Abstract
Sin Nombre virus (SNV; family Bunyaviridae, genus Hantavirus) causes a hemorrhagic fever known as hantavirus pulmonary syndrome (HPS) in North America. There have been approximately 200 fatal cases of HPS in the United States since 1993, predominantly in healthy working-age males (case fatality rate 35%). There are no FDA-approved vaccines or drugs to prevent or treat HPS. Previously, we reported that hantavirus vaccines based on the full-length M gene segment of Andes virus (ANDV) for HPS in South America, and Hantaan virus (HTNV) and Puumala virus (PUUV) for hemorrhagic fever with renal syndrome (HFRS) in Eurasia, all elicited high-titer neutralizing antibodies in animal models. HFRS is more prevalent than HPS (>20,000 cases per year) but less pathogenic (case fatality rate 1–15%). Here, we report the construction and testing of a SNV full-length M gene-based DNA vaccine to prevent HPS. Rabbits vaccinated with the SNV DNA vaccine by muscle electroporation (mEP) developed high titers of neutralizing antibodies. Furthermore, hamsters vaccinated three times with the SNV DNA vaccine using a gene gun were completely protected against SNV infection. This is the first vaccine of any kind that specifically elicits high-titer neutralizing antibodies against SNV. To test the possibility of producing a pan-hantavirus vaccine, rabbits were vaccinated by mEP with an HPS mix (ANDV and SNV plasmids), or HFRS mix (HTNV and PUUV plasmids), or HPS/HFRS mix (all four plasmids). The HPS mix and HFRS mix elicited neutralizing antibodies predominantly against ANDV/SNV and HTNV/PUUV, respectively. Furthermore, the HPS/HFRS mix elicited neutralizing antibodies against all four viruses. These findings demonstrate a pan-hantavirus vaccine using a mixed-plasmid DNA vaccine approach is feasible and warrants further development.
Keywords: Sin Nombre, virus Hantavirus, DNA vaccine, HPS, HFRS
1. Introduction
A 1993 outbreak of acute illness characterized by fever, myalgia, and pulmonary failure in the four corners region of the southwest United States led to the discovery of Sin Nombre virus (SNV), one of the etiological agents of hantavirus pulmonary syndrome (HPS) [1–3]. According to the Centers for Disease Control and Prevention, from 1993–2012, there have been 586 reported cases of HPS in the U.S. with a case fatality rate of 35%. SNV is the predominant hantavirus causing disease in North America including the most recent HPS outbreak in Yosemite Valley, California [4].
Hantaviruses cause two unique diseases targeting the lung (HPS) or the kidney hemorrhagic fever with renal syndrome (HFRS). HPS is primarily associated with New World hantaviruses (e.g. SNV and ANDV) found in the Americas, whereas Old World hantaviruses (e.g. HTNV, PUUV, Seoul virus, and Dobrava-Belgrade virus) cause HFRS in Europe and Asia [5,6]. There are currently no FDA-approved vaccines or therapeutics to treat hantavirus disease [7].
Hantaviruses are enveloped viruses with a trisegmented, negative-sense RNA genome. The S segment encodes for the nucleoprotein (N), M segment encodes the Gn and Gc glycoproteins, and L segment encodes the RNA-dependent RNA polymerase [8]. While both N and Gn/Gc have been shown to contribute to protective immunity (reviewed in [7]), only the glycoproteins have been shown to be the targets of neutralizing antibodies. Moreover, neutralizing antibodies have been shown to be sufficient to confer protection in passive transfer experiments using Gn/Gc-specific monoclonal and polyclonal antibodies [9–11].
We have previously reported on the construction and efficacy of gene gun-delivered DNA vaccines targeting HTNV and PUUV M segment in nonhuman primates, hamsters, and humans in a phase I clinical trial [12–14]. We have also reported that an ANDV M gene-based DNA vaccine, and a plasmid containing both the ANDV and HTNV M genes, could elicit high titer neutralizing antibodies in rabbits and nonhuman primates when delivered by mEP or gene gun [11,15,16]. Others have demonstrated that a N-based or glycoprotein fragment-based DNA vaccine were capable of eliciting detectable neutralizing antibody titers in BALB/c mice (Focus Reduction Neutralization Test80 = 20) and protecting deer mice against infection with SNV in the absence of high titer neutralizing antibodies [17,18]. There have been no reports of DNA vaccines, or any other type of vaccine, capable of eliciting high titer neutralizing antibodies against SNV. In the current study, we report on the development, immunogenicity, and protective efficacy of a full length M gene-based DNA vaccine to SNV. We expand on these findings and demonstrated the efficacy of multivalent DNA vaccines simultaneously targeting the causative agents of both HPS and HFRS.
2. Materials and methods
2.1. Viruses, cells, and medium
SNV strain CC107 [19], ANDV strain Chile-9717869 [20], HTNV strain 76–118 [21], and PUUV strain K27 [22] were propagated in Vero E6 cells (Vero C1008; ATCC CRL 1586). These cells were maintained in Eagle's minimum essential medium with Earle's salts containing 10% fetal bovine serum, 10 mM HEPES, pH 7.4, and Penicillin Streptomycin (Invitrogen) at 1X, and gentamicin sulfate (50 μg/ml) at 37 °C in a 5% CO2 incubator.
2.2. Animals
Female New Zealand white rabbits (Oryctolagus cuniculus) aged 11 weeks were used in the DNA vaccination studies. Female Syrian hamsters (Mesocricetus auratus) aged 6–8 weeks were used in the vaccination/challenge study.
2.3. Construction of hantavirus M gene vaccine plasmid
The SNV M gene DNA vaccine plasmid pWRG/SN-M(2a) was constructed by reverse transcription of viral RNA, followed by PCR amplification of cDNA, and standard cloning techniques. Forward and reverse primers were based on SNV sequences. The forward primer was SN-Fj (5′-GGCCGCGGCCGCGGATCTGCAGGAATTCGGCACGAGAGTAGTAGACTCCGCACGAAGAAGC) and the reverse primer was SN-Mrev (5′-GGCCTTCGAATAGTAGTAGACTCCGCAGGAAC). The forward primer included a NotI restriction site (underlined) and the reverse primer included a BstBI restriction site (underlined). cDNA was purified by use of a PCR purification kit (Qiagen) and used as a template in PCR. Primers were included in the PCR mix, which also included Platinum Taq High Fidelity DNA polymerase (Invitrogen); the PCR conditions were one 3-min cycle at 94 °C followed by 30 cycles of 94 °C for 30 s and 68 °C for 8 min. The PCR product was cut with NotI and BstBI and then ligated into NotI-BstBI-cut modified pWRG7077 vector to produce pWRG/SN-M(2a).
To produce an optimized plasmid, the SNV M gene open reading frame from pWRG/SN-M(2a) was codon-optimized [23] and four amino acids were changed to consensus residues based on alignments with published SNV M genes. The optimized gene was synthesized by GeneArt and subcloned into the NotI and BglII sites of the DNA vaccine vector pWRG7077, creating pWRG/SN-M(opt). This plasmid has been submitted to the ATCC, Patent Deposit Designation PTA-11660. The HTNV M gene DNA vaccine plasmid is designated as pWRG/HTN-M(x), the PUUV vaccine plasmid is pWRG/PUU-M(s2), and the ANDV is pWRG/AND-M, they have been described previously [13,14].
2.4. mEP vaccination
Anesthetized New Zealand white rabbits were vaccinated by mEP using either the Inovio Elgen delivery device or the Ichor TriGrid delivery device. DNA vaccination with the Inovio Elgen delivery device was conducted as previously described [16]. Each vaccination session was a single mEP event in one leg.
Rabbits vaccinated using the Ichor TriGrid delivery device was conducted as previously described [24]. The dose of DNA per injection is provided in figure legends.
2.5. Gene gun vaccination
Vaccinations using an XR particle-mediated epidermal delivery device (gene gun) (PowderJect-XR delivery device; PowderJect Vaccines, Inc.) have been described previously [13,14,25]. Anesthetized outbred female Syrian hamsters (6–8 weeks or older) were vaccinated with four administrations per vaccination. This procedure is non-painful with the only adverse effect being mild erythema at the vaccination site.
2.6. Challenge with hantavirus
Anesthetized Syrian hamsters were exposed to SNV or ANDV by intramuscular (i.m.) injection to the caudal thigh. 200 PFU SNV (100 ID50) or 200 PFU ANDV (25 LD50) was diluted in sterile phosphate-buffered saline (pH 7.4) and administered in a volume of 0.2 ml.
2.7. PRNT
Plaque-reduction neutralization tests (PRNT) were performed using Vero E6 as previously described [14]. The 50% (or 80%) PRNT titer (PRNT50 or PRNT80 titer) was the highest serum dilution reducing the number of plaques by 50% (or 80%) relative to the average number of plaques in control wells that received medium alone. For most of the experiments in this report, and in previous studies, we have reported PRNT50 titers because this gives the highest level of sensitivity when evaluating vaccines for the first time in vivo. PRNT80 titers are more stringent and are used when sensitivity is less important than obtaining a conservative determination of the neutralizing antibody titer.
2.8. N-specific ELISA
The enzyme-linked immunosorbent assay (ELISA) used to detect N-specific antibodies (N-ELISA) was described previously [14,25,26]. The endpoint titer was determined as the highest dilution that had an optical density (OD) greater than the mean OD for serum samples from negative-control wells plus 3 standard deviations. The PUUV N antigen was used to detect SNV N-specific antibodies as previously reported [22].
2.9. Ethics
Animal research was conducted under an IACUC approved protocol at USAMRIID (USDA Registration Number 51-F-00211728 & OLAW Assurance number A3473-01) in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 2011.
2.10. Statistical analysis
Comparison of neutralizing titers was done using Student's t test (two-tailed). P values of less than 0.05 were considered significant. Survival analyses were done using log-rank test. Analyses were conducted using GraphPad Prism (version 5).
3. Results
3.1. Individual hantavirus M-gene-based DNA vaccines elicited neutralizing antibodies when delivered by mEP in rabbits
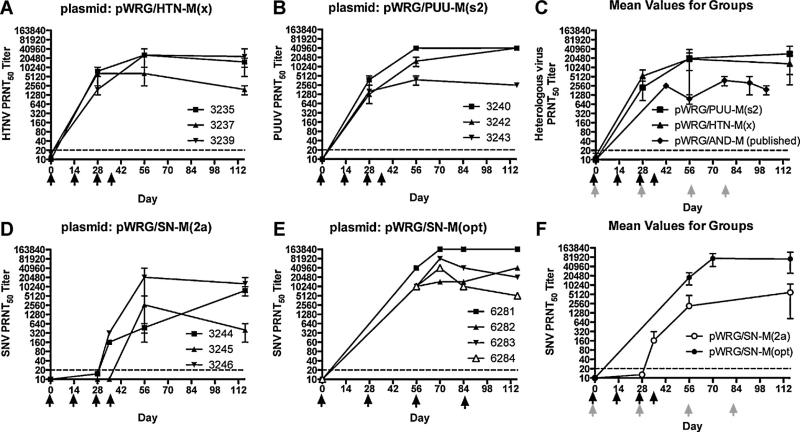
Groups of three rabbits were vaccinated four times by mEP using pWRG/HTN-M(x) and pWRG/PUU-M(s2), and neutralizing antisera titers were determined by plaque reduction neutralization test (PRNT) (Fig. 1A and B). A comparison with previously published titers produced in rabbits by mEP vaccination with an ANDV full-length M segment was included in Fig. 1C. Similarly, a SNV full-length M gene segment DNA vaccine, pWRG/SN-M(2a), was immunogenic in rabbits but only after three or four vaccinations (Fig. 1D) indicating this plasmid was less immunogenic than the HTNV, PUUV, or ANDV [16] vaccines. An optimized version of the SNV M gene open reading frame was synthesized and sub-cloned into the DNA vaccine vector to produce pWRG/SN-M(opt). pWRG/SN-M(2a) and pWRG/SN-M(opt) M genes are identical except for the open reading frame where optimization resulted in 980/3423 (29%) nucleotide changes and four amino acid changes: Q27K, A241T, G434D, P519S, where the first letter is the symbol for the amino acid in the product from pWRG/SN-M(2a), the number is the amino acid position, and the second letter is the amino acid in the product from pWRG/SN-M(opt). pWRG/SN-M(opt) elicited high-titer (>10,000) neutralizing antibodies after mEP detectable in sera on day 56, after two vaccinations (Fig. 1E). The mean PRNT titers produced by the two SNV DNA vaccines were plotted to illustrate the increased immunogenicity of the optimized SNV M gene plasmid to the original plasmid (Fig. 1F).
Fig. 1.
Hantavirus neutralizing antibodies produced in rabbits vaccinated with full-length hantavirus M gene-based DNA vaccines using mEP. Groups of 3 rabbits were vaccinated with DNA vaccines (A) pWRG/HTN-M(x) and (B) pWRG/PUU-M(s2) on days noted by black arrows by mEP (Inovio Elgen device, dose 0.4 mg DNA per injection). Sera collected were tested in homotypic PRNT. Symbols represent the mean of two separate PRNT50 ± SE. (C) The same data from (A) and (B) were combined to show mean titers for the groups. Previously published mean titers from rabbits vaccinated with pWRG/AND-M were shown for comparison. Note the vaccination days were different for the Andes DNA vaccine (shown in gray arrows). Groups of 3 or 4 rabbits were vaccinated with (D) the first generation SNV M gene-based DNA vaccine, pWRG/SN-M(2a) or (E) the optimized SNV M gene-based DNA vaccine, pWRG/SN-M(opt), on days noted by black arrows. Sera collected were tested in homotypic PRNT. (F) The same data from (D) and (E) were combined to show mean titers for the groups. Black arrows indicate vaccination days for pWRG/SN-M(2a) and gray arrows indicate vaccination days for pWRG/SN-M(opt). The PRNT limit of detection was a titer of 20 (dashed lines).
3.2. Immunogenicity of combination DNA vaccines targeting causative agents of HPS, HFRS, and HFRS/HPS
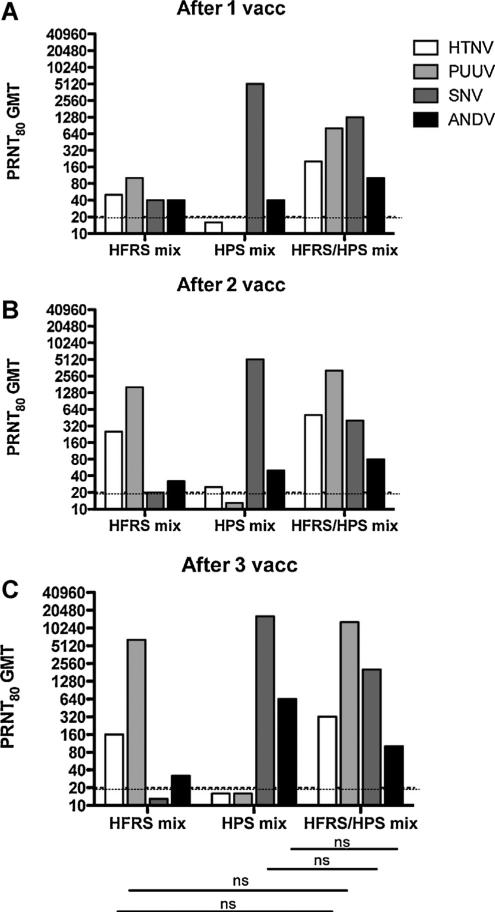
We next determined if the plasmids could be combined to produce vaccines eliciting specific neutralizing antibody responses against multiple hantaviruses causing HFRS (HTNV and PUUV), HPS (ANDV and SNV) and a combination targeting both HFRS and HPS agents (HTNV, PUUV, ANDV, and SNV). Groups of three rabbits were vaccinated with combinations of DNA vaccines targeting HFRS, HPS or HFRS/HPS delivered by mEP. Sera were collected, and neutralizing antibody titers were determined by SNV, ANDV, HTNV, and PUUV PRNT. Titers at different time points for individual rabbits (Fig. 2A), and mean titers for the groups (Fig. 2B), were plotted. All but one of the rabbits (#6320) developed homotypic neutralizing antibody responses after a single vaccination, and all were positive after the first boost. Additionally, both the HFRS and HPS DNA vaccines elicited some level of cross-neutralization against the HPS or HFRS hantavirus, respectively, based on geometric mean titers (GMT) for the PRNT80 values after a single vaccination in all by one rabbit (Fig. 3). Nevertheless, the highest SNV and ANDV mean titers were found in the HPS vaccine and the HFRS/HPS vaccine groups, and the highest HTNV and PUUV mean titers were found in the HFRS vaccine and HFRS/HPS vaccine groups. These data demonstrate that it is possible to mix hantavirus DNA vaccines into a single-injection vaccine and produce neutralizing antibodies against multiple hantaviruses. The neutralizing antibody titers did not significantly vary between the HFRS or HPS bivalent vaccines and the HFRS/HPS quadravalent combination (p > 0.05) indicating little interference between the targets in the larger combination vaccine.
Fig. 2.
Single-injection multiagent hantavirus DNA vaccines are feasible by mEP. Three mixtures of hantavirus DNA vaccine plasmids were delivered to rabbits by mEP (Ichor Tri-grid). Groups of 3 rabbits were vaccinated at 3-week intervals and sera were collected for PRNT analysis. The HFRS mixture was comprised of equal volumes of pWRG/HTN-M(x) and pWRG/PUU-M(s2), (2 mg DNA total, 1 mg/plasmid/injection, 1 injection/vaccination). The HPS mixture was comprised of equal volumes of pWRG/ANDM and pWRG/SN-M(opt) (2 mg DNA total, 1 mg/plasmid/injection, 1 injection/vaccination). The HFRS/HPS mixture was comprised of equal volumes of HTNV, PUUV, ANDV, and SNV DNA vaccine plasmids (4 mg DNA total, 1 mg/plasmid/injection, 2 injections/vaccination). (A) Neutralizing antibody titers for individual rabbits are shown. The virus used in the neutralization test is shown on the y-axis. (B) Mean neutralization titers for each group ± SE. The PRNT limit of detection was a titer of 20 (dashed lines).
Fig. 3.
PRNT80 GMT against HTNV, PUUV, ANDV, and SNV for each DNA vaccine formulation after 1, 2, or 3 vaccinations. These data are from the same experiment shown in Fig. 2; however, PRNT80 GMT are presented (A) after 1 vaccination, (B)after 2 vaccinations, and (C) after 3 vaccinations. The PRNT limit of detection was a titer of 20 (dashed lines). ns indicates a lack of statistical significance when titers were compared from HFRS or HPS mix to HFRS/HPS mix vaccine. Significance lines pertain to (A), (B), and (C).
SNV M gene-based DNA vaccine delivered by gene gun protects hamsters against SNV infection, but not lethal disease caused by ANDV. Currently, there is no animal model for disease caused by SNV, but there are models of SNV infection. Syrian hamsters injected with a dose as low as 2 PFU become infected with SNV as measured by seroconversion to the N protein [20]. Because the M gene-based DNA vaccines do not include the N protein, it is possible to monitor the development of anti-N antibodies as a measure of productive infection. To determine if our SNV vaccine was protective, groups of 7–8 hamsters were vaccinated by gene gun with pWRG/SN-M(opt) or a negative control plasmid either two times (0 and 3 weeks), three times (0, 3, and 6 weeks) or were not vaccinated at all. Neutralizing antibody titers were determined at 0, 3, 6, and 9 weeks by PRNT (Fig. 4A). pWRG/SN-M(opt) was immunogenic in hamsters, however, antibody titers were lower than rabbits vaccinated by mEP. After two vaccinations, a statistically significant antibody response was observed compared to negative control DNA vaccination group (p = 0.0026 and p = 0.0112, respectively). Titers increased after a third vaccination (week 9 sera), but this increase was not statistically significant (Fig. 4B).
Fig. 4.
pWRG/SN-M(opt) DNA vaccine (gene gun) is immunogenic and protective in hamsters. Groups of 7–8 hamsters received 2 or 3 vaccinations with the pWRG/SN-M(opt) SNV DNA vaccine, 3 vaccinations with a negative control DNA vaccine, or no vaccine. (A) Sera collected were tested for SNV neutralizing antibodies by PRNT. Mean titers ± SE are shown. (B) Individual PRNT50 titers from sera collected on week 9 are presented with the GMT and 95% confidence interval depicted. The PRNT limit of detection was a titer of 20 (dashed lines). (C) Sera collected on week 16 (5 weeks postchallenge) were tested by ELISA for evidence of SNV infection. All prechallenge sera samples were negative by ELISA (data not shown).<indicates titer was below level of detection for the assay. *indicates antibody responses were statistically significant when compared to negative DNA vaccination controls.
To evaluate the protective efficacy of the SNV DNA vaccine (5 weeks after the last vaccination), hamsters were challenged with SNV and were then monitored for seroconversion by N-ELISA. Analysis of sera collected four weeks after challenge revealed 5 of 8 hamsters receiving two vaccinations were protected from SNV infection (62.5%, p = 0.0392 when compared to negative control DNA vaccination group), 7 of 7 hamsters receiving three vaccinations were protected from SNV infection (100%, p = 0.0008 when compared to negative control DNA vaccination group), and no hamsters receiving negative control DNA or left unvaccinated were protected from SNV infection (Fig. 4C). This indicated that pWRG/SN-M(opt) could protect hamsters against SNV but required neutralizing antibody titers equivalent to those produced by three vaccinations.
We next hypothesized that this vaccine would be capable of cross-protecting against ANDV infection in the hamster disease model. Unlike SNV, ANDV infection of Syrian hamsters causes a lethal endothelium-leak disease that closely resembles human HPS [20]. To test this, 8 hamsters were vaccinated 3 times at 3-week intervals with pWRG/SN-M(opt) using gene gun. A group of 7 unvaccinated hamsters served as a negative control for the ANDV challenge. Five weeks after the last vaccination, hamsters were challenged with 200 PFU of ANDV by the i.m. route (25 LD50). Only 3 of 8 hamsters vaccinated with pWRG/SN-M(opt) survived despite the presence of SNV neutralizing antibodies in 6 of 8 hamsters (group GMT = 135, p = 0.0045 when compared to no vaccine controls) (Fig. 5B). One of 7 hamsters survived in the negative control group (p = 0.3108) (Fig. 5A). Results of an ANDV PRNT demonstrated that sera from vaccinated hamsters had little cross-neutralization activity (data not shown). Thus, the antibody response elicited by the SNV DNA vaccine failed to confer statistically significant protection against ANDV.
Fig. 5.
pWRG/SN-M(opt) DNA vaccine (gene gun) does not protect hamsters from ANDV challenge. A group of 8 hamsters received 3 vaccinations with pWRG/SNM(opt). (A) Vaccinated and unvaccinated hamsters were challenged with 200 PFU ANDV i.m. and observed for survival. (B) Sera collected prechallenge were tested by SNV PRNT for homotypic neutralizing antibodies. The PRNT limit of detection was a titer of 20 (dashed lines). *indicate antibody response was statistically significant when compared to no vaccine controls. s indicates the PRNT titer of a surviving hamster.
4. Discussion
SNV-associated HPS is a serious and unpredictable public health threat, as affirmed by the 2012 outbreak at Yosemite National Park, CA [4] that resulted in 3 fatalities and more than 260,000 park visitors potentially exposed to a lethal virus. Here, we present the construction, immunogenicity, and protective efficacy of a novel SNV DNA vaccine designated pWRG/SN-M(opt). To our knowledge, this is the first SNV vaccine of any kind that has been shown to elicit high-titer neutralizing antibodies (i.e., PRNT50 > 1000). The antibody response elicited by pWRG/SN-M(opt) is among the most potent per vaccination ever achieved with a hantavirus DNA vaccine. One to two vaccinations with pWRG/SN-M(opt) plasmid alone using mEP resulted in SNV PRNT50 titers as high as 40,280, and one vaccination with the combined SNV and ANDV plasmids resulted in SNV PRNT titers as high as 20,480. The PRNT80 GMT for rabbits vaccinated one or two times with pWRG/SN-M(opt) was 5120. These PRNT80 titers were higher than those reported for HPS survivors [27]. Studies were not performed to elucidate which of the 980 nucleotide and/or four amino acid changes improved the immunogenicity of the SNV DNA vaccine. We speculate that a combination of changes resulted in improved vaccine quality (e.g., plasmid stability, plasmid yield) and vaccine immunogenicity (e.g., mRNA stability, efficiency of expression, and protein folding). Neutralizing antibodies are not required for protection; however, we and others have shown that neutralizing antibodies are sufficient to protect [10,11,16,28–31]. Thus, a hantavirus vaccine capable of eliciting a potent neutralizing antibody response is an excellent candidate for evaluation as a SNV vaccine (e.g., Phase 1 clinical trials) and a means to produce a neutralizing antibody-based immunoprophylactic/therapeutic.
Hantaviruses are a neglected worldwide infectious disease problem and it would be beneficial to have a single vaccine capable of protecting against multiple pathogenic hantaviruses. This would reduce the burden of developing specific vaccines for the myriad different pathogenic hantaviruses around the world. Combinations of plasmids co-delivered separately or delivered as mixtures shows promise in animal models [15,32]. Data from these studies have demonstrated that DNA vaccines can also elicit cross-protection against other species of hantaviruses [13,14]. This cross-neutralizing activity appears to be highly variable and can depend on the vaccine, the route of delivery, the species, and the individual. For example, in nonhuman primates, the ANDV DNA vaccine elicited antibodies that demonstrated a high level of cross-neutralizing activity against SNV [15]. However, the same vaccine delivered to rabbits failed to elicit SNV cross-neutralizing antibodies [16]. Similarly, a combination of ANDV and HTNV M genes was capable of eliciting cross neutralizing antibodies against both HPS and HFRS hantaviruses in nonhuman primates; but titers were low [15]. Here, we tested the possibility of combining SNV and ANDV plasmids, PUUV and HTNV plasmids, or a combination of all four plasmids delivered by mEP in rabbits. We found that the HPS vaccine elicited potent responses against both SNV and ANDV, but not HTNV or PUUV. Conversely, the HFRS vaccine elicited a potent response against both HTNV and PUUV but not ANDV or SNV. When all four plasmids were combined, comparable levels of neutralizing antibody titers relative to the HPS or HFRS vaccines were produced against all four hantaviruses (Fig. 3). Given the importance of neutralizing antibodies in protection [9–11], the levels achieved with the quadravalent vaccine suggest it will protect against ANDV, SNV, PUUV, and HTNV.
Previous studies using gene gun technology to administer the HTNV/PUUV DNA vaccines in hamsters has shown significantly reduced anti-HTNV neutralizing antibody responses when the plasmids were mixed [32]. Interestingly, this interference was not observed if the HTNV and PUUV were delivered separately or when coated on different gold particles before delivery. While the mechanism is unknown, it is likely the interference occurs when the different plasmids are expressing the Gn/Gc proteins in the same cells. The data in Figs. 2 and 3 indicate that the anti-PUUV response was higher than the anti-HTNV responses and the anti-SNV response was higher than the anti-ANDV response. Despite this apparent dominance, the anti-HTNV and anti-ANDV responses were still impressive. Dominance of the PUUV and SNV plasmids over the HTNV and ANDV plasmid, respectively, could reflect the advantages of optimization because neither the HTNV nor ANDV vaccines used in this study were codon optimized.
To investigate the efficacy of the optimized SNV DNA vaccine, we used the Syrian hamster infection model. This model and a deer mouse infection model are the only systems available to evaluate protection against SNV [33,34]. For these studies, we used gene gun delivery since a gene gun had been previously shown to successfully deliver HTNV and PUUV DNA vaccines to hamsters and humans [12]. In addition, we were interested in demonstrating that the pWRG/SN-M(opt) vaccine could be effectively delivered using different delivery technologies. We found that the pWRG/SN-M(opt) vaccine was immunogenic in hamsters, although the titers were significantly lower than those achieved in rabbits (with orders of magnitude more DNA) using mEP. Despite lower titers, hamsters were still protected from SNV infection. In contrast, despite ANDV cross-neutralizing antibodies elicited by mEP in rabbits (Fig. 2), hamsters vaccinated with pWRG/SN-M(opt) did not cross-protect against ANDV disease (Fig. 4).
The experiments reported here consist of a first-look at the SNV DNA vaccine, and combinations thereof. These experiments involved different animals species (i.e., rabbits and hamsters), DNA doses (i.e., micrograms to milligrams), vaccination schedules (i.e., 2-, 3-, or 4-week intervals), and delivery technologies (i.e., gene gun and two types of muscle electroporation). Although no single experiment directly compared how changing these parameters affected immunogenicity, our findings that all of these vaccination conditions resulted in the production of neutralizing antibodies demonstrate the robustness of the SNV DNA vaccine. Future studies will directly test how dose, schedule, and device affect the quantity, quality, and duration of the immune responses.
The present report demonstrates active protection against SNV infection in hamsters. Protection following vaccination with the mulit-valent hantavirus vaccines was not tested. Previous findings that hantavirus neutralizing antibodies are sufficient to protect should allow a passive approach to efficacy testing involving active vaccination of one species followed by passive transfer of immunity to animal models of infection and/or disease. For example, sera from rabbits, nonhuman primates, or even humans vaccinated with the multivalent hantavirus vaccines could be tested for a capacity to protect hamsters against infection and/or disease caused by SNV, ANDV, PUUV, and HTNV. A limitation to a passive transfer approach is that it only evaluates the protection conferred by the humoral immune response. Although neutralizing antibodies have been shown to be sufficient to protect, there is a recent example of hantavirus DNA vaccines protecting against disease in the absence of detectable neutralizing antibodies. The PUUV DNA vaccine protected hamsters against lethal disease caused by ANDV in the absence of neutralizing antibodies [13]. Here, we found the SNV DNA vaccine did not protect hamsters against lethal disease caused by ANDV suggesting the cell-mediated immune response elicited by the PUUV DNA vaccine is more cross-reactive with epitopes on the ANDV glycoproteins than the SNV DNA vaccine. Thus, if the passive transfer approach to efficacy testing proves inadequate to evaluate protection and cross-protection against hantaviruses, then it will be necessary to develop new animal models of HPS and HFRS that allow direct efficacy testing of mono- and multi-valent hantavirus vaccines.
This report is the first to describe a SNV vaccine capable of eliciting high-titer neutralizing antibodies, and protection, in animal models. We demonstrated the feasibility of combining this vaccine with other hantavirus vaccines to expand the breadth of neutralizing activity. Current treatment for hantavirus disease is strictly supportive care [35,36]. But the endemic and episodic emergence of lethal HPS and HFRS warrants the development of a direct countermeasure to prevent disease caused by infection with SNV and other hantaviruses around the world.
Acknowledgments
The Elgen electroporation device was provided by Inovio. The TriGrid device was provided by Ichor. The research gene gun and pWRG7077 were provided by Powderject Vaccine, Inc. A modified version of pWRG7077 containing the BstB1 restriction site was provided by Alexander Dekonenko. The pPUUSXdelta plasmid used to produce the N-ELISA antigen in E. coli were provided by Fredrick Elgh, Umea, Sweden. This work was funded by the U.S. Army Medical Research and Material Command, Military Infectious Disease Research Program, Program Area T; NIH R01 AI098933-01 (Hooper); and Chemical, Biological Medical Systems (CBMS) Award Number W9113M-08-1-0008. Opinions, interpretations, conclusions, and recommendations are ours and are not necessarily endorsed by the U.S. Army or the Department of Defense.
Abbreviation
- mEP
muscle electroporation
References
- 1.Centers for Disease C Prevention. Outbreak of acute illness–southwestern United States, 1993. MMWR. 1993;42:421–4. [PubMed] [Google Scholar]
- 2.Centers for Disease C. Prevention update: outbreak of hantavirus infection–southwestern United States, 1993. MMWR. 1993;42:495–6. [PubMed] [Google Scholar]
- 3.Elliott LH, Ksiazek TG, Rollin PE, Spiropoulou CF, Morzunov S, Monroe M, et al. Isolation of the causative agent of hantavirus pulmonary syndrome. Am J Trop Med Hyg. 1994;51:102–8. doi: 10.4269/ajtmh.1994.51.102. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease C. Prevention. Hantavirus pulmonary syndrome in visitors to a national park–Yosemite Valley, California, 2012. MMWR. 2012;61:952. [PubMed] [Google Scholar]
- 5.Padula PJ, Colavecchia SB, Martinez VP, Gonzalez Della Valle MO, Edelstein A, Miguel SD, et al. Genetic diversity, distribution, and serological features of hantavirus infection in five countries in South America. J Clin Microbiol. 2000;38:3029–35. doi: 10.1128/jcm.38.8.3029-3035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters CJ, Simpson GL, Levy H. Spectrum of hantavirus infection: hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Annu Rev Med. 1999;50:531–45. doi: 10.1146/annurev.med.50.1.531. [DOI] [PubMed] [Google Scholar]
- 7.Hammerbeck CD, Hooper JW. Hantavirus vaccines. In: Levine MM, editor. New Generation Vaccines. Informa Healthcare; New York: 2010. pp. 905–13. [Google Scholar]
- 8.Schmaljohn C, Nichol S. Bunyaviridae. In: Knipe D, Howley P, editors. Fields Virology. 5th ed. Lippincott, Williams, and Wilkins; Philadelphia: 2006. pp. 1741–89. [Google Scholar]
- 9.Arikawa J, Yao JS, Yoshimatsu K, Takashima I, Hashimoto N. Protective role of antigenic sites on the envelope protein of Hantaan virus defined by monoclonal antibodies. Arch Virol. 1992;126:271–81. doi: 10.1007/BF01309700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brocato R, Josleyn M, Ballantyne J, Vial P, Hooper JW. DNA vaccine-generated duck polyclonal antibodies as a postexposure prophylactic to prevent hantavirus pulmonary syndrome (HPS). PloS one. 2012;7:e35996. doi: 10.1371/journal.pone.0035996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Custer DM, Thompson E, Schmaljohn CS, Ksiazek TG, Hooper JW. Active and passive vaccination against hantavirus pulmonary syndrome with Andes virus M genome segment-based DNA vaccine. J Virol. 2003;77:9894–905. doi: 10.1128/JVI.77.18.9894-9905.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boudreau EF, Josleyn M, Ullman D, Fisher D, Dalrymple L, Sellers-Myers K, et al. A Phase 1 clinical trial of Hantaan virus and Puumala virus M-segment DNA vaccines for hemorrhagic fever with renal syndrome. Vaccine. 2012;30:1951–8. doi: 10.1016/j.vaccine.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Brocato RL, Josleyn MJ, Wahl-Jensen V, Schmaljohn CS, Hooper JW. Construction and nonclinical testing of a puumala virus synthetic m gene-based DNA vaccine. Clin Vaccine Immunol CVI. 2013;20:218–26. doi: 10.1128/CVI.00546-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooper JW, Custer DM, Thompson E, Schmaljohn CS. DNA vaccination with the hantaan virus M gene protects hamsters against three of four HFRS hantaviruses and elicits a high-titer neutralizing antibody response in Rhesus monkeys. J Virol. 2001;75:8469–77. doi: 10.1128/JVI.75.18.8469-8477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooper JW, Custer DM, Smith J, Wahl-Jensen V. Hantaan/Andes virus DNA vaccine elicits a broadly cross-reactive neutralizing antibody response in non-human primates. Virology. 2006;347:208–16. doi: 10.1016/j.virol.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 16.Hooper JW, Ferro AM, Wahl-Jensen V. Immune serum produced by DNA vacci-nation protects hamsters against lethal respiratory challenge with Andes virus. J Virol. 2008;82:1332–8. doi: 10.1128/JVI.01822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bharadwaj M, Lyons CR, Wortman IA, Hjelle B. Intramuscular inoculation of Sin Nombre hantavirus cDNAs induces cellular and humoral immune responses in BALB/c mice. Vaccine. 1999;17:2836–43. doi: 10.1016/s0264-410x(99)00096-1. [DOI] [PubMed] [Google Scholar]
- 18.Bharadwaj M, Mirowsky K, Ye C, Botten J, Masten B, Yee J, et al. Genetic vaccines protect against Sin Nombre hantavirus challenge in the deer mouse (Peromyscus maniculatus). J Gen Virol. 2002;83:1745–51. doi: 10.1099/0022-1317-83-7-1745. [DOI] [PubMed] [Google Scholar]
- 19.Schmaljohn AL, Li D, Negley DL, Bressler DS, Turell MJ, Korch GW, et al. Isolation and initial characterization of a newfound hantavirus from California. Virology. 1995;206:963–72. doi: 10.1006/viro.1995.1019. [DOI] [PubMed] [Google Scholar]
- 20.Hooper JW, Larsen T, Custer DM, Schmaljohn CS. A lethal disease model for hantavirus pulmonary syndrome. Virology. 2001;289:6–14. doi: 10.1006/viro.2001.1133. [DOI] [PubMed] [Google Scholar]
- 21.Lee HW, Lee PW, Johnson KM. Isolation of the etiologic agent of Korean hemorrhagic fever. J Infect Dis. 1978;137:298–308. doi: 10.1093/infdis/137.3.298. [DOI] [PubMed] [Google Scholar]
- 22.Xiao SY, Spik KW, Li D, Schmaljohn CS. Nucleotide and deduced amino acid sequences of the M and S genome segments of two Puumala virus isolates from Russia. Virus Res. 1993;30:97–103. doi: 10.1016/0168-1702(93)90019-j. [DOI] [PubMed] [Google Scholar]
- 23.Fath S, Bauer AP, Liss M, Spriestersbach A, Maertens B, Hahn P, et al. Multiparameter RNA and codon optimization: a standardized tool to assess and enhance autologous mammalian gene expression. PloS one. 2011;6:e17596. doi: 10.1371/journal.pone.0017596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dupuy LC, Richards MJ, Ellefsen B, Chau L, Luxembourg A, Hannaman D, et al. A DNA vaccine for venezuelan equine encephalitis virus delivered by intramuscular electroporation elicits high levels of neutralizing antibodies in multiple animal models and provides protective immunity to mice and nonhuman primates. Clin Vaccine Immunol CVI. 2011;18:707–16. doi: 10.1128/CVI.00030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hooper JW, Kamrud KI, Elgh F, Custer D, Schmaljohn CS. DNA vaccination with hantavirus M segment elicits neutralizing antibodies and protects against seoul virus infection. Virology. 1999;255:269–78. doi: 10.1006/viro.1998.9586. [DOI] [PubMed] [Google Scholar]
- 26.Elgh F, Lundkvist A, Alexeyev OA, Stenlund H, Avsic-Zupanc T, Hjelle B, et al. Serological diagnosis of hantavirus infections by an enzyme-linked immunosorbent assay based on detection of immunoglobulin G and M responses to recombinant nucleocapsid proteins of five viral serotypes. J Clin Microbiol. 1997;35:1122–30. doi: 10.1128/jcm.35.5.1122-1130.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye C, Prescott J, Nofchissey R, Goade D, Hjelle B. Neutralizing antibodies and Sin Nombre virus RNA after recovery from hantavirus cardiopulmonary syndrome. Emerg Infect Dis. 2004;10:478–82. doi: 10.3201/eid1003.020821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klingstrom J, Stoltz M, Hardestam J, Ahlm C, Lundkvist A. Passive immunization protects cynomolgus macaques against Puumala hantavirus challenge. Antiviral Ther. 2008;13:125–33. [PubMed] [Google Scholar]
- 29.Liang M, Chu YK, Schmaljohn C. Bacterial expression of neutralizing mouse monoclonal antibody Fab fragments to Hantaan virus. Virology. 1996;217:262–71. doi: 10.1006/viro.1996.0113. [DOI] [PubMed] [Google Scholar]
- 30.Xu Z, Wei L, Wang L, Wang H, Jiang S. The in vitro and in vivo protective activity of monoclonal antibodies directed against Hantaan virus: potential application for immunotherapy and passive immunization. Biochem Biophys Res Commun. 2002;298:552–8. doi: 10.1016/s0006-291x(02)02491-9. [DOI] [PubMed] [Google Scholar]
- 31.Zhang XK, Takashima I, Hashimoto N. Characteristics of passive immunity against hantavirus infection in rats. Arch Virol. 1989;105:235–46. doi: 10.1007/BF01311360. [DOI] [PubMed] [Google Scholar]
- 32.Spik KW, Badger C, Mathiessen I, Tjelle T, Hooper JW, Schmaljohn C. Mixing of M segment DNA vaccines to Hantaan virus and Puumala virus reduces their immunogenicity in hamsters. Vaccine. 2008;26:5177–81. doi: 10.1016/j.vaccine.2008.03.097. [DOI] [PubMed] [Google Scholar]
- 33.Botten J, Mirowsky K, Kusewitt D, Ye C, Gottlieb K, Prescott J, et al. Persistent Sin Nombre virus infection in the deer mouse (Peromyscus maniculatus) model: sites of replication and strand-specific expression. J Virol. 2003;77:1540–50. doi: 10.1128/JVI.77.2.1540-1550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medina RA, Mirowsky-Garcia K, Hutt J, Hjelle B. Ribavirin, human convalescent plasma and anti-beta3 integrin antibody inhibit infection by Sin Nombre virus in the deer mouse model. J Gen Virol. 2007;88:493–505. doi: 10.1099/vir.0.82459-0. [DOI] [PubMed] [Google Scholar]
- 35.Dietl CA, Wernly JA, Pett SB, Yassin SF, Sterling JP, Dragan R, et al. Extracorporeal membrane oxygenation support improves survival of patients with severe Hantavirus cardiopulmonary syndrome. J Thorac Cardiovasc Surg. 2008;135:579–84. doi: 10.1016/j.jtcvs.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Wernly JA, Dietl CA, Tabe CE, Pett SB, Crandall C, Milligan K, et al. Extracorporeal membrane oxygenation support improves survival of patients with Hantavirus cardiopulmonary syndrome refractory to medical treatment. Eur J Cardio Thorac Surg. 2011;40:1334–40. doi: 10.1016/j.ejcts.2011.01.089. [DOI] [PubMed] [Google Scholar]