Abstract
Gastric adenocarcinoma is a leading cause of cancer-related death worldwide, and Helicobacter pylori infection is one of the strongest known risk factors for this malignancy. H. pylori strains exhibit a high level of genetic diversity, and the risk of gastric cancer is higher in persons carrying certain strain types (for example, those that contain a cag pathogenicity island or type s1 vacA alleles) than in persons carrying other strain types. Additional risk factors for gastric cancer include specific human genetic polymorphisms and specific dietary preferences (for example, a high-salt diet or a diet deficient in fruits and vegetables). Finally, iron-deficiency anemia is a risk factor for gastric cancer. Recent studies have provided evidence that several dietary risk factors for gastric cancer directly impact H. pylori virulence. In this review article, we discuss mechanisms by which diet can modulate H. pylori virulence and thereby influence gastric cancer risk.
Keywords: gastric cancer, gastric adenocarcinoma, diet, salt, iron, CagA, cag pathogenicity island, VacA, BabA
Epidemiology of Gastric Cancer
Gastric adenocarcinoma is the second leading cause of cancer-related death worldwide.1-4 Several different types of cancer can arise in the stomach, including adenocarcinoma, lymphoma, and leiomyosarcoma, but adenocarcinoma is by far the most common. Two types of gastric adenocarcinoma (intestinal-type and diffuse-type) can be differentiated histologically.5 The diagnosis of gastric adenocarcinoma often is not established until late in the course of disease, and therefore there is great interest in understanding the factors that contribute to the occurrence of this malignancy, identifying persons who are at highest risk, and developing means for gastric cancer prevention.
The incidence of gastric adenocarcinoma varies markedly throughout the world. Incidence rates are currently highest in East Asia, Central America, parts of South America, and Eastern Europe.1,2,6,7 Throughout the world, gastric adenocarcinomas of the distal stomach (body and antrum) occur more commonly in men than in women in a 2:1 ratio.1,7 There have been marked changes in the incidence of gastric adenocarcinoma over the past 100 y. A century ago, gastric cancer was a leading cause of cancer-related death in developed countries.8,9 Most gastric adenocarcinomas in the United States in the early 1900s occurred in the distal stomach and were of intestinal-type histology, but the incidence of these tumors in the distal stomach has steadily declined over the past century.1,8,9 Currently in the United States, distal gastric adenocarcinoma is diagnosed most commonly in elderly persons, and occurs more commonly in African-Americans, Hispanic-Americans, and Native Americans than in other ethnicities.10,11 In conjunction with the declining incidence of gastric adenocarcinomas of the distal stomach over the past century, there has been a steady increase in gastric adenocarcinomas of the proximal stomach and gastresophageal junction in the United States and Europe.12,13
Helicobacter pylori as a Risk Factor for Gastric Cancer
Histologic studies performed many decades ago suggested that intestinal-type gastric adenocarcinoma of the distal stomach was usually preceded by gastric inflammation (termed superficial gastritis) and several other histologic alterations, including intestinal metaplasia (presence of intestinal-type epithelium in the stomach), gastric atrophy (loss of specialized cell types such as parietal cells and chief cells), and dysplasia.5,14 In the early 1980s, the Gram-negative bacterium Helicobacter pylori was identified as a causative agent of superficial gastritis,15 and H. pylori colonization of the stomach is now widely recognized as the strongest known risk factor for distal gastric adenocarcinoma.16,17
H. pylori is typically acquired early in life, and persistently colonizes the human stomach in the absence of antimicrobial treatment.18,19H. pylori is present in about 50% of the global population worldwide, but its prevalence varies substantially throughout the world.20 The majority of persons in developing countries are colonized with H. pylori, whereas colonization rates are lower in developed countries.20
Numerous case-control studies throughout the world have shown that H. pylori is associated with an increased risk for intestinal-type adenocarcinoma of the distal stomach,21,22 but not cancer of the gastric cardia.23 The risk of gastric cancer conferred by H. pylori is similar to the risk of lung cancer conferred by smoking. A prospective study in Japan showed that gastric cancer (both intestinal and diffuse types) developed in a significantly higher number of H. pylori-colonized persons than in uninfected persons,24 and serologic analyses of stored serum specimens have also provided evidence that H. pylori colonization of the stomach precedes the development of cancer.25,26 Gastric inflammation (superficial gastritis) is consistently detected in H. pylori-infected persons,27 and persistent H. pylori colonization of the stomach increases the risk of each of the histologic abnormalities (atrophic gastritis, intestinal metaplasia, and dysplasia) that are considered precursors to gastric adenocarcinoma. There are probably multiple mechanisms by which H. pylori infection contributes to the development of gastric cancer, including alterations in DNA induced by chronic inflammation, alterations in cell proliferation or apoptosis, direct effects of H. pylori products on host cells, and alterations in gastric pH that lead to colonization of the stomach by nitrate-producing bacteria that are not typically found in the acidic stomach.16,17,28-30
H. pylori infection is considered one of the most common infectious causes of cancer, but only a small fraction of colonized persons develop gastric cancer. In this review, we consider the factors that are known to influence the risk of H. pylori-associated gastric cancer. These include strain-specific variations in H. pylori virulence, host genetic factors, and diet. We also discuss recently identified relationships among these individual risk factors, focusing in particular on relationships between diet and H. pylori virulence.
Microbial Virulence Constituents that Influence Gastric Cancer Risk
There is a high level of genetic variability among isolates of H. pylori from unrelated persons.31 The variability includes strain-specific variation in gene content as well as variation in the sequences of individual genes. Several strain-specific H. pylori constituents influence the risk of gastric adenocarcinoma.
One of the H. pylori determinants that influences the risk of gastric cancer is a 40 kb chromosomal region known as the cag pathogenicity island (PAI).32,33 This chromosomal region may be either present, present in an incomplete form, or absent in H. pylori strains.34 Genes within the cag PAI encode an antigenic effector protein (CagA) as well as proteins that form a type IV bacterial secretion system (T4SS) that exports CagA from adherent H. pylori into host cells.35-38 H. pylori strains containing the cag PAI (cag+ strains) are associated with a significantly higher risk of distal gastric cancer than are cag- strains.39,40 In contrast to wild-type H. pylori strains carrying an intact cag PAI, mutant strains lacking cagA or defective in cag T4SS function fail to cause gastric cancer in rodent models.41,42
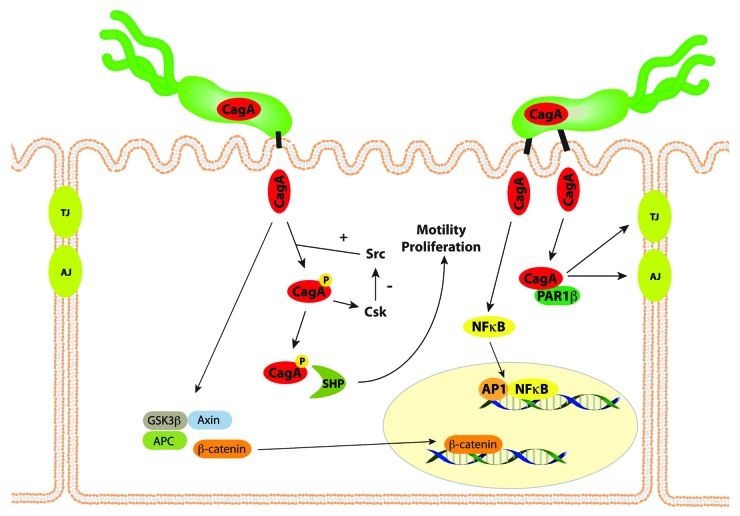
After CagA is translocated into host epithelial cells, it undergoes tyrosine phosphorylation by Src and Abl kinases at motifs containing the amino acid sequence EPIYA (Fig. 1).35,43 Phospho-CagA interacts with and activates several host cell proteins, including a host cellular phosphatase (SHP-2), leading to morphological alterations such as cell scattering and elongation.44 Non-phosphorylated CagA also exerts effects within the host cell. For example, non-phosphorylated CagA directly binds PAR1b, a central regulator of cell polarity, and inhibits its kinase activity, an interaction that promotes loss of cell polarity.45 Non-phosphorylated CagA associates with the epithelial tight-junction scaffolding protein ZO-1 and the transmembrane protein junctional adhesion molecule-A (JAM-A) to cause ineffective assembly of tight-junctions at sites of bacterial attachment,46 and also activates β-catenin, leading to transcriptional upregulation of genes implicated in cancer.47,48 The CagA protein of certain H. pylori strains also can induce IL-8 expression via NFκB activation,49 thereby contributing to neutrophil infiltration in the gastric mucosa. Thus, contact between cag+ strains and host cells activates multiple signaling pathways that may increase the risk for malignant transformation during the prolonged colonization that is typical of H. pylori infection. Compared with wild-type control mice, mice that transgenically express CagA develop increased gastric epithelial cell proliferation and carcinoma in the absence of inflammation.50 Because of these activities, CagA has been labeled a “bacterial oncoprotein”51.
Figure 1. CagA affects multiple signaling pathways within gastric epithelial cells. CagA is translocated into host epithelial cells by the cag type IV secretion system, and undergoes tyrosine phosphorylation by Src and Abl kinases. Phospho-CagA interacts with and activates several host cell proteins, including a host cellular phosphatase (SHP-2), leading to increased motility and proliferation. Non-phosphorylated CagA directly binds PAR1b, a central regulator of cell polarity, and inhibits its kinase activity, an interaction that promotes loss of cell polarity. CagA in an unphosphorylated form activates β-catenin, leading to transcriptional upregulation of genes implicated in cancer. The CagA protein of certain H. pylori strains also can induce NFκB activation. Thus, the entry of CagA into host cells activates multiple signaling pathways that may increase the risk for malignant transformation.
Another H. pylori constituent linked to the development of gastric cancer is the secreted VacA toxin.52,53 A 140 kDa precursor VacA protein undergoes proteolytic processing, resulting in an 88 kDa protein that is secreted through an autotransporter pathway.52,54 Similar to several other autotransporter passenger domains, the secreted VacA protein has a predominantly β-helical structure.55 VacA forms anion-conductive channels in lipid bilayers and the plasma membrane of cells,56-58 and therefore, it has been classified as a pore-forming toxin. VacA can cause a wide assortment of alterations in gastric epithelial cells, including cell vacuolation, alteration of plasma membrane permeability, alterations in the permeability of polarized epithelial cell monolayers, increased mitochondrial membrane permeability, autophagy, and cell death.52,53 VacA can also cause alterations in multiple types of immune cells, including T cells, B cells, neutrophils, mast cells, and macrophages.52,54,59-62
All H. pylori strains possess vacA, but there is marked variation in vacA sequences among strains. The regions of greatest diversity are localized to the 5′ region of the gene, which encodes the signal sequence and N-terminus of the secreted toxin (allele types s1a, s1b, s1c, or s2), an “intermediate region” (allele types i1 or i2), and a “mid-region” (allele types m1 or m2).63,64 Type s2 VacA proteins are inactive in most in vitro assays,63,65,66 and there are detectable differences in the activities of m1 and i1 VacA proteins compared with m2 and i2 VacA proteins, respectively.64,67,68 Strains containing type s1, i1 and m1 forms of vacA are associated with a higher risk of gastric cancer than are strains containing type s2, i2 and m2 forms of vacA.69-71
Another H. pylori constituent that has been linked to gastric cancer risk is an outer membrane protein (BabA) that binds sialyl-Lewis b.72-75 H. pylori strains that contain the cag PAI typically contain type s1 forms of vacA.63 Similarly, BabA is expressed more commonly by cag PAI-positive strains than cag PAI-negative strains.73,76 Thus, patients can be infected with strains harboring multiple constituents associated with increased gastric cancer risk, with strains lacking most of these constituents, or with strains containing an intermediate assortment of virulence determinants. In East Asia, where the incidence of gastric cancer is much higher than in the United States and Western Europe, most strains contain the cag PAI and type s1 vacA alleles.77 Moreover, the CagA proteins produced by East Asian strains contain distinctive EPIYA motifs that are associated with increased intracellular tyrosine phosphorylation and activity, compared with Western H. pylori strains.44,78
Host Genetic Factors that Influence Gastric Cancer Risk
Several host genetic factors influence the likelihood of gastric cancer arising in H. pylori-infected persons. IL-1β is a pro-inflammatory cytokine that inhibits gastric acid secretion, and production of IL-1β is increased in the gastric mucosa of H. pylori–infected persons compared with uninfected persons.79 Polymorphisms in the IL-1β gene cluster, specifically IL-1β-31 and IL-1β-511, are associated with increased IL-1β production, and are associated with a significantly increased risk for hypochlorhydria, gastric atrophy, and distal gastric adenocarcinoma compared other IL-1β genotypes.69,80 These relationships are observed among persons infected with H. pylori, but not among uninfected persons.69,80 Another genetic locus harboring polymorphisms that influence gastric cancer risk encodes TNFα, a pro-inflammatory, acid-suppressive cytokine that is present at higher levels in H. pylori-colonized human gastric mucosa than in the stomach of uninfected persons.81 TNFα polymorphisms linked to increased TNFα production are associated with an increased risk of gastric cancer and its precursors.82
Genetic polymorphisms associated with increased production of IL-1β and TNFα are associated with increased gastric cancer risk, and conversely, polymorphisms associated with decreased production of the anti-inflammatory cytokine IL-10 are associated with increased risk for distal gastric cancer.82 Investigations into the combinatorial effects of IL-1β, TNFα, and IL-10 polymorphisms on the development of cancer have revealed that the risk of cancer increases progressively with an increasing number of pro-inflammatory polymorphisms; in one study, the presence of three high-risk polymorphisms increased the risk of cancer 27-fold over baseline.82
Host genetic factors can influence not only the risk of gastric cancer among H. pylori-infected persons, but can also influence the likelihood that individuals become infected with H. pylori. Two independent genome-wide association studies (GWAS) and a subsequent meta-analysis showed that the TLR-1 and FCGR2A loci are associated with H. pylori sero-prevalence.83
The presence of a virulent strain of H. pylori in a host with genetic risk factors for gastric cancer results in a particularly high risk for development of gastric cancer. One study analyzed the risk for development of gastric cancer in persons harboring high- or low-expression -511 alleles of IL-1β, who were infected with H. pylori isolates that differed in virulence.69 Persons harboring a high-expression IL-1β allele who were infected with cagA+ H. pylori strains had a 25-fold increased risk for gastric cancer compared with uninfected persons. The risk for cancer in persons harboring a high-expression IL-1β allele who were colonized with an H. pylori vacA s1-type strain was increased 87-fold compared with uninfected persons.69
Tobacco Use and Gastric Cancer Risk
Tobacco use has been linked to an increased risk for gastric cancer.84 A meta-analysis of prospective studies concluded that the cumulative risk of gastric cancer risk in male smokers was 1.62 and in female smokers was 1.20, compared with persons who never smoked.85 The risk for gastric cancer increases in a linear fashion with tobacco exposure, whether measured by cigarettes smoked per day, pack-years, or smoking duration.85 Moreover, gastric cancer risk is lower in former smokers compared with current smokers. When analyzed in conjunction with H. pylori and CagA status, the risk for gastric cancer conferred by tobacco increases synergistically; the relative risks for smokers infected with H. pylori were 16.6 and 9.2 for persons harboring CagA+ or CagA- strains, respectively, when compared with non-smokers who were not infected.86 In H. pylori-infected non-smokers, the same relative risks for persons harboring CagA+ or CagA- strains were 6.1 and 2.4 respectively, compared with uninfected non-smokers.86
Dietary Risk Factors for Gastric Cancer
Epidemiologic studies throughout the world have shown a relationship between diet and gastric cancer risk. The diets that are most commonly linked to high gastric cancer risk are those that are rich in salted, pickled, smoked or poorly preserved foods, those with a high meat content, and those with low fruit and vegetable content.87-93
A link between high salt consumption and increased gastric cancer risk has been reported in numerous studies.87,94,95 Dietary salt intake varies widely among humans, and in some populations with a high incidence of gastric cancer, median dietary salt intakes of 46 g per day have been reported.96,97 One study analyzed urinary sodium excretion in persons from 24 different countries and showed a strong correlation between salt intake and gastric cancer mortality rates.98 In Colombia, the consumption of high levels of salt (as measured by high urinary sodium-to-creatinine ratios) was associated with an increased risk for precancerous gastric lesions (chronic atrophic gastritis, intestinal metaplasia, and dysplasia) compared with what is observed in persons who consume lower levels of salt.99 Additionally, a prospective study of a Japanese population, conducted over a 14 y period, reported that H. pylori-infected subjects consuming a high-salt diet had an increased risk of gastric cancer when compared with H. pylori-infected subjects who consumed lower levels of salt.100
Meta-analyses of case-control studies have shown that a high intake of fruits and non-starchy vegetables confers a significant benefit against the development of stomach cancer, an effect that is stronger in Asia than in the United States or Europe. Specifically, flavonoid intake is associated with a significant reduction (20%) in gastric cancer risk in women.101 A recent meta-analysis demonstrated that dietary fiber intake is inversely associated with gastric cancer risk.102 Vitamin C has also been studied as a potential protective factor against the development of gastric cancer, likely through its antioxidant effects. Higher plasma levels of vitamin C have been associated with a lower risk for gastric cancer, irrespective of anatomic site103,104; however, dietary intake of vitamin C has not been associated with a significantly reduced risk for cancer of the stomach.101 A weak positive association has been found between ingestion of alcohol and the development of distal, but not proximal, gastric cancer.105 Finally, non-steroidal anti-inflammatory drug use has been associated with a decreased risk of gastric cancer.106
Over the past century, there have been numerous changes in methods for storing and preserving food in developed countries. The availability of refrigeration has resulted in increased consumption of fresh fruit and vegetables, decreased reliance on older preservative methods (salt, curing or smoking), and a reduction in the consumption of spoiled food.107 The gradual decrease in gastric cancer rates in many populations over the last century may be at least partially attributable to the changes in diet that have accompanied refrigeration.
Iron Deficiency and Gastric Cancer Risk
Iron deficiency is associated with an increased risk for gastric cancer, as well as neoplasms that arise elsewhere in the gastrointestinal tract.108-113 There are multiple mechanisms through which iron deficiency may arise, including blood loss and dietary deficiency of iron. Among the many possible causes of blood loss, colonization by certain H. pylori strains has been associated with hemorrhagic gastritis and a resulting loss of iron.114 Long-term H. pylori infection can also lead to the development of gastric atrophy, which results in hypochlorhydria, lower ascorbic acid levels, and a concomitant reduced absorption of iron.115 Case-control studies have demonstrated an inverse relationship between dietary iron intake and gastric adenocarcinoma,108,109,111,116 which suggests that not only iron deficiency resulting from blood loss but also iron deficiency resulting from a low-iron diet is relevant.
Analysis of Diet in Animal Models of H. pylori Infection
Epidemiologic studies of diet in humans are subject to many limitations, including a reliance on patient reporting and difficulty in ascertaining the foods that were consumed decades prior to the development of gastric cancer. Moreover, it is difficult to determine whether dietary parameters are causally linked to the development gastric cancer or merely markers for other factors that are important in gastric cancer pathogenesis. To further investigate potential relationships between diet and gastric cancer risk, several studies have tested the role of diet in rodent models of H. pylori-induced gastric cancer.
Salt
The effects of a high-salt diet on H. pylori infection and gastric cancer have been investigated using both mouse and gerbil models. One study in mice showed that a high salt diet enhanced levels of H. pylori colonization in the stomach and resulted in increased parietal cell loss.117 Other studies reported that a high salt diet had relatively little effect on disease outcomes in H. pylori-infected mice.118,119 Most of these studies in mice were conducted using H. pylori strains that have a non-functional cag type IV secretion system.
Several studies have provided evidence indicating that a high salt diet contributes to gastric carcinogenesis in Mongolian gerbil models.120,121 One study reported that H. pylori infection and a high-salt diet could independently induce atrophic gastritis and intestinal metaplasia in Mongolian gerbils.122 Other studies provided evidence that the presence of H. pylori and a high-salt diet have a synergistic effect on gastric carcinogenesis in a Mongolian gerbil model when the animals also receive a chemical carcinogen.123,124
To further investigate the effects of a high-salt diet on H. pylori-induced carcinogenesis in a gerbil model, a recent study infected Mongolian gerbils with a wild-type cagA+ H. pylori strain and maintained the animals on a regular diet or a high-salt diet.42 At 4 mo postinfection, gastric adenocarcinoma was detected in a significantly higher proportion of the infected animals on a high-salt diet than in infected animals on a regular diet.42 Infected animals that were fed a high-salt diet had more severe gastric inflammation compared with those on a regular diet.42 Hypochlorhydria, parietal cell loss and high levels of gastric IL-1β were detected in the animals that developed gastric cancer. Animals infected with a cagA mutant strain and fed a high salt diet had low levels of gastric inflammation and did not develop hypochlorhydria or gastric cancer. Similarly, a high salt diet did not cause the development of gastric cancer in uninfected animals.42 These results indicate that a high-salt diet potentiates the carcinogenic effects of cagA+ H. pylori strains.
The high-salt diets used in these studies (containing 6% to 8% sodium chloride) approximate the concentration of sodium chloride in some foods consumed by humans. For example, dried fish is often preserved in 3 to 20% salt, pickled foods contain up to 25% salt, and soy sauce contains 19% salt.96,117 In contrast to human diets, which can vary considerably from day to day, the animals in these studies were fed a high-salt diet with no variation throughout the experiments.
Iron
Iron deficiency in rats accelerates carcinogen-induced gastrointestinal cancer and metastasis.125 To evaluate whether H. pylori infection is sufficient to induce iron deficiency, several studies have examined the effects of chronic Helicobacter infection on iron status in rodents.126,127 One system that has been extensively utilized to study Helicobacter-induced pathogenesis is the transgenic INS-GAS mouse model. INS-GAS mice overexpress human gastrin and spontaneously develop gastric cancer, but this requires the virtual lifetime of the animal (2 y).118 Concomitant infection with Helicobacter felis accelerates this process, suggesting that persistently elevated gastrin levels synergize with Helicobacter to augment cancer progression. Helicobacter infection in INS-GAS mice leads to corpus-predominant gastritis, and parietal cell loss,30 similar to changes that develop in humans; thus this model recapitulates many features of gastric cancer in humans. A recent study demonstrated that infection of INS-GAS mice with H. felis results in decreased serum iron concentrations, reduced numbers of parietal cells, and altered expression of host genes important in iron physiology such as hepcidin, ferroportin 1, and transferrin receptor 1.128 Notably, H. felis does not contain many of the virulence factors present in H. pylori, such as the cag pathogenicity island, which limits its applicability to studies of H. pylori pathogenesis.
A recent study analyzed the effect of dietary iron depletion on the development of H. pylori-induced cancer in gerbils infected with a cagA+ H. pylori strain.129 H. pylori infection induced more severe gastritis in iron-depleted gerbils than in iron-replete gerbils. Gastritis also developed earlier in H. pylori-infected iron-depleted gerbils compared with infected iron-replete gerbils.129 Consistent with the increased severity of gastric inflammation, dysplasia and gastric adenocarcinoma occurred significantly more frequently in H. pylori-infected iron-depleted gerbils compared with iron-replete gerbils.129 A low iron diet did not produce these effects in animals infected with an isogenic cagA-mutant strain. These data demonstrate that dietary iron depletion significantly increases the severity of gastric inflammation and accelerates the development of H. pylori-induced premalignant and malignant lesions in a rodent model of gastric cancer.
Cholesterol
H. pylori is auxotrophic for cholesterol,130 and therefore, the dietary content of cholesterol could potentially have an impact on H. pylori colonization of the stomach or H. pylori-induced gastric disease. In vitro experiments showed that H. pylori follows a cholesterol gradient and extracts lipid from plasma membranes of epithelial cells for subsequent glucosylation. Excessive cholesterol promoted phagocytosis of H. pylori by antigen-presenting cells, such as macrophages and dendritic cells, and enhances antigen-specific T cell responses.130 A cholesterol-rich diet led to T cell-dependent reduction of the H. pylori burden in the stomach. Thus, a diet with high cholesterol content may reduce the risk of subsequent inflammation and injury induced by H. pylori.
Possible Mechanisms by which Diet May Influence Gastric Cancer Risk
There are several potential mechanisms by which dietary constituents may protect against or augment the development of gastric cancer. One possibility is that dietary factors may exert direct effects on the host. For example, direct effects of dietary constituents on the gastric epithelium might either raise or lower the threshold for malignant transformation, or dietary components might damage the gastric mucosa, thereby allowing increased entry of carcinogens into gastric tissue. Certain dietary components are known to interact with intestinal immune receptors and thereby regulate intestinal immunity,131 and potentially similar phenomena might occur in the stomach. The composition of the diet might influence the composition of the gastric microbiota, or may favor the proliferation of H. pylori variants with adaptive properties. Dietary factors may also influence epigenetic alterations, as documented in a recent study which reported that dietary folic acid supplementation protected against loss of global DNA methylation and markedly reduced the development of gastric dysplasia and inflammation in Helicobacter-infected INS-GAS mice.132 Finally, dietary constituents might modulate gene expression in H. pylori, resulting in increased or decreased production of bacterial virulence factors. These potential mechanisms by which diet can modulate gastric cancer risk are not mutually exclusive, and it seems possible that several may be operative. Several recent studies have focused in particular on the concept that diet may directly influence the pathogenic potential of H. pylori by augmenting the expression and function of cancer-associated microbial virulence determinants. In the following sections, we review these recent findings.
Effect of Salt on H. pylori Virulence
Gene expression in several bacterial pathogens, including Vibrio cholerae, Salmonella enterica, Listeria monocytogenes, and Campylobacter jejuni is regulated in response to salt concentration or osmotic stress.133 Similarly, H. pylori gene expression is altered in response to changes in the concentrations of sodium chloride present in the bacterial culture medium.134-136 In response to high sodium chloride concentrations, H. pylori cells change from a typical spiral shape to a more elongated shape and form chains.135 The sodium chloride concentrations tested in these in vitro studies ranged from 0.25% (43 mmol/L) to 2.0% (342 mmol/L), which it hypothesized to be similar to the gastric luminal salt concentrations that are attained in some populations.134 However, salt concentrations in the gastric mucus layer (the natural niche for H. pylori) are likely to be lower than in the gastric lumen.
Both transcriptional studies and proteomic studies have revealed increased expression of cagA in response to high salt conditions.134,136 Concordant with the in vitro results, increased cagA transcription was detected in vivo in H. pylori-infected gerbils that were fed a high-salt diet compared with those on a regular diet.42 Analysis of 36 H. pylori strains isolated from unrelated persons revealed marked differences among strains in salt-responsive CagA expression.137 Sequence analysis of the cagA promoter region in these strains revealed a DNA motif (TAATGA) that was present in either one or two copies. Salt-induced upregulation of CagA expression was detected more commonly in strains containing two copies of the TAATGA motif than in strains containing one copy.137 Mutagenesis experiments confirmed that two copies of the TAATGA motif are required for salt-induced upregulation of CagA expression.
Effects of Iron on H. pylori Gene Expression and Virulence
Iron is an essential micronutrient for virtually all microorganisms, including H. pylori. Due to limited availability of free iron in the host, H. pylori has evolved multiple mechanisms for iron acquisition in vivo.138-140 In contrast to many mucosal pathogens, H. pylori does not synthesize siderophores,140 but can utilize several iron-containing host factors, including lactoferrin, transferrin and hemoglobin, as iron sources. Transferrin and lactoferrin in the iron-bound state can support growth of H. pylori as the sole iron source. H. pylori can utilize both Fe2+ (ferrous) iron, which is relatively soluble and readily available under anaerobic and acidic conditions, as well as Fe3+ (ferric) iron, which is typically complexed with binding molecules such as lactoferrin, transferrin, or heme.141 Several H. pylori proteins are known or predicted to have important roles in iron acquisition. These include three putative FecA homologs (which are predicted to recognize ferric iron), FrpB (which binds heme), TonB/ExbB/ExbD proteins (which aid in iron transport), and FeoNB (which is predicted to function as a ferrous iron transport).138-141 Iron storage proteins are produced by H. pylori to ensure that oxidative stress is not induced by the presence of free iron within the bacterial cell. These include NapA and Pfr, ferritin-like proteins that function to store iron in a non-reactive state.140,141
Experiments using H. pylori DNA microarrays have detected numerous growth phase-dependent gene expression changes that occur in response to iron starvation conditions.142 Several virulence genes were found to be differentially expressed, including cagA and vacA.142 One mechanism by which H. pylori regulates gene expression in response to environmental iron concentrations involves the global ferric uptake regulator (Fur). In comparison to Fur proteins in most other bacteria, H. pylori Fur appears to be unique in that it can function as either a transcriptional repressor or a transcriptional activator under iron-limiting or iron-replete conditions.140,141 This is due to the ability of Fur to bind to cognate promoters in either an iron-bound or an iron-free (apo) form. Iron-bound Fur can repress fur transcription, and thus, fur expression is governed by an autoregulatory mechanism.140,141 Global analyses have been conducted using DNA microarrays and proteomics techniques to identify members of the H. pylori Fur regulon; these include genes involved in iron storage and transport, as well as genes involved in motility, energy metabolism, and respiration.143-145 The consensus sequence recognized by Fur is a 7–1-7 motif with dyad symmetry, and a recent study used this sequence as a probe to identify multiple additional targets within the H. pylori genome that are regulated by Fur.146 Fur-regulated genes not only included those encoding previously identified iron acquisition systems but also genes encoding virulence proteins, such as CagA and gamma-glutamyltransferase (GGT).146
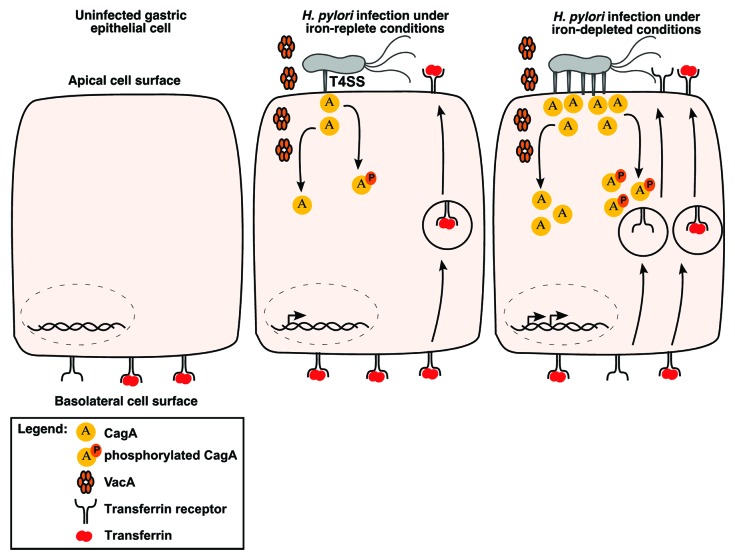
To evaluate the relationship between iron availability and virulence gene expression in the context of host cells, a recent study utilized a Transwell system containing polarized MDCK epithelial cells to demonstrate that wild-type H. pylori could replicate and form microcolonies on the apical surface of epithelial cell monolayers, whereas cagA- isogenic mutants did not.147 In contrast, the cagA- mutant strain was able to form microcolonies if ferric iron was added to the apical chamber.148 More in-depth mechanistic studies revealed that CagA, as well as VacA, coordinate an orchestrated mislocalization of transferrin and transferrin receptors from the basolateral surface to apical surface microniches where H. pylori were attached148 (Fig. 2). This action of CagA was dependent on the presence of EPIYA phosphorylation motifs. The role of CagA in promoting bacterial growth in co-culture systems mimics results observed in vivo, as H. pylori cagA- mutant strains exhibited a significant decrease in colonization density in Mongolian gerbils compared with wild-type H. pylori.148
Figure 2. Effect of low-iron conditions on interactions between H. pylori and host cells. CagA and VacA each induce mislocalization of transferrin receptors from the basolateral cell surface to the apical surface where H. pylori are localized. Under iron-deplete conditions, CagA production is increased, the formation of pili associated with the cag type IV secretion system is increased, and translocation of CagA into host cells occurs at increased levels. This potentially leads to a more robust mislocalization of transferrin receptors to the apical membrane.
To further investigate relationships between iron concentration and H. pylori-host cell interactions, H. pylori has been cultured in vitro under iron-replete or iron-restricted conditions induced by iron chelation with dipyridyl, and then co-cultured with gastric epithelial cells. Cells co-cultured with H. pylori grown under iron-restricted conditions contained significantly higher levels of phosphorylated CagA and produced significantly higher levels of IL-8 than did cells that were co-cultured with bacteria grown under iron-replete conditions.129 Scanning EM studies to analyze the formation of cag T4SS-associated pili at the bacteria-host cell interface revealed that the number of pili formed by H. pylori grown under iron-restricted conditions was significantly higher than the number formed by H. pylori grown under iron-replete conditions.129 The enhanced production of pili stimulated by dipyridyl was abrogated following the addition of exogenous iron. These experiments indicate that exposure of H. pylori to low iron conditions enhances the ability of the bacteria to cause alterations in gastric epithelial cells.
An important goal is to differentiate the specific effects of iron deficiency on H. pylori virulence vs. the effects of iron deficiency on the host. This topic has been addressed by examining proinflammatory cytokine levels in gastric tissue from gerbils. Infection of gerbils with H. pylori resulted in significantly increased gastric levels of IL-1β, IFNγ, and TNFα, compared with levels in uninfected control animals; however, there were no differences in the levels of these cytokines between H. pylori-infected gerbils under iron-replete vs. iron-depleted conditions.129 Although these results do not completely exclude effects exerted by iron deficiency on the host, these data, when viewed within the context of in vitro data, suggest that the direct effects of iron deficiency on H. pylori, particularly enhanced function of the cag T4SS, likely contribute to the increased severity and incidence of gastric disease observed in this model.
Selection of H. pylori Variants with Adaptive Properties in Response to Dietary Conditions
To test the hypothesis that H. pylori adapts to particular environmental conditions associated with changes in diet, H. pylori strains that were cultured from gerbils fed a low iron diet or a regular diet have been compared using two-dimensional (2D) DIGE/mass spectrometry. Multiple proteins differed in abundance when comparing H. pylori strains isolated from iron-deplete vs. iron-replete gerbils, including proteins that mediate survival, microbial adherence and function of the cag T4SS.129 For example, H. pylori FlaA and FlaB, the major flagellin subunits, were significantly upregulated in bacteria cultured from the iron-deplete animals. H. pylori strains isolated from iron-depleted gerbils also expressed significantly higher levels of CagA.129 To further investigate differences between these two types of H. pylori strains, gastric epithelial cells were co-cultured with H. pylori strains isolated from iron-depleted gerbils or iron-replete gerbils. The former cells contained significantly higher levels of phosphorylated CagA and produced significantly higher levels of IL-8 when compared with cells co-cultured with strains isolated from iron-replete gerbils.129 When co-cultured with gastric epithelial cells, the number of cag T4SS pili was significantly increased for strains isolated from iron-depleted vs. iron replete gerbils.129
As another approach for analyzing relationships between properties of the bacteria and the iron state of the host, the properties of H. pylori strains isolated from patients in a geographic region with a high risk of gastric cancer have been correlated with serum ferritin levels of the corresponding patients. Strains from patients with low serum ferritin levels induced more robust inflammatory responses compared with strains isolated from patients with high ferritin levels.129 Collectively, these results suggest that prolonged exposure of H. pylori to low iron conditions in vivo leads to stable changes in the bacteria, including increased activity of cag PAI-mediated phenotypes.
Conclusions
Gastric cancer risk is determined by many factors, including properties of the H. pylori strain, the host genotype, and environmental exposures such as diet, each affecting the level of long-term interactions between H. pylori and humans. Among the approximately one-half of the world’s population that is infected with H. pylori, fewer than 5% will ever develop gastric cancer. Side effects associated with H. pylori treatment, including the development of antibiotic resistance in H. pylori and other commensal flora, preclude the use of H. pylori eradication campaigns on a population-wide basis. Thus, there is great interest in developing techniques to identify sub-populations at high risk for disease. Such targeted therapy requires the use of biological markers other than simply detection of H. pylori per se. For example, iron deficient persons with high-expression IL-1β polymorphisms who are colonized by cag+ strains may represent a population that is most likely to derive benefit from H. pylori eradication, and treatment of such persons could result in a substantially reduced cancer risk. In future studies, it will be important to gain deeper insight into the pathogenesis of H. pylori-induced gastric adenocarcinoma, not only to develop more effective means for preventing and treating this cancer, but also because it might serve as a paradigm for understanding the role of chronic inflammation in the pathogenesis of other malignancies.
Acknowledgments
This study was supported in part by National Institutes of Health Grants CA-116087, DK-58587, DK-77955, DK-058404, AI-039657, AI- 068009, and the Department of Veterans Affairs.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/26262
References
- 1.Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:32–41. doi: 10.1056/NEJM199507063330107. [DOI] [PubMed] [Google Scholar]
- 2.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–15. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 5.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- 6.Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, Bray F. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–50. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 7.Forman D, Pisani P. Gastric cancer in Japan--honing treatment, seeking causes. N Engl J Med. 2008;359:448–51. doi: 10.1056/NEJMp0804354. [DOI] [PubMed] [Google Scholar]
- 8.Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1–27. doi: 10.1093/oxfordjournals.epirev.a036288. [DOI] [PubMed] [Google Scholar]
- 9.Sonnenberg A. Differences in the birth-cohort patterns of gastric cancer and peptic ulcer. Gut. 2010;59:736–43. doi: 10.1136/gut.2009.195008. [DOI] [PubMed] [Google Scholar]
- 10.Haile RW, John EM, Levine AJ, Cortessis VK, Unger JB, Gonzales M, Ziv E, Thompson P, Spruijt-Metz D, Tucker KL, et al. A review of cancer in U.S. Hispanic populations. Cancer Prev Res (Phila) 2012;5:150–63. doi: 10.1158/1940-6207.CAPR-11-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Chen VW, Andrews PA, Ruiz B, Correa P. Incidence of esophageal and gastric cancers among Hispanics, non-Hispanic whites and non-Hispanic blacks in the United States: subsite and histology differences. Cancer Causes Control. 2007;18:585–93. doi: 10.1007/s10552-007-9000-1. [DOI] [PubMed] [Google Scholar]
- 12.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF., Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–9. doi: 10.1001/jama.1991.03460100089030. [DOI] [PubMed] [Google Scholar]
- 13.Pera M, Cameron AJ, Trastek VF, Carpenter HA, Zinsmeister AR. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993;104:510–3. doi: 10.1016/0016-5085(93)90420-h. [DOI] [PubMed] [Google Scholar]
- 14.Correa P, Cuello C, Duque E, Burbano LC, Garcia FT, Bolanos O, Brown C, Haenszel W. Gastric cancer in Colombia. III. Natural history of precursor lesions. J Natl Cancer Inst. 1976;57:1027–35. doi: 10.1093/jnci/57.5.1027. [DOI] [PubMed] [Google Scholar]
- 15.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–5. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 16.Polk DB, Peek RM., Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–14. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–9. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863–73. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J Clin Invest. 2009;119:2475–87. doi: 10.1172/JCI38605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22:283–97. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 21.The EUROGAST Study Group An international association between Helicobacter pylori infection and gastric cancer. Lancet. 1993;341:1359–62. doi: 10.1016/0140-6736(93)90938-D. [DOI] [PubMed] [Google Scholar]
- 22.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–79. doi: 10.1016/S0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 23.Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, Abnet CC, Albanes D, Virtamo J, Taylor PR. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445–52. doi: 10.1093/jnci/djj393. [DOI] [PubMed] [Google Scholar]
- 24.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 25.Nomura A, Stemmermann GN, Chyou PH, Kato I, Pérez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–6. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 26.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–31. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 27.Dooley CP, Cohen H, Fitzgibbons PL, Bauer M, Appleman MD, Perez-Perez GI, Blaser MJ. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989;321:1562–6. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- 28.Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306–23. doi: 10.1053/j.gastro.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63–96. doi: 10.1146/annurev.pathol.1.110304.100125. [DOI] [PubMed] [Google Scholar]
- 30.Peek RM, Jr., Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 31.Blaser MJ, Berg DE. Helicobacter pylori genetic diversity and risk of human disease. J Clin Invest. 2001;107:767–73. doi: 10.1172/JCI12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996;93:14648–53. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akopyants NS, Clifton SW, Kersulyte D, Crabtree JE, Youree BE, Reece CA, Bukanov NO, Drazek ES, Roe BA, Berg DE. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 34.Olbermann P, Josenhans C, Moodley Y, Uhr M, Stamer C, Vauterin M, Suerbaum S, Achtman M, Linz B. A global overview of the genetic and functional diversity in the Helicobacter pylori cag pathogenicity island. PLoS Genet. 2010;6:e1001069. doi: 10.1371/journal.pgen.1001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 36.Fischer W, Püls J, Buhrdorf R, Gebert B, Odenbreit S, Haas R. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol Microbiol. 2001;42:1337–48. doi: 10.1046/j.1365-2958.2001.02714.x. [DOI] [PubMed] [Google Scholar]
- 37.Kwok T, Zabler D, Urman S, Rohde M, Hartig R, Wessler S, Misselwitz R, Berger J, Sewald N, König W, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449:862–6. doi: 10.1038/nature06187. [DOI] [PubMed] [Google Scholar]
- 38.Shaffer CL, Gaddy JA, Loh JT, Johnson EM, Hill S, Hennig EE, McClain MS, McDonald WH, Cover TL. Helicobacter pylori exploits a unique repertoire of type IV secretion system components for pilus assembly at the bacteria-host cell interface. PLoS Pathog. 2011;7:e1002237. doi: 10.1371/journal.ppat.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–5. [PubMed] [Google Scholar]
- 40.Nomura AM, Lee J, Stemmermann GN, Nomura RY, Perez-Perez GI, Blaser MJ. Helicobacter pylori CagA seropositivity and gastric carcinoma risk in a Japanese American population. J Infect Dis. 2002;186:1138–44. doi: 10.1086/343808. [DOI] [PubMed] [Google Scholar]
- 41.Franco AT, Johnston E, Krishna U, Yamaoka Y, Israel DA, Nagy TA, Wroblewski LE, Piazuelo MB, Correa P, Peek RM., Jr. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68:379–87. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaddy JA, Radin JN, Loh JT, Zhang F, Washington MK, Peek RM, Jr., Algood HM, Cover TL. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect Immun. 2013;81:2258–67. doi: 10.1128/IAI.01271-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller D, Tegtmeyer N, Brandt S, Yamaoka Y, De Poire E, Sgouras D, Wessler S, Torres J, Smolka A, Backert S. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. J Clin Invest. 2012;122:1553–66. doi: 10.1172/JCI61143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683–6. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- 45.Saadat I, Higashi H, Obuse C, Umeda M, Murata-Kamiya N, Saito Y, Lu H, Ohnishi N, Azuma T, Suzuki A, et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447:330–3. doi: 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- 46.Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–4. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, Neish AS, Collier-Hyams L, Perez-Perez GI, Hatakeyama M, et al. Activation of β-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:10646–51. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H, Aburatani H, Akiyama T, Peek RM, Jr., Azuma T, et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the β-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617–26. doi: 10.1038/sj.onc.1210251. [DOI] [PubMed] [Google Scholar]
- 49.Brandt S, Kwok T, Hartig R, König W, Backert S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci U S A. 2005;102:9300–5. doi: 10.1073/pnas.0409873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohnishi N, Yuasa H, Tanaka S, Sawa H, Miura M, Matsui A, Higashi H, Musashi M, Iwabuchi K, Suzuki M, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci U S A. 2008;105:1003–8. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688–94. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 52.Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3:320–32. doi: 10.1038/nrmicro1095. [DOI] [PubMed] [Google Scholar]
- 53.Boquet P, Ricci V. Intoxication strategy of Helicobacter pylori VacA toxin. Trends Microbiol. 2012;20:165–74. doi: 10.1016/j.tim.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Cover TL, Blaser MJ. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–5. [PubMed] [Google Scholar]
- 55.Gangwer KA, Mushrush DJ, Stauff DL, Spiller B, McClain MS, Cover TL, Lacy DB. Crystal structure of the Helicobacter pylori vacuolating toxin p55 domain. Proc Natl Acad Sci U S A. 2007;104:16293–8. doi: 10.1073/pnas.0707447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwamoto H, Czajkowsky DM, Cover TL, Szabo G, Shao Z. VacA from Helicobacter pylori: a hexameric chloride channel. FEBS Lett. 1999;450:101–4. doi: 10.1016/S0014-5793(99)00474-3. [DOI] [PubMed] [Google Scholar]
- 57.Szabò I, Brutsche S, Tombola F, Moschioni M, Satin B, Telford JL, Rappuoli R, Montecucco C, Papini E, Zoratti M. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. EMBO J. 1999;18:5517–27. doi: 10.1093/emboj/18.20.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McClain MS, Iwamoto H, Cao P, Vinion-Dubiel AD, Li Y, Szabo G, Shao Z, Cover TL. Essential role of a GXXXG motif for membrane channel formation by Helicobacter pylori vacuolating toxin. J Biol Chem. 2003;278:12101–8. doi: 10.1074/jbc.M212595200. [DOI] [PubMed] [Google Scholar]
- 59.Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301:1099–102. doi: 10.1126/science.1086871. [DOI] [PubMed] [Google Scholar]
- 60.Boncristiano M, Paccani SR, Barone S, Ulivieri C, Patrussi L, Ilver D, Amedei A, D’Elios MM, Telford JL, Baldari CT. The Helicobacter pylori vacuolating toxin inhibits T cell activation by two independent mechanisms. J Exp Med. 2003;198:1887–97. doi: 10.1084/jem.20030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sundrud MS, Torres VJ, Unutmaz D, Cover TL. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc Natl Acad Sci U S A. 2004;101:7727–32. doi: 10.1073/pnas.0401528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Torres VJ, VanCompernolle SE, Sundrud MS, Unutmaz D, Cover TL. Helicobacter pylori vacuolating cytotoxin inhibits activation-induced proliferation of human T and B lymphocyte subsets. J Immunol. 2007;179:5433–40. doi: 10.4049/jimmunol.179.8.5433. [DOI] [PubMed] [Google Scholar]
- 63.Atherton JC, Cao P, Peek RM, Jr., Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–7. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 64.Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, Eshagh Hosseini M, Atherton JC. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133:926–36. doi: 10.1053/j.gastro.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 65.Letley DP, Atherton JC. Natural diversity in the N terminus of the mature vacuolating cytotoxin of Helicobacter pylori determines cytotoxin activity. J Bacteriol. 2000;182:3278–80. doi: 10.1128/JB.182.11.3278-3280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McClain MS, Cao P, Iwamoto H, Vinion-Dubiel AD, Szabo G, Shao Z, Cover TL. A 12-amino-acid segment, present in type s2 but not type s1 Helicobacter pylori VacA proteins, abolishes cytotoxin activity and alters membrane channel formation. J Bacteriol. 2001;183:6499–508. doi: 10.1128/JB.183.22.6499-6508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pagliaccia C, de Bernard M, Lupetti P, Ji X, Burroni D, Cover TL, Papini E, Rappuoli R, Telford JL, Reyrat JM. The m2 form of the Helicobacter pylori cytotoxin has cell type-specific vacuolating activity. Proc Natl Acad Sci U S A. 1998;95:10212–7. doi: 10.1073/pnas.95.17.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.González-Rivera C, Algood HM, Radin JN, McClain MS, Cover TL. The intermediate region of Helicobacter pylori VacA is a determinant of toxin potency in a Jurkat T cell assay. Infect Immun. 2012;80:2578–88. doi: 10.1128/IAI.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, Carvalho R, Capelinha AF, Quint W, Caldas C, van Doorn LJ, et al. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680–7. doi: 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- 70.Basso D, Zambon CF, Letley DP, Stranges A, Marchet A, Rhead JL, Schiavon S, Guariso G, Ceroti M, Nitti D, et al. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135:91–9. doi: 10.1053/j.gastro.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 71.Miehlke S, Kirsch C, Agha-Amiri K, Günther T, Lehn N, Malfertheiner P, Stolte M, Ehninger G, Bayerdörffer E. The Helicobacter pylori vacA s1, m1 genotype and cagA is associated with gastric carcinoma in Germany. Int J Cancer. 2000;87:322–7. doi: 10.1002/1097-0215(20000801)87:3<322::AID-IJC3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 72.Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Borén T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–7. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 73.Gerhard M, Lehn N, Neumayer N, Borén T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci U S A. 1999;96:12778–83. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oliveira AG, Santos A, Guerra JB, Rocha GA, Rocha AM, Oliveira CA, Cabral MM, Nogueira AM, Queiroz DM. babA2- and cagA-positive Helicobacter pylori strains are associated with duodenal ulcer and gastric carcinoma in Brazil. J Clin Microbiol. 2003;41:3964–6. doi: 10.1128/JCM.41.8.3964-3966.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu J, Leung WK, Go MY, Chan MC, To KF, Ng EK, Chan FK, Ling TK, Chung SC, Sung JJ. Relationship between Helicobacter pylori babA2 status with gastric epithelial cell turnover and premalignant gastric lesions. Gut. 2002;51:480–4. doi: 10.1136/gut.51.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hennig EE, Mernaugh R, Edl J, Cao P, Cover TL. Heterogeneity among Helicobacter pylori strains in expression of the outer membrane protein BabA. Infect Immun. 2004;72:3429–35. doi: 10.1128/IAI.72.6.3429-3435.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ito Y, Azuma T, Ito S, Miyaji H, Hirai M, Yamazaki Y, Sato F, Kato T, Kohli Y, Kuriyama M. Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J Clin Microbiol. 1997;35:1710–4. doi: 10.1128/jcm.35.7.1710-1714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hatakeyama M. Anthropological and clinical implications for the structural diversity of the Helicobacter pylori CagA oncoprotein. Cancer Sci. 2011;102:36–43. doi: 10.1111/j.1349-7006.2010.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noach LA, Bosma NB, Jansen J, Hoek FJ, van Deventer SJ, Tytgat GN. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425–9. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- 80.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 81.Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;32:1473–7. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–201. doi: 10.1016/S0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 83.Mayerle J, den Hoed CM, Schurmann C, Stolk L, Homuth G, Peters MJ, Capelle LG, Zimmermann K, Rivadeneira F, Gruska S, et al. Identification of genetic loci associated with Helicobacter pylori serologic status. JAMA. 2013;309:1912–20. doi: 10.1001/jama.2013.4350. [DOI] [PubMed] [Google Scholar]
- 84.Shikata K, Doi Y, Yonemoto K, Arima H, Ninomiya T, Kubo M, Tanizaki Y, Matsumoto T, Iida M, Kiyohara Y. Population-based prospective study of the combined influence of cigarette smoking and Helicobacter pylori infection on gastric cancer incidence: the Hisayama Study. Am J Epidemiol. 2008;168:1409–15. doi: 10.1093/aje/kwn276. [DOI] [PubMed] [Google Scholar]
- 85.Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, Lunet N. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689–701. doi: 10.1007/s10552-008-9132-y. [DOI] [PubMed] [Google Scholar]
- 86.Brenner H, Arndt V, Bode G, Stegmaier C, Ziegler H, Stümer T. Risk of gastric cancer among smokers infected with Helicobacter pylori. Int J Cancer. 2002;98:446–9. doi: 10.1002/ijc.10201. [DOI] [PubMed] [Google Scholar]
- 87.Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 88.Epplein M, Nomura AM, Hankin JH, Blaser MJ, Perez-Perez G, Stemmermann GN, Wilkens LR, Kolonel LN. Association of Helicobacter pylori infection and diet on the risk of gastric cancer: a case-control study in Hawaii. Cancer Causes Control. 2008;19:869–77. doi: 10.1007/s10552-008-9149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.González CA, Jakszyn P, Pera G, Agudo A, Bingham S, Palli D, Ferrari P, Boeing H, del Giudice G, Plebani M, et al. Meat intake and risk of stomach and esophageal adenocarcinoma within the European Prospective Investigation Into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2006;98:345–54. doi: 10.1093/jnci/djj071. [DOI] [PubMed] [Google Scholar]
- 90.Gonzalez CA, Lujan-Barroso L, Bueno-de-Mesquita HB, Jenab M, Duell EJ, Agudo A, Tjønneland A, Boutron-Ruault MC, Clavel-Chapelon F, Touillaud M, et al. Fruit and vegetable intake and the risk of gastric adenocarcinoma: a reanalysis of the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study after a longer follow-up. Int J Cancer. 2012;131:2910–9. doi: 10.1002/ijc.27565. [DOI] [PubMed] [Google Scholar]
- 91.Kim HJ, Lim SY, Lee JS, Park S, Shin A, Choi BY, Shimazu T, Inoue M, Tsugane S, Kim J. Fresh and pickled vegetable consumption and gastric cancer in Japanese and Korean populations: a meta-analysis of observational studies. Cancer Sci. 2010;101:508–16. doi: 10.1111/j.1349-7006.2009.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ren JS, Kamangar F, Forman D, Islami F. Pickled food and risk of gastric cancer--a systematic review and meta-analysis of English and Chinese literature. Cancer Epidemiol Biomarkers Prev. 2012;21:905–15. doi: 10.1158/1055-9965.EPI-12-0202. [DOI] [PubMed] [Google Scholar]
- 93.Kim MK, Sasaki S, Sasazuki S, Tsugane S, Japan Public Health Center-based Prospective Study Group Prospective study of three major dietary patterns and risk of gastric cancer in Japan. Int J Cancer. 2004;110:435–42. doi: 10.1002/ijc.20132. [DOI] [PubMed] [Google Scholar]
- 94.Lee SA, Kang D, Shim KN, Choe JW, Hong WS, Choi H. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J Epidemiol. 2003;13:162–8. doi: 10.2188/jea.13.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsugane S. Salt, salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci. 2005;96:1–6. doi: 10.1111/j.1349-7006.2005.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oiso T. Incidence of stomach cancer and its relation to dietary habits and nutrition in Japan between 1900 and 1975. Cancer Res. 1975;35:3254–8. [PubMed] [Google Scholar]
- 97.You WC, Blot WJ, Chang YS, Ershow AG, Yang ZT, An Q, Henderson B, Xu GW, Fraumeni JF, Jr., Wang TG. Diet and high risk of stomach cancer in Shandong, China. Cancer Res. 1988;48:3518–23. [PubMed] [Google Scholar]
- 98.Joossens JV, Hill MJ, Elliott P, Stamler R, Lesaffre E, Dyer A, Nichols R, Kesteloot H, European Cancer Prevention (ECP) and the INTERSALT Cooperative Research Group Dietary salt, nitrate and stomach cancer mortality in 24 countries. Int J Epidemiol. 1996;25:494–504. doi: 10.1093/ije/25.3.494. [DOI] [PubMed] [Google Scholar]
- 99.Chen VW, Abu-Elyazeed RR, Zavala DE, Ktsanes VK, Haenszel W, Cuello C, Montes G, Correa P. Risk factors of gastric precancerous lesions in a high-risk Colombian population. I. Salt. Nutr Cancer. 1990;13:59–65. doi: 10.1080/01635589009514045. [DOI] [PubMed] [Google Scholar]
- 100.Shikata K, Kiyohara Y, Kubo M, Yonemoto K, Ninomiya T, Shirota T, Tanizaki Y, Doi Y, Tanaka K, Oishi Y, et al. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer. 2006;119:196–201. doi: 10.1002/ijc.21822. [DOI] [PubMed] [Google Scholar]
- 101.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253–6. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Z, Xu G, Ma M, Yang J, Liu X. Dietary fiber intake reduces risk for gastric cancer: a meta-analysis. Gastroenterology. 2013;145:113–20, e3. doi: 10.1053/j.gastro.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 103.You WC, Zhang L, Gail MH, Chang YS, Liu WD, Ma JL, Li JY, Jin ML, Hu YR, Yang CS, et al. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. J Natl Cancer Inst. 2000;92:1607–12. doi: 10.1093/jnci/92.19.1607. [DOI] [PubMed] [Google Scholar]
- 104.Jenab M, Riboli E, Ferrari P, Sabate J, Slimani N, Norat T, Friesen M, Tjønneland A, Olsen A, Overvad K, et al. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Carcinogenesis. 2006;27:2250–7. doi: 10.1093/carcin/bgl096. [DOI] [PubMed] [Google Scholar]
- 105.Tramacere I, Negri E, Pelucchi C, Bagnardi V, Rota M, Scotti L, Islami F, Corrao G, La Vecchia C, Boffetta P. A meta-analysis on alcohol drinking and gastric cancer risk. Ann Oncol. 2012;23:28–36. doi: 10.1093/annonc/mdr135. [DOI] [PubMed] [Google Scholar]
- 106.Wang WH, Huang JQ, Zheng GF, Lam SK, Karlberg J, Wong BC. Non-steroidal anti-inflammatory drug use and the risk of gastric cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2003;95:1784–91. doi: 10.1093/jnci/djg106. [DOI] [PubMed] [Google Scholar]
- 107.Correa P. Gastric cancer: overview. Gastroenterol Clin North Am. 2013;42:211–7. doi: 10.1016/j.gtc.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nomura A, Chyou PH, Stemmermann GN. Association of serum ferritin levels with the risk of stomach cancer. Cancer Epidemiol Biomarkers Prev. 1992;1:547–50. [PubMed] [Google Scholar]
- 109.Harrison LE, Zhang ZF, Karpeh MS, Sun M, Kurtz RC. The role of dietary factors in the intestinal and diffuse histologic subtypes of gastric adenocarcinoma: a case-control study in the U.S. Cancer 1997; 80:1021-1028. [PubMed]
- 110.Prá D, Rech Franke SI, Pegas Henriques JA, Fenech M. A possible link between iron deficiency and gastrointestinal carcinogenesis. Nutr Cancer. 2009;61:415–26. doi: 10.1080/01635580902803701. [DOI] [PubMed] [Google Scholar]
- 111.Akiba S, Neriishi K, Blot WJ, Kabuto M, Stevens RG, Kato H, Land CE. Serum ferritin and stomach cancer risk among a Japanese population. Cancer. 1991;67:1707–12. doi: 10.1002/1097-0142(19910315)67:6<1707::AID-CNCR2820670638>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 112.van Lee L, Heyworth J, McNaughton S, Iacopetta B, Clayforth C, Fritschi L. Selected dietary micronutrients and the risk of right- and left-sided colorectal cancers: a case-control study in Western Australia. Ann Epidemiol. 2011;21:170–7. doi: 10.1016/j.annepidem.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 113.Ioannou GN, Rockey DC, Bryson CL, Weiss NS. Iron deficiency and gastrointestinal malignancy: a population-based cohort study. Am J Med. 2002;113:276–80. doi: 10.1016/S0002-9343(02)01214-7. [DOI] [PubMed] [Google Scholar]
- 114.Yip R, Limburg PJ, Ahlquist DA, Carpenter HA, O’Neill A, Kruse D, Stitham S, Gold BD, Gunter EW, Looker AC, et al. Pervasive occult gastrointestinal bleeding in an Alaska native population with prevalent iron deficiency. Role of Helicobacter pylori gastritis. JAMA. 1997;277:1135–9. doi: 10.1001/jama.1997.03540380049030. [DOI] [PubMed] [Google Scholar]
- 115.Waring AJ, Drake IM, Schorah CJ, White KL, Lynch DA, Axon AT, Dixon MF. Ascorbic acid and total vitamin C concentrations in plasma, gastric juice, and gastrointestinal mucosa: effects of gastritis and oral supplementation. Gut. 1996;38:171–6. doi: 10.1136/gut.38.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Knekt P, Reunanen A, Takkunen H, Aromaa A, Heliövaara M, Hakulinen T. Body iron stores and risk of cancer. Int J Cancer. 1994;56:379–82. doi: 10.1002/ijc.2910560315. [DOI] [PubMed] [Google Scholar]
- 117.Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 1999;59:4823–8. [PubMed] [Google Scholar]
- 118.Fox JG, Rogers AB, Ihrig M, Taylor NS, Whary MT, Dockray G, Varro A, Wang TC. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res. 2003;63:942–50. [PubMed] [Google Scholar]
- 119.Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 2005;65:10709–15. doi: 10.1158/0008-5472.CAN-05-1846. [DOI] [PubMed] [Google Scholar]
- 120.Gamboa-Dominguez A, Ubbelohde T, Saqui-Salces M, Romano-Mazzoti L, Cervantes M, Domínguez-Fonseca C, de la Luz Estreber M, Ruíz-Palacios GM. Salt and stress synergize H. pylori-induced gastric lesions, cell proliferation, and p21 expression in Mongolian gerbils. Dig Dis Sci. 2007;52:1517–26. doi: 10.1007/s10620-006-9524-3. [DOI] [PubMed] [Google Scholar]
- 121.Toyoda T, Tsukamoto T, Hirano N, Mizoshita T, Kato S, Takasu S, Ban H, Tatematsu M. Synergistic upregulation of inducible nitric oxide synthase and cyclooxygenase-2 in gastric mucosa of Mongolian gerbils by a high-salt diet and Helicobacter pylori infection. Histol Histopathol. 2008;23:593–9. doi: 10.14670/HH-23.593. [DOI] [PubMed] [Google Scholar]
- 122.Bergin IL, Sheppard BJ, Fox JG. Helicobacter pylori infection and high dietary salt independently induce atrophic gastritis and intestinal metaplasia in commercially available outbred Mongolian gerbils. Dig Dis. Sci Mar. 2003;48:475–85. doi: 10.1023/a:1022524313355. [DOI] [PubMed] [Google Scholar]
- 123.Nozaki K, Shimizu N, Inada K, Tsukamoto T, Inoue M, Kumagai T, Sugiyama A, Mizoshita T, Kaminishi M, Tatematsu M. Synergistic promoting effects of Helicobacter pylori infection and high-salt diet on gastric carcinogenesis in Mongolian gerbils. Jpn J Cancer Res. 2002;93:1083–9. doi: 10.1111/j.1349-7006.2002.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kato S, Tsukamoto T, Mizoshita T, Tanaka H, Kumagai T, Ota H, Katsuyama T, Asaka M, Tatematsu M. High salt diets dose-dependently promote gastric chemical carcinogenesis in Helicobacter pylori-infected Mongolian gerbils associated with a shift in mucin production from glandular to surface mucous cells. Int J Cancer. 2006;119:1558–66. doi: 10.1002/ijc.21810. [DOI] [PubMed] [Google Scholar]
- 125.Jagadeesan V, Rao NJ, Sesikeran B. Effect of iron deficiency on DMH-induced gastrointestinal tract tumors and occurrence of hepatocyte abnormalities in Fischer rats. Nutr Cancer. 1994;22:285–91. doi: 10.1080/01635589409514354. [DOI] [PubMed] [Google Scholar]
- 126.Keenan JI, Peterson RA, Fraser R, Frampton CM, Walmsley TA, Allardyce RA, Roake JA. The effect of Helicobacter pylori infection and dietary iron deficiency on host iron homeostasis: a study in mice. Helicobacter. 2004;9:643–50. doi: 10.1111/j.1083-4389.2004.00278.x. [DOI] [PubMed] [Google Scholar]
- 127.Gøbel R, Symonds EL, Kritas S, Butler RN, Tran CD. Helicobacter felis infection causes an acute iron deficiency in nonpregnant and pregnant mice. Helicobacter. 2006;11:529–32. doi: 10.1111/j.1523-5378.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 128.Thomson MJ, Pritchard DM, Boxall SA, Abuderman AA, Williams JM, Varro A, Crabtree JE. Gastric Helicobacter infection induces iron deficiency in the INS-GAS mouse. PLoS One. 2012;7:e50194. doi: 10.1371/journal.pone.0050194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, Colvin DC, Romero-Gallo J, Suarez G, Loh J, Slaughter JC, et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest. 2013;123:479–92. doi: 10.1172/JCI64373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wunder C, Churin Y, Winau F, Warnecke D, Vieth M, Lindner B, Zähringer U, Mollenkopf HJ, Heinz E, Meyer TF. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat Med. 2006;12:1030–8. doi: 10.1038/nm1480. [DOI] [PubMed] [Google Scholar]
- 131.Tilg H. Diet and intestinal immunity. N Engl J Med. 2012;366:181–3. doi: 10.1056/NEJMcibr1113158. [DOI] [PubMed] [Google Scholar]
- 132.Gonda TA, Kim Y-I, Salas MC, Gamble MV, Shibata W, Muthupalani S, Sohn KJ, Abrams JA, Fox JG, Wang TC, et al. Folic acid increases global DNA methylation and reduces inflammation to prevent Helicobacter-associated gastric cancer in mice. Gastroenterology. 2012;142:824–33, e7. doi: 10.1053/j.gastro.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 133.Cameron A, Frirdich E, Huynh S, Parker CT, Gaynor EC. Hyperosmotic stress response of Campylobacter jejuni. J Bacteriol. 2012;194:6116–30. doi: 10.1128/JB.01409-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67:4709–15. doi: 10.1158/0008-5472.CAN-06-4746. [DOI] [PubMed] [Google Scholar]
- 135.Gancz H, Jones KR, Merrell DS. Sodium chloride affects Helicobacter pylori growth and gene expression. J Bacteriol. 2008;190:4100–5. doi: 10.1128/JB.01728-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Loh JT, Friedman DB, Piazuelo MB, Bravo LE, Wilson KT, Peek RM, Jr., Correa P, Cover TL. Analysis of Helicobacter pylori cagA promoter elements required for salt-induced upregulation of CagA expression. Infect Immun. 2012;80:3094–106. doi: 10.1128/IAI.00232-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Loh JT, Shaffer CL, Piazuelo MB, Bravo LE, McClain MS, Correa P, Cover TL. Analysis of cagA in Helicobacter pylori strains from Colombian populations with contrasting gastric cancer risk reveals a biomarker for disease severity. Cancer Epidemiol Biomarkers Prev. 2011;20:2237–49. doi: 10.1158/1055-9965.EPI-11-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gilbreath JJ, Cody WL, Merrell DS, Hendrixson DR. Change is good: variations in common biological mechanisms in the epsilonproteobacterial genera Campylobacter and Helicobacter. Microbiol Mol Biol Rev. 2011;75:84–132. doi: 10.1128/MMBR.00035-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pich OQ, Merrell DS. The ferric uptake regulator of Helicobacter pylori: a critical player in the battle for iron and colonization of the stomach. Future Microbiol. 2013;8:725–38. doi: 10.2217/fmb.13.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ge R, Sun X. Iron trafficking system in Helicobacter pylori. Biometals. 2012;25:247–58. doi: 10.1007/s10534-011-9512-8. [DOI] [PubMed] [Google Scholar]
- 141.Whitmire JM, Gancz H, Merrell DS. Balancing the double-edged sword: metal ion homeostasis and the ulcer bug. Curr Med Chem. 2007;14:469–78. doi: 10.2174/092986707779941069. [DOI] [PubMed] [Google Scholar]
- 142.Merrell DS, Thompson LJ, Kim CC, Mitchell H, Tompkins LS, Lee A, Falkow S. Growth phase-dependent response of Helicobacter pylori to iron starvation. Infect Immun. 2003;71:6510–25. doi: 10.1128/IAI.71.11.6510-6525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gancz H, Censini S, Merrell DS. Iron and pH homeostasis intersect at the level of Fur regulation in the gastric pathogen Helicobacter pylori. Infect Immun. 2006;74:602–14. doi: 10.1128/IAI.74.1.602-614.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ernst FD, Bereswill S, Waidner B, Stoof J, Mäder U, Kusters JG, Kuipers EJ, Kist M, van Vliet AH, Homuth G. Transcriptional profiling of Helicobacter pylori Fur- and iron-regulated gene expression. Microbiology. 2005;151:533–46. doi: 10.1099/mic.0.27404-0. [DOI] [PubMed] [Google Scholar]
- 145.Lee HW, Choe YH, Kim DK, Jung SY, Lee NG. Proteomic analysis of a ferric uptake regulator mutant of Helicobacter pylori: regulation of Helicobacter pylori gene expression by ferric uptake regulator and iron. Proteomics. 2004;4:2014–27. doi: 10.1002/pmic.200300740. [DOI] [PubMed] [Google Scholar]
- 146.Pich OQ, Carpenter BM, Gilbreath JJ, Merrell DS. Detailed analysis of Helicobacter pylori Fur-regulated promoters reveals a Fur box core sequence and novel Fur-regulated genes. Mol Microbiol. 2012;84:921–41. doi: 10.1111/j.1365-2958.2012.08066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tan S, Tompkins LS, Amieva MR. Helicobacter pylori usurps cell polarity to turn the cell surface into a replicative niche. PLoS Pathog. 2009;5:e1000407. doi: 10.1371/journal.ppat.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tan S, Noto JM, Romero-Gallo J, Peek RM, Jr., Amieva MR. Helicobacter pylori perturbs iron trafficking in the epithelium to grow on the cell surface. PLoS Pathog. 2011;7:e1002050. doi: 10.1371/journal.ppat.1002050. [DOI] [PMC free article] [PubMed] [Google Scholar]