The presence or absence of Rh antigens, abundant components of the red blood cell (RBC) plasma membrane, is a major cause of blood transfusion incompatibility and of hemolytic disease of the newborn. As deduced from studies on Rhnull erythrocytes, the Rh complex of RBC includes the Rh-associated glycoprotein RhAG, the Rh polypeptides, CD47, LW proteins, and glycophorin B (1). RhAG is essential for the assembly of the Rh complex, and mutations in RhAG result in the Rhnull syndrome (2). Despite their significance and intensive research, the biological role of the Rh protein complex remains controversial. This controversy may be accounted for by the fact that RhAG homologues are found in other mammalian tissues and in many lower organisms (3), including unicellular eukaryotes (4). Studies by Marini and colleagues (3) identified some homology (20–27% identity) between RhAG and Mep/Amt ammonium and methylammonium transporters in various organisms and suggested that the erythroid RhAG may function as an ammonium transporter (3). This finding was supported by the observation that RhAG (and its kidney-located homologue) could complement a yeast mutant impaired in ammonium uptake. Further, RhAG enhanced efflux of a preloaded methylammonium from yeast, suggesting that it might be involved in ammonium export as well (5). The notion that RhAG and its homologues are involved in 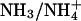 transfer gained additional support by the observation that expression of RhCG, a mammalian nonerythroid homologue of RhAG, along the rat nephron, matched that expected from ammonium excretion activities of the respective nephron sections (6). An exciting contribution from the laboratory of S. Kustu (7) in this issue of PNAS provides supporting evidence for their earlier suggestion (4) that RH1 of the green alga Chlamydomonas reinhardtii, which is highly homologous to RhAG, may in fact function as a CO2 channel. In their earlier study Soupene et al. (4) showed that the expression of rh1 was strongly up-regulated after exposure of Chlamydomonas cells to high (3% vol/vol) levels of CO2. In the present study (7) the authors used RNA interference technology to lower the abundance of RH1 mRNA. The three lines selected for further analysis did not express rh1 but induced low-CO2-dependent genes normally when grown in an air level of CO2 and showed reduced growth under high-CO2 conditions. Uptake of methylammonium hardly differed between the wild-type and the rh1 RNA interference strains.
transfer gained additional support by the observation that expression of RhCG, a mammalian nonerythroid homologue of RhAG, along the rat nephron, matched that expected from ammonium excretion activities of the respective nephron sections (6). An exciting contribution from the laboratory of S. Kustu (7) in this issue of PNAS provides supporting evidence for their earlier suggestion (4) that RH1 of the green alga Chlamydomonas reinhardtii, which is highly homologous to RhAG, may in fact function as a CO2 channel. In their earlier study Soupene et al. (4) showed that the expression of rh1 was strongly up-regulated after exposure of Chlamydomonas cells to high (3% vol/vol) levels of CO2. In the present study (7) the authors used RNA interference technology to lower the abundance of RH1 mRNA. The three lines selected for further analysis did not express rh1 but induced low-CO2-dependent genes normally when grown in an air level of CO2 and showed reduced growth under high-CO2 conditions. Uptake of methylammonium hardly differed between the wild-type and the rh1 RNA interference strains.
RH1 of the green alga Chlamydomonas reinhardtii may function as a CO2 channel.
Many photosynthetic microorganisms possess inducible mechanisms that concentrate CO2 at the carboxylation site, compensating for the relatively low affinity of ribulose-1,5-bisphosphate carboxylase/oxygenase for CO2 and allowing acclimation to a wide range of CO2 concentrations (see refs. 8–16 and references therein). The organization of the carboxysomes in prokaryotes and the pyrenoids in eukaryotes, and the presence of membrane mechanisms for inorganic carbon (Ci) uptake, are central to the activity of the CO2-concentrating mechanism. The presence of multiple Ci-transporting systems in cyanobacteria has been indicated (17), but little is known about the mechanism of Ci uptake in eukaryotes, such as the green alga Chlamydomonas (13). As in most other photosynthetic organisms examined to date, Chlamydomonas can use either CO2 or 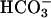 or both (13). Kinetic data based on initial rates of transport are available for Ci uptake in some organisms but difficult to obtain for Chlamydomonas, because the steadystate internal Ci concentration is attained rapidly. This finding is in agreement with the proposal that CO2 can cross the membrane quickly by specific channels (7).
or both (13). Kinetic data based on initial rates of transport are available for Ci uptake in some organisms but difficult to obtain for Chlamydomonas, because the steadystate internal Ci concentration is attained rapidly. This finding is in agreement with the proposal that CO2 can cross the membrane quickly by specific channels (7).
Facilitation of CO2 formation from 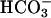 in the periplasmic space by carbonic anhydrase (CA) (18), encoded by Cah1; within the pyrenoid by thylakoid lumen-located CA (10, 19), encoded by Cah3 (ctCA); and possibly the involvement of a mitochondrial-located CA, encoded by mtCA (20), enhances the supply of CO2 to ribulose-1,5-bisphosphate carboxylase/oxygenase (Fig. 1). As opposed to CO2 supply, there is far less information regarding the mechanism of
in the periplasmic space by carbonic anhydrase (CA) (18), encoded by Cah1; within the pyrenoid by thylakoid lumen-located CA (10, 19), encoded by Cah3 (ctCA); and possibly the involvement of a mitochondrial-located CA, encoded by mtCA (20), enhances the supply of CO2 to ribulose-1,5-bisphosphate carboxylase/oxygenase (Fig. 1). As opposed to CO2 supply, there is far less information regarding the mechanism of 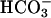 uptake in Chlamydomonas. Nevertheless, isolated chloroplasts of Chlamydomonas can accumulate
uptake in Chlamydomonas. Nevertheless, isolated chloroplasts of Chlamydomonas can accumulate 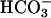 to levels higher than could be accounted for by passive diffusion (13). Furthermore, Ci uptake is higher in chloroplasts isolated from low- than from high-CO2-grown cells (8). Genes involved in Ci uptake by Chlamydomonas are strongly induced when high-CO2-grown cells are transferred to low-CO2 conditions (12, 13, 19, 20). Mutants in which these genes were impaired required high CO2 for growth. In contrast, expression of rh1 is induced under high CO2 and suppressed under low CO2 (4, 7). The function of RH1 as a CO2 channel, as suggested, would allow influx of CO2 when all other (known) means of mediated Ci influx are suppressed (i.e., under high levels of ambient CO2), whereas the absence of RH1 under low CO2 would minimize wasteful leakage of CO2 from the cells. Thus, assignment of RH1 as a CO2 channel is novel to our understanding of the mechanisms and components involved in CO2 acquisition by photo-synthetic microorganisms.
to levels higher than could be accounted for by passive diffusion (13). Furthermore, Ci uptake is higher in chloroplasts isolated from low- than from high-CO2-grown cells (8). Genes involved in Ci uptake by Chlamydomonas are strongly induced when high-CO2-grown cells are transferred to low-CO2 conditions (12, 13, 19, 20). Mutants in which these genes were impaired required high CO2 for growth. In contrast, expression of rh1 is induced under high CO2 and suppressed under low CO2 (4, 7). The function of RH1 as a CO2 channel, as suggested, would allow influx of CO2 when all other (known) means of mediated Ci influx are suppressed (i.e., under high levels of ambient CO2), whereas the absence of RH1 under low CO2 would minimize wasteful leakage of CO2 from the cells. Thus, assignment of RH1 as a CO2 channel is novel to our understanding of the mechanisms and components involved in CO2 acquisition by photo-synthetic microorganisms.
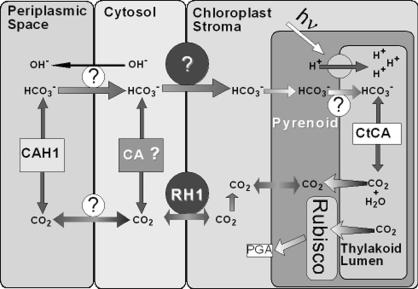
Fig. 1.
A simplified scheme of inorganic carbon uptake and accumulation in C. reinhardtii. The putative function of RH1 as a chloroplast envelope-located CO2 channel is emphasized. The means by which 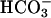 is taken up across the plasmalemma, the chloroplast envelope, and the thylakoid, if any, is not known. Possible involvement of other components, including the mitochondrial-located CA, LIP-36, and pmp-1 (8, 13), is not indicated. For the sake of simplicity, diffusion of CO2 across the lipid bilayer of the various membranes is not shown. Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase; PGA, 3-phosphoglyceric acid.
is taken up across the plasmalemma, the chloroplast envelope, and the thylakoid, if any, is not known. Possible involvement of other components, including the mitochondrial-located CA, LIP-36, and pmp-1 (8, 13), is not indicated. For the sake of simplicity, diffusion of CO2 across the lipid bilayer of the various membranes is not shown. Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase; PGA, 3-phosphoglyceric acid.
Although not yet examined experimentally, the presence of a typical N-terminal transit peptide suggested that RH1 is located within chloroplast envelope (4) (Fig. 1) where the low-CO2-induced LIP-36 also resides. Should RH1 be confined to the chloroplast envelope, transfer of CO2 across the plasmalemma of Chlamydomonas may limit Ci uptake under high-CO2 conditions.
The function of RH1 as a CO2 channel allows influx of CO2 when other means are suppressed.
The possibility that CO2 does not merely diffuse through the bulk lipids within membranes was indicated in experiments where an aquaporin blocker severely inhibited CO2 uptake by Synechococcus sp. strain PCC 7942 (21). Another means of passage of CO2 across the membrane was suggested by Forster and colleagues (22) who provided 4,4′-diisothiocyanato-stilbene-2,2′-disulfonate to RBCs. This treatment resulted in reduced CO2 permeability without affecting the CA activity. 4,4′-Diisothiocyanato-stilbene-2,2′-disulfonate is known to inhibit the 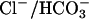 exchanger (band 3) in the RBC mem brane. Recent studies by Tanner (23) and Bruce et al. (24) showed that the two major complexes, Rh and the
exchanger (band 3) in the RBC mem brane. Recent studies by Tanner (23) and Bruce et al. (24) showed that the two major complexes, Rh and the 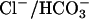 exchanger, are ciated closely assoforming a single macrocomplex that may function as an integrated CO2/O2 gas exchange unit in the erythrocyte membrane.
exchanger, are ciated closely assoforming a single macrocomplex that may function as an integrated CO2/O2 gas exchange unit in the erythrocyte membrane.
Finally, both RBCs and the soil alga Chlamydomonas must cope with high CO2 levels in their surroundings and have apparently adopted a similar channel mechanism to allow efficient exhaustion or uptake of CO2. It is plausible that ability of the alga to acclimate to low CO2 conditions was acquired simultaneously with development of the means to block the expression of rh.
Acknowledgments
This research was supported by grants from the Israel Science Foundation, the German Bundes Ministerium fur Bildung Wissenschaft, Forschung und Technologie, and the Avron-Evenari Minerva Center of Photosynthesis Research.
See companion article on page 7787.
References
- 1.Cartron, J.-P. (1999) Baillieres Clin. Haematol. 12, 655-699. [Google Scholar]
- 2.Avent, N. D. (2001) Trends Mol. Med. 7, 94-96. [DOI] [PubMed] [Google Scholar]
- 3.Marini, A. M., Urrestarazu, A., Beauwens, R. & Andre, B. (1997) Trends Biochem. Sci. 22, 460-461. [DOI] [PubMed] [Google Scholar]
- 4.Soupene, E., King, N., Feild, E., Liu, P., Niyogi, K. K., Huang, C. H. & Kustu, S. (2002) Proc. Natl. Acad. Sci. USA 99, 7769-7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marini, A. M., Matassi, G., Raynal, V., Andre, B., Cartron, J. P. & Cherif-Zahar, B. (2000) Nat. Genet. 26, 341-344. [DOI] [PubMed] [Google Scholar]
- 6.Eladari, D., Cheval, E., Quentin, F., Bertrand, O., Mouro, I., Cherif-Zahar, B., Cartron, J. P., Paillard, M., Doucet, A. & Chambrey, R. (2002) J. Am. Soc. Nephrol. 13, 1999-2008. [DOI] [PubMed] [Google Scholar]
- 7.Soupene, E., Inwood, W. & Kustu, S. (2004) Proc. Natl. Acad. Sci. USA 101, 7787-7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan, A. & Reinhold, L. (1999) Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 539-570. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan, A., Helman, Y., Tchernov, D. & Reinhold, L. (2001) Proc. Natl. Acad. Sci. USA 98, 4817-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raven, J. A. (1997) Plant Cell Environ. 20, 147-154. [Google Scholar]
- 11.Badger, M. R. & Price, G. D. (2003) J. Exp. Bot. 54, 609-622. [DOI] [PubMed] [Google Scholar]
- 12.Moroney, J. V. & Somanchi, A. (1999) Plant Physiol. 119, 9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spalding, M. H., Van, K., Wang, Y. & Nakamura, Y. (2002) Funct. Plant Biol. 29, 221-230. [DOI] [PubMed] [Google Scholar]
- 14.Yoshioka, S., Taniguchi, F., Miura, K., Inoue, T., Yamano, T. & Fukuzawa, H. (2004) Plant Cell, in press. [DOI] [PMC free article] [PubMed]
- 15.Xiang, Y., Zhang, J. & Weeks, D. P. (2001) Proc. Natl. Acad. Sci. USA 98, 5341-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuzawa, H., Miura, K., K., I., Kucho, K. I., Saito, T., Kohinata, T. & Ohyama, K. (2001) Proc. Natl. Acad. Sci. USA 98, 5347-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa, T. & Kaplan, A. (2003) Photosynth. Res. 77, 105-115. [DOI] [PubMed] [Google Scholar]
- 18.Aizawa, K. & Miyachi, S. (1986) FEMS Microbiol. Rev. 39, 215-233. [Google Scholar]
- 19.Karlsson, J., Clarke, A. K., Chen, Z. Y., Hugghins, S. Y., Park, Y. I., Husic, H. D., Moroney, J. V. & Samuelsson, G. (1998) EMBO J. 17, 1208-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eriksson, M., Villand, P., Gardestrom, P. & Samuelsson, G. (1998) Plant Physiol. 116, 637-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tchernov, D., Helman, Y., Keren, N., Luz, B., Ohad, I., Reinhold, L., Ogawa, T. & Kaplan, A. (2001) J. Biol. Chem. 276, 23450-23455. [DOI] [PubMed] [Google Scholar]
- 22.Forster, R. E., Gros, G., Lin, L., Ono, Y. & Wunder, M. (1998) Proc. Natl. Acad. Sci. USA 95, 15815-15820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanner, M. J. A. (2002) Curr. Opin. Hematol. 9, 133-139. [DOI] [PubMed] [Google Scholar]
- 24.Bruce, L. J., Beckmann, R., Ribeiro, M. L., Peters, L. L., Chasis, J. A., Delaunay, J., Mohandas, N., Anstee, D. J. & Tanner, M. J. A. (2003) Blood 101, 4180-4188. [DOI] [PubMed] [Google Scholar]