Abstract
Exercise training has emerged as an intervention for the primary and secondary prevention of coronary artery disease, but the mechanisms through which training reduces relative risk are not completely understood. The goal of this study was to investigate the impact of endurance exercise training on vasomotor function and vascular cell phenotype in coronary arteries and systemic conduit arteries and veins against a background of advanced atherosclerosis. We tested the hypothesis that exercise training restores endothelial vasomotor function and produces an anti-atherogenic endothelial and smooth muscle cell phenotype in familial hypercholesterolemic (FH) swine. The study included 30 FH (15 EX, 15 SED) and 13 non-FH control male, castrated swine. The exercise training intervention consisted of treadmill running 5 days/wk for 16–20 wks. Tissues sampled at sacrifice included vascular rings from the coronary circulation for vasomotor function experiments (dose-dependent bradykinin-induced vasorelaxation) and ECs from isolated segments of the thoracic aorta, the carotid, brachial, femoral, and renal arteries, and each of these vessel’s regionally associated vein, as well from the abdominal vena cava, the right coronary (RCA), and internal mammary arteries. Smooth muscle cells were sampled from the RCA only. Vascular cell phenotype was assessed by immunoblotting for a host of both pro- and anti-atherogenic markers (e.g., eNOS, p67phox, SOD-1, etc). Coronary artery endothelium-dependent vasomotor function was depressed in sedentary FH-pigs compared to sedentary controls, and exercise training did not change vasomotor function within FH. In contrast, only scattered effects of FH on EC phenotype were noted across the vasculature, which included both pro- and anti-atherogenic changes in EC protein expression (e.g., increased eNOS in carotid artery ECs, decreased p67phox in brachial artery ECs, but decreased expression of the antioxidant protein SOD1 in thoracic vena cava (all P<0.05)). In thoracic vena cava ECs, this deficit was corrected by EX, while no other effects of exercise were observed in conduit vessel EC phenotype. Thus, while EX abrogated the adverse effect of hypercholesterolaemia on thoracic vena cava SOD1 expression, it appears that EX does not produce a consistently improved EC phenotype in either coronary or systemic conduit vessels in this FH swine model.
Keywords: vasomotor function, endothelial cell, phenotype, gene expression, atherosclerosis
Introduction
The beneficial effects of exercise and increased physical activity on cardiovascular morbidity and mortality are well documented (Thompson et al., 2003). Less well understood, however, are the mechanisms through which exercise acts to reduce cardiovascular mortality risk. Numerous potential mechanisms have been suggested, including beneficial impacts of training on traditional and novel risk factors. However, recent data in this area suggest that no more than ~ 60% of the beneficial effect of exercise on cardiovascular risk can be attributed to known mechanisms (e.g., effects mediated through changes in cardiovascular risk factors; (Mora et al., 2007)). It is thought that a substantial fraction of the unidentified mechanisms through which exercise improves cardiovascular risk are the result of direct effects of exercise on the vasculature (Green, 2009), possibly mediated through chronic haemodynamic changes associated with exercise bouts (e.g., increased blood flow and shear stress).
In support of the ‘direct effects of exercise on the vasculature’ hypothesis, several investigations have demonstrated improved vascular endothelial health in human patients after exercise training, most commonly using peripheral assessments of vasomotor reactivity (i.e., flow-mediated vasodilation) (Gokce et al., 2002; Walsh et al., 2003). Few investigations, however, have directly assessed coronary vasomotor reactivity after exercise training (Hambrecht et al., 2000; Hambrecht et al., 2003), and fewer still have assayed endothelial health as assessed by markers of endothelial cell phenotype in cells harvested from human volunteers (Hambrecht et al., 2003; Pierce et al., 2011). Because endothelial vasomotor reactivity is only a single assay of endothelial health/function, it is important to study other metrics of endothelial cell health in response to training, and clearly the requirement of harvesting cells to achieve this presents an obstacle for conducting such studies in humans. In this regard, swine represent an optimal model for such studies because of similarities between humans and pigs in coronary anatomy, vascular structure (layers of smooth muscle) and systemic haemodynamics (Turk & Laughlin, 2004).
Although atherosclerosis exhibits regional selectivity in its severity, several areas of the vasculature may be affected in a given patient. As such, it is important to extend investigation of the mechanisms by which exercise is beneficial beyond the coronary circulation in order to understand how different regions respond to, and may be athero-protected by, exercise training. Therefore, it was the goal of this study to investigate the impact of endurance exercise training on the coronary arteries and systemic conduit arteries and veins against a background of advanced atherosclerosis (using familial hypercholesterolaemic [FH] swine). We tested the hypothesis that exercise training produces improved endothelial vasomotor function and an improved (i.e., anti-atherogenic) endothelial and smooth muscle cell phenotype in FH pigs, and that improved functional and phenotypic profiles would more closely resemble those of healthy control pigs.
Methods
This study was approved by the Animal Care and Use Committee of the University of Missouri, and pigs involved in this research were housed in rooms maintained at 20–23 ºC with a 12:12 hour light-dark cycle.
Experimental Animals
Castrated male adult pigs used in this study were either Rapacz familial hypercholesterolaemic swine (purchased from the University of Wisconsin Swine Research and Teaching Center; n = 30), miniature Yucatan pigs (n = 20), or standard breed farm pigs (n = 5). The Miniature Yucatan pig data are historical data from our laboratory that have been included to compare vasomotor function data against FH pigs, since vasomotor experiments were not performed in the farm pig control group. The Rapacz familial hypercholesterolaemic swine model is characterized by a single missense mutation in the Low Density Lipoprotein Receptor (LDLR) that decreases LDLR affinity for LDL, resulting in elevated total cholesterol levels between 180 and 240 mg/dl (Hasler-Rapacz et al., 1998). Pigs were fed either a standard chow diet (controls) or the University of Wisconsin gestation diet, a corn- and soybean-based cholesterol-free, 3% fat diet. All FH and Yucatan pigs were between 11–14 months of age at the time of sacrifice; farm pigs were younger (~ 6 mo) to achieve similar body weights.
Exercise training intervention
Within the group of 30 FH pigs included in this study, each was randomly assigned to either an exercise training group (EX; n = 15) or a sedentary group (SED; n = 15). Pigs in the sedentary group were confined to cage activity only. Pigs in the exercise-training group underwent a 16–20 wk exercise training intervention consisting of moderate-intensity (~70% of maximum heart rate) aerobic exercise on treadmills, one time per day, 5 days/wk. This exercise training program has been described previously (Bunker & Laughlin, 2010; Company et al., 2010) and used extensively by our research group for the past 2 decades (Padilla et al., 2010a). Briefly, the exercise protocol was shorter in duration and lower intensity at the beginning of the intervention (5-min warm up at 2–2.5 mph, 15 min at 4 mph, 20 min at 3 mph, and a 5 min cool-down at 2–2.5 mph) but subsequently increased in difficulty such that, by week 10 of training, pigs exercised for 85 min per day, consisting of a 5-min warm up at 2–2.5 mph, 15 min at 6.5–7 mph, 60 min at 4.5–5 mph, and a 5 min cool-down at 2–2.5 mph. The training program’s effectiveness was assessed by measurements of heart weight, heart weight/body weight ratio, and skeletal muscle citrate synthase activity.
Tissue collection
At the time of sacrifice, pigs were anesthetized with intramuscular ketamine (35mg/kg; Fort Dodge Animal Health, Fort Dodge, IA, USA) and xylazine (2.25 mg/kg; Lloyd Laboratories, Shenandoah, IA, USA), and intravenous pentothal (25mg/kg; Abbott Labs, Abbott Park, IL, USA) for deep anesthesia, and the heart was removed to achieve euthanasia. Subsequently, coronary vessels from the heart and additional systemic vessels were immediately dissected from the vasculature and placed in iced Krebs bicarbonate buffer (131.5mM NaCl, 5.0mM KCl, 1.2mM NaH2PO4, 1.2mM MgCl2, 2.5mM CaCl2, 25.0mM NaHCO3, and 11.2mM glucose).
In vitro assessment of endothelium-dependent and –independent relaxation
Vasomotor experiments were performed on the right coronary artery of FH pigs, and the left anterior descending coronary (LAD) artery of Yucatan pigs. These historical vasomotor function data collected in the LAD of Yucatan pigs were the only coronary data available for comparison of the FH group against control animals of the same age and gender, fed a normal diet. Although it would be preferable to compare our FH coronary function data against similar responses collected in the RCA of control animals, our laboratory has previously demonstrated similar responsiveness to an array of vasodilator drugs across the LAD and RCA when pre-contracted with PGF2α (Oltman et al., 1992).
Procedures used to assess vasoactive responses of arterial rings have been published previously in detail (Thompson et al., 2004; Woodman et al., 2004; Woodman et al., 2005). Coronary arteries from Yucatan and FH pigs were trimmed of fat and connective tissue and sectioned into 2- to 3-mm rings in cold Krebs bicarbonate buffer solution (see above). Vasomotor reactivity was examined with the rings stretched to the length that produced maximal active tension. Before dose-response curves were initiated, arterial rings were preconstricted with PGF2α (30 μM). Endothelium-dependent relaxation was assessed by using bradykinin (10−11 to 10−6 M; BK) diffused into a Krebs bicarbonate buffer solution containing 131.5 mM NaCl, 5.0 mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 2.5 mM CaCl2, 11.2 mM glucose, 20.8 mM NaHCO3, 0.003 mM propranolol, and 0.025 mM EDTA. Solutions were aerated with 95% O2–5% CO2 (pH 7.4) and maintained at 37°C.
Immunoblot analysis
Vessels harvested for immunoblot analysis included the right coronary artery (RCA), thoracic aorta, internal mammary, carotid, brachial, femoral, and renal arteries. In addition, segments of the thoracic and abdominal vena cava, as well as the jugular, brachial, femoral, and renal veins were harvested for analysis. After removal at the time of sacrifice, vessels were dissected free of connective tissue under a microscope, cut longitudinally, and the endothelium was mechanically scraped in the presence of Laemmli buffer (62.5 mM Tris, pH 6.8, 6 M urea, 160 mM dithiothreitol, 2% SDS, and 0.001% bromophenol blue) yielding both endothelial enriched and endothelial depleted samples of vascular cells, as described previously (Newcomer et al., 2007; Padilla et al., 2010a; Padilla et al., 2010b; Padilla et al., 2011; Simmons et al., 2012). The endothelial depleted portions were used as smooth muscle samples, and all samples were subsequently stored at −80°C until analysis of protein content.
In order to test the effects of exercise training on coronary conduit artery vascular cell phenotype, we studied a wide range of proteins in order to gain a broad view of expression profiles related to atherogenicity ((Laughlin et al., 2008)). Proteins investigated in this analysis were: endothelial nitric oxide synthase (eNOS), phospho-eNOSSer1177, the phospho-eNOS/eNOS ratio, heat shock protein 90 (HSP90), p67phox, superoxide dismutase 1 (SOD1), superoxide dismutase 3 (SOD3/ecSOD), protein kinase B (Akt), phospho-Akt, the phospho-Akt/Akt ratio, catalase, rac-1, arginase-1, adipophilin, caveolin-1, and the angiotensin-II receptor. This analysis was carried out on 29 of 30 FH pigs (n = 29; 14 EX, 15 SED) because insufficient sample remained from one of the pigs at the time of analysis. In order to test the effects of exercise training on systemic conduit vessels, the list of proteins investigated was shortened in order to expand the number of different vessels tested (both arteries and veins). Proteins investigated in this analysis were: eNOS, HSP90, p67phox, SOD1, and SOD3. This analysis was carried out on the final 14 FH pigs (7 EX, 7 SED) and the control farm pigs (n = 5).
Prior to immunoblot analysis, vascular cell samples were first boiled and sonicated to expose the intracellular contents. Total protein in each sample was then quantified using the NanoOrange Protein Quantitation Kit (Invitrogen; Grand Island, NY, USA) and 3μg of protein were loaded onto a polyacrylamide gel and separated by electrophoresis. Gels were loaded with only one vessel represented on each gel, having an equal number of samples (per gel) originating from sedentary and exercise trained animals. After protein separation, contents of the gel were transferred to a polyvinylidene diflouride (PVDF) membrane by the application of 34V for 1 hour, and this membrane was subsequently blocked with 5% non-fat milk in TBS-Tween (20 mM Tris • HCl, 137 mM NaCl, and 0.1% Tween 20) at room temperature for 1 hour. After 1 hour incubation in non-fat milk, a primary antibody against the protein of interest was applied overnight. Antibodies and concentrations used were as follows: eNOS (1:1000; BD Transduction; Franklin Lakes, NJ, USA), phospho-eNOS (1:250; BD Transduction), HSP90 (1:1000; BD Transduction), p67phox (1:1000; BD Transduction), SOD1 (1:5000; Assay Designs; Farmingdale, NY, USA), SOD3 (1:1000; Upstate/Millipore; Billerica, MA, USA), Akt (1:500; Cell Signaling; Danvers, MA, USA), phospho-Akt (1:250; Cell Signaling), catalase (1:5000; Sigma-Aldrich; St. Louis, MO, USA), rac-1 (1:500; Cytoskeleton; Denver, CO, USA), arginase-1 (1:1000; BD Transduction), adipophilin (1:500; Abcam; Cambridge, MA, USA), caveolin-1 (1:250; BD Transduction), and the angiotensin-II receptor (1:500; Santa Cruz; Santa Cruz, CA, USA). The following morning the secondary antibody was applied for 1 hour, and then protein content was then detected by enhanced chemiluminescence (ECL, Amersham; Pittsburgh, PA, USA) and quantified by densitometry.
Plasma Cytokines
Plasma samples were assayed in duplicate for concentrations of GM-CSF, IFN-γ, IL-1A, IL-1β, IL-1RA, IL-2, IL-4, IL-6, IL-8, IL-10, IL-18, TNF-α using a 13-plex porcine cytokine assay (Millipore Milliplex; Billerica, MA) on a MAGPIX instrument (Luminex Technologies; Luminex, Austin, TX) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using SigmaPlot (Systat Software, Chicago, IL, USA). Coronary artery vascular cell protein expression was compared between EX and SED by unpaired t-test. Systemic conduit vessel endothelial cell protein expression (and coronary artery endothelial cell protein expression in a subset of FH pigs) was compared between control, FH-SED, and FH-EX by 1-way ANOVA. Main effects of Group and Dose on vasomotor responses to BK were compared between Control, FH-SED, and FH-EX groups by 2-way ANOVA; drug doses associated with half-maximal relaxation were also compared by 1 way ANOVA across groups. Differences were considered statistically significant when p < 0.05; adjustments to alpha for multiple comparisons were done using the Bonferroni correction. All values are presented as means ± SE unless otherwise indicated.
Results
Experimental animal characteristics
Results of the serum lipid and glucose analysis as well as heart weights, body weights, and citrate synthase activity of the medial head of the triceps brachii are presented in table 1. Total cholesterol and triglycerides were higher in FH pigs compared to farm pigs (P < 0.05 for both FH-SED and FH-EX vs. control). Heart weight, heart weight:body weight ratio, and citrate synthase activity of the medial head of the triceps brachii muscle were all higher in FH-EX animals compared to FH-SEDs (all P < 0.05 vs. FH-SED). Table 2 displays the results of plasma cytokine analysis; circulating IL-8 was lower in FH-EX compared to FH-SED (P<0.05).
Table 1.
Pig characteristics
| Variable | Control (n=5) | FH-SED (n=15) | FH-EX (n=15) |
|---|---|---|---|
| total cholesterol (mg/dl) | 77.6 ± 4.8 | 327.4 ± 20.0* | 358.0 ± 19.1* |
| Triglycerides (mg/dl) | 13.2 ± 3.0 | 53.3 ± 6.0* | 53.0 ± 5.7* |
| Glucose (mg/dl) | 127.8 ± 19.0 | 112.1 ± 7.9 | 115.9 ± 7.9 |
| heart weight (g) | 283.4 ± 16.4 | 228.6 ± 6.9* | 260.9 ± 11# |
| body weight(kg) | 72.0 ± 2.0 | 66.8 ± 2.9 | 63.1 ± 2.7* |
| heart wght:bd wght(g/kg) | 3.9 ± 0.2 | 3.5 ± 0.1 | 4.1 ± 0.1# |
| citrate synthase activity of the medial head of the triceps brachii (μmol/min/g) | N/A | 12.32 ± 0.58 | 15.47 ± 0.76# |
P < 0.05 vs. control
P < 0.05 vs. FH-SED
Control refers to standard breed farm pigs
Table 2.
Plasma cytokines
| Variable | Control (n=12) | FH-SED (n=7) | FH-EX (n=7) |
|---|---|---|---|
| IFN-γ (pg/ml) | 0.01 ± 0.00 | 0.06 ± 0.02 | 0.02 ± 0.01 |
| IL-1α (pg/ml) | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.01 |
| IL-1β (pg/ml) | 0.25 ± 0.07 | 0.23 ± 0.06 | 0.11 ± 0.04 |
| IL-1RA (pg/ml) | 0.20 ± 0.08 | 0.36 ± 0.14 | 0.28 ± 0.16 |
| IL-2 (pg/ml) | 0.08 ± 0.03 | 0.08 ± 0.02 | 0.03 ± 0.01 |
| IL-4 (pg/ml) | 0.50 ± 0.23 | 0.55 ± 0.19 | 0.23 ± 0.11 |
| IL-6 (pg/ml) | 0.04 ± 0.02 | 0.05 ± 0.01 | 0.03 ± 0.01 |
| IL-8 (pg/ml) | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.00 ± 0.00# |
| IL-10 (pg/ml) | 0.15 ± 0.07 | 0.17 ± 0.03 | 0.10 ± 0.03 |
| IL-18 (pg/ml) | 0.88 ± 0.33 | 1.04 ± 0.19 | 0.46 ± 0.20 |
P < 0.05 vs. FH-SED
Control refers to male castrated, yucatan pigs
Effects of hypercholesterolaemia and exercise training on coronary artery vasomotor reactivity
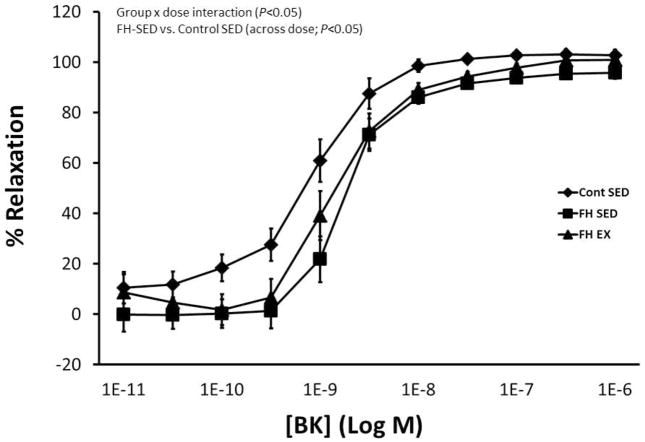
Figure 1 displays coronary vascular responses to increasing doses of BK in control Yucatan, FH-SED, and FH-EX animals. Responses differed among groups (P<0.05 for group x dose interaction effect) such that the dose-response curve was shifted rightward (less responsive) in sedentary FH pigs compared to control (P<0.05 for between group comparison). Responses were not different between exercise-trained and sedentary FH groups. Table 3 displays the EC50 values for each dose-response curve, which similarly demonstrate reduced sensitivity in FH-SED (compared to control; P<0.05) and no difference between FH-SED and FH-EX. Characteristics of the coronary artery segments used in these experiments are displayed in table 4.
Figure 1.
Conduit coronary vasomotor responses to increasing doses of BK in control sedentary (miniature Yucatan), FH sedentary, and FH exercise trained pigs. SED, sedentary; EX, exercise trained.
Table 3.
EC50 values for coronary artery vasomotor function curves
| Group | EC50 (−log M) |
|---|---|
| Control | −9.117 |
| FH-SED | −8.759* |
| FH-EX | −8.787 |
P < 0.05 vs. control
Table 4.
Characteristics of the coronary artery vascular rings used in the functional experiments
| Variable | Control (n=8) | FH-SED (n=7) | FH-EX (n=6) |
|---|---|---|---|
| Outer diameter (mm) | 2.57 ± 0.14 | 2.24 ± 0.10 | 2.39 ± 0.13 |
| Inner diameter (mm) | 1.58 ± 0.07 | 1.31 ± 0.05* | 1.19 ± 0.05* |
| Wall thickness (mm) | 0.5 ± 0.05 | 0.46 ± 0.03 | 0.6 ± 0.06 |
| Axial length (mm) | 3.48 ± 0.35 | 3.81 ± 0.18 | 3.83 ± 0.17 |
P < 0.05 vs. control
Control refers to male castrated, yucatan pigs
Effects of exercise training on coronary artery vascular cell phenotype
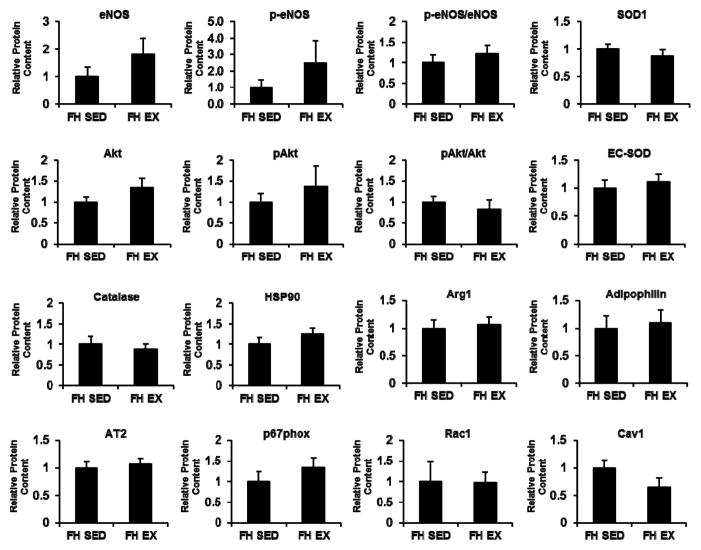
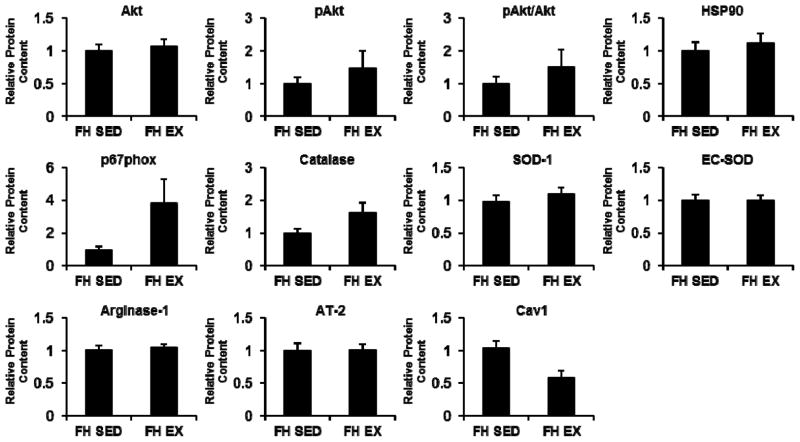
Figure 2 contrasts the protein contents of coronary artery endothelial cells from EX and SED FH pigs. Although several of the protein levels investigated tended to be higher in coronary artery endothelial cells of EX compared to SED (e.g., eNOS, phospho-eNOS), none of the sampled protein markers showed a statistically significant difference between EX and SED (all P>0.05). Figure 3 contrasts the protein contents of coronary artery smooth muscle cells from EX and SED FH pigs. Likewise, none of the sampled protein markers showed a statistically significant difference between EX and SED (all P>0.05).
Figure 2.
Differences in coronary artery endothelial cell protein content between sedentary and exercise trained FH pigs. Values represent protein expression measured in net intensity units and scaled to the average of the value for sedentary animals. Data are means ± SE. SED, sedentary; EX, exercise trained.
Figure 3.
Differences in coronary artery smooth muscle cell protein content between sedentary and exercise trained FH pigs. Values represent protein expression measured in net intensity units and scaled to the average of the value for sedentary animals. Data are means ± SE. SED, sedentary; EX, exercise trained.
Effects of exercise training on systemic conduit vessel endothelial cell phenotype
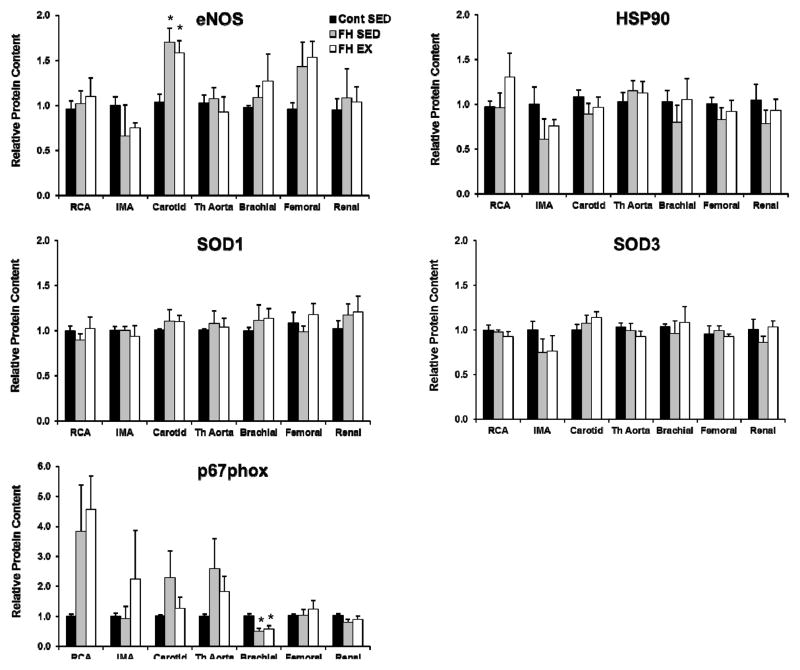
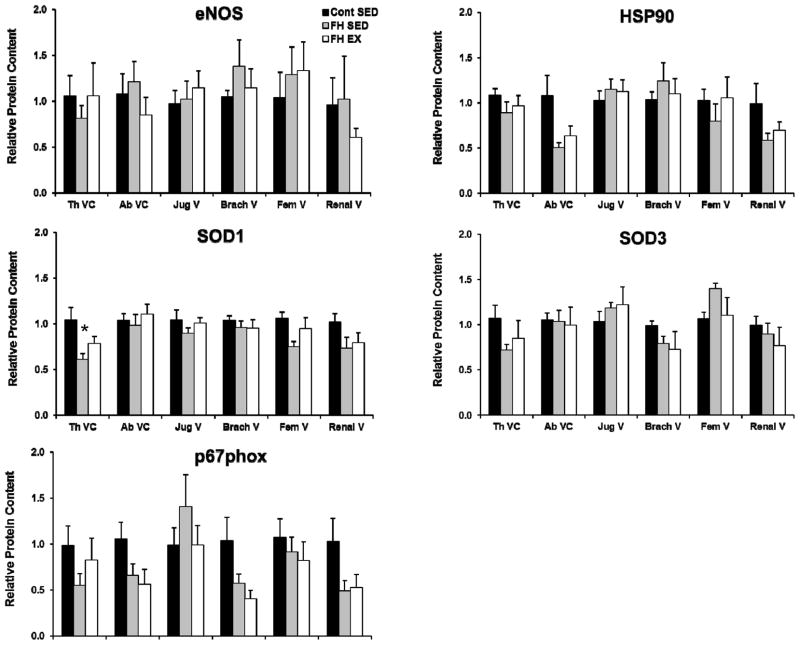
Figure 4 contrasts endothelial cell protein expression in the thoracic aorta, the right coronary, internal mammary, carotid, brachial, femoral, and renal arteries between control, FH-SED, and FH-EX pigs. Carotid artery endothelial cell eNOS content was increased and brachial artery p67phox content decreased in FH animals compared to control (both P<0.05). Although a trend was noted for increased p67phox in the RCA of FH animals (P=0.08 for effect of group), none of the other markers tested changed with FH or exercise training in the conduit arteries (all P>0.05 for ANOVA between groups). Figure 5 contrasts endothelial cell protein expression in the thoracic and abdominal vena cava, as well as the jugular, brachial, femoral, and renal veins between control, FH-SED, and FH-EX pigs. Endothelial cell SOD1 expression was lower in the thoracic vena cava of FH-SED compared to control (P<0.05), but was not different between control and FH-EX. Endothelial cell HSP90 expression tended to be lower in the brachial vein (P=0.09) as well as the thoracic vena cava (P=0.06) of FH-SED compared to control, but these differences did not meet the threshold for statistical significance. None of the other markers tested changed with FH or exercise training in these or other conduit veins (all P>0.05 for ANOVA between groups).
Figure 4.
Differences in protein content within the endothelial cells of the systemic conduit arteries between control sedentary, FH sedentary, and FH exercise trained pigs. Values represent protein expression measured in net intensity units and scaled to the average of the value for control animals. * p< 0.05 vs. control pigs. Data are means ± SE; n = 19. SED, sedentary; EX, exercise trained.
Figure 5.
Differences in protein content within the endothelial cells of the systemic conduit veins between control sedentary, FH sedentary, and FH exercise trained pigs. Values represent protein expression measured in net intensity units and scaled to the average of the value for control animals. * p< 0.05 vs. control pigs. Data are means ± SE; n = 19. SED, sedentary; EX, exercise trained.
Discussion
This is the first study to investigate the effect of endurance exercise training on coronary and systemic conduit artery and venous EC phenotype in a swine model of familial hypercholesterolaemia. Coronary artery endothelium-dependent vasomotor function was depressed in sedentary FH-pigs compared to sedentary controls, and exercise training did not have a statistically significant effect on vasomotor function within FH. Notably, however, only scattered effects of FH on EC phenotype were observed across the vasculature, which included both pro- and anti-atherogenic changes in EC protein expression (e.g., increased eNOS in carotid artery ECs, decreased p67phox in brachial artery ECs, but decreased expression of the antioxidant protein SOD1 in thoracic vena cava). In thoracic vena cava ECs, the deficit in SOD1 expression associated with FH was corrected by EX, while no other effects of exercise on conduit vessel EC phenotype were observed. These results suggest that EX does not produce a consistently improved EC phenotype in either coronary or systemic conduit vessels in this FH model.
Our group has previously investigated the effects of exercise on the endothelium using both healthy pigs and pigs fed a combination high-fat/cholesterol diet to produce early stage atherosclerotic disease. Findings from these studies demonstrate that, in the larger conduit arteries of healthy swine, short-term exercise training exerts beneficial effects on the endothelium but that more prolonged training leads to normalization of endothelial vasomotor function and protein expression, a process that coincides temporally with outward vascular remodeling (Oltman et al., 1995; McAllister et al., 1996; McAllister & Laughlin, 1997; LAUGHLIN et al., 1998; Laughlin et al., 2001; Laughlin et al., 2003; Padilla et al., 2010a; Laughlin et al., 2012). In contrast to the normalization of endothelial vasomotor function and phenotype after prolonged exercise training in healthy swine, pigs with early stage atherosclerosis (diet-induced hypercholesterolaemia) and impaired endothelial vasomotor function prior to training exhibit beneficial responses in the endothelium that are sustained even after 16–20 weeks of training (Woodman et al., 2003; Thompson et al., 2004; Woodman et al., 2004; Woodman et al., 2005; Woodman et al., 2006). The majority of these findings have now been documented in healthy and diseased humans as well (Gokce et al., 2002; Walsh et al., 2003; Green et al., 2004; Tinken et al., 2008; Green, 2009; Tinken et al., 2010; Birk et al., 2012).
In the present study, we sought to build upon the progress described above using an FH model known to develop extensive atherosclerotic lesions (Prescott et al., 1991; Company et al., 2010) in order to elucidate the mechanisms through which exercise training confers beneficial effects in patients with overt vascular pathology (e.g., (Hambrecht et al., 2000; Hambrecht et al., 2003)). On the basis of a multitude of studies suggesting that endothelial function/phenotype is impaired throughout the course of the atherosclerotic disease process (Casino et al., 1993; Celermajer et al., 1994; Sorensen et al., 1994; Enderle et al., 1998; Hambrecht et al., 2003; Thompson et al., 2004; Turk et al., 2005; Civelek et al., 2010), we supposed that EC phenotype in Rapacz FH swine would be atherogenic in nature (when compared to healthy pigs) and hypothesized that exercise training would beneficially impact EC phenotype. The bulk of the data here suggest that our initial supposition was incorrect i.e., EC phenotype in FH was not different for the majority of conduit vessels compared to control pigs, nor were circulating inflammatory cytokines elevated, as would be expected based on observations in humans with FH (El Messal et al., 2006; Gokalp et al., 2009). In fact, the only significant changes in conduit artery EC protein expression in response to FH were anti-atherogenic in nature (i.e., changes in EC phenotype in the carotid and brachial arteries). These paradoxical anti-atherogenic effects of this model of hypercholesterolaemia on conduit artery EC phenotype mirror results recently reported by our group in which p-Akt/Akt ratio was increased in coronary artery ECs from pigs with early stage atherosclerotic disease (Arce-Esquivel et al., 2012). In that study, anti-atherogenic effects on coronary conduit artery EC phenotype were observed even as evidence of disease progression was noted via immunohistochemical staining for markers of oxidative stress and adhesion molecules. Such a coexistence of pro- and anti-atherogenic EC expression profiles has been reported previously in the context of regional EC heterogeneity caused by disturbed blood fluid dynamics (Passerini et al., 2004). Thus these data suggest that paradoxical anti-atherogenic effects on conduit artery EC phenotype may be a common feature of swine models of atherosclerosis that employ hypercholesterolaemia (either genetic or diet-induced) in order to promote disease progression, and further that such effects may persist even as vascular pathophysiology worsens.
Considering the relatively healthy arterial EC phenotype of the FH pigs in this study, it is perhaps not surprising that the hypothesized beneficial effects of exercise training were not observed in this model. As noted in the paragraphs above, when EC phenotype is normal (as in healthy pigs), endurance exercise training sustained over a period of 16–20 weeks is not expected to change EC phenotype in coronary conduit (Laughlin et al., 2001) or systemic conduit arteries (Padilla et al., 2010a), including the brachial and femoral arteries. Thus, it is possible that the results of the present study were contrary to our hypothesis because an initial atherogenic arterial EC phenotype was lacking. Indeed, this may also explain negative findings published by our program regarding the effect of exercise on vascular inflammation, oxidative stress, and vasomotor function in FH swine (Bunker & Laughlin, 2010; Masseau et al., 2012). Conversely, in the thoracic vena cava, where hypercholesterolaemia had a pro-atherogenic effect on EC phenotype in the present study (i.e., reduced SOD1 protein content), exercise training abolished this effect. Taken together, the majority of the data in the present study suggest that systemic EC phenotype in FH pigs, as assessed by the markers presently used, is less atherogenic than expected and, overall, exercise training in FH pigs does not appear to alter vascular expression of pro- and anti-atherogenic proteins.
The relatively normal EC expression profile notwithstanding, there are some effects of exercise that would still be expected in this group of FH pigs. In the thoracic aortic endothelium, exercise training of similar duration to that undertaken in the present study was previously shown to increase SOD1 and SOD3 expression while reducing p67phox expression in ECs of healthy swine (Rush et al., 2003). In the coronary circulation, previous data suggest that the rightward shifted vasomotor function curve in hypercholesterolaemic pigs should have moved leftward in response to training, indicating increased sensitivity of the endothelium (Thompson et al., 2004; Woodman et al., 2004). It is not clear why these expected findings were not seen. Objective metrics of training status suggest that these pigs adapted to the training load in a manner similar to previous study groups (increased heart weight:body weight ratio, increased skeletal muscle citrate synthase activity, etc). One difference between this FH group and previous study samples however is the mutation in the LDLR gene, and therefore it is possible that functional LDL receptors are required in order to observe the beneficial effects of exercise on the arterial endothelium in hypercholesterolaemia. This concept warrants further investigation.
It is important to mention that some but not all of the cytokine values reported in table 2 are lower than those reported in other studies of swine. In order to clarify this issue, we compared the results of our cytokine assays with previously reported values for swine, and determined that many of the values reported in table 2 are in agreement with previous work. Specifically, we compared data from our control group to the control groups/time points found in two previous studies (Niemann et al., 2009; Baker et al., 2012), and found agreement between our results and these previously published reports for most (5 of 7) of cytokines for which direct comparisons were available. We do not currently have an explanation for the remaining discrepancy between the cytokine values that we report, and those reported by others, although it is possible that different breeds of swine are at least partially responsible.
In summary, coronary artery endothelium-dependent vasomotor function was depressed in sedentary FH-pigs compared to sedentary controls, and exercise training did not change vasomotor function within FH. In systemic conduit arteries and veins, few effects of FH on EC protein expression were noted, including both pro- and anti-atherogenic changes. In thoracic vena cava ECs, the deficit in SOD1 expression associated with FH was corrected by EX, but no other effects of exercise on conduit vessel EC phenotype were observed. These findings suggest that EX does not produce a consistently improved EC phenotype in either coronary or systemic conduit vessels in this FH swine model.
Supplementary Material
What is the central question of this study?
Does endurance exercise training cause anti-atherogenic effects on the endothelium in a familial hypercholesterolaemia swine model, and how are these effects distributed across veins, arteries, and multiple vascular territories within each system.
What is the main finding and what is its importance?
Coronary artery endothelium-dependent vasomotor function is depressed in sedentary Familial Hypercholesterolaemic (FH) pigs compared to sedentary controls, and exercise training does not change vasomotor function within FH. In systemic conduit arteries and veins, few effects of FH on EC protein expression were noted, including both pro- and anti-atherogenic changes. These findings suggest that EX does not produce a consistently improved EC phenotype in either coronary or systemic conduit vessels in this FH swine model.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Pam Thorne, Ann Melloh, David Harah, Miles Tanner, and Stacy Bruno. This study was supported by the following grants: NIH P01-HL052490 (M.H.L.), NIH-HL36088 (M.H.L.), NIH T32-AR048523 (G.H.S. and N.T.J.), and AHA 11POST5080002 (J.P.).
References
- Arce-Esquivel AA, Kreutzer KV, Rush JWE, Turk JR, Laughlin MH. Exercise Does Not Attenuate Early CAD Progression in a Pig Model. Medicine and Science in Sports and Exercise. 2012;44:27–38. doi: 10.1249/MSS.1240b1013e318228879b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TA, Romero J, Bach HH, Strom JA, Gamelli RL, Majetschak M. Systemic release of cytokines and heat shock proteins in porcine models of polytrauma and hemorrhage. Critical Care Medicine. 2012;40:876–885. doi: 10.1097/CCM.0b013e318232e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk GK, Dawson EA, Atkinson C, Haynes A, Cable NT, Thijssen DHJ, Green DJ. Brachial artery adaptation to lower limb exercise training: role of shear stress. Journal of Applied Physiology. 2012;112:1653–1658. doi: 10.1152/japplphysiol.01489.2011. [DOI] [PubMed] [Google Scholar]
- Bunker AK, Laughlin MH. Influence of exercise and perivascular adipose tissue on coronary artery vasomotor function in a familial hypercholesterolemic porcine atherosclerosis model. Journal of Applied Physiology. 2010;108:490–497. doi: 10.1152/japplphysiol.00999.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casino P, Kilcoyne C, Quyyumi A, Hoeg J, Panza J. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88:2541–2547. doi: 10.1161/01.cir.88.6.2541. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. Journal of the American College of Cardiology. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Civelek M, Grant GR, Irolla CR, Shi C, Riley RJ, Chiesa OA, Stoeckert CJ, Karanian JW, Pritchard WF, Davies PF. Prelesional arterial endothelial phenotypes in hypercholesterolemia: universal ABCA1 upregulation contrasts with region-specific gene expression in vivo. American Journal of Physiology - Heart and Circulatory Physiology. 2010;298:H163–H170. doi: 10.1152/ajpheart.00652.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Company JM, Booth FW, Laughlin MH, Arce-Esquivel AA, Sacks HS, Bahouth SW, Fain JN. Epicardial fat gene expression after aerobic exercise training in pigs with coronary atherosclerosis: relationship to visceral and subcutaneous fat. Journal of Applied Physiology. 2010;109:1904–1912. doi: 10.1152/japplphysiol.00621.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Messal M, Beaudeux J-L, Drissi A, Giral P, Chater R, Bruckert E, Adlouni A, Chapman MJ. Elevated serum levels of proinflammatory cytokines and biomarkers of matrix remodeling in never-treated patients with familial hypercholesterolemia. Clinica Chimica Acta. 2006;366:185–189. doi: 10.1016/j.cca.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Enderle M-D, Schroeder S, Ossen R, Meisner C, Baumbach A, Haering HU, Karsch KR, Pfohl M. Comparison of peripheral endothelial dysfunction and intimal media thickness in patients with suspected coronary artery disease. Heart. 1998;80:349–354. doi: 10.1136/hrt.80.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokalp D, Tuzcu A, Bahceci M, Arikan S, Pirinccioglu AG, Bahceci S. Levels of proinflammatory cytokines and hs-CRP in patients with homozygous familial hypercholesterolaemia. Acta Cardiologica. 2009;64:603–609. doi: 10.2143/AC.64.5.2042689. [DOI] [PubMed] [Google Scholar]
- Gokce N, Vita JA, Bader DS, Sherman DL, Hunter LM, Holbrook M, O’Malley C, Keaney JF, Balady GJ. Effect of exercise on upper and lower extremity endothelial function in patients with coronary artery disease. The American Journal of Cardiology. 2002;90:124–127. doi: 10.1016/s0002-9149(02)02433-5. [DOI] [PubMed] [Google Scholar]
- Green DJ. Exercise Training as Vascular Medicine: Direct Impacts on the Vasculature in Humans. Exercise and Sport Sciences Reviews. 2009;37:196–202. doi: 10.1097/JES.0b013e3181b7b6e3. [DOI] [PubMed] [Google Scholar]
- Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambrecht R, Adams V, Erbs S, Linke A, Kränkel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular Physical Activity Improves Endothelial Function in Patients With Coronary Artery Disease by Increasing Phosphorylation of Endothelial Nitric Oxide Synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. New England Journal of Medicine. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- Hasler-Rapacz J, Ellegren H, Fridolfsson A-K, Kirkpatrick B, Kirk S, Andersson L, Rapacz J. Identification of a mutation in the low density lipoprotein receptor gene associated with recessive familial hypercholesterolemia in swine. American Journal of Medical Genetics. 1998;76:379–386. [PubMed] [Google Scholar]
- Laughlin MH, Bowles DK, Duncker DJ. The coronary circulation in exercise training. American Journal of Physiology - Heart and Circulatory Physiology. 2012;302:H10–H23. doi: 10.1152/ajpheart.00574.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. Journal of Applied Physiology. 2008;104:588–600. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAUGHLIN MH, OLTMAN CL, BOWLES DK. Exercise training-induced adaptations in the coronary circulation. Medicine and Science in Sports and Exercise. 1998;30:352–360. doi: 10.1097/00005768-199803000-00004. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. Journal of Applied Physiology. 2001;90:501–510. doi: 10.1152/jappl.2001.90.2.501. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Rubin LJ, Rush JWE, Price EM, Schrage WG, Woodman CR. Short-term training enhances endothelium-dependent dilation of coronary arteries, not arterioles. Journal of Applied Physiology. 2003;94:234–244. doi: 10.1152/japplphysiol.00246.2002. [DOI] [PubMed] [Google Scholar]
- Masseau I, Davis MJ, Bowles DK. Carotid inflammation is unaltered by exercise in hypercholesterolemic swine. Medicine and Science in Sports and Exercise. 2012 doi: 10.1249/MSS.0b013e318266af0a. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister RM, Kimani JK, Webster JL, Parker JL, Laughlin MH. Effects of exercise training on responses of peripheral and visceral arteries in swine. Journal of Applied Physiology. 1996;80:216–225. doi: 10.1152/jappl.1996.80.1.216. [DOI] [PubMed] [Google Scholar]
- McAllister RM, Laughlin MH. Short-term exercise training alters responses of porcine femoral and brachial arteries. Journal of Applied Physiology. 1997;82:1438–1444. doi: 10.1152/jappl.1997.82.5.1438. [DOI] [PubMed] [Google Scholar]
- Mora S, Cook N, Buring JE, Ridker PM, Lee I-M. Physical Activity and Reduced Risk of Cardiovascular Events. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer SC, Taylor JC, Bowles DK, Laughlin MH. Endothelium-dependent and -independent relaxation in the forelimb and hindlimb vasculatures of swine. Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology. 2007;148:292–300. doi: 10.1016/j.cbpa.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Niemann J, Rosborough J, Youngquist S, Shah A, Lewis R, Phan Q, Filler S. Cardiac function and the proinflammatory cytokine response after recovery from cardiac arrest in swine. Journal of Interferon and Cytokine Research. 2009;29:749–758. doi: 10.1089/jir.2009.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltman CL, Parker JL, Adams HR, Laughlin MH. Effects of exercise training on vasomotor reactivity of porcine coronary arteries. American Journal of Physiology - Heart and Circulatory Physiology. 1992;263:H372–H382. doi: 10.1152/ajpheart.1992.263.2.H372. [DOI] [PubMed] [Google Scholar]
- Oltman CL, Parker JL, Laughlin MH. Endothelium-dependent vasodilation of proximal coronary arteries from exercise-trained pigs. Journal of Applied Physiology. 1995;79:33–40. doi: 10.1152/jappl.1995.79.1.33. [DOI] [PubMed] [Google Scholar]
- Padilla J, Newcomer SC, Simmons GH, Kreutzer KV, Laughlin MH. Long-term exercise training does not alter brachial and femoral artery vasomotor function and endothelial phenotype in healthy pigs. American Journal of Physiology - Heart and Circulatory Physiology. 2010a;299:H379–H385. doi: 10.1152/ajpheart.00294.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J, Simmons GH, Davis JW, Whyte JJ, Zderic TW, Hamilton MT, Bowles DK, Laughlin MH. Impact of exercise training on endothelial transcriptional profiles in healthy swine: a genome-wide microarray analysis. American Journal of Physiology - Heart and Circulatory Physiology. 2011;301:H555–H564. doi: 10.1152/ajpheart.00065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J, Simmons GH, Newcomer SC, Laughlin MH. Relationship between brachial and femoral artery endothelial vasomotor function/phenotype in pigs. Experimental Biology and Medicine. 2010b;235:1287–1291. doi: 10.1258/ebm.2010.010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011;10:1032–1037. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott MF, McBride CH, Hasler-Rapacz J, Von Linden J, Rapacz J. Development of complex atherosclerotic lesions in pigs with inherited hyper-LDL cholesterolemia bearing mutant alleles for apoliproprotein B. American Journal of Pathology. 1991;139:139–147. [PMC free article] [PubMed] [Google Scholar]
- Rush JWE, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. American Journal of Physiology - Heart and Circulatory Physiology. 2003;284:H1378–H1387. doi: 10.1152/ajpheart.00190.2002. [DOI] [PubMed] [Google Scholar]
- Simmons GH, Padilla J, Laughlin MH. Heterogeneity of endothelial cell phenotype within and amongst conduit vessels of the swine vasculature. Experimental Physiology. 2012;97:1074–1082. doi: 10.1113/expphysiol.2011.064006. [DOI] [PubMed] [Google Scholar]
- Sorensen KE, Celermajer DS, Georgakopoulos D, Hatcher G, Betteridge DJ, Deanfield JE. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein(a) level. The Journal of Clinical Investigation. 1994;93:50–55. doi: 10.1172/JCI116983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MA, Henderson KK, Woodman CR, Turk JR, Rush JWE, Price E, Laughlin MH. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. Journal of Applied Physiology. 2004;96:1114–1126. doi: 10.1152/japplphysiol.00768.2003. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Buchner D, Piña IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK. Exercise and Physical Activity in the Prevention and Treatment of Atherosclerotic Cardiovascular Disease: A Statement From the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- Tinken TM, Thijssen DHJ, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol. 2008;586:5003–5012. doi: 10.1113/jphysiol.2008.158014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinken TM, Thijssen DHJ, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear Stress Mediates Endothelial Adaptations to Exercise Training in Humans. Hypertension. 2010;55:312–318. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- Turk JR, Henderson KK, Vanvickle GD, Watkins J, Laughlin MH. Arterial endothelial function in a porcine model of early stage atherosclerotic vascular disease. International Journal of Experimental Pathology. 2005;86:335–345. doi: 10.1111/j.0959-9673.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk JR, Laughlin MH. Physical Activity and Atherosclerosis: Which Animal Model? Canadian Journal of Applied Physiology. 2004;29:657–683. doi: 10.1139/h04-042. [DOI] [PubMed] [Google Scholar]
- Walsh JH, Yong G, Cheetham C, Watts GF, O’Driscoll GJ, Taylor RR, Green DJ. Effects of exercise training on conduit and resistance vessel function in treated and untreated hypercholesterolaemic subjects. European Heart Journal. 2003;24:1681–1689. doi: 10.1016/s0195-668x(03)00384-1. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Ingram D, Bonagura J, Laughlin MH. Exercise training improves femoral artery blood flow responses to endothelium-dependent dilators in hypercholesterolemic pigs. American Journal of Physiology - Heart and Circulatory Physiology. 2006;290:H2362–H2368. doi: 10.1152/ajpheart.01026.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman CR, Thompson MA, Turk JR, Laughlin MH. Endurance exercise training improves endothelium-dependent relaxation in brachial arteries from hypercholesterolemic male pigs. Journal of Applied Physiology. 2005;99:1412–1421. doi: 10.1152/japplphysiol.00293.2005. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Turk JR, Rush JWE, Laughlin MH. Exercise attenuates the effects of hypercholesterolemia on endothelium-dependent relaxation in coronary arteries from adult female pigs. Journal of Applied Physiology. 2004;96:1105–1113. doi: 10.1152/japplphysiol.00767.2003. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Turk JR, Williams DP, Laughlin MH. Exercise training preserves endothelium-dependent relaxation in brachial arteries from hyperlipidemic pigs. Journal of Applied Physiology. 2003;94:2017–2026. doi: 10.1152/japplphysiol.01025.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.