Abstract
Pax3 and Pax7 paralogous genes have functionally diverged in vertebrate evolution, creating opportunity for a new distribution of roles between the two genes and the evolution of novel functions. Here we focus on the regulation and function of Pax7 in the brain and neural crest of amphibian embryos, which display a different pax7 expression pattern, compared to the other vertebrates already described. Pax7 expression is restricted to the midbrain, hindbrain and anterior spinal cord, and Pax7 activity is important for maintaining the fates of these regions, by restricting otx2 expression anteriorly. In contrast, pax3 displays broader expression along the entire neuraxis and Pax3 function is important for posterior brain patterning without acting on otx2 expression. Moreover, while both genes are essential for neural crest patterning, we show that they do so using two distinct mechanisms: Pax3 acts within the ectoderm which will be induced into neural crest, while Pax7 is essential for the inducing activity of the paraxial mesoderm towards the prospective neural crest.
Keywords: Pax3, Pax7, Brain, Neural crest, Mesoderm, FGF, WNT, Otx2, Krox20, Snail2, Patterning
Introduction
Pax3 and Pax7 are members of the Pax family of transcriptional regulators. They contain two DNA binding domains, a paired domain and a paired-type homeodomain (Chalepakis et al., 1994a,b; Gruss and Walther, 1992; Jostes et al., 1990). Human syndromes with Pax3 mutations (Waardenburg syndrome types I and III) and mouse mutants (Splotch/Pax3 mutant and Pax7 mutant) have highlighted their prominent roles in early embryogenesis and during adulthood (Chalepakis et al., 1994a; Epstein et al., 1991; Read and Newton, 1997; Tassabehji et al., 1992). In adults, they are essential in muscle homeostasis and repair (Buckingham, 2006; Kuang and Rudnicki, 2008; Le Grand and Rudnicki, 2007). In embryos, they are major regulators of central nervous system, neural crest and paraxial mesoderm patterning and differentiation. During vertebrate evolution, Pax3 and Pax7 duplicated from an ancestral gene (Holland et al., 1999; McCauley and Bronner-Fraser, 2002; Osorio et al., 2005). Further duplications have occurred in zebrafish, which has four genes: Pax3/Pax3b and Pax7/Pax7b (Thompson et al., 2008). While such gene duplications may retain functional overlap between Pax3 and Pax7, they also open the possibility for distribution of the various functions assumed by the ancestral gene and the evolution of novel functions for each paralog. Indeed, the comparison of Pax3 and Pax7 gene expression shows differences between vertebrates (see below). The analysis of the functional implications of such differences reveals distinct functions between Pax3 and Pax7 in various cell types, such as muscle or neuron progenitors (Relaix et al., 2004; Thompson et al., 2008).
Here we focus on the early mechanisms of neural and neural crest development. During mouse, chick and fish neurulation and organogenesis, Pax3 and Pax7 are both expressed in overlapping patterns in the central nervous system, including expression in the mesencephalon, the hindbrain and the spinal cord, with some subtle regional differences between the two genes (Borycki et al., 1999; Goulding et al., 1994a; Mansouri et al., 1996; Minchin and Hughes, 2008; Thompson et al., 2008). In their various locations along the anterior–posterior axis, Pax3 and Pax7 domains of expression are restricted to the dorsal part of the central nervous system. Ventral midline-derived signals, such as sonic hedgehog and noggin, restrict their expression to the dorsal half of the neural tube, while dorsal midline regulators, such as BMP4, promote their dorsal expression at early steps of neural patterning (Goulding et al., 1994a; Liem et al., 1997; McMahon et al., 1998; Monsoro-Burq et al., 1995; Monsoro-Burq et al., 1996). In turn, Pax3 is an essential regulator of neural tube dorsal–ventral patterning. Splotch mutants are strongly affected in dorsal neural tube development and present severe spina bifida (Borycki et al., 1999; Epstein et al., 1991). Reciprocally, Pax3 gain of function in mouse embryos alters ventral neural tube patterning (Tremblay et al., 1996). Strikingly, the Pax7 homozygous mutants do not show any abnormal phenotype in the central nervous system, suggesting a significant functional overlap between Pax3 and Pax7 activities within the neural tube of mouse embryos (Mansouri et al., 1996). Indeed, Pax7 knock-in into the Pax3 locus in Splotch mutants rescues the spina bifida phenotype (Relaix et al., 2004). At later stages of brain morphogenesis, in chick embryos, Pax3 and Pax7 expression is regulated at the isthmus by Fgf8 and En2/Pax2–5; in turn, Pax3 and Pax7 are involved in tectum organisation downstream of Fgf8/En2/Pax2–5 (Matsunaga et al., 2001). During later mouse mesencephalon development, Pax3 and Pax7 expression partially segregate and control the development of specific neuronal populations (Thompson et al., 2008).
Pax3 and Pax7 are also major regulators of neural crest early development. The neural crest is a transient vertebrate-specific population of pluripotent and migratory progenitors, from which are derived peripheral neurons and glia, melanocytes and other pigment cells, as well as craniofacial structures (Le Douarin and Kalcheim, 1999). The neural crest delaminates from the dorsalmost part of the neural tube, after being induced in the lateral neural plate (also named the neural border, (Meulemans and Bronner-Fraser, 2004) during gastrulation and early neurulation. Mutations in the human Pax3 gene, which can be heterozygous or rarely homozygous, affect a subset of neural crest derivatives, such as the melanocytes, which, in particular, contribute to ear development and to pigmentation of skin and hair (Waardenburg syndrome I and III), but initial neural crest development seems to occur normally since other neural crest structures are formed (Read and Newton, 1997). In the mouse, homozygous Pax3 mutants display reduced to absent neural crest derivatives, especially in posterior areas, while heterozygous mice only show reduced belly pigmentation (Auerbach, 1954; Franz and Kothary, 1993; Relaix et al., 2004). Strikingly, the craniofacial structures form rather normally in Pax3 homozygotes, suggesting that the initiation of neural crest development is not affected in the cephalic neural crest (Relaix et al., 2004). Mouse Pax7 mutants show only late cephalic neural crest defects (Mansouri et al., 1996), suggesting that Pax3 activity is sufficient for early neural crest. Overlap between Pax3 and Pax7 functions in the neural tube and neural crest is further evidenced by the rescue of the Pax3 mutant phenotype by knock-in of Pax7 into the Pax3 locus, showing that increasing Pax7 activity compensates for Pax3 loss (Relaix et al., 2004). Because of the overlapping expression and roles, and because the double mutant Pax3/Pax7 has not yet been described, the contributions of Pax3 or Pax7 to mammalian cranial neural crest induction remain unknown. However, in non-mammalian species, both Pax3 and Pax7 have been implicated in neural crest induction. In particular, Pax3 appears as the earliest neural border-specific marker in the Xenopus laevis gastrula (Monsoro-Burq et al., 2005), a similar pattern being assumed by Pax7 in chick embryos (Basch et al., 2006). In either species, Pax3 (Xenopus) or Pax7 (chick) cooperate with other regulators and induce the early neural crest marker Snail2 (Basch et al., 2006; Monsoro-Burq et al., 2005). By contrast, the respective roles of Xenopus Pax7 and chick Pax3 in neural crest induction remain to be explored. Moreover, besides interactions between the neural plate and the ectoderm, interactions between the paraxial mesoderm and the ectoderm are essential in neural crest induction (Bonstein et al., 1998; Monsoro-Burq et al., 2003). Although Pax3 and Pax7 are important regulators of paraxial mesoderm development (Borycki et al., 1999; Goulding et al., 1994b; Relaix et al., 2004, 2005), their potential participation in the neural crest inducing activity of the paraxial mesoderm remains to be explored.
During later neural crest development, Pax3 and Pax7 are found in migrating neural crest cells (NCC) with species-specific differences: cranial NCC in mouse, cranial and trunk NCC in chick and zebrafish embryos (Lacosta et al., 2005; Mansouri et al., 1996; Minchin and Hughes, 2008). When NCC condense into dorsal root ganglia in chick embryos, Pax3 remains expressed while Pax7 expression is extinguished (Lacosta et al., 2005). Later, both Pax3 and Pax7 are detected in the neural crest derived pigment cells, melanocytes or xanthophores in chick or zebrafish (Lacosta et al., 2005; Minchin and Hughes, 2008). However, only Pax3 was detected in mammalian melanoblasts (Lacosta et al., 2005). In conclusion, the survey of Pax3 and Pax7 expression profiles in various vertebrates outlines the variability of common and gene-specific patterns, especially in the neural crest lineage.
In this study, we have examined and compared the respective roles of Pax3 and Pax7 in neural and neural crest patterning in Xenopus embryos. We show striking differences in the expression domains of these two genes compared to other vertebrate species, notably in the neural tube and neural crest, that could indicate different functional specialization between the two genes in amphibians. Using a series of gain-of-function and loss of function experiments, we address the regulation of Pax7 expression by FGF, Wnt, retinoids and Pax3 during early neural patterning. We then analyze and compare Pax3 and Pax7 specific function in early anterior–posterior brain patterning. Finally, we explore their respective roles in neural crest induction by the paraxial mesoderm.
Materials and methods
Embryo and explant manipulation
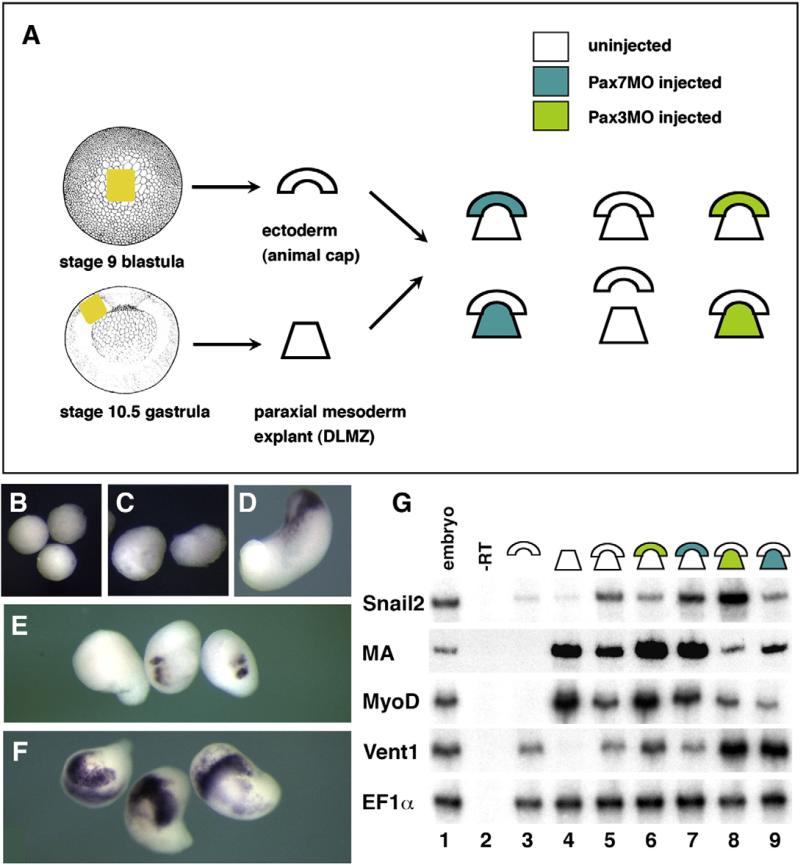
X. laevis embryos were obtained by in vitro fertilization using standard procedures and were staged according to Nieuwkoop and Faber developmental table (Nieuwkoop and Faber, 1994; Sive et al., 2000). Embryos were injected in one blastomere at the two to four-cell stage unless otherwise noted. Co-injection of mRNA encoding nuclear-targeted lacZ traced the progeny of the injected blastomere. Staining for beta-galactosidase activity was done prior to final fixation (Monsoro-Burq, 2007). The recombination of stage 9 ectoderm from the animal cap to stage 10.25 prospective paraxial mesoderm (dorsal–lateral marginal zone, DLMZ), as an assay for neural crest induction, was described in Bonstein et al. (1998) and Monsoro-Burq et al. (2003).
Semi-quantitative RT-PCR
Embryos were lysed in proteinase K-containing lysis buffer, followed by DNase treatment and reverse transcription (Sive et al., 2000), the minus-RT sample is a control sample amplified without the reverse transcription step, monitoring potential DNA contamination. EF1a was used to calibrate the reaction (21 cycles, Krieg et al., 1989). Muscle actin, snail2, vent1, myod, pax3 and pax7 primers (Mizuseki et al., 1998; Monsoro-Burq et al., 2005; Rupp and Weintraub, 1991; Shapira et al., 1999; Stutz and Spohr, 1986, and this study) were used in 25-cycle amplification 32P-dCTP-traced PCR (see Supplemental Fig. 2A for sequences).
Whole mount in situ hybridization (ISH) and immunostaining
Embryos were fixed and prepared for whole mount in situ hybridization (ISH) according to a shortened protocol optimized for superficial structures (Monsoro-Burq, 2007). Antisense digoxigenin-labelled RNA probes were used at a final concentration of 1 μg/ml. Probes were described elsewhere: snail2 (Grammer et al., 2000), pax3 (Monsoro-Burq et al., 2005), myoD (Hopwood et al., 1989), otx2 (Lamb et al., 1993), en2 (Hemmati-Brivanlou et al., 1991), krox20 (Bradley et al., 1993), gbx2 (PCR-amplified and cloned into pGEM-T Easy (Promega) using sequence in Tour et al. (2002a)), dct (Kumasaka et al., 2003) and hoxb9 (Sharpe et al., 1987). For immunostaining of myotome after ISH, the bleached embryos were saturated in 10% serum then incubated in 12/101 monoclonal antibody (Kintner and Brockes, 1984) before washes and peroxidase (HRP-DAB) staining. The embryos were examined after vibratome (30 μm) sectioning.
Messenger RNA synthesis, antisense morpholinos
Messenger RNAs used for microinjection were obtained by in vitro transcription of plasmids containing the desired cDNA using the mMessage mMachine SP6 or T7 kits (Ambion) and purified on G50 sephadex spin columns. The following plasmids were used: nlacZ (125–250 pg/cell), fgf8a (CS107-fgf8a;10–50 pg/cell) (Monsoro-Burq et al., 2003), wnt7b (50 pg/cell) (Chang and Hemmati-Brivanlou, 1998; Grammer et al., 2000), dnRARα (250 pg/cell) (Blumberg et al., 1997), X. laevis pax3 (AY725269; cloned into CS107-Pax3, 125 pg/cell) (Monsoro-Burq et al., 2005), mouse pax3 (CS107-mpax3, 125 pg/cell) (Goulding et al., 1991; Monsoro-Burq et al., 2005), mouse pax3-EnR (subcloned into pCS107 by R. Harland and B. Martin, 62–125 pg/cell; Ridgeway and Skerjanc, 2001), X. laevis pax7 (full-length cDNA subcloned into pCS107, 250–500 pg/cell, this study, Genbank AY725267), X. laevis pax7-myc (pax7 ORF fused to N-terminal myc tags and subcloned into pCS107, 62–125 pg/cell, this study), X. laevis pax7-EnR (pCDNA3-pax7-EnR, pax7 DNA binding domain fused to Engrailed repressor domain, 62 pg/cell (Chen et al., 2006). Silencing of selected genes was performed using translation blocking antisense morpholino oligonucleotides (GeneTools, see Supplemental Fig. 2B for sequences): Fgf8MO (Monsoro-Burq et al., 2003), beta-cateninMO (Genetools), Pax3MO (Monsoro-Burq et al., 2005), Pax7MO (translation blocking), Pax7 mismatch MO and Pax7 splice MO (this study). The specificity of Pax3MO and Pax7MO (translation blocking MOs) MOs was tested by 35S-labelled in vitro transcription-and-translation reaction using TNT kit (Promega). Relative morpholino concentration was maintained constant compared to the volume of the injected cell: whole embryo injections were done in all blastomeres at the two or four-cell stage with a total dose four times higher than injections done into one blastomere of four-cell stage embryos.
Results
pax3 and pax7 display both partially overlapping and distinct expression domains in mesoderm, neural plate and brain of X. laevis embryos
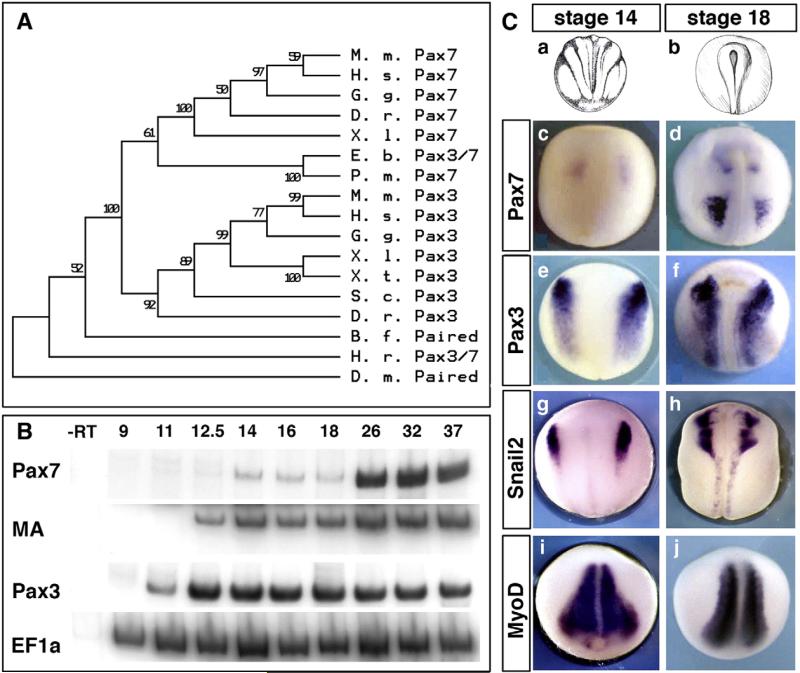
We previously subcloned and sequenced the full-length pax7 cDNA from NIBB EST library (http://xenopus.nibb.ac.jp, Genbank accession #AY725267). As expected, the encoded protein shows high similarity with other vertebrate Pax7 especially within the paired and octapeptide-homeodomain parts of the protein (Supplemental Figs. 1A–B) and synteny in Xenopus tropicalis (not shown). In contrast, Pax3 and Pax7 proteins, although very closely related, can be clearly distinguished by several amino acid sequence features and are quite distinct at the nucleotide level (Supplemental Figs. 1C and 2C), allowing construction of a non-ambiguous phylogenetic tree. We have analyzed the available Pax3/7 sequences in chordates, using neighbour-joining method (Clustal W in MacVector and MEGA4 software (Tamura et al., 2007). This tree groups the cyclostomes (lamprey and hagfish) genes in the Pax7 group, while both amphioxus and ascidian are found with a single Pax3/7 gene. This confirms that the duplication of the Pax3/7 ancestor has occurred at the base of the vertebrate lineage (Fig. 1A; O'Neill et al., 2007). Moreover, we have also compiled the available data on Pax7 gene organisation using both X. laevis BAC sequencing and X. tropicalis genome data (Supplemental Fig. 2).
Fig. 1.
Expression of pax7 and pax3 compared during neurulation in Xenopus laevis. (A) A bootstrap phylogenetic tree illustrates the clear grouping of pax7 and pax3 paralogs in vertebrates while the chordate have a single Pax3/7 gene. Accession numbers are given in Supplemental Fig. 1. (B) RT-PCR analysis on whole embryos shows the early onset of pax3 expression during gastrulation (i.e. at times of neural crest induction), whereas pax7 is detected at mid-neurulation, after muscle actin (MA) is detected. EF1α is used as a baseline control. (C) In situ hybridization on stage-matched sibling embryos confirms the late onset of pax7, in brain first (c) then in paraxial mesoderm, and the lack of expression in the neural crest progenitor area (c, d). In contrast, pax3 and snail2 label neural crest (e–h). Myod marks paraxial mesoderm (i, j).
We compared the onset of pax3, muscle actin, and pax7 transcription in vivo using semi-quantitative RT-PCR on whole embryos (Fig. 1B). As we described before, pax3 appears the earliest, at gastrula stage 11 (Monsoro-Burq et al., 2005), followed by muscle actin which is detected by stage 12.5. Pax7 appears last, being faintly detected at mid-neurula stage 14, then being reinforced in tadpoles (from stage 26 onwards). We have further compared expression patterns of pax3, pax7, snail2 and myoD by whole mount ISH, at neurula stages. In agreement with the RT-PCR data, we found that pax7 is faintly expressed by stage 14 in the anterior neural plate (Fig. 1C, a, c) and reinforced in the brain and trunk paraxial mesoderm by stage 18 (Fig. 1C, b, d). This expression is thus detected at a later developmental stage than pax3 (detected at the neural border at stage 14 and in neural plate and paraxial mesoderm later on, Fig. 1C, e, f), snail2 (detected in the neural crest at both stages 14 and 18, Fig. 1C, g, h) and myoD (expressed in the entire paraxial mesoderm at stages 14 and 18, Fig. 1C, i, j).
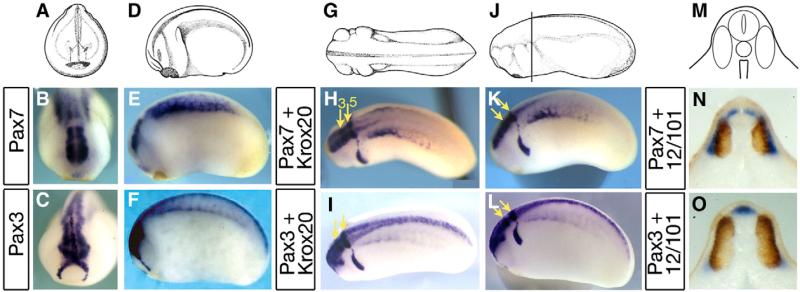
At tailbud stage, pax3 and pax7 remain expressed in overlapping but distinct domains in both neural tissue and somites. At stage 23, a strong pax7 staining is observed in the posterior part of the brain and in the paraxial mesoderm (Figs. 2A–B, D–E). This pattern differs from pax3 expression, which extends the entire length of the nervous system (Figs. 2C, F). In addition, pax3 stains the hatching gland in the ectoderm (Fig. 2C). Double staining with krox20 helps define the pax7-positive brain level: pax7 is expressed in a domain centered on rhombomeres 3 and 5 (Figs. 2G–H, J–K, yellow arrows), including mesencephalon and anterior spinal cord while pax3 labels the entire length of the neural tube (Figs. 2I, L). Transverse trunk sections (line in Fig. 2J), double-stained with 12–101 muscle marker show that pax7 labels the superficial part of the myotome as well as a dorsal spinal cord domain excluding the roof plate, while pax3 labels the hypaxial myotome and the entire dorsal domain of the spinal cord (Figs. 2M–O). Since the origins of the dermatome are unclear at this stage, we cannot rule out a contribution of gene expression to this domain. Neither of the two genes is expressed in the migrating neural crest (compare to krox20-positive neural crest from rhombomere 5 in Figs. 2K–L).
Fig. 2.
Distinct pax7 and pax3 patterns in central nervous system and paraxial mesoderm at tailbud stages. (A–C) Front views of stage 22 embryos (A) show pax7 expression in the caudal part of the brain (B), whereas pax3 labels both the whole brain and the hatching gland (C). (D–F) Side views (D) illustrate pax7 restriction to mesencephalon, hindbrain and anterior spinal cord whereas pax3 is found along the entire anterior–posterior length of the central nervous system. Pax7 labels the central paraxial mesoderm while pax3 is found in hypaxial cells. (G–L) Dorsal (G–I) and side (J–L) views of tailbud stage 24, with double staining for pax3 or pax7 and krox20, which labels hindbrain rhombomeres r3 and r5 (yellow arrows), confirms the limited extent of pax7 expression along the spinal cord. (M–O) Tailbud stage 24 transverse sections were double-stained with 12–101 myotome marker (brown). This shows pax7 expression in the alar plate of the anterior-most spinal cord and medial myotome (N) and pax3 in the roof plate, alar plates and in the hypaxial myotome (O).
Both pax7 and pax3 respond to FGF, WNT and retinoic acid pathways patterning activity
The differences observed in the expression of pax7 and pax3 might result from the cues that define the anterior–posterior neural pattern in the neurula embryos. During neural development, FGF signaling contributes to neural induction and anterior–posterior polarization of the neural tube (Delaune et al., 2005; Fletcher et al., 2006). Moreover, FGF8 is a major brain patterning signal at the midbrain–hindbrain boundary (Koebernick et al., 2006). FGF8 expression is first detected at this boundary by stage 16 in X. laevis neurulae, which is similar to the observed onset time of pax7 expression in the brain (Fig. 1C).
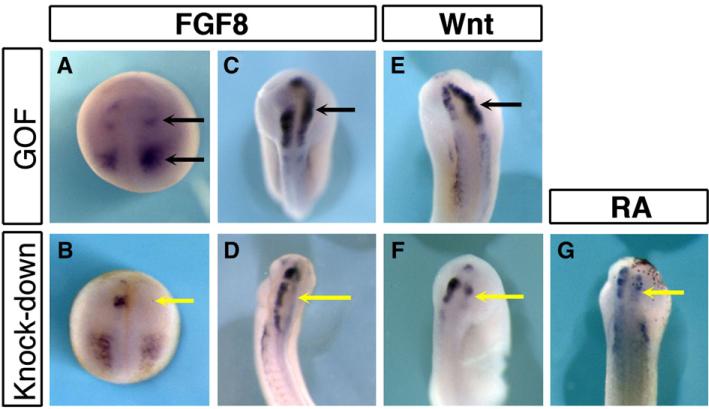
We analyzed the role of FGF signaling on pax7 patterning by gain and loss of function experiments. Fgf8a mRNA injections were done in one dorso-animal blastomere at the 4-cell stage, at a low dose (5 pg) that only marginally affects gene expression on the contralateral side as monitored by snail2 expression (n=8, 75% of unilateral expansion of snail2 at stage 18, 12% expansion in the anterior neural fold, not shown, (Monsoro-Burq et al., 2003). Increased FGF8 signaling resulted in expanded pax7 expression: at neurula stage 18, the anteriormost expression domain, located in the prospective forebrain was lost while the emerging rhombencephalon domain and the mesoderm domain were shifted anteriorly (black arrows) (Fig. 3A). At tailbud stage (stages 20–25), pax7 was found expressed in the whole brain on the injected side, extending anteriorly towards the forebrain (n=31, 84% expansion, Fig. 3C, arrow). Interestingly, the posterior boundary of pax7 expression in the rhombencephalon remained unchanged. In sibling embryos, FGF8 over-expression enlarged pax3 expression domain in the dorsal neural tube (n=27, 100% expansion, not shown, (Monsoro-Burq et al., 2005)) as well as krox20 expression in rhombomeres 3 and 5 (n=24, 83% expanded, not shown). Conversely, knocking down both FGF8a and FGF8b forms by a translation blocking morpholino against X. laevis FGF8 (FGF8MO, (Fletcher et al., 2006; Monsoro-Burq et al., 2005), Supplemental Fig. 2B) efficiently downregulated both pax7 and pax3 expression in the brain on the injected side (Pax7: n=20, 85% decrease to complete loss of expression, Figs. 3B, D; Pax3: n=10, 60% decrease, not shown). This was similar to the decrease observed after injection of a dominant-negative FGF receptor (XFD, not shown). This result shows that FGF signaling, and FGF8 in particular, play an essential part in initiating and patterning pax7 expression in the brain.
Fig. 3.
pax7 pattern in the brain is positively regulated by FGF, Wnt and retinoid signals. (A–D) FGF8 gain of function expands pax7 expression into forebrain, while FGF8 depletion leads to lack of pax7 at induction stage (stage18, A, B) and in later development (stage 22, C, D). (E–F) Wnt7b over-expression and beta-catenin morpholino injections demonstrate the requirement for Wnt signals in pax7 patterning. (G) Similarly, blocking retinoic acid signaling resulted in defective pax7 expression.
We have then asked at what time of neural patterning were FGF signals required to establish a proper pax7 pattern in the brain: either as part of the general neural anterior–posterior patterning or more specifically during isthmus formation. We blocked FGF signaling using the FGF receptor inhibitor SU5402 during three periods of development, in three groups of sibling embryos: group 1 was treated from late blastula to early tadpole stage (from stages 8 to 22), group 2 during neural induction only (from stages 8 to 12) and group 3 during neural patterning (from stages 12 to 22) (Delaune et al., 2005). The first two treatments inhibit neural induction and mesoderm formation as noted by the reduced sox2 expression (100% reduction, n=21; DMSO treated sibling are 92% normal, n=24) and the severe gastrulation defects observed in these embryos. The third treatment avoids perturbing general mesoderm and neural induction as shown by sox2 expression analysis: 96% of the embryos show normal sox2 staining (n=23) while 100% of DMSO treated siblings are normal (n=22). However, this treatment alters posteriorization of the neural plate as marked by decreased hoxb9 expression (Supplemental Fig. 3, n=23, 61% decreased expression; DMSO treated siblings are 100% normal (n=22); Fletcher and Harland, 2008; Roche et al., 2009). In all cases, pax7 expression was analyzed at stage 22. For all three periods of treatment, we observed pax7 expression in the brain in the majority of the embryos. There was an altered pax7 pattern in the anteriormost part of the nervous system that still forms when embryos are treated early (treatments done between stages 8–12 (group 1) and 8–22 (group 2): n=24, 71% of embryos showed slightly reduced to normal staining, 29% had a loss of staining, compared to DMSO treated siblings (n=23, 100% normal staining) (data not shown). This reduction correlated with the abnormal sox2 expression. There was no significant loss of pax7 expression in group-3 embryos: normal expression was observed in the majority of both in DMSO treated embryos, (n=23, 100% normal) and SU-treated embryos (n=35, 60% with normal staining and 31% with a moderate decrease in the extent of Pax7 domain and staining intensity (see Supplemental Fig. 3). This result indicates that FGF signaling plays a major role in regulating pax7 expression during early brain patterning but only marginally modulates pax7 pattern at later neurulation stages such as during isthmus formation.
Additionally, FGFs cooperate with Wnt and Retinoic acid signals to pattern the midbrain and hindbrain (Blumberg et al., 1997; McGrew et al., 1997). We have either over-expressed Wnt7b or injected a beta-catenin morpholino to activate or block the Wnt-beta-catenin pathway respectively. As observed for FGF signals, an increased Wnt signaling resulted in an enlarged pax7 expression domain (n=20, 65% increase, Fig. 3E), with a posterior border shift, similar to what is observed for pax3 in sibling embryos (n=13, 77% increased; not shown, (Monsoro-Burq et al., 2005)). Conversely, pax7 expression was strongly decreased to absent in beta-catenin morpholino-injected embryos (n=35, 77% decrease, Fig. 3F). Retinoic acid signaling was blocked using a dominant-negative form of retinoic acid receptor alpha (dnRARα, Blumberg et al., 1997), resulting in decreased pax7 expression in the injected area (n=13, 70% decrease, Fig. 3G). Together, these results show that the three main posterior neural patterning pathways, namely FGF, Wnt and RA, are active in defining the pax7-positive domain; that they act during early neurulation rather than mid–hindbrain boundary formation, and that the differences observed between pax3 and pax7 domains in X. laevis are not due to a loss of competence to respond to these patterning cues in pax7 gene regulatory elements.
Pax3 regulates pax7 expression in the brain
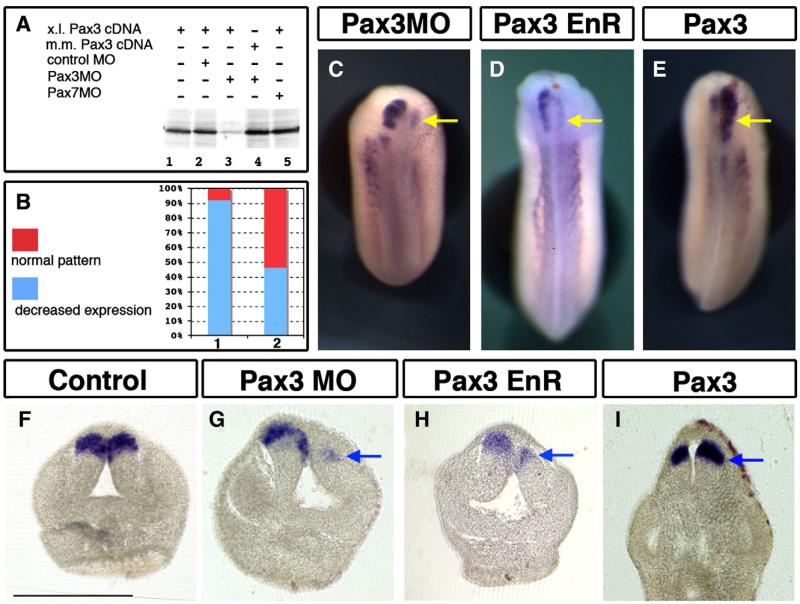
Focusing on neural patterning, we next asked if Pax3, which is expressed earlier and in a larger domain that pax7 in neural tissue, was involved in pax7 patterning. In order to analyze the regulation of pax7 expression by Pax3, we used an antisense morpholino-mediated depletion of Pax3 in conjunction with a rescue experiment (Monsoro-Burq et al., 2005). Using an in vitro reticulocyte lysate transcription-coupled-to-translation assay, we have first verified that Pax3MO efficiently and specifically blocked the translation of X. laevis Pax3 cDNA (Fig. 4A, lanes 1–3), while it affected neither the translation of mouse Pax3 cDNA (Fig. 4A, lane 4), nor that of the Pax7 cDNA (see Supplemental Fig. 2C and below Fig. 6A, lane 5).
Fig. 4.
Pax3 regulates pax7 expression and alar plate patterning in the brain. (A) Pax3 depletion using an antisense morpholino (Pax3MO) specifically prevents Xenopus laevis pax3 translation in vitro (lane 3). Pax7MO and a control MO have no effect on Xenopus laevis pax3 translation while Mus musculus pax3 is unaffected by Pax3MO. (B) Following Pax3 depletion in vivo (bar 1), pax7 expression is severely reduced, Mus musculus Pax3 mRNA efficiently rescues the decrease in pax7 expression (bar 2). (C) Unilateral depletion of Pax3 results in loss in pax7 expression; (D) a similar effect is observed after Pax3-EnR over-expression. (E) Gain in Pax3 activity increases and expands pax7 expression. (F–I) Transverse sections show that the loss in pax7 (C, D, G, H) is accompanied by reduced alar plates development, while Pax3 increase results in expanded pax7-expressing alar plates (I). Bar=500 μm.
Fig. 6.
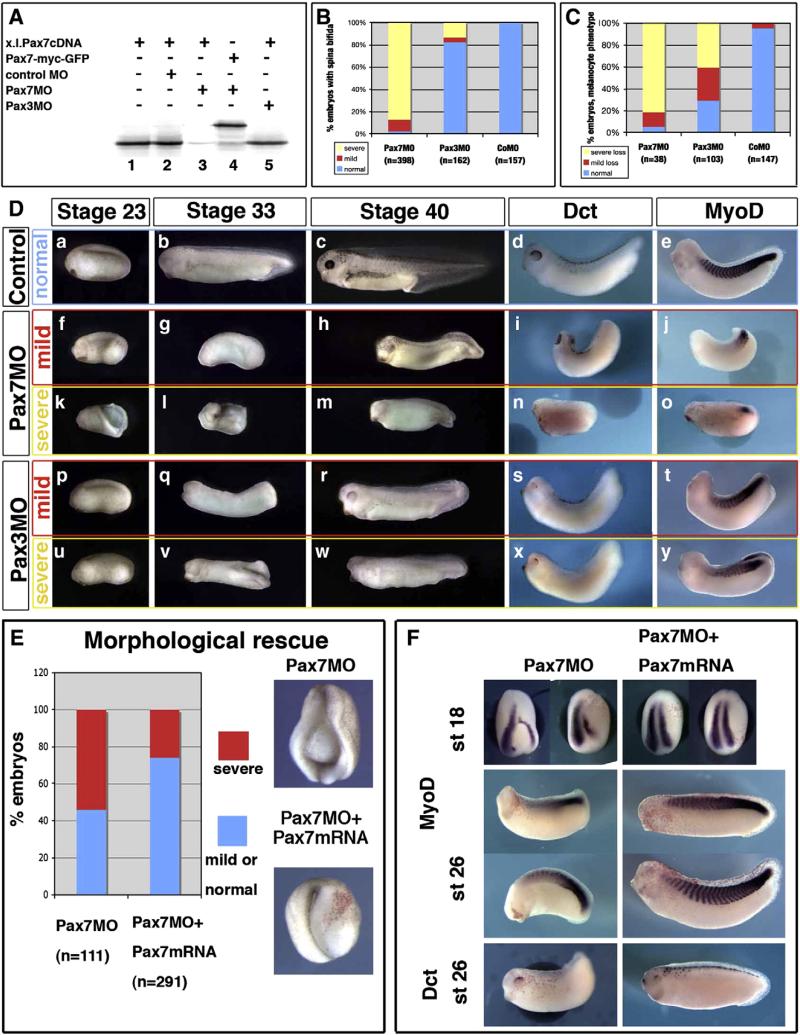
Pax7 morphants are affected in earlier stages than Pax3 morphants, although both display mesoderm and neural crest defects. (A) Pax7MO blocks in vitro translation of pax7 (lane 3) but neither that of a pax7-gfp fusion lacking the MO-binding sequence (lane 4), nor of pax3 (5). (B) Pax7 morphants display early and severe elongation defects shortly after gastrulation (yellow), while a few of them exhibit milder phenotypes allowing us to follow their development further (red). In contrast, Pax3 morphants are rather normal until the end of neurulation except for posterior spina bifida in the more severely affected ones (yellow). (C) Melanocyte development was analyzed in later stage embryos, among the mild phenotype for Pax7 morphants, and in Pax3 morphants. Severe loss refers to the lack of dct positive or pigmented cells, while “mild loss” refers to decreased melanocyte number associated to lack of melanocyte migration. (D) Sibling control embryos (a–e), Pax7 morphants with mild (f–j) or severe (k–o) phenotype, Pax3 morphants with mild (p–t) or severe (u–y) phenotype were analyzed at the end of neurulation (stage 23), tailbud stage 33 or swimming tadpole stage 45, and stained for dct and myod at stage 33. They display prominent head, brain, mesoderm and melanocytes defects. (E)The Pax7MO phenotype is rescued by co-injections with Pax7-myc mRNA, insensitive to the morpholino. See text for details. (F) Both myoD and dct expression are rescued by pax7 mRNA injection into Pax7 morphants (see text for details).
In vivo, pax7 expression is strongly decreased by Pax3 knockdown (20–30 ng) in the brain, analyzed at stages 22–25, while the contralateral side remains unaffected (81%, n=106) (Fig. 4C). This inhibitory effect is rescued when a moderate amount of mouse pax3 mRNA (100 pg), insensitive to the Pax3MO, used is co-injected with Pax3MO (30 ng): in experimental series where Pax3MO-injected embryos showed a 90% loss of pax7 (n=25) and expression in only 10% of the embryos, the sibling group, co-injected with Pax3MO and mouse pax3 mRNA, displayed restored pax7 expression in 46% of the embryos while 54% still showed decreased pax7 expression (n=39) (Fig. 4B). We then analyzed the phenotype of Pax3-depleted embryos on transverse sections to evaluate the extent of loss of pax7 expression in the dorsal–ventral neural axis. Compared to the normal situation (Fig. 4F) and to the contralateral side (Fig. 4G), the size of the dorsal brain domain is reduced and pax7 expression strongly impaired (Fig. 4G). A very similar phenotype was obtained by injecting a repressor form of Pax3 (Pax3-engrailed repressor domain fusion; Ridgeway and Skerjanc, 2001): pax7 expression was decreased both in wholemount views and on transverse sections, accompanied by a reduced size of the alar plate on the injected side (n=21, 95% decrease, Figs. 4D, H). The loss of pax7 expression seems independent from a potential mechanical or indirect effect due to the spina bifida phenotype caused by Pax3MO or Pax3EnR since some embryos with severe spina bifida still displayed Pax7 staining on the non-injected side, while the expression was lost in the injected cells (not shown).
We next examined the effect of increasing Pax3 activity in vivo by injection of Xenopus or mouse mRNA around the prospective neural plate (250 pg into one dorsal animal blastomere at 4-cell stage). A clear expansion of pax7 expression is observed in about half of the injected embryos (54% expansion, n=94 Fig. 4E), which is rather modest when compared to sibling embryos analyzed for Snail2 expression as a positive control (69% expansion, n=54, not shown). A similar effect was obtained using mouse Pax3 mRNA (expansion in 8 embryos out of 13, not shown). On transverse sections, it appeared that the expansion is due to an enlargement of the alar plates, accompanied by the expansion of pax7 expression dorsally (Fig. 4I). The sum of these observations suggest that Pax3 regulates general alar plate patterning, acts as an activator in pax7 regulation and is required for pax7 induction and patterning in the brain.
Pax7 is expressed later, in brain, myotome, lymph heart and melanocytes
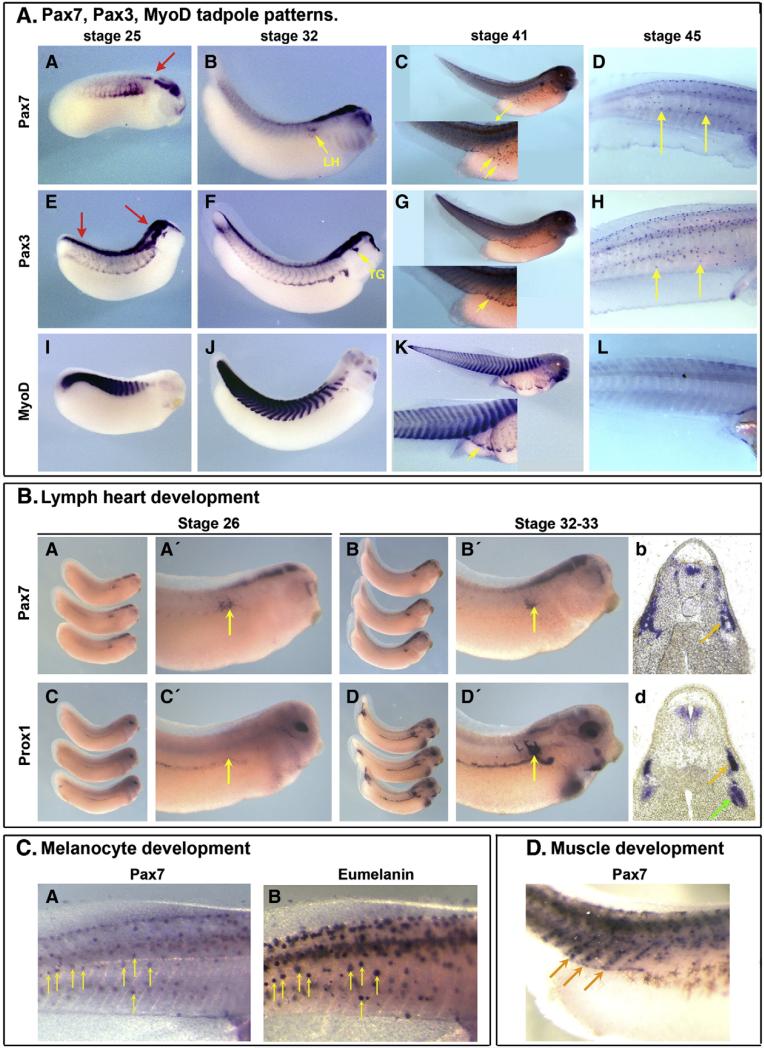
In tadpole stage embryos, pax7 is expressed in four main locations: the brain, the superficial part of the somite, the lymph heart and the melanocytes. As observed at earlier stage, at stage 25, pax7 is restricted to midbrain, hindbrain and the most anterior part of the spinal cord, contrasting to pax3 expression that extends along the entire neuraxis (Fig. 5A/A,E, red arrows). In the paraxial mesoderm, both pax7 and pax3 are found with patterns similar to those of stage 22 embryos (Figs. 2 and 5A/A,E, compare to myoD staining in I). At later stages (stage 32 onwards), a similar pax7 brain expression is observed, as well as a faint more posterior spinal cord staining (Fig. 5A/B,F). While pax3 marks the trigeminal ganglia (Fig. 5A/F, arrow), pax7 expression is not observed at this location but is found in the lymph heart (Ny et al., 2005), (Fig. 5A/B, arrow). At this stage, pax7 expression is no longer detected in the paraxial mesoderm (Fig. 5A/B) while pax3 still strongly labels the hypaxial muscle and intersomitic areas (Fig. 5A/F, compare to myoD in Fig. 5A/J) (Martin and Harland, 2001). We have further documented pax7 expression in the lymph heart by comparing it to lymph heart marker prox1 expression in stage-matched sibling embryos: we found that pax7 is expressed earlier than prox1, but both genes are expressed later (Fig. 5B/A–D). At swimming tadpole stage 41, pax7 is found in a dispersed population of cells in the body wall, while pax3 is found at the edge of the migrating paraxial muscle progenitors (Fig. 5A/C, G, yellow arrows, compare to myoD staining in Fig. 5A/K). Pax7 is detected again in the myotome, in cells that align at the edge of each myotome (Fig. 5D). We propose that these cells may constitute the future satellite cell population described in mature muscles (Chen et al., 2006). At stage 45, pax7 and pax3 are both expressed in the melanocytes (Fig. 5A/D, H). We have analyzed the colocalisation of pax7 expression with eumelanin by comparing pictures of the same embryos, taken either before bleaching or after in situ staining (Fig. 5C). While neither myoD nor prox1 — (used here as bleaching and in situ hybridization control) stain these cells (Fig. 5A/L), there is a striking co-localisation of pax7 staining and melanosomes (Fig. 5C/A,B, arrows).
Fig. 5.
Pax7 expression in tadpoles. A/ At tailbud stage (st. 25–32) pax7 exhibits a dynamic expression in the myotome, which vanishes at late stage, while brain and spinal cord expression remains restricted to hindbrain and anterior spinal cord (A–B). Pax7 is also found in the lymph heart (B, arrow). This contrasts to pax3 expression in the entire length of the spinal cord, in the hypaxial muscle and trigeminal ganglia (E–F, arrows) or to myod expression in the epaxial muscles (I–J). At swimming tadpole stage (st 41–45), pax7 is expressed in scattered cells on the side of the embryo (yellow arrows) and cells that align at the edge of the myotome cells (see D/), while pax3 and myod mark muscle (G, K). Both pax7 and pax3, but not myod, label melanocytes (D, H, L). B/ During lymph heart development, pax7 is detected earlier than the lymph heart marker prox1 (A, A′, C, C′). By stage 33, both pax7 and prox1 expression overlap in the lymph heart (B, B′, D, D′ and transverse sections b, d, yellow arrows), while prox1 is also expressed adjacent to the cardinal vein (green arrow; Ny et al., 2005). C/ Late tadpoles where photographed before bleaching to position the melanocytes (B) and then pax7 was revealed (A). Most melanocytes were labelled by pax7 (yellow arrows). D/ Some pax7-positive cells aligned at the edge of each myotome in stage 41 tadpoles and may constitute the prospective satellite cell population (arrows).
Pax7 loss of function affects brain, muscle and melanocyte development
We have designed a Pax7-specific translation blocking antisense morpholino oligonucleotide that has no complementarity to Pax3 cDNA sequence (Supplemental Fig. 2F). We have checked using in vitro transcription–translation reticulocyte lysate assay, that Pax7MO efficiently blocks Pax7 translation (Fig. 6A, lanes 1–3), but not translation from a construct lacking the MO complementary sequence (Pax7-myc-GFP fusion, Fig. 6A, lane 4) nor from a Pax3 construct (Fig. 4A, lane 5). Pax3MO did not block Pax7 translation either (Fig. 6A, lane 5). Embryos were injected bilaterally with either control, Pax7MO or Pax3MO. Until early-mid-neurula stages (stage 14), embryos injected with either Pax7MO (70 ng) or Pax3MO (70–80 ng) did not exhibit obvious morphological defects compared to sibling embryos (data not shown). This indicates that gastrulation and neurulation initiate normally, consistent with the lack of Pax7 expression at these stages. At late neurula and tadpole stages, three main phenotypes were observed after Pax7MO bilateral injection in vivo: head atrophy, shortened axis with (posterior) spina bifida and melanocyte loss. These phenotypes were compared to that of control and Pax3MO-injected siblings, which also display spina bifida and loss of melanocytes, but less severe head malformation (Fig. 6). The majority of the Pax7MO-injected tadpoles did not close the neural tube and exhibited a shortened axis with mild (brain is closed, 10.3%, n=398, Fig. 6D, f) to severe spina bifida (87.4%, n=398) (Fig. 6D, k). When embryos that exhibit severe spina bifida developed further, they died between stages 24 and 26 (i.e. too early for melanocyte lineage analysis). The embryos with less severe neural defects survived until stages 33–40, provided that the vitelline envelope was manually removed (Fig. 6D, g–j and l–o, n=38): these latter embryos showed severely altered anterior morphology, with reduced head, reduced eyes and few/no melanocytes (severe melanocyte loss, with no lateral migration, 81%, n=38). A similar phenotype was observed when a Pax7-EnR fusion was injected bilaterally (not shown).
At stage 23, Pax3 morphants were fairly normal, although cell death dorsal to the neural tube was observed (not shown), suggesting that unspecified neural crest cells die at the end of neurulation (Monsoro-Burq et al., 2005). These embryos did not hatch autonomously and were devitellinized manually around tailbud stages 22–23. At stages 26–33, Pax3MO-injections resulted in a shorter body axis than normal but more elongated than Pax7 morphants (Fig. 6D, q, v). In addition, head and fin defects were prominent, spina bifida was frequently observed especially in the posterior neural tube (18%, n=162) (Fig. 6D, p–r and u–w). Those features mimic the Splotch mutant phenotype (Auerbach, 1954; Epstein et al., 1991).
We have used embryos displaying the milder phenotype to assess muscle and pigment formation at tadpole stage by ISH. Stage 26–33 Pax7 morphants showed myoD staining only in a reduced posterior area (Fig. 6D, j, o), while Pax3MO injections resulted in a rather normally elongated myoD area along the side of the embryo (Fig. 6D, t, y), although the segmentation pattern was severely altered (Supplemental Fig. 4). Melanocyte differentiation was assessed in stage 26–40 embryos using either dct staining for melanocyte precursors (Fig. 6D) or eumelanin pigment as a marker in lightly pigmented eggs or albino eggs fertilized with sperm from a pigmented male (not shown). Pax7 morphants lacked dct expression (81.6%, n=38, Fig. 6D, h, m and i, n, compare to stage-matched control in Fig. 6D, c, d). Similarly, the number of melanocytes in Pax3MO-injected embryos was either reduced (i.e. some melanocytes are observed dorsal to the neural tube, but very few melanocytes have migrated laterally, 30%, n=103 Fig. 6H) to severely depleted (no melanocyte observed, Fig. 6O, 40%, n=103, compared to stage-matched control in Fig. 6D, c, d). Together, these observations suggest that head and brain development, muscle and neural crest derivative formation are affected by the depletion of Pax7 and Pax3 activity in vivo. However, the phenotypes observed present clear gene-specific features.
To address the specificity of this phenotype, we have rescued the Pax7MO phenotype by co-injecting the Pax7-myc fusion, insensitive to the MO. Embryos injected with Pax7MO exhibit the defects described above on the injected side at the end of neurulation (stage 18, 54% of defective embryos, n=111), but the sibling embryos co-injected with Pax7MO and Pax7-myc mRNA are fairly normal in 74% of cases, n=291) (Fig. 6E). The rescued embryos also displayed rescued expression of myoD and dct (Fig. 6F). To further address the specificity of the observed phenotype, we have injected a morpholino with 5-mismatches compared to Pax7MO (see Supplemental Fig. 2C). In this case, both morphology of the embryos and gene expression were normal (Supplemental Fig. 5). Furthermore, we have designed a splice-blocking morpholino, preventing splicing of the first intron (Supplemental Fig. 2). In this case, we evaluated the level of pax7 knock-down to 60% by semi-quantitative RT-PCR at stage 22, using embryos injected with the Pax7 splice MO in all four blastomeres (Supplemental Fig. 6A). Whole embryo injections resulted in a similar phenotype as described above for Pax7MO (although is a slightly lower proportion of the embryos with most severe phenotypes) and snail2, myod and dct expression were affected similarly (Supplemental Fig. 6).
Pax7 function is essential for neural crest formation
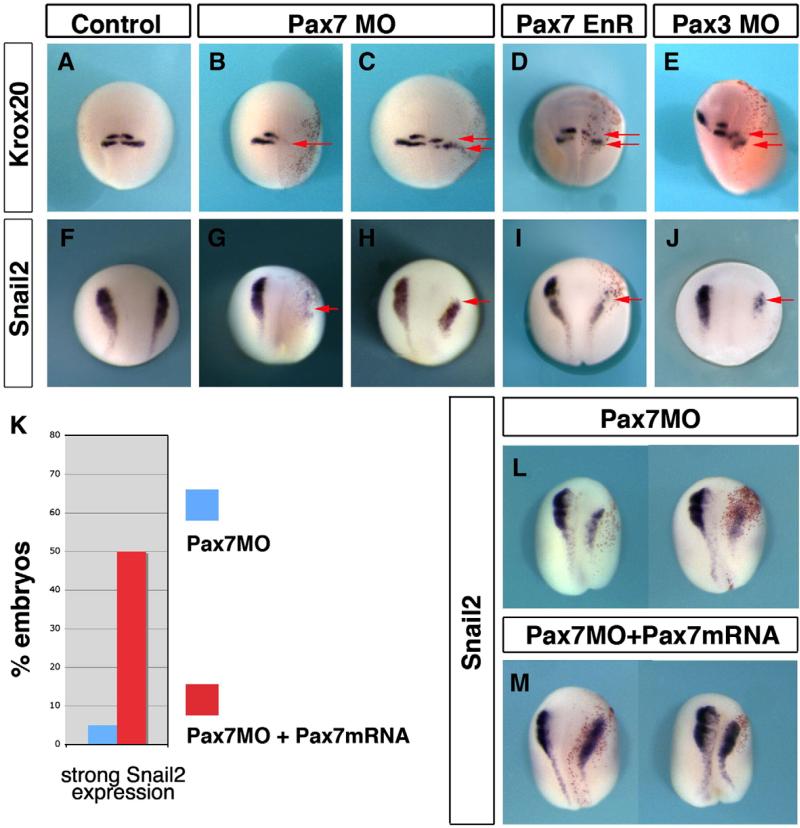
Since morphological alterations appear during neurulation, we examined if Pax7, like Pax3, is important in neural and neural crest development in Xenopus. We have analyzed the effects of Pax7 depletion on krox20 expression, (krox20 labels hindbrain rhombomeres 3 and 5, and neural crest emigrating from rhombomere 5, Fig. 7A) and on snail2 expression (Fig. 7F) in stage 18 neurulae. Krox20 expression was severely altered both in the brain and in the neural crest: rhombomere 3/5 staining was either shifted posteriorly or lost, while rhombomere 5 neural crest was diminished or lost (95%, n=61; Figs. 7B, C). Similarly, snail2 expression was strongly reduced or completely abolished on the injected side (74%, n=43; Figs. 7G, H). Similar phenotypes were observed with Pax7-EnR fusion (70%, n=57 for krox20 and 69.7%, n=76 for snail2; Figs. 7D, I) and Pax3MO (75%, n=45 for krox20 and 76% n=47 for snail2; Figs. 7E, J), suggesting redundant effects of Pax3 and Pax7 in this process.
Fig. 7.
Pax7 and Pax3 are essential for hindbrain and neural crest patterning. The early phenotype of Pax7 morphants was analyzed at stage 18. Control siblings show normal expression of krox20 (A) and snail2 (F). Pax7 morphants exhibit either a severe loss of krox20 (B) and snail2 (G), or a posterior shift of rhombomeres r3 and r5 (C, arrows) accompanied by reduced snail2 expression (H). A similar phenotype is observed in Pax7EnR injected embryos (D, I) or Pax3 morphants (E, J). Pax7MO phenotype is rescued by injection of Pax7-myc mRNA), insensitive to the morpholino (Fig. 6): snail2 expression is restored (L shows two morphants and (M) two siblings injected with MO and the rescue construct).
To assess the specificity of Pax7 morpholino phenotype, we have performed a rescue experiment, using a pax7-GFP-myc fusion. Sibling embryos were injected unilaterally with either Pax7MO, or co-injected by Pax7MO and the pax7-GFP-myc mRNA (125 pg). Snail2 rescue was observed. Embryos injected with Pax7MO lost snail2 expression (95% strong decrease, n=20, Fig. 7L). In contrast, co-injections resulted in restored snail2 expression (50% normal, 50% mild decrease, n=18, Fig. 7M).
Thus, we show here that Pax7 function is essential for full neural crest formation. However, as we did not observe pax7 expression at the neural border or in the neural crest progenitors themselves, we have proposed two hypotheses in order to explain how Pax7 may control neural crest formation: Pax7 may be important for central nervous system patterning along the anterior–posterior axis, and thus secondarily influence neural crest formation at the edge of the posterior domain; alternatively, Pax7 may be important for paraxial mesoderm development and thus secondarily in its inductive function in neural crest early development. We have explored these two hypotheses below.
Pax7 controls brain patterning by maintaining midbrain and hindbrain fates
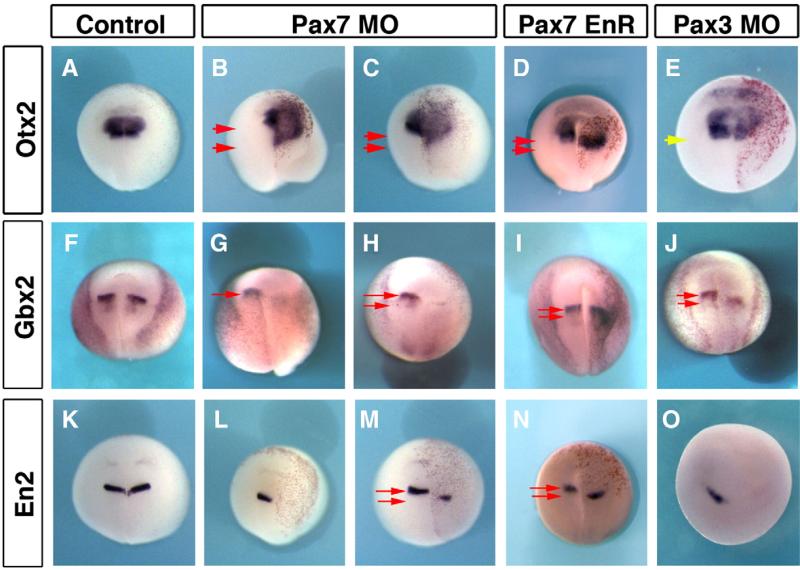
In order to analyze brain patterning, we have looked at four regional markers: otx2 which labels the forebrain and midbrain (Fig. 8A); gbx2 and engrailed2 (en2) which mark the mid–hindbrain boundary (Figs. 8F, K); and krox20 for the hindbrain (Fig. 7). At early neurula stage (stages 12–13), otx2 and gbx2 expression were normal after Pax7 unilateral depletion (not shown), suggesting that the initial onset of neural anterior–posterior patterning occurs normally (Tour et al., 2002a), and in agreement with the late onset of pax7 expression (Fig. 1). At mid-late neurula stages, unilateral Pax7MO injections resulted in otx2 expansion in more posterior areas (69.2%, n=65; Figs. 8B–D), while gbx2, en2 and krox20 were either shifted posteriorly or lost (respectively 89.3%, n=28 for gbx2, 69%, n=52 for en2 and 95%, n=61 for krox20; Figs. 7B, C and 8G–I, L–N). Pax7EnR mRNA injections resulted in a similar phenotype: otx2 expansion in 84.6% of the embryos (n=104; Fig. 8D) and diminished en2 (69%, n=52; Fig. 8N) and krox20 (70%, n=57; Fig. 7D). In this case, gbx2 expression was only slightly shifted posteriorly (Fig. 8I). In contrast to Pax7 depletion phenotype, Pax3MO did not perturb otx2 pattern (symmetrical expression in 72% of the injected embryos, n=50). However, Pax3 depletion had similar posterior shifting or loss on gbx2 (75%, n=28; Fig. 8J), en2 (73%, n=11; Fig. 8O) and krox20 (75%, n=45; Fig. 7E). In conclusion, these experiments uncover a novel role for Pax7 in maintaining the mid–hindbrain boundary (prospective isthmus) and hindbrain fates and preventing the posterior expansion of forebrain and midbrain fates during early brain patterning (at stage of neural closure). In relationship to neural crest patterning, this altered midbrain and hindbrain patterning could secondarily result into defective neural crest induction.
Fig. 8.
Pax7 and Pax3 regulate mid–hindbrain boundary maintenance. Pax7 depletion leads to forebrain–midbrain expansion as seen by otx2 posterior expansion (B, C). This is accompanied by loss or posterior shift of the mid–hindbrain boundary (Gbx2, G, H) and En2 (L, M). A similar phenotype is observed after PaxEnR (D, I, N). Pax3 morphants do not exhibit expanded otx2 domain (E, yellow arrow indicates similar posterior boundaries on each side), but they also display defective or shifted mid–hindbrain boundary (J, O). Red arrows indicate the extent of shift between the posterior boundary on control non-injected (left) side, and the shifted boundary on injected (right) side.
Pax7 is needed for neural crest induction by the paraxial mesoderm
Morphological and myoD analysis of Pax7 morphants suggests that Pax7 loss has profound effects on paraxial mesoderm development (Fig. 6). Paraxial mesoderm signaling activity is essential during neural crest induction (Bonstein et al., 1998; Monsoro-Burq et al., 2003). Defective paraxial mesoderm, after Pax7 depletion, could impair neural crest induction in the overlying ectoderm, and thus suggest how Pax7 depletion may affect neural crest induction. To address this issue, we have used heterochronic recombinants, using ectoderm from the blastocoel roof from a stage 9 blastula embryo, recombined with the dorsal–lateral marginal zone (prospective paraxial mesoderm) of a stage 10.25 early gastrula. This assay results in potent snail2 and other neural crest marker induction in the ectoderm, as well as melanocyte differentiation later on (Bonstein et al., 1998; Monsoro-Burq et al., 2003) (Figs. 9A, D; G, lane5). Ectoderm (Figs. 9B; G, lane 3) or paraxial mesoderm (Figs. 9C; G, lane 4) explants grown in isolation, do not express snail2. When the ectoderm was dissected from Pax3MO injected embryos, snail2 induction was strongly impaired (Fig. 9G, lane 6) while Pax7MO in the ectoderm did not prevent snail2 induction (Fig. 9G, lane 7). In sharp contrast, depletion of Pax3 in the paraxial mesoderm did not alter snail2 induction, despite defective muscle actin specification (Figs. 9F, G, lane 8). Moreover, Pax7 function in the paraxial mesoderm explant is essential for snail2 induction in the ectoderm, as well as for proper muscle actin expression (Figs. 9E; G, lane 9; F).
Fig. 9.
Pax7 is essential for the neural crest inducing activity of the paraxial mesoderm, while Pax3 acts in the ectoderm. (A) We have recombined the stage 9 blastocoel roof ectoderm to stage 10.25 prospective paraxial mesoderm (dorsal–lateral marginal zone), a classical assay for neural crest induction in the ectoderm. Ectoderm and DLMZ were dissected from either control uninjected or Pax3 or Pax7 morphants (see color code). Ectoderm (B) or DLMZ (C) grown alone until stage 18 do not express snail2, while the recombined explants (D) do. When the DLMZ comes from Pax7 morphant (E), snail2 induction is strongly impeded, while Pax3 depleted DLMZ do not perturb snail2 induction (F). RT-PCR analysis (G) further shows that ectoderm alone (lane 3) does not express snail2, myoD or muscle actin (MA), DLMZ alone expresses only myoD and MA (lane 4) while the recombinant expresses both (lane 5). Snail2 induction is impaired if the ectoderm is depleted for Pax3 (lane 6) but not for Pax7 (lane 7). In contrast, snail2 induction is normal if DLMZ comes from Pax3 morphants (lane 8) but is impaired for Pax7 depleted DLMZ (lane 9). In both Pax3- and Pax7-depleted DLMZ, myoD and MA expression are decreased while vent1 is abnormally upregulated.
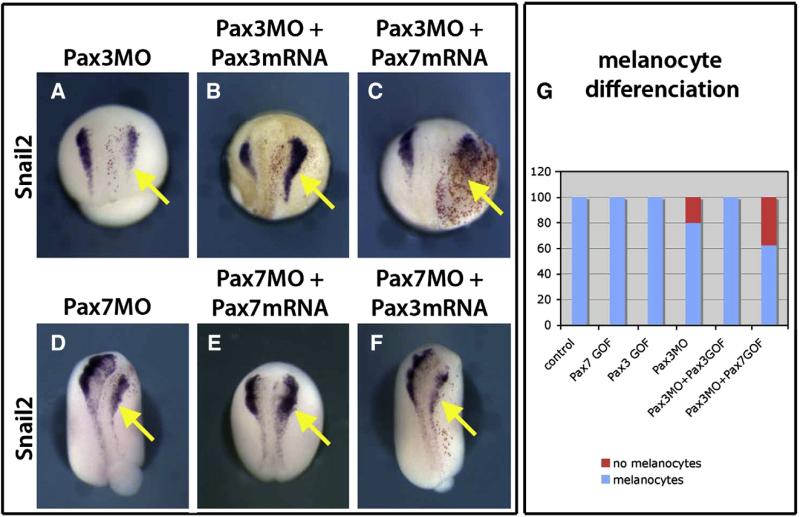
The results above suggested that Pax3 and Pax7 act in different tissues during neural crest induction. This does not rule out that the two proteins could have similar functions. To address if they display redundant functions, we have attempted to rescue Pax3 and Pax7 knock-down by Pax7 and Pax3 gain of function respectively. In both situations, while the homologous gain of function (Pax3 for Pax3MO and Pax7 for Pax7MO) restored snail2 expression, neither Pax3 nor Pax7 were able to compensate for the knock-down of Pax7 or Pax3 respectively (Fig. 10). This was also true when a later stage was considered: Pax7 did not rescue the loss of melanocytes observed in the Pax3 morphants. All these data suggest that Pax3 and Pax7 exert distinct functions, in distinct tissues in early and later neural crest development in Xenopus.
Fig. 10.
Pax3 and Pax7 do not display redundant functions in neural crest induction and development. Pax3 morphant phenotype (A) is rescued by gain of function for Pax3 (B) but not Pax7 (C). At a later stage, Pax7 does not restore melanocyte development in Pax3 morphants either (G). Conversely, Pax3 is not sufficient to rescue Pax7 depletion (D, F), in contrast to Pax7 gain of function (E). These data indicate that the two proteins do not display similar roles in neural crest induction and development. Injected side is on the right (yellow arrow).
Finally, as we observed that myod expression was defective in Pax7 morphants (Fig. 6), we asked if the effect of Pax7 in the paraxial mesoderm was due to altered mesoderm patterning. We examined the paraxial mesoderm markers myoD and muscle actin and the ventral mesoderm marker vent1 in the recombinants (Fig. 9G). We found that both in Pax3 and Pax7-depleted dorsal–lateral marginal zone mesoderm, paraxial patterning is defective and that ventral marker vent1 is higher than in controls at neurula stage. However, we did not find a molecular difference between Pax3 and Pax7 morphant mesoderm that could account for their distinct neural crest inducing ability.
In conclusion, these results show a dramatic difference in the mode of activity of Pax7 and Pax3 in neural crest induction, Pax7 being essential for the inductive signaling from the paraxial mesoderm, while Pax3 is needed in the ectoderm.
Discussion
In this work, we address pax7 pattern regulation in the brain of X. laevis embryos, and Pax7 function in neural and neural crest early development. We provide a parallel analysis of Pax3 and Pax7 function, using a specific antisense morpholino strategy, which uncovers both common and distinct functions for these two paralogous genes. In particular, both genes are important for neural crest induction, but they act by two strikingly different mechanisms.
Pax7 is expressed in a spatially restricted pattern in X. laevis central nervous system, and is activated by posteriorizing signals and Pax3
Pax3 and Pax7 have diverged early in vertebrate evolution; their sequences can be clearly grouped on a phylogenetic tree, and specific primers, probes and morpholinos can be designed (Fig. 1, Supplemental Figs. 1 and 2). Their respective expression in the alar plates of the central nervous system, in the migrating neural crest and in the paraxial mesoderm varies according to the different species described, suggesting that the respective functions within the Pax3/7 subfamily could vary in different species. We provide here the first precise comparison of Pax3 and Pax7 expression in X. laevis.
In X. laevis, pax7 and pax3 display clearly distinct expression patterns and pax7 pattern differs in several respects from pax7 expression in other vertebrates. During early-mid-neurulation, only pax3 is detected, either by RT-PCR using specific primers, or by in situ hybridization (Fig. 1). Pax3 is present in the prospective hatching gland, the lateral aspect (alar plates) of the neural plate comprising the prospective neural crest domain. In contrast, the pax7 expression appears in the paraxial mesoderm and in the midbrain and hindbrain at later neurulation stages, which differs from observations of Pax7 expression in the chick gastrula stages (Basch et al., 2006). Pax7 is neither detected in the prospective neural crest domain nor in the migrating neural crest. This later onset of pax7, after neural tube closure, is also observed in lamprey, zebrafish, chick and mouse neurulae (Mansouri et al., 1996; Matsunaga et al., 2001; McCauley and Bronner-Fraser, 2002; Minchin and Hughes, 2008). However, pax7 expression in a small subpopulation of migrating cranial neural crest cells is seen in zebrafish, chick and mouse embryos (Mansouri et al., 1996; Matsunaga et al., 2001; Minchin and Hughes, 2008).
These two initial expression patterns in the CNS resolve into two distinct domains, pax3 being detected along the entire anterior– posterior axis, while pax7 is expressed locally in the midbrain, hindbrain and anteriormost spinal cord, but not in rest of the spinal cord (Fig. 2). This strikingly differs from expression of both Pax3 and Pax7 all along the neuraxis in chick and mouse, but is similar to the described pattern in shark, zebrafish, and salmon (Borycki et al., 1999; Freitas et al., 2006; Goulding et al., 1993a; Minchin and Hughes, 2008; O'Neill et al., 2007; Sibthorpe et al., 2006). Additional differences include that pax7 does not label the trigeminal ganglia as pax3 does (Baker et al., 2002) and pax7 – but not pax3 – is expressed in the lymph heart (Ny et al., 2005) (Fig. 5). Both pax3 and pax7 are expressed in melanocytes in Xenopus (Fig. 5) whereas they differentially label both melanophores and xanthophores in zebrafish (Minchin and Hughes, 2008).
In contrast, the expression of pax3 and pax7 in the paraxial mesoderm, respectively in the hypaxial and the superficial myotome (Figs. 2 and 5), is in agreement with expression in other vertebrates (Borycki et al., 1999; Goulding et al., 1993b). In mouse embryos, distinct enhancers control the distinct regions of pax7 expression in brain and neural crest derivatives (Lang et al., 2003). Our expression pattern analysis in Xenopus suggests that evolution has acted differentially on these enhancers.
We have explored the possibility that the differences in pax7 expression compared to other species, in particular in the spinal cord, could be due to different response to the posteriorizing signals that pattern the neural plate, namely FGF, Wnt and retinoid signaling. We have found that pax7 expression is modulated by these three pathways, similarly to other genes expressed in the posterior neural cells, such as pax3, hoxb9 or snail2 (Fig. 3 and data not shown). The restricted pattern observed for pax7 could nonetheless depend on lower sensitivity to endogenous signaling levels rather than qualitatively different response. Additionally, we did not observe a significant role of FGF signaling at the time of isthmus formation (Supplemental Fig. 3), suggesting that general early anterior–posterior patterning broadly controls pax7 among other neural restricted markers.
In Splotch mutant mice, pax7 expression in the somites and spinal cord is upregulated (Borycki et al., 1999). Here we show that Pax3, which expression domain comprises that of pax7, positively controls pax7 expression in the brain, in parallel to general development of the alar plates (Fig. 4). We did not observe broad ectopic expression in the spinal cord after Pax3 misexpression (Fig. 4), suggesting that regulation by Pax3 is active only in the brain and anterior spinal cord, i.e. around pax7 normal domain, compared to the mouse situation.
Although not expressed in neural crest progenitors, Pax7 is essential for brain, neural crest and myotome patterning
Pax3 and Pax7 play an essential role in neural crest induction in Xenopus and chick embryos respectively (Basch et al., 2006; Monsoro-Burq et al., 2005; Sato et al., 2005). Moreover, Pax7 and Pax3 are thought to display redundant activities in the mouse neural crest, since the replacement of pax7 in the pax3 locus suffices to rescue the neural/neural crest phenotype, while the paraxial mesoderm phenotype remains severe (Relaix et al., 2004). Here, we have explored the possibility that Pax7 displays a similar function to Pax3 in Xenopus neurulae. Surprisingly, pax7 was not detected in the neural fold at the time of neural crest induction (late gastrula, early neurula) when pax3 is abundantly expressed (Fig. 1). At later stages, neither pax3 nor pax7 transcripts are detected in the migrating neural crest (Fig. 2). This expression contrasts with the pattern in chick and mouse embryos (Basch et al., 2006; Mansouri et al., 1996). Later on, however, both pax3 and pax7 are expressed in the melanocytes (Fig. 5) as described in several other species (Lacosta et al., 2005; Minchin and Hughes, 2008).
However, rather surprisingly, although we did not find pax7 expression in the neural crest progenitors, our knock-down experiments show that Pax7 depletion dramatically affects embryo elongation, brain, and neural crest development. Dct and myoD expression analysis confirmed that myotome and pigment formation were severely affected, the Pax7 phenotypes being even more dramatic than that of Pax3 depletion, and rescued by adding back Pax7 transcripts (Fig. 6). Additionally, neural crest and brain induction and early patterning are strongly affected (Figs. 7 and 8). This is in contrast to the Pax7 mouse mutants, or with zebrafish morphants, which only display a mild and late phenotype (Mansouri et al., 1996; Minchin and Hughes, 2008). In mouse, pax3 and pax7 patterns overlap largely and Pax3 and Pax7 exert redundant functions; in zebrafish, pax7 has a late onset after initial neural and mesoderm patterning. We have tested possible functional redundancy between Pax3 and Pax7 in Xenopus, by attempting to rescue Pax3 and Pax7 knock-downs by Pax7 and Pax3 respectively (Fig. 10): Pax3 did not rescue snail2 expression in Pax7 morphants, nor did Pax7 rescue snail2 expression or melanocyte differentiation in Pax3 morphants. Our results, compared to those in the other species, strongly suggest a re-distribution of functions between Pax3 and Pax7 in vertebrates. Such gene function reshuffling as has also been described for Snail family members (Sefton et al., 1998).
Pax7 participates in neural crest induction via its role in brain and paraxial mesoderm patterning
We have analyzed Pax3 and Pax7 respective roles in early brain patterning, at times of isthmus formation. We show that both genes are essential activators to allow the maintenance of the mid– hindbrain boundary (Gbx2, En2) and hindbrain (Krox20) patterning, and that Pax7 specifically prevents ectopic posterior expansion of otx2. Otx2 specifies forebrain–midbrain fates (Rhinn et al., 1999; Tour et al., 2002b). Otx2 and gbx2 domains are initially set up shortly after the start of neurulation and the two factors exert reciprocal negative regulation that defines the border between their respective domains, this border being the mid–hindbrain boundary (Acampora et al., 2001; Tour et al., 2002a; Ye et al., 2001). The onset of otx2 and gbx2 domains is initiated normally in presence of Pax7MO (stages 12–14), but the maintenance of this boundary is perturbed (en2) as well as hindbrain patterning (krox20) and cranial neural crest formation (krox20, snail2) (Figs. 7 and 8). Pax3 depletion has a similar outcome on hindbrain formation, although Pax3 does not seem to restrict otx2 expression (Fig. 8), in agreement with pax3 expression pattern in the forebrain (Figs. 1 and 2). Our results from depletion analysis correlate with the phenotypes obtained by gain of function in chicken embryos: Pax3/7 electroporation promotes ectopic anterior hindbrain and optic tectum development in the diencephalon (Matsunaga et al., 2001). Hence we show here that Pax3 and Pax7 cooperate to promote early hindbrain development, a major source of cranial neural crest progenitors (Creuzet et al., 2005).
In addition to interactions within the ectoderm–neurectoderm layer, neural crest induction requires signals from paraxial mesoderm (Bonstein et al., 1998; Monsoro-Burq et al., 2003). We observe here severe defects in mesoderm formation and axis elongation in the Pax7 morphants, suggesting that the neural crest inducing function of paraxial mesoderm might be altered as well (Fig. 6). By using an explant recombination strategy, we show that Pax3 and Pax7 participate in neural crest induction by two distinct mechanisms: Pax3 activity is essential in the ectoderm, confirming previous work on Pax3 role in Xenopus neural crest induction (Monsoro-Burq et al., 2005; Sato et al., 2005); while Pax7 acts in the paraxial mesoderm, which in turn promotes induction in the ectoderm (Fig. 9). Given the relatively late onset of Pax7 expression, compared to neural crest induction, our data also suggest that Pax7-mediated paraxial mesoderm induction participates in maintaining or amplifying the initial Pax3-mediated neural crest induction in the ectoderm. Thus, the two genes cooperate in neural crest induction, but by two distinct strategies (Figs. 9 and 10). In addition, the roles of Pax3 and Pax7 in the ectoderm have been exchanged between species, when Xenopus and chick are compared (Basch et al., 2006). Our observations also show that Pax3 and Pax7 do not play redundant roles in the paraxial mesoderm (Fig. 6 and Supplemental Fig. 3), similar to the mouse situation (Relaix et al., 2004), but here, their relative importance in the mesoderm seems to be swapped since the Pax7 depletion has earlier and more profound effects than Pax3 loss, which is opposite to the mouse mutant (Mansouri et al., 1996; Relaix et al., 2004).
In conclusion, our study analyzes how the functions of two closely related Pax3 and Pax7 paralogs have been distributed in the amphibian X. laevis, during neural and neural crest induction. We use their strikingly distinct expression patterns, in the brain, the spinal cord and in the paraxial mesoderm of Xenopus embryos to experimentally explore their specific function in each of these tissues, in the absence of redundant activity of the other paralog. We show that Pax3 and Pax7 cooperate in mid–hindbrain boundary formation, by promoting hindbrain fates, and that Pax7 specifically restricts otx2 expression. Additionally, Pax3 and Pax7 cooperate to trigger neural crest induction, by acting in the ectoderm and in the mesoderm respectively.
Supplementary Material
Acknowledgments
The authors are grateful to Dr Clare Baker for her critical reading of the manuscript, to Dr J. Slack for his generous gift of Pax7-EnR clone, and to the Monsoro-Burq laboratory members for their comments and support. This work was initiated in RMH's laboratory with funding from NIH GM42341. This work was funded by grants from the CNRS (ATIP Program), Ligue contre le Cancer, Association pour la Recherche contre le Cancer (ARC), and Fondation de France to A.H. M.-B. S. M. was funded by the Institut Curie. D. R. was funded by Fondation de France, Region Ile de France and Institut Curie.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ydbio.2010.01.022.
References
- Acampora D, Gulisano M, Broccoli V, Simeone A. Otx genes in brain morphogenesis. Prog. Neurobiol. 2001;64:69–95. doi: 10.1016/s0301-0082(00)00042-3. [DOI] [PubMed] [Google Scholar]
- Auerbach R. Analysis of the developmental effects of a lethal mutation in the house mouse. J. Exp. Zool. 1954;127:305–329. [Google Scholar]
- Baker CV, Stark MR, Bronner-Fraser M. Pax3-expressing trigeminal placode cells can crest sites but are committed to a cutaneous sensory. Dev. Biol. 2002;249:219–236. doi: 10.1006/dbio.2002.0767. [DOI] [PubMed] [Google Scholar]
- Basch ML, Bronner-Fraser M, Garcia-Castro MI. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature. 2006;441:218–222. doi: 10.1038/nature04684. [DOI] [PubMed] [Google Scholar]
- Blumberg B, Bolado J, Jr., Moreno TA, Kintner C, Evans RM, Papalopulu N. An essential role for retinoid signaling in anteroposterior neural patterning. Development. 1997;124:373–379. doi: 10.1242/dev.124.2.373. [DOI] [PubMed] [Google Scholar]
- Bonstein L, Elias S, Frank D. Paraxial-fated mesoderm is required for neural crest induction in Xenopus embryos. Dev. Biol. 1998;193:156–168. doi: 10.1006/dbio.1997.8795. [DOI] [PubMed] [Google Scholar]
- Borycki AG, Li J, Jin F, Emerson CP, Epstein JA. Pax3 functions in cell survival and in pax7 regulation. Development. 1999;126:1665–1674. doi: 10.1242/dev.126.8.1665. [DOI] [PubMed] [Google Scholar]
- Bradley LC, Snape A, Bhatt S, Wilkinson DG. The structure and expression of the Xenopus Krox-20 gene: conserved and divergent patterns of expression in rhombomeres and neural crest. Mech. Dev. 1993;40:73–84. doi: 10.1016/0925-4773(93)90089-g. [DOI] [PubMed] [Google Scholar]
- Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr. Opin. Genet. Dev. 2006;16:525–532. doi: 10.1016/j.gde.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Chalepakis G, Goulding M, Read A, Strachan T, Gruss P. Molecular basis of splotch and Waardenburg Pax-3. Proc. Natl. Acad. Sci. USA. 1994a;91:3685–3689. doi: 10.1073/pnas.91.9.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalepakis G, Jones FS, Edelman GM, Gruss P. Pax-3 contains domains for transcription activation and transcription inhibition. Proc. Natl. Acad. Sci. USA. 1994b;91:12745–12749. doi: 10.1073/pnas.91.26.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Hemmati-Brivanlou A. Neural crest induction by Xwnt7B in Xenopus. Dev. Biol. 1998;194:129–134. doi: 10.1006/dbio.1997.8820. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lin G, Slack JM. Control of muscle regeneration in the Xenopus tadpole tail by Pax7. Development. 2006;133:2303–2313. doi: 10.1242/dev.02397. [DOI] [PubMed] [Google Scholar]
- Creuzet S, Couly G, Le Douarin NM. Patterning the neural crest derivatives during development of the vertebrate head: insights from avian studies. J. Anat. 2005;207:447–459. doi: 10.1111/j.1469-7580.2005.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. [DOI] [PubMed] [Google Scholar]
- Epstein DJ, Vekemans M, Gros P. Splotch (Sp2H), a mutation affecting development of the mouse neural tube, shows a deletion within the paired homeodomain of Pax-3. Cell. 1991;67:767–774. doi: 10.1016/0092-8674(91)90071-6. [DOI] [PubMed] [Google Scholar]
- Fletcher RB, Harland RM. The role of FGF signaling in the establishment and maintenance of mesodermal gene expression in Xenopus. Dev. Dyn. 2008;237:1243–1254. doi: 10.1002/dvdy.21517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher RB, Baker JC, Harland RM. FGF8 spliceforms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development. 2006;133:1703–1714. doi: 10.1242/dev.02342. [DOI] [PubMed] [Google Scholar]
- Franz T, Kothary R. Characterization of the neural crest defect in Splotch (Sp1H) mutant mice using a lacZ transgene. Brain Res. Dev. Brain Res. 1993;72:99–105. doi: 10.1016/0165-3806(93)90163-5. [DOI] [PubMed] [Google Scholar]
- Freitas R, Zhang G, Cohn MJ. Evidence that mechanisms of fin development evolved in the midline of early vertebrates. Nature. 2006;442:1033–1037. doi: 10.1038/nature04984. [DOI] [PubMed] [Google Scholar]
- Goulding MD, Chalepakis G, Deutsch U, Erselius JR, Gruss P. Pax-3, a novel murine DNA binding protein expressed neurogenesis. EMBO J. 1991;10:1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding MD, Lumsden A, Gruss P. Signals from the notochord and floor plate regulate expression of two Pax genes in the developing spinal. Development. 1993a;117:1001–1016. doi: 10.1242/dev.117.3.1001. [DOI] [PubMed] [Google Scholar]
- Goulding MD, Lumsden A, Gruss P. Signals from the notochord and floor plate regulate the region-specific expression of two Pax genes in the developing spinal cord. Development. 1993b;117:1001–1016. doi: 10.1242/dev.117.3.1001. [DOI] [PubMed] [Google Scholar]
- Goulding M, Lumsden A, Paquette AJ. Regulation of Pax-3 expression in the dermomyotome and its role in muscle development. Development. 1994a;120:957–971. doi: 10.1242/dev.120.4.957. [DOI] [PubMed] [Google Scholar]
- Goulding M, Lumsden A, Paquette AJ. Regulation of Pax-3 expression in the dermomyotome development. Development. 1994b;120:957–971. doi: 10.1242/dev.120.4.957. [DOI] [PubMed] [Google Scholar]
- Grammer TC, Liu KJ, Mariani FV, Harland RM. Use of large-scale expression cloning screens in the Xenopus laevis tadpole to identify gene function. Dev. Biol. 2000;228:197–210. doi: 10.1006/dbio.2000.9945. [DOI] [PubMed] [Google Scholar]
- Gruss P, Walther C. Pax in development. Cell. 1992;69:719–722. doi: 10.1016/0092-8674(92)90281-g. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, de la Torre JR, Holt C, Harland RM. Cephalic expression and molecular characterization of Xenopus En-2. Development. 1991;111:715–724. doi: 10.1242/dev.111.3.715. [DOI] [PubMed] [Google Scholar]
- Holland LZ, Schubert M, Kozmik Z, Holland ND. AmphiPax3/7, an amphioxus paired box gene: insights into chordate myogenesis, neurogenesis, and the possible evolutionary precursor of definitive vertebrate neural crest. Evol. Dev. 1999;1:153–165. doi: 10.1046/j.1525-142x.1999.99019.x. [DOI] [PubMed] [Google Scholar]
- Hopwood ND, Pluck A, Gurdon JB. MyoD expression in the forming somites is an early response to mesoderm induction in Xenopus embryos. EMBO J. 1989;8:3409–3417. doi: 10.1002/j.1460-2075.1989.tb08505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostes B, Walther C, Gruss P. The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mech. Dev. 1990;33:27–37. doi: 10.1016/0925-4773(90)90132-6. [DOI] [PubMed] [Google Scholar]
- Kintner CR, Brockes JP. Monoclonal antibodies identify blastemal cells derived from dedifferentiating limb regeneration. Nature. 1984;308:67–69. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- Koebernick K, Kashef J, Pieler T, Wedlich D. Xenopus Teashirt1 regulates posterior identity in brain and cranial neural crest. Dev. Biol. 2006;298:312–326. doi: 10.1016/j.ydbio.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Krieg PA, Varnum SM, Wormington WM, Melton DA. The mRNA encoding elongation factor 1-alpha (EF-1 alpha) is a major transcript at the midblastula transition in Xenopus. Dev. Biol. 1989;133:93–100. doi: 10.1016/0012-1606(89)90300-x. [DOI] [PubMed] [Google Scholar]
- Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol. Med. 2008;14:82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Kumasaka M, Sato S, Yajima I, Yamamoto H. Isolation and developmental expression of tyrosinase family genes in Xenopus laevis. Pigment Cell Res. 2003;16:455–462. doi: 10.1034/j.1600-0749.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Lacosta AM, Muniesa P, Ruberte J, Sarasa M, Dominguez L. Novel expression patterns of Pax3/Pax7 in early trunk neural crest and its melanocyte and non-melanocyte lineages in amniote embryos. Pigment Cell Res. 2005;18:243–251. doi: 10.1111/j.1600-0749.2005.00238.x. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, Harland RM. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Lang D, Brown CB, Milewski R, Jiang YQ, Lu MM, Epstein JA. Distinct enhancers regulate neural expression of Pax7. Genomics. 2003;82:553–560. doi: 10.1016/s0888-7543(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Kalcheim C. The neural crest. Cambridge university Press; 1999. [Google Scholar]
- Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr. Opin. Cell Biol. 2007;19:628–633. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem KF, Jr., Tremml G, Jessell TM. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–138. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Stoykova A, Torres M, Gruss P. Dysgenesis of cephalic neural crest derivatives in Pax7−/− mutant mice. Development. 1996;122:831–838. doi: 10.1242/dev.122.3.831. [DOI] [PubMed] [Google Scholar]
- Martin BL, Harland RM. Hypaxial muscle migration during primary myogenesis in Xenopus laevis. Dev. Biol. 2001;239:270–280. doi: 10.1006/dbio.2001.0434. [DOI] [PubMed] [Google Scholar]
- Matsunaga E, Araki I, Nakamura H. Role of Pax3/7 in the tectum regionalization. Development. 2001;128:4069–4077. doi: 10.1242/dev.128.20.4069. [DOI] [PubMed] [Google Scholar]
- McCauley DW, Bronner-Fraser M. Conservation of Pax gene expression in ectodermal placodes of the lamprey. Gene. 2002;287:129–139. doi: 10.1016/s0378-1119(01)00894-0. [DOI] [PubMed] [Google Scholar]
- McGrew LL, Hoppler S, Moon RT. Wnt and FGF pathways cooperatively pattern anteroposterior neural ectoderm in Xenopus. Mech. Dev. 1997;69:105–114. doi: 10.1016/s0925-4773(97)00160-3. [DOI] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Dev. Cell. 2004;7:291–299. doi: 10.1016/j.devcel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Minchin JE, Hughes SM. Sequential actions of Pax3 and Pax7 drive xanthophore development in zebrafish neural crest. Dev. Biol. 2008;317:508–522. doi: 10.1016/j.ydbio.2008.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH. A Rapid Protocol for Whole-Mount In Situ Hybridization on Xenopus Embryos. Protocols; Cold Spring Harbor: 2007. %R 10.1101/pdb.prot4809. 2007, pdb.prot4809- [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Bontoux M, Vincent C, Le Douarin NM. The developmental relationships of the neural tube and the notochord: short and long term effects of the notochord on the dorsal spinal cord. Mech. Dev. 1995;53:157–170. doi: 10.1016/0925-4773(95)00426-2. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Duprez D, Watanabe Y, Bontoux M, Vincent C, Brickell P, Le Douarin N. The role of bone morphogenetic proteins in vertebral development. Development. 1996;122:3607–3616. doi: 10.1242/dev.122.11.3607. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Fletcher RB, Harland RM. Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development. 2003;130:3111–3124. doi: 10.1242/dev.00531. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Wang E, Harland R. Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev. Cell. 2005;8:167–178. doi: 10.1016/j.devcel.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin). Garland; New York: 1994. [Google Scholar]
- Ny A, Koch M, Schneider M, Neven E, Tong RT, Maity S, Fischer C, Plaisance S, Lambrechts D, Heligon C, Terclavers S, Ciesiolka M, Kalin R, Man WY, Senn I, Wyns S, Lupu F, Brandli A, Vleminckx K, Collen D, Dewerchin M, Conway EM, Moons L, Jain RK, Carmeliet P. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat. Med. 2005;11:998–1004. doi: 10.1038/nm1285. [DOI] [PubMed] [Google Scholar]
- O'Neill P, McCole RB, Baker CV. A molecular analysis of neurogenic placode and cranial sensory ganglion development in the shark, Scyliorhinus canicula. Dev. Biol. 2007;304:156–181. doi: 10.1016/j.ydbio.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio J, Mazan S, Retaux S. Organisation of the lamprey (Lampetra fluviatilis) embryonic brain: insights from LIM-homeodomain, Pax and hedgehog genes. Dev. Biol. 2005;288:100–112. doi: 10.1016/j.ydbio.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Read AP, Newton VE. Waardenburg syndrome. J. Med. Genet. 1997;34:656–665. doi: 10.1136/jmg.34.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev. 2004;18:1088–1105. doi: 10.1101/gad.301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Dierich A, Le Meur M, Ang S. Cell autonomous and non-cell autonomous functions of Otx2 in patterning the rostral brain. Development. 1999;126:4295–4304. doi: 10.1242/dev.126.19.4295. [DOI] [PubMed] [Google Scholar]
- Ridgeway AG, Skerjanc IS. Pax3 is essential for skeletal myogenesis and the expression of Six1 and Eya2. J. Biol. Chem. 2001;276:19033–19039. doi: 10.1074/jbc.M011491200. [DOI] [PubMed] [Google Scholar]
- Roche DD, Liu KJ, Harland RM, Monsoro-Burq AH. Dazap2 is required for FGF-mediated posterior neural patterning, independent of Wnt and Cdx function. Dev. Biol. 2009;333:26–36. doi: 10.1016/j.ydbio.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp RA, Weintraub H. Ubiquitous MyoD transcription at the midblastula transition precedes induction-dependent MyoD expression in presumptive mesoderm of X. laevis. Cell. 1991;65:927–937. doi: 10.1016/0092-8674(91)90545-a. [DOI] [PubMed] [Google Scholar]
- Sato T, Sasai N, Sasai Y. Neural crest determination by co-activation of Pax3 and Zic1 genes in Xenopus ectoderm. Development. 2005;132:2355–2363. doi: 10.1242/dev.01823. [DOI] [PubMed] [Google Scholar]
- Sefton M, Sanchez S, Nieto MA. Conserved and divergent roles for members of the Snail family of transcription factors in the chick and mouse embryo. Development. 1998;125:3111–3121. doi: 10.1242/dev.125.16.3111. [DOI] [PubMed] [Google Scholar]
- Shapira E, Marom K, Yelin R, Levy A, Fainsod A. A role for the homeobox gene Xvex-1 as part of the BMP-4 ventral signaling pathway. Mech. Dev. 1999;86:99–111. doi: 10.1016/s0925-4773(99)00120-3. [DOI] [PubMed] [Google Scholar]
- Sharpe CR, Fritz A, De Robertis EM, Gurdon JB. A homeobox-containing marker of posterior neural differentiation shows the importance of predetermination in neural induction. Cell. 1987;50:749–758. doi: 10.1016/0092-8674(87)90333-3. [DOI] [PubMed] [Google Scholar]
- Sibthorpe D, Sturlaugsdottir R, Kristjansson BK, Thorarensen H, Skulason S, Johnston IA. Characterisation and expression of the paired box protein 7 (Pax7) gene in polymorphic Arctic charr (Salvelinus alpinus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006;145:371–383. doi: 10.1016/j.cbpb.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: a Laboratory Manual. Cold Spring Harbor Press; Cold Spring Harbor, N.Y.: 2000. [Google Scholar]
- Stutz F, Spohr G. Isolation and characterization of sarcomeric actin genes expressed in Xenopus laevis embryos. J. Mol. Biol. 1986;187:349–361. doi: 10.1016/0022-2836(86)90438-9. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tassabehji M, Read AP, Newton VE, Harris R, Balling R, Gruss P, Strachan T. Waardenburg's syndrome patients have mutations in the human homologue of the Pax-3 paired box gene. Nature. 1992;355:635–636. doi: 10.1038/355635a0. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Zembrzycki A, Mansouri A, Ziman M. Pax7 is requisite for maintenance of a subpopulation of superior collicular neurons and shows a diverging expression pattern to Pax3 during superior collicular development. BMC Dev. Biol. 2008;8:62. doi: 10.1186/1471-213X-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tour E, Pillemer G, Gruenbaum Y, Fainsod A. Gbx2 interacts with Otx2 and patterns the anterior–posterior axis during gastrulation in Xenopus. Mech. Dev. 2002a;112:141–151. doi: 10.1016/s0925-4773(01)00653-0. [DOI] [PubMed] [Google Scholar]
- Tour E, Pillemer G, Gruenbaum Y, Fainsod A. Otx2 can activate the isthmic organizer genetic network in the Xenopus embryo. Mech. Dev. 2002b;110:3–13. doi: 10.1016/s0925-4773(01)00591-3. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Pituello F, Gruss P. Inhibition of floor plate differentiation by Pax3: evidence from ectopic expression in transgenic mice. Development. 1996;122:2555–2567. doi: 10.1242/dev.122.8.2555. [DOI] [PubMed] [Google Scholar]
- Ye W, Bouchard M, Stone D, Liu X, Vella F, Lee J, Nakamura H, Ang SL, Busslinger M, Rosenthal A. Distinct regulators control the expression of the mid–hindbrain organizer signal FGF8. Nat. Neurosci. 2001;4:1175–1181. doi: 10.1038/nn761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.