Abstract
Alternative splicing (AS) is tightly coupled to transcription for the majority of human genes. However, how these two processes are linked is not well understood. Here, we unveil a direct role for the transcription factor FBI-1 in the regulation of AS. FBI-1 interacts with the splicing factor SAM68 and reduces its binding to BCL-X mRNA. This, in turn, results in the selection of the proximal 5′ splice site in BCL-X exon 2, thereby favoring the anti-apoptotic BCL-XL variant and counteracting SAM68-mediated apoptosis. Conversely, depletion of FBI-1, or expression of a SAM68 mutant lacking the FBI-1 binding region, restores the ability of SAM68 to induce BCL-XS splicing and apoptosis. FBI-1's role in splicing requires the activity of histone deacetylases, whose pharmacological inhibition recapitulates the effects of FBI-1 knockdown. Our study reveals an unexpected function for FBI-1 in splicing modulation with a direct impact on cell survival.
Keywords: alternative splicing, apoptosis, BCL-X, FBI-1, SAM68
Introduction
Alternative splicing (AS) amplifies genome complexity in eukaryotes by allowing different combinations of exons in the pre-mRNAs transcribed from each gene. The large spectrum of protein variants produced by AS greatly contributes to fine-tune many complex biological processes 1, 2. Splicing of pre-mRNAs is operated by a ribonucleoprotein complex named spliceosome 3. Due to the lack of a strict consensus signature at the exon–intron junctions in the majority of eukaryotic genes, spliceosome recruitment requires the assistance of RNA-binding proteins (RBPs) that recognize specific elements in the vicinity of the splice sites (ss) 4. Thus, the relative balance of the activity or expression of these splicing factors modulates AS in each given cell and determines changes in mRNA variants that affect several biological processes, including cell proliferation, survival, and differentiation 1, 2.
Programmed cell death, or apoptosis, participates to cell homeostasis in development and disease and represents a typical example of biological process controlled by AS 5. The majority of apoptotic genes produce multiple splice variants that often function antagonistically in the cell 5. BCL-X represents a striking example of apoptotic protein whose function is tightly regulated by AS. Alternative usage of two 5′ ss in exon 2 of the BCL-X gene yields splice variants that play antagonistic effects on cell survival. Selection of the proximal 5′ ss at the end of the exon leads to expression of a long isoform (BCL-XL) with anti-apoptotic function, whereas selection of the distal 5′ ss within the exon yields a short variant that promotes cell death (BCL-XS) 6. Thus, cells can respond to external cues and opt for survival or death by fine-tuning the regulation of BCL-X splicing. Importantly, cancer cells frequently overexpress BCL-XL, and treatments with antisense oligonucleotides that promote splicing of the pro-apoptotic BCL-XS variant sensitize them to chemotherapy, suggesting that modulation of BCL-X splicing has potential therapeutic value 7.
A splicing regulator involved in BCL-X AS and apoptosis is SAM68 8, a member of the evolutionary conserved signal transduction and activator of RNA (STAR) family of RBPs, which regulate cell proliferation, survival, and differentiation in various organisms 9. In prostate cancer (PCa), SAM68 supports cell proliferation and survival 10. Intriguingly, whereas up-regulation of SAM68 generally causes apoptosis by promoting BCL-XS splicing 8, its depletion in PCa cells negatively affects the expression of the anti-apoptotic BCL-XL isoform 10. These observations suggest that the splicing activity of SAM68 is modulated by cell-specific factors.
Herein, we identified the transcription factor FBI-1 as a novel regulator of the splicing activity of SAM68 by two-hybrid screen. FBI-1 is an essential protein 11 that is overexpressed in human cancers 12, 13, like SAM68 14. We show that FBI-1 physically interacts with SAM68 and affects its interaction with the BCL-X mRNA, thereby promoting splicing of the anti-apoptotic BCL-XL variant and cell survival. These findings identify an unpredicted, transcription-independent function for FBI-1 in splicing regulation through the interaction with a splicing factor.
Results and Discussion
The transcription factor FBI-1 is a novel SAM68-interacting protein
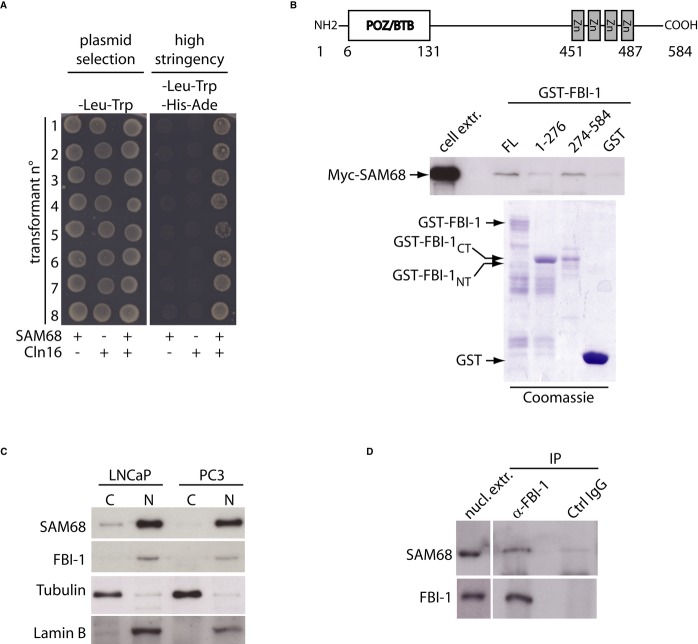
Earlier observations indicated that over-expression of SAM68 in non-transformed cells caused G1 arrest and triggered apoptosis 15. Notably, SAM68-dependent apoptosis required its splicing activity in HEK293T cells 8. However, in cancer cells, the pro-apoptotic activity of SAM68 is substantially repressed 14. Thus, we hypothesized that interaction with specific cofactors could modulate the splicing activity of SAM68 in cancer cells. To search for such functional partners, we carried out a yeast two-hybrid screen using a library amplified from the PCa cell line LNCaP. Among several novel SAM68-interacting proteins, we focused our attention on FBI-1, a transcription factor previously described as a proto-oncogene in mouse and humans 12, 13. Since FBI-1 was shown to regulate transcription of genes involved in cell proliferation and survival 12–18, we first verified that expression of FBI-1 alone did not activate reporter genes. Yeast cells were co-transformed with the plasmid recovered from the original clone (# 16) and either pGBKT7 alone or pGBKT7-SAM68. Eight independent transformants confirmed that the interaction between SAM68 and FBI-1 was required to support yeast growth under high-stringency conditions (Fig 1A).
Figure 1.
- Yeast two-hybrid assay of the interaction between FBI-1 and SAM68. Eight clones of the AH109 yeast strain were transformed with the plasmid expressing the fusion protein Gal4-AD-FBI-1278–584 (clone 16) and either Gal4-DBD-SAM68 or empty vector. Clones were plated in non-stringency (SD without Leu and Trp) and high-stringency (SD without Leu, Trp, His, and Ade) media and grown at 28°C for 4 days.
- Schematic representation of FBI-1 structure (upper panel). Western blot analysis of SAM68 in pull-down assay (bottom panel) with the indicated GST-FBI-1 fusion proteins and cell extract of HEK293T cells expressing Myc-SAM68. The Coomassie blue staining shows the purified GST-FBI-1 proteins.
- Western blot analysis of cytoplasmic (C) and nuclear (N) compartments fractionated from LNCaP and PC3 cells. Purity of fractions was assessed using tubulin (cytoplasm) and Lamin-B (nucleus) as markers.
- Western blot analysis of SAM68 and FBI-1 after immunoprecipitation (IP) of endogenous FBI-1 from LNCaP nuclear extracts with anti-FBI-1 or control rabbit IgGs.
FBI-1 contains an N-terminal POZ/BTB domain and a C-terminal region characterized by four Kruppel-like zinc finger motifs (Fig 1B). The clone retrieved from our screen encoded the C-terminal 306 amino acids (aa 278–584). In line with the result of the two-hybrid assay, GST pull-down assays showed that Myc-SAM68 interacts with full-length GST-FBI-1 and with GST-FBI-1CT (aa 274–584), but not with the POZ/BTB domain in GST-FBI-1NT (aa 1–276) nor with GST alone (Fig 1B). This interaction was also confirmed by using the CheckMate™ Mammalian System reporter (Promega Corporation, Madison, WI, USA) assay in live HEK293T cells (Supplementary Fig S1A). Moreover, we found that the endogenous FBI-1 and SAM68 proteins were mainly localized in the nucleus of PCa cells (Fig 1C), co-sedimented in sucrose gradient fractionations of nuclear extracts (Supplementary Fig S1B and C), and were co-immunoprecipitated from LNCaP cell extracts (Fig 1D). Lastly, endogenous SAM68 associated with Flag-FBI-1 in transfected HEK293T cells (Supplementary Fig S1D). Collectively, these results demonstrate that FBI-1 interacts with SAM68.
FBI-1 represses the ability of SAM68 to induce apoptosis
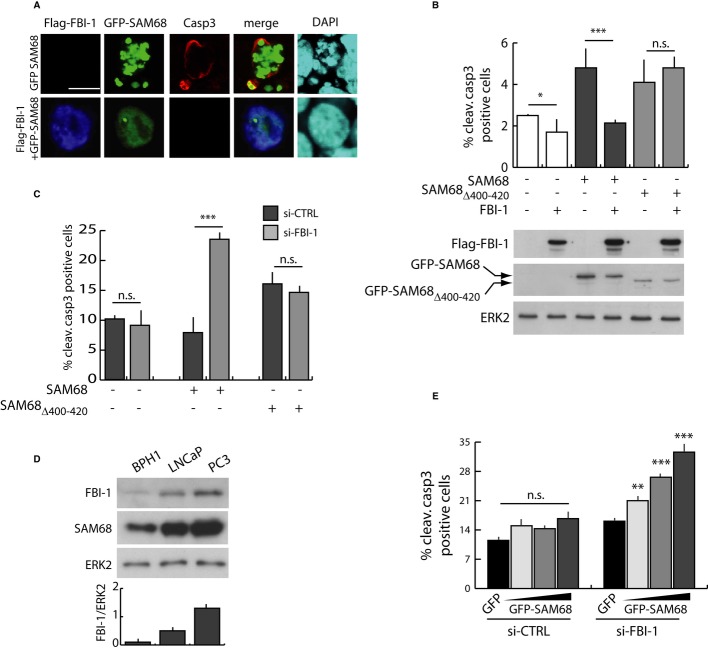
To test whether FBI-1 modulates the function of SAM68, we co-expressed the two proteins in HEK293T cells. As expected 8, expression of GFP-SAM68 augmented the number of apoptotic cells, but co-expression of Flag-FBI-1 completely suppressed this effect (Fig 2A and B). To investigate whether physical interaction between FBI-1 and SAM68 is required for this inhibition, we set out to identify the minimal region of SAM68 involved in the association. SAM68 is characterized by a GSG domain required for RNA binding and homo-dimerization, flanked by regulatory regions that are sites of protein–protein interactions and post-translational modifications affecting SAM68 localization and function (Supplementary Fig S2A) 13. GST pull-down assays showed that FBI-1 interacted with the full-length SAM68 and with its C-terminal region (aa 321–443), but not with the N-terminal region, including the GSG domain (aa 1–277) (Supplementary Fig S2B). Progressive deletions of the C-terminal region from both ends restricted the surface of interaction to 35 residues in SAM68 (aa 400–434) (Supplementary Fig S2B). Since the nuclear localization signal (NLS) in SAM68 partially overlaps with this region 19, we constructed a deletion mutant lacking residues 400–420 to avoid its mislocalization. SAM68Δ400–420 localized in the nucleus and interacted with poly-U and poly-A synthetic RNA similarly to full-length SAM68 (Supplementary Fig S2C and D), indicating that it is a functional protein. The two-hybrid CheckMate™ Mammalian System and pull-down assays showed that the interaction of SAM68Δ400–420 with FBI-1 is strongly reduced (Supplementary Fig S2E and F). Notably, while Sam68Δ400–420 triggered apoptosis like the full-length protein (Fig 2B), co-expression of FBI-1 did not suppress this activity (Fig 2B), indicating that FBI-1 inhibits SAM68-dependent apoptosis through physical interaction with it.
Figure 2.
- Representative immunofluorescence analysis of activated (cleaved) caspase-3 in HEK293T cells 72 h after transfection with the indicated plasmids. Scale bar, 10 μm.
- Quantitative analysis of cleaved caspase-3 in HEK293T transfected as in panel (A). The bar graph represents apoptotic cells scored as positive for cleaved caspase-3 staining within the GFP-positive cell population (mean ± s.d., n = 3). Western blot analysis of the expression levels of the recombinant proteins is shown in the bottom panels.
- Quantitative analysis of cleaved caspase-3 in HEK293T cells transfected with the indicated siRNAs and with suboptimal amount of GFP-SAM68 (100 ng plasmid). Cells were analyzed for cleaved caspase-3 as described in (B) (mean ± s.d., n = 3).
- Western blot analysis of endogenous SAM68 and FBI-1 in BPH1, LNCaP, and PC3 cells. ERK2 was used for normalization of extracts. Bar graph represents densitometric analysis of the bands (mean ± s.d., n = 3).
- Analysis of cleaved caspase-3 in PC3 cells transfected with FBI-1 (si-FBI-1) or control (si-CTRL) siRNAs and increasing amounts of GFP-SAM68. The bar graph represents the percentage of positive cells as described in (B) (mean ± s.d. from three experiments).
Data information: P-values of Student's t-test: *P < 0.05, **P < 0.01; ***P < 0.001; n.s., not significant.
Down-regulation of FBI-1 in prostate cancer cells restores SAM68-mediated apoptosis
We reasoned that depletion of FBI-1 should enhance SAM68-dependent apoptosis. Indeed, expression of suboptimal amounts of SAM68 did not trigger cell death in control cells (si-CTRL), but knockdown of FBI-1 restored SAM68-dependent apoptosis (si-FBI-1 in Fig 2C). In line with the requirement for physical interaction, Sam68Δ400–420 induced apoptosis at this lower dose also in control cells and depletion of FBI-1 did not affect its activity (Fig 2C).
Previous reports documented that FBI-1 and SAM68 are up-regulated in various cancer cells 12–14. Consistently, we found that both proteins were expressed at higher levels in PCa cells (LNCaP and PC3) with respect to benign prostate epithelial cells (BPH1) (Fig 2D). LNCaP cells derive from androgen-sensitive PCa lymph node metastases, whereas PC3 cells derive from androgen-insensitive PCa bone metastases and represent cells at advanced stage of the disease. Noteworthy, dose-dependent overexpression of SAM68 in PC3 cells did not trigger apoptosis (si-CTRL in Fig 2E), whereas a small effect was observed in LNCaP cells (si-CTRL in Supplementary Fig S3). Strikingly, depletion of endogenous FBI-1 restored the pro-apoptotic activity of SAM68 in PC3 cells (Fig 2E) and strongly enhanced its effect in LNCaP cells (Supplementary Fig S3). These results indicate that high levels of FBI-1 are required to mask the pro-apoptotic activity of SAM68 in PCa cells.
FBI-1 modulates BCL-X splicing through direct physical interaction with SAM68
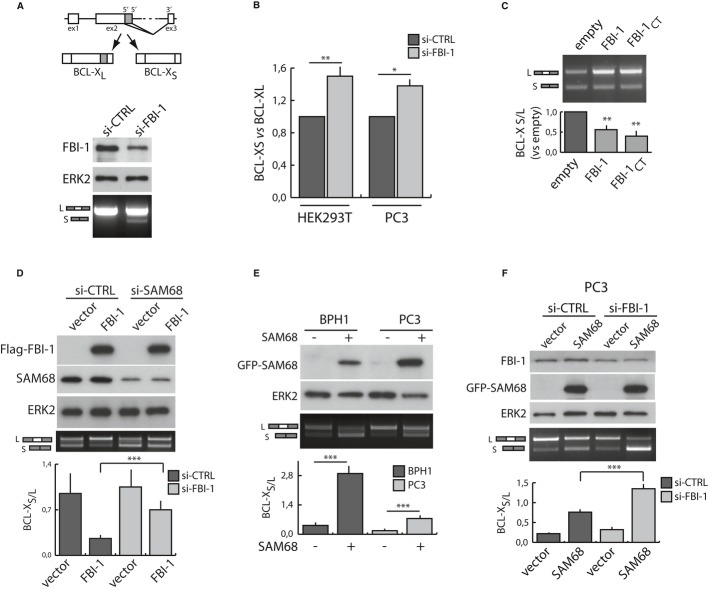
The ability of SAM68 to induce apoptosis correlates with its effect on BCL-X AS 8. The BCL-X gene encodes two splice variants that play antagonistic effects on cell survival (Fig 3A): the anti-apoptotic BCL-XL or the pro-apoptotic BCL-XS proteins 6. Moderate knockdown of FBI-1 in HEK293T promoted the expression of the pro-apoptotic BCL-XS variant, which was not detectable in control cells by conventional PCR (Fig 3A). Quantitative real-time PCR (qPCR) using exon junction-specific primers confirmed that knockdown of FBI-1 enhanced splicing of BCL-XS in HEK293T and PC3 cells (Fig 3B). This result suggests that splicing of the endogenous BCL-X pre-mRNA is modulated by FBI-1 in live cells.
Figure 3.
FBI-1 modulates BCL-X splicing in SAM68-dependent manner.
A Schematic representation of BCL-X splice variants (upper panel). Analysis of endogenous BCL-X splicing in HEK293T cells transfected with control (si-CTRL) or FBI-1 (si-FBI-1) siRNAs. Western blot analyses (lower panel) for the indicated proteins and agarose gel of the RT–PCR analysis are shown.
B qRT-PCR analysis of BCL-X variants using exon junction primers performed in HEK293T and PC3 cells transfected with control (si-CTRL) or FBI-1 (si-FBI-1) siRNAs. Fold variation of each sample was calculated by delta-delta Ct method as described in Supporting information.
C, D Analysis of in vivo splicing assay of BCL-X minigene in HEK293T cells transfected with Flag-FBI-1 (FBI-1) or Myc-FBI-1CT (C), or with control (si-CTRL) or SAM68 (si-SAM68) siRNAs and Flag-FBI-1 (D). In (C), the BCL-XS/BCL-XL ratio of the control sample (empty vector) was set to 1.
E, F Analysis of in vivo splicing assay of BCL-X minigene in BPH1 and PC3 cells (E) or in PC3 cells transfected with control (si-CTRL) or FBI-1 (si-FBI) siRNAs (F) in the presence of GFP-SAM68.
Data information: In (D–F): Western blots (upper panel), agarose gels of the RT–PCR analyses (middle panel), and bar graphs (bottom panel) of the BCL-XS/BCL-XL ratio. P-values of Student's t-test: *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant; mean ± s.d., n = 3.
To gain more insight into the regulation of BCL-X AS by FBI-1, we employed a minigene that recapitulates the splicing of the endogenous gene 8. Up-regulation of FBI-1, or FBI-1CT, favored splicing of the anti-apoptotic BCL-XL variant (Fig 3C). This effect required SAM68 expression, as its down-regulation by RNAi in HEK293T (Fig 3D), or by stable transfection of a different siRNA in PC3 cells (Supplementary Fig S4A), reduced or abolished the ability of FBI-1 to promote BCL-XL splicing. Moreover, FBI-1 was unable to modulate BCL-X splicing when the splicing-defective SAM68V229F 8 was co-transfected in HEK293T cells (Supplementary Fig S4B), indicating that the splicing activity of SAM68 was necessary.
We then asked whether a functional interaction with SAM68 was required for the effect of FBI-1 on BCL-X splicing. As previously reported 8, SAM68 induced splicing of the pro-apoptotic BCL-XS when it was expressed alone (Supplementary Fig S4C, lane 3). However, this effect was suppressed by co-expression of FBI-1 or its C-terminal domain (Supplementary Fig S4C, lane 4 and 6). Importantly, SAM68Δ400–420 strongly enhanced splicing of the BCL-XS variant, like full-length SAM68, but co-expression of FBI-1 did not affect its splicing activity (Supplementary Fig S4D), proving that physical interaction with SAM68 is required for the effect of FBI-1 on BCL-X AS.
The pro-apoptotic activity of SAM68 was suppressed by FBI-1 in PC3 cells (Fig 2E). Consistently, overexpression of SAM68 in PC3 cells only slightly affected BCL-X AS, whereas it strongly enhanced splicing of the pro-apoptotic BCL-XS in the benign BPH1 cells (Fig 3E), which express low levels of FBI-1 (Fig 2D) similarly to HEK293T cells (data not shown). On the other hand, knockdown of endogenous FBI-1 significantly restored SAM68-dependent splicing of BCL-XS in PC3 cells (Fig 3F) and strongly enhanced this splicing event in HEK293T cells (Supplementary Fig S4E). These results suggest that elevated expression of FBI-1 promotes cell survival through direct interaction with SAM68 and modulation of BCL-X splicing.
BCL-X splicing is involved in FBI-1-dependent suppression of apoptosis
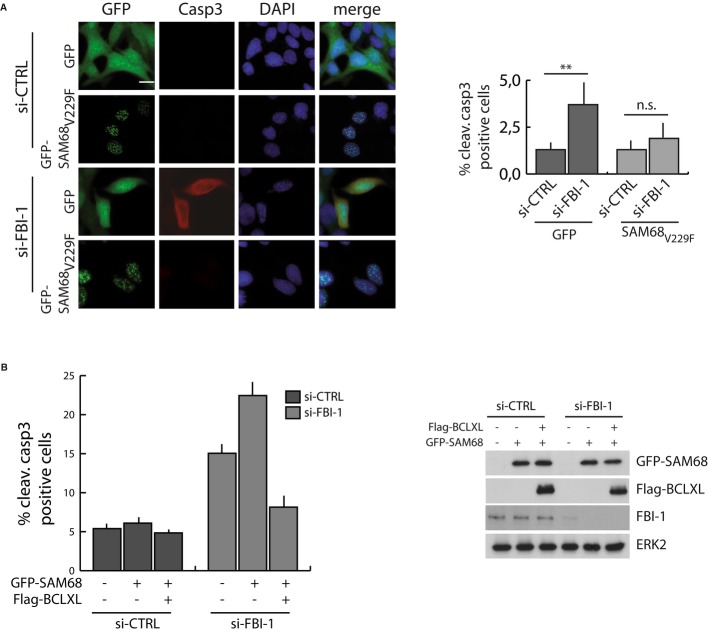
Next, we further evaluated whether the pro-apoptotic effect mediated by FBI-1 depletion was due to the splicing activity of SAM68. Expression of the RNA binding-defective SAM68V229F mutant exerts dominant-negative effect on the splicing activity of endogenous SAM68 20, while retaining the transcriptional activity of the protein 21. We infected LNCaP cells, whose apoptosis was strongly induced upon FBI-1 depletion (GFP samples in Fig 4A), with a retrovirus encoding GFP-SAM68V229F, or GFP as a control. Expression of the dominant-negative GFP-SAM68V229F almost completely abolished apoptosiscaused by FBI-1 knockdown in GFP-infected cells (Fig 4A), suggesting that FBI-1 counteracts SAM68-mediated apoptosis by modulating its splicing activity toward BCL-X. Accordingly, overexpression of the anti-apoptotic BCL-XL also blocked cell death induced by SAM68 overexpression in PC3 cells depleted of FBI-1 (Fig 4B). Thus, in our conditions, the anti-apoptotic effect of FBI-1 correlates with SAM68-dependent regulation of BCL-X splicing. Since FBI-1 was shown to promote cell survival in mouse erythroblasts by directly repressing transcription of the pro-apoptotic BIM gene products 16, it is likely that this protein can promote cell survival through at least two distinct mechanisms: direct repression of transcription of target genes or indirect modulation of their AS through SAM68.
Figure 4.
- LNCaP cells, stably infected with GFP or GFP-SAM68V229F retroviruses, were transfected with FBI-1 (si-FBI-1) or control (si-CTRL) siRNAs. Scale bar, 10 μm.
- Analysis of cleaved caspase-3 in PC3 cells transfected with FBI-1 (si-FBI-1) or control (si-CTRL) siRNAs, GFP-SAM68, and Flag-BCL-XL as indicated. Western blot analysis shows the expression levels of the recombinant proteins.
Data information: Bar graphs represent the percentage of positive cells as described in Fig 2B (mean ± s.d., n = 3). P-values of Student's t-test: **P < 0.01; n.s., not significant.
FBI-1 modulates SAM68 binding to BCL-X mRNA through histone deacetylases
FBI-1 interacts with residues 400–420 in the C-terminal region of SAM68, which was previously shown to bind other proteins involved in splicing 8. Moreover, FYN-dependent tyrosine phosphorylation of this region modulates SAM68 homodimerization and protein–protein interactions 9. However, we found that overexpression of FBI-1 did not perturb the ability of SAM68 to homodimerize and to interact with hnRNP A1 (Supplementary Fig S5A). Furthermore, FYN-dependent phosphorylation did not impair the interaction of SAM68 with FBI-1 (Supplementary Fig S5B). Thus, FBI-1 does not appear to modulate the splicing activity of SAM68 by interfering with its interaction with cofactors.
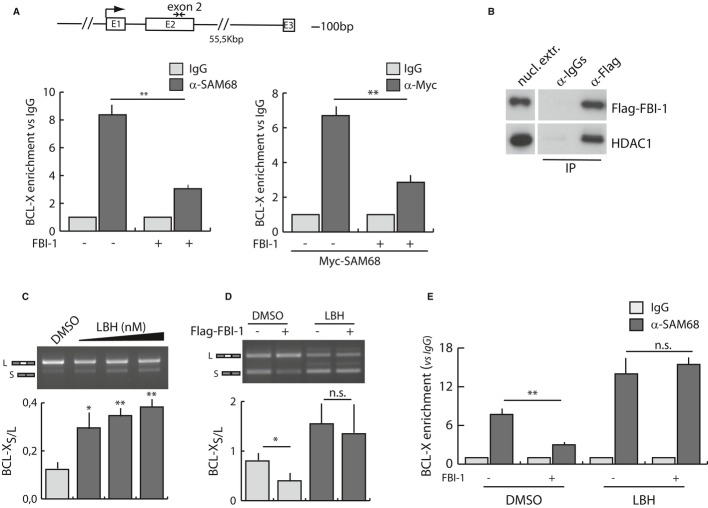
To investigate the mechanism by which FBI-1 modulates SAM68-dependent AS, we first analyzed the binding of SAM68 to BCL-X RNA. Cross-link immunoprecipitation (CLIP) experiments showed that SAM68 binds BCL-X in exon 2, while no binding was observed in the distal part of intron 2 (Supplementary Fig S5C). Notably, overexpression of FBI-1 strongly reduced binding of endogenous SAM68 or Myc-SAM68 to BCL-X mRNA (Fig 5A). However, FBI-1 did not generally reduce the affinity of SAM68 for RNA, as determined by binding to synthetic poly-U RNA in vitro (Supplementary Fig S5D).
Figure 5.
- Cross-link immunoprecipitation (CLIP) analysis of endogenous SAM68 and recombinant Myc-SAM68 in cells transfected or not with Flag-FBI-1. Associated BCL-X RNA was quantified by qRT-PCR and represented as fold enrichment relative to the IgG sample. The scheme indicates the position of primers used for the analysis.
- Western blot analysis of HDAC1 co-immunoprecipitated with Flag-FBI-1 or control immunoprecipitates (rabbit IgGs) from HEK293T nuclear extracts.
- RT–PCR analysis of endogenous BCL-X mRNA splicing in HEK293T cells treated for 40 h with 50, 100, and 200 nM of LBH589 or with DMSO.
- RT–PCR analysis of the BCL-X minigene splicing in HEK293T transfected with or without Flag-FBI-1 and treated for 40 h with LBH or DMSO.
- CLIP assay of endogenous SAM68 in HEK293T transfected as in (D). Associated BCL-X RNA was quantified by qRT-PCR and represented as fold enrichment relative to the IgG sample.
Data information: P-values of Student's t-test: *P < 0.05; **P < 0.01; n.s., not significant. Data were obtained from three independent experiments.
Since FBI-1 is known to associate with histone deacetylase (HDAC) 1, 2 and 3 18, 22 and HDAC inhibitors affect a wide spectrum of AS events 23, we asked whether HDAC activity influences BCL-X splicing. First, we confirmed the interaction of FBI-1 with HDAC1 by co-immunoprecipitation (Fig 5B). Next, we tested whether HDAC activity influences splicing of the endogenous BCL-X gene by using the pan-HDAC inhibitor LBH589 24. Treatment of HEK293T cells with LBH589 mimicked the effect of FBI-1 knockdown on AS of BCL-X mRNA, causing a dose-dependent accumulation of the BCL-XS variant (Fig 5C). To directly test whether HDAC activity is implicated in the effect of FBI-1 on BCL-X AS, we co-transfected FBI-1 and the BCL-X minigene in the presence or absence of LBH589. Notably, the increase in BCL-XL splicing elicited by FBI-1 was completely abolished when cellular HDAC activity was suppressed (Fig 5D). Moreover, the effect on splicing could be explained with modulation of SAM68 recruitment on the BCL-X mRNA. In fact, LBH589 enhanced binding of SAM68 to the BCL-X transcript and completely suppressed the ability of FBI-1 to inhibit its recruitment on this target mRNA (Fig 5E). Collectively, these results strongly suggest that FBI-1 affects recruitment of SAM68 on the BCL-X transcript through an HDAC-dependent mechanism, thereby modulating BCL-X AS and apoptosis in live cells.
Conclusions
Our studies suggest that FBI-1 modulates BCL-X splicing by directly interacting with SAM68 and inhibiting its recruitment on the BCL-X mRNA. Thus, FBI-1 appears to function by lowering the abundance on the pre-mRNA of a splicing factor that promotes BCL-XS splicing. Mechanistically, several observations point to an involvement of HDACs in FBI-1-mediated BCL-X splicing, as we found that an increase in the BCL-XS variant can be similarly obtained either by lowering the levels of FBI-1 or by blocking the activity of HDACs, which form a complex with this transcription factor 18, 22. These results, together with the notion that FBI-1 is a transcriptional regulator, suggest that the effect of FBI-1 on SAM68-dependent BCL-X splicing occurs co-transcriptionally. Whether HDACs influence FBI-1-mediated AS by altering chromatin structure or by affecting the SAM68/RNA polymerase II complex 25 (or both) remains to be elucidated.
In conclusion, our results highlight a new, unexpected function of FBI-1 in splicing regulation and unveil a new layer in the regulation of gene expression by this essential transcription factor through the physical interaction with a splicing factor.
Materials and Methods
Yeast two-hybrid screen
The yeast two-hybrid screen was performed according to the manufacturer's instruction (Clontech). Briefly, the Gal4 activation domain-fusion library was generated by using 1 μg polyA+ RNA isolated from LNCaP cells. The ds-cDNA was generated by SMART technology using a modified oligo(dT) primer (CDSIII primer) and cloned in pGADT7-rec2 vector (see also Supplementary information).
Cell cultures, transfections, and cell extract preparation
Cell cultures, transfections, and sample preparation were carried out by standard methods as described 10 (see also Supplementary information).
Co-immunoprecipitation and GST pull-down assays
GST pull-down assays and co-immunoprecipitation (co-IP) experiments were carried out in presence of RNase and DNase using either total cell extracts or nuclear extracts from LNCaP or HEK293T cells transfected with the indicated plasmids as previously described 10 (see also Supplementary information).
UV cross-link RNA/protein immunoprecipitation (CLIP)
Cells were washed with PBS, UV-irradiated (400 mJ/cm2), and used for CLIP experiments as described 26 (see also Supplementary information).
Statistical analysis
Statistical significance was calculated by Student's t-test on at least three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant).
Acknowledgments
The authors wish to thank Drs D. Del Bufalo, M.P. Paronetto, D. Farini, S. Pedrotti, M. Cappellari, and C. Verri for generation of reagents used in this study and for helpful suggestions. This work was supported by the Association for International Cancer Research (AICR), the Associazione Italiana Ricerca sul Cancro (AIRC) and the Ministry of Health Ricerca Corrente to the Fondazione Santa Lucia.
Author contribution
PB designed the study, performed most of the experiments, and wrote the manuscript; RB and FB performed immunofluorescence analyses; MJM, ARK, and SMDS provided critical reagents and contributed to writing the manuscript; CS designed the study and wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information for this article is available online: http://embor.embopress.org
References
- 1.Chen M, Manley JL. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat Rev Mol Cell Biol. 2009;10:741–754. doi: 10.1038/nrm2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalsotra A, Cooper TA. Functional consequences of developmentally regulated alternative splicing. Nat Rev Genet. 2011;12:715–729. doi: 10.1038/nrg3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wahl MC, Will CL, Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem. 2003;72:291–336. doi: 10.1146/annurev.biochem.72.121801.161720. [DOI] [PubMed] [Google Scholar]
- 5.Schwerk C, Schulze-Osthoff K. Regulation of apoptosis by alternative pre-mRNA splicing. Mol Cell. 2005;19:1–13. doi: 10.1016/j.molcel.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Boise LH, Gonzáles-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nuñez G, Thompson CB. bcl-x, a bcl-2-related gene that function as a dominant regulator of apoptotic cell death. Cell. 1993;74:507–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 7.Bauman JA, Li SD, Yang A, Huang L, Kole R. Anti-tumor activity of splice-switching oligonucleotides. Nucleic Acids Res. 2010;38:8348–8356. doi: 10.1093/nar/gkq731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paronetto MP, Achsel T, Massiellom A, Chalfant CE, Sette C. The RNA-binding protein Sam68 modulates the alternative splicing of Bcl-x. J Cell Biol. 2007;176:929–939. doi: 10.1083/jcb.200701005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lukong KE, Richard S. Sam68, the KH domain-containing superSTAR. Biochim Biophys Acta. 2003;653:73–86. doi: 10.1016/j.bbcan.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Busà R, Paronetto MP, Farini D, Pierantozzi E, Botti F, Angelici DF, Attisani F, Vespasiani G, Sette C. The RNA-binding protein Sam68 contributes to proliferation and survival of human prostate cancer cells. Oncogene. 2007;26:4372–4382. doi: 10.1038/sj.onc.1210224. [DOI] [PubMed] [Google Scholar]
- 11.Maeda T, Merghoub T, Hobbs RM, Dong L, Maeda M, Zakrzewski J, van den Brink MR, Zelent A, Shigematsu H, Akashi K, Teruya-Feldstein J, Cattoretti G, Pandolfi PP. Regulation of B versus T lymphoid lineage fate decision by the proto-oncogene LRF. Science. 2007;11:860–866. doi: 10.1126/science.1140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeda T, Hobbs RM, Merghoub T, Guernah I, Zelent A, Cordon-Cardo C, Teruya-Feldstein J, Pandolfi PP. Role of the proto-oncogene Pokemon in cellular transformation and ARF repression. Nature. 2005;433:278–285. doi: 10.1038/nature03203. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal A, Hunter WJ, III, Aggarwal H, Silva ED, Davey MS, Murphy RF, Agrawal DK. Expression of leukemia/lymphoma-related factor (LRF/POKEMON) in human breast carcinoma and other cancers. Exp Mol Pathol. 2010;89:140–148. doi: 10.1016/j.yexmp.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bielli P, Busà R, Paronetto MP, Sette C. The RNA-binding protein Sam68 is a multifunctional player in human cancer. Endocr Relat Cancer. 2011;18:R91–R102. doi: 10.1530/ERC-11-0041. [DOI] [PubMed] [Google Scholar]
- 15.Taylor SJ, Resnick RJ, Shalloway D. Sam68 exerts separable effects on cell cycle progression and apoptosis. BMC Cell Biol. 2004;5:5. doi: 10.1186/1471-2121-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda T, Ito K, Merghoub T, Poliseno L, Hobbs RM, Wang G, Dong L, Maeda M, Dore LC, Zelent A, Luzzatto L, Teruya-Feldstein J, Weiss MJ, Pandolfi PP. LRF is an essential downstream target of GATA1 in erythroid development and regulates BIM-dependent apoptosis. Dev Cell. 2009;17:527–540. doi: 10.1016/j.devcel.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon BN, Yoo JY, Choi WI, Lee CE, Yoon HG, Hur MW. Proto-oncogene FBI-1 (Pokemon/ZBTB7A) represses transcription of the tumor suppressor Rb gene via binding competition with Sp1 and recruitment of co-repressors. J Biol Chem. 2008;283:33199–33210. doi: 10.1074/jbc.M802935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi WI, Jeon BN, Yun CO, Kim PH, Kim SE, Choi KY, Kim SH, Hur MW. Proto-oncogene FBI-1 represses transcription of p21CIP1 by inhibition of transcription activation by p53 and Sp1. J Biol Chem. 2009;284:12633–12644. doi: 10.1074/jbc.M809794200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukong KE, Larocque D, Tyner AL, Richard S. Tyrosine phosphorylation of sam68 by breast tumor kinase regulates intranuclear localization and cell cycle progression. J Biol Chem. 2005;280:38639–38647. doi: 10.1074/jbc.M505802200. [DOI] [PubMed] [Google Scholar]
- 20.Pedrotti S, Bielli P, Paronetto MP, Ciccosanti F, Fimia GM, Stamm S, Manley JL, Sette C. The splicing regulator Sam68 binds to a novel exonic splicing silencer and functions in SMN2 alternative splicing in spinal muscular atrophy. EMBO J. 2010;29:1235–1247. doi: 10.1038/emboj.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paronetto MP, Cappellari M, Busà R, Pedrotti S, Vitali R, Comstock C, Hyslop T, Knudsen KE, Sette C. Alternative splicing of the cyclin D1 proto-oncogene is regulated by the RNA-binding protein Sam68. Cancer Res. 2010;70:229–239. doi: 10.1158/0008-5472.CAN-09-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laudes M, Bilkovski R, Oberhauser F, Droste A, Gomolka M, Leeser U, Udelhoven M, Krone W. Transcription factor FBI-1 acts as a dual regulator in adipogenesis by coordinated regulation of cyclin-A and E2F-4. J Mol Med (Berl) 2008;86:597–608. doi: 10.1007/s00109-008-0326-2. [DOI] [PubMed] [Google Scholar]
- 23.Hnilicová J, Hozeifi S, Dušková E, Icha J, Tománková T, Staněk D. Histone deacetylase activity modulates alternative splicing. PLoS ONE. 2011;6:e16727. doi: 10.1371/journal.pone.0016727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prince HM, Bishton MJ, Johnstone RW. Clinical studies of histone deacetylase inhibitors. Future Oncol. 2009;5:601–612. doi: 10.2217/fon.09.36. [DOI] [PubMed] [Google Scholar]
- 25.Paronetto MP, Messina V, Barchi M, Geremia R, Richard S, Sette C. Sam68 marks the transcriptionally active stages of spermatogenesis and modulates alternative splicing in male germ cells. Nucleic Acids Res. 2011;39:4961–4974. doi: 10.1093/nar/gkr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Tollervey J, Briese M, Turner D, Ule J. CLIP: construction of cDNA libraries for high-throughput sequencing from RNAs cross-linked to proteins in vivo. Methods. 2009;48:287–293. doi: 10.1016/j.ymeth.2009.02.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.