Abstract
Shiga toxin-producing Escherichia coli (STEC) is a major cause of severe food-borne disease worldwide, and two Shiga toxins, Stx1 and Stx2, are primarily responsible for the serious disease consequence, hemolytic-uremic syndrome (HUS). Here we report identification of a panel of heavy-chain-only antibody (Ab) VH (VHH) domains that neutralize Stx1 and/or Stx2 in cell-based assays. VHH heterodimer toxin-neutralizing agents containing two linked Stx1-neutralizing VHHs or two Stx2-neutralizing VHHs were generally much more potent at Stx neutralization than a pool of the two-component monomers tested in cell-based assays and in vivo mouse models. We recently reported that clearance of toxins can be promoted by coadministering a VHH-based toxin-neutralizing agent with an antitag monoclonal antibody (MAb), called the “effector Ab,” that indirectly decorates each toxin molecule with four Ab molecules. Decoration occurs because the Ab binds to a common epitopic tag present at two sites on each of the two VHH heterodimer molecules that bind to each toxin molecule. Here we show that coadministration of effector Ab substantially improved the efficacy of Stx toxin-neutralizing agents to prevent death or kidney damage in mice following challenge with Stx1 or Stx2. A single toxin-neutralizing agent consisting of a double-tagged VHH heterotrimer—one Stx1-specific VHH, one Stx2-specific VHH, and one Stx1/Stx2 cross-specific VHH—was effective in preventing all symptoms of intoxication from Stx1 and Stx2 when coadministered with effector Ab. Overall, the availability of simple, defined, recombinant proteins that provide cost-effective protection against HUS opens up new therapeutic approaches to managing disease.
INTRODUCTION
Shiga toxin (Stx)-producing Escherichia coli (STEC) bacteria cause both sporadic and major outbreaks of diarrheal disease through consumption of contaminated food or water. For example, in 2011, an outbreak of STEC in Germany was due to contaminated sprouts (1, 2). STEC (which includes enterohemorrhagic E. coli [EHEC]) infection typically causes acute bloody diarrhea and abdominal cramping. In 2 to 10% of patients, mostly children and the elderly, hemolytic-uremic syndrome (HUS), which is characterized by acute renal failure, hemolytic anemia, and thrombocytopenia, develops as a sequela. HUS is a severe complication requiring blood transfusion, kidney dialysis, and sometimes kidney transplantation. The major virulence determinants of STEC are attributed to the Shiga toxins Stx1 and Stx2 (3). Both toxins contribute to disease in animal models (4), but in humans Stx2 is more often linked to HUS (5–8).
Stx1 and Stx2 each consist of an A subunit N-glycosidase and five B subunits that bind to the Gb3 receptor, leading to cell internalization (9, 10) and inhibition of protein synthesis, which triggers apoptosis (4, 11–14). The toxins primarily affect the glomerular endothelial endothelium in humans (15) and the renal tubular epithelium in mice (16), which express the Gb3 receptor. The systemic consequences of intoxication are vascular dysfunction, leukocyte recruitment, and thrombus formation, which can lead to HUS (reviewed in reference 17).
Antibiotic treatment is not recommended for STEC infection (18), so treatment is limited to fluid replacement and supportive care (4, 19). Thus, there is a need for new treatment options. Currently, anti-Stx monoclonal antibodies (Abs) (MAbs) show promise in animal models (20–25), and clinical trials are ongoing (Thallion Pharmaceuticals). It remains unknown whether antitoxin antibodies administered after the onset of diarrheal symptoms will prevent or modify the outcome of HUS (23, 25). Even if effective, the use of MAb-based antitoxins may be too costly to stockpile them as a therapeutic option, since different MAbs are likely required to neutralize the two Shiga toxins and multiple different MAbs targeting each toxin may be needed to decorate the toxins and promote their clearance via low-affinity Fc receptors (FcRs) (26, 27).
We have developed an alternative antitoxin platform (28) that has advantages over current strategies. Our antitoxins contain just two simple proteins: a “VHH (heavy-chain-only Ab VH)-based neutralizing agent” (VNA) and an “effector Ab” (efAb) (28). The VNAs consist of linked VHHs, produced as heteromultimers, that bind and neutralize their toxin targets. The VHH components of VNAs are 14-kDa camelid heavy-chain-only Ab VH domains. VHHs are robustly expressed by recombinant E. coli and thus economical to produce (28, 29). To promote toxin clearance, the VNA can be coadministered with a single antitag MAb, the efAb, that binds to multiple epitopic tags engineered into each VNA molecule. When VNAs are bound at separate sites on the toxin and each VNA is bound to two or more efAbs through the tags, the toxin becomes decorated by sufficient efAbs to promote liver clearance (30), presumably by low-affinity FcRs.
Here we report the identification of Stx-binding VHHs that neutralize each of the Shiga toxins, Stx1 and Stx2, and some VHHs that neutralize both toxins. VHH heterotrimer VNAs in which a single VNA protein potently neutralizes both Stxs through binding at two separate sites on each toxin are described. The heterotrimeric VNAs have much greater antitoxin efficacy when the VNA is coadministered with the efAb. These simple antitoxin agents, effective against both Shiga toxins, should offer new therapeutic options for treating STEC infections to prevent HUS sequelae.
MATERIALS AND METHODS
Ethics statement.
All studies followed protocols approved by the Tufts University Institutional Animal Care and Use Committee (IACUC).
Toxins and reagents.
O157:H7 Stx1 purified from cell lysates of Stx1-producing E. coli HB101-H19B (31) and O157:H7 Stx2 from culture supernatants of Stx2-producing E. coli C600W (31) as previously described (32) were obtained from Phoenix Lab at Tufts Medical Center. The toxins were dissolved at 1 mg/ml in phosphate-buffered saline (PBS), aliquoted, and stored at −80°C. Purified anti-Stx1 MAb 4D3 and anti-Stx2 MAb 3D1 were obtained as we described previously (21, 22). Reagents for Western blotting were purchased from KPL. Antibodies used were anti-E-tag MAb (Phadia), horseradish peroxidase (HRP)–anti-E-tag MAb (Bethyl Labs), and HRP–anti-M13 Ab (GE Healthcare).
Preparation of Stx reagents for immunization.
Intact Stx1 B subunit (Stx1B) and Stx2 A unit (Stx2A) and Stx2 B subunit (Stx2B) were produced as recombinant proteins in E. coli. The DNAs encoding the subunits (GenBank accession no. M19473.1 and EF441614.1) were amplified by PCR and ligated into pET-25B in frame with C-terminal His tags, and plasmids were confirmed by sequencing. Expression and purification of recombinant Stx subunits were performed essentially as previously described for VHH expression (33). The purified proteins were dialyzed against PBS, sterilized using 0.22-μm filter, and stored at −70°C. Stx1 and Stx2 toxoids were prepared by formalin inactivation of the holotoxins followed by dialysis against PBS and storage at −70°C.
Alpaca immunization and VHH-display library preparation.
An alpaca was immunized by four successive multisite subcutaneous injections at 3-week intervals using an immunogen consisting of 50 μg of Stx1 toxoid and 50 μg of Stx2 toxoid in alum/CpG adjuvant. Serum at the completion of the immunization process contained Ab titers for Stx1 of approximately 1:10,000 and for Stx2 of approximately 1:100,000. Six days following the final boost, blood was obtained for lymphocyte preparation, and a VHH display phage library was prepared from the immunized alpaca as previously described (33, 34, 40). More than 106 independent clones were prepared from B cells of the alpaca successfully immunized with each of the immunogens.
ELISAs and Western blots.
Capture enzyme-linked immunosorbent assays (ELISAs) were performed by first coating plates with 0.5 μg/ml of 4D3 MAb for Stx1 and 3D1 MAb for Stx2 (32). After blocking, the plates were incubated with 0.3 μg/ml of Stx1 or Stx2. For standard ELISAs, plates were coated with 1.5 μg/ml of Stx1 or Stx2 or 2 μg/ml Stx subunits. Test VHH agents were serially diluted, incubated for 1 h at room temperature (RT), and washed, and bound agent was detected with HRP–anti-E tag. Bound HRP was detected using the tetramethylbenzidine (TMB) kit (Sigma), and values were plotted as a function of the input VHH concentration. Fifty percent effective concentrations (EC50s) were estimated from these plots as the VHH concentration that produced a signal equal to 50% of the peak binding signal. Competition ELISAs were performed as previously described (28). Western blotting to identify Stx subunit recognition of the purified VHHs was performed as previously described (28).
Anti-Stx VHH identification and preparation.
About 2 × 106 independent clones were prepared from B cells of the alpaca successfully immunized with the Stx immunogens. Panning, phage recovery, and clone fingerprinting were performed much as previously described (28, 33, 34) but with the following variations. Separate panning processes were always performed for Stx1 and Stx2. Panning for each toxin was initially performed using plastic coated with toxin (Nunc Immuno), and later another panning process was performed using the toxins captured on plastic with a MAb. For each process, three cycles of panning were performed: two cycles at “low stringency” and the third cycle at “high stringency.” For low-stringency panning, plastic wells were coated directly with the two Stx toxins at 10 μg/ml or the toxins were captured to plastic with 5 μg/ml of capture MAb (see above) followed by 1.5 μg/ml of toxin. Wells were incubated for 1 h with about 1012 input phage, followed by 15 rapid washes, a 15-min wash, and elution of bound phage. For high-stringency panning, plastic wells were coated with the two Stx toxins at 0.5 μg/ml or the toxins were captured to plastic with 5 μg/ml of capture MAb (see above) followed by 0.15 μg/ml of toxin. Wells were incubated for 10 min with about 1010 input phage, followed by 15 rapid washes, a final wash of 1 h, and elution.
After plating phage from the third panning cycle, individual colonies were picked and grown overnight at 37° in 96-well plates. A replica plate was then prepared by transferring 2 μl of culture to another 96-well plate containing 180 μl of culture medium. After 4 h of incubation at 37°C, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to 3 mM in all wells and incubation was continued at 30°C overnight. Bacteria were pelleted by centrifugation at 1,000 × g, and 50 μl of the supernatant was screened for Stx-binding soluble VHH by ELISA as described above.
For each panning regimen, about 10 to 20% of VHH clones were positives for binding to Stx1 and Stx2 based on ELISA signals of at least 2× the signal of negative controls. About 100 positives for each toxin were selected for “DNA fingerprinting.” For this, the VHH coding region was amplified from each of the clones by PCR and separately digested with HaeIII, BsaJ1, or BstNI. The products of the digests were resolved on gels in an effort to identify clones with distinctive digestion products. Eighteen unique DNA fingerprints were identified among the VHHs selected as positives for Stx1 and 25 for VHHs selected as positives for Stx2. One or two clones from each group of clones with apparently identical DNA fingerprints were selected for DNA sequence analysis of the VHH coding region. Generally, clones selected for sequencing were those from each fingerprint group that produced the strongest ELISA signals. DNA sequences of the VHH coding regions were obtained and analyzed by phylogenetic tree analysis to identify closely related VHHs likely to have common B cell clonal origins. Phylogenetic trees were obtained using Accelrys Gene 2.0 software following alignment of only the VHH amino acid sequences encoded internal to the PCR primers which were employed to amplify the VHH coding DNAs from alpaca B cells (i.e., primer binding sites and hinge regions were excluded). Based on this analysis, VHHs that appeared to be unrelated to any other VHH were selected for protein expression. In addition, some VHHs that produced particularly strong signals on ELISA but were distantly related to other VHHs, as well as VHHs that appeared to have interesting properties, such as cross-specificity to both Stxs, were also selected for protein expression.
Expression and purification of VHHs in E. coli as recombinant thioredoxin (Trx) fusion proteins containing hexahistidine was performed as previously described (33). VHH heteromultimers were engineered such that all VHHs were in the same reading frame separated by DNA encoding a 15-amino-acid flexible spacer [(GGGGS)3]. All monomer VHHs were expressed with a carboxyl-terminal E-tag epitope. All heteromultimer VHHs were engineered to contain a second copy of the E tag in frame between the Trx and VHH domains (28). Competition analysis was performed as previously described (28) to identify VHHs that may bind to identical or overlapping epitopes.
Kinetic analysis by surface plasmon resonance.
Studies to assess the kinetic parameters of the VHHs were performed using a ProteOn XPR36 protein interaction array system (Bio-Rad, Hercules, CA) after immobilization of Stx1 or Stx2 by amine coupling chemistry using the manufacturer's recommended protocol. Briefly, after activation of a ProteOn GLH (high protein immobilization capacity) chip surface with a mixture of 0.4 M EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) and 0.1 M sulfo-NHS (N-hydroxysulfosuccinimide) injected for 300 s at 30 μl/min, Stx1 or Stx2 was immobilized by passing a 90- or 30-μg/ml solution of the protein, respectively, at pH 5 over the surface for 150 s at 25 μl/min. The surface was deactivated with a 30-μl/min injection of 1 M ethanolamine for 300 s. A concentration series for each VHH (between 1.5625 nM and 400 nM, optimized for each antibody fragment) was passed over the surface at 100 μl/min for 60 s, and then dissociation was recorded for 600 s or 1,200 s. The surface was then regenerated with a 30-s injection of 50 mM HCl at 50 μl/min. Running buffer for these studies was 10 mM HEPES, pH 7.4, 150 mM NaCl, and 0.005% Tween 20. Data were evaluated with ProteOn Manager software (version 3.1.0.6) using the Langmuir interaction model. Reported values are the means for at least four runs.
Cell-based Shiga toxin neutralization assay.
Stx neutralization by VHH-based agents was assessed as previously described (35) with the following modifications. Vero cells (ATCC CCL-81) were cultured in 96-well plates in 100 μl of minimum essential medium (Mediatech Inc.), supplemented with 10% fetal bovine serum (FBS) (HyClone). Cells were plated at about 10,000 cells/well the day prior to the assay. Stx doses were determined by performing a dose-response assay with each batch of toxin. Serial dilutions of Stx were added to wells of near-confluent Vero cells, cultured for 48 h, and stained with crystal violet. The Stx dose selected for neutralization assays was the minimum dose that caused >90% cell death based on reduced well staining (A590). Typically these doses were ∼0.1 ng/well (∼15 pM) for Stx1 and ∼0.25 ng/well (∼35 pM) for Stx2. Control wells containing dilutions of toxin were included in each assay to confirm that the toxin potency on the cells was as previously measured. Serial dilutions of various test antitoxin agents were generated in culture medium, combined with toxin, and incubated for 1 h at 37°C. Toxin-only control wells were always included. Vero cell medium was removed and replaced with the mixture of test agents and toxin, followed by culture for 48 h prior to staining and reading of absorbance at 590 nm. Fifty percent inhibitory concentration (IC50) estimates were assessed as the agent concentration that produced a signal that was 50% of the difference between the peak signal and the baseline signal from wells having no agent.
In vivo mouse assay of Shiga toxin lethality.
Female CD1 mice, 15 to 17 g each (Charles River Labs), were weighed and sorted into groups of five mice each to minimize intergroup weight variation. The minimum lethal dose (MLD) of Stx1 and Stx2 was determined based on dose-response studies. For evaluation of test agents, a dose of 1.25× the MLD was utilized: 60 ng Stx2/mouse or 1.25 μg Stx1/mouse. Solutions of test agents and Stx were prepared at twice the final concentration required, and then 600 μl of test agent and 600 μl of the selected Stx in PBS were combined, resulting in the final desired concentration of each component. Following incubation at room temperature for 30 min, 200 μl of the mixture was administered by intravenous tail vein injection at time zero to mice in groups of five. Mice were monitored 4 to 6 times each day and individually scored for overall disposition, presence of central nervous system signs (trembling, ataxia, paralysis, and opisthotonos), activity level, and mortality. Mice that were moribund or exhibiting central nervous system signs were euthanized. The time to death was determined for each mouse. No relapse was found to occur through 18 days in VHH- or MAb-treated mice that survived the lethal dose of Stx1 or Stx2 in an early study, so surviving mice in subsequent studies were typically euthanized after a week. Mouse survival data were analyzed nonparametrically via Kaplan-Meier and log rank tests (SigmaPlot for Windows v. 12.3; Systat Software, Inc.).
Tissue evaluation by light microscopy.
Following euthanasia, right and left kidneys from each mouse were harvested, fixed in 10% neutral buffered formalin, dehydrated, paraffin embedded, sectioned at 3 μm, stained with hematoxylin and eosin, and evaluated by board-certified veterinary pathologists (R. Peters and G. Beamer) blinded to the treatment groups. Tubular lesions were quantified by counting the number of affected tubules in 6 random 20× fields in the cortex and corticomedullary junctions from the left and right kidneys.
Nucleotide sequence accession numbers.
The novel nucleotide sequences reported in this paper have been deposited in GenBank under accession numbers KF551949 to KF551958 .
RESULTS
Identification and binding properties of VHHs recognizing Stx1 and/or Stx2.
Heavy-chain-only Vh (VHH) binding agents were obtained from a VHH display phage library representing the VHH repertoire of an alpaca immunized with both Stx1 and Stx2 immunogens. Eighteen clearly distinct Stx1-binding VHHs and 25 Stx2-binding VHHs were identified using DNA fingerprinting. Coding DNA analysis of the Stx-binding VHHs (representatives are shown in Fig. S1 in the supplemental material) identified numerous unique VHHs and a large group of related VHHs (a dendrogram is shown in Fig. S2). The group of related VHHs contained clones selected on both Stx1 and Stx2, including some that were virtually identical (e.g., Stx-F1 and Stx-H3). These results suggested (confirmed below) that VHH members of this group recognize both Stx toxins. Eleven members of the large homology group and each of the unique Stx1- and Stx2-selected VHHs (all the VHHs in Fig. S1) were expressed as soluble proteins and purified for further characterization.
Anti-Stx VHH binding to Stx1 and Stx2 was assessed by dilution ELISA, and representative results are shown in Fig. 1 and 2. The ELISA results confirmed that all 11 members of the large VHH homology group (see Fig. S2 in the supplemental material) recognized both Stx1 and Stx2, although with wide variation in the relative EC50s for the two toxins. The two VHHs in this homology group having the lowest EC50 for both Stx1 and Stx2 (Stx-A4 and Stx-A5) were selected for further study. All of the remaining VHHs were highly specific for either Stx1 or Stx2. The two Stx1-specific VHHs with the lowest EC50s (Stx1-A9 and Stx1-D4) and the six Stx2-specific VHHs with lowest the EC50s (Stx2-A6, Stx2-D2, Stx2-D10, Stx2-G1, Stx2-G9, and Stx2-H6) were selected for further study.
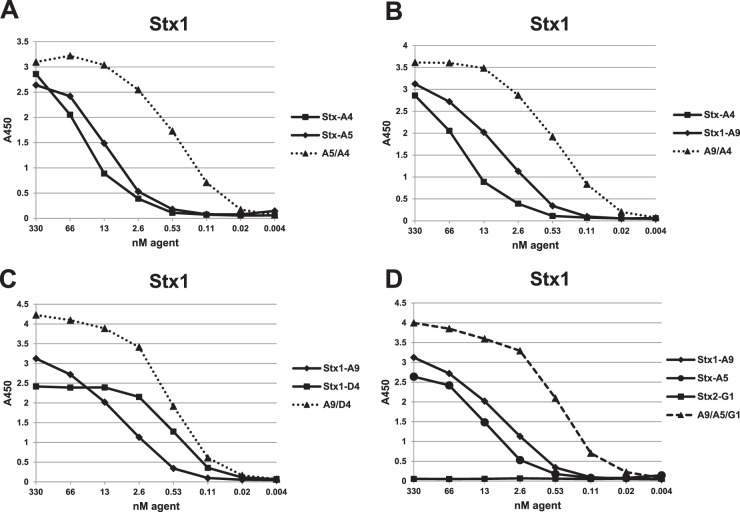
Fig 1.
Dilution ELISAs to assess VHH binding to the Stx1 toxin. ELISAs were performed using plates coated with 1.5 μg/ml of Stx1. Binding was plotted as a function of VHH concentration. Plots for VHH heterodimers are displayed by dotted lines, and the VHH heterotrimer is displayed as a dashed line. VHH names are as shown in Tables 1 and 2. Panels A, B, C, and D compare dilution ELISAs of related samples within the same assay. ELISA results shown are representative of at least one other study and are supported by SPR data shown in the tables.
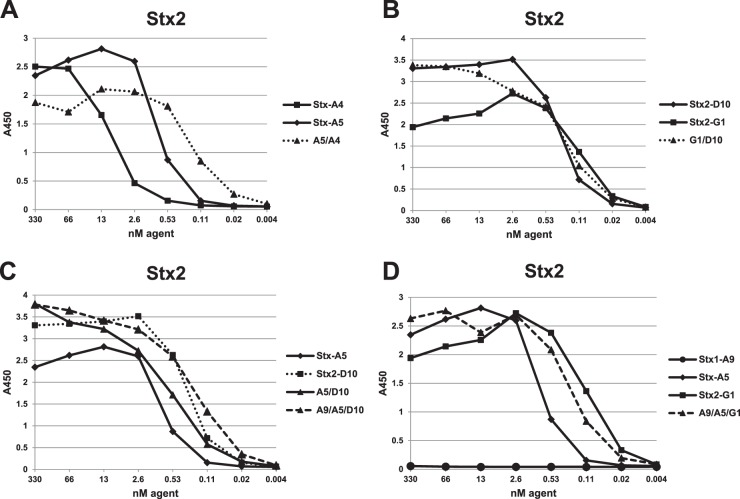
Fig 2.
Dilution ELISAs to assess VHH binding to the Stx2 toxin. ELISAs were performed using plates coated with 1.5 μg/ml of Stx2. Binding was plotted as a function of VHH concentration. Plots for VHH heterodimers are displayed by dotted lines, and the VHH heterotrimer is displayed as a dashed line. VHH names are as shown in Tables 1 and 2. Panels A, B, C, and D compare dilution ELISAs of related samples within the same assay. ELISA results shown are representative of at least one other study and are supported by SPR data shown in the tables.
Selected Stx-binding VHHs were further characterized for affinity and subunit recognition. Binding affinities (equilibrium dissociation constant [KD]) were determined by performing surface plasmon resonance (SPR). These data correlated well with EC50s (Table 1) and confirmed the Stx cross-specificity of Stx-A4 and Stx-A5. Several VHHs displayed KD values in the subnanomolar range, indicating very high affinity. Western blot analysis (see Fig. S3 in the supplemental material) detected binding to the Stx1 and Stx2 subunits following SDS-PAGE and specificity matched the ELISA and SPR data. Surprisingly, all VHHs recognize the Stx B subunits except for Stx1-D4, which recognizes the Stx1 A subunit. Despite the high affinity of these VHHs for native Stxs, binding to the denatured Stx1/2 B subunits on the Western blots was generally poor, suggesting that these VHHs recognize conformationally sensitive epitopes. Stx subunit binding for the VHHs as reported in Table 1 was confirmed by ELISAs using purified recombinant Stx subunits (not shown).
Table 1.
Properties of VHHs recognizing Stx1 and/or Stx2
| VHH name | Clone | Protein | Specificity | Subunita |
KD (nM) forb: |
Neutralizing activity (nM) against: |
GenBank accession no. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stx1 |
Stx2 |
||||||||||
| Stx1 | Stx2 | EC50c | IC50d | EC50c | IC50d | ||||||
| Stx1-A9 | JFA-26 | JET-A9 | Stx1 | B | 7.6 ± 0.9 | NB | 10 | 10 | >1,000 | >1,000 | KF551949 |
| Stx1-D4 | JGL-8 | JGG-D4 | Stx1 | A | 0.128 ± 0.006 | NB | 0.5 | >1,000 | >1,000 | >1,000 | KF551950 |
| Stx-A4 | JFL-17 | JFD-A4 | Stx1/Stx2 | B | 7.2 ± 0.8 | 12 ± 4 | 30 | >330 | 10 | 50 | KF551951 |
| Stx-A5 | JFL-29 | JFD-A5 | Stx1/Stx2 | B | 12.5 ± 0.9 | 7.7 ± 0.5 | 15 | 100 | 1 | 10 | KF551952 |
| Stx2-A6 | JFA-31 | JEU-A6 | Stx2 | B | NB | 5 ± 2 | >1,000 | ND | 1 | 5 | KF551953 |
| Stx2-D2 | JFA-36 | JEU-D2 | Stx2e | B | NB | 7.0 ± 0.9 | >1000 | ND | 2 | 20 | KF551954 |
| Stx2-D10 | JFL-47 | JEN-D10 | Stx2 | B | NB | 0.21 ± 0.01 | >1000 | >1,000 | 0.3 | 0.7 | KF551955 |
| Stx2-G1 | JGL-34 | JGH-G1 | Stx2 | B | NB | 0.023 ± 0.003 | >1,000 | >1,000 | 0.1 | 0.04 | KF551956 |
| Stx2-G9 | JGL-40 | JGH-G9 | Stx2e | B | NB | 19 ± 2 | >1,000 | >1,000 | 2 | 3 | KF551957 |
| Stx2-H6 | JFL-88 | JFG-H6 | Stx2 | B | NB | 0.41 ± 0.01 | >1,000 | >1,000 | 0.5 | 1 | KF551958 |
Stx binding studies are complicated by the fact that Stx1 and Stx2 both contain a pentameric B subunit and thus VHHs binding to the B subunit have the potential to bind at five separate sites on each toxin molecule. SPR analyses were unable to detect differences in the extent of binding between the various VHHs recognizing Stx A or B subunits, and no conclusions could be reached as to the binding valency. Consequently, we were unable to assess the influence of valency on the binding kinetics.
Competition ELISAs demonstrated that VHHs binding to the B subunit, including both the Stx-specific VHHs and the cross-specific VHHs, displayed some ability to compete for the binding of the other VHHs recognizing B subunits of the same toxinotype. VHHs with a lower KD for Stx, as expected, were stronger competitors than the other VHHs. The results imply that all VHHs recognizing the Stx B subunit bind at the same or overlapping epitopes or induce conformational changes that reduce binding by other VHHs.
Binding properties of Stx-binding VHH heterodimers.
We previously showed that linking of two toxin-neutralizing VHHs into heterodimers that also contain two epitopic tags (called a VHH-based neutralizing agent [VNA]) neutralized the toxin target and protected animals from intoxication. Antitoxin protection, especially with high-dose challenge, was enhanced by coadministration of an antitag effector antibody (efAb) (28). As shown in Fig. 3A, the doubly tagged heterodimer directs four efAb molecules to the toxin, leading to clearance from the serum (30). To test this strategy with Shiga toxins, several heterodimeric VNAs were generated by fusing different combinations of two VHHs that target Stx1 and/or Stx2. The five heterodimeric VNAs with highest affinities for Stx1 and Stx2 are listed in Table 2. The binding properties of these VNAs were assessed by dilution ELISAs (Fig. 1 and 2), and EC50s were estimated (Table 2).
Fig 3.
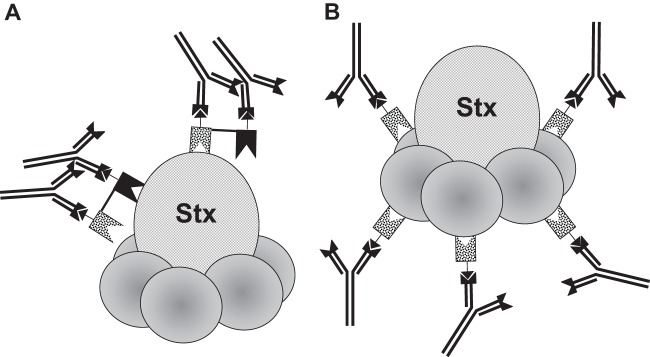
Binding of multiple efAb molecules to Shiga toxin directed by a double-tagged VHH heterodimer targeting two epitopes (called a VNA) or to a single-tagged VHH monomer, which binds the pentameric B subunit. (A) A VHH heterodimer VNA may bind to a toxin, such as Shiga toxin (Stx), at two separate, nonoverlapping epitopes. If the heterodimer contains two copies of an epitopic “tag,” then two molecules of the antitag efAb may bind each bound heterodimer molecule, leading to decoration of each toxin molecule by four efAb molecules. (B) A VHH monomer that binds to an epitope that is present at multiple sites on the toxin, such as the pentameric B subunit of Stx, may bind at multiple sites on the toxin. If the VHH contains an epitopic tag, the efAb may decorate each toxin molecule at five sites.
Table 2.
Properties of VHH heteromultimers recognizing Stx1 and/or Stx2
| Heteromultimer name | Clone | Specificity |
KD (nM)a for: |
Neutralizing activity (nM) against: |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stx1 |
Stx2 |
|||||||||
| VHH 1 | VHH 2 | VHH 3 | Stx1 | Stx2 | EC50b | IC50c | EC50b | IC50c | ||
| A5/A4 | JFX-10 | Stx-A5 | Stx-A4 | None | 0.74 ± 0.04 | 0.9 ± 0.1 | 0.4 | 0.3 | 0.3 | 0.05 |
| A9/A4 | JFX-27 | Stx1-A9 | Stx-A4 | None | 0.50 ± 0.03 | 80 ± 20 | 0.5 | 0.05 | 50 | >100 |
| A9/D4 | JGX-2 | Stx1-A9 | Stx1-D4 | None | 1.2 ± 0.4 | NB | 0.6 | 1 | ND | >100 |
| A5/D10 | JFX-16 | Stx-A5 | Stx2-D10 | None | 9.2 ± 0.8 | 0.20 ± 0.01 | 30 | 50 | 0.8 | 0.02 |
| G1/D10 | JGX-19 | Stx2-G1 | Stx2-D10 | None | NB | 0.004 ± 0.005 | >1,000 | >100 | 0.3 | 0.04 |
| A9/A5/D10 | JFZ-29 | Stx1-A9 | Stx-A5 | Stx2-D10 | 0.71 ± 0.03 | 0.7 ± 0.1 | 0.3 | 0.08 | 0.3 | 0.03 |
| A9/A5/G1 | JHO-2 | Stx1-A9 | Stx-A5 | Stx2-G1 | 0.46 ± 0.02 | 0.09 ± 0.02 | 0.5 | 0.05 | 0.3 | 0.04 |
In most cases, heterodimeric VNAs displayed substantially lower EC50 and KD values than either VHH alone (Table 2), suggesting that linking the VHHs together improves target affinity. Enhanced binding affinities were unambiguous when the two-component VHHs had lower target affinity, such as with the Stx cross-specific VHHs, Stx-A4 and Stx-A5. In this case, monomer VHHs displayed EC50 and KD values in the range of 1 to 30 nM for both Stx1 and Stx2, while the A5/A4 heterodimer displayed subnanomolar values, about 10× improvements. Similar improvements were observed with other heterodimers (e.g., A9/A4 and G1/D10) when both VHH components recognized the same Stx toxinotype. Some heterodimer combinations, such as A9/D4 and A5/D10, did not improve affinity compared to results with the component monomers, possibly because both VHHs did not recover full function expressed in linked form.
Shiga toxin neutralization properties of VHH-based agents recognizing Stx1 and/or Stx2.
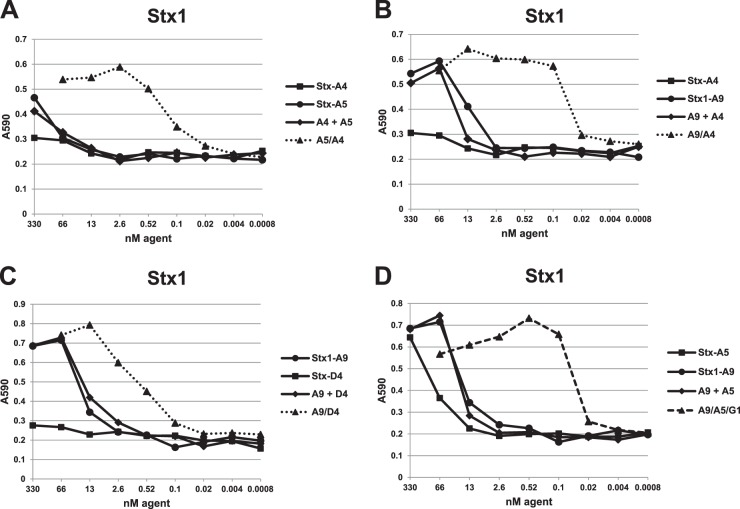
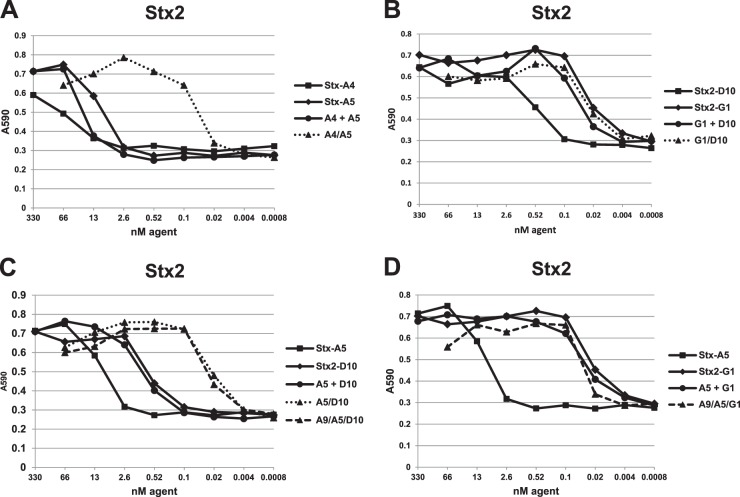
Stx1- and Stx2-binding VHHs were assessed for their toxin neutralization potency in a cell-based assay. Dilution assays are shown in Fig. 4 for Stx1 and in Fig. 5 for Stx2. The results, including the IC50 estimates from serial dilution assays, are summarized in Tables 1 and 2. All of the VHHs in Table 1, except Stx1-D4 (Fig. 4C), displayed some ability to neutralize one or both of the Stxs (Fig. 4 and 5). As expected, none of the VHHs tested showed neutralizing activity on an Stx for which no binding was detected by ELISA or SPR. VNAs with low ELISA EC50s displayed low cell-based neutralizing IC50s, indicating that toxin affinity plays an important role in neutralization. The cross-specific VHH monomers Stx-A4 and -A5 displayed substantially higher IC50s than EC50s and were poor toxin neutralizers as monomers. The Stx1- or Stx2-specific VNAs generally displayed IC50s that were equal to or slightly lower than the EC50s and were as low as 100 pM for Stx2-specific VHH Stx2-G1.
Fig 4.
Stx1 toxin neutralization by VHH-based agents in a cell-based assay. An Stx1 dose (∼15 pmol) that induced nearly 100% Vero cell killing after 48 h was selected. A VHH monomer, VHH monomer pool, or VHH heterodimer, as labeled, was premixed with Stx1 in culture medium and applied to Vero cells. Toxin neutralization was assessed after 48 h by cell staining at A590 as described in Materials and Methods. The extent of cell staining was plotted as a function of the VHH agent concentration employed. Plots for VHH heterodimers are displayed as dotted lines, and the VHH heterotrimer is displayed as a dashed line. Results shown are from one experiment and are representative of at least three independent experiments. VHH names are as shown in Tables 1 and 2.
Fig 5.
Stx2 toxin neutralization by VHH-based agents in a cell-based assay. An Stx2 dose (∼35 pmol) that induced nearly 100% Vero cell killing after 24 h was selected. Neutralization assays were performed as for Fig. 4. Plots for VHH heterodimers are displayed as dotted lines, and the VHH heterotrimers are displayed as dashed lines. Results shown are from one experiment and are representative of at least three independent experiments. VHH names are as shown in Tables 1 and 2.
Stx neutralization by the heterodimeric VNAs listed in Table 2 was assessed by comparing their IC50s with those of equimolar pools of their two-component monomers. As shown in Fig. 4 and 5, neutralization potency of monomer pools is never greater than that of the most potent monomer in the pool. In contrast, linking VHHs into a heterodimeric VNA almost always improved neutralization potency. This was most apparent with the poorly neutralizing Stx cross-specific VHHs, Stx-A4 and StxA5, for which the A4/A5 heterodimer potency on both Stx1 and Stx2 was 100-fold greater than that of the pool of monomer VHH components (Fig. 4A and 5A and Tables 1 and 2). Similar major improvements in potencies of heterodimeric VNAs compared to those of monomer pools were observed with Stx-A4 and Stx1-A9 (Fig. 4B) and Stx-A5 and Stx2-D10 (Fig. 5C). Interestingly, a heterodimer joining the nonneutralizing VHH, Stx1-D4, and the neutralizing VHH, Stx1-A9, was substantially more potent at neutralizing Stx1 than was an equimolar treatment with Stx1-D4 and Stx1-A9 monomers, suggesting that the improvement in affinity afforded by the A9/D4 heterodimer versus that with the Stx1-A9 monomer (Tables 1 and 2) is sufficient to improve the neutralizing potency.
Only one heterodimeric VNA, G1/D10, did not achieve Stx neutralization potency greater than those of the component monomers (Fig. 5B). This is likely because the neutralizing IC50 of the monomer, Stx2-G1, at 40 pM, is approximately the same as the Stx2 concentration (35 pM) used in the cell-based assay. Since neutralization is expected to require at least a 1:1 stoichiometric ratio of agent/toxin, further improvement in potency may not be possible even if higher-affinity VHHs are identified (Tables 1 and 2). By this analysis, many of the VHH-based anti-Stx VNAs in Table 2 were found to be effective at Stx neutralization when combined at equimolar ratios to the toxin target (e.g., A9/A4 with Stx1 and for A5/A4, A5/D10, and G1/D10 with Stx2).
VHH heteromultimers that recognize both Shiga toxins Stx1 and Stx2.
An ideal antitoxin agent for the Shiga toxins would be a single protein capable of neutralizing both Stx1 and Stx2. Because some neutralizing VHHs were cross-specific for both Stx1 and Stx2, we engineered VNAs that included one Stx cross-specific VHH and two neutralizing VHHs specific to either Stx1 or Stx2. Two such heterotrimeric VNAs were produced, one combining Stx1-A9, Stx-A5, and Stx2-D10 (A9/A5/D10) and another combining Stx1-A9, Stx-A5, and Stx2-G1 (A9/A5/G1). Each VHH in the VNAs was separated by a flexible spacer region (GGGGS)3, and a copy of the E-tag peptide was present at the amino and carboxyl sides of the VHH heterotrimer.
The Stx-binding properties of the two heterotrimer VNAs were characterized by ELISAs and neutralization assays. Figures 1D and 2C and D and Table 2 show that the VNAs have EC50 binding properties in the subnanomolar range for both toxins. The EC50 and KD values of the heterotrimeric VNAs (Table 2) were similar to those of corresponding heterodimer VNAs, indicating that the full binding functions of all three VHHs in the heterotrimers were retained. Both Stx-binding heterotrimer VNAs also showed excellent neutralization properties against both Stx1 and Stx2 in cell-based assays (Fig. 4D and 5C and D). In fact, the IC50 estimates for the heterotrimer VNAs were near the toxin concentrations for both Stx1 and Stx2, implying that each agent was able to neutralize both toxins when present at concentrations nearly equimolar to those of the toxins (Table 2) in these assays. Thus, a single heterotrimer VNA consisting of a high-affinity Stx1-binding VHH, a high-affinity Stx2-binding VHH, and a moderate-affinity Stx cross-specific VHH is capable of potent neutralization of both Stx1 and Stx2.
Protection from Shiga toxin intoxication in mice using VHH-based antitoxin agents.
Stx1- and Stx2-binding monomer VHHs and heteromultimeric VNAs were tested for the ability to protect mice from Stx lethality. For these studies, 40 pmol of VHH or VNA was coadministered with toxin. A 1.25× minimal lethal dose (MLD) of Stx1 was found to be about 20 pmol, while for Stx2, this was about 1 pmol. As a result of the different Stx potencies, the doses of VHH-based agents used were at about 2-fold molar excess to Stx1 and about 40-fold excess to Stx2.
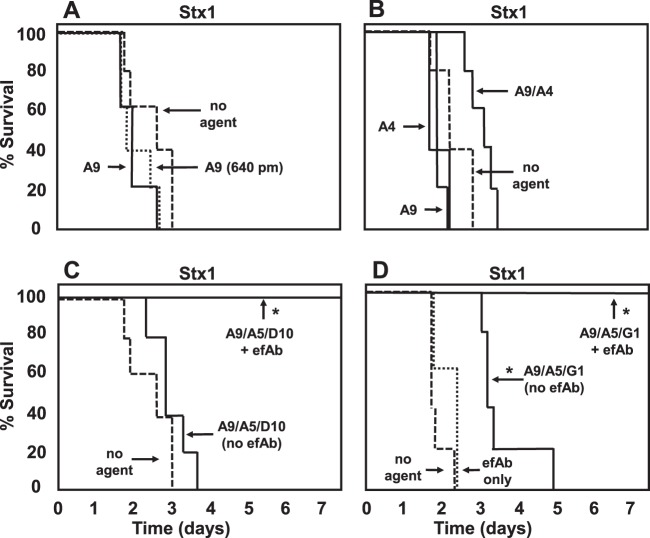
All Stx1-binding monomer VHHs in Table 1 were tested for in vivo efficacy, and none led to improved survival (examples are shown in Fig. 6A and B). To test whether this was due to the small molar excess employed, a series of 2-fold-higher doses of Stx1-A9 was employed, up to 16-fold (640 pmol), which led to no apparent improvement in efficacy (Fig. 6A). Use of heterodimer or heterotrimer VNAs resulted in a small yet reproducible extension in the time to death in mice intoxicated with Stx1 (some examples are shown in Fig. 6B to D). No significant improvement in survival was detected using a 2-fold-higher dose (not shown). To determine whether efficacy could be improved by promoting clearance of Stx1, the anti-E-tag efAb was coadministered with either of the two heterotrimer VNAs. An 80-pmol dose of this efAb was employed to provide sufficient Ab to bind to both copies of the tag present on each of the heterotrimer VNAs, thus leading to toxin decoration by up to four efAbs. Inclusion of the efAb resulted in complete protection of mice from clinical signs and death due to Stx1 (Fig. 6C and D). Administration of efAb alone had no effect on the survival of mice given 1.25 MLD of Stx1 or Stx2.
Fig 6.
Protection from Stx1 lethality in mice by treatment with VHH-based agents. Groups of five mice were injected with 20 pmol of Stx1 premixed with 40 pmol of the labeled VHH-based antitoxin agent (or 640 pmol of VHH-A9 where indicated) and monitored for illness and death for 1 week. The percent survival is plotted as a function of time. In some animals, an 80-pmol dose of efAb was included in the treatment. VHH names are as listed in Tables 1 and 2. Test agents that led to significant (P < 0.01) protection of mice compared to results for “no agent”-treated mice are indicated with an asterisk. Mice receiving agents plus efAb were significantly protected (P < 0.01) compared to mice receiving the same agent without efAb. Results shown are from one experiment and are representative of at least two independent experiments with each agent.
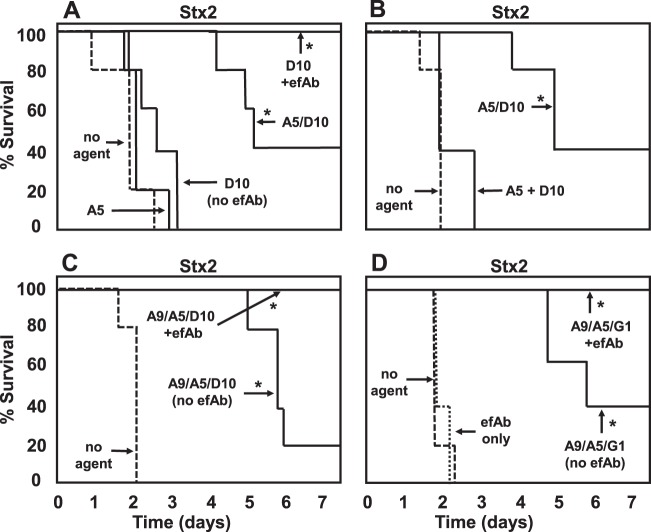
With Stx2 intoxication, monomer neutralizing VHHs did not improve survival (examples are shown in Fig. 7A and B). A beneficial effect on survival was observed with heteromultimeric VNAs for Stx2-intoxicated mice (Fig. 7B to D); however, these mice had signs of intoxication (lethargy, dehydration, and excessive urination). In contrast, when clearance of Stx2 from serum was promoted by coadministering efAb with the VNA, 100% of mice survived in all groups (e.g., Fig. 7C and D) and displayed no signs of intoxication.
Fig 7.
Protection from Stx2 lethality in mice by treatment with VHH-based agents. Groups of five mice were injected with 1 pmol of Stx2 premixed with 40 pmol of the indicated VHH-based antitoxin agent, and the protection assays were performed as for Fig. 6. In some animals, an 80-pmol dose of efAb was included in the treatment. VHH names are as listed in Tables 1 and 2. Test agents that led to significant (P < 0.01) protection of mice compared to results for “no agent”-treated mice are indicated with an asterisk. Mice receiving agents plus efAb were significantly protected (P < 0.01) compared to mice receiving the same agent without efAb. Results shown are from one experiment representative of at least two independent experiments. The Stx2-D10 + efAb agent was tested in only one experiment, but identical results were obtained in tests using two other B-subunit-binding VHHs (Stx2-G1 and Stx2-H6) administered with efAb. Of note, results with A9/A5/G1, with or without efAb, were replicated in four independent experiments, including one in which toxin and agent (with or without efAb) were administered separately (intravenous [i.v.] toxin, intraperitoneal [i.p.] agent).
Decorating Stx with efAb to promote clearance by targeting pentameric B subunits.
The Stxs consist of a single A subunit and five B subunits. VHHs that bind to the B subunit thus have the potential to bind at five separate sites on each Stx molecule. If each VHH binds to a single efAb, the toxin could become decorated by up to five Ab molecules (see Fig. 3B), which should be sufficient to promote serum clearance (30). In prior studies (28), we observed no improved protection when monomeric VHHs recognizing a single toxin epitope were coadministered with efAb. In contrast, coadministering efAb with monomeric VHHs recognizing the pentameric B subunit of Stx frequently provided substantial improvements in survival of toxin challenge. One example, employing monomeric Stx2-D10, is shown in Fig. 7A. In the absence of efAb, the monomeric, toxin-neutralizing VHH delayed death for a day or two, but the animals invariably died, while coadministration of efAb resulted in 100% survival. Virtually identical results were observed in separate studies testing two additional B-subunit-binding, single-tagged monomeric VHHs, Stx2-G1 and Stx2-H6 (not shown).
Treatment with VNAs and efAb protects mice from Stx2-induced kidney toxicity.
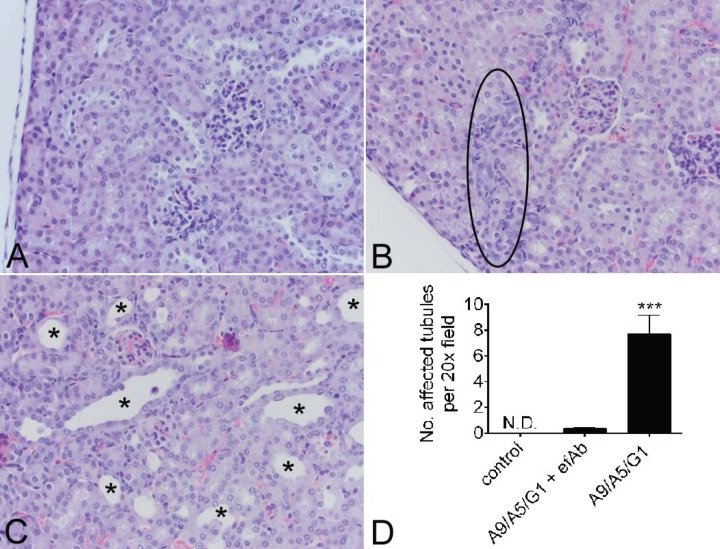
Stx2-intoxicated mice that survived due to treatment only with the heteromultimer VNA, A9/A5/G1, showed signs of kidney damage due to intoxication (lethargy, dehydration, and excessive urination). The kidneys from mice surviving 1.25 MLD of Stx2 treated with the A9/A5/G1 VNA alone had evidence of damage to distal tubular epithelial cells. Affected tubules demonstrated epithelial cell changes (apoptosis/necrosis, attenuation and restitution, hypertrophy, hyperplasia, and luminal dilation) and additional lesions (tubular atrophy/collapse, interstitial cell proliferation, and early interstitial fibrosis). In contrast, damaged tubules were difficult to identify and significantly reduced in mice that received A9/A5/G1 plus the efAb (Fig. 8D). A representative image from an untreated age- and sex-matched control kidney with no lesions is shown (Fig. 8A), revealing minimal kidney damage with A9/A5/G1 plus efAb (Fig. 8B) and severe distal tubular lesions in mice that received this VNA alone (Fig. 8C). The tubular epithelial lesions are consistent with the stereotypical reparative responses secondary to death of tubular epithelial cells due to Stx2 (unpublished observations) and with Stx2-induced tubular epithelial cell apoptosis (16). Together with previous results, this suggests that A9/A5/G1 VNA and efAb treatment averted kidney damage by promoting both toxin neutralization and clearance.
Fig 8.
VNA plus efAb protects mice from Stx2-induced renal damage. Formalin-fixed, paraffin-embedded, hematoxylin-and-eosin-stained 3-μm sections were examined by light microscopy from untreated age- and sex-matched controls (A) (representative image), mice receiving the A9/A5/G1 VNA plus efAb (B) (representative image), and mice receiving only this VNA (C) (representative image). The numbers of tubules with lesions (epithelial apoptosis/necrosis, attenuation and restitution, hypertrophy, hyperplasia, luminal dilation, tubular atrophy/collapse, interstitial cell proliferation, and early interstitial fibrosis) were quantified in 6 random 20× fields per mouse, totaling 114 measurements (D). Examples of lesions are highlighted by the black oval in panel B and the asterisks in panel C. ND, none detected. Results shown are from one experiment with 3 to 5 mice per group and reproduced results from one previous study.
DISCUSSION
We previously reported a novel antitoxin strategy that employs a VHH-based neutralizing agent (VNA), consisting of two antitoxin VHHs flanked by two copies of an epitopic tag, to direct the binding of up to four antitag effector Ab (efAb) molecules to the toxin and promote both toxin neutralization and toxin clearance from serum (28). Here we created and tested VNAs in which a single protein agent neutralizes both of the Shiga toxins produced by Shiga-like toxin-producing E. coli (STEC) infection. STEC disease can lead to serious, sometimes fatal complications, such as HUS and encephalopathy, for which no specific therapy currently exists. When these VNAs were administered together with the efAb to mice, Shiga toxin-induced mortality was mitigated and renal damage was minimal.
To develop an antitoxin agent effective against both Shiga toxins, we identified and expressed VHHs capable of binding Stx1 and/or Stx2. The VHHs were characterized for their subunit specificity and their toxin binding and neutralization properties. Most Shiga toxin-binding VHHs recognized the B subunit, and these VHHs neutralized their targets in cell assays. Surprisingly, one class of B-subunit-binding VHHs recognized both Stx1 and Stx2. Donohue-Rolfe et al. (32) described a MAb (4D1) with similar binding characteristics. Only one Shiga toxin-binding VHH, an Stx1-specific VHH (Stx1-D4), recognized the A subunit, and this proved incapable of neutralizing either toxin. In total, 9/10 of the unique VHHs tested (Table 1) proved capable of neutralizing their targets, a much higher proportion than previously observed with toxin-binding MAbs (36). This high proportion may be related to the reported ability of VHHs to bind preferentially to active site grooves on their targets (37).
Our antitoxin strategy uses VNAs consisting of two or more linked, toxin-neutralizing VHHs recognizing nonoverlapping epitopes on the toxin. VHH heteromultimers were initially developed to facilitate the decoration of toxins at multiple sites so as to promote clearance of the toxin from serum when the VNA is coadministered with efAb (28). Studies described here highlight another frequent advantage of linking VHHs together: increased toxin binding affinity and potency of neutralization. In every instance tested, VHH heterodimer VNAs functioned more effectively as antitoxins in cell and animal assays than did equimolar pools of the component VHHs. In some cases, linking VHHs into VNAs improved the antitoxin potency as much as 100-fold (Fig. 4 and 5 and Table 2) and substantially improved in vivo efficacy (Fig. 6 and 7).
The identification of cross-specific VHHs that recognized Stx1 and Stx2 made possible the development of a VHH heterotrimer VNA capable of binding to two separate epitopes on each of the two Shiga toxins. Although these cross-specific VHHs were relatively poor at toxin neutralization on their own, when these VHHs were linked to an Stx1- or Stx2-specific VHH, the resulting heterodimers proved to be extremely potent, displaying subnanomolar in vitro IC50s. Doubly tagged VHH heterotrimer VNAs consisting of a cross-specific VHH linked to an Stx1-specific VHH and an Stx2-specific VHH were prepared. These agents retained high toxin-neutralizing potency and were effective in protecting mice from exposure to both Shiga toxins, especially when coadministered with the efAb (Fig. 6 and 7).
These studies lead to a better appreciation of the importance of toxin clearance to the efficacy of antitoxin therapies. The contribution of serum clearance to improved efficacy was most apparent with Stx1, probably because this toxin is less potent in mice. Since a 20-fold-higher dose of Stx1 was required for an MLD than with Stx2, the molar excess of VNA to toxin was 20-fold less with Stx1, and this may have contributed to the poor efficacy of the antitoxin VNAs in protecting mice from toxemia and death. By including the efAb to promote serum clearance, Stx1 becomes decorated with up to four efAbs and is thus rapidly cleared through the liver (30), and this treatment resulted in the complete asymptomatic survival of all mice. The important role of serum clearance was less dramatically demonstrated with Stx2. In this model, mice often survived 1.25 MLD of toxin when given the VNA alone but developed demonstrable kidney damage. Coadministration of efAb fully protected the mice receiving Stx2 from death and kidney pathology.
Since Shiga toxins, which inactivate ribosomes, should be toxic to virtually all mammalian cells they enter, a concern existed that clearance of Shiga toxins using VNAs coadministered with efAb might lead to selective killing of cells responsible for the clearance. Previous results (30) demonstrated that agent clearance occurs in the liver, presumably by low-affinity Fc-receptor-mediated endocytosis primarily in Kupffer cells (27). Selective killing of these important cells could be a consequence of promoting Shiga toxin clearance. We found that mice treated with VNAs together with efAb did not display clinical signs or microscopic evidence of liver damage (not shown), perhaps because toxin neutralization by VNAs continued after cell uptake.
Our goal is to employ VNAs to treat the disease associated with STEC infection. Shiga toxins, especially Stx2, cause neurological signs and kidney damage in rodents and cause STEC-associated HUS in humans. Several groups generated and tested anti-Stx MAb-based treatments for STEC infection (20–24), and their use has shown promise in animal models (23, 25). However, to ensure protection against both Shiga toxins, such treatments will likely require at least two MAbs that potently neutralize each toxin, and further MAbs may be required to promote serum clearance. Therapeutic agents to prevent HUS consisting of multiple MAbs will likely be complicated and expensive to develop, manufacture, and test in clinical trials. We believe VNA antitoxins could lead to more practical and effective therapies for STEC infection.
A major consideration in development of treatments that prevent HUS must be the timing of the kidney injury in relation to the onset of gastrointestinal (GI) symptoms. If kidney injury occurs early in infection and prior or simultaneous to the onset of bloody diarrhea, as has been suggested (38), then inactivation of toxins is unlikely to improve the outcome unless it is administered prior to these symptoms. This might be possible, for example, by treating patients who display early signs of gastrointestinal upset or patients suspected to have ingested food contaminated by STEC. Treatment of large populations only considered to be at potential risk of STEC infection would be impractical unless the treatment was extremely safe and inexpensive.
A single VNA that neutralize both Shiga toxins makes possible new, more practical approaches to preventing STEC sequelae. One option would be to engineer gene therapy vehicles, such as adenoviruses, that promote transient secretion of the VNA (and efAb if enhanced potency was needed) into the circulation. Alternatively, strategies for oral delivery of a VNA that are sufficiently safe and economical to permit prophylactic use in at-risk populations may be possible. For example, a VNA could be expressed and secreted in the GI tract by genetically engineered commensal bacteria, similar to an approach employed to treat inflammatory bowel disease in an animal model (39). Alternatively, a VNA could be delivered to the GI tract in capsules or other vehicles that protect the agent through the stomach.
In summary, we have found that a single VNA that is capable of neutralizing both Shiga toxins, coadministered with efAb to promote toxin clearance, can effectively protect mice from lethal doses of Stx1 and Stx2. Since the single agent neutralizes both Shiga toxins, it should be capable of protecting patients from STEC sequelae, such as HUS. The simplicity of the agent and its ease of production make possible a variety of alternative treatment strategies, including genetic and oral delivery routes. Further studies will determine the optimal doses, timing, and route of delivery in STEC models. Ultimately, it is hoped that these agents will form the basis of treatments that reduce or eliminate the tissue damage due to Shiga toxin(s) and STEC-associated HUS.
Supplementary Material
ACKNOWLEDGMENTS
We thank Susan Chapman-Bonofiglio for her assistance in establishing the cell-based neutralization assay. We also thank Stephanie Reilly for excellent assistance with alpaca immunization and mouse assays and Ocean Cohen for technical assistance. We thank David Vance for critical reading of the manuscript.
This project has been supported in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract number N01-AI-30050 and award number U54 AI057159.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Published ahead of print 30 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01033-13.
REFERENCES
- 1.Buchholz U, Bernard H, Werber D, Bohmer MM, Remschmidt C, Wilking H, Delere Y, an der Heiden M, Adlhoch C, Dreesman J, Ehlers J, Ethelberg S, Faber M, Frank C, Fricke G, Greiner M, Hohle M, Ivarsson S, Jark U, Kirchner M, Koch J, Krause G, Luber P, Rosner B, Stark K, Kuhne M. 2011. German outbreak of Escherichia coli O104:H4 associated with sprouts. N. Engl. J. Med. 365:1763–1770 [DOI] [PubMed] [Google Scholar]
- 2.Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, Bernard H, Fruth A, Prager R, Spode A, Wadl M, Zoufaly A, Jordan S, Kemper MJ, Follin P, Muller L, King LA, Rosner B, Buchholz U, Stark K, Krause G. 2011. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N. Engl. J. Med. 365:1771–1780 [DOI] [PubMed] [Google Scholar]
- 3.Karmali MA, Petric M, Lim C, Fleming PC, Arbus GS, Lior H. 1985. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J. Infect. Dis. 151:775–782 [DOI] [PubMed] [Google Scholar]
- 4.Melton-Celsa A, Mohawk K, Teel L, O'Brien A. 2012. Pathogenesis of shiga-toxin producing Escherichia coli. Curr. Top. Microbiol. Immunol. 357:67–103 [DOI] [PubMed] [Google Scholar]
- 5.Boerlin P, McEwen SA, Boerlin-Petzold F, Wilson JB, Johnson RP, Gyles CL. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37:497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedrich AW, Bielaszewska M, Zhang WL, Pulz M, Kuczius T, Ammon A, Karch H. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J. Infect. Dis. 185:74–84 [DOI] [PubMed] [Google Scholar]
- 7.Hedican EB, Medus C, Besser JM, Juni BA, Koziol B, Taylor C, Smith KE. 2009. Characteristics of O157 versus non-O157 Shiga toxin-producing Escherichia coli infections in Minnesota, 2000–2006. Clin. Infect. Dis. 49:358–364 [DOI] [PubMed] [Google Scholar]
- 8.Kawano K, Okada M, Haga T, Maeda K, Goto Y. 2008. Relationship between pathogenicity for humans and stx genotype in Shiga toxin-producing Escherichia coli serotype O157. Eur. J. Clin. Microbiol. Infect. Dis. 27:227–232 [DOI] [PubMed] [Google Scholar]
- 9.Lingwood CA, Law H, Richardson S, Petric M, Brunton JL, De Grandis S, Karmali M. 1987. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J. Biol. Chem. 262:8834–8839 [PubMed] [Google Scholar]
- 10.Cohen A, Hannigan GE, Williams BR, Lingwood CA. 1987. Roles of globotriosyl- and galabiosylceramide in verotoxin binding and high affinity interferon receptor. J. Biol. Chem. 262:17088–17091 [PubMed] [Google Scholar]
- 11.Johannes L, Romer W. 2010. Shiga toxins—from cell biology to biomedical applications. Nat. Rev. Microbiol. 8:105–116 [DOI] [PubMed] [Google Scholar]
- 12.Spooner RA, Lord JM. 2012. How ricin and Shiga toxin reach the cytosol of target cells: retrotranslocation from the endoplasmic reticulum. Curr. Top. Microbiol. Immunol. 357:19–40 [DOI] [PubMed] [Google Scholar]
- 13.O'Brien AD, Tesh VL, Donohue-Rolfe A, Jackson MP, Olsnes S, Sandvig K, Lindberg AA, Keusch GT. 1992. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr. Top. Microbiol. Immunol. 180:65–94 [DOI] [PubMed] [Google Scholar]
- 14.Cherla RP, Lee SY, Tesh VL. 2003. Shiga toxins and apoptosis. FEMS Microbiol. Lett. 228:159–166 [DOI] [PubMed] [Google Scholar]
- 15.Obrig TG, Louise CB, Lingwood CA, Boyd B, Barley-Maloney L, Daniel TO. 1993. Endothelial heterogeneity in Shiga toxin receptors and responses. J. Biol. Chem. 268:15484–15488 [PubMed] [Google Scholar]
- 16.Psotka MA, Obata F, Kolling GL, Gross LK, Saleem MA, Satchell SC, Mathieson PW, Obrig TG. 2009. Shiga toxin 2 targets the murine renal collecting duct epithelium. Infect. Immun. 77:959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoja C, Buelli S, Morigi M. 2010. Shiga toxin-associated hemolytic uremic syndrome: pathophysiology of endothelial dysfunction. Pediatr. Nephrol. 25:2231–2240 [DOI] [PubMed] [Google Scholar]
- 18.Wong CS, Mooney JC, Brandt JR, Staples AO, Jelacic S, Boster DR, Watkins SL, Tarr PI. 2012. Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: a multivariable analysis. Clin. Infect. Dis. 55:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt JM. 2010. Shiga toxin-producing Escherichia coli (STEC). Clin. Lab Med. 30:21–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamagami S, Motoki M, Kimura T, Izumi H, Takeda T, Katsuura Y, Matsumoto Y. 2001. Efficacy of postinfection treatment with anti-Shiga toxin (Stx) 2 humanized monoclonal antibody TMA-15 in mice lethally challenged with Stx-producing Escherichia coli. J. Infect. Dis. 184:738–742 [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee J, Chios K, Fishwild D, Hudson D, O'Donnell S, Rich SM, Donohue-Rolfe A, Tzipori S. 2002. Human Stx2-specific monoclonal antibodies prevent systemic complications of Escherichia coli O157:H7 infection. Infect. Immun. 70:612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee J, Chios K, Fishwild D, Hudson D, O'Donnell S, Rich SM, Donohue-Rolfe A, Tzipori S. 2002. Production and characterization of protective human antibodies against Shiga toxin 1. Infect. Immun. 70:5896–5899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tzipori S, Sheoran A, Akiyoshi D, Donohue-Rolfe A, Trachtman H. 2004. Antibody therapy in the management of shiga toxin-induced hemolytic uremic syndrome. Clin. Microbiol. Rev. 17:926–941, table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowling TC, Chavaillaz PA, Young DG, Melton-Celsa A, O'Brien A, Thuning-Roberson C, Edelman R, Tacket CO. 2005. Phase 1 safety and pharmacokinetic study of chimeric murine-human monoclonal antibody c alpha Stx2 administered intravenously to healthy adult volunteers. Antimicrob. Agents Chemother. 49:1808–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauter KA, Melton-Celsa AR, Larkin K, Troxell ML, O'Brien AD, Magun BE. 2008. Mouse model of hemolytic-uremic syndrome caused by endotoxin-free Shiga toxin 2 (Stx2) and protection from lethal outcome by anti-Stx2 antibody. Infect. Immun. 76:4469–4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies KA, Robson MG, Peters AM, Norsworthy P, Nash JT, Walport MJ. 2002. Defective Fc-dependent processing of immune complexes in patients with systemic lupus erythematosus. Arthritis Rheum. 46:1028–1038 [DOI] [PubMed] [Google Scholar]
- 27.Lovdal T, Andersen E, Brech A, Berg T. 2000. Fc receptor mediated endocytosis of small soluble immunoglobulin G immune complexes in Kupffer and endothelial cells from rat liver. J. Cell Sci. 113(Part 18):3255–3266 [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee J, Tremblay JM, Leysath CE, Ofori K, Baldwin K, Feng X, Bedenice D, Webb RP, Wright PM, Smith LA, Tzipori S, Shoemaker CB. 2012. A novel strategy for development of recombinant antitoxin therapeutics tested in a mouse botulism model. PLoS One 7:e29941. 10.1371/journal.pone.0029941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibbs WW. 2005. Nanobodies. Sci. Am. 293:78–83 [DOI] [PubMed] [Google Scholar]
- 30.Sepulveda J, Mukherjee J, Tzipori S, Simpson LL, Shoemaker CB. 2010. Efficient serum clearance of botulinum neurotoxin achieved using a pool of small antitoxin binding agents. Infect. Immun. 78:756–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacewicz MS, Acheson DW, Binion DG, West GA, Lincicome LL, Fiocchi C, Keusch GT. 1999. Responses of human intestinal microvascular endothelial cells to Shiga toxins 1 and 2 and pathogenesis of hemorrhagic colitis. Infect. Immun. 67:1439–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donohue-Rolfe A, Acheson DW, Kane AV, Keusch GT. 1989. Purification of Shiga toxin and Shiga-like toxins I and II by receptor analog affinity chromatography with immobilized P1 glycoprotein and production of cross-reactive monoclonal antibodies. Infect. Immun. 57:3888–3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tremblay JM, Kuo CL, Abeijon C, Sepulveda J, Oyler G, Hu X, Jin MM, Shoemaker CB. 2010. Camelid single domain antibodies (VHHs) as neuronal cell intrabody binding agents and inhibitors of Clostridium botulinum neurotoxin (BoNT) proteases. Toxicon 56:990–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maass DR, Harrison GB, Grant WN, Shoemaker CB. 2007. Three surface antigens dominate the mucosal antibody response to gastrointestinal L3-stage strongylid nematodes in field immune sheep. Int. J. Parasitol. 37:953–962 [DOI] [PubMed] [Google Scholar]
- 35.Sheoran AS, Chapman-Bonofiglio S, Harvey BR, Mukherjee J, Georgiou G, Donohue-Rolfe A, Tzipori S. 2005. Human antibody against Shiga toxin 2 administered to piglets after the onset of diarrhea due to Escherichia coli O157:H7 prevents fatal systemic complications. Infect. Immun. 73:4607–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow SK, Casadevall A. 2012. Monoclonal antibodies and toxins—a perspective on function and isotype. Toxins (Basel) 4:430–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wesolowski J, Alzogaray V, Reyelt J, Unger M, Juarez K, Urrutia M, Cauerhff A, Danquah W, Rissiek B, Scheuplein F, Schwarz N, Adriouch S, Boyer O, Seman M, Licea A, Serreze DV, Goldbaum FA, Haag F, Koch-Nolte F. 2009. Single domain antibodies: promising experimental and therapeutic tools in infection and immunity. Med. Microbiol. Immunol. 198:157–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarr PI, Gordon CA, Chandler WL. 2005. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365:1073–1086 [DOI] [PubMed] [Google Scholar]
- 39.Vandenbroucke K, de Haard H, Beirnaert E, Dreier T, Lauwereys M, Huyck L, Van Huysse J, Demetter P, Steidler L, Remaut E, Cuvelier C, Rottiers P. 2010. Orally administered L. lactis secreting an anti-TNF nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol. 3:49–56 [DOI] [PubMed] [Google Scholar]
- 40.Maass DR, Sepulveda J, Pernthaner A, Shoemaker CB. 2007. Alpaca (Lama pacos) as a convenient source of recombinant camelid heavy chain antibodies (VHHs). J. Immunol. Methods 324:13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.