Abstract
Background
Lifetime risk estimates of chronic kidney disease (CKD) can motivate preventative behaviors at the individual level and forecast disease burden and health care utilization at the population level.
Study Design
Markov Monte Carlo model simulation study.
Setting & Population
Current U.S. black and white population.
Model, Perspective, & Timeframe
Markov models simulating kidney disease development, using an individual perspective and lifetime horizon.
Outcomes
Age-, sex- and race-specific residual lifetime risks of CKD stages 3a+ (eGFR<60 ml/min/1.73m2), 3b+ (eGFR<45 ml/min/1.73 m2), and 4+ (eGFR<30 ml/min/1.73m2), and end stage renal disease (ESRD).
Measurements
State transition probabilities of developing CKD and of dying prior to its development were modeled using: 1) mortality rates from National Vital Statistics Report, 2) mortality risk estimates from a 2-million person meta-analysis, and 3) CKD prevalence from National Health and Nutrition Examination Surveys. Incidence, prevalence, and mortality related to ESRD were supplied by the US Renal Disease System.
Results
At birth, the overall lifetime risks of CKD stages 3a+, 3b+, 4+, and ESRD were 59.1%, 33.6%, 11.5%, and 3.6%, respectively. Women experienced greater CKD risk yet lower ESRD risk than men; blacks of both sexes had markedly higher CKD stage 4+ and ESRD risk (lifetime risks for white men, white women, black men, and black women, respectively: 53.6%, 64.9%, 51.8%, and 63.6% [CKD stage 3a+]; 29.0%, 36.7%, 33.7%, and 40.2% [CKD stage 3b+]; 9.3%, 11.4%, 15.8%, and 18.5% [CKD stage 4+]; and 3.3%, 2.2%, 8.5%, and 7.8% [ESRD]). Risk of CKD increased with age, with approximately one-half of CKD stage 3a+ cases developing after 70 years of age.
Limitations
CKD incidence estimates were modeled from prevalence in the U.S. population.
Conclusions
In the U.S., the lifetime risk of developing CKD stage 3a+ is high, underscoring the importance of primary prevention and effective therapy to reduce CKD-related morbidity and mortality.
Individualized, long-term risk estimates are increasingly used in clinical practice, treatment guidelines, screening and education campaigns, health care utilization planning, and goal development for risk reduction and preventative behavior.1,2 While the absolute risk of disease development in a given year may be small, the lifetime risk (for an individual at birth) and residual lifetime risk (for an individual currently free of disease) of common diseases can be quite high. Indeed, the lifetime risk of diabetes is 33%–39%, and the residual lifetime risks of hypertension and diabetes for a middle-aged man are 83% and 18%, respectively.3, 4
Chronic kidney disease (CKD) is rising in prevalence, increasingly expensive, and associated with a high degree of morbidity and mortality.5–10 Reduced eGFR is a well-accepted risk factor for all-cause mortality, acute kidney injury, and end-stage renal disease (ESRD),7, 8,11 and CKD may carry a coronary heart disease risk similar to that of diabetes.12 ESRD, the most severe stage of CKD, is associated with a residual life expectancy of less than 5 years.9 Despite a national education campaign,13, 14 however, CKD awareness remains low, and little is known about a given individual’s lifetime risk for CKD.15
Previous risk forecasts have focused exclusively on ESRD16–18 (most recently, in a predominately white, Canadian population) or on CKD for cost-effectiveness models, with little exploration by baseline age, race, and sex.18, 19 Older adults experience dramatically greater incidence of kidney disease than do younger adults.20, 21 Blacks face greater risk of kidney disease than whites, particularly in the more severe stages.9, 22–26 Women live longer than men and may accordingly face a greater lifetime CKD burden.27 The objective of this study was thus to estimate age-, race-, and sex-specific residual lifetime risks of CKD stages 3a+, 3b+, and 4+, and to update U.S. estimates for the residual lifetime risk of ESRD.
METHODS
Lifetime Risk Estimates
The residual lifetime risks for four kidney disease outcomes were independently estimated: CKD Stage 3a+ (eGFR < 60 ml/min/1.73 m2, as estimated using the CKD-EPI [CKD Epidemiology Collaboration] creatinine [2009] equation), CKD stage 3b+ (eGFR < 45 ml/min/1.73 m2), CKD stage 4+ (eGFR < 30 ml/min/1.73 m2), and ESRD (chronic kidney failure treated by dialysis or transplantation). For each outcome, a separate Markov chain model was designed to simulate progression of an initially outcome-free individual of given sex, race, and baseline age through the mutually exclusive states of no kidney disease, kidney disease, and death, with kidney disease and death treated as absorbing states (Figure 1). State transition probabilities were specified as 1) the probability of dying prior to the development of kidney disease (defined as CKD stage 3a+, 3b+, 4+, or ESRD) (Qno_CKD), and 2) the probability of developing kidney disease (ICKD). The formulas for calculating each state transition probability are provided as online supplementary material; estimates are provided in Tables S1 (CKD stage 3a+), S2 (CKD stage 3b+), S3 (CKD stage 4+), and S4 (ESRD). Monte Carlo simulations were conducted in simulated cohort of 10,000 individuals of specified race, sex, and baseline age, with a lifetime horizon (capped at 90 years), a cycle length of one year, and an individual perspective. Age-specific transition probabilities were assumed unchanged throughout the lifetime of the simulated cohort; progression through CKD stages was not modeled.
Figure 1.
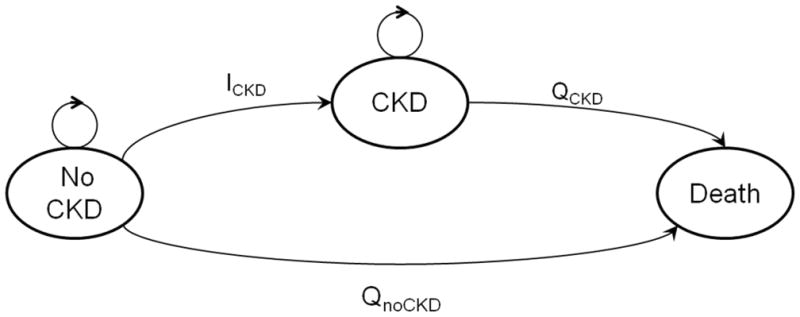
Markov chain model used to simulate the progression of an initially CKD-free individual through death or the development of CKD. ICKD=one-year probability of developing CKD, QnoCKD=one-year probability of dying prior to the development of CKD, QCKD=one-year probability of dying in an individual with CKD. Here, CKD is treated as a single outcome, representing the stage of interest (CKD 3a+, CKD 3b+, CKD 4+, or ESRD). Progression through CKD stages is not modeled.
Data Sources
Annual probabilities of death for the U.S. population by age, sex, and race were obtained from the National Vital Statistics Report.28 Sex- and race-adjusted estimates of the relative risk of mortality associated with CKD by category of eGFR and urine albumin-creatinine ratio (ACR) and in four age groups (18–54, 55–64, 65–74, ≥75 years) were obtained from a previously published meta-analysis encompassing over 2 million participants and 46 cohorts (Table S5).29
CKD prevalence was estimated using data from the National Health and Nutrition Examination Survey (NHANES), a multi-stage, population-level survey of community-dwelling U.S. civilians conducted by the National Center for Health Statistics (NCHS). The current study included surveys conducted from 1988–1994 (two phases, 1988–1991 and 1991–1994), from 1999–2004 (three phases, 1999–2000, 2001–2002, and 2003–2004), and from the continuous NHANES. The study population was limited to individuals ages 20 and older with non-missing serum creatinine, urine albumin, and urine creatinine. Data were analyzed using NCHS-provided sample weights, primary sampling units, and strata specific to each survey.
Treated ESRD incidence, prevalence, and mortality rates for the year 2009 were provided by the US Renal Data System (USRDS) in 5- to 10-year age intervals.9 All rates were specific to sex and race (white or black). Incidence and mortality rates (R) were converted to one-year probabilities (Q) using the following formula: Q=R/(1+ 0.5*R).30
U.S. population estimates for 2012 by age, sex, and race (non-Hispanic blacks and non-Hispanic whites) were obtained from U.S. Census Bureau projections.31
Disease Prevalence Estimates
Multinomial logistic regression was used to model the probability of CKD categories, defined by eGFR and ACR, to improve the precision of age-specific NHANES estimates of CKD prevalence. The base model for CKD stage 3a+ prevalence fit 3 eGFR categories (45 to <60, 30 to <45, and <30 ml/min/1.73 m2) and 4 ACR categories (<10, 10 to 30, >30 to 300, and >300 mg/g), for a total of 12 mutually exclusive CKD categories, as a function of age (a linear variable), race, and sex. No adjustment was made for survey year, a conservative approach given recent increases in CKD prevalence.5 For ESRD prevalence, actual sex- and race-specific figures supplied by the USRDS were used in the mortality calculations, assuming constant prevalence in each age interval provided.
State Transition Probabilities
The one-year probability of death among persons of given age, sex, and race, and without CKD (QnoCKD) were calculated using age-, sex-, and race-specific estimates of mortality in the overall population (i.e., among those with and without CKD), CKD prevalence, and hazard ratios (HRs) for mortality associated with CKD (Item S1, part A). All sex- and race-adjusted HRs used the referent category (eGFR, 90–105 ml/min/1.73 m2; ACR<10 mg/g) and were assumed constant within each age range (18–54, 55–64, 65–74, and ≥75 years). In addition, HRs for those under 18 were assumed equivalent to those in the 18–54 year age group (Table S5). Age-specific CKD incidence (ICKD,x) was estimated from the proportion of individuals with CKD at age x+1, less those with CKD at age x, and taking into account both the competing risk of death prior to CKD development (QnoCKD) as well as the one-year probability of death among prevalent cases of CKD (QCKD) (Item S1, part B).
For the model of lifetime risk of ESRD, mortality rates among persons of given age, sex, and race, and lacking ESRD were calculated using USRDS-supplied ESRD prevalence and mortality rates, and converted to probabilities (QnoESRD) using established methods.16 Age-specific incidence of ESRD (IESRD) was estimated using rates provided by the USRDS.
Sensitivity Analyses
Forecasts of lifetime CKD risk are highly sensitive to estimates of CKD incidence (ICKD). Our equation estimates CKD incidence as a function of CKD prevalence (PCKD) and the competing risk of death prior to kidney disease (Qno_CKD), which in turn is a function of CKD-associated mortality (HRCKD); all assume constant probabilities for a given age over time (i.e., ICKD among 30 year olds in 2000 is the same as ICKD among 30 year olds in 2050). We therefore evaluated the robustness of our models in the following ways. First, we tested the impact of a more complex estimation of CKD prevalence (PCKD), modeling prevalence as a function of age as a linear spline (knot at 70 years), race, sex, and two age-by-sex and age-by-race interaction terms. Second, we tested the effect of lower CKD-associated mortality (HRCKD), using a two CKD category model (eGFR <60 ml/min/1.73 m2 with and without ACR>30 mg/g) and the most conservative HRs in each category (eGFR 45–60 ml/min/1.73 m2 with ACR< 10 mg/g and ACR 30–300 mg/g, respectively). Third, we capped CKD stage 3a+ incidence at 5% (affecting white men aged 76 and older, white women aged 75 and older, black men aged 72 and older, and black women aged 73 and older). Finally, we tested the impact of increasing incidence rates by modeling CKD prevalence on survey year in addition to age, sex, and race.
To test the validity of modeling CKD incidence on estimates of CKD prevalence, we compared our modeled estimates of CKD incidence to those observed in the Atherosclerosis Risk in Communities (ARIC) Study, a population-based cohort of black and white middle-aged individuals with interval assessment of serum creatinine.
Statistical Analysis
All model input estimates were calculated using Stata SE, Version 11.2 (StataCorp LP, College Station, TX), and are available in Tables S1 and S2. Markov models were constructed and microsimulations conducted using C++ and TreeAge Pro 2012 (Williamstown, MA).
RESULTS
Residual Lifetime Risk of Kidney Disease
From birth, lifetime risk in the overall U.S. population was an estimated 59.1% for CKD stage 3a+, 33.6% for CKD stage 3b+, 11.5% for CKD stage 4+, and 3.6% for ESRD. In general, residual lifetime risk (i.e., remaining lifetime risk, conditional on disease-free status) for CKD increased until age 70, reflecting the generally late onset of kidney disease and the increase in life expectancy with attained baseline age (Table 1). In contrast, the residual lifetime risk of treated ESRD was highest among 20- to 30-year-olds, reflecting the relative decline in the probability of ESRD compared with death at older ages (Table S4).
Table 1.
Life expectancy and residual lifetime risk of CKD Stage 3a+, Stage 3b, Stage 4+, and ESRD by baseline age
| At birth | 10 y | 20 y | 30y | 40 y | 50 y | 60 y | 70 y | 80 y | |
|---|---|---|---|---|---|---|---|---|---|
| Life Expectancy* | |||||||||
| White Men | 76 | 77 | 77 | 78 | 78 | 79 | 81 | 84 | 88 |
| White Women | 81 | 81 | 81 | 82 | 82 | 83 | 84 | 86 | 89 |
| Black Men | 70 | 71 | 72 | 73 | 74 | 75 | 78 | 82 | 88 |
| Black Women | 77 | 78 | 78 | 79 | 79 | 80 | 82 | 85 | 89 |
| Residual Lifetime Risk** | |||||||||
| CKD stage 3a+** | |||||||||
| White Men | 53.6 | 54.2 | 54.6 | 55.1 | 55.6 | 56.8 | 58.5 | 60.6 | 59.0 |
| White Women | 64.9 | 65.2 | 65.6 | 65.9 | 66.0 | 67.0 | 67.6 | 68.1 | 63.3 |
| Black Men | 51.8 | 52.8 | 53.0 | 54.2 | 55.4 | 57.2 | 60.5 | 64.9 | 64.5 |
| Black Women | 63.6 | 64.3 | 64.6 | 64.8 | 65.4 | 67.0 | 68.9 | 70.7 | 66.9 |
| CKD stage 3b+^ | |||||||||
| White Men | 29.0 | 29.3 | 29.4 | 29.9 | 30.2 | 31.1 | 32.2 | 33.3 | 30.8 |
| White Women | 36.7 | 36.8 | 36.8 | 37.3 | 37.4 | 37.9 | 38.3 | 38.3 | 32.7 |
| Black Men | 33.7 | 34.3 | 34.5 | 35.0 | 36.2 | 37.4 | 39.3 | 41.0 | 36.9 |
| Black Women | 40.2 | 40.7 | 40.9 | 41.1 | 41.5 | 42.8 | 43.6 | 43.5 | 36.7 |
| CKD stage 4+ ^^ | |||||||||
| White Men | 9.3 | 9.5 | 9.4 | 9.5 | 9.6 | 9.9 | 10.2 | 10.5 | 10.4 |
| White Women | 11.4 | 11.6 | 11.5 | 11.5 | 11.6 | 11.8 | 11.8 | 11.8 | 11.1 |
| Black Men | 15.8 | 16.1 | 16.1 | 16.5 | 16.8 | 17.2 | 17.8 | 18.8 | 18.8 |
| Black Women | 18.5 | 18.7 | 18.7 | 18.8 | 18.9 | 19.1 | 19.2 | 19.4 | 17.9 |
| ESRD | |||||||||
| White Men | 3.3 | 3.4 | 3.4 | 3.3 | 3.2 | 3.2 | 3.0 | 2.5 | 1.6 |
| White Women | 2.2 | 2.3 | 2.3 | 2.2 | 2.2 | 2.1 | 1.9 | 1.4 | 0.7 |
| Black Men | 8.5 | 8.7 | 8.7 | 8.8 | 8.4 | 7.9 | 6.9 | 5.1 | 2.9 |
| Black Women | 7.8 | 7.9 | 7.9 | 7.7 | 7.5 | 7.1 | 6.3 | 4.6 | 2.4 |
Note: By race, sex, and decade of age until age 90 years.
Based on Arias28.
Values given as percentages.
eGFR < 60 ml/min/1.73m2
eGFR<45 ml/min/1.73m2
eGFR<30 ml/min/1.73m2
CKD, chronic kidney disease; ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate
Lifetime Risk of Kidney Disease by Sex and Race
Lifetime risk estimates differed by sex and race (Table 1). Women faced higher CKD stage 3a+ risk: from birth, the predicted lifetime risks for a white man, white woman, black man, and black woman were 53.6%, 64.9%, 51.8%, and 63.6%, respectively. Blacks faced higher risks of CKD stage 4+ and ESRD: from birth, the predicted lifetime risks for a white man, white woman, black man, and black woman were 9.3%, 11.4%, 15.8%, and 18.5% for CKD stage 4+ and 3.3%, 2.2%, 8.5%, and 7.8% for ESRD. Thus, within each race, women had higher lifetime risks of CKD, whereas men had the higher risk of ESRD.
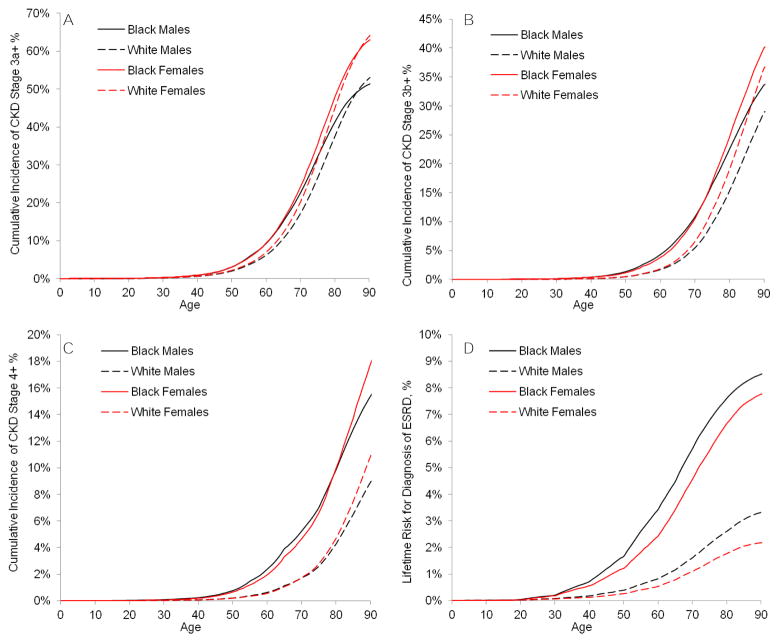
The estimated onset of kidney disease was earlier in blacks than whites (Figure 2). For example, by age 60 years, 10.3% of black men and 10.4% of black women were projected to have CKD stage 3a+, compared with 6.9% and 7.8% among white men and women. Whites “caught up” in CKD stage 3a+ risk at older ages, but the earlier onset of risk for CKD stage 4+ and ESRD among blacks persisted. As such, white women had the highest risk of CKD stage 3a+ (64.9%), black women had the highest risk of stage 4+ CKD (18.5%), and black men had the highest risk of ESRD (8.5%).
Figure 2.
Cumulative incidence from birth by race and sex of CKD stage (A) 3a+, (B) 3b+, (C) 4+, and (D) ESRD
Lifetime Risk Scaled to the Current US Population
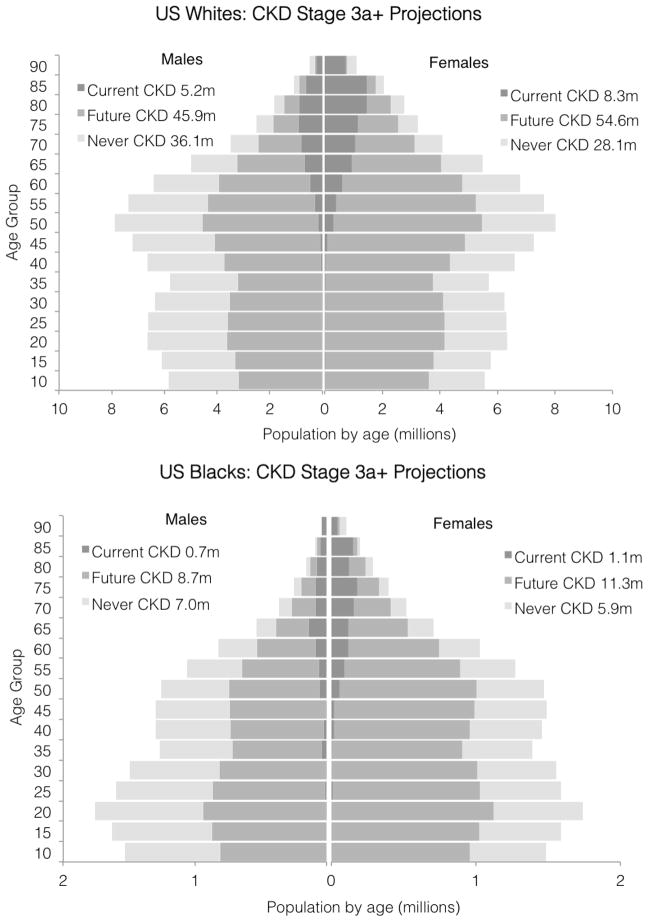
Among the 178.3 million non-Hispanic white U.S. citizens, an estimated 15.3 million have CKD stage 3a+, and another 120.5 million were predicted to develop it during their remaining lifetime (Figure 3; Fig S1). Among the 34.8 million black U.S. citizens, an estimated 1.8 million have CKD stage 3a+, and an additional 20.0 million were predicted to develop it during their lifetime. Overall, 135.8 million people (63.8%) in the current U.S. white and black population either have (7.2%) or are expected to develop CKD stage 3a+ during their lifetime (56.6%). Similarly, a projected 26.1 million (7.4%) either have or are expected to develop CKD stage 4+ (Figure S2–S3).
Figure 3.
CKD stage 3a+ projections by age, race, and sex, scaled by estimated 2012 U.S. population
Sensitivity Analyses
In sensitivity analyses using more complex models of CKD prevalence and lower rates of CKD-associated mortality, lifetime risk estimates for CKD stage 3+ ranged from 45.9% to 53.6% for white men, 54.5% to 64.9% for white women, 44.2% to 51.8% for black men, and 55.8% to 63.6% for black women (Table 2). In all scenarios, the population-weighted lifetime risk of kidney disease was greater than 50%. In a sensitivity analysis using survey year in the model of CKD prevalence (and thus allowing CKD incidence to vary by year), lifetime risk estimates were much higher, at 72.9%, 82.6%, 65.8%, and 77.5% for white men, white women, black men, and black women, respectively. Compared with age-, sex- and race-specific CKD incidence observed in the prospective middle-aged, population based ARIC study, our model-based estimates were similar and generally lower, resulting in a more conservative estimate of lifetime CKD incidence. For example, our estimates of 20-year CKD stage 3+ incidence for an initially disease-free 45 year old were slightly lower than those observed in ARIC among white men, white women, and black women (absolute differences of −2.6%, −0.9%, and −0.1%, respectively), and slightly higher than those observed among black men (absolute difference, 0.4%). Differences of similar magnitude were observed for the incidence of CKD stage 4+ (absolute differences: 0.6%, 0.9%, −1.5%, and 1.8% among white men, white women, black men, and black women, respectively).
Table 2.
Sensitivity analyses: lifetime risk from birth to age 90 years of CKD Stage 3a+
| Simple Prevalence Model* | Complex Prevalence Model | Increasing Prevalence Model | ||||
|---|---|---|---|---|---|---|
| 12 CKD Categories** | Capped Incidence*** | 2 CKD Categories** | 12 CKD Categories** | 2 CKD Categories** | 2 CKD Categories** | |
| White Men | 53.6 | 45.9 | 47.8 | 53.3 | 48.4 | 72.9 |
| White Women | 64.9 | 54.5 | 58.4 | 64.1 | 58.6 | 82.6 |
| Black Men | 51.8 | 45.3 | 45.5 | 50.8 | 44.2 | 65.8 |
| Black Women | 63.6 | 56.3 | 57.0 | 62.4 | 55.8 | 77.5 |
| Total**** | 59.1 | 50.4 | 53.0 | 58.5 | 53.1 | 76.9 |
Note: Values given as percentages.
Simple prevalence model covariates: age (linear), sex, and race; complex prevalence model covariates: age (linear spline with a knot at 70 y), sex, race, age-sex and age-race interaction terms; increasing prevalence model covariates: age (linear), sex, race, and survey year.
The number of CKD categories is reflected in the mortality risk: the 2 CKD category model is more conservative, classifying participants into eGFR<60 ml/min/1.73m2with or without ACR>30 mg/g and using the HRs associated with eGFR 45–60 ml/min/1.73m2, ACR<10 mg/g and eGFR 45–60 ml/min/1.73m2, ACR 30–300 mg/g. The 12-category model uses specific HRs associated with each ACR/eGFR category; because the HRs generally increase with more CKD, this results in higher estimates of CKD mortality.
The capped incidence model is the 12 CKD category, simple prevalence model, with annual incidence rates capped at 5%.
Total values reflect the combination of lifetime risks, weighted by race- and sex-specific prevalence in the U.S. population.
CKD, chronic kidney disease; ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate; ACR, albumin-creatinine ratio; HR, hazard ratio
DISCUSSION
In this simulation study, the estimated lifetime risk of CKD stage 3a+ was over 50%, lower than that of hypertension (83%–90% for a 55-year-old),3 but higher than that of diabetes (33%–39%),4 coronary heart disease (32%–49% for a 40-year-old),32 and invasive cancer (38%–45%).33 The lifetime risks of CKD stages 3b+ and 4+ and ESRD were also considerable, at 33.6%, 11.5%, and 3.6%, respectively. Consistent with previous studies,34–36 the risk of CKD stage 3a+ increased dramatically with age, with approximately half of incident cases occurring after age 70, an observation even more pronounced in incident CKD stage 4+. In contrast, cases of ESRD developed earlier, plateauing at older ages.
The strong relationship between age and incident CKD leads to an interesting result: those with the longest life expectancy had the highest risk of CKD stage 3a+. This observation – consistent with previous demonstrations of CKD incidence in older populations (eg, 20.5% incidence over a 24-month period in the Women’s Health and Aging Study I) 37– fuels the longstanding debate as to whether eGFR decline is “normal aging” or a pathological process.38 While the current study was not designed to address this controversy, it does rely on HRs from a recent meta-analysis that suggested that CKD-associated risks persist in older adults, albeit with diminished relative risks.29 In contrast to the relationship between age and CKD, the relationship between age and ESRD incidence was not monotonic: rates declined after age 75 years, perhaps reflecting a more frequent refusal of renal replacement therapy39 and a higher competing risk of pre-ESRD death among older adults.40
Lifetime estimates of kidney disease risk differed substantially by sex and race. White women faced the highest risk of CKD stage 3a+ yet the lowest risk of treated ESRD, possibly reflecting the older onset of kidney disease (with respect to blacks24), slower CKD progression, or differences in the acceptance of renal replacement therapy.39 Black individuals faced higher risk of kidney disease at earlier ages. For CKD stage 4+ and ESRD, the risk difference was dramatic and persistent over a lifetime, findings consistent with the seemingly paradoxical lower prevalence yet higher incidence of moderate CKD in the U.S. black population.22 This may suggest a susceptibility to CKD progression, whether due to diminished access to medical care, genetic predisposition (e.g., the prevalence of APOL1 high risk variants),41–43 differences in disease etiology, or lower competing risks of death in older age.28
Our results expand upon the published literature in several ways. Models were based on the current, U.S. general population, stratified by sex and race, and they estimated the lifetime risk of not only ESRD but also more moderate forms of CKD. Risk of CKD has been simulated for a study of cost-effectiveness in the US.19 However, the forecasts were solely among the overall population, with no stratification by sex and race, and few age groups. Previous studies of ESRD risk used different populations in nationality or era.16–18 Kiberd and Clase’s study of ESRD risk provided useful U.S. estimates based on mortality rates from 1998 and ESRD incidence from 1996–1998,16 but rates have changed significantly since that time.44 More recently published studies estimated ESRD risk among Canadian participants receiving medical care, where the majority of the population is likely to be white.17, 18 Our study updates risk estimates in U.S. whites as well as U.S. blacks, who shoulder a disproportionate amount of disease.9
The estimate that more than 50% of the U.S. population will develop CKD stage 3a+ is higher than that in a 2010 simulation study19 but consistent with the estimate that CKD prevalence nears 40% after age 70 years.5 Given that the average life expectancy is well above 70 years for most individuals (black men being the exception28), that the risk of low eGFR increases with older age,20, 21, 35 and that persons who develop CKD at younger ages have a much higher mortality risk than those without CKD, 27 the previously published U.S. population lifetime CKD risk estimate of 38.5% is too low to be consistent with current prevalence estimates. This discrepancy may be secondary to difficulties in estimating CKD incidence rates: the simulation study estimated incidence using projections of eGFR decline, assuming a fixed multiplier for CKD-associated mortality, irrespective of age.19 This method is highly sensitive to laboratory drift in serum creatinine assays, which influences eGFR decline and the modeled distribution of eGFR slopes. CKD incidence estimated from eGFR decline is driven primarily by those who rapidly progress, a tail of the distribution which can be difficult to estimate precisely.
Our estimates of lifetime ESRD risk, as well as the dramatic racial disparities in risk of kidney disease, are consistent with previous studies.9, 22, 23, 26, 45 Kiberd and Clase estimated a lifetime risk of 7.3% and 7.8% among black men and women, respectively, compared with 2.5% and 1.8% among white men and women.16 Using current ESRD incidence rates, we demonstrate an even higher lifetime risk for all but black women. Somewhat surprisingly, ESRD risk estimates among white Americans were only slightly higher than those recently published from Alberta, Canada.17 In their cohort of insured patients, residual lifetime risk of ESRD was estimated as 2.7% and 1.8% for 40-year-old men and women, respectively, compared with our estimates of 3.2% and 2.2%. The small differences may reflect differences in disease progression, the competing risk of non-ESRD death, or practice patterns, including eGFR at renal replacement therapy initiation and the use of conservative (non-dialysis) therapy, a practice that may be more prevalent in Canada.39
Unlike those generated from a prospective cohort, our estimates of CKD incidence were modeled on U.S. prevalence estimates. We chose this method for several reasons. First, national data on CKD incidence are sparse, and incidence estimates from a prospective cohort are extremely sensitive to laboratory drift, or instability in the serum creatinine assay over long periods. Our cross-sectional model is robust to this drift. Second, we need not extrapolate from follow-up times far shorter than the average lifetime, nor do we presume constant rates of eGFR decline. Third, using NHANES prevalence facilitates application to the general population. Our method relies on different assumptions but has the strength of providing lifetime CKD estimates that match the current U.S. population prevalence. Reassuringly, in sensitivity analysis, our prevalence-based estimates of CKD incidence were very similar to those observed in the ARIC cohort, a population-based sample of middle aged adults.
The central assumptions of our CKD models are: (1) a stable population (i.e., no change in population structure, age-specific life expectancy, and CKD incidence rates), and (2) irreversibility of disease (i.e., once CKD stage 3+ or 4+ develops, it is present until death). For a population with increasing life expectancy such as the U.S., the equation we use provides a conservative estimate of CKD incidence. Similarly, an increase in CKD incidence – a real possibility, given the projected increases in diabetes, hypertension, and obesity – would result in higher lifetime risk than we have presented. If CKD were to remit, we would underestimate CKD incidence, although estimates of lifetime risk might be less meaningful.
Additional limitations include the selection bias inherent in NHANES prevalence estimates – those with more severe disease may be less inclined to participate in the survey, particularly in older age groups, and a noted survey exclusion criterion is nursing home residence – however, this limitation would also apply to a prospective study and likely result in a conservative estimate of lifetime CKD risk. Finally, in estimating ESRD risk, we include those receiving dialysis or transplantation only, which may substantially underestimate those with untreated chronic kidney failure (eGFR<15 ml/min/1.73m2 and death).39
In summary, we estimate that over 50% of Americans born today will develop CKD stage 3+ during their lifetime. Racial disparities, particularly in severe disease, are marked. Among whites, approximately 9%–11% will develop CKD stage 4+, and 2%–3% will develop ESRD; among blacks, the risks of CKD stage 4+ and ESRD are 16%–18% and 8%, respectively. Future research is needed in methods of preventing the development of CKD and ameliorating its associated morbidity.
Supplementary Material
Supplementary Material
Table S1: State transition probabilities for CKD 3a+, by race, sex, and age.
Table S2: State transition probabilities for CKD 3b+, by race, sex, and age.
Table S3: State transition probabilities for CKD 4+, by race, sex, and age.
Table S4: State transition probabilities for ESRD, by race, sex, and age.
Table S5: Sex- and race-adjusted HRs of mortality associated with CKD, by age group.
Figure S1: Actual vs predicted US prevalence of CKD 3a+, by age, sex, and race.
Figure S2: CKD 4+ projections by age, race, and sex, scaled by estimated 2012 US population.
Figure S3: ESRD projections by age, race, and sex, scaled by estimated 2012 US population.
Item S1: State transition probability formulas.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Acknowledgments
Some of the data reported in this article have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Support: MG receives support from the National Institute of Diabetes and Digestive and Kidney Diseases (K08DK092287).
Financial Disclosure: JC has consulted for Amgen and Merck and has an investigator-initiated grant from Amgen. The other authors declare that they have no other relevant financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults: Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA: The Journal of the American Medical Association. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Turin TC, Hemmelgarn BR. Long-term risk projection and its application to nephrology research. J Nephrol. 2012;25(4):441–449. doi: 10.5301/jn.5000197. [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287(8):1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 4.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 5.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 6.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsushita K, van der Velde M, et al. Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80(1):93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U S Renal Data System. USRDS 2011 annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States. 2011. [DOI] [PubMed] [Google Scholar]
- 10.Plantinga LC, Johansen K, Crews DC, et al. Association of CKD with disability in the United States. Am J Kidney Dis. 2011;57(2):212–227. doi: 10.1053/j.ajkd.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James MT, Hemmelgarn BR, Wiebe N, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: A cohort study. Lancet. 2010;376(9758):2096–2103. doi: 10.1016/S0140-6736(10)61271-8. [DOI] [PubMed] [Google Scholar]
- 12.Tonelli M, Muntner P, Lloyd A, et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: A population-level cohort study. Lancet. 2012 doi: 10.1016/S0140-6736(12)60572-8. [DOI] [PubMed] [Google Scholar]
- 13.Hostetter TH, Lising M. National kidney disease education program. J Am Soc Nephrol. 2003;14(7 Suppl 2):S114–6. doi: 10.1097/01.asn.0000070156.78824.c7. [DOI] [PubMed] [Google Scholar]
- 14.Narva AS, Briggs M. The national kidney disease education program: Improving understanding, detection, and management of CKD. Am J Kidney Dis. 2009;53(3 Suppl 3):S115–20. doi: 10.1053/j.ajkd.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 15.Plantinga LC, Boulware LE, Coresh J, et al. Patient awareness of chronic kidney disease: Trends and predictors. Arch Intern Med. 2008;168(20):2268–2275. doi: 10.1001/archinte.168.20.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiberd BA, Clase CM. Cumulative risk for developing end-stage renal disease in the US population. J Am Soc Nephrol. 2002;13(6):1635–1644. doi: 10.1097/01.asn.0000014251.87778.01. [DOI] [PubMed] [Google Scholar]
- 17.Turin TC, Tonelli M, Manns BJ, et al. Lifetime risk of ESRD. J Am Soc Nephrol. 2012 doi: 10.1681/ASN.2012020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manns B, Hemmelgarn B, Tonelli M, et al. Population based screening for chronic kidney disease: Cost effectiveness study. BMJ. 2010;341:c5869. doi: 10.1136/bmj.c5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoerger TJ, Wittenborn JS, Segel JE, et al. A health policy model of CKD: 1. model construction, assumptions, and validation of health consequences. Am J Kidney Dis. 2010;55(3):452–462. doi: 10.1053/j.ajkd.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Bash LD, Astor BC, Coresh J. Risk of incident ESRD: A comprehensive look at cardiovascular risk factors and 17 years of follow-up in the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis. 2010;55(1):31–41. doi: 10.1053/j.ajkd.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291(7):844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 22.Muntner P, Newsome B, Kramer H, et al. Racial differences in the incidence of chronic kidney disease. Clin J Am Soc Nephrol. 2012;7(1):101–107. doi: 10.2215/CJN.06450611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans K, Coresh J, Bash LD, et al. Race differences in access to health care and disparities in incident chronic kidney disease in the US. Nephrol Dial Transplant. 2011;26(3):899–908. doi: 10.1093/ndt/gfq473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarver-Carr ME, Powe NR, Eberhardt MS, et al. Excess risk of chronic kidney disease among african-american versus white subjects in the United States: A population-based study of potential explanatory factors. J Am Soc Nephrol. 2002;13(9):2363–2370. doi: 10.1097/01.asn.0000026493.18542.6a. [DOI] [PubMed] [Google Scholar]
- 25.McClellan WM, Warnock DG, Judd S, et al. Albuminuria and racial disparities in the risk for ESRD. J Am Soc Nephrol. 2011;22(9):1721–1728. doi: 10.1681/ASN.2010101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peralta CA, Katz R, DeBoer I, et al. Racial and ethnic differences in kidney function decline among persons without chronic kidney disease. J Am Soc Nephrol. 2011;22(7):1327–1334. doi: 10.1681/ASN.2010090960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turin TC, Tonelli M, Manns BJ, Ravani P, Ahmed SB, Hemmelgarn BR. Chronic kidney disease and life expectancy. Nephrol Dial Transplant. 2012;27(8):3182–3186. doi: 10.1093/ndt/gfs052. [DOI] [PubMed] [Google Scholar]
- 27a.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arias E. United States life tables, 2007. national vital statistics reports. 9. Vol. 59. Hyattsville, MD: National center for health statistics; 2011. [PubMed] [Google Scholar]
- 29.Hallan SI, Matsushita K, Sang Y, et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308(22):2349–60. doi: 10.1001/jama.2012.16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szklo M, Nieto FJ. Epidemiology: Beyond the basics. 2. Sudbury, Massachusetts: Jones and Bartlett Publishers; 2007. [Google Scholar]
- 31.United States Census Bureau. US interim population projections, 2000–2050. 2012. Aug 16, [Google Scholar]
- 32.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353(9147):89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 33.National Cancer Institute. [Accessed August 20, 2012.];Surveillance epidemiology and end results cancer statistics review 1975–2009. [Google Scholar]
- 34.Bash LD, Coresh J, Kottgen A, et al. Defining incident chronic kidney disease in the research setting: The ARIC study. Am J Epidemiol. 2009;170(4):414–424. doi: 10.1093/aje/kwp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drey N, Roderick P, Mullee M, Rogerson M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis. 2003;42(4):677–684. doi: 10.1016/s0272-6386(03)00916-8. [DOI] [PubMed] [Google Scholar]
- 36.Shlipak MG, Katz R, Kestenbaum B, et al. Rate of kidney function decline in older adults: A comparison using creatinine and cystatin C. Am J Nephrol. 2009;30(3):171–178. doi: 10.1159/000212381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semba RD, Fink JC, Sun K, et al. Serum fibroblast growth factor-23 and risk of incident chronic kidney disease in older community-dwelling women. Clin J Am Soc Nephrol. 2012;7(1):85–91. doi: 10.2215/CJN.08070811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winearls CG, Glassock RJ. Classification of chronic kidney disease in the elderly: Pitfalls and errors. Nephron Clin Pract. 2011;119 (Suppl 1):c2–4. doi: 10.1159/000328013. [DOI] [PubMed] [Google Scholar]
- 39.Hemmelgarn BR, James MT, Manns BJ, et al. Rates of treated and untreated kidney failure in older vs younger adults. JAMA. 2012;307(23):2507–2515. doi: 10.1001/jama.2012.6455. [DOI] [PubMed] [Google Scholar]
- 40.Dalrymple LS, Katz R, Kestenbaum B, et al. Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med. 2011;26(4):379–385. doi: 10.1007/s11606-010-1511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedman BI, Hicks PJ, Bostrom MA, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int. 2009;75(7):736–745. doi: 10.1038/ki.2008.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kao WH, Klag MJ, Meoni LA, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40(10):1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman DJ, Pollak MR. Genetics of kidney failure and the evolving story of APOL1. J Clin Invest. 2011;121(9):3367–3374. doi: 10.1172/JCI46263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.US Renal Data System. USRDS 2000 annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States. 2000 [Google Scholar]
- 45.Peralta CA, Shlipak MG, Fan D, et al. Risks for end-stage renal disease, cardiovascular events, and death in hispanic versus non-hispanic white adults with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2892–2899. doi: 10.1681/ASN.2005101122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Table S1: State transition probabilities for CKD 3a+, by race, sex, and age.
Table S2: State transition probabilities for CKD 3b+, by race, sex, and age.
Table S3: State transition probabilities for CKD 4+, by race, sex, and age.
Table S4: State transition probabilities for ESRD, by race, sex, and age.
Table S5: Sex- and race-adjusted HRs of mortality associated with CKD, by age group.
Figure S1: Actual vs predicted US prevalence of CKD 3a+, by age, sex, and race.
Figure S2: CKD 4+ projections by age, race, and sex, scaled by estimated 2012 US population.
Figure S3: ESRD projections by age, race, and sex, scaled by estimated 2012 US population.
Item S1: State transition probability formulas.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org