Abstract
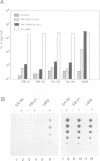
Tumor necrosis factor (TNF) has cytotoxic and gene-inductive activities on several cell types. Previous studies on L929 fibrosarcoma cells have revealed that the mitochondrial electron transport system plays a key role in inducing TNF cytotoxicity, presumably by the formation of reactive oxygen intermediates (ROI). Here we report that mitochondria-derived intermediates are not only cytotoxic but, in addition, function as signal transducers of TNF-induced gene expression. The activation of NF kappa B, which fulfills an important role in TNF-induced gene transcription, could be blocked by interference with the mitochondrial electron transport system. Furthermore, antimycin A, a mitochondrial inhibitor that increases the generation of ROI, potentiated TNF-triggered NF kappa B activation. The dual role of mitochondria-derived intermediates in cytotoxicity and immediate-early gene induction of TNF was further substantiated by isolating L929 subclones which lacked a functional respiratory chain. This depletion of the mitochondrial oxidative metabolism resulted in resistance towards TNF cytotoxicity, as well as in inhibition of NF kappa B activation and interleukin-6 gene induction by TNF. These findings suggest that mitochondria are the source of second messenger molecules and serve as common mediators of the TNF-cytotoxic and gene-regulatory signaling pathways.
Full text
PDF
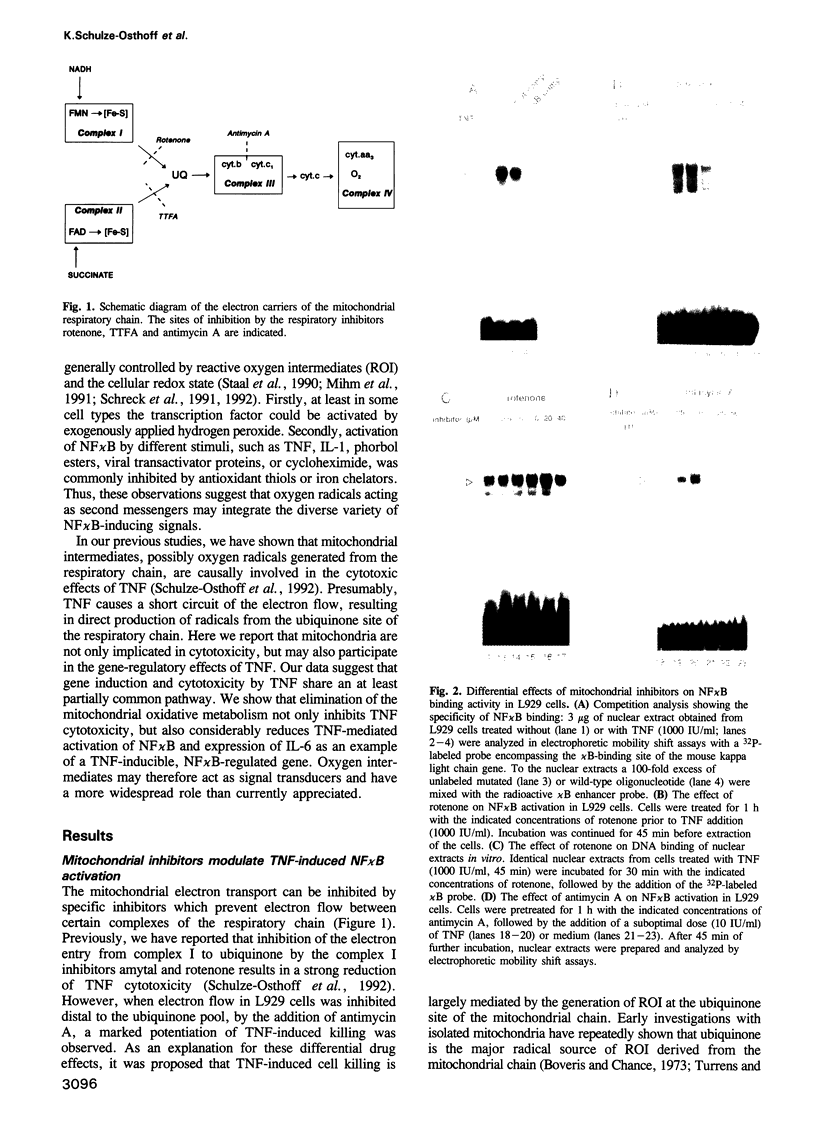







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Amstad P. A., Krupitza G., Cerutti P. A. Mechanism of c-fos induction by active oxygen. Cancer Res. 1992 Jul 15;52(14):3952–3960. [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Bauskin A. R., Alkalay I., Ben-Neriah Y. Redox regulation of a protein tyrosine kinase in the endoplasmic reticulum. Cell. 1991 Aug 23;66(4):685–696. doi: 10.1016/0092-8674(91)90114-e. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Beyaert R., Schulze-Osthoff K., Van Roy F., Fiers W. Lithium chloride potentiates tumor necrosis factor-induced and interleukin 1-induced cytokine and cytokine receptor expression. Cytokine. 1991 Jul;3(4):284–291. doi: 10.1016/1043-4666(91)90496-z. [DOI] [PubMed] [Google Scholar]
- Blank V., Kourilsky P., Israël A. NF-kappa B and related proteins: Rel/dorsal homologies meet ankyrin-like repeats. Trends Biochem Sci. 1992 Apr;17(4):135–140. doi: 10.1016/0968-0004(92)90321-y. [DOI] [PubMed] [Google Scholar]
- Bomsztyk K., Rooney J. W., Iwasaki T., Rachie N. A., Dower S. K., Sibley C. H. Evidence that interleukin-1 and phorbol esters activate NF-kappa B by different pathways: role of protein kinase C. Cell Regul. 1991 Apr;2(4):329–335. doi: 10.1091/mbc.2.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Cadenas E., Stoppani A. O. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem J. 1976 May 15;156(2):435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973 Jul;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brach M. A., Hass R., Sherman M. L., Gunji H., Weichselbaum R., Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J Clin Invest. 1991 Aug;88(2):691–695. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. A., O'Hara M., Angel P., Chojkier M., Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989 Feb 16;337(6208):661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- CONRAD H., SMITH L. A study of the kinetics of the oxidation of cytochrome c by cytochrome c oxidase. Arch Biochem Biophys. 1956 Aug;63(2):403–413. doi: 10.1016/0003-9861(56)90055-8. [DOI] [PubMed] [Google Scholar]
- Cadenas E., Boveris A. Enhancement of hydrogen peroxide formation by protophores and ionophores in antimycin-supplemented mitochondria. Biochem J. 1980 Apr 15;188(1):31–37. doi: 10.1042/bj1880031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Mariottini P., Cleeter M. W., Ragan C. I., Matsuno-Yagi A., Hatefi Y., Doolittle R. F., Attardi G. Six unidentified reading frames of human mitochondrial DNA encode components of the respiratory-chain NADH dehydrogenase. Nature. 1985 Apr 18;314(6012):592–597. doi: 10.1038/314592a0. [DOI] [PubMed] [Google Scholar]
- Cino M., Del Maestro R. F. Generation of hydrogen peroxide by brain mitochondria: the effect of reoxygenation following postdecapitative ischemia. Arch Biochem Biophys. 1989 Mar;269(2):623–638. doi: 10.1016/0003-9861(89)90148-3. [DOI] [PubMed] [Google Scholar]
- Cromlish J. A., Roeder R. G. Human transcription factor IIIC (TFIIIC). Purification, polypeptide structure, and the involvement of thiol groups in specific DNA binding. J Biol Chem. 1989 Oct 25;264(30):18100–18109. [PubMed] [Google Scholar]
- Dealtry G. B., Naylor M. S., Fiers W., Balkwill F. R. DNA fragmentation and cytotoxicity caused by tumor necrosis factor is enhanced by interferon-gamma. Eur J Immunol. 1987 May;17(5):689–693. doi: 10.1002/eji.1830170517. [DOI] [PubMed] [Google Scholar]
- Defilippi P., Poupart P., Tavernier J., Fiers W., Content J. Induction and regulation of mRNA encoding 26-kDa protein in human cell lines treated with recombinant human tumor necrosis factor. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4557–4561. doi: 10.1073/pnas.84.13.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Amábile-Cuevas C. F. Redox redux: the control of oxidative stress responses. Cell. 1991 Nov 29;67(5):837–839. doi: 10.1016/0092-8674(91)90355-3. [DOI] [PubMed] [Google Scholar]
- Desjardins P., Frost E., Morais R. Ethidium bromide-induced loss of mitochondrial DNA from primary chicken embryo fibroblasts. Mol Cell Biol. 1985 May;5(5):1163–1169. doi: 10.1128/mcb.5.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Lau L. F., Karin M. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol. 1991 May;11(5):2804–2811. doi: 10.1128/mcb.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh E. J., Maury W. J., Folks T. M., Fauci A. S., Rabson A. B. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh E. J., Maury W. J., Folks T. M., Fauci A. S., Rabson A. B. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991 Jul 22;285(2):199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- Fransen L., Müller R., Marmenout A., Tavernier J., Van der Heyden J., Kawashima E., Chollet A., Tizard R., Van Heuverswyn H., Van Vliet A. Molecular cloning of mouse tumour necrosis factor cDNA and its eukaryotic expression. Nucleic Acids Res. 1985 Jun 25;13(12):4417–4429. doi: 10.1093/nar/13.12.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadaleta G., Pepe G., De Candia G., Quagliariello C., Sbisà E., Saccone C. The complete nucleotide sequence of the Rattus norvegicus mitochondrial genome: cryptic signals revealed by comparative analysis between vertebrates. J Mol Evol. 1989 Jun;28(6):497–516. doi: 10.1007/BF02602930. [DOI] [PubMed] [Google Scholar]
- Gal A., Herrmann R. G., Lottspeich F., Ohad I. Phosphorylation of cytochrome b6 by the LHC II kinase associated with the cytochrome complex. FEBS Lett. 1992 Feb 17;298(1):33–35. doi: 10.1016/0014-5793(92)80016-a. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Grivell L. A. Nucleo-mitochondrial interactions in yeast mitochondrial biogenesis. Eur J Biochem. 1989 Jul 1;182(3):477–493. doi: 10.1111/j.1432-1033.1989.tb14854.x. [DOI] [PubMed] [Google Scholar]
- Guehmann S., Vorbrueggen G., Kalkbrenner F., Moelling K. Reduction of a conserved Cys is essential for Myb DNA-binding. Nucleic Acids Res. 1992 May 11;20(9):2279–2286. doi: 10.1093/nar/20.9.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Hohmann H. P., Kolbeck R., Remy R., van Loon A. P. Cyclic AMP-independent activation of transcription factor NF-kappa B in HL60 cells by tumor necrosis factors alpha and beta. Mol Cell Biol. 1991 Apr;11(4):2315–2318. doi: 10.1128/mcb.11.4.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook N. J., Fornace A. J., Jr Response to adversity: molecular control of gene activation following genotoxic stress. New Biol. 1991 Sep;3(9):825–833. [PubMed] [Google Scholar]
- Israël N., Gougerot-Pocidalo M. A., Aillet F., Virelizier J. L. Redox status of cells influences constitutive or induced NF-kappa B translocation and HIV long terminal repeat activity in human T and monocytic cell lines. J Immunol. 1992 Nov 15;149(10):3386–3393. [PubMed] [Google Scholar]
- King M. E., Godman G. C., King D. W. Respiratory enzymes and mitochondrial morphology of HeLa and L cells treated with chloramphenicol and ethidium bromide. J Cell Biol. 1972 Apr;53(1):127–142. doi: 10.1083/jcb.53.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. P., Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989 Oct 27;246(4929):500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- Konstantinov A. A., Peskin A. V., Popova EYu, Khomutov G. B., Ruuge E. K. Superoxide generation by the respiratory chain of tumor mitochondria. Biochim Biophys Acta. 1987 Oct 29;894(1):1–10. doi: 10.1016/0005-2728(87)90206-4. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Darnell J. E., Jr Cytoplasmic activation of ISGF3, the positive regulator of interferon-alpha-stimulated transcription, reconstituted in vitro. Genes Dev. 1989 Sep;3(9):1362–1371. doi: 10.1101/gad.3.9.1362. [DOI] [PubMed] [Google Scholar]
- Liao X. S., Small W. C., Srere P. A., Butow R. A. Intramitochondrial functions regulate nonmitochondrial citrate synthase (CIT2) expression in Saccharomyces cerevisiae. Mol Cell Biol. 1991 Jan;11(1):38–46. doi: 10.1128/mcb.11.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libermann T. A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990 May;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschen G., Azzi A., Richter C., Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974 May 15;42(1):68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- Mihm S., Ennen J., Pessara U., Kurth R., Dröge W. Inhibition of HIV-1 replication and NF-kappa B activity by cysteine and cysteine derivatives. AIDS. 1991 May;5(5):497–503. doi: 10.1097/00002030-199105000-00004. [DOI] [PubMed] [Google Scholar]
- Morais R., Desjardins P., Turmel C., Zinkewich-Péotti K. Development and characterization of continuous avian cell lines depleted of mitochondrial DNA. In Vitro Cell Dev Biol. 1988 Jul;24(7):649–658. doi: 10.1007/BF02623602. [DOI] [PubMed] [Google Scholar]
- Nass M. M. Differential effects of ethidium bromide on mitochondrial and nuclear DNA synthesis in vivo in cultured mammalian cells. Exp Cell Res. 1972 May;72(1):211–222. doi: 10.1016/0014-4827(72)90583-6. [DOI] [PubMed] [Google Scholar]
- Niki E. Antioxidants in relation to lipid peroxidation. Chem Phys Lipids. 1987 Jul-Sep;44(2-4):227–253. doi: 10.1016/0009-3084(87)90052-1. [DOI] [PubMed] [Google Scholar]
- Ostrove J. M., Gifford G. E. Stimulation of RNA synthesis in L-929 cells by rabbit tumor necrosis factor. Proc Soc Exp Biol Med. 1979 Mar;160(3):354–358. doi: 10.3181/00379727-160-40449. [DOI] [PubMed] [Google Scholar]
- Parikh V. S., Morgan M. M., Scott R., Clements L. S., Butow R. A. The mitochondrial genotype can influence nuclear gene expression in yeast. Science. 1987 Jan 30;235(4788):576–580. doi: 10.1126/science.3027892. [DOI] [PubMed] [Google Scholar]
- Ray A., Sassone-Corsi P., Sehgal P. B. A multiple cytokine- and second messenger-responsive element in the enhancer of the human interleukin-6 gene: similarities with c-fos gene regulation. Mol Cell Biol. 1989 Dec;9(12):5537–5547. doi: 10.1128/mcb.9.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C., Frei B. Ca2+ release from mitochondria induced by prooxidants. Free Radic Biol Med. 1988;4(6):365–375. doi: 10.1016/0891-5849(88)90088-3. [DOI] [PubMed] [Google Scholar]
- Rossi F. The O2- -forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and function. Biochim Biophys Acta. 1986 Nov 4;853(1):65–89. doi: 10.1016/0304-4173(86)90005-4. [DOI] [PubMed] [Google Scholar]
- Ruggiero V., Johnson S. E., Baglioni C. Protection from tumor necrosis factor cytotoxicity by protease inhibitors. Cell Immunol. 1987 Jul;107(2):317–325. doi: 10.1016/0008-8749(87)90240-1. [DOI] [PubMed] [Google Scholar]
- Schreck R., Meier B., Männel D. N., Dröge W., Baeuerle P. A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992 May 1;175(5):1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
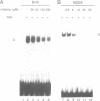
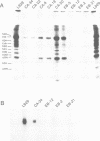
- Schulze-Osthoff K., Bakker A. C., Vanhaesebroeck B., Beyaert R., Jacob W. A., Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992 Mar 15;267(8):5317–5323. [PubMed] [Google Scholar]
- Schütze S., Potthoff K., Machleidt T., Berkovic D., Wiegmann K., Krönke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced "acidic" sphingomyelin breakdown. Cell. 1992 Nov 27;71(5):765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Mitomo K., Watanabe T., Okamoto S., Yamamoto K. Involvement of a NF-kappa B-like transcription factor in the activation of the interleukin-6 gene by inflammatory lymphokines. Mol Cell Biol. 1990 Feb;10(2):561–568. doi: 10.1128/mcb.10.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Mizel S. B. In vitro activation and nuclear translocation of NF-kappa B catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol Cell Biol. 1989 Jun;9(6):2424–2430. doi: 10.1128/mcb.9.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C. M., Cidlowski J. A. Direct evidence for intra- and intermolecular disulfide bond formation in the human glucocorticoid receptor. Inhibition of DNA binding and identification of a new receptor-associated protein. J Biol Chem. 1989 Apr 25;264(12):6638–6647. [PubMed] [Google Scholar]
- Spolsky C. M., Eisenstadt J. M. Chloramphenicol-resistant mutants of human HeLa cells. FEBS Lett. 1972 Sep 15;25(2):319–324. doi: 10.1016/0014-5793(72)80514-3. [DOI] [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B., Rahmsdorf H. J., Steffen A., Litfin M., Herrlich P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol Cell Biol. 1989 Nov;9(11):5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G., Tartaglia L. A., Ames B. N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990 Apr 13;248(4952):189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- Suffys P., Beyaert R., De Valck D., Vanhaesebroeck B., Van Roy F., Fiers W. Tumour-necrosis-factor-mediated cytotoxicity is correlated with phospholipase-A2 activity, but not with arachidonic acid release per se. Eur J Biochem. 1991 Jan 30;195(2):465–475. doi: 10.1111/j.1432-1033.1991.tb15727.x. [DOI] [PubMed] [Google Scholar]
- Suffys P., Beyaert R., Van Roy F., Fiers W. Involvement of a serine protease in tumour-necrosis-factor-mediated cytotoxicity. Eur J Biochem. 1988 Dec 1;178(1):257–265. doi: 10.1111/j.1432-1033.1988.tb14451.x. [DOI] [PubMed] [Google Scholar]
- Tada H., Shiho O., Kuroshima K., Koyama M., Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986 Nov 6;93(2):157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- Toledano M. B., Leonard W. J. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens J. F., Alexandre A., Lehninger A. L. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985 Mar;237(2):408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980 Nov 1;191(2):421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P. G., Rubira M. R., Simpson R. J. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevoorde V., Haegeman G., Fiers W. TNF-mediated IL6 gene expression and cytotoxicity are co-inducible in TNF-resistant L929 cells. FEBS Lett. 1992 May 18;302(3):235–238. doi: 10.1016/0014-5793(92)80449-q. [DOI] [PubMed] [Google Scholar]
- Vandevoorde V., Haegeman G., Fiers W. Tumor necrosis factor-induced interleukin-6 expression and cytotoxicity follow a common signal transduction pathway in L929 cells. Biochem Biophys Res Commun. 1991 Aug 15;178(3):993–1001. doi: 10.1016/0006-291x(91)90990-o. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Zheng X. X., Lott M. T., Shoffner J. M., Hodge J. A., Kelley R. I., Epstein C. M., Hopkins L. C. Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell. 1988 Nov 18;55(4):601–610. doi: 10.1016/0092-8674(88)90218-8. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Wirth T., Staudt L., Baltimore D. An octamer oligonucleotide upstream of a TATA motif is sufficient for lymphoid-specific promoter activity. Nature. 1987 Sep 10;329(6135):174–178. doi: 10.1038/329174a0. [DOI] [PubMed] [Google Scholar]
- Wiseman A., Attardi G. Reversible tenfod reduction in mitochondria DNA content of human cells treated with ethidium bromide. Mol Gen Genet. 1978 Nov 16;167(1):51–63. doi: 10.1007/BF00270321. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Elwell J. H., Oberley L. W., Goeddel D. V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989 Sep 8;58(5):923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- Xanthoudakis S., Miao G., Wang F., Pan Y. C., Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992 Sep;11(9):3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi N., Kuriyama H., Watanabe N., Neda H., Maeda M., Niitsu Y. Intracellular hydroxyl radical production induced by recombinant human tumor necrosis factor and its implication in the killing of tumor cells in vitro. Cancer Res. 1989 Apr 1;49(7):1671–1675. [PubMed] [Google Scholar]
- Yamauchi N., Watanabe N., Kuriyama H., Neda H., Maeda M., Himeno T., Tsuji Y., Niitsu Y. Suppressive effects of intracellular glutathione on hydroxyl radical production induced by tumor necrosis factor. Int J Cancer. 1990 Nov 15;46(5):884–888. doi: 10.1002/ijc.2910460522. [DOI] [PubMed] [Google Scholar]
- Yu L., Yang F. D., Yu C. A. Interaction and identification of ubiquinone-binding proteins in ubiquinol-cytochrome c reductase by azido-ubiquinone derivatives. J Biol Chem. 1985 Jan 25;260(2):963–973. [PubMed] [Google Scholar]
- Zabel U., Schreck R., Baeuerle P. A. DNA binding of purified transcription factor NF-kappa B. Affinity, specificity, Zn2+ dependence, and differential half-site recognition. J Biol Chem. 1991 Jan 5;266(1):252–260. [PubMed] [Google Scholar]
- Zhang Y. H., Lin J. X., Yip Y. K., Vilcek J. Enhancement of cAMP levels and of protein kinase activity by tumor necrosis factor and interleukin 1 in human fibroblasts: role in the induction of interleukin 6. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6802–6805. doi: 10.1073/pnas.85.18.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lin J. X., Vilcek J. Synthesis of interleukin 6 (interferon-beta 2/B cell stimulatory factor 2) in human fibroblasts is triggered by an increase in intracellular cyclic AMP. J Biol Chem. 1988 May 5;263(13):6177–6182. [PubMed] [Google Scholar]
- Zimmerman R. J., Chan A., Leadon S. A. Oxidative damage in murine tumor cells treated in vitro by recombinant human tumor necrosis factor. Cancer Res. 1989 Apr 1;49(7):1644–1648. [PubMed] [Google Scholar]