Abstract
The chronic use of nicotine, the main psychoactive ingredient of tobacco smoking, alters diverse physiological processes and consequently generates physical dependence. To understand the impact of chronic nicotine on neuropeptides, which are potential molecules associated with dependence, we conducted qualitative and quantitative neuropeptidomics on the rat dorsal striatum, an important brain region implicated in the preoccupation/craving phase of drug dependence. We used extensive LC-FT-MS/MS analyses for neuropeptide identification and LC-FT-MS in conjunction with stable isotope addition for relative quantification. The treatment with chronic nicotine for 3 months led to moderate changes in the levels of endogenous dorsal striatum peptides. Five enkephalin opioid peptides were up-regulated, although no change was observed for dynorphin peptides. Specially, nicotine altered levels of nine non-opioid peptides derived from precursors, including somatostatin and cerebellin, which potentially modulate neurotransmitter release and energy metabolism. This broad but selective impact on the multiple peptidergic systems suggests that apart from the opioid peptides, several other peptidergic systems are involved in the preoccupation/craving phase of drug dependence. Our finding permits future evaluation of the neurochemical circuits modulated by chronic nicotine exposure and provides a number of novel molecules that could serve as potential therapeutic targets for treating drug dependence.
Nicotine is the main psychoactive ingredient of tobacco (1). By acting on the nicotinic acetylcholine receptors located in diverse brain areas, nicotine generates psychoactive effects such as euphoria, reduced stress, increased energy, and enhanced cognitive functions (2). Chronic nicotine use alters various aspects of neurochemical transmission and has a strong impact on diverse physiological processes (2), resulting in drug-seeking and drug-taking behaviors for normal smokers and for a considerable number of patients suffering from schizophrenia and Alzheimer disease, who use nicotine for self-medication (3, 4). The dorsal striatum (DS)1 is one of the key brain regions that has been associated with neural regulation during chronic nicotine exposure (5). In particular, the DS is involved in habit formation during the preoccupation/craving (later) phase of nicotine dependence characterized by compulsive drug-taking (6). Behavioral changes associated with nicotine dependence have been linked to small molecule neurotransmitter systems, including the glutamate and dopamine system in the DS (7). The DS is also known to contain diverse neuropeptides, many of which are probably critical mediators of physiological processes that are associated with nicotine, such as the regulation of reinforcement and energy metabolism. However, neuropeptides have not been extensively investigated in the DS during long periods of nicotine administration.
Immunoassay studies have shown that neuropeptides, including substance P, neuropeptide Y, and opioid peptides, including the enkephalins, are expressed by inhibitory neurons (8), which make up a large majority of the neurons in the DS (9). Many of these inhibitory GABAergic neurons express nicotinic cholinergic receptors (10), suggesting that nicotine administration may regulate their activity, leading to variations in the release of neuropeptides, as well as the inhibitory neurotransmitter GABA. Previous investigations of peptide regulation during chronic nicotine administration in the striatum have exclusively focused on the class of opioid peptides, which are thought to play an important role in the control of diverse physiological processes, including reward processing, nociception, and regulation of emotions (11, 12). Available studies have focused on the analysis of three opioid peptides, their precursors, or receptors as follows: met-enkephalin, dynorphin, and β-endorphin, using conventional techniques like immunoassays (13, 14). There is considerable variability in reported changes of peptide levels in the striatum during chronic nicotine administration. For example, when animals are treated with 1 mg/kg free base nicotine (daily for 14 days), met-enkephalin increased in the striatum (15). By contrast, met-enkephalin is reduced in the striatum when rats are treated with 0.3 mg/kg nicotine (three times/day for 14 days) (16). A number of factors might contribute to this observed variability, including the exact dosing, daily frequency, time span of administration, and delivery method of nicotine. Furthermore, as individual studies have each so far generally examined a single opioid peptide, there is currently little reliable information about peptide co-regulation, even for these well studied opioid peptides. In addition to these opioid peptides, the DS expresses peptides from other peptide families, which are also potential targets under the regulation of chronic nicotine treatment. So far, however, there is no information available about changes of these non-opioid peptides during chronic nicotine administration.
In this study, our aim was to use a neuropeptidomics approach (17) to provide a comprehensive characterization of dorsal striatal neuropeptides after long term nicotine chronic treatment in adult rats using oral administration. The main advantage of this approach is that it allows the simultaneous monitoring of many peptides from the same brain tissue derived from a single drug protocol. We used a combination of a robust sample preparation method (18), high accuracy LC-MS analysis (19, 20), and the use of multiple synthetic internal standards (21) to compare peptide levels in the DS between chronic nicotine and control animals. Our peptidome analysis determined 14 peptides exhibiting significant changes following chronic nicotine administration. Among these peptides were members of the opioid family that had previously been associated with nicotine dependence, as well as a number of newly identified peptides, including members of the secretogranin, cholecystokinin, and somatostatin families. This greatly expands the present scope of peptide involvement in drug dependence in the dorsal striatum.
EXPERIMENTAL PROCEDURES
Materials
LC-MS-grade acetonitrile and formic acid were purchased from Fisher and Fluka, respectively. Acetic acid was purchased from Fluka (Buchs, Switzerland). Pure water was prepared by GenPure system (TKA, Niederelbert, Germany). Siliconized microcentrifuge tubes (2 ml) were purchased from Eppendorf (Hamburg, Germany). Microcon centrifugal filter devices (Vivacon 500) were purchased from Sartorius AG (Goettingen, Germany). The nicotine hydrogen tartrate salt and the saccharin sodium salt hydrate were purchased from Sigma-Aldrich. Thirteen internal standards of peptides were used: nociceptin, NH2-FGGFTGARKSA[Arg(13C6,15N4)]KLANQ-COOH; α-neoendorphin, NH2-YGG[Phe(13C9,15N)]LRKYPK-COOH; dynorphin A(1–17), YGGF[Lys(13C9,15N)]CRRIRPKLKWDNQ; rimorphin (dynorphin B), NH2-YGG[Phe(13C9,15N)]LRRQFKVVT-COOH; somatostatin28, NH2-SANSNPAMAP[Arg(13C,15N4)]ERKAGCKNFFWKTFTSC-CONH2; Met-enkephalin NH2-YGG[Phe(13C9,15N)]M-COOH; melanotrophin α, acetyl-NH-SYSMEHF[Arg(13C6,15N4)]WGKPV-CONH2; galanin, GWTLNSAGYLLGPHAIDNH[Arg(13C6,15N4)]SFSDKHGLT-CONH2; Leu-enkephalin, YGG[Phe(13C9,15N)]L; vasoactive intestinal peptide, NH2-HSDVAFTDNYTRL[Arg(13C6,15N4)]KQMAVKKYLNSILN-CONH2; β-endorphin, YGGFMTSEKSQTPLVTLFKNAIIKNAHK[Lys(13C6,15N2)]GQ; orexin-B, RPGPPGLQGRLQ[Arg(13C6,15N4)]LLQANGNHAAGILTM-CONH2; neuropeptide Y, YPSKPDNPGEDAPAEDMA[Arg(13C6,15N4)]YYSALRHYINLITRQRY-CONH2, and melanin-concentrating hormone, NH2-DFDMLRCMLGRVY-[Arg(13C6,15N4)]PCWQV-COOH. The heavy peptides of 95% purity were obtained from Thermo Fisher Scientific. These heavy peptides had different lengths and hydrophobicities. They thus had different retention times and were distributed in the different time windows during the LC-MS analysis, which made them work as analogous for the quantitation of endogenous peptides.
Animals and Nicotine Administration
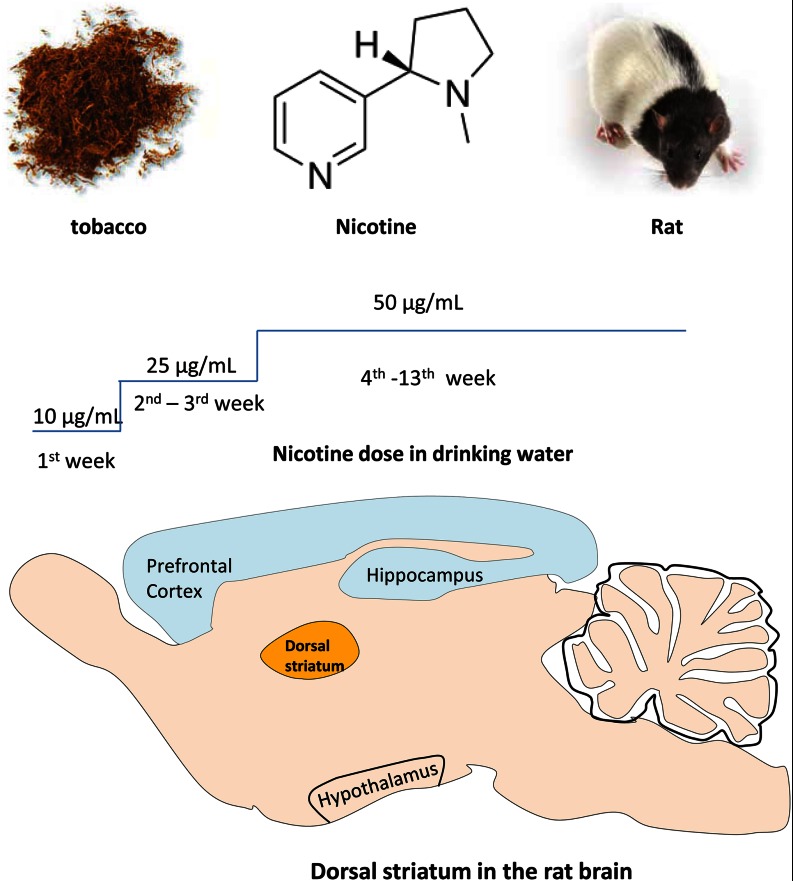
Fifteen Long Evans rats (male, 8 weeks old) were used in the experiments. All animals were pair-housed under constant temperature and humidity with free access to food and water. All procedures with live animals were conducted with protocol approved by the veterinary office in Fribourg, Switzerland, and complied with relevant European Union veterinary directives. Three naive adult rats were used for identifying prohormone precursor peptides from multiple samples, including peptide-rich brain areas. Twelve rats were randomly divided into nicotine group (n = 6) and control group (n = 6). Nicotine administration was conducted by adding it in the drinking water with a gradual increase in dose (10–50 μg/ml, free base) for a total period of 13 weeks (see Fig. 1). Because of the bitter taste of the nicotine solution, a dose of 1% of saccharine was added to the water. The control group was treated with a solution of 1% of saccharine for the entire experimental time. Average nicotine intake during the last week of administration was thus 1.5 mg/kg/day. The conversion of drug doses from animal studies to human studies was performed using the body surface area normalization method (22). According to this method, animal models, such as rodents, required a much higher dose of drugs than humans due to their large body surface area to weight ratios. In the case of our study, nicotine consumption in the drinking water by rats for an entire day was similar to the calculated nicotine dose for medium-to-high smokers who smoked 17 cigarettes daily. The nicotine dose in our study was thus within a physiologically relevant range for in vivo nicotine study (23).
Fig. 1.
Nicotine schedule and DS. The Nicotine dose schedule is shown, starting with a low dose and increasing stepwise to a 50 μg/ml dose that was maintained for a 10-week period. The location of the DS is shown on a sagittal slice of the rat brain.
Sample Preparation
To reduce the interference peptides produced from fragmentation of proteins (17), restricted temperature control was used to minimize the degeneration of the endogenous neuropeptides. For peptide identification, three naive adult rats were sacrificed by decapitation after anesthetizing with ketamine (100 mg/kg, Streuli Pharma AG, Uznach, Switzerland)); the brains were then removed and stabilized by using Denator heat irradiation (Denator AB, Gothenburg, Sweden) as described elsewhere (18). Dorsal striatum, hypothalamus, and pre-frontal cortex were dissected from the denaturized brains. For quantitative analysis, the brains of 12 adult rats were collected using the above-mentioned method, and the DS was dissected from right hemisphere.
Before extraction, 13 heavy neuropeptides were added as internal standards to each DS tissue sample (20 mg). We used the mixing on column method (19) for sample treatment. Each brain tissue was extracted four times using the following: (i) 0.2% acetic acid aqueous solution; (ii) 0.2% acetic acid aqueous solution; (iii) water/methanol/acetic acid solution (69.8:30:0.2, v/v/v), and (iv) water/methanol/acetic acid solution (49.8:50:0.2, v/v/v), respectively. Each step used 3.75 μl of solution per 1 mg of tissue. In each extraction step, the sample was homogenized twice (each time 20 s) within 1 min by a Precellys 24 homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France). Before filtration, the first two-step aqueous extractions, and the last two-step organic extractions were mixed, respectively. The last two steps used methanol to increase the capability in the extraction of hydrophobic peptides. The sample was then centrifuged at 22,000 × g for 60 min at 4°C. All supernatants filtered on a 10-kDa cutoff filter (Vivacon 500, Sartorius AG, Goettingen, Germany) by centrifuging for 90 min at 14,000 × g at 4°C. For each LC-MS analysis, the organic extract was loaded first on the trap column, and after a 3-min conditioning, the aqueous extract was loaded with the same volume (5 μl). The usage of organic solvent in samples was demonstrated to be necessary to increase the recovery of large peptides in injection, separation, and storage (19). For all of the brain samples, the concentration of each internal standard was 20 nm in the final solution.
LC-FT-MS Data Acquisition
The peptide extracts from hypothalamus, prefrontal cortex, and DS of the three naive brains were analyzed using an LTQ-Orbitrap Discovery (Thermo Fisher Scientific, Bremen, Germany) coupled to a two-dimensional NanoLC (Eksigent Technologies). In an LC-MS analysis, the extract was injected (5 μl/times) with a one-dimensional pump (channel 1) on a trap column (100 μm inner diameter and 3 cm long), which was packed with a ReproSil-Pur C18 AQ particles (5 μm, 100 Å; Dr. Maisch GmbH, Ammerbuch-Entringen, Germany) in a peak column holder (Upchurch, Oak Harbor). The trap column was kept eluting for 3 min with 2% acetonitrile and 98% water containing 0.2% formic acid. The elution direction of the trap column was then reversed through a 10-port valve when it was switched to couple with the analytical column. The analytical column used C18 AQ (3 μm, 100 Å; Dr. Maisch GmbH) as medium, which was packed in a Picofrit capillary with an emitter tip of 10 μm (NewObjective, Cambridge). The mobile phases A and B in a two-dimensional pump (channel 2) were 0.2% formic acid, and 95% acetonitrile containing 0.2% formic acid, respectively. The mobile phases were eluted on the analytical column at 300 nl/min with a gradient profile as follows: 0–3.5 min, 10% B; 3.5–6 min, 10–20% B; 6–35 min, 20–30% B; 35–55 min, 30–45% B; 55–60 min, 45–55% B; 60–65 min, 55–90% B; and 65–80 min, 90% B.
Differential Analysis of Endogenous DS Peptides
For quantitative analysis experiments of the nicotine group rats, the peptide extracts from the DS (right hemisphere, n = 6 for each animal group) were subjected to LC-FT-MS analysis. The run order in an LC-MS analysis sequence was arranged in blocks with each nicotine sample analysis followed by a control sample analysis. The sequence was run two times for obtaining the LC-MS analysis replicates of each sample. Quality control samples were also inserted into the sequence and repeatedly analyzed for examining the variation of peptides. In the experiments, each type of extract (20 μl) was taken from the samples of each animal group (n = 6) and then mixed for LC-MS analysis. The LC-FT-MS data were acquired with tandem MS functions turned off. Data acquisition on the LTQ-FT-MS instrument consisted of a full FT-MS scan event at a mass range of 350–2000 m/z. The lock mass (445.120025 from polydimethylcyclosiloxane) was used for real time internal recalibration (24). The LC-FT-MS analysis of each DS sample was repeated twice.
The differential analysis was conducted using Sieve software (2.0 Beta Thermo Scientific, San Jose, CA). In all the differential analyses, the parameters used were such values: frames = 20,000; m/z start = 300, m/z stop = 2000; retention time window = 10 min; ion m/z width = 10 ppm; retention time start = 5 min, retention time stop = 80 min; peak intensity threshold = 100,000. Using one of the nicotine treatment group samples as a reference, the differential analysis was conducted on 12 DS samples, six from the control group and six from the nicotine group. The peak intensity of each peptide was acquired using the software Sieve 2.0 and exported into a CSV file. In this CSV file, the retention times of all the peptides were ranked in an ascending order for comparison between the retention time of endogenous and heavy peptides. The mean peak intensity of each peptide of the 12 LC-FT-MS runs (6 DS sample × 2 technical replicates) was then normalized to a heavy peptide that had the closest retention time to this peptide. A t test between control and nicotine group was performed, and the level of statistical significance was set at p < 0.05 (n = 6). Peptides were rejected if the coefficient of variation was greater than 40% for quality control samples (n = 3). In addition, a time window of ±3 min was used for peptides of low hydrophobicity (retention time <35 min in our case), and a time window of ±5 min was used for peptides of medium-high hydrophobicity (retention time >35 min) to reject peptides that eluted too far from the internal standards. Moreover, the MS signal-to-noise ratio of all significantly changed peptides was at least larger than 50.
Targeted LC-FT-MS/MS Analysis for Peptide Identification
After differential analysis, the m/z and retention times of the changed peptides were added to an inclusion list for further targeted LC-FT-MS/MS analysis (19, 25), by which these peptides in the DS samples were selected for fragmentation. Data acquisition on the LTQ-FT-MS instrument consisted of a full FT-MS scan event and three FT-MS/MS scan events at mass range of 350–2000 m/z. Minimum signal thresholds were selected to 100,000 counts, and isolation width was selected to m/z = 2; maximum accumulation time was 300 ms, normalized collision energy, 30%; activation Q, 0.25; and activation time, 50 ms. Dynamic exclusion was set as a repeat count of 1, an exclusion duration of 30 s, and a repeat duration of 30 s, 25 ppm mass tolerance for precursor selection with disabled preview mode for FT master scan and disabled monoisotopic precursor selection mode. Finally, all the peptides identified using targeted LC-FT-MS/MS in this step were loaded back to software Sieve for identification of the significantly changed peptides.
Charge-state Directed LC-FT-MS/MS Analysis for Peptide Identification
Charge-state directed LC-FT-MS/MS was used for the identification of peptides from the dorsal striatum, hypothalamus, and prefrontal cortex. The samples from three brain areas were subjected to LC-FT-MS/MS analysis. Data acquisition on the LTQ-FT-MS instrument consisted of a full FT-MS scan event and five FT-MS/MS scan events at a mass range of 350–2000 m/z. Minimum signal thresholds were selected to 100,000 counts; isolation width was selected to m/z = 2; maximum accumulation time 300 ms, normalized collision energy 30%, activation Q 0.25, and activation time 50 ms. Dynamic exclusion was set as a repeat count of 1, an exclusion duration of 30 s, and a repeat duration of 30 s. Dynamic exclusion used 25 ppm mass tolerance. We used charge-state directed LC-FT-MS/MS analysis in the experiment (19). The selection of charge state 1, 2, 3, ≥4 was conducted by using the charge rejection function in the Xcalibur software (1.2 version, Thermo Fisher Scientific, San Jose, CA). To select the charge state of interest, the options for the rejection of other charge states are enabled. In this manner, four CSD-LC-FT-MS/MS analyses were conducted for each sample by the instrument automatically selecting the peptides of directed charge state(s) for fragmentation. All the raw data were subjected to database search for identification. The identified peptides were checked using software Sieve 2.0 to see if a peptide present in the DS. LC-MS/MS information, such as retention time, molecular mass, m/z, charge states, was used to recognize a targeted peptide. The parameters for Sieve 2.0 were the same as those used in the above-mentioned differential analysis.
Database Search and Spectral Interpretation
All the raw LC-FT-MS/MS data were subjected to Peaks Studio 5.3 (BSI, Waterloo, Ontario, Canada) for spectral interpretation (26). Peaks Studio offers functions such as Data Refinement, Auto De Novo, and Peaks Search (27). The Data Refinement program allows correcting of the parent mass and charge states to provide accurate monoisotopic mass of a peptide. The scans of quality value >0.5 were kept for further sequence analysis. Data processing, including peak centroiding, charge deconvolution, and deisotoping, was conducted for data refinement.
The refined data were subjected to the Auto De Novo program for sequencing with the mass tolerance of parent ions and product ions set at 10 ppm and 0.05 Da, respectively. No enzyme was specified for cleavage. Variable post-translational modifications, including amidation (C-terminal), acetylation (N-terminal), pyroglutamylation from glutamic acid, and glutamine (N-terminal) were selected in de novo sequencing. De novo sequencing-based protein ID search was used to sequence. The database search was conducted on a customized neuropeptide database (20) (480 entries for rats) and Swissprot database (7710 entries for rats) (downloaded on April 2nd, 2012). Estimation of false positives was conducted by searching all spectra against decoy databases. The cutoff of false discovery rate for peptide identification in Peaks search was <1%. To confirm sequences identified from database search, all the search results were subjected to manual inspection. A sequence was considered correct only if it matched all the following criteria: 1) the mass of a peptide must have been calculated from the monoisotopic ions of a peptide; 2) all the database search results were inspected with de novo sequencing results; 3) the peptide mass had to be within 10 ppm of the theoretical mass; 4) the major fragments observed in MS/MS have to match within 0.05 Da to predicted monoisotopic fragmentation ions; and 5) fragmentation information must be enough to recognize the alignment of amino acids, in particular if they fall in the substitution positions across the adjacent species. The predicted fragmentation ions were calculated using the MS-Product tool in ProteinProspector (version 5.7.2, Mass Spectrometry Facility, University of California, San Francisco). The sequence identity analysis was performed by searching the peptide sequences in Uniprot.
Statistical Analysis
The relative change of a peptide referred to the difference between its mean relative intensity for nicotine group compared with control group samples. The relative intensity of each peptide was calculated by normalizing its peak intensity to the peak intensity of an internal standard that had closest retention time to this peptide. The mean relative intensity of control samples was set to 100%. An unpaired t test (n = 6, p < 0.05) was used to examine the significance of difference for a peptide between the nicotine and control group animals.
RESULTS
Our study aimed to perform a comprehensive characterization of the DS peptides affected by chronic nicotine treatment. Taking the advantage of high accuracy MS and MS/MS, we performed extensive identification and quantitation of endogenous peptides in the DS using a dual LC-MS approach. First, we performed a differential LC-FT-MS analysis (28) to relatively quantify peptides between nicotine and control animal groups. To identify the significantly changed peptides, we loaded an inclusion list of m/z of changed peptides to perform a targeted LC-FT-MS/MS analysis. Second, we conducted a charge-state directed LC-FT-MS/MS analysis to detect peptides across different brain regions and then used comparative analysis to determine whether they were present in the DS. All the identified peptides were then reexamined using differential analysis to confirm which peptides changed significantly. This targeted and directed dual approach was optimized for detecting peptides that are involved in pathways regulated by nicotine, although at the same time allowing us to examine the effects of nicotine on as many known peptides as possible.
DS Peptide Identification Using Targeted LC-FT-MS/MS Analysis Following Differential Analysis
To identify peptides affected by chronic nicotine administration, we first conducted a differential analysis of all peptides, regardless of their precursor, between the control and nicotine animal groups. To increase the accuracy of quantitative analysis, we used 13 heavy peptides of different lengths as internal standards. The native forms of these synthetic peptides are classical peptides that can be found in databases such as Swiss-Prot and Swepep. Under LC-FT-MS analysis, these internal standards were distributed within a 10–65-min time window, during which most endogenous peptides were eluted from the column. The peak intensity of each peptide was normalized to the peak intensity of a target heavy peptide, where it functioned as an isotopically labeled internal standard for its endogenous form in the brain samples or, alternatively, where it functioned as a peptide analog internal standard for other endogenous peptides that had retention times close to this heavy peptide (see the retention times of heavy peptides in supplemental Table S1).
Neuropeptides are peptides that are of extreme diversity of lengths, hydrophobicities, and solvent solubility. For example, somatostatin 28 (amino acids 1–12) is a hydrophilic peptide with a hydrophobicity index of around 10, as calculated using peptide analyzing tool. By comparison, neuropeptide Y is a highly hydrophobic peptide with a hydrophobicity index around 48. Previous studies have shown that hydrophobicity and solubility of peptides remarkably influence the stability and content of peptides in samples (19). To cope with this issue, we selected an internal standard of similar hydrophobicity to each targeted endogenous peptide, which also has close retention time on the C18 column (29), to correct the variations of its content caused during the LC-MS data acquisition, in particular during sample treatment. Compared with the label-free method, previous studies have shown that the use of even a single labeled peptide as a reference standard allows for the increased precision for measuring all other measured endogenous peptides (30). Consistent with this conclusion, another study also showed that the use of synthetic heavy peptides as analog or isotopically labeled internal standards allows the improvement in precision and accuracy of peptide analysis (21). Our method thus served as an intermediate between the label-free method and label-based method to improve the precision of measurement.
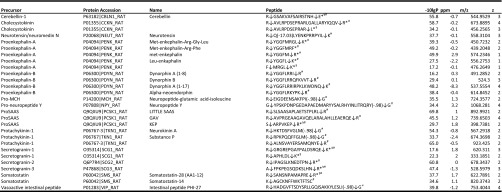
After the differential analysis, we prepared an inclusion list, which contained the m/z and retention times of the modulated peptides. With this inclusion list, targeted LC-FT-MS/MS analysis was conducted on the DS samples, and the LC-FT-MS/MS data were searched against the neuropeptide database. The database search allowed the identification of eight prohormone-derived peptides with significant modulations (see peptides marked with § in Table I), all which were up-regulated by chronic nicotine treatment.
Table I. Changed prohormone-derived peptides and unchanged known neuropeptides in rat dorsal striatum (DS).
The identification list of neuropeptides in the DS. The peptides include known neuropeptides, changed peptides, and putative neuropeptides identified using the dual LC-FT-MS/MS approach. PTMS means Δ mass −17.03, pyro-glutamate formation from glutamic acid or glutamine; Δ mass −0.98, amidation. * indicates significantly changed peptides due to chronic nicotine treatment. The sequence of dynorphin A is confirmed using de novo sequencing. § indicates the peptides identified using targeted LC-FT-MS/MS, and # means the peptides identified using directed LC-FT-MS.
DS Peptide Identification Using Charge-state Directed LC-FT-MS/MS Analysis on the Multiple Brain Area Samples
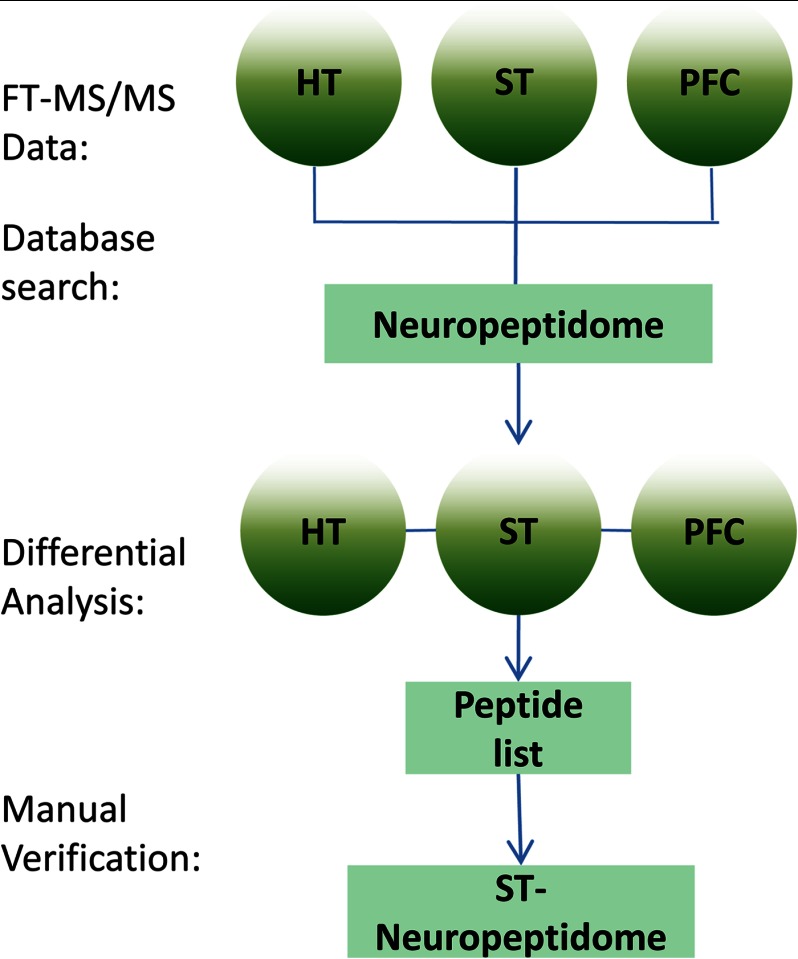
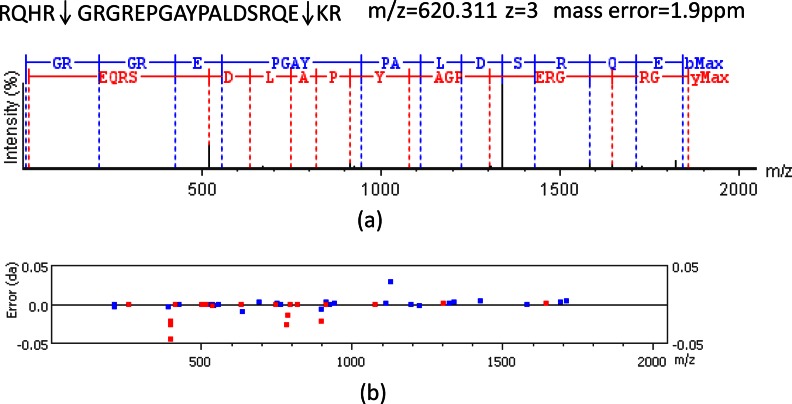
To enhance the identification of prohormone peptides modulated by nicotine, and also to examine if some classical neuropeptides such as neuropeptide Y are involved in nicotine application, we performed a comprehensive identification of known neuropeptides and potentially bioactive peptides derived from prohormone precursors using charge-state directed LC-FT-MS/MS (19), by which high accuracy tandem MS analysis was performed based on charge states to improve the peptide identification rate. Because some peptides tend to be at low abundance in the DS, it is difficult to identify them directly using their tandem MS information. For this reason, we used brain samples from two peptide-rich brain areas, the hypothalamus and the prefrontal cortex, together with the DS samples to prepare a rat neuropeptidome based on an independent set of six brain hemispheres. The LC-FT-MS/MS data for all the three brain areas were acquired and combined for database search (see Fig. 2). Following analyte separation using LC, we acquired both high accuracy mass information for precursor ions and fragmentation ions, permitting high confidence peptide identification (19, 31). Fig. 3 represents the identification of the peptide RQHR↓GRGREPGAYPALDSRQE↓KR from precursor secretogranin-1. This peptide was derived from cleavage at the dibasic cleavage site, which are the hallmark of neuropeptides. Using narrow mass tolerance for precursor ions and product ions (10 ppm and 0.05 Da, respectively), the database search allowed this peptide to be identified with high confidence (−log 10P indicates the P value of database search 56.45). With this method, we in total identified 345 peptides derived from 29 different precursors. These peptides included 19 known neuropeptides (see peptides with name in Table I) and a large number of potentially bioactive neuropeptide candidates (see supplemental Table S2).
Fig. 2.
Identification strategy for DS neuropeptides. The CSD LC-FT-MS/MS data from DS, hypothalamus (HT), and prefrontal cortex (PFC) were combined and subjected to database search. The peptides identified from three brain areas were then loaded to differential analysis software to check if each peptide was present in the DS according to its retention time, molecular mass, and charge states. This method allowed identification of low abundance DS peptides as well as the estimation of their expression levels.
Fig. 3.
High accuracy MS and MS/MS information allows peptide identified with high confidence. (a) and (b) indicate the aligned MS/MS spectrum and mass error of fragmentation ions of a representative peptide RQHR↓GRGREPGAYPALDSRQE↓KR, respectively. The sequence of this fragment with classical dibasic cleavage sites was RQHR↓GRGREPGAYPALDSRQE↓KR, corresponding to the secretogranin-1 precursor.
Having acquired a rat neuropeptidome, we proceeded to examine which of these peptides was present in the DS. Thus, all the data acquired for the three brain areas were further subjected to comparative analysis. The LC-MS/MS information, such as retention time, molecular mass, m/z, and charge states, was used to recognize a targeted peptide. Overall, this method facilitated the identification of a large number of striatal peptides despite their low abundance (see supplemental Table S2). Our results showed that most peptides expressed in peptide-rich brain areas such as the hypothalamus and PFC were also present in the DS (n = 325). The prohormone-derived peptides identified using charge-state directed LC-FT-MS/MS (n = 345) were then resubmitted to the software for relative quantitative analysis. By this way, we found that levels of 10 prohormone-derived peptides changed significantly (p < 0.05), and six of these peptides had not been detected using the targeted LC-MS/MS analysis approach. Finally, we verified the correctness of these changed peptide sequences by resubmitting DS samples to the targeted LC-FT-MS/MS analysis and database search.
Peptides Regulated by Chronic Nicotine Treatment
In total, using the two approaches, we observed that 14 peptides derived from several prohormones were significantly up-regulated following chronic nicotine treatment (see peptides marked with # in Table I). This finding remarkably expands on previous information, which has linked chronic nicotine treatment to only a few opioid peptides in the dorsal striatum as well as the ventral striatum (12). Despite the fact that most identified prohormone-derived peptides exhibited no significant changes, or could not be analyzed quantitatively due to the high signal variations, we observed a small fraction of the identified peptides (n = 14) changed significantly after nicotine administration. We also show in Table I the identification information for an additional 15 known neuropeptides, which are known to play an important role in the regulation of various physiological processes, but most of which have not been studied previously in the context of nicotine administration.
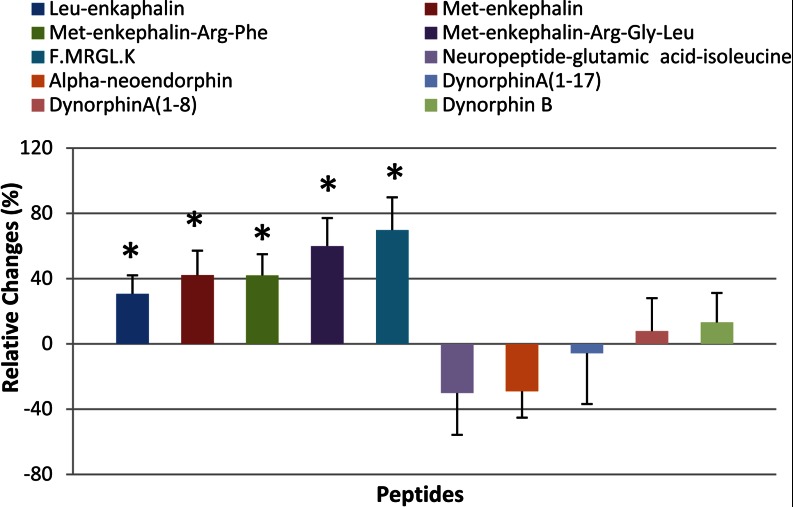
Changes of Five Peptides Derived from Opioid Precursors
Our results showed that four classical opioid peptides as well as a peptide fragment F↓MRGL↓K changed significantly in response to chronic nicotine application, although three other opioid peptides of interest did not show any changes (Fig. 4). The opioid neuropeptides are a class of neuropeptides that play a crucial role in a number of physiological functions, including pain perception and reward (32). In the brain, the opioid system consists of peptides from three precursors as follows: pro-opiomelanocortin (POMC), pro-enkephalin A (PENK A), and pro-dynorphin (PDYN). Of the four opioid peptides regulated by chronic nicotine, Met-enkephalin, Met-enkephalin-Arg-Phe, Met-enkephalin-Arg-Gly-Leu, and the peptide F↓MRGL↓K are derived exclusively from PENK A, whereas Leu-enkephalin can be derived from PENK A and PDYN. Interestingly, the levels for these peptides were all enhanced to a similar degree, with increases for Met-enkephalin, Leu-enkephalin, Met-enkephalin-Arg-Phe, Met-enkephalin-Arg-Gly-Leu, and FMRGLK of 42% (p = 0.013), 30.5% (p = 0.015), 41.9% (p = 0.005), 59.9% (p = 0.003), and 69.7% (p = 0.003) respectively. The other opioid peptides we examined all derived from the PDYN and POMC precursor and showed no nicotine-dependent changes.
Fig. 4.
Chronic nicotine effects on opioid family neuropeptides. Bars indicate the mean relative changes (%) of peptides following chronic nicotine treatment. Error bars indicate variability of the difference between sample means (standard error). Asterisk denotes statistical significance (p < 0.05).
Changes of Nine Peptides Derived from Non-opioid Precursors
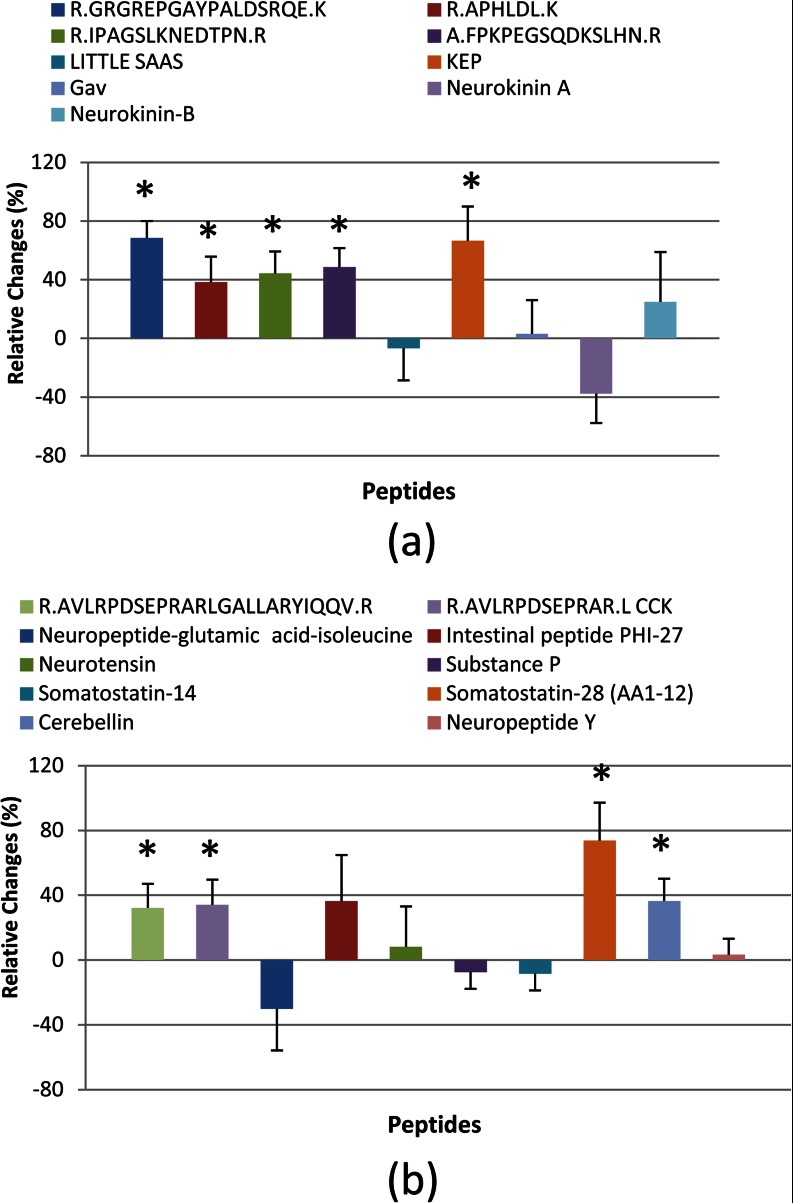
We also observed changes in a number of non-opioid peptides in our study. We found a significant increase in the expression level of three peptides from granin family (Fig. 5a). Granins are a group of acidic, soluble, and secretory proteins (33). As pro-hormones, they give rise to bioactive peptides through enzymatic processing. We identified a large number of peptide fragments from four precursors derived from this family (see supplemental Table S2). The differential analysis revealed that RQHR↓GRGREPGAYPALDSRQE↓KR (from secretogranin-1), R↓APHLDL↓KR (from secretogranin-1), KR↓IPAGSLKNEDTPN↓R (from secretogranin-2), and A↓FPKPEGSQDKSLHN↓R (from secretogranin-3), increased 68% (p = 8.96E-06), 38.3% (p = 0.0481), 44% (p = 0.0087), and 48% (p = 0.0016), respectively. Chronic nicotine altered these peptides to a similar degree, even though they belonged to different precursors. In addition, a peptide KEP (S↓ARPVKEP↓R), derived from granin-like Pro-SAAS, increased significantly after chronic nicotine treatment (66.7%, p = 0.0118).
Fig. 5.
Chronic nicotine effects on non-opioid family neuropeptides. a, peptides derived from granin or granin-like precursors and from protachykinin; b, peptides derived from the precursors cholecystokinin, neurotensin/neuromedin N, vasoactive intestinal peptide, somatostatin, cerebellin-1, and pro-neuropeptide Y. Bars indicate the mean relative changes (%) of neurochemicals following chronic nicotine treatment. Error bars indicate variability of the difference between sample means (standard error). Asterisk denotes statistical significance (p < 0.05).
We further detected the changes of several peptides derived from other prohormones, which have been associated with important functions such as regulating metabolism and emotion (34). Two cholecystokinin-derived peptides, R↓AVLRPDSEPRAR↓L and QLR↓AVLRPDSEPRARLGALLARYIQQV↓RVA were found to increase 34.1% (p = 0.0454) and 32% (p = 0.0481), respectively (Fig. 5b). Cholecystokinin is a pro-hormone expressed in the central nervous system (CNS) and in the gastrointestinal tract. Peptides from this precursor are involved in physiological mechanisms like nausea and anxiety (35, 36). Somatostatin, also called somatotropin-release inhibiting factor, is a multifunctional hormone involved in the inhibition of the release of other hormones and the exocrine secretions (37). A peptide RLELQR↓SANSNPAMAPRE↓RK derived from this precursor was up-regulated of 73% (p = 0.007) by nicotine. Similarly, we also found a significant increase of cerebellin (36%, p = 0.016). Cerebellin is a peptide derived from its precursor, which is required for synapse integrity and synaptic plasticity (38, 39).
DISCUSSION
Our present peptidome study explored the effects of chronic nicotine on neuropeptides levels in the DS. Using high resolution MS data and differential analysis, we were able to identify more than 300 prohormone-derived peptides from the rat DS. Of these identified peptides, our study revealed a significant change of 14 peptides in the rat DS. We confirmed the changes of Met-enkephalin in DS reported using previous immunoassays, and we also uncovered the involvement of several other enkephalins in chronic nicotine treatment. Moreover, our study showed for the first time that chronic nicotine treatment also altered DS peptides that were derived from granin family precursors, cholecystokinin and cerebellin-1.
Previous research found that chronic nicotine modulates several neuropeptides such as neuropeptide Y (40), galanin (41), and orexins (42) in the hypothalamus or in the mesolimbocortical dopamine regions. However, it was unclear if chronic nicotine altered these peptides in the DS. Using database search and manual examination, we found that galanin and orexins were undetectable in the rat DS, suggesting extremely low density of innervations of galanin and orexin neurons from the hypothalamus to the DS. By comparison, we reliably detected some known neuropeptides, including neuropeptide Y and neurotensin, whose expression in the DS was however unaffected by chronic nicotine administration. The difference in the changes of these neuropeptide between hypothalamus and DS reflected the brain region-specific regulatory effects of nicotine, because each brain region has specific anatomical structure, including expressing different types of neurons and different types of nicotinic acetylcholine receptors (43, 44).
In this study, we found the changes of multiple opioid peptides following chronic nicotine treatment. The four PENK-derived opioid peptides Met-enkephalin, Leu-enkephalin, Met-enkephalin-Arg-Phe, and Met-enkephalin-Arg-Gly-Leu all increased significantly after chronic nicotine application. Our results, obtained after 13 weeks of nicotine administration, are consistent with previous reports of elevated Met-enkephalin in the striatum following nicotine treatment for periods of 2 weeks, which was measured using immunoassay (15). These results parallel the persistent activation of the DS during long term nicotine exposure or the preoccupation/craving stage of drug dependence (5, 45), and the results further support a close functional link between dopamine and enkephalin systems, which are both under the regulation of nicotine (46). Enhancements of the remaining three PENK peptides have not been previously reported and suggest a broader involvement of the PENK pathway than has not been previously uncovered using immunoassay. These three enkephalins are all derived from the same precursor by the action of the same enzyme, enkephalinase. This suggests that the increase of these peptides may reflect elevated precursor synthesis, consistent with the elevated preproenkephalin mRNA levels in the striatum after chronic nicotine application (15). Another class of opioid peptides, dynorphins derived from PDYN including dynorphin(1–17) and dynorphin(1–8), was not modulated by chronic nicotine. This is consistent with previous literature linking PDYN peptides to aversive states associated with drug withdrawal (47), which were not induced in our experiment. These peptides are thus up-regulated during a drug withdrawal phase but appear to be less involved during the preoccupation/craving phase of drug dependence. Finally, β-endorphin, which is an opioid peptide derived from POMC, was not detectable in the DS, consistent with anatomical findings showing dense β-endorphin innervations only in the ventral but not the DS (12).
In addition to opioid peptides, our peptidome analysis further revealed a number of non-opioid peptides that were up-regulated by chronic nicotine application, for example peptides derived from chromograninA, chromograninB, and secretogranin II, which all belong to the granin peptide family. Granin family peptides are present in secretory granules of many types of neurons and endocrine cells and are involved in various functions such as regulating homeostatic processes, tissue assembly, cell growth, and regulation of the delivery of neurotransmitters and neuropeptides (48). These peptides are thus frequently co-localized with neuropeptides such as substance P, Leu-enkephalin, or prolactin (49, 50), consistent with a possible role of granin family peptides in the up-regulation of enkephalins and granin family peptides that we observed following chronic nicotine treatment. These granin family peptides may therefore play an active role in the maintenance of drug-related habits and associated compulsive behaviors during the preoccupation/craving stage of addiction. Indeed, many of the granin family peptides identified exhibit the dibasic or monobasic cleavage site characteristic of bioactive peptides. A number of additional non-opioid peptides from other peptide families also showed a significant modulation after nicotine administration, including peptides derived from the precursors cholecystokinin, cerebellin, and somatostatin. These peptides are thought to be involved in the regulation anxiety, nausea, and energy metabolism (35, 51). It is highly interesting to know how important these peptides would be for the formation of habitual and compulsive drug-taking. Further biological studies are thus required to reveal their specific roles in drug dependence.
Overall, the changes of peptides observed in our study reflect the profound neuronal response in the DS, which is a brain region playing a particular role in drug dependence. Compared with the brain regions associated with reward- and craving-related processes (6), the DS is a core brain center associated with habitual mechanisms, which are important for the later stage of drug dependence (52). Indeed, an increasing body of evidence has shown that the DS is involved in compulsive and automatic drug-taking (5, 52). Our neuropeptidomics study provides a comprehensive investigation on the peptide changes of the DS under the long-period challenging of nicotine. Some of the peptide changes likely support the DS to underlie aberrant behaviors in drug dependence, which is evidenced by the association between Met-enkephalin and nicotine addiction (12). Some of the changes may also reflect neuronal attempts to adapt or compensate for deficits in other neurotransmitters, such as acetylcholine, dopamine, glutamate, and GABA, because their neurons are directly or indirectly under the modulation of chronic nicotine (10). From a systemic view, these peptide modulations may play an important role in various physiological aspects of nicotine dependence, including reward processing, the reinforcement of drug-seeking habits, and the regulation of energy metabolism. Therefore, these peptides may serve as the important basis of a deeper understanding the neurochemical mechanism of drug dependence and also as novel drug targets for treating drug dependence.
Supplementary Material
Acknowledgments
We thank Prof. Robert Kretz for brain sample preparation.
Footnotes
* This work was supported by SNF R'Equip Grant 316000-121308 and a EURYI award (to G.R.).
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- DS
- dorsal striatum
- GABA
- γ-aminobutyric acid
- LTQ
- linear trap quadrupole
- MOC
- mixing on column
- PENK A
- pro-enkephalin A
- PDYN
- pro-dynorphin
- POMC
- pro-opiomelanocortin.
REFERENCES
- 1. De Biasi M., Dani J. A. (2011) Reward, addiction, withdrawal to nicotine. Annu. Rev. Neurosci. 34, 105–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benowitz N. L. (2010) Nicotine addiction. N. Engl. J. Med. 362, 2295–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sherr J. D., Myers C., Avila M. T., Elliott A., Blaxton T. A., Thaker G. K. (2002) The effects of nicotine on specific eye tracking measures in schizophrenia. Biol. Psychiatry 52, 721–728 [DOI] [PubMed] [Google Scholar]
- 4. Knott V., Mohr E., Mahoney C., Engeland C., Ilivitsky V. (2002) Effects of acute nicotine administration on cognitive event-related potentials in tacrine-treated and non-treated patients with Alzheimer disease. Neuropsychobiology 45, 156–160 [DOI] [PubMed] [Google Scholar]
- 5. Yalachkov Y., Kaiser J., Naumer M. J. (2009) Brain regions related to tool use and action knowledge reflect nicotine dependence. J. Neurosci. 29, 4922–4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koob G. F., Volkow N. D. (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiao C., Nashmi R., McKinney S., Cai H., McIntosh J. M., Lester H. A. (2009) Chronic nicotine selectively enhances α4β2* nicotinic acetylcholine receptors in the nigrostriatal dopamine pathway. J. Neurosci. 29, 12428–12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rice M. W., Roberts R. C., Melendez-Ferro M., Perez-Costas E. (2011) Neurochemical characterization of the tree shrew dorsal striatum. Front. Neuroanat. 5, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graveland G. A., DiFiglia M. (1985) The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 327, 307–311 [DOI] [PubMed] [Google Scholar]
- 10. Zhou F.-M., Wilson C. J., Dani J. A. (2002) Cholinergic interneuron characteristics and nicotinic properties in the striatum. J. Neurobiol. 53, 590–605 [DOI] [PubMed] [Google Scholar]
- 11. Bodnar R. J., Hadjimarkou M. M. (2003) Endogenous opiates and behavior: 2002. Peptides 24, 1241–1302 [DOI] [PubMed] [Google Scholar]
- 12. Hadjiconstantinou M., Neff N. H. (2011) Nicotine and endogenous opioids: neurochemical and pharmacological evidence. Neuropharmacology 60, 1209–1220 [DOI] [PubMed] [Google Scholar]
- 13. McCollum L. A., Roche J. K., Roberts R. C. (2012) Immunohistochemical localization of enkephalin in the human striatum: A postmortem ultrastructural study. Synapse 66, 204–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Isola R., Zhang H., Tejwani G. A., Neff N. H., Hadjiconstantinou M. (2008) Dynorphin and prodynorphin mRNA changes in the striatum during nicotine withdrawal. Synapse 62, 448–455 [DOI] [PubMed] [Google Scholar]
- 15. Dhatt R. K., Gudehithlu K. P., Wemlinger T. A., Tejwani G. A., Neff N. H., Hadjiconstantinou M. (1995) Preproenkephalin mRNA and methionine-enkephalin content are increased in mouse striatum after treatment with nicotine. J. Neurochem. 64, 1878–1883 [DOI] [PubMed] [Google Scholar]
- 16. Wewers M. E., Dhatt R. K., Snively T. A., Tejwani G. A. (1999) The effect of chronic administration of nicotine on antinociception, opioid receptor binding, and met-enkephalin levels in rats. Brain Res. 822, 107–113 [DOI] [PubMed] [Google Scholar]
- 17. Svensson M., Sköld K., Nilsson A., Fälth M., Nydahl K., Svenningsson P., Andrén P. E. (2007) Neuropeptidomics: MS applied to the discovery of novel peptides from the brain. Anal. Chem. 79, 15–16 [DOI] [PubMed] [Google Scholar]
- 18. Svensson M., Boren M., Sköld K., Fälth M., Sjögren B., Andersson M., Svenningsson P., Andren P. E. (2009) Heat stabilization of the tissue proteome: a new technology for improved proteomics. J. Proteome Res. 8, 974–981 [DOI] [PubMed] [Google Scholar]
- 19. Zhang X., Petruzziello F., Zani F., Fouillen L., Andren P. E., Solinas G., Rainer G. (2012) High identification rates of endogenous neuropeptides from mouse brain. J. Proteome Res. 11, 2819–2827 [DOI] [PubMed] [Google Scholar]
- 20. Petruzziello F., Fouillen L., Wadensten H., Kretz R., Andren P. E., Rainer G., Zhang X. (2012) Extensive characterization of Tupaia belangeri neuropeptidome using an integrated mass spectrometric approach. J. Proteome Res. 11, 886–896 [DOI] [PubMed] [Google Scholar]
- 21. Pailleux F., Beaudry F. (2012) Internal standard strategies for relative and absolute quantitation of peptides in biological matrices by liquid chromatography tandem mass spectrometry. Biomed. Chromatogr. 26, 881–891 [DOI] [PubMed] [Google Scholar]
- 22. Reagan-Shaw S., Nihal M., Ahmad N. (2008) Dose translation from animal to human studies revisited. FASEB J. 22, 659–661 [DOI] [PubMed] [Google Scholar]
- 23. Matta S. G., Balfour D. J., Benowitz N. L., Boyd R. T., Buccafusco J. J., Caggiula A. R., Craig C. R., Collins A. C., Damaj M. I., Donny E. C., Gardiner P. S., Grady S. R., Heberlein U., Leonard S. S., Levin E. D., Lukas R. J., Markou A., Marks M. J., McCallum S. E., Parameswaran N., Perkins K. A., Picciotto M. R., Quik M., Rose J. E., Rothenfluh A., Schafer W. R., Stolerman I. P., Tyndale R. F., Wehner J. M., Zirger J. M. (2007) Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 190, 269–319 [DOI] [PubMed] [Google Scholar]
- 24. Olsen J. V., de Godoy L. M., Li G., Macek B., Mortensen P., Pesch R., Makarov A., Lange O., Horning S., Mann M. (2005) Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol. Cell. Proteomics 4, 2010–2021 [DOI] [PubMed] [Google Scholar]
- 25. Schmidt A., Gehlenborg N., Bodenmiller B., Mueller L. N., Campbell D., Mueller M., Aebersold R., Domon B. (2008) An integrated, directed mass spectrometric approach for in-depth characterization of complex peptide mixtures. Mol. Cell. Proteomics 7, 2138–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma B., Zhang K., Hendrie C., Liang C., Li M., Doherty-Kirby A., Lajoie G. (2003) PEAKS: powerful software for peptide de novo sequencing by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 17, 2337–2342 [DOI] [PubMed] [Google Scholar]
- 27. Han Y., Ma B., Zhang K. (2005) SPIDER: software for protein identification from sequence tags with de novo sequencing error. J. Bioinform. Comput. Biol. 3, 697–716 [DOI] [PubMed] [Google Scholar]
- 28. Domon B., Aebersold R. (2010) Options and considerations when selecting a quantitative proteomics strategy. Nat. Biotechnol. 28, 710–721 [DOI] [PubMed] [Google Scholar]
- 29. Krokhin O. V., Spicer V. (2009) Peptide retention standards and hydrophobicity indexes in reversed-phase high performance liquid chromatography of peptides. Anal. Chem. 81, 9522–9530 [DOI] [PubMed] [Google Scholar]
- 30. Zhang H., Liu Q., Zimmerman L. J., Ham A. J., Slebos R. J., Rahman J., Kikuchi T., Massion P. P., Carbone D. P., Billheimer D., Liebler D. C. (2011) Methods for peptide and protein quantitation by liquid chromatography-multiple reaction monitoring mass spectrometry. Mol. Cell. Proteomics 10, M110 006593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee J. E., Atkins N., Jr., Hatcher N. G., Zamdborg L., Gillette M. U., Sweedler J. V., Kelleher N. L. (2010) Endogenous peptide discovery of the rat circadian clock: a focused study of the suprachiasmatic nucleus by ultrahigh performance tandem mass spectrometry. Mol. Cell. Proteomics 9, 285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akil H., Owens C., Gutstein H., Taylor L., Curran E., Watson S. (1998) Endogenous opioids: overview and current issues. Drug Alcohol. Depend. 51, 127–140 [DOI] [PubMed] [Google Scholar]
- 33. Zhao E., Zhang D., Basak A., Trudeau V. L. (2009) New insights into granin-derived peptides: evolution and endocrine roles. Gen. Comp. Endocrinol. 164, 161–174 [DOI] [PubMed] [Google Scholar]
- 34. Bartolomucci A., Possenti R., Mahata S. K., Fischer-Colbrie R., Loh Y. P., Salton S. R. (2011) The extended granin family: structure, function, and biomedical implications. Endocr. Rev. 32, 755–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Greenough A., Cole G., Lewis J., Lockton A., Blundell J. (1998) Untangling the effects of hunger, anxiety, and nausea on energy intake during intravenous cholecystokinin octapeptide (CCK-8) infusion. Physiol. Behav. 65, 303–310 [DOI] [PubMed] [Google Scholar]
- 36. Bradwejn J., Koszycki D. (1994) The cholecystokinin hypothesis of anxiety and panic disorder. Ann. N.Y. Acad. Sci. 713, 273–282 [DOI] [PubMed] [Google Scholar]
- 37. Viollet C., Lepousez G., Loudes C., Videau C., Simon A., Epelbaum J. (2008) Somatostatinergic systems in brain: networks and functions. Mol. Cell. Endocrinol. 286, 75–87 [DOI] [PubMed] [Google Scholar]
- 38. Kavety B., Morgan J. I. (1998) Characterization of transcript processing of the gene encoding precerebellin-1. Brain Res. Mol. Brain Res. 63, 98–104 [DOI] [PubMed] [Google Scholar]
- 39. Urade Y., Oberdick J., Molinar-Rode R., Morgan J. I. (1991) Precerebellin is a cerebellum-specific protein with similarity to the globular domain of complement C1q B chain. Proc. Natl. Acad. Sci. U.S.A. 88, 1069–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li M. D., Kane J. K., Parker S. L., McAllen K., Matta S. G., Sharp B. M. (2000) Nicotine administration enhances NPY expression in the rat hypothalamus. Brain Res. 867, 157–164 [DOI] [PubMed] [Google Scholar]
- 41. Jackson K. J., Chen X., Miles M. F., Harenza J., Damaj M. I. (2011) The Neuropeptide galanin and variants in the GalR1 gene are associated with nicotine dependence. Neuropsychopharmacology 36, 2339–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kane J. K., Parker S. L., Matta S. G., Fu Y., Sharp B. M., Li M. D. (2000) Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology 141, 3623–3629 [DOI] [PubMed] [Google Scholar]
- 43. Albuquerque E. X., Pereira E. F., Alkondon M., Rogers S. W. (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol. Rev. 89, 73–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Laviolette S. R., van der Kooy D. (2004) The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat. Rev. Neurosci. 5, 55–65 [DOI] [PubMed] [Google Scholar]
- 45. Everitt B. J., Belin D., Economidou D., Pelloux Y., Dalley J. W., Robbins T. W. (2008) Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 3125–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hadjiconstantinou M., Duchemin A. M., Zhang H., Neff N. H. (2011) Enhanced dopamine transporter function in striatum during nicotine withdrawal. Synapse 65, 91–98 [DOI] [PubMed] [Google Scholar]
- 47. Isola R., Zhang H., Tejwani G. A., Neff N. H., Hadjiconstantinou M. (2008) Dynorphin and prodynorphin mRNA changes in the striatum during nicotine withdrawal. Synapse 62, 448–455 [DOI] [PubMed] [Google Scholar]
- 48. Helle K. B. (2004) The granin family of uniquely acidic proteins of the diffuse neuroendocrine system: comparative and functional aspects. Biol. Rev. Camb. Philos. Soc. 79, 769–794 [DOI] [PubMed] [Google Scholar]
- 49. Ozawa H., Takata K. (1995) The granin family–Its role in sorting and secretory granule formation. Cell Struct. Funct. 20, 415–420 [DOI] [PubMed] [Google Scholar]
- 50. Klimaschewski L., Benndorf K., Kirchmair R., Fischer-Colbrie R., Heym C. (1995) Secretoneurin-immunoreactivity in nerve terminals apposing identified preganglionic sympathetic neurons in the rat: colocalization with substance P and enkephalin. J. Chem. Neuroanat. 9, 55–63 [DOI] [PubMed] [Google Scholar]
- 51. Gardiner J. V., Beale K. E., Roy D., Boughton C. K., Bataveljic A., Campbell D. C., Bewick G. A., Patel N. A., Patterson M., Leavy E. M., Ghatei M. A., Bloom S. R., Dhillo W. S. (2010) Cerebellin1 is a novel orexigenic peptide. Diabetes Obes. Metab. 12, 883–890 [DOI] [PubMed] [Google Scholar]
- 52. Gerdeman G. L., Partridge J. G., Lupica C. R., Lovinger D. M. (2003) It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 26, 184–192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.