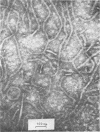
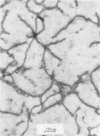
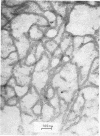
Abstract
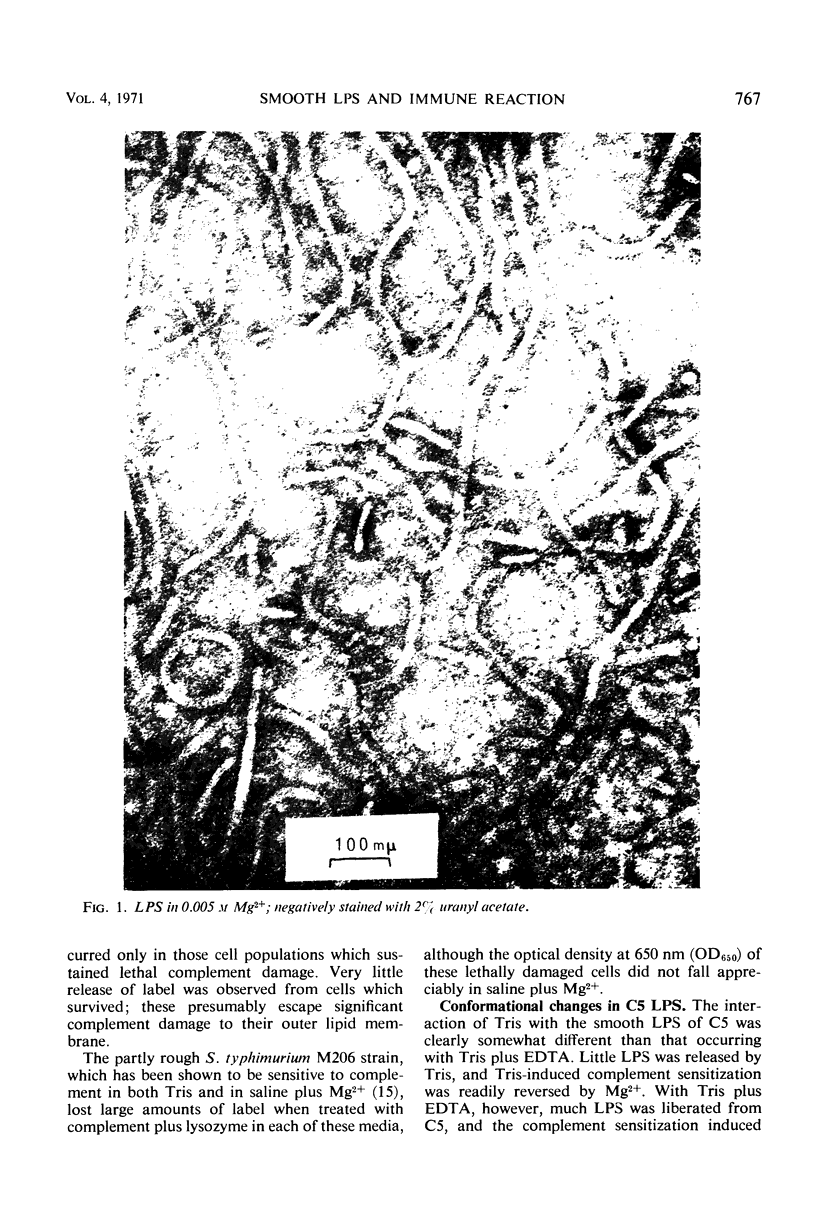
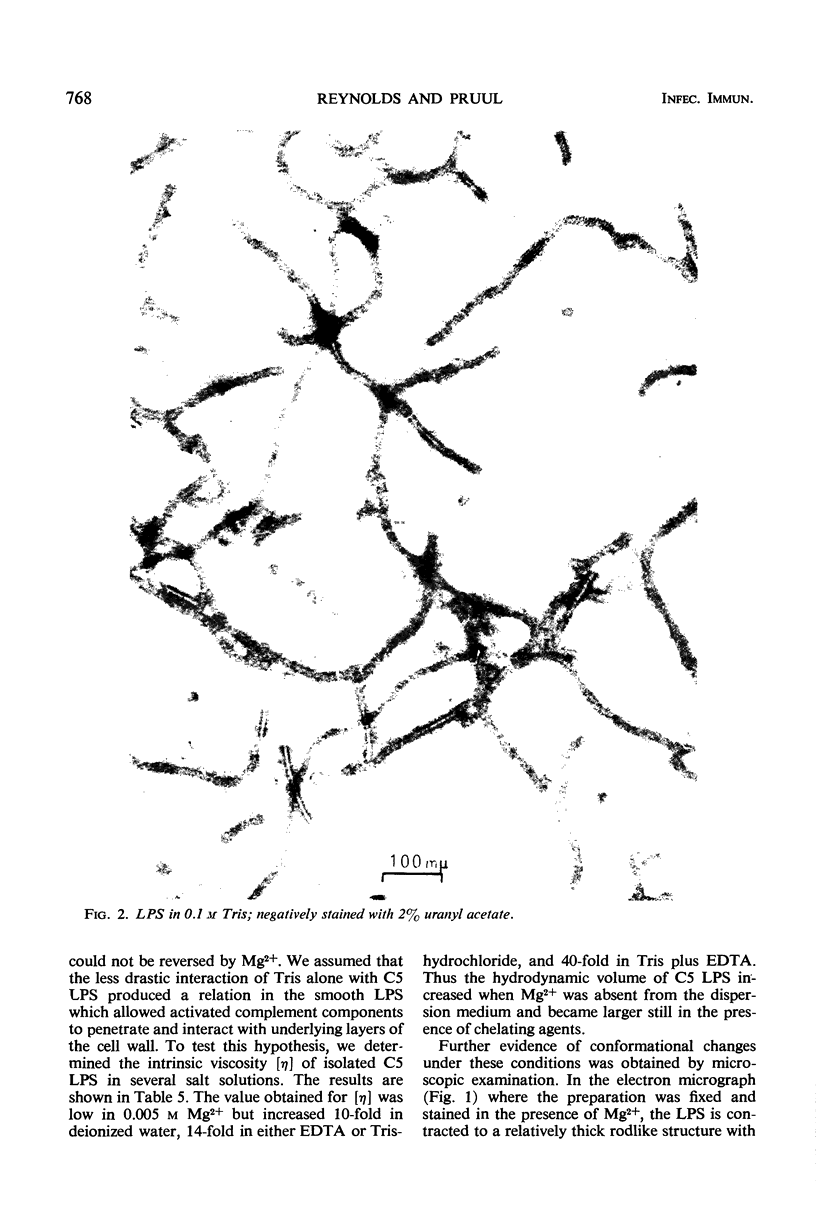
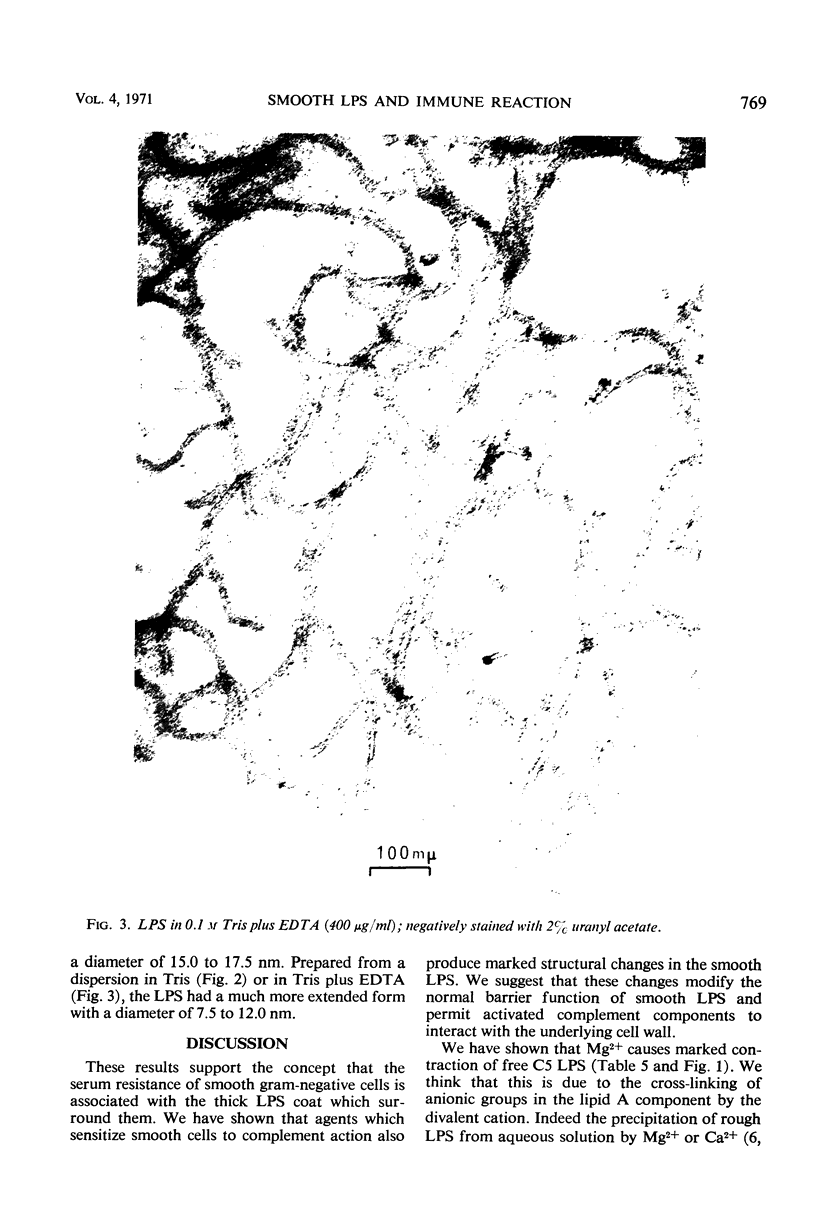
The mechanism by which smooth lipopolysaccharide (LPS) protects Salmonella typhimurium C5 from the lethal action of specific antibody and complement has been investigated. Tris(hydroxymethyl)aminomethane (Tris) and ethylenediaminetetraacetate (EDTA), which sensitize S. typhimurium C5 to the bactericidal action of immune serum, were shown to cause appreciable structural modification of the smooth LPS associated with these cells. Mg2+ was shown to reverse the sensitization of C5 by Tris, but this divalent cation was unable to reverse the sensitizing effect of Tris plus EDTA, which, unlike Tris, caused relatively large amounts of O-antigenic components to be released from the C5 cell wall. The possibility that smooth LPS blocks the interaction of activated complement components with receptors on the cell wall is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer J. R., Rowley D. A quantitative comparison of the antigenic structure of a virulent and an avirulent strain of Salmonella typhimurium. Immunology. 1969 Oct;17(4):551–558. [PMC free article] [PubMed] [Google Scholar]
- Asbell M. A., Eagon R. G. Role of Multivalent Cations in the Organization, Structure, and Assembly of the Cell Wall of Pseudomonas aeruginosa. J Bacteriol. 1966 Aug;92(2):380–387. doi: 10.1128/jb.92.2.380-387.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H. A., Gewurz H., Mergenhagen S. E. Interactions of the complement system with the surface and endotoxic lipopolysaccharide of Veillonella alcalescens. J Exp Med. 1967 May 1;125(5):767–786. doi: 10.1084/jem.125.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S. T., Jr, Eagon R. G. Action of ethylenediaminetetraacetic acid, tris(hydroxymethyl)-aminomethane, and lysozyme on cell walls of Pseudomonas aeruginosa. Can J Microbiol. 1968 Aug;14(8):913–922. doi: 10.1139/m68-153. [DOI] [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the lethal event in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2127–2131. doi: 10.1128/jb.96.6.2127-2131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O. Interaction of lipopolysaccharides and lipid A with complement. Eur J Biochem. 1971 Mar 1;19(1):143–152. doi: 10.1111/j.1432-1033.1971.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Glynn A. A. The complement lysozyme sequence in immune bacteriolysis. Immunology. 1969 Apr;16(4):463–471. [PMC free article] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Medearis D. N., Jr, Camitta B. M., Heath E. C. Cell wall composition and virulence in Escherichia coli. J Exp Med. 1968 Sep 1;128(3):399–414. doi: 10.1084/jem.128.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel L. H., Jackson J. E. Reversal of the bactericidal reaction of serum by magnesium ion. J Bacteriol. 1966 Apr;91(4):1399–1402. doi: 10.1128/jb.91.4.1399-1402.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Jackson K. E., Cross W. R. Correlation between pathogenicity of Shigella and intraperitoneal survival in mice. Infect Immun. 1970 Nov;2(5):570–573. doi: 10.1128/iai.2.5.570-573.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., HORECKER B. L. Biosynthesis of bacterial lipopolysaccharide. I. Enzymatic incorporation of galactose in a mutant strain of Salmonella. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1831–1838. doi: 10.1073/pnas.48.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. L., Pruul H. Sensitization of complement-resistant smooth gram-negative bacterial strains. Infect Immun. 1971 Mar;3(3):365–372. doi: 10.1128/iai.3.3.365-372.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. L., Rowley D. Sensitization of complement resistant bacterial strains. Nature. 1969 Mar 29;221(5187):1259–1261. doi: 10.1038/2211259a0. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Shin H. S., Wood W. B., Jr Natural immunity to bacterial infections: the relation of complement to heat-labile opsonins. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1151–1156. doi: 10.1073/pnas.63.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss J. G. Effects of organic cations on the gram-negative cell wall and their bactericidal activity with ethylenediaminetetra-acetate and surface active agents. J Gen Microbiol. 1967 Sep;48(3):391–400. doi: 10.1099/00221287-48-3-391. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. The nature of the ghosts obtained by lysozyme lysis of Bacillus megaterium. Exp Cell Res. 1956 Feb;10(1):214–221. doi: 10.1016/0014-4827(56)90087-8. [DOI] [PubMed] [Google Scholar]