828, 829). As well as activating Ih, hyperpolarizations result in deinactivation of low-threshold calcium channels, allowing their subsequent activation once the membrane potential reaches less negative potentials (828). Opening of these calcium channels leads to a low-threshold spike (LTS) and a burst of action potentials (556–558). Bursts of action potentials in thalamocortical neurons lead to a prominent burst in large numbers of cortical pyramidal neurons. Bursting of corticothalamic neurons potentiates intrinsic rhythms in thalamocortical neurons and entrains their firing through excitation of thalamic reticular neurons leading to rhythmic hyperpolarizations in thalamocortical neurons, creating increased network synchronization (25, 1225). Calcium influx through the low-threshold channels allows activation of calcium-dependent potassium conductances, restarting the cycle. Ascending influences during waking or REM sleep block this cycle by acting on PLC-coupled receptors that block a leak potassium conductance causing inactivation of the low-threshold calcium channels and bringing the membrane potential out of the range of the H-current (826, 828).
A) Role of Low-Threshold Calcium Channels in Delta Waves
Low-threshold bursts in thalamocortical neurons were abolished in mice constitutively lacking the Cav3.1 calcium-channel gene (695) or in mice with a targeted deletion of Cav3.1 in rostral-midline thalamus (32). Delta waves were abolished in these mice with knockouts in the whole brain or thalamus, whereas deletions of Cav3.1 channels in cortical neurons did not affect delta waves (32). Loss of delta waves was associated with fragmented sleep with a higher incidence of brief arousals. Similar to thalamocortical neurons, bursting in thalamic reticular neurons is regulated by calcium dynamics involving low-threshold calcium channels, endoplasmic reticulum calcium ATPases which sequester intracellular calcium, and small-conductance calcium-dependent potassium (SK) channels (266). Like Cav3.1 knockouts, mice lacking the SK2 channels responsible for slow after hyperpolarizations in thalamic reticular nucleus neurons had disrupted sleep and a threefold reduction of low-frequency rhythms during NREM sleep (266).
B) The Neocortical Slow Oscillation (0.5–1 HZ)
This phenomenon, discovered by Steriade in anesthetized cats (1230), was subsequently observed in naturally sleeping animals (28, 1237, 1289) and in humans (27, 272). Somewhat confusingly, despite its name, the so-called slow oscillation does not necessarily imply rhythmicity. High-density EEG recordings in humans revealed that each cycle of the slow oscillation represents a traveling wave originating most frequently in prefrontal-orbitofrontal regions and propagating towards more posterior cortical areas (809). The slow oscillation occurs throughout all NREM sleep stages and serves to bind together the other EEG phenomena of NREM sleep such as spindles and delta waves (1217, 1228, 1229). Slow-wave activity (SWA; 0.5–4 Hz), a combination of EEG power in the frequency bands reflecting the slow oscillation and delta oscillations, is widely considered to be a measure of sleep need and/or intensity (3, 1292). Periods of sleep deprivation cause increases of SWA in the subsequent sleep period in both animals and in humans. SWA is highest at the beginning of the sleep period and progressively decreases during sleep. Naps during the day also reduce SWA in the subsequent night (1292). The hypothesized relationship between SWA and synaptic strength is discussed in section IIID. The slow oscillation is generated within the cortex since it is abolished in thalamic neurons following removal of the cortex (1290), and it persists following disconnection of subcortical inputs (1231) and can occur in vitro in cortical slices, following manipulation of the ionic milieu bathing the slices (269, 1110). However, in intact animals, the slow oscillation strongly influences the activity of the thalamus through corticothalamic projections and conversely the thalamus influences the cortex through thalamocortical projections (264, 535, 1229, 1231).
C) Cellular Mechanism Causing up and Down States
Intracellular recordings from cortical neurons in vivo (259, 1374) and in vitro (1110) revealed that the slow oscillation consists of prolonged depolarizations associated with extracellular gamma frequency activity (UP states) separated by prolonged hyperpolarizations (DOWN states) when most cortical neurons are silent (255, 256). These states are well-synchronized over widespread areas of cortex (1374). The UP states are due to a barrage of excitatory synaptic inputs mediated by glutamatergic AMPA/kainate and NMDA receptors and activation of a persistent sodium current and usually begin in deeper cortical layers, possibly due to an increased frequency of excitatory spontaneous synaptic potentials (211). Consistent with this idea, the frequency and amplitude of miniature excitatory postsynaptic currents in pyramidal neurons of frontal cortex was enhanced following waking and decreased following sleep (737). Fast inhibitory GABAA receptor-mediated potentials also occur during the UP state due to input from GABAergic interneurons activated by the firing of principal glutamatergic neurons. The hyperpolarizing phase is due to withdrawal of excitatory input.
B. Generation and Maintenance of NREM Sleep
An involvement of the preoptic area (PO)/BF (FIGURE 2) in the control of sleep has been inferred since the observations by Constantin von Economo of damage to this area in the brains of patients with persistent insomnia following the influenza pandemic in the early years of the 20th century (1377). Extensive lesions of the PO/BF led to long-lasting insomnia in the cat (840, 1105), whereas warming caused increases in sleep (1068). Whereas most brain neurons exhibit a wake-On, a wake/REM-on or state-indifferent firing patterns across the sleep-wake cycle, the PO/BF is unusual in that it contains a large number of neurons utilizing the neurotransmitter GABA which have sleep-on, wake-off firing patterns. Many of the sleep-active neurons in the medial and lateral preoptic area are also temperature sensitive, likely explaining the coupling of body temperature and sleep (16). In the BF, caudally projecting, possibly sleep-active, GABA neurons (449) are intermingled with cortically projecting cholinergic, GABAergic, and glutamatergic neurons (451) which increase firing in association with cortical activation (481).
1. Ventrolateral preoptic nucleus
With the use of Fos immunohistochemistry to identify neurons that had been recently active, a cluster of sleep-active neurons was identified in the ventrolateral preoptic nucleus (VLPO) (FIGURE 2) of the rat (1162). These neurons contained the inhibitory neurotransmitters GABA and galanin and projected heavily to nuclei of the ARAS, especially the histaminergic tuberomammillary nucleus (406, 1161, 1162, 1210). Single-unit recordings targeting this area confirmed that it contains sleep-active neurons (1254). Extensive neurotoxic lesions of the central cluster of the VLPO in the rat led to a large decrease in delta power and NREM sleep time and a fragmentation of the sleep-wake cycle (756), effects which persisted for at least 3 wk postlesion. Furthermore, the number of remaining Fos-immunoreactive neurons was linearly related to NREM sleep time and EEG delta power.
In vitro recordings in the rat determined that many VLPO neurons are multipolar, triangular-shaped neurons, which exhibit a LTS. All of these were inhibited by norepinephrine and acetylcholine, and the majority were also inhibited by serotonin (5-HT1A) (403). Activation of non-α7 containing nicotinic receptors enhances the release of norepinephrine onto VLPO neurons, indicating a synergistic inhibitory action of acetylcholine and norepinephrine (1091). Other VLPO neurons, possibly local interneurons, had a fusiform/ bipolar shape, lacked an LTS, and were excited by serotonin and an adenosine A2a receptor agonist (403, 404). Initial experiments suggested that histamine and orexin did not affect the firing rate of VLPO neurons, but more recent experiments have revealed an indirect histaminergic inhibition due to excitation of local inhibitory interneurons (736). Retrograde tracing revealed surprisingly few cholinergic projections to VLPO but prominent projections from the histaminergic tuberomammillary nucleus, norepinephrine neurons in the LC and ventrolateral medulla, and serotonergic neurons in the dorsal median and central linear raphe nuclei (222). However, somatodendritic release of acetylcholine from nearby cholinergic neurons in the HDB and MCPO is a possibility. Interestingly, VLPO neurons also receive direct inputs from the retina (759) and indirect projections from the suprachiasmatic nucleus via the dorsomedial hypothalamus (222, 306, 1250), one pathway by which light exposure could affect sleep. In vitro studies also revealed that VLPO neurons are excited by adenosine through an indirect mechanism: A1 receptor-mediated presynaptic inhibition of inhibitory synaptic inputs (203, 888). In addition, activation of adenosine A2a receptors by infusion of an A2a agonist in the subarachnoid space underlying the VLPO area increases the activity of VLPO neurons in vivo (1128).
2. Median preoptic area
Single-unit recordings and Fos studies have defined another preoptic nucleus containing a large population of GABAergic sleep-active neurons, the median preoptic nucleus (MnPO), located just dorsal to the third ventricle (FIGURE 2) (432, 1094, 1252). Like VLPO neurons, MnPO neurons project to and inhibit wake-promoting neurons of the ARAS in the perifornical lateral hypothalamus, DRN, and LC (1251, 1321). Inactivation of the MnPO by infusion of the GABAA receptor agonist muscimol led to a prolonged waking state in rats (1251). On the other hand, high-frequency electrical stimulation or perfusion with glutamate or the GABAA receptor antagonist bicuculline enhanced NREM sleep and inhibited the activity of wake-active neurons in the perifornical hypothalamus (1251). MnPO neurons recorded in vitro were dose-dependently inhibited by norepinephrine via α2 receptor-mediated activation of inwardly rectifying potassium channels (63), possibly explaining their silence during waking. Experiments comparing the extent of Fos during spontaneous sleep, sleep deprivation, and recovery from sleep deprivation suggest that MnPO neurons are active during sleep deprivation, whereas VLPO neurons are mainly active during sleep (462, 871, 994). Thus MnPO neurons increase their activity in response to increased homeostatic sleep pressure, whereas VLPO neurons may function to consolidate and maintain sleep and regulate sleep depth (462).
3. Wake-NREM transitions and the flip-flop model
Von Economo (1378) was the first to propose the existence of an anterior hypothalamic sleep-promoting area and a posterior hypothalamic waking center. More recent anatomical tracing experiments revealed that neurons in the core of the VLPO project heavily to wake-promoting histamine neurons in the tuberomammillary nucleus (TMN) of the posterior hypothalamus and also to wake-promoting serotonin DRN neurons and norepinephrine LC neurons in the brain stem (1161). Around the same time, pharmacological and electrophysiological experiments showed that GABA and galanin inhibit TMN, DR, and LC neurons (420, 421, 726, 1003, 1141), whereas serotonin and norepinephrine inhibit most VLPO neurons (403). Similarly, histamine excites a subpopulation of inhibitory interneurons in the VLPO via H1 and H2 receptors and thereby causes an indirect inhibition of VLPO projection neurons (736). In addition, histamine neurons appear to utilize GABA as a cotransmitter (677); thus TMN neurons can potentially also inhibit VLPO neurons through release of GABA. These mutually inhibitory interactions between VLPO neurons and TMN/DRN/LC neurons were conceptualized in the form of a flip-flop switch (838, 1117) such that activation of VLPO leads to inactivity of TMN/ DRN/LC neurons and promotes sleep, whereas activation of TMN/DRN/LC leads to inactivity of VLPO neurons and promotes wakefulness (FIGURE 7). A crucial aspect of this model is that the two halves of the switch, by strongly inhibiting each other, create a feedback loop that is stable in only two states such that intermediate states of sleep and wakefulness are very brief. A further component to the model was the proposal that orexins stabilize behavioral state via their strong excitatory actions on wake-promoting neurons. Analysis of orexin knockout mice revealed that they have many more transitions between wake, NREM, and REM states than do wild-type mice, supporting this model (870). Although intuitively appealing, this model has a few weaknesses. First, the model does not well represent the changes in firing of all the neuronal subpopulations involved. While sleep-active preoptic neurons have fast transitions around state-transitions (1266), the firing rate of wake-active BF neurons changes more slowly (1266) and thus more closely resembles a latch than a switch. Furthermore, recordings of histamine neurons showed that they begin firing after the onset of EEG activation during NREM→Wake transitions (1264), which would seem inconsistent with them being involved in causing the state change. Second, the mechanism responsible for turning the switch on and off is unclear since a switch remains in one state unless a third mechanism causes a transition. Possible candidates for facilitators of the wake-sleep transition are sleep homeostatic factors that slowly build up during wakefulness and are discussed in the following section.
FIGURE 7.
The “flip-flop” switch model of transitions between sleep and wakefulness (1117). The wake state is stabilized by lateral hypothalamic (LH) excitatory orexinergic input to wake-related nuclei, including GABAergic/ histaminergic neurons of the tuberomammillary nucleus (TMN), serotonergic neurons of the dorsal raphe nucleus (DR), and noradrenergic neurons of the locus coeruleus (LC). During sleep, GABAergic/galaninergic ventrolateral preoptic (VLPO) neurons inhibit wake-promoting nuclei, including LH. Transitions between wake and sleep are due to mutual inhibition between these sleep- and wake-related nuclei. (Adapted from Saper et al. Nature 437: 1257–1263, 2005, with permission from MacMillan Publishers Ltd.
C. NREM Sleep Homeostasis
Homeostatic control of sleep refers to the increased propensity for sleep during prolonged waking and the prolonged sleep time and depth of sleep (reflected as increased EEG slow wave activity) following a period of sleep deprivation (123, 311). Sleep homeostasis is considered to reflect the accumulation of sleep homeostatic factors during waking, particularly in the BF and cortex, in a manner related to brain energy usage (see sect. IIID). Sleep homeostatic factors inhibit the activity of ARAS neurons as well as cortical neurons and thereby facilitate the slow oscillations typical of NREM sleep.
1. Sleep factors
The search for sleep-promoting factors dates back 100 years when Ishimori (548) and Legendre and Pieron (701) reported that injection of CSF from a sleep-deprived dog into the cisterna magna of a normal animal induced sleep. Later in 1967, Pappenheimer et al. (972) performed similar experiments where CSF from the cisterna magna of goats deprived of sleep for 72 h induced sleep in cats and rats following intraventricular application. These pioneering studies suggested that endogenous humoral factors are induced and accumulate during waking and generate a homeostatic sleep response. This led to a series of investigations in search of humoral sleep-promoting substances (545), leading to the identification of several substances including 1) delta sleep inducing peptide (438); 2) uridine (517); 3) oxidized glutathione, originally designated as SPS-B (516); 4) muramyl dipeptide (N-acetylmuramyl-l-alanyl-d-isoglutamine), originally described as Factor S (971); and 5) prostaglandin D2 (1317). In the following years, a variety of additional endogenous sleep-inducing substances were identified including peptides, growth factors, and cytokines as well as neuromodulators such as adenosine and NO. Homeostatic sleep factors should fulfill the following criteria: 1) administration of the substance induces sleep; 2) the levels of the substance in the brain should increase with increasing sleep propensity; and 3) the substance should act on brain regions and neurons involved in the regulation of sleep or wakefulness. Recent studies have focused extensively on the role of adenosine, nitric oxide, prostaglandin D2, and cytokines in sleep regulation and the following sections will review the latest research on these factors.
A) Adenosine
The neuromodulator adenosine links energy metabolism, neuronal activity, and sleep (79, 91, 319, 446). The hypnogenic effects of adenosine were first described in cats by Feldberg and Sherwood (359) and later in dogs by Haulica et al. (483). Systemic and central administrations of adenosine or adenosine A1 receptor agonists induced sleepiness and impaired vigilance (91, 226, 320, 1022, 1037, 1038, 1373) by inhibition of wake-active neurons. Adenosine A2A receptors are also implicated in mediating the somnogenic effects of adenosine by excitation of sleep active neurons (485, 531, 1123). Stimulants such as caffeine and theophylline counteract the sleep-inducing effects of adenosine by serving as antagonists at both A1 and A2A adenosine receptors (384, 1192).
Adenosine levels correlate with time spent awake. Endogenous, extracellular adenosine levels in the BF (102, 591, 843, 1017, 1018) and cortex (591, 1017) increase in proportion with time spent awake (FIGURE 8). Thus adenosine induces sleep and adenosine levels track sleep need, fulfilling the criteria for adenosine being a homeostatic sleep factor. Measurements of extracellular adenosine levels across the sleep-wake cycle and in response to sleep deprivation revealed that adenosine levels rise only in select regions of the brain (1017, 1247). In particular, adenosine levels correlate with time awake in the region of the caudal BF containing cortically projecting wake-active neurons, and in the cortex itself. In contrast, adenosine levels did not follow this pattern in other brain areas such as the preoptic area of the hypothalamus, ventral thalamus, DRN, or pedunculopontine tegmentum. BF adenosine levels also rise when rats are exposed to a sleep fragmentation protocol (844), possibly explaining excessive daytime sleepiness in sleep disorders where sleep is fragmented (see sect. VII).
FIGURE 8.
Investigations of the role of adenosine (AD) as a neuromodulatory sleep factor. A: extracellular AD concentrations in the feline basal forebrain (BF) for 10-min consecutive samples from an individual animal, showing elevated levels during wakefulness. Labels indicate behavioral state: W, wakefulness; S, slow wave (NREM) sleep; R, REM sleep. [Adapted from Porkka-Heiskanen et al. (1018). Reprinted with permission from AAAS.] B: AD concentrations in the feline BF rise during 6 h of sleep deprivation (SD) and decrease towards baseline levels during 3 h of spontaneous recovery sleep. [Adapted from Porkka-Heiskanen et al. (1018). Reprinted with permission from AAAS.] C: AD and nitric oxide (NOx, red) concentrations in the rat BF rise during 11 h of SD. The rise of NOx during SD precedes the rise of AD. AD levels are significantly elevated by hour 2 of SD and remain elevated until recovery sleep, when levels fall towards baseline levels. Levels are normalized to baseline levels in the 2 h preceding SD. [Adapted from Kalinchuk et al. (591), with permission from John Wiley and Sons.] D: AD and NOx levels in the rat frontal cortex also rise during SD. Again, the rise of NOx during SD precedes the rise of AD. The rise of AD is significant by hour 6 of SD and is delayed compared with the rise seen in BF, as shown in C. Levels are normalized to baseline levels in the 2 h preceding SD. [Adapted from Kalinchuk et al. (591), with permission from John Wiley and Sons.] E: graphic depiction of the intracellular signaling pathway of the AD A1 receptor in BF observed following sleep deprivation in rats. Steps of the pathway: 1) AD binds to the A1 receptor; 2) activation of PLC pathway, releasing inositol 1,4,5-trisphosphate (IP3); 3) IP3 receptor-mediated intracellular calcium mobilization and activation of protein kinase C; 4) phosphorylation of Iκ-B and release of nuclear factor-κB (NF-κB) dimer; 5) nuclear translocation of NF-κB dimer; 6) promoter DNA binding of NF-κB and transcriptional activation of target genes including A1 receptor; 7) protein synthesis (A1 receptor synthesis). This signaling cascade appears to be confined to cholinergic neurons of BF. (Adapted from Basheer et al. Neuroscience 104: 731–739, 2001, with permission from Elsevier.
I) Mechanisms underlying adenosine increases during wakefulness. Increased levels of extracellular adenosine during prolonged wakefulness are caused by interactions between neuronal and glial mechanisms. Glutamatergic stimulation of the BF elevates extracellular adenosine and increases sleep (1409). Selective activation of glutamatergic NMDA receptors on hippocampal pyramidal (790) or on brain stem cholinergic neurons (134) also leads to slow adenosine release and inhibition of neuronal activity. In the BF, cell-specific lesion of cholinergic neurons attenuates the sleep deprivation-induced increase of adenosine (102, 592), suggesting either that increases in extracellular adenosine are derived from these neurons or that they release an essential signal for extracellular adenosine accumulation. Such a signal may be NO (see next section). There is also strong recent evidence in support of an astrocytic origin of adenosine via so-called “gliotransmission.” Neurotransmitter release, especially glutamate release, triggers a rise in astrocytic calcium which triggers gliotransmision of ATP along with other neurotransmitters such as glutamate and d-serine (360, 979). In turn, breakdown of the extracellular ATP released by glia yields adenosine, which depresses neuronal activity (981, 1474). Blockade of vesicular release via transgenic expression of a dominant-negative SNARE domain specifically in astrocytes (dn-SNARE mice) blocked the accumulation of homeostatic sleep pressure following sleep deprivation as reflected by slow-wave activity and prevented the sleep-suppressing effects of an adenosine A1 receptor antagonist (360, 472), suggesting that blocking gliotransmission affected sleep by reducing the accumulation of extracellular adenosine.
II) Adenosine mediates its sleep-promoting effects through activation of both A1 and A2A receptors. Electrophysiological, behavioral, and molecular evidence suggest that in wake-active areas, the effects of adenosine are primarily mediated via A1 receptors. In vitro studies have demonstrated inhibitory postsynaptic effects mediated by activation of inwardly rectifying potassium channels on brain stem (48, 1041) and BF cholinergic neurons (45), orexin neurons (738), and hippocampal/neocortical pyramidal neurons (417, 834). A weaker postsynaptic inhibitory effect mediated via an A1 receptor-mediated shift of the activation threshold of the hyperpolarization-activated current Ih is observed in thalamic relay neurons (826) and BF noncholinergic neurons (45). Adenosine further dampens neuronal activity and promotes sleep via presynaptic inhibitory effects on excitatory glutamatergic inputs to cortical glutamatergic neurons (446) and wake-active cholinergic (48, 134, 897, 1332) and orexin (1444) neurons, as well as on inhibitory GABAergic inputs to sleep-active VLPO neurons (203, 888). Infusion of A1 receptor agonists in the BF, laterodorsal tegmentum, lateral hypothalamus, and prefrontal cortex increases sleep, whereas infusion of A1 receptor antagonists in the same areas increases waking (14, 1022, 1039, 1247, 1277, 1332). Although adenosine A1 receptors have effects in multiple regions of the brain controlling sleep-wakefulness, as mentioned above, to date adenosine levels have only been shown to increase with prolonged wakefulness in the BF and neocortex. Consistent with the BF being a crucial site mediating adenosine effects, local perfusion of an A1 receptor antagonist in this region activated wake-active neurons (17, 1276), and localized suppression of A1 receptor expression using antisense oligonucleotides significantly reduced spontaneous sleep time as well as the homeostatic sleep response (1282). In contrast, adenosine A1 receptor blockade in the lateral hypothalamus did not block the homeostatic sleep response (1277). While sleep homeostasis was intact in constitutive A1 receptor knockout mice (1214), conditional deletion of A1 receptor in forebrain and brain stem after 6–8 wk of age, circumventing developmental compensation, not only resulted in a decreased homeostatic sleep response after sleep restriction but also led to a failure in working memory consolidation (sect. V) (99).
Prolonged sleep deprivation upregulates A1 receptor mRNA and protein in BF and cortex in both rats and humans (74, 76, 340, 341). Upregulation of adenosine receptors provides an additional level of homeostatic control beyond rises in extracellular adenosine levels. The intracellular signaling pathway leading to this positive feedback regulation was revealed in experiments in rat BF (79) (FIGURE 8). The A1 adenosine receptor, coupled to the inhibitory Gi-3 G protein, is capable of “dual signaling,” i.e., inhibition of adenylyl cyclase and stimulation of PLC (422). Increased stimulation of the A1 receptor during sleep deprivation activates the PLC pathway, mobilizing intracellular calcium which in turn activates the transcription factor NF-kB and upregulates A1 receptor expression (73, 74).
What is the involvement of the adenosine A2A receptor in the homeostatic sleep response of adenosine? A2A receptors are coupled to the stimulatory Gs subunit and activate adenylyl cyclase. In contrast to the A1 receptor with its wide distribution in brain, the distribution of A2A receptor is more restricted to basal ganglia structures such as striatum, nucleus accumbens, and olfactory tubercle with much lower abundance in other areas such as the hippocampus, neocortex, BF, and other sleep-wake regulatory structures (1076). In rats, selective A2A receptor agonists such as CGS21680 administered to the subarachnoid space adjacent to BF and ventrolateral preoptic area (VLPO) induce NREM sleep (485, 1123, 1124, 1320). The local application of the A2A receptor selective agonist CGS21680 increases Fos expression in the VLPO (1128). Consistent with this are the observations that adenosine excites one subpopulation of sleep-promoting VLPO neurons via A2A receptors (404), and administration of CGS21680 into the lateral preoptic area close to the VLPO induces sleep (850). In A2A receptor knockout mice, the homeostatic sleep response following sleep deprivation (1320) and the wake-promoting effects of caffeine were blocked (529), although given the strong expression of this receptor in the basal ganglia, effects on motivation or motor behavior may confound these results. Accordingly, the locomotor stimulatory effects of caffeine mediated via A2A receptor blockade were shown to require the presence of A2A receptors in the nucleus accumbens (694).
The importance of adenosine as a sleep factor is supported by studies of enzymes involved in adenosine metabolism. Adenosine deaminase is involved in clearance of adenosine from the extracellular space. Blocking its activity using coformycin increased extracellular adenosine and sleep (949). The enzyme adenosine kinase phosphorylates adenosine to adenosine monophosphate, and blocking adenosine kinase activity with ABT-702 increased sleep in rat (1036). These data from rats are consistent with findings in mice that a genomic region encoding adenosine deaminase influences the rate at which NREM sleep need accumulates during wakefulness (sect. VI) (378). In humans, a genetic variation of the adenosine deaminase gene resulting in an amino acid substitution (asparagine for aspartic acid) results in decreased enzyme activity and is associated with increased sleep time and sleep intensity (687, 1059).
B) NO
NO is a small gaseous molecule with multiple roles in the control of sleep and wakefulness (407) (see also sects. II and IV). In the brain, it is predominantly synthesized under basal conditions by neuronal NO synthase (nNOS) and endothelial NO synthase (eNOS) in blood vessels. nNOS is highly expressed in brain stem cholinergic neurons and participates in their control of cortical activation and REM sleep. In this section we focus on the role of NO in the homeostatic regulation of NREM sleep.
I) NO promotes NREM sleep. The enzymatic activity of NOS is highest in the rat brain when animals are awake (57), and NO itself is detected in higher quantities in the cortex during waking (167). Systemic or intracerebroventricular (icv) administration of NOS inhibitors at light onset reduced NREM sleep within the first few hours in rats (199, 324, 325, 599, 882, 883, 1062) and in rabbits (601). Thus, although some studies (1347) provided contradictory evidence, most evidence suggests that NO promotes NREM sleep. Recent studies suggest that NO produced by the inducible isoform of NOS (iNOS), is responsible for this effect. Microdialysis infusion of an iNOS selective inhibitor, during sleep deprivation prevented NREM sleep rebound (593), while an inhibitor of nNOS decreased REM recovery but did not affect NREM recovery (593). Consistent with these findings, iNOS knockout mice had less NREM sleep during the dark period (218). The induction of sleep deprivation-induced iNOS occurs in neurons within the BF (590). The iNOS expressing neurons also express Fos during sleep deprivation, suggesting they are wake active and the number of iNOS/Fos double-labeled neurons positively correlates with the sleep pressure following prolonged sleep deprivation (590). Furthermore, NO itself also rises during the early stages of sleep deprivation as assessed by the dye 4,5-diaminofluorescein diacetate (DAF-2/DA), which fluoresces upon binding NO. Currently, the cellular signaling pathway by which sleep deprivation leads to the induction of iNOS is unknown.
II) How does NO produced by iNOS cause sleep in the BF?. NO has multiple cellular effectors, but one which may be particularly important in the context of the homeostatic sleep drive is release of adenosine (FIGURE 8). In vitro studies in culture and brain slices have shown that NO donors cause release of adenosine, which in turn inhibits neuronal activity (149, 352, 1075). Multiple pathways for NO-stimulated adenosine release may exist including inhibition of adenosine kinase (1075), inhibition of glycolysis and the mitochondrial electron-transport chain with a subsequent decrease in ATP/ADP ratio (150), and stimulation of neurotransmitter release leading to corelease of ATP which is subsequently degraded to adenosine (142). In vivo, NO production and adenosine increased concurrently in the BF during sleep deprivation (589, 593). Furthermore, under nondeprived conditions, microdialysis perfusion of an NO donor, DETA/NO, mimicked the ability of sleep deprivation to increase adenosine and NREM sleep (589). Recent in vitro electrophysiological evidence suggests that NO causes an initial excitation of cholinergic and cortically projecting GABAergic BF neurons which is followed by a long-lasting inhibition that can be reversed with an adenosine A1 receptor antagonist, suggesting mediation by adenosine (154). Similarly, in vivo, the effects of NO donors on BF neurons were found to be dose-dependent, with lower doses favoring excitation and higher doses leading to more inhibition (662, 663). Similar mechanisms may also be active in the perifornical lateral hypothalamus (661).
III) Sleep-active cortical interneurons contain nNOS. The presence of nNOS has been recently described in a small subset of cortical interneurons which are sleep active as determined by Fos immunohistochemistry (416, 620). In rats, mice, or hamsters, a majority of cortical GABAergic interneurons that express nNOS also express Fos during the recovery sleep that follows sleep deprivation. Fos expression in these sleep-active, nNOS-immunoreactive neurons parallels changes in the intensity of slow-wave activity in the EEG, and thus these neurons are suggested to be part of the neurobiological substrate that underlies homeostatic sleep regulation (416, 620, 984).
C) Prostaglandin D2
Prostaglandins are lipid signaling molecules produced from arachidonic acid through the cyclooxygenase pathway. The most abundant prostanoid in the brain, prostaglandin D2 (PGD2), meets all the criteria of a potent sleep-factor (485, 531, 943). Infusion of PGD2 into the third ventricle or preoptic area of rats (546, 1316, 1317) or the third ventricle of monkeys (953) increased sleep in a dose-dependent manner. Further investigations in rats showed that the levels of PGD2 in CSF increase with increasing time awake and propensity to sleep (963, 1043). The effects of PDG2 are mediated via the prostaglandin receptor 1 (DP1 R) as shown by the absence of sleep-inducing effects of PGD2 infusions in DP1R knockout mice (867). Blocking the PGD2 receptor with a selective antagonist, ONO-412, reduces sleep time (485). Lipocalin-type prostaglandin synthase (L-PGDS) is expressed in the brain and has been associated with sleep-wake regulation (1031). Animals that overexpress human L-PGDS show a significant increase in NREM sleep that is positively correlated with the increases in PGD2 produced in the brain (1004). Inhibitors of L-PGDS, such as selenium-based compounds, inhibit sleep, an effect that is reversed by subsequent administration of PGD2 (812). In L-PGDS knockout mice, the PDG2 levels do not increase following sleep deprivation (485).
The somnogenic effects of PGD2 are predominantly mediated via the membranes surrounding the brain in the leptomeninges/ arachnoid space (485). The expression of both L-PGDS and DP1R is mainly observed in the rostroventral area of the subarachnoid space near the BF which is the most potent site for the sleep promoting effects of PGD2; in this area, L-PGDS and DP1R expression is colocalized in arachnoid trabecular cells (95, 867) along with the synthesizing and degradation enzymes for adenosine (949). PGD2 infusion induces Fos expression in the sleep active neurons of the VLPO area (1126). Furthermore, the sleep-promoting effects of PGD2 have been shown to be mediated by A2A receptors in the VLPO. Subarachnoid administration of an A2A agonist induces Fos expression in the VLPO and increases NREM sleep (1128). The activation of DP1R by PGD2 in the meninges is followed by an increase in adenosine that acts on the A2A receptor in the sleep promoting preoptic area (867). Blocking the A2A receptor with the selective antagonist KF17837 also blocks the sleep-inducing effects of PGD2. The role of adenosine in the leptomeninges has been demonstrated by infusion of A2A selective agonist CGS 21680, leading to increased Fos expression in VLPO and increased sleep (518, 1128). Like adenosine, PGD2 is implicated in the homeostatic sleep response as animals that lack L-PDGS or PGD2 receptors fail to exhibit a sleep rebound in response to sleep deprivation (484, 485) and infusion of PGD2 mimics the effects of prolonged wakefulness in promoting sleep (1125).
Clinical data suggest that the PGD2-A2A sleep inducing system may be particularly important in mediating enhanced sleep in pathological states. For instance, synthesis of PGD2 was massively upregulated in the CSF of patients with African sleeping sickness (991). L-PGDS was also upregulated in sleep apnea patients exhibiting excessive daytime sleepiness (EDS) compared with controls or patients without EDS (70).
D) Cytokines
Cytokines are best known for their role in the immune system response to infection which includes enhanced sleep (543). Several cytokines and their receptors are present in the brain, and even in the absence of immune challenge, they are involved in sleep regulation (543, 669). Among the different cytokines, the most convincing evidence for a sleep-regulatory role is available for interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α). Administration of either of these cytokines increases NREM sleep in mice, rats, rabbits, cats, and sheep (603, 669, 670, 943). In humans, IL-1 administration results in fatigue and sleepiness (603, 669, 670, 943). Consistent with their somnogenic role, the endogenous brain and plasma levels of IL-1 and TNF-α increase with increased propensity to sleep. For example, in rat, the mRNA and protein expression of IL-1 and TNF-α in brain shows diurnal variation with changes paralleling the amount of NREM sleep (135, 368, 1262). In cat cerebrospinal fluid, the changes in IL-1-like activity correspond to sleep-wake behavior (762). In humans, plasma concentrations of IL-1 peak at sleep onset (874). The mRNA levels of these cytokines increase during sleep deprivation (1262).
The somnogenic effects of IL-1 and TNF-α are mediated through the IL-1 type 1 receptor and the TNF 55-kDa receptor. Mice lacking these receptors showed reduced sleep and failed to respond to IL-1 and TNF-α (353, 354). Antagonists against IL-1 and TNF-α receptors reduce sleep (954, 1267, 1368). Reduction of sleep in IL-1 receptor knockout mice was predominantly observed during the dark period, whereas TNF receptor knockout mice showed decreased sleep during the transition from the dark to the light period, suggesting involvement in the circadian component of sleep regulation. Accordingly, TNF-α has been shown to inhibit the expression of clock genes by interfering with the interaction of CLOCK-BMAL1 with the E-box regulatory element (198).
I) Mechanisms underlying cytokine-induced sleep. It has been suggested that the release of extracellular ATP associated with neurotransmitter release during waking promotes astrocytic production of IL-1 and TNF-α via activation of P2 purinergic receptors (97, 1253). In addition to effects on cortical synapses (see next section), cytokines may act to induce sleep by affecting monoaminergic neurons that express the IL-1 receptor. Administration of IL-1 into the DRN or LC (293) induces sleep (409, 788), and blocking the 5-HT2 receptor attenuates the sleep-inducing effects of IL-1 (542).
2. Local regulation of sleep homeostasis
Sleep is normally a global, coordinated phenomenon affecting the whole organism and nervous system. However, recent evidence suggests that slow waves and spindles can be induced or modulated locally in cortical areas (532, 671, 921, 1383). The most striking example of local sleep is the unihemispheric sleep exhibited by cetaceans (766). In rodents, multiunit recordings showed that groups of cortical neurons display coordinated off-states, associated with local slow waves as the duration of wakefulness increases, even while the scalp EEG shows a LVFA pattern typical of wakefulness (1383). Dissociation of different sleep/wake phenomena is also a feature of several sleep disorders (778). A number of cortically produced neuromodulators could be involved in local modulation of sleep intensity. These include 1) adenosine and NO (discussed above); 2) TNF-α, which is a regulator of sleep and sleep intensity and also regulates synaptic homeostasis (1213); 3) brain-derived neurotrophic factor (BDNF), which is involved in the establishment of neuronal circuitry during development and promotes synaptic plasticity in the adult. Cortical infusion of BDNF locally enhances NREM slow-wave activity, whereas infusion of a BDNF antibody or an inhibitor of trkB receptors causes the reverse effect (355). 4) Cortistatin is a recently discovered peptide neuromodulator structurally related to somatostatin which is expressed in cortical and hippocampal interneurons and hyperpolarizes hippocampal pyramidal neurons (289). Intraventricular administration of cortistatin increased cortical slow waves (127, 289). Furthermore, the cortistatin transcript was upregulated following sleep deprivation (127, 232). 5) Growth hormone releasing hormone (GHRH): local administration of a GHRH antagonist or siRNA targeting GHRH to the somatosensory cortex increased EEG delta power during NREM sleep but not during waking (718). Use-dependent alterations in the release of these neuromodulators may account for local cortical changes in synaptic weights and/or cortical region-dependent alterations in NREM delta activity (532, 609, 865, 1381).
D. Functional Aspects of NREM Sleep
1. Brain energy metabolism
The brain constitutes only 2% of the body mass. However, brain oxygen and glucose utilization account for ~20% of those of the whole organism (775), consistent with the high energetic costs incurred by neural tissue, both whilst processing information and at rest (54). Compared with wakefulness, sleep reduces brain energy demands, as suggested by the 44% reduction in the cerebral metabolic rate (CMR) of glucose (791) and a 25% reduction in the CMR of O2 (774) during sleep.
A) Local Changes in Atp Usage During the Sleep-Wake Cycle
Under normal physiological conditions, use-dependent variations in the CMR of glucose and O2 maintain a balance in brain energetics at all times to maintain a stable level of the high-energy molecule ATP. At the molecular level, this is achieved due to tightly coupled ATP biosynthesis/ usage and an efficient buffering of ATP energy via paired creatine kinase reactions to conserve ATP energy in the form of phosphocreatine and its release, as needed, in the cell (1477). However, recent studies indicate that transient changes in brain ATP levels can occur in a brain region specific- and treatment-dependent manner. For example, altered ATP levels have been observed in response to localized electrical stimulation, glucose deprivation, or manipulations of Na+-K+-ATPase activity (56, 225, 764, 773). In a recent rat study, ATP tissue levels showed a surge in the initial hours of spontaneous sleep in wake-active but not in sleep-active brain regions of rat (323). The surge was dependent on sleep but not on time of day, since preventing sleep by gentle handling of rats for 3 or 6 h delayed the surge in ATP. A significant positive correlation was observed between the surge in ATP and EEG NREM delta activity (0.5–4.5 Hz) during spontaneous sleep. Inducing sleep and delta activity by adenosine infusion into the BF during the normally active dark period also increased tissue levels of ATP. Taken together, these observations suggest that a surge in ATP occurs when the neuronal activity is reduced, as occurs during sleep. The levels of the cellular energy sensor, phosphorylated AMP-activated protein kinase (P-AMPK), show reciprocal changes to ATP levels. Thus P-AMPK levels are lower during the sleep-induced ATP surge than during periods of wakefulness or sleep deprivation. Together, these results suggest that the sleep-induced surge in ATP and the decrease in P-AMPK levels set the stage for increased anabolic processes during sleep and provide insight into the molecular events leading to the restorative biosynthetic processes occurring during sleep (323) (FIGURE 9).
FIGURE 9.
Sleep and energy metabolism. The interaction between state-dependent changes in ATP, AMPK, and AMPK-regulated anabolic and catabolic pathways is shown. Wakefulness and sleep deprivation are both characterized by increased neuronal activity and increased consumption of ATP. A higher AMP/ATP ratio results in and leads to increased phosphorylated-AMPK (P-AMPK), promoting catabolic processes. Sleep states are characterized by increased NREM delta activity, low neuronal activity, and a rise in ATP levels. The resulting lower AMP/ATP ratio leads to decreased phosphorylated AMPK, promoting anabolic processes, such as synthesis of proteins, glycogen, and fatty acids. [Adapted from Dworak et al. (323).]
B) Adenosine as a Regulator of Brain Energy
One of the proposed functional theories for adenosine’s role in sleep-wake behavior suggests that adenosine, a by product of energy metabolism, may serve as a homeostatic regulator of brain energy during sleep (91, 1130). Extracellular adenosine concentrations rise with increased metabolism and neural activity (1027). Wakefulness has a greater metabolic rate than NREM sleep (774, 791), and accordingly, extracellular adenosine levels in neostriatum and hippocampus were higher during the circadian active period and lower during the circadian inactive period in rats (541). Differential pulse voltammetry using a glucose sensor in cortex reveal that extracellular glucose levels are higher during NREM sleep compared with waking, an observation consistent with the idea that energy metabolism and glucose utilization/breakdown decrease during NREM sleep compared with waking (915). Thus changes in adenosine levels during spontaneous sleep-wake and sleep deprivation conditions may be direct reflections of ATP breakdown.
C) Hypothalamic Neuromodulators Linking Metabolism and the Sleep-Wake Cycle
Several hypothalamic neurotransmitter systems link energy usage and arousal (658). The role of the orexins was discussed in section II. Several recent studies have suggested that the GABAergic/MCH neurons may be involved in connecting metabolism and sleep (1353). Mice lacking MCH or the MCH 1 receptor eat less, are lean, and have a higher metabolic rate (804, 1164). Recordings from identified MCH neurons across the sleep-wake cycle revealed that they are silent during wake and fire occasionally during NREM sleep but maximally during REM sleep (482). This silence during waking is likely due to direct inhibition by norepinephrine, serotonin, and acetylcholine (1325). Intracerebroventricular injections of MCH increased REM sleep via an increase in the number of REM episodes and increased NREM sleep to a lesser extent (1353), while an MCH1 receptor antagonist decreased NREM and REM sleep (12). The mechanisms connecting the energy conservation and sleep promoting functions of this peptide remain to be established.
2. Synaptic homeostasis
Together, the slow oscillation and cortical delta waves are termed SWA. Recent evidence suggests that sleep intensity, as measured by SWA, can be modulated locally in the cortex in a use-dependent fashion (532, 671, 1298). What might be the function of such a local regulation? Learning and synaptic plasticity studies, together with the results of experiments investigating genomic and proteomic changes during sleep (see sect. V), led Cirelli and Tononi to propose the synaptic homeostasis theory of sleep (1298). This theory proposes the following:
1) Wakefulness is associated with net synaptic potentiation. Gene and protein studies showed that animals killed following prolonged periods of wakefulness have upregulated levels of genes/proteins implicated in long-term potentiation, whereas genes/proteins implicated in long-term depression were increased following periods of sleep (241, 242). A recent ex vivo slice study showed that the frequency and amplitude of miniature excitatory postsynaptic currents (EPSCs) recorded from pyramidal neurons in the prefrontal cortex were higher following a prolonged period of waking and decreased after sleep, independent of time of day (737). Although consistent with the hypothesis, it should be noted that changes in miniature EPSCs may not accurately reflect action potential-dependent changes in synaptic strength. Several other theories of sleep and memory are based on the premise that long-term potentiation is induced during sleep (53, 175, 1417), especially during the sharp-wave/ripple complexes and spindles of NREM sleep (175, 179) or in association with the theta rhythm during REM sleep (534) (sect. V). More direct evidence of synaptic potentiation was found in Drosophila (172), where synapse size and number increased with waking and decreased during sleep.
2) Synaptic potentiation is coupled with the homeostatic regulation of cortical slow-wave activity. This idea is supported by large-scale computational models of the thalamocortical system (350, 507). It is further bolstered by experiments in which application of neuromodulators previously shown to enhance LTP, locally enhanced SWA in subsequent sleep periods (see sect. IIIC above) and learning experiments showing local increases in SWA in cortical areas following learning of a particular task (532). For instance, in an experiment pairing transcranial magnetic stimulation of the motor cortex with median nerve stimulation, subjects showing a potentiation of local field potentials also had a local increase in slow-wave activity during subsequent sleep (533).
3) SWA leads to synaptic downscaling, allowing improved cognitive performance following NREM sleep. Local neocortical field potential recordings in rats (1385) and high-density EEG recordings in humans (1063) showed that the slope and amplitude of slow waves were lower at the end of the sleep phase compared with those during sleep at the beginning of the sleep phase, findings which are consistent with synaptic downscaling if, as suggested by modeling studies, slow wave slope and amplitude reflect synaptic strength. Furthermore, single and multiunit recordings showed that the firing rate of neocortical neurons was higher following a period of sustained wakefulness, whereas firing rates and synchrony decreased following a period of sustained sleep (1384). These findings are also consistent with the increased energetic cost of wakefulness discussed in the previous section.
Although the synaptic homeostasis theory is an attractive hypothesis supported by a large amount of evidence, several questions remain. 1) Many different forms of NMDA receptor-dependent and NMDA receptor-independent synaptic plasticity have been described. Which of these are involved in sleep-dependent changes? 2) Are homeostatic changes restricted to synapses between glutamatergic neurons? So far experiments have targeted cortical regions and not investigated other brain areas such as the striatum and cerebellum where GABA neurons predominate and homosynaptic long-term depression of glutamatergic synapses is the predominant form of synaptic modification associated with learning. Furthermore, how inhibitory synapses change across the sleep-wake cycle remains unclear. Experiments in zebrafish revealed circadian and homeostatic changes in synapses from orexin neurons (36), suggesting that neuromodulatory synapses also change according to the sleep-wake cycle. 3) What happens to synapses during REM sleep?
E. Synthesis
The induction of sleep is mediated by the build-up of homeostatic sleep factors such as adenosine and NO in the BF and cortex and by increased activity of median preoptic nucleus GABA neurons that inhibit the wake-promoting neurons of the ARAS. Circadian influences are mediated by retinal and indirect SCN projections to GABAergic sleep-promoting neurons in the VLPO and other regions of the preoptic area and BF. Once initiated, sleep and the silence of cortically projecting wake-active neurons is maintained by increased firing of VLPO and other preoptic/BF GABA neurons and subsequent postsynaptic inhibition of ARAS neurons via activation of GABA and galanin receptors. As ascending excitatory influences are progressively withdrawn, cortical and thalamic neurons become increasingly hyperpolarized entering into the range of membrane potentials conducive to rhythmic bursting due to activation of Ih and It currents and leading to the characteristic EEG phenomena of NREM sleep. During early NREM sleep, effective cortical connectivity (assessed by transcranial magnetic stimulation) begins to break down (808), and in deep sleep, the activity of the brain regions comprising the default network becomes decoupled, in particular the frontal cortex (523). Local cortical differences in delta power during NREM sleep reflect the extent to which that cortical area was active during the prior waking period and the duration of prior waking, possibly reflective of increased synaptic potentiation during waking with respect to sleep. Energy usage is high during waking and during sleep deprivation, due to increased neuronal firing, synaptic activity, and synaptic potentiation. This increased energy usage of waking is reflected in increased release of sleep homeostatic factors such as adenosine during waking and a surge of ATP production which occurs during the early NREM sleep that follows waking. Synaptic activity and plasticity are major components of brain energy usage (775), potentially tying together the proposed energetic and synaptic plasticity functions of NREM sleep. Under normal conditions, wakefulness transitions first into NREM sleep and then later transitions into REM sleep. The mechanisms underlying REM sleep and the cyclic oscillation of NREM and REM sleep during the night are described next.
IV. REM SLEEP
Dreaming, which occurs most frequently in REM sleep, has inspired and fascinated artists, writers, and scientists for centuries. However, REM sleep was only defined as a separate brain state relatively recently. Experiments by Aserinsky, Dement, and Kleitmann in humans (50, 299) and Jouvet in animals (580) showed that REM sleep is defined by a distinct constellation of tonic and phasic features of the EEG and EMG. The association of an activated cortical EEG reminiscent of waking, together with paralysis of antigravity muscles (FIGURE 1), led Jouvet to term this state Le sommeil paradoxal paradoxical sleep). In young animals, where REM is the predominant sleep state (585, 1070), it is also known as active sleep (NREM sleep is called quiet sleep in this terminology). Awakening human subjects during REM sleep commonly led to reports of dreaming (300); thus REM sleep has also been called dream sleep, although some dreaming also occurs in NREM sleep (509). Since the discovery of REM sleep, research on REM sleep can be subdivided into three main areas: 1) delineation of the neurons, circuits, and neurotransmitter effector systems responsible for the individual signs of REM sleep; 2) control of when and for how long REM sleep is expressed (REM sleep master control mechanisms); and 3) investigation of the relation of REM sleep to learning and memory. In this section we focus on the first two of these areas and the relation of REM sleep to dreaming; studies of REM sleep and learning and memory are covered in section V.
The terminology used to describe the reticular formation regions involved in REM-sleep control (FIGURE 2) differs between brain atlases, between species, and between different investigators. In the cat, the REM control area was defined as a region immediately ventral to the main cluster of noradrenergic neurons in the LC and dorsal to the gigantocellular tegmental field (FTG). This region was termed subcoeruleus (SubC) which is the term used in this review. A subset of this area, containing caudally projecting neurons involved in REM muscle atonia, and with tonic firing patterns very specific to REM sleep (1093) was termed the peri-LC alpha. Slightly lateral to this area, neurons with burst firing patterns correlated with PGO waves were found in the parabrachial area (276, 824, 1092), which at its most rostral extent also includes the cholinergic PPT area. In the rat and mouse brain atlases of Paxinos and colleagues (988, 989) the REM control region is also termed SubC (dorsal or alpha parts); however, in these species the region immediately ventral to the LC is cell-poor and the REM-control neurons (particularly those involved in muscle atonia) appear to be located slightly more rostrally, ventral to the cholinergic neurons of the laterodorsal tegmental nucleus (LDT). Hence, the functionally equivalent region in the rat or mouse has been termed the sublaterodorsal nucleus (SLD). This area corresponds to the rostral part of the subcoeruleus area as defined by Paxinos and Watson in the rat (160). REM-on neurons in this area are primarily glutamatergic, as indicated by vGLUT2 in situ hybridization and Fos immunohistochemistry (248). Other nearby reticular formation areas containing glutamatergic neurons such as the nucleus pontis oralis (PnO) and nucleus pontis caudalis (PnC) play a role in particular aspects of REM sleep such as theta rhythm generation or rapid eye movements.
I) Spike discharge characteristics of neurons in regions of the brain stem involved in REM control see also sect. IVB) Intracellular recordings from medullary and pontine reticular formation neurons in naturally sleeping cats revealed that the excitability of these neurons was greater during REM than during NREM sleep or waking, i.e., there is a tonic depolarization during REM (209, 549) (FIGURE 10). Phasic depolarizations associated with action potential firing were superimposed upon this tonic depolarization (549). In contrast, the membrane potential was more hyperpolarized during waking and NREM sleep with phasic depolarizations occurring with motor activity during waking. When recorded in vitro, SubC “reticular” neurons fire tonically at high rates with little adaptation when depolarized (160), as in other reticular areas (418, 934). Identified GABAergic neurons in this area show similar properties (156), making it difficult to distinguish GABAergic from glutamatergic neurons solely on the basis of intrinsic membrane properties. A subset of the reticular formation neurons fire stereotyped high-frequency bursts of action potentials due to the presence of low-threshold calcium channels that are deinactivated by hyperpolarization (160, 448). Cholinergic neurons also exhibit this property (whereas most aminergic brain stem neurons do not). These neurons may be involved in phasic phenomena of REM sleep such as PGO waves. Interestingly, many presumed REM-on neurons in the SubC exhibit “spikelets” (small alterations in membrane potential resembling low-pass filtered action potentials), indicating that they are likely to be electrically coupled (489) and suggesting a possible mechanism by which their firing may be synchronized.
FIGURE 10.
Pontine tegmental membrane depolarization and action potential activity increases prior to and during REM sleep. A: first trace is nuchal (neck) EMG in the cat, showing a lack of muscle tone during REM sleep; second trace is frontal cortex EEG, showing low-amplitude activity during REM sleep; third trace is lateral geniculate nucleus (LGN) neuronal activity, revealing PGO waves immediately preceding and during REM sleep; fourth trace is extraocular muscle EOG, showing eye movement during REM sleep; and fifth trace is the membrane potential record for one pontine tegmental neuron (MP). B: oscilloscope photographs depict the changes of action potential frequency that accompany MP depolarization. Arrows in the MP trace of A correspond to the eight oscilloscope photographs of B showing tonic neuronal firing during transition into REM sleep, the REM sleep episode, and transition out of REM sleep. REM, REM sleep; NREM, NREM sleep; T, transition; W, wake; Wm, wake with movement. [Adapted from Ito et al. (550).]
A. REM Sleep Signs
1. Electrographic signs of REM sleep
Ascending pathways from the brain stem areas that control activation of the neocortex during waking and REM sleep were covered in section II. We focus here on REM specific aspects of hippocampal theta rhythms and PGO waves, which appear prior to the REM state and define the transitional REM period (t-REM) in between NREM and REM sleep.
A) Theta Activity
Theta rhythmic activity is a prominent feature of the EEG during REM sleep in rodents (FIGURE 1) and other lower mammals (1069). Early studies in rats and Type I (4–7 Hz) was observed when the animals were under urethane or ether anesthesia and during behavioral immobility and was abolished by systemic administration of the muscarinic antagonist atropine sulfate (667). A higher frequency form of theta (type II theta, 7–12 Hz) was observed during waking associated with movement and was abolished by urethane anesthesia. Theta occurring during REM sleep appears to represent a combination of type I and type II, since atropine sulfate abolished continuous lower-frequency theta during tonic REM periods whilst leaving intact intermittent, higher frequency hippocampal theta during periods with muscle twitches (1069, 1166). Similarly, mice with a knockout of phospholipase β1 (an effector for M1-type muscarinic receptors) lacked type I theta activity and had only intermittent theta activity during REM sleep (1166). A recent genetic study suggests that theta generation during REM is different from theta during waking. A deficiency in short-chain fatty acid β-oxidation (affecting the enzyme short-chain acylcoenzyme A dehydrogenase, Acads) caused a slowing (by ~1 Hz) of peak theta frequency during REM sleep in mice but did not affect waking theta (1260).
I) Theta rhythm during REM sleep in humans. Low-frequency (4–7 Hz) theta activity has also been observed in the human hippocampus (of epileptic patients) during sleep but, in contrast to rodents, theta rhythm was not observed continuously but rather was limited to short (1 s) epochs (188). The occurrence of these short theta epochs in humans was not correlated with the occurrence of rapid eye movements. A further contrast to rodents was the finding that theta activity was not observed in the basal temporal lobe or frontal cortex during REM (188). Another study has demonstrated a type of rhythmic slow activity in the hippocampus during REM sleep with a slightly lower peak frequency (1.5–3 Hz; delta), leaving open the possibility that these EEG signals in humans are equivalent to the type I and type II theta but that the peak frequencies for the two types are shifted to lower values (113).
II) Brain stem generation of theta rhythm during REM sleep. The ascending pathways controlling forebrain theta rhythm are discussed in section IIA. One recent study (758) suggested that a region just dorsal to the LC termed precoeruleus is necessary for theta activity during REM sleep. This region was found to provide the major glutamatergic input to the MS/vDB and contained cells that were active during REM sleep (contained Fos immunoreactivity). Ibotenic acid lesions of this area abolished theta rhythm during REMsleep (758). However, this part of the study was based on a small number of animals, and no quantification of damage to surrounding regions containing cholinergic neurons (known to be important for control of theta rhythm) was reported. Therefore, the precise role of the precoeruleus in theta rhythm generation during REM sleep awaits further confirmation with more targeted lesions (i.e., lesions affecting only glutamatergic neurons).
B) Pgo Waves
Synchronized electrical field potentials in the pons, lateral geniculate nucleus, and occipital cortex (PGO waves) occur singly at high amplitude in the period immediately preceding the onset of REM sleep (transitional REM period; 30–90 s) and in bursts of lower amplitude during REM sleep itself (98, 146, 274, 580, 584, 1228). They are considered the source of dreaming episodes and visual imagery during REM sleep (1228) since they occur simultaneously with rapid eye movements associated with gaze direction in dream imagery (300, 825) and are prominent in visual thalamocortical circuits (144, 145, 901). PGO waves have been studied most intensively in cats, where the largest potentials can be recorded in the LGN and occipital cortex. However, more recent studies in both animals and humans describe a widespread activation of limbic, parahippocampal and many thalamocortical systems during these phasic REM events (26, 274, 1395). The pontine component of PGO waves has been recorded in rats (286, 356, 610, 1113), but the thalamic (dLGN) component is difficult or impossible to record in rodents, likely due to the smaller size of this region and the fact that rodents are not very visual animals. However, phasic potentials during REM sleep can be recorded in several other forebrain areas in this species (274). In non-human primates, PGO wavelike phasic field potentials have been recorded from the LGN and pons of macaques (251) and the LGN of baboons (1380). In humans, two recent studies recorded phasic field potentials during REM sleep in the pons (720) and subthalamic nucleus (364), respectively. Furthermore, event-related fMRI showed activation of the thalamus and occipital cortex, as well as limbic regions such as the hippocampus and amygdala phase-locked to rapid eye movements (866, 1395). Thus phasic activations of thalamocortical and limbicbrain regions generated by synchronized activation in the pons appear to be typical of both animals and humans, although the pattern of forebrain activation may vary according to the species studied.
I) The thalamocortical component of PGO waves. Thalamic PGO waves are biphasic field potentials with an initial negative-going component (1228). Although the thalamic component can be recorded most easily in the lateral geniculate nucleus (LGN) due to its laminated structure (and resulting summation of extracellular field potentials), they also occur in other thalamic nuclei such as the pulvinar, rostral intralaminar, and anterior nuclei. In humans, PGO waves have also been observed with depth recordings from the subthalamic nucleus (364). The cellular mechanisms underlying the thalamic component of PGO waves were investigated by Hu, Steriade and Deschenes using intracellular recordings in urethane-anesthetized and reserpine-treated cats (526). In these animals, each PGO wave was accompanied by a depolarization of relay neurons lasting 200–300 ms, interrupted by a short-lasting (50–60 ms) hyperpolarization occurring 40–80 ms after the onset of the depolarization due to the coactivation of GABAergic geniculate interneurons. Action potential firing may precede or follow the brief hyperpolarization. The negative-going component of the field component correlates with the early depolarization recorded intracellularly, whereas the subsequent positive-going component reflects the subsequent hyperpolarization. In recordings from naturally sleeping animals, single PGO waves of high amplitude can be recorded during the period of NREM immediately preceding REM when thalamic relay neurons are still hyperpolarized (1233). The firing of relay cells causes the depolarization of pyramidal neurons in the primary visual cortex (occipital cortex), the cortical component of PGO waves.
II) PGO waves require cholinergic input to the thalamus. The following evidence suggests that the induction of PGO waves in the thalamus is due to a strong cholinergic input from the brain stem, acting on ionotropic nicotinic receptors (274, 277, 1228): 1) brain stem cholinergic neurons send a massive projection to the LGN and other thalamic nuclei (291, 974, 1235). 2) Electrical stimulation of the brain stem in the region of cholinergic neurons elicits PGO-like waves in the LGN (1100). 3) Lesions in the region of brain stem cholinergic neurons abolish PGO waves (1394). 4) Neurons of the cholinergic LDT/PPT area fire in bursts that are correlated with thalamic PGO waves (336, 615, 665, 1099, 1234). These PGO burst neurons are inhibited by the cholinergic agonist carbachol, suggesting activation of inhibitory muscarinic autoreceptors (337, 665, 1097). 5) Systemic (1081) or iontophoretic (525) application of nicotinic antagonists into the lateral geniculate nucleus blocks the thalamic component of PGO waves. 6) Nicotinic receptor agonists depolarize LGN neurons (1476) and also pre-synaptically facilitate glutamatergic synaptic inputs (457). Together these pieces of evidence strongly support the idea that bursting of cholinergic brain stem neurons leads to a nicotinic receptor-mediated depolarization of LGN and other thalamic neurons which underlies the negative-going component of the field potential at the thalamic level.
III) Noncholinergic brain stem neurons generate the pontine component of PGO waves. The initial study of the pontine component of PGO field potentials (so-called P-waves) found that they can be recorded in a large swathe of the brain stem reticular formation, most prominently the part of the reticular formation close to the abducens nucleus (146). More recent studies in rats have focused on the SubC/parabrachial (PB) area (356, 610, 801) where there are relatively few cholinergic neurons (160) and on the equivalent caudolateral peribrachial region of the cat. This site is the most sensitive site for carbachol to enhance P-wave frequency (1033) and a concomitant facilitation of learning and memory formation (280, 286, 813). Conversely, neurotoxic lesions of this area abolish PGO waves (277). Neurons in the SubC/PB area fire synchronized bursts of action potentials preceding and phase-locked to PGO waves (274, 824, 1098, 1234). PGO-burst neurons in this region exhibit a longer latency (50–150 ms) to thalamic PGO waves than those in the regions with high concentration of cholinergic neurons projecting to the thalamus (10–25 ms). The stereotyped nature of the bursts in PGO burst neurons led Steriade and colleagues (973) to conclude that the bursts were mediated by a low-threshold calcium spike. Low-threshold bursts are present in brain stem cholinergic neurons (596, 763, 1410) and in noncholinergic neurons in the SubC region (160). Neurons with low-threshold calcium spikes in the SubC are hyperpolarized by carbachol (160). The inhibition of SubC neurons by acetylcholine during REM sleep will deinactivate their low-threshold calcium current and allow them to fire bursts of action potentials. This would also be consistent with the block of carbachol-induced PGO waves in the cat by a M2 receptor antagonist (283), since activation ofM2 receptors usually causes a hyperpolarizing response (332, 419). Low-threshold bursts in brain stem cholinergic neurons are inhibited by serotonin (763), likely explaining the inhibitory action of serotonin on PGO-wave generation (sect. IVB). Together, the data suggest that P-waves are initiated in the SubC/PB area and trigger bursts in cholinergic neurons projecting to the thalamus (274).
2. Muscle atonia and twitches
Atonia of skeletal muscles is one of the cardinal, defining features of REM sleep. The dramatic consequences of a malfunction in the brain mechanisms controlling atonia can be observed in the sleep disorders narcolepsy and REM sleep behavior disorder (RBD) (sect. VII). In narcolepsy, atonia is activated at inappropriate times, resulting in cataplexy (atonia triggered by emotional arousal) during waking, and sleep paralysis at the transition from sleep to wakefulness (923). Conversely, in RBD, atonia fails and patients act out their dreams, resulting in injury to themselves and their bed partners (1131).
A) Inhibition of Motoneurons During Rem Sleep
Intracellular recordings by Chase and colleagues in somatic (trigeminal) and spinal (lumbar) motoneurons of unanesthetized cats showed that REM atonia is accompanied by a hyperpolarization caused by a barrage of IPSPs, as well as a decrease in input resistance, making the motoneurons relatively insensitive to excitatory inputs (210, 889, 910). The IPSPs were completely blocked by local iontophoretic application of strychnine, an antagonist of glycine receptors but not by antagonists of GABAA receptors (picrotoxin or bicuculline methiodide), suggesting that glycine is the neurotransmitter involved (889, 1196). Opening of these chloride-permeable channels is likely responsible for the observed decrease in input resistance and reduced responsivity to synaptic inputs. Overall, the evidence that glycinergic inhibition is necessary for REM atonia remains strong (208, 1195). Nevertheless, although Chase and colleagues showed that local application of strychnine blocked all the effects observed during REM in the motoneurons they recorded, they did not show that an antagonism of glycine receptors blocks muscle atonia.
Recently, the well-accepted role of glycine in generating atonia has been challenged by Brooks and Peever (147), although their conclusions have been disputed (208, 398, 767, 1195). Brooks and Peever monitored the activity of masseter motoneurons extracellularly whilst using reverse microdialysis to apply pharmacological agents at the trigeminal motor pool (which innervates the masseter muscle). Antagonism of glycine receptors with strychnine or GABAA receptors with bicuculline increased masseter muscle tone during waking and non-REM sleep but not during the tonic periods of REM sleep. Thus they concluded that glycine and GABAA receptors are not involved in REM atonia (at least for somatic motoneurons). Similar results were described by others for respiratory hypoglossal motoneurons and associated genioglossus muscle activity (894, 895). Kubin and co-workers showed in the carbachol model of REM sleep that coantagonism of norepinephrine, serotonin, and GABA receptors abolished REM-sleep like depression of hypoglossal motor neuronal activity (361). However, Horner and colleagues (894) report contrasting results suggesting that GABAA receptors are important in control of hypoglossal muscle tone during NREM but not during REM sleep.
I) Disfacilitation of excitatory inputs during REM. Using the same methodology described above, Peever and colleagues (165) showed that trigeminal motoneurons receive a glutamatergic input during waking which helps maintain muscle tone. Motoneurons also receive excitatory input from brain stem norepinephrine and serotonin neurons during waking which depolarize them and increase their response to excitatory input (1403, 1404). During REM sleep, these neurons are silent so this excitatory influence is lost (disfacilitation). Indeed, antagonism of both norepinephrine and serotonin receptors at the hypoglossal motor nucleus abolished the REM-sleep like reduction of muscle tone induced by pontine carbachol in anesthetized rats (362). The shut-off of norepinephrine LC neurons (564, 1435) and reduced firing of DRN neurons (564) during REM-like cataplectic attacks in dogs support the importance of this mechanism.
Disfacilitation and active inhibition contribute to atonia. Although glycinergic inhibition may be the most important mechanism producing decreased firing of motoneurons during NREM and their silence during REM sleep, a combination of mechanisms including disfacilitation (reduced norepinephrine, serotonin, glutamate release) and active inhibition (increased glycine, GABA) is likely to be involved. In addition to these direct effects on motoneurons, a presynaptic inhibition of excitatory inputs could be important. In fact, in vitro evidence for a presynaptic inhibition mediated by muscarinic M2 receptors has been demonstrated for hypoglossal motoneurons (90). It is also worth recognizing the possibility that mechanisms controlling atonia and muscle twitches may differ between somatic (particularly respiratory) and spinal motoneuron pools (376, 398).
II) Muscle twitches are caused by phasic glutamatergic input. During REM, occasional bursts of muscle activity occur (muscle twitches). These twitches, particularly in developing animals, have been suggested to be important in the refinement of spinal cord connectivity (996). In intracellular recordings from lumbar motoneurons, twitches are accompanied by brief depolarizing events that can be blocked by the non-NMDA glutamate receptor antagonist kynurenate, but not the selective NMDA receptor antagonist APV (1197). Similarly, muscle twitches in masseter muscles were blocked by an AMPA/kainate receptor antagonist (CNQX) into the trigeminal motoneurons pool (165). Thus twitches during REM sleep are due to bursts of excitatory, glutamatergic AMPA/kainate receptor-mediated synaptic potentials.
B) Brain Stem Control of Atonia and Muscle Twitches
Increased activity of neurons in the SubC region of the pons causes muscle atonia. Lesions of several areas of the brain stem reticular formation cause a loss of muscle atonia during REM sleep and may lead to expression of motor behaviors during sleep (569, 893). Similarly, injections of carbachol at a variety of dorsal and ventral brain stem sites can lead to atonia (504, 798, 1057). However, the area most consistently implicated in atonia in both adult (119, 580, 758, 893, 1358) and neonatal animals (606) is the SubC/SLD of the dorsolateral pons (FIGURE 11): 1) small electrolytic lesions of the SubC abolish REM muscle atonia in the cat and in the rat, whilst larger lesions encompassing more widespread areas of the reticular formation are required for the expression of dreamlike (“oneiric”) behavior (606, 893, 900, 1111) typical of RBD (sect. VII). 2) Selective inactivation of glutamatergic neurotransmission of neurons in the SubC and neighboring LDT region in mice strongly reduced atonia and led to motor behaviors during REM sleep (668). 3) Rats receiving infusions of the fiber-sparing neurotoxin orexin-2-saporin into the SubC in the rat have increased limb movements during REM sleep (100), whereas injections of antisense oligonucleotides directed against the type II orexin receptor in the pontine reticular formation near the SubC cause cataplexy and increased REM sleep (1278). 4) Neurons have been recorded in the SubC in the cat (1099) and neonatal rat (606) which fire tonically at increased rates just prior to and during the muscle atonia of REM sleep. 5) Electrical stimulation of the SubC in rats which have had the forebrain removed (470) (i.e., decerebrate) elicits bilateral muscle atonia. 6) Application of the cholinergic agonist carbachol to this area in the cat causes, at short latency, a pharmacologically induced state virtually indistinguishable from REM sleep, including the muscle atonia component (62, 863, 1340, 1450). In the rat, carbachol does not consistently cause a REM-like state when injected into the SubC (119, 675), but a state similar to REM can be induced by SubC application of the GABAA receptor antagonists bicuculline and GABAzine (119, 1009).
FIGURE 11.
Descending circuitry responsible for muscle atonia during REM sleep. During REM sleep, descending pontine subcoeruleus (SubC) glutamatergic projections excite diffusely organized glycinergic neurons of the bulbar reticular formation, including the medullary ventral gigantocellular nucleus (GiV). GABAergic/glycinergic output from the GiV inhibits spinal motoneurons, producing muscle atonia. An alternative pathway consists of a direct SubC glutamatergic projection to the spinal cord, directly synapsing on inhibitory interneurons of the ventral horn. When activated, these interneurons inhibit the spinal cord motor neurons, again producing muscle atonia. Red lines denote excitation; black, inhibition. [Adapted from Pakinos and Watson (989), with permission from Elsevier.]
I) Descending projections of the SubC mediate atonia. Earlier work (119, 210, 681, 684, 776, 1458) suggested that the SubC region causes glycinergic inhibition of motoneurons via a glutamatergic activation of glycine neurons in the ventral medulla (1045) which project to the spinal cord (515) (FIGURE 11). Indeed, glycine-containing neurons in the nucleus reticularis gigantocellularis (NRGc)/nucleus magnocellularis (NMC) and nucleus paramedianus reticularis (nPR) express REM-sleep related Fos immunoreactivity (890), and electrical stimulation of the NRGc leads to IPSPs in spinal α-motoneurons (466, 1268). Glutamatergic stimulation of the NMC or cholinergic stimulation of the nPR in decerebrate cats is effective in eliciting muscle atonia (684). However, this pathway does not appear to be essential for REM atonia. In fact, transection at the pontomedullary junction abolishes inhibition of muscle tone produced by stimulation of the ventromedial medulla (1179), suggesting that stimulation of the medulla may in fact cause atonia via ascending activation of the SubC. Furthermore, recent experiments by Lu and colleagues found that functional lesions of GABA and glycine containing neurons in the medulla (through genetic inactivation of the gene for the vesicular GABA/glycine transporter, vGAT) did not abolish muscle atonia (758, 1367). Instead, these authors proposed that REM-on glutamatergic [vesicular glutamate transporter 2 (vGluT2) mRNA positive] neurons in the dorsal SubC project directly to glycinergic neurons in the spinal cord (758), which, in turn, inhibit spinal motoneurons during REM (FIGURE 11). Consistent with this interpretation, inactivation of glycinergic/GABAergic neurotransmission from local neurons in the ventral horn in the mouse reduced atonia (668). Inactivation of supraolivary medulla glutamatergic (VGluT2-positive) neurons caused exaggerated muscle twitches during REM sleep (1367), suggesting that glutamatergic projections from the medulla may participate in control of motoneuron excitability.
3. Rapid eye movements
The observation of rapid eye movements during sleep led to the discovery of this distinct brain state (50). Eye movements during REM sleep are characterized by both tonic and phasic components (347, 803, 1339). The tonic component consists of a strong downward and convergent movement of the two eyes due to a relaxation of the lateral recti muscles and a tonic contraction of the medial muscles. The relaxation of the lateral recti is due to a tonic inhibition and reduction of firing of the abducens motoneurons similar to that seen in other motoneurons during this phase of sleep (347). The phasic component consists of rapid eye movements that are either isolated or in bursts occurring simultaneously with PGO waves (347, 803, 1339). Lesion, stimulation, and recording experiments have identified the paramedian reticular formation regions immediately rostral and caudal to the abducens motor nucleus as being responsible for REMs. These fast movements are due to a burst of action potentials in abducens motoneurons as a result of inputs from excitatory and inhibitory burst neurons that are also responsible for saccades during alertness (1245, 1246). The mechanisms responsible for long-lasting bursts of action potentials in these “short-lead burst neurons” remain to be determined, but it is noteworthy they are much longer (1245, 1246) (4–24 action potentials) than the bursts typically produced by low-threshold calcium channel activation (typically 1–4 action potentials) seen in P-wave related neurons. The activity of burst neurons is normally inhibited by omnipause neurons (OPNs), which are characterized by pauses in firing that begin ~13–16 ms before saccades. These neurons are located on either side of the midline in the caudal pons (nucleus raphe interpositus in the monkey) at the same level as the descending fibers of the sixth nerve.
4. Other REM phenomena
Sleep-related penile erections (SRE) are a prominent feature of REM sleep in sexually potent males and can be a useful diagnostic tool to differentiate between psychogenic and organic erectile dysfunction (508). In healthy adult males the erection begins near the onset of REM sleep, persists throughout the REM episode, and then ends rapidly at the exit from REM sleep. Similarly, in females, increased blood pressure is observed in the vagina during REM sleep (1, 1072). The development of methods to measure erections in rats (1140) has allowed some delineation of the brain circuits involved, although research in this area is sparse. In contrast to most other REM sleep signs, SREs require the activation of the forebrain, in particular the lateral preoptic area (LPOA)/ventral bed nucleus of the stria terminalis since lesions of this area abolished SREs whilst leaving waking erections unchanged (1139). Current models (508) suggest two output pathways from the LPOA: one from the LPOA to serotonergic paragigantocellularis neurons in the ventral medulla, which in turn excite thoracolumbar (T11-L2) sympathetic preganglionic neurons innervating the penis, and another via the paraventricular nucleus of the hypothalamus which send oxytocinergic projections to parasympathetic preganglionic neurons in the sacral (S2-S4) spinal cord. Recent evidence also suggests a role for another forebrain site, the lateral septum, presumably acting through projections to the hypothalamic sites described above. Electrical stimulation in dorsal and intermediate aspects of the lateral septum was effective in triggering SRE during REM (456).
REM sleep is also associated with increased rate and variability of heart rate, breathing, and autonomic nervous system function as well as with altered body temperature regulation. These alterations are likely to be important in the context of cardiovascular disease, sleep apnea, and other disorders (see sect. VII).
B. REM Sleep Master Control Mechanisms
1. Location and neurotransmitter content of REM sleep controlling neurons in the brain stem
Seminal work by Jouvet (580) in cats revealed that a knife cut placed at the junction of the brain stem and midbrain eliminated forebrain signs of REM sleep (cortical LVFA, hippocampal theta) whereas muscle atonia and REMs were preserved, suggesting that the REM sleep generating neurons are located in the brain stem. Further work using electrolytic lesions or cell body selective neurotoxins showed that the most important region is the dorsolateral pons (570) (TABLE 1); in particular, the region surrounding and including the LC and laterodorsal tegmental nucleus as well as the reticular formation immediately ventral to these areas. Large lesions of this area led to substantial reductions in the amount of REM sleep (570), whereas smaller, more discrete lesions could abolish particular features of REM sleep such as muscle atonia (893, 1358). The development of histochemical techniques in the 1960s and immunohistochemical techniques in the 1970s/80s revealed the presence of norepinephrine, serotonin, and cholinergic neurons in the REM-sleep control area (570). The ability to visualize these neurons, together with their large size and clustering into distinct nuclei, led to an explosion of interest in their role in REM sleep control. Only relatively recently have improved immunohistochemical, in situ hybridization, and transgenic techniques allowed the visualization of GABA-ergic and glutamatergic neurons in the REM control area (118, 156, 248, 248, 372, 758, 784, 785). Thus less is understood of these GABAergic and glutamatergic systems, although recent studies suggest that they are likely to be of comparable importance.
2. Mechanisms controlling NREM-REM transitions
One of the most consistent features of human sleep is the alternation between NREM and REM sleep during the night. Early sleep is characterized by a progression from light NREM to deeper stages of NREM sleep with the first REM episode occurring ~90 min into sleep. The 90-min cycles of NREM and REM sleep continue through the night with the proportion of REM sleep steadily increasing and the proportion of deep NREM declining over the course of a night’s sleep. Lower mammals, including rats, mice, and cats, do not have such long consolidated periods of sleep as humans; each of the behavioral states is much shorter and more transitions occur between states. However, the general sleep architecture is similar in that, with the exception of disease states (especially narcolepsy), sleep following a period of prolonged wakefulness is initially NREM sleep. REM sleep follows a period of NREM and is not entered into directly from wakefulness.
A) Reciprocal Interaction Model (Cholinergic and Aminergic Mechanisms
Based on extracellular recordings of neuronal activity from the REM sleep zone in the dorsolateral pons, McCarley and Hobson proposed a mechanistic explanation for the alternation of NREM and REM during the night - the reciprocal interaction model (511, 819), involving two populations of neurons (FIGURE 12A). REM-on neurons increased their firing just prior to and during REM, whereas REM-off neurons showed the reverse pattern. REM-off neurons were proposed to be norepinephrine neurons (serotonin neurons show a similar pattern of firing), whereas REM-on reticular neurons were proposed (erroneously) o be cholinergic neurons. Subsequently, the REM-on cholinergic neurons were localized to the LDT and PPT regions and were proposed to direct the firing of glutamatergic effector neurons in the reticular formation responsible for the different aspects of REM sleep (e.g., muscle atonia) (FIGURE 13). A mathematical model using Lotka-Volterra equations (derived from population models of predator/prey interactions) was able to capture the basic structure of the oscillation between NREM and REM sleep (819) (FIGURE 12A). The essence of this model is that REM-off neurons (norepinephrine and serotonin neurons) inhibit REM-on neurons (cholinergic neurons) during waking and NREM sleep, but as these neurons reduce their firing during NREM, REM-on neurons are disinhibited and REM sleep is generated. Positive feedback between REM-on neurons (cholinergic and glutamatergic reticular neurons) stabilizes the REM state (Figs. 12 and 13). A key feature of this model, not reproduced in other more recent models of REM-sleep control [e.g., REM sleep flip-flop model of Lu et al. (758)] is that REM-on neurons are proposed to be excitatory to REM-off neurons so that as the REM state continues, REM-off neurons gradually become more active, terminating the REM bout and giving a mechanistic explanation for changes in state. A more sophisticated version of this model has been produced by McCarley and Massaquoi (821) (the limit cycle model) which incorporates circadian influences on the REM oscillator (these may be mediated by the orexins; see below) as well as local GABAergic neurons (next section, FIGURE 12B), which are likely to be important in shutting off REM-off neurons as well as in controlling the activity of REM-on neurons (807, 816, 821–823).
FIGURE 12.
The original (A) and modified (B) reciprocal interaction models of REM sleep control, originally proposed by McCarley and Hobson (819). A: the original reciprocal interaction model demonstrates increased REM activity as positive feedback of REM-on neuronal populations occurs. This activity leads to excitation of REM-off neuronal populations, which then inhibit REM-on activity. REM-off activity is self-inhibiting, and eventually wanes, releasing REM-on neurons as REM sleep again occurs. [Adapted from McCarley and Hobson (819). Reprinted with permission from AAAS.] B: LDT/PPT REM-on activity excites pontine reticular formation (PRF) glutamatergic REM-on cells, promoting REM sleep. LDT/PPT REM-on neurons also excite GABAergic interneurons adjacent to REM-off neurons, inhibiting REM-off neuronal activity. REM-on output also inhibits GABAergic REM-off interneurons, which in turn inhibit REM-on PRF neurons. As REM sleep progresses, REM-on cells begin to excite REM-off cells, leading to REM sleep cessation. Dorsal raphe (DR) and locus coeruleus (LC) REM-off neurons inhibit laterodorsal/pedunculopontine tegmental nuclei (LDT/PPT) REM-on neurons during waking and NREM sleep. Self-inhibition of these REM-off neurons leads to disinhibition of REM-on neurons, again allowing REM sleep. (Adapted from McCarley. Sleep Med 8: 302–330, 2007, with permission from Elsevier.)
FIGURE 13.
Pontine generation of REM sleep phenomena. Interaction between the pontine/mesencephalic reticular formation (PRF Glutamatergic) and cholinergic laterodorsal/pedunculopontine tegmental nuclei (LDT/PPT ACh) produces ascending and descending activation, resulting in REM sleep phenomena, including PGO waves, rapid eye movements, muscle atonia, hippocampal theta oscillations, and cortical activation. GiV, ventral gigantocellular nucleus; HDB, horizontal limb of the diagonal band; MCPO, magnocellular preoptic nucleus; MRF, medullary reticular formation; MS/vDB, medial septum/vertical limb of the diagonal band; PRF, pontine reticular formation; SI, substantia innominata.
I) Evidence supporting the reciprocal interaction model: state-dependent firing patterns and neurotransmitter release. A large body of evidence supports the reciprocal interaction model ofREMsleep (816) and is summarized here (contrary evidence is discussed subsequently). Confirming the initial recording data, multiple studies support the contention that the discharge of presumed cholinergic and aminergic neurons shows an activity profile consistent with causality, i.e., these neurons show an increase or decrease in firing rate, respectively, prior to the beginning of REM sleep (336, 511, 615, 768, 1280). In these studies, neurons were identified based on location, extracellular action potential characteristics, and antidromic activation. In the future it will be important to confirm the neurotransmitter phenotype of the cell recorded using juxtacellular labeling/post hoc immunohistochemistry techniques together with recordings of activity across sleep-wake cycles. So far, this has only been achieved in anesthetized preparations (126), which do not show the full range of sleep-wake behaviors. Confirming the single-unit recording data, measurements of extracellular neurotransmitter levels show that the firing pattern of the cholinergic and aminergic neurons are translated into the predicted pattern of release across the sleep-wake cycle (560, 704, 709, 1020, 1172, 1411, 1412). In contrast to the single-unit recording data, these measurements are limited in temporal resolution, so with current methodologies cannot provide evidence of causality.
II) Evidence supporting the reciprocal interaction model: cholinergic modulation of reticular formation neurons. Anatomical and electrophysiological experiments have confirmed the existence of many of the proposed interconnections of this model. Cholinergic modulation of reticular formation neurons: anatomical and electrical stimulation studies have demonstrated prominent cholinergic projections from the LDT and pedunculopontine tegmentum (PPT) to the reticular formation areas involved in REM sleep control (544, 861). Early in vitro intracellular recording studies in the PnO and PnC regions of the reticular formation revealed excitatory effects of cholinergic agonists mediated by nicotinic and muscarinic receptors in approximately two-thirds of reticular neurons (447, 1241), acting via nonselective cation channels and blockade of leak potassium, channels, respectively. Thus these neurons likely represent REM-on or wake/REM-on neurons. In contrast, one-quarter of the neurons were inhibited via activation of an inwardly rectifying potassium conductance (419), consistent with immunohistochemical evidence for the presence of inhibitoryM2 muscarinic receptors on a substantial proportion of non-GABAergic PnO and PnC reticular neurons (141). More recent studies specifically targeting the REM control SubC region in the rat (160, 490) showed that similar mechanisms also operate in this region. The majority of “reticular” (i.e., noncholinergic, nonnoradrenergic) neurons in the SubC were excited by carbachol. Pharmacological analysis revealed that this action is mediated by both ionotropic nicotinic receptors and muscarinic M1-type (likely M3) receptors (490). A large subset (~40%) of SubC neurons were silent at rest (i.e., in the absence of current injection through the recording electrode), were hyperpolarized further by carbachol, and fired in bursts of several action potentials at the offset of hyperpolarizations due to the presence of low-threshold calcium channels (160). Pharmacological analysis revealed that M2 muscarinic receptors and activation of a potassium current mediate the outward current responsible for this hyperpolarization (490). These neurons could play a role in PGO-wave generation since hyperpolarization by carbachol would allow deinactivation of the low-threshold calcium currents responsible for burst firing.
III) Evidence supporting the reciprocal interaction model: aminergic inhibition of brain stem cholinergic neurons. Extensive projections exist from brain stem serotonin neurons in the DR and MR to cholinergic LDT/PPT neurons (1149, 1211). Activation of 5-HT1A receptors leads to inhibition of cholinergic LDT neurons in vitro, via activation of inwardly rectifying potassium channels (763). Similarly, LC neurons project to and partially overlap neighboring cholinergic brain stem regions (571, 731, 1001), and norepinephrine inhibits mesopontine cholinergic neurons via activation of α2 adrenoceptors (1413). In vivo, the firing of REM-on LDT/PPT neurons in the cat was inhibited by application of the 5-HT1A receptor agonist 8-OH-DPAT (presumably explaining their silence during wake), whereas wake/REM-on neurons were unaffected by this agent (1280). Autoradiographic studies in the mouse did not find evidence for 5-HT1A receptors in brain stem cholinergic neurons (120), suggesting either a species difference or mediation of serotonin effects by other receptor subtypes or indirect mechanisms.
IV) Evidence supporting the reciprocal interaction model: cholinergic excitation of aminergic neurons. Cholinergic neurons directly excite LC neurons (1160). Cholinergic agonists acting on nicotinic receptors indirectly excite DRN serotonin neurons via a presynaptic facilitation of excitatory noradrenergic inputs (714).
V) Evidence supporting the reciprocal interaction model: pharmacological experiments. In animals, early studies suggested a REM-promoting role for acetylcholine (410, 504) particularly when monoamines had been previously depleted by use of the agent reserpine (605). In humans, enhancement of cholinergic tone by systemic application of acetylcholinesterase inhibitors or muscarinic agonists (93, 513, 692, 1065, 1185, 1186) consistently decreases REM sleep latency and enhances REM sleep amount, particularly phasic REM events, whereas muscarinic antagonists cause the reverse effect (428, 625, 1051). Enhancement of monoaminergic tone with antidepressants is well known to result in a long-lasting suppression of REM in humans and animals. Following the localization of REM generating sites to the brain stem by Jouvet, enhancement of cholinergic tone or stimulation of cholinergic receptors in the pontine reticular formation of cats or dogs was found to cause a REM-like state (675, 1055, 1228). In rats and mice, a similar effect can be induced, although it is often less robust in these species, perhaps as a result of difficulty in localizing the drug applications in the smaller brains of rodents and the interaction with circadian control or descending forebrain influences (675, 800, 1228). Activation of the inhibitory 5-HT1A receptor, via perfusion of the serotonin 5-HT1A agonist 8-OH-DPAT, caused increased REM sleep due to activation of autoreceptors when infused in the DRN (1021), whereas perfusion of serotonin in the LDT decreased REM via activation of inhibitory 5-HT1A receptors on cholinergic neurons (522).
VI) Evidence opposing the reciprocal interaction model. Although a large body of evidence supports the suggestion that cholinergic neurons promote REM and aminergic neurons inhibit REM, several lines of evidence appear to contradict this model. However, in each case alternative explanations are also possible: 1) lesions encompassing the PPT or LDT regions where the majority of brain stem cholinergic neurons are located did not significantly alter the amount of REM sleep (758). Combined lesions of both areas were not performed. Thus, in theory, one area could compensate for the other. We note that large lesions of the LDT/PPT and surrounding areas led to substantial reductions in REM sleep, and these reductions were correlated with the extent of loss of cholinergic neurons (1394). 2) Knockout mice lacking M2/M4, M3, or both M3 and M2/M4 muscarinic receptors (the main subtypes present in the brain stem) still show REM sleep (434), although REM sleep was reduced by 22% in M3 receptor knockouts. Since these are constitutive knockouts, compensatory responses of the other cholinergic receptors may occur. 3) Using Fos as a marker of neuronal activation, the Luppi lab did not find activation of ChAT-positive neurons in REM rebound following relatively selective REM sleep deprivation (1354), although these results apparently contradict those of the Jones group (784). Furthermore, Fos is acknowledged as an imperfect marker of neuronal activity, particularly for neurons with complex firing patterns (664). 4) Inconsistent effects of carbachol in promoting REM sleep in rats and mice may be due to close proximity of REM promoting and REM-inhibiting neurons in these species, making it difficult to restrict the effect to the neurons targeted and due to injections of carbachol during the daytime when rats and mice already sleep maximally, i.e., a ceiling effect. 5) Lesions/inactivation of norepinephrine neurons do not affect REM sleep amounts. Studies performing neurotoxic or electrolytic lesions of the LC reported no change in the daily amounts of REM sleep (100, 576, 758). Mice lacking dopamine-β-hydroxylase, the enzyme necessary to convert dopamine to norepinephrine, were shown to either have unchanged baseline sleep-wake parameters (539) or a decrease in REM sleep, opposite that predicted by the reciprocal-interaction theory (956). As with other knockouts, compensatory changes may occur, and the differences between these two studies suggest that genetic background or other technical factors may influence the effect. Contradictory effects were also found using neurotoxic lesions of norepinephrine neurons with DSP-4. Monti et al. (881) found an increase of REM sleep several days after administration, whereas Cirelli and Tononi (244) reported no baseline sleep-wake changes. In all of these studies, it is possible that the activity of serotonin neurons could have masked the loss of norepinephrine neurons since serotonin has similar effects on the REM control neurons as norepinephrine. In contrast to the rather drastic manipulations described above, we have recently found that a partial, reversible knockdown of orexin receptors in LC neurons using RNAi increased REM sleep during the dark period (219). Similarly, reversible inactivation of the LC or DR by cooling increases REM sleep (201).
VII) Alternative models for the contribution of cholinergic and aminergic neurons to REM sleep control. Overall, the question of whether activation of cholinergic neurons and silencing of aminergic neurons are essential for the induction of REM sleep is still not completely resolved. An alternative but related hypothesis is that cholinergic and aminergic neurons are not absolutely required for REM sleep generation but instead bias the system in one direction or another. Another possibility is that these neurotransmitter systems may control particular aspects of REM sleep. For instance, cholinergic agonists consistently promote theta rhythm and PGO waves in rats and cats. In contrast, serotonergic neurons inhibit theta rhythm and PGO waves. The discharge of serotonin neurons is very closely (inversely) correlated with the occurrence of PGO waves (768), and systemic application of the serotonin synthesis inhibitor p-chlorophenylalanine (PCPA) in the cat leads to the appearance of PGO waves during the waking state (555). Furthermore, injection of serotonin into the SubC region in the rat suppressed the pontine component of PGO waves (P-waves) without affecting other components of REM sleep (279). Similarly, the silencing of noradrenergic LC neurons has been closely linked to muscle atonia. Cataplexy seen in the sleep disorder narcolepsy is thought to represent inappropriate inactivation of REM muscle atonia. LC neurons completely shut off prior to attacks of cataplexy in genetically narcoleptic dogs, whereas other aminergic neurons (serotonergic and histaminergic) do not reduce their firing to the same extent (564, 1435). These attacks can be reduced by use of norepinephrine selective reuptake inhibitors increasing norepinephrine tone (923). In the future, new techniques (e.g., optogenetics) to selectively excite or inhibit cholinergic and aminergic neurons, in particular the caudally projecting cholinergic neurons, will be of great help in resolving their exact role in REM sleep control.
B) Gabaergic Control of Rem Sleep
Recently, there has been increased interest in the role of GABAergic neurons in the control of REM sleep. A large number of mainly small or medium-sized GABA neurons are present in the brain stem, and many of them project to, surround, or are located within brain stem areas involved in REM control (118, 156, 372, 784, 785, 1118). At least two functional groups of brain stem GABAergic neurons seem to be involved in REM control, namely, REM-off GABA neurons preventing activation of REM-on reticular neurons during wakefulness and REM-on GABA neurons inhibiting the activity of aminergic neurons (FIGURE 12B). GABAergic inhibition of cholinergic neurons during wake and/or NREM sleep is also a factor in regulating their state-dependent discharge (1303) and neurotransmitter release (1344). Other groups of GABA neurons outside the brain stem regulate the circadian timing and homeostatic control of REM sleep. Although mounting evidence supports a role for these different groups of GABA neurons in controlling REM sleep (reviewed below), we note that in most cases, direct evidence that selective stimulation or inhibition of particular subsets of GABA neurons affects REM sleep occurrence is lacking. Similarly, electrophysiological recordings of identified GABA neurons showing that they have the appropriate discharge patterns to cause a change in state are not available. Thus, as with the cholinergic and aminergic neurons, not all criteria required for firmly establishing the role of GABAergic neurons in REM control have been fulfilled.
I) REM-off GABA neurons. Application of the GABAA receptor antagonist bicuculline to the SubC or PnO/FTP regions induced a REM-like state in both cats (1441, 1443) and rats (119, 1009, 1112). These results suggested that the REM effector neurons located in this region are normally under inhibitory GABAergic control and are consistent with microdialysis measurement of extracellular GABA levels in the reticular formation across behavioral state which are ordered wake > NREM > REM (1281, 1337). Retrograde tracer injection into the SubC of rats revealed prominent GABAergic inputs from the PnO, ventrolateral periaqueductal grey matter (vlPAG), and the lateral pontine tegmentum (LPT) (118, 758). On the basis of Fos studies and anatomical tracing, it was suggested that GABAergic neurons in the vlPAG and in the LPT region (which encompasses mesencephalic components and is also termed the deep mesencephalic reticular nucleus; DpMe) might be particularly important (758, 1118). Early work in the cat showed that inhibition of this area with the GABAA receptor agonist muscimol led to a large increase in REM sleep (1120). These findings were confirmed more recently in the rat (1118) and guinea pig (1336). Cell body specific lesions of either the vlPAG or the LPT cause an increase in the amount of REM sleep in rats (758) and in mice (612). The vlPAG lesions increased both REM sleep duration and the number of REM sleep bouts during the dark (active period), whereas the LPT lesions increased the number of REM bouts during the light period and caused occasional episodes of sleep-onset REM/cataplexy-like states. Although the evidence for this region being a REM-inhibiting area is quite strong, a recent study suggests that GABAergic neurons in this region are not involved since inactivation of GABAergic (and glycinergic) neurotransmission did not alter REM sleep (668). Presumably glutamatergic neurons in this region excite GABAergic neurons located elsewhere in the brain stem, which then inhibit REM-on neurons.
II) REM-on GABA neurons. Another group of GABAergic neurons within the brain stem RF likely play a role in the silencing of REM-off neurons located within the LC and DRN. Microdialysis experiments showed that GABA levels rise in these areas during REM sleep compared with wakefulness (926, 927). Injections of GABA receptor antagonists into the DRN or LC reverse the cessation of firing of 5-HT and NE neurons normally seen during REM (420, 421). However, in the case of the DR, disfacilitation (withdrawal of excitatory noradrenergic, histaminergic, and orexinergic inputs) is also likely to be important (158, 713, 1095). Retrograde tracing studies combined with GAD immunohistochemistry showed that GABAergic neurons in the rostral PnO and vlPAG/LPT region project to the DRN (421) and may be involved in silencing them during REM. GABAergic neurons surrounding the LC norepinephrine neurons express Fos following recovery from REM deprivation (784) and may thus inhibit them during REM sleep. Consistent with this idea, identified SubC GABA neurons were excited by the cholinergic agonist carbachol (156). In addition, the LC receives input from more distally located REM-on GABAergic neurons (1118, 1352). In particular, GABAergic neurons in the dorsal paragigantocellular (DPGi) nucleus of the medulla may be important in silencing the LC (and DR) during REM sleep, since single-unit recordings in this area in unanesthetized rats revealed a population of (unidentified) tonically firing REM-on neurons whose activity preceded the onset of the REM state (435) and Fos studies revealed GABAergic, REM-on neurons in this area (1118).
III) Models of REM sleep control incorporating GABAergic neurons. How can these findings involving REM controlling GABA neurons be reconciled with the cholinergicaminergic reciprocal interaction theory? It has been hypothesized that acetylcholine and amines modulate the activity of REM-on and REM-off GABAergic neurons and thereby affect REM sleep occurrence (156, 574, 816, 1443) (FIGURE 12B). Several lines of evidence suggest that acetylcholine-excited GABA neurons in the SubC/PnO/LPT areas are REM-on neurons that inhibit LC and DRN neurons. Conversely, acetylcholine-inhibited GABA neurons in the LPT/PnO regions are REM-off neurons which inhibit REM-effector neurons in the PRF during waking/NREM sleep. Muscarinic inhibition of the GABAergic inhibitor neurons would thus disinhibit REM-promoting reticular neurons. This model also resolves the puzzling reports of muscarinic M2 receptor promotion of REM sleep (61, 252) and cataplexy in narcoleptic dogs (1056), findings that were puzzling in view of the generally hyperpolarizing, inhibitory M2 actions (332). Unlike the reciprocal-interaction model incorporating GABAergic neurons, two recent models of REM sleep control involve only GABAergic and glutamatergic neurons (758) (REM flip-flop model) or GABA, glutamate, and aminergic/orexin/MCH neurons (765). Aside from the evidence presented above which supports the cholinergic promotion of REM sleep (especially in the cat), these models suffer from the weakness that they do not provide any explanation for how a change in state is achieved or any explanation for the timing of REM-NREM transitions.
C) Nitric Oxide Control of Rem Sleep
nNOS is localized within REM sleep control structures such as the PPT, LDT, and DRN (702, 705). nNOS knockout mice have substantially lower levels of REM sleep than their wild-type controls (218). Microinjection of NG-nitro-l-arginine methyl ester (l-NAME), a nonspecific inhibitor of NOS, into the PPT reduced REM sleep (281), whereas another NOS inhibitor, NG-nitro-l-arginine p-nitroanilide (l-NAPNA), reduced both NREM and REM sleep (480). Moreover, microinjection of nNOS inhibitor 7-nitroindazole (7–NI) into DRN, in rats, decreased REM sleep (168), and microinjection of l-arginine, a precursor of NO, into PPT increased the duration of NREM sleep and the number of REM sleep episodes (480). Inhibition of NO production in mPRF reduced acetylcholine release and decreased the amount of REM sleep (708, 709). Overall these data suggest that NO, produced by nNOS, mainly in brain stem cholinergic neurons, promotes REM sleep.
3. Forebrain control of REM sleep timing
Although the brain stem appears to be sufficient for generation of REM sleep and NREM-REM cycles (580), other factors controlled by forebrain inputs to the brain stem affect the timing and amount of REM sleep such as the time of day, light exposure, stress, emotion, temperature, nutritional state, and sleep homeostasis (1116). We here focus on diurnal/circadian control. REM sleep occurs predominantly in the inactive period, i.e., night-time in humans and daytime in rats and mice (1439). In an extension of the reciprocal-interaction model of REM sleep (limit cycle model), McCarley and Massaquoi introduced an additional factor to account for the circadian variation in REM sleep across the day. This term provided an excitatory input to REM-off neurons, suppressing REM during the day (in humans) and causing a smaller amplitude and shorter initial first NREM-REM cycle (807, 821, 822).
A) Orexinergic Control of Rem Sleep
Several strong lines of evidence support orexin neurons in the lateral hypothalamus as providing this circadian factor (598). 1) Orexin neurons receive direct input from the SCN and indirect input via the dorsomedial hypothalamus (52, 224). 2) Orexin neurons show a wake-on, REM-off pattern of firing (except during phasic periods of REM) (699, 856). Measurements of orexin levels in the squirrel monkey (1472) and rat (296) are consistent with the proposed diurnal fluctuation. 3) As hypothesized in the model, orexins excite wake-active, REM inhibiting, serotonergic DRN neurons (157, 158, 735) and noradrenergic LC neurons (524). Surprisingly, they also seem to excite cholinergic brain stem neurons in vitro (169). 4) The loss of orexins causes narcolepsy (215, 727), a sleep disorder characterized by the presence of excessive daytime sleepiness and several symptoms related to abnormal timing of REM sleep signs (see sect. VII) and loss of diurnal REM control in humans (983). 5) Orexin loss-of-function experiments in animals (TABLE 1) result in increased REM during the normally REM-poor dark period, arguing strongly that orexinergic activity suppresses the occurrence of REM sleep during the diurnal active phase.
B) Preoptic Hypothalamus Control of Rem Sleep
In addition to the orexin systems, the preoptic hypothalamic area also plays a role in circadian control of sleep. The preoptic area receives indirect projections from the SCN via the medial preoptic area and dorsomedial hypothalamus (222, 224, 306). Lesion of the area medial and dorsal to the ventrolateral preoptic area (VLPO) of the hypothalamus, an important area in NREM control, was correlated with loss of REM sleep (756). The loss of REM sleep occurred predominantly during the light period (756) (the rat’s inactive period). Furthermore, after periods of dark exposure that triggered enrichment of REM sleep, the number of Fos-positive cells in this extended VLPO area was correlated with the amount of REM sleep (755). Thus diurnal variation in REM sleep is caused by an orexin-mediated suppression of REM during the active period and a promotion of REM by the extended VLPO during the inactive period.
C. Relation of REM to Dreams
The lay public is interested in REM sleep largely as a result of its close association with dreaming. Interpretation of dreams is ascribed great significance in many cultures and predates modern science. In western societies, the work of Freud and his counterparts led to a widespread acceptance of dreams as giving important insights into “psychic disturbance.” However, the findings of modern neuroscience have led to a view of dreaming which asserts that the features of dreams arise from internally generated patterns of brain activation and deactivation during REM sleep, and dream content does not necessarily have any meaning or message for the individual (509, 510, 815, 820).
A) Activation-Synthesis Hypothesis of Dream Generation
The modern neuroscience view of dreams was laid out by Hobson and McCarley as the activation-synthesis hypothesis (510, 815, 820). An expanded state-space version of this hypothesis, the AIM (activation, input gating, modulation) model was developed by Hobson to characterize all conscious states (588). In the activation-synthesis view of dreaming, during the REM state, the brain is activated internally by the activity of the brain stem (described above).
Sensory systems, in particular visual systems (activated by PGO activity), and vestibular systems are activated. Sensations and feedback from the neuronal command signals for muscular activity influence the dream experience, although motor output is inhibited by brain stem muscle atonia generating systems. This mismatch between motor programs and motor output may contribute to common dream experiences of floating, flying, or an inability to flee a dangerous situation. Brain areas involved in emotional behavior and memory formation such as the hippocampus and amygdala are “reactivated” during REM sleep, possibly reflecting memory consolidation processes (562, 1089, 1391) and provide content (especially emotional content) to the dream. The synthesis of these different elements of brain activity, together with their conscious awareness, causes the dream experience. The synthesis process occurring in dreams is in some ways similar to the “confabulation” of patients with various kinds of neurological injuries, which can also be bizarre and nonsensical and makes little sense to observers (1143). An intriguing recent theory of REM sleep and dreams suggests that they represent an evolutionarily early form of conscious awareness (proto-consciousness), a precursor of the conscious awareness seen during the waking state (509).
B) Brain Imaging of the Rem/Dreaming State
Positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) duringREMsleep and dreaming in humans have supported the scheme proposed in the activation-synthesis hypothesis, which was originally based on animal work (1143). These studies reported increased blood flow/oxygen utilization in a network of interconnected regions during REM sleep: the pontine tegmentum, thalamus, amygdala, basal ganglia and anterior cingulate and occipital cortex (512, 754, 792, 1143). Amygdala activation is likely responsible for the high percentage of dreams featuring negative emotions such as anxiety and fear. In contrast, decreased activity was seen in the dorsolateral prefrontal cortex, parietal cortex, posterior cingulate cortex, and precuneus. Deactivation of frontal areas likely accounts for the lack of insight, distortion of time perception, and difficulty in remembering dreams upon waking (512, 1143). The mechanism responsible for this deactivation is unclear since cholinergic projections to the cortex, thought to be responsible for activation during REM, target prefrontal as well as other cortical areas (500). Another, still to be resolved issue, is how particular dream elements are selected among the massive number of possibilities available to the brain.
C) Do Dreams Represent Internal Drives?
Unlike Freud’s model, the brain activity in the Hobson/McCarley activation-synthesis model of dreaming was considered motivationally neutral, since it was due to the turning on of a brain stem neural oscillator. However, brain stem cholinergic neurons activated during REM sleep provide a strong excitatory input to dopaminergic midbrain neurons involved in reward processes, and others have argued that dreaming is closely linked to activation of these pathways (1198). Although the average firing rate of dopamine neurons in the VTA does not vary with behavioral state (858), increased bursting occurs in VTA neurons during REM sleep (271), and an increased number of Fos-positive dopamine neurons was observed in the VTA during REM-rich periods following REM deprivation (786). Furthermore, REM involves a strong activation of limbic brain regions (792), which may feedback onto the brain stem oscillator, although there is no evidence for this. Thus processing of emotionally relevant events may occur during the REM state (1388), as do many other complex functions such as alterations in learning and memory. However, the regular generation of this cyclic state does not depend on motivationally relevant stimuli, such as protection against repressed and forbidden wishes (Freud’s hypothesis).
V. SLEEP LOSS AND COGNITION
In humans, sleep loss/disruption alters cognition and performance in a wide variety of behavioral domains including attention/vigilance, executive function, emotional reactivity, memory formation, decision making, risk taking behavior, and judgment (835). In addition, a substantial body of literature supports the intuitive notion that a good night’s sleep can facilitate human cognitive performance (339), a literature not to be covered in detail in this review. This section focuses on mechanistic aspects of sleep loss/sleep disruption. Methodological consideration related to rodent models of sleep disruption are covered in a recent review (835).
A. Attention and Executive Function
Executive function is a broad and poorly defined term that is used to describe a set of higher order cognitive functions that involve the prefrontal cortex. Impairments of attention and other aspects of executive function following sleep disruption have been well documented in humans in both experimental and patient populations (396, 621). The mechanisms by which sleep disruption alters executive function are unknown but likely involve functional impairment of the prefrontal cortex and/or its afferents (907). Sleep disturbances in humans alter normal functioning of the prefrontal cortex as well as the posterior parietal cortex, which has dense interconnections to the frontal lobe (1287). Imaging studies have revealed altered activity in the default network, resulting in a dissociation of anterior and posterior midline regions during sleep deprivation (212, 454, 1107, 1108). Sleep deprivation (SD) also increases activity in thalamic regions that project to the cortex and may be involved in maintaining cortical activity in the face of increasing sleep pressure (322, 1297, 1433). Lapses of attention during SD are associated with reduced thalamic and frontal/parietal cortical activity that contrast with activation of these same regions during successful performance (213). Possible mechanisms underlying impaired prefrontal cortex function during sleep disruption are described next.
1. Sleep loss-induced impairment of high-frequency oscillations
Slowing of the EEG during sleep deprivation is particularly prominent in the frontal cortex and is accompanied by a concurrent reduction in higher frequency beta/gamma rhythms important for normal cognition (see sect. II). Modeling, electrophysiological, and optogenetic studies all support the idea that beta/gamma oscillations and gamma-theta coupling have important functions in attention-dependent stimulus selection (193, 297, 391, 1194) via their ability to synchronize firing between functionally related cortical and subcortical brain areas, reduce noise, and enhance signal transmission. The firing of principal cells relative to the phase of the gamma rhythm has been proposed as a mechanism for coding of information (390, 732). In addition, the frequency of gamma oscillations affects the direction of information flow between brain areas (253).
Changes in the activity of neocortical or allocortical neurons themselves are likely to contribute to the disruption of EEG oscillations. For example, SD or sleep fragmentation (SF) in rats leads to reduced excitability of pyramidal neurons in area CA1 of the hippocampus (837, 1271). In the neocortex, a recent study found that as wake duration increases, individual cortical neurons can enter OFF states, accompanied by slow waves in the local field potentials, similar to NREM sleep, while other nearby neurons show normal wake activity and overall the EEG shows a wake pattern (1383). The incidence of these local OFF states increases with the duration of the wake state and is correlated with impairments in a sugar pellet reaching task (1383). Thus alterations in the excitability of cortical neurons lead to a disruption of higher frequency EEG rhythms, impacting attention and higher cognitive function.
2. Inhibition of cholinergic and other BF projections to prefrontal cortex
Inhibition of BF cholinergic neurons by adenosine and/or NOduring SD (see sect. IIIC) is likely to impair beta/gamma oscillations, since acetylcholine released from these neurons is a potent facilitator of such rhythms (see sect. IIC). Aside from their major role in basic cortical arousal and behavioral activation, evidence suggests that the BF and pontine cholinergic systems play a significant role in visual attention, short-term spatial (working) memory, and responsiveness to novel and motivationally relevant stimuli during the acquisition of new associations (1066). Thus the BF cholinergic system figures prominently in models of the effects of sleep disruption on behavior and cognition (1119). As reviewed in section III, an upregulation of BF adenosine and adenosine A1 receptors occurs during SD or SF, which may lead to inhibition of BF cholinergic and noncholinergic neurons. Also, during SD, adenosine A1 receptors are upregulated in the human PFC, which may consequently produce inhibition of PFC activity (341). The role of BF adenosine in attentional deficits following sleep disruption were directly investigated using a rat model of the human psychomotor vigilance task (PVT) (226). Microdialysis administration of adenosine directly into the BF mimicked the effect of 24 h of sleep deprivation, producing an increase in vigilance lapses relative to baseline, or to control perfusion of artificial cerebrospinal fluid (ACSF). This effect of AD was reversed by the coperfusion of AD with an A1 receptor antagonist. Thus an inhibition of cholinergic neurons by adenosine during sleep deprivation causes deficits in attention.
3. Alterations in catecholaminergic systems
Dopaminergic and noradrenergic arousal systems projecting to the PFC and striatum have also been strongly implicated in working memory and attention (1066, 1067). The level of dopamine required for optimal function follows an inverted U-shaped curve; thus increases or decreases may negatively impact function. Early animal studies suggested that SD causes an increase in dopamine levels (424) and a supersensitivity of dopamine receptors (1310). More recent human studies support increased striatal dopamine levels (1376) that correlate negatively with performance on a visual attention task (1375). Pharmacological treatment of the behavioral consequences of SD also suggests a role for dopaminergic mechanisms. Thus the stimulants commonly used for the treatment of sleep disorders (sect. VII), such as modafinil, inhibit the dopamine transporter leading to increased catecholamine levels (1425). Similarly, in Drosophila, dopamine is important in the control of arousal and the response to SD (679, 1153, 1438). Dopamine D1 receptor activation in the mushroom bodies rescues sleep loss-induced learning impairments (1154). Changes in the norepinephrine system may also be involved in sleep disruption-induced deficits. One day of REM SD produced a decrease of activity in the LC and a decrease of NA release (783, 1016). A more extended REM SD showed the opposite effect, as NA levels and turnover increased (77, 992, 1016). Studies monitoring changes in norepinephrine receptors report inconsistent results (745, 1423). Overall, the data suggest that changes in dopamine systems may be more important than changes in norepinephrine or its receptors in causing behavioral alaterations.
4. Interindividual differences
The neurobehavioral response to SD shows large interindividual differences in both humans and rats (1330, 1331). Very recent genetic studies suggest that polymorphisms in genes related to adenosine metabolism or signaling (59) affect interindividual differences in susceptibility to cognitive disruption following SD. In addition, a polymorphism of the gene that encodes catechol-O-methyltransferase (COMT), an enzyme which degrades catecholamines, was predictive of the interindividual variability in performance of an executive function task after SD, as well as EEG alpha frequency activity during wakefulness (112).
B. Emotion
1. Mood
Changes in mood and motivation are common subjective experiences during sleep disruption. Recent human studies have begun to investigate these changes under controlled experimental conditions. Mood is often elevated during SD (108, 521), particularly in subjects with a evening-type circadian profile (1147). The effect of acute SD can be used to treat severely depressed patients (sect. VII), although unfortunately the effect is reversible once the patients are allowed to sleep. At the same time as the basal mood changes, sleepdeprived subjects show increased reactivity to negative stimuli (31, 1274, 1334), reduced facial expressiveness (859), and impaired recognition of human emotions (622, 1328). The mechanisms underlying these changes are largely unknown but have been hypothesized to reflect a loss of REM sleep (453), since REM sleep is characterized by a strong activation of brain areas regulating emotional responses such as the amygdala, hippocampus, and frontal cortex (sect. IV). Emotional memory is enhanced across sleep intervals with high amounts of REM sleep (1387), and a recent imaging study showed that sleep enhanced the memory of emotional pictures by increasing functional connectivity between the ventromedial PFC and the precuneus, amygdala, and occipital cortex (1240). Increased amygdala activation due to a loss of top-down control from the medial prefrontal cortex may explain the increased response to negative emotional stimuli (455, 1465). At the same time, activity in brain mesolimbic reward networks is also enhanced (455), consistent with reports of increased dopamine release (1375, 1376) and positive effects on mood and motivation.
2. Anxiety/fear
Both anxiogenic (1088, 1180) and anxiolytic (806, 1248) effects have been reported in experimental investigations of sleep disruption. Self-reported increases in anxiety have been observed in humans after SD (58, 1088), consistent with reports of the increased reactivity to negative stimuli described above. Several studies have examined negative emotional memory formation in animals in association with sleep disruption. Contextual fear conditioning, a hippocampus-dependent task, was impaired by 72 h of SD before training (1085) or by REM (441) or total SD (468) 5–6 h following training. REM SD was also reported to impair extinction of cued fear extinction, a prefrontal cortex and amygdala-dependent task (1181). In humans, recollection of negative emotional stimuli 72 h later was associated with increased amygdala activation in subjects sleepdeprived on the night following initial exposure (1239). The relevant factors that predict whether sleep disturbance will increase or decrease anxiety are not known. Duration of exposure, method used to disrupt sleep, type of sleep disturbance, and how the findings are interpreted are potential factors that may help to explain seemingly contradictory findings.
C. Learning and Memory
A substantial body of evidence from both humans and experimental animals suggests that normal sleep facilitates certain forms of learning and memory (309, 339, 1007, 1389), although these data, particularly the link between REM sleep and memory, have been criticized (1173, 1361). A full review of the controversial topic of the role of sleep in learning and memory is beyond the scope of this review, and the reader is referred to the reviews cited above. A more consistent literature describes impairments in learning and memory produced by sleep disruption (339, 835). A variety of mechanisms could mediate deficits in learning and memory associated with sleep disruption. These mechanisms are discussed next.
1. Role of hormonal stress responses
A problem inherent in conducting experiments to assess behavioral and cognitive impairments associated with sleep disruption is that the reported deficits might be due to a stress response associated with the method employed to disrupt sleep. Sleep deprivation induced by the treadmill method (1272), rotating wheels (846), or the flowerpot method (1469) enhance corticosterone levels. It is important to point out, however, that the effects of stress on learning and memory are not always deleterious, but instead are dose-dependent. High levels of chronic stress (258) or chronic administration of the adrenal stress hormone corticosterone (114) impair spatial learning and memory. Conversely, stressors of lessor intensity sometimes facilitate learning and memory and long-term potentiation (307). Thus stressors may facilitate or inhibit learning and memory processes, depending on the type, magnitude, and timing of the stressor. Although an indirect effect of stress is always possible, several lines of evidence suggest that the effects of sleep disturbance on learning and memory are predominantly direct. For instance, there are REM sleep periods, or “windows” of time during which prior learning is highly sensitive to selective REM SD (1190, 1191). Such time-dependent sleep disturbances would not likely result from a nonspecific stress response.
The most compelling evidence that effects of sleep disturbance on learning and memory are independent of stress is from studies on adrenalectomized animals. In rats, 72 h of SD prior to training impaired acquisition on the water maze task (1084), but surgical removal of the adrenal glands (with corticosterone replacement to normal, nonstressed levels) did not significantly alter this effect. Similarly, at the cellular level, 96 h of SD was found to inhibit adult hippocampal neurogenesis, but this effect was also observed in adrenalectomized rats that were maintained via subcutaneous minipumps on continuous low-dose corticosterone replacement (902). Hence, the effect of sleep loss on adult neurogenesis was independent of adrenal stress hormones (902). Another study found that adrenalectomy and subsequent corticosterone replacement significantly altered sleep architecture but did not alter the homeostatic sleep response to 6 h of SD (131). Taken together, these studies suggest that both the homeostatic response to sleep disruption as well as the subsequent deficits in learning and memory are mediated by mechanisms that are largely independent of endocrine status.
2. Sleep loss impacts cellular mechanisms of learning and memory occurring during NREM sleep
Sleep disruption could prevent memory consolidation by impacting the following mechanisms that normally occur during NREM sleep: 1) synaptic homeostasis. The synaptic homeostasis theory of sleep posits that the amplitude and slope of delta and slow oscillations downregulate synapses so as to avoid a ceiling effect of synaptic potentiation occurring during waking (sect. III). Increases of delta waves during waking are a prominent feature of short-term SD and mounting sleep pressure, suggesting that they are important for maintaining function. Indeed, boosting slow oscillations during sleep potentiates memory (805) and EEG delta power during recovery sleep after SD predicts cognitive recovery (787). 2) Replay of firing patterns: hippocampal CA1 pyramidal place cells, which are known to fire together when an animal occupies a specific spatial location (939), were also found to fire together during subsequent sleep (985). Moreover, cells that were not active during wake, or that were active but had nonoverlapping spatial patterns of firing, did not show increased firing during subsequent sleep (1417). Hippocampal place cells also encode temporal information concerning the order of events; replay of the firing of these neurons in sleep has been shown to exhibit a pattern that reflects the temporal order in which these cells fired initially during waking exploration (1188). Hence, reactivation of these hippocampal neuronal ensembles during sleep is postulated to represent the “consolidation” of labile memories into more stable forms. This reactivation of hippocampal neurons occurs in a compressed manner during high-frequency ripples occurring in the hippocampus during NREM sleep, a possible endogenous mechanism for the generation of LTP (175, 179) and sleep loss will impact this process. 3) Sharp-wave ripple complexes: the large and high-frequency depolarization of CA1 neurons occurring during sharp-wave-ripple complexes (sect. III) induces synaptic potentiation in a Hebbian manner reminiscent of in vitro tetanic stimulation protocols used to induce long-term potentiation (LTP) (179, 628). LTP-like stimuli induce sharp waves in vitro (89), and LTP is occluded in hippocampal slices that produce spontaneous sharp waves (254), supporting the idea that sharp waves represent an endogenous trigger for LTP-like synaptic plasticity. Buzsaki (1989) proposed in his two-process model of memory formation that these events are involved in the consolidation and transfer of short-term memory traces from the hippocampus to the neocortex during sleep (175). Hippocampal sharp waves occur just prior to the transition from neocortical down states to up states, suggesting that they facilitate down-to-up transitions. Furthermore, in combined recordings from hippocampus and prefrontal cortex in naturally sleeping rats, prefrontal neurons consistently fired 100 ms following hippocampal neurons participating in hippocampal sharp waves, suggesting a hippocampal-neocortical dialogue (875, 1408). In animals, an elevated sleep spindle density has been observed after learning in rats (345, 346, 876). Several human studies suggest that disruption of stage 2 sleep, when spindles occur, is linked with impairments in procedural memory (1390). 4) Reduced acetylcholine levels: low acetylcholine levels occurring duringNREMsleep are important for declarative memory consolidation (402).
3. Sleep loss impacts cellular mechanisms of learning and memory occurring during REM sleep
Disruption of REM sleep neuronal processing events represents another potential mechanism affecting learning and memory formation. 1) Neuronal replay during REM sleep may reflect neocortical activation of hippocampal circuits during a later stage of the memory consolidation process (499, 1244) since the duration of replay of hippocampal neuronal ensemble activity during REM sleep was comparable to that during waking task performance (753) (i.e., on the order of tens of seconds to minutes), much longer than replay recorded during NREM sleep. The existence of replay of hippocampal ensemble activity during REM sleep leads to speculation concerning the information content in dream states (linked to REM sleep) and its potential significance in memory processing, perhaps especially for procedural memory which is known to be REM sleep-dependent (607). 2) Theta rhythms: behavior-dependent modifications of subcortically driven theta rhythms are also reproduced during REM sleep. 3) P-waves: work from Datta and colleagues has shown that activation of the P-wave generator in the dorsolateral pons (sect. IV) facilitates learning and memory, particularly involving dorsal hippocampus activation, whereas inhibition causes the reverse effect (275, 280, 284, 285, 813, 814). Disruption of any of these three processes during sleep disruption may affect memory formation.
4. Inhibition of synaptic plasticity
It is generally accepted that long-lasting changes in hippocampal synaptic efficacy, examined experimentally in LTP or LTD paradigms, underlie declarative memory formation (109). Multiple studies in recent years have shown an impairment of the induction or maintenance of LTP by periods of total SD (184, 1074, 1350), REM SD (288, 749, 797, 837, 1074, 1155), or sleep fragmentation (46, 1273). Interestingly, the sleep disruption effects on synaptic plasticity appear to be specific for LTP since short-term presynaptic plasticity (paired-pulse facilitation) and long-term depression were unaffected (837, 1273). In addition to these physiological changes in LTP and LTD, sleep disruption reduces hippocampal neurogenesis (459–461, 469).
A) Nmda Receptor Composition and Ltp/Ltd
One likely mechanism underlying the loss of LTP by sleep disruption is altered subunit composition ofNMDAreceptors (216, 655, 746). Following long-periods of REM SD (24 or 72 h), a reduction in the NMDA receptor-mediated component of excitatory synaptic currents was shown in the CA1 region of the hippocampus (836) and dentate gyrus (216). This reduction was associated with reductions in the surface expression of the obligatory NR1 subunit of the NMDA receptor (216, 836). An increased NR2A/NR2B subunit ratio was observed in the CA1 region using a milder form of SD (4–6 h) and electrophysiological analysis of isolated synaptic currents and immunoblot analysis of purified synaptosomes (655). Furthermore, immunogold labeling of NR2A and NR2B subunits and electron microscopy revealed a 1.6-fold increase in the amount of NR2A subunits at synaptic sites in the CA1 region. Greater NR2A receptor content was shown to facilitate the induction of LTD elicited by stimulation in the theta-frequency range (746). Thus the sliding threshold for inducing LTD or LTP was shifted in the direction of LTD by SD (655, 746). Changes in LTP/LTD were not observed following SD in NR2A knock-out mice, suggesting that the change in NR2A subunits was sufficient to explain the changes in synaptic plasticity by sleep loss (746). Theoretically, reduced LTP produced by NMDA receptor downregulation or altered composition could be overcome by increasing the amplitude or duration of depolarization produced by the AMPA/kainate subtype of glutamate receptors so that increased calcium enters the postsynaptic cell either via NMDA receptors or voltagedependent calcium channels. Along these lines, a positive allosteric modulator of AMPA receptors (AMPAkine CX717), which prolongs AMPA receptor-mediated EPSP decay time, was shown to strikingly enhance the performance of monkeys following a single night of sleep deprivation (477, 1019). In addition to alterations in NMDA receptors, SD also impairs the late-phase of hippocampal LTP via impaired cAMP signaling (1350), perhaps suggestive of impaired cellular energy (323). Consistent with this idea, the energy sensor AMPK regulates the late phase of hippocampal LTP (1023).
B) Ltp And Ltd During Normal Sleep and Wakefulness
In contrast to the well-replicated inhibition of LTP with sleep loss, there is less consensus regarding synaptic plasticity processes occurring during natural waking and different stages of sleep. Early studies showed that the strength of synaptic transmission varies according to the time of day (72). More recently, Cirelli and Tononi, based on an examination of gene transcripts expressed during waking and sleep, proposed that LTP occurs mainly during waking, whereas LTD occurs mainly during sleep (sect. III). Buzsaki, on the other hand, based on hippocampal neuronal activity patterns proposed a two-stage model of memory formation which postulated that sharp waves occurring during NREMsleep in the CA3 region would be an ideal trigger for LTP-like processes in the CA1 region (175). Also in line with the idea that synaptic strengthening can occur during sleep are findings of strengthened cortical responses in the visual system of developing cats which required NMDA receptors and PKA activity (53, 561).
5. Increases in extracellular adenosine
Adenosine can inhibit LTP via A1 receptors (38), and both extracellular adenosine levels and A1 receptors have been shown to increase in cortical areas during SD (sect. III). Several experiments have shown that disruption of this process leads to deficits in working or spatial memory: 1) conditional knock-out mice for the adenosine A1 receptor (99); 2) rats with VLPO lesions had disruption in LTP that could be partially rescued by adenosine A1 receptor antagonism (46); and 3) dn-SNARE mice which cannot release adenosine from astrocytes also had disrupted cortical slow waves (360) and memory formation (367).
E. Conclusion
Sleep disruption-induced impairments in cognitive performance have many real world consequences. Increased industrial and car accidents are an obvious correlate, but sleep disruption also has more subtle effects that affect individuals and the economy. For instance, recent studies showed that acute SD increases risk-taking behavior (623, 841, 1351) and impairs the ability to integrate emotion and cognition to guide moral, emotionally evocative, judgements (624). On the positive side, acute SD has a rapid mood-elevating effect in some depressed patients. Current evidence suggests changes in synaptic transmission, neuronal oscillations, and neuromodulatory projections to the cortex, in particular cholinergic and dopaminergic systems, may be particularly important in mediating the behavioral alterations which result from sleep loss.
VI. GENOMICS AND PROTEOMICS
The description of sleep and wakefulness described in sections II-V of this review has primarily relied on electrophysiological, behavioral, and pharmacological studies. However, there are multiple aspects of sleep and wakefulness that are regulated in concert, suggesting contributions of genes and their protein products. Thus many recent studies have focused on the genetics and proteomics of sleep.
The earliest indications of genetic influences on sleep were from studies of mono- and dizygotic twins in the 1930s (287). Although most early twin studies used relatively small numbers of subjects, many aspects of sleep, such as sleep latency, awakening measures, amount of REM sleep, and temporal pattern of eye movement, were significantly correlated in mono- but not in dizygotic twins. Moreover, the EEG spectral patterns showed striking similarities between monozygotic twins (287). More recent genomic studies have utilized various techniques including: 1) subtractive hybridization, 2) nylon membrane macroarrays, 3) microarrays used in conjunction with specific quantitative methods such as in situ hybridization and real time polymerase chain reaction, and 4) transgenic mice with constitutive or conditional loss or gain of gene function. Several animal model systems have been used to understand the fundamental nature of sleep including sparrows, mice, the fruit fly Drosophila melanogaster, the nematode round worm Caenorhabditis elegans, and zebrafish. In the past decade, several reviews have been written on this topic of genome-wide gene expression (230, 287, 1145). Similarities in the sleep markers (genes and proteins) across different species have not only underscored the universal nature and the need for sleep but also revealed a plethora of information on various functional categories of genes and proteins. In this section we review the genes and proteins associated with sleep and wakefulness in humans and animals, grouped according to function at the cellular or network level.
A. Genes Associated With Sleep-Wake Regulation
1. EEG
Zung and Wilson in 1966 (1479) were the first to perform polysomnographic studies in twins. These authors demonstrated striking similarities in the temporal pattern of sleep stages between monozygotic twins. One of the first genetic loci responsible for low voltage EEG was mapped to human chromosome 20q (1212). Later, several twin studies reported that EEG delta, theta, alpha, and beta frequencies are heritable traits that are highly correlated in monozygotic twins (24, 34, 292, 1324). These results were corroborated by findings in rodents, showing strong heritability in EEG traits in inbred mice (382, 383, 1258, 1322). Quantitative trait loci (QTL, stretches ofDNAthat have clusters of genes related to a trait, in this case “sleep”) studies among different strains of mice identified several heritable genes for specific sleep parameters (383, 938, 1258), especially different EEG frequencies. QTL analysis identified a single gene encoding the retinoic acid receptor beta (Rarb), located on chromosome 14, that controls delta frequency EEG activity (796). Targeted disruption of this gene confirmed the importance of retinoic acid signaling in control of EEG delta frequencies (796), although the mechanism remains unclear. Theta frequency (5–8 Hz) activity during REM sleep is controlled by a single autosomal recessive gene, known as acylcoenzyme A dehydrogenase for short chain fatty acids (Acads) that is localized to chromosome 5 in mice (1260). The ion channels that contribute significantly to the characteristic EEG frequency bands of NREM and REM sleep are discussed in detail below.
2. Neurotransmitter systems
A) Acetylcholine
Few genetic studies of sleep and wakefulness have targeted the cholinergic system, although, as described in other sections of this review this system has been extensively investigated with other methodologies. In muscarinic receptor 3 (M3) knockout mice, REM sleep was decreased, whereas M2/M4 double knockouts had normal sleep (434). Brain stem cholinergic neurons provide a strong innervation of midbrain dopamine neurons involved in brain reward processes (see sects. II and IV). The VTA dopamine neurons express exclusively the M5 muscarinic receptor (1207) and not other muscarinic receptor subtypes. A reduced activation of dopamine midbrain neurons projecting to the nucleus accumbens was seen in M5 knockout mice (374). These mice also showed a reduced locomotor response to morphine (1207). Similarly, rats that had M5 antisense oligonucleotides injected into the VTA had reduced lateral hypothalamic self-stimulation (1461). Cholinergic nicotinic receptor mutations have little impact on sleep wake behavior (371, 703), possibly due to the large number of alternative subunits. The complexity of the gene encoding the synthesizing enzyme choline acetyltransferase (ChAT) has precluded a genetic analysis of its role in sleep-wake control.
B) Serotonin
Genetic studies confirm that the serotonergic system has important roles in arousal, suppression of REM sleep, and the sleep response to restraint stress. Mice in which serotonin neurons are deleted by deletion of the LIM homeobox transcription factor Lmx1b revealed a deficit in thermoregulation that led to an increase in wakefulness due to increased movement to generate heat (162). Furthermore, in these animals there was an impaired arousal response to CO2 reminiscent of sudden infant death syndrome (sect. VII). Serotonin 5-HT1A and 5-HT1B receptor knockout mice exhibited higher amounts ofREMsleep during both the light and dark periods, consistent with the inhibitory role of serotonin in REM sleep control (sect. IV). In addition, a reduced rebound REM sleep response following restraint stress was observed in these animals (8, 130). Similarly, genetic ablation of the serotonin transporter in mice led to enhanced spontaneous REM sleep (19, 1427) and a blunted REM sleep response to stress. Attenuation of restraint stress-induced enhancement of REM sleep in these animals was attributed to an overproduction of orexin, since it was reversed by blocking orexin receptors (1034). 5-HT2A receptor knockout mice showed a significant decrease in NREM sleep, with an increase in wake that was attributed to a decreased sensitivity of 5-HT2B receptors due to developmental alterations (1014), an important consideration in constitutive knockout studies. The homeostatic sleep response following 6 h of sleep deprivation was also attenuated in 5-HT2A mutant mice while changes in REM sleep were minimal. In Drosophila, mutations of 5-HT1A receptors resulted in short and fragmented sleep that was rescued by expressing the receptor in the mushroom bodies, a structure associated with learning and memory. On the other hand, 5-HT1B and 5-HT2 receptor mutations did not impact sleep wake behavior in this species (1471).
C) Norepinephrine
The noradrenergic system modulates the expression of several immediate early genes in brain regions that receive projections from the LC. In rats, lesions of noradrenergic neurons of the LC using the neurotoxin 6-hydroxydopamine downregulated the expression of Fos, nerve growth factor-induced A (NGF-IA), and the phosphorylation of CREB protein, to levels similar to those observed during sleep (238). On the contrary, in transgenic mice with disinhibition of NE neurons by conditional expression of chlorotoxin (Cltx), a scorpion venom that partially blocks small-conductance chloride channels preventing inhibitory GABAergic and glycinergic input, the level of NGF-IA expression was increased (1104). In both of these conditions the sleep-wake behavior was not altered, but the wakefulness-associated gene expression patterns were changed, suggesting norepinephrine is important for coding for arousal-dependent gene expression that promotes synaptic plasticity.
D) Histamine
Genetic studies suggest that histamine neurons are involved in the control of cognitive aspects of wakefulness (29). Mice with a knockout of the histamine synthesizing enzyme histamine decarboxylase (HDC) displayed reduced brain histamine, shortened sleep latencies, and a deficiency of wakefulness and exploration when mice are faced with the behavioral challenge of a novel environment (978). During the light period, sleep in these mice is fragmented whereas REM sleep increases (29). These mice show impaired cortical activation and a reduction in the differentiation of EEG signals seen between NREM sleep and wakefulness.
E) Orexins
One of the most significant genetic contributions to sleep research is the identification of the gene and receptors of the orexin (hypocretin) peptide and their role in sleep. The orexins were discovered simultaneously by two groups in 1998 (290, 1101). The study of De Lecea (290) reported the isolation of two novel peptides expressed at high levels in the hypothalamus using directional tag PCR subtraction technology. They named these peptides hypocretins based on their hypothalamic localization and weak homology to the secretin/incretin family of peptides. Simultaneously Sakurai and colleagues used a reverse pharmacology approach to identify ligands for the orphan G proteincoupled receptor HFGAN72 (now renamed OX1R or Hcrtr1) and named the peptides orexin A and orexin B (from the Latin orexis = appetite) since their cell bodies were located within the lateral hypothalamic feeding area and because they stimulated feeding upon intracerebroventricular administration (1101). They also discovered a second receptor for these peptides: OX2R or hcrt2. Shortly after, two laboratories independently linked the orexin system to narcolepsy (sect. VII). In dogs, the gene responsible for narcolepsy was mapped to the gene encoding orexin receptor 2 (hcrtr2) (727). On the other hand in mice, orexin peptide mutation was associated with narcoleptic behavior (214). In humans, narcolepsy results from the selective loss of orexin-producing neurons in lateral hypothalamus (475, 924, 999, 1284). The prominent reduction in orexin peptides in the cerebrospinal fluid is associated with the human leukocyte antigen haplotype DQB1*0602, leading to the suggestion that narcolepsy in humans is the result of an autoimmune attack on orexin-producing neurons (270, 520, 855) (see sect. VII). For a detailed discussion of the effects of orexin knockout/knockdown on wakefulness and REM sleep in rodents, see sections II, IV, and VII.
I) Orexin system in zebrafish. Orexins also play an important role in fish behavior (966). The orexin gene from zebrafish was cloned in 2004 (608). As in mammals, this gene is expressed in a localized manner within a cluster of hypothalamic cells and encodes for two peptides. The orexin-expressing neurons innervate several aminergic nuclei involved in sleep-wake regulation (608), and orexins are needed for promoting wakefulness as well as sleep consolidation. Overexpression of the orexin peptides in transgenic fish increases wakefulness (1024), while orexin knockout mutants display sleep fragmentation (1464). Studies in zebrafish orexin knockouts suggest that orexin regulates sleep-wake via regulating the pineal melatonin production (37). In Drosophila melanogaster, no genes with close homology to preproorexin have been found, but a group of cells which may serve a similar functional role to the orexins express the pigment dispersing factor (PDF). PDF released from central clock neurons promotes waking and consolidates sleep (977, 1157). Mutation in the PDF gene or its receptors, or ablation of PDF neurons leads to reduced activity at the beginning of the day, resulting in increased sleep and increased transitions from wake to sleep (1157).
F) Dopamine
In mice, knocking out the D2 receptor gene leads to decreased wakefulness with a concomitant increase in NREM and REM sleep and an increase in NREM delta power. Sleep-wake durations were shorter during spontaneous sleep, but the homeostatic sleep response following 2, 4, or 6 h of sleep deprivation was not affected by the absence of the D2 receptor (1032). Conversely, in the absence of dopamine uptake from the synapse in dopamine transporter (DAT) knockout mice, waking was increased and NREM sleep was reduced (1425). Similarly, flies with DAT mutation were short sleepers and lacked a homeostatic sleep response (679).
G) Gaba
The receptors for the inhibitory neurotransmitter GABA are the major target of hypnotic agents. However, genetic manipulations of the GABAergic system have so far shown only minor effects on spontaneous sleep wake regulation. At the receptor level, the large number of possible subunit compositions seems to allow compensatory changes in other subunits in response to the absence of one subunit. Such a strong compensatory response reflects a resistance to change and possibly emphasizes the importance for normal functioning of the GABAergic system in the brain. Point mutations in GABAA receptor alpha1–3 subunits failed to alter sleep-wake pattern (656, 657, 1295), although gene linkage analysis indicates significant linkage between the β frequencies of the human EEG and GABAA receptor genes (1015). GABAA receptor alpha3 subunit knockout mice also showed no gross changes in the EEG spectral analysis during sleep and wake (1421). However, EEG power in the spindle frequency range (10–15 Hz) was significantly lower at NREM-REM transitions in mutants. The homeostatic sleep response was normal in these mice (1421). GABA receptors containing the delta subunit show a predominant extrasynaptic localization (936, 1249) and mediate nondesensitizing “tonic” inhibition, in contrast to “phasic” inhibition controlled by synaptic GABAA receptors (357). GABAA delta receptor subunit knockouts did not show any differences in EEG (1422). In one study, GABAA receptor beta3 subunit knockout mice showed no difference in 24-h baseline sleep-wake recordings compared with wild-type mice (689), although another group of researchers reported that NREM delta and REM sleep were significantly increased in knockouts (1424). Mice lacking GABAB receptor 1 or 2 had an altered distribution of sleep across the day, suggesting that GABAB receptors play a role in the diurnal regulation of sleep (1369).
3. Sleep factors
A) Adenosine
Considerable genetic evidence in mice and humans supports the role of adenosine in spontaneous sleep-wake control and in the homeostatic sleep response (discussed in sect. IIIC). QTL studies in mice demonstrated that a genomic region containing the genes of two adenosine metabolizing enzymes, adenosine deaminase and S-adenosyl-homocysteine hydrolase, impact the rate at which sleep need accumulates during wakefulness (378). In humans, a homologous region in chromosome 20 containing an adenosine deaminase gene polymorphism at nucleotide 22 (coding DNA 22G→A, leading to a substitution of asparagine for aspartic acid at codon 8) influences sleep. Individuals with the G/A genotype reported fewer awakenings at night, spent longer in NREM sleep, and showed higher delta power during sleep than individuals with the G/G genotype (1059). The enzyme adenosine kinase, responsible for conversion of adenosine to adenosine monophosphate, is also important in the regulation of adenosine metabolism and sleep-wake behavior. A transgenic mouse model, Adk-tg, with enhanced constitutive expression and activity of cytoplasmic adenosine kinase, had reduced adenosine tone, suggested to be due to enhanced intracellular conversion of adenosine to adenosine monophosphate, which in turn facilitates adenosine uptake into the cell resulting in lower levels of extracellular adenosine (358). A recent study of these mice (960) showed a significant reduction of EEG power at low frequencies in all vigilance states and in theta activity (6.25–11 Hz) in REM sleep and waking. These mice spent significantly less time in NREM and REMsleep compared with wild-type mice. The homeostatic delta power response following 6 h of sleep deprivation was attenuated compared with the wild-type mice (960).
I) Adenosine receptor knockouts and polymorphisms. Both A1 and A2A adenosine receptors are implicated in mediating the sleep-inducing effects of adenosine. Studies in receptor knockout mice highlight the possible confounds arising from developmental compensations for the absence of a specific gene. For example, despite ample physiological, pharmacological, and electrophysiological evidence for the role of A1 adenosine receptor in sleep wake regulation, constitutive A1 receptor knockout mice failed to show any changes in the sleep patterns and EEG parameters under baseline conditions or following sleep deprivation (1214). However, in the absence of any developmental compensatory changes, mice with a conditional A1 receptor deletion in forebrain and brain stem after 6–8 wk of age showed a decreased homeostatic sleep response after sleep disruption (99). In A2A receptor knockout mice, the homeostatic sleep response was attenuated (485). A comparative study of the A1 and A2A receptor knockout mice showed that the wakepromoting effect of caffeine was absent only in A2A knockout mice but not in A1 knockouts, suggesting that A2A has a more prominent role in sleep-wake regulation (529). However, in light of the pronounced A2A receptor-mediated locomotor effects of caffeine (338, 1460, 1470), the reported decrease in wakefulness in A2A but not A1 knockout mice in response to caffeine, needs careful interpretation. In humans, self-rated, caffeine-sensitive individuals showed impaired performance on a psychomotor vigilance task after one night without sleep. Individuals with the lowest sensitivity to caffeine were least sensitive to the detrimental effects of sleep debt (1058). Further investigations reported that a distinct polymorphism within the A2A receptor gene (c.1083T>C) determines the caffeine responsiveness to sleep in humans (1060). Together, these genetic studies strongly support the importance of adenosinergic mechanisms in sleep-wake regulation.
B) Nitric Oxide
As discussed in other sections of this review, nitric oxide produced by nNOS or iNOS plays an important role in several aspects of sleep-wake control. Mice lacking nNOS showed a significant reduction in REM sleep during 24 h baseline sleep recording. In contrast, iNOS knockout mice showed a significant increase in REM sleep during 24 h baseline sleep and a decrease in NREM sleep during the dark period (218). Consistent with these observations are the results from studies performed to evaluate the effect of selective inhibitors of nNOS and iNOS on recovery NREM and REM sleep that follows prolonged sleep deprivation (589, 593). In rats, in vivo microdialysis of specific inhibitors of iNOS, 1400W, prevented NREM recovery, while an inhibitor of nNOS, l-N-propyl-arginine, decreased REM recovery but did not affect NREM recovery (589, 593).
C) Cytokines and Other Humoral Factors
Cytokines involved in host defense mechanisms have been implicated as sleep factors (see sect. IIIC). The effect of the loss of function of many of these cytokines on sleep-wake pattern has been studied using specific gene knockout mouse models. Mice lacking the IL-1β receptor spent less time in NREM sleep during the light period (354), whereas IL-6-deficient mice spent 30% more time in REM sleep during 24 h of baseline without any significant change in NREM sleep or wakefulness (896). In response to SD, IL-6 knockout mice took much longer to recover from the sleep loss, suggesting a role for IL-6 in the dynamics of responses to SD. Like IL-1β receptor knockout mice, the TNF receptor 1 knockout mice also sleep less during the light period (353). The levels of TNF mRNA and protein increase during the light periods in rats suggesting their involvement in sleep (135, 368). TNF and lymphotoxin-α are the ligands for two TNF receptors (TNFR1, 55 kDa; TNFR2, 75 kDa in size). In mice deficient in both of the ligands, a 15% decrease in REM sleep during the baseline light period was observed. A similar reduction in REM sleep is also observed in TNFR2 knockout mice (295). The slow wave parameters of the recovery sleep that followed 6 h of sleep deprivation varied in different mice. In the double ligand knockout and TNFR2 knockout mice, the SWA activity selectively increased in the 2.75–4.0 Hz range, whereas in TNFR1-deficient mice, the intensity of low range SWA (0.75–2.5 Hz) increased during recovery sleep (295). In mice lacking both IL-1β type 1 receptor and TNFR1, the power spectra of the NREM sleep EEG showed differences compared with wildtype mice for 24 h. Following sleep deprivation, the increase in delta power during NREM sleep of IL-1R1/TNFR1 knockout mice was of greater magnitude and of longer duration than that observed in control mice (69). These genetic experiments suggest a role for these cytokines in normal sleep-wake regulation in addition to their more well-known role in the response to infection.
D) Growth Hormone and Growth Hormone Releasing Hormone
Growth hormone (GH) and GH releasing hormone (GHRH) are additional factors associated with sleep. Rats with mutation of the GH gene exhibit stunted growth and a higher expression of GHRH in the hypothalamus (995) as well as higher NREM sleep and decreased REM sleep during the light period. While the homeostatic NREM sleep response was normal in these rats, the NREM delta activity was not enhanced during the recovery sleep that follows sleep deprivation (995). On the other hand, dwarf rats (207) show reduced responsiveness to GHRH and thus exhibit moderate growth retardation and decreased plasma and pituitary GH (207). The dwarf rats show decreased spontaneous sleep compared with wild-type controls (942). Decreased spontaneous sleep was also reported for a mouse model with a point mutation in GHRH receptor (941). Thus genetic studies support a role for the GH/GHRH system in the control of sleep.
4. Transcription factors
Transcription factors, a large class of DNA binding proteins, respond to the transduced intracellular signals originating from membrane proteins (receptors, ion channels) and induce the expression of specific target gene(s) that code for different physiological processes.
A) Fos
One of the first, and most commonly studied transcription factors with respect to the sleep-wake cycle, is the immediate early gene Fos (243). Many studies have investigated the distribution of Fos protein in specific populations of neurons to determine their activity during spontaneous wake or sleep or in sleep homeostasis (78, 235–237, 440, 444, 842, 930, 1010, 1011, 1162, 1169–1171, 1459). Although lacking in temporal resolution, this technique has been extremely useful in identifying the location and neuronal phenotype of neurons involved in sleep-wake control. However, it should be noted that typically only a small subset of any particular neuronal subpopulation is labeled with Fos in any particular behavioral state (243). Fos expression primarily reflects intracellular calcium increases rather than neuronal firing per se (664). Fos controls the expression of other genes, some of which are listed below and may be involved in functions related to sleep-wakefulness control such as synaptic plasticity (243). Fos knockout mice have increased wakefulness with a concomitant reduction in NREM sleep, whereas REM sleep is not affected (1167). Knockout of another member of the Fos family, fosB, caused decreased REM sleep without other changes in sleep or wake (1167).
B) Camp Response Element-Binding Protein
Another stimulus-induced transcription factor, the cAMP responsive element binding protein (CREB), is implicated in memory formation and sleep in both flies (496) and rodents (442). Flies with mutations resulting in increased cAMP (resulting in increased CREB activity) rested significantly less, whereas mutations that abolish CREB activity rested more with increased recovery sleep (496). Similarly, mice lacking the alpha and delta isoforms of CREB had reduced time spent awake and more NREM sleep (442).
C) Transcription Factors Associated with the Circadian Pacemaker
Several transcription factors and inhibitors of transcription factors involved in circadian behavior also directly impact the sleep EEG parameters associated with sleep homeostasis (379). Homeostatic sleep regulation is considered independent of the circadian regulation since it is intact in animals with lesions of the SCN, the circadian pacemaker (860, 1293, 1305). However, in animals with intact SCN, several transcription factors originally recognized as circadian factors also impact homeostatic sleep parameters, as described next.
I) The neuronal Per-Arnt-Sim-type (NPAS) and cryptochromes. The NPAS domain protein 2 combines sensor and effector functions by sensing the redox state of the cell (1086) and regulating the transcription of metabolic genes such as lactose dehydrogenase1 ldh1) and the period gene (per-2 gene), the latter of which is also implicated in sleep homeostasis. Sleep characteristics are altered in the absence of Npas-2 as demonstrated by the study of NPAS2-GENE knockout mice. These mice showed a decrease in NREM sleep time, even after periods of sleep deprivation (380). During NREM sleep, the spindle activity was reduced and the EEG activity within the delta range was shifted to faster frequencies. Sleep is also altered in mice lacking cryptochromes (Cry), transcriptional regulators integral to circadian oscillations. Cry 1 and 2 knockout mice exhibit longer bouts of NREM sleep and higher EEG delta power during NREM sleep (1426). In these mutants, the absence of transcriptional inhibition of Clock and NPAS2 proteins is suggested to cause the increased sleep duration. In contrast, in clock gene knockout mice, NREM sleep durations are decreased, as was observed in NPAS-2 gene knockout mice (912). The deletion of yet another transcription factor associated with circadian rhythmicity, the albumin d-binding protein (Dbp), results in decreased sleep consolidation and NREM sleep delta power (381).
Regulators of circadian rhythms can impact sleep timing, duration, and intensity. The transcriptional factor NPAS2 regulates the expression of the Period proteins, per1 and per2. Per1 and per2 mutant mice have altered circadian rhythmicity (18), but sleep homeostasis is unaltered (654). In humans, per2 gene mutations were shown to contribute to 4 h advance of the sleep/wake rhythm in some cases of familial advanced sleep-phase syndrome (1296). A recent report further confirmed the role of Per proteins in sleep timing in relation to the light-dark cycle. A polymorphism in the Per3 gene has been associated with morning/evening sleep-wake preferences. Individuals with a 5-repeat allele (per35/5) show increases in alpha activity in REM sleep, theta/alpha activity during wakefulness, and slow wave activity in NREM sleep (310). Sleep deprivation results in greater cognitive deficits, as demonstrated by brain fMRI-assessed responses to executive tasks compared with individuals with the wild-type allele (310). In one family, a mutation in the casein kinase 1δ gene which interacts with the Per protein, and a per mutation in another family, were associated with familial advanced sleep phase syndrome (1296, 1447). Another protein, Dec2, suggested to repress the expression of Clock/Bmal1, was shown to regulate sleep time in humans. Point mutations in the dec2 gene were identified in a family of short sleepers (6 h sleep) (487). These reports in humans collectively suggest interactions between regulations of circadian rhythms and sleep timing, duration, and intensity.
5. Ion channels
A) Potassium Channels
Voltage-dependent potassium channels (Kv channels). The Kv channels are activated by depolarization and are present in a wide range of tissues. They are composed of four alpha units that form a pore and four beta units closely associated with the alpha units, forming an octameric channel. Nine Kv channel alpha subunit families (Kv1–9) have been described (250). Genetic studies have shown strong associations of some of these Kv channels with sleep-wake regulation.
I) The voltage-gated potassium channel, Shaker. This channel has been shown to play a major role in sleep in Drosophila melanogaster 231). Shaker mutants had reduced sleep and were resistant to the effects of sleep deprivation (231). Further studies in Drosophila identified a novel glycosylphosphatidyl-inositol-anchored protein/gene, which as indicated by the name, SLEEPLESS (SSS), prevents sleep (650) and regulates the function of Shaker (1437). Flies carrying a defective shaker gene, the null allele minisleep (Shakermns), or other null alleles of Shaker, are short sleepers (3–4 h/day), while the wild-type controls sleep 8–14 h/day (231). Similarly, loss-of-function mutations in another gene, Hyperkinetic, that codes for the regulatory (beta) subunit of the Shaker channel, results in reduced sleep (170). Thus there is ample evidence in flies demonstrating the importance of voltage-gated potassium channels in normal sleep-wake regulation. In mice, the Kv1 alpha subunit family of potassium channels is closely related to Shaker in flies. A null mutation in the Kv1.2 gene, Kcna2, in mice, when homozygous, generates seizures in pups after P17 and the pups die at a young age (postnatal day 28). However, sleep measured at P17 shows significantly less NREM sleep (315). The sleep studies in these mutant mice, while limited to young ages, are nevertheless indicative of the importance of the Kv1.2 channels in sleep regulation.
II) Delayed rectifier Kv3.1 and Kv3.3 channels. Subunits of fast-activating/deactivating, high-threshold voltage-gated potassium channels, encoded by the genes Kcnc1 and Kcnc3, are widely expressed in several brain regions including the thalamus, basal ganglia, and cerebellum, areas implicated in the control and modulation of arousal states and motor activity (1083). Although Kv3.1 and Kv3.3 subunits are expressed throughout the brain, their expression is restricted to distinct neuronal subpopulations (204, 1146, 1396, 1397). In neocortex, thalamus, hippocampus, and striatum, Kv3-type channels are found in GABAergic cells that also express the calcium-binding protein parvalbumin (PV), a marker for fast-spiking neurons. Kv3-type channels are involved in the rapid repolarization of the action potential, and their presence in neurons correlates with narrow action potentials, fast afterhyperpolarization, and high-frequency firing. Single Kv3.1 mutation or double Kv3.1/Kv3.3 mutations led to an increase in action potential duration of 20 and 60%, respectively (349). Mutation of these genes showed profound effects on sleep-wake patterns in mice. Mice with a knockout of Kv3.1 show increased gamma oscillations and markedly reduced delta oscillations (566). Mice with double mutations display severe sleep loss (40% decrease in the light period and 22% decrease in the dark period) as a result of unstable slow-wave sleep (349, 567). Absence of these two gene products led to a 70% reduction in the cortical spectral power at frequencies <15 Hz. In addition, the number of sleep spindles in vivo as well as rhythmic rebound firing of thalamic reticular neurons in vitro is diminished in double mutant mice (349). The mice show a 70% reduction in the absolute power in the delta band and fail to show a homeostatic sleep response following 6 h of acute SD (349). It was suggested that the Kv3.1 and Kv3.3 channels in the GABAergic thalamic reticular neurons play an important role in the thalamocortical network in generating oscillations typical for sleep (567).
Another member of the voltage-gated potassium channel family, Kv3.2, is also expressed abundantly in the thalamus, neocortex, and hippocampus and is moderately expressed in medial septum, LC, and basal ganglia (1082, 1397). Deletion of the Kv3.2 gene in mice results in reduced power in the NREM EEG frequency range 3.25–6 Hz, suggested to be a consequence of decreased efficacy of GABAergic interneurons that express Kv3.2 in cortex. Unlike the deletion of Kv3.1 subtype channels which increases wake EEG at gamma frequencies (20–60 Hz)(566), the Kv3.2 deletions do not affect waking EEG parameters (1382).
III) Potassium-selective leak channels. Potassium-selective leak channels possess two pore-forming domains in each subunit (431). The leak currents generated by these channels (also known as resting or background conductances) control the resting membrane potential and input conductance, thereby influencing the excitability of the neurons (431). As described in section II, many neurotransmitters excite neurons by inhibition of resting K+leak currents. This is crucial to the regulation of “state-switching” in cortical and thalamic neurons. Recently, one such acid-sensitive and anesthetic activated, two-pore domain potassium channel subtype, TASK-3, was shown to be involved in the regulation of cortical type II theta oscillation (also known as arousal theta) in the frequency range 4–9 Hz (965). Mice with TASK-3 gene knockout showed an absence of such type II theta oscillations. They displayed a slower progression into sleep (increased sleep latency) during spontaneous sleep in the lights-on period. In these mice sleep was also fragmented, with a higher number but shorter duration of sleep episodes.
B) Calcium Channels
Genetic evidence suggests that the lowthreshold, voltage-activated, T-type calcium channels are involved in the regulation of sleep and particularly in sleep rhythms. Low-threshold calcium channels are crucial for shaping subthreshold membrane fluctuations and thereby contribute to such behaviors as rebound burst firing (740) and rhythmic oscillation (64, 458). The α1G subunit of T-type calcium channels is widely expressed in the brain, including thalamocortical neurons, and thalamic reticular neurons and cortex (1269). Both constitutive and targeted deletions of the α1G (Cav3.1) subunit of T-type channels have been studied in the context of sleep regulation. Conflicting observations in sleep parameters were reported. In one study, power spectral analysis showed that the power of low frequencies (2– 6.5 Hz) in NREM sleep was significantly reduced compared with their wild-type littermates (695). Another study showed that in mice with either a α1G constitutive knockout or a localized thalamic specific knockout, the cortical EEG delta power during NREM sleep was increased (32). These mice have difficulty in initiating and maintaining sleep. The differences in the observations may reflect differences in the manner the knockout mice were constructed.
In the dendrites of thalamic reticular neurons, the calciumdependent small-conductance (SK)-type K+ SK2, Kcnn2) channels and the T-type calcium channels act in concert with the sarco/endoplasmic reticulum calcium-ATPases (SERCAs) to influence the characteristic frequency bands of NREM sleep (55, 64). In SK2−/− knockout mice NREM sleep is fragmented, with more frequent awakenings, indicative of decreased sleep depth (266). Consistent with this behavior, the EEG spectral profile showed a fourfold reduction in the delta (1–4 Hz) frequency range and a more than threefold reduction in the sleep spindle (10–15 Hz) band. During waking and REM sleep, a pronounced reduction was observed in the 10-Hz range, i.e., a slowing of EEG theta oscillations.
6. Genes implicated in sleep functions
A) Synaptic Plasticity, Learning, and Memory
Cellular functions such as transcription, cell signaling, and synaptic plasticity are integral to learning and memory. Alteration of these functions also affects sleep-wake regulation. Mutations in many of the genes associated with the molecular cascades underlying learning and memory have been shown to alter sleep-wake cycles. On the other hand, alterations in sleep-wake patterns impact learning and memory (see sect. V) (503).
One signaling pathway implicated in both processes involves the signaling molecule cAMP and cAMP-dependent protein kinase A (1350). Activation of the cAMP signaling pathway promotes waking. In flies, the expression of cAMP and protein kinase A in the mushroom bodies, an important region for memory formation (488), regulates sleep (568, 1006). In transgenic mice, with a dominant negative mutation in the regulatory subunit of protein kinase A in neurons, NREM sleep is fragmented with increased NREM delta activity and reduced spindle amplitude, whereas REM sleep is increased, suggesting protein kinase A is involved in sleep-wake regulation (491). cAMP affects gene expression via the cAMP response element binding protein (CREB). Mice lacking CREB have reduced LTP and memory formation and exhibit shorter wake bouts, whereas NREM bouts are longer (441, 442). These studies are broadly consistent with the idea that synaptic potentiation predominates during waking as discussed in section III, in the context of the synaptic homeostasis theory of sleep.
Another protein involved in synaptic plasticity that has been investigated in the context of sleep is Homer1a. The Homer proteins are a family of proteins broadly expressed in the brain where they serve as molecular scaffolds at synapses. Increased expression of Homer 1a reduces glutamate-induced intracellular calcium release and thus down-regulates synapse formation (1103, 1432). All the homer proteins are expressed constitutively except for Homer1a, an immediate early gene originally isolated as a neural activity-regulated gene product from seizure-stimulated rat hippocampus. In rodents, the expression of Homer1a is modulated during sleep and wakefulness and is highly upregulated during sleep deprivation (233, 914). Further evidence for sleep deprivation-induced upregulation of Homer1a comes from transcriptome profiling of inbred mouse strains where Homer1a is consistently increased in all strains following sleep deprivation (795). QTL analysis showed a significant association of Homer1a with sleep-wake regulation and sleep homeostasis (771).
A recent study (425) monitored the expression of synaptic genes over the course of the normal sleep-wake cycle, as well as following periods of sleep deprivation in Drosophila. The expression of synaptic proteins decreased in a sleep-dependent manner (425). The Drosophila Fragile X mental retardation gene (dfmr) is one synaptic plasticity related gene that has been shown to regulate sleep need, although the exact mechanism is unknown (171). This gene codes for a protein (dFMRP) that is present in dendritic spines. The expression of this gene is high during development when sleep is higher and synaptic plasticity is high. Increased expression of dFMRP, even if restricted to mushroom bodies, decreases daily sleep and decreased expression increases sleep. Both gain and loss of dFRMP expression result in loss of sleep homeostasis (171).
B) Sleep and Energy
Brain energy use varies between sleep and wakefulness. During waking, higher levels of energy are consumed compared with sleep (see sect. IIID). Gene expression studies have shown that increased ATP use during waking leads to the upregulation of enzymes involved in the synthesis of ATP by oxidative phosphorylation. Five, multsubunit enzyme complexes, complexes I to V (Cox I-V) constitute the mitochondrial oxidative phosphorylation system, and enzymes belonging to each complex are upregulated during waking, indicating an increased need for ATP synthesis. For example, the expression of Nadh2, cytochrome c oxidase (CoxI), Cox4, and Atp5a genes increase during sleep deprivation in mammalian cortex (233, 239, 240, 242). A concurrent increase in the activity of cytochrome c oxidase has been reported in mice and rats (919, 920). The expression of mitochondrial uncoupler protein 2 is also increased during sleep deprivation (245). The nuclear transcription factors Nrf1 and 2 (known as nuclear respiratory factors) involved in the transcription of the components of oxidative phosphorylation are also upregulated during sleep deprivation (919). The essential nature of the genes coding for oxidative phosphorylation prevents any studies using knockout mice, but conditional knockouts may prove useful in further examining the effect of these genes in the regulation of sleep and wakefulness.
B. Proteomic Studies
Changes in gene expression are suggestive of potential cellular alterations associated with different vigilance states. However, the complexity of the regulation of downstream processes such as translation of mRNA into protein, posttranslational modifications of translated proteins, and protein-protein interactions prevents a direct assessment of the effect of gene expression on physiological processes. Since proteins are the ultimate players capable of influencing vigilance states, it is important to understand their regulation. Proteomics is an experimental approach to examine the proteins involved in the control of biological processes and pathways. The proteome indicates the quantitative expression profile of a cell, an organism, or a tissue under defined conditions. In contrast to the temporally constant genome, the proteome is dependent on intracellular and extracellular parameters. Thus the analysis of a proteome represents an important supplement to the genome analysis.
The use of proteomic approaches for investigating the regulation of vigilance states is relatively new. As the efficiency of proteomic methodology is being refined for increased sensitivity and inclusion of a wider range of proteins, a few attempts have already been made to explore overall protein changes during sleep-wake cycle or during prolonged waking (75, 234, 986, 1008, 1345, 1346). Most of these studies in the cortex, BF, or hippocampus of rat brain have compared protein profiles during sleep with those during prolonged sleep deprivation. So far, only two studies have examined protein changes after 10 min of spontaneous sleep or wake (1345, 1346), and no studies have examined proteomic changes selective to REM sleep. Major challenges for such studies originate from the short durations of sleep-wake episodes in rodents, especially REM sleep. During spontaneous sleep-wakefulness and prolonged waking, the major groups of proteins in the rat BF and cerebral cortex that showed alterations were associated with the following four functions: 1) synaptic plasticity, e.g., SNAP25, Amphyphysin, and vesicular N-ethylmaleimide fusion (NSF) protein; 2) the cytoskeleton, e.g., RhoB and GTP binding protein rab3D, Cofilin; and 3) cellular energy metabolism, e.g., creatine kinase, NADH dehydrogenase, pyruvate dehydrogenase, glutathione synthase, and glyceradehyde-3-phosphate dehydrogenase (75, 1345). Surface-enhanced laser desorption-ionization (SELDI) studies identified increases in hemoglobin alpha 1/2 and beta as well as cytochrome c in rat cerebral cortex (234). 4) Cellular stress responses, as discussed next.
Taking a lead from the wide-spectrum genomic and proteomic screening, one group of proteins, the heat shock/chaperone proteins, has been studied in more detail. Short periods of sleep deprivation (3–12 h) result in an increase in these proteins that are involved in preventing misfolding of proteins in the endoplasmic reticulum (153, 909). This unfolded protein response is considered to be a protective action that counteracts the cellular stress associated with sleep deprivation (153). The mRNA level of one such protein, the immunoglobulin binding protein, BiP, the most abundant protein in the endoplasmic reticulum (also known as glucose regulated protein 78, GRP78 or heat shock protein 5A, HSP5A) increases with prolonged wakefulness (229, 233, 772, 795, 1275). BiP binds to the hydrophobic domains of the nascent peptides and helps to prevent misfolding while the rest of the protein is being synthesized (423). In Drosophila, BiP levels regulate the quantity of recovery sleep (908). Transgenic animals that overexpressed BiP displayed increased recovery sleep following deprivation compared with animals that had reduced BiP levels.
C. Summary
A variety of genes and proteins have been associated with sleep and wakefulness by means of genetic and proteomic studies. Comparative studies have underscored the universal nature of sleep-wake regulation in different species. Perhaps unsurprisingly, mutations or knockouts of genes encoding ion channels or proteins involved in neurotransmitter release/uptake/transduction exert major effects on the sleep-wake cycle. In addition, genetic techniques have been instrumental in the identification of novel peptide neurotransmitters controlling the sleep-wake cycle such as the orexins, and their connections to human sleep physiology and pathophysiology. Furthermore, genetic and proteomic studies have provided important clues as to sleep function by identifying the major classes of genes and proteins that vary according to the sleep-wake cycle, in particular those involved in synaptic plasticity, cytoskeletal function, and cellular stress and energy regulation.
VII. SLEEP PATHOLOGY AND TREATMENT
In this section we summarize current knowledge of disorders of wakefulness and sleep and how they relate to the brain mechanisms of waking as well as NREM and REM sleep described in previous sections. Although epilepsy can be considered a disorder of wakefulness, this large topic is beyond the scope of this review (for more information, see Steriade and McCarley, Ref. 1228).
A. Coma
Profound changes in wakefulness and consciousness occur in patients with brain injury resulting in comatose, vegetative, or minimally conscious states. The EEG shows variable changes depending on the type and stage of coma, with alpha/theta rhythms, spindles, and triphasic/delta waves most common (139, 602). In general, faster rhythms typical of wakefulness are diminished and unresponsive to external stimuli.
In those coma patients who do not have widespread, diffuse loss of forebrain neurons, coma is normally associated with loss of activity and neurons at the origin or along the pathways of the ARAS, as described in section II. In the upper brain stem, damage is observed along the midline at the origin of the ARAS encompassing raphe nuclei, LC, LDT, parabrachial nucleus, and PnO (980). In rodents, cell-specific lesions of parabrachial but not neighboring brain stem neurons caused a comalike state (see sect. IIB), suggesting that this nucleus may be particularly important in maintaining wakefulness (397). Glutamatergic neurons in this region were shown to provide a major input to the BF (397). Surprisingly, a cat model of unconsciousness induced by cerebral concussion reported increased glucose utilization in the dorsomedial tegmentum adjacent to the VTG, suggesting that neurons in this dorsomedial area were activated (486). Infusions of carbachol in this area produced behavioral suppression (486), similar to cat models of REM sleep utilizing this pharmacological agent (see sect. IV). Along the ascending dorsal pathway, coma is observed following bilateral lesions of the regions of the thalamus supplied by the paramedian artery, i.e., dorsomedial nuclei and intralaminar centromedial, parafascicular and centrolateral nuclei (1137). Surprisingly, in rodents, extensive lesions of the thalamus failed to have pronounced effects on the EEG (397).
In some brain-damaged patients, imaging studies have indicated appropriate brain responsiveness to external commands or stimuli, indicating some preservation of function (1135, 1136). In these patients, partial restoration of function has been observed following deep brain stimulation of the central thalamus, administration of dopaminergic agents, or administration of the sleep aid and GABAergic receptor modulator zolpidem (Ambien). It has recently been postulated (1135) that all of these interventions act by enhancing activity in nonspecific thalamic projections to the cortex either directly (deep brain stimulation) or by reducing the inhibitory input from the basal ganglia (dopaminergic agents, zolpidem) to nonspecific thalamic nuclei. The paradoxical alerting effect of zolpidem may be due to a preferential inhibition of globus pallidus neurons, which project to the thalamus and are potently inhibited by zolpidem (220, 1135). The strong wake-promoting effects of the dopaminergic system and the weaker arousal effects of adenosine A2A antagonists in normal humans/animals may also be explained by such interactions of the basal ganglia and intralaminar thalamus.
Coma in humans is rarely associated with damage to the final node of the ventral pathway, the BF, presumably because its blood supply arises from multiple cerebral arteries so it is less susceptible to stroke. However, recent studies in animals suggest that extensive cell-specific lesions of the BF can also result in a comalike state (397, 611).
B. Insomnia
Insomnia, defined as insufficient quantity or quality of sleep, is the most prevalent sleep disorder. Approximately 50% of adults complain of occasional insomnia, and 10–15% of chronic insomnia, persisting for at least 1mo (892). Insomnia can involve difficulty falling asleep, staying asleep, or poor quality of sleep. Consequences of insomnia including daytime sleepiness, lack of energy, and cognitive impairment (835). Insomnia may even precipitate or accompany the development of psychiatric disorders (913). Insomnia is classified into two types: comorbid, with other psychological and/or physical pathologies; and primary, existing independent of other conditions (931).
1. Animal models of insomnia
Clinical and animal studies have employed a variety of sleep disruption techniques to mimic the symptoms of various types of insomnia (835). Other animal models investigate the neural mechanisms causing insomnia (1061). Stress-related models include classical fear conditioning with foot shock, cage change, or disturbing stimuli such as noise, unpleasant odors, cold, and pain (987, 1061). Some forms of insomnia may be due to hyperarousal (121, 929). Thus insomnia may also be induced pharmacologically in animals through manipulations of the vigilance-state circuitry described in earlier sections, e.g., through serotonergic synthesis inhibition by parachlorophenylalanine (PCPA) (899), adenosine receptor antagonism with caffeine (384), or enhancement of histaminergic (1044), dopaminergic (129), or orexinergic tone (1024, 1073). Genetic models that involve manipulation of circadian clock genes may also be regarded as insomnia models (see sect. VI). A rare autosomal dominant genetic disorder, fatal familial insomnia, involves a gene mutation of the prion protein gene prnp 878). Transgenic mice with a mutation of the prnp gene exhibit very fragmented sleep, as well as an exaggerated response to sleep deprivation (313, 1294) correlating with damage to the thalamic branch of the ARAS.
2. Possible neural circuits mediating insomnia
Recent preclinical investigations have begun to unravel the neural mechanisms by which cognitive, emotional, and sleep neural circuitry may interact to generate insomnia (186, 1116). For example, the sleep-promoting VLPO as well as the wake-promoting orexinergic zone of the lateral hypothalamus receive projections from mnemonic and emotion-related regions such as the infralimbic cortex, lateral septum, bed nucleus of stria terminalis, amygdala (central nucleus), and ventral subiculum (222, 1102, 1466). In an insomnia model involving cage change as a stressor, Fos protein was expressed in arousal, autonomic, and surprisingly, also in sleep-promoting regions, suggesting coactivation of arousal and sleep systems (186). Consequently, it was suggested that, in contrast to development of sleep-promoting compounds, attenuation of arousal systems may be a more effective approach to treat insomnia.
3. Imaging of insomnia
Functional imaging studies in humans have described brain regions with altered activity in insomnia (303, 304, 929). SPECT and PET studies have revealed that, during transition into NREM sleep in the insomniac, the normal reduction of glucose consumption is attenuated in the anterior cingulate cortex, the medial prefrontal cortex (mPFC), and limbic/arousal systems. Furthermore, during wakefulness, decreased glucose metabolism was evident in the cortex, thalamus, hypothalamus, and reticular formation relative to controls (303, 304, 929).
4. Insomnia treatment
Pharmacological treatment of insomnia usually involves sleep aids acting as agonists of the α1 subunit of the GABA receptor, such as benzodiazepines and the “Z” drugs (zolpidem, zalpelon, zopiclone) (329) which potentiate the action of sleep-promoting GABAergic neurons. Newer compounds to promote sleep that do not primarily involve manipulation of GABAergic systems have been recently introduced, such as the melatonin receptor agonist ramelteon (964); ritanserin, an antagonist of both 5-HT2A and 5-HT2C receptors (1372); and antidepressants such as trazadone (848) and agomelatine, a unique compound that acts as both a serotonergic 5-HT2C antagonist and melatonin receptor agonist (879). A variety of other compounds used to treat insomnia antagonize histaminergic (676) and orexinergic wake-promoting nuclei. In preliminary studies, dual orexin receptor antagonists, almorexant and MK4305, were shown to enhance both NREM and REM sleep and reduce wakefulness in animals, healthy humans, and insomniacs (140, 505).
C. Sleep Apnea
In sleep apnea, the patient stops breathing (the apneic moment) during sleep, leading to blood oxygen desaturation (hypoxia), as well as an elevation of carbon dioxide levels (hypercarbia, also known as hypercapnia). Sleep apneas are divided into three categories: central, obstructive, and complex (a combination of obstructive and central sleep apneas). Central sleep apnea involves dysfunction of the central respiratory control centers in the brain (1401). Obstructive sleep apnea (OSA) affects nearly 7% of the general population (1468) and is characterized by episodic cessation in breathing during sleep due to closure of the airway, of at least 10 s in length, and usually accompanied by hypoxia and hypercarbia (301, 1401). The apneic moment is usually terminated by a slight arousal, as well as an increase in sympathetic tone, as airway patency is reestablished. OSA diagnostic criteria include a rate of 5 or more apneic episodes per hour, and the most severe cases may experience upwards of 70–80 moments per hour. The sleep profile of an OSA patient includes sleep fragmentation and decreased prevalence of both deep stage NREM and REM sleep.
1. Mechanism of apnea
A reduction of activity in motoneurons innervating the upper airway during sleep is the cause of sleep apnea, occurring during both NREM and REM sleep. As described in section IV, a reduction in the activity of motoneurons occurs during NREM sleep and progresses to complete silence during tonic periods of REM sleep so that the upper airway is more susceptible to collapse during inhalation in susceptible individuals (369, 614, 760, 852, 948, 1189). Respiratory motoneurons follow a similar pattern, with expiratory pharyngeal and upper airway (hypoglossal, XII) motoneurons being the most strongly suppressed whilst phrenic motoneurons that drive the diaphragm are the least affected (398). Following the apneic moment, the upper airway reflex occurs, during which dilator muscles activate, reestablishing the patency of the airway (1401).
2. Cognitive consequences of apnea-induced sleep disruption
The abnormal sleep profile of OSA patients leads to daytime sleepiness, which is often comorbid with impaired cognitive function (626). Deficits in executive function among apneic patients have been assumed to be related to prefrontal lobe dysfunction caused by intermittent hypoxia (IH) (88). Consistent with this idea, rats exposed to IH exhibited spatial learning and memory impairments (437). However, sleep disruption itself can affect both “lower” level processes, such as arousal, as well as “higher” cognitive processes, such as memory and executive function (835). Thus the extent of behavioral impairment in OSA patients correlates with both the degree of hypoxemia and with the degree of fragmentation (87). Animal models mimicking the SF that occurs during OSA (see sect. V) revealed increased sleepiness and cognitive decrements in attention and working memory-tasks (844, 1273) as well as impaired hippocampal LTP (1273). Thus one cannot assume that the deficits in higher cognitive function observed in apneic patients are due solely, or even primarily, to hypoxemia during sleep. In fact, a functional imaging study suggested that working memory deficits exhibited in patients with sleep apnea may be due to the sleep fragmentation, not nocturnal hypoxia (1288).
3. Pathological features of OSA
Apneic episodes during OSA are accompanied by abnormal blood gas levels of oxygen (hypoxia) and carbon dioxide (hypercarbia). Animal models of intermittent hypoxia revealed cognitive deficits, oxidative stress, an increase of gene products related to apoptosis, and a decreased number or functional activity of catecholaminergic and cholinergic BF neurons (301, 835). However, whether apoptosis actually occurs is controversial (301). Clinical and animal studies have determined that exposure to intermittent hypoxia promotes reactive oxygen species, and in turn oxidative stress (301, 693). Reactive oxygen species formation has been shown to lead to physiological pathology, including cardiovascular morbidity, sympathetic activation, and hypertension. Accordingly, OSA has been confirmed to be a risk factor for stroke, atherosclerosis, heart attack, and hypertension (301, 1156).
4. Brain imaging of OSA patients
Investigations of brain anatomical abnormalities in OSA patients have provided some evidence of structural injury. MRI studies have reported gray matter morphological abnormalities in the frontal, anterior cingulate, and parietal cortices, mammillary bodies, temporal lobe, hippocampus, and cerebellum (678, 769). Most recently, diffusion tensor imaging revealed extensive abnormalities in white matter, including the corpus callosum, cingulum bundle, fornix, cerebellar peduncle, as well as within structures such as the deep cerebellar nuclei and the cingulate, prefrontal, and parietal cortices (770). Thus neural damage may occur in OSA. However, such findings were not replicated by other investigators (937), a discrepancy that may be due to differences in analysis methodology and subject variation (770).
5. Apnea treatment
Effective treatments for apnea include the CPAP (continuous positive airway pressure) device, dental devices, weight loss, and surgery (672). Pharmacological interventions to treat OSA increase the activity of the upper airway dilator muscles, as well as the ventilatory drive, e.g., noradrenergic and serotonergic agents, progestogens, and bronchodilators. Some patients experience the majority of apneic moments during REM sleep. Therefore, treatment with serotonergic agents that suppress REM sleep may be useful (1349). The stimulant modafinil has been prescribed to address the daytime hypersomnia experienced by the OSA patient (616).
6. Sudden infant death syndrome
Although not strictly an apneic syndrome, sudden infant death syndrome (SIDS) may be considered under the umbrella term of sleep-disordered breathing. SIDS deaths are usually caused by hypotension and bradycardia, due to an abnormal response to respiratory challenge (634). SIDS involves aberrant brain stem control of cardiorespiratory mechanisms by the developing brain. In one study, attenuated muscarinic cholinergic receptor binding of the arcuate nucleus, which is involved in cardiorespiratory mechanisms, was documented in SIDS cases (632). Also, serotonergic abnormalities in SIDS cases were recently shown (633), including deficient receptor binding and decreased tissue levels of both serotonin and the serotonergic synthetic enzyme tryptophan hydroxylase (318). Due to such abnormalities, the infant may have an attenuated response to respiratory challenges such as hypoxia or hypercapnia. An unusually high reoccurrence rate of SIDS has been documented within families, suggesting genetic susceptibility (634).
D. Metabolic Syndrome
Metabolic syndrome (MetS) consists of a combination of symptoms (low high-density lipoprotein cholesterol levels, abdominal obesity, elevated triglycerides, hypertension, insulin resistance/glucose intolerance) which increase the likelihood of developing cardiovascular disease (330). If at least three of these five criteria are present, the patient is considered to be suffering from MetS (330). Epidemiological studies indicate that poor sleep is associated with MetS symptoms (1200), although the direction of causality in patients with MetS remains to be determined; thus sleep disturbances may cause MetS symptoms or vice versa. Conversely, treatment of sleep disorders may help alleviate glucose and energy metabolism abnormalities (1200). An association of MetS with sleep is perhaps not surprising considering that one function of sleep appears to be regulation of energy metabolism (see sect. III).
Behavioral alterations of sleep are also associated with increased risk for MetS (1333). Shift work enhances the risk for a number of MetS symptoms by increasing oxidative stress (1158). Short or long sleep duration is associated with increased likelihood of MetS symptoms (405), with the incidence doubling in those that slept less than 6 h a night. Disturbed circadian/diurnal regulation of sleep also leads to MetS in animals. Homozygous Clock mutant mice with attenuated diurnal rhythms of feeding ate more, gained more weight, and had increased peripheral metabolic hormones such as insulin and leptin and abnormalities in hypothalamic hormones which regulate food intake (1313). Knockout of other circadian regulatory genes causes similar metabolic defects (41).
E. Narcolepsy
Narcolepsy is defined by a tetrad of symptoms: excessive daytime sleepiness, cataplexy, hypnagogic hallucinations, and sleep paralysis (1467). Narcolepsy affects all three stages of sleep and wakefulness, with a prevalence of ~1 in 2,000 individuals and an onset in early adulthood (1127, 1261). As originally described by Westphal (1400) and Gelineau (408) at the end of the 19th century, in this disorder there is a pronounced fragmentation of wakefulness, disrupted night-time sleep and intrusion of REM signs into wakefulness. Particularly striking and disabling are involuntary sleep attacks and episodes of (REM-like) muscle atonia induced by emotional arousal (cataplexy) with preservation of consciousness resulting from both abnormal control of REM control mechanisms (see below) and alterations in emotional processing (202, 303, 1309). Additional REM-like symptoms are hypnagogic hallucinations (hallucinations occurring around sleep onset or awakening) and sleep paralysis (inability to move following awakening from REM sleep).
Narcolepsy is caused by loss of orexin neurons or orexin receptors. Following the seminal discoveries of the gene defect responsible for a heritable form of narcolepsy in dogs (alteration of orexin type 2 receptor) (727) and narcolepsy-like symptoms in orexin knockout mice (214), this disease is now known to be caused by a loss of orexin/hypocretin neurons, orexin peptides, or their receptors. A marked reduction in the CSF levels of these peptides is found in living narcoleptic patients, whereas post mortem brains of human narcoleptics show a loss of orexin neurons (924, 999, 1284). A mutation in the preproorexin gene was found in a rare early-onset case of narcolepsy (999). Orexin neurons can also be damaged following stroke (1129) or traumatic brain injury (82), leading to excessive daytime sleepiness. Animal studies (217) show a narcoleptic phenotype following 1) knockout of orexin peptides or orexin receptors (214, 640, 870, 1415), 2) nonspecific lesion of the lateral hypothalamic area where orexin neurons are concentrated (414), and 3) genetic modification introducing a toxic transgene (ataxin-3) specifically in the orexin neurons (96, 479). However, the complete narcoleptic phenotype including cataplexy is not observed following short-term pharmacological blockade of orexin receptors (140) or RNAi-mediated knockdown of orexin peptides (221), suggesting that a more prolonged loss of orexin signaling is required to observe the full narcoleptic phenotype. This idea is consistent with studies which revealed neurodegenerative changes (1178), alterations of emotional arousal, and upregulations of cholinergic and dopaminergic systems involved in REM sleep control in narcolepsy (923).
1. Mechanisms underlying cataplexy
The multiple mechanisms by which orexins promote consolidated wakefulness and suppress REM sleep were discussed in sections II and IV, respectively. Early pharmacological studies in narcoleptic dogs suggested increased activity of cholinergic REM-on and decreased activity of aminergic REM-off systems is important for causing cataplexy. Infusions of cholinergic agonists in the pontine reticular formation caused cataplexy in normal dogs and exacerbated symptoms in narcoleptic dogs (1055, 1056). Furthermore, enhanced acetylcholine release was detected in the pons during cataplexy (1054). Recent data report an association of the choline kinase β (CHKB) gene involved in acetylcholine synthesis with susceptibility to narcolepsy (864). Enhanced cholinergic enzyme levels were detected in brain stem cholinergic neurons in orexin knockout mice (594). Electrophysiological studies in narcoleptic dogs revealed a cessation of firing of REM-off LC norepinephrine neurons (1435) and an increase in firing of muscle atonia related neurons in the medial medulla (1176, 1177) preceding and during cataplexy. Behavioral arrest reminiscent of cataplexy was observed following intense activation of the locus coeruleus with optogenetic techniques (196). More recent recording studies confirmed the cessation of firing of LC neurons and demonstrated its selectivity, since histaminergic neurons do not change their firing and DRN serotonin neurons show only a reduction and not a complete cessation of activity (564, 1436). Thus neuronal activity in cataplexy does not exactly parallel that observed during REM sleep, a finding confirmed by recordings of neuronal activity in the lateral pontine tegmentum REM control area (1283).
2. Narcolepsy treatment
Current treatment of narcoleptic symptoms normally involves the use of stimulants acting on the dopaminergic systems (e.g., modafinil) to alleviate excessive daytime sleepiness and antidepressants (especially those promoting increased noradrenergic tone) for suppression of REM-like phenomena such as cataplexy (923). γ-Hydroxybutyrate (GHB, sodium oxybate) is prescribed to improve nighttime sleep in narcoleptics (151). GHB is a metabolite of GABA that modulates sleep via activation of GABAB receptors (1369). Pharmacological agonists of the orexin receptors and histamine H3 receptor antagonists/inverse agonists (increasing histaminergic tone) are under development for the treatment of narcolepsy (395, 723, 854). Transplantation of orexin neurons (42) and orexin gene therapy (733, 734) are also being explored in preclinical animal studies. A dual orexin receptor antagonist has recently been promoted as a potential sleep aid (140), although development of narcoleptic symptoms could be a potential side effect of such compounds (no cataplectic events have been observed as yet).
3. Evidence that narcolepsy is an autoimmune disorder
Narcolepsy has long been considered an autoimmune disorder (728) due to its late onset, its association with the human leukocyte antigen (LHA) class II allele DQB1*0602 (728, 1259), and more recently due to the observed loss of orexin neurons in the hypothalamus in most human cases of the disease (1261). However, direct connections to the immune system have been hard to demonstrate. The strongest evidence comes from very recent findings of a linkage to a polymorphism in the T-cell receptor alpha gene (475) encoding the major receptor for HLA peptide presentation, findings of autoantibodies against the Tribbles Homolog 2 (TRIB2) gene product which is produced in orexin neurons (270, 613, 1304), and findings of anti-streptococcal antibodies (39) in a proportion of patients in the early stages of the disease. Although tantalizing, whether these markers are related to the cause, the progression, or are incidental to the disease process remains to be determined (721).
F. Other Sleep Muscle Tone-Related Disorders
1. REM sleep behavior disorder
REM sleep behavior disorder (RBD) is a human manifestation of the syndrome originally observed in cats (583) following large lesions of the brain stem reticular formation including sites involved in muscle atonia (893)(sect. IV). Sporadic cases can also be observed in cats and dogs without experimenter-induced lesions (493, 494). This disorder was first formally described in humans in a landmark study by Schenck, Mahowald and colleagues in 1986 (1131) and has a reported prevalence of 0.5% (779). RBD involves uncontrolled movements and muscular expression of dream sequences leading to sleep fragmentation and injury to patients and their sleeping partners (1134). In the sleep laboratory such activity is correlated with enhanced EMG activity during sleep, whereas other aspects of REM sleep are normal. It is more common in people aged over 50 years old and affects more men than women. Acute RBD can be induced by various medications (e.g., antidepressants acting to increase serotonergic or noradrenergic tone) (401, 982, 1418), whereas the cause of the chronic form is unknown. The acute form of RBD is managed by withdrawal of the offending medication, whereas the chronic form can be well managed symptomatically by clonazepam (first choice) or melatonin treatment prior to bedtime (401, 779).
Importantly, RBD has been shown to precede and predict the later development of neurodegenerative diseases known as synucleinopathies (115, 400) including Parkinson’s disease (117, 399, 1132), dementia with Lewy bodies disease (116, 1314, 1315), and multiple system atrophy (547, 1291). These studies suggest that a neurodegenerative process in the generation of idiopathic RBD, presumably occurring in the brain stem REM muscle atonia generation zones in the dorsolateral pons and medulla. RBD may be an important marker allowing early-stage preventative treatments of synucleinopathies since RBD often occurs many years earlier than the other conditions (400, 547, 1132, 1270, 1291). In fact, post mortem studies in idiopathic RBD have revealed damage to the LC-SubC area (1314, 1315). MRI scans revealed a discrete infarct in the upper pons in several cases (267, 627), although not all studies have found such abnormalities. Surgery involving damage to the upper pons can also result in RBD (1025). A recent advance in RBD research is the identification of an animal model which recapitulates many of the features of RBD (148). This model consists of transgenic mice that have deficits in inhibitory glycinergic and GABAergic neurotransmission due to the expression of a mutant glycine receptor α1 subunit, consistent with the substantial evidence for a role of these neurotransmitters in controlling muscle atonia during REM sleep (see sect. IV).
2. Restless legs syndrome
Restless legs syndrome (RLS) is a compelling urge to move limbs, particularly the legs, in the evening, due to unpleasant sensations, such as tingling, which can be relieved by movement. RLS is a very common in older subjects, particularly in western countries such as the United States, where ~9% of adults are affected (946). RLS can lead to severe insomnia and subsequent daytime hypersomnia (335).
A) Pathogenesis of Rls
Recent investigations implicate iron deficiency and abnormalities of dopaminergic systems as possible root causes of RLS (21). Iron is a cofactor for tyrosine hydroxylase, required for synthesis of dopamine; thus iron deficiency may lead to dopaminergic dysfunction. CSF iron levels are low in a minority of RLS patients (327, 868), and iron supplementation decreased symptoms of RLS in some cases (328). Studies of post mortem brain tissue revealed reduced iron content (257), confirmed by neuroimaging techniques such as MRI (326). Furthermore, the severity of RLS symptoms correlates with low levels of ferritin, an intracellular protein that binds iron (327, 673, 868), as well as high levels of transferrin, a plasma glycoprotein involved in iron transport (327, 868). Experimentally induced iron deficiency in animal studies produced an increase of wakefulness during the inactive (light) period (294). This model, although incomplete, paralleled the circadian aspect of RLS, whereby symptoms mostly occur during the early part of the inactive/rest period (952). Genetic studies have identified polymorphisms in three loci encoding developmental factors as predisposing to RLS (1204, 1420) and in particular to periodic limb movements during sleep (see next section).
B) Treatment and Animal Models of Rls
Dopaminergic agonists alleviate RLS symptoms (497), and blocking dopaminergic transmission worsens symptoms (887, 1419). Dopaminergic cell-specific lesioning of the A11 region, by means of the toxin 6-hydroxydopamine, produced an increase in sleep latencies, as well as less sleeplike behaviors (951). Excitotoxic (NMDA) lesions of another dopaminergic region, at the ventral mesopontine junction, produced an increase of wakefulness, as well as periodic leg movements during NREM sleep, similar to that seen in the RLS patient (682). A combination of A11 lesions and an iron-deficient diet led to a significant increase in locomotor activity, which was elevated compared with either manipulation (lesion or diet) alone (1030). Furthermore, symptoms were improved following treatment with D2/D3 agonists (such as ropinirole), as well as worsened with a D2 antagonist (haloperidol). D3 receptors, specifically in the dorsal horn (246, 712, 1142), may play a role in RLS symptomatology (247). D3 antagonists and D3-receptor knockout mice both exhibited increased locomotor activity, and D3 antagonist treatment leads to a decrease of total sleep (2, 71). Therefore, dopaminergic systems may be abnormally affected in RLS patients, particularly the A11 dopaminergic cell group and its efferent projections to the spinal cord (1187).
3. Periodic limb movements
RLS is usually accompanied by stereotyped periodic limb movements (PLM) during sleep, which are involuntary repetitive extensions of the toes, feet, and occasionally the knee and hip. Interestingly, two genetic studies of RLS identified a locus, BTBD9, which also predispose individuals to PLM (1204, 1420). PLM may also be evident in narcolepsy (886), apnea (392), and RBD (688).
4. Other disorders of motor control during sleep
Inappropriate activation of motor programs during sleep also occurs in bruxism (grinding of teeth during sleep), sleepwalking (occurring during NREM sleep), nocturnal sleep-related eating disorder (NSRED), and sexsomnia (automated sexual behavior during sleep).
G. Posttraumatic Stress Disorder
The diagnostic criteria for posttraumatic stress disorder (PTSD) include hyperarousal and disturbed sleep including sleep-onset insomnia, inability to stay asleep, excessive daytime sleepiness, and traumatic nightmares (1183). Approximately 8% of the adult population suffers from PTSD (617) caused by exposure to either emotional or physical trauma. NREM-related symptoms include an increased latency to fall asleep, as well as a decrease of overall NREM amounts (429, 847). The majority of studies also report REM-related symptoms, including decreased REM episode length, increased episode number, and an increased number of arousals from REM sleep (847, 1078).
1. Nightmares in PTSD
The chief subjective sleep complaint reported by PTSD patients is an increased prevalence of frightening dreams/nightmares (1329), suggesting a pathological enhancement of the normal activation of brain areas involved in emotion during REM sleep (sect. IV). In particular, increased activation of the amygdala and its connections with the hippocampus and prefrontal cortex may be important (719). The amygdala is a key player in the formation of fearful memories, and amygdala activity is abnormally elevated in PTSD patients following exposure to visual trauma-related stimuli (538). PTSD is also associated with endocrinological alterations that may contribute to disturbed sleep, including an attenuation of catecholamine plasma levels and an elevation of corticotrophin-releasing factor (CRF) levels (917).
2. Medication for PTSD
Numerous medications have been prescribed to decrease the hyperarousal associated with PTSD, with varying degrees of improvement of sleep disturbances (389). Treatments include benzodiazepines, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, atypical antidepressants (such as nefazadone and trazodone), beta blockers (such as propranolol), and α-adrenergic antagonists (prazosin). Norepinephrine plays a key role in the brain’s response to stress and anxiety and in the suppression of REM sleep (sect. IV). Activation of α1 norepinephrine receptors is involved in the REM suppressing effect of norepinephrine (644, 782, 990, 1079). Therefore, manipulations of this receptor may lead to improvement of sleep-related PTSD disturbance. Administration of the α1 receptor antagonist prazosin to PTSD patients increased total sleep amounts, normalized the REM sleep profile, and decreased traumatic nightmares (674, 1049). Another system that may be targeted in the future is the orexin system. Recent work in a rat model demonstrated a role for the orexin system in panic anxiety (565).
H. Depression
There are several intriguing links between depression and sleep (1208, 1228). Both monopolar and bipolar depression are associated with sleep disturbances, and acute sleep deprivation has a potent and rapid antidepressant action in severely depressed individuals. Furthermore, commonly used antidepressants that enhance serotonergic and noradrenergic tone strongly inhibit the expression of REM sleep (sect. IV). Conversely, light deprivation produces damage to monoamine neurons and a depressive behavioral phenotype in rats (433). Major (monopolar) depression is associated with sleep fragmentation, decreases in NREM sleep intensity, and promotion of REM sleep (173, 1208, 1228). EEG delta power, a measure of sleep intensity (sect. III), is reduced in depressed patients (124). REM alterations most commonly observed are a decrease in REM latency, sometimes resulting in sleep-onset REM periods, a prolonged duration of the first REM period, and increased phasic REM events. Consistent with the reciprocal-interaction theory of REM sleep control (sect. IV), pharmacological challenge experiments with cholinergic or serotonergic agents suggested an increased sensitivity of cholinergic systems and/or decreased activity of aminergic systems may cause these REM abnormalities (426 – 428, 1228).
VIII. CONCLUSIONS
The past century witnessed an enormous explosion in our knowledge of the brain mechanisms that control wakefulness and sleep. Ethological and genetic studies have revealed the presence of NREM sleep-like states even in invertebrates such as Drosophila and have revealed that homologous genes/proteins control rest/sleep in flies, fish, mice, and humans. Multiple interacting neurotransmitter systems making up the ARAS arouse the brain and produce wakefulness in response to physiological challenges as diverse as increases in blood CO2decreases in ambient temperature, and the presence of rewarding stimuli. Increases in arousal are observed as an increase in low-amplitude fast EEG rhythms important for synchronization of neuronal assemblies involved in attention, working memory, and conscious awareness. Conversely, NREM sleep is associated with high-amplitude slow waves produced by the combination of a circadian inhibition of ARAS neurons mediated by GABAergic neurons in the preoptic hypothalamus and basal forebrain and by the action of a plethora of homeostatic sleep factors acting locally in the cortex or basal forebrain. Multiple interacting lines of evidence support a role for NREM sleep in the control of energy metabolism and synaptic plasticity/memory formation. REM sleep is induced by the increased firing of glutamatergic and cholinergic neurons in the dorsolateral pons, resulting in muscle atonia coupled with both tonic and phasic activation of the cortex. Phasic activation of visual cortex and limbic regions during REM sleep, coupled with deactivation of prefrontal cortex, is responsible for the bizarre imagery of dreams and may reflect a role for REM sleep in emotional regulation and/or memory consolidation in the adult or establishment of neural circuitry in the developing animal. Sleep deprivation leads to an inhibition of arousal mechanisms at subcortical and cortical sites, leading to impairments in cognitive function, which in turn can result in accidents at home and in the workplace. Deficits in arousal mechanisms and highfrequency rhythms are observed in sleep disorders and conditions such as coma, schizophrenia, Alzheimer’s disease, and epilepsy. Consistent with a role for sleep in energy metabolism, sleep deprivation is a major contributor to metabolic syndrome, a leading public health issue. Furthermore, dysregulation of sleep is a feature of depression, PTSD, and a variety of sleep disorders related to muscle control. Thus studies of the mechanisms controlling sleep and wakefulness can reasonably be expected to lay the groundwork for therapies to treat a multitude of afflictions affecting mankind.
ACKNOWLEDGMENTS
We thank the two anonymous reviewers for their time and helpful critiques which helped to improve the manuscript.
GRANTS
This work was supported by Department of Veterans Affairs Medical Research Service Awards (to R. W. McCarley, R. Basheer, and R. E. Strecker) and by National Institutes of Health Grants R01 MH-039683 (to R. W. McCarley), R21 MH-094803 (to R.E. Brown), and P01 HL-095491 (to R. W. McCarley and R. E. Strecker).
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Abel GG, Murphy WD, Becker JV, Bitar A. Women’s vaginal responses during REM sleep. J Sex Marital Ther. 1979;5:5–14. doi: 10.1080/00926237908403713. [DOI] [PubMed] [Google Scholar]
- 2.Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci USA. 1996;93:1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achermann P, Borbely AA. Mathematical models of sleep regulation. Front Biosci. 2003;8 doi: 10.2741/1064. s683-1945-s693. [DOI] [PubMed] [Google Scholar]
- 4.Adamantidis A, de Lecea L. The hypocretins as sensors for metabolism and arousal. J Physiol. 2009;587:33–40. doi: 10.1113/jphysiol.2008.164400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamantidis A, Salvert D, Goutagny R, Lakaye B, Gervasoni D, Grisar T, Luppi PH, Fort P. Sleep architecture of the melanin-concentrating hormone receptor 1-knockout mice. Eur J Neurosci. 2008;27:1793–1800. doi: 10.1111/j.1460-9568.2008.06129.x. [DOI] [PubMed] [Google Scholar]
- 6.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adrian ED, Yamagiwa K. The origin of the Berger rhythm. Brain. 1935;58:323–351. [Google Scholar]
- 8.Adrien J, Alexandre C, Boutrel B, Popa D. Contribution of the “knock-out” technology to understanding the role of serotonin in sleep regulations. Arch Ital Biol. 2004;142:369–377. [PubMed] [Google Scholar]
- 9.Aghajanian GK. Modulation of a transient outward current in serotonergic neurones by alpha 1-adrenoceptors. Nature. 1985;315:501–503. doi: 10.1038/315501a0. [DOI] [PubMed] [Google Scholar]
- 10.Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 11.Aghajanian GK, Marek GJ. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825:161–171. doi: 10.1016/s0006-8993(99)01224-x. [DOI] [PubMed] [Google Scholar]
- 12.Ahnaou A, Drinkenburg WH, Bouwknecht JA, Alcazar J, Steckler T, Dautzenberg FM. Blocking melanin-concentrating hormone MCH1 receptor affects rat sleep-wake architecture. Eur J Pharmacol. 2008;579:177–188. doi: 10.1016/j.ejphar.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–631. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alam MN, Kumar S, Rai S, Methippara M, Szymusiak R, McGinty D. Role of adenosine A(1) receptor in the perifornical-lateral hypothalamic area in sleep-wake regulation in rats. Brain Res. 2009;1304:96–104. doi: 10.1016/j.brainres.2009.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam MN, Kumar S, Suntsova N, Bashir T, Szymusiak R, McGinty D. GABAergic regulation of the perifornical-lateral hypothalamic neurons during non-rapid eye movement sleep in rats. Neuroscience. 2010;167:920–928. doi: 10.1016/j.neuroscience.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alam MN, McGinty D, Szymusiak R. Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: relation to NREM sleep. Am J Physiol Regul Integr Comp Physiol. 1995;269:R1240–R1249. doi: 10.1152/ajpregu.1995.269.5.R1240. [DOI] [PubMed] [Google Scholar]
- 17.Alam MN, Szymusiak R, Gong H, King J, McGinty D. Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis. J Physiol. 1999;521:679–690. doi: 10.1111/j.1469-7793.1999.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. MPer1 and mper2 are essential for normal resetting of the circadian clock. J Biol Rhythms. 2001;16:100–104. doi: 10.1177/074873001129001791. [DOI] [PubMed] [Google Scholar]
- 19.Alexandre C, Popa D, Fabre V, Bouali S, Venault P, Lesch KP, Hamon M, Adrien J. Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression- like behavior in adult knock-out mice lacking the serotonin transporter. J Neurosci. 2006;26:5554–5564. doi: 10.1523/JNEUROSCI.5156-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Nicotinic receptor activation in human cerebral cortical interneurons: a mechanism for inhibition and disinhibition of neuronal networks. J Neurosci. 2000;20:66–75. doi: 10.1523/JNEUROSCI.20-01-00066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen R. Dopamine and iron in the pathophysiology of restless legs syndrome (RLS) Sleep Med. 2004;5:385–391. doi: 10.1016/j.sleep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Alonso A, Faure MP, Beaudet A. Neurotensin promotes oscillatory bursting behavior and is internalized in basal forebrain cholinergic neurons. J Neurosci. 1994;14:5778–5792. doi: 10.1523/JNEUROSCI.14-10-05778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alreja M. Excitatory actions of serotonin on GABAergic neurons of the medial septum and diagonal band of Broca. Synapse. 1996;22:15–27. doi: 10.1002/(SICI)1098-2396(199601)22:1<15::AID-SYN2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 24.Ambrosius U, Lietzenmaier S, Wehrle R, Wichniak A, Kalus S, Winkelmann J, Bettecken T, Holsboer F, Yassouridis A, Friess E. Heritability of sleep electroencephalogram. Biol Psychiatry. 2008;64:344–348. doi: 10.1016/j.biopsych.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Amzica F, Nunez A, Steriade M. Delta frequency (1–4 Hz) oscillations of perigeniculate thalamic neurons and their modulation by light. Neuroscience. 1992;51:285–294. doi: 10.1016/0306-4522(92)90315-s. [DOI] [PubMed] [Google Scholar]
- 26.Amzica F, Steriade M. Progressive cortical synchronization of ponto-geniculo-occipital potentials during rapid eye movement sleep. Neuroscience. 1996;72:309–314. doi: 10.1016/0306-4522(96)00012-7. [DOI] [PubMed] [Google Scholar]
- 27.Amzica F, Steriade M. The K-complex: its slow (<1-Hz) rhythmicity and relation to delta waves. Neurology. 1997;49:952–959. doi: 10.1212/wnl.49.4.952. [DOI] [PubMed] [Google Scholar]
- 28.Amzica F, Steriade M. Cellular substrates and laminar profile of sleep K-complex. Neuroscience. 1998;82:671–686. doi: 10.1016/s0306-4522(97)00319-9. [DOI] [PubMed] [Google Scholar]
- 29.Anaclet C, Parmentier R, Ouk K, Guidon G, Buda C, Sastre JP, Akaoka H, Sergeeva OA, Yanagisawa M, Ohtsu H, Franco P, Haas HL, Lin JS. Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J Neurosci. 2009;29:14423–14438. doi: 10.1523/JNEUROSCI.2604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen P, Bland HB, Myhrer T, Schwartzkroin PA. Septo-hippocampal pathway necessary for dentate theta production. Brain Res. 1979;165:13–22. doi: 10.1016/0006-8993(79)90040-4. [DOI] [PubMed] [Google Scholar]
- 31.Anderson C, Platten CR. Sleep deprivation lowers inhibition and enhances impulsivity to negative stimuli. Behav Brain Res. 2011;217:463–466. doi: 10.1016/j.bbr.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Anderson MP, Mochizuki T, Xie J, Fischler W, Manger JP, Talley EM, Scammell TE, Tonegawa S. Thalamic Cav3.1-type Ca2+channel plays a crucial role in stabilizing sleep. Proc Natl Acad Sci USA. 2005;102:1743–1748. doi: 10.1073/pnas.0409644102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anokhin A, Steinlein O, Fischer C, Mao Y, Vogt P, Schalt E, Vogel F. A genetic study of the human low-voltage electroencephalogram. Hum Genet. 1992;90:99–112. doi: 10.1007/BF00210751. [DOI] [PubMed] [Google Scholar]
- 35.Anver H, Ward PD, Magony A, Vreugdenhil M. NMDA receptor hypofunction phase couples independent gamma-oscillations in the rat visual cortex. Neuropsychopharmacology. 2011;36:519–528. doi: 10.1038/npp.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appelbaum L, Wang G, Yokogawa T, Skariah GM, Smith SJ, Mourrain P, Mignot E. Circadian and homeostatic regulation of structural synaptic plasticity in hypocretin neurons. Neuron. 2010;68:87–98. doi: 10.1016/j.neuron.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Appelbaum L, Wang GX, Maro GS, Mori R, Tovin A, Marin W, Yokogawa T, Kawakami K, Smith SJ, Gothilf Y, Mignot E, Mourrain P. Sleep-wake regulation and hypocretin-melatonin interaction in zebrafish. Proc Natl Acad Sci USA. 2009;106:21942–21947. doi: 10.1073/pnas.906637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arai A, Kessler M, Lynch G. The effects of adenosine on the development of long-term potentiation. Neurosci Lett. 1990;119:41–44. doi: 10.1016/0304-3940(90)90750-4. [DOI] [PubMed] [Google Scholar]
- 39.Aran A, Lin L, Nevsimalova S, Plazzi G, Hong SC, Weiner K, Zeitzer J, Mignot E. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep. 2009;32:979–983. doi: 10.1093/sleep/32.8.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- 41.Arble DM, Ramsey KM, Bass J, Turek FW. Circadian disruption and metabolic disease: findings from animal models. Best Pract Res Clin Endocrinol Metab. 2010;24:785–800. doi: 10.1016/j.beem.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arias-Carrion O, Murillo-Rodriguez E, Xu M, Blanco-Centurion C, Drucker-Colin R, Shiromani PJ. Transplantation of hypocretin neurons into the pontine reticular formation: preliminary results. Sleep. 2004;27:1465–1470. doi: 10.1093/sleep/27.8.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arii J, Kanbayashi T, Tanabe Y, Ono J, Nishino S, Kohno Y. A hypersomnolent girl with decreased CSF hypocretin level after removal of a hypothalamic tumor. Neurology. 2001;56:1775–1776. doi: 10.1212/wnl.56.12.1775. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol. 1983;216:53–68. doi: 10.1002/cne.902160106. [DOI] [PubMed] [Google Scholar]
- 45.Arrigoni E, Chamberlin NL, Saper CB, McCarley RW. Adenosine inhibits basal forebrain cholinergic and noncholinergic neurons in vitro. Neuroscience. 2006;140:403–413. doi: 10.1016/j.neuroscience.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Arrigoni E, Lu J, Vetrivelan R, Saper CB. Long-term synaptic plasticity is impaired in rats with lesions of the ventrolateral preoptic nucleus. Eur J Neurosci. 2009;30:2112–2120. doi: 10.1111/j.1460-9568.2009.07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arrigoni E, Mochizuki T, Scammell TE. Activation of the basal forebrain by the orexin/hypocretin neurones. Acta Physiol. 2010;198:223–235. doi: 10.1111/j.1748-1716.2009.02036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arrigoni E, Rainnie DG, McCarley RW, Greene RW. Adenosine-mediated presynaptic modulation of glutamatergic transmission in the laterodorsal tegmentum. J Neurosci. 2001;21:1076–1085. doi: 10.1523/JNEUROSCI.21-03-01076.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asanuma C, Porter LL. Light and electron microscopic evidence for a GABAergic projection from the caudal basal forebrain to the thalamic reticular nucleus in rats. J Comp Neurol. 1990;302:159–172. doi: 10.1002/cne.903020112. [DOI] [PubMed] [Google Scholar]
- 50.Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- 51.Assaf SY, Miller JJ. The role of a raphe serotonin system in the control of septal unit activity and hippocampal desynchronization. Neuroscience. 1978;3:539–550. doi: 10.1016/0306-4522(78)90018-0. [DOI] [PubMed] [Google Scholar]
- 52.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 53.Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Naidoo N, Frank MG. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Avanzini G, Vergnes M, Spreafico R, Marescaux C. Calcium-dependent regulation of genetically determined spike and waves by the reticular thalamic nucleus of rats. Epilepsia. 1993;34:1–7. doi: 10.1111/j.1528-1157.1993.tb02369.x. [DOI] [PubMed] [Google Scholar]
- 56.Avsar E, Empson RM. Adenosine acting via A1 receptors controls the transition to status epilepticus-like behaviour in an in vitro model of epilepsy. Neuropharmacology. 2004;47:427–437. doi: 10.1016/j.neuropharm.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 57.Ayers NA, Kapas L, Krueger JM. Circadian variation of nitric oxide synthase activity and cytosolic protein levels in rat brain. Brain Res. 1996;707:127–130. doi: 10.1016/0006-8993(95)01362-8. [DOI] [PubMed] [Google Scholar]
- 58.Babson KA, Trainor CD, Feldner MT, Blumenthal H. A test of the effects of acute sleep deprivation on general and specific self-reported anxiety and depressive symptoms: an experimental extension. J Behav Ther Exp Psychiatry. 2010;41:297–303. doi: 10.1016/j.jbtep.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bachmann V, Klaus F, Bodenmann S, Schafer N, Brugger P, Huber S, Berger W, Landolt HP. Functional ADA polymorphism increases sleep depth and reduces vigilant attention in humans. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr173. [DOI] [PubMed] [Google Scholar]
- 60.Backberg M, Ultenius C, Fritschy JM, Meister B. Cellular localization of GABA receptor alpha subunit immunoreactivity in the rat hypothalamus: relationship with neurones containing orexigenic or anorexigenic peptides. J Neuroendocrinol. 2004;16:589–604. doi: 10.1111/j.1365-2826.2004.01207.x. [DOI] [PubMed] [Google Scholar]
- 61.Baghdoyan HA, Lydic R. M2 muscarinic receptor subtype in the feline medial pontine reticular formation modulates the amount of rapid eye movement sleep. Sleep. 1999;22:835–847. doi: 10.1093/sleep/22.7.835. [DOI] [PubMed] [Google Scholar]
- 62.Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Res. 1987;414:245–261. doi: 10.1016/0006-8993(87)90005-9. [DOI] [PubMed] [Google Scholar]
- 63.Bai D, Renaud LP. Median preoptic nucleus neurons: an in vitro patch-clamp analysis of their intrinsic properties and noradrenergic receptors in the rat. Neuroscience. 1998;83:905–916. doi: 10.1016/s0306-4522(97)00435-1. [DOI] [PubMed] [Google Scholar]
- 64.Bal T, McCormick DA. Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. J Physiol. 1993;468:669–691. doi: 10.1113/jphysiol.1993.sp019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balkin TJ, Braun AR, Wesensten NJ, Jeffries K, Varga M, Baldwin P, Belenky G, Herscovitch P. The process of awakening: a PET study of regional brain activity patterns mediating the re-establishment of alertness and consciousness. Brain. 2002;125:2308–2319. doi: 10.1093/brain/awf228. [DOI] [PubMed] [Google Scholar]
- 67.Bandyopadhya RS, Datta S, Saha S. Activation of pedunculopontine tegmental protein kinase A: a mechanism for rapid eye movement sleep generation in the freely moving rat. J Neurosci. 2006;26:8931–8942. doi: 10.1523/JNEUROSCI.2173-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baraban JM, Aghajanian GK. Suppression of firing activity of 5-HT neurons in the dorsal raphe by alpha-adrenoceptor antagonists. Neuropharmacology. 1980;19:355–363. doi: 10.1016/0028-3908(80)90187-2. [DOI] [PubMed] [Google Scholar]
- 69.Baracchi F, Opp MR. Sleep-wake behavior and responses to sleep deprivation of mice lacking both interleukin-1 beta receptor 1 and tumor necrosis factor-alpha receptor 1. Brain Behav Immun. 2008;22:982–993. doi: 10.1016/j.bbi.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barcelo A, de la PM, Barbe F, Pierola J, Bosch M, Agusti AG. Prostaglandin D synthase (beta trace) levels in sleep apnea patients with and without sleepiness. Sleep Med. 2007;8:509–511. doi: 10.1016/j.sleep.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 71.Barik S, de BR. Dopamine D3 modulation of locomotor activity and sleep in the nucleus accumbens and in lobules 9 and 10 of the cerebellum in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:718–726. doi: 10.1016/j.pnpbp.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 72.Barnes CA, McNaughton BL, Goddard GV, Douglas RM, Adamec R. Circadian rhythm of synaptic excitability in rat and monkey central nervous system. Science. 1977;197:91–92. doi: 10.1126/science.194313. [DOI] [PubMed] [Google Scholar]
- 73.Basheer R, Arrigoni E, Thatte HS, Greene RW, Ambudkar IS, McCarley RW. Adenosine induces inositol 1,4,5-trisphosphate receptor-mediated mobilization of intracellular calcium stores in basal forebrain cholinergic neurons. J Neurosci. 2002;22:7680–7686. doi: 10.1523/JNEUROSCI.22-17-07680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Basheer R, Bauer A, Elmenhorst D, Ramesh V, McCarley RW. Sleep deprivation upregulates A1 adenosine receptors in the rat basal forebrain. Neuroreport. 2007;18:1895–1899. doi: 10.1097/WNR.0b013e3282f262f6. [DOI] [PubMed] [Google Scholar]
- 75.Basheer R, Brown R, Ramesh V, Begum S, McCarley RW. Sleep deprivation-induced protein changes in basal forebrain: implications for synaptic plasticity. J Neurosci Res. 2005;82:650–658. doi: 10.1002/jnr.20675. [DOI] [PubMed] [Google Scholar]
- 76.Basheer R, Halldner L, Alanko L, McCarley RW, Fredholm BB, Porkka-Heiskanen T. Opposite changes in adenosine A1 and A2A receptor mRNA in the rat following sleep deprivation. Neuroreport. 2001;12:1577–1580. doi: 10.1097/00001756-200106130-00013. [DOI] [PubMed] [Google Scholar]
- 77.Basheer R, Magner M, McCarley RW, Shiromani PJ. REM sleep deprivation increases the levels of tyrosine hydroxylase and norepinephrine transporter mRNA in the locus coeruleus. Brain Res. 1998;57:235–240. doi: 10.1016/s0169-328x(98)00088-6. [DOI] [PubMed] [Google Scholar]
- 78.Basheer R, Sherin JE, Saper CB, Morgan JI, McCarley RW, Shiromani PJ. Effects of sleep on wake-induced c-fos expression. J Neurosci. 1997;17:9746–9750. doi: 10.1523/JNEUROSCI.17-24-09746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 80.Bassant MH, Apartis E, Jazat-Poindessous FR, Wiley RG, Lamour YA. Selective immunolesion of the basal forebrain cholinergic neurons: effects on hippocampal activity during sleep and wakefulness in the rat. Neurodegeneration. 1995;4:61–70. doi: 10.1006/neur.1995.0007. [DOI] [PubMed] [Google Scholar]
- 81.Bassant MH, Poindessous-Jazat F. Ventral tegmental nucleus of Gudden: a pontine hippocampal theta generator? Hippocampus. 2001;11:809–813. doi: 10.1002/hipo.1096. [DOI] [PubMed] [Google Scholar]
- 82.Baumann CR, Bassetti CL, Valko PO, Haybaeck J, Keller M, Clark E, Stocker R, Tolnay M, Scammell TE. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann Neurol. 2009;66:555–559. doi: 10.1002/ana.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bayer L, Eggermann E, Saint-Mleux B, Machard D, Jones BE, Muhlethaler M, Serafin M. Selective action of orexin (hypocretin) on nonspecific thalamocortical projection neurons. J Neurosci. 2002;22:7835–7839. doi: 10.1523/JNEUROSCI.22-18-07835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bayer L, Eggermann E, Serafin M, Grivel J, Machard D, Muhlethaler M, Jones BE. Opposite effects of norepinephrine and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience. 2005;130:807–811. doi: 10.1016/j.neuroscience.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 85.Bayer L, Eggermann E, Serafin M, Saint-Mleux B, Machard D, Jones B, Muhlethaler M. Orexins (hypocretins) directly excite tuberomammillary neurons. Eur J Neurosci. 2001;14:1571–1575. doi: 10.1046/j.0953-816x.2001.01777.x. [DOI] [PubMed] [Google Scholar]
- 86.Bayer L, Serafin M, Eggermann E, Saint-Mleux B, Machard D, Jones BE, Muhlethaler M. Exclusive postsynaptic action of hypocretin-orexin on sublayer 6b cortical neurons. J Neurosci. 2004;24:6760–6764. doi: 10.1523/JNEUROSCI.1783-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bedard MA, Montplaisir J, Richer F, Rouleau I, Malo J. Obstructive sleep apnea syndrome: pathogenesis of neuropsychological deficits. J Clin Exp Neuropsychol. 1991;13:950–964. doi: 10.1080/01688639108405110. [DOI] [PubMed] [Google Scholar]
- 88.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 89.Behrens CJ, van den Boom LP, de HL, Friedman A, Heinemann U. Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci. 2005;8:1560–1567. doi: 10.1038/nn1571. [DOI] [PubMed] [Google Scholar]
- 90.Bellingham MC, Berger AJ. Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J Neurophysiol. 1996;76:3758–3770. doi: 10.1152/jn.1996.76.6.3758. [DOI] [PubMed] [Google Scholar]
- 91.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 92.Berger H. Ueber das elektroenkephalogramm des Menschen. Arch Psychiatr Nervenkr. 1929;87:527–570. [Google Scholar]
- 93.Berkowitz A, Sutton L, Janowsky DS, Gillin JC. Pilocarpine, an orally active muscarinic cholinergic agonist, induces REM sleep and reduces delta sleep in normal volunteers. Psychiatry Res. 1990;33:113–119. doi: 10.1016/0165-1781(90)90064-c. [DOI] [PubMed] [Google Scholar]
- 94.Berntson GG, Shafi R, Sarter M. Specific contributions of the basal forebrain corticopetal cholinergic system to electroencephalographic activity and sleep/waking behaviour. Eur J Neurosci. 2002;16:2453–2461. doi: 10.1046/j.1460-9568.2002.02310.x. [DOI] [PubMed] [Google Scholar]
- 95.Beuckmann CT, Fujimori K, Urade Y, Hayaishi O. Identification of mu-class glutathione transferases M2-2 and M3-3 as cytosolic prostaglandin E synthases in the human brain. Neurochem Res. 2000;25:733–738. doi: 10.1023/a:1007579507804. [DOI] [PubMed] [Google Scholar]
- 96.Beuckmann CT, Sinton CM, Williams SC, Richardson JA, Hammer RE, Sakurai T, Yanagisawa M. Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat. J Neurosci. 2004;24:4469–4477. doi: 10.1523/JNEUROSCI.5560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 98.Bizzi E, Brooks DC. Functional connections between pontine reticular formation and lateral geniculate nucleus during deep sleep. Arch Ital Biol. 1963;101:666–680. [PubMed] [Google Scholar]
- 99.Bjorness TE, Kelly CL, Gao T, Poffenberger V, Greene RW. Control and function of the homeostatic sleep response by adenosine A1 receptors. J Neurosci. 2009;29:1267–1276. doi: 10.1523/JNEUROSCI.2942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blanco-Centurion C, Gerashchenko D, Salin-Pascual RJ, Shiromani PJ. Effects of hypocretin2-saporin and antidopamine-beta-hydroxylase-saporin neurotoxic lesions of the dorsolateral pons on sleep and muscle tone. Eur J Neurosci. 2004;19:2741–2752. doi: 10.1111/j.1460-9568.2004.03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci. 2007;27:14041–14048. doi: 10.1523/JNEUROSCI.3217-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blanco-Centurion C, Xu M, Murillo-Rodriguez E, Gerashchenko D, Shiromani AM, Salin-Pascual RJ, Hof PR, Shiromani PJ. Adenosine and sleep homeostasis in the basal forebrain. J Neurosci. 2006;26:8092–8100. doi: 10.1523/JNEUROSCI.2181-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blanco-Centurion CA, Shiromani A, Winston E, Shiromani PJ. Effects of hypocretin-1 in 192-IgG-saporin-lesioned rats. Eur J Neurosci. 2006;24:2084–2088. doi: 10.1111/j.1460-9568.2006.05074.x. [DOI] [PubMed] [Google Scholar]
- 104.Bland BH, Declerck S, Jackson J, Glasgow S, Oddie S. Septohippocampal properties of N-methyl-d-aspartate-induced theta-band oscillation and synchrony. Synapse. 2007;61:185–197. doi: 10.1002/syn.20357. [DOI] [PubMed] [Google Scholar]
- 105.Bland BH, Konopacki J, Kirk IJ, Oddie SD, Dickson CT. Discharge patterns of hippocampal theta-related cells in the caudal diencephalon of the urethan-anesthetized rat. J Neurophysiol. 1995;74:322–333. doi: 10.1152/jn.1995.74.1.322. [DOI] [PubMed] [Google Scholar]
- 106.Bland BH, Trepel C, Oddie SD, Kirk IJ. Intraseptal microinfusion of muscimol: effects on hippocampal formation theta field activity and phasic theta-ON cell discharges. Exp Neurol. 1996;138:286–297. doi: 10.1006/exnr.1996.0067. [DOI] [PubMed] [Google Scholar]
- 107.Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron. 2003;38:805–817. doi: 10.1016/s0896-6273(03)00300-3. [DOI] [PubMed] [Google Scholar]
- 108.Bliss EL, Clark LD, West CD. Studies of sleep deprivation-relationship to schizophrenia. AMA Arch Neurol Psychiatry. 1959;81:348–359. doi: 10.1001/archneurpsyc.1959.02340150080009. [DOI] [PubMed] [Google Scholar]
- 109.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 110.Blumberg MS, Karlsson KA, Seelke AM, Mohns EJ. The ontogeny of mammalian sleep: a response to Frank and Heller (2003) J Sleep Res. 2005;14:91–98. doi: 10.1111/j.1365-2869.2004.00430_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blumberg MS, Seelke AM, Lowen SB, Karlsson KA. Dynamics of sleep-wake cyclicity in developing rats. Proc Natl Acad Sci USA. 2005;102:14860–14864. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bodenmann S, Rusterholz T, Durr R, Stoll C, Bachmann V, Geissler E, Jaggi-Schwarz K, Landolt HP. The functional Val158Met polymorphism of COMT predicts interin-dividual differences in brain alpha oscillations in young men. J Neurosci. 2009;29:10855–10862. doi: 10.1523/JNEUROSCI.1427-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bodizs R, Kantor S, Szabo G, Szucs A, Eross L, Halasz P. Rhythmic hippocampal slow oscillation characterizes REM sleep in humans. Hippocampus. 2001;11:747–753. doi: 10.1002/hipo.1090. [DOI] [PubMed] [Google Scholar]
- 114.Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boeve BF, Saper CB. REM sleep behavior disorder: a possible early marker for synucleinopathies. Neurology. 2006;66:796–797. doi: 10.1212/01.wnl.0000209264.61035.bb. [DOI] [PubMed] [Google Scholar]
- 116.Boeve BF, Silber MH, Ferman TJ, Kokmen E, Smith GE, Ivnik RJ, Parisi JE, Olson EJ, Petersen RC. REM sleep behavior disorder and degenerative dementia: an association likely reflecting Lewy body disease. Neurology. 1998;51:363–370. doi: 10.1212/wnl.51.2.363. [DOI] [PubMed] [Google Scholar]
- 117.Boeve BF, Silber MH, Parisi JE, Dickson DW, Ferman TJ, Benarroch EE, Schmeichel AM, Smith GE, Petersen RC, Ahlskog JE, Matsumoto JY, Knopman DS, Schenck CH, Mahowald MW. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology. 2003;61:40–45. doi: 10.1212/01.wnl.0000073619.94467.b0. [DOI] [PubMed] [Google Scholar]
- 118.Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi PH. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. Eur J Neurosci. 2003;18:1627–1639. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- 119.Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat pontomedullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- 120.Bonnavion P, Bernard JF, Hamon M, Adrien J, Fabre V. Heterogeneous distribution of the serotonin 5-HT(1A) receptor mRNA in chemically identified neurons of the mouse rostral brain stem: implications for the role of serotonin in the regulation of wakefulness and REM sleep. J Comp Neurol. 2010;518:2744–2770. doi: 10.1002/cne.22331. [DOI] [PubMed] [Google Scholar]
- 121.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 122.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 123.Borbely AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–495. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 124.Borbely AA, Tobler I, Loepfe M, Kupfer DJ, Ulrich RF, Grochocinski V, Doman J, Matthews G. All-night spectral analysis of the sleep EEG in untreated depressives and normal controls. Psychiatry Res. 1984;12:27–33. doi: 10.1016/0165-1781(84)90135-5. [DOI] [PubMed] [Google Scholar]
- 125.Borhegyi Z, Varga V, Szilagyi N, Fabo D, Freund TF. Phase segregation of medial septal GABAergic neurons during hippocampal theta activity. J Neurosci. 2004;24:8470–8479. doi: 10.1523/JNEUROSCI.1413-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Boucetta S, Jones BE. Activity profiles of cholinergic and intermingled GABAergic and putative glutamatergic neurons in the pontomesencephalic tegmentum of urethane- anesthetized rats. J Neurosci. 2009;29:4664–4674. doi: 10.1523/JNEUROSCI.5502-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bourgin P, Fabre V, Huitron-Resendiz S, Henriksen SJ, Prospero-Garcia O, Criado JR, de Lecea L. Cortistatin promotes and negatively correlates with slow-wave sleep. Eur J Neurosci. 2007;26:729–738. doi: 10.1111/j.1460-9568.2007.05696.x. [DOI] [PubMed] [Google Scholar]
- 128.Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Boutrel B, Koob GF. What keeps us awake: the neuropharmacology of stimulants and wakefulness-promoting medications. Sleep. 2004;27:1181–1194. doi: 10.1093/sleep/27.6.1181. [DOI] [PubMed] [Google Scholar]
- 130.Boutrel B, Monaca C, Hen R, Hamon M, Adrien J. Involvement of 5-HT1A receptors in homeostatic and stress-induced adaptive regulations of paradoxical sleep: studies in 5-HT1A knock-out mice. J Neurosci. 2002;22:4686–4692. doi: 10.1523/JNEUROSCI.22-11-04686.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bradbury MJ, Dement WC, Edgar DM. Effects of adrenalectomy and subsequent corticosterone replacement on rat sleep state and EEG power spectra. Am J Physiol Regul Integr Comp Physiol. 1998;275:R555–R565. doi: 10.1152/ajpregu.1998.275.2.R555. [DOI] [PubMed] [Google Scholar]
- 132.Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 133.Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brambilla D, Chapman D, Greene R. Adenosine mediation of presynaptic feedback inhibition of glutamate release. Neuron. 2005;46:275–283. doi: 10.1016/j.neuron.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 135.Bredow S, Guha-Thakurta N, Taishi P, Obal F, Jr, Krueger JM. Diurnal variations of tumor necrosis factor alpha mRNA and alpha-tubulin mRNA in rat brain. Neuroimmunomodulation. 1997;4:84–90. doi: 10.1159/000097325. [DOI] [PubMed] [Google Scholar]
- 136.Bremer F. Cerveau “isole” et physiologie du sommeil. C R Soc Biol Paris. 1935;118:1235–1242. [Google Scholar]
- 137.Bremer F. L’activite cerebrale et physiolgie du sommeil. Bull Acad Royale Medicine Belgique. 1937;4:68–86. [Google Scholar]
- 138.Bremer F. L’activite electrique de l’ecorce cerebrale et le probleme physiologique du sommeil. Boll Soc Ital Biol Sper. 1938;13:271–290. [Google Scholar]
- 139.Brenner RP. The interpretation of the EEG in stupor and coma. Neurologist. 2005;11:271–284. doi: 10.1097/01.nrl.0000178756.44055.f6. [DOI] [PubMed] [Google Scholar]
- 140.Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, Mueller C, Nayler O, van GJ, de Haas SL, Hess P, Qiu C, Buchmann S, Scherz M, Weller T, Fischli W, Clozel M, Jenck F. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- 141.Brischoux F, Mainville L, Jones BE. Muscarinic-2 and orexin-2 receptors on GABAergic and other neurons in the rat mesopontine tegmentum and their potential role in sleep-wake state control. J Comp Neurol. 2008;510:607–630. doi: 10.1002/cne.21803. [DOI] [PubMed] [Google Scholar]
- 142.Broad RM, Fallahi N, Fredholm BB. Nitric oxide interacts with oxygen free radicals to evoke the release of adenosine and adenine nucleotides from rat hippocampal slices. J Auton Nerv Syst. 2000;81:82–86. doi: 10.1016/s0165-1838(00)00124-7. [DOI] [PubMed] [Google Scholar]
- 143.Broicher T, Wettschureck N, Munsch T, Coulon P, Meuth SG, Kanyshkova T, Seidenbecher T, Offermanns S, Pape HC, Budde T. Muscarinic ACh receptor-mediated control of thalamic activity via Gq/G11-family G-proteins. Pflügers Arch. 2008;456:1049–1060. doi: 10.1007/s00424-008-0473-x. [DOI] [PubMed] [Google Scholar]
- 144.Brooks DC. Localization and characteristics of the cortical waves associated with eye movement in the cat. Exp Neurol. 1968;22:603–613. doi: 10.1016/0014-4886(68)90152-0. [DOI] [PubMed] [Google Scholar]
- 145.Brooks DC. Waves associated with eye movement in the awake and sleeping cat. Electroencephalogr Clin Neurophysiol. 1968;24:532–541. doi: 10.1016/0013-4694(68)90042-4. [DOI] [PubMed] [Google Scholar]
- 146.Brooks DC, Bizzi E. Brain stem electrical activity during deep sleep. Arch Ital Biol. 1963;101:648–665. [PubMed] [Google Scholar]
- 147.Brooks PL, Peever JH. Glycinergic and GABA(A)-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia. J Neurosci. 2008;28:3535–3545. doi: 10.1523/JNEUROSCI.5023-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brooks PL, Peever JH. Impaired GABA and glycine transmission triggers cardinal features of rapid eye movement sleep behavior disorder in mice. J Neurosci. 2011;31:7111–7121. doi: 10.1523/JNEUROSCI.0347-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Broome MR, Collingridge GL, Irving AJ. Activation of the NO-cGMP signalling pathway depresses hippocampal synaptic transmission through an adenosine receptor-dependent mechanism. Neuropharmacology. 1994;33:1511–1513. doi: 10.1016/0028-3908(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 150.Brorson JR, Schumacker PT, Zhang H. Nitric oxide acutely inhibits neuronal energy production. The Committees on Neurobiology and Cell Physiology. J Neurosci. 1999;19:147–158. doi: 10.1523/JNEUROSCI.19-01-00147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Broughton R, Mamelak M. Effects of nocturnal gamma-hydroxybutyrate on sleep/ waking patterns in narcolepsy-cataplexy. Can J Neurol Sci. 1980;7:23–31. [PubMed] [Google Scholar]
- 152.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brown MK, Naidoo N. The UPR and the anti-oxidant response: relevance to sleep and sleep loss. Mol Neurobiol. 2010;42:103–113. doi: 10.1007/s12035-010-8114-8. [DOI] [PubMed] [Google Scholar]
- 154.Brown RE, Franciosi S, McKenna JT, Winston S, Yanagawa Y, McCarley RW. Electrophysiological and pharmacological characterization of cortically projecting basal forebrain neurons in the mouse. Soc Neurosci Abstr. 2008;384:103–316. [Google Scholar]
- 155.Brown RE, Franciosi S, Yanagawa Y, McCarley RW. Cellular mechanisms underlying theta rhythm in a mammillary body-tegmentum circuit. Soc Neurosci Abstr. 2007;734:103–115. [Google Scholar]
- 156.Brown RE, McKenna JT, Winston S, Basheer R, Yanagawa Y, Thakkar MM, McCarley RW. Characterization of GABAergic neurons in rapid-eye-movement sleep controlling regions of the brain stem reticular formation in GAD67-green fluorescent protein knock-in mice. Eur J Neurosci. 2008;27:352–363. doi: 10.1111/j.1460-9568.2008.06024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40:457–459. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 158.Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline) J Neurosci. 2002;22:8850–8859. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 160.Brown RE, Winston S, Basheer R, Thakkar MM, McCarley RW. Electrophysiological characterization of neurons in the dorsolateral pontine rapid-eye-movement sleep induction zone of the rat: intrinsic membrane properties and responses to carbachol and orexins. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bubser M, Fadel JR, Jackson LL, Meador-Woodruff JH, Jing D, Deutch AY. Dopaminergic regulation of orexin neurons. Eur J Neurosci. 2005;21:2993–3001. doi: 10.1111/j.1460-9568.2005.04121.x. [DOI] [PubMed] [Google Scholar]
- 162.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2 . Proc Natl Acad Sci USA. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Buhl EH, Tamas G, Fisahn A. Cholinergic activation and tonic excitation induce persistent gamma oscillations in mouse somatosensory cortex in vitro. J Physiol. 1998;513:117–126. doi: 10.1111/j.1469-7793.1998.117by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O’Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 165.Burgess C, Lai D, Siegel J, Peever J. An endogenous glutamatergic drive onto somatic motoneurons contributes to the stereotypical pattern of muscle tone across the sleep-wake cycle. J Neurosci. 2008;28:4649–4660. doi: 10.1523/JNEUROSCI.0334-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Burk JA, Sarter M. Dissociation between the attentional functions mediated via basal forebrain cholinergic and GABAergic neurons. Neuroscience. 2001;105:899–909. doi: 10.1016/s0306-4522(01)00233-0. [DOI] [PubMed] [Google Scholar]
- 167.Burlet S, Cespuglio R. Voltammetric detection of nitric oxide (NO) in the rat brain: its variations throughout the sleep-wake cycle. Neurosci Lett. 1997;226:131–135. doi: 10.1016/s0304-3940(97)00247-4. [DOI] [PubMed] [Google Scholar]
- 168.Burlet S, Leger L, Cespuglio R. Nitric oxide and sleep in the rat: a puzzling relationship. Neuroscience. 1999;92:627–639. doi: 10.1016/s0306-4522(99)00031-7. [DOI] [PubMed] [Google Scholar]
- 169.Burlet S, Tyler CJ, Leonard CS. Direct and indirect excitation of laterodorsal tegmental neurons by Hypocretin/Orexin peptides: implications for wakefulness and narcolepsy. J Neurosci. 2002;22:2862–2872. doi: 10.1523/JNEUROSCI.22-07-02862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bushey D, Huber R, Tononi G, Cirelli C. Drosophila Hyperkinetic mutants have reduced sleep and impaired memory. J Neurosci. 2007;27:5384–5393. doi: 10.1523/JNEUROSCI.0108-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bushey D, Tononi G, Cirelli C. The Drosophila fragile X mental retardation gene regulates sleep need. J Neurosci. 2009;29:1948–1961. doi: 10.1523/JNEUROSCI.4830-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: structural evidence in Drosophila . Science. 2011;332:1576–1581. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Buysse DJ, Frank E, Lowe KK, Cherry CR, Kupfer DJ. Electroencephalographic sleep correlates of episode and vulnerability to recurrence in depression. Biol Psychiatry. 1997;41:406–418. doi: 10.1016/S0006-3223(96)00041-8. [DOI] [PubMed] [Google Scholar]
- 174.Buzsaki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- 175.Buzsaki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 176.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 177.Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 179.Buzsaki G, Haas HL, Anderson EG. Long-term potentiation induced by physiologically relevant stimulus patterns. Brain Res. 1987;435:331–333. doi: 10.1016/0006-8993(87)91618-0. [DOI] [PubMed] [Google Scholar]
- 180.Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- 181.Buzsaki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- 182.Buzsaki G, Ponomareff GL, Bayardo F, Ruiz R, Gage FH. Neuronal activity in the subcortically denervated hippocampus: a chronic model for epilepsy. Neuroscience. 1989;28:527–538. doi: 10.1016/0306-4522(89)90002-x. [DOI] [PubMed] [Google Scholar]
- 183.Camacho-Arroyo I, Alvarado R, Manjarrez J, Tapia R. Microinjections of muscimol and bicuculline into the pontine reticular formation modify the sleep-waking cycle in the rat. Neurosci Lett. 1991;129:95–97. doi: 10.1016/0304-3940(91)90728-c. [DOI] [PubMed] [Google Scholar]
- 184.Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2002;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- 185.Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- 186.Cano G, Mochizuki T, Saper CB. Neural circuitry of stress-induced insomnia in rats. J Neurosci. 2008;28:10167–10184. doi: 10.1523/JNEUROSCI.1809-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cantero JL, Atienza M, Stickgold R, Kahana MJ, Madsen JR, Kocsis B. Sleep-dependent theta oscillations in the human hippocampus and neocortex. J Neurosci. 2003;23:10897–10903. doi: 10.1523/JNEUROSCI.23-34-10897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cape EG, Jones BE. Differential modulation of high-frequency gamma-electroencephalogram activity and sleep-wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. J Neurosci. 1998;18:2653–2666. doi: 10.1523/JNEUROSCI.18-07-02653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cape EG, Jones BE. Effects of glutamate agonist versus procaine microinjections into the basal forebrain cholinergic cell area upon gamma and theta EEG activity and sleep-wake state. Eur J Neurosci. 2000;12:2166–2184. doi: 10.1046/j.1460-9568.2000.00099.x. [DOI] [PubMed] [Google Scholar]
- 191.Cape EG, Manns ID, Alonso A, Beaudet A, Jones BE. Neurotensin-induced bursting of cholinergic basal forebrain neurons promotes gamma and theta cortical activity together with waking and paradoxical sleep. J Neurosci. 2000;20:8452–8461. doi: 10.1523/JNEUROSCI.20-22-08452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Carden WB, Bickford ME. Location of muscarinic type 2 receptors within the synaptic circuitry of the cat visual thalamus. J Comp Neurol. 1999;410:431–443. [PubMed] [Google Scholar]
- 193.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Carlsson A, Falck B, Hillarp NA. Cellular localization of brain monoamines. Acta Physiol Scand, Suppl. 1962;56:1–28. [PubMed] [Google Scholar]
- 195.Carter ME, Adamantidis A, Ohtsu H, Deisseroth K, de Lecea L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J Neurosci. 2009;29:10939–10949. doi: 10.1523/JNEUROSCI.1205-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cash SS, Halgren E, Dehghani N, Rossetti AO, Thesen T, Wang C, Devinsky O, Kuzniecky R, Doyle W, Madsen JR, Bromfield E, Eross L, Halasz P, Karmos G, Csercsa R, Wittner L, Ulbert I. The human K-complex represents an isolated cortical down-state. Science. 2009;324:1084–1087. doi: 10.1126/science.1169626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A. TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci USA. 2007;104:12843–12848. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cavas M, Navarro JF. Effects of selective neuronal nitric oxide synthase inhibition on sleep and wakefulness in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:56–67. doi: 10.1016/j.pnpbp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 200.Celesia GG, Jasper HH. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology. 1966;16:1053–1063. doi: 10.1212/wnl.16.11.1053. [DOI] [PubMed] [Google Scholar]
- 201.Cespuglio R, Gomez ME, Faradji H, Jouvet M. Alterations in the sleep-waking cycle induced by cooling of the locus coeruleus area. Electroencephalogr Clin Neurophysiol. 1982;54:570–578. doi: 10.1016/0013-4694(82)90042-6. [DOI] [PubMed] [Google Scholar]
- 202.Chabas D, Habert MO, Maksud P, Tourbah A, Minz M, Willer JC, Arnulf I. Functional imaging of cataplexy during status cataplecticus. Sleep. 2007;30:153–156. doi: 10.1093/sleep/30.2.153. [DOI] [PubMed] [Google Scholar]
- 203.Chamberlin NL, Arrigoni E, Chou TC, Scammell TE, Greene RW, Saper CB. Effects of adenosine on GABAergic synaptic inputs to identified ventrolateral preoptic neurons. Neuroscience. 2003;119:913–918. doi: 10.1016/s0306-4522(03)00246-x. [DOI] [PubMed] [Google Scholar]
- 204.Chang SY, Zagha E, Kwon ES, Ozaita A, Bobik M, Martone ME, Ellisman MH, Heintz N, Rudy B. Distribution of Kv3.3 potassium channel subunits in distinct neuronal populations of mouse brain. J Comp Neurol. 2007;502:953–972. doi: 10.1002/cne.21353. [DOI] [PubMed] [Google Scholar]
- 205.Chapin EM, Andrade R. A 5-HT(7) receptor-mediated depolarization in the anterodorsal thalamus. I. Pharmacological characterization. J Pharmacol Exp Ther. 2001;297:395–402. [PubMed] [Google Scholar]
- 206.Chapin EM, Andrade R. A 5-HT(7) receptor-mediated depolarization in the anterodorsal thalamus. II. Involvement of the hyperpolarization-activated current I(h) J Pharmacol Exp Ther. 2001;297:403–409. [PubMed] [Google Scholar]
- 207.Charlton HM, Clark RG, Robinson IC, Goff AE, Cox BS, Bugnon C, Bloch BA. Growth hormone-deficient dwarfism in the rat: a new mutation. J Endocrinol. 1988;119:51–58. doi: 10.1677/joe.0.1190051. [DOI] [PubMed] [Google Scholar]
- 208.Chase MH. Confirmation of the consensus that glycinergic postsynaptic inhibition is responsible for the atonia of REM sleep. Sleep. 2008;31:1487–1491. doi: 10.1093/sleep/31.11.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chase MH, Enomoto S, Murakami T, Nakamura Y, Taira M. Intracellular potential of medullary reticular neurons during sleep and wakefulness. Exp Neurol. 1981;71:226–233. doi: 10.1016/0014-4886(81)90084-4. [DOI] [PubMed] [Google Scholar]
- 210.Chase MH, Morales FR. The atonia and myoclonia of active (REM) sleep. Annu Rev Psychol. 1990;41:557–584. doi: 10.1146/annurev.ps.41.020190.003013. [DOI] [PubMed] [Google Scholar]
- 211.Chauvette S, Volgushev M, Timofeev I. Origin of active states in local neocortical networks during slow sleep oscillation. Cereb Cortex. 2010;20:2660–2674. doi: 10.1093/cercor/bhq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–4567. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chee MW, Tan JC, Zheng H, Parimal S, Weissman DH, Zagorodnov V, Dinges DF. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci. 2008;28:5519–5528. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 215.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 216.Chen C, Hardy M, Zhang J, LaHoste GJ, Bazan NG. Altered NMDA receptor trafficking contributes to sleep deprivation-induced hippocampal synaptic and cognitive impairments. Biochem Biophys Res Commun. 2006;340:435–440. doi: 10.1016/j.bbrc.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 217.Chen L, Brown RE, McKenna JT, McCarley RW. Animal models of narcolepsy. CNS Neurol Disord Drug Targets. 2009;8:296–308. doi: 10.2174/187152709788921717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chen L, Majde JA, Krueger JM. Spontaneous sleep in mice with targeted disruptions of neuronal or inducible nitric oxide synthase genes. Brain Res. 2003;973:214–222. doi: 10.1016/s0006-8993(03)02484-3. [DOI] [PubMed] [Google Scholar]
- 219.Chen L, McKenna JT, Bolortuya Y, Winston S, Thakkar MM, Basheer R, Brown RE, McCarley RW. Knockdown of orexin type 1 receptor in rat locus coeruleus increases REM sleep during the dark period. Eur J Neurosci. 2010;32:1528–1536. doi: 10.1111/j.1460-9568.2010.07401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chen L, Savio CC, Yung WH. Electrophysiological and behavioral effects of zolpidem in rat globus pallidus. Exp Neurol. 2004;186:212–220. doi: 10.1016/j.expneurol.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 221.Chen L, Thakkar MM, Winston S, Bolortuya Y, Basheer R, McCarley RW. REM sleep changes in rats induced by siRNA-mediated orexin knockdown. Eur J Neurosci. 2006;24:2039–2048. doi: 10.1111/j.1460-9568.2006.05058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22:977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Christian SL, Ross AP, Zhao HW, Kristenson HJ, Zhan X, Rasley BT, Bickler PE, Drew KL. Arctic ground squirrel (Spermophilus parryii) hippocampal neurons tolerate prolonged oxygen-glucose deprivation and maintain baseline ERK1/2 and JNK activation despite drastic ATP loss. J Cereb Blood Flow Metab. 2008;28:1307–1319. doi: 10.1038/jcbfm.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Christie MA, Bolortuya Y, Chen LC, McKenna JT, McCarley RW, Strecker RE. Microdialysis elevation of adenosine in the basal forebrain produces vigilance impairments in the rat psychomotor vigilance task. Sleep. 2008;31:1393–1398. [PMC free article] [PubMed] [Google Scholar]
- 227.Chrobak JJ, Buzsaki G. Selective activation of deep layer (V-VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat. J Neurosci. 1994;14:6160–6170. doi: 10.1523/JNEUROSCI.14-10-06160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chrobak JJ, Buzsaki G. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J Neurosci. 1996;16:3056–3066. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cirelli C. How sleep deprivation affects gene expression in the brain: a review of recent findings. J Appl Physiol. 2002;92:394–400. doi: 10.1152/jappl.2002.92.1.394. [DOI] [PubMed] [Google Scholar]
- 230.Cirelli C. The genetic and molecular regulation of sleep: from fruit flies to humans. Nat Rev Neurosci. 2009;10:549–560. doi: 10.1038/nrn2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, Tononi G. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–1092. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- 232.Cirelli C, Faraguna U, Tononi G. Changes in brain gene expression after long-term sleep deprivation. J Neurochem. 2006;98:1632–1645. doi: 10.1111/j.1471-4159.2006.04058.x. [DOI] [PubMed] [Google Scholar]
- 233.Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 234.Cirelli C, Pfister-Genskow M, McCarthy D, Woodbury R, Tononi G. Proteomic profiling of the rat cerebral cortex in sleep and waking. Arch Ital Biol. 2009;147:59–68. [PMC free article] [PubMed] [Google Scholar]
- 235.Cirelli C, Pompeiano M, Arrighi P, Tononi G. Fos-positive cells associated with forced wakefulness in the hypothalamus of the rat are not GABAergic. Arch Ital Biol. 1995;133:143–148. [PubMed] [Google Scholar]
- 236.Cirelli C, Pompeiano M, Tononi G. Fos-like immunoreactivity in the rat brain in spontaneous wakefulness and sleep. Arch Ital Biol. 1993;131:327–330. [PubMed] [Google Scholar]
- 237.Cirelli C, Pompeiano M, Tononi G. Sleep deprivation and c-fos expression in the rat brain. J Sleep Res. 1995;4:92–106. doi: 10.1111/j.1365-2869.1995.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 238.Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: a role for the locus coeruleus. Science. 1996;274:1211–1215. doi: 10.1126/science.274.5290.1211. [DOI] [PubMed] [Google Scholar]
- 239.Cirelli C, Tononi G. Differences in gene expression between sleep and waking as revealed by mRNA differential display. Brain Res. 1998;56:293–305. doi: 10.1016/s0169-328x(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 240.Cirelli C, Tononi G. Differences in gene expression during sleep and wakefulness. Ann Med. 1999;31:117–124. doi: 10.3109/07853899908998787. [DOI] [PubMed] [Google Scholar]
- 241.Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000;20:9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–321. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 243.Cirelli C, Tononi G. On the functional significance of c-fos induction during the sleep-waking cycle. Sleep. 2000;23:453–469. [PubMed] [Google Scholar]
- 244.Cirelli C, Tononi G. Locus ceruleus control of state-dependent gene expression. J Neurosci. 2004;24:5410–5419. doi: 10.1523/JNEUROSCI.0949-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cirelli C, Tononi G. Uncoupling proteins and sleep deprivation. Arch Ital Biol. 2004;142:541–549. [PubMed] [Google Scholar]
- 246.Clemens S, Hochman S. Conversion of the modulatory actions of dopamine on spinal reflexes from depression to facilitation in D3 receptor knock-out mice. J Neurosci. 2004;24:11337–11345. doi: 10.1523/JNEUROSCI.3698-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67:125–130. doi: 10.1212/01.wnl.0000223316.53428.c9. [DOI] [PubMed] [Google Scholar]
- 248.Clement O, Sapin E, Berod A, Fort P, Luppi PH. Evidence that neurons of the sublaterodorsal tegmental nucleus triggering paradoxical (REM) sleep are glutamatergic. Sleep. 2011;34:419–423. doi: 10.1093/sleep/34.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept. 2002;104:131–144. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- 250.Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de ME, Rudy B. Molecular diversity of K+ channels. Ann NY Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 251.Cohen B, Feldman M. Relationship of electrical activity in pontine reticular formation and lateral geniculate body to rapid eye movements. J Neurophysiol. 1968;31:806–817. doi: 10.1152/jn.1968.31.6.806. [DOI] [PubMed] [Google Scholar]
- 252.Coleman CG, Lydic R, Baghdoyan HA. M2 muscarinic receptors in pontine reticular formation of C57BL/6J mouse contribute to rapid eye movement sleep generation. Neuroscience. 2004;126:821–830. doi: 10.1016/j.neuroscience.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 253.Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 254.Colgin LL, Kubota D, Jia Y, Rex CS, Lynch G. Long-term potentiation is impaired in rat hippocampal slices that produce spontaneous sharp waves. J Physiol. 2004;558:953–961. doi: 10.1113/jphysiol.2004.068080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Compte A, Reig R, Descalzo VF, Harvey MA, Puccini GD, Sanchez-Vives MV. Spontaneous high-frequency (10–80 Hz) oscillations during up states in the cerebral cortex in vitro. J Neurosci. 2008;28:13828–13844. doi: 10.1523/JNEUROSCI.2684-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Compte A, Sanchez-Vives MV, McCormick DA, Wang XJ. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol. 2003;89:2707–2725. doi: 10.1152/jn.00845.2002. [DOI] [PubMed] [Google Scholar]
- 257.Connor JR, Wang XS, Patton SM, Menzies SL, Troncoso JC, Earley CJ, Allen RP. Decreased transferrin receptor expression by neuromelanin cells in restless legs syndrome. Neurology. 2004;62:1563–1567. doi: 10.1212/01.wnl.0000123251.60485.ac. [DOI] [PubMed] [Google Scholar]
- 258.Conrad CD, Galea LA, Kuroda Y, Mcewen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 259.Contreras D, Timofeev I, Steriade M. Mechanisms of long-lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks. J Physiol. 1996;494:251–264. doi: 10.1113/jphysiol.1996.sp021488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. 1990;25:271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- 261.Cornwall J, Phillipson OT. Afferent projections to the parafascicular thalamic nucleus of the rat, as shown by the retrograde transport of wheat germ agglutinin. Brain Res Bull. 1988;20:139–150. doi: 10.1016/0361-9230(88)90171-2. [DOI] [PubMed] [Google Scholar]
- 262.Crochet S, Sakai K. Effects of microdialysis application of monoamines on the EEG and behavioural states in the cat mesopontine tegmentum. Eur J Neurosci. 1999;11:3738–3752. doi: 10.1046/j.1460-9568.1999.00760.x. [DOI] [PubMed] [Google Scholar]
- 263.Crochet S, Sakai K. Dopaminergic modulation of behavioral states in mesopontine tegmentum: a reverse microdialysis study in freely moving cats. Sleep. 2003;26:801–806. doi: 10.1093/sleep/26.7.801. [DOI] [PubMed] [Google Scholar]
- 264.Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci. 2010;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Cudeiro J, Rivadulla C, Grieve KL. A possible role for nitric oxide at the sleep/wake interface. Sleep. 2000;23:829–835. [PubMed] [Google Scholar]
- 266.Cueni L, Canepari M, Lujan R, Emmenegger Y, Watanabe M, Bond CT, Franken P, Adelman JP, Luthi A. T-type Ca(2+) channels, SK2 channels and SERCAs gate sleeprelated oscillations in thalamic dendrites. Nat Neurosci. 2008;11:683–692. doi: 10.1038/nn.2124. [DOI] [PubMed] [Google Scholar]
- 267.Culebras A, Moore JT. Magnetic resonance findings in REM sleep behavior disorder. Neurology. 1989;39:1519–1523. doi: 10.1212/wnl.39.11.1519. [DOI] [PubMed] [Google Scholar]
- 268.Cuna-Goycolea C, Van Den Pol A. Glucagon-like peptide 1 excites hypocretin/ orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. J Neurosci. 2004;24:8141–8152. doi: 10.1523/JNEUROSCI.1607-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Cunningham MO, Pervouchine DD, Racca C, Kopell NJ, Davies CH, Jones RS, Traub RD, Whittington MA. Neuronal metabolism governs cortical network response state. Proc Natl Acad Sci USA. 2006;103:5597–5601. doi: 10.1073/pnas.0600604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Cvetkovic-Lopes V, Bayer L, Dorsaz S, Maret S, Pradervand S, Dauvilliers Y, Lecendreux M, Lammers GJ, Donjacour CE, Du Pasquier RA, Pfister C, Petit B, Hor H, Muhlethaler M, Tafti M. Elevated Tribbles homolog 2-specific antibody levels in narcolepsy patients. J Clin Invest. 2010;120:713–719. doi: 10.1172/JCI41366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32:1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- 272.Dang-Vu TT, Schabus M, Desseilles M, Albouy G, Boly M, Darsaud A, Gais S, Rauchs G, Sterpenich V, Vandewalle G, Carrier J, Moonen G, Balteau E, Degueldre C, Luxen A, Phillips C, Maquet P. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci USA. 2008;105:15160–15165. doi: 10.1073/pnas.0801819105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Datta S. Cellular basis of pontine ponto-geniculo-occipital wave generation and modulation. Cell Mol Neurobiol. 1997;17:341–365. doi: 10.1023/a:1026398402985. [DOI] [PubMed] [Google Scholar]
- 275.Datta S. Avoidance task training potentiates phasic pontine-wave density in the rat: a mechanism for sleep-dependent plasticity. J Neurosci. 2000;20:8607–8613. doi: 10.1523/JNEUROSCI.20-22-08607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Datta S, Hobson JA. Neuronal activity in the caudolateral peribrachial pons: relationship to PGO waves and rapid eye movements. J Neurophysiol. 1994;71:95–109. doi: 10.1152/jn.1994.71.1.95. [DOI] [PubMed] [Google Scholar]
- 277.Datta S, Hobson JA. Suppression of ponto-geniculo-occipital waves by neurotoxic lesions of pontine caudo-lateral peribrachial cells. Neuroscience. 1995;67:703–712. doi: 10.1016/0306-4522(95)00081-s. [DOI] [PubMed] [Google Scholar]
- 278.Datta S, Li G, Auerbach S. Activation of phasic pontine-wave generator in the rat: a mechanism for expression of plasticity-related genes and proteins in the dorsal hippocampus and amygdala. Eur J Neurosci. 2008;27:1876–1892. doi: 10.1111/j.1460-9568.2008.06166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Datta S, Mavanji V, Patterson EH, Ulloor J. Regulation of rapid eye movement sleep in the freely moving rat: local microinjection of serotonin, norepinephrine, and adenosine into the brain stem. Sleep. 2003;26:513–520. doi: 10.1093/sleep/26.5.513. [DOI] [PubMed] [Google Scholar]
- 280.Datta S, Mavanji V, Ulloor J, Patterson EH. Activation of phasic pontine-wave generator prevents rapid eye movement sleep deprivation-induced learning impairment in the rat: a mechanism for sleep-dependent plasticity. J Neurosci. 2004;24:1416–1427. doi: 10.1523/JNEUROSCI.4111-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Datta S, Patterson EH, Siwek DF. Endogenous and exogenous nitric oxide in the pedunculopontine tegmentum induces sleep. Synapse. 1997;27:69–78. doi: 10.1002/(SICI)1098-2396(199709)27:1<69::AID-SYN7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 282.Datta S, Prutzman SL. Novel role of brain stem pedunculopontine tegmental adenylyl cyclase in the regulation of spontaneous REM sleep in the freely moving rat. J Neurophysiol. 2005;94:1928–1937. doi: 10.1152/jn.00272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Datta S, Quattrochi JJ, Hobson JA. Effect of specific muscarinic M2 receptor antagonist on carbachol induced long-term REM sleep. Sleep. 1993;16:8–14. [PubMed] [Google Scholar]
- 284.Datta S, Saha S, Prutzman SL, Mullins OJ, Mavanji V. Pontine-wave generator activation- dependent memory processing of avoidance learning involves the dorsal hippocampus in the rat. J Neurosci Res. 2005;80:727–737. doi: 10.1002/jnr.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Datta S, Siwek DF, Huang MP. Improvement of two-way active avoidance memory requires protein kinase a activation and brain-derived neurotrophic factor expression in the dorsal hippocampus. J Mol Neurosci. 2009;38:257–264. doi: 10.1007/s12031-009-9206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Datta S, Siwek DF, Patterson EH, Cipolloni PB. Localization of pontine PGO wave generation sites and their anatomical projections in the rat. Synapse. 1998;30:409–423. doi: 10.1002/(SICI)1098-2396(199812)30:4<409::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 287.Dauvilliers Y, Maret S, Tafti M. Genetics of normal and pathological sleep in humans. Sleep Med Rev. 2005;9:91–100. doi: 10.1016/j.smrv.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 288.Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Res. 2003;973:293–297. doi: 10.1016/s0006-8993(03)02508-3. [DOI] [PubMed] [Google Scholar]
- 289.De Lecea L, Criado JR, Prospero-Garcia O, Gautvik KM, Schweitzer P, Danielson PE, Dunlop CL, Siggins GR, Henriksen SJ, Sutcliffe JG. A cortical neuropeptide with neuronal depressant and sleep-modulating properties. Nature. 1996;381:242–245. doi: 10.1038/381242a0. [DOI] [PubMed] [Google Scholar]
- 290.De Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.De Lima AD, Singer W. The brainstem projection to the lateral geniculate nucleus in the cat: identification of cholinergic and monoaminergic elements. J Comp Neurol. 1987;259:92–121. doi: 10.1002/cne.902590107. [DOI] [PubMed] [Google Scholar]
- 292.De GL, Marzano C, Fratello F, Moroni F, Pellicciari MC, Ferlazzo F, Costa S, Couyoumdjian A, Curcio G, Sforza E, Malafosse A, Finelli LA, Pasqualetti P, Ferrara M, Bertini M, Rossini PM. The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Ann Neurol. 2008;64:455–460. doi: 10.1002/ana.21434. [DOI] [PubMed] [Google Scholar]
- 293.De SG, Gareri P, Sinopoli VA, David E, Rotiroti D. Comparative, behavioural and electrocortical effects of tumor necrosis factor-alpha and interleukin-1 microinjected into the locus coeruleus of rat. Life Sci. 1997;60:555–564. doi: 10.1016/s0024-3205(96)00692-3. [DOI] [PubMed] [Google Scholar]
- 294.Dean T, Jr, Allen RP, O’Donnell CP, Earley CJ. The effects of dietary iron deprivation on murine circadian sleep architecture. Sleep Med. 2006;7:634–640. doi: 10.1016/j.sleep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 295.Deboer T, Fontana A, Tobler I. Tumor necrosis factor (TNF) ligand and TNF receptor deficiency affects sleep and the sleep EEG. J Neurophysiol. 2002;88:839–846. doi: 10.1152/jn.2002.88.2.839. [DOI] [PubMed] [Google Scholar]
- 296.Deboer T, Overeem S, Visser NA, Duindam H, Frolich M, Lammers GJ, Meijer JH. Convergence of circadian and sleep regulatory mechanisms on hypocretin-1. Neuroscience. 2004;129:727–732. doi: 10.1016/j.neuroscience.2004.07.049. [DOI] [PubMed] [Google Scholar]
- 297.Deco G, Thiele A. Attention: oscillations and neuropharmacology. Eur J Neurosci. 2009;30:347–354. doi: 10.1111/j.1460-9568.2009.06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Dejean C, Gross CE, Bioulac B, Boraud T. Synchronous high-voltage spindles in the cortex-basal ganglia network of awake and unrestrained rats. Eur J Neurosci. 2007;25:772–784. doi: 10.1111/j.1460-9568.2007.05305.x. [DOI] [PubMed] [Google Scholar]
- 299.Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr Clin Neurophysiol, Suppl. 1957;9:673–690. doi: 10.1016/0013-4694(57)90088-3. [DOI] [PubMed] [Google Scholar]
- 300.Dement W, Kleitman N. The relation of eye movements during sleep to dream activity: an objective method for the study of dreaming. J Exp Psychol. 1957;53:339–346. doi: 10.1037/h0048189. [DOI] [PubMed] [Google Scholar]
- 301.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Denoyer M, Sallanon M, Buda C, Kitahama K, Jouvet M. Neurotoxic lesion of the mesencephalic reticular formation and/or the posterior hypothalamus does not alter waking in the cat. Brain Res. 1991;539:287–303. doi: 10.1016/0006-8993(91)91633-c. [DOI] [PubMed] [Google Scholar]
- 303.Desseilles M, Dang-Vu T, Schabus M, Sterpenich V, Maquet P, Schwartz S. Neuroimaging insights into the pathophysiology of sleep disorders. Sleep. 2008;31:777–794. doi: 10.1093/sleep/31.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Desseilles M, Dang-Vu T, Maquet P. Functional neuroimaging in sleep, sleep deprivation, and sleep disorders. Handb Clin Neurol. 2011;98:71–94. doi: 10.1016/B978-0-444-52006-7.00006-X. [DOI] [PubMed] [Google Scholar]
- 305.Detari L, Rasmusson DD, Semba K. The role of basal forebrain neurons in tonic and phasic activation of the cerebral cortex. Prog Neurobiol. 1999;58:249–277. doi: 10.1016/s0301-0082(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 306.Deurveilher S, Burns J, Semba K. Indirect projections from the suprachiasmatic nucleus to the ventrolateral preoptic nucleus: a dual tract-tracing study in rat. Eur J Neurosci. 2002;16:1195–1213. doi: 10.1046/j.1460-9568.2002.02196.x. [DOI] [PubMed] [Google Scholar]
- 307.Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- 308.Diano S, Horvath B, Urbanski HF, Sotonyi P, Horvath TL. Fasting activates the nonhuman primate hypocretin (orexin) system and its postsynaptic targets. Endocrinology. 2003;144:3774–3778. doi: 10.1210/en.2003-0274. [DOI] [PubMed] [Google Scholar]
- 309.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 310.Dijk DJ, Archer SN. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Med Rev. 2010;14:151–160. doi: 10.1016/j.smrv.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 311.Dijk DJ, Brunner DP, Beersma DG, Borbely AA. Electroencephalogram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep. 1990;13:430–440. doi: 10.1093/sleep/13.5.430. [DOI] [PubMed] [Google Scholar]
- 312.Doroshenko P, Renaud LP. Acid-sensitive TASK-like K(+) conductances contribute to resting membrane potential and to orexin-induced membrane depolarization in rat thalamic paraventricular nucleus neurons. Neuroscience. 2009;158:1560–1570. doi: 10.1016/j.neuroscience.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 313.Dossena S, Imeri L, Mangieri M, Garofoli A, Ferrari L, Senatore A, Restelli E, Balducci C, Fiordaliso F, Salio M, Bianchi S, Fioriti L, Morbin M, Pincherle A, Marcon G, Villani F, Carli M, Tagliavini F, Forloni G, Chiesa R. Mutant prion protein expression causes motor and memory deficits and abnormal sleep patterns in a transgenic mouse model. Neuron. 2008;60:598–609. doi: 10.1016/j.neuron.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 314.Dossi RC, Nunez A, Steriade M. Electrophysiology of a slow (0.5–4 Hz) intrinsic oscillation of cat thalamocortical neurones in vivo. J Physiol. 1992;447:215–234. doi: 10.1113/jphysiol.1992.sp018999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Douglas CL, Vyazovskiy V, Southard T, Chiu SY, Messing A, Tononi G, Cirelli C. Sleep in Kcna2 knockout mice. BMC Biol. 2007;5:42. doi: 10.1186/1741-7007-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Dringenberg HC, Vanderwolf CH. Involvement of direct and indirect pathways in electrocorticographic activation. Neurosci Biobehav Rev. 1998;22:243–257. doi: 10.1016/s0149-7634(97)00012-2. [DOI] [PubMed] [Google Scholar]
- 317.Duangdao DM, Clark SD, Okamura N, Reinscheid RK. Behavioral phenotyping of neuropeptide S receptor knockout mice. Behav Brain Res. 2009;205:1–9. doi: 10.1016/j.bbr.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 320.Dunwiddie TV, Worth T. Sedative and anticonvulsant effects of adenosine analogs in mouse and rat. J Pharmacol Exp Ther. 1982;220:70–76. [PubMed] [Google Scholar]
- 321.Duque A, Balatoni B, Detari L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. J Neurophysiol. 2000;84:1627–1635. doi: 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- 322.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 323.Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010;30:9007–9016. doi: 10.1523/JNEUROSCI.1423-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Dzoljic E, van LR, De VR, Dzoljic MR. Vigilance and EEG power in rats: effects of potent inhibitors of the neuronal nitric oxide synthase. Naunyn-Schmiedebergs Arch Pharmacol. 1997;356:56–61. doi: 10.1007/pl00005028. [DOI] [PubMed] [Google Scholar]
- 325.Dzoljic MR, De VR, van LR. Sleep and nitric oxide: effects of 7-nitro indazole, inhibitor of brain nitric oxide synthase. Brain Res. 1996;718:145–150. doi: 10.1016/0006-8993(96)00102-3. [DOI] [PubMed] [Google Scholar]
- 326.Earley CJ, Barker B, Horska A, Allen RP. MRI-determined regional brain iron concentrations in early- and late-onset restless legs syndrome. Sleep Med. 2006;7:458–461. doi: 10.1016/j.sleep.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 327.Earley CJ, Connor JR, Beard JL, Malecki EA, Epstein DK, Allen RP. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54:1698–1700. doi: 10.1212/wnl.54.8.1698. [DOI] [PubMed] [Google Scholar]
- 328.Earley CJ, Heckler D, Allen RP. The treatment of restless legs syndrome with intravenous iron dextran. Sleep Med. 2004;5:231–235. doi: 10.1016/j.sleep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 329.Ebert B, Wafford KA, Deacon S. Treating insomnia: current and investigational pharmacological approaches. Pharmacol Ther. 2006;112:612–629. doi: 10.1016/j.pharmthera.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 330.Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375:181–183. doi: 10.1016/S0140-6736(09)61794-3. [DOI] [PubMed] [Google Scholar]
- 331.Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci. 2009;29:15721–15726. doi: 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Egan TM, North RA. Acetylcholine hyperpolarizes central neurones by acting on an M2 muscarinic receptor. Nature. 1986;319:405–407. doi: 10.1038/319405a0. [DOI] [PubMed] [Google Scholar]
- 333.Eggermann E, Bayer L, Serafin M, Saint-Mleux B, Bernheim L, Machard D, Jones BE, Muhlethaler M. The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J Neurosci. 2003;23:1557–1562. doi: 10.1523/JNEUROSCI.23-05-01557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, Muhlethaler M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- 335.Ekbom K, Ulfberg J. Restless legs syndrome. J Intern Med. 2009;266:419–431. doi: 10.1111/j.1365-2796.2009.02159.x. [DOI] [PubMed] [Google Scholar]
- 336.El Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp Brain Res. 1989;76:519–529. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- 337.El Mansari M, Sakai K, Jouvet M. Responses of presumed cholinergic mesopontine tegmental neurons to carbachol microinjections in freely moving cats. Exp Brain Res. 1990;83:115–123. doi: 10.1007/BF00232199. [DOI] [PubMed] [Google Scholar]
- 338.El YM, Ledent C, Menard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. Br J Pharmacol. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Ellenbogen JM. Cognitive benefits of sleep and their loss due to sleep deprivation. Neurology. 2005;64:E25–E27. doi: 10.1212/01.wnl.0000164850.68115.81. [DOI] [PubMed] [Google Scholar]
- 340.Elmenhorst D, Basheer R, McCarley RW, Bauer A. Sleep deprivation increases A(1) adenosine receptor density in the rat brain. Brain Res. 2009;1258:53–58. doi: 10.1016/j.brainres.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Elmenhorst D, Meyer PT, Winz OH, Matusch A, Ermert J, Coenen HH, Basheer R, Haas HL, Zilles K, Bauer A. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J Neurosci. 2007;27:2410–2415. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Eriksson KS, Sergeeva OA, Stevens DR, Haas HL. Neurotransmitter-induced activation of sodium-calcium exchange causes neuronal excitation. Ann NY Acad Sci. 2002;976:405–407. doi: 10.1111/j.1749-6632.2002.tb04767.x. [DOI] [PubMed] [Google Scholar]
- 344.Eriksson KS, Stevens DR, Haas HL. Serotonin excites tuberomammillary neurons by activation of Na+/Ca2+-exchange. Neuropharmacology. 2001;40:345–351. doi: 10.1016/s0028-3908(00)00175-1. [DOI] [PubMed] [Google Scholar]
- 345.Eschenko O, Molle M, Born J, Sara SJ. Elevated sleep spindle density after learning or after retrieval in rats. J Neurosci. 2006;26:12914–12920. doi: 10.1523/JNEUROSCI.3175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Eschenko O, Ramadan W, Molle M, Born J, Sara SJ. Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learn Mem. 2008;15:222–228. doi: 10.1101/lm.726008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Escudero M, Marquez-Ruiz J. Tonic inhibition and ponto-geniculo-occipital-related activities shape abducens motoneuron discharge during REM sleep. J Physiol. 2008;586:3479–3491. doi: 10.1113/jphysiol.2008.153254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Espana RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 349.Espinosa F, Torres-Vega MA, Marks GA, Joho RH. Ablation of Kv3.1 and Kv3.3 potassium channels disrupts thalamocortical oscillations in vitro and in vivo. J Neurosci. 2008;28:5570–5581. doi: 10.1523/JNEUROSCI.0747-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Esser SK, Hill SL, Tononi G. Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep. 2007;30:1617–1630. doi: 10.1093/sleep/30.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Fallahi N, Broad RM, Jin S, Fredholm BB. Release of adenosine from rat hippocampal slices by nitric oxide donors. J Neurochem. 1996;67:186–193. doi: 10.1046/j.1471-4159.1996.67010186.x. [DOI] [PubMed] [Google Scholar]
- 353.Fang J, Wang Y, Krueger JM. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNFalpha treatment. J Neurosci. 1997;17:5949–5955. doi: 10.1523/JNEUROSCI.17-15-05949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Fang J, Wang Y, Krueger JM. Effects of interleukin-1 beta on sleep are mediated by the type I receptor. Am J Physiol Regul Integr Comp Physiol. 1998;274:R655–R660. doi: 10.1152/ajpregu.1998.274.3.R655. [DOI] [PubMed] [Google Scholar]
- 355.Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28:4088–4095. doi: 10.1523/JNEUROSCI.5510-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Farber J, Marks GA, Roffwarg HP. Rapid eye movement sleep PGO-type waves are present in the dorsal pons of the albino rat. Science. 1980;209:615–617. doi: 10.1126/science.6994229. [DOI] [PubMed] [Google Scholar]
- 357.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 358.Fedele DE, Gouder N, Guttinger M, Gabernet L, Scheurer L, Rulicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- 359.Feldberg A, Sherwood PD. Injections of drugs into the lateral ventricle of the cat. J Physiol. 1954;123:148–167. doi: 10.1113/jphysiol.1954.sp005040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Fellin T, Halassa MM, Terunuma M, Succol F, Takano H, Frank M, Moss SJ, Haydon PG. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc Natl Acad Sci USA. 2009;106:15037–15042. doi: 10.1073/pnas.0906419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Fenik VB, Davies RO, Kubin L. Noradrenergic, serotonergic and GABAergic antagonists injected together into the XII nucleus abolish the REM sleep-like depression of hypoglossal motoneuronal activity. J Sleep Res. 2005;14:419–429. doi: 10.1111/j.1365-2869.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- 362.Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005;172:1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Ferguson J, Henriksen S, Cohen H, Mitchell G, Barchas J, Dement W. “Hypersexuality” and behavioral changes in cats caused by administration of p-chlorophenylalanine. Science. 1970;168:499–501. doi: 10.1126/science.168.3930.499. [DOI] [PubMed] [Google Scholar]
- 364.Fernandez-Mendoza J, Lozano B, Seijo F, Santamarta-Liebana E, Ramos-Platon MJ, Vela-Bueno A, Fernandez-Gonzalez F. Evidence of subthalamic PGO-like waves during REM sleep in humans: a deep brain polysomnographic study. Sleep. 2009;32:1117–1126. doi: 10.1093/sleep/32.9.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Finelli LA, Baumann H, Borbely AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–529. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 366.Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- 367.Florian C, Vecsey CG, Halassa MM, Haydon PG, Abel T. Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J Neurosci. 2011;31:6956–6962. doi: 10.1523/JNEUROSCI.5761-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Floyd RA, Krueger JM. Diurnal variation of TNF alpha in the rat brain. Neuroreport. 1997;8:915–918. doi: 10.1097/00001756-199703030-00020. [DOI] [PubMed] [Google Scholar]
- 369.Fogel RB, Trinder J, White DP, Malhotra A, Raneri J, Schory K, Kleverlaan D, Pierce RJ. The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. J Physiol. 2005;564:549–562. doi: 10.1113/jphysiol.2005.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Fogel SM, Smith CT, Beninger RJ. Increased GABAergic activity in the region of the pedunculopontine and deep mesencephalic reticular nuclei reduces REM sleep and impairs learning in rats. Behav Neurosci. 2010;124:79–86. doi: 10.1037/a0018244. [DOI] [PubMed] [Google Scholar]
- 371.Fonck C, Cohen BN, Nashmi R, Whiteaker P, Wagenaar DA, Rodrigues-Pinguet N, Deshpande P, McKinney S, Kwoh S, Munoz J, Labarca C, Collins AC, Marks MJ, Lester HA. Novel seizure phenotype and sleep disruptions in knock-in mice with hypersensitive alpha 4* nicotinic receptors. J Neurosci. 2005;25:11396–11411. doi: 10.1523/JNEUROSCI.3597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Ford B, Holmes CJ, Mainville L, Jones BE. GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- 373.Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000;12:3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- 374.Forster GL, Yeomans JS, Takeuchi J, Blaha CD. M5 muscarinic receptors are required for prolonged accumbal dopamine release after electrical stimulation of the pons in mice. J Neurosci. 2002;22:RC190. doi: 10.1523/JNEUROSCI.22-01-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Fort P, Khateb A, Pegna A, Muhlethaler M, Jones BE. Noradrenergic modulation of cholinergic nucleus basalis neurons demonstrated by in vitro pharmacological and immunohistochemical evidence in the guinea-pig brain. Eur J Neurosci. 1995;7:1502–1511. doi: 10.1111/j.1460-9568.1995.tb01145.x. [DOI] [PubMed] [Google Scholar]
- 376.Fraigne JJ, Orem JM. Phasic motor activity of respiratory and non-respiratory muscles in REM sleep. Sleep. 2011;34:425–434. doi: 10.1093/sleep/34.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Frank MG, Heller HC. The ontogeny of mammalian sleep: a reappraisal of alternative hypotheses. J Sleep Res. 2003;12:25–34. doi: 10.1046/j.1365-2869.2003.00339.x. [DOI] [PubMed] [Google Scholar]
- 378.Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001;21:2610–2621. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Franken P, Dijk DJ. Circadian clock genes and sleep homeostasis. Eur J Neurosci. 2009;29:1820–1829. doi: 10.1111/j.1460-9568.2009.06723.x. [DOI] [PubMed] [Google Scholar]
- 380.Franken P, Dudley CA, Estill SJ, Barakat M, Thomason R, O’Hara BF, McKnight SL. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: genotype and sex interactions. Proc Natl Acad Sci USA. 2006;103:7118–7123. doi: 10.1073/pnas.0602006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Franken P, Lopez-Molina L, Marcacci L, Schibler U, Tafti M. The transcription factor DBP affects circadian sleep consolidation and rhythmic EEG activity. J Neurosci. 2000;20:617–625. doi: 10.1523/JNEUROSCI.20-02-00617.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Franken P, Malafosse A, Tafti M. Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1127–R1137. doi: 10.1152/ajpregu.1998.275.4.R1127. [DOI] [PubMed] [Google Scholar]
- 383.Franken P, Malafosse A, Tafti M. Genetic determinants of sleep regulation in inbred mice. Sleep. 1999;22:155–169. [PubMed] [Google Scholar]
- 384.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 385.Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- 386.Freund TF, Meskenaite V. gamma-Aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc Natl Acad Sci USA. 1992;89:738–742. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Friedman L, Jones BE. Computer graphics analysis of sleep-wakefulness state changes after pontine lesions. Brain Res Bull. 1984;13:53–68. doi: 10.1016/0361-9230(84)90008-x. [DOI] [PubMed] [Google Scholar]
- 388.Friedman L, Jones BE. Study of sleep-wakefulness states by computer graphics and cluster analysis before and after lesions of the pontine tegmentum in the cat. Electroencephalogr Clin Neurophysiol. 1984;57:43–56. doi: 10.1016/0013-4694(84)90007-5. [DOI] [PubMed] [Google Scholar]
- 389.Friedman MJ. Future pharmacotherapy for post-traumatic stress disorder: prevention and treatment. Psychiatr Clin North Am. 2002;25:427–441. doi: 10.1016/s0193-953x(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 390.Fries P, Nikolic D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 391.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 392.Fry JM, DiPhillipo MA, Pressman MR. Periodic leg movements in sleep following treatment of obstructive sleep apnea with nasal continuous positive airway pressure. Chest. 1989;96:89–91. doi: 10.1378/chest.96.1.89. [DOI] [PubMed] [Google Scholar]
- 393.Fu LY, Acuna-Goycolea C, van den Pol AN. Neuropeptide Y inhibits hypocretin/ orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J Neurosci. 2004;24:8741–8751. doi: 10.1523/JNEUROSCI.2268-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Fuentealba P, Steriade M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog Neurobiol. 2005;75:125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 395.Fujiki N, Yoshida Y, Ripley B, Mignot E, Nishino S. Effects of IV and ICV hypocretin-1 (orexin A) in hypocretin receptor-2 gene mutated narcoleptic dogs and IV hypocretin-1 replacement therapy in a hypocretin-ligand-deficient narcoleptic dog. Sleep. 2003;26:953–959. doi: 10.1093/sleep/26.8.953. [DOI] [PubMed] [Google Scholar]
- 396.Fulda S, Schulz H. Cognitive dysfunction in sleep disorders. Sleep Med Rev. 2001;5:423–445. doi: 10.1053/smrv.2001.0157. [DOI] [PubMed] [Google Scholar]
- 397.Fuller P, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Funk GD. Are all motoneurons created equal in the eyes of REM sleep and the mechanisms of muscle atonia? Sleep. 2008;31:1479–1482. doi: 10.1093/sleep/31.11.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Gagnon JF, Bedard MA, Fantini ML, Petit D, Panisset M, Rompre S, Carrier J, Montplaisir J. REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease. Neurology. 2002;59:585–589. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]
- 400.Gagnon JF, Postuma RB, Mazza S, Doyon J, Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5:424–432. doi: 10.1016/S1474-4422(06)70441-0. [DOI] [PubMed] [Google Scholar]
- 401.Gagnon JF, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67:742–747. doi: 10.1212/01.wnl.0000233926.47469.73. [DOI] [PubMed] [Google Scholar]
- 402.Gais S, Born J. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci USA. 2004;101:2140–2144. doi: 10.1073/pnas.0305404101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, Audinat E, Muhlethaler M, Serafin M. Identification of sleep-promoting neurons in vitro. Nature. 2000;404:992–995. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- 404.Gallopin T, Luppi PH, Cauli B, Urade Y, Rossier J, Hayaishi O, Lambolez B, Fort P. The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A2A receptors in the ventrolateral preoptic nucleus. Neuroscience. 2005;134:1377–1390. doi: 10.1016/j.neuroscience.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 405.Gangwisch JE. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes Rev. Suppl 2. 2009;10:37–45. doi: 10.1111/j.1467-789X.2009.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115:285–294. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 407.Gautier-Sauvigne S, Colas D, Parmantier P, Clement P, Gharib A, Sarda N, Cespuglio R. Nitric oxide and sleep. Sleep Med Rev. 2005;9:101–113. doi: 10.1016/j.smrv.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 408.Gelineau J. De La narcolepsie. Gaz des Hop (Paris) 1880;55:635–637. [Google Scholar]
- 409.Gemma C, Imeri L, De Simoni MG, Mancia M. Interleukin-1 induces changes in sleep, brain temperature, and serotonergic metabolism. Am J Physiol Regul Integr Comp Physiol. 1997;272:R601–R606. doi: 10.1152/ajpregu.1997.272.2.R601. [DOI] [PubMed] [Google Scholar]
- 410.George R, Haslett WL, Jenden DJ. A cholinergic mechanism in the brainstem reticular formation: induction of paradoxical sleep. Int J Neuropharmacol. 1964;3:541–552. doi: 10.1016/0028-3908(64)90076-0. [DOI] [PubMed] [Google Scholar]
- 411.Gerashchenko D, Blanco-Centurion C, Greco MA, Shiromani PJ. Effects of lateral hypothalamic lesion with the neurotoxin hypocretin-2-saporin on sleep in Long- Evans rats. Neuroscience. 2003;116:223–235. doi: 10.1016/s0306-4522(02)00575-4. [DOI] [PubMed] [Google Scholar]
- 412.Gerashchenko D, Blanco-Centurion CA, Miller JD, Shiromani PJ. Insomnia following hypocretin2-saporin lesions of the substantia nigra. Neuroscience. 2006;137:29–36. doi: 10.1016/j.neuroscience.2005.08.088. [DOI] [PubMed] [Google Scholar]
- 413.Gerashchenko D, Chou TC, Blanco-Centurion CA, Saper CB, Shiromani PJ. Effects of lesions of the histaminergic tuberomammillary nucleus on spontaneous sleep in rats. Sleep. 2004;27:1275–1281. doi: 10.1093/sleep/27.7.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Gerashchenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Gerashchenko D, Salin-Pascual R, Shiromani PJ. Effects of hypocretin-saporin injections into the medial septum on sleep and hippocampal theta. Brain Res. 2001;913:106–115. doi: 10.1016/s0006-8993(01)02792-5. [DOI] [PubMed] [Google Scholar]
- 416.Gerashchenko D, Wisor JP, Burns D, Reh RK, Shiromani PJ, Sakurai T, De LI, Kilduff TS. Identification of a population of sleep-active cerebral cortex neurons. Proc Natl Acad Sci USA. 2008;105:10227–10232. doi: 10.1073/pnas.0803125105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Gerber U, Greene RW, Haas HL, Stevens DR. Characterization of inhibition mediated by adenosine in the hippocampus of the rat in vitro. J Physiol. 1989;417:567–578. doi: 10.1113/jphysiol.1989.sp017819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Gerber U, Greene RW, McCarley RW. Repetitive firing properties of medial pontine reticular formation neurones of the rat recorded in vitro. J Physiol. 1989;410:533–560. doi: 10.1113/jphysiol.1989.sp017548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Gerber U, Stevens DR, McCarley RW, Greene RW. Muscarinic agonists activate an inwardly rectifying potassium conductance in medial pontine reticular formation neurons of the rat in vitro. J Neurosci. 1991;11:3861–3867. doi: 10.1523/JNEUROSCI.11-12-03861.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Gervasoni D, Darracq L, Fort P, Souliere F, Chouvet G, Luppi PH. Electrophysiological evidence that noradrenergic neurons of the rat locus coeruleus are tonically inhibited by GABA during sleep. Eur J Neurosci. 1998;10:964–970. doi: 10.1046/j.1460-9568.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- 421.Gervasoni D, Peyron C, Rampon C, Barbagli B, Chouvet G, Urbain N, Fort P, Luppi PH. Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J Neurosci. 2000;20:4217–4225. doi: 10.1523/JNEUROSCI.20-11-04217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Gerwins P, Fredholm BB. ATP and its metabolite adenosine act synergistically to mobilize intracellular calcium via the formation of inositol 1,4,5-trisphosphate in a smooth muscle cell line. J Biol Chem. 1992;267:16081–16087. [PubMed] [Google Scholar]
- 423.Gething MJ. Role and regulation of the ER chaperone BiP. Semin Cell Dev Biol. 1999;10:465–472. doi: 10.1006/scdb.1999.0318. [DOI] [PubMed] [Google Scholar]
- 424.Ghosh PK, Hrdina PD, Ling GM. Effects of REMS deprivation on striatal dopamine and acetylcholine in rats. Pharmacol Biochem Behav. 1976;4:401–405. doi: 10.1016/0091-3057(76)90055-1. [DOI] [PubMed] [Google Scholar]
- 425.Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila . Science. 2009;324:109–112. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Gillin JC, Sitaram N, Duncan WC. Muscarinic supersensitivity: a possible model for the sleep disturbance of primary depression? Psychiatry Res. 1979;1:17–22. doi: 10.1016/0165-1781(79)90023-4. [DOI] [PubMed] [Google Scholar]
- 427.Gillin JC, Sitaram N, Mendelson WB. Acetylcholine, sleep, and depression. Hum Neurobiol. 1982;1:211–219. [PubMed] [Google Scholar]
- 428.Gillin JC, Sutton L, Ruiz C, Golshan S, Hirsch S, Warmann C, Shiromani P. Dose dependent inhibition of REM sleep in normal volunteers by biperiden, a muscarinic antagonist. Biol Psychiatry. 1991;30:151–156. doi: 10.1016/0006-3223(91)90169-m. [DOI] [PubMed] [Google Scholar]
- 429.Glaubman H, Mikulincer M, Porat A, Wasserman O, Birger M. Sleep of chronic post-traumatic patients. J Trauma Stress. 1990;3:225–263. [Google Scholar]
- 430.Goaillard JM, Vincent P. Serotonin suppresses the slow afterhyperpolarization in rat intralaminar and midline thalamic neurones by activating 5-HT(7) receptors. J Physiol. 2002;541:453–465. doi: 10.1113/jphysiol.2001.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Goldstein SA, Bockenhauer D, O’Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- 432.Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Gonzalez MM, Ston-Jones G. Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats. Proc Natl Acad Sci USA. 2008;105:4898–4903. doi: 10.1073/pnas.0703615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Goutagny R, Comte JC, Salvert D, Gomeza J, Yamada M, Wess J, Luppi PH, Fort P. Paradoxical sleep in mice lacking M3 and M2/M4 muscarinic receptors. Neuropsychobiology. 2005;52:140–146. doi: 10.1159/000087560. [DOI] [PubMed] [Google Scholar]
- 435.Goutagny R, Luppi PH, Salvert D, Lapray D, Gervasoni D, Fort P. Role of the dorsal paragigantocellular reticular nucleus in paradoxical (rapid eye movement) sleep generation: a combined electrophysiological and anatomical study in the rat. Neuroscience. 2008;152:849–857. doi: 10.1016/j.neuroscience.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 436.Govindaiah G, Cox CL. Modulation of thalamic neuron excitability by orexins. Neuropharmacology. 2006;51:414–425. doi: 10.1016/j.neuropharm.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 437.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21:2442–2450. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Graf MV, Kastin AJ. Delta-sleep-inducing peptide (DSIP): a review. Neurosci Biobehav Rev. 1984;8:83–93. doi: 10.1016/0149-7634(84)90022-8. [DOI] [PubMed] [Google Scholar]
- 439.Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El MS. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Grassi-Zucconi G, Giuditta A, Mandile P, Chen S, Vescia S, Bentivoglio M. c-fos spontaneous expression during wakefulness is reversed during sleep in neuronal subsets of the rat cortex. J Physiol. 1994;88:91–93. doi: 10.1016/0928-4257(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 441.Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Graves LA, Hellman K, Veasey S, Blendy JA, Pack AI, Abel T. Genetic evidence for a role of CREB in sustained cortical arousal. J Neurophysiol. 2003;90:1152–1159. doi: 10.1152/jn.00882.2002. [DOI] [PubMed] [Google Scholar]
- 443.Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 444.Greco MA, Lu J, Wagner D, Shiromani PJ. c-Fos expression in the cholinergic basal forebrain after enforced wakefulness and recovery sleep. Neuroreport. 2000;11:437–440. doi: 10.1097/00001756-200002280-00002. [DOI] [PubMed] [Google Scholar]
- 445.Green JD, Arduini A. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17:533–537. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- 446.Greene RW, Haas HL. The electrophysiology of adenosine in the mammalian central nervous system. Prog Neurobiol. 1991;36:329–341. doi: 10.1016/0301-0082(91)90005-l. [DOI] [PubMed] [Google Scholar]
- 447.Greene RW, Gerber U, McCarley RW. Cholinergic activation of medial pontine reticular formation neurons in vitro. Brain Res. 1989;476:154–159. doi: 10.1016/0006-8993(89)91549-7. [DOI] [PubMed] [Google Scholar]
- 448.Greene RW, Haas HL, McCarley RW. A low threshold calcium spike mediates firing pattern alterations in pontine reticular neurons. Science. 1986;234:738–740. doi: 10.1126/science.3775364. [DOI] [PubMed] [Google Scholar]
- 449.Gritti I, Mainville L, Jones BE. Projections of GABAergic and cholinergic basal forebrain and GABAergic preoptic-anterior hypothalamic neurons to the posterior lateral hypothalamus of the rat. J Comp Neurol. 1994;339:251–268. doi: 10.1002/cne.903390206. [DOI] [PubMed] [Google Scholar]
- 450.Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol. 1997;383:163–177. [PubMed] [Google Scholar]
- 451.Gritti I, Manns ID, Mainville L, Jones BE. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J Comp Neurol. 2003;458:11–31. doi: 10.1002/cne.10505. [DOI] [PubMed] [Google Scholar]
- 452.Grivel J, Cvetkovic V, Bayer L, Machard D, Tobler I, Muhlethaler M, Serafin M. The wake-promoting hypocretin/orexin neurons change their response to noradrenaline after sleep deprivation. J Neurosci. 2005;25:4127–4130. doi: 10.1523/JNEUROSCI.0666-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Gujar N, McDonald SA, Nishida M, Walker MP. A role for rem sleep in recalibrating the sensitivity of the human brain to specific emotions. Cereb Cortex. 2011;21:115–123. doi: 10.1093/cercor/bhq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Gujar N, Yoo SS, Hu P, Walker MP. The unrested resting brain: sleep deprivation alters activity within the default-mode network. J Cogn Neurosci. 2010;22:1637–1648. doi: 10.1162/jocn.2009.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Gujar N, Yoo SS, Hu P, Walker MP. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J Neurosci. 2011;31:4466–4474. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Gulia KK, Jodo E, Kawauchi A, Miki T, Kayama Y, Mallick HN, Koyama Y. The septal area, site for the central regulation of penile erection during waking and rapid eye movement sleep in rats: a stimulation study. Neuroscience. 2008;156:1064–1073. doi: 10.1016/j.neuroscience.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 457.Guo JZ, Liu Y, Sorenson EM, Chiappinelli VA. Synaptically released and exogenous ACh activates different nicotinic receptors to enhance evoked glutamatergic transmission in the lateral geniculate nucleus. J Neurophysiol. 2005;94:2549–2560. doi: 10.1152/jn.00339.2005. [DOI] [PubMed] [Google Scholar]
- 458.Gutnick MJ, Yarom Y. Low threshold calcium spikes, intrinsic neuronal oscillation and rhythm generation in the CNS. J Neurosci Methods. 1989;28:93–99. doi: 10.1016/0165-0270(89)90014-9. [DOI] [PubMed] [Google Scholar]
- 459.Guzman-Marin R, Bashir T, Suntsova N, Szymusiak R, McGinty D. Hippocampal neurogenesis is reduced by sleep fragmentation in the adult rat. Neuroscience. 2007;148:325–333. doi: 10.1016/j.neuroscience.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Guzman-Marin R, Suntsova N, Methippara M, Greiffenstein R, Szymusiak R, McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur J Neurosci. 2005;22:2111–2116. doi: 10.1111/j.1460-9568.2005.04376.x. [DOI] [PubMed] [Google Scholar]
- 461.Guzman-Marin R, Suntsova N, Stewart DR, Gong H, Szymusiak R, McGinty D. Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J Physiol. 2003;549:563–571. doi: 10.1113/jphysiol.2003.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Gvilia I, Xu F, McGinty D, Szymusiak R. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci. 2006;26:9426–9433. doi: 10.1523/JNEUROSCI.2012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Haas HL, Konnerth A. Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. Nature. 1983;302:432–434. doi: 10.1038/302432a0. [DOI] [PubMed] [Google Scholar]
- 464.Haas HL, Reiner PB. Membrane properties of histaminergic tuberomammillary neurones of the rat hypothalamus in vitro. J Physiol. 1988;399:633–646. doi: 10.1113/jphysiol.1988.sp017100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 466.Habaguchi T, Takakusaki K, Saitoh K, Sugimoto J, Sakamoto T. Medullary reticulospinal tract mediating the generalized motor inhibition in cats: II. Functional organization within the medullary reticular formation with respect to postsynaptic inhibition of forelimb and hindlimb motoneurons. Neuroscience. 2002;113:65–77. doi: 10.1016/s0306-4522(02)00149-5. [DOI] [PubMed] [Google Scholar]
- 467.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Hagewoud R, Bultsma LJ, Barf RP, Koolhaas JM, Meerlo P. Sleep deprivation impairs contextual fear conditioning and attenuates subsequent behavioural, endocrine and neuronal responses. J Sleep Res. 2011;20:259–266. doi: 10.1111/j.1365-2869.2010.00895.x. [DOI] [PubMed] [Google Scholar]
- 469.Hairston IS, Little MT, Scanlon MD, Barakat MT, Palmer TD, Sapolsky RM, Heller HC. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–4233. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- 470.Hajnik T, Lai YY, Siegel JM. Atonia-related regions in the rodent pons and medulla. J Neurophysiol. 2000;84:1942–1948. doi: 10.1152/jn.2000.84.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Hajos M, Allers KA, Jennings K, Sharp T, Charette G, Sik A, Kocsis B. Neurochemical identification of stereotypic burst-firing neurons in the rat dorsal raphe nucleus using juxtacellular labelling methods. Eur J Neurosci. 2007;25:119–126. doi: 10.1111/j.1460-9568.2006.05276.x. [DOI] [PubMed] [Google Scholar]
- 472.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol. 1987;262:105–124. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- 474.Hallanger AE, Wainer BH. Ascending projections from the pedunculopontine tegmental nucleus and the adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;274:483–515. doi: 10.1002/cne.902740403. [DOI] [PubMed] [Google Scholar]
- 475.Hallmayer J, Faraco J, Lin L, Hesselson S, Winkelmann J, Kawashima M, Mayer G, Plazzi G, Nevsimalova S, Bourgin P, Hong SC, Honda Y, Honda M, Hogl B, Longstreth WT, Jr, Montplaisir J, Kemlink D, Einen M, Chen J, Musone SL, Akana M, Miyagawa T, Duan J, Desautels A, Erhardt C, Hesla PE, Poli F, Frauscher B, Jeong JH, Lee SP, Ton TG, Kvale M, Kolesar L, Dobrovolna M, Nepom GT, Salomon D, Wichmann HE, Rouleau GA, Gieger C, Levinson DF, Gejman PV, Meitinger T, Young T, Peppard P, Tokunaga K, Kwok PY, Risch N, Mignot E. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. 2009;41:708–711. doi: 10.1038/ng.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Hampson RE, Espana RA, Rogers GA, Porrino LJ, Deadwyler SA. Mechanisms underlying cognitive enhancement and reversal of cognitive deficits in nonhuman primates by the ampakine CX717. Psychopharmacology. 2009;202:355–369. doi: 10.1007/s00213-008-1360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Hanbury J, Ajmone-Marsan C, Dilworth M. Pathways of non-specific thalamocortical projection system. Electroencephalogr Clin Neurophysiol. 1954;6:103–118. doi: 10.1016/0013-4694(54)90010-3. [DOI] [PubMed] [Google Scholar]
- 479.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 480.Hars B. Endogenous nitric oxide in the rat pons promotes sleep. Brain Res. 1999;816:209–219. doi: 10.1016/s0006-8993(98)01183-4. [DOI] [PubMed] [Google Scholar]
- 481.Hassani OK, Lee MG, Henny P, Jones BE. Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J Neurosci. 2009;29:11828–11840. doi: 10.1523/JNEUROSCI.1259-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci USA. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Haulica I, Ababei LBD, Topoliceanu F. Preliminary data on the possible hypnogenic role of adenosine. J Neurochem. 1973;21:1019–1020. doi: 10.1111/j.1471-4159.1973.tb07549.x. [DOI] [PubMed] [Google Scholar]
- 484.Hayaishi O. Molecular genetic studies on sleep-wake regulation, with special emphasis on the prostaglandin D(2) system. J Appl Physiol. 2002;92:863–868. doi: 10.1152/japplphysiol.00766.2001. [DOI] [PubMed] [Google Scholar]
- 485.Hayaishi O, Urade Y, Eguchi N, Huang ZL. Genes for prostaglandin d synthase and receptor as well as adenosine A2A receptor are involved in the homeostatic regulation of nrem sleep. Arch Ital Biol. 2004;142:533–539. [PubMed] [Google Scholar]
- 486.Hayes RL, Pechura CM, Katayama Y, Povlishock JT, Giebel ML, Becker DP. Activation of pontine cholinergic sites implicated in unconsciousness following cerebral concussion in the cat. Science. 1984;223:301–303. doi: 10.1126/science.6701514. [DOI] [PubMed] [Google Scholar]
- 487.He Y, Jones CR, Fujiki N, Xu Y, Guo B, Holder JL, Jr, Rossner MJ, Nishino S, Fu YH. The transcriptional repressor DEC2 regulates sleep length in mammals. Science. 2009;325:866–870. doi: 10.1126/science.1174443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 489.Heister DS, Hayar A, Charlesworth A, Yates C, Zhou YH, Garcia-Rill E. Evidence for electrical coupling in the subcoeruleus (SubC) nucleus. J Neurophysiol. 2007;97:3142–3147. doi: 10.1152/jn.01316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Heister DS, Hayar A, Garcia-Rill E. Cholinergic modulation of GABAergic and glutamatergic transmission in the dorsal subcoeruleus: mechanisms for REM sleep control. Sleep. 2009;32:1135–1147. doi: 10.1093/sleep/32.9.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Hellman K, Hernandez P, Park A, Abel T. Genetic evidence for a role for protein kinase A in the maintenance of sleep and thalamocortical oscillations. Sleep. 2010;33:19–28. doi: 10.1093/sleep/33.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 493.Hendricks JC, Lager A, O’Brien D, Morrison AR. Movement disorders during sleep in cats and dogs. J Am Vet Med Assoc. 1989;194:686–689. [PubMed] [Google Scholar]
- 494.Hendricks JC, Morrison AR, Farnbach GL, Steinberg SA, Mann G. A disorder of rapid eye movement sleep in a cat. J Am Vet Med Assoc. 1981;178:55–57. [PubMed] [Google Scholar]
- 495.Hendricks JC, Morrison AR, Mann GL. Different behaviors during paradoxical sleep without atonia depend on pontine lesion site. Brain Res. 1982;239:81–105. doi: 10.1016/0006-8993(82)90835-6. [DOI] [PubMed] [Google Scholar]
- 496.Hendricks JC, Williams JA, Panckeri K, Kirk D, Tello M, Yin JC, Sehgal A. A noncircadian role for cAMP signaling and CREB activity in Drosophila rest homeostasis. Nat Neurosci. 2001;4:1108–1115. doi: 10.1038/nn743. [DOI] [PubMed] [Google Scholar]
- 497.Hening W, Walters AS, Allen RP, Montplaisir J, Myers A, Ferini-Strambi L. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med. 2004;5:237–246. doi: 10.1016/j.sleep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 498.Henley K, Morrison AR. A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat. Acta Neurobiol Exp. 1974;34:215–232. [PubMed] [Google Scholar]
- 499.Hennevin E, Hars B, Maho C, Bloch V. Processing of learned information in paradoxical sleep: relevance for memory. Behav Brain Res. 1995;69:125–135. doi: 10.1016/0166-4328(95)00013-j. [DOI] [PubMed] [Google Scholar]
- 500.Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Hentschke H, Perkins MG, Pearce RA, Banks MI. Muscarinic blockade weakens interaction of gamma with theta rhythms in mouse hippocampus. Eur J Neurosci. 2007;26:1642–1656. doi: 10.1111/j.1460-9568.2007.05779.x. [DOI] [PubMed] [Google Scholar]
- 502.Herculano-Houzel S, Munk MH, Neuenschwander S, Singer W. Precisely synchronized oscillatory firing patterns require electroencephalographic activation. J Neurosci. 1999;19:3992–4010. doi: 10.1523/JNEUROSCI.19-10-03992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Hernandez PJ, Abel T. A molecular basis for interactions between sleep and memory. Sleep Med Clin. 2011;6:71–84. doi: 10.1016/j.jsmc.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Hernandez-peon R, Chavez-Ibarra G, Morgane PJ, Timo-Iaria C. Limbic cholinergic pathways involved in sleep and emotional behaviour. Exp Neurol. 1963;8:93–111. [Google Scholar]
- 505.Herring WJ, Budd KS, Hutzelmann J, Snyder E, Snavely D, Liu K, Lines C, Michelson D, Roth T. Efficacy and tolerability of the dual orexin receptor antagonist MK-4305 in patients with primary insomnia: randomized, controlled, adaptive crossover polysomnography study. Sleep. 2010;33:A591. [Google Scholar]
- 506.Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- 507.Hill S, Tononi G. Modeling sleep and wakefulness in the thalamocortical system. J Neurophysiol. 2005;93:1671–1698. doi: 10.1152/jn.00915.2004. [DOI] [PubMed] [Google Scholar]
- 508.Hirshkowitz M, Schmidt MH. Sleep-related erections: clinical perspectives and neural mechanisms. Sleep Med Rev. 2005;9:311–329. doi: 10.1016/j.smrv.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 509.Hobson JA. REM sleep and dreaming: towards a theory of protoconsciousness. Nat Rev Neurosci. 2009;10:803–813. doi: 10.1038/nrn2716. [DOI] [PubMed] [Google Scholar]
- 510.Hobson JA, McCarley RW. The brain as a dream state generator: an activationsynthesis hypothesis of the dream process. Am J Psychiatry. 1977;134:1335–1348. doi: 10.1176/ajp.134.12.1335. [DOI] [PubMed] [Google Scholar]
- 511.Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- 512.Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3:679–693. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- 513.Hohagen F, Riemann D, Spiegel R, Holzhauer M, Berger M. Influence of the cholinergic agonist SDZ 210-086 on sleep in healthy subjects. Neuropsychopharmacology. 1993;9:225–232. doi: 10.1038/npp.1993.58. [DOI] [PubMed] [Google Scholar]
- 514.Holmes CJ, Jones BE. Importance of cholinergic, GABAergic, serotonergic and other neurons in the medial medullary reticular formation for sleep-wake states studied by cytotoxic lesions in the cat. Neuroscience. 1994;62:1179–1200. doi: 10.1016/0306-4522(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 515.Holstege JC, Bongers CM. A glycinergic projection from the ventromedial lower brainstem to spinal motoneurons. An ultrastructural double labeling study in rat. Brain Res. 1991;566:308–315. doi: 10.1016/0006-8993(91)91715-d. [DOI] [PubMed] [Google Scholar]
- 516.Honda K, Komoda Y, Inoue S. Oxidized glutathione regulates physiological sleep in unrestrained rats. Brain Res. 1994;636:253–258. doi: 10.1016/0006-8993(94)91024-3. [DOI] [PubMed] [Google Scholar]
- 517.Honda K, Okano Y, Komoda Y, Inoue S. Sleep-promoting effects of intraperitoneally administered uridine in unrestrained rats. Neurosci Lett. 1985;62:137–141. doi: 10.1016/0304-3940(85)90297-6. [DOI] [PubMed] [Google Scholar]
- 518.Hong ZY, Huang ZL, Qu WM, Eguchi N, Urade Y, Hayaishi O. An adenosine A receptor agonist induces sleep by increasing GABA release in the tuberomammillary nucleus to inhibit histaminergic systems in rats. J Neurochem. 2005;92:1542–1549. doi: 10.1111/j.1471-4159.2004.02991.x. [DOI] [PubMed] [Google Scholar]
- 519.Hopkins WF, Johnston D. Frequency-dependent noradrenergic modulation of longterm potentiation in the hippocampus. Science. 1984;226:350–352. doi: 10.1126/science.6091272. [DOI] [PubMed] [Google Scholar]
- 520.Hor H, Kutalik Z, Dauvilliers Y, Valsesia A, Lammers GJ, Donjacour CE, Iranzo A, Santamaria J, Peraita AR, Vicario JL, Overeem S, Arnulf I, Theodorou I, Jennum P, Knudsen S, Bassetti C, Mathis J, Lecendreux M, Mayer G, Geisler P, Beneto A, Petit B, Pfister C, Burki JV, Didelot G, Billiard M, Ercilla G, Verduijn W, Claas FH, Vollenweider P, Waeber G, Waterworth DM, Mooser V, Heinzer R, Beckmann JS, Bergmann S, Tafti M. Genome-wide association study identifies new HLA class II haplotypes strongly protective against narcolepsy. Nat Genet. 2010;42:786–789. doi: 10.1038/ng.647. [DOI] [PubMed] [Google Scholar]
- 521.Horne JA. Human sleep, sleep loss and behaviour. Implications for the prefrontal cortex and psychiatric disorder. Br J Psychiatry. 1993;162:413–419. doi: 10.1192/bjp.162.3.413. [DOI] [PubMed] [Google Scholar]
- 522.Horner RL, Sanford LD, Annis D, Pack AI, Morrison AR. Serotonin at the laterodorsal tegmental nucleus suppresses rapid-eye-movement sleep in freely behaving rats. J Neurosci. 1997;17:7541–7552. doi: 10.1523/JNEUROSCI.17-19-07541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, Duyn JH. Decoupling of the brain’s default mode network during deep sleep. Proc Natl Acad Sci USA. 2009;106:11376–11381. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Horvath TL, Peyron C, Diano S, Ivanov A, Aston JG, Kilduff TS, Van-den Pol A. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- 525.Hu B, Bouhassira D, Steriade M, Deschenes M. The blockage of ponto-geniculooccipital waves in the cat lateral geniculate nucleus by nicotinic antagonists. Brain Res. 1988;473:394–397. doi: 10.1016/0006-8993(88)90873-6. [DOI] [PubMed] [Google Scholar]
- 526.Hu B, Steriade M, Deschenes M. The cellular mechanism of thalamic ponto-geniculo- occipital waves. Neuroscience. 1989;31:25–35. doi: 10.1016/0306-4522(89)90028-6. [DOI] [PubMed] [Google Scholar]
- 527.Huang H, Ghosh P, van den Pol AN. Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J Neurophysiol. 2006;95:1656–1668. doi: 10.1152/jn.00927.2005. [DOI] [PubMed] [Google Scholar]
- 528.Huang ZL, Mochizuki T, Qu WM, Hong ZY, Watanabe T, Urade Y, Hayaishi O. Altered sleep-wake characteristics and lack of arousal response to H3 receptor antagonist in histamine H1 receptor knockout mice. Proc Natl Acad Sci USA. 2006;103:4687–4692. doi: 10.1073/pnas.0600451103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 530.Huang ZL, Qu WM, Li WD, Mochizuki T, Eguchi N, Watanabe T, Urade Y, Hayaishi O. Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci USA. 2001;98:9965–9970. doi: 10.1073/pnas.181330998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Huang ZL, Urade Y, Hayaishi O. Prostaglandins and adenosine in the regulation of sleep and wakefulness. Curr Opin Pharmacol. 2007;7:33–38. doi: 10.1016/j.coph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 532.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 533.Huber R, Maatta S, Esser SK, Sarasso S, Ferrarelli F, Watson A, Ferreri F, Peterson MJ, Tononi G. Measures of cortical plasticity after transcranial paired associative stimulation predict changes in electroencephalogram slow-wave activity during subsequent sleep. J Neurosci. 2008;28:7911–7918. doi: 10.1523/JNEUROSCI.1636-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Huerta PT, Lisman JE. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature. 1993;364:723–725. doi: 10.1038/364723a0. [DOI] [PubMed] [Google Scholar]
- 535.Hughes SW, Cope DW, Blethyn KL, Crunelli V. Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron. 2002;33:947–958. doi: 10.1016/s0896-6273(02)00623-2. [DOI] [PubMed] [Google Scholar]
- 536.Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist. 2005;11:357–372. doi: 10.1177/1073858405277450. [DOI] [PubMed] [Google Scholar]
- 537.Hughes SW, Lorincz M, Cope DW, Blethyn KL, Kekesi KA, Parri HR, Juhasz G, Crunelli V. Synchronized oscillations at alpha and theta frequencies in the lateral geniculate nucleus. Neuron. 2004;42:253–268. doi: 10.1016/s0896-6273(04)00191-6. [DOI] [PubMed] [Google Scholar]
- 538.Hull AM. Neuroimaging findings in post-traumatic stress disorder. Systematic review. Br J Psychiatry. 2002;181:102–110. [PubMed] [Google Scholar]
- 539.Hunsley MS, Palmiter RD. Norepinephrine-deficient mice exhibit normal sleep-wake states but have shorter sleep latency after mild stress and low doses of amphetamine. Sleep. 2003;26:521–526. [PubMed] [Google Scholar]
- 540.Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization. J Comp Neurol. 2005;483:351–373. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- 541.Huston JP, Haas HL, Boix F, Pfister M, Decking U, Schrader J, Schwarting RK. Extracellular adenosine levels in neostriatum and hippocampus during rest and activity periods of rats. Neuroscience. 1996;73:99–107. doi: 10.1016/0306-4522(96)00021-8. [DOI] [PubMed] [Google Scholar]
- 542.Imeri L, Mancia M, Opp MR. Blockade of 5-hydroxytryptamine (serotonin)-2 receptors alters interleukin-1-induced changes in rat sleep. Neuroscience. 1999;92:745–749. doi: 10.1016/s0306-4522(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 543.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Imon H, Ito K, Dauphin L, McCarley RW. Electrical stimulation of the cholinergic laterodorsal tegmental nucleus elicits scopolamine-sensitive excitatory postsynaptic potentials in medial pontine reticular formation neurons. Neuroscience. 1996;74:393–401. doi: 10.1016/0306-4522(96)00134-0. [DOI] [PubMed] [Google Scholar]
- 545.Inoue S. Sleep and sleep substances. Brain Dev. 1986;8:469–473. doi: 10.1016/s0387-7604(86)80071-7. [DOI] [PubMed] [Google Scholar]
- 546.Inoue S, Honda K, Komoda Y, Uchizono K, Ueno R, Hayaishi O. Differential sleep-promoting effects of five sleep substances nocturnally infused in unrestrained rats. Proc Natl Acad Sci USA. 1984;81:6240–6244. doi: 10.1073/pnas.81.19.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Iranzo A, Molinuevo JL, Santamaria J, Serradell M, Marti MJ, Valldeoriola F, Tolosa E. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–577. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 548.Ishimori K. True cause of sleep: a hypnogenic substance as evidenced in the brain of sleep-deprived animals. Tokyo Igakkai Zasshi. 1909;23:429–457. [Google Scholar]
- 549.Ito K, McCarley RW. Alterations in membrane potential and excitability of cat medial pontine reticular formation neurons during changes in naturally occurring sleep-wake states. Brain Res. 1984;292:169–175. doi: 10.1016/0006-8993(84)90903-x. [DOI] [PubMed] [Google Scholar]
- 550.Ito K, Yanagihara M, Imon H, Dauphin L, McCarley RW. Intracellular recordings of pontine medial gigantocellular tegmental field neurons in the naturally sleeping cat: behavioral state-related activity and soma size difference in order of recruitment. Neuroscience. 2002;114:23–37. doi: 10.1016/s0306-4522(02)00253-1. [DOI] [PubMed] [Google Scholar]
- 551.Itowi N, Yamatodani A, Kiyono S, Hiraiwa ML, Wada H. Effect of histamine depletion on the circadian amplitude of the sleep-wakefulness cycle. Physiol Behav. 1991;49:643–646. doi: 10.1016/0031-9384(91)90293-w. [DOI] [PubMed] [Google Scholar]
- 552.Ivanov A, Aston-Jones G. Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. Neuroreport. 2000;11:1755–1758. doi: 10.1097/00001756-200006050-00031. [DOI] [PubMed] [Google Scholar]
- 553.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 554.Jacobs BL, Fornal CA. Activity of brain serotonergic neurons in the behaving animal. Pharmacol Rev. 1991;43:563–578. [PubMed] [Google Scholar]
- 555.Jacobs BL, Henriksen SJ, Dement WC. Neurochemical bases of the PGO wave. Brain Res. 1972;48:406–411. doi: 10.1016/0006-8993(72)90200-4. [DOI] [PubMed] [Google Scholar]
- 556.Jahnsen H, Llinas R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Jahnsen H, Llinas R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Jahnsen H, Llinas R. Voltage-dependent burst-to-tonic switching of thalamic cell activity: an in vitro study. Arch Ital Biol. 1984;122:73–82. [PubMed] [Google Scholar]
- 559.Jasper HH. Diffuse projection systems: the integrative action of the thalamic reticular system. Electroencephalogr Clin Neurophysiol. 1949;1:405–419. [PubMed] [Google Scholar]
- 560.Jasper HH, Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science. 1971;172:601–602. doi: 10.1126/science.172.3983.601. [DOI] [PubMed] [Google Scholar]
- 561.Jha SK, Jones BE, Coleman T, Steinmetz N, Law CT, Griffin G, Hawk J, Dabbish N, Kalatsky VA, Frank MG. Sleep-dependent plasticity requires cortical activity. J Neurosci. 2005;25:9266–9274. doi: 10.1523/JNEUROSCI.2722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 563.John J, Kumar VM. Effect of NMDA lesion of the medial preoptic neurons on sleep and other functions. Sleep. 1998;21:587–598. doi: 10.1093/sleep/21.6.587. [DOI] [PubMed] [Google Scholar]
- 564.John J, Wu MF, Boehmer LN, Siegel JM. Cataplexy-active neurons in the hypothalamus; implications for the role of histamine in sleep and waking behavior. Neuron. 2004;42:619–634. doi: 10.1016/s0896-6273(04)00247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Joho RH, Ho CS, Marks GA. Increased gamma- and decreased delta-oscillations in a mouse deficient for a potassium channel expressed in fast-spiking interneurons. J Neurophysiol. 1999;82:1855–1864. doi: 10.1152/jn.1999.82.4.1855. [DOI] [PubMed] [Google Scholar]
- 567.Joho RH, Marks GA, Espinosa F. Kv3 potassium channels control the duration of different arousal states by distinct stochastic and clock-like mechanisms. Eur J Neurosci. 2006;23:1567–1574. doi: 10.1111/j.1460-9568.2006.04672.x. [DOI] [PubMed] [Google Scholar]
- 568.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 569.Jones BE. Elimination of paradoxical sleep by lesions of the pontine gigantocellular tegmental field in the cat. Neurosci Lett. 1979;13:285–293. doi: 10.1016/0304-3940(79)91508-8. [DOI] [PubMed] [Google Scholar]
- 570.Jones BE. Paradoxical sleep and its chemical/structural substrates in the brain. Neuroscience. 1991;40:637–656. doi: 10.1016/0306-4522(91)90002-6. [DOI] [PubMed] [Google Scholar]
- 571.Jones BE. The role of noradrenergic locus coeruleus neurons and neighboring cholinergic neurons of the pontomesencephalic tegmentum in sleep-wake states. Prog Brain Res. 1991;88:533–543. doi: 10.1016/s0079-6123(08)63832-7. [DOI] [PubMed] [Google Scholar]
- 572.Jones BE. The organization of central cholinergic systems and their functional importance in sleep-waking states. Prog Brain Res. 1993;98:61–71. doi: 10.1016/s0079-6123(08)62381-x. [DOI] [PubMed] [Google Scholar]
- 573.Jones BE. Arousal systems. Front Biosci. 2003;8:s438–s451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- 574.Jones BE. Paradoxical REM sleep promoting and permitting neuronal networks. Arch Ital Biol. 2004;142:379–396. [PubMed] [Google Scholar]
- 575.Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 576.Jones BE, Harper ST, Halaris AE. Effects of locus coeruleus lesions upon cerebral monoamine content, sleep-wakefulness states and the response to amphetamine in the cat. Brain Res. 1977;124:473–496. doi: 10.1016/0006-8993(77)90948-9. [DOI] [PubMed] [Google Scholar]
- 577.Jones BE, Webster HH. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. I. Effects upon the cholinergic innervation of the brain. Brain Res. 1988;451:13–32. doi: 10.1016/0006-8993(88)90745-7. [DOI] [PubMed] [Google Scholar]
- 578.Jones EG, Leavitt RY. Retrograde axonal transport and the demonstration of nonspecific projections to the cerebral cortex and striatum from thalamic intralaminar nuclei in the rat, cat and monkey. J Comp Neurol. 1974;154:349–377. doi: 10.1002/cne.901540402. [DOI] [PubMed] [Google Scholar]
- 579.Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol. 1997;504:603–610. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Jouvet M. Recherches sur les structures nerveuses et les mecanismes responsables des differentes phases du sommeil physiologique. Arch Ital Biol. 1962;100:125–206. [PubMed] [Google Scholar]
- 581.Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163:32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- 582.Jouvet M, Bobillier P, Pujol JF, Renault J. Permanent insomnia and diminution of cerebral serotonin due to lesion of the raphe system in cats. J Physiol. 1967;59:248. [PubMed] [Google Scholar]
- 583.Jouvet M, Delorme JF. Locus coeruleus et sommeil paradoxal. Comptes Rendus de la Societe de Biologie (Paris) 1965;159:895–899. [Google Scholar]
- 584.Jouvet M, Jeannerod M, Delorme F. Organization of the system responsible for phase activity during paradoxal sleep. C R Seances Soc Biol Fil. 1965;159:1599–1604. [PubMed] [Google Scholar]
- 585.Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- 586.Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Kahana MJ. The cognitive correlates of human brain oscillations. J Neurosci. 2006;26:1669–1672. doi: 10.1523/JNEUROSCI.3737-05c.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Kahn D, Pace-Schott EF, Hobson JA. Consciousness in waking and dreaming: the roles of neuronal oscillation and neuromodulation in determining similarities and differences. Neuroscience. 1997;78:13–38. doi: 10.1016/s0306-4522(96)00550-7. [DOI] [PubMed] [Google Scholar]
- 589.Kalinchuk AV, Lu Y, Stenberg D, Rosenberg PA, Porkka-Heiskanen T. Nitric oxide production in the basal forebrain is required for recovery sleep. J Neurochem. 2006;99:483–498. doi: 10.1111/j.1471-4159.2006.04077.x. [DOI] [PubMed] [Google Scholar]
- 590.Kalinchuk AV, McCarley RW, Porkka-Heiskanen T, Basheer R. Sleep deprivation triggers inducible nitric oxide-dependent nitric oxide production in wake-active basal forebrain neurons. J Neurosci. 2010;30:13254–13264. doi: 10.1523/JNEUROSCI.0014-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Kalinchuk AV, McCarley RW, Porkka-Heiskanen T, Basheer R. The time course of adenosine, nitric oxide (NO) and inducible NO synthase changes in the brain with sleep loss and their role in the non-rapid eye movement sleep homeostatic cascade. J Neurochem. 2011;116:260–272. doi: 10.1111/j.1471-4159.2010.07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Kalinchuk AV, McCarley RW, Stenberg D, Porkka-Heiskanen T, Basheer R. The role of cholinergic basal forebrain neurons in adenosine-mediated homeostatic control of sleep: lessons from 192 IgG-saporin lesions. Neuroscience. 2008;157:238–253. doi: 10.1016/j.neuroscience.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Kalinchuk AV, Stenberg D, Rosenberg PA, Porkka-Heiskanen T. Inducible and neuronal nitric oxide synthases (NOS) have complementary roles in recovery sleep induction. Eur J Neurosci. 2006;24:1443–1456. doi: 10.1111/j.1460-9568.2006.05019.x. [DOI] [PubMed] [Google Scholar]
- 594.Kalogiannis M, Grupke SL, Potter PE, Edwards JG, Chemelli RM, Kisanuki YY, Yanagisawa M, Leonard CS. Narcoleptic orexin receptor knockout mice express enhanced cholinergic properties in laterodorsal tegmental neurons. Eur J Neurosci. 2010;32:130–142. doi: 10.1111/j.1460-9568.2010.07259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Kameyama M, Yamaguchi I, Ichikawa K, Sugiyama T, Hirono M, Hori H, Ikeda M, Kuwahata Y, Eguchi N, Urade Y, Yoshioka T. Effect of phospholipase Cbeta4 lacking in thalamic neurons on electroencephalogram. Biochem Biophys Res Commun. 2003;304:153–159. doi: 10.1016/s0006-291x(03)00536-9. [DOI] [PubMed] [Google Scholar]
- 596.Kamondi A, Williams JA, Hutcheon B, Reiner PB. Membrane properties of mesopontine cholinergic neurons studied with the whole-cell patch-clamp technique: implications for behavioral state control. J Neurophysiol. 1992;68:1359–1372. doi: 10.1152/jn.1992.68.4.1359. [DOI] [PubMed] [Google Scholar]
- 597.Kanbayashi T, Kodama T, Kondo H, Satoh S, Inoue Y, Chiba S, Shimizu T, Nishino S. CSF histamine contents in narcolepsy, idiopathic hypersomnia and obstructive sleep apnea syndrome. Sleep. 2009;32:181–187. doi: 10.1093/sleep/32.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Kantor S, Mochizuki T, Janisiewicz AM, Clark E, Nishino S, Scammell TE. Orexin neurons are necessary for the circadian control of REM sleep. Sleep. 2009;32:1127–1134. doi: 10.1093/sleep/32.9.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Kapas L, Fang J, Krueger JM. Inhibition of nitric oxide synthesis inhibits rat sleep. Brain Res. 1994;664:189–196. doi: 10.1016/0006-8993(94)91969-0. [DOI] [PubMed] [Google Scholar]
- 600.Kapas L, Obal F, Jr, Book AA, Schweitzer JB, Wiley RG, Krueger JM. The effects of immunolesions of nerve growth factor-receptive neurons by 192 IgG-saporin on sleep. Brain Res. 1996;712:53–59. doi: 10.1016/0006-8993(95)01431-4. [DOI] [PubMed] [Google Scholar]
- 601.Kapas L, Shibata M, Kimura M, Krueger JM. Inhibition of nitric oxide synthesis suppresses sleep in rabbits. Am J Physiol Regul Integr Comp Physiol. 1994;266:R151–R157. doi: 10.1152/ajpregu.1994.266.1.R151. [DOI] [PubMed] [Google Scholar]
- 602.Kaplan PW. The EEG in metabolic encephalopathy and coma. J Clin Neurophysiol. 2004;21:307–318. [PubMed] [Google Scholar]
- 603.Kapsimalis F, Richardson G, Opp MR, Kryger M. Cytokines and normal sleep. Curr Opin Pulm Med. 2005;11:481–484. doi: 10.1097/01.mcp.0000183062.98665.6b. [DOI] [PubMed] [Google Scholar]
- 604.Karashima A, Katayama N, Nakao M. Enhancement of synchronization between hippocampal and amygdala theta waves associated with pontine wave density. J Neurophysiol. 2010;103:2318–2325. doi: 10.1152/jn.00551.2009. [DOI] [PubMed] [Google Scholar]
- 605.Karczmar AG, Longo VG, De Carolis AS. A pharmacological model of paradoxical sleep: the role of cholinergic and monoamine systems. Physiol Behav. 1970;5:175–182. doi: 10.1016/0031-9384(70)90061-2. [DOI] [PubMed] [Google Scholar]
- 606.Karlsson KA, Gall AJ, Mohns EJ, Seelke AM, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biol. 2005;3:e143. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 608.Kaslin J, Nystedt JM, Ostergard M, Peitsaro N, Panula P. The orexin/hypocretin system in zebrafish is connected to the aminergic and cholinergic systems. J Neurosci. 2004;24:2678–2689. doi: 10.1523/JNEUROSCI.4908-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Kattler H, Dijk DJ, Borbely AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–164. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 610.Kaufman LS, Morrison AR. Spontaneous and elicited PGO spikes in rats. Brain Res. 1981;214:61–72. doi: 10.1016/0006-8993(81)90438-8. [DOI] [PubMed] [Google Scholar]
- 611.Kaur S, Junek A, Black MA, Semba K. Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats. J Neurosci. 2008;28:491–504. doi: 10.1523/JNEUROSCI.1585-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Kaur S, Thankachan S, Begum S, Liu M, Blanco-Centurion C, Shiromani PJ. Hypocretin-2 saporin lesions of the ventrolateral periaquaductal gray (vlPAG) increase REM sleep in hypocretin knockout mice. PLoS One. 2009;4:e6346. doi: 10.1371/journal.pone.0006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Kawashima M, Lin L, Tanaka S, Jennum P, Knudsen S, Nevsimalova S, Plazzi G, Mignot E. Anti-Tribbles homolog 2 (TRIB2) autoantibodies in narcolepsy are associated with recent onset of cataplexy. Sleep. 2010;33:869–874. doi: 10.1093/sleep/33.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Kay A, Trinder J, Bowes G, Kim Y. Changes in airway resistance during sleep onset. J Appl Physiol. 1994;76:1600–1607. doi: 10.1152/jappl.1994.76.4.1600. [DOI] [PubMed] [Google Scholar]
- 615.Kayama Y, Ohta M, Jodo E. Firing of “possibly” cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569:210–220. doi: 10.1016/0006-8993(92)90632-j. [DOI] [PubMed] [Google Scholar]
- 616.Keating GM, Raffin MJ. Modafinil: a review of its use in excessive sleepiness associated with obstructive sleep apnoea/hypopnoea syndrome and shift work sleep disorder. CNS Drugs. 2005;19:785–803. doi: 10.2165/00023210-200519090-00005. [DOI] [PubMed] [Google Scholar]
- 617.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 618.Khateb A, Fort P, Alonso A, Jones BE, Muhlethaler M. Pharmacological and immunohistochemical evidence for serotonergic modulation of cholinergic nucleus basalis neurons. Eur J Neurosci. 1993;5:541–547. doi: 10.1111/j.1460-9568.1993.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 619.Khateb A, Fort P, Pegna A, Jones BE, Muhlethaler M. Cholinergic nucleus basalis neurons are excited by histamine in vitro. Neuroscience. 1995;69:495–506. doi: 10.1016/0306-4522(95)00264-j. [DOI] [PubMed] [Google Scholar]
- 620.Kilduff TS, Cauli B, Gerashchenko D. Activation of cortical interneurons during sleep: an anatomical link to homeostatic sleep regulation? Trends Neurosci. 2011;34:10–19. doi: 10.1016/j.tins.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 622.Killgore WD, Kahn-Greene ET, Lipizzi EL, Newman RA, Kamimori GH, Balkin TJ. Sleep deprivation reduces perceived emotional intelligence and constructive thinking skills. Sleep Med. 2008;9:517–526. doi: 10.1016/j.sleep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 623.Killgore WD, Kamimori GH, Balkin TJ. Caffeine protects against increased risk-taking propensity during severe sleep deprivation. J Sleep Res. 2010 doi: 10.1111/j.1365-2869.2010.00893.x. [DOI] [PubMed] [Google Scholar]
- 624.Killgore WD, Killgore DB, Day LM, Li C, Kamimori GH, Balkin TJ. The effects of 53 hours of sleep deprivation on moral judgment. Sleep. 2007;30:345–352. doi: 10.1093/sleep/30.3.345. [DOI] [PubMed] [Google Scholar]
- 625.Kim EJ, Jeong DU. Transdermal scopolamine alters phasic REM activity in normal young adults. Sleep. 1999;22:515–520. doi: 10.1093/sleep/22.4.515. [DOI] [PubMed] [Google Scholar]
- 626.Kim HC, Young T, Matthews CG, Weber SM, Woodward AR, Palta M. Sleep-disordered breathing and neuropsychological deficits. A population-based study. Am J Respir Crit Care Med. 1997;156:1813–1819. doi: 10.1164/ajrccm.156.6.9610026. [DOI] [PubMed] [Google Scholar]
- 627.Kimura K, Tachibana N, Kohyama J, Otsuka Y, Fukazawa S, Waki R. A discrete pontine ischemic lesion could cause REM sleep behavior disorder. Neurology. 2000;55:894–895. doi: 10.1212/wnl.55.6.894. [DOI] [PubMed] [Google Scholar]
- 628.King C, Henze DA, Leinekugel X, Buzsaki G. Hebbian modification of a hippocampal population pattern in the rat. J Physiol. 1999;521:159–167. doi: 10.1111/j.1469-7793.1999.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Kinney GG, Kocsis B, Vertes RP. Injections of excitatory amino acid antagonists into the median raphe nucleus produce hippocampal theta rhythm in the urethaneanesthetized rat. Brain Res. 1994;654:96–104. doi: 10.1016/0006-8993(94)91575-x. [DOI] [PubMed] [Google Scholar]
- 630.Kinney GG, Kocsis B, Vertes RP. Injections of muscimol into the median raphe nucleus produce hippocampal theta rhythm in the urethane anesthetized rat. Psychopharmacology. 1995;120:244–248. doi: 10.1007/BF02311170. [DOI] [PubMed] [Google Scholar]
- 631.Kinney GG, Kocsis B, Vertes RP. Medial septal unit firing characteristics following injections of 8-OH-DPAT into the median raphe nucleus. Brain Res. 1996;708:116–122. doi: 10.1016/0006-8993(95)01296-6. [DOI] [PubMed] [Google Scholar]
- 632.Kinney HC, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, White WF. Decreased muscarinic receptor binding in the arcuate nucleus in sudden infant death syndrome. Science. 1995;269:1446–1450. doi: 10.1126/science.7660131. [DOI] [PubMed] [Google Scholar]
- 633.Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 2009;4:517–550. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Kirk IJ. Supramammillary neural discharge patterns and hippocampal EEG. Brain Res Bull. 1997;42:23–26. doi: 10.1016/s0361-9230(96)00094-9. [DOI] [PubMed] [Google Scholar]
- 636.Kirk IJ. Frequency modulation of hippocampal theta by the supramammillary nucleus, and other hypothalamo-hippocampal interactions: mechanisms and functional implications. Neurosci Biobehav Rev. 1998;22:291–302. doi: 10.1016/s0149-7634(97)00015-8. [DOI] [PubMed] [Google Scholar]
- 637.Kirk IJ, McNaughton N. Supramammillary cell firing and hippocampal rhythmical slow activity. Neuroreport. 1991;2:723–725. doi: 10.1097/00001756-199111000-00023. [DOI] [PubMed] [Google Scholar]
- 638.Kirk IJ, McNaughton N. Mapping the differential effects of procaine on frequency and amplitude of reticularly elicited hippocampal rhythmical slow activity. Hippocampus. 1993;3:517–525. doi: 10.1002/hipo.450030411. [DOI] [PubMed] [Google Scholar]
- 639.Kirk IJ, Oddie SD, Konopacki J, Bland BH. Evidence for differential control of posterior hypothalamic, supramammillary, and medial mammillary theta-related cellular discharge by ascending and descending pathways. J Neurosci. 1996;16:5547–5554. doi: 10.1523/JNEUROSCI.16-17-05547.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Kisanuki YY, Chemelli RM, Tokita S, Willie JT, Sinton CM, Yanagisawa Y. Behavioral and polysomnographic characterization of orexin-1 receptor and orexin-2 receptor double knockout mice. Sleep. 2001;24:A22. [Google Scholar]
- 641.Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Kiyono S, Seo ML, Shibagaki M, Watanabe T, Maeyama K, Wada H. Effects of alpha-fluoromethylhistidine on sleep-waking parameters in rats. Physiol Behav. 1985;34:615–617. doi: 10.1016/0031-9384(85)90057-5. [DOI] [PubMed] [Google Scholar]
- 643.Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 644.Kleinlogel H. Effects of the selective alpha 1-adrenoceptor blocker prazosin on EEG sleep and waking stages in the rat. Neuropsychobiology. 1989;21:100–103. doi: 10.1159/000118560. [DOI] [PubMed] [Google Scholar]
- 645.Kleitman N, Engelmann TG. Sleep characteristics of infants. J Appl Physiol. 1953;6:269–282. doi: 10.1152/jappl.1953.6.5.269. [DOI] [PubMed] [Google Scholar]
- 646.Klink R, de Kerchove dA, Zoli Changeux MJP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Knoche A, Yokoyama H, Ponomarenko A, Frisch C, Huston J, Haas HL. Highfrequency oscillation in the hippocampus of the behaving rat and its modulation by the histaminergic system. Hippocampus. 2003;13:273–280. doi: 10.1002/hipo.10057. [DOI] [PubMed] [Google Scholar]
- 648.Kocsis B, Di Prisco GV, Vertes RP. Theta synchronization in the limbic system: the role of Gudden’s tegmental nuclei. Eur J Neurosci. 2001;13:381–388. [PubMed] [Google Scholar]
- 649.Kocsis B, Vertes RP. Characterization of neurons of the supramammillary nucleus and mammillary body that discharge rhythmically with the hippocampal theta rhythm in the rat. J Neurosci. 1994;14:7040–7052. doi: 10.1523/JNEUROSCI.14-11-07040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Koh K, Joiner WJ, Wu MN, Yue Z, Smith CJ, Sehgal A. Identification of SLEEPLESS, a sleep-promoting factor. Science. 2008;321:372–376. doi: 10.1126/science.1155942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Kohlmeier KA, Watanabe S, Tyler CJ, Burlet S, Leonard CS. Dual orexin actions on dorsal raphe and laterodorsal tegmentum neurons: noisy cation current activation and selective enhancement of Ca2+transients mediated by L-type calcium channels. J Neurophysiol. 2008;100:2265–2281. doi: 10.1152/jn.01388.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Kohyama J, Lai YY, Siegel JM. Inactivation of the pons blocks medullary-induced muscle tone suppression in the decerebrate cat. Sleep. 1998;21:695–699. doi: 10.1093/sleep/21.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Kopp C, Albrecht U, Zheng B, Tobler I. Homeostatic sleep regulation is preserved in mPer1 and mPer2 mutant mice. Eur J Neurosci. 2002;16:1099–1106. doi: 10.1046/j.1460-9568.2002.02156.x. [DOI] [PubMed] [Google Scholar]
- 655.Kopp C, Longordo F, Nicholson JR, Luthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J Neurosci. 2006;26:12456–12465. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Kopp C, Rudolph U, Keist R, Tobler I. Diazepam-induced changes on sleep and the EEG spectrum in mice: role of the alpha3-GABA(A) receptor subtype. Eur J Neurosci. 2003;17:2226–2230. doi: 10.1046/j.1460-9568.2003.02651.x. [DOI] [PubMed] [Google Scholar]
- 657.Kopp C, Rudolph U, Low K, Tobler I. Modulation of rhythmic brain activity by diazepam: GABA(A) receptor subtype and state specificity. Proc Natl Acad Sci USA. 2004;101:3674–3679. doi: 10.1073/pnas.0306975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- 659.Korotkova TM, Haas HL, Brown RE. Histamine excites GABAergic cells in the rat substantia nigra and ventral tegmental area in vitro. Neurosci Lett. 2002;320:133–136. doi: 10.1016/s0304-3940(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 660.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Kostin A, Rai S, Kumar S, Szymusiak R, McGinty D, Alam MN. Nitric oxide production in the perifornical-lateral hypothalamic area and its influences on the modulation of perifornical-lateral hypothalamic area neurons. Neuroscience. 2011;179:159–169. doi: 10.1016/j.neuroscience.2011.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Kostin A, Stenberg D, Kalinchuk AV, Porkka-Heiskanen T. Nitric oxide modulates the discharge rate of basal forebrain neurons. Psychopharmacology. 2008;201:147–160. doi: 10.1007/s00213-008-1257-x. [DOI] [PubMed] [Google Scholar]
- 663.Kostin A, Stenberg D, Porkka-Heiskanen T. Nitric oxide modulates the discharge rate of basal forebrain neurones: a study in freely moving rats. J Sleep Res. 2009;18:447–453. doi: 10.1111/j.1365-2869.2009.00761.x. [DOI] [PubMed] [Google Scholar]
- 664.Kovacs KJ. Measurement of immediate-early gene activation: c-fos and beyond. J Neuroendocrinol. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 665.Koyama Y, Sakai K. Modulation of presumed cholinergic mesopontine tegmental neurons by acetylcholine and monoamines applied iontophoretically in unanesthetized cats. Neuroscience. 2000;96:723–733. doi: 10.1016/s0306-4522(00)00004-x. [DOI] [PubMed] [Google Scholar]
- 666.Koyama Y, Takahashi K, Kodama T, Kayama Y. State-dependent activity of neurons in the perifornical hypothalamic area during sleep and waking. Neuroscience. 2003;119:1209–1219. doi: 10.1016/s0306-4522(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 667.Kramis R, Vanderwolf CH, Bland BH. Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Exp Neurol. 1975;49:58–85. doi: 10.1016/0014-4886(75)90195-8. [DOI] [PubMed] [Google Scholar]
- 668.Krenzer M, Anaclet C, Vetrivelan R, Wang N, Vong L, Lowell BB, Fuller PM, Lu J. Brainstem and spinal cord circuitry regulating REM sleep and muscle atonia. PLoS One. 2011;6:e24998. doi: 10.1371/journal.pone.0024998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14:3408–3416. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Krueger JM, Rector DM, Churchill L. Sleep and cytokines. Sleep Med Clin. 2007;2:161–169. doi: 10.1016/j.jsmc.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Kryger MH. Management of obstructive sleep apnea-hypopnea syndrome: overview. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia, PA: Saunders; 2000. pp. 940–954. [Google Scholar]
- 673.Kryger MH, Otake K, Foerster J. Low body stores of iron and restless legs syndrome: a correctable cause of insomnia in adolescents and teenagers. Sleep Med. 2002;3:127–132. doi: 10.1016/s1389-9457(01)00160-5. [DOI] [PubMed] [Google Scholar]
- 674.Krystal AD, Davidson JR. The use of prazosin for the treatment of trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:925–927. doi: 10.1016/j.biopsych.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 675.Kubin L. Carbachol models of REM sleep: recent developments and new directions. Arch Ital Biol. 2001;139:147–168. [PubMed] [Google Scholar]
- 676.Kudo Y, Kurihara M. Clinical evaluation of diphenhydramine hydrochloride for the treatment of insomnia in psychiatric patients: a double-blind study. J Clin Pharmacol. 1990;30:1041–1048. doi: 10.1002/j.1552-4604.1990.tb03592.x. [DOI] [PubMed] [Google Scholar]
- 677.Kukko-Lukjanov TK, Panula P. Subcellular distribution of histamine, GABA and galanin in tuberomamillary neurons in vitro. J Chem Neuroanat. 2003;25:279–292. doi: 10.1016/s0891-0618(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 678.Kumar R, Birrer BV, Macey PM, Woo MA, Gupta RK, Yan-Go FL, Harper RM. Reduced mammillary body volume in patients with obstructive sleep apnea. Neurosci Lett. 2008;438:330–334. doi: 10.1016/j.neulet.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 679.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25:7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Lacey MG, Calabresi P, North RA. Muscarine depolarizes rat substantia nigra zona compacta and ventral tegmental neurons in vitro through M1-like receptors. J Pharmacol Exp Ther. 1990;253:395–400. [PubMed] [Google Scholar]
- 681.Lai YY, Clements JR, Wu XY, Shalita T, Wu JP, Kuo JS, Siegel JM. Brainstem projections to the ventromedial medulla in cat: retrograde transport horseradish peroxidase and immunohistochemical studies. J Comp Neurol. 1999;408:419–436. doi: 10.1002/(sici)1096-9861(19990607)408:3<419::aid-cne8>3.0.co;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Lai YY, Hsieh KC, Nguyen D, Peever J, Siegel JM. Neurotoxic lesions at the ventral mesopontine junction change sleep time and muscle activity during sleep: an animal model of motor disorders in sleep. Neuroscience. 2008;154:431–443. doi: 10.1016/j.neuroscience.2008.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Lai YY, Shalita T, Hajnik T, Wu JP, Kuo JS, Chia LG, Siegel JM. Neurotoxic N-methyl-d-aspartate lesion of the ventral midbrain and mesopontine junction alters sleep-wake organization. Neuroscience. 1999;90:469–483. doi: 10.1016/s0306-4522(98)00429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Lai YY, Siegel JM. Medullary regions mediating atonia. J Neurosci. 1988;8:4790–4796. doi: 10.1523/JNEUROSCI.08-12-04790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Lambe EK, Aghajanian GK. Hypocretin (orexin) induces calcium transients in single spines postsynaptic to identified thalamocortical boutons in prefrontal slice. Neuron. 2003;40:139–150. doi: 10.1016/s0896-6273(03)00598-1. [DOI] [PubMed] [Google Scholar]
- 686.Lamberty Y, Margineanu DG, Dassesse D, Klitgaard H. H3 agonist immepip markedly reduces cortical histamine release, but only weakly promotes sleep in the rat. Pharmacol Res. 2003;48:193–198. doi: 10.1016/s1043-6618(03)00094-x. [DOI] [PubMed] [Google Scholar]
- 687.Landolt HP. Sleep homeostasis: a role for adenosine in humans? Biochem Pharmacol. 2008;75:2070–2079. doi: 10.1016/j.bcp.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 688.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–1374. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 689.Laposky AD, Homanics GE, Basile A, Mendelson WB. Deletion of the GABA(A) receptor beta 3 subunit eliminates the hypnotic actions of oleamide in mice. Neuroreport. 2001;12:4143–4147. doi: 10.1097/00001756-200112210-00056. [DOI] [PubMed] [Google Scholar]
- 690.Laurent JP, Guerrero FA, Jouvet M. Reversible suppression of the geniculate PGO waves and of the concomitant increase of excitability of the intrageniculate optic nerve terminals in cats. Brain Res. 1974;81:558–563. doi: 10.1016/0006-8993(74)90852-x. [DOI] [PubMed] [Google Scholar]
- 691.Laurent JP, Rondouin G, Benita M, Jouvet M. Reversible blockade of PGO waves and concomitant modifications of thalamic unit activity in chronic cats. Brain Res. 1977;137:305–322. doi: 10.1016/0006-8993(77)90341-9. [DOI] [PubMed] [Google Scholar]
- 692.Lauriello J, Kenny WM, Sutton L, Golshan S, Ruiz C, Kelsoe J, Rapaport M, Gillin JC. The cholinergic REM sleep induction test with pilocarpine in mildly depressed patients and normal controls. Biol Psychiatry. 1993;33:33–39. doi: 10.1016/0006-3223(93)90275-i. [DOI] [PubMed] [Google Scholar]
- 693.Lavie L, Polotsky V. Cardiovascular aspects in obstructive sleep apnea syndrome: molecular issues, hypoxia and cytokine profiles. Respiration. 2009;78:361–370. doi: 10.1159/000243552. [DOI] [PubMed] [Google Scholar]
- 694.Lazarus M, Shen HY, Cherasse Y, Qu WM, Huang ZL, Bass CE, Winsky-Sommerer R, Semba K, Fredholm BB, Boison D, Hayaishi O, Urade Y, Chen JF. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci. 2011;31:10067–10075. doi: 10.1523/JNEUROSCI.6730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Lee J, Kim D, Shin HS. Lack of delta waves and sleep disturbances during non-rapid eye movement sleep in mice lacking alpha1G-subunit of T-type calcium channels. Proc Natl Acad Sci USA. 2004;101:18195–18199. doi: 10.1073/pnas.0408089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Lee KH, McCormick DA. Abolition of spindle oscillations by serotonin and norepinephrine in the ferret lateral geniculate and perigeniculate nuclei in vitro. Neuron. 1996;17:309–321. doi: 10.1016/s0896-6273(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 697.Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 698.Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25:4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Lee RS, Steffensen SC, Henriksen SJ. Discharge profiles of ventral tegmental area GABA neurons during movement, anesthesia, and the sleep-wake cycle. J Neurosci. 2001;21:1757–1766. doi: 10.1523/JNEUROSCI.21-05-01757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Legendre R, Peiron H. Recherches sur le besoin de sommeil consecutive a une vielle prolongee. Z Allgem Physiol. 1913;14:235–262. [Google Scholar]
- 702.Leger L, Gay N, Burlet S, Charnay Y, Cespuglio R. Localization of nitric oxidesynthesizing neurons sending projections to the dorsal raphe nucleus of the rat. Neurosci Lett. 1998;257:147–150. doi: 10.1016/s0304-3940(98)00826-x. [DOI] [PubMed] [Google Scholar]
- 703.Lena C, Popa D, Grailhe R, Escourrou P, Changeux JP, Adrien J. Beta2-containing nicotinic receptors contribute to the organization of sleep and regulate putative micro-arousals in mice. J Neurosci. 2004;24:5711–5718. doi: 10.1523/JNEUROSCI.3882-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Lena I, Parrot S, Deschaux O, Muffat-Joly S, Sauvinet V, Renaud B, Suaud-Chagny MF, Gottesmann C. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep-wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J Neurosci Res. 2005;81:891–899. doi: 10.1002/jnr.20602. [DOI] [PubMed] [Google Scholar]
- 705.Leonard CS, Kerman I, Blaha G, Taveras E, Taylor B. Interdigitation of nitric oxide synthase-, tyrosine hydroxylase-, and serotonin-containing neurons in and around the laterodorsal and pedunculopontine tegmental nuclei of the guinea pig. J Comp Neurol. 1995;362:411–432. doi: 10.1002/cne.903620309. [DOI] [PubMed] [Google Scholar]
- 706.Leonard CS, Llinas R. Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study. Neuroscience. 1994;59:309–330. doi: 10.1016/0306-4522(94)90599-1. [DOI] [PubMed] [Google Scholar]
- 707.Leonard CS, Michaelis EK, Mitchell KM. Activity-dependent nitric oxide concentration dynamics in the laterodorsal tegmental nucleus in vitro. J Neurophysiol. 2001;86:2159–2172. doi: 10.1152/jn.2001.86.5.2159. [DOI] [PubMed] [Google Scholar]
- 708.Leonard TO, Lydic R. Nitric oxide synthase inhibition decreases pontine acetylcholine release. Neuroreport. 1995;6:1525–1529. doi: 10.1097/00001756-199507310-00015. [DOI] [PubMed] [Google Scholar]
- 709.Leonard TO, Lydic R. Pontine nitric oxide modulates acetylcholine release, rapid eye movement sleep generation, and respiratory rate. J Neurosci. 1997;17:774–785. doi: 10.1523/JNEUROSCI.17-02-00774.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Leranth C, Kiss J. A population of supramammillary area calretinin neurons terminating on medial septal area cholinergic and lateral septal area calbindin-containing cells are aspartate/glutamatergic. J Neurosci. 1996;16:7699–7710. doi: 10.1523/JNEUROSCI.16-23-07699.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Leresche N, Jassik-Gerschenfeld D, Haby M, Soltesz I, Crunelli V. Pacemaker-like and other types of spontaneous membrane potential oscillations of thalamocortical cells. Neurosci Lett. 1990;113:72–77. doi: 10.1016/0304-3940(90)90497-w. [DOI] [PubMed] [Google Scholar]
- 712.Levant B, McCarson KE. D(3) dopamine receptors in rat spinal cord: implications for sensory and motor function. Neurosci Lett. 2001;303:9–12. doi: 10.1016/s0304-3940(01)01692-5. [DOI] [PubMed] [Google Scholar]
- 713.Levine ES, Jacobs BL. Neurochemical afferents controlling the activity of serotonergic neurons in the dorsal raphe nucleus: microiontophoretic studies in the awake cat. J Neurosci. 1992;12:4037–4044. doi: 10.1523/JNEUROSCI.12-10-04037.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Li X, Rainnie DG, McCarley RW, Greene RW. Presynaptic nicotinic receptors facilitate monoaminergic transmission. J Neurosci. 1998;18:1904–1912. doi: 10.1523/JNEUROSCI.18-05-01904.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715.Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 716.Li Y, van den Pol AN. Direct and indirect inhibition by catecholamines of hypocretin/ orexin neurons. J Neurosci. 2005;25:173–183. doi: 10.1523/JNEUROSCI.4015-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Li Y, van den Pol AN. Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci. 2006;26:13037–13047. doi: 10.1523/JNEUROSCI.3380-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Liao F, Taishi P, Churchill L, Urza MJ, Krueger JM. Localized suppression of cortical growth hormone-releasing hormone receptors state-specifically attenuates electroencephalographic delta waves. J Neurosci. 2010;30:4151–4159. doi: 10.1523/JNEUROSCI.6047-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 720.Lim AS, Lozano AM, Moro E, Hamani C, Hutchison WD, Dostrovsky JO, Lang AE, Wennberg RA, Murray BJ. Characterization of REM-sleep associated ponto-geniculo- occipital waves in the human pons. Sleep. 2007;30:823–827. doi: 10.1093/sleep/30.7.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Lim AS, Scammell TE. The trouble with Tribbles: do antibodies against TRIB2 cause narcolepsy? Sleep. 2010;33:857–858. doi: 10.1093/sleep/33.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Lin JS. Brain structures and mechanisms involved in the control of cortical activation and wakefulness, with emphasis on the posterior hypothalamus and histaminergic neurons. Sleep Med Rev. 2000;4:471–503. doi: 10.1053/smrv.2000.0116. [DOI] [PubMed] [Google Scholar]
- 723.Lin JS, Dauvilliers Y, Arnulf I, Bastuji H, Anaclet C, Parmentier R, Kocher L, Yanagisawa M, Lehert P, Ligneau X, Perrin D, Robert P, Roux M, Lecomte JM, Schwartz JC. An inverse agonist of the histamine H(3) receptor improves wakefulness in narcolepsy: studies in orexin−/− mice and patients. Neurobiol Dis. 2008;30:74–83. doi: 10.1016/j.nbd.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 724.Lin JS, Sakai K, Jouvet M. Evidence for histaminergic arousal mechanisms in the hypothalamus of cat. Neuropharmacology. 1988;27:111–122. doi: 10.1016/0028-3908(88)90159-1. [DOI] [PubMed] [Google Scholar]
- 725.Lin JS, Sakai K, Vanni Mercier G, Arrang JM, Garbarg M, Schwartz JC, Jouvet M. Involvement of histaminergic neurons in arousal mechanisms demonstrated with H3-receptor ligands in the cat. Brain Res. 1990;523:325–330. doi: 10.1016/0006-8993(90)91508-e. [DOI] [PubMed] [Google Scholar]
- 726.Lin JS, Sakai K, Vanni MG, Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479:225–240. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- 727.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de JP, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 728.Lin L, Hungs M, Mignot E. Narcolepsy and the HLA region. J Neuroimmunol. 2001;117:9–20. doi: 10.1016/s0165-5728(01)00333-2. [DOI] [PubMed] [Google Scholar]
- 729.Lin SC, Gervasoni D, Nicolelis MA. Fast modulation of prefrontal cortex activity by basal forebrain noncholinergic neuronal ensembles. J Neurophysiol. 2006;96:3209–3219. doi: 10.1152/jn.00524.2006. [DOI] [PubMed] [Google Scholar]
- 730.Lindsley DB, Bowden JW, Magoun HW. Effect upon the EEG of acute injury to the brain stem activating system. Electroencephalogr Clin Neurophysiol. 1949;1:475–486. [PubMed] [Google Scholar]
- 731.Lindvall O, Bjorklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand, Suppl. 1974;412:1–48. [PubMed] [Google Scholar]
- 732.Lisman J. The theta/gamma discrete phase code occuring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus. 2005;15:913–922. doi: 10.1002/hipo.20121. [DOI] [PubMed] [Google Scholar]
- 733.Liu M, Blanco-Centurion C, Konadhode R, Begum S, Pelluru D, Gerashchenko D, Sakurai T, Yanagisawa M, van den Pol AN, Shiromani PJ. Orexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic mice. J Neurosci. 2011;31:6028–6040. doi: 10.1523/JNEUROSCI.6069-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Liu M, Thankachan S, Kaur S, Begum S, Blanco-Centurion C, Sakurai T, Yanagisawa M, Neve R, Shiromani PJ. Orexin (hypocretin) gene transfer diminishes narcoleptic sleep behavior in mice. Eur J Neurosci. 2008;28:1382–1393. doi: 10.1111/j.1460-9568.2008.06446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Liu YW, Li J, Ye JH. Histamine regulates activities of neurons in the ventrolateral preoptic nucleus. J Physiol. 2010;588:4103–4116. doi: 10.1113/jphysiol.2010.193904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J Neurosci. 2010;30:8671–8675. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Liu ZW, Gao XB. Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypothalamus: a possible sleep-promoting effect. J Neurophysiol. 2007;97:837–848. doi: 10.1152/jn.00873.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 739.Llinas R, Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci USA. 1993;90:2078–2081. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Llinas R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Llinas RR, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc Natl Acad Sci USA. 2002;99:449–454. doi: 10.1073/pnas.012604899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Lo CC, Chou T, Penzel T, Scammell TE, Strecker RE, Stanley HE, Ivanov PC. Common scale-invariant patterns of sleep-wake transitions across mammalian species. Proc Natl Acad Sci USA. 2004;101:17545–17548. doi: 10.1073/pnas.0408242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci USA. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Longo VG. Behavioral and electroencephalographic effects of atropine and related compounds. Pharmacol Rev. 1966;18:965–996. [PubMed] [Google Scholar]
- 745.Longordo F, Kopp C, Luthi A. Consequences of sleep deprivation on neurotransmitter receptor expression and function. Eur J Neurosci. 2009;29:1810–1819. doi: 10.1111/j.1460-9568.2009.06719.x. [DOI] [PubMed] [Google Scholar]
- 746.Longordo F, Kopp C, Mishina M, Lujan R, Luthi A. NR2A at CA1 synapses is obligatory for the susceptibility of hippocampal plasticity to sleep loss. J Neurosci. 2009;29:9026–9041. doi: 10.1523/JNEUROSCI.1215-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 747.Lopes da Silva FH, Storm Van LW. The cortical source of the alpha rhythm. Neurosci Lett. 1977;6:237–241. doi: 10.1016/0304-3940(77)90024-6. [DOI] [PubMed] [Google Scholar]
- 748.Lopes da Silva FH, Vos JE, Mooibroek J, Van RA. Relative contributions of intracortical and revealed by partial coherence analysis thalamo-cortical processes in the generation of alpha rhythms. Electroencephalogr Clin Neurophysiol. 1980;50:449–456. doi: 10.1016/0013-4694(80)90011-5. [DOI] [PubMed] [Google Scholar]
- 749.Lopez J, Roffwarg HP, Dreher A, Bissette G, Karolewicz B, Shaffery JP. Rapid eye movement sleep deprivation decreases long-term potentiation stability and affects some glutamatergic signaling proteins during hippocampal development. Neuroscience. 2008;153:44–53. doi: 10.1016/j.neuroscience.2008.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Lorente de No R. Cerebral cortex: architecture, intracortical connections, motor projections. In: Fulton J, editor. Physiology of the Nervous System. London: Oxford Univ. Press; 1938. pp. 291–340. [Google Scholar]
- 751.Lorincz ML, Crunelli V, Hughes SW. Cellular dynamics of cholinergically induced alpha (8–13 Hz) rhythms in sensory thalamic nuclei in vitro. J Neurosci. 2008;28:660–671. doi: 10.1523/JNEUROSCI.4468-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Lorincz ML, Kekesi KA, Juhasz G, Crunelli V, Hughes SW. Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron. 2009;63:683–696. doi: 10.1016/j.neuron.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 754.Lovblad KO, Thomas R, Jakob PM, Scammell T, Bassetti C, Griswold M, Ives J, Matheson J, Edelman RR, Warach S. Silent functional magnetic resonance imaging demonstrates focal activation in rapid eye movement sleep. Neurology. 1999;53:2193–2195. doi: 10.1212/wnl.53.9.2193. [DOI] [PubMed] [Google Scholar]
- 755.Lu J, Bjorkum AA, Xu M, Gaus SE, Shiromani PJ, Saper CB. Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci. 2002;22:4568–4576. doi: 10.1523/JNEUROSCI.22-11-04568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 756.Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757.Lu J, Jhou TC, Saper CB. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci. 2006;26:193–202. doi: 10.1523/JNEUROSCI.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 759.Lu J, Shiromani P, Saper CB. Retinal input to the sleep-active ventrolateral preoptic nucleus in the rat. Neuroscience. 1999;93:209–214. doi: 10.1016/s0306-4522(99)00094-9. [DOI] [PubMed] [Google Scholar]
- 760.Lu JW, Mann GL, Ross RJ, Morrison AR, Kubin L. Differential effect of sleep-wake states on lingual and dorsal neck muscle activity in rats. Respir Physiol Neurobiol. 2005;147:191–203. doi: 10.1016/j.resp.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 761.Lucas EA, Sterman MB. Effect of a forebrain lesion on the polycyclic sleep-wake cycle and sleep-wake patterns in the cat. Exp Neurol. 1975;46:368–388. doi: 10.1016/0014-4886(75)90142-9. [DOI] [PubMed] [Google Scholar]
- 762.Lue FA, Bail M, Jephthah-Ochola J, Carayanniotis K, Gorczynski R, Moldofsky H. Sleep and cerebrospinal fluid interleukin-1-like activity in the cat. Int J Neurosci. 1988;42:179–183. doi: 10.3109/00207458808991595. [DOI] [PubMed] [Google Scholar]
- 763.Luebke JI, Greene RW, Semba K, Kamondi A, McCarley RW, Reiner PB. Serotonin hyperpolarizes cholinergic low-threshold burst neurons in the rat laterodorsal tegmental nucleus in vitro. Proc Natl Acad Sci USA. 1992;89:743–747. doi: 10.1073/pnas.89.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.Luo L, MacLean DB. Effects of thyroid hormone on food intake, hypothalamic Na/K ATPase activity and ATP content. Brain Res. 2003;973:233–239. doi: 10.1016/s0006-8993(03)02514-9. [DOI] [PubMed] [Google Scholar]
- 765.Luppi PH, Clement O, Sapin E, Gervasoni D, Peyron C, Leger L, Salvert D, Fort P. The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Med Rev. 2011;15:153–163. doi: 10.1016/j.smrv.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 766.Lyamin OI, Mukhametov LM, Siegel JM, Nazarenko EA, Polyakova IG, Shpak OV. Unihemispheric slow wave sleep and the state of the eyes in a white whale. Behav Brain Res. 2002;129:125–129. doi: 10.1016/s0166-4328(01)00346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.Lydic R. The motor atonia of REM sleep: a critical topics forum. Introduction Sleep. 2008;31:1471–1472. doi: 10.1093/sleep/31.11.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 768.Lydic R, McCarley RW, Hobson JA. The time-course of dorsal raphe discharge, PGO waves, and muscle tone averaged across multiple sleep cycles. Brain Res. 1983;274:365–370. doi: 10.1016/0006-8993(83)90720-5. [DOI] [PubMed] [Google Scholar]
- 769.Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, Harper RK, Yan-Go FL, Harper RM. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:1382–1387. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- 770.Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–977. [PMC free article] [PubMed] [Google Scholar]
- 771.Mackiewicz M, Paigen B, Naidoo N, Pack AI. Analysis of the QTL for sleep homeostasis in mice: Homer1a is a likely candidate. Physiol Genomics. 2008;33:91–99. doi: 10.1152/physiolgenomics.00189.2007. [DOI] [PubMed] [Google Scholar]
- 772.Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, Baldwin DA, Jensen ST, Churchill GA, Pack AI. Macromolecule biosynthesis: a key function of sleep. Physiol Genomics. 2007;31:441–457. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- 773.MacLean DB, Luo LG. Increased ATP content/production in the hypothalamus may be a signal for energy-sensing of satiety: studies of the anorectic mechanism of a plant steroidal glycoside. Brain Res. 2004;1020:1–11. doi: 10.1016/j.brainres.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 774.Madsen PL, Vorstrup S. Cerebral blood flow and metabolism during sleep. Cerebrovasc Brain Metab Rev. 1991;3:281–296. [PubMed] [Google Scholar]
- 775.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776.Magoun HW, Rhines R. An inhibitory mechanism in the bulbar reticular formation. J Neurophysiol. 1946;9:165–171. doi: 10.1152/jn.1946.9.3.165. [DOI] [PubMed] [Google Scholar]
- 777.Mahon S, Vautrelle N, Pezard L, Slaght SJ, Deniau JM, Chouvet G, Charpier S. Distinct patterns of striatal medium spiny neuron activity during the natural sleep-wake cycle. J Neurosci. 2006;26:12587–12595. doi: 10.1523/JNEUROSCI.3987-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778.Mahowald MW. What state dissociation can teach us about consciousness and the function of sleep. Sleep Med. 2009;10:159–160. doi: 10.1016/j.sleep.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 779.Mahowald MW, Schenck CH. Rem sleep without atonia–from cats to humans. Arch Ital Biol. 2004;142:469–478. [PubMed] [Google Scholar]
- 780.Mahowald MW, Schenck CH. Insights from studying human sleep disorders. Nature. 2005;437:1279–1285. doi: 10.1038/nature04287. [DOI] [PubMed] [Google Scholar]
- 781.Maier N, Guldenagel M, Sohl G, Siegmund H, Willecke K, Draguhn A. Reduction of high-frequency network oscillations (ripples) and pathological network discharges in hippocampal slices from connexin 36-deficient mice. J Physiol. 2002;541:521–528. doi: 10.1113/jphysiol.2002.017624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.Makela JP, Hilakivi IT. Effect of alpha-adrenoceptor blockade on sleep and wakefulness in the rat. Pharmacol Biochem Behav. 1986;24:613–616. doi: 10.1016/0091-3057(86)90566-6. [DOI] [PubMed] [Google Scholar]
- 783.Mallick BN, Siegel JM, Fahringer H. Changes in pontine unit activity with REM sleep deprivation. Brain Res. 1990;515:94–98. doi: 10.1016/0006-8993(90)90581-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Maloney KJ, Mainville L, Jones BE. Differential c-Fos expression in cholinergic, monoaminergic, and GABAergic cell groups of the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery. J Neurosci. 1999;19:3057–3072. doi: 10.1523/JNEUROSCI.19-08-03057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 785.Maloney KJ, Mainville L, Jones BE. c-Fos expression in GABAergic, serotonergic, and other neurons of the pontomedullary reticular formation and raphe after paradoxical sleep deprivation and recovery. J Neurosci. 2000;20:4669–4679. doi: 10.1523/JNEUROSCI.20-12-04669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.Maloney KJ, Mainville L, Jones BE. c-Fos expression in dopaminergic and GABAergic neurons of the ventral mesencephalic tegmentum after paradoxical sleep deprivation and recovery. Eur J Neurosci. 2002;15:774–778. doi: 10.1046/j.1460-9568.2002.01907.x. [DOI] [PubMed] [Google Scholar]
- 787.Mander BA, Reid KJ, Baron KG, Tjoa T, Parrish TB, Paller KA, Gitelman DR, Zee PC. EEG measures index neural and cognitive recovery from sleep deprivation. J Neurosci. 2010;30:2686–2693. doi: 10.1523/JNEUROSCI.4010-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 788.Manfridi A, Brambilla D, Bianchi S, Mariotti M, Opp MR, Imeri L. Interleukin-1beta enhances non-rapid eye movement sleep when microinjected into the dorsal raphe nucleus and inhibits serotonergic neurons in vitro. Eur J Neurosci. 2003;18:1041–1049. doi: 10.1046/j.1460-9568.2003.02836.x. [DOI] [PubMed] [Google Scholar]
- 789.Manns ID, Alonso A, Jones BE. Rhythmically discharging basal forebrain units comprise cholinergic, GABAergic, and putative glutamatergic cells. J Neurophysiol. 2003;89:1057–1066. doi: 10.1152/jn.00938.2002. [DOI] [PubMed] [Google Scholar]
- 790.Manzoni OJ, Manabe T, Nicoll RA. Release of adenosine by activation of NMDA receptors in the hippocampus. Science. 1994;265:2098–2101. doi: 10.1126/science.7916485. [DOI] [PubMed] [Google Scholar]
- 791.Maquet P. Sleep function(s) and cerebral metabolism. Behav Brain Res. 1995;69:75–83. doi: 10.1016/0166-4328(95)00017-n. [DOI] [PubMed] [Google Scholar]
- 792.Maquet P, Peters J, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- 793.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 794.Marek GJ, Aghajanian GK. The electrophysiology of prefrontal serotonin systems: therapeutic implications for mood and psychosis. Biol Psychiatry. 1998;44:1118–1127. doi: 10.1016/s0006-3223(98)00036-5. [DOI] [PubMed] [Google Scholar]
- 795.Maret S, Dorsaz S, Gurcel L, Pradervand S, Petit B, Pfister C, Hagenbuchle O, O’Hara BF, Franken P, Tafti M. Homer1a is a core brain molecular correlate of sleep loss. Proc Natl Acad Sci USA. 2007;104:20090–20095. doi: 10.1073/pnas.0710131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Maret S, Franken P, Dauvilliers Y, Ghyselinck NB, Chambon P, Tafti M. Retinoic acid signaling affects cortical synchrony during sleep. Science. 2005;310:111–113. doi: 10.1126/science.1117623. [DOI] [PubMed] [Google Scholar]
- 797.Marks CA, Wayner MJ. Effects of sleep disruption on rat dentate granule cell LTP in vivo. Brain Res Bull. 2005;66:114–119. doi: 10.1016/j.brainresbull.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 798.Marks GA, Birabil CG. Enhancement of rapid eye movement sleep in the rat by cholinergic and adenosinergic agonists infused into the pontine reticular formation. Neuroscience. 1998;86:29–37. doi: 10.1016/s0306-4522(98)00005-0. [DOI] [PubMed] [Google Scholar]
- 799.Marks GA, Birabil CG. Infusion of adenylyl cyclase inhibitor SQ22,536 into the medial pontine reticular formation of rats enhances rapid eye movement sleep. Neuroscience. 2000;98:311–315. doi: 10.1016/s0306-4522(00)00093-2. [DOI] [PubMed] [Google Scholar]
- 800.Marks GA, Birabil CG. Carbachol induction of REM sleep in the rat is more effective at lights-out than lights-on. Brain Res. 2007;1142:127–134. doi: 10.1016/j.brainres.2007.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Marks GA, Farber J, Roffwarg HP. Metencephalic localization of ponto-geniculooccipital waves in the albino rat. Exp Neurol. 1980;69:667–677. doi: 10.1016/0014-4886(80)90059-x. [DOI] [PubMed] [Google Scholar]
- 802.Marks GA, Roffwarg HP. Spontaneous activity in the thalamic reticular nucleus during the sleep/wake cycle of the freely-moving rat. Brain Res. 1993;623:241–248. doi: 10.1016/0006-8993(93)91434-t. [DOI] [PubMed] [Google Scholar]
- 803.Marquez-Ruiz J, Escudero M. Tonic and phasic phenomena underlying eye movements during sleep in the cat. J Physiol. 2008;586:3461–3477. doi: 10.1113/jphysiol.2008.153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 804.Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, Guan XM, Jiang MM, Feng Y, Camacho RE, Shen Z, Frazier EG, Yu H, Metzger JM, Kuca SJ, Shearman LP, Gopal-Truter S, MacNeil DJ, Strack AM, MacIntyre DE, Van der Ploeg LH, Qian S. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci USA. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 806.Martinez-Gonzalez D, Obermeyer W, Fahy JL, Riboh M, Kalin NH, Benca RM. REM sleep deprivation induces changes in coping responses that are not reversed by amphetamine. Sleep. 2004;27:609–617. [PubMed] [Google Scholar]
- 807.Massaquoi SG, McCarley RW. Extension of the limit cycle reciprocal interaction model of REM cycle control. An integrated sleep control model. J Sleep Res. 1992;1:138–143. doi: 10.1111/j.1365-2869.1992.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 808.Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 809.Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 810.Mathis J, Hess CW, Bassetti C. Isolated mediotegmental lesion causing narcolepsy and rapid eye movement sleep behaviour disorder: a case evidencing a common pathway in narcolepsy and rapid eye movement sleep behaviour disorder. J Neurol Neurosurg Psychiatry. 2007;78:427–429. doi: 10.1136/jnnp.2006.099515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811.Matsuki T, Nomiyama M, Takahira H, Hirashima N, Kunita S, Takahashi S, Yagami K, Kilduff TS, Bettler B, Yanagisawa M, Sakurai T. Selective loss of GABA(B) receptors in orexin-producing neurons results in disrupted sleep/wakefulness architecture. Proc Natl Acad Sci USA. 2009;106:4459–4464. doi: 10.1073/pnas.0811126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 812.Matsumura H, Takahata R, Hayaishi O. Inhibition of sleep in rats by inorganic selenium compounds, inhibitors of prostaglandin D synthase. Proc Natl Acad Sci USA. 1991;88:9046–9050. doi: 10.1073/pnas.88.20.9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 813.Mavanji V, Datta S. Activation of the phasic pontine-wave generator enhances improvement of learning performance: a mechanism for sleep-dependent plasticity. Eur J Neurosci. 2003;17:359–370. doi: 10.1046/j.1460-9568.2003.02460.x. [DOI] [PubMed] [Google Scholar]
- 814.Mavanji V, Ulloor J, Saha S, Datta S. Neurotoxic lesions of phasic pontine-wave generator cells impair retention of 2-way active avoidance memory. Sleep. 2004;27:1282–1292. doi: 10.1093/sleep/27.7.1282. [DOI] [PubMed] [Google Scholar]
- 815.McCarley RW. Dreams: disguise of forbidden wishes or transparent reflections of a distinct brain state? Ann NY Acad Sci. 1998;843:116–133. doi: 10.1111/j.1749-6632.1998.tb08210.x. [DOI] [PubMed] [Google Scholar]
- 816.McCarley RW. Mechanisms and models of REM sleep control. Arch Ital Biol. 2004;142:429–467. [PubMed] [Google Scholar]
- 817.McCarley RW, Benoit O, Barrionuevo G. Lateral geniculate nucleus unitary discharge in sleep and waking: state- and rate-specific aspects. J Neurophysiol. 1983;50:798–818. doi: 10.1152/jn.1983.50.4.798. [DOI] [PubMed] [Google Scholar]
- 818.McCarley RW, Hobson JA. Single neuron activity in cat gigantocellular tegmental field: selectivity of discharge in desynchronized sleep. Science. 1971;174:1250–1252. doi: 10.1126/science.174.4015.1250. [DOI] [PubMed] [Google Scholar]
- 819.McCarley RW, Hobson JA. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science. 1975;189:58–60. doi: 10.1126/science.1135627. [DOI] [PubMed] [Google Scholar]
- 820.McCarley RW, Hobson JA. The neurobiological origins of psychoanalytic dream theory. Am J Psychiatry. 1977;134:1211–1221. doi: 10.1176/ajp.134.11.1211. [DOI] [PubMed] [Google Scholar]
- 821.McCarley RW, Massaquoi SG. A limit cycle mathematical model of the REM sleep oscillator system. Am J Physiol Regul Integr Comp Physiol. 1986;251:R1011–R1029. doi: 10.1152/ajpregu.1986.251.6.R1011. [DOI] [PubMed] [Google Scholar]
- 822.McCarley RW, Massaquoi SG. Further discussion of a model of the REM sleep oscillator. Am J Physiol Regul Integr Comp Physiol. 1986;251:R1033–R1036. doi: 10.1152/ajpregu.1986.251.6.R1033. [DOI] [PubMed] [Google Scholar]
- 823.McCarley RW, Massaquoi SG. Neurobiological structure of the revised limit cycle reciprocal interaction model of REM cycle control. J Sleep Res. 1992;1:132–137. doi: 10.1111/j.1365-2869.1992.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 824.McCarley RW, Nelson JP, Hobson JA. Ponto-geniculo-occipital (PGO) burst neurons: correlative evidence for neuronal generators of PGO waves. Science. 1978;201:269–272. doi: 10.1126/science.663656. [DOI] [PubMed] [Google Scholar]
- 825.McCarley RW, Winkelman JW, Duffy FH. Human cerebral potentials associated with REM sleep rapid eye movements: links to PGO waves and waking potentials. Brain Res. 1983;274:359–364. doi: 10.1016/0006-8993(83)90719-9. [DOI] [PubMed] [Google Scholar]
- 826.McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- 827.McCormick DA. Actions of acetylcholine in the cerebral cortex and thalamus and implications for function. Prog Brain Res. 1993;98:303–308. doi: 10.1016/s0079-6123(08)62412-7. [DOI] [PubMed] [Google Scholar]
- 828.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 829.McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization- activated cation current in thalamic relay neurones. J Physiol. 1990;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 830.McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 831.McCormick DA, Prince DA. Acetylcholine induces burst firing in thalamic reticular neurones by activating a potassium conductance. Nature. 1986;319:402–405. doi: 10.1038/319402a0. [DOI] [PubMed] [Google Scholar]
- 832.McCormick DA, Prince DA. Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. J Physiol. 1987;392:147–165. doi: 10.1113/jphysiol.1987.sp016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833.McCormick DA, Wang Z. Serotonin and noradrenaline excite GABAergic neurones of the guinea-pig and cat nucleus reticularis thalami. J Physiol. 1991;442:235–255. doi: 10.1113/jphysiol.1991.sp018791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834.McCormick DA, Williamson A. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci USA. 1989;86:8098–8102. doi: 10.1073/pnas.86.20.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835.McCoy JG, Strecker RE. The cognitive cost of sleep lost. Neurobiol Learn Mem. 2011;96:564–582. doi: 10.1016/j.nlm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 836.McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J Physiol. 2006;570:553–565. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 838.McGinty D, Szymusiak R. The sleep-wake switch: a neuronal alarm clock. Nat Med. 2000;6:510–511. doi: 10.1038/74988. [DOI] [PubMed] [Google Scholar]
- 839.McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 840.McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160:1253–1255. doi: 10.1126/science.160.3833.1253. [DOI] [PubMed] [Google Scholar]
- 841.McKenna BS, Dickinson DL, Orff HJ, Drummond SP. The effects of one night of sleep deprivation on known-risk and ambiguous-risk decisions. J Sleep Res. 2007;16:245–252. doi: 10.1111/j.1365-2869.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- 842.McKenna JT, Cordeira JW, Jeffrey BA, Ward CP, Winston S, McCarley RW, Strecker RE. c-Fos protein expression is increased in cholinergic neurons of the rodent basal forebrain during spontaneous and induced wakefulness. Brain Res Bull. 2009;80:382–388. doi: 10.1016/j.brainresbull.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 843.McKenna JT, Dauphin LJ, Mulkern KJ, Stronge AM, McCarley RW, Strecker RE. Nocturnal elevation of extracellular adenosine in the basal forebrain. Sleep Res Online. 2003;5:155–160. [Google Scholar]
- 844.McKenna JT, Tartar JL, Ward CP, Thakkar MM, Cordeira JW, McCarley RW, Strecker RE. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience. 2007;146:1462–1473. doi: 10.1016/j.neuroscience.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 845.McNaughton N, Logan B, Panickar KS, Kirk IJ, Pan WX, Brown NT, Heenan A. Contribution of synapses in the medial supramammillary nucleus to the frequency of hippocampal theta rhythm in freely moving rats. Hippocampus. 1995;5:534–545. doi: 10.1002/hipo.450050605. [DOI] [PubMed] [Google Scholar]
- 846.Meerlo P, Koehl M, van der BK, Turek FW. Sleep restriction alters the hypothalamicpituitary- adrenal response to stress. J Neuroendocrinol. 2002;14:397–402. doi: 10.1046/j.0007-1331.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- 847.Mellman TA, Nolan B, Hebding J, Kulick-Bell R, Dominguez R. A polysomnographic comparison of veterans with combat-related PTSD, depressed men, and non-ill controls. Sleep. 1997;20:46–51. doi: 10.1093/sleep/20.1.46. [DOI] [PubMed] [Google Scholar]
- 848.Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469–476. doi: 10.4088/jcp.v66n0409. [DOI] [PubMed] [Google Scholar]
- 849.Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- 850.Methippara MM, Kumar S, Alam MN, Szymusiak R, McGinty D. Effects on sleep of microdialysis of adenosine A1 and A2a receptor analogs into the lateral preoptic area of rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1715–R1723. doi: 10.1152/ajpregu.00247.2005. [DOI] [PubMed] [Google Scholar]
- 851.Meynard MM, Valdes JL, Recabarren M, Seron-Ferre M, Torrealba F. Specific activation of histaminergic neurons during daily feeding anticipatory behavior in rats. Behav Brain Res. 2005;158:311–319. doi: 10.1016/j.bbr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 852.Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med. 1996;153:1880–1887. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- 853.Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T. Differential roles of orexin receptor-1 and-2 in the regulation of non-REM and REM sleep. J Neurosci. 2011;31:6518–6526. doi: 10.1523/JNEUROSCI.6506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 854.Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci USA. 2004;101:4649–4654. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 855.Mignot E, Thorsby E. Narcolepsy and the HLA system. N Engl J Med. 2001;344:692. doi: 10.1056/NEJM200103013440918. [DOI] [PubMed] [Google Scholar]
- 856.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 857.Miller AM, Obermeyer WH, Behan M, Benca RM. The superior colliculus-pretectum mediates the direct effects of light on sleep. Proc Natl Acad Sci USA. 1998;95:8957–8962. doi: 10.1073/pnas.95.15.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 858.Miller JD, Farber J, Gatz P, Roffwarg H, German DC. Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain Res. 1983;273:133–141. doi: 10.1016/0006-8993(83)91101-0. [DOI] [PubMed] [Google Scholar]
- 859.Minkel J, Htaik O, Banks S, Dinges D. Emotional expressiveness in sleep-deprived healthy adults. Behav Sleep Med. 2011;9:5–14. doi: 10.1080/15402002.2011.533987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 860.Mistlberger RE, Bergmann BM, Waldenar W, Rechtschaffen A. Recovery sleep following sleep deprivation in intact and suprachiasmatic nuclei-lesioned rats. Sleep. 1983;6:217–233. doi: 10.1093/sleep/6.3.217. [DOI] [PubMed] [Google Scholar]
- 861.Mitania, Ito K, Hallanger AE, Wainer BH, Kataoka K, McCarley RW. Cholinergic projections from the laterodorsal and pedunculopontine tegmental nuclei to the pontine gigantocellular tegmental field in the cat. Brain Res. 1988;451:397–402. doi: 10.1016/0006-8993(88)90792-5. [DOI] [PubMed] [Google Scholar]
- 862.Mitchell DJ, McNaughton N, Flanagan D, Kirk IJ. Frontal-midline theta from the perspective of hippocampal “theta”. Prog Neurobiol. 2008;86:156–185. doi: 10.1016/j.pneurobio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 863.Mitler MM, Dement WC. Cataplectic-like behavior in cats after micro-injections of carbachol in pontine reticular formation. Brain Res. 1974;68:335–343. doi: 10.1016/0006-8993(74)90402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 864.Miyagawa T, Kawashima M, Nishida N, Ohashi J, Kimura R, Fujimoto A, Shimada M, Morishita S, Shigeta T, Lin L, Hong SC, Faraco J, Shin YK, Jeong JH, Okazaki Y, Tsuji S, Honda M, Honda Y, Mignot E, Tokunaga K. Variant between CPT1B and CHKB associated with susceptibility to narcolepsy. Nat Genet. 2008;40:1324–1328. doi: 10.1038/ng.231. [DOI] [PubMed] [Google Scholar]
- 865.Miyamoto H, Katagiri H, Hensch T. Experience-dependent slow-wave sleep development. Nat Neurosci. 2003;6:553–554. doi: 10.1038/nn1064. [DOI] [PubMed] [Google Scholar]
- 866.Miyauchi S, Misaki M, Kan S, Fukunaga T, Koike T. Human brain activity time-locked to rapid eye movements during REM sleep. Exp Brain Res. 2009;192:657–667. doi: 10.1007/s00221-008-1579-2. [DOI] [PubMed] [Google Scholar]
- 867.Mizoguchi A, Eguchi N, Kimura K, Kiyohara Y, Qu WM, Huang ZL, Mochizuki T, Lazarus M, Kobayashi T, Kaneko T, Narumiya S, Urade Y, Hayaishi O. Dominant localization of prostaglandin D receptors on arachnoid trabecular cells in mouse basal forebrain and their involvement in the regulation of non-rapid eye movement sleep. Proc Natl Acad Sci USA. 2001;98:11674–11679. doi: 10.1073/pnas.201398898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 868.Mizuno S, Mihara T, Miyaoka T, Inagaki T, Horiguchi J. CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14:43–47. doi: 10.1111/j.1365-2869.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 869.Mochizuki T, Arrigoni E, Marcus JN, Clark EL, Yamamoto M, Honer M, Borroni E, Lowell BB, Elmquist JK, Scammell TE. Orexin receptor 2 expression in the posterior hypothalamus rescues sleepiness in narcoleptic mice. Proc Natl Acad Sci USA. 2011;108:4471–4476. doi: 10.1073/pnas.1012456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 870.Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24:6291–6300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 871.Modirrousta M, Mainville L, Jones BE. GABAergic neurons with alpha2-adrenergic receptors in basal forebrain and preoptic area express c-Fos during sleep. Neuroscience. 2004;129:803–810. doi: 10.1016/j.neuroscience.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 872.Modirrousta M, Mainville L, Jones BE. Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur J Neurosci. 2005;21:2807–2816. doi: 10.1111/j.1460-9568.2005.04104.x. [DOI] [PubMed] [Google Scholar]
- 873.Mohns EJ, Karlsson KA, Blumberg MS. The preoptic hypothalamus and basal forebrain play opposing roles in the descending modulation of sleep and wakefulness in infant rats. Eur J Neurosci. 2006;23:1301–1310. doi: 10.1111/j.1460-9568.2006.04652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 874.Moldofsky H, Lue FA, Eisen J, Keystone E, Gorczynski RM. The relationship of interleukin-1 and immune functions to sleep in humans. Psychosom Med. 1986;48:309–318. doi: 10.1097/00006842-198605000-00001. [DOI] [PubMed] [Google Scholar]
- 875.Molle M, Born J. Hippocampus whispering in deep sleep to prefrontal cortex–for good memories? Neuron. 2009;61:496–498. doi: 10.1016/j.neuron.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 876.Molle M, Eschenko O, Gais S, Sara SJ, Born J. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur J Neurosci. 2009;29:1071–1081. doi: 10.1111/j.1460-9568.2009.06654.x. [DOI] [PubMed] [Google Scholar]
- 877.Monckton JE, McCormick DA. Neuromodulatory role of serotonin in the ferret thalamus. J Neurophysiol. 2002;87:2124–2136. doi: 10.1152/jn.00650.2001. [DOI] [PubMed] [Google Scholar]
- 878.Montagna P, Gambetti P, Cortelli P, Lugaresi E. Familial and sporadic fatal insomnia. Lancet Neurol. 2003;2:167–176. doi: 10.1016/s1474-4422(03)00323-5. [DOI] [PubMed] [Google Scholar]
- 879.Montgomery SA, Kasper S. Severe depression and antidepressants: focus on a pooled analysis of placebo-controlled studies on agomelatine. Int Clin Psychopharmacol. 2007;22:283–291. doi: 10.1097/YIC.0b013e3280c56b13. [DOI] [PubMed] [Google Scholar]
- 880.Montgomery SM, Sirota A, Buzsaki G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J Neurosci. 2008;28:6731–6741. doi: 10.1523/JNEUROSCI.1227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 881.Monti JM, D’Angelo L, Jantos H, Barbeito L, Abo V. Effect of DSP-4, a noradrenergic neurotoxin, on sleep and wakefulness and sensitivity to drugs acting on adrenergic receptors in the rat. Sleep. 1988;11:370–377. doi: 10.1093/sleep/11.4.370. [DOI] [PubMed] [Google Scholar]
- 882.Monti JM, Hantos H, Ponzoni A, Monti D, Banchero P. Role of nitric oxide in sleep regulation: effects of l-NAME, an inhibitor of nitric oxide synthase, on sleep in rats. Behav Brain Res. 1999;100:197–205. doi: 10.1016/s0166-4328(98)00131-4. [DOI] [PubMed] [Google Scholar]
- 883.Monti JM, Jantos H. Microinjection of the nitric oxide synthase inhibitor l-NAME into the lateral basal forebrain alters the sleep/wake cycle of the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:239–247. doi: 10.1016/j.pnpbp.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 884.Monti JM, Jantos H. The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking. Prog Brain Res. 2008;172:625–646. doi: 10.1016/S0079-6123(08)00929-1. [DOI] [PubMed] [Google Scholar]
- 885.Monti JM, Jantos H, Boussard M, Altier H, Orellana C, Olivera S. Effects of selective activation or blockade of the histamine H3 receptor on sleep and wakefulness. Eur J Pharmacol. 1991;205:283–287. doi: 10.1016/0014-2999(91)90911-9. [DOI] [PubMed] [Google Scholar]
- 886.Montplaisir J, Godbout R. Nocturnal sleep of narcoleptic patients: revisited. Sleep. 1986;9:159–161. doi: 10.1093/sleep/9.1.159. [DOI] [PubMed] [Google Scholar]
- 887.Montplaisir J, Lorrain D, Godbout R. Restless legs syndrome and periodic leg movements in sleep: the primary role of dopaminergic mechanism. Eur Neurol. 1991;31:41–43. doi: 10.1159/000116643. [DOI] [PubMed] [Google Scholar]
- 888.Morairty S, Rainnie D, McCarley R, Greene R. Disinhibition of ventrolateral preoptic area sleep-active neurons by adenosine: a new mechanism for sleep promotion. Neuroscience. 2004;123:451–457. doi: 10.1016/j.neuroscience.2003.08.066. [DOI] [PubMed] [Google Scholar]
- 889.Morales FR, Chase MH. Intracellular recording of lumbar motoneuron membrane potential during sleep and wakefulness. Exp Neurol. 1978;62:821–827. doi: 10.1016/0014-4886(78)90289-3. [DOI] [PubMed] [Google Scholar]
- 890.Morales FR, Sampogna S, Rampon C, Luppi PH, Chase MH. Brainstem glycinergic neurons and their activation during active (rapid eye movement) sleep in the cat. Neuroscience. 2006;142:37–47. doi: 10.1016/j.neuroscience.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 891.Morison RS, Dempsey. EW. A study of thalamo-cortical relations. Am J Physiol. 1942;135:281–292. [Google Scholar]
- 892.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30:274–280. [PubMed] [Google Scholar]
- 893.Morrison AR. Paradoxical sleep without atonia. Arch Ital Biol. 1988;126:275–289. [PubMed] [Google Scholar]
- 894.Morrison JL, Sood S, Liu H, Park E, Liu X, Nolan P, Horner RL. Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. J Physiol. 2003;552:975–991. doi: 10.1113/jphysiol.2003.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 895.Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL. GABAAreceptor antagonism at the hypoglossal motor nucleus increases genioglossus muscle activity in NREM but not REM sleep. J Physiol. 2003;548:569–583. doi: 10.1113/jphysiol.2002.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 896.Morrow JD, Opp MR. Sleep-wake behavior and responses of interleukin-6-deficient mice to sleep deprivation. Brain Behav Immun. 2005;19:28–39. doi: 10.1016/j.bbi.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 897.Morton RA, Davies CH. Regulation of muscarinic acetylcholine receptor-mediated synaptic responses by adenosine receptors in the rat hippocampus. J Physiol. 1997;502:75–90. doi: 10.1111/j.1469-7793.1997.075bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 898.Moruzzi G, Magoun HW. Brainstem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 899.Mouret J, Bobillier P, Jouvet M. Insomnia following parachlorophenylalanine in the rat. Eur J Pharmacol. 1968;5:17–22. doi: 10.1016/0014-2999(68)90151-9. [DOI] [PubMed] [Google Scholar]
- 900.Mouret J, Delorme F, Jouvet M. Lesions of the pontine tegmentum and sleep in rats. C R Seances Soc Biol Fil. 1967;161:1603–1606. [PubMed] [Google Scholar]
- 901.Mouret J, Jeannerod M, Jouvet M. Electrical activity of the visual system during the paradoxical phase of sleep in the cat. J Physiol. 1963;55:305–306. [PubMed] [Google Scholar]
- 902.Mueller AD, Pollock MS, Lieblich SE, Epp JR, Galea LA, Mistlberger RE. Sleep deprivation can inhibit adult hippocampal neurogenesis independent of adrenal stress hormones. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1693–R1703. doi: 10.1152/ajpregu.00858.2007. [DOI] [PubMed] [Google Scholar]
- 903.Munk MH, Roelfsema PR, Konig P, Engel AK, Singer W. Role of reticular activation in the modulation of intracortical synchronization. Science. 1996;272:271–274. doi: 10.1126/science.272.5259.271. [DOI] [PubMed] [Google Scholar]
- 904.Murai Y, Akaike T. Orexins cause depolarization via nonselective cationic and K+channels in isolated locus coeruleus neurons. Neurosci Res. 2005;51:55–65. doi: 10.1016/j.neures.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 905.Muraki Y, Yamanaka A, Tsujino N, Kilduff TS, Goto K, Sakurai T. Serotonergic regulation of the orexin/hypocretin neurons through the 5-HT1A receptor. J Neurosci. 2004;24:7159–7166. doi: 10.1523/JNEUROSCI.1027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 906.Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci USA. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 907.Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends Cogn Sci. 2002;6:475–481. doi: 10.1016/s1364-6613(02)01992-7. [DOI] [PubMed] [Google Scholar]
- 908.Naidoo N, Casiano V, Cater J, Zimmerman J, Pack AI. A role for the molecular chaperone protein BiP/GRP78 in Drosophila sleep homeostasis. Sleep. 2007;30:557–565. doi: 10.1093/sleep/30.5.557. [DOI] [PubMed] [Google Scholar]
- 909.Naidoo N, Giang W, Galante RJ, Pack AI. Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem. 2005;92:1150–1157. doi: 10.1111/j.1471-4159.2004.02952.x. [DOI] [PubMed] [Google Scholar]
- 910.Nakamura Y, Goldberg LJ, Chandler SH, Chase MH. Intracellular analysis of trigeminal motoneuron activity during sleep in the cat. Science. 1978;199:204–207. doi: 10.1126/science.202025. [DOI] [PubMed] [Google Scholar]
- 911.Nauta WJH. Hypothalamic regulation of sleep in rats. An experimental study. J Neurophysiol. 1946;9:285–361. doi: 10.1152/jn.1946.9.4.285. [DOI] [PubMed] [Google Scholar]
- 912.Naylor E, Bergmann BM, Krauski K, Zee PC, Takahashi JS, Vitaterna MH, Turek FW. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci. 2000;20:8138–8143. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 913.Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30:873–880. doi: 10.1093/sleep/30.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 914.Nelson SE, Duricka DL, Campbell K, Churchill L, Krueger JM. Homer1a and 1bc levels in the rat somatosensory cortex vary with the time of day and sleep loss. Neurosci Lett. 2004;367:105–108. doi: 10.1016/j.neulet.2004.05.089. [DOI] [PubMed] [Google Scholar]
- 915.Netchiporouk L, Shram N, Salvert D, Cespuglio R. Brain extracellular glucose assessed by voltammetry throughout the rat sleep-wake cycle. Eur J Neurosci. 2001;13:1429–1434. doi: 10.1046/j.0953-816x.2001.01503.x. [DOI] [PubMed] [Google Scholar]
- 916.Newman DB, Ginsberg CY. Brainstem reticular nuclei that project to the thalamus in rats: a retrograde tracer study. Brain Behav Evol. 1994;44:1–39. doi: 10.1159/000113566. [DOI] [PubMed] [Google Scholar]
- 917.Neylan TC, Otte C, Yehuda R, Marmar CR. Neuroendocrine regulation of sleep disturbances in PTSD. Ann NY Acad Sci. 2006;1071:203–215. doi: 10.1196/annals.1364.015. [DOI] [PubMed] [Google Scholar]
- 918.Nicoll RA, Malenka RC, Kauer JA. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990;70:513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- 919.Nikonova EV, Naidoo N, Zhang L, Romer M, Cater JR, Scharf MT, Galante RJ, Pack AI. Changes in components of energy regulation in mouse cortex with increases in wakefulness. Sleep. 2010;33:889–900. doi: 10.1093/sleep/33.7.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 920.Nikonova EV, Vijayasarathy C, Zhang L, Cater JR, Galante RJ, Ward SE, Avadhani NG, Pack AI. Differences in activity of cytochrome c oxidase in brain between sleep and wakefulness. Sleep. 2005;28:21–27. doi: 10.1093/sleep/28.1.21. [DOI] [PubMed] [Google Scholar]
- 921.Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, Fried I, Tononi G. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 922.Nishino S, Fujiki N, Ripley B, Sakurai E, Kato M, Watanabe T, Mignot E, Yanai K. Decreased brain histamine content in hypocretin/orexin receptor-2 mutated narcoleptic dogs. Neurosci Lett. 2001;313:125–128. doi: 10.1016/s0304-3940(01)02270-4. [DOI] [PubMed] [Google Scholar]
- 923.Nishino S, Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol. 1997;52:27–78. doi: 10.1016/s0301-0082(96)00070-6. [DOI] [PubMed] [Google Scholar]
- 924.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 925.Nishino S, Sakurai E, Nevsimalova S, Yoshida Y, Watanabe T, Yanai K, Mignot E. Decreased CSF histamine in narcolepsy with and without low CSF hypocretin-1 in comparison to healthy controls. Sleep. 2009;32:175–180. doi: 10.1093/sleep/32.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 926.Nitz D, Siegel J. GABA release in the dorsal raphe nucleus: role in the control of REM sleep. Am J Physiol Regul Integr Comp Physiol. 1997;273:R451–R455. doi: 10.1152/ajpregu.1997.273.1.R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 927.Nitz D, Siegel JM. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience. 1997;78:795–801. doi: 10.1016/s0306-4522(96)00549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 928.Nofzinger EA. Functional neuroimaging of sleep. Semin Neurol. 2005;25:9–18. doi: 10.1055/s-2005-867070. [DOI] [PubMed] [Google Scholar]
- 929.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–2128. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 930.Novak CM, Nunez AA. Daily rhythms in Fos activity in the rat ventrolateral preoptic area and midline thalamic nuclei. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1620–R1626. doi: 10.1152/ajpregu.1998.275.5.R1620. [DOI] [PubMed] [Google Scholar]
- 931.Nowell PD, Buysse DJ, Reynolds CF, III, Hauri PJ, Roth T, Stepanski EJ, Thorpy MJ, Bixler E, Kales A, Manfredi RL, Vgontzas AN, Stapf DM, Houck PR, Kupfer DJ. Clinical factors contributing to the differential diagnosis of primary insomnia and insomnia related to mental disorders. Am J Psychiatry. 1997;154:1412–1416. doi: 10.1176/ajp.154.10.1412. [DOI] [PubMed] [Google Scholar]
- 932.Nunez A, Amzica F, Steriade M. Intrinsic and synaptically generated delta (1–4 Hz) rhythms in dorsal lateral geniculate neurons and their modulation by light-induced fast (30–70 Hz) events. Neuroscience. 1992;51:269–284. doi: 10.1016/0306-4522(92)90314-r. [DOI] [PubMed] [Google Scholar]
- 933.Nunez A, Cervera-Ferri A, Olucha-Bordonau F, Ruiz-Torner A, Teruel V. Nucleus incertus contribution to hippocampal theta rhythm generation. Eur J Neurosci. 2006;23:2731–2738. doi: 10.1111/j.1460-9568.2006.04797.x. [DOI] [PubMed] [Google Scholar]
- 934.Nunez A, De la RC, Rodrigo-Angulo ML, Buno W, Reinoso-Suarez F. Electrophysiological properties and cholinergic responses of rat ventral oral pontine reticular neurons in vitro. Brain Res. 1997;754:1–11. doi: 10.1016/s0006-8993(97)00035-8. [DOI] [PubMed] [Google Scholar]
- 935.Nunez A, De AI, Garcia-Austt E. Relationships of nucleus reticularis pontis oralis neuronal discharge with sensory and carbachol evoked hippocampal theta rhythm. Exp Brain Res. 1991;87:303–308. doi: 10.1007/BF00231847. [DOI] [PubMed] [Google Scholar]
- 936.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAAreceptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 937.O’Donoghue FJ, Briellmann RS, Rochford PD, Abbott DF, Pell GS, Chan CH, Tarquinio N, Jackson GD, Pierce RJ. Cerebral structural changes in severe obstructive sleep apnea. Am J Respir Crit Care Med. 2005;171:1185–1190. doi: 10.1164/rccm.200406-738OC. [DOI] [PubMed] [Google Scholar]
- 938.O’Hara BF, Ding J, Bernat RL, Franken P. Genomic and proteomic approaches towards an understanding of sleep. CNS Neurol Disord Drug Targets. 2007;6:71–81. doi: 10.2174/187152707779940745. [DOI] [PubMed] [Google Scholar]
- 939.O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 940.Oakman SA, Faris PL, Cozzari C, Hartman BK. Characterization of the extent of pontomesencephalic cholinergic neurons’ projections to the thalamus: comparison with projections to midbrain dopaminergic groups. Neuroscience. 1999;94:529–547. doi: 10.1016/s0306-4522(99)00307-3. [DOI] [PubMed] [Google Scholar]
- 941.Obal F, Jr, Alt J, Taishi P, Gardi J, Krueger JM. Sleep in mice with nonfunctional growth hormone-releasing hormone receptors. Am J Physiol Regul Integr Comp Physiol. 2003;284:R131–R139. doi: 10.1152/ajpregu.00361.2002. [DOI] [PubMed] [Google Scholar]
- 942.Obal F, Jr, Fang J, Taishi P, Kacsoh B, Gardi J, Krueger JM. Deficiency of growth hormone-releasing hormone signaling is associated with sleep alterations in the dwarf rat. J Neurosci. 2001;21:2912–2918. doi: 10.1523/JNEUROSCI.21-08-02912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 943.Obal F, Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003;8 doi: 10.2741/1033. d520-2912–d550. [DOI] [PubMed] [Google Scholar]
- 944.Oddie SD, Bland BH, Colom LV, Vertes RP. The midline posterior hypothalamic region comprises a critical part of the ascending brainstem hippocampal synchronizing pathway. Hippocampus. 1994;4:454–473. doi: 10.1002/hipo.450040408. [DOI] [PubMed] [Google Scholar]
- 945.Oddie SD, Stefanek W, Kirk IJ, Bland BH. Intraseptal procaine abolishes hypothalamic stimulation-induced wheel-running and hippocampal theta field activity in rats. J Neurosci. 1996;16:1948–1956. doi: 10.1523/JNEUROSCI.16-05-01948.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 946.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53:547–554. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 947.Ohno K, Hondo M, Sakurai T. Cholinergic regulation of orexin/hypocretin neurons through M(3) muscarinic receptor in mice. J Pharmacol Sci. 2008;106:485–491. doi: 10.1254/jphs.fp0071986. [DOI] [PubMed] [Google Scholar]
- 948.Okabe S, Hida W, Kikuchi Y, Taguchi O, Takishima T, Shirato K. Upper airway muscle activity during REM and non-REM sleep of patients with obstructive apnea. Chest. 1994;106:767–773. doi: 10.1378/chest.106.3.767. [DOI] [PubMed] [Google Scholar]
- 949.Okada T, Mochizuki T, Huang ZL, Eguchi N, Sugita Y, Urade Y, Hayaishi O. Dominant localization of adenosine deaminase in leptomeninges and involvement of the enzyme in sleep. Biochem Biophys Res Commun. 2003;312:29–34. doi: 10.1016/j.bbrc.2003.09.220. [DOI] [PubMed] [Google Scholar]
- 950.Oke OO, Magony A, Anver H, Ward PD, Jiruska P, Jefferys JG, Vreugdenhil M. High-frequency gamma oscillations coexist with low-frequency gamma oscillations in the rat visual cortex in vitro. Eur J Neurosci. 2010;31:1435–1445. doi: 10.1111/j.1460-9568.2010.07171.x. [DOI] [PubMed] [Google Scholar]
- 951.Ondo WG, He Y, Rajasekaran S, Le WD. Clinical correlates of 6-hydroxydopamine injections into A11 dopaminergic neurons in rats: a possible model for restless legs syndrome. Mov Disord. 2000;15:154–158. doi: 10.1002/1531-8257(200001)15:1<154::aid-mds1025>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 952.Ondo WG, Zhao HR, Le WD. Animal models of restless legs syndrome. Sleep Med. 2007;8:344–348. doi: 10.1016/j.sleep.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 953.Onoe H, Ueno R, Fujita I, Nishino H, Oomura Y, Hayaishi O. Prostaglandin D2, a cerebral sleep-inducing substance in monkeys. Proc Natl Acad Sci USA. 1988;85:4082–4086. doi: 10.1073/pnas.85.11.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 954.Opp MR, Krueger JM. Interleukin 1-receptor antagonist blocks interleukin 1-induced sleep and fever. Am J Physiol Regul Integr Comp Physiol. 1991;260:R453–R457. doi: 10.1152/ajpregu.1991.260.2.R453. [DOI] [PubMed] [Google Scholar]
- 955.Oren I, Hajos N, Paulsen O. Identification of the current generator underlying cholinergically induced gamma frequency field potential oscillations in the hippocampal CA3 region. J Physiol. 2010;588:785–797. doi: 10.1113/jphysiol.2009.180851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 956.Ouyang M, Hellman K, Abel T, Thomas SA. Adrenergic signaling plays a critical role in the maintenance of waking and in the regulation of REM sleep. J Neurophysiol. 2004;92:2071–2082. doi: 10.1152/jn.00226.2004. [DOI] [PubMed] [Google Scholar]
- 957.Pais I, Hormuzdi SG, Monyer H, Traub RD, Wood IC, Buhl EH, Whittington MA, Lebeau FE. Sharp wave-like activity in the hippocampus in vitro in mice lacking the gap junction protein connexin 36. J Neurophysiol. 2003;89:2046–2054. doi: 10.1152/jn.00549.2002. [DOI] [PubMed] [Google Scholar]
- 958.Pal D, Mallick BN. Role of noradrenergic and GABAergic inputs in pedunculopontine tegmentum for regulation of rapid eye movement sleep in rats. Neuropharmacology. 2006;51:1–11. doi: 10.1016/j.neuropharm.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 959.Pal D, Mallick BN. GABA in pedunculopontine tegmentum increases rapid eye movement sleep in freely moving rats: possible role of GABAergic inputs from substantia nigra pars reticulata. Neuroscience. 2009;164:404–414. doi: 10.1016/j.neuroscience.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 960.Palchykova S, Winsky-Sommerer R, Shen HY, Boison D, Gerling A, Tobler I. Manipulation of adenosine kinase affects sleep regulation in mice. J Neurosci. 2010;30:13157–13165. doi: 10.1523/JNEUROSCI.1359-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 961.Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 962.Pan ZZ, Grudt TJ, Williams JT. Alpha 1-adrenoceptors in rat dorsal raphe neurons: regulation of two potassium conductances. J Physiol. 1994;478:437–447. doi: 10.1113/jphysiol.1994.sp020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 963.Pandey HP, Ram A, Matsumura H, Hayaishi O. Concentration of prostaglandin D2 in cerebrospinal fluid exhibits a circadian alteration in conscious rats. Biochem Mol Biol Int. 1995;37:431–437. [PubMed] [Google Scholar]
- 964.Pandi-Perumal SR, Trakht I, Spence DW, Srinivasan V, Dagan Y, Cardinali DP. The roles of melatonin and light in the pathophysiology and treatment of circadian rhythm sleep disorders. Nat Clin Pract Neurol. 2008;4:436–447. doi: 10.1038/ncpneuro0847. [DOI] [PubMed] [Google Scholar]
- 965.Pang DS, Robledo CJ, Carr DR, Gent TC, Vyssotski AL, Caley A, Zecharia AY, Wisden W, Brickley SG, Franks NP. An unexpected role for TASK-3 potassium channels in network oscillations with implications for sleep mechanisms and anesthetic action. Proc Natl Acad Sci USA. 2009;106:17546–17551. doi: 10.1073/pnas.0907228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 966.Panula P, Chen YC, Priyadarshini M, Kudo H, Semenova S, Sundvik M, Sallinen V. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol Dis. 2010;40:46–57. doi: 10.1016/j.nbd.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 967.Pape HC, Mager R. Nitric oxide controls oscillatory activity in thalamocortical neurons. Neuron. 1992;9:441–448. doi: 10.1016/0896-6273(92)90182-d. [DOI] [PubMed] [Google Scholar]
- 968.Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- 969.Pape HC, McCormick DA. Electrophysiological and pharmacological properties of interneurons in the cat dorsal lateral geniculate nucleus. Neuroscience. 1995;68:1105–1125. doi: 10.1016/0306-4522(95)00205-w. [DOI] [PubMed] [Google Scholar]
- 970.Papez JW. Path for projection of non-specific diffuse impulses to cortex for EEG, related to consciousness. Dis Nerv Syst. 1956;17:103–108. [PubMed] [Google Scholar]
- 971.Pappenheimer JR, Koski G, Fencl V, Karnovsky ML, Krueger J. Extraction of sleeppromoting factor S from cerebrospinal fluid and from brains of sleep-deprived animals. J Neurophysiol. 1975;38:1299–1311. doi: 10.1152/jn.1975.38.6.1299. [DOI] [PubMed] [Google Scholar]
- 972.Pappenheimer JR, Miller TB, Goodrich CA. Sleep-promoting effects of cerebrospinal fluid from sleep-deprived goats. Proc Natl Acad Sci USA. 1967;58:513–517. doi: 10.1073/pnas.58.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 973.Pare D, Curro DR, Datta S, Steriade M. Brainstem genesis of reserpine-induced ponto-geniculo-occipital waves: an electrophysiological and morphological investigation. Exp Brain Res. 1990;81:533–544. doi: 10.1007/BF02423502. [DOI] [PubMed] [Google Scholar]
- 974.Pare D, Smith Y, Parent A, Steriade M. Projections of brainstem core cholinergic and non-cholinergic neurons of cat to intralaminar and reticular thalamic nuclei. Neuroscience. 1988;25:69–86. doi: 10.1016/0306-4522(88)90007-3. [DOI] [PubMed] [Google Scholar]
- 975.Pare D, Steriade M, Deschenes M, Oakson G. Physiological characteristics of anterior thalamic nuclei, a group devoid of inputs from reticular thalamic nucleus. J Neurophysiol. 1987;57:1669–1685. doi: 10.1152/jn.1987.57.6.1669. [DOI] [PubMed] [Google Scholar]
- 976.Parent A, Pare D, Smith Y, Steriade M. Basal forebrain cholinergic and noncholinergic projections to the thalamus and brainstem in cats and monkeys. J Comp Neurol. 1988;277:281–301. doi: 10.1002/cne.902770209. [DOI] [PubMed] [Google Scholar]
- 977.Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, Griffith LC. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 978.Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002;22:7695–7711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 979.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 980.Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain. 2003;126:1524–1536. doi: 10.1093/brain/awg166. [DOI] [PubMed] [Google Scholar]
- 981.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 982.Passouant P, Cadilhac J, Ribstein M. Sleep privation with eye movements using antidepressive agents. Rev Neurol. 1972;127:173–192. [PubMed] [Google Scholar]
- 983.Passouant P, Halberg F, Genicot R, Popoviciu L, Baldy-Moulinier M. Periodicity of narcoleptic attacks and the circadian rhythm of rapid sleep. Rev Neurol. 1969;121:155–164. [PubMed] [Google Scholar]
- 984.Pasumarthi RK, Gerashchenko D, Kilduff TS. Further characterization of sleep-active neuronal nitric oxide synthase neurons in the mouse brain. Neuroscience. 2010;169:149–157. doi: 10.1016/j.neuroscience.2010.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 985.Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J Neurosci. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 986.Pawlyk AC, Ferber M, Shah A, Pack AI, Naidoo N. Proteomic analysis of the effects and interactions of sleep deprivation and aging in mouse cerebral cortex. J Neurochem. 2007;103:2301–2313. doi: 10.1111/j.1471-4159.2007.04949.x. [DOI] [PubMed] [Google Scholar]
- 987.Pawlyk AC, Morrison AR, Ross RJ, Brennan FX. Stress-induced changes in sleep in rodents: models and mechanisms. Neurosci Biobehav Rev. 2008;32:99–117. doi: 10.1016/j.neubiorev.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 988.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic; 2001. [Google Scholar]
- 989.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- 990.Pellejero T, Monti JM, Baglietto J, Jantos H, Pazos S, Cichevski V, Hawkins M. Effects of methoxamine and alpha-adrenoceptor antagonists, prazosin and yohimbine, on the sleep-wake cycle of the rat. Sleep. 1984;7:365–372. doi: 10.1093/sleep/7.4.365. [DOI] [PubMed] [Google Scholar]
- 991.Pentreath VW, Rees K, Owolabi OA, Philip KA, Doua F. The somnogenic T lymphocyte suppressor prostaglandin D2 is selectively elevated in cerebrospinal fluid of advanced sleeping sickness patients. Trans R Soc Trop Med Hyg. 1990;84:795–799. doi: 10.1016/0035-9203(90)90085-s. [DOI] [PubMed] [Google Scholar]
- 992.Perez NM, Benedito MA. Activities of monoamine oxidase (MAO) A and B in discrete regions of rat brain after rapid eye movement (REM) sleep deprivation. Pharmacol Biochem Behav. 1997;58:605–608. doi: 10.1016/s0091-3057(97)10002-8. [DOI] [PubMed] [Google Scholar]
- 993.Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22:273–280. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- 994.Peterfi Z, Churchill L, Hajdu I, Obal JF, Krueger JM, Parducz A. Fos-immunoreactivity in the hypothalamus: dependency on the diurnal rhythm, sleep, gender, and estrogen. Neuroscience. 2004;124:695–707. doi: 10.1016/j.neuroscience.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 995.Peterfi Z, Obal F, Jr, Taishi P, Gardi J, Kacsoh B, Unterman T, Krueger JM. Sleep in spontaneous dwarf rats. Brain Res. 2006;1108:133–146. doi: 10.1016/j.brainres.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 996.Petersson P, Waldenstrom A, Fahraeus C, Schouenborg J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature. 2003;424:72–75. doi: 10.1038/nature01719. [DOI] [PubMed] [Google Scholar]
- 997.Petsche H, Gogolak G, Vanzwieten PA. Rhythmicity of septal cell discharges at various levels of reticular excitation. Electroencephalogr Clin Neurophysiol. 1965;19:25–33. doi: 10.1016/0013-4694(65)90004-0. [DOI] [PubMed] [Google Scholar]
- 998.Petsche H, Stumpf C, Gogolak G. The significance of the rabbit’s septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells. Electroencephalogr Clin Neurophysiol. 1962;14:202–211. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- 999.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 1000.Peyron C, Tighe DK, van-den Pol AN, de LL, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1001.Pickel VM, Segal M, Bloom FE. A radioautographic study of the efferent pathways of the nucleus locus coeruleus. J Comp Neurol. 1974;155:15–42. doi: 10.1002/cne.901550103. [DOI] [PubMed] [Google Scholar]
- 1002.Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- 1003.Pieribone VA, Xu ZQ, Zhang X, Grillner S, Bartfai T, Hokfelt T. Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience. 1995;64:861–874. doi: 10.1016/0306-4522(94)00450-j. [DOI] [PubMed] [Google Scholar]
- 1004.Pinzar E, Kanaoka Y, Inui T, Eguchi N, Urade Y, Hayaishi O. ProstaglandinDsynthase gene is involved in the regulation of non-rapid eye movement sleep. Proc Natl Acad Sci USA. 2000;97:4903–4907. doi: 10.1073/pnas.090093997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1005.Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur J Neurosci. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 1006.Pitman JL, McGill JJ, Keegan KP, Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila . Nature. 2006;441:753–756. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- 1007.Poe GR, Walsh CM, Bjorness TE. Cognitive neuroscience of sleep. Prog Brain Res. 2010;185:1–19. doi: 10.1016/B978-0-444-53702-7.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1008.Poirrier JE, Guillonneau F, Renaut J, Sergeant K, Luxen A, Maquet P, Leprince P. Proteomic changes in rat hippocampus and adrenals following short-term sleep deprivation. Proteome Sci. 2008;6:14. doi: 10.1186/1477-5956-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1009.Pollock MS, Mistlberger RE. Rapid eye movement sleep induction by microinjection of the GABA-A antagonist bicuculline into the dorsal subcoeruleus area of the rat. Brain Res. 2003;962:68–77. doi: 10.1016/s0006-8993(02)03956-2. [DOI] [PubMed] [Google Scholar]
- 1010.Pompeiano M, Cirelli C, Tononi G. Effects of sleep deprivation on fos-like immunoreactivity in the rat brain. Arch Ital Biol. 1992;130:325–335. [PubMed] [Google Scholar]
- 1011.Pompeiano M, Cirelli C, Tononi G. Immediate-early genes in spontaneous wakefulness and sleep: expression of c-fos and NGFI-A mRNA and protein. J Sleep Res. 1994;3:80–96. doi: 10.1111/j.1365-2869.1994.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 1012.Ponomarenko AA, Knoche A, Korotkova TM, Haas HL. Aminergic control of highfrequency (~200 Hz) network oscillations in the hippocampus of the behaving rat. Neurosci Lett. 2003;348:101–104. doi: 10.1016/s0304-3940(03)00742-0. [DOI] [PubMed] [Google Scholar]
- 1013.Ponomarenko AA, Korotkova TM, Sergeeva OA, Haas HL. Multiple GABAAreceptor subtypes regulate hippocampal ripple oscillations. Eur J Neurosci. 2004;20:2141–2148. doi: 10.1111/j.1460-9568.2004.03685.x. [DOI] [PubMed] [Google Scholar]
- 1014.Popa D, Lena C, Fabre V, Prenat C, Gingrich J, Escourrou P, Hamon M, Adrien J. Contribution of 5-HT2 receptor subtypes to sleep-wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2A receptors. J Neurosci. 2005;25:11231–11238. doi: 10.1523/JNEUROSCI.1724-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1015.Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, Goate A, Rice JP, O’Connor SJ, Rohrbaugh J, Kuperman S, Bauer LO, Crowe RR, Schuckit MA, Hesselbrock V, Conneally PM, Tischfield JA, Li TK, Reich T, Begleiter H. Linkage disequilibrium between the beta frequency of the human EEG and a GABAAreceptor gene locus. Proc Natl Acad Sci USA. 2002;99:3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1016.Porkka-Heiskanen T, Smith SE, Taira T, Urban JH, Levine JE, Turek FW, Stenberg D. Noradrenergic activity in rat brain during rapid eye movement sleep deprivation and rebound sleep. Am J Physiol Regul Integr Comp Physiol. 1995;268:R1456–R1463. doi: 10.1152/ajpregu.1995.268.6.R1456. [DOI] [PubMed] [Google Scholar]
- 1017.Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 1018.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1019.Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005;3:e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1020.Portas CM, Bjorvatn B, Ursin R. Serotonin and the sleep/wake cycle: special emphasis on microdialysis studies. Prog Neurobiol. 2000;60:13–35. doi: 10.1016/s0301-0082(98)00097-5. [DOI] [PubMed] [Google Scholar]
- 1021.Portas CM, Thakkar M, Rainnie D, McCarley RW. Microdialysis perfusion of 8-hydroxy- 2-(di-n-propylamino)tetralin (8-OH-DPAT) in the dorsal raphe nucleus decreases serotonin release and increases rapid eye movement sleep in the freely moving cat. J Neurosci. 1996;16:2820–2828. doi: 10.1523/JNEUROSCI.16-08-02820.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1022.Portas CM, Thakkar M, Rainnie DG, Greene RW, McCarley RW. Role of adenosine in behavioral state modulation: a microdialysis study in the freely moving cat. Neuroscience. 1997;79:225–235. doi: 10.1016/s0306-4522(96)00640-9. [DOI] [PubMed] [Google Scholar]
- 1023.Potter WB, O’Riordan KJ, Barnett D, Osting SM, Wagoner M, Burger C, Roopra A. Metabolic regulation of neuronal plasticity by the energy sensor AMPK. PLoS One. 2010;5:e8996. doi: 10.1371/journal.pone.0008996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1024.Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1025.Provini F, Vetrugno R, Pastorelli F, Lombardi C, Plazzi G, Marliani AF, Lugaresi E, Montagna P. Status dissociatus after surgery for tegmental ponto-mesencephalic cavernoma: a state-dependent disorder of motor control during sleep. Mov Disord. 2004;19:719–723. doi: 10.1002/mds.20027. [DOI] [PubMed] [Google Scholar]
- 1026.Puig MV, Ushimaru M, Kawaguchi Y. Two distinct activity patterns of fast-spiking interneurons during neocortical UP states. Proc Natl Acad Sci USA. 2008;105:8428–8433. doi: 10.1073/pnas.0712219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1027.Pull I, McIlwain H. Adenine derivatives as neurohumoral agents in the brain. The quantities liberated on excitation of superfused cerebral tissues. Biochem J. 1972;130:975–981. doi: 10.1042/bj1300975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1028.Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia A-S, McNamara JO. Neuroscience. Sunderland, MA: Sinauer; 1997. [Google Scholar]
- 1029.Qiu MH, Vetrivelan R, Fuller PM, Lu J. Basal ganglia control of sleep-wake behavior and cortical activation. Eur J Neurosci. 2010;31:499–507. doi: 10.1111/j.1460-9568.2009.07062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1030.Qu S, Le W, Zhang X, Xie W, Zhang A, Ondo WG. Locomotion is increased in a11-lesioned mice with iron deprivation: a possible animal model for restless legs syndrome. J Neuropathol Exp Neurol. 2007;66:383–388. doi: 10.1097/nen.0b013e3180517b5f. [DOI] [PubMed] [Google Scholar]
- 1031.Qu WM, Huang ZL, Xu XH, Aritake K, Eguchi N, Nambu F, Narumiya S, Urade Y, Hayaishi O. Lipocalin-type prostaglandin D synthase produces prostaglandin D2 involved in regulation of physiological sleep. Proc Natl Acad Sci USA. 2006;103:17949–17954. doi: 10.1073/pnas.0608581103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1032.Qu WM, Xu XH, Yan MM, Wang YQ, Urade Y, Huang ZL. Essential role of dopamine D2 receptor in the maintenance of wakefulness, but not in homeostatic regulation of sleep, in mice. J Neurosci. 2010;30:4382–4389. doi: 10.1523/JNEUROSCI.4936-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1033.Quattrochi JJ, Hobson JA. Carbachol microinjection into the caudal peribrachial area induces long-term enhancement of PGO wave activity but not REM sleep. J Sleep Res. 1999;8:281–290. doi: 10.1046/j.1365-2869.1999.00173.x. [DOI] [PubMed] [Google Scholar]
- 1034.Rachalski A, Alexandre C, Bernard JF, Saurini F, Lesch KP, Hamon M, Adrien J, Fabre V. Altered sleep homeostasis after restraint stress in 5-HTT knock-out male mice: a role for hypocretins. J Neurosci. 2009;29:15575–15585. doi: 10.1523/JNEUROSCI.3138-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1035.Racz A, Ponomarenko AA, Fuchs EC, Monyer H. Augmented hippocampal ripple oscillations in mice with reduced fast excitation onto parvalbumin-positive cells. J Neurosci. 2009;29:2563–2568. doi: 10.1523/JNEUROSCI.5036-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1036.Radek RJ, Decker MW, Jarvis MF. The adenosine kinase inhibitor ABT-702 augments EEG slow waves in rats. Brain Res. 2004;1026:74–83. doi: 10.1016/j.brainres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 1037.Radulovacki M, Virus RM, Djuricic-Nedelson M, Green RD. Adenosine analogs and sleep in rats. J Pharmacol Exp Ther. 1984;228:268–274. [PubMed] [Google Scholar]
- 1038.Radulovacki M, Virus RM, Rapoza D, Crane RA. A comparison of the dose response effects of pyrimidine ribonucleosides and adenosine on sleep in rats. Psychopharmacology. 1985;87:136–140. doi: 10.1007/BF00431796. [DOI] [PubMed] [Google Scholar]
- 1039.Rai S, Kumar S, Alam MA, Szymusiak R, McGinty D, Alam MN. A1 receptor mediated adenosinergic regulation of perifornical-lateral hypothalamic area neurons in freely behaving rats. Neuroscience. 2010;167:40–48. doi: 10.1016/j.neuroscience.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1040.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1041.Rainnie DG, Grunze HC, McCarley RW, Greene RW. Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal. Science. 1994;263:689–692. doi: 10.1126/science.8303279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1042.Raizen DM, Zimmerman JE, Maycock MH, Ta UD, You YJ, Sundaram MV, Pack AI. Lethargus is a Caenorhabditis elegans sleep-like state. Nature. 2008;451:569–572. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- 1043.Ram A, Pandey HP, Matsumura H, Kasahara-Orita K, Nakajima T, Takahata R, Satoh S, Terao A, Hayaishi O. CSF levels of prostaglandins, especially the level of prostaglandin D2, are correlated with increasing propensity towards sleep in rats. Brain Res. 1997;751:81–89. doi: 10.1016/s0006-8993(96)01401-1. [DOI] [PubMed] [Google Scholar]
- 1044.Ramesh V, Thakkar MM, Strecker RE, Basheer R, McCarley RW. Wakefulnessinducing effects of histamine in the basal forebrain of freely moving rats. Behav Brain Res. 2004;152:271–278. doi: 10.1016/j.bbr.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 1045.Rampon C, Luppi PH, Fort P, Peyron C, Jouvet M. Distribution of glycine-immunoreactive cell bodies and fibers in the rat brain. Neuroscience. 1996;75:737–755. doi: 10.1016/0306-4522(96)00278-3. [DOI] [PubMed] [Google Scholar]
- 1046.Ranson SW. Somnolence caused by hypothalamic lesions in the monkey. Arch Neurol Psychiatr. 1939;41:1–23. [Google Scholar]
- 1047.Rao Y, Liu ZW, Borok E, Rabenstein RL, Shanabrough M, Lu M, Picciotto MR, Horvath TL, Gao XB. Prolonged wakefulness induces experience-dependent synaptic plasticity in mouse hypocretin/orexin neurons. J Clin Invest. 2007;117:4022–4033. doi: 10.1172/JCI32829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1048.Rao Y, Lu M, Ge F, Marsh DJ, Qian S, Wang AH, Picciotto MR, Gao XB. Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus. J Neurosci. 2008;28:9101–9110. doi: 10.1523/JNEUROSCI.1766-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1049.Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, Dobie DJ, Hoff D, Rein RJ, Straits-Troster K, Thomas RG, McFall MM. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 1050.Rasmussen K, Morilak DA, Jacobs BL. Single unit activity of locus coeruleus neurons in the freely moving cat. I. During naturalistic behaviors and in response to simple and complex stimuli. Brain Res. 1986;371:324–334. doi: 10.1016/0006-8993(86)90370-7. [DOI] [PubMed] [Google Scholar]
- 1051.Rauniar GP, Gitanjali B, Shashindran C. Comparative effects of hyoscine butylbromide and atropine sulphate on sleep architecture in healthy human volunteers. Indian J Physiol Pharmacol. 1998;42:395–400. [PubMed] [Google Scholar]
- 1052.Ray WJ, Cole HW. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science. 1985;228:750–752. doi: 10.1126/science.3992243. [DOI] [PubMed] [Google Scholar]
- 1053.Rechtschaffen A, Kales A. A manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects, US Goverment Printing Office, National Institue of Health Publication. Washington, DC: NIH; 1968. [Google Scholar]
- 1054.Reid MS, Siegel JM, Dement WC, Mignot E. Cholinergic mechanisms in canine narcolepsy–II. Acetylcholine release in the pontine reticular formation is enhanced during cataplexy. Neuroscience. 1994;59:523–530. doi: 10.1016/0306-4522(94)90174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1055.Reid MS, Tafti M, Geary JN, Nishino S, Siegel JM, Dement WC, Mignot E. Cholinergic mechanisms in canine narcolepsy–I. Modulation of cataplexy via local drug administration into the pontine reticular formation. Neuroscience. 1994;59:511–522. doi: 10.1016/0306-4522(94)90173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1056.Reid MS, Tafti M, Nishino S, Siegel JM, Dement WC, Mignot E. Cholinergic regulation of cataplexy in canine narcolepsy in the pontine reticular formation is mediated by M2 muscarinic receptors. Sleep. 1994;17:424–435. [PMC free article] [PubMed] [Google Scholar]
- 1057.Reinoso-Suarez F, De A, I, Rodrigo-Angulo, ML Rodriguez-Veiga E. Location and anatomical connections of a paradoxical sleep induction site in the cat ventral pontine tegmentum. Eur J Neurosci. 1994;6:1829–1836. doi: 10.1111/j.1460-9568.1994.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 1058.Retey JV, Adam M, Gottselig JM, Khatami R, Durr R, Achermann P, Landolt HP. Adenosinergic mechanisms contribute to individual differences in sleep deprivationinduced changes in neurobehavioral function and brain rhythmic activity. J Neurosci. 2006;26:10472–10479. doi: 10.1523/JNEUROSCI.1538-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1059.Retey JV, Adam M, Honegger E, Khatami R, Luhmann UF, Jung HH, Berger W, Landolt HP. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc Natl Acad Sci USA. 2005;102:15676–15681. doi: 10.1073/pnas.0505414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1060.Retey JV, Adam M, Khatami R, Luhmann UF, Jung HH, Berger W, Landolt HP. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin Pharmacol Ther. 2007;81:692–698. doi: 10.1038/sj.clpt.6100102. [DOI] [PubMed] [Google Scholar]
- 1061.Revel FG, Gottowik J, Gatti S, Wettstein JG, Moreau JL. Rodent models of insomnia: a review of experimental procedures that induce sleep disturbances. Neurosci Biobehav Rev. 2009;33:874–899. doi: 10.1016/j.neubiorev.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 1062.Ribeiro AC, Kapas L. Day- and nighttime injection of a nitric oxide synthase inhibitor elicits opposite sleep responses in rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R521–R531. doi: 10.1152/ajpregu.00605.2004. [DOI] [PubMed] [Google Scholar]
- 1063.Riedner BA, Vyazovskiy VV, Huber R, Massimini M, Esser S, Murphy M, Tononi G. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep. 2007;30:1643–1657. doi: 10.1093/sleep/30.12.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1064.Riekkinen P, Jr, Riekkinen M, Sirvio J, Riekkinen P. Neurophysiological consequences of combined cholinergic and noradrenergic lesions. Exp Neurol. 1992;116:64–68. doi: 10.1016/0014-4886(92)90176-q. [DOI] [PubMed] [Google Scholar]
- 1065.Riemann D, Hohagen F, Bahro M, Lis S, Stadmuller G, Gann H, Berger M. Cholinergic neurotransmission, REM sleep and depression. J Psychosom Res. 1994;38(Suppl 1):15–25. doi: 10.1016/0022-3999(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 1066.Robbins TW. Arousal systems and attentional processes. Biol Psychol. 1997;45:57–71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- 1067.Robbins TW. Chemistry of the mind: neurochemical modulation of prefrontal cortical function. J Comp Neurol. 2005;493:140–146. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- 1068.Roberts WW, Robinson TC. Relaxation and sleep induced by warming of preoptic region and anterior hypothalamus in cats. Exp Neurol. 1969;25:282–294. doi: 10.1016/0014-4886(69)90051-x. [DOI] [PubMed] [Google Scholar]
- 1069.Robinson TE, Kramis RC, Vanderwolf CH. Two types of cerebral activation during active sleep: relations to behavior. Brain Res. 1977;124:544–549. doi: 10.1016/0006-8993(77)90954-4. [DOI] [PubMed] [Google Scholar]
- 1070.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 1071.Rogawski MA, Aghajanian GK. Norepinephrine and serotonin: opposite effects on the activity of lateral geniculate neurons evoked by optic pathway stimulation. Exp Neurol. 1980;69:678–694. doi: 10.1016/0014-4886(80)90060-6. [DOI] [PubMed] [Google Scholar]
- 1072.Rogers GS, Van de Castle RL, Evans WS, Critelli JW. Vaginal pulse amplitude response patterns during erotic conditions and sleep. Arch Sex Behav. 1985;14:327–342. doi: 10.1007/BF01550848. [DOI] [PubMed] [Google Scholar]
- 1073.Rolls A, Colas D, Adamantidis A, Carter M, Lanre-Amos T, Heller HC, De Lecea L. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1015633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1074.Romcy-Pereira R, Pavlides C. Distinct modulatory effects of sleep on the maintenance of hippocampal and medial prefrontal cortex LTP. Eur J Neurosci. 2004;20:3453–3462. doi: 10.1111/j.1460-9568.2004.03808.x. [DOI] [PubMed] [Google Scholar]
- 1075.Rosenberg PA, Li Y, Le M, Zhang Y. Nitric oxide-stimulated increase in extracellular adenosine accumulation in rat forebrain neurons in culture is associated with ATP hydrolysis and inhibition of adenosine kinase activity. J Neurosci. 2000;20:6294–6301. doi: 10.1523/JNEUROSCI.20-16-06294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1076.Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol. 1998;401:163–186. [PubMed] [Google Scholar]
- 1077.Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol. 2003;465:593–603. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- 1078.Ross RJ, Ball WA, Dinges DF, Kribbs NB, Morrison AR, Silver SM, Mulvaney FD. Rapid eye movement sleep disturbance in posttraumatic stress disorder. Biol Psychiatry. 1994;35:195–202. doi: 10.1016/0006-3223(94)91152-5. [DOI] [PubMed] [Google Scholar]
- 1079.Ross RJ, Gresch PJ, Ball WA, Sanford LD, Morrison AR. REM sleep inhibition by desipramine: evidence for an alpha-1 adrenergic mechanism. Brain Res. 1995;701:129–134. doi: 10.1016/0006-8993(95)00984-x. [DOI] [PubMed] [Google Scholar]
- 1080.Ruch-Monachon MA, Jalfre M, Haefely W. Drugs and PGO waves in the lateral geniculate body of the curarized cat. II. PGO wave activity and brain 5-hydroxytryptamine. Arch Int Pharmacodyn Ther. 1976;219:269–286. [PubMed] [Google Scholar]
- 1081.Ruch-Monachon MA, Jalfre M, Haefely W. Drugs and PGO waves in the lateral geniculate body of the curarized cat. IV. The effects of acetylcholine, GABA and benzodiazepines on PGO wave activity. Arch Int Pharmacodyn Ther. 1976;219:308–325. [PubMed] [Google Scholar]
- 1082.Rudy B, Kentros C, Weiser M, Fruhling D, Serodio P, Vega-Saenz de ME, Ellisman MH, Pollock JA, Baker H. Region-specific expression of a K+channel gene in brain. Proc Natl Acad Sci USA. 1992;89:4603–4607. doi: 10.1073/pnas.89.10.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1083.Rudy B, McBain CJ. Kv3 channels: voltage-gated K+channels designed for highfrequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- 1084.Ruskin DN, Dunn KE, Billiot I, Bazan NG, LaHoste GJ. Eliminating the adrenal stress response does not affect sleep deprivation-induced acquisition deficits in the water maze. Life Sci. 2006;78:2833–2838. doi: 10.1016/j.lfs.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 1085.Ruskin DN, Liu C, Dunn KE, Bazan NG, LaHoste GJ. Sleep deprivation impairs hippocampus-mediated contextual learning but not amygdala-mediated cued learning in rats. Eur J Neurosci. 2004;19:3121–3124. doi: 10.1111/j.0953-816X.2004.03426.x. [DOI] [PubMed] [Google Scholar]
- 1086.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2DNAbinding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 1087.Rye DB. The two faces of Eve: dopamine’s modulation of wakefulness and sleep. Neurology. 2004;63:S2–S7. doi: 10.1212/wnl.63.8_suppl_3.s2. [DOI] [PubMed] [Google Scholar]
- 1088.Sagaspe P, Sanchez-Ortuno M, Charles A, Taillard J, Valtat C, Bioulac B, Philip P. Effects of sleep deprivation on Color-Word, Emotional, and Specific Stroop interference and on self-reported anxiety. Brain Cogn. 2006;60:76–87. doi: 10.1016/j.bandc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 1089.Saha S, Datta S. Two-way active avoidance training-specific increases in phosphorylated cAMP response element-binding protein in the dorsal hippocampus, amygdala, and hypothalamus. Eur J Neurosci. 2005;21:3403–3414. doi: 10.1111/j.1460-9568.2005.04166.x. [DOI] [PubMed] [Google Scholar]
- 1090.Sainsbury RS, Bland BH. The effects of selective septal lesions on theta production in CA1 and the dentate gyrus of the hippocampus. Physiol Behav. 1981;26:1097–1101. doi: 10.1016/0031-9384(81)90214-6. [DOI] [PubMed] [Google Scholar]
- 1091.Saint-Mleux B, Eggermann E, Bisetti A, Bayer L, Machard D, Jones BE, Muhlethaler M, Serafin M. Nicotinic enhancement of the noradrenergic inhibition of sleep-promoting neurons in the ventrolateral preoptic area. J Neurosci. 2004;24:63–67. doi: 10.1523/JNEUROSCI.0232-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1092.Saito H, Sakai K, Jouvet M. Discharge patterns of the nucleus parabrachialis lateralis neurons of the cat during sleep and waking. Brain Res. 1977;134:59–72. doi: 10.1016/0006-8993(77)90925-8. [DOI] [PubMed] [Google Scholar]
- 1093.Sakai K. Executive mechanisms of paradoxical sleep. Arch Ital Biol. 1988;126:239–257. [PubMed] [Google Scholar]
- 1094.Sakai K. Sleep-waking discharge profiles of median preoptic and surrounding neurons in mice. Neuroscience. 2011;182:144–161. doi: 10.1016/j.neuroscience.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 1095.Sakai K, Crochet S. Serotonergic dorsal raphe neurons cease firing by disfacilitation during paradoxical sleep. Neuroreport. 2000;11:3237–3241. doi: 10.1097/00001756-200009280-00037. [DOI] [PubMed] [Google Scholar]
- 1096.Sakai K, Crochet S. Role of the locus coeruleus in the control of paradoxical sleep generation in the cat. Arch Ital Biol. 2004;142:421–427. [PubMed] [Google Scholar]
- 1097.Sakai K, El Mansari M, Jouvet M. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res. 1990;527:213–223. doi: 10.1016/0006-8993(90)91140-c. [DOI] [PubMed] [Google Scholar]
- 1098.Sakai K, Jouvet M. Brain stem PGO-on cells projecting directly to the cat dorsal lateral geniculate nucleus. Brain Res. 1980;194:500–505. doi: 10.1016/0006-8993(80)91231-7. [DOI] [PubMed] [Google Scholar]
- 1099.Sakai K, Koyama Y. Are there cholinergic and non-cholinergic paradoxical sleep-on neurones in the pons? Neuroreport. 1996;7:2449–2453. doi: 10.1097/00001756-199611040-00009. [DOI] [PubMed] [Google Scholar]
- 1100.Sakai K, Petitjean F, Jouvet M. Effects of ponto-mesencephalic lesions and electrical stimulation upon PGO waves and EMPs in unanesthetized cats. Electroencephalogr Clin Neurophysiol. 1976;41:49–63. doi: 10.1016/0013-4694(76)90214-5. [DOI] [PubMed] [Google Scholar]
- 1101.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 1102.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/ hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 1103.Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- 1104.Salbaum JM, Cirelli C, Walcott E, Krushel LA, Edelman GM, Tononi G. Chlorotoxinmediated disinhibition of noradrenergic locus coeruleus neurons using a conditional transgenic approach. Brain Res. 2004;1016:20–32. doi: 10.1016/j.brainres.2004.03.078. [DOI] [PubMed] [Google Scholar]
- 1105.Sallanon M, Denoyer M, Kitahama K, Aubert C, Gay N, Jouvet M. Long-lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat. Neuroscience. 1989;32:669–683. doi: 10.1016/0306-4522(89)90289-3. [DOI] [PubMed] [Google Scholar]
- 1106.Sallanon M, Sakai K, Buda C, Puymartin M, Jouvet M. Increase of paradoxical sleep induced by microinjections of ibotenic acid into the ventrolateral part of the posterior hypothalamus in the cat. Arch Ital Biol. 1988;126:87–97. [PubMed] [Google Scholar]
- 1107.Samann PG, Tully C, Spoormaker VI, Wetter TC, Holsboer F, Wehrle R, Czisch M. Increased sleep pressure reduces resting state functional connectivity. MAGMA. 2010;23:375–389. doi: 10.1007/s10334-010-0213-z. [DOI] [PubMed] [Google Scholar]
- 1108.Samann PG, Wehrle R, Hoehn D, Spoormaker VI, Peters H, Tully C, Holsboer F, Czisch M. Development of the brain’s default mode network from wakefulness to slow wave sleep. Cereb Cortex. 2011 doi: 10.1093/cercor/bhq295. [DOI] [PubMed] [Google Scholar]
- 1109.Sanchez-Vives MV, Bal T, Kim U, von KM, McCormick DA. Are the interlaminar zones of the ferret dorsal lateral geniculate nucleus actually part of the perigeniculate nucleus? J Neurosci. 1996;16:5923–5941. doi: 10.1523/JNEUROSCI.16-19-05923.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1110.Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- 1111.Sanford LD, Cheng CS, Silvestri AJ, Tang X, Mann GL, Ross RJ, Morrison AR. Sleep and behaviour in rats with pontine lesions producing REM without atonia. Sleep Res Online. 2001;4:1–5. [Google Scholar]
- 1112.Sanford LD, Tang X, Xiao J, Ross RJ, Morrison AR. GABAergic regulation of REM sleep in reticularis pontis oralis and caudalis in rats. J Neurophysiol. 2003;90:938–945. doi: 10.1152/jn.00993.2002. [DOI] [PubMed] [Google Scholar]
- 1113.Sanford LD, Tejani-Butt SM, Ross RJ, Morrison AR. Elicited PGO waves in rats: lack of 5-HT1A inhibition in putative pontine generator region. Pharmacol Biochem Behav. 1996;53:323–327. doi: 10.1016/0091-3057(95)02029-2. [DOI] [PubMed] [Google Scholar]
- 1114.Sanford LD, Yang L, Tang X, Ross RJ, Morrison AR. Tetrodotoxin inactivation of pontine regions: influence on sleep-wake states. Brain Res. 2005;1044:42–50. doi: 10.1016/j.brainres.2005.02.079. [DOI] [PubMed] [Google Scholar]
- 1115.Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus. J Comp Neurol. 1984;222:313–342. doi: 10.1002/cne.902220302. [DOI] [PubMed] [Google Scholar]
- 1116.Saper CB, Cano G, Scammell TE. Homeostatic, circadian, and emotional regulation of sleep. J Comp Neurol. 2005;493:92–98. doi: 10.1002/cne.20770. [DOI] [PubMed] [Google Scholar]
- 1117.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 1118.Sapin E, Lapray D, Berod A, Goutagny R, Leger L, Ravassard P, Clement O, Hanriot L, Fort P, Luppi PH. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS One. 2009;4:e4272. doi: 10.1371/journal.pone.0004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1119.Sarter M, Bruno JP. Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: differential afferent regulation of the basal forebrain by telencephalic and brainstem afferents. Neuroscience. 2000;95:933–952. doi: 10.1016/s0306-4522(99)00487-x. [DOI] [PubMed] [Google Scholar]
- 1120.Sastre JP, Buda C, Kitahama K, Jouvet M. Importance of the ventrolateral region of the periaqueductal gray and adjacent tegmentum in the control of paradoxical sleep as studied by muscimol microinjections in the cat. Neuroscience. 1996;74:415–426. doi: 10.1016/0306-4522(96)00190-x. [DOI] [PubMed] [Google Scholar]
- 1121.Sastre JP, Sakai K, Jouvet M. Persistence of paradoxical sleep in the cat after destruction of the pontine gagantocellular tegmental field with kainic acid. C R Seances Acad Sci D. 1979;289:959–964. [PubMed] [Google Scholar]
- 1122.Sastre JP, Sakai K, Jouvet M. Are the gigantocellular tegmental field neurons responsible for paradoxical sleep? Brain Res. 1981;229:147–161. doi: 10.1016/0006-8993(81)90752-6. [DOI] [PubMed] [Google Scholar]
- 1123.Satoh S, Matsumura H, Hayaishi O. Involvement of adenosine A2A receptor in sleep promotion. Eur J Pharmacol. 1998;351:155–162. doi: 10.1016/s0014-2999(98)00302-1. [DOI] [PubMed] [Google Scholar]
- 1124.Satoh S, Matsumura H, Koike N, Tokunaga Y, Maeda T, Hayaishi O. Region-dependent difference in the sleep-promoting potency of an adenosine A2A receptor agonist. Eur J Neurosci. 1999;11:1587–1597. doi: 10.1046/j.1460-9568.1999.00569.x. [DOI] [PubMed] [Google Scholar]
- 1125.Satoh S, Matsumura H, Suzuki F, Hayaishi O. Promotion of sleep mediated by the A2a-adenosine receptor and possible involvement of this receptor in the sleep induced by prostaglandin D2 in rats. Proc Natl Acad Sci USA. 1996;93:5980–5984. doi: 10.1073/pnas.93.12.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1126.Scammell T, Gerashchenko D, Urade Y, Onoe H, Saper C, Hayaishi O. Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2 . Proc Natl Acad Sci USA. 1998;95:7754–7759. doi: 10.1073/pnas.95.13.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1127.Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol. 2003;53:154–166. doi: 10.1002/ana.10444. [DOI] [PubMed] [Google Scholar]
- 1128.Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA, Saper CB, Urade Y, Hayaishi O. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience. 2001;107:653–663. doi: 10.1016/s0306-4522(01)00383-9. [DOI] [PubMed] [Google Scholar]
- 1129.Scammell TE, Nishino S, Mignot E, Saper CB. Narcolepsy and low CSF orexin (hypocretin) concentration after a diencephalic stroke. Neurology. 2001;56:1751–1753. doi: 10.1212/wnl.56.12.1751. [DOI] [PubMed] [Google Scholar]
- 1130.Scharf MT, Naidoo N, Zimmerman JE, Pack AI. The energy hypothesis of sleep revisited. Prog Neurobiol. 2008;86:264–280. doi: 10.1016/j.pneurobio.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1131.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 1132.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46:388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 1133.Schenck CH, Garcia-Rill E, Skinner RD, Anderson ML, Mahowald MW. A case of REM sleep behavior disorder with autopsy-confirmed Alzheimer’s disease: postmortem brain stem histochemical analyses. Biol Psychiatry. 1996;40:422–425. doi: 10.1016/0006-3223(96)00070-4. [DOI] [PubMed] [Google Scholar]
- 1134.Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep. 2002;25:120–138. doi: 10.1093/sleep/25.2.120. [DOI] [PubMed] [Google Scholar]
- 1135.Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33:1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1136.Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, Fritz B, Eisenberg B, O’Connor J, Kobylarz EJ, Farris S, Machado A, McCagg C, Plum F, Fins JJ, Rezai AR. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 1137.Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003;34:2264–2278. doi: 10.1161/01.STR.0000087786.38997.9E. [DOI] [PubMed] [Google Scholar]
- 1138.Schmidt MH, Valatx JL, Sakai K, Fort P, Jouvet M. Role of the lateral preoptic area in sleep-related erectile mechanisms and sleep generation in the rat. J Neurosci. 2000;20:6640–6647. doi: 10.1523/JNEUROSCI.20-17-06640.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1140.Schmidt MH, Valatx JL, Schmidt HS, Wauquier A, Jouvet M. Experimental evidence of penile erections during paradoxical sleep in the rat. Neuroreport. 1994;5:561–564. doi: 10.1097/00001756-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 1141.Schonrock B, Busselberg D, Haas HL. Properties of tuberomammillary histamine neurones and their response to galanin. Agents Actions. 1991;33:135–137. doi: 10.1007/BF01993148. [DOI] [PubMed] [Google Scholar]
- 1142.Schwartz JC, Griffon N, Diaz J, Levesque D, Sautel F, Sokoloff P, Simon P, Costentin J, Garrido F, Mann A. The D3 receptor and its relevance in psychiatry. Int Clin Psychopharmacol. 1995;10(Suppl 3):15–20. [PubMed] [Google Scholar]
- 1143.Schwartz S, Maquet P. Sleep imaging and the neuro-psychological assessment of dreams. Trends Cogn Sci. 2002;6:23–30. doi: 10.1016/s1364-6613(00)01818-0. [DOI] [PubMed] [Google Scholar]
- 1144.Seelke AM, Blumberg MS. The microstructure of active and quiet sleep as cortical delta activity emerges in infant rats. Sleep. 2008;31:691–699. doi: 10.1093/sleep/31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1145.Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011;146:194–207. doi: 10.1016/j.cell.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1146.Sekirnjak C, Martone ME, Weiser M, Deerinck T, Bueno E, Rudy B, Ellisman M. Subcellular localization of the K+channel subunit Kv3.1b in selected rat CNS neurons. Brain Res. 1997;766:173–187. doi: 10.1016/s0006-8993(97)00527-1. [DOI] [PubMed] [Google Scholar]
- 1147.Selvi Y, Gulec M, Agargun MY, Besiroglu L. Mood changes after sleep deprivation in morningness-eveningness chronotypes in healthy individuals. J Sleep Res. 2007;16:241–244. doi: 10.1111/j.1365-2869.2007.00596.x. [DOI] [PubMed] [Google Scholar]
- 1148.Semba K. Multiple output pathways of the basal forebrain: organization, chemical heterogeneity, and roles in vigilance. Behav Brain Res. 2000;115:117–141. doi: 10.1016/s0166-4328(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 1149.Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- 1150.Sergeeva OA, Eriksson KS, Sharonova IN, Vorobjev VS, Haas HL. GABA(A) receptor heterogeneity in histaminergic neurons. Eur J Neurosci. 2002;16:1472–1482. doi: 10.1046/j.1460-9568.2002.02221.x. [DOI] [PubMed] [Google Scholar]
- 1151.Sergeeva OA, Korotkova TM, Scherer A, Brown RE, Haas HL. Co-expression of non-selective cation channels of the transient receptor potential canonical family in central aminergic neurones. J Neurochem. 2003;85:1547–1552. doi: 10.1046/j.1471-4159.2003.01806.x. [DOI] [PubMed] [Google Scholar]
- 1152.Servos P, Barke KE, Hough LB, Vanderwolf CH. Histamine does not play an essential role in electrocortical activation during waking behavior. Brain Res. 1994;636:98–102. doi: 10.1016/0006-8993(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 1153.Seugnet L, Suzuki Y, Thimgan M, Donlea J, Gimbel SI, Gottschalk L, Duntley SP, Shaw PJ. Identifying sleep regulatory genes using a Drosophila model of insomnia. J Neurosci. 2009;29:7148–7157. doi: 10.1523/JNEUROSCI.5629-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1154.Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila . Curr Biol. 2008;18:1110–1117. doi: 10.1016/j.cub.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1155.Shaffery JP, Sinton CM, Bissette G, Roffwarg HP, Marks GA. Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats. Neuroscience. 2002;110:431–443. doi: 10.1016/s0306-4522(01)00589-9. [DOI] [PubMed] [Google Scholar]
- 1156.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 1157.Shang Y, Griffith LC, Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci USA. 2008;105:19587–19594. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1158.Sharifian A, Farahani S, Pasalar P, Gharavi M, Aminian O. Shift work as an oxidative stressor. J Circadian Rhythms. 2005;3:15. doi: 10.1186/1740-3391-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1159.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster . Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 1160.Shen KZ, North RA. Muscarine increases cation conductance and decreases potassium conductance in rat locus coeruleus neurones. J Physiol. 1992;455:471–485. doi: 10.1113/jphysiol.1992.sp019312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1161.Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1162.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 1163.Shigemoto Y, Fujii Y, Shinomiya K, Kamei C. Participation of histaminergic H1 and noradrenergic alpha 1 receptors in orexin A-induced wakefulness in rats. Brain Res. 2004;1023:121–125. doi: 10.1016/j.brainres.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 1164.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melaninconcentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 1165.Shimono K, Brucher F, Granger R, Lynch G, Taketani M. Origins and distribution of cholinergically induced beta rhythms in hippocampal slices. J Neurosci. 2000;20:8462–8473. doi: 10.1523/JNEUROSCI.20-22-08462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1166.Shin J, Kim D, Bianchi R, Wong RK, Shin HS. Genetic dissection of theta rhythm heterogeneity in mice. Proc Natl Acad Sci USA. 2005;102:18165–18170. doi: 10.1073/pnas.0505498102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1167.Shiromani PJ, Basheer R, Thakkar J, Wagner D, Greco MA, Charness ME. Sleep and wakefulness in c-fos and fos B gene knockout mice. Brain Res. 2000;80:75–87. doi: 10.1016/s0169-328x(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 1168.Shiromani PJ, Fishbein W. Continuous pontine cholinergic microinfusion via minipump induces sustained alterations in rapid eye movement (REM) sleep. Pharmacol Biochem Behav. 1986;25:1253–1261. doi: 10.1016/0091-3057(86)90120-6. [DOI] [PubMed] [Google Scholar]
- 1169.Shiromani PJ, Kilduff TS, Bloom FE, McCarley RW. Cholinergically induced REM sleep triggers Fos-like immunoreactivity in dorsolateral pontine regions associated with REM sleep. Brain Res. 1992;580:351–357. doi: 10.1016/0006-8993(92)90968-f. [DOI] [PubMed] [Google Scholar]
- 1170.Shiromani PJ, Malik M, Winston S, McCarley RW. Time course of Fos-like immunoreactivity associated with cholinergically induced REM sleep. J Neurosci. 1995;15:3500–3508. doi: 10.1523/JNEUROSCI.15-05-03500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1171.Shiromani PJ, Winston S, McCarley RW. Pontine cholinergic neurons show Fos-like immunoreactivity associated with cholinergically induced REM sleep. Brain Res. 1996;38:77–84. doi: 10.1016/0169-328x(95)00325-m. [DOI] [PubMed] [Google Scholar]
- 1172.Shouse MN, Staba RJ, Saquib SF, Farber PR. Monoamines and sleep: microdialysis findings in pons and amygdala. Brain Res. 2000;860:181–189. doi: 10.1016/s0006-8993(00)02013-8. [DOI] [PubMed] [Google Scholar]
- 1173.Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–1063. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1174.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1175.Siegel JM, Manger PR, Nienhuis R, Fahringer HM, Pettigrew JD. Monotremes and the evolution of rapid eye movement sleep. Philos Trans R Soc Lond B Biol Sci. 1998;353:1147–1157. doi: 10.1098/rstb.1998.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1176.Siegel JM, Nienhuis R, Fahringer HM, Chiu C, Dement WC, Mignot E, Lufkin R. Activity of medial mesopontine units during cataplexy and sleep-waking states in the narcoleptic dog. J Neurosci. 1992;12:1640–1646. doi: 10.1523/JNEUROSCI.12-05-01640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1177.Siegel JM, Nienhuis R, Fahringer HM, Paul R, Shiromani P, Dement WC, Mignot E, Chiu C. Neuronal activity in narcolepsy: identification of cataplexy-related cells in the medial medulla. Science. 1991;252:1315–1318. doi: 10.1126/science.1925546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1178.Siegel JM, Nienhuis R, Gulyani S, Ouyang S, Wu MF, Mignot E, Switzer RC, McMurry G, Cornford M. Neuronal degeneration in canine narcolepsy. J Neurosci. 1999;19:248–257. doi: 10.1523/JNEUROSCI.19-01-00248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1179.Siegel JM, Nienhuis R, Tomaszewski KS. Rostral brainstem contributes to medullary inhibition of muscle tone. Brain Res. 1983;268:344–348. doi: 10.1016/0006-8993(83)90501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1180.Silva RH, Kameda SR, Carvalho RC, Takatsu-Coleman AL, Niigaki ST, Abilio VC, Tufik S, Frussa-Filho R. Anxiogenic effect of sleep deprivation in the elevated plusmaze test in mice. Psychopharmacology. 2004;176:115–122. doi: 10.1007/s00213-004-1873-z. [DOI] [PubMed] [Google Scholar]
- 1181.Silvestri AJ. REM sleep deprivation affects extinction of cued but not contextual fear conditioning. Physiol Behav. 2005;84:343–349. doi: 10.1016/j.physbeh.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 1182.Simon AP, Poindessous-Jazat F, Dutar P, Epelbaum J, Bassant MH. Firing properties of anatomically identified neurons in the medial septum of anesthetized and unanesthetized restrained rats. J Neurosci. 2006;26:9038–9046. doi: 10.1523/JNEUROSCI.1401-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1183.Singareddy RK, Balon R. Sleep in posttraumatic stress disorder. Ann Clin Psychiatry. 2002;14:183–190. doi: 10.1023/a:1021190620773. [DOI] [PubMed] [Google Scholar]
- 1184.Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsaki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1185.Sitaram N, Gillin JC. Development and use of pharmacological probes of the CNS in man: evidence of cholinergic abnormality in primary affective illness. Biol Psychiatry. 1980;15:925–955. [PubMed] [Google Scholar]
- 1186.Sitaram N, Wyatt RJ, Dawson S, Gillin JC. REM sleep induction by physostigmine infusion during sleep. Science. 1976;191:1281–1283. doi: 10.1126/science.176724. [DOI] [PubMed] [Google Scholar]
- 1187.Skagerberg G, Lindvall O. Organization of diencephalic dopamine neurones projecting to the spinal cord in the rat. Brain Res. 1985;342:340–351. doi: 10.1016/0006-8993(85)91134-5. [DOI] [PubMed] [Google Scholar]
- 1188.Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 1189.Skatrud JB, Dempsey JA, Badr S, Begle RL. Effect of airway impedance on CO2 retention and respiratory muscle activity during NREM sleep. J Appl Physiol. 1988;65:1676–1685. doi: 10.1152/jappl.1988.65.4.1676. [DOI] [PubMed] [Google Scholar]
- 1190.Smith C. Sleep states and memory processes. Behav Brain Res. 1995;69:137–145. doi: 10.1016/0166-4328(95)00024-n. [DOI] [PubMed] [Google Scholar]
- 1191.Smith C, Rose GM. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol Behav. 1996;59:93–97. doi: 10.1016/0031-9384(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 1192.Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW. Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci USA. 1981;78:3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1193.Sofroniew MV, Priestley JV, Consolazione A, Eckenstein F, Cuello AC. Cholinergic projections from the midbrain and pons to the thalamus in the rat, identified by combined retrograde tracing and choline acetyltransferase immunohistochemistry. Brain Res. 1985;329:213–223. doi: 10.1016/0006-8993(85)90527-x. [DOI] [PubMed] [Google Scholar]
- 1194.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1195.Soja PJ. Glycine-mediated postsynaptic inhibition is responsible for REM sleep atonia. Sleep. 2008;31:1483–1486. doi: 10.1093/sleep/31.11.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1196.Soja PJ, Lopez-Rodriguez F, Morales FR, Chase MH. The postsynaptic inhibitory control of lumbar motoneurons during the atonia of active sleep: effect of strychnine on motoneuron properties. J Neurosci. 1991;11:2804–2811. doi: 10.1523/JNEUROSCI.11-09-02804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1197.Soja PJ, Lopez-Rodriguez F, Morales FR, Chase MH. Effects of excitatory amino acid antagonists on the phasic depolarizing events that occur in lumbar motoneurons during REM periods of active sleep. J Neurosci. 1995;15:4068–4076. doi: 10.1523/JNEUROSCI.15-05-04068.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1198.Solms M. Dreaming and REM sleep are controlled by different brain mechanisms. Behav Brain Sci. 2000;23:843–850. doi: 10.1017/s0140525x00003988. [DOI] [PubMed] [Google Scholar]
- 1199.Soltesz I, Lightowler S, Leresche N, Jassik-Gerschenfeld D, Pollard CE, Crunelli V. Two inward currents and the transformation of low-frequency oscillations of rat and cat thalamocortical cells. J Physiol. 1991;441:175–197. doi: 10.1113/jphysiol.1991.sp018745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1200.Spiegel K, Tasali E, Leproult R, Van CE. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1201.Srividya R, Mallick HN, Kumar VM. Differences in the effects of medial and lateral preoptic lesions on thermoregulation and sleep in rats. Neuroscience. 2006;139:853–864. doi: 10.1016/j.neuroscience.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 1202.Stanton PK, Sarvey JM. Depletion of norepinephrine, but not serotonin, reduces long-term potentiation in the dentate gyrus of rat hippocampal slices. J Neurosci. 1985;5:2169–2176. doi: 10.1523/JNEUROSCI.05-08-02169.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1203.Starzl TE, Magoun HW. Organization of the diffuse thalamic projection system. J Neurophysiol. 1951;14:133–146. doi: 10.1152/jn.1951.14.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1204.Stefansson H, Rye DB, Hicks A, Petursson H, Ingason A, Thorgeirsson TE, Palsson S, Sigmundsson T, Sigurdsson AP, Eiriksdottir I, Soebech E, Bliwise D, Beck JM, Rosen A, Waddy S, Trotti LM, Iranzo A, Thambisetty M, Hardarson GA, Kristjansson K, Gudmundsson LJ, Thorsteinsdottir U, Kong A, Gulcher JR, Gudbjartsson D, Stefansson K. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357:639–647. doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- 1205.Steffensen SC, Lee RS, Stobbs SH, Henriksen SJ. Responses of ventral tegmental area GABA neurons to brain stimulation reward. Brain Res. 2001;906:190–197. doi: 10.1016/s0006-8993(01)02581-1. [DOI] [PubMed] [Google Scholar]
- 1206.Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1207.Steidl S, Yeomans JS. M5 muscarinic receptor knockout mice show reduced morphine- induced locomotion but increased locomotion after cholinergic antagonism in the ventral tegmental area. J Pharmacol Exp Ther. 2009;328:263–275. doi: 10.1124/jpet.108.144824. [DOI] [PubMed] [Google Scholar]
- 1208.Steiger A, Kimura M. Wake and sleep EEG provide biomarkers in depression. J Psychiatr Res. 2010;44:242–252. doi: 10.1016/j.jpsychires.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 1209.Steinfels GF, Heym J, Strecker RE, Jacobs BL. Behavioral correlates of dopaminergic unit activity in freely moving cats. Brain Res. 1983;258:217–228. doi: 10.1016/0006-8993(83)91145-9. [DOI] [PubMed] [Google Scholar]
- 1210.Steininger TL, Gong H, McGinty D, Szymusiak R. Subregional organization of preoptic area/anterior hypothalamic projections to arousal-related monoaminergic cell groups. J Comp Neurol. 2001;429:638–653. [PubMed] [Google Scholar]
- 1211.Steininger TL, Wainer BH, Blakely RD, Rye DB. Serotonergic dorsal raphe nucleus projections to the cholinergic and noncholinergic neurons of the pedunculopontine tegmental region: a light and electron microscopic anterograde tracing and immunohistochemical study. J Comp Neurol. 1997;382:302–322. doi: 10.1002/(sici)1096-9861(19970609)382:3<302::aid-cne2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 1212.Steinlein O, Anokhin A, Yping M, Schalt E, Vogel F. Localization of a gene for the human low-voltage EEG on 20q and genetic heterogeneity. Genomics. 1992;12:69–73. doi: 10.1016/0888-7543(92)90408-k. [DOI] [PubMed] [Google Scholar]
- 1213.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 1214.Stenberg D, Litonius E, Halldner L, Johansson B, Fredholm BB, Porkka-Heiskanen T. Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J Sleep Res. 2003;12:283–290. doi: 10.1046/j.0962-1105.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 1215.Steriade M. Thalamic origin of sleep spindles: Morison and Bassett (1945) J Neurophysiol. 1995;73:921–922. doi: 10.1152/jn.1995.73.3.921. [DOI] [PubMed] [Google Scholar]
- 1216.Steriade M. Acetylcholine systems and rhythmic activities during the waking-sleep cycle. Prog Brain Res. 2004;145:179–196. doi: 10.1016/S0079-6123(03)45013-9. [DOI] [PubMed] [Google Scholar]
- 1217.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 1218.Steriade M, Amzica F. Intracortical and corticothalamic coherency of fast spontaneous oscillations. Proc Natl Acad Sci USA. 1996;93:2533–2538. doi: 10.1073/pnas.93.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1219.Steriade M, Amzica F, Contreras D. Synchronization of fast (30–40 Hz) spontaneous cortical rhythms during brain activation. J Neurosci. 1996;16:392–417. doi: 10.1523/JNEUROSCI.16-01-00392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1220.Steriade M, Amzica F, Nunez A. Cholinergic and noradrenergic modulation of the slow (~03 Hz) oscillation in neocortical cells. J Neurophysiol. 1993;70:1385–1400. doi: 10.1152/jn.1993.70.4.1385. [DOI] [PubMed] [Google Scholar]
- 1221.Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1222.Steriade M, Deschenes M. The thalamus as a neuronal oscillator. Brain Res. 1984;320:1–63. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- 1223.Steriade M, Deschenes M, Domich L, Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985;54:1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- 1224.Steriade M, Domich L, Oakson G, Deschenes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57:260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- 1225.Steriade M, Dossi RC, Nunez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholinergic suppression. J Neurosci. 1991;11:3200–3217. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1226.Steriade M, Dossi RC, Pare D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Natl Acad Sci USA. 1991;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1227.Steriade M, Glenn LL. Neocortical and caudate projections of intralaminar thalamic neurons and their synaptic excitation from midbrain reticular core. J Neurophysiol. 1982;48:352–371. doi: 10.1152/jn.1982.48.2.352. [DOI] [PubMed] [Google Scholar]
- 1228.Steriade M, McCarley RW. Brain Control of Wakefulness and Sleep. New York: Plenum; 2005. [Google Scholar]
- 1229.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 1230.Steriade M, Nunez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1231.Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1232.Steriade M, Oakson G, Ropert N. Firing rates and patterns of midbrain reticular neurons during steady and transitional states of the sleep-waking cycle. Exp Brain Res. 1982;46:37–51. doi: 10.1007/BF00238096. [DOI] [PubMed] [Google Scholar]
- 1233.Steriade M, Pare D, Bouhassira D, Deschenes M, Oakson G. Phasic activation of lateral geniculate and perigeniculate thalamic neurons during sleep with ponto-geniculo- occipital waves. J Neurosci. 1989;9:2215–2229. doi: 10.1523/JNEUROSCI.09-07-02215.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1234.Steriade M, Pare D, Datta S, Oakson G, Curro DR. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990;10:2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1235.Steriade M, Pare D, Parent A, Smith Y. Projections of cholinergic and non-cholinergic neurons of the brainstem core to relay and associational thalamic nuclei in the cat and macaque monkey. Neuroscience. 1988;25:47–67. doi: 10.1016/0306-4522(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 1236.Steriade M, Sakai K, Jouvet M. Bulbo-thalamic neurons related to thalamocortical activation processes during paradoxical sleep. Exp Brain Res. 1984;54:463–475. doi: 10.1007/BF00235472. [DOI] [PubMed] [Google Scholar]
- 1237.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 1238.Steriade MM, McCarley RW. Brain Control of Wakefulness and Sleep. New York: Kluwer Academic/Plenum; 2005. [Google Scholar]
- 1239.Sterpenich V, Albouy G, Boly M, Vandewalle G, Darsaud A, Balteau E, ng-Vu TT, Desseilles M, D’Argembeau A, Gais S, Rauchs G, Schabus M, Degueldre C, Luxen A, Collette F, Maquet P. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biol. 2007;5:e282. doi: 10.1371/journal.pbio.0050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1240.Sterpenich V, Albouy G, Darsaud A, Schmidt C, Vandewalle G, ng Vu TT, Desseilles M, Phillips C, Degueldre C, Balteau E, Collette F, Luxen A, Maquet P. Sleep promotes the neural reorganization of remote emotional memory. J Neurosci. 2009;29:5143–5152. doi: 10.1523/JNEUROSCI.0561-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1241.Stevens DR, Birnstiel S, Gerber U, McCarley RW, Greene RW. Nicotinic depolarizations of rat medial pontine reticular formation neurons studied in vitro. Neuroscience. 1993;57:419–424. doi: 10.1016/0306-4522(93)90073-o. [DOI] [PubMed] [Google Scholar]
- 1242.Stevens DR, Eriksson KS, Brown RE, Haas HL. The mechanism of spontaneous firing in histamine neurons. Behav Brain Res. 2001;124:105–112. doi: 10.1016/s0166-4328(01)00219-4. [DOI] [PubMed] [Google Scholar]
- 1243.Stevens DR, Kuramasu A, Eriksson KS, Selbach O, Haas HL. Alpha 2-adrenergic receptor-mediated presynaptic inhibition of GABAergic IPSPs in rat histaminergic neurons. Neuropharmacology. 2004;46:1018–1022. doi: 10.1016/j.neuropharm.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 1244.Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: a multi-step process occurring during sleep. J Cogn Neurosci. 2000;12:246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- 1245.Strassman A, Highstein SM, McCrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J Comp Neurol. 1986;249:337–357. doi: 10.1002/cne.902490303. [DOI] [PubMed] [Google Scholar]
- 1246.Strassman A, Highstein SM, McCrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. II. Inhibitory burst neurons. J Comp Neurol. 1986;249:358–380. doi: 10.1002/cne.902490304. [DOI] [PubMed] [Google Scholar]
- 1247.Strecker RE, Morairty S, Thakkar MM, Porkka-Heiskanen T, Basheer R, Dauphin LJ, Rainnie DG, Portas CM, Greene RW, McCarley RW. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res. 2000;115:183–204. doi: 10.1016/s0166-4328(00)00258-8. [DOI] [PubMed] [Google Scholar]
- 1248.Suchecki D, Tiba PA, Tufik S. Hormonal and behavioural responses of paradoxical sleep-deprived rats to the elevated plus maze. J Neuroendocrinol. 2002;14:549–554. doi: 10.1046/j.1365-2826.2002.00812.x. [DOI] [PubMed] [Google Scholar]
- 1249.Sun C, Sieghart W, Kapur J. Distribution of alpha1, alpha4, gamma2, and delta subunits of GABAAreceptors in hippocampal granule cells. Brain Res. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1250.Sun X, Whitefield S, Rusak B, Semba K. Electrophysiological analysis of suprachiasmatic nucleus projections to the ventrolateral preoptic area in the rat. Eur J Neurosci. 2001;14:1257–1274. doi: 10.1046/j.0953-816x.2001.0001755.x. [DOI] [PubMed] [Google Scholar]
- 1251.Suntsova N, Guzman-Marin R, Kumar S, Alam MN, Szymusiak R, McGinty D. The median preoptic nucleus reciprocally modulates activity of arousal-related and sleep-related neurons in the perifornical lateral hypothalamus. J Neurosci. 2007;27:1616–1630. doi: 10.1523/JNEUROSCI.3498-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1252.Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol. 2002;543:665–677. doi: 10.1113/jphysiol.2002.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1253.Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1254.Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- 1255.Szymusiak R, McGinty D. Sleep suppression following kainic acid-induced lesions of the basal forebrain. Exp Neurol. 1986;94:598–614. doi: 10.1016/0014-4886(86)90240-2. [DOI] [PubMed] [Google Scholar]
- 1256.Szymusiak R, McGinty D, Fairchild MD, Jenden DJ. Sleep-wake disturbances in an animal model of chronic cholinergic insufficiency. Brain Res. 1993;629:141–145. doi: 10.1016/0006-8993(93)90492-6. [DOI] [PubMed] [Google Scholar]
- 1257.Taddese A, Bean BP. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron. 2002;33:587–600. doi: 10.1016/s0896-6273(02)00574-3. [DOI] [PubMed] [Google Scholar]
- 1258.Tafti M, Chollet D, Valatx JL, Franken P. Quantitative trait loci approach to the genetics of sleep in recombinant inbred mice. J Sleep Res. 1999;8(Suppl 1):37–43. doi: 10.1046/j.1365-2869.1999.00007.x. [DOI] [PubMed] [Google Scholar]
- 1259.Tafti M, Nishino S, Aldrich MS, Liao W, Dement WC, Mignot E. Major histocompatibility class II molecules in the CNS: increased microglial expression at the onset of narcolepsy in canine model. J Neurosci. 1996;16:4588–4595. doi: 10.1523/JNEUROSCI.16-15-04588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1260.Tafti M, Petit B, Chollet D, Neidhart E, de BF, Kiss JZ, Wood PA, Franken P. Deficiency in short-chain fatty acid beta-oxidation affects theta oscillations during sleep. Nat Genet. 2003;34:320–325. doi: 10.1038/ng1174. [DOI] [PubMed] [Google Scholar]
- 1261.Taheri S, Zeitzer JM, Mignot E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu Rev Neurosci. 2002;25:283–313. doi: 10.1146/annurev.neuro.25.112701.142826. [DOI] [PubMed] [Google Scholar]
- 1262.Taishi P, Chen Z, Obal F, Jr, Hansen MK, Zhang J, Fang J, Krueger JM. Sleep-associated changes in interleukin-1beta mRNA in the brain. J Interferon Cytokine Res. 1998;18:793–798. doi: 10.1089/jir.1998.18.793. [DOI] [PubMed] [Google Scholar]
- 1263.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1264.Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci. 2006;26:10292–10298. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1265.Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–870. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 1266.Takahashi K, Lin JS, Sakai K. Characterization and mapping of sleep-waking specific neurons in the basal forebrain and preoptic hypothalamus in mice. Neuroscience. 2009;161:269–292. doi: 10.1016/j.neuroscience.2009.02.075. [DOI] [PubMed] [Google Scholar]
- 1267.Takahashi S, Kapas L, Fang J, Seyer JM, Wang Y, Krueger JM. An interleukin-1 receptor fragment inhibits spontaneous sleep and muramyl dipeptide-induced sleep in rabbits. Am J Physiol Regul Integr Comp Physiol. 1996;271:R101–R108. doi: 10.1152/ajpregu.1996.271.1.R101. [DOI] [PubMed] [Google Scholar]
- 1268.Takakusaki K, Kohyama J, Matsuyama K, Mori S. Medullary reticulospinal tract mediating the generalized motor inhibition in cats: parallel inhibitory mechanisms acting on motoneurons and on interneuronal transmission in reflex pathways. Neuroscience. 2001;103:511–527. doi: 10.1016/s0306-4522(00)00586-8. [DOI] [PubMed] [Google Scholar]
- 1269.Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (Ttype) calcium channels. J Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1270.Tan A, Salgado M, Fahn S. Rapid eye movement sleep behavior disorder preceding Parkinson’s disease with therapeutic response to levodopa. Mov Disord. 1996;11:214–216. doi: 10.1002/mds.870110216. [DOI] [PubMed] [Google Scholar]
- 1271.Tartar JL, McKenna JT, Ward CP, McCarley RW, Strecker RE, Brown RE. Sleep fragmentation reduces hippocampal CA1 pyramidal cell excitability and response to adenosine. Neurosci Lett. 2010;469:1–5. doi: 10.1016/j.neulet.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1272.Tartar JL, Ward CP, Cordeira JW, Legare SL, Blanchette AJ, McCarley RW, Strecker RE. Experimental sleep fragmentation and sleep deprivation in rats increases exploration in an open field test of anxiety while increasing plasma corticosterone levels. Behav Brain Res. 2009;197:450–453. doi: 10.1016/j.bbr.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1273.Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, Brown RE, Strecker RE. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1274.Tempesta D, Couyoumdjian A, Curcio G, Moroni F, Marzano C, De GL, Ferrara M. Lack of sleep affects the evaluation of emotional stimuli. Brain Res Bull. 2010;82:104–108. doi: 10.1016/j.brainresbull.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 1275.Terao A, Steininger TL, Hyder K, Apte-Deshpande A, Ding J, Rishipathak D, Davis RW, Heller HC, Kilduff TS. Differential increase in the expression of heat shock protein family members during sleep deprivation and during sleep. Neuroscience. 2003;116:187–200. doi: 10.1016/s0306-4522(02)00695-4. [DOI] [PubMed] [Google Scholar]
- 1276.Thakkar MM, Delgiacco RA, Strecker RE, McCarley RW. Adenosinergic inhibition of basal forebrain wakefulness-active neurons: a simultaneous unit recording and microdialysis study in freely behaving cats. Neuroscience. 2003;122:1107–1113. doi: 10.1016/j.neuroscience.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 1277.Thakkar MM, Engemann SC, Walsh KM, Sahota PK. Adenosine and the homeostatic control of sleep: effects of A1 receptor blockade in the perifornical lateral hypothalamus on sleep-wakefulness. Neuroscience. 2008;153:875–880. doi: 10.1016/j.neuroscience.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 1278.Thakkar MM, Ramesh V, Cape EG, Winston S, Strecker RE, McCarley RW. REM sleep enhancement and behavioral cataplexy following orexin (hypocretin)-II receptor antisense perfusion in the pontine reticular formation. Sleep Res Online. 1999;2:112–120. [PubMed] [Google Scholar]
- 1279.Thakkar MM, Ramesh V, Strecker RE, McCarley RW. Microdialysis perfusion of orexin-A in the basal forebrain increases wakefulness in freely behaving rats. Arch Ital Biol. 2001;139:313–328. [PubMed] [Google Scholar]
- 1280.Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J Neurosci. 1998;18:5490–5497. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1281.Thakkar MM, Tao R, Yunren B, Winston S, Chen L, McCarley RW. GABA release in the pontine reticular formation is lowest during REM sleep. Soc Neurosci Abstr. 2004 895.5490-5. [Google Scholar]
- 1282.Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J Neurosci. 2003;23:4278–4287. doi: 10.1523/JNEUROSCI.23-10-04278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1283.Thankachan S, Kaur S, Shiromani PJ. Activity of pontine neurons during sleep and cataplexy in hypocretin knock-out mice. J Neurosci. 2009;29:1580–1585. doi: 10.1523/JNEUROSCI.5151-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1284.Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1285.Thannickal TC, Nienhuis R, Siegel JM. Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep. 2009;32:993–998. doi: 10.1093/sleep/32.8.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1286.Thinschmidt JS, Kinney GG, Kocsis B. The supramammillary nucleus: is it necessary for the mediation of hippocampal theta rhythm? Neuroscience. 1995;67:301–312. doi: 10.1016/0306-4522(95)00045-k. [DOI] [PubMed] [Google Scholar]
- 1287.Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 1288.Thomas RJ, Rosen BR, Stern CE, Weiss JW, Kwong KK. Functional imaging of working memory in obstructive sleep-disordered breathing. J Appl Physiol. 2005;98:2226–2234. doi: 10.1152/japplphysiol.01225.2004. [DOI] [PubMed] [Google Scholar]
- 1289.Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study. Proc Natl Acad Sci USA. 2001;98:1924–1929. doi: 10.1073/pnas.041430398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1290.Timofeev I, Steriade M. Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. J Neurophysiol. 1996;76:4152–4168. doi: 10.1152/jn.1996.76.6.4152. [DOI] [PubMed] [Google Scholar]
- 1291.Tison F, Wenning GK, Quinn NP, Smith SJ. REM sleep behaviour disorder as the presenting symptom of multiple system atrophy. J Neurol Neurosurg Psychiatry. 1995;58:379–380. doi: 10.1136/jnnp.58.3.379-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1292.Tobler I, Borbely AA. Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol. 1986;64:74–76. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- 1293.Tobler I, Borbely AA, Groos G. The effect of sleep deprivation on sleep in rats with suprachiasmatic lesions. Neurosci Lett. 1983;42:49–54. doi: 10.1016/0304-3940(83)90420-2. [DOI] [PubMed] [Google Scholar]
- 1294.Tobler I, Deboer T, Fischer M. Sleep and sleep regulation in normal and prion protein-deficient mice. J Neurosci. 1997;17:1869–1879. doi: 10.1523/JNEUROSCI.17-05-01869.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1295.Tobler I, Kopp C, Deboer T, Rudolph U. Diazepam-induced changes in sleep: role of the alpha 1 GABA(A) receptor subtype. Proc Natl Acad Sci USA. 2001;98:6464–6469. doi: 10.1073/pnas.111055398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1296.Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptacek LJ, Fu YH. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 1297.Tomasi D, Wang RL, Telang F, Boronikolas V, Jayne MC, Wang GJ, Fowler JS, Volkow ND. Impairment of attentional networks after 1 night of sleep deprivation. Cereb Cortex. 2009;19:233–240. doi: 10.1093/cercor/bhn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1298.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 1299.Tononi G, Cirelli C. Staying awake puts pressure on brain arousal systems. J Clin Invest. 2007;117:3648–3650. doi: 10.1172/JCI34250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1300.Tononi G, Pompeiano M, Cirelli C. Suppression of desynchronized sleep through microinjection of the alpha 2-adrenergic agonist clonidine in the dorsal pontine tegmentum of the cat. Pflügers Arch. 1991;418:512–518. doi: 10.1007/BF00497780. [DOI] [PubMed] [Google Scholar]
- 1301.Tononi G, Pompeiano M, Pompeiano O. Modulation of desynchronized sleep through microinjection of beta-adrenergic agonists and antagonists in the dorsal pontine tegmentum of the cat. Pflügers Arch. 1989;415:142–149. doi: 10.1007/BF00370584. [DOI] [PubMed] [Google Scholar]
- 1302.Torres GE, Chaput Y, Andrade R. Cyclic AMP and protein kinase A mediate 5-hydroxytryptamine type 4 receptor regulation of calcium-activated potassium current in adult hippocampal neurons. Mol Pharmacol. 1995;47:191–197. [PubMed] [Google Scholar]
- 1303.Torterolo P, Morales FR, Chase MH. GABAergic mechanisms in the pedunculopontine tegmental nucleus of the cat promote active (REM) sleep. Brain Res. 2002;944:1–9. doi: 10.1016/s0006-8993(02)02475-7. [DOI] [PubMed] [Google Scholar]
- 1304.Toyoda H, Tanaka S, Miyagawa T, Honda Y, Tokunaga K, Honda M. Anti-Tribbles homolog 2 autoantibodies in Japanese patients with narcolepsy. Sleep. 2010;33:875–878. doi: 10.1093/sleep/33.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1305.Trachsel L, Edgar DM, Seidel WF, Heller HC, Dement WC. Sleep homeostasis in suprachiasmatic nuclei-lesioned rats: effects of sleep deprivation and triazolam administration. Brain Res. 1992;589:253–261. doi: 10.1016/0006-8993(92)91284-l. [DOI] [PubMed] [Google Scholar]
- 1306.Traub RD, Bibbig A, Fisahn A, Lebeau FE, Whittington MA, Buhl EH. A model of gamma-frequency network oscillations induced in the rat CA3 region by carbachol in vitro. Eur J Neurosci. 2000;12:4093–4106. doi: 10.1046/j.1460-9568.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 1307.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 1308.Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- 1309.Tucci V, Stegagno L, Vandi S, Ferrillo F, Palomba D, Vignatelli L, Ferini-Strambi L, Montagna P, Plazzi G. Emotional information processing in patients with narcolepsy: a psychophysiologic investigation. Sleep. 2003;26:558–564. doi: 10.1093/sleep/26.5.558. [DOI] [PubMed] [Google Scholar]
- 1310.Tufik S, Lindsey CJ, Carlini EA. Does REM sleep deprivation induce a supersensitivity of dopaminergic receptors in the rat brain? Pharmacology. 1978;16:98–105. doi: 10.1159/000136753. [DOI] [PubMed] [Google Scholar]
- 1311.Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci. 2007;27:8184–8189. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1312.Tully K, Bolshakov VY. Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. Mol Brain. 2010;3:15. doi: 10.1186/1756-6606-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1313.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee- Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1314.Turner RS, D’Amato CJ, Chervin RD, Blaivas M. The pathology of REM sleep behavior disorder with comorbid Lewy body dementia. Neurology. 2000;55:1730–1732. doi: 10.1212/wnl.55.11.1730. [DOI] [PubMed] [Google Scholar]
- 1315.Uchiyama M, Isse K, Tanaka K, Yokota N, Hamamoto M, Aida S, Ito Y, Yoshimura M, Okawa M. Incidental Lewy body disease in a patient with REM sleep behavior disorder. Neurology. 1995;45:709–712. doi: 10.1212/wnl.45.4.709. [DOI] [PubMed] [Google Scholar]
- 1316.Ueno R, Honda K, Inoue S, Hayaishi O. Prostaglandin D2, a cerebral sleep-inducing substance in rats. Proc Natl Acad Sci USA. 1983;80:1735–1737. doi: 10.1073/pnas.80.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1317.Ueno R, Ishikawa Y, Nakayama T, Hayaishi O. Prostaglandin D2 induces sleep when microinjected into the preoptic area of conscious rats. Biochem Biophys Res Commun. 1982;109:576–582. doi: 10.1016/0006-291x(82)91760-0. [DOI] [PubMed] [Google Scholar]
- 1318.Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci USA. 2009;106:9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1319.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 1320.Urade Y, Eguchi N, Qu WM, Sakata M, Huang ZL, Chen JF, Schwarzschild MA, Fink JS, Hayaishi O. Sleep regulation in adenosine A2A receptor-deficient mice. Neurology. 2003;61:S94–S96. doi: 10.1212/01.wnl.0000095222.41066.5e. [DOI] [PubMed] [Google Scholar]
- 1321.Uschakov A, Gong H, McGinty D, Szymusiak R. Efferent projections from the median preoptic nucleus to sleep- and arousal-regulatory nuclei in the rat brain. Neuroscience. 2007;150:104–120. doi: 10.1016/j.neuroscience.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1322.Valatx JL, Bugat R, Jouvet M. Genetic studies of sleep in mice. Nature. 1972;238:226–227. doi: 10.1038/238226a0. [DOI] [PubMed] [Google Scholar]
- 1323.Valdes JL, Farias P, Ocampo-Garces A, Cortes N, Seron-Ferre M, Torrealba F. Arousal and differential Fos expression in histaminergic neurons of the ascending arousal system during a feeding-related motivated behaviour. Eur J Neurosci. 2005;21:1931–1942. doi: 10.1111/j.1460-9568.2005.04013.x. [DOI] [PubMed] [Google Scholar]
- 1324.Van Beijsterveldt CE, Boomsma DI. Genetics of the human electroencephalogram (EEG) and event-related brain potentials (ERPs): a review. Hum Genet. 1994;94:319–330. doi: 10.1007/BF00201587. [DOI] [PubMed] [Google Scholar]
- 1325.Van den Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42:635–652. doi: 10.1016/s0896-6273(04)00251-x. [DOI] [PubMed] [Google Scholar]
- 1326.Van den Pol AN, Ghosh PK, Liu RJ, Li Y, Aghajanian GK, Gao XB. Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J Physiol. 2002;541:169–185. doi: 10.1113/jphysiol.2002.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1327.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 1328.Van der HE, Gujar N, Walker MP. Sleep deprivation impairs the accurate recognition of human emotions. Sleep. 2010;33:335–342. doi: 10.1093/sleep/33.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1329.Van der KB, Blitz R, Burr W, Sherry S, Hartmann E. Nightmares and trauma: a comparison of nightmares after combat with lifelong nightmares in veterans. Am J Psychiatry. 1984;141:187–190. doi: 10.1176/ajp.141.2.187. [DOI] [PubMed] [Google Scholar]
- 1330.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- 1331.Van Dongen HP, Maislin G, Dinges DF. Dealing with inter-individual differences in the temporal dynamics of fatigue and performance: importance and techniques. Aviat Space Environ Med. 2004;75 A147-423–A154. [PubMed] [Google Scholar]
- 1332.Van Dort CJ, Baghdoyan HA, Lydic R. Adenosine A(1) and A(2A) receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. J Neurosci. 2009;29:871–881. doi: 10.1523/JNEUROSCI.4111-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1333.Van CE, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S23–S28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1334.Vandekerckhove M, Cluydts R. The emotional brain and sleep: an intimate relationship. Sleep Med Rev. 2010;14:219–226. doi: 10.1016/j.smrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 1335.Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- 1336.Vanini G, Torterolo P, McGregor R, Chase MH, Morales FR. GABAergic processes in the mesencephalic tegmentum modulate the occurrence of active (rapid eye movement) sleep in guinea pigs. Neuroscience. 2007;145:1157–1167. doi: 10.1016/j.neuroscience.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 1337.Vanini G, Wathen BL, Lydic R, Baghdoyan HA. Endogenous GABA levels in the pontine reticular formation are greater during wakefulness than during rapid eye movement sleep. J Neurosci. 2011;31:2649–2656. doi: 10.1523/JNEUROSCI.5674-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1338.Vanni-Mercier G, Gigout S, Debilly G, Lin JS. Waking selective neurons in the posterior hypothalamus and their response to histamine H3-receptor ligands: an electrophysiological study in freely moving cats. Behav Brain Res. 2003;144:227–241. doi: 10.1016/s0166-4328(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 1339.Vanni-Mercier G, Pelisson D, Goffart L, Sakai K, Jouvet M. Eye saccade dynamics during paradoxical sleep in the cat. Eur J Neurosci. 1994;6:1298–1306. doi: 10.1111/j.1460-9568.1994.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 1340.Vanni-Mercier G, Sakai K, Lin JS, Jouvet M. Mapping of cholinoceptive brainstem structures responsible for the generation of paradoxical sleep in the cat. Arch Ital Biol. 1989;127:133–164. [PubMed] [Google Scholar]
- 1341.Vanni-Mercier G, Sakai K, Lin JS, Jouvet M. Carbachol microinjections in the mediodorsal pontine tegmentum are unable to induce paradoxical sleep after caudal pontine and prebulbar transections in the cat. Neurosci Lett. 1991;130:41–45. doi: 10.1016/0304-3940(91)90222-f. [DOI] [PubMed] [Google Scholar]
- 1342.Varela C, Sherman SM. Differences in response to serotonergic activation between first and higher order thalamic nuclei. Cereb Cortex. 2009;19:1776–1786. doi: 10.1093/cercor/bhn208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1343.Varga V, Hangya B, Kranitz K, Ludanyi A, Zemankovics R, Katona I, Shigemoto R, Freund TF, Borhegyi Z. The presence of pacemaker HCN channels identifies theta rhythmic GABAergic neurons in the medial septum. J Physiol. 2008;586:3893–3915. doi: 10.1113/jphysiol.2008.155242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1344.Vazquez J, Baghdoyan HA. GABAAreceptors inhibit acetylcholine release in cat pontine reticular formation: implications for REM sleep regulation. J Neurophysiol. 2004;92:2198–2206. doi: 10.1152/jn.00099.2004. [DOI] [PubMed] [Google Scholar]
- 1345.Vazquez J, Hall SC, Greco MA. Protein expression is altered during spontaneous sleep in aged Sprague Dawley rats. Brain Res. 2009;1298:37–45. doi: 10.1016/j.brainres.2009.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1346.Vazquez J, Hall SC, Witkowska HE, Greco MA. Rapid alterations in cortical protein profiles underlie spontaneous sleep and wake bouts. J Cell Biochem. 2008;105:1472–1484. doi: 10.1002/jcb.21970. [DOI] [PubMed] [Google Scholar]
- 1347.Vazquez J, Lydic R, Baghdoyan HA. The nitric oxide synthase inhibitor NG-Nitro-l-arginine increases basal forebrain acetylcholine release during sleep and wakefulness. J Neurosci. 2002;22:5597–5605. doi: 10.1523/JNEUROSCI.22-13-05597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1348.Veale EL, Kennard LE, Sutton GL, MacKenzie G, Sandu C, Mathie A. Gαq-mediated regulation of TASK3 two-pore domain potassium channels: the role of protein kinase C. Mol Pharmacol. 2007;71:1666–1675. doi: 10.1124/mol.106.033241. [DOI] [PubMed] [Google Scholar]
- 1349.Veasey SC. Serotonin agonists and antagonists in obstructive sleep apnea: therapeutic potential. Am J Respir Med. 2003;2:21–29. doi: 10.1007/BF03256636. [DOI] [PubMed] [Google Scholar]
- 1350.Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li XY, Descalzi G, Kim SS, Chen T, Shang YZ, Zhuo M, Houslay MD, Abel T. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–1125. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1351.Venkatraman V, Chuah YM, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30:603–609. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- 1352.Verret L, Fort P, Gervasoni D, Leger L, Luppi PH. Localization of the neurons active during paradoxical (REM) sleep and projecting to the locus coeruleus noradrenergic neurons in the rat. J Comp Neurol. 2006;495:573–586. doi: 10.1002/cne.20891. [DOI] [PubMed] [Google Scholar]
- 1353.Verret L, Goutagny R, Fort P, Cagnon L, Salvert D, Leger L, Boissard R, Salin P, Peyron C, Luppi PH. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1354.Verret L, Leger L, Fort P, Luppi PH. Cholinergic and noncholinergic brainstem neurons expressing Fos after paradoxical (REM) sleep deprivation and recovery. Eur J Neurosci. 2005;21:2488–2504. doi: 10.1111/j.1460-9568.2005.04060.x. [DOI] [PubMed] [Google Scholar]
- 1355.Vertes RP. Brain stem gigantocellular neurons: patterns of activity during behavior and sleep in the freely moving rat. J Neurophysiol. 1979;42:214–228. doi: 10.1152/jn.1979.42.1.214. [DOI] [PubMed] [Google Scholar]
- 1356.Vertes RP. An analysis of ascending brain stem systems involved in hippocampal synchronization and desynchronization. J Neurophysiol. 1981;46:1140–1159. doi: 10.1152/jn.1981.46.5.1140. [DOI] [PubMed] [Google Scholar]
- 1357.Vertes RP. A lectin horseradish peroxidase study of the origin of ascending fibers in the medial forebrain bundle of the rat. The upper brainstem. Neuroscience. 1984;11:669–690. doi: 10.1016/0306-4522(84)90052-6. [DOI] [PubMed] [Google Scholar]
- 1358.Vertes RP. Brainstem control of the events of REM sleep. Prog Neurobiol. 1984;22:241–288. doi: 10.1016/0301-0082(84)90020-0. [DOI] [PubMed] [Google Scholar]
- 1359.Vertes RP. Brainstem afferents to the basal forebrain in the rat. Neuroscience. 1988;24:907–935. doi: 10.1016/0306-4522(88)90077-2. [DOI] [PubMed] [Google Scholar]
- 1360.Vertes RP. PHA-L analysis of projections from the supramammillary nucleus in the rat. J Comp Neurol. 1992;326:595–622. doi: 10.1002/cne.903260408. [DOI] [PubMed] [Google Scholar]
- 1361.Vertes RP. Memory consolidation in sleep; dream or reality. Neuron. 2004;44:135–148. doi: 10.1016/j.neuron.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 1362.Vertes RP. Hippocampal theta rhythm: a tag for short-term memory. Hippocampus. 2005;15:923–935. doi: 10.1002/hipo.20118. [DOI] [PubMed] [Google Scholar]
- 1363.Vertes RP, Colom LV, Fortin WJ, Bland BH. Brainstem sites for the carbachol elicitation of the hippocampal theta rhythm in the rat. Exp Brain Res. 1993;96:419–429. doi: 10.1007/BF00234110. [DOI] [PubMed] [Google Scholar]
- 1364.Vertes RP, Kinney GG, Kocsis B, Fortin WJ. Pharmacological suppression of the median raphe nucleus with serotonin1A agonists, 8-OH-DPAT and buspirone, produces hippocampal theta rhythm in the rat. Neuroscience. 1994;60:441–451. doi: 10.1016/0306-4522(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 1365.Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- 1366.Vertes RP, McKenna JT. Collateral projections from the supramammillary nucleus to the medial septum and hippocampus. Synapse. 2000;38:281–293. doi: 10.1002/1098-2396(20001201)38:3<281::AID-SYN7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 1367.Vetrivelan R, Fuller PM, Tong Q, Lu J. Medullary circuitry regulating rapid eye movement sleep and motor atonia. J Neurosci. 2009;29:9361–9369. doi: 10.1523/JNEUROSCI.0737-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1368.Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab. 2004;89:4409–4413. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 1369.Vienne J, Bettler B, Franken P, Tafti M. Differential effects of GABABreceptor subtypes, γ-hydroxybutyric acid, and Baclofen on EEG activity and sleep regulation. J Neurosci. 2010;30:14194–14204. doi: 10.1523/JNEUROSCI.3145-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1370.Villablanca J, Salinas-Zeballos ME. Sleep-wakefulness, EEG and behavioral studies of chronic cats without the thalamus: the “athalamic” cat. Arch Ital Biol. 1972;110:383–411. [PubMed] [Google Scholar]
- 1371.Vincent SR, Hokfelt T, Skirboll LR, Wu JY. Hypothalamic gamma-aminobutyric acid neurons project to the neocortex. Science. 1983;220:1309–1311. doi: 10.1126/science.6857253. [DOI] [PubMed] [Google Scholar]
- 1372.Viola AU, Brandenberger G, Toussaint M, Bouhours P, Paul MJ, Luthringer R. Ritanserin, a serotonin-2 receptor antagonist, improves ultradian sleep rhythmicity in young poor sleepers. Clin Neurophysiol. 2002;113:429–434. doi: 10.1016/s1388-2457(02)00014-7. [DOI] [PubMed] [Google Scholar]
- 1373.Virus RM, Djuricic-Nedelson M, Radulovacki M, Green RD. The effects of adenosine and 2’-deoxycoformycin on sleep and wakefulness in rats. Neuropharmacology. 1983;22:1401–1404. doi: 10.1016/0028-3908(83)90231-9. [DOI] [PubMed] [Google Scholar]
- 1374.Volgushev M, Chauvette S, Mukovski M, Timofeev I. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave oscillations. J Neurosci. 2006;26:5665–5672. doi: 10.1523/JNEUROSCI.0279-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1375.Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Wang RL, Logan J, Wong C, Jayne M, Swanson JM. Hyperstimulation of striatal D2 receptors with sleep deprivation: Implications for cognitive impairment. Neuroimage. 2009;45:1232–1240. doi: 10.1016/j.neuroimage.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1376.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Wong C, Ma J, Pradhan K, Tomasi D, Thanos PK, Ferre S, Jayne M. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008;28:8454–8461. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1377.Von Economo C. Die Pathologie des Schlafes. In: Von Bethe A, Von Bergmann G, Embden G, Ellinger A, editors. Handbuch des Normalen und Pathologischen Physiologie. Berlin: Springer; 1926. pp. 591–610. [Google Scholar]
- 1378.Von Economo C. Sleep as a problem of localization. J Nerv Ment Dis. 1930;71:249–259. [Google Scholar]
- 1379.Vreugdenhil M, Jefferys JG, Celio MR, Schwaller B. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol. 2003;89:1414–1422. doi: 10.1152/jn.00576.2002. [DOI] [PubMed] [Google Scholar]
- 1380.Vuillon-Cacciuttolo G, Seri B. Effects of optic nerve section in baboons on the geniculate and cortical spike activity during various states of vigilance. Electroencephalogr Clin Neurophysiol. 1978;44:754–768. doi: 10.1016/0013-4694(78)90210-9. [DOI] [PubMed] [Google Scholar]
- 1381.Vyazovskiy V, Borbely AA, Tobler I. Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J Sleep Res. 2000;9:367–371. doi: 10.1046/j.1365-2869.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 1382.Vyazovskiy VV, Deboer T, Rudy B, Lau D, Borbely AA, Tobler I. Sleep EEG in mice that are deficient in the potassium channel subunit K.v32. Brain Res. 2002;947:204–211. doi: 10.1016/s0006-8993(02)02925-6. [DOI] [PubMed] [Google Scholar]
- 1383.Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–447. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1384.Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1385.Vyazovskiy VV, Riedner BA, Cirelli C, Tononi G. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep. 2007;30:1631–1642. doi: 10.1093/sleep/30.12.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1386.Vyazovskiy VV, Tobler I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005;1050:64–71. doi: 10.1016/j.brainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 1387.Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8:112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1388.Walker MP. The role of sleep in cognition and emotion. Ann NY Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 1389.Walker MP. Sleep, memory and emotion. Prog Brain Res. 2010;185:49–68. doi: 10.1016/B978-0-444-53702-7.00004-X. [DOI] [PubMed] [Google Scholar]
- 1390.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 1391.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 1392.Watson CJ, Soto-Calderon H, Lydic R, Baghdoyan HA. Pontine reticular formation (PnO) administration of hypocretin-1 increases PnO GABA levels and wakefulness. Sleep. 2008;31:453–464. doi: 10.1093/sleep/31.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1393.Webster HH, Friedman L, Jones BE. Modification of paradoxical sleep following transections of the reticular formation at the pontomedullary junction. Sleep. 1986;9:1–23. doi: 10.1093/sleep/9.1.1. [DOI] [PubMed] [Google Scholar]
- 1394.Webster HH, Jones BE. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. II. Effects upon sleep-waking states. Brain Res. 1988;458:285–302. doi: 10.1016/0006-8993(88)90471-4. [DOI] [PubMed] [Google Scholar]
- 1395.Wehrle R, Kaufmann C, Wetter TC, Holsboer F, Auer DP, Pollmacher T, Czisch M. Functional microstates within human REM sleep: first evidence from fMRI of a thalamocortical network specific for phasic REM periods. Eur J Neurosci. 2007;25:863–871. doi: 10.1111/j.1460-9568.2007.05314.x. [DOI] [PubMed] [Google Scholar]
- 1396.Weiser M, Bueno E, Sekirnjak C, Martone ME, Baker H, Hillman D, Chen S, Thornhill W, Ellisman M, Rudy B. The potassium channel subunit KV3.1b is localized to somatic and axonal membranes of specific populations of CNS neurons. J Neurosci. 1995;15:4298–4314. doi: 10.1523/JNEUROSCI.15-06-04298.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1397.Weiser M, Vega-Saenz de ME, Kentros C, Moreno H, Franzen L, Hillman D, Baker H, Rudy B. Differential expression of Shaw-related K+channels in the rat central nervous system. J Neurosci. 1994;14:949–972. doi: 10.1523/JNEUROSCI.14-03-00949.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1398.Wenk GL. The nucleus basalis magnocellularis cholinergic system: one hundred years of progress. Neurobiol Learn Mem. 1997;67:85–95. doi: 10.1006/nlme.1996.3757. [DOI] [PubMed] [Google Scholar]
- 1399.Wenk GL, Stoehr JD, Quintana G, Mobley S, Wiley RG. Behavioral, biochemical, histological, and electrophysiological effects of 192 IgG-saporin injections into the basal forebrain of rats. J Neurosci. 1994;14:5986–5995. doi: 10.1523/JNEUROSCI.14-10-05986.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1400.Westphal C. Eigentuemliche mit Einschlafen verbundene Anfaelle. Arch Psychiatr Nervenkr. 1877;7:631–635. [Google Scholar]
- 1401.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 1402.White JM, Rumbold GR. Behavioural effects of histamine and its antagonists: a review. Psychopharmacology. 1988;95:1–14. doi: 10.1007/BF00212757. [DOI] [PubMed] [Google Scholar]
- 1403.White SR, Fung SJ, Barnes CD. Norepinephrine effects on spinal motoneurons. Prog Brain Res. 1991;88:343–350. doi: 10.1016/s0079-6123(08)63821-2. [DOI] [PubMed] [Google Scholar]
- 1404.White SR, Fung SJ, Jackson DA, Imel KM. Serotonin, norepinephrine and associated neuropeptides: effects on somatic motoneuron excitability. Prog Brain Res. 1996;107:183–199. doi: 10.1016/s0079-6123(08)61865-8. [DOI] [PubMed] [Google Scholar]
- 1405.Whittington MA, Cunningham MO, Lebeau FE, Racca C, Traub RD. Multiple origins of the cortical gamma rhythm. Dev Neurobiol. 2010 doi: 10.1002/dneu.20814. [DOI] [PubMed] [Google Scholar]
- 1406.Whittington MA, Jefferys JG, Traub RD. Effects of intravenous anaesthetic agents on fast inhibitory oscillations in the rat hippocampus in vitro. Br J Pharmacol. 1996;118:1977–1986. doi: 10.1111/j.1476-5381.1996.tb15633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1407.Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 1408.Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron. 2009;61:587–596. doi: 10.1016/j.neuron.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1409.Wigren HK, Schepens M, Matto V, Stenberg D, Porkka-Heiskanen T. Glutamatergic stimulation of the basal forebrain elevates extracellular adenosine and increases the subsequent sleep. Neuroscience. 2007;147:811–823. doi: 10.1016/j.neuroscience.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 1410.Wilcox KS, Grant SJ, Burkhart BA, Christoph GR. In vitro electrophysiology of neurons in the lateral dorsal tegmental nucleus. Brain Res Bull. 1989;22:557–560. doi: 10.1016/0361-9230(89)90111-1. [DOI] [PubMed] [Google Scholar]
- 1411.Wilkinson LO, Auerbach SB, Jacobs BL. Extracellular serotonin levels change with behavioral state but not with pyrogen-induced hyperthermia. J Neurosci. 1991;11:2732–2741. doi: 10.1523/JNEUROSCI.11-09-02732.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1412.Williams JA, Comisarow J, Day J, Fibiger HC, Reiner PB. State-dependent release of acetylcholine in rat thalamus measured by in vivo microdialysis. J Neurosci. 1994;14:5236–5242. doi: 10.1523/JNEUROSCI.14-09-05236.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1413.Williams JA, Reiner PB. Noradrenaline hyperpolarizes identified rat mesopontine cholinergic neurons in vitro. J Neurosci. 1993;13:3878–3883. doi: 10.1523/JNEUROSCI.13-09-03878.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1414.Williams JA, Vincent SR, Reiner PB. Nitric oxide production in rat thalamus changes with behavioral state, local depolarization, and brainstem stimulation. J Neurosci. 1997;17:420–427. doi: 10.1523/JNEUROSCI.17-01-00420.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1415.Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, Marcus JN, Lee C, Elmquist JK, Kohlmeier KA, Leonard CS, Richardson JA, Hammer RE, Yanagisawa M. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 1416.Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 1417.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 1418.Winkelman JW, James L. Serotonergic antidepressants are associated with REM sleep without atonia. Sleep. 2004;27:317–321. doi: 10.1093/sleep/27.2.317. [DOI] [PubMed] [Google Scholar]
- 1419.Winkelmann J, Schadrack J, Wetter TC, Zieglgansberger W, Trenkwalder C. Opioid and dopamine antagonist drug challenges in untreated restless legs syndrome patients. Sleep Med. 2001;2:57–61. doi: 10.1016/s1389-9457(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 1420.Winkelmann J, Schormair B, Lichtner P, Ripke S, Xiong L, Jalilzadeh S, Fulda S, Putz B, Eckstein G, Hauk S, Trenkwalder C, Zimprich A, Stiasny-Kolster K, Oertel W, Bachmann CG, Paulus W, Peglau I, Eisensehr I, Montplaisir J, Turecki G, Rouleau G, Gieger C, Illig T, Wichmann HE, Holsboer F, Muller-Myhsok B, Meitinger T. Genome- wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet. 2007;39:1000–1006. doi: 10.1038/ng2099. [DOI] [PubMed] [Google Scholar]
- 1421.Winsky-Sommerer R, Knapman A, Fedele DE, Schofield CM, Vyazovskiy VV, Rudolph U, Huguenard JR, Fritschy JM, Tobler I. Normal sleep homeostasis and lack of epilepsy phenotype in GABA A receptor alpha3 subunit-knockout mice. Neuroscience. 2008;154:595–605. doi: 10.1016/j.neuroscience.2008.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1422.Winsky-Sommerer R, Vyazovskiy VV, Homanics GE, Tobler I. The EEG effects of THIP (Gaboxadol) on sleep and waking are mediated by the GABA(A)delta-subunitcontaining receptors. Eur J Neurosci. 2007;25:1893–1899. doi: 10.1111/j.1460-9568.2007.05455.x. [DOI] [PubMed] [Google Scholar]
- 1423.Wirz-Justice A, Tobler I, Kafka MS, Naber D, Marangos PJ, Borbely AA, Wehr TA. Sleep deprivation: effects on circadian rhythms of rat brain neurotransmitter receptors. Psychiatry Res. 1981;5:67–76. doi: 10.1016/0165-1781(81)90062-7. [DOI] [PubMed] [Google Scholar]
- 1424.Wisor JP, DeLorey TM, Homanics GE, Edgar DM. Sleep states and sleep electroen-cephalographic spectral power in mice lacking the beta 3 subunit of the GABA(A) receptor. Brain Res. 2002;955:221–228. doi: 10.1016/s0006-8993(02)03467-4. [DOI] [PubMed] [Google Scholar]
- 1425.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1426.Wisor JP, O’Hara BF, Terao A, Selby CP, Kilduff TS, Sancar A, Edgar DM, Franken P. A role for cryptochromes in sleep regulation. BMC Neurosci. 2002;3:20. doi: 10.1186/1471-2202-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1427.Wisor JP, Wurts SW, Hall FS, Lesch KP, Murphy DL, Uhl GR, Edgar DM. Altered rapid eye movement sleep timing in serotonin transporter knockout mice. Neuroreport. 2003;14:233–238. doi: 10.1097/00001756-200302100-00015. [DOI] [PubMed] [Google Scholar]
- 1428.Woo TU, Spencer K, McCarley RW. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry. 2010;18:173–189. doi: 10.3109/10673221003747609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1429.Woolf NJ. A possible role for cholinergic neurons of the basal forebrain and pontomesencephalon in consciousness. Conscious Cogn. 1997;6:574–596. doi: 10.1006/ccog.1997.0319. [DOI] [PubMed] [Google Scholar]
- 1430.Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- 1431.Woolf NJ, Eckenstein F, Butcher LL. Cholinergic projections from the basal forebrain to the frontal cortex: a combined fluorescent tracer and immunohistochemical analysis in the rat. Neurosci Lett. 1983;40:93–98. doi: 10.1016/0304-3940(83)90285-9. [DOI] [PubMed] [Google Scholar]
- 1432.Worley PF, Zeng W, Huang G, Kim JY, Shin DM, Kim MS, Yuan JP, Kiselyov K, Muallem S. Homer proteins in Ca2+signaling by excitable and non-excitable cells. Cell Calcium. 2007;42:363–371. doi: 10.1016/j.ceca.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1433.Wu JC, Gillin JC, Buchsbaum MS, Hershey T, Hazlett E, Sicotte N, Bunney WE., Jr The effect of sleep deprivation on cerebral glucose metabolic rate in normal humans assessed with positron emission tomography. Sleep. 1991;14:155–162. [PubMed] [Google Scholar]
- 1434.Wu M, Zhang Z, Leranth C, Xu C, van den Pol AN, Alreja M. Hypocretin increases impulse flow in the septohippocampal GABAergic pathway: implications for arousal via a mechanism of hippocampal disinhibition. J Neurosci. 2002;22:7754–7765. doi: 10.1523/JNEUROSCI.22-17-07754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1435.Wu MF, Gulyani SA, Yau E, Mignot E, Phan B, Siegel JM. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience. 1999;91:1389–1399. doi: 10.1016/s0306-4522(98)00600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1436.Wu MF, John J, Boehmer LN, Yau D, Nguyen GB, Siegel JM. Activity of dorsal raphe cells across the sleep-waking cycle and during cataplexy in narcoleptic dogs. J Physiol. 2004;554:202–215. doi: 10.1113/jphysiol.2003.052134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1437.Wu MN, Joiner WJ, Dean T, Yue Z, Smith CJ, Chen D, Hoshi T, Sehgal A, Koh K. SLEEPLESS, a Ly-6/neurotoxin family member, regulates the levels, localization and activity of Shaker. Nat Neurosci. 2010;13:69–75. doi: 10.1038/nn.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1438.Wu MN, Koh K, Yue Z, Joiner WJ, Sehgal A. A genetic screen for sleep and circadian mutants reveals mechanisms underlying regulation of sleep in Drosophila . Sleep. 2008;31:465–472. doi: 10.1093/sleep/31.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1439.Wurts SW, Edgar DM. Circadian and homeostatic control of rapid eye movement (REM) sleep: promotion of REM tendency by the suprachiasmatic nucleus. J Neurosci. 2000;20:4300–4310. doi: 10.1523/JNEUROSCI.20-11-04300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1440.Xi MC, Morales FR, Chase MH. A GABAergic pontine reticular system is involved in the control of wakefulness and sleep. Sleep Res Online. 1999;2:43–48. [PubMed] [Google Scholar]
- 1441.Xi MC, Morales FR, Chase MH. Evidence that wakefulness and REM sleep are controlled by a GABAergic pontine mechanism. J Neurophysiol. 1999;82:2015–2019. doi: 10.1152/jn.1999.82.4.2015. [DOI] [PubMed] [Google Scholar]
- 1442.Xi MC, Morales FR, Chase MH. Effects on sleep and wakefulness of the injection of hypocretin-1 (orexin-A) into the laterodorsal tegmental nucleus of the cat. Brain Res. 2001;901:259–264. doi: 10.1016/s0006-8993(01)02317-4. [DOI] [PubMed] [Google Scholar]
- 1443.Xi MC, Morales FR, Chase MH. Interactions between GABAergic and cholinergic processes in the nucleus pontis oralis: neuronal mechanisms controlling active (rapid eye movement) sleep and wakefulness. J Neurosci. 2004;24:10670–10678. doi: 10.1523/JNEUROSCI.1987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1444.Xia J, Chen F, Ye J, Yan J, Wang H, Duan S, Hu Z. Activity-dependent release of adenosine inhibits the glutamatergic synaptic transmission and plasticity in the hypothalamic hypocretin/orexin neurons. Neuroscience. 2009;162:980–988. doi: 10.1016/j.neuroscience.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 1445.Xie X, Crowder TL, Yamanaka A, Morairty SR, Lewinter RD, Sakurai T, Kilduff TS. GABA(B) receptor-mediated modulation of hypocretin/orexin neurones in mouse hypothalamus. J Physiol. 2006;574:399–414. doi: 10.1113/jphysiol.2006.108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1446.Xu C, Datta S, Wu M, Alreja M. Hippocampal theta rhythm is reduced by suppression of the H-current in septohippocampal GABAergic neurons. Eur J Neurosci. 2004;19:2299–2309. doi: 10.1111/j.0953-816X.2004.03316.x. [DOI] [PubMed] [Google Scholar]
- 1447.Xu Y, Padiath QS, Shapiro RE, Jones CR, Wu SC, Saigoh N, Saigoh K, Ptacek LJ, Fu YH. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 1448.Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007;500:84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- 1449.Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 1450.Yamamoto K, Mamelak AN, Quattrochi JJ, Hobson JA. A cholinoceptive desynchronized sleep induction zone in the anterodorsal pontine tegmentum: spontaneous and drug-induced neuronal activity. Neuroscience. 1990;39:295–304. doi: 10.1016/0306-4522(90)90268-9. [DOI] [PubMed] [Google Scholar]
- 1451.Yamamoto T, Watanabe S, Oishi R, Ueki S. Effects of midbrain raphe stimulation and lesion on EEG activity in rats. Brain Res Bull. 1979;4:491–495. doi: 10.1016/0361-9230(79)90033-9. [DOI] [PubMed] [Google Scholar]
- 1452.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 1453.Yamanaka A, Muraki Y, Ichiki K, Tsujino N, Kilduff TS, Goto K, Sakurai T. Orexin neurons are directly and indirectly regulated by catecholamines in a complex manner. J Neurophysiol. 2006;96:284–298. doi: 10.1152/jn.01361.2005. [DOI] [PubMed] [Google Scholar]
- 1454.Yamanaka A, Muraki Y, Tsujino N, Goto K, Sakurai T. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun. 2003;303:120–129. doi: 10.1016/s0006-291x(03)00299-7. [DOI] [PubMed] [Google Scholar]
- 1455.Yamanaka A, Tabuchi S, Tsunematsu T, Fukazawa Y, Tominaga M. Orexin directly excites orexin neurons through orexin 2 receptor. J Neurosci. 2010;30:12642–12652. doi: 10.1523/JNEUROSCI.2120-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1456.Yamanaka A, Tsujino N, Funahashi H, Honda K, Guan JL, Wang QP, Tominaga M, Goto K, Shioda S, Sakurai T. Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Commun. 2002;290:1237–1245. doi: 10.1006/bbrc.2001.6318. [DOI] [PubMed] [Google Scholar]
- 1457.Yamuy J, Fung SJ, Xi M, Chase MH. Hypocretinergic control of spinal cord motoneurons. J Neurosci. 2004;24:5336–5345. doi: 10.1523/JNEUROSCI.4812-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1458.Yamuy J, Jimenez I, Morales F, Rudomin P, Chase M. Population synaptic potentials evoked in lumbar motoneurons following stimulation of the nucleus reticularis gigantocellularis during carbachol-induced atonia. Brain Res. 1994;639:313–319. doi: 10.1016/0006-8993(94)91745-0. [DOI] [PubMed] [Google Scholar]
- 1459.Yamuy J, Mancillas JR, Morales FR, Chase MH. C-fos expression in the pons and medulla of the cat during carbachol-induced active sleep. J Neurosci. 1993;13:2703–2718. doi: 10.1523/JNEUROSCI.13-06-02703.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1460.Yang JN, Bjorklund O, Lindstrom-Tornqvist K, Lindgren E, Eriksson TM, Kahlstrom J, Chen JF, Schwarzschild MA, Tobler I, Fredholm BB. Mice heterozygous for both A1 and A(2A) adenosine receptor genes show similarities to mice given long-term caffeine. J Appl Physiol. 2009;106:631–639. doi: 10.1152/japplphysiol.90971.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1461.Yeomans JS, Takeuchi J, Baptista M, Flynn DD, Lepik K, Nobrega J, Fulton J, Ralph MR. Brain-stimulation reward thresholds raised by an antisense oligonucleotide for the M5 muscarinic receptor infused near dopamine cells. J Neurosci. 2000;20:8861–8867. doi: 10.1523/JNEUROSCI.20-23-08861.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1462.Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G. Sharp waveassociated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1463.Yoder RM, Pang KC. Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus. 2005;15:381–392. doi: 10.1002/hipo.20062. [DOI] [PubMed] [Google Scholar]
- 1464.Yokogawa T, Marin W, Faraco J, Pezeron G, Appelbaum L, Zhang J, Rosa F, Mourrain P, Mignot E. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 2007;5:e277. doi: 10.1371/journal.pbio.0050277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1465.Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep—a prefrontal amygdala disconnect. Curr Biol. 2007;17:R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 1466.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2005;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1467.Yoss RE, Daly DD. Criteria for the diagnosis of the narcoleptic syndrome. Proc Staff Mtg Mayo. 1957;32:320–328. [PubMed] [Google Scholar]
- 1468.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 1469.Youngblood BD, Zhou J, Smagin GN, Ryan DH, Harris RB. Sleep deprivation by the “flower pot” technique and spatial reference memory. Physiol Behav. 1997;61:249–256. doi: 10.1016/s0031-9384(96)00363-0. [DOI] [PubMed] [Google Scholar]
- 1470.Yu L, Coelho JE, Zhang X, Fu Y, Tillman A, Karaoz U, Fredholm BB, Weng Z, Chen JF. Uncovering multiple molecular targets for caffeine using a drug target validation strategy combining A 2A receptor knockout mice with microarray profiling. Physiol Genomics. 2009;37:199–210. doi: 10.1152/physiolgenomics.90353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1471.Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 1472.Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23:3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1473.Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de LL, Deisseroth K. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 2010;5:439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1474.Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL,WuCP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- 1475.Zhang ZW, Arsenault D. Gain modulation by serotonin in pyramidal neurones of the rat prefrontal cortex. J Physiol. 2005;566:379–394. doi: 10.1113/jphysiol.2005.086066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1476.Zhu JJ, Uhlrich DJ. Nicotinic receptor-mediated responses in relay cells and interneurons in the rat lateral geniculate nucleus. Neuroscience. 1997;80:191–202. doi: 10.1016/s0306-4522(97)00095-x. [DOI] [PubMed] [Google Scholar]
- 1477.Zhu XH, Du F, Zhang N, Zhang Y, Lei H, Zhang X, Qiao H, Ugurbil K, Chen W. Advanced in vivo heteronuclear MRS approaches for studying brain bioenergetics driven by mitochondria. Methods Mol Biol. 2009;489:317–357. doi: 10.1007/978-1-59745-543-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1478.Zimmerman JE, Naidoo N, Raizen DM, Pack AI. Conservation of sleep: insights from non-mammalian model systems. Trends Neurosci. 2008;31:371–376. doi: 10.1016/j.tins.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1479.Zung WW, Wilson WP. Sleep and dream patterns in twins. Markov analysis of a genetic trait. Recent Adv Biol Psychiatry. 1966;9:119–130. doi: 10.1007/978-1-4684-8228-7_8. [DOI] [PubMed] [Google Scholar]