Abstract
This study aims to provide understanding of the macroscopic viscoelastic behavior of collagen matrices through studying the relaxation time distribution spectrum obtained from stress relaxation tests. Hydrated collagen gel and dehydrated collagen thin film was exploited as two different hydration levels of collagen matrices. Genipin solution was used to induce crosslinking in collagen matrices. Biaxial stress relaxation tests were performed to characterize the viscoelastic behavior of collagen matrices. The rate of stress relaxation of both hydrated and dehydrated collagen matrices shows a linear initial stress level dependency. Increased crosslinking reduces viscosity in collagen gel, but the effect is negligible for thin film. Relaxation time distribution spectrum was obtained from the stress relaxation data by inverse Laplace transform. For most of the collagen matrices, three peaks at the short (0.3s ~1 s), medium (3s ~90 s), and long relaxation time (> 200 s) were observed in the continuous spectrum, which likely corresponds to relaxation mechanisms involve fiber, inter-fibril, and fibril sliding. Splitting of the middle peak was observed at higher initial stress levels suggesting increased structural heterogeneity at the fibril level with mechanical loading. The intensity of the long-term peaks increases with higher initial stress levels indicating the engagement of collagen fibrils at higher levels of tissue strain.
Keywords: collagen gel, collagen thin film, crosslinking, hydration, inverse Laplace transform, relaxation time, stress relaxation, viscoelastic
Introduction
Collagen is the most abundant protein in the body. It plays critical roles in many supporting and connecting tissues such as tendon, ligament, bone, blood vessels, skin, etc. Collagen gel prepared from commercially available collagen solution have been broadly used as a biomaterial in tissue engineering, drug delivery, and wound healing for its biocompatibility, low toxicity, and well-documented physical, chemical, and immunological properties.1-3 Collagen gel is also used as three-dimensional model systems of extracellular matrix (ECM) in numerous studies of cell-ECM interactions under physiological and pathological conditions.4-7 Collagen thin film, or dehydrated collagen gel, has been used as a two-dimensional platform in a number of studies to examine cell-ECM interactions.8
As a biphasic material, collagen matrices contain a solid phase representing by collagen network and an interstitial fluid phase.9 This special structure makes collagen a viscoelastic material. The interstitial water can be assorted into two different types: tightly bound with collagen molecules and “free” or bulk like.10 The tightly bound water is believed to play an important role in stabilizing collagen structure by forming hydrogen bonds between collagen molecules and is not easily lost. The free water are the ones usually exchanges. The hierarchical structure of collagen, first unveiled by Kastelic et al.,11 is believed to be responsible for the necessary elastic strength and viscoelastic responses. Viscoelasticity is important for force/energy storage, transmission, and dissipation in biological tissues.12
To study the mechanical properties of collagen gel, direct measurements using uniaxial tensile testing,13-15 rheological method,16,17 dynamic mechanical analysis,18 and noninvasive microscopy approaches17,19,20 have been used in previous studies. Uniaxial tensile test has also been used to study the elastic property of collagen thin film.21,22 Tensile stress relaxation test has been broadly used to understand the viscoelastic properties of many materials. Previous studies have shown that it can effectively demonstrate the chemical and microstructural changes on the macroscopic viscoelastic properties.23 However the relationship between tissue level mechanical responses and micro-level structural changes is still not well understood. Further studies are needed to better understand the stress relaxation mechanisms.
Viscoelastic biomaterials likely contain a continuous spectrum of relaxation time constants.24 In the present study, the time-dependent distribution spectrum σ(τ) is obtained through numerical inverse Laplace transform. Investigating the spectrum in terms of the number of peaks, time constants, and peak intensity was found to appropriately demonstrate the main properties of viscoelastic behaviors.25,26 The intensity of the peak reflects the amount of dissipated energy during relaxation. The number of peaks and time constants are often correlated with specific molecular architectures; as a result it can be used as an approach to understand the structural behavior of biomaterials, as well as a useful tool to distinguish materials.27,28
The present study is designed to understand the stress relaxation mechanisms in collagen matrices with the effects of crosslinking and hydration on the viscoelastic properties of collagen matrices. We have previously studied the effects of crosslinking on the elastic properties of collagen gel.29 Some of the experimental approaches were adopted in the present study. Genipin (GP) solution was used to induce crosslinking in collagen matrices. Biaxial stress relaxation tests were performed to characterize the viscoelastic behavior of collagen matrices. Viscoelasticity was also studied with the effect of initial stress levels. The relaxation time distribution spectrum is obtained from stress relaxation data by means of inverse Laplace transform. This spectrum is employed to understand the mechanisms of stress relaxation in collagen network.
Results
Figure 1 shows the averaged (n = 3) tangent stiffness calculated from the equi-biaxial tensile stress-strain data of collagen matrices. The tangent modulus of dehydrated collagen thin film is about one order of magnitude higher than collagen gel. The effect of GP concentration on the stiffness of the matrix is more obvious at strains below ~2%, although the tangent moduli of collagen matrices crosslinked with 0.25% GP remains to be the highest for the entire strain range.
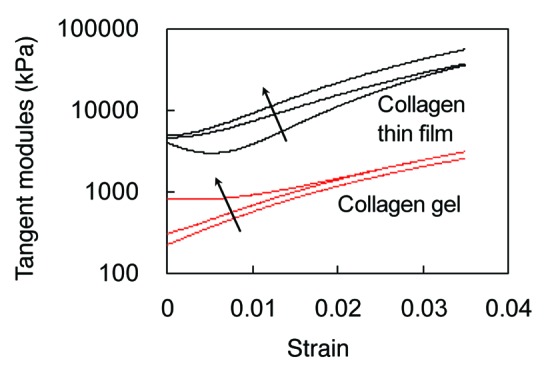
Figure 1. Averaged tangent moduli obtained from biaxial tensile tests of collagen gel and thin film crosslinked with 0.03%, 0.1% and 0.25% GP. Direction of arrows indicates increased crosslinking.
Figure 2 shows the representative stress relaxation curves obtained from three repeated testing of the same collagen gel sample. Collagen gel exhibits significantly more stress relaxation in the first test. The repeatability is highly improved in the subsequent cycles of stress relaxation tests. Although not shown here, collagen thin film shows similar behavior with the initial stress relaxation behavior significantly different from subsequent testing.
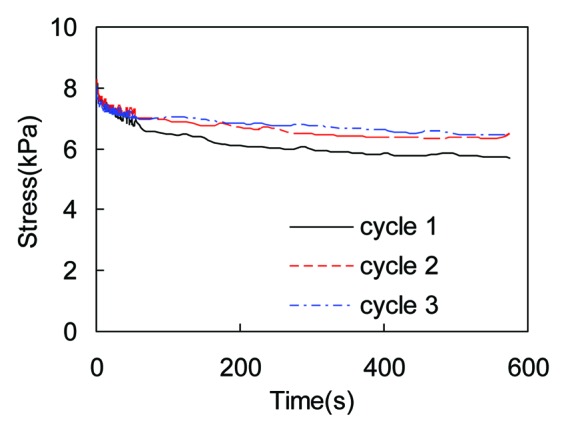
Figure 2. Stress relaxation preconditioning results showing the relaxation behavior of collagen gel (crosslinked with 0.3% GP) subjected to three cycles of repeated stress relaxation tests.
Effect of initial stress levels on stress relaxation behavior of collagen matrices was investigated. Each sample was tested at multiple initial stress levels. To better illustrate the initial stress level dependency of stress relaxation, the rate of stress relaxation at each initial stress level was obtained by taking the slope of the semi-log fit of the stress relaxation plots. As shown in Figure 3, multiple stress relaxation tests demonstrate a linear increase in the rate of stress relaxation with higher initial stresses for both collagen gel and thin film (n = 2).
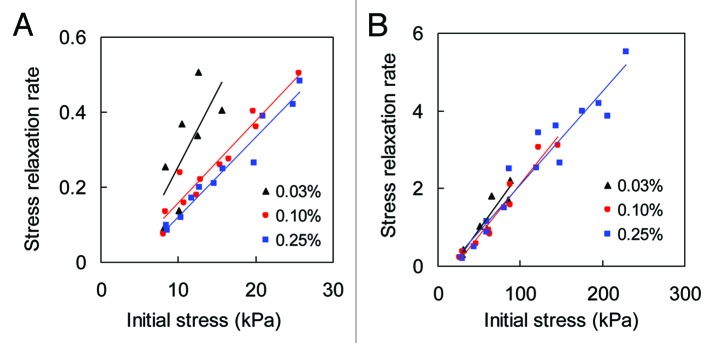
Figure 3. The rate of stress relaxation vs. initial stress levels for (A) collagen gel, and (B) collagen thin film crosslinked with 0.03%, 0.1%, and 0.25% GP. Solid lines are linear fit to aid viewing.
The continuous relaxation spectrum obtained from CONTIN analysis is plotted in Figures 4 and 5 for collagen gel and thin film, respectively. The effect of crosslinking on stress relaxation was studied by varying the GP concentration at 0.03%, 0.1%, and 0.25%. At each GP concentration, collagen matrices were tested at different initial stress levels. It is noted from the relaxation spectrum that the intensity of the peaks as well as the area under the spectrum increases with increasing initial stress level for both collagen gel and thin film. Usually there are three peaks in the continuous distribution curve located at short relaxation time (0.3 s ~1 s), medium relaxation time (3 s ~90 s), and long relaxation time (> 200 s). However, the number of peaks can increase with higher initial stress levels, as shown for the 0.1% and 0.25% GP crosslinked collagen matrices in Figures 6 and 7.
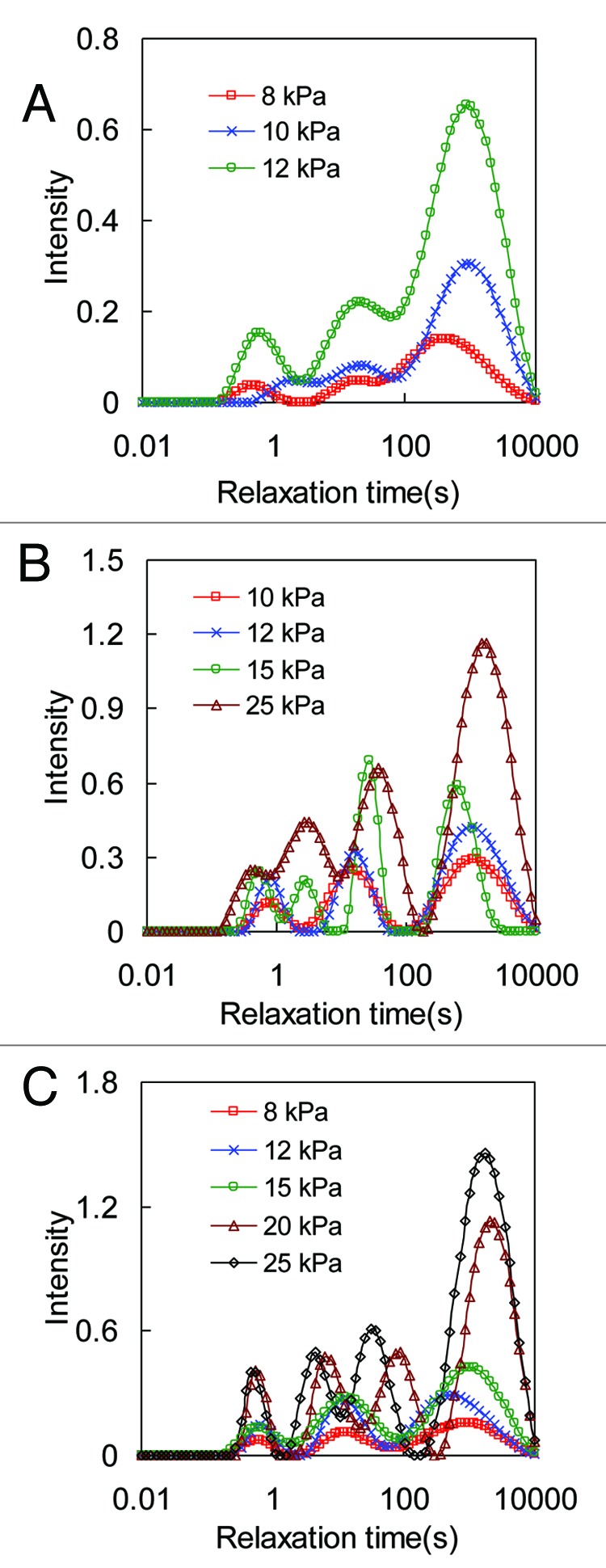
Figure 4. Relaxation time distribution spectra obtained from biaxial stress relaxation tests of collagen gel under different initial stress levels. Collagen gel is crosslinked with (A) 0.03%, (B) 0.1% and (C) 0.25% GP.
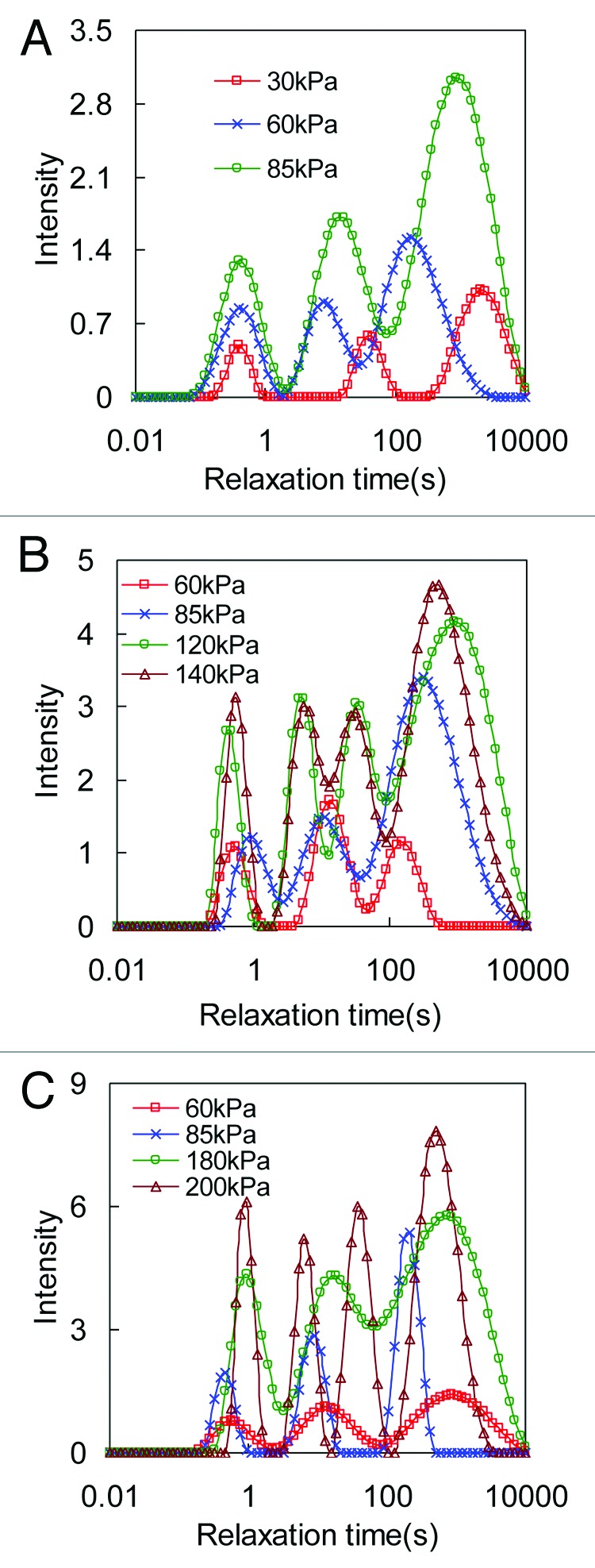
Figure 5. Relaxation time distribution spectra obtained from biaxial stress relaxation tests of collagen thin film under different initial stress levels. Collagen gel is crosslinked with (A) 0.03%, (B) 0.1%, and (C) 0.25% GP.
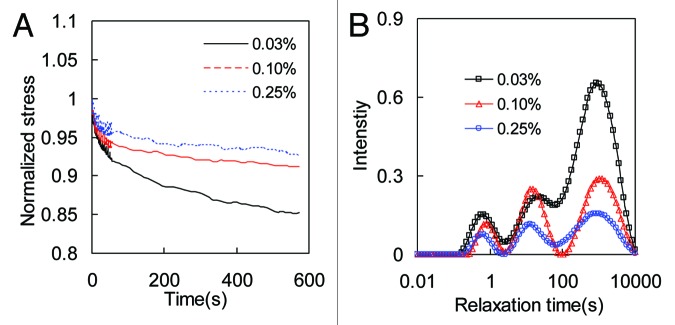
Figure 6. (A) Effect of crosslinking on the stress relaxation behavior of collagen gel crosslinked with 0.03%, 0.1%, and 0.25% GP. For each sample, the relaxation data in the x-and y-direction are averaged. Stresses were normalized to the initial stresses at time t = 0. (B) The corresponding relaxation time distribution spectra.
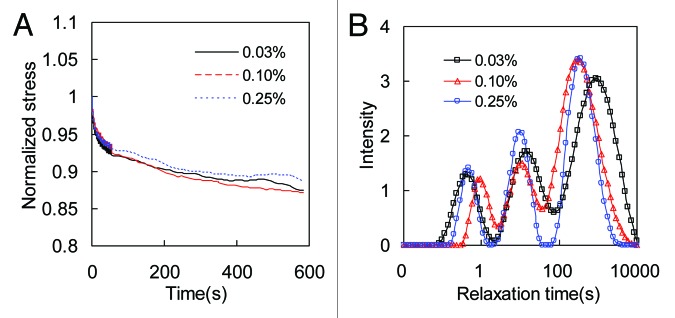
Figure 7. (A) Effect of crosslinking on the stress relaxation behavior of collagen thin film crosslinked with 0.03%, 0.1%, and 0.25% GP. For each sample, the relaxation data in the x-and y-direction are averaged. Stresses were normalized to the initial stresses at time t = 0. (B) The corresponding relaxation time distribution spectra.
To study the effect of crosslinking on the stress relaxation behavior of collagen matrices, in Figures 6A and 7A we plotted the normalized biaxial stress relaxation curves. Stresses were normalized to the initial stresses at time t = 0. To eliminate the effect of initial stress levels on the rate of stress relaxation, samples with different crosslinking were tested at the initial stresses of 12 ± 0.2 kPa and 85 ± 2 kPa for collagen gel and thin film, respectively.30 For collagen thin film in Figure 7A, the rate of stress relaxation is almost independent on crosslinking. For collagen gel in Figure 6A, however, the rate of stress-relaxation shows obvious inverse dependency on crosslinking. More crosslinked collagen gel relaxes slower than the less crosslinked ones, which suggests that less crosslinked collagen gels are more viscous.
The effect of crosslinking on the stress relaxation behavior of collagen matrices can also be seen from the relaxation time spectrum in Figures 6B and 7B. For both collagen matrices, the relaxation times at each peak are similar for different crosslinking. For collagen gel, there is a decrease in peak intensity for higher GP concentration in general, although the last peak shows the most prominent decrease in intensity. However collagen thin film shows little variation of peak intensity as the GP concentration changes.
Discussion
Hydration level is important to many connective tissues in order to maintain their normal biomechanical functions.31-33 The dehydration process may change the structure of collagen network by deducing the space between molecules as well as affecting the inter-and intra-molecular chemical bonds.34 Our macroscopic mechanical testing results show that the stiffness of collagen thin film is about one order of magnitude higher than the hydrated collagen gel (Fig. 1). McDaniel et al. (2007) found that the contact stiffness of collagen fibrils increases an order of magnitude when dehydrated. The changes of hydrogen bonds and network structure during dehydration were believed to cause the increased stiffness. Infrared reflection spectroscopy showed a strengthening and shortening of hydrogen bonds within the triple helix during the dehydration process.35 Using Raman spectroscopy, Leikin et al.36 demonstrated the structural role for hydration layers in keeping the spacing between collagen fibrils. The tighter packing of fibrils during dehydration resulted in enhanced mechanical rigidity. Also, molecular dynamics simulations of a collagen like peptide showed that the number of intra-molecular hydrogen bonds increased due to the absence of water and the molecule tended to be stiffer.34
Both experimental and modeling efforts have been made to determine the mechanisms by which strain is dispersed within the tissue. Tendon has received considerable interest in many studies for its simple aligned structure and well documented viscoelastic nature. Puxkandl et al. used in situ X-ray diffraction to measure simultaneously the elongation of the collagen fibrils inside the tendon and of the tendon as a whole.37 Their study demonstrates that the deformation takes place in the individual fibrils as well as in the matrix between fibrils. They also modeled tendon as an interacting viscoelastic system of the fibrils and the proteoglycan matrix described by two different Kelvin-Voigt models in series. The viscosity of tendon collagen was assumed to be due to the viscosity of the fibril and the matrix. Screen used confocal microscopy in conjunction with mechanical testing to examine the mechanisms of stress relaxation in tendon.38 Their study suggests that the relaxation behavior is predominated by fiber sliding mechanisms, with possible fibril sliding as the applied loads become greater. In a recent study Gupta et al. employed high time resolutions synchrotron X-ray diffraction and confocal microscopy to investigate the structural reorganization at the nano-and micro-length scales of tendon during stress relaxation.39 The viscoelastic behavior of tendon was modeled by serially coupling three viscoelastic elements at the fibril, inter-fibril, and inter-fiber levels. A stiff Kelvin-Voigt element represents the collagen fibrils and two Maxwell elements correspond to the inter-fibril and inter-fiber matrices. Molecular modeling results by Gautieri et al. suggest the viscoelastic behavior of collagen fibril may involve molecular sliding within the fibril.40
In the present study, three peaks were observed in the relaxation spectra for most of the collagen matrices. The time constants corresponding to these peaks are between 0.3 s ~1 s, 3 s ~90 s, and > 200 s. Following previous microstructural observations, it is reasonable to hypothesis that the three peaks in the relaxation spectra indicate that the relaxation behavior of collagen matrices through hierarchy can be depicted as inter fiber relaxation; inter fibril relaxation, and fibril relaxation with increased order of relaxation time constants. This is in agreement with the fact that intra fibril crosslinks is the most stable part under long-term load,39,41 and therefore the fibril relaxation is likely to be the slowest. Previous studies have shown that the stress–relaxation behavior of collagen based material was well described by a function with three exponential decay terms which reflecting the short-, medium- and long-term relaxation components in the tissue.42-44 According to study by Sundararaghavan et al., collagen fibers are observed in GP crosslinked collagen gel.45 However it is important to note that crosslinking can affect the relaxation rate and thus the time constants. In a recent work, atomic force was used to assess the viscoelastic properties of collagen fibrils (Yang et al., 2012). The authors found that the two time constants for native collagen fibrils, attributing to inter-fibril and molecular sliding, are roughly 1s and 60s, but for crosslinked collagen fibrils they are roughly 3s and 250s, which are roughly in the range of time constants for inter-and intra-fibril molecular sliding mechanisms suggested by our study.
Initial stress/strain level can also change the relaxation rate. The relaxation spectra provide insights on the effects of mechanical stresses on the major relaxation components in collagen matrices. Our results show that the rate of stress relaxation of collagen matrices increases linearly with initial stress level (Fig. 4). Similar trend has been observed in previous studies on skin tissue46 and tendon.47 It is noted that opposite relationship between stress level and stress relaxation was reported in ligament,47,48 i.e., the rate of stress relaxation decreases with higher stress level. While the reason is currently unclear, studies combining stress relaxation distribution spectrum analysis and microstructural observations may provide some insight.
The relaxation spectra demonstrate an increase in the intensity of peaks with higher initial stress level (Figs. 4 and 5). It is also noted that there is a more significant increase in the intensity of the long-term peak with higher initial stress levels. The long-term relaxation component has been attributed to more stable polymer networks.28,49 For collagen matrices, this long-term relaxation component is associated with the relaxation in the fibrils. The covalent crosslinks between collagen molecules and microfibrils plays an important role in stabilizing the fibrils and the collagen network. Periodic banding and fibril diameter was observed to change significantly only at higher tissue strains.50 The appreciable increase in the intensity of the long-term peak in the relaxation spectra at higher stress levels from our results further suggests the engagement of collagen fibrils at higher levels of tissue strain. For some collagen matrices the number of peaks increases from three to four at higher initial stress levels. Specifically, the middle peak from the relaxation spectra splits into two peaks resulting in the increase of peak numbers. The number of peaks in the spectrum reflects the amount of heterogeneity of the material.26,51 From polymer science studies, it is widely accepted that molecular weight and structure of polymers are linked to the viscoelastic behavior of the material. The splitting of the middle peak in the relaxation spectrum indicates increased structural heterogeneity at the fibril level with mechanical loading. Such correlations may shed light on understanding the complex structure-function relationship in collagen matrices.
Collagen crosslinking plays important roles in the biological and biomechanical functions of connective tissues. Recent fundamental and clinical studies have found that collagen crosslinking in native tissues have a great close correlation with osteoporosis and cardiovascular diseases.52,53 In the present study, the dependence of relaxation on crosslinking was studied by varying GP concentration. Increase of crosslinking would develop lateral network linkages between collagen molecules and microfibrils54 and cause dehydration of the fibers by drawing the molecules closer,55 which prevents slippage of inter and intra fibrils. Crosslinking also reduces the swelling56 and increases fiber volume fraction,57 which prevent the relaxation induced by fiber sliding. Consequently, less crosslinked collagen gel demonstrates faster stress relaxation, as shown by the results from biaxial stress relaxation (Fig. 6). Similar relationships between crosslinking and viscosity of collagen gel have been reported in previous studies.19,56,57 Furthermore, the variance among the intensity of long time relaxation peaks is much more significant than the other two, which indicate that crosslinking has a greater effect on preventing the slippage between molecules or microfibrils. However the rate of stress relaxation of collagen thin film doesn’t seem to have any obvious dependency on crosslinking or GP concentration (Fig. 7). The relaxation spectra are similar for different GP concentration, which demonstrates that the contribution from different relaxation components are not affected much by GP crosslinking. It is possible that the hierarchical structures of the collagen thin film is already extremely tight due to dehydration, which would prevent fiber and fibril sliding. Such kind of effects may weaken the contribution from chemical crosslinking. In the present study, we contribute the stress relaxation mechanisms to fiber sliding, inter-fibril sliding and intra-fibril sliding. However the movement/rearrangement of water molecules upon applied mechanical stresses is important for these events.
Conclusions
Understanding the mechanisms controlling the viscoelastic properties of collagen matrices has profound importance for biomaterial research. Here we performed a systematic study on the effects of hydration, crosslinking, and mechanical loading on the stress relaxation behavior of collagen matrices using coupled experiment-modeling method. Relaxation time distribution spectrum obtained from inverse Laplace transform provides useful information on understanding the underlying microstructural mechanisms. Our study shows that relaxation at the fiber, inter-fibril, and fibril level plays important roles in the viscoelastic behavior of collagen matrices. The rate of stress relaxation increase linearly with initial stress levels for both collagen gel and thin film. The appreciable increase in the intensity of the long-term peak in the relaxation spectra at higher stress levels suggests the engagement of collagen fibrils at higher levels of tissue strain. The relaxation spectrum also suggests increased fibril structural heterogeneity at higher initial stress level. Future studies are needed to confirm such microstructural changes.
Materials and Methods
Sample preparation
Collagen gel
Nutragen Type I collagen solution (6 mg/ml) was purchased from Advanced BioMatirx. Collagen was dissolved in 0.01N HCl with a pH value of approximately
2.0. Neutralized collagen solution was prepared by quickly mixing Nutragen collagen solution, 10X PBS (Fisher Scientific) and 0.1M NaOH (Fisher Scientific) solution with a ratio of 8:1:1 at 4°C with a final collagen concentration of 4.8mg/ml. The pH value of the solution was adjusted to between 7.2~7.4. The neutralized solution was transferred into a custom made square reservoir that sits in a Petri dish. On each side of the reservoir, a notch was cut to fit the polyethylene bars (Fisher Scientific) pre-threaded with nylon sutures. The solution was incubated at 37°C for 12 h for gelation. During gelation, the polyethylene bars were polymerized into the collagen gel (Fig. 8A). The collagen gel was then immersed in 0.03%, 0.1% and 0.25% GP solutions for another 6 h in the incubator for crosslinking.28 The collagen gel was then rinsed with distilled water to remove the residual GP solutions. The dimension of the collagen gel samples are approximately 20 × 20 × 1 mm.
Figure 8.
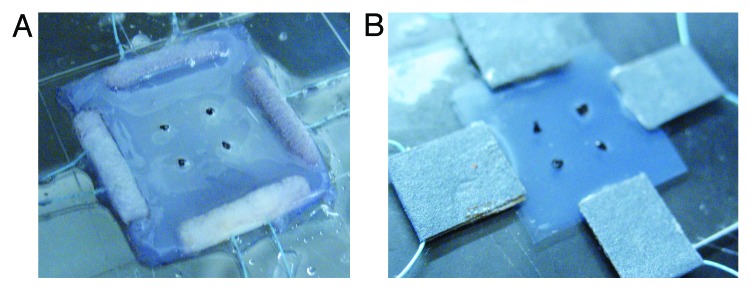
Images of (A) collagen gel with pre-threaded polyethylene bars at the edges for biaxial mechanical testing; and (B) collagen thin film sample with sand paper glued to the edges and sutures looping through the sandpaper tab.
Collagen thin film
Neutralized collagen solution was prepared as described above. The solution was poured into Petri dishes and incubated at 37°C for 12 h for gelation. Genipin solutions of 0.03%, 0.1%, and 0.25% were added into the dishes for further crosslinking for another 6 h. The collagen gel was then rinsed with distilled water to remove the residual GP solutions, and dried in air at room temperature. Collagen thin film, about 0.3mm in thickness, was cut into square pieces of about 20 × 20 mm. Each side of the thin film samples was glued with sand paper at the edges, which was connected to nylon sutures for biaxial tensile loading (Fig. 8B).
Mechanical testing
Equi-biaxial tensile test
The elastic properties of collagen gel (n = 3) and thin film (n = 3) were characterized using a planar biaxial tensile tester.58 During biaxial tensile testing, a roughly square-shaped sample was mounted so that it could be stretched along the x-and y in-plane directions simultaneously. Four carbon dot markers were placed at the center of the sample, and a CCD camera was used to track the position of markers from which the tissue strains in both directions can be determined throughout the deformation. The load applied to the specimen was measured and recorded using load cells during the loading and unloading processes. Samples were preconditioned equi-biaxially for 8 cycles to achieve a repeatable mechanical response. A half cycle time of 10s was used. The samples were then subjected to 8 cycles of equi-biaxial loads with the maximum loads varying from 40 g to 70 g for hydrated collagen gel and from 70 g to 100 g for collagen thin film. Cauchy stress and logarithm strain was calculated59 and used for the description of the mechanical behavior of collagen matrices under biaxial tensile test. Tangent stiffness was obtained by taking the derivative of the stress-strain curve. To do so, a six-order polynomial is fit to the loading-unloading stress-strain curves. Tangent stiffness from the x-and y-directions are then averaged for each sample. Three samples were tested under each hydration and crosslinking condition.
Stress relaxation test
Biaxial tensile test was first performed as described above to reach sychronization. Immediately after the sample was loaded to the target stretch with a rise time of 5 s and held at this constant stretch for 600 s. The load in both loading directions was recorded during the holding period. Stress relaxation preconditioning tests were first performed to achieve repeatable stress relaxation behavior. Specifically, Three cycles of stress relaxation tests were performed to confirm the repeatability of the viscoelastic behavior. Stress relaxation experiments were performed at different initial strain levels. Stress at each strain level are reported during the holding period.30 The relaxation time distribution spectrum was obtained from the stress relaxation experiment data through inverse Laplace transform using the CONTIN program.60 Usually there are multiple peaks in the relaxation spectrum owing to the different stress relaxation components. The peaks and the corresponding relaxation time in the relaxation spectrum reflect the dominant relaxation processes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
This work was supported by National Science Foundation grant CMMI 0954825.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/24651
References
- 1.Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221:1–22. doi: 10.1016/S0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 2.Stone KR, Steadman JR, Rodkey WG, Li ST. Regeneration of meniscal cartilage with use of a collagen scaffold. Analysis of preliminary data. J Bone Joint Surg Am. 1997;79:1770–7. doi: 10.2106/00004623-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Mudera VC, Pleass R, Eastwood M, Tarnuzzer R, Schultz G, Khaw P, et al. Molecular responses of human dermal fibroblasts to dual cues: contact guidance and mechanical load. Cell Motil Cytoskeleton. 2000;45:1–9. doi: 10.1002/(SICI)1097-0169(200001)45:1<1::AID-CM1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 4.Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14:633–9. doi: 10.1016/S0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 5.Hegerfeldt Y, Tusch M, Bröcker EB, Friedl P. Collective cell movement in primary melanoma explants: plasticity of cell-cell interaction, beta1-integrin function, and migration strategies. Cancer Res. 2002;62:2125–30. [PubMed] [Google Scholar]
- 6.Bunyaratavej P, Wang HL. Collagen membranes: a review. J Periodontol. 2001;72:215–29. doi: 10.1902/jop.2001.72.2.215. [DOI] [PubMed] [Google Scholar]
- 7.Behring J, Junker R, Walboomers XF, Chessnut B, Jansen JA. Toward guided tissue and bone regeneration: morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes. A systematic review. Odontology. 2008;96:1–11. doi: 10.1007/s10266-008-0087-y. [DOI] [PubMed] [Google Scholar]
- 8.Plant AL, Bhadriraju K, Spurlin TA, Elliott JT. Cell response to matrix mechanics: focus on collagen. Biochim Biophys Acta. 2009;1793:893–902. doi: 10.1016/j.bbamcr.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Chandran PL, Barocas VH. Microstructural mechanics of collagen gels in confined compression: poroelasticity, viscoelasticity, and collapse. J Biomech Eng. 2004;126:152–66. doi: 10.1115/1.1688774. [DOI] [PubMed] [Google Scholar]
- 10.Chapman GE, Danyluk SS, McLauchlan KA. a model for collagen hydration. Proc R Soc Lond B Biol Sci. 1971;178:465–76. doi: 10.1098/rspb.1971.0076. [DOI] [PubMed] [Google Scholar]
- 11.Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- 12.Fratzl Peter. 2008. Collagen: structure and mechanics, Springer, New York. [Google Scholar]
- 13.Roeder BA, Kokini K, Sturgis JE, Robinson JP, Voytik-Harbin SL. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. J Biomech Eng. 2002;124:214–22. doi: 10.1115/1.1449904. [DOI] [PubMed] [Google Scholar]
- 14.Duan X, Sheardown H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: mechanical properties and corneal epithelial cell interactions. Biomaterials. 2006;27:4608–17. doi: 10.1016/j.biomaterials.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Olde Damink LHH, Dijkstra PJ, Van Luyn MJA, Van Wachem PB, Nieuwenhuis P, Feijen J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J Mater Sci Mater Med. 1995;6:460–72. doi: 10.1007/BF00123371. [DOI] [Google Scholar]
- 16.Yang YL, Kaufman LJ. Rheology and confocal reflectance microscopy as probes of mechanical properties and structure during collagen and collagen/hyaluronan self-assembly. Biophys J. 2009;96:1566–85. doi: 10.1016/j.bpj.2008.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundararaghavan HG, Monteiro GA, Lapin NA, Chabal YJ, Miksan JR, Shreiber DI. Genipin-induced changes in collagen gels: correlation of mechanical properties to fluorescence. J Biomed Mater Res A. 2008;87:308–20. doi: 10.1002/jbm.a.31715. [DOI] [PubMed] [Google Scholar]
- 18.Sheu MT, Huang JC, Yeh GC, Ho HO. Characterization of collagen gel solutions and collagen matrices for cell culture. Biomaterials. 2001;22:1713–9. doi: 10.1016/S0142-9612(00)00315-X. [DOI] [PubMed] [Google Scholar]
- 19.Raub CB, Suresh V, Krasieva T, Lyubovitsky J, Mih JD, Putnam AJ, et al. Noninvasive assessment of collagen gel microstructure and mechanics using multiphoton microscopy. Biophys J. 2007;92:2212–22. doi: 10.1529/biophysj.106.097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang YL, Leone LM, Kaufman LJ. Elastic moduli of collagen gels can be predicted from two-dimensional confocal microscopy. Biophys J. 2009;97:2051–60. doi: 10.1016/j.bpj.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charulatha V, Rajaram A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials. 2003;24:759–67. doi: 10.1016/S0142-9612(02)00412-X. [DOI] [PubMed] [Google Scholar]
- 22.Cheng X, Gurkan UA, Dehen CJ, Tate MP, Hillhouse HW, Simpson GJ, et al. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials. 2008;29:3278–88. doi: 10.1016/j.biomaterials.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 23.Sung HW, Chang Y, Chiu CT, Chen CN, Liang HC. Crosslinking characteristics and mechanical properties of a bovine pericardium fixed with a naturally occurring crosslinking agent. J Biomed Mater Res. 1999;47:116–26. doi: 10.1002/(SICI)1097-4636(199911)47:2<116::AID-JBM2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 24.Fung YC. 1993. Biomechanics: Mechanical properties of living tissues Springer, New York. [Google Scholar]
- 25.Malkin YA. The use of a continuous relaxation spectrum for describing the viscoelastic properties of polymers. Polymer Science. 2006;A48:39–45. [Google Scholar]
- 26.Sodhi NS, Sasaki T, Lu Zh, Kohyama K. Phenomenological viscoelasticity of some rice starch gels. Food Hydrocoll. 2010;24:512–7. doi: 10.1016/j.foodhyd.2009.12.009. [DOI] [Google Scholar]
- 27.Mao R, Tang J, Swanson BG. Relaxation time spectrum of hydrogels by CONTIN analysis. J Food Sci. 2000;65:374–81. doi: 10.1111/j.1365-2621.2000.tb16010.x. [DOI] [Google Scholar]
- 28.Li W, Dobraszczyk BJ, Schofield JD. Stress relaxation behavior of wheat dough, gluten and gluten protein fractions. Cereal Chemistry. 2003;80:333–8. doi: 10.1094/CCHEM.2003.80.3.333. [DOI] [Google Scholar]
- 29.Xu B, Chow MJ, Zhang Y. Experimental and modeling study of collagen scaffolds with the effects of crosslinking and fiber alignment. Int J Biomater. 2011;2011:172389. doi: 10.1155/2011/172389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou Y, Zhang Y. The orthotropic viscoelastic behavior of aortic elastin. Biomech Model Mechanobiol. 2011;10:613–25. doi: 10.1007/s10237-010-0260-4. [DOI] [PubMed] [Google Scholar]
- 31.Daxer A, Misof K, Grabner B, Ettl A, Fratzl P. Collagen fibrils in the human corneal stroma: structure and aging. Invest Ophthalmol Vis Sci. 1998;39:644–8. [PubMed] [Google Scholar]
- 32.Price RI, Lees S, Kirschner DA. X-ray diffraction analysis of tendon collagen at ambient and cryogenic temperatures: role of hydration. Int J Biol Macromol. 1997;20:23–33. doi: 10.1016/S0141-8130(97)01148-3. [DOI] [PubMed] [Google Scholar]
- 33.Wachtel E, Maroudas A. The effects of pH and ionic strength on intrafibrillar hydration in articular cartilage. Biochim Biophys Acta. 1998;1381:37–48. doi: 10.1016/S0304-4165(97)00158-X. [DOI] [PubMed] [Google Scholar]
- 34.Mogilner IG, Ruderman G, Grigera JR. Collagen stability, hydration and native state. J Mol Graph Model. 2002;21:209–13. doi: 10.1016/S1093-3263(02)00145-6. [DOI] [PubMed] [Google Scholar]
- 35.George A, Veis A. FTIRS in H2O demonstrates that collagen monomers undergo a conformational transition prior to thermal self-assembly in vitro. Biochemistry. 1991;30:2372–7. doi: 10.1021/bi00223a011. [DOI] [PubMed] [Google Scholar]
- 36.Leikin S, Parsegian VA, Yang WH, Walrafen GE. Raman spectral evidence for hydration forces between collagen triple helices. Proc Natl Acad Sci U S A. 1997;94:11312–7. doi: 10.1073/pnas.94.21.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puxkandl R, Zizak I, Paris O, Keckes J, Tesch W, Bernstorff S, et al. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philos Trans R Soc Lond B Biol Sci. 2002;357:191–7. doi: 10.1098/rstb.2001.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Screen HRC. Investigating load relaxation mechanics in tendon. J Mech Behav Biomed Mater. 2008;1:51–8. doi: 10.1016/j.jmbbm.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Gupta HS, Seto J, Krauss S, Boesecke P, Screen HRC. In situ multi-level analysis of viscoelastic deformation mechanisms in tendon collagen. J Struct Biol. 2010;169:183–91. doi: 10.1016/j.jsb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Gautieri A, Vesentini S, Redaelli A, Buehler MJ. Viscoelastic properties of model segments of collagen molecules. Matrix Biol. 2012;31:141–9. doi: 10.1016/j.matbio.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev. 2001;122:735–55. doi: 10.1016/S0047-6374(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 42.Toms SR, Dakin GJ, Lemons JE, Eberhardt AW. Quasi-linear viscoelastic behavior of the human periodontal ligament. J Biomech. 2002;35:1411–5. doi: 10.1016/S0021-9290(02)00166-5. [DOI] [PubMed] [Google Scholar]
- 43.Wagenseil JE, Wakatsuki T, Okamoto RJ, Zahalak GI, Elson EL. One-dimensional viscoelastic behavior of fibroblast populated collagen matrices. J Biomech Eng. 2003;125:719–25. doi: 10.1115/1.1614818. [DOI] [PubMed] [Google Scholar]
- 44.Komatsu K. Mechanical strength and viscoelastic response of the periodontal ligament in relation to structure. J Dent Biomech. 2010;2010:1–18. doi: 10.4061/2010/502318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sundararaghavan HG, Monteiro GA, Firestein BL, Shreiber DI. Neurite growth in 3D collagen gels with gradients of mechanical properties. Biotechnol Bioeng. 2009;102:632–43. doi: 10.1002/bit.22074. [DOI] [PubMed] [Google Scholar]
- 46.Purslow PP, Wess TJ, Hukins DWL. Collagen orientation and molecular spacing during creep and stress-relaxation in soft connective tissues. J Exp Biol. 1998;201:135–42. doi: 10.1242/jeb.201.1.135. [DOI] [PubMed] [Google Scholar]
- 47.Duenwald SE, Vanderby RJ, Jr., Lakes RS. Stress relaxation and recovery in tendon and ligament: experiment and modeling. Biorheology. 2010;47:1–14. doi: 10.3233/BIR-2010-0559. [DOI] [PubMed] [Google Scholar]
- 48.Provenzano P, Lakes R, Keenan T, Vanderby R., Jr. Nonlinear ligament viscoelasticity. Ann Biomed Eng. 2001;29:908–14. doi: 10.1114/1.1408926. [DOI] [PubMed] [Google Scholar]
- 49.Kontogiorgos V, Kasapis S. Temperature dependence of relaxation spectra for highly hydrated gluten networks. J Cereal Sci. 2010;52:100–5. doi: 10.1016/j.jcs.2010.04.001. [DOI] [Google Scholar]
- 50.Rigozzi S, Stemmer A, Müller R, Snedeker JG. Mechanical response of individual collagen fibrils in loaded tendon as measured by atomic force microscopy. J Struct Biol. 2011;176:9–15. doi: 10.1016/j.jsb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Ptaszek A, Berski W, Ptaszek P, WItczak T, Repelewicz U, Grzesik M. Viscoelastic properties of waxy maize starch and selected non-starch hydrocolloids gels. Carbohydr Polym. 2009;76:567–77. doi: 10.1016/j.carbpol.2008.11.023. [DOI] [Google Scholar]
- 52.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21:195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 53.Redfield MM. Treating diastolic heart failure with AGE crosslink breakers: thinking outside the heart failure box. J Card Fail. 2005;11:196–9. doi: 10.1016/j.cardfail.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Yang L, van der Werf KO, Koopman BFJM, Subramaniam V, Bennink ML, Dijkstra PJ, et al. Micromechanical bending of single collagen fibrils using atomic force microscopy. J Biomed Mater Res A. 2007;82:160–8. doi: 10.1002/jbm.a.31127. [DOI] [PubMed] [Google Scholar]
- 55.Miles CA, Avery NC, Rodin VV, Bailey AJ. The increase in denaturation temperature following cross-linking of collagen is caused by dehydration of the fibres. J Mol Biol. 2005;346:551–6. doi: 10.1016/j.jmb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Friess W, Schlapp M. Effects of processing conditions on the rheological behavior of collagen dispersions. Eur J Pharm Biopharm. 2001;51:259–65. doi: 10.1016/S0939-6411(01)00136-9. [DOI] [PubMed] [Google Scholar]
- 57.Francis-Sedlak ME, Uriel S, Larson JC, Greisler HP, Venerus DC, Brey EM. Characterization of type I collagen gels modified by glycation. Biomaterials. 30:1851–6. doi: 10.1016/j.biomaterials.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 58.Sacks MS. Biaxial mechanical evaluation of planar biological materials. J Elast. 2009;61:199–246. [Google Scholar]
- 59.Zou Y, Zhang Y. An experimental and theoretical study on the anisotropy of elastin network. Ann Biomed Eng. 2009;37:1572–83. doi: 10.1007/s10439-009-9724-z. [DOI] [PubMed] [Google Scholar]
- 60.Provencher SW. CONTIN: a general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput Phys Commun. 1982;27:229–42. doi: 10.1016/0010-4655(82)90174-6. [DOI] [Google Scholar]


