Abstract Abstract
We present the first comprehensive taxonomic revision and review the biology of the olingos, the endemic Neotropical procyonid genus Bassaricyon, based on most specimens available in museums, and with data derived from anatomy, morphometrics, mitochondrial and nuclear DNA, field observations, and geographic range modeling. Species of Bassaricyon are primarily forest-living, arboreal, nocturnal, frugivorous, and solitary, and have one young at a time. We demonstrate that four olingo species can be recognized, including a Central American species (Bassaricyon gabbii), lowland species with eastern, cis-Andean (Bassaricyon alleni) and western, trans-Andean (Bassaricyon medius) distributions, and a species endemic to cloud forests in the Andes. The oldest evolutionary divergence in the genus is between this last species, endemic to the Andes of Colombia and Ecuador, and all other species, which occur in lower elevation habitats. Surprisingly, this Andean endemic species, which we call the Olinguito, has never been previously described; it represents a new species in the order Carnivora and is the smallest living member of the family Procyonidae. We report on the biology of this new species based on information from museum specimens, niche modeling, and fieldwork in western Ecuador, and describe four Olinguito subspecies based on morphological distinctions across different regions of the Northern Andes.
Keywords: Andes, Bassaricyon, biogeography, Neotropics, new species, olingo, Olinguito
Introduction
“New Carnivores of any sort are always few and far between…”
Oldfield Thomas (1894:524)
Olingos (genus Bassaricyon J.A. Allen, 1876) are small to medium-sized (0.7 to 2 kg) arboreal procyonids found in the forests of Central America and northern South America. No comprehensive systematic revision of the genus has ever been undertaken, such that species boundaries in Bassaricyon are entirely unclear, and probably more poorly resolved than in any other extant carnivoran genus. There are various reasons for limited knowledge of Bassaricyon. For such a widespread genus of Carnivora, olingos were discovered surprisingly late (first described from Central America in 1876 and from South America in 1880; Allen 1876, Thomas 1880); they were long known by few specimens in museum collections; they are often overlooked in the field because they are regularly confused with another better known procyonid, the kinkajou, Potos flavus (Schreber, 1774) (e.g., Thomas 1880, Manville 1956, Ford and Hoffmann 1988, Sampaio et al. 2011); and they are often largely or entirely omitted from both authoritative and popular references on Neotropical wildlife and natural history (e.g., Janzen 1983, Henderson 2002, Lord 2007). In the absence of a detailed systematic review, five species of Bassaricyon are tentatively recognized in most recent taxonomic references, including three species in Central America (Bassaricyon gabbii Allen, 1876; Bassaricyon lasius Harris, 1932; Bassaricyon pauli Enders, 1936) and three species in South America (with Bassaricyon gabbii recognized as occurring west of the Andes, and Bassaricyon alleni Thomas, 1880 and Bassaricyon beddardi Pocock, 1921a east of the Andes), but most authors have explicitly identified a longstanding need for a detailed taxonomic overview to clarify species diversity and distributions in this genus (Cabrera 1958, Decker and Wozencraft 1991, Eisenberg 1989, Eisenberg and Redford 1999, Eizirik 2012, Emmons 1990, 1997, Ewer 1973, Glatston 1994, González-Maya et al. 2011, Hall 1981, Hall and Kelson 1959, Helgen et al. 2008c, Hunter 2011, Kays 2009, Kays and Russell 2001, Nowak 1999, Poglayen-Neuwall and Poglayen-Neuwall 1965, Prange and Prange 2009, Reid 1997, 2009, Reid and Helgen 2008a, 2008b, 2008c, Russell 1984, Samudio et al. 2008, Stains 1967, Wozencraft 1989, 1993, 2005, Zeveloff 2002).
Here we review the taxonomic standing of all named forms of Bassaricyon based on morphological, morphometric, and molecular comparisons of voucher specimens in museums; we clarify the distribution and conservation status of each valid taxon; and, as far as possible, we enable information from published literature on olingo anatomy (e.g., Beddard 1900, Mivart 1885, 1886, Pocock 1921a, 1921b, Segall 1943, Stains 1973, Story 1951), ecology and behavior (e.g., Aquino and Encarnación 1986, Emmons 1990, 1991, Glanz 1990, Goldman 1920, Hunter and Caro 2008, Janson and Emmons 1990, Kays 2000, Loyola et al. 2008, Patton et al. 1982, Peres 2001, Poglayen-Neuwall 1966, 1973, 1976, 1989, Poglayen-Neuwall and Poglayen-Neuwall 1965, Prange and Prange 2009, Reid 1997, Rodríguez and Amanzo 2001, Wainwright 2002), and parasites and disease (e.g., Grimaldi and Tesh 1993, Hendricks 1977, Herrer and Christensen 1975, Jewell et al. 1972, Magalhães-Pinto et al. 2009) to be associated with particular olingo taxa now recognized as valid.
All previously described olingo taxaoccur in lower to middle-elevation tropical or subtropical forests (≤ 2000 meters in elevation). Remarkably, our morphological, morphometric, molecular, and field studies document the existence of an undescribed species in the genus, endemic to higher-elevation cloud forests (1500 to 2750 meters) in the Western and Central Andes of Colombia and Ecuador, which we describe here as a new species. (This species has been discussed preliminarily, in advance of its formal description, by Kays [2009] and Hunter [2011].) This species, upon which we bestow the common name of Olinguito (oh-ling-GHEE’-toh), is the sister taxon to a lineage comprising all previously described species of Bassaricyon; is the smallest living procyonid; and is the first new species of American carnivore described since the discovery of the Colombian weasel (Mustela felipei) in similar habitats in the same region of the Andes more than three decades ago (Izor and de la Torre 1978). We discuss what is known to date of the biology of this remarkable new procyonid, the Olinguito.
Materials and methods
Museum specimens and comparisons
We examined all Bassaricyon specimens in the collections of the (AMNH); (ANSP); (BMNH); (EPN); (FMNH); (KU); (LACM); (MCZ); (MECN); (MVZ); (NMS); (QCAZ); (ROM); (TCWC); (UMMZ); (USNM); (YPM); and (ZMB). These holdings include all type specimens in the genus and represent the great majority (well over 95%) of olingo specimens in world museums. We also had access to published information on a few additional specimens in museum collections in Colombia and Bolivia (Saavedra-Rodríguez and Velandia-Perilla 2011, Anderson 1997). Tissue samples are stored in the frozen tissue collections of the MVZ, ROM, USNM (including specimens to be accessioned at QCAZ), the (NYSM), and the (TTU) (Table 1).
Table 1.
List of samples (and associated information) used in phylogenetic analysis. Boldfaced entries represent samples newly sequenced in this study.
| SPECIES | Identifier in Figure 1 | Specific locality | Source (catalog reference) | Genbank Accession Numbers | |
|---|---|---|---|---|---|
| Cytochrome b | CHRNA1 | ||||
| Bassaricyon medius orinomus | Panama | Limbo plot | NYSM ZT105 | EF107703 | KC773757 |
| Bassaricyon medius orinomus | Panama | Rio Juan Grande | NYSM ZT106 | EF107704 | KC773758 |
| Bassaricyon medius orinomus | Panama | Limbo plot | Koepfli et al. (2007) | DQ660300 | DQ660210 |
| Bassaricyon medius medius | Ecuador | Las Pampas | QCAZ 8659; tk149097 | EF107706 | KC773759 |
| Bassaricyon medius medius | Ecuador | Las Pampas | QCAZ 8658; tk149094 | EF107707 | KC773760 |
| Bassaricyon alleni | Guyana | Iwokrama | ROM 107380 | EF107710 | KC773763 |
| Bassaricyon alleni | Peru | Rio Cenapa | MVZ 155219; Koepfli et al. (2007) | DQ660299 | DQ660209 |
| Bassaricyon gabbii | Costa Rica | Monteverde | KU 165554 | JX948744 | --- |
| Bassaricyon neblina neblina | Ecuador | La Cantera | QCAZ 8662; tk149108 | EF107708 | KC773761 |
| Bassaricyon neblina neblina | Ecuador | Otonga Reserve | QCAZ 8661; tk149001 | EF107709 | KC773762 |
| Bassaricyon neblina osborni | Colombia | Vicinity of Cali | Genbank | X94931 | DQ533950 |
| Potos flavus | Potos flavus | Costa Rica | Koepfli et al. (2007) | DQ660304 | DQ660214 |
| Procyon cancrivorus | Procyon cancrivorus | Paraguay | Koepfli et al. (2007) | DQ660305 | DQ660215 |
| Procyon lotor | Procyon lotor | Montana, USA | Koepfli et al. (2007) | DQ660306 | AF498152 |
| Bassariscus astutus | Bassariscus astutus | Arizona, USA | Koepfli et al. (2007) | AF498159 | AF498151 |
| Bassariscus sumichrasti | Bassariscus sumichrasti | Mexico | Koepfli et al. (2007) | DQ660301 | DQ660211 |
| Nasua nasua | Nasua nasua | Bolivia | Koepfli et al. (2007) | DQ660303 | DQ660213 |
| Nasua narica | Nasua narica | Panama | Koepfli et al. (2007) | DQ660302 | DQ660212 |
| Enhydra lutris | Mustelidae | Attu Island, Alaska, USA | Koepfli et al. (2007) | AF057120 | AF498131 |
| Eira barbara | Mustelidae | Bolivia | Koepfli et al. (2007) | AF498154 | AF498144 |
| Taxidea taxus | Mustelidae | New Mexico, USA | Koepfli et al. (2007) | AF057132 | AF498148 |
| Neovison vison | Mustelidae | Texas, USA | Koepfli et al. (2007) | AF057129 | AF498140 |
| Martes americana | Mustelidae | Rocky Mtn Research Station, USA | Koepfli et al. (2007) | AF057130.1 | AF498141 |
| Lontra longicaudis | Mustelidae | Kagka, Peru | Koepfli et al. (2007) | AF057123.1 | AF498134 |
| Ictonyx libyca | Mustelidae | Brookfield Zoo | Genbank | EF987739.1 | EF987699 |
| Meles meles | Mustelidae | No voucher infromation | Koepfli et al. (2007) | AM711900.1 | AF498147 |
| Mephitis mephitis | Mephitidae | San Diego Zoo | Eizirik et al. (2010), Yu et al. (2011) | HM106332.1 | GU931029.1 |
| Spilogale putorius | Mephitidae | Arnason et al. (2007), Eizirik et al. (2010) | NC_010497.1 | GU931030.1 | |
| Ailurus fulgens | Ailuridae | Arnason et al. (2007), Eizirik et al. (2010) | AM711897.1 | GU931037.1 | |
| Arctocephalus australis | Otariidae | Davis et al. (2004), Fulton and Strobeck (2006) | AY377329.1 | DQ205738.1 | |
| Odobenus rosmarus | Odobenidae | Bardeleben et al. (2005), Fulton and Strobeck (2010) | GU174611.1 | DQ093076.1 | |
| Phoca fasciata | Phocidae | Fulton and Strobeck (2010) | GU167294.1 | GU167764.1 | |
| Mirounga angustirostris | Phocidae | Bardeleben et al. (2005), Peng et al. (2007) | AY377325.1 | DQ093075.1 | |
| Canis lupus | Canis lupus | Delisle and Strobeck (2005), Fulton and Strobeck (2006) | AY598499 | DQ205757 | |
| Nyctereutes procyonoides | other Canidae | Eizirik et al. (2010), Chen and Zhang (2012) | GU256221 | GU931027.1 | |
| Urocyon cinereoargenteus | other Canidae | Eizirik et al. (2010), Naidu et al. (2012) | JF489121.1 | GU931028.1 | |
| Ailuropoda melanoleuca | Ursidae | Bardeleben et al. (2005), Peng et al. (2007) | NC_009492 | DQ093074.1 | |
| Ursus americanus | Ursidae | Delisle and Strobeck (2002), Fulton and Strobeck (2006) | NC_003426.1 | DQ205726.1 | |
Values from external measurements of 95 specimens are presented to provide an appreciation of general body size and lengths and proportions of appendages. Values (in mm) for total length and length of tail are those recorded by collectors on labels attached to skins; subtracting length of tail (abbreviated TV) from total length produced a value for (HB). Values for (HF), which includes claws, were either obtained from skin labels or from our measurements of dry study skins; those for (E), or pinna, come from collector’s measurements recorded on skin labels or in field journals (we assume, but are not certain for all specimens, that ear-length measurements represent the greatest length from the notch to the distal margin of the pinna).
Morphological terminology follows Evans (1993) and Ahrens (2012). Craniodental variables were measured by the first author with digital calipers to the nearest 0.1 mm. Single-tooth measurements are measured on the crown. All measurements of length are in millimeters, and measurements of mass are given in grams. Only fully adult, wild-collected specimens that are sufficiently intact were included in our morphometric analyses. A total of 115 specimens were included (51 male, 64 female). The classification of ‘‘adult’’ was applied generally only to skulls in which the full dentition is completely erupted, and in which the basilar (basioccipital-basisphenoid) suture (synchondrosis) in particular is obliterated via ossification. Variables measured include (W) of (p1, p2, p3, p4, P2, P3, P4, with lower case designating lower teeth and uppercase designating upper teeth) and (m1, m2, M1, and M2); (L) of the larger premolars and molars (P4, M1, M2, m1, and m2); (CBL), (ZYG), (BBC), (CC), and (MTR), all as defined by Kennedy and Lindsay (1984); and four posterior skull measurements: greatest width across the postdental palatal shelf (WPP), length of the postdental palate behind an imaginary line delineated by the back of the second molars (LPP), anteroposterior length of the auditory bullae including the eustachian tube (LAB), and the dorsoventral diameter inside the (EAM). Unless explicitly noted, all reported metrics (and resulting statistical and multivariate comparisons) refer only to fully mature (adult) specimens, as judged by direct examination of skulls. Because some olingo taxa demonstrate significant sexual dimorphism in cranial measurements, patterns of morphometric variation in males and females were compiled and analyzed separately. (PCA) and (DFA) were undertaken using a combination of cranial and dental measurements indicated in tables and in the text, selected to sample craniodental size and shape, and to maximize sample size. All measurement values were transformed to natural logarithms prior to multivariate analysis. Principal components were extracted from the covariance matrix. The software program Statistica 8.0 (Statsoft Inc., Tulsa, Oklahoma, USA) was used for all multivariate analyses.
The taxa and sequences included in our analysis are listed in Table 1. Our choice of taxa outside of Bassaricyon was guided by the findings of Koepfli et al. (2007), Fulton and Strobeck (2007), and Eizirik et al. (2010). These studies provide strong statistical support for relationships and divergence dates within Procyonidae and Carnivora based on >6,000 bases of DNA and fossil evidence. We chose one mitochondrial marker and one nuclear marker used in these and many other mammalian studies, in order to capture the evolutionary histories of these distinct genetic systems. Although deeper relationships within the order Carnivora cannot be resolved solely by using these two genes, we are confident that they provide the appropriate level of support to resolve species-level relationships within this group of procyonids (Koepfli et al. 2007). As our specific goal was to estimate the timing of divergence within Bassaricyon, and our reduced dataset did not provide enough support to resolve deeper nodes in Caniformia, we decided to use highly supported divergence date estimates from Koepfli et al. (2007) and Eizirik et al. (2010) as priors in our analysis.
DNA extraction
Tissues from fresh and frozen specimens were processed using a Qiagen DNeasy kit (QIAGEN, Valencia, CA, USA) to obtain genomic DNA. The sample from the skull of KU 165554, a museum specimen of Bassaricyon gabbii, was taken from the turbinate bones and extracted following the method of Wisely et al. (2004). Including this turbinate sample of Bassaricyon gabbii, we successfully extracted DNA from eight individuals of Bassaricyon (four Bassaricyon medius, one Bassaricyon alleni, and two Bassaricyon neblina sp. n.). All pre-PCR protocols were conducted in an isolated ancient DNA laboratory facility located in a separate building from the one containing the primary DNA laboratory.
DNA Sequencing
Mitochondrial gene, cytochrome b: For cytochrome b (1140 bp), polymerase chain reaction (PCR) and sequencing reactions were carried out with primers LGL 765 and LGL 766 from Bickham et al. (2004) and using a thermal cycler (MJ Research, Waltham, MA, USA) under the following conditions, repeated for 35 cycles: denaturation at 92°C for 1 min, annealing at 50°C for 1 min, extension at 72°C for 1 min. The PCR reagents in a 25 μL reaction were 0.2 μL AmpliTaq (5 units μL-1, Applied Biosystems, Foster City, CA, USA), 1μL per primer (10 μM), 2.5 μL dNTP (2 μM), 2 μL MgCl2 (25 mM), 2.5 μL AmpliTaq Buffer (Applied Biosystems), 2μL BSA (0.01 mg/μL), 1 μL gDNA and 12.8 μL sterile water. To amplify DNA from turbinate samples, PCR and sequencing were carried out with internal primers designed for this study using sequences generated from the tissue samples; the reverse primer, H151949Pro (5’ CTCCTCAAAAGGATATTTGYCCTCA 3’), located at 14611 – 14636 on the Nasua nasua mitochondrial genome, was used with LGL 765. A new forward primer, BAS420F (5’ TCAGACAAAATCCCCTTCCA 3’), position 14825 - 14845 on the Nasua nasua mitochondrial genome, was used with LGL 766. The thermal cycle regime was modified to 50 cycles; reagents were as above.
Nuclear intron, Cholinergic Receptor Nicotinic Alpha Polypeptide 1 precursor (CHRNA1): For CHRNA1 (347 bp), we used the primers described by Lyons et al. (1997) and the thermocycling conditions consisted of an initial denaturation (95°C for 10 min), followed by 30 cycles of denaturation at 95°C for 30 s, annealing at 54°C for 30 s and extension for 72°C for 45 s, and final extension of 72°C for 5 min. Reagent volumes were the same as for cytochrome b amplification (above), except 2μL of gDNA was added for CHRNA1 amplification, decreasing sterile water volume to 10.8μL. We were unable to sequence the nuclear intron from the turbinate bone sample.
Each PCR was conducted with negative and positive controls to minimize risk of spurious results from contamination or failure of the reaction. A 2μL sample of the PCR product was stained with ethidium bromide and run on an agarose gel with a 1 kb ladder. The gel was placed under UV light to visualize the PCR products. Polymerase chain reaction products were amplified for sequencing using a 10 μL reaction mixture of 2 μL of PCR product, 0.8 μL of primer (10 μM), 1.5 μL Big Dye 5 x Buffer (Applied Biosystems), 1 μL Big Dye version 3 (Applied Biosystems), and 4.7 μL sterile water. The reaction was run using a thermal cycler (MJ Research) with denaturation at 96°C for 10 s, annealing at 50°C for 10 s and extension at 60°C for 4 min: this was repeated for 25 cycles. The product was cleaned using sephadex filtration method and sequences for both strands were run on a 50 cm array using the ABI PRISM 3100 Genetic Analyzer (Applied Biosystems).
Molecular analysis
Sequences were aligned and edited in Sequencher version 4.1.2 using the implemented Clustal algorithm and the default gap penalty parameters (Gene Codes Corporation, Ann Arbor, MI, USA, http://www.genecodes.com).
For Bassaricyon, we included all newly sequenced and previously available sequences for cytochrome b and CHRNA1 (for cytochrome b this included five individuals of Bassaricyon medius, two Bassaricyon alleni, one Bassaricyon gabbii and three Bassaricyon neblina sp. n.; for CHRNA1 this included five individuals of Bassaricyon medius, two Bassaricyon alleni, and three Bassaricyon neblina sp. n.) (Table 1).
Maximum Parsimony, Maximum Likelihood and Bayesian analyses were performed for each gene and a concatenation of the two genes to check for any incongruence in structure and support of the Bassaricyon clade. All Bayesian and Maximum Likelihood phylogenetic inferences were carried out using the Cipres Portal (Miller et al. 2009). Indels were treated as missing data or non-informative data for all of the analyses as in previous molecular phylogenetic studies of procyonids (Koepfli et al. 2007; Eizirik et al. 2010).
Pairwise distances for cytochrome b were generated using the Kimura 2-parameter model using MEGA4 (Tamura et al. 2007).
The branch and bound search method implemented in the software package TNT (Goloboff et al. 2008) was used for the maximum parsimony analyses. Parsimony bootstrap support was generated using the heuristic search method with 100 random stepwise additions for 1000 replicates.
Maximum Likelihood analysis was conducted using the software package GARLI 0.96b (Zwickl 2006). The genetic-like algorithm was used to simultaneously find the topology, branch lengths and substitution model parameters with the greatest log-likelihood (lnL) for each dataset. Bootstrap support was generated with 1000 replicates and two independent searches per replicate.
jModeltest version 0.1.1 (Posada 2008) was used to find the best model of sequence evolution. We chose to partition the cytochrome b data in order to minimize the number of parameters and to account for differences in base composition and substitution rates among the different codon positions (Corse et al. 2013). The software PartitionFinder (Lanfear et al. 2012) was used to determine the best partitioning scheme, and for the cytochrome b, the scheme with 1st, 2nd, and 3rd codon positions partitioned separately was selected. The best fit model under the Bayesian information criterion (BIC) for cytochrome b for the first and second codon position partitions was HKY + G + I (Hasegawa et al. 1985), and for the third codon position, the best model under BIC was TrN + I + G (Tamura and Nei 1993). The model chosen for CHRNA1 was K80 + G (Kimura 1980). The parameters were then applied in MrBayes version 3.1p (Huelsenbeck and Ronquist 2001). The model parameters were set to nst = 2 using a gamma distribution for CHRNA1, nst = 2 and the rate parameter invariant with a gamma distribution for the cytochrome b 1st and 2nd codon partitions, and nst = 6 with a gamma distribution and rate parameter invariant for cytochrome b 3rd codon partition. Since this version of MrBayes did not include the specific model selected for the cytochrome b 3rd codon position partition, we opted for using a more complex model (nst = 6) following the results of Huelsenbeck and Rannala (2004). The Bayesian analysis was run using 5,000,000 generations along four chains with 2 replicates at a temperature of 0.05. The convergence between the two replicates run in MrBayes was assessed by the average standard deviation of split frequencies (ASDSF) between runs. After 5,000,000 generations, the ASDSF was 0.003. Sample frequency was set to 1000 with a burn-in of 1,250.
Molecular divergence estimates were generated in BEAST (Drummond and Rambaut 2007). The following calibration nodes were included based on Eizirik et al. (2010): Nasua – Bassaricyon truncated normal mean 7.2 million years ago (mya) (± 1.7 s.d.); -Potos truncated normal mean 16.2 mya (± 2.5 s.d.); Procyonidae normal mean 20.7 mya (± 4.0 s.d.); Procyonidae-Mustelidae-Ailuridae-Mephitidae normal mean 30 mya (± 7.0 s.d.); Phoca-Mirounga normal mean 20 mya (± 6 s.d.); Caniformia normal mean 48 mya (± 6.5 s.d.). The molecular clock was estimated using the uncorrelated lognormal setting, operators were left to the default setting, and trees were searched using the Yule process. The substitution and clock models were left unlinked, partition tree model was linked, and the models for the two gene partitions were: cytochrome b (1), (2), and (3) => TN93 + I + G (all parameters unlinked) and CHRNA1 K80 + G (HKY + G). In order to evaluate the effects of the priors on the divergence time estimates, we carried out a run using an empty alignment but with the same settings and compared it to our results,with the outcome indicating that the priors are not having an especially strong effect on the estimated divergence times (Drummond et al. 2006).
To infer geographical range evolution of procyonids we used the Maximum Likelihood model of dispersal-extinction-cladogenesis (DEC) implemented in Lagrange v. 20130526 (Ree and Smith 2008). The BEAST chronogram tree was trimmed to keep one representative per procyonid species, and two additional lineages, one representing Mustelidae and one representing Mephitidae. Six general geographic areas were used to characterize the distribution ranges: Eurasia, North America, Central America, Chocó, Andes, and Amazonia. The branches of the mustelid and mephitid lineages were treated as belonging to the ancestors of the families and their hypothesized distributions are according to previous ancestral range estimations (Koepfli et al. 2008, Sato et al. 2009). Reconstruction of potential ancestral area combinations and dispersal scenarios took into account realistic dispersal routes (e.g., allowing Eurasia to connect only with North America) and the geological history of the region (e.g., formation of the Panama Isthmus during the late Miocene and Pliocene; Weyl 1980, Almendra and Rogers 2012).
Bioclimatic range modeling
Vouchered localities of occurrence for Bassaricyon used in our analyses were extracted from museum specimen labels, often as clarified by associated field notes and journals, and from definitive published accounts. Gazetteers published by Paynter (1982, 1993, 1997), Stephens and Taylor (1983, 1985), Fairchild and Handley (1966), Handley (1976), Voss (1988), and Voss et al. (2002) were especially helpful in georeferencing Neotropical expedition and collecting localities represented in museum collections.
We used Maximum Entropy Modeling (Maxent) (Phillips et al. 2006) to predict the geographic range of the geographic range of the four Bassaricyon species at broad scales based on vouchered localities (Appendix 2) and 20 environmental variables representing potential vegetation and climate. For potential vegetation we used the 15 major habitat types classified as ecological biomes (Olson et al. 2001). For climate we used 19 BIOCLIM variables representing annual trends, seasonality, and extremes in temperature and precipitation across Central and South America (derived from Hijmans et al. [2005] as described at http://www.worldclim.org/bioclim.htm). We used all vouchered specimen localities to train the final model (excluding published records based only on visual observations). We also tested overall performance by running 10 model iterations while randomly withholding 20% of the points as test locations. To produce geographic ranges showing presence/absence of a species we used the average equal training sensitivity and specificity for the 10 test models as our probability cutoff value (Phillips et al. 2006).
Results
Phylogenetics
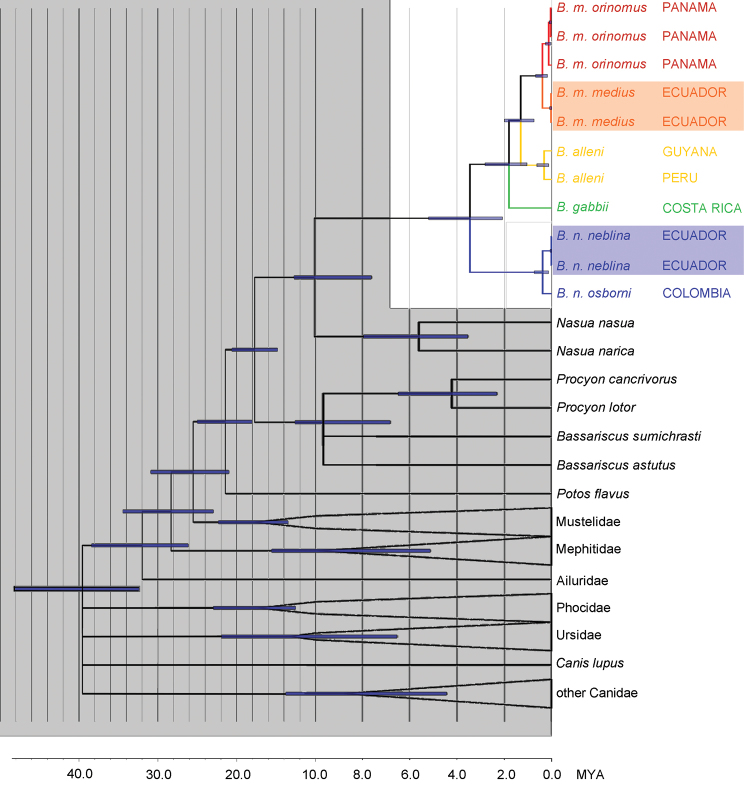
With the largest molecular sampling effort to date, we show that Bassaricyon is well resolved as a monophyletic genus (cf. Nyakatura and Bininda-Emonds 2012) within the family Procyonidae. All of our analyses resolve Bassaricyon as a clade with bootstrap and probability values of 100%. The sister genus to Bassaricyon is Nasua, a relationship consistently recovered in our analyses with 100% support. The divergence between Bassaricyon and Nasua was estimated at 10.2 million years old (mya) (95% Confidence Interval [CI] = 7.6 – 12.7 mya), consistent with previously published results (Koepfli et al. 2007, Eizirik et al. 2010).
The family Procyonidae is well resolved as monophyletic (100% bootstrap and probability values) with a divergence date of 21.4 mya (CI 18.1 – 25.0 mya), in agreement with the divergence estimate of 22.6 mya (CI 19.4 – 25.5 mya) by Eizirik et al. (2010). Eizirik et al. (2010) had a more constrained confidence interval on the age of this divergence, due to the incorporation of genes that are more informative at deeper nodes in the tree. We chose CHRNA1 and cytochrome b with a focus toward resolving relationships within Bassaricyon; these markers are far more useful for determining relationships in recent radiations within Procyonidae than the deeper relationships within Carnivora. The only part of the Procyonidae where CHRNA1 and cytochrome b did not provide sufficient resolution to re-construct recently published multi-gene topologies (Koepfli et al. 2007, Eizirik et al. 2010) was the divergence between the two species of Bassariscus, and Procyon. In our BEAST chronogram the divergence for Bassariscus is 7.6 mya (CI 4.8 – 10.6 mya) but the branch leading to their divergence has no support, and therefore is collapsed in the phylogeny (Figure 1; see also Koepfli et al. 2007, Eizirik et al. 2010). The other procyonid genera are well-supported monophyletic groups; according to our chronogram Procyon lotor and Procyon cancrivorus diverged 4.2 mya (CI 2.3 – 6.5 mya) and Nasua narica and Nasua nasua diverged 5.6 mya (CI 3.5 – 7.9 mya).
Figure 1.
Phylogeny of the genus Bassaricyon. Phylogeny generated from the concatenated CHRNA1 and cytochrome b sequences. All analyses consistently recovered the same relationships with high support. Divergence dating was generated in BEAST; bars show the 95% confidence interval at each node. Branches without support are collapsed and outgroup clades have been collapsed, leaving monophyletic groupings with 100% support. Data for CHRNA1 are missing for Bassaricyon gabbii, for which DNA was extracted from a museum skull. All nodes in Bassaricyon have 1.00 Bayesian posterior probability, except the split between Bassaricyon gabbii and Bassaricyon alleni/Bassaricyon medius (0.97 Bayesian posterior probability). Non-focal and outgroup taxa are shaded in gray, Bassaricyon species and subspecies are color coded, samples of Bassaricyon medius medius and Bassaricyon neblina neblina that were collected within 5 km of each other in Ecuador are shaded.
The concordance of our recovered topology and estimates of genetic divergence with previous phylogenetic studies of the Procyonidae suggests that data from cytochrome b and CHRNA1 across sampled taxa have provided a well-supported framework in which the species relationships and divergence dates within Bassaricyon can be reliably assessed. Previous molecular phylogenetic studies have included either only one species (e.g., Ledje and Arnason 1996a, 1996b, and further studies using the same sequences, see below), identified as “Bassaricyon gabbii” (Genbank identifier X94931), but actually representing Bassaricyon neblina sp. n.; or, two species (Koepfli et al. 2007), identified as Bassaricyon alleni (correctly, sample from Amazonian Peru) and “Bassaricyon gabbii” (actually Bassaricyon medius orinomus,from Panama). Koepfli et al. (2007) gave the divergence estimate for these latter two taxa (i.e. Bassaricyon alleni and Bassaricyon medius orinomus) as 2.5–2.8 mya (CI 1.2–5.0 mya). Our results indicate that the earliest divergence within Bassaricyon, corresponding to the split between the ancestors of Bassaricyon neblina sp. n. and other Bassaricyon, occurred 3.5 mya (CI = 2.1 – 5.2 mya). Sequence divergence in cytochrome b between Bassaricyon neblina sp. n. and other Bassaricyon (including specimens of Bassaricyon medius medius collected in regional sympatry with Bassaricyon neblina sp. n. in the Western Andes of Ecuador) is 9.6-11.3% (Table 2). Cytochrome b sequence divergences between Bassaricyon gabbii, Bassaricyon medius, and Bassaricyon alleni are 6-7% (Table 2). Subspecific distances (see Systematics, below, for discussion of subspecies boundaries) are 1.6-2.0% within Bassaricyon medius (between Bassaricyon medius medius and Bassaricyon medius orinomus) and 1.6% within Bassaricyon neblina sp. n. (between Bassaricyon neblina neblina subsp. n. and Bassaricyon neblina osborni subsp. n., the two subspecies for which we have molecular data).
Table 2.
Percentage sequence divergence in cytochrome b sequences (Kimura 2-Parameter) among specimens of Bassaricyon (numbers 1-11) and other Procyonidae (numbers 12-18) in our analyses (see Table 1, Figure 1). Numbers across the top row match numbered samples in the vertical column.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Bassaricyon medius orinomus (Panama) | |||||||||||||||||
| 2. Bassaricyon medius orinomus (Panama) | 0.2 | ||||||||||||||||
| 3. Bassaricyon medius orinomus (Panama) | 0.3 | 0.4 | |||||||||||||||
| 4. Bassaricyon medius medius (Ecuador) | 1.9 | 1.9 | 1.6 | ||||||||||||||
| 5. Bassaricyon medius medius (Ecuador) | 1.9 | 2.0 | 1.6 | 0.1 | |||||||||||||
| 6. Bassaricyon alleni (Guyana) | 6.9 | 7.0 | 6.6 | 7.2 | 7.4 | ||||||||||||
| 7. Bassaricyon alleni (Peru) | 6.3 | 6.4 | 6.0 | 6.3 | 6.5 | 1.3 | |||||||||||
| 8. Bassaricyon gabbii (Costa Rica) | 7.3 | 7.1 | 7.0 | 6.9 | 6.7 | 6.3 | 6.6 | ||||||||||
| 9. Bassaricyon neblina neblina (Ecuador) | 10.1 | 10.1 | 9.8 | 10.4 | 10.6 | 11.3 | 11.0 | 9.9 | |||||||||
| 10. Bassaricyon neblina neblina (Ecuador) | 10.1 | 10.1 | 9.8 | 10.5 | 10.6 | 11.3 | 11.0 | 9.9 | 0.0 | ||||||||
| 11. Bassaricyon neblina osborni (Colombia) | 10.0 | 9.9 | 9.6 | 10.3 | 10.4 | 11.2 | 10.6 | 10.4 | 1.6 | 1.6 | |||||||
| 12. Potos flavus | 28.7 | 28.9 | 28.7 | 29.5 | 29.5 | 29.8 | 29.0 | 28.1 | 29.8 | 29.9 | 28.9 | ||||||
| 13. Procyon lotor | 34.8 | 34.3 | 34.3 | 35.2 | 34.9 | 35.6 | 34.9 | 33.0 | 33.8 | 33.7 | 32.7 | 27.3 | |||||
| 14. Procyon cancrivorus | 31.9 | 31.2 | 31.2 | 32.2 | 32.0 | 32.1 | 29.9 | 31.9 | 32.0 | 31.8 | 30.4 | 29.4 | 13.1 | ||||
| 15. Bassariscus astutus | 30.7 | 30.5 | 30.0 | 29.8 | 30.0 | 30.8 | 30.0 | 29.4 | 29.3 | 29.1 | 29.5 | 29.6 | 20.7 | 17.8 | |||
| 16. Bassariscus sumichrasti | 28.1 | 27.4 | 27.7 | 27.7 | 27.9 | 27.7 | 25.7 | 28.3 | 26.2 | 26.1 | 25.6 | 26.8 | 17.1 | 18.3 | 15.8 | ||
| 17. Nasua nasua | 26.8 | 26.7 | 26.7 | 28.1 | 28.4 | 25.4 | 24.1 | 25.7 | 25.0 | 24.8 | 24.1 | 35.6 | 35.8 | 30.3 | 30.5 | 29.1 | |
| 18. Nasua narica | 30.3 | 29.7 | 30.0 | 30.2 | 30.0 | 29.0 | 29.2 | 28.8 | 25.1 | 25.1 | 24.2 | 31.3 | 29.7 | 26.4 | 27.3 | 26.3 | 20.4 |
We obtained the highest bootstrap and posterior probability support values (100% and 1.0 respectively) for relationships within Bassaricyon with every method of phylogenetic inference that was used in this study. The single exception was that the topology that recovered the node uniting Bassaricyon alleni and Bassaricyon medius to the exclusion of Bassaricyon gabbii was assigned a slightly lower Bayesian posterior probability value of 0.97, but all other methods lent full support to this topology (Bassaricyon gabbii,(Bassaricyon medius, Bassaricyon alleni)). These results were also well-supported by our comparisons of morphological characters and together lend strong support for this scenario as being an accurate representation of the evolutionary history of diversification within Bassaricyon.
Biogeography
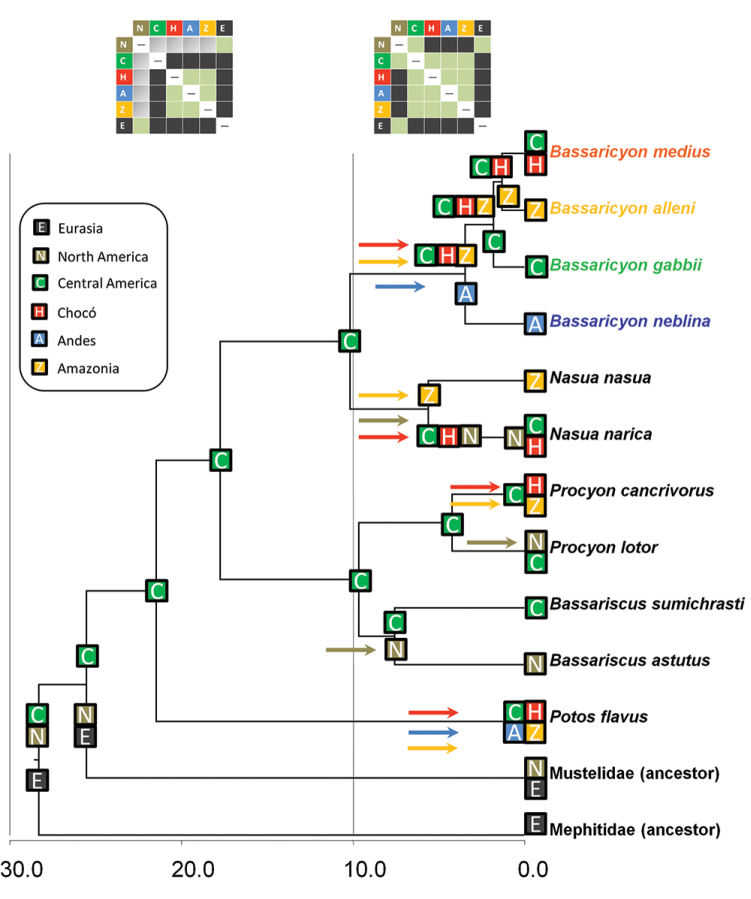
The historical biogeographic reconstruction for the Procyonidae using the DEC model sets Central America as the likely center of origin of crown-group procyonids (Figure 2) (though we note that the family has many extinct, Eocene to Miocene representatives in North America and Europe). Major splits within the family appear to have occurred in Central America previous to the formation of the Panamanian Isthmus, and all the dispersal events resulting in the extant species have occurred within the last 10 million years. All those dispersal events involving southward movements seem to have occurred up to circa 6 mya, coinciding with the initial uplift of the Panamanian Isthmus, and, presumably once it was consolidated, with the Great American Biotic Interchange (GABI) (Figures 1–2). The clade containing all olingo species is likely to have originated directly as a result of the formation of the Panamanian Isthmus, and provides evidence of a complex pattern of dispersal events out of Central America (Figure 2).
Figure 2.
Historical biogeography of procyonids. Reconstructed under the DEC model implemented in Lagrange. See legend for geographical areas used in the analysis. Colored squares at the tip of the branches reflect the distribution of taxa, and previously inferred distributions of the ancestors of mustelids and mephitids. Colored squares at the nodes represent the geographic ranges with the highest probabilites in the DEC model inherited by each descendant branch. Colored arrows reflect dispersal events between ancestral and derived areas, with colors matching with recipient areas. Upper boxes: different dispersal constraints at time intervals 0–10 mya and 10–30 mya, the former to simulate the effect of the land bridge formation between Central and South America, the latter restricted dispersal due to the absence of the land bridge; the cells in green indicate no restriction to dispersal, cells in gray indicate a reduction by half in dispersal capability, and cells in black do not allow dispersal. Timescale in millions of years before present (mya).
Morphology and morphometrics
Our study of Bassaricyon taxonomy originally began with close examination of craniodental traits of museum specimens, which quickly revealed to the first author the existence of Bassaricyon neblina sp. n., which is highly distinctive morphologically. Close examinations of skins and skulls revealed clear differences in qualitative traits, and in external and craniodental measurements and proportions, between the four principal Bassaricyon lineages identified in this paper (which we recognize taxonomically as Bassaricyon neblina sp. n., Bassaricyon gabbii, Bassaricyon alleni, and Bassaricyon medius; Figures 3–5). Externally, these especially include differences in body size, pelage coloration, pelage length, relative length of the tail, and relative size of the ears (Figure 3, Table 5). Craniodentally, these especially include differences in skull size, relative size of the premolars and molars, configuration of molar cusps, relative size of the auditory bullae and external auditory meati, and the shape of the postdental palatal shelf (Figures 4–5, Tables 3–4). These and other differences are discussed in greater detail in the species accounts provided later in the paper.
Figure 3.
Illustrations of the species of Bassaricyon. From top to bottom, Bassaricyon neblina sp. n. (Bassaricyon neblina ruber subsp. n. of the western slopes of the Western Andes of Colombia), Bassaricyon medius (Bassaricyon medius orinomus of eastern Panama), Bassaricyon alleni (Peru), and Bassaricyon gabbii (Costa Rica, showing relative tail length longer than average). Artwork by Nancy Halliday.
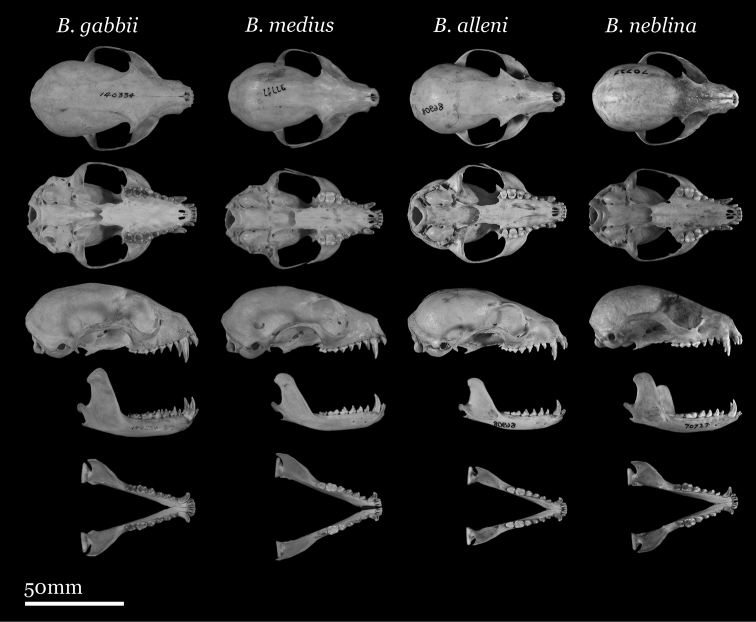
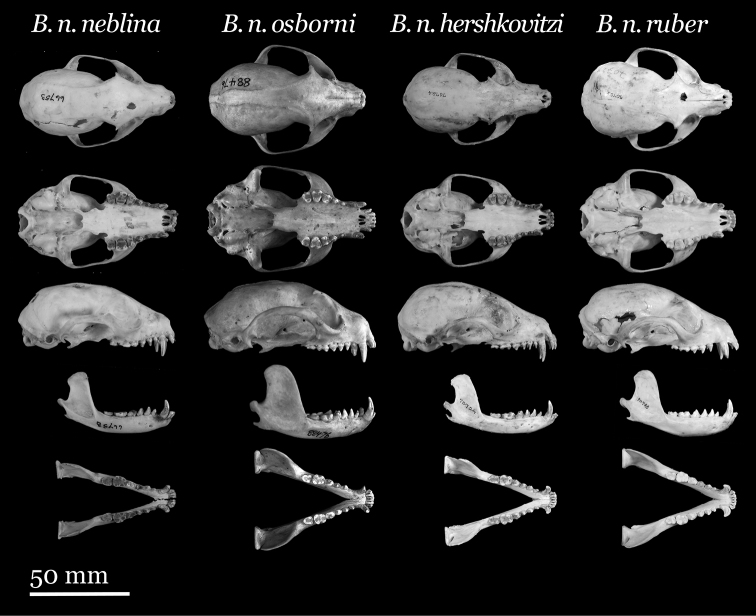
Figure 5.
Skulls of adult female Bassaricyon. From left to right: Bassaricyon gabbii (AMNH 140334, Lajas Villa, Costa Rica); Bassaricyon medius orinomus (AMNH 37797, Puerta Valdivia, Antioquia District, Colombia); Bassaricyon alleni (FMNH 86908, Santa Rita, Rio Nanay, Maynas, Loreto Region, Peru); Bassaricyon neblina hershkovitzi (FMNH 70727, San Antonio, Agustin, Huila District, Colombia). Scale bar = 50 mm.
Table 5.
External measurements of olingo species. For each measurement, means are provided, ± standard deviation, with ranges in parentheses.
|
Bassaricyon gabbii n= 13 |
Bassaricyon medius n= 36 |
Bassaricyon alleni n= 27 |
Bassaricyon neblina n= 19 |
|
|---|---|---|---|---|
| TL | 873 ± 54.8 (785 - 970) |
819 ± 60.5 (680 - 905) |
842 ± 50.6 (705 - 985) |
745 ± 33.7 (660 - 820) |
| Tail | 445 ± 40.3 (400 - 521) |
441 ± 44.6 (350 - 520) |
450 ± 28.8 (401 - 530) |
390 ± 21 (335 - 424) |
| HF | 84 ± 8.7 (65 - 100) |
81 ± 7.3 (58 - 92) |
81 ± 5.8 (70 - 92) |
76 ± 6.9 (60 - 86) |
| Ear | 36 ± 4.7 (25 - 44) |
37 ± 5.4 (25 - 44) |
37 ± 3.4 (30 - 43) |
34 ± 4.3 (25 - 39) |
| Mass (g) | 1382 ± 165 (1136 - 1580) |
1076 ± 71.6 (915 - 1200) |
1336 ± 152 (1100 - 1500) |
872 ± 169 (750 - 1065) |
| HB | 428 ± 27.9 (373 - 470) |
379 ± 23.2 (310 - 415) |
391 ± 29.3 (304 - 455) |
355 ± 21.1 (325 - 400) |
| Tail/HB | 1.04 ± 0.1 (0.9 - 1.2) |
1.16 ± 0.1 (1.0 - 1.4) |
1.15 ± 0.08 (1.0 - 1.3) |
1.10 ± 0.08 (1.0 - 1.2) |
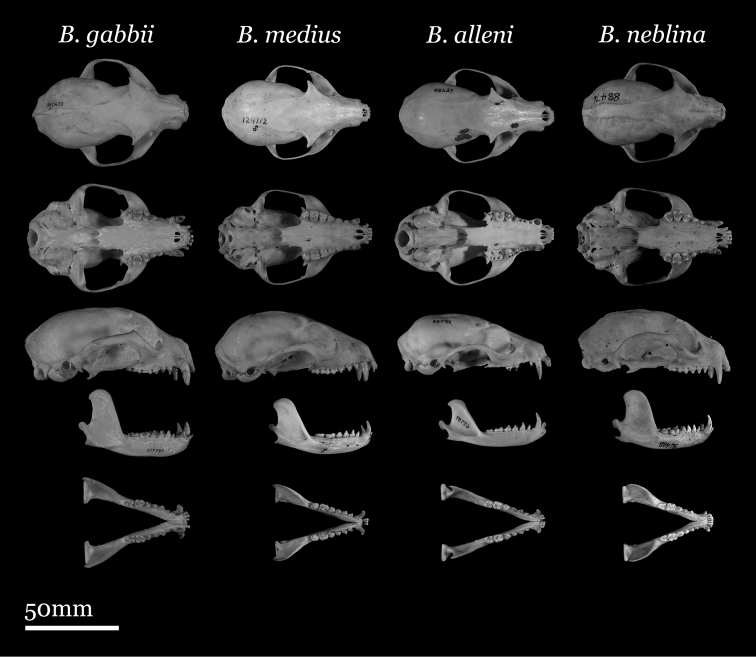
Figure 4.
Skulls of adult male Bassaricyon. From left to right: Bassaricyon gabbii (USNM 324293, Cerro Punta, 1700 m, Chiriqui Mountains, Panama); Bassaricyon medius medius (MVZ 124112, Dagua, 1800 m, Colombia); Bassaricyon alleni (FMNH 65789, Chanchamayo, 1200 m, Junin, Peru); Bassaricyon neblina osborni (FMNH 88476, Munchique, 2000 m, Cauca, Colombia). Scale bar = 50 mm.
Table 3.
Cranial measurements for olingo species (compiled separately by sex). For each measurement, means are provided, ± standard deviation, with ranges in parentheses.
|
Bassaricyon gabbii n= 11 ♂♂, 11 ♀♀ |
Bassaricyon medius n= 18 ♂♂, 27 ♀♀ |
Bassaricyon alleni n= 12 ♂♂, 17 ♀♀ |
Bassaricyon neblina n= 10 ♂♂, 9 ♀♀ |
||
|---|---|---|---|---|---|
| CBL | ♂♂ | 80.8 ± 1.50 (78.1 - 83.0) |
79.4 ± 2.67 (74.5 - 85.1) |
79.4 ± 1.81 (76.5 - 82.8) |
74.5 ± 3.26 (70.1 - 79.5) |
| ♀♀ | 78.2 ± 1.75 (75.0 - 80.2) |
77.3 ± 2.70 (70.8 - 82.3) |
77.0 ± 2.24 (73.1 - 80.5) |
75.1 ± 1.49 (72.9 - 77.9) |
|
| ZYG | ♂♂ | 55.2 ± 2.76 (49.5 - 58.7) |
52.0 ± 2.66 (48.3 - 56.7) |
51.6 ± 1.02 (49.0 - 52.8) |
50.1 ± 3.02 (46.2 - 54.4) |
| ♀♀ | 51.3 ± 1.90 (48.1 - 54.4) |
50.0 ± 2.50 (44.4 - 54.0) |
50.2 ± 0.99 (48.6 - 52.2) |
49.0 ± 2.69 (44.6 - 53.0) |
|
| BBC | ♂♂ | 36.1 ± 0.86 (34.3 - 37.6) |
35.1 ± 1.16 (32.9 - 37.5) |
35.4 ± 0.80 (34.2 - 36.8) |
34.6 ± 1.62 (32.4 - 37.5) |
| ♀♀ | 35.7 ± 1.34 (33.1 - 37.5) |
34.6 ± 1.20 (32.0 - 37.2) |
34.9 ± 0.91 (33.3 - 36.8) |
34.2 ± 1.62 (31.0 - 36.6) |
|
| HBC | ♂♂ | 28.7 ± 0.88 (26.4 - 29.7) |
27.6 ± 0.84 (26.5 - 29.3) |
27.4 ± 0.73 (26.2 - 28.5) |
27.4 ± 0.61 (26.5 - 28.3) |
| ♀♀ | 27.9 ± 0.74 (26.9 - 28.8) |
26.9 ± 0.90 (25.4 - 28.5) |
26.9 ± 0.63 (26.0 - 28.1) |
26.5 ± 0.93 (24.9 - 27.8) |
|
| MTR | ♂♂ | 28.5 ± 0.50 (27.8 - 29.3) |
28.6 ± 0.87 (27.0 - 30.4) |
28.4 ± 0.83 (26.5 - 29.5) |
26.5 ± 1.38 (24.5 - 28.7) |
| ♀♀ | 27.3 ± 1.02 (26.0 - 29.0) |
27.7 ± 0.90 (25.6 - 29.1) |
27.3 ± 0.69 (26.1 - 28.5) |
26.9 ± 0.88 (25.8 - 28.3) |
|
| CC | ♂♂ | 18.7 ± 1.12 (17.2 - 20.4) |
16.4 ± 0.92 (15.0 - 17.9) |
16.8 ± 0.51 (15.8 - 17.6) |
15.9 ± 0.94 (14.7 - 17.1) |
| ♀♀ | 16.9 ± 0.76 (15.6 - 17.9) |
15.7 ± 0.80 (14.5 - 17.2) |
15.9 ± 0.55 (14.8 - 16.8) |
15.7 ± 0.47 (14.9 - 16.4) |
|
| WPP | ♂♂ | 11.3 ± 1.27 (9.0 - 12.9) |
10.3 ± 0.95 (8.4 - 12.1) |
10.4 ± 0.82 (8.7 - 11.8) |
11.7 ± 1.05 (10.6 - 14.0) |
| ♀♀ | 10.7 ± 0.99 (9.3 - 12.7) |
10.3 ± 0.90 (9.0 - 13.0) |
9.9 ± 0.89 (8.2 - 11.7) |
11.6 ± 0.87 (10.5 - 12.7) |
|
| LPP | ♂♂ | 12.3 ± 0.99 (10.7 - 14.0) |
10.2 ± 0.88 (7.9 - 11.7) |
10.8 ± 1.21 (9.3 - 12.9) |
11.2 ± 1.24 (9.2 - 12.7) |
| ♀♀ | 10.8 ± 0.77 (9.7 - 12.0) |
10.1 ± 0.90 (8.1 - 11.8) |
10.4 ± 0.67 (8.7 - 11.6) |
11.1 ± 0.82 (9.7 - 12.3) |
|
| LAB | ♂♂ | 13.8 ± 0.63 (12.9 - 14.7) |
14.0 ± 0.81 (12.8 - 15.6) |
15.1 ± 0.76 (14.1 - 16.8) |
11.8 ± 0.76 (10.9 - 13.3) |
| ♀♀ | 13.8 ± 0.67 (12.9 - 14.8) |
14.0 ± 0.80 (12.6 - 15.2) |
14.4 ± 0.81 (13.0 - 15.6) |
12.2 ± 0.51 (11.0 - 12.7) |
|
| EAM | ♂♂ | 3.6 ± 0.47 (2.6 - 4.2) |
3.9 ± 0.33 (3.4 - 4.5) |
3.8 ± 0.40 (3.2 - 4.5) |
2.9 ± 0.22 (2.5 - 3.1) |
| ♀♀ | 3.6 ± 0.39 (3.0 - 4.2) |
3.9 ± 0.30 (3.5 - 4.7) |
3.8 ± 0.36 (3.2 - 4.4) |
3.2 ± 0.33 (2.6 - 3.5) |
|
Table 4.
Selected dental measurements of olingo species. For each measurement, means are provided, ± standard deviation, with ranges in parentheses.
|
Bassaricyon gabbii n= 22 |
Bassaricyon medius n= 45 |
Bassaricyon alleni n= 34 |
Bassaricyon neblina n= 19 |
|
|---|---|---|---|---|
| p1 width | 1.7 ± 0.17 (1.4 - 2.1) |
1.7 ± 0.13 (1.4 - 2.0) |
1.7 ± 0.12 (1.5 - 1.9) |
1.6 ± 0.13 (1.4 - 1.8) |
| p2 width | 2.4 ± 0.24 (2.0 - 2.8) |
2.2 ± 0.18 (1.8 - 2.6) |
2.2 ± 0.15 (1.9 - 2.5) |
2.1 ± 0.17 (1.9 - 2.5) |
| p3 width | 2.7 ± 0.21 (2.3 - 3.0) |
2.5 ± 0.18 (2.2 - 2.9) |
2.6 ± 0.16 (2.2 - 2.9) |
2.4 ± 0.22 (2.1 - 2.9) |
| p4 width | 3.4 ± 0.27 (3.0 - 3.9) |
3.2 ± 0.18 (2.8 - 3.6) |
3.4 ± 0.21 (2.8 - 3.7) |
3.3 ± 0.15 (3.0 - 3.7) |
| P2 width | 2.4 ± 0.24 (2.1 - 2.9) |
2.3 ± 0.19 (1.9 - 2.8) |
2.2 ± 0.17 (1.9 - 2.7) |
2.1 ± 0.19 (1.8 - 2.5) |
| P3 width | 2.9 ± 0.22 (2.5 - 3.3) |
3.0 ± 0.29 (2.5 - 3.6) |
3.0 ± 0.22 (2.6 - 3.5) |
2.9 ± 0.21 (2.6 - 3.4) |
| P4 length | 4.4 ± 0.24 (3.9 - 4.8) |
4.2 ± 0.27 (3.6 - 4.9) |
4.2 ± 0.20 (3.8 - 4.6) |
4.5 ± 0.24 (4.1 - 4.9) |
| P4 width | 5.1 ± 0.35 (4.5 - 5.6) |
4.7 ± 0.26 (4.2 - 5.4) |
4.8 ± 0.23 (4.4 - 5.6) |
5.0 ± 0.40 (4.5 - 5.9) |
| M1 length | 5. 0 ± 0.27 (4.4 - 5.4) |
5.0 ± 0.29 (4.3 - 5.6) |
5.1 ± 0.21 (4.6 - 5.5) |
5.3 ± 0.35 (4.8 - 6.1) |
| M1 width | 5.5 ± 0.30 (4.7 - 5.9) |
5.3 ± 0.32 (4.7 - 5.9) |
5.5 ± 0.28 (4.9 - 6.0) |
5.8 ± 0.31 (5.4 - 6.4) |
| M2 length | 3.7 ± 0.32 (2.8 - 4.1) |
4.0 ± 0.25 (3.2 - 4.4) |
3.8 ± 0.27 (3.3 - 4.4) |
3.8 ± 0.35 (3.3 - 4.4) |
| M2 width | 4.6 ± 0.38 (4.0 - 5.3) |
4.7 ± 0.27 (4.1 - 5.2) |
4.7 ± 0.28 (4.0 - 5.2) |
4.8 ± 0.24 (4.4 - 5.4) |
| m1 length | 5.6 ± 0.31 (5.0 - 6.3) |
5.7 ± 0.26 (4.9 - 6.2) |
5.6 ± 0.22 (5.2 - 6.0) |
5.8 ± 0.29 (5.4 - 6.3) |
| m1 width | 4.3 ± 0.29 (3.8 - 4.9) |
4.3 ± 0.21 (3.9 - 4.7) |
4.3 ± 0.23 (3.7 - 4.8) |
4.8 ± 0.22 (4.5 - 5.3) |
| m2 length | 4.8 ± 0.25 (4.4 - 5.3) |
5.1 ± 0.36 (4.2 - 5.7) |
4.8 ± 0.25 (4.4 - 5.4) |
5.0 ± 0.35 (4.4 - 5.6) |
| m2 width | 3.8 ± 0.24 (3.3 - 4.2) |
3.7 ± 0.24 (3.2 - 4.2) |
3.7 ± 0.19 (3.3 - 4.0) |
3.8 ± 0.17 (3.5 - 4.1) |
Principal component analyses of cranial and dental measurements support our molecular results in clearly identifying a fundamental morphometric separation between the Olinguito (Bassaricyon neblina sp. n.) and all other Bassaricyon taxa, in separate comparisons involving both males and females (Figures 6–7, Appendix 1). When first and second principal components are juxtaposed in a bivariate plot, Olinguito specimens demonstrate clear morphometric separation from all other Bassaricyon, despite overlap between these clusters in body size (as indicated by overlap in the first principal component, on which all or most variables in the analysis show positive [males] or negative [females] loadings). Despite smaller average body size compared to other Bassaricyon, the morphometric distinctness of Olinguito specimens is reflected not especially in small size but rather primarily by separation along the second principal component, indicating trenchant differences in overall shape and proportion, especially reflecting consistent differences in the molars, auditory bullae, external auditory meati, and palate, in which the Olinguito differs strongly and consistently from other Bassaricyon (Figures 6–7, Tables 3–4, Appendix 1).
Figure 6.
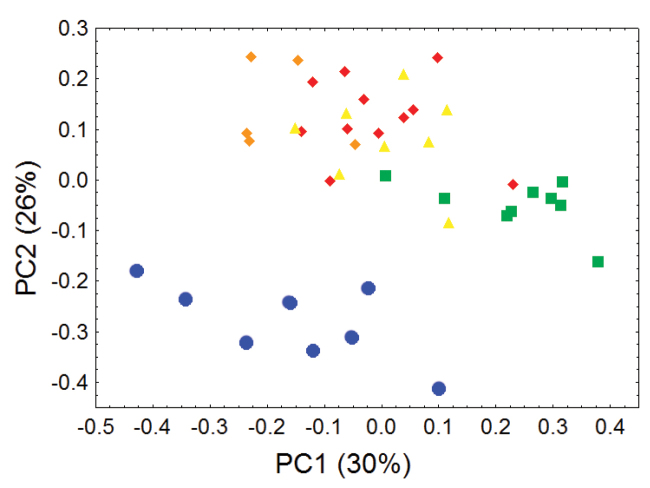
Morphometric distinction between Olinguitos and other Bassaricyon, males. Morphometric dispersion (first two components of a principal component analysis) of 41 adult male Bassaricyon skulls based on 21 craniodental measurements (see Appendix 1, Table A1). The most notable morphometric distinction is between the Olinguito (blue circles) and all other Bassaricyon taxa. The plot also demonstrates substantial morphometric variability across geographic populations of the Olinguito, which we characterize with the description of four subspecies across different Andean regions. Symbols: blue circles (Bassaricyon neblina), green squares (Bassaricyon gabbii), yellow triangles (Bassaricyon alleni), orange diamonds (Bassaricyon medius medius), red diamonds (Bassaricyon medius orinomus).
Figure 7.
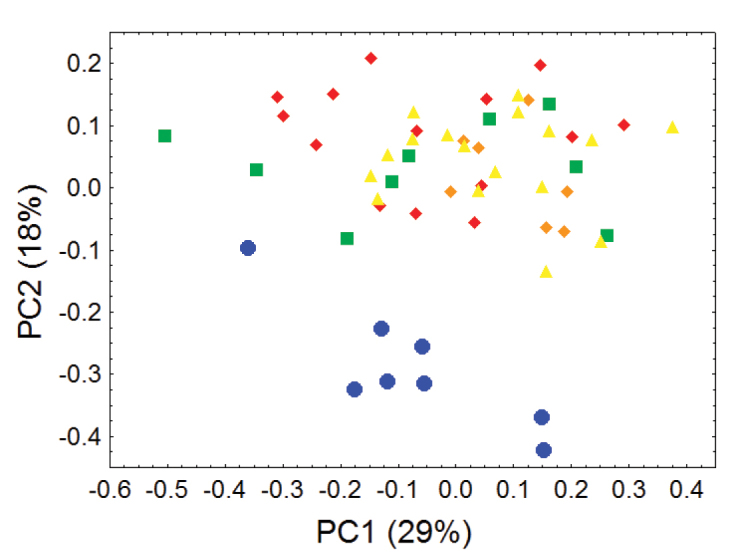
Morphometric distinction between Olinguitos and other Bassaricyon, females. Morphometric dispersion (first two components of a principal component analysis) of 55 adult female Bassaricyon skulls based on 24 craniodental measurements (see Appendix 1, Table A2). The most notable morphometric distinction is between the Olinguito (blue circles) and all other Bassaricyon taxa. The plot also demonstrates substantial morphometric variability across geographic populations of the Olinguito, which we characterize with the description of four subspecies across different Andean regions. Symbols: blue circles (Bassaricyon neblina), green squares (Bassaricyon gabbii), yellow triangles (Bassaricyon alleni), orange diamonds (Bassaricyon medius medius), red diamonds (Bassaricyon medius orinomus).
The lower elevation olingo taxa Bassaricyon gabbii, Bassaricyon medius, and Bassaricyon alleni are not separable in most principal component analyses of craniodental measurements (e.g., Figures 6–7), but discriminant function analyses of craniodental measurements (e.g., Figure 8, showing separation of male skulls) separates them into discrete clusterings with few misclassifications, and identifies some of the more important craniodental traits that help to distinguish between them (Appendix 1). These (and other, qualitative) craniodental distinctions are complemented by differences in pelage features and genetic divergences that we discuss below.
Figure 8.
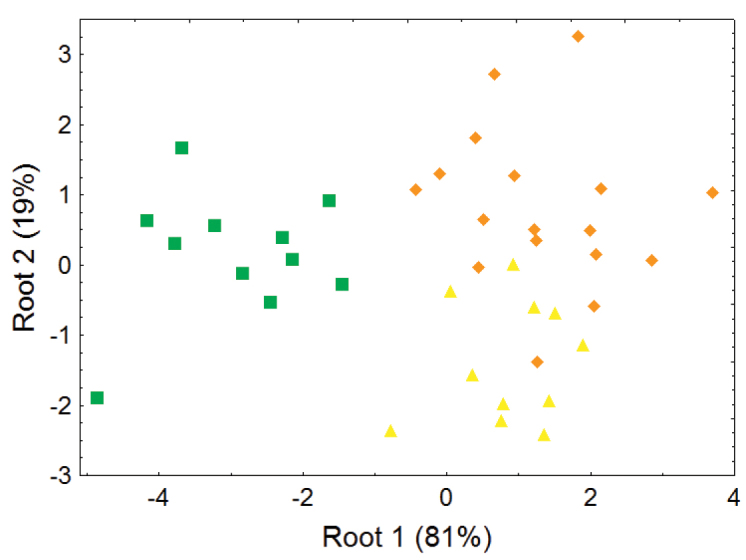
Morphometric distinction between species of Bassaricyon, excluding the Olinguito, adult males. Morphometric dispersion (first two variates of a discriminant function analysis) of 39 adult male Bassaricyon skulls based on 8 craniodental measurements (see Appendix 1, Table A3). Symbols: green squares (Bassaricyon gabbii), yellow triangles (Bassaricyon alleni), orange diamonds (Bassaricyon medius).
Because of marked and consistent differences in body size between the two regional populations of Bassaricyon medius (one distributed in western South America, the other primarily distributed in Panama), we choose to recognize these two as separate subspecies (Bassaricyon medius medius and Bassaricyon medius orinomus, respectively, Tables 6–7). The Olinguito likewise shows a clear pattern of geographic variation, with different regional populations in the Northern Andes showing consistent differences in craniodental size and morphology (Figures 9–10, Table 8, Appendix 1), as well as pelage coloration and length. We recognize four distinctive subspecies of the Olinguito throughout its recorded distribution, as discussed in the description of Bassaricyon neblina sp. n., below. Two of these subspecies are included in our genetic comparisons; genetic comparisons involving the remaining two subspecies remain a goal for the future.
Table 6.
Cranial measurements for the two subspecies of Bassaricyon medius. For each measurement, means are provided, ± standard deviation, with ranges in parentheses.
| Bassaricyon medius medius | Bassaricyon medius orinomus | ||
|---|---|---|---|
| W Colombia, W Ecuador | C Panama to N Colombia | ||
| n = 5 ♂♂, 7 ♀♀ | n = 12 ♂♂, 17 ♀♀ | ||
| CBL | ♂♂ | 77.2 ± 1.81 (74.5 - 78.8) | 80.3 ± 2.50 (76.2 - 85.1) |
| ♀♀ | 75.4 ± 1.65 (72.4 - 76.7) | 78.8 ± 1.72 (75.5 - 82.3) | |
| ZYG | ♂♂ | 50.2 ± 1.14 (48.9 - 51.2) | 53.0 ± 2.57 (48.9 - 56.7) |
| ♀♀ | 48.5 ± 1.69 (46.5 – 51.0) | 51.2 ± 1.98 (47.4 – 54.0) | |
| BBC | ♂♂ | 34.0 ± 0.80 (32.9 - 34.8) | 35.6 ± 0.98 (34.0 - 37.5) |
| ♀♀ | 34.4 ± 0.41 (33.7 – 35.0) | 35.0 ± 1.15 (32.8 - 37.2) | |
| HBC | ♂♂ | 28.2 ± 1.06 (27.1 - 29.3) | 27.4 ± 0.62 (26.6 - 28.3) |
| ♀♀ | 26.8 ± 0.89 (26.1 - 28.5) | 27.0 ± 0.89 (25.4 - 28.5) | |
| MTR | ♂♂ | 28.5 ± 0.97 (27.3 - 29.8) | 28.7 ± 0.90 (27.0 - 30.4) |
| ♀♀ | 27.1 ± 0.78 (25.6 - 27.9) | 28.0 ± 0.77 (26.4 - 29.1) | |
| CC | ♂♂ | 15.9 ± 0.69 (15.1 - 17.0) | 16.7 ± 0.94 (15.0 - 17.9) |
| ♀♀ | 15.0 ± 0.46 (14.5 - 15.8) | 16.1 ± 0.71 (14.6 - 17.2) | |
| WPP | ♂♂ | 9.7 ± 0.95 (8.4 - 10.8) | 10.6 ± 0.91 (8.6 - 12.1) |
| ♀♀ | 10.0 ± 0.57 (9.1 - 10.6) | 10.3 ± 1.04 (9.0 - 13.0) | |
| LPP | ♂♂ | 9.4 ± 1.03 (7.9 - 10.6) | 10.5 ± 0.64 (9.8 - 11.7) |
| ♀♀ | 9.8 ± 0.84 (8.9 - 11.3) | 10.2 ± 1.01 (8.1 - 11.8) | |
| LAB | ♂♂ | 13.6 ± 0.72 (12.8 - 14.6) | 14.2 ± 0.84 (13.1 - 15.6) |
| ♀♀ | 13.4 ± 0.45 (12.6 - 13.9) | 14.3 ± 0.73 (12.8 - 15.2) | |
| EAM | ♂♂ | 3.9 ± 0.47 (3.4 - 4.5) | 3.9 ± 0.27 (3.5 - 4.4) |
| ♀♀ | 3.9 ± 0.34 (3.5 - 4.4) | 3.9 ± 0.28 (3.6 - 4.7) | |
Table 7.
External measurements for the two subspecies of Bassaricyon medius. For each measurement, means are provided, ± standard deviation, with ranges in parentheses.
|
Bassaricyon medius medius W Colombia, W Ecuador n= 12 |
Bassaricyon medius orinomus C Panama to N Colombia n= 24 |
|
|---|---|---|
| TL | 754 ± 49.7 (680 - 819) | 844 ± 42.9 (770 - 905) |
| Tail | 392 ± 29.1 (350 - 435) | 460 ± 33.6 (400 - 520) |
| HF | 73 ± 5.4 (58 - 79) | 85 ± 3.5 (77 - 92) |
| Ear | 32 ± 4.8 (25 - 40) | 39 ± 4 (30 - 44) |
| Mass (g) | 1058 ± 146 (915 - 1200) | 1090 ± 19.2 (1050 - 1100) |
| HB | 362 ± 29.5 (310 - 415) | 385 ± 17.2 (355 - 410) |
| Tail/HB | 1.1 ± 0.09 (0.97 - 1.24) | 1.2 ± 0.08 (1.04 - 1.35) |
Figure 9.
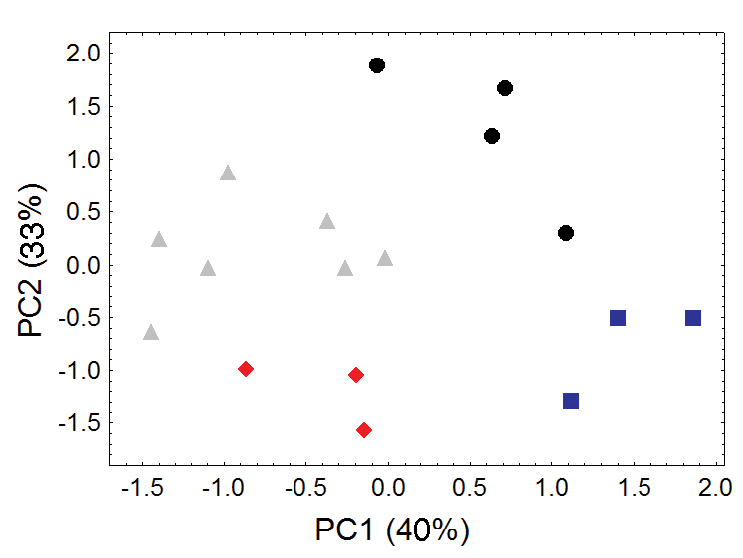
Morphometric distinction between Olinguito subspecies. Both sexes combined. Morphometric dispersion (first two components of a principal component analysis) of 17 adultskulls based on 13 cranial measurements (see Appendix 1, Table A4). (Dental measurements also discretely partition these subspecies in a separate principal component analysis, not shown.) Black dots = Bassaricyon neblina neblina; gray triangles = Bassaricyon neblina osborni; red diamonds = Bassaricyon neblina ruber; blue squares = Bassaricyon neblina hershkovitzi.
Figure 10.
Skulls of Olinguito subspecies. From left to right: Bassaricyon neblina neblina (AMNH 66753, holotype, old adult female, Las Maquinas, Ecuador); Bassaricyon neblina osborni (FMNH 88476, holotype, adult male, Munchique, 2000 m, Cauca Department, Colombia); Bassaricyon neblina hershkovitzi (FMNH 70724, paratype, adult male, San Antonio, Agustin, Huila District, Colombia); Bassaricyon neblina ruber (FMNH 70723, paratype, adult male, Guapantal, 2200 m, Urrao, Antioquia Department, Colombia). Scale bar = 50 mm.
Table 8.
Dental and cranial measurements of Olinguito (Bassaricyon neblina) subspecies. For each measurement, means are provided, ± standard deviation, with ranges in parentheses.
|
Bassaricyon neblina ruber n= 3 |
Bassaricyon neblina hershkovitzi n= 4 |
Bassaricyon neblina osborni n= 8 |
Bassaricyon neblina neblina n= 4 |
|
|---|---|---|---|---|
| p1 width | 1.4 ± 0.06 (1.4 - 1.5) |
1.5 ± 0.12 (1.4 - 1.6) |
1.6 ± 0.09 (1.6 - 1.8) |
1.7 ± 0.11 (1.5 - 1.8) |
| p2 width | 2.1 ± 0.14 (1.9 - 2.2) |
1.9 ± 0.06 (1.9 – 2.0) |
2.2 ± 0.15 (2.0 - 2.5) |
2.2 ± 0.17 (2.1 - 2.4) |
| p3 width | 2.4 ± 0.08 (2.3 - 2.5) |
2.2 ± 0.06 (2.1 - 2.2) |
2.5 ± 0.16 (2.4 - 2.8) |
2.4 ± 0.32 (2.2 - 2.9) |
| p4 width | 3.3 ± 0.11 (3.2 - 3.4) |
3.1 ± 0.12 (3.0 - 3.3) |
3.4 ± 0.13 (3.2 - 3.7) |
3.4 ± 0.09 (3.3 - 3.5) |
| P2 width | 2.0 (2.0 – 2.0) |
1.9 ± 0.05 (1.8 – 2.0) |
2.2 ± 0.17 (2.1 - 2.5) |
2.3 ± 0.15 (2.2 - 2.5) |
| P3 width | 2.9 ± 0.17 (2.7 - 3.1) |
2.7 ± 0.10 (2.6 - 2.8) |
3.0 ± 0.19 (2.8 - 3.4) |
3.1 ± 0.15 (2.9 - 3.3) |
| P4 length | 4.3 ± 0.21 (4.1 - 4.5) |
4.2 ± 0.13 (4.1 - 4.3) |
4.5 ± 0.17 (4.3 - 4.8) |
4.7 ± 0.17 (4.5 - 4.9) |
| P4 width | 4.6 ± 0.14 (4.5 - 4.8) |
5.0 ± 0.23 (4.8 - 5.3) |
4.9 ± 0.20 (4.6 - 5.1) |
5.7 ± 0.13 (5.6 - 5.9) |
| M1 length | 5.0 ± 0.12 (5.0 - 5.2) |
5.0 ± 0.25 (4.8 - 5.4) |
5.3 ± 0.23 (5.0 - 5.6) |
5.7 ± 0.4 (5.2 - 6.1) |
| M1 width | 5.5 ± 0.14 (5.4 - 5.6) |
5.5 ± 0.10 (5.4 - 5.6) |
5.8 ± 0.20 (5.5 - 6.1) |
6.2 ± 0.13 (6.1 - 6.4) |
| M2 length | 3.6 ± 0.22 (3.5 - 3.9) |
3.5 ± 0.16 (3.3 - 3.7) |
4.1 ± 0.29 (3.6 - 4.4) |
3.9 ± 0.4 (3.3 - 4.2) |
| M2 width | 4.5 ± 0.13 (4.4 - 4.6) |
4.7 ± 0.03 (4.7 - 4.8) |
4.8 ± 0.20 (4.6 - 5.2) |
4.9 ± 0.3 (4.7 - 5.4) |
| m1 length | 5.5 ± 0.05 (5.4 - 5.5) |
5.8 ± 0.21 (5.6 – 6.0) |
5.8 ± 0.18 (5.6 – 6.0) |
6.2 ± 0.03 (6.2 - 6.3) |
| m1 width | 4.7 ± 0.12 (4.6 - 4.8) |
4.8 ± 0.17 (4.7 – 5.0) |
4.8 ± 0.26 (4.5 - 5.3) |
5.0 ± 0.22 (4.7 - 5.2) |
| m2 length | 4.7 ± 0.39 (4.4 - 5.1) |
5.0 ± 0.37 (4.5 - 5.2) |
5.2 ± 0.26 (4.9 - 5.6) |
4.8 ± 0.22 (4.5 - 5.1) |
| m2 width | 3.7 ± 0.09 (3.6 - 3.8) |
3.7 ± 0.19 (3.5 - 3.9) |
3.9 ± 0.10 (3.7 - 4.0) |
3.9 ± 0.16 (3.7 - 4.1) |
| CBL | 73.0 ± 0.58 (72.4 - 73.5) |
71.4 ± 1.13 (70.1 - 72.9) |
76.6 ± 1.64 (75.1 - 79.5) |
75.9 ± 1.4 (74.6 - 77.9) |
| ZYG | 51.1 ± 2.28 (48.9 - 53.4) |
46.7 ± 0.60 (46.2 - 47.5) |
51.7 ± 1.73 (49.1 - 54.4) |
46.9 ± 1.59 (44.6 - 48) |
| BBC | 36.0 ± 1.44 (34.7 - 37.5) |
32.9 ± 0.54 (32.4 - 33.6) |
35.1 ± 0.90 (33.9 - 36.6) |
33.2 ± 1.62 (31.0 - 34.9) |
| HBC | 27.7 ± 0.55 (27.2 - 28.3) |
27.6 ± 0.38 (27.1 - 27.9) |
27.2 ± 0.58 (26.5 - 28.2) |
25.8 ± 0.63 (24.9 - 26.2) |
| MTR | 25.9 ± 0.22 (25.7 - 26.1) |
25.1 ± 0.56 (24.5 - 25.8) |
27.4 ± 0.78 (26.0 - 28.7) |
27.5 ± 0.56 (27 - 28.3) |
| CC | 15.7 ± 0.52 (15.4 - 16.3) |
14.9 ± 0.15 (14.7 – 15.0) |
16.4 ± 0.54 (15.5 - 17.1) |
15.6 ± 0.25 (15.4 - 15.9) |
| WPP | 12.1 ± 0.25 (11.8 - 12.3) |
11.8 ± 1.54 (10.6 – 14.0) |
11.8 ± 0.74 (10.8 - 12.8) |
10.9 ± 0.8 (10.5 - 12.1) |
| LPP | 10.9 ± 0.54 (10.3 - 11.4) |
9.7 ± 0.34 (9.2 - 9.9) |
11.9 ± 0.56 (11.0 - 12.7) |
11.2 ± 1.05 (9.7 - 12.3) |
| LAB | 11.7 ± 0.38 (11.4 - 12.1) |
11.2 ± 0.40 (10.9 - 11.8) |
12.3 ± 0.60 (11.2 - 13.3) |
12.5 ± 0.18 (12.3 - 12.7) |
| EAM | 2.7 (2.7 - 2.7) |
3.2 ± 0.16 (3.1 - 3.4) |
2.9 ± 0.29 (2.5 - 3.3) |
3.4 ± 0.05 (3.4 - 3.5) |
Bioclimatic range modeling
Distribution models for all species are judged to have performed well based on their high values for ‘area under the curve’ (AUC) and unregularized test gain (Table 9), as well as their fit of the final prediction to the locality data (Figures 11–12). There was relatively low impact of withholding test data from these models, as indicated by the low Mean Test AUC values. These values are lowest for Bassaricyon alleni, probably reflecting its larger distribution relative to the variation of environmental data (Phillips et al. 2006). The strongest environmental predictors for Bassaricyon neblina sp. n. were seasonal variation in temperature (suitability declines with higher variation, after sharp threshold) and the temperature of the wettest quarter (negative relationship). The annual range of temperatures was the most important predictor for the Bassaricyon gabbii and Bassaricyon medius distributions (both sharp negative relationships). Bassaricyon alleni was the only one of the four species to have an ecological biome ranked as one of the top predictors (Tropical Moist Broadleaf Forests as highly suitable).
Table 9.
Performance of bioclimatic distribution models for four Bassaricyon species using vouchered specimen localities. Mean values are averages of 10 models run, each withholding 20% of data as test localities, while the Full Model AUC used all available data. The mean value for equal training sensitivity and specificity was used as a logistic threshold to create a range map predicting presence/absence.
| Localities | Mean Test AUC (stdev) | Full Model AUC | Mean Unregularized Training Gain | Mean equal training sensitivity and specificity (logistic threshold) | |
|---|---|---|---|---|---|
| Bassaricyon alleni | 43 | 0.901 (0.036) | 0.939 | 1.85 | 0.302 |
| Bassaricyon gabbii | 18 | 0.977 (0.012) | 0.993 | 4.09 | 0.222 |
| Bassaricyon medius | 31 | 0.952 (0.028) | 0.988 | 3.76 | 0.119 |
| Bassaricyon neblina | 16 | 0.996 (0.002) | 0.998 | 4.77 | 0.160 |
Figure 11.

Bioclimatic distribution models and localities for Bassaricyon species. Models from MAXENT using all vouchered occurrence records, 19 bioclimatic variables, and one potential habitat variable.
Figure 12.
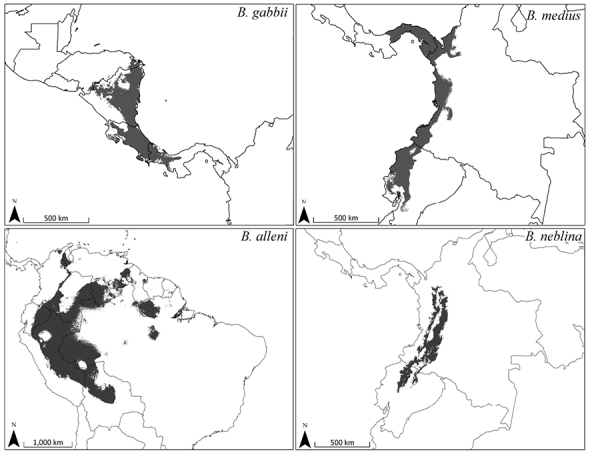
Predicted distribution for Bassaricyon species based on bioclimatic models. To create these binary maps we used the average minimum training presence for 10 test models as our cutoff. In addition, we excluded areas of high probability that were outside of the known range of the species if they were separated by unsuitable habitat.
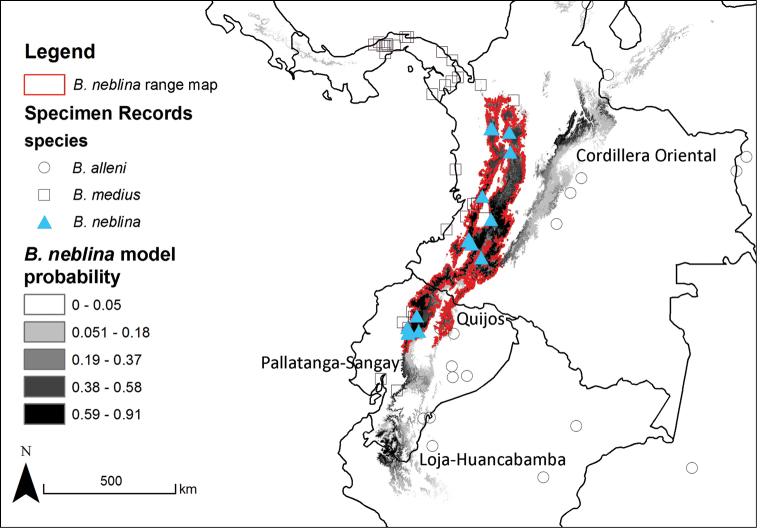
The full Maxent distribution models predict the suitability of habitat across South and Central America (Figure 11). To make the binary prediction maps (Figure 12) we excluded areas with high probability that were disjunct from areas where specimens have been recorded (e.g., western Venezuela excluded from the map for Bassaricyon neblina sp. n., central and eastern Brazil excluded from the Bassaricyon alleni map, northern Central America excluded from the Bassaricyon medius map, South America excluded from the Bassaricyon gabbii map). For Bassaricyon neblina sp. n. we excluded areas of high probability from the Eastern Cordillera of Colombia and the Andes of southern Ecuador and northern Peru because of the lack of specimens. Likewise, predicted suitable habitat for Bassaricyon gabbii in northern Central America (Honduras, Guatemala) remains unverified by specimen data. Although there are two recent unconfirmed records in the region (Ordóñez Garza et al. 1999–2000), the specific locations of these sightings did not fall in areas predicted as suitable habitat by our models. Finally, the exact area of transition between Bassaricyon gabbii and Bassaricyon medius in Panama remains unclear. All of these regions should be considered high priority areas for future surveys, especially areas identified as potential Bassaricyon neblina sp. n. habitat (see Discussion, below).
The range of Bassaricyon neblina sp. n. is typical of many Andean species in being restricted to wet cloud forest habitats, which are limited in area and also under heavy development pressure. In comparing recent land use (Eva et al. 2004) of suitable historical Bassaricyon neblina habitat, we found that 42% of suitable habitats have been converted to agriculture or urban areas, and 21% remain in natural but largely unforested conditions. Thus we predict that only 37% (40,760 km2) of appropriate Olinguito habitats remain forested.
Systematics
Bassaricyon neblina sp. n.
http://zoobank.org/94DDB038-2111-44D1-A940-766BF8F15E51
http://species-id.net/wiki/Bassaricyon_neblina
Holotype.
We designate as the holotype of neblina specimen number 66753 in the mammalogy collection of the American Museum of Natural History, New York, a skin and complete skull of an old adult female, from Las Máquinas (= Las Machinas [see Voss 1988:474], circa 00°32’S, 78°39’W, 2130 m), Pichincha Province, Ecuador, collected 21 September 1923 by G.H.H. Tate.
Referred specimens.
QCAZ 0159, partial skin, Otonga Reserve, 1800 m, Cotopaxi Province, Ecuador; MECN 2177, adult female, skin and skull, La Cantera 2300 m, Cotopaxi Province, Ecuador; QCAZ 8661, young adult female, skin, skull, and postcranial skeleton, Otonga Reserve, 2100 m, Cotopaxi Province, Ecuador (collected by K. Helgen et al., August 2006); QCAZ 8662, young adult female, skin, skull, and postcranial skeleton, [“forested gully near”] La Cantera, 2260 m, Cotopaxi Province, Ecuador (collected by M. Pinto et al., August 2006). We have also seen photographs of this species from Tandayapa, 2350 m, Pichincha Province (Figure 13).
Figure 13.

The Olinguito, Bassaricyon neblina neblina, in life, in the wild. Taken at Tandayapa Bird Lodge, Ecuador (for mammalogical background of Tandayapa, see Lee et al. 2006). Photograph by Mark Gurney.
Below, we identify additional referred specimens when we describe three additional subspecies of Bassaricyon neblina from the cordilleras of Colombia (Figures 9–10, 13–16).
Figure 16.
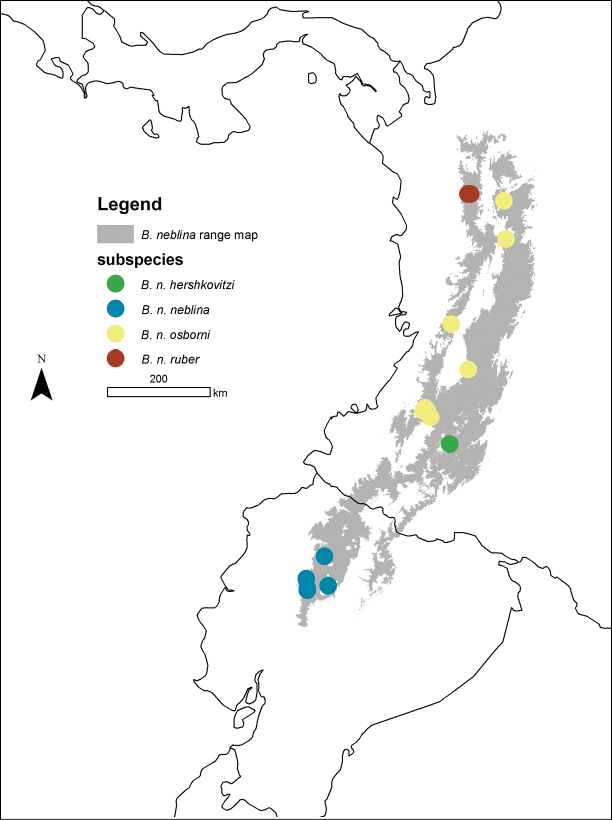
Distributions (localities) of the four Olinguito subspecies in the Andes of Colombia and Ecuador.
Diagnosis.
Bassaricyon neblina can be easily identified on the basis of both external and craniodental characteristics (Figures 3–7, Tables 3–5). It differs from other Bassaricyon in its smaller body and cranial size; longer, denser, and more richly coloured dorsal pelage (black-tipped, tan to strikingly orange- to reddish-brown); indistinctly banded, bushier, and proportionally shorter tail (at least compared to the lowland olingos, Bassaricyon alleni and Bassaricyon medius, Table 5); (externally) more rounded face with a blunter, less tapering muzzle; smaller and more heavily furred external ears, and considerably reduced auditory bullae, with a markedly smaller external auditory meatus; broadened and more elongate postdental palate (‘palatal shelf’), bearing more prominent lateral ‘flanges’ (sometimes developed to the point where it nearly closes off the “palatal notch” sensu Asher 2007); and proportionally much larger first molars (M1and m1), achieved especially by the development of more massive and bulbous principal molar cusps (protocone, paracone, metacone, hypocone) in M1, and for m1 by the widening of the talonid with the expansion in particular of the entoconid and hypoconid. The m1paraconid is reduced relative to other Bassaricyon.
Where Bassaricyon medius and Bassaricyon neblina occur in regional sympatry on the western slopes of the Andes, Bassaricyon neblina is smaller and more richly rufous and/or blackish in coloration, and is distinguished by all of the characteristics noted above. Externally, Bassaricyon neblina can only be confused with the highest elevation populations of Bassaricyon alleni, from forests above 1000 m on the eastern slopes of the Andes (specimens from Pozuzo and Chanchamayo in Peru), which, like Bassaricyon neblina, also have long, black-tipped dorsal pelage (though not so strongly rufous as in Bassaricyon neblina), ears that are especially furry (though not so small as in Bassaricyon neblina), and tails averaging slightly shorter than in lowland populations of Bassaricyon alleni (but not as short as in Bassaricyon neblina). The craniodental characteristics of Bassaricyon neblina (especially of the palate, bullae, and molars) are unmistakable.
Etymology.
The specific epithet neblina (Spanish, “fog or mist”), a noun in apposition, references the cloud forest habitat of the Olinguito.
Distribution.
The recorded distribution of Bassaricyon neblina comprises humid montane rainforests (“cloud forests”) from 1500 m to 2750 m in the Northern Andes, specifically along the western and eastern slopes of the Western Andes of Colombia and Ecuador, and along the western and eastern slopes of the Central Andes of Colombia (Figure 16). Bassaricyon neblina occurs in regional sympatry with Bassaricyon medius medius on the western slopes of the Ecuadorian Andes, where we have encountered the two species at localities less than 5 km apart. On the basis of our museum and field research, we document Bassaricyon neblina from 16 localities (representing 19 elevational records) in the Western Andes of Ecuador and the Western and Central Andes of Colombia. All sites are situated between 1500 and 2750 m (mean 2100 m, median 2130 m, ± 280 s.d.) and are associated with humid montane forest (“cloud forest”, Churchill et al. 1995). We used bioclimatic modeling to predict the global geographic distribution of Bassaricyon neblina, which comprises wet, forested ecoregions typical of the habitats where Olinguitos have been recorded (Figures 11–12). As noted above, of the entire land area predicted to be suitable for Olinguito occurrence, 42% has been converted to agriculture or urban areas and 21% comprises other unforested landscapes; only 37% (40,760 km2) of this land area is currently forested.
Geographic variation.
Geographic variation in the Olinguito is remarkable, reflecting consistent regional differences in color, size, and craniodental features associated with differential distributions in disjunct areas of the Andes. This is unsurprising given that the montane forests of the Central and Western Cordilleras of the Northern Andes are a region where major evolutionary differentiation has unfolded in many endemic Andean vertebrate groups (e.g., Benham 2012, Graham et al. 2010, Voss et al. 2002, Velasco et al. 2010). Below we diagnose four distinctive subspecies of Bassaricyon neblina and describe their geographic ranges as so far understood.
Subspecies of Bassaricyon neblina
Bassaricyon neblina neblina subsp. n.
http://species-id.net/wiki/Bassaricyon_neblina_neblina
(western slopes of Western Andes of Ecuador)
Diagnosis.
This subspecies is (in skull length) smaller than Bassaricyon neblina osborni subsp. n., but larger than Bassaricyon neblina hershkovitzi subsp. n. and Bassaricyon neblina ruber subsp. n. (though Bassaricyon neblina ruber subsp. n. is more robust cranially, with a wider skull). It has proportionally very large teeth, especially P4 and the first molars, and a narrow skull, with a narrow and low-domed braincase (Figures 9–10, Table 8). In color it most closely resembles Bassaricyon neblina osborni subsp. n., but is the least rufous of the subspecies, usually with the greatest preponderance of black tipping to the fur (e.g., Figure 13).
Distribution.
The nominate subspecies is endemic to Ecuador, where it is recorded from the western slopes of the Andes, in Pichincha and Cotopaxi Provinces, in forests at elevations from 1800 to 2300 m (Figure 16).
Referred specimens.
As listed for Bassaricyon neblina, above.
Bassaricyon neblina osborni subsp. n.
http://species-id.net/wiki/Bassaricyon_neblina_osborni
(eastern slopes of Western Andes and western slopes of Central Andes of Colombia)
Diagnosis.
This is the largest subspecies of Bassaricyon neblina, with a short rostrum, widely splayed zygomata, wide rostrum and braincase, and very large molars and posterior premolars; the dorsal pelage is of moderate length, tan to orangish-brown in overall color, with prominent black and gold tipping, with a more grayish face and limbs, with the limbs bearing relatively short fur, and a tail usually grizzled with golden-brown fur tipping.
Distribution.
This is the representative of Bassaricyon neblina on the eastern slopes of the Western Andes of Colombia (e.g., Castilla Mountains [AMNH]; Sabanetas [FMNH]; El Tambo [NMS]; the vicinity of Cali [Poglayen-Neuwall 1976]; El Duende [Saavedra-Rodríguez and Velandia-Perilla 2011]; Gallera: “western slope of most eastern ridge of southern Western Andes” [AMNH, Paynter 1997:222]) and the western slopes of the Central Andes of Colombia (Cerro Munchique [FMNH]). One specimen (AMNH 42351, from Santa Elena, Antioquia Department) derives from the eastern slopes of the Central Andes in northern Colombia (habitat described as “deforested, grassy, and bushy (Chapman 1917:61)”; Paynter 1997:403); this shows that this subspecies also crosses to the eastern slopes of the Central Andes in Antioquia. Further south, in the department of Huila, the smaller subspecies Bassaricyon neblina hershkovitzi subsp. n. (see below) occurs on the eastern slopes of the Central Andes.
Records to date of Bassaricyon neblina osborni are from 1500 to at least 2750 m elevation in Cauca, Valle del Cauca, and Antioquia Departments of Colombia (Figure 16). Bassaricyon medius medius is also recorded from the Cauca Valley (east slopes of Western Andes and western slopes of Central Andes) at elevations up to at least 725 m (UV-3774: Saavedra-Rodríguez and Velandia-Perilla 2011; see account of Bassaricyon medius below), so these two taxa (Bassaricyon medius medius and Bassaricyon neblina osborni) are presumably regionally sympatric (and probably elevationally stratified) across the range of this Olinguito subspecies on the slopes of the Western and Central Andes.
Etymology.
The name honors Henry Fairfield Osborn (1857–1935), paleontologist, faculty of Princeton and Columbia Universities, and Curator of Vertebrate Paleontology (1891–1909) and President (1909–1933) of the American Museum of Natural History (Gregory 1937, Colbert 1996). “Bassaricyon osborni” is a manuscript name (never formally published) associated with a specimen of this taxon (AMNH 32609, with “Type” written on the skull), demonstrating a century-old intention, later discarded (probably by J.A. Allen or H.E. Anthony, see below), to name this taxon after Osborn. Here we validate this unpublished name as a newly described subspecies of Bassaricyon neblina, but we choose a more complete specimen than AMNH 32609, which has a damaged mandible and various broken teeth, as holotype.
Holotype.
FMNH 88476, adult male, skin and skull, Munchique, 2000 m, Cauca Department, Colombia (collected by K. von Sneidern, 3 June 1957).
Paratypes.
AMNH 32608, adult female, skin and skull, and AMNH 32609, adult male, skin and skull, Gallera (Chapman 1912:155; = “La Gallera” of Paynter 1997:222), 5000 feet (=1524 m), Cauca Department, Colombia (both collected by L. Miller, 13 July 1911); NMS A59-5083, adult female, skin and skull, El Tambo, 1700 m, Cauca Department, Colombia (collected by K. von Sneidern); FMNH 85818, adult male, skin and skull, Munchique, 2000 m, Cauca Department, Colombia (collected by K. von Sneidern, 19 January 1956); FMNH 89220, adult female, skin and skull, Sabanetas, 2000 m, Cauca Department, Colombia (collected by K. von Sneidern, 26 September 1957); FMNH 90052, adult female, skin and skull, Sabanetas, 1900 m, Cauca Department, Colombia (collected by K. von Sneidern, 12 February 1959).
Referred specimens.
AMNH 14185, skin (skull not found), adult male, Castilla Mountains (“La Castilla” of Paynter 1997), Valle del Cauca Department (collected by J.H. Batty, 9 June 1898); AMNH 42351, adult male, skin and skull, Santa Elena, apparently at 9000 feet (= 2750 m), Antioquia Department, Colombia (collected by H. Niceforo Maria, 10 January 1919) (Paynter 1997:403); USNM 598996, adult male, skin, skull, and postcranial skeleton, from Colombia, specific locality unknown (received from Tulane University).
Bassaricyon neblina hershkovitzi subsp. n.
http://species-id.net/wiki/Bassaricyon_neblina_hershkovitzi
(eastern slopes of Central Andes of Colombia)
Diagnosis.
This is the smallest subspecies of Bassaricyon neblina, with the fur of the dorsum and tail very long, and richly orange-brown (brown with strong golden and black tipping) in coloration, and more golden brown face and limbs, with the limbs well-furred. The skull, braincase, and rostrum are especially narrowed, the posterior palatal shelf is extremely broad, and the molars are proportionally very large.
Distribution.
This is the representative of Bassaricyon neblina on the eastern slopes of the Central Andes of southern Colombia (Figure 16). Records to date are from 2300 to 2400 m elevation in the vicinity of San Antonio (Huila Department), a forested locality “on eastern slope of Central Andes at headwaters of Rio Magdalena, near San Agustin” (Paynter 1997:380) (see Kattan et al. 1994).
Etymology.
The name honors American mammalogist Philip Hershkovitz (1909–1997), collector of the type series, Curator of Mammals at the Field Museum of Natural History (1947–1974; Emeritus Curator until 1997), and authority on South American mammals (Patterson 1987, 1997).
Holotype.
FMNH 70727, adult female, skin, skull, and postcranial skeleton, San Antonio, 2300 m, San Agustin, Huila Department, Colombia (collected by P. Hershkovitz, 6 September 1951) (see Figure 18).
Figure 18.
Type series of an Olinguito subspecies, Bassaricyon neblina hershkovitzi, in the field. Two Olinguito specimens (FMNH 70726, paratype of hershkovitzi, and FMNH 70727, holotype of hershkovitzi, along with a Long-tailed weasel, Mustela frenata, FMNH 70998) brought in by a local hunter, 6 September 1951, at San Antonio, San Agustín, Huila District, Colombia. Photo by P. Hershkovitz, courtesy of the Field Museum of Natural History.
Paratypes.
FMNH 70724, adult male, skin, skull, and postcranial skeleton, San Antonio, 2400 m, San Agustin, Huila Department, Colombia (collected by P. Hershkovitz, 20 August 1951); FMNH 70725, adult male, skin, skull, and postcranial skeleton, San Antonio, 2400 m, San Agustin, Huila Department, Colombia (collected by P. Hershkovitz, 25 August 1951); FMNH 70726, adult male, skin, skull, and postcranial skeleton, San Antonio, 2300 m, San Agustin, Huila Department, Colombia (collected by P. Hershkovitz, 6 September 1951).
Bassaricyon neblina ruber subsp. n.
http://species-id.net/wiki/Bassaricyon_neblina_ruber
(Urrao District, western slope of Western Andes of Colombia)
Diagnosis.
This subspecies is markedly smaller (at least in skull length) than Bassaricyon neblina neblina and Bassaricyon neblina osborni, with the fur longest and most strikingly reddish of all the Olinguito populations (reddish with golden and black tipping), and more golden brown face and and reddish brown limbs, with the limbs well-furred. Though similar in overall skull length to Bassaricyon neblina hershkovitzi, the skull is especially wide for its size (Table 8), with broad zygomata, braincase, and rostrum compared to that subspecies.
Distribution.
This subspecies is recorded from the Urrao District of Colombia (2200–2400 m in Huila and Antioquia Departments), on the western slope of the Western Andes, where it is documented by specimens collected in 1951 by Philip Hershkovitz.
Etymology.
The name refers to the rich reddish-brown pelage of this subspecies (Figures 3, 14).
Figure 14.
Olinguito skins from different regions of the Colombian Andes. Left, Bassaricyon neblina ruber, of the western slopes of the Western Andes of Colombia (FMNH 70722, adult male); Middle, Bassaricyon neblina hershkovitzi, of the eastern slopes of the Central Andes of Colombia (FMNH 70727, adult female); Right, Bassaricyon neblina osborni,of the eastern slopes of the Western Andes and eastern slopes of the Central Andes of Colombia (FMNH 90052, adult female).
Holotype.
FMNH 70722, adult male, skin, skull, and postcranial skeleton, Rio Urrao, 2400 m, Urrao, Huila Department, Colombia (collected by P. Hershkovitz, 24 April 1951).
Paratypes.
FMNH 70721, adult female, skin, skull, and postcranial skeleton, Rio Ana, 2200 m, Urrao, Huila Department, Colombia (collected by P. Hershkovitz, 19 April 1951); FMNH 70723, adult male, skin, skull, and postcranial skeleton, Guapantal, 2200 m, Urrao, Antioquia Department, Colombia (collected by P. Hershkovitz, 28 April 1951).
Reproductive isolation and genetic divergence of Bassaricyon neblina
Information from sympatric occurrences and captive breeding demonstrates that the Olinguito, Bassaricyon neblina, is reproductively isolated from other species of Bassaricyon and clearly constitutes a distinct “biological species” (i.e., sensu Mayr 1940, 1942).
In Ecuador we documented the Olinguito (Bassaricyon neblina neblina) in regional sympatry with the Western lowland olingo, Bassaricyon medius medius; we recorded the two species at localities less than 5 km apart (i.e., at Otonga and San Francisco de las Pampas) during fieldwork in August 2006. The ecogeographic relationship between the two species is probably one of elevational parapatry or limited elevational overlap along the western slopes of the Andes. Bassaricyon medius medius extends into the elevational range of Bassaricyon neblina,perhaps especially in areas where cloud forests have been cleared for human settlement, agriculture, and pastoralism (Figure 17).
Figure 17.
Area of sympatric occurrence between Bassaricyon species in western Ecuador. Farmland cutting into cloud forest habitat at Las Pampas, approximately 1800 m, on the western slopes of the Western Andes, Ecuador, along the boundary of Otonga, a protected forest reserve. It is at this elevational and environmental boundary that Bassaricyon medius medius (lower elevations, including more anthropogenically disturbed habitats) and Bassaricyon neblina neblina (higher elevations, less disturbed forests) co-occur in regional sympatry on the western slopes of the Andes.
Ingeborg Poglayen-Neuwall (pers comm. to R. Kays, 2006) informed us that an adult female zoo animal named “Ringerl” (Figure 15; also figured by Poglayen-Neuwall 1976), which we can now identify as an Olinguito (Bassaricyon neblina osborni), was moved among several zoos during the 1970s because it would not successfully breed with other captive olingos (i.e., not Bassaricyon neblina), most of which were apparently Bassaricyon alleni (see Poglayen-Neuwall 1976).
Figure 15.
The Olinguito, Bassaricyon neblina osborni, in life. Photograph taken in captivity, at the Louisville Zoo (see Poglayen-Neuwall 1976). This animal, named “Ringerl”, was received as an adult in 1967 from the mountains of Colombia near Cali, and exhibited in various zoos, including the National Zoo in Washington, D.C. (see text). Photographs by I. Poglayen-Neuwall, previously unpublished (additional photographs published by Poglayen-Neuwall 1976).
The Olinguito differs from congeners (Bassaricyon alleni, Bassaricyon medius, and Bassaricyon gabbii) by 9.6–11.3% in base-pair composition of the (mitochondrial) cytochrome b gene (Table 2), a level of divergence consistent with that separating biological species in many groups of mammals, including carnivores (Baker and Bradley 2006). For comparison with other procyonids, this level of genetic distinction is equivalent to the 10–11% divergence between Procyon lotor and Procyon cancrivorus, sympatrically-occurring raccoons traditionally classified in separate subgenera (Goldman 1950, Helgen and Wilson 2005), and comparable to the 9–13% divergence between Nasua narica and Nasuella olivacea (Helgen et al. 2009), coatis traditionally classified in separate genera (Hollister 1915, Decker and Wozencraft 1991, Wozencraft 1993, 2005).
Karyotype: The karyotype of an adult female Olinguito(Bassaricyon neblina osborni, then identified as “Bassaricyon gabbii”, with 2n = 38, as in all procyonids) was reported and discussed (but not described in detail) by Wurster-Hill and Gray (1975), and figured by Nash (2006). This was based on a captive animal originally captured from mountains in the vicinity of Cali in Colombia (Figure 15).
Description: The Olinguito is the smallest species of Bassaricyon, both in skull and body size (Tables 3, 5), and is thus, on average, the smallest living procyonid (matched only by small individuals of the Ringtail, Bassariscus astutus). The tail averages 10% longer than the head-body length (Table 5). The pinnae are proportionally much smaller in Bassaricyon neblina than in other Bassaricyon, appearing shorter and rounder, and standing out less conspicuously on the head; they are also more heavily furred and usually fringed with a paler, contrasting border of buffy or golden fur. The dorsal fur is dense, long, and luxurious, with the longer hairs measuring 30–40 mm in length (usually much shorter in other Bassaricyon, at least in the predominantly lowland taxa Bassaricyon medius and Bassaricyon alleni, but reaching 25 mm in the highest-elevation populations of Bassaricyon alleni on the eastern versant of the Andes). The hairs of the dorsum, crown, upper limbs, and tail are golden-orange, with grey bases and dark red-brown or blackish-brown tips, generating a distinctly dark, often red-brown appearance, more striking than the relatively drab fur colors (more tan or yellowish-brown to grayish-brown) of other Bassaricyon (Figure 3). The fur of the cheeks, chin, venter, and underside of the limbs is yellow to the bases, often washed with orange. The fur of the face in front of the eyes is shorter and gray or buff with black tipping, sometimes with a pale cream ring around the eyes. The hairs of the tail are strongly tipped with gold, or with both golden and blackish-brown tipping. In contrast to specimens of other Bassaricyon, the tail is not conspicuously banded, though when viewed in the right light, a banding pattern of alternating golden and brown hues is weakly apparent in some specimens. A white terminal tail tip is present in a minority of individuals.
Like other Bassaricyon, the cranium of Bassaricyon neblina is long relative to its width, with a moderately long and broad rostrum, an elongate and somewhat globose braincase with a smooth dorsal surface, and moderately developed postorbital processes.
In Bassaricyon neblina, the temporal ridges do not meet to form a sagittal crest, even in older animals. The postdental palate is usually flared laterally, but is smoothly parallel-sided, tapers posteriorly, or bears only weaker bony flaring in other Bassaricyon (Figures 4–7, 19). At its more extreme development (e.g., in FMNH 70726), the portion of the bony palate sitting behind M2 is almost continuously joined to the postdental palate by a continuous shelf of bone, rather than bearing a deep excavation separating the molar-bearing portion of the bony palate from the postdental shelf (Figure 19). The auditory bullae are very small in the Olinguito relative to other Bassaricyon, both in length and vertical inflation, and the external auditory meatus is considerably narrower in diameter, on average (Figures 4–7). The median septal foramen of the anterior palate (Steno’s Foramen), between the paired incisive (or anterior palatal) foramina, is usually well-developed. The mandible is proportionally less elongate than in other Bassaricyon, with a proportionally larger and more vertically-oriented coronoid process (Figures 4–5). The first two upper premolars are caniniform, similar in size and shape to those of other Bassaricyon. P3 is usually relatively smaller in Bassaricyon neblina than in other Bassaricyon. P4 is similar in structure to congenersbut is relatively larger with a more bulbous protocone and more prominent metacone. M1 and M2 are proportionally lengthened and considerably more massive in appearance, especially relative to skull size, than in other Bassaricyon. p4 is variable in size among Bassaricyon neblina subspecies, generally smaller than other Bassaricyon in Bassaricyon neblina ruber and Bassaricyon neblina hershkovitzi, but proportionally quite large in Bassaricyon neblina neblina. m1 is relatively much larger in Bassaricyon neblina than in other Bassaricyon;each of the four major cusps that define the subrectangular shape of this tooth are massive and bulbous, and the posterior portion is especially broadened, with the metaconid and hypoconid particularly large and laterally expanded relative to congeners. m2 is also often expanded in size in Bassaricyon neblina relative to other Bassaricyon.
Figure 19.
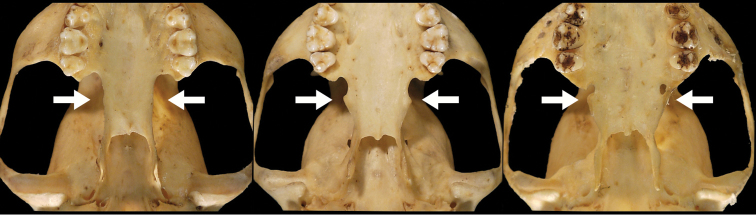
Lateral flare of the postpalatal shelf in Bassaricyon. Lateral extension of the postpalatal shelf (shown by white arrows) is usually absent or little-developed in other Bassaricyon (e.g., left, Bassaricyon alleni, FMNH 41501), but is well-developed in Bassaricyon neblina (e.g., center, Bassaricyon neblina ruber, FMNH 70721, and right, Bassaricyon neblina hershkovitzi, FMNH 70726).
Natural history: Our field observations document that Bassaricyon neblina is nocturnal, arboreal, frugivorous, and probably largely solitary (compiled during July and August 2006 at Otonga Forest Reserve in Ecuador: 00°41’S, 79°00’W; for faunal and floral context see Freiberg and Freiberg 2000, Nieder and Barthlott 2001, Jarrín-V 2001). It occupies cloud forest canopies and is an adept leaper. It has a single pair of mammae and probably raises one young at a time. Notes associated with AMNH 14185, the first specimen to arrive in a museum, mention that it was “shot at 2 pm [an error for 2 am?] in high trees while coming down mountain to feed on guavas; strictly nocturnal.”
An adult female Olinguito (an animal named “Ringerl”, Bassaricyon neblina osborni, Figure 15) that lived at the Louisville Zoological Park and the National Zoological Park in Washington during 1967–1974 made vocalizations different from those of other Bassaricyon according to Poglayen-Neuwall (1976). Poglayen-Neuwall (1976) figured a picture of this animal in characteristic estrus behavior and in various other circumstances (see below for more discussion of this captive Olinguito).
Previous identifications and references:
Though described taxonomically for the first time in this paper, the Olinguito (heretofore misidentified as other species of Bassaricyon) has been represented in museum collections for more than a century, has been exhibited in zoos, has had its karyotype published, and has been included in published molecular phylogenetic studies.
Olinguito museum specimens previously reported in the literature include specimens from Gallera, Colombia, mentioned by Allen (1912, 1916) (AMNH 32608 and 32609, as “Bassaricyon medius”); a specimen from Santa Elena, Colombia, reported by Anthony (1923) (AMNH 42351, as “Bassaricyon medius”), specimens from “El Duende Regional Reserve” (2200 m asl; 04°02'55.6"N, 76°27'28.4"W)” and “Los Alpes, Florida, 2250 m asl” in Valle del Cauca Department, Colombia (mammal collection of the Universidad del Valle, Cali, Colombia, specimen numbers 12736, 13700) discussed by Saavedra-Rodríguez and Velandia-Perilla (2011) (as “Bassaricyon gabbii”); and a skull from San Antonio, Huila Department, Colombia (FMNH 70727) figured by Prange and Prange (2009) (as “Bassaricyon gabbii”, designated above as the holotype of Bassaricyon neblina hershkovitzi). One Olinguito specimen, AMNH 32609, bears an unpublished scientific name, “Bassaricyon osborni”, written on the skull and on the tags, apparently during the early twentieth century—correctly reflecting an understanding that the specimen represented an undescribed species. This appellation (a “manuscript name”) is likely attributable to J.A. Allen or H.E. Anthony (seemingly too early to be G.H.H. Tate). In any case, the name was never published, and by 1923, Allen had passed away (in 1921) and Anthony had decided that the specimen in question was best referable to Bassaricyon medius (see Anthony 1923). We have chosen to validate this name under our own authorship, above, as a subspecies of Bassaricyon neblina.
Mejía Correa (2009) reported camera-trap photos of a species of Bassaricyon at Munchique in Colombia; these records presumably represent Bassaricyon neblina osborni, the only Bassaricyon recorded at Munchique.
Molecular data for Bassaricyon neblina from a cell line were first generated and used in a phylogenetic study of carnivore relationships by Ledje and Arnason (1996a, 1996b), apparently the same animal whose karyotype was reported and discussed by Wurster-Hill and Gray (1975) (also Nash 2006). DNA sequence data (12S rRNA, cytochrome b) from this sample, available on Genbank, have been used in various other published studies (e.g., Flynn and Nedbal 1998, Koepfli and Wayne 1998, Emerson et al. 1999, Flynn et al. 2000, Gaubert et al. 2004, Marmi et al. 2004, Flynn et al. 2005, Fulton and Strobeck 2007, Yonezawa et al. 2007, Wolsan and Sato 2010, Nyakatura and Bininda-Emonds 2012, but not in some important studies, e.g., Koepfli et al. 2007, Agnarsson et al. 2010). This cell line apparently originated from the zoo animal “Ringerl” (discussed by Poglayen-Neuwall [1976]), an adult female Olinguito (Bassaricyon neblina osborni, originally from mountains near Cali, Colombia), apparently exhibited at the Louisville Zoo, National Zoo, Tucson Zoo, Bronx Zoo, and possibly Salt Lake City Zoo during the late 1960s and 1970s (Ingeborg Poglayen-Neuwall, pers. comm. to R. Kays, 2006). Ivo Poglayen-Neuwall (in litt. to C.O. Handley, Jr., 6 November 1964) mentioned another Bassaricyon, a young adult male at the Louisville Zoo, also from Cali, received in 1964, that seems also to have been an Olinguito (“shows the following unusual physical features: (1) strikingly round head… (2) very short, round ears! (3) rather short tail (no amputation!)”). This latter animal seems not to be discussed in Poglayen-Neuwall’s various publications on olingos, and it is unclear what became of it.
Bassaricyon gabbii
J. A. Allen, 1876:23.
http://species-id.net/wiki/Bassaricyon_gabbii
Northern Olingo
Bassaricyon richardsoni J.A. Allen, 1908:662.
Bassaricyon lasius Harris, 1932:3.
Bassaricyon pauli Enders, 1936:365.
Type specimens and localities.
The holotype of gabbii is USNM A14214, an unsexed adult skull (with dimensions, in this sexually dimorphic species, that indicate that the specimen is female). The holotype skull, collected by William Gabb, was figured by Allen (1908). No specific locality other than Costa Rica was given in the original description (Allen 1876), but Allen (1877) later specified its origin as “Talamanca” (i.e., the Talamanca Mountains of southeastern Costa Rica; see also Allen 1908:667). The skin associated with the specimen was lost before the species was described (Allen 1876). Allen (1877) later incorrectly associated the skin of a coati, Nasua narica, with the holotype skull, but corrected this mistake soon after (Allen 1879).
The holotype of richardsoni is AMNH 28486, an adult female (skin and skull), from “Rio Grande (altitude below 1,000 feet), Atlantic Slope”, Nicaragua (Allen 1908). The skull of the type was figured by Allen (1908).
The holotype of lasius is UMMZ 64103, an adult male (skin and skull), from “Estrella de Cartago… six to eight miles south of Cartago near the source of the Rio Estrella, … about 4500 feet”, Costa Rica (Harris 1932).
The holotype of pauli is ANSP 17911, an adult male (skin and skull), from “between Rio Chiriqui Viejo and Rio Colorado, on a hill known locally as Cerro Pando, elevation 4800 feet, about ten miles from El Volcan, Province de Chiriqui”, Panama (Enders 1936).
Diagnosis.
This is the largest olingo, measuring larger than all other taxa in most measurements, and is the most sexually dimorphic species of Bassaricyon in cranial characters and measurements (Table 3). It can be distinguished externally from other olingo species by its coloration, which is grayish-brown (less rufous than in other Bassaricyon), with the face usually dominated by gray, the belly fur cream-colored (sometimes washed with orange), and the tail showing a faint banding pattern (Figures 3, 20). Fur length on the dorsum varies noticeably with elevation (longer at higher elevation). The tail is similar in length to the head-body length, averaging shorter relative to total length than in other olingos (Table 5), perhaps an indication of less complete arboreal habits than in other Bassaricyon (an aspect unfortunately not captured well in our Figure 3).
Figure 20.

Northern Olingo, Bassaricyon gabbii, in life, in the wild. Photographed at Monteverde, Costa Rica by Greg Basco (left) and Samantha Burke (right).
The skull is large compared to other Bassaricyon (Table 3), with the zygomata more widely splayed, particularly in males (Figures 4–5, Table 3), and a wide rostrum. Uniquely in Bassaricyon, a sagittal crest develops in older males. The cheekteeth and auditory bullae are proportionally quite small compared to the size of the skull, relatively smaller than in Bassaricyon medius and Bassaricyon alleni, and the postpalatal shelf tends to be broadened relative to Bassaricyon medius and Bassaricyon alleni. The canines are more massive than in other Bassaricyon. The first lower molar (m1) is distinctively shaped relative to other Bassaricyon, with the paraconid usually situated right at the midpoint of the front of the tooth and often jutting out anteriorly (the m1 paraconid is less prominent and/or situated more antero-medially in other Bassaricyon).
Distribution.
This species occurs in the central portion of Central America, in montane and foothill forests, from northern Nicaragua to Costa Rica and into the Chiriqui Mountains in western Panama, possibly also extending north into Honduras and Guatemala (Reid 2009; see below). Northern olingos are recorded at elevations as low as sea level, but are most commonly encountered in forests above 1000 m, and extend elevationally at least as high as 1700 m (USNM 324293), and probably as high as the upper limit of forest on the highest peaks in Costa Rica. Forested areas above 1000 m in Central America can be understood to be the core distribution of this species. Vouchered records are from the north-central mountains of Nicaragua (Allen 1908, AMNH, USNM); the mountains of Costa Rica, including the slopes stretching down to the Atlantic coast (Allen 1877, Allen 1908, Harris 1932, Goodwin 1946, Wilson 1983, Timm et al. 1989, Reid 1997, Timm and LaVal 2000, de la Rosa and Nocke 2000, Wainwright 2007, Reid 2009) and a few records of observations from the Pacific slopes (Puntarenas Province: Daily et al. 2003; Guanacaste Province: González-Maya and Belant 2010); and in the Chiriqui Mountains of western Panama (Enders 1936, ANSP, USNM). Reid (2009) included the Azuero Peninsula of Panama in a distribution map for Bassaricyon, but we can trace no record from this region and the basis of this record is unclear (F. Reid, R. Samudio, J. Pino, in litt., 2012–2013). The eastern limits of occurrence for this species are not yet firmly established, but the boundary of occurrence between Bassaricyon gabbii and Bassaricyon medius orinomus apparently lies between 81 and 80 degrees longitude in central Panama. Ours is the first study to document the marked taxonomic distinction between Bassaricyon gabbii of (especially montane) central Mesoamerica, including western Panama, and Bassaricyon medius orinomus of eastern Panama, the Central American member of a group of closely related lowland taxa that also includes Bassaricyon medius medius (of northern South America west of the Andes) and Bassaricyon alleni (of South America east of the Andes). The nature of the interactions between Bassaricyon gabbii and Bassaricyon medius orinomus at this boundary (whether involving, e.g., parapatry, sympatric overlap, or limited hybridization) is unknown and a priority for field study (see Figures 11–12).
There are unverified records of olingos occurring north of Nicaragua, in Honduras and Guatemala, and these records may represent Bassaricyon gabbii. Ordóñez Garza et al. (1999–2000) reported a night sighting of an olingo in Honduras at “La Picucha, Montaña de Babilonia, 1380 m, Parque Nacional Sierra de Agalta, Departamento de Olancho” and discussed a museum specimen of an olingo (later apparently lost) obtained from hunters in Guatemala near the Honduras border at “Montaña Cerro Negro Norte… Río Bobos… 300–500 m” in the Sierra del Merendón, Departamento de Izabal” (Ordóñez Garza et al. 1999–2000, McCarthy and Pérez 2006). Neither of these localities is immediately adjacent to large contiguous areas of Bassaricyon gabbii occurrence as predicted by our range modeling analyses (Figure 11), but both areas could represent relevant habitats for the Northern Olingo, and verifying the occurrence of olingo populations in Honduras or Guatemala should be considered an important goal in Mesoamerican mammalogy.
Geographic variation.
The nominal taxa richardsoni, lasius, and pauli, synonymized here with Bassaricyon gabbii, were all originally diagnosed based on distinctions in pelage coloration and fur length, in small samples (Allen 1908, Harris 1932, Enders 1936), and their taxonomic status has never been closely reviewed.
Specimens from northern Nicaragua have fur that is slightly more suffused with orange tones than animals from Costa Rica or western Panama. Nicaraguan populations may deserve recognition as a distinct subspecies, Bassaricyon gabbii richardsoni, as sometimes classified (e.g., Goodwin 1946, Hall 1981), but specimens from Nicaragua are too rare in collections for a detailed assessment, and Nicaraguan animals are otherwise very similar to specimens from Costa Rica and Panama. A young adult female specimen of Bassaricyon gabbii from Almirante in Panama’s Bocas del Toro Province (USNM 316320) is one of few low-elevation records for Bassaricyon gabbii, and is notable in having much smaller teeth than specimens from higher-elevation forests in the adjacent Chiriqui Mountains, and deserves close study in the future. We have carefully examined the type series of the nominal taxa Bassaricyon lasius and Bassaricyon pauli, the morphological attributes of which clearly fall into the range of variation seen in series we refer to Bassaricyon gabbii. We confidently relegate these names, often previously recognized as distinct species known only from the type localities (e.g., Hall 1981, Nowak 1999, Wozencraft 2005), to the synonymy of Bassaricyon gabbii, although we note that the only specimen of gabbii that we have seen from the area of the type locality, the Talamanca Range, is the holotype, which is an adult with worn teeth and no accompanying skin. Without further investigation, ideally involving the compilation and study of greater number of museum specimens from throughout the range of this species, we do not yet advocate recognizing subspecies of Bassaricyon gabbii, though we note that names are available for several of the major sections of the Middle American Highlands (Cordillera de Talamanca: gabbii, Chiriqui: pauli, Cordillera Central: lasius, Nicaraguan Highlands: richardsoni).
Notes.
This is the olingo speciesmost commonly seen and photographed by visitors to the Neotropics, especially because it is present at Monteverde and several other protected areas in Costa Rica that are frequently visited by both tourists and biologists (e.g., Forsyth 2008, Kays 2009, Reid 2009). It is larger, more sexually dimorphic, and has a shorter tail than other olingo species, suggesting a different ecology and behavior compared to the slightly better studied Bassaricyon medius and Bassaricyon alleni (see accounts below). Bassaricyon gabbii has been reported feeding on fruit and nectar in rainforest trees, but no details have been published on its diet. Olingos in Monteverde, Costa Rica, are often seen during the day, typically as solitary animals; it is unclear if diurnal activity is typical for the species or if this is in response to being fed by humans at the tourist lodge (Reid 2009, Kays 2009). Relevant field notes associated with Bassaricyon gabbii include: “fruit in stomach” (ANSP 18851); “shot in fruit tree at night” (ANSP 18852); “lactating” on 4 June 1937 (ANSP 18894); “shot at 8:00 pm in small trees” (ANSP 17911); mother with accompanying young on 20 August 1909 (AMNH 30748).
Specimens examined.
Costa Rica: AMNH 140334, LACM 26480, 64837, UMMZ 64103 (holotype of lasius), 112321, 112322, USNM A14214 (holotype of gabbii). Nicaragua: AMNH 28486 (holotype of richardsoni), 30748, 30749, USNM 337632, 338859. Panama: AMNH 147772, ANSP 17911 (holotype of pauli), 18850, 18851, 18852, 18893, 18894, BMNH 3.12.6.3, 5.5.4.5, KU 165554, MCZ 38506, TCWC 12941, USNM 316230, 324293, 324294, 516945, 516946.
Bassaricyon alleni
Thomas, 1880:397.
http://species-id.net/wiki/Bassaricyon_alleni
Eastern Lowland Olingo
Bassaricyon beddardi Pocock, 1921a: 231.
Bassaricyon medius siccatus Thomas, 1927:80.
Type specimens and localities.
The holotype of alleni is BMNH 80.5.6.37, an adult female (skin and skull), from “Sarayacu, on the Bobonasa River, Upper Pastasa River …this must not be confused with the far larger and better known Sarayacu on the Ucayali in Peru”, in Amazonian Ecuador (Thomas 1880). An image of the holotype as a living animal was figured by Thomas (1880: plate XXXVIII), and the anatomy of this specimen was further discussed by Mivart (1885, 1886).
The holotype of beddardi was an adult female, from “Bastrica Woods, Essequibo River”, Guyana (Flower 1895; Pocock 1921a), and was an animal that lived in the London Zoological Gardens from 1894 to 1900 (Beddard 1900; Allen 1908). Aspects of the internal anatomy of this specimen were described in detail by Beddard (1900), and the skull of this specimen was figured and discussed by Pocock (1921a), who also mentioned the specimen was prepared as a skeleton (Pocock 1921b), but apparently this specimen was not retained as a museum specimen in the collections of the BMNH, and now cannot be found (D. Hills, BMNH, in litt., 2004).
The holotype of siccatus is BMNH 27.5.3.2, an adult female (skin and skull), from “Guaicaramo, on the Llanos of Villavicencio, east of Bogata, about 1800 feet”, Colombia (Thomas 1927).
Diagnosis.
Bassaricyon alleni is a medium-sized olingo, smaller than Bassaricyon gabbii of Mesoamerica, and larger than Bassaricyon neblina of the Andes. It requires closest comparison with the closely-related and allopatrically-distributed taxon Bassaricyon medius, from which it differs especially in having (externally) more strikingly black-tipped dorsal pelage (giving the pelage a slightly darker appearance in Bassaricyon alleni), (cranially) in its proportionally wider and (on average) shorter rostrum, and in having more inflated auditory bullae (Table 3), and (dentally) in its generally larger p4 (Table 4). Bassaricyon alleni is considerably larger than Bassaricyon medius medius (of South America west of the Andes), such that there is a clear body size contrast between the two lowland olingo taxa of South America (Bassaricyon alleni east of Andes vs. B. medius medius west of Andes), but is very similar in size to Bassaricyon medius orinomus (of eastern Panama). Bassaricyon medius orinomus often has a reddish tail that contrasts somewhat with the less rufous head and body; Bassaricyon alleni tends to be more uniformly colored head to tail. In life, Bassaricyon alleni usually has a darkly pigmented nose, whereas in Bassaricyon medius the nose is often pink (Ivo Poglayen-Neuwall to C.O. Handley Jr., in litt., 9 February 1973; Figures 21–22). Sequence divergence in cytochrome b in these sister species (Bassaricyon alleni, Bassaricyon medius), separated by the Andes, is 6–7% (Table 2).
Figure 21.

Eastern Lowland Olingo, Bassaricyon alleni, in life, in the wild. Top, photographed at night (accentuating the dark tones in the pelage) at La Esperanza (Distrito de Yambrasbamba, Provincia de Bongará, Departamento Amazonas), 2000 m, northern Peru; Middle, color camera trap photo in forest canopy, from confluence of the Camisea and Urubamba Rivers (11°42'S, 72°48'W). Peru; Bottom, infrared camera trap photo in forest canopy (same locality as middle photo), showing an olingo carrying a baby in its mouth. Top photograph by César M. Aguilar; middle and bottom camera trap photos courtesy of Smithsonian Conservation Biology Institute.
Figure 22.
Western Lowland Olingo, Bassaricyon medius medius, in life. A wild animal photographed under studio conditions at Las Pampas, adjacent to Otonga Reserve, Ecuador. Photographs courtesy of P. Asimbaya and L. Velásquez.
Distribution.
This is the only species of Bassaricyon found east of the Andes. Bassaricyon alleni has a wide distribution in forests on the eastern slopes of the Andes and in lowland forests east of the Andes, with records from forested areas of Venezuela (Thomas 1920, Handley 1976, Bisbal 1989, 1993, Ochoa et al. 1993, Linares 1998, BMNH, USNM), Guyana (Pocock 1921a, Lim and Engstrom 2005, ROM), eastern Colombia (Thomas 1927, Donegan and Salaman 1999, AMNH, BMNH, USNM), eastern Ecuador (Thomas 1880, 1920, Schulenberg and Aubrey 1997, Pitman et al. 2002, Tirira 2007, Borman et al. 2007, Alverson et al. 2008, Pinto and Tirira 2011b, BMNH, EPN, FMNH, MCZ, QCAZ), eastern Peru (Thomas 1920, Grimwood 1969, Patton et al. 1982, Terborgh et al. 1984, Aquino and Encarnación 1986, Janson and Emmons 1990, Woodman et al. 1991, Pacheco et al. 1993, Pitman et al. 2003, 2004, Emmons et al. 1994a, 1994b, Emmons and Romo 1994, Boddicker 1997, Emmons 2001, Rodríguez and Amanzo 2001, Emmons et al. 2001, Vriesendorp et al. 2004, Alverson et al. 2008, Gilmore et al. 2010, BMNH, FMNH, MVZ, UMMZ, USNM, ZMB), northwestern Bolivia (Crespo 1959, Emmons 1991, Redford and Stearman 1993, Anderson 1997, Alverson et al. 2000, 2003, Alverson 2003, Ríos-Uzeda and Arispe 2010), and western Brazil (Calouro 1999, Kays and Russell 2001, Vaz 2004, Oliveira 2009, Magalhães-Pinto et al. 2009, Sampaio et al. 2010).
In Guyana, Bassaricyon alleni is recorded only from two specimens, the type of beddardi (Pocock 1921a, see above) and a specimen from Iwokrama Forest (Lim and Engstrom 2005, at ROM); there are no records to date from either Suriname or French Guiana, where it might be expected to occur (Tate 1939, Husson 1978, Voss et al. 2001, Lim et al. 2005).
In Brazil, the only firm records are from southwestern Amazonia (the states of Amazonas and Acre) (Calouro 1999, Kays and Russell 2001, Vaz 2004, Oliveira 2009, Magalhães-Pinto et al. 2009, Sampaio et al. 2010), though it is likely to occur also in Roraima and Pará (Figures 11–12). Brazilian Amazonian records of olingos from the state of Roraima, as “Bassaricyon beddardi” (Mendes Pontes and Chivers 2002, Mendes Pontes et al. 2002, Mendes Pontes 2004, 2009, Cheida et al. 2006), are thus far apparently based on misidentifications of kinkajous, Potos (Sampaio et al. 2011).
The elevational range of Bassaricyon alleni as documented by museum specimens extends from sea level to 2000 m. The great majority of records originate from lowland forests below 1000 m, but specimens from Ecuador and Peru (especially from Chanchamayo) have been collected from 1100 to 2000 m (specimens at BMNH, FMNH, USNM). It seems likely that the distribution of Bassaricyon alleni extends higher on the eastern slopes of the Andes than that of Bassaricyon medius does on the western slopes because of the apparent absence of Bassaricyon neblina on the eastern versant of the Andes.
Karyotype.
The karyotype of a male Bassaricyon alleni (2n = 38, NF = 68; then identified as “Bassaricyon gabbii”) was reported and described by Wurster and Benirschke (1967, 1968) based on an animal at the National Zoo (Washington, D.C.)—most likely USNM 395837, an adult male received from Leticia, Amazonas District, Colombia (the only male olingo at the zoo at the time).
Geographic variation.
Some geographic variation is apparent in Bassaricyon alleni, and several taxonomic names have been applied to different regional representatives of this species, including in the western Amazon (typical alleni Thomas 1880), Guyana (beddardi Pocock 1921a), and the Eastern Andes of Colombia (siccatus Thomas 1927).
The most notable morphological distinction that we have observed within Bassaricyon alleni is between lowland specimens (from forests below 1000 m) and specimens collected in montane forests above 1000 m in the Eastern Andes (e.g., Chanchamayo and Pozuzo in Peru). Specimens from these higher elevations have somewhat shorter tails and are more brownish (less orange tones in pelage), with notably longer fur, and greater development of black tipping to the fur, though the pelage is not as long and luxurious as in Bassaricyon neblina. Press reports of a possibly new species of Bassaricyon discovered in the Tabaconas – Namballe National Sanctuary in the Eastern Andes of Peru (e.g., Hance 2012), where Bassaricyon alleni is predicted to occur (Figure 11–12), may refer to such a highland population of Bassaricyon alleni.
Bassaricyon beddardi of Guyana has often been recognized as a species distinct from Bassaricyon alleni in checklists and inventories (e.g., Lim and Engstrom 2005, Reid and Helgen 2008c, Sampaio et al. 2011, Wozencraft 1993, 2005), but supporting justification has been lacking. The holotype of beddardi, originally a zoo animal, appears to be lost (see above). However, both the holotype (as described by Beddard [1900] and Pocock [1921a]) and a second (and the only additional) specimen from Guyana (ROM 107380, from Iwokrama Forest) closely correspond in their morphological characteristics to Amazonian and Andean specimens of Bassaricyon alleni, and our molecular comparisons demonstrate little molecular divergence between the ROM specimen and a specimen of Bassaricyon alleni from the Peruvian Amazon (Table 1; 1.3% sequence divergence in cytochrome b), such that we suggest that Bassaricyon beddardi can be regarded as a synonym of Bassaricyon alleni. We allocate the name siccatus to the synonymy of Bassaricyon alleni based on geography and craniodental morphology of the type specimen, but further, more detailed study of geographic variation across the range of Bassaricyon alleni would be welcome, perhaps focused in particular on variation across different regions of the Eastern Andes (cf. Thomas 1920, 1927). At present, we recognize no subspecies within Bassaricyon alleni.
Notes.
Though this is the most widely distributed member of the genus (Figure 12), relatively little is known of this species in the wild. Brief notes about the ecology and behavior of wild Bassaricyon alleni are included in the publications of Aquino and Encarnación (1986), Emmons (1990, 1991), Janson and Emmons (1990), and Patton et al. (1982). However, captive olingos described and discussed in detail by Poglayen-Neuwall and Poglayen-Neuwall (1965) (also Poglayen-Neuwall 1966, 1989) were all (or almost all) Bassaricyon alleni, originally from the vicinity of Iquitos (Amazonian Peru), such that for behavior under captive conditions, Bassaricyon alleni is the best studied member of the genus. Most olingos discussed by Poglayen-Neuwall (1976) were probably also Bassaricyon alleni, though one animal, an adult female named “Ringerl” (Figure 15), was an Olinguito, Bassaricyon neblina osborni (see account of Bassaricyon neblina, above). Poglayen-Neuwall’s (1973) delightful popular article, “The Odorous Olingo,” remains one of the most concentrated sources of firsthand information for this species (and olingos in general), discussing how Bassaricyon alleni is highly arboreal but will cross open spaces on the ground, is active from sunset to dawn, is predominantly frugivorous but also eats some animal matter (small rodents and lizards, nestling birds, insects, and eggs), has little social organization beyond mother-offspring pairs, displays a high intensity of scent marking in both sexes, flees and releases a foul-smelling odor when threatened, has one young following a 72–74 day gestation period, and that males are aggressive with one another and cannot be housed together. Relevant (and limited) field notes associated with Bassaricyon alleni include: “stomach contents fruits and a green vegetable pulp” (USNM 194315); “lactating” on 7 April 1967 (USNM 443717).
Specimens examined.
Colombia: AMNH 70532, 142223, BMNH 27.5.3.2 (type of siccatus), USNM 281482, 281483, 281484, 281485, 395837, 544415. Ecuador: AMNH 67706, BMNH 14.4.25.38, 80.5.6.37 (holotype of alleni), EPN “4”, RM0151, FMNH 41501, 41502, MCZ 37920, 37921, 37922, 37923, QCAZ 3371, YPM 1458, 1459. Guyana: ROM 107380. Peru: AMNH 98653, 98654, 98662, 98709, BMNH 5.11.2.6, 27.1.1.70, 1912.1.15.3, 1922.1.1.17, FMNH 34717, 65787, 65789, 65805, 86908, 86909, 98709, MVZ 153646, 155219, 155220, UMMZ 107907, USNM 194315, 194316, 255121, 255122, ZMB 63197. Venezuela: BMNH 99.9.11.25, USNM 443279, 443717, 443718.
Bassaricyon medius
Thomas, 1909
http://species-id.net/wiki/Bassaricyon_medius
Western Lowland Olingo
Bassariscyon [sic] gabbi [sic] orinomus Goldman, 1912:16.
Type specimens and localities.
The holotype of medius is BMNH 9.7.17.10, an adult male (skin and skull) from “Jimenez, mountains inland of Chocó, W. Colombia, 2400 feet” (Thomas 1909).
The holotype of orinomus is USNM 179157, an adult male (skin and skull), from “Cana (altitude 1,800 feet), in the mountains of eastern Panama” (Goldman 1912). Goldman (1912) figured the holotype skull.
Diagnosis.
Bassaricyon medius is a medium-sized olingo, smaller (on average) than Bassaricyon gabbii of Mesoamerica and larger than Bassaricyon neblina of the Andes. It requires closest comparison with the closely-related, allopatrically-distributed taxon Bassaricyon alleni, from which it differs especially in having (externally) less strikingly black-tipped dorsal pelage (which gives the pelage a slightly darker appearance in Bassaricyon alleni), (cranially) in its proportionally narrower and (on average) longer rostrum, and in having less inflated auditory bullae (Table 3), and (dentally) in its generally smaller p4 (Table 4). Bassaricyon medius medius is considerably smaller than Bassaricyon alleni (of South America east of the Andes), such that there is a clear body-size contrast between the two lowland olingo taxa of South America (Bassaricyon alleni east of Andes vs. B. medius medius west of Andes), but Bassaricyon medius orinomus (of eastern Panama and northwestern Colombia) is very similar in size to Bassaricyon alleni. Bassaricyon medius orinomus often has a reddish tail that contrasts somewhat with the less rufous head and body; Bassaricyon alleni tends to be more uniformly colored head to tail. In life, Bassaricyon alleni usually has a darkly pigmented nose, whereas in Bassaricyon medius the nose is often pink (Ivo Poglayen-Neuwall to C.O. Handley Jr., in litt., 9 February 1973; Figures 21–22). Sequence divergence in cytochrome b in these sister species (Bassaricyon medius, Bassaricyon alleni), separated by the Andes, is 6–7% (Table 2).
Distribution and geographic variation.
Bassaricyon medius occurs in forests from Central Panama to Colombia and Ecuador west of the Andes, where it is recorded from sea level up to about 1800 m elevation. We recognize two distinctive subspecies of Bassaricyon medius, distinguished especially by clear differences in size (Tables 6–7).
Bassaricyon medius medius (Figure 22) occurs in most of the South American portion of the range, where it is recorded west of the Andes in western Colombia (Thomas 1909) and western Ecuador (Lönnberg 1921, Parker and Carr 1992, Tirira 2008, Pinto and Tirira 2011a), in the Chocó region, on the western slopes of the Andes, and in outlying western ranges. It occurs in regional sympatry with Bassaricyon neblina at Otonga–San Francisco de las Pampas and probably elsewhere along the western versant of the Andes.
Bassaricyon medius orinomus (Figure 23) occurs primarily in the Central American portion of the range, where it is recorded from central and eastern Panama, from the vicinity of the Canal Zone in the west to the Darien Mountains in the east (Goldman 1912, 1920, Handley 1966, Mendez 1970, Kays 2000). In the Darien, records include Cerro Tacarcuna and Cerro Pirri (USNM series), and as these mountain blocks extend across the Colombian border, there can be no doubt this subspecies enters South America in northwestern Colombia. The two north-westernmost records of Bassaricyon medius in Colombia, from Villa Arteaga, Antioquia District (FMNH 69578) and from the Cauca Valley (AMNH 37797) have large anterior premolars relative to Bassaricyon medius medius and we tentatively attribute them to Bassaricyon medius orinomus, with Bassaricyon medius medius then recorded further south in forests of the Chocó and Western Andean slopes. The specimen from the Cauca Valley (from Puerta Valdivia, Antioquia District: Allen 1916) demonstrates that Bassaricyon medius also occurs in lowland forests in between the Western and Central Andes; another low elevation specimen from the Cauca Valley of Colombia reported by Saavedra-Rodríguez and Velandia-Perilla (2011) (UV-3774, Río Agua Sucia, Río Cajambre, 725 m) that we have not examined is also presumably Bassaricyon medius.
Figure 23.
Western Lowland Olingo, Bassaricyon medius orinomus, in life. Wild animals captured, radio-collared, released, and studied by Roland Kays in Limbo Plot, Pipeline Road, Gamboa, Panama (Kays 2000). Photographs courtesy of M. Guerra and R. Kays.
As noted above for Bassaricyon gabbii, the nature of interactions with the distribution of Bassaricyon gabbii on the western margin of the range of Bassaricyon medius in Panama (whether characterized by allopatry, parapatry, or sympatry) is unknown and worthy of study.
Notes.
The first detailed description of representatives of this species was published in French by Huet (1883), based on two specimens from Chorrera, near Panama City (Thomas 1909, Goldman 1920), the first specimens to become available after the original discovery of Bassaricyon gabbii in Costa Rica in 1876 (Allen 1876). Thomas (1909) was first to name it (Bassaricyon medius), based on specimens from western Colombia, and Goldman (1912, 1920) described the larger Panamanian subspecies (orinomus) and provided brief notes on the species, noting its arboreal, nocturnal, and frugivorous habits, and its association in fruiting trees with Potos. More recent field studies in Panama (Kays 2000, Kays et al. 2012, Figure 23) are limited but likewise show Bassaricyon medius to be nocturnal, arboreal, frugivorous (and nectarivorous), and to feed in the same trees as kinkajous, which sometimes displace the smaller olingos. It is mostly solitary at night, spends its days in tree holes or other arboreal hide-outs, and usually has one young at a time. One olingo was found to have a home range of 37 hectares. Field workers have described this species’ vocalizations as “whey-chuck”, “wer-toll”, or “wake-up.”
Relevant field notes associated with Bassaricyon medius include: “shot at dusk in high tree in forest” (FMNH 29180); “shot at 8 pm, 40 feet up in large tree, active and agile, but curious, eyes shine brightly” (USNM 305748); “shot at 8:30 pm in avocado plantation” (USNM 305749); “shot near banana plantation (at night), stomach with banana” (USNM 305750); ” “shot at 8:30 pm in large tree in cafetal [coffee plantation], stomach with soft fruit with tomato-like seed” (USNM 305751); “shot at 8 pm in forest” (USNM 307037); “lactating” and pregnant with “1 embryo”, “stomach: fruit pulp” (USNM 310666); “shot in tree at night” (USNM 335767, 338348); “shot at night in tree in forest” (USNM 335769); “shot at night in tree in cocoa grove” (USNM 335770); “shot in small tree in plantain patch at night” (USNM 335771); “one embryo” in a pregnant female “shot in forest” (USNM 363342); “shot in banana tree” (USNM 363343)
Specimens examined.
Bassaricyon medius medius
Colombia: BMNH 9.7.17.10 (holotype of medius), 9.7.17.11, FMNH 29180, 86852, 90049, 90051, MVZ 124112, USNM 598997. Ecuador: AMNH 66752, BMNH 34.9.10.81, 34.9.10.82, EPN 841, 900, MECN DAP37, NMS A59-5081, A59-5082, QCAZ 8758, 8659.
Bassaricyon medius orinomus
Panama: USNM 171138, 179053, 179157 (holotype of orinomus), 179158, 179779, 179917, 206123, 284773, 284903, 284933, 284934, 284935, 305748, 305749, 305750, 305751, 305752, 305753, 305754, 307035, 307036, 307037, 310666, 310667, 310668, 324295, 324296, 335767, 335768, 335769, 335770, 335771, 338348, 338894, 363342, 363343, 363344. Colombia (tentatively attributed): AMNH 37797, FMNH 69578.
Discussion
Carnivore taxonomy
Descriptions of new species of carnivores are especially rare, and the order Carnivora is generally considered one of the most completely characterized groups across the entire tree of life (Collen et al. 2004, Reeder et al. 2007). Bassaricyon neblina is a deeply divergent lineage within its genus, a very morphologically distinctive member of the family Procyonidae, and even shows signs of evolutionary diversification across its geographic range. It thus adds significantly to current understanding of taxonomic, phylogenetic, and ecomorphological evolution in the family Procyonidae. It has presumably been overlooked by taxonomists for several reasons—principally the lack of close taxonomic attention paid to Neotropical procyonids for nearly a century (Helgen and Wilson 2003, Helgen et al. 2009), but probably also because of its nocturnal and arboreal habits, relatively limited geographic distribution, and the small number of specimens scattered across various museum collections (see Patterson 1994, 2000).
The description of the Olinguito highlights how incompletely known the taxonomy of almost all kinds of mammals remains, including the Carnivora (Gutiérrez and Helgen 2013). Our study of olingo taxonomy is part of a series of studies that have better clarified species diversity in insufficiently studied genera of Carnivora, especially in Neotropical small carnivores (e.g., Procyon: Helgen and Wilson 2002, 2003, 2005; Helgen et al. 2008b; Nasuella: Helgen et al. 2009; Galictis: Bornholdt et al. 2013), but also in other little-known genera (Arctonyx: Helgen et al. 2008a; Eupleres: Goodman and Helgen 2010), often revealing considerable overlooked biodiversity in poorly studied groups. Many additional carnivore genera have not been the subject of modern integrative systematic reviews, especially in the Neotropics (e.g., Potos, Nasua, Conepatus). Detailed reviews of these groups are likely to reveal additional overlooked diversity.
Conservation
The rapid and ongoing discovery of endemic mammals and birds in northern Andean cloud forests (e.g., Robbins and Stiles 1999, Anderson and Jarrín-V 2002, Cuervo et al. 2001, 2005, Lara et al. 2012, Ojala-Barbour et al. in press) reaffirms the evolutionary importance of these unique habitats and betrays how incompletely inventoried this biota remains. Though a center of diversity and endemism for many groups (e.g., Young et al. 2002, Brehm et al. 2005, Mittermeier et al. 2005, Hughes and Eastwood 2006, Patterson et al. 2012), northern Andean cloud forests are among the most threatened ecosystems in the Neotropics (Young 1994, Myers et al. 2000, Mittermeier et al. 2005, Schipper et al. 2008). Drawing on the criteria used by the International Union for the Conservation of Nature (IUCN; Schipper et al. 2008; in this case, based on inferred population declines due to habitat declines over last three generations), we suggest classifying the Olinguito under the IUCN category of “Near Threatened.” Given that Olinguitos are directly dependent on cloud forest for habitat and food, deforestation appears to be the primary threat to Olinguito populations, and this IUCN categorization reflects our concerns about habitat destruction across its relatively restricted geographic range. Based on our distribution model, it appears that 42% of potential Olinguito habitat in Colombia and Ecuador has already been converted to agriculture or urban environments. Remaining habitat is highly fragmented and faces increasing threats from farming, grazing, deforestation for drug cultivation, logging, and climate change (Kattan et al. 1994, Myers et al. 2000, Brooks et al. 2002, Sarmiento 2002, Armenteras et al. 2003). The long-term survival of Bassaricyon neblina will depend on the preservation of those upland forest fragments that remain, and restoration of degraded habitat to maintain connectivity between populations. Its discovery introduces a novel flagship species around which to rally conservation initiatives in the region. Preserving cloud forests in this region would benefit the long-term conservation of the Olinguito, and many other Northern Andean cloud forest endemics.
Based on their relatively expansive distributional ranges, all of which include various protected areas (Figures 11, 12), we suggest IUCN Red List rankings of “Least Concern” for Bassaricyon alleni, Bassaricyon medius, and Bassaricyon gabbii, for the present.
Biogeography
A well-resolved taxonomy for olingos has never been available, such that biogeographic patterns within the genus, and their origins, have never before been critically reviewed (Eizirik 2012). Our overview of Bassaricyon allows us to glimpse these patterns for the first time, unveiling both anticipated and unexpected biogeographic patterns.
Previous overviews of procyonid biogeography have focused especially on the important potential role of the Great American Biotic Interchange (GABI) in the diversification of the family (Marshall et al. 1979, Koepfli et al. 2007, Eizirik 2012). We complement this focus by suggesting that northern Andean uplift, proceeding in greatest part since the middle Miocene (Gregory-Wodzicki et al. 2000, Ollier 2006, Weir 2006), has played an almost equally important role in procyonid diversification.
The most detailed previous phylogenetic comparisons of olingos (Koepfli et al. 2007) highlighted the genetic divergence between taxa originating from North America and from South America (Bassaricyon medius from Panama [then called “Bassaricyon gabbii” by Koepfli et al. 2007] and Bassaricyon alleni from Peru), finding that this split apparently postdated the GABI. This comparison was undertaken prior to the discovery of the Olinguito lineage, the deepest split in the genus, and could not resolve the question of whether the radiation of crown group olingos unfolded first in North or in South America. Our phylogenetic comparisons indicate that Bassaricyon neblina, an Andean cloud forest endemic, is the sister taxon to all other Bassaricyon and last shared a common ancestor with congeners3–4 million years ago, a timescale concordant with the timing of both the GABI and Northern Andean mountain-building. That Bassaricyon mainly occurs in South America, with only one species, Bassaricyon gabbii, endemic to Central America, and that the earliest divergence in Bassaricyon is between Bassaricyon neblina and the other three species allows us to suggest that the most important events in the diversification of crown group Bassaricyon occurred in northwestern South America (as suggested by Poglayen-Neuwall 1973) (see Velazco and Patterson [2013] for particularly clear example of this same biogeographic pattern). That the two earliest divergences within the genus involve what are today montane (Bassaricyon neblina of the Andes) or mostly montane (Bassaricyon gabbii of the Costa Rican, Nicaraguan, and western Panama highlands) taxa provides an indication that the isolation of upland Neotropical habitats was likely important in the early diversification of the genus. Uplift of the Andes simultaneously created a barrier to dispersal that is ultimately reflected in the speciation event between the allopatric pair of lowland olingos, Bassaricyon alleni (eastern, cis-Andean) and Bassaricyon medius (western, trans-Andean) (cis- and trans-Andean sensu Haffer 1967). In addition to promoting evolutionary diversification within Bassaricyon, Northern Andean uplift has fostered the evolution of other endemic montane procyonids (the Mountain coatis Nasuella olivacea and Nasuella meridensis [Helgen et al. 2009] as well ascurrently unrecognized montane species of Nasua, synonymized uncritically with Nasua nasua under current taxonomic checklists [following Decker 1991]). These mountains also served as a key barrier to dispersal of presumed recent North American procyonid immigrants (Procyon lotor and Nasua narica) to South America, which penetrate South America only west of the Andes, primarily in western Colombia (Marín et al. 2012), with Nasua narica perhaps extending also to western Ecuador (Decker 1991) and Procyon lotor perhaps also to western Venezuela (Helgen and Wilson 2005).
The phylogenetic topology seen in Bassaricyon, with an Andean species sister to a clade of lowland congeners, is unusual among mammals, but seen in some groups with lowland representatives restricted to the Amazon. For example, the echimyid rodent genera Dactylomys and Isothrix present this pattern, with Isothrix barbarabrownae and Dactylomys peruanus restricted to the Andes and their congeners to the Amazon lowlands (Patterson and Velazco 2008, Lim 2012, Patterson et al. 2012). In olingos, the time estimates for this diversification are broadly equivalent with the estimated Pliocene divergence timing (2–5 mya) proposed between Isothrix barbarabrownae and its lowland congeners (Upham and Patterson 2012). A similar pattern of inferred colonization from the Andes to the Amazonian lowlands was proposed for dendrobatid frogs, but occurred earlier, during the late Miocene (11.2 – 5.3 mya), when the Andes were considerably lower in elevation (Santos et al. 2009).
One species of olingo, Bassaricyon alleni,is endemic to habitats east of the Andes, especially the Amazon. The Amazon is arguably the most diverse region of the planet (e.g. Bass et al. 2010, Malhado et al. 2013; but see Solari et al. 2012), and it has been postulated that its high current diversity is a result of an accumulation of lineages for a prolonged period of time, covering mostly the Pliocene and Miocene, with subsequent local divergences (e.g., Hoorn et al. 2010; Leite and Rogers 2013). However, Bassaricyon alleni appears to be a considerably more recent immigrant to this region, likely arriving in the Pleistocene, during the past 1–2 mya (Figures 1–2), well after the last major uplift of the Andes, which occurred until circa 3.0 mya (Gregory-Wodzicki 2000). Thus, it is likely that a dispersal event across the North Andes is responsible for the cis-Andean distribution of Bassaricyon alleni. This supports the idea that the Andes and the trans-Andean Neotropics (the western side of South America, and Central America) serve as continuous pumps of diversity into the Amazon, as proposed in other vertebrate groups such as tanagers and woodcreepers (e.g. Sedano and Burns 2010, Weir and Price 2011). The western boundary of the Amazon with the Andes and close proximity to the Chocó and Central America contribute to an influx of species from these regions into the Amazon and this influx seems to be a principal driver of the high diversity of the western Amazon and the eastern slopes of the Andes (Patterson et al. 2012).
One species of olingo, Bassaricyon medius, is distributed in the Chocó forests to the west of the Western Andes of Colombia and Ecuador, as well as in tropical forests of eastern Panama in Central America (Figure 12). For vertebrates, this is a common pattern: the Chocó has closer biogeographic affinities with Central America than with other areas of South America (Ron 2000). Mammalian examples of a Chocó + Central America distributional pattern include many medium-sized species in the region, including Nasua narica, Procyon lotor, Coendou rothschildi, Tamandua mexicana, Caluromys derbianus, and Philander opossum (Eisenberg 1989, Brown 2004, Voss 2011, Voss et al. 2013, Marín et al. 2012). That these various distributions result from multiple biogeographic events is evidenced by the dissimilar evolutionary divergence timings involved, but the GABI and Northern Andean uplift no doubt are key events that collaborated to generate these co-distributions. The divergence between the two subspecies of Bassaricyon medius is recent (circa 1.0 mya, Figure 1), but considering both subspecies are recorded in Colombia, it seems possible that Bassaricyon medius entered Panama quite recently, perhaps penetrating the North American continent as far as the distribution of the Mesoamerican endemic taxon Bassaricyon gabbii. The location of the geographic boundary between Bassaricyon medius and Bassaricyon gabbii in Panama is not yet clear, and the nature of interaction between these species, if any, at this boundary, will be a very interesting subject for further investigation.
The last species of olingo to consider, Bassaricyon gabbii, is a Mesoamerican endemic, distributed from Nicaragua to western Panama and recorded primarily in montane contexts: the Nicaraguan highlands, Costa Rican cordilleras, and Chiriqui Mountains. The eludication of the phylogenetic relationship, depth of divergence (we estimate a circa 2.0 mya divergence between Bassaricyon gabbii and the lowland species-pair Bassaricyon medius/Bassaricyon alleni) and the distinctive morphological features of Bassaricyon gabbii allow us to recognize it as the only carnivore species endemic to this region of Central America, although many vertebrate species, especially birds, reptiles and amphibians, and small mammals, are endemic to this same region (Savage 1966, 1982, Slud 1964, Stiles and Skutch 1989, Carleton and Musser 1995). As noted by Carleton and Musser (1995:357–358), “some have attributed the high endemism to the possible isolation of the Talamanca-Chiriquí region as an island, or a series of islands, within the Panamanian portal prior to complete closure and late-Pliocene formation of the landbridge” (citing McPherson 1985, 1986, among others). This vision of insular or archipelagic diversification in Bassaricyon during the GABI may provide insight into the early splits in the genus that ultimately gave rise to the principal modern lineages so far identified in the genus: Bassaricyon neblina in the Andes of northwestern South America, Bassaricyon gabbii in the Nicaragua-W Panama highlands, and Bassaricyon medius/Bassaricyon alleni in the Neotropical lowlands primarily in South America (southward from eastern Panama). Additional geographic surveys, specimen collecting, and specimen-based comparisons are needed to better understand the nature of differentiation in Bassaricyon gabbii across different Central American cordilleras, and the true easternmost extent of its distribution, where it may co-occur with or abut the range of Bassaricyon medius.
Additional Olinguito study priorities
Our studies of Olinguito specimens in museums reveal a remarkable pattern of geographic variation, allowing for the delineation of four distinctive subspecific taxa distributed in separate biogeographic regions of the Andes of Colombia and Ecuador. Additional study is needed to more fully evaluate the level of genetic divergence between different Olinguito subspecies, especially for Bassaricyon neblina ruber, perhaps the most isolated and distinctive of the four (Figures 3, 9–10, 13–16).
Our bioclimatic analyses (Figures 11–12) also identify a number of high-priority candidate regions where further exploration is needed to assess whether additional populations of the Olinguito, or other distinctive high-elevation Bassaricyon populations, are present (Figure 24). One of these is the Colombian Eastern Andes, or Cordillera Oriental, the eastern branch of the Andes in Colombia. Olinguitos are recorded from the Western and Central Andes of Colombia, but not yet from the Eastern Andes, an area of substantial montane biotic endemism, where only Bassaricyon alleni is known to occur. Another survey priority is the Quijos region of Ecuador, a county and river situated on the eastern side of the Andes, which comprises relevant cloud forest habitats (Quijos is an old, pre-Spaniard name for the indigenous community in the area). This region deserves greater attention and contains the important Papallacta region discussed by Voss (2003). The Pallatanga-Sangay region in the Central Andes of Ecuador is another important priority study area; Pallatanga is an important mammal type locality (Tomes 1860), and Sangay is a national park with peculiar cloud forest mammal representation (Fonseca et al. 2003, Lee et al. 2011). Finally the Loja-Huancabamba, a low elevation region of the Andes in southern Ecuador and northern Peru has potential as Olinguito habitat. Though situated on the eastern side of the Andes, this region was recognized as biogeographically important by Chapman (1926). Patterson et al. (1992) showed little differentiation between Artibeus from the western slope of the Andes and the Marañón valley in this area of northern Peru, and Pinto (2009) inferred potential cases of east-west dispersal in vampire bats across both slopes of the Andes in this region of southern Ecuador, suggesting this could well be an area where the Olinguito could cross from the western to the eastern versant of the Andes.
Figure 24.
Selected priority areas to search for Olinguitos. Areas mentioned in the text with appropriate cloud forest habitats. A) Cordillera Oriental, the eastern branch of the Andes in Colombia. B) Quijos, a county on the eastern side of the Andes in Ecuador. C) Pallatanga-Sangay in central Ecuador. D) The Loja-Huancabamba region of the Andes in southern Ecuador and northern Peru.
Much remains to be learned about the Olinguito, including its distribution. The taxonomic description of this species is the first step toward further studies of its biology, and we look forward to future reports of additional discoveries from Andean cloud forests regarding this beautiful procyonid.
Supplementary Material
Acknowledgments
For assistance in Ecuador we thank E. Tapia, C. Tapia, G. Onore, P. Asimbaya, L. Velásquez, P. Rosero, M. Grijalva, G. Acosta, P. Pinto, M. Gallo, A. Padilla, M. Vargas, P. Vargas, I. Rodriguez, C. Byrne, and Fundación Otonga. For access to specimens under their care, loan of tissues, and other assistance during museum visits we are grateful to D. Lunde, E. Westwig, R. Voss, W. Stanley, B. Patterson, L. Heaney, R. Banasiak, S. Burneo, R. Timm, J. Patton, C. Conroy, M. Engstrom, B. Lim, J. Eger, P. Jenkins, R. Portela Miguez, D. Hills, J. Chupasko, N. Gilmore, J. Dines, J. Light, W. Murphy, R. Asher, I. Thomas, L. Albuja-V., O. Groenwall, R. Anderson, E. Sargis, K. Zyskowski, P. Myers, S. Jansa, R. Baker, K. Koepfli, R. Fleischer, R. Arcos, C. Handley, Jr., L. Gordon, and H. Kafka. For assistance in the registrar’s office at the National Zoo, we thank L. Morse. For helpful reviews, helpful discussion, and other assistance, we thank W. Bogdanowicz, B. Patterson, R. Sampaio, E. Gutiérrez, G. Slater, K. Koepfli, R. Anderson, I. Poglayen-Neuwall, J. Gibbons, P. Engelbrektsson, F. Reid, J. Pino, R. Samudio, M. Bass, T. Flannery, E. Eizirik, R. Baker, H. Mantilla, R. Ree, R. Voss, and L. Emmons. This work was partially funded by the Smithsonian Institution. M.P. was supported in part by a grant from the Texas Tech University Association of Biologists. We thank Nancy Halliday for original artwork. For photographic contributions we thank P. Asimbaya and L. Velásquez of Finding Species, and M. Gurney, I. Poglayen-Neuwall, T. Gregory (Smithsonian Conservation Biology Institute), C. Aguilar, G. Basco, M. Guerra, R. Banasiak, R. Bornholdt, and S. Burke.
Appendix 1
Morphometry
Table A1.
PCA for male Bassaricyon skulls: variable contributions for the PCA axes that are graphically represented in Figure 6.
| Variable | PC1 | PC2 |
|---|---|---|
| p2W | 0.877 | -0.077 |
| p3W | 0.768 | 0.004 |
| p4W | 0.600 | -0.267 |
| P4L | 0.306 | -0.412 |
| P4W | 0.526 | -0.245 |
| M1L | 0.136 | -0.427 |
| M1W | 0.336 | -0.475 |
| m1L | 0.079 | -0.241 |
| m1W | -0.069 | -0.750 |
| m2L | -0.344 | 0.196 |
| m2W | 0.164 | -0.330 |
| CBL | 0.733 | 0.317 |
| ZYG | 0.806 | 0.011 |
| BBC | 0.549 | -0.050 |
| HBC | 0.458 | -0.022 |
| MTR | 0.544 | 0.411 |
| CC | 0.912 | -0.013 |
| WPP | 0.226 | -0.464 |
| LPP | 0.704 | -0.464 |
| LAB | 0.366 | 0.736 |
| EAM | 0.368 | 0.825 |
| Eigenvalue | 0.035 | 0.030 |
| Variance (%) | 30.1 | 25.8 |
Table A2.
PCA for female Bassaricyon skulls: variable contributions for the PCA axes that are graphically represented in Figure 7.
| Variable | PC1 | PC2 |
|---|---|---|
| p1W | -0.389 | 0.424 |
| p2W | -0.765 | 0.341 |
| p3W | -0.663 | 0.560 |
| p4W | -0.783 | 0.068 |
| P2W | -0.745 | 0.342 |
| P3W | -0.724 | 0.221 |
| P4L | -0.649 | -0.314 |
| P4W | -0.631 | -0.370 |
| M1L | -0.565 | -0.275 |
| M2L | -0.550 | -0.003 |
| M2W | -0.606 | -0.324 |
| m1L | -0.637 | -0.200 |
| m1W | -0.644 | -0.524 |
| m2L | -0.452 | -0.166 |
| m2W | -0.665 | -0.302 |
| CBL | -0.348 | 0.579 |
| ZYG | -0.231 | 0.402 |
| BBC | -0.317 | 0.210 |
| MTR | -0.557 | 0.389 |
| CC | -0.511 | 0.249 |
| WPP | -0.342 | -0.474 |
| LPP | -0.170 | -0.216 |
| LAB | -0.018 | 0.733 |
| EAM | 0.066 | 0.743 |
| Eigenvalue | 0.034 | 0.021 |
| Variance (%) | 29.1 | 18.2 |
Table A3.
DFA for male skulls of Bassaricyon gabbii, Bassaricyon medius, and Bassaricyon alleni, including correlation coefficients for the axes that are graphically represented in Figure 8. (Wilks’ Lambda: 0.11054 approx. F (16,58) = 7.2781 p < 0.0000).
| CV 1 | CV 2 | Wilks’ Lambda | Partial Lambda | F-remove (2,29) | p-value | Toler. | 1-Toler. (R-sq) | |
|---|---|---|---|---|---|---|---|---|
| p1W | 0.249 | -0.598 | 0.125 | 0.885 | 1.887 | 0.170 | 0.526 | 0.474 |
| P3W | -0.644 | -0.349 | 0.156 | 0.708 | 5.971 | 0.007 | 0.757 | 0.243 |
| M2L | 0.215 | 0.317 | 0.115 | 0.958 | 0.635 | 0.537 | 0.500 | 0.500 |
| m1W | 0.089 | 0.062 | 0.111 | 0.996 | 0.063 | 0.939 | 0.540 | 0.460 |
| HBC | -0.855 | 0.207 | 0.181 | 0.610 | 9.267 | 0.001 | 0.653 | 0.347 |
| ZYG | -0.274 | 0.174 | 0.119 | 0.933 | 1.048 | 0.363 | 0.916 | 0.084 |
| WPP | 0.289 | -0.890 | 0.181 | 0.612 | 9.191 | 0.001 | 0.878 | 0.122 |
| LPP | 0.551 | 0.213 | 0.134 | 0.828 | 3.016 | 0.065 | 0.660 | 0.340 |
| Eigenvalue | 3.750 | 0.904 | ||||||
| Variance (%) | 80.5 | 19.5 |
Table A4.
PCA for Bassaricyon neblina skulls: variable contributions for the PCA axes that are graphically represented in Figure 9.
| Variable | PC1 | PC2 |
|---|---|---|
| CBL | -0.682 | 0.632 |
| ZYG | -0.804 | -0.185 |
| BBC | -0.671 | -0.373 |
| HBC | -0.214 | -0.689 |
| MTR | -0.644 | 0.664 |
| CC | -0.879 | 0.186 |
| WPP | -0.194 | -0.622 |
| LPP | -0.854 | 0.461 |
| LAB | -0.332 | 0.562 |
| EAM | 0.531 | 0.762 |
| Eigenvalue | 0.016 | 0.013 |
| Variance (%) | 39.7 | 33.0 |
Appendix 2
List of localities discussed in text and used in niche modeling analyses (visual records excluded from modeling).
| taxon | latitude, longitude | locality |
|---|---|---|
| Bassaricyon gabbii | 10.367, -83.850 | Costa Rica: 9 mi NNW Roxana on Rio Chirripo |
| 9.800, -83.900 | Costa Rica: Estrella de Cartago, 6 or 8 miles south of Cartago near the source of the Rio Estrella, altitude of ca. 4,500 feet. | |
| 9.717, -84.267 | Costa Rica: Fila Geronimo | |
| 10.250, -84.433 | Costa Rica: Lajas Villa Quesada | |
| 8.783, -82.950 | Costa Rica: Las Cruces Reserve (visual record only) | |
| 10.100, -83.433 | Costa Rica: Monteverde, Puntarenas Prov., T Guindez pasture | |
| 10.467, -84.000 | Costa Rica: Puerto Viejo, Rio Sarapiqui | |
| 10.467, -84.017 | Costa Rica: Rio Sarapiqui,Puerto Viejo, 300FT | |
| 9.950, -84.050 | Costa Rica: San Gerardo | |
| 9.600, -82.783 | Costa Rica: Talamanca (see Allen 1908) | |
| 15.367, -88.733 | Guatemala: La Sierra del Merendón (visual record only) | |
| 14.983, -85.933 | Honduras: Sierra de Agalta (visual record only) | |
| 13.150, -86.150 | Nicaragua: Jinotega, 16 km E, 5.5 km N Hacienda la Trampa | |
| 12.917, -85.917 | Nicaragua: Matagalba | |
| 12.733, -85.733 | Nicaragua: Rio Grande, south of Tuma, altitude below 1000 ft, Atlantic slope | |
| 13.017, -86.000 | Nicaragua: Tepeyac Matagalpa | |
| 8.783, -82.417 | Panama: Chiriqui, Boquete | |
| 8.850, -82.567 | Panama: Chiriqui, Cerro Punta, Casa Tilley | |
| 8.850, -82.767 | Panama: Chiriqui, Osta Clara | |
| 8.883, -82.567 | Panama: Chirirqui, between Rio Chiriqui Viejo and Rio Colorado, on a hill known locally as Cerro Pando, elevation 4800 feet, ca. 10 miles from El Volcan. | |
| 9.300, -82.400 | Panama: Almirante, Bocas del Toro | |
| Bassaricyon medius orinomus | 7.300, -75.383 | Colombia: Puerta Valdivia |
| 7.833, -76.500 | Colombia: Antioquia, Usaba, Villa Arteage, 130 m | |
| 9.150, -79.850 | Panama: Barro Colorado Island | |
| 8.867, -79.717 | Panama: Caimito | |
| 8.950, -77.817 | Panama: Campo Sasardi, 4 miles, W Mulatupo, Camarca de San Blas | |
| 7.817, -77.717 | Panama: Cana, in the mountains of the east | |
| 9.283, -79.967 | Panama: Canal Zone Rio Indio near Yatun | |
| 9.167, -79.417 | Panama: Cerro Azul, La Zumbadora | |
| 9.200, -80.133 | Panama: Colón Salud | |
| 7.517, -78.167 | Panama: Darien Jaque, near junction Rio Jaque and Rio Imamado | |
| 7.950, -77.500 | Panama: Darien Rio Paya (mouth) | |
| 8.083, -77.283 | Panama: Darien Tacarcuna, casita camp | |
| 9.450, -79.067 | Panama: Mandinga | |
| 9.167, -79.667 | Panama: Mouth of Rio Hondo, near Juan Mina | |
| 7.817, -77.717 | Panama: Mt Pirri, near head of Rio Limon | |
| 9.217, -79.683 | Panama: near Palenque | |
| 9.150, -79.733 | Panama: Parque National Soberania, lowland forest. | |
| 9.450, -78.967 | Panama: Rio Carti | |
| 8.667, -77.467 | Panama: San Blas Armila, Quebrada Venado | |
| Bassaricyon medius medius | 3.350, -77.000 | Colombia: Agua Sucia, Rio Cajambre |
| 4.950, -77.367 | Colombia: Chocó, Rio Baudo, Rio Saudo | |
| 3.667, -76.400 | Colombia: Dagua | |
| 3.783, -76.767 | Colombia: Jimenez, mountains inland of Chocó | |
| 2.883, -77.667 | Colombia: Rio Saija, La Boca | |
| 3.800, -76.633 | Colombia: Valle del Cauca, Sabaleta | |
| -0.433, -78.967 | Ecuador: Cotopaxi, Las Pampas | |
| -2.167, -79.900 | Ecuador: Guayas, Guayaquil | |
| -2.567, -79.350 | Ecuador: Manta Real | |
| 0.117, -78.833 | Ecuador: Pichincha, near Gualea, W side Pichincha | |
| -0.050, -78.767 | Ecuador: Pichincha, near Mindo | |
| -0.250, -79.150 | Ecuador: Santo Domingo de los Tsachilas | |
| Bassaricyon alleni | -13.633, -68.750 | Bolivia: Alto Madidi |
| -17.000, -64.000 | Bolivia: Buena Vista | |
| -16.800, -64.950 | Bolivia: Campamento Yuqui | |
| -11.567, -67.133 | Bolivia: Cotoca Camp | |
| -17.100, -64.783 | Bolivia: Sajta | |
| -11.400, -69.017 | Bolivia: San Sebastien | |
| -17.450, -63.850 | Bolivia: Santa Cruz, Ichilo, Rio Isamo, 400 m | |
| -4.150, -60.117 | Brazil: Amazonas, Campina do Tupana (visual record only) | |
| -8.667, -72.783 | Brazil: Acre | |
| -7.432, -73.661 | Brazil: Acre, Serra do Divisor N.P. | |
| -5.083, -61.467 | Brazil: Amazonas, Capina do Igapo Acu, 256km s of Manaus | |
| -5.200, -69.316 | Brazil: Amazonas, Cujubim S.D.R., Curuena | |
| -7.467, -71.100 | Brazil: Amazonas, Gregorio River E.R. | |
| -8.417, -72.400 | Brazil: Amazonas, Igarape´ Porangaba | |
| -3.883, -60.617 | Brazil: Amazonas, Modulo PPBio Manaquiri (visual record only) | |
| -5.450, -69.267 | Brazil: Amazonas, Uacari S.D.R. (visual record only) | |
| 4.667, -73.067 | Colombia: Guaicaramo, on the Llanos of Villavicencio, east of Bogota | |
| 3.100, -73.917 | Colombia: Macarena Mountains | |
| 10.583, -72.867 | Colombia: Magdalena, Valledupar, Villanueva, Rio Cesar | |
| 4.150, -73.450 | Colombia: Medina | |
| 10.583, -72.867 | Colombia: Sierra Negra, Magdalena, Valledupar, Villanueva | |
| -3.517, -78.450 | Ecaudor: Gualaquiza | |
| -3.483, -78.233 | Ecuador/Peru: Coangos | |
| -0.633, -77.417 | Ecuador: Avila | |
| -1.733, -77.483 | Ecuador: Jatun Yacu, Rio Rutuno | |
| -0.450, -77.883 | Ecuador: Napo, Baeza | |
| -2.067, -76.967 | Ecuador: Pastaza, Rio Bobonaza, Montalvo | |
| -2.117, -77.450 | Ecuador: Road Sarayacu, Copataz | |
| 5.783, -57.633 | Guyana: Bastrica (= Bartica) on the Essequibo River in British Guiana | |
| 4.733, -58.850 | Guyana: Burro-Burro River, Iwokrama Forest | |
| -10.667, -73.900 | Peru Uvini, Rio Cosireni | |
| -8.833, -74.683 | Peru: Aguas Calientes, etc. | |
| -10.133, -71.217 | Peru: Balta | |
| -11.867, -72.783 | Peru: Cashiriari | |
| -11.900, -71.367 | Peru: Cocha Cashu | |
| -12.833, -69.300 | Peru: Explorer’s Inn Reserve | |
| -11.050, -75.317 | Peru: Junin, Chanchamayo, 1200 m | |
| -3.767, -73.250 | Peru: Loreto, Iquitos, Santa Rita 120 m | |
| -5.517, -74.367 | Peru: Loreto: Rio Ucayali, Yarinacocha | |
| -10.383, -75.450 | Peru: Pasco, Cerros de Yanachagua | |
| -7.633, -75.017 | Peru: Pisqui R, Ucalayi | |
| -10.067, -75.533 | Peru: Pozuzo | |
| -11.783, -72.700 | Peru: San Martin | |
| -13.150, -69.600 | Peru: Tambopata | |
| -4.450, -78.150 | Peru: Tseasim (Aguaruna village), Rio Huampami of Rio Cenepa | |
| 4.900, -67.800 | Venezuela: Munduapo, R. Orinoco, Venezuela | |
| 2.250, -65.283 | Venezuela: T. F. Amazonas, 108 km SE, Rio Mavaca, | |
| 5.400, -67.650 | Venezuela: T. F. Amazonas, 32 km SSE Puerto Ayacucho, Raya | |
| 8.167, -72.167 | Venezuela: Tachira 45 km N & 6 km E San Cristobal | |
| Bassaricyon neblina | 3.253, -76.160 | Colombia, Los Alpes, Florida |
| 6.333, -76.117 | Colombia: Antioquia, Urrao, Rio Ana | |
| 6.333, -76.167 | Colombia: Antioquia, Urrao, Rio Urrao | |
| 5.545, -75.496 | Colombia: Castillo Mts | |
| 2.583, -76.917 | Colombia: Cauca, Gallera | |
| 2.533, -76.950 | Colombia: Cauca, Munchique, 2000 m | |
| 2.533, -76.883 | Colombia: Cauca, Sabanetas | |
| 4.049, -76.458 | Colombia: El Duende Regional Reserve | |
| 2.417, -76.817 | Colombia: El Tambo | |
| 1.950, -76.483 | Colombia: Huila, San Agustin, San Antonio | |
| 6.217, -75.533 | Colombia: Santa Elena | |
| -0.617, -78.983 | Ecuador: Cotopaxi, La Cantera in the trail to Sigchos | |
| -0.583, -78.983 | Ecuador: Cotopaxi, near La Cantera in the trail to Sigchos | |
| -0.417, -79.000 | Ecuador: Cotopaxi, Otonga | |
| -0.533, -78.650 | Ecuador: S Domingo Trail, Las Maquinas | |
| -0.021, -78.684 | Ecuador: Tandayapa |
References
- Agnarsson I, Kuntner M, May-Collado LJ. (2010) Dogs, cats, and kin: a molecular species-level phylogeny of Carnivora. Molecular Phylogenetics and Evolution 54: 726-745. doi: 10.1016/j.ympev.2009.10.033 [DOI] [PubMed] [Google Scholar]
- Ahrens HE. (2012) Craniodental characters and the relationships of Procyonidae (Mammalia: Carnivora). Zoological Journal of the Linnean Society 164: 669-713. doi: 10.1111/j.1096-3642.2011.00778.x [DOI] [Google Scholar]
- Allen JA. (1876) Description of a new generic type (Bassaricyon) of Procyonidae from Costa Rica. Proceedings of the Academy of Natural Sciences of Philadelphia 28: 20-23. [Google Scholar]
- Allen JA. (1877) Additional note on Bassaricyon gabbii. Proceedings of the Academy of Natural Sciences of Philadelphia 29: 267-268. [Google Scholar]
- Allen JA. (1879) On the coatis (genus Nasua, Storr). Bulletin of the United States Geological and Geographical Survey of the Territories 5: 153-174. [Google Scholar]
- Allen JA. (1908) Mammals from Nicaragua. Bulletin of the American Museum of Natural History 24: 647-668. [Google Scholar]
- Allen JA. (1912) Mammals from western Colombia. Bulletin of the American Museum of Natural History 31: 71-95. [Google Scholar]
- Allen JA. (1916) List of mammals collected in Colombia by the American Museum of Natural History Expeditions, 1910–1915. Bulletin of the American Museum of Natural History 35: 191-238. [Google Scholar]
- Almendra AL, Rogers DS. (2012) Biogeography of Central American mammals: patterns and processes. In: Patterson BD, Costa LP. (Eds) Bones, clones, and biomes: the history and geography of Recent Neotropical mammals. University of Chicago Press, Chicago, Illinois, 203-229. doi: 10.7208/chicago/9780226649214.003.0010 [DOI] [Google Scholar]
- Alverson WS. (Ed) (2003) Bolivia: Pando, Madre de Dios. Rapid Biological Inventories, Number 5, Field Museum of Natural History, Chicago, Illinois. [Google Scholar]
- Alverson WS, Moskovits DK, Hahn IC. (Eds) (2003) Bolivia: Pando, Federico Román. Rapid Biological Inventories, Number 6, Field Museum of Natural History, Chicago, Illinois. [Google Scholar]
- Alverson WS, Moskovits DK, Shoplan JM. (Eds) (2000) Bolivia: Pando, Río Tahuamanu. Rapid Biological Inventories, Number 1, Field Museum of Natural History, Chicago, Illinois. [Google Scholar]
- Alverson WS, Vriesendorp C, del Campo Á, Moskovits DK, Stotz DF, Donayre MG, Borbor L LA. (Eds) (2008) Ecuador, Perú: Cuyabeno-Güeppi. Rapid Biological Inventories, Number 20, Field Museum of Natural History, Chicago, Illinois. [Google Scholar]
- Anderson RP, Jarrín-V P. (2002) A new species of spiny pocket mouse (Heteromyidae: Heteromys) endemic to Western Ecuador. American Museum Novitates 3382: 1-26. doi: [DOI] [Google Scholar]
- Anderson S. (1997) Mammals of Bolivia: taxonomy and distribution. Bulletin of the American Museum of Natural History 231: 1-652. [Google Scholar]
- Anthony HE. (1923) Mammals from Mexico and South America. American Museum Novitates 54: 1-10. [Google Scholar]
- Aquino R, Encarnación F. (1986) Characteristics and use of sleeping sites in Aotus (Cebidae: Primates) in the Amazon lowlands of Peru. American Journal of Primatology 11: 319-331. doi: 10.1002/ajp.1350110403 [DOI] [PubMed] [Google Scholar]
- Armenteras D, Gast F, Villareal H. (2003) Andean forest fragmentation and the representativeness of protected natural areas in the eastern Andes, Colombia. Biological Conservation 113: 245-256. doi: 10.1016/S0006-3207(02)00359-2 [DOI] [Google Scholar]
- Arnason U, Gullberg A, Janke A, Kullberg M. (2007) Mitogenomic analyses of caniform relationships. Molecular Phylogenetics and Evolution 45: 863-874. doi: 10.1016/j.ympev.2007.06.019 [DOI] [PubMed] [Google Scholar]
- Asher RJ. (2007) A web-database of mammalian morphology and a reanalysis of placental phylogeny. BMC Evolutionary Biology 2007, 7: 108. doi: 10.1186/1471-2148-7-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RJ, Bradley RD. (2006) Speciation in mammals and the Genetic Species Concept. Journal of Mammalogy 87: 643-662. doi: 10.1644/06-MAMM-F-038R2.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeleben C, Moore RL, Wayne RK. (2005) A molecular phylogeny of the Canidae based on six nuclear loci. Molecular Phylogenetics and Evolution 37: 815-831. doi: 10.1016/j.ympev.2005.07.019 [DOI] [PubMed] [Google Scholar]
- Bass MS, Finer M, Jenkins CN, Kreft H, Cisneros-Heredia DF, et al. (2010) Global conservation significance of Ecuador’s Yasuní National Park. PLoS ONE 5(1): e8767. doi: 10.1371/journal.pone.0008767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham PH. (2012) The roles of geological history, topography, and environmental heterogeneity in the diversification of an endemic Andean radiation of the Metallura hummingbirds. Master’s Thesis, University of New Mexico, Albuquerque, New Mexico. [Google Scholar]
- Bickham JW, Patton JC, Schlitter DA, Rautenbach IL, Honeycutt RL. (2004) Molecular phylogenetics, karyotypic diversity, and partition of the genus Myotis (Chiroptera: Vespertilionidae). Molecular Phylogenetics and Evolution 33: 333-338. doi: 10.1016/j.ympev.2004.06.012 [DOI] [PubMed] [Google Scholar]
- Bisbal FJ. (1989) Distribution and habitat association of the carnivores in Venezuela. In: Redford KH, Eisenberg JF. (Eds) Advances in neotropical mammalogy. Sandhill Crane Press, Gainesville, Florida, 339-362. [Google Scholar]
- Bisbal FJ. (1993) Impacto humano sobre los carnívoros de Venezuela. Studies on Neotropical Fauna and Environment 28: 145-156. doi: 10.1080/01650529309360899 [DOI] [Google Scholar]
- Boddicker M. (1997) Medium and large mammals: biodiversity assessment in the Lower Urubamba region. In: Dallmeier F, Alonso A. (Eds) Biodiversity assessment and monitoring of the Lower Urubamba Region, Peru. Smithsonian MAB Biodiversity Program Series 1: 311–340.
- Borman R, Vriesendorp C, Alverson WS, Moskovits DK, Stotz DF, del Campo Á. (Eds) (2007) Ecuador: Territorio Cofan Dureno. Rapid Biological Inventories, Number 19, Field Museum of Natural History, Chicago, Illinois. [Google Scholar]
- Bornholdt R, Helgen K, Koepfli KP, de Oliveira LR, Lucherini M, Eizirik E. (2013) Taxonomic revision of genus Galictis (Carnivora: Mustelidae): species delimitation, morphological diagnosis and refined mapping of geographic distribution. Zoological Journal of the Linnean Society 167: 449-472. doi: 10.1111/j.1096-3642.2012.00859.x [DOI] [Google Scholar]
- Brehm G, Pitkin LM, Hilt N, Fiedler K. (2005) Montane Andean rain forests are a global diversity hotspot of geometrid moths. Journal of Biogeography 32: 1621-1627. doi: 10.1111/j.1365-2699.2005.01304.x [DOI] [Google Scholar]
- Brooks TM, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Rylands AB, Konstant WR, Flick P, Pilgrim J, Oldfield S, Magin G, Hilton-Taylor C. (2002) Habitat loss and extinction in the hotspots of biodiversity. Conservation Biology 16: 909-923. doi: 10.1046/j.1523-1739.2002.00530.x [DOI] [Google Scholar]
- Brown BE. (2004) Atlas of New World marsupials. Fieldiana Zoology 102: 1-308. [Google Scholar]
- Cabrera A. (1957 [1958]-1961) Catálogo de los mamíferos de América del Sur. Revista del Museo Argentino de Ciencias Naturales “Bernardino Rivadavia.” Ciencias Zoológicas 4(1): iv + 308 pp. [1958]; 4(2): v-xxii+309–732 [1961]. [Google Scholar]
- Calouro AM. (1999) Riqueza de mamíferos de grande e médio porte do Parque Nacional da Serra do Divisor (Acre, Brasil). Revista Brasileira de Zoologia 16: 195-213. doi: 10.1590/S0101-81751999000600020 [DOI] [Google Scholar]
- Carleton MD, Musser GG. (1995) Systematic studies of oryzomyine rodents (Muridae: Sigmodontinae): definition and distribution of Oligoryzomys vegetus (Bangs, 1902). Proceedings of the Biological Society of Washington 108: 338-369. [Google Scholar]
- Chapman FM. (1912) Diagnoses of apparently new Colombian birds. Bulletin of the American Museum of Natural History 31: 139-166. [Google Scholar]
- Chapman FM. (1917) The distribution of bird-life in Colombia: a contribution to a biological survey of South America. Bulletin of the American Museum of Natural History 36: 1-729. [Google Scholar]
- Chapman FM. (1926) The distribution of bird-life in Ecuador: a contribution to a study of the origin of Andean bird-life. Bulletin of the American Museum of Natural History 55: 1-784. [Google Scholar]
- Cheida CC, Nakano-Oliveira E, Fusco-Costa R, Rocha-Mendes F, Quadros J. (2006) Ordem Carnivora. In: Reis NR, Peracchi AL, Pedro WA, Lima IP. (Eds) Mamíferos do Brasil. Universidade Estadual de Londrina, Londrina, 231–275. [Google Scholar]
- Chen L, Zhang H. (2012) The complete mitochondrial genome and phylogenetic analysis of Nyctereutes procyonoides. Acta Ecologica Sinica 32: 232-239. doi: 10.1016/j.chnaes.2012.07.003 [DOI] [Google Scholar]
- Churchill SP, Balslev H, Forero E, Luteyn JL. (Eds) (1995) Biodiversity and conservation of Neotropical montane forests. New York Botanical Garden, New York. [Google Scholar]
- Colbert EH. (1996) Henry Fairfield Osborn and the Proboscidea. In: Shoshani J, Tassy P. (Eds) The Proboscidea: evolution and palaeoecology of the elephants and their relatives. Oxford University Press, Oxford, xxiii-xxvii. [Google Scholar]
- Collen B, Purvis A, Gittleman JL. (2004) Biological correlates of description date in carnivores and primates. Global Ecology and Biogeography 13: 459-467. doi: 10.1111/j.1466-822X.2004.00121.x [DOI] [Google Scholar]
- Corse E, Rampal J, Cuoc C, Pech N, Perez Y, Gilles A. (2013) Phylogenetic analysis of Thecosomata Blainville, 1824 (Holoplanktonic Opisthobranchia) using morphological and molecular data. PLoS ONE 8(4): e59439. doi: 10.1371/journal.pone.0059439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo JA. (1959) Tres géneros de mamíferos nuevos para la fauna de Bolivia (Mammalia, Carnivora, Rodentia). Neotropica 5: 9-12 [Google Scholar]
- Cuervo AM, Cadena CD, Krabbe N, Renjifo LM. (2005) Scytalopus stilesi, a new species of tapaculo (Rhinocryptidae) from the Cordillera Central of Colombia. Auk 122: 445-463. doi: 10.1642/0004-8038(2005)122[0445:SSANSO]2.0.CO;2 [DOI] [Google Scholar]
- Cuervo AM, Salaman P, Donegan T, Ochoa JM. (2001) A new species of piha (Cotingidae: Lipaugus) from the Cordillera Central of Colombia. Ibis 143: 353-368. doi: 10.1111/j.1474-919X.2001.tb04937.x [DOI] [Google Scholar]
- Daily G, Ceballos G, Pacheco J, Suzán G, Sánchez-Azofeifa A. (2003) Countryside biogeography of Neotropical mammals: conservation opportunities in agricultural landscapes of Costa Rica. Conservation Biology 17: 1814-1826. doi: 10.1111/j.1523-1739.2003.00298.x [DOI] [Google Scholar]
- Davis CS, Delisle I, Stirling I, Siniff DB, Strobeck C. (2004) A phylogeny of the extant Phocidae inferred from complete mitochondrial DNA coding regions. Molecular Phylogenetics and Evolution 33: 363-377. doi: 10.1016/j.ympev.2004.06.006 [DOI] [PubMed] [Google Scholar]
- de la Rosa CI, Nocke CC. (2000) A guide to the carnivores of Central America. University of Texas Press, Austin. [Google Scholar]
- Decker DM. (1991) Systematics of the coatis, genus Nasua (Mammalia: Procyonidae). Proceedings of the Biological Society of Washington 104: 370-386. [Google Scholar]
- Decker DM, Wozencraft WC. (1991) Phylogenetic analysis of recent procyonid genera. Journal of Mammalogy 72: 42-55. doi: 10.2307/1381979 [DOI] [Google Scholar]
- Delisle I, Strobeck C. (2002) Conserved primers for rapid sequencing of the complete mitochondrial genome from carnivores, applied to three species of bears. Molecular Biology and Evolution 19: 357-361. doi: 10.1016/j.ympev.2005.04.025 [DOI] [PubMed] [Google Scholar]
- Delisle I, Strobeck C. (2005) A phylogeny of the Caniformia (order Carnivora) based on 12 complete protein-coding mitochondrial genes. Molecular Phylogenetics and Evolution 37: 192-201. doi: 10.1093/oxfordjournals.molbev.a004090 [DOI] [PubMed] [Google Scholar]
- Donegan TM, Salaman PGW. (Eds) (1999) Colombian EBA Project: rapid biodiversity assessments and conservation evaluations in the Colombian Andes: northeast Antioquia and highlands of Serranía de los Churumbelos. Colombian EBA Project Report Series No. 2. Fundación Proaves, Colombia.
- Drummond AJ, Ho SYW, Phillips MJ, Rambaut A. (2006) Relaxed phylogenetics and dating with confidence. PLoS Biology 4(5): e88. doi: 10.1371/journal.pbio.0040088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Rambaut A. (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology 2007, 7: 214. doi: 10.1186/1471-2148-7-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg JF. (1989) Mammals of the Neotropics: The Northern Neotropics Vol. 1. University of Chicago Press, Chicago, Illinois. [Google Scholar]
- Eisenberg JF, Redford KH. (1999) Mammals of the Neotropics: The Central Neotropics Vol. 3. University of Chicago Press, Chicago, Illinois. [Google Scholar]
- Eizirik E. (2012) A molecular view on the evolutionary history and biogeography of Neotropical carnivores (Mammalia, Carnivora). In: Patterson BD, Costa LP. (Eds) Bones, clones and biomes: the history and geography of recent Neotropical mammals. University of Chicago Press, Chicago, Illinois, 123-142. doi: 10.7208/chicago/9780226649214.003.0007 [DOI] [Google Scholar]
- Eizirik E, Murphy WJ, Koepfli KP, Johnson WE, Dragoo JW, Wayne RK, O’Brien SJ. (2010) Pattern and timing of diversification of the mammalian order Carnivora inferred from multiple nuclear gene sequences. Molecular Phylogenetics and Evolution 56: 49-63. doi: 10.1016/j.ympev.2010.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson GL, Kilpatrick CW, McNiff BE, Ottenwalder J, Allard MW. (1999) Phylogenetic relationships of the order Insectivora based on complete 12S rRNA sequences from mitochondria. Cladistics 15: 221-230. doi: 10.1111/j.1096-0031.1999.tb00265.x [DOI] [PubMed] [Google Scholar]
- Emmons LH. (1990) Neotropical rainforest mammals, a field guide. University of Chicago Press, Chicago, Illinois. [Google Scholar]
- Emmons LH. (1991) Mammals of Alto Madidi. Conservation International, Rapid Assessment Program Working Papers 1: 23–25, 72–73. [Google Scholar]
- Emmons LH. (1997) Neotropical rainforest mammals, a field guide. Second edition. University of Chicago Press, Chicago, Illinois. [Google Scholar]
- Emmons LH. (2001) Mammal species collected in 1915 by E. Heller in the upper Urubamba Valley. Conservation International, Rapid Assessment Program Working Papers (SI/MAB series 6) 12: 259–261. [Google Scholar]
- Emmons LH, Ascorra C, Romo M. (1994a) Mammals of the Río Heath and Peruvian Pampas. Conservation International, Rapid Assessment Program Working Papers 6: 69–71, 146–149. [Google Scholar]
- Emmons LH, Barkley LJ, Romo M. (1994b) Mammals of the Explorer’s Inn Reserve. Conservation International, Rapid Assessment Program Working Papers 6: 144-145. [Google Scholar]
- Emmons LH, Luna WL, Romo RM. (2001) Mammals of the Northern Vilcabamba Mountain Range, Peru. Conservation International, Rapid Assessment Program Working Papers (SI/MAB series 6) 12: 105–109, 255–257. [Google Scholar]
- Emmons LH, Romo M. (1994) Mammals of the Upper Tambopata/Távara. Conservation International, Rapid Assessment Program Working Papers 6: 46–47, 140–143. [Google Scholar]
- Enders RK. (1936) Bassaricyon pauli, a new species from Panama. Proceedings of the Academy of Natural Sciences of Philadelphia 88: 365-366. [Google Scholar]
- Eva HD, Belward AS, De Miranda EE, Di Bella CM, Gond V, Huber O, Jones S, Sgrenzaroli M, Fritz S. (2004) A land cover map of South America. Global Change Biology 10: 731-744. doi: 10.1111/j.1529-8817.2003.00774.x [DOI] [Google Scholar]
- Evans HE. (1993) Miller’s anatomy of the dog. Third edition W. B. Saunders Company, Philadelphia. [Google Scholar]
- Ewer RF. (1973) The carnivores. Cornell University Press, Ithaca, New York. [Google Scholar]
- Fairchild GB, Handley CO Jr. (1966) Gazetteer of collecting localities in Panama. In: Wenzel RL, Tipton VJ. (Eds) Ectoparasites of Panama. Field Museum of Natural History, Chicago, Illinois, 9-20. [Google Scholar]
- Flynn JJ, Finarelli JA, Zehr S, Hsu J, Nedbal MA. (2005) Molecular phylogeny of the Carnivora (Mammalia): assessing the impact of increased sampling on resolving enigmatic relationships. Systematic Biology 54: 317-337. doi: 10.1080/10635150590923326 [DOI] [PubMed] [Google Scholar]
- Flynn JJ, Nedbal MA. (1998) Phylogeny of the Carnivora (Mammalia): congruence vs incompatibility among multiple data sets. Molecular Phylogenetics and Evolution 9: 414-426. doi: 10.1006/mpev.1998.0504 [DOI] [PubMed] [Google Scholar]
- Flynn JJ, Nedbal MA, Dragoo JW, Honeycutt RL. (2000) Whence the Red Panda? Molecular Phylogenetics and Evolution 17: 190–199. doi: 10.1006/mpev.2000.0819 [DOI] [PubMed] [Google Scholar]
- Flower WH. (1895) Untitled note [note on a Bassaricyon specimen from British Guiana]. Proceedings of the Zoological Society of London 1895: 520-521. [Google Scholar]
- Fonseca RM, Carrera JP, Enríquez T, Lasso DO, Pinto CM, Tello S, Viteri X. (2003) Identificación preliminar de un corredor ecológico para mamíferos entre los parques nacionales Llanganates y Sangay. Revista de la Pontificia Universidad Católica del Ecuador 71: 201-216. [Google Scholar]
- Ford LS, Hoffmann RS. (1988) Potos flavus. Mammalian Species 321: 1–9. doi: 10.2307/3504086 [DOI] [Google Scholar]
- Forsyth A. (2008) Rainforests: Costa Rica and beyond. Cornell University Press, Ithaca, New York. [Google Scholar]
- Freiberg M, Freiberg E. (2000) Epiphyte diversity and biomass in the canopy of lowland and montane forests in Ecuador. Journal of Tropical Ecology 16: 673-688. doi: 10.1017/S0266467400001644 [DOI] [Google Scholar]
- Fulton TL, Strobeck C. (2006) Molecular phylogeny of the Arctoidea (Carnivora): Effect of missing data on supertree and supermatrix analyses of multiple gene data sets. Molecular Phylogenetics and Evolution 41: 165-181. doi: 10.1016/j.ympev.2006.05.025 [DOI] [PubMed] [Google Scholar]
- Fulton TL, Strobeck C. (2007) Novel phylogeny of the raccoon family (Procyonidae, Carnivora) based on nuclear and mitochondrial DNA evidence. Molecular Phylogenetics and Evolution 43: 1171-1177. doi: 10.1016/j.ympev.2006.10.019 [DOI] [PubMed] [Google Scholar]
- Fulton TL, Strobeck C. (2010) Multiple fossil calibrations, nuclear loci, and mitochondrial genomes provide new insight into biogeography and divergence timing for true seals (Phocidae, Pinnipedia). Journal of Biogeography 37: 814-829 doi: 10.1111/j.1365-2699.2010.02271.x [DOI] [Google Scholar]
- Gaubert P, Tranier M, Delmas A-S, Colyn M, Veron G. (2004) First molecular evidence for reassessing phylogenetic affinities between genets (Genetta) and the enigmatic genet‐like taxa Osbornictis, Poiana and Prionodon (Carnivora, Viverridae). Zoologica Scripta 33: 117-129. doi: 10.1111/j.1463-6409.2004.00140.x [DOI] [Google Scholar]
- Gilmore MP, Vriesendorp C, Alverson WS, del Campo Á, von May R, Wong CL, Ochoa SR. (Eds) (2010) Perú: Maijuna. Rapid Biological Inventories, Number 22, Field Museum of Natural History, Chicago, Illinois. [Google Scholar]
- Glanz WE. (1990) Neotropical mammal densities: how unusual is the community on Barro Colorado Island, Panama? In: Gentry AH. (Ed) Four Neotropical rainforests. Yale University Press, New Haven, Connecticut, 287-313. [Google Scholar]
- Glatston AR. (Ed) (1994) The red panda, olingos, coatis, raccoons, and their relatives. Status survey and conservation action plan for procyonids and ailurids. International Union for Conservation of Nature, Gland, Switzerland. [Google Scholar]
- Goldman EA. (1912) New mammals from eastern Panama. Smithsonian Miscellaneous Collections 60(2): 1-18. [Google Scholar]
- Goldman EA. (1920) Mammals of Panama. Smithsonian Miscellaneous Collections 69(5): 1-309. [Google Scholar]
- Goldman EA. (1950) Raccoons of North and Middle America. North American Fauna 60: 1-153. doi: 10.3996/nafa.60.0001 [DOI] [Google Scholar]
- Goloboff PA, Farris JS, Nixon KC. (2008) TNT, a free program for phylogenetic analysis. Cladistics 24: 774-786. doi: 10.1111/j.1096-0031.2008.00217.x [DOI] [Google Scholar]
- González-Maya JF, Belant JL. (2010) Range extension and sociality of bushy-tailed olingo Bassaricyon gabbii in Costa Rica. Small Carnivore Conservation 43: 37-39. [Google Scholar]
- González-Maya JF, Cepeda AA, Belant JL, Zárrate-Charry DA, Balaguera-Reina SA, Rodríguez-Bolaños A. (2011) Research priorities for the small carnivores of Colombia. Small Carnivore Conservation 44: 7-13. [Google Scholar]
- Goodman SM, Helgen KM. (2010) Species limits and distribution of the Malagasy carnivoran genus Eupleres (family Eupleridae). Mammalia 74: 177-185. doi: 10.1515/mamm.2010.018 [DOI] [Google Scholar]
- Goodwin GG. (1946) Mammals of Costa Rica. Bulletin of the American Museum of Natural History 87: 271-478. [Google Scholar]
- Graham CH, Silva N, Velásquez-Tibatá J. (2010) Evaluating the potential causes of range limits of birds of the Colombian Andes. Journal of Biogeography 37: 1863-1875. doi: 10.1111/j.1365-2699.2010.02356.x [DOI] [Google Scholar]
- Gregory WK. (1937) Biographical memoir of Henry Fairfield Osborn, 1857–1935. Biographical Memoirs of the National Academy of Sciences of the United States of America 19: 53-119. [Google Scholar]
- Gregory-Wodzicki KM. (2000) Uplift history of the Central and Northern Andes: a review. Geological Society of America Bulletin 112: 1091-1105. doi: [DOI] [Google Scholar]
- Grimaldi G Jr., Tesh RB. (1993) Leishmaniases of the New World: current concepts and implications for future research. Clinical Microbiology Reviews 6: 230-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood IR. (1969) Notes on the distribution and status of some Peruvian mammals, 1968. American Committee for International Wild Life Protection and New York Zoological Society, Special Publication 21: 1-86. [Google Scholar]
- Gutiérrez EE, Helgen KM. (2013) Outdated taxonomy blocks conservation. Nature 495: 314. doi: 10.1038/495314e [DOI] [PubMed] [Google Scholar]
- Haffer J. (1967) Speciation in Colombian forest birds west of the Andes. American Museum Novitates 2294: 1-57. [Google Scholar]
- Hall ER. (1981) The mammals of North America. Second edition. Two volumes. John Wiley & Sons, New York. [Google Scholar]
- Hall ER, Kelson KR. (1959) The mammals of North America. Two volumes. Ronald Press, New York. [Google Scholar]
- Hance J. (2012) Photos: new mammal menagerie uncovered in remote Peruvian cloud forest. http://news.mongabay.com/2012/1003-hance-newmammals-peru.html
- Handley CO Jr. (1966) Checklist of the mammals of Panama. In: Wenzel RL, Tipton VJ. (Eds) Ectoparasites of Panama. Field Museum of Natural History, Chicago, Illinois, 753-795. [Google Scholar]
- Handley CO Jr. (1976) Mammals of the Smithsonian Venezuelan Project. Brigham Young University Science Bulletin 20: 1-91. [Google Scholar]
- Harris WP Jr. (1932) Four new mammals from Costa Rica. Occasional Papers of the Museum of Zoology University of Michigan 248: 1-6. [Google Scholar]
- Hasegawa M, Kishino H, Yano TA. (1985) Dating of the human–ape splitting by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution 22: 160-174. doi: 10.1007/BF02101694 [DOI] [PubMed] [Google Scholar]
- Helgen KM, Kays R, Helgen LE, Tsuchiya-Jerep MTN, Pinto CM, Koepfli K, Eizirik E, Maldonado JE. (2009) Taxonomic boundaries and geographic distributions revealed by an integrative systematic overview of the mountain coatis, Nasuella (Carnivora: Procyonidae). Small Carnivore Conservation 41: 65-74. [Google Scholar]
- Helgen KM, Lim NT-L, Helgen LE. (2008a) The hog-badger is not an edentate: systematics and evolution of Arctonyx (Mammalia: Mustelidae). Zoological Journal of the Linnean Society 154: 353-385. doi: 10.1111/j.1096-3642.2008.00416.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgen KM, Maldonado JE, Wilson DE, Buckner SD. (2008b) Molecular confirmation of the origin and invasive status of West Indian raccoons. Journal of Mammalogy 89: 282-291. doi: 10.1644/07-MAMM-A-155R.1 [DOI] [Google Scholar]
- Helgen K, Timm R, Schipper J. (2008c) Bassaricyon lasius. In: IUCN 2012. IUCN Red List of Threatened Species. Version 2012.2. www.iucnredlist.org [accessed on 5 May 2013]
- Helgen KM, Wilson DE. (2002) The history of the raccoons of the West Indies. Journal of the Barbados Museum and Historical Society 48: 1-11. [Google Scholar]
- Helgen KM, Wilson DE. (2003) Taxonomic status and conservation relevance of the raccoons (Procyon spp.) of the West Indies. Journal of Zoology (London) 259: 69-76. doi: 10.1017/S0952836902002972 [DOI] [Google Scholar]
- Helgen KM, Wilson DE. (2005) A systematic and zoogeographic overview of the raccoons of Mexico and Central America. In: Sánchez-Cordero V, Medellin RA. (Eds) Contribuciones Mastozoológicas en Homenaje a Bernardo Villa. Instituto de Biologia e Instituto de Ecologia, UNAM, Mexico, 221-236. [Google Scholar]
- Henderson C. (2002) Field guide to the wildlife of Costa Rica. University of Texas Press, Austin, Texas. [Google Scholar]
- Hendricks LD. (1977) Host range characteristics of the primate coccidian, Isospora arctopitheci Rodhain 1933 (Protozoa: Eimeriidae). Journal of Parasitology 63: 32-35. doi: 10.2307/3280100 [DOI] [PubMed] [Google Scholar]
- Herrer A, Christensen HA. (1975) Infrequency of gross skin lesions among Panamanian forest mammals with cutaneous leishmaniasis. Parasitology 71: 87-92. doi: 10.1017/S0031182000053178 [DOI] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. (2005) Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965-1978. doi: 10.1002/joc.1276 [DOI] [Google Scholar]
- Hollister N. (1915) The genera and subgenera of raccoons and their allies. Proceedings of the United States National Museum 49: 143-150. doi: 10.5479/si.00963801.49-2100.143 [DOI] [Google Scholar]
- Hoorn C, Wesselingh FP, ter Steege H, Bermudez MA, Mora A, Sevink J, Sanmartín I, Sanchez-Meseguer A, Anderson CL, Figueiredo JP, Jaramillo C, Riff D, Negri FR, Hooghiemstra HH, Lundberg J, Stadler T, Särkinen T, Antonelli A. (2010) Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330: 927-931. doi: 10.1126/science.1194585 [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754-755. doi: 10.1093/bioinformatics/17.8.754 [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Rannala B. (2004) Frequentist properties of Bayesian posterior probabilities of phylogenetic trees under simple and complex substitution models. Systematic Biology 53: 904-913. doi: 10.1080/10635150490522629 [DOI] [PubMed] [Google Scholar]
- Huet M. (1883) Note sur les Carnassiers du genre Bassaricyon. Nouvelles archives du Muséum d’histoire naturelle de Paris (ser. 2) 5: 1–12. [Google Scholar]
- Hughes C, Eastwood R. (2006) Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proceedings of the National Academy of Sciences U.S.A. 103: 10334-10339. doi: 10.1073/pnas.0601928103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter L. (2011) Carnivores of the world. Princeton University Press, Princeton, New Jersey. [Google Scholar]
- Hunter J, Caro TM. (2008) Interspecific competition and predation in American carnivore families. Ethology Ecology and Evolution 20: 295-324. doi: 10.1080/08927014.2008.9522514 [DOI] [Google Scholar]
- Husson AM. (1978) The mammals of Suriname. E. J. Brill, Leiden, Netherlands. [Google Scholar]
- Izor RJ, de la Torre L. (1978) A new species of weasel (Mustela) from the highlands of Colombia, with comments on the evolution and distribution of South American weasels. Journal of Mammalogy 59: 92-102. doi: 10.2307/1379878 [DOI] [Google Scholar]
- Janson CH, Emmons LH. (1990) Ecological structure of the nonflying mammal community at Cocha Cashu Biological Station, Manu National Park, Peru. In: Gentry AH. (Ed) Four Neotropical rainforests. Yale University Press, New Haven, Connecticut, 314-338. [Google Scholar]
- Janzen DH. (Ed) (1983) Costa Rican natural history. University of Chicago Press, Chicago, Illinois.
- Jarrín-V P. (2001) Mamíferos en la niebla: Otonga, un bosque nublado del Ecuador. Publicación Especial 5 Museo de Zoología, Pontificia Universidad Católica del Ecuador, Quito, Ecuador. [Google Scholar]
- Jewell ML, Frenkel JK, Johnson KM, Reed V, Ruiz A. (1972) Development of Toxoplasma oocysts in Neotropical Felidae. American Journal of Tropical Medicine and Hygiene 21: 512-517. [DOI] [PubMed] [Google Scholar]
- Kattan GH, Alvarez-Lopez H, Giraldo M. (1994) Forest fragmentation and bird extinctions—San Antonio 80 years later. Conservation Biology 8: 138-146. doi: 10.1046/j.1523-1739.1994.08010138.x [DOI] [Google Scholar]
- Kays RW. (2000) The behavior and ecology of olingos (Bassaricyon gabbii) and their competition with kinkajous (Potos flavus) in central Panama. Mammalia 64: 1-10. doi: 10.1515/mamm.2000.64.1.1 [DOI] [Google Scholar]
- Kays RW. (2009) Family Procyonidae (raccoons). In: Wilson DE, Mittermeier RA. (Eds) Handbook of the mammals of the world.1. Carnivores. Lynx Edicions, Barcelona, Spain, 504-530. [Google Scholar]
- Kays R, Rodríguez ME, Valencia LM, Horan R, Smith AR, Ziegler C. (2012) Animal visitation and pollination of flowering balsa trees (Ochroma pyramidale) in Panama. Mesoamericana 16(3): 56-70. [Google Scholar]
- Kays R, Russell JK. (2001) Other procyonids. In: Macdonald D. (Ed) The new encyclopedia of mammals. Oxford University Press, 94-95. [Google Scholar]
- Kennedy ML, Lindsay SL. (1984) Morphological variation in the raccoon, Procyon lotor, and its relationship to genic and environmental variation. Journal of Mammalogy 65: 195-205. doi: 10.2307/1381159 [DOI] [Google Scholar]
- Kimura M. (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16: 111-120. doi: 10.1007/BF01731581 [DOI] [PubMed] [Google Scholar]
- Koepfli K-P, Deere KA, Slater GJ, Begg C, Begg K, Grassman L, Lucherini M, Veron G, Wayne RK. (2008) Multigene phylogeny of the Mustelidae: resolving relationships, tempo and biogeographic history of a mammalian adaptive radiation. BMC Biology 2008, 6: 10. doi: 10.1186/1741-7007-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepfli K-P, Gompper ME, Eizirik E, Ho C-C, Linden L, Maldonado JE, Wayne RK. (2007) Phylogeny of the Procyonidae (Mammalia: Carnivora): Molecules, morphology, and the Great American Interchange. Molecular Phylogenetics and Evolution 43: 1076-1095. doi: 10.1016/j.ympev.2006.10.003 [DOI] [PubMed] [Google Scholar]
- Koepfli K-P, Wayne RK. (1998) Phylogenetic relationships of otters (Carnivora: Mustelidae) based on mitochondrial cytochrome b sequences. Journal of Zoology, London, 246: 401-416. doi: 10.1111/j.1469-7998.1998.tb00172.x [DOI] [Google Scholar]
- Lanfear R, Calcott B, Ho SYW, Guindon S. (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29: 1695-1701. doi: 10.1093/molbev/mss020 [DOI] [PubMed] [Google Scholar]
- Lara CE, Cuervo AM, Valderrama SV, Calderón-F D, Cadena CD. (2012) A new species of wren (Troglodytidae: Thryophilus) from the dry Cauca River canyon, northwestern Colombia. Auk 129: 537−550. doi: 10.1525/auk.2012.12028 [DOI] [Google Scholar]
- Ledje C, Arnason U. (1996a) Phylogenetic analyses of complete cytochrome b genes of the order Carnivora with particular emphasis on the Caniformia. Journal of Molecular Ecology 42: 135-144. doi: 10.1007/BF02202112 [DOI] [PubMed] [Google Scholar]
- Ledje C, Arnason U. (1996b) Phylogenetic relationships within caniform carnivores based on analyses of the mitochondrial 12S rRNA gene. Journal of Molecular Ecology 43: 641-649. doi: 10.1007/BF02198839 [DOI] [PubMed] [Google Scholar]
- Lee TE, Boada-Terán C, Scott AM, Burneo SF, Hanson JD. (2011) Small mammals of Sangay National Park, Chimborazo province and Morona Santiago province, Ecuador. Occasional Papers of the Museum of Texas Tech University 305: 1-14. [Google Scholar]
- Lee TE Jr., Packer JB, Alvarado-Serrano D. (2006) Results of a mammal survey of the Tandayapa Valley, Ecuador. Occasional Papers of the Museum of Texas Tech University 250: 1-8. [Google Scholar]
- Leite RN, Rogers DS. (2013) Revisiting Amazonian phylogeography: insights into diversification hypotheses and novel perspectives. Organisms Diversity and Evolution. doi: 10.1007/s13127-013-0140-8 [DOI]
- Lim BK. (2012) Biogeography of mammals from the Guianas of South America. In: Patterson BD, Costa LP. (Eds) Bones, clones and biomes: the history and geography of recent Neotropical mammals. University of Chicago Press, Chicago, Illinois, 230-258. doi: 10.7208/chicago/9780226649214.003.0011 [DOI] [Google Scholar]
- Lim BK, Engstrom MD. (2005) Mammals of Iwokrama Forest. Proceedings of the Academy of Natural Sciences of Philadelphia 154: 71-108. doi: 10.1635/0097-3157(2004)154[0071:MOIF]2.0.CO;2 [DOI] [Google Scholar]
- Lim BK, Engstrom MD, Ochoa G J. (2005) Mammals. In: Hollowell T, Reynolds RP. (Eds) Checklist of the terrestrial vertebrates of the Guiana Shield. Bulletin of the Biological Society of Washington 13: 76–92, 98.
- Linares OJ. (1998) Mamíferos de Venezuela. Sociedad Conservacionista Audubon de Venezuela, Caracas, Venezuela. [Google Scholar]
- Lönnberg E. (1921) A second contribution to the mammalogy of Ecuador with some remarks on Caenolestes. Arkiv för Zoologi 14: 1-104. [Google Scholar]
- Lord R. (2007) Mammals of South America. Johns Hopkins University Press, Baltimore, Maryland. [Google Scholar]
- Loyola RD, de Oliveira G, Diniz-Filho JAF, Lewinsohn TM. (2008) Conservation of Neotropical carnivores under different prioritization scenarios: mapping species traits to minimize conservation conflicts. Diversity and Distributions 14: 949-960. doi: 10.1111/j.1472-4642.2008.00508.x [DOI] [Google Scholar]
- Lyons LA, Laughlin TF, Copeland NG, Jenkins NA, Womack JE, O’Brien SJ. (1997) Comparative anchor tagged sequences (CATS) for integrative mapping of mammalian genomes. Nature Genetics 15: 47-56. doi: 10.1038/ng0197-47 [DOI] [PubMed] [Google Scholar]
- Magalhães-Pinto R, Knoff M, Queiroga-Gonçalves A, Sanches M, Noronha D. (2009) First report of Taenia mustelae (Eucestoda, Taeniidae) parasitizing the bushy-tailed olingo, Bassaricyon gabbii (Carnivora, Procyonidae) in South America with an updated checklist of cestodes from other American procyonid hosts. Neotropical Helminthology 3: 7-14. [Google Scholar]
- Malhado ACM, Ladle RJ, Whittaker RJ, Neto JAO, Malhi Y, ter Steege H. (2013) The ecological biogeography of Amazonia. Frontiers of Biogeography 5.2: 103–112. [Google Scholar]
- Manville R. (1956) This “kinkajou” was really the very rare olingo. Animal Kingdom 59: 109-111. [Google Scholar]
- Marín D, Ramírez-Chaves HE, Suárez-Castro AF. (2012) Revisión cráneo-dentaria de Procyon (Carnivora: Procyonidae) en Colombia y Ecuador, con notas sobre su taxonomía y distribución. Mastozoología Neotropical 19: 259-270. [Google Scholar]
- Marmi J, López‐Giráldez JF, Domingo‐Roura X. (2004) Phylogeny, evolutionary history, and taxonomy of the Mustelidae based on sequences of the cytochrome b gene and a complex repetitive flanking region. Zoologica Scripta 33: 481-499. doi: 10.1111/j.0300-3256.2004.00165.x [DOI] [Google Scholar]
- Marshall LG, Butler RF, Drake RE, Curtis GH, Tedford RH. (1979) Calibration of the Great American Interchange. Science 204: 272-279. doi: 10.1126/science.204.4390.272 [DOI] [PubMed] [Google Scholar]
- Mayr E. (1940) Speciation phenomena in birds. American Naturalist 74: 249-278. doi: 10.1086/280892 [DOI] [Google Scholar]
- Mayr E. (1942) Systematics and the origin of species. Columbia University Press, New York. [Google Scholar]
- McCarthy TJ, Perez SJ. (2006) Land and freshwater mammals of Guatemala: faunal documentation and diversity. In: Cano EB. (Ed) Biodiversidad de Guatemala, Vol. 1. Universidad del Valle de Guatemala, Guatemala, 625-674. [Google Scholar]
- McPherson AB. (1985) A biogeographical analysis of factors influencing the distribution of Costa Rican rodents. Brenesia 23: 97-273. [Google Scholar]
- McPherson AB. (1986) The biogeography of Costa Rican rodents: an ecological, geological, and evolutionary approach. Brenesia 25–26: 229–244. [Google Scholar]
- Mejía Correa S. (2009) Inventario de mamíferos grandes y medianos en el Parque Nacional Natural Munchique, Colombia. Mastozoología Neotropical 16: 264-266. [Google Scholar]
- Mendes Pontes AR. (2004) Ecology of a community of mammals in a seasonally dry forest in Roraima, Brazilian Amazon. Zeitschrift für Säugetierkunde 69: 319-336. [Google Scholar]
- Mendes Pontes AR. (2009) Amazonia and other forests of Brazil. Janus Publishing Company, London. [Google Scholar]
- Mendes Pontes AR, Chivers DJ. (2002) Abundance, habitat use, and conservation of the olingo Bassaricyon sp. in Maracá Ecological Station, Roraima, Brazilian Amazonia. Studies in Neotropical Fauna and Environment 37: 105-109. doi: 10.1076/snfe.37.2.105.8577 [DOI] [Google Scholar]
- Mendes Pontes AR, Rosas Ribeiro PF, Mendonça TM. (2002) Olingos, Bassaricyon beddardi Pocock, 1921, in Brazilian Amazonia: status and recommendations. Small Carnivore Conservation 26: 768-769. [Google Scholar]
- Mendez E. (1970) Los principales mamíferos silvestres de Panamá. Privately printed in Panamá.
- Miller MA, Holder M, Vos R, Midford PE, Liebowitz T, Chan L, Hoover P, Warnow T. (2009) The CIPRES Portals. CIPRES. http://www.phylo.org/sub_sections/portal
- Mittermeier RA, Gil PR, Hoffman M, Pilgrim J, Brooks T, Mittermeier CG, Lamoreux J, Da Fonseca GAB. (2005) Hotspots revisited: earth’s biologically richest and most endangered terrestrial ecoregions. Conservation International, Cemex, Mexico City. [Google Scholar]
- Mivart SG. (1885) On the anatomy, classification, and distribution of the Arctoidea. Proceedings of the Zoological Society of London 1885: 340-404. [Google Scholar]
- Mivart SG. (1886) Notes on the cerebral convolutions of the Carnivora. Journal of the Linnean Society 19: 1-25. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. (2000) Biodiversity hotspots for conservation priorities. Nature 403: 853-858. doi: 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Naidu A, Fitak RR, Munguia-Vega A, Culver M. (2012) Novel primers for complete mitochondrial cytochrome b gene sequencing in mammals. Molecular Ecology Resources 12: 191-196. [DOI] [PubMed] [Google Scholar]
- Nash WG. (2006) Order Carnivora. In: O’Brien SJ, Menninger JC, Nash WG. (Eds) Atlas of mammalian chromosomes. John Wiley & Sons, Hoboken, New Jersey, 445-538. [Google Scholar]
- Nieder J, Barthlott W. (Eds) (2001) Epiphytes and canopy fauna of the Otonga rain forest (Ecuador). Botanisches Institut der Universität Bonn, Bonn, Germany. doi: 10.1111/j.1755-0998.2011.03078.x [DOI] [Google Scholar]
- Nowak R. (1999) Walker’s mammals of the world. Sixth edition Johns Hopkins University Press, Baltimore, Maryland. [Google Scholar]
- Nyakatura K, Bininda-Emonds ORP. (2012) Updating the evolutionary history of Carnivora (Mammalia): a new species-level supertree complete with divergence time estimates. BMC Biology 2012, 10: 12. doi: 10.1186/1741-7007-10-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa G J, Molina C, Giner S. (1993) Inventario y estudio comunitario de los mamíferos del Parque Nacional Canaima, con un lista de las especies registradas para la Guayana Venezolana. Acta Científica Venezolana 44: 244-261. [Google Scholar]
- Ojala-Barbour R, Pinto CM, Brito J, Albuja-V L, Lee Jr TE, Patterson BD. (in press) A new species of shrew-opossum (Paucituberculata: Caenolestidae) with a phylogeny of extant caenolestids. Journal of Mammalogy 94. [Google Scholar]
- Oliveira TG de. (2009) Notes on the distribution, status, and research priorities of little-known small carnivores in Brazil. Small Carnivore Conservation 41: 22-24. [Google Scholar]
- Ollier CD. (2006) Mountain uplift and the neotectonic period. Annals of Geophysics 49: 437-450. [Google Scholar]
- Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC, D’Amico JA, Strand HE, Morrison JC, Loucks CJ, Allnutt TF, Lamoreux JF, Ricketts TH, Itoua I, Wettengel WW, Kura Y, Hedao P, Kassem K. (2001) Terrestrial ecoregions of the world: a new map of life on Earth. BioScience 51: 933-938. doi: 10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2 [DOI] [Google Scholar]
- Ordóñez Garza N, McCarthy TJ, Monzon Sierra J, Matson JO, Eckerlin RP. (1999–2000) Amplicación del área de distribución de Bassaricyon gabbii J.A. Allen, 1876 (Carnivora: Procyonidae) en el norte de América Central. Revista Mexicana de Mastozoología 4: 114-116. [Google Scholar]
- Pacheco V, Patterson BD, Patton JL, Emmons LE, Solari S, Ascorra CF. (1993) List of mammal species known to occur in Manu Biosphere Reserve, Peru. Publicaciones del Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Serie A Zoologia 44: 1-12. [Google Scholar]
- Parker TA III, Carr JL. (Eds) (1992) Status of forest remnants in the Cordillera de la Costa and adjacent areas of southwestern Ecuador. Conservation International, Rapid Assessment Program Working Papers 2, Washington, D.C. [Google Scholar]
- Patterson BD. (1987) A biographical sketch of Philip Hershkovitz, with a complete scientific bibliography. Fieldiana Zoology 39: 1-10. [Google Scholar]
- Patterson BD. (1994) Accumulating knowledge on the dimensions of biodiversity: systematic perspectives on Neotropical mammals. Biodiversity Letters 2: 79-86. doi: 10.2307/2999761 [DOI] [Google Scholar]
- Patterson BD. (1997) Philip Hershkovitz: 1909–1997. Journal of Mammalogy 78: 978-981. [Google Scholar]
- Patterson BD. (2000) Patterns and trends in the discovery of new Neotropical mammals. Diversity and Distributions 6: 145-151. doi: 10.1046/j.1472-4642.2000.00080.x [DOI] [Google Scholar]
- Patterson BD, Ceballos G, Sechrest W, Tognelli MF, Brooks T, Luna L, Ortega P, Salazar I, Young BE. (2005) Digital distribution maps of the mammals of the western hemisphere, version 2.0. NatureServe, Arlington, Virginia. [Google Scholar]
- Patterson BD, Pacheco V, Ashley M. (1992) On the origins of the western slope region of endemism: systematics of fig-eating bats, genus Artibeus. Memorias del Museo de Historia Natural, UNMSM (Lima) 21: 189-205. [Google Scholar]
- Patterson BD, Solari S, Velazco PM. (2012) The role of the Andes in the diversification and biogeography of Neotropical mammals. In: Patterson BD, Costa LP. (Eds) Bones, clones and biomes: the history and geography of recent Neotropical mammals. University of Chicago Press, Chicago, Illinois, 351-378. doi: 10.7208/chicago/9780226649214.003.0015 [DOI] [Google Scholar]
- Patterson BD, Velazco PM. (2008) Phylogeny of the rodent genus Isothrix (Hystricognathi, Echimyidae) and its diversification in Amazonia and the eastern Andes. Journal of Mammalian Evolution 15: 181-201. doi: 10.1007/s10914-007-9070-6 [DOI] [Google Scholar]
- Patton JL, Berlin B, Berlin EA. (1982) Aboriginal perspectives of a mammal community in Amazonian Perú: knowledge and utilization patterns among the Aguaruna Jívaro. In: Mares MA, Genoways HH. (Eds) Mammalian biology in South America, University of Pittsburgh, Pittsburgh, Pennsylvania, 111–128. [Google Scholar]
- Paynter RA Jr. (1993) Ornithological gazetteer of Ecuador, second edition. Museum of Comparative Zoology, Cambridge, Massachusetts. [Google Scholar]
- Paynter RA Jr. (1997) Ornithological gazetteer of Colombia, second edition. Museum of Comparative Zoology, Cambridge, Massachusetts. [Google Scholar]
- Paynter RA Jr. (1982) Ornithological gazetteer of Venezuela. Museum of Comparative Zoology, Cambridge, Massachusetts. [Google Scholar]
- Peng R, Zeng B, Meng X, Yue B, Zhang Z, Zou F. (2007) The complete mitochondrial genome and phylogenetic analysis of the giant panda (Ailuropoda melanoleuca). Gene 397: 76-83. doi: 10.1016/j.gene.2007.04.009 [DOI] [PubMed] [Google Scholar]
- Peres CA. (2001) Synergistic effects of subsistence hunting and habitat fragmentation on Amazonian forest vertebrates. Conservation Biology 15: 1490-1505. doi: 10.1046/j.1523-1739.2001.01089.x [DOI] [Google Scholar]
- Phillips SJ, Anderson RP, Schapire RE. (2006) Maximum entropy modeling of species geographic distributions. Ecological Modeling 190: 231-259. doi: 10.1016/j.ecolmodel.2005.03.026 [DOI] [Google Scholar]
- Pinto CM. (2009) Genetic diversity of the common vampire bat Desmodus rotundus in Ecuador: testing cross-Andean gene flow. MSc thesis, Texas Tech University, Lubbock, Texas. [Google Scholar]
- Pinto CM, Tirira DG. (2011a) Olingo de la costa Bassaricyon gabbii Carnivora, Procyonidae. In: Tirira DG. (Ed) Libro Rojo de los Mamíferos del Ecuador, Second edition. Fundación Mamíferos y Conservación, Pontificia Universidad Católica del Ecuador and Ministerio del Ambiente del Ecuador, Publicación especial sobre los mamíferos del Ecuador 8, Quito, 138-139. [Google Scholar]
- Pinto CM, Tirira DG. (2011b) Olingo de oriente Bassaricyon alleni Carnivora, Procyonidae. In: Tirira DG. (Ed) Libro Rojo de los Mamíferos del Ecuador, Second edition Fundación Mamíferos y Conservación, Pontificia Universidad Católica del Ecuador and Ministerio del Ambiente del Ecuador, Publicación especial sobre los mamíferos del Ecuador 8, Quito, 223. [Google Scholar]
- Pitman N, Moskovits DK, Alverson WS, Borman A R. (2002) Ecuador: Serranías Cofán-Bermejo, Sinangoe. Rapid Biological Inventories, Number 3, Field Museum of Natural History, Chicago, Illinois. [Google Scholar]
- Pitman N, Smith RC, Vriesendorp C, Moskovits D, Piana R, Knell G, Wachter T. (Eds) (2004) Perú: Ampiyacu, Apayacu, Yaguas, Medio Putumayo. Rapid Biological Inventories, Number 12, Field Museum of Natural History, Chicago, Illinois. [Google Scholar]
- Pitman N, Vriesendorp C, Moskovits D. (Eds) (2003) Perú: Yavarí. Rapid Biological Inventories, Number 11, Field Museum of Natural History, Chicago, Illinois. [Google Scholar]
- Pocock RI. (1921a) A new species of Bassaricyon. Annals and Magazine of Natural History (series 9) 7: 229–234. [Google Scholar]
- Pocock RI. (1921b) The external characters and classification of the Procyonidae. Proceedings of the Zoological Society of London 1921: 389-422. [Google Scholar]
- Poglayen-Neuwall I. (1966) Notes on care, display and breeding of olingos. International Zoo Yearbook 6: 169-171. [Google Scholar]
- Poglayen-Neuwall I. (1973) The odorous olingo. Animal Kingdom 76(5): 10-14. doi: 10.1111/j.1748-1090.1966.tb01739.x [DOI] [Google Scholar]
- Poglayen-Neuwall I. (1976) Fortpflanzung, Geburt und Aufzucht, nebst anderen Beobachtungen von Makibären (Bassaricyon Allen, 1876). Zoologische Beiträge 22: 179-233. [Google Scholar]
- Poglayen-Neuwall I. (1989) Notes on reproduction, aging and longevity of Bassaricyon sp. (Procyonidae). Zoologische Garten 59: 122-128. [Google Scholar]
- Poglayen-Neuwall I, Poglayen-Neuwall I. (1965) Gefangenschaftsbeobachtungen an Makibären (Bassaricyon Allen, 1876). Zeitschrift für Säugetierkunde 30: 321-366. [Google Scholar]
- Posada D. (2008) jModelTest: phylogenetic model averaging. Molecular Biology and Evolution 25(7): 1253-1256. doi: 10.1093/molbev/msn083 [DOI] [PubMed] [Google Scholar]
- Prange S, Prange TJ. (2009) Bassaricyon gabbii (Carnivora: Procyonidae). Mammalian Species 826: 1-7. doi: 10.1644/826.1 [DOI] [Google Scholar]
- Redford K, Stearman AML. (1993) Notas sobre la biología de tres procyonidos simpátricos bolivianos (Mammalia, Procyonidae). Ecología en Bolivia 21: 35-44. [Google Scholar]
- Ree RH, Smith SA. (2008) Maximum likelihood inference of geographical range evolution by dispersal, local extinction and cladogenesis. Systematic Biology 57: 4-14. doi: 10.1080/10635150701883881 [DOI] [PubMed] [Google Scholar]
- Reeder DM, Helgen KM, Wilson DE. (2007) Global trends and biases in new mammal species discoveries. Occasional Papers of the Museum at Texas Tech University 269: 1-36. [Google Scholar]
- Reid FA. (1997) A field guide to the mammals of Central America and southeast Mexico. Oxford University Press, New York. [Google Scholar]
- Reid FA. (2009) A field guide to the mammals of Central America and southeast Mexico. Second edition. Oxford University Press, New York.
- Reid F, Helgen K. (2008a) Bassaricyon gabbii. In: IUCN 2012. IUCN Red List of Threatened Species. Version 2012.2. www.iucnredlist.org [accessed on 5 May 2013]
- Reid F, Helgen K. (2008b) Bassaricyon alleni. In: IUCN 2012. IUCN Red List of Threatened Species. Version 2012.2. www.iucnredlist.org [accessed on 5 May 2013]
- Reid F, Helgen K. (2008c) Bassaricyon beddardi. In: IUCN 2012. IUCN Red List of Threatened Species. Version 2012.2. www.iucnredlist.org [accessed on 5 May 2013]
- Ríos-Uzeda B, Arispe R. (2010) Procyonidae. In: Wallace RB, Gómez H, Porcel ZR, Rumiz DI. (Eds) Distribución, ecología y conservación de los mamíferos medianos y grandes de Bolivia. Centro de Ecología Difusión Simón I. Patino, Santa Cruz de la Sierra, Bolivia, 497-517. [Google Scholar]
- Robbins MB, Stiles HG. (1999) A new species of pygmy-owl (Strigidae: Glaucidium) from the Pacific slope of the Northern Andes. Auk 116: 305-315. doi: 10.2307/4089365 [DOI] [Google Scholar]
- Rodríguez JJ, Amanzo JM. (2001) Medium and large mammals of the southern Vilcabamba region, Peru. Conservation International, Rapid Assessment Program Working Papers (SI/MAB series 6) 12: 117–126. [Google Scholar]
- Ron SR. (2000) Biogeographic area relationships of lowland Neotropical rainforest based on raw distributions of vertebrate groups. Biological Journal of the Linnean Society 71: 379-402. doi: 10.1111/j.1095-8312.2000.tb01265.x [DOI] [Google Scholar]
- Russell JK. (1984) Other procyonids. In: Macdonald D. (Ed) The encyclopedia of mammals. Facts on File, New York, 106-107. [Google Scholar]
- Saavedra-Rodríguez CA, Velandia-Perilla JH. (2011) Bassaricyon gabbii Allen, 1876 (Carnivora: Procyonidae): new distribution point on western range of Colombian Andes. Check List 7: 505-507. [Google Scholar]
- Sampaio R, da Silva MNF, Cohn-Haft M. (2011) Reassessment of the occurrence of the kinkajou (Potos flavus Schreber, 1774) and olingo (Bassaricyon beddardi Pocock, 1921) in the northern Brazilian Amazon. Studies on Neotropical Fauna and Environment 46: 85-90. doi: 10.1080/01650521.2011.572678 [DOI] [Google Scholar]
- Sampaio R, Munaril DP, Röhe F, Ravetta AL, Rubim P, Farias IP, da Silva MNF, Cohn-Haft M. (2010) New distribution limits of Bassaricyon alleni Thomas 1880 and insights on an overlooked species in the Western Brazilian Amazon. Mammalia 74: 323-327. doi: 10.1515/mamm.2010.008 [DOI] [Google Scholar]
- Samudio R, Pino JL, Helgen K. (2008) Bassaricyon pauli. In: IUCN 2012. IUCN Red List of Threatened Species. Version 2012.2. www.iucnredlist.org [accessed on 5 May 2013] doi: 10.1371/journal.pbio.1000056 [DOI]
- Santos JC, Coloma LA, Summers K, Caldwell JP, Ree R, Cannatella DC. (2009) Amazonian amphibian diversity is primarily derived from Late Miocene Andean lineages. PLoS Biology 7(3): e1000056. doi: 10.1371/journal.pbio.1000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento FO. (2002) Anthropogenic change in the landscapes of highland Ecuador. Geographical Review 92: 213-234. doi: 10.2307/4140971 [DOI] [Google Scholar]
- Sato JJ, Woslan M, Minami S, Hosoda T, Sinaga MH, Hiyama K, Yamaguchi Y, Suzuki H. (2009) Deciphering and dating the red panda’s ancestry and early adaptive radiation of Musteloidea. Molecular Phylogenetics and Evolution 53: 907-922. doi: 10.1016/j.ympev.2009.08.019 [DOI] [PubMed] [Google Scholar]
- Savage JM. (1966) The origins and history of the Central American herpetofauna. Copeia 1966: 719-766. doi: 10.2307/1441404 [DOI] [Google Scholar]
- Savage JM. (1982) The enigma of the Central American herpetofauna: dispersals or vicariance? Annals of the Missouri Botanical Garden 69: 464–597. doi: 10.2307/2399082 [DOI] [Google Scholar]
- Schipper J, et al. (2008) The status of the world’s land and marine mammals: diversity, threat, and knowledge. Science 322: 225-230. doi: 10.1126/science.1165115 [DOI] [PubMed] [Google Scholar]
- Schulenberg TS, Awbrey K. (1997) The Cordillera del Cóndor region of Ecuador and Peru: a biological assessment. Conservation International, Rapid Assessment Program Working Papers 7, Washington, D.C. [Google Scholar]
- Sedano RE, Burns KJ. (2010) Are the Northern Andes a species pump for Neotropical birds? Phylogenetics and biogeography of a clade of Neotropical tanagers (Aves: Thraupini). Journal of Biogeography 37: 325-343. doi: 10.1111/j.1365-2699.2009.02200.x [DOI] [Google Scholar]
- Segall W. (1943) The auditory region of the arctoid carnivores. Field Museum of Natural History, Zoological Series 29: 33-59. [Google Scholar]
- Slud P. (1964) The birds of Costa Rica: distribution and ecology. Bulletin of the American Museum of Natural History 128: 1-430. [Google Scholar]
- Solari S, Velazco PM, Patterson BD. (2012) Hierarchical organization of Neotropical mammal diversity and its historical basis. In: Patterson BD, Costa LP. (Eds) Bones, clones, and biomes: the history and geography of Recent Neotropical mammals. University of Chicago Press, Chicago, Illinois, 145-156. doi: 10.7208/chicago/9780226649214.003.0008 [DOI] [Google Scholar]
- Stains HJ. (1967) Carnivores and pinnipeds. In: Anderson S, Jones JK Jr. (Eds) Recent mammals of the world: a synopsis of families. Ronald Press Company, New York, 325-354. [Google Scholar]
- Stains HJ. (1973) Comparative study of the calcanea of members of the Ursidae and Procyonidae. Bulletin of the Southern California Academy of Sciences 72: 137-148. [Google Scholar]
- Stephens L, Traylor MA Jr. (1983) Ornithological gazetteer of Peru. Museum of Comparative Zoology, Cambridge, Massachusetts.
- Stephens L, Traylor MA Jr. (1985) Ornithological gazetteer of the Guianas. Museum of Comparative Zoology, Cambridge, Massachusetts.
- Stiles FG, Skutch AF. (1989) A guide to the birds of Costa Rica. Comstock/Cornell University Press, Ithaca, New York. [Google Scholar]
- Story HE. (1951) The carotid arteries in the Procyonidae. Fieldiana Zoology 32: 477-557. [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596-1599. doi: 10.1093/molbev/msm092 [DOI] [PubMed] [Google Scholar]
- Tamura K, Nei M. (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10: 512-526. [DOI] [PubMed] [Google Scholar]
- Tate GHH. (1939) The mammals of the Guiana region. Bulletin of the American Museum of Natural History 76: 151-229. [Google Scholar]
- Terborgh JW, Fitzpatrick JW, Emmons LH. (1984) Annotated checklist of the bird and mammal species of Cocha Cashu Biological Station, Manu National Park, Peru. Fieldiana Zoology (new series) 21: 1-29. [Google Scholar]
- Thomas O. (1880) On mammals from Ecuador. Proceedings of the Zoological Society of London 1880: 393-400. [Google Scholar]
- Thomas O. (1894) On a new African genus of Mustelidae. Annals and Museum of Natural History (series 6) 13: 522–524. [Google Scholar]
- Thomas O. (1909) Notes on some South American mammals, with descriptions of new species. Annals and Magazine of Natural History (series 8) 4: 230–242. [Google Scholar]
- Thomas O. (1920) Report on the Mammalia collected by Mr. Edmund Heller during the Peruvian Expedition of 1915 under the auspices of Yale University and the National Geographic Society. Proceedings of the United States National Museum 58: 217-249. doi: 10.5479/si.00963801.58-2333.217 [DOI] [Google Scholar]
- Thomas O. (1927) A new subspecies of Bassaricyon from Colombia. Annals and Magazine of Natural History (series 9) 20: 80. [Google Scholar]
- Timm RM, LaVal RK. (2000) Observations on Monteverde’s mammals. In: Nadkarni NM, Wheelwright NT. (Eds) Monteverde: ecology and conservation of a tropical cloud forest. Oxford University Press, New York, 235–236, 553-557. [Google Scholar]
- Timm RM, Wilson DE, Clauson BL, LaVal RK, Vaughan CS. (1989) Mammals of La Selva-Braulio Carrillo Complex, Costa Rica. North American Fauna 75: 1-162. doi: 10.3996/nafa.75.0001 [DOI] [Google Scholar]
- Tirira D. (2007) Guía de campo de los mamíferos del Ecuador. Ediciones Murciélago Blanco, Quito, Ecuador. [Google Scholar]
- Tirira D. (2008) Mamíferos de los bosques húmedos del noroccidente de Ecuador. Ediciones Murciélago Blanco, Quito, Ecuador. [Google Scholar]
- Tomes RF. (1860) Notes on a third collection of Mammalia made by Mr. Fraser in the Republic of Ecuador. Proceedings of the Zoological Society of London 28: 260-268. [Google Scholar]
- Upham NS, Patterson BD. (2012) Diversification and biogeography of the Neotropical caviomorph lineage Octodontoidea (Rodentia: Hystricognathi). Molecular Phylogenetics and Evolution 63: 417-429. [DOI] [PubMed] [Google Scholar]
- Velasco JA, Gutiérrez-Cárdenas PDA, Quintero-Angel A. (2010) A new species of Anolis of the aequatorialis group (Squamata: Iguania) from the central Andes of Colombia. Herpetological Journal 20: 231-236. [Google Scholar]
- Velazco PM, Patterson BD. (2013) Diversification of the Yellow-shouldered bats, genus Sturnira (Chiroptera: Phyllostomidae) in the New World tropics. Molecular Phylogenetics and Evolution 68: 683-698. doi: 10.1016/j.ympev.2013.04.016 [DOI] [PubMed] [Google Scholar]
- Voss RS. (1988) Systematics and ecology of ichthyomyine rodents (Muroidea): patterns of morphological evolution in a small adaptive radiation. Bulletin of the American Museum of Natural History 188: 259-493. [Google Scholar]
- Voss RS. (2003) A new species of Thomasomys (Rodentia: Muridae) from Eastern Ecuador, with remarks on mammalian diversity and biogeography in the Cordillera Oriental. American Museum Novitates 3421: 1-47. doi: [DOI] [Google Scholar]
- Voss RS. (2011) Revisionary notes on Neotropical porcupines (Rodentia: Erethizontidae) 3. An annotated checklist of the species of Coendou Lacépède, 1799. American Museum Novitates 3720: 1-36. doi: 10.1206/3720.2 [DOI] [Google Scholar]
- Voss RS, Gómez-Laverde M, Pacheco V. (2002) A new genus for Aepeomys fuscatus Allen, 1912, and Oryzomys intectus Thomas, 1921: enigmatic murid rodents from Andean cloud forests. American Museum Novitates 3373: 1-42. doi: [DOI] [Google Scholar]
- Voss RS, Hubbard C, Jansa SA. (2013) Phylogenetic relationships of New World porcupines (Rodentia, Erethizontidae): Implications for taxonomy, morphological evolution, and biogeography. American Museum Novitates 3769: 1-36. doi: 10.1206/3769.2 [DOI] [Google Scholar]
- Voss RS, Lunde DP, Simmons NB. (2001) Mammals of Paracou, French Guiana: a Neotropical lowland rainforest fauna. Part 2: nonvolant species. Bulletin of the American Museum of Natural History 263: 1-236. doi: [DOI] [Google Scholar]
- Vriesendorp C, Chávez Lr, Moskovits D, Shopland J. (Eds) (2004) Perú: Megantoni. Rapid Biological Inventories, Number 15, Field Museum of Natural History, Chicago, Illinois. [Google Scholar]
- Wainwright M. (2002) The natural history of Costa Rican mammals. Zona Tropical, San Jose, Costa Rica. [Google Scholar]
- Wainwright M. (2007) The mammals of Costa Rica, a natural history and field guide. Zona Tropical, San José, Costa Rica. [Google Scholar]
- Weir JT. (2006) Divergent timing and patterns of species accumulation in lowland and highland Neotropical birds. Evolution 60: 842-855. [PubMed] [Google Scholar]
- Weir JT, Bermingham E, Schluter D. (2009) The Great American Biotic Interchange in birds. Proceedings of the National Academy of Sciences 106: 21737-21742. doi: 10.1073/pnas.0903811106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir JT, Price M. (2011) Andean uplift promotes lowland speciation through vicariance and dispersal in Dendrocincla woodcreepers. Molecular Ecology 20: 4550-4563. doi: 10.1111/j.1365-294X.2011.05294.x [DOI] [PubMed] [Google Scholar]
- Weyl R. (1980) Geology of Central America. Gebrüder Borntraeger, Berlin. [Google Scholar]
- Wilson DE. (1983) Checklist of mammals. In: Janzen DH. (Ed) Costan Rican Natural History. University of Chicago Press, Chicago, Illinois, 443-447. [Google Scholar]
- Wisely SM, Maldonado JE, Fleischer RC. (2004) A technique for sampling ancient DNA that minimizes damage to museum specimens. Conservation Genetics 5: 105-107. doi: 10.1023/B:COGE.0000014061.04963.da [DOI] [Google Scholar]
- Wolsan M, Sato JJ. (2010) Effects of data incompleteness on the relative performance of parsimony and Bayesian approaches in a supermatrix phylogenetic reconstruction of Mustelidae and Procyonidae (Carnivora). Cladistics 26: 168-194. doi: 10.1111/j.1096-0031.2009.00281.x [DOI] [PubMed] [Google Scholar]
- Woodman N, Timm RM, Arana C R, Pacheco V, Schmidt CA, Hooper ED, Pacheco A C. (1991) Annotated checklist of the mammals of Cuzco Amazonico, Peru. Occasional Papers of the Museum of Natural History, University of Kansas, 145: 1-12. [Google Scholar]
- Wozencraft WC. (1989) Classification of the recent Carnivora. In: Gittleman J. (Ed) Carnivore behavior, ecology, and conservation. Cornell University Press, Ithaca, New York, 569-593. doi: 10.1007/978-1-4613-0855-3_19 [DOI] [Google Scholar]
- Wozencraft WC. (1993) Order Carnivora. In: Wilson DE, Reeder DM. (Eds) Mammal species of the world: a taxonomic and geographic reference, second edition. Smithsonian Institution Press, Washington, D.C., 279-348. [Google Scholar]
- Wozencraft WC. (2005) Order Carnivora. In: Wilson DE, Reeder DM. (Eds) Mammal species of the world: a taxonomic and geographic reference, third edition. Johns Hopkins University Press, Baltimore, Maryland, 532-628. [Google Scholar]
- Wurster DH, Benirschke K. (1967) Chromosome numbers in thirty species of carnivores. Mammalian Chromosome Newsletter 8: 195-196. [Google Scholar]
- Wurster DH, Benirschke K. (1968) Comparative cytogenetic studies in the order Carnivora. Chromosoma 24: 336-382. doi: 10.1007/BF00336201 [DOI] [PubMed] [Google Scholar]
- Wurster-Hill DH, Gray CW. (1975) The interrelationships of chromosome banding patterns in procyonids, viverrids, and felids. Cytogenetics and Cell Genetics 15: 306-331. doi: 10.1159/000130528 [DOI] [PubMed] [Google Scholar]
- Yonezawa T, Nikaido M, Kohno N, Fukumoto Y, Okada N, Hasegawa M. (2007) Molecular phylogenetic study on the origin and evolution of Mustelidae. Gene 396: 1-12. doi: 10.1016/j.gene.2006.12.040 [DOI] [PubMed] [Google Scholar]
- Young KR. (1994) Roads and the environmental degradation of tropical montane forests. Conservation Biology 8: 972-976. doi: 10.1046/j.1523-1739.1994.08040972.x [DOI] [Google Scholar]
- Young KR, Ulloa CU, Luteyn JL, Knapp S. (2002) Plant evolution and endemism in Andean South America: an introduction. Botanical Review 68: 4-21. doi: 10.1663/0006-8101(2002)068[0004:PEAEIA]2.0.CO;2 [DOI] [Google Scholar]
- Yu L, Peng D, Liu J, Luan P, Liang L, Lee H, Lee M, Ryder OA, Zhang Y. (2011) On the phylogeny of Mustelidae subfamilies: analysis of seventeen nuclear non-coding loci and mitochondrial complete genomes. BMC Evolutionary Biology 2011, 11: 92. doi: 10.1186/1471-2148-11-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeveloff S. (2002) Raccoons: a natural history. Smithsonian Institution Press, Washington, D.C. [Google Scholar]
- Zwickl DJ. (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Ph.D. dissertation, University of Texas, Austin, Texas. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.