SUMMARY
Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is a pattern recognition receptor for a variety of endogenous and exogenous ligands. However, LOX-1 function in the host immune response is not fully understood. Here, we report that LOX-1 expressed on dendritic cells (DCs) and B cells promotes humoral responses. On B cells LOX-1 signaling upregulated CCR7, promoting cellular migration towards lymphoid tissues. LOX-1 signaling on DCs licensed the cells to promote B cell differentiation into class-switched plasmablasts, and led to downregulation of chemokine receptor CXCR5 and upregulation of chemokine receptor CCR10 on plasmablasts, which enabled their exit from germinal centers and migration towards local mucosa and skin. Finally, we found that targeting influenza hemagglutinin 1 (HA1) subunit to LOX-1 elicited HA1-specific protective antibody responses in rhesus macaques. Thus, LOX-1 expressed on B cells and DC cells has complementary functions to promote humoral immune responses.
Keywords: Dendritic cells, B cells, LOX-1, Lectin, Oxidized-LDL, Immunity, IgG, IgA, Influenza virus, Hemagglutinin, HA1
INTRODUCTION
Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is a pattern recognition receptor (Huysamen and Brown, 2009). LOX-1 is expressed on the surface of endothelial cells, smooth muscle cells, platelets, fibroblasts, dendritic cells (DCs), B cells, and macrophages (Delneste et al., 2002; Dunn et al., 2008; Huysamen and Brown, 2009; Jeannin et al., 2005; Li et al., 2012; Nickel et al., 2009). LOX-1 expression can be regulated by a variety of stimuli, including proinflammatory and inflammatory cytokines (Chen et al., 2007; Dunn et al., 2008). LOX-1 also belongs to the scavenger receptor family and can recognize both endogenous and exogenous ligands, including modified lipoproteins such as oxidized-LDL (ox-LDL), as well as apoptotic cells, activated platelets and certain bacteria (Huysamen and Brown, 2009; Kakutani et al., 2000; Oka et al., 1998; Parlato et al., 2010; Shimaoka et al., 2001).
The involvement of LOX-1 in host immune responses, especially T cell responses, was previously reported (Delneste et al., 2002; Jeannin et al., 2005; Parlato et al., 2010). LOX-1 expressed on DCs can capture bacterial components, which then co-localize with toll-like receptor 2 (TLR2) to activate DCs, leading to enhanced cellular responses (Jeannin et al., 2005). In addition, antigen delivered to mouse DCs via LOX-1 using antigen conjugated to either anti-mouse LOX-1 (Delneste et al., 2002) or heat shock protein 70 (HSP70) (Xie et al., 2010) elicited antigen-specific CD8+ T cell responses. Parlato et al. (Parlato et al., 2010) also showed that human monocyte-derived DCs capture antigens via LOX-1 and cross-present them to CD8+ T cells. More recently, we have shown that targeting antigens to DCs via LOX-1 can elicit antigen-specific T cell responses (Li et al., 2012).
In consideration of its wide expression and the currently known biological functions of LOX-1 as a c-type lectin-like pattern recognition receptor (PRR), we surmised that LOX-1 could have many more diverse functions in host immune responses. While LOX-1 expression on DCs and B cells is known, the role of LOX-1 in humoral immune responses remains unknown. We therefore explored novel functions of LOX-1 expressed on DCs and B cells in humoral responses. DCs are major antigen-presenting cells that can induce and control the quantity and quality of cellular immune responses. DCs also play an important role in both T-dependent (TD) and T-independent (TI) humoral responses.
In this study, we found that LOX-1 can program DCs and B cells to promote class-switched antibody (Ab) responses. This unique ability of LOX-1 makes it stand out from other lectin-like receptors. We have also investigated the mechanisms by which LOX-1 promotes DC-mediated class-switched B cell responses. Finally, we tested whether targeting influenza HA1 antigen to LOX-1 could elicit HA1-specific protective Ab responses in rhesus macaques.
RESULTS
αLOX-1-Treated DCs Promote B Cell Differentiation into Plasmablasts (PBs)
To study the biology of human LOX-1 (hLOX-1), we generated an αLOX-1 (clone 8B4; IgG1κ) monoclonal antibody (mAb) specific for the ectodomain of hLOX-1, as previously described (Li et al., 2012). The specificity of αLOX-1 mAb was tested by staining 293F cells transfected with a full-length hLOX-1 expression vector (Figure S1A and S1B). This was confirmed by the data showing that αLOX-1 mAb bound to a recombinant hLOX-1 ectodomain-Fc, but not a control fusion protein (human dendritic cell immunoreceptor (hDCIR) ectodomain-Fc fusion; Figure S1C).
αLOX-1 mAb bound to fractions of CD11c+, CD14+, and CD19+, but not CD3+ T cells, in peripheral blood mononuclear cells (PBMCs) (Figure 1A). hLOX-1 was not expressed in human plasmacytoid DCs (pDCs) (Li et al., 2012). We also found that many of the CD11c+ cells localized at marginal zones and extrafollicular areas in human spleen expressed LOX-1 (Figure 1B and Figure S1D). Interestingly, CD11c+LOX-1+ cells in the marginal zone interacted with IgD+ B cells, suggesting that LOX-1 on DCs could contribute to humoral responses.
Figure 1. LOX-1 Promotes DC-Mediated PB Differentiation and Antibody Responses.
(A) Binding of αLOX-1 mAb to CD11c+, CD14+, CD19+ and CD3+ cells in PBMCs from healthy donors.
(B) Immunofluorescence of spleens. Frozen sections of human spleens were stained for αCD11c (green), αLOX-1 (red), αIgD (blue). The outlined area on the left corresponds to the enlargement on the right. Original magnification, 10x (left) or 63x (right).
(C) LOX-1 expression on in vitro monocyte-derived IL-4DCs.
(D) Different numbers of IL-4DCs were incubated overnight in plates coated with 2 μg/ml αLOX-1 (8B4) or control IgG. 1–2×105 CFSE-labeled CD19+ B cells were co-cultured for 12 days in the presence of IL-2. Concentrations of Ig in the culture supernatants were assessed by sandwich ELISA. Three independent experiments using cells from three healthy donors resulted in similar data.
(E) On day 6 of the culture in (D), CD38 expression and CFSE dilution were measured (left). CD19+CFSE−CD38+ B cells were FACS-sorted and cell morphology was examined (middle). B cells were also stained for CD20 (right).
(F) DC and B cell co-cultures in (D) were performed in transwells or single wells. CD38 expression and CFSE dilution were assessed. Numbers of CD38+CD20− B cells were assessed using counting beads. Four independent experiments using cells from two healthy donors were performed. Error bars indicate SD.
(G) On day 6 of the culture in (D), surface expression of CD38 and CD20 on B cells were assessed. Expression of intracellular Pax5 and Blimp-1 was measured. Three independent experiments using cells from different healthy donors showed similar results.
(H) 5×103 IL-4DCs were cultured overnight in plates coated with αLOX-1 (8B4) or control IgG. The amount of IL-6 in the culture supernatants was analyzed by the BeadLyte assay kit (left). Purified CD19+ B cells were incubated in the culture supernatant of αLOX-1- or control IgG-treated DCs for 15 min (right). Cells were lysed and used for western blot analysis of STAT3 and phospho-STAT3 (pSTAT3).
We next tested the roles of LOX-1 in DC-mediated B cell responses. IL-4DCs were generated by culturing monocytes in the presence of GM-CSF and interleukin 4 (IL-4). IL-4DCs expressed surface LOX-1 (Figure 1C). Different numbers of IL-4DCs were incubated overnight in the presence of αLOX-1 or control IgG. CFSE-labeled CD19+ total B cells were then co-cultured for 12 days in the presence of interleukin-2 (IL-2). Figure 1D shows that B cells co-cultured with αLOX-1-treated DCs secreted greater amounts of IgM, IgG, and IgA than B cells co-cultured with control IgG-treated DCs. Furthermore, the amounts of IgM, IgG and IgA in the supernatants correlated with the numbers of DCs in the culture. In addition, αLOX-1-treated DCs were more efficient than control IgG-treated DCs at inducing B cell proliferation and CD38+CD20− PB differentiation (Figure 1E). These PBs displayed eccentric nuclei, which is a typical morphology of plasma cells, but they did not express CD138 (Figure S1E). Figure 1F shows that intercellular interactions between αLOX-1-treated DCs and B cells are important for PB differentiation. In addition to IL-4DCs, IFNDCs generated by culturing monocytes in the presence of GM-CSF and IFNα also expressed LOX-1, but TNFDCs generated with GM-CSF and TNFα did not (upper left, Figure S1F). αLOX-1-treated IFNDCs also enhanced PB differentiation (lower left, Figure S1F) and immunoglobulin (Ig) secretion (right, Figure S1F). αLOX-1 mAb was also able to promote PB differentiation (left, Figure S1G) and Ig secretion (right, Figure S1G) from B cells in PBMC cultures.
Taken together, αLOX-1-treated DCs can promote B cell responses by enhancing PB differentiation and Ig secretion. In addition, intercellular interactions between αLOX-1-treated DCs and B cells play an important role in enhancing B cell responses.
αLOX-1-Treated DCs Control Expression of Pax5 and Blimp-1 Transcription Factors to Generate PBs
Both paired box 5 (Pax5) and B lymphocyte-induced maturation protein 1 (Blimp-1) play important roles in B cell differentiation into plasma cells (Martins and Calame, 2008). B cells co-cultured with αLOX-1-treated DCs downregulated Pax5, but upregulated Blimp-1 expression (Figure 1G). Furthermore, αLOX-1-treated DCs secreted IL-6 (left, Figure 1H). In addition, B cells incubated in the culture supernatant of αLOX-1-treated DCs showed more signal transducer and activator of transcription 3 (STAT3) phosphorylation than B cells incubated in the culture supernatant of control IgG-treated DCs (right, Figure 1H). STAT3 activation is involved in plasma cell differentiation by inducing Blimp-1 expression in B cells (Diehl et al., 2008). αLOX-1-treated DCs did not secrete IL-4, IL-10 or type 1 IFN. Taken together, the enhanced PB differentiation by αLOX-1-treated DCs is supported by Pax5 downregulation and Blimp-1 upregulation along with STAT3 activation in B cells.
B Cells Express Functional LOX-1
Total (CD19+), naïve (CD19+IgD+CD27−), and memory B cells (CD19+IgD−CD27+ and CD19+IgD+CD27+) in peripheral blood expressed similar amounts of surface LOX-1 (Figure 2A). However, LOX-1 expression on total B cells was downregulated by αBCR, αCD40 and CpG treatments (Figure 2B). Figure 2C shows that αLOX-1 mAb alone did not promote PB differentiation, as measured by CD38 expression, with minimal B cell proliferation. However, it did induce B cells to secrete some IgM and IgG, but minimal IgA (Figure 2D); although IgM and IgG concentrations in the supernatants of B cell cultures (Figure 2D) were much lower than those observed in the supernatants of B cells co-cultured with DCs (Figure 1D).
Figure 2. Human B Cells Express Functional LOX-1.
(A) LOX-1 expression on peripheral B cell subsets.
(B) Purified total B cells were cultured overnight in the presence or absence of 10 μg/ml αIgM, 100 ng/ml αCD40 or 50 nM CpG ODN2006. LOX-1 expression was assessed.
(C) Purified and CFSE-labeled total B cells were cultured in plates coated with αLOX-1 or control IgG for 7 days. Cells were stained with CD19 and CD38.
(D) On day 12 of the B cell culture in (C), culture supernatants were analyzed for measuring Igs by ELISA.
(E) Purified total B cells were cultured overnight in plates coated with αLOX-1 or control IgG. B cells were stained for CD40, CCR6, CXCR5 and CCR7.
(F) B cells in (E) were added to the upper wells of transwells containing the indicated concentrations of CCL27 or CCL19 in the lower wells. After 2 hours, the number of cells that migrated into the lower wells was determined using counting beads. Two independent experiments using cells from two different donors showed similar results. In D and F, error bars indicate mean±SD of triplicate assays in individual experiments.
To further investigate the immunological function of LOX-1 expression on B cells, total B cells were cultured overnight with αLOX-1 or control IgG and then stained for CD40, C-X-C chemokine receptor type 5 (CXCR5), chemokine receptor 6 (CCR6) and chemokine receptor 7 (CCR7) (Figure 2E). αLOX-1 mAb slightly upregulated both CCR6 and CXCR5, which could contribute to B cell migration into and retention within germinal centers (GCs). B cells incubated with αLOX-1 mAb expressed CCR7, a lymphoid organ homing receptor. B cells cultured with αLOX-1 mAb also migrated more efficiently towards chemokine ligand 19 (CCL19) than B cells cultured with control IgG (Figure 2F). The two groups of B cells did not respond to chemokine ligand 27 (CCL27), a ligand for chemokine receptor 10 (CCR10). Collectively, LOX-1 expressed on B cells is functional and could therefore contribute to humoral immune responses.
LOX-1 Programs DCs to Induce Naïve B Cell Differentiation into Class-Switched PBs
Although IgG and IgA class-switching can occur in a TI manner at local tissues, including mucosa (Fagarasan et al., 2001; He et al., 2007; Macpherson et al., 2000; Uematsu et al., 2008), TD GC reactions are central for clonal expansion, Ig class-switching and high-affinity plasma cell generation (Liu and Arpin, 1997). Thus, we tested whether LOX-1 on DCs could promote naïve B cell differentiation into IgG- and IgA-secreting PBs in a TD manner. CFSE-labeled naïve B cells (CD19+IgD+CD27−) were co- cultured for 6 days with αLOX-1- or control IgG-treated DCs. In order to mimic a TD B cell response, naïve B cells were activated with a combination of αBCR, CpG (TLR9 engagement by microbial DNA), αCD40 and IL-2 (cognate interactions with CD4+ T cells). DCs treated with 2 μg/ml αLOX-1 induced greater naïve B cell proliferation than DCs treated with 2 μg/ml control IgG. The αLOX-1-treated DCs also induced greater PB (CD38+CD20−) differentiation (Figure 3A). These PBs also expressed decreased amounts of HLA-DR (Figure S2A) (Joo et al., 2012). Naïve B cell differentiation into PBs was also dependent on the amount of αLOX-1 mAb. PB differentiation data from four independent experiments with 2 μg/ml αLOX-1 or control IgG are summarized in Figure 3B. Without DCs, naïve B cell differentiation into PBs was minimal (Figure S2B).
Figure 3. LOX-1 Promotes Naïve B Cell Differentiation into PBs and Ig Class- Switching.
(A) IL-4DCs (5×103/well) were incubated overnight in plates coated with αLOX-1 or control IgG. CFSE-labeled naïve B cells (1×105, CD19+IgD+CD27−) were stimulated with αIgM and then co-cultured with the DCs in the presence of 20 units/ml IL-2, 50 nM CpG and 100 ng/ml agonistic αCD40 mAb. On day 6, B cells were stained with CD38 and CD20. Four independent experiments using cells from different donors showed similar results.
(B) Summary of PB differentiation from four independent experiments in (A).
(C) Culture supernatants in (A, B) were harvested on day 12 and the amounts of Igs were measured by ELISA.
(D–F) Naïve B cells co-cultured with the DCs (A) were harvested on day 4. Real time RT-PCR was performed for activation-induced cytidine deaminase (AICDA) (D), switch circle transcripts Iγ-Cμ and Iα-Cμ (E), germline (left) and mature transcripts (right) (F). The expression of AICDA was normalized to ACTB expression. Iγ-Cμ and Iα-Cμ expression was normalized to Iμ-Cμ expression (He et al., 2007). Data shown are mean±SD of three independent experiments using cells from two different healthy donors. PCR products of the germline mature transcripts were analyzed on agarose gels.
Consistent with the PB differentiation data, αLOX-1-treated DCs resulted in greater amounts of both IgM and IgG than did control IgG-treated DCs (upper, Figure 3C). Naïve B cells co-cultured with control IgG-treated DCs secreted low or minimal amounts of IgA1 and IgA2, whereas αLOX-1-treated DCs greatly enhanced both IgA1 and IgA2 secretion from B cells (lower, Figure 3C). Data in Figure S2C further demonstrates the correlation between the amount of Igs and the concentrations of αLOX-1 mAb. Thus, LOX-1 plays an important role in enhanced B cell responses by promoting naïve B cell differentiation into PBs that secrete class-switched Igs.
Quantitative real time RT-PCR data showed that naive B cells co-cultured with αLOX-1-treated DCs induced increased AICDA expression, a hallmark of B cells undergoing active class-switching (Muramatsu et al., 2000), than B cells co-cultured with control IgG-treated or untreated DCs (Figure 3D). Figure 3E shows that naïve B cells co-cultured with αLOX-1-treated DCs expressed increased Iγ-Cμ and Iα-Cμ switch circle transcripts. Naïve B cells co-cultured with αLOX-1-treated DCs also expressed higher amounts of germline and mature transcripts for IgA1, IgA2, IgG1-4 and IgM (Figure 3F). Taken together, LOX-1 can program DCs to promote naïve B cell proliferation, PB differentiation and Ig class-switching.
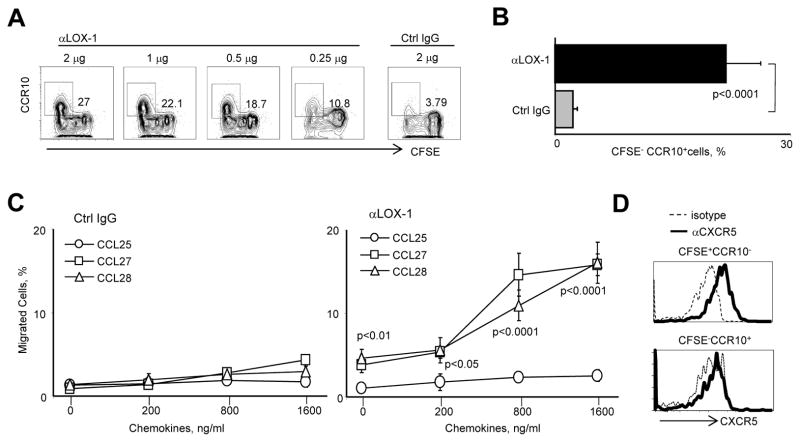
αLOX-1-Treated DCs Upregulate CCR10 but Downregulate CXCR5 Expression on B Cells
We next measured expression of chemokine receptors on B cells co-cultured with the DCs. αLOX-1-treated DCs induced 20–30% of activated naïve B cells to express CCR10 (Figure 4A, B). The induced CCR10 was functional as the B cells migrated, in a chemotaxis assay, towards the two CCR10 ligands: CCL28 and CCL27 (Figure 4C). There was no upregulation of CCR6, CCR9 or β7 integrin (Figure S3). Consequently, naïve B cells cultured with αLOX-1-treated DCs did not migrate in response to CCL25, a ligand for CCR9. CFSE−CCR10+ B cells induced with αLOX-1-treated DCs expressed less CXCR5 than did CFSE+CCR10− naïve B cells in the same cultures (Figure 4D). Thus, upregulation of CCR10, along with downregulation of CXCR5, could allow PBs to migrate out of the GCs en route to mucosal sites. This is supported by our previous study, showing that the majority of IgA+ cells in a tonsil GCs express CCR10 (Dullaers et al., 2009).
Figure 4. αLOX-1-Treated DCs Upregulate CCR10 but Downregulate CXCR5 on B Cells.
(A) CFSE-labeled naïve B cells were co-cultured for 6 days with IL-4DCs treated with either αLOX-1 or control IgG as in Figure 3A. B cells were stained for CCR10 expression.
(B) Summary of the data from eight experiments in (A).
(C) On day 6 of the DC-B cell co-cultures, FACS-sorted CFSE− B cells were added to the upper wells of transwell containing the indicated concentrations of CCL25, CCL27 and CCL28 in the lower wells. After 2 hours, the numbers of cells that migrated into the lower wells was determined using counting beads. Results shown are the means of three independent experiments. Error bars indicate mean±SD of triplicate assays.
(D) Expression of CXCR5 on CFSE−CCR10− and CFSE−CCR10+ B cells co-cultured with α LOX-1-treated IL-4DCs in (A).
LOX-1-Activated DCs Secrete TNF family ligands to Promote IgA- and IgG- Secreting B Cell Responses
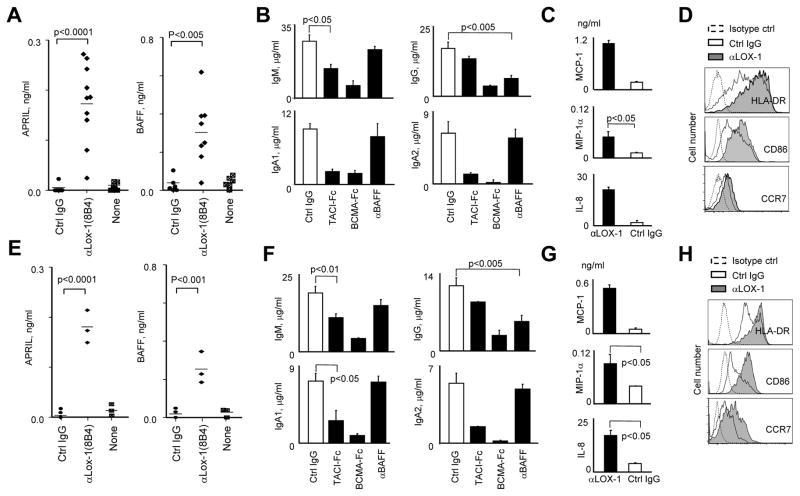
To further investigate the mechanisms by which αLOX-1-treated DCs promote IgA and IgG responses, overnight culture supernatants of DCs were analyzed for the amounts of a proliferation-inducing ligand (APRIL) and B cell activating factor (BAFF), cytokines that promote B cell proliferation, differentiation, class- switching and plasma cell survival (Cerutti, 2008; Joo et al., 2012; Litinskiy et al., 2002; Xu et al., 2007). Both IL-4DCs (Figure 5A) and blood myeloid DCs (mDCs) (Figure 5E) secreted APRIL and BAFF, but not TGF-β (not shown), in response to αLOX-1 mAb.
Figure 5. LOX-1 Induces DCs to Secrete APRIL and BAFF That Promote IgA- and IgG-Secreting B Cell Responses, Respectively.
(A and E) 1× 105 IL-4DCs (A) or blood mDCs (E) were cultured 72h in plates coated with the indicated mAbs (2 μg/ml). The amounts of APRIL and BAFF in the culture supernatants were assessed by ELISA. Each dot represents the data from experiments using cells from different healthy donors.
(B and F) FACS-sorted naïve B cells were co-cultured for 12 days with αLOX-1-activated IL-4DCs (B) and mDCs in the presence of the indicated antibodies or recombinant proteins (10 μg/ml). The amounts of Igs in the supernatants were measured by ELISA. Data are mean±SD of four (B) and three (F) independent experiments using cells from four healthy donors.
(C and G) Culture supernatants of IL-4DCs (C) and mDCs (G) in (A and E) were analyzed for the amounts of chemokines by the BeadLyte cytokine assay kit.
(D and H) Phenotypes of IL-4DCs (D) and mDCs (H) treated with mAbs in (A and E).
To test the roles of APRIL and BAFF in the αLOX-1-activated DC-mediated B cell responses, recombinant proteins that neutralize APRIL and BAFF were added to co- cultures. Transmembrane activator and calcium-modulator and cytophilin ligand interactor-Fc (TACI-Fc) and B cell maturation antigen-Fc (BCMA-Fc) (which can neutralize both APRIL and BAFF) or αBAFF Ab (which neutralizes BAFF) were added at the beginning of co-cultures of αLOX-1-activated DCs and naïve B cells. After 12 days, concentrations of Ig in culture supernatants were measured by ELISA (Figure 5B, 5F). Compared to control antibody, both TACI-Fc and BCMA-Fc significantly reduced IgM and IgA concentrations, but BCMA-Fc was more efficient than TACI-Fc, particularly for IgM and IgA2. αBAFF Ab decreased IgG secretion, but not IgM or IgA secretion. Data generated with either IL-4DCs (Figure 5B) or blood mDCs gave similar results (Figure 5F). We also found that both IL-4DCs (Figure 5C) and mDCs (Figure 5G) secreted more monocyte chemotactic protein 1 (MCP-1), macrophage inhibitory protein 1α (MIP-1α) and IL-8 in response to αLOX-1 than to control IgG. LOX-1-activated IL-4DCs (Figure 5D) and mDCs (Figure 5H) also expressed more HLA-DR, CD86 and CCR7. Therefore, we concluded that activation of DCs via LOX-1 promotes antibody-secreting B cell responses. In particular, LOX-1-induced APRIL and BAFF secretion from DCs plays an important role in enhancing B cell responses, which is supported by data from previous studies by us and others (Joo et al., 2012; Litinskiy et al., 2002).
αDC inhibitory receptor (DCIR), αDC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), αDectin-1 or αDEC205 mAb did not increase the amounts of APRIL or BAFF secreted from DCs (Figure S4A). However, αDectin-1-treated DCs slightly enhanced IgM- and IgG-, but not IgA-, secreting B cell responses (Figure S4B). This was probably due to the roles of cytokines, including IL-6, TNFα, IL-1β and IL-10, that were secreted from αDectin-1-treated DCs (Ni et al., 2010). We further compared αLOX-1 (8B4) with three other commercially available αLOX-1 mAbs for their ability to induce APRIL and BAFF secretion from DCs (Figure S4C). αLOX-1 (8B4) mAb was clearly the most efficient at inducing BAFF and APRIL from DCs.
ox-LDL Induces DCs to Secrete APRIL and BAFF Leading to Enhanced IgA and IgG Responses
Blood mDCs were cultured overnight in the presence of 2 μg/ml αLOX-1 mAb or various concentrations of ox-LDL. The amounts of APRIL and BAFF in the supernatants were measured by ELISA (Figure 6A). ox-LDL (30 μg/ml) and αLOX-1 mAb (2 μg/ml) induced similar amounts of both APRIL and BAFF secretion from DCs. In addition, the amount of APRIL and BAFF secreted from the DCs were ox-LDL dose-dependent. DCs activated with either αLOX-1 or ox-LDL also expressed surface APRIL and BAFF (Figure 6B), supporting the notion that DC-B cell interactions are important for the enhanced B cell response (Figure 1F) (Joo et al., 2012).
Figure 6. ox-LDL Induces Blood mDCs to Secrete APRIL and BAFF Leading to Enhanced IgA- and IgG-Secreting B Cell Responses, Respectively.
(A) 1×105 DCs were cultured overnight in the presence of αLOX-1 (2 μg/ml) and different concentrations of ox-LDL. The amounts of APRIL and BAFF in the supernatants were measured by ELISA. Data are mean±SD of three independent experiments using cells from different healthy donors.
(B) DCs were stained for surface APRIL and BAFF expression.
(C) DCs were incubated in plates coated with 2 μg/ml αLOX-1 mAb or control IgG (left). DCs were incubated in the presence or absence of 30 μg/ml ox-LDL (right). Cells were harvested at indicated time points for western blots. Two independent experiments showed similar results.
(D) DCs were treated first with 2 μM Wedelolactone for 3h and then cultured for 72h in plates coated with αLOX-1 (2 μg/ml) or control IgG. The amount of APRIL in the supernatants was assessed by ELISA. Error bars indicate SD of triplicate assay. Three independent experiments using cells from different donors showed similar results.
(E) 5×103 blood mDCs were incubated overnight with 2 μg/ml αLOX-1 or 30 ng/ml ox-LDL. 1×105 FACS-sorted and CFSE-labeled naïve B cells were added and cultured for 6 days in the presence of 20 units/ml IL-2, 50 nM CpG and 100 ng/ml agonistic αCD40 mAb. Data from four independent experiments showed similar results.
(F) FACS-sorted naïve B cells were co-cultured for 12 days with αLOX-1-activated mDCs in the presence of the indicated antibodies or recombinant proteins (10 μg/ml). The amounts of Igs in the supernatants were measured by ELISA. Data are mean±SD of three independent experiments using cells from three healthy donors.
Figure 6C shows that both αLOX-1 and ox-LDL results in the phosphorylation of p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK1/2), as well as IκBα phosphorylation in DCs. Control IgG-induced phosphorylation of p38 and ERK at the late time points was probably due to signals via Fc receptors. Furthermore, the nuclear factor-kappa B (NF-κB) inhibitor Wedelolactone (Carneiro et al., 2009; Kuroda et al., 2011) blocked APRIL secretion from the αLOX-1-treated DCs (Figure 6D).
Similar to αLOX-1-treated DCs, ox-LDL-treated DCs were able to promote B cell responses by enhancing naïve B cell proliferation and differentiation into PBs (Figure 6E). Blocking both APRIL and BAFF or blocking BAFF alone in the co-cultures of ox-LDL-treated DCs and naïve B cells resulted in outcomes that were similar to those seen in the co-cultures of αLOX-1-activated DCs with naïve B cells (Figure 6F). αBAFF mAb mainly decreased IgG responses. BCMA-Fc and TACI-Fc (to a lesser extent) decreased IgA1, IgA2 and IgM. ox-LDL could also stimulate B cells to secrete IgM and IgG as did αLOX-1 mAb, but the amount of Igs secreted from B cells alone (Figure S5) were far smaller than the amount of Igs secreted from B cells co-cultured with DCs. These data support our findings that LOX-1 plays an important role in the DC-mediated class-switched B cell responses in humans.
Targeting Influenza HA1 to LOX-1 Elicits HA1-specific Protective Antibody Responses in Macaques
We tested whether recombinant fusion proteins of influenza HA1 and αLOX-1 mAb (αLOX-1-HA1) (Li et al., 2012) could elicit HA1-specific protective Ab responses in rhesus macaques. The αLOX-1 mAb was made in mice and thus does not bind to mouse LOX-1. αLOX-1 mAb bound to rhesus macaque LOX-1 (Figure S6A). It also bound to CD11c+ and CD14+ cells, but not CD3+ cells, in the blood of rhesus macaques (upper, Figure S6B). In human in vitro experiments, we also observed that αLOX-1-HA1 could promote PB differentiation (upper, Figure S7).
Five animals were primed and boosted twice intradermally (i.d.) with 100 μg αLOX-1-HA1, which carried approximately 15 μg HA1 (corresponding to the amount of HA1 for each component of the Trivalent Inactivated Influenza Vaccine). LOX-1+CD1c+ DCs were mainly found in the dermis of human skin (Li et al., 2012). As shown in Figure 7A, αLOX-1-HA1 elicited HA1-specific humoral responses, as assessed by measuring serum hemagglutination inhibition (HAI) and virus neutralization titers. Two animals showed HAI>40, which is a protective in humans. Serum antibodies in all animals could neutralize H1N1 (Cal 04) virus infection, with higher neutralization titers in the two animals showing HAI>40.
Figure 7. α LOX-1-HA1 Fusion Proteins Elicit HA1-Specific Protective Antibody Responses in Rhesus Macaques.
(A) Five rhesus macaques were immunized i.d. with 100 μg αLOX-1-HA1 on weeks 0, 6 and 12. Serum HAI (left) and micro-neutralization (right) titers on weeks 0 and 14 were assessed. Each dot displays the data generated with individual macaques.
(B) Macaques were exposed with aerosolized 2.3 x 107 pfu of H1N1 (Cal 04) virus. At 3 months post-exposure, animals were divided into two groups and boosted i.d. with either UV-inactivated H1N1 (Cal 04) virus (5×107 pfu) or 100 μg αLOX-1-HA1. Serum HAI (upper) and micro-neutralization (lower) titers were measured.
(C) Macaques were primed (on week 0) and boosted twice (on weeks 6 and 12) i.d. with 100 μg αLOX-1-HA1 or αDectin-1-HA1 in combination of 1 mg poly ICLC. Serum HAI (upper) and neutralization (lower) titers on weeks 0 and 14 were measured.
(D) Animals in (C) were challenged with aerosolized H1N1 (Cal 04) as in (A). Serum HAI (upper) and micro-neutralization (lower) titers were measured. Each dot represents data generated with individual animals.
We next tested whether αLOX-1-HA1 could efficiently boost HA1-specific antibody responses. Animals were challenged with aerosolized virus particles and then boosted with 100 μg αLOX-1-HA1 or UV-inactivated virus (5×107 pfu). Both UV-inactivated virus and αLOX-1-HA1 were able to boost HA1-specific Ab responses as assessed by measuring serum HAI (upper, Figure 7B) and neutralization (lower, Figure 7B) titers. However, αLOX-1-HA1 was more efficient than the UV-inactivated viruses at boosting HA1-specific humoral responses.
We next compared the immunogenicity of αLOX-1-HA1 with that of αDectin-1-HA1 (Figure 7D). Although αDectin-1 did not induce APRIL or BAFF expression by DCs (Figure S4A), it was able to promote antibody responses (Figure S4B). We have shown that αDectin-1 and αDectin-1-HA1 could induce DCs to secrete IL-6, TNFα, IL-1β and IL-10, which could contribute to B cell responses (Duluc et al., 2014; Ni et al., 2010). αDectin-1 mAb also bound to CD11c+ and CD14+ cells in the PBMCs of rhesus macaques (lower, Figure S6B). In this experiment, we employed poly ICLC as an adjuvant because 1) it is currently being tested in several clinical trials and 2) it could enhance αLOX-1-HA1-induced PB differentiation in human in vitro experiments (lower, Figure S7). Two groups of animals were immunized with 100 μg of the proteins plus 1 mg poly ICLC on weeks 0, 6, and 12. Animals immunized with αLOX-1-HA1 plus poly ICLC showed higher serum HA1 titers than animals immunized with αDectin-1-HA1 plus poly ICLC (upper, Figure 7C). Animals immunized with αLOX-1-HA1 also showed slightly greater neutralization titers than animals immunized with αDectin-1-HA1 (lower, Figure 7C). The same animals in the two groups (Figure 7C) were challenged with aerosolized virus after 3 months of the second boosting. Notably, animals immunized with αLOX-1-HA1 showed higher serum HA1 titers than animals immunized with αDectin-1-HA1 (upper, Figure 7D). Although there was no significant difference in viral neutralization titers in the two groups (lower, Figure 7D), two out of three animals that received αLOX-1-HA1 showed greater neutralization titers than the others.
Taken together, we concluded that αLOX-1-HA1 could elicit HA1-specific antibody responses. The immunogenicity of αLOX-1-HA1 is greatly enhanced by poly ICLC. αLOX-1-HA1 plus poly ICLC was also more efficient than αDectin-1-HA1 plus poly ICLC at eliciting HA1-specific serum antibody responses in non-human primates (NHPs).
DISCUSSION
Much of the interest in LOX-1 has been focused on its involvement in vascular diseases, including atherosclerosis. This is mainly due to its expression on endothelial cells and its pathophysiologic functions in atherosclerosis. In this study, we report for the first time that LOX-1 expressed on DCs and B cells has novel functions to promote humoral immune responses.
DCs can induce and activate B cell responses in a TD as well as TI fashion (Dubois et al., 1997; He et al., 2007; Jego et al., 2003; Joo et al., 2012; Litinskiy et al., 2002; Tezuka et al., 2011; Tezuka et al., 2007). DCs are able to secrete both APRIL and BAFF, and thus can support B cell survival, proliferation and class-switching (Joo et al., 2012; Litinskiy et al., 2002; Schneider, 2005; Stohl et al., 2003; Xu et al., 2007). In this study, we first found that LOX-1 can induce DCs to secrete both APRIL and BAFF, permitting enhanced humoral immune responses. Consistent with our previous data (Joo et al., 2012), both APRIL alone and APRIL together with BAFF played important roles in enhancing IgM and IgA responses. APRIL-induced IgA2 class-switching and PB differentiation were previously reported (He et al., 2007; Litinskiy et al., 2002). In support of these findings, our data also showed that TACI-Fc was more efficient at decreasing both IgM and IgA responses than it was at decreasing IgG responses, whereas αBAFF antibody was more efficient at decreasing IgG responses than IgM or IgA responses (Joo et al., 2012).
The ability of LOX-1 to induce DCs to express APRIL and BAFF distinguishes it from other lectin receptors. Unlike Dectin-1, CLEC1B and CLEC9A, LOX-1 does not carry a known cytoplasmic signaling motif (YxxL§) (Geijtenbeek and Gringhuis, 2009; Hardison and Brown, 2012). However, both αLOX-1 mAb and ox-LDL induced the activation of p38 and ERK1/2, as well as IκBα phosphorylation in DCs. In addition, NF- κB inhibitor (Carneiro et al., 2009; Kuroda et al., 2011) could block APRIL secretion from αLOX-1-activated DCs, suggesting that NFκB activation by LOX-1 engagement plays an important role in enhancing B cell responses. In addition, DC-SIGN does not carry a known signaling motif, but it can activate NF-κB mainly in conjunction with TLR2 or TLR4 (Geijtenbeek and Gringhuis, 2009). It is also known that Dectin-1 can induce MAPK and NF-κB activation (Geijtenbeek and Gringhuis, 2009). However, neither Dectin-1 nor DC-SIGN induced DCs to express significant amounts of APRIL or BAFF. Although both Dectin-1 and DC-SIGN can induce IL-6 secretion by DCs (Geijtenbeek and Gringhuis, 2009), as can LOX-1(Figure 1H), Dectin-1-activated DCs can also secrete multiple cytokines, including IL-6, IL-1β, TNFα, and IL-10, as well as chemokines (Geijtenbeek and Gringhuis, 2009; Huysamen and Brown, 2009; Ni et al., 2010) that could further modulate downstream signaling pathways to interfere with the expression of other proteins, including APRIL and BAFF.
LOX-1 expressed on B cells does not greatly enhance B cell proliferation, PB differentiation or antibody secretion. However, it directs B cells to upregulate CCR7, which plays an important role in B cell migration into lymphoid tissues. CD40 plays an essential role in B cell proliferation, class-switching and the development of high affinity memory B cells (Arpin et al., 1995; Liu and Arpin, 1997). It is also interesting to note that the expression of LOX-1 on B cells was less when B cells were activated via three major receptors: B cell receptors (BCRs), CD40 or Toll-like receptor 9 (TLR9). These data suggest that LOX-1 expressed on human B cells can contribute to humoral responses.
Many of the CD11c+LOX-1+ cells localized in the marginal zones of human spleens interact with B cells. In fact, it is known that DCs in marginal zones can produce large amounts of APRIL and BAFF in response to immune stimuli (MacLennan and Vinuesa, 2002) that contribute to humoral responses, particularly TI responses. Although the types of natural ligands for LOX-1 need to be further characterized, one can assume that LOX-1+DCs could secrete not only APRIL and BAFF, but also IL-6 by sensing LOX-1 ligands, including ox-LDL, HSPs and ligands provided by different cell types, and could thus contribute to B cell responses in a steady state. Our data generated with poly ICLC also suggest that LOX-1 signaling can collaborate with microbial stimuli to promote humoral responses. Elevated serum ox-LDL concentrations and ox-LDL-mediated DC activation in systemic lupus erythematosus (SLE) patients have been reported (Bassi et al., 2009; McMahon and Hahn, 2007). Thus, the ox-LDL-induced DC activation could also be responsible, at least in part, for the higher serum concentrations of APRIL and BAFF that were followed by enhanced autoreactive B cell responses in SLE patients.
CCR10 is known to be expressed mainly on terminally differentiated B cells, as the expression of CCR10 had not been described at any of the developmental or differentiation stages of B cells (Bowman et al., 2000). A substantial fraction of plasma cells derived from human bone marrow expresses CCR10 and efficiently migrates to its ligands, CCL27 and CCL28 (Nakayama et al., 2003). A previous study showed that the active metabolites of vitamin D3 could induce CCR10 expression on terminally differentiating B cells (Shirakawa et al., 2008). In addition, a recent study has shown that DCs in the caecal patch, but not Peyer’s patches, can induce CCR10 on in vitro cultured murine B cells (Masahata et al., 2014). Although it is still unclear how LOX-1-activated DCs induce pan-mucosal homing receptor CCR10 (Kunkel et al., 2003; Lazarus et al., 2003; Shirakawa et al., 2008) on B cells, this study suggests that LOX-1 can be a novel target to enhance mucosal immunity against many microbial infections. The CCL28-CCR10 system is an important element in the common mucosal immune system by promoting the wide distribution of locally induced IgA-secreting plasma cells to various mucosal tissues in the body (Brandtzaeg and Johansen, 2005; Kunkel et al., 2003; Lazarus et al., 2003; Shirakawa et al., 2008).
Mucosal surfaces are the most frequent port of entry for microorganisms (Brandtzaeg and Johansen, 2005; Fagarasan and Honjo, 2003). IgA, the predominant antibody isotype in mucosal secretions, is of paramount importance in the immune defense of these surfaces (Brandtzaeg, 2007; Renegar et al., 2004; Yel, 2010). Because mucosal IgA is mainly secreted locally in subepithelial areas, IgA-plasma cells (PC) need to acquire appropriate homing receptors and migrate towards the effector site. Thus, the generation of mucosal homing PCs is essential for mounting potent immunity in local mucosa. It is also known that tissue microenvironments where B cells undergo differentiation are important factors that determine the expression of tissue-specific homing receptors by B cells (Mora et al., 2006; Shirakawa et al., 2008). Therefore, it would be interesting to target LOX-1 in local mucosa to see if it could promote mucosal immunity in the local tissues. Nonetheless, we demonstrated that αLOX-1 mAb fused to influenza HA1 was able to elicit protective HA1-specific Ab responses in a preclinical animal model.
In conclusion, this study demonstrates a novel function of LOX-1 expressed on DCs and B cells that could be applied for the design of new vaccines against many microbial infections. Data from this study also suggest that LOX-1 could play an important role in the host immune responses in both healthy and diseased states.
EXPERIMENTAL PROCEDURES
Antibodies, Reagents, and Flow Cytometry
Antibodies specific for the following molecules were used for flow cytometric analysis and cell sorting; CD3, CD4, CD8, CD19, CD20, CD27, CD38, CD40, CD86, CD138 and HLA-DR were from BD BioSciences. CCR6, CCR7, CCR9, CCR10, CXCR5 and α4β7 were from R&D Systems. αCD40 (clone 12E12, in-house), αBlimp-1 (Novus biological), αPax5 (eBioscience), αimmunoglobulins (Southern Biotech), αAPRIL (BioLegend) and αBAFF (R&D Systems) were used. The Live/Dead Fixable dead cell stain kit (Invitrogen) and the CellTrace CFSE Cell Proliferation kit (Invitrogen) were used. Cytofix/wash buffer kit (BD) was used for an intracellular Pax5 and Blimp-1 staining. Cells were acquired on an LSRII™ or FACSCanto II™ (BD Biosciences), and analysis was performed using FlowJo software (Tree Star Inc). Antibodies specific for pSTAT3 (Y705), phospho-p38, phospho-ERK, phospho-IκBα, STAT3, p38, ERK and IκBα were from Cell Signaling Technology. GAPDH was from Sigma-Aldrich. ox-LDL was from BTI Biomedical Technology. CCL19, CCL25, CCL27 and CCL28 were from eBioscience. TACI-Fc, BCMA-Fc and αBAFF mAb were from R&D Systems. CpG ODN2006 was from Invivogen. Poly ICLC was provided by Dr. Andres M. Salazar (Oncovir, Inc.)
Enzyme-Linked Immunosorbent Assay (ELISA)
Sandwich ELISAs were performed to measure total IgM, IgG, IgA, APRIL and BAFF (Dullaers et al., 2009; Joo et al., 2012), as described in Supplemental Experimental Procedures.
B Cell Morphology
B cells were stained using the DiffQuick™ Stain Set (Siemens, IL) (Joo et al., 2012), as described in Supplemental Experimental Procedures.
mAbs to C-Type Lectins
αhLOX-1 (23C11) mAb was purchased from Hycult Biotech. Clones 331212 and 331219 were from R&D Systems. Mouse mAbs specific for LOX-1 and DC-ASGPR (Li et al., 2012), Dectin-1 (Ni et al., 2010) and DCIR (Klechevsky et al., 2010) were generated. αDC-SIGN was generated in-house, as described in Supplemental Experimental Procedures. αDEC-205 was provided by Dr. Ralph Steinman (Rockefeller Univ.). All mAbs specific for c-type lectins contained less than 0.02 ng/ml endotoxin. 293F cells were transfected with mock plasmid (pIRES2-DsRed2) or the same plasmid carrying hLOX-1 full length (Li et al., 2012; Ni et al., 2010). Cells were stained with the indicated mAbs and analyzed by flow cytometry. Recombinant fusion proteins of Fc with hLOX-1 and hDCIR ectodomain (Klechevsky et al., 2010; Li et al., 2012) were used to determine the specificity of αLOX-1 mAb by ELISA.
Dendritic Cells (DCs), B Cells, Medium, and Cultures
The collection of peripheral blood mononuclear cells from healthy individuals was approved by the Institutional Review Board of Baylor Research Institute. Blood mDCs (Lin−HLA-DR+CD11c+CD123−) were FACS-sorted (FACSAria™, BD). Monocyte-derived IL-4DCs, IFNDCs and TNFDCs were generated as previously described (Ni et al., 2010). B cells were isolated using Human B Cell Enrichment Kit (STEMCELL Technology). Naïve B cells (CD19+IgD+CD27−) were sorted on a FACSAria™ (BD). The purity of sorted mDCs and naïve B cells was generally >98%. DC, B, DC-B and PBMC medium and cultures are described in Supplemental Experimental Procedures.
Conventional and Quantitative Real-Time PCR
RNA was isolated from stimulated B cells on day 4 using Trizol (Invitrogen) and cDNA was synthesized with Reverse Transcription System (Promega). AICDA, switch circle transcripts and germline and mature transcripts (Dullaers et al., 2009; Joo et al., 2012; Litinskiy et al., 2002) were analyzed as described in Supplemental Experimental Procedures.
Tissue Sections and Immunofluorescence
Human spleens were obtained from chronic pancreatitis patients undergoing total pancreatectomy and splenectomy surgeries at Baylor University Medical Center. This study was approved by the Institutional Review Board of Baylor Research Institute. Detailed methods are described in Supplemental Experimental Procedures.
Western Blot
Cell extracts were separated by SDS-PAGE and transferred to a nitrocellulose membrane (Bio-Rad). Immunodetection was performed by incubation with anti-primary antibodies followed by HRP-conjugated secondary antibodies. The bands were then visualized using the ECL chemiluminescence Western blotting detection system (Amersham Biosciences).
Cytokine and Chemokine Multiplex Analysis
Cytokines and chemokines were assessed using the BeadLyte Cytokine Assay Kit (Upstate), as per the manufacturer’s protocol. Fluorescence was analyzed with a Bio-Plex Luminex 100 XYP instrument (Bio-Rad), with the Bio-Plex Manager 4.1 software, using a 5-parameter curve-fitting algorithm applied for standard curve calculations.
Recombinant Fusion Proteins of mAbs and HA1 Antigens
Recombinant fusion proteins of αLOX-1-HA1 and αDectin-1-HA1 were made as described (Duluc et al., 2014; Li et al., 2012). Detailed methods are described in
Animals, Immunization, Viral Challenge, and Serum Antibody Assays
Male rhesus macaques (Macaca mulatta) (4–5 years old) weighing 4–8 kg were used. All animal experiments were conducted at the Tulane National Primate Research Center (TNPRC), with the approval of the Animal Care and Use Committees at the Baylor Research Institute and TNPRC. Hemagglutination inhibition (HAI) and microneutralization assays were performed at a BSL3 laboratory as previously described. Detailed methods are described in Supplemental Experimental Procedures.
Statistical Analysis
All bar graphs represent mean ±SD. Data were analyzed using GraphPad Prism 4 software. The significance of difference between experimental variables was determined using the Student’s t-test and ANOVA. The significance was set at P<0.05.
Supplementary Material
Figure S1. α LOX-1 mAb specific for human LOX-1 promotes B cell responses. (A) Mock- (left) and hLOX-1-transfected (right) 293F cells were stained with 1μg/ml αLOX-1 (8B4) or isotype control. (B) hLOX-1-transfected 293 cells were stained with various amounts of αLOX-1 (8B4) or isotype control. Mean fluorescent intensity (MFI) values are plotted. (C) Recombinant proteins, hLOX-1-Fc and hDCIR-Fc, were coated in the plates. Different concentrations of αLOX-1 (8B4) mAb were incubated. The amount of αLOX-1 mAb bound to hLOX-1-Fc or DCIR-Fc proteins were detected using αGoat mouse IgG-HRP. (D) CD11c (green), LOX-1(red) and IgD (blue) expression as well as isotype control staining in human spleen sections. Original magnification, x10. (E) IL-4DCs (5×103/well) were incubated overnight in plates coated with 2μg/ml αLOX-1 (8B4) or control IgG. CFSE-labeled B cells (1×105) were co-cultured with the DCs in the presence of 20 units/ml IL-2. On day 6, cells were stained with αCD19, αCD38, and αCD138. CD138 expression on CD19+CD38+CFSE− cells were assessed. (F) Staining of IFNDCs and TNFDCs with αLOX-1 (8B4) mAb (upper left). B cells were co-cultured with αLOX-1-treated IFNDCs, as in (E). On day 6, cells proliferation and PB differentiation were assessed. (lower left). On day12, culture supernatants were analyzed to measure the amount of Igs by ELISA (right). (G) CFSE-labeled 5×105 PBMCs were cultured for 7 days in plates coated with 2μg/ml αLOX-1 or control IgG. PB differentiation was assessed (left). On day 12, the amounts of Igsin the supernatants were assessed by ELISA. Error bars indicate SD of triplicate assays from two independent experiments.
Figure S2. α LOX-1-treated DCs promote naïve B cell differentiation into Ig-secreting PBs. (A) IL-4DCs (5×103/well) were incubated overnight in plates coated with αLOX-1 (8B4) or control IgG. FACS-sorted and CFSE-labeled naïve B cells (1×105, CD19+IgD+CD27−) were stimulated with αIgM then co-cultured with the DCs in the presence of 20 units/ml IL-2, 50 nM CpG and 100 ng/ml agonistic αCD40 mAb (clone 12E12). On day 6, B cells were stained for HLA-DR. (B) Naïve B cell culture in (A) were performed in the absence or presence of DCs. On day 6, B cells were stained and assessed for PB differentiation. Two independent experiments using cells from different donors showed similar results. (C) Culture supernatants of the DC-B cell co-culture in (A) were harvested on day 12 and the amounts of Igs were measured by ELISA.
Figure S3. αLOX-1 mAb does not induce β7 integrin, CCR6, or CCR9 expression on naïve B cells co-cultured with DCs. IL-4DCs (5×103/well) were incubated overnight in plates coated with 2μg/mlαLOX-1 or control IgG. FACS-sorted and CFSE-labeled naïve B cells (1×105, CD19+IgD+CD27−) were stimulated with αIgM then co-cultured with the DCs in the presence of 20 units/ml IL-2, 50 nM CpG and 100 ng/ml agonisticαCD40 mAb (clone 12E12). On day 6, cells were stained withαCD19 andαCD38 along with indicated antibodies. CD19+CD38+ live cells were gated to assess the surface expression levels of β7 integrin, CCR6, and CCR9.
Figure S4. α LOX-1 (8B4) mAb can induce DCs to secrete APRIL and BAFF and further promotes Ig-secreting B cell responses. (A) 1×105 IL-4DCs were cultured 72h in plates coated with the indicated mAbs (2μg/ml). The amounts of APRIL and BAFF in the supernatants were measured by ELISA. Each dot represents data generated with cells from different healthy donors. (B) IL-4DCs (5×103/well) were incubated overnight in plates coated with 2 μg/ml αLOX-1 (8B4), αDCIR (9E8),αDectin-1 (15E2),αDC-SIGN (24G3), or control IgG. FACS-sorted and CFSE-labeled naïve B cells (1×105, CD19+IgD+CD27−) were stimulated with αIgM then co-cultured with the DCs in the presence of 20 units/ml IL-2, 50 nM CpG, and 100 ng/ml agonisticαCD40 mAb (clone 12E12). Culture supernatants were harvested on day 12 and the amounts of Igs were measured by ELISA. Error bars indicate SD of triplicate assays. Two independent experiments using cells from different healthy donors showed similar results. (C) 1× 105 IL-4DCs were cultured 72h in plates coated with the indicated mAbs (2μg/ml). The amounts of APRIL and BAFF in the supernatants were measured by ELISA. Each dot represents data generated with cells from different healthy donors.
Figure S5. ox-LDL can activate B cells. Purified CD19+B cells (1×105/well) were cultured in the presence or absence of 30μg/ml ox-LDL for 12 days. 20 units/ml IL-2 was added into the culture. Culture supernatants were analyzed to measure the amount of Igs by ELISA. Two independent experiments using cells from different donors were performed. Each experiment was performed with a triplicate assay. Error bars indicate SD.
Figure S6. αLOX-1 mAb binds to rhesus macaque LOX-1 and also binds to the surface of CD11c+ and CD14+ cells, but not CD3+ cells, in the blood of rhesus macaques. (A)αLOX-1 binds to LOX-1 expressed in rhesus macaques. Recombinant proteins, rhesus macaque LOX-1-Fc and DCIR-Fc, were coated in plates. Different concentrations of αLOX-1 (8B4) mAb were incubated. The amount ofαLOX-1 bound to macaque LOX-1-Fc or DCIR-Fc proteins were detected using mouse αGoat IgG labeled with horseradish peroxidase (HRP). (B) Peripheral blood mononuclear cells from the blood of rhesus macaques were stained first withαLOX-1,αDectin-1 and control IgG mAbs. Cells were then stained with PE-labeled goat α Mouse IgG. Cells were finally stained for CD11c, CD14 and CD3 expression. CD11c+, CD14+ and CD3+ cells were gated and the expression levels of LOX-1 and Dectin-1 were assessed.
Figure S7. αLOX-1-HA1 fusion protein can promote B cell differentiation into PBs. IL-4DCs (5×103/well) were incubated overnight with 5μg/mlαLOX-1-HA1 or control IgG-HA1 in the absence (upper) or presence (lower) of 500 ng/ml poly ICLC. Purified and CFSE-labeled total B cells (1×105) were co-cultured with the DCs for 6 days in the presence of 20 units/ml IL-2. PB differentiation was measured.
Acknowledgments
We thank Drs. Andres M. Salazar (Oncovir, Inc.) and Adolfo Garcia-Sastre (Icahn School of Medicine at Mount Sinai) for providing Poly ICLC and influenza viruses, respectively. We thank the FACS Core, Cell Processing Core, Biobank & Project Management Core, Imaging Core and Luminex Core at the Baylor Institute for Immunology Research. We thank Amy O’Bar and Dr. Xiao-Hua Li for providing monoclonal antibodies specific for lectins. We thank Dr. Carson Harrod and Mr. Jerome Ellis for editing the manuscript. We also thank Drs. Jacques Banchereau, Andrew Lackner and Michael Ramsay for supporting this study. This study was funded by the BHCS Foundation and NIH U19 AI057234.
Footnotes
Supplemental Information includes seven figures and Supplemental Experimental Procedures.
The authors have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arpin C, Dechanet J, Van Kooten C, Merville P, Grouard G, Briere F, Banchereau J, Liu YJ. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720–722. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- Bassi N, Zampieri S, Ghirardello A, Tonon M, Zen M, Beggio S, Matsuura E, Doria A. oxLDL/beta2GPI complex and anti-oxLDL/beta2GPI in SLE: prevalence and correlates. Autoimmunity. 2009;42:289–291. doi: 10.1080/08916930902828247. [DOI] [PubMed] [Google Scholar]
- Bowman EP, Campbell JJ, Soler D, Dong Z, Manlongat N, Picarella D, Hardy RR, Butcher EC. Developmental switches in chemokine response profiles during B cell differentiation and maturation. J Exp Med. 2000;191:1303–1318. doi: 10.1084/jem.191.8.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–5484. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Johansen FE. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev. 2005;206:32–63. doi: 10.1111/j.0105-2896.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- Carneiro LA, Travassos LH, Soares F, Tattoli I, Magalhaes JG, Bozza MT, Plotkowski MC, Sansonetti PJ, Molkentin JD, Philpott DJ, Girardin SE. Shigella induces mitochondrial dysfunction and cell death in nonmyleoid cells. Cell Host Microbe. 2009;5:123–136. doi: 10.1016/j.chom.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XP, Xun KL, Wu Q, Zhang TT, Shi JS, Du GH. Oxidized low density lipoprotein receptor-1 mediates oxidized low density lipoprotein-induced apoptosis in human umbilical vein endothelial cells: role of reactive oxygen species. Vascul Pharmacol. 2007;47:1–9. doi: 10.1016/j.vph.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, Kawakami-Honda N, Goetsch L, Sawamura T, Bonnefoy J, Jeannin P. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- Diehl SA, Schmidlin H, Nagasawa M, van Haren SD, Kwakkenbos MJ, Yasuda E, Beaumont T, Scheeren FA, Spits H. STAT3-mediated up-regulation of BLIMP1 Is coordinated with BCL6 down-regulation to control human plasma cell differentiation. J Immunol. 2008;180:4805–4815. doi: 10.4049/jimmunol.180.7.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Vanbervliet B, Fayette J, Massacrier C, Van Kooten C, Briere F, Banchereau J, Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J Exp Med. 1997;185:941–951. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dullaers M, Li D, Xue Y, Ni L, Gayet I, Morita R, Ueno H, Palucka KA, Banchereau J, Oh S. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity. 2009;30:120–129. doi: 10.1016/j.immuni.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duluc D, Joo H, Ni L, Yin W, Upchurch K, Li D, Xue Y, Klucar P, Zurawski S, Zurawski G, Oh S. Induction and activation of human Th17 by targeting antigens to dendritic cells via dectin-1. J Immunol. 2014;192:5776–5788. doi: 10.4049/jimmunol.1301661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S, Vohra RS, Murphy JE, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. The lectin-like oxidized low-density-lipoprotein receptor: a pro-inflammatory factor in vascular disease. Biochem J. 2008;409:349–355. doi: 10.1042/BJ20071196. [DOI] [PubMed] [Google Scholar]
- Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639–643. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol. 2012;13:817–822. doi: 10.1038/ni.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Huysamen C, Brown GD. The fungal pattern recognition receptor, Dectin-1, and the associated cluster of C-type lectin-like receptors. FEMS Microbiol Lett. 2009;290:121–128. doi: 10.1111/j.1574-6968.2008.01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannin P, Bottazzi B, Sironi M, Doni A, Rusnati M, Presta M, Maina V, Magistrelli G, Haeuw JF, Hoeffel G, et al. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 2005;22:551–560. doi: 10.1016/j.immuni.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- Joo H, Coquery C, Xue Y, Gayet I, Dillon SR, Punaro M, Zurawski G, Banchereau J, Pascual V, Oh S. Serum from patients with SLE instructs monocytes to promote IgG and IgA plasmablast differentiation. J Exp Med. 2012;209:1335–1348. doi: 10.1084/jem.20111644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakutani M, Masaki T, Sawamura T. A platelet-endothelium interaction mediated by lectin-like oxidized low-density lipoprotein receptor-1. Proc Natl Acad Sci U S A. 2000;97:360–364. doi: 10.1073/pnas.97.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechevsky E, Flamar AL, Cao Y, Blanck JP, Liu M, O’Bar A, Agouna-Deciat O, Klucar P, Thompson-Snipes L, Zurawski S, et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116:1685–1697. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel EJ, Kim CH, Lazarus NH, Vierra MA, Soler D, Bowman EP, Butcher EC. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J Clin Invest. 2003;111:1001–1010. doi: 10.1172/JCI17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda E, Ishii KJ, Uematsu S, Ohata K, Coban C, Akira S, Aritake K, Urade Y, Morimoto Y. Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms. Immunity. 2011;34:514–526. doi: 10.1016/j.immuni.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, Butcher EC. A Common Mucosal Chemokine (Mucosae-Associated Epithelial Chemokine/CCL28) Selectively Attracts IgA Plasmablasts. J Immunol. 2003;170:3799–3805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- Li D, Romain G, Flamar AL, Duluc D, Dullaers M, Li XH, Zurawski S, Bosquet N, Palucka AK, Le Grand R, et al. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J Exp Med. 2012;209:109–121. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Arpin C. Germinal center development. Immunol Rev. 1997;156:111–126. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- MacLennan I, Vinuesa C. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity. 2002;17:235–238. doi: 10.1016/s1074-7613(02)00398-9. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- Martins G, Calame K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol. 2008;26:133–169. doi: 10.1146/annurev.immunol.26.021607.090241. [DOI] [PubMed] [Google Scholar]
- Masahata K, Umemoto E, Kayama H, Kotani M, Nakamura S, Kurakawa T, Kikuta J, Gotoh K, Motooka D, Sato S, et al. Generation of colonic IgA-secreting cells in the caecal patch. Nat Commun. 2014;5:3704. doi: 10.1038/ncomms4704. [DOI] [PubMed] [Google Scholar]
- McMahon M, Hahn BH. Atherosclerosis and systemic lupus erythematosus: mechanistic basis of the association. Curr Opin Immunol. 2007;19:633–639. doi: 10.1016/j.coi.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hieshima K, Izawa D, Tatsumi Y, Kanamaru A, Yoshie O. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J Immunol. 2003;170:1136–1140. doi: 10.4049/jimmunol.170.3.1136. [DOI] [PubMed] [Google Scholar]
- Ni L, Gayet I, Zurawski S, Duluc D, Flamar AL, Li XH, O’Bar A, Clayton S, Palucka AK, Zurawski G, et al. Concomitant activation and antigen uptake via human dectin-1 results in potent antigen-specific CD8+ T cell responses. J Immunol. 2010;185:3504–3513. doi: 10.4049/jimmunol.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel T, Schmauss D, Hanssen H, Sicic Z, Krebs B, Jankl S, Summo C, Fraunberger P, Walli AK, Pfeiler S, Weis M. oxLDL uptake by dendritic cells induces upregulation of scavenger-receptors, maturation and differentiation. Atherosclerosis. 2009;205:442–450. doi: 10.1016/j.atherosclerosis.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Oka K, Sawamura T, Kikuta K, Itokawa S, Kume N, Kita T, Masaki T. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci U S A. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlato S, Romagnoli G, Spadaro F, Canini I, Sirabella P, Borghi P, Ramoni C, Filesi I, Biocca S, Gabriele L, Belardelli F. LOX-1 as a natural IFN-alpha-mediated signal for apoptotic cell uptake and antigen presentation in dendritic cells. Blood. 2010;115:1554–1563. doi: 10.1182/blood-2009-07-234468. [DOI] [PubMed] [Google Scholar]
- Renegar KB, Small PA, Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173:1978–1986. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–289. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Kume N, Minami M, Hayashida K, Sawamura T, Kita T, Yonehara S. LOX-1 supports adhesion of Gram-positive and Gram-negative bacteria. J Immunol. 2001;166:5108–5114. doi: 10.4049/jimmunol.166.8.5108. [DOI] [PubMed] [Google Scholar]
- Shirakawa AK, Nagakubo D, Hieshima K, Nakayama T, Jin Z, Yoshie O. 1, 25-Dihydroxyvitamin D3 Induces CCR10 Expression in Terminally Differentiating Human B Cells. J Immunol. 2008;180:2786–2795. doi: 10.4049/jimmunol.180.5.2786. [DOI] [PubMed] [Google Scholar]
- Stohl W, Metyas S, Tan SM, Cheema GS, Oamar B, Xu D, Roschke V, Wu Y, Baker KP, Hilbert DM. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum. 2003;48:3475–3486. doi: 10.1002/art.11354. [DOI] [PubMed] [Google Scholar]
- Tezuka H, Abe Y, Asano J, Sato T, Liu J, Iwata M, Ohteki T. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity. 2011;34:247–257. doi: 10.1016/j.immuni.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, Shiohara T, Akira S, Ohteki T. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- Xie J, Zhu H, Guo L, Ruan Y, Wang L, Sun L, Zhou L, Wu W, Yun X, Shen A, Gu J. Lectin-like oxidized low-density lipoprotein receptor-1 delivers heat shock protein 60-fused antigen into the MHC class I presentation pathway. J Immunol. 2010;185:2306–2313. doi: 10.4049/jimmunol.0903214. [DOI] [PubMed] [Google Scholar]
- Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, Ding A, Knowles DM, Santini PA, Cerutti A. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30:10–16. doi: 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. α LOX-1 mAb specific for human LOX-1 promotes B cell responses. (A) Mock- (left) and hLOX-1-transfected (right) 293F cells were stained with 1μg/ml αLOX-1 (8B4) or isotype control. (B) hLOX-1-transfected 293 cells were stained with various amounts of αLOX-1 (8B4) or isotype control. Mean fluorescent intensity (MFI) values are plotted. (C) Recombinant proteins, hLOX-1-Fc and hDCIR-Fc, were coated in the plates. Different concentrations of αLOX-1 (8B4) mAb were incubated. The amount of αLOX-1 mAb bound to hLOX-1-Fc or DCIR-Fc proteins were detected using αGoat mouse IgG-HRP. (D) CD11c (green), LOX-1(red) and IgD (blue) expression as well as isotype control staining in human spleen sections. Original magnification, x10. (E) IL-4DCs (5×103/well) were incubated overnight in plates coated with 2μg/ml αLOX-1 (8B4) or control IgG. CFSE-labeled B cells (1×105) were co-cultured with the DCs in the presence of 20 units/ml IL-2. On day 6, cells were stained with αCD19, αCD38, and αCD138. CD138 expression on CD19+CD38+CFSE− cells were assessed. (F) Staining of IFNDCs and TNFDCs with αLOX-1 (8B4) mAb (upper left). B cells were co-cultured with αLOX-1-treated IFNDCs, as in (E). On day 6, cells proliferation and PB differentiation were assessed. (lower left). On day12, culture supernatants were analyzed to measure the amount of Igs by ELISA (right). (G) CFSE-labeled 5×105 PBMCs were cultured for 7 days in plates coated with 2μg/ml αLOX-1 or control IgG. PB differentiation was assessed (left). On day 12, the amounts of Igsin the supernatants were assessed by ELISA. Error bars indicate SD of triplicate assays from two independent experiments.
Figure S2. α LOX-1-treated DCs promote naïve B cell differentiation into Ig-secreting PBs. (A) IL-4DCs (5×103/well) were incubated overnight in plates coated with αLOX-1 (8B4) or control IgG. FACS-sorted and CFSE-labeled naïve B cells (1×105, CD19+IgD+CD27−) were stimulated with αIgM then co-cultured with the DCs in the presence of 20 units/ml IL-2, 50 nM CpG and 100 ng/ml agonistic αCD40 mAb (clone 12E12). On day 6, B cells were stained for HLA-DR. (B) Naïve B cell culture in (A) were performed in the absence or presence of DCs. On day 6, B cells were stained and assessed for PB differentiation. Two independent experiments using cells from different donors showed similar results. (C) Culture supernatants of the DC-B cell co-culture in (A) were harvested on day 12 and the amounts of Igs were measured by ELISA.
Figure S3. αLOX-1 mAb does not induce β7 integrin, CCR6, or CCR9 expression on naïve B cells co-cultured with DCs. IL-4DCs (5×103/well) were incubated overnight in plates coated with 2μg/mlαLOX-1 or control IgG. FACS-sorted and CFSE-labeled naïve B cells (1×105, CD19+IgD+CD27−) were stimulated with αIgM then co-cultured with the DCs in the presence of 20 units/ml IL-2, 50 nM CpG and 100 ng/ml agonisticαCD40 mAb (clone 12E12). On day 6, cells were stained withαCD19 andαCD38 along with indicated antibodies. CD19+CD38+ live cells were gated to assess the surface expression levels of β7 integrin, CCR6, and CCR9.
Figure S4. α LOX-1 (8B4) mAb can induce DCs to secrete APRIL and BAFF and further promotes Ig-secreting B cell responses. (A) 1×105 IL-4DCs were cultured 72h in plates coated with the indicated mAbs (2μg/ml). The amounts of APRIL and BAFF in the supernatants were measured by ELISA. Each dot represents data generated with cells from different healthy donors. (B) IL-4DCs (5×103/well) were incubated overnight in plates coated with 2 μg/ml αLOX-1 (8B4), αDCIR (9E8),αDectin-1 (15E2),αDC-SIGN (24G3), or control IgG. FACS-sorted and CFSE-labeled naïve B cells (1×105, CD19+IgD+CD27−) were stimulated with αIgM then co-cultured with the DCs in the presence of 20 units/ml IL-2, 50 nM CpG, and 100 ng/ml agonisticαCD40 mAb (clone 12E12). Culture supernatants were harvested on day 12 and the amounts of Igs were measured by ELISA. Error bars indicate SD of triplicate assays. Two independent experiments using cells from different healthy donors showed similar results. (C) 1× 105 IL-4DCs were cultured 72h in plates coated with the indicated mAbs (2μg/ml). The amounts of APRIL and BAFF in the supernatants were measured by ELISA. Each dot represents data generated with cells from different healthy donors.
Figure S5. ox-LDL can activate B cells. Purified CD19+B cells (1×105/well) were cultured in the presence or absence of 30μg/ml ox-LDL for 12 days. 20 units/ml IL-2 was added into the culture. Culture supernatants were analyzed to measure the amount of Igs by ELISA. Two independent experiments using cells from different donors were performed. Each experiment was performed with a triplicate assay. Error bars indicate SD.
Figure S6. αLOX-1 mAb binds to rhesus macaque LOX-1 and also binds to the surface of CD11c+ and CD14+ cells, but not CD3+ cells, in the blood of rhesus macaques. (A)αLOX-1 binds to LOX-1 expressed in rhesus macaques. Recombinant proteins, rhesus macaque LOX-1-Fc and DCIR-Fc, were coated in plates. Different concentrations of αLOX-1 (8B4) mAb were incubated. The amount ofαLOX-1 bound to macaque LOX-1-Fc or DCIR-Fc proteins were detected using mouse αGoat IgG labeled with horseradish peroxidase (HRP). (B) Peripheral blood mononuclear cells from the blood of rhesus macaques were stained first withαLOX-1,αDectin-1 and control IgG mAbs. Cells were then stained with PE-labeled goat α Mouse IgG. Cells were finally stained for CD11c, CD14 and CD3 expression. CD11c+, CD14+ and CD3+ cells were gated and the expression levels of LOX-1 and Dectin-1 were assessed.
Figure S7. αLOX-1-HA1 fusion protein can promote B cell differentiation into PBs. IL-4DCs (5×103/well) were incubated overnight with 5μg/mlαLOX-1-HA1 or control IgG-HA1 in the absence (upper) or presence (lower) of 500 ng/ml poly ICLC. Purified and CFSE-labeled total B cells (1×105) were co-cultured with the DCs for 6 days in the presence of 20 units/ml IL-2. PB differentiation was measured.