Aphid-derived protein elicitors trigger distinct plant innate immune responses that are dependent on BAK1 and PAD3.
Abstract
The importance of pathogen-associated molecular pattern-triggered immunity (PTI) against microbial pathogens has been recently demonstrated. However, it is currently unclear if this layer of immunity mediated by surface-localized pattern recognition receptors (PRRs) also plays a role in basal resistance to insects, such as aphids. Here, we show that PTI is an important component of plant innate immunity to insects. Extract of the green peach aphid (GPA; Myzus persicae) triggers responses characteristic of PTI in Arabidopsis (Arabidopsis thaliana). Two separate eliciting GPA-derived fractions trigger induced resistance to GPA that is dependent on the leucine-rich repeat receptor-like kinase BRASSINOSTEROID INSENSITIVE1-ASSOCIATED KINASE1 (BAK1)/SOMATIC-EMBRYOGENESIS RECEPTOR-LIKE KINASE3, which is a key regulator of several leucine-rich repeat-containing PRRs. BAK1 is required for GPA elicitor-mediated induction of reactive oxygen species and callose deposition. Arabidopsis bak1 mutant plants are also compromised in immunity to the pea aphid (Acyrthosiphon pisum), for which Arabidopsis is normally a nonhost. Aphid-derived elicitors induce expression of PHYTOALEXIN DEFICIENT3 (PAD3), a key cytochrome P450 involved in the biosynthesis of camalexin, which is a major Arabidopsis phytoalexin that is toxic to GPA. PAD3 is also required for induced resistance to GPA, independently of BAK1 and reactive oxygen species production. Our results reveal that plant innate immunity to insects may involve early perception of elicitors by cell surface-localized PRRs, leading to subsequent downstream immune signaling.
Close to a million insect species have so far been described, and nearly one-half of them feed on plants (Wu and Baldwin, 2010). Within these plant-feeding insects, most feed on a few related plant species, with only 10% feeding upon multiple plant families (Schoonhoven et al., 2005). Plant defense to insects include several layers (Bos and Hogenhout, 2011; Hogenhout and Bos, 2011). Physical barriers, volatile cues, and composition of secondary metabolites of plants are important components that determine insect host choice (Howe and Jander, 2008; Bruce and Pickett, 2011). In addition, plants induce a variety of plant defense responses upon perception of herbivore oral secretions (OS), saliva, and eggs (De Vos and Jander, 2009; Bruessow et al., 2010; Ma et al., 2010; Wu and Baldwin, 2010). These responses may provide full protection against the majority of insect herbivores, and insects that are able to colonize specific plant species likely produce effectors in their saliva or during egg laying that suppress these induced defense responses (Bos and Hogenhout, 2011; Hogenhout and Bos, 2011; Pitino and Hogenhout, 2013).
Aphids are sap-feeding insects of the order Hemiptera and are among the most destructive pests in agriculture, particularly in temperate regions (Blackman and Eastop, 2000). More than 4,000 aphid species in 10 families are known (Dixon, 1998). Most aphid species are specialists and use one or a few closely related plant species within one family as host for feeding and reproduction. Examples are pea aphid (Acyrthosiphon pisum), cabbage aphid (Brevicoryne brassicae), and English grain aphid (Sitobion avenae) that colonize plant species within the legumes (family Fabaceae), brassicas (Brassicaceae), and grasses (Gramineae), respectively. The green peach aphid (GPA; Myzus persicae) is one of few aphid species with a broad host range and can colonize hundreds of plants species in over 40 plant families, including brassicas (Blackman and Eastop, 2000). Aphids possess mouthparts composed of stylets that navigate to the plant vascular system, predominantly the phloem, for long-term feeding. However, before establishing a long-term feeding site, these insects display a host selection behavior by probing the upper leaf cell layers with their stylets, a behavior seen on host and nonhost plants of the aphid (Nam and Hardie, 2012). When the plant is judged unsuitable, the aphid takes off to find an alternative plant host. It is not yet clear what happens in the initial stages of insect interactions with plants.
Plants sense microbial organisms (including bacteria, fungi, and oomycetes) through perception of conserved molecules, named microbe-associated molecular patterns and pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors (PRRs) to induce the first stage of plant immunity, termed PAMP-triggered immunity (PTI). PTI is effective against the majority of plant pathogens. Bacterial and fungal PAMPs characterized so far include bacterial flagellin (or its derived peptide flg22), bacterial elongation factor (EF)-Tu (or its derived peptide elf18), bacterial lipopolysaccharides and bacterial cold shock protein, chitin oligosaccharides, and the oomycete elicitin INF1 (Boller and Felix, 2009)
Plant PRRs are either receptor-like kinases (RLKs) or receptor-like proteins. Most leucine-rich repeat (LRR)-type PRRs associate with and rely for their function on the small regulatory LRR-RLK BRASSINOSTEROID INSENSITIVE1-ASSOCIATED KINASE1 (BAK1)/SOMATIC-EMBRYOGENESIS RECEPTOR-LIKE KINASE3 (SERK3; Monaghan and Zipfel, 2012). For example, in Arabidopsis (Arabidopsis thaliana), flg22 and elf18 bind to the LRR-RLKs FLAGELLIN SENSITIVE2 (FLS2) and EF-TU RECEPTOR (EFR), respectively, leading to a quasi-instant association with BAK1 (Gómez-Gómez and Boller, 2000; Zipfel et al., 2006; Chinchilla et al., 2007; Heese et al., 2007; Schulze et al., 2010; Roux et al., 2011; Sun et al., 2013). BAK1 is required for optimal downstream immune signaling events, such as mitogen-activated protein kinase (MAPK) activation, reactive oxygen species (ROS) bursts, callose depositions, induction of immune genes, and induced resistance. Similarly, BAK1 is a positive regulator of innate immune responses triggered by the Arabidopsis LRR-RLKs PLANT ELICITOR PEPTIDE1 RECEPTOR1 (PEPR1) and PEPR2 that bind the Arabidopsis-derived damage-associated molecular pattern A. thaliana Peptide1 (AtPep1; Krol et al., 2010; Postel et al., 2010; Roux et al., 2011) and by the tomato (Solanum lycopersicum) LRR receptor-like protein Ve1 that recognizes Ave1 derived from Verticillium spp. (Fradin et al., 2009; de Jonge et al., 2012). Consistent with the role of BAK1 downstream of numerous PRRs, BAK1 is required for full immunity to a number of bacterial, fungal, oomycete, and viral pathogens (Heese et al., 2007; Kemmerling et al., 2007; Fradin et al., 2009; Chaparro-Garcia et al., 2011; Roux et al., 2011; Kørner et al., 2013).
Notably, it has been recently shown that the ortholog of BAK1 in Nicotiana attenuata regulates the induction of jasmonic acid (JA) accumulation upon herbivory (Yang et al., 2011a). However, immunity to insects was not affected when BAK1 was silenced, and the observed effect on JA accumulation may be due to an indirect effect on brassinosteroid (BR) responses, for which BAK1 is also an important positive regulator (Li et al., 2002; Nam and Li, 2002). Therefore, it is currently unclear if BAK1 is involved in the early recognition of insect-derived elicitors leading to immunity.
We discovered that the key regulatory LRR-RLK BAK1 participates in plant defense to an insect herbivore. We found that extracts of GPA trigger plant defense responses in Arabidopsis that are characteristic of PTI. Arabidopsis bak1 mutant plants are compromised in defense to GPA, which colonizes Arabidopsis, and to pea aphid, for which Arabidopsis is a nonhost. BAK1 is required for ROS bursts, callose deposition, and induced resistance in Arabidopsis upon perception of aphid-derived elicitors. One of the defense genes induced by GPA-derived extracts encodes PHYTOALEXIN DEFICIENT3 (PAD3), a cytochrome P450 that catalyzes the conversion of dihydrocamalexic acid to camalexin, which is a major Arabidopsis phytoalexin that is toxic to GPA (Kettles et al., 2013). PAD3 expression is required for Arabidopsis-induced resistance to GPA, independently of BAK1 and ROS. Our results provide evidence that innate immunity to insect herbivores may rely on the early perception of elicitors by cell surface-localized PRR.
RESULTS
We first investigated if GPA-derived elicitors trigger cellular responses characteristic of PTI responses, including the induction of PTI marker genes, ROS bursts, and callose depositions (Boller and Felix, 2009). Aphids secrete saliva into the plant while probing and feeding; however, the plant is not only exposed to aphid saliva, but also aphid mouthparts and honeydew. In addition, aphid saliva collected from feeding membranes differs in composition depending on the medium into which it is secreted (Cherqui and Tjallingii, 2000; Cooper et al., 2010). Studies of aphid saliva have identified proteins that were not detected in the salivary gland (Carolan et al., 2011), did not possess secretion signals (Harmel et al., 2008), or originated from bacterial endosymbionts (Filichkin et al., 1997). Therefore, the composition of aphid saliva is complex and unlikely to be entirely represented by collecting secretions from feeding membranes. Aphid honeydew contains proteins from the aphid plus its endosymbiotic bacteria and gut flora, including known PAMPs (Sabri et al., 2013). In light of this, we opted to expose the plant to whole aphid extracts rather than aphid saliva only.
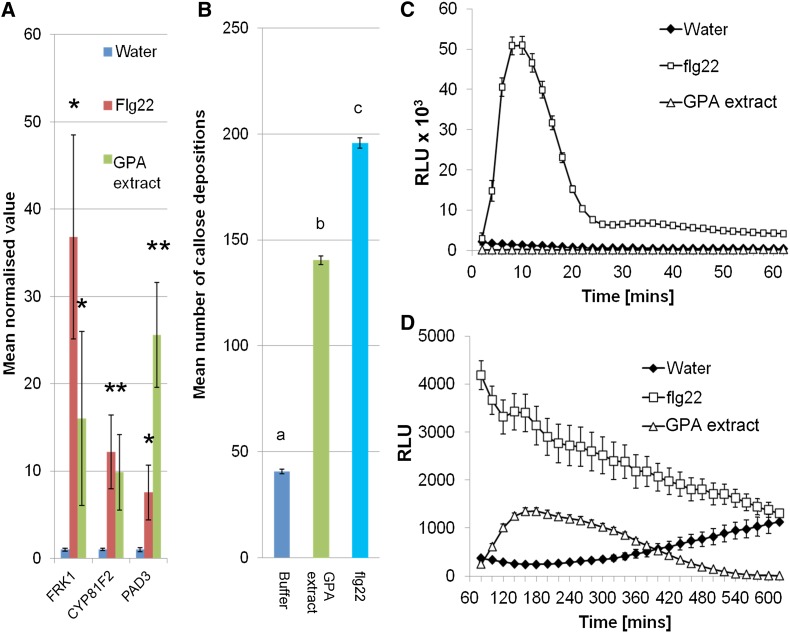
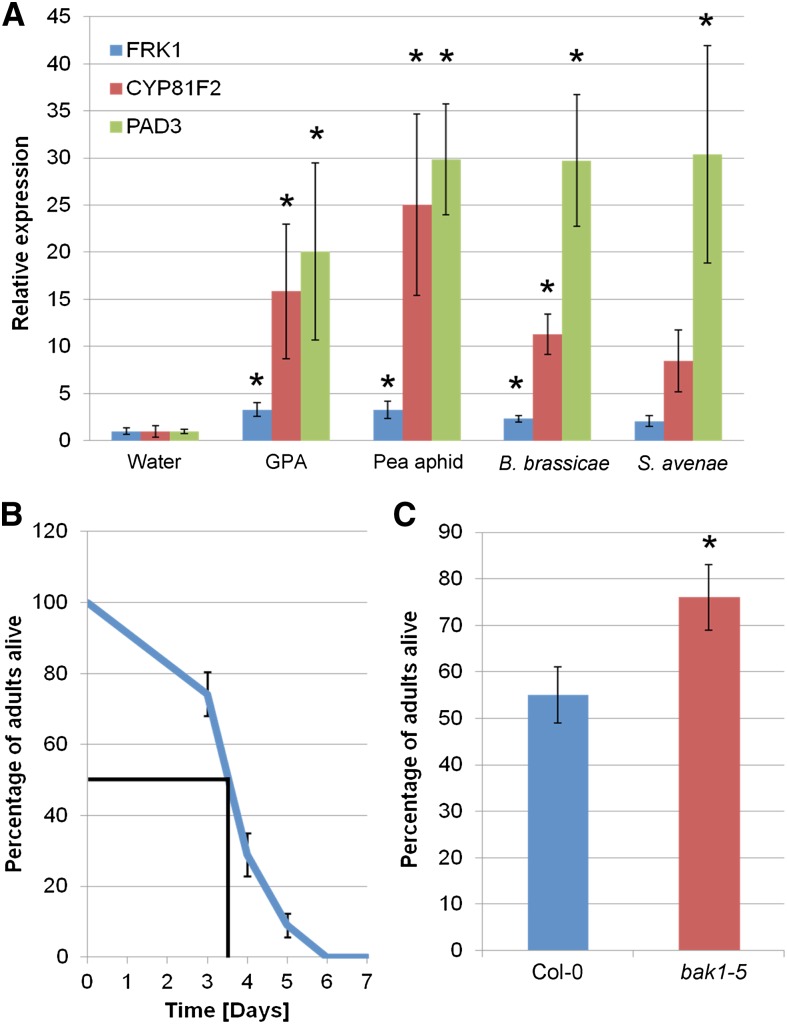
Treatment of Arabidopsis leaves with a GPA-derived extract up-regulates transcript levels of genes encoding FLG22-INDUCED RECEPTOR-LIKE KINASE1 (FRK1), CYTOCHROME P450, FAMILY 81, SUBFAMILY F, POLYPEPTIDE2 (CYP81F2), and PAD3/CYP71B15 (Fig. 1A), which are markers for early immune signaling, indolic glucosinolate production, and camalexin biosynthesis, respectively (Zhou et al., 1999; Asai et al., 2002; Bednarek et al., 2009). These genes have been previously shown to be induced by both protein and carbohydrate elicitors (Gust et al., 2007; Denoux et al., 2008). The levels of gene inductions to GPA-derived extract and flg22 were similar, except for pad3, which was more up-regulated in GPA-derived extract than in flg22-treated leaves (Fig. 1A). Callose deposition is a commonly observed plant response to elicitors, the timing of which depends on the elicitor used (Luna et al., 2011). We assayed callose deposition 24 h after elicitor treatment and observed increased numbers of callose deposits in Arabidopsis leaves treated with GPA-derived extract compared with a buffer control, although not quite as high as in flg22-treated leaves (Fig. 1B). Similarly, an ROS burst was observed in Arabidopsis leaves treated with GPA-derived extract (Fig. 1D). This ROS burst was however delayed compared with that of the flg22 treatment; the ROS burst to flg22 occurred within 10 to 20 min (Fig. 1C), while that to GPA-derived extract occurred after 1 h. At this time, the flg22-induced ROS levels were returning to base level (Fig. 1D). Nonetheless, these data show that GPA-derived extract contains one or several elicitors that trigger PTI-like plant responses.
Figure 1.
Plant defense elicitations to GPA-derived extract are characteristic of PTI. A, GPA-derived extract elicits the expression of PTI marker genes. Bars show the means ± se of target gene expression levels of four independent experiments (n = three per experiment). Asterisks indicate significant differences in GPA fraction compared to water (Student’s t probabilities calculated within GLM), with *P < 0.05 compared to water control for each gene and **P < 0.05 between flg22 and GPA-derived extract treatment. B, GPA-derived extract elicits callose deposition. Data shown are mean callose deposits produced per 1.34 mm2 of leaf upon each treatment with means ± se of three independent experiments (n = 12 leaf discs per experiment). Different letters indicate significant differences between the treatments (Student’s t probabilities calculated within GLM) at P < 0.05 (n = 36, F2,103 = 2039.93). C and D, Col-0 leaf discs were elicited with water, 12.5 nm flg22 (in water), and GPA-derived extract (in water), and ROS bursts in these leaf discs were measured using luminol-based assays at 0 to 60 min (C) and 60 to 600 min (D) after elicitation. Graphs show means ± se of n = 32 leaf discs per replicate. Data of one representative experiment are shown. The experiment was repeated three times with similar results. RLU, Relative light units. [See online article for color version of this figure.]
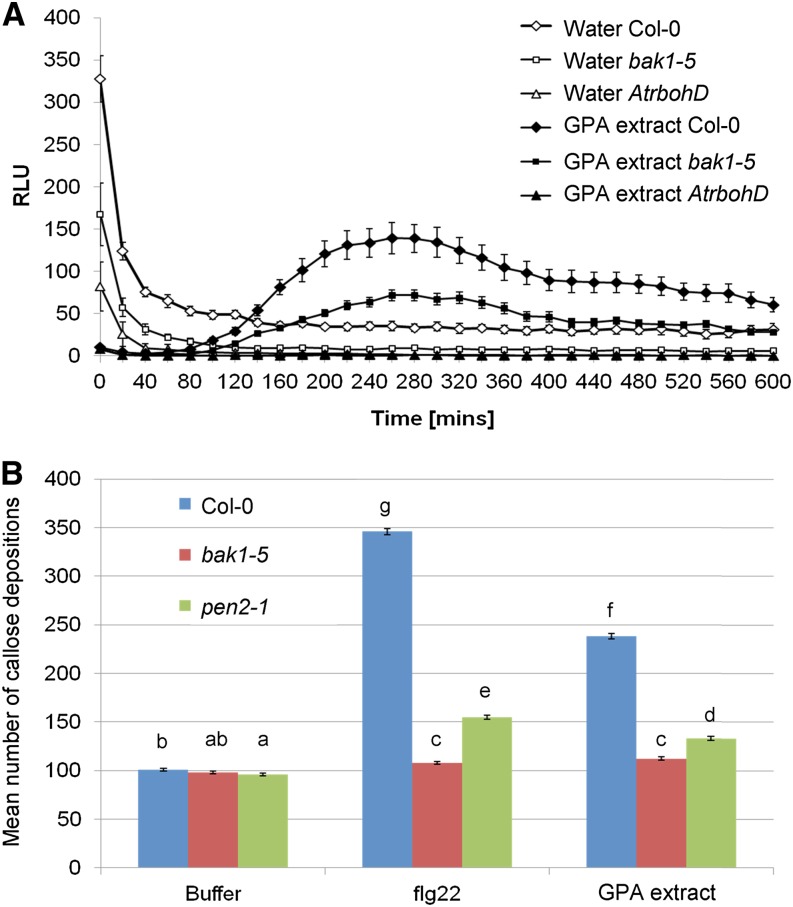
We next investigated whether PTI-like responses triggered by GPA-derived extract required components involved in PTI. Flg22-triggered ROS burst is dependent on the NADPH-oxidase A. thaliana respiratory burst oxidase homolog D (AtRbohD; Nühse et al., 2007; Zhang et al., 2007). We previously found that an aphid candidate effector M. persicae candidate effector10 suppresses the flg22-mediated ROS burst (Bos et al., 2010), a response that also requires BAK1 (Chinchilla et al., 2007; Heese et al., 2007). Because BAK1 is an essential regulator of many PTI responses characterized so far (Monaghan and Zipfel, 2012), we also investigated if BAK1 was required for the PTI-like responses to GPA-derived extract. The GPA-derived extract-triggered ROS burst was reduced in the semidominant bak1-5 mutant and was completely absent in AtrbohD (Fig. 2A). Flg22-triggered callose deposition requires biosynthesis of 4-methoxylated indole glucosinolates, mediated by CYP81F2 (Clay et al., 2009), and is diminished in mutants of PENETRATION2 (PEN2), which encodes a myrosinase involved in glucosinolate metabolism (Lipka et al., 2005; Bednarek et al., 2009; Clay et al., 2009; Luna et al., 2011). As GPA-derived extract induces CYP81F2 expression (Fig. 1A), we investigated whether PEN2 and BAK1 were required for GPA-triggered callose depositions. The number of callose deposits was significantly reduced in bak1-5 and pen2-1 mutants compared with ecotype Columbia (Col-0) after treatment with GPA-derived extract (Fig. 2B). Together, these data provide evidence that PTI-like responses to GPA-derived extract require components involved in PTI responses.
Figure 2.
Plant defense elicitations to GPA-derived extract require components of PTI. A, GPA-derived extract elicits an ROS burst in wild-type Col-0 that is reduced in bak1-5 and absent in the AtrbohD mutant. ROS bursts were measured over a 600-min period. Graph shows means ± se of n = 16 leaf discs per replicate. White symbols represent water-treated leaf discs, and black symbols represent GPA-derived extract-treated leaf discs. Data of one representative experiment are shown. The experiment was repeated three times with similar results. B, GPA-derived extract-elicited callose deposition is significantly reduced in bak1-5 and pen2-1. Data shown are mean callose deposits produced per 1.34 mm2 of leaf upon each treatment with means ± se of three independent experiments (n = 12 leaf discs per replicate). Different letters indicate significant differences between the treatments (Student’s t probabilities calculated within GLM) at P < 0.05 (n = 36, F10,323 = 1388.15). [See online article for color version of this figure.]
As very little is known about plant cell surface perception of insect-derived elicitors, we further investigated the role of BAK1 in immunity to aphids. In addition to its role in PTI signaling, BAK1 is also involved in BR responses (Li et al., 2002; Nam and Li, 2002), light signaling (Whippo and Hangarter, 2005), and cell death control (He et al., 2007; Kemmerling et al., 2007). Null bak1 mutants are compromised in all of these areas. The ethyl methane sulfonate mutant bak1-5 has a substitution in the cytoplasmic kinase domain that leads to compromised innate immune signaling but is not impaired in BR or cell death control (Schwessinger et al., 2011), allowing its use to investigate the relevance of BAK1 in resistance to pathogens with different lifestyles (Roux et al., 2011). We investigated GPA performance on bak1-5, the null mutant bak1-4 (He et al., 2007), and a null mutant of BAK1-LIKE1 (BKK1)/SERK4, bkk1-1, which is the closest paralog of BAK1 and similarly controls PTI, BR, and cell death responses (He et al., 2007; Roux et al., 2011). GPA reproduction on wild-type Col-0 and bak1-5 plants were more similar than the reproduction rates of this aphid on bak1-4 and bkk1-1 plants (Supplemental Fig. S1). This suggests that the pleiotropic phenotypes, such as deregulated cell death, of the null mutants affect aphid performance (He et al., 2007; Kemmerling et al., 2007). These results are consistent with the response of the obligate biotrophic oomycetes Hyaloperonospora arabidopsidis, which showed decreased reproduction on bak1-4 plants but no increase in reproduction on bak1-5 plants for three H. arabidopsidis isolates (Roux et al., 2011). Therefore, we continued our investigation with the Arabidopsis bak1-5 mutant alone.
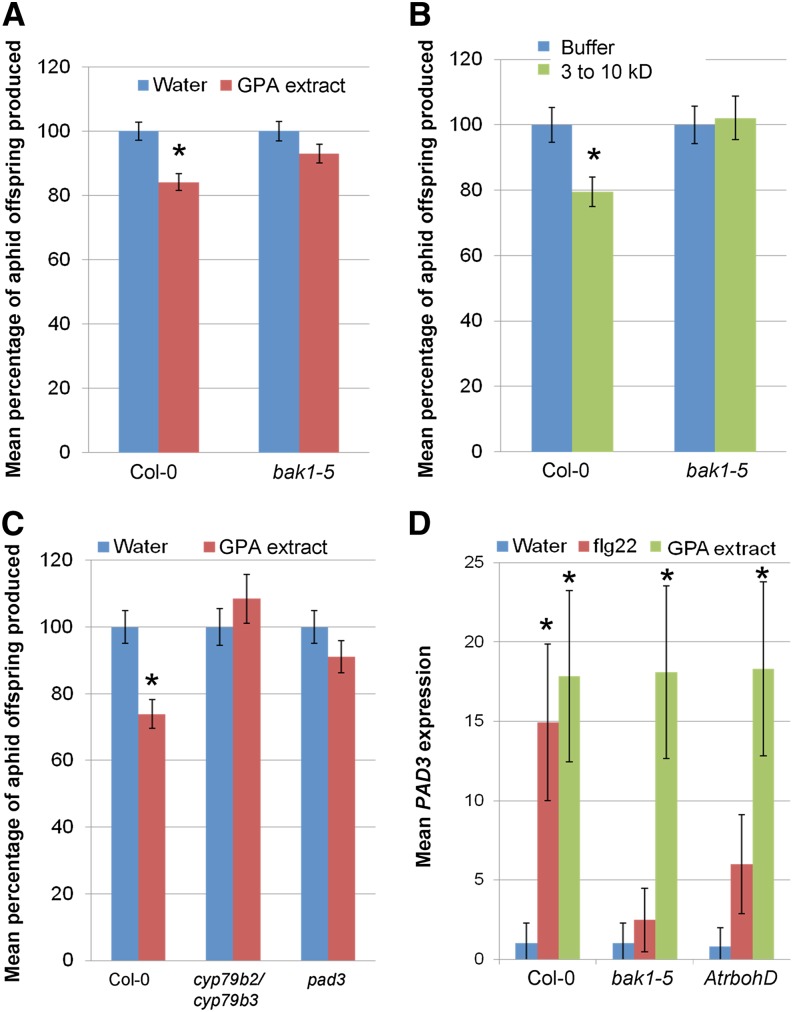
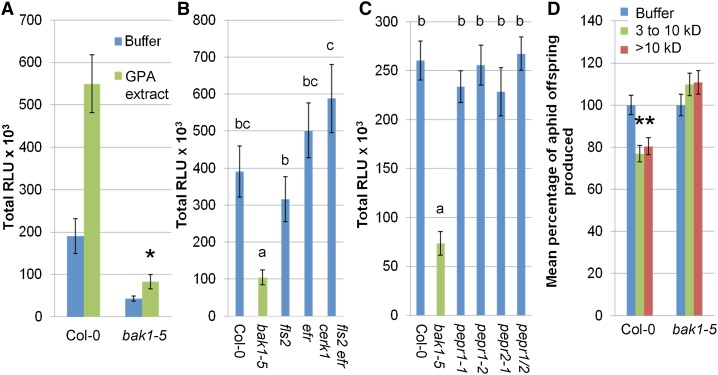
Treatment with exogenous PAMPs enhances plant resistance to pathogens, and this is also known as induced resistance (Zipfel et al., 2004; Balmer et al., 2013). De Vos and Jander (2009) previously observed that GPA saliva proteins between 3 and 10 kD in molecular mass elicit induced resistance to GPA in Arabidopsis (De Vos and Jander, 2009). To investigate if BAK1 is involved in this response, wild type Col-0 plants were treated with GPA-derived extract, and GPA reproduction on these leaves was then assessed over a period of 10 d. Induced resistance was triggered by whole GPA-derived extract (Fig. 3A), the GPA-derived 3- to 10-kD fraction (Fig. 3B), and the 3- to 10-kD GPA saliva fraction (Supplemental Fig. S2). Induced resistance was reduced in the bak1-5 mutant (Fig. 3, A and B; Supplemental Fig. S2). These demonstrate that aphid elicitors present in whole GPA-derived extract and saliva are recognized in a BAK1-dependent manner, leading to immunity to GPA.
Figure 3.
Plant defense responses elicited by GPA-derived extract are dependent on BAK1 and PAD3. A and B, Induced-resistance to GPA-derived extract (A) and GPA 3- to 10-kD fraction (B) is dependent on BAK1. Bars show the means ± se of total nymphs produced per plant of six (A) and three (B) independent experiments. The nymph counts were normalized with the water or buffer controls set at 100%. Asterisks indicate significant differences to GPA fraction compared with water/buffer (Student’s t probabilities calculated within GLM) with *P < 0.001 (Col-0 wild type, n = 60, F1,19 = 17.88) and P = 0.063 (A; bak1-5 mutant, n ≥ 57, F1,115 = 3.45) and *P = 0.005 (Col-0 wild type, n ≥ 28, F1,56 = 8.065) and P = 0.835 (B; bak1-5 mutant, n ≥ 25, F1,53 = 0.043). C, Induced-resistance to GPA-derived extract is dependent on PAD3. Bars show the means ± se of total nymphs produced per plant of three independent experiments. Nymph counts were normalized with the water control set at 100%. *P < 0.001 (Col-0, n ≥ 23, F1,46 = 15.5), P = 0.384 (cyp79b2/cyp79b3 mutants, n ≥ 16, F1,36 = 0.76), and P = 0.188 (pad3 mutant, n ≥ 19, F1,41 = 1.73). D, GPA-derived extract-triggered PAD3 expression is not dependent on BAK1 or AtRbohD. Bars show the means ± se of target gene expression levels of three independent experiments (n = three per experiment). Expression levels were normalized with the water control of Col-0 set at 1. Asterisks indicate significant differences compared with water control (Student’s t probabilities calculated within GLM) with *P < 0.05. [See online article for color version of this figure.]
Next, we investigated if PAD3 is involved in Arabidopsis-induced resistance to GPA. The cytochrome P450 PAD3 catalyzes the conversion of dihydrocamalexic acid to camalexin, the major Arabidopsis phytoalexin, and acts downstream of CYP79B2 and CYP79B3 enzymes in the glucosinolate biosynthetic pathway (Zhao et al., 2002; Schuhegger et al., 2006). We previously demonstrated that camalexin is toxic to GPA (Kettles et al., 2013). Moreover, PAD3 expression is induced upon perception of aphid elicitors (Fig. 1A), GPA saliva (De Vos and Jander, 2009), and GPA feeding (De Vos et al., 2005; Kettles et al., 2013).
We found that Arabidopsis pad3 and cyp79b2/cyp79b3 mutants do not show induced resistance to GPA upon treatment of plants with GPA-derived extract (Fig. 3C). To determine whether the PAD3-dependent induced resistance requires BAK1 and apoplastic ROS production, we measured PAD3 induction in bak1-5 and AtrbohD plants in response to GPA-derived extract. PAD3 expression was reduced in bak1-5 and AtrbohD in response to flg22 but not GPA-derived extract (Fig. 3D), suggesting that PAD3-dependent induced resistance to GPA-derived extract is independent of BAK1 and apoplastic ROS production. Therefore, Arabidopsis-induced resistance to GPA is dependent on BAK1 and PAD3 through separate signaling pathways.
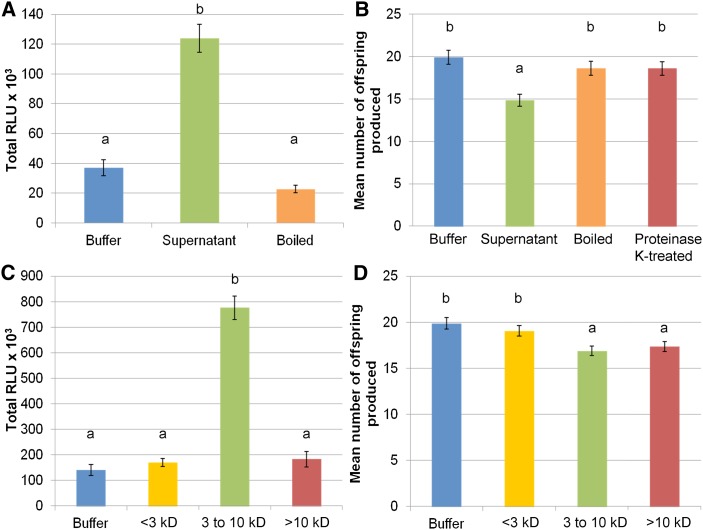
We sought to characterize further the biochemical properties of the GPA-derived elicitors. The ROS burst and induced-resistance responses disappeared when GPA-derived extract was boiled (Fig. 4, A and B). The proteinase K-treated GPA-derived extract did not generate an induced-resistance response to GPA (Fig. 4B), even though proteinase K itself induced an ROS burst in Arabidopsis Col-0 that started at about 1 h after treatment and disappeared upon boiling of proteinase K (Supplemental Fig. S3, A and B). The 3- to 10-kD fraction induced an ROS burst, while fractions that are smaller than 3 kD and larger than 10 kD did not (Fig. 4C). Induced resistance to GPA was, however, observed for both the 3- to 10-kD and larger-than-10-kD fractions but not for the smaller-than-3-kD fraction (Fig. 4D). Altogether, these results indicate the presence of at least two eliciting fractions in GPA-derived extract, which are likely to contain heat-sensitive proteins or peptides.
Figure 4.
GPA-derived extract-eliciting activities disappear upon boiling and proteinase K treatments. A, Boiled GPA-derived extract does not elicit an ROS burst. ROS bursts were measured over a 600-min period. Bars show means ± se of n = 16 leaf discs per replicate. Data of one representative experiment are shown. The experiment was repeated three times with similar results. Bars marked with different letters indicate significant differences at P < 0.05 using ANOVA. B, Boiled and proteinase K-treated GPA-derived extract do not elicit induced resistance. Bars show the means ± se of total nymphs produced per plant of three independent experiments. Bars marked with different letters indicate significant differences at P < 0.05 (Student’s t probabilities calculated within GLM; n = 30, F3,119 = 7.688). C, The 3- to 10 kD fraction of GPA-derived extract elicits ROS bursts. ROS bursts were measured over an 800-min period. Bars show means ± se of n = 16 leaf discs per replicate. Data of one representative experiment are shown. The experiment was repeated three times with similar results. Letters indicates significant differences at P < 0.05 using ANOVA. D, Three- to ten-kilodalton and larger-than-10-kD GPA-derived extracts elicit induced resistance. Bars show the means ± se of total nymphs produced per plant of six independent experiments. Letters indicate significant differences at P < 0.05 (Student’s t probabilities calculated within GLM; n = 60, F3,237 = 6.051). [See online article for color version of this figure.]
Arabidopsis bak1-5 mutant plants produce significantly less ROS in response to the GPA-derived 3- to 10-kD extract (Fig. 5A). BAK1 is a coreceptor that associates with several LRR-RLK-type PRRs, such as FLS2, EFR, PEPR1, and PEPR2 (Chinchilla et al., 2007; Heese et al., 2007; Postel et al., 2010; Roux et al., 2011), which perceive bacterial flagellin, bacterial EF-Tu, and the damage-associated molecular patterns AtPeps, respectively (Gómez-Gómez and Boller, 2000; Yamaguchi et al., 2006; Zipfel et al., 2006; Yamaguchi et al., 2010). However, Arabidopsis mutant lines in these PRRs did not show reduced ROS bursts to the 3- to 10-kD extract (Fig. 5, B and C). While the lysine-motif-RLK CHITIN ELICITOR RECEPTOR KINASE1 (CERK1) does not require BAK1 for signaling, this receptor is involved in the perception of chitin (Miya et al., 2007; Wan et al., 2008), which is abundant in the aphid cytoskeleton, including the aphid mouthparts that are in contact with the plant during feeding. Nonetheless, the response to GPA-derived extract was not reduced in an Arabidopsis fls2 efr cerk1 triple mutant (Fig. 5B). Thus, aphid elicitor-induced ROS burst is dependent on BAK1 and a thus-far unknown PRR.
Figure 5.
Plant immune responses to individual GPA-derived elicitor fractions are BAK1 dependent. A, BAK1 is involved in Arabidopsis ROS burst to GPA-derived elicitors. ROS bursts were measured in response to buffer and 2.5 mg mL–1 3- to 10-kD GPA-derived extract over an 800-min period. Bars show means ± se of n = eight leaf discs per replicate. Data of one representative experiment are shown. The experiment was repeated three times with similar results. Asterisk indicates significant differences at P < 0.05 between GPA-derived extract ROS burst in Col-0 and bak1-5 using Student’s t test. B and C, The ROS burst of Arabidopsis to GPA-derived elicitors is not reduced in mutants of known PRR genes. ROS bursts were measured in response to 2.5 mg mL–1 3- to 10-kD GPA-derived extract over an 800-min period. Bars show means ± se of n = 16 leaf discs per replicate. Data of one representative experiment are shown. The experiment was repeated three times with similar results. Letters indicates significant differences at P < 0.05 using ANOVA. D, Induced resistance to GPA 3- to 10-kD and larger-than-10-kD fractions is dependent on BAK1. Bars show the means ± se of total nymphs produced per plant of four independent experiments (n = eight per experiment). Nymph counts were normalized with the buffer control set at 100%. Asterisks indicate significant differences at P < 0.05 (Student’s t probabilities calculated within GLM; Col-0, n ≥ 28, F2,86 = 8.14; bak1-5, n ≥ 25, F2,80 = 1.53). [See online article for color version of this figure.]
We also investigated whether BAK1 was involved in the induced resistance to the larger-than-10-kD eliciting fraction. Induced resistance was observed on Col-0 Arabidopsis plants but not on the bak1-5 mutant plants for the 3- to 10-kD and larger-than-10-kD fractions (Fig. 5D). Therefore, BAK1 is involved in the signaling pathways to both of these eliciting fractions.
Elicitors perceived by PRRs are often conserved among groups of pathogens (Medzhitov and Janeway, 1997). To investigate if this is also the case for aphids, we examined the expression levels of the PTI marker genes FRK1, CYP81F2, and PAD3 in Arabidopsis plants treated with extracts of various aphid species (pea aphid, cabbage aphid, and English grain aphid). The expression of these genes were induced to similar levels after treatment with aphid-derived extracts from the three other species tested, although the induction of FRK1 and CYP81F2 was not statistically significant upon treatment with English grain aphid-derived extract (Fig. 6A). These results provide evidence that aphid-derived elicitors perceived by Arabidopsis are potentially conserved among different aphid genera/species.
Figure 6.
BAK1 is involved in pea aphid resistance. A, Elicitors derived from several aphid species trigger up-regulation of PTI marker genes. Bars show the means ± se of target gene expression levels of four biological replicates (n = three per replicate). Asterisks indicate significant differences in aphid-derived extracts compared with water (Student’s t probabilities calculated within GLM) with *P < 0.05. B, Pea aphids do not survive beyond 6 d on Col-0 Arabidopsis. Data show the percentage of aphids alive at a given time point with means ± se of four biological replicates with n = five per replicate. The time point at which 50% of pea aphids are still alive is indicated. C, Pea aphids survive better on Arabidopsis bak1-5 plants. Bars show the percentage of aphids alive between days 3 and 4 with means ± se of six biological replicates with n = five per replicate. Asterisk indicates significant difference in aphid survival (Student’s t probabilities calculated within GLM; n = 30, F1,59 = 5.028; *P = 0.025). [See online article for color version of this figure.]
The pea aphid host range is mostly restricted to plants of the legume family; these insects do not like to feed on brassicas, such as Arabidopsis. Because PRRs regulate the first active line of plant defense response and are proposed to be involved in nonhost resistance in plant species distantly related to the natural host (Schulze-Lefert and Panstruga, 2011), we investigated if the pea aphid survives better on Arabidopsis bak1-5 mutant plants. About 50% of the pea aphids on Arabidopsis Col-0 are still alive between 3 and 4 d (Fig. 6B). Remarkably, at this time, the survival rates of pea aphids were significantly higher, about 75%, on the Arabidopsis bak1-5 mutant plants (Fig. 6C). Thus, nonhost resistance of Arabidopsis to the pea aphid appears compromised in the bak1-5 background, further reflecting an important contribution of BAK1 (and by extension PRR-mediated immunity) to plant immunity against aphids.
DISCUSSION
Our research provides an increased understanding of plant perception of insects, by showing that BAK1 is required for the ROS burst, callose deposition, and induced resistance triggered by GPA-derived elicitors. GPA-derived elicitors trigger plant immunity characteristic of PTI, including the induction of PTI marker genes, AtRbohD-dependent ROS burst, PEN2-dependent callose deposition, and induced resistance. The GPA-derived eliciting fractions are likely to contain heat-sensitive peptides of 3 to 10 kD and larger than 10 kD in which the 3- to 10-kD fraction induces the ROS burst and both 3- to 10-kD and larger-than-10-kD fractions elicit induced resistance to GPA. Induced resistance is also dependent on PAD3, the expression of which is induced upon Arabidopsis perception of aphid-derived elicitors and is independent of BAK1 and ROS. Finally, the legume specialist pea aphid survives better on the Arabidopsis bak1-5 mutant than on wild-type Col-0 plants.
Our results are in agreement with those of De Vos and Jander (2009), who found that the 3- to 10-kD GPA saliva fraction generates induced resistance, which is lost upon boiling and proteinase K treatments of the fraction (De Vos and Jander, 2009). In addition, Arabidopsis colonization by another aphid species, the cabbage aphid, triggers an ROS burst and the expression of PAD3, CYP81F2, and FRK1 genes (Kuśnierczyk et al., 2008; Barah et al., 2013). These findings and our observation that multiple aphids induce PAD3, CYP81F2, and FRK1 expression (Fig. 5A) suggest that the eliciting components are conserved among aphids. Our study shows evidence that there are at least two eliciting fractions derived from aphids: the GPA 3- to 10-kD fraction that triggers an ROS burst and induced resistance and the larger than 10-kD fraction that does not induce ROS burst but nonetheless triggers induced resistance. The eliciting activities of both fractions require BAK1 and are lost upon boiling and proteinase K treatments, indicating that the elicitors are likely proteins with enzymatic activities. It is possible that the two eliciting fractions contain different concentrations of the same elicitor due to incomplete separation by the Mr cutoff columns. Therefore, the elicitor may be in sufficient quantity to trigger an ROS burst in the 3- to 10-kD fraction but not the larger-than-10-kD fraction. It is important to note that the elicitors perceived by Arabidopsis are either derived directly from aphids or from their endosymbionts. However, the possibility remains that elicitors in GPA-derived extract may not normally come into contact with plants. Further investigation is required to identify the elicitors and their origin. This will then allow the availability of the GPA-derived elicitors to be perceived by the plant during the plant-aphid interaction to be assessed.
The ROS burst triggered by flg22 is an early transient response, which starts very soon after addition of the PAMP and finishes within 30 min. By contrast, the ROS burst triggered by the GPA-derived 3- to 10-kD fraction occurs much later, starting more than an hour after addition of the extract. Its duration is also longer compared with flg22, as the burst takes nearly 9 h to reach basal level again. These kinetics are consistent with potential enzymatic activities of the GPA-derived elicitors. However, the kinetics of plant immune responses triggered by distinct elicitors can be highly variable. For example, Phytophthora infestans elicitin INF1 triggers a BAK1-dependent ROS burst in Nicotiana benthamiana that is also much longer than that of flg22 (Chaparro-Garcia et al., 2011). While there is a delay in the GPA-derived elicitor ROS burst compared with that of flg22, there is no delay in GPA-derived gene expression of PAD3, CYP81F2, and FRK1. We show that PAD3 expression to GPA-derived elicitors does not require ROS (Fig. 3D). CYP81F2 and FRK1 are MAPK-activated genes (Boudsocq et al., 2010), and MAPK activation in PTI does not require ROS (Ranf et al., 2011; Segonzac et al., 2011). Consistent with this, FRK1 expression upon flg22 treatment is not reduced in AtrbohD (Macho et al., 2012).
GPA elicitation is specific, as proteinase K triggers an ROS burst in Arabidopsis that is lost upon boiling, but this ROS burst does not generate induced resistance to GPA. Arabidopsis can generate induced resistance to GPA without a measurable ROS burst, as evidenced by the induced resistance triggered by the larger-than-10-kD GPA fraction. Nonetheless, the ROS burst plays a role in Arabidopsis innate immunity to GPA given that Arabidopsis mutants in RbohD, which is required for PTI- and effector-triggered immunity ROS bursts (Torres et al., 2002; Zhang et al., 2007), are more susceptible to GPA (Miller et al., 2009). Thus, aphid-derived elicitors are likely to trigger different immune pathways in plants, some of which involve ROS bursts and others that do not. All these pathways together likely contribute to an effective immunity against aphids.
BAK1 is required for the establishment of PTI by ligand-induced heteromerization with surface-localized PRRs. Characterized PRRs that require BAK1 for signaling include FLS2, EFR, and PEPR1/PEPR2 (Chinchilla et al., 2007; Heese et al., 2007; Postel et al., 2010; Roux et al., 2011). However, Arabidopsis mutants for FLS2, EFR, PEPR1, and PEPR2 are not affected in ROS bursts to the 3- to 10-kD GPA fraction. Therefore, elicitors in the 3- to 10-kD GPA fraction are likely to interact with thus-far unknown Arabidopsis PRRs, which form ligand-induced heteromers with BAK1 for triggering an ROS burst upon perception of aphid-derived elicitors.
The involvement of BAK1 in plant-herbivore interactions was previously investigated in N. attenuata (Yang et al., 2011a). Plants are likely to perceive insect elicitors, often referred to as herbivory-associated molecular patterns, in insect OS and egg-associated molecular patterns in egg fluid (Wu and Baldwin, 2010; Gouhier-Darimont et al., 2013). Application of OS into wounds activates two MAPKs, salicylic acid (SA)-induced protein kinase and wound-induced protein kinase, which are required for the accumulation of JA, JA-Ile, and ethylene (ET), phytohormones that are important for mediating plant immunity to insects (Wu and Baldwin, 2010). The LECTIN-RECEPTOR KINASES LecRK1 and LecRK-I.8 act upstream or downstream of phytohormone signaling events (Gilardoni et al., 2011; Gouhier-Darimont et al., 2013). While silencing of BAK1 in N. attenuata leads to attenuated JA and JA-Ile levels in wounded and OS-treated plants, activities of the two MAPKs were not impaired (Yang et al., 2011a). This indicated that BR signaling but not innate immunity may be compromised in these BAK1-silenced plants (Yang et al., 2011b). The Arabidopsis bak1-5 mutant used in our study is severely compromised in PTI signaling but is not impaired in BR signaling and cell death control (Schwessinger et al., 2011). In addition, the saliva-induced resistance to GPA in Arabidopsis is not dependent on JA, SA, and ET signaling (De Vos and Jander, 2009). This is in agreement with a study of Arabidopsis responses to the necrotrophic fungus Botrytis cinerea showing that plant-derived oligogalacturonides induce a resistance that is not dependent on JA, SA, and ET (Ferrari et al., 2007). Similarly to aphids, the induction of resistance to B. cinerea requires PAD3 (Ferrari et al., 2007). Thus, BAK1 contributes most likely to innate immunity to GPA in a manner that is independent of BR, JA, SA, and ET signaling in Arabidopsis.
Arabidopsis is a nonhost to the pea aphid. We observed that these aphids nonetheless attempt to feed on Arabidopsis leaves but do not adopt a settled feeding behavior and often walk to the top of the leaf cages, where they die within 6 d. Notably, pea aphids survive longer on Arabidopsis bak1-5 plants compared with Col-0, indicating that they may obtain more nutrition from the mutant plant or receive fewer toxic compounds. While BAK1 has a role in plant immune signaling upon pea aphid perception, the observation that pea aphids do not fully survive on Arabidopsis bak1-5 plants suggests that other BAK1-independent receptor complexes and/or additional downstream components also contribute to the triggering of plant immunity to aphids. Studying of pea aphid-Arabidopsis interactions will be useful for the identification of such components. Aphids that use brassicas, including Arabidopsis, as hosts, such as GPA and the cabbage aphid, are likely to possess specific effectors that suppress the PTI-like plant immune responses. We identified about 50 candidate effectors in GPA (Bos et al., 2010) and found that three promote GPA colonization on Arabidopsis, whereas the pea aphid homologs of these three effectors do not promote GPA colonization on this plant (Pitino and Hogenhout, 2013). It remains to be investigated if the GPA effectors, but not pea aphid effectors, suppress PTI-like plant defenses.
In summary, we identified an upstream (BAK1) and downstream (camalexin) component of two independent pathways in plant innate immunity to aphids. This is in agreement with earlier findings that camalexin is involved in plant defense to aphids (Kuśnierczyk et al., 2008; Kettles et al., 2013). Aphids are likely to suppress innate immunity to colonize plants. This is in agreement with the identification of a GPA effector that suppress PTI (Bos et al., 2010) and aphid effectors that promote colonization of the plant (Atamian et al., 2013; Pitino and Hogenhout, 2013).
MATERIALS AND METHODS
Aphids
GPAs (Myzus persicae; Rothamsted Research genotype O; Bos et al., 2010) were reared on Chinese cabbage (Brassica rapa, subspecies chinensis), and pea aphids (Acyrthosiphon pisum) were reared on broad bean (Vicia faba) in 52-cm × 52-cm × 50-cm cages. Cabbage aphids (Brevicoryne brassicae) were reared on Chinese cabbage, and English grain aphids (Sitobion avenae) were reared on oat (Avena sativa) in 24-cm × 54-cm × 47-cm cages. All species were reared in controlled-environment conditions with a 14-h-day (90 μmol m–2 s–1 at 18°C) and a 10-h-night (15°C) photoperiod.
Plant Growth Conditions
All plants were germinated and grown in Scotts Levington F2 compost. Arabidopsis (Arabidopsis thaliana) seeds were vernalized for 1 week at 5°C to 6°C and then grown in a controlled-environment room (CER) with a 10-h-day (90 μmol m–2 s–1) and a 14-h-night photoperiod and at a constant temperature of 22°C.
All Arabidopsis mutants used in this study were generated in Col-0 background, except pen2-1, which is in the glabrous1 background. The bak1-5, bak1-4, bkk1-1, efr-1 (efr), fls2c (fls2), and fls2 efr cerk1 mutants were previously described (Zipfel et al., 2004, 2006; He et al., 2007; Gimenez-Ibanez et al., 2009; Schwessinger et al., 2011). The pepr1-1, pepr1-2, and pepr2-1 mutants (Yamaguchi et al., 2010) were obtained from the Nottingham Arabidopsis Stock Centre. The pepr1/pepr2 double mutant (Krol et al., 2010) was obtained from Dirk Becker (Department of Molecular Plant Physiology and Biophysics, University of Wuerzburg). The pen2-1 (Lipka et al., 2005) and AtrbohD (Torres et al., 2002) mutants were obtained from Jonathan Jones (The Sainsbury Laboratory). The pad3 and cyp79b2/cyb79b3 double mutants (Glazebrook and Ausubel, 1994; Zhao et al., 2002) were used in a previous study (Kettles et al., 2013).
Preparation of Aphid-Derived Extract and Fractions for Elicitation Experiments
Apterous late instar and adult aphids were collected using a moist paintbrush, placed in a 2-mL Eppendorf tube, and snap frozen in liquid nitrogen. The aphids were ground to a fine powder using a prechilled mortar and pestle. The powder was then transferred to a 50-mL Corning tube on ice using a prechilled spoon. Sterile, distilled water was added to the ground powder and thoroughly mixed with a pipette to generate 20 mg (wet weight) mL–1 of whole aphid-derived extract.
GPA-derived extracts were further processed as described (De Vos and Jander, 2009; Schäfer et al., 2011). The ground aphid powder was resuspended in sterile 0.025 m potassium phosphate buffer (KH2PO4, pH 6.8). The extract was centrifuged at 13,200 rpm for 15 min at 4°C, and the supernatant was collected. For fractionation of GPA-derived extract, the supernatant was filtered by centrifuging at 13,200 rpm for 15 min at 4°C using a 10-kD cutoff column (Ultracel 10K membrane, Millipore). The fraction remaining in the upper part of the column was the larger-than-10-kD fraction. The fraction that passed through the column was retrieved by placing the column upside down in a fresh centrifuge tube and centrifuging it at 1,000g for 2 min. It was then filtered by centrifuging at 13,200 rpm for 15 min at 4°C using a 3-kD cutoff column (Ultracel 3K membrane, Millipore). The fraction that passed through the column was the smaller-than-3-kD fraction, while the fraction that remained in upper part of the column was the 3- to 10-kD fraction. The 3- to 10-kD fraction was retrieved by placing the column upside down in a fresh centrifuge tube at centrifuging at 1,000g for 2 min. After filtering, all fractions were adjusted to their original volume using potassium phosphate buffer.
GPA-derived extract was denatured by boiling for 10 min or degraded in a final concentration of 0.2 μg μL–1 of proteinase K (Sigma-Aldrich) at 37°C for 30 min.
Saliva Collection
GPA saliva was collected using a Parafilm sachet. Two 500-mL plastic tumblers (Sainsbury’s Supermarkets) had several small holes pierced in them with a hot syringe (Terumo). Approximately 1,000 adult GPA from the Chinese cabbage stock cage, amounting to a weight of 0.2 g (50 adult GPA weighed 0.01 g), were added to one of the tumblers. The other tumbler served as a no-aphid control. A thin layer of Parafilm (Brand GMBH) was stretched over each tumbler, and 1 mL of sterile, distilled water was pipetted onto the Parafilm. A second layer of Parafilm was then stretched over each tumbler. The tumblers were placed underneath a sheet of yellow plastic (Lincoln Polythene) to enhance feeding activity in a CER with a 14-h-day (90 μmol m–2 s–1 at 18°C) and 10-h-night (15°C) photoperiod. After 24 h, the saliva/water was collected from both tumblers under sterile conditions. The 3- to 10-kD fraction of the saliva and control was obtained using centrifugal filters as described above. After filtering, the saliva and control were adjusted to their original volume using sterile, distilled water.
Induced Resistance Assays
Induced-resistance fecundity assays were carried out using a modified protocol as described (De Vos and Jander, 2009). Experiments were conducted in a CER with an 8-h-day (90 μmol m–2 s–1 at 18°C) and 16-h-night (16°C) photoperiod. To obtain aphids of the approximately the same age, 5-week old Col-0 Arabidopsis plants were potted into 1-L round black pots (13-cm diameter, 10 cm tall) that were caged inside clear plastic tubing (10-cm diameter, 15 cm tall; Jetran tubing, Bell Packaging), which was pushed inside the soil of the pot and capped at the top with a white gauze-covered plastic lid. Each plant was seeded with 20 adult GPA. After 24 h, all adults were removed from the Col-0 plants, while the nymphs remained on the plants for 10 d.
For treatment of plants with aphid elicitors, 5-week old Arabidopsis plants in black plastic pots (base measurement, 3.5 cm × 3.5 cm; top measurement, 5.5 cm × 5.5 cm; height, 5.5 cm) were infiltrated with the GPA-derived extracts on the first fully expanded leaf using a needleless 1-mL syringe (Terumo). The extracts being tested were diluted 1:10 with distilled water or potassium phosphate buffer as appropriate. The 3- to 10-kD fraction of GPA saliva was diluted 1:2 with distilled water. Control plants were infiltrated with distilled water or potassium phosphate buffer without GPA-derived extract. The infiltrated leaves were marked. The plants were used for aphid reproduction assays after 24 h.
To assay aphid reproduction on the infiltrated leaves, one aged adult of 10 d was placed in a clip cage using a moist paintbrush, and the cage was placed on the infiltrated leaf at one aphid per plant. Plants were returned to the experimental CER and left for 10 d. After 10 d, the number of aphids in each clip cage was counted. Each experiment included 10 plants per condition and/or genotype unless otherwise stated. Each plant was randomly placed in a tray of 42 cm × 52 cm × 9 cm. Each experiment was repeated at least three times on different days to generate data from at least three independent biological replicates. Leaves that had shriveled up and died, thus killing all the aphids, were removed from the analysis.
GPA Whole-Plant Fecundity Assays
GPA whole-plant fecundity assays were carried out as previously described (Kettles et al., 2013). Experiments were conducted in a CER with an 8-h-day (90 μmol m–2 s–1 at 18°C) and 16-h-night (16°C) photoperiod. Four-week-old Arabidopsis plants were potted into 1-L round black pots and caged in clear plastic tubing as described above. Each plant was seeded with five adult GPA. After 48 h, all adults were removed from test plants, while the nymphs remained at five nymphs per plant. These nymphs developed into adults and started producing their own nymphs at about day 8. The number of nymphs and surviving adults were counted on days 11 and 14, in which the nymphs were removed at each count. The total number of nymphs produced per live adult was calculated for each time point and combined. Each experiment included five plants per genotype, and each plant was randomly placed in a tray of 42 cm × 52 cm × 9 cm. Each experiment was repeated three times on different days to generate data from three independent biological replicates.
Pea Aphid Survival Assays
To obtain pea aphid adults of the same age, 50 adult pea aphids were transferred to three mature broad bean plants between 3 and 4 weeks old and placed in 24-cm × 54-cm × 47-cm cages. Each cage was placed in a CER with a 14-h-day (90 μmol m–2 s–1 at 18°C) and 10-h-night (15°C) photoperiod. After 24 h, all adults were removed from the plants, while the nymphs remained. Pea aphid adults 10 to 14 d old were used for survival experiments on Arabidopsis. The survival experiments on Arabidopsis were conducted in a CER with an 8-h-day (90 μmol m–2 s–1 at 18°C) and 16-h-night (16°C) photoperiod. Five 10- to 14-d adult pea aphids were placed in one clip cage using a moist paintbrush. The clip cages were clipped on one leaf per plant of 7-week-old Arabidopsis plants potted in black plastic pots (base measurement, 3.5 cm × 3.5 cm; top measurement, 5.5 cm × 5.5 cm; height, 5.5 cm). To ascertain pea aphid survival on Col-0 Arabidopsis, the number of aphids remaining alive on days 3 to 7 was counted. To compare survival on Col-0 and bak1-5 Arabidopsis, the number of adult aphids remaining alive on days 3 and 4 were recorded, and the average of these two readings were taken. Each experiment consisted of five plants per genotype. Each plant was randomly placed in a tray of 42 cm × 52 cm × 9 cm. The experiments were repeated at least four times on different days to generate data from at least four independent biological replicates.
Measurements of ROS Bursts
Measurements of ROS bursts to the peptide flg22 (QRLSTGSRINSAKDDAAGLQIA; Felix et al., 1999; Peptron) and GPA-derived extracts were carried out as previously described (Bos et al., 2010). One leaf disc was taken from each of the two youngest fully expanded leaves of 5-week-old Arabidopsis plants using a circular cork borer (diameter, 4 mm). The leaf discs were floated on water overnight in 96-well plates (Grenier Bio-One). Flg22 (final concentration 100 nm unless stated otherwise) or GPA-derived extract (final concentration, 5 mg mL–1 unless otherwise stated) were added to a solution containing 20 μg mL–1 horseradish peroxidase (Sigma-Aldrich) and 21 nm of the luminol derivative 8-amino-5-chloro-7-phenylpyrido[3,4-d]pyridazine-1,4(2H,3H)dione (Nishinaka et al., 1993; Wako). Before the experiment began, the water was removed from the wells and replaced with 100 μL of horseradish peroxidase and 8-amino-5-chloro-7-phenylpyrido[3,4-d]pyridazine-1,4(2H,3H)dione solution containing flg22, GPA-derived extract, or water/buffer controls. ROS burst assays to proteinase K were conducted with 100 μg of proteinase K (Sigma-Aldrich) or 100 μg of proteinase K boiled for 10 min. Luminescence was captured using a Photek camera system and analyzed using company software and Microsoft Office Excel. Experiments were repeated at least three times on different days to generate independent biological replicates.
Quantitative Reverse Transcriptase (qRT)-PCR Assays
Two Arabidopsis leaf discs were taken from each of the two youngest fully expanded leaves of the 5-week-old Col-0 plant using a circular cork borer with a diameter of 6 mm. The leaf discs were floated on water overnight in 96-well plates (Grenier Bio-One). Before the experiment began, the water was removed, and leaf discs were exposed to 100 μL of water (control), 100 nm flg22 (in water), and 20 mg mL–1 GPA-derived extract (in water) for 1 h. Eight leaf discs under the same treatment were pooled generating one sample. Samples were ground in chilled 1.5-mL Eppendorf tubes using disposable pellet pestles (Sigma-Aldrich). Total RNA was extracted using Tri-Reagent (Sigma-Aldrich) and included a DNase I treatment (RQ1 DNase set; Promega). Complementary DNA (cDNA) was synthesized from 1 µg RNA using the M-MLV-RT Kit (Invitrogen) and oligo(dT) primer, following the manufacturer’s instructions. cDNA from these reactions was diluted 1:10 with distilled water before qRT-PCR.
Each reaction consisted of 20 µL containing 25 ng of cDNA and 0.5 µm of each primer (Supplemental Table S1) added to SYBR Green JumpStart Taq ReadyMix (Sigma-Aldrich) in a single well of a 96-well plate white ABgene PCR plate (Thermo Scientific). Reactions for the target and reference genes and corresponding controls were combined in one 96-well plate, which was placed in a CFX96 Real-Time System with a C1000 Thermal Cycler (Bio-Rad). PCRs were carried out using the following thermocycle: 3 min at 95°C, followed by 40 cycles of 30 s at 95°C, 30 s at 60°C, and 30 s at 72°C and melt curve analysis for 30 s at 50°C (65°C–95°C at 0.5°C increments, 5 s for each).
Using a selection of candidates previously identified as superior reference genes (Czechowski et al., 2005), we selected Arabidopsis genes GLYCERAL DEHYDE-3-PHOSPHATE DEHYDROGENASE C2 (At1g13440) and TWO A AND RELATED PHOSPHATASE-ASSOCIATED PROTEIN42-INTERACTING PROTEIN OF 41 KD (At4g34270) as the most stable across a range of mock, flg22, and GPA-derived extract-exposed Arabidopsis leaf disc RNA samples by geNORM analysis (Vandesompele et al., 2002). All primers are listed in Supplemental Table S1.
To calculate the relative expression levels of target genes, mean cycle threshold (Ct) values for each sample-primer pair combination were calculated from three replicate reaction wells. Mean Ct values were then converted to relative expression values using efficiency of primer pair –∆Ct. The geometric mean of the relative expression values of the reference genes was calculated to produce a normalization factor unique to each sample that was used to calculate the relative expression values for each gene of interest in each sample. These values from independent biological replicates were compared using a described method (Willems et al., 2008).
Callose Staining
The first two fully expanded leaves of 5-week-old Arabidopsis plants were infiltrated using a 1-mL syringe with buffer (control), 100 nm flg22 (in buffer), and 20 mg mL–1 GPA-derived extract (in buffer). After 24 h, one leaf disc was taken from each infiltrated leaf using a circular cork borer with a diameter of 5 mm. To remove chlorophyll from the leaf discs, the discs were placed in 70% (v/v) ethanol for 1 h, 95% (v/v) ethanol with chloroform overnight (18 h), and 100% (v/v) ethanol for 2 h. The discs were then rehydrated for 30 min in 70% (v/v) ethanol, 30 min in 50% (v/v) ethanol, and 30 min in 67 mm K2HPO4 at pH 9.5. Staining with 0.1% (w/v) aniline blue in 67 mm K2HPO4 at pH 9.5 was carried out for 1 h. Leaf discs were mounted in glycerol and viewed under a Nikon Eclipse 800 microscope using a UV filter (Bandpass, 340–380 nm; Longpass, 425 nm). An image was taken of the entire field of view of the center of each leaf disc under 10× magnification (1.34 mm2–1.34 mm by 1 mm). The images were analyzed using ImageJ (National Institutes of Health) to count the number of callose deposits.
Statistical Analyses
Statistical analyses were conducted using Genstat version 12 (VSN International). Aphid survival or fecundity assays and callose deposition were analyzed by classical linear regression analysis using a Poisson distribution within a generalized linear model (GLM). ROS burst assays comparing two conditions were analyzed with Student’s t tests, and those comparing more than two conditions were analyzed with ANOVA. The qRT-PCR data were analyzed using classical linear regression analysis within a GLM in which the means were compared by calculating Student’s t probabilities within the GLM.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. GPA reproduction on bak1 and bkk1 Arabidopsis mutants.
Supplemental Figure S2. Induced resistance in Arabidopsis to the 3-10 kD fraction of GPA saliva is BAK1 dependent.
Supplemental Figure S3. Proteinase K triggers an ROS burst in Arabidopsis.
Supplemental Table S1. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Simon Lloyd and Lucy Gannon for help with the callose assays, members of the Hogenhout and Zipfel laboratories for materials and useful discussions, Graham McGrann and Alexander Coleman for help with the qRT-PCR experiments, Ian Bedford, Gavin Hatt, and Anna Jordan for rearing the insects, and the John Innes Horticultural Services for taking care of plants.
Glossary
- PTI
pathogen-associated molecular pattern-triggered immunity
- PAMP
pathogen-associated molecular pattern
- PRR
pattern recognition receptor
- GPA
green peach aphid
- qRT
quantitative reverse transcriptase
- OS
oral secretion
- ROS
reactive oxygen species
- RLK
receptor-like kinase
- LRR
leucine-rich repeat
- MAPK
mitogen-activated protein kinase
- JA
jasmonic acid
- BR
brassinosteroid
- Col-0
ecotype Columbia
- SA
salicylic acid
- ET
ethylene
- CER
controlled-environment room
- cDNA
complementary DNA
- GLM
generalized linear model
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council (grant nos. BB/J004553/1 to S.A.H. and BB/G024936/1 to C.Z.), the John Innes Foundation (to S.A.H.), the Gatsby Charitable Foundation (to C.Z.), and Biotechnology and Biological Sciences Research Council studentships (to D.C.P. and C.D.).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Atamian HS, Chaudhary R, Cin VD, Bao E, Girke T, Kaloshian I. (2013) In planta expression or delivery of potato aphid Macrosiphum euphorbiae effectors Me10 and Me23 enhances aphid fecundity. Mol Plant Microbe Interact 26: 67–74 [DOI] [PubMed] [Google Scholar]
- Balmer D, Planchamp C, Mauch-Mani B. (2013) On the move: induced resistance in monocots. J Exp Bot 64: 1249–1261 [DOI] [PubMed] [Google Scholar]
- Barah P, Winge P, Kusnierczyk A, Tran DH, Bones AM. (2013) Molecular signatures in Arabidopsis thaliana in response to insect attack and bacterial infection. PLoS ONE 8: e58987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek P, Piślewska-Bednarek M, Svatoš A, Schneider B, Doubský J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, et al. (2009) A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323: 101–106 [DOI] [PubMed] [Google Scholar]
- Blackman RL, Eastop VF (2000) Aphids on the World’s Crops: An Identification and Information Guide. John Wiley and Sons, Chichester, UK [Google Scholar]
- Boller T, Felix G. (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Bos JIB, Hogenhout SA (2011) Effectors in Plant-Insect Interactions. In F Martin, S Kamoun, eds, Effectors in Plant–Microbe Interactions. Wiley-Blackwell, Oxford, pp 355–375 [Google Scholar]
- Bos JIB, Prince D, Pitino M, Maffei ME, Win J, Hogenhout SA. (2010) A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). PLoS Genet 6: e1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng SH, Sheen J. (2010) Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 464: 418–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce TJA, Pickett JA. (2011) Perception of plant volatile blends by herbivorous insects—finding the right mix. Phytochemistry 72: 1605–1611 [DOI] [PubMed] [Google Scholar]
- Bruessow F, Gouhier-Darimont C, Buchala A, Metraux JP, Reymond P. (2010) Insect eggs suppress plant defence against chewing herbivores. Plant J 62: 876–885 [DOI] [PubMed] [Google Scholar]
- Carolan JC, Caragea D, Reardon KT, Mutti NS, Dittmer N, Pappan K, Cui F, Castaneto M, Poulain J, Dossat C, et al. (2011) Predicted effector molecules in the salivary secretome of the pea aphid (Acyrthosiphon pisum): a dual transcriptomic/proteomic approach. J Proteome Res 10: 1505–1518 [DOI] [PubMed] [Google Scholar]
- Chaparro-Garcia A, Wilkinson RC, Gimenez-Ibanez S, Findlay K, Coffey MD, Zipfel C, Rathjen JP, Kamoun S, Schornack S. (2011) The receptor-like kinase SERK3/BAK1 is required for basal resistance against the late blight pathogen Phytophthora infestans in Nicotiana benthamiana. PLoS ONE 6: e16608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherqui A, Tjallingii WF. (2000) Salivary proteins of aphids, a pilot study on identification, separation and immunolocalisation. J Insect Physiol 46: 1177–1186 [DOI] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JDG, Felix G, Boller T. (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM. (2009) Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323: 95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper WR, Dillwith JW, Puterka GJ. (2010) Salivary proteins of Russian wheat aphid (Hemiptera: Aphididae). Environ Entomol 39: 223–231 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge R, van Esse HP, Maruthachalam K, Bolton MD, Santhanam P, Saber MK, Zhang Z, Usami T, Lievens B, Subbarao KV, et al. (2012) Tomato immune receptor Ve1 recognizes effector of multiple fungal pathogens uncovered by genome and RNA sequencing. Proc Natl Acad Sci USA 109: 5110–5115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M, Jander G. (2009) Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana. Plant Cell Environ 32: 1548–1560 [DOI] [PubMed] [Google Scholar]
- De Vos M, Van Oosten VR, Van Poecke RMP, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Métraux JP, Van Loon LC, Dicke M, et al. (2005) Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact 18: 923–937 [DOI] [PubMed] [Google Scholar]
- Denoux C, Galletti R, Mammarella N, Gopalan S, Werck D, De Lorenzo G, Ferrari S, Ausubel FM, Dewdney J. (2008) Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol Plant 1: 423–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon AFG (1998) Aphid Ecology: An Optimization Approach, Ed 2. Chapman and Hall, London [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18: 265–276 [DOI] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Denoux C, De Lorenzo G, Ausubel FM, Dewdney J. (2007) Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol 144: 367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filichkin SA, Brumfield S, Filichkin TP, Young MJ. (1997) In vitro interactions of the aphid endosymbiotic SymL chaperonin with barley yellow dwarf virus. J Virol 71: 569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin EF, Zhang Z, Juarez Ayala JC, Castroverde CDM, Nazar RN, Robb J, Liu CM, Thomma BPHJ. (2009) Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol 150: 320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardoni PA, Hettenhausen C, Baldwin IT, Bonaventure G. (2011) Nicotiana attenuata LECTIN RECEPTOR KINASE1 suppresses the insect-mediated inhibition of induced defense responses during Manduca sexta herbivory. Plant Cell 23: 3512–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Ibanez S, Ntoukakis V, Rathjen JP. (2009) The LysM receptor kinase CERK1 mediates bacterial perception in Arabidopsis. Plant Signal Behav 4: 539–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J, Ausubel FM. (1994) Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc Natl Acad Sci USA 91: 8955–8959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T. (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Gouhier-Darimont C, Schmiesing A, Bonnet C, Lassueur S, Reymond P. (2013) Signalling of Arabidopsis thaliana response to Pieris brassicae eggs shares similarities with PAMP-triggered immunity. J Exp Bot 64: 665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust AA, Biswas R, Lenz HD, Rauhut T, Ranf S, Kemmerling B, Götz F, Glawischnig E, Lee J, Felix G, et al. (2007) Bacteria-derived peptidoglycans constitute pathogen-associated molecular patterns triggering innate immunity in Arabidopsis. J Biol Chem 282: 32338–32348 [DOI] [PubMed] [Google Scholar]
- Harmel N, Létocart E, Cherqui A, Giordanengo P, Mazzucchelli G, Guillonneau F, De Pauw E, Haubruge E, Francis F. (2008) Identification of aphid salivary proteins: a proteomic investigation of Myzus persicae. Insect Mol Biol 17: 165–174 [DOI] [PubMed] [Google Scholar]
- He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J. (2007) BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol 17: 1109–1115 [DOI] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AME, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. (2007) The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA 104: 12217–12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenhout SA, Bos JIB. (2011) Effector proteins that modulate plant—insect interactions. Curr Opin Plant Biol 14: 422–428 [DOI] [PubMed] [Google Scholar]
- Howe GA, Jander G. (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59: 41–66 [DOI] [PubMed] [Google Scholar]
- Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Qamar SA, Mengiste T, Betsuyaku S, Parker JE, Müssig C, et al. (2007) The BRI1-associated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr Biol 17: 1116–1122 [DOI] [PubMed] [Google Scholar]
- Kettles GJ, Drurey C, Schoonbeek HJ, Maule AJ, Hogenhout SA. (2013) Resistance of Arabidopsis thaliana to the green peach aphid, Myzus persicae, involves camalexin and is regulated by microRNAs. New Phytol 198: 1178–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kørner CJ, Klauser D, Niehl A, Domínguez-Ferreras A, Chinchilla D, Boller T, Heinlein M, Hann DR. (2013) The immunity regulator BAK1 contributes to resistance against diverse RNA viruses. Mol Plant Microbe Interact 26: 1271–1280 [DOI] [PubMed] [Google Scholar]
- Krol E, Mentzel T, Chinchilla D, Boller T, Felix G, Kemmerling B, Postel S, Arents M, Jeworutzki E, Al-Rasheid KAS, et al. (2010) Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J Biol Chem 285: 13471–13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuśnierczyk A, Winge P, Jørstad TS, Troczyńska J, Rossiter JT, Bones AM. (2008) Towards global understanding of plant defence against aphids—timing and dynamics of early Arabidopsis defence responses to cabbage aphid (Brevicoryne brassicae) attack. Plant Cell Environ 31: 1097–1115 [DOI] [PubMed] [Google Scholar]
- Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC. (2002) BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110: 213–222 [DOI] [PubMed] [Google Scholar]
- Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M, Landtag J, Brandt W, Rosahl S, Scheel D, et al. (2005) Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310: 1180–1183 [DOI] [PubMed] [Google Scholar]
- Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, Ton J. (2011) Callose deposition: a multifaceted plant defense response. Mol Plant Microbe Interact 24: 183–193 [DOI] [PubMed] [Google Scholar]
- Ma R, Chen JL, Cheng DF, Sun JR. (2010) Activation of defense mechanism in wheat by polyphenol oxidase from aphid saliva. J Agric Food Chem 58: 2410–2418 [DOI] [PubMed] [Google Scholar]
- Macho AP, Boutrot F, Rathjen JP, Zipfel C. (2012) Aspartate oxidase plays an important role in Arabidopsis stomatal immunity. Plant Physiol 159: 1845–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr (1997) Innate immunity: the virtues of a nonclonal system of recognition. Cell 91: 295–298 [DOI] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R. (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2: ra45. [DOI] [PubMed] [Google Scholar]
- Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N. (2007) CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA 104: 19613–19618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan J, Zipfel C. (2012) Plant pattern recognition receptor complexes at the plasma membrane. Curr Opin Plant Biol 15: 349–357 [DOI] [PubMed] [Google Scholar]
- Nam KH, Li J. (2002) BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110: 203–212 [DOI] [PubMed] [Google Scholar]
- Nam KJ, Hardie J. (2012) Host acceptance by aphids: probing and larviposition behaviour of the bird cherry-oat aphid, Rhopalosiphum padi on host and non-host plants. J Insect Physiol 58: 660–668 [DOI] [PubMed] [Google Scholar]
- Nishinaka Y, Aramaki Y, Yoshida H, Masuya H, Sugawara T, Ichimori Y. (1993) A new sensitive chemiluminescence probe, L-012, for measuring the production of superoxide anion by cells. Biochem Biophys Res Commun 193: 554–559 [DOI] [PubMed] [Google Scholar]
- Nühse TS, Bottrill AR, Jones AME, Peck SC. (2007) Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J 51: 931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitino M, Hogenhout SA. (2013) Aphid protein effectors promote aphid colonization in a plant species-specific manner. Mol Plant Microbe Interact 26: 130–139 [DOI] [PubMed] [Google Scholar]
- Postel S, Küfner I, Beuter C, Mazzotta S, Schwedt A, Borlotti A, Halter T, Kemmerling B, Nürnberger T. (2010) The multifunctional leucine-rich repeat receptor kinase BAK1 is implicated in Arabidopsis development and immunity. Eur J Cell Biol 89: 169–174 [DOI] [PubMed] [Google Scholar]
- Ranf S, Eschen-Lippold L, Pecher P, Lee J, Scheel D. (2011) Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. Plant J 68: 100–113 [DOI] [PubMed] [Google Scholar]
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tör M, de Vries S, Zipfel C. (2011) The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23: 2440–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri A, Vandermoten S, Leroy PD, Haubruge E, Hance T, Thonart P, De Pauw E, Francis F. (2013) Proteomic investigation of aphid honeydew reveals an unexpected diversity of proteins. PLoS ONE 8: e74656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer M, Fischer C, Meldau S, Seebald E, Oelmüller R, Baldwin IT. (2011) Lipase activity in insect oral secretions mediates defense responses in Arabidopsis. Plant Physiol 156: 1520–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonhoven L, van Loon JJ, Dicke M (2005) Insect-Plant Biology, Ed 2. Oxford University Press, Oxford [Google Scholar]
- Schuhegger R, Nafisi M, Mansourova M, Petersen BL, Olsen CE, Svatoš A, Halkier BA, Glawischnig E. (2006) CYP71B15 (PAD3) catalyzes the final step in camalexin biosynthesis. Plant Physiol 141: 1248–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze B, Mentzel T, Jehle AK, Mueller K, Beeler S, Boller T, Felix G, Chinchilla D. (2010) Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem 285: 9444–9451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Lefert P, Panstruga R. (2011) A molecular evolutionary concept connecting nonhost resistance, pathogen host range, and pathogen speciation. Trends Plant Sci 16: 117–125 [DOI] [PubMed] [Google Scholar]
- Schwessinger B, Roux M, Kadota Y, Ntoukakis V, Sklenar J, Jones A, Zipfel C. (2011) Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet 7: e1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonzac C, Feike D, Gimenez-Ibanez S, Hann DR, Zipfel C, Rathjen JP. (2011) Hierarchy and roles of pathogen-associated molecular pattern-induced responses in Nicotiana benthamiana. Plant Physiol 156: 687–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Li L, Macho AP, Han Z, Hu Z, Zipfel C, Zhou JM, Chai J. (2013) Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 342: 624–628 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG. (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: H0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Zhang XC, Neece D, Ramonell KM, Clough S, Kim SY, Stacey MG, Stacey G. (2008) A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 20: 471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whippo CW, Hangarter RP. (2005) A brassinosteroid-hypersensitive mutant of BAK1 indicates that a convergence of photomorphogenic and hormonal signaling modulates phototropism. Plant Physiol 139: 448–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems E, Leyns L, Vandesompele J. (2008) Standardization of real-time PCR gene expression data from independent biological replicates. Anal Biochem 379: 127–129 [DOI] [PubMed] [Google Scholar]
- Wu J, Baldwin IT. (2010) New insights into plant responses to the attack from insect herbivores. Annu Rev Genet 44: 1–24 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Huffaker A, Bryan AC, Tax FE, Ryan CA. (2010) PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell 22: 508–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Pearce G, Ryan CA. (2006) The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc Natl Acad Sci USA 103: 10104–10109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DH, Hettenhausen C, Baldwin IT, Wu J. (2011a) BAK1 regulates the accumulation of jasmonic acid and the levels of trypsin proteinase inhibitors in Nicotiana attenuata’s responses to herbivory. J Exp Bot 62: 641–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DH, Hettenhausen C, Baldwin IT, Wu J. (2011b) The multifaceted function of BAK1/SERK3: plant immunity to pathogens and responses to insect herbivores. Plant Signal Behav 6: 1322–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shao F, Li Y, Cui H, Chen L, Li H, Zou Y, Long C, Lan L, Chai J, et al. (2007) A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe 1: 175–185 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JR, Normanly J, Chory J, Celenza JL. (2002) Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev 16: 3100–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Tootle TL, Glazebrook J. (1999) Arabidopsis PAD3, a gene required for camalexin biosynthesis, encodes a putative cytochrome P450 monooxygenase. Plant Cell 11: 2419–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G. (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JDG, Felix G, Boller T. (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428: 764–767 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.