Abstract
Altered brain metabolism is likely to be an important contributor to normal cognitive decline and brain pathology in elderly individuals. To characterize the metabolic changes associated with normal brain aging, we used high-field proton magnetic resonance spectroscopy in vivo to quantify 20 neurochemicals in the hippocampus and sensorimotor cortex of young adult and aged rats. We found significant differences in the neurochemical profile of the aged brain when compared with younger adults, including lower aspartate, ascorbate, glutamate, and macromolecules, and higher glucose, myo-inositol, N-acetylaspartylglutamate, total choline, and glutamine. These neurochemical biomarkers point to specific cellular mechanisms that are altered in brain aging, such as bioenergetics, oxidative stress, inflammation, cell membrane turnover, and endogenous neuroprotection. Proton magnetic resonance spectroscopy may be a valuable translational approach for studying mechanisms of brain aging and pathology, and for investigating treatments to preserve or enhance cognitive function in aging.
Keywords: Aging, Biomarker, Proton magnetic resonance spectroscopy, Magnetic resonance imaging, In vivo, Neurochemical profile, Inflammation, Bioenergetics, Membrane turnover, Oxidative stress
1. Introduction
Understanding the neurobiological changes that occur with normal brain aging may aid in the prevention, diagnosis, and treatment of age-related cognitive decline and disease. In vivo proton magnetic resonance spectroscopy (1H-MRS) allows noninvasive quantification of neurochemicals related to specific cellular mechanisms. These neurochemicals can serve as imaging biomarkers of cellular and molecular changes, both in the context of normal brain development and aging, and under pathologic conditions (Harris et al., 2012; Lee et al., 2012; Tkac et al., 2003). For example, N-acetylaspartate (NAA) is synthesized in neuronal mitochondria in an energy-dependent manner, so a decrease in NAA may indicate neuronal loss or metabolic dysfunction. Because the total choline (tCho) signal measured with 1H-MRS is comprised largely of membrane phospholipids, increased tCho suggests cell membrane damage or increased turnover. Changes measured in glutamate (Glu) and/or its metabolic precursor glutamine (Gln) are interpreted as a sign of altered excitatory neurotransmission.
In humans, 1H-MRS studies have reported that advancing age is associated with several neurochemical changes in the brain, including lower concentrations of NAA particularly in the frontal lobe, higher tCho in frontal and parietal regions, and higher total creatine (tCr) in the parietal and occipital lobes (reviewed in Haga et al., 2009). In addition, lower striatal Glu and higher myo-inositol (Ins) in the subcortical white matter, posterior cingulate, and hippocampus have been described in elderly individuals (Gruber et al., 2008; Kaiser et al., 2005; Reyngoudt et al., 2012; Zahr et al., 2013). However, it is not feasible to confirm the links between these brain chemicals and specific cellular and molecular mechanisms in humans.
In animal models of aging, not only can the potential variability in brain neurochemistry from genetic and environmental factors be strictly controlled, but the underlying mechanisms associated with altered brain neurochemistry can be established through histologic and biochemical assays. However, in vivo 1H-MRS studies of aged animal models have been limited, and at times contradictory. A study by Katz-Brull et al. (2002) reported increased tCho in the hippocampus of aged rats, whereas Driscoll et al. (2006) observed no changes in NAA, tCr, or tCho in the aged rat brain. In vitro MRS studies of brain tissue extracts from rodents have measured additional neurochemical biomarkers. For example, an age-related increase in Ins suggests change in the density or metabolism of astroglial cells (Macri et al., 2006; Paban et al., 2010; Zhang et al., 2009) and altered Glu, aspartate (Asp), and gamma-aminobutyric acid (GABA), suggest a shift in the balance of excitatory and inhibitory neurotransmission in the aging brain (Paban et al., 2010; Zhang et al., 2009). However, one caveat of ex vivo MRS studies is that neurochemicals might be altered by the tissue extraction and homogenization process. A comprehensive in vivo assessment of neurochemical biomarkers of brain aging would be useful to facilitate translation of findings to clinical applications.
Compared with human studies, additional 1H-MRS biomarkers can be measured in laboratory animals using higher magnetic field strengths and advanced spectral fitting techniques. Routine quantification of at least 18 neurochemicals in the rodent brain is now feasible with in vivo 1H-MRS (Harris et al., 2012; Lei et al., 2009; Mlynarik et al., 2006; Pfeuffer et al., 1999). Such an expanded neurochemical profile has the potential to provide insight into cellular mechanisms, such as oxidative stress, inhibitory neurotransmission, and mitochondrial bioenergetics that might relate to changes in brain function with age. Thus, in the present study we characterized the metabolic effects of normal brain aging with in vivo 1H-MRS at 9.4 tesla (9.4 T). Using validated, noninvasive methods (Harris et al., 2012), we measured 20 neurochemicals in young and aged Fischer (F344) rats. Because impaired memory and motor functions are common aspects of neurologic decline in normal aging (Schuff et al., 1999; Seidler et al., 2010; West, 1993), we acquired MRS from the hippocampus and the sensorimotor cortex.
2. Methods
2.1. Animals
Male F344 rats 2 to 3 months old (“young adults”, n = 30) and 20 to 22 months old (“aged”, n = 20) were used in the study. Animals were housed in pairs on a 12 hour light-dark cycle with free access to rat chow and water. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center and are consistent with standards of animal care set forth in the guidelines of the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
2.2. In vivo magnetic resonance imaging and spectroscopy
All magnetic resonance assessments were performed on a 9.4 T horizontal magnetic resonance (MR) system (Varian; Palo Alto, CA, USA). During imaging, anesthesia was maintained with 1.5%–3% isoflurane delivered via nosecone to maintain a respiration rate of 40–80 cycles/min. Respiration was monitored with a pressure pad (SA Instruments; Stony Brook, NY, USA). Animals were placed on a heating pad in the scanning cradle, and body temperature monitored rectally was maintained at 37 °C via feedback control (Cole Parmer; Vernon Hills, IL, USA).
Coronal and sagittal localizer MR images were acquired using a gradient echo multi-slice sequence to position the animal’s head in the magnet (repetition time [TR] = 100 ms, echo time [TE] = 2.8 ms, number of slices = 10, slice thickness = 1 mm). Next coronal and sagittal T2-weighted MR images were acquired using a rapid acquisition with relaxation enhancement sequence (TR = 4000 ms, TE = 18 ms, echo train length = 8; averages = 2; field of view 2.56 × 2.56 cm2; resolution = 256 × 256 pixels; number of slices = 20; slice thickness = 1 mm) to position the voxels for 1H-MRS.
1H-MRS was acquired from a 2.7 mm × 1.3 mm × 2.7 mm voxel containing sensorimotor cortex, and a 3.0 mm × 2.5 mm × 3.0 mm voxel containing primarily hippocampus. Voxel positioning was based on anatomic landmarks. All spectra were acquired from the right hemisphere.
We used a custom-made quadrature dual-coil transmitter-receiver surface coil (each coil was 18 mm in diameter; Tkac et al., 1999). 1H-MRS was performed using a stimulated echo acquisition mode (STEAM) sequence with variable pulse power and optimized relaxation delays (VAPOR) water suppression (TE = 2 ms, TR = 4000 ms; Tkac et al., 1999). First and second order shims were adjusted using FASTMAP (Gruetter, 1993) to achieve water linewidths of <18 Hz. Data were acquired as a series of free induction decays (each of which averaged 16 transients), corrected for frequency drift, averaged and corrected for eddy current effects. In the hippocampal voxel we averaged 320 transients over a period of approximately 20 minutes. In the smaller cortical voxel, we averaged 640 transients over approximately 40 minutes. We also acquired unsuppressed water scans (16 transients) from each voxel to use for concentration calculations described in the following.
2.3. Spectral fitting and quantification
Spectra were analyzed with LCModel (Provencher, 1993). LCModel uses a basis set of spectra to calculate the in vivo neurochemical concentrations, and the unsuppressed water signal from the prescribed voxel as a reference for each scan to correct for small variations in coil sensitivity as described previously (Pfeuffer et al., 1999). For specific neurochemicals, our basis set was acquired from in vitro samples of pure chemicals. The macromolecule basis set was measured empirically. Peak assignments for individual metabolites in the neurochemical profile were based on previous reports (Pfeuffer et al., 1999; Tkac et al., 2003). It has been confirmed that measurements of brain neurochemicals using this 1H-MRS protocol are in good agreement with measurements from traditional invasive methods (Pfeuffer et al., 1999; Tkac et al., 2003). The following 20 neurochemicals were quantified: Ala, alanine; Asc, ascorbate; Asp, aspartate; Cr, creatine; GABA, gamma-aminobutyric acid; Glc, glucose; Gln, glutamine; Glu, glutamate; GPC, glycerophosphocholine; GSH, glutathione; Ins, myo-inositol; Lac, lactate; MM, macromolecules; NAA, N-acetylaspartate; NAAG, N-acetylaspartyl glutamate; PCho, phosphocholine; PCr, phosphocreatine; PE, phosphoethanolamine; Ser, serine; and Tau, taurine. Because certain neurochemicals that overlap at lower field strengths are often reported together, particularly in human studies, we report the following sums: tCr = Cr + PCr, tCho = GPC + PCho, returned by LCModel. We also calculated concentration ratios for certain pairs of metabolically-linked neurochemicals (i.e., Glu to Gln, and PCr to Cr).
2.4. Statistical analysis
Analysis of neurochemical concentrations was based on a weighted averages method. The LCModel software provides neurochemical concentration estimates and reliability measures expressed as Cramèr-Rao lower bounds (CRLB). Let xi denote the measured concentration of a neurochemical X and ri the corresponding reliability measure (CRLB), i = 1, 2, …, N. Then the weight is computed as:
When the reliability measure is infinitely low (indicated by CRLB = 999 in LCModel), the weight function is defined as 0. Suppose, n of N measurements have finite reliability (CRLB < 999) and a positive concentration in LCModel. Then the weighted mean and weighted standard deviation are determined by:
where df may take different forms such as or . Because no speci formula for weighted standard error exists, we used the conventional formula of , which produces weighted standard error values similar to those obtained from bootstrapping. These formulae were used to summarize the neurochemicals in each group, and to make between-group comparisons by the weighted t-test at each voxel of interest.
The weighted averages method accounts for differences in fitting reliability between samples and allows us to use all observations with CRLB < 999, giving lower weight to those with lower reliability. However, we decided a priori, that any metabolite for which >50% of observations in a group had very low reliability (i.e., CRLB > 100) would be excluded from further analysis.
Considering the number of comparisons, we adopted Holm’s sequential Bonferroni procedure (Holm, 1979) to control the family-wise type I error rate at the 0.05 level. For descriptive purposes, we indicate the magnitude of the between-group differences as a percentage of the young adult concentrations.
We also explored the concentration differences between hippocampus and cortex. To account for within-animal correlations, we applied mixed-effects analysis of variance models, weighted by CRLB as previously mentioned, using factors of age, region, and age-by-region interaction, and a compound symmetry correlation structure. Considering the small sample size, the degrees of freedom were calculated by the Kenward-Roger method (Kenward and Roger, 1997). The between-region differences were calculated for each age group, and the family-wise type I error rate was again controlled at 0.05 level by Holm’s procedure.
3. Results
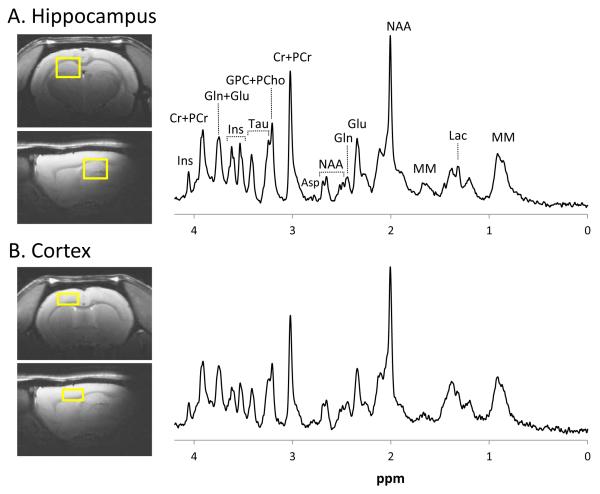
Locations and sizes of the voxels used for MRS are shown in Fig. 1. Sample spectra illustrate the spectral quality consistently obtained in both brain regions. Linewidths measured from the unsuppressed water signal in the hippocampus in young animals were 11.4 ± 0.6 Hz compared with 13.1 ± 0.9 Hz in the aged animals. In the young animals, cortex linewidths were 12.5 ± 1.3 Hz compared with 15.6 ± 1.3 Hz in the aged animals. Overall, about 90% of our measurements had CRLB <30, a frequently used inclusion criterion indicating high spectral resolution and good fitting reliability. However, for Ala, PCho, and PE in the aged cortex, >50% of samples returned CRLB >100 and could not be reliably quantified (see Supplementary Table 1).
Fig. 1.
Voxel placement for proton magnetic resonance spectroscopy and sample spectra from the hippocampus (A) and cortex (B). Images and spectra shown are from an aged (20-month-old) rat.
In the hippocampus, 9 of 20 neurochemicals were significantly different between young adult and aged animals (Table 1). We observed lower concentrations of Asc (−11%), Asp (−18%), PE (−21%), and MM (−12%) in the older animals compared with young adults. By contrast, concentrations of several neurochemicals were significantly higher in the aged hippocampus, including Ins (+12%), Glc (+48%), Gln (+23%), PCho (+43%), and NAAG (+15%).
Table 1.
Neurochemical concentrations in the young and aged brain
| Hippocampus |
Cortex |
|||||
|---|---|---|---|---|---|---|
| Young | Aged | p-value | Young | Aged | p-value | |
| Ala | 0.45 ± 0.02 | 0.38 ± 0.03 | 0.497 | 0.23 ± 0.03 | -- | -- |
| Asc | 2.80 ± 0.04 | 2.48 ± 0.07 | 0.004 | 2.99 ± 0.08 | 3.11 ± 0.12 | 0.876 |
| Asp | 1.83 ± 0.05 | 1.50 ± 0.10 | 0.041 | 2.39 ± 0.10 | 1.42 ± 0.15 | < 0.0001 |
| Cr | 3.99 ± 0.05 | 3.77 ± 0.09 | 0.269 | 3.63 ± 0.07 | 3.95 ± 0.17 | 0.498 |
| PCr | 5.41 ± 0.06 | 5.64 ± 0.08 | 0.253 | 4.99 ± 0.10 | 4.61 ± 0.15 | 0.499 |
| tCr | 9.45 ± 0.07 | 9.46 ± 0.10 | 0.911 | 8.61 ± 0.12 | 8.70 ± 0.20 | 0.710 |
| GABA | 1.33 ± 0.02 | 1.26 ± 0.03 | 0.774 | 0.95 ± 0.04 | 0.82 ± 0.05 | 0.407 |
| Glc | 2.11 ± 0.12 | 3.11 ± 0.22 | 0.002 | 3.73 ± 0.19 | 4.82 ± 0.25 | 0.030 |
| Gln | 2.51 ± 0.05 | 3.08 ± 0.08 | < 0.0001 | 2.91 ± 0.06 | 3.09 ± 0.12 | 1.000 |
| Glu | 9.97 ± 0.08 | 9.60 ± 0.16 | 0.269 | 10.44 ± 0.16 | 9.27 ± 0.22 | 0.003 |
| GPC | 0.86 ± 0.02 | 0.93 ± 0.02 | 0.28 | 0.75 ± 0.04 | 1.22 ± 0.05 | < 0.0001 |
| PCho | 0.24 ± 0.01 | 0.34 ± 0.03 | 0.002 | 0.21 ± 0.02 | -- | -- |
| tCho | 1.10 ± 0.01 | 1.26 ± 0.02 | < 0.0001 | 0.89 ± 0.03 | 1.23 ± 0.04 | < 0.0001 |
| GSH | 0.87 ± 0.02 | 0.91 ± 0.04 | 1.000 | 0.81 ± 0.04 | 0.64 ± 0.05 | 0.164 |
| Ins | 7.44 ± 0.08 | 8.34 ± 0.11 | < 0.0001 | 5.60 ± 0.10 | 7.26 ± 0.26 | < 0.0001 |
| Lac | 1.71 ± 0.10 | 1.45 ± 0.11 | 0.729 | 1.11 ± 0.11 | 0.94 ± 0.12 | 1.000 |
| MM | 2.01 ± 0.02 | 1.77 ± 0.03 | < 0.0001 | 1.83 ± 0.02 | 1.53 ± 0.03 | < 0.0001 |
| NAA | 9.24 ± 0.07 | 9.30 ± 0.09 | 1.000 | 9.99 ± 0.13 | 9.68 ± 0.53 | 1.000 |
| NAAG | 0.91 ± 0.02 | 1.04 ± 0.04 | 0.017 | 0.98 ± 0.04 | 1.23 ± 0.06 | 0.046 |
| PE | 1.72 ± 0.03 | 1.37 ± 0.07 | 0.0001 | 1.19 ± 0.08 | -- | -- |
| Ser | 0.94 ± 0.05 | 0.95 ± 0.10 | 0.989 | 1.64 ± 0.12 | 2.31 ± 0.28 | 0.219 |
| Tau | 6.40 ± 0.06 | 6.25 ± 0.10 | 0.913 | 5.29 ± 0.13 | 4.76 ± 0.16 | 0.234 |
Neurochemical concentrations are expressed as μmol/g wet tissue weight. Values are weighted group means ± weighted standard error. p < 0.05 is denoted in bold. Key: Ala, alanine; Asc, ascorbate, Asp, aspartate; Cr, creatine; GABA, gamma aminobutyric acid; Glc, glucose; Gln, glutamine; Glu, glutamate; GPC, glycerophosphocholine; GSH, glutathione; Ins, myo-inositol; Lac, lactate; MM, macromolecules; NAA, N-acetylaspartate; NAAG, N-acetylaspartyl glutamate; PCho, phosphocholine; PCr, phosphocreatine; PE, phosphoethanolamine; Ser, serine; Tau, taurine; tCho, total choline (GPC + PCho); tCr, total creatine (Cr + PCr).
In the cortex, 7 of 20 neurochemicals were significantly different with age (Table 1). Older animals had lower Asp (−41%), Glu (−11%), and MM (−16%). We observed higher concentrations of Ins (+30%), Glc (+29%), GPC (+62%), and NAAG (+25%) in the cortex of older rats compared with young adults. There was no significant difference in NAA levels in the young versus aged groups in either of the 2 brain regions studied (Table 1).
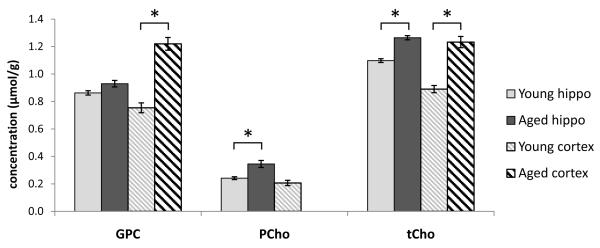
In both brain regions, tCho was significantly elevated with age (+15% in hippocampus, +38% in cortex; Fig. 2). In the hippocampus of older rats, we found that this was because of the contribution from PCho, which was significantly higher (+43%), and not from GPC, which was not significantly different. Conversely, GPC was higher in the cortex of aged animals (+62%) while PCho could not be quantified reliably.
Fig. 2.
Choline differences with age. Total choline (tCho), the sum of phosphocholine (PCho), and glycerophosphocholine (GPC), was elevated in the hippocampus and cortex of aged rats compared with young adults. In the cortex this increase appears to be driven by a significant increase in GPC, while in the hippocampus the significant increase in PCho predominates. As discussed in the text, PCho could not be quantified in the aged cortex. Plots show weighted group means and weighted standard error, * indicates p < 0.005. Abbreviation: Hippo, hippocampus.
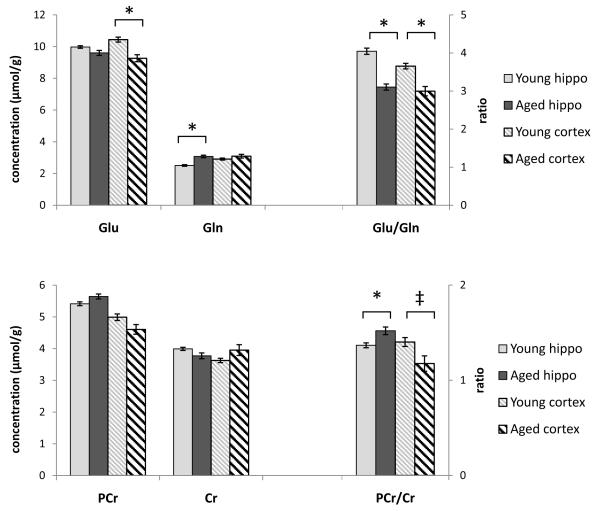
The ratio of Glu to Gln was significantly lower in both of the examined brain regions in older animals (−23% in the hippocampus, −18% in the cortex; Fig. 3). However, Glu and Gln each showed different significant changes in the 2 regions. Glu in the cortex was significantly lower in the aged animals compared with young adults, although a trend toward lower hippocampal Glu did not reach statistical significance. Hippocampal Gln was significantly higher in the aged animals, while there were no age-related differences in cortical Gln.
Fig. 3.
Ratios of metabolically linked neurochemicals in the young and aged brain. Glutamate (Glu) was significantly lower in the aged hippocampus while glutamine (Gln) was significantly higher in the cortex of aged animals compared with younger adults. The ratio of Glu to Gln was decreased in aged animals in both brain regions. Neither phosphocreatine (PCr) nor creatine (Cr) was significantly different across age in the hippocampus and cortex. However, the ratio of PCr to Cr was altered with age in both brain regions. Plots show weighted group means and weighted standard error, * indicates p < 0.005, ‡ indicates p < 0.05. Abbreviation: Hippo, hippocampus.
The ratio of PCr to Cr was also different in older animals in both the hippocampus and cortex, although PCr and Cr each were not significantly changed (Fig. 3). PCr to Cr was higher in the hippocampus (+11%) and lower in the cortex (−16%) of older animals compared with young adults. Levels of tCr were not significantly different between the young adult and aged brain (Table 1).
We also compared neurochemicals between the cortex and hippocampus within each age group. In the younger animals, 17 of 20 neurochemicals were different between regions, and in the older animals 13 were different (Supplementary Table 2).
4. Discussion
4.1. 1H-MRS biomarkers of brain health and pathology in aging
We used high field in vivo 1H-MRS with ultrashort TE and efficient water suppression to identify neurochemical changes associated with normal brain aging in rats. Our investigation of the neurometabolic effects of age focused on brain regions subserving memory and motor function, systems which show progressive functional impairment with age (Schuff et al., 1999; Seidler et al., 2010; West, 1993). Previous in vivo spectroscopy studies in humans and animal models have quantified 7 neurochemicals in the aging brain (Driscoll et al., 2006; Emir et al., 2011; Haga et al., 2009; Katz-Brull et al., 2002). We have measured an additional 13 neurochemicals that have not been reported in the aging brain in vivo: Ala, Asp, Cr, PCr, GABA, Glc, GPC, PCho, MM, NAAG, PE, Ser, and Tau. This expanded neurochemical profile of noninvasive biomarkers provides quantitative data related to putative mechanisms of brain health and pathology in aging. These biomarkers and their possible associated mechanisms are described in brief, in the following.
4.2. Age-related biomarker changes in both the hippocampus and cortex
We found age-related changes in several neurochemicals in both the hippocampal and cortical regions, suggesting that certain physiological mechanisms of brain aging may be global, or at least common to multiple brain areas.
4.2.1. Myo-inositol
We measured higher Ins in both the aged hippocampus and cortex. Because Ins is found at high concentrations in astrocytes, these results support age-related changes in the astroglial population, consistent with previous reports in the aging brain (Amenta et al., 1998; David et al., 1997; Sandhir et al., 2008). It has been proposed that changes in astrocytes in the older brain reflect chronic low-level inflammatory and/or oxidative cellular stressors (Cotrina and Nedergaard, 2002; Godbout and Johnson, 2009; Salminen et al., 2011).
4.2.2. Glucose
We also recorded higher Glc levels in aged rats. Higher brain Glc might result from higher circulating blood Glc, which can enter the brain through facilitated diffusion (Choi et al., 2001). Although not measured in the present study, blood levels of Glc, leptin, and insulin have been linked with changes in body fat mass (Barzilai & Gupta, 1999), and we note that aged rats in the present study had more body fat than young adults. One previous study reported an age-related increase in blood Glc (Asghar and Lokhandwala, 2006), but others found no difference in blood Glc between young and aged F344 rats (Barzilai & Rossetti, 1995; Mooridian & Chehade, 2000).
An alternative interpretation of higher Glc measured in the aged brain might be reduced glycolytic activity. This possibility is supported by positron emission tomography evidence of lower cerebral Glc metabolism in older humans (Petit-Taboue et al., 1998; Shen et al., 2012), and by lower gene expression related to Glc oxidation pathways in aged rats and mice (Rowe et al., 2007; Xu et al., 2007). Although isoflurane anesthesia influences Glc metabolism, this is unlikely to account for the observed age-related Glc difference because the same average concentration of isoflurane was used in young and old animals. Moreover, we found no correlation (R2 < 0.1) between the anesthesia duration for each animal and Glc levels measured in the hippocampus or cortex. Clearly, additional studies are needed to better understand age-related changes in brain Glc metabolism.
4.2.3. Aspartate and glutamate
Further evidence of altered bioenergetics is provided by the lower Asp and Glu, we observed in the brains of aged rats. A transaminase reaction in neuronal mitochondria normally converts Glu and oxaloacetate into Asp and α-ketoglutarate (Moffett et al., 2006). Thus, lower concentrations of Glu and Asp might point to decreased production of α-ketoglutarate to feed into the citric acid cycle. Notably, the decrease in both Asp and Glu was larger in the cortical voxel of aged versus younger animals (Asp −41%, Glu −11% in cortex versus Asp −18%, Glu −4% [n.s.] in the hippocampus), which might suggest a more severe impairment in citric acid cycle energy production in the aging neocortex.
4.2.4. Macromolecules
Although MM, attributed to free cytosolic proteins (Behar and Ogino, 1993), are part of the LCModel basis set there have been few 1H-MRS studies reporting MM data, and none in aging. We found that the MM signal was lower in both the hippocampus and cortex of aged rats. We previously reported a decrease in MM levels after traumatic brain injury, possibly reflecting increased or prolonged activity of intracellular proteases known to occur after brain trauma (Harris et al., 2012). If proteolysis degrades MR-visible intracellular proteins, then the well-documented increase in caspase activity in the aging brain (Gemma and Bickford, 2007; Snigdha et al., 2012) might similarly account for the lower MM levels in aged animals. Alternatively, MM levels measured with 1H-MRS have been proposed to reflect total cell mass (Lei et al., 2009). However, it seems unlikely that the observed MM decrease would indicate a decrease in total cell mass with age, given that we found no changes in the neuronal marker NAA and higher concentrations of the astrocytic marker Ins in aged rats.
4.2.5. N-acetylaspartylglutamate
In light of accumulating evidence, including the present study, pointing to potentially detrimental biological processes in the aging brain (e.g., chronic inflammation, bioenergetic impairment, and elevated protease activity), it may not be surprising to find that some compensatory neuroprotective mechanisms are also activated. One candidate neuroprotective compound is the neurotransmitter NAAG, which we found to be significantly higher in both the hippocampus and cortex in older animals. NAAG can exert neuroprotective effects through its actions at presynaptic group II metabotropic glutamate receptors (Sanabria et al., 2004; Thomas et al., 2001; Zhong et al., 2006). Specifically, NAAG has been shown to protect neurons in vitro from cell death after exposure to high glucose (Berent-Spillson and Russell, 2007; Berent-Spillson et al., 2004). Thus, higher NAAG levels might represent a protective, compensatory response to the constitutively elevated Glc we observed in aged brains. Further studies are needed to test whether a similar mechanism might be active in the aging human brain.
4.3. Other age-related biomarker changes
In addition to the neurochemical differences we observed in both the hippocampus and cortex of aged versus younger rats, other neurochemical differences were specific to one region only. Such regional variation is not unexpected, given the cytoarchitectural and functional differences between the 2 brain areas of interest.
4.3.1. Ascorbate and glutathione
Notably, we found a significant age-related decrease in Asc in the hippocampal but not in the cortical voxel. Microdialysis studies in aged rats have also revealed lower extracellular Asc in the hippocampus (Moor et al., 2006). Asc is an important endogenous antioxidant, and decreases in antioxidant defense mechanisms have been well documented in the aging rodent and human brain (Calabrese et al., 2008). Hippocampal Asc depletion may be related to the selective vulnerability of the hippocampal neurons to oxidative stress in the context of aging (Wang and Michaelis, 2010).
High-field 1H-MRS is also capable of measuring the antioxidant GSH. Although not reaching statistical significance, we observed 21% lower GSH in the aged cortex. Emir et al. (2011) have previously documented lower GSH, but not Asc, in the occipital cortex of elderly humans. Because Asc is found at higher concentrations in neurons, whereas GSH predominates in astrocytes (Rice and Russo-Menna, 1998), we speculate that accumulating reactive oxygen species in aging may selectively target neurons in the hippocampus (resulting in lower hippocampal Asc), while having a relatively larger effect on glial cells in other cortical areas (resulting in lower cortical GSH).
4.3.2. Total choline, phosphocholine, glycerophosphocholine, and phosphethanolamine
Our finding of increased tCho in older rats is consistent with several MRS studies of human brain aging (Gruber et al., 2008; Haga et al., 2009; Maudsley et al., 2012; Zahr et al., 2013). The tCho signal is comprised of membrane phospholipid derivatives, which are more mobile and thus more “visible” to 1H-MRS when free in the cytosol than when incorporated into cellular membranes. Thus, an increase in the tCho signal is thought to reflect increased cell membrane turnover and breakdown. The greater spectral resolution available at 9.4 T provides unambiguous separation and quantification of the main components of tCho, that is, PCho and GPC. We found higher levels of PCho in the aged hippocampus and higher levels of GPC in the aged cortex, although the biological significance of the regional difference in PCho and GPC remains to be clarified. In contrast, we found that PE, another membrane phospholipid, which does not contribute to tCho, was lower in the aged hippocampus compared with younger adults. In the aging human brain, a lower ratio of PE to glycerophosphoethanolamine was proposed to reflect slower membrane synthesis at older ages (Wijnen et al., 2010). Lower PE might also be tied to a decline in cellular bioenergetics with age, because a smaller fraction of PE in brain mitochondria of older rats was shown to correlate with lower mitochondrial state III respiration (Modi et al., 2008).
4.3.3. Glutamate and glutamine
Our primary experimental approach was to measure absolute neurochemical concentrations. However, examining metabolite ratios can provide added insight, particularly in understanding changes in compounds that are directly metabolically linked. Glu and Gln illustrate this point. We found that Gln levels were higher in the hippocampus of older rats, but remained unchanged with age in the cortex. On the contrary, Glu levels were unchanged in the hippocampus, but were significantly lower in the aging cortex. In both brain regions, however, the ratio of Glu to Gln was significantly lower in the aged rats, suggesting a shift in the balance of the Glu-Gln cycle. This shift may be the result of a relative increase in the astrocyte population with age, demonstrated in both animal models and humans (Amenta et al., 1998; David et al., 1997; Sandhir et al., 2008). An alternative explanation might be increased citric acid cycle flux in astrocytes in the aging brain, which would generate more metabolic intermediates including glutamine (Boumezbeur et al., 2010).
4.3.4. Total creatine, phosphocreatine, and creatine
The pools of intracellular Cr and PCr are also closely metabolically linked, with PCr providing an energy reserve essential for normal cellular function. We did not find significant differences with age in tCr, PCr, or Cr levels. Although some previous studies have shown higher tCr in older humans, others found no change (Haga et al., 2009). Similarly, human and rat studies with phosphorous magnetic resonance spectroscopy (31P-MRS) have yielded mixed results, with some evidence of increased PCr (Forester et al., 2010; Pettegrew et al., 1990) and other results indicating no age-related change (Aureli et al., 1990).
We did, however, find differences in the PCr to Cr ratio between young and aged brains. Specifically, PCr to Cr was higher in the aged hippocampus, and lower in the aged cortex, compared with younger animals. The significance of higher PCr to Cr in the aged hippocampus is currently not clear. However, a lower PCr to Cr ratio in the cortex suggests that the PCr pool is donating more phosphates to maintain cellular adenosine triphosphate supply, and is consistent with our Glu and Asp findings suggesting potentially greater energy impairment in the cortical voxel than the hippocampal voxel in the present model of brain aging.
4.4. No change in N-acetylaspartate and lactate in the aged rat brain
Although there is an extensive literature on in vivo quantification of NAA and Lac in neuropathology, previous results in normal brain aging are more modest and mixed. NAA is considered a marker of neuronal health and mitochondrial function, whereas increased Lac indicates anaerobic metabolism. The literature on NAA changes in the healthy aging brain is inconsistent, with some human studies finding lower NAA with advancing age (Boumezbeur et al., 2010; Gruber et al., 2008; Maudsley et al., 2012; Schuff et al., 1999) and others finding no difference (Chang et al., 1996; Saunders et al., 1999; Wu et al., 2012). Consistent with these latter studies, we found similar NAA concentrations in the young and aged rat brain. It has been suggested that studies describing NAA as a ratio to tCr or tCho were more likely to report decreased NAA with age (Haga et al., 2009), implying that tCr and/or tCho increases drive the findings in ratio studies. Using absolute quantification, our finding of maintained NAA, but higher tCho, in aged animals, is in agreement with previous in vivo MRS studies in rats (Driscoll et al., 2006; Katz-Brull et al., 2002) and supports concerns regarding the use of ratios for interpreting spectroscopic data.
Although 2 ex vivo MRS studies in rats found increased brain Lac with age (Macri et al., 2006; Zhang et al., 2009), 1 in vivo study found no age-related differences in resting state Lac (Urrila et al., 2004). Likewise, we found no difference in Lac levels between the young adult and aged rat brain. Our Glc, Asp, and Glu findings suggest altered bioenergetics in the aged brain, which might predict altered Lac. Therefore, the absence of a significant age-related Lac change in vivo requires further investigation.
4.5. Comparison of biomarkers between hippocampus and cortex
The primary goal of this study was to characterize the effect of age on the neurochemical profile. However, because previous studies have shown anatomic variability in neurochemical concentrations (Harris et al., 2012; Hong et al., 2011; Tkac et al., 2003; Zahr et al., 2013), we also compared metabolite concentrations measured in the hippocampus and cortex within each age group. As expected, most neurochemicals showed inter-regional differences: 17 of 20 in young animals and 13 of 20 in aged animals as detailed in Supplementary Table 2. These differences are likely because of anatomic variability in cellular architecture, function, and/or metabolic status; however, confirmation will require additional studies.
4.6. 1H-MRS in the aging brain: technical considerations
Fitting reliability of 1H-MRS data is dependent on spectral resolution (linewidth) and signal-to noise ratio (SNR) (Lin et al., 2013). We found broader linewidths in older animals than in young adults, consistent with recent findings in older humans (Maudsley et al., 2012). This could result from greater iron concentration in the brain. Age-related increases in brain iron concentration have been documented in rats (Focht et al., 1997) and in humans (Hallgren and Sourander, 1958; Rodrigue et al., 2011), although we did not measure iron in the present study. Alternatively, the lower water content of the aging brain could shorten T2. We also found that older rats have slightly thicker skulls. The resulting greater distance between the surface coil and the MRS voxel (~0.5 mm) might have contributed to lower SNR. However, as shown in Supplementary Table 1, the vast majority of neurochemical fits from both older and younger animals met our study criteria.
The CRLB values provided by the LCModel software indicate reliability of the linear combination fit used to estimate neurochemical concentrations from in vivo spectra. Higher CRLB values indicate less reliable measures, and the most common analysis approach in the literature is to exclude all neurochemical measures with CRLB higher than a designated cutoff. Because fitting reliability is dependent on spectral resolution and SNR, lower neurochemical concentrations are more likely to have high CRLB. Thus, excluding these measures could lead to an overestimation bias in results. An alternate approach is to estimate values using such methods as multiple imputation. This approach is appropriate for longitudinal studies when some neurochemicals fall below detection limits at some timepoints (Harris et al., 2012; Schafer, 1997). In the present cross-sectional study, we used a weighted averages approach to reduce potential overestimation bias. This approach includes all values when calculating a group mean, but gives greater weight to measures with low CRLB, that is, those with a higher confidence. Although this strategy allowed us to include more data points in our estimates of concentration, we found that a few neurochemicals did not have adequate spectra for meaningful interpretation across all experimental groups. Accordingly, Ala, PCho, and PE could not be measured in the aged cortex.
Previous studies in human and rodent brains point to a decrease of up to 5% in total brain water content with aging (Chang et al., 1996; Desbordes and Cohadon, 1987; Neeb et al., 2006; Unterberg et al., 1994). To account for issues that might affect MRS signal (such as coil sensitivity, voxel definition, and receiver gain), we used the unsuppressed water signal from each scan as a correction factor (Provencher, 1993). It is therefore possible that decreased water in the aged brain resulted in overestimation of the neurochemical concentrations in aged animals. However, our observation that some neurochemicals are significantly higher in the aged brain, while others are lower suggests that any potential effects of water referencing would be small relative to the metabolic changes that occur in the brain with aging. An age-related difference between water and metabolite relaxation time could also affect metabolite quantification; to minimize T2 effects we used an ultra-short echo time (TE = 2 ms) and a recycle time of 4000 ms.
Many of the present results support findings from previous ex vivo MRS studies on tissue extracted from the aged rat brain (Macri et al., 2006; Paban et al., 2010; Zhang et al., 2009). However, extrapolation from ex vivo to in vivo findings is not always possible, particularly for highly labile species such as GSH and neurochemicals such as Lac that accumulate rapidly after cessation of tissue oxygenation (Chang et al., 1996; Macri et al., 2006). In addition, it is important to consider rodent strain and sex differences when comparing animal studies. We used male F344 rats, the most commonly used rat strain for aging research (Nadon, 2005). Previous rodent studies of brain aging have used male (Katz-Brull et al., 2002; Paban et al., 2010) and female (Driscoll et al., 2006; Macri et al., 2006; Zhang et al., 2009) rats. Data from humans suggest that aging might differentially affect brain chemistry in men versus women (Zahr et al., 2013), however, this has yet to be tested with in vivo MRS in animal models.
To date, all 1H-MRS studies of aging in both humans and animals have been cross-sectional in design. From observed group differences, inference can be drawn about increases or decreases in neurochemical concentrations, but longitudinal studies are needed to truly confirm changes with age. Longitudinal study design is feasible in animal models because of shorter life spans, and we emphasize that future longitudinal studies are warranted.
4.7. Conclusions
An understanding of the metabolic changes associated with brain aging is important for further investigation of normal cognitive decline and brain pathology in elderly individuals. The current findings demonstrate significant metabolic differences in the hippocampus and cortex of aged versus younger adult rats. Previous studies have demonstrated both cognitive (Frick et al., 1995) and motor (Spangler et al., 1994) decline in F344 rats with increasing age, which are likely due to declining cellular function in specific brain regions including hippocampus and neocortex. The changes we report in 1H-MRS-visible biomarkers in these locations suggest specific cellular mechanisms that may be involved in brain aging, including inflammation, altered bioenergetics, oxidative stress, altered protease activity, cell membrane turnover, and an endogenous neuroprotective response.
Although many of the neurochemicals we quantify in the present study are not routinely measured on clinical scanners, technical developments continue to expand the flexibility of 1H-MRS in humans. For example, sequences are now available to quantify NAAG, GABA, Asc, and GSH on 3T clinical scanners (Choi et al., 2011; Edden et al., 2007; Mescher et al., 1998). Thus, it would be feasible to translate noninvasive 1H-MRS findings in animal models of aging to human studies using existing clinical scanners and sequences. Noninvasive 1H-MRS quantification of neurochemical biomarkers represents a promising approach for identifying therapeutic targets, and for testing treatments to preserve or enhance cognitive function in aging.
Supplementary Material
Acknowledgements
Supported by a Pilot Grant from the KU Alzheimer’s Disease Center P30 AG035982 to Dr Harris, a University of Kansas Lied Endowed Basic Science grant to Dr Brooks, R03 NS077852 from the National Institute of Neurological Disorders and Stroke to Dr. Swerdlow, and the Frank and Evangeline Thompson Alzheimer’s Treatment Program Fund. The Hoglund Brain Imaging Center is supported by a generous gift from Forrest and Sally Hoglund. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or its institutes.
Footnotes
Disclosure statement The authors disclose no conflicts of interest.
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neurobiolaging.2014.01.018.
References
- Amenta F, Bronzetti E, Sabbatini M, Vega JA. Astrocyte changes in aging cerebral cortex and hippocampus: a quantitative immunohistochemical study. Microsc. Res. Tech. 1998;43:29–33. doi: 10.1002/(SICI)1097-0029(19981001)43:1<29::AID-JEMT5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Asghar M, Lokhandwala MF. Antioxidant tempol lowers age-related increases in insulin resistance in Fischer 344 rats. Clin. Exp. Hypertens. 2006;28:533–541. doi: 10.1080/10641960600798697. [DOI] [PubMed] [Google Scholar]
- Aureli T, Miccheli A, Ricciolini R, Di Cocco ME, Ramacci MT, Angelucci L, Ghirardi O, Conti F. Aging brain: effect of acetyl-L-carnitine treatment on rat brain energy and phospholipid metabolism. A study by 31P and 1H NMR spectroscopy. Brain Res. 1990;526:108–112. doi: 10.1016/0006-8993(90)90255-a. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Gupta G. Revisiting the role of fat mass in the life extension induced by caloric restriction. J. Gerontol. A Biol. Sci. Med. Sci. 1999;54:B89–B98. doi: 10.1093/gerona/54.3.b89. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Rossetti L. Relationship between changes in body composition and insulin responsiveness in models of the aging rat. Am. J. Physiol. 1995;269:E591–E597. doi: 10.1152/ajpendo.1995.269.3.E591. [DOI] [PubMed] [Google Scholar]
- Behar KL, Ogino T. Characterization of macromolecule resonances in the 1H NMR spectrum of rat brain. Magn. Reson. Med. 1993;30:38–44. doi: 10.1002/mrm.1910300107. [DOI] [PubMed] [Google Scholar]
- Berent-Spillson A, Robinson AM, Golovoy D, Slusher B, Rojas C, Russell JW. Protection against glucose-induced neuronal death by NAAG and GCP II inhibition is regulated by mGluR3. J. Neurochem. 2004;89:90–99. doi: 10.1111/j.1471-4159.2003.02321.x. [DOI] [PubMed] [Google Scholar]
- Berent-Spillson A, Russell JW. Metabotropic glutamate receptor 3 protects neurons from glucose-induced oxidative injury by increasing intracellular glutathione concentration. J. Neurochem. 2007;101:342–354. doi: 10.1111/j.1471-4159.2006.04373.x. [DOI] [PubMed] [Google Scholar]
- Boumezbeur F, Mason GF, de Graaf RA, Behar KL, Cline GW, Shulman GI, Rothman DL, Petersen KF. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J. Cereb. Blood Flow Metab. 2010;30:211–221. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese VB, D.A, Stella AMG. Aging and oxidative stress response in the CNS. In: Lajtha A, editor. Handbook of Neurochemistry and Molecular Neurobiology. Springer; New York, NY, USA: 2008. pp. 103–146. [Google Scholar]
- Chang L, Ernst T, Poland RE, Jenden DJ. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sci. 1996;58:2049–2056. doi: 10.1016/0024-3205(96)00197-x. [DOI] [PubMed] [Google Scholar]
- Choi IY, Lee SP, Denney DR, Lynch SG. Lower levels of glutathione in the brains of secondary progressive multiple sclerosis patients measured by 1H magnetic resonance chemical shift imaging at 3 T. Mult. Scler. 2011;17:289–296. doi: 10.1177/1352458510384010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Lee SP, Kim SG, Gruetter R. In vivo measurements of brain glucose transport using the reversible Michaelis-Menten model and simultaneous measurements of cerebral blood flow changes during hypoglycemia. J. Cereb. Blood Flow Metab. 2001;21:653–663. doi: 10.1097/00004647-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Nedergaard M. Astrocytes in the aging brain. J. Neurosci. Res. 2002;67:1–10. doi: 10.1002/jnr.10121. [DOI] [PubMed] [Google Scholar]
- David JP, Ghozali F, Fallet-Bianco C, Wattez A, Delaine S, Boniface B, Di Menza C, Delacourte A. Glial reaction in the hippocampal formation is highly correlated with aging in human brain. Neurosci. Lett. 1997;235:53–56. doi: 10.1016/s0304-3940(97)00708-8. [DOI] [PubMed] [Google Scholar]
- Desbordes P, Cohadon F. Brain water and aging. J. Gerontol. 1987;42:655–659. doi: 10.1093/geronj/42.6.655. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, Sutherland RJ. The aging hippocampus: a multi-level analysis in the rat. Neuroscience. 2006;139:1173–1185. doi: 10.1016/j.neuroscience.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Edden RA, Pomper MG, Barker PB. In vivo differentiation of N-acetyl aspartyl glutamate from N-acetyl aspartate at 3 tesla. Magn. Reson. Med. 2007;57:977–982. doi: 10.1002/mrm.21234. [DOI] [PubMed] [Google Scholar]
- Emir UE, Raatz S, McPherson S, Hodges JS, Torkelson C, Tawfik P, White T, Terpstra M. Noninvasive quantification of ascorbate and glutathione concentration in the elderly human brain. NMR Biomed. 2011;24:888–894. doi: 10.1002/nbm.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focht SJ, Snyder BS, Beard JL, Van Gelder W, Williams LR, Connor JR. Regional distribution of iron, transferrin, ferritin, and oxidativelymodified proteins in young and aged Fischer 344 rat brains. Neuroscience. 1997;79:255–261. doi: 10.1016/s0306-4522(96)00607-0. [DOI] [PubMed] [Google Scholar]
- Forester BP, Berlow YA, Harper DG, Jensen JE, Lange N, Froimowitz MP, Ravichandran C, Iosifescu DV, Lukas SE, Renshaw PF, Cohen BM. Age-related changes in brain energetics and phospholipid metabolism. NMR Biomed. 2010;23:242–250. doi: 10.1002/nbm.1444. [DOI] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol. Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Gemma C, Bickford PC. Interleukin-1beta and caspase-1: players in the regulation of age-related cognitive dysfunction. Rev. Neurosci. 2007;18:137–148. doi: 10.1515/revneuro.2007.18.2.137. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Immunol. Allergy Clin. North Am. 2009;29:321–337. doi: 10.1016/j.iac.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Gruber S, Pinker K, Riederer F, Chmelik M, Stadlbauer A, Bittsansky M, Mlynarik V, Frey R, Serles W, Bodamer O, Moser E. Metabolic changes in the normal ageing brain: consistent findings from short and long echo time proton spectroscopy. Eur. J. Radiol. 2008;68:320–327. doi: 10.1016/j.ejrad.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn. Reson. Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- Haga KK, Khor YP, Farrall A, Wardlaw JM. A systematic review of brain metabolite changes, measured with 1H magnetic resonance spectroscopy, in healthy aging. Neurobiol. Aging. 2009;30:353–363. doi: 10.1016/j.neurobiolaging.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J. Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Harris JL, Yeh HW, Choi IY, Lee P, Berman NE, Swerdlow RH, Craciunas SC, Brooks WM. Altered neurochemical profile after traumatic brain injury: (1)H-MRS biomarkers of pathological mechanisms. J. Cereb. Blood Flow Metab. 2012;32:2122–2134. doi: 10.1038/jcbfm.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- Hong ST, Balla DZ, Pohmann R. Determination of regional variations and reproducibility in in vivo 1H NMR spectroscopy of the rat brain at 16.4 T. Magn. Reson. Med. 2011;66:11–17. doi: 10.1002/mrm.22943. [DOI] [PubMed] [Google Scholar]
- Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol. Aging. 2005;26:665–672. doi: 10.1016/j.neurobiolaging.2004.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz-Brull R, Koudinov AR, Degani H. Choline in the aging brain. Brain Res. 2002;951:158–165. doi: 10.1016/s0006-8993(02)03155-4. [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- Lee MR, Denic A, Hinton DJ, Mishra PK, Choi DS, Pirko I, Rodriguez M, Macura SI. Preclinical (1)H-MRS neurochemical profiling in neurological and psychiatric disorders. Bioanalysis. 2012;4:1787–1804. doi: 10.4155/bio.12.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Berthet C, Hirt L, Gruetter R. Evolution of the neurochemical profile after transient focal cerebral ischemia in the mouse brain. J. Cereb. Blood Flow Metab. 2009;29:811–819. doi: 10.1038/jcbfm.2009.8. [DOI] [PubMed] [Google Scholar]
- Lin P-C, Lee P, Wang W-T, Brooks WM, Choi IY. Statistical strategy to overcome estimation bias in CRLB threshold for LCModel analysis of MRS. Intl. Soc. Mag. Reson. Med. Annual Meeting Salt Lake City; Utah, USA. 2013. [Google Scholar]
- Macri MA, D’Alessandro N, Di Giulio C, Di Iorio P, Di Luzio S, Giuliani P, Bianchi G, Esposito E. Regional changes in the metabolite profile after long-term hypoxia-ischemia in brains of young and aged rats: a quantitative proton MRS study. Neurobiol. Aging. 2006;27:98–104. doi: 10.1016/j.neurobiolaging.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Maudsley AA, Govind V, Arheart KL. Associations of age, gender and body mass with 1H MR-observed brain metabolites and tissue distributions. NMR Biomed. 2012;25:580–593. doi: 10.1002/nbm.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Mlynarik V, Gambarota G, Frenkel H, Gruetter R. Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn. Reson. Med. 2006;56:965–970. doi: 10.1002/mrm.21043. [DOI] [PubMed] [Google Scholar]
- Modi HR, Katyare SS, Patel MA. Ageing-induced alterations in lipid/phospholipid profiles of rat brain and liver mitochondria: implications for mitochondrial energy-linked functions. J. Membr. Biol. 2008;221:51–60. doi: 10.1007/s00232-007-9086-0. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Tieman SB, Weinberger DR, Coyle JT, Namboodiri AM. N-Acetylaspartate: a unique neuronal molecule in the central nervous system. In: Back N, Cohen IR, Kritchevsky D, Lajtha A, Paoletti R, editors. Adv. Exp. Med. Biol. Springer; New York, NY: 2006. [Google Scholar]
- Moor E, Shohami E, Kanevsky E, Grigoriadis N, Symeonidou C, Kohen R. Impairment of the ability of the injured aged brain in elevating urate and ascorbate. Exp. Gerontol. 2006;41:303–311. doi: 10.1016/j.exger.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Chehade JM. Serum leptin response to endogenous hyperinsulinemia in aging rats. Mech. Ageing Dev. 2000;115:101–106. doi: 10.1016/s0047-6374(00)00110-x. [DOI] [PubMed] [Google Scholar]
- Nadon NL. Gerontology and age-associated lesions. In: Suckow MA, Weisbroth SH, Franklin CL, editors. The Laboratory Rat. Elsevier; London: 2005. pp. 761–772. [Google Scholar]
- Neeb H, Zilles K, Shah NJ. Fully-automated detection of cerebral water content changes: study of age- and gender-related H2O patterns with quantitative MRI. Neuroimage. 2006;29:910–922. doi: 10.1016/j.neuroimage.2005.08.062. [DOI] [PubMed] [Google Scholar]
- Paban V, Fauvelle F, Alescio-Lautier B. Age-related changes in metabolic profiles of rat hippocampus and cortices. Eur. J. Neurosci. 2010;31:1063–1073. doi: 10.1111/j.1460-9568.2010.07126.x. [DOI] [PubMed] [Google Scholar]
- Petit-Taboue MC, Landeau B, Desson JF, Desgranges B, Baron JC. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage. 1998;7:176–184. doi: 10.1006/nimg.1997.0318. [DOI] [PubMed] [Google Scholar]
- Pettegrew JW, Panchalingam K, Withers G, McKeag D, Strychor S. Changes in brain energy and phospholipid metabolism during development and aging in the Fischer 344 rat. J. Neuropathol. Exp. Neurol. 1990;49:237–249. doi: 10.1097/00005072-199005000-00005. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, Tkac I, Provencher SW, Gruetter R. Toward an in vivo neuro-chemical profile: quantification of 18 metabolites in short-echo-time (1)H NMR spectra of the rat brain. J. Magn. Reson. 1999;141:104–120. doi: 10.1006/jmre.1999.1895. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Reyngoudt H, Claeys T, Vlerick L, Verleden S, Acou M, Deblaere K, De Deene Y, Audenaert K, Goethals I, Achten E. Age-related differences in metabolites in the posterior cingulate cortex and hippocampus of normal ageing brain: a 1H-MRS study. Eur. J. Radiol. 2012;81:e223–e231. doi: 10.1016/j.ejrad.2011.01.106. [DOI] [PubMed] [Google Scholar]
- Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Haacke EM, Raz N. Differential effects of age and history of hypertension on regional brain volumes and iron. Neuroimage. 2011;54:750–759. doi: 10.1016/j.neuroimage.2010.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, Thibault O, Porter NM, Rose GM, Landfield PW. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J. Neurosci. 2007;27:3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur. J. Neurosci. 2011;34:3–11. doi: 10.1111/j.1460-9568.2011.07738.x. [DOI] [PubMed] [Google Scholar]
- Sanabria ER, Wozniak KM, Slusher BS, Keller A. GCP II (NAALADase) inhibition suppresses mossy fiber-CA3 synaptic neurotransmission by a pre-synaptic mechanism. J. Neurophysiol. 2004;91:182–193. doi: 10.1152/jn.00465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp. Neurol. 2008;213:372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders DE, Howe FA, van den Boogaart A, Griffiths JR, Brown MM. Aging of the adult human brain: in vivo quantitation of metabolite content with proton magnetic resonance spectroscopy. J. Magn. Reson. Imaging. 1999;9:711–716. doi: 10.1002/(sici)1522-2586(199905)9:5<711::aid-jmri14>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Schafer JL. Analysis of Incomplete Multivariate Data. Chapman & Hall; Boca Raton, FL: 1997. [Google Scholar]
- Schuff N, Amend DL, Knowlton R, Norman D, Fein G, Weiner MW. Age-related metabolite changes and volume loss in the hippocampus by magnetic resonance spectroscopy and imaging. Neurobiol. Aging. 1999;20:279–285. doi: 10.1016/s0197-4580(99)00022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Liu H, Hu Z, Hu H, Shi P. The relationship between cerebral glucose metabolism and age: report of a large brain PET data set. PLoS One. 2012;7:e51517. doi: 10.1371/journal.pone.0051517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snigdha S, Smith ED, Prieto GA, Cotman CW. Caspase-3 activation as a bifurcation point between plasticity and cell death. Neurosci. Bull. 2012;28:14–24. doi: 10.1007/s12264-012-1057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler EL, Waggie KS, Hengemihle J, Roberts D, Hess B, Ingram DK. Behavioral assessment of aging in male Fischer 344 and brown Norway rat strains and their F1 hybrid. Neurobiol. Aging. 1994;15:319–328. doi: 10.1016/0197-4580(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Thomas AG, Olkowski JL, Slusher BS. Neuroprotection afforded by NAAG and NAALADase inhibition requires glial cells and metabotropic glutamate receptor activation. Eur. J. Pharmacol. 2001;426:35–38. doi: 10.1016/s0014-2999(01)01198-0. [DOI] [PubMed] [Google Scholar]
- Tkac I, Rao R, Georgieff MK, Gruetter R. Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo 1H NMR spectroscopy. Magn. Reson. Med. 2003;50:24–32. doi: 10.1002/mrm.10497. [DOI] [PubMed] [Google Scholar]
- Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn. Reson. Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Unterberg A, Schneider GH, Gottschalk J, Lanksch WR. Development of traumatic brain edema in old versus young rats. Acta Neurochir. Suppl. (Wien.) 1994;60:431–433. doi: 10.1007/978-3-7091-9334-1_117. [DOI] [PubMed] [Google Scholar]
- Urrila AS, Hakkarainen A, Heikkinen S, Vuori K, Stenberg D, Hakkinen AM, Lundbom N, Porkka-Heiskanen T. Stimulus-induced brain lactate: effects of aging and prolonged wakefulness. J. Sleep Res. 2004;13:111–119. doi: 10.1111/j.1365-2869.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. Regionally specific loss of neurons in the aging human hippo-campus. Neurobiol. Aging. 1993;14:287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- Wijnen JP, Scheenen TW, Klomp DW, Heerschap A. 31P magnetic resonance spectroscopic imaging with polarisation transfer of phosphomono- and diesters at 3 T in the human brain: relation with age and spatial differences. NMR Biomed. 2010;23:968–976. doi: 10.1002/nbm.1523. [DOI] [PubMed] [Google Scholar]
- Wu WE, Gass A, Glodzik L, Babb JS, Hirsch J, Sollberger M, Achtnichts L, Amann M, Monsch AU, Gonen O. Whole brain N-acetylaspartate concentration is conserved throughout normal aging. Neurobiol. Aging. 2012;33:2440–2447. doi: 10.1016/j.neurobiolaging.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhan M, Duan W, Prabhu V, Brenneman R, Wood W, Firman J, Li H, Zhang P, Ibe C, Zonderman AB, Longo DL, Poosala S, Becker KG, Mattson MP. Gene expression atlas of the mouse central nervous system: impact and interactions of age, energy intake and gender. Genome Biol. 2007;8:R234. doi: 10.1186/gb-2007-8-11-r234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Chanraud S, Gu M, Sullivan EV, Pfefferbaum A. In vivo glutamate measured with magnetic resonance spectroscopy: behavioral correlates in aging. Neurobiol. Aging. 2013;34:1265–1276. doi: 10.1016/j.neurobiolaging.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Liu H, Wu J, Liu M, Wang Y. Metabonomic alterations in hippocampus, temporal and prefrontal cortex with age in rats. Neurochem. Int. 2009;54:481–487. doi: 10.1016/j.neuint.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Zhong C, Zhao X, Van KC, Bzdega T, Smyth A, Zhou J, Kozikowski AP, Jiang J, O’Connor WT, Berman RF, Neale JH, Lyeth BG. NAAG peptidase inhibitor increases dialysate NAAG and reduces glutamate, aspartate and GABA levels in the dorsal hippocampus following fluid percussion injury in the rat. J. Neurochem. 2006;97:1015–1025. doi: 10.1111/j.1471-4159.2006.03786.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.