Abstract
Mitosis is promoted and regulated by reversible protein phosphorylation catalyzed by the essential NIMA and CDK1 kinases in the model filamentous fungus Aspergillus nidulans. Protein methylation mediated by the Set1/COMPASS methyltransferase complex has also been shown to regulate mitosis in budding yeast with the Aurora mitotic kinase. We uncover a genetic interaction between An-swd1, which encodes a subunit of the Set1 protein methyltransferase complex, with NIMA as partial inactivation of nimA is poorly tolerated in the absence of swd1. This genetic interaction is additionally seen without the Set1 methyltransferase catalytic subunit. Importantly partial inactivation of NIMT, a mitotic activator of the CDK1 kinase, also causes lethality in the absence of Set1 function, revealing a functional relationship between the Set1 complex and two pivotal mitotic kinases. The main target for Set1-mediated methylation is histone H3K4. Mutational analysis of histone H3 revealed that modifying the H3K4 target residue of Set1 methyltransferase activity phenocopied the lethality seen when either NIMA or CDK1 are partially functional. We probed the mechanistic basis of these genetic interactions and find that the Set1 complex performs functions with CDK1 for initiating mitosis and with NIMA during progression through mitosis. The studies uncover a joint requirement for the Set1 methyltransferase complex with the CDK1 and NIMA kinases for successful mitosis. The findings extend the roles of the Set1 complex to include the initiation of mitosis with CDK1 and mitotic progression with NIMA in addition to its previously identified interactions with Aurora and type 1 phosphatase in budding yeast.
Keywords: NIMA, CDK1, Set1, histone H3K4, mitosis
DURING the transition from interphase into mitosis, chromatin undergoes dramatic global restructuring to go from a relaxed interphase configuration amenable to gene expression to a condensed form characteristic of mitotic chromosomes. Chromatin condensation not only marks mitosis but is also essential for the normal segregation of the duplicated sister chromatids into daughter nuclei. On the other hand, during mitotic exit, decondensation of chromatin needs to be triggered in a correct temporal manner to enable successful transition into interphase. In Aspergillus nidulans, a model filamentous fungus that enabled discovery of cell cycle-specific genes, mitotic initiation requires the function of the essential NIMA mitotic kinase (Oakley and Morris 1983; Osmani et al. 1987). Early evidence pointed to a role for NIMA in regulating chromatin compaction through the cell cycle since overexpression of NIMA was sufficient to induce chromatin condensation independent of cell cycle phase (Osmani et al. 1988; Osmani and Ye 1996). Importantly, NIMA is required for, and can promote, the phosphorylation of histone H3 at serine 10 (De Souza et al. 2000), a universal marker of mitotic chromatin. In addition, NIMA has been shown to regulate the partial disassembly of nuclear pore complexes, allowing the access of mitotic regulators to the nucleus (Wu et al. 1998; De Souza et al. 2003, 2004; Govindaraghavan et al. 2014a). Using cells that have partial NIMA function, roles for NIMA in establishing a bipolar spindle as well as in promoting anaphase completion were identified, indicating that NIMA is required not only for mitotic initiation but also for successful transition through mitosis (Govindaraghavan et al. 2014a). In agreement with this conclusion, cells with partial NIMA function have a strict requirement for a functional spindle assembly checkpoint (SAC) for survival. Furthermore, the dynamic localization of NIMA to spindle pole bodies at the initiation of mitosis and then to nuclear pore complexes, the mitotic apparatus, and back to spindle pole bodies during mitotic exit lends further support to the overarching role that NIMA plays in regulating successful nuclear division (De Souza et al. 2000; Shen and Osmani 2013). The study of yeast two-hybrid NIMA-interacting proteins and the examination of cells with partial NIMA function have also indicated a role for this kinase in regulating normal nuclear envelope dynamics at telophase (Davies et al. 2004; Govindaraghavan et al. 2014a).
The mitotic functions of NIMA are conserved in eukaryotic cells, with NIMA being the founding member of the family of NIMA-related kinases called the Nek kinases (Fry et al. 2012). Human Nek kinases have been shown to share NIMA’s function in regulating the bipolar spindle formation as well as the permeability of the nuclear envelope at mitotic entry (Fry et al. 1998; Bahe et al. 2005; Bahmanyar et al. 2008; Bertran et al. 2011; Laurell et al. 2011). Consistent with these conserved functions, ectopic expression of NIMA in organisms such as fission yeast, Xenopus, and human cells results in premature mitotic characteristics, including chromatin condensation (O’Connell et al. 1994; Lu and Hunter 1995). Interestingly human Nek2 plays an important role in regulating the kinetochore protein Hec1, a function that is conserved with the budding yeast NIMA ortholog, kin3 (Chen et al. 2002).
Given the importance of the NIMA family of kinases and mitotic regulation, we undertook a genetic approach to gain further insights into NIMA function. A synthetic lethal screen was conducted using the deletion of the nonessential Saccharomyces cerevisiae NIMA ortholog Kin3 (Govindaraghavan et al. 2014b). Here we reveal that the novel genetic interaction between nimA and the gene encoding a subunit of the Set1 protein methyltransferase complex (also called the COMPASS complex for Complex Proteins Associated with Set1) (Miller et al. 2001) An-swd1 (Roguev et al. 2001) is conserved in A. nidulans. A conserved substrate of the Set1 complex is histone 3 lysine 4 (H3K4) and the An-swd1 subunit is essential for Set1 complex function (Krogan et al. 2002; Nagy et al. 2002). Moreover, we find that the genetic interaction with the Set1 complex extends to the universal CDK1 mitotic kinase. Set1 complex-mediated methylation of H3K4 is found most commonly on actively transcribed genes (Dehe and Geli 2006). In addition, H3K4 methylation is also involved in maintaining the repression of telomere proximal genes in S. cerevisiae and Schizosaccharomyces pombe and the suppression of secondary metabolite production in A. nidulans and A. fumigatus (Krogan et al. 2002; Kanoh et al. 2003; Bok et al. 2009; Palmer et al. 2013). Growing evidence indicates that the Set1 complex is involved in a gamut of additional cellular functions, many of which may be independent of its roles in gene expression, including kinetochore maintenance, DNA repair, and replication checkpoints (Faucher and Wellinger 2010; Bergmann et al. 2011). Importantly the Set1 complex regulates mitosis in budding yeast with the Aurora mitotic kinase Ipl1 and the type1 phosphatase Glc7 (Zhang et al. 2005; Latham et al. 2011). This mitotic function involves a second Set1 target substrate, the inner kinetochore protein Dam1. Methylation of Dam1 by the Set1 complex inhibits Ipl1-mediated phosphorylation and therefore helps modulate the phosphorylation state of Dam1 and chromosome segregation fidelity in yeast (Zhang et al. 2005). Finally, the rearrangement of the human ortholog of Set1 gene (mixed lineage leukemia, MLL) is widely seen in several aggressive human leukemias, further underlining the importance of fully understanding the network of proteins that function along with Set1 complexes (Shilatifard 2012).
Our studies provide genetic, biochemical, as well as cell biological evidence for Set1 complex functions in the initiation and completion of mitosis, which are performed together with the NIMA and CDK1 mitotic kinases.
Materials and Methods
A. nidulans strains were grown using standard protocols, as described previously, (Pontecorvo et al. 1953) with minimal modifications. pBLAST searches were performed at http://aspgd.org/ (Arnaud et al. 2012) or National Center for Biotechnology Information BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Gene deletions were performed using constructs generated by fusion PCR and introduced into the genome via homologous recombination (Yang et al. 2004; Osmani et al. 2006; Szewczyk et al. 2006). Genetic crossing was used to generate strains with different combinations of gene deletions and fluorescent mitotic markers (Todd et al. 2007). Genotypes of strains used in this study are given in Supporting Information, Table S1. Synthetic lethality was assessed by examining the formation of colonies of the strains to be compared, at the appropriate semipermissive temperature, from isolated conidiospores. An E800 microscope (Nikon) and an UltraPix digital camera (Life Science Resources) were used to examine fixed cells. For confocal imaging, a ×60 1.49 NA total internal reflection fluorescence objective lens on a Nikon 484 Eclipse TE 2000-U (Nikon) microscope was used, which was equipped with an UltraView ERS spinning disk 485 confocal system (PerkinElmer) and a Hamamatsu ORCA-AG 486 camera. For live cell imaging of mitosis at the semipermissive temperatures 35° or 37.5°, cells were grown in Bioptechs Delta T dishes in humidity chambers for 4.5 hr and then observed under a temperature-controlled environment using equipment from Bioptechs. Images and movies were analyzed using either Ultraview (PerkinElmer) or ImageJ version 1.46m (http://rsbweb.nih.gov/ij/) with all confocal images presented as maximum intensity projections. Western blot analysis employing methylation state-specific antibodies against histone H3K4 [Active Motif histone H3K4me1 antibody, catalog (cat.) no. 39298; Millipore histone H3K4me2 antibody, cat. no. 07-030; and Active Motif histone H3K4me3 antibody, cat. no. 39160] was completed using total protein extracts as previously described (Liu et al. 2010).
Results
The Set1 complex is essential when the function of NIMA or NIMT (and hence mitotic CDK1) is partially impaired
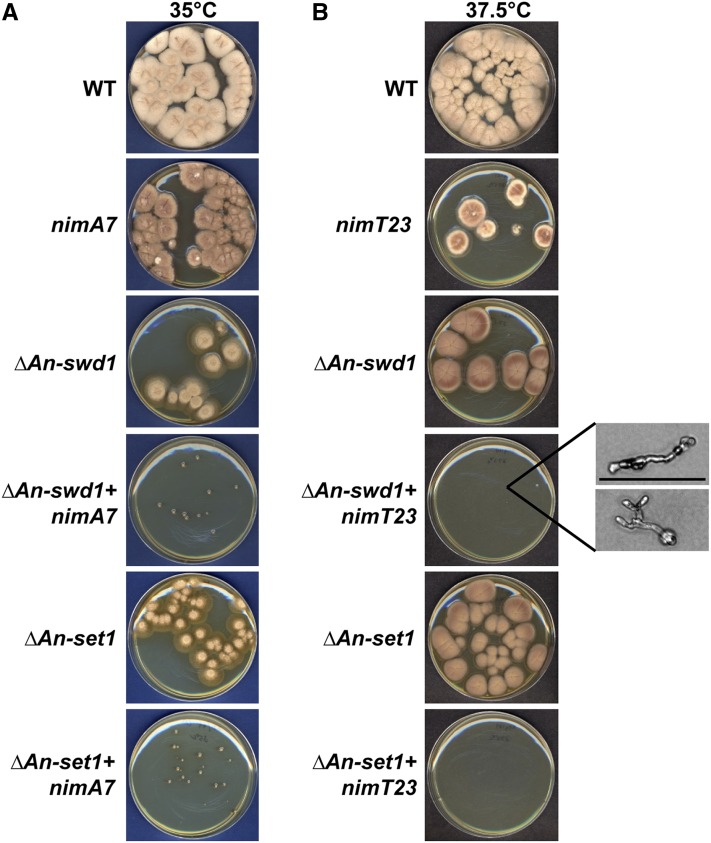
By extending the results of a synthetic lethal screen conducted using the nonessential S. cerevisiae NIMA ortholog Kin3 to A. nidulans (Govindaraghavan et al. 2014b), a genetic interaction was identified between NIMA and An-swd1 (Figure 1), the gene encoding the A. nidulans ortholog of yeast Swd1, a WD40 repeat containing subunit of the Set1 methyltransferase complex, also called the COMPASS complex (locus AN0808; see http://www.aspergillusgenome.org/cgi-bin/locus.pl?locus=AN0808). Deletion of An-swd1 generated haploid null strains having growth and conidiation defects that displayed cold sensitivity (Figure S1). Although the conidiation defects were somewhat remediated by high sucrose (1 M) media, the growth defects and cold sensitivity were not (Figure S1). To test for synthetic genetic interactions between nimA and An-swd1, we compared the colony growth of strains carrying both nimA7 and ΔAn-swd1 with that of the single mutants at 35°, a semipermissive temperature for nimA7 (Figure 1A). The temperature sensitivity of nimA7 strains allows restricted colony growth at this temperature compared to wild-type (WT) cells. Strikingly, in contrast to nimA7 or ΔAn-swd1, the double mutants are severely impaired for colony formation at 35°, revealing enhanced growth defects compared to either single mutant (Figure 1A). It is possible that the growth defects in cells that lack An-swd1 in combination with partial NIMA function are specific to NIMA or are caused by more general mitotic regulatory defects. To test this, we employed a mutant allele of the Cdc25 phosphatase, nimT23, an activator of the mitotic CDK1 kinase (termed NIMX in A. nidulans) (Osmani et al. 1991; O’Connell et al. 1992). This enabled us to investigate the mitotic defects caused by impairing the mitotic specific functions of CDK1 rather than its interphase functions. At a semipermissive temperature for nimT23, 37.5°, the function of nimT is reduced and mitotic CDK1 activation is partially defective. In contrast to the single mutants, the double mutant nimT23 + ΔAn-swd1 cannot form colonies but instead formed severely growth-restricted cells visible only under the microscope (Figure 1B), revealing an enhanced lethal interaction between nimT23 and ΔAn-swd1. Taken together, our data show that An-swd1 is essential when the function of either NIMA or CDK1 mitotic kinase is partially inhibited.
Figure 1.
The function of the Set1 complex is essential for cells that have partially functional NIMA or CDK1 mitotic kinases. (A) Colony growth of strains of the indicated genotype at the semipermissive temperature of nimA7 (35°) after 96 hr and (B) at the semipermissive temperature of nimT23 (37.5°, resulting in partial NIMT and hence mitotic CDK1 function) after 96 hr. The inset shows magnified images of cells on the plate (Bar, 0.1 mm). Strains: WT = R153, nimA7 = MG71, nimT23 = MG99, ΔAn-swd1 = MG41, ΔAn-swd1 + nimA7 = MG65, ΔAn-swd1 + nimT23 = MG104; ΔAn-set1 = MG177, ΔAn-set1 + nimA7 = MG179, and ΔAn-set1 + nimT23 = MG181.
Swd1 is essential for the function of the Set1 complex (Krogan et al. 2002; Nagy et al. 2002), and therefore it is likely that the genetic interaction between An-swd1 and the mitotic kinases is due to the absence of Set1 complex-mediated methylation. However it is possible that An-swd1 plays other functions independent of the Set1 complex. To determine whether the loss of Set1 complex function in ΔAn-swd1 cells is responsible for the enhanced growth defects in ΔAn-swd1 + nimT23 and ΔAn-swd1 + nimA7 strains, we identified the A. nidulans ortholog of Set1 (locus AN5795; see http://www.aspergillusgenome.org/cgi-bin/locus.pl?locus=AN5795) that encodes the catalytic methyltransferase protein of the Set1 complex and deleted it in WT strains as well as strains carrying nimA7 or nimT23. The colony growth of the double mutants that lack the An-set1 gene in combination with nimA7 or nimT23 at the respective semipermissive temperatures was assessed. We found that the deletion of An-set1 causes markedly enhanced growth defects in combination with partial inactivation of either the NIMA or CDK1 mitotic kinases (Figure 1B), indicating that the Set1 complex performs important functions together with the NIMA and CDK1 mitotic kinases.
Modification of the histone H3K4 Set1 substrate phenocopied the genetic interactions between Set1 and NIMA or NIMT
A conserved substrate of the Set1 complex is the K4 of histone H3 involving mono-, di-, or trimethylation. To confirm that H3K4 is a substrate for the Set1 complex in A. nidulans, we probed by Western blot the requirement of An-set1 and An-swd1 for mono-, di-, or trimethylation at H3K4. For a wild-type strain, H3K4 mono-, di-, and trimethylation was readily detected in total cell extracts but no methylation was detected in the ΔAn-set1 or ΔAn-swd1 strains (Figure S2). This result is consistent with the Set1 complex mediating mono-, di-, and trimethylation at histone H3K4 in A. nidulans, similar to other organisms (Krogan et al. 2002; Noma and Grewal 2002; Dehe and Geli 2006; Shilatifard 2012; Palmer et al. 2013).
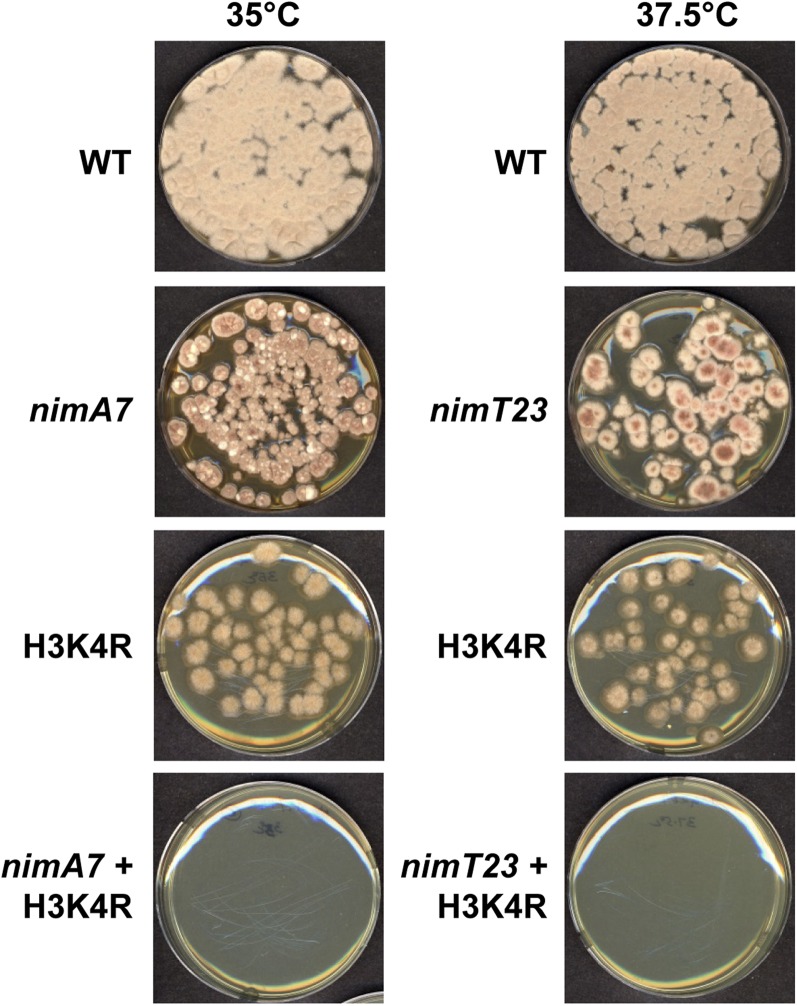
In addition to the methylation of H3 at K4, the Set1 complex has also been shown to methylate the kinetochore protein Dam1 in S. cerevisiae (Zhang et al. 2005). To determine whether the histone H3K4 target site of the Set1 complex was involved in the synthetic growth defects with partial NIMA or CDK1 function (Figure 1), we generated strains carrying a mutant version of histone H3, where the lysine K4 was changed to arginine (H3K4R). In these cells, although the Set1 complex is fully functional, the methylation of the H3 substrate cannot occur. Importantly, in combination with nimA7 or nimT23 at their respective semirestrictive temperatures, H3K4R caused marked growth defects when compared to the single mutants (Figure 2), phenocopying the genetic interactions observed between partial NIMA and CDK1 in combination with a deficiency in Set1 complex function.
Figure 2.
Mutation of lysine 4 (K4) of histone H3 is lethal when NIMA or mitotic CDK1 function is reduced. Colony growth of the strains of the indicated genotype at the semipermissive temperature for nimA7 or nimT23 is shown after 96 hr. Strains: WT = R153, nimA7 = MG71, nimT23 = MG99, H3K4R = MG316, H3K4R + nimA7 = MG318, and H3K4R + nimT23 = MG267.
If the synthetic lethality between ΔAn-swd1 and mitotic CDK1 involves methylation of proteins in addition to histone H3K4 the degree of genetic interaction between ΔAn-swd1 and nimT23 should be greater than between H3K4R and nimT23. This is because lack of An-swd1 would affect not only the methylation of H3K4 but also other potential substrates of the Set1 complex, while the H3K4R mutation would leave other Set1 complex substrates to be methylated as usual. We therefore tested if the growth defects caused by ΔAn-swd1 with nimT23 were greater than between H3K4R and nimT23 by observing colony growth at a more permissive temperature. The results indicate that the genetic interaction between ΔAn-swd1 and nimT23 was not more severe than between H3K4R and nimT23. In fact the opposite was observed because slightly enhanced growth defects were caused at the lower temperature (32°) between H3K4R and nimT23 than between ΔAn-swd1 and nimT23 (Figure S3). This result suggests that the H3K4R mutation might cause defects in addition to preventing H3K4 methylation, such as H3K4 acetylation for example (Kim and Workman 2010; Xhemalce and Kouzarides 2010).
To further investigate if histone H3 is the relevant Set1 complex substrate involved in the genetic interactions, we investigated further the growth defects of the ΔAn-swd1 and H3K4R mutants. We reasoned that if H3K4 is the pertinent substrate for the Set1 complex then the H3K4R mutation should not cause worse growth defects than the ΔAn-swd1 mutant. Conversely, if the Set1 complex had important substrates in addition to H3K4, then the ΔAn-swd1 mutant would be expected to cause greater defects than H3K4R. We found that the H3K4R mutation impaired spore viability although the ΔAn-swd1 mutant did not and that the double ΔAn-swd1 + H3K4R strain also had impaired spore viability (Figure S4). Thus the H3K4R mutation causes defects that are worse than ΔAn-swd1, indicating that defects in Set1 complex-mediated methylation probably do not include substrates beyond H3K4.
Histone H3 is a substrate of not only the Set1 complex but also the NIMA kinase. The serine 10 residue of histone H3 (H3S10) is phosphorylated during mitosis and NIMA is required for this phosphorylation and can promote this modification out of cell cycle phase upon overexpression (De Souza et al. 2000). Therefore, the combination of loss of Set1 complex function with the loss of phosphorylation at H3S10 might underlie the synthetic genetic interaction between An-swd1 and nimA7. To test this, we generated strains carrying a mutant version of H3 that had a nonphosphorylatable alanine instead of serine at position 10 (H3-S10A) as well as a version of H3 that had both K4 and S10 mutated to arginine and alanine, respectively (H3K4R-S10A). We find that preventing the phosphorylation of H3S10 does not compromise viability. In combination, the H3K4R and H3-S10A alleles caused growth defects but not lethality (Figure S5). This indicates that the loss of H3S10 phosphorylation is not causative of the genetic interaction between the Set1 complex and the mitotic kinases.
In the absence of sufficient NIMT (hence mitotic CDK1) function, the Set1 complex is essential for nuclear divisions but not cell growth
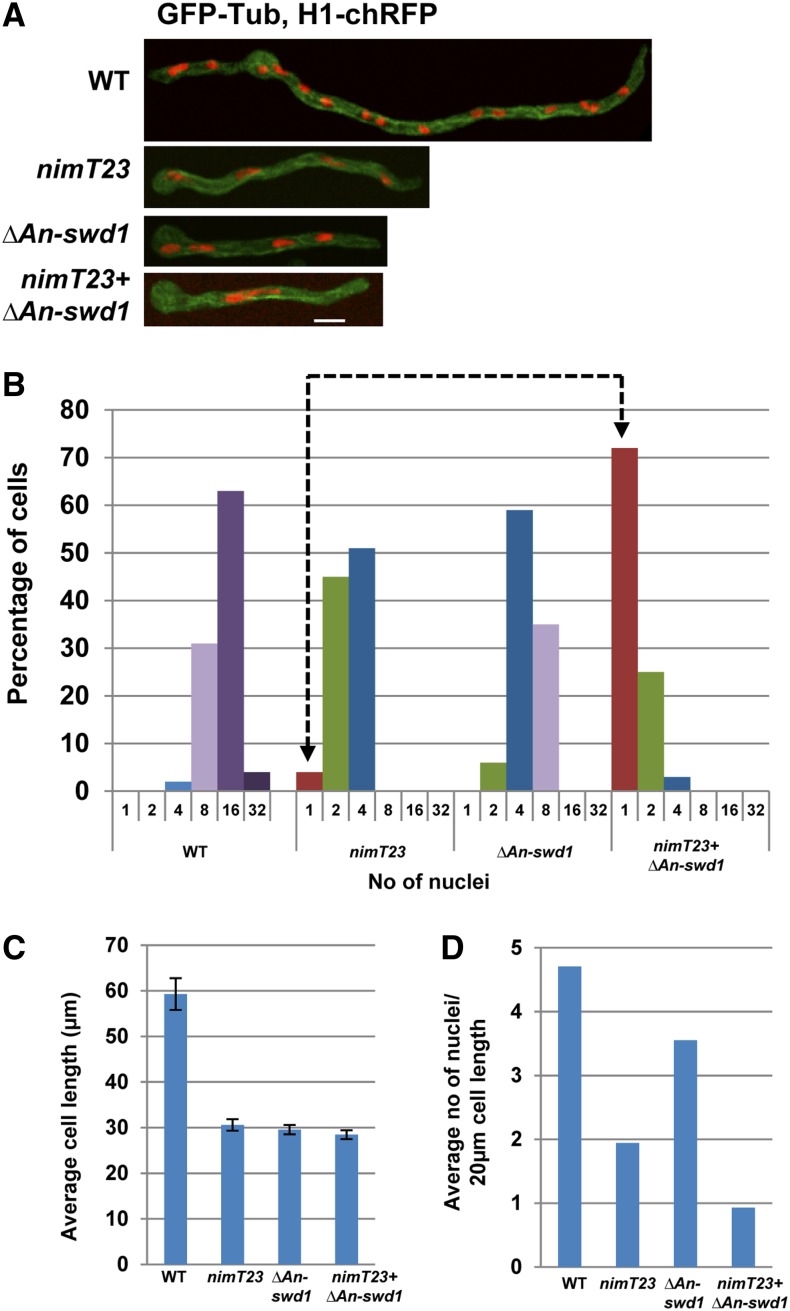
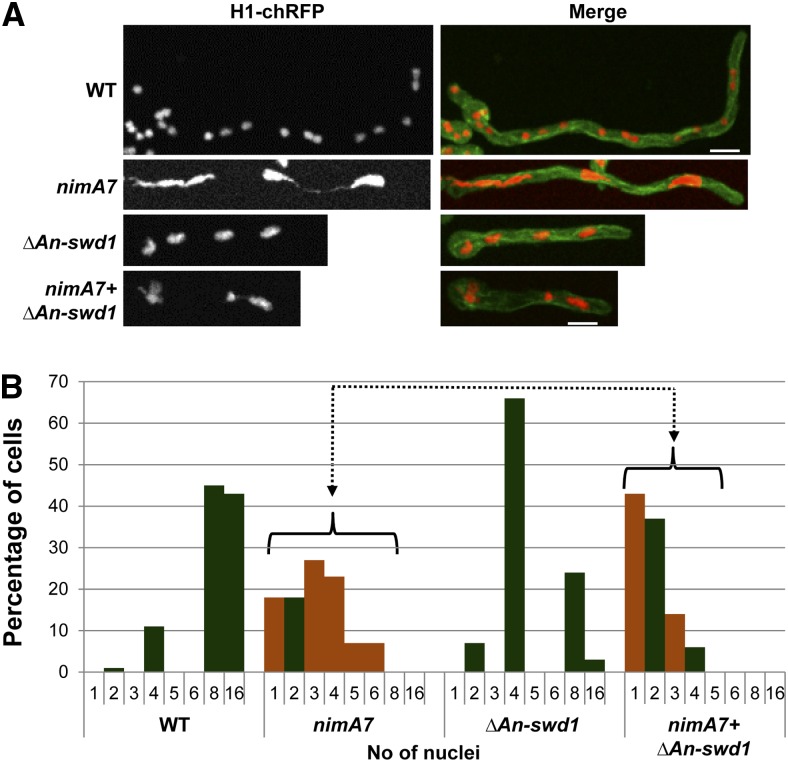
To gain further insights into the potential cause for the synthetic lethal interaction between NIMT and the Set1 complex, we examined microscopically the double mutants that lacked An-swd1 in combination with partial CDK1 function in comparison to the single mutants, using histone H1-chRFP to mark chromatin and GFP-tubulin (Ovechkina et al. 2003) to visualize microtubules. The strains were germinated at the semipermissive temperature, fixed, and observed. As shown in Figure 3, the majority of WT cells had 8–16 nuclei after 7 hr of growth, whereas most nimT23 cells under identical conditions contained 2–4 nuclei, consistent with a delay in mitotic entry (Figure 3, A and B). In contrast to WT cells, cells deleted for An-swd1 contained fewer nuclei (Figure 3, A and B). Strikingly, the majority of cells lacking An-swd1 with partial NIMT function contained a single nucleus (Figure 3, A and B). This increased occurrence of uninucleated cells among the double mutant population and fewer nuclei in single ΔAn-swd1 cells may be reflective of defects in cell growth and/or mitotic initiation. To discern between these two possibilities, we calculated the average cell length and found that the cell growth of the double mutant does not differ significantly from either single mutant (Figure 3C). This suggests that the synthetic lethality of double mutant cells that lack Set1 complex function in combination with reduced NIMT function is not due to defects in cell growth but to a defect in entry into mitosis.
Figure 3.
The G2–M transition in cells having partial mitotic CDK1 function requires An-swd1 and thus Set1 complex function. (A) Representative images of cells of the indicated genotypes carrying histone H1-chRFP and GFP-Tub grown at the semipermissive temperature for nimT23 (37.5°) and fixed for microscopic analysis. (B) Categorization of cells of different genotypes based on the number of nuclei. (C) Quantitation of average cell length of cells of each genotype as indicated. WT, n = 49; nimT23, n = 121; ΔAn-swd1, n = 110; and nimT23 + ΔAn-swd1, n = 114. (D) Calculation of the number of nuclei in 20 μm of cell length in WT cells compared to the single mutants and the double mutant. Strains: WT = HA365, nimT23 = HA375, ΔAn-swd1 = MG160, and nimT23 + ΔAn-swd1 = MG161.
The continued short-term growth in A. nidulans mutants that are defective in the G2–M transition results in fewer nuclei per unit length compared to WT cells (Osmani and Ye 1996). Since the double mutants lacking An-swd1 and partial nimT function had fewer nuclei but grew to the same extent as the single mutants, we calculated the average number of nuclei per cell length after 7 hr of growth. WT cells had on average four to five nuclei in a span of 20 μm, while there were on average two nuclei within the same length in cells with reduced nimT function. Although cells lacking An-swd1 have four to eight nuclei on average, which is fewer than WT cells (Figure 3, A and B), they also exhibit reduced cell growth (Figure 3C). This suggests that the reduced number of nuclei in ΔAn-swd1 cells is potentially an indirect effect of reduced cell growth. However, cells lacking An-swd1 in combination with partial nimT function only had a single nucleus within a span of 20 μm (Figure 3D). These data reveal that there is an enhanced defect in mitotic entry in the double mutant such that abolishing Set1 complex function makes the nim (never in mitosis) phenotype of nimT23 cells (normally seen at 42°) tighter and therefore manifest at a lower temperature. The results indicate the Set1 complex plays roles in promoting cells into mitosis.
Cells lacking Set1 complex function in addition to partial NIMT function exhibit a delay in initiating mitosis
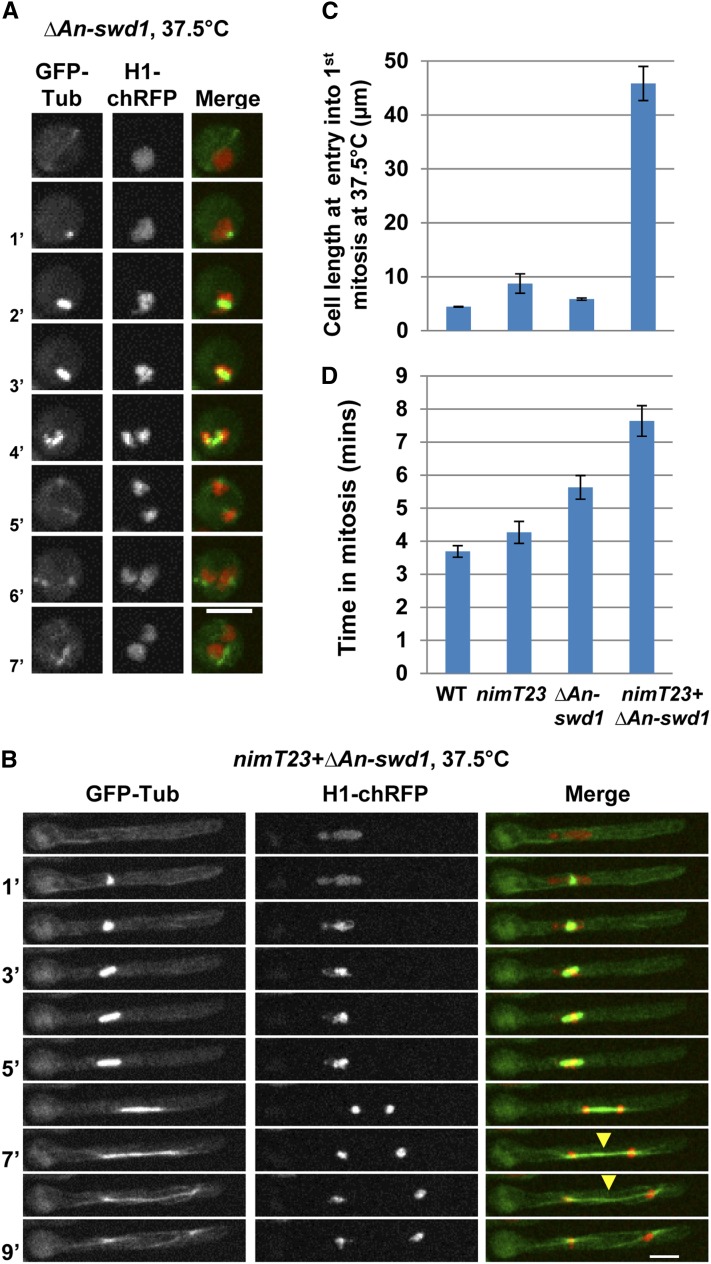
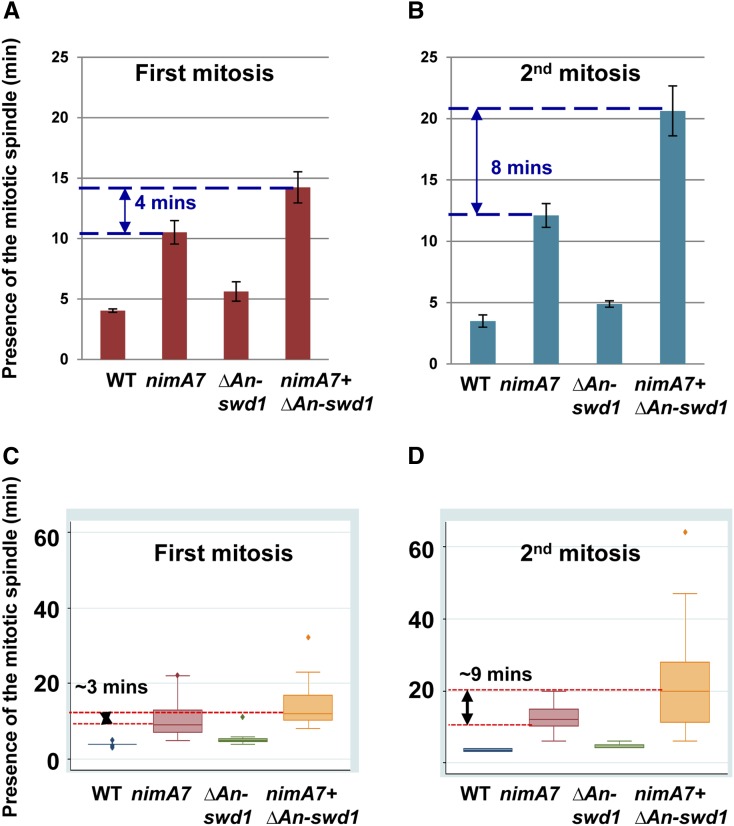
To further define the earliest defects in cells lacking the functions of both the Set1 complex and NIMT, we examined the first mitosis during spore (conidia) germination using live cell confocal microscopy at the semipermissive temperature. In WT and An-swd1-null cells, the first mitosis was triggered in the swollen conidia before germ tube emergence (Figure 4, A and C; Figure S6A), further supporting our previous analysis that An-swd1-deleted cells likely do not have defects in mitotic entry. We note that An-swd1-deleted cells show a slightly elongated period of time spent in mitosis compared to WT cells (Figure 4D). However, ΔAn-swd1 mitoses were always completed successfully and we did not observe any obvious defects in chromatin segregation or spindle formation (Figure 4A). In contrast to WT and An-swd1-null cells, nimT23 cells reached an increased average cell length before entering their first mitosis (Figure 4C; Figure S6B). However, the cell length of the ΔAn-swd1 + nimT23 double mutant was far greater than either single mutant, indicating that there is an enhanced defect in the double mutants in their ability to initiate the first mitosis (Figure 4, B and C). Importantly, in spite of this G2–M delay, the resulting mitosis was completed successfully to give two daughter nuclei (Figure 4B).
Figure 4.
Cells lacking An-swd1 in addition to having partial mitotic CDK1 function exhibit a pronounced delay in initiating the first mitosis. (A) Live cell imaging of mitosis in a representative ΔAn-swd1 cell (strain MG276) resulting in two daughter nuclei. (B) Live cell imaging of mitosis in a representative cell lacking An-swd1 in addition to having partial CDK1 function (strain MG302) is successful in generating two daughter nuclei. Arrowhead indicates the spindle that continues to be visible in telophase. Images of representative live cell imaging of WT and nimT23 cells carrying H1-chRFP and GFP-Tub progressing through mitosis at the semipermissive temperature for nimT23 (37.5°) are shown in Figure S6. (C) Cell length at the time of entry into the first mitosis. WT (MG300), n = 13; nimT23 (MG304), n = 13; ΔAn-swd1 (MG276), n = 17; and nimT23 + ΔAn-swd1 (MG302), n = 15. (D) Time in mitosis as measured using GFP-Tub to monitor spindle formation and H1-chRFP chromosome condensation as markers for mitosis. WT, n = 12; nimT23, n = 13; ΔAn-swd1, n = 19; and nimT23 + ΔAn-swd1, n = 14. Bars, 5 μm.
In WT cells, the mitotic spindle is typically disassembled soon after chromatin segregation in anaphase. During our analysis of fixed ΔAn-swd1 + nimT23 cells, we detected the presence of long spindle-like microtubule arrays between segregated daughter nuclei (data not shown). Consistent with this observation, live cell imaging of double mutant cells revealed the same phenotype (Figure 4B, arrowheads), suggesting a delay in disassembling the mitotic spindle during telophase and progression out of mitosis into G1. In agreement with this, we found that the nimT23 + ΔAn-swd1 double mutants take longer to complete mitosis compared to either single mutant (Figure 4D).
Absence of Set1 complex function in addition to partial NIMA function results in mitotic defects
We next sought to determine the potential cause for the genetic interaction between NIMA and the Set1 complex by examining WT, nimA7, ΔAn-swd1, and nimA7 + ΔAn-swd1 cells that were engineered to also carry the chromatin marker, histone H1-chRFP and the microtubule marker GFP-TubA. After growth at the semipermissive temperature for nimA7 (35°) for 7 hr, cells were fixed for microscopic analysis. We found that nimA7 cells at the semipermissive temperature display penetrant chromatin segregation defects suggestive of mitotic failures (Figure 5A). In WT cells the distribution of the number of nuclei per cell is reflective of successful rounds of synchronous mitoses (Figure 5B). In contrast, nimA7 cells were quite distinctive, having abnormal odd numbers of nuclei (orange bars) but lacking cells with 8 or 16 nuclei. This indicates that partial NIMA function caused mitotic defects rather than just delaying mitotic entry as seen after reduction in NIMT function. Closer examination of mitosis in cells with partial NIMA function recently revealed that NIMA is required for the successful progression of specific mitotic events in addition to mitotic entry (Govindaraghavan et al. 2014a). On quantitating the numbers of nuclei in ΔAn-swd1 cells, we found a majority had 4 nuclei (Figure 5B). Importantly, the double mutant nimA7 + ΔAn-swd1 cells had a higher number of uni- and binucleated cells, a lower proportion of 3 and 4 nucleated cells and no cells with 5 or 6 nuclei (Figure 5B).
Figure 5.
Synthetic growth defects in cells that lack Set1 complex function in addition to partial NIMA function. (A) Representative images of cells of the indicated genotypes carrying H1-chRFP and GFP-Tub grown at the semipermissive temperature for nimA7 (35°) and fixed for microscopic analysis. (B) Categorization of cells of different genotypes based on the number of nuclei. Orange bars represent cells containing one or abnormal numbers of nuclei. WT, n = 65; nimA7, n = 72; ΔAn-swd1, n = 70; and nimA7 + ΔAn-swd1, n = 79. Strains: WT = HA365, nimA7 = MG153, ΔAn-swd1 = MG160, and nimA7 + ΔAn-swd1 = MG159.
One potential cause for the reduced nuclear divisions in double mutants is a more pronounced delay in completing mitosis successfully compared to either single mutant. To test this, we collected time lapse images of WT, nimA7, ΔAn-swd1, and nimA7 + ΔAn-swd1 cells at the semipermissive temperature and calculated the length of time that the mitotic spindle is visible during first mitosis. Consistent with our data indicating that nimA7 cells have mitotic defects, we find the mitotic spindle to be present for twice as long in nimA7 cells as in WT cells (Figure 6A and Govindaraghavan et al. 2014a). Importantly, although cells deleted for An-swd1 take similar time as WT cells to complete mitosis, in cells that lack An-swd1 in addition to having partial NIMA function, the time taken to complete the first mitosis is greater than nimA7 cells (Figure 6A). In addition, we see that there is more variability in the time taken to complete the first mitosis in nimA7 and the double mutants compared to ΔAn-swd1 or WT cells (Figure 6C). Interestingly, when we examine the second mitosis, we found that the double mutants exhibit a mitotic delay that is more enhanced compared to either single mutant (Figure 6B). Also, the high variability in the length of the second mitosis is more evident in the double mutant compared to either single mutant (Figure 6D). There are therefore more severe mitotic defects in cells lacking the Set1 complex function in combination with having partial NIMA function as compared to either single mutant.
Figure 6.
The absence of Set1 complex function in combination with partial NIMA function results in enhanced mitotic delays. (A) The time spent in the first mitosis by each of the mutant strains is indicated compared to the WT strain. WT, n = 13; nimA7, n = 21; ΔAn-swd1, n = 8; and nimT23 + ΔAn-swd1, n = 21. (B) The time spent in the second mitosis by each of the mutant strains is indicated compared to the WT strain. WT, n = 2; nimA7, n = 14; ΔAn-swd1, n = 9; and nimT23 + ΔAn-swd1, n = 37. (C and D) Variability in the time spent in mitosis within the cell population in each of the indicated strains is represented by a box and whiskers plot for the first mitosis (C) and subsequent mitosis (D). Spindle formation was used as a measure of determining time in mitosis. Strains used to compute the average time in mitosis: WT = MG224; nimA7 = MG190, MG227, and MG229; ΔAn-swd1 = MG244; and nimA7 + ΔAn-swd1 = MG213 and MG243. The data for WT and nimA7 cells in A and C were previously reported in Govindaraghavan et al. (2014a).
Absence of Set1 complex function makes cells with partial NIMA function more dependent on the spindle assembly checkpoint
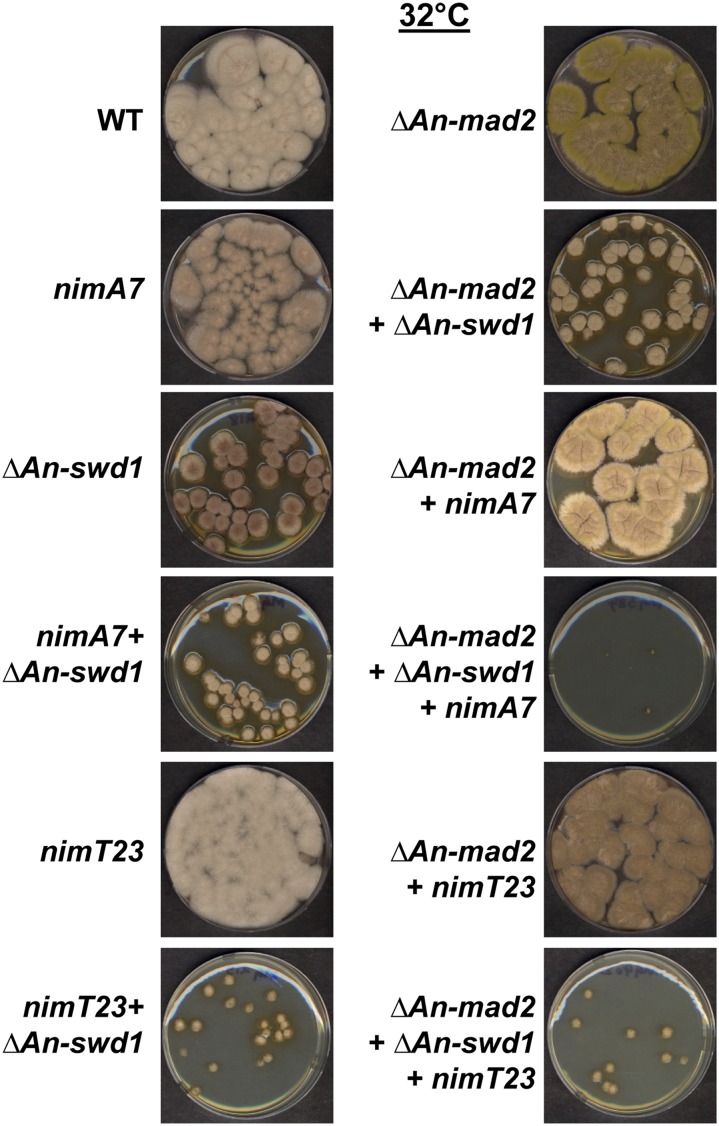
The SAC monitors defects in mitosis, including correct kinetochore–spindle attachment to control the transition from metaphase into anaphase (Musacchio and Salmon 2007). Mutants that cause mitotic defects are often dependent on the SAC for successful mitotic progression, and hence survival. Therefore, the fidelity of mitosis in a mutant background can be assessed by testing the requirement of SAC function for growth. We have recently determined that nimA7 cells are dependent on the function of the SAC for survival at the semipermissive temperature (35°) but not at the permissive temperatures of 32° (Govindaraghavan et al. 2014a). Since the nimA7 + ΔAn-swd1 double mutant cells exhibit enhanced mitotic delays compared to either single mutant, we considered that they might be dependent on the SAC for survival at a more permissive temperature than nimA7. To test this, we generated strains that lack An-mad2 and therefore SAC functions (De Souza et al. 2009), in the background of WT, nimA7, ΔAn-swd1, and nimA7 + ΔAn-swd1 and compared their colony growth. We found that the absence of Mad2 does not affect the growth of either nimA7 or ΔAn-swd1 significantly at 32° (Figure 7). However, strains lacking Mad2 in nimA7 + ΔAn-swd1 mutants had severely inhibited colony growth (Figure 7). The dependence of nimA7 + ΔAn-swd1 double mutants on the SAC for colony growth at a temperature where both single mutants are not affected by the absence of SAC, points to the presence of more pronounced mitotic defects in the nimA7 + ΔAn-swd1 double mutants compared to either single mutant. Importantly, we find that the cells that lack Set1 complex function in combination with reduced NIMT function are not dependent on the SAC for colony growth (Figure 7). This is consistent with our prior results (Figure 3 and Figure 4) that indicate that defects in mitotic initiation, but not mitotic progression, contribute to the synthetic growth defects between lack of Set1 complex and mitotic CDK1 functions.
Figure 7.
Cells lacking Set1 complex function and carrying the nimA7ts allele have enhanced mitotic defects. Absence of An-swd1 or An-set1 confers dependence on the SAC in nimA7 cells but not in nimT23 cells at the permissive temperature (32°) as seen by the colony growth of strains of the indicated genotypes after 96 hr. Strains: WT = R153, nimA7 = CDS790, ΔAn-swd1 = MG218, nimA7 + ΔAn-swd1 = MG215, ΔAn-mad2 = CDS629, ΔAn-swd1 + ΔAn-mad2 = MG383, nimA7 + ΔAn-mad2 = MG381, nimT23 + ΔAn-mad2 = MG405, nimA7 + ΔAn-swd1 + ΔAn-mad2 = MG384, nimT23 + ΔAn-swd1 = MG219, nimT23 = SO53, and nimT23 + ΔAn-swd1 + ΔAn-mad2 = MG402.
Discussion
We identified synthetic genetic interactions between the Set1 methyltransferase complex (as well as its histone H3K4 substrate) and the mitotic kinases, NIMA and CDK1 in A. nidulans. We used cell biological techniques to define that defects in cell cycle regulation underlie the genetic interactions between the Set1 complex and the mitotic kinases. The findings implicate the Set1 complex in regulating mitotic entry with mitotic CDK1 activation and in regulating progression through mitosis with NIMA. Our results extend the mitotic regulatory systems in which the Set1 complex is involved, beyond its previously defined functions with the budding yeast Aurora kinase at the kinetochore involving Dam1 methylation (Zhang et al. 2005; Latham et al. 2011).
The synthetic lethality caused by Set1 mutation and impairment of mitotic CDK1 or NIMA kinase activity involves the Set1 substrate histone H3K4
The synthetic genetic interaction of the gene encoding the catalytic protein An-set1 in addition to the Set1 complex subunit An-swd1 with the NIMA and CDK1 mitotic kinases indicates that the loss of methyltransferase activity of the Set1 complex is responsible for the genetic interactions. Our analysis confirmed that Swd1 and Set1 were both required for histone H3K4 mono-, di-, and trimethylation in A. nidulans. Although the most well conserved substrate of the Set1 complex is the histone H3K4 site, Set1 is also known to methylate the kinetochore protein Dam1 in S. cerevisiae (Zhang et al. 2005; Latham et al. 2011) to regulate kinetochore functions. If preventing methylation at H3K4 showed similar genetic interactions with NIMA and CDK1 as the absence of An-set1 or An-swd1, this would support the hypothesis that the absence of Set1 complex-mediated H3K4 methylation causes the genetic interactions. Indeed, using site-directed mutational analysis to prevent H3K4 methylation, we show this to be true. Therefore the synthetic lethality caused by absence of Set1 complex function in cells with partial NIMA or mitotic CDK1 is most likely due to the absence of H3K4 methylation rather than via methylation of other substrates. Additional analysis of the growth defects caused by lack of An-swd1 compared to the H3K4R mutation further support the conclusion that the Set1 complex likely works through H3K4 methylation in combination with mitotic CDK1 and NIMA.
Set1 is required with mitotic CDK1 for normal transition from G2 into mitosis
Cells that lack Set1 complex function in combination with partial NIMT function show an enhanced delay in initiating mitosis. That this delay is in the G2 phase of interphase is indicated by the observation that the majority of the double mutant cells enter mitosis promptly on being downshifted to permissive temperature from the semipermissive temperature (data not shown). This suggests that the Set1 complex is a positive regulator of mitotic entry through a mechanism that involves CDK1 activation. For instance, the Set1 complex might play a role in the expression of positive mitotic regulators such as NIMT. Alternatively, it is possible that the Set1 complex is involved in a process that is monitored by a G2–M checkpoint. In support of this, we find that An-swd1-deleted cells are sensitive to DNA damaging agents such as 1,2,7,8-Diepoxyoctane and MMS (data not shown). This is consistent with studies in S. cerevisiae and human cells that have shown that the Set1 complex functions in the DNA damage response (Corda et al. 1999; Faucher and Wellinger 2010). Moreover, partial CDK1 activity in A. nidulans also results in sensitivity to DNA damaging agents (data not shown), potentially due to a reduced efficiency in recovering from DNA damage-mediated G2–M checkpoint arrest (Van Vugt et al. 2005; Karlsson-Rosenthal and Millar 2006; Lindqvist et al. 2009). It is possible then that a combination of lack of Set1 complex function and partial CDK1 function results in an increased sensitivity to naturally occurring DNA damage in the absence of external agents of DNA damage, thus causing a G2 delay.
Set1 complex function is required with NIMA for normal transition through mitosis
Our data indicate that the Set1 complex has a function in ensuring proper mitotic progression, which is uncovered upon partial inactivation of NIMA. Further supporting this role, we found that the absence of the Set1 complex in cells with reduced NIMA makes them more sensitive to the absence of the SAC. The reliance of nimA7 + ΔAn-swd1 cells on SAC function is greater than either single mutant, suggesting kinetochore–microtubule attachments might be compromised in these cells. One possibility is that NIMA-mediated phosphorylation and Set1 complex-mediated H3K4 methylation are required to jointly regulate the function of the kinetochore. In fact, the kinetochore protein Hec1 (A. nidulans Ndc80) is phosphorylated by the human NIMA-related kinase, Nek2, thereby contributing to proper chromosome segregation (Chen et al. 2002; Wei et al. 2011). In addition, it is possible that deficiencies in Set1-mediated modulation of Aurora target phosphorylation (Zhang et al. 2005) also contributes to the genetic interactions with NIMA.
Importantly, although H3K4 methylation was considered a mark of euchromatin, it is present at heterochromatic centromeric DNA in fission yeast, Drosophila, and human cells (Sullivan and Karpen 2004; Cam et al. 2005). Diminished levels of H3K4 methylation affects the recruitment and deposition of the histone CENPA at centromeres of human artificial chromosomes, providing direct evidence for the involvement of Set1-mediated methylation in kinetochore function (Bergmann et al. 2011). Therefore misregulation of centromere–kinetochore functions could contribute to the synthetic lethality and SAC-monitored mitotic defects in cells lacking adequate NIMA and Set1 complex function. Alternatively, since our data show that Set1 complex function is required along with mitotic CDK1 to promote mitosis, absence of An-swd1 function alone might cause defects in the G2–M transition that are not manifested when NIMA and CDK1 are fully functional. However when cells lacking An-swd1 undergo mitosis in the absence of sufficient NIMA (nimA7 + An-swd1 at 35°), downstream defects in mitosis may be triggered because of the G2–M transition defects caused by lack of Set1 complex function.
H3K4 methylation is retained on mitotic chromatin in S. pombe, Dictyostelium, and human cells (Noma and Grewal 2002; Muramoto et al. 2010), suggesting that this epigenetic mark might be involved in the regulation of chromatin through the cell cycle. Consistent with this idea, MLL complexes (human orthologs of Set1 complexes) have been shown to act as mitotic bookmarks, facilitating the resumption of gene expression at mitotic exit (Blobel et al. 2009). Moreover, in human cells, methylated H3K4 has been proposed to mark the site for the return of transcription factors to target genes at the end of mitosis (Kelly et al. 2010). In addition, H3K4 methylation has been implicated not in determining the level of transcription, but the inheritance of transcription states from the mother cell to daughter Dictyostelium cells (Muramoto et al. 2010; Peterson 2010). Therefore, another potential explanation for the enhanced mitotic defects in nimA7 + ΔAn-swd1 cells is that the Set1 complex is required to mediate the resetting of gene expression to an interphase-like state after mitosis. If this was true, then we might expect that defects in resuming G1 interphase state after first mitosis might give rise to more pronounced defects in the second mitosis. In agreement with this idea, we find that nimA7 + ΔAn-swd1 cells show enhanced defects in successive mitoses compared to the first mitosis.
Set1 helps modulate mitosis with three different mitotic kinases
Temperature-sensitive mutations in the budding yeast Aurora kinase, Ipl1, can be suppressed by deletion of Set1 complex subunits as well as cause lethality in combination with mutations in the type 1 phosphatase (PP1) Glc7 (Zhang et al. 2005). These genetic interactions are due to the absence of Set1 complex-mediated methylation on an inner kinetochore protein Dam1. Intriguingly, however, the mutation of H3K4 to a residue that cannot be methylated by the Set1 complex can also partially suppress the growth defects of the ts Ipl1 allele (Zhang et al. 2005). These data, taken together with our results, indicate that a complex interplay between the reversible phosphorylation mediated by NIMA, CDK1, and Aurora kinases with PP1 and Set1-mediated H3K4 methylation act to not only regulate kinetochore function but also the initiation of mitosis.
Although the absence of H3K4 methylation mediated by the Set1 complex is not tolerated when either NIMA or CDK1 is partially functional, our data show that the basis of these two synthetic genetic interactions is different and therefore likely reflects specific functional relationships between Set1 complex and CDK1, and Set1 complex and NIMA. Our studies showing that the Set1 complex methyltransferase and the CDK1/NIMA protein kinases regulate mitotic entry and progression lay the foundation for further understanding the interplay between these two critical classes of regulatory enzymes.
Supplementary Material
Acknowledgments
We thank all the members of the Osmani laboratory for insightful feedback into the project. We also thank Jin Woo Bok and Nancy P. Keller (University of Wisconsin) for sharing their findings and helpful discussions about Set1 functions. This study was funded by National Institutes of Health (NIH) grant GM042564 to S.A.O., a Pelotonia graduate fellowship to M.G., and Mississippi NIH IDeA Networks of Biomedical Research Excellence grant P20GM103476 to S.L.A.
Footnotes
Communicating editor: O. Cohen-Fix
Literature Cited
- Arnaud M. B., Cerqueira G. C., Inglis D. O., Skrzypek M. S., Binkley J., et al. , 2012. The Aspergillus Genome Database (AspGD): recent developments in comprehensive multispecies curation, comparative genomics and community resources. Nucleic Acids Res. 40: D653–D659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahe S., Stierhof Y. D., Wilkinson C. J., Leiss F., Nigg E. A., 2005. Rootletin forms centriole-associated filaments and functions in centrosome cohesion. J. Cell Biol. 171: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmanyar S., Kaplan D. D., Deluca J. G., Giddings T. H., Jr, O’toole E. T., et al. , 2008. beta-Catenin is a Nek2 substrate involved in centrosome separation. Genes Dev. 22: 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann J. H., Rodriguez M. G., Martins N. M., Kimura H., Kelly D. A., et al. , 2011. Epigenetic engineering shows H3K4me2 is required for HJURP targeting and CENP-A assembly on a synthetic human kinetochore. EMBO J. 30: 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran M. T., Sdelci S., Regue L., Avruch J., Caelles C., et al. , 2011. Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5. EMBO J. 30: 2634–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. A., Kadauke S., Wang E., Lau A. W., Zuber J., et al. , 2009. A reconfigured pattern of MLL occupancy within mitotic chromatin promotes rapid transcriptional reactivation following mitotic exit. Mol. Cell 36: 970–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok J. W., Chiang Y. M., Szewczyk E., Reyes-Dominguez Y., Davidson A. D., et al. , 2009. Chromatin-level regulation of biosynthetic gene clusters. Nat. Chem. Biol. 5: 462–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cam H. P., Sugiyama T., Chen E. S., Chen X., Fitzgerald P. C., et al. , 2005. Comprehensive analysis of heterochromatin- and RNAi-mediated epigenetic control of the fission yeast genome. Nat. Genet. 37: 809–819. [DOI] [PubMed] [Google Scholar]
- Chen Y., Riley D. J., Zheng L., Chen P. L., Lee W. H., 2002. Phosphorylation of the mitotic regulator protein Hec1 by Nek2 kinase is essential for faithful chromosome segregation. J. Biol. Chem. 277: 49408–49416. [DOI] [PubMed] [Google Scholar]
- Corda Y., Schramke V., Longhese M. P., Smokvina T., Paciotti V., et al. , 1999. Interaction between Set1p and checkpoint protein Mec3p in DNA repair and telomere functions. Nat. Genet. 21: 204–208. [DOI] [PubMed] [Google Scholar]
- Davies J. R., Osmani A. H., De Souza C. P., Bachewich C., Osmani S. A., 2004. Potential link between the NIMA mitotic kinase and nuclear membrane fission during mitotic exit in Aspergillus nidulans. Eukaryot. Cell 3: 1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza C. P., Osmani A. H., Wu L. P., Spotts J. L., Osmani S. A., 2000. Mitotic histone H3 phosphorylation by the NIMA kinase in Aspergillus nidulans. Cell 102: 293–302. [DOI] [PubMed] [Google Scholar]
- De Souza C. P., Horn K. P., Masker K., Osmani S. A., 2003. The SONB(NUP98) nucleoporin interacts with the NIMA kinase in Aspergillus nidulans. Genetics 165: 1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza C. P., Osmani A. H., Hashmi S. B., Osmani S. A., 2004. Partial nuclear pore complex disassembly during closed mitosis in Aspergillus nidulans. Curr. Biol. 14: 1973–1984. [DOI] [PubMed] [Google Scholar]
- De Souza C. P., Hashmi S. B., Nayak T., Oakley B., Osmani S. A., 2009. Mlp1 acts as a mitotic scaffold to spatially regulate spindle assembly checkpoint proteins in Aspergillus nidulans. Mol. Biol. Cell 20: 2146–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehe P. M., Geli V., 2006. The multiple faces of Set1. Biochem. Cell Biol. 84: 536–548. [DOI] [PubMed] [Google Scholar]
- Faucher D., Wellinger R. J., 2010. Methylated H3K4, a transcription-associated histone modification, is involved in the DNA damage response pathway. PLoS Genet. 6: e1001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry A. M., Mayor T., Meraldi P., Stierhof Y. D., Tanaka K., et al. , 1998. C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J. Cell Biol. 141: 1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry A. M., O’regan L., Sabir S. R., Bayliss R., 2012. Cell cycle regulation by the NEK family of protein kinases. J. Cell Sci. 125: 4423–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraghavan M., Lad A. A., Osmani S. A., 2014a The NIMA kinase is required to execute stage-specific mitotic functions after initiation of mitosis. Eukaryot. Cell 13: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraghavan M., Mcguire Anglin S. L., Shen K. F., Shukla N., De Souza C. P., et al. , 2014b Identification of interphase functions for the NIMA kinase involving microtubules and the ESCRT pathway. PLoS Genet. 10: e1004248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh J., Francesconi S., Collura A., Schramke V., Ishikawa F., et al. , 2003. The fission yeast spSet1p is a histone H3–K4 methyltransferase that functions in telomere maintenance and DNA repair in an ATM kinase Rad3-dependent pathway. J. Mol. Biol. 326: 1081–1094. [DOI] [PubMed] [Google Scholar]
- Karlsson-Rosenthal C., Millar J. B., 2006. Cdc25: mechanisms of checkpoint inhibition and recovery. Trends Cell Biol. 16: 285–292. [DOI] [PubMed] [Google Scholar]
- Kelly T. K., Miranda T. B., Liang G., Berman B. P., Lin J. C., et al. , 2010. H2A.Z maintenance during mitosis reveals nucleosome shifting on mitotically silenced genes. Mol. Cell 39: 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Workman J. L., 2010. Histone acetylation in heterochromatin assembly. Genes Dev. 24: 738–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan N. J., Dover J., Khorrami S., Greenblatt J. F., Schneider J., et al. , 2002. COMPASS, a histone H3 (Lysine 4) methyltransferase required for telomeric silencing of gene expression. J. Biol. Chem. 277: 10753–10755. [DOI] [PubMed] [Google Scholar]
- Latham J. A., Chosed R. J., Wang S., Dent S. Y., 2011. Chromatin signaling to kinetochores: transregulation of Dam1 methylation by histone H2B ubiquitination. Cell 146: 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell E., Beck K., Krupina K., Theerthagiri G., Bodenmiller B., et al. , 2011. Phosphorylation of Nup98 by multiple kinases is crucial for NPC disassembly during mitotic entry. Cell 144: 539–550. [DOI] [PubMed] [Google Scholar]
- Lindqvist A., Rodriguez-Bravo V., Medema R. H., 2009. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J. Cell Biol. 185: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. L., Osmani A. H., Ukil L., Son S., Markossian S., et al. , 2010. Single-step affinity purification for fungal proteomics. Eukaryot. Cell 9: 831–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K. P., Hunter T., 1995. Evidence for a NIMA-like mitotic pathway in vertebrate cells. Cell 81: 413–424. [DOI] [PubMed] [Google Scholar]
- Miller T., Krogan N. J., Dover J., Erdjument-Bromage H., Tempst P., et al. , 2001. COMPASS: a complex of proteins associated with a trithorax-related SET domain protein. Proc. Natl. Acad. Sci. USA 98: 12902–12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto T., Muller I., Thomas G., Melvin A., Chubb J. R., 2010. Methylation of H3K4 Is required for inheritance of active transcriptional states. Curr. Biol. 20: 397–406. [DOI] [PubMed] [Google Scholar]
- Musacchio A., Salmon E. D., 2007. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8: 379–393. [DOI] [PubMed] [Google Scholar]
- Nagy P. L., Griesenbeck J., Kornberg R. D., Cleary M. L., 2002. A trithorax-group complex purified from Saccharomyces cerevisiae is required for methylation of histone H3. Proc. Natl. Acad. Sci. USA 99: 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma K., Grewal S. I., 2002. Histone H3 lysine 4 methylation is mediated by Set1 and promotes maintenance of active chromatin states in fission yeast. Proc. Natl. Acad. Sci. USA 99(Suppl 4): 16438–16445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell M. J., Norbury C., Nurse P., 1994. Premature chromatin condensation upon accumulation of NIMA. EMBO J. 13: 4926–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell M. J., Osmani A. H., Morris N. R., Osmani S. A., 1992. An extra copy of nimEcyclinB elevates pre-MPF levels and partially suppresses mutation of nimTcdc25 in Aspergillus nidulans. EMBO J. 11: 2139–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Morris N. R., 1983. A mutation in Aspergillus nidulans that blocks the transition from interphase to prophase. J. Cell Biol. 96: 1155–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani A. H., Mcguire S. L., Osmani S. A., 1991. Parallel activation of the NIMA and p34cdc2 cell cycle-regulated protein kinases is required to initiate mitosis in A. nidulans. Cell 67: 283–291. [DOI] [PubMed] [Google Scholar]
- Osmani A. H., Oakley B. R., Osmani S. A., 2006. Identification and analysis of essential Aspergillus nidulans genes using the heterokaryon rescue technique. Nat. Protoc. 1: 2517–2526. [DOI] [PubMed] [Google Scholar]
- Osmani S. A., Ye X. S., 1996. Cell cycle regulation in Aspergillus by two protein kinases. Biochem. J. 317(Pt 3): 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani S. A., May G. S., Morris N. R., 1987. Regulation of the mRNA levels of nimA, a gene required for the G2-M transition in Aspergillus nidulans. J. Cell Biol. 104: 1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani S. A., Pu R. T., Morris N. R., 1988. Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase. Cell 53: 237–244. [DOI] [PubMed] [Google Scholar]
- Ovechkina Y., Maddox P., Oakley C. E., Xiang X., Osmani S. A., et al. , 2003. Spindle formation in Aspergillus is coupled to tubulin movement into the nucleus. Mol. Biol. Cell 14: 2192–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. M., Bok J. W., Lee S., Dagenais T. R., Andes D. R., et al. , 2013. Loss of CclA, required for histone 3 lysine 4 methylation, decreases growth but increases secondary metabolite production in Aspergillus fumigatus. PeerJ 1: e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C. L., 2010. Transcriptional memory: mothers SET the table for daughters. Curr. Biol. 20: R240–R242. [DOI] [PubMed] [Google Scholar]
- Pontecorvo G., Roper J. A., Hemmons L. M., Macdonald K. D., Bufton A. W., 1953. The genetics of Aspergillus nidulans. Adv. Genet. 5: 141–238. [DOI] [PubMed] [Google Scholar]
- Roguev A., Schaft D., Shevchenko A., Pijnappel W. W., Wilm M., et al. , 2001. The Saccharomyces cerevisiae Set1 complex includes an Ash2 homologue and methylates histone 3 lysine 4. EMBO J. 20: 7137–7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K. F., Osmani S. A., 2013. Regulation of mitosis by the NIMA kinase involves TINA and its newly discovered partner An-WDR8 at spindle pole bodies. Mol. Biol. Cell 24: 3842–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilatifard A., 2012. The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annu. Rev. Biochem. 81: 65–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan B. A., Karpen G. H., 2004. Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat. Struct. Mol. Biol. 11: 1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk E., Nayak T., Oakley C. E., Edgerton H., Xiong Y., et al. , 2006. Fusion PCR and gene targeting in Aspergillus nidulans. Nat. Protoc. 1: 3111–3120. [DOI] [PubMed] [Google Scholar]
- Todd R. B., Davis M. A., Hynes M. J., 2007. Genetic manipulation of Aspergillus nidulans: heterokaryons and diploids for dominance, complementation and haploidization analyses. Nat. Protoc. 2: 822–830. [DOI] [PubMed] [Google Scholar]
- Van Vugt M. A., Bras A., Medema R. H., 2005. Restarting the cell cycle when the checkpoint comes to a halt. Cancer Res. 65: 7037–7040. [DOI] [PubMed] [Google Scholar]
- Wei R., Ngo B., Wu G., Lee W. H., 2011. Phosphorylation of the Ndc80 complex protein, HEC1, by Nek2 kinase modulates chromosome alignment and signaling of the spindle assembly checkpoint. Mol. Biol. Cell 22: 3584–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Osmani S. A., Mirabito P. M., 1998. A role for NIMA in the nuclear localization of cyclin B in Aspergillus nidulans. J. Cell Biol. 141: 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xhemalce B., Kouzarides T., 2010. A chromodomain switch mediated by histone H3 Lys 4 acetylation regulates heterochromatin assembly. Genes Dev. 24: 647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Ukil L., Osmani A., Nahm F., Davies J., et al. , 2004. Rapid production of gene replacement constructs and generation of a green fluorescent protein-tagged centromeric marker in Aspergillus nidulans. Eukaryot. Cell 3: 1359–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Lin W., Latham J. A., Riefler G. M., Schumacher J. M., et al. , 2005. The Set1 methyltransferase opposes Ipl1 aurora kinase functions in chromosome segregation. Cell 122: 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.