Abstract
The heteromeric Kir4.1-Kir5.1 channel is a candidate sensing molecule for CO2 central chemoreception. Since it is known that the CO2 central chemoreception is subject to neural modulations, we performed studies to test the hypothesis that the Kir4.1-Kir5.1 channel is modulated by neurotransmitters critical for respiratory control, including serotonin (5-HT), substance-P (SP), and thyrotropin releasing hormone (TRH). Immunocytochemistry showed coexpression of Kir4.1 and Kir5.1 in cultured brainstem neurons. The heteromeric Kir4.1-Kir5.1 channel was strongly inhibited by SP, TRH, and 5-HT when expressed in Xenopus oocytes. Such inhibitions were not seen in the homomeric Kir4.1 channel. The inhibition was specific and had clear dose dependence. The effect relied on activation of G-proteins and protein kinase C (PKC), a downstream second messenger of Gαq coupled receptors. The neural modulation did not compromise the channel sensitivity to CO2/pH, as the channel remained to be inhibited by acidic pH with a pre-exposure to the neurotransmitters. Firing rate of cultured brainstem neurons in microelectrode arrays was augmented by application of SP or DOI to the culture medium. The augmentation of the firing rate was blocked by PKC inhibitors suggesting that PKC underscored the inhibitory effect in brainstem neurons as well. These results therefore indicate that the Kir4.1-Kir5.1 channel is modulated by neurotransmitters critical for respiratory control, and the neural modulation appears to enhance channel sensitivity to high PCO2 and acidic pH.
Keywords: central CO2 chemoreception, Kir4.1-Kir5.1, substance-P, 5-HT, PKC
INTRODUCTION
Inward rectifier K+ (Kir) channels regulate resting membrane potential, cellular excitability and neurotransmission (Bajic et al., 2002; Jan and Jan, 1997; Nichols and Lopatin, 1997). The heteromeric Kir4.1-Kir5.1 channel is a unique member in the Kir channel family. This channel is composed of Kir4.1 and Kir5.1 subunits with distinct functional properties such as singlechannel conductance, time-dependent activation and pH sensitivity (Casamassima et al., 2003; Konstas et al., 2003; Pessia et al., 2001; Tanemoto et al., 2000; Yang et al., 2000). We have previously shown that the Kir4.1-Kir5.1 channel is sensitive to physiological PCO2 and pH with pKa 7.45, coupling changes in CO2/pH to membrane excitability (Cui et al., 2001; Xu et al., 2000a). In addition to the pH sensitivity, we have found high levels of expression of Kir4.1 and Kir5.1 mRNAs in various brainstem nuclei (Wu et al., 2004). These findings suggest that the Kir4.1-Kir5.1 channel may be involved in the function and modulation of brainstem neurons.
It is known that the brainstem control of respiration relies on several neurotransmitters including serotonin (5-HT), substance-P (SP) and thyrotropin releasing hormone (TRH) (Cream et al., 1997; Dekin et al., 1985; Moss et al., 1986; Mutolo et al., 1999; Nattie et al., 2004; Nink et al., 1991; Pete et al., 2002; Richerson, 2004; Richerson et al., 2005; Schulz et al., 1996; Severson et al., 2003; Taylor et al., 2005; Wang et al., 2001). Site-specific injections of these neurotransmitters can potently stimulate ventilation. Systemic CO2 response is reduced by selective disruption of serotonergic neurons and neurons expressing the neurokinin-1 receptor (NK1R) (Hodges et al., 2004; Nattie and Li, 2002; Wenninger et al., 2004a; Wenninger et al., 2004b). Receptors for these neurotransmitters have been identified in the ventral and dorsal respiratory groups (VRG, DRG). Also containing these neurotransmitters are midline raphe neurons that are known to be CO2 chemosensitive and project to the DRG and VRG, suggesting that these neurotransmitters may modulate neuronal response to hypercapnia (Richerson, 2004; Richerson et al., 2005; Severson et al., 2003). Therefore, identification of target molecules of these neurotransmitters becomes necessary for the understanding of CO2 central chemoreception and the neural control of breathing.
Several candidate K+ channels are regulated by 5-HT, SP or TRH. For example, G-protein coupled inward rectifier K+ (GIRK) channels have been shown to be regulated by SP, 5-HT and TRH whereas TWIK-related acid-sensitive K+ (TASK) channels are modulated by TRH and 5-HT (Koike-Tani et al., 2005; Lei et al., 2001; Mao et al., 2004; Talley et al., 2000; Talley and Bayliss, 2002). Since the Kir4.1-Kir5.1 channel is highly sensitive to CO2 and exists in chemosensitive brainstem nuclei, it is possible that this channel is one of the downstream target molecules of these neurotransmitters, a hypothesis that we proposed to test in the present study. Our results suggest that proteins of the Kir4.1-Kir5.1 channel are expressed in brainstem neurons, and the channel is subject to modulation by SP, TRH, and 5-HT through activation of Gαq-coupled receptors. Such neural modulation has major effects on brainstem neuronal responses to hypercapnia.
MATERIALS AND METHODS
Oocyte preparation and injection
All experimental procedures were carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals by the NIH and Georgia State University. Frogs were anesthetized by bathing them in 0.3% 3-aminobenzoic acid ethyl ester. A few lobes of ovaries were removed through a small abdominal incision (~5 mm). Xenopus oocytes were treated with 1 mg/ml of collagenase (Type IA, Sigma Chemicals) in the OR2 solution containing (in mM): NaCl 82, KCl 2, MgCl2 1 and HEPES 5 (pH 7.4) for 60 min at room temperature. After five washes (10 min each) of the oocytes with the OR2 solution, cDNAs (25–50 ng or 5–10 femtomoles in 50 nl DD water) were injected into the oocytes. The oocytes were then incubated at 18 °C in the ND-96 solution containing (in mM): NaCl 96, KCl 2, MgCl2 1, CaCl2 1.8, HEPES 5, and sodium pyruvate 2.5 with 100 mg/l geneticin and 50 mg/l tetracycline added (pH 7.4).
Molecular biology
Rat Kir4.1 cDNA (GenBank #X83585) and rat Kir5.1 cDNA (GenBank #AF249676) are gifts from Dr. John Adelman at Oregon Health and Science University, Portland, Oregon. Rat neurokinin-1 receptor (NK1R) cDNA (Genbank #J05097) was generously provided by Dr. Shigetada Nakanishi at Kyoto University Faculty of medicine, Kyoto, Japan. Rat serotonin 2A receptor (5-HT2A) cDNA (Genbank #M30705) was generously provided by Dr. David Julius at the University of California at San Francisco, San Francisco, CA. Mouse thyrotropin-releasing hormone receptors R1 (mTRH-R1) (Genbank #M59811) and R2 (mTRH-R2) (Genbank #NM_133202) were generously provided by Dr. Marvin Gershengorn at the National Institutes of Health, Bethesda, MD. Rat mu-opioid receptor cDNA (MOR) (Genbank #L22455) was generously provided by Dr. Stanley Watson at the University of Michigan, Ann Arbor, MI. These cDNAs were sub-cloned into the eukaryotic expression vector pcDNA3.1 (Invitrogen, Carlsbad, CA) and used for Xenopus oocyte expression without cRNA synthesis. For co-expression of Kir4.1 and Kir5.1, a tandem dimer of these two cDNAs was constructed using the overlapping extension technique, in which full length Kir4.1 and Kir5.1 sequences were obtained using Pfu DNA polymerase (Stratagene, La Jolla, CA) chain reaction (PCR). The PCR products were joined to each other at the 3’ end of Kir4.1 and 5’ end of Kir5.1. Correct constructions were confirmed with DNA sequencing.
Electrophysiology
Whole-cell currents were studied on oocytes 2–4 days post cDNA injection. Two-electrode voltage clamp was performed using an amplifier (Geneclamp 500, Axon Instruments Inc., Foster City, CA) at room temperature (~25 °C). The extracellular recording solution contained (in mM): KCl 90, MgCl2 3, and HEPES 5 (pH 7.4). The recording pipettes were filled with 3M KCl. Oocytes were accepted for further study if they did not show leakage in membrane currents. CO2 experiments were performed in a semi-closed recording chamber (BSC-HT, Medical System, Greenvale, NY), in which oocytes were placed on a supporting nylon mesh, and the perfusion solution bathed both the top and bottom surfaces of the oocytes. Exposure of the oocytes to CO2 was carried out by switching to a perfusate that was bubbled with 15% CO2 balanced with air, and superfused with the same gas. The CO2 resulted in a detectable change in intra- or extracellular pH as fast as 10 sec in these oocytes. We have previously measured intra- and extracellular pH at various PCO2 levels and found that the increase in PCO2 leads to corresponding decreases in intra- and extracellular pH. In experiments when a bicarbonate buffer was used, the cells were perfused with 90mM KHCO3 in an oocyte recording chamber (RC-3Z, Warner Instruments, Hamden, CT) using a gravity-driven perfusion system. The baseline solution was the same in all experiments.
Patch clamp experiments were performed at room temperature (~25 °C) using the Axopatch 200B amplifier (Axon Instruments). In brief, the vitelline membranes were mechanically removed after exposing the oocytes to a hypertonic solution (400 mOsm). The stripped oocytes were placed in a petri dish containing the regular bath solution (see below). Fire-polished patch pipettes (2–4 MΩ) were made of 1.2 mm borosilicate glass capillaries (Sutter Instruments, Novato, CA). The pipette tip was ~2 µm. The bath solution (FVPP) contained (in mM): 40 KCl, 75 potassium gluconate, 5 potassium fluoride, 0.1 sodium vanadate, 10 potassium pyrophosphate, 1 ethylene glycol-bis-β-aminoethylether-N,N,N’,N’-tetraacetic acid (EGTA), 0.2 adenosine diphosphate (ADP), 10 piperazine-N,N’-bis-2-ethanesulfonic acid (PIPES), 10 glucose, and 0.1 spermine (FVPP solution, pH=7.4). The pipette was filled with the same FVPP solution used in the bath or a solution containing: 40 KCl, 110 potassium gluconate, 0.2 ADP, 1 EGTA, 10 N-2- hydroxyethyl-piperazine-N’-2-ethanesulfonic acid (HEPES), 10 glucose, 2 MgCl2 (pH 7.4). Single channel currents were recorded from cell-attached patches. Current records were low-pass filtered (2,000 Hz, Bessel, 4-pole filter, −3 dB), digitized (10 kHz, 12-bit resolution), and stored on computer disk for later analysis using the pClamp 9 software. Junction potentials between bath and pipette solutions were appropriately nulled before seal formation. The open-state probability (NPo) was calculated by first measuring the time, tj, spent at current levels corresponding to j=0, 1, 2,(…)N channels open. The Popen was then obtained as Popen = (∑Nj=1 tj j)/TN, where N is the number of channels active in the patch and T is the duration of recordings. Popen values were calculated from one or a few stretches of data having a duration of 20 s each.
Primary culture of brainstem neurons
The medulla oblongata and pons of fetal (P16–20) sprague dawley rat embryos were surgically removed and rapidly placed in ice cold Earle’s Balanced Salt Solution (EBSS). The tissue was dissociated by treatment using a papain preparation kit (Worthington, Lakewood, NJ). The neurons were then plated on a polyethyleneimine, polyornithine, and laminin coated MEA dish which has 64 planar microelectrodes (ALA Scientific, Westbury, NY) containing dulbecco's modified minimum essential medium (Invitrogen-Gibco, Carlsbad, CA), with 5% horse serum (Invitrogen-Gibco), and 5% fetal calf serum (Invitrogen-Gibco) added. Two-thirds of the culture medium was replaced twice weekly. The neurons showed a similar morphology as in regular cell culture plates. The dissociated neurons were cultured at 37 °C in 5% CO2/95% air at saturating humidity.
Recording of neuronal activity in multi-electrode arrays
Extracellular recordings were carried out in the DMEM medium using a preamplifier (MCS MEA1060, Reutingen, Germany) 10–60 days post culture at 37 °C. The spikes were digitized at 40 kHz with a 64-channel A-D converter and the MEA workstation software (Plexoninc. Dallas, TX). Single units were identified using the Offline Sorter software (Plexon Inc.) based on the Principal Component Analysis method, with which the likelihood to achieve pure single unit recording is greatly improved over the traditional window discriminators (Horn and Friedman, 2003). Single-unit recordings were also determined by the absence of action potentials in the initial period (5–100 ms) of the inter-spike histogram.
Immunocytochemistry
Brainstem neurons were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS, 0.1 M, pH 7.4) for 30 min. The cells were washed three times with PBS and then blocked for 30 min in PBS containing 1% bovine serum albumin (BSA), 10% normal donkey serum (NDS) or 10% normal goat serum (NGS), and 0.3% Triton X-100. The cells were then incubated overnight with the primary antibodies: rabbit polyclonal anti-Kir4.1 (1:1000) (Alomone Lab, Israel), goat polyclonal anti-Kir5.1 (1:500), (Santa Cruz Biotech, Santa Cruz, CA), and mouse monoclonal anti-MAP2 (1:400, Sigma, St. Louis, MO) diluted in antibody dilution solution (ADS), containing 0.1% gelatin, 0.1% NaN3, and 0.3% Triton X-100 in PBS. After washing five times with ADS (5 min each), the cultured cells were incubated at 25 °C with the secondary antibodies for 2 hrs: AlexaFluor488-conjugated donkey anti-rabbit IgG for demonstration of Kir4.1 (1:1000) (Molecular Probes, Eugene, OR), AlexaFluor594-conjugated donkey anti-goat IgG for Kir5.1 (1:1000) (Molecular Probes, Eugene, OR), and AMCA (7-amino-4-methylcoumarin-3-acetic acid) conjugated donkey anti-mouse IgG (1:100, Jackson ImmunoRes, West Grove, PA) for MAP2. In control experiments, the primary antibodies were omitted for Kir5.1 and MAP2 or pre-absorbed with a 3-fold excess of the epitope for Kir4.1 (Alomone Lab). All of these control experiments showed negative stainings. The fluorescence reaction was first visualized using a Zeiss axioscope 200 fluorescence microscope (Zeiss, Oberkochen, Germany). Subsequently, fluorescence imaging was performed with a confocal microscope (LSM 510) (Zeiss, Jena, Germany). The confocal images were taken using a 20x and 40x objective lens.
Chemical administration and exposure
4-α-phorbol 12-myristate 13-acetate (PMA) was purchased from Calbiochem (La Jolla, CA). Chelerythrine chloride and Calphostin-C were purchased from Sigma (St. Louis, MO). Substance-P (SP) (acetate salt), Spantide I, L-703,606, 4-Iodo-2, 5-dimethoxyamphetamine (DOI), serotonin (5-HT), thyrotropin releasing hormone (TRH), and [D-Ala2, N-Me-Phe4, Gly5-ol]-Enkephalin (DAMGO) were also purchased from sigma. Spiperone and Ketanserin Tartate were purchased from Tocris (Ellisville, MO). PMA, chelerythrine, calphostin-C, and spiperone were dissolved in dimethylsulfoxide (DMSO) as stocks and mixed with a recording solution reaching a final concentration as indicated in the results. Other chemicals were dissolved in double-distilled water or experimental solutions. Exposures to these chemicals were done after baseline currents were stabilized and maintained until the plateau effect.
Data analysis
Data are presented as means ± s.e. (standard error). Student t test or single-factor ANOVA was used. Differences of chemical effects before versus during chemical exposures were considered to be statistically significant if P ≤ 0.05.
RESULTS
Inhibition of the Kir4.1-Kir5.1 channel by SP, DOI, and TRH
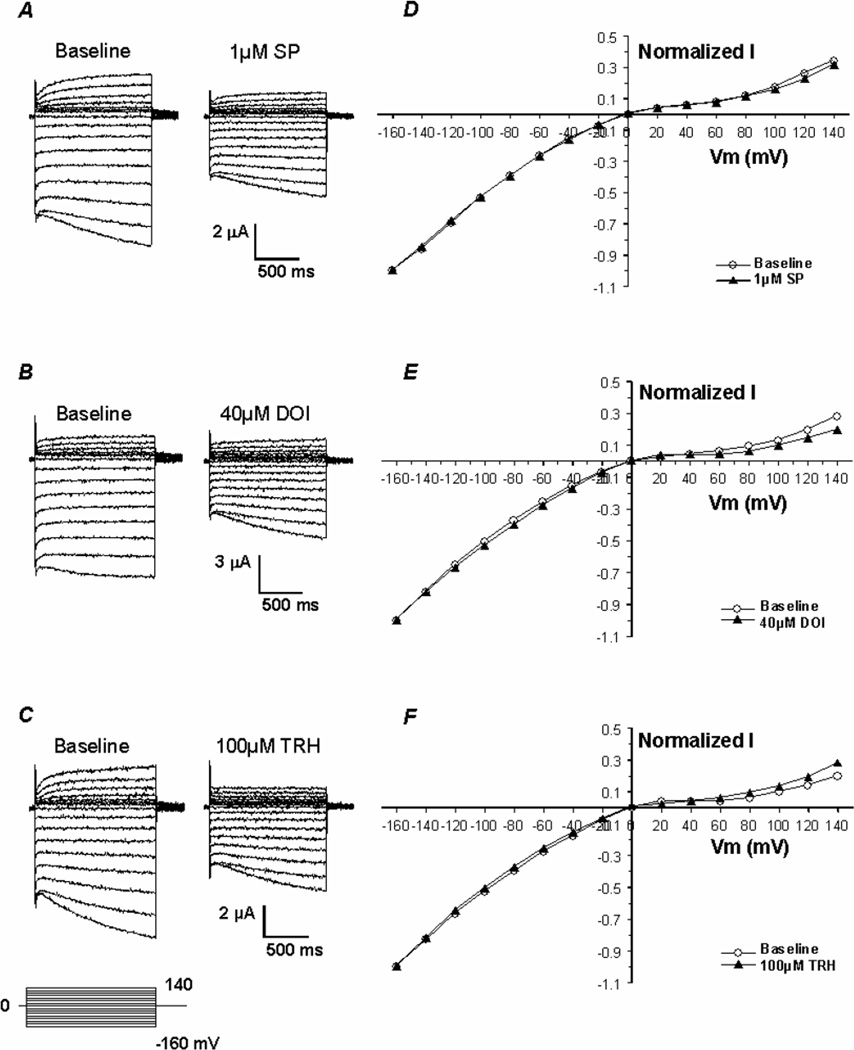
The NK1R receptor cDNA was co-injected into Xenopus oocytes together with the Kir4.1-Kir5.1 tandem dimer cDNA. Inward rectifying currents were recorded from the oocytes 2–3 days post injection using two-electrode voltage clamp with the bath solution containing 90 mM K+. Typical Kir4.1-Kir5.1 currents were revealed: small outward currents and large inward currents with slow activation at highly negative membrane potentials (Fig. 1A, B, C). Following stabilization of the baseline, the Kir4.1-Kir5.1 currents were inhibited by an exposure to 1 µM SP (Fig. 1A). Although the exposure was carried out by application of the neurotransmitter to the bath solution, the time-dependent inhibition occurred rapidly. The inhibition started within the first minute and reached a maximal effect in ~15 min (Fig. 3A, B). Thereafter, all neurotransmitter experiments were done with a 10–20 min exposure. At the maximal effect, 36.4±3.6% (n=10) of the inward rectifying currents were inhibited by 1 µM SP.
Fig 1. Kir4.1-Kir5.1 channel is inhibited by SP, DOI, and TRH.
A, B, C. Using TEVC whole-cell Kir4.1-Kir5.1 currents were recorded from an oocyte 3 days post-injection of the Kir4.1-Kir5.1 dimer cDNA along with the NK1R, 5-HT2A, and mTRH-R1 receptor cDNAs. With 90 mM K+ in the extracellular solution inward rectifying currents were recorded at baseline. Membrane potential (Vm) was held at 0 mV. A series of command pulse potentials from −160 mV to 140 mV with a 20-mV increment was applied to the cell. Note that in highly negative membrane potentials, there was slow activation of the currents. Exposure to 1 µM SP, 40 µM DOI, and 100 µM TRH inhibited the currents by 36, 43, and 30%, respectively. D, E, F. When baseline and peak PMA affected currents were scaled to the same magnitude at −160 mV, the I/V relationship of the currents recorded under these two conditions were superimposed, suggesting that the effects were voltage-independent.
Fig 3. SP and DOI time-dependence.
A. Using TEVC whole-cell Kir4.1-Kir5.1 currents were recorded from an oocyte 3 days post-injection of the Kir4.1-Kir5.1 dimer cDNA along with the NK1R receptor cDNA. With 90 mM K+ in the extracellular solution inward rectifying currents were recorded at baseline. Membrane potential (Vm) was held at 0 mV. A series of command pulse potentials from −160 mV to 140 mV with a 20-mV increment was applied to the cell. Exposure to 1µM SP inhibited the channel currents by ~36%. B. The time profile showed that the current amplitude decreased rapidly when SP was present in the bath solution, and reached maximum inhibition in ~15 min. C. Whole cell currents were recorded from an oocytes 3 days post injection of the Kir4.1-Kir5.1 tandem-dimer along with the 5-HT2A as described in A. D. The time profile shows that DOI decreased the current amplitude rapidly when applied to the bath solution. The maximum inhibition was reached in ~10 min.
Similar experiments were done by expressing the Kir4.1-Kir5.1 with the 5-HT2A or mTRH-R1 receptor. The currents were inhibited by 43.0±5.9% (n=8) and 30.1±5.1% (n=5) with 40 µM DOI (a specific 5-HT2A receptor agonist) and 100 µM TRH, respectively (Fig. 1B, C). The Kir4.1-Kir5.1 co-expressed with the MOR failed to be regulated by 1 µM DAMGO (an enkephalin analog) which is a potent activator of the MOR (Online-Fig. 1). The effect of DOI on the Kir4.1-Kir5.1 currents was also time dependent. The inhibition occurred rapidly, starting within the first minute after exposure to the chemicals and reaching a maximal effect in ~12 min (Fig. 3C, D). Since DOI is not the natural ligand of the 5-HT2A receptor, experiments were also carried out with 5-HT. In the presence of 40 µM 5-HT the Kir4.1-Kir5.1 currents were inhibited by 35.0±4.1% (n=6) (Online-Fig. 2).
The current-voltage (I–V) relationship was examined for the neurotransmitter effects. Currents were recorded at baseline as well as at the plateau after exposure to 1 µM SP, 40 µM DOI, and 100 µM TRH, respectively. Both currents were then normalized to −160 mV and plotted against the membrane potential. The I-V plot indicated that current inhibition occurred in all negative membrane potentials and did not show evident voltage-dependence (Fig. 1D, E, F).
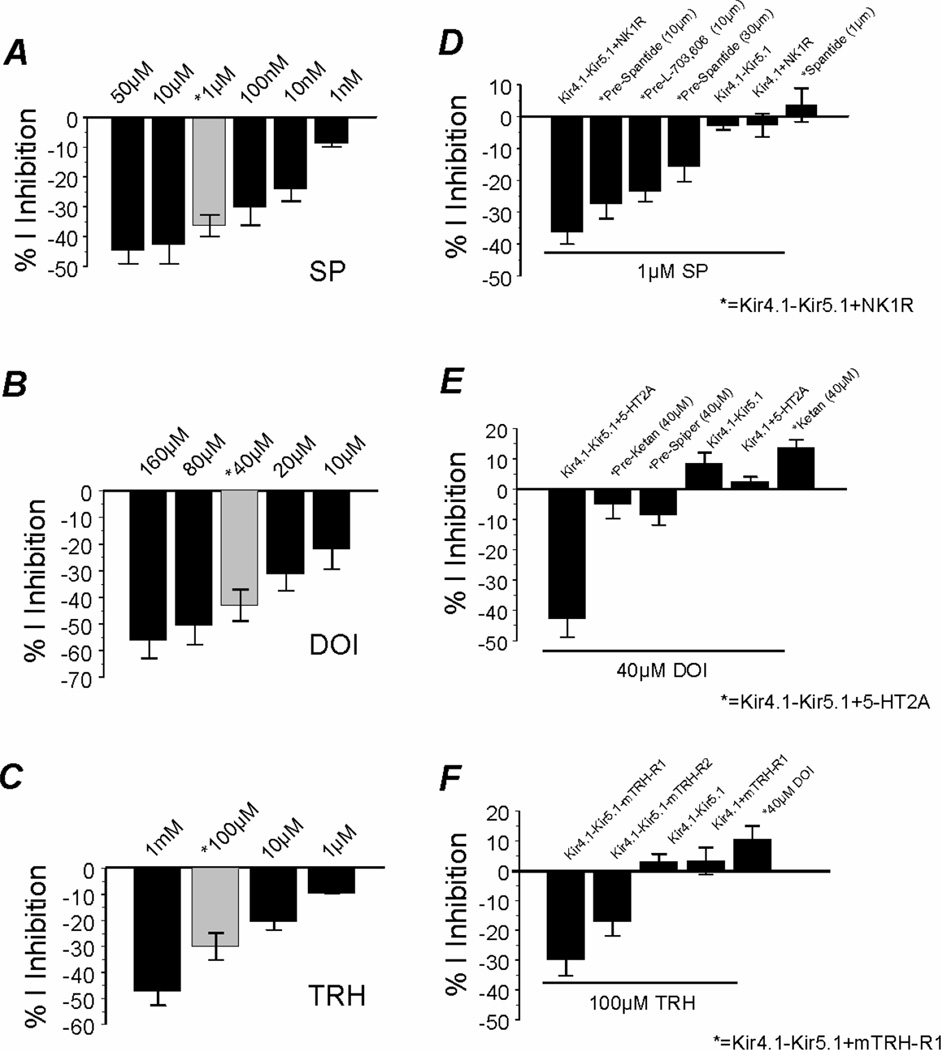
The response to SP, DOI, and TRH showed clear dose dependence. The maximum inhibition (44.6±4.6% (n=9) occurred with 50 µM SP (Fig. 2A). Channel inhibition (8.7±1.2%, n=4) remained to be seen with SP concentrations as low as 1 nM. Similarly, 160 µM DOI produced the maximum inhibition of the Kir4.1-Kir5.1 currents by 56.1±6.8% (n=4), while significant channel inhibition was seen with 10 µM DOI (21.9±7.5%, n=7) (Fig. 2B). Dosedependent inhibition was also observed with TRH (Fig. 2C).
Fig 2. Specificity and concentration-dependence of the neurotransmitter effect.
A, B, C. The effect of SP, DOI, and TRH on the Kir4.1-Kir5.1 channel was clearly concentration-dependent (n≥4). The Kir4.1-Kir5.1 channel was inhibited by very low concentrations of each agonist. Exposure to higher concentrations of the agonists resulted in greater channel inhibition. The gray bars represent the concentrations used for further experiments. D, E, F. Specificity for the receptor-mediated channel inhibition was studied. The channel inhibition was attenuated in the presence of specific antagonists of the neurotransmitters or receptors. The Kir4.1-Kir5.1 channel expressed without receptors failed to be inhibited by the neurotransmitters. Also, SP, DOI, and TRH failed to inhibit the homomeric Kir4.1 channel expressed with the respective receptors. n≥4 for each experiment.
Specificity of the neurotransmitter effects was examined using specific antagonists. Preincubation of oocytes expressing the Kir4.1-Kir5.1/NK1R with 10 µM spantide I, a competitive peptide antagonist for SP, markedly attenuated the effect of 1 µM SP (Fig. 2D). Higher concentrations of spantide I (30 µM) more potently attenuated the effect of 1 µM SP by over 60% (15.9±4.6% inhibition, n=4) (Fig. 2D). Similar attenuation was seen with 10 µM L-703,606, a non-competitive receptor blocker (23.7±3.0%, n=4) (Fig. 2D). Pre-incubation of oocytes expressing the Kir4.1-Kir5.1+5-HT2A with 40 µM ketanserin or 40 µM spiperone, two 5-HT2A receptor antagonists, almost completely abolished the effect of 40 µM DOI that inhibited the currents by 5.3±4.5% (n=6) and 8.9±3.1% (n=5), respectively (Fig. 2E). Since there is no commercially available antagonist for the mTRH-R1 receptor, we used the mTRH-R2 receptor to demonstrate the specificity as it is less sensitive to TRH than the mTRH-R1 receptor. Indeed, 100 µM TRH inhibited the Kir4.1-Kir5.1+mTRH-R1 to a greater degree than the Kir4.1-Kir5.1+mTRH-R2 (Fig. 2F).
Injection of the Kir4.1 cDNA along with the NK1R, 5-HT2A, or mTRH-R1 expressed typical Kir4.1 currents. Strikingly, the homomeric Kir4.1 channel was not capable of being inhibited by SP, DOI, or TRH (Fig. 2D, E, F). Furthermore, the Kir4.1-Kir5.1 channel expressed without exogenous receptors failed to be inhibited by SP, DOI and TRH (Fig. 2D, E, F). Taken together these results strongly suggest that inhibition of Kir4.1-Kir5.1 currents by SP, DOI, and TRH is specific depending on their receptors and requiring the expression of both Kir4.1 and Kir5.1 subunits.
PKC involvement in the modulation of the Kir4.1-Kir5.1 channel by the neurotransmitters
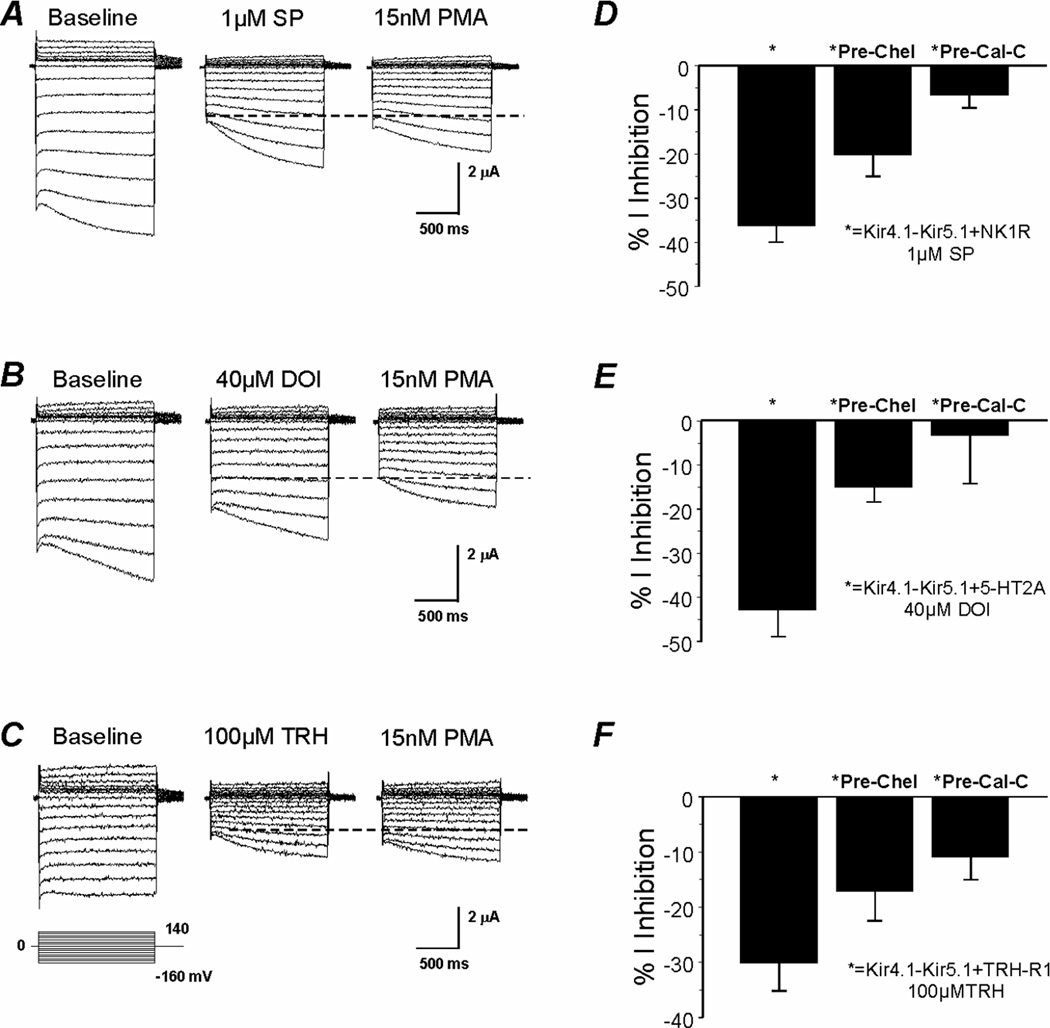
The NK1, 5-HT2A, and TRH-R1 are G-protein coupled receptors (GPCRs). These receptors share a common cascade following ligand binding involving activations of Gαq, phospholipase C and protein kinase C (PKC). The latter has been shown to phosphorylate several Kir channels and regulate their activity (Fakler et al., 1994; Henry et al., 1996; Light et al., 2000; Zhu et al., 1999). For instance, we have previously shown that protein kinase C (PKC) phosphorylation underscores the inhibition of GIRK channels by SP (Mao et al., 2004). Therefore, we conducted experiments to determine whether PKC was involved in the Kir4.1-Kir5.1 channel inhibition by SP, DOI, and TRH. Pre-incubation of oocytes expressing Kir4.1-Kir5.1+NK1R, Kir4.1-Kir5.1+5-HT2A, or Kir4.1-Kir5.1+mTRH-R1 with the specific PKC inhibitor chelerythrine showed a clear attenuation of the SP, DOI, and TRH effects (Fig. 4D, E, F). Calphostin-C, another specific and potent PKC inhibitor, showed strong attenuations of the channel inhibition to 6.7±2.8% (n=6), 3.4±10.8% (n=6), and 11±4.0% (n=8) by SP, DOI, and TRH, respectively (Fig. 4D, E, F).
Fig 4. Involvement of PKC in the Kir4.1-Kir5.1 inhibition by SP, DOI and TRH.
A, B, C. Currents were recorded from an oocyte in the same condition as in Figure 1A. Following exposure to SP, DOI or TRH the cells were subsequently treated with 15 nM PMA. Prior exposure to the neurotransmitters resulted in a reduced response to PMA. Instead of 40% current inhibition as indicated by the dashed lines, the Kir4.1-Kir5.1 currents were inhibited by 0~20%. D, E, F. Pre-incubation of oocytes with specific PKC inhibitors (50 µM chelerythrine or 3 µM calphostin-C) also strongly attenuated the effect of SP, DOI, and TRH (n≥4).
Exposure of oocytes expressing Kir4.1-Kir5.1/NK1R to 15 nM PMA, a specific and potent PKC activator, inhibited Kir4.1-Kir5.1 currents by 39.8±5.5% (n=5) (Table 1). Such an inhibition was significantly lessened (11.0±3.6%, n=5) when the oocytes were prior exposed to SP (Fig. 4A). Similar effects were observed for DOI and TRH (Fig. 4B, C). The attenuation of PKC activation was more profound when the order of the neurotransmitter and PMA exposure was reversed, suggesting that the effects of the neurotransmitters and PMA are mediated via a common mechanism (Online-Fig. 3).
Table 1.
Effect of PMA, SP, DOI, and TRH on wild-type Kir4.1 and Kir4.1-Kir5.1 channels
| Agonist | Baseline I (µA) | Peak effect I (µA) | % effect | n | |
|---|---|---|---|---|---|
| Kir4.1-Kir5.1 + NK1R | 1 µM SP | 3.9±0.5 | 2.5±0.3 | −36.4±3.6 | 10 |
| Kir4.1-Kir5.1 + 5-HT2A | 40 µM DOI | 6.1±0.6 | 3.4±0.3 | −43.0±5.9 | 8 |
| Kir4.1-Kir5.1 + 5-HT2A | 40 µM 5-HT | 11.1±1.8 | 7.1±0.9 | −34.8±4.1 | 6 |
| Kir4.1-Kir5.1 + mTRH-R1 | 100 µM TRH | 7.9±1.8 | 5.8±1.7 | −30.1±5.1 | 5 |
| Kir4.1-Kir5.1 + MOR | 1 µM DAMGO | 3.4±0.4 | 3.4±0.4 | 0.8±7.7 | 4 |
| Kir4.1-Kir5.1 | 1 µM SP | 4.0±0.2 | 4.1±0.2 | 3.0±1.2 | 4 |
| Kir4.1-Kir5.1 | 40 µM DOI | 5.6±0.5 | 6.0±0.6 | 8.08±3.87 | 12 |
| Kir4.1-Kir5.1 | 100 µM TRH | 7.0±1.0 | 7.1±1.0 | 2.86±2.65 | 5 |
| Kir4.1 + NK1R | 1 µM SP | 8.8±1.2 | 8.7±1.5 | −2.8±3.6 | 4 |
| Kir4.1 + 5-HT2A | 40 µM DOI | 7.7±1.5 | 7.9±1.6 | 2.1±2.0 | 5 |
| Kir4.1 + mTRH-R1 | 100 µM TRH | 5.1±0.9 | 5.2±0.8 | 3.2±4.5 | 6 |
| Kir4.1-Kir5.1 | 15 nM PMA | 6.5±0.6 | 3.8±0.4 | −41.4±2.2 | 13 |
| Kir4.1-Kir5.1 + NK1R | 15 nM PMA | 7.3±0.9 | 4.2±0.3 | −39.8±5.5 | 5 |
| Kir4.1-Kir5.1 + 5-HT2A | 15 nM PMA | 9.1±2.5 | 4.8±1.2 | −47.5±1.2 | 4 |
| Kir4.1-Kir5.1 + NK1R | SP/PMA | 9.4±2.4 | 8.1±1.9 | −11.0±3.6 | 5 |
| Kir4.1-Kir5.1 + 5-HT2A | DOI/PMA | 4.7±0.7 | 3.9±0.6 | −16.4±2.2 | 5 |
| Kir4.1-Kir5.1 + mTRH-R1 | TRH/PMA | 3.4±0.4 | 3.5±0.5 | 4.8±5.3 | 9 |
Abbreviations: n, number of observation; I, current;
Effects of SP on single-channel properties
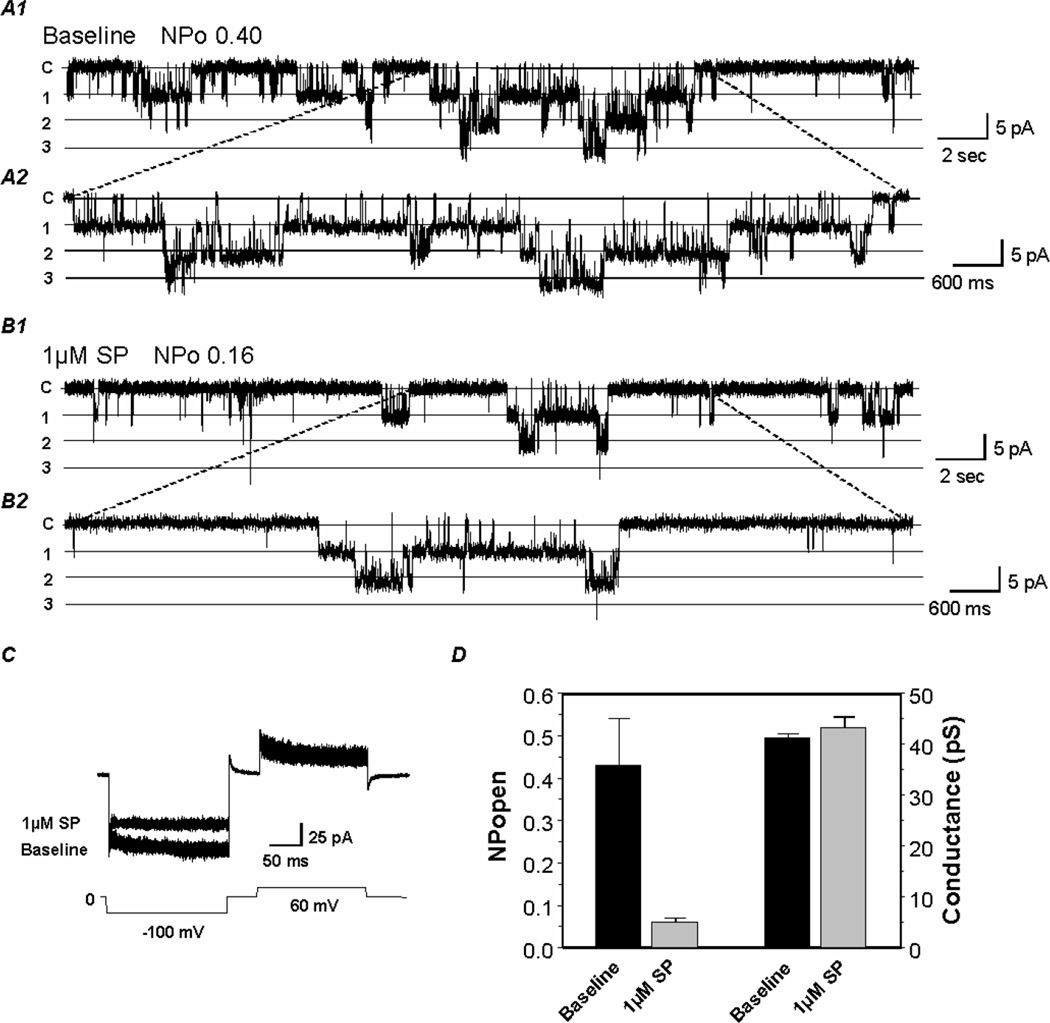
The effect of SP on the single-channel biophysical properties was studied in cellattached patches with 145 mM K+ applied to the extracellular solution at a membrane potential of −80 mV. Inward rectifying currents with a single-channel conductance of ~40 pS (Pessia et al., 1996; Tanemoto et al., 2000) showing long-lasting openings and closures were recorded from oocytes expressing the Kir4.1-Kir5.1+NK1R (Fig. 5). These currents were inhibited with an exposure to 1 µM SP (Fig. 5A, B). The current inhibition was mainly produced by a suppression of the channel open-state probability (NPo). Plotting the baseline NPo versus SP NPo shows a clear reduction in the NPo (0.43±0.11 at baseline vs. 0.06±0.01 with SP, n=7), while the single-channel conductance did not show any significant changes (Fig. 5D).
Fig 5. Effects of SP on the single channel activity of Kir4.1-Kir5.1.
A. Single Kir4.1-Kir5.1 currents were recorded from an oocyte in a cell-attached patch configuration with 145 mM K+ in the patch pipette. At Vm of −80 mV, two active channels are seen at pH 7.4. B. Following stabilization, exposure to 1 µM SP reduced the channel activity mainly by a decrease in the NPo. Labels on the left: c, closure; 1, the first opening; 2, the second opening; etc. Labels on the top represent NPo at baseline and NPo at SP exposure level. C. Reduction of the macroscopic inward currents can be seen in the cell-attached patch configuration. D. Bargraph showing that 1 µM SP drastically reduced the NPo, but only slightly affected the single-channel conductance (n≥6)
Relationship with CO2/pH Sensitivity
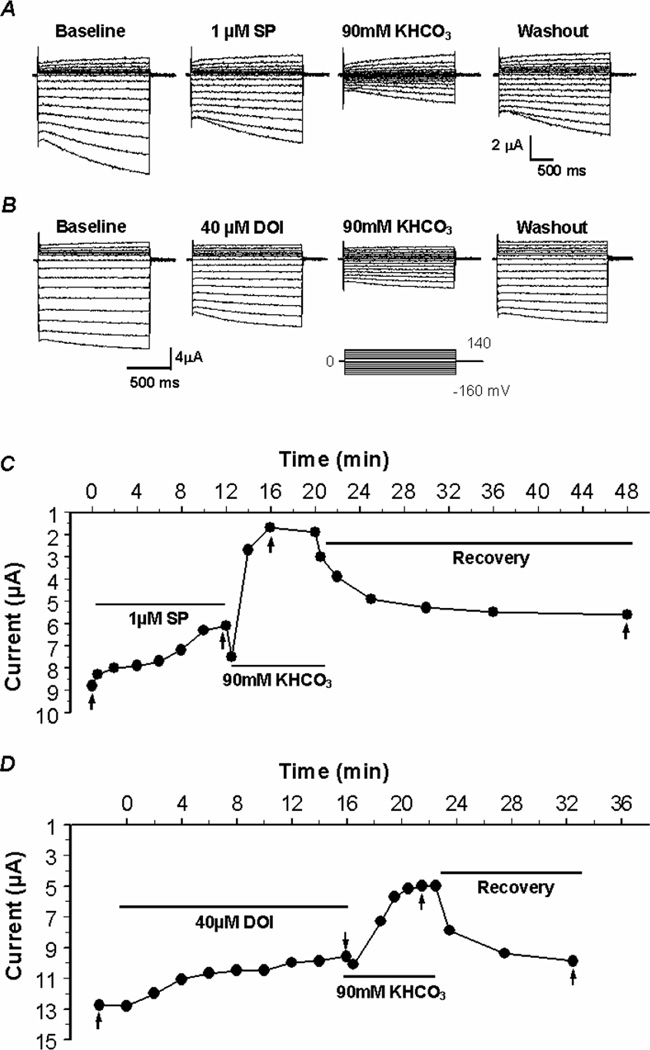
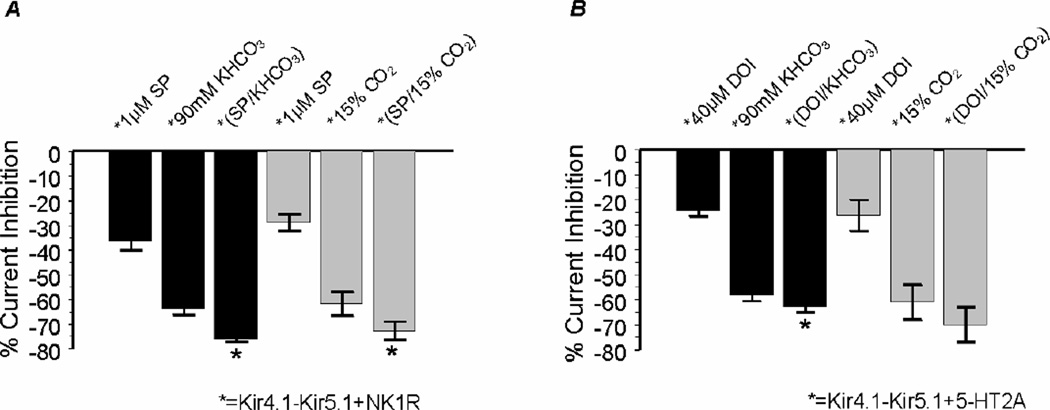
We have previously shown that the Kir4.1-Kir5.1 channel is strongly inhibited by intracellular acidosis (Cui et al., 2001; Xu et al., 2000a; Yang et al., 2000). To understand whether the neural modulation affects the channel sensitivity to pH, the Kir4.1-Kir5.1 channel was co-expressed in Xenopus oocytes with the NK1R or 5-HT2A. Following stabilization the cells were perfused with 1 µM SP or 40 µM DOI until the plateau effect was reached. Subsequently, the cells were exposed to 90 mM KHCO3 that has been shown to reduce intracellular pH (pHi) to ~6.6. Following SP and DOI exposure all of the oocytes tested were further inhibited by intracellular acidosis (Fig. 6A, B), and recovered with washout. The time course showed that following SP and DOI inhibition, the channel was further inhibited by intracellular acidosis (Fig. 6C, D), suggesting that the neurotransmitters and CO2 inhibit the channel through two independent mechanisms. Intracellular acidosis inhibited the Kir4.1-Kir5.1 channel expressed without receptors by 58.2±2.4% (n=8). In the presence of the NK1R and 5-HT2A receptors, the Kir4.1-Kir5.1 channel remained to be inhibited by 60.8±1.5% (n=9) and 55.0±3.7% (n=5) by intracellular acidosis, respectively. It is clear that both neurotransmitter and CO2 have a partial additive effect on the channel which leads to a stronger channel inhibition when both are present together than either one separately (P<0.05, n≥4; Fig. 7A, B). The same experiments were repeated with 15% CO2 and similar results were obtained (Fig. 7A, B).
Fig 6. Independent regulation of the Kir4.1-Kir5.1 channel by neurotransmitters and pHi.
A, B. Using TEVC whole-cell Kir4.1-Kir5.1 currents were recorded from an oocyte 3 days post-injection of the Kir4.1-Kir5.1 dimer cDNA along with the NK1R or 5-HT2A receptor cDNA. With 90 mM K+ in the extracellular solution inward rectifying currents were recorded at baseline. Membrane potential (Vm) was held at 0 mV. A series of command pulse potentials from −160 mV to 140 mV with a 20-mV increment was applied to the cell. Following exposure to 1 µM SP or 40 µM DOI, the cells were perfused with 90 mM KHCO3 (pHi≈6.6). Such an exposure resulted in a further inhibition of the Kir4.1-Kir5.1 currents to the degree identical to that of KHCO3 exposure alone, suggesting that the two channel inhibitors are independent of each other. Washout allowed the currents to recover to the level prior to KHCO3 exposure. C, D. Shows a plot of time course for the effect of the neurotransmitters and 90 mM KHCO3 (pHi≈6.6). The SP and DOI effects are relatively slow and long-lasting, while the effect of 90 mM KHCO3 is rapid and fully reversible.
Fig 7. Additive effects of neurotransmitters and CO2.
A. The percent effect shows that exposure to SP/KHCO3 gives a larger inhibition of the Kir4.1-Kir5.1 currents than either modulator alone. B. Similar results were found for DOI. Both sets of experiments were repeated with 15% CO2 represented by the gray bars. n≥4 for each experiment. The asterisk represents the significant difference between KHCO3 and NT/KHCO3. Differences were considered to be statistically significant if P ≤ 0.05.
Modulation of brainstem neurons by neurotransmitters
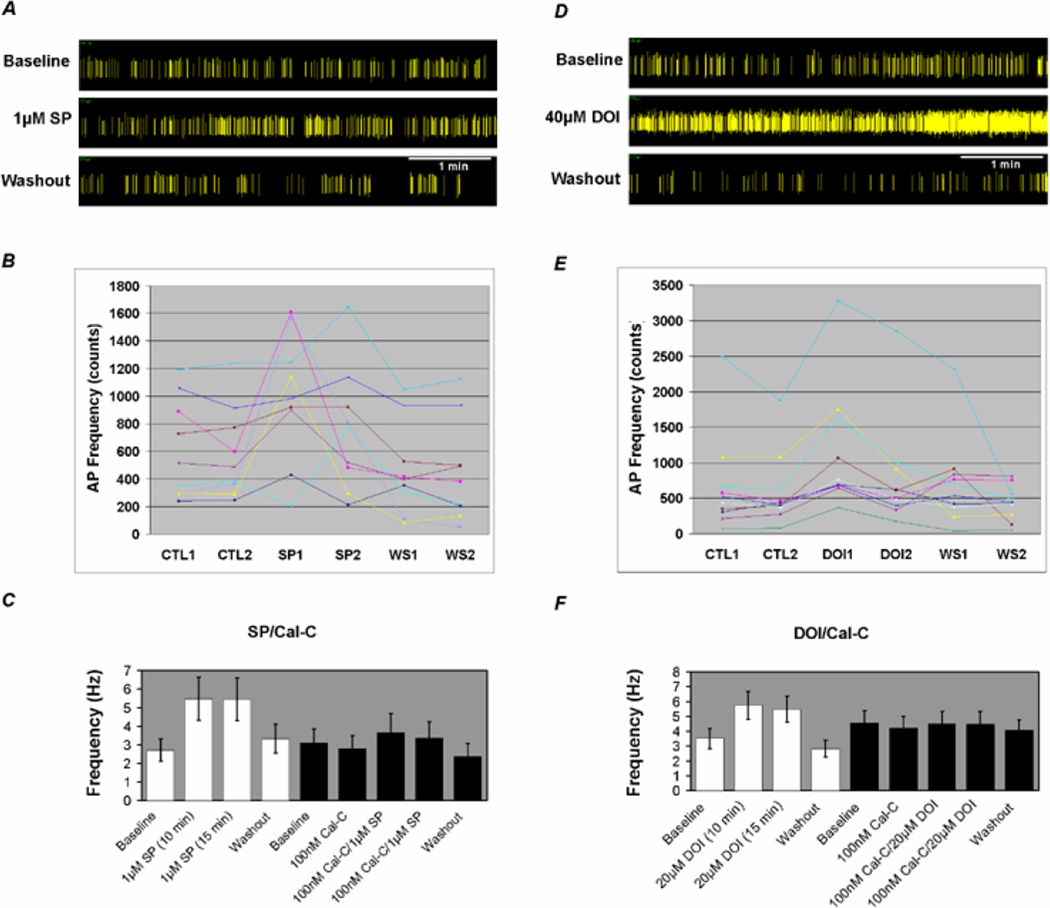
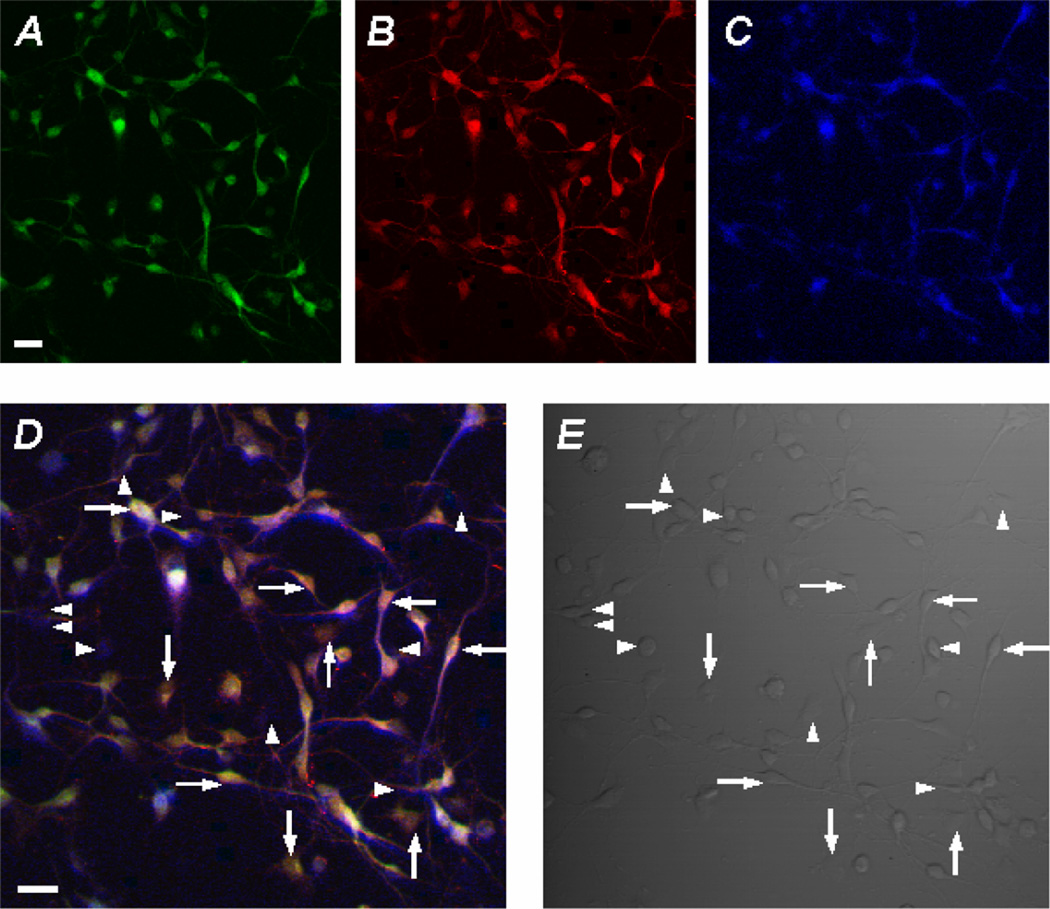
To demonstrate the modulation of brainstem neurons by the neurotransmitters SP and 5-HT we took advantage of multi-electrode array (MEA) technology. Neurons were isolated from the brainstem of fetal rats and cultured on MEA dishes as detailed in the methods section. Extracellular recording was carried out in the DMEM medium at 37 °C. Single-unit recordings were identified using the Offline Sorter software based on the principal component analysis methods (Horn and Friedman, 2003). Single-unit recordings were also determined by the absence of action potentials in the initial period (5–100ms) of the inter-spike histogram. Most spikes showed a negative-positive waveform with a duration > 1 ms, suggesting that they were recorded from the soma (Gustafsson and Jankowska, 1976; Jiang and Lipski, 1990). In these studies, neuronal responses to SP and DOI were observed. SP and DOI augmented the firing frequency of a group of neurons. Washout led to complete recovery (Fig. 8A–E). To determine whether PKC played a role, 100 nM calphostin-C was applied to the MEA dish for 1 hour prior to application of SP and DOI. Action potentials were recorded before and after application of calphostin-C. In the presence of calphostin-C, SP and DOI only modestly augmented the action potential frequency (Fig. 8C, F). The presence of the Kir4.1 and Kir5.1 channels in these neurons was demonstrated with immunocytochemistry showing that both Kir4.1 and Kir5.1 subunits were expressed together in brainstem neurons that displayed positive immunoreactivity of the microtubule-associate protein 2 (Fig. 9). Control experiments were performed to demonstrate the specificity of the neurotransmitter effects. These results suggest that brainstem neurons are modulated by substance-P and serotonin through a PKC mediated mechanism.
Fig 8. Modulation of brainstem neurons by neurotransmitters.
A. A single unit was recorded from a 14-day brainstem neuronal culture using the multiple electrode arrays technique. The spikes had a negative-positive waveform with a duration >1 ms, and the 2D cluster plot and the interspike interval (ISI) histogram indicated single-unit recording. Application of 1 µM SP augmented the firing frequency shown in a period of 5 min. B. The effect of SP on the average firing frequency from a 5 min recording. Nine units were stimulated during SP exposure. Washout led to complete recovery. C. Following pre-incubation with 100 nM calphostin-C for 1 hour the stimulatory effect of SP was greatly reduced. D. A single unit was recorded from a 14-day brainstem neuronal culture using multiple electrode arrays. The spikes had a negative-positive waveform with a duration >1 ms, and the 2D cluster plot and the interspike interval (ISI) histogram indicated single-unit recording. Application of 40 µM DOI augmented the firing frequency shown in a period of 5 min. E. The effect of DOI on the average firing frequency from a 5 min recording. Ten units were stimulated during DOI exposure. Washout led to complete recovery. C. Following pre-incubation with 100 nM calphostin-C for 1 hour the stimulatory effect of DOI was greatly reduced.
Fig 9. Expression of Kir4.1-Kir5.1 in brainstem neurons.
A. Immunocytochemistry was performed on cultured brainstem neurons. A. Shows positive immunoassaying for Kir4.1. B. Shows positive immunoassaying for Kir5.1. C. Blue fluorescence indicates the presence of the neuronal marker microtubule associated protein 2 (MAP2). D. Overlay of A, B, C. The horizontal arrows represent typical neurons expressing the Kir4.1 and Kir5.1 subunits. Vertical arrows represent typical glia cells expressing Kir4.1 and Kir5.1. Horizontal arrowheads indicate neurons void of Kir4.1 and Kir5.1. The vertical arrowheads indicate glia void of Kir4.1 and Kir5.1. E. Shows the phase contrast (note that majority of cells are neurons). Bar=20 µm.
DISCUSSION
In the present study, we have demonstrated a novel property of the Kir4.1-Kir5.1 channel but not the Kir4.1, i.e., the capability to be modulated by several neurotransmitters. This finding provides another evidence for the functional significance of the heteromultimerization, and suggests that the Kir4.1-Kir5.1 channel is likely to be involved in more cellular functions than currently believed.
Unlike other members in the Kir channel family, the Kir4.1-Kir5.1 channel is formed by the heteromultimerization of two inter-subfamily members. While normal channel activity is not seen in the homomeric Kir5.1, the Kir4.1 is fully functional. The members in the Kir4 subfamily have been shown to play important roles in renal epithelium, retinal müller cells, glia cells in the central nervous system, etc. (Hibino et al., 1999; Hibino et al., 2004; Higashi et al., 2001; Ishii et al., 2003; Ito et al., 1996; Kusaka et al., 1999; Tucker et al., 2000). The Kir4x channels are sensitive to extremely acidic pH, which may underscore their functions, at least in part, in these cells (Xu et al., 2000b, Xu et al., 2000c). Several new biophysical properties emerge with the heteromultimerization with Kir5.1, such as the time-dependent activation, larger unitary conductance and higher pH sensitivity (Casamassima et al., 2003; Konstas et al., 2003; Pessia et al., 2001; Tanemoto et al., 2000; Yang et al., 2000).
One important functional property of the Kir4.1-Kir5.1 channel is its pH sensitivity in the physiological range. The channel has a pKa at 7.45 allowing it to detect both alkaline and acidic pH levels (Cui et al., 2001; Xu et al., 2000a). This property has led to the hypothesis that this channel may act as a molecular sensor in central CO2 chemoreception. The central CO2 chemoreceptors are highly sensitive to CO2. It has been shown that a change in PCO2 as low as 1–2 torr is sufficient to produce a marked increase in ventilation rate (by 20–30%) (Feldman et al., 2003; Nattie, 1999). However, none of pH sensitive molecules identified to date including the Kir4.1-Kir5.1 channel can produce a change in cellular activity by 20–30%. Therefore, signal amplifications are crucial (Jiang et al., 2005). Our current studies suggest that the Kir4.1-Kir5.1 channel may be involved in such signal amplification.
The Kir4.1 and Kir5.1 subunits are expressed in brainstem neurons. The mRNAs of both Kir subunits have been detected in various nuclei within the brainstem (Wu et al., 2004). Several studies have demonstrated specific expression of Kir4.1 and Kir5.1 at the protein and mRNA level in oligodendrocytes and astrocytes in the CNS (Hibino et al., 1999; Hibino et al., 2004; Higashi et al., 2001). Here, we have shown expression of both the Kir4.1 and Kir5.1 proteins in brainstem neurons. Although the co-expression of the Kir4.1 and Kir5.1 subunits in the same neurons strongly suggests that the heteromeric Kir4.1-Kir5.1 channel may form in these cells and play a role in central CO2 chemoreception, the involvement of the Kir4.1-Kir5.1 channel depends on its action in neurotransmission.
Several neurotransmitters are particularly important for the brainstem control of respiration including substance-P, serotonin, and thyrotropin releasing hormone. These neuromodulators have been shown to regulate central respiratory activity in vitro and in vivo (Cream et al., 1997; Dekin et al., 1995; Hodges et al., 2004; Moss et al., 1986; Mutolo et al., 1997; Nattie and Li, 2002; Nattie et al., 2004; Nink et al., 1991; Pete et al., 2002; Richerson, 2004; Richerson et al., 2005; Schulz et al., 1996; Severson et al., 2003; Taylor et al., 2005; Wang et al., 2001; Wenninger et al., 2004a; Wenninger et al., 2004b). In the present study, we have shown evidence for the inhibition of the heteromeric Kir4.1-Kir5.1 channel by these neurotransmitters. Exposures to SP, 5-HT, and TRH resulted in inhibition of the Kir4.1-Kir5.1 channel currents when the channel was expressed with the corresponding receptors in Xenopus oocytes. The neurotransmitter effects were reversible, specific and dependent on ligand concentrations. The current inhibition is voltage-independent and is mediated by selective inhibition of Po with no effect on single-channel conductance.
One commonality shared by these three neurotransmitters is that they act on G-protein coupled receptors. Ligand binding to these receptors activates Gαq which in turn propagates multiple second messenger cascades. One such cascade involves activation of phospholipase-C that cleaves PIP2 into IP3 and DAG. The PIP2 derivative DAG activates PKC whereas IP3 increases intracellular calcium which is also necessary for PKC activation. We previously showed that PKC underscored the inhibition of GIRK channels by substance-P (Mao et al., 2004). Therefore, we assayed for the involvement of PKC in the inhibition of the heteromeric Kir4.1-Kir5.1 channel by SP, DOI and TRH. Our results suggest that PKC activation also underscores the inhibition of the Kir4.1-Kir5.1 channel by these neurotransmitters. In cultured brainstem neurons, inhibition of PKC led to a dramatic attenuation of the augmentation of the firing frequency in neurons by SP and DOI, suggesting that PKC activation also underscores the SP and DOI dependent increase in the firing rate of brainstem neurons.
The modulation of the Kir4.1-Kir5.1 channel by SP, 5-HT, and TRH does not compromise the channel sensitivity to pH, as we have shown that both modulators combined have a greater inhibitory effect on the channel than either modulator alone. This suggests that application of both modulators to brainstem respiratory nuclei expressing the Kir4.1-Kir5.1 channel may allow for a stronger depolarization of these chemosensitive neurons and an amplification of the response to CO2 changes. This is important physiologically as we know that during central CO2 chemoreception, there is an amplification of the CO2 signal.
The neural modulation described in the present study seems to enable the Kir4.1-Kir5.1 channel to function as a distinct member in the Kir channel family. It is known that GIRK channels play a role in neurotransmission (Jan and Jan, 1997; Luscher et al., 1997; Yamada et al., 1998). A common characteristic of these channels is that their activity is controlled by neurotransmitters and hormones. Clearly such a property is shared by the Kir4.1-Kir5.1 channel, as shown in our current studies. Unlike GIRK channels, however, the Kir4.1-Kir5.1 channel is not directly regulated by membrane-bound G-proteins, which renders the Kir4.1-Kir5.1 to be modulated in a way that is different from the GIRK channels. Without recruiting G-proteins, the neural modulation of the Kir4.1-Kir5.1 channel may be more local and specific. Furthermore, the effect may last even after the neurotransmitters are cleared from the synaptic cleft, since the modulation appears to takes place through protein phosphorylation. Thus, the time course of the Kir4.1-Kir5.1 channel seems also different from the conventional neural modulation. The neural modulation shown in the present study does not occur in the homomeric Kir4.1 channel and relies on the Kir5.1 that is known to form heteromeric channels only with Kir4x (Casamassima et al., 2003; Konstas et al., 2003; Pessia et al., 2001; Tanemoto et al., 2000). Thus, the expression of the Kir4.1 in cells allows two functional properties that both can control membrane potential and cellular excitability with one of them regulated by extracellular messengers. All of these functional properties therefore allow diverse cellular responses that appear to fit well to the diverse cellular functions in the brainstem and other systems.
Supplementary Material
ACKNOWLEDGEMENTS
Special thanks to Mr. Jean-Pierre Muhumuza and Ms. Carmen Adams for their technical assistance. We are grateful to Dr. John Adelman for the gifts of Kir4.1 and Kir5.1. We are also grateful to Dr. Shigetada Nakanishi, Dr. David Julius, Dr. Marvin Gershengorn, and Dr. Stanley Watson for the gifts of the NK1R, 5-HT2A, mTRH-R1/mTRH-R2, and MOR, respectively. This work is supported by the NIH (HL067890).
Abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- 5-HT2A
serotonin receptor 2A
- DAB
diaminobenzidine
- DAG
diacylglycerol
- DOI
2,5-dimethoxy-4-iodophenyl-2-aminopropane
- GIRK
G protein-coupled inward rectifier K+ channels
- IC50
concentration required for 50% inhibition
- IP3
inositol 1,4,5 trisphosphate
- Kir
inward rectifier K+ channel
- LC
locus coeruleus
- MOR
mu-opioid receptor
- NK1R
neurokinin-1 receptor
- PIP2
Phosphatidylinositol 1,4 bisphosphate
- PKC
protein kinase C
- PMA
4-α-phorbol 12-myristate 13-acetate
- Po
open-state probability
- SP
substance-P
- TEVC
two-electrode voltage clamp
- TH
tyrosine hydroxylase
- TRH
thyrotropin releasing hormone
- TRH-R1
thyrotropin releasing hormone receptor 1
REFERENCES
- Bajic D, Koike M, Albsoul-Younes AM, Nakajima S, Nakajima Y. Two different inward rectifier K+ channels are effectors for transmitter-induced slow excitation in brain neurons. Proc Natl Acad Sci USA. 2002;99:14494–14499. doi: 10.1073/pnas.222379999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamassima M, D'Adamo MC, Pessia M, Tucker SJ. Identification of a heteromeric interaction that influences the rectification, gating, and pH sensitivity of Kir4.1/Kir5.1 potassium channels. J Biol Chem. 2003;278:43533–43540. doi: 10.1074/jbc.M306596200. [DOI] [PubMed] [Google Scholar]
- Cream CL, Li A, Nattie EE. RTN TRH causes prolonged respiratory stimulation. J Appl Physiol. 1997;83:792–799. doi: 10.1152/jappl.1997.83.3.792. [DOI] [PubMed] [Google Scholar]
- Cui N, Giwa LR, Xu H, Rojas A, Abdulkadir L, Jiang C. Modulation of the heteromeric Kir4.1-Kir5.1 channels by P(CO2) at physiological levels. J Cell Physiol. 2001;189:229–236. doi: 10.1002/jcp.10021. [DOI] [PubMed] [Google Scholar]
- Dekin MS, Richerson GB, Getting PA. Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science. 1985;229:67–69. doi: 10.1126/science.3925552. [DOI] [PubMed] [Google Scholar]
- Fakler B, Brandle U, Glowatzki E, Zenner HP, Ruppersberg JP. Kir2.1 inward rectifier K+ channels are regulated independently by protein kinases and ATP hydrolysis. Neuron. 1994;13:1413–1420. doi: 10.1016/0896-6273(94)90426-x. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol. 1976;258:33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry P, Pearson WL, Nichols CG. Protein kinase C inhibition of cloned inward rectifier (HRK1/KIR2.3) K+ channels expressed in Xenopus oocytes. J Physiol. 1996;495(Pt 3):681–688. doi: 10.1113/jphysiol.1996.sp021625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Horio Y, Fujita A, Inanobe A, Doi K, Gotow T, Uchiyama Y, Kubo T, Kurachi Y. Expression of an inwardly rectifying K+ channel, Kir4.1, in satellite cells of rat cochlear ganglia. Am J Physiol. 1999;277:C638–C644. doi: 10.1152/ajpcell.1999.277.4.C638. [DOI] [PubMed] [Google Scholar]
- Hibino H, Fujita A, Iwai K, Yamada M, Kurachi Y. Differential assembly of inwardly rectifying K+ channel subunits, Kir4.1 and Kir5.1, in brain astrocytes. J Biol Chem. 2004;279:44065–44073. doi: 10.1074/jbc.M405985200. [DOI] [PubMed] [Google Scholar]
- Higashi K, Fujita A, Inanobe A, Tanemoto M, Doi K, Kubo T, Kurachi Y. An inwardly rectifying K+ channel, Kir4.1, expressed in astrocytes surrounds synapses and blood vessels in brain. Am J Physiol Cell Physiol. 2001;281:C922–C931. doi: 10.1152/ajpcell.2001.281.3.C922. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Opansky C, Qian B, Davis S, Bonis J, Bastasic J, Leekley T, Pan LG, Forster HV. Transient attenuation of CO2 sensitivity after neurotoxic lesions in the medullary raphe area of awake goats. J Appl Physiol. 2004;97:2236–2247. doi: 10.1152/japplphysiol.00584.2004. [DOI] [PubMed] [Google Scholar]
- Horn CC, Friedman MI. Detection of single unit activity from the rat vagus using cluster analysis of principal components. J Neurosci Methods. 2003;122:141–147. doi: 10.1016/s0165-0270(02)00304-7. [DOI] [PubMed] [Google Scholar]
- Ishii M, Fujita A, Iwai K, Kusaka S, Higashi K, Inanobe A, Hibino H, Kurachi Y. Differential expression and distribution of Kir5.1 and Kir4.1 inwardly rectifying K+ channels in retina. Am J Physiol Cell Physiol. 2003;285:C260–C267. doi: 10.1152/ajpcell.00560.2002. [DOI] [PubMed] [Google Scholar]
- Ito M, Inanobe A, Horio Y, Hibino H, Isomoto S, Ito H, Mori K, Tonosaki A, Tomoike H, Kurachi Y. Immunolocalization of an inwardly rectifying K+ channel, K(AB)-2 (Kir4.1), in the basolateral membrane of renal distal tubular epithelia. FEBS Lett. 1996;388:11–15. doi: 10.1016/0014-5793(96)00502-9. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Voltage-gated and inwardly rectifying potassium channels. J Physiol. 1997;505(Pt 2):267–282. doi: 10.1111/j.1469-7793.1997.267bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Botzinger complex in the cat. Exp Brain Res. 1990;81:639–648. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- Jiang C, Rojas A, Wang R, Wang X. CO2 central chemosensitivity: why are there so many sensing molecules? Respir Physiol Neurobiol. 2005;145:115–126. doi: 10.1016/j.resp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Koike-Tani M, Collins JM, Kawano T, Zhao P, Zhao Q, Kozasa T, Nakajima S, Nakajima Y. Signal transduction pathway for the substance P-induced inhibition of rat Kir3 (GIRK) channel. J Physiol. 2005;564:489–500. doi: 10.1113/jphysiol.2004.079285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstas AA, Korbmacher C, Tucker SJ. Identification of domains that control the heteromeric assembly of Kir5.1/Kir4.0 potassium channels. Am J Physiol Cell Physiol. 2003;284:C910–C917. doi: 10.1152/ajpcell.00479.2002. [DOI] [PubMed] [Google Scholar]
- Kusaka S, Horio Y, Fujita A, Matsushita K, Inanobe A, Gotow T, Uchiyama Y, Tano Y, Kurachi Y. Expression and polarized distribution of an inwardly rectifying K+ channel, Kir4.1, in rat retinal pigment epithelium. J Physiol. 1999;520(Pt 2):373–381. doi: 10.1111/j.1469-7793.1999.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Q, Talley EM, Bayliss DA. Receptor-mediated inhibition of G protein-coupled inwardly rectifying potassium channels involves Gαq family subunits, phospholipase C, and a readily diffusible messenger. J Biol Chem. 2001;276:16720–16730. doi: 10.1074/jbc.M100207200. [DOI] [PubMed] [Google Scholar]
- Light PE, Bladen C, Winkfein RJ, Walsh MP, French RJ. Molecular basis of protein kinase C-induced activation of ATP-sensitive potassium channels. Proc Natl Acad Sci USA. 2000;97:9058–9063. doi: 10.1073/pnas.160068997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Mao J, Wang X, Chen F, Wang R, Rojas A, Shi Y, Piao H, Jiang C. Molecular basis for the inhibition of G protein-coupled inward rectifier K+ channels by protein kinase C. Proc Natl Acad Sci USA. 2004;101:1087–1092. doi: 10.1073/pnas.0304827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss IR, Denavit-Saubie M, Eldridge FL, Gillis RA, Herkenham M, Lahiri S. Neuromodulators and transmitters in respiratory control. Fed Proc. 1986;45:2133–2147. [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Carfi M, Pantaleo T. Respiratory responses to thyrotropin-releasing hormone microinjected into the rabbit medulla oblongata. Am J Physiol. 1999;277:R1331–R1338. doi: 10.1152/ajpregu.1999.277.5.R1331. [DOI] [PubMed] [Google Scholar]
- Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol. 2002;544:603–616. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A, Richerson G, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol. 2004;556:235–253. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- Nink M, Krause U, Lehnert H, Heuberger W, Huber I, Schulz R, Hommel G, Beyer J. Thyrotropin-releasing hormone has stimulatory effects on ventilation in humans. Acta Physiol Scand. 1991;141:309–318. doi: 10.1111/j.1748-1716.1991.tb09086.x. [DOI] [PubMed] [Google Scholar]
- Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO J. 1996;15:2980–2987. [PMC free article] [PubMed] [Google Scholar]
- Pessia M, Imbrici P, D'Adamo MC, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerisation with Kir5.1. J Physiol. 2001;532:359–367. doi: 10.1111/j.1469-7793.2001.0359f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pete G, Mack SO, Haxhiu MA, Walbaum S, Gauda EB. CO2-induced c-Fos expression in brainstem preprotachykinin mRNA containing neurons. Respir Physiol Neurobiol. 2002;130:265–274. doi: 10.1016/s0034-5687(02)00013-0. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol. 2005;90:259–266. doi: 10.1113/expphysiol.2005.029843. [DOI] [PubMed] [Google Scholar]
- Schulz R, Nink M, Werner GS, Andreas S, Kreuzer H, Beyer J, Lehnert H. Human corticotropin-releasing hormone and thyrotropin-releasing hormone modulate the hypercapnic ventilatory response in humans. Eur J Clin Invest. 1996;26:989–995. doi: 10.1046/j.1365-2362.1996.2130573.x. [DOI] [PubMed] [Google Scholar]
- Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Talley EM, Bayliss DA. Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action. J Biol Chem. 2002;277:17733–17742. doi: 10.1074/jbc.M200502200. [DOI] [PubMed] [Google Scholar]
- Tanemoto M, Kittaka N, Inanobe A, Kurachi Y. In vivo formation of a proton-sensitive K+ channel by heteromeric subunit assembly of Kir5.1 with Kir4.1. J Physiol. 2000;525(Pt 3):587–592. doi: 10.1111/j.1469-7793.2000.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol. 2005;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SJ, Imbrici P, Salvatore L, D'Adamo MC, Pessia M. pH dependence of the inwardly rectifying potassium channel, Kir5.1, and localization in renal tubular epithelia. J Biol Chem. 2000;275:16404–16407. doi: 10.1074/jbc.C000127200. [DOI] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. Large lesions in the pre-Botzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol. 2004a;97:1629–1636. doi: 10.1152/japplphysiol.00953.2003. [DOI] [PubMed] [Google Scholar]
- Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah T, Davis S, Forster HV. Small reduction of neurokinin-1 receptor-expressing neurons in the pre-Botzinger complex area induces abnormal breathing periods in awake goats. J Appl Physiol. 2004b;97:1620–1628. doi: 10.1152/japplphysiol.00952.2003. [DOI] [PubMed] [Google Scholar]
- Wu J, Xu H, Shen W, Jiang C. Expression and coexpression of CO2-sensitive Kir channels in brainstem neurons of rats. J Membr Biol. 2004;197:179–191. doi: 10.1007/s00232-004-0652-4. [DOI] [PubMed] [Google Scholar]
- Xu H, Cui N, Yang Z, Qu Z, Jiang C. Modulation of kir4.1 and kir5.1 by hypercapnia and intracellular acidosis. J Physiol. 2000a;524(Pt 3):725–735. doi: 10.1111/j.1469-7793.2000.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Yang Z, Cui N, Giwa LR, Abdulkadir L, Patel M, Sharma P, Shan G, Shen W, Jiang C. Molecular determinants for the distinct pH sensitivity of Kir1.1 and Kir4.1 channels. Am J Physiol Cell Physiol. 2000b;279:C1464–C1471. doi: 10.1152/ajpcell.2000.279.5.C1464. [DOI] [PubMed] [Google Scholar]
- Xu H, Yang Z, Cui N, Chanchevalap S, Valesky WW, Jiang C. A single residue contributes to the difference between Kir4.1 and Kir1.1 channels in pH sensitivity, rectification and single channel conductance. J Physiol. 2000c;528(Pt 2):267–277. doi: 10.1111/j.1469-7793.2000.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Inanobe A, Kurachi Y. G protein regulation of potassium ion channels. Pharmacol Rev. 1998;50:723–760. [PubMed] [Google Scholar]
- Yang Z, Xu H, Cui N, Qu Z, Chanchevalap S, Shen W, Jiang C. Biophysical and molecular mechanisms underlying the modulation of heteromeric Kir4.1-Kir5.1 channels by CO2 and pH. J Gen Physiol. 2000;116:33–45. doi: 10.1085/jgp.116.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Qu Z, Cui N, Jiang C. Suppression of Kir2.3 activity by protein kinase C phosphorylation of the channel protein at threonine 53. J Biol Chem. 1999;274:11643–11646. doi: 10.1074/jbc.274.17.11643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.