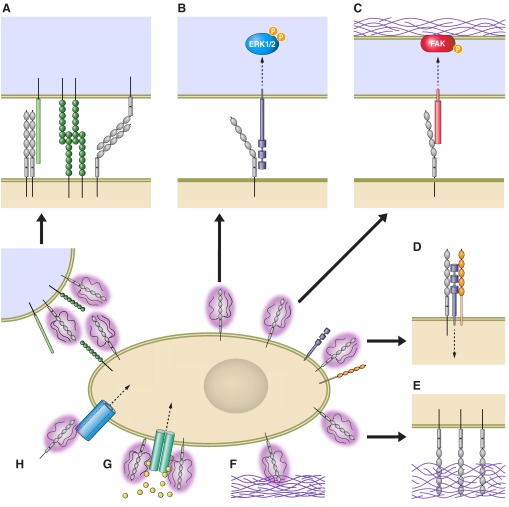
Abstract
Every cell in nature carries a rich surface coat of glycans, its glycocalyx, which constitutes the cell's interface with its environment. In eukaryotes, the glycocalyx is composed of glycolipids, glycoproteins, and proteoglycans, the compositions of which vary among different tissues and cell types. Many of the linear and branched glycans on cell surface glycoproteins and glycolipids of vertebrates are terminated with sialic acids, nine-carbon sugars with a carboxylic acid, a glycerol side-chain, and an N-acyl group that, along with their display at the outmost end of cell surface glycans, provide for varied molecular interactions. Among their functions, sialic acids regulate cell-cell interactions, modulate the activities of their glycoprotein and glycolipid scaffolds as well as other cell surface molecules, and are receptors for pathogens and toxins. In the brain, two families of sialoglycans are of particular interest: gangliosides and polysialic acid. Gangliosides, sialylated glycosphingolipids, are the most abundant sialoglycans of nerve cells. Mouse genetic studies and human disorders of ganglioside metabolism implicate gangliosides in axon-myelin interactions, axon stability, axon regeneration, and the modulation of nerve cell excitability. Polysialic acid is a unique homopolymer that reaches >90 sialic acid residues attached to select glycoproteins, especially the neural cell adhesion molecule in the brain. Molecular, cellular, and genetic studies implicate polysialic acid in the control of cell-cell and cell-matrix interactions, intermolecular interactions at cell surfaces, and interactions with other molecules in the cellular environment. Polysialic acid is essential for appropriate brain development, and polymorphisms in the human genes responsible for polysialic acid biosynthesis are associated with psychiatric disorders including schizophrenia, autism, and bipolar disorder. Polysialic acid also appears to play a role in adult brain plasticity, including regeneration. Together, vertebrate brain sialoglycans are key regulatory components that contribute to proper development, maintenance, and health of the nervous system.
I. INTRODUCTION
Evolution has yet to generate a living cell without a dense and complex coating of sugars (513). In eukaryotes, cell surface sugars exist as glycoproteins, glycolipids, and proteoglycans, previously called complex carbohydrates but now commonly referred to as glycans (to distinguish them from dietary sugars). Invisible using standard microscopic techniques, glycans are dominant chemical and physical features of the extracellular surface of all cells, forming a deep, rich, and diverse glycocalyx (Figure 1). Glycans are the cell's interface with their outside world (159, 186, 481, 515); cell surface glycans (among other functions) mediate cell-cell recognition and regulate interactions between cells and other components in their local environment (e.g., pathogens, toxins, hormones, etc.). Evolution selected glycans to serve these roles based on their physical characteristics and structural diversity. Because they are predominantly hydrophilic and often negatively charged, glycans are hydrated and spread out in space (395). Compared with proteins, which fold in on themselves, glycans occupy much more space per unit mass and their conformations are very sensitive to even minor changes in chemical structure. Because each sugar-sugar bond can exist in two configurations (α and β), at any of multiple hydroxyls and in branched arrays, a relatively small number of fundamental building blocks (monosaccharides) combine to create a wide diversity of oligosaccharide structures. As the technologies required to determine glycan structure, expression, and function have improved, glycobiology has grown in its contributions to an enhanced understanding of physiology and pathology.
FIGURE 1.
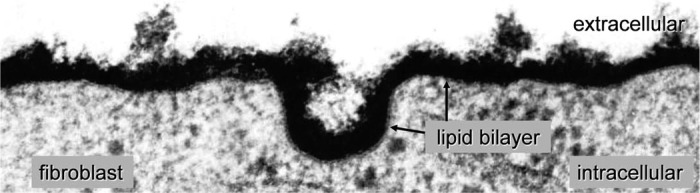
Glycocalyx. All cells carry a surface glycan coat which varies in size and composition. In the electron micrograph shown (magnification ×120,000), the glycan coat of a fibroblast in culture (BHK-21 cell) appears black after staining with ruthenium red. The underlying lipid bilayer of the plasma membrane is distinctly visible as a gray strand. The glycocalyx extends outward ∼40 nm, dwarfing the ∼6-nm-thick bilayer. [From Martinez-Palomo et al. (286). Reprinted by permission of the American Association for Cancer Research.]
Cell surface glycans modulate cell functions by several distinct mechanisms (508). Because they are often large, hydrated, and anionic, glycans dominate the architecture and physical properties of cell surfaces. Proteoglycans (such as heparan and chondroitin sulfates) with their long glycosaminoglycan chains and mucins (high-molecular-weight heavily glycosylated anionic glycoproteins) are common examples of glycans that define the physical milieu of the cell surfaces on which they reside. Regardless of their size, addition of glycans to proteins as cotranslational or posttranslational modifications regulates the protein's folding, steady-state cellular distribution, stability, and functions. Alterations of glycoprotein glycosylation can result in changes as dramatic as misfolding and delivery to the proteasome, to altered cellular or tissue distributions, or loss of function (e.g., for enzymes and ion channels). Such changes are the basis for a class of human diseases called congenital disorders of glycosylation (CDG) that result in severe developmental defects (150). Another major way in which cell surface glycans regulate cell function is by specific interactions with complementary glycan binding proteins (518). There are several families of glycan binding proteins, termed lectins, each of which carries a distinct carbohydrate recognition polypeptide domain evolved to bind specific target glycans. In humans there are >80 lectins in at least 12 distinct families (127). They mediate events as diverse as protein sorting and cell-cell recognition. The physical and biochemical functions of glycans are not mutually exclusive; glycans of large mucins or defined sequences on large glycosaminoglycans can act as receptors for lectins, for example. Finally, as shown for the role of polysialic acid in brain development, glycosylation can serve as a modifier of protein function (195) (see sect. VB3). The diversity in glycan structures and their various mechanisms of action have provided a wealth of evolutionary opportunities to engineer molecular events and interactions.
Evolution favored glycans as cell surface molecules, at least in part, because they have the ability to encode diverse structures with very few building blocks (37). This structural and functional diversity and complexity is a challenge to those in and out of the field, and in the past limited the advancement of glycomics compared with genomics and proteomics. Unlike nucleic acids and proteins, which are template-based and linearly constructed, glycans are built by suites of biosynthetic enzymes termed glycosyltransferases (59, 253), each of which adds a saccharide building block to the growing glycan chain. The glycosidic linkage formed between two sugars can, in theory, involve any of a number of hydroxyls on the penultimate sugar giving either linear or branched arrays. Whereas three different amino acids or nucleic acids can combine to form six distinct structures, three different hexoses can combine to form over a thousand distinct structures. With many glycans having a dozen or more saccharides, the potential structural diversity becomes truly daunting. Luckily for glycoscientists working to functionally decode the glycome, there are biosynthetic limitations and rules that confine the number of possible structures, and the analytical power of mass spectrometry and NMR have greatly enhanced our ability to sequence glycans (40). Advances in the biochemistry, cell biology, and genetics of glycobiology have provided the means to relate glycan structures to physiology and pathology (351). The result is that glycoscience is emerging as a major contributor to the understanding of biology and medicine.
The functional specificity of glycans is often dominated by the sugars at the outermost ends of glycan chains. In mammals, glycoproteins and glycolipids are often terminated with sialic acids, nine-carbon backbone sugars with distinctive chemical properties that determine their functions (86). Sialic acids play a particularly important role in cell and molecular interactions of glycans in mammals. Interruption of sialic acid biosynthesis is embryonic lethal in mice, and more subtle mutations in sialic acid metabolism result in a variety of diseases in humans (196). In this review, we discuss the structures and roles of sialic acids, the diverse family of sialic acid-bearing glycans (the “sialome”), and draw particular attention to two subclasses of sialoglycans, gangliosides and polysialic acids, that play decisive roles in nervous system physiology and pathology.
II. SIALIC ACIDS: PROMINENT DETERMINANTS OF THE CELL SURFACE
A. Sialic Acid Structure and Diversity
Sialic acids (Sia) are nine-carbon backbone sugars bearing chemical moieties well suited for their functions at the cell surface (77, 413) (Figure 2). The most common Sia in humans by far is N-acetylneuraminic acid (NeuAc). It is a relatively strong acid (pKa 2.6) due to the C-1 carboxylate vicinal to the C-2 anomeric carbon. The exocyclic glycerol side chain (C-7, C-8, C-9) provides the opportunity for extensive hydrogen bonding. The N-acetyl group provides opportunities for hydrophobic interactions. Each of these moieties plays important roles in the binding specificities and functions of sialoglycans.
FIGURE 2.
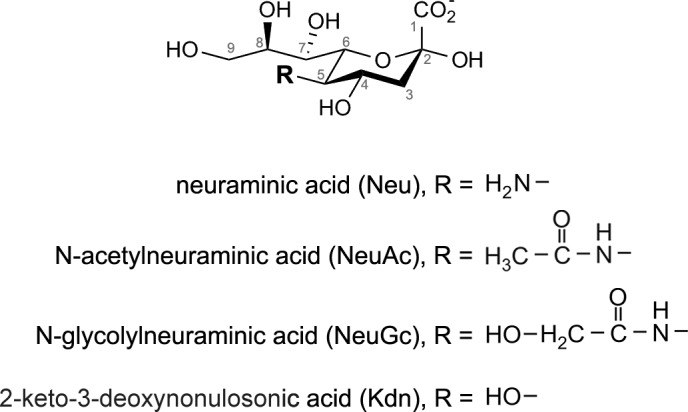
Sialic acids. The sialic acids of animals are based on neuraminic acid (Neu), which is typically found in its N-acetyl (NeuAc) or N-glycolyl (NeuGc) forms, or on 2-keto-3-deoxy-nonulosonic acid (Kdn). Other modifications (O-acylation, sulfation, phosphorylation, cyclization) result in >50 naturally occurring structures (412).
The nomenclature of the sialic acids is complicated because of their concomitant discovery as components of bovine salivary mucins by Blix et al. (44) and human brain gangliosides by Klenk and Lauenstein (237). Blix named his substance “sialic acid” after saliva (Greek σάλıο), and Klenk named his substance “neuraminic acid” after the neurons of brain gray matter. To further complicate matters, Blix isolated an N,O-diacetylated substance, whereas Klenk characterized the unacetylated free amine (174). By the time the relationship between these substances was sorted out, there were two names that have persisted to this day. Sialic acid (Sia) now refers to any member of the inclusive nonulosonic acid family (Figure 2). Neuraminic acid (Neu, 5-amino-3,5-dideoxy-d-glycero-d-galacto-non-2-ulosonic acid) refers to the C-5 free amine form, which is rarely encountered in nature. The most abundant sialic acids in nature are the N-acetyl and N-glycolyl derivatives of Neu, NeuAc, and NeuGc, respectively. An additional family member, ketodeoxynonulosonic acid (Kdn, 3-deoxy-d-glycero-d-galacto-non-2-ulosonic acid), has no amine, instead bearing a hydroxyl at C-5. Although the term sialic acid is properly used to refer to any member of this family, it is also commonly used to refer to NeuAc, which is far and away the most abundant sialic acid in humans.
Sialic acids have an interesting natural history. Only in the deuterostome lineage (vertebrates and a few higher invertebrates) are sialic acids expressed abundantly (15). In other lineages (bacteria, archaea, plants, fungi, protozoa, and protostomes), Sia expression is detected in only a small minority of species (525). Gram-negative bacteria that are associated with human or animal hosts are by far the majority in this latter group and often the causative agent for serious illness (510). Some neuroinvasive bacteria (e.g., Escherichia coli K1 and Neisseria meningitidis serogroup B) express high-molecular-weight homopolymers of α2–8- and/or α2–9-linked NeuAc called polysialic acid (polySia) (525). The size and charge of these Sia polymers support bacterial dissemination by downmodulation of complement activity (6, 425). Moreover, the structural similarity of the α2–8-linked polySia capsule produced by Neisseria meningitidis serogroup B to polySia synthesized in the human host hampers the initiation of an immune response (589). A few other pathogenic bacteria (e.g., Campylobacter jejuni) express remarkably complex sialoglycans on lipooligosaccharides of their outer membranes (575). Some of these glycans are so similar to vertebrate brain sialoglycans that they induce autoimmune neuropathies, as will be discussed later in this review (577).
Sialic acids are rare in invertebrates, so much so that they were long considered to be absent except in a few higher species (15). This changed with the sequencing of the Drosophila genome, which revealed sequences similar to mammalian sialoglycan biosynthetic genes (244), including a single sialyltransferase (DsiaT). Glycan analysis confirmed the presence of NeuAc on Drosophila glycoproteins (17, 243). Notably, DsiaT is expressed only in a subset of differentiated central nervous system (CNS) neurons (381). Disruption of the DsiaT gene results in pronounced locomotor defects, progressive loss of coordination, and progressive temperature-sensitive paralysis. These phenotypes may be related to apparent defects in the development and physiology of neuromuscular junctions and alterations in the function of voltage-gated sodium channels. These data reveal perhaps the most ancient functions of sialic acid, and focus evolutionary attention on sialylation in the nervous system.
More recent events in evolution hold hints as to sialic acid's roles in physiology and pathology inside and outside of the nervous system. Whereas most vertebrates, including our closest evolutionary relatives (bonobos and chimps), express significant amounts of NeuGc as well as NeuAc (see Figure 2), humans only synthesize NeuAc (81). The inability of humans to synthesize NeuGc is due to an exon deletion in the gene responsible for converting the N-acetyl to the N-glycolyl form, CMP-N-acetylneuraminic acid hydroxylase (CMAH). Molecular clock comparison of the disrupted human and intact chimpanzee genes places the insertion at ∼3 million years ago. What caused the species to diverge? Since sialic acid recognition is a virulence factor for some pathogens, it has been speculated that evolutionary selection against expression of functional CMAH in the human lineage was due to a catastrophic pandemic by a pathogen that targeted NeuGc (512). Varki (511) has termed this theoretical event the “sialoquake.” His hypothesis is consistent with the glycan binding specificities of related human and non-human pathogens, and with evolutionary changes in immune system sialic acid binding proteins that postdate the loss of CMAH. Notably, even among species in which NeuGc is the major sialic acid of most tissues, it is conspicuously diminished in the brain, where NeuAc greatly predominates. The Cmah gene in mice, for example, is expressed robustly in liver, kidney, thymus, and spleen, but transcripts are undetectable in brain. The reason that NeuAc is selected over NeuGc in the brains of non-human mammals remains a mystery, but may relate to specific functions of NeuAc in molecular recognition in the brain (106).
Whereas variations at C-5 define core sialic acid structures (Figure 2), O-substitutions at C-4, C-7, C-8, and/or C-9 (including acetyl, methyl, lactyl, sulfate, and phosphate groups) generate further diversity in the Sia family (15, 412). In addition, the anionic C-1 carboxylate can form lactones and lactams, neutralizing its charge. Together, over 50 naturally occurring sialic acid structures have been identified. In some cases, Sia diversity has been tied to Sia function. For example, the influenza C virus hemagglutinin binds only to NeuAc bearing a 9-O-acetyl group (387). The budding virus releases itself from cells using a Sia-specific O-acetylesterase (190). In non-human primates, Siglec-9, a sialic acid-binding immune regulatory protein, binds preferentially or exclusively to sialoglycans terminated with NeuGc, the most abundant Sia in those species. In contrast, human Siglec-9 has a broader specificity, binding both NeuAc and NeuGc. One can infer that after the “sialoquake,” human-lineage Siglec-9 variants that had the ability to bind NeuAc as well as NeuGc provided a selective advantage (455).
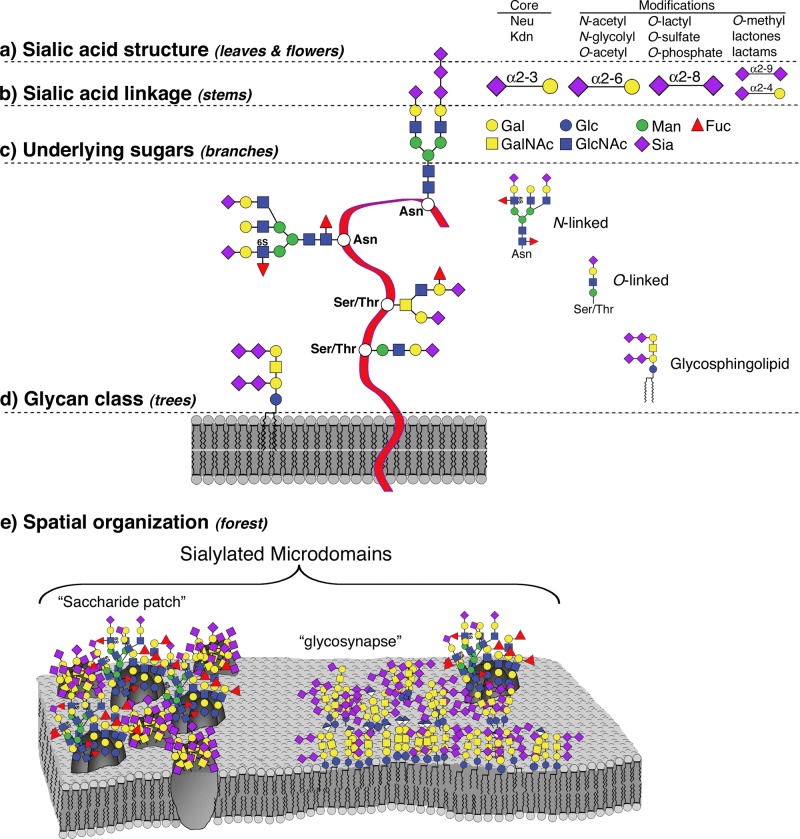
The functions of sialic acids at the cell surface depend on sialoglycan structures at several levels, from the modifications on each sialic acid carbon to their context within oligosaccharides, larger glycans, and multiglycan complexes. Cohen and Varki (86) introduced an insightful conceptualization of the cell surface “sialome” on five hierarchical levels, making an analogy to the forest canopy (Figure 3). At the outermost level are the sialic acid residues (the leaves), which are in glycosidic linkage to underlying linear and branched oligosaccharides (the stems and branches), which in turn are components of glycoproteins and glycolipids (the trees). At the cell surface, glycolipids and glycoproteins organize into lateral domains (forests). Just as the forest terrain varies greatly from place to place, from boreal to jungle, depending on the types of vegetation, the sialome varies among cell types and among lateral domains on the cell surface. Probing the structures and functions of the glycocalyx forests that dominate the chemical and physical landscape of all cell surfaces is integral to a full understanding of cell physiology and pathology.
FIGURE 3.
Hierarchical levels of sialome complexity. The sialome can be analyzed at the following complexity levels. A: sialic acid core and core modifications: esterification (with various groups), O-methylation, lactonization, or lactamization yielding >50 different structures. B: linkage to the underlying sugar (four major and many minor linkages). C: identity and arrangement of the underlying sugars that can also be further modified by fucosylation or sulfation. D: glycan class (N-linked, O-linked, or glycosphingolipids). E: spatial organization of the Sia in sialylated microdomains, which have been referred to as “clustered saccharide patches” (509) or “the glycosynapse” (182). Gal, galactose (Gal), GalNAc, N-acetylgalactosamine; Glc, glucose; GlcNAc, N-acetylglucosamine; Man, mannose; Sia, sialic acid; Fuc, fucose; Asn, asparagine; Ser, serine; Thr, threonine. [Adapted from Cohen and Varki (86), with permission from Mary Ann Liebert, Inc.]
B. Sialoglycans
Sialic acids are enzymatically added to the growing end of glycan chains in α-anomeric configuration from the C-2 carbon of the Sia, typically in one of five linkages: α2–3- or α2–6-linked to galactose (Gal), α2–6-linked to N-acetylgalactosamine (GalNAc) or N-acetylglucosamine (GlcNAc), or α2–8-linked to another sialic acid (185). Whereas addition of a single sialic acid often terminates a glycan chain, some major sialoglycans carry short (NeuAc2), medium (NeuAc3–7), or long (NeuAc8–90+) sialic acid oligomers or polymers (408). The functions of sialic acids depend on the linkage to the underlying sugars of oligosaccharide chains (the “branches,” Figure 3) and to the glycan class on which they are carried (the “trees”; glycoproteins or glycolipids).
Sialic acids occupy the outermost termini of many N-linked (asparagine-linked) and O-linked (serine- or threonine-linked) glycoprotein glycans in mammals, some examples of which are shown in (Figure 3). The multiple antennae of classical “complex type” N-linked glycans are often terminated with “NeuAc α2–3 (or α2–6) Gal β1–4 GlcNAc” sequences, which may be clustered on multiple adjacent branches of the same N-linked glycan (459). Many O-linked glycans are also sialylated, typically on short linear or branched glycans. The most abundant O-glycans are bound to proteins via a GalNAc-Ser/Thr linkage (62). Hundreds of densely packed sialylated O-linked glycans can be found on cell surface mucins, large proteins with extracellular domains rich in glycosylated serine and threonine residues. Mucins provide a thick lawn of terminal Sia residues that have profound physical and biochemical effects. Other classes of sialylated O-glycans on glycoproteins are structurally less diverse, but no less important (151). For example, the sialylated trisaccharide “Sia α2–3(6) Gal β1–4 GlcNAc β” is found in linkage to fucose (Fuc) or mannose (Man) residues that are themselves O-linked to serines and/or threonines on selected glycoproteins. O-Fuc glycans function on Notch and Cripto family proteins involved in development and cell differentiation, whereas O-Man glycans are relatively abundant in the brain (and at neuromuscular junctions), where they are found on dystroglycan (among other proteins) and regulate cell-cell interactions key to proper brain development (120). Disruption of protein O-fucosylation or protein O-mannosylation results in severe developmental abnormalities.
Sialic acids are rarely extended by further glycosylation except with another sialic acid. Structures containing Sia-Sia linkages are denoted as diSia [degree of polymerization (DP) = 2], oligoSia (DP = 3–7) and polySia (DP =8) (408). DiSia structures are abundant on brain glycolipids (gangliosides) and less so on brain glycoproteins. Notably, polySia is a prominent structural feature on a highly select group of proteins that are modified by extension of a terminal α2–3-linked or α2–6-linked Sia with additional α2–8-linked Sia residues, resulting in long chains that can reach DP >90 (193) (Figure 4). The expression of polySia provides exceptional physical and biochemical properties to the carrier protein and the cell surface on which it is expressed. This topic is discussed in detail below.
FIGURE 4.
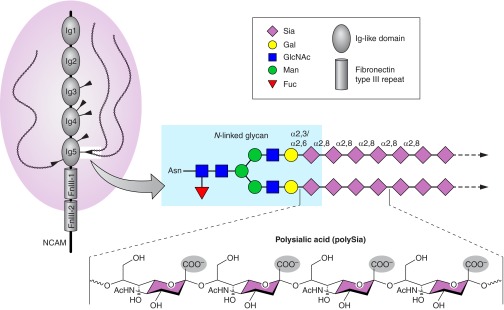
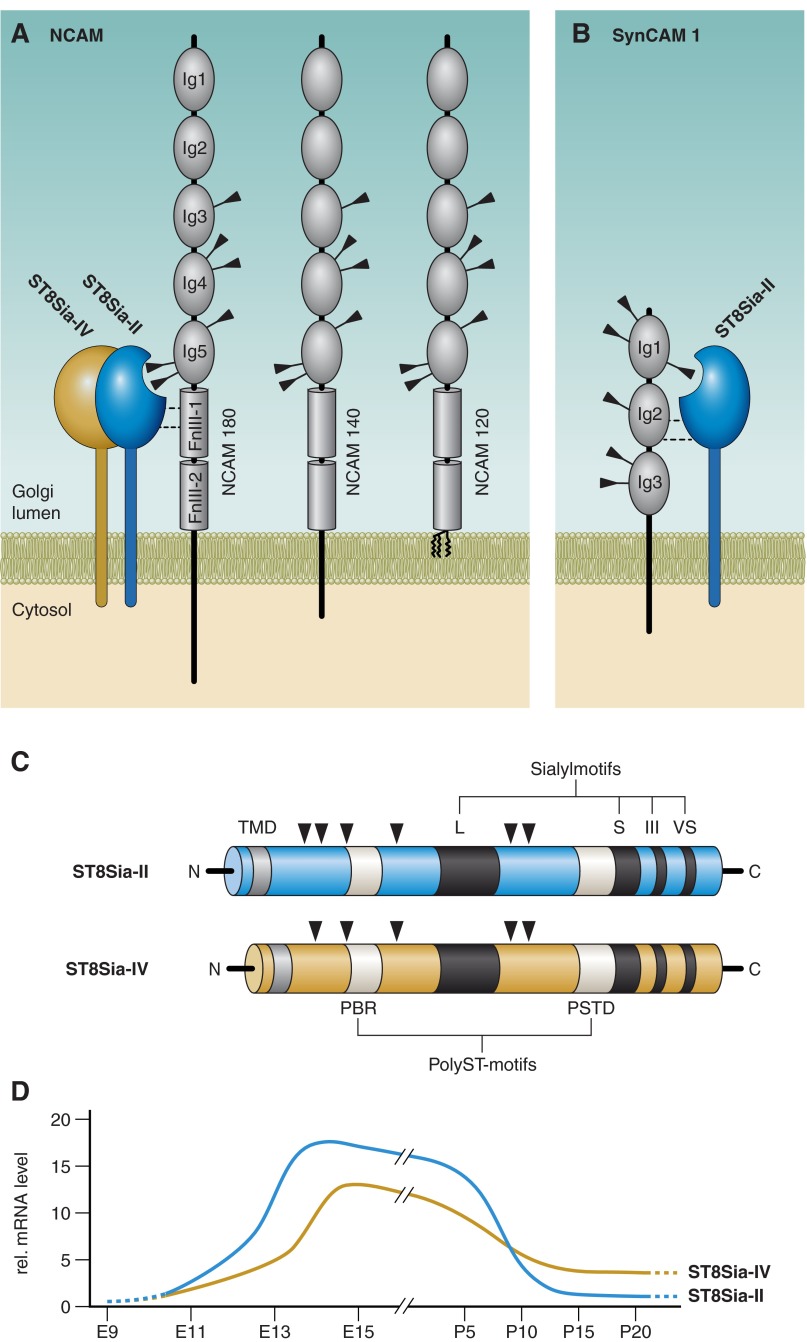
Schematic representation of polysialylated NCAM. NCAM is a prototypic member of the immunoglobulin family of cell adhesion molecules with five immunoglobulin-like modules (Ig1 to Ig5) and two fibronectin type III repeats (FnIII-1 and FnIII-2) in the extracellular domain. Of the six N-glycosylation sites (indicated by black arrowheads on Ig3, Ig4, and Ig5), only two, located in the 5th Ig-like domain, can be extended by the addition of polySia, a homopolymer of α2–8-linked sialic acid residues. One of several possible core glycans is depicted (see text for details). PolySia chains are linked to complex glycans with the first sialic acid in α2–3 or α2–6 linkage. PolySia chains can comprise >90 monomer units, leading to polyanions (negatively charged carboxylate groups are highlighted with gray spheres) with high water binding capacity. The large hydration shell formed by polySia markedly increases the hydrodynamic volume of its carrier molecule and thus interferes with its natural binding capabilities. Bound to NCAM, polySia is believed to invert an adhesive into a repelling molecule.
Whereas sialic acids in many tissues are most abundant on N- and O-linked glycoproteins, this is not true of the vertebrate brain, where sialoglycolipids dominate (420). These invariably occur in the brain in the form of sialylated glycosphingolipids, for which the term gangliosides was coined by Klenk in 1942 (236). Although gangliosides are found on all vertebrate cells and tissues, they are much more abundant and more complex in the brain, as will be detailed later in this review.
At the level of the cell surface “forest,” glycans associate into sialoglycan-rich lateral membrane microdomains (Figure 3) with distinct characteristics and functions. In some cases, extracellular lectins crosslink cell surface glycoproteins via carbohydrate recognition (53, 60). Disrupting these interactions leads to changes in cell surface residency, lifetime, and function of sialoglycans (352). Another example of lateral association is the spontaneous formation of membrane rafts (448, 458). Sphingolipids, including gangliosides, have long saturated carbohydrate chains on their ceramide (lipid) moieties that associate with each other, with cholesterol and/or with selected intracellular and transmembrane proteins to create small (nanometer scale) and dynamic lateral membrane microdomains. These domains concentrate and enrich selected membrane components. They provide interaction sites (landing pads) for extracellular ganglioside-interacting proteins and enhance the functions of interacting cell surface signaling molecules. The structure, formation, dynamics, and functions of cell surface microdomains remain one of the least well understood levels of the sialome, despite broad appreciation of their functional significance.
C. Sialyltransferases and Sialidases
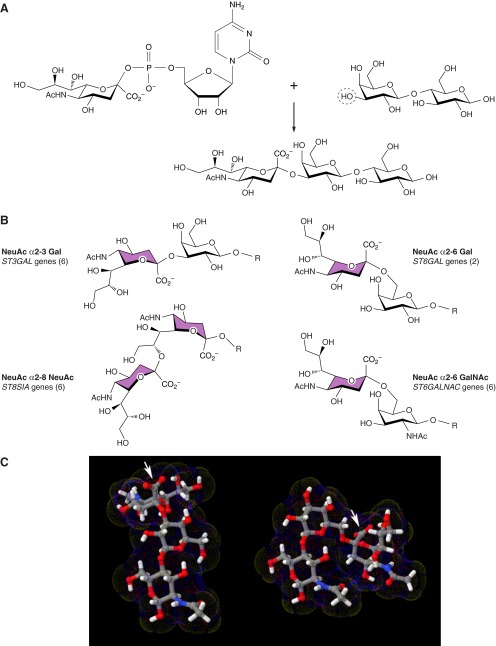
Sialoglycans are biosynthesized by the enzymatic transfer of sialic acid from its activated nucleotide sugar donor (CMP-Sia) to the terminus of an oligosaccharide chain of a glycoprotein or glycolipid (Figure 5). The enzymes responsible, sialyltransferases, have been cloned from organisms ranging from bacteria to human (185). There are 20 mammalian sialyltransferases, each of which has high sequence similarity between human and mouse. Animal sialyltransferases are characterized by two major and two minor conserved amino acid sequences (sialylmotifs; see sect. IIIB2) involved in donor (CMP-Sia) binding (22). Their discovery aided the search for sialyltransferases in a wide variety of species. Bacterial sialyltransferases appear to have evolved separately, but also exhibit conserved motifs, “bacterial sialyl-motifs,” which appear to serve functions similar to those in eukaryotic sialyltransferases (152, 224, 340, 557).
FIGURE 5.
Sialyltransferases. A: sialoglycans are synthesized by the enzymatic transfer of sialic acid from the activated nucleotide sugar donor CMP-sialic acid, to an acceptor (e.g., the 3-carbon hydroxyl of the galactose of the disaccharide lactose, circled) to form a glycosidic linkage (e.g., NeuAc α2–3 Gal). B: there are 20 human sialyltransferase genes in four families designated by the major linkages they form. C: an example of the structural differences between α2–3 and α2–6 linked sialic acids. Each structure contains the acceptor disaccharide Gal β1–4 GlcNAc in the same orientation with a terminal sialic acid linked to the 3-carbon hydroxyl (left) or 6-carbon hydroxyl (right) of the terminal galactose. The position of the sialic acid carboxylate is designated by an arrow to assist in visualization. Three-dimensional structures were generated using Glycam-Web (www.glycam.org).
The 20 mammalian sialyltransferases (STs) are categorized into four families (ST3Gal, ST6Gal, ST6GalNAc, or ST8Sia) based on the linkage they generate (α2–3, α2–6, or α2–8) and their primary saccharide acceptor (Gal, GalNAc, or Sia) (Figure 5). Family members are arbitrarily denoted by Roman numerals (e.g., ST3Gal-I through ST3Gal-VI), and the genes that encode them by numbers (e.g., ST3GAL1-ST3GAL6 in human, St3gal1-St3gal6 in mice) (495). There are six vertebrate ST3Gal enzymes, two ST6Gal enzymes, six ST6GalNAc enzymes, and six ST8Sia enzymes. The reasons for evolving multiple enzymes for each linkage are not fully understood but are presumed to account for fine specificities based on the underlying sugars (branches) and glycan class (trees) of their acceptor glycans. Since sialyltransferases coinhabit the Golgi apparatus, they may have evolved multiple distinct acceptor specificities to allow independent regulation of different sialoglycans. Genetic observations in human diseases and studies of engineered mouse mutants are helping to reveal the in vivo specificities and functions of sialyltransferases and their products (517).
Enzymatic modification of sialic acid residues to generate diverse Sia species occurs at both the CMP-Sia and sialoglycan level (15). Hydroxylation of the C5 N-acetyl group to convert NeuAc to NeuGc (by CMAH) occurs only at the CMP-Sia level, prior to transfer of Sia to the glycan. In contrast, O-acetylation and other Sia modifications may occur at either the CMP-Sia level or after the Sia is transferred depending on the specific modification and enzyme involved. The genetics and enzymology of Sia diversity and its functions remain active areas of inquiry.
Sialic acids are removed from sialoglycans by the enzymatic actions of sialidases, of which there are four in humans generated by the genes NEU1-NEU4 (164, 309). In contrast to sialyltransferases, the NEU enzymes are not strictly linkage specific and have a variety of subcellular distributions. NEU1, which is the most abundant mammalian sialidase, is primarily intralysosomal and is active on glycopeptides and oligosaccharides but not on gangliosides. Mutations in the human NEU1 gene are responsible for sialidosis, a congenital lysosomal storage disorder that disproportionally affects the nervous system (445). NEU2 is cytoplasmic, is active at near-neutral pH, and has the ability to remove sialic acids from different glycan classes. NEU3 is found on the plasma membrane and is ganglioside-selective (requires a hydrophobic aglycone), whereas NEU4 is predominantly on intracellular membranes and has broad glycan class specificity. Beyond their role in catabolism, sialidases have recently been recognized as active regulators of sialoglycan functions (304). NEU3, for example, has been implicated in hippocampal neuron outgrowth (102), whereas NEU1 is upregulated and targeted to the plasma membrane in differentiating monocytes (266) and activated T-cells (333) and positively impacts insulin receptor signaling in peripheral tissues (128). Moreover, NEU1-NEU3 are implicated in skeletal muscle differentiation (136). More studies are needed to better understand the different roles of these four enzymes.
The term sialidase is synonymous with the term neuraminidase. Since “neuraminic acid” refers to the C-5 free amine form (which is rarely found in nature) whereas “sialic acid” refers to the entire family of nonulosonic acids (Figure 2), the term sialidase is strictly more accurate. However, since the term neuraminidase is firmly established as the nomenclature used to describe the sialidases of influenza viruses and other pathogens, both terms are widely used.
D. Sialic Acid Binding Proteins
There are several mechanisms by which the sialome regulates cells and tissues (412, 516). Based on their relatively strong anionic charge, sialic acids regulate glycoprotein secondary and tertiary structure and contribute to the hydration of proteins (such as mucins) at the cell surface. They shield glycoproteins from interactions with enzymes (e.g., proteases) as well as with protein receptors on other cells, regulating intracellular communication. In addition to these general mechanisms, sialic acids are specific receptors for complementary sialic acid binding proteins.
Their prominence on cell surfaces and their selective expression on the outermost branches of glycoprotein and glycolipid glycans make sialic acids well suited as specific receptors for complementary binding proteins that can “read” the sialome code. This was first discovered in the study of pathogens that evolved to bind to these abundant and readily accessible cell surface targets (259), of which influenza viruses are the best studied (338, 450, 462). Influenza virions bind to terminal sialic acids on target cells (e.g., upper airway epithelial cells in humans) via their surface hemagglutinin. After entering target cells and proliferating, the daughter virions bud from the host cell surface, then release themselves via surface sialidases (viral neuraminidases) that act as “receptor destroying enzymes” (529). The viral hemagglutinin is specific for the Sia linkage (α2–3 versus α2–6), and the strain specificity dictates species tropism (462). Thus α2–6-NeuAc-targeting strains are typically transmissible between humans because they bind to the major sialoglycans of the human upper airway, whereas α2–3-targeting strains infect birds, which prominently express that linkage on their gut epithelial cells. Other pathogens and pathogen protein toxins also take advantage of cell surface sialoglycans for binding and entry, including Helicobacter pylori, Escherichia coli K99, Vibrio cholerae (toxin), Clostridium tetani (toxin), Plasmodium falciparum, and others (259). The discovery of pathogen Sia-binding proteins predated and anticipated the discovery of vertebrate counterparts.
Selectins and siglecs are two important families of Sia-binding proteins in mammals. The three selectins (E-, L-, and P-selectin) mediate various types of leukocyte and platelet trafficking (292). Most of the 14 human siglecs (sialic acid-binding immunoglobulin-like lectins) are also involved in immune cell regulation (98, 362). However, the family includes one nervous system member (Siglec-4, myelin-associated glycoprotein, MAG) that is described in detail later in this review (422).
The three selectins each recognize α2–3-linked Sia on a fucosylated core structure, such as sialyl Lewis x [NeuAc α2–3 Gal β1–4 (Fuc α1–3) GlcNAc] and related structures in the context of larger glycoproteins and glycolipids (292). In contrast, siglecs have diversified to take advantage of the many linkages and contexts in which sialic acids are found on tissues (98). For example, Siglec-2 binds exclusively to α2–6-linked Sia, whereas Siglec-8 binds to a Sia that is α2–3-linked to a galactose that also carries a 6-position sulfate group. Siglec-7 and Siglec-11 prefer α2–8-linked Sia, with the latter functionally binding polysialic acid (14, 24; see sect. VA). Detailed structure-function studies reveal that selectins primarily recognize the appropriately positioned anionic moiety of sialic acid, which can be replaced with other anionic groups (73), whereas siglecs typically require the entire sialic acid (92, 514). In both the selectin and siglec families, sialic acid binding proteins have evolved to recognize and initiate responses to particular sialoglycans that are recognized at several levels of molecular organization: the “leaves, stems, branches, and trees” of the cell surface sialome forest.
III. BRAIN-ENRICHED SIALOGLYCANS
A. Gangliosides: Dominant Molecular Determinants in the Brain
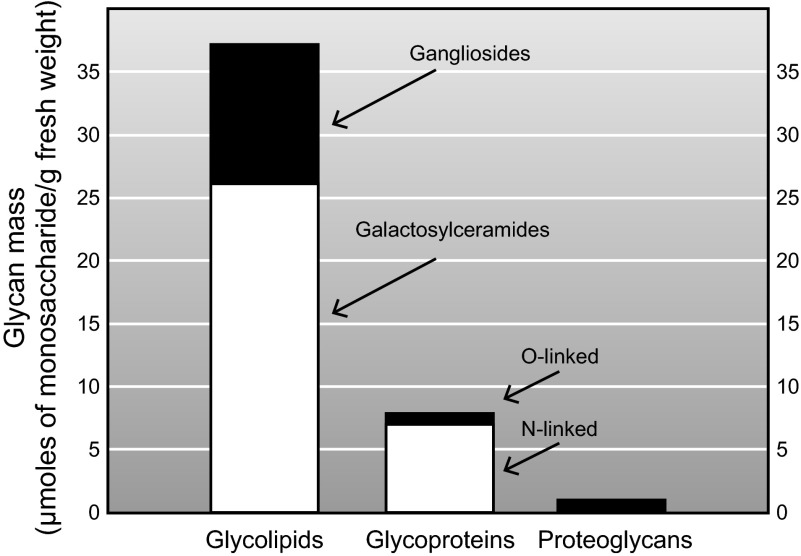
The glycome of each animal tissue is comprised of glycoproteins, glycolipids, and proteoglycans in varying proportions. The brain is unusual in that its glycome is dominated by glycolipids (421) (Figure 6). These include galactosylceramide and its 3-O-sulfated form sulfatide, which are major myelin lipids, and gangliosides, which are enriched on nerve cells. Together, glycosphingolipids represent ∼80% of the total glycan mass in the brain, and gangliosides carry ∼75% of the brain's sialic acid (Figure 7). In addition to expressing a much higher proportion of its sialome as gangliosides, the adult brain expresses gangliosides of higher complexity than those typical in other tissues (420). This section describes the structures, biosynthesis, and expression of brain gangliosides; their functions are discussed later in this review.
FIGURE 6.
Glycans in the adult rat brain. The mass of constituent monosaccharides (μmol/g fresh brain wt) for each glycan class and subclass were calculated from published data as follows. 1) Galactosylceramide and sulfatide together represent 19.4 mg/g brain fresh wt, with 76% (by weight) being galactosylceramide (346). This represents ∼20.3 μmol galactosylceramide and ∼5.8 μmol sulfatide/g fresh wt. 2) Ganglioside sialic acid is expressed at 1.15 mg (3.9 μmol)/g fresh wt. Since there is an average of 2.9 ganglioside monosaccharides per ganglioside sialic acid, gangliosides represent 11.2 μmol monosaccharide/g fresh wt (482). 3) Glycoproteins represent 71 μmol monosaccharide/g lipid-free dry wt. Since lipid-free dry weight is ∼11% of brain fresh weight, glycoproteins represent ∼7.8 μmol monosaccharide/g fresh wt (281). Of these, O-linked GalNAc is expressed at 0.28 μmol/g fresh wt. Since there is an average of 3.2 O-linked monosaccharides per O-linked GalNAc, O-linked glycoproteins represent 0.88 μmol/g fresh wt (139). Proteoglycan hexosamine is expressed at 4.1 μmol/g lipid-free dry wt in the adult rat brain (∼8.1 μmol total monosaccharide/g dry wt). Based on lipid-free dry weight being 11% of fresh weight, proteoglycans represent 0.89 μmol/g fresh wt (282). [From Schnaar (421), by permission of Oxford University Press.]
FIGURE 7.
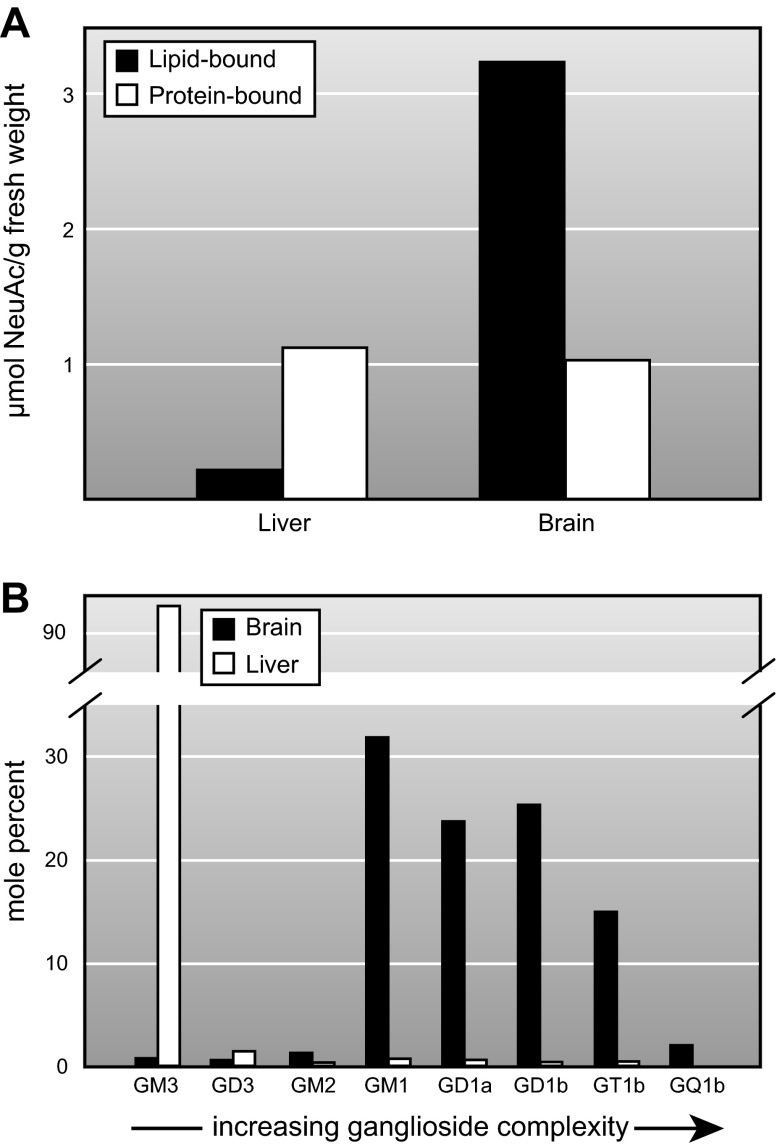
Sialoglycoproteins and gangliosides in liver and brain. A: protein-bound sialic acid is equivalent in the two tissues, whereas expression of lipid-bound sialic acids (gangliosides) is >10-fold higher in the brain. B: ganglioside patterns (mol%) in human brain and liver. Data compiled from References 5, 342, 482. [Adapted from Schnaar (420), with permission from Elsevier.]
B. Brain Ganglioside Structures and Metabolism
Gangliosides are members of the larger glycosphingolipid family, consisting of glycans attached to a ceramide lipid (Figure 8). Ceramides are composed of a long-chain amino alcohol, sphingosine, with a C2 primary amine that is N-acylated with a long-chain fatty acid amide. Brain ganglioside ceramides typically have C1- and C3-hydroxyl groups and a C4-C5 double bond, with the C1-hydroxyl linked via glycosidic linkage to the glycan chain (181). The properties of sphingolipids are unusual due to the nature of their lipid moieties. The sphingosine chain of mammalian brain gangliosides is long (18 or 20 carbons), and the fatty acid amide is saturated (typically C18:0). This results in a relatively rigid structure within the plane of the outer leaflet of the plasma membrane, and thereby results in enhanced lateral self-association (e.g., see Figure 3, spatial organization). Ganglioside-enriched domains may also be enriched in other sphingolipids (notably sphingomyelin), cholesterol, glycosylphosphatidylinositol-linked cell surface proteins, fatty acylated intracellular signaling proteins (e.g., Src family members), and selected transmembrane scaffold and/or signaling proteins (448, 458). Together, these lateral associations within the plasma membrane have been called lipid rafts, glycolipid-enriched microdomains, or detergent-resistant membranes. For perspective, gangliosides constitute ∼0.6% of total brain lipids, but are more highly enriched in neurons. The lipid composition of the whole brain is as follows (in μmol/g wet wt, adult rat): phosphoglycerolipids, 89; cholesterol, 69; galactosylceramides, 23; sphingomyelin, 7; gangliosides, 1.1 (347, 573).
FIGURE 8.
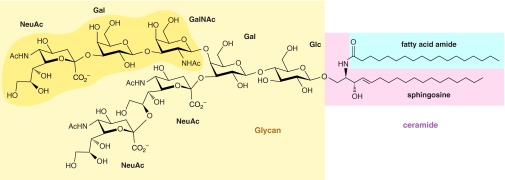
Ganglioside GT1b. The glycan (shaded yellow) is in glycosidic linkage to the ceramide lipid which is comprised of a long-chain base (sphingosine, pink) bearing a fatty acid amide (blue). Within the glycan, the main binding site for myelin-associated glycoprotein is shaded (92, 565).
Gangliosides reside primarily in the outer leaflet of the plasma membrane, with the ceramide and much of the first sugar (glucose) engaged with the lipid leaflet, and the glycan extending out into the surrounding milieu (110) (Figure 9). The dual properties of intraleaflet lipid associations and glycan extension outward from the membrane bilayer provide the ability of gangliosides to act as cell surface recognition and regulation molecules.
FIGURE 9.
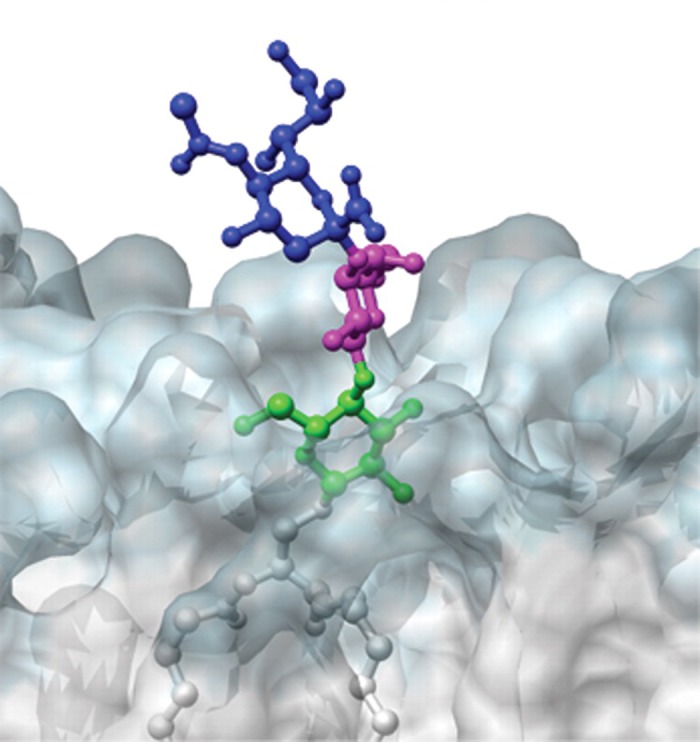
Atomic-resolution conformational analysis of ganglioside GM3 in a lipid bilayer. The image represents a 20-ns snapshot taken perpendicular to the plane of the bilayer near the head group of GM3. The ganglioside is shown as a ball-and-stick model and the membrane as a transparent space-filling model with the membrane hydrophilic region in blue and membrane hydrophobic region in white. [From DeMarco and Woods (110), by permission from Oxford University Press.]
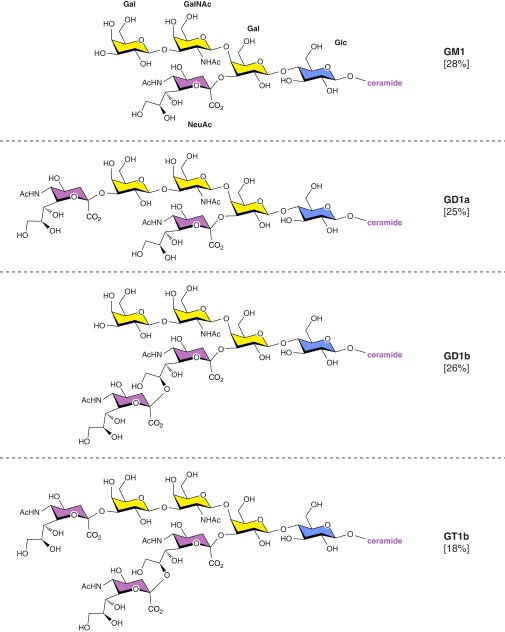
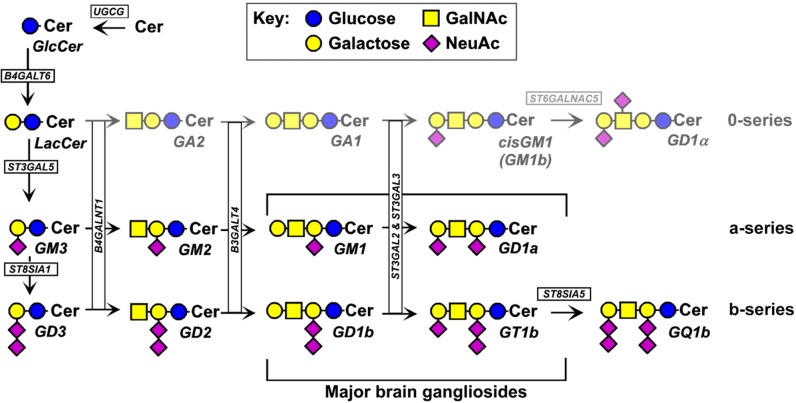
Although many ganglioside structures have been identified in a variety of tissues and organisms, adult mammalian brain gangliosides are dominated by just four closely related structures (GM1, GD1a, GD1b, and GT1b) that together represent the vast majority (97%) of gangliosides in the adult human brain (482) (Figure 10). The same four structures comprise the major sialoglycans in the brains of all adult mammals and birds, indicating a selective advantage for expression of these particular structures. These four gangliosides share the same neutral glycan core (Gal β1–3 GalNAc β1–4 Gal β1–4 Glc β1–1 Cer) with varying numbers of sialic acids attached to the internal and terminal galactose residues. The four major brain gangliosides are synthesized stepwise (Figure 11) by glycosyltransferases that transfer each monosaccharide from its activated form (UDP-Glc, UDP-Gal, UDP-GalNAc, or CMP-NeuAc) to the growing glycan chain (Table 1). A key branch point in the biosynthetic chain is GM3, which can be extended by addition of an α2–8-linked NeuAc to form GD3 or by a β1–4-linked GalNAc to form GM2. Once the GalNAc is transferred, internal α2–8-linked NeuAc residues cannot be added, dedicating this pathway to biosynthesis of GM1 and GD1a, so-called “a-series” gangliosides. GD3, in contrast, can be extended by addition of GalNAc and subsequent sugars to form GD1b and GT1b, “b-series” gangliosides. There are many less abundant gangliosides in the brain that may have important functions in physiology and pathology. Among these are the “0-series” gangliosides that arise from transfer of GalNAc to lactosylceramide (no Sia on the internal Gal), the “c-series” gangliosides (not shown) that have a trisialyl moiety (NeuAc α2–8 NeuAc α2–8 NeuAc α2–3) on the internal Gal. GQ1b carries a diSia moiety on the outermost Gal residue, whereas the rare “α-series” gangliosides carry an α2–6-linked Sia on the GalNAc residue (e.g., GD1α; Figure 11). Very rare gangliosides may carry up to seven total Sia residues (403), including linear oligomers of up to five sialic acids (460). Gangliosides bearing longer oligo- or polysialic acid chains have not been reported.
FIGURE 10.
The four major brain gangliosides of mammals and birds share the same neutral tetrasaccharide core (Gal β1–3 GalNAc β1–4 Gal β1–4 Glc) attached to ceramide, with varying numbers and linkage positions of sialic acids. The sugars are color-coded: yellow, Gal/GalNAc; blue, Glc; and purple, NeuAc. Molar percentages are for total human brain gangliosides.
FIGURE 11.
Brain ganglioside biosynthesis. Complex brain gangliosides are biosynthesized stepwise by the action of a suite of glycosyltransferases. The genes responsible for the expression of each glycosyltransferase are boxed (see Table 1).
Table 1.
Brain ganglioside biosynthetic gene nomenclature
| Official Gene Name | Ganglioside(s) Synthesized* | Also Known As† |
|---|---|---|
| St3gal5 | GM3 | Siat9, SAT I |
| St8sia1 | GD3 | Siat8a, GD3S, SAT II |
| B4galnt1 | GM2, GD2 | Galgt1, GalNAc T |
| B3galt4 | GM1, GD1b | GALT2, GALT4, GalT II |
| St3gal2 | GD1a, GT1b | Siat4b, SAT IV |
| St3gal3 | GD1a, GT1b | Siat6, ST3N |
| St8sia5 | GQ1b | Siat8e, SAT V |
Only major brain gangliosides (or their synthetic intermediates) listed (see Figure 11).
Each enzyme (or gene) has also been referred to as the “synthase” associated with that ganglioside (e.g., “GM3 synthase” for St3gal5).
Ganglioside catabolism occurs stepwise by the actions of glycan-specific exoglycosidases (406). Removal of specific terminal glycans must proceed prior to further ganglioside degradation. Mutations in specific ganglioside glycosidases result in devastating lysosomal storage diseases. For example, mutation of the β-galactosidase gene GLB1 results in GM1 gangliosidosis, whereas mutation of either of the two genes coding the β-N-acetylhexosaminidase subunits (HEXA or HEXB) results in GM2 gangliosidosis (406).
C. Brain Ganglioside Development and Expression
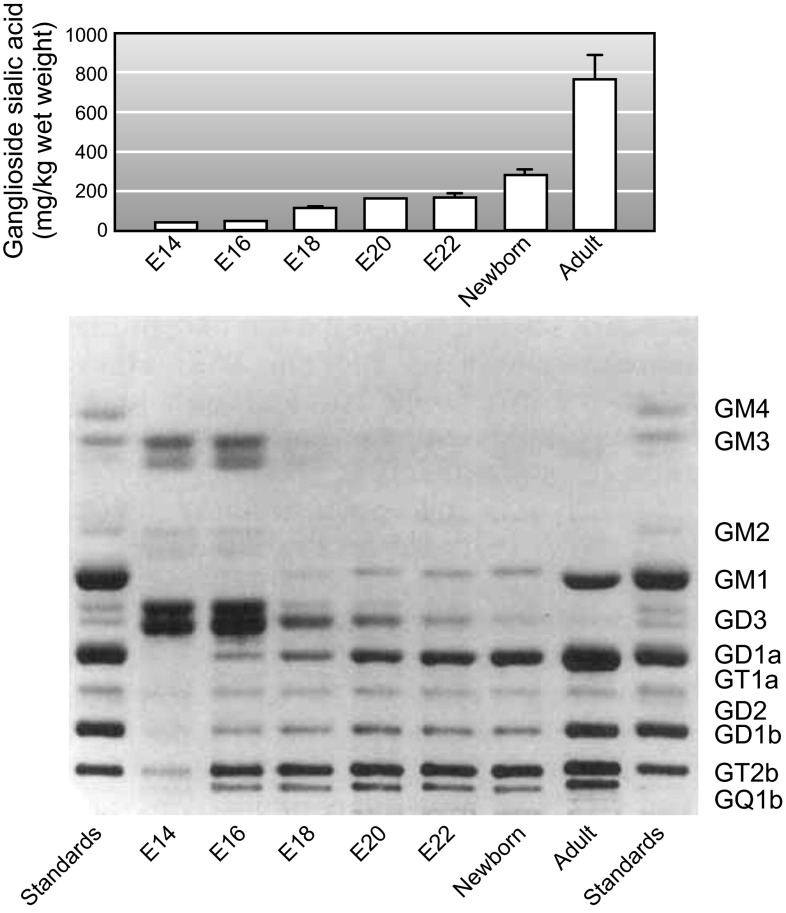
The simple ganglioside structures GM3 and GD3 (see structures; Figure 11) dominate the glycome of the brain early in development (573, 574). At embryonic day 14 (E14) in the rat, GM3 and GD3 comprise nearly all of the brain gangliosides (Figure 12). Soon thereafter, more complex gangliosides arise such that by E20 they dominate the glycome. At birth, GM3 and GD3 are minor components, with GD1a and GT1b taking their place as the major sialoglycans in the brain. As the brain matures further to adulthood, GM1 and GD1b expression increase until the four major brain gangliosides (GM1, GD1a, GD1b, and GT1b) are at comparable levels and together represent the vast majority of total brain gangliosides. Over this same time period, the total brain ganglioside concentration is increasing sharply (Figure 12). Expressed as the amount of total ganglioside sialic acid per gram fresh brain weight, ganglioside density increases fivefold between E14 and birth, and another threefold between newborn and adult in the rat. Likewise, ganglioside density in the human cerebral cortex increases sharply during the last trimester of fetal development and continues to increase during the first 2 years of life (470). Thus both ganglioside density and structural complexity increase as the brain develops and matures.
FIGURE 12.
Ganglioside expression during brain development. Top: rat brain ganglioside expression (ganglioside sialic acid density as mg/kg wet wt) during brain development. Bottom: equal amounts of extracted brain gangliosides from each developmental age indicated were resolved by thin-layer chromatography and stained using a sialic acid-specific colorimetric reagent (resorcinol-HCl). Gangliosides GM3, GM2, and GD3 migrate as double bands due to ceramide lipid diversity. [Adapted from Yu et al. (573), with permission from John Wiley and Sons, Inc.]
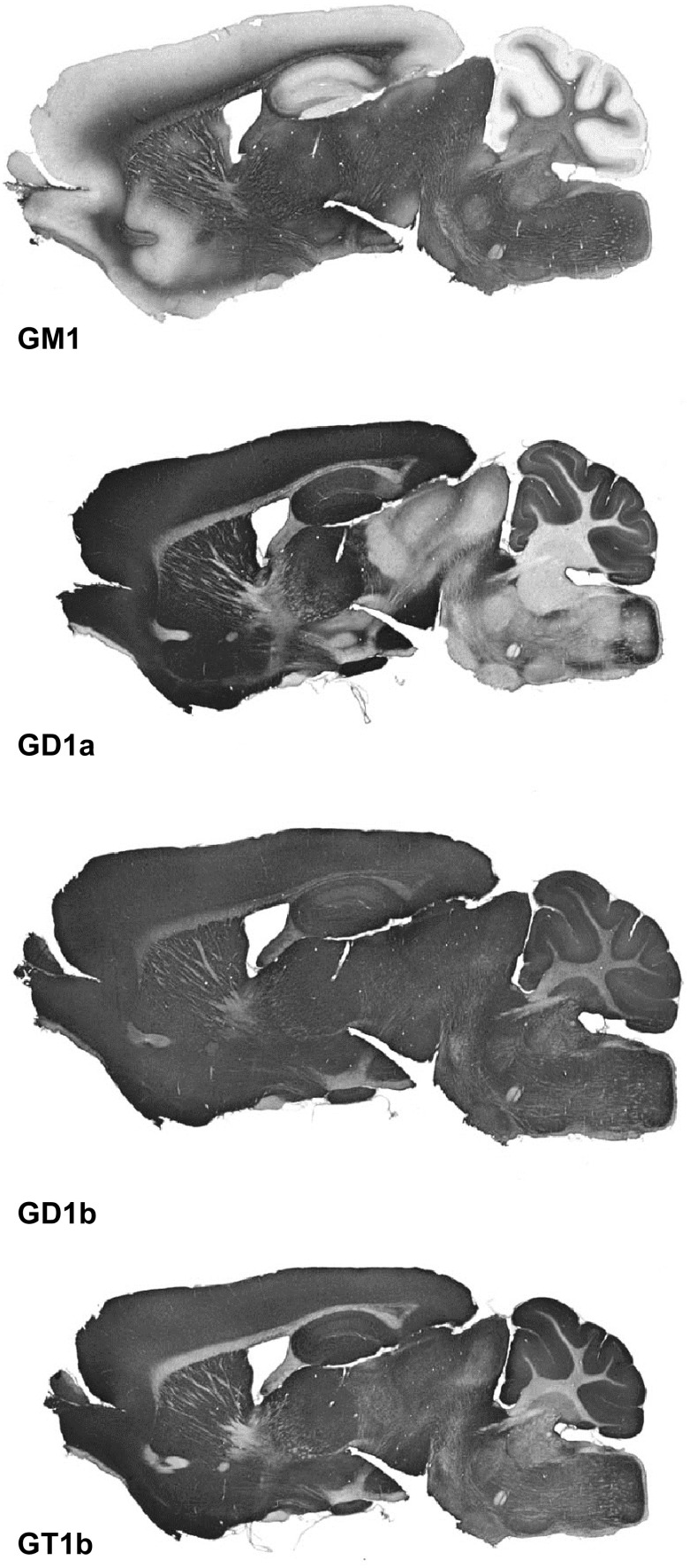
Although gangliosides are major cell surface determinants throughout the nervous system, the four major gangliosides distribute nonuniformly in different brain structures (248, 467). In the adult mouse brain, for example, GM1 is concentrated in white matter tracts throughout the brain, GD1a distributes in a largely complementary pattern in gray matter (Figure 13), whereas GT1b and GD1b are found in both white and gray matter (467). Certain scarce gangliosides have remarkable cell-type restricted expression. The α-series gangliosides, which have an additional sialic acid in α2–6-linkage to the ganglioside GalNAc residue, are found selectively on cholinergic neurons (198, 212). They were discovered as the antigen of an antiserum raised against the cholinergic electroplax organ of the electric fish Torpedo marmorata, and cross-react selectively with cholinergic neurons in the mammalian brain (112, 384). The functional relationship between rare ganglioside structures and cholinergic neurotransmission is as yet unknown.
FIGURE 13.
Ganglioside immunohistochemistry on adult wild-type C57Bl/6 mouse mid-sagittal brain sections. [Adapted from Sturgill et al. (467), by permission of Oxford University Press.]
An even more subtle ganglioside structural variation has been linked to migrating cells in the developing brain. The simple ganglioside GD3 (see structure; Figure 11) is the major ganglioside in the early fetal brain, where it is widely distributed. GD3 modified with an O-acetyl group on the C9 hydroxyl of the terminal sialic acid, however, has highly restricted expression that has been linked, for example, to migrating neuroblasts of the rostral migratory stream that arise from the subventricular zone during brain development (305, 418). O-Acetylated and lactone forms of gangliosides may constitute several percent of total adult brain gangliosides (306, 467), and their expression may be underestimated due to loss of acetyl groups under commonly used (alkaline) conditions for brain ganglioside purification (419). How ganglioside acetylation affects cell function is an area for further research (393).
D. Polysialic Acid and Polysialyltransferases
A major protein-bound sialoglycan in the brain is polysialic acid (polySia), a linear homopolymer of α2–8-linked sialic acid residues. Predominantly linked to the neural cell adhesion molecule (NCAM) (Figure 4), polySia provides an ideal example to highlight how versatile glycans expand the information content of their protein scaffolds.
1. Polysialic acid
The term polysialic acid, when used in vertebrates, describes α2–8-linked chains with DP ≥8 (Figure 4). This terminology is based on the immunological properties of polySia chains. Whereas a single antibody binding site can typically encompass at most six saccharide units (222), a polySia epitope comprises between 8 and 10 (140, 218). Based on biophysical analyses, polySia chains form extended helices with eight to nine residues per turn, the conformation recognized by polySia-specific antibodies (30, 61, 135). With development of the first monoclonal antibody (mAb) directed against polySia (mAb735; Ref. 154) ligand binding studies in solution confirmed Sia8 (a linear octamer of α2,8-linked Sia residues) as the minimal epitope (187, 188). Considering that polySia recognition by antibodies is temperature sensitive (114) and, as recently shown by NMR, even the tetramer (Sia4) attains helical conformation at low temperature (263°K) in solution (29), it is understandable that helical structures in the polymer are transient and form dynamically along the chain (299). With increasing polymer length (the threshold seems to be DP8) and under physiological conditions, extended helices of 8–10 Sia residues seem to be the preferred structures (558) and have been co-crystallized with interacting proteins (360). Crystallization of polySia in complex with an enzymatically inactive form of a bacteriophage-derived polySia degradase endosialidaseNF (endo-N-acetylneuraminidaseF; endoNF), demonstrated the existence of conformational freedom in polySia chains (426, 427). EndoNF possesses an extended interface (path of positively charged amino acids) for the interaction with polySia (427), which at two sites converts into high binding sites (Figure 14). Electron density obtained at these positions demonstrated two different helical conformations for polySia. The distance between the C2 atoms of the first and fourth sialic acid of a Sia4 fragment bound to the stalk domain of endoNF was 14.7 Å, corresponding to a translation of 4.9 Å/residue and thus highly reminiscent of the extended helical conformation described in solution (558). In contrast, a distance of only 12.4 Å was determined between C2 of the first and the fifth sialic acid of a Sia5 fragment bound to the head domain of the protein, indicating a compressed helical conformation with a translation of 3.1 Å per residue. These data indicate that the binding partner constrains the conformation of the secondary structure organization in polySia chains, implying that no significant energy barriers exist between the different polymer conformations. This flexibility, in turn, may be a key feature that explains polySia binding to different proteins (see sect. VA).
FIGURE 14.
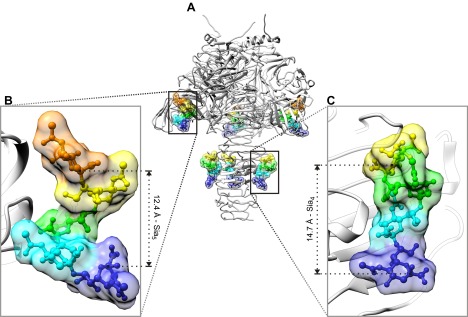
The conformational freedom inherent in polySia chains has been instructively displayed by cocrystallization with an inactive from of the polySia degrading endosialidaseNF. A: endosialidases are homotrimeric enzymes with an overall mushroomlike outline (466). In addition to the active site (not shown), each monomer contains two peripheral high-affinity binding sites for polySia in the head (B) and the stalk (C) of the mushroom (431). The polySia structures cocrystallized with the enzyme clearly indicated two different conformations of the polymer. The Sia5 molecule bound to the head domain attains a compressed conformation with the distance between the C2 carbons of the most distant sugars being only 12.4 Å. In contrast, in the Sia4 fragment at the stalk domain, the distance from the C2 carbon at the reducing end sugar to the C2 carbon at the nonreducing end sugar is 14.7 Å. This relaxed helical pitch is similar to the soluble state structure described for polySia (558). These data demonstrate that the binding partner imposes structural information onto polySia chains.
In discussing the physiological features of polySia, most studies focus on the size and charge of the molecule (for review, see Refs. 316, 397). In biological samples, polySia chains were shown to reach DPs >90 (161, 330, 354). Their polyanionic nature and, most probably, other yet unidentified chemical properties equip polySia with an unusual capacity to bind water. The resulting large hydration volume increases the size of the carrier molecule and by stereochemical means confers repulsive forces to polysialylated surface molecules, as shown for the major polySia carrier, the NCAM (Figure 4). Consistent with this model, physicochemical measurements carried out in cell culture demonstrated an increased distance between two cell membranes expressing polySia-NCAM (566, 567). The presence of polySia on the cell surface is thus believed to generally weaken contacts between neighboring cells. The model agrees with a variety of polySia functions but fails to fully describe the many pathophysiological aspects that have been identified in mouse models with impaired polySia biosynthesis. This issue will be discussed in detail in section VA.
2. Polysialyltransferases, polySia acceptors, and the structural basis of polysialylation
In 1995 the genes encoding the enzymes responsible for polySia biosynthesis, the polysialyltransferases (polyST), were independently characterized by four groups (130, 242, 332, 415). Initially named STX and PST-1, the enzymes were later designated ST8Sia-II and ST8Sia-IV, respectively, according to a systematic nomenclature (495). The official gene names are ST8SIA2 and ST8SIA4 in humans and St8sia2 and St8sia4 in mice.
In mice (as well as in other mammals), the two enzymes share ∼60% identity at the amino acid level and are typical representatives of the mammalian sialyltransferase family with the conserved sialylmotifs L, S, III, and VS (105, 168) (Figure 15C). In addition, polySTs are characterized by two additional motifs termed the polysialyltransferase domain (PSTD) and the polybasic region (PBR). The PSTD motif consists of 32 amino acids and is essential to establish polysialylation capacity. The finding that arginine residues in this stretch are indispensable for enzymatic function led to the postulation that this region provides an extended binding site for the growing polySia chain and thereby confers processivity to polySTs (104, 331). The PBR motif in contrast is part of the stem regions of the enzymes, and data obtained for ST8Sia-IV revealed that amino acids located in this area make specific contacts with NCAM the major polySia acceptor protein (144, 581). NCAM in turn seems to possess a complementary binding site (488) and an additional polyST recognition site in its 5th Ig-like domain (487). The biological significance of protein-protein interactions between polySTs and NCAM is not fully understood. However, because completeness of NCAM polysialylation is of utmost importance during brain morphogenesis (see sect. V), it is tempting to speculate that these contacts favor NCAM over potential concurrent polySia acceptors like the synaptic cell adhesion molecule 1 (SynCAM 1). Of note, SynCAM 1 shows a broadly overlapping expression with NCAM during brain development, but receives polySia only in a distinct cellular subset (162).
FIGURE 15.
A: the predominant polySia acceptor is the neural cell adhesion molecule (NCAM). The three major isoforms NCAM-180, -140, and -120 share identical extracellular domain structures but vary with respect to the size of the intracellular domain. In cell culture, all isoforms are polysialylated, and polySia is selectively transferred onto two N-glycans in the 5th immunoglobulin like module (Ig5). The absence of region-specific core glycans in these positions (see text) prompted the suggestion that membrane-bound polysialyltransferases require specific spacing between acceptor glycans and their active sites (8). Later studies confirmed the model by demonstrating that the minimal polySia acceptor encompasses the tandem domains Ig5 and FnIII-1. Black triangles indicate N-glycosylation sites. B: the spacing model is also suited to explain the transfer of polySia onto selective N-glycans in other acceptors shown here SynCAM 1. Of note, both polySTs are independently able to polysialylate NCAM, while SynCAM 1 is specifically recognized by ST8Sia-II. The molecular basis of specific acceptor recognition is not known. C: schematic representation of the polysialyltransferases ST8Sia-II and ST8Sia-IV. Both polySTs consist of a short NH2-terminal cytosolic domain, a transmembrane domain (TMD), a stem region, and a COOH-terminal catalytic domain. The sialyl motifs large (L), short (S), motif III (III), and very short (VS) are conserved in all mammalian sialyltransferases (476) and are depicted as black boxes. The two polyST specific domains, termed polybasic region (PBR) and polyST specific domain (PSTD), are shown as white boxes. Black triangles indicate N-glycosylation sites. D: overview of ST8Sia-II and ST8Sia-IV expression during mouse brain development as determined by quantitative real-time RT-PCR from whole brain mRNA extracts (354, 417).
Another issue of intensive investigation is the selectivity with which polySTs transfer polySia onto specific N-glycans on their acceptors. As illustrated in Figure 15A, NCAM is a prototypic member of the immunoglobulin (Ig) family of adhesion molecules, with six N-glycan attachment sites, all modified with complex core structures (4, 267). PolySia is selectively added to N-glycans 5 and 6, both of which are in the fifth Ig-like domain (160, 528, 555). Extensive work has been carried out to determine if this selectivity is conferred by specific structural features of the N-glycan core structures at the two positions. However, the isolation of polysialylated glycopeptides from NCAM expressed in cultured cells and from natural sources (i.e., postnatal mouse and bovine brain) and their detailed structural analysis ruled out the theory that site selectivity for polySia addition is determined by region-specific N-glycan core glycosylation (160, 528, 555). The N-linked structures at these polySia addition sites included neutral, partially truncated di-, tri-, and tetra-antennary fucosylated and nonfucosylated cores as well as acidic species and the glycopeptide analysis clearly demonstrated roughly equal frequency of all identified core structures at both polySia attachment sites (528, 555). Considering that a distinct type of glycan structure is not required to prime the polyST reaction, Angata and Fukuda (8) suggested that polyST activity requires a proper spacing between acceptor glycans and the polyST active sites. Subsequent studies searched for the “minimal NCAM” that serves as polySia acceptor. Deletion and replacement studies carried out in different laboratories identified a variant encompassing the tandem domains Ig5 and FnIII-1 to be efficiently polysialylated, while no polysialylation was seen when the FnIII-1 was deleted from this construct (89, 337, 488).
For more than 20 years, the prominence of polySia on NCAM (in the nervous system >95% of polySia are linked to NCAM) obscured the fact that other proteins are also polysialylated inside and outside the nervous system (for a recent review, see Ref. 316). Initially, evidence for the existence of additional polySia acceptors in the CNS was obtained in a biochemical study carried out in synaptosomal fractions of adult rat brain. Using the polySia specific mAb735 in NCAM-depleted brain lysates, Roth and Zuber (590) obtained evidence for sodium channel α-subunit polysialylation. Although this finding has not been confirmed by other methods, it is notable that the sodium channel α-subunit in Electrophorus electricus carries polySia (215) and that mAb735 has been successfully used to enrich for this molecule. More recent approaches used perinatal brain from Ncam knockout mice, which clearly express residual amounts of polySia (95) to find additional polySia carriers. Application of high-resolution glycoproteomic approaches to these specimens identified SynCAM 1 (162). Like NCAM, SynCAM 1 is a member of the Ig superfamily with a short cytosolic domain, a single membrane span and extracellular Ig-like domains containing potential N-glycosylation sites (39) (Figure 15B). N-glycans in SynCAM 1 Ig1 are essential for its function as a synapse inducer (142). Polysialylation of SynCAM 1 under in vivo conditions fully depends on the activity of ST8Sia-II (392). As shown in a bead aggregation assay in vitro, polysialylation completely abrogates homophilic adhesion of SynCAM 1 (162), indicating that polySia serves as a regulator of SynCAM 1 interactions, as it does for NCAM (see sect. VA). Although broadly expressed in the developing rodent brain with patterns overlapping NCAM expression, only a small subfraction of SynCAM 1 is polysialylated when analyzed at postnatal day 1. Moreover, polySia-SynCAM 1 is confined to few brain areas, mainly the pontomedullary hindbrain, where it is found on so-called NG2 cells (162). NG2 cells are multifunctional progenitor cells that form unique synaptic associations with neurons through which they receive excitatory synaptic inputs (343, 361, 494). It is, however, not known if polySia impacts the functions of these cells. The transfer of polySia onto SynCAM 1 occurs selectively to the third N-glycan in the first Ig-module. As in NCAM, the next downstream module, in this case the Ig2 domain, is indispensable to make SynCAM 1 an acceptor for ST8Sia-II, suggesting an important role of this module as a docking site for the enzyme (392). Together with proper spacing between the membrane and the particular glycosylation site, this provides a reasonable basis to explain the selective transfer of polySia onto SynCAM 1 (Figure 15B).
The above example indicates that polySTs have developed individual protein substrate specificities and have the capability to recognize acceptors in a context-dependent manner (392). This general conclusion is supported by the finding that ST8Sia-IV also has a specific protein substrate (in addition to NCAM), neuropilin-2 (NRP2) (316). Although highly expressed in the developing brain in patterns widely overlapping with the expression of ST8Sia-IV, polysialylation of NRP2 has thus far only been seen in mature dendritic cells of the immune system (31, 100, 382, 383).
It remains an open question whether specializations in substrate recognition provided the basis for the evolution of two polySTs. Compelling evidence, however, exists that proper time and tissue specific NCAM polysialylation requires the concerted activity of both ST8Sia-II and ST8Sia-IV (315). Analyses carried out in isogenic mouse strains, differing only in the number of active polysialyltransferase alleles, highlighted differences in the contributions of the two enzymes. The in vivo studies corrected previous in vitro data that ST8Sia-IV was the polyST with higher activity (13, 234, 241). The animal models unequivocally showed that ST8Sia-II is the dominant enzyme during brain development. If analyzed at postnatal day 1, ∼50% of the NCAM pool remained polySia-free in St8sia2 knockout mice, while no change was detected in St8sia4 knockouts compared with the wild type, indicating compensation and a high degree of redundancy between the two enzymes in vivo. In contrast, the length of polySia chains produced by ST8Sia-IV (in St8sia2 knockouts) was identical to wild type, whereas a significant drop in the amount of long polySia chains (DP >36) was found in mice lacking ST8Sia-IV (160). These findings reveal one level of polySia chain-length control. Another plausible mechanism of polySia chain-length control may be destabilization of the catalytically active ternary complex after the polyanionic chains have reached a critical size (DP 80–100). However, direct evidence for this is not yet available.
To start building polySia chains on their glycoprotein acceptors, polySTs do not require the help of a “priming enzyme.” The sole prerequisite for the enzymes to start is the presence of appropriate core glycans with a single terminal sialic acid linked α2–3 or α2–6 to the penultimate sugar (160, 313). As shown for ST8Sia-II- and ST8Sia-IV-catalyzed NCAM polysialylation in vitro, this terminal sialic acid can be linked either α2–3 or α2–6 to the penultimate sugar (12, 313) (Figure 4). Whereas one study found that the type of linkage had no measured effect on the subsequent polysialylation step (313), a second study indicated that the α2–3-linked sialic acid may be the preferred acceptor, at least in vitro (12). The finding that polySTs build polySia on themselves has been referred to as auto-polysialylation (84, 312), which led to the hypothesis that preformed chains are transferred en bloc to acceptors. Supporting experimental evidence for this possibility, however, has not been obtained (312). PolyST auto-polysialylation occurs on specific N-glycans that are highly conserved in all α2–8-STs and is a prerequisite for enzyme function (314, 546). Much more work is needed to comprehend the catalytic mechanism of mammalian polySTs, for which crystal structures do not yet exist.
3. Regulation of polysialic acid expression
The expression of polySia on the cell surface primarily represents the balance between synthesis of polysialylated structures and their degradation by internalization and lysosomal degradation (115, 161, 417). Moreover, the chemical stability of polySia is pH dependent. Under mildly acidic conditions, accelerated fragmentation of polySia has been observed, which has been explained by an intramolecular self-cleavage mechanism and might facilitate lysosomal degradation of polySia (279). On the other hand, acidic conditions promote the formation of intramolecular lactones which are formed by condensation of the carboxyl group of one sialic acid residue with the hydroxyl group in position C9 of the adjacent residue to yield a six-membered ring (268). Lactonization reduces charge and flexibility of polySia and increases the resistance to both enzyme- and acid-catalyzed glycosidic bond cleavage (586). At neutral pH, however, almost all polySia is lactone free (25), and the role of lactone formation for chemical stability and physiological properties of polySia in vivo has not been established.
Enzymes that specifically and robustly degrade polySia at the cell surface have not been convincingly demonstrated in vertebrates. The observation that Neu1-Neu4 each has limited activity on polySia corresponds well with the long half-life of polySia in circulation (270, 530). However, extracellular functions of vertebrate sialidases are largely unexplored, which may be partly due to their instability and low expression (304). It therefore remains possible that some of these enzymes impact expression of polySia on intact cells. In this light, evidence that Neu4 may be functional in polySia degradation warrants further exploration (474).
Another way to release polySia from the cell surface is shedding of the extracellular domain of the polySia carrier protein. In fact, elevated levels of shed NCAM fragments have been detected in cerebrospinal fluid and post-mortem brain samples of schizophrenic patients (468, 519) as discussed in section VIE. Shedding of polysialylated NCAM has been confirmed in cell culture, and the responsible enzymes were identified as members of the ADAM (A Disintegrin and metalloproteinase) family of metalloproteases (205, 225). Since the shed fragment is stable and may retain biological functions, shedding by itself cannot be considered a form of polySia degradation. As shown recently, cleavage of polySia-NCAM by ADAM10 is a prerequisite for ephrinA5-induced growth cone collapse (58). In this case the loss of steric hindrance after removal of the polySia-NCAM ectodomain, and not the shed fragment itself, appears functionally important (194). A biological function for shed polySia-NCAM, per se, has yet to be established.
The major control level of polySia expression appears to be transcription and translation of the polySTs and NCAM (11, 191, 192, 250, 355, 369). The mRNAs of the three genes peak in the late embryonic and early postnatal mouse brain together with the maximal levels of polySia immunoreactivity (354, 417) (Figure 15D). Quantitative analyses in brains from mice lacking either St8Sia2 or St8sia4 indicated that the two genes are independently expressed and, as discussed above, are capable of at least partial cross-compensation (161). Remarkably, the data indicate a perinatal overcapacity of the polysialylation machinery (354). This excess may be necessary to guarantee the completeness of NCAM polysialylation during the critical phases of neural development and wiring (further discussed in sect. VB3). Despite their overlap during brain development, the mRNA levels of the polySTs show considerable differences in their tissue patterns if determined at later times (11, 192, 354, 355). Combined with the observed differences that polySTs have in terms of recognizing protein substrates (391, 392) and in their capacity to elongate polySia chains (160, 230), these data stress the advantage of having two polySTs.
Experimentally, it is worth noting that native phage-borne endosialidases (endoN) coded by bacteriophages that penetrate bacterial polysialic acid capsules are key tools for polySia removal (see below) and that enzymatically inactive forms of these enzymes, which retain polySia binding activity, are useful artificial lectins to identify and/or enrich polySia and polysialylated proteins (214).
4. Polysialylation during brain development: overview
In the mouse embryo, the first expression of the two polyST mRNAs is detected at E8.5, at the time of neural tube closure (355, 374), and polySia immunoreactivity appears half a day later at E9 (355, 374). PolySia is expressed throughout embryonic brain development and reaches peak expression levels perinatally (250, 355). Recent progress made in HPLC-based detection of polySia (160, 161, 330, 354) allowed the profiling of their patterns both quantitatively and qualitatively. During postnatal mouse brain development (354), the amounts of polySia remain high during the first postnatal week, before they rapidly decline between postnatal days 9–17 to ∼30% of the level at birth. This is followed by a further decrease to ∼10% in adulthood (>6 mo of age) (354). Perinatally, >95% of polySia is added to the isoforms NCAM-140 and -180, leading to the quantitative concealing of these proteins under a polySia shell. As these isoforms stay constantly expressed during the first three postnatal weeks, polySia reduction correlates with an increase in the appearance of polySia-free (“naked”) NCAM-140 and -180 (354). A decline of polySia and constancy of NCAM expression was also found in the prefrontal and visual cortices (34, 56, 113). Of note, a similar developmental profile has been described for the human prefrontal cortex (94).
Northern blotting (250, 355) and quantitative real-time RT-PCR (qPCR) (354, 417) were used to assess the expression of the polyST-genes during embryonic and postnatal mouse brain development. In accordance with the earlier Northern blot results, qPCR indicates a steep increase of St8sia2 and St8sia4 mRNA from E10.5 to E13.5 when plateau levels were reached and maintained until birth (417) (Figure 15D). The same method applied to the mesencephalic dopaminergic system gave identical results and in addition showed that upregulation of the two polySTs is paralleled by a comparable increase in Ncam transcript levels (417). Postnatally, downregulation of the polySTs preceded the decline of polySia; however, in this developmental window the two mRNAs showed different profiles. The St8sia2 mRNA dropped sharply between postnatal days 5–11 on whole brain level, and between postnatal days 9–12 in the visual cortex, while the decrease of St8sia4 mRNA proceeded more slowly (34, 354) (Figure 15D). The results were highly consistent with previous Northern blot and in situ hybridization studies that identified ST8Sia-IV as the prevailing polyST of the adult rodent brain (11, 192, 355). In humans, microarray data indicated an age-dependent decline of ST8SIA2 expression in the dorsolateral prefrontal cortex. This could be validated and quantified by qPCR. Between the group of neonates and infants up to the age of 1 yr and the age group of toddlers and children up to 13 yr of age, mRNA levels dropped by ∼50%. As in mice, this steep decline was followed by a gradual decrease from teenagers to adults, who retained ∼8% of the neonatal ST8SIA2 mRNA level in this cortical region (291).
5. Polysialylation at different stages of neurogenesis
During formation of the mammalian brain, neuronal and glial cells are derived from stem cells in the neuroepithelium. These stem cells give rise to radial glia, the proliferating precursors that generate neurons by asymmetric and symmetric divisions and serve as a scaffold for radial migration of newborn neurons (370). The majority of polySia-positive cells in the embryonic brain belong to the neuronal lineage (41) and most, if not all, neurons seem to be positive for polySia at some stage of differentiation (48). To lay the ground for discussing the major developmental functions of polySia in section VB, we review the occurrence of polySia during neurogenesis from the radial glia stage to migration of polySia-positive precursors to axon growth and synaptogenesis.
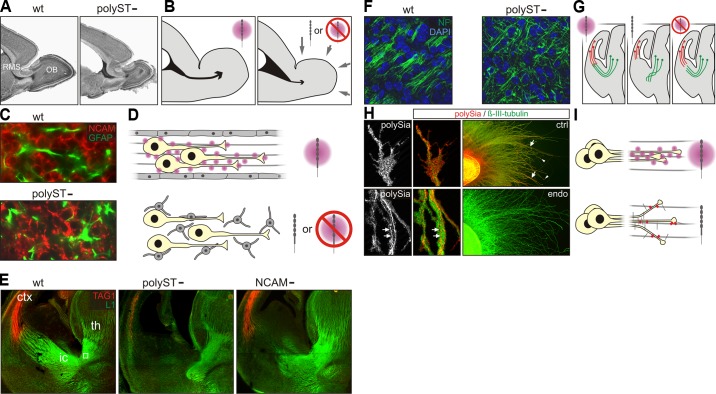
PolySia has been detected on radial glia of the developing cortex and mesencephalon (264, 265, 417). Likewise, polySia is found on Bergmann and Müller glia, the radial glia of cerebellum and retina, respectively (27, 189, 251). The pattern is consistent with a role of polySia in radial migration as suggested by Angata et al. (9), who observed aberrantly localized pyramidal cells in the neocortex of polyST-negative mice. At the next step of neurogenesis, polySia is associated with long-distance cell migration of various types of neuroblasts. The most prominent example is the migration of olfactory interneuron precursors, which are generated throughout life from dividing astrocytic precursors in the anterior subventricular zone of the lateral ventricle (SVZ) and migrate tangentially in a process called chain migration to the olfactory bulb, where they differentiate into granular and periglomerular interneurons (51, 122, 123, 394) (Figure 16). The neurogenic niche of the adult SVZ is derived from the embryonic lateral ganglionic eminence and a chain-like pattern of polySia-positive neuroblasts, the hallmark of the postnatal rostral migratory stream (RMS), has also been described in the rat embryonic forebrain (366). However, tangential migration in the embryonic and early postnatal RMS differs from chain migration in the mature brain. In the adult, the polySia-positive chains move through longitudinally oriented glial tubes, which develop from astrocytic networks in the third postnatal week (367). Thus the chain-like migration pattern of polySia-positive neuroblasts at earlier stages is independent from the glial scaffold, and this is consistent with in vitro data showing that chain migration of SVZ explants from 5-day-old mice, i.e., the sliding of neuroblasts along each other, occurs in the absence of glial guidance (542). As further discussed in section VB, the efficiency of this migration process, but not chain migration per se, depends on the presence of polySia. In contradiction to the prevailing view of polySia as just a steric inhibitor of cell-cell interactions, loss of polySia delays the formation and causes reduced compaction or even a dispersal of isolated migratory chains in vitro (76, 203).
FIGURE 16.
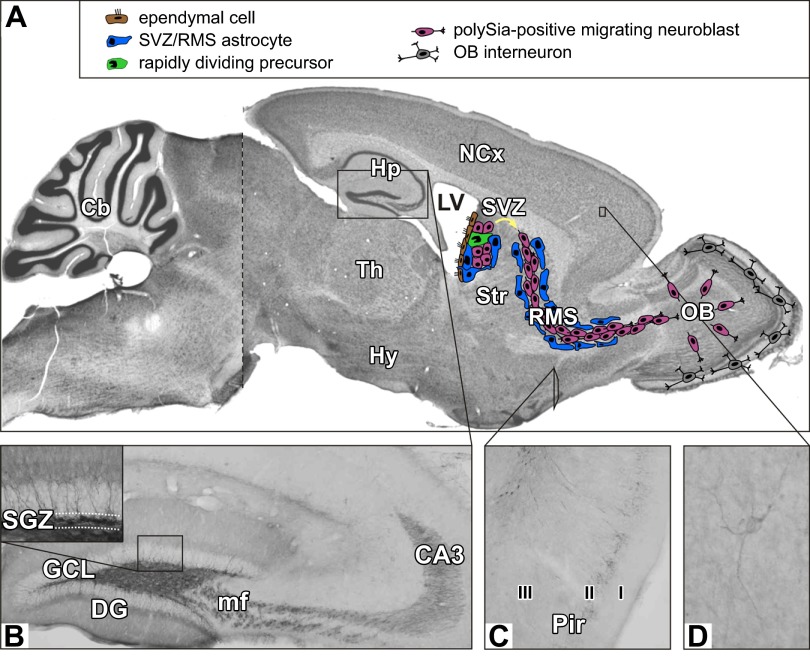
Hotspots of polySia immunoreactivity in the adult mouse brain. A: Nissl-stained sagittal section with a schematic representation of the neurogenic system of the anterior subventricular zone (SVZ) of the lateral ventricle (LV, lined by ependymal cells) and the rostral migratory stream (RMS) producing new interneurons of the olfactory bulb (OB). The neurogenic niche consists of slowly dividing astrocytes giving rise to rapidly dividing precursors, which in turn generate the migratory, polySia-positive neuroblasts. [Based on Doetsch et al. (121–123).] B: polySia expression on mossy fibers (mf) projecting from the granule cell layer (GCL) towards the CA3 region of the hippocampus (Hp) and in the subgranular zone (SGZ, inset), the neurogenic niche of the dentate gyrus (DG). C: pattern of polySia immunoreactivity in the piriform cortex (Pir; layer I-III). D: example of a polySia-positive interneuron in the prefrontal part of the neocortex (NCx). [B and C from Natcher et al. (327).] To facilitate orientation the approximate positions are indicated in A, but note that the micrographs shown in B and C were obtained from coronal sections. Cb, cerebellum; Hy, hypothalamus; Str, striatum; TH, thalamus.
Based on the knowledge that polySia is a marker of adult neurogenesis in the rodent SVZ, immunostaining for polySia has been used to identify the presence of the SVZ/RMS system in rabbits (274, 373), non-human primates (165, 246, 365, 410), and humans (36, 101, 379, 404, 405, 540). While the organization of the SVZ-RMS in adult rabbits and monkeys seems largely comparable to rodents, results on the human brain differ. First reports described clusters or chains of polySia-positive cells in the SVZ of adult humans (36, 540). Work by the Alvarez-Buylla group also identified polySia-positive cells in the SVZ, but these were not arranged in chains (379, 405). A later study then made the heavily disputed claim that significant numbers of cells generated in the SVZ of adult humans migrate towards the olfactory bulb in a rostral migratory stream (101). More recently, an RMS with massive chain migration was found in infants, but in contrast to the situation in rodents, only a subfraction of the migrating neuroblasts seems to express polySia (404). In newborns, a high number of polySia-positive immature neurons was detected in the RMS at the level of the olfactory tract, which declines rapidly, and in a 7-yr-old child, chains of migrating cells were no longer observed (404). In specimens obtained from 4- to 6-mo-old humans, polySia immunoreactivity was additionally detected in clusters of immature neurons forming a previously unknown medial migratory stream that deviates from the RMS and moves towards the ventromedial prefrontal cortex (404).
Further types of migrating polySia-positive neuroblasts comprise precursors of cortical interneurons (111, 437, 439), which originate from the embryonic medial ganglionic eminence and migrate tangentially into the pallium (283). During their tangential migration, cortical interneurons are not in close contact with each other and move independent of interactions with glia through the polySia-positive environment of the developing cortex (111, 264, 283, 284).
One of the most intricate patterns of long distance migration through the brain is performed by the gonadotropin-releasing hormone (GnRH; also known as luteinizing hormone-releasing hormone, LHRH) neurons originating from the olfactory placode, migrating along the olfactory or vomeronasal nerve to the olfactory bulb and from there into the developing forebrain (430, 547). Migration of GnRH neurons during embryonic states is closely related to the presence of polySia (148). In chickens (321, 323), rodents (571), and humans (429), the GnRH neurons migrate along polySia-positive fascicles of the embryonic vomeronasal nerve (olfactory nerve in chicken) into the olfactory bulb, but it remains a matter of debate whether the migrating cells themselves are polySia positive or if migration is assisted by polySia-positive olfactory ensheathing cells. These latter cells represent a unique glial cell type associated with the olfactory nerve and are known to express polySia (148). In mouse (571) and chicken (322), injection of the polySia degrading enzyme endoN inhibits migration of GnRH neurons. After polySia removal by endoN, only half of the cells crossed the cribriform plate and entered the olfactory bulb in the mouse model. Most interestingly, genetic deletion of NCAM has no such effect (571), alluding to the existence of an alternative polySia carrier in these cells.
Prominent polySia expression is associated with neurogenesis of granule cells in the dentate gyrus of the adult hippocampus (438, 440) (Figure 16B). Similar to the SVZ-RMS, polySia-positive neuroblasts are generated from astrocytic stem cells in this second major neurogenic niche of the adult brain (121, 229). The newly generated cells of the subgranular zone arrange in clusters from which they migrate tangentially before extending neurites and dispersing into the granule cell layer (436, 441). Early, tangentially oriented processes of immature granule cells stay in touch with the proliferating clusters and provide a polySia-positive microenvironment which is believed to support the neurogenic niche (436). Adult neurogenesis in the dentate gyrus has been observed across mammalian species including non-human primates and humans (134, 175, 238, 246), and polySia provides a useful, though not definitive, marker for assessing changes in hippocampal neurogenesis associated with, e.g., exercise (453) or pathological conditions (96, 219, 539). As judged by expression of polySia and other markers, the pattern of dentate gyrus neurogenesis in humans (238) is comparable to the situation in rodents (435, 444) and undergoes similar qualitative and quantitative age-related changes.
In the process of neurogenesis, polySia is associated with neurite outgrowth, axon pathfinding, and fasciculation. First described in chickens, polySia was shown to be present on axons and growth cones of retina ganglion cells and motoneurons (124, 255, 478, 479). In rodents, developing axons of Cajal Retzius cells, and axon tracts including the optic nerve, corticospinal tract, and thalamocortical projections display strong polySia immunoreactivity (27, 83, 103, 416, 437).
Finally, polySia has been implicated in positive and negative regulation of synaptogenesis (113, 116, 117, 442). How these still controversial functions are realized is a matter of intensive research and needs a much better understanding of the time course and spatial localization of polySia during synapse formation. An immunoelectron microscopic study of the topology of polySia in rat striatum during postnatal development demonstrated considerable dynamics. Intense polySia labeling of the entire synapse was followed by progressive restriction of polySia to the pre- and postsynaptic membranes and subsequent loss of polySia (496). In contrast, a study on the formation of oculomotor synapses in the chick embryo indicated that polySia is absent from the actual sites of synaptic contact formation, although it is expressed on ciliary neurons (64). Similarly, polySia was shown to be absent from mossy fiber synapses, although the presynaptic axonal membrane was immunoreactive (428). Some mature synapses, however, such as a subset of spine synapses in the outer molecular layer of the dentate gyrus, are polySia positive (428). During synaptogenesis of cultured hippocampal neurons, NCAM accumulates rapidly at sites of contact (472). Although these cells are known to express polySia (117, 126), the membrane distribution of polySia has not been addressed in this system.
6. Polysialic acid on immature and mature neurons of the adult brain
Apart from the neurogenic niches of the adult brain, three types of neurons display prominent polySia immunoreactivity. The first population of polySia-positive cells is found mainly in layer II of the paleocortex. These cells coexpress doublecortin, a marker of immature neurons and are mostly devoid of neuronal nuclear antigen (NeuN), a marker for mature nerve cells (171, 273, 325, 503). In rodents, these immature neurons are particularly abundant in the piriform cortex (Figure 16C), but in other mammals, including non-human primates, the same cell type has also been detected in the neocortex (273, 503). Although expressing immature features in the adult brain, the polySia-positive layer II neurons are born prenatally (171, 273) and their numbers decline significantly with age (2, 505). Thus the increase of polySia-positive cells after administration of an N-methyl-d-aspartate (NMDA) receptor antagonist suggests an activity-dependent modulation of glycosylation in existing neurons (325). Furthermore, a potential for maturation in the context of olfactory processing is implicated by the increase of differentiated, NeuN-expressing neurons at the expense of polySia- and doublecortin-positive cells in the piriform cortex of bulbectomized rats (173).
Second, mature (NeuN-positive) polySia-positive neurons are present in different cortical areas, including the prefrontal cortex (504, 507) (Figure 16D), piriform cortex (325), and hippocampus (326) as well as in the septum (143) and amygdala (166, 328). These cells are inhibitory interneurons characterized by the presence of the GABAergic marker glutamic acid decarboxylase. Depending on the area under consideration, they show divergent expression of markers indicative of different interneuron subtypes, such as calbindin, somatostatin, parvalbumin or the vasoactive intestinal peptide (143, 172). PolySia-positive interneurons receive less synaptic input than their polySia-negative counterparts and show reduced dendritic arborization and spine numbers. These morphological features are consistent with the notion that polySia is a negative regulator of interneuron connectivity and may support plasticity of inhibitory cortical networks (172). Corroborating this hypothesis a recent report showing a transient increase in dendritic spines of interneurons after removal of polySia (178).
Third, some differentiated neurons of the mature brain are characterized by polySia-negative somata while displaying polySia on their neurites. Hippocampal mossy fibers are unmyelinated axons of dentate gyrus granule cells and most, if not all, mossy fibers show intense polySia staining, but their somata in the granule cell layer are polySia negative (439) (Figure 16B). Similarly, polySia immunoreactivity is detected on axons and dendrites of pyramidal cells in the hippocampal CA1 region (32, 349), whereas the cell layer itself is polySia negative (50).
As reviewed in great detail elsewhere (48, 485), wide areas of the adult brain retain a diffuse pattern of polySia staining, which as yet has not been assigned to defined cell populations. In the hypothalamo-neurohypophysial system, some of the diffuse polySia immunoreactivity is associated with neurons of hypothalamic magnocellular nuclei, but fine perineuronal processes of astrocytes are the major source of polySia (201, 307, 348, 454, 484, 486). Remodeling of glia and synapses during physiological regulation of neurohormone release is associated with changes of polySia patterns, and enzymatic removal of polySia prevents the rearrangement of synapses and astrocytic processes indicating that polySia is a prerequisite for these changes (201, 307, 484).
7. Polysialic acid on nonneuronal brain cells
In addition to the astrocytes of the hypothalamus, polySia is expressed on other astrocytic cells of the adult brain. Among these are the pituicytes of the neurohypophysis (232) and the radial glia-like tanycytes in the ependymal layer of the third ventricular wall, which send processes into the mediobasal hypothalamus (47). Furthermore, polySia is found on reactive astrocytes, activated in response to various insults in brain and spinal cord (21, 49, 69, 256, 329, 345).
Most recently, a subpopulation of NG2 cells, the pluripotent early stage of the oligodendrocyte lineage, was shown to be positive for polySia and SynCAM 1 in the perinatal brain (162). While the further characterization of polySia expressing NG2 cells is pending, it is known that oligodendrocyte precursor cells are polySia positive during their migratory phase. However, in this stage polySia is linked to the NCAM isoforms NCAM-180 and -140. During maturation into myelinating oligodendrocytes polySia as well as NCAM-140 and -180 are downregulated (27, 329, 493, 535). Inversely, NCAM-120 is upregulated in mature myelinating oligodendrocytes (38). In contrast to in vitro data (147) NCAM-120 is not polysialylated during myelination in vivo (354). Questions remain open as to whether the switch in the NCAM isoform expression or the downregulation of polySTs causes the disappearance of polySia during oligodendrocyte maturation, and the functional consequences of these changes.
IV. BRAIN GANGLIOSIDE FUNCTIONS
A. Genetic Models of Ganglioside Expression
Because gangliosides are major molecular determinants at the nerve cell surface and their structures undergo major changes during brain development, gangliosides were hypothesized to be regulators of intracellular interactions in the brain. Genetic models in mice address this hypothesis and provide a nuanced and more accurate understanding of brain ganglioside functions (216, 375, 421).
Several genetically engineered mouse lines targeting ganglioside biosynthetic genes have been created and studied (Table 2). These fall into two major categories, mutations that block all ganglioside biosynthesis (Ugcg-null, B4galnt1/St3gal5-double null) and those that block within the ganglioside biosynthetic pathway but retain ganglioside expression (St3gal5, St8sia1, B4galnt1, St3gal2, St3gal3). In the latter set of mutants, gangliosides build up behind the block (see Figure 11) such that the total ganglioside density in the adult mouse brain remains essentially unchanged. Excellent examples of this phenomenon are found in B4galnt1-null, St8sia1-null, and B4galnt1/St8sia1-double null mice (227) (Figure 17). Wild-type mouse brains express GM1, GD1a, GD1b, and GT1b. Mice with disrupted GD3 synthesis (St8sia1-null) lack b-series gangliosides and express commensurately increased a-series gangliosides (GM1 and GD1a). Mice lacking the enzyme that extends simple gangliosides by addition of GalNAc (B4galnt1-null) express commensurately increased amounts of GM3 and GD3. Finally, mice with both genes disrupted are biosynthetically “stuck” at GM3, which is overexpressed to levels comparable to total brain ganglioside concentration in wild-type mice. The phenotypes of these mutant mice reflect specifically on the functions of gangliosides, since these glycosyltransferases are glycosphingolipid-specific.
Table 2.
Ganglioside expression in genetically engineered mice
| Gene(s) D | Major Brain Gangliosides | Major Nervous System Phenotype(s) | Reference Nos. |
|---|---|---|---|
| None (wild type) | GM1, GD1a, GD1b, GT1b | Normal | |
| B4galnt1 | GM3, GD3 (“complex ganglioside knockout”) | Axon degeneration, dysmyelination, progressive motor behavioral deficits, hyperactivity, seizure susceptibility | 78, 358, 447, 475, 552 |
| St8sia1 | GM1, GD1a | Mostly normal (selective reduction in axon regeneration) | 227, 353 |
| St3gal2/St3gal3 | GM1, GD1b | Short lived, early dysreflexia | 467 |
| St3gal5 | GM1b, GD1α | Mostly normal | 560 |
| B4galnt1/St8sia1 | GM3 (“GM3 only”) | Motor and sensory deficits, audiogenic seizure | 227, 473 |
| B4galnt1/St3gal5 | None (“ganglioside knockout”) | Short lived, severe early neurodegeneration, perturbed axon-myelin interactions | 563 |
| Ugcg | No brain development | Death at gastrulation | 562 |
| Ugcg in neurons (Nes-Cre) | Uniform ≥75% decrease | Short lived, ataxic, progressive dysreflexia and motor deficits, dysmyelination, loss of Purkinje neurons | 559 |
| Ugcg in oligodendrocytes (Cnp-Cre) | Normal | Normal | 400 |
| Ugcg in Purkinje neurons (L7-Cre) | Not quantitatively determined | Axon degeneration, dysmyelination, progressive loss of Purkinje neurons | 538 |
FIGURE 17.
Brain ganglioside patterns from wild-type and ganglioside mutant mice. Brain gangliosides were extracted, and an amount equivalent to 10 mg brain fresh wt was resolved by thin-layer chromatography. Glycolipids were detected colorimetrically. Migration positions of standard brain gangliosides are indicated on the sides of the panel. [Adapted from Kawai et al. (227), with permission from American Society for Biochemistry and Molecular Biology.]
1. Complex ganglioside knockout mice
The developmental switch from simple (GM3, GD3) to complex gangliosides (GM1, GD1a, GD1b, GT1b) late in fetal brain development (Figure 12) starts with the addition of GalNAc to the simple gangliosides GM3 and GD3 to synthesize GM2 and GD2, respectively (see Figure 11). A single glycolipid-specific N-acetylgalactosaminyltransferase (GM2/GD2 synthase), coded by the B4galnt1 gene, is responsible. B4galnt1-null mice express GM3 and GD3 as major brain gangliosides throughout life, never making the switch to complex gangliosides (271, 475). On that basis, B4galnt1-null mice can be considered “complex ganglioside knockout” mice. Given the observations that all adult mammals and birds express the same four major complex brain ganglioside structures, that gangliosides are among the most abundant cell surface molecular determinants in the brain, and that ganglioside expression undergoes a remarkable simple-to-complex conversion during brain development, it was notable that the first published study of B4galnt1-null mice reported only subtle phenotypic defects (475). Mutant pups were born at expected frequencies and developed grossly normal nervous system anatomy and behavior, at least into young adulthood. Fine-structural analyses indicate that mice lacking complex gangliosides have normal nerve cell morphology including dendritic length, complexity, and spine density (119). These data demonstrate that complex gangliosides are not required for basic nerve cell differentiation, migration, or synapse formation. Nevertheless, B4galnt1-null mice display distinctive nervous system deficits that support a role for complex gangliosides in axon-myelin interactions and neuronal excitability.
The earliest deficit noted in B4galnt1-null mice (at 10 wk of age) was a modest decrease in nerve conduction velocity in somatosensory evoked potentials measured from the tibial nerve near the Achilles tendon to the somatosensory cortex, the longest distance measured (475). Subsequent studies provided a mechanistic basis for this deficit. Complex gangliosides are required for optimal myelin formation, axon-myelin interactions, and peripheral and central axon stability (276, 358, 447, 469). In young adult mice, dysmyelination was responsible for reduced long-distance nerve conduction velocity, whereas in older mice more serious phenotypic deficits were noted (see below).
Myelin, elaborated by oligodendrocytes in the CNS and Schwann cells in the peripheral nervous system (PNS), wraps long segments of axons in a multilayered membrane sheath interrupted only by well-sealed and exquisitely organized periodic gaps, the nodes of Ranvier (334). Myelin insulates axons and directs action potentials to nodes of Ranvier, allowing rapid (saltatory) nerve conduction and greatly enhancing the speed and density of information processing in the brain. Although myelin function was once thought to be limited to insulation and conduction velocity, it is now appreciated that axon-myelin interactions are required to establish and maintain appropriate axon cytoarchitecture and to support long- and short-term axon stability and integrity. Myelin nurtures the axons it enwraps, and when axon-myelin interactions are compromised, axons suffer (492). Functional interactions between axons and myelin are choreographed by complementary cell surface recognition molecules on the innermost myelin wrap and the apposing axon surface. Complex gangliosides act as receptors in axon-myelin interactions, and mice with altered gangliosides display phenotypes consistent with this function.
Although normal myelin is abundant in complex ganglioside knockout mice, they display progressive central and peripheral axon degeneration characteristic of a failure of myelin support of axons (Figure 18) and also display uncharacteristically large unmyelinated fibers in the CNS, indicative of a myelination defect (276, 447). Axons in complex ganglioside knockout mice have decreased intra-axonal neurofilament spacing, leading to significantly reduced axon calibers (358, 447) that are consistent with a failure in axon-myelin interactions (202). Defects at nodes of Ranvier (Figure 19) provide additional evidence of a failure in axon-myelin interactions (469). In contrast to the uniform regularly spaced paranodal loops of myelin with well-defined transverse bands found in wild-type mice, B4galnt1-null mice display enlarged and disorganized loops, some of which fail to form axo-glial junctions. A significant number of nodes also display disorganization of the well-defined molecular expression of adhesion molecules and ion channels at or near the nodes, changes consistent with reduced nerve conduction velocities.
FIGURE 18.
Axon degeneration in complex ganglioside knockout (B4galnt1-null) mice. Top: pathological features in PNS (sciatic nerve). Low-power electron microscopic image showing axon degeneration with collapsed myelin (red asterisks) and a thinly myelinated fiber surrounded by supernumerary Schwann cell process (red arrow). Normal myelin is denoted with a blue asterisk for comparison. Scale bar = 2.5 μm. Bottom: pathological features in the CNS (optic nerve). Electron microscopic image showing axonal degeneration and myelin collapse (red arrow), and a large unmyelinated axon (red asterisk). Normal myelin is denoted with a blue asterisk for comparison. Scale bar = 200 nm. [Adapted from Sheikh et al. (447).]
FIGURE 19.
Node of Ranvier defects in complex ganglioside knockout (B4galnt1-null) mice. A and B: electron micrographs of 12-wk-old paranodal loops from wild-type (A) and B4galnt1-null (B) mouse optic nerves. Some paranodal loops face away from the axon in the mutant mice (arrowheads). Scale bars = 0.5 μm. C and D: immunostained molecules at nodes of Ranvier from wild-type (C) and B4galnt1-null (D) mouse ventral roots. Sodium channel immunostaining (Nav, red) delineates the node, Caspr (green) delineates the paranode, and potassium channels (Kv1.2, green) delineate the juxtaparanode. In mutant mice, potassium channels invade the paranode, extending toward the node (arrowheads), and there is abnormal protrusion of sodium channels (arrow). [Adapted from Susuki et al. (469), with permission from John Wiley and Sons, Inc.]
As complex ganglioside knockout mice age, progressive neuropathy results in motor behavioral deficits, including defective hindlimb reflexes, coordination, and gait (78, 358). By 1 year of age, the mice uniformly display severe loss of hindlimb function, along with other behavioral changes that are less readily ascribed to axonal loss or changes in conduction velocity. Notably, the mice display whole-body tremor and significant hyperactivity, despite their motor deficits (358).
2. a-Series only, 0-series only, GM3 only, and total ganglioside knockout mice
Mice lacking other ganglioside biosynthetic enzymes reveal additional details about the functions of complex gangliosides in the nervous system. St8sia1-null mice, which lack all b-series gangliosides but overexpress a-series gangliosides (Table 2), are nearly normal in that they do not display the axon-myelin deficits of B4galnt1-null mice (227, 353). Mice with a disrupted St3gal5 gene (lacking GM3 synthase; see Figure 11) were expected to lack all ganglio-series brain gangliosides. Surprisingly, these mice instead expressed high concentrations of the normally rare 0-series gangliosides GM1b and GD1α (563, 572) and displayed none of the axon-myelin deficits of complex ganglioside knockout mice (although they were deaf due to loss of the organ of Corti; Ref. 572). Taken together, these studies imply that complex brain gangliosides are required for axon-myelin interactions and long-term axon stability and that complex 0-series and a-series gangliosides alone are equally efficient in supporting axon-myelin interactions as are the normal complement of a- and b-series gangliosides. An additional test of this hypothesis came with evaluation of double null mice in this pathway. B4galnt1/St8sia1-double null mice (so called “GM3 only” mice; Table 2) lack GD3 as well as all complex brain gangliosides. Like B4galnt1-null mice, they display nerve degeneration and progressive motor behavioral and sensory deficits (211, 473). Notably, GM3-only mice also were found to suffer a seizure disorder, rendering them susceptible to lethal audiogenic seizures (227). B4galnt1/St3gal5-double null mice were particularly instructive (563). As expected from the biosynthetic block (Figure 11), these mice lacked all ganglio-series gangliosides and had increased LacCer expression. What little sialylated glycosphingolipid was found in the brain had a different neutral glycan core than normal brain gangliosides. These “ganglioside null” mice were born with an intact nervous system, consistent with the conclusion that gangliosides are not required for neuronal development or gross brain morphogenesis. However, they were short-lived, had small brains, vacuolization of the spinal and cerebellar white matter, marked degeneration of myelinated axons, malformed nodes of Ranvier, and severe hindlimb weakness. Their phenotype indicates defects in axon-myelin interactions, along with other nervous system deficits, that are similar but more severe than those in complex ganglioside (B4galnt1-null) knockout mice.
3. Glucosylceramide knockout mice
Deletion of the single gene responsible for glucosylceramide synthesis (Figure 11), Ugcg, results in interruption of embryonic development during gastrulation (562). Therefore, conditional Ugcg mutants have been used to test the roles of GlcCer-based glycosphingolipids (primarily gangliosides) in the nervous system. Ugcg deficiency driven by the nestin (neural) promoter resulted in nearly complete loss of gangliosides in the nervous system (but not other major tissues) in one model (217), and a 75% decrease in brain ganglioside expression in a second model (559). Mice lacking brain gangliosides were born and appeared normal, but within days displayed severe ataxia and died within the month. Affected mice displayed deficient hindlimb reflexes and lack of coordination. Their peripheral nerves displayed axon degeneration and dysmyelination, and central neurons showed excessive axonal pruning, although the length of the remaining axons was maintained. Mice with a 75% decrease in total brain gangliosides had a progressive neuropathy with dysreflexia by 3 mo of age, gait disorders, and progressive loss of Purkinje neurons. A more subtle conditional mutation was created by knockout of Ugcg selectively in mature Purkinje neurons using Cre under the control of the L7 (Pcp2) promoter (538). These mice showed progressive Purkinje cell loss, but more notably this was preceded by axon degeneration in the absence of dendritic changes. This was accompanied by abnormalities at nodes of Ranvier, including disrupted paranodal loops and altered distributions of nodal ion channels and adhesion molecules. In comparison, knockout of Ugcg in myelinating cells (using Cre driven by a Cnp promoter) did not result in notable changes in brain ganglioside expression, axonal/myelin histology, or phenotype (400). Together, these data are consistent with the interpretation that gangliosides are not required for nerve cell differentiation or nervous system anatomic development, but their expression on nerve cells is required to maintain healthy intracellular interactions essential to nervous system stability and function, especially axon-myelin interactions.
4. GD1a/GT1b knockout mice
As described above, mice lacking complex gangliosides, or neurons lacking all gangliosides, display varying degrees and onset of axon-myelin dysregulation, among other nervous system disorders. In contrast, mice expressing only a-series or 0-series gangliosides do not display such severe phenotypes, indicating that different complex gangliosides might compensate for each other to support axon-myelin stabilization. One test of this hypothesis was to block the sialyltransferase(s) that add(s) the terminal α2–3 sialic acids to GM1 (GD1a synthase) and GD1b (GT1b synthase). Experiments using single- and double-null mice revealed the sialyltransferases involved (467). Of the six α2–3 sialyltransferases in mice (and humans), four (St3gal1 through St3gal4) can synthesize GD1a/GT1b from GM1/GD1b in vitro. In the study of each of these knockout mouse strains, all of which are viable, only St3gal2-null mice had altered brain ganglioside expression. They expressed half the amount of brain GD1a and GT1b with GM1 and GD1b building up commensurately behind the partial block. St3gal2-null mice had no evident nervous system deficits. Testing of double-null mice revealed that the St3gal3 gene product was compensating for the absence of St3gal2, in that GD1a or GT1b were nearly absent in St3gal2/3-double null mice, with GM1 and GD1b proportionally increased. Notably, these mice were short-lived and displayed early dysreflexia. Although supportive of a role for GD1a and GT1b, per se, in nervous system stability, the St3gal2/3-double-null mice also had reduced total glycoprotein sialylation, complicating the molecular interpretation of their phenotype. Nevertheless, they support the essential functions of α2–3 sialic acid-terminated glycans, which include GD1a and GT1b, in the nervous system.
B. Molecular Mechanisms of Ganglioside Function
Membrane gangliosides at the cell surface function in two distinct modes, cis and trans (182). In the cis mode, gangliosides associate laterally with other membrane molecules, including receptors and ion channels, to modulate their activities. In the trans mode, ganglioside glycans, which extend into the extracellular milieu, interact with complementary glycan binding proteins to mediate recognition, including cell-cell recognition. Separately, these cis and trans modes of ganglioside molecular interactions are regulators of cell recognition and cell signaling; together they can translate cell surface recognition into changes in cell physiology.
1. Cis-regulation
Gangliosides associate laterally in the plane of the external leaflet of the plasma membrane with each other and with selected lipids and proteins in structures referred to as lipid rafts (458). Lipid rafts are thought to be small (nanometer scale) highly dynamic associations with a variety of lipid and protein substructures (448). Ganglioside-containing lipid rafts are enriched in other raft lipids such as sphingomyelin, cholesterol, and phosphatidylinositols; in lipid-modified proteins such as glycophosphatidylinositol-linked surface proteins and lipid-modified signaling molecules (such as those of the Src family); and in selected transmembrane proteins. Either in or out of rafts, gangliosides may associate laterally with signaling molecules at the cell surface, regulate their lateral distribution and associations with other molecules, and regulate their signaling capacity (489). In this way, the expression and distribution of gangliosides provides an additional layer of control in cell surface signaling. An example of this is ganglioside-mediated regulation of receptor tyrosine kinases (RTKs).
Ganglioside GM3, a common ganglioside in many cell types outside of the nervous system, associates laterally with the EGF receptor (55) and with the insulin receptor (344) to downregulate their protein tyrosine kinase activities in response to agonist binding. Evidence for the physiological significance of ganglioside-RTK regulation comes from St3gal5-null mice, which lack GM3 and its downstream metabolites (560). These mice displayed enhanced insulin sensitivity characterized by increased insulin-induced insulin receptor phosphorylation in skeletal muscle. Remarkably, St3gal5-null mice were protected from high-fat diet-induced insulin resistance, suggesting that GM3 plays an important role in metabolic control via this specific RTK association. A model for GM3 regulation of differential insulin receptor lateral distribution provides a framework for understanding its control of insulin sensitivity. Insulin receptors associate with caveolin-1, an interaction required for their responsiveness (85), and separately with ganglioside GM3. Immunoprecipitation, colocalization, and fluorescence recovery after photobleaching studies support a model in which these interactions are functionally competitive (223). In the absence of GM3, insulin receptors associate with caveolin-1 and are more insulin sensitive. When GM3 is increased, insulin receptors associate selectively with GM3, are released from their interaction with caveolin-1, and are more insulin resistant.
A CNS correlate to the above model is the enhancement of high-affinity nerve growth factor receptor (TrkA) activity by GM1 (125). Immunoprecipitates of TrkA contained GM1, and GM1 addition activated TrkA's protein tyrosine kinase activity (324). A multireceptor system linking the neurite-outgrowth activating activity of laminin-1 to GM1 and TrkA was recently described (208). Treatment of dorsal root ganglion neurons with laminin clustered GM1, TrkA, and β1-integrin, presumably via direct binding of laminin to GM1. Knockdown of individual components via siRNA suggested a model in which GM1 facilitates the association of laminin, β1-integrin, TrkA, and the intracellular Src family member Lyn to activate neurite outgrowth (Figure 20). Although mice lacking GM1 and other complex gangliosides (B4galnt1-null mice) elaborate a well-functioning nervous system, evidence for loss of regenerative capacity in selected nerve pathways in these mice may relate to cis-regulation of neurotrophic factor receptors by gangliosides (235).
FIGURE 20.
Model for laminin-1-induced clustering of GM-1, TrkA, β1 integrin, and signaling molecules in lipid rafts to stimulate neurite outgrowth. Laminin-1 directly binds to GM1 and induces its focal aggregation, enhancing the relocation of TrkA in lipid rafts and the subsequent activation of signaling molecules downstream of TrkA, such as Lyn. Clustering of GM1 with laminin-1, along with laminin-1 self-assembly, also promotes relocation and enrichment of β1 integrin in lipid rafts and enhances combined laminin-integrin signaling to trigger neurite outgrowth. [From Ichikawa et al. (208), with permission from The Company of Biologists, Ltd.]
2. Gangliosides as receptors for neurotropic bacterial toxins
Brain gangliosides were first identified as receptor molecules in the context of bacterial toxins (500). The family of potent protein neurotoxins secreted by certain clostridial strains includes several types of botulinum neurotoxins and the structurally related tetanus neurotoxin (471). Each toxin is comprised of a heavy chain that binds to the neuronal cell surface and an enzymatic light chain that is inserted into the cytoplasm, proteolyzes key vesicle fusion proteins (SNARE proteins), and thereby disrupts synaptic transmission. Given the neuromuscular symptoms of tetanus, brain tissue extracts were tested for their ability to bind and remove the toxin from solution. The “toxin-fixing” component was extracted from brain tissue using ethanol and was present primarily in brain gray matter. Its identification as ganglioside was confirmed by van Heyningen using brain gangliosides purified by leaders in the early ganglioside field, E. Klenk of Cologne and J. Folch-Pi of Harvard (500). Subsequently, van Heyningen and Miller (501) purified and fractionated gangliosides from bovine brain and found a major brain ganglioside species with a ratio of sialic acid to hexosamine of 3:1 (now known to be GT1b) to be particularly potent at “fixing” tetanus toxin. Subsequent studies demonstrated that tetanus toxin binding to brain synaptosomal membranes was most potently reversed by GT1b followed by GD1b, GD1a, and GM1, which was 100-fold less potent than GT1b (388). Subsequent studies indicate that all of the clostridial neurotoxins bind to gangliosides with differing specificities that depend on multiple potential binding sites near the COOH terminus of the heavy (binding) subunit (35, 471). Crystallography reveals that the clostridial toxins evolved patches of amino acid residues that form a network of hydrogen bonds and hydrophobic stacking interactions that specify ganglioside glycan binding (461) (Figure 21). Proof of the role of gangliosides in clostridial toxicity comes from studies using complex ganglioside knockout (B4galnt1-null) mice. Despite retaining simple gangliosides, these mice display reduced sensitivity to tetanus and botulinum toxins (233); the potency of the clostridial toxins in inhibiting neuromuscular activity is reduced 100-fold in the absence of complex gangliosides (65, 396).
FIGURE 21.
Crystal structure of botulinum neurotoxin type A in complex with the cell surface co-receptor GT1b. Top: close-up of the GT1b binding site. GT1b represented as sticks with yellow carbons. The GT1b coordinating residues are shown as sticks with gray carbons. Bottom: schematic picture of GT1b and its hydrogen bonds to botulinum neurotoxin type A. The hydrogen bonds between the protein (blue) and GT1b (black) are shown as dotted red lines and the GT1b internal hydrogen bonds as dotted black lines. Distances of key hydrogen bonds are displayed in Ångstroms. The α2,8-linked sialic acid (Sia7) that is disordered in the complex is shaded gray. Numbered monosaccharide names are shown; Glc, glucose; Gal, galactose; GalNAc, N-acetylgalactosamine; Sia, sialic acid. [Adapted from Stenmark et al. (461).]
In addition to the neurotropic toxins, gangliosides are receptors for other multi-subunit protein toxins. Cholera toxin and E. coli enterotoxin LT-I bind selectively to GM1, whereas E. coli enterotoxin LT-IIb binds to GD1a (93, 158). As with the clostridial neurotoxins, each toxin is multi-subunit, with its binding subunit mediating toxin binding to gangliosides at the cell surface and an enzymatic subunit then entering the cytoplasm. These toxins target the intestinal epithelium and dysregulate cAMP signaling, resulting in severe diarrhea. As with the clostridial toxins, ganglioside-binding enterotoxins have evolved protein binding sites that provide a network of hydrogen bond and hydrophobic stacking interactions engineered to specifically bind ganglioside glycans (200).
3. Gangliosides as receptors in axon-myelin interactions
The evolution of bacterial toxins that specifically bind to brain gangliosides suggested that endogenous vertebrate lectins may have evolved to do the same. A search for ganglioside binding proteins in the brain led to myelin-associated glycoprotein (MAG), and to an enhanced understanding of ganglioside functions in axon-myelin interactions (228, 423).
MAG is a single-pass transmembrane protein that is selectively expressed by myelinating cells: oligodendrocytes in the CNS and Schwann cells in the PNS (377). In the CNS, MAG is found only on the innermost (periaxonal) wrap of myelin, directly apposed to the axon surface. In the PNS it is also found on periaxonal myelin, as well as on Schmidt-Lanterman incisures, lateral loops, and the inner and outer mesaxons (491). Based on its expression, MAG was long proposed to be involved in axon-myelin interactions. Although gene deletion of MAG in mice did not result in severe demyelination as some had expected (263, 308), the study of Mag-null mice reveals important roles for MAG in axon-myelin interactions relating to axon cytoarchitecture, axon stability, and axon regeneration. Mag-null mice myelinate axons throughout the nervous system, and most myelin in Mag-null mice appears grossly normal. However, myelination is delayed, and there is a higher proportion of myelin structural abnormalities (155, 368). Mag-null mice also have increased numbers of poorly formed nodes of Ranvier and disruption of the spatial patterns of nodal ion channels and adhesion molecules (280, 368). Normally, myelination induces local increases in neurofilament phosphorylation in ensheathed axons, resulting in increased axon diameter and increased conduction velocity (202). Mag-null mice display reduced neurofilament phosphorylation and spacing and reduced axon diameter, even in myelinated axons (568). Myelination also nurtures axons; dysmyelination results in axon degeneration (335). MAG is part of this nurturing effect in that Mag-null mice display axon degeneration in both central and peripheral nerves, resulting in progressive axonal loss over their lifetimes (339). Notably, MAG also protects axons from short-term toxic insults; Mag-null mice are more sensitive to axonopathic neurotoxins, and soluble forms of MAG can protect axons from neurotoxic insults (339). In addition to its broad role in supporting healthy long- and short-term axon-myelin interactions, MAG also inhibits axon regeneration (293, 317). MAG is one of a suite of molecules, including those on residual myelin, that limit recovery from traumatic nervous system injury by signaling axons to halt outgrowth (258, 407, 570). Although genetic deletion of these factors has, thus far, failed to generate broad and robust regeneration after CNS injury, Mag-null mice display increased axon sprouting after injury in some studies (257, 411). From long-term axon-myelin stabilization to inhibition of regeneration, all of the physiological roles of MAG have, at least in part, been linked to its recognition of brain gangliosides (422).
MAG was a founding member of the siglec family of sialic acid binding proteins (97, 228), with an NH2-terminal (outermost) V-type Ig domain that has distinctive structural elements shared among the 14-member human siglec family (514). Different siglecs have evolved to take advantage of the various linkages and subterminal structures of sialoglycans to provide a high degree of binding specificity (278). Initial specificity studies discovered that MAG bound preferentially to the trisaccharide “NeuAc α2–3 Gal β1–3 GalNAc” (228), the shared terminal sequence of gangliosides GD1a and GT1b (see Figure 8). Direct testing of gangliosides for their ability to support MAG binding confirmed GD1a/GT1b specificity among the major brain gangliosides (Figure 22) and revealed enhanced binding to the rare α-series gangliosides (90–92, 565). These data provide a context to consider the relationship between the physiological functions of MAG and those of complex gangliosides, particularly GD1a and GT1b.
FIGURE 22.
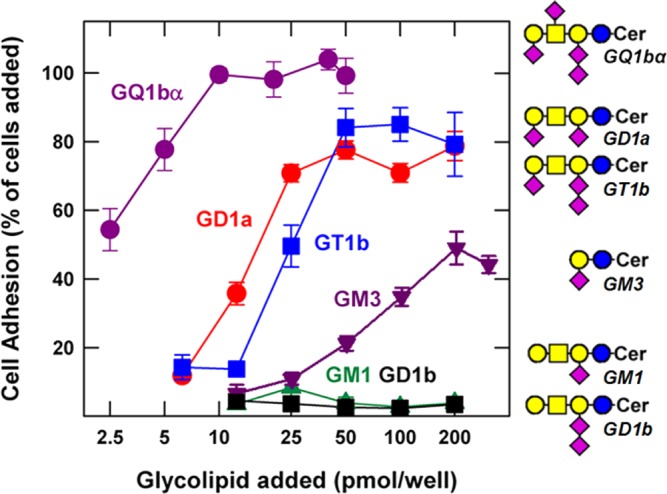
MAG binding to gangliosides: structural specificity (91, 565). COS cells (fibroblasts) transfected to express full-length MAG were placed in microwells adsorbed with the indicated concentrations of the indicated gangliosides. MAG-mediated cell adhesion is expressed as a percent of the MAG-transfected cells added to each well. Ganglioside structures are shown schematically using the key in Figure 11. [Adapted from Collins et al. (91), with permission from American Society for Biochemistry and Molecular Biology, and Yang et al. (565).]
The phenotypes of mutant mice with altered ganglioside expression, as described above, are consistent with a role for GD1a and GT1b in the functions of MAG (422). Mice lacking complex gangliosides (B4galnt1-null) display many of the same phenotypic traits as Mag-null mice, including myelin anomalies, progressive axon degeneration in the central and peripheral nervous systems, reduced neurofilament spacing, reduced axon diameters of myelinated axons, and disrupted nodes of Ranvier (358, 447, 469). Behaviorally, Mag-null and B4galnt1-null mice display similar disruptions in hindlimb reflexes and coordination, as well as similar patterns of whole-body tremor and hyperactivity. Double-null mice (B4galnt1/Mag) have similar defects as each single null strain (358). In contrast to the B4galnt1-null mice, St8sia1-null mice do not display altered axon-myelin interactions, nor do St3sia5-null mice. Since these mouse strains express high amounts of GD1a (St8sia1) and GD1α (St3sia5), respectively, and both of these gangliosides are high-affinity MAG receptors (409), the lack of axon-myelin deficits is consistent with a phenotype based on MAG-ganglioside recognition. B4galnt1/St8sia1 double null (GM3 only) and B4galnt1/St3gal5 double null (ganglioside knockout) mice are consistent with this hypothesis, in that they lack high-affinity MAG receptor gangliosides and display significant defects in axon-myelin interactions.
In addition to a role in stabilizing long-term axon-myelin interactions, complex gangliosides mediate the ability of MAG to protect axons from short-term toxic insults (339). For example, addition of soluble expressed forms of MAG to neurons in culture protects them from the effects of vincristine, an axonopathic neurotoxin. A mutant form of MAG lacking the shared arginine in siglecs required for sialic acid binding fails to protect neurons, and neurons from complex ganglioside knockout mice (B4galnt1-null) are not protected by wild-type MAG. Treatment of nerve cells with sialidase or with an inhibitor of the ganglioside biosynthetic pathway reverses the ability of MAG to protect neurons (298). Together, the data imply that, among other potential ganglioside functions, the major brain gangliosides GD1a and GT1b are essential to healthy axon-myelin interactions due to their actions as receptors for MAG. However, the stabilizing effects of MAG are not always beneficial, in that MAG also inhibits axon regeneration.
4. Gangliosides as regulators of neuronal survival and axon regeneration
It has long been observed that exogenous addition of gangliosides to nerve cells in culture results in neurite sprouting and outgrowth (67, 389), and enhances survival in the face of specific toxic insults (137). The mechanisms of these well-established observations have been elusive and range from specific interactions with growth factor receptors such as TrkA (208, 324), to modification of calcium flux (107, 553), to generalized and structurally nonspecific effects on the membrane milieu (457). Regardless of the mechanism(s), the results were sufficiently consistent to warrant human trials of injectable GM1 in a variety of human diseases including spinal cord injury (163), Parkinson's disease (424), and stroke (70). The results have been variable, with effects on recovery from spinal cord injury and stroke limited, and those in Parkinson's disease more encouraging. Animal models of ganglioside deficiency display some Parkinson-like symptoms that are alleviated by administration of l-DOPA or cell-permeable ganglioside mimetics (550), and gangliosides may be reduced in Parkinson's disease patients (551). Rare reports of sporadic treatment-related anti-ganglioside immune responses resulting in Guillain-Barré syndrome have raised concerns (209). With ganglioside delivery involving intravenous and/or intramuscular delivery, the pharmacokinetics and pharmacodynamics of gangliosides as drugs, and their potential to enhance outcomes in human disease, are a matter for further investigation.
MAG inhibition of axon regeneration (see above) is also mediated, at least in part, by complex gangliosides. MAG has several receptors on axons that act cooperatively or independently to generate intracellular signals that halt axon outgrowth (71, 423). When studied in vitro, MAG inhibition of axon outgrowth from some nerve cell types (but not others) was reversed by cleaving the terminal sialic acids from gangliosides with sialidase, by inhibiting ganglioside biosynthesis, by exogenously added GT1b and/or GD1a, or by sialoglycan inhibitors of MAG binding (297, 523, 526, 533, 534). MAG inhibition of axon outgrowth from cerebellar granule neurons was found to be completely ganglioside dependent, from hippocampal neurons was partially ganglioside dependent, and from dorsal root ganglion neurons and retinal ganglion cells was ganglioside independent, emphasizing the diverse pathways MAG uses in this context (297, 523). Ganglioside-mediated MAG inhibition of axon outgrowth, like other modes of axon inhibition, is dependent on activation of the small intracellular GTPase RhoA. The data are consistent with the hypothesis that MAG binding to gangliosides initiates a signaling pathway that results in RhoA activation, at least in some types of nerve cells. This hypothesis is supported by the observation that clustering GD1a or GT1b with anti-ganglioside antibodies mimics MAG-mediated axon inhibition in a RhoA-dependent manner (526, 534, 543), a mechanism that may have implications in autoimmune neuropathologies involving anti-ganglioside antibodies as described in a subsequent section of this review (446, 582).
Since gangliosides are on the outer leaflet of the axon plasma membrane, recruitment of other membrane molecules is likely to be involved in MAG signaling. Although such transducing molecule(s) have yet to be fully established, the precedent of cis-regulation by gangliosides is instructive. One cis-interaction that may influence MAG/ganglioside-mediated axon inhibition is the interaction of gangliosides with another MAG receptor on axons, NgR1 (543). However, in other experiments ganglioside-mediated and NgR-mediated MAG inhibition are clearly independent (297, 523). Gangliosides have also been reported to interact with a transmembrane inhibitory coreceptor, p75NTR (157, 561), although axon outgrowth inhibition from some neurons is insensitive to loss of p75 (and the related TROY), indicating other ganglioside-related transducers must be present (523). Together, the data support a role for gangliosides as receptors for MAG on some nerve cell types, transducing signals that inhibit axon outgrowth after injury (423). The extent to which regeneration of any particular nerve pathway is sialoglycan dependent requires direct testing.
Treatment of cultured nerve cells with sialidases, which modify sialoglycans including gangliosides, modulates neurite/axon outgrowth either positively or negatively depending on the neurons and sialidase (102, 474, 549). A role for endogenous sialoglycans in restricting axon regeneration in vivo was demonstrated by testing sialidase as a treatment to enhance axon outgrowth after CNS injury in animal models (310, 311, 564). Delivery of bacterial sialidases to the site of spinal cord injuries enhanced axon regeneration as well as behavioral and physiological outcomes. In one model of brachial plexus injury, nerve roots were cut flush to the spinal cord in rats, a peripheral nerve graft was inserted into the cord, and sialidase pumped to the injury site using an implanted osmotic pump (564). Motoneuron outgrowth into the grafts was enhanced 2.5-fold by sialidase treatment. In another injury model, rats were subjected to a thoracic spinal cord contusion by blunt impact (310, 311). Delivery of sialidase to the injury site by osmotic pump increased axon sprouting distal to the lesion, and enhanced recovery of hindlimb motor functions, coordination, and spinal-mediated physiological function. Consistent with the well-established specificity of bacterial sialidases, the treatments (primarily with exogenous V. cholerae sialidase) resulted in conversion of complex gangliosides, including GD1a and GT1b, to GM1 (414). The data are consistent with gangliosides being mechanistic targets of therapeutic sialidase, either by loss of inhibitory gangliosides (GD1a and GT1b), generation of stimulatory ganglioside GM1, or both. Since sialidases act on sialoglycoproteins as well as gangliosides, roles for other sialoglycans are not ruled out. Regardless of the underlying mechanism(s), the ability of sialidase to enhance outcomes from nerve injury identifies nervous system sialoglycans as important factors controlling axon regeneration.
V. POLYSIALIC ACID FUNCTIONS
A. Polysialic Acid as Modulator of Cell Interactions
After the initial in vitro finding that polysialylation decreases NCAM binding (99, 401), the prevailing “steric hindrance model” was shaped mainly by work in the Rutishauser laboratory (397, 399). According to this model, depicted in Figure 23A, polySia affects not only specific functions of its carrier proteins but generally increases the intercellular space (566, 567) and abrogates adhesive binding of other cell surface molecules such as cadherins and integrins (156, 220, 221). Although consistent with some of the biological functions ascribed to polySia, the model is not sufficient to explain many of the defects identified in animals with genetically induced polySia deficiency, and falls short of explaining the results of other cellular and molecular studies. Additional models of polySia function are summarized in Figure 23, B–H, and are discussed below.
FIGURE 23.
PolySia and cell interactions. A: polySia as a negative regulator of cell-cell apposition. Loss of polySia allows for trans-interactions of NCAM and other cell surface proteins. B and C: loss of polySia induces heterophilic trans-interactions and activates the protein kinase ERK1/2 via FGF receptor signaling (B), or promotes focal adhesion at the cell-substrate interface by interactions with a yet unknown heterophilic binding partner and signaling to the focal adhesion kinase (FAK) (C). D: polySia may interfere with cis-interactions of NCAM and signaling complex formation. E and F: loss of polySia enables interactions of NCAM with proteoglycans of the ECM (E), or polySia-NCAM itself interacts with proteoglycans of the ECM (F). G: polySia as a scavenger of soluble factors facilitating receptor activation. H: polySia modulates activation of ionotropic glutamate receptors. See text for details.
In a series of in vitro studies, polySia was shown to control NCAM signaling (132, 191, 386, 433, 434). EndoN-mediated removal of polySia from human neuroblastoma cells initiated NCAM interactions at cell-cell contacts and was followed by reduced cell proliferation and a parallel activation of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) (Figure 23B). Activated ERK1/2, in turn, promoted survival and neuronal differentiation with enhanced formation of neuritelike processes (191, 433, 434). These same responses were obtained in a coculture system of NCAM-negative neuroblasts exposed to polySia-negative, NCAM-positive membranes, demonstrating that the polySia-free NCAM acts as ligand of a heterophilic interaction partner (434). Similarly, neuronal precursors isolated from the subventricular zone of early postnatal mice responded with enhanced differentiation to the removal of polySia or to exposure with polySia-negative NCAM. A higher degree of differentiation was obtained by adding polySia-negative NCAM to precursors from Ncam knockout mice, demonstrating that this NCAM function is also mediated via heterophilic binding (386).
NCAM-induced signaling is believed to involve its association with fibroblast growth factor (FGF) receptors, causing receptor activation and downstream signaling through the mitogen-activated protein kinase ERK1/2 pathway (72, 146, 197, 245, 341, 402). Most data are compatible with the idea that homophilic trans-interactions of NCAM are a prerequisite for the association with the FGF receptor in the plane of the membrane (in cis). However, one study demonstrates FGF receptor-mediated signaling induced by application of soluble NCAM to NCAM-negative cells (146). Consistent with this mode of heterophilic NCAM binding, the activation of ERK1/2 in response to a loss of polySia could be linked to FGF receptor activity (132) (Figure 23B). The activation could be reproduced by the stimulation of NCAM-negative cells with a NCAM-mimetic peptide representing the binding site of the FGF receptor (231, 336) and by contacting these cells with soluble NCAM-forms containing at least one of the two FGF receptor binding sites localized in the FnIII-1 and FnIII-2 domains (see Figure 15) (82, 231).
Notably, other cellular responses evoked by the removal of polySia occur independent of FGF receptor activation (132). The loss of polySia causes an increase in the number of focal adhesions and promotes association of the Src family kinase p59Fyn with the focal adhesion scaffolding protein paxillin and the focal adhesion kinase (FAK). Again, this effect is mimicked by soluble forms of NCAM applied to NCAM-negative cells, but cannot be induced with the FNIII domains or the mimetic peptide. Instead, a fragment comprising the third and fourth Ig-like domain of NCAM is necessary and sufficient to enhance focal adhesion without a detectable activation of ERK1/2. Because polySia and NCAM are enriched at sites of cell-cell contact and NCAM is never detected in association with the focal adhesions, these data imply that loss of polySia triggers an FGF receptor-independent mechanism by which NCAM-induced signaling at cell-cell contacts leads to enhanced cell-matrix interactions by focal adhesions (132) (Figure 23C).
NCAM-dependent signaling via cis interactions with the FGF receptor may occur in a cell autonomous way. In β-cells from pancreatic tumors, NCAM is essential for the assembly of a signaling complex including N-cadherin and FGF receptor 4, and activation of the FGF receptor in this complex promotes integrin-mediated matrix adhesion (72). As for other possible cis interactions of NCAM, the role of polySia has not yet been considered in this model. However, β-cell tumors are known to express polySia (252), and it is tempting to speculate that its presence affects the NCAM-dependent signaling complex (Figure 23D).
Last in this series of indirect polySia functions is the modulation of NCAM binding to components of the extracellular matrix (ECM) (Figure 23, E and F). “Naked” NCAM binds with high affinity (dissociation constants ∼0.1 nM) to the prominent chondroitin sulfate proteoglycans neurocan and phosphacan (153, 177, 303), two prominent constituents of the brain ECM (118), and it can be assumed that the presence of polySia inhibits this interaction. In sharp contrast, NCAM binding to heparan sulfate proteoglycans (87, 88) is promoted by polySia (464). Together with cellular assays showing that polySia-driven synapse formation is abolished by heparinase treatment (117), the data indicate that polySia itself or combined interactions of polySia and NCAM may bind to the heparan sulfate proteoglycans in the ECM or at the cell surface.
It is not clear how NCAM-expressing cells regulate the establishment of homo- versus heterophilic NCAM binding. Apparently, both types of interactions coexist and are integrated on the cellular level (433). Possibly, as shown for the high-affinity interactions with proteoglycans (177), heterophilic interactions outcompete homophilic NCAM-NCAM binding.
In the above examples, polySia acts to block interactions. In contrast, models of direct polySia function are shown in Figure 23, F–H. Among these, the best studied are interactions of polySia with soluble ligands. Interactions with the brain-derived growth factor BDNF were initially proposed based on the observation that sensitivity towards the brain-derived growth factor BDNF requires polySia in two models: 1) long-term potentiation of synaptic transmission in hippocampal slice cultures and 2) survival and differentiation of cultured cortical neurons (318, 531). Increased BDNF responsiveness may be explained by polySia capturing the neurotrophic factor from the extracellular milieu, enhancing its local concentration, increasing its availability, and facilitating its interactions with BDNF receptors (Figure 23G). In cholinergic neurons of the septum, however, polySia limits the binding of BDNF and reduces BDNF-induced activation of acetylcholine synthesis, suggesting that polySia has a shielding function, which hampers interactions between receptor and ligand (66). Similar to the responsiveness of cortical neurons, hypothalamic neurons were more sensitive to ciliary neurotrophic factor in the presence of polySia (532), and oligodendrocyte precursor cells need polySia to sense a gradient of platelet-derived growth factor (583). Indeed, evidence for direct interactions of polySia with the neurotrophins BDNF, NT3, and NT4 as well as with FGF2 and the neurotransmitter dopamine was obtained by biochemical approaches using electrophoretic and chromatographic methods (213, 226, 357).
Finally, polySia may interact directly with cell surface proteins (Figure 23H). Of high interest in this field, soluble polySia as well as soluble NCAM-fragment carrying polySia were capable of potentiating the opening of AMPA-type glutamate receptors and inhibiting activation of GluN2B-containing NMDA receptors (184, 497). Consistent with the latter, two recent studies provide evidence that polySia regulates synaptic plasticity by setting a balance in signaling via extrasynaptic GluN2B and synaptic GluN2A receptors (239, 240). In addition, polySia binds directly to human Siglec-11 expressed by human brain microglia (537). Siglec-11-mediated interaction with polySia reduced inflammatory and neurotoxic responses in an experimental system of ectopic human Siglec-11 expression in mouse microglia (537).
B. Lessons From Mouse Genetic Models
The first polySia-deficient mouse models were obtained by complete inactivation of Ncam, deleting all isoforms (95), or by ablation of exon 19 to generate a NCAM-180 knockout (490). In fact, the resulting NCAM-deficient mice were almost completely negative for polySia, but in marked contrast to the predicted central roles of NCAM and polySia in neurodevelopment and plasticity of the mature nervous system, Ncam knockouts showed a surprisingly mild phenotype, appeared healthy and fertile, and had an overall fairly normal brain architecture. Although these data indicated that the complex and energetically “expensive” polysialylation machinery had evolved primarily to produce polySia on NCAM, grossly normal brain development of Ncam knockout mice was paradoxically taken as evidence that polySia has no vital function. Only later, with the identification of the polySTs, were mouse models generated that allowed the biological functions of NCAM and its polySia modification to be addressed separately. As detailed below, these mouse models impressively document the importance of a strictly controlled NCAM homeostasis and open up exciting new vistas towards understanding the relevance of protein glycosylation for the complex process of building the vertebrate brain.
1. St8sia4 and St8sia2 single knockout mice
St8sia4 knockout mice were the first polyST-deficient animal model to be created (129). Consistent with the potential of ST8Sia-II to almost entirely compensate for loss of ST8Sia-IV during development (see sect. IIIB3), St8sia4 knockouts had no overt neuroanatomical defects. However, based on its developmental expression pattern, St8sia4 knockout mice had markedly reduced polySia levels in selected parts of the postnatal and adult brain (129, 327). Hippocampal mossy fibers were devoid of polySia by immunohistochemistry, but their lamination was normal (129). Conversely, St8sia2 knockouts expressed normal polySia levels on mature mossy fibers but, similar to Ncam knockouts, axons showed guidance errors and formed ectopic synapses in the hippocampus (10). Taken together, these data show that malformations in the mossy fiber tract originate from a lack of polySia during development. Since both polySTs are colocalized in the developing dentate gyrus (192), the absence of ST8Sia-II is likely to cause only a partial reduction of polySia (161, 354), which is, however, sufficient to damage mossy fiber formation as severely as the loss of NCAM.
Additional evidence for the importance of polySia homeostasis during mossy fiber tract formation was obtained in a hippocampal slice culture model (527). When slices derived from wild-type brains were treated with N-propanoyl-d-mannosamine (ManNProp), a chemically modified sialic acid precursor, polysialylation of NCAM was reduced, resulting in aberrant mossy fiber projections and formation of ectopic synapses similar to those seen in the Ncam and St8sia2 knockouts.
In young rats St8sia2 mRNA was localized to polySia-positive cells in the subgranular zone of the dentate gyrus (192). Consistent with this, St8sia2 knockouts possess a diminished number of polySia-positive cells in this neurogenic niche (10). The frequency of mitotic neural progenitor cells was not altered (10), but many of the immature granule neurons showed aberrant location and altered morphology, suggesting a role of ST8Sia-II in their differentiation (327). In St8sia2-null (−/−) mice, a reduction of polySia-positive cells was also observed in the posterior part of the SVZ (10). In both polyST single knockout lines, however, the levels of polySia on the neuroblasts of the anterior SVZ and RMS were unaffected (10, 129, 327).
2. St8sia2, St8sia4 double-knockout mice
The simultaneous deletion of both polySTs generates mice that are polySia-negative, but retain normal levels of NCAM isoform expression (9, 541). Since Ncam−/− mice are almost completely devoid of polySia, it is not surprising that their two major neurodevelopmental defects, delamination of mossy fibers and the migration deficit of RMS neuroblasts with smaller olfactory bulbs (Figure 24, A and B), are recapitulated in St8sia2, St8sia4 double-knockout mice (2−/−4−/−) (9, 541). This is in good agreement with previous findings showing that both defects can also be induced by endosialidase treatment removing the sugar polymer and leaving the NCAM protein backbone unaltered (356, 442). The phenotype of 2−/−4−/− mice, therefore, corroborates that the loss of polySia causes these defects independent of the presence or absence of NCAM (Figure 24B). The migration deficit of neuroblasts is linked to marked changes of cellular arrangement within the RMS (Figure 24, C and D). As discussed in section IIIB5, a reduced compaction or even dispersal of the migratory chains is induced by removal of polySia in vitro (76, 203). In addition, in Ncam knockouts and after endosialidase injection, the glial tubes, which serve as a scaffold for migratory neuroblast chains, disintegrate and a massive astrogliosis develops (28, 76). As illustrated in Figure 24C, similar changes are also evident in the RMS of 2−/−4−/− mice (H. Hildebrandt, unpublished observations). In addition to reduced homotypic contacts between the migratory neuroblasts, ultrastructural analyses in Ncam knockouts revealed the formation of zona-adherens-like heterotypic contacts of precursors with astrocytes and an accumulation of axons in the RMS (76). Thus altered neuroblast migration in the absence of polySia or NCAM involves direct perturbations of cell-cell interactions as well as an extensive rearrangement of cytoarchitecture.
FIGURE 24.
Two categories of neurodevelopmental defects in polySia-negative mice. A–D: shared features of 2−/−4−/− and Ncam knockout (N−/−) mice are caused by loss of polySia. A and B: migration deficits of olfactory interneuron precursors in the rostral migratory stream (RMS) and smaller olfactory bulbs (OB) in polysialyltransferase-negative (polyST−) 2−/−4−/− mice and polySia-NCAM-negative Ncam knockout mice. Wt, wild type. C: altered cytoarchitecture of the RMS in polyST− mice. The ordered arrangement of GFAP-positive astrocyte tunnels around migratory chains of NCAM-positive neuroblasts as observed in the wild-type (wt) is severely disturbed (H. Hildebrandt, unpublished data). D: schematic representation of the situation in the presence or absence of polySia, based on the work of Chazal et al. (76). E–I: defects that manifest exclusively in 2−/−4−/− and are absent from Ncam knockout and 2−/−4−/− N−/− triple-knockout mice are caused by a gain of polySia-free NCAM. E–G: misrouting of thalamocortical (green) and corticofugal fibers (red) in polyST- but not in 2−/−4−/− N−/− triple-knockout mice (NCAM−). Only in the polyST− situation, thalamocortical axons fail to pass through the internal capsule (ic) at embryonic day 14.5 (E and G) and fibers in the ventral thalamus (boxed in E) are reduced and highly disorganized at postnatal day 1 (F; high magnification views of the ventral thalamus at postnatal day 1 corresponding to the site boxed in E). Immunofluorescence with TAG1-, L1-, and neurofilament-specific antibodies and nuclear counterstain with DAPI as indicated. Th, thalamus; ctx, cortex. H: polySia on growth cones (arrowheads) and at the interface between fasciculated axons (arrows) in explants of embryonic mammillary body grown on laminin. Endosialidase treatment (endo) causes defasciculation (bottom right). Immunofluorescence with polySia- and β-III-tubulin-specific antibodies as indicated (H. Hildebrandt, unpublished data). I: schematic representation of the situation in the presence or absence of polySia on NCAM. [Micrographs in A, E, and F from Schiff et al. (416) and Weinhold et al. (541), with permission from American Society for Biochemistry and Molecular Biology.]
In marked contrast to single knockout mice (Ncam−/−, St8sia2−/−, or St8sia4−/−), double-knockout 2−/−4−/− mice have severe developmental deficits. Although born at Mendelian ratio and without overt morphological defects, 2−/−4−/− mice fail to thrive and more than 80% die within 4 wk after birth (541). With regard to brain development, many, but not all, of the 2−/−4−/− mice develop a progressive, noncommunicating form of hydrocephalus associated with cortical thinning, corpus callosum hypoplasia, and deformation of the hippocampal formation and fimbria (541). This was confirmed in an independent study, which in addition described defects of cortical organization associated with apoptotic cell death and reduced neuron numbers in 1-mo-old mice (9). At least some of the changes in the postnatal cortex are associated with ventricular dilatation and therefore may be secondary to a developing hydrocephalus. Another cause of cortical derangement may be altered migration of neural precursors observed during embryonic development in 2−/−4−/− mice (9), but lineage identity of these cells and underlying mechanisms await further analyses.
Prominent among the neurodevelopmental defects of 2−/− 4−/− mice are malformations of a specific set of commissural and noncommissural brain axon tracts. Most pronounced is the complete absence of the anterior commissure, which connects the olfactory nuclei and lateral parts of the temporal lobe. The anterior and posterior limbs of the commissure rarely approach and never cross the midline. Tracing of the anterior limb disclosed fasciculation defects and early deviation of fibers from their normal trajectory. These deficits were never observed in Ncam-null mice but occurred with 100% penetrance in the 2−/−4−/− double-knockout, including 2−/−4−/− mice that escape from hydrocephalus and have normal-sized ventricles. It is therefore safe to say that these axonal defects develop independent from ventricular dilatation (541).
Other fiber tract deficits involve the internal capsule and, as detailed below, are at least partially caused by defects of thalamocortical and corticothalamic pathfinding (195, 416, 541). In addition, 2−/−4−/− mice show a marked hypoplasia of the corticospinal tract at the level of the medullary hindbrain but normal midline crossing of the remaining fibers in the pyramidal decussation. The rostrocaudal length of the corpus callosum is reduced with a smaller splenium and a loss of fibers crossing the midline in the posterior part. Moreover, the diameter of the mammillothalamic tract is reduced (195, 541). This tract projects from the mammillary body of the hypothalamus to nuclei of the dorsal thalamus. It closes a circuit involving the thalamus, cortex, hippocampus, and mammillary body (Papez' circuit) and is essential for spatial working memory in the mouse (380). Importantly, other ipsilaterally projecting and commissural tracts such as the lateral olfactory tract, the fasciculus retroflexus, the optic tract, and the posterior commissure are regularly shaped and properly positioned. Thus, contrasting with the widespread expression of polySia, the wiring defects are specific and it remains open why only a selected set of axon tracts is affected by the loss of polysialylation.
3. Polysialic acid as the key regulator of NCAM
The differences in the phenotype of 2−/−4−/− and Ncam knockout mice may be caused by loss of NCAM polysialylation, i.e., the untimely appearance of polySia-negative NCAM. Another possibility is the lack of polySia on other proteins, which is largely neglected but has received new impetus from recent discoveries of alternative polySia carriers with potential neurodevelopmental impact (316). In addition, the absence of polySia synthesis may affect the cells' sialic acid metabolism causing changes in other sialoglycoconjugates with unforeseen consequences. To address these uncertainties, it was of paramount importance to determine whether the specific phenotype of the polySia-negative but NCAM-positive 2−/−4−/− mice could be rescued in triple-knockout mice with additional ablation of NCAM (2−/−4−/−N−/−) (541). The 2−/−4−/−N−/− triple-knockout mice recapitulate the phenotype of the Ncam knockout with normal viability and body weight development, a slight and constant enlargement of the lateral ventricles, delaminated mossy fibers, expanded SVZ-RMS and smaller olfactory bulbs (541). In contrast, the anterior commissure, internal capsule, corpus callosum, and mammillothalamic tract are completely normal in the mature brain of 2−/−4−/−N−/− mice and the severe hypoplasia of the corticospinal tract is reverted to a milder deficit as seen in the Ncam knockout mouse brain (390). This provides strong evidence that a gain of polySia-free NCAM causes developmental deficits in 2−/−4−/− mice.
A developmental study was performed to explore the cause of the internal capsule deficit (416). As the major gateway of fibers to and from the cerebral cortex, the internal capsule consists of a large collection of different fiber bundles. These include corticofugal fibers, such as corticospinal axons, which pass into the cerebral peduncle, fibers projecting to nuclei of the extrapyramidal system, as well as corticothalamic and thalamocortical axons. During normal embryogenesis, these two fiber systems grow towards each other and intermingle to form the reciprocal connections between cortex and thalamus (Figure 24, E–G). In the wild-type embryo, thalamocortical axons cross the primordium of the reticular thalamic nucleus in the ventral thalamus in a highly ordered fashion, before making a sharp turn to enter into the internal capsule. In the polyST-negative 2−/−4−/− embryos, the fibers are present but highly disorganized, and they fail to turn into the internal capsule (Figure 24, E and F). In 2−/−4−/−N−/− mice, the additional deletion of NCAM restored the normal growth pattern and, despite a minor developmental delay, the thalamocortical axons turn correctly into the internal capsule giving rise to a completely normal-sized fiber structure in the postnatal brain. Although less prominent, the growth of corticofugal axons is also affected, and deficiencies of corticothalamic connections contribute to the hypoplasia of the internal capsule in polysialylation-deficient mice (416) (TAG1 immunoreactive fibers in Figure 24E).
Taken together, the aberrant growth patterns of polySia-deficient, NCAM-positive axons in 2−/−4−/− mice are associated with altered fasciculation and caused by the untimely appearance of polySia-negative NCAM. The impact of polySia on axon outgrowth and fasciculation is a long-standing observation. In the motor plexus of the chicken hindlimb, endosialidase, but also treatment with NCAM antibodies, prevents correct nerve branching by increasing axon-axon contacts (254, 255, 479). In contrast, in spinal cord explant cultures and in the chicken optic tract in vivo, endosialidase treatment causes defasciculation (3, 569). Both of these phenomena have been explained by an altered balance between axon-axon and axon-environment interactions, based on the model of polySia as an unspecific steric inhibitor (398, 399) (Figure 23A). For the case of altered axon tract development in 2−/−4−/− mice, it is also reasonable to assume that the pathfinding defects involve altered interactions between growing axons and their environment. In this context, however, these interactions must be specifically caused by the gain of polySia-free NCAM.
Similar to prior studies with chick spinal cord (3), mammillary body explants of embryonic mice grow out fascicles when cultured on laminin. They show high levels of polySia immunoreactivity on their axons and growth cones, and defasciculate upon removal of polySia by endosialidase treatment (Figure 24H). Remarkably, polySia is highly enriched between the fasciculated fibers, i.e., at axon-axon rather than axon-matrix contacts. Thus, reminiscent of the cellular model described above (see Figure 23C), loss of polySia may result in NCAM signaling at axon-axon contacts that increases adhesion to the laminin matrix in preference to axon-axon interactions. In support of this model, it is of note that focal adhesion kinase-dependent point contacts regulate growth cone motility (385). It is currently not clear whether this model, based on in vitro data, applies to gain of NCAM function causing altered axon tract development in vivo. As indicated in the schematic representation (Figure 24I), NCAM signaling as well as direct, high-affinity interactions of polySia-negative NCAM with ECM compounds (see sect. VA and Figure 23E) may contribute to the defects of axon fasciculation and guidance observed in the 2−/−4−/− embryo.
Deficits of axon connectivity may cause secondary effects due to reduced synaptic supply or innervation of inappropriate targets. As an example, the striking degeneration of the reticular thalamic nucleus (Rt) in 2−/−4−/− mice seems to be caused by defects in thalamocortical and corticothalamic axon development (416). Rt neurons develop in normal numbers before dying by apoptosis in a narrow time-window right after birth, which, under healthy conditions, matches the onset of glutamatergic innervation by thalamocortical and corticothalamic fibers. Apoptotic death of Rt neurons could also be induced by lesioning corticothalamic fibers on whole brain slice cultures, suggesting that the degeneration of the Rt in 2−/−4−/− mice is a consequence of defective afferent innervation.
To substantiate the assumption that the malformation of brain axon tracts are a direct consequence of the untimely appearance of polySia-negative NCAM, the available Ncam, St8sia2, and St8sia4 knockout mouse models were used to breed mice with different levels of polySia-negative NCAM during brain development (195). Nine allelic combinations with different levels of polySia, NCAM, and polySia-free NCAM at postnatal day one were selected for morphometric evaluation at the age of 4 wk. As shown in (Figure 25) for the example of the internal capsule, the degree of the axon tract defects correlates precisely with the amounts of polySia-free NCAM and not with the overall polySia or NCAM level at postnatal day one (195). Similar results were obtained for the other axon tracts, anterior commissure, internal capsule, and corpus callosum, for which morphological deficits have been identified in polyST-negative mice. This strengthens the view that concealing NCAM is the key regulatory mechanism that makes polySia essential for appropriate brain development. Considering a possible role of particularly ST8Sia-II in genetic predisposition to psychiatric disorders (see sect. VI), the stronger impact of ST8Sia-II in the developing brain together with the strictly dose-dependent relation between polySia-deprived NCAM and neurodevelopmental defects has the important implication that even minor quantitative imbalances of NCAM polysialylation during brain development lead to defects in connectivity.
FIGURE 25.
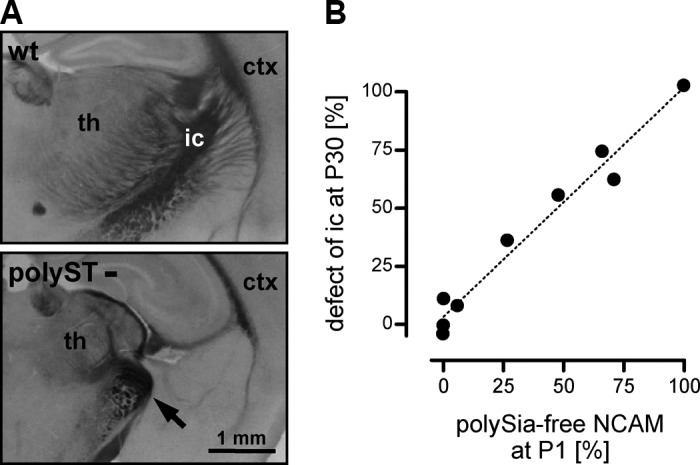
Gain of polySia-free NCAM determines axon tract deficits. A: compared with the wild-type situation (wt), 2−/−4−/− mice without functional polysialyltransferases (polyST−) show severe malformations of the internal capsule (ic, arrow) affecting fiber connections between thalamus (th) and cortex (ctx). B: the degree of the defect, based on morphometric evaluation at postnatal day 30 (P30), correlates with the relative levels of polySia-deficient NCAM determined during the developmental phase at postnatal day 1 (P1). Each of the data points stands for one of the nine mouse lines investigated (see text for details). The polyST-negative 2−/−4−/− mice show the strongest defect and the highest levels of polySia-negative NCAM, set to 100%, respectively. [Adapted from Hildebrandt et al. (195), by permission from Oxford University Press.]
4. Polysialic acid and synaptic plasticity
Several studies address the role of polySia in long-term potentiation (LTP) in the hippocampus, the primary experimental model to study synaptic plasticity as the basis of learning and memory (43). First, a role of NCAM in LTP was deduced from the observation that NCAM antibodies block LTP in the CA1 region (272). Shortly afterwards, this was corroborated by experiments with Ncam knockout mice (320), and at the same time the involvement of polySia was demonstrated (32, 320). Removal of polySia inhibits LTP and the related paradigm of long-term depression in the CA1 region (32, 320). In addition, local injections of endosialidase into the brain reduce spatial memory, which depends on plasticity in the hippocampus (32). Analyses of St8sia2 and St8sia4 knockout mice provide evidence that LTP in the CA1 region requires polySia synthesis by ST8Sia-IV, whereas the loss of ST8Sia-II has no effect (10, 129). Despite abnormal lamination and ectopic synapse formation of mossy fibers, LTP at the mossy fiber synapses in the CA3 region is normal in the St8sia2 knockout (10). CA3 LTP is also not affected in St8sia4 knockout mice, which lack polySia on their mossy fibers (129). As measured in vivo, LTP at synapses of the perforant path in the dentate gyrus is impaired in the Ncam knockout but not in either of the polyST single knockout mice (239, 463). This implies that this form of LTP requires the presence of NCAM, either acutely or during development, while the role of polySia remains open; polySia is either not required, or the remaining enzyme in each of the two polyST knockout lines produces sufficient polySia to maintain LTP in the dentate gyrus. It therefore would be interesting to study dentate gyrus LTP in 2−/−4−/− mice, but their severely disturbed development prevents a meaningful analysis. The role of polySia in CA1 LTP may be linked to effects of polySia on glutamate receptors of the AMPA and NMDA type, which are independent from NCAM and possibly caused by direct protein-binding of polySia (as in Figure 23H) (184, 497). Consistent with this idea, impaired LTP in Ncam knockout mice can be rescued by application of soluble polySia (443). As a potential mechanism, polySia may inhibit NMDA receptors containing the GluN2B subunit changing the balance between extrasynaptic and synaptic NMDA receptor signaling (239, 240).
5. Overexpression of ST8Sia-IV
The role of polySia on migrating oligodendrocyte precursor cells and its downregulation during maturation (see sect. IIIB7) suggest a role for polySia in myelination. In vitro data indicate that polySia promotes oligodendrocyte precursor cell migration in response to chemoattractive guidance cues (169, 535, 583), while downregulation of polySia enhances their differentiation into mature oligodendrocytes (108, 109). A transgenic mouse model has been established to address the question of whether downregulation of polySia is required for myelination in vivo (138). The expression of ST8Sia-IV under an oligodendrocyte-specific promoter prevents the postnatal reduction of polySia in oligodendrocytes and causes a delay of myelin formation and defects of myelin structure. In another approach, ST8Sia-IV-overexpressing neural precursors were transplanted into the brain of hypomyelinated shiverer mice (149). The engineered cells displayed widespread integration and myelination in the host, but differentiated more slowly than controls. Thus downregulation of polysialylation during oligodendrocyte differentiation is a prerequisite for optimal myelin formation and maintenance.
VI. SIALOGLYCANS AND DISEASES OF THE NERVOUS SYSTEM
A. Human Diseases of Sialic Acid Metabolism
Rare but serious human congenital disorders of sialic acid metabolism lead to sialic acid deficiency and sialic acid excess (207, 465) (Table 3). The first and rate-limiting step of sialic acid synthesis is conversion of UDP-N-acetylglucosamine to N-acetylmannosamine-6-phosphate by the bifunctional enzyme UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase, encoded by the GNE gene in humans (207). Loss-of-function mutations in this gene are the basis of Hereditary Inclusion Body Myopathy (HIBM), a serious progressive muscle wasting disease. More than 60 mutations in the epimerase or kinase domains of GNE result in sialic acid deficiency and HIBM. Most mutations are autosomal recessive and retain reduced but significant enzyme activity, whereas a few have no activity. Mutations with no activity are found as heterozygotes only, consistent the finding that complete disruption of the Gne gene (in mice) results in early embryonic lethality (432). The basis for the muscle wasting associated with reduced sialic acid biosynthesis has not been established. Although it is reasonable to propose that hyposialylation, either in general or of a specific sialoglycan, is the basis for HIBM, data have yet to establish that relationship. Mutations in the allosteric site of the GNE gene product result in sialouria, a very rare disorder in which overproduction of sialic acid occurs due to loss of allosteric feedback control of the epimerase/kinase by a downstream product, CMP-sialic acid (260). This disease course is variable but typically lacks severe nervous system defects, although delayed neuromuscular development has been noted.
Table 3.
Human diseases of sialic acid metabolism
| Enzyme/Protein Deficit | Gene Mutation | Biochemical Outcome | Notes |
|---|---|---|---|
| UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase | GNE | Sialic acid production is reduced (loss of function) or increased (loss of feedback inhibition) | Loss of function results in hereditary inclusion body myopathy (HIBM), loss of feedback inhibition results in sialouria |
| Solute carrier family 17 member 5 (sialin) | SLC17A5 | Lysosomal accumulation of sialic acid | Salla disease and infantile free sialic acid storage disease (ISSD) |
| β-Galactoside α-2,3-sialyltransferase 3 | ST3GAL3 | Undetermined in humans | Associated with intellectual disability |
| α-N-acetylneuraminide α-2,8-sialyltransferase 2 | ST8SIA2 | Undetermined in humans | Associated with schizophrenia |
| Lysosomal sialidase | NEU1 | Lysosomal accumulation of sialoglycans | Sialidosis |
Only disorders mentioned in the text are listed. For human diseases of ganglioside metabolism, see Table 4.
In contrast to HIBM and sialuria, Salla disease and the allelic but more severe infantile free sialic acid storage disease (ISSD) are lysosomal sialic acid storage disorders that result in debilitating nervous system pathology (548). The milder Salla disease, named after the Finnish district in which it arose (23), is marked by hypotonia, ataxia, and delayed motor development that may be accompanied by seizures. Severe CNS hypomyelination is a consistent finding in Salla disease, with thin corpus callosum observed by magnetic resonance imaging in all patients (456). ISSD is much more severe, with multiorgan system failure and early death. Both are caused by mutations in a lysosomal membrane sialic acid exporter called sialin, which is encoded by the SLC17A5 gene (524), and disease severity is inversely correlated with residual sialic acid transport activity (548). SLC17A5 mutation results in lysosomal sequestration of sialic acid. It remains unresolved whether lysosomal sialic acid storage, lack of cytoplasmic (recycled) sialic acid, or loss of other transport activities of sialin are causative in Salla and ISSD. A mouse model of sialin deficiency (Slc17a5-null) phenocopied the human disorders. The mutant mice displayed poor coordination, tremor, seizures, and early death (376). Analysis of one brain sialoglycan, polySia-NCAM, revealed delayed developmental downregulation. It will be interesting to evaluate the effect of lysosomal sialic acid buildup on other members of the brain sialome, since Slc17a5-null mice and ganglioside-deficient mice share some phenotypic characteristics (227, 376, 563).
B. Human Diseases of Ganglioside Metabolism
Human diseases of ganglioside metabolism (Table 4) are rare, with congenital errors in biosynthesis reported in only a few families and errors in catabolism occurring in 1 of ∼100,000 live births. Both anabolic and catabolic disorders are dominated by severe neurological deficits (52).
Table 4.
Congenital diseases of brain ganglioside metabolism
| Enzyme/Protein Deficit | Gene Mutation | Major Brain Gangliosides | Notes |
|---|---|---|---|
| GM2/GD2 synthase | B4GALNT1 | Loss of GM1, GD1a, GD1b, GT1b with partial replacement by GM3, GD3* | 10 independent families |
| GM3 synthase | ST3GAL5 | Undetermined | 4 independent families |
| Hexosaminidase A | HEXA | GM2 storage | Tay Sachs disease |
| Hexosaminidase A and B | HEXB | GM2 storage | Sandhoff disease |
| GM2 activator protein | GM2A | GM2 storage | |
| β-Galactosidase | GLB1 | GM1 storage | GM1 gangliosidosis |
| Niemann-Pick C1 protein | NPC1 | GM2, GM3 storage | UniProtKB/Swiss-Prot: O15118.2 |
| Niemann-Pick C2 protein | NPC2 | GM2, GM3 storage | UniProtKB/Swiss-Prot: P61916.1 |
Postmortem brain ganglioside analysis from a single case study lacking genetic confirmation (289).
In one case study, an infant presented with severe mental impairment from birth and failure to thrive, and died at 3 mo of age (289). Postmortem analysis revealed a complete lack of complex brain gangliosides, and instead expression of GM3 and GD3. Enzyme assays indicated sharply diminished GM2/GD2 synthase activity (UDP-GalNAc:GM3 N-acetylgalactosaminyl-transferase) in brain homogenates (141). Although the genetic basis was not determined, the biosynthetic deficiency was proposed to be lack of GM2/GD2 synthase (the B4GALNT1 gene product), and patient history indicated a familial disorder. Histological and ultrastructural studies of the brain noted major abnormalities in myelin (477), supporting a role for gangliosides in axon-myelin interactions in humans. The patient, however, had much more severe pathology than B4galnt1-null mice, with mental impairment evident from birth including hypotonia, lack of responsiveness, generalized seizures, and death in infancy (277). In contrast to that very severe neurological phenotype, more recent genetic studies linked several independent mutations in human B4GALNT1 to hereditary spastic paraplegia in 10 families (54). Analysis of ∼30 affected individuals revealed early-onset progressive spasticity of the lower extremities resulting from axonal degeneration. Unlike the earlier infant case study, these patients were relatively long-lived and typically ambulatory, although some became confined to a wheelchair later in life. Mild to moderate cognitive impairment and developmental delay was noted, as well as white matter hyperintensities by MRI (dysmyelination) and low testosterone levels in men. These deficits are phenocopied by B4galnt1-null mice, including the male testosterone deficit. Understanding the differences between different forms of B4GALNT1 deficiency in humans, and the relationship between brain ganglioside expression and neural deficits in these cases, awaits further biochemical and histological analyses.
Recently, deficiencies of GM3 synthase (ST3GAL5 gene mutations) were also discovered by genome-wide linkage analyses (46, 145, 449, 536). Affected individuals in several families presented with infantile seizures that were resistant to treatment accompanied by hypotonia and neurological deterioration. These deficits are not phenocopied in St3gal5-null mice, but are similar to those in “GM3 only” (St8Sia1/B4galnt1-doulbe null) and ganglioside-knockout (St3gal5/B4galnt1-double null) mice. St3gal5-single null mice express alternate complex brain gangliosides (GM1b and GD1α) that may compensate for the loss of other complex gangliosides. It has not been determined whether the human loss of GM3 synthase results in comparable biosynthetic compensation in the brains of ST3GAL5 patients, although studies on patient fibroblasts reveal lower rather than higher GM1b expression (46). Regardless, seizure susceptibility appears to be a common feature of human diseases and mouse genetic models of altered brain ganglioside expression.
Disorders of ganglioside catabolism result in lysosomal storage diseases marked by progressive neurodegeneration (52, 556). Normally, exoglycosidases sequentially remove the outer-most (nonreducing terminal) ganglioside saccharides. Genetic defects in exoglycosidases result in lysosomal buildup of the precursors, leading to severe neuronal pathology. Among these disorders, GM2 gangliosidoses have been studied most extensively. GM2 catabolism in humans requires removal of the terminal GalNAc by hexosaminidase A, an enzyme comprised of two subunits (α and β) coded by separate genes, HEXA and HEXB, respectively. Defects in either of the two gene products result in GM2 gangliosidosis, the extensive lysosomal buildup of GM2 in neurons. HEXA mutations are designated as Tay-Sachs disease and HEXB mutations as Sandhoff disease. The natural history of the two diseases varies with the amount of residual enzyme activity, with the course of disease indistinguishable between Tay-Sachs and Sandhoff diseases (42). In addition to hexosaminidase, GM2 catabolism requires an activator protein that facilitates access of the enzyme to the lipid substrate. Rare mutations in the human activator protein gene (GM2A) result in GM2 gangliosidosis without loss of hexosaminidase enzyme activity (measured using synthetic substrate), and patients display a disease course similar to Tay Sachs and Sandhoff diseases (452). Affected infants develop and progress normally for several weeks, then are subject to profound progressive neurodegeneration marked by increased startle response, hypotonia, and seizures. Mean survival is 4 years, with few individuals living beyond 8 years. Although the mechanisms of neurodegeneration are not fully understood, a role for microglia, macrophages, and neuroinflammation has been proposed. Genetic deletion of the immune mediators MIP-1α (554) or TNF-α (1) in Sandhoff disease mice (Hexb-null) reduced neurodegeneration and increased lifespan.
Human gangliosidosis can also result from defects in the β-galactosidase responsible for hydrolysis of the terminal galactose of GM1, coded by the GLB1 gene in humans (63, 68). Neurological deficits are invariably present in GM1 gangliosidosis patients. Different GLB1 mutations result in infantile (type I), juvenile (type II), and adult (type III) forms of the disease that are associated with severe psychomotor regression by 6 mo of age (type I), progressive locomotor deficits and seizures by 3 years of age (type II), and cerebellar dysfunction and dystonia later in childhood or into adulthood (type III). Mouse models of GM1 gangliosidosis have been useful for both pathophysiological and preclinical therapeutic studies (287, 288).
C. Anti-ganglioside Immunity
Guillain-Barré syndromes (GBS) are the leading cause of acute neuromuscular paralysis, with >75,000 cases per year worldwide (206, 499, 577). Although GBS is multifaceted, a large subset of cases involve anti-ganglioside antibodies (545).
GBS is an acquired disease characterized by rapidly progressive bilateral and relatively symmetrical limb weakness (499). Disease severity typically worsens for days or weeks until most patients cannot walk and may require artificial ventilation as respiratory muscles weaken. Although many patients recover, ∼5% of GBS patients die of the disease and 20% remain unable to walk 6 mo after disease onset. Many patients report ongoing symptoms including functionally significant fatigue lasting years after acute disease resolution. The efficacy of anti-immune interventions (plasma exchange and IVIG) (371, 483, 498), as well as histopathology (179, 180, 544), support the conclusion that peripheral nerves are subject to acute complement-mediated antibody attack.
Based on the affected nerves and conductance pathophysiology, GBS is classified into three major subtypes (294, 499, 577): acute inflammatory demyelinating polyneuropathy (AIDP), acute motor (or motor/sensory) axonal neuropathy (AMAN or AMSAN), and Miller-Fisher Syndrome (MFS), the last of which displays cranial nerve involvement resulting in opthalmoplegia and ataxia. In AIDP, the immune attack is on Schwann cells (180) and the antigens are not well established. In AMAN and MFS, the immune attack is on axons (179) and/or nerve terminals (183), and the antigens are often gangliosides. The study of anti-ganglioside antibodies in GBS was prompted by the discovery that some patients with an otherwise unrelated disorder, IgM paraproteinemia associated with peripheral neuropathy, expressed anti-ganglioside IgM antibodies (378). Subsequent studies discovered that a subset of North American GBS patients (∼20%) also expressed anti-ganglioside antibodies (210). These findings set the stage for the discovery of the pathophysiology of AMAN and MFS, both of which are caused by immune crossover between ganglioside-like epitopes on pathogens and endogenous gangliosides on axons and nerve terminals.
The onset of GBS often follows recovery from an infectious disease, typically an upper respiratory tract infection or diarrhea (206). Epidemiology, histology, and molecular studies support the conclusion that an infection-induced humoral immune response results in production of antibodies that cross react with endogenous epitopes on Schwann cells or axons, recruit the immune membrane attack complex and macrophages, disrupt peripheral myelin and axons, and cause paralysis (499, 577) (Table 5). This type of immune crossover attack, termed “molecular mimicry,” is well established in AMAN and MFS (Figure 26). The AMAN form of GBS was first recognized as a summertime disease of primarily rural children and young adults in northern China with acute axonal loss rather than the demyelinating electrophysiological hallmarks of the GBS most common in Europe and North America (294, 295). Although AMAN patients were afebrile at the time of admission, many reported recent illness, and over 90% had robust serum antibodies against Campylobacter jejuni, a common cause of human gastroenteritis. Histological studies indicated immune attack, with complement, immune membrane attack complexes, and macrophages at the axonal surface (176). Remarkably, chemical studies of C. jejuni strains associated with GBS revealed that bacterial surface lipooligosaccharides mimicked the structures of human gangliosides associated with anti-ganglioside antibodies in GBS, notably GM1 and GD1a (19, 170, 199, 575, 576, 579) (Figure 27). Campylobacter sialyltransferase polymorphisms control which ganglioside mimics are expressed (170, 575). Further evidence that GBS pathology involves ganglioside molecular mimicry comes from the observation that rabbits immunized with purified C. jejuni lipooligosaccharide (from a GBS-related strain) produced circulating anti-ganglioside antibodies and experience flaccid paralysis along with the histochemical hallmarks of GBS (578). With appropriate immune stimulation, an induced immune response to purified gangliosides also resulted in flaccid paralysis (580). These data establish ganglioside molecular mimicry in the pathophysiology of post-C. jejuni AMAN, with anti-GM1 and anti-GD1a immunity of special note.
Table 5.
Anti-ganglioside antibodies in GBS subtypes
| Subtype | Anti-ganglioside Antibodies (Minor) |
|---|---|
| AIDP | Unknown |
| AMAN and AMSAN | GM1, GD1a (GM1b, GalNAc-GD1a, GT1a) |
| MFS | GQ1b, GT1a, GD3 |
FIGURE 26.
Immunopathogenesis of the AMAN form of Guillain-Barré syndrome. Gangliosides GM1 and GD1a are strongly expressed at nodes of Ranvier, where the voltage-gated sodium (Nav) channels are localized. Anti-GM1 or anti-GD1a antibodies bind to the nodal axolemma, leading to formation of the complement membrane attack complex (MAC). This results in the disappearance of Nav clusters and the detachment of paranodal myelin, which can lead to nerve-conduction failure and muscle weakness. Axonal degeneration may follow at a later stage. Macrophages subsequently invade from the nodes into the periaxonal space, scavenging the injured axons. [Adapted from Yuki and Hartung (577), with permission from Massachusetts Medical Society.]
FIGURE 27.
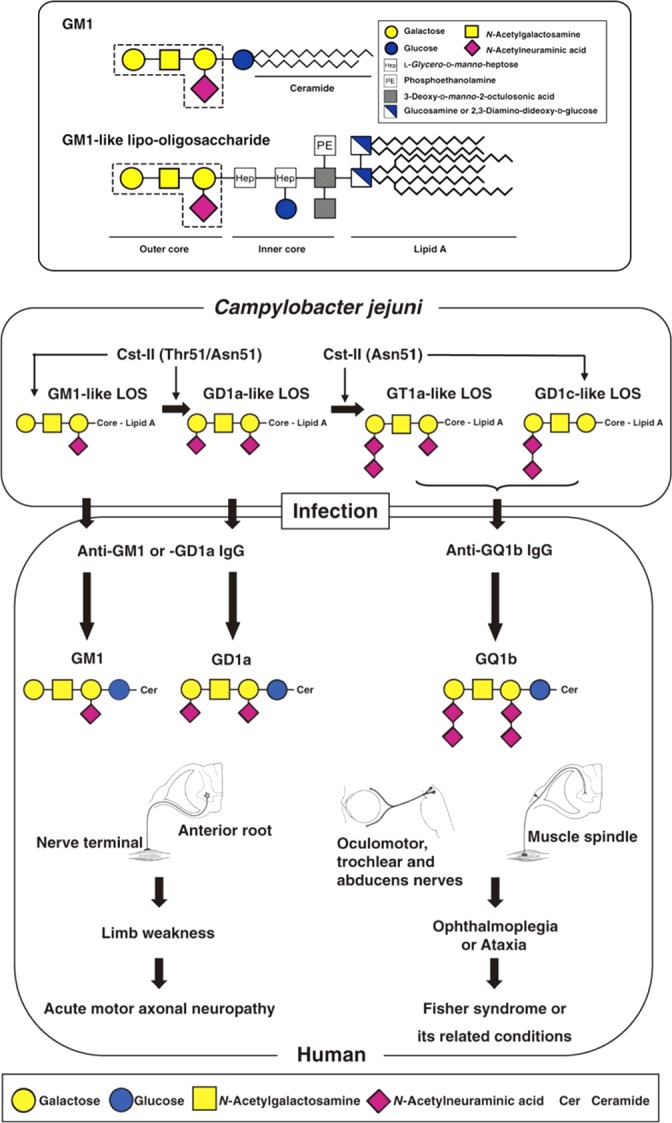
Campylobacter jejuni lipooligosaccharides and molecular mimicry in GBS. Top: carbohydrate mimicry between a ganglioside (GM1) and a Campylobacter jejuni lipooligosaccharide (LOS). The terminal tetrasaccharides are identical (dashed lines). Bottom: Campylobacter jejuni gene polymorphism as a determinant of clinical neuropathies after infection. C. jejuni that carries cst-II (Thr51) can express GM1-like and GD1a-like LOS on its cell surface, which upon infection may induce anti-GM1, anti-GD1a, or anti-GM1/GD1a complex IgG production. Since these products are expressed on motor nerves of the four limbs, binding induces acute motor axonal neuropathy. In contrast, C. jejuni that carries cst-II (Asn51) expresses GT1a-like or GD1c-like LOS on its cell surface, which upon infection may induce anti-GQ1b IgG production. Anti-GQ1b IgG antibody binds to GQ1b on oculomotor nerves and primary sensory neurons, inducing Fisher syndrome and related conditions. [Adapted from Yuki (575), with permission from John Wiley and Sons, Inc., and (576), with permission from Proceedings of the Japan Academy, Ser. B.]
A similar association was established for MFS, but specifically with the complex ganglioside GQ1b (350) (Figure 27). Studies of patients suffering from typical MFS (opthalmoplegia and ataxia), atypical MFS (opthalmoplegia without ataxia), and GBS with opthalmoplegia revealed that 28 of 30 had serum antibodies against GQ1b, whereas none of the GBS patients without opthalmoplegia (or normal controls) had anti-GQ1b antibodies (79, 80). Most of these patients reported antecedent infections, either upper respiratory or diarrhea. Whereas lipooligosaccharides from C. jejuni strains isolated from GBS patients (without opthalmoplegia) expressed mimics of GM1 and GD1a (see above), strains from MFS and related patients expressed terminal disialylated structures akin to GQ1b that invoked anti-GQ1b antibodies (170). GQ1b is expressed on the oculomotor, trochlear, and abducens nerves that control eye movement, but rarely on other peripheral nerves, in part explaining the characteristic neuropathy of MFS. MFS-associated C. jejuni strains express sialyltransferase polymorphisms that promote disialyl extensions of ganglioside-like lipooligosaccharides (170, 575). Patients with opthalmoplegia associated with anti-GQ1b antibodies have been grouped into a related spectrum of disorders including MFS, Bickerstaff's brain stem encephalitis, and acute ophthalmoparesis (350).
Ganglioside molecular mimicry has been established as a pathogen-induced, complement-mediated humoral immune response leading to peripheral nerve attack and resulting in motor deficits. Whereas many cases can be traced to specific pathogen structures (notably lipooligosaccharides on C. jejuni), the causative agent in other cases remains unknown. Enhanced knowledge of these pathogens and mechanisms of nerve damage may provide ways to lessen the worldwide burden of GBS.
D. ST3GAL3 and Cognition
Genome-wide linkage analysis implicates sialylation, particularly via the enzyme ST3Gal-III (the product of the ST3GAL3 gene), as a factor in higher cognitive functions (131, 204). Nonsyndromic autosomal recessive intellectual disability is a familial disorder characterized by a low intelligence quotient (IQ) associated with difficulty in learning basic language and motor skills during childhood and significantly diminished intellectual capacity as an adult. Two Iranian pedigrees including 12 affected individuals whose average IQ was <40 (<0.1 percentile) from four sets of consanguineous parents were subjected to genetic linkage analyses (204). This led to identification of two mutations responsible (one in each pedigree), both in the ST3GAL3 gene. One mutation was in the catalytic region and resulted in loss of sialyltransferase activity, whereas another was in the transmembrane domain, did not affect enzyme activity, but appeared to result in mistargeting of the enzyme to the endoplasmic reticulum where the donors and acceptors are absent.
West Syndrome is a severe infantile seizure disorder associated with developmental regression and intellectual disability that can occur as an autosomal recessive familial disorder. Members of a Palestinian pedigree including four affected individuals (three from consanguineous parents) were subjected to genetic linkage analysis (131). A single mutation in the “small sialylmotif” of the ST3GAL3 gene, a domain required for enzyme activity, was found to be responsible for the disorder.
The above studies do not identify which classes of brain sialoglycans are altered in human ST3GAL3 deficiency syndromes. The enzyme ST3Gal-III has broad acceptor specificity, transferring sialic acids to terminal Gal residues in β1–3 or β1–4 linkage to GlcNAc or in β1–3 linkage to GalNAc characteristic of O- and N-linked glycoproteins and gangliosides, respectively (45). Although evoked in the sialylation of Lewis epitopes, the specificity of ST3Gal-III is much broader. In the mouse brain, ST3Gal-III synthesizes both sialoglycoproteins and gangliosides (467). Further studies will be required to understand the brain sialoglycans affected in ST3GAL3 deficiency disorders and why these disorders present with and without severe infantile seizures. Enhanced genetic linkage analyses bring a new focus to research on brain sialoglycans in higher cognitive function in humans.
E. Polysialic Acid and Psychiatric Disorders
Dysregulation of polySia and NCAM have been frequently observed in association with psychiatric disorders, mainly schizophrenia, bipolar disorder, and autism (57, 519). In schizophrenia, the described changes in NCAM range from altered concentrations of NCAM fragments in either serum or cerebrospinal fluid (275, 372, 502, 521, 522) to increased levels of a roughly 110-kDa band in Western blot analyses of hippocampus and prefrontal cortex from postmortem brains of patients (520). A specific reduction of polySia-positive cells was found in the hilus region of the hippocampus (26) and, most recently, lower levels of polySia immunoreactivity, probably associated with interneuron changes, were detected in layers IV and V of the dorsolateral prefrontal cortex (PFC) of schizophrenics, while levels were unchanged in samples from patients with major depression and bipolar disorder (167). In contrast, polySia immunoreactivity was not altered in the amygdala of schizophrenics, but was reduced in depressed patients and increased in bipolar disorder (506).
In addition to changes of NCAM protein and polySia, important genetic associations have been discovered. NCAM1 and both polysialyltransferase genes map to chromosomal regions that harbor susceptibility loci for schizophrenia (11q23.1, 15q26, and 5q21 for NCAM1, ST8SIA2, and ST8SIA4, respectively) (261, 269, 290). Analyses of genetic variation in NCAM1 indicate associations of five contiguous single nucleotide polymorphisms (SNPs) with schizophrenia and different risk haplotypes in the NCAM1 gene are linked to schizophrenia or bipolar disorder (20, 468). Two independent studies performed with schizophrenic patients from Japan or China found significant associations of different polymorphisms in the promoter region of ST8SIA2, but not ST8SIA4, and a recent study with Australian patients describes a risk haplotype in the ST8SIA2 gene (18, 480). ST8SIA2 variants have also been associated with autism spectrum and bipolar disorder (16, 291). In vitro promoter assays were performed with a risk haplotype of ST8SIA2 in the Japanese sample of schizophrenic patients, and the results point towards enhanced transcriptional activity (18). In contrast, the risk haplotype identified in the Australian cohort is associated with reduced ST8SIA2 mRNA expression (291). Moreover, a point mutation near the sialylmotif L, detected heterozygously in one of the Japanese patients (18), leads to reduced ST8SIA2 activity and production of shorter polySia chains (213). Together, the available data provide strong evidence that variation in the ST8SIA2 gene is linked to increased risk of mental illness.
Schizophrenia has a high heritability. Additionally, disruptions in neural development resulting in altered brain connectivity is increasingly recognized as a risk factor (33, 262). In light of the strong neurodevelopmental phenotype of polyST-deficient mice and the clear dose-response relationship between loss of polySia and defects of brain connectivity (see sect. VB3), St8sia2-knockout mice are particularly well-suited to explore potential links between polysialylation deficits and pathophysiological features of schizophrenia. Indeed, a recent study demonstrates a schizophrenia-like phenotype of these mice (249). St8sia2-null mice displayed reduced prepulse inhibition of the startle response as an operational measure of sensorimotor gating and significant working memory deficits, indicating cognitive impairments. Both traits resemble characteristic features of schizophrenic patients and may be linked to the neurodevelopmental disturbance of thalamocortical connectivity leading to reduced glutamatergic input of thalamic afferents to the frontal and prefrontal cortex of St8sia2-knockout mice (249).
F. Polysialic Acid in Epilepsy, Neurodegeneration, and Nervous System Repair
Structural changes observed in epilepsy and neurodegenerative disease may be part of a pathological cascade or indicate attempts of repair. With regard to the role of polySia in developmental and adult plasticity, it is assumed that the increased expression of polySia as observed in the hippocampus and entorhinal cortex of patients suffering from temporal lobe epilepsy is part of such disease-related plasticity (301). The same holds true for the higher polySia levels detected in the molecular layer of the dentate gyrus in patients with Alzheimer's disease (300, 302). There is experimental evidence for polySia as a positive modulator of hippocampal neurogenesis in the amygdala kindling model of chronic temporal lobe epilepsy in the rat, which leads to ectopic neurons in the hilus (364). Endosialidase treatment has no beneficial effect on epilepsy development, but counteracts the increase of newborn neurons during the kindling process. These findings suggest that enhanced neurogenesis reflects an attempt of repair and implicates that stimulation of polySia synthesis may reinforce endogenous mechanisms of brain repair (363, 364).
Beyond neurogenesis, an increase of polySia may promote axon regeneration. Following lesions, reexpression of polySia is found on sprouting and regenerating CNS axons (7, 21, 319, 584). The presence of polySia on these axons may play a permissive role. However, even more important as a permissive factor may be the presence of polySia in reactive gliosis associated with a CNS lesion (21, 69). Support of regeneration by a polySia-positive environment is firmly established by the results of several studies showing that engineered overexpression of polySTs in astrocytes or grafted Schwann cells promotes axonal regeneration in the brain and spinal cord (133, 359, 585, 587, 588). Complementary, application of polySia mimetic peptides improves functional recovery after spinal cord injury (285, 296).
In contrast to these favorable effects of polySia overproduction, a reduction of polySia may be a strategy to promote myelin repair. The presence of polySia on axons hampers myelin formation in vitro (74), and chronically demyelinated axons in multiple sclerosis lesions reexpress polySia (75). In contrast, polySia is absent from so-called shadow plaques with partial repair, suggesting that the presence of polySia could act as an inhibitor of remyelination. In a first attempt to address this experimentally, remyelination after cuprizone-induced demyelination (451) was studied in St8sia4 knockout mice. A slight but significant acceleration in the reexpression of several myelin markers indicates improved remyelination in the absence of ST8Sia-IV (247). The mechanisms responsible for this improvement are yet to be explored. Nevertheless, the data indicate that targeting polysialyltransferases has the potential to support remyelination.
In this review, we have considered the significant contributions of two classes of brain sialoglycans, gangliosides and polysialic acid, to brain development, function, health, and disease. The rapid development of new methods and new knowledge in the field of glycobiology promises to bring enhanced understanding of how glycans help to manage the massive flow of information that underpins brain morphogenesis, maintenance, and functions, with the potential of meaningfully improving our understanding of human physiology and pathology.
GRANTS
We acknowledge the support of National Institute of Neurological Disorders and Stroke Grants NS037096 and NS057338 (to R. L. Schnaar). Work at Hannover Medical School was supported by funds from the Deutsche Forschungsgemeinschaft, Deutsche Krebshilfe, the Sixth Framework Program of the European Commission (FP6 PROMEMORIA), and the German Ministry for Research and Education in the frame of ERA-NET NEURON (NeuConnect).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are grateful to Martina Mühlenhoff, Beate Schwinzer, Tim Kröcher, and Johannes Cramer for help with figure preparation and critical reading of the manuscript.
Address for reprint requests and other correspondence: R. L. Schnaar, Dept. of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, 725 N. Wolfe St., Baltimore, MD 21205 (e-mail: schnaar@jhu.edu).
REFERENCES
- 1.Abo-Ouf H, Hooper AW, White EJ, Janse van Rensburg HJ, Trigatti BL, Igdoura SA. Deletion of tumor necrosis factor-alpha ameliorates neurodegeneration in Sandhoff disease mice. Hum Mol Genet 22: 3960–3975, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Abrous DN, Montaron MF, Petry KG, Rougon G, Darnaudery M, Le Moal M, Mayo W. Decrease in highly polysialylated neuronal cell adhesion molecules and in spatial learning during ageing are not correlated. Brain Res 744: 285–292, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Acheson A, Sunshine JL, Rutishauser U. NCAM polysialic acid can regulate both cell-cell and cell-substrate interactions. J Cell Biol 114: 143–153, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albach C, Damoc E, Denzinger T, Schachner M, Przybylski M, Schmitz B. Identification of N-glycosylation sites of the murine neural cell adhesion molecule NCAM by MALDI-TOF and MALDI-FTICR mass spectrometry. Anal Bioanal Chem 378: 1129–1135, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Alhadeff JA, Holzinger RT. Sialyltransferase, sialic acid and sialoglycoconjugates in metastatic tumor and human liver tissue. Int J Biochem 14: 119–126, 1982 [DOI] [PubMed] [Google Scholar]
- 6.Allen PM, Roberts I, Boulnois GJ, Saunders JR, Hart CA. Contribution of capsular polysaccharide and surface properties to virulence of Escherichia coli K1. Infect Immun 55: 2662–2668, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonso G, Prieto M, Legrand A, Chauvet N. PSA-NCAM and B-50/GAP-43 are coexpressed by specific neuronal systems of the adult rat mediobasal hypothalamus that exhibit remarkable capacities for morphological plasticity. J Comp Neurol 384: 181–199, 1997 [PubMed] [Google Scholar]
- 8.Angata K, Fukuda M. Polysialyltransferases: major players in polysialic acid synthesis on the neural cell adhesion molecule. Biochimie 85: 195–206, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Angata K, Huckaby V, Ranscht B, Terskikh A, Marth JD, Fukuda M. Polysialic acid-directed migration and differentiation of neural precursors is essential for mouse brain development. Mol Cell Biol 27: 6659–6668, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angata K, Long JM, Bukalo O, Lee W, Dityatev A, Wynshaw-Boris A, Schachner M, Fukuda M, Marth JD. Sialyltransferase ST8Sia-II assembles a subset of polysialic acid that directs hippocampal axonal targeting and promotes fear behavior. J Biol Chem 279: 32603–32613, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Angata K, Nakayama J, Fredette B, Chong K, Ranscht B, Fukuda M. Human STX polysialyltransferase forms the embryonic form of the neural cell adhesion molecule: tissue-specific expression, neurite outgrowth, and chromosomal localization in comparison with another polysialyltransferase, PST. J Biol Chem 272: 7182–7190, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Angata K, Suzuki M, Fukuda M. Differential and cooperative polysialylation of the neural cell adhesion molecule by two polysialyltransferases, PST and STX. J Biol Chem 273: 28524–28532, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Angata K, Suzuki M, Fukuda M. ST8Sia II and ST8Sia IV polysialyltransferases exhibit marked differences in utilizing various acceptors containing oligosialic acid and short polysialic acid. The basis for cooperative polysialylation by two enzymes. J Biol Chem 277: 36808–36817, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Angata T, Kerr SC, Greaves DR, Varki NM, Crocker PR, Varki A. Cloning and characterization of human Siglec-11. A recently evolved signaling molecule that can interact with SHP-1 and SHP-2 and is expressed by tissue macrophages, including brain microglia. J Biol Chem 277: 24466–24474, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Angata T, Varki A. Chemical diversity in the sialic acids and related alpha-keto acids: an evolutionary perspective. Chem Rev 102: 439–469, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Anney R, Klei L, Pinto D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, Sykes N, Pagnamenta AT, Almeida J, Bacchelli E, Bailey AJ, Baird G, Battaglia A, Berney T, Bolshakova N, Bolte S, Bolton PF, Bourgeron T, Brennan S, Brian J, Carson AR, Casallo G, Casey J, Chu SH, Cochrane L, Corsello C, Crawford EL, Crossett A, Dawson G, de Jonge M, Delorme R, Drmic I, Duketis E, Duque F, Estes A, Farrar P, Fernandez BA, Folstein SE, Fombonne E, Freitag CM, Gilbert J, Gillberg C, Glessner JT, Goldberg J, Green J, Guter SJ, Hakonarson H, Heron EA, Hill M, Holt R, Howe JL, Hughes G, Hus V, Igliozzi R, Kim C, Klauck SM, Kolevzon A, Korvatska O, Kustanovich V, Lajonchere CM, Lamb JA, Laskawiec M, Leboyer M, Le Couteur A, Leventhal BL, Lionel AC, Liu XQ, Lord C, Lotspeich L, Lund SC, Maestrini E, Mahoney W, Mantoulan C, Marshall CR, McConachie H, McDougle CJ, McGrath J, McMahon WM, Melhem NM, Merikangas A, Migita O, Minshew NJ, Mirza GK, Munson J, Nelson SF, Noakes C, Noor A, Nygren G, Oliveira G, Papanikolaou K, Parr JR, Parrini B, Paton T, Pickles A, Piven J, Posey DJ, Poustka A, Poustka F, Prasad A, Ragoussis J, Renshaw K, Rickaby J, Roberts W, Roeder K, Roge B, Rutter ML, Bierut LJ, Rice JP, Salt J, Sansom K, Sato D, Segurado R, Senman L, Shah N, Sheffield VC, Soorya L, Sousa I, Stoppioni V, Strawbridge C, Tancredi R, Tansey K, Thiruvahindrapduram B, Thompson AP, Thomson S, Tryfon A, Tsiantis J, Van Engeland H, Vincent JB, Volkmar F, Wallace S, Wang K, Wang Z, Wassink TH, Wing K, Wittemeyer K, Wood S, Yaspan BL, Zurawiecki D, Zwaigenbaum L, Betancur C, Buxbaum JD, Cantor RM, Cook EH, Coon H, Cuccaro ML, Gallagher L, Geschwind DH, Gill M, Haines JL, Miller J, Monaco AP, Nurnberger JI, Jr, Paterson AD, Pericak-Vance MA, Schellenberg GD, Scherer SW, Sutcliffe JS, Szatmari P, Vicente AM, Vieland VJ, Wijsman EM, Devlin B, Ennis S, Hallmayer J. A genome-wide scan for common alleles affecting risk for autism. Hum Mol Genet 19: 4072–4082, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoki K, Perlman M, Lim JM, Cantu R, Wells L, Tiemeyer M. Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J Biol Chem 282: 9127–9142, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Arai M, Yamada K, Toyota T, Obata N, Haga S, Yoshida Y, Nakamura K, Minabe Y, Ujike H, Sora I, Ikeda K, Mori N, Yoshikawa T, Itokawa M. Association between polymorphisms in the promoter region of the sialyltransferase 8B (SIAT8B) gene and schizophrenia. Biol Psychiatry 59: 652–659, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Aspinall GO, McDonald AG, Pang H, Kurjanczyk LA, Penner JL. Lipopolysaccharides of Campylobacter jejuni serotype O:19: structures of core oligosaccharide regions from the serostrain and two bacterial isolates from patients with the Guillain-Barre syndrome. Biochemistry 33: 241–249, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Atz ME, Rollins B, Vawter MP. NCAM1 association study of bipolar disorder and schizophrenia: polymorphisms and alternatively spliced isoforms lead to similarities and differences. Psychiatr Genet 17: 55–67, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aubert I, Ridet JL, Schachner M, Rougon G, Gage FH. Expression of L1 and PSA during sprouting and regeneration in the adult hippocampal formation. J Comp Neurol 399: 1–19, 1998 [PubMed] [Google Scholar]
- 22.Audry M, Jeanneau C, Imberty A, Harduin-Lepers A, Delannoy P, Breton C. Current trends in the structure-activity relationships of sialyltransferases. Glycobiology 21: 716–726, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Aula P, Autio S, Raivio KO, Rapola J, Thoden CJ, Koskela SL, Yamashina I. “Salla disease”: a new lysosomal storage disorder. Arch Neurol 36: 88–94, 1979 [DOI] [PubMed] [Google Scholar]
- 24.Avril T, North SJ, Haslam SM, Willison HJ, Crocker PR. Probing the cis interactions of the inhibitory receptor Siglec-7 with alpha2,8-disialylated ligands on natural killer cells and other leukocytes using glycan-specific antibodies and by analysis of alpha2,8-sialyltransferase gene expression. J Leukoc Biol 80: 787–796, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Azurmendi HF, Vionnet J, Wrightson L, Trinh LB, Shiloach J, Freedberg DI. Extracellular structure of polysialic acid explored by on cell solution NMR. Proc Natl Acad Sci USA 104: 11557–11561, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbeau D, Liang JJ, Robitalille Y, Quirion R, Srivastava LK. Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc Natl Acad Sci USA 92: 2785–2789, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartsch U, Kirchhoff F, Schachner M. Highly sialylated N-CAM is expressed in adult mouse optic nerve and retina. J Neurocytol 19: 550–565, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Battista D, Rutishauser U. Removal of polysialic acid triggers dispersion of subventricularly derived neuroblasts into surrounding CNS tissues. J Neurosci 30: 3995–4003, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Battistel MD, Shangold M, Trinh L, Shiloach J, Freedberg DI. Evidence for helical structure in a tetramer of alpha2–8 sialic acid: unveiling a structural antigen. J Am Chem Soc 134: 10717–10720, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baumann H, Brisson JR, Michon F, Pon R, Jennings HJ. Comparison of the conformation of the epitope of alpha(2–>8) polysialic acid with its reduced and N-acyl derivatives. Biochemistry 32: 4007–4013, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Bax M, van Vliet SJ, Litjens M, Garcia-Vallejo JJ, van Kooyk Y. Interaction of polysialic acid with CCL21 regulates the migratory capacity of human dendritic cells. PLoS One 4: e6987, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker CG, Artola A, Gerardy-Schahn R, Decker T, Welzl H, Schachner M. The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J Neurosci Res 45: 143–152, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Begre S, Koenig T. Cerebral disconnectivity: an early event in schizophrenia. Neuroscientist 14: 19–45, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Belanger MC, Di Cristo G. Sensory experience differentially modulates the mRNA expression of the polysialyltransferases ST8SiaII and ST8SiaIV in postnatal mouse visual cortex. PLoS One 6: e24874, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benson MA, Fu Z, Kim JJ, Baldwin MR. Unique ganglioside recognition strategies for clostridial neurotoxins. J Biol Chem 286: 34015–34022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernier PJ, Vinet J, Cossette M, Parent A. Characterization of the subventricular zone of the adult human brain: evidence for the involvement of Bcl-2. Neurosci Res 37: 67–78, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Bertozzi CR, Rabuka D. Structural basis of glycan diversity. In: Essentials of Glycobiology (2nd ed.), edited by Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. New York: Cold Spring Harbor Laboratory Press, 2009, p. 23–36 [PubMed] [Google Scholar]
- 38.Bhat S, Silberberg DH. Developmental expression of neural cell adhesion molecules of oligodendrocytes in vivo and in culture. J Neurochem 50: 1830–1838, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297: 1525–1531, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Bielik AM, Zaia J. Historical overview of glycoanalysis. Methods Mol Biol 600: 9–30, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Blass-Kampmann S, Reinhardt-Maelicke S, Kindler-Rohrborn A, Cleeves V, Rajewsky MF. In vitro differentiation of E-N-CAM expressing rat neural precursor cells isolated by FACS during prenatal development. J Neurosci Res 37: 359–373, 1994 [DOI] [PubMed] [Google Scholar]
- 42.Bley AE, Giannikopoulos OA, Hayden D, Kubilus K, Tifft CJ, Eichler FS. Natural history of infantile G(M2) gangliosidosis. Pediatrics 128: e1233–e1241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Blix G, Lindberg E, Odin L, Werner I. Sialic acids. Nature 175: 340–341, 1955 [DOI] [PubMed] [Google Scholar]
- 45.Blixt O, Allin K, Bohorov O, Liu X, Andersson-Sand H, Hoffmann J, Razi N. Glycan microarrays for screening sialyltransferase specificities. Glycoconj J 25: 59–68, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Boccuto L, Aoki K, Flanagan-Steet H, Chen CF, Fan X, Bartel F, Petukh M, Pittman A, Saul R, Chaubey A, Alexov E, Tiemeyer M, Steet R, Schwartz CE. A mutation in a ganglioside biosynthetic enzyme, ST3GAL5, results in salt & pepper syndrome, a neurocutaneous disorder with altered glycolipid and glycoprotein glycosylation. Hum Mol Genet 23: 418–433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolborea M, Laran-Chich MP, Rasri K, Hildebrandt H, Govitrapong P, Simonneaux V, Pevet P, Steinlechner S, Klosen P. Melatonin controls photoperiodic changes in tanycyte vimentin and neural cell adhesion molecule expression in the Djungarian hamster (Phodopus sungorus). Endocrinology 152: 3871–3883, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol 80: 129–164, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Bonfanti L, Merighi A, Theodosis DT. Dorsal rhizotomy induces transient expression of the highly sialylated isoform of the neural cell adhesion molecule in neurons and astrocytes of the adult rat spinal cord. Neuroscience 74: 619–623, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Bonfanti L, Olive S, Poulain DA, Theodosis DT. Mapping of the distribution of polysialylated neural cell adhesion molecule throughout the central nervous system of the adult rat: an immunohistochemical study. Neuroscience 49: 419–436, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Bonfanti L, Theodosis DT. Expression of polysialylated neural cell adhesion molecule by proliferating cells in the subependymal layer of the adult rat, in its rostral extension and in the olfactory bulb. Neuroscience 62: 291–305, 1994 [DOI] [PubMed] [Google Scholar]
- 52.Boomkamp SD, Butters TD. Glycosphingolipid disorders of the brain. Subcell Biochem 49: 441–467, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Boscher C, Dennis JW, Nabi IR. Glycosylation, galectins and cellular signaling. Curr Opin Cell Biol 23: 383–392, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Boukhris A, Schule R, Loureiro JL, Lourenco CM, Mundwiller E, Gonzalez MA, Charles P, Gauthier J, Rekik I, costa Lebrigio RF, Gaussen M, Speziani F, Ferbert A, Feki I, Caballero-Oteyza A, onne-Laporte A, Amri M, Noreau A, Forlani S, Cruz VT, Mochel F, Coutinho P, Dion P, Mhiri C, Schols L, Pouget J, Darios F, Rouleau GA, Marques W, Jr, Brice A, Durr A, Zuchner S, Stevanin G. Alteration of ganglioside biosynthesis responsible for complex hereditary spastic paraplegia. Am J Hum Genet 93: 118–123, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bremer EG, Schlessinger J, Hakomori S. Ganglioside-mediated modulation of cell growth: specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem 261: 2434–2440, 1986 [PubMed] [Google Scholar]
- 56.Brennaman LH, Maness PF. Developmental regulation of GABAergic interneuron branching and synaptic development in the prefrontal cortex by soluble neural cell adhesion molecule. Mol Cell Neurosci 37: 781–793, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brennaman LH, Maness PF. NCAM in neuropsychiatric and neurodegenerative disorders. Adv Exp Med Biol 663: 299–317, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Brennaman LH, Moss ML, Maness PF. EphrinA/EphA-induced ectodomain shedding of neural cell adhesion molecule regulates growth cone repulsion through ADAM10 metalloprotease. J Neurochem 128: 267–279, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Breton C, Fournel-Gigleux S, Palcic MM. Recent structures, evolution and mechanisms of glycosyltransferases. Curr Opin Struct Biol 22: 540–549, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Brewer CF, Miceli MC, Baum LG. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr Opin Struct Biol 12: 616–623, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Brisson JR, Baumann H, Imberty A, Perez S, Jennings HJ. Helical epitope of the group B meningococcal alpha(2–8)-linked sialic acid polysaccharide. Biochemistry 31: 4996–5004, 1992 [DOI] [PubMed] [Google Scholar]
- 62.Brockhausen I, Schachter H, Stanley PO. GalNAc glycans. In: Essentials of Glycobiology (2nd ed.), edited by Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. New York: Cold Spring Harbor Laboratory Press, 2009, p. 115–128 [PubMed] [Google Scholar]
- 63.Brunetti-Pierri N, Scaglia F. GM1 gangliosidosis: review of clinical, molecular, and therapeutic aspects. Mol Genet Metab 94: 391–396, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Bruses JL, Chauvet N, Rubio ME, Rutishauser U. Polysialic acid and the formation of oculomotor synapses on chick ciliary neurons. J Comp Neurol 446: 244–256, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Bullens RW, O'Hanlon GM, Wagner E, Molenaar PC, Furukawa K, Furukawa K, Plomp JJ, Willison HJ. Complex gangliosides at the neuromuscular junction are membrane receptors for autoantibodies and botulinum neurotoxin but redundant for normal synaptic function. J Neurosci 22: 6876–6884, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burgess A, Aubert I. Polysialic acid limits choline acetyltransferase activity induced by brain-derived neurotrophic factor. J Neurochem 99: 797–806, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Byrne MC, Ledeen RW, Roisen FJ, Yorke G, Sclafani JR. Ganglioside-induced neuritogenesis: verification that gangliosides are the active agents, and comparison of molecular species. J Neurochem 41: 1214–1222, 1983 [DOI] [PubMed] [Google Scholar]
- 68.Caciotti A, Garman SC, Rivera-Colon Y, Procopio E, Catarzi S, Ferri L, Guido C, Martelli P, Parini R, Antuzzi D, Battini R, Sibilio M, Simonati A, Fontana E, Salviati A, Akinci G, Cereda C, onisi-Vici C, Deodato F, D'Amico A, d'Azzo A, Bertini E, Filocamo M, Scarpa M, di RM, Tifft CJ, Ciani F, Gasperini S, Pasquini E, Guerrini R, Donati MA, Morrone A. GM1 gangliosidosis and Morquio B disease: an update on genetic alterations and clinical findings. Biochim Biophys Acta 1812: 782–790, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Camand E, Morel MP, Faissner A, Sotelo C, Dusart I. Long-term changes in the molecular composition of the glial scar and progressive increase of serotoninergic fibre sprouting after hemisection of the mouse spinal cord. Eur J Neurosci 20: 1161–1176, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Candelise L, Ciccone A. Gangliosides for acute ischemic stroke. Stroke 33: 2336, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Cao Z, Gao Y, Deng K, Williams G, Doherty P, Walsh FS. Receptors for myelin inhibitors: structures and therapeutic opportunities. Mol Cell Neurosci 43: 1–14, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Cavallaro U, Niedermeyer J, Fuxa M, Christofori G. N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat Cell Biol 3: 650–657, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Chang J, Patton JT, Sarkar A, Ernst B, Magnani JL, Frenette PS. GMI-1070, a novel pan-selectin antagonist, reverses acute vascular occlusions in sickle cell mice. Blood 116: 1779–1786, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Charles P, Hernandez MP, Stankoff B, Aigrot MS, Colin C, Rougon G, Zalc B, Lubetzki C. Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc Natl Acad Sci USA 97: 7585–7590, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Charles P, Reynolds R, Seilhean D, Rougon G, Aigrot MS, Niezgoda A, Zalc B, Lubetzki C. Re-expression of PSA-NCAM by demyelinated axons: an inhibitor of remyelination in multiple sclerosis? Brain 125: 1972–1979, 2002 [DOI] [PubMed] [Google Scholar]
- 76.Chazal G, Durbec P, Jankovski A, Rougon G, Cremer H. Consequences of neural cell adhesion molecule deficiency on cell migration in the rostral migratory stream of the mouse. J Neurosci 20: 1446–1457, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen X, Varki A. Advances in the biology and chemistry of sialic acids. ACS Chem Biol 5: 163–176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chiavegatto S, Sun J, Nelson RJ, Schnaar RL. A functional role for complex gangliosides: motor deficits in GM2/GD2 synthase knockout mice. Exp Neurol 166: 227–234, 2000 [DOI] [PubMed] [Google Scholar]
- 79.Chiba A, Kusunoki S, Obata H, Machinami R, Kanazawa I. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain-Barre syndrome: clinical and immunohistochemical studies. Neurology 43: 1911–1917, 1993 [DOI] [PubMed] [Google Scholar]
- 80.Chiba A, Kusunoki S, Shimizu T, Kanazawa I. Serum IgG antibody to ganglioside GQ1b is a possible marker of Miller Fisher syndrome. Ann Neurol 31: 677–679, 1992 [DOI] [PubMed] [Google Scholar]
- 81.Chou HH, Hayakawa T, Diaz S, Krings M, Indriati E, Leakey M, Paabo S, Satta Y, Takahata N, Varki A. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci USA 99: 11736–11741, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Christensen C, Lauridsen JB, Berezin V, Bock E, Kiselyov VV. The neural cell adhesion molecule binds to fibroblast growth factor receptor 2. FEBS Lett 580: 3386–3390, 2006 [DOI] [PubMed] [Google Scholar]
- 83.Chung KY, Leung KM, Lin CC, Tam KC, Hao YL, Taylor JS, Chan SO. Regionally specific expression of L1 and sialylated NCAM in the retinofugal pathway of mouse embryos. J Comp Neurol 471: 482–498, 2004 [DOI] [PubMed] [Google Scholar]
- 84.Close BE, Colley KJ. In vivo autopolysialylation and localization of the polysialyltransferases PST and STX. J Biol Chem 273: 34586–34593, 1998 [DOI] [PubMed] [Google Scholar]
- 85.Cohen AW, Razani B, Wang XB, Combs TP, Williams TM, Scherer PE, Lisanti MP. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol 285: C222–C235, 2003 [DOI] [PubMed] [Google Scholar]
- 86.Cohen M, Varki A. The sialome–far more than the sum of its parts. OMICS 14: 455–464, 2010 [DOI] [PubMed] [Google Scholar]
- 87.Cole GJ, Burg M. Characterization of a heparan sulfate proteoglycan that copurifies with the neural cell adhesion molecule. Exp Cell Res 182: 44–60, 1989 [DOI] [PubMed] [Google Scholar]
- 88.Cole GJ, Loewy A, Glaser L. Neuronal cell-cell adhesion depends on interactions of N-CAM with heparin-like molecules. Nature 320: 445–447, 1986 [DOI] [PubMed] [Google Scholar]
- 89.Colley KJ. Structural basis for the polysialylation of the neural cell adhesion molecule. Adv Exp Med Biol 663: 111–126, 2010 [DOI] [PubMed] [Google Scholar]
- 90.Collins BE, Ito H, Sawada N, Ishida H, Kiso M, Schnaar RL. Enhanced binding of the neural siglecs, myelin-associated glycoprotein and Schwann cell myelin protein, to Chol-1 (α-series) gangliosides and novel sulfated Chol-1 analogs. J Biol Chem 274: 37637–37643, 1999 [DOI] [PubMed] [Google Scholar]
- 91.Collins BE, Kiso M, Hasegawa A, Tropak MB, Roder JC, Crocker PR, Schnaar RL. Binding specificities of the sialoadhesin family of I-type lectins. Sialic acid linkage and substructure requirements for binding of myelin-associated glycoprotein, Schwann cell myelin protein, and sialoadhesin. J Biol Chem 272: 16889–16895, 1997 [DOI] [PubMed] [Google Scholar]
- 92.Collins BE, Yang LJS, Mukhopadhyay G, Filbin MT, Kiso M, Hasegawa A, Schnaar RL. Sialic acid specificity of myelin-associated glycoprotein binding. J Biol Chem 272: 1248–1255, 1997 [DOI] [PubMed] [Google Scholar]
- 93.Connell TD. Cholera toxin, LT-I, LT-IIa and LT-IIb: the critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins. Expert Rev Vaccines 6: 821–834, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cox ET, Brennaman LH, Gable KL, Hamer RM, Glantz LA, LaMantia AS, Lieberman JA, Gilmore JH, Maness PF, Jarskog LF. Developmental regulation of neural cell adhesion molecule in human prefrontal cortex. Neuroscience 162: 96–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, Barthels D, Rajewsky K, Willie W. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 367: 455–459, 1994 [DOI] [PubMed] [Google Scholar]
- 96.Crespel A, Rigau V, Coubes P, Rousset MC, de Bock F, Okano H, Baldy-Moulinier M, Bockaert J, Lerner-Natoli M. Increased number of neural progenitors in human temporal lobe epilepsy. Neurobiol Dis 19: 436–450, 2005 [DOI] [PubMed] [Google Scholar]
- 97.Crocker PR, Clark EA, Filbin M, Gordon S, Jones Y, Kehrl JH, Kelm S, Le Douarin N, Powell L, Roder J, Schnaar RL, Sgroi DC, Stamenkovic K, Schauer R, Schachner M, van den Berg TK, van der Merwe PA, Watt SM, Varki A. Siglecs: a family of sialic-acid binding lectins. Glycobiology 8: v, 1998 [DOI] [PubMed] [Google Scholar]
- 98.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol 7: 255–266, 2007 [DOI] [PubMed] [Google Scholar]
- 99.Cunningham BA, Hoffman S, Rutishauser U, Hemperly JJ, Edelman GM. Molecular topography of the neural cell adhesion molecule N-CAM: surface orientation and location of sialic acid-rich and binding regions. Proc Natl Acad Sci USA 80: 3116–3120, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Curreli S, Arany Z, Gerardy-Schahn R, Mann D, Stamatos NM. Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J Biol Chem 282: 30346–30356, 2007 [DOI] [PubMed] [Google Scholar]
- 101.Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, Roon-Mom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 315: 1243–1249, 2007 [DOI] [PubMed] [Google Scholar]
- 102.Da Silva JS, Hasegawa T, Miyagi T, Dotti CG, Abad-Rodriguez J. Asymmetric membrane ganglioside sialidase activity specifies axonal fate. Nat Neurosci 8: 606–615, 2005 [DOI] [PubMed] [Google Scholar]
- 103.Daston MM, Bastmeyer M, Rutishauser U, Oleary DDM. Spatially restricted increase in polysialic acid enhances corticospinal axon branching related to target recognition and innervation. J Neurosci 16: 5488–5497, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Datta AK. Comparative sequence analysis in the sialyltransferase protein family: analysis of motifs. Curr Drug Targets 10: 483–498, 2009 [DOI] [PubMed] [Google Scholar]
- 105.Datta AK, Paulson JC. The sialyltransferase “sialylmotif” participates in binding the donor substrate CMP-NeuAc. J Biol Chem 270: 1497–1500, 1995 [DOI] [PubMed] [Google Scholar]
- 106.Davies LR, Varki A. Why Is N-glycolylneuraminic acid rare in the vertebrate brain? Top Curr Chem. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Erausquin GA, Manev H, Guidotti A, Costa E, Brooker G. Gangliosides normalize distorted single-cell intracellular free Ca2+ dynamics after toxic doses of glutamate in cerebellar granule cells. Proc Natl Acad Sci USA 87: 8017–8021, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Decker L, Avellana-Adalid V, Nait-Oumesmar B, Durbec P, Baron-van Evercooren A. Oligodendrocyte precursor migration and differentiation: combined effects of PSA residues, growth factors, and substrates. Mol Cell Neurosci 16: 422–439, 2000 [DOI] [PubMed] [Google Scholar]
- 109.Decker L, Durbec P, Rougon G, Baron-van Evercooren A. Loss of polysialic residues accelerates CNS neural precursor differentiation in pathological conditions. Mol Cell Neurosci 19: 225–238, 2002 [DOI] [PubMed] [Google Scholar]
- 110.DeMarco ML, Woods RJ. Atomic-resolution conformational analysis of the GM3 ganglioside in a lipid bilayer and its implications for ganglioside-protein recognition at membrane surfaces. Glycobiology 19: 344–355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Denaxa M, Kyriakopoulou K, Theodorakis K, Trichas G, Vidaki M, Takeda Y, Watanabe K, Karagogeos D. The adhesion molecule TAG-1 is required for proper migration of the superficial migratory stream in the medulla but not of cortical interneurons. Dev Biol 288: 87–99, 2005 [DOI] [PubMed] [Google Scholar]
- 112.Derrington EA, Masco D, Whittaker VP. Confirmation of the cholinergic specificity of the Chol-1 gangliosides in mammalian brain using affinity-purified antisera and lesions affecting the cholinergic input to the hippocampus. J Neurochem 53: 1686–1692, 1989 [DOI] [PubMed] [Google Scholar]
- 113.Di Cristo G, Chattopadhyaya B, Kuhlman SJ, Fu Y, Belanger MC, Wu CZ, Rutishauser U, Maffei L, Huang ZJ. Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat Neurosci 10: 1569–1577, 2007 [DOI] [PubMed] [Google Scholar]
- 114.Diaz RJ, Outschoorn I. Selective biotinylation of Neisseria meningitidis group B capsular polysaccharide and application in an improved ELISA for the detection of specific antibodies. J Immunol Methods 160: 35–47, 1993 [DOI] [PubMed] [Google Scholar]
- 115.Diestel S, Schaefer D, Cremer H, Schmitz B. NCAM is ubiquitylated, endocytosed and recycled in neurons. J Cell Sci 120: 4035–4049, 2007 [DOI] [PubMed] [Google Scholar]
- 116.Dityatev A, Dityateva G, Schachner M. Synaptic strength as a function of post- versus presynaptic expression of the neural cell adhesion molecule NCAM. Neuron 26: 207–217, 2000 [DOI] [PubMed] [Google Scholar]
- 117.Dityatev A, Dityateva G, Sytnyk V, Delling M, Toni N, Nikonenko I, Muller D, Schachner M. Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J Neurosci 24: 9372–9382, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci 11: 735–746, 2010 [DOI] [PubMed] [Google Scholar]
- 119.Dobrovic B, Curic G, Petanjek Z, Heffer M. Dendritic morphology and spine density is not altered in motor cortex and dentate granular cells in mice lacking the ganglioside biosynthetic gene B4galnt1: a quantitative Golgi cox study. Coll Antropol 35 Suppl 1: 25–30, 2011 [PubMed] [Google Scholar]
- 120.Dobson CM, Hempel SJ, Stalnaker SH, Stuart R, Wells L. O-Mannosylation and human disease. Cell Mol Life Sci 70: 2849–2857, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev 13: 543–550, 2003 [DOI] [PubMed] [Google Scholar]
- 122.Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci USA 93: 14895–14900, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 17: 5046–5061, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Doherty P, Cohen J, Walsh FS. Neurite outgrowth in response to transfected N-CAM changes during development and is modulated by polysialic acid. Neuron 5: 209–219, 1990 [DOI] [PubMed] [Google Scholar]
- 125.Doherty P, Dickson JG, Flanigan TP, Walsh FS. Ganglioside GM1 does not initiate, but enhances neurite regeneration of nerve growth factor-dependent sensory neurones. J Neurochem 44: 1259–1265, 1985 [DOI] [PubMed] [Google Scholar]
- 126.Doherty P, Skaper SD, Moore SE, Leon A, Walsh FS. A developmentally regulated switch in neuronal responsiveness to NCAM and N-cadherin in the rat hippocampus. Development 115: 885–892, 1992 [DOI] [PubMed] [Google Scholar]
- 127.Drickamer K, Fadden AJ. Genomic analysis of C-type lectins. Biochem Soc Symp 59–72, 2002 [DOI] [PubMed] [Google Scholar]
- 128.Dridi L, Seyrantepe V, Fougerat A, Pan X, Bonneil E, Thibault P, Moreau A, Mitchell GA, Heveker N, Cairo CW, Issad T, Hinek A, Pshezhetsky AV. Positive regulation of insulin signaling by neuraminidase 1. Diabetes 62: 2338–2346, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eckhardt M, Bukalo O, Chazal G, Wang L, Goridis C, Schachner M, Gerardy-Schahn R, Cremer H, Dityatev A. Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J Neurosci 20: 5234–5244, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eckhardt M, Mühlenhoff M, Bethe A, Koopman J, Frosch M, Gerardy-Schahn R. Molecular characterization of eukaryotic polysialyltransferase-1. Nature 373: 715–718, 1995 [DOI] [PubMed] [Google Scholar]
- 131.Edvardson S, Baumann AM, Mühlenhoff M, Stephan O, Kuss AW, Shaag A, He L, Zenvirt S, Tanzi R, Gerardy-Schahn R, Elpeleg O. West syndrome caused by ST3Gal-III deficiency. Epilepsia 54: e24–e27, 2013 [DOI] [PubMed] [Google Scholar]
- 132.Eggers K, Werneburg S, Schertzinger A, Abeln M, Schiff M, Scharenberg MA, Burkhardt H, Mühlenhoff M, Hildebrandt H. Polysialic acid controls NCAM signals at cell-cell contacts to regulate focal adhesion independent from FGF receptor activity. J Cell Sci 124: 3279–3291, 2011 [DOI] [PubMed] [Google Scholar]
- 133.El Maarouf A, Petridis AK, Rutishauser U. Use of polysialic acid in repair of the central nervous system. Proc Natl Acad Sci USA 103: 16989–16994, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med 4: 1313–1317, 1998 [DOI] [PubMed] [Google Scholar]
- 135.Evans SV, Sigurskjold BW, Jennings HJ, Brisson JR, To R, Tse WC, Altman E, Frosch M, Weisgerber C, Kratzin HD. Evidence for the extended helical nature of polysaccharide epitopes. The 28 A resolution structure and thermodynamics of ligand binding of an antigen binding fragment specific for alpha-(2–>8)-polysialic acid. Biochemistry 34: 6737–6744, 1995 [DOI] [PubMed] [Google Scholar]
- 136.Fanzani A, Zanola A, Faggi F, Papini N, Venerando B, Tettamanti G, Sampaolesi M, Monti E. Implications for the mammalian sialidases in the physiopathology of skeletal muscle. Skeletal Muscle 2: 23, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Favaron M, Manev H, Alho H, Bertolino M, Ferret B, Guidotti A, Costa E. Gangliosides prevent glutamate and kainate neurotoxicity in primary neuronal cultures of neonatal rat cerebellum and cortex. Proc Natl Acad Sci USA 85: 7351–7355, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fewou SN, Ramakrishnan H, Bussow H, Gieselmann V, Eckhardt M. Down-regulation of polysialic acid is required for efficient myelin formation. J Biol Chem 282: 16700–16711, 2007 [DOI] [PubMed] [Google Scholar]
- 139.Finne J. Structure of the O-glycosidically linked carbohydrate units of rat brain glycoproteins. Biochim Biophys Acta 412: 317–325, 1975 [DOI] [PubMed] [Google Scholar]
- 140.Finne J, Makela PH. Cleavage of the polysialosyl units of brain glycoproteins by a bacteriophage endosialidase. Involvement of a long oligosaccharide segment in molecular interactions of polysialic acid. J Biol Chem 260: 1265–1270, 1985 [PubMed] [Google Scholar]
- 141.Fishman PH, Max SR, Tallman JF, Brady RO, Maclaren NK, Cornblath M. Deficient ganglioside biosynthesis: a novel human sphingolipidosis. Science 187: 68–70, 1975 [DOI] [PubMed] [Google Scholar]
- 142.Fogel AI, Li Y, Giza J, Wang Q, Lam TT, Modis Y, Biederer T. N-glycosylation at the SynCAM (synaptic cell adhesion molecule) immunoglobulin interface modulates synaptic adhesion. J Biol Chem 285: 34864–34874, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Foley AG, Ronn LC, Murphy KJ, Regan CM. Distribution of polysialylated neural cell adhesion molecule in rat septal nuclei and septohippocampal pathway: transient increase of polysialylated interneurons in the subtriangular septal zone during memory consolidation. J Neurosci Res 74: 807–817, 2003 [DOI] [PubMed] [Google Scholar]
- 144.Foley DA, Swartzentruber KG, Colley KJ. Identification of sequences in the polysialyltransferases ST8Sia II and ST8Sia IV that are required for the protein-specific polysialylation of the neural cell adhesion molecule, NCAM. J Biol Chem 284: 15505–15516, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fragaki K, it-El-Mkadem S, Chaussenot A, Gire C, Mengual R, Bonesso L, Beneteau M, Ricci JE, squiret-Dumas V, Procaccio V, Rotig A, Paquis-Flucklinger V. Refractory epilepsy and mitochondrial dysfunction due to GM3 synthase deficiency. Eur J Hum Genet 21: 528–534, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Francavilla C, Cattaneo P, Berezin V, Bock E, Ami D, de Marco A, Christofori G, Cavallaro U. The binding of NCAM to FGFR1 induces a specific cellular response mediated by receptor trafficking. J Cell Biol 187: 1101–1116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Franceschini I, Angata K, Ong E, Hong A, Doherty P, Fukuda M. Polysialyltransferase ST8Sia II (STX) polysialylates all of the major isoforms of NCAM and facilitates neurite outgrowth. Glycobiology 11: 231–239, 2001 [DOI] [PubMed] [Google Scholar]
- 148.Franceschini I, Desroziers E, Caraty A, Duittoz A. The intimate relationship of gonadotropin-releasing hormone neurons with the polysialylated neural cell adhesion molecule revisited across development and adult plasticity. Eur J Neurosci 32: 2031–2041, 2010 [DOI] [PubMed] [Google Scholar]
- 149.Franceschini I, Vitry S, Padilla F, Casanova P, Tham TN, Fukuda M, Rougon G, Durbec P, Dubois-Dalcq M. Migrating and myelinating potential of neural precursors engineered to overexpress PSA-NCAM. Mol Cell Neurosci 27: 151–162, 2004 [DOI] [PubMed] [Google Scholar]
- 150.Freeze HH. Understanding human glycosylation disorders: biochemistry leads the charge. J Biol Chem 288: 6936–6945, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Freeze HH, Haltiwanger RS. Other classes of ER/Golgi-derived glycans. In: Essentials of Glycobiology (2nd ed.), edited by Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. New York: Cold Spring Harbor Laboratory Press, 2009, p. 163–74 [Google Scholar]
- 152.Freiberger F, Claus H, Gunzel A, Oltmann-Norden I, Vionnet J, Mühlenhoff M, Vogel U, Vann WF, Gerardy-Schahn R, Stummeyer K. Biochemical characterization of a Neisseria meningitidis polysialyltransferase reveals novel functional motifs in bacterial sialyltransferases. Mol Microbiol 65: 1258–1275, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol 125: 669–680, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Frosch M, Gorgen I, Boulnois GJ, Timmis KN, Bitter-Suermann D. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc Natl Acad Sci USA 82: 1194–1198, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fruttiger M, Montag D, Schachner M, Martini R. Crucial role for the myelin-associated glycoprotein in the maintenance of axon-myelin integrity. Eur J Neurosci 7: 511–515, 1995 [DOI] [PubMed] [Google Scholar]
- 156.Fujimoto I, Bruses JL, Rutishauser U. Regulation of cell adhesion by polysialic acid: effects on cadherin, IgCAM and integrin function and independence from NCAM binding or signaling activity. J Biol Chem 276: 31745–31751, 2001 [DOI] [PubMed] [Google Scholar]
- 157.Fujitani M, Kawai H, Proia RL, Kashiwagi A, Yasuda H, Yamashita T. Binding of soluble myelin-associated glycoprotein to specific gangliosides induces the association of p75NTR to lipid rafts and signal transduction. J Neurochem 94: 15–21, 2005 [DOI] [PubMed] [Google Scholar]
- 158.Fukuta S, Magnani JL, Twiddy EM, Holmes RK, Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect Immun 56: 1748–1753, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gabius HJ. The Sugar Code. Weinheim, Germany: Wiley-VCH, 2009 [Google Scholar]
- 160.Galuska SP, Geyer R, Gerardy-Schahn R, Mühlenhoff M, Geyer H. Enzyme-dependent variations in the polysialylation of the neural cell adhesion molecule (NCAM) in vivo. J Biol Chem 283: 17–28, 2008 [DOI] [PubMed] [Google Scholar]
- 161.Galuska SP, Oltmann-Norden I, Geyer H, Weinhold B, Kuchelmeister K, Hildebrandt H, Gerardy-Schahn R, Geyer R, Mühlenhoff M. Polysialic acid profiles of mice expressing variant allelic combinations of the polysialyltransferases ST8SiaII and ST8SiaIV. J Biol Chem 281: 31605–31615, 2006 [DOI] [PubMed] [Google Scholar]
- 162.Galuska SP, Rollenhagen M, Kaup M, Eggers K, Oltmann-Norden I, Schiff M, Hartmann M, Weinhold B, Hildebrandt H, Geyer R, Mühlenhoff M, Geyer H. Synaptic cell adhesion molecule SynCAM 1 is a target for polysialylation in postnatal mouse brain. Proc Natl Acad Sci USA 107: 10250–10255, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Geisler FH, Coleman WP, Grieco G, Poonian D. The Sygen multicenter acute spinal cord injury study. Spine 26: S87–S98, 2001 [DOI] [PubMed] [Google Scholar]
- 164.Giacopuzzi E, Bresciani R, Schauer R, Monti E, Borsani G. New insights on the sialidase protein family revealed by a phylogenetic analysis in metazoa. PLoS One 7: e44193, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gil-Perotin S, Duran-Moreno M, Belzunegui S, Luquin MR, Garcia-Verdugo JM. Ultrastructure of the subventricular zone in Macaca fascicularis and evidence of a mouse-like migratory stream. J Comp Neurol 514: 533–554, 2009 [DOI] [PubMed] [Google Scholar]
- 166.Gilabert-Juan J, Castillo-Gomez E, Perez-Rando M, Molto MD, Nacher J. Chronic stress induces changes in the structure of interneurons and in the expression of molecules related to neuronal structural plasticity and inhibitory neurotransmission in the amygdala of adult mice. Exp Neurol 232: 33–40, 2011 [DOI] [PubMed] [Google Scholar]
- 167.Gilabert-Juan J, Varea E, Guirado R, Blasco-Ibanez JM, Crespo C, Nacher J. Alterations in the expression of PSA-NCAM and synaptic proteins in the dorsolateral prefrontal cortex of psychiatric disorder patients. Neurosci Lett 530: 97–102, 2012 [DOI] [PubMed] [Google Scholar]
- 168.Giordanengo V, Bannwarth S, Laffont C, Van MV, Harduin-Lepers A, Delannoy P, Lefebvre JC. Cloning and expression of cDNA for a human Gal(beta1–3)GalNAc alpha2,3-sialyltransferase from the CEM T-cell line. Eur J Biochem 247: 558–566, 1997 [DOI] [PubMed] [Google Scholar]
- 169.Glaser T, Brose C, Franceschini I, Hamann K, Smorodchenko A, Zipp F, Dubois-Dalcq M, Brustle O. Neural cell adhesion molecule polysialylation enhances the sensitivity of embryonic stem cell-derived neural precursors to migration guidance cues. Stem Cells 25: 3016–3025, 2007 [DOI] [PubMed] [Google Scholar]
- 170.Godschalk PC, Kuijf ML, Li J, St MF, Ang CW, Jacobs BC, Karwaski MF, Brochu D, Moterassed A, Endtz HP, van BA, Gilbert M. Structural characterization of Campylobacter jejuni lipooligosaccharide outer cores associated with Guillain-Barre and Miller Fisher syndromes. Infect Immun 75: 1245–1254, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gomez-Climent MA, Castillo-Gomez E, Varea E, Guirado R, Blasco-Ibanez JM, Crespo C, Martinez-Guijarro FJ, Nacher J. A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood. Cereb Cortex 18: 2229–2240, 2008 [DOI] [PubMed] [Google Scholar]
- 172.Gomez-Climent MA, Guirado R, Castillo-Gomez E, Varea E, Gutierrez-Mecinas M, Gilabert-Juan J, Garcia-Mompo C, Vidueira S, Sanchez-Mataredona D, Hernandez S, Blasco-Ibanez JM, Crespo C, Rutishauser U, Schachner M, Nacher J. The polysialylated form of the neural cell adhesion molecule (PSA-NCAM) is expressed in a subpopulation of mature cortical interneurons characterized by reduced structural features and connectivity. Cereb Cortex 21: 1028–1041, 2011 [DOI] [PubMed] [Google Scholar]
- 173.Gomez-Climent MA, Hernandez-Gonzalez S, Shionoya K, Belles M, Alonso-Llosa G, Datiche F, Nacher J. Olfactory bulbectomy, but not odor conditioned aversion, induces the differentiation of immature neurons in the adult rat piriform cortex. Neuroscience 181: 18–27, 2011 [DOI] [PubMed] [Google Scholar]
- 174.Gottschalk A. Virus enzymes and virus templates. Physiol Rev 37: 66–83, 1957 [DOI] [PubMed] [Google Scholar]
- 175.Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA 96: 5263–5267, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Griffin JW, Li CY, Ho TW, Tian M, Gao CY, Xue P, Mishu B, Cornblath DR, Macko C, McKhann GM, Asbury AK. Pathology of the motor-sensory axonal Guillain-Barre syndrome. Ann Neurol 39: 17–28, 1996 [DOI] [PubMed] [Google Scholar]
- 177.Grumet M, Flaccus A, Margolis RU. Functional characterization of chondroitin sulfate proteoglycans of brain: interactions with neurons and neural cell adhesion molecules. J Cell Biol 120: 815–824, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guirado R, Perez-Rando M, Sanchez-Matarredona D, Castillo-Gomez E, Liberia T, Rovira-Esteban L, Varea E, Crespo C, Blasco-Ibanez JM, Nacher J. The dendritic spines of interneurons are dynamic structures influenced by PSA-NCAM expression. Cereb Cortex. In press [DOI] [PubMed] [Google Scholar]
- 179.Hafer-Macko C, Hsieh ST, Li CY, Ho TW, Sheikh K, Cornblath DR, McKhann GM, Asbury AK, Griffin JW. Acute motor axonal neuropathy: an antibody-mediated attack on axolemma. Ann Neurol 40: 635–644, 1996 [DOI] [PubMed] [Google Scholar]
- 180.Hafer-Macko CE, Sheikh KA, Li CY, Ho TW, Cornblath DR, McKhann GM, Asbury AK, Griffin JW. Immune attack on the Schwann cell surface in acute inflammatory demyelinating polyneuropathy. Ann Neurol 39: 625–635, 1996 [DOI] [PubMed] [Google Scholar]
- 181.Hakomori S. Chemistry of glycosphingolipids. In: Sphingolipid Biochemistry, edited by Kanfer JN, Hakomori S. New York: Plenum, 1983, p. 1–165 [Google Scholar]
- 182.Hakomori S. The glycosynapse. Proc Natl Acad Sci USA 99: 225–232, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Halstead SK, Humphreys PD, Goodfellow JA, Wagner ER, Smith RA, Willison HJ. Complement inhibition abrogates nerve terminal injury in Miller Fisher syndrome. Ann Neurol 58: 203–210, 2005 [DOI] [PubMed] [Google Scholar]
- 184.Hammond MS, Sims C, Parameshwaran K, Suppiramaniam V, Schachner M, Dityatev A. NCAM associated polysialic acid inhibits NR2B-containing NMDA receptors and prevents glutamate-induced cell death. J Biol Chem 281: 34859–34869, 2006 [DOI] [PubMed] [Google Scholar]
- 185.Harduin-Lepers A, Mollicone R, Delannoy P, Oriol R. The animal sialyltransferases and sialyltransferase-related genes: a phylogenetic approach. Glycobiology 15: 805–817, 2005 [DOI] [PubMed] [Google Scholar]
- 186.Hart GW, Copeland RJ. Glycomics hits the big time. Cell 143: 672–676, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hayrinen J, Bitter-Suermann D, Finne J. Interaction of meningococcal group B monoclonal antibody and its Fab fragment with alpha 2–8-linked sialic acid polymers: requirement of a long oligosaccharide segment for binding. Mol Immunol 26: 523–529, 1989 [DOI] [PubMed] [Google Scholar]
- 188.Hayrinen J, Haseley S, Talaga P, Mühlenhoff M, Finne J, Vliegenthart JF. High affinity binding of long-chain polysialic acid to antibody, and modulation by divalent cations and polyamines. Mol Immunol 39: 399–411, 2002 [DOI] [PubMed] [Google Scholar]
- 189.Hekmat A, Bitter-Suermann D, Schachner M. Immunocytological localization of the highly polysialylated form of the neural cell adhesion molecule during development of the murine cerebellar cortex. J Comp Neurol 291: 457–467, 1990 [DOI] [PubMed] [Google Scholar]
- 190.Herrler G, Rott R, Klenk HD, Müller HP, Shukla AK, Schauer R. The receptor-destroying enzyme of influenza C virus is neuraminate-O-acetylesterase. EMBO J 4: 1503–1506, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hildebrandt H, Becker C, Glüer S, Rösner H, Gerardy-Schahn R, Rahmann H. Polysialic acid on the neural cell adhesion molecule correlates with expression of polysialyltransferases and promotes neuroblastoma cell growth. Cancer Res 58: 779–784, 1998 [PubMed] [Google Scholar]
- 192.Hildebrandt H, Becker C, Mürau M, Gerardy-Schahn R, Rahmann H. Heterogeneous expression of the polysialyltransferases ST8Sia II and ST8Sia IV during postnatal rat brain development. J Neurochem 71: 2339–2348, 1998 [DOI] [PubMed] [Google Scholar]
- 193.Hildebrandt H, Dityatev A. Polysialic acid in brain development and synaptic plasticity. Top Curr Chem. In press [DOI] [PubMed] [Google Scholar]
- 194.Hildebrandt H, Dityatev A. With a little help from EphA3 and polysialic acid: ectodomain shedding of NCAM is gaining momentum. J Neurochem 128: 206–209, 2014 [DOI] [PubMed] [Google Scholar]
- 195.Hildebrandt H, Mühlenhoff M, Oltmann-Norden I, Röckle I, Burkhardt H, Weinhold B, Gerardy-Schahn R. Imbalance of neural cell adhesion molecule and polysialyltransferase alleles causes defective brain connectivity. Brain 132: 2831–2838, 2009 [DOI] [PubMed] [Google Scholar]
- 196.Hinderlich S, Weidemann W, Yardeni T, Horstkorte R, Huizing M. UDP-GlcNAc 2-epimerase/ManNAc kinase (GNE): a master regulator of sialic acid synthesis. Top Curr Chem. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hinsby AM, Lundfald L, Ditlevsen DK, Korshunova I, Juhl L, Meakin SO, Berezin V, Bock E. ShcA regulates neurite outgrowth stimulated by neural cell adhesion molecule but not by fibroblast growth factor 2: evidence for a distinct fibroblast growth factor receptor response to neural cell adhesion molecule activation. J Neurochem 91: 694–703, 2004 [DOI] [PubMed] [Google Scholar]
- 198.Hirabayashi Y, Nakao T, Irie F, Whittaker VP, Kon K, Ando S. Structural characterization of a novel cholinergic neuron-specific ganglioside in bovine brain. J Biol Chem 267: 12973–12978, 1992 [PubMed] [Google Scholar]
- 199.Ho TW, Willison HJ, Nachamkin I, Li CY, Veitch J, Ung H, Wang GR, Liu RC, Cornblath DR, Asbury AK, Griffin JW, McKhann GM. Anti-GD1a antibody is associated with axonal but not demyelinating forms of Guillain-Barre syndrome. Ann Neurol 45: 168–173, 1999 [DOI] [PubMed] [Google Scholar]
- 200.Holmner A, Mackenzie A, Okvist M, Jansson L, Lebens M, Teneberg S, Krengel U. Crystal structures exploring the origins of the broader specificity of escherichia coli heat-labile enterotoxin compared to cholera toxin. J Mol Biol 406: 387–402, 2011 [DOI] [PubMed] [Google Scholar]
- 201.Hoyk Z, Parducz A, Theodosis DT. The highly sialylated isoform of the neural cell adhesion molecule is required for estradiol-induced morphological synaptic plasticity in the adult arcuate nucleus. Eur J Neurosci 13: 649–656, 2001 [DOI] [PubMed] [Google Scholar]
- 202.Hsieh ST, Kidd GJ, Crawford TO, Xu Z, Lin WM, Trapp BD, Cleveland DW, Griffin JW. Regional modulation of neurofilament organization by myelination in normal axons. J Neurosci 14: 6392–6401, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hu H. Polysialic acid regulates chain formation by migrating olfactory interneuron precursors. J Neurosci Res 61: 480–492, 2000 [DOI] [PubMed] [Google Scholar]
- 204.Hu H, Eggers K, Chen W, Garshasbi M, Motazacker MM, Wrogemann K, Kahrizi K, Tzschach A, Hosseini M, Bahman I, Hucho T, Mühlenhoff M, Gerardy-Schahn R, Najmabadi H, Ropers HH, Kuss AW. ST3GAL3 mutations impair the development of higher cognitive functions. Am J Hum Genet 89: 407–414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hubschmann MV, Skladchikova G, Bock E, Berezin V. Neural cell adhesion molecule function is regulated by metalloproteinase-mediated ectodomain release. J Neurosci Res 80: 826–837, 2005 [DOI] [PubMed] [Google Scholar]
- 206.Hughes RA, Cornblath DR. Guillain-Barre syndrome. Lancet 366: 1653–1666, 2005 [DOI] [PubMed] [Google Scholar]
- 207.Huizing M, Krasnewich DM. Hereditary inclusion body myopathy: a decade of progress. Biochim Biophys Acta 1792: 881–887, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ichikawa N, Iwabuchi K, Kurihara H, Ishii K, Kobayashi T, Sasaki T, Hattori N, Mizuno Y, Hozumi K, Yamada Y, Arikawa-Hirasawa E. Binding of laminin-1 to monosialoganglioside GM1 in lipid rafts is crucial for neurite outgrowth. J Cell Sci 122: 289–299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Illa I, Ortiz N, Gallard E, Juarez C, Grau JM, Dalakas MC. Acute axonal Guillain-Barre syndrome with IgG antibodies against motor axons following parenteral gangliosides. Ann Neurol 38: 218–224, 1995 [DOI] [PubMed] [Google Scholar]
- 210.Ilyas AA, Willison HJ, Quarles RH, Jungalwala FB, Cornblath DR, Trapp BD, Griffin DE, Griffin JW, McKhann GM. Serum antibodies to gangliosides in Guillain-Barre syndrome. Ann Neurol 23: 440–447, 1988 [DOI] [PubMed] [Google Scholar]
- 211.Inoue M, Fujii Y, Furukawa K, Okada M, Okumura K, Hayakawa T, Furukawa K, Sugiura Y. Refractory skin injury in complex knock-out mice expressing only the GM3 ganglioside. J Biol Chem 277: 29881–29888, 2002 [DOI] [PubMed] [Google Scholar]
- 212.Irie F, Kurono S, Li YT, Seyama Y, Hirabayashi Y. Isolation of three novel cholinergic neuron-specific gangliosides from bovine brain and their in vitro syntheses. Glycoconj J 13: 177–186, 1996 [DOI] [PubMed] [Google Scholar]
- 213.Isomura R, Kitajima K, Sato C. Structural and functional impairments of polysialic acid by a mutated polysialyltransferase found in schizophrenia. J Biol Chem 286: 21535–21545, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jakobsson E, Schwarzer D, Jokilammi A, Finne J. Endosialidases: versatile tools for the study of polysialic acid. Top Curr. In press [DOI] [PubMed] [Google Scholar]
- 215.James WM, Agnew WS. Alpha-(2—-8)-polysialic acid immunoreactivity in voltage-sensitive sodium channel of eel electric organ. Proc R Soc Lond B Biol Sci 237: 233–245, 1989 [DOI] [PubMed] [Google Scholar]
- 216.Jennemann R, Grone HJ. Cell-specific in vivo functions of glycosphingolipids: lessons from genetic deletions of enzymes involved in glycosphingolipid synthesis. Prog Lipid Res 52: 231–248, 2013 [DOI] [PubMed] [Google Scholar]
- 217.Jennemann R, Sandhoff R, Wang S, Kiss E, Gretz N, Zuliani C, Martin-Villalba A, Jager R, Schorle H, Kenzelmann M, Bonrouhi M, Wiegandt H, Grone HJ. Cell-specific deletion of glucosylceramide synthase in brain leads to severe neural defects after birth. Proc Natl Acad Sci USA 102: 12459–12464, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jennings HJ, Roy R, Michon F. Determinant specificities of the groups B and C polysaccharides of Neisseria meningitidis. J Immunol 134: 2651–2657, 1985 [PubMed] [Google Scholar]
- 219.Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci USA 101: 343–347, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Johnson CP, Fragneto G, Konovalov O, Dubosclard V, Legrand JF, Leckband DE. Structural studies of the neural-cell-adhesion molecule by X-ray and neutron reflectivity. Biochemistry 44: 546–554, 2005 [DOI] [PubMed] [Google Scholar]
- 221.Johnson CP, Fujimoto I, Rutishauser U, Leckband DE. Direct evidence that neural cell adhesion molecule (NCAM) polysialylation increases intermembrane repulsion and abrogates adhesion. J Biol Chem 280: 137–145, 2005 [DOI] [PubMed] [Google Scholar]
- 222.Kabat EA. Basic principles of antigen-antibody reactions. Methods Enzymol 70: 3–49, 1980 [DOI] [PubMed] [Google Scholar]
- 223.Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, Sonnino S, Kinjo M, Igarashi Y, Inokuchi J. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci USA 104: 13678–13683, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kakuta Y, Okino N, Kajiwara H, Ichikawa M, Takakura Y, Ito M, Yamamoto T. Crystal structure of Vibrionaceae photobacterium sp. JT-ISH-224 alpha2,6-sialyltransferase in a ternary complex with donor product CMP and acceptor substrate lactose: catalytic mechanism and substrate recognition. Glycobiology 18: 66–73, 2008 [DOI] [PubMed] [Google Scholar]
- 225.Kalus I, Bormann U, Mzoughi M, Schachner M, Kleene R. Proteolytic cleavage of the neural cell adhesion molecule by ADAM17/TACE is involved in neurite outgrowth. J Neurochem 98: 78–88, 2006 [DOI] [PubMed] [Google Scholar]
- 226.Kanato Y, Kitajima K, Sato C. Direct binding of polysialic acid to a brain-derived neurotrophic factor depends on the degree of polymerization. Glycobiology 18: 1044–1053, 2008 [DOI] [PubMed] [Google Scholar]
- 227.Kawai H, Allende ML, Wada R, Kono M, Sango K, Deng C, Miyakawa T, Crawley JN, Werth N, Bierfreund U, Sandhoff K, Proia RL. Mice expressing only monosialoganglioside GM3 exhibit lethal audiogenic seizures. J Biol Chem 276: 6885–6888, 2001 [DOI] [PubMed] [Google Scholar]
- 228.Kelm S, Pelz A, Schauer R, Filbin MT, Song T, de Bellard ME, Schnaar RL, Mahoney JA, Hartnell A, Bradfield P, Crocker PR. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr Biol 4: 965–972, 1994 [DOI] [PubMed] [Google Scholar]
- 229.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27: 447–452, 2004 [DOI] [PubMed] [Google Scholar]
- 230.Keys TG, Freiberger F, Ehrit J, Krueger J, Eggers K, Buettner FF, Gerardy-Schahn R. A universal fluorescent acceptor for high-performance liquid chromatography analysis of pro- and eukaryotic polysialyltransferases. Anal Biochem 427: 107–115, 2012 [DOI] [PubMed] [Google Scholar]
- 231.Kiselyov VV, Skladchikova G, Hinsby AM, Jensen PH, Kulahin N, Soroka V, Pedersen N, Tsetlin V, Poulsen FM, Berezin V, Bock E. Structural basis for a direct interaction between FGFR1 and NCAM and evidence for a regulatory role of ATP. Structure 11: 691–701, 2003 [DOI] [PubMed] [Google Scholar]
- 232.Kiss JZ, Wang C, Rougon G. Nerve-dependent expression of high polysialic acid neural cell adhesion molecule in neurohypophysial astrocytes of adult rats. Neuroscience 53: 213–221, 1993 [DOI] [PubMed] [Google Scholar]
- 233.Kitamura M, Takamiya K, Aizawa S, Furukawa K, Furukawa K. Gangliosides are the binding substances in neural cells for tetanus and botulinum toxins in mice. Biochim Biophys Acta 1441: 1–3, 1999 [DOI] [PubMed] [Google Scholar]
- 234.Kitazume-Kawaguchi S, Kabata S, Arita M. Differential biosynthesis of polysialic or disialic acid structure by ST8Sia II and ST8Sia IV. J Biol Chem 276: 15696–15703, 2001 [DOI] [PubMed] [Google Scholar]
- 235.Kittaka D, Itoh M, Ohmi Y, Kondo Y, Fukumoto S, Urano T, Tajima O, Furukawa K, Furukawa K. Impaired hypoglossal nerve regeneration in mutant mice lacking complex gangliosides: down-regulation of neurotrophic factors and receptors as possible mechanisms. Glycobiology 18: 509–516, 2008 [DOI] [PubMed] [Google Scholar]
- 236.Klenk E. über die Ganglioside, eine neue Gruppe von zuckerhaltigen Gehirnlipoiden. Hoppe-Seyler's Z Physiol Chem 273: 76–86, 1942 [Google Scholar]
- 237.Klenk E, Lauenstein K. Zur Kenntnis der Kohlenhydratgruppen des Submaxillarismucins und Harnmucoproteids : Die Isolierung von Neuraminsäure als Spaltprodukt. Hoppe Seylers Z Physiol Chem 291: 147–152, 1952 [PubMed] [Google Scholar]
- 238.Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One 5: e8809, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kochlamazashvili G, Bukalo O, Senkov O, Salmen B, Gerardy-Schahn R, Engel AK, Schachner M, Dityatev A. Restoration of synaptic plasticity and learning in young and aged NCAM-deficient mice by enhancing neurotransmission mediated by GluN2A-containing NMDA receptors. J Neurosci 32: 2263–2275, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kochlamazashvili G, Senkov O, Grebenyuk S, Robinson C, Xiao MF, Stummeyer K, Gerardy-Schahn R, Engel AK, Feig L, Semyanov A, Suppiramaniam V, Schachner M, Dityatev A. Neural cell adhesion molecule-associated polysialic acid regulates synaptic plasticity and learning by restraining the signaling through GluN2B-containing NMDA receptors. J Neurosci 30: 4171–4183, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kojima N, Kono M, Yoshida Y, Tachida Y, Nakafuku M, Tsuji S. Biosynthesis and expression of polysialic acid on the neural cell adhesion molecule is predominantly directed by ST8Sia II/STX during in vitro neuronal differentiation. J Biol Chem 271: 22058–22062, 1996 [DOI] [PubMed] [Google Scholar]
- 242.Kojima N, Yoshida Y, Tsuji S. A developmentally regulated member of the sialyltransferase family (ST8Sia II, STX) is a polysialic acid synthase. FEBS Lett 373: 119–122, 1995 [DOI] [PubMed] [Google Scholar]
- 243.Koles K, Lim JM, Aoki K, Porterfield M, Tiemeyer M, Wells L, Panin V. Identification of N-glycosylated proteins from the central nervous system of Drosophila melanogaster. Glycobiology 17: 1388–1403, 2007 [DOI] [PubMed] [Google Scholar]
- 244.Koles K, Repnikova E, Pavlova G, Korochkin LI, Panin VM. Sialylation in protostomes: a perspective from Drosophila genetics and biochemistry. Glycoconj J 26: 313–324, 2009 [DOI] [PubMed] [Google Scholar]
- 245.Kolkova K, Novitskaya V, Pedersen N, Berezin V, Bock E. Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein kinase C and the ras-mitogen-activated protein kinase pathway. J Neurosci 20: 2238–2246, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci USA 98: 4752–4757, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Koutsoudaki PN, Hildebrandt H, Gudi V, Skripuletz T, Skuljec J, Stangel M. Remyelination after cuprizone induced demyelination is accelerated in mice deficient in the polysialic acid synthesizing enzyme St8siaIV. Neuroscience 171: 235–244, 2010 [DOI] [PubMed] [Google Scholar]
- 248.Kracun I, Rosner H, Cosovic C, Stavljenic A. Topographical atlas of the gangliosides of the adult human brain. J Neurochem 43: 979–989, 1984 [DOI] [PubMed] [Google Scholar]
- 249.Kröcher T, Malinovskaja K, Jürgenson M, Aonurm-Helm A, Zharkovskaya T, Kalda A, Röckle I, Schiff M, Weinhold B, Gerardy-Schahn R, Hildebrandt H, Zharkovsky A. Schizophrenia-like phenotype of polysialyltransferase ST8SIA2-deficient mice. Brain Struct Funct. In press [DOI] [PubMed] [Google Scholar]
- 250.Kurosawa N, Yoshida Y, Kojima N, Tsuji S. Polysialic acid synthase (ST8Sia II STX) mRNA expression in the developing mouse central nervous system. J Neurochem 69: 494–503, 1997 [DOI] [PubMed] [Google Scholar]
- 251.Kustermann S, Hildebrandt H, Bolz S, Dengler K, Kohler K. Genesis of rods in the zebrafish retina occurs in a microenvironment provided by polysialic acid-expressing Muller glia. J Comp Neurol 518: 636–646, 2010 [DOI] [PubMed] [Google Scholar]
- 252.Lahr G, Mayerhofer A, Bucher S, Barthels D, Wille W, Gratzl M. Neural cell adhesion molecules in rat endocrine tissues and tumor cells: distribution and molecular analysis. Endocrinology 132: 1207–1217, 1993 [DOI] [PubMed] [Google Scholar]
- 253.Lairson LL, Henrissat B, Davies GJ, Withers SG. Glycosyltransferases: structures, functions, mechanisms. Annu Rev Biochem. 77: 521–555, 2008 [DOI] [PubMed] [Google Scholar]
- 254.Landmesser L, Dahm L, Schultz K, Rutishauser U. Distinct roles for adhesion molecules during innervation of embryonic chick muscle. Dev Biol 130: 645–670, 1988 [DOI] [PubMed] [Google Scholar]
- 255.Landmesser L, Dahm L, Tang JC, Rutishauser U. Polysialic acid as a regulator of intramuscular nerve branching during embryonic development. Neuron 4: 655–667, 1990 [DOI] [PubMed] [Google Scholar]
- 256.Le Gal La Salle G, Rougon G, Valin A. The embryonic form of neural cell surface molecule (E-NCAM) in the rat hippocampus and its reexpression on glial cells following kainic acid-induced status epilepticus. J Neurosci 12: 872–882, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron 66: 663–670, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lee JK, Zheng B. Role of myelin-associated inhibitors in axonal repair after spinal cord injury. Exp Neurol 235: 33–42, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lehmann F, Tiralongo E, Tiralongo J. Sialic acid-specific lectins: occurrence, specificity and function. Cell Mol Life Sci 63: 1331–1354, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Leroy JG, Seppala R, Huizing M, Dacremont G, De SH, Van Coster RN, Orvisky E, Krasnewich DM, Gahl WA. Dominant inheritance of sialuria, an inborn error of feedback inhibition. Am J Hum Genet 68: 1419–1427, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, Brzustowicz LM, Kaufmann CA, Garver DL, Gurling HM, Lindholm E, Coon H, Moises HW, Byerley W, Shaw SH, Mesen A, Sherrington R, O'Neill FA, Walsh D, Kendler KS, Ekelund J, Paunio T, Lonnqvist J, Peltonen L, O'Donovan MC, Owen MJ, Wildenauer DB, Maier W, Nestadt G, Blouin JL, Antonarakis SE, Mowry BJ, Silverman JM, Crowe RR, Cloninger CR, Tsuang MT, Malaspina D, Harkavy-Friedman JM, Svrakic DM, Bassett AS, Holcomb J, Kalsi G, McQuillin A, Brynjolfson J, Sigmundsson T, Petursson H, Jazin E, Zoega T, Helgason T. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: schizophrenia. Am J Hum Genet 73: 34–48, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 25: 409–432, 2002 [DOI] [PubMed] [Google Scholar]
- 263.Li C, Tropak MB, Gerial R, Clapoff S, Abramow-Newerly W, Trapp B, Peterson A, Roder J. Myelination in the absence of myelin-associated glycoprotein. Nature 369: 747–750, 1994 [DOI] [PubMed] [Google Scholar]
- 264.Li H, Babiarz J, Woodbury J, Kane-Goldsmith N, Grumet M. Spatiotemporal heterogeneity of CNS radial glial cells and their transition to restricted precursors. Dev Biol 271: 225–238, 2004 [DOI] [PubMed] [Google Scholar]
- 265.Li H, Grumet M. BMP and LIF signaling coordinately regulate lineage restriction of radial glia in the developing forebrain. Glia 55: 24–35, 2007 [DOI] [PubMed] [Google Scholar]
- 266.Liang F, Seyrantepe V, Landry K, Ahmad R, Ahmad A, Stamatos NM, Pshezhetsky AV. Monocyte differentiation up-regulates the expression of the lysosomal sialidase, Neu1, and triggers its targeting to the plasma membrane via major histocompatibility complex class II-positive compartments. J Biol Chem 281: 27526–27538, 2006 [DOI] [PubMed] [Google Scholar]
- 267.Liedtke S, Geyer H, Wuhrer M, Geyer R, Frank G, Gerardy-Schahn R, Zahringer U, Schachner M. Characterization of N-glycans from mouse brain neural cell adhesion molecule. Glycobiology 11: 373–384, 2001 [DOI] [PubMed] [Google Scholar]
- 268.Lifely MR, Gilbert AS, Moreno C. Sialic acid polysaccharide antigens of Neisseria meningitidis and Escherichia coli: esterification between adjacent residues. Carbohydr Res 94: 193–203, 1981 [DOI] [PubMed] [Google Scholar]
- 269.Lindholm E, Aberg K, Ekholm B, Pettersson U, Adolfsson R, Jazin EE. Reconstruction of ancestral haplotypes in a 12-generation schizophrenia pedigree. Psychiatr Genet 14: 1–8, 2004 [DOI] [PubMed] [Google Scholar]
- 270.Lindhout T, Iqbal U, Willis LM, Reid AN, Li J, Liu X, Moreno M, Wakarchuk WW. Site-specific enzymatic polysialylation of therapeutic proteins using bacterial enzymes. Proc Natl Acad Sci USA 108: 7397–7402, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Liu Y, Wada R, Kawai H, Sango K, Deng C, Tai T, McDonald MP, Araujo K, Crawley JN, Bierfreund U, Sandhoff K, Suzuki K, Proia RL. A genetic model of substrate deprivation therapy for a glycosphingolipid storage disorder. J Clin Invest 103: 497–505, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lüthi A, Laurent JP, Figurov A, Muller D, Schachner M. Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature 372: 777–779, 1994 [DOI] [PubMed] [Google Scholar]
- 273.Luzzati F, Bonfanti L, Fasolo A, Peretto P. DCX and PSA-NCAM expression identifies a population of neurons preferentially distributed in associative areas of different pallial derivatives and vertebrate species. Cereb Cortex 19: 1028–1041, 2009 [DOI] [PubMed] [Google Scholar]
- 274.Luzzati F, Peretto P, Aimar P, Ponti G, Fasolo A, Bonfanti L. Glia-independent chains of neuroblasts through the subcortical parenchyma of the adult rabbit brain. Proc Natl Acad Sci USA 100: 13036–13041, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lyons F, Martin ML, Maguire C, Jackson A, Regan CM, Shelley RK. The expression of an N-CAM serum fragment is positively correlated with severity of negative features in type II schizophrenia. Biol Psychiatry 23: 769–775, 1988 [DOI] [PubMed] [Google Scholar]
- 276.Ma Q, Kobayashi M, Sugiura M, Ozaki N, Nishio K, Shiraishi Y, Furukawa K, Furukawa K, Sugiura Y. Morphological study of disordered myelination and the degeneration of nerve fibers in the spinal cord of mice lacking complex gangliosides. Arch Histol Cytol 66: 37–44, 2003 [DOI] [PubMed] [Google Scholar]
- 277.Maclaren NK, Max SR, Cornblath M, Brady RO, Ozand PT, Campbell J, Rennels M, Mergner WJ, Garcia JH. GM3 gangliosidosis: a novel human sphingolipodystrophy. Pediatrics 57: 106–110, 1976 [PubMed] [Google Scholar]
- 278.Magesh S, Ando H, Tsubata T, Ishida H, Kiso M. High-affinity ligands of Siglec receptors and their therapeutic potentials. Curr Med Chem 18: 3537–3550, 2011 [DOI] [PubMed] [Google Scholar]
- 279.Manzi AE, Higa HH, Diaz S, Varki A. Intramolecular self-cleavage of polysialic acid. J Biol Chem 269: 23617–23624, 1994 [PubMed] [Google Scholar]
- 280.Marcus J, Dupree JL, Popko B. Myelin-associated glycoprotein and myelin galactolipids stabilize developing axo-glial interactions. J Cell Biol 156: 567–577, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Margolis RK, Preti C, Lai D, Margolis RU. Developmental changes in brain glycoproteins. Brain Res 112: 363–369, 1976 [DOI] [PubMed] [Google Scholar]
- 282.Margolis RU, Margolis RK, Chang LB, Preti C. Glycosaminoglycans of brain during development. Biochemistry 14: 85–88, 1975 [DOI] [PubMed] [Google Scholar]
- 283.Marin O. Cellular and molecular mechanisms controlling the migration of neocortical interneurons. Eur J Neurosci 38: 2019–2029, 2013 [DOI] [PubMed] [Google Scholar]
- 284.Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci 26: 441–483, 2003 [DOI] [PubMed] [Google Scholar]
- 285.Marino P, Norreel JC, Schachner M, Rougon G, Amoureux MC. A polysialic acid mimetic peptide promotes functional recovery in a mouse model of spinal cord injury. Exp Neurol 219: 163–174, 2009 [DOI] [PubMed] [Google Scholar]
- 286.Martinez-Palomo A, Braislovsky C, Bernhard W. Ultrastructural modifications of the cell surface and intercellular contacts of some transformed cell strains. Cancer Res 29: 925–937, 1969 [PubMed] [Google Scholar]
- 287.Matsuda J, Suzuki O, Oshima A, Ogura A, Noguchi Y, Yamamoto Y, Asano T, Takimoto K, Sukegawa K, Suzuki Y, Naiki M. Beta-galactosidase-deficient mouse as an animal model for GM1-gangliosidosis. Glycoconj J 14: 729–736, 1997 [DOI] [PubMed] [Google Scholar]
- 288.Matsuda J, Suzuki O, Oshima A, Yamamoto Y, Noguchi A, Takimoto K, Itoh M, Matsuzaki Y, Yasuda Y, Ogawa S, Sakata Y, Nanba E, Higaki K, Ogawa Y, Tominaga L, Ohno K, Iwasaki H, Watanabe H, Brady RO, Suzuki Y. Chemical chaperone therapy for brain pathology in G(M1)-gangliosidosis. Proc Natl Acad Sci USA 100: 15912–15917, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Max SR, Maclaren NK, Brady RO, Bradley RM, Rennels MB, Tanaka J, Garcia JH, Cornblath M. GM3 (hematoside) sphingolipodystrophy. N Engl J Med . 291: 929–931, 1974 [DOI] [PubMed] [Google Scholar]
- 290.Maziade M, Roy MA, Chagnon YC, Cliche D, Fournier JP, Montgrain N, Dion C, Lavallee JC, Garneau Y, Gingras N, Nicole L, Pires A, Ponton AM, Potvin A, Wallot H, Merette C. Shared and specific susceptibility loci for schizophrenia and bipolar disorder: a dense genome scan in Eastern Quebec families. Mol Psychiatry 10: 486–499, 2005 [DOI] [PubMed] [Google Scholar]
- 291.McAuley EZ, Scimone A, Tiwari Y, Agahi G, Mowry BJ, Holliday EG, Donald JA, Weickert CS, Mitchell PB, Schofield PR, Fullerton JM. Identification of sialyltransferase 8B as a generalized susceptibility gene for psychotic and mood disorders on chromosome 15q25–26. PLoS One 7: e38172, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol 14: 581–586, 2002 [DOI] [PubMed] [Google Scholar]
- 293.McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron 13: 805–811, 1994 [DOI] [PubMed] [Google Scholar]
- 294.McKhann GM, Cornblath DR, Griffin JW, Ho TW, Li CY, Jiang Z, Wu HS, Zhaori G, Liu Y, Jou LP. Acute motor axonal neuropathy: a frequent cause of acute flaccid paralysis in China. Ann Neurol 33: 333–342, 1993 [DOI] [PubMed] [Google Scholar]
- 295.McKhann GM, Cornblath DR, Ho T, Li CY, Bai AY, Wu HS, Yei QF, Zhang WC, Zhaori Z, Jiang Z. Clinical and electrophysiological aspects of acute paralytic disease of children and young adults in northern China. Lancet 338: 593–597, 1991 [DOI] [PubMed] [Google Scholar]
- 296.Mehanna A, Jakovcevski I, Acar A, Xiao M, Loers G, Rougon G, Irintchev A, Schachner M. Polysialic acid glycomimetic promotes functional recovery and plasticity after spinal cord injury in mice. Mol Ther 18: 34–43, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mehta NR, Lopez PH, Vyas AA, Schnaar RL. Gangliosides and Nogo receptors independently mediate myelin-associated glycoprotein inhibition of neurite outgrowth in different nerve cells. J Biol Chem 282: 27875–27886, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Mehta NR, Nguyen T, Bullen JW, Griffin JW, Schnaar RL. Myelin-associated glycoprotein (MAG) protects neurons from acute toxicity using a ganglioside-dependent mechanism. ACS Chem Neurosci 1: 215–222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Michon F, Brisson JR, Jennings HJ. Conformational differences between linear alpha (2–8)-linked homosialooligosaccharides and the epitope of the group B meningococcal polysaccharide. Biochemistry 26: 8399–8405, 1987 [DOI] [PubMed] [Google Scholar]
- 300.Mikkonen M, Soininen H, Alafuzof I, Miettinen R. Hippocampal plasticity in Alzheimer's disease. Rev Neurosci 12: 311–325, 2001 [DOI] [PubMed] [Google Scholar]
- 301.Mikkonen M, Soininen H, Kalvianen R, Tapiola T, Ylinen A, Vapalahti M, Paljarvi L, Pitkanen A. Remodeling of neuronal circuitries in human temporal lobe epilepsy: increased expression of highly polysialylated neural cell adhesion molecule in the hippocampus and the entorhinal cortex. Ann Neurol 44: 923–934, 1998 [DOI] [PubMed] [Google Scholar]
- 302.Mikkonen M, Soininen H, Tapiola T, Alafuzoff I, Miettinen R. Hippocampal plasticity in Alzheimer's disease: changes in highly polysialylated NCAM immunoreactivity in the hippocampal formation. Eur J Neurosci 11: 1754–1764, 1999 [DOI] [PubMed] [Google Scholar]
- 303.Milev P, Friedlander DR, Sakurai T, Karthikeyan L, Flad M, Margolis RK, Grumet M, Margolis RU. Interactions of the chondroitin sulfate proteoglycan phosphacan, the extracellular domain of a receptor-type protein tyrosine phosphatase, with neurons, glia, and neural cell adhesion molecules. J Cell Biol 127: 1703–1715, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Miyagi T, Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology 22: 880–896, 2012 [DOI] [PubMed] [Google Scholar]
- 305.Miyakoshi LM, Todeschini AR, Mendez-Otero R, Hedin-Pereira C. Role of the 9-O-acetyl GD3 in subventricular zone neuroblast migration. Mol Cell Neurosci 49: 240–249, 2012 [DOI] [PubMed] [Google Scholar]
- 306.Mlinac K, Fabris D, Vukelic Z, Rozman M, Heffer M, Bognar SK. Structural analysis of brain ganglioside acetylation patterns in mice with altered ganglioside biosynthesis. Carbohydr Res. 382C: 1–8, 2013 [DOI] [PubMed] [Google Scholar]
- 307.Monlezun S, Ouali S, Poulain DA, Theodosis DT. Polysialic acid is required for active phases of morphological plasticity of neurosecretory axons and their glia. Mol Cell Neurosci 29: 516–524, 2005 [DOI] [PubMed] [Google Scholar]
- 308.Montag D, Giese KP, Bartsch U, Martini R, Lang Y, Bluthmann H, Karthingasan J, Kirschner DA, Wintergerst ES, Nave KA, Zielasek J, Toyka KV, Lipp HP, Schachner M. Mice deficient for the myelin-associated glycoprotein show subtle abnormalities in myelin. Neuron 13: 229–246, 1994 [DOI] [PubMed] [Google Scholar]
- 309.Monti E, Miyagi T. Structure and function of mammalian sialidases. Top Curr Chem. In press [DOI] [PubMed] [Google Scholar]
- 310.Mountney A, Zahner MR, Lorenzini I, Oudega M, Schramm LP, Schnaar RL. Sialidase enhances recovery from spinal cord contusion injury. Proc Natl Acad Sci USA 107: 11561–11566, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Mountney A, Zahner MR, Sturgill ER, Riley CJ, Aston JW, Oudega M, Schramm LP, Hurtado A, Schnaar RL. Sialidase, chondroitinase ABC, and combination therapy after spinal cord contusion injury. J Neurotrauma 30: 181–190, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Mühlenhoff M, Eckhardt M, Bethe A, Frosch M, Gerardy-Schahn R. Autocatalytic polysialylation of polysialyltransferase-1. EMBO J 15: 6943–6950, 1996 [PMC free article] [PubMed] [Google Scholar]
- 313.Mühlenhoff M, Eckhardt M, Bethe A, Frosch M, Gerardy-Schahn R. Polysialylation of NCAM by a single enzyme. Curr Biol 6: 1188–1191, 1996 [DOI] [PubMed] [Google Scholar]
- 314.Mühlenhoff M, Manegold A, Windfuhr M, Gotza B, Gerardy-Schahn R. The impact of N-glycosylation on the functions of polysialyltransferases. J Biol Chem 276: 34066–34073, 2001 [DOI] [PubMed] [Google Scholar]
- 315.Mühlenhoff M, Oltmann-Norden I, Weinhold B, Hildebrandt H, Gerardy-Schahn R. Brain development needs sugar: the role of polysialic acid in controlling NCAM functions. Biol Chem 390: 567–574, 2009 [DOI] [PubMed] [Google Scholar]
- 316.Mühlenhoff M, Rollenhagen M, Werneburg S, Gerardy-Schahn R, Hildebrandt H. Polysialic acid: versatile modification of NCAM, SynCAM 1 and Neuropilin-2. Neurochem Res 38: 1134–1143, 2013 [DOI] [PubMed] [Google Scholar]
- 317.Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron 13: 757–767, 1994 [DOI] [PubMed] [Google Scholar]
- 318.Muller D, Djebbara-Hannas Z, Jourdain P, Vutskitz L, Durbec P, Rougon G, Kiss JZ. Brain-derived neurotrophic factor restores long-term potentiation in polysialic acid-neural cell adhesion molecule-deficient hippocampus. Proc Natl Acad Sci USA 97: 4315–4320, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Muller D, Stoppini L, Wang C, Kiss JZ. A role for polysialylated neural cell adhesion molecule in lesion-induced sprouting in hippocampal organotypic cultures. Neuroscience 61: 441–445, 1994 [DOI] [PubMed] [Google Scholar]
- 320.Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ. PSA-NCAM is required for activity-induced synaptic plasticity. Neuron 17: 413–422, 1996 [DOI] [PubMed] [Google Scholar]
- 321.Murakami S, Kamiya M, Akutsu S, Seki T, Kuwabara Y, Arai Y. Straying phenomenon of migrating LHRH neurons and highly polysialylated NCAM in the chick embryo. Neurosci Res 22: 109–115, 1995 [DOI] [PubMed] [Google Scholar]
- 322.Murakami S, Seki T, Rutishauser U, Arai Y. Enzymatic removal of polysialic acid from neural cell adhesion molecule perturbs the migration route of luteinizing hormone-releasing hormone neurons in the developing chick forebrain. J Comp Neurol 420: 171–181, 2000 [DOI] [PubMed] [Google Scholar]
- 323.Murakami S, Seki T, Wakabayashi K, Arai Y. The ontogeny of luteinizing hormone-releasing hormone (LHRH) producing neurons in the chick embryo: possible evidence for migrating LHRH neurons from the olfactory epithelium expressing a highly polysialylated neural cell adhesion molecule. Neurosci Res 12: 421–431, 1991 [DOI] [PubMed] [Google Scholar]
- 324.Mutoh T, Tokuda A, Miyadai T, Hamaguchi M, Fujiki N. Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc Natl Acad Sci USA 92: 5087–5091, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Nacher J, Alonso-Llosa G, Rosell D, McEwen B. PSA-NCAM expression in the piriform cortex of the adult rat. Modulation by NMDA receptor antagonist administration. Brain Res 927: 111–121, 2002 [DOI] [PubMed] [Google Scholar]
- 326.Nacher J, Blasco-Ibanez JM, McEwen BS. Non-granule PSA-NCAM immunoreactive neurons in the rat hippocampus. Brain Res 930: 1–11, 2002 [DOI] [PubMed] [Google Scholar]
- 327.Nacher J, Guirado R, Varea E, Alonso-Llosa G, Röckle I, Hildebrandt H. Divergent impact of the polysialyltransferases ST8SiaII and ST8SiaIV on polysialic acid expression in immature neurons and interneurons of the adult cerebral cortex. Neuroscience 167: 825–837, 2010 [DOI] [PubMed] [Google Scholar]
- 328.Nacher J, Lanuza E, McEwen BS. Distribution of PSA-NCAM expression in the amygdala of the adult rat. Neuroscience 113: 479–484, 2002 [DOI] [PubMed] [Google Scholar]
- 329.Nait-Oumesmar B, Vignais L, Duhamel-Clerin E, Avellana-Adalid V, Rougon G, Baron-van Evercooren A. Expression of the highly polysialylated neural cell adhesion molecule during postnatal myelination and following chemically induced demyelination of the adult mouse spinal cord. Eur J Neurosci 7: 480–491, 1995 [DOI] [PubMed] [Google Scholar]
- 330.Nakata D, Troy FA. Degree of polymerization (DP) of polysialic acid (polySia) on neural cell adhesion molecules (N-CAMS): development and application of a new strategy to accurately determine the DP of polySia chains on N-CAMS. J Biol Chem 280: 38305–38316, 2005 [DOI] [PubMed] [Google Scholar]
- 331.Nakata D, Zhang L, Troy FA. Molecular basis for polysialylation: a novel polybasic polysialyltransferase domain (PSTD) of 32 amino acids unique to the alpha 2,8-polysialyltransferases is essential for polysialylation. Glycoconj J 23: 423–436, 2006 [DOI] [PubMed] [Google Scholar]
- 332.Nakayama J, Fukuda MN, Fredette B, Ranscht B, Fukuda M. Expression cloning of a human polysialyltransferase that forms the polysialylated neural cell adhesion molecule present in embryonic brain. Proc Natl Acad Sci USA 92: 7031–7035, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Nan X, Carubelli I, Stamatos NM. Sialidase expression in activated human T lymphocytes influences production of IFN-gamma. J Leukoc Biol 81: 284–296, 2007 [DOI] [PubMed] [Google Scholar]
- 334.Nave KA. Myelination and support of axonal integrity by glia. Nature 468: 244–252, 2010 [DOI] [PubMed] [Google Scholar]
- 335.Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci 31: 535–561, 2008 [DOI] [PubMed] [Google Scholar]
- 336.Neiiendam JL, Kohler LB, Christensen C, Li S, Pedersen MV, Ditlevsen DK, Kornum MK, Kiselyov VV, Berezin V, Bock E. An NCAM-derived FGF-receptor agonist, the FGL-peptide, induces neurite outgrowth and neuronal survival in primary rat neurons. J Neurochem 91: 920–935, 2004 [DOI] [PubMed] [Google Scholar]
- 337.Nelson RW, Bates PA, Rutishauser U. Protein determinants for specific polysialylation of the neural cell adhesion molecule. J Biol Chem 270: 17171–17179, 1995 [DOI] [PubMed] [Google Scholar]
- 338.Neu U, Bauer J, Stehle T. Viruses and sialic acids: rules of engagement. Curr Opin Struct Biol 21: 610–618, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Nguyen T, Mehta NR, Conant K, Kim K, Jones M, Calabresi PA, Melli G, Hoke A, Schnaar RL, Ming GL, Song H, Keswani SC, Griffin JW. Axonal protective effects of the myelin associated glycoprotein. J Neurosci 29: 630–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Ni L, Chokhawala HA, Cao H, Henning R, Ng L, Huang S, Yu H, Chen X, Fisher AJ. Crystal structures of Pasteurella multocida sialyltransferase complexes with acceptor and donor analogues reveal substrate binding sites and catalytic mechanism. Biochemistry 46: 6288–6298, 2007 [DOI] [PubMed] [Google Scholar]
- 341.Niethammer P, Delling M, Sytnyk V, Dityatev A, Fukami K, Schachner M. Cosignaling of NCAM via lipid rafts and the FGF receptor is required for neuritogenesis. J Cell Biol 157: 521–532, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Nilsson O, Svennerholm L. Characterization and quantitative determination of gangliosides and neutral glycosphingolipids in human liver. J Lipid Res 23: 327–334, 1982 [PubMed] [Google Scholar]
- 343.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 10: 9–22, 2009 [DOI] [PubMed] [Google Scholar]
- 344.Nojiri H, Stroud M, Hakomori S. A specific type of ganglioside as a modulator of insulin-dependent cell growth and insulin receptor tyrosine kinase activity. Possible association of ganglioside-induced inhibition of insulin receptor function and monocytic differentiation induction in HL-60 cells. J Biol Chem 266: 4531–4537, 1991 [PubMed] [Google Scholar]
- 345.Nomura T, Yabe T, Rosenthal ES, Krzan M, Schwartz JP. PSA-NCAM distinguishes reactive astrocytes in 6-OHDA-lesioned substantia nigra from those in the striatal terminal fields. J Neurosci Res 61: 588–596, 2000 [DOI] [PubMed] [Google Scholar]
- 346.Norton WT, Poduslo SE. Myelination in rat brain: changes in myelin composition during brain maturation. J Neurochem 21: 759–773, 1973 [DOI] [PubMed] [Google Scholar]
- 347.Norton WT, Poduslo SE. Myelinisation in rat brain: changes in myelin composition during brain maturation. J Neurochem 21: 759–773, 1973 [DOI] [PubMed] [Google Scholar]
- 348.Nothias F, Vernier P, Von Boxberg Y, Mirman S, Vincent JD. Modulation of NCAM polysialylation is associated with morphofunctional modifications in the hypothalamo-neurohypophysial system during lactation. Eur J Neurosci 9: 1553–1565, 1997 [DOI] [PubMed] [Google Scholar]
- 349.O'Connell AW, Fox GB, Barry T, Murphy KJ, Fichera G, Foley AG, Kelly J, Regan CM. Spatial learning activates neural cell adhesion molecule polysialytion in a corticohippocampal pathway within the medial temporal lobe. J Neurochem 68: 2538–2546, 1997 [DOI] [PubMed] [Google Scholar]
- 350.Odaka M, Yuki N, Hirata K. Anti-GQ1b IgG antibody syndrome: clinical and immunological range. J Neurol Neurosurg Psychiatry 70: 50–55, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell 126: 855–867, 2006 [DOI] [PubMed] [Google Scholar]
- 352.Ohtsubo K, Takamatsu S, Minowa MT, Yoshida A, Takeuchi M, Marth JD. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell 123: 1307–1321, 2005 [DOI] [PubMed] [Google Scholar]
- 353.Okada M, Itoh MM, Haraguchi M, Okajima T, Inoue M, Oishi H, Matsuda Y, Iwamoto T, Kawano T, Fukumoto S, Miyazaki H, Furukawa K, Aizawa S, Furukawa K. β-Series ganglioside deficiency exhibits no definite changes in the neurogenesis and the sensitivity to Fas-mediated apoptosis but impairs regeneration of the lesioned hypoglossal nerve. J Biol Chem 277: 1633–1636, 2002 [DOI] [PubMed] [Google Scholar]
- 354.Oltmann-Norden I, Galuska SP, Hildebrandt H, Geyer R, Gerardy-Schahn R, Geyer H, Mühlenhoff M. Impact of the polysialyltransferases ST8SiaII and ST8SiaIV on polysialic acid synthesis during postnatal mouse brain development. J Biol Chem 283: 1463–1471, 2008 [DOI] [PubMed] [Google Scholar]
- 355.Ong E, Nakayama J, Angata K, Reyes L, Katsuyama T, Arai Y, Fukuda M. Developmental regulation of polysialic acid synthesis in mouse directed by two polysialyltransferases, PST and STX. Glycobiology 8: 415–424, 1998 [DOI] [PubMed] [Google Scholar]
- 356.Ono K, Tomasiewicz H, Magnuson T, Rutishauser U. N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron 13: 595–609, 1994 [DOI] [PubMed] [Google Scholar]
- 357.Ono S, Hane M, Kitajima K, Sato C. Novel regulation of fibroblast growth factor 2 (FGF2)-mediated Cell growth by polysialic acid. J Biol Chem 287: 3710–3722, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Pan B, Fromholt SE, Hess EJ, Crawford TO, Griffin JW, Sheikh KA, Schnaar RL. Myelin-associated glycoprotein and complementary axonal ligands, gangliosides, mediate axon stability in the CNS and PNS: neuropathology and behavioral deficits in single- and double-null mice. Exp Neurol 195: 208–217, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Papastefanaki F, Chen J, Lavdas AA, Thomaidou D, Schachner M, Matsas R. Grafts of Schwann cells engineered to express PSA-NCAM promote functional recovery after spinal cord injury. Brain 130: 2159–2174, 2007 [DOI] [PubMed] [Google Scholar]
- 360.Patenaude SI, Vijay SM, Yang QL, Jennings HJ, Evans SV. Crystallization and preliminary X-ray diffraction analysis of antigen-binding fragments which are specific for antigenic conformations of sialic acid homopolymers. Acta Crystallogr D Biol Crystallogr 54: 1005–1007, 1998 [DOI] [PubMed] [Google Scholar]
- 361.Paukert M, Bergles DE. Synaptic communication between neurons and NG2+ cells. Curr Opin Neurobiol 16: 515–521, 2006 [DOI] [PubMed] [Google Scholar]
- 362.Paulson JC, Macauley MS, Kawasaki N. Siglecs as sensors of self in innate and adaptive immune responses. Ann NY Acad Sci 1253: 37–48, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Pekcec A, Fuest C, Mühlenhoff M, Gerardy-Schahn R, Potschka H. Targeting epileptogenesis-associated induction of neurogenesis by enzymatic depolysialylation of NCAM counteracts spatial learning dysfunction but fails to impact epilepsy development. J Neurochem 105: 389–400, 2008 [DOI] [PubMed] [Google Scholar]
- 364.Pekcec A, Mühlenhoff M, Gerardy-Schahn R, Potschka H. Impact of the PSA-NCAM system on pathophysiology in a chronic rodent model of temporal lobe epilepsy. Neurobiol Dis 27: 54–66, 2007 [DOI] [PubMed] [Google Scholar]
- 365.Pencea V, Bingaman KD, Freedman LJ, Luskin MB. Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp Neurol 172: 1–16, 2001 [DOI] [PubMed] [Google Scholar]
- 366.Pencea V, Luskin MB. Prenatal development of the rodent rostral migratory stream. J Comp Neurol 463: 402–418, 2003 [DOI] [PubMed] [Google Scholar]
- 367.Peretto P, Giachino C, Aimar P, Fasolo A, Bonfanti L. Chain formation and glial tube assembly in the shift from neonatal to adult subventricular zone of the rodent forebrain. J Comp Neurol 487: 407–427, 2005 [DOI] [PubMed] [Google Scholar]
- 368.Pernet V, Joly S, Christ F, Dimou L, Schwab ME. Nogo-A and myelin-associated glycoprotein differently regulate oligodendrocyte maturation and myelin formation. J Neurosci 28: 7435–7444, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Phillips GR, Krushel LA, Crossin KL. Developmental expression of two rat sialyltransferases that modify the neural cell adhesion molecule, N-CAM. Dev Brain Res 102: 143–155, 1997 [DOI] [PubMed] [Google Scholar]
- 370.Pinto L, Götz M. Radial glial cell heterogeneity–the source of diverse progeny in the CNS. Prog Neurobiol 83: 2–23, 2007 [DOI] [PubMed] [Google Scholar]
- 371.Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barre syndrome. Lancet 349: 225–230, 1997 [PubMed] [Google Scholar]
- 372.Poltorak M, Khoja I, Hemperly JJ, Williams JR, el Mallakh R, Freed WJ. Disturbances in cell recognition molecules (N-CAM and L1 antigen) in the CSF of patients with schizophrenia. Exp Neurol 131: 266–272, 1995 [DOI] [PubMed] [Google Scholar]
- 373.Ponti G, Aimar P, Bonfanti L. Cellular composition and cytoarchitecture of the rabbit subventricular zone and its extensions in the forebrain. J Comp Neurol 498: 491–507, 2006 [DOI] [PubMed] [Google Scholar]
- 374.Probstmeier R, Bilz A, Schneider-Schaulies J. Expression of the neural cell adhesion molecule and polysialic acid during early mouse embryogenesis. J Neurosci Res 37: 324–335, 1994 [DOI] [PubMed] [Google Scholar]
- 375.Proia RL. Glycosphingolipid functions: insights from engineered mouse models. Philos Trans R Soc Lond B Biol Sci 358: 879–883, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Prolo LM, Vogel H, Reimer RJ. The lysosomal sialic acid transporter sialin is required for normal CNS myelination. J Neurosci 29: 15355–15365, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Quarles RH. Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem 100: 1431–1448, 2007 [DOI] [PubMed] [Google Scholar]
- 378.Quarles RH, Ilyas AA, Willison HJ. Antibodies to glycolipids in demyelinating diseases of the human peripheral nervous system. Chem Phys Lipids 42: 235–248, 1986 [DOI] [PubMed] [Google Scholar]
- 379.Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol 494: 415–434, 2006 [DOI] [PubMed] [Google Scholar]
- 380.Radyushkin K, Anokhin K, Meyer BI, Jiang Q, Alvarez-Bolado G, Gruss P. Genetic ablation of the mammillary bodies in the Foxb1 mutant mouse leads to selective deficit of spatial working memory. Eur J Neurosci 21: 219–229, 2005 [DOI] [PubMed] [Google Scholar]
- 381.Repnikova E, Koles K, Nakamura M, Pitts J, Li H, Ambavane A, Zoran MJ, Panin VM. Sialyltransferase regulates nervous system function in Drosophila. J Neurosci 30: 6466–6476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Rey-Gallardo A, Delgado-Martin C, Gerardy-Schahn R, Rodriguez-Fernandez JL, Vega MA. Polysialic acid is required for neuropilin-2a/b-mediated control of CCL21-driven chemotaxis of mature dendritic cells and for their migration in vivo. Glycobiology 21: 655–662, 2011 [DOI] [PubMed] [Google Scholar]
- 383.Rey-Gallardo A, Escribano C, Delgado-Martin C, Rodriguez-Fernandez JL, Gerardy-Schahn R, Rutishauser U, Corbi AL, Vega MA. Polysialylated neuropilin-2 enhances human dendritic cell migration through the basic C-terminal region of CCL21. Glycobiology 20: 1139–1146, 2010 [DOI] [PubMed] [Google Scholar]
- 384.Richardson PJ, Walker JH, Jones RT, Whittaker VP. Identification of a cholinergic-specific antigen Chol-1 as a ganglioside. J Neurochem 38: 1605–1614, 1982 [DOI] [PubMed] [Google Scholar]
- 385.Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat Neurosci 9: 1274–1283, 2006 [DOI] [PubMed] [Google Scholar]
- 386.Röckle I, Seidenfaden R, Weinhold B, Mühlenhoff M, Gerardy-Schahn R, Hildebrandt H. Polysialic acid controls NCAM-induced differentiation of neuronal precursors into calretinin-positive olfactory bulb interneurons. Dev Neurobiol 68: 1170–1184, 2008 [DOI] [PubMed] [Google Scholar]
- 387.Rogers GN, Herrler G, Paulson JC, Klenk HD. Influenza C virus uses 9-O-acetyl-N-acetylneuraminic acid as a high affinity receptor determinant for attachment to cells. J Biol Chem 261: 5947–5951, 1986 [PubMed] [Google Scholar]
- 388.Rogers TB, Snyder SH. High affinity binding of tetanus toxin to mammalian brain membranes. J Biol Chem 256: 2402–2407, 1981 [PubMed] [Google Scholar]
- 389.Roisen FJ, Bartfeld H, Nagele R, Yorke G. Ganglioside stimulation of axonal sprouting in vitro. Science 214: 577–578, 1981 [DOI] [PubMed] [Google Scholar]
- 390.Rolf B, Bastmeyer M, Schachner M, Bartsch U. Pathfinding errors of corticospinal axons in neural cell adhesion molecule-deficient mice. J Neurosci 22: 8357–8362, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Rollenhagen M, Buettner FF, Reismann M, Jirmo AC, Grove M, Behrens GM, Gerardy-Schahn R, Hanisch FG, Mühlenhoff M. Polysialic acid on neuropilin-2 is exclusively synthesized by the polysialyltransferase ST8SiaIV and attached to mucin-type O-glycans located between the b2 and c Domain. J Biol Chem 288: 22880–22892, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Rollenhagen M, Kuckuck S, Ulm C, Hartmann M, Galuska SP, Geyer R, Geyer H, Mühlenhoff M. Polysialylation of the synaptic cell adhesion molecule 1 (SynCAM 1) depends exclusively on the polysialyltransferase ST8SiaII in vivo. J Biol Chem 287: 35170–35180, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Romero-Ramirez L, Nieto-Sampedro M. Inhibiting human astrocytoma growth: structure-activity relationships in neurostatin related glycolipids. J Med Chem 47: 4983–4984, 2004 [DOI] [PubMed] [Google Scholar]
- 394.Rousselot P, Nottebohm F. Expression of polysialylated N-CAM in the central nervous system of adult canaries and its possible relation to function. J Comp Neurol 356: 629–640, 1995 [DOI] [PubMed] [Google Scholar]
- 395.Rudd PM, Merry AH, Wormald MR, Dwek RA. Glycosylation and prion protein. Curr Opin Struct Biol 12: 578–586, 2002 [DOI] [PubMed] [Google Scholar]
- 396.Rummel A, Eichner T, Weil T, Karnath T, Gutcaits A, Mahrhold S, Sandhoff K, Proia RL, Acharya KR, Bigalke H, Binz T. Identification of the protein receptor binding site of botulinum neurotoxins B and G proves the double-receptor concept. Proc Natl Acad Sci USA 104: 359–364, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci 9: 26–35, 2008 [DOI] [PubMed] [Google Scholar]
- 398.Rutishauser U, Landmesser L. Polysialic acid on the surface of axons regulates patterns of normal and activity-dependent innervation. Trends Neurosci 14: 528–532, 1991 [DOI] [PubMed] [Google Scholar]
- 399.Rutishauser U, Landmesser L. Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell-cell interactions. Trends Neurosci 19: 422–427, 1996 [DOI] [PubMed] [Google Scholar]
- 400.Saadat L, Dupree JL, Kilkus J, Han X, Traka M, Proia RL, Dawson G, Popko B. Absence of oligodendroglial glucosylceramide synthesis does not result in CNS myelin abnormalities or alter the dysmyelinating phenotype of CGT-deficient mice. Glia 58: 391–398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Sadoul R, Hirn M, Deagostini-Bazin H, Rougon G, Goridis C. Adult and embryonic mouse neural cell adhesion molecules have different binding properties. Nature 304: 347–349, 1983 [DOI] [PubMed] [Google Scholar]
- 402.Saffell JL, Williams EJ, Mason IJ, Walsh FS, Doherty P. Expression of a dominant negative FGF receptor inhibits axonal growth and FGF receptor phosphorylation stimulated by CAMs. Neuron 18: 231–242, 1997 [DOI] [PubMed] [Google Scholar]
- 403.Saito M, Kitamura H, Sugiyama K. A novel heptasialosyl c-series ganglioside in embryonic chicken brain: its structure and stage-specific expression. Biochim Biophys Acta 1571: 18–26, 2002 [DOI] [PubMed] [Google Scholar]
- 404.Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, Rowitch DH, Alvarez-Buylla A. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478: 382–386, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia VJ, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427: 740–744, 2004 [DOI] [PubMed] [Google Scholar]
- 406.Sandhoff K, Harzer K. Gangliosides and gangliosidoses: principles of molecular and metabolic pathogenesis. J Neurosci 33: 10195–10208, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia 46: 225–251, 2004 [DOI] [PubMed] [Google Scholar]
- 408.Sato C, Kitajima K. Disialic, oligosialic and polysialic acids: distribution, functions and related disease. J Biochem 154: 115–136, 2013 [DOI] [PubMed] [Google Scholar]
- 409.Sawada N, Ishida H, Collins BE, Schnaar RL, Kiso M. Ganglioside GD1α analogs as high affinity ligands for myelin-associated glycoprotein. Carbohydr Res 316: 1–5, 1999 [DOI] [PubMed] [Google Scholar]
- 410.Sawamoto K, Hirota Y, Alfaro-Cervello C, Soriano-Navarro M, He X, Hayakawa-Yano Y, Yamada M, Hikishima K, Tabata H, Iwanami A, Nakajima K, Toyama Y, Itoh T, Alvarez-Buylla A, Garcia-Verdugo JM, Okano H. Cellular composition and organization of the subventricular zone and rostral migratory stream in the adult and neonatal common marmoset brain. J Comp Neurol 519: 690–713, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Schäfer M, Fruttiger M, Montag D, Schachner M, Martini R. Disruption of the gene for the myelin-associated glycoprotein improves axonal regrowth along myelin in C57BL/Wlds mice. Neuron 16: 1107–1113, 1996 [DOI] [PubMed] [Google Scholar]
- 412.Schauer R. Sialic acids: fascinating sugars in higher animals and man. Zoology 107: 49–64, 2004 [DOI] [PubMed] [Google Scholar]
- 413.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol 19: 507–514, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Schauer R, Rudiger WV, Sander M, Corfield AP, Wiegandt H. “Neuraminidase-resistant” sialic acid residues of gangliosides. Adv Exp Med Biol 125: 283–294, 1980 [PubMed] [Google Scholar]
- 415.Scheidegger EP, Sternberg LR, Roth J, Lowe JB. A human STX cDNA confers polysialic acid expression in mammalian cells. J Biol Chem 270: 22685–22688, 1995 [DOI] [PubMed] [Google Scholar]
- 416.Schiff M, Röckle I, Burkhardt H, Weinhold B, Hildebrandt H. Thalamocortical pathfinding defects precede degeneration of the reticular thalamic nucleus in polysialic acid-deficient mice. J Neurosci 31: 1302–1312, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Schiff M, Weinhold B, Grothe C, Hildebrandt H. NCAM and polysialyltransferase profiles match dopaminergic marker gene expression but polysialic acid is dispensable for development of the midbrain dopamine system. J Neurochem 110: 1661–1673, 2009 [DOI] [PubMed] [Google Scholar]
- 418.Schlosshauer B, Blum AS, Mendez-Otero R, Barnstable CJ, Constantine-Paton M. Developmental regulation of ganglioside antigens recognized by the JONES antibody. J Neurosci 8: 580–592, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Schnaar RL. Isolation of glycosphingolipids. Methods Enzymol 230: 348–370, 1994 [DOI] [PubMed] [Google Scholar]
- 420.Schnaar RL. Glycolipid-mediated cell-cell recognition in inflammation and nerve regeneration. Arch Biochem Biophys 426: 163–172, 2004 [DOI] [PubMed] [Google Scholar]
- 421.Schnaar RL. Brain glycolipids: insights from genetic modifications of biosynthetic enzymes. In: Neuroglycobiology, edited by Fukuda M, Rutishauser U, Schnaar RL, Yamaguchi Y. Oxford, UK: Oxford Univ. Press, 2005, p. 95–113 [Google Scholar]
- 422.Schnaar RL. Brain gangliosides in axon-myelin stability and axon regeneration. FEBS Lett 584: 1741–1747, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Schnaar RL, Lopez PH. Myelin-associated glycoprotein and its axonal receptors. J Neurosci Res 87: 3267–3276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Schneider JS, Gollomp SM, Sendek S, Colcher A, Cambi F, Du W. A randomized, controlled, delayed start trial of GM1 ganglioside in treated Parkinson's disease patients. J Neurol Sci 324: 140–148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Schneider MC, Exley RM, Ram S, Sim RB, Tang CM. Interactions between Neisseria meningitidis and the complement system. Trends Microbiol 15: 233–240, 2007 [DOI] [PubMed] [Google Scholar]
- 426.Schulz EC, Neumann P, Gerardy-Schahn R, Sheldrick GM, Ficner R. Structure analysis of endosialidase NF at 0.98 A resolution. Acta Crystallogr D Biol Crystallogr 66: 176–180, 2010 [DOI] [PubMed] [Google Scholar]
- 427.Schulz EC, Schwarzer D, Frank M, Stummeyer K, Mühlenhoff M, Dickmanns A, Gerardy-Schahn R, Ficner R. Structural basis for the recognition and cleavage of polysialic acid by the bacteriophage K1F tailspike protein EndoNF. J Mol Biol 397: 341–351, 2010 [DOI] [PubMed] [Google Scholar]
- 428.Schuster T, Krug M, Stalder M, Hackel N, Gerardy-Schahn R, Schachner M. Immunoelectron microscopic localization of the neural recognition molecules L1, NCAM, and its isoform NCAM180, the NCAM-associated polysialic acid, beta1 integrin and the extracellular matrix molecule tenascin-R in synapses of the adult rat hippocampus. J Neurobiol 49: 142–158, 2001 [DOI] [PubMed] [Google Scholar]
- 429.Schwanzel-Fukuda M, Crossin KL, Pfaff DW, Bouloux PMG, Hardelin JP, Petit C. Migration of luteinizing hormone-releasing hormone (LHRH) neurons in early human embryos. J Comp Neurol 366: 547–557, 1996 [DOI] [PubMed] [Google Scholar]
- 430.Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature 338: 161–164, 1989 [DOI] [PubMed] [Google Scholar]
- 431.Schwarzer D, Stummeyer K, Haselhorst T, Freiberger F, Rode B, Grove M, Scheper T, von IM, Mühlenhoff M, Gerardy-Schahn R. Proteolytic release of the intramolecular chaperone domain confers processivity to endosialidase F. J Biol Chem 284: 9465–9474, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Schwarzkopf M, Knobeloch KP, Rohde E, Hinderlich S, Wiechens N, Lucka L, Horak I, Reutter W, Horstkorte R. Sialylation is essential for early development in mice. Proc Natl Acad Sci USA 99: 5267–5270, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Seidenfaden R, Krauter A, Hildebrandt H. The neural cell adhesion molecule NCAM regulates neuritogenesis by multiple mechanisms of interaction. Neurochem Int 49: 1–11, 2006 [DOI] [PubMed] [Google Scholar]
- 434.Seidenfaden R, Krauter A, Schertzinger F, Gerardy-Schahn R, Hildebrandt H. Polysialic acid directs tumor cell growth by controlling heterophilic neural cell adhesion molecule interactions. Mol Cell Biol 23: 5908–5918, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Seki T. Expression patterns of immature neuronal markers PSA-NCAM, CRMP-4 and NeuroD in the hippocampus of young adult and aged rodents. J Neurosci Res 70: 327–334, 2002 [DOI] [PubMed] [Google Scholar]
- 436.Seki T. Hippocampal adult neurogenesis occurs in a microenvironment provided by PSA-NCAM-expressing immature neurons. J Neurosci Res 69: 772–783, 2002 [DOI] [PubMed] [Google Scholar]
- 437.Seki T, Arai Y. Expression of highly polysialylated NCAM in the neocortex and piriform cortex of the developing and the adult rat. Anat Embryol 184: 395–401, 1991 [DOI] [PubMed] [Google Scholar]
- 438.Seki T, Arai Y. The persistent expression of a highly polysialylated NCAM in the dentate gyrus of the adult rat. Neurosci Res 12: 503–513, 1991 [DOI] [PubMed] [Google Scholar]
- 439.Seki T, Arai Y. Distribution and possible roles of the highly polysialylated neural cell adhesion molecule (NCAM-H) in the developing and adult central nervous system. Neurosci Res 17: 265–290, 1993 [DOI] [PubMed] [Google Scholar]
- 440.Seki T, Arai Y. Highly polysialylated neural cell adhesion molecule (NCAM- H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci 13: 2351–2358, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Seki T, Namba T, Mochizuki H, Onodera M. Clustering, migration, and neurite formation of neural precursor cells in the adult rat hippocampus. J Comp Neurol 502: 275–290, 2007 [DOI] [PubMed] [Google Scholar]
- 442.Seki T, Rutishauser U. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J Neurosci 18: 3757–3766, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Senkov O, Sun M, Weinhold B, Gerardy-Schahn R, Schachner M, Dityatev A. Polysialylated neural cell adhesion molecule is involved in induction of long-term potentiation and memory acquisition and consolidation in a fear-conditioning paradigm. J Neurosci 26: 10888–10898, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol 478: 359–378, 2004 [DOI] [PubMed] [Google Scholar]
- 445.Seyrantepe V, Poupetova H, Froissart R, Zabot MT, Maire I, Pshezhetsky AV. Molecular pathology of NEU1 gene in sialidosis. Hum Mutat 22: 343–352, 2003 [DOI] [PubMed] [Google Scholar]
- 446.Sheikh KA. Autoantobodies activate small GTPase RhoA to modulate neurite outgrowth. Small GTPases 2: 233–238, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Sheikh KA, Sun J, Liu Y, Kawai H, Crawford TO, Proia RL, Griffin JW, Schnaar RL. Mice lacking complex gangliosides develop Wallerian degeneration and myelination defects. Proc Natl Acad Sci USA 96: 7532–7537, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol 3: a004697, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Simpson MA, Cross H, Gurtz K, Priestman DA, Neville DCA, Reinkensmeier G, Wiznitzer M, Proukakis C, Verganelaki A, Pryde A, Patton MA, Dwek RA, Butters TD, Platt FM, Crosby AH. Infantile onset symptomatic epilepsy syndrome caused by homozygous loss of function mutations in GM3 synthase. Nat Genet 36: 1225–1229, 2004 [DOI] [PubMed] [Google Scholar]
- 450.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem 69: 531–569, 2000 [DOI] [PubMed] [Google Scholar]
- 451.Skripuletz T, Gudi V, Hackstette D, Stangel M. De- and remyelination in the CNS white and grey matter induced by cuprizone: the old, the new, and the unexpected. Histol Histopathol 26: 1585–1597, 2011 [DOI] [PubMed] [Google Scholar]
- 452.Smith NJ, Winstone AM, Stellitano L, Cox TM, Verity CM. GM2 gangliosidosis in a UK study of children with progressive neurodegeneration: 73 cases reviewed. Dev Med Child Neurol 54: 176–182, 2012 [DOI] [PubMed] [Google Scholar]
- 453.Snyder JS, Radik R, Wojtowicz JM, Cameron HA. Anatomical gradients of adult neurogenesis and activity: young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus 19: 360–370, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Soares S, Von Boxberg Y, Ravaille-Veron M, Vincent JD, Nothias F. Morphofunctional plasticity in the adult hypothalamus induces regulation of polysialic acid-neural cell adhesion molecule through changing activity and expression levels of polysialyltransferases. J Neurosci 20: 2551–2557, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Sonnenburg JL, Altheide TK, Varki A. A uniquely human consequence of domain-specific functional adaptation in a sialic acid-binding receptor. Glycobiology 14: 339–346, 2004 [DOI] [PubMed] [Google Scholar]
- 456.Sonninen P, Autti T, Varho T, Hamalainen M, Raininko R. Brain involvement in Salla disease. Am J Neuroradiol 20: 433–443, 1999 [PMC free article] [PubMed] [Google Scholar]
- 457.Sonnino S, Mauri L, Ciampa MG, Prinetti A. Gangliosides as regulators of cell signaling: ganglioside-protein interactions or ganglioside-driven membrane organization? J Neurochem 124: 432–435, 2013 [DOI] [PubMed] [Google Scholar]
- 458.Sonnino S, Prinetti A. Membrane domains and the “lipid raft” concept. Curr Med Chem 20: 4–21, 2013 [PubMed] [Google Scholar]
- 459.Stanley P, Schachter H, Taniguchi N. N-glycans. In: Essentials of Glycobiology (2nd ed.), edited by Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. New York: Cold Spring Harbor Laboratory Press, 2009, p. 101–14 [Google Scholar]
- 460.Steenackers A, Vanbeselaere J, Cazet A, Bobowski M, Rombouts Y, Colomb F, Le B, X, Guerardel Y, Delannoy P. Accumulation of unusual gangliosides G(Q3) and G(P3) in breast cancer cells expressing the G(D3) synthase. Molecules 17: 9559–9572, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Stenmark P, Dupuy J, Imamura A, Kiso M, Stevens RC. Crystal structure of botulinum neurotoxin type A in complex with the cell surface co-receptor GT1b-insight into the toxin-neuron interaction. PLoS Pathog 4: e1000129, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Stevens J, Blixt O, Paulson JC, Wilson IA. Glycan microarray technologies: tools to survey host specificity of influenza viruses. Nat Rev Microbiol 4: 857–864, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Stoenica L, Senkov O, Gerardy-Schahn R, Weinhold B, Schachner M, Dityatev A. In vivo synaptic plasticity in the dentate gyrus of mice deficient in the neural cell adhesion molecule NCAM or its polysialic acid. Eur J Neurosci 23: 2255–2264, 2006 [DOI] [PubMed] [Google Scholar]
- 464.Storms SD, Rutishauser U. A role for polysialic acid in neural cell adhesion molecule heterophilic binding to proteoglycans. J Biol Chem 273: 27124–27129, 1998 [DOI] [PubMed] [Google Scholar]
- 465.Strehle EM. Sialic acid storage disease and related disorders. Genet Test 7: 113–121, 2003 [DOI] [PubMed] [Google Scholar]
- 466.Stummeyer K, Dickmanns A, Mühlenhoff M, Gerardy-Schahn R, Ficner R. Crystal structure of the polysialic acid-degrading endosialidase of bacteriophage K1F. Nat Struct Mol Biol 12: 90–96, 2005 [DOI] [PubMed] [Google Scholar]
- 467.Sturgill ER, Aoki K, Lopez PH, Colacurcio D, Vajn K, Lorenzini I, Majic S, Yang WH, Heffer M, Tiemeyer M, Marth JD, Schnaar RL. Biosynthesis of the major brain gangliosides GD1a and GT1b. Glycobiology 22: 1289–1301, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Sullivan PF, Keefe RS, Lange LA, Lange EM, Stroup TS, Lieberman J, Maness PF. NCAM1 and neurocognition in schizophrenia. Biol Psychiatry 61: 902–910, 2007 [DOI] [PubMed] [Google Scholar]
- 469.Susuki K, Baba H, Tohyama K, Kanai K, Kuwabara S, Hirata K, Furukawa K, Furukawa K, Rasband MN, Yuki N. Gangliosides contribute to stability of paranodal junctions and ion channel clusters in myelinated nerve fibers. Glia 55: 746–757, 2007 [DOI] [PubMed] [Google Scholar]
- 470.Svennerholm L, Bostrom K, Fredman P, Mansson JE, Rosengren B, Rynmark BM. Human brain gangliosides: developmental changes from early fetal stage to advanced age. Biochim Biophys Acta 1005: 109–117, 1989 [DOI] [PubMed] [Google Scholar]
- 471.Swaminathan S. Molecular structures and functional relationships in clostridial neurotoxins. FEBS J 278: 4467–4485, 2011 [DOI] [PubMed] [Google Scholar]
- 472.Sytnyk V, Leshchyns'ka I, Delling M, Dityateva G, Dityatev A, Schachner M. Neural cell adhesion molecule promotes accumulation of TGN organelles at sites of neuron-to-neuron contacts. J Cell Biol 159: 649–661, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Tajima O, Egashira N, Ohmi Y, Fukue Y, Mishima K, Iwasaki K, Fujiwara M, Inokuchi J, Sugiura Y, Furukawa K, Furukawa K. Reduced motor and sensory functions and emotional response in GM3-only mice: emergence from early stage of life and exacerbation with aging. Behav Brain Res 198: 74–82, 2009 [DOI] [PubMed] [Google Scholar]
- 474.Takahashi K, Mitoma J, Hosono M, Shiozaki K, Sato C, Yamaguchi K, Kitajima K, Higashi H, Nitta K, Shima H, Miyagi T. Sialidase NEU4 hydrolyzes polysialic acids of neural cell adhesion molecules and negatively regulates neurite formation by hippocampal neurons. J Biol Chem 287: 14816–14826, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Takamiya K, Yamamoto A, Furukawa K, Yamashiro S, Shin M, Okada M, Fukumoto S, Haraguchi M, Takeda N, Fujimura K, Sakae M, Kishikawa M, Shiku H, Aizawa S. Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc Natl Acad Sci USA 93: 10662–10667, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Takashima S, Matsumoto T, Tsujimoto M, Tsuji S. Effects of amino acid substitutions in the sialylmotifs on molecular expression and enzymatic activities of alpha2,8-sialyltransferases ST8Sia-I and ST8Sia-VI. Glycobiology. 23: 603–612, 2013 [DOI] [PubMed] [Google Scholar]
- 477.Tanaka J, Garcia JH, Max SR, Viloria JE, Kamijyo Y, McLaren NK, Cornblath M, Brady RO. Cerebral sponginess and GM3 gangliosidosis; ultrastructure and probable pathogenesis. J Neuropathol Exp Neurol 34: 249–262, 1975 [DOI] [PubMed] [Google Scholar]
- 478.Tang J, Landmesser L, Rutishauser U. Polysialic acid influences specific pathfinding by avian motoneurons. Neuron 8: 1031–1044, 1992 [DOI] [PubMed] [Google Scholar]
- 479.Tang J, Rutishauser U, Landmesser L. Polysialic acid regulates growth cone behavior during sorting of motor axons in the plexus region. Neuron 13: 405–414, 1994 [DOI] [PubMed] [Google Scholar]
- 480.Tao R, Li C, Zheng Y, Qin W, Zhang J, Li X, Xu Y, Shi YY, Feng G, He L. Positive association between SIAT8B and schizophrenia in the Chinese Han population. Schizophr Res 90: 108–114, 2007 [DOI] [PubMed] [Google Scholar]
- 481.Taylor ME, Drickamer K. Introduction to Glycobiology (3rd ed.). Oxford, UK: Oxford Univ. Press, 2011 [Google Scholar]
- 482.Tettamanti G, Bonali F, Marchesini S, Zambotti V. A new procedure for the extraction, purification and fractionation of brain gangliosides. Biochim Biophys Acta 296: 160–170, 1973 [DOI] [PubMed] [Google Scholar]
- 483.The Guillain-Barre syndrome Study Group. Plasmapheresis and acute Guillain-Barre syndrome. Neurology 35: 1096–1104, 1985 [PubMed] [Google Scholar]
- 484.Theodosis DT, Bonhomme R, Vitiello S, Rougon G, Poulain DA. Cell surface expression of polysialic acid on NCAM is a prerequisite for activity-dependent morphological neuronal and glial plasticity. J Neurosci 19: 10228–10236, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev 88: 983–1008, 2008 [DOI] [PubMed] [Google Scholar]
- 486.Theodosis DT, Rougon G, Poulain DA. Retention of embryonic features by an adult neuronal system capable of plasticity: polysialylated neural cell adhesion molecule in the hypothalamo-neurohypophysial system. Proc Natl Acad Sci USA 88: 5494–5498, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Thompson MG, Foley DA, Colley KJ. The polysialyltransferases interact with sequences in two domains of the neural cell adhesion molecule to allow its polysialylation. J Biol Chem 288: 7282–7293, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Thompson MG, Foley DA, Swartzentruber KG, Colley KJ. Sequences at the interface of the fifth immunoglobulin domain and first fibronectin type III repeat of the neural cell adhesion molecule are critical for its polysialylation. J Biol Chem 286: 4525–4534, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Todeschini AR, Hakomori SI. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim Biophys Acta 1780: 421–433, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Tomasiewicz H, Ono K, Yee D, Thompson C, Goridis C, Rutishauser U, Magnuson T. Genetic deletion of a neural cell adhesion molecule variant (N-CAM-180) produces distinct defects in the central nervous system. Neuron 11: 1163–1174, 1993 [DOI] [PubMed] [Google Scholar]
- 491.Trapp BD, Andrews SB, Cootauco C, Quarles R. The myelin-associated glycoprotein is enriched in multivesicular bodies and periaxonal membranes of actively myelinating oligodendrocytes. J Cell Biol 109: 2417–2426, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci 31: 247–269, 2008 [DOI] [PubMed] [Google Scholar]
- 493.Trotter J, Bitter-Suermann D, Schachner M. Differentiation-regulated loss of the polysialylated embryonic form and expression of the different polypeptides of the neural cell adhesion molecule by cultured oligodendrocytes and myelin. J Neurosci Res 22: 369–383, 1989 [DOI] [PubMed] [Google Scholar]
- 494.Trotter J, Karram K, Nishiyama A. NG2 cells: properties, progeny and origin. Brain Res Rev 63: 72–82, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Tsuji S, Datta AK, Paulson JC. Systematic nomenclature for sialyltransferases. Glycobiology 6: v–vii, 1996 [DOI] [PubMed] [Google Scholar]
- 496.Uryu K, Butler AK, Chesselet MF. Synaptogenesis and ultrastructural localization of the polysialylated neural cell adhesion molecule in the developing striatum. J Comp Neurol 405: 216–232, 1999 [DOI] [PubMed] [Google Scholar]
- 497.Vaithianathan T, Matthias K, Bahr B, Schachner M, Suppiramaniam V, Dityatev A, Steinhauser C. Neural cell adhesion molecule-associated polysialic acid potentiates alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor currents. J Biol Chem 279: 47975–47984, 2004 [DOI] [PubMed] [Google Scholar]
- 498.Van der Meche FG, Schmitz PI. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barre syndrome. Dutch Guillain-Barre Study Group. N Engl J Med 326: 1123–1129, 1992 [DOI] [PubMed] [Google Scholar]
- 499.Van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barre syndrome. Lancet Neurol 7: 939–950, 2008 [DOI] [PubMed] [Google Scholar]
- 500.Van Heyningen WE. Tentative identification of the tetanus toxin recptor in nervous tissue. J Gen Microbiol 20: 310–320, 1959 [DOI] [PubMed] [Google Scholar]
- 501.Van Heyningen WE, Miller PA. The fixation of tetanus toxin by ganglioside. J Gen Microbiol 24: 107–119, 1961 [DOI] [PubMed] [Google Scholar]
- 502.Van Kammen DP, Poltorak M, Kelley ME, Yao JK, Gurklis JA, Peters JL, Hemperly JJ, Wright RD, Freed WJ. Further studies of elevated cerebrospinal fluid neuronal cell adhesion molecule in schizophrenia. Biol Psychiatry 43: 680–686, 1998 [DOI] [PubMed] [Google Scholar]
- 503.Varea E, Belles M, Vidueira S, Blasco-Ibanez JM, Crespo C, Pastor AM, Nacher J. PSA-NCAM is expressed in immature, but not recently generated, neurons in the adult cat cerebral cortex layer II. Front Neurosci 5: 17, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Varea E, Castillo-Gomez E, Gomez-Climent MA, Blasco-Ibanez JM, Crespo C, Martinez-Guijarro FJ, Nacher J. PSA-NCAM expression in the human prefrontal cortex. J Chem Neuroanat 33: 202–209, 2007 [DOI] [PubMed] [Google Scholar]
- 505.Varea E, Castillo-Gomez E, Gomez-Climent MA, Guirado R, Blasco-Ibanez JM, Crespo C, Martinez-Guijarro FJ, Nacher J. Differential evolution of PSA-NCAM expression during aging of the rat telencephalon. Neurobiol Aging 30: 808–818, 2009 [DOI] [PubMed] [Google Scholar]
- 506.Varea E, Guirado R, Gilabert-Juan J, Marti U, Castillo-Gomez E, Blasco-Ibanez JM, Crespo C, Nacher J. Expression of PSA-NCAM and synaptic proteins in the amygdala of psychiatric disorder patients. J Psychiatr Res 46: 189–197, 2012 [DOI] [PubMed] [Google Scholar]
- 507.Varea E, Nacher J, Blasco-Ibanez JM, Gomez-Climent MA, Castillo-Gomez E, Crespo C, Martinez-Guijarro FJ. PSA-NCAM expression in the rat medial prefrontal cortex. Neuroscience 136: 435–443, 2005 [DOI] [PubMed] [Google Scholar]
- 508.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology 3: 97–130, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Varki A. Selectin ligands. Proc Natl Acad Sci USA 91: 7390–7397, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Varki A. Sialic acids in human health and disease. Trends Mol Med 14: 351–360, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Varki A. Multiple changes in sialic acid biology during human evolution. Glycoconj J 26: 231–245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Varki A. Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc Natl Acad Sci USA 107 Suppl 2: 8939–8946, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Varki A. Evolutionary forces shaping the Golgi glycosylation machinery: why cell surface glycans are universal to living cells. Cold Spring Harb Perspect Biol 3: a005462, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Varki A, Angata T. Siglecs–the major subfamily of I-type lectins. Glycobiology 16: 1R–27R, 2006 [DOI] [PubMed] [Google Scholar]
- 515.Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. Essentials of Glycobiology (2nd ed.). New York: Cold Spring Harbor Laboratory Press, 2009 [PubMed] [Google Scholar]
- 516.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann NY Acad Sci 1253: 16–36, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Varki NM, Varki A. Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab Invest 87: 851–857, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Vasta GR, Ahmed H. Animal Lectins: A Functional View. Boca Raton, FL: Taylor & Francis Group, 2013 [Google Scholar]
- 519.Vawter MP. Dysregulation of the neural cell adhesion molecule and neuropsychiatric disorders. Eur J Pharmacol 405: 385–395, 2000 [DOI] [PubMed] [Google Scholar]
- 520.Vawter MP, Cannon-Spoor HE, Hemperly JJ, Hyde TM, VanderPutten DM, Kleinman JE, Freed WJ. Abnormal expression of cell recognition molecules in schizophrenia. Exp Neurol 149: 424–432, 1998 [DOI] [PubMed] [Google Scholar]
- 521.Vawter MP, Frye MA, Hemperly JJ, VanderPutten DM, Usen N, Doherty P, Saffell JL, Issa F, Post RM, Wyatt RJ, Freed WJ. Elevated concentration of N-CAM VASE isoforms in schizophrenia. J Psychiatr Res 34: 25–34, 2000 [DOI] [PubMed] [Google Scholar]
- 522.Vawter MP, Hemperly JJ, Freed WJ, Garver DL. CSF N-CAM in neuroleptic-naive first-episode patients with schizophrenia. Schizophr Res 34: 123–131, 1998 [DOI] [PubMed] [Google Scholar]
- 523.Venkatesh K, Chivatakarn O, Sheu SS, Giger RJ. Molecular dissection of the myelin-associated glycoprotein receptor complex reveals cell type-specific mechanisms for neurite outgrowth inhibition. J Cell Biol 177: 393–399, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Verheijen FW, Verbeek E, Aula N, Beerens CE, Havelaar AC, Joosse M, Peltonen L, Aula P, Galjaard H, van der Spek PJ, Mancini GM. A new gene, encoding an anion transporter, is mutated in sialic acid storage diseases. Nat Genet 23: 462–465, 1999 [DOI] [PubMed] [Google Scholar]
- 525.Vimr ER, Kalivoda KA, Deszo EL, Steenbergen SM. Diversity of microbial sialic acid metabolism. Microbiol Mol Biol Rev 68: 132–153, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Vinson M, Strijbos PJ, Rowles A, Facci L, Moore SE, Simmons DL, Walsh FS. Myelin-associated glycoprotein interacts with ganglioside GT1b: a mechanism for neurite outgrowth inhibition. J Biol Chem 276: 20280–20285, 2001 [DOI] [PubMed] [Google Scholar]
- 527.Vogt J, Glumm R, Schluter L, Schmitz D, Rost BR, Streu N, Rister B, Suman BB, Gagiannis D, Hildebrandt H, Weinhold B, Mühlenhoff M, Naumann T, Savaskan NE, Brauer AU, Reutter W, Heimrich B, Nitsch R, Horstkorte R. Homeostatic regulation of NCAM polysialylation is critical for correct synaptic targeting. Cell Mol Life Sci 69: 1179–1191, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Von der Ohe M, Wheeler SF, Wuhrer M, Harvey DJ, Liedtke S, Mühlenhoff M, Gerardy-Schahn R, Geyer H, Dwek RA, Geyer R, Wing DR, Schachner M. Localization and characterization of polysialic acid-containing N-linked glycans from bovine NCAM. Glycobiology 12: 47–63, 2002 [DOI] [PubMed] [Google Scholar]
- 529.Von IM. The war against influenza: discovery and development of sialidase inhibitors. Nat Rev Drug Discov 6: 967–974, 2007 [DOI] [PubMed] [Google Scholar]
- 530.Vorobiev I, Matskevich V, Kovnir S, Orlova N, Knorre V, Jain S, Genkin D, Gabibov A, Miroshnikov A. Chemical polysialylation: design of conjugated human oxyntomodulin with a prolonged anorexic effect in vivo. Biochimie 95: 264–270, 2013 [DOI] [PubMed] [Google Scholar]
- 531.Vutskits L, Djebbara-Hannas Z, Zhang H, Paccaud JP, Durbec P, Rougon G, Muller D, Kiss JZ. PSA-NCAM modulates BDNF-dependent survival and differentiation of cortical neurons. Eur J Neurosci 13: 1391–1402, 2001 [DOI] [PubMed] [Google Scholar]
- 532.Vutskits L, Gascon E, Kiss JZ. Removal of PSA from NCAM affects the survival of magnocellular vasopressin- and oxytocin-producing neurons in organotypic cultures of the paraventricular nucleus. Eur J Neurosci 17: 2119–2126, 2003 [DOI] [PubMed] [Google Scholar]
- 533.Vyas AA, Blixt O, Paulson JC, Schnaar RL. Potent glycan inhibitors of myelin-associated glycoprotein enhance axon outgrowth in vitro. J Biol Chem 280: 16305–16310, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Vyas AA, Patel HV, Fromholt SE, Heffer-Lauc M, Vyas KA, Dang J, Schachner M, Schnaar RL. Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration. Proc Natl Acad Sci USA 99: 8412–8417, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Wang C, Rougon G, Kiss JZ. Requirement of polysialic acid for the migration of the O-2A glial progenitor cell from neurohypophyseal explants. J Neurosci 14: 4446–4457, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Wang H, Bright A, Xin B, Bockoven JR, Paller AS. Cutaneous dyspigmentation in patients with ganglioside GM3 synthase deficiency. Am J Med Genet A 161: 875–879, 2013 [DOI] [PubMed] [Google Scholar]
- 537.Wang Y, Neumann H. Alleviation of neurotoxicity by microglial human Siglec-11. J Neurosci 30: 3482–3488, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Watanabe S, Endo S, Oshima E, Hoshi T, Higashi H, Yamada K, Tohyama K, Yamashita T, Hirabayashi Y. Glycosphingolipid synthesis in cerebellar Purkinje neurons: roles in myelin formation and axonal homeostasis. Glia 58: 1197–1207, 2010 [DOI] [PubMed] [Google Scholar]
- 539.Weber M, Modemann S, Schipper P, Trauer H, Franke H, Illes P, Geiger KD, Hengstler JG, Kleemann WJ. Increased polysialic acid neural cell adhesion molecule expression in human hippocampus of heroin addicts. Neuroscience 138: 1215–1223, 2006 [DOI] [PubMed] [Google Scholar]
- 540.Weickert CS, Webster MJ, Colvin SM, Herman MM, Hyde TM, Weinberger DR, Kleinman JE. Localization of epidermal growth factor receptors and putative neuroblasts in human subependymal zone. J Comp Neurol 423: 359–372, 2000 [DOI] [PubMed] [Google Scholar]
- 541.Weinhold B, Seidenfaden R, Röckle I, Mühlenhoff M, Schertzinger F, Conzelmann S, Marth JD, Gerardy-Schahn R, Hildebrandt H. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J Biol Chem 280: 42971–42977, 2005 [DOI] [PubMed] [Google Scholar]
- 542.Wichterle H, Garcia-Verdugo JM, Alvarez-Buylla A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron 18: 779–791, 1997 [DOI] [PubMed] [Google Scholar]
- 543.Williams G, Wood A, Williams EJ, Gao Y, Mercado ML, Katz A, Joseph-McCarthy D, Bates B, Ling HP, Aulabaugh A, Zaccardi J, Xie Y, Pangalos MN, Walsh FS, Doherty P. Ganglioside inhibition of neurite outgrowth requires Nogo receptor function: identification of interaction sites and development of novel antagonists. J Biol Chem 283: 16641–16652, 2008 [DOI] [PubMed] [Google Scholar]
- 544.Willison HJ. The translation of the pathological findings described in humans to experimental models of acute motor axonal neuropathy. J Peripher Nerv Syst 17 Suppl 3: 3–8, 2012 [DOI] [PubMed] [Google Scholar]
- 545.Willison HJ, Goodyear CS. Glycolipid antigens and autoantibodies in autoimmune neuropathies. Trends Immunol 34: 453–459, 2013 [DOI] [PubMed] [Google Scholar]
- 546.Windfuhr M, Manegold A, Mühlenhoff M, Eckhardt M, Gerardy-Schahn R. Molecular defects that cause loss of polysialic acid in the complementation group 2A10. J Biol Chem 275: 32861–32870, 2000 [DOI] [PubMed] [Google Scholar]
- 547.Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA 86: 8132–8136, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Wreden CC, Wlizla M, Reimer RJ. Varied mechanisms underlie the free sialic acid storage disorders. J Biol Chem 280: 1408–1416, 2005 [DOI] [PubMed] [Google Scholar]
- 549.Wu G, Ledeen RW. Stimulation of neurite outgrowth in neuroblastoma cells by neuraminidase: putative role of GM1 ganglioside in differentiation. J Neurochem 56: 95–104, 1991 [DOI] [PubMed] [Google Scholar]
- 550.Wu G, Lu ZH, Kulkarni N, Amin R, Ledeen RW. Mice lacking major brain gangliosides develop parkinsonism. Neurochem Res 36: 1706–1714, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Wu G, Lu ZH, Kulkarni N, Ledeen RW. Deficiency of ganglioside GM1 correlates with Parkinson's disease in mice and humans. J Neurosci Res 90: 1997–2008, 2012 [DOI] [PubMed] [Google Scholar]
- 552.Wu G, Lu ZH, Wang J, Wang Y, Xie X, Meyenhofer MF, Ledeen RW. Enhanced susceptibility to kainate-induced seizures, neuronal apoptosis, and death in mice lacking gangliotetraose gangliosides: protection with LIGA 20, a membrane-permeant analog of GM1. J Neurosci 25: 11014–11022, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Wu G, Xie X, Lu ZH, Ledeen RW. Sodium-calcium exchanger complexed with GM1 ganglioside in nuclear membrane transfers calcium from nucleoplasm to endoplasmic reticulum. Proc Natl Acad Sci USA 106: 10829–10834, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Wu YP, Proia RL. Deletion of macrophage-inflammatory protein 1 alpha retards neurodegeneration in Sandhoff disease mice. Proc Natl Acad Sci USA 101: 8425–8430, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Wuhrer M, Geyer H, von der Ohe M, Gerardy-Schahn R, Schachner M, Geyer R. Localization of defined carbohydrate epitopes in bovine polysialylated NCAM. Biochimie 85: 207–218, 2003 [DOI] [PubMed] [Google Scholar]
- 556.Xu YH, Barnes S, Sun Y, Grabowski GA. Multi-system disorders of glycosphingolipid and ganglioside metabolism. J Lipid Res 51: 1643–1675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Yamamoto T, Ichikawa M, Takakura Y. Conserved amino acid sequences in the bacterial sialyltransferases belonging to glycosyltransferase family 80. Biochem Biophys Res Commun 365: 340–343, 2008 [DOI] [PubMed] [Google Scholar]
- 558.Yamasaki R, Bacon B. Three-dimensional structural analysis of the group B polysaccharide of Neisseria meningitidis 6275 by two-dimensional NMR: the polysaccharide is suggested to exist in helical conformations in solution. Biochemistry 30: 851–857, 1991 [DOI] [PubMed] [Google Scholar]
- 559.Yamashita T, Allende ML, Kalkofen DN, Werth N, Sandhoff K, Proia RL. Conditional LoxP-flanked glucosylceramide synthase allele controlling glycosphingolipid synthesis. Genesis 43: 175–180, 2005 [DOI] [PubMed] [Google Scholar]
- 560.Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, Kono M, Tsuji S, Daniotti JL, Werth N, Sandhoff R, Sandhoff K, Proia RL. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci USA 100: 3445–3449, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Yamashita T, Higuchi H, Tohyama M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J Cell Biol 157: 565–570, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Yamashita T, Wada R, Sasaki T, Deng C, Bierfreund U, Sandhoff K, Proia RL. A vital role for glycosphingolipid synthesis during development and differentiation. Proc Natl Acad Sci USA 96: 9142–9147, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Yamashita T, Wu YP, Sandhoff R, Werth N, Mizukami H, Ellis JM, Dupree JL, Geyer R, Sandhoff K, Proia RL. Interruption of ganglioside synthesis produces central nervous system degeneration and altered axon-glial interactions. Proc Natl Acad Sci USA 102: 2725–2730, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Yang LJ, Lorenzini I, Vajn K, Mountney A, Schramm LP, Schnaar RL. Sialidase enhances spinal axon outgrowth in vivo. Proc Natl Acad Sci USA 103: 11057–11062, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Yang LJS, Zeller CB, Shaper NL, Kiso M, Hasegawa A, Shapiro RE, Schnaar RL. Gangliosides are neuronal ligands for myelin-associated glycoprotein. Proc Natl Acad Sci USA 93: 814–818, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Yang P, Major D, Rutishauser U. Role of charge and hydration in effects of polysialic acid on molecular interactions on and between cell membranes. J Biol Chem 269: 23039–23044, 1994 [PubMed] [Google Scholar]
- 567.Yang P, Yin X, Rutishauser U. Intercellular space is affected by the polysialic acid content of NCAM. J Cell Biol 116: 1487–1496, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Yin X, Crawford TO, Griffin JW, Tu P, Lee VM, Li C, Roder J, Trapp BD. Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J Neurosci 18: 1953–1962, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Yin X, Watanabe M, Rutishauser U. Effect of polysialic acid on the behavior of retinal ganglion cell axons during growth into the optic tract and tectum. Development 121: 3439–3446, 1995 [DOI] [PubMed] [Google Scholar]
- 570.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7: 617–627, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Yoshida K, Rutishauser U, Crandall JE, Schwarting GA. Polysialic acid facilitates migration of luteinizing hormone-releasing hormone neurons on vomeronasal axons. J Neurosci 19: 794–801, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Yoshikawa M, Go S, Takasaki K, Kakazu Y, Ohashi M, Nagafuku M, Kabayama K, Sekimoto J, Suzuki S, Takaiwa K, Kimitsuki T, Matsumoto N, Komune S, Kamei D, Saito M, Fujiwara M, Iwasaki K, Inokuchi J. Mice lacking ganglioside GM3 synthase exhibit complete hearing loss due to selective degeneration of the organ of Corti. Proc Natl Acad Sci USA 106: 9483–9488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Yu RK, Macala LJ, Taki T, Weinfeld HM, Yu FS. Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J Neurochem 50: 1825–1829, 1988 [DOI] [PubMed] [Google Scholar]
- 574.Yu RK, Tsai YT, Ariga T. Functional roles of gangliosides in neurodevelopment: an overview of recent advances. Neurochem Res 37: 1230–1244, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Yuki N. Ganglioside mimicry and peripheral nerve disease. Muscle Nerve 35: 691–711, 2007 [DOI] [PubMed] [Google Scholar]
- 576.Yuki N. Guillain-Barre syndrome and anti-ganglioside antibodies: a clinician-scientist's journey. Proc Jpn Acad Ser B Phys Biol Sci 88: 299–326, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Yuki N, Hartung HP. Guillain-Barre syndrome. N Engl J Med 366: 2294–2304, 2012 [DOI] [PubMed] [Google Scholar]
- 578.Yuki N, Susuki K, Koga M, Nishimoto Y, Odaka M, Hirata K, Taguchi K, Miyatake T, Furukawa K, Kobata T, Yamada M. Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barre syndrome. Proc Natl Acad Sci USA 101: 11404–11409, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Yuki N, Taki T, Inagaki F, Kasama T, Takahashi M, Saito K, Handa S, Miyatake T. A bacterium lipopolysaccharide that elicits Guillain-Barre syndrome has a GM1 ganglioside-like structure. J Exp Med 178: 1771–1775, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Yuki N, Yamada M, Koga M, Odaka M, Susuki K, Tagawa Y, Ueda S, Kasama T, Ohnishi A, Hayashi S, Takahashi H, Kamijo M, Hirata K. Animal model of axonal Guillain-Barre syndrome induced by sensitization with GM1 ganglioside. Ann Neurol 49: 712–720, 2001 [PubMed] [Google Scholar]
- 581.Zapater JL, Colley KJ. Sequences prior to conserved catalytic motifs of the polysialyltransferase, ST8SiaIV, are required for substrate recognition. J Biol Chem 287: 6441–6453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Zhang G, Lehmann HC, Manoharan S, Hashmi M, Shim S, Ming GL, Schnaar RL, Lopez PH, Bogdanova N, Sheikh KA. Anti-ganglioside antibody-mediated activation of RhoA induces inhibition of neurite outgrowth. J Neurosci 31: 1664–1675, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Zhang H, Vutskits L, Calaora V, Durbec P, Kiss JZ. A role for the polysialic acid-neural cell adhesion molecule in PDGF-induced chemotaxis of oligodendrocyte precursor cells. J Cell Sci 117: 93–103, 2004 [DOI] [PubMed] [Google Scholar]
- 584.Zhang Y, Campbell G, Anderson PN, Martini R, Schachner M, Lieberman AR. Molecular basis of interactions between regenerating adult rat thalamic axons and schwann cells in peripheral nerve grafts. 1. Neural cell adhesion molecules. J Comp Neurol 361: 193–209, 1995 [DOI] [PubMed] [Google Scholar]
- 585.Zhang Y, Ghadiri-Sani M, Zhang X, Richardson PM, Yeh J, Bo X. Induced expression of polysialic acid in the spinal cord promotes regeneration of sensory axons. Mol Cell Neurosci 35: 109–119, 2007 [DOI] [PubMed] [Google Scholar]
- 586.Zhang Y, Lee YC. Acid-catalyzed lactonization of alpha2,8-linked oligo/polysialic acids studied by high performance anion-exchange chromatography. J Biol Chem 274: 6183–6189, 1999 [DOI] [PubMed] [Google Scholar]
- 587.Zhang Y, Zhang X, Wu D, Verhaagen J, Richardson PM, Yeh J, Bo X. Lentiviral-mediated expression of polysialic acid in spinal cord and conditioning lesion promote regeneration of sensory axons into spinal cord. Mol Ther 15: 1796–1804, 2007 [DOI] [PubMed] [Google Scholar]
- 588.Zhang Y, Zhang X, Yeh J, Richardson P, Bo X. Engineered expression of polysialic acid enhances Purkinje cell axonal regeneration in L1/GAP-43 double transgenic mice. Eur J Neurosci 25: 351–361, 2007 [DOI] [PubMed] [Google Scholar]
- 589.Ziak M, Qu B, Zuo X, Zuber C, Kanamori A, Kitajima K, Inoue S, Inoue Y, Roth J. Occurrence of poly(alpha2,8-deaminoneuraminic acid) in mammalian tissues: widespread and developmentally regulated but highly selective expression on glycoproteins. Proc Natl Acad Sci USA 93: 2759–2763, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Zuber C, Lackie PM, Catterall WA, Roth J. Polysialic acid is associated with sodium channels and the neural cell adhesion molecule N-CAM in adult rat brain. J Biol Chem 267: 9965–9971, 1992 [PubMed] [Google Scholar]