Abstract
Primary culture of human Schwann cells (SCs) and vestibular schwannoma (VS) cells are invaluable tools to investigate SC physiology and VS pathobiology, and to devise effective pharmacotherapies against VS, which are sorely needed. However, existing culture protocols, in aiming to create robust, pure cultures, employ methods that can lead to loss of biological characteristics of the original cells, potentially resulting in misleading biological findings. We have developed a minimally manipulative method to culture primary human SC and VS cells, without the use of selective mitogens, toxins, or time-consuming and potentially transformative laboratory techniques. Schwann cell purity was quantified longitudinally using S100 staining in SC cultures derived from the great auricular nerve and VS cultures followed for 7 and 12 weeks, respectively. SC cultures retained approximately ≥85% purity for 2 weeks. VS cultures retained approximately ≥80% purity for the majority of the span of 12 weeks, with maximal purity of 87% at 2 weeks. The VS cultures showed high level of biological similarity (68% on average) to their respective parent tumors, as assessed using a protein array featuring 41 growth factors and receptors. Apoptosis rate in vitro negatively correlated with tumor volume. Our results, obtained using a faster, simplified culturing method than previously utilized, indicate that highly pure, primary human SC and VS cultures can be established with minimal manipulation, reaching maximal purity at 2 weeks of culture. The VS cultures recapitulate the parent tumors' biology to a great degree, making them relevant models to investigate VS pathobiology.
Keywords: Schwann cell, schwannoma cell, vestibular schwannoma, primary human culture
1. Introduction
Schwann cells are the principal glia of the peripheral nervous system, supporting neuronal function and regeneration. Neoplastic growth of Schwann cells leads to schwannomas, with the most common type being vestibular schwannomas (VSs) arising from the vestibular nerves. Histological incidence of VS is 1:100 (Ohtani, Tada, & Omori, 2007) while clinical incidence ranges from 1:10,000 for unilateral and sporadic VS (Stangerup & Caye-Thomasen, 2012) to 1:30,000 for bilateral VSs occurring in association with neurofibromatosis type II syndrome (Evans et al., 2010). VSs typically cause hearing loss, tinnitus, and imbalance, and can lead to death from brainstem compression (Matthies & Samii, 1997; Thomsen, Zilstorff, & Tos, 1983). Treatment options available today are essentially limited to surgical resection via craniotomy or stereotactic radiation therapy, both of which carry substantial risks (Demetriades et al., 2010; Hardy, Macfarlane, Baguley, & Moffat, 1989). There is an unmet medical need for an effective pharmacotherapy against VS; a representative culture model of VS cells and healthy SCs can address this need by expediting testing of promising compounds. The existing culture models have limitations, particularly in their complex and potentially transformative purification procedures. Further, many studies describe the VS and SC culture systems at a given time point, lacking data that characterize the ideal time points to utilize the cultures.
Among existing SC culture methods, some have utilized SC mitogens such as forskolin, and fibroblast cytotoxins such as cytosine arabinoside (Calderon-Martinez, Garavito, Spinel, & Hurtado, 2002; Casella, Bunge, & Wood, 1996; Niapour et al., 2010), which can alter SC physiology (Hood, Levene, & Levi, 2009), and potentially cause SC cytotoxicity or selection for a subset of SCs (Armati, Constable, & Llewellyn, 1990). Although highly pure SCs can be cultured by fluorescent-activated cell sorting (Spiegel & Peles, 2009) and by exploitation of differential SC attachment using collagenase treatment (Jin, Liu, Hong, & Cao, 2008), these techniques require expensive materials, special facilities and substantial cell manipulation. Other methods to achieve high SC purity rely on time-consuming explantations of cells (Hood, Levene, & Levi, 2009; Morrissey, Kleitman, & Bunge, 1991). Additionally, cultures derived from adult rat sciatic and other peripheral nerves (Mauritz, Grothe, & Haastert, 2004; Morrissey, Kleitman, & Bunge, 1991; Niapour et al., 2010) may have limited applicability to humans, as cultured animal and human SCs can behave differently (Morrissey, Kleitman, & Bunge, 1991). Primary cultures derived from human VS are established in a similar manner as SCs to prevent fibroblast contamination (Nair, Leung, Collins, Ramsden, & Wilson, 2007), using methods that may alter VS pathobiology. Further, those who have successfully cultured VS cells have not characterized these cultures over time (Bush et al., 2012; Neff et al., 2012).
We describe an improved, minimally-manipulative method, based on modification of techniques previously applied in animal and cadaveric human tissue (Casella, Bunge, & Wood, 1996; Mauritz, Grothe, & Haastert, 2004; Morrissey, Kleitman, & Bunge, 1991; Niapour et al., 2010) to efficiently and affordably establish human SC and VS cultures. By following the SC and VS cultures longitudinally for 7 and 12 weeks, respectively, we define 2 weeks of culture as the optimal time point to maximize cell purity. We demonstrate that our culture system is representative of the parent tissue as the derived VS cultures showed a high level biological similarity to the respective parent tumors, reinforcing the cultures to be relevant models of VS pathobiology.
2. Methods
2.1 Specimen collection
Great auricular nerves (GANs) were used as the source for healthy human SCs as they are routinely sacrificed for access during parotidectomies and neck dissections. Importantly, schwannomas and diseases of the GAN are exceptionally rare, making it an excellent source of healthy SCs. Immediately after GAN resection, nerve specimens measuring 1 cm (from parotidectomies) to 5 cm (from neck dissections) were placed in sterile saline on ice and transported to the laboratory. Similarly, human VS tumor specimens were collected immediately after resection and were transported to the laboratory in sterile saline on ice. The total time from resection to processing was approximately 20 min for GANs and VSs. Specimens were handled according to the institutional review board's study protocol approved by the Human Studies Committee of Massachusetts General Hospital (Protocol No. 2004- P2297/2, PI: K.M.S) and Massachusetts Eye and Ear Infirmary (Protocol No. 05-02-009X, PI: K.M.S).
2.2 Schwann and schwannoma cell isolation
GAN samples were washed with sterile PBS thrice to remove accompanying blood or scar tissue, and transferred to supplemented DMEM/F12 medium, consisting of 39% Dulbecco's Modified Eagle's Medium (DMEM; Life Technologies, NY, #10313), 39% F12 Nutrient Mixture (ThermoScientific, MA, #SH30026.01), 10% Fetal Bovine Serum (Life Technologies, NY, #16140-071), 1% Penicillin/Streptomycin mix (ThermoScientific, MA, 15140-122) and 1% L-Glutamate (Life Technologies, NY,#55050). Under a dissecting microscope, the fascicles were isolated from the epineurium by tugging on the perineurium using no. 5 forceps (Fine Science Tools, CA, #11251-20), while clasping the epineurium with no. 3 forceps (Fine Science Tools, CA, #11231-30). A scalpel blade (#10) was used to cut the nerve into 1-2 mm segments, which were then incubated in an enzymatic mixture containing 250 U/mL Hyaluronidase Type I-S (Sigma-Aldrich, MO, #C1639) and 160 U/mL Collagenase Type I (Sigma-Aldrich, MO, #3506) in DMEM/F12 medium. No further growth factors were added. GAN pieces were incubated for 24 h at 37° C with 5% CO 2 levels. In the meantime, in a sterile environment, 12-well dishes (USA Scientific, Inc., FL, # CC7682-7512) were coated with poly-L-ornithine (Sigma-Aldrich, MO,#P4957) overnight at room temperature (RT), rinsed with sterile PBS thrice and coated with laminin (BD Biosciences, MA, #354232) diluted (1:400) in DMEM/F12 medium for at least 1 h at room temperature (RT). After the enzymatic incubation of the culture, the cell culture-containing medium was triturated using an 18-gauge needle (BD Biosciences, MA, #309574). The cells were recovered by centrifugation at 1000 g for 5 min at RT. The pellet was resuspended in supplemented DMEM/F12 medium and plated on poly-L-Lysine and laminin pre-coated coverslips (BD Biosciences, MA, #334087) within the 12-well dishes coated with poly-L-ornithine and laminin. Culture medium was replaced with fresh medium after 24 h, then every 3 days.
The same protocol was followed for VS cell cultures with two notable changes. Firstly, during initial tissue dissection, cauterized portions (white and opaque) and blood vessels were carefully separated and removed from the main specimen (yellow and clear, fascia-like). The cleaned specimen was minced into approximately 1 mm3 pieces by using two no. 5 forceps. Secondly, the tumor cells were incubated in media with enzyme mixture for 18 h (versus 24 h for GAN). This length of time was found to be ideal for separating cells while also retaining some tumor cell clusters to augment the growth of the culture.
2.3 Culture characterization
Longitudinal culture growth was assessed qualitatively through light microscopy. Differential interference contrast microscopy images were obtained weekly in select GAN-derived and VS-derived cultures for up to 10 and 12 weeks, respectively.
2.4 Immunofluorescence
Longitudinal SC purity was quantified using immunofluorescence. Cultured cells were washed in PBS, fixed with 4% paraformaldehyde (Electron Microscopy Sciences, PA) in PBS for 20 min, washed with PBS, treated with 0.4% Triton X (Integra Chemical, WA, #T756.30.30) for 5 min, exposed to a blocking buffer consisting of 5% Normal Horse Serum (NHS, Sigma-Aldrich, MO, #H1270), and incubated in primary anti-S100 antibody (Dako, CA, #Z0311, 1:400) diluted in 1% NHS overnight at 4 °C to mark SCs. According to the manufacturer, this antibody strongly labels S100B, an isoform expressed by glial cells and highly enriched in SCs (Spreca et al., 1989), and very weakly labels S100A6, an isoform found in fibroblasts and epithelial cells. At the dilution used, we did not find S100 labeling in morphologically fibroblast-like cells. The cells were washed and an anti-rabbit IgG (Jackson Immuno Research, PA, #711-095-152, 1:200) diluted in 1% NHS was applied for 2 h at RT. Nuclear staining was performed with two 5-min washes in Hoechst stain 33342 (Life Technologies, NY, #H1399, 1 nM dilution) followed by two 5-min PBS washes. The coverslips were mounted on glass slides using Vectashield (Vector Laboratories, CA, #H-1000). The edges of the coverslips were sealed using clear nail polish (Electron Microscopy Sciences, PA). Cells were observed under the Axioskop 2 mot plus differential interference contrast microscope (Carl Zeiss, Germany) and photographed with the Axiocamera (Carl Zeiss, Germany) attached to the microscope. The fraction of Schwann and schwannoma cells present in the culture was quantified using manual counting. Cells were counted in ≥3 random fields per culture per time point. SC purity was reported as the ratio of S100 positive cells (cytoplasmic stain) to Hoechst positive cells (nuclear stain). The quantification was done for ≥3 different cultures for each time point. The data for each time point were not necessarily obtained from the same culture, although the majority of the measurements were done by following a given culture longitudinally. Slides were stored in the dark at -20°C to minimize photobleaching.
2.5 Growth Factor Protein Arrays
Part of the fresh tumor specimens, after being washed in fresh sterile phosphate-buffered saline (PBS) thrice, were placed into cold RIPA buffer fortified with protease and phosphatase inhibitors for protein extraction. Protein was also extracted from VS cultures, aged approximately 2 weeks. Human growth factor array membranes printed with 41 specific antibodies in replicate (Human Growth Factor Array C1, RayBiotech, Inc., GA, Catalog #AAH-GF-1) were probed with tissue lysate from 3 parent VSs and corresponding cell culture lysates. The manufacturer's protocol was followed for experimental procedures. Briefly, samples were dialyzed and protein concentrations, measured spectrophotometrically, were normalized and then conjugated with biotin. The membranes were exposed to the blocking buffer, incubated with biotin-conjugated sample at 4°C overnight, washed and incubated with HRP-conjugated streptavidin at 4°C overnight. The membranes were incubated in detection buffer for 1 min, and exposed in Chemidoc (BioRad Laboratories, Hercules, CA). Optical density for the growth factor arrays was measured using Quantity One (BioRad Laboratories, Hercules, CA) and was analyzed and normalized for all samples using the RayBiotech Growth Factor Array analysis tool (RayBiotech, Inc., GA).
2.6 Proliferation assay
Proliferation rate of 12 VS cultures was assessed and correlated with the tumor volume, measured by investigator G.M. B. and G. J. H., in the latest gadolinium enhanced T1-weighted magnetic resonance imaging (MRI) scan prior to surgical resection, and with tumor growth in vivo, measured as changes in the tumor's volume over time calculated from serial MRI scans. Tumor growth was standardized by dividing the growth rate by the initial tumor volume. Separate analyses were performed for solid tumors, which generally account for approximately 96% of VSs (Charabi et al., 1994), versus all studied tumors, which included 4 out of 13 total tumors with a visible cystic component, because cystic components could misrepresent true tumor volume (Charabi et al., 1994). To determine the level of cell proliferation in the cultures, Bromodeoxyuridine (BrdU) was added to the cells at a concentration of 10 μg/ml 20 h before the cells were fixed. The cells were kept in the dark after the addition of BrdU. Immunofluorescence protocol was followed as described (section 2.4), and cell and nuclear membranes were permeablized by incubation in 1% Triton-X for 10 min and by incubation in 2N Hydrochloric acid for 20 min, respectively, after fixation. Primary antibody against BrdU (AbD Serotec, NC, #OBT0030G, 1:200) and anti-rat IgG (Life Technologies, NY, #A21209, 1:1000) were used. BrdU- and Hoechst-stained nuclei were counted in 3-5 fields and the ratio of BrdU positive to Hoechst positive nuclei was used to determine the proliferation rate in vitro.
2.7 Apoptosis assay
Rate of apoptosis in 6 VS cultures was assessed and correlated with tumor growth in vivo and tumor volume. Two out of the six VS had cystic components. Apoptosis was measured using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL, Roche Applied Sciences, NY, #11684795910) following manufacturer's instructions. Briefly, immunofluorescence protocol was followed as described (section 2.4) until fixation, then the cells were washed with PBS thrice and incubated in 1% Triton-X for 10 min on shaker. The cells were washed with PBS once and incubated in TUNEL mix for 1 h at 37°C, then for 30 min at RT on shaker. The cells were then incubated in rhodamine phalloidin (Life Technologies, NY, #R415, 1:40) and Hoechst stain for 20 minutes, washed with PBS thrice and mounted onto slides for imaging. TUNEL and Hoechst stained nuclei were counted in ≥3 fields and the ratio of TUNEL positive to Hoechst positive nuclei was used to determine apoptosis rate in vitro. A positive control of 10 min-DNAse (Roche Applied Sciences, NY, #04536282001) treatment prior to TUNEL labeling was utilized.
2.8 Statistical Analyses
Microsoft Excel 2010 (licensed to MEEI) was utilized for statistical analyses pertaining to Schwann cell purity, proliferation and apoptosis assays. Schwann cell purity was compared between different time points using a two-tailed t-test followed by Benjamini-Hochberg adjustment to obtain p-values. Non-parametric spearman's rank correlations were utilized when correlating VS culture proliferation and apoptosis rates to tumor growth rate in vivo and tumor volume as recommended for small sample sizes (N<15). Standard error of means (SEM) are provided for S100, proliferation and apoptosis cell counts, where mean of each culture (counted in ≥3 different fields) was compared across cultures from different specimens. Standard deviations (SD) are provided for all other measures. To analyze growth factor array expression, R software (Free Software Foundation) was utilized for hierarchical clustering (with Manhattan distance measurement and complete linkage). Additionally, repeated measures ANOVA and Excel were utilized for paired t-tests followed by Benjamini-Hochberg adjustment to obtain p-values. For all statistical analyses, a p-value (p) <0.05 was considered significant.
3. Results
3.1 Human nerve-derived primary Schwann cell culture
Fifteen GAN specimens, each from a different patient, were acquired, yielding healthy SCs for culture. Cells isolated after enzymatic digestion were cultured in media and adhered onto coverslips in less than 24 hours. Dissections with the most clear and successful isolation of the fascicles gave rise to the purest SC cultures. The cultured cells demonstrated distinct morphologies whose distribution changed significantly overtime (Fig A.1). The morphologies seen were SC-like with a small cell body and bipolar processes versus fibroblast-like with flat and polygonal cell body accompanied by a larger nucleus than that of SC-like cells. SC-like morphology predominated in the culture until week 2 (Fig. 1A (a), 1A (b)) at a confluence of approximately 40%, at which point fibroblast-like cells began to predominate. Although the confluence increased significantly after week 2 (Fig. 1A(c)), progressively reaching 99%, most of this increase could be attributed to fibroblast-like cell infiltration and proliferation (Fig. A.1). This interceding phase of fibroblast-predominance reverted around week 7, at which time proliferation subsided and fibroblast-like cells appeared to be dying faster than SCs (Fig. 1A (d)). The culture retained a high SC-like cell distribution in weeks 8 through 10, similar to the cellular distribution seen before 2 weeks of growth (Fig. A.1). Culture growth was not assessed after 10 weeks in vitro as very few cells remained.
Figure 1.
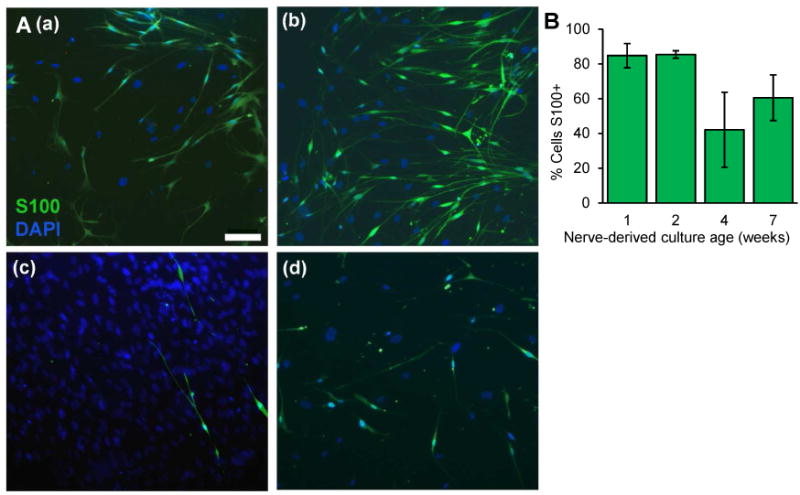
Growth and purity of the great auricular nerve-derived schwann cell-enriched culture. A. Representative images of longitudinal progression of culture at: (a) 1, (b) 2, (c) 4, (d) 7 weeks. Green: S100 immunoreactivity, Blue: Hoechst nuclear stain (DAPI). Scale bar = 100 μm applies to all images. B. Quantification of S100 positive Schwann cells in the culture at corresponding time points (N≥3 different cultures for each time point); mean ± SEM shown.
3.2 Human schwannoma-derived primary cell culture
Twenty-four VS specimens, each from a different patient, were acquired and used for VS cell culture. Specimens that were minimally cauterized before resection and were processed for culture immediately post-resection seemed to yield the purest and most robust cultures. Cellular morphology seen was similar to nerve-derived cultures, although the cells were larger (Fig. 2A). Longitudinally, the cells could be characterized by sustained growth, lacking contact-mediated inhibition and cell loss seen in week 7 of nerve-derived cultures. These characteristics are consistent with neoplastic growth. For VS cultures, it was important to retain few cell clusters (Fig. 2C) for many of the cultures, or else the cultures were not as robust. The cell density was noted to be increasing until week 3, after which the total number of cells decreased as the cultures aged (Table A.1), suggesting that culture proliferation peaks at approximately week 3.
Figure 2.
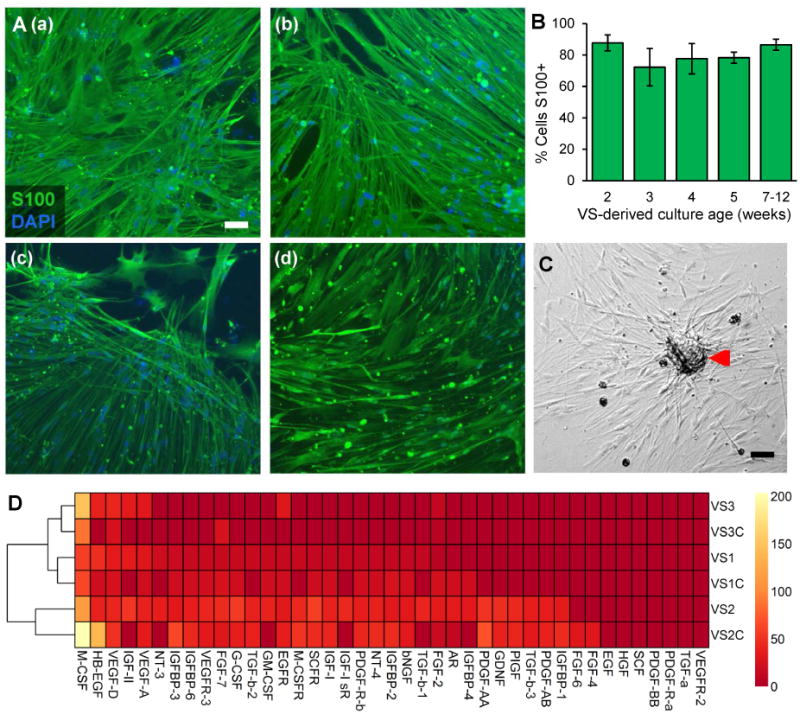
Growth and purity of the vestibular schwannoma-derived culture. A. Representative images of longitudinal progression of culture at: (a) 2, (b) 3, (c) 5, (d) 12 weeks. Green: S100 immunoreactivity, Blue: Hoechst nuclear stain (DAPI). B. Quantification of S100 positive Schwann cells in the culture at corresponding time points (N≥3 different cultures for each time point); mean ± SEM shown. C. A schwannoma-derived cell cluster (red arrow) that is observed to augment growth of the culture. D. Dendogram and heat map showing relative expression of 41 proteins analyzed in three VSs, VS1, VS2, VS3, and their derived cultures, VS1C, VS2C, and VS3C, respectively. Color reflects normalized protein expression: yellow indicates high expression, orange indicated low expression, and dark red indicated no detectable expression. Scale bar = 100 μm applies to all images in panel A and C.
3.3 Schwann cell purity
Schwann cell purity was assessed by immunostaining for cytoplasmic S100, a well-established marker for SCs (Spreca et al., 1989). Actual values for fraction of S100 positive cells from the nerve-derived and schwannoma-derived cultures are provided in Table A.1. In the SC cultures followed in vitro over time, we demonstrate a high level of SC purity, averaging 85% for up to 2 weeks; after that fibroblast-like cells predominate (Fig. 1A, 1B, Table A.1). For weeks 1 through 7, our qualitative observations (Fig. A.1) were in concert with the quantitative measurements based on the fraction of S100 positive cells (Fig. 1B, Table A.1). Although most SC cultures demonstrated ≥70% purity throughout the duration of the experiments, two out of nine cultures retained approximately 10% SCs over time.
Schwannoma-derived cells retained 80% purity on average for the majority of 12 weeks in vitro (Fig. 2A, 2B, Table A.1). There was a decrease in S100 positivity at week 3, which could be partly attributed to the fact that different cultures were used to quantify percentage of S100-positive cells at 3 weeks than at other time points (Table A.1). Similar to the nerve-derived cultures, two out of seventeen VS cultures retained many more fibroblast-like cells than SCs, potentially due to the extent of cauterization of the tumor immediately prior to resection. S100-based SC or VS purity did not differ significantly between subsequent weeks of growth (p>0.05 for all comparisons).
3.4 Correlation of parent VS biology to derived cultures
Out of the 41 growth factors and receptors analyzed, VS1, 2 and 3 had 31, 25 and 7 proteins expressed, respectively (Fig. 2D, Table A.2). VS1's protein expression was most similar to its derived culture, having 25 proteins expressed, with 83% proteins overlapping (Fig. 2D, Table A.2). VS2, having 19 proteins expressed, and VS3, having 4 proteins expressed, had 76% and 43% proteins overlapping with their respective derived cultures (Fig. 2D, Table A.2). On average, 68% proteins present in a tumor were also present in the corresponding derived culture, with only a few new proteins being detected in the culture that were not present in the tumors, on average 13.4% (Table A.2). Three proteins, namely macrophage colony stimulating factor (M-CSF), vascular endothelial growth factor D (VEGF-D) and fibroblast growth factor 2 (FGF2), were present in all VS and VS cultures. Hierarchical clustering demonstrated that a given VS and its derived culture were most closely related (Fig. 2D). Although we did find that the relative level of different proteins differed between the original tumor and cultures, the most highly expressed proteins in the tumors were also highly expressed by the cultures (Fig. 2D, Table A.2). Conducting a repeated measures ANOVA, significant expression difference among the entire set of tumors and derived cultures was found for the 41 proteins (p<0.001). Paired t-tests indicated that the parent tumors were not significantly different from their cultures, with p-values for VS1, VS2 and VS3 being 0.99, 0.99 and 0.41, respectively. The rest of the comparisons, e.g. VS1 with VS2 or VS1 with VS2 culture, were significant (p<0.01), except for VS3 with VS1 (p=0.55) and VS1 culture (p=0.55). This trend of similarity between VS1 and VS3 is also reflected in the dendogram (Fig. 2D). Although most proteins found in the VS were present in the cultures, three proteins, being the insulin growth factor 2 (IGF-II), insulin-like growth factor 1 receptor (IGF-I sR) and neurotrophin-3 (NT-3), were not found in the cultures although being present in atleast two out of three VSs analyzed (Fig. 2D, Table A.2). Members of the fibroblast growth factor family, fibroblast growth factor 6 (FGF6) and 7 (FGF7), although not being present in the parent tumors, were expressed in the derived cultures VS2C and VS3C, respectively (Fig. 2D, Table A.2). Probing with RIPA only did not produce positive staining except at the positive control spots coated with the biotinylated IgGs.
3.5 Correlation of tumor growth rate in vivo to culture characteristics
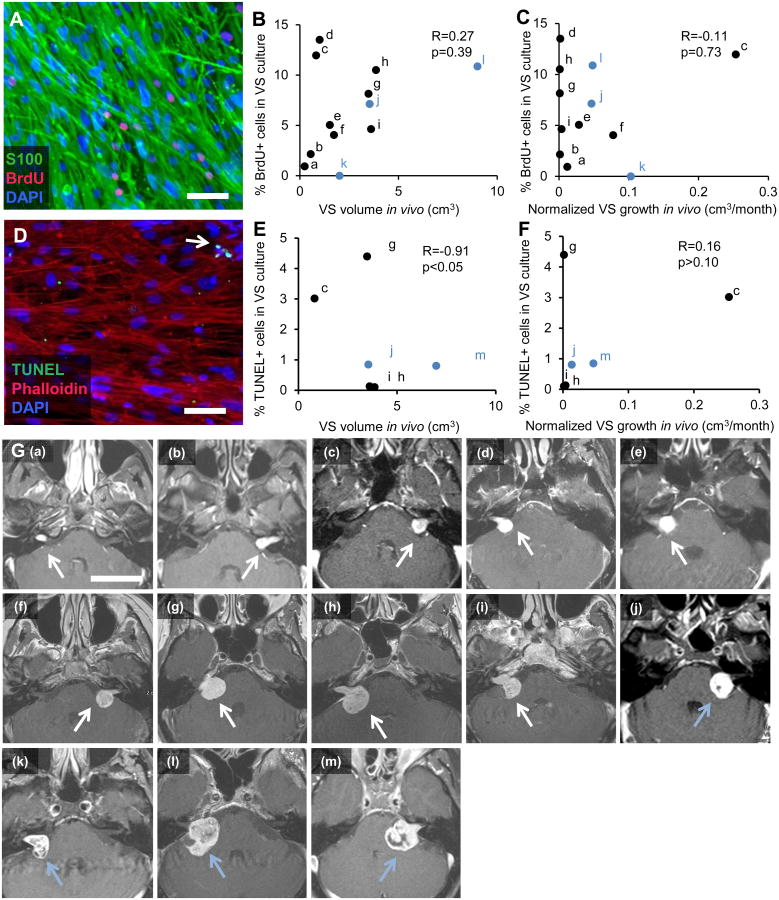
To determine whether the growth patterns noted in vivo were recapitulated in the cultures, we studied how VS volume and growth in vivo, as assessed by MRI, correlated with VS cell proliferation (Fig. 3A) and apoptosis in vitro (Fig. 3D). Thirteen VS patients had tumor growth rates available because their tumors were followed by serial imaging prior to resection; 12 of these tumors were used for assessing proliferation rate in vitro and 6 for apoptosis rate in vitro in the derived cultures. MRI sections of the 13 VS (Fig. 3G) demonstrate that 4 tumors had an apparent cystic component (tumors labeled (j)-(m)). Spearman's coefficient of rank correlation is indicated by R, with number of specimens being N. The range of VS proliferation in vitro was 0% to 13.51% for all 12 VS analyzed. When including all tumors (N=12), VS proliferation in vitro, expressed as mean ± SEM, was 6.58 ± 1.29% and did not correlate with tumor volume, expressed as mean + SD, being 2.61 ± 2.39 cm3 (R=0.27, p=0.39, Fig. 3B) or the normalized tumor growth rate in vivo, being 0.05 ± 0.07 cm3/month (R=-0.11, p=0.73, Fig. 3C). When including only solid tumors (N=9, black dots in Figs. 3B and 3C), VS proliferation in vitro was 6.77 ± 1.48% and still did not correlate with tumor volume, being 1.87 ± 1.41 cm3 (R=0.33, p>0.10) or the normalized tumor growth rate in vivo, being 0.04 ± 0.08 cm3/month (R=-0.10, p>0.10). Analyzing a subset of the tumors (N=4), 64.0% ± 4.7% (SEM) of the BrdU-positive cells were also S100-positive (Fig. 3A), suggesting that majority of the proliferation is arising from the schwannoma cells in the culture. Tumor growth and tumor volume in vivo did not correlate when including all VS (N=12 different VS, R=-0.08, p=0.81) or when including only solid VS (N=9 different VS, R=-0.37, p>0.10) in the analysis. Apoptosis was measured in VS cultured cells using TUNEL and found to be occurring at low rates (1.55 ± 0.72% SEM) in VS cultures (N=6 different VS, Fig. 3D). Apoptosis rates in vitro negatively correlated with tumor volume (R=-0.91, 0.025<p<0.05, Fig. 3E) and did not correlate with tumor growth in vivo (R=0.16, p>0.10, Fig. 3F) when analyzing all VS. When including only solid VS (N=4, black markers in Figs. 3E and 3F), the negative correlation between apoptosis rate in vitro and tumor volume was no longer present (R=-0.8 p=0.20).
Figure 3.
Comparison of parent tumor growth characteristics with derived primary cultures. A. Representative image of VS cultured cells with labeled proliferating cells. Green: S100, schwannoma cells; Red: BrdU, proliferating cells; Blue: Hoechst nuclear stain (DAPI). B. Tumor size (cm3) is plotted against VS proliferation rates in vitro (based on % nuclei that are BrdU-positive) (N=12 different VS). C. Tumor growth in vivo (cm3/month) calculated from serial MRIs is plotted against VS proliferation rates in vitro (N=12 different VS). D. Representative image of VS cultured cells with labeled cell death. Green: TUNEL; Red: Phalloidin; Blue: Hoechst nuclear stain (DAPI). White arrow marks an apoptotic cell. E. Tumor size (cm3) is plotted against VS apoptosis rates in vitro (based on % nuclei that are TUNEL-positive) (N=6 different VS). F. Tumor growth in vivo (cm3/month) calculated from serial MRIs is plotted against VS apoptosis rates in vitro (N=6 different VS). G. Gadolinium-enhanced VSs, which appear white on T1 weighted images, used in the proliferation and apoptosis assay, with (a)-(m) images representing the tumors labeled a-m in plots shown in panels B, C, E and F. White arrows, shown in images (a)-(i), point to solid VSs and light blue arrows, shown in images (j)-(m), point to VS with cystic components. In panels B and C, out of 12 VSs, 3 VSs with a cystic component are marked in light blue and in panels E and F, out of 6 VSs, 2 VSs with a cystic component are marked in light blue. Spearman's coefficient of rank correlation (R) and p-value (p) in given for analyses conducted on all VSs, including cystic VSs, in panels B, C, E and F. Scale bar = 100 μm applies for panels A and D. Scale bar = 4 cm for all images in panel G.
4. Discussion
Primary cultures of VS cells and control non-neoplastic SCs are important tools to investigate VS pathobiology and its divergences from healthy SCs, and thereby enable effective drug development against VS. To overcome some of the manipulations currently utilized in VS and SC cultures, which could transform cells and alter their true biology, we established a reproducible and technically easier method to culture primary human SCs and VS cells. We avoided currently popular practices of using cell specific-mitogens or toxins, and we circumvented time-consuming, resource-intensive protocols. Additionally, we did not passage the cultured cells, a procedure known to alter the cells' biology (Neumann et al., 2010). Our modified protocol employs techniques such as laminin-coated coverslips and mild collagenase treatment, as suggested by previous successful work in isolating SCs (Pannunzio et al., 2005). Our study is the first to longitudinally and quantitatively characterize primary human nerve-derived culture (over 7 weeks) and schwannoma-derived culture (over 12 weeks). This provides estimates for the most effective time windows to use healthy and neoplastic Schwann cells to investigate biological pathways in vitro. Additionally, we investigated biological similarity between the parent VSs' and derived cultures' pathobiology, validating our culture system to be representative of the tumor in vivo.
The SC purity that we achieved (85%) from surgically sacrificed human GANs is superior to previously described minimally manipulative SC culture methods, which demonstrated SC purities ranging from 35-64% (Morrissey, Kleitman, & Bunge, 1991; Niapour et al., 2010). In line with other studies demonstrating fibroblast infiltration in Schwann and VS cultures (Calderon-Martinez, Garavito, Spinel & Hurtado, 2002; Niapour et al., 2010), we note fibroblast-like cells infiltrating the cultures over time, although at lower levels than previously reported. Our success may partly result from careful dissection of the nerve to remove the epineurium. In fact, in the two outlier SC cultures with very low purity, we believe that impurities may have been introduced at the time of nerve dissection due to incomplete removal of fibrous sheath or contamination of cell culture with discarded pieces. We realized over the duration of the study that precision in removing the epineurium cleanly and carefully to avoid damage to the fascicles was an important factor for a high SC purity longitudinally. While we were unable to overcome eventual fibroblast-like cell contamination, as has been possible with potentially highly manipulative and transformative methodologies, e.g. 95% purity gradually achieved over 20 days with antimitotic cytosine arabinoside (Ara-C) treatment (Calderon-Martinez, Garavito, Spinel & Hurtado, 2002), we did establish a relatively pure population (85% purity) of minimally transformed SCs for up to 2 weeks. Our data demonstrating a drop in SCs after 2 weeks, followed by resurgence of SCs at 7 weeks, are consistent with fibroblast proliferation and eventual death in culture, with more stable SCs over time.
Our protocol was also effective in establishing a robust schwannoma cell culture (80% pure) for the majority of 12 weeks, in contrast to others who noted significant fibroblast contamination when using a minimally manipulative protocol (Nair, Leung, Collins, Ramsden, & Wilson, 2007). Our purity was highest at 2 weeks (87% pure), suggesting that this time point is optimal for use of the culture to investigate schwannoma pathobiology.
Although an immortalized schwannoma cell line is available, i.e. HEI-193, a major advantage of primary cell cultures over cell lines is that the primary cultures are not transformed, and that they can capture the inherent biological diversity between different patients and tumors, which facilitates development of therapies that are generally effective. We attempted to explore the similarity between the parent tumors' biology and their derived cultures using a protein array, a novel comparison to the best of our knowledge. This microarray, consisting of 41 proteins, included several receptor tyrosine kinases (e.g. epidermal growth factor receptor (EGFR), Ahmed et al., 2011) and growth factors (e.g. fibroblast growth factor 2 (FGF2), Koutsimpelas et al., 2007; Dilwali et al., 2013) that have already been implicated in VS pathobiology. Such correlations are important to critically evaluate how representative a culture system is of the VS pathobiology in vivo. We found, on average, 68% biological similarity between the parent tumor and the derived culture, as assessed with protein arrays. Our studies are comparable to those of other cells, namely hepatocytes, which showed 77% similarity in gene expression between parent tissue and primary cultures (Olsavsky et al., 2007) using mRNA microarrays. Our analysis demonstrates that the significant intertumor heterogeneity present in VS is maintained in our culture system since the parent VSs and their derived cultures segregated into pairs in an unbiased hierarchical clustering analysis. Although most of the biology in parent tumors was noted in the derived cultures, the diminished expression of specific proteins, such as some components of the insulin growth factor pathway or neutrotrophin-3, as well as new expression of proteins in culture reinforces that one should validate the aberrant expression of a given biological protein in primary tissue before studying its role in culture. Interestingly, some components of the IGF pathway, i.e. IGF-I, IGFBP-1 and IGFBP-3, were still detected in the culture if present in the parent VS, suggesting the diminished expression to be protein-specific rather than pathway-specific. Similarly, there was new expression of some members of the fibroblast growth family. These changes are expected as we alter the microenvironment of these cells from in vivo to a much simpler in vitro system. Nonetheless, it is reassuring that our VS culture system still provides a representative model to a great extent.
We attempted to correlate VS cultures' growth patterns with the clinical features of the parent tumors in vivo. We observed a large range of VS proliferation in vitro among different cultures, consistent with the heterogeneous VS growth rates in vivo and in vitro (Utermark, Kaempchen, Antoniadis, & Hanemann, 2005). The low rates of apoptosis that we observed in VS cultures are consistent with the neoplastic nature of these cells. Although our rate, averaging 1.55%, is lower than a study conducted by Utermark et al. describing apoptosis rate in primary VS cultures (8.5% using TUNEL), it was closer to their findings in paraffin-embedded tumor specimens (0.65%) (Utermark, Kaempchen, Antoniadis, & Hanemann, 2005) and similar to other studies noting 1.16% apoptosis rate in primary VS cultures (Cioffi et al., 2010). Interestingly, we did find that the apoptosis rate was negatively correlated with tumor volume (0.025<p<0.05), suggesting that smaller tumors exhibit higher rates of cell death. Future work is needed to affirm this correlation. Removing the VSs with cystic component, the correlation's significance was lost. Since the spread of the data points did not change considerably (Fig. 3E), it is probable that this change in significance was due to a decrease in sample size from N=6 to N=4, rather that due to exclusion of cystic tumors. We did not find proliferation rates of cultured VS cells to correlate with tumor growth rate or volume in vivo – this held even if only solid tumors were analyzed, if tumor growth was not standardized to initial volume or if growth rate was measured linearly, rather than volumetrically. This could be partially due to the intratumor heterogeneity as we could have obtained the specimen from a portion of the tumor that is proliferating differently than other areas within the tumor. Additionally, sample manipulation prior to surgical removal could have led to the cells' behavioral changes from in vivo to in vitro. However, we could not find any specific characteristic such as potential time delay in extracting and processing the sample or the level of cauterization to be indicative of the culture's proliferation rate. Further, since on average 64% of the BrdU-positive cells were S100-positive, it could be that the remainder of proliferating cells, including fibroblasts and other cell types that may not be as proliferative in vivo, are actually altering the proliferation rate of the culture in comparison to if it was solely comprised of schwannoma cells. We found no correlation between tumor growth in vivo versus tumor volume, in line with studies that indicate that there is a negative or weak correlation between tumor volume and growth rate (Herwadker, Vokurka, Evans, Ramsden, & Jackson, 2005, Nutik & Babb, 2001; Yoshimoto, 2005). Importantly, our study employed mostly large tumors because smaller tumors are typically not surgically removed (Smouha, Yoo, Mohr, & Davis, 2005) and therefore size could have been a confounder leading to the lack of correlations noted. Additionally, although approximately 96% of all VS are usually cystic in nature (Charabi et al., 1994), our study had 31% VS (4 out of 13) with a cystic component, a characteristic that has been associated with large and symptomatic tumors that are targeted for surgical removal (Nutik & Babb, 2001). In our study, cystic VSs' growth patterns in vivo or in culture could not be distinguished from solid VSs.
5. Conclusion
The ability to grow human SCs and VS cells with minimal manipulation provides a way to expand a population of such cells without the risk of transformation or loss of functional characteristics. Here we longitudinally characterize pure primary human SC and VS cultures, established using a methodology that combines clinical and practical feasibility with technical simplicity and speed. We demonstrate a high level of biological similarity between the derived VS cultures and their respective parent tumors, suggesting the culture system to be a more representative tool to study the pathobiology of neoplastic SCs.
Supplementary Material
Table A.1. Longitudinal growth of nerve- and schwannoma-derived cultures. For each type of culture, first column describes the age at which the cultures were assessed. Second column details the total cells counted per field on average per time point. Third column details the average fraction of immunofluorescently marked S100 positive cells over total Hoechst stain marked nuclei as seen in ≥3 different fields with the number of cultures derived from different surgical specimens shown with N in parentheses. Fourth column details the standard error of mean (SEM) calculated within the cultures at a given time point.
Table A.2. Protein names, abbreviations and expression values (optical density units) of 41 proteins analyzed in 3 VS, namely VS1, VS2, VS3, and their derived cultures VS1C, VS2C and VS3C, respectively. All optical densities were normalized to VS1. Zeros represent no protein detected, i.e. when signal detected was below the negative control on the array.
Figure A.1. Light microscopy-based images of longitudinal growth of nerve-derived Schwann cell culture at 1 (A), 2 (B), 3 (C), 4 (D), 5 (E), 6 (F), 7 (G), 8 (H), 9 (I), 10 (J) weeks. Scale bar = 200 μm applies to all panels.
Highlights.
Minimally manipulative method for primary human schwann and schwannoma cell culture from freshly harvested surgical specimens
Avoidance of mitogens, cytotoxins and potentially transformative techniques
High level of biological similarity between parent VS and the derived primary culture
A more representative in vitro model to study Schwann and schwannoma cell biology
Acknowledgments
This research was supported by NIDCD Grants T32 DC00038 (S.D., K.M.S) and K08DC010419 (K.M.S.), and the Bertarelli Foundation (K.M.S.). We are grateful to Drs. McKenna and Barker for assisting in VS specimen collection.
Abbreviations
- VS
vestibular schwannoma
- SC
Schwann cell
- GAN
great auricular nerve
Footnotes
Contributions: S.D., P.B.P. and K.M.S designed the protocol. S.D., P.B.P and D.S.R conducted the experiments. S.D., P.B.P., D.S.R. and K.M.S. analyzed the data. K.E. provided GAN surgical specimens. G.M.B and G.J.H. conducted the volumetrics analysis. S.D. and K.M.S. wrote the manuscript. S.D., P.B.P., D.S.R., G.M.B, G.J.H., K.E. and K.M.S. edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad ZK, Brown CM, Cueva RA, Ryan AF, Doherty JK. ErbB expression, activation, and inhibition with lapatinib and tyrphostin (AG825) in human vestibular schwannomas. Otol Neurotol. 2011 Jul;32(5):841–7. doi: 10.1097/MAO.0b013e31821f7d88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armati PJ, Constable AL, Llewellyn F. A new medium for in vitro peripheral nervous tissue myelination without the use of antimitotics. Journal of Neuroscience Methods. 1990;33(2):149–155. doi: 10.1016/0165-0270(90)90018-b. [DOI] [PubMed] [Google Scholar]
- Artz JJM, Timmer FA, Mulder JS, Cremers CRJ, Graamans K. Predictors of future growth of sporadic vestibular schwannomas obtained by history and radiologic assessment of the tumor. European Archives of Oto-Rhino-Laryngology. 2009;266(5):641–646. doi: 10.1007/s00405-008-0791-9. [DOI] [PubMed] [Google Scholar]
- Bush ML, Burns SS, Oblinger J, Davletova S, Chang L, Welling DB, Jacob A. Treatment of vestibular schwannoma cells with ErbB inhibitors. Otology & Neurotology. 2012;33(2) doi: 10.1097/MAO.0b013e31823e287f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Martinez D, Garavito Z, Spinel C, Hurtado H. Schwann cell-enriched cultures from adult human peripheral nerve: A technique combining short enzymatic dissociation and treatment with cytosine arabinoside (ara-C) Journal of Neuroscience Methods. 2002;114(1):1–8. doi: 10.1016/s0165-0270(01)00493-9. [DOI] [PubMed] [Google Scholar]
- Casella GTB, Bunge RP, Wood PM. Improved method for harvesting human schwann cells from mature peripheral nerve and expansion in vitro. Glia. 1996;17(4):327–338. doi: 10.1002/(SICI)1098-1136(199608)17:4<327∷AID-GLIA7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Charabi S, Klinken L, Tos M, Thomsen J. Histopathology and growth pattern of cystic acoustic neuromas. Laryngoscope. 1994 Nov;104(11 Pt 1):1348–52. doi: 10.1288/00005537-199411000-00006. [DOI] [PubMed] [Google Scholar]
- Cioffi JA, Yue WY, Mendolia-Loffredo S, Hansen KR, Wackym PA, Hansen MR. MicroRNA-21 overexpression contributes to vestibular schwannoma cell proliferation and survival. Otol Neurotol. 2010 Dec;31(9):1455–62. doi: 10.1097/MAO.0b013e3181f20655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriades AK, Saunders N, Rose P, Fisher C, Rowe J, Tranter R, Hardwidge C. Malignant transformation of acoustic neuroma/vestibular schwannoma 10 years after gamma knife stereotactic radiosurgery. Skull Base. 2010;20(05):381–387. doi: 10.1055/s-0030-1253576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilwali S, Lysaght A, Roberts D, Barker FG, 2nd, McKenna MJ, Stankovic KM. Sporadic vestibular schwannomas associated with good hearing secrete higher levels of fibroblast growth factor 2 than those associated with poor hearing irrespective of tumor size. Otol Neurotol. 2013 Jun;34(4):748–54. doi: 10.1097/MAO.0b013e31828048ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DG, Howard E, Giblin C, Clancy T, Spencer H, Huson SM, Lalloo F. Birth incidence and prevalence of tumor-prone syndromes: Estimates from a UK family genetic register service. American Journal of Medical Genetics Part A. 2010;152A(2):327–332. doi: 10.1002/ajmg.a.33139. [DOI] [PubMed] [Google Scholar]
- Hardy DG, Macfarlane R, Baguley D, Moffat DA. Surgery for acoustic neurinoma. Journal of Neurosurgery. 1989;71(6):799–804. doi: 10.3171/jns.1989.71.6.0799. [DOI] [PubMed] [Google Scholar]
- Herwadker A, Vokurka EA, Evans DG, Ramsden RT, Jackson A. Size and growth rate of sporadic vestibular schwannoma: Predictive value of information available at presentation. Otology & Neurotology. 2005;26(1) doi: 10.1097/00129492-200501000-00015. [DOI] [PubMed] [Google Scholar]
- Hood B, Levene HB, Levi AD. Transplantation of autologous schwann cells for the repair of segmental peripheral nerve defects. Neurosurgical Focus. 2009;26(2):E4. doi: 10.3171/FOC.2009.26.2.E4. [DOI] [PubMed] [Google Scholar]
- Jin Y, Liu W, Hong T, Cao Y. Efficient schwann cell purification by differential cell detachment using multiplex collagenase treatment. Journal of Neuroscience Methods. 2008;170(1):140–148. doi: 10.1016/j.jneumeth.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Koutsimpelas D, Stripf T, Heinrich UR, Mann WJ, Brieger J. Expression of vascular endothelial growth factor and basic fibroblast growth factor in sporadic vestibular schwannomas correlates to growth characteristics. Otol Neurotol. 2007 Dec;28(8):1094–9. doi: 10.1097/MAO.0b013e31814b2787. [DOI] [PubMed] [Google Scholar]
- Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery. 1997 Jan;40(1):1–9. doi: 10.1097/00006123-199701000-00001. discussion 9-10. [DOI] [PubMed] [Google Scholar]
- Mauritz C, Grothe C, Haastert K. Comparative study of cell culture and purification methods to obtain highly enriched cultures of proliferating adult rat schwann cells. Journal of Neuroscience Research. 2004;77(3):453–461. doi: 10.1002/jnr.20166. [DOI] [PubMed] [Google Scholar]
- Morrissey TK, Kleitman N, Bunge RP. Isolation and functional characterization of schwann cells derived from adult peripheral nerve. The Journal of Neuroscience. 1991;11(8):2433–2442. doi: 10.1523/JNEUROSCI.11-08-02433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Leung H, Collins A, Ramsden R, Wilson J. Primary cultures of human vestibular schwannoma: Selective growth of schwannoma cells. Otology & Neurotology. 2007;28(2) doi: 10.1097/01.mao.0000247811.93453.6a. [DOI] [PubMed] [Google Scholar]
- Neff BA, Voss SG, Schmitt WR, Driscoll CLW, Link MJ, Beatty CW, Kita H. Inhibition of MEK pathway in vestibular schwannoma cell culture. The Laryngoscope. 2012;122(10):2269–2278. doi: 10.1002/lary.23472. [DOI] [PubMed] [Google Scholar]
- Neumann E, Riepl B, Knedla A, Lefèvre S, Tarner IH, Grifka J, Steinmeyer J, Schölmerich J, Gay S, Müller-Ladner U. Cell culture and passaging alters gene expression pattern and proliferation rate in rheumatoid arthritis synovial fibroblasts. Arthritis Res Ther. 2010;12(3):R83. doi: 10.1186/ar3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niapour A, Karamali F, Karbalaie K, Kiani A, Mardani M, Nasr-Esfahani M, Baharvand H. Novel method to obtain highly enriched cultures of adult rat schwann cells. Biotechnology Letters. 2010;32(6):781–786. doi: 10.1007/s10529-010-0230-z. [DOI] [PubMed] [Google Scholar]
- Nutik SL, Babb MJ. Determinants of tumor size and growth in vestibular schwannomas. Journal of Neurosurgery. 2001;94(6):922–926. doi: 10.3171/jns.2001.94.6.0922. [DOI] [PubMed] [Google Scholar]
- Ohtani I, Tada Y, Omori K. Incidence of asymptomatic vestibular schwannoma and origin of the tumor in japanese. Otology Japan. 2007;17(5):615–620. [Google Scholar]
- Olsavsky KM, Page JL, Johnson MC, Zarbl H, Strom SC, Omiecinski CJ. Gene expression profiling and differentiation assessment in primary human hepatocyte cultures, established hepatoma cell lines, and human liver tissues. Toxicol Appl Pharmacol. 2007 Jul 1;222(1):42–56. doi: 10.1016/j.taap.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannunzio ME, Jou I, Long A, Wind TC, Beck G, Balian G. A new method of selecting schwann cells from adult mouse sciatic nerve. Journal of Neuroscience Methods. 2005;149(1):74–81. doi: 10.1016/j.jneumeth.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Smouha EE, Yoo M, Mohr K, Davis RP. Conservative management of acoustic neuroma: A meta-analysis and proposed treatment algorithm. The Laryngoscope. 2005;115(9):1704–1704. doi: 10.1097/01.mlg.0000175681.52517.cf. [DOI] [PubMed] [Google Scholar]
- Spiegel I, Peles E. A novel method for isolating schwann cells using the extracellular domain of Necl1. Journal of Neuroscience Research. 2009;87(15):3288–3296. doi: 10.1002/jnr.21985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreca A, Rambotti MG, Rende M, Saccardi C, Aisa MC, Giambanco I, Donato R. Immunocytochemical localization of S-100b protein in degenerating and regenerating rat sciatic nerves. Journal of Histochemistry & Cytochemistry. 1989;37(4):441–446. doi: 10.1177/37.4.2926122. [DOI] [PubMed] [Google Scholar]
- Stangerup S, Caye-Thomasen P. Epidemiology and natural history of vestibular schwannomas. Otolaryngologic Clinics of North America; Vestibular Schwannoma Evidence-Based Treatment. 2012;45(2):257–268. doi: 10.1016/j.otc.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Thomsen J, Zilstorff K, Tos M. Acoustic neuromas (diagnostic value of testing the function of the trigeminal nerve, the cerebellum and optokinetic nystagmus. The Journal of Laryngology & Otology. 1983;97(09):801–812. doi: 10.1017/s0022215100095037. [DOI] [PubMed] [Google Scholar]
- Utermark T, Kaempchen K, Antoniadis G, Hanemann CO. Reduced apoptosis rates in human schwannomas. Brain Pathology. 2005;15(1):17–22. doi: 10.1111/j.1750-3639.2005.tb00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto Y. Systematic review of the natural history of vestibular schwannoma. Journal of Neurosurgery. 2005;103(1):59–63. doi: 10.3171/jns.2005.103.1.0059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A.1. Longitudinal growth of nerve- and schwannoma-derived cultures. For each type of culture, first column describes the age at which the cultures were assessed. Second column details the total cells counted per field on average per time point. Third column details the average fraction of immunofluorescently marked S100 positive cells over total Hoechst stain marked nuclei as seen in ≥3 different fields with the number of cultures derived from different surgical specimens shown with N in parentheses. Fourth column details the standard error of mean (SEM) calculated within the cultures at a given time point.
Table A.2. Protein names, abbreviations and expression values (optical density units) of 41 proteins analyzed in 3 VS, namely VS1, VS2, VS3, and their derived cultures VS1C, VS2C and VS3C, respectively. All optical densities were normalized to VS1. Zeros represent no protein detected, i.e. when signal detected was below the negative control on the array.
Figure A.1. Light microscopy-based images of longitudinal growth of nerve-derived Schwann cell culture at 1 (A), 2 (B), 3 (C), 4 (D), 5 (E), 6 (F), 7 (G), 8 (H), 9 (I), 10 (J) weeks. Scale bar = 200 μm applies to all panels.