Abstract
The anaerobic gastrointestinal pathogen Clostridium difficile must form a metabolically dormant spore to survive in oxygenic environments and be transmitted from host to host. The regulatory factors by which C. difficile initiates and controls the early stages of sporulation in C. difficile are not highly conserved in other Clostridium or Bacillus species. Here, we investigated the role of two conserved oligopeptide permeases, Opp and App, in the regulation of sporulation in C. difficile. These permeases are known to positively affect sporulation in Bacillus species through the import of sporulation-specific quorum-sensing peptides. In contrast to other spore-forming bacteria, we discovered that inactivating these permeases in C. difficile resulted in the earlier expression of early sporulation genes and increased sporulation in vitro. Furthermore, disruption of opp and app resulted in greater virulence and increased the amounts of spores recovered from feces in the hamster model of C. difficile infection. Our data suggest that Opp and App indirectly inhibit sporulation, likely through the activities of the transcriptional regulator SinR and its inhibitor, SinI. Taken together, these results indicate that the Opp and App transporters serve a different function in controlling sporulation and virulence in C. difficile than in Bacillus subtilis and suggest that nutrient availability plays a significant role in pathogenesis and sporulation in vivo. This study suggests a link between the nutritional status of the environment and sporulation initiation in C. difficile.
INTRODUCTION
Clostridium difficile is an anaerobic, Gram-positive pathogen that causes a significant gastrointestinal disease in humans and other mammals. Because C. difficile is an obligate anaerobe, its survival outside the host requires the formation of a metabolically inactive spore, which can withstand harsh environmental conditions. The ability of C. difficile to form a spore is critical to the spread and recurrence of the disease (1). Spore formation also contributes to C. difficile resistance to traditional antibiotic therapies. Despite the importance of spore formation in the pathogenesis of C. difficile, little is understood about how the initiation of sporulation is regulated.
Sporulation is a complex process governed by multiple regulators and feedback processes in bacteria; however, the regulatory components and environmental signals that control the initiation of sporulation in well-studied sporeformers are not well conserved in C. difficile (2–7). In all studied spore-forming bacteria, including C. difficile (1, 8), the initiation of sporulation is controlled by the activity of the highly conserved transcription factor Spo0A, which functions as the master regulator of sporulation. In Bacillus species, Spo0A activity is tightly regulated through a phosphorylation-mediated signal transduction pathway in response to nutrient availability (9). One mechanism that controls Spo0A phosphorylation and, thereby, the activity of Spo0A in Bacillus subtilis and other species is the uptake of small, quorum-signaling peptides known as Phr peptides (10). The Phr peptides are imported by the Opp (Spo0K) and App oligopeptide permeases and contribute to the induction of sporulation or genetic competence when bacterial population densities are high (11–13). The Phr peptides are encoded by multiple phr genes and are initially secreted as proproteins that are subsequently processed to form pentapeptides (10, 13). Once imported by Opp or App, the Phr peptides directly target the Rap phosphatases, which would otherwise inhibit sporulation by dephosphorylating components of the Spo0A phosphorelay pathway (10, 14).
Opp (also known as Spo0K in spore-forming bacteria and Ami in Streptococcus species) and App are membrane-associated five-protein complexes of the ABC transporter family and are found in both Gram-positive and Gram-negative species. These transporters contain an extracellular ligand-binding lipoprotein (OppA/AppA), two transmembrane proteins (OppBC/AppBC) that form a membrane-spanning pore, and two cytoplasmic ATPases (OppDF/AppDF) that drive the transport of the peptide into the cell (15, 16). The Opp and App oligopeptide transport systems influence many cellular processes besides sporulation, including competence in Bacillus and Streptococcus species (11, 17, 18), plasmid transfer in Enterococcus faecalis (19, 20), and the expression of virulence factors in Bacillus thuringiensis (21, 22). However, the primary function of Opp and App in bacteria is to import small, heterogeneous peptides varying from 3 to 20 amino acids relatively nonspecifically as nutrient sources (23–27). These imported peptides may be utilized as carbon or nitrogen sources and can also serve as the substrates for the generation of ATP in clostridial species via the Stickland reaction (28–32).
The C. difficile genome encodes orthologs of the Opp and App oligopeptide transporter systems and two Rap phosphatase orthologs; however, no clear Phr ortholog is detectable by sequence analysis. In this study, we asked whether the conserved oligopeptide transporter systems have a role in regulating the initiation of sporulation of C. difficile. By studying opp- and app-null mutants, we determined that the absence of App or both Opp and App results in increased sporulation in vitro, as well as earlier and increased expression of sporulation genes. Our results indicate that the loss of either or both putative transporters results in a hypervirulent phenotype as well as increased spore formation during infection in a hamster model. Further, we observed that the increase in sporulation in opp app mutants is associated with the differential expression of the genes encoding SinR, a transcriptional regulator, and the predicted SinI, a putative inhibitor of SinR. Together these data suggest that the C. difficile Opp and App transporters indirectly inhibit sporulation by facilitating the uptake of peptides and, thus, the availability of intracellular nutrients.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Clostridium difficile strains were routinely cultured in brain heart infusion-supplemented (BHIS) broth or on BHIS agar plates (33). Media for the growth of C. difficile were supplemented with 2 to 10 μg thiamphenicol ml−1, 5 μg erythromycin ml−1, or 30 μg lincomycin ml−1 (Sigma-Aldrich), as needed. Counterselection of Escherichia coli after conjugation with C. difficile was done using 50 μg kanamycin ml−1, as detailed previously (34). Taurocholate was added to cultures (0.1%) to induce the germination of C. difficile spores, as indicated below (Sigma-Aldrich) (35, 36). C. difficile strains were cultured in an anaerobic chamber maintained at 37°C (Coy Laboratory Products) with an atmosphere of 10% H2, 5% CO2, and 85% N2, as previously described (37, 38). In minimal-medium growth experiments, oligopeptides composed of 5 or 10 random amino acids (Peptide 2.0) were added to minimal defined medium (39) containing no other amino acid sources. Escherichia coli strains were grown at 37°C in LB (40) or BHIS medium and supplemented with 20 μg chloramphenicol ml−1 or 100 μg ampicillin ml−1 as needed.
TABLE 1.
Bacterial strains and plasmids
| Plasmid or strain | Relevant genotype or features | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α Max Efficiency | F− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 (rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1 | Invitrogen |
| MC101 | HB101/pRK24 | B. Dupuy |
| MC135 | HB101 containing pRK24 and pMC123 | 48 |
| MC253 | HB101 containing pRK24 and pMC199 | This study |
| MC263 | HB101 containing pRK24 and pMC213 | This study |
| MC274 | HB101 containing pRK24 and pMC217 | This study |
| MC277 | HB101 containing pRK24 and pMC211 | This study |
| MC295 | HB101 containing pRK24 and pMC230 | This study |
| MC306 | HB101 containing pRK24 and pJS107::spo0A178::TargeTron | 69 |
| MC329 | HB101 containing pRK24 and pMC238 | This study |
| MC330 | HB101 containing pRK24 and pMC240 | This study |
| C. difficile | ||
| 630 | Clinical isolate | 97 |
| 630Δerm | Erms derivative of strain 630 | N. Minton and 98 |
| R20291 | Clinical isolate | 41 |
| MC282 | 630Δerm pMC211 | This study |
| MC283 | 630Δerm pMC217 | This study |
| MC296 | 630Δerm oppB::ermB | This study |
| MC301 | 630Δerm appA::ermB | This study |
| MC307 | 630Δerm oppB::ermB appA::ermB | This study |
| MC310 | 630Δerm spo0A::ermB | This study |
| MC322 | 630Δerm oppB::ermB/pMC213 | This study |
| MC323 | 630Δerm oppB::ermB appA::ermB/pMC213 | This study |
| MC324 | 630Δerm/pMC123 | This study |
| MC331 | 630Δerm appA::ermB/pMC238 | This study |
| MC332 | 630 Δerm oppB::ermB/pMC240 | This study |
| MC334 | 630Δerm oppB::ermB appA::ermB/pMC238 | This study |
| MC337 | 63 Δerm oppB::ermB/pMC123 | This study |
| MC338 | 630Δerm appA::ermB/pMC123 | This study |
| MC339 | 630Δerm oppB::ermB appA::ermB/pMC123 | This study |
| Plasmids | ||
| pRK24 | Tra+ Mob+ bla tet | 99 |
| pBL100 | Untargeted group II intron-TargeTron vector | 29 |
| pCR2.1 | bla kan | Invitrogen |
| pCE240 | C. difficile TargeTron construct based on pJIR750ai (group II intron, ermB::RAM ltrA) catP | C. Ellermeier (44) |
| pJS107-spo0A-178TT | Group II intron targeted to spo0A | 69 |
| pMC123 | E. coli-C. difficile shuttle vector, bla catP | 48 |
| pMC199 | pBL100 with group II intron targeted to oppB | This study |
| pMC211 | pMC123 with cprA promoter | This study |
| pMC213 | pMC123 with PoppBCAD operon | This study |
| pMC217 | pMC211 with CD0852 | This study |
| pMC220 | pCR2.1 with group II intron targeted to appA | This study |
| pMC226 | pCE240 with appA-targeted intron | This study |
| pMC230 | pMC123 with appA-targeted intron, ermB::RAM ltrA catP | This study |
| pMC237 | pCR2.1 with PappFDABC operon | This study |
| pMC238 | pMC123 with PappFDABC operon | This study |
| pMC239 | pCR2.1 with PoppBCADF from R20291 | This study |
| pMC240 | pMC123 with PoppBCADF from R20291 | This study |
Sporulation efficiency assays.
Sporulation assays were performed in 70:30 sporulation broth medium (36). C. difficile cultures were started in BHIS medium supplemented with 0.1% taurocholate and 0.5% fructose until mid-log phase (active growth) to allow the germination of any spores within the starting inoculum. Cultures were then back-diluted and equilibrated to an optical density at 600 nm (OD600) of 0.1 in 70:30 medium. These cultures were further diluted 1:10 in 70:30 medium, incubated at 37°C, and monitored for the production of spores. Samples were taken at the point of maximum cell density (approximately 2 h after the onset of stationary phase [T2]), serially diluted, and plated onto BHIS medium with 0.1% taurocholate to determine the total number of CFU from which spores could form. To determine the number of spores present, 500-μl samples were taken from each culture, mixed 1:1 with 95% ethanol, and incubated for 1 h to kill all nonspores (vegetative cells) present. Ethanol-treated samples were then serially diluted, plated onto BHIS medium containing 0.1% taurocholate, and incubated for 24 to 48 h to enumerate the spore CFU. The sporulation frequency was determined by dividing the number of spores by the total number of CFU at T2 for each culture (spore ratio) and multiplying that value by 100 (to obtain the percentage of spores formed). The sporulation-defective spo0A mutant was used as a negative control to ensure that all vegetative cells were killed during ethanol treatment. Cultures were assessed at the time of inoculation and at the start of stationary phase (time zero [T0]) to ensure that no contaminating spores were present in the starter cultures.
Strain and plasmid construction.
The oligonucleotides used in this study are listed in Table 2. Details of DNA cloning and vector construction are outlined in File S1 in the supplemental material. C. difficile strain 630 (GenBank accession NC_009089.1) was used as the template for primer design, unless otherwise specified. Strain 630Δerm was used as the template for PCR amplification, except where use of strain R20291 (GenBank accession number NC_013316.1) as the template is specified (41). Isolation of plasmid DNA, PCR, and cloning were performed using standard protocols. Genomic DNA was prepared and genetic manipulation of C. difficile was performed as previously described (37, 42). Null mutations in C. difficile were created by retargeting the group II intron from pCE240 using the intron-retargeting primers listed in Table 2, as previously described (43–45). The double opp app mutant (MC307) was created by using the oppB-specific group II intron in MC301 (app single mutant) and selecting for the second intron integration event on BHIS medium plates containing lincomycin (30 μg ml−1). To complement the opp and app disruptions, plasmids containing the oppBCAD (pMC213) or appFDABC (pMC238) operons and upstream promoter regions were transferred into C. difficile mutants from E. coli by conjugation as previously described, except that 50 μg kanamycin ml−1 was used to counterselect against E. coli postconjugation (46). Cloned DNA fragments were verified by sequencing (Eurofins MWG Operon) prior to use.
TABLE 2.
Oligonucleotides
| Primer | Sequencea (5′ → 3′) | Purpose (operon) | Source or reference |
|---|---|---|---|
| EBS universal | 5′-CGAAATTAGAAACTTGCGTTCAGTAAAC-3′ | Sigma-Aldrich | |
| oMC44 | 5′-CTAGCTGCTCCTATGTCTCACATC-3′ | rpoC qPCR (CD0067) | 46 |
| oMC45 | 5′-CCAGTCTCTCCTGGATCAACTA-3′ | rpoC qPCR (CD0067) | 46 |
| oMC112 | 5′ -GGCAAATGTAAGATTTCGTACTCA- 3′ | tcdB qPCR (CD0660) | |
| oMC113 | 5′ -TCGACTACAGTATTCTCTGAC- 3′ | tcdB qPCR (CD0660) | |
| oMC152 | 5′-GTTATGGAAGTCAAGGACATGCAC-3′ | ilvC qPCR (CD1565) | 42 |
| oMC153 | 5′-GCTTCTGCTACACTCTTAACTTCA-3′ | ilvC qPCR (CD1565) | 42 |
| oMC158 | 5′-CCAGAAGAATCTATGGTAAAGGTTG-3′ | hisZ qPCR (CD1547) | 42 |
| oMC159 | 5′-ACCATCTTCCCATCTTGGTACA-3′ | hisZ qPCR (CD1547) | 42 |
| oMC215 | 5′-GCGAATTCGACATGGAAGTAGAAGTTAAGG-3′ | PcprA cloning | |
| oMC331 | 5′-CTCAAAGCGCAATAAATCTAGGAGC-3′ | spo0A qPCR (CD1214) | |
| oMC332 | 5′-TTGAGTCTCTTGAACTGGTCTAGG-3′ | spo0A qPCR (CD1214) | |
| oMC339 | 5′-GGGCAAATATACTTCCTCCTCCAT-3′ | sigE qPCR (CD2643) | |
| oMC340 | 5′-TGACTTTACACTTTCATCTGTTTCTAGC-3′ | sigE qPCR (CD2643) | |
| oMC346 | 5′-GCGGATCCAATTTTCATCCTTTCTTTTGTCAAAC-3′ | PcprA cloning | |
| oMC349 | 5′-CCTTTGTGCTAGCCTTATTGTTAGG-3′ | oppB qPCR (CD0853) | |
| oMC350 | 5′-AAGTATGAGTACTAAGGCAACCCA-3′ | oppB qPCR (CD0853) | |
| oMC363 | 5′-GACTTGCTCTGTGTTAGTTCCATC-3′ | spoIID qPCR (CD0124) | |
| oMC364 | 5′-TGTTTATAGATACTGGGCTCTCTGG-3′ | spoIID qPCR (CD0124) | |
| oMC365 | 5′-GGAAGTAACTGTTGCCAGAGAAGA-3′ | sigF qPCR (CD0772) | |
| oMC366 | 5′-CGCTCCTAACTAGACCTAAATTGC-3′ | sigF qPCR (CD0772) | |
| oMC371 | 5′-AAAAGCTTTTGCAACCCACGTCGATCGTGAAATATACAGTCAAGTGCGCCCAGATAGGGTG-3′ | oppB (CD0853) intron retargeting | |
| oMC372 | 5′- CAGATTGTACAAATGTGGTGATAACAGATAAGTCAGTCAATATAACTTACCTTTCTTTGT-3′ | oppB (CD0853) intron retargeting | |
| oMC373 | 5′-CGCAAGTTTCTAATTTCGGTTTATATTCGATAGAGGAAAGTGTCT-3′ | oppB (CD0853) intron retargeting | |
| oMC427 | 5′-GTGGTGTTAATACATCAGAACTTCC-3′ | sigG qPCR (CD2642) | |
| oMC428 | 5′-CAAACTGTTGTCTGGCTTCTTC-3′ | sigG qPCR (CD2642) | |
| oMC429 | 5′-GCCTGTGCTTCCAATGATAAAG-3′ | appA qPCR (CD2672) | |
| oMC430 | 5′-ATATCTGGGTCACTTGCCATAG-3′ | appA qPCR (CD2672) | |
| oMC431 | 5′-GTTGGAGAATCAGGATGTGGTA-3′ | appD qPCR (CD2671) | |
| oMC432 | 5′-GCTGGGTTTAAAGATGTCATTGG-3′ | appD qPCR (CD2671) | |
| oMC439 | 5′- GCCGGATCCCGTTTGCTTTTATGTGGTTT-3′ | oppB::erm verification | |
| oMC440 | 5′- GCCGGATCCCTGTTGGTGTGTTAATCCATTTTC-3′ | oppBCAD cloning | |
| oMC441 | 5′-GCCTGCAGGCTTTTACAGTAGTACTTTTGTCC-3′ | oppBCAD cloning | |
| oMC506 | 5′-GCCGGATCCAACCATTTAAGAGGTGATTT-3′ | CD0852 cloning | |
| oMC507 | 5′-GACCTGCAGCTTTCTCCAAATACACCTAT-3′ | CD0852 cloning | |
| oMC527 | 5′-AGGCAGGTTTACATCCAACATA-3′ | sinR qPCR (CD2214) | |
| oMC528 | 5′-AGTGGTATGTCTAAAGCAGTAGC-3′ | sinR qPCR (CD2214) | |
| oMC544 | 5′-AAAAGCTTTTGCAACCCACGTCGATCGTGAAGTTAACGTAGAAGTGCGCCCAGATAGGGTG-3′ | CD2672 (appA) intron retargeting | |
| oMC545 | 5′-CAGATTGTACAAATGTGGTGATAACAGATAAGTCGTAGAAAATAACTTACCTTTCTTTGT-3′ | CD2672 (appA) intron retargeting | |
| oMC546 | 5′-CGCAAGTTTCTAATTTCGGTTTTAACTCGATAGA GGAAAGTGTCT-3′ | CD2672 (appA) intron retargeting | |
| oMC547 | 5′-TGGATAGGTGGAGAAGTCAGT-3′ | tcdA qPCR (CD0663) | |
| oMC548 | 5′-GCTGTAATGCTTCAGTGGTAGA-3′ | tcdA qPCR (CD0663) | |
| oMC553 | 5′-TGAATGCACCAGAACCAACT-3′ | appA::ermB verification | |
| oMC587 | 5′-GTCGGATCCGGCTTTACATAACATGGTAGAGTAGTA-3′ | appFDABC cloning | |
| oMC588 | 5′-AGCGAATTCAACAGCATGAAATCCCATCTTAATC-3′ | appFDABC cloning | |
| oMC589 | 5′-GACACATGAACCAACATACATGG-3′ | oppC qPCR (CD0854) | |
| oMC590 | 5′-AGATGCTGAACCACTTATACTACC-3′ | oppC qPCR (CD0854) | |
| oMC591 | 5′-GATAAGAAGGCAGATACGCCTAAA-3′ | oppA qPCR (CD0855) | |
| oMC592 | 5′-GCTAGACAAGGAACCACCTTATTA-3′ | oppA qPCR (CD0855) | |
| oMC593 | 5′-ATTTCAAGACCCTATGACATCTCTC-3′ | oppD qPCR (CD0856) | |
| oMC594 | 5′-TCTCAGGATTATCAATTCCAACCA-3′ | oppD qPCR (CD0856) | |
| oMC595 | 5′-CATGAATCTACTGGTGGAGAAGTAA-3′ | oppF qPCR (CD0857) | |
| oMC596 | 5′-CTGCAATTGTCATACGTGGATTT-3′ | oppF qPCR (CD0857) | |
| oMC597 | 5′-TGCAGGAAGAACCATTCTTAGAT-3′ | appF qPCR (CD2670) | |
| oMC598 | 5′-ATGTTCTATAACTGCCTCGGATATT-3′ | appF qPCR (CD2670) | |
| oMC599 | 5′-GGTTAATATGGGACTTGATAAACCAG-3′ | appB qPCR (CD2673) | |
| oMC600 | 5′-CATAATTTGTTCTGTAACGGGCATA-3′ | appB qPCR (CD2673) | |
| oMC601 | 5′-CTTCAAGAACCTAGCTTAAAGCATATC-3′ | appC qPCR (CD2674) | |
| oMC602 | 5′-ATAACACCTACAGCAGTACCAAATA-3′ | appC qPCR (CD2674) | |
| oMC612 | 5′-ACAGAAGAAGGTGTTGAAGGATATG-3′ | appA (3′) qPCR | |
| oMC613 | 5′-TTATGCCCAAATCTACCTGTGTTAT-3′ | appA (3′) qPCR | |
| oMC654 | 5′-GCCTGCAGCCTGTTCAAGTATTTTTATACTAAAC-3′ | oppF cloning | |
| oMC683 | 5′-GTATCTGACAACATCAATTGCCTAAA-3′ | CD0341 qPCR | |
| oMC684 | 5′-TCAGCTTGAGATTCAATTTCTTCATT-3′ | CD0341 qPCR | |
| oMC733 | 5′-AGGTTTGGAGCAATCAAGAAAGA-3′ | murG qPCR (CD2651) | |
| oMC734 | 5′-TGCTCATGTATTATGGCTGGTATTT-3′ | murG qPCR (CD2651) | |
| oMC735 | 5′-CCTGGTAATTGGTCTTGGAAATAGA-3′ | gpr qPCR (CD2470) | |
| oMC736 | 5′-CCTCAGTGAAATCATCATCATAGTCTTTA-3′ | gpr qPCR (CD2470) | |
| oMC739 | 5′-AAGTGTGGCATTTGTAGACCATAATA-3′ | acnB qPCR (CD0833) | |
| oMC740 | 5′-ACCATTTCCTGGCTTAGAGAAATAAA-3′ | acnB qPCR (CD0833) | |
| oMC741 | 5′-CTCTGCAAATGTAGGTCATGGTAATAA-3′ | gabT qPCR (CD2158) | |
| oMC742 | 5′-TTCCACAAGCTTCTCAGCTAATTT-3′ | gabT qPCR (CD2158) | |
| oMC745/oBD522 | 5′-ATCTGTAGGAGAACCTATGGGAAC-3′ | Intron forward primer for Southern blotting | 47 |
| oMC746/oBD523 | 5′-CACGTAATAAATATCTGGACGTAAAA-3′ | Intron reverse primer for Southern blotting | 47 |
| oMC747 | 5′-TCAACGGAAGATCAGGATGATTTA-3′ | sigK qPCR (CD1230) | |
| oMC748 | 5′-CATATGTTGCTAATCGAGTTCCTTTAT-3′ | sigK qPCR (CD1230) | |
| oMC756 | 5′-TGGCAAGTAACAATAACAACAACAG-3′ | sspA qPCR (CD2688) | |
| oMC757 | 5′-CCATTTGTTGTTCAGCCATTTCA-3′ | sspA qPCR (CD2688) |
Underlining indicates sequence-specific sites within gene of interest for intron retargeting.
Phase-contrast microscopy.
C. difficile strains were grown in 70:30 sporulation medium as described above. At the indicated time points, 1 ml of culture was removed from the anaerobic chamber, centrifuged at full speed for 30 s, and resuspended in ∼10 μl of supernatant. Slides were prepared by placing 2 μl of the concentrated culture onto a thin layer of 0.7% agarose applied directly to the surface of the slide. Phase-contrast microscopy was performed using a ×100 Ph3 oil immersion objective on a Nikon Eclipse Ci-L microscope. At least three fields of view for each strain were acquired with a DS-Fi2 camera and used to calculate the percentage of spores (the number of spores divided by the total number of spores, prespores, and vegetative cells) from two independent experiments.
Fluorescence microscopy.
C. difficile strains were grown in 70:30 sporulation medium as described above. At approximately 4 h after the onset of stationary phase (∼T4), 2 ml of culture was removed from the anaerobic chamber, pelleted, and resuspended in 50 μl of brain heart infusion medium. The membrane-specific dyes FM4-64 and MitoTracker green were added to a final concentration of 165 μM and 100 nM, respectively, and the cultures were incubated for 30 min at room temperature. Slides were prepared by first applying a thin layer of 1.0% agarose to the slide, followed by application of 8 μl of the concentrated culture onto the agarose bed. Fluorescence microscopy was performed using a ×100 oil immersion objective (numerical aperture, 1.49) on a Nikon structured illumination microscope (N-SIM). At least three fields of view for each strain were used to calculate the percentage of cells entering sporulation from two independent experiments. The percentage of sporulating cells was defined as the number of cells possessing polar septa or partially/completely engulfed forespores divided by the total number of cells.
Southern blot analysis.
Genomic DNA was isolated from overnight cultures of C. difficile strains 630Δerm, MC296, MC301, and MC307 as previously described (42). Six micrograms of DNA from each strain was digested with HindIII (NEB), separated on a 0.7% agarose gel, and transferred onto Hybond-N+ nylon membranes (GE Healthcare). DNA was then fixed to the membranes by UV cross-linking. Southern blot analysis was performed using a DIG High Prime labeling and detection kit (Roche). An intron-specific probe was prepared by PCR using primers OBD522 and OBD523 as previously described (47).
Spore preparation and quantification.
To prepare spores for animal studies, strains for spore preparation were grown in BHIS broth overnight and 100 μl was spread onto 70:30 agar plates (36). The plates were then incubated for 48 h to allow spores to form. Following incubation, cells were scraped from the plates, resuspended in 5 ml of phosphate-buffered saline (PBS), pelleted at 3,000 × g for 15 min, and resuspended in 5 ml of PBS. The resuspended cells were then combined 1:1 with 95% ethanol and incubated at room temperature for 1 h to kill all nonspores present. The spores were then pelleted, washed twice in PBS, and resuspended in 5 ml of fresh PBS. Spore suspensions were then heated to 70°C for 20 min, followed by addition of 1% bovine serum albumin (BSA) to prevent spores from sticking to each other and plastic or glass surfaces. Spore preparations were serially plated in PBS with 1% BSA to determine the number of CFU present and diluted prior to use. Spores were stored in glass tubes at room temperature.
Quantitative reverse transcription-PCR (qRT-PCR) analysis.
Samples of C. difficile grown in 70:30 sporulation medium were harvested into 1:1 ethanol-acetone, and RNA was purified as previously described (42, 48). RNA isolated from hamster cecal contents was treated identically, except that cells were mechanically disrupted, using a bead beater, for twice as long (a total of 6 min per sample). cDNA synthesis was performed with random hexamers using a Tetro cDNA synthesis kit (Bioline), and either 50 ng (samples isolated from in vitro cultures) or 200 ng (samples isolated from cecal contents) cDNA per reaction mixture was used for quantitative PCR (qPCR) analysis. qPCR analysis was performed using a SensiMix SYBR & Fluorescein kit (Bioline) and a Bio-Rad CFX96 real-time system as previously described (49). Mock cDNA synthesis reaction mixtures containing no reverse transcriptase were used to control for genomic contamination in subsequent amplifications. The primers used for qPCRs were designed using the PrimerQuest tool by Integrated DNA Technologies, and primer efficiencies were determined for each primer set prior to use. qPCRs were performed in technical triplicate for each cDNA sample and primer pair. qPCR was performed on cDNA isolated from a minimum of three biological replicates. Results are presented as the means and standard errors of the means of the data obtained. Results were calculated by the comparative cycle threshold method (50), with the expression of the amplified target sequence being normalized to that of the internal control transcript, rpoC. A two-tailed Student's t test was used to compare the transcriptional ratios of the variable to the control sets, as indicated below.
Animal studies.
Female Syrian golden hamsters (Mesocricetus auratus) weighing between 70 and 120 g were purchased from Charles River Laboratories and maintained in an animal biosafety level 2 facility in the Emory University Division of Animal Resources. Animals were housed individually in sterile cages and were fed a standard rodent diet and provided water ad libitum. Five days prior to inoculation with C. difficile, the hamsters were administered a single dose of clindamycin (30 mg kg of body weight−1) by oral gavage to perturb the intestinal microbiota and induce susceptibility to infection (51, 52). At 5 days after antibiotic treatment, the hamsters were administered approximately 500 spores of a single strain of C. difficile and monitored for symptoms of disease (weight loss, lethargy, diarrhea, and wet tail). The hamsters were weighed at least once per day, and fecal samples were collected daily for enumeration of spores. Cohorts of 5 to 6 animals were tested per strain, and experiments were performed a minimum of two times. Negative-control animals that were treated with clindamycin but not administered C. difficile were included in all experiments. Animals were considered moribund if (i) they had lost 15% or more of their starting weight or (ii) if they presented with diarrhea, lethargy, and wet tail. Animals meeting either of these criteria were euthanized to prevent unnecessary suffering. Hamsters were euthanized by CO2 asphyxiation, followed by thoracotomy as a secondary method of euthanasia. At the time of death, animals infected with either the 630Δerm or MC307 strain were necropsied, and cecal contents were collected and stored in a 1:1 acetone-ethanol solution at −80°C. All animal studies were performed with prior approval from the Emory University Institutional Animal Care and Use Committee (IACUC). Differences in spores recovered from fecal samples were analyzed by analysis of variance (single factor; Excel; Microsoft). Differences in hamster survival between those infected with C. difficile 630Δerm and those infected with either MC296 (opp), MC301 (app), or MC307 (opp app) were analyzed using the log rank test (GraphPad Prism).
Nucleotide sequence accession numbers.
The nucleotide sequences of C. difficile strains 630 and R20291 have been deposited in GenBank under accession numbers NC_009089.1 and NC_013316.1, respectively.
RESULTS
Identification of Opp and App transporters in C. difficile.
The opp and app operons were identified on the chromosome of sequenced strain 630 as CD0853 to CD0857 and CD2670 to CD2674, respectively (53, 54). The app genes are arranged in two apparently divergently transcribed operons (appABC and appDF), while the opp genes appear to be organized as a single transcription unit (oppBCADF) (Fig. 1). Similar to the oligopeptide transporters found in other Gram-positive bacteria, both the app and opp regions encode an apparent extracellular substrate binding protein (appA and oppA), two apparent integral membrane permeases (appBC and oppBC), and two apparent cytoplasmic ATP-binding proteins (appDF and oppDF). Orthologs of the opp and app oligopeptide transporters are common to many bacterial species, and often, two or more analogous transport systems are encoded by the genomes of Gram-positive bacteria (17, 55–58). In the latter cases, the systems may have overlapping or redundant functions (57). Although the predicted App and Opp protein sequences are orthologous to the oligopeptide transport systems found in other bacteria (Fig. 1), the individual App and Opp proteins of C. difficile have only modest amino acid identity to one another (e.g., 29% identity between OppA and AppA); therefore, the specific functions of these transporters in C. difficile could not be assumed from their sequence similarities. In previous C. difficile studies, the opp operon was found to be repressed by the global transcriptional regulators CodY and CcpA and by glucose, implying that the Opp transporter is involved in adaptation to nutrient-limiting conditions (42, 59). In addition, SigH, the alternative sigma factor involved in the transition to stationary phase, was shown to differentially affect transcription of the app and opp genes (60). In a sigH mutant, transcription of the opp genes was decreased, while app transcripts were increased, suggesting that the transporters are regulated differently during the adaptation to stationary phase in TY medium (60). However, our transcriptional analysis of app and opp expression in cells grown in sporulation medium (70:30 broth) (36) revealed that the expression of appA and oppB genes increases similarly throughout the transition to stationary phase (see Fig. S2A in the supplemental material).
FIG 1.
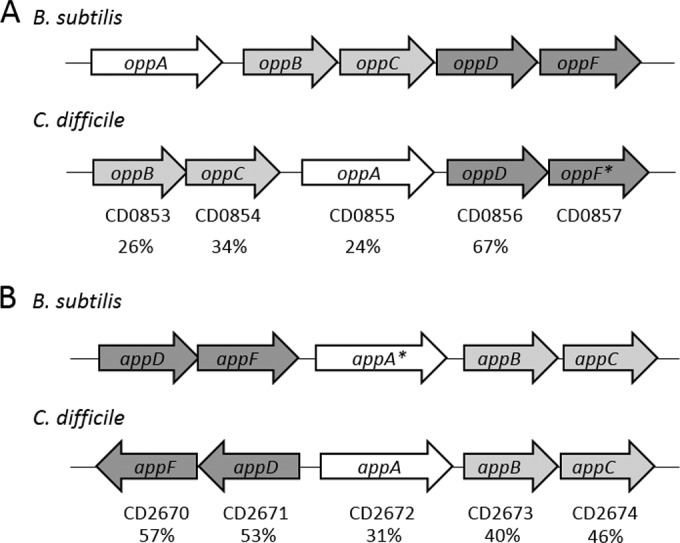
Identification of oligopeptide transporter systems in C. difficile. Comparison of the opp (A) and app (B) operons in B. subtilis 168 (GenBank accession no. AL009126) and C. difficile 630 (GenBank accession no. AM180355). OppA/AppA are solute-binding lipoproteins, OppBC/AppBC are transmembrane proteins, and OppDF/AppDF are ATP-binding proteins. *, a pseudogene; OppF is annotated as a pseudogene in C. difficile 630 as well as AppA in B. subtilis 168 (57). Numbers below the arrows represent percent identity to proteins in the corresponding B. subtilis sequences.
Disruption of the opp and app operons results in increased spore formation.
In the model spore-forming firmicute Bacillus subtilis, the Opp (Spo0K) and App transporters are responsible for the uptake of small, quorum-signaling peptides that stimulate competence and sporulation (12, 57). In B. subtilis, the absence of functional App and Opp transporters results in a significant sporulation defect (61, 62). C. difficile does not carry orthologs of either the PhrA peptide or the competence-stimulating factor (CSF) PhrC (18), but unrelated peptides with similar activities could potentially be transported by the oligopeptide transporters. To test whether the Opp and App transporter systems have a role in regulating the entry into sporulation in C. difficile, we introduced insertional mutations into the first genes of the app and opp operons (appA and oppB, respectively). To do so, we used a TargeTron-based group II intron, which was retargeted for integration into appA at nucleotide 439 and into oppB at nucleotide 178 of the coding sequences (see Materials and Methods). The locations of the insertions were confirmed by Southern blotting (see Fig. S3 in the supplemental material). Both the appA and oppB insertional mutations greatly reduced the expression of all the downstream genes in each operon, as evidenced by qRT-PCR analysis (see Fig. S2B and C in the supplemental material)
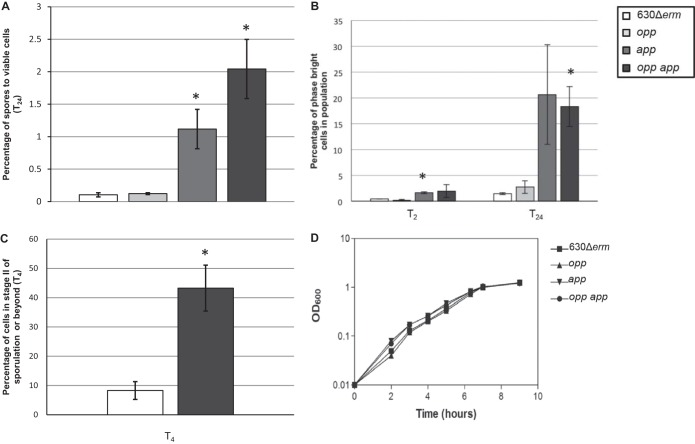
The app- and opp-null mutants (MC296 and MC301, respectively) were tested for the frequency of spore formation after 24 h of growth in 70:30 sporulation medium by measuring the number of ethanol-resistant spores recovered and normalizing this to the number of viable cells present at T2, as described in Materials and Methods (36). We observed a sporulation frequency of ∼0.1% in the parent strain, 630Δerm (Fig. 2A). Low sporulation frequencies in C. difficile have been observed in other studies (1, 63, 64); however, the percentage of spore formation reported is widely variable, depending on the strain, medium, and methods used to enumerate spores (63). As shown in Fig. 2A, the opp mutant had the same low frequency of spore formation as the parent strain. In contrast, the app-null mutant produced an average of 10-fold more ethanol-resistant spores than did the parent strain (Fig. 2A). To determine if the Opp and App transporter systems have overlapping or redundant roles in mediating sporulation frequency, a double mutant strain containing both the oppB and appA insertional disruptions was created (MC307). The opp app double mutant produced ∼20-fold more spores than did the parent strain (Fig. 2A). These data suggest that the App transporter influences the sporulation frequency in C. difficile, while the Opp transporter does not appear to affect sporulation in vitro.
FIG 2.
Disruption of the opp and app operons results in an increase in sporulation frequencies. (A) Sporulation frequency (CFU ml−1 ethanol-resistant spores) of 630Δerm, MC296 (opp), MC301 (app), and MC307 (opp app) grown in 70:30 sporulation medium at T24. (B) Percentage of phase-bright spores assessed by phase-contrast microscopy. (C) Percentage of cells that have a polar septum or partially/completely engulfed forespores, assessed by fluorescence microscopy using the membrane-specific dye FM4-64. (D) Representative growth curve of 630Δerm, MC296 (opp), MC301 (app), and MC307 (opp app) grown in 70:30 sporulation medium. The means and standard errors of the means for spore counts of at least two biological replicates are shown. *, P ≤ 0.05 by two-tailed Student's t test.
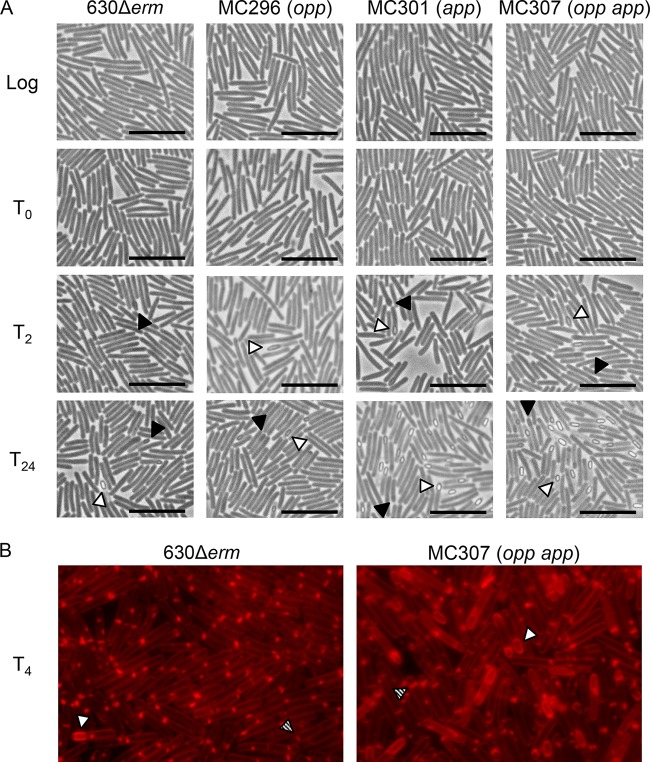
Using phase-contrast microscopy, we followed the rate of sporulation in the parent and the transporter mutant strains (Fig. 3A). Phase-dark prespores and phase-bright spores were visible by the second hour of stationary phase (T2) in all strains; however, the app and opp app mutants produced more spores than the wild type by T2 (3.7-fold and 4.3-fold higher, respectively) and T24 (14.1-fold and 12.5-fold higher, respectively) (Fig. 2B). To further define sporulation initiation in the parent strain and the opp app mutant, we performed fluorescence microscopy using the membrane-specific dye FM4-64 (65, 66). This technique enables the identification of cells that have asymmetric sporulation septa (stage II) as well as partial or complete engulfment of the prespore (stage III). At T4, more cells of the transporter mutant (43.2% ± 7.8%) than of the wild-type strain (8.3% ± 3.0%) were in stage II or beyond (Fig. 2C and 3B). There was no significant difference in the growth rate of the parent, app, opp, or opp app strain in 70:30 or BHIS medium (Fig. 2C; data not shown). These results indicate that these oligopeptide transporters are not essential for growth or for detection of population density under the conditions tested. Surprisingly, the increased sporulation rate of C. difficile App transporter mutants contrasts dramatically with the roles of analogous transporters in other spore-forming species, suggesting that peptide transport plays a distinctly different role in C. difficile spore formation (21, 57).
FIG 3.
Oligopeptide transporter mutants hypersporulate and form spores earlier than the wild-type strain. (A) Phase-contrast microscopy of 630Δerm, MC296 (opp), MC301 (app), and MC307 (opp app) grown in 70:30 sporulation medium. Filled arrowheads, phase-dark prespores; open arrowheads, phase-bright mature spores. Bars, 10 μm. (B) Fluorescence microscopy of 630Δerm and MC307 (opp app) grown in 70:30 sporulation medium and stained with FM4-64. Open arrowheads, partially or completely engulfed forespores; patterned arrowheads, cells with polar septa. For both phase-contrast and fluorescence microscopy, samples were removed at the indicated times and prepared for microscopy as described in Materials and Methods.
The sporulation phenotypes of the opp and opp app transporter mutants were fully rescued when plasmid copies of the opp operon were used to complement the disrupted transporters. Expression of the app operons from their native promoters on a plasmid did not effectively complement either the app or the opp app double mutant (see Fig. S4 in the supplemental material). We performed transcriptional analysis to determine the levels of opp and app expression from plasmids in the complemented strains. Transcript levels of oppB were increased ∼25-fold in the respective complemented strains (MC322 and MC323), while appA and appD transcript levels were upregulated ∼60- to 120-fold in the respective complemented strains (MC331 and MC334; see Fig. S5 in the supplemental material). These data reveal that expression of the app operons in trans results in significantly increased levels of the app transcript compared to the levels of the native app transcript during log-phase growth. Increased app transcription may result in a dysfunctional App system that may cause additional stress on the strains harboring plasmid copies of the app operons. We were unable to complement the transporter mutants in a single copy on the chromosome with current technologies due to size constraints (>5 kb) and toxicity issues. Notably, the sporulation frequency was increased in all strains containing a plasmid, including those with only the vector control (data not shown). This increase in sporulation may be due to the energy burden of the plasmid or the addition of thiamphenicol to the medium for plasmid maintenance, although these possibilities are not mutually exclusive.
Loss of Opp and App affects the expression of genes required for initiation of spore formation.
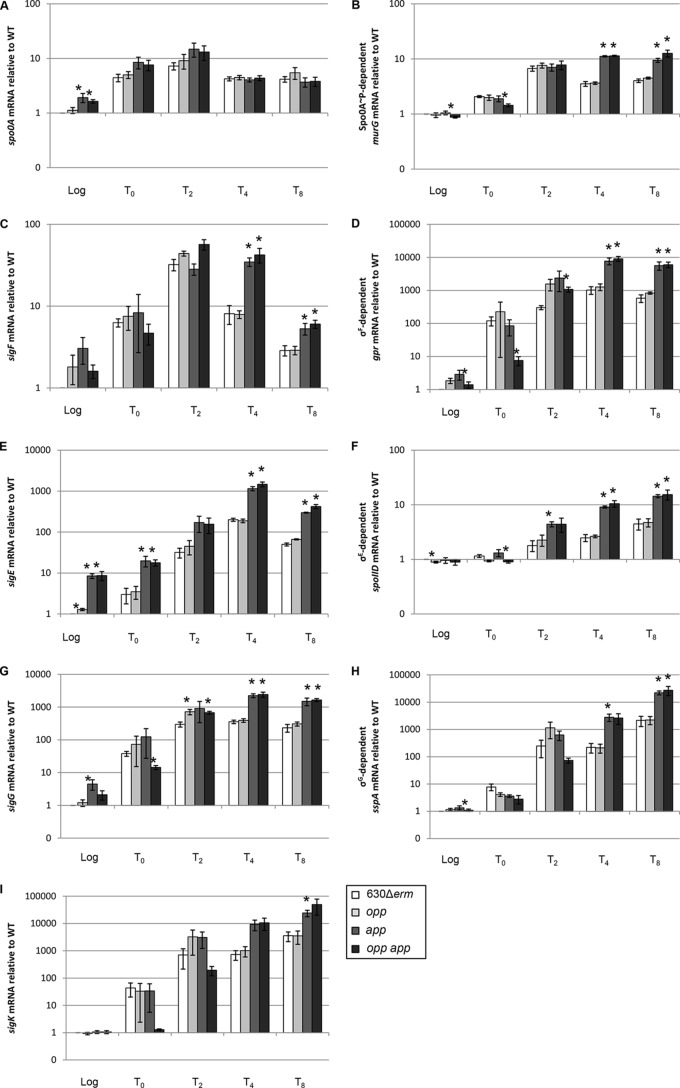
The increased sporulation frequencies of the app and opp app transporter mutants may result from earlier and/or higher transcription of key sporulation-specific genes or other post-exponential-phase control genes during growth. To determine if sporulation-dependent gene expression correlates with the increased sporulation phenotype of the app and opp app mutants, we analyzed early sporulation gene expression during growth in 70:30 sporulation medium, starting with the master regulator of sporulation, Spo0A. Spo0A is a DNA-binding protein that directly and indirectly regulates many stationary-phase processes, including sporulation. spo0A transcription is regulated by multiple global regulators, including positive autoregulation of its own transcription, and Spo0A activity in other sporeformers is tightly controlled at multiple levels by transcriptional, posttranscriptional, and posttranslational mechanisms (3, 59, 60, 67, 68). Expression of spo0A was slightly elevated (∼1.5-fold) in the opp app double mutant during exponential growth phase compared to that in the parent strain (Fig. 4A). We also analyzed the expression of the murG gene, which is a direct target of active Spo0A (69). murG transcript levels increased in the app and opp app mutants at the T4 and T8 time points (∼3-fold compared to those for the parent strain; Fig. 4B), indicating that more Spo0A is active and Spo0A-dependent gene expression is upregulated in the absence of the App transporter.
FIG 4.
Effects of oligopeptide transporters on the regulation of sporulation-specific gene transcription. qRT-PCR analysis of spo0A (A), the Spo0A-dependent gene murG (B), sigF (C), the σF-dependent gene gpr (D), sigE (E), the σE-dependent gene spoIID (F), sigG (G), the σG-dependent gene sspA (H), and sigK (I) expression in 630Δerm, MC296 (opp), MC301 (app), and MC307 (opp app) grown in 70:30 sporulation medium to OD600s of 0.5 (log phase) and 1.0 (start of stationary phase; T0) and 2, 4, and 8 h after T0 (T2, T4, and T8, respectively), as described in Materials and Methods. The means and standard errors of the means of at least three biological replicates are shown. WT, wild type. *, P ≤ 0.05 by a two-tailed Student's t test.
To determine if subsequent sporulation-specific gene transcription differs in the transporter mutants, we used qRT-PCR to analyze the expression of sporulation sigma factors and genes dependent upon activation of each sigma factor. qRT-PCR analysis was performed to assess the expression and activities of the sporulation sigma factors. SigF (σF) controls early forespore-specific sporulation gene expression (47, 69, 70). Transcript analysis revealed that the level of sigF mRNA is increased in the app and the opp app transporter mutants at T4 and T8 (∼4-fold and ∼2-fold, respectively; Fig. 4C), and expression of a σF-dependent gene, gpr, is also up at T4 (∼8-fold) and T8 (∼10-fold) compared to that in the parent strain (Fig. 4D). gpr transcript levels were ∼4- to 8-fold higher in the app single mutant and the opp app double mutant at T2. These data indicate that σF gene expression and activity are increased in the App transporter mutants in stationary phase. SigE (σE) is responsible for transcription of early sporulation-specific genes in the mother cell compartment (71), and sigE transcription is directly upregulated only by active Spo0A (47, 69, 70, 72). The app and opp app mutants expressed more sigE transcript than the parent cells during logarithmic and stationary phases of growth (∼5- to 10-fold; Fig. 4E). In addition, analysis of the σE-dependent transcript, spoIID, showed that σE-dependent gene expression is increased ∼3-fold at stationary phase in the app and opp app mutants compared to that in the parent or opp mutant strain (Fig. 4F) (73). These data indicate that early sporulation-specific gene expression proceeds earlier and is increased at stationary phase in the app and opp app mutants.
To further understand sporulation progression in the transporter mutants, we analyzed expression of additional mid- to late-stage sporulation genes. In contrast to B. subtilis, the order and synchronization of sporulation sigma factor expression and activation are not tightly regulated in C. difficile (47, 69, 70), but increased expression of these sigma factors and genes dependent on their activation is positively correlated with the later stages of spore development. Transcription of sigG, the forespore-specific late sigma factor, was higher (∼2- to 3-fold) in the App transporter mutants by T2 and was increased even further (∼6- to 7-fold) by later time points (Fig. 4G). Transcription of the σG-dependent gene, sspA, was also increased in stationary phase in the app and opp app mutants (∼10- to 13-fold; Fig. 4H). σK, the late-stage mother cell sigma factor, contains a 14.6-kb skincd element that must be excised prior to translation (74). σK activity is necessary for efficient sporulation but is not strictly required for the formation of heat-resistant spores (70). Expression of sigK in the App transporter mutants was upregulated ∼7- to 15-fold at T4 and T8 (Fig. 4I). Together, these results demonstrate that sporulation genes are expressed earlier and at higher levels in the app and opp app mutants, consistent with their increased sporulation phenotype observed in vitro.
In B. subtilis, transcription of opp and app is repressed by the DNA-binding negative regulator of sporulation, ScoC (75). C. difficile contains a putative scoC ortholog (CD0852) encoded upstream of the opp operon. Preliminary data suggest that overexpression of CD0852 in C. difficile negatively impacts sporulation (A. N. Edwards and S. M. McBride, unpublished data). qRT-PCR analysis revealed that oppB, appA, and appD transcript levels at stationary phase of growth (T2) were unchanged between the wild-type strain and a CD0852-overexpressing strain (see Fig. S2D in the supplemental material), suggesting that the opp and app operons are not under the same regulatory control in C. difficile as they are in B. subtilis. Altogether, these results indicate that the Opp and App transporters play a different role in controlling sporulation in C. difficile than in other spore-forming bacteria, such as B. subtilis.
The absence of Opp and App transporters influences expression of the transcriptional regulator SinR and its putative inhibitor, SinI.
As the Opp and App transporters are not predicted to have direct DNA-binding domains or regulatory capabilities, the changes in gene expression observed in the transporter mutants likely result from the lack of peptides feeding into nutritional and/or regulatory pathways. The two global regulatory proteins known to control cellular responses to nutrient availability in C. difficile are CodY and CcpA (39, 42, 76–78). CodY is a DNA-binding transcriptional regulator that controls the expression of hundreds of genes in response to the intracellular availability of GTP and branched-chain amino acids (76, 79). CcpA mediates carbon catabolite repression in many Gram-positive bacteria, including C. difficile, and controls the expression of a wide range of genes involved in sugar uptake, fermentation, and amino acid metabolism (59, 77). Both CodY and CcpA directly control the transcription of gene products required for sporulation initiation and toxin synthesis in C. difficile and other sporulating pathogens (59, 76, 77, 80, 81). To determine if either of these global regulatory proteins is involved in mediating the increase in spore formation observed in the app and opp app mutants, we investigated the effects of the transporter mutations on CodY-mediated and CcpA-mediated gene regulation. qRT-PCR analysis of samples taken during logarithmic- and stationary-phase growth in 70:30 sporulation medium revealed that the expression of multiple CodY- and CcpA-dependent genes was not greatly influenced in the transporter mutants (see Fig. S6 in the supplemental material), suggesting that inactivation of the transporters is likely not signaling through CodY or CcpA to control sporulation. However, we found that expression of the CcpA-dependent sinR transcript was greatly elevated (∼30- to 80-fold) in logarithmic and stationary phases in the app and opp app mutants (Fig. 5A) (59). In other species, SinR acts as a repressor of sporulation by directly inhibiting Spo0A transcription (82). Although transcription of sinR is elevated, the SinR protein can be held inactive in a complex with its repressor, SinI (83). C. difficile encodes a sinI-like regulator adjacent to sinR. Transcription of the putative sinI was also amplified in the app and opp app mutants (∼80- to 125-fold; Fig. 5B), suggesting that the inhibitory effects of SinR on sporulation are negated by SinI in C. difficile. These results suggest that the function of the App transporter influences sinR and sinI gene expression and, potentially, the initiation of spore formation under the conditions tested.
FIG 5.
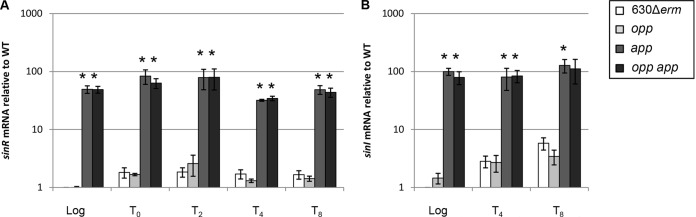
Effects of oligopeptide transporters on regulation of sinR and sinI gene expression. qRT-PCR analysis of expression of sinR (A) and sinI (B) in 630Δerm, MC296 (opp), MC301 (app), and MC307 (opp app) grown in 70:30 sporulation medium to OD600s of 0.5 (log phase) and 1.0 (start of stationary phase; T0) and 2, 4, or 8 h after T0 (T2, T4, and T8), as described in Materials and Methods. The means and standard errors of the means of at least three biological replicates are shown. *, P ≤ 0.05 by a two-tailed Student's t test.
Loss of Opp and App transporters results in increased virulence and spore formation in an animal model of CDI.
The conditions that lead to the sporulation of C. difficile within the intestinal environment are not known. What is known is that C. difficile initiates sporulation in vivo within hours after germination and that de novo spores can be detected within 12 h postinoculation (84, 85). To determine if the Opp and App transporters influence sporulation frequency in the intestinal environment, we examined the effects of the opp, app, and opp app mutations on spore production and virulence in the hamster model of C. difficile infection (CDI), a model that has been used for more than 35 years to study acute disease caused by C. difficile (52, 86, 87). Female Syrian golden hamsters were infected by gavage with approximately 500 spores of either the parental strain or the transporter mutants. Animals were closely monitored for symptoms of disease, and fecal samples were retrieved to quantify the number of spores shed during infection, as described in Materials and Methods. As shown in Fig. 6A, hamsters infected with the opp-, app-, and both opp- and app-null mutants shed greater numbers of spores in feces than those infected with the parent strain at 24 h postinfection. On average, opp app mutant-infected animals shed approximately 8.5 times the number spores per gram of feces than wild-type-infected animals. The number of vegetative cells present in feces was not calculated, as exposure to oxygen could not be eliminated from our test conditions. Likewise, the number of spores located throughout the gastrointestinal tract was not assessed.
FIG 6.
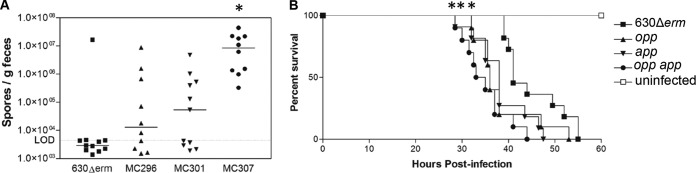
Effects of oligopeptide transporters on fecal spore output and virulence in the hamster model of CDI. (A) Total number of C. difficile spores per gram of feces recovered at 24 h postinfection from two independent experiments of clindamycin-treated Syrian golden hamsters inoculated with 500 spores of C. difficile 630Δerm, MC296 (opp), MC301 (app), or MC307 (opp app). Fecal samples were weighed, resuspended in 1× PBS, and plated onto TCCFA plates (95, 96), and C. difficile colonies were enumerated after 48 h. Solid lines, the median for each strain; dotted line, limit of detection (4.51 × 103 CFU/g). (B) Kaplan-Meier survival curve representing the cumulative results from the same two independent experiments described in the legend to panel A. Hamsters infected with the mutant strains succumbed more rapidly than those infected with the parent strain. Mean times to morbidity were as follows: 630Δerm, 45.1 ± 6.4 h; MC296 (opp), 38.4 ± 6.6 h; MC301 (app), 38.2 ± 5.8 h; and MC307 (opp app), 35.0 ± 4.9 h (P < 0.02 for all mutants, log rank test).
All of the hamsters infected with transporter mutant strains succumbed to disease more rapidly than those infected with the parent strain (P < 0.02, log rank test; mean times to morbidity, 45.1 ± 6.4 h for 630Δerm, 38.4 ± 6.6 h for MC296 [opp], 38.2 ± 5.8 h for MC301 [app], and 35.0 ± 4.9 h for MC307 [opp app]; Fig. 6B). Hamsters infected with the transporter mutant strains lost weight and displayed symptoms earlier than wild-type-infected animals, but no statistically significant difference in weight loss at the time of death between hamsters infected with different strains was observed (data not shown). All animals lost a minimum of 6% of their body weight during infection. As described in other studies, a few animals did not exhibit wet tail prior to becoming moribund (88). The hypervirulent phenotypes of the transporter mutants demonstrate that these transporters are not required for growth in vivo but instead may indirectly function to influence toxin production or other virulence phenotypes in the host. Interestingly, the opp mutant exhibited a phenotype similar to that of the app mutant in the hamster model of CDI, despite the fact that no significant phenotype was observed in vitro. These data demonstrate that the strain 630 opp operon, containing oppF as a pseudogene, has an important function in vivo.
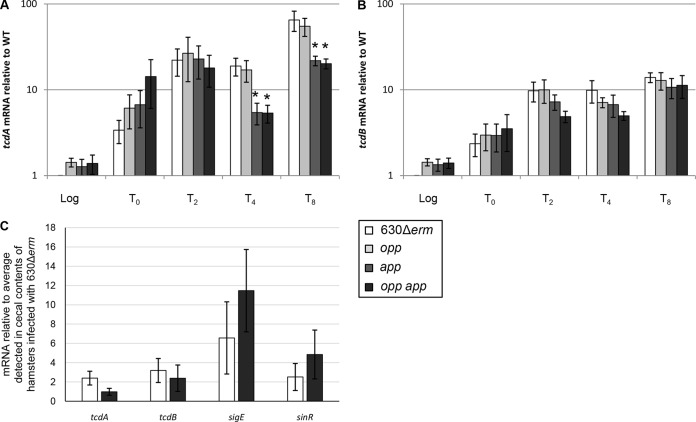
Production of both toxin A and toxin B by C. difficile 630 is required for full virulence in the hamster model of CDI; however, toxin A or toxin B alone is sufficient to cause disease (89). It was previously established that toxin production in C. difficile is upregulated in response to nutrient limitation, including amino acid starvation (39, 76–78, 90). We asked whether expression of the toxin genes tcdA and tcdB was upregulated in the transporter mutants in vitro, potentially explaining the observed hypervirulent phenotype. qRT-PCR analysis of the tcdA and tcdB transcripts revealed that there was no difference in toxin expression between the parent strain and the opp, app, and opp app mutants, except for a modest decrease in tcdA transcript levels in the app and the opp app mutants in late stationary phase, when grown in 70:30 medium (Fig. 7A and B). To determine if toxin production is upregulated in vivo, RNA was isolated from cecal contents obtained at the time of death from hamsters infected with the parental or opp app mutant strain. As a control, we analyzed RNA isolated from the cecal contents of an uninfected animal. We recovered insignificant levels of the rpoC transcript and were unable to detect specific C. difficile gene transcripts in uninfected controls (data not shown). qRT-PCR analysis revealed that tcdA and tcdB gene expression was slightly decreased in hamsters infected with the opp app double mutant compared to those infected with the parent strain (Fig. 7C). Additionally, the transcript levels of the sporulation-specific gene sigE and the negative transcriptional regulator sinR in RNA isolated from cecal contents were slightly increased in the opp app double mutant (Fig. 7C). These results suggest that the hypervirulent phenotype observed in hamsters infected with the oligopeptide transporter mutants is not directly correlated with toxin production but may be related to the increased sporulation phenotype characterized in vitro and in vivo.
FIG 7.
Effects of oligopeptide transporters on toxin gene expression. (A, B) qRT-PCR analysis of tcdA (A) and tcdB (B) expression in 630Δerm, MC296 (opp), MC301 (app), and MC307 (opp app) grown in 70:30 sporulation medium to OD600s of 0.5 (log phase) and 1.0 (start of stationary phase; T0) and 2, 4, or 8 h after T0 (T2, T4, and T8), as described in Materials and Methods. The means and standard errors of the means of four biological replicates are shown. *, P ≤ 0.05 by a two-tailed Student's t test. The key at the bottom right applies to panels A and B. (C) qRT-PCR analysis of tcdA, tcdB, sigE, and sinR gene expression in cecal contents from hamsters infected with either 630Δerm or MC307 (opp app). The means and standard errors of the means of samples from at least 10 animals per strain are shown. *, P ≤ 0.05 by a two-tailed Student's t test.
DISCUSSION
As an obligate anaerobe, the ability of C. difficile to form dormant spores is required for the effective spread of this pathogen from host to host. Although spore morphology and the stages of spore formation are similar among the spore-forming Firmicutes, the regulatory pathways and environmental triggers that control the initiation of sporulation are not conserved in C. difficile (2). While spore formation begins in response to nutrient deprivation and cell density in the well-studied model organism B. subtilis (91), these signals were not previously proven to influence the sporulation of C. difficile (59, 77). Oligopeptide transporters have been shown to activate sporulation in other bacteria through the import of specific quorum-sensing peptides that indirectly upregulate early sporulation-specific gene transcription (11–13). In the current work, we demonstrate that the Opp and App transporters play a significant role in C. difficile sporulation and pathogenesis in vitro and in vivo, but the role of these transporters in C. difficile sporulation differs from that in B. subtilis.
Our initial hypothesis was that the C. difficile oligopeptide transporters could be stimulating sporulation in a cell density-dependent manner through the uptake of small secreted quorum-signaling peptides, similar to the Phr peptides found in spore-forming Bacillus species (11–13). Surprisingly, the Opp and App transporters negatively impact both the initiation of spore formation and the total number of spores formed both in vitro and in vivo (Fig. 2A to C, 3, and 6A). The data indicate that in C. difficile these transporters are not required for cell density-dependent signaling. This conclusion is supported by the absence of phr orthologs in the C. difficile genome (2, 53, 54). Furthermore, addition of spent medium to exponentially growing wild-type C. difficile did not affect early sporulation gene expression (data not shown). One hypothesis is that C. difficile could import a signaling peptide that inhibits sporulation at low cell density; however, this is unlikely, given the ecological niche that C. difficile inhabits. Alternatively, other quorum-sensing mechanisms, such as agr, may potentially fill the role of the Phr system. C. difficile may not maintain a high population density within the gastrointestinal tract due to frequent diarrhea, which results in the transient availability of nutrients and other metabolites. We favor the hypothesis that the Opp and App transporters serve to import general oligopeptides that influence the intracellular nutrient availability (Fig. 8). In an effort to demonstrate directly the function of the transporters, we examined the efficacy of peptide uptake (random 5- and 10-amino-acid peptides) in the wild-type and mutant strains in the absence of other amino acid sources; however, both the wild-type and transporter mutants were unable to utilize 5- or 10-amino-acid peptides as their sole source of amino acids in minimal defined medium (data not shown) (39). Our data suggest that without the Opp and App transporters, sporulation-specific gene programming is initiated, likely due to intracellular signals of poor nutritional and environmental conditions. Finally, the regulation of opp and app expression is different in C. difficile and B. subtilis (see Fig. S2D in the supplemental material), suggesting that opp and app are not influencing sporulation similarly in both species.
FIG 8.
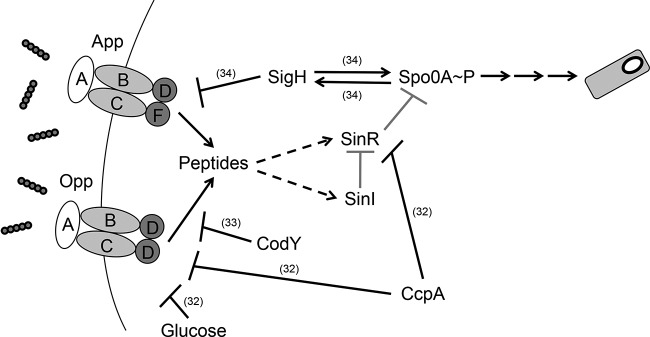
Proposed model of Opp and App influence on sporulation initiation in C. difficile. Opp and App mediate the uptake of peptides as a nutrient source. The acquisition of peptides indirectly influences both sinR and sinI gene expression through an unknown mechanism which represses sporulation-specific gene expression. Gray arrows, putative regulatory effects, hatched arrows, indirect effects. References for experimentally proven interactions are denoted in parentheses.
In contrast to B. subtilis, mutants defective in the Opp and App transporters express the genes for certain sporulation regulatory proteins (Spo0A, σF, and σE) prematurely, suggesting that their normal activities cause the uptake of peptide nutrients that block the initiation of sporulation (Fig. 4). Although the complete genetic pathway through which transporter activity signals sporulation initiation remains unclear, our data suggest that these signals may involve both SinR and SinI (Fig. 5). In B. subtilis, SinR functions as a homotetramer and directly represses a number of genes involved in sporulation initiation, including spo0A (82). However, SinR repression is relieved by SinI, which inhibits SinR-mediated repression by disrupting the SinR homotetramer and forming an inactive heterodimer with SinR (83). In C. difficile, sinR and sinI (CD2214 and CD2215, respectively) appear to form an operon. Our preliminary data indicate that the increase in sinI transcript levels was greater than the increase in sinR levels in the app and opp app mutants as cells entered sporulation (Fig. 5). These data suggest that sinR and sinI are differentially expressed, as they are in B. subtilis (92). A greater increase in SinI levels than SinR levels would allow the efficient deactivation of SinR-mediated spo0A repression if these proteins function like the B. subtilis orthologs. Even a minimal increase in spo0A transcription and subsequent activation may lead to the increases in transcription observed for the phosphorylated Spo0A (Spo0A∼P)-dependent gene, sigE (Fig. 4E), and influence overall sporulation frequencies in the transporter mutants.
The regulatory pathway through which the intracellular amino acid available is signaled to initiate sporulation is unknown. Our data suggest that CodY and CcpA are not involved in this regulatory pathway, as expression of many direct targets, including the tcdA and tcdB genes (76, 77), are unchanged or nonuniformly changed in the transporter mutants (Fig. 7; see also Fig. S6 in the supplemental material). Determining the intermediates of the genetic pathway linking Opp and App transporter activity and sporulation initiation would provide further evidence of the environmental signals that regulate the initiation of spore formation and would fill a gap in the regulatory mechanisms governing the early stages of sporulation in C. difficile as well as nutrient acquisition.
The Opp and App transporters are not expected to be the only mechanisms by which C. difficile could obtain extracellular amino acids. C. difficile has orthologs of several individual amino acid importer proteins found in other species, as well as putative di- and tripeptide transporters. Additional oligopeptide transporters present in other spore-forming species include the Dpp and DtpT transporter systems (93, 94). However, the C. difficile genome does not contain a full dpp transporter complement. The C. difficile genome possesses two DtpT orthologs (CD2260 and CD3036), but their role in peptide uptake is unknown.
In our assays, the Opp transporter did not have a significant effect on sporulation in vitro. However, overexpression of the opp operon from its native promoter in the opp app mutant fully restored wild-type levels of sporulation, indicating that this operon encodes a functional oligopeptide transporter (see Fig. S4 in the supplemental material). In addition, the effects of the loss of opp function in vivo were similar to those of the loss of app function, and the opp app double mutant resulted in more fecal spores and a shorter time to morbidity compared to the number of fecal spores and time to morbidity obtained with the single mutants in the hamster model of infection (Fig. 6). Interestingly, a functional oppF gene is required in B. subtilis for competence but not for sporulation (61, 62). However, no natural competence system in C. difficile has been described, nor does the genome contain many of the orthologs required for competence in Bacillus species (53).
It is unclear why the transporter mutants are hypervirulent, despite no apparent increase in toxin expression within the population, in vitro or in vivo (Fig. 7). Our data do not account for differences in secreted toxin between the transporter mutants and the parent strain. When these experiments were performed, we did not anticipate the increased virulence of the mutants or the need to acquire additional samples for toxin assays. However, no difference in the rate of lysis, which would result in toxin release, was observed between the parent strain and the transporter mutants in vitro (see Fig. S7 in the supplemental material). It is possible that the inability to import peptides as a nutrient source affects growth rates or results in the upregulation of other virulence factors, such as motility or adherence, in vivo. We did not observe a difference in growth rates (Fig. 2D) or in motility (data not shown) between the parent strain and the transporter mutants in vitro. Although inactivation of Opp and App results in increased sporulation and increased virulence in vivo, it does not necessarily suggest that these phenotypes are controlled through the same regulatory pathways. Further studies are needed to determine how nutrient limitation and increased sporulation contribute to more severe disease in vivo.
Finally, differences in ecological niches could explain much of the divergence in the genetic mechanisms of spore initiation and patterns of gene expression between bacterial sporeformers. We hypothesize that C. difficile does not require a quorum-sensing system to signal sporulation because C. difficile never inhabits an environment where cell population density directly correlates with nutrient availability. Rather, C. difficile relies on directly linking the availability of environmental nutrients, such as amino acids, to the regulatory mechanisms controlling sporulation initiation. This direct link between nutrient availability and sporulation allows C. difficile to quickly respond to changing conditions as the organism transits through the gastrointestinal tract. The findings presented here provide the first evidence of a nutritional link to spore formation in C. difficile and represent a mechanism that could potentially be exploited to prevent spore formation and, thereby, the spread of the pathogen.
Supplementary Material
ACKNOWLEDGMENTS
We give special thanks to Charles Moran, Linc Sonenshein, and members of the S. M. McBride lab for helpful suggestions and discussions during the course of this work, Aimee Shen for the spo0A plasmid, and Jeremy Boss for use of the Bio-Rad CFX96 real-time PCR detection system.
This research was supported by the U.S. National Institutes of Health through research grants DK087763, DK101870, and AI109526 to S.M.M. and training grant AI106699 to K.L.N. Microscopy studies were supported in part by the Emory University Integrated Cellular Imaging Microscopy Core.
The content of this article is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Footnotes
Published ahead of print 28 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.02323-14.
REFERENCES
- 1. Deakin LJ, Clare S, Fagan RP, Dawson LF, Pickard DJ, West MR, Wren BW, Fairweather NF, Dougan G, Lawley TD. 2012. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect. Immun. 80:2704–2711. 10.1128/IAI.00147-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paredes CJ, Alsaker KV, Papoutsakis ET. 2005. A comparative genomic view of clostridial sporulation and physiology. Nat. Rev. Microbiol. 3:969–978. 10.1038/nrmicro1288 [DOI] [PubMed] [Google Scholar]
- 3. Underwood S, Guan S, Vijayasubhash V, Baines SD, Graham L, Lewis RJ, Wilcox MH, Stephenson K. 2009. Characterization of the sporulation initiation pathway of Clostridium difficile and its role in toxin production. J. Bacteriol. 191:7296–7305. 10.1128/JB.00882-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones SW, Paredes CJ, Tracy B, Cheng N, Sillers R, Senger RS, Papoutsakis ET. 2008. The transcriptional program underlying the physiology of clostridial sporulation. Genome Biol. 9:R114. 10.1186/gb-2008-9-7-r114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steiner E, Scott J, Minton NP, Winzer K. 2012. An agr quorum sensing system that regulates granulose formation and sporulation in Clostridium acetobutylicum. Appl. Environ. Microbiol. 78:1113–1122. 10.1128/AEM.06376-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Durre P, Hollergschwandner C. 2004. Initiation of endospore formation in Clostridium acetobutylicum. Anaerobe 10:69–74. 10.1016/j.anaerobe.2003.11.001 [DOI] [PubMed] [Google Scholar]
- 7. Paredes-Sabja D, Shen A, Sorg JA. 2014. Clostridium difficile spore biology: sporulation, germination, and spore structural proteins. Trends Microbiol. 22:406–416. 10.1016/j.tim.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edwards AN, McBride SM. 9 June 2014. Initiation of sporulation in Clostridium difficile: a twist on the classic model. FEMS Microbiol. Lett. 10.1111/1574-6968.12499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sonenshein AL. 2000. Control of sporulation initiation in Bacillus subtilis. Curr. Opin. Microbiol. 3:561–566. 10.1016/S1369-5274(00)00141-7 [DOI] [PubMed] [Google Scholar]
- 10. Perego M, Hoch JA. 1996. Cell-cell communication regulates the effects of protein aspartate phosphatases on the phosphorelay controlling development in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 93:1549–1553. 10.1073/pnas.93.4.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Magnuson R, Solomon J, Grossman AD. 1994. Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell 77:207–216. 10.1016/0092-8674(94)90313-1 [DOI] [PubMed] [Google Scholar]
- 12. Lazazzera BA, Solomon JM, Grossman AD. 1997. An exported peptide functions intracellularly to contribute to cell density signaling in B. subtilis. Cell 89:917–925. 10.1016/S0092-8674(00)80277-9 [DOI] [PubMed] [Google Scholar]
- 13. Perego M. 1997. A peptide export-import control circuit modulating bacterial development regulates protein phosphatases of the phosphorelay. Proc. Natl. Acad. Sci. U. S. A. 94:8612–8617. 10.1073/pnas.94.16.8612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perego M, Hanstein C, Welsh KM, Djavakhishvili T, Glaser P, Hoch JA. 1994. Multiple protein-aspartate phosphatases provide a mechanism for the integration of diverse signals in the control of development in B. subtilis. Cell 79:1047–1055. 10.1016/0092-8674(94)90035-3 [DOI] [PubMed] [Google Scholar]
- 15. Hiles ID, Gallagher MP, Jamieson DJ, Higgins CF. 1987. Molecular characterization of the oligopeptide permease of Salmonella typhimurium. J. Mol. Biol. 195:125–142. 10.1016/0022-2836(87)90332-9 [DOI] [PubMed] [Google Scholar]
- 16. Pearce SR, Mimmack ML, Gallagher MP, Gileadi U, Hyde SC, Higgins CF. 1992. Membrane topology of the integral membrane components, OppB and OppC, of the oligopeptide permease of Salmonella typhimurium. Mol. Microbiol. 6:47–57. 10.1111/j.1365-2958.1992.tb00836.x [DOI] [PubMed] [Google Scholar]
- 17. Pearce BJ, Naughton AM, Masure HR. 1994. Peptide permeases modulate transformation in Streptococcus pneumoniae. Mol. Microbiol. 12:881–892. 10.1111/j.1365-2958.1994.tb01076.x [DOI] [PubMed] [Google Scholar]
- 18. Solomon JM, Lazazzera BA, Grossman AD. 1996. Purification and characterization of an extracellular peptide factor that affects two different developmental pathways in Bacillus subtilis. Genes Dev. 10:2014–2024. 10.1101/gad.10.16.2014 [DOI] [PubMed] [Google Scholar]
- 19. Ruhfel RE, Manias DA, Dunny GM. 1993. Cloning and characterization of a region of the Enterococcus faecalis conjugative plasmid, pCF10, encoding a sex pheromone-binding function. J. Bacteriol. 175:5253–5259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leonard BA, Podbielski A, Hedberg PJ, Dunny GM. 1996. Enterococcus faecalis pheromone binding protein, PrgZ, recruits a chromosomal oligopeptide permease system to import sex pheromone cCF10 for induction of conjugation. Proc. Natl. Acad. Sci. U. S. A. 93:260–264. 10.1073/pnas.93.1.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gominet M, Slamti L, Gilois N, Rose M, Lereclus D. 2001. Oligopeptide permease is required for expression of the Bacillus thuringiensis plcR regulon and for virulence. Mol. Microbiol. 40:963–975. 10.1046/j.1365-2958.2001.02440.x [DOI] [PubMed] [Google Scholar]
- 22. Slamti L, Lereclus D. 2002. A cell-cell signaling peptide activates the PlcR virulence regulon in bacteria of the Bacillus cereus group. EMBO J. 21:4550–4559. 10.1093/emboj/cdf450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guyer CA, Morgan DG, Staros JV. 1986. Binding specificity of the periplasmic oligopeptide-binding protein from Escherichia coli. J. Bacteriol. 168:775–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tame JR, Murshudov GN, Dodson EJ, Neil TK, Dodson GG, Higgins CF, Wilkinson AJ. 1994. The structural basis of sequence-independent peptide binding by OppA protein. Science 264:1578–1581. 10.1126/science.8202710 [DOI] [PubMed] [Google Scholar]
- 25. Kunji ER, Hagting A, De Vries CJ, Juillard V, Haandrikman AJ, Poolman B, Konings WN. 1995. Transport of beta-casein-derived peptides by the oligopeptide transport system is a crucial step in the proteolytic pathway of Lactococcus lactis. J. Biol. Chem. 270:1569–1574. 10.1074/jbc.270.4.1569 [DOI] [PubMed] [Google Scholar]
- 26. Sleigh SH, Seavers PR, Wilkinson AJ, Ladbury JE, Tame JR. 1999. Crystallographic and calorimetric analysis of peptide binding to OppA protein. J. Mol. Biol. 291:393–415. 10.1006/jmbi.1999.2929 [DOI] [PubMed] [Google Scholar]
- 27. Lanfermeijer FC, Detmers FJ, Konings WN, Poolman B. 2000. On the binding mechanism of the peptide receptor of the oligopeptide transport system of Lactococcus lactis. EMBO J. 19:3649–3656. 10.1093/emboj/19.14.3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nisman B, Raynaud M, Cohen GN. 1948. Extension of the Stickland reaction to several bacterial species. Arch. Biochem. 16:473. [PubMed] [Google Scholar]
- 29. Bouillaut L, Self WT, Sonenshein AL. 2013. Proline-dependent regulation of Clostridium difficile Stickland metabolism. J. Bacteriol. 195:844–854. 10.1128/JB.01492-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stickland LH. 1934. Studies in the metabolism of the strict anaerobes (genus Clostridium): the chemical reactions by which Cl. sporogenes obtains its energy. Biochem. J. 28:1746–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stickland LH. 1935. Studies in the metabolism of the strict anaerobes (genus Clostridium): the oxidation of alanine by Cl. sporogenes. IV. The reduction of glycine by Cl. sporogenes. Biochem. J. 29:889–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stickland LH. 1935. Studies in the metabolism of the strict anaerobes (genus Clostridium): the reduction of proline by Cl. sporogenes. Biochem. J. 29:288–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith CJ, Markowitz SM, Macrina FL. 1981. Transferable tetracycline resistance in Clostridium difficile. Antimicrob. Agents Chemother. 19:997–1003. 10.1128/AAC.19.6.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Purcell EB, McKee RW, McBride SM, Waters CM, Tamayo R. 2012. Cyclic diguanylate inversely regulates motility and aggregation in Clostridium difficile. J. Bacteriol. 194:3307–3316. 10.1128/JB.00100-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sorg JA, Dineen SS. 2009. Laboratory maintenance of Clostridium difficile. Curr. Protoc. Microbiol. Chapter 9:Unit 9A.1. 10.1002/9780471729259.mc09a01s12 [DOI] [PubMed] [Google Scholar]
- 36. Putnam EE, Nock AM, Lawley TD, Shen A. 2013. SpoIVA and SipL are Clostridium difficile spore morphogenetic proteins. J. Bacteriol. 195:1214–1225. 10.1128/JB.02181-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bouillaut L, McBride SM, Sorg JA. 2011. Genetic manipulation of Clostridium difficile. Curr. Protoc. Microbiol. Chapter 9:Unit 9A.2. 10.1002/9780471729259.mc09a02s20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Edwards AN, Suarez JM, McBride SM. 2013. Culturing and maintaining Clostridium difficile in an anaerobic environment. J. Vis. Exp. 2013:e50787. 10.3791/50787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karlsson S, Burman LG, Akerlund T. 1999. Suppression of toxin production in Clostridium difficile VPI 10463 by amino acids. Microbiology 145(Pt 7):1683–1693. 10.1099/13500872-145-7-1683 [DOI] [PubMed] [Google Scholar]
- 40. Luria SE, Burrous JW. 1957. Hybridization between Escherichia coli and Shigella. J. Bacteriol. 74:461–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stabler RA, He M, Dawson L, Martin M, Valiente E, Corton C, Lawley TD, Sebaihia M, Quail MA, Rose G, Gerding DN, Gibert M, Popoff MR, Parkhill J, Dougan G, Wren BW. 2009. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol. 10:R102. 10.1186/gb-2009-10-9-r102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dineen SS, McBride SM, Sonenshein AL. 2010. Integration of metabolism and virulence by Clostridium difficile CodY. J. Bacteriol. 192:5350–5362. 10.1128/JB.00341-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Karberg M, Guo H, Zhong J, Coon R, Perutka J, Lambowitz AM. 2001. Group II introns as controllable gene targeting vectors for genetic manipulation of bacteria. Nat. Biotechnol. 19:1162–1167. 10.1038/nbt1201-1162 [DOI] [PubMed] [Google Scholar]
- 44. Ho TD, Ellermeier CD. 2011. PrsW is required for colonization, resistance to antimicrobial peptides, and expression of extracytoplasmic function sigma factors in Clostridium difficile. Infect. Immun. 79:3229–3238. 10.1128/IAI.00019-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP. 2007. The ClosTron: a universal gene knock-out system for the genus Clostridium. J. Microbiol. Methods 70:452–464. 10.1016/j.mimet.2007.05.021 [DOI] [PubMed] [Google Scholar]
- 46. McBride SM, Sonenshein AL. 2011. The dlt operon confers resistance to cationic antimicrobial peptides in Clostridium difficile. Microbiology 157:1457–1465. 10.1099/mic.0.045997-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saujet L, Pereira FC, Serrano M, Soutourina O, Monot M, Shelyakin PV, Gelfand MS, Dupuy B, Henriques AO, Martin-Verstraete I. 2013. Genome-wide analysis of cell type-specific gene transcription during spore formation in Clostridium difficile. PLoS Genet. 9:e1003756. 10.1371/journal.pgen.1003756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McBride SM, Sonenshein AL. 2011. Identification of a genetic locus responsible for antimicrobial peptide resistance in Clostridium difficile. Infect. Immun. 79:167–176. 10.1128/IAI.00731-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Suarez JM, Edwards AN, McBride SM. 2013. The Clostridium difficile cpr locus is regulated by a noncontiguous two-component system in response to type A and B lantibiotics. J. Bacteriol. 195:2621–2631. 10.1128/JB.00166-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3:1101–1108. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 51. Bartlett JG, Onderdonk AB, Cisneros RL, Kasper DL. 1977. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J. Infect. Dis. 136:701–705. 10.1093/infdis/136.5.701 [DOI] [PubMed] [Google Scholar]
- 52. Chang TW, Bartlett JG, Gorbach SL, Onderdonk AB. 1978. Clindamycin-induced enterocolitis in hamsters as a model of pseudomembranous colitis in patients. Infect. Immun. 20:526–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sebaihia M, Wren BW, Mullany P, Fairweather NF, Minton N, Stabler R, Thomson NR, Roberts AP, Cerdeno-Tarraga AM, Wang H, Holden MT, Wright A, Churcher C, Quail MA, Baker S, Bason N, Brooks K, Chillingworth T, Cronin A, Davis P, Dowd L, Fraser A, Feltwell T, Hance Z, Holroyd S, Jagels K, Moule S, Mungall K, Price C, Rabbinowitsch E, Sharp S, Simmonds M, Stevens K, Unwin L, Whithead S, Dupuy B, Dougan G, Barrell B, Parkhill J. 2006. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 38:779–786. 10.1038/ng1830 [DOI] [PubMed] [Google Scholar]
- 54. Monot M, Boursaux-Eude C, Thibonnier M, Vallenet D, Moszer I, Medigue C, Martin-Verstraete I, Dupuy B. 2011. Reannotation of the genome sequence of Clostridium difficile strain 630. J. Med. Microbiol. 60:1193–1199. 10.1099/jmm.0.030452-0 [DOI] [PubMed] [Google Scholar]
- 55. Doeven MK, Kok J, Poolman B. 2005. Specificity and selectivity determinants of peptide transport in Lactococcus lactis and other microorganisms. Mol. Microbiol. 57:640–649. 10.1111/j.1365-2958.2005.04698.x [DOI] [PubMed] [Google Scholar]
- 56. Monnet V. 2003. Bacterial oligopeptide-binding proteins. Cell. Mol. Life Sci. 60:2100–2114. 10.1007/s00018-003-3054-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Koide A, Hoch JA. 1994. Identification of a second oligopeptide transport system in Bacillus subtilis and determination of its role in sporulation. Mol. Microbiol. 13:417–426. 10.1111/j.1365-2958.1994.tb00436.x [DOI] [PubMed] [Google Scholar]
- 58. Peltoniemi K, Vesanto E, Palva A. 2002. Genetic characterization of an oligopeptide transport system from Lactobacillus delbrueckii subsp. bulgaricus. Arch. Microbiol. 177:457–467. 10.1007/s00203-002-0411-9 [DOI] [PubMed] [Google Scholar]
- 59. Antunes A, Camiade E, Monot M, Courtois E, Barbut F, Sernova NV, Rodionov DA, Martin-Verstraete I, Dupuy B. 2012. Global transcriptional control by glucose and carbon regulator CcpA in Clostridium difficile. Nucleic Acids Res. 40:10701–10718. 10.1093/nar/gks864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saujet L, Monot M, Dupuy B, Soutourina O, Martin-Verstraete I. 2011. The key sigma factor of transition phase, SigH, controls sporulation, metabolism, and virulence factor expression in Clostridium difficile. J. Bacteriol. 193:3186–3196. 10.1128/JB.00272-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Perego M, Higgins CF, Pearce SR, Gallagher MP, Hoch JA. 1991. The oligopeptide transport system of Bacillus subtilis plays a role in the initiation of sporulation. Mol. Microbiol. 5:173–185. 10.1111/j.1365-2958.1991.tb01838.x [DOI] [PubMed] [Google Scholar]
- 62. Rudner DZ, LeDeaux JR, Ireton K, Grossman AD. 1991. The spo0K locus of Bacillus subtilis is homologous to the oligopeptide permease locus and is required for sporulation and competence. J. Bacteriol. 173:1388–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Burns DA, Heap JT, Minton NP. 2010. The diverse sporulation characteristics of Clostridium difficile clinical isolates are not associated with type. Anaerobe 16:618–622. 10.1016/j.anaerobe.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 64. Boudry P, Gracia C, Monot M, Caillet J, Saujet L, Hajnsdorf E, Dupuy B, Martin-Verstraete I, Soutourina O. 30 June 2014. Pleiotropic role of the RNA chaperone protein Hfq in the human pathogen Clostridium difficile. J. Bacteriol. 10.1128/JB.01923-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pogliano J, Osborne N, Sharp MD, Abanes-De Mello A, Perez A, Sun YL, Pogliano K. 1999. A vital stain for studying membrane dynamics in bacteria: a novel mechanism controlling septation during Bacillus subtilis sporulation. Mol. Microbiol. 31:1149–1159. 10.1046/j.1365-2958.1999.01255.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McBride SM, Rubio A, Wang L, Haldenwang WG. 2005. Contributions of protein structure and gene position to the compartmentalization of the regulatory proteins sigma(E) and SpoIIE in sporulating Bacillus subtilis. Mol. Microbiol. 57:434–451. 10.1111/j.1365-2958.2005.04712.x [DOI] [PubMed] [Google Scholar]
- 67. Chastanet A, Losick R. 2011. Just-in-time control of Spo0A synthesis in Bacillus subtilis by multiple regulatory mechanisms. J. Bacteriol. 193:6366–6374. 10.1128/JB.06057-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Errington J. 2003. Regulation of endospore formation in Bacillus subtilis. Nat. Rev. Microbiol. 1:117–126. 10.1038/nrmicro750 [DOI] [PubMed] [Google Scholar]
- 69. Fimlaid KA, Bond JP, Schutz KC, Putnam EE, Leung JM, Lawley TD, Shen A. 2013. Global analysis of the sporulation pathway of Clostridium difficile. PLoS Genet. 9:e1003660. 10.1371/journal.pgen.1003660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pereira FC, Saujet L, Tome AR, Serrano M, Monot M, Couture-Tosi E, Martin-Verstraete I, Dupuy B, Henriques AO. 2013. The spore differentiation pathway in the enteric pathogen Clostridium difficile. PLoS Genet. 9:e1003782. 10.1371/journal.pgen.1003782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Haldenwang WG. 1995. The sigma factors of Bacillus subtilis. Microbiol. Rev. 59:1–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Satola S, Kirchman PA, Moran CP., Jr 1991. Spo0A binds to a promoter used by sigma A RNA polymerase during sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 88:4533–4537. 10.1073/pnas.88.10.4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rong S, Rosenkrantz MS, Sonenshein AL. 1986. Transcriptional control of the Bacillus subtilis spoIID gene. J. Bacteriol. 165:771–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Haraldsen JD, Sonenshein AL. 2003. Efficient sporulation in Clostridium difficile requires disruption of the sigmaK gene. Mol. Microbiol. 48:811–821. 10.1046/j.1365-2958.2003.03471.x [DOI] [PubMed] [Google Scholar]
- 75. Koide A, Perego M, Hoch JA. 1999. ScoC regulates peptide transport and sporulation initiation in Bacillus subtilis. J. Bacteriol. 181:4114–4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dineen SS, Villapakkam AC, Nordman JT, Sonenshein AL. 2007. Repression of Clostridium difficile toxin gene expression by CodY. Mol. Microbiol. 66:206–219. 10.1111/j.1365-2958.2007.05906.x [DOI] [PubMed] [Google Scholar]
- 77. Antunes A, Martin-Verstraete I, Dupuy B. 2011. CcpA-mediated repression of Clostridium difficile toxin gene expression. Mol. Microbiol. 79:882–899. 10.1111/j.1365-2958.2010.07495.x [DOI] [PubMed] [Google Scholar]
- 78. Dupuy B, Sonenshein AL. 1998. Regulated transcription of Clostridium difficile toxin genes. Mol. Microbiol. 27:107–120. 10.1046/j.1365-2958.1998.00663.x [DOI] [PubMed] [Google Scholar]
- 79. Sonenshein AL. 2007. Control of key metabolic intersections in Bacillus subtilis. Nat. Rev. Microbiol. 5:917–927. 10.1038/nrmicro1772 [DOI] [PubMed] [Google Scholar]
- 80. Chateau A, van Schaik W, Joseph P, Handke LD, McBride SM, Smeets FM, Sonenshein AL, Fouet A. 2013. Identification of CodY targets in Bacillus anthracis by genome-wide in vitro binding analysis. J. Bacteriol. 195:1204–1213. 10.1128/JB.02041-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chiang C, Bongiorni C, Perego M. 2011. Glucose-dependent activation of Bacillus anthracis toxin gene expression and virulence requires the carbon catabolite protein CcpA. J. Bacteriol. 193:52–62. 10.1128/JB.01656-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mandic-Mulec I, Doukhan L, Smith I. 1995. The Bacillus subtilis SinR protein is a repressor of the key sporulation gene spo0A. J. Bacteriol. 177:4619–4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bai U, Mandic-Mulec I, Smith I. 1993. SinI modulates the activity of SinR, a developmental switch protein of Bacillus subtilis, by protein-protein interaction. Genes Dev. 7:139–148. 10.1101/gad.7.1.139 [DOI] [PubMed] [Google Scholar]
- 84. Janoir C, Deneve C, Bouttier S, Barbut F, Hoys S, Caleechum L, Chapeton-Montes D, Pereira F, Henriques A, Collignon A, Monot M, Dupuy B. 2013. Adaptive strategies and pathogenesis of Clostridium difficile from in vivo transcriptomics. Infect. Immun. 81:3757–3769. 10.1128/IAI.00515-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wilson KH, Sheagren JN, Freter R. 1985. Population dynamics of ingested Clostridium difficile in the gastrointestinal tract of the Syrian hamster. J. Infect. Dis. 151:355–361. 10.1093/infdis/151.2.355 [DOI] [PubMed] [Google Scholar]
- 86. Douce G, Goulding D. 2010. Refinement of the hamster model of Clostridium difficile disease. Methods Mol. Biol. 646:215–227. 10.1007/978-1-60327-365-7_14 [DOI] [PubMed] [Google Scholar]
- 87. Best EL, Freeman J, Wilcox MH. 2012. Models for the study of Clostridium difficile infection. Gut Microbes 3:145–167. 10.4161/gmic.19526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Razaq N, Sambol S, Nagaro K, Zukowski W, Cheknis A, Johnson S, Gerding DN. 2007. Infection of hamsters with historical and epidemic BI types of Clostridium difficile. J. Infect. Dis. 196:1813–1819. 10.1086/523106 [DOI] [PubMed] [Google Scholar]
- 89. Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. 2010. The role of toxin A and toxin B in Clostridium difficile infection. Nature 467:711–713. 10.1038/nature09397 [DOI] [PubMed] [Google Scholar]
- 90. Yamakawa K, Karasawa T, Ikoma S, Nakamura S. 1996. Enhancement of Clostridium difficile toxin production in biotin-limited conditions. J. Med. Microbiol. 44:111–114. 10.1099/00222615-44-2-111 [DOI] [PubMed] [Google Scholar]
- 91. Errington J. 1993. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol. Rev. 57:1–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gaur NK, Cabane K, Smith I. 1988. Structure and expression of the Bacillus subtilis sin operon. J. Bacteriol. 170:1046–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mathiopoulos C, Mueller JP, Slack FJ, Murphy CG, Patankar S, Bukusoglu G, Sonenshein AL. 1991. A Bacillus subtilis dipeptide transport system expressed early during sporulation. Mol. Microbiol. 5:1903–1913. 10.1111/j.1365-2958.1991.tb00814.x [DOI] [PubMed] [Google Scholar]
- 94. Caldwell R, Sapolsky R, Weyler W, Maile RR, Causey SC, Ferrari E. 2001. Correlation between Bacillus subtilis scoC phenotype and gene expression determined using microarrays for transcriptome analysis. J. Bacteriol. 183:7329–7340. 10.1128/JB.183.24.7329-7340.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. George WL, Sutter VL, Citron D, Finegold SM. 1979. Selective and differential medium for isolation of Clostridium difficile. J. Clin. Microbiol. 9:214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wilson KH, Silva J, Fekety FR. 1981. Suppression of Clostridium difficile by normal hamster cecal flora and prevention of antibiotic-associated cecitis. Infect. Immun. 34:626–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wust J, Hardegger U. 1983. Transferable resistance to clindamycin, erythromycin, and tetracycline in Clostridium difficile. Antimicrob. Agents Chemother. 23:784–786. 10.1128/AAC.23.5.784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hussain HA, Roberts AP, Mullany P. 2005. Generation of an erythromycin-sensitive derivative of Clostridium difficile strain 630 (630deltaerm) and demonstration that the conjugative transposon Tn916DeltaE enters the genome of this strain at multiple sites. J. Med. Microbiol. 54:137–141. 10.1099/jmm.0.45790-0 [DOI] [PubMed] [Google Scholar]
- 99. Thomas CM, Smith CA. 1987. Incompatibility group P plasmids: genetics, evolution, and use in genetic manipulation. Annu. Rev. Microbiol. 41:77–101. 10.1146/annurev.mi.41.100187.000453 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.