Abstract
Bidirectional signals mediated by Eph receptor tyrosine kinases and their membrane-bound ligands, ephrins, play pivotal roles in the formation of neural networks by induction of both collapse and elongation of neurites. However, the downstream molecular modules to deliver these cues are largely unknown. We report here that the interaction of a Rac1-specific guanine nucleotide-exchanging factor, Tiam1, with ephrin-B1 and EphA2 mediates neurite outgrowth. In cells coexpressing Tiam1 and ephrin-B1, Rac1 is activated by the extracellular stimulation of clustered soluble EphB2 receptors. Similarly, soluble ephrin-A1 activates Rac1 in cells coexpressing Tiam1 and EphA2. Cortical neurons from the E14 mouse embryos and neuroblastoma cells significantly extend neurites when placed on surfaces coated with the extracellular domain of EphB2 or ephrin-A1, which were abolished by the forced expression of the dominant-negative mutant of ephrin-B1 or EphA2. Furthermore, the introduction of a dominant-negative form of Tiam1 also inhibits neurite outgrowth induced by the ephrin-B1 and EphA2 signals. These results indicate that Tiam1 is required for neurite outgrowth induced by both ephrin-B1-mediated reverse signaling and EphA2-mediated forward signaling.
Keywords: cortical neuron, Eph, ephrin, neurite outgrowth, Tiam1
Introduction
The members of the Eph receptors and their ligands are variously involved in neural development: regulating axon guidance, axon fasciculation and synaptogenesis. The interaction of Eph and ephrin regulates axon guidance by a repulsive function. Retinal axons expressing EphA receptors are guided to their target tectal area according to interactions with a repellent ephrin-A gradient (Cheng et al, 1995). The pathfinding of mouse anterior commissure is also regulated by the repulsive function between ephrin-B1 and EphB2 receptor (Henkemeyer et al, 1996). On the other hand, the interaction of Eph and ephrin also induces attractive axon guidance in certain settings. Vomeronasal axons expressing ephrin-A5 are attractively elongated by interaction with target EphA receptors in the accessory olfactory bulb, implying that ephrin-A mediates attractive guidance mechanisms (Knoll et al, 2001). Another example of attractive axon guidance is also reported in terms of the interaction of the EphB receptor and ephrin-B. Mann et al (2002) show that Xenopus dorsal retinal axons expressing ephrin-B preferentially project to the tectal area where EphB1 is highly expressed, while the ventral ones that express EphB2 project to the dorsal area of the tectum where ephrin-Bs are highly expressed. Therefore, Eph receptors and ephrins are involved in both repulsive and attractive guidance mechanisms during the establishment of neuronal connections. Gao et al have previously shown the two opposing effects of Eph receptors and ephrins on neurite outgrowth in vitro by a series of experiments. Primary cultured rat neurons extended or retracted neurites when they were plated on cells stably expressing various Eph or ephrins on their surface (Gao et al, 1996, 1998, 1999, 2000). However, the molecular basis of such morphological change requires investigation.
Rho GTPases are important regulators of the actin cytoskeleton. Activation of RhoA and its effector protein Rho-kinase (ROCK) leads to growth cone collapse, neurite retraction or neurite growth inhibition by inducing the contraction of actomyosin (Wahl et al, 2000). On the other hand, activation of Rac1 induces neurite elongation. Tiam1, a specific guanine-nucleotide exchange factor (GEF) for Rac1 (Habets et al, 1994), affects neuronal morphology (Leeuwen et al, 1997; Kunda et al, 2001). Moreover, STEF, another GEF for Rac1, which has highly homologous regions with Tiam1, is also effective in neurite outgrowth (Matsuo et al, 2002). The cellular localization of Tiam1 and EphA2 is similar. When cells were cultured sparsely, both EphA2 and activated Tiam1 are highly expressed at the cell periphery containing membrane ruffles. However, when cells were adhered to each other, they are highly expressed at the site of cell-to-cell adhesion (Sander et al, 1998; Zantek et al, 1999). These observations led us to focus on the examination whether Tiam1 and EphA2 interact, and Tiam1 could be a mediator of EphA2 receptor. During the examination of the interaction of Tiam1 with several Eph receptors and ephrins, we have found that ephrin-B1 also associates with Tiam1.
In this study, we describe the interaction of Tiam1 with ephrin-B1 and EphA2. A part of Tiam1 was accumulated to the sites including clustered ephrin-B1 and EphA2 after the cells were stimulated with EphB2 and ephrin-A1, respectively. Neurite outgrowth was observed in primary cortical neurons from mouse embryos in response to the stimulation of EphB2-Fc, and NB1 neuroblastoma cells in response to ephrin-a1-Fc. Coexpression of the dominant-negative mutant of Tiam1, or mutant of ephrin-B1 or EphA2, which lacks its cytoplasmic region prevented the neurite extension described above. These results suggest that Tiam1 is a mediator of ephrin-B1- and EphA2-induced neurite outgrowth.
Results
Tiam1 interacts with the cytoplasmic domain of ephrin-B1 and EphA2
We have examined the association between Tiam1 with ephrin-B1 and EphA2 in vivo. Coexpression and co-precipitation analysis in COS1 cells revealed that Tiam1 was co-precipitated with ephrin-B1 and EphA2 by specific antibodies (Figure 1A, lanes 1 and 3, arrowheads, respectively), but not by the normal goat serum or mouse IgG1 (Figure 1A, lanes 2 and 4, arrowheads, respectively). These results were further confirmed by experiments using the antibodies in reverse order. Ephrin-B1 and EphA2 were co-precipitated with Tiam1 (Figure 1A, lanes 5 and 7, arrowheads, respectively). Next, we have generated several truncated mutants of Tiam1 to determine the region within Tiam1, which is required for the interaction with ephrin-B1 or EphA2 (Figure 1B). Among the truncated mutants of Tiam1, N-terminal-deleted Tiam1 (C1199) tightly bound to ephrin-B1, but Tiam1 encoding 392 amino-terminal amino acids (N392) did not associate with ephrin-B1 (Figure 1C, lanes 1–4). Reciprocally, EphA2 interacted with Tiam1 (N392), but did not associate with Tiam1 (C1199) (Figure 1C, lanes 5–8). These results indicate that ephrin-B1 and EphA2 interact with different regions of Tiam1.
Figure 1.
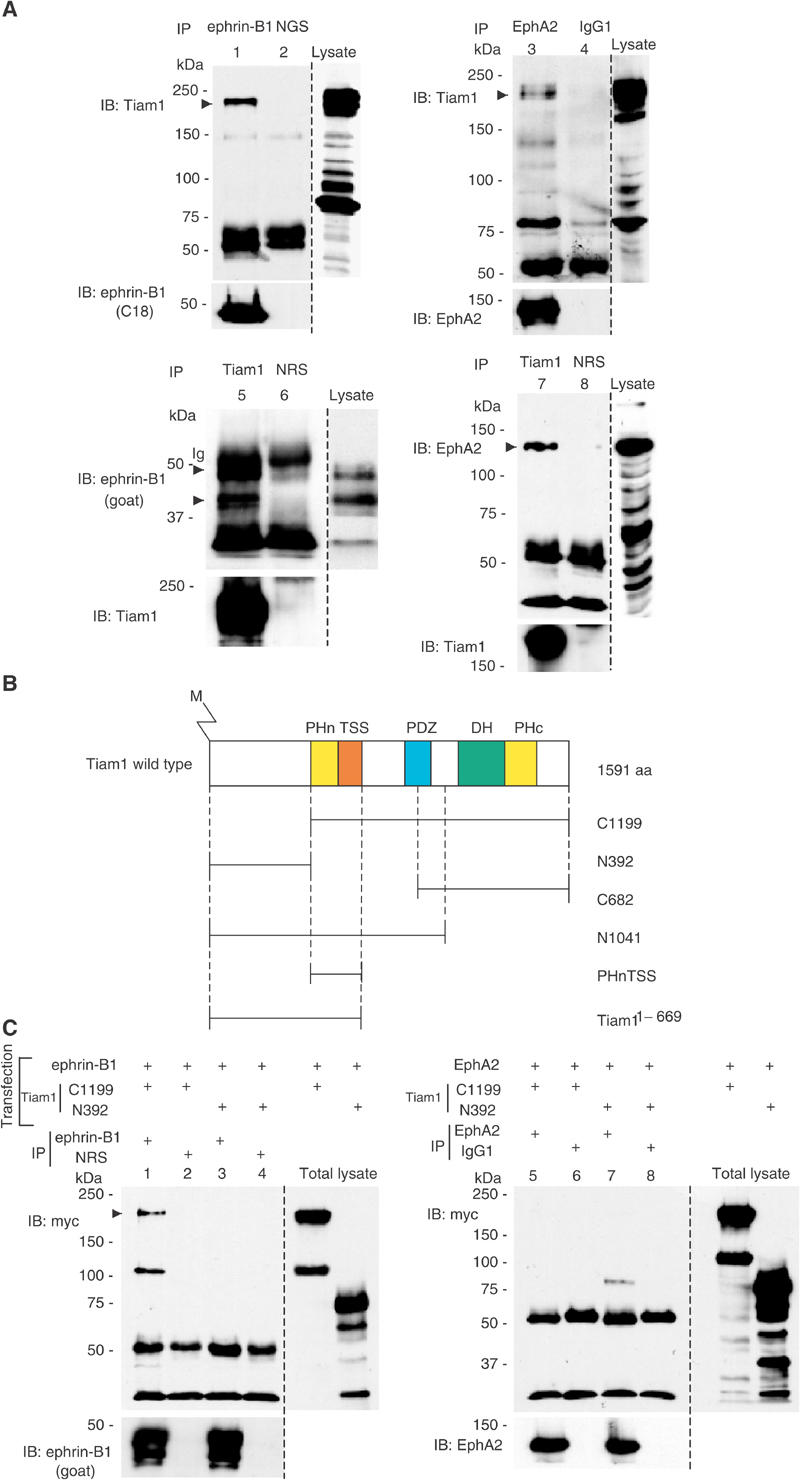
Tiam1 forms a complex with ephrin-B1 and EphA2 receptor. (A) COS1 cells were transiently transfected with a plasmid encoding wild-type Tiam1 together with that encoding ephrin-B1 (lanes 1, 2, 5, 6) or EphA2 (lanes 3, 4, 7, 8). Cells were lysed and immunoprecipitated (IP) with anti-ephrin-B1 (goat polyclonal, lane 1), normal goat serum (NGS, lane 2), anti-EphA2 (lane 3), mouse IgG1 (lane 4), anti-Tiam1 (lanes 5, 7) or normal rabbit serum (NRS, lanes 6, 8). The precipitates were subjected to immunoblotting (IB) with the indicated antibodies. The same membranes were reblotted with the antibodies indicated (bottom panels). The expression of Tiam1, ephrin-B1 and EphA2 in the cell lysate was confirmed by immunoblotting. When wild-type ephrin-B1 is overexpressed in COS1 cells, at least two major bands were observed by anti-ephrin-B1 goat polyclonal antibody. Ig, immunoglobulin. The prominent band of 80 kDa in lane 3 is an unknown protein, which associates with EphA2 and may have a related structure with Tiam1. (B) Schematic representation of a wild-type and the truncated Tiam1 cDNA constructs used in this study. Proteins are depicted to scale; M, myristoylation signal; PHn and PHc, NH2- and COOH-terminal pleckstrin homology domains; TSS, otherwise known as coiled-coil region and an additional adjacent region (CC-Ex); PDZ, PSD-95/DlgA/ZO-1 domain; DH, Dbl homology domain. (C) COS1 cells were transiently transfected with the plasmids as indicated in the above lanes. The cell lysates were immunoprecipitated with anti-ephrin-B1 C18 (lanes 1, 3), EphA2 (lanes 5, 7), normal rabbit serum (lanes 2, 4) or mouse IgG1 (lanes 6, 8) and immunoblotted with anti-myc antibody. The same membranes were reblotted with anti-ephrin-B1 (goat) or anti-EphA2 as indicated. The expression of myc-tagged Tiam1 constructs in these cell lysates (total lysate) was confirmed by immunoblotting. The additional band at 110 kDa in lane 1 may be an artificially produced fragment of overexpressed Tiam1 construct.
To identify the region of the Tiam1 protein essential for the interaction with ephrin-B1 or EphA2, we performed an in vitro glutathione S-transferase (GST) fusion protein pull-down assay. In vitro-translated Tiam1 was co-precipitated with the GST-tagged cytoplasmic region of ephrin-B1 (ephrin-B1264−346) or EphA2 (EphA2563−977) but not by the control GST alone (Figure 2, top). The cytoplasmic region of ephrin-B1 did not bind to Tiam1 (N392). As shown in Figure 2 (bottom), ephrin-B1 clearly associated with the PHnTSS region of Tiam1, which is also included in C1199 and N1041. Therefore, we concluded that the cytoplasmic region of ephrin-B1 binds to Tiam1 via its PHnTSS region. The PHnTSS region contains amino-terminal PH domain, which is known to involve in membrane targeting of Tiam1 protein, and TSS domain, which is conserved among Tiam1, STEF and SIF proteins (Matsuo et al, 2002). On the other hand, the GST-tagged cytoplasmic region of EphA2 did not associate with the PHnTSS region of Tiam1, but instead associated with Tiam1 constructs harboring the amino-terminal region of Tiam1 (N392). We assume that EphA2 binds to Tiam1 via its amino-terminal region (Tiam11–392). Although the association of Tiam1 with ephrin-B1 was detected without activation of ephrin-B1 by the extracellular domain (ECD) of EphB2, we found that the GST-tagged PHnTSS domain of Tiam1 expressed in 293T cells was co-precipitated with ephrin-B1 more effectively after the incubation with EphB2-Fc (Supplementary information 3A).
Figure 2.
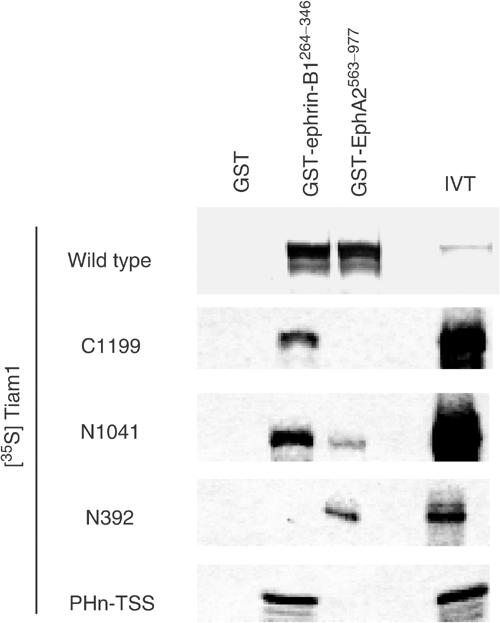
Tiam1 binds to ephrin-B1 and EphA2 in vitro: [35S]methionine -labeled wild type or truncated mutants of Tiam1 (C1199, N1041, N392, PHnTSS) translated products were incubated with glutathione–agarose-conjugated GST, GST-ephrin-B1264−346 and GST-EphA2563−977, respectively. IVT, the input of in vitro translation reaction before the beads binding.
Finally, we examined the in vivo status of Tiam1 with ephrin-B1/EphA2 in the E14 mouse brain, where Tiam1 and ephrin-B1 are highly expressed. Tiam1 was co-precipitated with ephrin-B1 from an extract of E14 mouse whole brain by the specific antibody, but not by normal goat serum (Figure 3A). Although EphA2 in the whole brain of E14 mouse is expressed at a low level, endogenously expressed Tiam1 protein was co-immunoprecipitated with EphA2 but not with the control mouse IgG1 (Figure 3B). The interaction of ephrin-B1 and EphA2 with Tiam1 in the mouse brain was further confirmed by experiments using the antibodies in reverse order. Ephrin-B1 and EphA2 were co-precipitated with Tiam1 by the specific antibodies, but not by normal rabbit serum (Figure 3C and D). The size of endogenous ephrin-B1 protein in the mouse brain was slightly larger than transiently expressed ephrin-B1 in COS1 cells.
Figure 3.
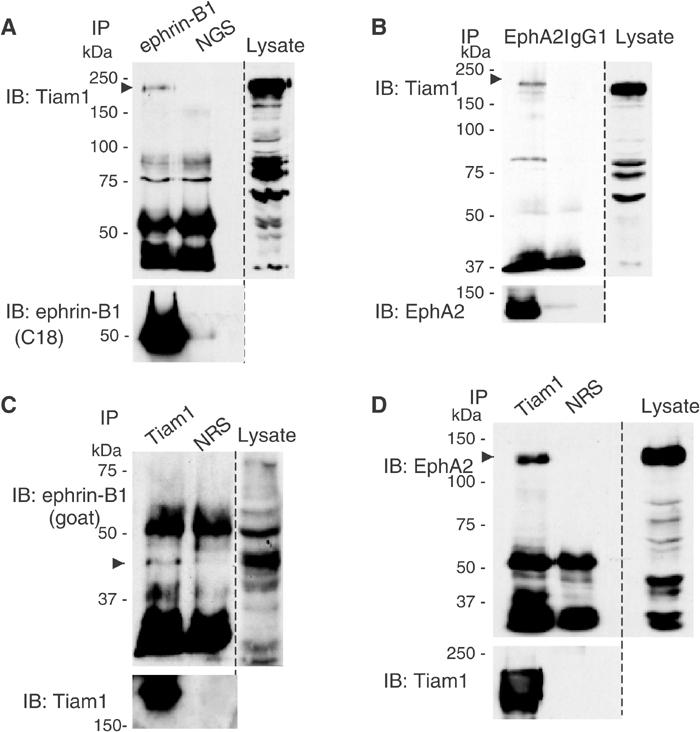
Tiam1 physiologically interacts with ephrin-B1 and EphA2 in the brain of an E14 mouse embryo. Whole brains from E14 mice embryos were lysed and immunoprecipitated (IP) with anti-ephrin-B1 (goat), anti-EphA2, anti-Tiam1, normal goat serum (NGS), normal rabbit serum (NRS) or mouse IgG1 respectively as indicated in the above lanes. The precipitates were subjected to immunoblotting (IB) with the indicated antibodies. Co-precipitated Tiam1, ephrin-B1 and EphA2 are indicated by arrowheads. In (C), the upper band of ephrin-B1 was overlapped with the band of immunoglobulin. The membranes were reblotted with antibodies indicated (bottom panels).
Tiam1 is translocated after stimulation with the extracellular domain of EphB2 or ephrin-A1
Next, we examined the cellular localization of Tiam1, ephrin-B1 and EphA2. Ephrin-B reverse signaling is known to induce the formation of large membrane patches containing several proteins after stimulation with preclustered EphB2-Fc (Cowan and Henkemeyer, 2002; Palmer et al, 2002). In SK-N-MC cells, intense staining of endogenous ephrin-B1 protein is observed in the cell membrane at the contact of cell-to-cell adhesion (Figure 4A, column c, top). Concomitantly with the stimulation of EphB2-Fc, patches containing ephrin-B1 were formed mostly in the cell membrane (Figure 4A, column a, top panel). As expected, the colocalization of ephrin-B1 in patches containing EphB2-Fc was also confirmed in SK-N-MC cells (Supplementary information 2, column a). Tiam1 protein is homogenously expressed in the cytoplasm before stimulation with EphB2-Fc (Figure 4A, column c, middle). However, at least a part of Tiam1 was clearly found to colocalize to the patches after exposure to preclustered EphB2-Fc (Figure 4A, column a, middle and bottom). Although there were a few spotty stains of both ephrin-B1 and Tiam1 in the cells without stimulation of EphB2-Fc, they never colocalized as shown in the merged panel (Figure 4A, column c). In order to exclude the possibility that the second antibodies used directly recognize the clustered Fc fusion proteins, we have shown that there is almost no staining of Tiam1 protein by staining with the secondary antibody without prior incubation with the primary anti-Tiam1 antibody (Figure 4A, column b). We also confirmed that the preincubation of anti-Tiam1 antibody with the blocking peptide (Tiam1 C16) or the bacterially produced GST fusion protein of Tiam11430−1591 containing its C-terminal region abolished Tiam1 staining (data not shown). In the primary cortical neurons from E14 mouse embryos, ephrin-B1 is highly expressed on the cell membrane and neurites, and Tiam1 protein is distributed in the cytoplasm of the cell body and neurites (Figure 4B, columns c and d). Tiam1 and ephrin-B1 were also copatched in E14 cortical neurons 1 h after stimulation with clustered EphB2-Fc (Figure 4B, columns a and b). We observe many patches containing Tiam1 and ephrin-B1 on the elongated neurites and the periphery of cell bodies of these neurons, while there was no such patchy localization of Tiam1 in the neurons without stimulation with EphB2-Fc. In Supplementary information 2, we show the precise colocalization of ephrin-B1 with EphB2-Fc patches, and that in the control there was no crossreaction of the rhodamine-labeled secondary antibody to clustered Fc fusion protein in primary cortical neurons (columns b and c, respectively).
Figure 4a.
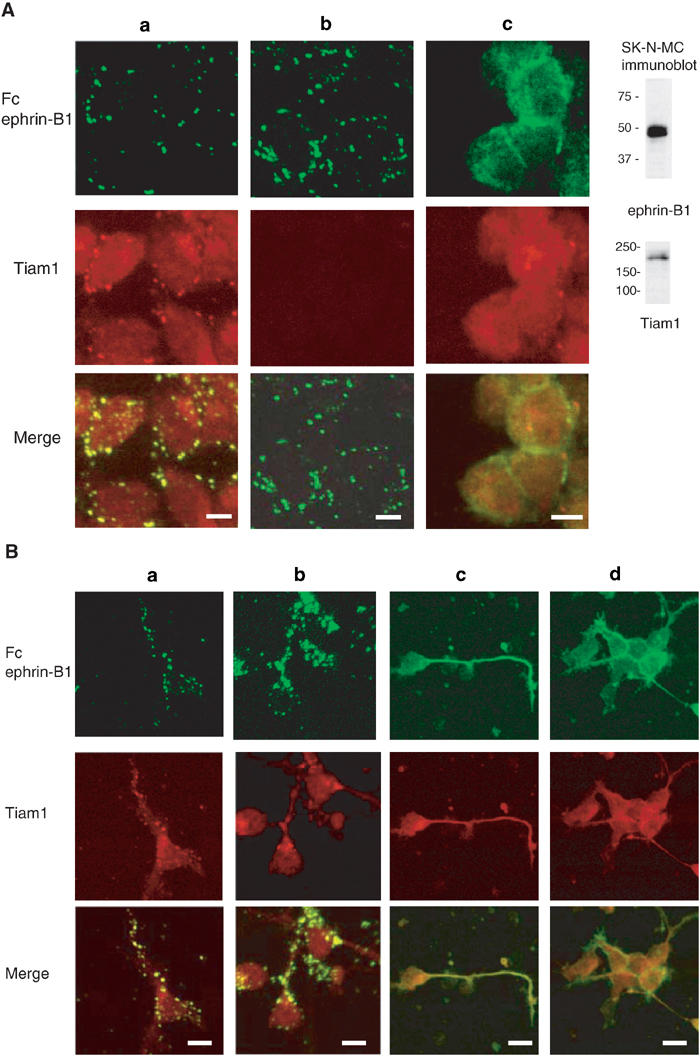
Tiam1 is recruited to patches induced by EphB2-Fc- or ephrin-A1-Fc stimulation. (A) SK-N-MC cells expressing ephrin-B1 were stimulated with 4 μg/ml of clustered EphB2-Fc (columns a, b) or left untreated (column c). After 10 min of stimulation, the cells were washed and incubated in a medium without EphB2-Fc for 1 h prior to fixation. The cells were immunostained with anti-Fc (green) and anti-Tiam1 (red) antibodies (column a). In column b, incubation with primary anti-Tiam1 antibody was omitted prior to incubation with the rhodamine-labeled secondary antibody. Column c shows the signals with anti-ephrin-B1 staining (green) and anti-Tiam1 staining (red). Expressions of Tiam1 and ephrin-B1 in SK-N-MC cells are shown by immunoblot. (B) (Columns a, b) E14 mouse cortical neurons were cultured for 20 h on poly-L-lysine-coated slides, and then stimulated with EphB2-Fc for 1 h as described above. The cells were fixed and immunostained with anti-Fc (green) and anti-Tiam1 (red) antibodies. (Columns c, d) Localization of ephrin-B1 (green) and Tiam1 protein (red) without stimulation of EphB2-Fc is shown.
NB1 neuroblastoma cells express EphA2 and Tiam1 endogenously as described later. Before stimulation of NB1 cells with ephrin-A1-Fc, there was no patchy localization of EphA2 detected by immunostaining. Intense staining of EphA2 was located on the cell membrane, especially at the sites of cell-to-cell contact (Figure 4C, column c, top). In the same manner as is the case with cortical neurons, Tiam1, initially distributed homogenously in the cytoplasm (Figure 4C, column c, middle), was partly translocated to patches containing EphA2 after stimulation with soluble ephrin-A1 (Figure 4C, column a, middle and bottom). The patches containing EphA2 localized not only on the cell membrane but also in the cytoplasm, which may be a consequence of the internalization of EphA2 from the cell membrane after stimulation of its ligand. Such internalization of ligand-stimulated EphA2, and more recently ephrin-B1, has been reported (Walker-Daniels et al., 2002; Zimmer et al., 2003). There was no crossreaction of the secondary antibody to the clustered ephrin-A1-Fc protein (Figure 4C, column b). These results together demonstrate the colocalization of Tiam1 with ephrin-B1 and EphA2, and the distribution of Tiam1 is at least partly translocated after stimulation of ephrin-B1 and EphA2.
Figure 4c.
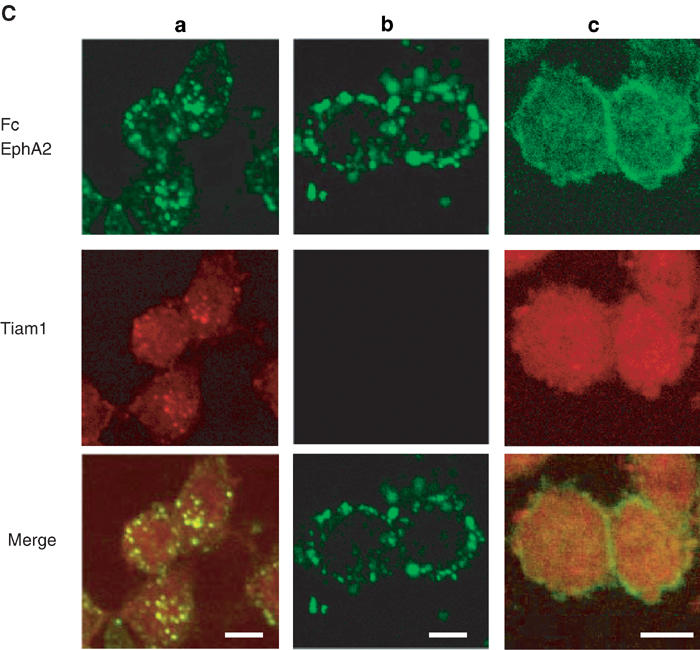
(C) (Columns a, b) NB1 neuroblastoma cells were stimulated with 4 μg/ml of clustered ephrin-A1-Fc as described in (A). The cells were immunostained with anti-Fc (green) and anti-Tiam1 (red) antibodies. In column b, incubation with primary anti-Tiam1 antibody was omitted prior to incubation with the rhodamine-labeled secondary antibody. (Column c) Localization of EphA2 (green) or Tiam1 protein (red) without stimulation of ephrin-A1-Fc is shown. Scale bar, 10 μm.
Tiam1 is involved in Rac1 activation induced by Eph/ephrin signaling
To analyze whether Eph/ephrin signaling mediates Tiam1 activation, we examined the modification of Tiam1-induced Rac1 activation by the affinity precipitation of GTP-bound Rac1 with the GST-tagged p21-binding domain of PAK1 (GST-PBD). Rac1 was slightly activated by the overexpression of wild-type Tiam1 (Figure 5A, compare lanes 1 and 2). Although Rac1 was also slightly activated after stimulation with EphB2-Fc in ephrin-B1-expressing cells (Figure 5A, lane 3), the amount of GTP-bound Rac1 was markedly increased in response to the stimulation by EphB2-Fc when both Tiam1 and ephrin-B1 are expressed (Figure 5A, compare lanes 4 and 5). Because the PHnTSS region of Tiam1 works as a dominant-negative mutant of Tiam1 (Stam et al, 1997), we next examined whether PHnTSS of Tiam1 blocks the Rac1 activity induced by the activation of ephrin-B1. The activation of Rac1 induced by the stimulation of EphB2-Fc was completely abolished by the coexpression of PHnTSS of Tiam1, but not by the coexpression of Tiam1 (N392) (Figure 5A, lanes 8 and 9). The coexpression of EphA2 and Tiam1 did not induce Rac1 activation significantly without stimulation of ephrin-A1-Fc (Figure 5A, lane 10). The overexpression of EphA2 alone is not effective in activating Rac1 even though the cells were stimulated by ephrin-A1 (Figure 5A, lane 15). The stimulation of ephrin-A1-Fc activated Rac1 in cells expressing EphA2 and Tiam1, which was blocked by the coexpression of Tiam11–669 (Figure 5A, compare lanes 11 and 12). The coexpression of PHnTSS partly inhibited the ephrin-A1-Fc-induced Rac1 activation, and Tiam11–392 blocked the Rac1 activation very effectively but not completely (Figure 5A, lanes 13 and 14).
Figure 5.
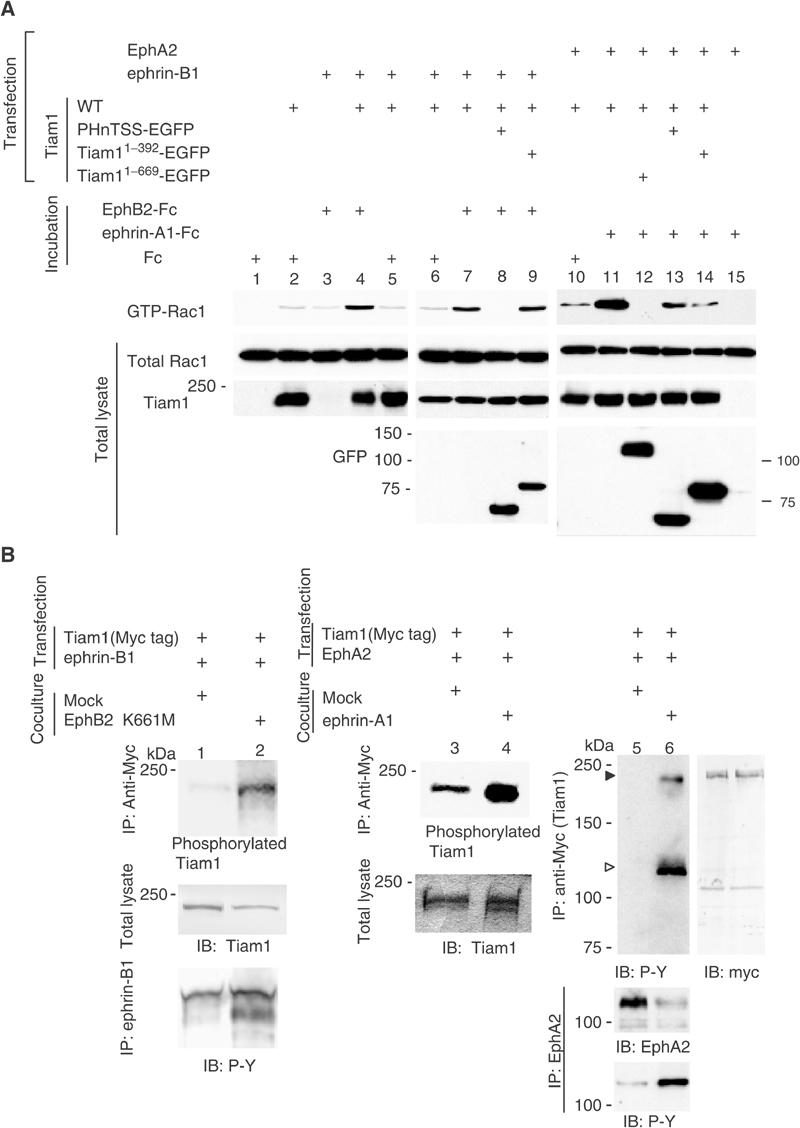
Tiam1 mediates Eph/ephrin-mediated signaling cascades leading to Rac1 activation. (A) COS1 cells were transiently transfected with 2 μg of a plasmid encoding wild-type Rac1, together with plasmids indicated in the above lanes. Mock plasmid was used to adjust the amount of DNA to total 10 μg for each transfection. The transfected cells were incubated in media containing clustered EphB2-Fc, ephrin-A1-Fc or control Fc each at a concentration of 5 μg/ml as indicated above the lanes for 15 min before harvesting the cell lysates for affinity precipitation with immobilized GST-PBD. Precipitated GTP-bound Rac1 was detected by immunoblotting with anti-Rac1 antibody. Expression of total Rac1 and Tiam1 is shown at the bottom. (B) Phosphorylation of Tiam1 induced by the activation of ephrin-B1 or EphA2 in vivo. 293T cells transfected with the plasmids indicated at the top were cocultured with the 293T cells (lane 1), the 293T cells stably expressing EphB2 K661M (lane 2), the NIH3T3 cells (lanes 3, 5) or the NIH3T3 cells stably expressing ephrin-A1 (lanes 4, 6). Lanes 1–4: The coculture was performed in media containing 32Pi for 4 h, and then the cells were lysed for immunoprecipitation with anti-myc, separated by SDS–PAGE and visualized by autoradiography. Lanes 1, 2 bottom: The lysates for immunoprecipitation were prepared from the cells as described above except for the labeling with 32Pi. Lanes 5, 6: The coculture was performed for 30 min before cell lysates were immunoprecipitated with anti-myc and immunoblotted with anti-phospho-tyrosine antibody (4G10). The phosphorylation of Tiam1 is indicated by filled triangle. Co-precipitated EphA2 is indicated by open triangle. The same membrane was reblotted with anti-myc antibody (right panel). The phosphorylation of ephrin-B1 and EphA2 on tyrosine residues is shown at the bottom of lanes 1, 2 and lanes 5, 6, respectively.
We next examined the phosphorylation of Tiam1 by the activation of ephrin-B1 or EphA2. When ephrin-B1-expressing cells were cocultured with cells expressing kinase-inactivated EphB2 (EphB2K661M), ephrin-B1 was highly phosphorylated on tyrosine residues, but not when cocultured with control mock-transfected cells as we have described before (Figure 5B, lanes 1 and 2, bottom) (Tanaka et al, 2003). Similarly, the tyrosine phosphorylation of EphA2 was increased when EphA2-expressing cells were cocultured with cells expressing ephrin-A1 (Figure 5B, lanes 5 and 6, bottom). The activation of ephrin-B1 by overlaying the EphB2-expressing cells leads to weak phosphorylation of Tiam1 as detected by labeling cells with orthophosphate (Figure 5B, lanes 1 and 2). Tiam1 is also phosphorylated upon stimulation of EphA2 by coculturing with cells expressing ephrin-A1 (Figure 5B, lanes 3 and 4). Moreover, Tiam1 is phosphorylated on tyrosine residues by the stimulation of EphA2-mediated signaling, as detected by immunoblotting with anti-phosphotyrosine antibody (Figure 5B, lanes 5 and 6). On the other hand, we did not clearly detect phosphorylation of Tiam1 in response to activation of ephrin-B1 on either tyrosine residues (examined by the same method as above) or on serine/threonine residues by immunoblotting with the antibodies described in Supplementary information 1 (data not shown).
Neurite outgrowth by ephrin-B1–Tiam1 and EphA2–Tiam1 interaction
We first confirmed that the dissociated primary cultured cells of E14 cortical neurons still maintain the expression of Tiam1, ephrin-B1, EphB2 and EphA4 in vitro, although the expression levels of these proteins were higher in the cortical tissue compared to dissociated cortical cells (Figure 6E). When E14 cortical neurons were seeded on preclustered EphB2-Fc-coated plates, ephrin-B1 is phosphorylated on tyrosine residues at 30 min and 4 h after plating (Figure 6E, bottom). Around 31% of the cells seeded on EphB2-Fc-coated plates show neurites longer than one cell body in length (Figure 6A, Table I), which is statistically different from the numbers when plated on the clustered Fc only or on the albumin (Figure 6B and C). The effect of ephrin-A1-Fc on neurite outgrowth of E14 mouse cortical neurons was weaker than the stimulation of EphB2-Fc (Figure 6D, Table I). In particular, the number of cells bearing longer neurites (longer than three cell bodies in length) was much smaller when cells were seeded on ephrin-A1-Fc-coated plates than on EphB2-Fc-coated plates (Table I). EphA2 expression in the cortex of E14-16 was monitored in brain lysates, because we suspect that the weak expression in this area and the developmental stage may be one of the reasons for the less dramatic effect on neurite outgrowth. Actually, the expression of EphA2 was barely detectable in the cortex region of this stage (Figure 6E), while it was detectable in the whole brain lysate (Figure 3). We did not observe any elongated neurites on ephrin-B1-Fc-coated plates (data not shown).
Figure 6.
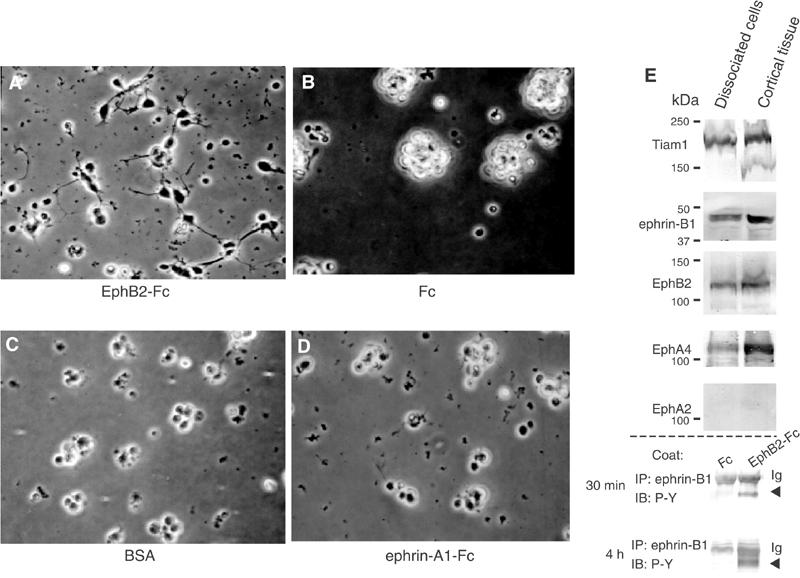
EphB2-Fc induces neurite outgrowth of E14 mouse cortical neurons. Primary cultured cortical neurons from E14 mouse cerebral cortex were seeded on plates coated with EphB2-Fc (A), Fc fragment (B), bovine serum albumin (BSA) (C) or ephrin-A1-Fc (D), as described in Materials and methods (Neurite elongation assay). Representative pictures are shown after incubation for 20 h (A–D). (E) Expression of Tiam1, ephrin-B1 and various Eph receptors in dissociated primary cultured cells after 20 h incubation on poly-L-lysine-coated dishes, or in the cortical tissues was monitored by immunoblotting. (E, bottom) E14 primary precursors plated on an EphB2-Fc-coated dish were lysed for the indicated period, and immunoprecipitated with anti-ephrin-B1. Tyrosine phosphorylation of ephrin-B1 is shown by immunoblotting.
Table 1.
Quantification of the effects of EphB2-Fc and ephrin-A1-Fc on neurite formation
| Three cell bodies/total counteda | One cell body/total counteda | |
|---|---|---|
| E14 primary cortical neuronb | ||
| Fc | 0/527 (0%) | 0/527 (0%) |
| BSA | 0/531 (0%) | 31/531 (5.8%) |
| EphB2-Fc | 61/552 (11.1%) | 173/552 (31.3%) |
| ephrin-A1-Fc | 10/523 (1.95) | 99/523 (18.9%) |
| NB1 neuroblastoma cell linec | ||
| Fc | 0/323 (0%) | |
| ephrin-A1-Fc | 115/349 (33.0%) | |
| EphA2-Fc |
|
14/304 (4.6%) |
| aThe number of cells possessing neuritis three cell bodies or one cell body was counted 20 h after plating the cells. | ||
| bTotal number of cells counted in four independent experiments. | ||
| cTotal number of cells counted in three independent experiments. | ||
In the neuroblastoma cell line NB1, a considerable expression of Tiam1 and EphA2 was detected (Figure 7D). We also confirmed that NB1 cells do not express detectable level of EphA4 (Figure 7D). We used this NB1 system in the following experiments for evaluation of EphA2 forward signaling. The tyrosine phosphorylation of EphA2 was clearly demonstrated at 30 min and 4 h after plating on ephrin-A1-Fc-coated dish (Figure 7D, bottom). The decreased expression level of EphA2 after stimulation is considered to be a result of negative regulation of activated EphA2 by Cbl-mediated ubiquitination (Wang et al, 2002). When NB1 cells were plated on a surface coated with preclustered ephrin-A1-Fc, many cells exhibited long thin neurites after 16 h of incubation (Figure 7B), whereas few cells bearing such neurites were observed in cells plated on the control Fc-coated plates (Figure 7A). The elongated neurites were not observed on preclustered EphA2-Fc-coated plate, either, as shown in Figure 7C.
Figure 7.
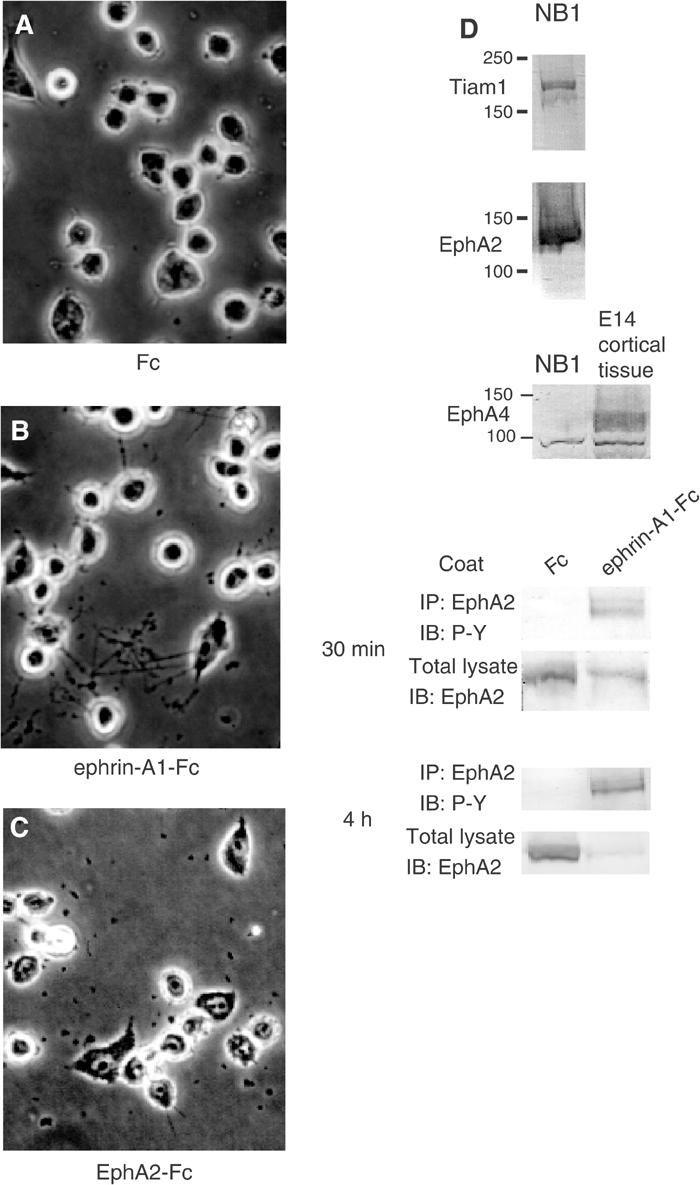
Ephrin-A1-Fc induces neurite outgrowth of NB1 neuroblastoma cells. NB1 neuroblastoma cells were seeded on plates coated with Fc fragment (A), ephrin-A1-Fc (B) or EphA2-Fc (C) as described in Materials and methods. Representative cells are shown after incubation for 20 h (A–C). (D) Expression of Tiam1 and Eph receptors in NB1 cells is shown by immunoblotting. (D, bottom) The NB1 cells plated on ephrin-A1-Fc-coated dishes were lysed for the indicated period, and immunoprecipitated with anti-EphA2. Tyrosine phosphorylation of EphA2 is shown by immunoblotting.
To confirm that the outgrowth of neurites observed above depends on the interaction of EphB2 or ephrin-A1 with its cognate ligand or receptor, we did several experiments interfering with this pathway by a soluble ligand or receptor and a dominant-negative or defective competitor in the following experiments. When the cells were plated on Fc fusion protein-coated surface as above with an excess volume of the soluble ephrin-B1-Fc or EphA2-Fc in the medium, the neurite outgrowth of E14 cortical neurons plated on the EphB2-Fc surface and neurite outgrowth of NB1 cells plated on the ephrin-A1-Fc surface were blocked, respectively. However, the addition of soluble ephrin-B1 or EphA2 in the medium had no effect on the nonspecific neurite outgrowth of E14 cortical neurons and NB1 cells induced by plating cells on a poly-L-lysine-coated surface (data not shown). Furthermore, the forced expression of an ephrin-B1 mutant lacking its cytoplasmic region (ephrin-B11−265) in E14 mouse cortical neurons lessened the effect both in numbers and length of neurites, while the expression of control EGFP did not affect the neurite outgrowth (Figure 8, compare A and B). Likewise, the expression of mutants of EphA2 lacking its cytoplasmic region (EphA21−540) in neuroblastoma cells reduced the number of these cells bearing neurites on an ephrin-A1-coated surface (Figure 8, compare E and F).
Figure 8.
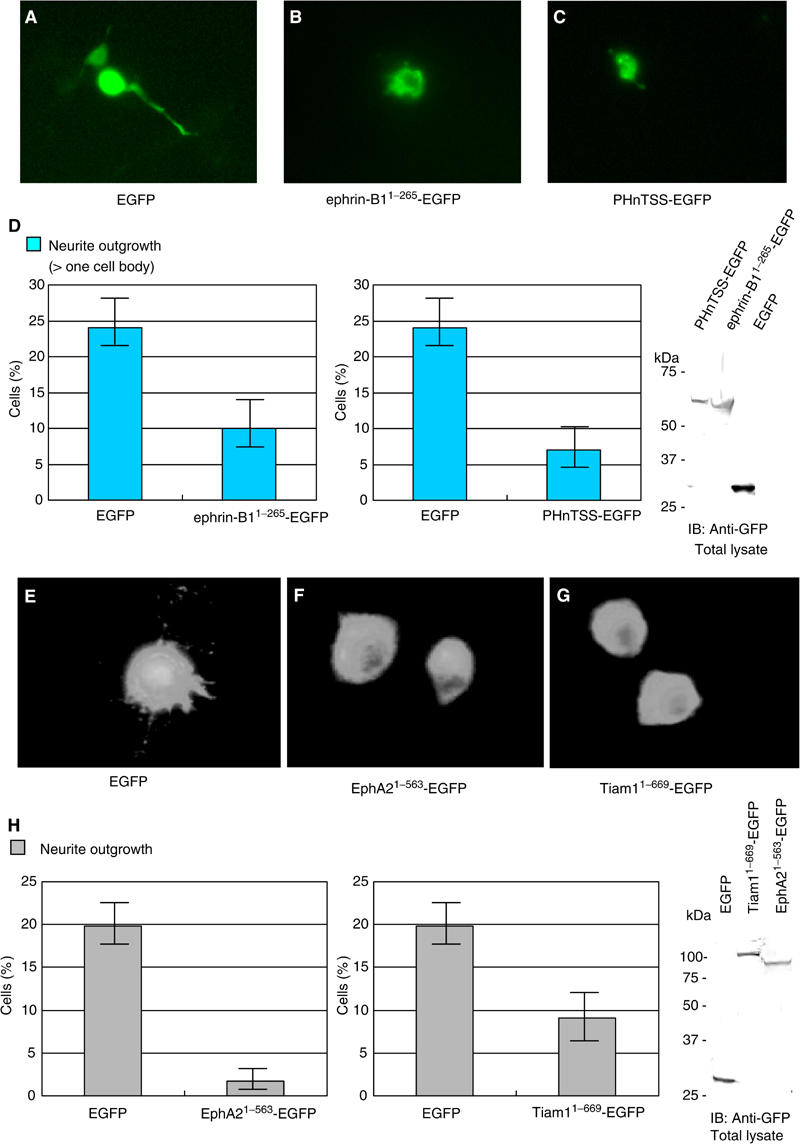
Expression of dominant-negative mutants of ephrin-B1, EphA2 and Tiam1 affects EphB2-Fc- or ephrin-A1-Fc-induced neurite outgrowth. Primary cultured cortical neurons from E14 mouse embryos (A–D), or NB1 cells (E–H) were transfected with plasmids encoding either EGFP, EGFP-tagged mutants of ephrin-B1 or EphA2, which lack the cytoplasmic regions (ephrin-B11−265-EGFP, EphA21−563-EGFP), or EGFP-tagged fragments of Tiam1 (PHnTSS-EGFP or Tiam11−669-EGFP). The transfected cells were fixed after 20 h of incubation on slides coated with either EphB2-Fc (A–D) or ephrin-A1-Fc (E–H), and observed through a fluorescence microscope. Representative cells are shown (A–C, E–G). (D, H) Percentage of transfected cells bearing neurites longer than one cell body. Exogenously expressed proteins in these cells were monitored by immunoblotting.
Finally, we examined whether dominant-negative mutants of Tiam1 inhibit the neurite outgrowth induced by the ephrin-B1- or EphA2-mediated signaling. E14 cortical neurons on the EphB2-Fc surface were transiently transfected with the PHnTSS region of Tiam1 tagged with EGFP. On the other hand, we used Tiam11−669 to test whether it could block the neurite outgrowth induced by EphA2-mediated signaling, because it effectively blocked the Rac1 activation induced by the activation of EphA2 (Figure 5A). As shown in Figure 8C, the neurite outgrowth of cortical neurons on the EphB2-Fc surface was significantly inhibited by the expression of PHnTSS protein. Similarly, NB1 cells expressing Tiam11–669 did not show apparent neurites on the ephrin-A1-Fc-coated surface (Figure 8G). On the other hand, the expression of these Tiam1 fragments did not effectively inhibit the nonspecific neurite outgrowth of cortical neurons and NB1 cells induced by plating the cells on poly-L-lysine-coated plates (data not shown). Moreover, the inhibitory effect of PHnTSS protein on the neurite outgrowth of NB1 cells on the ephrin-A1-Fc-coated surface was modest, and we did not observe any inhibition of neurite outgrowth by Tiam11−392 in cortical neurons on the EphB2-Fc-coated surface (data not shown). Taken together, these results (Figure 8D and H) indicate that Tiam1 locates downstream of ephrin-B1- or EphA2-mediated signaling and is involved in the neurite outgrowth.
Discussion
Examination of the association between Tiam1 with ephrin-B1 and EphA2 receptor revealed that Tiam1 interacts with ephrin-B1 and EphA2. Interaction of Tiam1 with EphA2 was not apparently modified by the phosphorylation status of EphA2, because EphA2 with a mutation in its kinase domain and an abolished catalytic activity also associated with Tiam1 as well as wild-type EphA2 (data not shown). However, we cannot completely exclude the possibility that the association of Tiam1 with ephrin-B1 is increased by the stimulation of EphB2-Fc (Supplementary information 3A). The observations that staining of Tiam1 in the cytoplasm did not appear to diminish in the stimulated cells (Figure 4) reveal that only a part of Tiam1 protein translocated to the patches colocalizing with ephrin-B1 or EphA2. Therefore, the localized activation of Tiam1 and Rac1 should be monitored spatially and temporally in these cells in order to evaluate the significance of the translocation of Tiam1. In primary cultured cortical neurons, patches containing ephrin-B1 and Tiam1 often locate along the extended neurites. The biological significance of such colocalization along the neurites requires elucidation. Although Tiam1 is clearly phosphorylated on tyrosine residues by EphA2 activation, we detected phosphorylation of Tiam1 in ephrin-B1-activated cells at low level only by the orthophosphate labeling (Figure 5B). Because the phosphorylation of Tiam1 on threonine residues has been detected by the same antibody in response to the stimulation of platelet-derived growth factor, Tiam1 was not phosphorylated at least on the same positions by the activation of ephrin-B1 (Fleming et al, 1998). The significance of the phosphorylation of Tiam1 induced by the activation of ephrin-B1 and EphA2 remains to be further studied.
Among proteins reported to be putatively associated with the cytoplasmic regions of Ephs or ephrins, Ephexin, which is a GEF for the rho family GTPases, preferentially interacts with EphA receptors (Shamah et al, 2001). Stimulation of ephrin-A regulates growth cone collapse or retraction through Ephexin. Although A-class ephrins are not highly expressed in the developing mouse cortex (Yun et al, 2003), it is important to examine the expression of Tiam1 and Ephexin, two exchange factors having opposing effects on the neurites motility, in the cortical region. Furthermore, we also have to consider a homologous protein of Tiam1, STEF, another GEF for Rac1, because STEF is also expressed in the cerebral cortex of developing mice (Matsuo et al, 2002; Kawauchi et al, 2003). We detected the association of the PHnTSS domain of STEF with ephrin-B1 by immunoprecipitation analysis (Supplementary information 3B). Therefore, the neurite outgrowth may be regulated by exchange factors other than or in addition to Tiam1 in a certain region and stage. In this report, we have used Tiam1 fragments containing the PHnTSS region (Tiam1431−669 and Tiam11−669) to block wild-type Tiam1. Because the PHnTSS region of Tiam1 is highly homologous to the PHnTSS domain of STEF, these Tiam1 fragments may also work as dominant-negative mutants for STEF. In addition, we cannot exclude the possibility that Tiam1 fragments containing PHnTSS might antagonize the binding of ephrin-B1 or EphA2 to Tiam1 in addition to acting as a dominant-negative for Tiam1.
The molecular mechanism of the activation of Tiam1 in ephrin-B1- and EphA2-mediated signaling is not clear. Although PHnTSS region is not involved in the association of Tiam1 with EphA2, the expression of PHnTSS inhibited EphA2-induced Rac1 activation mildly, but evidently (Figure 5A). This result may suggest the possibility that EphA2 also activates Tiam1 by a mechanism independent of their association. EphA2 interacts with the p85 subunit of phosphatidylinositol-3-kinase (PI3-kinase) and induces the activation of PI3-kinase (Pandey et al, 1994). On the other hand, Tiam1 has been reported to activate by the lipid products of PI3-kinase in vitro (Fleming et al, 2000). Because PHn (amino-terminal PH domain) is required for the membrane localization and the binding of Tiam1 to the lipid products of PI3-kinase (Stam et al, 1997), the overexpression of PHnTSS is considered to block PI3-kinase-dependent Tiam1 activation. Therefore, the inhibitory effect by N392-PHnTSS (Tiam11−669) on the neurite outgrowth of NB1 cells may include a mechanism that may not depend on the association of Tiam1 with EphA2 (Figure 8). However, as shown in Figure 5A, overexpression of N392 alone abolished the Rac1 activation more effectively than PHnTSS. Therefore, the significance of the PI3-kinase for the activation of Tiam1 in EphA2-mediated signaling should be further elucidated.
Concerning the ephrin-B1-mediated reverse signaling on retinal axon growth, both repulsive and attractive guidance has been previously suggested. Birgbauer et al (2001) have shown that the ECD of EphB receptors as soluble proteins induced growth cone collapse of ephrin-B-expressing retinal ganglion cells. They also show that axon outgrowth of ephrin-B-expressing dorsal retina explants of E14 mouse was inhibited on the laminin substratum containing EphB-ECD. On the other hand, Mann et al (2002) propose an attractive axon guidance that requires the ephrin-B cytoplasmic domain. Retinal axons of Xenopus expressing ephrin-Bs prefer to grow on laminin substratum containing EphB receptor stripes in vitro. These apparently inconsistent observations may be due to the differences in the experimental procedures, and not to those in the species used between these reports. EphB-Fc protein was used as a dimer in the former (Birgbauer et al, 2001) and as a multimer by preclustering with immunoglobulin in the latter (Mann et al, 2002); the concentration of laminin substratum used in these reports was also different. In the present study, we followed a procedure similar to that of Mann et al, and devised a simplified system for evaluating the outputs of Eph/ephrin-induced signaling. We found that ephrin-B1-expressing cortical neurons markedly extend neurites on surfaces coated with preclustered EphB2-Fc without any other substratum. As to the laminin concentration, the substratum we used contained no laminin, more similar to that used by Mann et al (1 μg/ml) than that used by Birgbauer et al (10 μg/ml). The lower concentration of laminin seems to prefer the more attractive or progrowth reaction of these systems.
Cell adhesion itself may promote neurite growth, and there are reports concerning adhesive interactions between ephrin-B-expressing axons and the substrate-bound Eph receptor (Holash et al, 1997). We also noticed faster attachment of dissociated E14 cortical neurons on EphB2-Fc-coated plates compared to that on Fc-coated plate. We have not directly examined whether Tiam1 mediates this cell-to-substrate adhesion of the cortical neurons, but the adhesion of cortical neurons on the EphB2-Fc surface was accompanied by the phosphorylation of the tyrosine residues of p130Cas and focal adhesion kinase (Supplementary information 4). The primary cultured cortical neurons of E14 mouse embryos adhered to the plates to almost the same degree as a few hours after plating and thereafter, irrespective of whether plates were coated with preclustered-EphB2-Fc, -ephrin-A1-Fc or bovine serum albumin (BSA). Similarly, NB1 cells adhered almost equally to plates precoated with Fc, ephrin-A1-Fc or EphA2-Fc after a few hours of seeding and thereafter. However, we cannot completely exclude the possibility that the differences in neurite outgrowth could depend on differential adhesiveness of the substrates.
Among other members of Eph receptors and ephrins, considerable expression of ephrin-B2 and ephrin-B3 mRNAs is also present in the cortical neurons (Stuckmann et al, 2001). Moreover, EphA4 protein is broadly expressed within the cortex of E14 and E16 mouse (Liebl et al, 2003; Yun et al, 2003). Because the amino-terminal region of Tiam1 (Tiam11−392) also associates with EphA4 leading to the phosphorylation of Tiam1 on tyrosine residues (data not shown), EphA4 receptor may also regulate Rac1 activation by interaction with Tiam1. Further studies are necessary to examine whether Tiam1 also regulates signals mediated by ephrin-B2, ephrin-B3 and EphA4 in the neurons, which may position Tiam1 as a general mediator of Eph/ephrin signals. In NB1 cells, we cannot exclude the possibility that neurite outgrowth of NB1 cells was not exclusively mediated by EphA2.
Our results suggest the importance of Tiam1 and Rac1 as downstream molecules in ephrin-B1- or EphA2-mediated signaling in neurite outgrowth. According to previous information, Eph receptors/ephrins signals, both forward and reverse, have exhibited various, sometimes paradoxical effects on the regulation of neurite extension, that is, retraction and elongation. Our discovery of the involvement of Tiam1 downstream with both the forward and reverse signals of Eph/ephrin system in these neurite responses provides insight that the selection of the GEFs actually working downstream to Ephs/ephrins may determine the fate of neurites.
Materials and methods
Plasmids and antibodies
Plasmids and antibodies used in these series of experiments are described in Supplementary information 1.
Cell culture and transfection
SK-N-MC neuroepithelioma cells and NB1 neuroblastoma cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum. The cerebral cortex from E14 DDY mouse embryos were dissected, dissociated and plated on chamber slides as described (Ueki et al, 1993), and cultured in DMEM supplemented with D-glucose (4.5 g/l) and 10% fetal bovine serum. For transient expression assays, NB1 cells were transfected with plasmid DNA using Lipofectamine 2000 (GIBCO-BRL), and 293T cells and COS1 cells were transfected with plasmid DNA either by a calcium phosphate co-precipitation method essentially as described previously (Otsuki et al, 2001) or using FuGENE6 reagent (Roche). For the transfection of plasmid DNA into E14 primary cortical neurons, a total of 3 μg of plasmid DNA and 4 μl Lipofectamine 2000 reagent were each diluted in 50 μl of OPTI-MEM™ (GIBCO-BRL), and applied to the cells cultured in 400 μl of NEUROBASAL with B27 supplement 3 h after plating. The medium was completely exchanged at 4.5 h after transfection, and cells were maintained in DMEM supplemented with D-glucose (4.5 g/l) and 10% fetal bovine serum. NIH3T3 cells stably expressing ephrin-A1 and 293T cells stably expressing EphB2 were established by transfection of pAlterMax encoding EphA2 or EphB2 as described above. Cells were cultured and selected in DMEM containing puromycin at a concentration of 2 μg/ml (for 293T cells) or G418 (Gibco BRL) at 0.6 mg/ml (for NIH3T3 cells) for 2–3 weeks. Well-isolated colonies were characterized further.
Immunoprecipitation and affinity precipitation
Transfected cells were harvested 48 h after transfection, and cell lysates were prepared with protease inhibitors in TXB buffer (10 mM Tris (pH 7.6), 150 mM NaCl, 5 mM EDTA (pH 8.0), 10% glycerol, 1 mM Na3VO4 and 1% Triton X-100). Whole brain tissue from E14 DDY mice was also lysed in TXB buffer with a Dounce homogenizer. The lysates were precleared by incubating them with protein G agarose (Boehringer Mannheim) for 1 h at 4°C. To purify the proteins, 1 μg of monoclonal or affinity-purified polyclonal antibodies was added and incubated with 500 μl of cell lysate for 1 h at 4°C. The antibodies were precipitated with protein G agarose for 2 h at 4°C. Immunoprecipitates were extensively washed with TXB buffer, separated by SDS–polyacrylamide gel electrophoresis (PAGE) and immunoblotted.
Affinity precipitation with GST-PBD was performed as described previously (Otsuki et al, 2001). Briefly, 293T cells were lysed in the lysis buffer (20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 20 mM MgCl2, 1 mM Na3VO4, 5% Triton X-100, 5 μg/ml aprotinin and 1 mM PMSF), and incubated with GST-PBD on sepharose for 1 h at 4°C. The precipitants were washed four times in the same buffer, and endogenous Rac1 protein was detected by immunoblotting.
In vivo phosphate labeling
Plasmids encoding Myc-tagged Tiam1 with ephrin-B1 or EphA2 were transfected into 293T cells in a 35 mm diameter dish. At 48 h after transfection, the transfected cells were cocultured with cells stably expressing EphB2 K661M or ephrin-A1. Transfected cells were preincubated in phosphate-free medium (GIBCO-BRL) for 15 h, and then coculture was performed in 0.5 ml of phosphate-free MEM containing 0.09 mCi of 32Pi (NEN) for a further 4 h. The cells were lysed in the lysis buffer (20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 20 mM MgCl2, 1 mM Na3VO4, 0.5% Triton X-100, 5 μg/ml aprotinin and 1 mM PMSF). Myc-tagged Tiam1 was purified by immunoprecipitation using an anti-Myc antibody and separated on SDS–PAGE. 32Pi-labeled Tiam1 was visualized with a Bio Imaging Analyzer (BAS1000, Fuji) (lanes 1 and 2).
Cell staining
To examine the localization of Tiam1 after activation of ephrin-B1 or EphA2, cortical neurons from E14 DDY mice or NB1 cells were seeded on chamber slides. Cells were incubated with 4 μg/ml of clustered EphB2-Fc or ephrin-A1-Fc for 10 min, and then the medium was removed and further incubated in DMEM containing 10% FBS for the indicated period. Cells were fixed for 5 min at room temperature with 4% paraformaldehyde (PFA) in PBS and permeabilized for 5 min with 0.2% Triton X-100. The cells were preincubated in 2% BSA with 5% normal serum for 1 h, and incubated with the FITC-conjugated anti-Fc fragment of IgG antibody for 1 h at room temperature. In some experiments, cells were further stained for Tiam1 protein by sequential incubation with anti-Tiam1 (C-16) antibody and rhodamine-conjugated secondary antibody for 1 h for each. Photos were taken with Radiance 2100 confocal microscope (BioRad).
Neurite elongation assay
Plates and glass chamber slides were coated with Fc fusion proteins, by being filled with 5 μg/ml of Fc fusion proteins or the control Fc fragment plus 50 μg/ml of goat anti-IgG Fc (ICN) in sterile PBS(−) with gentle rocking for 1–2 h at room temperature before the protein solution was aspirated. Plates and chamber slides were washed three times with sterile PBS, and then incubated in PBS containing 2% (w/v) BSA for 30 min at room temperature to block the remaining protein-binding sites. Cortical neurons from the E14 mouse embryos were plated on the coated surface, and then fixed in 4% PFA in PBS after 20 h of incubation. In some experiments, cortical neurons were transfected with EGFP-tagged plasmids indicated as described above 3 h after plating on chamber slides, and then fixed after 20 h of transfection. For the statistical calculations, cells bearing neurites longer than one or three cell bodies in length were considered as cells with neurite outgrowth. In counting, only cells positive for EGFP were included in the analysis.
Supplementary Material
Supplementary Information 1
Supplementary Information 2
Supplementary Information 3
Supplementary Information 4
Acknowledgments
We thank Drs T Pawson (Mount Sinai Hospital), R Klein (European Molecular Biology Laboratory), N Kawazoe and N Nakaya (Laboratory of Biological Chemistry, School of Pharmaceutical Sciences, Showa University), M Hoshino (Department of Pathology and Tumor Biology, Graduate School of Medicine, Kyoto University) and Y Dobashi (Yamanashi University) for providing plasmids and cells used in this study. We thank Dr Ueki (First Department of Anatomy, Hamamatsu University School of Medicine) for assisting with the primary neuron culture technique from mouse embryos, and Associate Prof. Yamamoto (Laboratory of Cell Imaging, Photon Medical Research Center, Hamamatsu University School of Medicine) for assisting with the operation of the fluorescent microscope. We thank Prof. Nakahara (Department of Psychology, Hamamatsu University School of Medicine) for careful reading of this paper. We thank Prof. Sato (First Department of Anatomy, Hamamatsu University School of Medicine), Prof. Tsutsui and Dr Kosugi (Second Department of Pathology, Hamamatsu University School of Medicine) for useful discussion. This work was supported by the Smoking Research Foundation, Grants-in-Aid for Cancer Research from the Ministry of Health and Welfare of Japan, a Grant-in Aid-for Scientific Research (B, 10470056) on Priority areas (C-2, 12218215; C-2, 13216044), and COE (Medical Photonics, Hamamatsu University School of Medicine) from the Ministry of Education, Culture, Sports Technology and Science of Japan, and a Grant-in Aid-for Scientific Research (C2, 14570183) from the Japan Society for the Promotion of Science.
References
- Birgbauer E, Oster SF, Severin CG, Sretavan DW (2001) Retinal axon growth cones respond to EphB extracellular domains as inhibitory axon guidance cues. Development 128: 3041–3048 [DOI] [PubMed] [Google Scholar]
- Cheng HJ, Nakamoto M, Bergemann AD, Flanagan JG (1995) Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell 82: 371–381 [DOI] [PubMed] [Google Scholar]
- Cowan CA, Henkemeyer M (2002) Ephrins in reverse, park and drive. Trends Cell Biol 12: 339–346 [DOI] [PubMed] [Google Scholar]
- Fleming IN, Elliott CM, Exton JH (1998) Phospholipase C-γ, protein kinase C and Ca2+/calmodulin-dependent protein kinase II are involved in platelet-derived grpwth factor-induced phosphorylation of Tiam1. FEBS Lett 429: 229–233 [DOI] [PubMed] [Google Scholar]
- Fleming IN, Gray A, Downes CP (2000) Regulation of the Rac1-specific exchange factor Tiam1 involves both phosphoinositide 3-kinase-dependent and-independent components. Biochem J 351: 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao PP, Sun CH, Zhou XF, DiCicco-Bloom E, Zhou R (2000) Ephrins stimulate or inhibit neurite outgrowth and survival as a function of neuronal cell type. J Neurosci Res 60: 427–436 [DOI] [PubMed] [Google Scholar]
- Gao PP, Yue Y, Cerretti DP, Dreyfus C, Zhou R (1999) Ephrin-dependent growth and pruning of hippocampal axons. Proc Natl Acad Sci USA 96: 4073–4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao PP, Yue Y, Zhang JH, Cerretti DP, Levitt P, Zhou R (1998) Regulation of thalamic neurite outgrowth by the Eph ligand ephrin-A5: implications in the development of thalamocortical projections. Proc Natl Acad Sci USA 95: 5329–5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao PP, Zhang JH, Yokoyama M, Racey B, Dreyfus CF, Black IB, Zhou R (1996) Regulation of topographic projection in the brain: Elf-1 in the hippocamposeptal system. Proc Natl Acad Sci USA 93: 11161–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets GG, Scholtes EH, Zuydgeest D, van der Kammen RA, Stam JC, Berns A, Collard JG (1994) Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell 77: 537–549 [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, Orioli D, Henderson JT, Saxton TM, Roder J, Pawson T, Klein R (1996) Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell 86: 35–46 [DOI] [PubMed] [Google Scholar]
- Holash JA, Soans C, Chong LD, Shao H, Dixit VM, Pasquale EB (1997) Reciprocal expression of the Eph receptor Cek5 and its ligand(s) in the early retina. Dev Biol 182: 256–269 [DOI] [PubMed] [Google Scholar]
- Kawauchi T, Chihama K, Nabeshima Y, Hoshino M (2003) The in vivo roles of STEF/Tiam1, Rac1 and JNK in cortical neuronal migration. EMBO J 22: 4190–4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll B, Zarbalis K, Wurst W, Drescher U (2001) A role for the EphA family in the topographic targeting of vomeronasal axons. Development 128: 895–906 [DOI] [PubMed] [Google Scholar]
- Kunda P, Paglini G, Quiroga S, Kosik K, Caceres A (2001) Evidence for the involvement of Tiam1 in axon formation. J Neurosci 21: 2361–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuwen FN, Kain HE, Kammen RA, Michiels F, Kranenburg OW, Collard JG (1997) The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J Cell Biol 139: 797–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl DJ, Morris CJ, Henkemeyer M, Parada LF (2003) mRNA expression of ephrins and Eph receptor tyrosine kinases in the neonatal and adult mouse central nervous system. J Neurosci Res 71: 7–22 [DOI] [PubMed] [Google Scholar]
- Mann F, Ray S, Harris W, Holt C (2002) Topographic mapping in dorsoventral axis of the Xenopus retinotectal system depends on signaling through ephrin-B ligands. Neuron 35: 461–473 [DOI] [PubMed] [Google Scholar]
- Matsuo N, Hoshino M, Yoshizawa M, Nabeshima Y (2002) Characterization of STEF, a guanine nucleotide exchange factor for Rac1, required for neurite growth. J Biol Chem 277: 2860–2868 [DOI] [PubMed] [Google Scholar]
- Otsuki Y, Tanaka M, Yoshii S, Kawazoe N, Nakaya K, Sugimura H (2001) Tumor metastasis suppressor nm23H1 regulates Rac1 GTPase by interaction with Tiam1. Proc Natl Acad Sci USA 98: 4385–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A, Zimmer M, Erdmann KS, Eulenburg V, Porthin A, Heumann R, Deutsch U, Klein R (2002) Ephrin B phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase. Mol Cell 9: 725–737 [DOI] [PubMed] [Google Scholar]
- Pandey A, Lazar DF, Saltiel AR, Dixit VM (1994) Activation of the Eck receptor protein tyrosine kinase stimulates phosphatidylinositol 3-kinase activity. J Biol Chem 269: 30154–30157 [PubMed] [Google Scholar]
- Sander EE, van Delft S, ten Klooster JP, Reid T, van der Kammen RA, Michiels F, Collard JG (1998) Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell–cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol 143: 1385–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A, Greenberg ME (2001) EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 105: 233–244 [DOI] [PubMed] [Google Scholar]
- Stam JC, Sander EE, Michiels F, van Leeuwen FN, Kain HE, van der Kammen RA, Collard JG (1997) Targeting of Tiam1 to the plasma membrane requires the cooperative function of the N-terminal pleckstrin homology domain and an adjacent protein interaction domain. J Biol Chem 272: 28447–28454 [DOI] [PubMed] [Google Scholar]
- Stuckmann I, Weigmann A, Shevchenko A, Mann M, Huttner WB (2001) Ephrin B1 is expressed on neuroepithelial cells in correlation with neocortical neurogenesis. J Neurosci 21: 2726–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Kamo T, Ota S, Sugimura H (2003) Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. EMBO J 22: 847–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki T, Nakanishi K, Asai K, Okouchi Y, Isobe I, Eksioglu YZ, Kato T, Kohno K (1993) Neurotrophic action of gliostatin on cocultured neurons with glial cells. Brain Res 622: 299–302 [DOI] [PubMed] [Google Scholar]
- Wahl S, Barth H, Ciossek T, Aktories K, Mueller BK (2000) Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J Cell Biol 149: 263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Daniels J, Riese DJ, Kinch MS (2002) c-Cbl-dependent EphA2 protein degradation is induced by ligand binding. Mol cancer Res 1: 79–87 [PubMed] [Google Scholar]
- Wang Y, Ota S, Kataoka H, Kanamori M, Li Z, Band H, Tanaka M, Sugimura H (2002) Negative regulation of EphA2 receptor by Cbl. Biochem Biophys Res Commun 296: 214–220 [DOI] [PubMed] [Google Scholar]
- Yun ME, Johnson RR, Antic A, Donoghue MJ (2003) EphA family gene expression in the developing mouse neocortex: regional patterns reveal intrinsic programs and extrinsic influence. J Comp Neurol 456: 203–216 [DOI] [PubMed] [Google Scholar]
- Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS (1999) E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ 10: 629–638 [PubMed] [Google Scholar]
- Zimmer M, Palmer A, Kohler J, Klein R (2003) EphB–ephrin-B bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol 5: 869–878 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information 1
Supplementary Information 2
Supplementary Information 3
Supplementary Information 4


