Abstract
Aldehyde dehydrogenase 2 (ALDH2) is a mitochondrial enzyme that metabolizes ethanol and toxic aldehydes such as 4-hydroxy-2-nonenal (4-HNE). Using an unbiased proteomic search, we identified ALDH2 deficiency in stroke-prone spontaneously hypertensive rats (SHR-SP) as compared with spontaneously hypertensive rats (SHR). We concluded the causative role of ALDH2 deficiency in neuronal injury as overexpression or activation of ALDH2 conferred neuroprotection by clearing 4-HNE in in vitro studies. Further, ALDH2-knockdown rats revealed the absence of neuroprotective effects of PKCε. Moderate ethanol administration that is known to exert protection against stroke was shown to enhance the detoxification of 4-HNE, and to protect against ischemic cerebral injury through the PKCε-ALDH2 pathway. In SHR-SP, serum 4-HNE level was persistently elevated and correlated inversely with the lifespan. The role of 4-HNE in stroke in humans was also suggested by persistent elevation of its plasma levels for at least 6 months after stroke. Lastly, we observed that 21 of 1 242 subjects followed for 8 years who developed stroke had higher initial plasma 4-HNE levels than those who did not develop stroke. These findings suggest that activation of the ALDH2 pathway may serve as a useful index in the identification of stroke-prone subjects, and the ALDH2 pathway may be a potential target of therapeutic intervention in stroke.
Keywords: ALDH2, 4-HNE, stroke, ethanol
Introduction
Stroke is an age-related disease and the second most common cause of death1,2. Ischemic stroke is the largest subtype, with an estimated incidence of > 80% of all strokes3. The incidence of stroke could be reduced by control of risk factors, such as high blood pressure, elevated blood cholesterol, cigarette smoking, carotid stenosis, diabetes mellitus and heart failure state. However, these risk factors explain only ∼60% of the risk4, suggesting the existence of undiscovered or undefined risk factors. In contrast, > 90% of ischemic heart disease can be explained by clearly identifiable risk factors5. The unidentified risk factors for stroke, combined with unsatisfactory control of known risk factors (e.g., hypertension), may explain the continued high prevalence of stroke6. Identification of new risk factors may result in the development of new strategies for prevention and treatment of stroke.
Oxidative stress is one of the most important factors that contribute to ischemic injury. Reactive aldehydes generated from oxidized lipids, such as malondialdehyde (MDA) and 4-hydroxy-trans-2-nonenal (4-HNE), have been detected in almost all tissues that are subjected to ischemia. These aldehydes in turn form adducts with lipids, proteins and DNA, leading to their inactivation7,8,9,10.
Mitochondrial aldehyde dehydrogenase 2 (ALDH2) is a key enzyme that metabolizes acetaldehyde (a toxic intermediate of ethanol metabolism) to acetic acid, and also detoxifies ROS-generated aldehyde adducts11. ALDH2 is expressed abundantly in the liver and lung, and is also present in organs that require high mitochondrial content, such as heart and brain12. ALDH2 has a wide range of functional implications from cancer to heart disease13,14,15,16. Many of previous studies have been focused on heart disease. Recent data show that ALDH2 is phosphorylated and activated by the survival kinase, protein kinase C epsilon (PKCε), and inversely correlated to myocardial infarct size following ischemia-reperfusion16. Overexpression of ALDH2 appears to confer multiple beneficial effects on cardiac function15. Whether ALDH2 protects against stroke and whether PKCε is involved in this protection are unclear. Furthermore, the possible substrates of ALDH2 have not been fully defined.
Both genomics and proteomics have the potential to define stroke risk factors and to individualize stroke treatment. The contribution of any single gene in a multifactorial disease such as stroke is modest. Proteomics, the study of the entire protein content of a cell or tissue, may be a promising approach to identify new biomarkers for stroke17.
In this study, we used fluorescent two-dimensional difference gel electrophoresis (2D-DIGE) to detect the differentially-expressed proteins in the brain of spontaneously hypertensive rats (SHR), and their substrain, the stroke-prone spontaneously hypertensive rats (SHR-SP). Both SHR and SHR-SP are hypertensive, but their susceptibility to stroke varies greatly18,19,20,21. SHR-SP develops a lethal stroke at an early age. As a result, the lifespan of SHR-SP is considerably short. Therefore, proteomic comparison between SHR-SP and SHR would be helpful in finding one or more new risk factors for stroke except hypertension. Among the > 2 000 proteins included in our analysis, the reduction of ALDH2 in SHR-SP was the most prominent in our unbiased search. We further show that ALDH2 is neuroprotective both in vivo and in vitro by clearing 4-HNE, and mediates the protective effect of ethanol against stroke through the PKCε-ALDH2 pathway. The results also suggest that elevation of 4-HNE level is a risk factor for stroke.
Results
ALDH2 is downregulated in SHR-SP
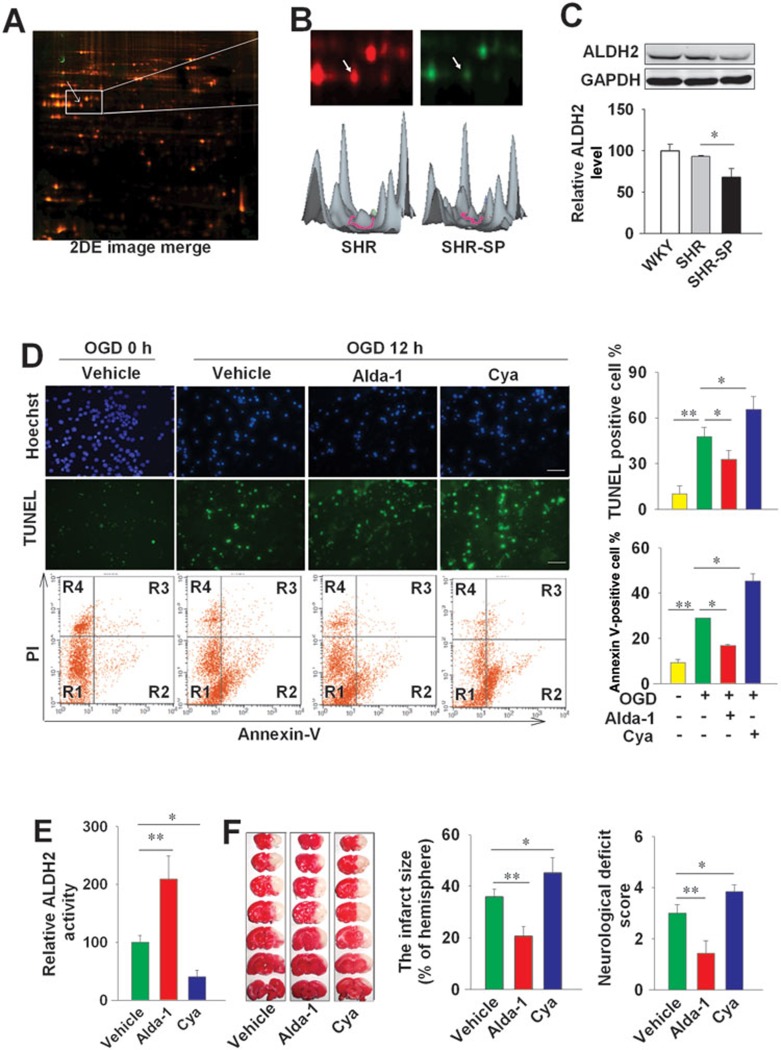
Fluorescent 2D-DIGE identified a number of differentially-expressed proteins in the brain of male SHR and SHR-SP aged 5 months (Figure 1A). Among ∼2 000 spots, 25 proteins were found to be downregulated and 18 were upregulated in SHR-SP (P < 0.05 vs SHR) using tandem matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF/TOF MS). As compared with SHR, ALDH2 was downregulated in SHR-SP (Figure 1B), which was confirmed by western blotting (Figure 1C). ALDH2 expression did not differ between SHR and the normotensive control Wistar Kyoto (WKY) rats (Figure 1C).
Figure 1.
The activation of ALDH2 protects against ischemic injuries in vivo and in vitro. (A) The expression level of ALDH2 was measured in SHR and SHR-SP aged 5 months, using 2D-DIGE. The image was scanned by typhoon Trio variable imager. The arrow points to the ALDH2 protein spot. (B) The sample from SHR was labeled with red, and SHR-SP with green. Pixel values from the images of fluorescent stained gels were converted into 3D representations to illustrate the differential quantification of ALDH2 between SHR and SHR-SP. (C) Western blotting analysis showing expression of ALDH2 in the brain of SHR and SHR-SP (n = 3 in each group). The ALDH2 level of control group was arbitrarily set as 100. GAPDH is used as a loading control. (D) The neurons were treated with Alda-1 (10 μM) or Cya (1 mM) under OGD for 12 h. The cells were stained with TUNEL staining kit (green) (for dead cells) and Hoechst 33342 to visualize the nuclei. The cell death (Annexin V staining, R2 + R3; PI staining, R3 + R4) was also analyzed by flow cytometer. Scale bar, 20 μm. n = 3 in each group. (E) In SD rats, the intracerebroventricular (i.c.v) injection of Alda-1 (50 μg in 5 μl) or Cya (1 mg in 5 μl) affected the activity of ALDH2. n = 8. (F) The left panel shows representative 1% TTC staining of 7 corresponding coronal brain sections of rats with vehicle, Alda-1 (50 μg in 5 μl, i.c.v) or Cya (1 mg in 5 μl, i.c.v) treatment on day 1 after MCAO for 2 h. The ischemic infarct region is white. n = 6. Data are shown as mean ± SD. Data are analyzed by ANOVA followed by LSD post-hoc testing. *P < 0.05, **P < 0.01.
Activation of ALDH2 confers neuroprotection
In cultured primary neurons, pre-treatment of ALDH2 activator Alda-1 (10 μM) reduced cell death induced by oxygen-glucose deprivation (OGD) for 12 h as assessed by flow cytometric analysis of annexin V and propidium iodide (PI) stainings (annexin V-positive cells, 16.7% ± 0.6% vs 28.9% ± 0.1% in the control neuron population subjected to OGD, P < 0.05, the lower panels of Figure 1D). The inhibitor cyanamide (Cya, 1 mM) enhanced neuronal cell death induced by OGD (45.2% ± 3.3% vs 28.9% ± 0.1% in the control neuron population, P < 0.05, the lower panels of Figure 1D). Similar results were obtained with TUNEL analysis (the upper panels of Figure 1D).
To further define the role of ALDH2, a group of Sprague-Dawley (SD) rats were subjected to middle cerebral artery occlusion (MCAO) after the intracerebroventricular (i.c.v.) injection of Alda-1 (50 μg in 5 μl) or Cya (1 mg in 5 μl, i.c.v). The injection of Alda-1 increased ALDH2 activity by 109% in the brain of SD rats, while Cya decreased ALDH2 activity by ∼60% (Figure 1E). Two hours after MCAO, the occluding filament was withdrawn to allow reperfusion. Twenty-four hours after MCAO, rats were killed and the brains were separated for quantitation of cerebral injury. As shown in Figure 1F, Alda-1 treatment decreased the infarct size by 42%, and decreased the neurological deficit score by 53%; whereas Cya treatment increased the infarct size by 28%, and increased the neurological deficit score by 27%.
Transfection of ALDH2 protects against ischemic cerebral injury
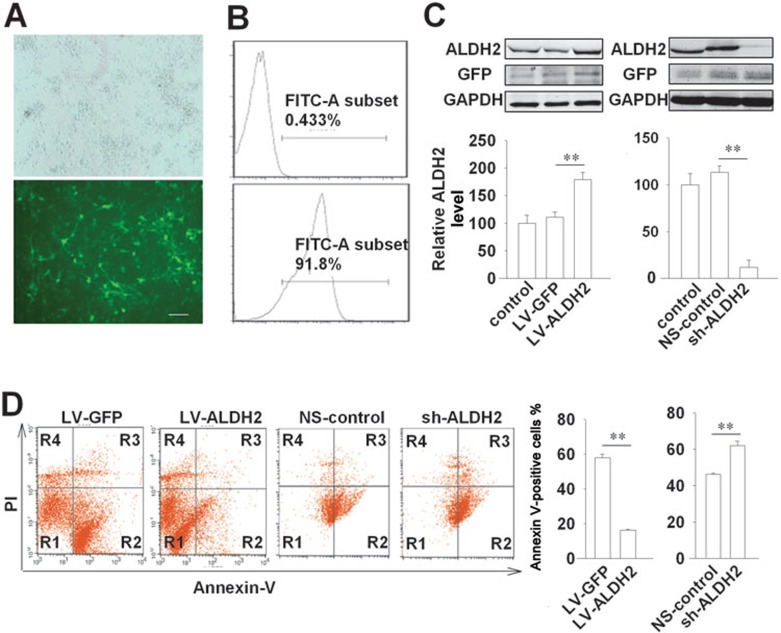
We also determined the role of ALDH2 in neuron survival by ALDH2 overexpression and knockdown in cultured neurons. Primary neurons were transfected with lentiviral vector encoding ALDH2 (LV-ALDH2) or short hairpin RNA (shRNA) targeting ALDH2 (sh-ALDH2). The control samples were transfected with corresponding empty lentiviral vector encoding green fluorescent protein (LV-GFP) and non-silencing control siRNA (NS-control), respectively. For all experiments, the transfection efficiency was maintained over 90% as determined by flow cytometry, with no detectable cellular toxicity (Figure 2A and 2B). Transfection with LV-ALDH2 enhanced the expression of ALDH2 (179 ± 13 vs 111 ± 9 in LV-GFP group, P < 0.01, Figure 2C), and attenuated OGD-induced cell death (16.2% ± 0.2% vs 57.8% ± 2.1% in LV-GFP group, P < 0.01, Figure 2D). In contrast, transfection with sh-ALDH2 decreased the expression of ALDH2 (12 ± 8 vs 113 ± 7 in NS-control group, P < 0.01, Figure 2C), and exacerbated OGD-induced cell death (62.1% ± 2.4% vs 46.1% ± 0.7% in NS-control group, P < 0.01, Figure 2D).
Figure 2.
Lentivirus-mediated overexpression and knockdown of ALDH2 regulate neuron survival under OGD. (A) Lentiviral delivery system efficiently transfected the cultured neurons. Representative image of neurons transfected with GFP-containing lentivirus (The upper panel from fluorescent light field, the lower panel from dark field) for 2 days (MOI = 10). Scale bar, 20 μm. (B) Flow cytometry analysis of neurons with LV-GFP (the lower panel) or without LV-GFP transfection (the upper panel) after incubation for 2 days. (C) Immunoblotting and quantification showing efficient overexpression of ALDH2 by LV-ALDH2 and knockdown by sh-ALDH2 in cultured neurons. n = 3. The ALDH2 level of control group was set as 100. Data are shown as mean ± SD. Data are analyzed by ANOVA followed by LSD post-hoc testing. **P < 0.01. (D) Primary neurons transfected with LV-ALDH2 (LV-GFP as control) or sh-ALDH2 (NS-control as control) were exposed to OGD for 12 h. The cell death (Annexin V staining, R2 + R3; PI staining, R3 + R4) was analyzed by flow cytometer. Data are shown as mean ± SD. Data are analyzed by Student's t test. **P < 0.01; n = 3. LV-GFP, lentiviral delivery system encoding GFP; LV-ALDH2, lentiviral delivery system encoding ALDH2; sh-ALDH2, shRNA-mediated ALDH2 knockdown vectors; NS-control, Non-silencing control siRNA.
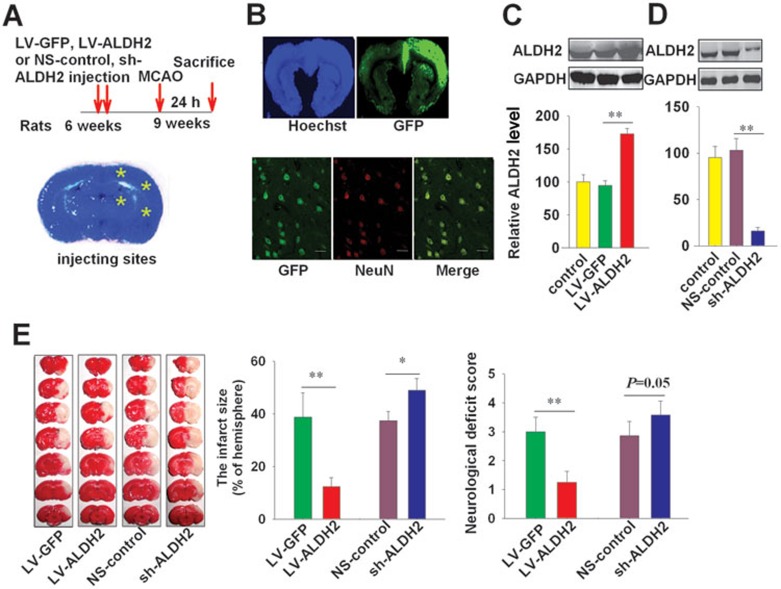
The protective effect of ALDH2 against ischemic stroke was further investigated by overexpression and knockdown of ALDH2 in the brain of SD rats aged 5 weeks. LV-ALDH2, sh-ALDH2 and their controls (LV-GFP or NS-control) were stereotaxically injected into the left hemisphere of rats at four different sites (2 × 106 TU/site, shown in Figure 3A and 3B). Three weeks after injection, MCAO was performed on the left side of brain. Local injection of LV-ALDH2 or sh-ALDH2 led to an approximately 2-fold (overexpression) or 0.16-fold (knockdown) change in ALDH2 protein levels, respectively (Figure 3C and 3D). ALDH2 overexpression reduced the infarct size (12% ± 3.3% vs 38.8% ± 9.2% in LV-GFP control), and improved neurological function (neurological deficit score: 1.3 ± 0.4 vs 3.0 ± 0.5). The knockdown of ALDH2 by sh-ALDH2 increased infarct size (from 37% to 49%), and compromised neurological function (Figure 3E).
Figure 3.
Effects of lentivirus-mediated ALDH2 overexpression/knockdown on cerebral injury induced by MCAO. (A) The upper panel: time schedule for lentivirus injection, MCAO operation and sacrifice for examinations. The lower panel: Four sites for injection (yellow asterisk). Lentivirus (2 × 106TU/site) was injected into the left cortex and hippocampus at 4 sites. (B) Upper panel: general view of lentivirus transfection in rat brain one week after injection (left, the brain was stained by Hoechst; right, GFP-containing transfection site). Lower panel: LV-GFP efficiently transfected neurons, as detected by colocalization with the neuron-specific marker NeuN. Scale bar, 20 μm. (C, D) Immunoblotting and quantification show efficient overexpression and knockdown of ALDH2 in local brain tissue of rats after 3 weeks. n = 3. The ALDH2 level of control group was set as 100. (E) The left panel shows representative 1% TTC staining of 7 corresponding coronal brain sections. The ischemic infarct region is white. The middle panel shows the infarct size and the right panel shows the neurological deficit scores (n = 5). Data are shown as mean ± SD. Data are analyzed by ANOVA followed by LSD post-hoc testing. *P < 0.05, **P < 0.01.
4-HNE level is high in SHR-SP and correlates inversely with the lifespan of SHR-SP
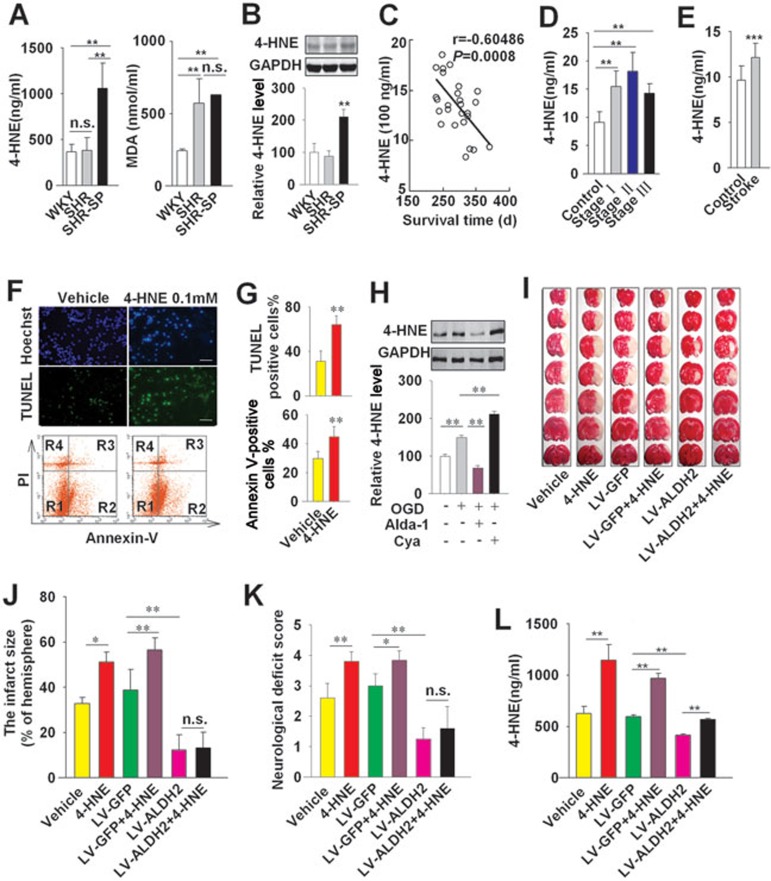
Both 4-HNE and MDA are very common aldehydes produced during oxidative stress as major end-products of lipid peroxidation and accumulate during ischemia-reperfusion7,8,9,10. To investigate the role of aldehydes in stroke, we measured MDA and 4-HNE levels in sera of male WKY rats, SHR and SHR-SP (6-month-old) (Figure 4A). Serum MDA levels did not differ between SHR and SHR-SP, but were significantly higher than that in age-matched WKY rats (6-month-old) (Figure 4A). The 4-HNE level was significantly higher in both the serum and brain of SHR-SP, but not in those of SHR, in comparison to the WKY control (Figure 4A and 4B). Furthermore, we found that serum 4-HNE level in SHR-SP (n = 27; at 6 months of age) was inversely correlated with the survival time (r = −0.60486, P = 0.0008, Figure 4C).
Figure 4.
Elevation of 4-HNE level may be a risk factor for ischemic stroke and ALDH2 abolishes 4-HNE-induced cerebral injury. (A) 4-HNE and MDA levels in serum of WKY, SHR and SHR-SP aged 6 months; n = 8. (B) Western blotting analysis showing the levels of 4-HNE in brains of 6-month-old WKY, SHR and SHR-SP. n = 3. The 4-HNE level of control group was set as 100. (C) Representative scatter plots showing the significant and inverse correlation between serum 4-HNE level (detected at 6-month old) and survival time of SHR-SP. r = −0.60486. P = 0.0008; n = 27. (D) The clinical observation of plasma 4-HNE levels of control subjects (n = 30) and ischemic stroke patients at three stages: (1) Stage I, within 3 days after onset of ischemic stroke (n = 23); (2) stage II, 7-14 days after onset of stroke (n = 15) and (3) stage III, 6 months after onset of stroke (n = 13). (E) The clinical observation of 4-HNE levels in the plasma samples selected from a cohort study with 1 242 participants during 8-year follow-up. The 21 individuals who eventually developed stroke were defined as stroke group. The randomly selected 45 subjects without stroke during follow-up were used as control. (F, G) The neurons were treated with 4-HNE (0.1 mM) under OGD for 12 h. Upper panel: the cells were stained with TUNEL staining kit and Hoechst 33342. Lower panel: the cell death (Annexin V staining, R2 + R3; PI staining, R3 + R4) was also analyzed by flow cytometer. Scale bar, 20 μm. n = 3 in each group. (H) Western blot showing 4-HNE level in cultured neurons treated with Alda-1 (10 μM) or Cya (1 mM) under OGD (n = 3). (I) Representative 1% TTC staining of 7 corresponding coronal brain sections of vehicle- or 4-HNE-treated rats with local transfection of LV-ALDH2 or LV-GFP control on day 1 after MCAO for 2 h. The ischemic infarct region is white. (J, K) The infarct size and the neurological deficit score in MCAO rats. (L) The serum 4-HNE level was measured on day 1 after 4-HNE (31.25 μg/μl, i.c.v.) injection into rats with MCAO (n = 6). Data are shown as mean ± SD. Data in A, B, J, K and L are analyzed by ANOVA followed by LSD post-hoc testing. Data in E and G are analyzed by Student's t test. *P < 0.05, **P < 0.01, ***P < 0.001, n.s. not significant.
Elevation of 4-HNE level in human subjects with stroke
We conducted a preliminary clinical trial in 51 patients with ischemic stroke and 30 healthy human subjects matched for age and sex. Plasma 4-HNE level was measured 3 days (n = 23), 7-14 days (n = 15) and 6 months (n = 13) after stroke. Demographic information of patients is shown in Supplementary information, Table S1. 4-HNE level was higher in stroke patients at all three time points than in subjects without stroke (15.5 ± 2.8 ng/ml, 18.2 ± 3.3 ng/ml, 14.2 ± 1.7 ng/ml, respectively, vs 9.1 ± 1.9 ng/ml, all P < 0.01, Figure 4D). In a separate cohort study of 1 242 community-based participants aged > 40 years followed for 8 years (described in22), 21 subjects developed stroke during follow-up. Randomly selected 45 subjects matched for gender and age were included as control in the analysis of 4-HNE levels at enrollment (Supplementary information, Table S2). The sociodemographic and healthcare characteristics are shown in Supplementary information, Table S3. As shown in Figure 4E, the 21 individuals who developed stroke had significantly higher 4-HNE levels than the control at the start of the study (12.1 ± 1.6 vs 9.6 ± 1.6 ng/ml, P < 0.001).
4-HNE-induced cerebral injury is abolished by ALDH2
In cultured primary neurons, 4-HNE (0.1 mM) increased the cell death induced by OGD as assessed by flow cytometric analysis of annexin V staining (44.6% ± 7.2% vs 29.8% ± 4.8% in the control with OGD alone, P < 0.01) (the lower panels of Figure 4F and 4G). Similar results were obtained with TUNEL analysis (the upper panels of Figure 4F and 4G).
As 4-HNE can be detoxified by ALDH2, we hypothesized that activating ALDH2 would reduce the accumulation of 4-HNE, which would in turn alleviate cerebral injury induced by 4-HNE. Increase of 4-HNE level in cultured neurons exposed to OGD was completely abolished by ALDH2 activator Alda-1, and enhanced by ALDH2 inhibitor Cya (Figure 4H). 4-HNE treatment (31 μg in 5 μl, i.c.v.) at 30 min before MCAO increased infarct size. Similar results were obtained in LV-GFP control groups (Figure 4I and 4J). Overexpression of ALDH2 by local injection of LV-ALDH2 reduced infarct size following MCAO (12.3% ± 6.7% vs 38.8% ± 9.2% in LV-GFP group), abolished the cerebral injury induced by 4-HNE (Figure 4I to 4K), and prevented the rise in 4-HNE level (Figure 4L).
Moderate ethanol consumption protects against stroke and prolongs the lifespan of SHR-SP
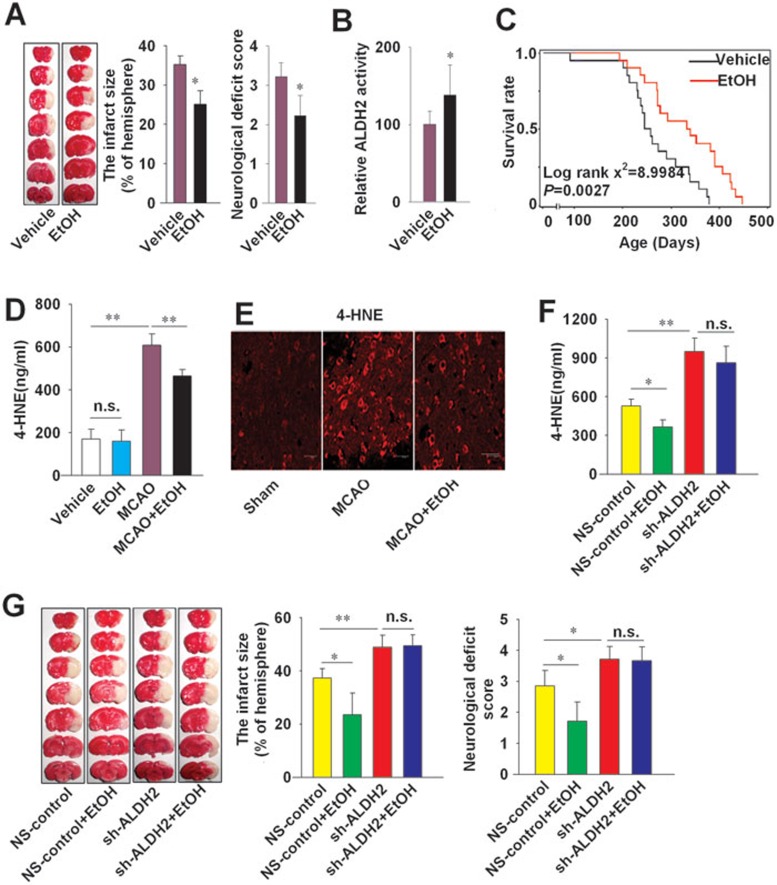
Ethanol is thought to confer protection against stroke23. To assess whether the effects of ethanol are mediated through ALDH2 pathway, SD rats were administered regular liquid diet with 6% ethanol (vol/vol) or isocaloric pair-feeding regimen (control) during a 6-week period according to the literature24. Although 36% ethanol consumption aggravated the cerebral injury induced by MCAO (data not shown), moderate ethanol intake (6%, vol/vol) significantly decreased infarct size (P < 0.05) and attenuated the deterioration in neurological function (P < 0.05, Figure 5A). Moderate intake of ethanol (6%) increased the activity of ALDH2 (138 ± 39 vs 100 ± 17, P < 0.05, Figure 5B), but did not affect the expression of ALDH2 in the brain or liver (Supplementary information, Figure S1A). Further, we observed a shift of isoelectric point of cerebral ALDH2 in rats receiving 6% ethanol (Supplementary information, Figure S1B), most likely reflecting ALDH2 phosphorylation. Long-term consumption of 6% ethanol prolonged the lifespan of SHR-SP (log rank χ2 = 8.9984, P = 0.0027 vs vehicle control, Figure 5C). Ethanol (6%) intake decreased 4-HNE levels both in serum (464 ± 29 vs 608 ± 53 ng/ml, P < 0.01, Figure 5D) and brain (Figure 5E and Supplementary information, Figure S2) of SD rats upon MCAO, but did not affect the 4-HNE level in serum of SD rats without MCAO (Figure 5D).
Figure 5.
The protective effect of ethanol against ischemic stroke is mediated by ALDH2 in MCAO rats. (A) The left panel shows representative 1% TTC staining of 7 corresponding coronal brain sections of rats with vehicle or ethanol (6%) intake on day 1 after MCAO for 2 h. The ischemic infarct region is white. The middle panel shows the infarct size and the right panel shows the neurological deficit scores. n = 10. (B) ALDH2 activity in cerebral cortex of SD rats. Data are shown as mean ± SD. Data were analyzed by Student's t test in A and B. n = 10. (C) Lifelong treatment of 6% ethanol prolonged the lifespan of SHR-SP. Log rank χ2 = 8.9984, P = 0.0027; n = 22. (D) Serum 4-HNE levels in rats with or without MCAO for 2 h. Data are shown as mean ± SD. Data are analyzed by Student's t test; n = 8. (E) Representative images of 4-HNE in the infarct penumbra after MCAO under a confocal fluorescent microscope. Scale bar, 20 μm. (F) Serum 4-HNE levels were measured one day after MCAO. (G) The left panel shows representative 1% TTC staining images of coronal brain sections of rats one day after MCAO for 2 h. The middle panel shows the infarct size and the right panel shows the neurological deficit scores. All animals were treated with vehicle or ethanol for 6 weeks before MCAO. Data are shown as mean ± SD. Data are analyzed by ANOVA followed by LSD post-hoc testing. n = 6. *P < 0.05, **P < 0.01, n.s. not significant. EtOH, ethanol.
ALDH2 mediates the protective effect of ethanol against ischemic stroke
The protective effect of 6% ethanol intake (reduction in infarct size and improvement of neurological function following MCAO; Figure 5A) was abolished by the ALDH2 inhibitor Cya (1 mg in 5 μl, i.c.v) (Supplementary information, Figure S3). Knockdown of ALDH2 by sh-ALDH2 injection increased infarct size and neurological deficit score induced by MCAO (P < 0.01, Figure 5G). Furthermore, ALDH2 knockdown also abolished the reduction of infarct size and neurological deficit score by ethanol (Figure 5G), and attenuated ethanol-induced 4-HNE reduction (Figure 5F). Taken together, these results indicate that the protective effect of ethanol against ischemic stroke is ALDH2 dependent.
PKCε confers neuroprotective effect and regulates the ALDH2 activity
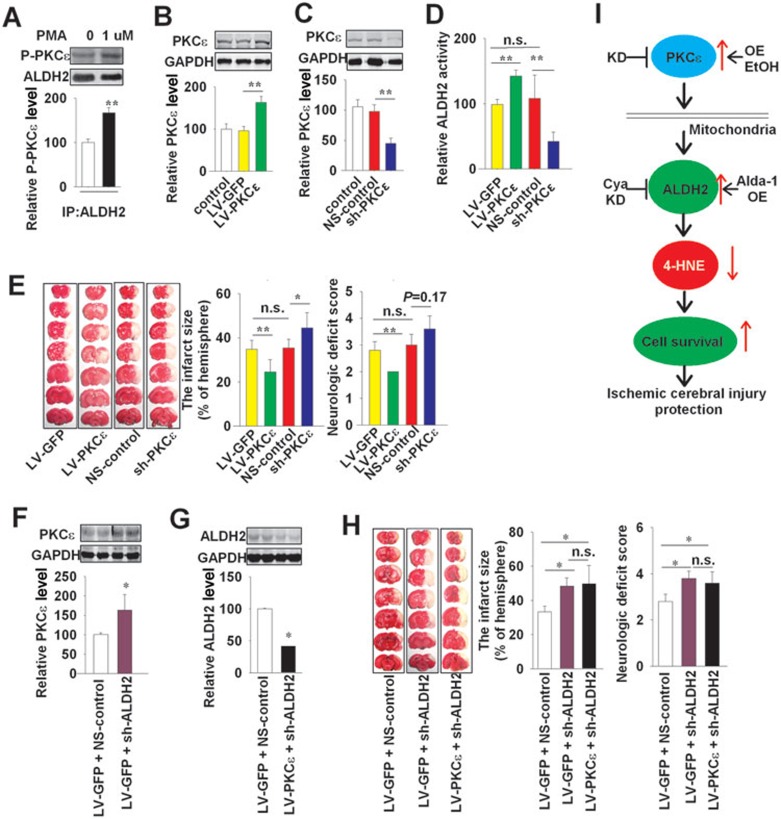
It has been reported that PKC directly phosphorylates ALDH2 in vitro and induces activation of ALDH216. In light of the interaction between PKC and ALDH2, we designed a series of experiments to investigate whether PKC is involved in the protective effect of ALDH2 or ethanol against stroke. In cultured neurons, ethanol (30 mM) enhanced the phosphorylation of PKCε by 68% and promoted the binding of ALDH2 to PKCε (Supplementary information, Figure S4A), but did not affect the binding of ALDH2 to PKCα (Supplementary information, Figure S4B). The next set of experiments was focused on PKCε in cultured neurons. Briefly, ALDH2 co-precipitated with PKCε (Figure 6A). The PKC activator phorbol 12-myristate 13-acetate (PMA) (1 μM) promoted this binding (Figure 6A). In cultured PC12 cells, ALDH2 activity was increased by 40% with PMA (1 μM) and reduced by 66% with the PKC inhibitor staurosporine (0.1 μM) (Supplementary information, Figure S5A). In rats subjected to MCAO, PMA (3 μg in 5 μl, i.c.v.) increased ALDH2 activity by 179% and decreased infarct size by 30%. In contrast, staurosporine (1 μg in 5 μl, i.c.v.) decreased ALDH2 activity by 58% and increased the infarct size by 50% (Supplementary information, Figure S5B and S5C).
Figure 6.
PKCε is upstream to ALDH2 for neuroprotective effect. (A) Immunoprecipitation showing the PKCε phosphorylation (P-PKCε) and the binding of ALDH2 to PKCε in cultured neurons treated with or without the activator of PKC (PMA, 1 μM). (B, C) Western blotting and quantification showing efficient overexpression of PKCε by LV-PKCε, and knockdown by sh-PKCε in left brain of SD rats; n = 3. Lentivirus (2 × 106TU/site) was injected into the left cortex and hippocampus at 4 sites. (D) PKCε overexpression (LV-PKCε) or knockdown (sh-PKCε) affected the ALDH2 activity. n = 5. (E) Representative 1% TTC staining images of coronal brain sections. The middle panel shows the infarct size and the right panel shows the neurological deficit scores. (F, G) Immunoblotting and quantification show efficient transfection of sh-ALDH2 plus LV-PKCε in local brain tissue of rats after 3 weeks. n = 2. (H) Representative 1% TTC staining images of coronal brain sections (left) with sh-ALDH2 plus LV-PKCε. The middle panel shows the infarct size and the right panel shows the neurological deficit scores; n = 5. (I) Proposed mechanism underlying the activation of ALDH2 against ischemic stroke. Data are shown as mean ± SD. Data in B, C, D, E and H are analyzed by ANOVA followed by LSD post-hoc testing. Data in A, F and G are analyzed by Student's t test. *P < 0.05, **P < 0.01, n.s. not significant. IP, immunoprecipitation; KD, knockdown; OE, overexpression; EtOH, ethanol. LV-PKCε, lentiviral delivery system encoding PKCε sh-PKCε, shRNA-mediated PKCε knockdown vectors; NS-control, Non-silencing control siRNA.
We investigated the role of PKCε in neuronal survival using PKCε overexpression and knockdown methods in cultured neurons. Primary neurons were transfected with a lentiviral delivery system encoding PKCε (LV-PKCε) or shRNA targeting PKCε (sh-PKCε) (Supplementary information, Figure S6A). Transfection with LV-PKCε decreased the cell death induced by OGD (12.5% ± 0.1% vs 38.2% ± 0.1%, Supplementary information, Figure S6B). In contrast, knockdown of PKCε by sh-PKCε increased susceptibility to cell injury (55.6% ± 2.9% vs 46.3% ± 0.7%, Supplementary information, Figure S6B).
PKCε overexpression protects against ischemic stroke
To further investigate the neural protective effect of PKCε in vivo and its function on ALDH2, we used LV-PKCε to increase the expression of PKCε, and sh-PKCε to decrease it in the brain of SD rats (Figure 6B and 6C). All lentiviral preparations mentioned above were stereotaxically injected into the cortex and hippocampus of rats at four different sites (2 × 106 TU/site), and MCAO was performed 3 weeks after the injection. Overexpression of PKCε increased ALDH2 activity by 44% and decreased infarct size by 33% (vs LV-GFP control, Figure 6D and 6E) in rats subjected to MCAO. Further, neurological function was also improved (2.0 ± 0.0 vs 2.8 ± 0.3 in LV-GFP control, Figure 6E). In contrast, the sh-PKCε injection decreased ALDH2 activity by 61% and increased infarct size by 26% (vs NS-control, Figure 6D and 6E). The neurological function was also slightly deteriorated by sh-PKCε injection (3.6 ± 0.5 vs 3.2 ± 0.4 in NS-control, P = 0.17, Figure 6E). These results suggest that PKCε confers protective effect against ischemic stroke and increases the activity of ALDH2.
PKCε is upstream to ALDH2 for neuroprotective effect
Injection of both LV-PKCε and sh-ALDH2 in the left cortex and hippocampus of SD rats led to 1.6-fold overexpression of PKCε and 0.42-fold knockdown of ALDH2 (vs control transfected by LV-GFP + NS-control, Figure 6F and 6G). ALDH2 knockdown increased the infarct size, and abolished the protective effect of PKCε against stroke (Figure 6H).
Knockdown of PKCε decreased ALDH2 activity in the brain, prevented the activation of ALDH2 induced by ethanol (Supplementary information, Figure S7A), abolished the ethanol-induced protection against ischemic cerebral injury (infarct size 42.0% ± 12.7% with ethanol vs 38.8% ± 6.9% without ethanol, Supplementary information, Figure S7B), and prevented the decrease in 4-HNE level induced by ethanol intake (1 054 ± 108 ng/ml with ethanol vs 952 ± 72 ng/ml without ethanol, Supplementary information, Figure S7C).
Discussion
ALDH2 is implicated in a wide range of physiological functions13,14,15,16. For example, ALDH2 modulates the impact of smoking on lung cancer risk13, and is a potential target for treating cocaine addiction14. ALDH2 involvement in the pathophysiology of ischemic heart disease has also been described15,16. Approximately 40% of the East Asian population carries an ALDH2*2 mutant allele with dramatic reduction in the enzymatic activity25. A study conducted in aged Korean men showed an association of higher risk of myocardial infarction with the ALDH2 E487K mutation26. A study conducted in Japan also identified ALDH2*2/*2 genotype as a risk factor for myocardial infarction27. Collectively, these observations suggest that ALDH2 is a critical factor in ischemic heart disease15,28,29.
Different from the situation in the heart, the studies on the effects of ALDH2 in the brain are largely missing. Two clinical observations described in the literature are interesting. A study that compared 65 stroke patients with a history of heavy drinking and 83 stroke patients without history of drinking in Taiwan suggested that ALDH2*2 may be a risk factor for excessive alcohol consumption30. A study from Japan reported an association between the ALDH2 *1/*1 genotype and the number of lacunar infarcts31. In vitro studies indicated that activation or overexpression of ALDH2 could protect neurons from aldehyde-induced cell death32,33.
The data in this study show that activation or overexpression of ALDH2 can protect the brain against stroke. Our findings also suggest that ALDH2 plays a critical role in removing endogenous aldehydes during brain ischemia. The results from the clinical parts indicate that ALDH2 dysfunction is a risk factor for stroke.
Moderate ethanol intake might be an economical and effective method to activate ALDH2. Recently, a systematic review and meta-analysis concluded that there is curvilinear relationship between ethanol consumption and ischemic stroke, with a protective effect of ethanol consumption in low to moderate amounts and a detrimental effect of large amounts of ethanol consumption23. Our results showed that low dose of ethanol can alleviate ischemic cerebral injury in the MCAO rat model through upregulation of the ALDH2 pathway, and prolong the lifespan of stroke-prone rats. Notably, activation of ALDH2 by ethanol to protect against stroke requires PKCε. PKCε is known for its oncogenic properties34, and even proposed as a tumor marker35. PKCε has been implicated in heart protection and ventricular hypertrophy34,36. In this study, we showed that PKCε is protective against stroke. Our results also identified PKCε as a molecule upstream of ALDH2 in stroke protection (Figure 6I), as suggested by Chen et al.16. These findings suggest that activation of PKCε or ALDH2 may underlie the cerebral protective effect of ethanol. Indeed, protective effects were achieved by activating ALDH2 with Alda-1 or activating PKC with PMA.
ALDH2 is expressed in mitochondria but barely detectable in the blood. However, the levels of aldehydes can be measured in the blood and used to estimate ALHD2 activity7. Reactive aldehydes, including MDA, 4-HNE and 1-palmitoyl-2-oxovaleroyl phosphatidyl choline (POVPC), were detected in almost all tissues that experienced ischemic injury10. All of them might be the potential substrates of ALDH2. MDA and 4-HNE are representative reactive aldehydes involved in cardiovascular diseases37. Therefore, in this study we mainly focused on 4-HNE and MDA. Higher levels of 4-HNE and MDA were found in the serum of SHR-SP than that of WKY rats. It is of note that only the level of 4-HNE, but not MDA, was elevated in SHR-SP when compared with SHR, suggesting that 4-HNE is a biomarker for stroke. In literature, 4-HNE adducts inhibit key metabolic enzymes and aggravate accumulation of damaged proteins in myocardium. 4-HNE also impairs ATP-generating ability of mitochondria, directly inhibits myocardial contractility38, exerts pro-arrhythmic effects39, and causes tissue damage after myocardial ischemia40.
4-HNE induces cell death in cultured primary neurons and immortalized neuronal cell lines32,33. 4-HNE level is elevated upon acute cerebral injury in both animal and human41,42,43. Due to easily recognizable symptoms and signs of stroke, the significance of 4-HNE as a biomarker for stroke is limited. The identification of risk factors to predict the occurrence of stroke, however, is important. This study made the following findings: (1) The serum level of 4-HNE negatively correlates with the lifespan of SHR-SP; (2) 4-HNE remained persistently elevated after stroke; (3) Subjects with high 4-HNE levels are more likely to develop stroke. These observations suggest that 4-HNE is an important predictor of stroke.
In summary, our results show that ALDH2 activation could be explored as a strategy to treat or to prevent ischemic stroke. Our results also indicate that elevation of 4-HNE level is a risk factor for ischemic stroke.
Materials and Methods
Animals
Male SD rats were purchased from Sino-British SIPPR/BK Lab Animal Ltd. (Shanghai, China). Male SHR-SP, SHR and WKY rats were provided by the animal center of Second Military Medical University. All rats were housed in controlled temperature (23 to 25 °C) and lighting (8:00 AM to 8:00 PM light, 8:00 PM to 8:00 AM dark) and with free access to standard food and drinking water. All animals used in this experiment received humane care in compliance with institutional guidelines of Second Military Medical University for health and care of experimental animals.
Cells and reagents
PC12 cells were purchased from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. Alda-1 was purchased from Calbiochem (Merck KGaA, Darmstadt, Germany). Cya, 4-HNE, PMA and staurosporine were purchased from Sigma-Aldrich (St Louis, MO, USA). Anti-ALDH2, anti-PKCε, anti-p-PKCε (Ser729) and anti-GFP antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-NeuN antibody was purchased from Millipore (Billerica, CA, USA). Anti-4-HNE antibody was purchased from Abcom (NT Hong Kong, China). Rat 4-HNE and MDA detecting kit was purchased from WEIAO BioTech (Shanghai, China). Human OxiSelectTM HNE-His Adduct ELISA Kit was purchased from Cell Biolabs Inc (San Diego, CA, USA). Cy3- and FITC-conjugated secondary antibodies were purchased from Beyotime Institute of Biotechnology (Haimen, Jiangsu, China). IRDye800CW-conjugated secondary antibody was purchased from Rockland (Gilbertsville, PA, USA). Liquid diet with or without ethanol were made by TROPHIC Animal Feed High-tech Co., Ltd (Nantong, Jiangsu, China).
Neuron culture and drug/lentivirus administration
Primary neuronal cells were obtained from the cerebral cortex of neonatal SD rats within 6 h after birth, as described previously44. One day after isolation, the cultures were replenished with Neurobasal medium (Invitrogen, Carlsbad, CA, USA) supplemented with 2% B27 (Invitrogen). Glial growth was suppressed by addition of uridine (10 μM). Staining for NeuN (neuron marker, Millipore) was performed to show that cultured cells contained > 90% neurons. After 7-day culture in vitro, the neurons were treated with Alda-1 (10 μM), Cya (1 mM), 4-HNE (0.1 mM) or vehicle for 30 min, prior to OGD. In some experiments, after 7-day culture in vitro, the neurons were transfected with lentivirus (multiplicity of infection (MOI) = 10) for overexpression or knockdown of ALDH2 or PKCε. The incubation time for transfection was 2 days.
OGD model and cell injury assay
To establish OGD condition, the cultured neurons were washed three times, cultured in Dulbecco's Modified Eagle Medium (DMEM) with no glucose and incubated for 12 h in a hypoxic chamber (Thermo Fisher Scientific, OH, USA) that was continuously flushed with 94% N2 and 5% CO2 at 37 °C to obtain 1% O2. Control neuron cultures were grown in DMEM containing glucose (25 mM) and incubated under normal culture conditions for the same period. After 7-day culture in vitro, cultured cells were exposed to OGD for 12 h prior to apoptosis assay. Cell survival and death was examined by manually counting the cells double-stained with Hoechst (Beyotime) and by in situ cell death detection kit (TUNEL staining kit, Roche, Mannheim, Germany), respectively. The cell nuclei were counterstained with Hoechst 33342 (1 μg/ml). The dead or apoptotic cells were labeled green with the TUNEL staining kit. Images were acquired under a fluorescent microscope (IX-71; Olympus, Tokyo, Japan) with 12.8 M pixel recording digital color cooled camera (DP72; Olympus). The ratio of cells labeled green vs blue is used to define death or apoptosis rate45. The cell death was also detected by Annexin V/PI staining analysis (Annexin V staining for early apoptotic cells, and PI staining for late apoptotic or necrotic cells). Alexa Fluor 488 labeled-Annexin V and PI (Invitrogen) were used according to the manufacturer's instruction. Flow cytometric analysis was performed with a BD FACScalibur flow cytometer (BD FACSCalibur TM Flow Cytometer, USA) to count 10 000 cells for each experiment.
MCAO
The animals were anesthetized with chloral hydrate (300 mg/kg). MCAO surgery in rats was performed as described46. Briefly, the core temperature (rectum) was maintained at 36.5 °C to 37.5 °C by use of a temperature controller pad (Nanjing Xin Xiao Yuan Biotech, Nanjing, China) throughout the surgery. Cerebral focal ischemia was produced by intraluminal occlusion of the left middle cerebral artery using a silicone rubber-coated nylon monofilament. Achievement of ischemia was confirmed by monitoring regional cerebral blood flow (CBF) in the area of the left middle cerebral artery. CBF was monitored through a laser Doppler transducer (MNP110XP, ADInstruments, Australia) to a laser Doppler computerized main unit (ML191, ADInstruments). The microtip was attached to the skull of the rat through cyanoacrilate glue. Animals that did not show a CBF reduction of at least 70% were excluded from the experimental group, as were animals that died after ischemia induction. Two hours after MCAO, the occluding filament was withdrawn to allow reperfusion. Twenty-four hours after MCAO, rats were killed for various examinations after neurological deficit scoring.
Neurological deficit scoring and 2,3,5-triphenyltetrazolium chloride (TTC) staining
Rats were examined for neurological deficits using a 5-point scale as described46. Briefly, animals with normal motor function were scored as 0, flexion of the contralateral torso and forearm on lifting the animal by the tail as 1, circling to the contralateral side but normal posture at rest as 2, leaning to the contralateral side as 3, and no spontaneous motor activity as 4. Brain slices were prepared with brain-cutting matrix (ASI Instruments, Warren, MI, USA). The slices were incubated in 1% TTC solution (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China) at 37 °C for 30 min, and then mounted on dry paper and photographed with a digital camera. The infarct size and hemisphere size of each section were traced and quantified with ImageJ software (NIH, MD, USA). The possible interference of a brain edema in assessing the infarct size was corrected with a standard method of subtracting the volume of the nonischemic ipsilateral hemisphere from that of the contralateral hemisphere. The infarct size is expressed as a percentage of the contralateral hemisphere.
Drug and lentivirus administrations
For Alda-1 or Cya administration, rats were fixed in ALC-H motorized digital stereotaxic instrument (Shanghai Alcott Biotech Co. Ltd., Shanghai, China) and were injected with 5 μl of vehicle, dimethyl sulfoxide (DMSO), Alda-1 (10 mg/ml) or Cya (200 mg/ml) into the left lateral ventricle for 15 min followed by MCAO. During the infusion (i.c.v.) and the following MCAO, a temperature controller pad was used to maintain the core temperature (rectum) at 37 °C.
For 4-HNE administration, rats were injected with 5 μl of vehicle (hexane solution) or 4-HNE (6.25 mg/ml) into the left lateral ventricle, which was similar to Alda-1 or Cya administration. For lentivirus infusion, lentiviral vectors encoding ALDH2 (1-2 μl per site; 2 × 106 transduction units [TU]/site), empty lentiviral vectors or vehicle control were injected into the cortex and hippocampus of 5-week-old rats at 4 sites by microliter syringes (Hamilton CO., Reno, NV, USA). Four sites of injection: site 1, 0.3 mm anterior to bregma, lateral 3 mm to midline; depth, 2 mm to skull surface; site 2, 0.3 mm anterior to bregma, lateral 3 mm to midline; depth, 5 mm to skull surface; site 3, 0.3 mm anterior to bregma, lateral 5 mm to midline; depth, 3 mm to skull surface; site 4, 0.3 mm anterior to bregma, lateral 5 mm to midline; depth, 6 mm to skull surface. Three weeks after injection, MCAO was performed in the left side of brain.
For administration of ethanol, rats were fed with a nutritionally complete liquid diet (TROPHIC Animal Feed High-tech Co., Ltd.) for a one-week acclimation period as described47. Upon completion of the acclimation period, the rats of control group were maintained with the regular liquid diet (without ethanol), and the rats of 6% ethanol group began a 6-week period of isocaloric 6% (vol/vol) ethanol diet feeding. An isocaloric pair-feeding regimen was employed to eliminate the possibility of nutritional deficits. Control rats were offered the same quantity of diet as the ethanol-consuming rats drunk on the previous day.
Immunoblotting
The total protein from tissue/cells was prepared as described48. Tissue/cell extract was boiled in 4× loading buffer, subjected to SDS-PAGE, and transferred onto the pure nitrocellulose blotting membranes. To determine the isoelectric point of ALDH2, tissue extract was separated by 2D-DIGE. The membranes were incubated with one of the following antibodies: anti-ALDH2 antibody (1:200), anti-GFP antibody (1:200), anti-4-HNE antibody (1:1 000, Abcom) prior to incubation with IRDye800CW-conjugated secondary antibody (Rockland). The image was captured by the Odyssey infrared imaging system (Li-Cor Bioscience, Lincoln, NE, USA). The data were analyzed using ImageJ software (NIH). All immunoblotting experiments were repeated three times. The mean value from three experiments was used to indicate the value for each animal.
Immunoprecipitation
Immunoprecipitation was performed as described49. Mitochondria were isolated from cultured neurons using a mitochondria isolation kit (Beyotime). The mitochondrial protein (500 μg) was used for immunoprecipitation in the presence or absence of ALDH2-specific antibodies (2 μg). After 3-h incubation, protein A/G agarose beads (EMD Chemicals Inc., Darmstadt, Germany) were added for an additional 1 h before pull-down. Immunocomplexes were washed three times with phosphate-buffered saline (PBS), eluted with 2× sample buffer, and subjected to a 10% SDS-PAGE. We analyzed the immunocomplexes for the presence of the associated proteins by western blotting using antibodies against ALDH2 (Santa Cruz Biotechnology).
Immunofluorescence
Frozen 20-μm-thick brain sections and cultured neurons were fixed in 4% paraformaldehyde, blocked by 8% normal goat serum, and incubated in specific primary antibodies as follows: anti-NeuN (1:200, Millpore) and anti-4-HNE (1:100, Abcom). After being washed three times with PBS, the sections and cells were incubated with Alexa 488- or Cy3-conjugated secondary antibodies.
ALDH2 enzymatic activity
Mitochondria were isolated from tissue (100 mg) or PC12 cells (3 × 106) using a mitochondria isolation kit (Beyotime). Enzymatic activity of ALDH2 was determined spectrophotometrically by monitoring the reductive reaction of NAD+ to NADH at A340 nm, as described previously50,51. The assays were carried out at 25 °C in 20 mM sodium pyrophosphate buffer (pH 8.5) with 10 mM acetaldehyde (Sangon Biotech Co., Ltd, Shanghai, China) and 200 μg of mitochondrial lysate protein added. To start the reaction, 2.5 mM NAD (Sangon Biotech Co., Ltd) was added and the accumulation of NADH was monitored for 2 min using clinical chemistry analyzer (Shanghai Xunda Medical Instrument Co., Ltd., Shanghai, china). ALDH2 activity was expressed as nmol NADH/min/mg protein. The ALDH2 activity of control group was set as 100.
Measurement of 4-HNE and MDA concentrations
The concentrations of 4-HNE and MDA in rat serum were measured using 4-HNE Detecting kit and MDA Detecting kit (WEIAO BioTech), respectively, according to the manufacturer's instructions. The 4-HNE level in human plasma was measured using OxiSelectTM HNE-His Adduct ELISA Kit (Cell Biolabs) according to the manufacturer's instructions.
Difference in gel electrophoresis (DIGE)
To prepare the total protein extract, frozen brain tissues were homogenized in liquid nitrogen and lyzed in lysis buffer. After sonicated with 5× 3 s bursts on ice, the crude extracts underwent ultracentrifugation for 30 min at 12 000× g at 4 °C. The obtained supernatants were purified with 2-D Clean-Up Kit (Bio-Rad Laboratories, CA, USA) and the protein concentration was determined by 2-D Quant Kit (Bio-Rad Laboratories). Then proteins were minimally labeled according to the manufacturer's instructions (CyDye DIGE fluor minimal labeling kit, GE Healthcare, General Electric Company, CT, USA). For the isoelectric focusing electrophoresis, the labeled samples were placed in a strip holder using a step gradient protocol (30 v 6 h, 60 v 6 h, 200 v 1 h, 500 v 1 h, 1 000 v 1 h, 8 000 v 6 h). After the Immobilized pH Gradient (IPG) strips were equilibrated in SDS equilibration solution, proteins were further separated on the 12.5% homogeneous SDS-PAGE gels (80 mv/gel for 30 min and 280 mv/gel for 4.5 h). Then, scanning and image analysis were carried out. The labeled protein spots in the gel were visualized with Typhoon Trio variable imager (GE Healthcare). The sample from SHR was labeled with red and SHR-SP with green. DIGE images were analyzed using DeCyder v6.5 software (GE Healthcare). The ratios of the log-standardized protein spot abundances (differences in expression) between the groups were computed (one-way ANOVA, P < 0.05). Differentially expressed protein spots were excised from the post-stained gel for further mass spectrometry and protein identification. The manually excised protein spots were then digested with trypsin and diluted with matrix solution for analysis by 4700 Matrix-unterstützte Laser-Desorption/Ionisation-time of flight (MALDI-TOF/TOF) Proteomics Analyzer (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions.
Construction of lentiviral vectors
The rat Aldh2 (GenBank accession number NM_032416) and Prkce (GenBank accession number NM_017171) cDNAs were PCR amplified and subcloned into a pLenO-DCE vector (Invabio, Shanghai Innovation Biotechnology Co. Ltd, Shanghai, China) with Nhe I/Not I restriction sites to construct pLenO-DCE-Aldh2 and pLenO-DCE-Prkce, respectively. Primers designed for Prkce and Aldh2 cDNA cloning are listed as PKCε-F, 5′-TACGAAGTGCGCTGGGCTAA-3′, and PKCε-R, 5′-GGAGCCACAGTGGTCACAGAA-3′ ALDH2-F, 5′-TGATGAAAGTGGCCGAGCAG-3′, and ALDH2-R, 5′-TAGGGCCGAATCCAGGAACA-3′.
siRNAs targeting the ORFs of the rat Aldh2 and Prkce genes were designed with sequences as follows: PKCε-SH1, 5′-GACGACCGATCCAAGTCAG-3′ PKCε-SH2, 5′-ACAGCCACCAAGCAGAAGA-3′ PKCε-SH3, 5′-AGAAGGACGTCATCCTGCA-3′ and PKCε-SH4, 5′-CTCTCATGTTTCTCCACCA-3′. ALDH2-SH1, 5′-GGAGGACGTAGACAAGGCA-3′ ALDH2-SH2, 5′-GGAACAAGGAGGACGTAGA-3′ ALDH2-SH3, 5′-CAGATCATTCCGTGGAACT-3′ and ALDH2-SH4, 5′-GCAACCAGATCTTCATTAA-3′. Real-time PCR and western blotting were performed to determine the RNA interference efficacy. We selected ALDH2-SH4 (sh-ALDH2) for knockdown of ALDH2, and PKCε-SH3 (sh-PKCε) for knockdown of PKCε. Non-silencing control siRNA (NS-control, 5′-TTCTCCGAACGTGTCACGT-3′) was used as control. Oligos were annealed and cloned into the Mu l/Cla l site of pLenO-THM vector (Invabio).
Production of lentiviral vectors
Lentiviral vectors were prepared by co-transfecting 293T cells (Invitrogen) with helper plasmid pRsv-REV, pMDlg-pRRE and pMD2G (Invabio) using the calcium phosphate technique. The transfection efficiency was monitored by flow cytometry analysis.
Clinical observations
In the first observation, ischemic stroke patients were collected from Changhai Hospital and Xinhua Hospital from March 2011 to March 2012, including three stages of stroke: (1) Stage I, within 3 days after onset of stroke (n = 23); (2) Stage II, 7-14 days after onset of stroke (n = 15) and (3) Stage III, 6 months after onset of stroke (n = 13). The control group was the healthy subjects with matched age, sex, etc. (n = 30).
In the second observation, the study population has been described in detail previously20. In brief, 1 242 community-based participants aged > 40 years were followed from 2004 to 2011. During 8-year follow-up, 21 subjects suffered from stroke. From the participants without stroke during follow-up, we randomly selected 45 subjects as control.
Information collection and covariate evaluation
The ethics committee in Second Military Medical University approved the study for sample collection. All participants gave written informed consent prior to data collection. Information of sociodemographic status (e.g., age and sex), personal health history (e.g., hypertension, diabetes and myocardial infarction) was obtained through a questionnaire. Each participant underwent weight and height measurement, using a calibrated scale. The body mass index (BMI) was calculated as weight (in kilograms) divided by height squared (in square meters). Blood pressure (BP) was measured according to the guidelines presented in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood pressure52. Hypertension was defined as systolic BP > 140 mmHg or diastolic BP > 90 mmHg or by the use of anti-hypertensive medications in the previous 2 weeks irrespective of the BP or by self-reported history of hypertension. Diabetes was defined as fasting plasma glucose > 7.0 mmol/l or 2-h plasma glucose > 11.1 mmol/l or by the use of hypoglycemic agents or by self-reported history of diabetes according to Standard World Health Organization criteria53. Serum total cholesterol, triglyceride and high density lipoprotein (HDL) were measured by Hitachi 7170 autoanalyzer (Hitachi, Tokyo, Japan). Indicators of liver and kidney function were also tested, including alanine transarninase (ALT), blood urea nitrogen (BUN), creatinine, uric acid, etc.
Statistical analysis
The investigators were blinded to the procedures when they assessed the infarct size and neurological deficit score of MCAO animals, and recorded the TUNEL-positive cells, etc. The animals were randomly assigned by using the random permutations table. Data are expressed as the mean ± SD. Data are analyzed with two-tailed Student's t-test or one-way analysis of variance (ANOVA). Kaplan-Meier analysis is used to estimate survival probabilities. Log-rank testing is used to evaluate equality of survival curves. P < 0.05 is considered statistically significant. The relationships between 4-HNE and the survival time of SHR-SP are assessed by univariate regression analysis. The Pearson r values are calculated.
Acknowledgments
This study was supported by the National Basic Research Program of China (2009CB521901) and the National Natural Science Foundation of China (81273505, 81230083).
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
Sociodemographic and health care characteristics of ischemic stroke patients at different stages and control subjects.
Sociodemographic and health care characteristics of 1242 participants and their randomly selected subjects.
Sociodemographic and health care characteristics of population before follow-up.
Ethanol affects the activity of ALDH2.
Ethanol treatment attenuates MCAO-induced rise of 4-HNE.
The protective effect of ethanol on ischemic stroke is mediated by ALDH2 in rats with MCAO.
Ethanol increases the binding of PKCε to ALDH2.
The activation of PKCε protects against ischemic injuries in vivo and in vitro.
Overexpression of PKCε confers neuroprotective effect in cultured neurons.
The knockdown of PKCε abolishes the neuroprotective effect of ethanol.
References
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Jauch EC, Cucchiara B, Adeoye O, et al. Part 11: adult stroke: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S818–S828. doi: 10.1161/CIRCULATIONAHA.110.971044. [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics: 2008 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Whisnant JP. Modeling of risk factors for ischemic stroke. The Willis Lecture. Stroke. 1997;28:1840–1844. doi: 10.1161/01.str.28.9.1840. [DOI] [PubMed] [Google Scholar]
- Rosengren A, Hawken S, Ounpuu S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- World Health Organization. World health statistics 2008Geneva, Switzerland: World Health Organization; 2008
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- He L, Liu B, Dai Z, et al. Alpha lipoic acid protects heart against myocardial ischemia-reperfusion injury through a mechanism involving aldehyde dehydrogenase 2 activation. Eur J Pharmacol. 2012;678:32–38. doi: 10.1016/j.ejphar.2011.12.042. [DOI] [PubMed] [Google Scholar]
- Berg RM, Møller K, Bailey DM. Neuro-oxidative-nitrosative stress in sepsis. J Cereb Blood Flow Metab. 2011;31:1532–1544. doi: 10.1038/jcbfm.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill BG, Bhatnagar A. Beyond reactive oxygen species: aldehydes as arbitrators of alarm and adaptation. Circ Res. 2009;105:1044–1046. doi: 10.1161/CIRCRESAHA.109.209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagranha CJ, Deschamps A, Aponte A, et al. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res. 2010;106:1681–1691. doi: 10.1161/CIRCRESAHA.109.213645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MJ, Malek K, Crabb DW. Distribution of messenger RNAs for aldehyde dehydrogenase 1, aldehyde dehydrogenase 2, and aldehyde dehydrogenase 5 in human tissues. J Investig Med. 1996;44:42–46. [PubMed] [Google Scholar]
- Park JY, Matsuo K, Suzuki T, et al. Impact of smoking on lung cancer risk is stronger in those with the homozygous aldehyde dehydrogenase 2 null allele in a Japanese population. Carcinogenesis. 2010;31:660–665. doi: 10.1093/carcin/bgq021. [DOI] [PubMed] [Google Scholar]
- Yao L, Fan P, Arolfo M, et al. Inhibition of aldehyde dehydrogenase-2 suppresses cocaine seeking by generating THP, a cocaine use-dependent inhibitor of dopamine synthesis. Nat Med. 2010;16:1024–1028. doi: 10.1038/nm.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Sun L, Mochly-Rosen D. Mitochondrial aldehyde dehydrogenase and cardiac diseases. Cardiovasc Res. 2010;88:51–57. doi: 10.1093/cvr/cvq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Budas GR, Churchill EN, et al. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco F, Martín-Ventura JL, Duran MC, et al. Quest for novel cardiovascular biomarkers by proteomic analysis. J Proteome Res. 2005;4:1181–1191. doi: 10.1021/pr0500197. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Yamori Y, Nagaoka A. Establishment of the stroke-prone spontaneously hypertensive rats (SHR) Circ Res. 1974;34:I-143-I-145. [Google Scholar]
- Yamori Y, Horie R, Handa H, et al. Pathogenic similarity of strokes in stroke-prone spontaneously hypertensive rats and humans. Stroke. 1976;7:46–55. doi: 10.1161/01.str.7.1.46. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Lei H, Liu AJ, et al. Increased oxidative stress is responsible for severer cerebral infarction in stroke-prone spontaneously hypertensive rats. CNS Neursci Ther. 2011;17:590–598. doi: 10.1111/j.1755-5949.2011.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou YX, Zhang XH, Su FY, Liu X. Importance of riboflavin kinase in the pathogenesis of stroke. CNS Neurosci Ther. 2012;18:834–840. doi: 10.1111/j.1755-5949.2012.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang F, Wang X, et al. The association between plasma uric acid and renal function decline in a Chinese population-based cohort. Nephrol Dial Transplant. 2012;27:1836–1839. doi: 10.1093/ndt/gfr597. [DOI] [PubMed] [Google Scholar]
- Patra J, Taylor B, Irving H, et al. Alcohol consumption and the risk of morbidity and mortality for different stroke types — a systematic review and meta-analysis. BMC Public Health. 2010;10:258. doi: 10.1186/1471-2458-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill EN, Disatnik MH, Mochly-Rosen D. Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of varepsilonPKC and activation of aldehyde dehydrogenase 2. J Mol Cell Cardiol. 2009;46:278–284. doi: 10.1016/j.yjmcc.2008.09.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Huang IY, Ikawa M. Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals. Proc Natl Acad Sci USA. 1984;81:258–261. doi: 10.1073/pnas.81.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo SA, Kim EK, Park MH, et al. A Glu487Lys polymorphism in the gene for mitochondrial aldehyde dehydrogenase 2 is associated with myocardial infarction in elderly Korean men. Clin Chim Acta. 2007;382:43–47. doi: 10.1016/j.cca.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Takagi S, Iwai N, Yamauchi R, et al. Aldehyde dehydrogenase 2 gene is a risk factor for myocardial infarction in Japanese men. Hypertens Res. 2002;25:677–681. doi: 10.1291/hypres.25.677. [DOI] [PubMed] [Google Scholar]
- Budas GR, Disatnik MH, Mochly-Rosen D. Aldehyde dehydrogenase 2 in cardiac protection: a new therapeutic target. Trends Cardiovasc Med. 2009;19:158–164. doi: 10.1016/j.tcm.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Guo R, Yu L, et al. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J. 2011;32:1025–1038. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CT, Cheng CA, Wang HK, et al. The role of ALDH2 and ADH1B polymorphism in alcohol consumption and stroke in Han Chinese. Hum Genomics. 2011;5:569–576. doi: 10.1186/1479-7364-5-6-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa H, Wada M, Arawaka S, et al. A polymorphism of the aldehyde dehydrogenase 2 gene is a risk factor for multiple lacunar infarcts in Japanese men: the Takahata Study. Eur J Neurol. 2007;14:428–434. doi: 10.1111/j.1468-1331.2007.01700.x. [DOI] [PubMed] [Google Scholar]
- Kong D, Kotraiah V. Modulation of aldehyde dehydrogenase activity affects (±)-4-hydroxy-2E-nonenal (HNE) toxicity and HNE-protein adduct levels in PC12 cells. J Mol Neurosci. 2012;47:595–603. doi: 10.1007/s12031-011-9688-y. [DOI] [PubMed] [Google Scholar]
- Bai J, Mei Y. Overexpression of aldehyde dehydrogenase-2 attenuates neurotoxicity induced by 4-hydroxynonenal in cultured primary hippocampal neurons. Neurotox Res. 2011;19:412–422. doi: 10.1007/s12640-010-9183-1. [DOI] [PubMed] [Google Scholar]
- Duquesnes N, Lezoualc'h F, Crozatier B. PKC-delta and PKC-epsilon: foes of the same family or strangers. J Mol Cell Cardiol. 2011;51:665–673. doi: 10.1016/j.yjmcc.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Gorin MA, Pan Q. Protein kinase C epsilon: an oncogene and emerging tumor biomarker. Mol Cancer. 2009;8:9. doi: 10.1186/1476-4598-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res. 2006;70:222–230. doi: 10.1016/j.cardiores.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292:2083–2086. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- Aberle NS, Picklo MJ, Sr, Amarnath V, Ren J. Inhibition of cardiac myocyte contraction by 4-hydroxy-trans-2-nonenal. Cardiovasc Toxicol. 2004;4:21–28. doi: 10.1385/ct:4:1:21. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res. 2006;99:692–705. doi: 10.1161/01.RES.0000243586.99701.cf. [DOI] [PubMed] [Google Scholar]
- Lucas DT, Szweda LI. Cardiac reperfusion injury: aging, lipid peroxidation, and mitochondrial dysfunction. Proc Natl Acad Sci USA. 1998;95:510–514. doi: 10.1073/pnas.95.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagotani S, Hayashi T, Sato K, et al. Reduction of cerebral infarction in stroke-prone spontaneously hypertensive rats by statins associated with amelioration of oxidative stress. Stroke. 2005;36:670–672. doi: 10.1161/01.STR.0000155732.27333.3c. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Cutler RG, et al. Neuroprotective actions of a histidine analogue in models of ischemic stroke. J Neurochem. 2007;101:729–736. doi: 10.1111/j.1471-4159.2006.04412.x. [DOI] [PubMed] [Google Scholar]
- Lee WC, Wong HY, Chai YY, et al. Lipid peroxidation dysregulation in ischemic stroke: Plasma 4-HNE as a potential biomarker. Biochem Biophys Res Commun. 2012;425:842–847. doi: 10.1016/j.bbrc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Tamatani M, Matsuyama T, Yamaguchi A, et al. ORP150 protects against hypoxia/ischemia-induced neuronal death. Nat Med. 2001;7:317–323. doi: 10.1038/85463. [DOI] [PubMed] [Google Scholar]
- Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- Liu AJ, Zang P, Guo JM, et al. Involvement of acetylcholine-α7nAChR in the protective effects of arterial baroreflex against ischemic stroke. CNS Neurosci Ther. 2012;18:918–926. doi: 10.1111/cns.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Babcock SA, Li Q, et al. Aldehyde dehydrogenase-2 transgene ameliorates chronic alcohol ingestion-induced apoptosis in cerebral cortex. Toxicol Lett. 2009;187:149–156. doi: 10.1016/j.toxlet.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wang HB, Lu YJ, Hu JW, Bao L, Zhang X. Transport of receptors, receptor signaling complexes and ion channels via neuropeptide-secretory vesicles. Cell Res. 2011;21:741–753. doi: 10.1038/cr.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Wang Y, Li QF, Björn LO, He JX, Li SS. Arabidopsis STO/BBX24 negatively regulates UV-B signaling by interacting with COP1 and repressing HY5 transcriptional activity. Cell Res. 2012;22:1046–1057. doi: 10.1038/cr.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SY, Gomelsky M, Duan J, et al. Overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene prevents acetaldehyde-induced cell injury in human umbilical vein endothelial cells: role of ERK and p38 mitogenactivated protein kinase. J Biol Chem. 2004;279:11244–11252. doi: 10.1074/jbc.M308011200. [DOI] [PubMed] [Google Scholar]
- Xu D, Guthrie JR, Mabry S, et al. Mitochondrial aldehyde dehydrogenase attenuates hyperoxia-induced cell death through activation of ERK/MAPK and PI3K-Akt pathways in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L966–L975. doi: 10.1152/ajplung.00045.2006. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National committee on Prevention, Detection, Evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sociodemographic and health care characteristics of ischemic stroke patients at different stages and control subjects.
Sociodemographic and health care characteristics of 1242 participants and their randomly selected subjects.
Sociodemographic and health care characteristics of population before follow-up.
Ethanol affects the activity of ALDH2.
Ethanol treatment attenuates MCAO-induced rise of 4-HNE.
The protective effect of ethanol on ischemic stroke is mediated by ALDH2 in rats with MCAO.
Ethanol increases the binding of PKCε to ALDH2.
The activation of PKCε protects against ischemic injuries in vivo and in vitro.
Overexpression of PKCε confers neuroprotective effect in cultured neurons.
The knockdown of PKCε abolishes the neuroprotective effect of ethanol.